Abstract
Immunity to malaria has been linked to the availability and function of helper CD4+ T cells, cytotoxic CD8+ T cells and γδ T cells that can respond to both the asymptomatic liver-stage and the symptomatic blood-stage of Plasmodium sp. infection. These T cell responses are also thought to be modulated by regulatory T cells. However, the precise mechanisms governing the development and function of Plasmodium-specific T cells and their capacity to form tissue-resident and long-lived memory populations are less well understood. The field has arrived at a point where the push for vaccines that exploit T cell-mediated immunity to malaria has made it imperative to define and reconcile the mechanisms that regulate the development and functions of Plasmodium-specific T cells. Here, we review our current understanding of the mechanisms by which T cell subsets orchestrate host resistance to Plasmodium infection, based on observational and mechanistic studies in humans, non-human primates and rodent models. We also examine the potential of new experimental strategies and human infection systems to inform a new generation of approaches to harness T cell responses against malaria.
Introduction
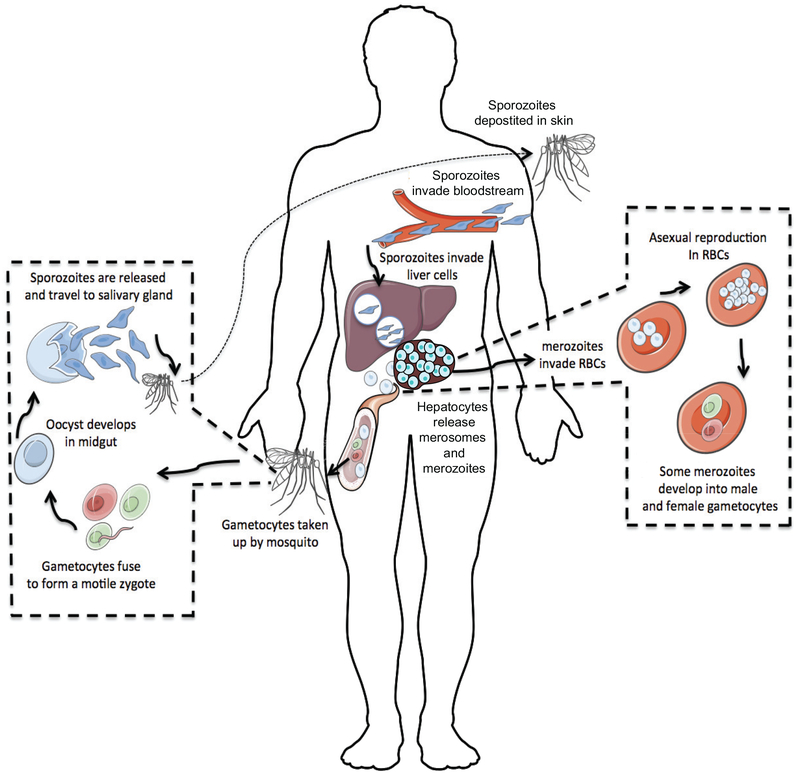
Plasmodium species are the causative agents of malaria, a devastating disease responsible for more than 200 million infections and approximately 450,000 deaths annually1. Malaria is transmitted when Plasmodium sporozoite [G] forms are deposited into the dermis during Anopheles mosquito blood-meals (Fig. 1). Parasites exit the dermis and transit through the circulation to infect hepatocytes in the liver. Over the next several days of asymptomatic liver-stage infection, parasites undergo amplification and differentiation into merozoites [G]. Merozoites emerge from infected hepatocytes either singly or as part of a merosome [G] and represent an antigenically distinct form of the parasite that targets host erythrocytes to establish blood-stage infection, the phase responsible for all clinical signs and symptoms associated with malaria.
FIGURE 1: Plasmodium life cycle.
The life cycle begins when a Plasmodium-infected female Anopheles mosquito takes a blood meal from a human host and deposits Plasmodium sporozoites into the skin. Motile sporozoites exit the dermis and travel through the blood to access hepatocytes. Sporozoites invade liver cells via interactions between Plasmodium circumsporozoite protein (CSP) and heparin sulfate molecules expressed on hepatocytes. One P. falciparum sporozoite will undergo differentiation over 6–7 days and amplify into ~10,000 merozoites. Infected hepatocytes release merozoites and merosomes, which are membrane bound packets of merozoites, into the blood stream where they proceed to invade erythrocytes. Merozoites undergo repeated rounds of asexual replication. A minor proportion of merozoites will differentiate into either male or female gametocytes that can be ingested by other female Anopheles mosquitos. In the mosquito midgut, male and female gametocytes fuse and develop into a motile ookinete. Ookinetes embed within the mosquito midgut wall and develop further into oocysts. Each oocyst produces thousands of sporozoites over a period of two weeks. Sporozoites eventually migrate to the salivary glands and poise the mosquito to transmit malaria to a new host.
Both cellular and humoral adaptive immune responses are essential for limiting Plasmodium parasite replication and the severity of malaria (Fig. 2). As detailed below, in immune animals and partially immune humans, parasite-specific, cytotoxic CD8+ T cells likely eliminate infected hepatocytes following recognition of parasite antigens presented on MHC class I molecules, whereas CD4+ T cell-dependent antibody responses can prevent sporozoite invasion of hepatocytes. Both of these immune mechanisms effectively prevent the progression from asymptomatic to clinical disease. During the blood-stage of Plasmodium infection in naïve or partially immune hosts, parasitized erythrocytes (which lack functional MHC expression) are indirectly targeted by CD4+ helper T cells and possibly γδ T cells that may orchestrate secreted antibody responses or the anti-parasitic activity of phagocytes.
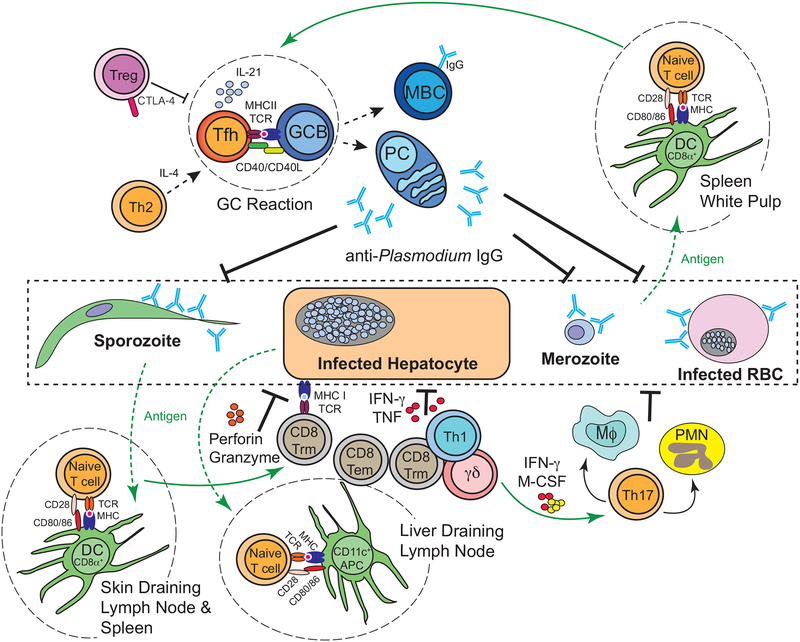
FIGURE 2: Overview of tissue-specific, T cell-mediated immune resistance networks during Plasmodium infection.
CD8α+ dendritic cells (DC) in the skin-draining lymph nodes and spleen, as well as CFS1R+ CD11c+ cells in the liver-draining lymph nodes, serve as antigen presenting cells and play an important role in bridging innate and adaptive immune responses during malaria. Upon phagocytosis of merozoites, parasitized RBC (pRBC), sporozoites, debris from infected hepatocytes, or circumsporozoite protein formulated as part of the RTS,S vaccine, DCs will process and present Plasmodium antigens to activate naïve CD4+ and CD8+ T cells. DC production of specific cytokines, such as IL-12 and IL-6, skew CD4+ T cell differentiation toward T helper 1 (Th1) and T follicular helper (Tfh) lineages. Th1 cells produce the cytokine IFN-γ that activates macrophages to enhance their phagocytic function and stimulates production of reactive oxygen species that are toxic to the parasite. Tfh cells engage parasite-specific B cells and orchestrate the germinal centre (GC) reaction, where they express co-stimulatory factors (CD40L) and secreted soluble factors (IL-4 and IL-21) that promote GC B cell (GCB) antibody isotype switching, affinity maturation, and somatic hypermutation, as well as the generation of memory B cells (MBC) and long-lived antibody-secreting plasma cells (PC). Parasite-specific antibodies potentially function to immobilize or target sporozoites for antibody dependent cellular cytotoxicity (ADCC), block merozoite invasion of RBCs, opsonize pRBC to enhance their phagocytosis, target merozoites and pRBC for ADCC, and activate the classical complement-pathway. Sporozoite- or liver-stage-specific, tissue-resident (Trm) CD8+ T cells elaborate the cytokines IFN-γ and TNF and trigger extrinsic cell death pathways via expression of perforin and granzyme to kill infected hepatocytes. Cytotoxic CD4+ T cells may function similarly to kill infected target cells expressing MHC class II. Cytotoxic CD8+ T cells also have the potential to kill infected reticulocytes that transiently retain expression of MHC. B cells and CD4+ and CD8+ T cells are subject to regulation by other αβ T cells, including Tregs, IL-27-secreting CD4+ T cells, and Tr1 cells (the latter two subsets are not depicted). γδ T cells are activated in response to liver and blood-stage infection in response to unknown cues. These cells express cytokines that may include IFN-γ and myeloid activating factors like M-CSF. γδ T cells likely promote blood and liver stage parasite clearance by orchestrating and amplifying the activity of phagocytes. The contributions of Th17 and Th2 cells are less clear, but may be related to either recruiting and activating phagocytes or promoting Plasmodium-specific GC B cell reactions.
Experimental malaria models in rodents and non-human primates have expanded our understanding of T cell-mediated protection against malaria and provided mechanistic insights that have guided the development of multiple experimental anti-malarial vaccine platforms. However, major challenges to immune-mediated elimination of malaria remain. The lead subunit vaccine candidate, RTS,S (Mosquirix™) [G] provides only short-lived, partial protection against malaria2, 3, 4. Thus, despite the current conceptual frameworks for αβ and γδ T cell-mediated protection against Plasmodium, we still lack sufficient mechanistic understanding of their formation and function, which has hampered the design of efficacious vaccines that can be deployed in malaria-endemic regions. The development of innovative anti-malarial vaccine platforms and the potential application of immunotherapies that stimulate or enhance resistance to malaria will require deeper insights into the cellular and molecular mechanisms that govern anti-Plasmodium T cell responses.
In this Review, we examine recent advances in our understanding of Plasmodium-specific αβ and γδ T cell subsets with specific emphasis on the mechanisms that these populations use to facilitate or hamper immune control of malaria. We discuss the secreted mediators that αβ and γδ T cell subsets employ to orchestrate resistance to malaria, including cytokines and pro-apoptotic factors. Although much of the mechanistic data describing both infection- and vaccine-induced T cells were generated in mouse studies, we also highlight key associations identified in human field studies and controlled human malaria infection (CHMI) models. However, missing critical information and technical limitations continue to impede progress in the field (Box 1). Despite this, we also highlight technical innovations and recent experimental advances that have facilitated critical insights into the biology of Plasmodium-specific T cells (Box 2) and illustrate the major gaps that remain to be addressed through future clinical and experimental studies.
Box 1: Unravelling T cell responses in human malaria.
Below, we summarize some of the main challenges to determining the mechanisms of T cell-mediated protection in natural and controlled human Plasmodium infections.
Specificity:
High-resolution studies of Plasmodium-specific T cell responses generally requires knowledge of the identity of the peptide epitopes and MHC restriction elements. Identifying immunodominant subsets of CD4+ and CD8+ T cells will necessitate a greater emphasis on deciphering immunodominant T cell epitopes that are targeted by liver- or blood-stage-specific T cells. Notably, some dominant T cell epitopes targeted by substantial populations of T cells do not represent protective epitopes205; this is likely due to large numbers of precursor T cells bearing T cell receptors (TCRs) that can recognize the epitope presented by professional antigen-presenting cells (APCs), but a lack of this epitope on infected cells. Determining which of the detectable epitope-specific T cell responses are the most protective represents a major goal.
Location:
With regard to liver-stage Plasmodium T cell-mediated protection, experimental data suggests that liver-resident T cells are key to eliminating Plasmodium-infected hepatocytes before merozoites emerge to establish clinical malaria. This presents a substantial hurdle for sampling and studying these cells in patients with malaria who have been either naturally exposed or exposed through controlled human malaria infections (see Box 3) or experimental vaccination platforms. Identifying circulating T cell populations that serve as a surrogate for protective tissue-resident T cells represents a major limitation for understanding the mechanisms by which liver-stage, and potentially blood-stage, T cells mediate protective immunity.
Regulation:
Malaria is well known to engage various co-stimulatory, co-inhibitory, and immunoregulatory circuits that alter the function of parasite-specific effector T cells. The number, complexity, and temporally overlapping expression patterns of these pathways represent significant challenges to understanding how such circuits govern the development and function of Plasmodium-specific memory T cells.
Polyfunctionality:
Plasmodium-specific effector and memory CD4 T cells commonly exhibit mixed characteristics of both Th1, Tfh, and Tr1 cells, including the simultaneous expression of T-bet, Bcl-6, IFNγ, IL-10 and IL-21. The extent to which these multifunctional cells either promote or hinder the development and function of long-lived adaptive immune responses remains a question.
Box 2: Tools, systems, and approaches to study Plasmodium-specific T cell responses.
In recent years, multiple new tools have been developed to enhance resolution and focus studies on mechanisms of T cell function during experimental malaria. Technologies now exists to interrogate the development, persistence, and precise effector functions of bona fide Plasmodium-specific T cells. Novel T cell receptor (TCR) transgenic mouse models have stimulated new insight into the biology and regulation of Plasmodium-specific T cells. Investigators can also generate fluorescently labeled multimeric peptide–MHC complexes to directly identify, quantify, and phenotype Plasmodium-specific, effector and memory CD4+ and CD8+ T cells using flow cytometric methods. Already such tools have been used to describe the single-cell transcriptomic profile of Plasmodium-specific, effector CD4+ T cells68 and critical factors that regulate the formation and protective mechanisms of effector and memory blood- and liver stage T cell responses33, 70, 185, 246, 247. Combining these methods with functional assays has greatly expanded our understanding of the quantitative, qualitative, and functional features of Plasmodium-specific CD4+ T cells and CD8+ T cells.
CD4+ T cells in malaria
CD4+ helper T (Th) cells are activated following engagement of pathogen-specific peptides presented on MHC class II molecules and are central to orchestrating key aspects of both innate and adaptive immunity during Plasmodium infection. The presence of Plasmodium-specific CD4+ T cells has been identified as a correlate of protective immunity following either natural exposure or anti-malarial vaccination5, 6, 7, 8. When activated in the presence of specific polarizing cytokines, CD4+ T cells have the capacity to differentiate into one of several functionally distinct subsets. Given the complexity of the Plasmodium parasite lifecycle (Fig. 1), it is not surprising that a number of functionally diverse CD4+ T cell subsets have been identified in both experimental and clinical malaria studies and that their mechanism(s) of protection are distinctly linked to specific Plasmodium developmental stages (Fig. 2). Notably, accumulating evidence supports the idea that long-lived, sterilizing immunity does not develop in humans following repeated exposure to Plasmodium parasites and there is also evidence of reduced efficacy of childhood vaccination in malaria-exposed individuals2, 8, 9, 10. These studies highlight the complexity of the regulatory circuits and immune checkpoints that become engaged during malaria. Rather than examine in detail the differentiation and regulation of CD4+ T cells, topics that have been recently reviewed11, 12, 13, we focus our discussion here on the mechanisms by which functionally distinct CD4+ T cell subsets shape resistance to liver- and blood-stage malaria.
Th1 cells.
The presence of Th1 cells and an elevated IFNγ response are signatures of both human14 and rodent15, 16, 17, 18 malaria. In addition to IL-12, the formation of Plasmodium-specific Th1 cells has been linked to CD4+ T cell-intrinsic sensing of extracellular ATP by P2X719, and P. falciparum-activated human DCs may be uniquely programmed to promote Th1 cell differentiation20. The activity of T-bet upregulates IFNγ and approximately half of all Th1 cell-associated genes, in addition to repressing the transcriptional programmes of other CD4+ T cell subsets (reviewed in detail elsewhere21), including Th2 cell, Th17 cell and T follicular helper (Tfh) cell subsets that are described below. During experimental blood-stage infection, T-bet (Tbx21)-deficient mice exhibited the loss of IFNγ-producing splenic CD4+ T cells, which directly correlated with elevated parasite burdens22. Similarly, Plasmodium infection of Il12−/− mice reduced T-bet and IFNγ expression in CD4+ T cells and abrogated control of Plasmodium replication15.
The exact mechanisms by which IFNγ and effector Th1 cells contribute to host protection during blood-stage Plasmodium infection remain largely speculative23, although experimental data suggests that IFNγ is critical for activating macrophages24, 25, 26 and may tune class-switch recombination in Plasmodium-specific B cells15. IL-2, another CD4+ T cell-derived, Th1-associated cytokine is important for activating natural killer (NK) cells, which may participate in protective immune responses by direct cytolysis of Plasmodium-infected erythrocytes27. IFNγ-producing, TBET+ Th1 cells can also express macrophage colony stimulating factor (M-CSF, also known as CSF1), and CD4+ T cell-specific deficiency of M-CSF exacerbates the loss of CD169+ macrophages and abrogates the control of blood-stage infection28. Notably, deletion of CD169+ macrophages phenocopied the reduced parasite control seen in mice with a T cell-restricted deficiency in M-CSF, suggesting a key mechanistic role for M-CSF-expressing Th1-like cells in promoting the function or antigen presentation capacity of protective myeloid cells29. Regarding memory responses, Th1-like CD4+ T cells are maintained following resolution of acute experimental malaria and exhibit protective capacity upon recall in rodent models30,31, 32, 33. Deciphering the precise mechanisms by which memory Th1 cells orchestrate protective recall responses to blood-stage malaria remain important lines of investigation.
Th1 cell responses and IFNγ secretion have also been linked to host resistance during liver-stage Plasmodium infection. In Plasmodium exposed34 and experimentally vaccinated34 individuals, the presence of IFNγ-expressing, circumsporozoite [G] (CSP)-specific Th1 cells was linked to reduced parasite burdens and disease severity. Although the precise mechanisms by which these liver-stage-specific CD4+ T cells orchestrated immunity was not explored in detail, Th1 cell-associated IFNγ35, 36 may either directly activate inducible nitric oxide synthase in infected hepatocytes37 or potentiate the cytotoxic activity of Plasmodium-specific CD8+ T cells via upregulation of MHC class I molecules on infected liver cells38. In controlled human malaria infection models39 (CHMI, see also Box 3) and following chemoprophylaxis and sporozoite (CPS) immunization [G] of malaria-naïve volunteers40, reduced blood-stage parasite burdens were associated with the presence of CD4+ T cells exhibiting characteristics of cytotoxic Th1-like cells. These cells exhibited elevated expression of CD38, IFNγ, CD107a, and granzyme B. The exact mechanisms by which cytotoxic CD4+ T cells participate in anti-Plasmodium immunity are not defined, but are potentially linked to the recognition and elimination of infected targets cells during either blood- or liver-stage infection (Fig. 2). In rodent models, Plasmodium-specific cytotoxic CD4+ T cells have also been identified35, 36, 41, 42 that may recognize and kill infected hepatocytes. The extent to which these cytotoxic Th1-like CD4+ T cells form in malaria exposed individuals remains a question and their relative contribution to controlling either blood- or liver-stage Plasmodium requires further study.
Box 3: Controlled human malaria infection models.
Major advances in our understanding Plasmodium pathogenesis, host immunity, and correlates of protection are projected to come from controlled human malaria infection (CHMI) models. CHMI involves either the delivery of live sporozoites by mosquito bites or by direct injection, or via delivery of Plasmodium-infected erythrocytes by needle injection. The development of fulminant blood-stage infection and clinical disease are blocked by the use of defined parasite strains with well-established drug sensitivity profiles in combination with the timely delivery of effective and appropriate antimalarial drugs. Studies typically cure infections at either a pre-determined parasite density or when the infection becomes detectable by microscopic examination of blood smears or PCR. The utility of such seemingly risky studies is apparent when we consider that candidate vaccines may be rapidly evaluated for efficacy before costly clinical trials are initiated in malaria endemic areas. CHMI models also facilitate examination of immune correlates of resistance and susceptibility. In malaria-naïve adults from non-endemic regions, CHMI has made it possible to examine dynamic changes in myeloid and lymphoid populations that arise in response to a first exposure to Plasmodium. Such studies have already revealed unexpected and potentially critical expansion of clonal populations of γδ T cells and cytotoxic CD4 T cells. In malaria-exposed individuals, CHMI should also reveal whether specific populations of effector or regulatory immune cell subsets are “boosted” following subsequent parasite exposures. Several such CHMI trials have taken place in malaria endemic settings248, 249, 250, and future CHMI studies will continue to expand our understanding of how the host responds and coordinates immune responses against Plasmodium parasites. These studies will also better define the precise mechanisms by which T cells contribute to controlling liver- and blood-stage Plasmodium infections.
Studies have also identified pathological roles for Th1 cell responses and IFNγ secretion during Plasmodium infection. IFNγ has been linked to atypical memory B cell formation in Plasmodium-exposed humans43 and rodent models show that elevated Th1 cell and IFNγ responses44, 45 and B cell-intrinsic IFNγ signalling46 can either impair humoral immunity or expand phosphatidylserine-specific, self-reactive B cells that can exacerbate anemia47. Notably, multiple CD4+ T cell subsets, including Tfh cells express IFNγ during experimental malaria. Thus, fundamental questions remain regarding the relative contribution of key cellular sources of IFNγ during Plasmodium infection, including γδ and CD8+ αβ T cells. Future experimental studies that exploit conditional allelic deletions of IFNγ or its receptor should help resolve long-standing questions regarding the cell type-specific sources and targets of IFNγ and help determine whether the protective versus pathological roles of this key Th1 cell-associated cytokine evolve as the infection progresses.
Th2 cells.
Th2 cells are primarily characterized by expression of the GATA3 transcription factor and by the production of IL-4 and IL-548. The role of Th2 cells in malaria is relatively unknown, as GATA3+ CD4+ T cells are rare or absent during Plasmodium infections49 and strong Th1-type polarization of CD4+ T cells may limit Th2 cell differentiation50. However, IL-4, the major cytokine expressed by Th2 cells, can promote B cell class switching51, 52 and modulate macrophage responses53 during Plasmodium infection. Moreover, IL-4 has been identified as a correlate of enhanced humoral immunity in Plasmodium exposed humans54. By contrast, experimental infections showed that WT and Il4−/− mice clear P. chabaudi-infected erythrocytes at identical rates52, suggesting that IL-4 may be dispensable for protection against blood-stage malaria. Regarding liver-stage infection, as discussed in detail below, CD4+ T cell-derived IL-4 is important for promoting potent effector CD8+ T cell responses against infected hepatocytes55, as well as maintaining functional Plasmodium-specific memory CD8+ T cell populations56. Thus, there may be potentially important context-dependent roles for IL-4 and Th2 cells during malaria and further work is required to define the precise role of Th2 cells, independent of their secretion of IL-4.
T follicular helper cells.
Both vaccination and infection-induced Tfh cells are broadly characterized by expression of the transcriptional repressor BCL-6, the chemokine receptor CXCR5, and the inhibitory receptor programmed cell death protein 1 (PD1)57. Multiple experimental systems have shown that expression of CXCR5 licenses pathogen-specific CD4+ T cells to undergo step-wise Tfh cell differentiation within lymph nodes; Tfh cells first localize to the ‘T-B border’ of the follicle, engage in cognate interactions with B cells that reinforce their differentiation, and then migrate into the light zone of the germinal centre (GC) where they provide selection, survival, and maturation signals to differentiating GC B cells58. CXCR5+PD-1+ Tfh cells expand during both human and rodent blood-stage Plasmodium infections and are essential for promoting protective antibody responses that aid in the resolution of malaria44, 45, 59, 60, 61, 62. Plasmodium-specific Tfh cells express IL-2163 and inducible T cell costimulator (ICOS)64, 65, which promote maturation of Plasmodium-specific GC B cells and the generation of long-lived plasma cells and memory B cells (Fig. 2). As described for virus-specific Tfh cells66, Plasmodium-specific Tfh cells downregulate BCL-6 and PD1 as they transition from effector to memory populations33, leaving CXCR5 as the most reliable marker for Plasmodium-specific memory Tfh-like cells. Reports also suggest that virus-specific Tfh cells retain greater plasticity and secondary proliferative potential compared to their terminally differentiated Th1-like counterparts66, 67. However, we and others showed that Plasmodium-specific Tfh-like memory cells are less protective than Th1-like memory cells on a per-cell basis following adoptive transfer32, 33. The mechanisms by which either Tfh- or Th1-like memory CD4+ T cells orchestrate secondary immune reactions in malaria remain unknown.
The differentiation of Plasmodium-specific Tfh cells was examined in a recent single cell RNA sequencing (scRNA-seq) study that revealed early bifurcation of Th1 and Tfh cell differentiation68. Recent studies also showed that Plasmodium-specific Tfh cell development and function are promoted by IL-669 and countered by the activity of IRF370. Notably, effector CD4+ Tfh cells exhibiting mixed characteristics of either Th1 or T regulatory 1 (Tr1) cells (described below) have also been reported during experimental malaria45, 31, 33, 71. These cells often co-express NK1.1, CXCR5, IFNγ, IL-21, and IL-10; and in one study, their formation and function did not depend on the function of BCL-631. This mixed ‘helper’ phenotype is perhaps not surprising because like many other CD4+ T cell subsets, Tfh cells are also highly plastic and can adopt characteristics of either Th1, Th2, Th17, or Treg cells. In Plasmodium-infected humans59 and rodents45, BCL-6-expressing Tfh cells adopt a Th1-like phenotype59, 72, secrete IFNγ and provide relatively inferior help to B cells46. Additionally, Tfh-like cells can express FOXP3 and exert regulatory function; such cells are referred to as T follicular regulatory (Tfr) cells and localize to the GC, where they suppress humoral immunity via expression of either CTLA-473 or PD174. Notably, the role of Tfr cells during Plasmodium infection may be temporally distinct or context-dependent, as Tfr cells are reported to be a critical source of IL-10 that supports GC B cell responses during viral infections75. Although the key source of IL-10 that supports humoral immunity during malaria46 has not been reported, it will be of interest to determine whether either Tfh, Tfr or T regulatory type 1 (Tr1) cells regulate humoral immunity via provision of IL-10. The importance of Tfh cells during acute and chronic malaria is now well documented. Although our understanding of Tfr cells continues to expand74, 76, 77, we still know very little about Tfr cell differentiation and function during either human or experimental Plasmodium infection. Aberrant Plasmodium-specific effector or memory Tfh or Tfr cell responses represent one possible explanation for the delayed acquisition or deficient maintenance of antibody-mediated anti-malarial immunity. Thus, future studies aimed at deciphering the phenotype, function, and role of Tfh and Tfr cells following either single or repeated Plasmodium exposures will be of interest.
Th17 cells.
In the presence of IL-6, IL-23, and TGFβ, naïve CD4+ T cells can adopt a functional programme governed by RORγt, an essential transcription factor regulating IL-17 expression. Th17 cells have been linked to orchestrating neutrophil recruitment and function during multiple scenarios of microbial infection (reviewed in ref.78). Although pathological neutrophil responses have been associated with severe malaria79, 80, only a limited number of studies describe the presence of Plasmodium infection-induced Th17 cells. The first study described CD4+ T cells in malaria exposed individuals from Mali with the capacity to express IL-17A following in vitro stimulation81. In experimental models, only a very limited number of Th17 cells are detected in the spleens of Plasmodium-infected mice82, 83, 84, 85. Mechanistic studies failed to identify any role for Th17 cells and the genetic deficiency in Il17a did not influence either disease severity82 or protective immunity83. However, Th17 cells can also secrete IL-2186, suggesting that Th17 cells, when present, may play a modest role in supporting GC reactions. IL-21 may also support CD8+ T cell responses, as has been described in Toxoplasma gondii infection87, 88. Moreover, rodent models have shown that the Th17 cell-associated cytokine IL-22 was essential for protecting against inflammatory pathology in the lung and liver in mice with malaria83. CD8+ T cells were found to express IL-22, although whether Th17 cells also served as a source of IL-22 was not determined. Further work is necessary to define the role of Th17 cells during Plasmodium infections. Whether Th17 cell populations can also be expanded in anti-malarial vaccinations and meaningfully contribute to host protection also remains to be addressed.
IL-27-producing CD4+ T cells.
IL-27 is a cytokine that has been linked to both pro- and anti-inflammatory immune functions. Initial analyses of the role of IL-27 during experimental malaria revealed its importance in limiting immunopathology in either primary or secondary blood-stage malaria89, 90, 91. First described as a myeloid-derived immunoregulatory factor, recent work using experimental P. berghei infection in mice revealed that IL-27 can also be produced by parasite-specific CD4+ T cells92. This study showed that IL-27 is produced by a subset of IL-10−IFNγ− CD4+ T cells during acute Plasmodium infection and that the primary effect of IL-27 is to suppress IL-2 production by other CD4+ T cells92. Given their immunosuppressive function, determining whether the expansion of IL-27-expressing CD4+ T cells is a potential signature of either acute or chronic Plasmodium infection warrants further investigation.
IL-10-producing CD4+ T cells.
T regulatory type 1 (Tr1) cells express high levels of both BLIMP1 and T-BET, are FOXP3-negative, and co-express IFNγ and IL-1093, 94, 95. These cells are widely regarded as a subset of terminally differentiated Th1 cells that have been reprogrammed to express IL-10. The precise mechanisms by which this transition occurs remain incompletely understood. However, experimental data support the view that CD4+ T cell-intrinsic IL-2790, IL-1096, and type I IFN signaling94 can promote Tr1 cell formation in Plasmodium-infected mice, whereas unspecified signals from CD8a+ cDC1s restrain the development of Tr1 cells97,. Type I IFNs also appear to promote rapid Tr1 cell formation following CHMI98. Tr1 cells have been established as the primary source of IL-1093, 94, 95 that both prevent immunopathology and facilitate parasite persistence during protozoan infections93, 99, 100. Malaria is no exception and Tr1 cells appear to limit protective immunity and parasite control93, 94, by either suppressing humoral immune responses94 or possibly by reducing the capacity of antigen presenting cells (APCs) to sustain T cell activity. An early cross-sectional study in Gambia identified that children with mildly symptomatic malaria had an increased proportion of IL-10-producing CD4+ T cells compared to children with severe clinical malaria101. More recently, the presence of CD4+ Tr1 cells was associated with increased parasitemia during human Plasmodium infections95, yet was also associated with a decreased risk of severe clinical disease102. Therefore, while Tr1 cells can down-regulate pro-inflammatory responses during malaria, IL-10-production by Tr1 cells may impair parasite control93. Thus, both FOXP3+ Treg cells (discussed below) and FOXP3− Tr1 cells are critical regulators of host anti-malarial immunity, with FOXP3− Tr1 cells primarily serving to limit malarial disease severity. The precise mechanisms by which Tr1-derived IFNγ and IL-10 modulate protective versus pathological responses remain to be deciphered. Understanding of the factors that regulate Tr1 cell activity during Plasmodium infection will have important implications for controlling malaria-associated immunopathology.
Treg cells.
Treg cells are a class of CD4+ T cells delineated by their expression of the transcription factor FOXP3. Although Treg cells were originally identified as expressing high levels of CD25 and low levels of CD45RB on their cell surface, we now know that there is no single surface marker that demarcates all Treg cells. Along with FOXP3, the current definition of Treg cells encompasses the epigenetic ‘Treg signature’, of CpG demethylation in Foxp3 conserved non-coding region 2, Tnfrsf18, Ctla4, Ikzf4 and Il2ra genes103, 104, 105. Treg cells are known to define host immune responses to human and mouse malaria, yet their impact and mode of action have remained controversial and contentious106.
Most of the inconsistencies observed in the mouse models of malaria (elaborated in various reviews106, 107, 108) can be traced back to the incomplete characterizations of various T cell subsets, limitations in the methodologies used to deplete Treg cells, or variations in the infection models used. For example, CD25 is expressed by activated conventional CD4+ and CD8+ T cells and is not expressed by a significant proportion of Treg cells109, yet anti-CD25 (PC61) has been commonly used to ‘deplete’ Treg cells106, 107, 108 in mice. When mice were treated with an anti-CD25 antibody at the onset of infection, better control of P. yoelii infection was seen in BALB/c mice110, but not in C57BL/6 mice93. Furthermore, treatment with anti-CD25 exacerbated P. chabaudi infection in BALB/c mice111. In addition, the timing of depletion of Treg cells in mouse studies has been inconsistent, with these studies often failing to consider the Treg cell kinetics associated with blood-stage malaria73. When Treg cells were depleted using anti-CD25 treatment at the peak of their population expansion (as opposed to at the onset of infection93) in P. yoelii-infected C57BL/6 mice73, parasitemia was better controlled. Given that Treg cells rapidly repopulate the host in 1–2 days after their complete depletion112, 113, informed Treg cell-depletion strategies may need to be tailored to the kinetics of Treg cell expansion in malaria. For instance, C57BL/6 mice treated with anti-CD25 antibody before the onset of P. berghei (ANKA) infection were better protected from experimental cerebral malaria (ECM)114, 115. But ECM symptoms remained unchanged with precise depletion of Treg cells in FOXP3-Diphtheria toxin receptor (DTR) transgenic (DEREG) C57BL/6 mice116, suggesting a minimal role for Treg cells in the ECM model. Malaria is a complex disease in which Treg cell numbers and function are kinetically modulated during its course. A better understanding of the Treg cell kinetics and employment of precise Treg cell-depletion strategies accordingly might help understand the underlying mechanisms of immunoregulation in malaria, using the mouse model.
Longitudinal or cross-sectional studies in human subjects from endemic areas naturally or experimentally infected with Plasmodium consistently show that Treg cell populations expand in blood-stage malaria; and lower Treg cell frequencies are associated with lower parasite loads along with more favorable disease outcomes73, 108, 117, 118, 119. Our inability to manipulate the immune system in humans has made it difficult to know if the expansion of Treg cell populations is a cause or consequence of enhanced parasite loads. Lower frequencies of functionally deficient Treg cells are associated with lower parasitemia levels in the African Fulani people, who are naturally more resistant to malaria, compared to the sympatric Mossi people118. This suggested that Treg cell expansion in humans may be a consequence, rather than the cause, of increasing parasite loads in malaria. In addition, longitudinal studies showed that clinical malaria was associated with higher Treg cell frequencies compared to pre-infection or convalescent levels73. Also, higher pre-infection Treg cell frequencies correlated with increased risk of febrile malaria120. Further longitudinal, controlled infection studies with human malaria will help draw better parallels on the nature of Treg cell responses and functions in the human and mouse model of malaria; and might advance our mechanistic understanding of immunoregulation by Treg cells in blood-stage malaria.
Treg cells can be broadly classified into two subsets based on their origins: thymically derived Treg (tTreg) cells, which differentiate in the thymus from immature CD4+CD8+ precursors, and peripheral Treg (pTreg) cells, which originate from conventional CD4+ T cells in peripheral tissues that have upregulated FOXP3 in response to chronic or sub-optimal T cell receptor (TCR) stimulation, homeostatic cues or commensal bacteria121, 122, 123, 124, 125. The presence of a diverse array of non-overlapping TCRs in tTreg and pTreg cells, indicates a distinct, non-redundant role for either Treg cell subsets in the recognition of antigens105, 126. Yet, the relative contributions of pTreg or tTreg cells in most infections, including blood-stage malaria, remain unresolved. This is partly due to the absence of precise markers that can distinguish pTreg cells from tTreg cells. Although the expression of Neuropilin-1 in mice and Helios in humans are sometimes used to separate tTreg cells from pTreg cells105, their lack of accuracy has confounded our abilities to segregate their functional roles in various infection models122, 127. Some earlier studies with experimental human malaria have suggested that pTreg cells may be induced in response to blood-stage malaria, through TGFβ activation107, 119, 128. However, the role of TGFβ is now considered to be mostly limited to inducing Treg cells in vitro122, 129, 130. Other studies have suggested that tTreg cell populations may expand in response to blood-stage malaria in both humans and mice93, 111, 114, 131. Therefore, the relative contributions of, one, antigen-driven proliferation, two, cytokine-mediated differentiation, and three, recruitment to peripheral tissues, to the generation and function of tTreg or pTreg cells in blood-stage malaria remain unsettled. More research needs to be done to help understand the relative contributions of tTreg or pTreg cells to the overall impact of Treg cells in combating blood-stage malaria.
The mechanism by which Treg cells function in malaria is also a relatively understudied area. Recent work from our own laboratory using a mouse model of malaria infection showed that Treg cells use CTLA-4 expression to hinder productive interactions between Tfh cells and B cells in GC reactions in lymphoid tissues; this resulted in compromised humoral and cell-mediated immune responses to blood-stage malaria73. Populations of Treg cells that expand in febrile malaria in humans and mice express higher levels of CTLA4; and therapeutic blockade of CTLA4 tailored to the kinetics of CTLA4 expression or Treg cell expansion in blood-stage malaria resulted in better control of the infection in the mouse model. In addition to expressing CTLA4, Treg cell populations that expand in P. yoelii- infected mice transcriptionally upregulate genes that are associated with Treg function, including Gpr83, Penk1, GzmB, Socs2 and Il10132. More extensive investigations in the future will help determine the roles of these genes, as well as the molecular mechanisms of immunoregulation in blood-stage malaria.
The immuno-regulatory responses to malaria, spearheaded by Treg cells help rein in the immune system from damaging the host. Malaria is a protracted disease with multiple phases of complex host-pathogen interactions leading to discrete waves of cellular and humoral immune responses. Only mechanistic studies using the mouse model, with concurrent observational studies in humans may help decipher the mechanistic underpinnings of immunoregulation by Treg cells in primary or subsequent blood-stage malaria infections.
CD8+ T cells in malaria
CD8+ T cells recognize pathogen-derived peptides bound to surface MHC class I molecules on APCs or infected cells and contribute to the clearance and immune memory against many intracellular pathogens. Malaria parasite-specific CD8+ T cells have been described in the blood of humans living in endemic areas133, 134 and after vaccination135, 136, 137. In experimental malaria, CD8+ T cells specific for sporozoite antigens, liver-stage antigens (the pre-erythrocytic stages) and blood-stage antigens (erythrocytic stage) (Fig. 2) have been described after infection or vaccination138, 139, 140. Although CD8+ T cells are primed against the various pre-erythrocytic stages of malaria in the vertebrate host, their relevance to protection in a primary infection is contentious140, 141. This is largely because an infected mosquito delivers only a few hundred sporozoites into the host dermis142, leading to a very low proportion of infected hepatocytes prior to release of blood-stage merozoites140, 143. Further, liver-stage malaria offers a short window of opportunity (~7 days in humans and ~2 days in mice)144, 145 to mount optimally functional146 effector CD8+ T cell responses106, 144, 147. Additionally, repeated exposure to Plasmodium infections does not generate sufficient immunity against the liver-stages in humans despite eliciting disease-limiting humoral immunity against the pathogenic blood stage148, 149, 150; the precise reasons for this remain a major knowledge gap. However, if sufficient antigen-specific CD8+ T cell responses are generated against the pre-erythrocytic stages of Plasmodium by immunization, progression to blood-stage malaria can be prevented in humans, non-human primates and the mouse models5, 151, 152, 153. Hence the underlying mechanisms of priming, dynamics and function of CD8+ T cell responses to malaria are areas of intense research in the quest for an anti-malarial vaccine.
For a long time it was assumed that CD8+ T cell responses against pre-erythrocytic stages of Plasmodium were primed by infected hepatocytes154. Yet, the unlikelihood of rare naïve CD8+ T cells, encountering infrequently infected hepatocytes in the liver made this event improbable based on the existing paradigms of T cell priming mechanisms155. Early studies revealed that CD11c+ dendritic cells (DCs) played a vital role in priming CD8+ T cell responses to pre-erythrocytic developmental stages of Plasmodium156. Sporozoite antigen-specific CD8+ T cell responses are generated in the skin-draining lymph nodes at the site of inoculation, primarily by the uptake and cross-presentation of sprorozoites by CD8+ CD11c+ DCs157, 158. However, the observation that late liver-stage arresting genetically attenuated Plasmodium parasites (GAPs) elicited better protective CD8+ T cell responses compared to early liver-stage arresting GAPs or radiation attenuated Plasmodium sporozoites (RAS) suggested that the developmental progression of Plasmodium in infected hepatocytes had a decisive role in generating better, perhaps antigenically broader CD8+ T cell responses159. Recent findings from our lab identified a class of monocyte-derived CD11b+CSF1R+CD207+F4/80+CD11c+ APCs in the liver that acquired Plasmodium following hepatocyte infection, to prime CD8+ T cell responses against liver-stage specific antigens, in the liver-draining lymph nodes (Kurup et al submitted). This finding uncovers how a broad spectrum of CD8+ T cell responses are primed against the bona fide liver stages of development in malaria. The precise mechanisms by which APCs acquire Plasmodium liver-stage antigens from infected hepatocytes remain to be elucidated, and may involve extrinsic or cell-intrinsic innate immune pathways. In contrast to natural infections, live-attenuated vaccines using RAS generate sterilizing CD8+ T cell mediated immune responses only after intravenous inoculation of the parasites160, 161. Here, CD8+ T cell responses may be primed in the spleen, in addition to the liver-draining lymph nodes162. Furthermore, other immune cells including NK cells, helper T cells and regulatory T cells influence the generation and maintenance of productive CD8+ T cell responses55, 163, 164, 165. A deeper understanding of the dynamics of CD8+ T cell priming in natural Plasmodium infections or live-attenuated malaria vaccines will help us devise strategies that can help engender stronger and long-lasting protective immunity to malaria.
Intravital and in vivo imaging studies showed that adoptively transferred sporozoite-specific effector CD8+ T cells clustered around infected hepatocytes, resulting in loss of Plasmodium-GFP signal indicating the likely destruction of the parasite and infected host cell166, 167; and it is assumed that memory CD8+ T cells may function similarly. Activated CD8+ T cells are capable of elaborating a number of anti-microbial effector mechanisms, broadly divided into cytolytic and cytokine pathways168, 169. Among the various effector pathways and molecules associated with CD8+ T cell function, while granzymes and Fas/FasL mediated pathways appeared dispensable for the effector functions of CD8+ T cells against liver-stage malaria in mice, IFNγ, TNF and perforin contributed to protective immunity, depending on the Plasmodium species and genetic background of the host170, 171, 172, 173, 174, 175. IFNγ is known to directly impair Plasmodium development in human hepatocytes in culture176. Mechanistic studies with the mouse models of malaria indicated that IFN-γ produced by CD8+ T cells induced nitric oxide synthase (and hence nitric oxide) in the infected hepatocytes to help eliminate them177. However, adoptively transferred Plasmodium circumsporozoite protein (CSP)-specific CD8+ T cells deficient in IFNγ were able to confer protection from P. yoelii liver-stage malaria178. The variation in the functional roles of CD8+ T cells depending on the parasite species and the genetic background of the host179, 180 indicates that effective CD8+ T cell responses in malaria may be influenced by cell-extrinsic and natural host defence mechanisms. The precise mechanisms by which CD8+ T cells function to limit liver-stage infection in human malaria remain unknown, and a better understanding of those will help further our ability to tailor sterilizing immunity to malaria.
As indicated above, the protective role of CD8+ T cell responses generated against pre-erythrocytic developmental stages of a primary Plasmodium infection are possibly relevant in subsequent infections or in vaccinations. Although the higher frequencies of peripheral CD8+ T cell responses elicited by various immunization strategies against Plasmodium may reflect the higher chances of protection from subsequent challenges with live sporozoites9, 153, 180, 181, if and how they functionally and numerically contribute directly to protection against liver-stage malaria is currently unknown. Nevertheless, a tangible pool of liver-resident CD8+ T cell populations specific for the liver-stage antigens of malaria, that might directly target infected hepatocytes, are generated and maintained after immunizations with RAS182 or through ‘prime and trap/target’ subunit vaccinations that target the liver182, 183, 184. These LFA1hi CXCR6+ CD69+ CXCR3+ CD8+ T cells that establish a niche within the hepatic sinusoids can be primed outside of the liver and offer protective immunity to subsequent sporozoite challenges182, 184, 185, 186. Although liver-resident memory CD8+ T cells appeared essential for immunity after RAS immunization of mice182, whether these cells alone are sufficient for sterilizing immunity, or provide a ‘sensing and alarm’ function187 to recruit circulating Plasmodium-specific CD8+ T cells remains an important knowledge gap. A thorough understanding of the origin, dynamics and functional mechanics of Plasmodium specific liver-resident CD8+ T cells may help tailor this population to better protect from future liver-stage Plasmodium infections.
Although CD8+ T cells are vital for protection from liver-stage malaria, they are thought to contribute little to control of the blood-stage of malaria infection, mostly owing to the lack of MHC class I on erythrocytes188, 189, 190. Yet, robust CD8+ T cell responses directed at various Plasmodium erythrocytic-stage antigens are primed, primarily in the spleen, in blood-stage malaria191, 192. Of note, human CD8+ T cells generated in infection with P. vivax that preferentially colonizes reticulocytes, specifically eliminated parasitized reticulocytes though an HLA-I mediated, granulysin driven mechanism, possibly contributing to protection193. Although the breadth of their role in protection to blood-stage malaria with other Plasmodium species may be limited, CD8+ T cells are speculated to drive the highly pathogenic cerebral malaria in humans, based on parallel studies in the mouse model194, 195, 196, 197. The pathogenic role of CD8+ T cells in murine experimental cerebral malaria is mediated directly by perforin and Granzyme B, and indirectly by IFN-γ-driven accumulation of parasitized RBC in the brain196, 198, 199. Cerebrovascular endothelial cells are possibly targeted by CD8+ T cells in an antigen-specific manner, to drive blood-brain barrier dysfunction, subsequent vascular leakage and neuronal death194. Of note, cerebral malaria is limited to certain mouse strains, Plasmodium species or isolates200, 201, 202, 203, 204. Recent experiments from our laboratory suggest that the magnitude of certain blood-stage specific CD8+ T cell responses, which is largely predicated on the size of the precursor pool in specific mouse strains, may contribute to disease specificity205. Although the functional role of CD8+ T cells in orchestrating cerebral malaria in humans remains unresolved206, 207, mostly owing to the ethical implications of invasive studies, only further research can enhance our understanding in this relatively understudied yet clinically critical area of malaria pathogenesis.
γδ T cells in malaria
γδ T cells are a subgroup of T cells that express distinct TCRγ and TCRδ chains and account for around 4% of all T cells in healthy adult humans208, 209, 210, 211. The precise contributions of γδ T cells to host immunity remain unresolved, primarily owing to the wide spectrum of effector functions they possess, that may be governed by the immediate tissue microenvironments212. Predictably, the contributions of γδ T cells to anti-malarial immunity also remain poorly understood and disputed213. Although γδ T cell populations, specifically those expressing the Vγ9+Vδ2+ chains (which constitute ~75% of all γδ T cells in humans), expand in primary P. falciparum or P. vivax infections214, 215, 216, 217 and correlate with protection218, how they function during Plasmodium infection remains to be determined. It is remarkable that in human malaria, Vγ9+Vδ2+ γδ T cell populations appear to expand during acute, primary infections, but possibly contract with each subsequent exposure to malaria, despite reactivation each time216, 217, 219. Although the progressive improvement in tolerance to clinical malaria with multiple exposures in endemic regions has been attributed to the decline of Vγ9+Vδ2+ γδ T cells220, it is hard to ascertain if the γδ T cell kinetics is also a cause or an effect in this relationship. Considering that the frequencies of Vγ9+Vδ2+ γδ T cells naturally increase with age221, and most humans are exposed to malaria from childhood in endemic regions, recurrent Plasmodium challenges that hinder γδ T cell expansion220 may indeed help control clinical malaria with age in endemic regions. It is noteworthy that severity of symptomatic malaria (excluding cerebral malaria) and mortality in primary infections increase with age222, 223 and could be a direct function of naturally increasing frequencies of γδ T cells. However, based on studies using the mouse model of malaria, γδ T cells were observed to undergo clonal expansion, albeit disproportionately, in various tissues as a consequence of blood-stage malaria. γδ T cells are reported to serve as a source of IL-21 that may support Tfh cell responses224, and γδ T cells helped control Plasmodium recrudescence in a TCR-dependent fashion, possibly via by their production of colony stimulating factor-1 (M-CSF)225. Of note, γδ T cells are also a correlate of protective efficacy in experimental RAS immunizations5, 8, 226. Depletion of γδ T cells at the time of RAS vaccination in mice hindered the influx of CD11c+ DCs into the liver, prevented optimal effector CD8+ T cell responses and sterilizing immunity to future sporozoite challenges. In contrast, γδ T cell ablation immediately before challenge of RAS immunized mice did not diminish protection226. These data suggest that γδ T cells may help facilitate effective CD8+ T cell responses that provide immunity to liver-stage malaria, although the underlying mechanisms remain a major knowledge gap. Future mechanistic studies that segregate the pre-erythrocytic and erythrocytic developmental stages of malaria will help improve our understanding of γδ T cell function in Plasmodium infections and anti-malarial vaccines in humans.
The fundamental mechanisms of γδ T cells function in the context of infections are also poorly understood. γδ T cells are thought to be among the first lines of defence against infections, with abilities to contextually stimulate or repress immune responses through distinct natural or induced cell subsets212, 227, 228, 229, 230. γδ T cells can also be recalled in reinfections, target pathogens directly in a TCR dependent or independent manner, or indirectly by enlisting other cell subsets208, 212, 231. These properties that bridge the distinctions of innate and adaptive responses make γδ T cells functionally unique. Vγ9+Vδ2+ γδ T cells may help control primary Plasmodium infections in humans through the production of various immune mediators, such as IFNγ, TNF or granzyme B, in addition to possibly killing the merozoites directly in blood-stage malaria72, 214, 232, 233. In human malaria, Vγ9+Vδ2+ γδ T cell populations undergo polyclonal expansion upon sensing of phosphoantigens derived from P. falciparum or P. vivax apicoplast [G], is independent of classical antigen presentation, but requires the presence of monocytes, CD4+ T cells or exogenous cytokines214, 215, 220, 225, 234, 235, 236, 237. By contrast, in P. chabaudi blood-stage malaria in mice, Vδ6.3+ (also known as TRAV15N-1+) γδ T cells specifically undergo clonal expansion and exhibit a unique transcriptional and functional profile that contributes to protection225. In summary, there is emerging evidence for key roles of γδ T cells in the control and pathogenesis of malaria and these should prompt further studies to decipher the mechanisms involved.
T cells and vaccine design
Multiple anti-malarial vaccine platforms are being evaluated in humans for their capacity to effectively target and block either sporozoite- or liver-stage progression, or trigger humoral responses that reduce the severity or delay the onset of blood-stage malaria and clinical disease (recently reviewed in238, 239). RTS,S, the most clinically advanced anti-malaria subunit vaccine, is believed to stimulate the production of antibodies that may either target sporozoites for destruction or prevent their ability to reach the liver. The humoral immune response to RTS,S likely involves T cell help and there is some evidence of CD4 T cell responses in vaccinees, although much work remains to characterize these responses and determine why immunity after RTS,S vaccination is of short duration238, 239. While whole parasite vaccines, including RAS, GAP, CPS, and chemically attenuated blood-stage platforms, are likely to trigger the expansion and function of Plasmodium-specific CD8+ and CD4+ T cells and antibodies responses, similar to rodent models discussed above, information on which of these platforms provides the best T cell responses in humans remains limited. Despite the many correlates observed in experimentally vaccinated humans, the true requirements for either Tfh, Th1, or cytotoxic CD4+ and CD8+ T cells and the precise effector mechanism by which these T cell subsets might mediate protection in humans are not understood. Advances in basic mechanistic insights into the role of T cells in malaria should provide a roadmap to address these issues in human vaccines.
Due to the low parasite burden and lack of disease association, the liver-stage of Plasmodium infection remains a particularly attractive target for T cell-based vaccines. Attenuated whole parasite vaccines such as RAS elicit potent protective liver-stage immunity in animals and humans240, 241. The protective mechanisms of these vaccines are likely a function of their ability to generate T resident memory in the liver targeting multiple parasite antigens162. However, such whole parasite vaccines are complicated by the cumbersome production process, the need to deliver large numbers of sporozoites multiple times via intravenous immunizations to achieve potent immunity and reduced efficacy in the field242. Prime-boost approaches with viral vectored antigens designed to elicit T cells have shown some promise in vaccine trials in malaria naïve subjects, although the number of antigens investigated to date is small and it is unclear how potent the current viral vectored immunization approaches are at eliciting liver T resident memory populations243.
An intriguing possible solution to the latter issue, that has had success in animal models, is to initially prime circulating T cell responses and then pull or trap these cells in the liver during the boosting phase, with liver-tropic viral antigen delivery systems such as adeno-associated virus182 or liver-targeted antigen and adjuvant containing nanoparticles244. Another potential solution is to combine subunit and RAS vaccinations, to take advantage of the ability to RAS to generate liver T resident memory but limit the number of cumbersome RAS immunizations184. Further work to optimize these systems and evaluate them in clinical trials should be a priority.
Additionally, it is unclear from human vaccine trials whether targeting a single antigen will permit generation of sufficient T cells to provide sterilizing liver-stage immunity to Plasmodium.
The field of malaria subunit vaccines is desperately in need of new target antigens to evaluate and the capacity to carry out whole parasite immunization studies in human volunteers could be a fertile basis for such antigen discovery. Importantly, recent work in animal models shows that detection of a T cell response to a Plasmodium antigen does not ensure that antigen will elicit T cells that can protect against infection245. Thus, additional screening approaches, perhaps based on demonstrated antigen-specific recognition of infected hepatocytes, must be developed to identify protective antigens and determine if protective antigens share characteristics such as surface or secreted localization or differentiation stage specificity, that will facilitate their identification and potential incorporation into a successful malaria vaccine.
In summary, recent mechanistic studies of the T cell response to malaria from the combination of animal models and human vaccine/challenge studies have provided new insights and identified critical knowledge gaps to overcome in developing potent, T cell-based vaccines for malaria. Emphasis should be placed on identifying of the mechanisms that determine the formation and persistence of liver-resident memory CD8+ T cells, as well as circulating memory CD4+ Th1 and Tfh cells with the capacity to orchestrate phagocytic and humoral responses. New mechanistic insight should facilitate refinements in the formulation, delivery, and induction of protection by next-generation anti-malarial vaccines.
Conclusions and perspectives
Cell-mediated immune responses are critical for immunity against malaria. Here, we have summarized our current understanding of the roles of various T cell subsets that contribute to protection against both pre-erythrocytic and erythrocytic stages of malaria, as understood from experimental or natural infections in humans or animal models. However, there are many fundamental questions that remain unanswered (Box 4). Addressing these gaps should help advance our understanding of the mechanical underpinnings of the immunology of malaria and move the field closer to developing more practical, feasible, and reliable immunization and therapeutic interventions that would help control or eliminate malaria.
Box 4. Key outstanding questions on T cell mediated immunity to malaria.
To what extent do phagocytes mediate clearance of circulating parasites and does IFNγ directly regulate these processes?
What factors govern the generation, maintenance, and function of Tfh cells during malaria? Does repeated Plasmodium parasite exposure distinctly program effector and memory Tfh cell responses?
Which sets of signals regulate the differentiation and function of IL-27- and IL-10-expressing effector CD4+ T cells during malaria? Do these functionally distinct subsets persist as memory cells and do they also regulate recall responses? Are IL-27-expressing cells a signature of malaria? What are the primary cellular targets of IL-27 and IL-10 during malaria? Do the effects of IL-27 and IL-10 evolve as infection progresses?
What are the contributions of distinct populations Th1 and Tfh memory CD4+ T cells in protection against repeated exposure to malaria? How do inflammation, antigen persistence, and the presence of specific immunoregulatory circuits impact the formation and function of Plasmodium-specific memory CD4 T cells?
What are the relative roles of either self- or antigen-specific peripheral and thymic Tregs in primary and repeated Plasmodium infections?
What signals regulate liver resident CD8+ T formation, dynamics, mechanisms of maintenance and protection in malaria?
What are the key functional dynamics of CD8+ T cells in human cerebral malaria?
What antigens stimulate oligoclonal γδ T cell expansions and do memory γδ T cell populations persist? By what mechanisms do γδ T cells protect against liver-stage and blood-stage malaria? Do the γδ T cells elicited by vaccination meaningfully contribute to host resistance to malaria?
Acknowlegements
We apologize to the countless researchers whose contributions are not discussed in this manuscript due to space limitations. We also thank the Butler and Harty laboratory members for helpful discussions. Work in the NSB lab is supported by grants from the NIH (AI125446 and AI127481). Work in the JTH lab is supported by grants from the NIH (AI42767, AI85515, AI95178 and AI100527)
Glossary terms:
- Sporozoite
Plasmodium parasite life form transmitted by mosquito bite capable of initiating the asexual cycle of growth and differentiation in the vertebrate host
- Merozoite
Plasmodium parasite life form that first develops in infected hepatocytes and is capable of initiating either sexual or asexual cycles of development in host red blood cells
- Merosome
Host cell-derived, membrane-bound structures containing multiple merozoites that bud from infected hepatocytes during Plasmodium egress from the liver. Merosomes release merozoites into circulation after rupture
- RTS,S (Mosquirix™)
Candidate anti-malarial vaccine furthest along in global development. RTS,S is comprised of two subdomains of the Plasmodium falciparum circumsporozoite protein that are associated with units of the hepatitis B surface antigen and formulated with the adjuvant AS01 (3-O-desacyl-4’-monophosphoryl lipid A and the saponin QS-21). Infection is prevented by inducing antibodies that either immobilize sporozoites in the skin or prevent sporozoites from infecting the liver
- Circumsporozoite protein (CSP)
Immunodominant, high-density surface antigen expressed by Plasmodium sporozoites that is the target of humoral and cellular immune responses that either block sporozoite infection of the liver or eliminate infected hepatocytes, respectively
- Chemoprophylaxis and sporozoite (CPS) immunization
Vaccination strategy whereby virulent sporozoites are delivered by either mosquito bites or needle injection with prophylactic delivery of a drug targeting Plasmodium blood-stages. Parasites initiate and complete liver-stage development, release merozoites, and initiate the first wave of blood-stage infection before being eliminated by the drug. Vaccinated individuals are thereby exposed to antigens that derive from multiple parasite lifecycle stages while remaining protected against clinical disease by the anti-blood-stage drug
- Apicoplasts
Non-photosynthetic organelles that characterize protists within the Phylum Apicomplexa that are likely derived from an algal endosymbiont. The composite of apicoplast functions are not fully known but defined activities primarily relate to essential metabolic pathways necessary for the viability of Plasmodium and other Apicomplexans
References
- 1.Cibulskis RE et al. Malaria: Global progress 2000 – 2015 and future challenges. Infect Dis Poverty 5, 61 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Regules JA et al. Fractional Third and Fourth Dose of RTS,S/AS01 Malaria Candidate Vaccine: A Phase 2a Controlled Human Malaria Parasite Infection and Immunogenicity Study. J Infect Dis 214, 762–771 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Rts SCTP Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386, 31–45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White MT et al. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis 15, 1450–1458 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishizuka AS et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 22, 614–623 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mordmuller B et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 542, 445–449 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roestenberg M et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 361, 468–477 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Seder RA et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341, 1359–1365 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Ewer KJ et al. Protective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat Commun 4, 2836 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sissoko MS et al. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis 17, 498–509 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wykes MN & Lewin SR Immune checkpoint blockade in infectious diseases. Nat Rev Immunol 18, 91–104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soon MSF & Haque A Recent Insights into CD4(+) Th Cell Differentiation in Malaria. J Immunol 200, 1965–1975 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Montes de Oca M, Good MF, McCarthy JS & Engwerda CR The Impact of Established Immunoregulatory Networks on Vaccine Efficacy and the Development of Immunity to Malaria. J Immunol 197, 4518–4526 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Troye-Blomberg M et al. Production of IL 2 and IFN-gamma by T cells from malaria patients in response to Plasmodium falciparum or erythrocyte antigens in vitro. J Immunol 135, 3498–3504 (1985). [PubMed] [Google Scholar]
- 15.Su Z & Stevenson MM Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect Immun 68, 4399–4406 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meding SJ, Cheng SC, Simon-Haarhaus B & Langhorne J Role of gamma interferon during infection with Plasmodium chabaudi chabaudi. Infect Immun 58, 3671–3678 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang KY, Schultz WW & Gordon FB Interferon induced by Plasmodium berghei. Science 162, 123–124 (1968). [DOI] [PubMed] [Google Scholar]
- 18.Shear HL, Srinivasan R, Nolan T & Ng C Role of IFN-gamma in lethal and nonlethal malaria in susceptible and resistant murine hosts. J Immunol 143, 2038–2044 (1989). [PubMed] [Google Scholar]
- 19.Salles EM et al. P2X7 receptor drives Th1 cell differentiation and controls the follicular helper T cell population to protect against Plasmodium chabaudi malaria. PLoS Pathog 13, e1006595 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotz A et al. Atypical activation of dendritic cells by Plasmodium falciparum. Proc Natl Acad Sci U S A 114, E10568–E10577 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazarevic V, Glimcher LH & Lord GM T-bet: a bridge between innate and adaptive immunity. Nat Rev Immunol 13, 777–789 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oakley MS et al. T-bet modulates the antibody response and immune protection during murine malaria. Eur J Immunol 44, 2680–2691 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Riley EM & Stewart VA Immune mechanisms in malaria: new insights in vaccine development. Nature medicine 19, 168–178 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Bastos KR et al. Impaired macrophage responses may contribute to exacerbation of blood-stage Plasmodium chabaudi chabaudi malaria in interleukin-12-deficient mice. J Interferon Cytokine Res 22, 1191–1199 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Jaramillo M, Gowda DC, Radzioch D & Olivier M Hemozoin increases IFN-gamma-inducible macrophage nitric oxide generation through extracellular signal-regulated kinaseand NF-kappa B-dependent pathways. J Immunol 171, 4243–4253 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Blanchette J, Jaramillo M & Olivier M Signalling events involved in interferon-gamma-inducible macrophage nitric oxide generation. Immunology 108, 513–522 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horowitz A et al. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol 184, 6043–6052 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Fontana MF et al. Macrophage Colony Stimulating Factor Derived from CD4+ T Cells Contributes to Control of a Blood-Borne Infection. PLoS Pathog 12, e1006046 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss WR et al. A plasmid encoding murine granulocyte-macrophage colony-stimulating factor increases protection conferred by a malaria DNA vaccine. J Immunol 161, 2325–2332 (1998). [PubMed] [Google Scholar]
- 30.Stephens R & Langhorne J Effector memory Th1 CD4 T cells are maintained in a mouse model of chronic malaria. PLoS Pathog 6, e1001208 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opata MM et al. Early effector cells survive the contraction phase in malaria infection and generate both central and effector memory T cells. J Immunol 194, 5346–5354 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Opata MM et al. Protection by and maintenance of CD4 effector memory and effector T cell subsets in persistent malaria infection. PLoS Pathog 14, e1006960 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zander RA et al. Th1-like Plasmodium-Specific Memory CD4(+) T Cells Support Humoral Immunity. Cell Rep 21, 1839–1852 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reece WH et al. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med 10, 406–410 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Oliveira GA et al. Class II-restricted protective immunity induced by malaria sporozoites. Infect Immun 76, 1200–1206 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Renia L et al. Effector functions of circumsporozoite peptide-primed CD4+ T cell clones against Plasmodium yoelii liver stages. J Immunol 150, 1471–1478 (1993). [PubMed] [Google Scholar]
- 37.Doolan DL et al. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ cell-, interferon gamma-, and nitric oxide-dependent immunity. J Exp Med 183, 1739–1746 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun P et al. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-gamma. J Immunol 171, 6961–6967 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Burel JG et al. Reduced Plasmodium Parasite Burden Associates with CD38+ CD4+ T Cells Displaying Cytolytic Potential and Impaired IFN-gamma Production. PLoS Pathog 12, e1005839 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bijker EM et al. Cytotoxic markers associate with protection against malaria in human volunteers immunized with Plasmodium falciparum sporozoites. J Infect Dis 210, 1605–1615 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuji M, Romero P, Nussenzweig RS & Zavala F CD4+ cytolytic T cell clone confers protection against murine malaria. J Exp Med 172, 1353–1357 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takita-Sonoda Y et al. Plasmodium yoelii: peptide immunization induces protective CD4+ T cells against a previously unrecognized cryptic epitope of the circumsporozoite protein. Exp Parasitol 84, 223–230 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Obeng-Adjei N et al. Malaria-induced interferon-gamma drives the expansion of Tbethi atypical memory B cells. PLoS Pathog 13, e1006576 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zander RA et al. PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity. Cell Host Microbe 17, 628–641 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryg-Cornejo V et al. Severe Malaria Infections Impair Germinal Center Responses by Inhibiting T Follicular Helper Cell Differentiation. Cell Rep 14, 68–81 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Guthmiller JJ, Graham AC, Zander RA, Pope RL & Butler NS Cutting Edge: IL-10 Is Essential for the Generation of Germinal Center B Cell Responses and Anti-Plasmodium Humoral Immunity. J Immunol 198, 617–622 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rivera-Correa J et al. Plasmodium DNA-mediated TLR9 activation of T-bet(+) B cells contributes to autoimmune anaemia during malaria. Nat Commun 8, 1282 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker JA & McKenzie ANJ TH2 cell development and function. Nature reviews. Immunology 18, 121–133 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Perez-Mazliah D & Langhorne J CD4 T-cell subsets in malaria: TH1/TH2 revisited. Front Immunol 5, 671 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coomes SM et al. IFNgamma and IL-12 Restrict Th2 Responses during Helminth/Plasmodium Co-Infection and Promote IFNgamma from Th2 Cells. PLoS pathogens 11, e1004994 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimoda K et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature 380, 630–633 (1996). [DOI] [PubMed] [Google Scholar]
- 52.von der Weid T, Kopf M, Kohler G & Langhorne J The immune response to Plasmodium chabaudi malaria in interleukin-4-deficient mice. European journal of immunology 24, 2285–2293 (1994). [DOI] [PubMed] [Google Scholar]
- 53.Kumaratilake LM & Ferrante A IL-4 inhibits macrophage-mediated killing of Plasmodium falciparum in vitro. A possible parasite-immune evasion mechanism. J Immunol 149, 194–199 (1992). [PubMed] [Google Scholar]
- 54.Troye-Blomberg M et al. Production by activated human T cells of interleukin 4 but not interferon-gamma is associated with elevated levels of serum antibodies to activating malaria antigens. Proc Natl Acad Sci U S A 87, 5484–5488 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carvalho LH et al. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat Med 8, 166–170 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Overstreet MG, Chen YC, Cockburn IA, Tse SW & Zavala F CD4+ T cells modulate expansion and survival but not functional properties of effector and memory CD8+ T cells induced by malaria sporozoites. PLoS One 6, e15948 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vinuesa CG & Cyster JG How T cells earn the follicular rite of passage. Immunity 35, 671–680 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Victora GD & Nussenzweig MC Germinal centers. Annu Rev Immunol 30, 429–457 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Obeng-Adjei N et al. Circulating Th1-Cell-type Tfh Cells that Exhibit Impaired B Cell Help Are Preferentially Activated during Acute Malaria in Children. Cell Rep 13, 425–439 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Butler NS et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 13, 188–195 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perez-Mazliah D et al. Follicular Helper T Cells are Essential for the Elimination of Plasmodium Infection. EBioMedicine 24, 216–230 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Figueiredo MM et al. T follicular helper cells regulate the activation of B lymphocytes and antibody production during Plasmodium vivax infection. PLoS Pathog 13, e1006484 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perez-Mazliah D et al. Disruption of IL-21 signaling affects T cell-B cell interactions and abrogates protective humoral immunity to malaria. PLoS Pathog 11, e1004715 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sebina I et al. IFNAR1-Signalling Obstructs ICOS-mediated Humoral Immunity during Non-lethal Blood-Stage Plasmodium Infection. PLoS Pathog 12, e1005999 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wikenheiser DJ, Ghosh D, Kennedy B & Stumhofer JS The Costimulatory Molecule ICOS Regulates Host Th1 and Follicular Th Cell Differentiation in Response to Plasmodium chabaudi chabaudi AS Infection. J Immunol 196, 778–791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hale JS et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity 38, 805–817 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ & Jenkins MK Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 35, 583–595 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lonnberg TSV; James KR; Fernandez-Ruiz D; Sebine I; Montandon R;Soon MSF; Fogg LG; Nair AS; Liligeto UN; Stubbington MJT; Ly L; Bagger FO; Zwiessele M; Lawrence ND; Souza-Fonseca-Guimarares F; Bunn PT; Engwerda CR; Heath WR; Billker O; Stegle O; Haque A; Teichmann SA Single-cell RNA-seq and computational analysis using temporal micture modeling resolves TH1/TFHfate bifurcation in malaria. Science Immunology 2 eaal2192 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sebina I et al. IL-6 promotes CD4(+) T-cell and B-cell activation during Plasmodium infection. Parasite Immunol 39 (2017). [DOI] [PubMed] [Google Scholar]
- 70.James KR et al. IFN Regulatory Factor 3 Balances Th1 and T Follicular Helper Immunity during Nonlethal Blood-Stage Plasmodium Infection. J Immunol 200, 1443–1456 (2018). [DOI] [PubMed] [Google Scholar]
- 71.Wikenheiser DJ, Brown SL, Lee J & Stumhofer JS NK1.1 Expression Defines a Population of CD4(+) Effector T Cells Displaying Th1 and Tfh Cell Properties That Support Early Antibody Production During Plasmodium yoelii Infection. Front Immunol 9, 2277 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Troye-Blomberg M et al. Human gamma delta T cells that inhibit the in vitro growth of the asexual blood stages of the Plasmodium falciparum parasite express cytolytic and proinflammatory molecules. Scand J Immunol 50, 642–650 (1999). [DOI] [PubMed] [Google Scholar]
- 73.Kurup SP et al. Regulatory T cells impede acute and long-term immunity to blood-stage malaria through CTLA-4. Nat Med 23, 1220–1225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sage PT & Sharpe AH T follicular regulatory cells. Immunological reviews 271, 246–259 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Laidlaw BJ et al. Interleukin-10 from CD4(+) follicular regulatory T cells promotes the germinal center response. Sci Immunol 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wing JB, Tekguc M & Sakaguchi S Control of Germinal Center Responses by T-Follicular Regulatory Cells. Front Immunol 9, 1910 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie MM & Dent AL Unexpected Help: Follicular Regulatory T Cells in the Germinal Center. Front Immunol 9, 1536 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandquist I & Kolls J Update on regulation and effector functions of Th17 cells. F1000Res 7, 205 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sercundes MK et al. Targeting Neutrophils to Prevent Malaria-Associated Acute Lung Injury/Acute Respiratory Distress Syndrome in Mice. PLoS Pathog 12, e1006054 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feintuch CM et al. Activated Neutrophils Are Associated with Pediatric Cerebral Malaria Vasculopathy in Malawian Children. MBio 7, e01300–01315 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Metenou S et al. Filarial infection suppresses malaria-specific multifunctional Th1 and Th17 responses in malaria and filarial coinfections. J Immunol 186, 4725–4733 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ishida H et al. Development of experimental cerebral malaria is independent of IL-23 and IL-17. Biochem Biophys Res Commun 402, 790–795 (2010). [DOI] [PubMed] [Google Scholar]
- 83.Mastelic B et al. IL-22 Protects Against Liver Pathology and Lethality of an Experimental Blood-Stage Malaria Infection. Front Immunol 3, 85 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keswani T & Bhattacharyya A Differential role of T regulatory and Th17 in Swiss mice infected with Plasmodium berghei ANKA and Plasmodium yoelii. Exp Parasitol 141, 82–92 (2014). [DOI] [PubMed] [Google Scholar]
- 85.Hu WC Human immune responses to Plasmodium falciparum infection: molecular evidence for a suboptimal THalphabeta and TH17 bias over ideal and effective traditional TH1 immune response. Malar J 12, 392 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei L, Laurence A, Elias KM & O’Shea JJ IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem 282, 34605–34610 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moretto MM, Hwang S & Khan IA Downregulated IL-21 Response and T Follicular Helper Cell Exhaustion Correlate with Compromised CD8 T Cell Immunity during Chronic Toxoplasmosis. Front Immunol 8, 1436 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stumhofer JS, Silver JS & Hunter CA IL-21 is required for optimal antibody production and T cell responses during chronic Toxoplasma gondii infection. PLoS One 8, e62889 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Findlay EG et al. Essential role for IL-27 receptor signaling in prevention of Th1-mediated immunopathology during malaria infection. J Immunol 185, 2482–2492 (2010). [DOI] [PubMed] [Google Scholar]
- 90.Freitas do Rosario AP et al. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol 188, 1178–1190 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gwyer Findlay E et al. IL-27 receptor signaling regulates memory CD4+ T cell populations and suppresses rapid inflammatory responses during secondary malaria infection. Infect Immun 82, 10–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kimura D et al. Interleukin-27-Producing CD4(+) T Cells Regulate Protective Immunity during Malaria Parasite Infection. Immunity 44, 672–682 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Couper KN et al. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog 4, e1000004 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zander RA et al. Type I Interferons Induce T Regulatory 1 Responses and Restrict Humoral Immunity during Experimental Malaria. PLoS Pathog 12, e1005945 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jagannathan P et al. IFNgamma/IL-10 co-producing cells dominate the CD4 response to malaria in highly exposed children. PLoS Pathog 10, e1003864 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loevenich K et al. DC-Derived IL-10 Modulates Pro-inflammatory Cytokine Production and Promotes Induction of CD4(+)IL-10(+) Regulatory T Cells during Plasmodium yoelii Infection. Front Immunol 8, 152 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Draheim M et al. Profiling MHC II immunopeptidome of blood-stage malaria reveals that cDC1 control the functionality of parasite-specific CD4 T cells. EMBO Mol Med 9, 1605–1621 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Montes de Oca M et al. Type I Interferons Regulate Immune Responses in Humans with Blood-Stage Plasmodium falciparum Infection. Cell Rep 17, 399–412 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jankovic D et al. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med 204, 273–283 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Montes de Oca M et al. Blimp-1-Dependent IL-10 Production by Tr1 Cells Regulates TNF-Mediated Tissue Pathology. PLoS Pathog 12, e1005398 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walther M et al. Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog 5, e1000364 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boyle MJ et al. The Development of Plasmodium falciparum-Specific IL10 CD4 T Cells and Protection from Malaria in Children in an Area of High Malaria Transmission. Front Immunol 8, 1329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miyao T et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity 36, 262–275 (2012). [DOI] [PubMed] [Google Scholar]
- 104.Ohkura N et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 37, 785–799 (2012). [DOI] [PubMed] [Google Scholar]
- 105.Yadav M, Stephan S & Bluestone JA Peripherally induced tregs - role in immune homeostasis and autoimmunity. Front Immunol 4, 232 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Van Braeckel-Budimir N, Kurup SP & Harty JT Regulatory issues in immunity to liver and blood-stage malaria. Curr Opin Immunol 42, 91–97 (2016). [DOI] [PubMed] [Google Scholar]
- 107.Finney OC, Riley EM & Walther M Regulatory T cells in malaria--friend or foe? Trends Immunol 31, 63–70 (2010). [DOI] [PubMed] [Google Scholar]
- 108.Hansen DS & Schofield L Natural regulatory T cells in malaria: host or parasite allies? PLoS Pathog 6, e1000771 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Couper KN et al. Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice. J Immunol 178, 4136–4146 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hisaeda H et al. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat Med 10, 29–30 (2004). [DOI] [PubMed] [Google Scholar]
- 111.Cambos M, Belanger B, Jacques A, Roulet A & Scorza T Natural regulatory (CD4+CD25+FOXP+) T cells control the production of pro-inflammatory cytokines during Plasmodium chabaudi adami infection and do not contribute to immune evasion. International journal for parasitology 38, 229–238 (2008). [DOI] [PubMed] [Google Scholar]
- 112.Feuerer M, Shen Y, Littman DR, Benoist C & Mathis D How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity 31, 654–664 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim JM, Rasmussen JP & Rudensky AY Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol 8, 191–197 (2007). [DOI] [PubMed] [Google Scholar]
- 114.Vigario AM et al. Regulatory CD4+ CD25+ Foxp3+ T cells expand during experimental Plasmodium infection but do not prevent cerebral malaria. International journal for parasitology 37, 963–973 (2007). [DOI] [PubMed] [Google Scholar]
- 115.Amante FH et al. A role for natural regulatory T cells in the pathogenesis of experimental cerebral malaria. Am J Pathol 171, 548–559 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Steeg C, Adler G, Sparwasser T, Fleischer B & Jacobs T Limited role of CD4+Foxp3+ regulatory T cells in the control of experimental cerebral malaria. J Immunol 183, 7014–7022 (2009). [DOI] [PubMed] [Google Scholar]
- 117.Jangpatarapongsa K et al. Plasmodium vivax parasites alter the balance of myeloid and plasmacytoid dendritic cells and the induction of regulatory T cells. Eur J Immunol 38, 2697–2705 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Torcia MG et al. Functional deficit of T regulatory cells in Fulani, an ethnic group with low susceptibility to Plasmodium falciparum malaria. Proc Natl Acad Sci U S A 105, 646–651 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Walther M et al. Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 23, 287–296 (2005). [DOI] [PubMed] [Google Scholar]
- 120.Todryk SM et al. Correlation of memory T cell responses against TRAP with protection from clinical malaria, and CD4 CD25 high T cells with susceptibility in Kenyans. PLoS One 3, e2027 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Apostolou I & von Boehmer H In vivo instruction of suppressor commitment in naive T cells. J Exp Med 199, 1401–1408 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Benoist C & Mathis D Treg cells, life history, and diversity. Cold Spring Harb Perspect Biol 4, a007021 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Finney CA, Taylor MD, Wilson MS & Maizels RM Expansion and activation of CD4(+)CD25(+) regulatory T cells in Heligmosomoides polygyrus infection. Eur J Immunol 37, 1874–1886 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haribhai D et al. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol 182, 3461–3468 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Haribhai D et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity 35, 109–122 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lathrop SK et al. Peripheral education of the immune system by colonic commensal microbiota. Nature 478, 250–254 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Elkord E Helios Should Not Be Cited as a Marker of Human Thymus-Derived Tregs. Commentary: Helios(+) and Helios(−) Cells Coexist within the Natural FOXP3(+) T Regulatory Cell Subset in Humans. Front Immunol 7, 276 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Scholzen A, Mittag D, Rogerson SJ, Cooke BM & Plebanski M Plasmodium falciparum-mediated induction of human CD25Foxp3 CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFbeta. PLoS Pathog 5, e1000543 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu Y et al. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol 9, 632–640 (2008). [DOI] [PubMed] [Google Scholar]
- 130.Zheng Y et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463, 808–812 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abel S et al. Plasmodium yoelii infection of BALB/c mice results in expansion rather than induction of CD4(+) Foxp3(+) regulatory T cells. Immunology 148, 197–205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abel S et al. Strong impact of CD4+ Foxp3+ regulatory T cells and limited effect of T cell-derived IL-10 on pathogen clearance during Plasmodium yoelii infection. J Immunol 188, 5467–5477 (2012). [DOI] [PubMed] [Google Scholar]
- 133.Sedegah M et al. Naturally acquired CD8+ cytotoxic T lymphocytes against the Plasmodium falciparum circumsporozoite protein. J Immunol 149, 966–971 (1992). [PubMed] [Google Scholar]
- 134.Doolan DL et al. Degenerate cytotoxic T cell epitopes from P. falciparum restricted by multiple HLA-A and HLA-B supertype alleles. Immunity 7, 97–112 (1997). [DOI] [PubMed] [Google Scholar]
- 135.Romero P et al. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature 341, 323–326 (1989). [DOI] [PubMed] [Google Scholar]
- 136.Doolan DL et al. HLA-DR-promiscuous T cell epitopes from Plasmodium falciparum pre-erythrocytic-stage antigens restricted by multiple HLA class II alleles. J Immunol 165, 1123–1137 (2000). [DOI] [PubMed] [Google Scholar]
- 137.Doolan DL et al. Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proc Natl Acad Sci U S A 100, 9952–9957 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Epstein JE et al. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science 334, 475–480 (2011). [DOI] [PubMed] [Google Scholar]
- 139.Hafalla JC et al. Identification of targets of CD8(+) T cell responses to malaria liver stages by genome-wide epitope profiling. PLoS Pathog 9, e1003303 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Van Braeckel-Budimir N & Harty JT CD8 T-cell-mediated protection against liver-stage malaria: lessons from a mouse model. Front Microbiol 5, 272 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Villarino N & Schmidt NW CD8(+) T Cell Responses to Plasmodium and Intracellular Parasites. Curr Immunol Rev 9, 169–178 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Medica DL & Sinnis P Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected anopheline mosquitoes. Infect Immun 73, 4363–4369 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Churcher TS et al. Probability of Transmission of Malaria from Mosquito to Human Is Regulated by Mosquito Parasite Density in Naive and Vaccinated Hosts. PLoS Pathog 13, e1006108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cowman AF, Healer J, Marapana D & Marsh K Malaria: Biology and Disease. Cell 167, 610–624 (2016). [DOI] [PubMed] [Google Scholar]
- 145.Crompton PD et al. Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annu Rev Immunol 32, 157–187 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Miller JD et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity 28, 710–722 (2008). [DOI] [PubMed] [Google Scholar]
- 147.Sturm A et al. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 313, 1287–1290 (2006). [DOI] [PubMed] [Google Scholar]
- 148.Tran TM et al. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin Infect Dis 57, 40–47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Offeddu V, Thathy V, Marsh K & Matuschewski K Naturally acquired immune responses against Plasmodium falciparum sporozoites and liver infection. International journal for parasitology 42, 535–548 (2012). [DOI] [PubMed] [Google Scholar]
- 150.Doolan DL, Dobano C & Baird JK Acquired immunity to malaria. Clin Microbiol Rev 22, 13–36, Table of Contents (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weiss WR & Jiang CG Protective CD8+ T lymphocytes in primates immunized with malaria sporozoites. PLoS One 7, e31247 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Weiss WR, Sedegah M, Beaudoin RL, Miller LH & Good MF CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A 85, 573–576 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schmidt NW, Butler NS, Badovinac VP & Harty JT Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS Pathog 6, e1000998 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Corradin G & Levitskaya J Priming of CD8(+) T Cell Responses to Liver Stage Malaria Parasite Antigens. Front Immunol 5, 527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang N & Bevan MJ CD8(+) T cells: foot soldiers of the immune system. Immunity 35, 161–168 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jung S et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chakravarty S et al. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med 13, 1035–1041 (2007). [DOI] [PubMed] [Google Scholar]
- 158.Radtke AJ et al. Lymph-node resident CD8alpha+ dendritic cells capture antigens from migratory malaria sporozoites and induce CD8+ T cell responses. PLoS Pathog 11, e1004637 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Butler NS et al. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe 9, 451–462 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Douradinha B et al. Genetically attenuated P36p-deficient Plasmodium berghei sporozoites confer long-lasting and partial cross-species protection. International journal for parasitology 37, 1511–1519 (2007). [DOI] [PubMed] [Google Scholar]
- 161.Kramer LD & Vanderberg JP Intramuscular immunization of mice with irradiated Plasmodium berghei sporozoites. Enhancement of protection with albumin. Am J Trop Med Hyg 24, 913–916 (1975). [DOI] [PubMed] [Google Scholar]
- 162.Holz LE, Fernandez-Ruiz D & Heath WR Protective immunity to liver-stage malaria. Clin Transl Immunology 5, e105 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ryg-Cornejo V et al. NK cells and conventional dendritic cells engage in reciprocal activation for the induction of inflammatory responses during Plasmodium berghei ANKA infection. Immunobiology 218, 263–271 (2013). [DOI] [PubMed] [Google Scholar]
- 164.Morrot A, Hafalla JC, Cockburn IA, Carvalho LH & Zavala F IL-4 receptor expression on CD8+ T cells is required for the development of protective memory responses against liver stages of malaria parasites. J Exp Med 202, 551–560 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.da Silva HB et al. Early skin immunological disturbance after Plasmodium-infected mosquito bites. Cell Immunol 277, 22–32 (2012). [DOI] [PubMed] [Google Scholar]
- 166.Kimura K et al. CD8+ T cells specific for a malaria cytoplasmic antigen form clusters around infected hepatocytes and are protective at the liver stage of infection. Infect Immun 81, 3825–3834 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cockburn IA et al. In vivo imaging of CD8+ T cell-mediated elimination of malaria liver stages. Proceedings of the National Academy of Sciences of the United States of America 110, 9090–9095 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Harty JT, Tvinnereim AR & White DW CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol 18, 275–308 (2000). [DOI] [PubMed] [Google Scholar]
- 169.Halle S, Halle O & Forster R Mechanisms and Dynamics of T Cell-Mediated Cytotoxicity In Vivo. Trends Immunol 38, 432–443 (2017). [DOI] [PubMed] [Google Scholar]
- 170.Butler NS, Schmidt NW & Harty JT Differential effector pathways regulate memory CD8 T cell immunity against Plasmodium berghei versus P. yoelii sporozoites. J Immunol 184, 2528–2538 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Morrot A & Zavala F Regulation of the CD8+ T cell responses against Plasmodium liver stages in mice. International journal for parasitology 34, 1529–1534 (2004). [DOI] [PubMed] [Google Scholar]
- 172.Renggli J et al. Elimination of P. berghei liver stages is independent of Fas (CD95/Apo-I) or perforin-mediated cytotoxicity. Parasite Immunol 19, 145–148 (1997). [DOI] [PubMed] [Google Scholar]
- 173.Nganou-Makamdop K, van Gemert GJ, Arens T, Hermsen CC & Sauerwein RW Long term protection after immunization with P. berghei sporozoites correlates with sustained IFNgamma responses of hepatic CD8+ memory T cells. PLoS One 7, e36508 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Clark IA, Hunt NH, Butcher GA & Cowden WB Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-gamma or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. J Immunol 139, 3493–3496 (1987). [PubMed] [Google Scholar]
- 175.Jobe O et al. Genetically attenuated Plasmodium berghei liver stages induce sterile protracted protection that is mediated by major histocompatibility complex Class I-dependent interferon-gamma-producing CD8+ T cells. J Infect Dis 196, 599–607 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mellouk S et al. Inhibitory activity of interferons and interleukin 1 on the development of Plasmodium falciparum in human hepatocyte cultures. J Immunol 139, 4192–4195 (1987). [PubMed] [Google Scholar]
- 177.Seguin MC et al. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon gamma and CD8+ T cells. J Exp Med 180, 353–358 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chakravarty S, Baldeviano GC, Overstreet MG & Zavala F Effector CD8+ T lymphocytes against liver stages of Plasmodium yoelii do not require gamma interferon for antiparasite activity. Infect Immun 76, 3628–3631 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Doolan DL & Hoffman SL The complexity of protective immunity against liver-stage malaria. J Immunol 165, 1453–1462 (2000). [DOI] [PubMed] [Google Scholar]
- 180.Schmidt NW, Butler NS & Harty JT Plasmodium-host interactions directly influence the threshold of memory CD8 T cells required for protective immunity. J Immunol 186, 5873–5884 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schmidt NW et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci U S A 105, 14017–14022 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fernandez-Ruiz D et al. Liver-Resident Memory CD8(+) T Cells Form a Front-Line Defense against Malaria Liver-Stage Infection. Immunity 45, 889–902 (2016). [DOI] [PubMed] [Google Scholar]
- 183.Gola A et al. Prime and target immunization protects against liver-stage malaria in mice. Sci Transl Med 10 (2018). [DOI] [PubMed] [Google Scholar]
- 184.Olsen TM, Stone BC, Chuenchob V & Murphy SC Prime-and-Trap Malaria Vaccination To Generate Protective CD8(+) Liver-Resident Memory T Cells. J Immunol 201, 1984–1993 (2018). [DOI] [PubMed] [Google Scholar]
- 185.Holz LE et al. CD8(+) T Cell Activation Leads to Constitutive Formation of Liver Tissue-Resident Memory T Cells that Seed a Large and Flexible Niche in the Liver. Cell Rep 25, 68–79 e64 (2018). [DOI] [PubMed] [Google Scholar]
- 186.McNamara HA et al. Up-regulation of LFA-1 allows liver-resident memory T cells to patrol and remain in the hepatic sinusoids. Sci Immunol 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schenkel JM, Fraser KA, Vezys V & Masopust D Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol 14, 509–513 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kumar S & Miller LH Cellular mechanisms in immunity to blood stage infection. Immunol Lett 25, 109–114 (1990). [DOI] [PubMed] [Google Scholar]
- 189.Vinetz JM et al. Adoptive transfer of CD8+ T cells from immune animals does not transfer immunity to blood stage Plasmodium yoelii malaria. J Immunol 144, 1069–1074 (1990). [PubMed] [Google Scholar]
- 190.Miyakoda M et al. Development of memory CD8+ T cells and their recall responses during blood-stage infection with Plasmodium berghei ANKA. J Immunol 189, 4396–4404 (2012). [DOI] [PubMed] [Google Scholar]
- 191.Chandele A, Mukerjee P, Das G, Ahmed R & Chauhan VS Phenotypic and functional profiling of malaria-induced CD8 and CD4 T cells during blood-stage infection with Plasmodium yoelii. Immunology 132, 273–286 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lundie RJ et al. Blood-stage Plasmodium infection induces CD8+ T lymphocytes to parasite-expressed antigens, largely regulated by CD8alpha+ dendritic cells. Proc Natl Acad Sci U S A 105, 14509–14514 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Junqueira C et al. Cytotoxic CD8(+) T cells recognize and kill Plasmodium vivax-infected reticulocytes. Nat Med 24, 1330–1336 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Swanson PA 2nd et al. CD8+ T Cells Induce Fatal Brainstem Pathology during Cerebral Malaria via Luminal Antigen-Specific Engagement of Brain Vasculature. PLoS Pathog 12, e1006022 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Belnoue E et al. On the pathogenic role of brain-sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J Immunol 169, 6369–6375 (2002). [DOI] [PubMed] [Google Scholar]
- 196.Nitcheu J et al. Perforin-dependent brain-infiltrating cytotoxic CD8+ T lymphocytes mediate experimental cerebral malaria pathogenesis. J Immunol 170, 2221–2228 (2003). [DOI] [PubMed] [Google Scholar]
- 197.Randall LM et al. Common strategies to prevent and modulate experimental cerebral malaria in mouse strains with different susceptibilities. Infect Immun 76, 3312–3320 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Claser C et al. CD8+ T cells and IFN-gamma mediate the time-dependent accumulation of infected red blood cells in deep organs during experimental cerebral malaria. PLoS One 6, e18720 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Haque A et al. Granzyme B expression by CD8+ T cells is required for the development of experimental cerebral malaria. J Immunol 186, 6148–6156 (2011). [DOI] [PubMed] [Google Scholar]
- 200.Farooq U, Dubey ML, Shrivastava SK & Mahajan RC Genetic polymorphism in Plasmodium falciparum: differentiation of parasite isolates of high & low virulence by RAPD. Indian J Med Res 136, 292–295 (2012). [PMC free article] [PubMed] [Google Scholar]
- 201.Kyriacou HM et al. Differential var gene transcription in Plasmodium falciparum isolates from patients with cerebral malaria compared to hyperparasitaemia. Mol Biochem Parasitol 150, 211–218 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bertin GI et al. Proteomic analysis of Plasmodium falciparum parasites from patients with cerebral and uncomplicated malaria. Sci Rep 6, 26773 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mazier D, Nitcheu J & Idrissa-Boubou M Cerebral malaria and immunogenetics. Parasite Immunol 22, 613–623 (2000). [DOI] [PubMed] [Google Scholar]
- 204.Mackey LJ, Hochmann A, June CH, Contreras CE & Lambert PH Immunopathological aspects of Plasmodium berghei infection in five strains of mice. II. Immunopathology of cerebral and other tissue lesions during the infection. Clin Exp Immunol 42, 412–420 (1980). [PMC free article] [PubMed] [Google Scholar]
- 205.Van Braeckel-Budimir N et al. A T Cell Receptor Locus Harbors a Malaria-Specific Immune Response Gene. Immunity 47, 835–847 e834 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.White NJ, Turner GD, Medana IM, Dondorp AM & Day NP The murine cerebral malaria phenomenon. Trends Parasitol 26, 11–15 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hunt NH et al. Murine cerebral malaria: the whole story. Trends Parasitol 26, 272–274 (2010). [DOI] [PubMed] [Google Scholar]
- 208.Chien YH, Meyer C & Bonneville M gammadelta T cells: first line of defense and beyond. Annu Rev Immunol 32, 121–155 (2014). [DOI] [PubMed] [Google Scholar]
- 209.Holtmeier W & Kabelitz D gammadelta T cells link innate and adaptive immune responses. Chem Immunol Allergy 86, 151–183 (2005). [DOI] [PubMed] [Google Scholar]
- 210.Hayday AC [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol 18, 975–1026 (2000). [DOI] [PubMed] [Google Scholar]
- 211.Carding SR & Egan PJ Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol 2, 336–345 (2002). [DOI] [PubMed] [Google Scholar]
- 212.Zhao Y, Niu C & Cui J Gamma-delta (gammadelta) T cells: friend or foe in cancer development? J Transl Med 16, 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kurup SP & Harty JT gammadelta T cells and immunity to human malaria in endemic regions. Ann Transl Med 3, S22 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hviid L et al. Perturbation and proinflammatory type activation of V delta 1(+) gamma delta T cells in African children with Plasmodium falciparum malaria. Infect Immun 69, 3190–3196 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Langhorne J, Morris-Jones S, Casabo LG & Goodier M The response of gamma delta T cells in malaria infections: a hypothesis. Res Immunol 145, 429–436 (1994). [DOI] [PubMed] [Google Scholar]
- 216.Roussilhon C, Agrapart M, Ballet JJ & Bensussan A T lymphocytes bearing the gamma delta T cell receptor in patients with acute Plasmodium falciparum malaria. J Infect Dis 162, 283–285 (1990). [DOI] [PubMed] [Google Scholar]
- 217.Teirlinck AC et al. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLoS Pathog 7, e1002389 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.D’Ombrain MC et al. Association of early interferon-gamma production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin Infect Dis 47, 1380–1387 (2008). [DOI] [PubMed] [Google Scholar]
- 219.Goodier M et al. Gamma delta T cells in the peripheral blood of individuals from an area of holoendemic Plasmodium falciparum transmission. Trans R Soc Trop Med Hyg 87, 692–696 (1993). [DOI] [PubMed] [Google Scholar]
- 220.Jagannathan P et al. Loss and dysfunction of Vdelta2(+) gammadelta T cells are associated with clinical tolerance to malaria. Sci Transl Med 6, 251ra117 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.De Rosa SC et al. Ontogeny of gamma delta T cells in humans. J Immunol 172, 1637–1645 (2004). [DOI] [PubMed] [Google Scholar]
- 222.Dondorp AM et al. The relationship between age and the manifestations of and mortality associated with severe malaria. Clin Infect Dis 47, 151–157 (2008). [DOI] [PubMed] [Google Scholar]
- 223.Schwartz E, Sadetzki S, Murad H & Raveh D Age as a risk factor for severe Plasmodium falciparum malaria in nonimmune patients. Clin Infect Dis 33, 1774–1777 (2001). [DOI] [PubMed] [Google Scholar]
- 224.Inoue SI, Niikura M, Asahi H, Kawakami Y & Kobayashi F gammadelta T cells modulate humoral immunity against Plasmodium berghei infection. Immunology (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mamedov MR et al. A Macrophage Colony-Stimulating-Factor-Producing gammadelta T Cell Subset Prevents Malarial Parasitemic Recurrence. Immunity 48, 350–363 e357 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zaidi I et al. gammadelta T Cells Are Required for the Induction of Sterile Immunity during Irradiated Sporozoite Vaccinations. J Immunol 199, 3781–3788 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zeng X et al. gammadelta T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity 37, 524–534 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cai Y et al. Differential developmental requirement and peripheral regulation for dermal Vgamma4 and Vgamma6T17 cells in health and inflammation. Nat Commun 5, 3986 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Paul S & Lal G Regulatory and effector functions of gamma-delta (gammadelta) T cells and their therapeutic potential in adoptive cellular therapy for cancer. Int J Cancer 139, 976–985 (2016). [DOI] [PubMed] [Google Scholar]
- 230.Constant P et al. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science 264, 267–270 (1994). [DOI] [PubMed] [Google Scholar]
- 231.Sheridan BS et al. gammadelta T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity 39, 184–195 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Elloso MM, van der Heyde HC, vande Waa JA, Manning DD & Weidanz WP Inhibition of Plasmodium falciparum in vitro by human gamma delta T cells. J Immunol 153, 1187–1194 (1994). [PubMed] [Google Scholar]
- 233.Hensmann M & Kwiatkowski D Cellular basis of early cytokine response to Plasmodium falciparum. Infect Immun 69, 2364–2371 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Behr C et al. Plasmodium falciparum stimuli for human gammadelta T cells are related to phosphorylated antigens of mycobacteria. Infect Immun 64, 2892–2896 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Elloso MM, van der Heyde HC, Troutt A, Manning DD & Weidanz WP Human gamma delta T cell subset-proliferative response to malarial antigen in vitro depends on CD4+ T cells or cytokines that signal through components of the IL-2R. J Immunol 157, 2096–2102 (1996). [PubMed] [Google Scholar]
- 236.Jones SM, Goodier MR & Langhorne J The response of gamma delta T cells to Plasmodium falciparum is dependent on activated CD4+ T cells and the recognition of MHC class I molecules. Immunology 89, 405–412 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pichyangkul S, Saengkrai P, Yongvanitchit K, Stewart A & Heppner DG Activation of gammadelta T cells in malaria: interaction of cytokines and a schizont-associated Plasmodium falciparum antigen. J Infect Dis 176, 233–241 (1997). [DOI] [PubMed] [Google Scholar]
- 238.Cockburn IA & Seder RA Malaria prevention: from immunological concepts to effective vaccines and protective antibodies. Nat Immunol 19, 1199–1211 (2018). [DOI] [PubMed] [Google Scholar]
- 239.Draper SJ et al. Malaria Vaccines: Recent Advances and New Horizons. Cell Host Microbe 24, 43–56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Butler NS, Vaughan AM, Harty JT & Kappe SH Whole parasite vaccination approaches for prevention of malaria infection. Trends Immunol 33, 247–254 (2012). [DOI] [PubMed] [Google Scholar]
- 241.Hoffman SL, Vekemans J, Richie TL & Duffy PE The March Toward Malaria Vaccines. Am J Prev Med 49, S319–333 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hill AV Vaccines against malaria. Philos Trans R Soc Lond B Biol Sci 366, 2806–2814 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ewer KJ et al. Progress with viral vectored malaria vaccines: A multi-stage approach involving “unnatural immunity”. Vaccine 33, 7444–7451 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Li Y et al. Enhancing immunogenicity and transmission-blocking activity of malaria vaccines by fusing Pfs25 to IMX313 multimerization technology. Sci Rep 6, 18848 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Doll KL, Pewe LL, Kurup SP & Harty JT Discriminating Protective from Nonprotective Plasmodium-Specific CD8+ T Cell Responses. J Immunol 196, 4253–4262 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lau LS et al. CD8+ T cells from a novel T cell receptor transgenic mouse induce liver-stage immunity that can be boosted by blood-stage infection in rodent malaria. PLoS Pathog 10, e1004135 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Fernandez-Ruiz D et al. Development of a Novel CD4(+) TCR Transgenic Line That Reveals a Dominant Role for CD8(+) Dendritic Cells and CD40 Signaling in the Generation of Helper and CTL Responses to Blood-Stage Malaria. J Immunol 199, 4165–4179 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Shekalaghe S et al. Controlled human malaria infection of Tanzanians by intradermal injection of aseptic, purified, cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg 91, 471–480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hodgson SH et al. Evaluating controlled human malaria infection in Kenyan adults with varying degrees of prior exposure to Plasmodium falciparum using sporozoites administered by intramuscular injection. Front Microbiol 5, 686 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Obiero JM et al. Impact of malaria preexposure on antiparasite cellular and humoral immune responses after controlled human malaria infection. Infect Immun 83, 2185–2196 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]