Abstract
BACKGROUND:
Obesity is a mechanical risk factor for osteoarthritis. In individuals with obesity, knee joint pain is prevalent. Weight loss reduces joint loads, and therefore potentially delays disease progression; however, how the knee joint responds to weight loss in individuals with obesity and knee pain is not clear.
RESEARCH QUESTION:
To assess the effect of weight loss on knee joint kinematics during gait in individuals with obesity and knee pain.
METHODS:
We recruited individuals with obesity (BMI ≥ 35) and knee pain who were participating in a weight loss program which included bariatric surgery or medical management. At baseline before and at 1 year after treatment, participants walked on a treadmill, and their knee joint kinematics were assessed using a dual-fluoroscopic imaging system and subject-specific magnetic resonance imaging knee joint models. Gait changes were represented by change in range of tibiofemoral motion, i.e., excursions in flexion-extension, adduction-abduction, internal-external rotation, anterior-posterior translation, medial-lateral translation, and superior-inferior translation during gait.
RESULTS:
Twelve individuals with obesity and knee pain completed the gait analysis at baseline and 1 year follow-up. Participants lost on average 10.4 % (standard deviation: 17.2%) of their baseline body weight. Reduction in body weight was associated with increased range of flexion-extension (r = −0.75, p < 0.01) and decreased range of adduction-abduction (r = 0.60, p = 0.04) during gait. The reduction in body weight was also associated with self-reported pain decrease (r= 0.62, p = 0.04); however, the change in pain was not significantly associated with kinematic changes.
SIGNIFICANCE:
Weight loss was associated with improved gait kinematics in the sagittal and frontal planes. The change in gait pattern in individuals with obesity and knee pain was not associated with the change in pain given a reduction in body weight.
Keywords: weight loss, gait, kinematics, knee pain, fluoroscopic imaging
INTRODUCTION
Obesity is an established risk factor for the development of knee osteoarthritis (OA), one of the most disabling diseases affecting quality of life 1, 2. In the US, more than one-third of adults are obese, and one in three obese adults has arthritis3, 4. In individuals with obesity, knee pain is highly prevalent 5 and is often thought to be the first symptom of knee OA 6. For patients with knee OA who are overweight or obese, weight loss is recommended by the American College of Rheumatology (ACR) to reduce joint loads and potentially delay knee OA progression7–12. Weight loss is effective in reducing pain and improving function in patients with obesity and knee OA11, 13–15, and in patients with obesity alone5, 16–18.
Walking is an important function of daily living and is suggested by the Arthritis Foundation and the ACR for knee OA patients to promote healthy living 7, 19, 20. This safe exercise is also promoted for individuals with obesity to increase energy expenditure to manage body weight 21, 22. However, reduced daily walking steps 23 and gait alterations were found in individuals with obesity when compared to healthy counterparts 24–26. In individuals with obesity and knee pain, gait function is further impaired 23, 27. While some biomechanical studies have demonstrated that weight loss improves spatiotemporal parameters and kinematics during gait in individuals with obesity 28–31, it is still not clear how weight loss affects knee kinematics of individuals with obesity and knee pain and whether any change in kinematics is more closely related to pain reduction than to weight loss. If there were a relationship between change in kinematics and knee pain after weight loss, then it might suggest that the gait alterations seen represent compensatory gait changes to lessen pain or vice versa.
The instrumentation (i.e., motion capture system) used in kinematic studies may threaten the accuracy of the measured parameters due to the uncertainty of marker placement when the subcutaneous tissue has significantly changed after weight loss 31. In the past decade, new instrumentation has been developed to measure the kinematics of the knee with higher accuracy and repeatability32, 33. This technique uses dynamic X-ray/fluoroscopic imaging to track bone motions directly, instead of capturing the markers on the skin, and then registers the subject-specific magnetic resonance imaging/computed tomography bone models to measure knee kinematics. This technology eliminates the artifacts created by subcutaneous tissue and improves the repeatability of marker placement in 3-dimensional (3D) motion capture systems after weight loss 32, 33.
We applied dual fluoroscopic imaging herein to assess the effect of body weight change on knee kinematics during gait in individuals with obesity and knee pain who were undergoing either bariatric surgery or medical weight loss treatment. We expected a wide variation in weight loss experiences with some participants losing a lot of weight and others experiencing less loss. We focused on the correlation of weight loss, knee pain, and knee kinematics, trying to determine, using this novel technology, what kinematic changes occurred and whether kinematic changes were more strongly correlated with weight loss or pain reduction in a setting in which both were likely to occur. We hypothesized that change in gait kinematics would be associated with the amount of weight loss and change in knee pain.
METHODS
Participants
Participants were recruited from The Nutrition and Weight Management Program at Boston Medical Center. The baseline recruitment period was from February 2014 to August 2015. Inclusion criteria consisted of BMI ≥ 35, knee pain, aching or stiffness on most of the past 30 days, age between 25-60 years old, ability to walk without any assistance, and eligibility for magnetic resonance (MR) imaging. For participants scheduled for surgery, baseline was within 2 weeks prior to surgery. Exclusion criteria included rheumatoid or inflammatory arthritis, and any prior knee surgeries or plan to receive knee surgery during the follow-up period. The study protocol was approved by Boston University School of Medicine Institutional Review Board, and each participant signed a consent form before the study procedure.
To promote weight loss, participants had bariatric surgery or dietary prescriptions (with or without a combination of medications including phentermine, lorcaserin, phentermine/topiramate, bupropion/naltrexone, or liraglutide). The dietary prescription was designed to control the total energy intake (1200-1500 kilocalories/day for women and 1500-1800 kilocalories/day for men) using a high-protein, low-fat diet with meal replacements as substitute meals. All participants were recommended to walk at least 30 minutes/day and perform resistance exercise at least twice a week.
Experimental procedures
Overall knee pain status was evaluated by a self-reported visual analog scale (VAS) rated on a 0-100 scale with the extremes anchored in 0 (no pain) and 100 (worst imaginable pain). We used a 3-Tesla MR machine (Philips, Achieva, Eindhoven, The Netherlands) with a 16-channel knee coil to acquire high resolution images of the knee (sequence: Proton Density-Weighted (PDW), Spectral Attenuated Inversion Recovery (SPAIR) sequence, FOV: 160mm × 160mm, TR = 1800ms, TE = 30ms, flip angle = 90°, thickness = 1mm, in-plane resolution = 512 × 512). All the MR images were reviewed and manually segmented to construct the 3D subject-specific knee joint models. Participants were asked to walk on a treadmill at 1.5 mph (0.67 m/s) and knee joint motion was captured using a validated dual fluoroscopic imaging system (Philips, BV Pulsera, Eindhoven, The Netherlands) (Fig 1A). This system captured knee motion at 30 frames per second 32.
Fig 1.
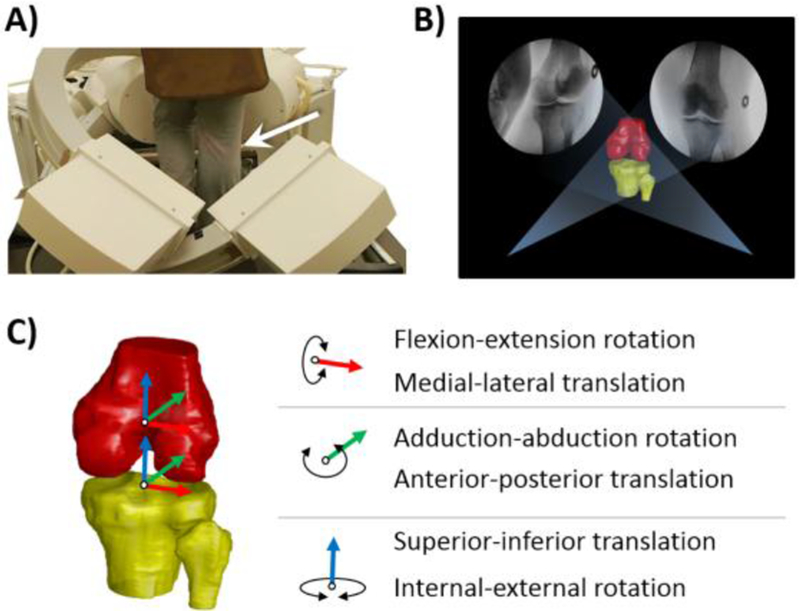
A) knee joint motion captured using a dual fluoroscopic imaging system ; B) two-dimensional (2D) to 3D registration procedure of the subject-specific bone models and the fluoroscopic images; C) knee kinematics in six degree of freedom (6DOF), i.e., flexion-extension, adduction-abduction, internal-external rotation, anterior-posterior translation, medial-lateral translation, and superior-inferior translation.
To determine the position of each bone at each time frame, we created a virtual environment for the two-dimensional (2D) to 3D registration procedure of the subject-specific bone models and the fluoroscopic images (Fig 1B)32. Once the projection of the 3D knee model was matched to the 2D silhouette of the corresponding bones in the fluoroscopic images, knee kinematics in six degree of freedom (6DOF), i.e., flexion-extension, adduction-abduction, internal-external rotation, anterior-posterior translation, medial-lateral translation, and superior-inferior translation, were derived based on the coordinate systems of the tibia and femur (Fig 1C). The details of the kinematic calculations were described in our previous article27.
The participants returned for a follow-up visit 1 year after the baseline visit. We used the same protocol from the baseline visit to evaluate pain status and measure knee kinematics during gait at the follow-up visit. We used the 6DOF range of knee motion (excursion), defined as the maximum minus the minimum values during the stance phase of the gait cycle, to represent the knee kinematics for each visit. We also calculated the changes in spatiotemporal parameters of interests, including stride length, duration of the stance phase, and cadence, to present change in gait characteristics between the two visits.
Statistical Analysis
A paired t-test was used to determine the difference of each variable between the baseline and follow-up visits. Although we technically had no cases and controls, we included both individuals who received bariatric surgery and individuals who received medical management to capture a range of weight loss in our participants (so that some would have more weight loss and others have less weight loss) 34, and we anticipated that this range would allow us to examine the correlations of change in pain and of weight with gait parameters. Pearson’s correlation analyses were performed to assess the relationship between the change in range of motion in 6DOF during the stance phase of the gait cycle, change in weight, and change in pain. A two sided significance level was 0.05 for all analyses.
RESULTS
Eighteen participants (15 females and 3 males) completed the baseline visit, of whom 12 (67%) completed the follow-up visit. The participants lost, on average, 10.4% of their baseline body weight (P = 0.05) at follow-up (Table 1). Of the 12 participants with follow-up, 4 had undergone bariatric surgery (mean (standard deviation) weight loss 29.6 (10.9) % of their baseline body weight) and 8 had medical management (mean (standard deviation) weight loss 0.8 (9.9) % of their baseline body weight). On average, knee pain at the follow-up visit was significantly lower than that at the baseline visit (P = 0.04).
Table 1.
Subject characteristics at baseline and follow-up
| Baseline | Follow-up | P value | |
|---|---|---|---|
| Women (%) | 75% | – | |
| Age (years) | 45.4 (8.8) | 46.5 (8.9) | |
| Mean Body Weight (kg) | 106.0 (12.2) | 95.3 (22.2) | 0.05 |
| Mean Body Weight change (%)a | −10.4 (17.2) | ||
| Mean BMI (km/m2) | 39.0 (2.9) | 34.8 (6.7) | 0.05 |
| Mean VAS Pain (0-100mm)b | 64.8 (17.3) | 42.0 (31.9) | 0.04 |
| VAS Pain change (%)a | −32.5 (54.8) | ||
| Mean WOMAC pain (0-20) | 10.0 (4.4) | 8.1 (7.3) | 0.19 |
| Knee OA Kellgren and Lawrence grade | 1.2 (0.9) | N/A | |
| Bariatric surgery/medical management (n/n) | 4/8 | – | |
| Mean Body Weight change (%,Bariatric surgery/medical management)a | −29.6(10.9)/−0.8 (9.9) |
percentage change was calculated as [(follow-up - baseline)/baseline]
VAS stands for visual analog scale
In terms of knee joint kinematics, the range of flexion-extension at the follow-up visit was significantly increased compared with the baseline visit (see appendix for 6DOF kinematics). Significant differences between the two visits were also found in internal-external rotation and superior-inferior translation (Table 2). As for the spatiotemporal parameters, the changes in stride length, duration of the stance phase, and cadence between the two visits were 0.5 (11.1) cm, 0.00 (0.10) second, and 0.7 (14.7) steps/min, respectively. None of these changes was statistically significant.
Table 2.
6DOF range of motion during gait at baseline and follow-up
| Range of motion | Baseline | Follow-up | Change | P-value |
|---|---|---|---|---|
| Flexion-extension | 33.8 (5.6) | 39.5 (8.0) | 5.7 (7.4) | 0.02 |
| Adduction-abduction | 2.7 (1.4) | 3.2 (1.4) | 0.5 (1.4) | 0.27 |
| Internal-external rotation | 7.4 (3.1) | 9.1 (3.8) | 1.7 (2.6) | 0.04 |
| Medial-lateral translation | 3.0 (1.2) | 2.9 (1.0) | −0.1 (1.2) | 0.79 |
| Anterior-posterior translation | 6.8 (2.2) | 5.8 (2.1) | −1.0 (3.0) | 0.26 |
| Superior-inferior translation | 1.9 (0.7) | 2.6 (1.0) | 0.8 (0.9) | 0.01 |
The percentage change in body weight was associated with the change in range of motion in flexion-extension (r = −0.75, p = 0.005) and in adduction-abduction (r = 0.60, p = 0.04) during gait (Table 3) such that the greater the weight loss, the greater the increase in both flexion-extension and decrease in adduction-abduction rotation excursion. The percentage change in body weight was associated with the reduction in self-reported VAS pain (r= 0.62, P = 0.04); however, the change in self-reported pain was not significantly associated with any changes in kinematics (Table 3).
Table 3.
The relationship between weight loss (% body weight change), the change in range of motion during gait, and the change in joint pain.
| Change in motion | Correlation coefficient body weight change (%) vs. Motion | P value | Correlation coefficient VAS pain change (%) vs. Motion | P value |
|---|---|---|---|---|
| Flexion-extension | −0.75 | 0.005 | −0.19 | 0.59 |
| Adduction-abduction | 0.60 | 0.04 | 0.36 | 0.28 |
| Internal-external rotation | 0.25 | 0.43 | −0.42 | 0.20 |
| Medial-lateral translation | 0.49 | 0.11 | 0.17 | 0.62 |
| Anterior-posterior translation | −0.08 | 0.79 | 0.20 | 0.55 |
| Superior-inferior translation | 0.02 | 0.94 | 0.25 | 0.46 |
All measurements were calculated as [follow-up - baseline]
DISCUSSION
We used a novel approach to accurately measure knee joint kinematics during gait before and after 1 year for individuals with obesity and knee pain in a weight loss program. We also tested the association between the changes in gait pattern, body weight, and pain. In general, the participants with weight loss walked with a greater range of flexion-extension and internal-external rotation of the knee joint. Some of the kinematic changes were found to be associated with the change in body weight, but were not associated with change in knee pain severity. To the best of our knowledge, this study is the first to investigate the 6DOF knee kinematics undergoing weight loss.
Our participants demonstrated an increased range of knee flexion-extension during the stance phase of the gait cycle after weight loss, suggesting that weight loss can effectively modify knee kinematics. However, our finding is not consistent with some studies showing that weight loss has no significant effect on knee kinematics during walking 28–31. For example, Hortobagyi et al. 30 and Vartiainen et al. 31 reported that the knee motion in the sagittal plane (i.e., flexion-extension) in stance phase was not significantly changed after weight loss when walking at a standardized speed. One possible explanation for why our results differ is that the instrumentation used in previous studies was prone to soft tissue artefacts. Instead, our study improved the measurement accuracy by using fluoroscopy technology. Another factor could be the pain status; participants analyzed in our study had pain that was rated as being more severe than individuals analyzed in previous studies 30–31.
Transverse plane motion, i.e., axial rotation, is thought to be a key component in OA development; however, this kinematic outcome is rarely reported in persons with obesity 35, 36. Our participants demonstrated an increased range of internal-external rotation that could be interpreted as an improvement in motion in the transverse plane, as individuals with obesity and knee pain have been shown to have smaller range of internal-external rotation compared to a healthy group 27. In addition, the combined effect of an increased range of motion in flexion-extension and internal-external rotation after weight loss may allow for greater distribution of stress at the knee joint during the stance phase of the gait cycle 37, 38. Although we found that the mean changes in spatiotemporal parameters between the two visits were minimal, the large variation in stride length and cadence suggests that a within-participant difference exists after weight loss. The same participant may adapt to a different stride length and cadence while walking after weight loss.
Our results demonstrated a negative association between the change in range of flexion-extension and the change in body weight, indicating that those participants who experienced more weight loss had more kinematic change than those who did not lose weight. In addition, we found a positive association between the decrease in range of adduction-abduction and weight loss, suggesting stability of the knee may change in the frontal plane after weight loss, as more body sway in the frontal plane is reported in individuals with obesity 26. This may reduce stresses on the knee through more even distribution of loads between the medial and lateral compartments.
Our results also showed a correlation between the reduction in self-report VAS knee pain after weight loss and change in body weight. This is in agreement with previous studies 5, 17, 39, 40, 41. Current evidence on pain reduction by weight loss is mostly through bariatric surgery. Vincent et al. 17 showed that knee pain and back pain were rapidly relieved by nearly one-third and more than one-half, respectively, of their pre-surgical pain with ~15% body weight loss in the first three months after surgery. Similarly, Abu-abeid et al. 41 reported that knee pain in patients with obesity was significantly reduced after weight loss of 6.2 BMI units.
Our findings have certain implications for knee OA in individuals with obesity. Based on the three variables in our study, i.e., body weight, knee kinematics, and pain (Fig 2), we found that, for those who lost greater than ~10% of their baseline body weight, the kinematics and pain both changed. Such combined effect of reduced total amount of loads, altered kinematics and pain may enhance the mechanical environment of the knee joint37. On the contrary, kinematic and pain changes in those who had consistent body weight did not show a clear tendency for change in kinematics or knee pain. This implies weight loss has both biomechanical and analgesic effects.
Fig 2.
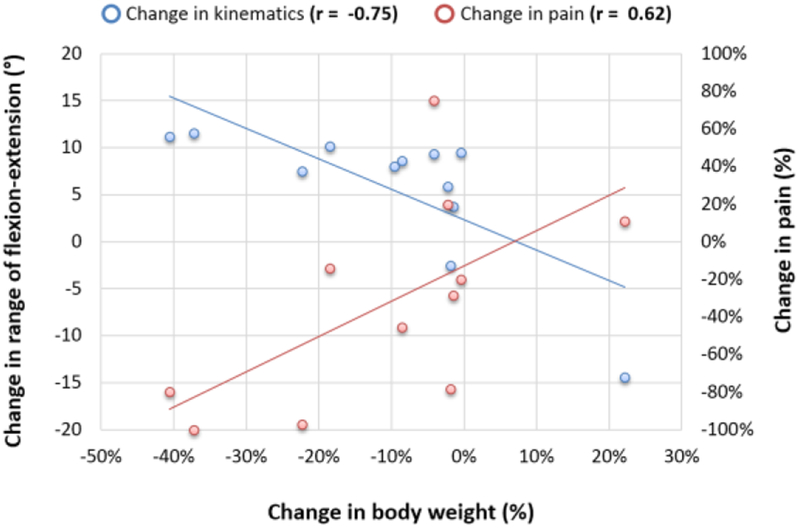
Illustration of distribution of change in body weight and corresponded body weight and pain changes. Change in each variable is calculated as [follow-up - baseline]
Among limitations of our study, the sample was small with higher ratio of females to males. This limited us in performing analysis with adjustment for potential confounders. In addition, we could not randomize participants to surgery. As expected, we found that patients with medical management did not lose much weight nor experience much reduction in pain, and we took advantage of this to create a sample with a broad range of experiences. This broad range made it possible for us to examine correlations of weight loss and pain reduction with change in gait. Our results may not generalize to all persons with obesity, as the BMI in our participants was ≥ 35 at baseline. We did not measure muscular activities using electromyography and ground reaction force data, so the kinematic data could not be further processed to calculate joint moments and estimate the joint force. The fluoroscopy imaging system in this study is designed for treadmill gait and the gait kinematics may not generalize to overground conditions. Despite these limitations, our study has some strengths. We used a technology that significantly improves kinematic measurement error by subcutaneous adipose tissue in obese individuals to track knee motion in a longitudinal study. The high precision in measurement allows us to assess the knee kinematics in 6DOF for individuals with obesity.
CONCLUSION
Weight loss was associated with improved gait kinematics in the sagittal and frontal planes. The change in gait pattern in individuals with obesity and knee pain was not associated with the change in pain. Therefore, weight loss should be addressed in individuals with obesity and knee pain to improve both gait pattern and knee pain.
Supplementary Material
Highlights.
Weight loss improves knee kinematics during gait in the sagittal and frontal plane
Weight loss improves knee pain
The kinematic change after weight loss was not associated with change in pain
ACKNOWLEDGEMENTS
The authors would like to thank the support from National Institutes of Health (P60 AR47785 and AR063235).
ROLE OF THE FUNDING SOURCE
This work was supported by the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41(8):1343–1355. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT. Does excess weight cause osteoarthritis and, if so, why? Ann Rheum Dis. 1996;55(9):668–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour KE, Helmick CG, Theis KA, Murphy LB, Hootman JM, Brady TJ, Cheng YJ. Prevalence of Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation — United States, 2010–2012. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6244a1.htm.
- 4.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooper MM, Stellato TA, Hallowell PT, Seitz BA, Moskowitz RW. Musculoskeletal findings in obese subjects before and after weight loss following bariatric surgery. Int J Obes (Lond). 2007;31(1):114–120. [DOI] [PubMed] [Google Scholar]
- 6.Thorstensson CA, Andersson ML, Jonsson H, Saxne T, Petersson IF. Natural course of knee osteoarthritis in middle-aged subjects with knee pain: 12-year follow-up using clinical and radiographic criteria. Ann Rheum Dis. 2009;68(12):1890–1893. [DOI] [PubMed] [Google Scholar]
- 7.Altman RD, Hochberg MC, Moskowitz RW, Schnitzer TJ. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43(9):1905–1915. [DOI] [PubMed] [Google Scholar]
- 8.Felson DT, Zhang Y, Anthony JM, Naimark A, Anderson JJ. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann Intern Med. 1992;116(7):535–539. [DOI] [PubMed] [Google Scholar]
- 9.Aaboe J, Bliddal H, Messier SP, Alkjaer T, Henriksen M. Effects of an intensive weight loss program on knee joint loading in obese adults with knee osteoarthritis. Osteoarthritis Cartilage. 2011;19(7):822–828. [DOI] [PubMed] [Google Scholar]
- 10.Gersing AS, Schwaiger BJ, Nevitt MC, Joseph GB, Chanchek N, Guimaraes JB, Mbapte Wamba J, Facchetti L, McCulloch CE, Link TM. Is Weight Loss Associated with Less Progression of Changes in Knee Articular Cartilage among Obese and Overweight Patients as Assessed with MR Imaging over 48 Months? Data from the Osteoarthritis Initiative. Radiology. 2017:161005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, Ettinger WH Jr., Pahor M, Williamson JD. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501–1510. [DOI] [PubMed] [Google Scholar]
- 12.Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52(7):2026–2032. [DOI] [PubMed] [Google Scholar]
- 13.Riecke BF, Christensen R, Christensen P, Leeds AR, Boesen M, Lohmander LS, Astrup A, Bliddal H. Comparing two low-energy diets for the treatment of knee osteoarthritis symptoms in obese patients: a pragmatic randomized clinical trial. Osteoarthritis Cartilage. 2010;18(6):746–754. [DOI] [PubMed] [Google Scholar]
- 14.Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, Beavers DP, Hunter DJ, Lyles MF, Eckstein F, Williamson JD, Carr JJ, Guermazi A, Loeser RF. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2007;66(4):433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent HK, Ben-David K, Conrad BP, Lamb KM, Seay AN, Vincent KR. Rapid changes in gait, musculoskeletal pain, and quality of life after bariatric surgery. Surg Obes Relat Dis. 2012;8(3):346–354. [DOI] [PubMed] [Google Scholar]
- 17.Vincent HK, Ben-David K, Cendan J, Vincent KR, Lamb KM, Stevenson A. Effects of bariatric surgery on joint pain: a review of emerging evidence. Surg Obes Relat Dis. 2010;6(4):451–460. [DOI] [PubMed] [Google Scholar]
- 18.Gill SV, Walsh MK, Pratt JA, Toosizadeh N, Najafi B, Travison TG. Changes in spatiotemporal gait patterns during flat ground walking and obstacle crossing 1 year after bariatric surgery. Surg Obes Relat Dis. 2016;12(5):1080–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callahan LF, Shreffler JH, Altpeter M, Schoster B, Hootman J, Houenou LO, Martin KR, Schwartz TA. Evaluation of group and self-directed formats of the Arthritis Foundation’s Walk With Ease Program. Arthritis Care Res (Hoboken). 2011;63(8):1098–1107. [DOI] [PubMed] [Google Scholar]
- 20.Foundation Arthritis. Walk with ease: your guide to walking for better health, improved fitness and less pain. Atlanta; 1999. [Google Scholar]
- 21.Saris WH, Blair SN, van Baak MA, Eaton SB, Davies PS, Di Pietro L, Fogelholm M, Rissanen A, Schoeller D, Swinburn B, Tremblay A, Westerterp KR, Wyatt H. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the IASO 1st Stock Conference and consensus statement. Obes Rev. 2003;4(2):101–114. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt S Obesity and Exercise. Available at: http://www.acsm.org/public-information/articles/2016/10/07/obesity-and-exercise.
- 23.White DK, Neogi T, Zhang Y, Felson D, Lavalley M, Niu J, Nevitt M, Lewis CE, Torner J, Douglas Gross K. The association of obesity with walking independent of knee pain: the multicenter osteoarthritis study. J Obes. 2012;2012:261974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vismara L, Romei M, Galli M, Montesano A, Baccalaro G, Crivellini M, Grugni G. Clinical implications of gait analysis in the rehabilitation of adult patients with “Prader-Willi” Syndrome: a cross-sectional comparative study (“Prader-Willi” Syndrome vs matched obese patients and healthy subjects). J Neuroeng Rehabil. 2007;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeVita P, Hortobagyi T. Obesity is not associated with increased knee joint torque and power during level walking. J Biomech. 2003;36(9):1355–1362. [DOI] [PubMed] [Google Scholar]
- 26.Lai PP, Leung AK, Li AN, Zhang M. Three-dimensional gait analysis of obese adults. Clin Biomech (Bristol, Avon). 2008;23 Suppl 1:S2–6. [DOI] [PubMed] [Google Scholar]
- 27.Li JS, Tsai TY, Felson DT, Li G, Lewis CL. Six degree-of-freedom knee joint kinematics in obese individuals with knee pain during gait. PLoS One. 2017;12(3):e0174663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herring LY, Stevinson C, Carter P, Biddle SJ, Bowrey D, Sutton C, Davies MJ. The effects of supervised exercise training 12-24 months after bariatric surgery on physical function and body composition: a randomised controlled trial. Int J Obes (Lond). 2017. [DOI] [PubMed] [Google Scholar]
- 29.Herring LY, Stevinson C, Davies MJ, Biddle SJ, Sutton C, Bowrey D, Carter P. Changes in physical activity behaviour and physical function after bariatric surgery: a systematic review and meta-analysis. Obes Rev. 2016;17(3):250–261. [DOI] [PubMed] [Google Scholar]
- 30.Hortobagyi T, Herring C, Pories WJ, Rider P, Devita P. Massive weight loss-induced mechanical plasticity in obese gait. J Appl Physiol. 2011;111(5):1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vartiainen P, Bragge T, Lyytinen T, Hakkarainen M, Karjalainen PA, Arokoski JP. Kinematic and kinetic changes in obese gait in bariatric surgery-induced weight loss. J Biomech. 2012;45(10):1769–1774. [DOI] [PubMed] [Google Scholar]
- 32.Li G, Van de Velde SK, Bingham JT. Validation of a non-invasive fluoroscopic imaging technique for the measurement of dynamic knee joint motion. J Biomech. 2008;41(7):1616–1622. [DOI] [PubMed] [Google Scholar]
- 33.Farrokhi S, Voycheck CA, Gustafson JA, Fitzgerald GK, Tashman S. Knee joint contact mechanics during downhill gait and its relationship with varus/valgus motion and muscle strength in patients with knee osteoarthritis. Knee. 2016;23(1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capristo E, Panunzi S, De Gaetano A, Raffaelli M, Guidone C, Iaconelli A, L’Abbate L, Birkenfeld AL, Bellantone R, Bornstein SR, Mingrone G. Intensive lifestyle modifications with or without liraglutide 3mg vs. sleeve gastrectomy: A three-arm non-randomised, controlled, pilot study. Diabetes Metab. 2018;44(3):235–242. [DOI] [PubMed] [Google Scholar]
- 35.Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39–44. [DOI] [PubMed] [Google Scholar]
- 36.Koo S, Rylander JH, Andriacchi TP. Knee joint kinematics during walking influences the spatial cartilage thickness distribution in the knee. J Biomech. 2011;44(7):1405–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutter EG, Widmyer MR, Utturkar GM, Spritzer CE, Garrett WE Jr., DeFrate LE. In vivo measurement of localized tibiofemoral cartilage strains in response to dynamic activity. Am J Sports Med. 2015;43(2):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lad NK, Liu B, Ganapathy PK, Utturkar GM, Sutter EG, Moorman CT 3rd, Garrett WE, Spritzer CE, DeFrate LE. Effect of normal gait on in vivo tibiofemoral cartilage strains. J Biomech. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White DK, Neogi T, Rejeski WJ, Walkup MP, Lewis CE, Nevitt MC, Foy CG, Felson DT, Look ARG. Can an intensive diet and exercise program prevent knee pain among overweight adults at high risk? Arthritis Care Res (Hoboken). 2015;67(7):965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foy CG, Lewis CE, Hairston KG, Miller GD, Lang W, Jakicic JM, Rejeski WJ, Ribisl PM, Walkup MP, Wagenknecht LE, Look ARG. Intensive lifestyle intervention improves physical function among obese adults with knee pain: findings from the Look AHEAD trial. Obesity (Silver Spring). 2011;19(1):83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abu-Abeid S, Wishnitzer N, Szold A, Liebergall M, Manor O. The influence of surgically-induced weight loss on the knee joint. Obes Surg. 2005;15(10):1437–1442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


