Abstract
PURPOSE
Sensorineural hearing loss (SNHL) is associated with intellectual and academic declines in children treated for embryonal brain tumors. This study expands upon existing research by examining core neurocognitive processes that may result in reading difficulties in children with treatment-related ototoxicity.
PATIENTS AND METHODS
Prospectively gathered, serial, neuropsychological and audiology data for 260 children and young adults age 3 to 21 years (mean, 9.15 years) enrolled in a multisite research and treatment protocol, which included surgery, risk-adapted craniospinal irradiation (average risk, n = 186; high risk, n = 74), and chemotherapy, were analyzed using linear mixed models. Participants were assessed at baseline and up to 5 years after diagnosis and grouped according to degree of SNHL. Included were 196 children with intact hearing or mild to moderate SNHL (Chang grade 0, 1a, 1b, or 2a) and 64 children with severe SNHL (Chang grade 2b or greater). Performance on eight neurocognitive variables targeting reading outcomes (eg, phonemics, fluency, comprehension) and contributory cognitive processes (eg, working memory, processing speed) was analyzed.
RESULTS
Participants with severe SNHL performed significantly worse on all variables compared with children with normal or mild to moderate SNHL (P ≤ .05), except for tasks assessing awareness of sounds and working memory. Controlling for age at diagnosis and risk-adapted craniospinal irradiation dose, performance on the following four variables remained significantly lower for children with severe SNHL: phonemic skills, phonetic decoding, reading comprehension, and speed of information processing (P ≤ .05).
CONCLUSION
Children with severe SNHL exhibit greater reading difficulties over time. Specifically, they seem to struggle most with phonological skills and processing speed, which affect higher level skills such as reading comprehension.
INTRODUCTION
Survivors of pediatric brain tumors are at risk for cognitive declines as a result of disease- and treatment-related factors.1,2 Difficulties have been described in intelligence and reasoning, attention and executive functioning, and processing speed,2-7 which emerge several years after diagnosis and persist throughout adulthood.8 Younger age and high-risk disease (ie, requiring greater treatment intensity) have been identified as risks for neurocognitive dysfunction.9,10
Children treated for embryonal brain tumors may receive chemotherapy (eg, platinum agents) and craniospinal irradiation (CSI), both of which are associated with ototoxicity (ie, sensorineural hearing loss [SNHL]) that can be permanent, progressive, and worsen months after treatment.11-13 Although often a result of peripheral damage, SNHL potentially alters neural development as a result of reduced sensory input during critical periods.14
Disparities between deaf and hearing peers on standardized academic measures are well documented.15 Pediatric SNHL in children without cancer is associated with decreased performance on intellectual and academic measures, with language skills being particularly vulnerable.16-18 Despite high rates of SNHL in children undergoing cancer-directed therapy, research regarding neurocognitive and functional implications of ototoxicity is scarce. In a subset of current participants, Schreiber et al19 prospectively examined cognitive and academic outcomes for patients treated for medulloblastoma. In comparison with those with intact hearing or mild to moderate SNHL, patients with severe SNHL demonstrated significant declines 5 years after diagnosis on global intellect, reading, and math measures. Similarly, Orgel et al20 described increased risk of intellectual and executive functioning declines in survivors of pediatric brain tumors with SNHL. Finally, Gurney et al21 suggested that survivors of pediatric neuroblastomas with SNHL were at risk for poorer academic and psychosocial outcomes on parent-report measures.
The primary aim of the current study was to identify neurocognitive skills contributing to poor reading outcomes in children with SNHL as result of cancer-directed treatment. We used prospective, longitudinal audiologic and cognitive assessments to examine ototoxicity effects on core reading skills, outcomes, and supportive neurocognitive abilities in a large sample of children and young adults with embryonal brain tumors observed up to 5 years after diagnosis. We hypothesized that those with severe SNHL would perform worse than those with better hearing on all measures 5 years after diagnosis. We expected more significant deficits for younger children who received more intensive treatment. Finally, we hypothesized that deficits may emerge several years after diagnosis.
PATIENTS AND METHODS
Participants
Eligible patients included 408 children or young adults, who were age 3 to 21 years at diagnosis, with histologically confirmed embryonal brain tumors enrolled in a multisite clinical trial (Appendix Fig A1 [online only]; St Jude Medulloblastoma 03 [SJMB03]; ClinicalTrials.gov identifier: NCT00085202) at collaborating institutions in the United States (n = 4; primary site was St Jude Children’s Research Hospital [SJCRH]), Australia (n = 4), and Canada (n = 1). Institutional review board approval was granted. Adult patients or caregivers provided informed consent before enrollment; children provided assent (ie, verbal, age 7 to 13 years; written, age 14 years or older).
Patients were considered for inclusion if they had completed two neurocognitive assessments between baseline and 5 years after diagnosis, as required for analysis. Of 408 potential participants, 94 did not undergo neurocognitive evaluations for the following reasons: did not provide informed consent or refused (n = 23), disease progression (n = 23), English was secondary language (n = 20), site did not participate in neurocognitive evaluations (n = 10), other diagnoses or reasons (eg, autism, unresolved posterior fossa syndrome [PFS], blindness, intellectual disability; n = 10), or lost to follow-up (n = 8). An additional 54 participants did not participate in at least two neurocognitive evaluations. The final group consisted of 260 children or young adults. Diagnoses primarily included medulloblastoma (80.38%), although other diagnoses included primitive neuroectodermal tumors (6.92%), atypical teratoid or rhabdoid tumors (6.15%), pineoblastomas (5.77%), and others (less than 1%). Approximately 17% of patients (n = 45) were identified as having PFS.
Treatment was protocol driven and consistent across sites. Patients underwent surgical resection, risk-adapted CSI within 31 days of resection, and high-dose chemotherapy, including stem-cell rescue. Treatment was stratified given disease status (ie, average v high risk). Patients were considered average risk if the tumor did not invade the brainstem, gross total resection was achieved (residual disease of 1.5 cm2 or less), and there was no evidence of metastatic disease.
Average-risk patients received a lower dose of CSI compared with high-risk patients (23.4 v 36 to 39.6 Gy, respectively); otherwise, treatment was similar between risk groups. After CSI, patients received a supplemental, conformal boost to the primary tumor site to 55.8 Gy (+ 1 cm margin). If appropriate, high-risk patients received supplemental, focal radiation therapy (50.4 to 54 Gy) for metastases. Six weeks after radiation therapy, patients began the first of four 28-day cycles of chemotherapy (ie, cyclophosphamide, cisplatin, vincristine), followed by autologous hematopoietic stem-cell transplantation and filgrastim. Cisplatin was dosed at 75 mg/m2 to a cumulative dose of 300 mg/m2. Amifostine (600 mg/m2) was administered during administration of cisplatin to reduce ototoxicity.22 Patients underwent medical follow-up every 3 months for 2 years during treatment; after treatment, medical follow-up was scheduled every 6 months.
Neurocognitive and Audiology Evaluations
Patients underwent neurocognitive and audiology evaluations throughout enrollment. Neurocognitive assessments were completed at baseline and up to 5 years after diagnosis. Patients at SJCRH participated in neurocognitive testing each year for 5 years after diagnosis; those at collaborating sites participated in testing at years 1, 3, and 5 after diagnosis. Audiology evaluations were conducted at baseline, before each chemotherapy cycle with high-dose cisplatin, and at specific time points after treatment (eg, 9, 12, 15, 24, 36, 48, and 72 months).
Neurocognitive assessment.
Patients were administered a standard battery of measures that were consistent across time points. Specific measures were selected for analysis and included variables germane to language and/or reading abilities (ie, single-word decoding or recognition, fluency, comprehension, phonological skills), in addition to neurocognitive processes that can affect reading and are known to be at risk in children treated for brain tumors (ie, working memory, processing speed). Subtests from the Woodcock-Johnson Third Edition, Tests of Achievement and Cognition23,24 were analyzed. Scores were age standardized and validated using large representative normative samples. Standardized scores have a mean ± standard deviation (SD) of 100 ± 15. Scores of 85 to 115 are broadly average; higher scores represent better performance.
Audiology assessment.
Audiometric data were gathered through tympanometry, pure-tone air and bone conduction thresholds, auditory brainstem response, auditory steady-state response, and/or distortion-product otoacoustic measurements. Data were assigned ototoxicity grades by an SJCRH audiologist using the Chang ototoxicity grading scale.25 Grades of 2a/2b or greater are considered to represent severe SNHL, warranting intervention. Consistent with previous research,19 a Chang grade of 2b or greater was considered to represent severe SNHL in the current study. Bilateral Chang grades were examined for time points correlating with neurocognitive assessment time points. Final Chang grades used in analyses were based on the better ear, providing estimates of participants’ best potential hearing status.
Statistical Analyses
Descriptive analyses examined demographic and clinical characteristics of participants and assessed relationships with SNHL. Random coefficient models (linear mixed effect models) with patient-specific intercepts and slopes, modeled with unstructured variance-covariance, were used to model trends of neurocognitive scores over time since diagnosis. The primary variable, SNHL, was dichotomized. Chang grades of 0, 1a, 1b, or 2a were considered normal hearing or mild to moderate SNHL; grades of 2b or greater were considered severe SNHL. Single-variable and multivariable models were used to describe data. Multivariable models used a backward elimination approach guided by the Akaike information criterion (AIC) and Bayesian information criterion (BIC) to simplify models by removing nonsignificant variables. Results were considered significant at P ≤ .05.
RESULTS
Demographic and Clinical Comparisons
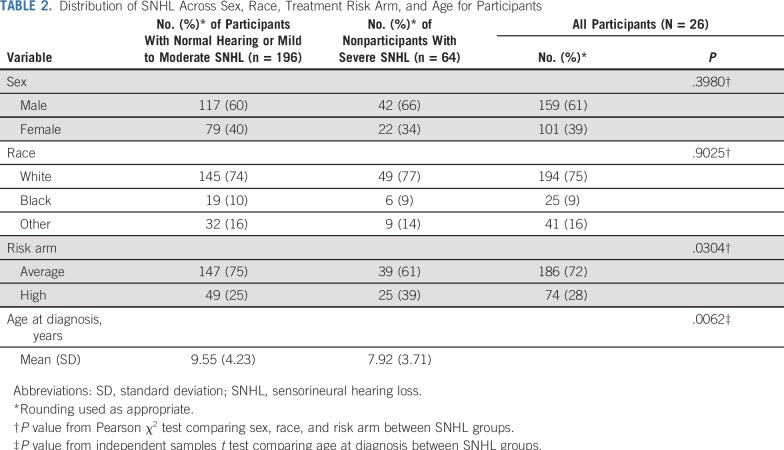
Comparisons between participants (n = 260) and nonparticipants (n = 148) are included in Table 1. Results indicate no statistically significant differences in sex, race, PFS status, or age at diagnosis (P > .05) between participants and nonparticipants. Differences regarding risk arm indicate a greater proportion of high-risk patients in the nonparticipant group.
TABLE 1.
Characteristics of Participants and Nonparticipants
Descriptive statistics for participants are provided in Table 2. Included in the sample were 260 individuals with a mean age at diagnosis of 9.15 years, divided into two groups based on degree of SNHL as determined by Chang grade. There were 196 patients with normal or mild to moderate SNHL (mean age, 9.55 years; SD, 4.23 years) and 64 patients with severe SNHL (mean age, 7.92 years; SD, 3.71 years). The sample included primarily white patients (75%) and average-risk patients (72%). Participants in the severe SNHL group were, on average, significantly younger at diagnosis than those in the normal or mild to moderate group (P = .0062). The SNHL groups were balanced regarding sex and race, although there was a greater proportion of high-risk patients in the severe SNHL group. Sample sizes varied for cognitive variables based on factors such as age, with final analyses including 180 to 257 patients per model (Table 3).
TABLE 2.
Distribution of SNHL Across Sex, Race, Treatment Risk Arm, and Age for Participants
TABLE 3.
Parameter Estimates of Univariable Linear Mixed Models With Hearing Loss Status and Neurocognitive Change Over Time
Hearing Loss
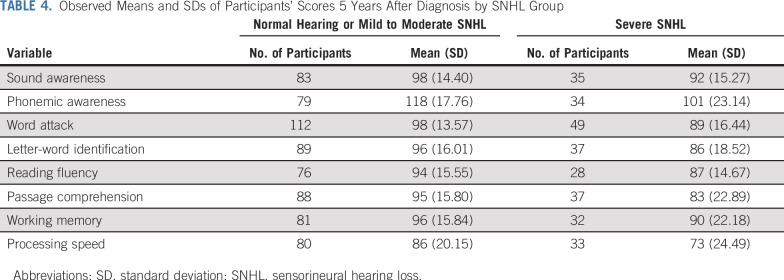
Linear mixed effect models examined effects of SNHL on language-related neurocognitive variables over time (Table 3; see Data Supplement for initial models without removal of insignificant variables or interactions). Results indicate declines in performance on all variables over time (time, P < .05), regardless of SNHL status. Children with severe SNHL had a significantly greater decline (time × hearing) than those with normal or mild to moderate SNHL on phonemic awareness (P < .001), word attack (P = .001), letter-word identification (P = .0036), reading fluency (P = .0365), passage comprehension (P < .001), and processing speed (P < .001). Descriptive data regarding the eight neurocognitive variables 5 years after diagnosis are listed in Table 4.
TABLE 4.
Observed Means and SDs of Participants’ Scores 5 Years After Diagnosis by SNHL Group
Hearing Loss, Risk Status, and Age at Diagnosis
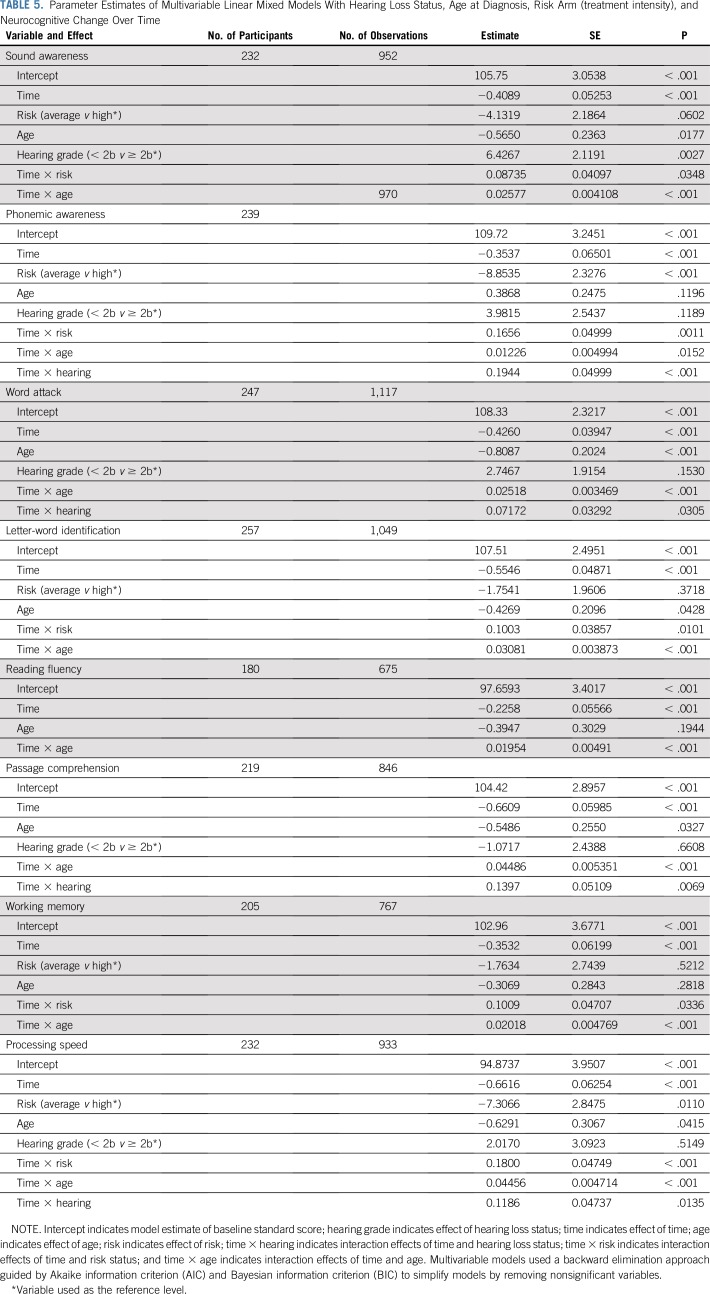
Treatment intensity (risk) and age at diagnosis are well-documented risks for neurocognitive difficulties. Given significant relationships between these risks and SNHL, risk and age were entered into the model as additional variables, with SNHL. These multivariable models indicate that participants with severe SNHL experienced greater cognitive decline over time on the following four variables: phonemic awareness (P < .001), word attack (P = .0305), passage comprehension (P = .0069), and processing speed (P = .0135), after accounting for effects of treatment intensity and age at diagnosis (Table 5; see Data Supplement for initial models without removal of insignificant variables or interactions). Results also indicate that the greatest mean standardized score declines occurred between 2 and 5 years after diagnosis (Fig 1). Follow-up analyses examining the slopes of the eight variables over time for the two SNHL groups revealed that both groups declined on three variables (ie, word attack, letter-word identification, and passage comprehension), with the severe SNHL group declining more significantly. Both groups declined on sound awareness and working memory, although the difference in slopes was not significant. The normal or mild to moderate group did not decline on reading fluency and processing speed, whereas the severe group did experience decline; the difference in the slopes was significant. The normal or mild to moderate group’s phonemic awareness scores increased over time, whereas the severe group’s scores declined, and the difference in slopes was significant.
TABLE 5.
Parameter Estimates of Multivariable Linear Mixed Models With Hearing Loss Status, Age at Diagnosis, Risk Arm (treatment intensity), and Neurocognitive Change Over Time
FIG 1.
Actual participant mean neurocognitive standard scores over time by hearing loss status. Scores have a mean of 100 and a standard deviation of 15; higher scores represent better performance. Error bars represent 95% CIs. RT, radiation therapy; WJ-III Ach, Woodcock-Johnson Third Edition, Tests of Achievement; WJ-III Cog, Woodcock-Johnson Third Edition, Tests of Cognitive Abilities.
Reading Intervention
Of the 260 included participants in this study, 53 (20.4%) were documented as having been randomly assigned in an imbedded reading intervention study26 targeting reading decoding skills (ie, Fast ForWord; Scientific Learning Corporation, Oakland, CA). Univariable linear mixed models comparing those in the reading intervention group to standard-of-care controls were conducted with regard to the eight outcome variables included in the current study. No significant associations were found based on reading intervention participation status, such that intervention participants are included for all presented analyses.
DISCUSSION
Study findings indicate that pediatric patients treated for embryonal brain tumors show declines on many language-based skills (ie, phonological, single-word decoding and recognition, reading comprehension) and supportive neurocognitive abilities (ie, working memory, processing speed) over time. Notably, compared with children with normal hearing or mild to moderate SNHL, patients with severe SNHL demonstrated a significantly greater decline on measures of phonological skills, single-word reading and decoding, reading fluency, reading comprehension, and processing speed. When controlling for disease risk status or treatment intensity and age at diagnosis, patients with severe SNHL still performed more poorly on measures of phonemic awareness and decoding, reading comprehension, and processing speed.
In contrast to our primary hypothesis, declines in most, but not all, investigated neurocognitive processes were more significant in the severe SNHL group. Age at diagnosis and risk arm or treatment intensity predicted most cognitive outcomes, consistent with previous literature.8,10,27-32 After accounting for age at diagnosis and risk, patients with severe SNHL demonstrated the steepest mean standardized score declines between 2 and 5 years after diagnosis, which occurred after the typical onset of hearing loss (ie, between the end of cisplatin administration and plateauing by 9 months)22 and were consistent with previous research documenting emergence of neurocognitive late effects.9,33
Much has been published about congenital SNHL and the need for universal newborn hearing screening (see the evidence summary by Thompson et al34), yet few rigorous scientific studies document the effects of acquired pediatric SNHL on developing neurocognition.20 Nevertheless, broader pediatric SNHL literature describes difficulties in speech and language and academic skills, suggesting underlying neurocognitive concerns. In contrast, much has been published about the effects of age-related SNHL on older adults’ cognition.35 There is evidence that even mild SNHL can have deleterious cognitive effects. Greater SNHL has also been associated with declines on nonverbal measures assessing processing speed and executive functions.36
To our knowledge, this study was novel in the primary aim to identify underlying neurocognitive skills that contribute to poor reading outcomes in children with SNHL as result of brain tumor treatment. After controlling for age at diagnosis and treatment exposure, results suggest that children with severe SNHL may struggle most with phonological skills and slowed cognitive processing speed, which affect higher level language skills, such as reading comprehension.37,38 These findings expand on previous literature outlining the relative contribution of processing speed to intellectual and academic declines.39 Because medulloblastoma was the primary diagnosis in this study, it is possible that, for some participants, deficits in processing speed and hearing are a result of ascending brainstem motor pathway and eighth cranial nerve (ie, vestibulocochlear) nuclei damage, respectively, rather than a result of nonspecific white matter changes (processing speed) and damage to basilar membrane cilia in the cochlea (hearing). However, regardless of etiology, difficulties with fundamental phonological skills, which are considered part of the building blocks of successful development of reading skills,40 in combination with slowed processing speed, put development of critical reading comprehension skills at risk.
Two unexpected but notable findings emerged. First, despite a significant decline in the severe SNHL group’s phonemic awareness scores, mean scores for both groups remained well within (or above, for the normal or mild to moderate SNHL group) the average range. This could be attributable in part to the relatively older sample size, in that some of these children would already have well-developed phonemic awareness skills, thus representing a potential ceiling effect of the measure. Second, the finding of decreased processing speed in the severe SNHL group, even after controlling for age at diagnosis and risk status or treatment intensity, is interesting, given that the tasks relate primarily to graphomotor skills, not auditory processing speed. It is possible that these impairments reflect damage to motor nuclei and ascending pathways in close proximity to the eighth cranial nerve, thereby explaining why those with greater SNHL exhibited greater processing speed declines.
Early scholastic instruction focuses on teaching children to learn to read, but a fundamental shift in instruction occurs around the third grade, such that children are no longer explicitly taught how to read and, instead, are expected to read to learn.41 Reading, therefore, becomes a gateway through which children learn nearly every other subject, and difficulties keeping pace with peers in this regard can significantly affect academic functioning over time. This expectation continues throughout one’s academic career and impacts functional learning beyond the classroom. Future neurocognitive rehabilitation efforts, therefore, should target underlying neurocognitive (ie, processing speed) and language-based (ie, phonological skills) deficits before attempting to remediate more complex literacy skills, such as reading comprehension. Although proactive efforts to bolster reading skills in a subset of these participants did not seem to be initially effective,26 follow-up analyses indicated positive neuroimaging findings with regard to trending patterns of brain activation for reading-related tasks,42 thus supporting prophylactic intervention. In addition, post hoc analyses (Data Supplement) within the current study suggest the need for intervention within the youngest age groups experiencing hearing loss (ie, younger than age 7 years).
Geriatric studies have suggested that hearing aid use is associated with positive cognitive outcomes,36 although there have been no known rigorous pediatric oncology studies examining this association. Theoretically, neurocognitive development will differ for children with SNHL who have better access to sound through assistive devices (eg, hearing aids, cochlear implants), compared with those who are unaided. Of the 169 participants from the primary study site, hearing aids were recommended for 36 patients, although only 19 patients self-reported use of hearing aids. Data regarding the use of hearing aids were unavailable for other study sites. Additional analyses were considered to examine differences between aided versus unaided children with SNHL, although small sample sizes lacked power to detect significant changes. Therefore, incomplete data regarding hearing aid adherence (including use during testing) are the primary limitation of the current study. In addition, despite a large sample, a number of clinical trial patients were not included in analyses given the lack of participation in serial neurocognitive assessments. Finally, current data were limited with respect to the examination of mild to moderate SNHL apart from normal hearing. Small group sizes did not facilitate the management of complex relationships between hearing loss and age at diagnosis, as well as hearing loss and treatment intensity. However, post hoc modeling suggested minimal risk in the mild to moderate group, a finding that requires replication.
This study documents important findings regarding specific language-based neurocognitive outcomes that are affected in children with acquired SNHL secondary to ototoxicity. Future research determining which interventions (and at what time in treatment) are most efficacious in bolstering reading skills in children undergoing cancer-directed treatment is crucial and may have important implications for others with SNHL. In addition, future research regarding the neurocognitive effects of SNHL should carefully document variables such as whether an aided device was recommended, age at first use, typical day-to-day use, and whether it was used during cognitive testing. Prospective studies investigating hearing aid adherence and neurocognitive outcomes would be particularly interesting.
ACKNOWLEDGMENT
We thank the patients and their families who volunteered their time to participate in neurocognitive and audiology assessments during their enrollment in the St Jude Medulloblastoma 03 (SJMB03) protocol. We also thank our psychological examiners (Maggi Dunavant, MS; Charlotte Fineberg-Buchner, MA; David Hopper, EdS; and Deborah Stewart, MEd) and clinical research associate, Lacey Hall, MS, MS, CCRP, CPA, for their valuable contributions to this work.
Appendix
FIG A1.
CONSORT diagram. SNHL, sensorineural hearing loss.
Footnotes
Presented, in part, at the 37th Annual Conference of the National Academy of Neuropsychology, Boston, MA, October 25-28, 2017, and the 46th Annual Meeting of the International Neuropsychological Society, Washington, DC, February 14-17, 2018.
Supported in part by the National Cancer Institute (St Jude Cancer Center Support [Core] Grant No. P30 CA21765) and the American Lebanese Syrian Associated Charities.
AUTHOR CONTRIBUTIONS
Conception and design: Traci W. Olivier, Johnnie K. Bass, Jane E. Schreiber, Melanie Bonner, Amar Gajjar, Heather M. Conklin
Administrative support: Jason M. Ashford
Provision of study materials or patients: Donald J. Mabbott, Michelle A. Swain, Karen D. Evankovich, Carol L. Armstrong, Amar Gajjar
Collection and assembly of data: Traci W. Olivier, Johnnie K. Bass, Jason M. Ashford, Rebecca Beaulieu, Sarah M. Scott, Jane E. Schreiber, Shawna Palmer, Donald J. Mabbott, Michelle A. Swain, Melanie Bonner, Robyn Boyle, Mary Lynn Chapeiski, Karen D. Evankovich, Carol L. Armstrong, Sarah J. Knight, Amar Gajjar
Data analysis and interpretation: Traci W. Olivier, Johnnie K. Bass, Shawna Palmer, Melanie Bonner, Shengjie Wu, Arzu Onar-Thomas, Amar Gajjar, Heather M. Conklin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cognitive Implications of Ototoxicity in Pediatric Patients With Embryonal Brain Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Traci W. Olivier
Consulting or Advisory Role: CogState
Patents, Royalties, Other Intellectual Property: Second author of a book published by Academic Press in 2017
Arzu Onar-Thomas
Honoraria: Eli Lilly
Research Funding: Novartis (Inst), Apexigen (Inst), Pfizer (Inst), Celgene (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Eli Lilly
Amar Gajjar
Research Funding: Genentech (Inst), Kazia Pharmaceutical (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ullrich NJ. Neurologic sequelae of brain tumors in children. J Child Neurol. 2009;24:1446–1454. doi: 10.1177/0883073809342491. [DOI] [PubMed] [Google Scholar]
- 2.Mulhern RK, Merchant TE, Gajjar A, et al. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 3.Conklin HM, Ashford JM, Howarth RA, et al. Working memory performance among childhood brain tumor survivors. J Int Neuropsychol Soc. 2012;18:996–1005. doi: 10.1017/S1355617712000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer SL, Armstrong C, Onar-Thomas A, et al. Processing speed, attention, and working memory after treatment for medulloblastoma: An international, prospective, and longitudinal study. J Clin Oncol. 2013;31:3494–3500. doi: 10.1200/JCO.2012.47.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mabbott DJ, Penkman L, Witol A, et al. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology. 2008;22:159–168. doi: 10.1037/0894-4105.22.2.159. [DOI] [PubMed] [Google Scholar]
- 6.Reeves CB, Palmer SL, Reddick WE, et al. Attention and memory functioning among pediatric patients with medulloblastoma. J Pediatr Psychol. 2006;31:272–280. doi: 10.1093/jpepsy/jsj019. [DOI] [PubMed] [Google Scholar]
- 7.Moxon-Emre I, Taylor MD, Bouffet E, et al. Intellectual outcome in molecular subgroups of medulloblastoma. J Clin Oncol. 2016;34:4161–4170. doi: 10.1200/JCO.2016.66.9077. [DOI] [PubMed] [Google Scholar]
- 8.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: A report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705–717. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulhern RK, Butler RW. Neurocognitive sequelae of childhood cancers and their treatment. Pediatr Rehabil. 2004;7:1–14. doi: 10.1080/13638490310001655528. [DOI] [PubMed] [Google Scholar]
- 10.Fouladi M, Gilger E, Kocak M, et al. Intellectual and functional outcome of children 3 years old or younger who have CNS malignancies. J Clin Oncol. 2005;23:7152–7160. doi: 10.1200/JCO.2005.01.214. [DOI] [PubMed] [Google Scholar]
- 11.van As JW, van den Berg H, van Dalen EC. Platinum-induced hearing loss after treatment for childhood cancer. Cochrane Database Syst Rev. 2016;8:CD010181. doi: 10.1002/14651858.CD010181.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bass JK, Hua CH, Huang J, et al. Hearing loss in patients who received cranial radiation therapy for childhood cancer. J Clin Oncol. 2016;34:1248–1255. doi: 10.1200/JCO.2015.63.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenna MA. Acquired hearing loss in children. Otolaryngol Clin North Am. 2015;48:933–953. doi: 10.1016/j.otc.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Flexer C. Cochlear implants and neuroplasticity: Linking auditory exposure and practice. Cochlear Implants Int. 2011;12(suppl 1):S19–S21. doi: 10.1179/146701011X13001035752255. [DOI] [PubMed] [Google Scholar]
- 15.Johnson R, Mitchell RE.(eds)Academic achievement of deaf studentsinTesting Deaf Students in an Age of Accountability Washington, DC: Gallaudet University Press; 2008. pp3850 [Google Scholar]
- 16.Farinetti A, Raji A, Wu H, et al. International consensus (ICON) on audiological assessment of hearing loss in children. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135(suppl 1):S41–S48. doi: 10.1016/j.anorl.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Suskind D. Thirty Million Words: Building a Child’s Brain. New York, NY: Dutton; 2015. [Google Scholar]
- 18.Grasty MA, Ittenbach RF, Knightly C, et al. Hearing loss after cardiac surgery in infancy: An unintended consequence of life-saving care. J Pediatr. 2018;192:144–151.e1. doi: 10.1016/j.jpeds.2017.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schreiber JE, Gurney JG, Palmer SL, et al. Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro Oncol. 2014;16:1129–1136. doi: 10.1093/neuonc/nou006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orgel E, O’Neil SH, Kayser K, et al. Effect of sensorineural hearing loss on neurocognitive functioning in pediatric brain tumor survivors. Pediatr Blood Cancer. 2016;63:527–534. doi: 10.1002/pbc.25804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurney JG, Tersak JM, Ness KK, et al. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: A report from the Children’s Oncology Group. Pediatrics. 2007;120:e1229–e1236. doi: 10.1542/peds.2007-0178. [DOI] [PubMed] [Google Scholar]
- 22.Gurney JG, Bass JK, Onar-Thomas A, et al. Evaluation of amifostine for protection against cisplatin-induced serious hearing loss in children treated for average-risk or high-risk medulloblastoma. Neuro Oncol. 2014;16:848–855. doi: 10.1093/neuonc/not241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodcock R, McGraw K, Mather N. Woodcock-Johnson Third Edition, Tests of Achievement (WJ-III Ach) Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 24.Woodcock R, McGraw K, Mather N. Woodcock-Johnson Third Edition, Tests of Cognitive Abilities (WJ-III Cog) Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 25.Chang KW, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. J Clin Oncol. 2010;28:1788–1795. doi: 10.1200/JCO.2009.24.4228. [DOI] [PubMed] [Google Scholar]
- 26.Palmer SL, Leigh L, Ellison SC, et al. Feasibility and efficacy of a computer-based intervention aimed at preventing reading decoding deficits among children undergoing active treatment for medulloblastoma: Results of a randomized trial. J Pediatr Psychol. 2014;39:450–458. doi: 10.1093/jpepsy/jst095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kieffer-Renaux V, Bulteau C, Grill J, et al. Patterns of neuropsychological deficits in children with medulloblastoma according to craniospatial irradiation doses. Dev Med Child Neurol. 2000;42:741–745. doi: 10.1017/s0012162200001377. [DOI] [PubMed] [Google Scholar]
- 28.Grill J, Renaux VK, Bulteau C, et al. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys. 1999;45:137–145. doi: 10.1016/s0360-3016(99)00177-7. [DOI] [PubMed] [Google Scholar]
- 29.Radcliffe J, Bunin GR, Sutton LN, et al. Cognitive deficits in long-term survivors of childhood medulloblastoma and other noncortical tumors: Age-dependent effects of whole brain radiation. Int J Dev Neurosci. 1994;12:327–334. doi: 10.1016/0736-5748(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 30.Ris MD, Packer R, Goldwein J, et al. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: A Children’s Cancer Group study. J Clin Oncol. 2001;19:3470–3476. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- 31.Palmer SL, Goloubeva O, Reddick WE, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: A longitudinal analysis. J Clin Oncol. 2001;19:2302–2308. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- 32.Mulhern RK, Palmer SL. Neurocognitive late effects in pediatric cancer. Curr Probl Cancer. 2003;27:177–197. doi: 10.1016/s0147-0272(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 33.Butler RW, Mulhern RK. Neurocognitive interventions for children and adolescents surviving cancer. J Pediatr Psychol. 2005;30:65–78. doi: 10.1093/jpepsy/jsi017. [DOI] [PubMed] [Google Scholar]
- 34.Thompson DC, McPhillips H, Davis RL, et al. Universal newborn hearing screening: Summary of evidence. JAMA. 2001;286:2000–2010. doi: 10.1001/jama.286.16.2000. [DOI] [PubMed] [Google Scholar]
- 35.Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173:293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66:1131–1136. doi: 10.1093/gerona/glr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joshi RM, Aaron P. The component model of reading: Simple view of reading made a little more complex. Read Psychol. 2000;21:85–97. [Google Scholar]
- 38.Engen L, Høien T. Phonological skills and reading comprehension. Read Writ. 2002;15:613–631. [Google Scholar]
- 39.Ris MD, Ryan PM, Lamba M, et al. An improved methodology for modeling neurobehavioral late-effects of radiotherapy in pediatric brain tumors. Pediatr Blood Cancer. 2005;44:487–493. doi: 10.1002/pbc.20251. [DOI] [PubMed] [Google Scholar]
- 40. Shankweiler D, Liberman IY (eds): The alphabetic principle and learning to read, in Phonology and Reading Disability: Solving the Reading Puzzle. Ann Arbor, MI, University of Michigan Press, 1989, pp 1-13. [Google Scholar]
- 41.Chall J. Stages of Reading Development. New York, NY: McGraw Hill; 1983. [Google Scholar]
- 42.Zou P, Conklin HM, Scoggins MA, et al. Functional MRI in medulloblastoma survivors supports prophylactic reading intervention during tumor treatment. Brain Imaging Behav. 2016;10:258–271. doi: 10.1007/s11682-015-9390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]