Abstract
PURPOSE
Human papillomavirus–positive oropharynx cancer incidence has increased rapidly in cohorts of US white men born during the 1930s to 1950s. It is unknown how the trajectory of the oropharynx cancer epidemic may be changing in the United States.
METHODS
Using US cancer registry information, we investigated whether increases in oropharynx cancer have continued into recent birth cohorts and forecasted the future burden across age, sex, and race/ethnicity subgroups. Log-linear Joinpoint regression and age-period-cohort models were used to evaluate incidence trends during 1992 to 2015 and projections through 2029.
RESULTS
Among white men, oropharynx cancer incidence increased rapidly in individuals born during 1939 to 1955 (5.3% per 2-year birth cohort; 95% CI, 4.8% to 5.7%), but this rate of increase significantly moderated in individuals born during 1955 to 1969 (1.7% per 2-year birth cohort; 95% CI, 1.0% to 2.4%). Should these birth-cohort trends continue, from 2016 to 2029 we forecast that incidence will increase dramatically in older white men 65 to 74 years of age (from 40.7 to 71.2 per 100,000) and 75 to 84 years of age (from 25.7 to 50.1 per 100,000), moderately in white men 55 to 64 years of age (from 40.3 to 52.0 per 100,000), and remain stable in white men 45 to 54 years of age (approximately 18 per 100,000). Accounting for population growth, we project an increase in annual number of cases in the United States from 20,124 (95% CI, 19,779 to 20,469) in 2016 to 30,629 (95% CI, 29,413 to 31,845) in 2029, primarily driven by older individuals (age ≥ 65 years; from 7,976 [95% CI, 7,782 to 8,172] to 18,072 [95% CI, 17,271 to 18,895]) and white men (from 14,453 [95% CI, 14,142 to 14,764] to 22,241 [95% CI, 21,119 to 23,364]).
CONCLUSION
The exponential increase in oropharynx cancer incidence in young white US men has ebbed, and modest increases are occurring/anticipated in cohorts born after 1955. Continued strong increases in incidence in cohorts born before 1955, and an approximate 50% increase in size of the US population age 65 years or older through 2029, portend a substantial shift in burden to elderly white men.
INTRODUCTION
Human papillomavirus (HPV) infection has caused an epidemic increase in the incidence of oropharynx cancers among white men in the United States.1-4 Changes in sexual behaviors in cohorts of individuals born during the 1930s to 1950s are believed to be responsible for this increase.1-5 It is, however, unclear if the increasing incidence has continued into cohorts born more recently, after the sexual revolution of the 1950s and 1960s.
Despite initial presentations of the epidemic increase in oropharynx cancer incidence in younger individuals (age < 60 years),3,4 as predicted in the initial studies,3 aging of the affected birth cohorts has now led to a rapid increase at older ages as well.6-10 This increase in oropharynx cancer incidence in older individuals has high clinical relevance. The number of US seniors older than 65 years is anticipated to increase by approximately 50% over the next decade, which portends a substantial increase in the annual number of oropharynx cancer cases in seniors.11 No study to date has quantified the anticipated burden of oropharynx cancers in older individuals.
Importantly, HPV-positive oropharynx cancer has historically been considered a disease of the young. In view of this young age at onset, good performance status, and favorable long-term survival (given the superior survival) of patients with HPV-positive oropharynx cancer, the field has moved toward modified/de-intensified treatment protocols.12,13 It is uncertain whether similar treatment protocols can also be used for older patients, who often present with comorbidities, have poorer outcomes, and experience greater treatment toxicities.6-10
We investigated the trajectory of the oropharynx cancer epidemic in the United States, with a specific emphasis on quantitative changes in incidence across birth cohorts. We also estimated the current and future burden of this disease across age, sex, and race/ethnicity subgroups using novel age-period-cohort projection methods.
METHODS
Data Sources
We obtained cancer incidence data for 1992 to 2015 from the US National Cancer Institute’s SEER Program.14,15 SEER cancer registries are considered the gold standard for cancer registration, with near-complete case ascertainment and microscopic verification. Data were included from the SEER 13 Registries Database14 for the years 1992 to 1999 (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, Utah, Los Angeles, San Jose–Monterey, Rural Georgia, and Alaska Native Tumor Registry) and the SEER 18 Registries Database15 for the years 2000 to 2015 (SEER 13 regions plus greater California, Kentucky, Louisiana, New Jersey, and greater Georgia), covering 17% and 28% of the US population, respectively.
Oropharynx cancers included base of tongue, lingual tonsil, soft palate and uvula, tonsil, oropharynx, and Waldeyer ring (International Classification of Diseases for Oncology version-3 topography codes C019, C024, C051-052, C090-099, C100-109, and C142, respectively),3 and are restricted to squamous cell histologies (codes 8050-8076, 8078, 8083, 8084, and 8094). Annual incidence rates for age 33 to 84 years (1992 to 2015; age standardized to the US 2000 standard population) were obtained using SEER*Stat version 8.3.5 software (November 2017 submission).
Statistical Analyses
All analyses were stratified by sex and race/ethnicity (non-Hispanic whites, non-Hispanic blacks, Hispanics, and other races including American Indian/Alaska Natives and Asian/Pacific Islanders) using SEER information abstracted from medical records and submitted to cancer registries. We initially evaluated temporal trends in oropharynx cancer incidence during 1992 to 2015 using log-linear regression models; results from these analyses are presented as the estimated annual percentage change (EAPC) in incidence.
We used age-period-cohort models to simultaneously evaluate the effects of age, period, and birth year/cohort on incidence rates. We note that because of linear dependency (ie, cohort = period-age), effects for age, period, and cohort are nonidentifiable. However, we estimated identifiable and interpretable parameters, as previously shown by Rosenberg and Anderson.16 In addition, our analyses focused on higher-order derivatives (eg, the second derivative of changes in the slopes of age, period, and cohort), all of which are uniquely identifiable. These models used 26 2-year age-groups (33 to 34, . . .,83 to 84) and 14 2-year calendar periods (1992 to 1993, . . .,2014 to 2015), spanning 40 partially overlapping 4-year birth cohorts referenced by midyear of birth (1910, . . .,1981). Additional details regarding the age-period-cohort methods are available in the Supplementary Methods of Best et al.17 From these models, we calculated cohort-specific incidence rate ratios for each birth cohort, with the 1942 cohort as the reference. Hence, incidence rate ratios less than 1.0 for a specific birth cohort indicate lower oropharynx cancer incidence when compared with the 1942 cohort, whereas ratios greater than 1.0 indicate higher incidence.16
We then investigated changes across the cohort-specific incidence rate ratios by fitting Joinpoint log-linear regression models.18 The number and location of the Joinpoints was determined using a permutation test (n = 10,000 permutations). Positive slopes for Joinpoint segments indicate that oropharynx cancer incidence increased across successive birth cohorts during an interval, whereas negative slopes indicate declines in incidence. Also, the model-selected Joinpoints indicate a change in the rate of increase/decrease at the specific birth cohorts (P value < .05).
Goodness-of-fit of the age-period-cohort models was assessed through the overdispersion statistic and heterogeneity of residuals.19,20 All models had negligible overdispersion, and there was no evidence of systematic lack of fit (Data Supplement).
We forecasted the future burden (incidence and annual number of cases) of oropharynx cancers through 2029 by projecting the observed cohort-specific age-specific incidence rates, after accounting for any period effects.17,21 Briefly, to estimate the future age-specific rate for a birth cohort observed during 1992 to 2015, the longitudinal age incidence in 1942 was multiplied by the cohort-specific rate ratio relative to the cohort of interest.17,19 Rate ratios for persons born during 1982 to 1996 were extrapolated from the most recent Joinpoint model segment for each sex-race/ethnicity. We then estimated the annual number of oropharynx cancer cases by multiplying the projected incidence rate (cases per 100,000 person-years) by the projected population size from the US Census Bureau, using previously described methods.17,19
Our forecasting methodology relies on the key assumption that the observed birth cohort effects would continue into the future. To refrain from projecting far beyond the observed data (1992 to 2015: 24 years), we restricted the forecast period to 14 years, an approximate ratio of 2:1 for observed: forecast rates. We validated our methodology by using a similar ratio of observed: forecast rates; cancer incidence rates for 1992 to 2007 (training set) were used to predict rates for 2008 to 2015 (testing set). As shown in the Data Supplement, we observed good validation. Given the combination of registries across SEER13 and SEER18, we also repeated key analyses restricted to SEER13 to show that results were similar (Data Supplement).
This study did not involve interaction with patients or the use of personal identifying information from the SEER data; therefore, institutional review and informed consent were not required. Analyses were conducted using MATLAB version R2017a (Mathworks; Natick, MA).
RESULTS
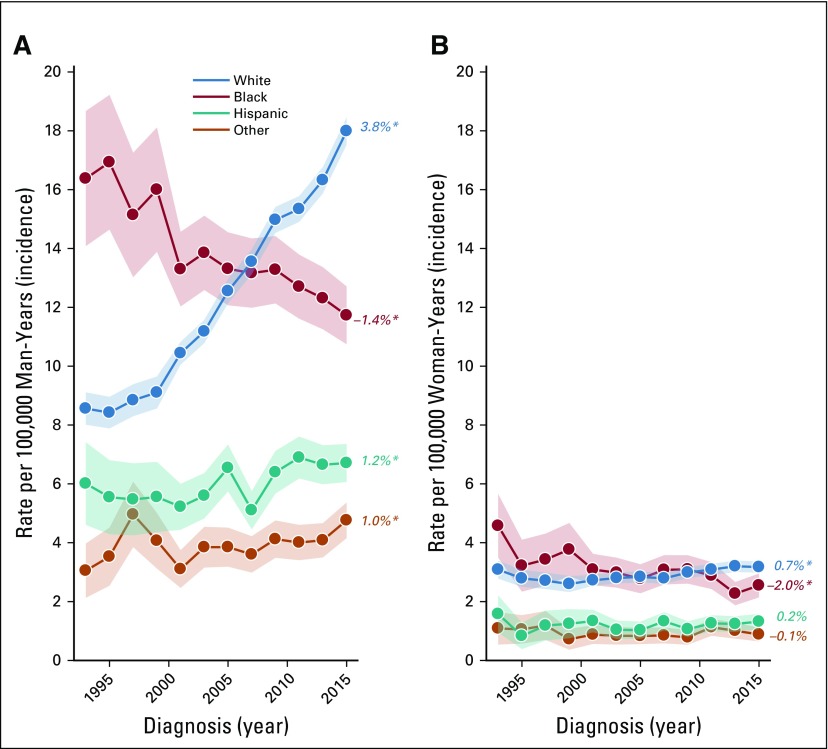
From 1992 to 2015, oropharynx cancer incidence increased significantly in white men (EAPC = 3.8%; Fig 1A), Hispanic men (EAPC = 1.2%), and men of other races/ethnicities (EAPC = 1.0%) but significantly decreased in black men (EAPC = −1.4%). In women, rates increased significantly in white women (EAPC = 0.7%), declined in black women (EAPC = −2.0%), and remained stable in Hispanics and other races (Fig 1B).
FIG 1.
Incidence trends for oropharynx cancer in the United States, stratified by race and sex. In both (A) men and (B) women there was an increase among non-Hispanic white individuals and decline among non-Hispanic black individuals. There was also an increase among Hispanic men and men of other races (American Indian/Alaska Natives and Asian/Pacific Islanders; A), whereas incidence remained stable among Hispanic women and women of other races (B). (*) Indicates statistically significant incidence trend (P < .05).
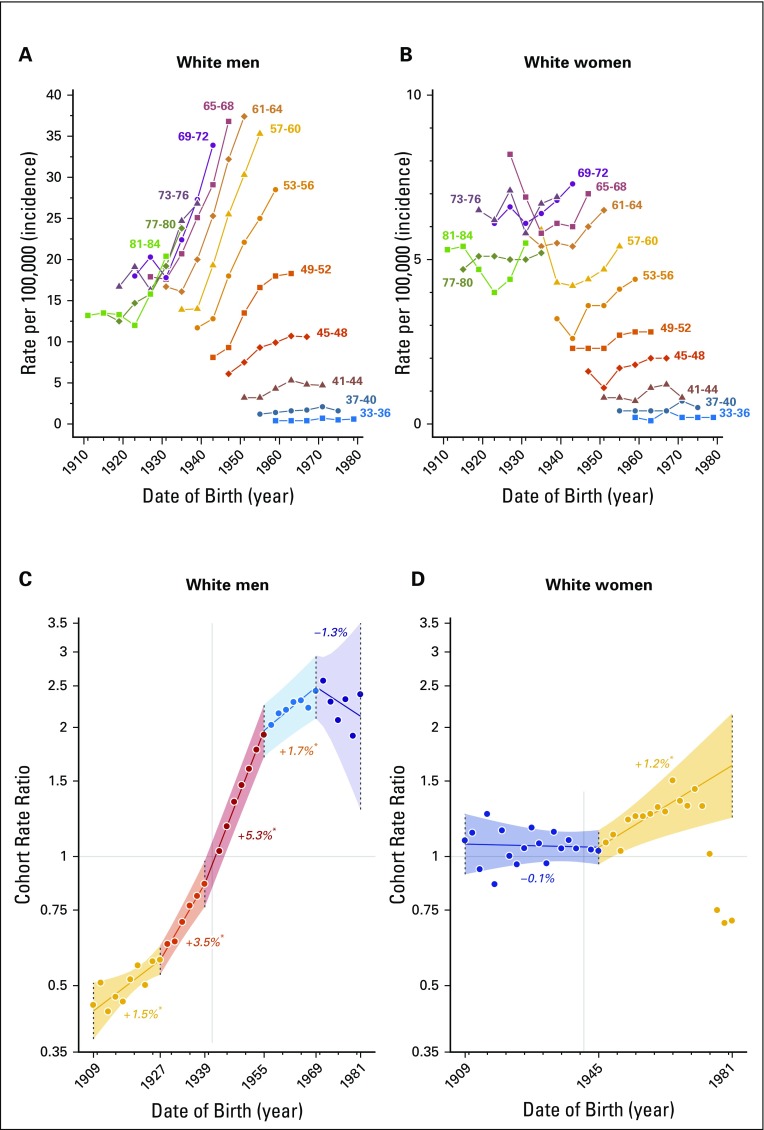
Increasing incidence in white men and women was generally observed in each successive birth cohort, but the degree of increase was variable by age and birth cohort (Figs 2A and 2B). In white men, oropharynx cancer incidence increased 1.5% per successive 2-year birth cohort from 1909 to 1927. This increase accelerated to 3.5% per 2-year birth cohort from 1927 to 1939 and further accelerated to 5.3% per 2-year birth cohort from 1939 to 1955. The magnitude of increase, however, substantially moderated to an increase of 1.7% per 2-year birth cohort from 1955 to 1969 and further stabilized in cohorts born until 1981 (Fig 2C), although data were sparse in the cohorts born after 1969.
FIG 2.
(A, B) Age-specific incidence rates for oropharynx cancers across birth cohort in non-Hispanic white individuals, stratified by sex. Increasing incidence was generally observed in successive birth cohorts born after the 1930s (men, A) or 1940s (women, B). (C, D) Cohort rate ratios (estimated percent change in rate annually by birth cohort) in non-Hispanic white individuals, stratified by sex. (C) Among white men, oropharynx cancer incidence increased most rapidly in the 1939 to 1955 birth cohorts and moderated significantly in cohorts born after 1955. (D) Among white women, incidence increased modestly in cohorts born after 1945. (*) Indicates statistically significant change in incidence in each successive birth cohort (P < 0.05).
In white women, incidence was stable for the 1909 to 1945 birth cohorts and increased by 1.2% per 2-year birth cohort from 1945 to 1981 (Fig 2D). Incidence of oropharynx cancers substantially declined among successive birth cohorts in blacks (men and women, all cohorts), Hispanics (1959 to 1981 birth cohorts in men, all cohorts in women), and in other races (women only, all cohorts; Data Supplement).
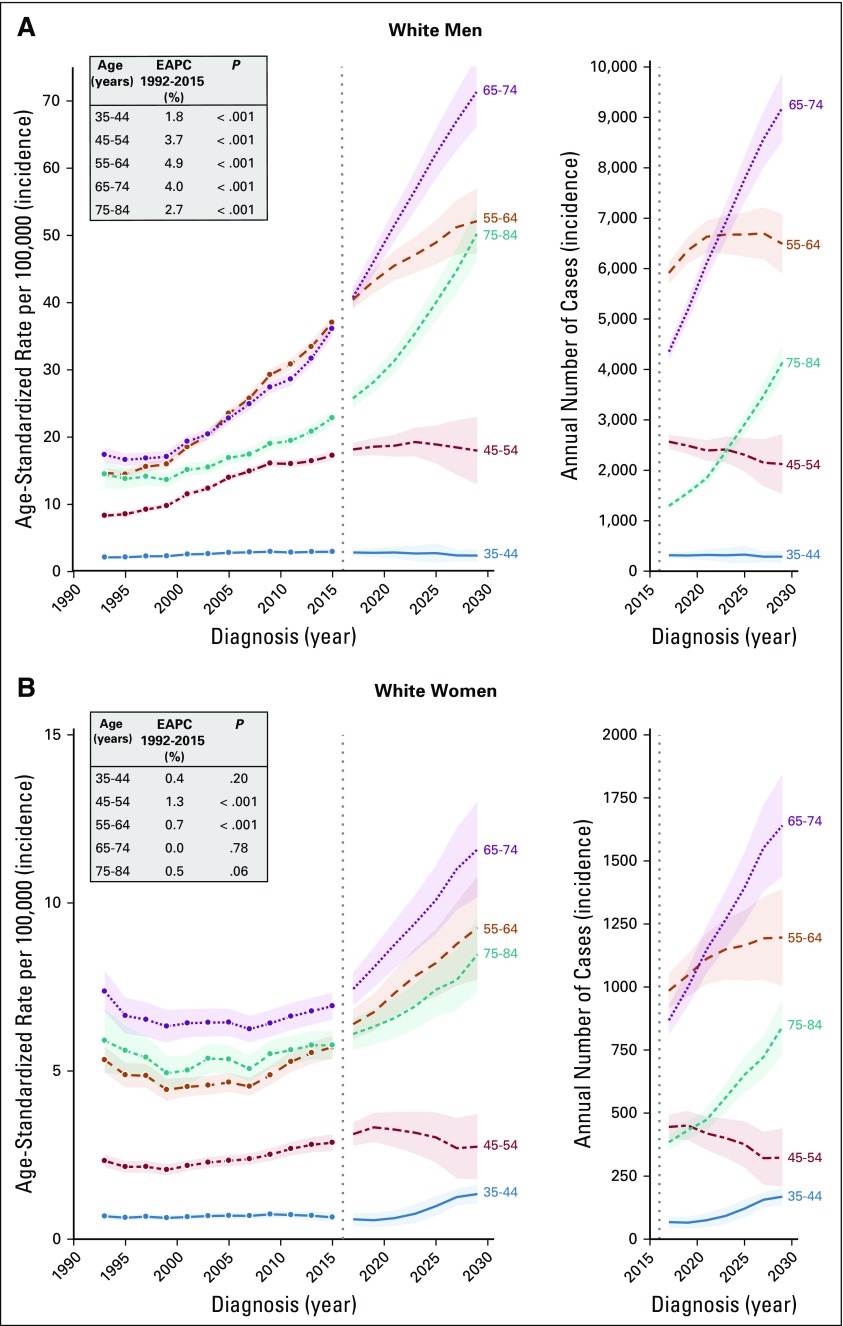
The differential rate of increase in oropharynx cancer incidence across birth cohorts also manifested as differential age-specific incidence trends across calendar periods (Data Supplement), underscoring an age-by-period/by-birth cohort interaction. From 1992 to 2015, oropharynx cancer incidence increased strongly in white men age 55 to 64 years (4.9% per year, from 14.7 to 37.0 per 100,000; Fig 3A), 65 to 74 years (4.0% per year, from 17.4 to 36.0 per 100,000), and 45 to 54 years (3.7% per year, from 8.3 to 17.2 per 100,000) and modestly for age 35 to 44 years (1.7% per year, from 2.1 to 2.9 per 100,000).
FIG 3.
Age-specific oropharynx cancer incidence trends, projections, and projected annual number of cases among non-Hispanic white men and women in the United States. From 1992 to 2015, incidence of oropharynx cancer increased significantly (P < .001) among (A) men of all ages, and (B) middle-age women age 45 to 54 and 55 to 64. In both men and women, incidence and annual number of oropharynx cases is projected to increase in older age groups from 2016 to 2029. EAPC, estimated annual percent change.
In white women, rates increased slightly from 1992 to 2015 in age 45 to 54 years (1.3% per year, from 2.3 to 2.9 per 100,000; Fig 3B) and 55 to 64 years (0.7% per year, from 5.3 to 5.7 per 100,000), whereas rates were generally stable for age 35 to 44 years (< 1 per 100,000), 65 to 74 years (approximately six to seven per 100,000), and 75 to 84 years (approximately five to six per 100,000). We also observed modest increases in Hispanics and other races (men only, age 55 to 74 years; EAPC = approximately 1%) and older black men (age 75 to 84 years, EAPC = 1.4%). Consistent with overall trends, we observed substantial declines in younger/middle-age black men and black women of all ages (Data Supplement).
We project that oropharynx cancer incidence will increase dramatically in older white men from 2016 to 2029 (Fig 3A: age 65 to 74 years, from 40.7 to 71.2 per 100,000; age 75 to 84 years, from 25.7 to 50.1 per 100,000) and moderately in middle-age white men (age 55 to 64 years, from 40.3 to 52.0 per 100,000) and will remain stable in white men age 45 to 54 years (approximately 18 per 100,000). Data were too sparse for reliable interpretation of projections for white men age 35 to 44 years. In white women, we project modest increases in incidence, with the largest absolute increase in older women (age 65 to 74 years, 7.4 to 11.6 per 100,000; Fig 3B). Except for older Hispanic men, no substantial increases are projected in other races (Data Supplement). Projected trends and annual number of cases are also presented for all men, all women, and all men and women combined (Data Supplement).
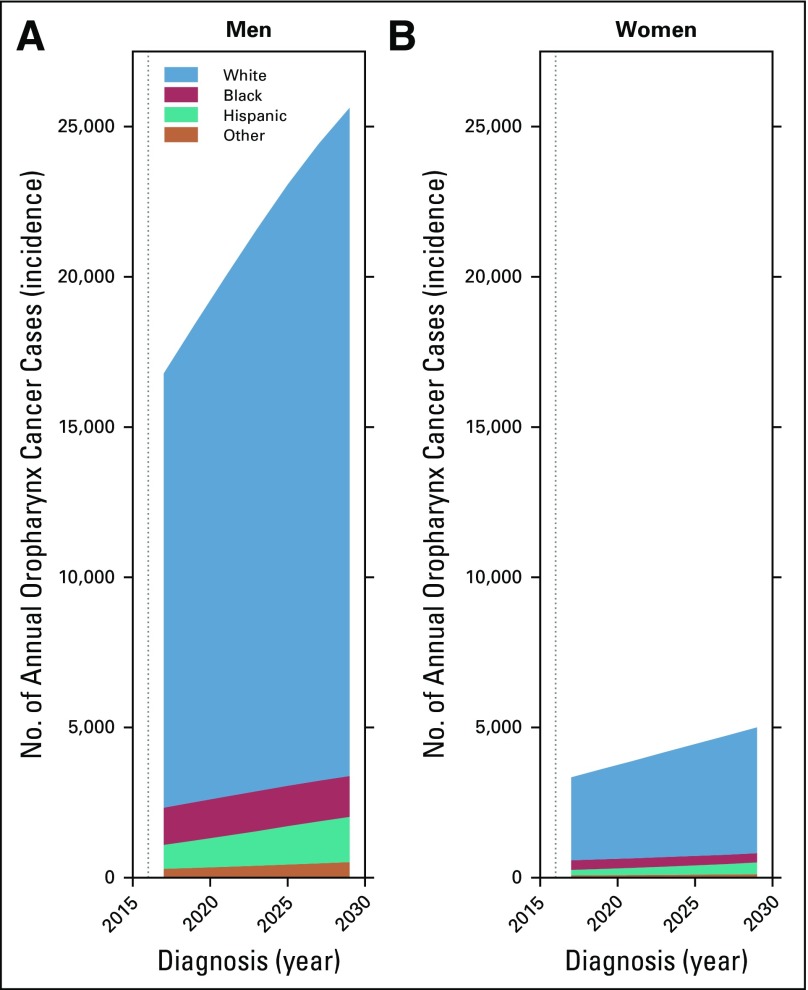
The annual number of oropharynx cancer cases is projected to increase dramatically from 20,124 (95% CI, 19,779 to 20,469) in 2016 to 30,629 (95% CI, 29,413 to 31,845) in 2029, because of sizeable increases in oropharynx cancer incidence in older white men and women and, importantly, the anticipated increase in the size of the US population age 65 years and older over the next decade (Data Supplement). Notably, the annual number of cases is projected to remain relatively stable in younger individuals (< 65 years, approximately 12,500 cases), with an increase almost exclusively in older individuals (≥ 65 years, from 7,976 [95% CI, 7,782 to 8,172] to 18,072 [95% CI, 17,271 to 18,895]) and primarily in older white men and women (≥ 65 years, from 5,645 [95% CI, 5,470 to 5,822] to 13,331 [95% CI, 12,593 to 14,072] and from 1,252 [95% CI, 1,188 to 1,317] to 2,484 [95% CI, 2,255 to 2,712], respectively; Figs 3A and 3B). In summary, the majority of oropharynx cancer cases in 2029 are projected to occur in older individuals and in white individuals (Fig 4A and 4B).
FIG 4.
Projected annual number of oropharynx cancer cases in the United States, stratified by sex. Within each panel, the annual number of projected cases is subdivided according to race. The majority of oropharynx cancer cases through 2029 are projected to occur among (A) non-Hispanic white men, and (B) non-Hispanic white women.
DISCUSSION
The rapid increase in the incidence of oropharynx cancers in young white men over the past two decades,3 since confirmed as caused by HPV,4 has come to be characterized as a virus-related epidemic of young individuals.22,23 Our study provides several clarifying observations regarding the observed and projected trajectory of the oropharynx cancer epidemic in the United States.
First, we show that the extent of increase in oropharynx cancer incidence has moderated in white males born after 1955. Although the incidence of oropharynx cancers continues to increase in white men born since the mid-1950s, the magnitude of increase has substantially attenuated when compared with the rapid increases experienced by white men born during 1940s to mid-1950s. Second, as predicted in prior studies3 and confirmed in recent studies,6-9 aging of these distant birth cohorts that have previously experienced exponential increases in incidence at younger ages is now manifesting as rapidly increasing incidence in white men older than 65 years. Third, we show that, in addition to white men, oropharynx cancer incidence has significantly increased, albeit modestly, in men of Hispanic or other races/ethnicities and in white women. Fourth, we project an exponential increase in the future burden of oropharynx cancers over the next decade (> 30,000 annual incident cancers in the year 2029), with an increasingly dominant burden in white men older than 65 years of age (n = 13,331; 44% of all cases).
This study is the first, to our knowledge, to identify a substantial attenuation of the increase in oropharynx cancer incidence in young white men (ie, those born during 1955 to 1969 and currently age 46 to 60 years) when compared with those born during 1939 to 1955 and currently age 61 to 76 years. Given the registry-based nature of our study, information was unavailable on key oropharynx cancer risk factors, such as sexual behavior and smoking. Thus, the reasons for this moderation remain unclear. Nevertheless, a likely explanation is changes in sexual behaviors in recent birth cohorts, given the presumed correlation between sexual behaviors and the course of the oropharynx cancer epidemic.5 In support of this hypothesis, and closely mirroring the oropharynx cancer incidence trends across birth cohorts in white men, US-representative data show a steady increase in the average number of recent sexual partners (a surrogate for high-risk lifetime sexual behavior) through the 1960 birth cohort and a significant moderation/decline thereafter.24 Likewise, seroprevalence of herpes simplex virus-2, another surrogate for high-risk sexual behaviors, has also declined in recent US birth cohorts.25 The emergence of the HIV epidemic has been proposed as a potential explanation for these moderations in high-risk sexual behaviors in recent birth cohorts. It is also possible that significant reductions in smoking, a known oropharynx cancer risk factor, in the recent birth cohorts could partly explain the moderation of the increasing incidence.26 Of note, we caution against overinterpretation of the trends and projections for individuals age 35 to 44 years, given sparse data due to low incidence rates as well as the lack of substantial observed data for these cohorts.
Our results point to the joint role of age and birth cohort in HPV-induced oropharyngeal carcinogenesis. The strong, yet differential, effects of both age and birth cohort (ie, increasing incidence in the affected birth cohorts as they age) suggest the joint role of older age as well as continued persistence of oral HPV infections, which perhaps have greater penetrance at older ages.
Our observations have important clinical implications, particularly regarding treatment of older patients with oropharynx cancer.6-10 Assuming the observed cohort effects through 2015 continue over the near future, our projections portend a significant shift in the burden of oropharynx cancers to older individuals (age ≥ 65 years) over the next decade. This shift arises from a combination of the moderation of increasing incidence in young individuals, robust increases in incidence in older individuals, and the anticipated 50% increase of the US population age 65 years or older through 2029.11
The profile of the typical patient with oropharynx cancer during the early 2000s included HPV-positive, young (in early to mid-50s), nonsmoker, with few comorbidities, good performance status, and long life expectancy.12,22 This low-risk profile and the substantially superior survival/treatment response of patients with HPV-positive oropharynx cancer has led to a consideration of treatment de-escalation to reduce short- and long-term treatment- toxicities.12,13 Current de-escalation strategies include reduction in the dose of chemotherapy/radiation or modification of the chemotherapy regimen.27-29 The recently completed NRG RTOG1016 and De-ESCALaTE trials demonstrated that replacement of cisplatin with cetuximab led to inferior overall and progression-free survival and did not significantly reduce the overall toxicity experience for patients.30,31 Therefore, next-generation trials in this patient population will focus on reduction in total radiation dose or replacement of cisplatin with immune checkpoint inhibitors (eg, NRG HN005).
In older patient populations (eg, > 70 years), the benefit of the addition of chemotherapy to radiotherapy is a matter of considerable controversy. Limited emerging evidence suggests that older patients with oropharynx cancer, although just as likely to be HPV positive as younger patients, experience an attenuated survival benefit.6-10 However, more studies with uniform HPV testing and adequate adjustment for confounders (eg, smoking) are needed. It is likely that the biology of HPV-positive tumors is similar in younger versus older patients; nonetheless, older patients have poorer survival outcomes because of competing comorbidities, treatment-associated acute and chronic toxicity with chemoradiation, or an inability to receive maximally effective therapies.32,33 Thus, older patients with oropharynx cancer may have different risks and benefits when receiving de-intensified regimens than younger patients. The emergence of immunotherapies, whose efficacy may be more age invariant than cytotoxic chemotherapies, could provide a promising treatment avenue for older patients with oropharynx cancer.34 These issues collectively underscore the growing relevance of ongoing trials focused on older patients with HPV-positive oropharynx cancer, such as NRGHN004 (radiation therapy with durvalumab v cetuximab in patients who cannot take cisplatin).
Our results also have important prevention implications. We show that oropharynx cancer incidence rates will likely continue to increase in the near future. Prophylactic HPV vaccines hold great promise in reducing HPV-positive oropharynx cancers in the vaccine-eligible birth cohorts (those born after 1990).35,36 Therefore, continued emphasis on increasing HPV vaccination uptake in men and women is needed. However, the birth cohorts currently experiencing increasing oropharynx cancer incidence (individuals born through 1970) are unlikely to benefit from vaccination. In these birth cohorts, screening and early detection remain the only viable prevention options. Unfortunately, screening for HPV-positive oropharynx cancers is not currently feasible, given the absence of an identifiable HPV-induced precancer/early cancer.5,37 Thus, studies are needed to identify such a lesion as well as to develop efficacious screening methods and risk-mitigation strategies.
In conclusion, our observations underscore a significant shift in the trajectory of the oropharynx cancer epidemic in the United States. Our results suggest an ebbing of the oropharynx cancer epidemic in younger individuals, exaggeration of the epidemic in older individuals, and a continued exponential increase in the annual number of oropharynx cancers over the next decade.
Footnotes
Presented at the 2018 International Papillomavirus Conference, Sydney, Australia, October 2-6, 2018.
Supported by the Intramural Research Program of the US National Institutes of Health/National Cancer Institute.
Processed as a Rapid Communication manuscript.
AUTHOR CONTRIBUTIONS
Conception and design: Joseph E. Tota, Zachary S. Zumsteg, Maura L. Gillison, Philip S. Rosenberg, Anil K. Chaturvedi
Financial support: Anil K. Chaturvedi
Administrative support: Anil K. Chaturvedi
Collection and assembly of data: Joseph E. Tota, Anil K. Chaturvedi
Data analysis and interpretation: Joseph E. Tota, Ana F. Best, Zachary S. Zumsteg, Philip S. Rosenberg, Anil K. Chaturvedi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Evolution of the Oropharynx Cancer Epidemic in the United States: Moderation of Increasing Incidence in Younger Individuals and Shift in the Burden to Older Individuals
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Joseph E. Tota
Travel, Accommodations, Expenses: Merck
Other Relationship: Merck
Zachary S. Zumsteg
Consulting or Advisory Role: EMD Serono
Consulting or Advisory Role: Scripps Proton Therapy Center
Other Relationship: King and Spalding (I)
Maura L. Gillison
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, EMD Serono, Roche, Genocea Biosciences, BioMimetix Pharmaceutical
Research Funding: Bristol-Myers Squibb (Inst), Genocea Biosciences (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Frisch M, Hjalgrim H, Jaeger AB, et al. Changing patterns of tonsillar squamous cell carcinoma in the United States. Cancer Causes Control. 2000;11:489–495. doi: 10.1023/a:1008918223334. [DOI] [PubMed] [Google Scholar]
- 2.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: An emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110:1429–1435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillison ML, Chaturvedi AK, Anderson WF, et al. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33:3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zumsteg ZS, Cook-Wiens G, Yoshida E, et al. Incidence of oropharyngeal cancer among elderly patients in the United States. JAMA Oncol. 2016;2:1617–1623. doi: 10.1001/jamaoncol.2016.1804. [DOI] [PubMed] [Google Scholar]
- 7.Windon MJ, D’Souza G, Rettig EM, et al. Increasing prevalence of human papillomavirus-positive oropharyngeal cancers among older adults. Cancer. 2018;124:2993–2999. doi: 10.1002/cncr.31385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rettig EM, Zaidi M, Faraji F, et al. Oropharyngeal cancer is no longer a disease of younger patients and the prognostic advantage of human papillomavirus is attenuated among older patients: Analysis of the National Cancer Database. Oral Oncol. 2018;83:147–153. doi: 10.1016/j.oraloncology.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu DJ, Luu M, Mita A, et al. Human papillomavirus-associated oropharyngeal cancer among patients aged 70 and older: Dramatically increased prevalence and clinical implications. Eur J Cancer. 2018;103:195–204. doi: 10.1016/j.ejca.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Chaturvedi AK, Zumsteg ZS. A snapshot of the evolving epidemiology of oropharynx cancers. Cancer. 2018;124:2893–2896. doi: 10.1002/cncr.31383. [DOI] [PubMed] [Google Scholar]
- 11.Vespa J, Armstrong DM, Medina L. Demographic Turning Points for the United States: Population Projections for 2020 to 2060, Current Population Reports. US Census Bureau; 2018. https://www.census.gov/content/dam/Census/library/publications/2018/demo/P25_1144.pdf [Google Scholar]
- 12.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 13.O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–550. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 14. SEER-13: Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 13 Regs Research Data, Nov 2017 Sub (1992-2015) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission.
- 15. SEER-18: Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data, Nov 2017 Sub (2000-2015) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission.
- 16.Rosenberg PS, Anderson WF. Age-period-cohort models in cancer surveillance research: Ready for prime time? Cancer Epidemiol Biomarkers Prev. 2011;20:1263–1268. doi: 10.1158/1055-9965.EPI-11-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Best AF, Haozous EA, Berrington de Gonzalez A, et al. Premature mortality projections in the USA through 2030: A modelling study. Lancet Public Health. 2018;3:e374–e384. doi: 10.1016/S2468-2667(18)30114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19. doi: 10.1093/jnci/djv159. Rosenberg PS, Barker KA, Anderson WF: Estrogen receptor status and the future burden of invasive and in situ breast cancers in the United States. J Natl Cancer Inst . , 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg PS, Check DP, Anderson WF. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2014;23:2296–2302. doi: 10.1158/1055-9965.EPI-14-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chernyavskiy P, Little MP, Rosenberg PS. A unified approach for assessing heterogeneity in age-period-cohort model parameters using random effects. Stat Methods Med Res. 2019;28:20–34. doi: 10.1177/0962280217713033. [DOI] [PubMed] [Google Scholar]
- 22.Marur S, D’Souza G, Westra WH, et al. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramqvist T, Dalianis T. Oropharyngeal cancer epidemic and human papillomavirus. Emerg Infect Dis. 2010;16:1671–1677. doi: 10.3201/eid1611.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twenge JM, Sherman RA, Wells BE. Changes in American adults’ sexual behavior and attitudes, 1972-2012. Arch Sex Behav. 2015;44:2273–2285. doi: 10.1007/s10508-015-0540-2. [DOI] [PubMed] [Google Scholar]
- 25.McQuillan G, Kruszon-Moran D, Flagg EW, et al. Prevalence of herpes simplex virus type 1 and type 2 in persons aged 14-49: United States, 2015-2016. NCHS Data Brief. 2018;304:1–8. [PubMed] [Google Scholar]
- 26.Anderson CM, Burns DM, Dodd KW, et al. Chapter 2: Birth-cohort-specific estimates of smoking behaviors for the U.S. population. Risk Anal. 2012;32(suppl 1):S14–S24. doi: 10.1111/j.1539-6924.2011.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marur S, Li S, Cmelak AJ, et al. E1308: Phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx- ECOG-ACRIN Cancer Research Group. J Clin Oncol. 2017;35:490–497. doi: 10.1200/JCO.2016.68.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen AM, Felix C, Wang PC, et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: A single-arm, phase 2 study. Lancet Oncol. 2017;18:803–811. doi: 10.1016/S1470-2045(17)30246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mesía R, Taberna M. HPV-related oropharyngeal carcinoma de-escalation protocols. Lancet Oncol. 2017;18:704–705. doi: 10.1016/S1470-2045(17)30250-4. [DOI] [PubMed] [Google Scholar]
- 30.Mehanna H, Robinson M, Hartley A, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet. 2019;393:51–60. doi: 10.1016/S0140-6736(18)32752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet. 2019;393:40–50. doi: 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Argiris A, Li Y, Murphy BA, et al. Outcome of elderly patients with recurrent or metastatic head and neck cancer treated with cisplatin-based chemotherapy. J Clin Oncol. 2004;22:262–268. doi: 10.1200/JCO.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 33.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferris RL. Immunology and immunotherapy of head and neck cancer. J Clin Oncol. 2015;33:3293–3304. doi: 10.1200/JCO.2015.61.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrero R, Quint W, Hildesheim A, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8:e68329. doi: 10.1371/journal.pone.0068329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J Clin Oncol. 2018;36:262–267. doi: 10.1200/JCO.2017.75.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreimer AR, Shiels MS, Fakhry C, et al. Screening for human papillomavirus-driven oropharyngeal cancer: Considerations for feasibility and strategies for research. Cancer. 2018;124:1859–1866. doi: 10.1002/cncr.31256. [DOI] [PubMed] [Google Scholar]