Abstract
Objective
In humans, offspring of women who are overweight or obese are more likely to develop metabolic disease later in life. Studies in lower animal species reveal that a calorically-dense maternal diet is associated with alterations in islet cell mass and function. The long-term effects of maternal diet on the structure and function of offspring islets with characteristics similar to humans are unknown. We used a well-established non-human primate (NHP) model to determine the consequences of exposure to Western-Style Diet (WSD) in utero and during lactation on islet cell mass and function in the offspring.
Methods
Female Japanese Macaques (Macaca fuscata) were fed either control (CTR) or WSD before and throughout pregnancy and lactation. Offspring were weaned onto CTR or WSD to generate four different groups based on maternal/offspring diets: CTR/CTR, WSD/CTR, CTR/WSD, and WSD/WSD. Offspring were analyzed at three years of age. Pancreatic tissue sections were immunolabelled to measure α- and β-cell mass and proliferation as well as islet vascularization. Live islets were also isolated to test the effects of WSD-exposure on islet function ex vivo. Offspring glucose tolerance was correlated with various maternal characteristics.
Results
α-cell mass was reduced as a result of maternal WSD exposure. α-cell proliferation was reduced in response to offspring WSD. Islet vasculature did not differ among the diet groups. Islets from WSD/CTR offspring secreted a greater amount of insulin in response to glucose ex vivo. We also found that maternal glucose tolerance and parity correlated with offspring glucose tolerance.
Conclusions
Maternal WSD exposure results in persistently decreased α-cell mass in the three-year old offspring. WSD/CTR islets secreted greater amounts of insulin ex vivo, suggesting that these islets are primed to hyper-secrete insulin under certain metabolic stressors. Although WSD did not induce overt impaired glucose tolerance in dams or offspring, offspring born to mothers with higher glucose excursions during a glucose tolerance test were more likely to also show higher glucose excursions.
Keywords: Developmental origins, Diabetes, Beta cell, Alpha cell, Fetal programming
Abbreviations: DOHaD, Developmental origins of health and disease; NHP, Non-human primate; CTR, Control diet; WSD, Western style diet; GTT, Glucose tolerance test; GAUC, Glucose area under the curve; IAUC, Insulin area under the curve; PECAM, Platelet and endothelial cell adhesion molecule
Highlights
-
•
Maternal Western Style Diet (WSD) results in decreased offspring α-cell mass.
-
•
Both maternal and offspring WSD lead to increased β:α cell ratio.
-
•
Islets from maternal WSD-exposed offspring secrete more insulin ex vivo.
-
•
Maternal parity and glucose tolerance correlated with offspring glucose tolerance.
-
•
Maternal factors modify the effect of WSD on offspring glucose tolerance.
1. Introduction
The Developmental Origins of Health and Disease (DOHaD) hypothesis states that the gestational and immediate postnatal environments influence long-term offspring health and play a role in adult disease etiology. Early evidence for the DOHaD hypothesis stemmed from epidemiological studies on offspring conceived during the Dutch Hunger Winter--a period of famine in the Netherlands during World War II where birth registries were maintained and records of rations were kept [1]. Children of the women who were exposed to famine during pregnancy were more likely to be obese as adults than their siblings born outside the period of famine [1], [2].
In addition to studies in humans evaluating the effects of in utero exposure to undernutrition on offspring [1], [2], it is clear that maternal obesity also leads to an increased risk of metabolic diseases in the offspring, such as obesity and/or Type 2 Diabetes (T2D) [1], [3], [4], [5]. In the US, it is estimated that over 50% of women are overweight or obese at the start of pregnancy [6], [7]. Findings from human cohort studies have been replicated with high fat diet feeding in animal models and collectively suggest that a calorically dense diet, in both the presence and absence of maternal obesity, results in an unfavorable in utero metabolic environment rendering risk of offspring obesity, glucose intolerance, and diabetes. Thus, future generations could face the consequences of maternal calorically dense diets and obesity, highlighting the need to understand the mechanisms by which maternal diet and metabolism lead to increased risk of T2D.
Studies in rodent models have described structural and functional changes to the pancreatic islets of offspring exposed to maternal obesity or high fat diet in utero [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]. In general, exposure to overnutrition leads to alterations in endocrine cell mass and diminished islet function, although outcomes vary from study to study and appear to be dependent on the specific obesity model and mouse strain used [6]. These parameters can result in vastly different offspring phenotypes in response to exposure to overnutrition, from impaired glucose tolerance persisting to the second generation [13], to a complete absence of islet structural or functional changes [19]. The discrepancies in rodent models highlight the need for animal models that more closely mimic human physiology to better understand how exposure to maternal overnutrition might affect human islet development and postnatal function, as well as increased risk for T2D.
Pregnancy in the Japanese Macaque (Macaca fuscata) more closely approximates human gestation than rodent and other litter models of gestational exposure. Dams typically give birth to a single offspring at a time, and macaque colonies are genetically heterogeneous. Islets from the macaque are well characterized and are similar to human islets in terms of structure, insulin secretion during perifusion, and expression of endocrine hormones and key transcription factors [20]. We utilized an established macaque model of maternal high fat, calorically dense diet to investigate the effects of maternal overnutrition on the offspring [21]. Female macaques were fed control diet (CTR) or Western-style diet (WSD) before and during pregnancy and through lactation. Offspring were weaned onto CTR or WSD, resulting in four different maternal/offspring diet groups: CTR/CTR, WSD/CTR, CTR/WSD, and WSD/WSD. As in human populations, maternal obesity and glucose tolerance varied within the same diet group; thus, one can independently analyze the effects of maternal metabolic phenotype as well as diet composition on the offspring.
In previous studies using this model, offspring of WSD-fed mothers had increased liver triglycerides and, when mothers were obese, histologic evidence of nonalcoholic fatty liver disease [22]. Additionally, maternal WSD exposure resulted in gut dysbiosis in the offspring, which was only partially corrected when offspring were weaned to a CTR diet [23]. Fetuses exposed to WSD had normal β-cell mass but reduced α-cell mass at late gestation [24]. At 13 months of age (five months post-weaning) offspring exposed to maternal WSD and weaned onto WSD (WSD/WSD) had an elevated β:α cell ratio compared with all other groups (CTR/CTR, WSD/CTR, CTR/WSD). Thirteen-month-old offspring exposed to maternal WSD also had impairments in islet vascular expansion in response to post-weaning WSD [25].
Here, we report on the longer-term consequences of maternal and postnatal WSD exposure on offspring islet morphology and function. We investigated whether the effects of maternal WSD persist when offspring are fed a healthy diet for 2.5 years post-weaning. Islets from WSD/CTR offspring secreted more insulin in response to a glucose challenge ex vivo compared with CTR/CTR islets. We show that α-cell mass reduction as a result of maternal WSD persists to three years of age even when offspring are weaned onto CTR diet. While maternal WSD did not alter β-cell mass or offspring glucose tolerance, offspring born to mothers with higher glucose excursions, across maternal diets, were more likely to also show higher glucose excursions.
2. Methods
2.1. Animal care
All animal procedures were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the Oregon National Primate Research Center (ONPRC) and Oregon Health and Science University and were approved by the ONPRC IACUC. The ONPRC abides by the Animal Welfare Act and Regulations enforced by the USDA and the Public Health Service Policy on Humane Care and Use of Laboratory Animals in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
2.2. Animal housing and diet
Adult female Japanese macaques (M. fuscata), starting at four to seven years of age, were placed on either a control (CTR- Fiber Balanced Diet 5000; Purina Mills) or Western-style diet (WSD- TAD Diet no. 5LOP, Test Diet, Purina Mills) for a minimum of two years. The CTR diet is made up of 15% of calories from fat, whereas the WSD has approximately 36% of calories from fat and animals in this group received calorically dense treats once per day as previously described [22].
All animals were housed in social environments comprised of several females to a male to facilitate optimal breeding. Females were sedated two to three times during pregnancy for fetal dating and third trimester measures. Pregnant females gave birth naturally in their social groups and most offspring began independently ingesting the maternal diet by four months of age but were allowed to stay with their mothers until weaning at between seven and nine months of age. Because of the length of the breeding season, multiple offspring groups are formed each year to keep offspring within the desired age range. Birth weight did not differ at one month of age between male and female offspring exposed to either CTR or WSD diet (data not shown). Post weaning diet group was determined by the needs of the study and the maternal diet of the juveniles in each weaning group. Weaned offspring were placed in social groups with similarly aged offspring and one to two adult females. Offspring either remained on their maternal diet after weaning or were placed on the opposite diet to generate four groups based on maternal and offspring diet as previously described [21], [22], [23]. Islets and tissue samples utilized in the current study were limited based on the number of offspring of the correct age available during the study period. Only CTR/CTR and WSD/CTR offspring were available for the past two seasons.
2.3. Intravenous glucose tolerance testing (ivGTT)
Intravenous glucose tolerance tests were performed on pregnant adult females during the early third trimester (gestational day ∼123) and on offspring at approximately 36 months of age, just prior to necropsy, for a measurement of insulin sensitivity as previously described [22]. Briefly, animals were fasted overnight and sedated with Telazol (3–8 mg/kg IM Tiletamine HCl/Zolazepam HCl, Fort Dodge Animal Health, Fort Dodge, Iowa, USA). If needed, additional anesthesia was accomplished with Ketamine (3–10 mg/kg IM, Abbott Laboratories, North Chicago, Illinois, USA). Once sedated, animals received an IV glucose bolus (50% dextrose solution) at a dose of 0.6 g/kg via the saphenous vein. Baseline blood samples were obtained prior to the infusion and at 1, 3, 5, 10, 20, 40, and 60 min after infusion. Glucose was measured immediately using OneTouch Ultra Blood Glucose Monitor (LifeScan), and the remainder of the blood was kept in heparinized tubes on ice for insulin measurement. After centrifugation, samples were stored at −80 °C until assayed. Insulin measurements were performed by the Endocrine Technologies Support Core (ETSC) at the ONPRC using a chemiluminescence-based automatic clinical platform (Roche Diagnostics Cobas e411, Indianapolis, IN, USA).
2.4. Percent body fat
Total body fat mass, lean mass, percent body fat, bone mineral content (BMC), and bone mineral density (BMD) for each animal were measured using dual-energy X-ray absorptiometry (DEXA; Hologic QDR Discovery A; Hologic, Inc., Bedford, MA, USA). After an overnight fast, animals were sedated with 3–5 mg/kg Telazol (Tiletamine HCl/Zolazepam HCl, Fort Dodge Animal Health, Fort Dodge, Iowa, USA) and then supplemented with small doses of Ketamine (5–10 mg/kg, Abbott Laboratories, North Chicago, Illinois, USA) if needed to maintain sedation during the procedure and positioned prone on the bed of the scanner. Total body scans were performed on each animal. QDR software (Hologic) was used to calculate body composition, BMC, and BMD.
2.5. Isolation of pancreatic islets
Primates were sedated with 15–20 mg/kg ketamine and humanely euthanized with sodium pentobarbital followed by exsanguination. Pancreata were excised from surrounding tissues and placed in ice-cold phosphate-buffered saline (PBS, Sigma–Aldrich) prior to islet isolations. Pancreata were removed from PBS and inflated with collagenase P (0.5 mg/mL, 80 mL total volume, Sigma–Aldrich) via cannulation of the pancreatic duct using 18-20-gauge catheters. Once inflated, each pancreas was divided into 12 sections and digested for 27–30 min at 37 °C in a collagenase solution. Using a histopaque gradient (Sigma–Aldrich), islets were collected at the interphase between histopaque and medium. Islets were cultured overnight in supplemented RMPI 1640 media (Sigma–Aldrich) at 37 °C and 5% CO2.
2.6. Glucose-stimulated insulin secretion (GSIS) assay
After overnight recovery, islets were transferred into prepared columns and placed in a perifusion system (PERI-4.2, Biorep Technologies, Miami Lakes, FL) maintained at 37 °C. Islets were pre-incubated in Krebs–Ringer bicarbonate HEPES buffer (KRBH) containing 4.0 mM glucose for one hour at a flow rate of 100 μL/min. After pre-incubation, islets underwent 4 × 15-minute washes in 4.0 or 16.7 mM glucose-supplemented KRBH for a total of 60 min, with collections every three minutes. Collections were stored at 4 °C and processed the same day. All perifusion GSIS assays were done in triplicate. Insulin was assayed using a Human Insulin ELISA kit (ALPCO, Salem, NH) and normalized to the number of islets.
2.7. Tissue processing
The pancreas was divided into 10 pieces from head to tail, with one being head and 10 being tail as follows: 1–4 head, 5–8 body, and 9–10 tail. Every other piece was used for fresh frozen or for paraffin/OCT embedding. The pancreas was weighed and then sectioned. For paraffin embedding, tissue was placed in a cassette and submerged in 10% zinc formalin for 48 h at room temperature, then transferred to 70% ethanol at 4 °C until embedded. For frozen sections, tissue was fixed in 4% paraformaldehyde for three hours, then washed four times and equilibrated overnight in 30% sucrose/10 mM PBS at 4 °C. Tissue was embedded in OCT in a cryomold (VWR, cat. # 25608-916) and frozen at −80 °C.
2.8. β-cell mass
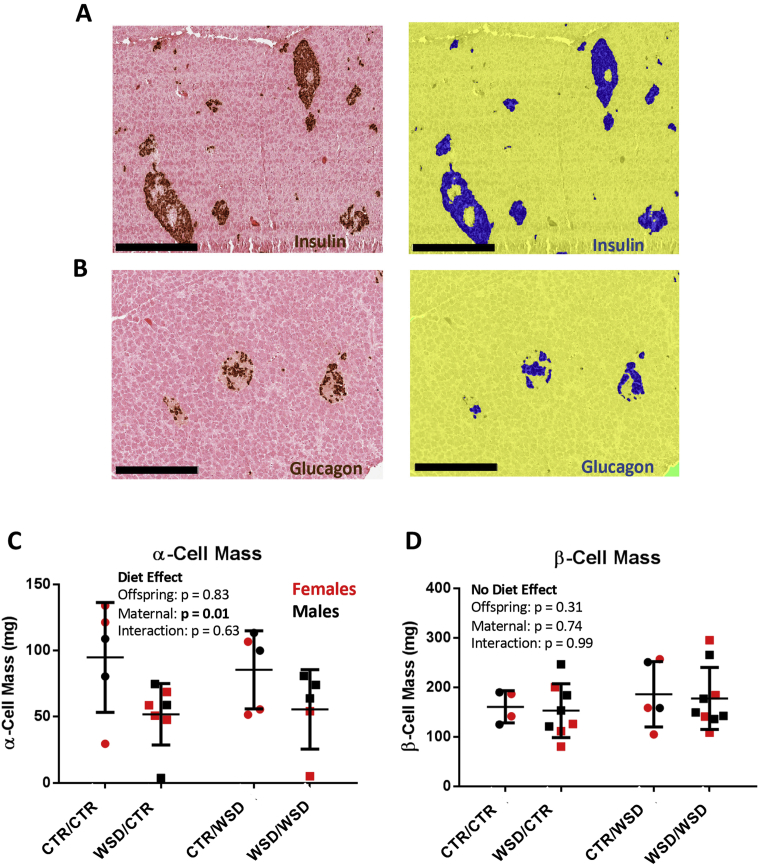
Paraffin embedded pancreata were sectioned at 5 μm. Six to nine slides (2–3 each from the head, body, and tail of the pancreas) were immunolabelled for insulin with a guinea pig anti-insulin primary antibody (Dako; 1:500), and a peroxidase-conjugated anti-guinea pig secondary antibody (Jackson ImmunoResearch; 1:400). β cells were visualized after incubation with DAB (Vector Laboratories), and the remaining tissue was counter-stained with eosin. Slides were imaged using a Scanscope CS bright field microscope (Aperio Technologies). An Aperio-based classifier was created using sample images which could accurately quantify DAB + area and total tissue area (Figure 1A), as previously described [26]. β-cell mass was calculated by multiplying the insulin-positive fraction (total DAB-positive area/total tissue area) by the weight of the pancreas. N = 4–9 animals per group.
Figure 1.
Pancreas sections were immunolabelled for either (A) insulin (brown) or (B) glucagon (brown) and counterstained with eosin (pink). Hormone-positive area was measured using a customized Aperio-based algorithm. Algorithm markup images are shown on the right in (A) and (B); 20X magnification, scale bar = 300 um. Hormone-positive tissue is marked in blue, hormone-negative tissue is in yellow, and the glass slide is green. (C) α-cell mass is significantly reduced as a result of maternal WSD; p = 0.01. (D) β-cell mass was unaffected by maternal or offspring diet. Groups are named by maternal diet/offspring diet. WSD = Western-Style Diet, CTR = Control Diet. N = 4–9 per group. Two-way ANOVA.
2.9. α-cell mass
Slides were immunolabelled, stained, imaged, and quantified as above (Figure 1B) with the following exceptions. Glucagon was labelled using a mouse anti-glucagon primary antibody (Millipore; 1:400) and an HRP-conjugated anti-mouse secondary antibody (Jackson ImmunoResearch; 1:400). N = 5–9 animals per group.
2.10. β-cell size
Paraffin embedded sections were immunolabelled for insulin as above and a Cy2-congugated anti-guinea pig secondary antibody (Jackson ImmunoResearch; 1:400), then stained with DAPI. Slides were scanned using a Scanscope FL microscope (Aperio Technologies). Insulin-positive area was annotated on the resulting images. An Aperio-based algorithm calculated total annotated area and the number of nuclei within the annotated area, which was then used to calculate average β-cell size in μm2. Over 12,000 cells were included per sample. N = 5 animals per group.
2.11. Islet vascularization
Paraffin embedded sections were immunolabelled for insulin and glucagon as above, and sheep anti-PECAM-1 (R&D Systems; 1:25). Cy2-congugated anti-guinea pig and anti-mouse secondary antibodies (Jackson ImmunoResearch; 1:400) were used to label insulin- and glucagon-positive cells and a Cy3-congugated secondary antibody was used to label PECAM-1-positive cells (Jackson ImmunoResearch; 1:400). PECAM-1-positive area was measured from at least 150 islets per sample from at least three slides >150 μm apart. Proportion of islet vascularization was determined by dividing PECAM-1-positive area by combined insulin- and glucagon-positive area. N = 4–5 animals per group.
2.12. α- and β-cell proliferation and ratio
OCT-embedded frozen (CTR/CTR and WSD/CTR groups) and paraffin embedded (CTR/WSD and WSD/WSD groups) slides were immunolabelled for insulin and glucagon as above, and Ki67 using rabbit anti-Ki67 (Abcam; 1:500), followed by Cy5-conjugated anti guinea pig, Cy3-conjugated anti-mouse, and Cy2-conjugated anti-Ki67 secondary antibodies. At least 10,000 endocrine cells were counted for each sample. For CTR/CTR and WSD/CTR groups samples, at least three slides >150 μm apart from head, body, and tail of the pancreas were analyzed. For CTR/WSD and WSD/WSD samples, at least three slides were analyzed from the head, body, and tail of the pancreas. The percentage of proliferating cells was determined by dividing the number of Ki67+/hormone+ cells by the total number of hormone+ cells (either insulin+ or glucagon+ cells). β:α cell ratio was determined by dividing the number of insulin+ cells by the number of glucagon+ cells. N = 5–9 animals per group.
2.13. Somatostatin and pancreatic polypeptide (PP)
Slides were incubated with guinea pig anti-insulin and mouse anti-glucagon antibodies as above, plus goat anti-somatostatin (1:250) and rabbit anti-PP (1:500). This was followed by incubation with Cy5 anti-guinea pig, Cy5 anti-mouse, Cy3 anti-rabbit, and Cy2 anti-goat (all 1:400). Percent somatostatin+ and PP+ cells were quantified by dividing respective cell numbers by the total number of islet cells. Between two and five thousand cells were counted per samples. N = 4 per group.
2.13.1. Statistics
Data were analyzed utilizing GraphPad Prism Software (Graphpad Software, Inc., La Jolla, CA, USA) for calculating glucose and insulin area under the curve from zero. For immunohistochemical analyses, two-way ANOVA was performed to test for effects of maternal diet, offspring diet, or interaction. For direct comparisons of continuous measures between two groups, unpaired t-test was used for normally-distributed measures and the Wilcoxon rank-sum test was used for non-parametric measures. For comparisons of categorical measures, the Pearson chi-squared test was used. For comparison of continuous measures among all four diet groups, two-way ANOVA and Tukey's multiple comparisons test were used to determine statistical significance (data are expressed as mean ± SD). For GSIS assay, a two-way ANOVA followed by Sidak post-hoc test with multiple comparisons was used to evaluate differences between treatment and time (data are expressed as mean ± SEM).
2.13.2. Multivariate analyses
We used linear regression to evaluate whether maternal factors including glucose area under the curve (GAUC) during pregnancy glucose tolerance test (GTT), diet history (years dam was on WSD), age, and parity were independently associated with offspring metabolic health, with and without adjustment for other factors. 26–36 animals were included in each analysis. We also used linear regression to calculate coefficients and 95% confidence intervals (CIs) to determine whether maternal WSD was associated with impaired glucose tolerance in offspring, stratified by offspring diet (N = 11–21 per group). Maternal parity and GAUC were selected as a priori confounders based on the literature and were included separately in adjusted models to avoid model inflation. The sandwich estimate of variance is used to obtain robust variance that accounts for correlation among siblings [27], [28].
All statistical analyses were performed at a 2-sided significance level of 0.05 using STATA 14.2 (StataCorp, Texas, USA).
3. Results
3.1. Reductions in α-cell mass persist in three year old offspring exposed to maternal WSD
We first investigated whether the decrease in α-cell mass observed in fetuses exposed to WSD persisted in offspring at three years of age. α-cell mass was indeed significantly reduced by nearly 50% as a result of maternal WSD (Figure 1C). There was no difference in α-cell size (not shown). Interestingly, a few animals had severely reduced, but detectable, α-cell mass. Offspring α-cell mass did not correlate with maternal metabolic health during pregnancy as assessed by intravenous glucose tolerance testing (data not shown). β-cell mass was unaffected by maternal diet in both WSD/CTR and WSD/WSD offspring (Figure 1D). There was no difference between male and female offspring in α-cell or β-cell mass outcomes in response to maternal diet.
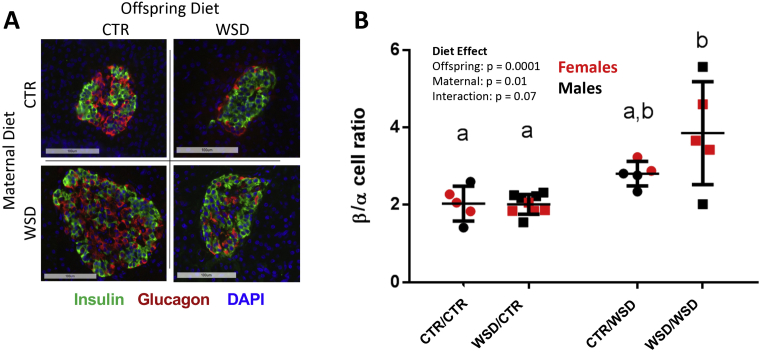
Like human islets, non-human primate (NHP) islets show heterogeneity in the proportions of the different endocrine cell types (Figure 2A) and lack the distinct mantle/core architecture observed in rodent islets, where β cells localize to the core of the islet and α cells are located at the periphery [20]. WSD-exposed fetuses had a greater than 50% increase β:α cell ratio due to a 50% decrease in α-cell mass, and 13-month-old offspring in the WSD/WSD group also had an increased β:α cell ratio (from ∼1.5 to 2.1) and decreased α-cell number [24]. At three years of age, we observed a significant increase in β:α cell ratio as a result of both maternal and offspring WSD (Figure 2B), with the WSD/WSD group having nearly double the β:α cell ratio of CTR/CTR animals. The proportion of both δ and PP cells was not affected by maternal or offspring diet (data not shown).
Figure 2.
Non-human primate islets display a heterogeneous distribution of β and α cells (A); 20X magnification, scale bar = 100 um. (B) β:α cell ratio was increased as a result of maternal diet; p = 0.01, two way ANOVA. WSD/WSD offspring had significantly greater β:α cell ratio than CTR/CTR (p = 0.0017) and WSD/CTR (p = 0.0004) offspring. N = 5–9 per group.
3.2. WSD/WSD animals tend to have reduced endocrine cell proliferation
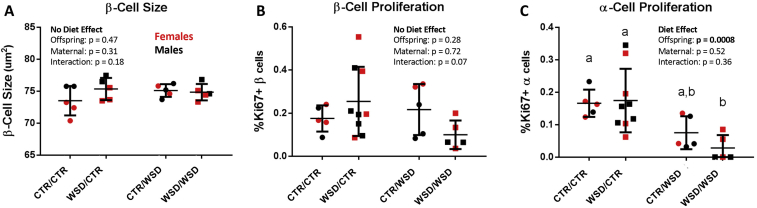
We next examined whether mechanisms of endocrine cell expansion, namely hypertrophy and proliferation, were affected by maternal diet and could explain the reduction in α-cell mass in WSD/CTR offspring. We found no evidence for β-cell hypertrophy, as β-cell size was not affected by maternal diet (Figure 3A). In general, proliferation of both α and β cells was extremely low (<0.6%) in samples from all four diet groups (Figure 3B,C). Interestingly, offspring WSD led to a significant decrease in α-cell proliferation (Figure 3C). Exposure to maternal WSD had no significant effect on proliferation of either cell type when offspring were weaned onto CTR diet or WSD. Thus, the persistent effects of in utero and early postnatal WSD exposure on α-cell mass cannot be explained by differences in α-cell proliferation at this time point.
Figure 3.
(A) β-cell size was not affected by maternal or offspring WSD. (B) β-cell proliferation trended toward reduction as a result of interaction between maternal and offspring WSD; p = 0.07, two way ANOVA. (C) α-cell proliferation was reduced as a result of offspring WSD (p = 0.0008), but not affected by maternal diet. WSD/WSD had significantly less α-cell proliferation than CTR/CTR (p = 0.03) and WSD/CTR (p = 0.01) offspring. N = 5–9 per group.
3.3. Maternal WSD has no lasting effects on islet vascularization
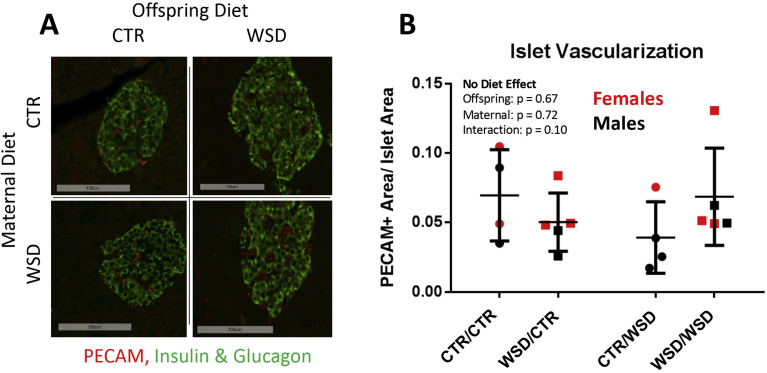
Pancreatic islets are highly vascularized [29], and the proportion of the islet that is vascularized increases in response to insulin resistance [30]. Indeed, in 13-month-old offspring, post-weaning exposure to WSD resulted in increased islet vascularization in CTR/WSD offspring relative to CTR/CTR animals [25]. This compensatory increase was absent in WSD/WSD offspring, suggesting that exposure to maternal WSD impairs the islet vascular expansion that should occur in response to WSD. We quantified islet vascularization at three years of age by utilizing the endothelial cell marker PECAM-1 (Figure 4A). Overall, islets were highly vascularized as expected. However, the CTR/WSD group no longer had elevated vascularization compared to WSD/WSD animals (Figure 4B). Thus, previous differences in islet vascularization due to maternal WSD are no longer present at three years of age.
Figure 4.
NHP islets are well vascularized, as labelled with the endothelial marker PECAM-1 (A; green = insulin + glucagon); 20X magnification, scale bar = 100 um. At three years of age, there were no differences in islet vascularization among the different diet groups (B); two-way ANOVA. N = 4–5 per group.
3.4. WSD/CTR islets hyper-secrete insulin ex vivo
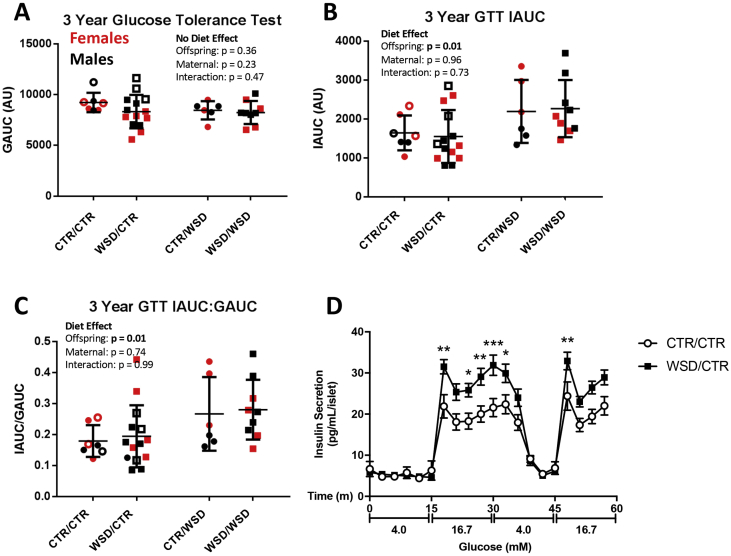
Although the long-term effects of maternal WSD exposure on islet morphology and vascularization are subtle, offspring islet cell function could still be compromised due to maternal diet exposure without obvious changes in whole islet morphology. We first investigated the effects of maternal diet on whole body glucose metabolism using intravenous glucose tolerance tests (GTTs) conducted before necropsy. Figure 5A shows the glucose area under the curve (GAUC) for the offspring GTTs. We found that offspring GAUC was not affected by either maternal or post-weaning diet. However, regardless of maternal diet, offspring weaned onto WSD had higher insulin area under the curve (IAUC) and higher insulin-to-glucose ratio by about 1.5 fold (Figure 5B,C), consistent with WSD-induced insulin resistance. We then directly tested islet whether maternal WSD exposure had an effect on islet function using an ex vivo perifusion assay to asses glucose-stimulated insulin secretion (GSIS). We predicted that islets from WSD/CTR offspring would secrete less insulin in response to a glucose challenge if maternal WSD exposure negatively affected offspring islet development. To our surprise, WSD/CTR islets secreted more insulin in response to elevated glucose than CTR/CTR islets (Figure 5D). Both first- and second-phase insulin secretion were elevated in WSD/CTR islets.
Figure 5.
Glucose area under the curve (GAUC) was not affected by maternal or post-weaning diet (A). (B) Insulin area under the curve (IAUC) during glucose tolerance tests was higher in post-weaning WSD animals (p = 0.012) but not affected by maternal diet. (C) The ratio of insulin to blood glucose was similarly elevated by offspring WSD (p = 0.0143), but unaffected by maternal diet. Two way ANOVA. N = 6–14 per group. (D) Perifusion of isolated islets reveals that WSD/CTR islets (dark squares) secreted significantly more insulin when stimulated with high glucose ex vivo; *p < 0.05, **p < 0.001, ***p < 0.0001. N = 3 per group. Open symbols in A-C represent data from animals used for the perifusions in D.
3.5. Identification of maternal factors that correlate with offspring glucose metabolism
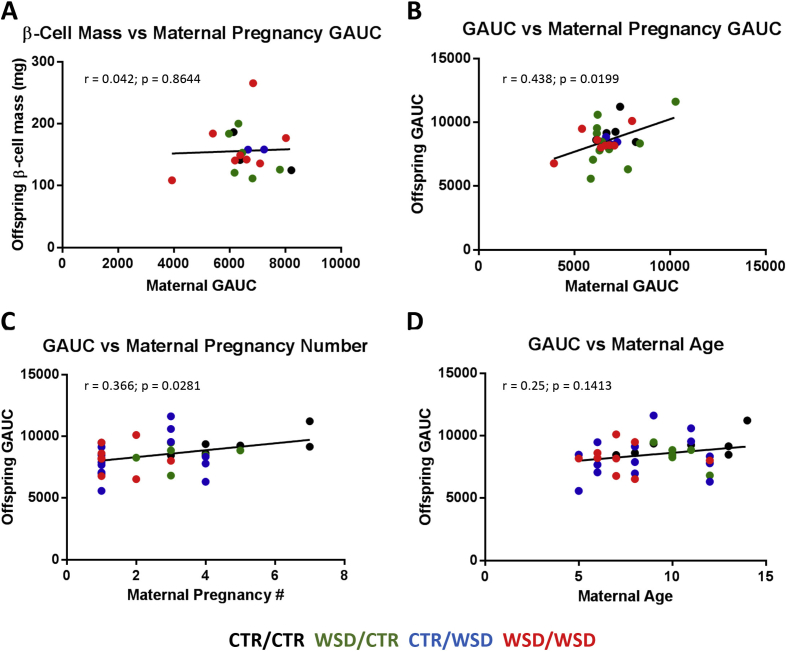
Because maternal diet alone appeared to have minimal effects on offspring glucose tolerance (Figure 5A), we wondered whether other maternal factors that may negatively affect the in utero environment were associated with poor metabolic health in the offspring. Maternal factors including age, parity, and glucose tolerance were examined for correlations with offspring phenotypes. Maternal GAUC did not correlate with β-cell mass regardless of maternal or offspring diet (Figure 6A). However, offspring GAUC was significantly correlated with maternal GAUC (Figure 6B) as well as maternal parity (Figure 6C). GAUC was not significantly correlated with maternal age (Figure 6D). Other correlations including maternal body fat percentage vs offspring GAUC or β-cell mass, maternal IAUC vs offspring or maternal GAUC, maternal years on WSD vs maternal or offspring GAUC, and offspring β-cell mass vs offspring GAUC were not significant (data not shown).
Figure 6.
Maternal GAUC during pregnancy was not correlated with β-cell mass in the offspring (A). Increases in both maternal glucose tolerance (B) and parity (C) were correlated with increasing GAUC in the offspring; p = 0.02 and p = 0.03, respectively. Maternal age trended toward correlation with impaired offspring GAUC, but this was not significant (D); p = 0.14. N = 26–36.
3.6. Maternal GAUC is independently associated with offspring GAUC
To identify independent risk factors associated with offspring metabolic health, we performed multivariate regression analyses adjusting for maternal factors including age, parity, and GAUC during pregnancy (Table 1). Characteristics of maternal/offspring pairs included in the analysis are shown in Supplementary Table 1. Maternal GAUC was significantly correlated with offspring GAUC (Figure 6B). However, this significance was no longer observed when adjusting for age or parity (Table 1A). Maternal parity was also correlated with offspring GAUC (Figure 6C). While this correlation was significant when adjusting for age, it was no longer significant when adjusting for maternal GAUC (Table 1B). Maternal age was not significantly correlated with offspring GAUC (Figure 6D), regardless of adjustment for other maternal factors (data not shown).
Table 1.
Maternal factors associated with offspring GAUC.
| Covariates in model | No.2 | Coefficient | 95% CI | P-value |
|---|---|---|---|---|
| A. Influence of covariates on the relationship between maternal GAUC and offspring GAUC (N = 36)1 | ||||
| Maternal GAUC only | 28 | 0.51 | 0.07, 0.95 | 0.02 |
| Maternal GAUC, age | 28 | 0.45 | −0.01, 0.92 | 0.05 |
| Maternal GAUC, parity | 28 | 0.42 | −0.07, 0.90 | 0.09 |
| Maternal GAUC, parity, age | 28 | 0.43 | −0.08, 0.93 | 0.09 |
| B. Influence of covariates on the relationship between parity and offspring GAUC (N = 36)1 | ||||
| Parity only | 36 | 280.36 | 59.62, 501.09 | 0.02 |
| Parity, age | 36 | 386.41 | 57.35, 715.46 | 0.02 |
| Parity, maternal GAUC | 28 | 184.56 | −69.22, 438.34 | 0.15 |
| Parity, age, maternal GAUC | 28 | 111.30 | −286.34, 508.94 | 0.57 |
Abbreviations: No., number; CI, confidence interval; GAUC, glucose tolerance test area under the curve.
Bold indicates the significant numbers.
One offspring missing GAUC value.
Eight offspring missing maternal GAUC value.
Crude and adjusted models comparing the effect of maternal diet on offspring GAUC were stratified by offspring diet (Table 2). Although maternal diet was not statistically associated with offspring GAUC, some patterns deserve note. Among offspring placed on CTR at weaning (Table 2A), maternal WSD diet appeared to be associated with a slight improvement in offspring GAUC (Figure 5A and Table 2A); however, this was not statistically significant (p = 0.12). The point estimate further shifted towards the null when adjusting for parity (coefficient went from −897 to +33) or maternal GAUC (coefficient went from −897 to −778), suggesting that maternal WSD is unlikely to improve offspring metabolic health. Among offspring weaned onto WSD (Table 2B), the direction of estimate shifted towards increased GAUC as a result of maternal WSD when adjusting for parity (coefficient went from −219 to −76) or maternal GAUC (coefficient went from −219 to +231). Thus, as hypothesized, it is likely that offspring exposed to maternal WSD would have impaired glucose tolerance, on either post-weaning diet, though we were underpowered to observe this effect.
Table 2.
Influence of covariates on the relationship between maternal WSD and offspring GAUC, stratified by offspring diet (N = 36)1.
|
A | ||||
|---|---|---|---|---|
| Covariates in model | No.2 | GAUC of offspring on CTR (n = 21) |
||
| Coefficient | 95% CI | P-value | ||
| Diet only | 21 | −897.39 | −2038.54, 243.75 | 0.12 |
| Diet, parity | 21 | 33.29 | −1858.65, 1925.24 | 0.97 |
| Diet, maternal GAUC | 17 | −777.90 | −2151.12, 595.32 | 0.25 |
| B | ||||
| Covariates in model | No.2 | GAUC of offspring on WSD (n = 15) |
||
| Coefficient | 95% CI | P-value | ||
| Diet only | 15 | −219.22 | −1565.04, 1126.59 | 0.73 |
| Diet, parity | 15 | −75.63 | −1962.25, 1810.98 | 0.93 |
| Diet, maternal GAUC | 11 | 231.48 | −686.32, 1149.28 | 0.58 |
Abbreviations: No., number; WSD, western-style diet; CTR, control diet; CI, confidence interval; GAUC, glucose tolerance test area under the curve.
One offspring on CTR is missing GAUC value.
Four offspring on CTR and four offspring on WSD are missing maternal GAUC value.
4. Discussion
In this study, we identified persistent effects of maternal WSD on offspring islet function in an animal model closely related to humans. Consistent with the phenotype observed in this model at earlier time points [24], α-cell mass is reduced at three years of age as a result of maternal WSD exposure. The reduction in α-cell mass may be due to impairments in differentiation during embryogenesis as a result of maternal diet. Indeed, fetuses exposed to WSD had decreases in the expression of transcription factors important for α-cell differentiation [24]. Interestingly, this deficit in α-cell mass was not overcome even after 2.5 years on a healthy diet.
The long-term functional consequences of α-cell loss remain unclear. Mice exposed to a high fat diet for 12 weeks also show reduced α-cell mass [31]. However, in mice, near complete ablation of α cells had no observable effects on insulin secretion and glucose tolerance [32]. Additionally, small β-cell-only pseudoislets formed from dispersed and sorted mouse islets display near-normal glucose stimulated insulin secretion [33]. However, nearly 50 years ago, rat pancreata were shown to release more insulin in response to glucose when glucagon was present [34]. This effect has more recently been observed in mice, where IV glucose plus glucagon led to higher insulin secretion in vivo than IV glucose alone [35]. In addition to glucagon, α cells also produce insulin secretagogues such as ghrelin [36] and GLP-1 [37], which act in a paracrine manner to stimulate insulin secretion. However, we observe increased insulin secretion in GSIS in islets from WSD/CTR offspring despite reduced α-cell mass.
Importantly, studies investigating the impact of maternal diet and metabolic health on islet cell mass are currently not possible in humans with available imaging techniques, highlighting the significance of the NHP model. Future studies in this model can elucidate the physiological consequences of decreased α-cell mass in a species more closely related to humans. Both NHP and human islets demonstrate a higher degree of intermingling between the different endocrine cell types, compared to rodents, with α cells making up a larger proportion of the islet in primates [20]. This suggests that paracrine interactions between α and β cells may be even more important in these species than in rodents.
Islet vascularization can increase in response to insulin resistance [30], and is also increased in T2D [38]. Islet vasculature was increased in CTR/WSD animals relative to WSD/WSD at 13 months of age [25]. The increase in vascularization at that time point was likely a response to acute exposure to WSD, while this response was absent in WSD/WSD animals, potentially due to prior exposure to the WSD. We observed that at three years of age, CTR/WSD animals no longer displayed an increase in islet vascularization (Figure 4), perhaps due to compensatory changes that re-establish homeostasis. Further studies are needed to determine whether vascular compensation in WSD/WSD offspring is impaired in other models of metabolic stress such as pregnancy.
Islets from WSD/CTR animals secreted higher amounts of insulin when stimulated with high glucose ex vivo. Interestingly, islets from individuals with higher BMI also hyper-secrete insulin ex vivo [39]. Insulin hyper-secretion is associated with worse clinical and metabolic phenotypes in humans, and predicts future deterioration of glucose control [40]. However, plasma insulin levels during GTT were not different in the offspring whose islets were used in the perifusion assay (Figure 5B, open symbols). Neither WSD/CTR nor WSD/WSD offspring had higher IAUC compared to CTR/CTR and CTR/WSD, respectively. Importantly, IAUC is affected by whole body glucose usage and disposal, and as such is not a measure of insulin secretion. These results suggest that exposure to WSD in utero and during lactation may prime islets to hyper-secrete insulin under certain conditions. However, differences in plasma insulin as a result of WSD-exposure may only manifest with more severe metabolic stressors, such as increasing age, prolonged WSD-feeding, or obesity.
One limitation of the study was that a small proportion of mothers (N = 7) gave birth more than once during the study period and thus multiple offspring from the same dam are included in the study. Given the small number of animals in our study, we used robust variance to account for correlation among siblings rather than performing a sensitivity analysis excluding subsequent pregnancies. In this genetically variable animal model, heterogeneity existed among dams in terms of age, parity, glucose tolerance, and response to glucose administration during a GTT (raw values are provided in Supplemental Table 2). This heterogeneity could be viewed as an additional limitation to the study design; however, it is more similar to the human situation and has allowed us to probe for independent maternal factors that affect offspring metabolic health.
We show that increased maternal GAUC is a potential predictor of elevated offspring GAUC. It should be noted that for completion, all dam/offspring pairs in our cohort were retained in this analysis, despite the spread in sample values. All GTTs used in the study were determined to be valid and true values based on a clear response to glucose stimulation (see Supplemental Table 2). Thus, there was no scientific basis for discarding any of the values. The robustness of our observation was significant but did rely on a limited number of dams, limiting our generalization to other experimental settings and populations. However, despite these limitations, it is worth noting that several studies in humans also demonstrate the impact of worsening maternal glucose tolerance on the offspring. A study on offspring of 1,049 Pima Indian women found that offspring of women with impaired glucose tolerance (but not gestational diabetes) during pregnancy were more likely to weigh more and have impaired glucose tolerance [41]. Another study in humans showed that increasing maternal glucose levels (even in the absence of overt diabetes) during glucose tolerance tests in pregnancy correlate with neonatal adiposity [42]. When this same cohort of offspring was assessed seven years later, increases in maternal glucose levels--both fasting and during glucose tolerance testing–were significantly associated with offspring glucose tolerance as assessed by area under the curve, independent of childhood obesity or being born large for gestation age [43]. Indeed, a currently ongoing randomized clinical trial aims to improve maternal glucose tolerance during pregnancy in order to improve health outcomes in both mother and offspring [44].
In addition to effects of the in utero environment, the relationship between maternal and offspring GAUC may be due to genetic factors. Indeed, previous work in the Japanese macaque model identified three single nucleotide polymorphisms associated with weight stability and insulin sensitivity in the dams [45]. These genomic variants that affect maternal GAUC may have been passed to the offspring that show strong correlations between maternal and offspring GAUC.
Maternal parity was also a significant predictor of worsened offspring glucose tolerance. A study in humans found that neither maternal age nor parity was associated with increased offspring adiposity, but offspring adiposity was independently associated with maternal BMI [46]. However, a larger study conducted in The Netherlands showed that multiparity was associated with maternal obesity, which was in turn associated with offspring childhood obesity [47]. Thus, the consequences of maternal parity on the offspring in humans remains somewhat unclear. Another human study showed an association between maternal age (above 35 or below 25) and poor metabolic health in the offspring [48]. We saw no statistically significant association with maternal age and offspring metabolic health in our study.
Together, these results demonstrate that longer-term effects of maternal WSD exposure on offspring islet composition and morphology are evident. WSD-exposed animals continue to display reduced α-cell mass at three years of age, while previous effects of WSD-exposure on islet vascularization are no longer present. The impact of maternal WSD feeding on the offspring cannot be offset with weaning onto a control diet, since islets of WSD/CTR animals hyper-secrete insulin ex vivo, but differences in IAUC are not observed as a result of maternal diet. Additionally, we identify maternal parity and GAUC during pregnancy as potential independent predictors of worsened glucose tolerance in the offspring. These data suggest that the in utero and early postnatal environment produced by elevated maternal GAUC impacts the metabolic health of juvenile offspring, and that maternal WSD exposure alters offspring islet function.
Acknowledgements
We would like to thank Drs. Richard O'Brien, David Wasserman, Heidi Silver, John Stafford, and David Aronoff (Vanderbilt University) for helpful discussions. J.M.E. was supported by NIGMS of the National Institutes of Health under award number T32GM007347. R.C.P. was supported by an American Heart Association Postdoctoral Fellowship (14POST20380262). D.L.T., T.A.D., C.E.M., K.M.A., A.C.P., J.E.F., P.K., and M.G. were supported by the NIH/NIDDK (R24DK090964-06).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2019.03.010.
Conflicts of interest
The authors declare no conflicts of interest.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Schulz L.C. The Dutch Hunger Winter and the developmental origins of health and disease. Proceedings of the National Academy of Sciences. 2010;107(39):16757–16758. doi: 10.1073/pnas.1012911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravelli G.P., Stein Z.A., Susser M.W. Obesity in young men after famine exposure in utero and early infancy. New England Journal of Medicine. 1976;295(7):349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 3.Drake A.J., Reynolds R.M. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010;140(3):387–398. doi: 10.1530/REP-10-0077. [DOI] [PubMed] [Google Scholar]
- 4.PM C., HM E. Review article: the short- and long-term implications of maternal obesity on the mother and her offspring. BJOG: An International Journal of Obstetrics and Gynaecology. 2006;113(10):1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 5.Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes. 1980;29(12):1023–1035. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- 6.Elsakr J.M., Gannon M. Developmental programming of the pancreatic islet by in utero overnutrition. Trends in Developmental Biology. 2017;10:79–95. [PMC free article] [PubMed] [Google Scholar]
- 7.Pregnancy Risk Assessment Monitoring Systems, Centers for Disease Control.
- 8.Cerf M.E., Louw J. Islet cell response to high fat programming in neonate, weanling and adolescent Wistar rats. Jop. 2014;15(3):228–236. doi: 10.6092/1590-8577/1534. [DOI] [PubMed] [Google Scholar]
- 9.Cerf M.E. Hyperglycaemia and reduced glucokinase expression in weanling offspring from dams maintained on a high-fat diet. British Journal of Nutrition. 2006;95(2):391–396. doi: 10.1079/bjn20051632. [DOI] [PubMed] [Google Scholar]
- 10.Cerf M.E. Islet cell response in the neonatal rat after exposure to a high-fat diet during pregnancy. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2005;288(5):R1122–R1128. doi: 10.1152/ajpregu.00335.2004. [DOI] [PubMed] [Google Scholar]
- 11.Dyrskog S.E., Gregersen S., Hermansen K. High-fat feeding during gestation and nursing period have differential effects on the insulin secretory capacity in offspring from normal Wistar rats. The Review of Diabetic Studies. 2005;2(3):136–145. doi: 10.1900/RDS.2005.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elena Zambrano T.S.-L., Calzada Lizbeth, Ibanez Carlos A., Mendoza-Rodriguez Carmen A., Morales Angelica, Morimoto Sumiko. Decreased basal insulin secretion from pancreatic islets of pups in a rat model of maternal obesity. Journal of Endocrinology. 2016;(231):49–57. doi: 10.1530/JOE-16-0321. [DOI] [PubMed] [Google Scholar]
- 13.Graus-Nunes F. Pregestational maternal obesity impairs endocrine pancreas in male F1 and F2 progeny. Nutrition. 2015;31(2):380–387. doi: 10.1016/j.nut.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Guo F., Jen K.L. High-fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiology & Behavior. 1995;57(4):681–686. doi: 10.1016/0031-9384(94)00342-4. [DOI] [PubMed] [Google Scholar]
- 15.Han J. Long-term effect of maternal obesity on pancreatic beta cells of offspring: reduced beta cell adaptation to high glucose and high-fat diet challenges in adult female mouse offspring. Diabetologia. 2005;48(9):1810–1818. doi: 10.1007/s00125-005-1854-8. [DOI] [PubMed] [Google Scholar]
- 16.Srinivasan M. Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. American Journal of Physiology Endocrinology and Metabolism. 2006;291(4):E792–E799. doi: 10.1152/ajpendo.00078.2006. [DOI] [PubMed] [Google Scholar]
- 17.Taylor P.D. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2005;288(1):R134–R139. doi: 10.1152/ajpregu.00355.2004. [DOI] [PubMed] [Google Scholar]
- 18.Yokomizo H.,I.T., Sonoda N., Sakaki Y., Maeda Y., Inoue T., Hirata E. Maternal high-fat diet induces insulin resistance and deterioration of pancreatic beta-cell function in adult offspring with sex differences in mice. American Journal of Physiology. Endocrinology and Metabolism. 2014;306(10):E1163–E1165. doi: 10.1152/ajpendo.00688.2013. [DOI] [PubMed] [Google Scholar]
- 19.Platt K.M., Charnigo R.J., Pearson K.J. Adult offspring of high-fat diet-fed dams can have normal glucose tolerance and body composition. Journal of Developmental Origins of Health and Disease. 2014;5(3):229–239. doi: 10.1017/S2040174414000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conrad E. The MAFB transcription factor impacts islet alpha-cell function in rodents and represents a unique signature of primate islet beta-cells. American Journal of Physiology. Endocrinology and Metabolism. 2016;310(1):e91–e102. doi: 10.1152/ajpendo.00285.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman J.E. Developmental programming of obesity and diabetes in mouse, monkey, and man in 2018: where are we headed? Diabetes. 2018;67(11):2137–2151. doi: 10.2337/dbi17-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCurdy C.E. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. Journal of Clinical Investigation. 2009;119(2):323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma J. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nature Communications. 2014;5:3889. doi: 10.1038/ncomms4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comstock S.M. High-fat diet consumption during pregnancy and the early post-natal period leads to decreased alpha cell plasticity in the nonhuman primate. Molecular Metabolism. 2012;2(1):10–22. doi: 10.1016/j.molmet.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pound L.D., Comstock S.M., Grove K.L. Consumption of a Western-style diet during pregnancy impairs offspring islet vascularization in a Japanese macaque model. American Journal of Physiology. Endocrinology and Metabolism. 2014;307(1):E115–E123. doi: 10.1152/ajpendo.00131.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golson M.L., Bush W.S., Brissova M. Automated quantification of pancreatic beta-cell mass. American Journal of Physiology. Endocrinology and Metabolism. 2014;306(12):E1460–E1467. doi: 10.1152/ajpendo.00591.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams R.L. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56(2):645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 28.Rogers W. Regression standard errors in clustered samples. Stata Technical Bulletin. 1993;(13):19–23. [Google Scholar]
- 29.Brissova M. Pancreatic islet production of vascular endothelial growth factor--a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55(11):2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 30.Dai C. Pancreatic islet vasculature adapts to insulin resistance through dilation and not angiogenesis. Diabetes. 2013;62(12):4144–4153. doi: 10.2337/db12-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merino B. Pancreatic alpha-cells from female mice undergo morphofunctional changes during compensatory adaptations of the endocrine pancreas to diet-induced obesity. Scientific Reports. 2015;5:11622. doi: 10.1038/srep11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorel F. Normal glucagon signaling and β-cell function after near-total α-cell ablation in adult mice. Diabetes. 2011;60(11):2872–2882. doi: 10.2337/db11-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reissaus C.A., Piston D.W. Reestablishment of glucose inhibition of glucagon secretion in small pseudoislets. Diabetes. 2017;66(4):960–969. doi: 10.2337/db16-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curry D.L. Glucagon potentiation of insulin secretion by the perfused rat pancreas. Diabetes. 1970;19(6):420. doi: 10.2337/diab.19.6.420. [DOI] [PubMed] [Google Scholar]
- 35.Song G. Glucagon increases insulin levels by stimulating insulin secretion without effect on insulin clearance in mice. Peptides. 2017;88:74–79. doi: 10.1016/j.peptides.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Date Y. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51(1):124–129. doi: 10.2337/diabetes.51.1.124. [DOI] [PubMed] [Google Scholar]
- 37.Hansen A.M. Upregulation of alpha cell glucagon-like peptide 1 (GLP-1) in Psammomys obesus--an adaptive response to hyperglycaemia? Diabetologia. 2011;54(6):1379–1387. doi: 10.1007/s00125-011-2080-1. [DOI] [PubMed] [Google Scholar]
- 38.Brissova M. Human islets have fewer blood vessels than mouse islets and the density of islet vascular structures is increased in type 2 diabetes. Journal of Histochemistry and Cytochemistry. 2015;63(8):637–645. doi: 10.1369/0022155415573324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henquin J.C. Influence of organ donor attributes and preparation characteristics on the dynamics of insulin secretion in isolated human islets. Physiological Reports. 2018;6(5) doi: 10.14814/phy2.13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trico D. Identification, pathophysiology, and clinical implications of primary insulin hypersecretion in nondiabetic adults and adolescents. JCI Insight. 2018;3(24) doi: 10.1172/jci.insight.124912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pettitt D.J. Gestational diabetes mellitus and impaired glucose tolerance during pregnancy: long-term effects on obesity and glucose tolerance in the offspring. Diabetes. 1985;34(Supplement 2):119–122. doi: 10.2337/diab.34.2.s119. [DOI] [PubMed] [Google Scholar]
- 42.Hyperglycemia and adverse pregnancy outcome (HAPO) study. Associations with neonatal anthropometrics. 2009;58(2):453–459. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tam W.H. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care. 2017;40(5):679–686. doi: 10.2337/dc16-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Godfrey K.M. Nutritional intervention preconception and during pregnancy to maintain healthy glucose metabolism and offspring health (“NiPPeR”): study protocol for a randomised controlled trial. Trials. 2017;18(1):131. doi: 10.1186/s13063-017-1875-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris R.A. Genomic variants associated with resistance to high fat diet induced obesity in a primate model. Scientific Reports. 2016;6:36123. doi: 10.1038/srep36123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynolds R.M. Maternal BMI, parity, and pregnancy weight gain: influences on offspring adiposity in young adulthood. Journal of Clinical Endocrinology & Metabolism. 2010;95(12):5365–5369. doi: 10.1210/jc.2010-0697. [DOI] [PubMed] [Google Scholar]
- 47.Gaillard R. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring) 2013;21(5):1046–1055. doi: 10.1002/oby.20088. [DOI] [PubMed] [Google Scholar]
- 48.Myrskylä M., Fenelon A. Maternal age and offspring adult health: evidence from the health and retirement study. Demography. 2012;49(4):1231–1257. doi: 10.1007/s13524-012-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.