Abstract
In the mammalian neocortex, an area typically receives inputs from, and projects to, dozens of other areas. Mechanisms are needed to flexibly route information to the right place at the right time, which we term “pathway gating”. For instance, a region in your brain that receives signals from both visual and auditory pathways may want to “gate in” the visual pathway while “gating out” the auditory pathway when you try to read a book surrounded by people in a noisy cafe. In this review, we marshall experimental and computational evidence in support of a circuit mechanism for flexible pathway gating realized by a disinhibitory motif. Moreover, recent work shows an increasing preponderance of this disinhibitory motif from sensory areas to association areas of the mammalian cortex. Pathway input gating is briefly compared with alternative or complementary gating mechanisms. Predictions and open questions for future research on this puzzle about the complex brain system will be discussed.
Keywords: diversity of inhibitory interneurons, disinhibitory motif, pathway gating, computational modeling
Introduction
Recent years have witnessed substantial progress in the neuroscience of large-scale brain circuits. Notably, a series of papers reported high-quality directed- and weighted inter-areal connectivity of cortex in macaque monkey [1–3] and mouse [4, 5]. These datasets provided an anatomical foundation for the development of computational models of the global cortical dynamics [6–8]. With this advance, a new set of questions have gained urgency, one of which is concerned with gating in the brain. In the mammalian neocortex, an area typically receives inputs from several dozens of other areas, and projects to similarly numerous areas downstream. Mechanisms are needed to flexibly route information to the right place at the right time, which we term “pathway gating”.
A parallel development in recent years is a dramatic increase in our knowledge about a diversity of GABAergic inhibitory neurons in the cortex. Thanks to the availability of genetic tools, researchers can label specific subtypes of GABAergic cells, quantify their molecular fingerprints, measure their morphological and physiological properties, record their activity from behaving animals and assess their functional role by optogenetic manipulations. Whereas classification of GABAergic interneurons continues to be refined and debated, a consensus has emerged with regard to a canonical disinhibitory motif that involves three non-overlapping sub-classes of interneurons, in empirical support of a model prediction [9]. A first type of parvalbumin (PV) positive interneurons target perisomatic regions of excitatory pyramidal (P) cells and control their spiking outputs; a second type of somatostatin (SST) positive interneurons target pyramidal dendrites and are in an ideal position to control their inputs; a third type of interneurons express vasoactive intestinal peptide (VIP) and preferentially target SST cells. When VIP neurons are activated, they inhibit SST cells, thereby disinhibiting pyramidal dendrites. Following the initial breakthroughs (for a review of the work prior to 2015, see [10]), our knowledge about these different inhibitory cell types [11–14], their interactions [15, 16] and their functions in behaving mice [14, 17–25] continue to grow over the last few years.
Built on the new barrage of experimental data, a computational model was developed to test the hypothesis that the canonical disinhibitory motif provides a circuit substrate for pathway gating [26]. In this short review, we first summarize recent experimental research on this canonical disinhibitory motif. Then, we will discuss requirements and supporting evidence for this circuit motif to implement pathway gating. Finally, we will contrast this mechanism with other, potentially complimentary, gating scenarios within cortex and involving subcortical structures, and suggest open questions for future research on the dynamical operation and flexible functions of the cell-type specific large-scale brain systems.
A disinhibitory motif
One of the cognitive functions that depend on input gating is working memory, the brain’s ability to internally store and manipulate information in the absence of sensory stimulation. A cardinal requirement for a working memory circuit to function properly is that only behavioral relevant stimuli are “gated in” while irrelevant distractors are filtered out and ignored. Computational considerations of this problem have led to the publication, in 2004, of a biologically-based local circuit model endowed with three subtypes of inhibitory neurons (Figure 1A) [9]. The model was inspired by three lines of anatomical evidence. First, a subpopulation of GABAergic cells labeled by VIP or calcium-binding protein calretinin (CR) preferentially target other interneurons rather than pyramidal cells in the hippocampus [27], as well as neocortex of rodents [28, 29] and monkeys [30–32]. Second, statistically, VIP and CR cells preferentially target dendrite-targeting inhibitory neurons expressing cal-bindin (CB) or SST, rather than PV cells (Figure 1B) [32]. Third, unlike primary sensory areas where PV cells are the majority of GABAergic neurons, CB and CR interneurons are predominant in the prefrontal cortex which plays a central role in working memory (Figure 1C) [33]. The model has several predictions. In particular, dendrite-targeting interneurons should have a significant spontaneous activity, which was later supported by empirical data [34]. Moreover, these cells display an “inverted tuning”, i.e. a decrease in activity for specific stimulus features, which was found in single-unit recording from behaving monkeys [9]. More direct support came recently in a mice experiment showing that activation of VIP or SST neurons of dorsomedial frontal cortex, respectively enhanced or impaired working memory retention and behavioral performance [25].
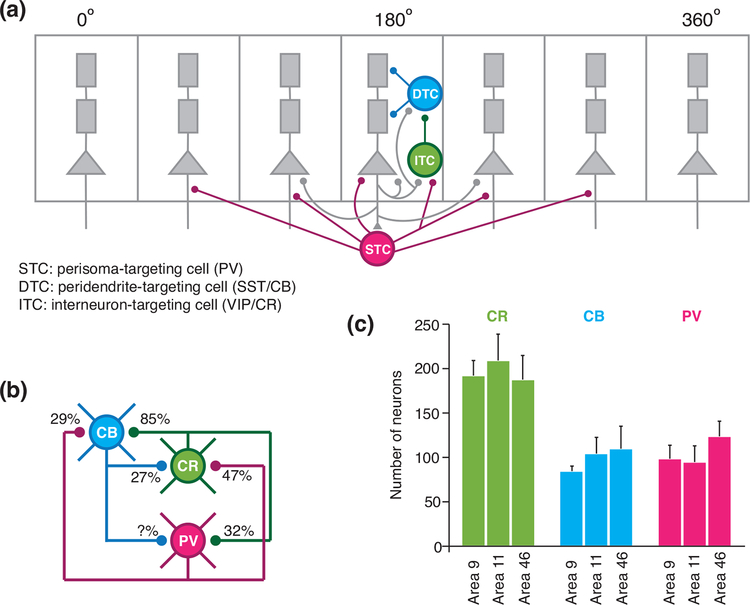
Figure 1:
A disinhibitory circuit motif. (a) A neural circuit model for working memory with three types of inhibitory neurons, i.e. perisoma targeting, peridendrite-targeting, and interneuron-targeting neurons. Dendrite-targeting inhibitory neurons (blue) control the resistance to distractors (adapted from [9]). (b) The circuit diagram of PV, CB, and CR neurons. The connection probabilities between different types of neurons are measured in inferior temporal cortex of maca que monkey (adapted from [32]). (c) Number of CR, CB, and PV neurons in three subregions of the macaque monkey prefrontal cortex, showing that PV are not predominant among the three interneuron types in the prefrontal cortex. (adapted from [33]).
Although the theoretical proposal of a disinhibitory microcircuit motif was originally motivated by the need of gating for a working memory network, the local circuit organization (Figure 1A) is in principle general for all cortical areas. Note that it appears that PV, CB and CR positive interneurons do not have significant overlap in macaque monkey; whereas the overlaps are more substantial in mice, for which PV, SST and VIP are better markers of non-overlapping interneuron sub-populations. Importantly, Wang et al. explicitly stated that “We emphasize that the three interneuron types in our model should be more appropriately interpreted according to their synaptic targets rather than calcium-binding protein expressions” [9].
With the invention of genetic labeling and optogenetic tools, an avalanche of papers have been published in recent years, demonstrating this disinhibitory motif in various cortical areas of mouse cortex [35–38]. In layer 2/3 of primary somatosensory cortex of behaving animals, onset of locomotion is correlated with an increase of activity in both pyramidal cells and VIP interneurons, concomitantly with a decrease of activity in SST cells [37]. This observation can be explained by the disinhibitory motif, which was rigorously established for the same circuit by intracellular recording from pairs of neurons in vitro [35]. Similar concurrent changes of activity in pyramidal cells, SST and VIP cells are observed in primary visual cortex [38] and medial frontal cortex [36] of awake behaving mice.
In a recurrent system where multiple cell types interact with each other, the collective behavior depends on many factors, including their baseline states and the strengths of their interconnectivity. In particular, the disinhibitory circuit may behave in counterintuitive ways. Recently, Pakan et al. and Dipoppa et al. [21, 39] reported that, as mice made a transition from rest to movement, SST cells in L2/3 of V1 showed an increase of activity, instead of a previously reported decrease [38]. This apparent inconsistency can be explained computationally by synaptic interactions between multiple neural populations and a nonlinear neuronal input-output relationship [40]. Therefore, in the same disinhibitory circuit, behavioral modulation of SST neural activity may change the sign depending on the circuit state. In contrast to L2/3, activity of SST cells in the deep layers of somatosensory cortex increases rather than decreases when mice started whisking, which could arise from laminar differences in the strength of VIP to SST projections, among other factors [24]. A notable recent work along these lines was that of Jiang et al. [15, 41, 42], where connections between different cell types were assessed physiologically with simultaneous intracellular recording from up to 8 neurons in slices of mouse V1. Layers within a local circuit may show markedly different properties thus operate in distinct dynamical regimes.
Another important aspect of the disinhibitory motif is concerned with the input signals. Within a cortical area, long-range clustered horizontal connections from pyramidal cells target SST cells [43], contributing to surround suppression [43, 44]. In addition, PV, SST and VIP interneurons are all targets of long-distance cortico-cortical projections [45], but finer grained differences remain to be elucidated. Particularly interesting is empirical evidence that top-down excitation of VIP interneurons contributes to attention induced amplification of neural responses in sensory areas [46]. Finally, variations may exist across different cortical areas, we will come back to this point in a later section.
The dendritic disinhibitory gating hypothesis
Whereas the disinhibitory motif has been proposed as a gating mechanism for working memory [9] or attention [47], can it also implement pathway routing of information in a multi-regional brain system? Consider a cortical area that receives inputs from multiple areas upstream, and assume that these cortico-cortical long-distance projections target overlapping pyramidal neurons so that any individual cell is the recipient of inputs from multiple pathways. In order for the disinhibitory motif to realize pathway gating, the following four requirements need to be fulfilled.
First, dendritic responses need to be nonlinear so that dendritic inhibition mediated by SST cells and its suppression would have a strong impact. This is supported by ample evidence of NMDA and calcium spikes in pyramidal dendrites that “is tightly controlled by local microcircuits of inhibitory neurons targeting subcellular compartments” [48]. Second, for a given target pyramidal cell, synaptic inputs from different pathways must be segregated onto different parts of its dendritic tree, consistent with recent findings of synapse clustering in sensory and motor cortices [49, 50]. Wilson et al. [50] showed that synapses with similar orientation tunings tend to cluster onto the same dendrites, and this clustering is important for explaining orientation selectivity observed in V1. Although direct experimental evidence supporting synaptic clustering of different pathways is still lacking, it is not difficult to imagine the principle of synaptic clustering being extended from sensory and motor areas to association areas, and from feature- to pathway-specificity. Third, dendrite-targeting interneurons should be able to exert selective control over individual dendritic branches. Cichon and Gan [51] observed that different motor tasks induced neural activities on different dendritic branches of pyramidal neurons in the motor cortex, and inactivating SST neurons drastically reduced the branch-specificity in dendritic activity. This work strongly suggests that SST neurons can provide branch-specific inhibition onto dendrites of pyramidal neurons. Fourth and finally, there need to be an alignment between the clustering of long-range synaptic inputs from different pathways and the branch-specific disinhibition. In order to selectively open the gate for one pathway, dendrites targeted by that pathway need to be disinhibited, while other dendrites remain inhibited.
In a computational work, Yang et al. [26] showed that all four requirements can be fulfilled with minimal assumptions about the underlying neural circuity (Figure 2). Surprisingly, this model revealed that to achieve branch-specific disinhibition, it is unnecessary to assume tailored connections from SST neurons to pyramidal neurons and from VIP to SST interneurons. Even random connectivity between them, together with random activation/deactivation of SST neurons, suffice to provide branch-specific inhibition/disinhibition. The core intuition is that, compared to the dense neuron-to-neuron connectivity [52], the connectivity from SST neurons to dendrites of pyramidal neurons is much sparser, due to the large number of thin dendritic branches per neuron. This sparse neuron-to-dendrite connectivity easily allows for branch-specific disinhibition over the dendritic tree. The alignment between excitation and disinhibition can also arise naturally through synaptic plasticity, because excitatory synapses targeting disinhibited thus depolarized branches tend to get strengthened, whereas those targeting hyperpolarized branches are depressed. In support of this idea, a recent work showed that dendritic disinhibition is crucial for synaptic potentiation in vivo [53]. In addition, the connections from SST neurons to dendrites are subject to inhibitory plasticity [54] that can continue to improve SST neurons’ control over individual dendritic branches.
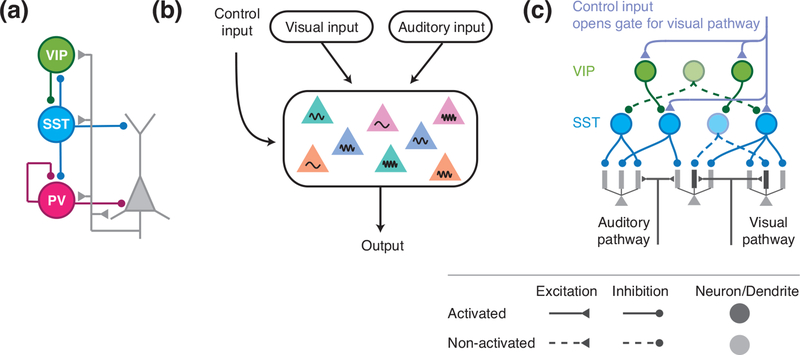
Figure 2:
A disinhibitory circuit motif for pathway gating. (a) The disinhibitory circuit diagram with PV, SST, and VIP neurons. (b) An area receives converging inputs from visual and auditory pathways. The control input selectively opens the gate for one pathway. (c) To open the gate for the visual pathway, the control input can target a subset of VIP and SST neurons, leading to disinhibition of dendrites targeted by the visual pathway (adapted from [26]).
Besides being a plausible mechanism for pathway gating, dendritic disinhibition could also be computationally favorable compared to a dendritic excitatory mechanism. When gating is implemented with excitation, the latter depolarizes the dendritic membrane thus reduces its dynamic range for information specific responses. In contrast, with an disinhibitory gating mechanism, dendrites are inhibited in the default state, allowing for a wider dynamic range from hyperpolarization to de-polarization. Another advantage of dendritic disinhibition is that it is permissive. Disinhibition can not activate dendrites by itself. However, if gating is implemented with direct dendritic (or somatic) excitation, the control inputs could get overly strong, leading to spurious responses.
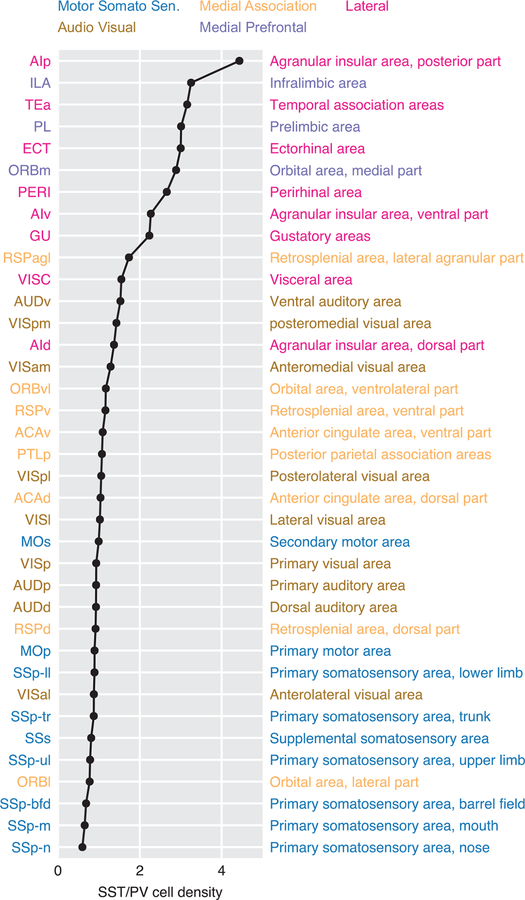
Inasmuch as the disinhibitory motif offers a mechanism for pathway gating, it follows that cortical areas with a greater need of input gating should be endowed with more inhibitory neurons dedicated to such a disinhibitory motif. Frontal and association cortices receive converging inputs from many different pathways in comparison to primary sensory or motor areas, therefore these areas may need a larger repertoire of dendrite-targeting interneurons to selectively gate inputs from different pathways. Anatomical data from monkeys hinted that dendrite-targeting interneurons are more prevalent in prefrontal cortices [33]. The dominance of SST neurons in mouse association and prefrontal cortices is recently established by Kim et al. [55], who measured the number of PV, SST, and VIP neurons in individual areas throughout the whole mouse brain. Remarkably, they found that there is a more than 7-times change in the balance between SST and PV neurons across the cortex (Figure 3). In primary somatosensory and motor cortices, SST neurons are less abundant than PV neurons. However, in higher-order cortical areas, like the prelimbic area, there are 3 to 5 times more SST neurons than PV neurons.
Figure 3:
A cortical hierarchy based on SST/PV cell density ratio. Mouse cortical areas are ranked by the ratio between their PV and SST cell densities. Primary somatosensory areas are abundant in PV neurons, while prefrontal areas are dominated by SST neurons (adapted from [55]). The color code indicates the cortical subnetwork each area belongs to [5].
Taken together, a strong case can be made that the disinhibitory motif provides a plausible circuit substrate within the cortex for routing information flow.
Other gating mechanisms
The current short review focuses on input gating by a disinhibitory motif. There are alternative and/or complementary, gating mechanisms in the brain (Figure 4). One possibility is that excitatory-inhibitory synaptic balance prevents input from entering a cortical area thus must be broken to enable “gate in” [56]; another is that synchrony is required whereas asynchronous inputs are “gated out” [57–59]. In contrast to input gating, a different scenario involves output gating. In a recent paper [60], the authors conducted an anatomical analysis in the mice prelimbic cortex focusing on chandelier cells which selectively target the initial segment of axons of pyramidal cells where action potentials are generated. They found that a subset of chandelier cells specifically target pyramidal cells projecting to amygdala while avoiding pyramidal cells projecting to the contralateral side of the same cortical area. Furthermore, these chandelier cells receive differential inputs from the two populations of excitatory neurons, therefore can be selectively activated, which would lead to suppression of output from the prelimbic area to one down-stream area but not another. Such an output gating mechanism would require not only different groups of chandelier cells dedicated to different output pathways, but also that principal neurons dedicated to different projection pathways are largely non-overlapping. With the advance of cell-type specific connectivity analysis, new information is expected in the coming years that will support or disapprove such output gating proposal.
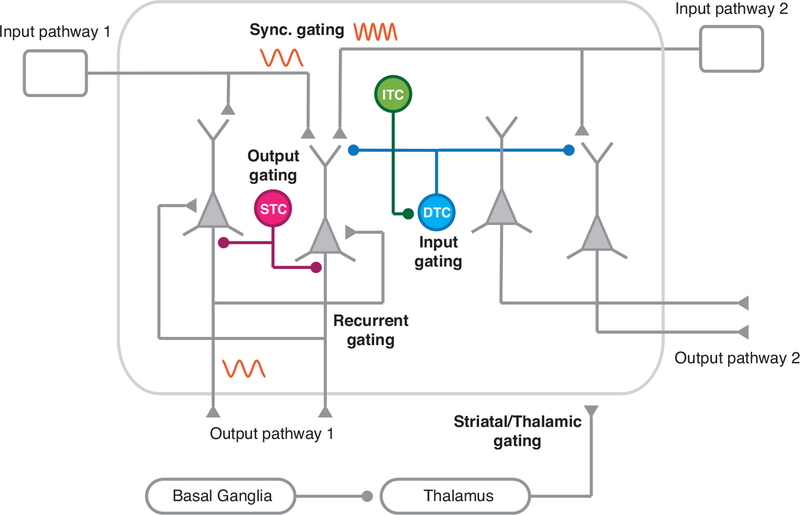
Figure 4:
Various mechanisms for information gating in the brain. Input gating can be achieved by dendrite-targeting interneurons that selectively control inputs to pyramidal dendrites. In the synchronous gating mechanism, communication between two areas depends on the degree of temporal synchrony of neural activity between the source and target areas. Recurrent gating mechanism involves selective integration of inputs based on context-dependent dynamics of the network. Output gating is instantiated with perisoma-targeting interneurons that specifically inhibit pyramidal neurons projecting to one pathway but not others. Gating may also involve subcortical structures, especially basal ganglia and thalamus.
In addition to input-gating and output-gating, it is conceivable that recurrent dynamics within a local circuit can selectively process information from one pathway but not the other. This was proposed in a study combining computational modeling and neurophysiology, in which monkeys were trained to make a decision based on either color or direction of a colored visual motion stimulus [61]. In each behavioral trial, the relevant feature, color or direction, is indicated by a rule cue. The authors’ analysis suggested that the rule cue input yields a “selection vector” to guide time integration of the relevant feature but not the irrelevant one in a recurrent network. Yang et al. [26] showed that the same task can be accomplished with moderate input gating by the disinhibitory motif mechanism. In principle, recurrent network dynamic can work cooperatively with input and output gating.
Finally, gating in the brain more than likely engages subcortical structures as well, including the thalamus [62], and basal ganglia [63, 64]. Their experimental support at the circuit level remains lacking and could be an interesting direction for future research. Moreover, it is worth keeping in mind that there are several kinds of gating in the brain. For instance selective attention to a spatial location but not other locations represent a form of gating within the same pathway, which is different from pathway gating. Distinct gating mechanisms may be suitable for various forms of information routing in the brain.
Concluding remarks
The complexity of inhibitory cells, which Cajal called “butterflies of the soul”, continues to fascinate neuroscientists. Transcriptome analysis promises to systematically define subclasses of inhibitory interneurons [12, 65]. It may also be proven as a powerful tool to understand area-to-area variations. Even among sensory areas, locomotion induces an enhancement of excitatory neurons in primary visual cortex [38] and somatosensory cortex [37], but suppression in primary auditory cortex [66, 67]. This finding highlights the idea that quantitative differences in a canonical disinhibitory motif could give rise to distinct dynamical operation regimes. As shown by the work of Kim et al. [55], there are marked differences between early sensory areas and association areas. This macroscopic gradient may have evolved to accommodate varying degrees of functional needs for input control across the brain’s hierarchy, the computational and functional implications await to be elucidated.
The disinhibitory motif model has several testable predictions. In particular, it suggests that cognitive control signals, such as those representing behavioral rule or context, act through targeting specific subclasses of inhibitory neurons like VIP- and SST-expressing interneurons, rather than pyramidal cells. Although research results summarized above present a strong case that the disinhibitory motif offers a circuit mechanism for pathway gating, a direct test of this theoretical prediction requires that, in future experiments, cell-type specific neurophysiology and manipulation be carried out in animals performing tasks that depend on flexible routing of information in the cortex. Decisive progress in this area will not only shed insights into this major puzzle, but also into related deficits associated with psychiatric illness [68].
Acknowledgments.
This work was supported by the NIH grant MH062349, ONR grant N00014–17–1–2041, STCSM grants 14JC1404900 and 15JC1400104.
Reference annotations
Special interests (*)
Outstanding interests (**)
- 1.Ercsey-Ravasz M et al. A predictive network model of cerebral cortical connectivity based on a distance rule. Neuron 80, 184–197 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markov NT et al. A weighted and directed interareal connectivity matrix for macaque cerebral cortex. Cereb. Cortex 24, 17–36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song HF, Kennedy H & Wang X-J Spatial embedding of similarity structure in the cerebral cortex. Proc. Natl. Acad. Sci. (USA) 111, 16580–16585 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh SW et al. A mesoscale connectome of the mouse brain. Nature 508, 207–214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zingg B et al. Neural networks of the mouse neocortex. Cell 156, 1096–1111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhuri R, Knoblauch K, Gariel MA, Kennedy H & Wang X-J A Large-Scale Circuit Mechanism for Hierarchical Dynamical Processing in the Primate Cortex. Neuron 88, 419–431 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mejias JF, Murray JD, Kennedy H & Wang XJ Feedforward and feedback frequency-dependent interactions in a large-scale laminar network of the primate cortex. Sci Adv 2, e1601335 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joglekar MR, Mejias JF, Yang GR & Wang X-J Inter-areal balanced amplification enhances signal propagation in a large-scale circuit model of the primate cortex. bioRxiv. doi:10.1101/186007 . eprint: https://www.biorxiv.org/content/early/2017/09/07/186007.full.pdf . <10.1101/186007https://www.biorxiv.org/content/early/2017/09/07/186007.full.pdfhttps://www.biorxiv.org/content/early/2017/09/07/186007. eprint: https://www.biorxiv.org/content/early/2017/09/07/186007.full.pdf . < https://www.biorxiv.org/content/early/2017/09/07/186007 > (2017). [DOI] [PubMed] [Google Scholar]
- 9.Wang X-J, Tegnér J, Constantinidis C & Goldman-Rakic PS Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci USA 101, 1368–1373 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kepecs A & Fishell G Interneuron cell types are fit to function. Nature 505, 318–326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeisel A et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–42 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Tasic B et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci 19, 335–46 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawrylycz M et al. Canonical genetic signatures of the adult human brain. Nat Neurosci 18, 1832–44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tremblay R, Lee S & Rudy B GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 91, 260–292 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang X et al. Principles of connectivity among morphologically defined cell types in adult neocortex. Science 350, aac9462 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper provides quantitative statistical connectivity between 15 morphologically-identified types of interneurons in V1 of mature mouse cortex.
- 16.Karnani MM et al. Cooperative Subnetworks of Molecularly Similar In-terneurons in Mouse Neocortex. Neuron 90, 86–100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Letzkus JJ, Wolff SBE & Lüthi A Disinhibition, a Circuit Mechanism for Associative Learning and Memory. Neuron 88, 264–76 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Urban-Ciecko J & Barth AL Somatostatin-expressing neurons in cortical networks. Nat. Rev. Neurosci 17, 401–409 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim D et al. Distinct Roles of Parvalbumin- and Somatostatin-Expressing Interneurons in Working Memory. Neuron 92, 902–915 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Jackson J, Ayzenshtat I, Karnani MM & Yuste R VIP+ interneurons control neocortical activity across brain states. J Neurophysiol 115, 3008–17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dipoppa M et al. Vision and locomotion shape the interactions between neuron types in mouse visual cortex. bioRxiv. doi: 10.1101/058396 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper showed that locomotion can increase the activity of both VIP and SST neurons in mouse V1, seemingly contradicting the VIP-SST disinhibitory circuit model. However, Garcia et al. [39] showed that a disinhibitory circuit can operate in different regimes, including one consistent with the findings of [19].
- 22.Kuchibhotla KV et al. Parallel processing by cortical inhibition enables context-dependent behavior. Nat Neurosci 20, 62–71 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JH, Koch C & Mihalas S A Computational Analysis of the Function of Three Inhibitory Cell Types in Contextual Visual Processing. Front Comput Neurosci 11, 28 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munoz W, Tremblay R, Levenstein D & Rudy B Layer-specific modulation of neocortical dendritic inhibition during active wakefulness. Science 355, 954–959 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Kamigaki T & Dan Y Delay activity of specific prefrontal interneuron sub-types modulates memory-guided behavior. Nat Neurosci 20, 854–863 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang GR, Murray JD & Wang X-J A dendritic disinhibitory circuit mechanism for pathway-specific gating. Nat Commun 7, 12815 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The authors proposed that the dendritic disinhibitory circuit motif can support pathway gating. They used computational models to show that assumptions about the circuit are minimal and plausible for the proposed mechanism to work.
- 27.Gulyás AI, Hájos N & Freund T Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J. Neurosci 16, 3397–3411 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawaguchi Y & Kubota Y GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex 7, 476–486 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Gonchar Y & Burkhalter A Connectivity of GABAergic calretinin-immunoreactive neurons in rat primary visual cortex. Cereb. Cortex 9, 683–696 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Gabbott PLA & Bacon SJ Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey. J. Comp. Neurol 364, 567–636 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Meskenaite V Calretinin-immunoreactive local circuit neurons in the area 17 of the cynomolgus monkey, Macaca fascicularis. J. Comp. Neurol 379, 113–132 (1997). [PubMed] [Google Scholar]
- 32.DeFelipe J, Gonzalez-Albo MC, Del Rio MR & Elston GN Distribution and patterns of connectivity of interneurons containing calbindin, calretinin, and parvalbumin in visual areas of occipital and temporal lobes of the macaque monkeys. J. Comp. Neurol 412, 515–26 (1999). [DOI] [PubMed] [Google Scholar]
- 33.Condé F, and Jacobowitz DM, Baimbridge JSL, G. K & Lewis DA Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbu-min in monkey prefrontal cortex: distribution and morphology. J. Comp. Neurol 341, 95–116 (1994). [DOI] [PubMed] [Google Scholar]
- 34.Gentet LJ et al. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat. Neurosci 15, 607–612 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Pfeffer CK, Xue M, He M, Huang ZJ & Scanziani M Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci 16, 1068–1076 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pi HJ et al. Cortical interneurons that specialize in disinhibitory control. Nature 503, 521–524 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S, Kruglikov I, Huang ZJ, Fishell G & Rudy B A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci 16, 1662–70 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu Y et al. A cortical circuit for gain control by behavioral state. Cell 156, 1139–1152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pakan JM et al. Behavioral-state modulation of inhibition is context-dependent and cell type specific in mouse visual cortex. Elife 5. doi: 10.7554/eLife.14985 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia Del Molino LC, Yang GR, Mejias JF & Wang X-J Paradoxical response reversal of top-down modulation in cortical circuits with three interneuron types. Elife 6. doi: 10.7554/eLife.29742 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barth L et al. Comment on “Principles of connectivity among morphologically defined cell types in adult neocortex”. Science 353, 1108 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Jiang X et al. Response to Comment on ‘Principles of connectivity among morphologically defined cell types in adult neocortex”. Science 353, 1108(2016). [DOI] [PubMed] [Google Scholar]
- 43.Adesnik H, Bruns W, Taniguchi H, Huang ZJ & Scanziani M A neural circuit for spatial summation in visual cortex. Nature 490, 226–231 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Litwin-Kumar A, Rosenbaum R & Doiron B Inhibitory stabilization and visual coding in cortical circuits with multiple interneuron subtypes. J. Neurophysiol 115, 1399–1409 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wall NR et al. Brain-Wide Maps of Synaptic Input to Cortical Interneurons. J. Neurosci 36, 4000–4009 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; * The authors mapped the brain-wide synaptic inputs onto PV, SST, and VIP neurons in mouse V1. This data provides a starting point for building large-scale models with multiple types of interneurons.
- 46.Zhang S et al. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345, 660–665 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sridharan D & Knudsen EI Selective disinhibition: A unified neural mechanism for predictive and post hoc attentional selection. Vision Res. 116, 194–209 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Larkum M A cellular mechanism for cortical associations: an organizing principle for the cerebral cortex. Trends Neurosci. 36, 141–151 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Yang G et al. Sleep promotes branch-specific formation of dendritic spines after learning. Science 344, 1173–1178 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson DE, Whitney DE, Scholl B & Fitzpatrick D Orientation selectivity and the functional clustering of synaptic inputs in primary visual cortex. Nature neuroscience 19, 1003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cichon J & Gan WB Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity. Nature 520, 180–185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The authors reported that different motor tasks activate different dendritic branches of the same pyramidal neuron. Silencing SST neurons strongly reduce this branch-specificity, suggesting branch-specific inhibition from SST neurons to dendrites of pyramidal neurons.
- 52.Fino E & Yuste R Dense inhibitory connectivity in neocortex. Neuron 69, 1188–1203 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu Y, Kaneko M, Tang Y, Alvarez-Buylla A & Stryker MP A cortical disinhibitory circuit for enhancing adult plasticity. Elife 4, e05558 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen SX, Kim AN, Peters AJ & Komiyama T Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning. Nature neuroscience 18, 1109–1115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim Y et al. Brain-wide Maps Reveal Stereotyped Cell-Type-Based Cortical Architecture and Subcortical Sexual Dimorphism. Cell 171, 456–469.e22(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The first study to quantify the density of specific types of inhibitory neurons across the mouse brain. The authors found that SST neurons are dominant in frontal and other associational cortices.
- 56.Vogels TP, Rajan K & Abbott LF Neural network dynamics. Annu Rev Neurosci 28, 357–376 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Palmigiano A, Geisel T, Wolf F & Battaglia D Flexible information routing by transient synchrony. Nat Neurosci 20, 1014–1022 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Fries P A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in Cogn. Sci 9, 474–480 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Wang X-J Neurophysiological and computational principles of cortical rhythms in cognition. Physiol. Rev 90, 1195–1268 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu J et al. Selective inhibitory control of pyramidal neuron ensembles and cortical subnetworks by chandelier cells. Nat. Neurosci 20, 1377–1383 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; * The authors showed that a subset of Chandelier cells in mouse prelimbic cortex selectively inhibit pyramidal neurons targeting amygdala, while sparing those targeting contralateral cortex. This work demonstrates the exquisite output-control inhibitory neurons can exert over pyramidal neurons.
- 61.Mante V, Sussillo D, Shenoy KV & Newsome WT Context-dependent computation by recurrent dynamics in prefrontal cortex. Nature 503, 78–84 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wimmer RD et al. Thalamic control of sensory selection in divided attention. Nature 526, 705–709 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frank MJ, Loughry B & O’Reilly RC Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci 1, 137–160 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Chatham CH & Badre D Multiple gates on working memory. Curr Opin Behav Sci 1, 23–31 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paul A et al. Transcriptional Architecture of Synaptic Communication Delineates GABAergic Neuron Identity. Cell (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper measured the transcriptional profile of six distinct types of interneurons. They found that different cell types can be best distinguished by expressions of genes linked to synaptic communications.
- 66.Schneider DM, Nelson A & Mooney R A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature 513, 189–194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou M et al. Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. Nat. Neurosci 17, 841–850 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hattori R, Kuchibhotla KV, Froemke RC & Komiyama T Functions and dysfunctions of neocortical inhibitory neuron subtypes. Nat. Neurosci 20, 1199–1208 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]