Abstract
Microglial cells are the predominant parenchymal immune cell of the brain. Recent evidence suggests that like peripheral immune cells, microglia patrol the brain in health and disease. Reviewing these data, we first examine the evidence that microglia invade the brain mesenchyme early in embryonic development, establish residence therein, proliferate and subsequently maintain their numbers throughout life. We, then, summarize established and novel evidence for microglial process surveillance in the healthy and injured brain. Finally, we discuss emerging evidence for microglial cell body dynamics that challenge existing assumptions of their sessile nature. We conclude that microglia are long-lived immune cells that patrol the brain through both cell body and process movements. This recognition has significant implications for neuroimmune interactions throughout the animal lifespan.
Keywords: microglia, surveillance, microglial landscape, neuroimmune interaction, epilepsy
Microglia are the predominant resident immune cells of the brain (Kofler and Wiley, 2011; Mrdjen et al., 2018) and regulate brain homeostasis by their immune surveillance (Casano and Peri, 2015; Kierdorf and Prinz, 2017; Nayak et al., 2014). Traditional immune cells exhibit robust migrations by which they patrol peripheral tissues. However, microglia are thought to be stationary with dynamic processes. Nevertheless, recent findings challenge the assumption that microglia are sessile and provide evidence for microglia migration and patrol of the brain like peripheral immune cells (Eyo et al., 2018b; Fuhrmann et al., 2010; Hefendehl et al., 2014; Stowell et al., 2018). Particularly, changes in the microglial landscape, i.e. how the microglial population is positionally arranged, increase in response to altered neuronal activities (Eyo et al., 2018b). In this review, we first summarize the evidence for microglial embryonic origins, postnatal expansion and adult maintenance. Then, we discuss recent findings on the regulation of microglial surveillance including their process dynamism and cell body movements in brain patrol.
Microglial Origins, Expansion and Maintenance.
Microglial Developmental Origins
There has been interest in the developmental origins of microglia for decades. Early competing hypotheses for microglial origins included their generation from the yolk sac or hematopoietic stem cells (HSCs). Significant progress was made when fate-mapping showed that adult murine microglia descended from yolk sac-derived cells (Ginhoux et al., 2010). Subsequently, Myb, a transcription factor that controls HSC maturation was found to be dispensable for microglial development. Conversely, Pu.1, a macrophage development transcription factor is required for microglial development (Schulz et al., 2012). Interestingly, only a third of adult microglia are labelled when fate-mapped at E7 – E7.5 (Ginhoux et al., 2010). This limited labelling of fate-mapped adult microglia might reflect a limitation in the labelling of embryonic precursors. However, an alternative hypothesis suggests that microglial progenitors distinct from yolk sac-derived cells may exist and this hypothesis is receiving some support discussed below.
In the zebrafish, distinct microglial populations colonize the embryonic and adult brain from regions homologous to the mouse yolk sac and HSC-derived cells, respectively (Xu et al., 2015). A similar two wave pattern of tissue macrophage colonization has been reported for skin-resident macrophages in mammals (Hoeffel et al., 2012). Liver-derived cells transiently infiltrate the murine brain during the first postnatal week but do not contribute to the adult microglial pool (Askew et al., 2017). Similarly, the microglial population at P1 was reduced by 40% when HSCs cells were depleted suggesting contributions to early postnatal microglia from non-yolk sac sources. However, whether these sources are required to generate the adult microglial pool was not determined (Fehrenbach et al., 2018). Nevertheless, the function of these alternate-to-yolk-sac-derived microglia in early development has not been determined. Recently, ontogenically distinct Hoxb8+ and Hoxb8− microglia were discovered in the murine brain. Hoxb8 is a transcription factor that belongs to the Hox gene family that play important roles in early development and various functions in adults. Although the majority of adult microglia are Hoxb8− and presumably emanate from the yolk sac, Hoxb8+ microglia were suggested to infiltrate the brain from the fetal liver (Chen et al., 2010; De et al., 2018). Future work is thus necessary to ascertain conclusively whether alternative sources from the yolk sac contribute to the adult microglial population.
Microglial Expansion and Maintenance in Postnatal Development
Microglia expand in the mouse brain following their entry. Indeed, their numbers increase during the first two postnatal weeks then begin to decline in the fourth postnatal week and stabilize to mature levels by the sixth postnatal week (Alliot et al., 1999; Kim et al., 2015; Nikodemova et al., 2015). In rats, abundant proliferating microglia were observed between late prenatal and early postnatal stages with peak labelling at P9 (Dalmau et al., 2003). Similarly, proliferating microglia were visualized in early postnatal rat brain slices (Petersen and Dailey, 2004). However, microglial proliferation occurred at a low frequency when studied by real time imaging in mice (Eyo et al., 2016). In addition, microglia in the early postnatal mouse subventricular zone are not proliferative (Xavier et al., 2015) though proliferating microglia were detected in the mouse basal ganglia at P8 (De Biase et al., 2017). Therefore, the role of proliferative mechanisms, the precise timing, and the regional specification of such events in early postnatal microglial expansion need to be better clarified.
Subsequent to the early postnatal microglial expansion, microglial numbers undergo significant reductions after the second postnatal week in mice (Kim et al., 2015; Nikodemova et al., 2015). The simplest and predominant mechanism proposed for this observation is apoptosis though this has been poorly documented. In the E18-P9 rat brain, microglial apoptosis was rarely detected (Dalmau et al., 2003) and microglia exhibited similar densities in Bax- (a pro-apoptotic protein) deficient mice (Eyo et al., 2016). Nevertheless, a surge in apoptotic microglia was reported at the beginning of the third postnatal week corresponding to the trimming of microglial numbers (Nikodemova et al., 2015).
Molecular mechanisms for microglial expansion and subsequent reduction have begun to be studied. Runx1, a transcription factor that regulates myeloid cell development, regulates amoeboid microglial proliferation in the postnatal forebrain (Zusso et al., 2012) and P2X7R regulates microglial proliferation in the embryonic spinal cord (Rigato et al., 2012) but not in the early postnatal hippocampus (Eyo et al., 2013). Although exogenous activation of microglial CSF1R increases microglial proliferation in the mature brain and spinal cord (De et al., 2014; Guan et al., 2016), its contribution to endogenous postnatal microglial proliferation has not been explored. Similarly, while TREM2 controls microglial proliferation in the mature brain (Eyo et al., 2018b; Zheng et al., 2017), its control of microglial proliferation in the developing brain remains to be determined and initial reports suggest that it may not regulate microglial proliferation in the development (Filipello et al., 2018). P2Y12R and CX3CR1 also contribute to microglial proliferation in the mature spinal dorsal horn after peripheral nerve injury (Gu et al., 2015; Gu et al., 2016). In summary, microglial reduction following their initial expansion has been understudied. Specifically, precise apoptotic mechanisms by which microglial numbers are trimmed need to be further clarified.
Microglial Maintenance in the Mature CNS
Once established by the sixth postnatal week, microglial density is maintained throughout life. The hypothesis that microglia, like other resident macrophages, are replenished by on-going turnover of infiltrating monocytes has now been abandoned given the results of several studies (Ajami etal., 2011; Ajami et al., 2007; Ginhoux et al., 2010; Gu et al., 2016). If self-contained and persistent throughout life, the question arises as to the mechanisms employed to maintain microglial numbers. Several recent studies have shed light on these mechanisms.
First, using multiple approaches including chronic two photon imaging, cell fate labelling and pharmacological techniques, Gomez-Nicola’s group showed that microglia undergo both proliferation and apoptosis in the adult mouse and human brain (Askew et al., 2017). Next, using a novel mouse fluorescent fate-mapping approach coupled with mathematical modeling, Prinz’s group reported varying proliferative capacities for microglia from different brain regions (Tay et al., 2017). Cortical and mid-brain microglia were minimally proliferative while microglia in the neurogenic olfactory bulb were most proliferative. Hippocampal and cerebellar microglia showed intermediate levels of proliferation. Third, using a chronic in vivo single-cell imaging approach, Jucker’s group revealed that microglia in the cortex are extremely long-lived with minimal death (Fuger et al., 2017). Since this study only examined adult microglia, it indicates that about 50% of cortical mouse microglia are maintained throughout adult life. Fourth, using carbon dating, Frisen’s group found that human microglia live an average of 4 years though some could live for up to two decades and approximately 28% of human microglia renew annually (Reu et al., 2017). Finally, Wu’s group’s recent study using chronic in vivo imaging in mice also observed that microglia exhibited little proliferation and cell death (Eyo et at, 2018b). Together, the emerging consensus of recent results suggest that microglia have a slow turnover in mammals. This implies a longevity in individual microglial presence in the brain. The implication and mechanism of this slow turnover for innate immune surveillance and response will need to be explored in future.
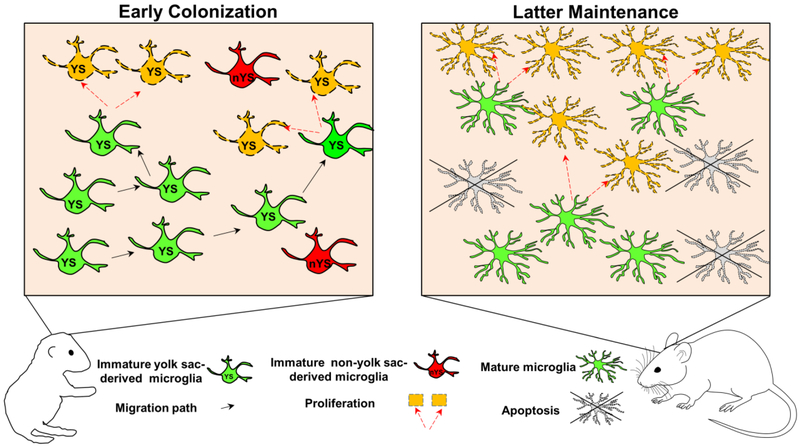
In summary, microglia originate predominantly from the embryonic yolk sac in mice. Alternative microglial sources exist in fish but whether they exist in mice has yet to be conclusively documented. These cells then colonize the brain by proliferative expansion in early pre- and postnatal development followed by latter apoptotic cell death. In adulthood, microglial numbers are maintained by the coupling of limited proliferation and apoptosis (Figure 1).
Figure 1: Microglial brain colonization and maintenance.
Microglia colonize the developing brain by a combination of migratory invasion and proliferation. While microglia originate predominantly from the embryonic yolk sac, there is some lingering debate as to whether some microglia originate from an alternative source during development. In the mature brain, the microglial population is maintained by a coupling of proliferative expansion and reductive apoptosis.
Microglia: Dynamic Process Surveillance of the CNS.
Microglial Process Surveillance in the Healthy Brain
Microglial surveillance, a process by which microglial processes are continually scanning the CNS, is important for inducing synapse formation in development (Miyamoto et al., 2016) as well as monitoring the functional state of synapses in adulthood (Tremblay et al., 2010; Wake et al., 2009). Although constitutive microglial surveillance has been established for over a decade, its underlying mechanisms have remained debated. Initially, microglial process dynamism was thought to be regulated by a constitutive release of ATP, presumably from astroglial cells. This hypothesis was developed from the observation that apyrase, which degrades extracellular ATP, significantly reduced microglial motility (Davalos et al., 2005; Wu et al., 2007). In addition, ATP receptor antagonist RB2 significantly reduced microglial basal motility (Wu et al., 2007). Moreover, astrocytes contribute to microglial morphologies (Bohlen et al., 2017; Schilling et al., 2001; Tanaka and Maeda, 1996) and astrocytic regional density correlates with microglial regional density (De Biase et al., 2017). These results suggest that microglial process dynamism may be in part directed towards astrocytes though robust evidence for this is lacking in the literature. In addition, recent data has challenged the hypothesis of constitutive astrocytic release of microglial motility-inducing factors.
One study from Barres’ group showed that microglia cultured in isolation exhibit robust process ramifications and dynamism in a cell autonomous manner (Bohlen et al., 2017). Furthermore, Attwell’s group showed that at the concentrations used, the motility-inhibiting effects of apyrase stem, not from its enzymatic activity to breakdown ATP, but rather from the presence of a higher concentration of K+ ions present in commercially available apyrase (Madry et al., 2018a). This study demonstrated that high K+ rapidly depolarizes the microglial membrane thereby inhibiting microglial motility. However, the use of other drugs such as flufenamic acid, PPADS and RB2 to block microglial process dynamics (Davalos et al., 2005; Wu et al., 2007) raised the open possibility for roles of ATP in regulating microglial motility. In addition, an alternative hypothesis has recently been proposed for the regulation of the basal microglial motility independent of ATP mechanisms (Madry et al., 2018b). THIK-1, a two-pore domain K+ channel, was identified as a regulator of basal microglial tissue surveillance and ramification. Microglial process ramification is significantly reduced when THIK-1 function is blocked pharmacologically or abolished genetically. As a result of this reduced process ramification, the area of tissue surveyed by microglia is also reduced (Madry et al., 2018b).
Microglial Process Chemotaxis in Response to Injury
In response to an acute injury, the pan-directional microglial process activity rapidly polarizes in a unidirectional manner toward the injury site (Davalos et al., 2005; Nimmerjahn et al., 2005). This is a protective phenotype as inhibition of this polarized extension of microglial processes toward the injury prevents the expansion of the injury (Hines et al., 2009). Similarly, microglial processes facilitate the resealing of damaged blood vessels to restore the integrity of the compromised blood-brain barrier (BBB) (Lou et al., 2016). This indicates that, as bona fide immune sentinels, microglia represent the first line of physical defense to injury in the brain.
The mechanism of this directed microglial process activity or chemotactic response to brain injury has been extensively studied with purinergic signaling taking center stage. P2Y12 receptors (P2Y12Rs) were identified as the primary regulators of injury-induced chemotaxis (Haynes et al., 2006). Indeed, local delivery of ATP through a pipette failed to elicit microglial process responses in P2Y12R-deficient microglia in vivo (Haynes et al., 2006). This result argues against P2Y12R-independent purine mechanisms for microglial process chemotaxis to ATP.
However, whether P2Y12Rs are the sole contributors to purine-dependent microglial directional process response to injury remains to be determined. In P2Y12R-deficient tissues, although ATP-dependent microglial process chemotaxis fails to occur, injury-induced microglial process chemotaxis persists with much slower pace (Haynes et al., 2006). These results suggest that though P2Y12R signaling is the principal microglial mechanism for the injury response, microglia possess P2Y12R-independent mechanisms which have yet to be elucidated. Candidate signaling pathways include fractalkine and cannabinoid signaling. In the mouse retina, fractalkine signaling contributes to the injury-induced microglial process chemotaxis (Liang et al., 2009). However, microglial chemotaxis to cannabinoids has thus far only been documented in dissociated cell culture contexts (Franklin et al., 2003; Franklin and Stella, 2003; Walter et al., 2003). Other candidates include serotonin, which induces microglial process chemotaxis (Kolodziejczak et al., 2015) and enhances microglial chemotaxis to injury (Krabbe et al., 2012) as well as fibrinogen, which can be released from the blood to trigger microglial process chemotaxis (Davalos et al., 2012).
Microglia Process Dynamics in Response to Neuronal Activities
In addition to injury-induced chemotaxis, microglial process dynamics also target neuronal elements as recent studies show. Microglial dynamic responses to glutamatergic signaling was first reported in the rodent retina and is dependent on ATP release (Fontainhas et al., 2011). In response to hyperactive neurons in the zebrafish or mice, microglial processes polarized towards neuronal somata or dendrites (Eyo et al., 2015; Eyo et al., 2014; Li et al., 2012). In the zebrafish, microglial interaction reduced the previous hyperactivity in contacted neurons suggesting some calming effect on the neurons (Li et al., 2012). A similar neuroprotective effect in mice was found when seizures were induced by kainic acid (Eyo et al., 2014). Here, microglia produced more processes and when such seizure-induced process increase was abolished in P2Y12-deficient mice, seizures were exacerbated supporting a calming effect of microglia specifically through P2Y12Rs (Eyo et al., 2014). Moreover, microglia display increasing contacts with neuronal dendrites in mouse and human epilepsy (Wyatt et al., 2017).
To elucidate potential mechanisms underlying this microglial activity, a simulated hyperactivity model where glutamate was bath applied to slices was adopted. Microglial process extension was observed and microglia robustly contacted neuronal somata and dendrites (Eyo et al., 2014). Following glutamate removal, a different phenomenon that was called microglial process convergence was observed whereby multiple microglial process contacted specific regions on dendrites (Eyo et al., 2017). Pharmacological interrogations of this mechanism revealed a requirement for NMDARs, specifically through their GluN2A subunit, subsequent ATP release through as-yet-unidentified avenues and activation of P2Y12R (Dissing-Olesen et al., 2014; Eyo et al., 2018a; Eyo et al., 2017; Eyo et al., 2014). Similar reductions in neuronal hyperactivity upon microglial contact of axons were reported in response to strongly depolarized neurons (Kato et al., 2016). Whether this action of microglia also required P2Y12R was not assessed. Nevertheless, the precise mechanism by which (P2Y12R-dependent) microglial contact on neurons downregulate neuronal hyperactivity remains to be determined.
Microglia also interact with synaptic elements in an activity- and experience-dependent manner. This was first shown in the mouse cortex using in vivo imaging where microglial frequently but transiently contacted synaptic spines and terminals (Wake et al., 2009). These interactions were reduced with inhibited neuronal activity and reduced body temperature. However, they were significantly prolonged following ischemia and occasionally associated with subsequent synaptic elimination (Wake et al., 2009). Similar combined electron and two photon microscopy revealed intimate interactions between microglial processes and synapses in the visual cortex that were altered with visual experience (Tremblay et al., 2010). However, it should be noted that correlated light and electron microscopy approaches have challenged previous reports on the extent of microglial elimination of synapses by traditional phagocytic engulfment (Weinhard et al., 2018). Finally, in an ex vivo slice context, microglia increased their interactions with dendritic spines following high frequency stimulation in an NMDAR-dependent manner (Pfeiffer et al., 2016). Nevertheless, precise molecular mechanisms underlying these fascinating interactions should be further elucidated.
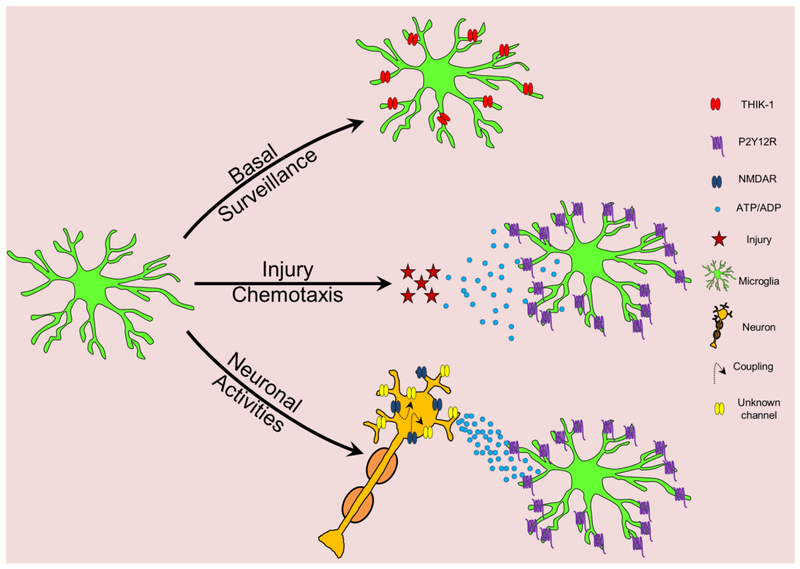
In summary, microglial process surveillance consist of basal motility regulated by the action of THIK-1 channels, chemotaxis towards injury under the control of P2Y12R and interactions with neuronal elements in which NMDAR and P2Y12R signaling has been strongly implicated (Figure 2).
Figure 2: Microglial process dynamics in the brain.
Microglial processes are very active during basal surveillance of the brain tissue. This surveillance is controlled by THIK-1 potassium channels. In response to an acute injury, microglial processes move towards the ATP/ADP source through P2Y12Rs to protect the brain from the injury. Especially during conditions of hyperactivity, NMDAR activation of neurons is coupled to the release of ATP/ADP through (an) unidentified channel(s) that recruits microglia through its P2Y12Rs.
Microglia: Dynamic Cell Body Patrol of the CNS.
Patrolling Microglia in the Developing Brain
Although less appreciated than their process dynamism, microglial somata exhibit varying capacities for translocation. This has been documented in the developing brain where murine microglia in the embryonic cortex (Swinnen et al., 2013) and postnatal hippocampus (Eyo et al., 2016) display migratory capacities in freshly prepared brain tissues. Remarkably, microglial migration is preserved even during simulated ischemia (Eyo and Dailey, 2012). As with rodents, zebrafish microglia display robust migration during early development (Svahn et al., 2013). Interestingly, murine and zebrafish microglia downregulate their migratory capacities with age (Eyo et al., 2016; Svahn et al., 2013). Thus, microglial migration in the developing brain is conserved among vertebrate species from fish to mammals suggesting microglial brain patrol during this period.
In rat brain slices, microglia home towards accumulated neurons in a purine-dependent manner (Kurpius et al., 2007). These purines are presumably released from dead / dying cells generated during the brain slicing process. This indicates that neonatal microglia are capable of purine-induced cell body migration. Since purines can be released from apoptotic cells as “find me” signals (Elliott et al., 2009) to trigger phagocytosis (Koizumi et al., 2007) and apoptosis occurs robustly during the developmental period (Eyo et al., 2016; Marin-Teva et al., 2004), one attractive hypothesis is that microglial migration in the developing brain is initiated by the release of apoptotic signals such as purines. This hypothesis has recently been tested in both mice and fish.
In the postnatal mouse hippocampus, developmental apoptosis increased from birth through the middle of the first postnatal week after which it declined (Eyo et al., 2016; Murase et al., 2011). This pattern of apoptosis corresponded with a pattern of microglial migration in freshly prepared hippocampal slices. To test the potential role for apoptotic signals regulating microglial migration, Bax-deficient mice were studied. While hippocampal apoptosis was significantly abolished in these mice, microglial density and migration were preserved indicating that at least in the early postnatal hippocampus, Bax-dependent apoptotic mechanisms are insufficient to account for developmental changes in microglial patrol of the developing brain (Eyo et al., 2016). Signals other than purines that drive microglial migration during rodent brain development are still largely unknown and warrant further investigations.
In the developing zebrafish, a similar hypothesis investigating a role for apoptosis in microglial patrol of the brain has been tested (Casano et al., 2016; Xu et al., 2016). Here, a spatio-temporal correlation between apoptotic cell presence and microglial brain entry was reported. Furthermore, genetically abrogating or increasing developmental apoptosis increased or diminished microglial entry into the brain, respectively (Casano et al., 2016; Xu et al., 2016). Using pharmacological approaches, one study confirmed that microglial entry into the brain was dependent on endogenous purinergic signaling (Casano et al., 2016) while the second study identified lysophosphatidylcholine (Xu et al., 2016), presumably released by apoptotic neurons, as instructive signals for microglial entry into the brain. Together, these studies suggest that neuronal apoptosis provides cues for attracting microglia into the brain.
Although the available data from mice and zebrafish seem to be in conflict, it is possible that the differences observed could reflect different developmental time windows since the zebrafish studies were performed at 2-5 days post-fertilization when microglia first enter the brain which would possibly correspond to the embryonic period in mice, whereas the studies in mice were performed postnatally. Moreover, the mouse studies were performed in the hippocampus while the zebrafish studies were performed in the optic tectum, which would be equivalent to the murine cortex. Therefore, spatio-temporal or species differences could account for the potential discrepancy in these studies and further studies should be undertaken to resolve this issue.
Patrolling Microglia in the Adult Brain
Microglia in the adult brain are not characteristically migratory. However, recent research suggests that they retain capacities for migration. Using chronic in vivo imaging, adult microglia translocated weekly and this increased with aging (Hefendehl et al., 2014). Weekly microglial migration was also increased in Alzheimer’s Disease (Fuhrmann et al., 2010). Finally, recent results noted that microglia patrol the healthy brain daily (Eyo et al., 2018b). Here, 10-15% of cortical microglia showed physical displacement independent of proliferation. Furthermore, local and global manipulations increased the microglial patrol of the brain (Eyo et al., 2018b).
Ex vivo studies are consistent with these in vivo findings showing increased capacities for migration following a stab injury lesion (Carbonell et al., 2005a). Microglia are non-migratory in the uninjured hemisphere but begin to display increased migration within a day that peaks at 3 days and declines to pre-injury levels by 5 days after the single stab injury (Carbonell et al., 2005a). This observation parallels features observed in microglial patrol following seizures in vivo when microglial migration is increased within the first 2 days of seizure induction and then returns to basal levels thereafter (Eyo et al., 2018b). In addition, in the subependymal zone, subventricular microglia migrate and phagocytize dead ependymal cells within 24 hours of drug treatment (Carbonell et al., 2005b). Likewise, following an acute injury, microglial migration was most dramatic within the first 24 hours of the localized injury after which it returned to normal (Eyo et al., 2018b). These results indicate that while not as elaborate as during the developmental period, adult microglia patrol the brain in health and following acute injury.
Microglial patrol of the brain also appears to differ between brain regions. Given the limitation of the in vivo imaging approaches used so far to investigate microglial patrol in the living animal, only outer brain regions have been investigated. We showed that microglial patrol is similar between superficial layer I and deeper layer II/III regions of the somatosensory cortex. In addition, regional differences exist even in the somatosensory cortex e.g. between the limb/trunk and barrel cortex regions (Eyo et al., 2018b). In a similar way, differences were reported in the weekly somal displacement between microglia in the visual cortex and those in the cerebellum with the latter exhibiting greater patrol (Stowell et al., 2018). The underlying factors regulating differences in regional migratory patterns are yet to be determined. One attractive hypothesis could be that the different degrees of migration reflect differences in the basal degree of neuronal activity in the region. Although this hypothesis is yet to be adequately tested, one study in brain slices found that inhibiting neuronal activity globally increased microglial migration following spreading depression while simulating increased neuronal activity decreased such migration (Grinberg et al., 2011). Although the precise molecular mechanisms for this were not identified, other studies indicate that spreading depression activates microglial potassium currents through NMDAR activation (Wendt et al., 2016). It is tempting, therefore, to speculate that microglial responses during spreading depressing may be mediated by an NMDAR-P2Y12R coupling.
Molecular regulation of microglial patrol is beginning to be clarified. In the healthy brain, the P2Y12R, which has emerged as a signature protein for microglial identity (Butovsky et al., 2014; Hickman et al., 2013), regulates basal microglial patrol (Eyo et al., 2018b). In addition, following seizures and sensory deprivation, the receptor was necessary for the increased patrol by microglia in these manipulated conditions. Furthermore, while fractalkine (CX3CL1-CX3CR1) signaling did not regulate basal microglial patrol in the mature brain (Eyo et al., 2018b), it was shown to regulate such patrol in Alzheimer’s Disease (Fuhrmann et al., 2010). Finally, CCR5-dependent mechanisms control the increased microglial migration following a stab injury (Carbonell et al., 2005a). Thus, purinergic and chemokine mechanisms have been implicated in microglial patrol of the brain.
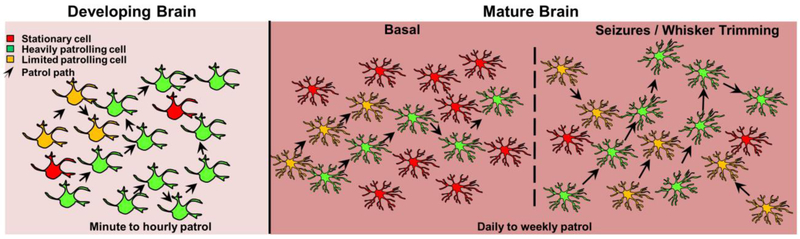
In summary, microglia patrol the developing and adult brains. During development the robust patrol is detectable at minute to hour time scales. However, caution should be employed for these conclusions from the mouse brain since they have been obtained from ex vivo studies. As the brain matures, microglial patrol is maintained but reduced and only detectable in time scales that span days to weeks. Nevertheless, differences exist between brain regions and during aging, pathology, or certain experimental manipulations when microglial patrol is increased (Figure 3).
Figure 3: Patrolling microglia in the developing and mature brain.
Microglia exhibit cell body translocations (patrol) in the healthy developing (left) and mature (right) brain. These cells display varying levels of patrol from relatively stationary cells (red cells) to cells with limited cell body translocations (yellow cells) to cells with very significant cell body translocations (green cells). In the developing brain, microglial cell body travel distances are detectable on an minute to hourly basis while in the mature brain detectable travel distances can be observed only on a daily to weekly basis. Changes to the adult patrol levels can be induced by experimental manipulations such as seizures and whisker trimming.
Conclusions and Future Perspectives.
Microglia originate predominantly from the embryonic yolk sac, migrate into the brain during pre- and postnatal development and attain adult numbers by coupling proliferative and apoptotic mechanisms. The adult microglial pool is not replenished by circulating monocytes but are rather long-lived with limited amounts of proliferation and apoptosis. During both development and adulthood, microglia are effective at patrolling the brain by both dynamic process surveillance and somal translocations. While process surveillance occurs robustly in both the developing and mature brain, somal migration though robust in the developing brain is decreased in adulthood and increased subsequently with aging. Therefore, microglia are bona fide patrolling immune cells of the brain. While process motility is exhibited by all microglia at any given period, soma migrations in adult microglia is exhibited by only 5-10% of microglia at any given period. Moreover, process motility (surveillance) is robust and within time scale of minutes in all ramified microglia, but soma migration (patrol) differs between developing, adult and aging microglia as well as in different brain regions within time range from minutes to days. Despite these results to increase our understanding of microglia as patrolling cells, several lines of investigation are pertinent for the future of the field.
First, although dramatic migratory microglia have been confirmed repeatedly in developing zebrafish microglia in vivo (Peri and Nusslein-Volhard, 2008; Svahn et al., 2013) and in murine cortical (Swinnen et al., 2013) and hippocampal microglia (Eyo et al., 2016; Kurpius et al., 2007) ex vivo, evidence of migratory mammalian microglia in vivo is lacking as are mechanisms controlling such endogenous microglial patrol in the developing brain. Moreover, the precise role of apoptotic (and other) signals in regulating microglial entry and subsequent patrol of the developing brain should be rigorously investigated especially in the mammalian system.
Second, microglial migratory capacities are rapidly downregulated with increasing age in the developing zebrafish brain (Svahn et al., 2013) as well as the mouse embryonic cortex (Swinnen et al., 2013) and early postnatal hippocampus (Eyo et al., 2016). Moreover, microglial patrol increases with aging (Hefendehl et al., 2014). However, the underlying mechanisms for these dramatic changes have not been determined. Possible hypotheses include (i) intrinsic downregulation of migratory genes during maturation and upregulation with aging, (ii) environmental instruction e.g. from the extracellular matrix or released factors from other neural cells to downregulate microglial migration with maturation or increase migration with aging and (iii) a combination of both intrinsic and extrinsic mechanisms. Approaches to identify which mechanism(s) are in play here will be critical in understanding the regulation of microglial patrol from development into maturity and then aging.
Third, microglial patrol has been investigated solely in the gray matter in the brain. The evidence for and potential mechanisms guiding such patrol (if it exists) in the white matter has not received sufficient experimental attention. Similarly, the regulation of microglial patrol in different brain regions e.g. between superficial cortex and deeper brain regions such as the hippocampus or between neurogenic regions like the olfactory bulb and non-neurogenic regions has not been undertaken. A comprehensive understanding of the regional regulation of microglial brain patrol remains to be determined.
Fourth, the extent of microglial patrol in pathology has yet to be fully explored. We have discussed the emerging data that’s available for microglial patrol in response to acute localized and epileptic injures as well as chronic conditions such as aging and Alzheimer’s Disease (see “Patrolling Microglia in the Adult Brain” section). However, whether microglial patrol also occurs in (i) other acute injuries such as ischemic or traumatic brain injuries, (ii) other chronic neurodegenerative pathologies such as Parkinson’s Disease, Amyotrophic Lateral Sclerosis or Huntington’s Disease and/or (iii) neuropsychiatric disorders such as schizophrenia, autism and depression should also be of interest.
Fifth, although P2Y12R have been implicated in microglial patrol in health and following seizures, the neural source(s) of the signal to direct microglial patrol remain unknown. Given that P2Y12R is activated by purines like ATP which is a ubiquitous cell source, all neural cell types residing in the brain parenchyma (neurons, astrocytes, oligodendrocytes and possible other microglia) as well as in the vasculature (circulating peripheral cells, pericytes and endothelial cells) could be candidate cell sources.
Finally, the existence of long-lived, patrolling microglia (Eyo et al., 2018b; Fuger et al., 2017; Stowell et al., 2018) raise the possibility that individual microglia may have “memory” of previous brain conditions (e.g. a previous injury, seizure episode or response to a vaccine). The consequence of this potential “microglia memory” on future brain conditions, function or pathology would be a subject of interest. These are exciting times to elucidate the mechanisms, significance and implications of such microglial patrol.
Highlights:
Microglia colonize the brain primarily from yolk sac sources during embryonic development though alternative source(s) may exist.
Microglial surveillance consist of its basal ubiquitous process dynamism, directed process chemotaxis towards injury sites and interactions with neuronal elements.
Microglial patrol involves its cell body movements that are robust during development, downregulated during maturity but increased in response to injury or pathology.
Acknowledgements
This work is supported by National Institute of Health (R01NS088627, R21DE025689 and K22NS104392)
Footnotes
Conflict of Interest: The authors declare no competing interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM, 2011. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nature neuroscience 14, 1142–1149. [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM, 2007. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 10, 1538–1543. [DOI] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B, 1999. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res 117, 145–152. [DOI] [PubMed] [Google Scholar]
- Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P, Tipton T, Chapman MA, Riecken K, Beccari S, Sierra A, Molnar Z, Cragg MS, Garaschuk O, Perry VH, Gomez-Nicola D, 2017. Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Rep 18, 391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen CJ, Bennett FC, Tucker AF, Collins HY, Mulinyawe SB, Barres BA, 2017. Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures. Neuron 94, 759–773 e758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL, 2014. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci 17, 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell WS, Murase S, Horwitz AF, Mandell JW, 2005a. Migration of perilesional microglia after focal brain injury and modulation by CC chemokine receptor 5: an in situ time-lapse confocal imaging study. J Neurosci 25, 7040–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell WS, Murase SI, Horwitz AF, Mandell JW, 2005b. Infiltrative microgliosis: activation and long-distance migration of subependymal microglia following periventricular insults. J Neuroinflammation 2, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casano AM, Albert M, Peri F, 2016. Developmental Apoptosis Mediates Entry and Positioning of Microglia in the Zebrafish Brain. Cell Rep 16, 897–906. [DOI] [PubMed] [Google Scholar]
- Casano AM, Peri F, 2015. Microglia: multitasking specialists of the brain. Dev Cell 32, 469–477. [DOI] [PubMed] [Google Scholar]
- Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G, Capecchi MR, 2010. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell 141, 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau I, Vela JM, Gonzalez B, Finsen B, Castellano B, 2003. Dynamics of microglia in the developing rat brain. J Comp Neurol 458, 144–157. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB, 2005. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8, 752–758. [DOI] [PubMed] [Google Scholar]
- Davalos D, Ryu JK, Merlini M, Baeten KM, Le Moan N, Petersen MA, Deerinck TJ, Smirnoff DS, Bedard C, Hakozaki H, Gonias Murray S, Ling JB, Lassmann H, Degen JL, Ellisman MH, Akassoglou K, 2012. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun 3, 1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biase LM, Schuebel KE, Fusfeld ZH, Jair K, Hawes IA, Cimbro R, Zhang HY, Liu QR, Shen H, Xi ZX, Goldman D, Bonci A, 2017. Local Cues Establish and Maintain Region-Specific Phenotypes of Basal Ganglia Microglia. Neuron 95, 341–356 e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De I, Nikodemova M, Steffen MD, Sokn E, Maklakova VI, Watters JJ, Collier LS, 2014. CSF1 overexpression has pleiotropic effects on microglia in vivo. Glia 62, 1955–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S, Van Deren D, Peden E, Hockin M, Boulet A, Titen S, Capecchi MR, 2018. Two distinct ontogenies confer heterogeneity to mouse brain microglia. Development 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissing-Olesen L, LeDue JM, Rungta RL, Hefendehl JK, Choi HB, MacVicar BA, 2014. Activation of neuronal NMDA receptors triggers transient ATP-mediated microglial process outgrowth. J Neurosci 34, 10511–10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS, 2009. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo U, Dailey ME, 2012. Effects of oxygen-glucose deprivation on microglial mobility and viability in developing mouse hippocampal tissues. Glia 60, 1747–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Bispo A, Liu J, Sabu S, Wu R, DiBona VL, Zheng J, Murugan M, Zhang H, Tang Y, Wu LJ, 2018a. The GluN2A Subunit Regulates Neuronal NMDA receptor-Induced Microglia-Neuron Physical Interactions. Sci Rep 8, 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Gu N, De S, Dong H, Richardson JR, Wu LJ, 2015. Modulation of microglial process convergence toward neuronal dendrites by extracellular calcium. J Neurosci 35, 2417–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Miner SA, Ahlers KE, Wu LJ, Dailey ME, 2013. P2X7 receptor activation regulates microglial cell death during oxygen-glucose deprivation. Neuropharmacology 73C, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Miner SA, Weiner JA, Dailey ME, 2016. Developmental changes in microglial mobilization are independent of apoptosis in the neonatal mouse hippocampus. Brain Behav Immun 55, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Mo M, Yi MH, Murugan M, Liu J, Yarlagadda R, Margolis DJ, Xu P, Wu LJ, 2018b. P2Y12R-Dependent Translocation Mechanisms Gate the Changing Microglial Landscape. Cell Rep 23, 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Peng J, Murugan M, Mo M, Lalani A, Xie P, Xu P, Margolis DJ, Wu LJ, 2017. Regulation of Physical Microglia-Neuron Interactions by Fractalkine Signaling after Status Epilepticus. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Peng J, Swiatkowski P, Mukherjee A, Bispo A, Wu LJ, 2014. Neuronal Hyperactivity Recruits Microglial Processes via Neuronal NMDA Receptors and Microglial P2Y12 Receptors after Status Epilepticus. J Neurosci 34, 10528–10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach MK, Tjwa M, Bechmann I, Krueger M, 2018. Decreased microglial numbers in Vav1-Cre(+):dicer knock-out mice suggest a second source of microglia beyond yolk sac macrophages. Ann Anat 218, 190–198. [DOI] [PubMed] [Google Scholar]
- Filipello F, Morini R, Corradini I, Zerbi V, Canzi A, Michalski B, Erreni M, Markicevic M, Starvaggi-Cucuzza C, Otero K, Piccio L, Cignarella F, Perrucci F, Tamborini M, Genua M, Rajendran L, Menna E, Vetrano S, Fahnestock M, Paolicelli RC, Matteoli M, 2018. The Microglial Innate Immune Receptor TREM2 Is Required for Synapse Elimination and Normal Brain Connectivity. Immunity 48, 979–991 e978. [DOI] [PubMed] [Google Scholar]
- Fontainhas AM, Wang M, Liang KJ, Chen S, Mettu P, Damani M, Fariss RN, Li W, Wong WT, 2011. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS One 6, e15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin A, Parmentier-Batteur S, Walter L, Greenberg DA, Stella N, 2003. Palmitoylethanolamide increases after focal cerebral ischemia and potentiates microglial cell motility. J Neurosci 23, 7767–7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin A, Stella N, 2003. Arachidonylcyclopropylamide increases microglial cell migration through cannabinoid CB2 and abnormal-cannabidiol-sensitive receptors. Eur J Pharmacol 474, 195–198. [DOI] [PubMed] [Google Scholar]
- Fuger P, Hefendehl JK, Veeraraghavalu K, Wendeln AC, Schlosser C, Obermuller U, Wegenast-Braun BM, Neher JJ, Martus P, Kohsaka S, Thunemann M, Feil R, Sisodia SS, Skodras A, Jucker M, 2017. Microglia turnover with aging and in an Alzheimer’s model via long-term in vivo single-cell imaging. Nat Neurosci 20, 1371–1376. [DOI] [PubMed] [Google Scholar]
- Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, Haass C, LaFerla FM, Kretzschmar H, Herms J, 2010. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease. Nature neuroscience 13, 411–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M, 2010. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg YY, Milton JG, Kraig RP, 2011. Spreading depression sends microglia on Levy flights. PLoS One 6, e19294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Eyo UB, Murugan M, Peng J, Matta S, Dong H, Wu LJ, 2015. Microglial P2Y12 receptors regulate microglial activation and surveillance during neuropathic pain. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Peng J, Murugan M, Wang X, Eyo UB, Sun D, Ren Y, DiCicco-Bloom E, Young W, Dong H, Wu LJ, 2016. Spinal Microgliosis Due to Resident Microglial Proliferation Is Required for Pain Hypersensitivity after Peripheral Nerve Injury. Cell Rep 16, 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, Guan AK, Evans-Reinsch Z, Braz J, Devor M, Abboud-Werner SL, Lanier LL, Lomvardas S, Basbaum AI, 2016. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci 19, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D, 2006. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nature neuroscience 9, 1512–1519. [DOI] [PubMed] [Google Scholar]
- Hefendehl JK, Neher JJ, Suhs RB, Kohsaka S, Skodras A, Jucker M, 2014. Homeostatic and injury- induced microglia behavior in the aging brain. Aging Cell 13, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J, 2013. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci 16, 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines DJ, Hines RM, Mulligan SJ, Macvicar BA, 2009. Microglia processes block the spread of damage in the brain and require functional chloride channels. Glia 57, 1610–1618. [DOI] [PubMed] [Google Scholar]
- Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, Choy SH, Grisotto M, Renia L, Conway SJ, Stanley ER, Chan JK, Ng LG, Samokhvalov IM, Merad M, Ginhoux F, 2012. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med 209, 1167–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G, Inada H, Wake H, Akiyoshi R, Miyamoto A, Eto K, Ishikawa T, Moorhouse AJ, Strassman AM, Nabekura J, 2016. Microglial Contact Prevents Excess Depolarization and Rescues Neurons from Excitotoxicity. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf K, Prinz M, 2017. Microglia in steady state. J Clin Invest 127, 3201–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Mlsna LM, Yoon S, Le B, Yu S, Xu D, Koh S, 2015. A postnatal peak in microglial development in the mouse hippocampus is correlated with heightened sensitivity to seizure triggers. Brain Behav 5, e00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler J, Wiley CA, 2011. Microglia: key innate immune cells of the brain. Toxicol Pathol 39, 103–114. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K, 2007. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446, 1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejczak M, Bechade C, Gervasi N, Irinopoulou T, Banas SM, Cordier C, Rebsam A, Roumier A, Maroteaux L, 2015. Serotonin Modulates Developmental Microglia via 5-HT2B Receptors: Potential Implication during Synaptic Refinement of Retinogeniculate Projections. ACS Chem Neurosci 6, 1219–1230. [DOI] [PubMed] [Google Scholar]
- Krabbe G, Matyash V, Pannasch U, Mamer L, Boddeke HW, Kettenmann H, 2012. Activation of serotonin receptors promotes microglial injury-induced motility but attenuates phagocytic activity. Brain Behav Immun 26, 419–428. [DOI] [PubMed] [Google Scholar]
- Kurpius D, Nolley EP, Dailey ME, 2007. Purines induce directed migration and rapid homing of microglia to injured pyramidal neurons in developing hippocampus. Glia 55, 873–884. [DOI] [PubMed] [Google Scholar]
- Li Y, Du XF, Liu CS, Wen ZL, Du JL, 2012. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell 23, 1189–1202. [DOI] [PubMed] [Google Scholar]
- Liang KJ, Lee JE, Wang YD, Ma W, Fontainhas AM, Fariss RN, Wong WT, 2009. Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling. Investigative ophthalmology & visual science 50, 4444–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou N, Takano T, Pei Y, Xavier AL, Goldman SA, Nedergaard M, 2016. Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc Natl Acad Sci U S A 113, 1074–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madry C, Arancibia-Carcamo IL, Kyrargyri V, Chan VTT, Hamilton NB, Attwell D, 2018a. Effects of the ecto-ATPase apyrase on microglial ramification and surveillance reflect cell depolarization, not ATP depletion. Proc Natl Acad Sci U S A 115, E1608–E1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madry C, Kyrargyri V, Arancibia-Carcamo IL, Jolivet R, Kohsaka S, Bryan RM, Attwell D, 2018b. Microglial Ramification, Surveillance, and Interleukin-1beta Release Are Regulated by the Two-Pore Domain K(+) Channel THIK-1. Neuron 97, 299–312 e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M, 2004. Microglia promote the death of developing Purkinje cells. Neuron 41, 535–547. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, Koizumi S, Moorhouse AJ, Yoshimura Y, Nabekura J, 2016. Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun 7, 12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, Lelios I, Heppner FL, Kipnis J, Merkler D, Greter M, Becher B, 2018. High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease. Immunity 48, 380–395 e386. [DOI] [PubMed] [Google Scholar]
- Murase S, Poser SW, Joseph J, McKay RD, 2011. p53 controls neuronal death in the CA3 region of the newborn mouse hippocampus. Eur J Neurosci 34, 374–381. [DOI] [PubMed] [Google Scholar]
- Nayak D, Roth TL, McGavern DB, 2014. Microglia development and function. Annu Rev Immunol 32, 367–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodemova M, Kimyon RS, De I, Small AL, Collier LS, Watters JJ, 2015. Microglial numbers attain adult levels after undergoing a rapid decrease in cell number in the third postnatal week. J Neuroimmunol 278, 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F, 2005. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. [DOI] [PubMed] [Google Scholar]
- Peri F, Nusslein-Volhard C, 2008. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 133, 916–927. [DOI] [PubMed] [Google Scholar]
- Petersen MA, Dailey ME, 2004. Diverse microglial motility behaviors during clearance of dead cells in hippocampal slices. Glia 46, 195–206. [DOI] [PubMed] [Google Scholar]
- Pfeiffer T, Avignone E, Nagerl UV, 2016. Induction of hippocampal long-term potentiation increases the morphological dynamics of microglial processes and prolongs their contacts with dendritic spines. Sci Rep 6, 32422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reu P, Khosravi A, Bernard S, Mold JE, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Druid H, Frisen J, 2017. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep 20, 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigato C, Swinnen N, Buckinx R, Couillin I, Mangin JM, Rigo JM, Legendre P, Le Corronc H, 2012. Microglia proliferation is controlled by P2X7 receptors in a Pannexin-1-independent manner during early embryonic spinal cord invasion. J Neurosci 32, 11559–11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling T, Nitsch R, Heinemann U, Haas D, Eder C, 2001. Astrocyte-released cytokines induce ramification and outward K+ channel expression in microglia via distinct signalling pathways. Eur J Neurosci 14, 463–473. [DOI] [PubMed] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F, 2012. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90. [DOI] [PubMed] [Google Scholar]
- Stowell RD, Wong EL, Batchelor HN, Mendes MS, Lamantia CE, Whitelaw BS, Majewska AK, 2018. Cerebellar microglia are dynamically unique and survey Purkinje neurons in vivo. Dev Neurobiol 78, 627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svahn AJ, Graeber MB, Ellett F, Lieschke GJ, Rinkwitz S, Bennett MR, Becker TS, 2013. Development of ramified microglia from early macrophages in the zebrafish optic tectum. Dev Neurobiol 73, 60–71. [DOI] [PubMed] [Google Scholar]
- Swinnen N, Smolders S, Avila A, Notelaers K, Paesen R, Ameloot M, Brone B, Legendre P, Rigo JM, 2013. Complex invasion pattern of the cerebral cortex bymicroglial cells during development of the mouse embryo. Glia 61, 150–163. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Maeda N, 1996. Microglial ramification requires nondiffusible factors derived from astrocytes. Exp Neurol 137, 367–375. [DOI] [PubMed] [Google Scholar]
- Tay TL, Mai D, Dautzenberg J, Fernandez-Klett F, Lin G, Sagar, Datta M, Drougard A, Stempfl T, Ardura-Fabregat A, Staszewski O, Margineanu A, Sporbert A, Steinmetz LM, Pospisilik JA, Jung S, Priller J, Grun D, Ronneberger O, Prinz M, 2017. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci 20, 793–803. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK, 2010. Microglial interactions with synapses are modulated by visual experience. PLoS Biol 8, e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J, 2009. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 29, 3974–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N, 2003. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci 23, 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, Exiga M, Vadisiute A, Raggioli A, Schertel A, Schwab Y, Gross CT, 2018. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun 9, 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt S, Wogram E, Korvers L, Kettenmann H, 2016. Experimental Cortical Spreading Depression Induces NMDA Receptor Dependent Potassium Currents in Microglia. J Neurosci 36, 6165–6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Vadakkan KI, Zhuo M, 2007. ATP-induced chemotaxis of microglial processes requires P2Y receptor-activated initiation of outward potassium currents. Glia 55, 810–821. [DOI] [PubMed] [Google Scholar]
- Wyatt SK, Witt T, Barbaro NM, Cohen-Gadol AA, Brewster AL, 2017. Enhanced classical complement pathway activation and altered phagocytosis signaling molecules in human epilepsy. Exp Neurol 295, 184–193. [DOI] [PubMed] [Google Scholar]
- Xavier AL, Lima FR, Nedergaard M, Menezes JR, 2015. Ontogeny of CX3CR1-EGFP expressing cells unveil microglia as an integral component of the postnatal subventricular zone. Front Cell Neurosci 9, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang T, Wu Y, Jin W, Wen Z, 2016. Microglia Colonization of Developing Zebrafish Midbrain Is Promoted by Apoptotic Neuron and Lysophosphatidylcholine. Dev Cell 38, 214–222. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhu L, He S, Wu Y, Jin W, Yu T, Qu JY, Wen Z, 2015. Temporal-Spatial Resolution Fate Mapping Reveals Distinct Origins for Embryonic and Adult Microglia in Zebrafish. Dev Cell 34, 632–641. [DOI] [PubMed] [Google Scholar]
- Zheng H, Jia L, Liu CC, Rong Z, Zhong L, Yang L, Chen XF, Fryer JD, Wang X, Zhang YW, Xu H, Bu G, 2017. TREM2 Promotes Microglial Survival by Activating Wnt/beta-Catenin Pathway. J Neurosci 37, 1772–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusso M, Methot L, Lo R, Greenhalgh AD, David S, Stifani S, 2012. Regulation of postnatal forebrain amoeboid microglial cell proliferation and development by the transcription factor Runx1. J Neurosci 32, 11285–11298. [DOI] [PMC free article] [PubMed] [Google Scholar]