Abstract
Sipuleucel-T is a therapeutic cancer vaccine that has shown improved survival in men with metastatic castration-resistant prostate cancer. As a first-in-class agent, it has been met with both fanfare and controversy. A broad review of immune-based therapies may reveal the delayed clinical impact of sipuleucel-T to be a class effect. As new strategies of immune-based therapy are developed, their effects can be optimized through better understanding of how they affect disease differently from more standard therapeutics. Furthermore, combination therapy with agents that can either work synergistically with immune-activating therapies or deplete immune-regulating cells may result in more vigorous immune responses and improved clinical outcomes. In addition, therapeutic vaccines may be ideal candidates to safely combine with standard-of-care therapies because of their nonoverlapping toxicity profile. The ultimate role of immunotherapy may not be to supplant standard therapies, but rather to work in concert with them to maximize clinical benefit for patients.
For several decades, medical oncologists and researchers have debated the potential role of immunotherapy in the clinical management of human malignancies. The recent FDA approval of the first therapeutic cancer vaccine, sipuleucel-T (Provenge, Dendreon Corporation, Seattle, WA), has taken the discussion to a more practical level.
Immunotherapy seeks to harness the immune system’s ability to recognize and destroy cancer cells. Over the years, 3 main treatment strategies were investigated: 1) using cytokines or chemokines to activate a nonspecific immune response; 2) selectively removing immune checkpoint inhibitors; and 3) using therapeutic vaccines (Table 1). An example of using a therapeutic vaccine is autologous immune effector cells propagated ex vivo and reinfused into the patient with the goal of killing specific cancer cells. Clinical trials using autologous antitumor lymphocytes in patients with melanoma provided initial proof of principle.1 However, this approach has not been widely available for randomized multicenter trials because of the intensive resource requirements. Another option, represented by sipuleucel-T, is to use antigen-presenting cells (APCs) to initiate a diverse, lasting, tumor-specific immune response.2 In this process, peripheral blood mononuclear cells are isolated via leukapheresis, then sent to a central facility where APCs are enriched and cocultured with an antigen “cassette” that contains the immunostimulatory cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) and the target tumor antigen prostatic acid phosphatase (PAP). This cell product is then reinfused into the patient.3
Table 1.
Strategies for Optimizing Therapeutic Cancer Vaccines
| Immune-based therapies early in the disease process | Given the limited toxicity of vaccines, it may be feasible to start these therapies early in the disease process with the goal of modulating long-term growth patterns and potentially significantly improving survival. |
| Combination therapy | Vaccines used in rational combinations with immune-enhancing therapies may result in more immediate cytotoxic effects and a slower growth rate resulting from a sustained immunologic effect. |
| Immune-modulating therapies | Some therapies, including standard therapies such as sunitinib, may alter the immune regulatory mechanisms that can suppress a vaccine-induced immune response. Combining these therapies with vaccines may result in enhanced immune responses. |
Sipuleucel-T Raises Hopes and Questions
After 2 small phase III trials in minimally symptomatic metastatic castration-resistant prostate cancer (mCRPC), sipuleucel-T showed no change in time to progression (the primary end point of each study). However, a preplanned secondary analysis suggested an improvement in overall survival.4,5 A third larger trial was then initiated, with survival as the primary end point. This trial enrolled essentially the same patient population as the previous trials: those with good functional status and asymptomatic or minimally symptomatic mCRPC. The patients (n=512) were randomized 2:1 in favor of sipuleucel-T. The results of this study showed an improvement in overall survival (25.8 vs. 21.7 months; P=.032) and subsequently led to FDA approval, representing the first approval of a therapeutic cancer vaccine.6 Once again, no change in time to progression was seen.
Despite its status as a first-in-class agent and its negligible toxicity profile, sipuleucel-T has not yet been widely embraced by practitioners who treat prostate cancer.
Although some investigators have raised speculative concerns about the impact on the control arm of both age (based on subgroup analysis) and possible immune depletion,7 more pragmatic studies have suggested that sipuleucel-T was beneficial for all subgroups (including those classified according to age6). Furthermore, only a clinically insignificant number of lymphocytes are removed during apheresis, and no increased infections were noted in the registration trial.8,9 Along with more practical issues involving logistics, cost, and reimbursement, the lack of early markers of therapeutic benefit has been a major concern. Unlike standard cytotoxic therapies or modern hormonal therapies, sipuleucel-T has not shown an ability to shrink tumor or decrease prostate-specific antigen (PSA) levels in most patients, in apparent contradiction to the ultimate outcome of 2 phase III studies that showed improved survival.4,6 Closer analysis, however, shows that 2 other immune-based therapies have also shown a paradoxical improvement in overall survival without near-term changes in tumor characteristics.
Survival Trumps Progression With Immunotherapies as Monotherapy
PSA-TRICOM (PROSTVAC; Bavarian Nordic, Mountain View, CA; developed through a Collaborative Research and Development Agreement with the NCI) is another therapeutic cancer vaccine that is now in phase III testing in minimally symptomatic mCRPC.10 This offthe-shelf vaccine is administered subcutaneously and uses modified poxviruses to deliver targeting information to the immune system to initiate a focused, antitumor, immunologic effect.11 As the viruses infect immune cells, including APCs, their modified genetic material codes for PSA and T-cell costimulatory molecules within the immune cell. The PSA is expressed and presented in the context of the major histocompatibility complex molecules, and in addition to the costimulatory molecules are expressed on the surface of the primed APCs. These APCs travel to draining lymph nodes, where they engage relevant cytotoxic T lymphocytes (CTLs), then activate CTLs that can travel throughout the body and destroy PSA-expressing tumor cells.12 Early studies of PSA-TRICOM showed minimal toxicity.13 When this agent was evaluated in a randomized, multicenter, placebo-controlled trial in mCRPC, no difference was seen in time to progression, but a significant improvement in overall survival was noted (25.1 vs. 16.6 months; P=.0061).14
Similarly, ipilimumab (YERVOY; Bristol-Myers Squibb, New York, NY) has shown delayed effects in metastatic melanoma.15 This monoclonal antibody serves as an immune checkpoint inhibitor by blocking T-cell interactions with the CTLA-4 molecule on APCs after T-cell activation.16,17 Because CTLA-4 serves to regulate the body’s immune response, diminishing this interaction has been shown to enhance antitumor immune responses while also increasing the risk of autoimmune reactions. In a phase III study of ipilimumab in melanoma, 3 cohorts received ipilimumab alone, ipilimumab with an active control (GP100), and GP100 alone, respectively. The results showed no significant difference in median progression-free survival among the 3 arms, but overall survival favored the ipilimumab-containing arms (10.0 and 10.1 months) over the active control arm (6.4 months; P<.001 and P=.003, respectively).15
Taken together, these data suggest that the delay in therapeutic impact seen with the use of modern immunotherapeutics in metastatic cancer may not be an artifact associated with sipuleucel-T alone. Rather, this apparent class effect of emerging immunologic agents may be from the nature of this type of therapy. Unlike cytotoxic therapies that have a direct impact on tumor, immune-based therapies work indirectly on the immune system, which in turn targets the tumor. This may take time and may partially explain why short-term changes in disease course (within a few months when restaging scans are conventionally performed) may not demonstrate an impact of therapy.18 Furthermore, tumor infiltration with activated immune cells can impair the ability to assess progression and may actually disguise an immune response as progressive disease. This phenomenon has been documented with ipilimumab, wherein patients with apparently enlarging liver lesions on scans ultimately experienced significant tumor responses, suggesting a transient increase in the size of lesions from an active immune response.19,20 This has resulted in the proposal of new guidelines to evaluate response in clinical trials involving immune-based therapies.21
Another confounding factor in the use of immunologic agents relates to the ultimate antitumor effect once it is generated. Given the nature of immune responses, their impact on tumor is more likely to be a moderation in growth rate rather than dramatic shrinkage. Although slowing a tumor’s growth may not seem as important as reducing its mass, it may ultimately have a greater impact on outcome, because an immune response is likely to be maintained long after treatment is terminated (much like the effect of childhood vaccines), unlike cytotoxic therapies, whose transient impact on tumor size is limited to the treatment period.18,22
Immune-Based Therapies May Alter Tumor Growth Patterns
This alteration in tumor growth kinetics was initially suggested by a retrospective analysis of 5 NCI trials in mCRPC over the past decade, 4 involving cytoreductive therapy and 1 involving the vaccine PSA-TRICOM.22 Using a mathematical model to predict disease mortality (a model developed and used in other cancers by using disease-appropriate markers), PSA values were used to evaluate tumor growth kinetics and to predict death.22,23 Patients initially treated with chemotherapy-based regimens showed decreases in tumor burden for variable periods. However, once treatment was discontinued, the tumor growth rate returned to pretreatment velocity and time to death was predictable along this trajectory. Patients treated with PSA-TRICOM showed little measurable change in tumor growth rate while on-study (median 3 months), but death occurred well beyond the predicted time point, based on the off-treatment tumor growth rate.12,22 These data suggest that patients treated with vaccine had a slowing of tumor growth rate that may ultimately have prolonged their survival.
This factor may also have been at work in 2 other trials using different vaccines in patients with rising PSA levels after definitive therapy for newly diagnosed prostate cancer (castration-sensitive, nonmetastatic). In one study, patients were treated with PSA-TRICOM as part of an ECOG trial. Preliminary results of this study suggest that PSA doubling time (PSA DT; another measure of tumor growth rate) was prolonged relative to on-study values, extending from 4.4 months at enrollment to 7.7 months after treatment, an improvement of 43% (P=.002).24 Similarly, a trial in the same patient population treated patients with androgen-deprivation therapy (ADT) and then randomized them to receive either sipuleucel-T or placebo. As PSA levels began to rise after the single dose of ADT (as expected when testosterone returns to normal), a significant difference in PSA DT was noted in patients who received sipuleucel-T compared with those who received placebo. This effect only became apparent when all PSA values were evaluated at least 30 days after ADT initiation (≈35% improvement with vaccine vs. placebo; P=.037), and remained even more evident after testosterone recovery (≈47.6% improvement with vaccine vs. placebo; P=.038).25
Although results of early trials support the hypothesis that therapeutic cancer vaccines can alter tumor growth rate, further randomized prospective trials with growth rate kinetics as a primary end point are required. Furthermore, these trials will need to link changes in growth rate kinetics to a relevant, FDA-accepted, clinical outcome. These trials are difficult to conduct in prostate cancer because a follow-up of nearly 10 years is required when survival is the primary end point. However, other ways exist to capitalize on therapeutic cancer vaccines’ ability to alter the growth rate of tumors. One approach would involve regimens that combine vaccine with standard therapeutics. Radiation, ADT, and chemotherapy could all work in concert with therapeutic cancer vaccines via numerous mechanisms to enhance clinical outcomes.26
Immune-Based Therapies May Be Combined With Standard Therapies for More Immediate Clinical Impact
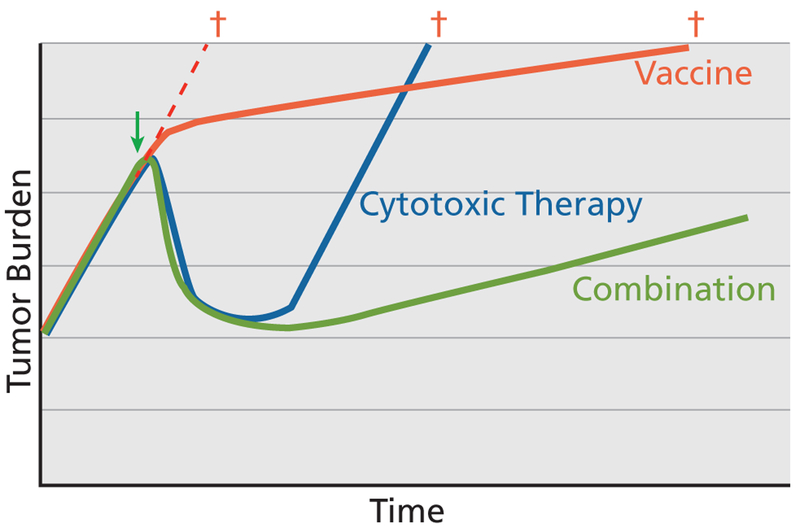
The tumor growth rate model developed by Stein et al22 shows that although cytoreductive therapy significantly debulks tumor mass, when the agents are inevitably discontinued because of toxicity or diminished efficacy, tumor growth resumes at pretreatment rates. Subsequent therapies with similar cytotoxic agents may reduce the growth trajectory, but these changes will invariably be transient. If therapeutic cancer vaccines can produce a lasting reduction in tumor growth rates, as the data suggest, then an ideal approach may be to combine a cancer vaccine with a cytoreductive therapy, such as chemotherapy and ADT in prostate cancer. The potential implications are 2-fold: 1) cytoreductive therapy would buy time (perhaps 2–3 months) necessary to initiate an immune response, and 2) once cytoreductive therapy has debulked the tumor, vaccine could be administered with the goal of altering the rate of tumor growth. The end result could be a smaller tumor growing at a slower rate. This combination would likely have a more significant impact on survival than the transient reduction in tumor volume or altered growth kinetics that might be expected from either therapy alone (Figure 1).
Figure 1.
Potential impact of vaccine and cytotoxic therapy on tumor growth. Although cytotoxic therapies have transient tumor-debulking effects, vaccines may slow tumor growth rate over the long term. If these therapies are appropriately combined, patients may benefit from both mechanisms, resulting in a smaller tumor that grows at a slower rate.
Adapted with permission from Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst 2012;104:599–613, by permission of Oxford University Press.
Besides the mathematical rationale for combining vaccines with cytoreductive therapies, based on the combined impact on tumor volume and tumor growth kinetics, a scientific rationale exists for combining these therapies based on their potential to further enhance immune responses. Notably, ADT, a cornerstone of therapy for patients with prostate cancer, has several mechanisms for enhancing an anti–prostate cancer immune response.27 ADT has been shown to enhance thymic production of naïve T cells, which can then be activated by vaccines to target the tumor.28 Additional data suggest that T cells traffic to the prostate in the setting of ADT.29 Furthermore, ADT can decrease immune tolerance for tumor antigens, thereby increasing the likelihood of antitumor immune stimulation.30 The combination of ADT and vaccine has been evaluated in clinical trials with promising results. However, with the advent of MDV3100, a modern androgen receptor antagonist (ARA), previous trials combining vaccine and older ARAs have assumed greater importance. Those studies, which combined vaccines with ARAs, have suggested benefit in patients who received both therapies, although more data from larger randomized trials are required.31,32
Docetaxel (along with a second taxane, cabazitaxel) is the primary chemotherapy used in patients with mCRPC.33–35 Although conventional wisdom is that potentially myelosuppressive therapy is immunosuppressive and thus not compatible with vaccines, a previous clinical trial showed that, in patients with mCRPC, vaccine combined with chemotherapy generated the same magnitude of T-cell–specific immune responses as vaccine alone.36 Preclinical data also show that vaccine plus docetaxel induces enhanced immune activity compared with either treatment alone.37 One possible explanation for this is that chemotherapy-induced cancer cell lysis in the setting of an activated immune response produces an antigen cascade wherein the immune system processes additional antigens from dying cancer cells, leading to a broader immune response. Additional effects include the release of cellular molecular and immunologically relevant “death signals” from dying cancer cells that enhance the immune response.38,39 Both of these effects of combining vaccine with certain chemotherapies are likely important factors in enhanced clinical outcomes.
Although conventional external-beam radiation therapy lacks the systemic effects of chemotherapy, it may still render tumors more amenable to immune-mediated attack. Even low levels of radiation can enhance immune response by altering the phenotype of cancer cells.40,41 Alternatively, and similar to chemotherapy, radiation may kill tumor cells through an immunologically relevant mechanism, also resulting in the release of antigens and/or molecular “danger signals” that enhance the immune response.42–44 The potential value of this effect in prostate cancer was demonstrated in a clinical trial that combined vaccine with definitive radiation therapy in patients with newly diagnosed disease. Patients treated with vaccine plus radiation had a significantly enhanced prostate cancer–specific immune response compared with those who received standard radiation alone.45,46
An ongoing phase III clinical trial combining ipilimumab with radiation is further investigating this hypothesis in mCRPC.47 If vaccines can be combined with radiation in patients with newly diagnosed prostate cancer, the immune response thus generated may have a sustained impact on tumor growth rate for the 20% to 40% of patients who develop recurrent disease, and may even significantly delay symptomatic disease progression. For patients with mCRPC, bone-seeking radionuclides such as 153Sm-EDTMP (Quadramet, Schering AG, Berlin, Germany, and Cytogen Co., Princeton, NJ) or Alpharadin (Algeta ASA, Oslo, Norway, and Bayer AG, Leverkusen, Germany) could be combined with vaccine.48
In addition to its aforementioned benefits in prostate cancer when combined with standard therapies, ipilimumab has been shown to improve survival in metastatic melanoma, based on a purported mechanism of enhanced T-cell activity.16,17 Thus, as 2 completed studies have shown,49,50 vaccines that stimulate T cells are logical candidates for combination with ipilimumab. The promising clinical data from these 2 studies suggest clinical benefit, albeit at the expense of greater toxicity than vaccine alone. Larger randomized trials are required to validate these hypothesis-generating data. Perhaps the apparently less toxic anti-PD1 molecules will lead to better results in combination with vaccine in future trials.51
Standard therapies can also be used to overcome immune suppression, which has been shown to increase with tumor volume.52,53 One strategy has been to overcome host immune inhibition through adding immunostimulatory cytokines, such as GM-CSF or IL-2, to the regimen. An alternative approach to improving immunocompetence is to downregulate immune inhibitory cells, such as T-regulatory cells (Tregs) or myeloid-derived suppressor cells (MDSCs). Although the biology of MDSCs in human cancers is not well understood, data on the immune inhibitory role of Tregs are compeling. Several immunotherapeutic approaches have been used that downregulate or even completely ablate Tregs.54 Emerging preclinical data suggest that anticancer agents such as sunitinib and BCL-2 inhibitors may also have this effect, indicating the need for further investigation of the combination of these agents with vaccine.55–57 The possibility exists that using standard agents such as chemotherapy or targeted molecular therapies for their therapeutic and immune-enhancing effects in combination with vaccines may optimize both therapies.
Assessing Immune Response
Although immune-based combinations may result in a more immediate clinical impact and obviate the need to develop intermediate markers of response, the challenge of identifying a mechanism of action remains. The apparent lack of understanding of this mechanism fuels much of the skepticism regarding this emerging class of therapy. To this end, comprehensive immune monitoring is a large component of many ongoing clinical trials.
Fortunately, in the past decades a plethora of biotechnological advances has provided researchers with the techniques needed to closely monitor antigen-specific immune responses and immune effector-cell populations. Assay and patient variability, however, has prevented these techniques from being developed into surrogate markers of immune response. The ELISPOT assay, for example, is a commonly used technique to assess antigen-specific T-cell immune response. Unfortunately, several studies have highlighted the significant variability of this assay in multiple laboratories using the same techniques.58,59
Perhaps a more relevant confounding variable is the discrepancies seen among patients themselves. Although the purpose of immune therapies is to activate an immune response, this response may manifest differently from patient to patient. For example, for nonspecific immune therapies such as ipilimumab, which antigen-specific T-cell response is most important? Given the multitude of antigens in a given tumor, this is likely to vary significantly among patients. Even for therapeutic cancer vaccines that initially focus the immune response on a single antigen, the ultimate and perhaps most significant immune response may focus on secondary antigens, via a process described as “antigen spreading” or “antigen cascade.”45,60 Other assays that evaluate specific humoral or cellular immune responses (eg, natural killer cells or Tregs) might play a relevant role in a proportion of patients.46,60 For this reason, one must accept the very real possibility that standardized biomarkers of immune response will remain elusive and impractical given the variability among patients.
Despite these difficulties, additional mechanisms for assessing immune response are being evaluated in intriguing studies, such as those evaluating genetic profiles of immune cells. Recent research in genomic profiling of immune cell populations may shed light on immune system defects in patients with cancer and help identify ideal patient populations for cancer immunotherapy. A genomic analysis of peripheral blood lymphocytes from patients with kidney cancer treated with a dendritic cell vaccine and high-dose IL–2 showed significant differences in the genomic profiles of patients and healthy age-matched donors. Patients showed upregulation of gene pathways consistent with immune stimulation. In addition, patients who subsequently benefited from immunotherapy showed upregulation of an entirely different subset of genes than those for whom treatment would eventually fail.61
Another intriguing approach would be to assess tumor-infiltrating immune cells after immune-based therapies. Although this may prove clinically challenging for some tumor locations, intriguing data are emerging showing that immune cell infiltration positively correlates with survival in patients treated with conventional therapies.62 Studies in common tumor types, including colorectal, ovarian, and pancreatic cancers,63–65 suggest that patients with enhanced immune cell infiltration of tumor have superior outcomes.62 One would expect that this would also be the case with immune-based therapies in these tumor types, and as image-guidance systems continue to improve, access to tumors will become increasingly feasible and safe. Correlative studies in future immunotherapy trials are required to further evaluate this hypothesis.
Conclusions
Ideally, immunologic correlates of immune response would be consistent predictors of clinical outcomes. However, the complexity and variability of the immune response may preclude the development of universal strategies for assessing outcomes of patients treated with immunologic agents. Future studies will focus on optimizing vaccine strategies, perhaps through deployment in combination with cytotoxic therapies, leading to standard measures of improvement, such as time to progression. This could maximize clinical effect while obviating the need for a prognostic evaluation of vaccine response. Long-range studies could also be designed to evaluate the effect of vaccine on tumor growth rate and overall survival, with the goal of validating short-term growth rates as an indicator of response. Thus, although sipuleucel-T has set in motion a revolution in cancer treatment, future strategies, along with next-generation immune-based treatments, will cement its legacy.
Footnotes
The authors have disclosed that they have no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or their competitors.
References
- 1.Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci U S A 2004;101(Suppl 2):14639–14645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwaab T, Ernstoff MS. Therapeutic vaccines in renal cell carcinoma. Therapy 2011;4:369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.So-Rosillo R, Small EJ. Sipuleucel-T (APC8015) for prostate cancer. Expert Rev Anticancer Ther 2006;6:1163–1167. [DOI] [PubMed] [Google Scholar]
- 4.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 2006;24:3089–3094. [DOI] [PubMed] [Google Scholar]
- 5.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 2009;115:3670–3679. [DOI] [PubMed] [Google Scholar]
- 6.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411–422. [DOI] [PubMed] [Google Scholar]
- 7.Huber ML, Haynes L, Parker C, et al. Interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst 2012;104:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolf C, Bolan C, Wesley R, et al. Interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. Transfusion 2003;43:28a. [Google Scholar]
- 9.Mark D, Samson D, Bonnell C, et al. Outcomes of sipuleucel-T therapy: technology assessment report. Available at: www.cms.gov/determinationprocess/downloads/id77TA.pdf. Accessed January 25, 2012.
- 10.A phase 3 efficacy study of a recombinant vaccinia virus vaccine to treat metastatic prostate cancer (Prospect). Available at: http://clinicaltrials.gov/ct2/show/NCT01322490?term=PROSPECT&rank=6. Accessed May 7, 2012.
- 11.Madan RA, Arlen PM, Mohebtash M, et al. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs 2009;18:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother 2010;59:663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arlen PM, Skarupa L, Pazdur M, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol 2007;178:1515–1520. [DOI] [PubMed] [Google Scholar]
- 14.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010;28:1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 2002;3:611–618. [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz AA, Sullivan TJ, Sobel RA, et al. Cytotoxic T lymphocyte antigen-4 (CTLA-4) limits the expansion of encephalitogenic T cells in experimental autoimmune encephalomyelitis (EAE)-resistant BALB/c mice. Proc Natl Acad Sci U S A 2002;99:3013–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madan RA, Gulley JL, Fojo T, et al. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist 2010;15:969–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Giacomo AM, Danielli R, Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother 2009;58:1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ledezma B, Binder S, Hamid O. Atypical clinical response patterns to ipilimumab. Clin J Oncol Nurs 2011;15:393–403. [DOI] [PubMed] [Google Scholar]
- 21.Hoos A, Eggermont AM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 2010;102:1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein WD, Gulley JL, Schlom J, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res 2011;17:907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein WD, Figg WD, Dahut W, et al. Tumor growth rates derived from data for patients in a clinical trial correlate strongly with patient survival: a novel strategy for evaluation of clinical trial data. Oncologist 2008;13:1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiPaola R, Chen Y, Bubley G, et al. A phase II study of PROSTVAC-V (vaccinia)/TRICOM and PROSTVAC-F (fowlpox)/TRICOM with GM-CSF in patients with PSA progression after local therapy for prostate cancer: results of ECOG 9802 [abstract] Presented at the 2009 ASCO Genitourinary Cancers Symposium; February 26–28, 2009; Orlando, Florida: Abstract 108. [Google Scholar]
- 25.Beer TM, Bernstein GT, Corman JM, et al. Randomized trial of autologous cellular immunotherapy with sipuleucel-T in androgen-dependent prostate cancer. Clin Cancer Res 2011;17:4558–4567. [DOI] [PubMed] [Google Scholar]
- 26.Bilusic M, Heery C, Madan RA. Immunotherapy in prostate cancer: emerging strategies against a formidable foe. Vaccine 2011;29:6485–6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonarakis ES, Drake CG. Combining immunological and androgen-directed approaches: an emerging concept in prostate cancer immunotherapy. Curr Opin Oncol 2012;24:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci 2007;12:4957–4971. [DOI] [PubMed] [Google Scholar]
- 29.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A 2001;98:14565–14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drake CG, Doody AD, Mihalyo MA, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell 2005;7:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madan RA, Gulley JL, Schlom J, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res 2008;14:4526–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bilusic M, Gulley JL, Heery C, et al. A randomized phase II study of flutamide with or without PSA-TRICOM in nonmetastatic castration-resistant prostate cancer (CRPC) [abstract]. J Clin Oncol 2011;29(Suppl):Abstract 163. [Google Scholar]
- 33.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502–1512. [DOI] [PubMed] [Google Scholar]
- 34.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351:1513–1520. [DOI] [PubMed] [Google Scholar]
- 35.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010;376:1147–1154. [DOI] [PubMed] [Google Scholar]
- 36.Arlen PM, Gulley JL, Parker C, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res 2006;12:1260–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res 2008;14:3536–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tesniere A, Panaretakis T, Kepp O, et al. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ 2008;15:3–12. [DOI] [PubMed] [Google Scholar]
- 39.Aymeric L, Apetoh L, Ghiringhelli F, et al. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res 2010;70:855–858. [DOI] [PubMed] [Google Scholar]
- 40.Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol 2003;170:6338–6347. [DOI] [PubMed] [Google Scholar]
- 41.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007;13:1050–1059. [DOI] [PubMed] [Google Scholar]
- 43.Bianchi ME, Beltrame M, Paonessa G. Specific recognition of cruciform DNA by nuclear protein HMG1. Science 1989;243:1056–1059. [DOI] [PubMed] [Google Scholar]
- 44.Friedman E Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr Pharm Des 2002;8:1765–1780. [DOI] [PubMed] [Google Scholar]
- 45.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res 2005;11:3353–3362. [DOI] [PubMed] [Google Scholar]
- 46.Nesslinger NJ, Ng A, Tsang KY, et al. A viral vaccine encoding prostate-specific antigen induces antigen spreading to a common set of self-proteins in prostate cancer patients. Clin Cancer Res 2010;16:4046–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phase 3 study of immunotherapy to treat advanced prostate cancer. Available at: http://clinicaltrials.gov/ct2/show/NCT01057810?term=NCT01057810&rank=1. Accessed May 7, 2012.
- 48.Heery C, Madan R, Bilusic M, et al. Interim analysis of a phase II randomized clinical trial of samarium-153 (Sm-153) with or without PSA-TRICOM vaccine in metastatic castration-resistant prostate cancer after docetaxel [abstract]. J Clin Oncol 2012;30(Suppl):Abstract 2526.23169502 [Google Scholar]
- 49.Madan RA, Mohebtash M, Arlen PM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 2012;13:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van den Eertwegh AJ, Versluis J, van den Berg HP, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 2012;13:509–517. [DOI] [PubMed] [Google Scholar]
- 51.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu T, Shen Y, Fujimoto S. Tumor-specific CD4(+) suppressor T-cell clone capable of inhibiting rejection of syngeneic sarcoma in A/J mice. Int J Cancer 2000;87:680–687. [PubMed] [Google Scholar]
- 53.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 2004;34:336–344. [DOI] [PubMed] [Google Scholar]
- 54.Vermeij R, Leffers N, Hoogeboom BN, et al. Potentiation of a p53-SLP vaccine by cyclophosphamide in ovarian cancer: a single-arm phase II study. Int J Cancer 2012;131:E670–680. [DOI] [PubMed] [Google Scholar]
- 55.Farsaci B, Sabzevari H, Higgins JP, et al. Effect of a small molecule BCL-2 inhibitor on immune function and use with a recombinant vaccine. Int J Cancer 2010;127:1603–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farsaci B, Higgins JP, Hodge JW. Consequence of dose scheduling of sunitinib on host immune response elements and vaccine combination therapy. Int J Cancer 2012;130:1948–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bose A, Taylor JL, Alber S, et al. Sunitinib facilitates the activation and recruitment of therapeutic anti-tumor immunity in concert with specific vaccination. Int J Cancer 2011;129:2158–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cox JH, Ferrari G, Kalams SA, et al. Results of an ELISPOT proficiency panel conducted in 11 laboratories participating in international human immunodeficiency virus type 1 vaccine trials. AIDS Res Hum Retroviruses 2005;21:68–81. [DOI] [PubMed] [Google Scholar]
- 59.Ryan JE, Ovsyannikova IG, Dhiman N, et al. Inter-operator variation in ELISPOT analysis of measles virus-specific IFN-gamma-secreting T cells. Scand J Clin Lab Invest 2005;65:681–689. [DOI] [PubMed] [Google Scholar]
- 60.Disis ML, Goodell V, Schiffman K, et al. Humoral epitope-spreading following immunization with a HER-2/neu peptide based vaccine in cancer patients. J Clin Immunol 2004;24:571–578. [DOI] [PubMed] [Google Scholar]
- 61.Schwarzer A, Wolf B, Fisher JL, et al. Regulatory T-cells and associated pathways in metastatic renal cell carcinoma (mRCC) patients undergoing DC-vaccination and cytokine-therapy. PLoS One, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jochems C, Schlom J. Tumor-infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity. Exp Biol Med (Maywood) 2011;236:567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pages F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 2009;27:5944–5951. [DOI] [PubMed] [Google Scholar]
- 64.Fukunaga A, Miyamoto M, Cho Y, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 2004;28:e26–31. [DOI] [PubMed] [Google Scholar]
- 65.Tomsova M, Melichar B, Sedlakova I, et al. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol 2008;108:415–420. [DOI] [PubMed] [Google Scholar]