Abstract
Background
Thermogenic adipocytes reorganize their metabolism during cold exposure. Metabolic reprogramming requires readily available bioenergetics substrates, such as glucose and fatty acids, to increase mitochondrial respiration and produce heat via the uncoupling protein 1 (UCP1). This condition generates a finely-tuned production of mitochondrial reactive oxygen species (ROS) that support non-shivering thermogenesis.
Scope of review
Herein, the findings underlining the mechanisms that regulate ROS production and control of the adaptive responses tuning thermogenesis in adipocytes are described. Furthermore, this review describes the metabolic responses to substrate availability and the consequence of mitochondrial failure to switch fuel oxidation in response to changes in nutrient availability. A framework to control mitochondrial ROS threshold to maximize non-shivering thermogenesis in adipocytes is provided.
Major conclusions
Thermogenesis synchronizes fuel oxidation with an acute and transient increase of mitochondrial ROS that promotes the activation of redox-sensitive thermogenic signaling cascade and UCP1. However, an overload of substrate flux to mitochondria causes a massive and damaging mitochondrial ROS production that affects mitochondrial flexibility. Finding novel thermogenic redox targets and manipulating ROS concentration in adipocytes appears to be a promising avenue of research for improving thermogenesis and counteracting metabolic diseases.
Keywords: Adipose tissue, Mitochondrial metabolism, Obesity, Type 2 diabetes, Adipocyte
Highlights
-
•
Mitochondrial ROS support non-shivering thermogenesis.
-
•
Thermogenic ROS are tightly related to mitochondrial metabolic reorganization.
-
•
Uncontrolled mitochondrial ROS production is causative of metabolic inflexibility.
1. Introduction
Non-shivering thermogenesis refers to the generation of heat by the body in response to cold stimuli. This physiological process was critical for the success of the earliest mammals as it allowed for occupation of cooler niches that could not be inhabited by ectoderms [1], [2]. Moreover, adaptive thermogenesis has been proposed to offer to the first small night active mammals the possibility to avoid the attention of predators [3], [4]. As such, the investigation of the mechanisms underlying non-shivering thermogenesis has been energized by the discovery of the presence of brown adipose tissue (BAT) in humans [5].
BAT is a mitochondria-rich tissue with high oxidative capacity and, when fully activated, synchronizes mainly glucose and fatty acids catabolism to finally dissipate the mitochondrial proton gradient through uncoupling protein 1 (UCP1 or thermogenin) [6]. It is now known that a considerable amount of active BAT persists in most adult humans and likely plays a metabolic homeostatic role that is exerted via a controlled modulation of nutrient and metabolite catabolism [5], [6], [7]. Adrenergically-stimulated BAT consumes large amounts of circulating fatty acids that are catabolized through mitochondrial β-oxidation [8]. In parallel, glucose is taken up and catabolized by stimulated brown adipocytes, thus impinging mitochondrial oxidative activity through the partial breakdown in the tricarboxylic acid cycle (TCA) [9], [10]. Relative to BAT's capacity to massively catabolize glucose and fatty acids during thermogenesis, glucose (2-deoxy-2-fluorine-18fluoro-d-glucose) and acetate (11C-acetate) tracers were used to unequivocally demonstrate the existence of metabolically active BAT in human also in response to cold exposure [8], [11]. As a consequence of its glucose and fatty acids avidity, BAT has gathered increasing attention as a therapeutic target for fighting human obesity and related metabolic disorders [12]. Importantly, an inverse relationship between the BAT activity and adiposity suggests that, by virtue of its dissipating activity, BAT is protective against body fatness in humans. In particular, retrospective studies have revealed that BAT amount and activity are lower in individuals with higher body mass index [7].
For a long time, adipose tissue has been categorized as BAT and white adipose tissue (WAT) in relation to the function and morphology. In contraposition to the thermogenic role of BAT, WAT was first recognized as an energetic sink that liberates stored fatty acids in response to energetic demands of high oxidative tissues. Specialized cells interspersed in WAT, the so-called beige adipocytes, have been discovered so far that, in a way similar to brown adipocytes and upon precise circumstances (e.g. cold exposure, physical exercise, fasting), are recruited and capable of dissipating energy in the form of heat, thus contributing to maintenance of systemic energetic homeostasis [13], [14]. A white-to-brown conversion is therefore triggered (a process also named “browning”) that can be recognized by the appearance of UCP1 positive adipocytes finely resembling brown adipocytes [14]. A metabolic switch also characterizes WAT undergoing browning that consists in the enhancement of mitochondrial mass and oxidative capacity in adipocytes [15], [16]. For this reason, inducing a robust WAT remodeling towards beige adipocytes and igniting thermogenesis in BAT constitute promising strategies for interventions aiming at preventing and/or treating obesity and its metabolic complications. However, further research is needed to better understand the molecular and metabolic pathways underlying and modulating these physiological processes.
Reactive oxygen species (ROS) represent at the same time important signaling and damaging molecules depending on their concentration levels [17]. It is now emerging that mitochondria produce ROS during thermogenesis [18], [19]. Moreover, a change in thiol redox state also occurs with a shift towards pro-oxidizing conditions. Both events are finely controlled through feedback mechanisms to overwhelm oxidative stress but are mandatory for thermogenesis. Actually, buffering ROS production or thiol oxidation significantly abrogates cold tolerance and WAT browning [18], [20], [21]. BAT thermogenesis is also under the control of circulating nutrients. In particular, after meal consumption, BAT activity contributes to production of heat and increases energy expenditure. By contrast, upon excessive calorie intake, a large number of oxidizable substrates converge on adipocyte mitochondria leading to uncontrolled mitochondrial ROS and redox imbalance that could irreversibly damage their thermogenic machinery.
The purpose of this review is to give a comprehensive framework of the relationship between ROS/redox status and thermogenesis. Moreover, going through this review, a new class of redox-sensitive molecular targets, which may be manipulated to enhance the function of thermogenic adipose tissue, could be envisaged by the readers.
2. Mitochondrial ROS and thermogenic program of adipose cells
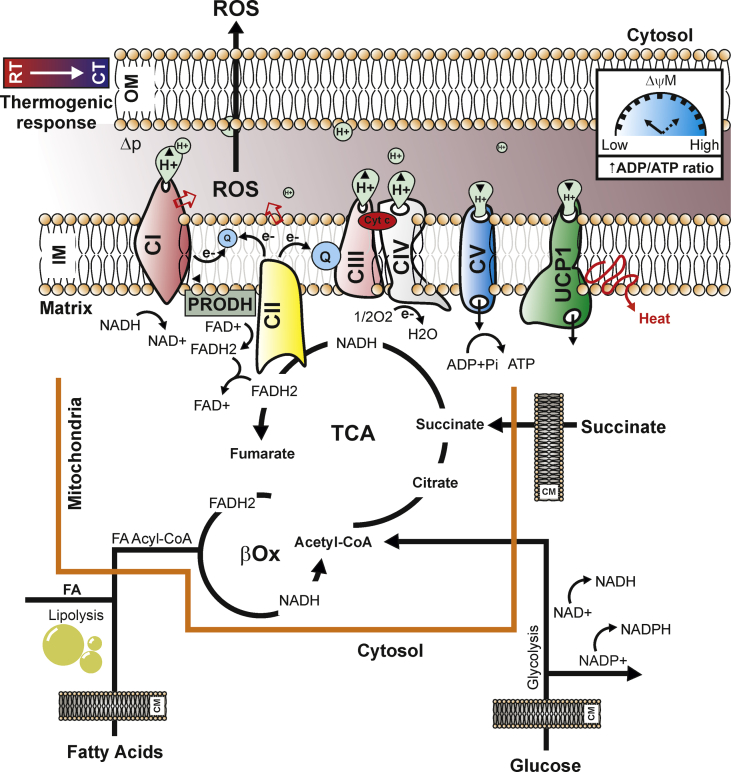
It is worth noting that, besides UCP1-mediated thermogenesis, other forms of BAT-mediated thermogenesis have been demonstrated to exist, including creatine [22], [23], [24] and lipid cycling [25] as well as mitochondrial calcium cycling [26]. In line with this, UCP1 has been shown to be dispensable for thermoregulation in certain congenic mouse strains [27]. At the molecular level, each molecule of acetyl-CoA produced by glucose, fat, or amino acid oxidation is accompanied by the generation and delivery of reducing equivalents to the electron transport chain (ETC) of mitochondria. In activated brown adipocytes, electron flux throughout the ETC functions to generate heat by proton-motive force (Δp) dissipation, which is a demand-driven process mainly regulated by ADP. Indeed, the addition of ADP as an acceptor for oxidative phosphorylation leads to a decrease in membrane potential and an increase in respiration, while ADP is being converted into ATP [28], [29]. It has been suggested that there are at least 10 sites that are capable of producing ROS within mitochondria [30]. The magnitude of ROS production is dependent on both the concentration of ETC complexes and their redox state. How effectively these electrons can go through the ETC also depends on the intensity of Δp generation by the same ETC [31]. Electrons that prematurely escape from the ETC partially reduce oxygen generating superoxide anion that is rapidly converted to hydrogen peroxide (H2O2) by superoxide dismutase [32]. It is emerging that mitochondrial ROS are molecular mediators to support adipocyte thermogenic identity and function [21], [33]. Acute activation of BAT as well as adrenergically-stimulated adipose cells elevate mitochondrial superoxide production concomitantly to UCP1-related uncoupled respiration [18]. Recently, succinate dehydrogenase (SDH) activation was demonstrated to be involved in the triggering of non-shivering thermogenesis through mitochondrial ROS production [19]. SDH or mitochondrial complex II oxidizes succinate to fumarate and reduces ubiquinone, thereby creating a direct link between the tricarboxylic acid (TCA) cycle and the respiratory chain. In 1961, it was demonstrated that high succinate concentrations lead to generation of NADH from NAD+ at complex I, reducing NAD+ to NADH with electrons received from ubiquinol via reverse electron transport (RET) [34], [35]. Interestingly, RET from complex II to complex I was observed to contribute to superoxide production upon high succinate addition in purified mitochondria [36], [37]. Likely, thermogenically activated adipocytes increase succinate uptake from the bloodstream and selectively accumulate it into mitochondrial compartment, suggesting that succinate is the genuine source of thermogenic ROS in brown and beige fat. Of note, higher circulating succinate levels were detected in obese than lean human [38]. In brown adipocytes, the treatment with malonate, a competitive inhibitor of succinate oxidation by the SDH flavin, abrogates both succinate-dependent ROS production as well as mitochondrial respiration. Remarkably, the inhibition at the SDH Q-site fully block succinate-dependent respiration, indicating that electron transfer between SDH and ubiquinone (Q) is required to drive mitochondrial Q-pool reduction during thermogenesis [19]. Mitochondrial ROS are also produced by alternative mechanisms to ETC and SDH. Several reports have indeed demonstrated that the activation of proline dehydrogenase (PRODH/POX) correlates with mitochondrial ROS production [39], [40]. PRODH is a mitochondrial inner-membrane flavoenzyme that catalyzes the rate-limiting two-electron oxidation of proline to Δ1-pyrroline-5-carboxylate (P5C) and the subsequent transfer of reducing equivalents from the reduced flavin cofactor to the ETC [41]. Importantly, PRODH activity is modulated by metabolic stress such as nutrient shortage or hypoxia. In white/beige adipose cells, PRODH-derived ROS act as signaling molecules that improve the flexibility of mitochondria by dictating fatty acid oxidation and mitochondrial oxidative capacity [42], [43]. Accordingly, impaired mitochondrial ROS production and the expression of thermogenic as well as lipolytic genes was observed in PRODH down-regulating adipocytes [42]. Mitochondrial proteomics data show that components of proline metabolism were significantly enriched in thermogenic fat cells thus further supporting a role of PRODH in thermogenic and/or browning program [24]. Furthermore, PRODH-derived P5C enters into TCA cycle as α-ketoglutarate through glutamate [44], thus allowing proline to be used to sustain ETC and anaplerosis during non-shivering thermogenesis. The TCA enzyme α-ketoglutarate dehydrogenase (α-KGDH) showed the competence to generate ROS, which are strongly dependent on the NADH/NAD+ ratio [45], suggesting that in activated brown adipocytes mitochondrial ROS may also originate by α-KGDH (Figure 1).
Figure 1.
Cold exposure induces mitochondrial metabolism reprogramming in adipose cells. Exposure to cool temperatures enhances glucose, fatty acids, and succinate uptake in brown adipocytes, which boosts tricarboxylic acid cycle (TCA) providing reducing equivalents (NADH and FADH2) and acetyl-CoA. In parallel, intracellular lipolysis helps funnel fatty acids towards β-oxidation producing additional NADH, FADH2, and acetyl-CoA units. The enhanced metabolite availability is coupled to an increased redox pressure (high NADH/NAD+, FADH2/FAD+) generating the proton motive force (Δp) and electron transport flux, which are mandatory for heat production through uncoupled respiration (low ΔψM). During thermogenesis, the massive oxygen consumption and the low energy production caused by UCP1 activation could activate proline dehydrogenase (PRODH) thus producing other FADH2 molecules that donate electrons to Complex II (CII). Under such metabolic circumstance the ubiquinone (Q) receives electrons from complex I (CI) and CII, and the electrons mainly leak to produce superoxide from the CI during the oxidation of NADH to NAD+. The consequent, transient production of functional mitochondrial ROS represents an epiphenomenon of the mitochondrial reprogramming required to sustain thermogenesis. RT: Room Temperature; CT: Cool Temperature; IM: Inner Mitochondrial Membrane; OM: Outer Mitochondrial Membrane; CM: Cell Membrane; UCP1: Uncoupling Protein 1.
The importance of mitochondrial ROS in thermogenesis is also highlighted by experiments carried out on adipocyte-specific knock-out mouse of mitochondrial superoxide dismutase (AdSOD2KO) [46]. Mitochondrial superoxide dismutase (SOD2) is a key enzyme involved in the dismutation of mitochondrial superoxide to hydrogen peroxide. The absence of SOD2 in adipose tissue elevated superoxide levels and showed increased thermogenesis. Moreover, AdSOD2KO also showed an increased abundance of UCP1 expression in subcutaneous adipose tissue, which was associated with elevated mitochondrial content [46]. Accordingly, treatment with the mitochondria-targeted antioxidant MitoQ prior to cold exposure is able to restrain thermogenesis program [18].
3. Thiol redox changes in thermogenesis
Mitochondrial ROS are now well recognized as signaling molecules able to modulate reversible oxidation/reduction of critical cysteine residues within numerous proteins. The redox reactions on protein thiols affect the activities and/or functions of key thermogenic proteins in adipocytes [18], [20], [47]. The importance of thiol redox reactions in thermogenesis was also demonstrated by experiments carried out on nuclear factor-erythroid 2-related factor 2 (NRF2) knock-out mice. NRF-2 is a transcription factor controlling a broad range of ROS and thiol targeted antioxidant enzymes [48]. NRF2 knock-out mice show a prominent oxidation of thiol molecules and a white-to-brown conversion of adipose cells [49], [50]. Furthermore, an increased cysteine thiol hyperoxidation to SO3− was observed following both adrenergic stimulation and succinate treatment [18], [19]. Supplementation with antioxidant N-acetyl cysteine (NAC) reduces adipose protein thiols and reverses the increased cellular respiration detected in NRF2KO mice [50]. In line with this evidence, several authors have revealed that the pharmacological depletion of glutathione (GSH) in mice triggers a significant reduction of adipose mass [20], [51]. Reduced (GSH) and oxidized (GSSG) glutathione represent the major thiol-disulphide redox couple within all cell types [52]. Variations in GSH/GSSG towards oxidizing conditions accompany adipogenesis [53], [54], [55], [56]. Interestingly, all conditions that have been reported to impinge BAT activation and/or WAT browning (e.g. acute physical exercise, cold exposure, inflammation and cancer) represent per se oxidative stress conditions that could cause GSH oxidation. More recently, it has been demonstrated that reducing body GSH levels promotes a rapid body weight and fat mass loss in mice (within the first day of treatment) that is associated with the ignition of BAT thermogenesis [20]. Moreover, GSH depletion promotes the white-to-brown conversion of adipocytes and changes in bioenergetics similar to canonical thermogenic stimuli. Remarkably, the increase of cysteine oxidation state through diamide treatment is sufficient to drive respiration in brown adipocytes [19]. In line with the beneficial effects associated with a pro-oxidant milieu, the over-accumulation of GSH in obese was involved in the pathogenesis of insulin resistance [57].
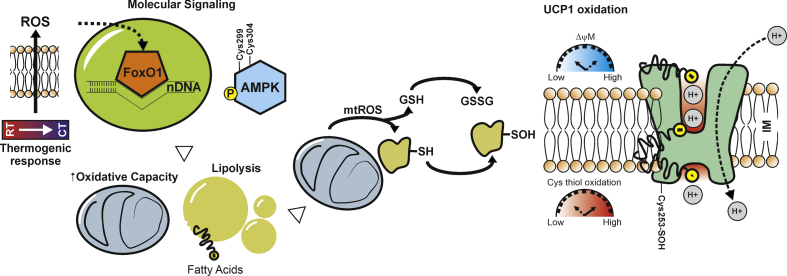
Notably, UCP1−/− mice are protected against the hypothermic effects of MitoQ, indicating that mitochondrial ROS are particularly important for UCP1-dependent thermogenesis [18]. In particular, upon cold stimulation, UCP1 undergoes sulfenylation of Cys253, thus indicating that mitochondrial ROS support thermogenesis by cysteine thiol oxidation of UCP1 (Figure 2). Limiting UCP1 thiol oxidation through NAC, a cell-permeable cysteine precursor shown to increase the intracellular pool of reduced thiols, causes significant decrease in core body temperature upon cold exposure as well as inhibits the elevation in oxygen consumption upon β3-adrenergic stimulation in mice [18].
Figure 2.
Thermogenic mitochondrial ROS drive metabolic and molecular pathways in adipocytes. Thermal shift toward cooler temperature leads to a transient production of mitochondrial ROS that induces FoxO1 nuclear redistribution and AMPK activation. This molecular signaling increases fatty acids availability (increased lipolysis and fatty acids uptake) to enhance the conductance of UCP1 to H+ sustaining the uncoupled respiration though mitochondrial oxidative flux. The thermogenic action of mitochondrial ROS seems to be mediated by reversible thiol (−SH) oxidations such as sulfenylation (−SOH) of effector mitochondrial proteins (e.g. UCP1). RT: Room Temperature; CT: Cool Temperature; GSH: reduced glutathione; GSSG: oxidized glutathione.
A recent work demonstrated that mice genetically lacking UCP1 show a dramatic reduction in electron transport chain abundance in brown fat [58]. In particular, the consequent mitochondrial dysfunction in brown adipocytes depends on ROS production by reverse electron transport through complex I and can be rescued by pharmacologic depletion of ROS levels. Notably, it has been suggested that UCP1 activity contributes to diminish the rate of ROS production (“mild-uncoupling” hypothesis) ameliorating the mitochondrial flexibility of brown fat cells [59]. Even though the effects of UCP1 on ROS production in brown fat mitochondria is still a debated matter of research, it has been suggested that ROS may directly activate UCP1, thus providing a feedback mechanism to avoid overcoming the mitochondrial ROS threshold [59], [60]. In this scenario, the mitochondrial NAD-dependent deacetylase Sirt3 could be also involved in the fine modulation of ROS production in order to maintain thermogenesis in brown fat cells [61].
4. Redox sensitive FoxO1 and Ampk in metabolic rearrangements of thermogenic adipocytes
The above described findings point to the relevance of mitochondrial ROS in adipocyte metabolic flexibility that becomes operative through key molecular sensors. Metabolic flexibility describes the ability of an organism to respond or adapt according to changes in metabolic or energy demand [62]. It is widely recognized that dietary restrictions (e.g. fasting, calorie restrictions) promote the acquisition of a brown-like phenotype in white adipose cells [21], [63], [64]. Contrasting results were observed in human wherein caloric restriction did not trigger the browning of subcutaneous white adipose tissue in obese individuals [65]. Such discrepancy could be explained by the fact that, during obesity, adipose tissue could become refractory to browning due to metabolic inflexibility of adipocyte mitochondria [62]. The transcription factor FoxO1 participates in browning by coordinating the thermogenic-like response [66], [67]. Recent findings revealed that during nutrient restriction mitochondria increase the production of ROS that are responsible for the nuclear FoxO1 redistribution, which in turn increases the transcription of mitochondrial ETC complexes (OxPHOS), a peculiar event of white-to-brown fat cell transition [21]. Notably, adipose cells lacking FoxO1 fail in igniting the metabolic reorganization of mitochondria upon nutrient restriction or thermogenic stimulus [42], [68]. FoxO1 may facilitate the fatty acid utilization by an induction of plasma membrane level of the fatty acid translocase FAT/CD36 [69], which is generally up-regulated in adipocytes during thermogenesis [70], [71]. Similarly, the pleiotropic energy sensor AMP-activated protein kinase (AMPK) [72] undergoes phospho-activation in thermogenically activated BAT [73]. AMPK responds to metabolic perturbations, which mediate the inactivation of Acetyl-CoA carboxylase (ACC) release from the inhibitory action on carnitine palmitoyltransferase 1 (CPT1), the rate-limiting enzyme for fatty acids entry into mitochondria for oxidation [74]. As a consequence, the AMPK activators inhibit ACC and enhance the expression of regulators of mitochondrial biogenesis and OxPHOS gene transcripts in BAT [75]. To confirm the role of AMPK in thermogenic responses of adipose cells, an inducible model for deletion of the two AMPK β subunits in adipocytes (iβ1β2AKO) was developed that showed cold intolerance and resistance to β-adrenergic activation [76]. Along these lines, adipocyte-specific deletion of AMPK (AMPKα-AKO) greatly reduces AMPK activity in both white and brown adipocytes impairing their thermogenic function [77]. Accordingly, the pharmacological activation of AMPK is able to induce the browning of white fat cells [78]. β3-adrenergic stimulation increases AMPK phosphorylation in UCP1 KO adipocytes to the same extent as in WT adipocytes, indicating that AMPK activation was independent of energy charge in brown adipocytes and its activation serves as an alternative thermogenic pathway. It is possible to hypothesize that AMPK could promote a “futile cycle” that becomes a supportive metabolic component of non-shivering thermogenesis. Indeed, intracellular free fatty acids can undergo a recycling into triglycerides in adrenergically stimulated fat cells [79]. Such an event is essential for the control of fatty acids levels to prevent cellular lipotoxicity [80]. Futile cycles also represent ATP sinks that drive an additional need for the regeneration of adenosine diphosphate through the mitochondrial oxidative phosphorylation and give rise to higher metabolic flux accompanied by increased heat dissipation [24], [79], [81], [82]. Other work pointed to an UCP1-independent thermogenesis via calcium cycling in mitochondria of beige adipocytes that is triggered through a Serca2b-mediated mechanism [26]; however, ATP-linked respiration was not shown in the published data, questioning the direct role of Serca2b in adipose energy turnover.
Several findings have demonstrated that AMPK senses redox imbalance. Previous reports proposed that AMPK activity responds to ROS levels by acting on redox sensitive cysteine residues (Cys-299/Cys-304) on the AMPK α subunit [83]. More recently, mitochondrial ROS have been found to promote AMPK activation and shape AMPK-dependent metabolic reprogramming in both a direct [84] and indirect manner [85]. Actually, mitochondrial ROS, in addition to directly oxidizing redox sensitive cysteines, could also affect mitochondrial ATP production and activate AMPK indirectly. These findings highlight a physiological role for mitochondrial ROS in coupling mitochondrial flexibility to AMPK-dependent programs that help maintain cellular metabolic fitness (Figure 2). However, even if it has been demonstrated that AMPK responds to mitochondrial ROS, this metabolic interaction in the thermogenic programming of adipose cells is still lacking.
5. ROS as foes of thermogenic program
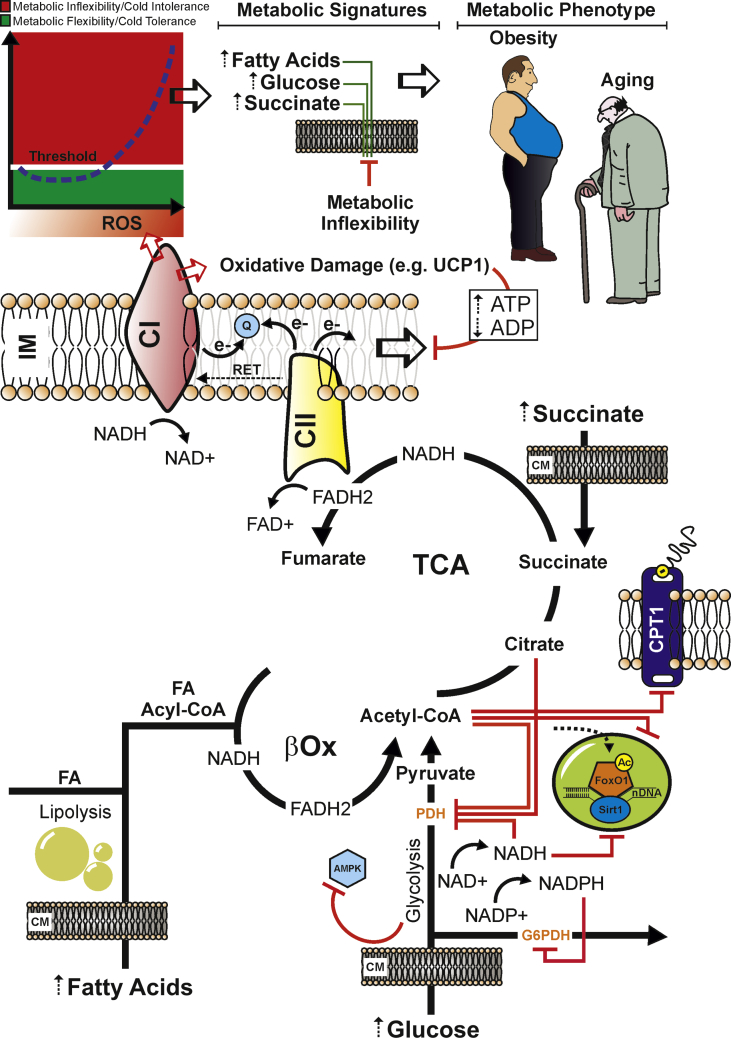
The physiological picture that accompanies the metabolic and molecular reprogramming in thermogenically active adipocytes is associated with a fine-tuned control of glucose and fatty acids catabolism leading to the accumulation of reducing equivalents (NADH and FADH2). This initiates electron flow, proton pumping, and a massive O2 consumption. The limited ATP synthesis of brown fat cells due to uncoupled respiration, however, maintains an elevated proton gradient and electron flux, thus limiting the reducing equivalent (NADH and FADH2) and substrate (mainly acetyl-CoA) accumulation into mitochondria. Meanwhile, as delivery of carbons persists and the level of excess fuel mounts, the redox pressure increases in the mitochondrial lumen leading to the inhibition of several TCA enzymes leading to mitochondrial substrate congestion and risk of metabolic damage. This phenomenon of blunted fuel switching has now been described during pathological states such as insulin resistance in which the highest circulating glucose and free fatty acid levels cause a competition of substrate (Randle cycle) leading to metabolic inflexibility [86]. The ensuing mismatch between glucose and fatty acid catabolism and TCA proceeding promotes acetyl-CoA accumulation into mitochondria. As such, a limitation at the level of oxaloacetate and/or accumulation of succinyl-CoA due to amino-acids catabolism adds further pressure on the expanding mitochondrial pool of acetyl-CoA. Furthermore, the massive substrate catabolism is also accompanied by NADH and FADH2 accumulation thus generating a massive mitochondrial flux of electrons feeding directly the coenzyme Q pool, which likely increases the ratio of reduced to oxidized Q (QH2/Q). In addition, unlike complex I, entry of electrons into the Q pool is not constrained by the membrane potential and the oxidation of FADH2 and further reduction of the Q pool proceeds [32]. Under these circumstances, the main escape route for incoming electrons occurs via the reduction of molecular oxygen and generation of mitochondrial ROS that could be detrimental at high levels. Indeed, continuous production of large amounts of mitochondrial ROS induces an irreversible oxidative damage to cellular components [87], affecting the integrity and plasticity of the metabolic network. High glucose and lipid levels also converge on acetyl-CoA production in the cytosol that is then carboxylated and converted to malonyl-CoA by one of the two isoforms of ACC. Malonyl-CoA allosterically inhibits carnitine palmitoyltransferase-1 (CPT1), the gateway for entry of fatty acids into the mitochondrial matrix, thus restraining β-oxidation [88]. Furthermore, an accumulation of acetyl-CoA in overall cellular compartment may force a hyperacetylation of key metabolic transcription factors such as FoxO1. Indeed, nuclear acetyl-FoxO1 results in a limited transcriptional activity [89] that could lead to an impairment in the expression of thermogenic and catabolic genes [90] (Figure 3).
Figure 3.
Persistent nutrient overload promotes mitochondrial exhaustion and an uncontrolled ROS production that culminates in a systemic metabolic inflexibility. Dietary nutrient overload (e.g. fats and carbohydrates excess) enhances mitochondrial redox pressure (high NADH and FADH2) and electron flow. Under such stressful conditions, Q pool becomes over-reduced, and a high membrane potential drives the reverse transfer of electrons from Q to complex I (CI) in a process called reverse electron transport (RET). During RET electrons leak at CI generating a significant amount of superoxide. Overcoming the functional redox threshold induces mitochondrial oxidative damage in the redox sensitive proteins (e.g. UCP1), affecting the uncoupled respiration. This could increase the ATP levels, thus slowing-down (negative feed-back) the mitochondria electron transfer and proton pump. Nutrient overload also causes acetyl-CoA, citrate, NADH, FADH2, and NADPH accumulation, which allosterically inhibits the metabolic checkpoints, i.e. pyruvate dehydrogenase (PDH), glucose-6-posphate dehydrogenase (G6PDH), and carnitine palmitoyltransferase I (CPT1). Hence, AMPK could be phospho-inactivated and the nuclear FoxO1 could be hyperacetylated (as consequence of NAD+-dependent Sirt1 inhibition). This metabolic and molecular setting could promote the overwhelming of the redox threshold (red zone), which is causative of cold intolerance, metabolic inflexibility and mitochondrial substrate indecision. This condition leads to a systemic impairment of fuel utilization and elevation of circulating levels of glucose, fatty acids, and succinate, which represent typical metabolic hallmarks of obesity, type 2 diabetes and aging. IM: Inner Mitochondrial Membrane; CM: Cell Membrane.
Previous experimental models of rats fed with cafeteria diet for 2 weeks show elevated energy expenditure associated with increased BAT activity [91]. The temporal association between food consumption and increased metabolic rate has prompted the hypothesis of a diet-induced thermogenesis as a physiological defense mechanism against diet-induced obesity [92], [93]. However, even if short periods of high fat diets are associated with enhanced mitochondrial activity and thermogenic capacity of BAT [94], a plethora of evidence has demonstrated that a persistent dietary fat excess leads to a systemic metabolic perturbation. It was observed that chronic high fat diets exhaust mitochondrial functionality and massively promote an uncontrolled mitochondrial ROS production in high oxidative tissues including skeletal muscles [95], [96], [97] and BAT [98], [99]. These results are in line with studies linking ROS-mediated mitochondrial dysfunctions to the metabolic perturbations observed during aging and type 2 diabetes [100], [101] and with data showing that anti-oxidant NAC supplementation in obese mice improves oxygen consumption in body fat [102].
6. Concluding remarks
A pathological and an uncontrolled mitochondrial ROS production such as that occurring upon high fat/calorie dietary patterns seem to be a causal factor of mitochondrial damage and impairment of thermogenic function in adipocytes. Such mitochondrial damage is associated with a systemic metabolic resilience characterized by increased circulating levels of energetic substrates (e.g. glucose, fatty acids and succinate) that represent a typical metabolic footprint of obesity and its related pathologies (e.g. type 2 diabetes). It is interesting that ROS also increase in a physiological range during caloric restriction, implying that ROS levels vary depending on the state of caloric intake as previously reviewed [43]. Once thought exclusively as damaging molecules, ROS, especially those physiologically produced by mitochondria, are now becoming increasingly appreciated for their integral role in cell signaling. Mitochondrial ROS are critical in driving UCP1-dependent adaptive thermogenesis in adipocytes in response to mild stressful conditions such as exposure to cool temperature or nutrient limitation (e.g. fasting, calorie restrictions). Limiting mitochondrial ROS production through genetic or pharmacological approaches blunts non-shivering thermogenesis in adipocytes; conversely, favoring controlled mitochondrial ROS production or shifting thiol redox equilibrium towards less reducing conditions ignites thermogenesis with favorable effects on body weight and systemic metabolism. Overall, this evidence underscores the strong effect of ROS modulation and redox changes on the thermogenic capacity of adipocytes. Therefore, finding novel redox targets that may be manipulated to limit oxidative damage or enhance the function of thermogenic adipose tissue represents a promising avenue of research for the development of anti-obesity and anti-diabetic drugs.
Competing interests
The author has not competing interests.
Conflict of interest
The author declares that there is no conflict of interest.
Funding
This work was supported by European Foundation for the Study of Diabetes (EFSD).
References
- 1.Chouchani E.T., Kazak L., Spiegelman B.M. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metabolism. 2019;29:27–37. doi: 10.1016/j.cmet.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Jastroch M., Oelkrug R., Keipert S. Insights into brown adipose tissue evolution and function from non-model organisms. Journal of Experimental Biology. 2018;221 doi: 10.1242/jeb.169425. [DOI] [PubMed] [Google Scholar]
- 3.Gerkema M.P., Davies W.I., Foster R.G., Menaker M., Hut R.A. The nocturnal bottleneck and the evolution of activity patterns in mammals. Proceedings of the Royal Society B: Biological Sciences. 2013;280:20130508. doi: 10.1098/rspb.2013.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genin F., Nibbelink M., Galand M., Perret M., Ambid L. Brown fat and nonshivering thermogenesis in the gray mouse lemur (Microcebus murinus) American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2003;284:R811–R818. doi: 10.1152/ajpregu.00525.2002. [DOI] [PubMed] [Google Scholar]
- 5.van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M., Kemerink G.J., Bouvy N.D. Cold-activated brown adipose tissue in healthy men. New England Journal of Medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 6.Saito M. Human brown adipose tissue: regulation and anti-obesity potential. Endocrine Journal. 2014;61:409–416. doi: 10.1507/endocrj.ej13-0527. [DOI] [PubMed] [Google Scholar]
- 7.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B. Identification and importance of brown adipose tissue in adult humans. New England Journal of Medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labbe S.M., Caron A., Bakan I., Laplante M., Carpentier A.C., Lecomte R. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. The FASEB Journal. 2015;29:2046–2058. doi: 10.1096/fj.14-266247. [DOI] [PubMed] [Google Scholar]
- 9.Carpentier A.C., Blondin D.P., Virtanen K.A., Richard D., Haman F., Turcotte E.E. Brown adipose tissue energy metabolism in humans. Frontiers in Endocrinology. 2018;9:447. doi: 10.3389/fendo.2018.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouellet V., Labbe S.M., Blondin D.P., Phoenix S., Guerin B., Haman F. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. Journal of Clinical Investigation. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hankir M.K., Klingenspor M. Brown adipocyte glucose metabolism: a heated subject. EMBO Reports. 2018;19 doi: 10.15252/embr.201846404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng Y.H., Cypess A.M., Kahn C.R. Cellular bioenergetics as a target for obesity therapy. Nature Reviews Drug Discovery. 2010;9:465–482. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kissig M., Shapira S.N., Seale P. SnapShot: Brown and beige adipose thermogenesis. Cell. 2016;166:258. doi: 10.1016/j.cell.2016.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J., Cohen P., Spiegelman B.M. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes & Development. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen P., Spiegelman B.M. Brown and beige fat: molecular parts of a thermogenic machine. Diabetes. 2015;64:2346–2351. doi: 10.2337/db15-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J., Bostrom P., Sparks L.M., Ye L., Choi J.H., Giang A.H. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ristow M., Schmeisser S. Extending life span by increasing oxidative stress. Free Radical Biology and Medicine. 2011;51:327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Chouchani E.T., Kazak L., Jedrychowski M.P., Lu G.Z., Erickson B.K., Szpyt J. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature. 2016;532:112–116. doi: 10.1038/nature17399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills E.L., Pierce K.A., Jedrychowski M.P., Garrity R., Winther S., Vidoni S. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature. 2018;560:102–106. doi: 10.1038/s41586-018-0353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lettieri Barbato D., Tatulli G., Maria Cannata S., Bernardini S., Aquilano K., Ciriolo M.R. Glutathione decrement drives thermogenic program in adipose cells. Scientific Reports. 2015;5:13091. doi: 10.1038/srep13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lettieri Barbato D., Tatulli G., Aquilano K., Ciriolo M.R. Mitochondrial hormesis links nutrient restriction to improved metabolism in fat cell. Aging. 2015;7:869–881. doi: 10.18632/aging.100832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller S., Balaz M., Stefanicka P., Varga L., Amri E.Z., Ukropec J. Proteomic analysis of human Brown adipose tissue reveals utilization of coupled and uncoupled energy expenditure pathways. Scientific Reports. 2016;6:30030. doi: 10.1038/srep30030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazak L., Chouchani E.T., Lu G.Z., Jedrychowski M.P., Bare C.J., Mina A.I. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metabolism. 2017;26:660–671. doi: 10.1016/j.cmet.2017.08.009. e663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazak L., Chouchani E.T., Jedrychowski M.P., Erickson B.K., Shinoda K., Cohen P. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015;163:643–655. doi: 10.1016/j.cell.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mottillo E.P., Balasubramanian P., Lee Y.H., Weng C., Kershaw E.E., Granneman J.G. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic beta3-adrenergic receptor activation. The Journal of Lipid Research. 2014;55:2276–2286. doi: 10.1194/jlr.M050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda K., Kang Q., Yoneshiro T., Camporez J.P., Maki H., Homma M. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nature Medicine. 2017;23:1454–1465. doi: 10.1038/nm.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofmann W.E., Liu X., Bearden C.M., Harper M.E., Kozak L.P. Effects of genetic background on thermoregulation and fatty acid-induced uncoupling of mitochondria in UCP1-deficient mice. Journal of Biological Chemistry. 2001;276:12460–12465. doi: 10.1074/jbc.M100466200. [DOI] [PubMed] [Google Scholar]
- 28.Nedergaard J., Golozoubova V., Matthias A., Asadi A., Jacobsson A., Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochimica et Biophysica Acta. 2001;1504:82–106. doi: 10.1016/s0005-2728(00)00247-4. [DOI] [PubMed] [Google Scholar]
- 29.Shabalina I.G., Petrovic N., de Jong J.M., Kalinovich A.V., Cannon B., Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Reports. 2013;5:1196–1203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 30.Starkov A.A. The role of mitochondria in reactive oxygen species metabolism and signaling. Annals of the New York Academy of Sciences. 2008;1147:37–52. doi: 10.1196/annals.1427.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial ROS-induced ROS release: an update and review. Biochimica et Biophysica Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 32.Jastroch M., Divakaruni A.S., Mookerjee S., Treberg J.R., Brand M.D. Mitochondrial proton and electron leaks. Essays in Biochemistry. 2010;47:53–67. doi: 10.1042/bse0470053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chouchani E.T., Kazak L., Spiegelman B.M. Mitochondrial reactive oxygen species and adipose tissue thermogenesis: bridging physiology and mechanisms. Journal of Biological Chemistry. 2017;292:16810–16816. doi: 10.1074/jbc.R117.789628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scialo F., Fernandez-Ayala D.J., Sanz A. Role of mitochondrial reverse electron transport in ROS signaling: potential roles in health and disease. Frontiers in Physiology. 2017;8:428. doi: 10.3389/fphys.2017.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scialo F., Sriram A., Fernandez-Ayala D., Gubina N., Lohmus M., Nelson G. Mitochondrial ROS produced via reverse electron transport extend animal lifespan. Cell Metabolism. 2016;23:725–734. doi: 10.1016/j.cmet.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drose S. Differential effects of complex II on mitochondrial ROS production and their relation to cardioprotective pre- and postconditioning. Biochimica et Biophysica Acta. 2013;1827:578–587. doi: 10.1016/j.bbabio.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Chance B., Hollunger G. The interaction of energy and electron transfer reactions in mitochondria. I. General properties and nature of the products of succinate-linked reduction of pyridine nucleotide. Journal of Biological Chemistry. 1961;236:1534–1543. [PubMed] [Google Scholar]
- 38.Serena C., Ceperuelo-Mallafre V., Keiran N., Queipo-Ortuno M.I., Bernal R., Gomez-Huelgas R. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. The ISME Journal. 2018;12:1642–1657. doi: 10.1038/s41396-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagano T., Nakashima A., Onishi K., Kawai K., Awai Y., Kinugasa M. Proline dehydrogenase promotes senescence through the generation of reactive oxygen species. Journal of Cell Science. 2017;130:1413–1420. doi: 10.1242/jcs.196469. [DOI] [PubMed] [Google Scholar]
- 40.Goncalves R.L., Rothschild D.E., Quinlan C.L., Scott G.K., Benz C.C., Brand M.D. Sources of superoxide/H2O2 during mitochondrial proline oxidation. Redox Biology. 2014;2:901–909. doi: 10.1016/j.redox.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y., Borchert G.L., Donald S.P., Diwan B.A., Anver M., Phang J.M. Proline oxidase functions as a mitochondrial tumor suppressor in human cancers. Cancer Research. 2009;69:6414–6422. doi: 10.1158/0008-5472.CAN-09-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lettieri Barbato D., Aquilano K., Baldelli S., Cannata S.M., Bernardini S., Rotilio G. Proline oxidase-adipose triglyceride lipase pathway restrains adipose cell death and tissue inflammation. Cell Death & Differentiation. 2014;21:113–123. doi: 10.1038/cdd.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lettieri Barbato D., Aquilano K. Feast and famine: adipose tissue adaptations for healthy aging. Ageing Research Reviews. 2016;28:85–93. doi: 10.1016/j.arr.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Phang J.M., Liu W., Zabirnyk O. Proline metabolism and microenvironmental stress. Annual Review of Nutrition. 2010;30:441–463. doi: 10.1146/annurev.nutr.012809.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxidants & Redox Signaling. 2005;7:1140–1149. doi: 10.1089/ars.2005.7.1140. [DOI] [PubMed] [Google Scholar]
- 46.Han Y.H., Buffolo M., Pires K.M., Pei S., Scherer P.E., Boudina S. Adipocyte-specific deletion of manganese superoxide dismutase protects from diet-induced obesity through increased mitochondrial uncoupling and biogenesis. Diabetes. 2016;65:2639–2651. doi: 10.2337/db16-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collins Y., Chouchani E.T., James A.M., Menger K.E., Cocheme H.M., Murphy M.P. Mitochondrial redox signalling at a glance. Journal of Cell Science. 2012;125:801–806. doi: 10.1242/jcs.098475. [DOI] [PubMed] [Google Scholar]
- 48.Baldelli S., Aquilano K., Ciriolo M.R. Punctum on two different transcription factors regulated by PGC-1alpha: nuclear factor erythroid-derived 2-like 2 and nuclear respiratory factor 2. Biochimica et Biophysica Acta. 2013;1830:4137–4146. doi: 10.1016/j.bbagen.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Chartoumpekis D.V., Ziros P.G., Psyrogiannis A.I., Papavassiliou A.G., Kyriazopoulou V.E., Sykiotis G.P. Nrf2 represses FGF21 during long-term high-fat diet-induced obesity in mice. Diabetes. 2011;60:2465–2473. doi: 10.2337/db11-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider K., Valdez J., Nguyen J., Vawter M., Galke B., Kurtz T.W. Increased energy expenditure, Ucp1 expression, and resistance to diet-induced obesity in mice lacking nuclear factor-erythroid-2-related transcription factor-2 (Nrf2) Journal of Biological Chemistry. 2016;291:7754–7766. doi: 10.1074/jbc.M115.673756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ortega S.P., Chouchani E.T., Boudina S. Stress turns on the heat: regulation of mitochondrial biogenesis and UCP1 by ROS in adipocytes. Adipocyte. 2017;6:56–61. doi: 10.1080/21623945.2016.1273298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giustarini D., Galvagni F., Tesei A., Farolfi A., Zanoni M., Pignatta S. Glutathione, glutathione disulfide, and S-glutathionylated proteins in cell cultures. Free Radical Biology and Medicine. 2015;89:972–981. doi: 10.1016/j.freeradbiomed.2015.10.410. [DOI] [PubMed] [Google Scholar]
- 53.Vigilanza P., Aquilano K., Baldelli S., Rotilio G., Ciriolo M.R. Modulation of intracellular glutathione affects adipogenesis in 3T3-L1 cells. Journal of Cellular Physiology. 2011;226:2016–2024. doi: 10.1002/jcp.22542. [DOI] [PubMed] [Google Scholar]
- 54.Lee H., Lee Y.J., Choi H., Ko E.H., Kim J.W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. Journal of Biological Chemistry. 2009;284:10601–10609. doi: 10.1074/jbc.M808742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu X., Zhu L., Zilbering A., Mahadev K., Motoshima H., Yao J. Hyperglycemia potentiates H(2)O(2) production in adipocytes and enhances insulin signal transduction: potential role for oxidative inhibition of thiol-sensitive protein-tyrosine phosphatases. Antioxidants & Redox Signaling. 2005;7:526–537. doi: 10.1089/ars.2005.7.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tormos K.V., Anso E., Hamanaka R.B., Eisenbart J., Joseph J., Kalyanaraman B. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metabolism. 2011;14:537–544. doi: 10.1016/j.cmet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobayashi H., Matsuda M., Fukuhara A., Komuro R., Shimomura I. Dysregulated glutathione metabolism links to impaired insulin action in adipocytes. American Journal of Physiology. Endocrinology and Metabolism. 2009;296:E1326–E1334. doi: 10.1152/ajpendo.90921.2008. [DOI] [PubMed] [Google Scholar]
- 58.Kazak L., Chouchani E.T., Stavrovskaya I.G., Lu G.Z., Jedrychowski M.P., Egan D.F. UCP1 deficiency causes brown fat respiratory chain depletion and sensitizes mitochondria to calcium overload-induced dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:7981–7986. doi: 10.1073/pnas.1705406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shabalina I.G., Vrbacky M., Pecinova A., Kalinovich A.V., Drahota Z., Houstek J. ROS production in brown adipose tissue mitochondria: the question of UCP1-dependence. Biochimica et Biophysica Acta. 2014;1837:2017–2030. doi: 10.1016/j.bbabio.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 60.Jastroch M. Uncoupling protein 1 controls reactive oxygen species in brown adipose tissue. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:7744–7746. doi: 10.1073/pnas.1709064114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi T., Wang F., Stieren E., Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. Journal of Biological Chemistry. 2005;280:13560–13567. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- 62.Goodpaster B.H., Sparks L.M. Metabolic flexibility in health and disease. Cell Metabolism. 2017;25:1027–1036. doi: 10.1016/j.cmet.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fabbiano S., Suarez-Zamorano N., Rigo D., Veyrat-Durebex C., Stevanovic Dokic A., Colin D.J. Caloric restriction leads to browning of white adipose tissue through type 2 immune signaling. Cell Metabolism. 2016;24:434–446. doi: 10.1016/j.cmet.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 64.Li G., Xie C., Lu S., Nichols R.G., Tian Y., Li L. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metabolism. 2017;26:672–685. doi: 10.1016/j.cmet.2017.08.019. e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barquissau V., Leger B., Beuzelin D., Martins F., Amri E.Z., Pisani D.F. Caloric restriction and diet-induced weight loss do not induce browning of human subcutaneous white adipose tissue in women and men with obesity. Cell Reports. 2018;22:1079–1089. doi: 10.1016/j.celrep.2017.12.102. [DOI] [PubMed] [Google Scholar]
- 66.Lettieri Barbato D., Aquilano K., Ciriolo M.R. FoxO1 at the nexus between fat catabolism and longevity pathways. Biochimica et Biophysica Acta. 2014;1841:1555–1560. doi: 10.1016/j.bbalip.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 67.Lettieri Barbato D., Tatulli G., Aquilano K., Ciriolo M.R. FoxO1 controls lysosomal acid lipase in adipocytes: implication of lipophagy during nutrient restriction and metformin treatment. Cell Death & Disease. 2013;4:e861. doi: 10.1038/cddis.2013.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ortega-Molina A., Efeyan A., Lopez-Guadamillas E., Munoz-Martin M., Gomez-Lopez G., Canamero M. Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell Metabolism. 2012;15:382–394. doi: 10.1016/j.cmet.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 69.Bastie C.C., Nahle Z., McLoughlin T., Esser K., Zhang W., Unterman T. FoxO1 stimulates fatty acid uptake and oxidation in muscle cells through CD36-dependent and -independent mechanisms. Journal of Biological Chemistry. 2005;280:14222–14229. doi: 10.1074/jbc.M413625200. [DOI] [PubMed] [Google Scholar]
- 70.Putri M., Syamsunarno M.R., Iso T., Yamaguchi A., Hanaoka H., Sunaga H. CD36 is indispensable for thermogenesis under conditions of fasting and cold stress. Biochemical and Biophysical Research Communications. 2015;457:520–525. doi: 10.1016/j.bbrc.2014.12.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lynes M.D., Leiria L.O., Lundh M., Bartelt A., Shamsi F., Huang T.L. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nature Medicine. 2017;23:631–637. doi: 10.1038/nm.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinberg G.R., Kemp B.E. AMPK in health and disease. Physiological Reviews. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 73.van Dam A.D., Kooijman S., Schilperoort M., Rensen P.C., Boon M.R. Regulation of brown fat by AMP-activated protein kinase. Trends in Molecular Medicine. 2015;21:571–579. doi: 10.1016/j.molmed.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 74.Bijland S., Mancini S.J., Salt I.P. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clinical Science. 2013;124:491–507. doi: 10.1042/CS20120536. [DOI] [PubMed] [Google Scholar]
- 75.Geerling J.J., Boon M.R., van der Zon G.C., van den Berg S.A., van den Hoek A.M., Lombes M. Metformin lowers plasma triglycerides by promoting VLDL-triglyceride clearance by brown adipose tissue in mice. Diabetes. 2014;63:880–891. doi: 10.2337/db13-0194. [DOI] [PubMed] [Google Scholar]
- 76.Mottillo E.P., Desjardins E.M., Crane J.D., Smith B.K., Green A.E., Ducommun S. Lack of adipocyte AMPK exacerbates insulin resistance and hepatic steatosis through brown and beige adipose tissue function. Cell Metabolism. 2016;24:118–129. doi: 10.1016/j.cmet.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Desjardins E.M., Steinberg G.R. Emerging role of AMPK in Brown and beige adipose tissue (BAT): implications for obesity, insulin resistance, and type 2 diabetes. Current Diabetes Reports. 2018;18:80. doi: 10.1007/s11892-018-1049-6. [DOI] [PubMed] [Google Scholar]
- 78.Wu L., Zhang L., Li B., Jiang H., Duan Y., Xie Z. AMP-activated protein kinase (AMPK) regulates energy metabolism through modulating thermogenesis in adipose tissue. Frontiers in Physiology. 2018;9:122. doi: 10.3389/fphys.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Braun K., Oeckl J., Westermeier J., Li Y., Klingenspor M. Non-adrenergic control of lipolysis and thermogenesis in adipose tissues. Journal of Experimental Biology. 2018;221 doi: 10.1242/jeb.165381. [DOI] [PubMed] [Google Scholar]
- 80.Hauck A.K., Bernlohr D.A. Oxidative stress and lipotoxicity. The Journal of Lipid Research. 2016;57:1976–1986. doi: 10.1194/jlr.R066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Flachs P., Adamcova K., Zouhar P., Marques C., Janovska P., Viegas I. Induction of lipogenesis in white fat during cold exposure in mice: link to lean phenotype. International Journal of Obesity. 2017;41:372–380. doi: 10.1038/ijo.2016.228. [DOI] [PubMed] [Google Scholar]
- 82.Rohm M., Schafer M., Laurent V., Ustunel B.E., Niopek K., Algire C. An AMP-activated protein kinase-stabilizing peptide ameliorates adipose tissue wasting in cancer cachexia in mice. Nature Medicine. 2016;22:1120–1130. doi: 10.1038/nm.4171. [DOI] [PubMed] [Google Scholar]
- 83.Shao D., Oka S., Liu T., Zhai P., Ago T., Sciarretta S. A redox-dependent mechanism for regulation of AMPK activation by Thioredoxin1 during energy starvation. Cell Metabolism. 2014;19:232–245. doi: 10.1016/j.cmet.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rabinovitch R.C., Samborska B., Faubert B., Ma E.H., Gravel S.P., Andrzejewski S. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Reports. 2017;21:1–9. doi: 10.1016/j.celrep.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 85.Hinchy E.C., Gruszczyk A.V., Willows R., Navaratnam N., Hall A.R., Bates G. Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. Journal of Biological Chemistry. 2018;293:17208–17217. doi: 10.1074/jbc.RA118.002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muoio D.M. Metabolic inflexibility: when mitochondrial indecision leads to metabolic gridlock. Cell. 2014;159:1253–1262. doi: 10.1016/j.cell.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frohnert B.I., Sinaiko A.R., Serrot F.J., Foncea R.E., Moran A., Ikramuddin S. Increased adipose protein carbonylation in human obesity. Obesity. 2011;19:1735–1741. doi: 10.1038/oby.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Foster D.W. Malonyl-CoA: the regulator of fatty acid synthesis and oxidation. Journal of Clinical Investigation. 2012;122:1958–1959. doi: 10.1172/JCI63967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daitoku H., Sakamaki J., Fukamizu A. Regulation of FoxO transcription factors by acetylation and protein-protein interactions. Biochimica et Biophysica Acta. 2011;1813:1954–1960. doi: 10.1016/j.bbamcr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 90.Lettieri-Barbato D., D'Angelo F., Sciarretta F., Tatulli G., Tortolici F., Ciriolo M.R. Maternal high calorie diet induces mitochondrial dysfunction and senescence phenotype in subcutaneous fat of newborn mice. Oncotarget. 2017;8:83407–83418. doi: 10.18632/oncotarget.19948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rothwell N.J., Stock M.J. A role for insulin in the diet-induced thermogenesis of cafeteria-fed rats. Metabolism. 1981;30:673–678. doi: 10.1016/0026-0495(81)90082-2. [DOI] [PubMed] [Google Scholar]
- 92.Bachman E.S., Dhillon H., Zhang C.Y., Cinti S., Bianco A.C., Kobilka B.K. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 93.Rothwell N.J., Stock M.J. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- 94.Hibi M., Oishi S., Matsushita M., Yoneshiro T., Yamaguchi T., Usui C. Brown adipose tissue is involved in diet-induced thermogenesis and whole-body fat utilization in healthy humans. International Journal of Obesity. 2016;40:1655–1661. doi: 10.1038/ijo.2016.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anderson E.J., Lustig M.E., Boyle K.E., Woodlief T.L., Kane D.A., Lin C.T. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. Journal of Clinical Investigation. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fisher-Wellman K.H., Neufer P.D. Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends in Endocrinol Metabolism. 2012;23:142–153. doi: 10.1016/j.tem.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lettieri-Barbato D., Cannata S.M., Casagrande V., Ciriolo M.R., Aquilano K. Time-controlled fasting prevents aging-like mitochondrial changes induced by persistent dietary fat overload in skeletal muscle. PLoS One. 2018;13:e0195912. doi: 10.1371/journal.pone.0195912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lettieri Barbato D., Tatulli G., Vegliante R., Cannata S.M., Bernardini S., Ciriolo M.R. Dietary fat overload reprograms brown fat mitochondria. Frontiers in Physiology. 2015;6:272. doi: 10.3389/fphys.2015.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liang X., Yang Q., Zhang L., Maricelli J.W., Rodgers B.D., Zhu M.J. Maternal high-fat diet during lactation impairs thermogenic function of brown adipose tissue in offspring mice. Scientific Reports. 2016;6:34345. doi: 10.1038/srep34345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bhatti J.S., Bhatti G.K., Reddy P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders - a step towards mitochondria based therapeutic strategies. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2017;1863:1066–1077. doi: 10.1016/j.bbadis.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang T., Si Y., Shirihai O.S., Si H., Schultz V., Corkey R.F. Respiration in adipocytes is inhibited by reactive oxygen species. Obesity. 2010;18:1493–1502. doi: 10.1038/oby.2009.456. [DOI] [PMC free article] [PubMed] [Google Scholar]