Yellow fever (YF) is an acute disease caused by a flavivirus that infects the liver. It can cause jaundice, bleeding, kidney damage, and death. No antiviral therapy exists. A vaccine does exist, however, and fortunately confers life-long immunity after a single dose (Monath et al.2016; WHO 2017a, b).
YF is transmitted by mosquitoes in two main cycles. In the sylvatic cycle the virus is spread by forest-dwelling mosquitoes, such as Aedes africanus and Haemagogus mosquitoes, between non-human primates and humans. In the urban cycle the virus is spread primarily by Aedes aegypti mosquitoes between humans. These Aedes mosquitoes can also transmit Dengue, Zika, and Chikungunya viruses.
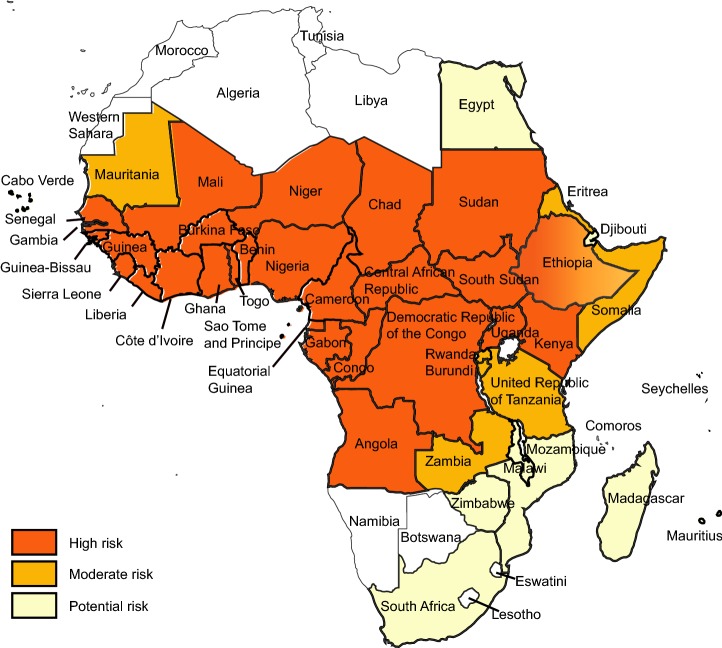
YF exists in multiple countries in sub-Saharan Africa and in Latin America and Caribbean countries (Figs. 1, 2) (WHO 2017a, 2018b). For reasons that are still not well understood, YF has not caused epidemics anywhere in Asia, despite the widespread presence of Aedes-transmitted dengue epidemics (WHO 2017a, b; Wasserman et al.2016; Wilder-Smith and Leong 2017; Lucey and Donaldson 2017).
Fig. 1.
Yellow fever risk regions in Africa 2018 (data from WHO Weekly Epidemiological Record, update to August 2018).
Fig. 2.
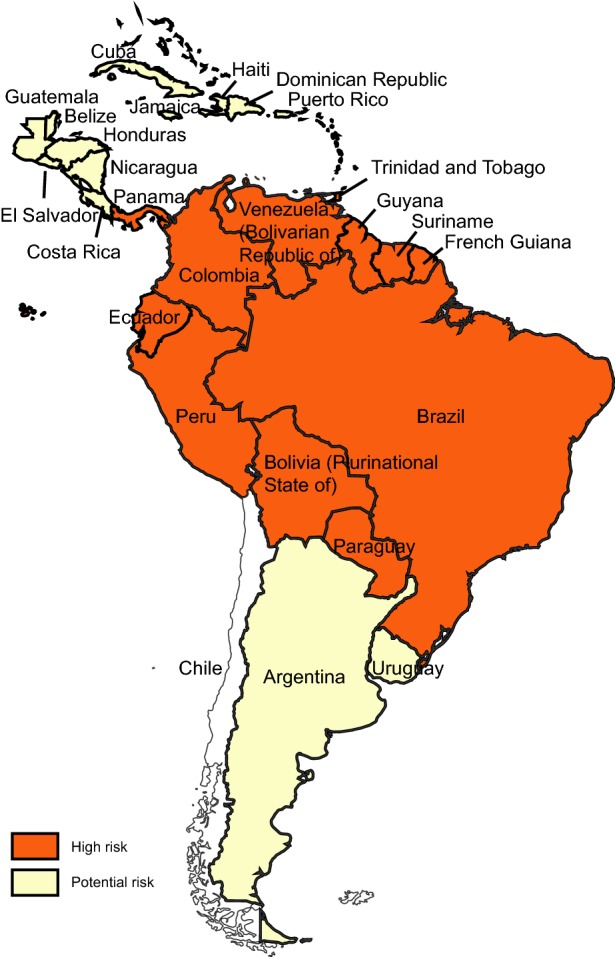
Yellow fever risk regions in Latin America and Caribbean countries 2018 (data from WHO Weekly Epidemiological Record, update to August 2018).
In 2016, two unprecedented events occurred regarding yellow fever flavivirus infection and vaccine. First, in China 11 persons were diagnosed with yellow fever virus after being infected while working in Angola and returning to China in March–April (WHO 2016a; Wang et al.2016; Chen et al.2016). No transmission within China occurred (Chen and Lu 2016). These were the first persons anywhere in Asia known to have laboratory-documented yellow fever infection. Second, a global shortage of yellow fever vaccine resulted in the first-ever use of a fractional (1/5) normal dose of yellow fever vaccine anywhere in the world when it was given to 7.5 million people in Kinshasa, Democratic Republic of the Congo (DRC) in August (Monath et al.2016; WHO 2016b).
These two events catalyzed multiple reports about the potential risk and implications of yellow fever epidemics occurring for the first-time ever in China or anywhere else in Asia where the Aedes aegypti vector for yellow fever and dengue exists, e.g. India, Pakistan, Thailand, Indonesia and beyond (Wasserman et al.2016: Baumgaertner 2016; The Economist 2016; Schlagenhauf and Chen 2017; Wilder-Smith and Leong 2017; Lucey and Donaldson 2017; Brey et al.2018; Shearer et al.2018; Musso et al.2018; Wilder-Smith and Massad 2018; Brent et al.2018). Moreover, this fractional dosing of vaccine in Kinshasa in 2016 signified that if additional outbreaks occurred soon after, anywhere in the world, e.g., Africa, Latin America or Asia, then shortages of full-dose vaccine would likely occur as well.
May 19, 2016 the World Health Organization (WHO) convened their Emergency Committee to advise the Director-General Dr. Chan on whether yellow fever epidemics in Angola and DRC, and the potential yellow fever vaccine supply problem constituted a Public Health Emergency of Concern (PHEIC) (WHO 2016c; Lucey and Gostin 2016). A PHEIC was not declared then nor when the Emergency Committee met again on August 31, 2016 (WHO 2016c). WHO did, however, create the first-ever “Global Strategy for Eliminating Yellow fever Epidemics (“EYE”) 2017–2026” program in September 2016 (WHO 2016d). This program estimated that 1.38 billion doses of yellow fever vaccine would be required to eliminate yellow fever epidemics from endemic areas in sub-Saharan Africa and in Latin America and the Caribbean (WHO 2016d). This projected 1.38 billion doses of vaccine did not include any doses for Asia a continent with no prior yellow fever transmission or vaccination campaigns, and therefore immunologically-susceptible to yellow fever epidemics.
Of concern, in Brazil throughout 2018 and into 2019, a YF vaccine shortage required use of the fractional (1/5) dose involving at least 21 million persons (PAHO 2018, 2019). Due to their national vaccine shortage, Brazil was no longer able to export YF vaccine, as they had done for use in Angola and DRC in 2016. Of note, only the Brazilian vaccine has been used for fractional dosing. Thus, vaccine being used outside of Brazil, e.g., sub-Saharan Africa, in 2018 was full-dose vaccine.
Brazil is the largest of the four vaccine manufacturers in the world that have vaccine that has been prequalified (PQ) by the World Health Organization (WHO) and thus can be used outside their own four nations. The other three are France, Russia, and Senegal (Monath et al.2016; WHO 2018a, b, c).
Only two other nations, China and the USA, produce any additional YF vaccine. Importantly, however, these two vaccines are not prequalified by the WHO. Thus, they cannot be used outside of China and the USA as part of the international YF vaccine stockpile administered by the WHO, UNICEF, Medecins Sans Frontieres, International Federation of Red Cross and Red Crescent, and GAVI under International Coordinating Group (ICG) that is authorized to provide yellow fever (and other) vaccines to many dozens of nations around the world (ICG YF 2017b; WHO 2016d, 2018b). Of note, however, during the widespread YF epidemic in Angola in 2016 when the government of Angola asked China to bring their vaccine to vaccinate Chinese citizens working in Angola (WHO 2016a). Updated WHO prequalification process and standards guidance are available on the WHO website (WHO 2016e, 2018a; Coyne 2018). China and the USA have prequalified other vaccines with the WHO. For example, China’s first prequalified vaccine was in 2013 with an attenuated Japanese Encephalitis vaccine (SA14-14-2) that could help prevent infection with this virus in children in developing countries (WHO 2013).
We advocate three steps for both the USA and China. First, to have their yellow fever vaccines prequalified by the WHO in order for their vaccines to be available for use both inside and outside their borders around the world under the authority of the multi-partnered ICG (WHO 2018a, b, c). Having six rather than four yellow fever vaccines prequalified by WHO would help alleviate the recent YF vaccine shortage in Africa (2016) and current shortage in Latin America (2018–2019) as well as the likely crisis caused by international YF vaccine shortage if yellow fever epidemics occur in Asia during or after 2019.
The second step we propose, after WHO prequalification of the YF vaccine in China and USA, would be to sharply boost production of the current small number of doses of YF vaccine in China (~ 300,000 doses/year currently) and in the USA (~ 1.1 million doses/year prior to 2017) (Monath et al.2016; CDC 2017; Lucey and Donaldson 2017). The third step we advocate for by both the USA and China would be to study the effect of fractional dosing (1/5 of full dose) of these two vaccines.
Taking these three steps as soon as possible by both China and the USA would augment international YF vaccine doses above the 1.38 billion doses estimated in 2016 to eliminate YF epidemics (“EYE”) worldwide by 2026, i.e., in Africa and Latin America (but not including any vaccine doses if YF epidemics occurred for the first time anywhere in Asia). We postulate that this EYE program is much more likely to succeed by 2026 if both China and the USA initiate the above three steps in 2019–2020 to boost YF vaccine production and continue until 2026, or as long as is needed by the global community.
Infections in China 2016 and Risk to Asia 2019 from Africa and Latin America
Since 2016 multiple authors have published work modelling the potential for yellow fever epidemics in Asia (Wilder-Smith and Leong 2017; Shearer et al.2018; Brent et al.2018; Wilder-Smith and Massad 2018). Others have emphasized the urgent need to prepare for such epidemics (Wasserman et al.2016; Baumgaertner 2016; Brey et al. 2018). New and ongoing YF epidemics in sub-Saharan Africa and Brazil 2017–2019 have emphasized the urgency to boost vaccine production for international use as quickly as possible. If YF epidemics are triggered for the first time anywhere in Asia as a result of linkages to these ongoing epidemics in Africa and Latin America, then this vaccine shortage urgency will become an emergency.
Since the Angola-DRC epidemic of 2016, multiple other YF epidemics have been reported from sub-Saharan Africa. Of most concern is the large and ongoing epidemic in Nigeria from September 2017 until now in 2019 (WHO 2018c). Smaller outbreaks requiring vaccination campaigns have also occurred in 2018 in Republic of Congo, Ethiopia, Central Africa Republic, and Liberia (WHO 2018b). The epidemic in Nigeria has resulted in the largest YF vaccination campaign ever undertaken in that nation. An estimated 25 million people have received or are scheduled to receive a full-dose vaccine between 2017 and 2019 (WHO 2018c).
Of international importance, Brazil reported unexpectedly large outbreaks of YF in 2017 and 2018. Small numbers of unvaccinated travelers with YF infection from Brazil to Europe, the Caribbean, and the US have been reported in 2018. These outbreaks in Brazil were non-urban i.e., the sylvatic or jungle-forest cycle including several areas on the outskirts of large metropolitan areas with international airports (e.g., Sao Paolo, Rio de Janeiro, Bahia). Thus, in 2018 the Brazilian government announced a plan to vaccinate the entire country including all major cities given the high-risk of urban yellow fever epidemics occurring in Brazil for the first time since 1942. Brazil boosted production of their vaccine starting in 2018. Nevertheless, fractional dosing has been necessary for over 21 million persons as the nationwide vaccination campaign continues into 2019 (PAHO 2018, 2019). Large increases in human cases and monkeys linked to sylvatic outbreaks occurred between December and April in Brazil in 2016–2017 and 2017–2018, and again has begun in December 2018–January 2019 (PAHO 2019).
The risk is evident for exportation of YF from Latin America and sub-Saharan Africa to places in Asia where the Aedes mosquito vector for yellow fever (the same as the vector for dengue, Zika, and chikungunya viruses) thrives.
Yellow Fever Vaccine Production and Fractional Dosing
The current vaccine uses a live-attenuated virus and technology developed 80 years ago. The vaccine is produced only in “Special Pathogen Free (SPF)” eggs. Thus, vaccine production is labor-intensive and time-consuming. Consequently, it is very difficult to produce large numbers of doses in a short period of time.
On the other hand, strongly in support of the global effort to eliminate YF epidemics by 2026 is the fact that that a single vaccination using full-dose YF vaccine confers protection for life (WHO 2016e). Prior to July 2016, the WHO stated that a booster dose of vaccine had to be given every 10 years (WHO 2016e).
In the USA, YF vaccine production was temporarily halted in 2017 due to manufacturing issues and the need to rebuild the only US manufacturing plant for the single producer (CDC 2017; Lucey et al.2017). Renewed production of the vaccine, called “YF-VAX”, in the USA is predicted to begin by August 2019.
The duration of protection from fractional dosing (1/5) is uncertain (Roukens et al.2008; Martins et al. 2013). Initially in Kinshasa 2016 recipients of the 1/5 does were told that immunity would last for at least 1 year (Ahuka-Mundeke et al.2018; Vannice et al.2018). Thus, another vaccination would be required. In addition, the standard WHO yellow fever vaccination card valid for travel across international borders would not be given to person who received a fractional dose (WHO 2016e). In late 2018, two follow-up studies of small numbers of persons were published suggesting that duration of protection could be as long as 8–10 years using either the Brazilian or French fractional-dosed vaccine (Roukens et al.2018; de Menezes Martins et al.2018).
Actions to Boost YF Vaccine Supply by China and the USA
We advocate for three actions to be taken by the USA and China to help boost global supply of YF vaccine and increase epidemic preparedness and response. First, both nations would work with the WHO to have their YF vaccines prequalified. Thus, their vaccines could be used outside their own borders as part of the international stockpile of YF vaccine as traditionally administrated by the WHO, UNICEF and other members of the multi-partner International Coordinating Group. Second, production of this vaccine would be sharply scaled-up by both countries. These vaccines could be made available nationally, regionally, and globally through the ICG, e.g., to help stop and prevent epidemics anywhere in the world. Third, clinical studies would be coordinated with the WHO to assess the efficacy and safety of fractional dosing using 1/5 normal dose and if needed, even a 1/10 dose.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Ahuka-Mundeke S, Casey RM, Harris JB, Dixon MG, Nsele PM, Kizito GM, Umutesi G, Laven J, Paluku G, Gueye AS, Hyde TB, Sheria GKM, Muyembe-Tanfum JJ, Staples JE. Immunogenicity of fractional-dose vaccine during a yellow fever outbreak—preliminary report. N Engl J Med. 2018 doi: 10.1056/nejmoa1710430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgaertner E (2016) Could yellow fever become the next pandemic? Sci Am. https://www.scientificamerican.com/article/could-yellow-fever-become-the-next-pandemic/
- Brent SE, Watts A, Cetron M, German M, Kraemer MUG, Bogoch II, Brady OJ, Hay SI, Creatore MI, Khan K. International travel between global urban centres vulnerable to yellow fever transmission. Bull World Health Organ. 2018;96:343–354B. doi: 10.2471/BLT.17.205658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brey PT, Fontenille D, Tang H. Yellow fever risk in Asia-Pacific region. Nature. 2018;554:31. doi: 10.1038/d41586-018-01305-w. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2017) Clinical update announcement: temporary total depletion of US licensed yellow fever vaccine addressed by availability of Stamaril vaccine at selected clinics. National Center for Emerging and Zoonotic Infectious Diseases (NCEZID) and Division of Vector-Borne Diseases (DVBD). https://wwwnc.cdc.gov/travel/news-announcements/yellow-fever-vaccine-access
- Chen J, Lu H. Yellow fever in China is still an imported disease. Biosci Trends. 2016;10:158–162. doi: 10.5582/bst.2016.01051. [DOI] [PubMed] [Google Scholar]
- Chen Z, Liu L, Lv Y, Zhang W, Li J, Zhang Y, Di T, Zhang S, Liu J, Li J, Qu J, Hua W, Li C, Wang P, Zhang Q, Xu Y, Jiang R, Wang Q, Chen L, Wang S, Pang X, Liang M, Ma X, Li X, Wang Q, Zhang F, Li D. A fatal yellow fever virus infection in China: description and lessons. Emerg Microbes Infect. 2016;5:e69. doi: 10.1038/emi.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne P (2018) The World Health Organization Prequalification Programme—playing an essential role in assuring quality medical products. Royal Society of Tropical Medicine and Hygiene (International Health). 10.1093/inthealth/ihy/095 [DOI] [PubMed]
- de Menezes Martins R, Maia MLS, de Lima SMB, de Noronha TG, Xavier JR, Camacho LAB, de Albuquerque EM, Farias RHG, da Matta de Castro T, Homma A, Collaborative Group for Studies on Duration of Immunity from Yellow Fever Vaccine Duration of post-vaccination immunity to yellow fever in volunteers eight years after a dose-response study. Vaccine. 2018;36:4112–4117. doi: 10.1016/j.vaccine.2018.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey DR, Donaldson H. Yellow fever vaccine shortages in the United States and abroad: a critical issue. Ann Intern Med. 2017;167:664–666. doi: 10.7326/M17-1337. [DOI] [PubMed] [Google Scholar]
- Lucey D, Gostin LO. A yellow fever epidemic: a new global health emergency? JAMA. 2016;315:2661–2662. doi: 10.1001/jama.2016.6606. [DOI] [PubMed] [Google Scholar]
- Martins RM, Maia Mde L, Farias RH, Camacho LA, Freire MS, Galler R, Yamamura AM, Almeida LF, Lima SM, Nogueira RM, Sá GR, Hokama DA, de Carvalho R, Freire RA, Pereira Filho E, Leal Mda L, Homma A. 17DD yellow fever vaccine: a double blind, randomized clinical trial of immunogenicity and safety on a dose-response study. Hum Vaccin Immunother. 2013;9:879–888. doi: 10.4161/hv.22982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monath TP, Woodall JP, Gubler DJ, Yuill TM, Mackenzie JS, Martins RM, Reiter P, Heymann DL. Yellow fever vaccine supply: a possible solution. Lancet. 2016;387:1599–1600. doi: 10.1016/S0140-6736(16)30195-7. [DOI] [PubMed] [Google Scholar]
- Musso D, Parola P, Raoult D. Yellow fever: the Pacific should be prepared. Lancet. 2018;392:2347. doi: 10.1016/S0140-6736(18)32520-0. [DOI] [PubMed] [Google Scholar]
- Pan American Health Organization/World Health Organization (2018) Epidemiological update: yellow fever. 7 Dec 2018. PAHO/WHO, Washington, DC
- Pan American Health Organization/World Health Organization (2019) Epidemiological update: yellow fever. 25 Jan 2019. PAHO/WHO, Washington, DC
- Roukens AH, Vossen AC, Bredenbeek PJ, van Dissel JT, Visser LG. Intradermally administered yellow fever vaccine at reduced dose induces a protective immune response: a randomized controlled non-inferiority trial. PLoS ONE. 2008;3:e1993. doi: 10.1371/journal.pone.0001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roukens AHE, van Halem K, de Visser AW, Visser LG. Long-term protection after fractional-dose yellow fever vaccination: follow-up study of a randomized, controlled, noninferiority trial. Ann Intern Med. 2018;169:761–766. doi: 10.7326/M18-1529. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf P, Chen LH. Yellow fever importation to China—a failure of pre- and post-travel control systems? Int J Infect Dis. 2017;60:91–92. doi: 10.1016/j.ijid.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Shearer FM, Longbottom J, Browne AJ, Pigott DM, Brady OJ, Kraemer MUG, Marinho F, Yactayo S, de Araújo VEM, da Nóbrega AA, Fullman N, Ray SE, Mosser JF, Stanaway JD, Lim SS, Reiner RC, Jr, Moyes CL, Hay SI, Golding N. Existing and potential infection risk zones of yellow fever worldwide: a modelling analysis. Lancet Glob Health. 2018;6:e270–e278. doi: 10.1016/S2214-109X(18)30024-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Economist (2016) Yellow fever: a preventable tragedy. The Economist Newspaper Limited: Print edition, Leaders Editorial. https://www.economist.com/leaders/2016/05/14/a-preventable-tragedy
- Vannice K, Wilder-Smith A, Hombach J. Fractional dose yellow fever vaccine—advancing the evidence base. N Engl J Med. 2018;379:603–605. doi: 10.1056/NEJMp1803433. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhou P, Fu X, Zheng Y, Huang S, Fang B, Zhang G, Jia K, Li S. Yellow fever virus: increasing imported cases in China. J Infect. 2016;73:377–380. doi: 10.1016/j.jinf.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Wasserman S, Tambyah PA, Lim PL. Yellow fever cases in Asia: primed for an epidemic. Int J Infect Dis. 2016;48:98–103. doi: 10.1016/j.ijid.2016.04.025. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith A, Leong WY. Importation of yellow fever into China: assessing travel patterns. J Travel Med. 2017;24:1–4. doi: 10.1093/jtm/tax018. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith A, Massad E. Estimating the number of unvaccinated Chinese workers against yellow fever in Angola. BMC Infect Dis. 2018;18:185. doi: 10.1186/s12879-018-3084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2013) Newly accessible Japanese encephalitis vaccine will make saving children easier in developing countries. https://www.who.int/mediacentre/news/releases/2013/japanese_encephalitis_20131009/en/
- World Health Organization (WHO) (2016a) Emergencies preparedness, response: yellow fever—China. WHO, Geneva. Disease Outbreak News. https://www.who.int/csr/don/22-april-2016-yellow-fever-china/en/
- World Health Organization (WHO) (2016b) Yellow Fever mass vaccination campaign using fractional dose in Kinshasa, DRC https://www.who.int/immunization/sage/meetings/2016/october/4_Yellow_fever_mass_vaccination_campaign_using_fractional_dose_in_Kinshasa.pdf
- World Health Organization (WHO) (2016c) Meeting of the Emergency Committee under the International Health Regulations (2005) concerning Yellow Fever. https://www.who.int/mediacentre/news/statements/2016/ec-yellow-fever/en/
- World Health Organization (WHO) (2016d) Global strategy to eliminate yellow fever epidemics (EYE). https://www.who.int/immunization/sage/meetings/2016/october/2_EYE_Strategy.pdf
- World Health Organization (WHO) (2016e) New yellow fever vaccination requirements for travelers. WHO, Geneva. International travel and health. https://www.who.int/ith/updates/20160727/en/
- World Health Organization (WHO) (2016f) Immunization standards: the WHO prequalification of vaccines procedure. WHO, Geneva. https://www.who.int/immunization_standards/vaccine_quality/pq_revision2010/en/
- World Health Organization (WHO) (2017a) Eliminate Yellow Fever Epidemics (EYE): a global strategy, 2017–2026. WHO, Geneva. https://apps.who.int/iris/bitstream/handle/10665/272408/9789241513661-eng.pdf?ua=1
- World Health Organization (WHO) (2017b) International Coordinating Group (ICG) on Vaccine Provision for Yellow Fever. Annual meeting, Geneva, 11–12 May 2017. https://www.who.int/csr/disease/icg/yellow-fever-may-2017/en/
- World Health Organization (WHO) (2018a) Immunization standards: vaccine PQ. WHO, Geneva. https://www.who.int/immunization_standards/vaccine_quality/vq_index/en/
- World Health Organization (WHO) (2018b) Yellow fever in Africa and the Americas, 2017. WHO, Geneva. https://apps.who.int/iris/bitstream/handle/10665/273782/WER9332.pdf?ua=1
- World Health Organization (WHO) (2018c) The second phase of Nigeria’s biggest-ever yellow fever vaccination campaign gets underway. Regional Office for Africa. WHO, Geneva. http://afro.who.int/news/second-phase-nigerias-biggest-ever-yellow-fever-vaccination-campaign-gets-underway