Abstract
Soft rot is an economically significant disease in potato and one of the major threats to sustainable potato production. This study aimed at isolating lytic bacteriophages and evaluating methods for and the efficacy of applying phages to control potato soft rot caused by Pectobacterium carotovorum. Eleven bacteriophages isolated from soil and water samples collected in Wuhan, China, were used to infect P. carotovorum host strains isolated from potato tubers showing soft rot symptoms in Nakuru county, Kenya. The efficacy of the phages in controlling soft rot disease was evaluated by applying individual phage strains or a phage cocktail on potato slices and tubers at different time points before or after inoculation with a P. carotovorum strain. The phages could lyse 20 strains of P. carotovorum, but not Pseudomonas fluorescens control strains. Among the 11 phages, Pectobacterium phage Wc5r, interestingly showed cross-activity against Pectobacterium atrosepticum and two phage-resistant P. carotovorum strains. Potato slice assays showed that the phage concentration and timing of application are crucial factors for effective soft rot control. Phage cocktail applied at a concentration of 1 × 109 plaque-forming units per milliliter before or within an hour after bacterial inoculation on potato slices, resulted in ≥ 90% reduction of soft rot symptoms. This study provides a basis for the development and application of phages to reduce the impact of potato soft rot disease.
Electronic supplementary material
The online version of this article (10.1007/s12250-019-00091-7) contains supplementary material, which is available to authorized users.
Keywords: Pectobacterium carotovorum, Potato soft rot, Bacteriophages, Phage resistance
Introduction
Phytopathogenic bacterial species belonging to the genus Pectobacterium (family, Enterobacteriaceae) are gram-negative facultative anaerobes (Toth et al.2003). One of the economically most important species in this genus is Pectobacterium carotovorum, which characteristically produces plant cell wall-degrading enzymes, including cellulase, pectinase, and polygalacturonase, which cause plant tissue maceration during infection (Pérombelon 2002; Toth et al.2003). P. carotovorum can infect tomatoes, potatoes, carrots, cabbages, and a variety of ornamental plants (Ma et al.2007). In potatoes, P. carotovorum causes tuber soft rot in stored farm produce and blackleg disease in the field. Infection can occur during cultivation, harvesting, handling, and transportation of farm produce or during postharvest storage, and results in considerable yield reduction, poor quality of produce, and economic loss (del Pilar Marquez-Villavicencio et al.2011). Potato (Solanum tuberosum) is a globally important high-yield crop that produces nutrient-rich tubers. This non-grain crop is the third most important food crop, after wheat and rice (Patil et al.2017), with a global production of 364.8 million tons in 2012 (FAO STAT 2013).
Different approaches to plant disease control have been developed and applied. Potato soft rot control measures involve the implementation of proper handling practices, including careful sorting to remove diseased tubers, observing sanitary practices during transportation, proper storage (Davidson 1948; De Boer 2008), and appropriate disposal of infected tissues to prevent the spread of the phytopathogen. The use of bacteriophages (phages) as biocontrol agents to target phytopathogens has gained increased interest in recent years (Frampton et al.2012; Buttimer et al.2017). Phages are bacterial viruses that specifically invade and lyse bacterial cells, thus effectively reducing bacterial populations (Sulakvelidze et al.2001), and have no direct negative effects on eukaryotic cells. Upon infection, lytic phages undergo rapid replication followed by lysis of the host bacterial cells and the release of numerous progeny viruses (Frampton et al.2012) into the environment. When the bacterial population is large and the environmental conditions are favorable (Hadas et al.1997), infection by phages results in significant phage amplification. A good phage cocktail is highly effective in controlling phytopathogenic bacteria and continues to work long after chemicals have lost their potency (Loc-Carrillo and Abedon 2011; Iriarte et al.2012). This is a distinctive advantage over other control measures. Another advantage of phages is that they can infect antibiotic- or heavy metal-resistant bacteria (Frampton et al.2012).
In our previous study, we isolated P. carotovorum and Pseudomonas fluorescens from potatoes showing soft-rot symptoms in Nakuru county, Kenya (Muturi et al.2018). The current study aimed at isolating and characterizing lytic bacteriophages suitable for the control of potato soft rot caused by P. carotovorum by using the Kenyan strains. The effectiveness of the phages in preventing infection of potato tubers by P. carotovorum was tested in laboratory experiments.
Materials and Methods
Bacteria Isolates and Culture Conditions
Pectobacterium carotovorum strains were isolated from rotting potato tubers collected from farms in Nakuru county, Kenya, between 2016 and 2017. Initial isolation was done using crystal violet pectate medium (CVPM). Cavity-forming colonies were picked and were identified by 16S rDNA sequencing, by using a BIOLOG microbial ID system (Biolog, Inc., Hayward, CA, USA), by PCR with Y1/Y2 primers specific for Pectobacterium (Darrasse et al.1994), and by comparison with published data (Muturi et al.2018). The bacteria were grown routinely at 28 °C in Luria–Bertani (LB) broth and on LB agar plates.
Phage Isolation, Amplification, and Purification
Water and soil samples for phage isolation were collected from different environments in Wuhan, Hubei, China, between 2016 and 2017, as previously described (Lim et al.2013), with slight modifications. Briefly, 10 mL of water or 10 g of soil sample was homogenized in 10 mL of phage buffer (containing 50 mmol/L Tris–HCl, pH 7.5, 150 mmol/L NaCl, 10 mmol/L MgCl2, 2 mmol/L CaCl2) in a sterile 100-mL conical flask. A 2-mL inoculum of host bacteria in mid-exponential growth phase (cultured in LB broth) and 5 mL of LB broth were added to the flask. The culture was incubated at 28 °C under shaking at 160 rpm in an incubator for 2–3 days. Then, the cells were filtered through a 0.22-µm membrane filter (Merck Millipore Ltd., Ireland), and tenfold serial dilutions were prepared. One milliliter of each dilution was mixed with 1 mL of host bacterial culture, and the culture was incubated at 28 °C under shaking at 160 rpm for 20 min. Then, the mixture was added to 4 mL of 0.7% soft agar (at 50 °C), swirled gently, and poured in LB agar plates. The plates were incubated at 28 °C for 1–2 days to allow plaque formation. Single plaques were picked from the plates and were purified at least five times by serial dilution and single-plaque isolation to ensure that the final suspension was from a single virion. Phages that consistently formed stable plaques were considered lytic and were selected for further analysis.
To amplify purified phages, 4 mL of buffer and 4 mL of LB broth were mixed in a sterile 100-mL conical flask inoculated with a single colony of the host bacterium (P. carotovorum). The culture was incubated at 28 °C under shaking at 160 rpm for 6 h. Then, 1 mL of pure phage solution was added into the flask and the culture was incubated for another 12 h under the same conditions. The culture containing amplified phages was centrifuged at 3000 ×g for 5 min and the supernatant was filtered through a sterile 0.22-µm filter to isolate the phages. The phage titer was determined by serial dilution and culturing of the phage-bacteria mixture on double-layered agar to count single plaque-forming units (PFUs).
Phage Morphology Assessment by Transmission Electron Microscope
To observe phage morphology, 6 mL of phage suspension (~ 1 × 109 PFUs/mL) was purified further by gradient centrifugation in a cesium chloride (CsCl) gradient (2 mL of each of 1.45 g/mL, 1.50 g/mL, 1.70 g/mL of CsCl in phage buffer) using a SW41 rotor (Beckman LE-80 K, USA) at 130,000 ×g for 2 h. The blue band, shown in Supplementary Figure S1, containing phages, was pipetted into a new sterile Eppendorf tube and dialyzed before negative staining on copper grids using 2% phosphotungstic acid. Stained samples were observed under a transmission electron microscope (Tecnai G2 20 TWIN) at the Wuhan Institute of Virology.
Lytic Activity of Phages against Pectobacterium carotovorum
The ability of the phages to lyse host bacteria was assessed as described previously (Wei et al.2017) using individual phage strains and a phage cocktail. All procedures were carried out at 28 °C. Briefly, 250 µL of P. carotovorum broth culture in mid-exponential growth phase was mixed with 250 µL of phage solution at a multiplicity of infection (MOI) of 0.1 PFU/bacteria and 500 µL of LB broth. Mixed bacteria/phage cultures were aseptically transferred to 96-well clear-bottom plates (200 µL/well) and incubated. Changes in the optical density at 600 nm (OD600) of the cultures were monitored over 12 h using a microplate reader (SynergyH1, BioTek, USA). To observe the growth pattern of uninfected bacteria, 250 µL of P. carotovorum broth culture was mixed with 250 µL of phage buffer and 500 µL of LB broth.
Bacteriophage One-Step Growth Assay
A one-step growth assay, initially described by Ellis and Delbrück (1939), was conducted as reported by Czajkowski et al. (2014), with a few modifications. Phage suspensions in phage buffer were incubated at 28 °C for 1 h and then mixed with LB broth (28 °C) containing P. carotovorum in mid-exponential growth phase at a MOI of 0.1 PFU/bacteria. The cultures were gently mixed and 100 µL was collected from each mixture to determine the initial phage titers. The remaining solution was incubated at 28 °C under shaking at 180 rpm. After 15 min of adsorption time, 100 µL of culture was sampled at 10-min intervals for 2 h, added immediately into 900 µL of cold phage buffer to limit the possibility of non-adsorbed phages to infect viable host cells, and centrifuged at 12,000 ×g for 5 min (Wei et al.2017) to separate free virions from bacteria. The supernatant was transferred into a sterile 2-mL tube. Phage titers were determined by serial dilution and culturing of 10 µL from each dilution with 1 mL of host bacterium at mid-exponential growth phase on LB agar plates to count PFUs.
Antibacterial Activity and Phage Specificity
The ability of the phages to infect and lyse host bacterial cells was tested by spot-on-lawn assays (Czajkowski et al.2014) on LB agar plates. The antibacterial effect of the bacteriophages was tested in 20 strains of P. carotovorum, all isolated in Nakuru county, Kenya. To test phage specificity, two strains of P. fluorescens, also isolated from rotten potato tubers in Nakuru county (Muturi et al.2018), and one strain of Pectobacterium atrosepticum isolated in China were also subjected to phage susceptibility tests.
Development of Phage Resistance in P. carotovorum
A single bacterial colony picked from an LB agar plate was inoculated in a conical flask containing 3 mL of LB broth and 3 mL of phage buffer and the mixture was incubated at 28 °C under shaking at 160 rpm for 6 h. One hundred microliters of phage suspension (1 × 107 PFUs/mL) was added into the flask and the culture was further incubated for 12 h. When turbidity was observed in the flask, 10 µL of the culture was streaked on LB agar to obtain single bacterial colonies for analysis using the spot-on-lawn assay (Czajkowski et al.2014).
Effectiveness of Bacteriophages in Controlling Potato Soft Rot
The effectiveness of the phages in preventing the spreading of potato soft rot was tested using potato slice assays and whole potato tubers. Healthy potato tubers were washed under running tap water to remove adhering soil, disinfected by spraying with 70% ethanol, and air-dried on a sterile surface for 30 min. Some of the disinfected potato tubers were aseptically cut into slices of approximately 5 mm thick that were placed in sterile petri dishes. In a first experiment, 200 µL of phage solution (1 × 109 PFUs/mL) was applied on the potato slices, which were left to stand for 10 min to allow the fluid to diffuse into the tissues. Sterile 13-mm diameter paper discs were soaked in an overnight P. carotovorum bacterial broth culture (1 × 108 colony forming units (CFU)/mL) for 10 min and then placed on the potato slices. Phage buffer and sterile ddH2O were used as negative controls. In a second experiment, the filter paper disks impregnated with P. carotovorum host bacteria were placed on the potato slices prior to phage application. The phage suspensions were then applied on one group of the potato slices after 1 h, on the next group of the potato slices after 2 h, on the third group after 3 h, and so on until after 8 h, before and after the onset of disease symptoms. The phages were applied up to 8 h post bacterial inoculation (hpi) because in preliminary control experiments, disease symptoms appeared at 7–9 hpi. In all cases, observations were made and recorded at 24 hpi. Disease prevention or treatment efficacy was calculated by dividing the difference between the diameter of the area of the potato slice showing soft rot symptoms and the diameter of the paper disc impregnated with the bacteria, by the diameter of the paper disc (13 mm). The results were expressed as a percentage. Experiments to examine phage therapy on whole potato tubers were performed as described by Adriaenssens et al. (2012). Briefly, inoculation was done aseptically in 1-cm-deep wounds made on potato tubers, and the tubers were incubated at 28 °C for 24 h to allow for the development of soft rot symptoms.
Results
Phage Isolation, Amplification, and Purification
In total, 11 bacteriophage strains were successfully isolated; seven (Pectobacterium phages Wc1, Wc2, Wc6, Wc7, Wc8, Wc9, and Wc10) from water samples and four (Pectobacterium phages Wc3, Wc4, Wc5, and Wc5r) from soil samples. All purified phages formed small lytic plaques of approximately 1 mm in diameter on P. carotovorum lawn on LB agar after 24 h incubation at 28 °C (supplementary Figure S2).
Phage Morphology
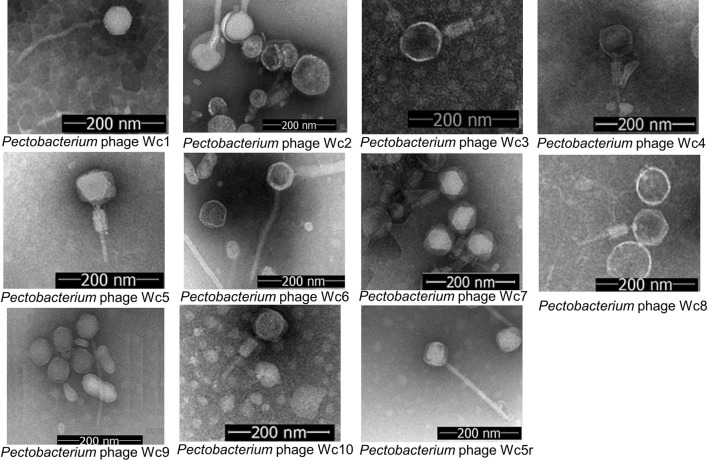
Transmission electron microscopy revealed that the isolated phages belong to two different groups of phages. The first group, including Wc2, Wc3, Wc4, Wc5, Wc7, Wcp9, and Wc10, produced virions with a contractile tail and an isometric capsid. Virions in the second group, including Wc1, Wc5r, Wc6, and Wc9, had a long, non-contractile tail and an isometric capsid (Fig. 1, Table 1).
Fig. 1.
Transmission electron micrographs of isolated P. carotovorum bacteriophages.
Table 1.
Partial characterization of 11 phages displaying antibacterial activity against plant-pathogenic Pectobacterium carotovorum
| Phage | Phage morphology | Latency period (h) | Burst size | Antibacterial activity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Head capsid diameter (nm) | Tail length (nm) | Tail capsid diameter (nm) | Family | Pectobacterium carotovorum | Pseudomonas fluorescens | Pectobacterium Atrosepticum Strain WHG10001 | |||||
| Strain KPM01 | Strain KPM17 | Strain KPM06 | Strain KPM37 | ||||||||
| Wc1 | 52 | 191 | 9 | Siphoviridae | 1.5 | 150 | + | + | − | − | − |
| Wc6 | 60 | 195 | 12 | 1.0 | 196 | + | + | − | − | − | |
| Wc9 | 61 | 218 | 12 | 1.2 | 157 | + | + | − | − | − | |
| Wc5r | 55 | 209 | 10 | 1.5 | 109 | + | + | − | − | + | |
| Wc2 | 75 | 115 | 21 | Myoviridae | 1.5 | 122 | + | + | − | − | − |
| Wc3 | 68 | 107 | 26 | 1.5 | 68 | + | + | − | − | − | |
| Wc4 | 64 | 97 | 20 | 1.2 | 106 | + | + | − | − | − | |
| Wc5 | 67 | 71 | 18 | 1.2 | 89 | + | + | − | − | − | |
| Wc7 | 65 | 72 | 24 | 1.0 | 82 | + | + | − | − | − | |
| Wc8 | 74 | 108 | 26 | 1.5 | 161 | + | + | − | − | − | |
| Wc10 | 61 | 112 | 26 | 1.2 | 125 | + | + | − | − | − | |
Latent periods and burst sizes were obtained from one-step growth assays and lytic curves. Burst size is reported as the number of plaque-forming units per infective center. “+” indicates the phage has lytic activity “−” indicates the phage does not have lytic activity
Lytic Activity of Phages Against P. carotovorum
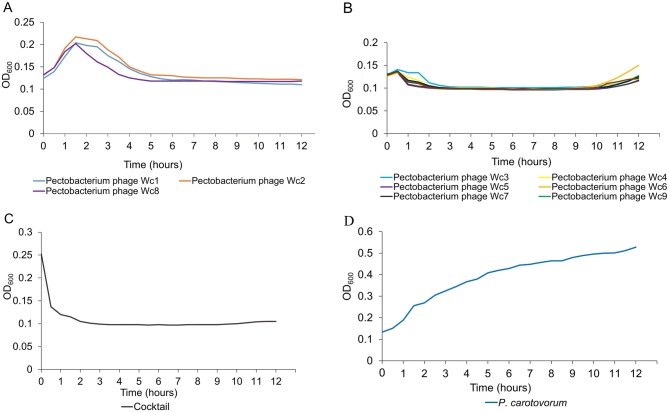
Lytic curves generated on the basis of the experimental data revealed some differences in bacterial lysis dynamics (Fig. 2). The OD600 in wells containing phages Wc1, Wc2, and Wc8, increased until approximately 1.5 h, and then decreased to the baseline, whereas in wells containing the other phages, the OD600 increased until approximately 0.5 h and then decreased gradually to the baseline after 1 h, but gradually increased again after 10 h of incubation. Lysis was more effective in microplate wells containing the phage cocktail (Fig. 2C). No increase in turbidity was recorded in the wells during the course of the experiment. In the control experiment, exponential bacterial growth was observed (Fig. 2D).
Fig. 2.
Lytic curves of individual bacteriophage strains infecting P. carotovorum (A, B), lytic curve of 11 bacteriophages in a cocktail (C), and growth pattern of uninfected P. carotovorum strain KPM17 (D).
Bacteriophage One-Step Growth Assay
The burst sizes of the phages ranged from 69 to 196 PFUs per infectious cycle, with latency periods ranging from 1 to 1.5 h in LB broth at 28 °C (Table 1).
Antibacterial Activity and Phage Specificity
All 11 phage strains exhibited antibacterial activity against 20 strains of P. carotovorum available in our lab. The phages did not infect the two P. fluorescens strains, and only one phage, Wc5r, showed lytic activity against P. atrosepticum (Table 1). Wc5r could also infect two phage-resistant strains of P. carotovorum (Supplementary Figure S1).
Development of Resistance Against Bacteriophage Infection
During phage amplification, some bacteria developed resistance against phage infection. Two phage-resistant P. carotovorum strains were isolated from flask cultures inoculated with phages Wc3 and Wc8. The two phage-resistant strains KPM01R1 and KPM01R2 were found to be resistant to ten of the isolated bacteriophages, but remained susceptible to Wc5r.
Effectiveness of Bacteriophages in Controlling Potato Soft Rot
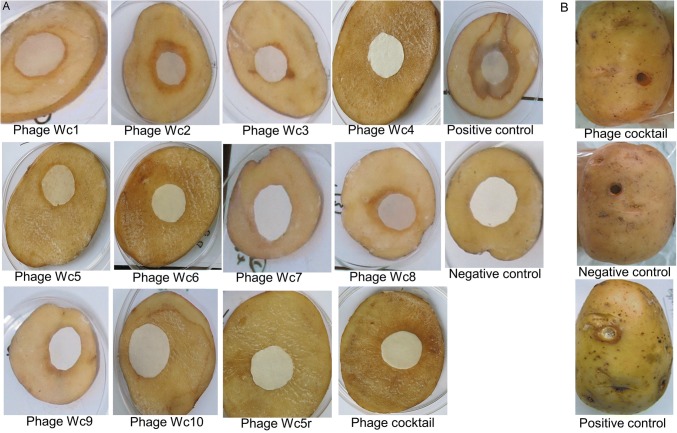
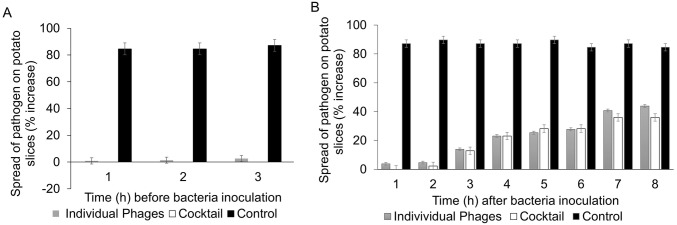
The experimental data showed that the isolated phages can effectively control potato soft rot caused by P. carotovorum. Application of phages to potato slices at 1.0 × 109 PFUs/mL before inoculation with the bacterial pathogen resulted in a reduction of disease symptom development by more than 50% (Fig. 3). Disease symptoms on potato slices were more effectively suppressed when the phage cocktail was used; up to 100% symptom prevention was observed. When the phages were applied after bacterial inoculation, treatment was more effective if phages were applied 1 or 2 hpi than at later time points, as shown in Fig. 4. Similar results were obtained in whole potato tubers (Fig. 3B).
Fig. 3.
Phage efficacy/therapy test results. A Prevention of soft rot development on potato slices by individual phage strains. B Phage treatment of whole tubers, results of soft rot prevention by bacteriophages (phage cocktail), negative control (buffer applied to wounded potato tuber), and positive control (bacteria plus phage buffer).
Fig. 4.
Efficacy tests of bacteriophages in preventing spread of P. carotovorum on the surface of potato slices. A Phages were applied once before inoculation of bacteria on the surface of each slice. B Potato slices were treated with individual phages or phage cocktail once at different times post bacterial infection and incubated at 28 °C. Results were recorded after 24 h and are based on the percentage increase in the diameter of the infected area on each potato slice.
Discussion
Soft rot disease of potatoes has been reported globally. In Kenya, it causes up to 50% total crop loss (Onkendi and Moleleki 2014; Muturi et al.2018). Control measures include field regulatory inspections (de Haan et al.2008) based on isolation of infected portions from healthy crops, the use of resistant varieties, and crop rotation. In an effort to provide biological disease control measures, we successfully isolated 11 bacteriophages from Wuhan, China with bactericidal activity against Kenyan P. carotovorum isolates. Finding bacteriophages in a region different from that of the host indicates a significant diversity of bacteriophages in nature. Morphological characterization revealed that seven of the phages belong to the family Myoviridae, and four belong to the family Siphoviridae. All the phages showed promising results in efficacy tests because they could lyse the bacteria infected, displaying the ability to control the development of soft rot disease in potatoes. The latent periods of all the phages were less than 2 h, and more than 60 virions were released from each infected cell, thus providing more infectious particles to infect other host bacterial cells.
Notably, we observed development of resistance to phage infection in our experiments. Two resistant bacterial strains were successfully obtained from the flask cultures used for phage amplification. Resistance development by the pathogen can explain the gradual increase in turbidity after 10 h in the plates containing Pectobacterium phages Wc3, Wc4, Wc5, Wc5r, Wc6, Wc7, Wc9, and Wc10, as can be seen in the lytic activity curves. Considering that different phages recognize different host receptors (Rakhuba et al.2010), the use of phage cocktails would increase the possibility of infection and hence, treatment efficacy (Chan and Abedon 2012). This was indeed observed in the experiments using phage cocktail, in lytic activity curves (Fig. 2C), and in phage efficacy tests (Figs. 3 and 4). Phage cocktails can lyse multiple bacteria within a short time, minimize the probability of resistance development, and, in some cases, widen the host spectrum. Further analysis of the two phage-resistant strains obtained in this study might shed light on the mechanism underlying resistance development. Pectobacterium phage Wc5r also seems to have unique characteristics because unlike the other phages, it infected the two phage-resistant P. carotovorum strains. The multivalent phage Wc5r indicated that there might be common phage receptors in closely related species of the genus Pectobacterium. Further studies on this phage will provide more information on the mechanism underlying the broad host range.
Application of the phage cocktail before inoculation of the potato tubers with bacteria provided more effective disease control, as indicated by the significantly reduced infection rate (Fig. 4). No symptoms were observed on slices on which the phage cocktail had been applied before or within an hour after bacterial inoculation. Therefore, it seems that the best way to control soft rot disease in contaminated tubers using phages is by spraying them on potatoes as soon as possible after harvest or before storage. Alternatively, potato seeds can be inoculated with phages before planting to protect the seed tubers from infection. However, as the stability of bacteriophages is influenced by environmental factors, including temperature (Fister et al.2016), suitable formulations will be required to maintain phage viability long after application.
To the best of our knowledge, this is the first report of isolation of bacteriophages with the potential to control soft rot caused by P. carotovorum strains occurring in Kenya. Our findings pave the way to the development of suitable phage cocktails for potato seed inoculation. This will help reduce the impact of soft rot disease on potato farming and trading in the country.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Pei Zhang and An-Na Du from Core Facility and Technical Support, Wuhan Institute of Virology, for their assistance in providing TEM micrographs. This study was supported financially by the Sino-Africa Joint Research Centre (SAJC201605) and the Chinese Academy of Sciences (ZDRW-ZS-2016-4).
Author Contributions
PM, FBM and HPW came up with the concept and designed the experiments. PM, JPY and ANM did the experiments. PM and JPY analysed the data. HPW, SK and FBM guided in developing the manuscript. All authors read and approved the final manuscript.
Compliance with Ethical Standards
Conflict of interest
All the authors declare that they have no conflict of interest.
Animal and Human Rights Statement
This article does not contain any studies with human or animal subjects performed by any of the Authors
References
- Adriaenssens EM, Van Vaerenbergh J, Vandenheuvel D, Dunon V, Ceyssens P-J, De Proft M, Kropinski AM, Noben J-P, Maes M, Lavigne R. T4-related bacteriophage LIMEstone isolates for the control of soft rot on potato caused by ‘Dickeya solani’. PLoS ONE. 2012;7:e33227. doi: 10.1371/journal.pone.0033227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttimer C, McAuliffe O, Ross RP, Hill C, O’Mahony J, Coffey A. Bacteriophages and bacterial plant diseases. Front Microbiol. 2014 doi: 10.3389/fmicb.2017.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BK, Abedon ST. Phage therapy pharmacology: phage cocktails. Adv Appl Microbiol. 2012 doi: 10.3389/fmicb.2017.00034. [DOI] [PubMed] [Google Scholar]
- Czajkowski R, Ozymko Z, Lojkowska E. Isolation and characterization of novel soilborne lytic bacteriophages infecting Dickeya spp. biovar 3 (‘D. solani’) Plant Pathol. 2014;63:758–772. doi: 10.1111/ppa.12157. [DOI] [Google Scholar]
- Darrasse A, Priou S, Kotoujansky A, Bertheau Y. PCR and restriction fragment length polymorphism of a pel gene as a tool to identify Erwinia carotovora in relation to potato diseases. Appl Environ Microbiol. 1994;60:1437–1443. doi: 10.1128/aem.60.5.1437-1443.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RS (1948) Factors affecting the development of bacterial soft rot of potato tuber initials. Dissertation, University of Minnesota
- De Boer S. Managing soft rot and ring rot. In: Johnson DA, editor. Potato health management. St Paul: APS Press; 2008. pp. 171–181. [Google Scholar]
- de Haan EG, Dekker-Nooren TC, van den Bovenkamp GW, Speksnijder AG, van der Zouwen PS, van der Wolf JM. Pectobacterium carotovorum subsp. carotovorum can cause potato blackleg in temperate climates. Eur J Plant Pathol. 2008;122:561. doi: 10.1007/s10658-008-9325-y. [DOI] [Google Scholar]
- del Pilar Marquez-Villavicencio M, Weber B, Witherell RA, Willis DK, Charkowski AO. The 3-hydroxy-2-butanone pathway is required for Pectobacterium carotovorum pathogenesis. PLoS ONE. 2011;6:e22974. doi: 10.1371/journal.pone.0022974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis EL, Delbrück M. The growth of bacteriophage. J Gen Physiol. 1939;22:365–384. doi: 10.1085/jgp.22.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO STAT (2013) Food and agriculture organization of the United Nations. Statistical database
- Fister S, Robben C, Witte AK, Schoder D, Wagner M, Rossmanith P. Influence of environmental factors on phage-bacteria interaction and on the efficacy and infectivity of phage P100. Front Microbiol. 2016;7:1152. doi: 10.3389/fmicb.2016.01152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton RA, Pitman AR, Fineran PC. Advances in bacteriophage-mediated control of plant pathogens. Int J Microbiol. 2012;2012:326452. doi: 10.1155/2012/326452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadas H, Einav M, Fishov I, Zaritsky A. Bacteriophage T4 development depends on the physiology of its host Escherichia coli. Microbiology. 1997;2012:326452. doi: 10.1099/00221287-143-1-179. [DOI] [PubMed] [Google Scholar]
- Iriarte FB, Obradović A, Wernsing MH, Jackson LE, Balogh B, Hong JA, Vallad GE. Soil-based systemic delivery and phyllosphere in vivo propagation of bacteriophages: two possible strategies for improving bacteriophage persistence for plant disease control. Bacteriophage. 1997;2(4):e23530. doi: 10.4161/bact.23530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J-A, Jee S, Lee DH, Roh E, Jung K, Oh C, Heu S. Biocontrol of Pectobacterium carotovorum subsp. carotovorum using bacteriophage PP1. J Microbiol Biotechnol. 2013;23:1147–1153. doi: 10.4014/jmb.1304.04001. [DOI] [PubMed] [Google Scholar]
- Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy. Bacteriophage. 2011;1:111–114. doi: 10.4161/bact.1.2.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Hibbing ME, Kim H-S, Reedy RM, Yedidia I, Breuer J, Breuer J, Glasner JD, Perna NT, Kelman A. Host range and molecular phylogenies of the soft rot enterobacterial genera Pectobacterium and Dickeya. Phytopathology. 2007;97:1150–1163. doi: 10.1094/PHYTO-97-9-1150. [DOI] [PubMed] [Google Scholar]
- Muturi P, Yu J, Li J, Jiang M, Maina AN, Kariuki S, Mwaura FB, Wei H. Isolation and characterization of pectolytic bacterial pathogens infecting potatoes in Nakuru County, Kenya. J Appl Microbiol. 2018;124:1580–1588. doi: 10.1111/jam.13730. [DOI] [PubMed] [Google Scholar]
- Onkendi EM, Moleleki LN. Characterization of Pectobacterium carotovorum subsp. carotovorum and brasiliense from diseased potatoes in Kenya. Eur J Plant Pathol. 2014;139:557–566. doi: 10.1007/s10658-014-0411-z. [DOI] [PubMed] [Google Scholar]
- Patil VU, Sharma NN, Chakrabarti SK. High-throughput sequencing of the potato genome. In: Chakrabarti SK, Xie C, Tiwari JK, editors. The potato genome. Berlin: Springer; 2017. pp. 95–107. [Google Scholar]
- Pérombelon M. Potato diseases caused by soft rot erwinias: an overview of pathogenesis. Plant Pathol. 2002;51:1–12. doi: 10.1046/j.0032-0862.2001.Shorttitle.doc.x. [DOI] [Google Scholar]
- Rakhuba D, Kolomiets E, Dey ES, Novik G. Bacteriophage receptors, mechanisms of phage adsorption and penetration into host cell. Pol J Microbiol. 2010;59:145–155. [PubMed] [Google Scholar]
- Sulakvelidze A, Alavidze Z, Morris JG. Bacteriophage therapy. Antimicrob Agents Chemother. 2001;45:649–659. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth IK, Bell KS, Holeva MC, Birch PR. Soft rot erwiniae: from genes to genomes. Mol Plant Pathol. 2003;4:17–30. doi: 10.1046/j.1364-3703.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- Wei C, Liu J, Maina AN, Mwaura FB, Yu J, Yan C, Zhang R, Wei H. Developing a bacteriophage cocktail for biocontrol of potato bacterial wilt. Virol Sin. 2017;32:476–484. doi: 10.1007/s12250-017-3987-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.