Abstract
Generation of superoxide by xanthine oxidase can be stimulated under ischemic and aberrant calcium homeostasis. Because patients and mice with Duchenne muscular dystrophy (DMD) suffer from ischemia and excessive calcium influx, we tested the hypothesis that xanthine oxidase activity is elevated and contributes to disease pathology. Xanthine oxidase activity was measured by urinary isoxanthopterin in DMD patients at rest and in response to exercise. Urinary isoxanthopterin/creatinine was elevated compared to age-matched controls and Becker muscular dystrophy (BMD) patients. Concentrations were also increased after a six minute walk test in ambulatory patients. We also measured urinary isoxanthopterin in wildtype mice and a number of dystrophic mouse models; the DMD mouse model (mdx), mdx mice overexpressing a variety of transgenic miniaturized and chimeric skeletal muscle-specific dystrophins and utrophin and the ß-sarcoglycan deficient (Scgb−/−) mouse which represents type 2E human limb-girdle muscular dystrophy. Mdx and Scgb−/− mice had greater urinary isoxanthopterin/creatinine than wildtype mice while mdx mice expressing dystrophin or utrophin linking the extracellular matrix to the actin cytoskeleton were not different than wildtype. We also measured higher levels of urinary ortho-tyrosine in humans and mice deficient for dystrophin to confirm elevated oxidative stress. Surprisingly, mdx had lower xanthine oxidase protein levels and higher mRNA in gastrocnemius muscle compared to wildtype mice, however, the enzymatic activity of skeletal muscle xanthine oxidase was elevated above wildtype and a transgenic rescued mdx mouse (Dys ΔMTB-mdx). Downhill treadmill running also caused significant increases in mdx urinary isoxanthopterin that was prevented with the xanthine oxidase inhibitor allopurinol. Similarly, in vitro eccentric contraction-induced force drop of mdx muscle was attenuated by the allopurinol metabolite, oxypurinol. Together, our data suggests hyper-activity of xanthine oxidase in DMD, identifies xanthine oxidase activity as a contributing factor in eccentric contraction-induced force drop of dystrophin-deficient skeletal muscle and highlights the potential of isoxanthopterin as a noninvasive biomarker in DMD.
Keywords: Allopurinol, Duchenne muscular dystrophy, dystrophin, gene therapy, isoxanthopterin, mdx, oxidative stress, pterin, xanthine oxidase
1. Introduction
Reactive oxygen species (ROS) have been implicated in the pathophysiology of Duchenne muscular dystrophy (DMD) [1–4], a fatal muscle wasting disease caused by the loss of the protein dystrophin [5]. A significant source of ROS includes inflammatory cells, NADP(H) oxidase and mitochondria, all of which have abnormal function in DMD [6–8]. It has also been suggested that aberrations in calcium homeostasis result in activation of the xanthine dehydrogenase/oxidase system (XDH/XO) in DMD with the subsequent production of superoxide [9]. In healthy tissue, XDH/XO is normally found as XDH, which uses NAD+ as a co-factor. In diseased tissue, calcium-activated proteinases cleave XDH to XO [10], which instead uses molecular oxygen as a co-factor [11], The conversion of XDH to XO is accelerated under ischemic conditions [12], Because patients with DMD fail to localize neuronal nitric oxide synthase to the membrane [13], patients suffer from both central and peripheral ischemia [14]. Thus, ischemia-induced XO activity may provide a further mechanism contributing to the oxidative environment of dystrophic tissue.
Measurement of superoxide in biological systems remains challenging. An in vitro approach to measure XO activity utilizes the fluorescent conversion of pterin to isoxanthopterin. Isoxanthopterin is part of the pteridine family, which represents important co-factors utilized in several essential biological pathways. Isoxanthopterin has been used as a noninvasive detection and diagnostic biomarker for cancer [15–17] because of its relative ease of detection and measurement in human urine [18]. Changes in isoxanthopterin can represent both superoxide formation and activity of xanthine oxidase and thus may serve as a potential indicator of oxidative stress in DMD patients.
In our first study, we tested the hypothesis that xanthine oxidase is a source of oxidative stress in patients with DMD and the milder form Becker muscular dystrophy (BMD) by measuring urinary isoxanthopterin at rest and after the six minute walk test (6MWT). We separated urinary isoxanthopterin concentrations in DMD patients at rest based on age, ambulatory status, and use of corticosteroids at time of urine collection. We also measured urinary ortho-tyrosine as a control for oxidative stress. In our second study, we measured urinary isoxanthopterin in mdx mice at rest and in response to treadmill exercise. We also determined whether the hypothesized elevation in isoxanthopterin in mdx mice compared to healthy wildtype mice is corrected when dystrophin or utrophin expression is restored to mdx skeletal muscle. Finally, because skeletal muscle contractions have been shown to activate XO-derived superoxide [19], we tested the effect of allopurinol on treadmill running and eccentric contraction-induced force drop in mdx mice.
2. Material and Methods
Ethical approval -
We made measurements in urine samples that had been collected from male DMD patients and healthy age-matched controls as previously reported [20]. The experimental protocol was approved by the Internal Review Board, University of Minnesota (1207M18081), and all participants were informed of the risks involved in the study before their written consent was obtained. The studies conformed to the standards set by the latest revision of the Declaration of Helsinki, except for registration in a database.
Experimental mice -
All animals were housed and treated in accordance with the standards set by the University of Minnesota Institutional Animal Care and Use Committee. Male mdx (C57BI/10ScSn-DMDmdx) and C57BL/10 (C57BI/10ScSn) were generated using founders purchased from Jackson Laboratory (Bar Harbor, ME, USA). Generation of Dys ΔMTBD-mdx [21], Dys ΔR4 23/ ΔCT-mdx [22], Dys ΔR2-15/ΔR18-23/ΔCT-mdx [22], Fiona-mdx [21], Scgb−/− [23], Dp-116-mdx [24] were previously reported. All mice used in this study were 3 month old adult males. All mice were housed in groups of 3 – 4 per cage on a 14/10 h light/dark cycle with food and water provided ad libitum. All animal protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee (1707–34941A).
Human participants -
Participants were male DMD patients (N = 47; 11.4 ± 0.6 yr), male BMD patients (N = 6; 19.7 ± 1.2 yr) and healthy unaffected male controls (N = 34; 13.9 ± 1.2 yr). Single catch urine samples were collected at several clinics and camps over three years. For 10 of the DMD patients, a repeat urine sample was collected at a different time point at least one year apart and incorporated into the total pool. At time of urine collection, participants completed a questionnaire which included information about age and ambulation but because of difficulties associated with ambulation, height and weight could not be collected. Because DMD patients are routinely administered corticosteroids, we also compared urinary isoxanthopterin concentrations based on known steroid use at time of collection. Each urine sample was collected mid-stream and placed on ice immediately before being transported to the laboratory, aliquoted and frozen at −80 °C until analysis.
Six minute walk test -
Urine samples were collected pre and post a 6MWT. The 6MWT for this study was similar to that performed by McDonald et al. [25]. Briefly, a 25-m taped line marked with 1-m intervals and cones at each end of the tape were used to mark the ambulatory path for patients with DMD. Continuous moderate verbal encouragement was allowed throughout the testing period. In addition, a “safety chaser” followed the child to keep him on the correct path, be in close proximity in case of a fall, to help initiate a standing position, or tend to the child quickly if an injury occurred. Urine was collected immediately pre and within 1 h of completion of the test.
Ex vivo muscle preparation and physiology -
Baseline contractile functions of EDL muscles were assessed according to the methods of Moran et al. [26]. Mice were anesthetized with sodium pentobarbital (100 mg/kg body mass (BM) for WT and 75 mg/kg BM for mdx). EDL muscles were removed and mounted on a dual-mode muscle lever system (300B-LR; Aurora Scientific Inc., Aurora, ON, Canada) with 5–0 suture in a 1.2 mL bath assembly and filled with oxygenated (95:5 % 02:C02) Krebs-Ringer bicarbonate (Krebs) buffer maintained at 25 °C. Muscles were adjusted to their anatomical optimal length (L0) based on resting tension. Muscle length was then measured from myotendonous junction to myotendonous junction using digital callipers. Prior to performing eccentric contractions, muscles remained quiescent in the bath for 5 min before beginning a protocol for testing contractile function. Maximal isometric tetanic force (P0) was measured every 2 min by stimulating the muscle to contract for 200 ms at 175 Hz until force plateaued (within 5 mN from one contraction to the next).
After plateau was attained, oxypurinol dissolved directly in Krebs buffer [19] was added to the bathing media (40 or 400 μM) and the muscle remained quiescent for 30 min as previously reported [20]. P0 was then re-measured at 30 and 32 min after addition of oxypurinol. Next, 10 eccentric contractions were performed [20] and the force loss associated with each eccentric contraction was recorded. For each eccentric contraction the muscle was passively shortened to 95% L0 and then stimulated for 200 ms while the muscle was simultaneously lengthened to 105% L0 at 0.5 L0/s. Each eccentric contraction was separated by 3 min of rest to prevent fatigue [27]. The force measured at each eccentric contraction was expressed as a percentage of the force produced during the first (“initial”) contraction.
Western blot analysis -
Gastrocnemius muscle was pulverized with mortar and pestle in liquid nitrogen. Tissue was then lysed with 1% SDS solution with added protease inhibitors (100 nM Aprotinin, 10 mg/ml E-64, 100 μM Leupeptin, 1 mM PMSF, 1 μg/ml Pepstatin) proportional to mass of the tissue pellet. Protein concentration was measured by A280 absorbance. Equal concentrations of lysate were then separated by electrophoresis at 150 V for 1 h and transferred to PVDF membrane at 100 V/0.7 Amp for 1.5 hrs. Membrane was blocked in 5% non-fat milk in PBS for 1 h and then incubated in blocking buffer with primary antibody overnight at 4 C. Primary antibodies used were anti-xanthine oxidase at 1:1000 (Abl09235, Abcam) and anti-GAPDH at 1:10,000 (Sigma G9545). Blots were then incubated in secondary antibodies anti-mouse and anti-rabbit IgG Dylight® 800 (Cell Signaling) at 1:10 000 in blocking buffer. Secondary antibody signal was visualized on Licor’s Odyssey* Infrared Imaging System and band density calculated with Odyssey Software v2.1.
qRT-PCR -
Total RNA was extracted from 3 mo old C57BL/10, mdx and Dys ΔMTBD-mcix gastrocnemius muscle samples using the Bio-Rad-Aurum Total RNA Mini Kit following the manufacturer’s instructions. RNA concentration and purity (260/280 ratio) were determined using a NanoDrop spectrophotometer (Wilmington, DE). First-strand cDNA was synthesized with a Bio-Rad iScript Advanced cDNA Synthesis Kit for qRT-PCR using the same initial RNA amount for all samples. Muscle samples were amplified in parallel with each specific qRT-PCR primer set using Bio-Rad SsoAdvanced Universal SYBR polymerase on the Bio-Rad CFX96 Real Time System C1000 Touch Thermal Cycler to measure xanthine oxidase transcript amount.
Treadmill assay -
We utilized a similar protocol to that previously reported as a “proof of concept” in mdx mice [28]. The protocol was modified because we found that mdx mice suffer from a stress-induced physical inactivity response and do not often voluntarily complete the proposed exercise prescription. Thus, 4–6 mice that were not familiarized with the treadmill were placed in individual running lanes. Over 5 min, the treadmill which was on a −10° decline, was increased in speed every minute from 10 to 15 m/min. Once at 15 m/min, the protocol began and mice were timed and distance calculated over 30 min. Mice either completed the protocol (450 m run) or became exhausted/unwilling to run which corresponded to termination. Urine was collected using a sterile 1.5 mL tube and gently massaging the lower back of the mouse while being elevated by the base of the tail immediately pre and at 5 time points post-exercise. Changes in urinary isoxanthopterin were measured as a percentage of initial. For mdx mice only, a separate cohort were anaesthetized with isoflurane and injected with 50 mg/kg allopurinol 1 h before treadmill exercise. A separate cohort was used to prevent any familiarization.
Xanthine oxidase activity assay-
Xanthine oxidase activity was measured according to the methods of Gomez-Cabrera [19]. Frozen gastrocnemius muscle or liver was pulverized with mortar and pestle in liquid nitrogen. Gastrocnemius muscle was chosen as the skeletal muscle for analysis because it possesses a variety of oxidative and glycolytic fibers [29]. Tissue was then lysed in 0.25M sucrose, 10 mM DTT, 0.2 mM PMSF, 0.1 mM EDTA, and 50 mM potassium phosphate, pH 7.4. Homogenates were centrifuged for 30 min at 14,000 g, and activities were measured in supernatants. The reaction was initiated by addition of pterin (100 μmol/I) as a substrate, and XO activity was obtained from the rate of increase in fluorescence due to the conversion of pterin to isoxanthopterin over 1 h. The reaction was stopped by addition of allopurinol (50 μmol/l). Protein concentration was measured by A280 absorbance.
High-performance liquid chromatography
Media collected following the xanthine oxidase activity assay were thawed on ice and an equal concentration of acetonitrile added, vortexed, and left on ice for 10 min. Samples were then spun down and the supernatant transferred to a microcentrifuge tube with a 3 kDa filter and spun at 14,000 g for 10 min. Thirty μL of the concentrate was transferred to a HPLC vial (Chromtech, MN, USA) and 10 μL directly injected onto the HPLC for isoxanthopterin and pterin measurement.
All human urine samples were thawed in the dark on ice immediately before analysis. Samples for pterin and isoxanthopterin were diluted 1 in 40 with 20 mM ammonium phosphate (pH 2.5) and 10 μL directly injected into the HPLC. Urine samples for ortho-tyrosine were prepared and measured with the same HPLC instrument and according to the methods of Molnar et al. as previously described [30].
Isoxanthopterin and pterin measurement was performed on a Shimadzu l-series 2030C with UV detector and auto-sampler, RF-20A florescence detector and on-line degasser with a 20 mM ammonium phosphate (pH 2.5) mobile phase, pumped isocratically at 1 mL/min as described previously [31]. Samples were maintained at 4 °C via the HPLC cooling unit and separated by positive charge using a Luna 5 μm SCX 100 Å, 250 × 4.6 mm column (Phenomenex, CA, USA). Pterin and isoxanthopterin (Schirck’s Laboratory, Jona, Switzerland) were detected by fluorescence at 353 nm excitation and 438 nm emission. Creatinine was measured using at its natural absorbance of 234 nm. The concentration and identity of each pterin and creatinine was compared to their corresponding standard (freshly prepared each day and spaced evenly throughout each analytical run) and quantified by peak area using the software Shimadzu Class VP. We chose to correct urinary isoxanthopterin concentrations with creatinine because it is possible to accurately compare mouse to human data [20].
Statistics -
Differences between groups were analyzed by t-tests, paired t-test, one-way ANOVA, repeated measures two-way ANOVA or linear regression where appropriate. When a difference was measured between groups using a one-way or two-way ANOVA, post-hoc analyses using Tukey and Dunnett, respectively, were used to identify individual differences. All data are presented as mean ± standard error of the mean (SEM) with significance set at p < 0.05.
3. Results
3.1. Urinary isoxanthopterin is elevated and associated with ambulation in DMD patients
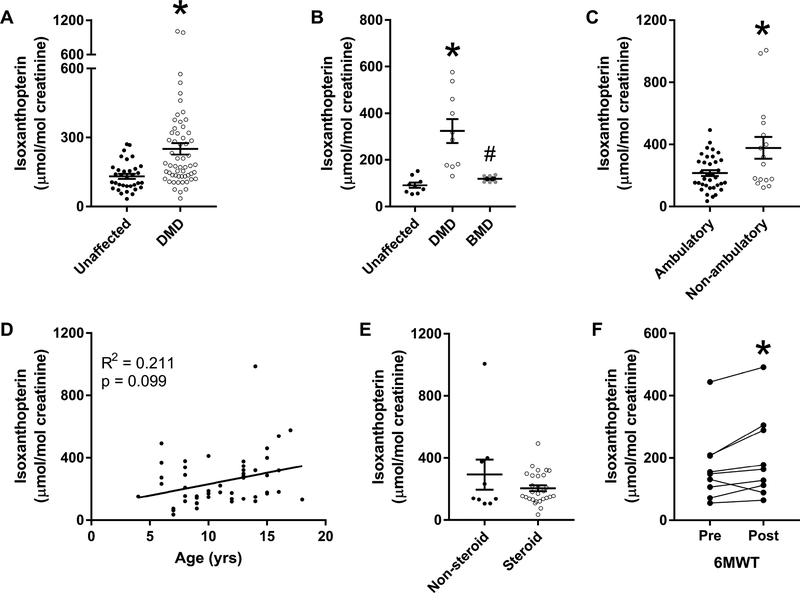
We determined whether xanthine oxidase activity was elevated in DMD patients by measuring urinary isoxanthopterin. Compared to age-matched controls, DMD patients had greater urinary isoxanthopterin/creatinine (131 ± 11 vs. 251 ± 25 μmol/mol creatinine; Fig. 1A; p < 0.001). Urinary isoxanthopterin was also significantly greater in age-matched DMD patients when compared to a group of 16–22 year old BMD patients (Fig. 1B; p ≤ 0.003). Because DMD is a progressive disease where patients become wheelchair bound by their early teens, we also assessed urinary isoxanthopterin as a function of age and ambulation. Urinary isoxanthopterin/creatinine was elevated in non-ambulant compared to ambulant DMD patients (215 ± 19 vs. 377 ± 71 μmol/mol creatinine; Fig. 1C; p < 0.040) but we did not measure a relationship between isoxanthopterin and age (Fig. 1D; p = 0.099). Next, we assessed urinary isoxanthopterin concentrations as a function of corticosteroid use because they are one effective therapy for DMD patients [32], We measured no effect of corticosteroid administration on urinary isoxanthopterin/creatinine concentrations (Fig. 1E). We also measured the response of urinary isoxanthopterin in nine DMD patients who performed the 6MWT because it has been utilized to test the efficacy of various therapies and treatments [33]. DMD patients completed 385 ± 31 m and urinary isoxanthopterin/creatinine increased in 8 of the 9 participants from before to after the 6MWT (170 ± 39 vs. 202 ± 46 pmol/mol creatinine; Fig. 1F; p = 0.044). There was no association between distance completed during the 6MWT and percent change in urinary isoxanthopterin (R2 = 0.032; p = 0.623).
Figure 1.
(A) Urinary isoxanthopterin/creatinine in unaffected controls vs. DMD patients aged 4 −18 y. *different from unaffected. (B) Urinary isoxanthopterin/creatinine in unaffected controls, BMD and DMD patients aged 16 – 23 y; *different from unaffected,# different from DMD. (C) Urinary isoxanthopterin/creatinine in DMD patients separated by ambulation status; *different from ambulatory. (D) Urinary isoxanthopterin/creatinine relationship with age in DMD patients. (E) Urinary isoxanthopterin/creatinine in DMD patients separated by steroid use at time of urine collection. (F) Urinary isoxanthopterin/creatinine before and following the six-minute walk test (6MWT); *different from pre, n = 9.
3.2. Skeletal muscle-specific dystrophin and utrophin expression normalizes elevated urinary isoxanthopterin in dystrophin-deficient mdx mice.
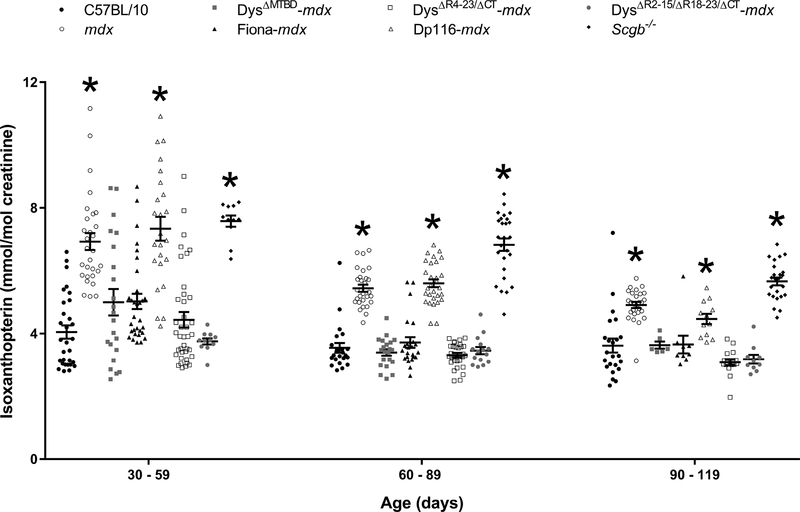
Because the DMD mouse model (mdx) recapitulates numerous pathological and physiological manifestations observed in humans [34], we assessed whether the mdx mouse had elevated urinary isoxanthopterin compared to healthy wildtype mice (C57BL/10). Between the ages of 30 and 120 days, mdx mice had greater urinary isoxanthopterin/creatinine than C57BL/10 (Fig. 2). For comparison, we also measured urinary isoxanthopterin in a mouse model of human Type 2E limb girdle muscular dystrophy. The limb girdle muscular dystrophy model (Scgb−/−) has C57BL/10 levels of dystrophin and utrophin but has a perturbed dystrophin-glycoprotein complex (DGC) because they are null for ß-sarcoglycan [35,36]. Scgb−/− also had elevated urinary isoxanthopterin/creatinine compared to C57BL/10 (Fig. 2).
Figure 2.
Urinary isoxanthopterin/creatinine in C57BL/10, mdx, a mouse model of limb girdle muscular dystrophy (Scgb−/−) and mdx mice expressing various truncated or chimeric dystrophins or utrophin. Data from mouse models are compared within each age range; *different from C57BL/10, n ≥ 6/genotype.
Muscle specific transgenic expression of dystrophin or utrophin rescue nearly all known phenotypes of the mdx mouse [37,38]. Because DMD is primarily a skeletal muscle disease with associated cardiac and cognitive phenotypes, we tested the hypothesis that skeletal muscle-specific expression of therapeutically relevant miniaturized dystrophins and utrophin could ameliorate the elevation in urinary isoxanthopterin. Urinary isoxanthopterin/creatinine were normalized to C57BL/10 levels in mdx mice expressing two therapeutically-relevant miniaturized dystrophins, or utrophin, all of which provide a link between the extracellular matrix (ECM) and actin cytoskeleton (Fig. 2). Mice expressing the Dpll6 isoform which is primarily expressed in Schwann cells [39] and restores the sarcolemmal glycoprotein complex [24], but fails to bind actin [24], had mdx concentrations of urinary isoxanthopterin/creatinine. There was also a significant effect of age for mdx, Dpll6-mdx and Scgb−/− mice (p < 0.001 for each genotype).
3.3. Urinary ortho-tyrosine in healthy and dystrophin-deficient humans and mice
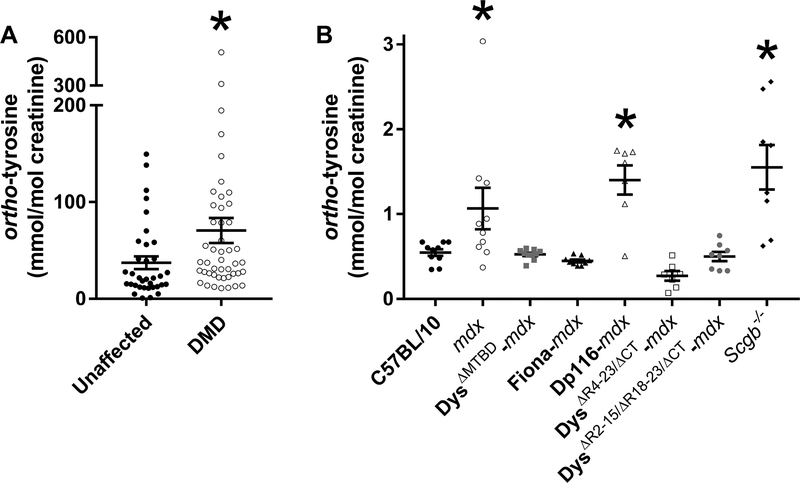
To confirm an elevated level of oxidative stress in DMD patients compared to unaffected controls, we measured urinary ortho-tyrosine which is the hydroxyl-radical-induced oxidized product of tyrosine. Compared to age-matched controls, DMD patients had greater urinary ortho-tyrosine/creatinine (37 ± 7 vs. 71 ± 13 mmol/mol creatinine; Fig. 3A; p < 0.039). Next we compared urinary ortho-tyrosine levels between C57BL/10, mdx and transgenic mice. Urinary ortho-tyrosine/creatinine was restored to C57BL/10 levels in mdx mice that expressed miniaturized or chimeric dystrophins, or utrophin. Dp-116 and Scgb−/− mice were not different than mdx (Fig. 3B; p < 0.001).
Figure 3.
(A) Urinary ortho-tyrosine/creatinine in unaffected controls vs. DMD patients aged 4 −18 y. *different from unaffected. (B) Urinary ortho-tyrosine creatinine in C57BL/10, mdx, a mouse model of limb girdle muscular dystrophy (Scgb−/−) and mdx mice expressing various truncated or chimeric dystrophins or utrophin. *different from C57BL/10, n ≥7/genotype.
3.4. Hyper-activity of xanthine oxidase in mdx skeletal muscle
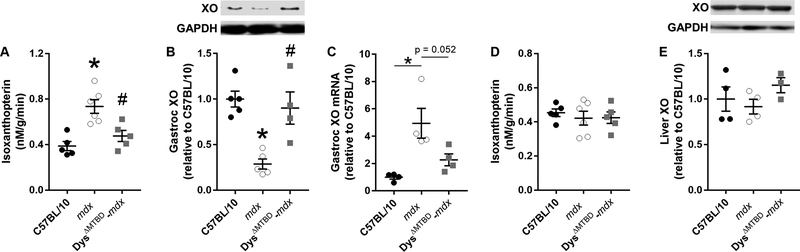
To understand the elevation in urinary isoxanthopterin in mdx mice (Fig. 2), we measured xanthine oxidase activity in gastrocnemius muscles from C57BL/10, mdx and Dys ΔMTBD-mdx mice. Dys ΔMTBD-mdx mice were chosen as the comparative mouse model because it is rescued for all known skeletal muscle phenotypes [23] and urinary isoxanthopterin concentrations are not different from C57BL/10 (Fig. 2). Following administration of pterin to the muscle lysate, mdx gastrocnemius muscle generated greater isoxanthopterin concentrations/g protein than C57BL/10 (p = 0.001), while DysΔMTBD-mcix mice did not differ from C57BL/10 but lowered concentrations compared to mdx (Fig. 4A; p = 0.009).
Figure 4.
(A) Isoxanthopterin concentrations in gastrocnemius muscle lysates following a 1 h incubation with 100 μM pterin. (B) Gastrocnemius xanthine oxidase protein and (C) gene expression and (D) liver xanthine oxidase protein levels and (E) isoxanthopterin concentrations in liver lysates following a 1 h incubation with 100 μM pterin in C57BL/10, mdx and Dys ΔMTBD-mdx mice; *different from C57BL/10, # different from mdx, n ≥3/group. XO: xanthine oxidase.
Because xanthine oxidase activity was elevated in the mdx mouse (Fig. 4A), we measured xanthine oxidase protein levels in skeletal muscle and liver from C57BL10, mdx and Dys ΔMTBD-mdx mice. Contrary to our hypothesis, xanthine oxidase protein levels in skeletal muscle of mdx mice were 71% lower than C57BL/10 (Fig. 4B). Dys ΔMTBD-mdx mice which are rescued for all other skeletal muscle phenotypes [23] normalized xanthine oxidase concentrations to C57BL/10 levels (Fig. 4B). To identify whether gastrocnemius muscle protein levels were affected by post-transcriptional change, we measured mRNA expression of xanthine oxidase in the same mouse strains. We measured a 4.9-fold increase in xanthine oxidase mRNA content in mdx compared to C57BL/10 that was normalized to C57BL/10 levels in Dys ΔMTBD-mc/x mice (Fig. 4C). We then measured liver xanthine oxidase activity to try and explain why systemic isoxanthopterin measured in the urine was elevated in mdx mice because xanthine oxidase is most abundantly expressed in the liver [40]. We measured no difference in xanthine oxidase activity in the liver between C57BL/10, mdx and Dys ΔMTBD-mdx (Fig. 4D), nor did we measure any difference in xanthine oxidase protein content (Fig. 4E).
3.5. Inhibition of xanthine oxidase attenuates exercise-induced isoxanthopterin increases in mdx mice
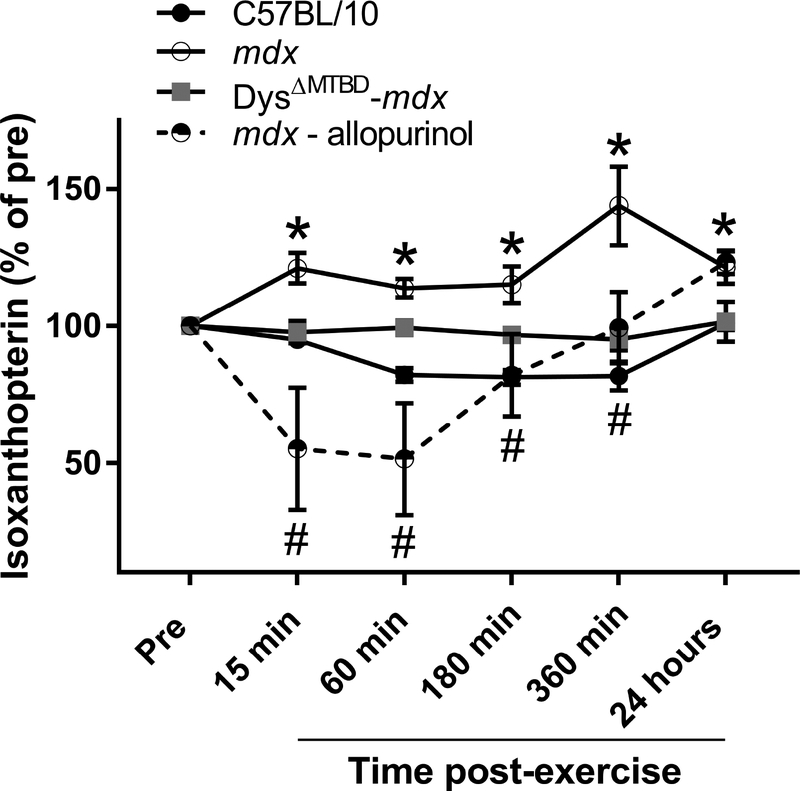
DMD patients presented with elevated urinary isoxanthopterin following the 6MWT (Fig. 1F). To determine if a similar effect occurred in mdx mice, we subjected C57BL/10, mdx and Dys ΔMTBD-mdx mice to treadmill exercise. Treadmill exercise did not cause any change in urinary isoxanthopterin/creatinine in C57BL/10 mice (Fig. 5). In the mdx mouse, we measured an immediate increase following exercise, which remained elevated up to 24 h post. As expected, Dys ΔMTBD-mdx mice were normalized to the C57BL/10 response. To confirm whether the increase in isoxanthopterin was directly associated with xanthine oxidase activity, we administered the XO inhibitor allopurinol to mdx mice 1 h before exercise. In response, urinary isoxanthopterin of mdx mice dramatically decreased immediately following exercise (to 51% of Pre) which remained depressed until 6 hours post exercise.
Figure 5.
Urinary isoxanthopterin following forced downhill treadmill exercise in C57BL/10, mdx with or without allopurinol, and Dys ΔMTBD-mdx mice; * different from C57BL/10, # different from mdx, n ≥4/group.
3.6. Inhibition of xanthine oxidase protects mdx skeletal muscle from eccentric contraction-induced force loss in vitro
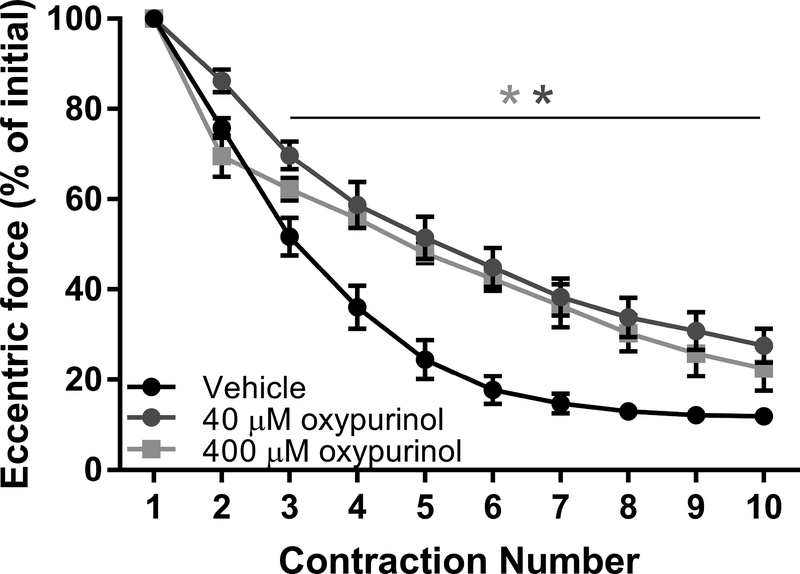
Dystrophic skeletal muscle is extremely susceptible to eccentric contraction-induced force loss [41]. There is also evidence that skeletal muscle contractions induce xanthine oxidase superoxide production that is attenuated with the allopurinol metabolite, oxypurinol [19]. To test the hypothesis that eccentric contraction-induced force loss in dystrophin-deficient skeletal muscle is affected by xanthine oxidasegenerated superoxide, we completed in vitro eccentric contractions in the mdx mouse. We completed eccentric contractions only in mdx mice because extensor digitorum longus (EDL) muscle from C57BI/10 mice is insensitive to force loss in our assay [20,37], Force production in isolated EDL muscles was not affected by 40 or 400 μM oxypurinol as measured by peak twitch and tetanic forces (Table. 1; p > 0.426). During the eccentric contraction protocol, EDL muscle lost 75% eccentric force by the 5th contraction (Fig. 6). In the presence of 40 or 400 μM oxypurinol, EDL muscle lost less eccentric force (48 and 52%, respectively; p < 0.001) and preserved some of the post-ECC protocol isometric force at 40 μM only (Table. 1; p = 0.036).
Table 1.
Physiological parameters of isolated EDL muscles used in ex vivo force measurements.
| Parameter | Control | Oxypurinol (μM) | p value | |
|---|---|---|---|---|
| 40 | 400 | |||
| Mouse mass (g) | 32.0 ± 1.3 | 31.9 ± 1.0 | 32.0 ± 1.0 | 0.997 |
| EDL mass (mg) | 15.9 ± 1.0 | 15.1 ± 0.5 | 14.3 ± 0.8 | 0.411 |
| Lo (mm) | 13.7 ± 0.2 | 13.2 ± 0.1 | 13.4 ± 0.2 | 0.166 |
| CSA (mm2) | 2.5 ± 0.1 | 2.4 ± 0.1 | 2.3 ± 0.1 | 0.389 |
| Peak twitch (mN) | 139 ± 8.5 | 137 ± 5.8 | 129 ± 11 | 0.701 |
| Po (mN) | 484 ± 12 | 451 ± 21 | 442 ± 36 | 0.426 |
| Specific Po (N/cm2) | 19.8 ± 1.4 | 18.6 ± 1.1 | 19.1 ± 0.7 | 0.760 |
| ΔPo (%) | 88.6 ± 1.0 | 74.9 ± 3.9* | 80.9 ± 4.7 | 0.036 |
| Eccentric force loss (%) | 88.1 ± 1.5 | 72.4 ± 3.8* | 77.6 ± 5.0 | 0.020 |
Values are mean ± SE.
Lo = optimal muscle length, CSA = physiological cross sectional area, Po = maximal isometric tetanic force. ΔPo = change in Po after eccentric contractions. n ≥ 5 for each line. Statistics were performed using unpaired t-test analyses with p < 0.05 considered significant.
different from control.
Figure 6.
Eccentric force tracings of mdx EDL muscle in vitro during eccentric contractions with or without xanthine oxidase inhibition. *different from vehicle, n ≥ 5/group.
4. Discussion
The current study was designed to assess the contribution of xanthine oxidase-derived ROS in DMD pathology. We focused on both patients and mice null for dystrophin and measured urinary isoxanthopterin at rest and in response to exercise. We also determined whether skeletal muscle-specific dystrophin or utrophin expression restored urinary isoxanthopterin concentrations in mdx mice. DMD patients had elevated urinary isoxanthopterin that was higher in non-ambulant patients and which increased in response to the 6MWT. Similarly, a DMD mouse model (mdx) had elevated isoxanthopterin, which increased further in response to treadmill exercise. Inhibition of xanthine oxidase prevented the exercise-induced increase in isoxanthopterin and attenuated eccentric contraction-induced force drop in vitro, while transgenic expression of miniaturized dystrophins, chimeric dystrophin and utrophin normalized urinary isoxanthopterin concentrations. We found that the difference in isoxanthopterin between healthy and mdx mice was associated not with xanthine oxidase protein content, but by the activity of the enzyme in skeletal muscle. Together the data suggests hyper-activation of xanthine oxidase in skeletal muscle of mdx mice which contributes to exercise-induced perturbations which can be mitigated by skeletal muscle specific dystrophin expression.
Oxidative stress is known to contribute to the pathophysiology of DMD [1,42]. Our data now suggests xanthine oxidase has increased activity in the skeletal muscle of the mdx mouse, corroborating elevated activity in dystrophic cardiomyocytes [43], and which contributes systemically to ROS-generation as indicated by elevated urinary isoxanthopterin in both patients and mice null for dystrophin. We further confirm the presence of oxidative stress in dystrophin null mice and humans by elevated urinary ortho-tyrosine which is produced through oxidation of tyrosine by hydroxyl radicals. While xanthine oxidase-derived ROS production seems to be due to skeletal muscle protein activity rather than protein content, the increased activity can be further amplified in response to a stress stimulus such as exercise. Interestingly, gastrocnemius muscle xanthine oxidase mRNA content was 5-fold higher in mdx than C57BL/10 yet the protein content was 4-fold lower. Evidence has shown that inflammatory and hypoxic conditions like that measured in DMD [14,44] can stimulate marked increases in xanthine oxidase mRNA and activity [45], thus potentially explaining the elevation in mdx mouse xanthine oxidase mRNA. It is therefore intriguing that the ischemic state and aberrant calcium perturbations associated with DMD [13,14], which are prominent in the conversion of xanthine dehydrogenase to xanthine oxidase [12], would not elevate xanthine oxidase protein levels above C57BL/10 - perhaps because of post-transcriptional regulation of xanthine oxidase in the mdx environment or perhaps the lack of correlations between mRNA and protein content [46]. Regardless, our data suggests dystrophin-deficiency increases activity of xanthine oxidase in mdx skeletal muscle, thus contributing to the high oxidative environment of dystrophin-deficient tissue [1].
Under ischemic conditions and aberrant calcium regulation, XDH is converted to xanthine oxidase [9,12] which generates superoxide. Superoxide accelerates oxidative modification of essential biomolecules including DNA [47], Because dystrophin-deficiency causes greater urinary isoxanthopterin and mdx skeletal muscle has increased xanthine oxidase activity which is exacerbated by exercise, inhibition of xanthine oxidase could provide a new avenue of therapeutic investigation. However, long term administration of allopurinol to DMD patients has been conducted with equivocal results. While some research shows no effect on functional impairment with allopurinol treatment [48–51], other clinical studies have found improvements in strength [52], muscular abilities according to the Zellwager and Harson clinical scale [53] and purine metabolism [54], In contrast to studies in DMD patients, minimal research has been conducted to assess effects of allopurinol in the mdx mouse model of DMD [55], possibly because the mdx mouse had not been identified until after the equivocal clinical trials had been completed [34]. Interestingly, chronic administration of allopurinol can preserve cardiac function in a mouse model of cardiomyopathy [56]. Therefore, because DMD patients die from cardiac and respiratory failure and certain DMD mouse models suffer from cardiomyopathy [57], there is justification for examining the activity and inhibition of xanthine oxidase in DMD as a potential therapeutic treatment.
Eccentric contraction-induced force drop of dystrophic skeletal muscle is a robust phenotype impacted by cellular oxidative status [20,58]. There is also evidence that isometric contractions of healthy muscle generates superoxide by xanthine oxidase [19]. By inhibiting xanthine oxidase, we show that eccentric contraction-induced force drop can be attenuated in mdx skeletal muscle in vitro. Our data corroborates the role of ROS in eccentric force drop and now offers a new mechanism by which force drop is affected.
Recently neopterin and 7,8-dihydroneopterin were measured in DMD patients and found to be elevated above unaffected controls [20]. Although both neopterin and 7,8-dihydroneopterin were elevated, these metabolites did not correlate with age and only 7,8-dihydroneopterin was greater in non-ambulatory compared to ambulatory patients. Here we measured a greater concentration of urinary isoxanthopterin/creatinine in DMD patients, which increased in response to the 6MWT, and was greater in non-ambulatory than ambulatory patients. Because urinary isoxanthopterin is measureable by HPLC and can be collected by non-invasive and stress-free methods, isoxanthopterin may offer an alternative sensitive pterin biomarker for DMD that is capable of monitoring specific perturbations in DMD patients independent of BMD patients.
In summary, our data indicate that patients and mice deficient for the protein dystrophin have elevated urinary isoxanthopterin concentrations. Isoxanthopterin concentrations appear to be dependent on the stability of the DGC which may impact the activity of the xanthine oxidase enzyme in skeletal muscle and thus the level of oxidative stress in DMD patients. Encouragingly, skeletal muscle-specific dystrophin expression restores xanthine oxidase perturbations in mdx mice while inhibition prevents further stress-induced activation.
6. Acknowledgements
This work was supported by the Muscular Dystrophy Association (349549), a University of Minnesota AHC Seed Grant, gifts from John and Cheryl Gunvalson and the Gregory Marzolf Jr. Foundation, and the National Institutes of Health (ROI AR042423 and ROI AR049899).
Abbreviations:
- 6MWT
six minute walk test
- BMD
Becker muscular dystrophy
- DMD
Duchenne muscular dystrophy
- ECM
extracellular matrix
- EDL
extensor digitorum longus
- HPLC
high performance liquid chromatography
- mdx
DMD mouse model
- ROS
reactive nitrogen species
- scgb−/−
ß-sarcoglycan knock out mouse and limb girdle muscular dystrophy model
- XDH
xanthine dehydrogenase
- XO
xanthine oxidase
Footnotes
Competing Interests
All authors have no financial or personal conflict with other people or organizations that could inappropriately influence our work.
7. References
- [1].Tidball JG, Wehling-Henricks M, The role of free radicals in the pathophysiology of muscular dystrophy, J. Appl. Physiol 102 (2007) 1677–1686. [DOI] [PubMed] [Google Scholar]
- [2].Disatnik M-H, Dhawan J, Yu Y, Beal MF, Whirl MM, Franco AA, Rando TA, Evidence of oxidative stress in mdx mouse muscle: studies of the pre-necrotic state, J. Neurol. Sei 161 (1998) 77–84. [DOI] [PubMed] [Google Scholar]
- [3].Haycock JW, MacNeil S, Jones P, Harris JB, Mantle D, Oxidative damage to muscle protein in Duchenne muscular dystrophy., Neuroreport 8 (1996) 357–361. [DOI] [PubMed] [Google Scholar]
- [4].Rando TA, Disatnik M-H, Yu Y, Franco A, Muscle cells from mdx mice have an increased susceptibility to oxidative stress, Neuromuscul. Disord 8 (1998) 14–21. [DOI] [PubMed] [Google Scholar]
- [5].Hoffman EP, Brown RH, Kunkel LM, Dystrophin: the protein product of the Duchenne muscular dystrophy locus, Cell 51 (1987) 919–928. [DOI] [PubMed] [Google Scholar]
- [6].Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG, Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy, Hum. Mol. Genet 18 (2009) 482–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Whitehead NP, Yeung EW, Froehner SC, Allen DG, Skeletal muscle NADPH oxidase is increased and triggers stretch-induced damage in the mdx mouse, PLoS One 5 (2010) el5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Percival JM, Siegel MP, Knowels G, Marcinek DJ, Defects in mitochondrial localization and ATP synthesis in the mdx mouse model of Duchenne muscular dystrophy are not alleviated by PDE5 inhibition, Hum. Mol. Genet 22 (2013) 153–167. doi: 10.1093/hmg/dds415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Baker MS, Austin L, The pathological damage in Duchenne muscular dystrophy may be due to increased intracellular OXY-radical generation caused by the absence of dystrophin and subsequent alterations in Ca2+ metabolism., Med. Hypotheses 29 (1989) 187–93. doi: 10.1016/0306-9877(89)90193-X. [DOI] [PubMed] [Google Scholar]
- [10].Schaffer SW, Roy RS, McMcord JM, Possible role for calmodulin in calcium paradox-induced heart failure, Eur. Heart J 4 (1983) 81–87. doi: 10.1093/eurheartj/4.suppl_H.81. [DOI] [PubMed] [Google Scholar]
- [11].Corte ED, Stirpe F, The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme., Biochem. J 126 (1972) 739–45. doi: 10.1042/BJ1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Engerson TD, McKelvey TG, Rhyne DB, Boggio EB, Snyder SJ, Jones HP, Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues., J. Clin. Invest 79 (1987) 1564–70. doi: 10.1172/JCI112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS, Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy, Cell 82 (1995)743–752. [DOI] [PubMed] [Google Scholar]
- [14].Sander M, Chavoshan B, Harris SA, lannaccone ST, Stull JT, Thomas GD, Victor RG, Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy, Proc. Natl. Acad. Sei 97 (2000) 13818–13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ma Y, Burton C, Pteridine detection in urine: the future of cancer diagnostics?, Biomark. Med 7 (2013) 679–681. doi: 10.2217/bmm.13.88. [DOI] [PubMed] [Google Scholar]
- [16].Burton C, Shi H, Ma Y, Normalization of urinary pteridines by urine specific gravity for early cancer detection, Clin. Chim. Acta 435 (2014) 42–47. doi: 10.1016/J.CCA.2014.04.022. [DOI] [PubMed] [Google Scholar]
- [17].Gamagedara S, Gibbons S, Ma Y, Investigation of urinary pteridine levels as potential biomarkers for noninvasive diagnosis of cancer, Clin. Chim. Acta 412 (2011) 120–128. [DOI] [PubMed] [Google Scholar]
- [18].Košlmski P, Jarzemski P, Markuszewski MJ, Kaliszan R, Determination of pterins in urine by HPLC with UV and fluorescent detection using different types of chromatographic stationary phases (HILIC, RP C8, RP C18), J. Pharm. Biomed. Anal 91 (2014) 37–45. doi: 10.1016/J.JPBA.2013.12.012. [DOI] [PubMed] [Google Scholar]
- [19].Gomez-Cabrera MC, Close GL, Kayani A, McArdle A, Vina J, MJ. Jackson, Effect of xanthine oxidase-generated extracellular superoxide on skeletal muscle force generation, Am. J. Physiol. Integr. Comp. Physiol 298 (2010) R2–R8. doi: 10.1152/ajpregu.00142.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lindsay A, Schmiechen A, Chamberlain CM, Ervasti JM, Lowe DA, Neopterin/7,8-dihydroneopterin is elevated in Duchenne muscular dystrophy patients and protects mdx skeletal muscle function, Exp. Physiol (2018). doi: 10.1113/EP087031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Belanto JJ, Mader TL, Eckhoff MD, Strandjord DM, Banks GB, Gardner MK, Lowe DA, Ervasti JM, Microtubule binding distinguishes dystrophin from utrophin., Proc. Natl. Acad. Sci. U. S. A Ill (2014) 5723–8. doi: 10.1073/pnas.1323842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li D, Yue Y, Lai Y, Hakim CH, Duan D, Nitrosative stress elicited by nNOSp delocalization inhibits muscle force in dystrophin-null mice, J. Pathol 223 (2011) 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Belanto JJ, Olthoff JT, Mader TL, Chamberlain CM, Nelson DM, McCourt PM, Talsness DM, Gundersen GG, Lowe DA, Ervasti JM, Independent variability of microtubule perturbations associated with dystrophy in the mdx mouse, Hum. Mol. Genet 25 (2016) 4951–4961. doi: 10.1093/hmg/ddw318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Judge LM, Haraguchiln M, Chamberlain JS, Dissecting the signaling and mechanical functions of the dystrophin-glycoprotein complex., J. Cell Sei 119 (2006) 1537–46. doi: 10.1242/jcs.02857. [DOI] [PubMed] [Google Scholar]
- [25].McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Elfring GL, Atkinson L, Reha A, Hirawat S, Miller LL, The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy, Muscle Nerve 41 (2010) 500–510. doi: 10.1002/mus.21544. [DOI] [PubMed] [Google Scholar]
- [26].Moran AL, Warren GL, Lowe DA, Soleus and EDL muscle contractility across the lifespan of female C57BL/6 mice, Exp. Gerontol 40 (2005) 966–975. [DOI] [PubMed] [Google Scholar]
- [27].Lowe DA, Warren GL, Hayes DA, Farmer MA, Armstrong RB, Eccentric contraction-induced injury of mouse soleus muscle: effect of varying [Ca2+lo, (n.d.) http://jap.physi0l0gy.0rg/c0ntent/jap/76/4/1445.full.pdf (accessed October 12, 2017). [DOI] [PubMed]
- [28].Radley-Crabb H, Terrill J, Shavlakadze T, Tonkin J, Arthur P, Grounds M, A single 30min treadmill exercise session is suitable for “proof-of concept studies” in adult mdx mice: A comparison of the early consequences of two different treadmill protocols, Neuromuscul. Disord 22 (2012) 170–182. [DOI] [PubMed] [Google Scholar]
- [29].Burkholder TJ, Fingado B, Baron S, Lieber RL, Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb, J. Morphol 221 (1994) 177–190. [DOI] [PubMed] [Google Scholar]
- [30].Molnâr GA, Wagner Z, Marko L, Mohâs M, Kocsis B, Matus Z, Wagner L, Tamaskô M, Mazâk I, Laczy B, Urinary ortho-tyrosine excretion in diabetes mellitus and renal failure: evidence for hydroxyl radical production, Kidney Int 68 (2005) 2281–2287. [DOI] [PubMed] [Google Scholar]
- [31].Lindsay A, Janmale T, Draper N, Gieseg SP, Measurement of changes in urinary neopterin and total neopterin in body builders using SCX HPLC, Pteridines 25 (2014) 53–63. [Google Scholar]
- [32].Manzur AY, Kuntzer T, Pike M, Swan AV, Glucocorticoid corticosteroids for Duchenne muscular dystrophy, in: Manzur AY (Ed.), Cochrane Database Syst. Rev, John Wiley & Sons, Ltd, Chichester, UK, 2008. doi: 10.1002/14651858.CD003725.pub3. [DOI] [PubMed] [Google Scholar]
- [33].McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Elfring GL, Atkinson L, Reha A, Hirawat S, Miller LL, The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy, Muscle Nerve 41 (2010) 500–510. [DOI] [PubMed] [Google Scholar]
- [34].Bulfield G, Siller WG, Wight PA, Moore KJ, X chromosome-linked muscular dystrophy (mdx) in the mouse, Proc. Natl. Acad. Sei 81 (1984) 1189–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Araishi K, Sasaoka T, Imamura M, Noguchi S, Hama H, Wakabayashi E, Yoshida M, Hori T, Ozawa E, Loss of the sarcoglycan complex and sarcospan leads to muscular dystrophy in ß-sarcoglycan-deficient mice, Hum. Mol. Genet 8 (1999) 1589–1598. [DOI] [PubMed] [Google Scholar]
- [36].Durbeej M, Cohn RD, Hrstka RF, Moore SA, Allamand V, Davidson BL, Williamson RA, Campbell KP, Disruption of the ß-sarcoglycan gene reveals pathogenetic complexity of limb-girdle muscular dystrophy type 2E, Mol. Cell 5 (2000) 141–151. [DOI] [PubMed] [Google Scholar]
- [37].Nelson DM, Lindsay A, Judge LM, Duan D, Chamberlain JS, Lowe DA, Ervasti JM, Variable rescue of microtubule and physiological phenotypes in mdx muscle expressing different miniaturized dystrophins, Hum. Mol. Genet (2018). doi: 10.1093/hmg/ddyll3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, Judge L, Bostick B, Chamberlain JS, Terjung RL, Duan D, Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy, J. Clin. Invest 119 (2009) 624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Imamura M, Araishi K, Noguchi S, Ozawa E, A sarcoglycan-dystroglycan complex anchors Dpll6 and utrophin in the peripheral nervous system, Hum. Mol. Genet 9 (2000) 3091–3100. doi: 10.1093/hmg/9.20.3091. [DOI] [PubMed] [Google Scholar]
- [40].Linder N, Rapola J, Raivio KO, Cellular expression of xanthine oxidoreductase protein in normal human tissues., Lab. Invest 79 (1999) 967–74. http://www.ncbi.nlm.nih.gov/pubmed/10462034 (accessed May 7, 2018). [PubMed] [Google Scholar]
- [41].Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL, Dystrophin protects the sarcolemma from stresses developed during muscle contraction, Proc. Natl. Acad. Sei 90 (1993) 3710–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Allen DG, Whitehead NP, Froehner SC, Absence of Dystrophin Disrupts Skeletal Muscle Signaling: Roles of Ca2+, Reactive Oxygen Species, and Nitric Oxide in the Development of Muscular Dystrophy, Physiol. Rev 96 (2016) 253–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Afzal MZ, Reiter M, Gastonguay C, McGivern JV, Guan X, Ge Z-D, Mack DL, Childers MK, Ebert AD, Strande JL, Nicorandil, a Nitric Oxide Donor and ATP-Sensitive Potassium Channel Opener, Protects Against Dystrophin-Deficient Cardiomyopathy., J. Cardiovasc. Pharmacol. Ther 21 (2016) 549–562. doi: 10.1177/1074248416636477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR, Leahy P, Li J, Guo W, Andrade FH, A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice, Hum. Mol. Genet 11 (2002) 263–272. [DOI] [PubMed] [Google Scholar]
- [45].Battelli MG, Bolognesi A, Polito L, Pathophysiology of circulating xanthine oxidoreductase: New emerging roles for a multi-tasking enzyme, Biochim. Biophys. Acta - Mol. Basis Dis 1842 (2014) 1502–1517. doi: 10.1016/J.BBADIS.2014.05.022. [DOI] [PubMed] [Google Scholar]
- [46].Liu Y, Beyer A, Aebersold R, On the Dependency of Cellular Protein Levels on mRNA Abundance, Cell 165 (2016) 535–550. doi: 10.1016/j.cell.2016.03.014. [DOI] [PubMed] [Google Scholar]
- [47].Keyer K, Imlay JA, Superoxide accelerates DNA damage by elevating free-iron levels., Proc. Natl. Acad. Sci. U. S. A 93 (1996) 13635–40. doi: 10.1073/PNAS.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Griffiths RD, Cady EB, Edwards RHT, Wilkie DR, Muscle energy metabolism in duchenne dystrophy studied by31P-NMR: Controlled trials show no effect of allopurinol or ribose, Muscle Nerve 8 (1985) 760–767. doi: 10.1002/mus.880080904. [DOI] [PubMed] [Google Scholar]
- [49].Bertorini TE, Palmieri GM, Griffin J, Chesney C, Pifer D, Verling L, Airozo D, Fox IH, Chronic allopurinol and adenine therapy in Duchenne muscular dystrophy: effects on muscle function, nucleotide degradation, and muscle ATP and ADP content., Neurology 35 (1985) 61–5. doi: 10.1212/WNL.35.1.61. [DOI] [PubMed] [Google Scholar]
- [50].Mendell JR, Wiechers DO, Lack of benefit of allopurinol in duchenne dystrophy, Muscle Nerve 2 (1979) 53–56. doi: 10.1002/mus.880020108. [DOI] [PubMed] [Google Scholar]
- [51].Stern LM, Fewings JD, Bretag AH, Ballard FJ, Tomas FM, Cooper DM, Goldblatt E, The progression of Duchenne muscular dystrophy: clinical trial of allopurinol therapy., Neurology 31 (1981) 422–6. doi: 10.1212/WNL.31.4.422. [DOI] [PubMed] [Google Scholar]
- [52].Thomson WH, Smith I, X-linked recessive (Duchenne) muscular dystrophy (DMD) and purine metabolism: effects of oral allopurinol and adenylate., Metabolism 27 (1978) 151–63. doi: 10.1016/0026-0495(78)90161-0. [DOI] [PubMed] [Google Scholar]
- [53].Tamari H, Ohtani Y, Higashi A, Miyoshino S, Matsuda I, Xanthine oxidase inhibitor in duchenne muscular dystrophy, Brain Dev 4 (1982) 137–143. doi: 10.1016/S0387-7604(82)80007-7. [DOI] [PubMed] [Google Scholar]
- [54].Castro-Gago M, Lojo S, Novo I, del Rio R, Pena J, Rodriguez-Segade S, Effects of chronic allopurinol therapy on purine metabolism in Duchenne muscular dystrophy, Biochem. Biophys. Res. Commun 147 (1987) 152–157. doi: 10.1016/S0006-291X(87)80100-6. [DOI] [PubMed] [Google Scholar]
- [55].Shkryl VM, Martins AS, Ullrich ND, Nowycky MC, Niggli E, Shirokova N, Reciprocal amplification of ROS and Ca2+ signals in stressed mdx dystrophic skeletal muscle fibers, Pflügers Arch. J. Physiol 458 (2009) 915–928. [DOI] [PubMed] [Google Scholar]
- [56].Duncan JG, Ravi R, Stull LB, Murphy AM, Chronic xanthine oxidase inhibition prevents myofibrillar protein oxidation and preserves cardiac function in a transgenic mouse modelof cardiomyopathy, 10.1152/ajpheart.00168.2005. (2005). doi:. [DOI] [PubMed] [Google Scholar]
- [57].Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR, Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy, Cell 90 (1997) 729–738. [DOI] [PubMed] [Google Scholar]
- [58].Loehr JA, Stinnett GR, Hernandez-Rivera M, Roten WT, Wilson LJ, Pautler RG, Rodney GG, Eliminating Nox2 reactive oxygen species production protects dystrophic skeletal muscle from pathological calcium influx assessed in vivo by manganese-enhanced magnetic resonance imaging, J. Physiol 594 (2016) 6395–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]