Abstract
Spinal cord injury (SCI) afflicts hundreds of thousands of Americans, and most SCI (∼80%) occurs in males. In experimental animal models, however, many studies used females. Funding agencies like the National Institutes of Health recommend that new proposed studies should include both genders due to variations in gender response to injuries, diseases, and treatments. However, cost and considerations for some animal models, such as SCI, affect investigators in adapting to this recommendation. Research has increased comparing gender effects in various disease and injury models, including SCI. However, most studies use weight-matched animals, which poses issues in comparing results and outcomes. The present study compared histologic and functional outcomes between age-matched male and female Sprague-Dawley rats in a moderate thoracic contusion SCI model. Cresyl violet and eosin staining showed no significant differences in lesion volume between genders after 9 weeks post-SCI (p > 0.05). Luxol fast blue–stained spared myelin was similar between genders, although slightly greater (∼6%) in spared myelin, compared with cord volume (p = 0.044). Glial reactivity and macrophage labeling in the lesion area was comparable between genders, as well. Basso, Beattie, Bresnahan (BBB) functional scores were not significantly different between genders, and Hargreaves thermal hyperalgesia and Gridwalk sensorimotor analyses also were similar between genders, compared with uninjured gender controls. Analysis of covariance showed weight did not influence functional recovery as assessed through BBB (p = 0.65) or Gridwalk assessment (p = 0.63) in this study. In conclusion, our findings suggest age-matched male and female rats recover similarly in a common clinically relevant SCI model.
Keywords: functional recovery, gender, gender effects, spinal cord injury
Introduction
Nearly 300,000 individuals in the United States have sustained some form of spinal cord injury (SCI), and there are approximately 12,000 new cases per year in the U.S. alone.1 In addition to the mechanical injury caused by physical trauma, further damage to the neurological system proceeds via secondary injury processes. Secondary injury results from inflammatory responses induced by cytokines and chemokines, and is comprised of pathology such as delayed programmed cell death of neurons and glia surrounding and remote to the initial site of injury. Although the inflammatory response may have some anatomical benefits,2,3 it is mostly characterized by promotion of apoptosis, necrosis, and scar tissue formation, which can be detrimental to functional recovery.4,5
A recently published article discussed the future implementation of guidelines for oversight for pre-clinical research intended to limit gender bias in both in vivo and in vitro models.6 Significant progress in the reduction of gender bias in clinical studies has been achieved; however, concerns remain regarding basic research and specifically the common practices of using a single gender for animal models or failing to report gender of host subjects for in vitro studies. The National Institutes of Health has demonstrated commitment by providing $10.1 million in additional funding for researchers wanting to explore the effects of gender in both clinical and pre-clinical studies.7 The impending need to provide proper justification for single-gender studies, as well as the efforts already being exerted concerning government funding, has prompted an increase in gender-associated studies.
The gender bias found in many pre-clinical and clinical SCI studies is likely due to the fact that men account for around 80% of SCI cases.1 Some studies have shown that females exhibit better recovery in certain measured outcomes,8,9 while others suggest that there is no difference between genders.10,11 Evidence suggests that sex hormones may be responsible for differences in the magnitude of the immune response and immune cell activity, and thus the impact on secondary injury progression following SCI.12,13 In light of this multitude of issues surrounding gender-related outcomes in SCI research, the present study aimed to identify whether gender influences functional outcome in widely utilized functional assessments, and lesion and pathology expansion in a thoracic contusive SCI model in age-matched Sprague-Dawley (SD) rats.
Methods
Animals and surgery
Adult male (250-300 g; Harlan) and female SD rats (200-250 g; Harlan; n = 26) were housed in controlled conditions with a 12:12 light:dark schedule, and ad libitum access to food and water. Prior to surgery, the animals were anesthetized with ketamine (40 mg/kg)/xylazine (5 mg/kg, intraperitoneally), and inflicted with either a laminectomy only or a moderate midline contusive SCI as described previously.14,15 Briefly, a customized vertebral stabilizer16 was set to support the 10th thoracic vertebrae (T10) and a laminectomy was performed to expose the spinal cord surface. With dura intact, the NYU/MASCIS Impactor17 (2.5 mm tip, 10 g weight) dropped from a height of 12.5 mm was used to inflict a moderate bilateral injury. Parameters of displacement and velocity ensuring spinal cord contusion using the NYU/MASCIS impactor are shown in Figure 1. Sham-operated animals received laminectomy only. Following surgery, animals received an injection of 3 mL 0.9% saline subcutaneously for hydration and were placed in temperature-controlled housing overnight for monitoring recovery. All surgical and animal handling procedures were performed as approved under the Guide for the Care and Use of Laboratory Animals (National Research Council) and the Guidelines of the Indiana University School of Medicine Institutional Animal Care and Use Committee.
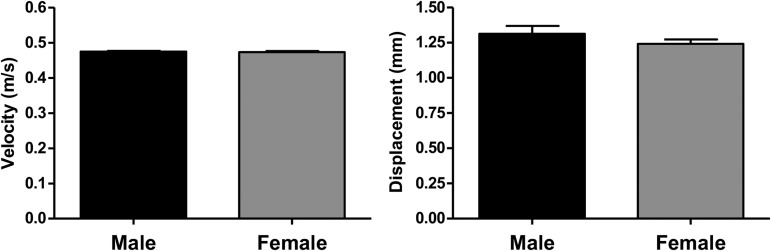
FIG. 1.
Male and female spinal cord injury (SCI) impact data from the NYU/MASCIS impactor. During spinal cord contusion impact, males and females showed similar average velocity values at cord impact (A; p > 0.05) and spinal cord displacement values (B; p > 0.05). In addition, little variation in values within groups was observed for these parameters. These findings indicate that contusions were consistent within and between groups. Sham: n = 5/gender/group. SCI: n = 8/gender/group.
Behavioral assessments
To assess long-term functional recovery or deficit differences and similarities between age-matched male and female SD rats post-SCI, animals were divided into four groups: male sham (n = 5), male SCI (n = 8), female sham (n = 5), and female SCI (n = 8). We conducted behavioral tests including the Basso, Beattie, Bresnahan (BBB)18 locomotor rating scale, Hargreaves test of nociceptive thermal sensation of the hindpaws,19 and Gridwalk assessments of sensorimotor coordination for 9 weeks post-SCI. Animals were weighed weekly through Week 6 of the study (Fig. 2). At the end of the study, all animals were sacrificed, their spinal cords harvested, and tissues prepared for histologic analysis.
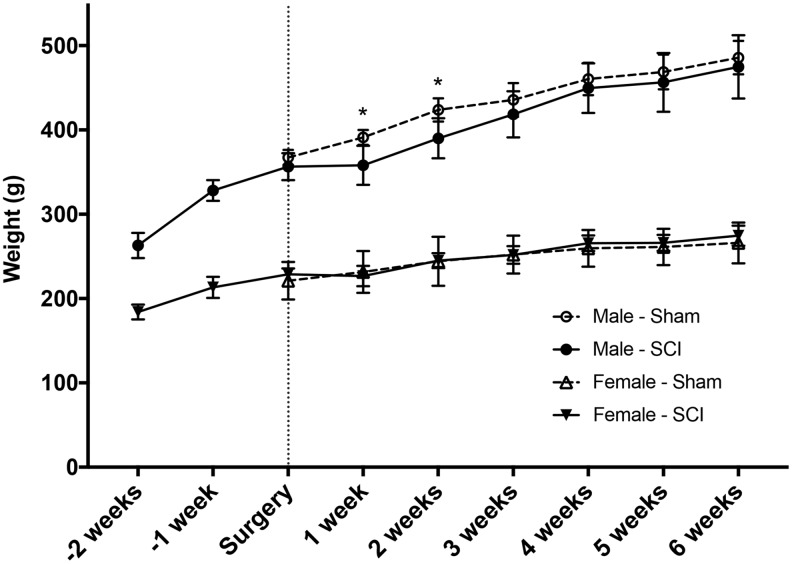
FIG. 2.
Male and female weight changes before and after spinal cord injury (SCI). Males weighed more at the start of the study (7 weeks old, ∼260 g) compared with females (7 weeks old, ∼180 g). Males continued to increase in weight at a greater rate than females until SCI at 9 weeks of age. For the first 2 weeks following SCI, males dropped a small but significant percentage of weight (p < 0.05) and recovered it compared with uninjured males by Week 3 post-injury. By comparison, female rats did not lose any weight following SCI with a similar growth trend as uninjured female rats until Week 6 post-SCI. *p < 0.05 compared with male SCI rats. Sham: n = 5/gender/group. SCI: n = 8/gender/group.
BBB locomotor rating scale
Hindlimb locomotor functional recovery was assessed using the BBB locomotor rating scale.18 BBB testing was performed prior to surgery as a baseline measurement, and was assessed at 2 days and weekly thereafter until 9 weeks following SCI. Briefly, all rats were allowed to roam freely in a 42-inch diameter open field and observed for 4 min by two trained individuals blinded to the experimental groups. The scores determined for the rats during observation ranged from 0 (no hindlimb movement) to 21 (normal locomotor function) based on overall hindlimb movement, joint movement, stepping, coordinated locomotion, and other factors.
Gridwalk analysis
Gridwalk analysis was performed to assess sensorimotor function as previously described.20–22 For this test, rats were allowed to freely navigate over a 3 foot × 3 foot plastic mesh grid with 4.5 × 5.0 cm spaces. The rats were required to walk for a minimum of 30 sec up to 3 min maximum for the testing period. All groups were tested at Weeks 2, 4, 6, and 9 post-SCI. For this assessment, rats were observed by two individuals blind to the experimental groups and the total number of hindlimb footfalls were documented. One footfall was defined as a drop of the hindlimb through a space (including the ankle joint) below the level of the grid.
Hargreaves test
At 2, 4, 6, and 9 weeks post-SCI or sham operation, thermal hindpaw sensation was measured in the rats using a plantar heat testing device (IITC Life Sciences, Inc., Woodland, CA) as described by Hargreaves and colleagues.19 Briefly, the rats were placed in clear rectangular Plexiglas containers on a transparent glass plate. After allowing the rats to acclimate to the environment for 10 min, the plantar surface of the hindpaw was exposed to a noxious infrared light beam, and latency in paw withdrawal was recorded in seconds. To avoid injury to the paw tissue, the light stimulus had a maximum exposure time of 30 sec before automatic shut off. Five trials, with a minimum 1-min interval between trials, were performed for both right and left hindpaws of each rat. The highest and lowest latency score for each paw were discarded to minimize variability caused by initial and final stimulus exposures and the remaining middle three scores averaged for one mean latency score per paw per animal at each time-point.
Histological analyses
We used cresyl violet–eosin stain to quantify spinal cord lesion volume, and Luxol fast blue to determine spared myelin volume surrounding the lesion in the injured cord according to previously published methods.23,24
Immunofluorescence labeling and analysis of astrocyte, microglia, and macrophage response
Immunofluorescence labeling of lesioned spinal cord tissue was performed as previously described.23,24 In brief, a section at the epicenter and 1.5 mm rostral and caudal to the epicenter were chosen per animal and adjacent serial sections were selected for each cellular immunolabeling procedure. For immunofluorescence labeling, sections were hydrated in 0.1 M phosphate-buffered saline, permeabilized with PBS +0.1% TritonX-100 (PBST; Sigma-Aldrich, St. Louis, MO), and blocked with PBST +10% normal goat serum (blocking buffer) to inhibit non-specific antibody labeling. Then, sections spanning the lesion epicenter was incubated with one of the following primary antibodies in blocking buffer overnight at 4°C: chicken anti-glial fibrillary acidic protein (GFAP; 1:1000; Aves Inc., Tigard, OR) to label reactive astrocytes, rabbit anti-ionized calcium-binding adapter molecule 1 (Iba1; 1:500; Wako, Japan) to label reactive microglia, and mouse anti-ED1 (1:500, Millipore) to label macrophages. The next day, the tissues were washed and incubated with respective fluorophore-conjugated goat-anti chicken, rabbit, or mouse secondary antibodies (1:200; Jackson Immunoresearch, West Grove, PA) for 1 h at room temperature. The sections were then washed and mounted with Prolong Gold Anti-Fade mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes, Carlsbad, CA).
For quantifying GFAP and Iba1 labeling, the intensity of labeling of the selected sections was measured using Image J software (National Institutes of Health [NIH], Bethesda, MD). In brief, a section at the epicenter, one section 1.5 mm rostral and one 1.5 mm caudal to the epicenter were selected for immunolabeling intensity measurement. Immunofluorescent images of the selected sections were acquired at equal exposure settings for each animal. To measure immunolabeling intensity, the labeled tissue section was outlined in ImageJ, converted to grayscale and mean gray value measured in the selected region of interest (the outlined tissue section). The values of all animals per gender at each location were averaged and graphed in GraphPad Prism 7.0 software (GraphPad Software Inc., La Jolla, CA). For quantifying ED1 labeling of macrophages, ED1+ area relative to the spinal cord section area was quantified with Image J. Briefly, selection of ED1-labeled tissue sections followed the protocol described for GFAP and Iba1 labeling. To quantify ED1 labeling, we measured percent ED1+ labeled area relative to the outlined tissue section area by setting threshold values (equivalent for all selected sections) and measuring all positive-labeled areas. Image J provided percent ED1+ area per tissue section. Percent ED1+ area per group and location were then averaged and graphed using GraphPad Prism 7.0 software. All images were taken on an Olympus BX60 epifluorescent microscope and Neurolucida software (MBF Bioscience, Williston, VT).
Statistical analysis
All data are represented as mean ± standard error of the mean. Histological data between Sham and SCI injured groups were assessed via Student's t-test. Behavioral data were analyzed using repeated measures two-way analysis of variance with Tukey post hoc test. To determine if weight influenced functional recovery scores, an analysis of covariance (ANCOVA) was performed using BBB scores as the dependent variable. Statistical significance was determined when p < 0.05. Most data were analyzed using Graph Pad Prism 7.0 software (GraphPad Software Inc.), although ANCOVA of weight influence on BBB score outcomes was performed using XLStat software (Addinsoft, New York, NY).
Results
Weight changes by gender following SCI
No significant difference in impact velocity or displacement was found for the male and female groups (Fig. 1; p > 0.05), indicating that NYU/MASCIS impactor produced a consistent moderate contusive SCI in adult rats in both males and females. Figure 2 shows the starting weights of both gender groups at 7 weeks of age (−2 weeks) and weekly thereafter until Week 6 post-SCI. Surgery was performed when the animals were 9 weeks of age (indicated by the vertical dashed line). Males started the study weighing more than females (263.0 ± 14.9 g vs. 184.0 ± 8.7 g), and this trend continued throughout the study, with both males and females increasing in weight. One week post-surgery, males that received T10 SCI weighed significantly less than Sham operated males (Sham 391.2 ± 8.7 g vs. SCI 358.0 ± 8.7 g; p = 0.003). This difference in male weights also was observed 2 weeks post-surgery (Sham 423.8 ± 13.8 vs. SCI 390.1 ± 23.7g; p = 0.003), although no difference was observed between sham-operated and SCI animals in both male and female groups from this point onward.
Lesion and spared myelin area were similar between genders post-SCI
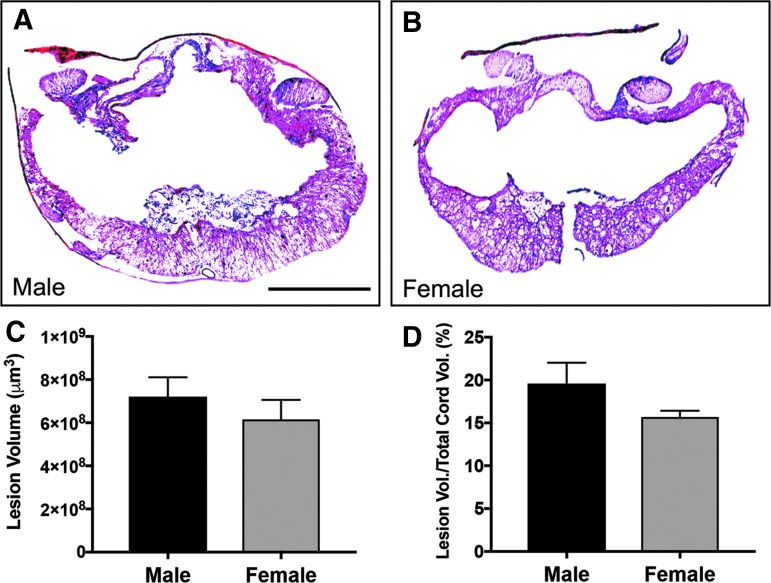
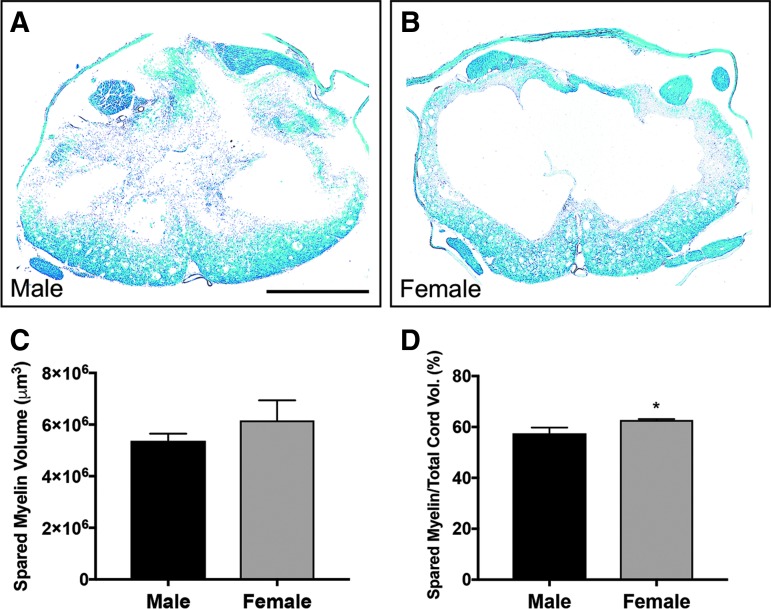
Following quantification of lesion volume using cresyl violet–eosin staining, we found no significant difference in total or relative lesion volume between genders (Fig. 3; p > 0.05). Likewise, total spared myelin volume surrounding the lesion was also not significantly different between male and female groups (p > 0.05), though spared myelin volume relative to cord area was approximately 6% greater in females than males (p = 0.044; Fig. 4).
FIG. 3.
Male and female lesion volumes following spinal cord injury (SCI). Representative images show cross sections of the injury epicenter, stained with cresyl violet–eosin, in male (A) and female (B) rats. Males and female lesion volumes were statistically similar (p > 0.05) at the end of the study both in absolute area (C) and relative to total cord area (D). Sham: n = 5/gender/group. SCI: n = 8/gender/group. Scale bar = 250 μm.
FIG. 4.
Male and female spared myelin volume following spinal cord injury (SCI). Representative images show cross sections of the injury epicenter, stained with Luxol fast blue, in male (A) and female (B) rats. Spared myelin volume values between male and female rats were statistically similar in absolute spared area (C). However, relative to total cord area, female spared myelin was marginally significantly greater (6%; p = 0.044; D) at the end of the study. Sham: n = 5/gender/group. SCI: n = 8/gender/group. Scale bar = 250 μm.
Glial reactivity and macrophage labeling between genders after injury
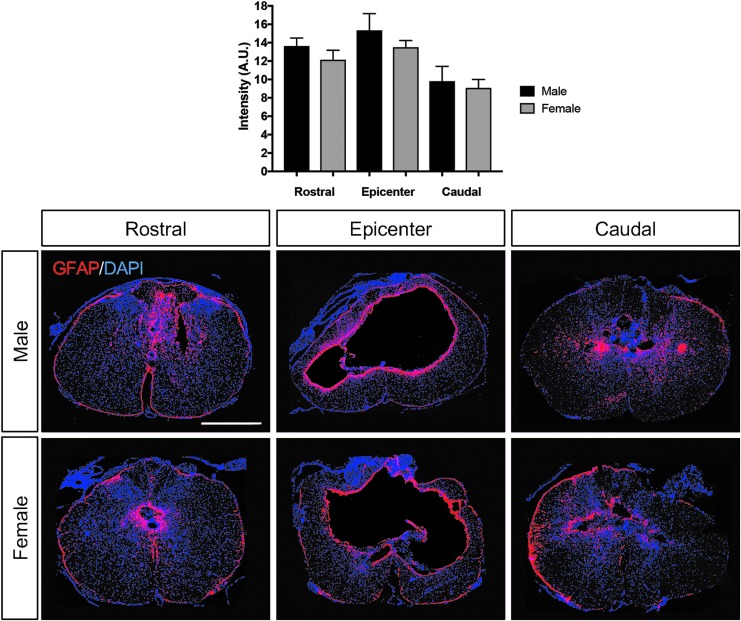
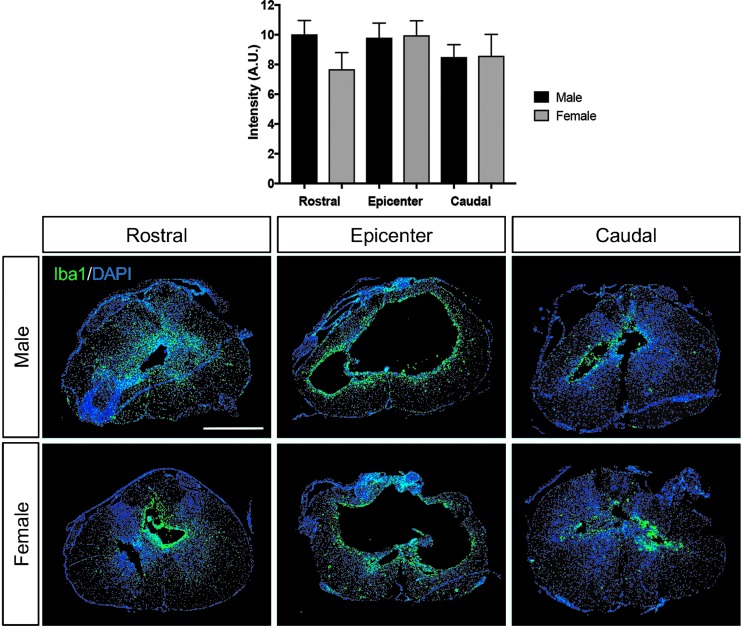
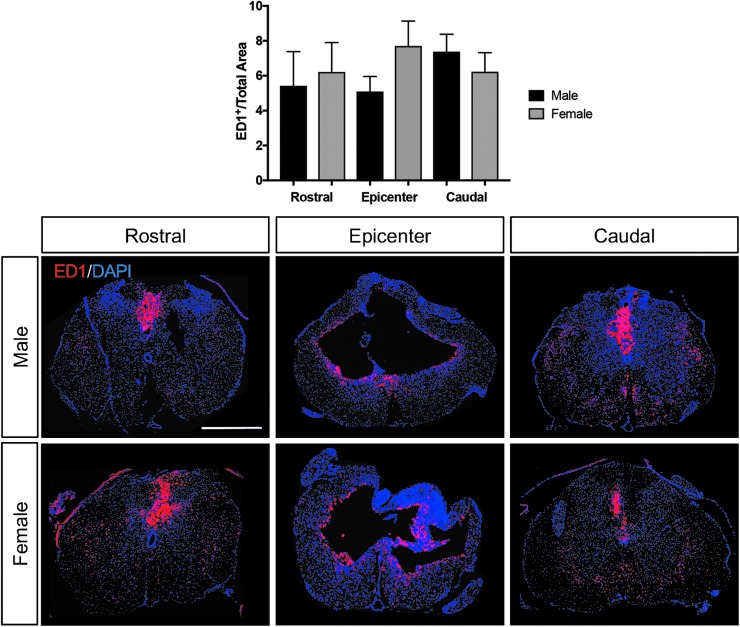
Astrocyte reactivity and influences on tissue sparing have been reported to be influenced by estrogen and estrogen receptor–mediated signaling in astrocytes following SCI.25 In addition, estrogens are suggested to have anti-inflammatory properties in central nervous system (CNS) damage, in part through microglia and related responses.26 To assess whether gender contributed to differences in long-term effects on astrogliosis, microglial reactivity, or macrophage response, GFAP and Iba1 reactivity intensity and ED1+ macrophage labeling were quantified at the epicenter and rostral and caudal to the lesion epicenter of the spinal cord in all animals at the end of the study. No significant differences were documented in GFAP intensity between genders (Fig. 5). Likewise, no major significant differences were observed in microglial reactivity (Fig. 6) or macrophage labeling (Fig. 7) through our measurements in this study.
FIG. 5.
Astrocyte reactivity in male and female rats post-SCI. Through glial fibrillary acidic protein immunofluorescent labeling, no considerable differences in astrogliosis was observed between males and females 1.5 mm rostral, at the lesion epicenter and 1.5 mm caudal to the epicenter. Scale bar = 250 μm.
FIG. 6.
Microglial reactivity in male and female rats post–spinal cord injury. As determined through assessment of ionized calcium-binding adapter molecule 1 immunofluorescence intensity, no significant differences in microglial reactivity were observed rostral, at the epicenter or caudal to the epicenter between male and female rats following T10 SCI. Scale bar = 250 μm.
FIG. 7.
Male and female macrophage spinal cord labeling after spinal cord injury (SCI). As observed for ionized calcium-binding adapter molecule 1 analysis, the amount of ED1+ area labeled rostral, at the epicenter and caudal to the lesion epicenter was not significantly different between male and female rats post-SCI. Scale bar = 250 μm.
Progression of functional deficit and recovery was not significantly different between genders following SCI
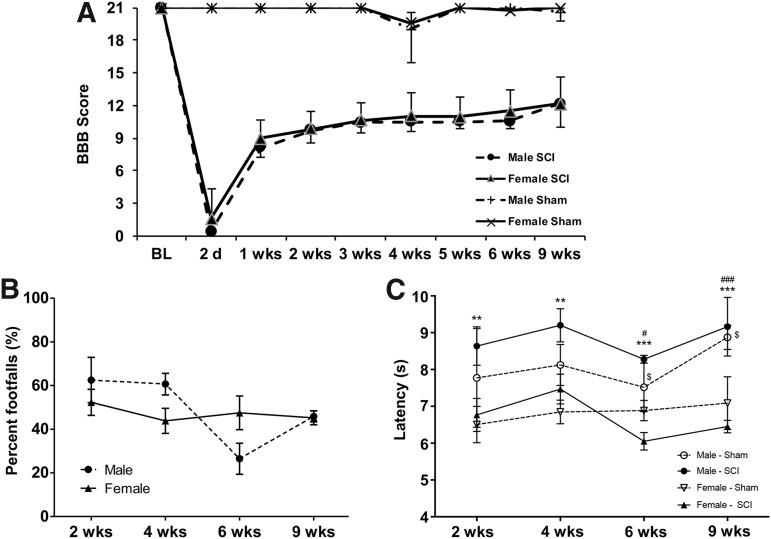
No differences in recovery post-SCI between male and female rats in BBB locomotor rating scales (Fig. 8A) and Gridwalk footfall errors (Fig. 8B) were observed. In general, sham males exhibited increased Hargreaves thermal response latency compared with sham females (p < 0.05 per time-point, except Week 6). Likewise, males with T10 SCI exhibited greater latency in response at all time-points compared with female rats with SCI (p < 0.01). Neither gender showed significant differences in latency response compared with their gender-specific sham counterparts at any time-point (Fig. 8C).
FIG. 8.
Functional assessments of male and female rats following spinal cord injury (SCI). Both male and female SCI rats responded similarly to SCI and showed a similar rate and extent of locomotor recovery throughout the course of the study with a maximum score of ∼12 on the Basso, Beattie, Bresnahan scale (A). In addition, rats of both genders performed similarly between 2 weeks and 9 weeks post-SCI in the Gridwalk assessment, demonstrating persisting sensorimotor deficits at the same level for both genders after SCI (B). Male rats exhibited significant thermal hyperalgesia latency response during the Hargreaves test of the hindpaws at 2, 4, 6, and 9 weeks post-SCI, compared with female SCI rats. Male SCI rats did not exhibit significant differences in response compared with sham-operated male rats. Female SCI rats responded with similar latency to uninjured female rats at these time-points, as well. In general, males with and without SCI responded with significantly greater latency of hindpaw response to thermal stimulus compared with both female Sham and SCI rats (p < 0.05). At Weeks 6 and 9, male Sham rats responded more slowly than female SCI rats (p < 0.05). Also at these time-points, male SCI rats exhibited slower thermal response than female Sham rats (C). **p < 0.01 vs. female SCI rats; ***p < 0.001 vs. female SCI rats; #p < 0.05 vs. female Sham rats; ###p < 0.001 vs. female Sham rats; $p < 0.05 vs. female SCI rats. Sham: n = 5/gender/group. SCI: n = 8/gender/group.
Age-matched gender differences in weight did not influence spontaneous functional recovery outcomes post-SCI
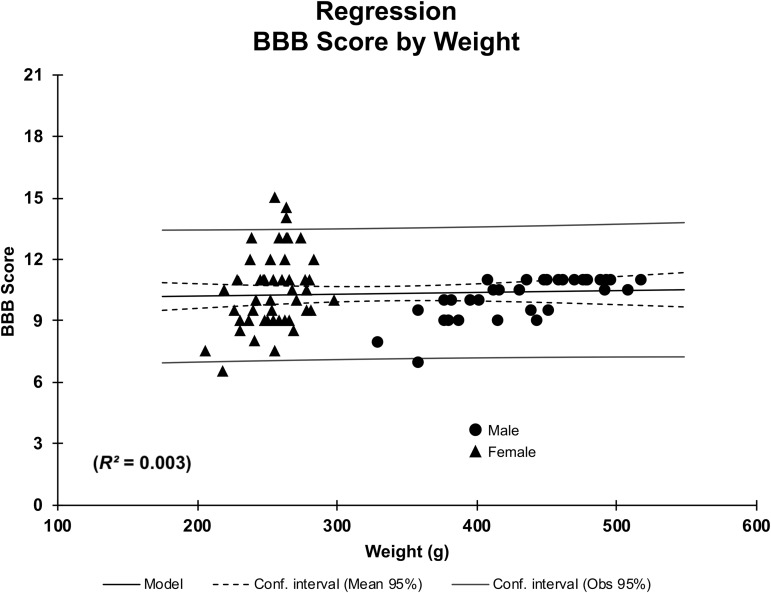
As described, age-matched males began the study weighing approximately 60 g more than females and this difference increased throughout the study. To address this potential influencing factor in functional outcome, ANCOVA analysis of the probability of weight differences contributing to BBB score outcomes throughout the study was performed. BBB assessment was the most robust test of functional recovery that correlated with weighing periods of the rats. Based on this analysis, weight was not found to be a significant factor in contributing to BBB scores in this study (p = 0.65). Regression analysis further highlighted this lack of influence (R2 = 0.003; Fig. 9). By comparison, corresponding with the overall response results in Figure 8C, gender and weight both significantly contributed to the latency of response to thermal stimulus (p < 0.001, R2 = 0.942). Lastly, the effect of weight on Gridwalk performance over time also was assessed using ANCOVA. Like, BBB scores, weight did not significantly contribute to footfall outcomes (p = 0.063), and there was no correlation (R2 = 0.01) between weight and Gridwalk performance, as determined through regression analysis.
FIG. 9.
Regression analysis of weight and Basso, Beattie, Bresnahan (BBB) scores in male and female rats following spinal cord injury (SCI). Following analysis of covariance, weight was determined not to have influenced BBB scores in male and female rats after T10 contusive SCI (p = 0.65). Regression analysis also showed no correlation between weight and BBB scores in the study (R2 = 0.003).
Discussion
Rodents are the most prevalent animals used in experimental SCI studies. Most studies historically have used animals of a single-gender, but some studies have recently used two genders to gather comparative information. A few of these studies have reported that female rodents have significantly more spared tissue post-SCI.9,12 Studies that utilize both male and female subjects are posed with a difficult choice to pair experimental groups by age or by weight. Weight-matching results in younger males and older females, possibly skewing data; while age-matching results in a weight/size discrepancy, but may be developmentally more comparable.27 Our study compared age-matched male and female rats through common behavioral and histological quantifications in order to explore the significance of gender in these experimental parameters. We believe that age matching is a more useful method to study gender-differences.
Comparison of spinal cord impact data
The impact data collected by the NYU/MASCIS device showed no significant difference between the velocity or displacement of the impactor tip for males and females (Fig. 1). This confirms that our impact was consistent across trials, and may suggest that aspects such as difference in cord volume and elasticity between genders had no discernable effect on the resulting velocity or displacement. Most other studies do not include such consistent results for SCI impacts and this consistent data highlights the quality of technique in this study and strengthens the comparison of data between the groups.
Lesion area and demyelination were similar between male and female rats
We used histological analysis to determine lesion area and area of spared myelin. Female rats exhibited a lesser but insignificant average lesion volume, but did show a minor significant increase in relative spared myelin volume. Despite this increase, this effect was not evident in outcomes of other measures in the study. We also compared the lesion volume between male and female rats. As expected, both genders had decreased cord volume after injury. Unsurprisingly, male rats tended to have larger calculated cord volume than females, but both genders showed a similar decrease in cord volume after injury relative to the uninjured cord (data not shown). The histologic data presented here indicate that, though there is a slight significant increase in spared myelin in females compared with males, there were no major significant differences between genders in terms of spinal cord damage and spared tissue in our rat model of thoracic contusive SCI.
Datto and colleagues28 reported a significant difference in the amount of spared gray and white matter between male and female Fischer rats. Although the data is compelling due to the large sample size, the sectioning and staining techniques used in the study may not be optimal for lesion quantification. Our cross-section technique gives a clear image that maintains the shape and proportion of the cord, which lends itself to accurate lesion volume construction of serial sections, in our opinion. Our data suggests that there is no difference in relative lesion volume between male and female rats. It cannot be discounted that our results may be due to a difference in lesion quantification techniques or due to differences in breed of rat. Fischer rats are inbred rats while Sprague-Dawley rats used in the present study are outbred. Genetic influences via strain differences are known to contribute to behavioral traits,29 immune and autoimmune responses,30–32 nervous system response to chemical toxicity,33 among other characteristics between rodent strains.
In assessing immunofluorescent labeling of astrogliosis, microglial reactivity and macrophage response, we observed no major differences between males in females. Nevertheless, as estrogen-mediated reduction in glial reactivity has been documented and this could increase spared white matter,25 the slightly increased spared relative myelinated tissue volume in females may be associated with this finding.
Behavioral outcomes showed no significant differences between genders
Our BBB data showed no significant difference between genders in both SCI and Sham groups. The SCI groups showed a maximum BBB score of around 12 at 9 weeks post-injury, confirming the desired injury severity. Large multi-center studies suggest that women and men have different levels of recovery or no difference in recovery based on functional independence measurement scores after SCI.8,11 Age at injury, level and type of recovery, and timing of recovery all must be taken into consideration when determining gender-based influences on outcomes following SCI. In our age-matched comparison of male and female rats, no significant locomotor recovery differences were observed. Other studies that show differences in locomotor recovery between male and female animals following SCI are often weight-matched. As such, older females and younger males may be compared with each other, which could involve factors such as hormonal levels and strength that impact the functional outcomes assessed in such studies. By age-matching, we believe we reduced the risk of confounding factors; however, as is evident by the comparison in progressive weight increases over time between males and females (Fig. 2) in this study, we cannot avoid all physiologic influences using an age-matched approach. In addressing the influence of weight differences on functional outcomes, we found that weight was not a significant contributing factor to BBB score outcomes in the present study (Fig. 9).
Concerning our sensory assessment of response to thermal paw stimulus, despite the clearly observed separation of male and female latencies of response, it is noteworthy that no differences were seen in paw withdrawal latencies in either gender compared with non-injured controls, as at least 40% of all SCI patients experience some type of chronic pain.34,35 Our Hargreaves assessment findings revealed that the male rats had a greater latency than females for both SCI and sham groups, which may be simply due to physiologic differences in size of the nerve (associated with increased size and weight of males over females) in this study. However, these findings also agree with clinical evidence that women exhibit higher prevalence of nociceptive pain following SCI,36 which may be attributed to the stage of estrus the females are in during testing.37 Estrogen and estradiol have demonstrated neuroprotective effects,37–40 and the ability to diminish autonomic dysfunction post-SCI.41 However, benefits are not limited to female sex hormones. Testosterone also has shown neuroprotection42,43 and functional benefits44 following SCI.
Although our data suggest that while baseline latency is higher in males than females, SCI had no effect on the comparative difference across genders.45 This does, however, contrast with recent research indicating moderate T9 SCI elicits thermal hyperalgesia using the Hargreaves test in both male and female rats.46 However, differences in impactor system used (NYU MASCIS in this study and the Infinite Horizons Impactor in the other) or other factors, including stage of estrus in females were not specifically addressed in either study. Otherwise, the pattern and extent of BBB locomotor recovery for both genders was similar to that observed in the present study, which indicates the sensory differences observed between genders by Gaudet and colleagues46 did not negatively affect motor function.
Our Gridwalk analysis also showed no significant difference between male and female functional recovery post-injury. This was consistent with Datto and colleagues, where despite presenting a significant difference in BBB scores, there was no difference in many of their behavioral assessments, including Gridwalk performance.28 The behavioral analyses support our hypothesis that the mechanical and secondary injury does not significantly differ resulting in similar outcomes in functional recovery post-SCI between male and female rats. Though we cannot reconcile our lack of pain response differences between male and female rats with other studies, we conclude that in aggregate, our findings provide compelling evidence for more similarities than differences between age-matched male and female Sprague-Dawley rats following thoracic SCI.
Conclusions
Our data provide evidence that single sex studies in Sprague-Dawley rats may be appropriate for similar spinal cord injury studies. Should a study use any pharmaceutical or procedures that interact uniquely with sex hormone production or receptors, then it follows that this should be tested in both male and female subjects. Male and female rats show no significant difference in terms of pathology following a moderate T10 midline injury according to accepted histological and behavioral analyses. In light of the continued efforts by funding agencies such as the NIH to promote use of both genders and the described need to examine therapeutic effects on both genders in experimental studies, the use of both male and female animals in research will remain an area of emphasis. However, male aggression and subsequent housing needs and complications that can arise with bladder expression in males in most SCI models are issues that require consideration in this area of research. In our age-matched study presented here, no clear evidence was revealed to suggest that using both genders is necessary for lower-thoracic contusive SCI research. Nevertheless, male and female animals are physiologically different and further research is necessary to determine if specific genders or both are required for studies assessing particular aspects of SCI not investigated in the present study.
Acknowledgments
We thank Ms. Qingbo Lu, Mr. Matthew Hamilton, and Mr. Alex Gianaris for their excellent technical assistance.
Funding: This work was supported in part by NIH R01 NS103481, NS100531, Merit Review Award I01 BX003705, I01 BX002356 from the U.S. Department of Veterans Affairs, Craig H. Neilsen Foundation #296749, Indiana State Department of Health #019919, Indiana Spinal Cord and Brain Injury Research Foundation, and Mari Hulman George Endowment Funds (XMX).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. National Spinal Cord Injury Statistical Center. (2011). Facts and figures at a glance. https://www.nscisc.uab.edu/PublicDocuments/fact_figures_docs/Facts%202012%20Feb%20Final.pdf (Last accessed December22, 2018)
- 2. David S. and Kroner A. (2011). Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 12, 388–399 [DOI] [PubMed] [Google Scholar]
- 3. Kigerl K.A., Gensel J.C., Ankeny D.P., Alexander J.K., Donnelly D.J., and Popovich P.G. (2009). Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29, 13435–13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. David S., Lopez-Vales R., and Wee Yong V. (2012). Harmful and beneficial effects of inflammation after spinal cord injury: potential therapeutic implications. Handb. Clin. Neurol. 109, 485–502 [DOI] [PubMed] [Google Scholar]
- 5. Hausmann O.N. (2003). Post-traumatic inflammation following spinal cord injury. Spinal cord 41, 369–378 [DOI] [PubMed] [Google Scholar]
- 6. Clayton J.A. and Collins F.S. (2014). Policy: NIH to balance sex in cell and animal studies. Nature 509, 282–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Institutes of Health. (2014). News Release. New supplemental awards apply sex and gender lens to NIH-funded research: Investment sets the stage for a transformative shift in science. https://www.nih.gov/news-events/news-releases/new-supplemental-awards-apply-sex-gender-lens-nih-funded-research (Last accessed December22, 2018
- 8. Sipski M.L., Jackson A.B., Gomez-Marin O., Estores I., and Stein A. (2004). Effects of gender on neurologic and functional recovery after spinal cord injury. Arch. Phys. Med. Rehabil. 85, 1826–1836 [DOI] [PubMed] [Google Scholar]
- 9. Farooque M., Suo Z., Arnold P.M., Wulser M.J., Chou C.T., Vancura R.W., Fowler S., and Festoff B.W. (2006). Gender-related differences in recovery of locomotor function after spinal cord injury in mice. Spinal cord 44, 182–187 [DOI] [PubMed] [Google Scholar]
- 10. Furlan J.C., Krassioukov A.V., and Fehlings M.G. (2005). The effects of gender on clinical and neurological outcomes after acute cervical spinal cord injury. J. Neurotrauma 22, 368–381 [DOI] [PubMed] [Google Scholar]
- 11. Greenwald B.D., Seel R.T., Cifu D.X., and Shah A.N. (2001). Gender-related differences in acute rehabilitation lengths of stay, charges, and functional outcomes for a matched sample with spinal cord injury: a multicenter investigation. Arch. Phys. Med. Rehabil. 82, 1181–1187 [DOI] [PubMed] [Google Scholar]
- 12. Hauben E., Mizrahi T., Agranov E., and Schwartz M. (2002). Sexual dimorphism in the spontaneous recovery from spinal cord injury: a gender gap in beneficial autoimmunity? Eur. J. neurosci. 16, 1731–1740 [DOI] [PubMed] [Google Scholar]
- 13. Garcia-Ovejero D., Gonzalez S., Paniagua-Torija B., Lima A., Molina-Holgado E., De Nicola A.F., and Labombarda F. (2014). Progesterone reduces secondary damage, preserves white matter, and improves locomotor outcome after spinal cord contusion. J. Neurotrauma 31, 857–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gensel J.C., Tovar C.A., Hamers F.P., Deibert R.J., Beattie M.S., and Bresnahan J.C. (2006). Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J. Neurotrauma 23, 36–54 [DOI] [PubMed] [Google Scholar]
- 15. Basso D.M., Beattie M.S., and Bresnahan J.C. (1996). Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 139, 244–256 [DOI] [PubMed] [Google Scholar]
- 16. Walker M.J., Walker C.L., Zhang Y.P., Shields L.B., Shields C.B., and Xu X.M. (2015). A novel vertebral stabilization method for producing contusive spinal cord injury. J. Vis. Exp. e50149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gruner J.A. (1992). A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma 9, 123–128 [DOI] [PubMed] [Google Scholar]
- 18. Basso D.M., Beattie M.S., and Bresnahan J.C. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 [DOI] [PubMed] [Google Scholar]
- 19. Hargreaves K., Dubner R., Brown F., Flores C., and Joris J. (1988). A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88 [DOI] [PubMed] [Google Scholar]
- 20. Hu J.G., Wang X.F., Deng L.X., Liu N.K., Gao X., Chen J.H., Zhou F.C., and Xu X.M. (2013). Cotransplantation of glial restricted precursor cells and Schwann cells promotes functional recovery after spinal cord injury. Cell Transplant 22, 2219–2236 [DOI] [PubMed] [Google Scholar]
- 21. Behrmann D.L., Bresnahan J.C., Beattie M.S., and Shah B.R. (1992). Spinal cord injury produced by consistent mechanical displacement of the cord in rats: behavioral and histologic analysis. J. Neurotrauma 9, 197–217 [DOI] [PubMed] [Google Scholar]
- 22. Gianaris A., Liu N.K., Wang X.F., Oakes E., Brenia J., Gianaris T., Ruan Y., Deng L.X., Goetz M., Vega-Alvarez S., Lu Q.B., Shi R., and Xu X.M. (2016). Unilateral microinjection of acrolein into thoracic spinal cord produces acute and chronic injury and functional deficits. Neuroscience 326, 84–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu N.K., Zhang Y.P., Titsworth W.L., Jiang X., Han S., Lu P.H., Shields C.B., and Xu X.M. (2006). A novel role of phospholipase A2 in mediating spinal cord secondary injury. Ann. Neurol. 59, 606–619 [DOI] [PubMed] [Google Scholar]
- 24. Walker C.L. and Xu X.M. (2014). PTEN inhibitor bisperoxovanadium protects oligodendrocytes and myelin and prevents neuronal atrophy in adult rats following cervical hemicontusive spinal cord injury. Neurosci. Lett. 573, 64–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spence R.D., Hamby M.E., Umeda E., Itoh N., Du S., Wisdom A.J., Cao Y., Bondar G., Lam J., Ao Y., Sandoval F., Suriany S., Sofroniew M.V., and Voskuhl R.R. (2011). Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc. Natl. Acad. Sci. U. S. A. 108, 8867–8872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Villa A., Gelosa P., Castiglioni L., Cimino M., Rizzi N., Pepe G., Lolli F., Marcello E., Sironi L., Vegeto E., and Maggi A. (2018). Sex-Specific Features of Microglia from Adult Mice. Cell. Rep. 23, 3501–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan W.M., Mohammed Y., Lee I., and Pearse D.D. (2013). Effect of gender on recovery after spinal cord injury. Transl. stroke res. 4, 447–461 [DOI] [PubMed] [Google Scholar]
- 28. Datto J.P., Bastidas J.C., Miller N.L., Shah A.K., Arheart K.L., Marcillo A.E., Dietrich W.D., and Pearse D.D. (2015). Female rats demonstrate improved locomotor recovery and greater preservation of white and gray matter after traumatic spinal cord injury compared to males. J. Neurotrauma 32, 1146–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yilmazer-Hanke D.M. (2008). Morphological correlates of emotional and cognitive behaviour: insights from studies on inbred and outbred rodent strains and their crosses. Behav. Pharmacol. 19, 403–434 [DOI] [PubMed] [Google Scholar]
- 30. Zdravkovic N., Shahin A., Arsenijevic N., Lukic M.L., and Mensah-Brown E.P. (2009). Regulatory T cells and ST2 signaling control diabetes induction with multiple low doses of streptozotocin. Mol. Immunol. 47, 28–36 [DOI] [PubMed] [Google Scholar]
- 31. Stanisavljevic S., Dedovic N., Vujicic M., Saksida T., Jevtic B., Milovanovic B., Momcilovic M., Miljkovic D., and Stojanovic I. (2017). Strain-specific helper T cell profile in the gut-associated lymphoid tissue. Immunol. Lett. 190, 282–288 [DOI] [PubMed] [Google Scholar]
- 32. Fournie G.J., Cautain B., Xystrakis E., Damoiseaux J., Mas M., Lagrange D., Bernard I., Subra J.F., Pelletier L., Druet P., and Saoudi A. (2001). Cellular and genetic factors involved in the difference between Brown Norway and Lewis rats to develop respectively type-2 and type-1 immune-mediated diseases. Immunol. Rev. 184, 145–160 [DOI] [PubMed] [Google Scholar]
- 33. Yu Q., Hui R., Park J., Huang Y., Kusnecov A.W., Dreyfus C.F., and Zhou R. (2017). Strain differences in cuprizone induced demyelination. Cell. Biosci. 7, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Siddall P.J., McClelland J.M., Rutkowski S.B., and Cousins M.J. (2003). A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103, 249–257 [DOI] [PubMed] [Google Scholar]
- 35. Siddall P.J., Taylor D.A., McClelland J.M., Rutkowski S.B., and Cousins M.J. (1999). Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain 81, 187–197 [DOI] [PubMed] [Google Scholar]
- 36. Norrbrink Budh C., Lund I., Hultling C., Levi R., Werhagen L., Ertzgaard P., and Lundeberg T. (2003). Gender related differences in pain in spinal cord injured individuals. Spinal cord 41, 122–128 [DOI] [PubMed] [Google Scholar]
- 37. Hubscher C.H., Fell J.D., and Gupta D.S. (2010). Sex and hormonal variations in the development of at-level allodynia in a rat chronic spinal cord injury model. Neurosci. Lett. 477, 153–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roof R.L. and Hall E.D. (2000). Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J. Neurotrauma 17, 367–388 [DOI] [PubMed] [Google Scholar]
- 39. Stein D.G. (2001). Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 24, 386–391 [DOI] [PubMed] [Google Scholar]
- 40. Datto J.P., Yang J., Dietrich W.D., and Pearse D.D. (2015). Does being female provide a neuroprotective advantage following spinal cord injury? Neural Regen. Res. 10, 1533–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Webb A.A., Chan C.B., Brown A., and Saleh T.M. (2006). Estrogen reduces the severity of autonomic dysfunction in spinal cord-injured male mice. Behav. Brain Res. 171, 338–349 [DOI] [PubMed] [Google Scholar]
- 42. Byers J.S., Huguenard A.L., Kuruppu D., Liu N.K., Xu X.M., and Sengelaub D.R. (2012). Neuroprotective effects of testosterone on motoneuron and muscle morphology following spinal cord injury. J. Comp. Neurol. 520, 2683–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gurer B., Kertmen H., Kasim E., Yilmaz E.R., Kanat B.H., Sargon M.F., Arikok A.T., Erguder B.I., and Sekerci Z. (2015). Neuroprotective effects of testosterone on ischemia/reperfusion injury of the rabbit spinal cord. Injury 46, 240–248 [DOI] [PubMed] [Google Scholar]
- 44. Clark M.J., Petroski G.F., Mazurek M.O., Hagglund K.J., Sherman A.K., Lammy A.B., Childers M.K., and Acuff M.E. (2008). Testosterone replacement therapy and motor function in men with spinal cord injury: a retrospective analysis. Am. J. Phys. Med. Rehabil. 87, 281–284 [DOI] [PubMed] [Google Scholar]
- 45. Dominguez C.A., Strom M., Gao T., Zhang L., Olsson T., Wiesenfeld-Hallin Z., Xu X.J., and Piehl F. (2012). Genetic and sex influence on neuropathic pain-like behaviour after spinal cord injury in the rat. Eur. J. Pain 16, 1368–1377 [DOI] [PubMed] [Google Scholar]
- 46. Gaudet A.D., Ayala M.T., Schleicher W.E., Smith E.J., Bateman E.M., Maier S.F., and Watkins L.R. (2017). Exploring acute-to-chronic neuropathic pain in rats after contusion spinal cord injury. Exp. Neurol. 295, 46–54 [DOI] [PubMed] [Google Scholar]