Abstract
Background:
Glucagon-like peptide-1 (GLP-1) is a gut derived incretin hormone that stimulates insulin secretion, cellular glucose uptake and has immune-regulatory functions. GLP-1 is markedly altered following trauma and sepsis, but the implications remain unclear.
Study design:
We performed an analysis of a prospective, longitudinal cohort study of critically-ill surgical patients with sepsis. Patient characteristics, and clinical data were collected, as well as peripheral blood sampling for biomarker analysis, out to 28 days after sepsis onset. We prospectively adjudicated sepsis diagnosis, severity, clinical outcomes and 6-month follow-up.
Results:
The cohort included 157 septic surgical patients with significant physiologic derangement (Max. SOFA score 8, IQR 4–11), a high rate of multiple organ failure (50.3%) and septic shock (24.2%). Despite high disease severity, both early death (<14 days; n=4, 2.9%) and overall inpatient mortality was low (n=12, 7.6%). However, post-discharge 6-month mortality was nearly 3-fold higher (19.7%). Both GLP-1 and IL-6 levels were significantly elevated for 21 days (p≤0.01) in patients that developed CCI compared to patients with a rapid recovery. Elevated GLP-1 at 24 hours was a significant independent predictor for the development of CCI after controlling for IL-6 and glucose levels (p=0.027), and at day 14 for death or severe functional disability at 6 months (WHO/Zubrod score 4–5, p=0.014).
Conclusions:
Elevated GLP-1 within 24 hours of sepsis is a predictor of early death or persistent organ dysfunction. Among early survivors, persistently elevated GLP-1 levels at day 14 are strongly predictive of death or severe functional disability at 6-months. Persistently elevated GLP-1 levels may be a marker of a non-resolving catabolic state which is associated with muscle wasting and dismal outcomes after sepsis and chronic critical illness.
Precis
Glucagon-like peptide-1 (GLP-1) is a gut-derived incretin hormone. Elevated GLP-1 after sepsis is a predictor of death or persistent organ dysfunction. Persistently elevated GLP-1 levels are predictive of death or severe functional disability at 6-months after operation. Persistently elevated GLP-1 levels may be a marker of a non-resolving catabolic state.
Introduction
Glucagon-like peptide-1 (GLP-1) is a gut derived incretin hormone that plays an important role in glucose homeostasis including stimulating insulin secretion, suppressing glucagon secretion, inhibiting gastric emptying and decreasing appetite. Loss of glucose homeostasis as a result of the injury stress response and ongoing pro-inflammation is a frequent occurrence in critically ill surgical intensive care unit (ICU) patients (1–3). Numerous studies have demonstrated the association between failure to control hyperglycemia and poor outcomes in the ICU patients including increased incidence of deaths, nosocomial infections, wound complications, prolonged ICU stays and the neuropathy of critical illness (4–5). Additionally, glycemic control is variable despite aggressive implementation of insulin protocols even in patients without previous evidence or history of diabetes (6–9). The pathophysiologic mechanisms for this variability and the role GLP-1 are poorly understood. While the role of GLP-1 in glycemic control has been studied in trauma (10), it has not been well studied in sepsis and the epidemiology of sepsis has changed in recent years. With rapid delivery of evidence based care, early hospital mortality has decreased substantially, but unfortunately, roughly one third of the survivors progress into chronic critical illness (CCI) with dismal long-term outcomes.(11–13) Our ongoing studies indicate that this is often due to a failure to achieve immune homeostasis leading to a persistent inflammation, immunosuppression and catabolism syndrome (PICS).(14–18) In this study, we serially determined GLP-1 levels after sepsis with the intent of determining how they were related to development of CCI-PICS and its dismal long-term outcome. We hypothesized that abnormal GLP-1 levels as a biomarker of metabolic stress and loss of glycemic homeostasis would be associated with poor outcomes, specifically the development of CCI and death or disability at 6-months.
Methods
Study design, site and patients
This was a retrospective subgroup analysis of an ongoing prospective longitudinal, observational cohort study of trauma and surgical ICU patients treated for sepsis at an academic, quaternary medical and Level-1 Trauma center (UF Health - Gainesville, FL). The parent study started in January 2015 and overall study design and protocols for the University of Florida (UF) Sepsis and Critical Illness Research Center (SCIRC) program have been previously published.(19) GLP-1 biomarker analysis was performed on a sub-group of 157 consecutively enrolled patients from the parent cohort between January 2015 and September 2016, which delineates the analytic cohort for this study. The GLP-1 analytic cohort was similar and representative of the parent program cohort across all domains (eTable 1). It should be noted that GLP-1 was not an a priori component or hypothesis of the parent sepsis cohort study. GLP-1 was added to the program’s serial biomarker panel and analytic batches after the senior author (RSS) joined the UF faculty, based on his interest and previous work on investigating GLP-1 in trauma patients.(11) Although GLP-1 was added in ad hoc fashion after initiation of the parent cohort, from that point on the patients were consecutively enrolled, sampled, batch analyzed (including GLP-1) and followed longitudinally in prospective fashion. The parent study received Institutional Review Board (IRB) approval upon program initiation in 2015 and IRB reapproval was obtained when GLP-1 was added as an ad hoc analyte. With an established IRB-approved precedent of initial enrollment with delayed consent for critically-ill patients, we obtained signed informed consent from the patient or legal proxy within 96 hours of study enrollment. Patients included in this study were those ICU patients that screened positive for sepsis and were placed on our ICU sepsis protocol either upon admission with, or subsequent development of sepsis. Sepsis screening was performed using the Modified Early Warning Signs-Sepsis Recognition System (MEWS-SRS) (12). All enrolled patients were managed using a standardized sepsis management protocol based on the Surviving Sepsis guidelines, supplemented by standardized evidenced-based ICU clinical care protocols, and executed with the assistance of electronic medical record (EMR)-based clinical care order sets to ensure high compliance and timely intervention.
Patient Enrollment, classification and outcomes
Parent cohort inclusion criteria consisted of the following: (1) age ≥18 years; (2) presence in the Surgical or Trauma ICU; and (3) clinical diagnosis of sepsis, severe sepsis, or septic shock as defined by the 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference (20). Sepsis diagnosis, severity, and source were clinically adjudicated in prospective fashion by SCIRC program investigators at weekly adjudication meetings. Exclusion criteria included: (1) refractory shock (death <24 hours) or inability to achieve source control (e.g. unresectable dead bowel); (2) pre-sepsis expected lifespan <3 months; (3) patient goals of care not consistent with aggressive management; (4) severe CHF (NYHA Class IV); (5) Child-Pugh Class C liver disease or pre-liver transplant; (6) HIV with CD4+ count <200 cells/mm3; (7) chronic corticosteroids or immunosuppressive agent use, including organ transplant recipients; (8) pregnancy; (9) chemotherapy or radiotherapy within past 30 days; (10) severe traumatic brain injury (evidence of neurologic injury on CT scan and GCS <8); or, (11) spinal cord injury resulting in permanent sensory and/or motor deficits. Baseline and inpatient clinical data collected prospectively during initial hospitalization included patient and infection characteristics, sepsis severity, EMR-based clinical and laboratory data, complications and inpatient disposition.
Based on established definitions, post-sepsis clinical trajectories were categorized as ‘early death’, ‘rapid recovery’ (RAP) or CCI. Early death was defined as those patients who died before day 14. CCI is defined as an ICU length of stay (LOS) greater than or equal to 14 days with evidence of persistent organ dysfunction, determined using components of the Sequential Organ Failure Assessment (SOFA) score (cardiovascular SOFA ≥1, or score in any other organ system ≥2). RAP patients were the remaining patients who had organ function recovery and discharge from the ICU within 14 days of sepsis onset. Inpatient outcomes included in-hospital mortality, hospital and ICU LOS, ventilator days, presence and severity of organ dysfunction, development of CCI, and discharge disposition. All patients enrolled in the parent cohort that survived to discharge underwent scheduled for follow-up visits, which were conducted at the UFInstitute on Aging, the patient’s home, or via telephone, with a tiered priority structure, respectively. Six-month outcomes included performance status and mortality. Performance status was measured by the WHO/Zubrod scale, a 5-point scale that measures the performance status of a patient’s ambulatory nature: 0) Asymptomatic (fully active), 1) Symptomatic but completely ambulatory (restricted in physically strenuous activity), 2) Symptomatic, <50% in bed during the day (ambulatory and capable of all self-care but unable to perform any work activities), 3) Symptomatic, >50% in bed, but not bedbound (capable of only limited self-care), 4) Bedbound (completely disabled, incapable of any self-care), and 5) Death. Six-month mortality incidence underwent cross-check validation via the United States Social Security Death Index (SSDI).
Biomarker analyses
All subjects enrolled in the parent cohort underwent peripheral blood sampling at 12 hours, one, four, seven, and 14 days after sepsis onset, and weekly thereafter while hospitalized. These samples were processed and stored for subsequent program a priori and ad hoc biomarker analysis. This included the biomarker of interest for this analysis GLP-1 (Luminex; MilliporeSigma, Massachusetts, USA) and interleukin-6 [IL-6] (ELISA; MilliporeSigma, Massachusetts, USA). We chose to utilize IL-6 as a covariate biomarker in this analysis because it is a well-established measure of the magnitude of the host innate pro-inflammatory response. We have also shown previously that IL-6 is a predictive biomarker for CCI after sepsis (16).
Statistical Analysis
Data are presented as either frequency and percentage, or mean and standard deviation, or median and 25th/75th percentiles. Student’s t-test, ANOVA and Kruskal-Wallis tests were used for comparison of continuous variables as appropriate. Chi-square test and fisher’s exact test were used for comparison of categorical variables. Measured biomarkers were compared using non-parametric rank tests to determine significant differences between groups at each time point. Due to similarity of clinical trajectory, the small number (n=4) of early death patients were combined with the CCI cohort for all biomarker analyses in this study. In order to determine if GLP-1 was associated with adverse outcomes independent of inflammation and glucose dysregulation, two multivariate logistic regression models were constructed to determine if GLP-1 was associated at 24 hours of the development of CCI, and at day 14 of death or severe disability (Zubrod score 4–5) at 6-months after sepsis onset after controlling for both IL-6 and peak glucose level on those days. Adjusted odds ratios (OR) with 95% confidence intervals (95% CI) and the area under the receiver operating characteristics curve values (AUC) and Hosmer-Lemeshow goodness-of-fit test were used. Due to suspicion of high collinearity of GLP-1 with the covariates total SOFA and Charlson comorbidity scores, we performed Spearman analysis to determine correlations between these continuous and ordinal variables. All significance tests were two-sided, with p-value ≤0.05 considered statistically significant. Statistical analyses were performed with SAS (v.9.4, Cary, NC).
Results
Over an 18-month period ending September 2016, 157 consecutively enrolled study patients underwent serial GLP-1 biomarker analyses. Demographics, baseline characteristics, in-patient outcomes and long-term outcomes of these study patients are depicted in Table 1. The overall study cohort consistent primarily of middle-aged and older adults (median age 62 years) with an equal biologic sex distribution, significant comorbidity burden, a high incidence and severity of physiologic derangement as measured by vasopressor requirements, APACHE II scores (Table 1). Roughly one-third carried a pre-existing diagnosis of diabetes mellitus requiring either oral or subcutaneous (i.e. insulin) hyperglycemic control medications. The majority of patients were admitted to the hospital with an acute, infection-related diagnosis and had intra-abdominal infection as the septic source (Table 1). The severity of organ dysfunction in this septic cohort was high with a 50 percent incidence of multiple organ failure and a low inhospital mortality of approximately 8 percent (Table 1).
Table 1:
Demographics, Baseline Characteristics and Outcomes for All Study Patients
| Parameter | Overall cohort (n=157) |
|---|---|
| Age, y, median (25th, 75th) | 62 (51, 70) |
| Male sex, n (%) | 85 (54) |
| Race/ethnicity, n (%) | |
| Black or African American | 13 (8) |
| Caucasian | 139 (89) |
| Other | 5 (3) |
| Charlson comorbidity index, median (25th, 75th) | 4 (2, 6) |
| Inter-facility hospital transfer, n (%) | 68 (43) |
| Hospital admission diagnosis, n (%) | |
| Planned surgical procedure | 28 (18) |
| Intra-abdominal sepsis | 24 (16) |
| NSTI | 23 (15) |
| Surgical site infection | 16 (10) |
| Trauma | 14 (9) |
| Other - non-infectious | 12 (8) |
| Vascular disease - aorta/mesenteric | 10 (7) |
| Other acute infection | 7 (4.5) |
| Necrotizing pancreatitis | 5 (3.2) |
| UTI | 5 (3.2) |
| Pneumonia | 4 (2.6) |
| Cholecystitis | 3 (1.9) |
| Planned other surgery not specified | 2 (1.3) |
| Vascular disease, extremity | 1 (0.6) |
| Primary sepsis diagnosis, n (%) | |
| Intra-abdominal sepsis | 65 (42) |
| Pneumonia | 28 (18) |
| NSTI | 25 (16) |
| Surgical Site Infection | 21 (14) |
| UTI | 9 (6) |
| Catheter-related bloodstream infection | 2 (1.3) |
| Bacteremia | 1 (0.6) |
| Empyema | 1 (0.6) |
| Other | 2 (1.3) |
| Sepsis severity, n (%) | |
| Sepsis | 57 (36) |
| Severe Sepsis | 62 (40) |
| Septic Shock | 38 (24) |
| APACHE II, median (25th, 75th) | 17 (12, 23) |
| Maximum SOFA score, median (25th, 75th) | 8 (4, 11) |
| Multiple organ failure*, n (%) | 79 (50) |
| Ventilator days, median (25th, 75th) | 2 (0, 6) |
| ICU LOS, median (25th, 75th) | 8 (4, 17) |
| Hospital LOS, median (25th, 75th) | 17 (8, 28) |
| In-hospital mortality, n (%) | 12 (7.6) |
| 6-month mortality, n (%) | 31 (19.7) |
Denver Multiple Organ Failure score ≥3
UTI, urinary tract infection; NSTI, necrotizing soft tissue infection; SOFA, sequential organ failure assessment; LOS, length of stay
The characterization of post-sepsis clinical trajectories are depicted in Table 2. There were 4 (2.5 %) early deaths, 98 (62.5%) RAP and 55 (35%) CCI patients. There were no significant differences in hospital admission diagnosis or septic source between these groups. CCI compared to RAP patients were older, had significantly higher incidence of septic shock, and a greater severity of organ dysfunction (Table 2). While CCI patients carried a higher comorbidity burden than RAP patients, there was no difference in the rate of medication-dependent diabetes mellitus between CCI and RAP groups. CCI patients had significantly worse clinical outcomes including a greater number of ventilator days, longer ICU length of stay, and higher inpatient mortality (Table 2). While approximately 85 percent of patients that developed CCI survived to discharge, 90 percent of these were discharged with a disposition to facilities associated with poor long-term outcomes (LTAC, SNF, another hospital or hospice). Additionally, prospective post-discharge follow up revealed a much higher 6-month mortality for the CCI patients (40%) compared to RAP patients (5%, Table 2).
Table 2:
Post-Sepsis Clinical Trajectory Comparisons
| Parameter | Early death (n=4) |
CCI (n=55) |
RAP (n=98) |
P Value* |
|---|---|---|---|---|
| Age, y, median (25th, 75th) | 62 (51, 70) | 65 (58, 72) | 59 (48, 69) | 0.010 |
| Male sex, n (%) | 2 (50) | 36 (65.5) | 47 (48) | 0.045 |
| Race, n (%) | 0.54 | |||
| Black or African American | 1 (25) | 4 (7.3) | 8 (8.2) | |
| White | 3 (75) | 50 (90.9) | 86 (87.8) | |
| Other | 0 (0) | 1 (1.8) | 4 (4.0) | |
| Charlson comorbidity index, median (25th, 75th) | 4.5 (3.5, 6) | 5 (3, 8) | 3.5 (2, 6) | 0.014 |
| Comorbidity, diabetes, n (%) | 1 (25) | 21 (38.2) | 31 (31.6) | 0.38 |
| Inter-facility hospital transfer, n (%) | 3 (75) | 29 (52.7) | 36 (36.7) | 0.032 |
| Sepsis severity, n (%) | ||||
| Sepsis | 1 (25) | 11 (20) | 45 (45.9) | 0.001 |
| Severe sepsis | 0 (0) | 25 (45.5) | 37 (37.8) | |
| Septic shock | 3 (75) | 19 (34.5) | 16 (16.3) | |
| Lactic acid (mmol/L), median (25th, 75th) | 2.5 (1.6, 5.2) | 2 (1.3, 3.6) | 1.6 (1.2, 2.3) | 0.033 |
| APACHE II, median (25th, 75th) | 27 (19, 38) | 21 (15, 26) | 14 (10, 21) | <0.001 |
| Maximum SOFA score, median (25th, 75th) | 17 (11, 22) | 10 (8, 13) | 6 (3, 8) | <0.001 |
| Multiple organ failure†, n (%) | 4 (100) | 43 (78.2) | 32 (32.7) | <0.001 |
| Ventilator days, median (25th, 75th) | 5.5 (5, 10) | 7 (3, 15) | 0 (0, 2) | <0.001 |
| ICU LOS, median (25th, 75th) | 5.5 (5, 9) | 20 (15, 29) | 5 (3, 9) | <0.001 |
| Hospital LOS, median (25th, 75th) | 6 (5.5, 9.5) | 28 (20, 41) | 12 (7, 20) | <0.001 |
| Hospital mortality, n (%) | 4 (100) | 8 (14.5) | 0 (0) | <0.001 |
| Discharge disposition, n (%) | <0.001 | |||
| Good | 0 (0) | 6 (11) | 74 (76) | |
| Home | 0 (0) | 0 (0) | 29 (30) | |
| Rehab | 0 (0) | 1 (1.8) | 3 (3.1) | |
| Home healthcare services | 0 (0) | 5 (9.1) | 42 (43) | |
| Poor disposition | 4 (100) | 49 (89) | 24 (24) | |
| LTAC | 0 (0) | 24 (44) | 1 (1) | |
| SNF | 0 (0) | 7 (13) | 23 (24) | |
| Another inpatient hospital | 0 (0) | 8 (15) | 0 (0) | |
| Hospice | 0 (0) | 5 (9.1) | 0 (0) | |
| Death | 4 (100) | 5 (9.1) | 0 (0) | |
| Six-month mortality, n (%) | 4 (100) | 22 (40) | 5 (5.1) | <0.001 |
Comparison of CCI and RAP groups.
Denver Multiple Organ Failure score ≥3
SOFA, sequential organ failure assessment score; LOS, length of stay; Rehab, inpatient rehabilitation facility; LTAC, long-term acute care facility; SNF, skilled nursing facility.
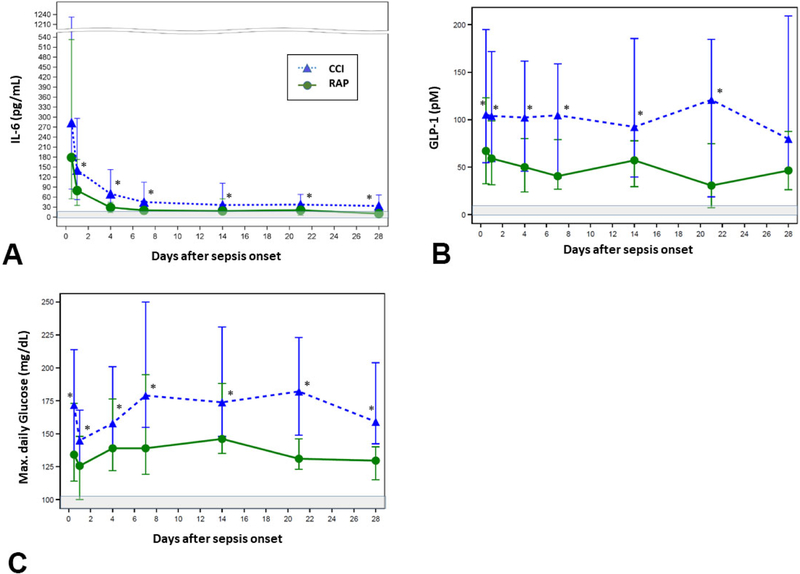
Figure 1 depicts serial IL-6, GLP-1 and maximum measured daily glucose levels. Consistent with previously published data on the known dysfunctional inflammatory response to sepsis, IL-6 levels were significantly elevated among all study patients as compared to healthy age, gender and ethnicity matched controls out to 28 days after sepsis onset (data not shown). Additionally, IL-6 levels were significantly elevated in CCI patients as compared to the RAP group at all measured time-points between 24 hours and 28 days after sepsis onset (Figure 1A). GLP-1 levels were significantly elevated among CCI patients at all measured time points from sepsis onset out to 21 days as compared to RAP (Figure 1B). Maximum daily blood glucose levels were significantly elevated in CCI patients as compared to the RAP group across all measured time points (Figure 1C).
Figure 1.
Post-sepsis biomarker trajectories. Select biomarker levels measured from peripheral blood samples collected at 0.5, 1, 4, 7, 14 and 28 days after sepsis onset, while hospitalized. (A) Interleukin-6 (IL-6, (B) Glucagon-like peptide-1 (GLP-1), (C) Maximum measured daily glucose. Grey-shaded areas represent standard reported normal ranges.
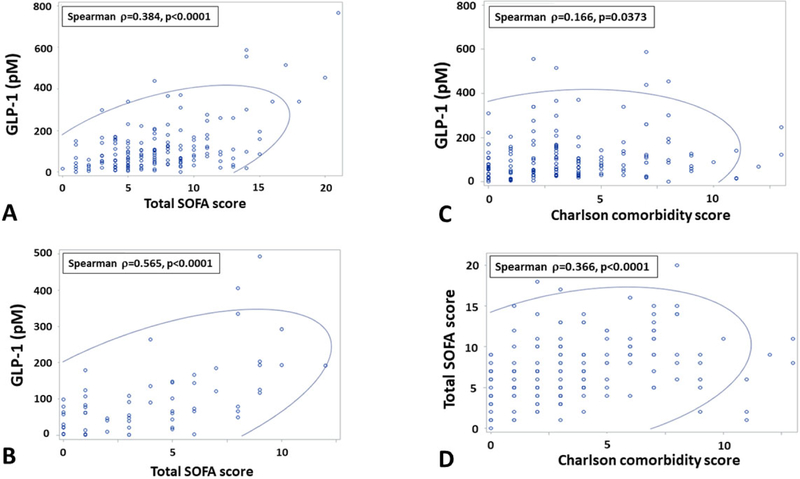
Table 3 depicts the results of the multivariate models for association of GLP-1 with CCI and 6-month outcomes. Across all time points, GLP-1 and maximal daily blood glucose levels were modestly, but significantly correlated (Spearman correlation=0.27, p<0.0001). However, GLP-1 level at 24 hours after sepsis onset was independently associated with CCI after controlling for IL-6 and blood glucose levels. Similarly, after controlling for IL-6 and blood glucose levels, persistently elevated GLP-1 level at day 14 was associated with death or severe disability (i.e., Zubrod score 4 or 5) at 6-months (Table 3). Inclusion of total insulin as a covariate did not affect these models. Of note, Charlson comorbidity index score and total SOFA score at 24 hours and 14 days are strong composite (i.e., multidomain) predictors of both CCI and 6 month mortality and eliminate all serum biomarkers (IL-6, glucose, GLP-1) when included in the stepwise selection models. We therefore tested correlations of GLP-1 with baseline Charlson comorbidity index and total SOFA score at 24 hours and 14 days. GLP-1 levels were strongly correlated with total SOFA score at 24 hours (Spearman rho=0.384, p<0.0001; Figure 2A) and 14 days (Spearman rho=0.59, p<0.0001; Figure 2B). GLP-1 levels were modestly correlated with baseline Charlson comorbidity score (Spearman rho=0.37, p<0.0001; Figure 2C). Total SOFA Score at 24 hours and 14 days was also significantly correlated with baseline comorbidity (Figure 2D).
Table 3.
Multivariate Models for Association of GLP-1 with Chronic Critical Illness and 6-Month Outcomes
| Model/covariate | Odds ratio (95% CI)† | P Value | AUC |
|---|---|---|---|
| 24-hour level predicting chronic critical illness | 0.689 | ||
| GLP-1 | 1.004 (1.000–1.007) | 0.027 | |
| IL-6 | 1.000 (1.000–1.000) | 0.076 | |
| Glucose | 1.005 (1.000–1.010) | 0.035 | |
| Day-14 level predicting 6-month death/severe disability* | 0.717 | ||
| GLP-1 | 1.011 (1.002–1.020) | 0.014 | |
| IL-6 | 0.995 (0.986–1.004) | 0.27 | |
| Glucose | 1.003 (0.994–1.013) | 0.50 |
Zubrod score of 4 (severe disability) or 5 (Death)
Odds ratio represents the odds increase for an increase in a 1-unit increase in biomarker level: GLP-1 (nM), IL-6 (pg/mL), glucose (md/dL).
AUC, multivariate model area under the curve.
Figure 2.
Correlation analysis of GLP-1, organ dysfunction and comorbidity burden. (A) Correlation plot of GLP-1 level and total Sequential Organ Failure Assessment (SOFA) score at 24 hours. (B) Correlation plot of GLP-1 level and total SOFA score at 14 days. (C) Corrleation plot of GLP-1 level at 24 hours and Charlson comorbidity score. (D) Correlation plot of Total SOFA score and Charlson comorbidity score. Blue oval represents 95% prediction ellipse.
Discussion
Gut hormones, such as GLP-1, facilitate metabolism of glucose through stimulation of insulin secretion and various other mechanisms. It has been well established that trauma and sepsis result in loss of glycemic homeostasis and that the resulting hyperglycemia adversely impacts immune function, wound healing and metabolism resulting in poor outcomes.(1–9, 21–24) The mechanisms responsible for this glucose dysregulation are complex. However, based on the normal physiologic role of GLP-1, it should play an important role regarding metabolic regulation in septic ICU patients.(25–31) Indeed, Deane and colleagues have demonstrated that the exogenous administration of GLP-1 analogues in critically ill populations has a modulating effect on glucose homeostasis (32–34). Our evaluation of septic surgical patients confirms these observations. Both GLP-1 levels and glucose levels were consistently and persistently elevated in patients that develop CCI. GLP-1 and blood glucose levels showed modest, but significant correlation in this group. The most straightforward explanation for this finding is that elevated GLP-1 levels were in response to high blood glucose. This is the well documented function of the incretin system, as GLP-1 receptors present on pancreatic islet cells increase insulin secretion. However, extrapancreatic functions of GLP-1 appear to be involved in the response to sepsis. Additionally, our multivariate models clearly demonstrated that an elevated GLP-1 level at 24 hours were independent of both IL-6 and maximum glucose levels, and is associated with the development of CCI. Similarly, elevated GLP-1 levels at 14 days after the onset of sepsis independently is associated with death or severe disability in the subsequent 6 months. This suggests that GLP-1 response after sepsis is independent of both systemic inflammation and glucose regulation. To our surprise, this analysis showed that elevated GLP-1 levels is a better predictor of outcome than IL-6. While CCI compared to RAP patients were older, more likely to require vasopressor support and had more medical comorbidities, they did not have a greater incidence of pre-sepsis diabetes requiring pharmacologic control. Again, this is suggestive of factors other than simple hyperglycemia causing compensatory elevation of GLP-1 levels.
GLP-1 secretion has been demonstrated to rapidly increase in response to cytokines, particularly IL-6. Ellingaard et al, showed that administration of IL-6 stimulate GLP-1 secretion from intestinal L cells and pancreatic alpha cells.(35) This mechanism increased insulin secretion and improved glycemic control. This group concluded that IL-6 mediates crosstalk between insulin sensitive tissues, intestinal L cells and pancreatic islet cells to adapt to changes in insulin demand. LeBrun et al, noted that GLP-1 suppresses inflammation and promotes gut mucosal integrity.(36) Furthermore this group demonstrated that GLP-1 levels increased rapidly after the administration of lipopolysaccharide (LPS) in mice. This phenomenon was detected prior to measurable changes in cytokine levels or LPS. A similar response was noted after gut ischemia (34). Lebherz et al, have previously demonstrated the predictive importance of GLP-1 levels in critically ill patients.(37) This group measured GLP-1 levels in critically ill patients admitted to an ICU, patients with chronic kidney disease on hemodialysis and a control group without acute inflammation or kidney disease. Critically ill patients had a 6-fold increase in GLP-1 levels compared to the control group. Those requiring hemodialysis exhibited a 4-fold higher GLP-1 level compared to controls. This group concluded that both chronic and acute inflammatory states, including sepsis, increase circulating GLP-1 levels. Furthermore, this group demonstrated in vivo that serum from critically ill patients had a strong potential for inducing GLP-1 secretion. This group concluded that elevated GLP-1 levels independently predicted mortality in critically ill and end-stage renal disease patients.
Given these aforementioned findings, it is easy to hypothesize that elevated GLP-1 levels are merely another compensatory biomarker of dysregulated glucose control after an acute, severe pro-inflammatory stressor. However, another possibility may be that GLP-1 elevation is representative of (or even contributory to) persistently deranged metabolism and a catabolic state after sepsis. Given the findings here of high GLP-1 correlation with both early and persistent organ dysfunction severity and comorbidity burden, it is possible that elevated GLP-1 levels after sepsis may represent non-insulin dependent metabolic dysregulation driven by organ dysfunction and exacerbated by pre-existing comorbidities.
Based on substantial clinical and basic research data available in 2012, we proposed that failure to achieve immune homeostasis after a septic insult results in a pathophysiologic syndrome of persistent inflammation, immunosuppression and catabolism (PICS).(14) We believe PICS is the driving mechanism for the development of CCI and its dismal long-term outcomes after surgical sepsis. Our subsequent and ongoing work, strongly supports this hypothesis.(15–18) Of note, our studies show that non-recovery from acute kidney injury (AKI) is the strongest predictor dismal outcomes in CCI patients and believe this perpetuates inflammation and immunosuppression through the sustained local and systemic release of damage associated molecular patterns (DAMPS), cytokines and the expansion of myeloid derived suppressor cells (MDSCs) and through metabolic reprograming (i.e. aerobic glycolysis). Normal cells metabolize glucose through glycolysis, and under normoxic conditions, the pyruvate that is generated is further oxidized in mitochondria to produce adenosine triphosphate (ATP). When oxygen is limited, mitochondrial oxidative metabolism is restricted and pyruvate is only converted to lactate, with minimal ATP production. In tumor cells, this latter process is dominant even when oxygen is plentiful and is known as “the Warburg effect” or aerobic glycolysis. As active innate immune effector cells rely on glucose primarily for cellular fuel, a switch to aerobic glycolysis is thought to occur as a compensatory mechanism. After sepsis persistently elevated levels of GLP-1 may represent an underlying shift in metabolic programming towards aerobic glycolysis, and serve as a metabolic marker of the development of PICS pathophysiology and prognosticate a high risk of poor long-term CCI outcomes.
Conclusions
Elevated circulating GLP-1 levels within 24 hours of sepsis are strongly associated with early death or the development of CCI independent of the known modulating effects of IL-6 and glucose dysregulation. Similarly among early survivors, persistently elevated GLP-1 levels at day 14 was also independently associated with death or severe functional disability at 6 months. Of note, elevated GLP-1 levels appear to be more strongly associated with a poor outcome than IL-6. Given significant correlation with organ dysfunction severity and pre-existing comorbidities, persistently elevated GLP-1 levels may be a marker of ongoing stress metabolism and a non-resolving catabolic state, associated with muscle wasting and dismal CCI outcomes after sepsis. Future work should focus on elaborating these underlying mechanisms.
Supplementary Material
Support:
This study was supported in part by National Institute of General Medical Sciences (NIGMS) grants: R01 GM-113945 (PE), T32 GM008721 (MC) and P50 GM-111152 (SB, PE, FM) awarded by the National Institute of General Medical Sciences (NIGMS). Support was also provided by National Institute on Aging (NIA) grants R03 AG056444 (SB), P30 AG028740 (SB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
ClinicalTrials.gov Identifier: NCT02276417
Presented at the Western Surgical Association 126th Scientific Session, San Jose del Cabo, Mexico, November 2018.
References
- 1.Yendamuri S, Fulda GJ, Tinkoff G. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma 2003; 55: 33–38. [DOI] [PubMed] [Google Scholar]
- 2.Laird AM, Miller PR, Kilgo PD, et al. Relationship of early hyperglycemia to mortality in trauma patients. J Trauma 2004; 56: 1058–62. [DOI] [PubMed] [Google Scholar]
- 3.Bochcchio GV, Sung J, Joshi M, et al. Persistent hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma 2005; 58: 921–24. [DOI] [PubMed] [Google Scholar]
- 4.Vanhorebeek, Langouche L, Van den Berghe G. Tight blood glucose control: what is the evidence? Crit Care Med 2007; S496–502. [DOI] [PubMed] [Google Scholar]
- 5.Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345: 1359–67. [DOI] [PubMed] [Google Scholar]
- 6.Van den Berghe G, Wouters PJ, Bouillon R, et al. Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemia control. Crit Care Med 2003; 31: 359–66. [DOI] [PubMed] [Google Scholar]
- 7.Mohr AM, Lavery RF, Sifri ZC et al. Gender differences in glucose variability after severe trauma. Am Surg 2010;76(8):896–902. [PubMed] [Google Scholar]
- 8.Wiener RS, Wiener DC, Larson RKJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300(8):933–44. [DOI] [PubMed] [Google Scholar]
- 9.Dubose JJ, Scalea TM. Glucose elevations and outcomes in critically injured trauma patients. Adv Surg 2011; 45: 187–196. [DOI] [PubMed] [Google Scholar]
- 10.Smith RS, Fry WR, Philp FH, Philp AS, Berry SD, Helmer S. Mild hyperglycemia, but not glucagon-like peptide 1 predicts poor outcome after injury. Am J Surg 2012; 204:915–920. [DOI] [PubMed] [Google Scholar]
- 11.McKinley BA, Moore LJ, Sucher JF, Todd SR, Turner KL, Valdivia A, Sailors RM, Moore FA. Computer Protocol Facilitates Evidence-Based Care of Sepsis in the Surgical Intensive Care Unit. JTrauma May 2011;70(5):1153–67. [DOI] [PubMed] [Google Scholar]
- 12.Croft CA, Moore FA, Efron PA, et al. Computer versus paper system for recognition and management of sepsis in surgical intensive care. J Trauma Acute Care Surg. 2014;76(2):311–7. [DOI] [PubMed] [Google Scholar]
- 13.Gardner AK, Ghita GL, Wang Z, et al. The Development of Chronic Critical Illness Determines Physical Function, Quality of Life, and Long-Term Survival Among Early Survivors of Sepsis in Surgical ICUs.Crit Care Med. 2019. April;47(4):566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentile LF1, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. JTrauma Acute Care Surg. 2012. June;72(6):1491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mira JC, Gentile LF, Mathias BJ, et al. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit Care Med. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stortz JA, Mira JC, Raymond SL, et al. Benchmarking clinical outcomes and the immunocatabolic phenotype of chronic critical illness after sepsis in surgical intensive care unit patients. J Trauma Acute Care Surg 2018; 84(2):342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkins RB, Raymond SL, Stortz JA, et al. Chronic Critical Illness and the Persistent Inflammation, Immunosuppression and Catabolism Syndrome. Front Immunol 2018; 9:1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Efron PA, Mohr AM, Bihorac A, et al. Persistent inflammation, immunosuppression and catabolism and the development of chronic critical illness after surgery. Surgery 2018; 164(2):178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loftus TJ, Mira JC, Ozrazgat-Baslanti T, et al. Sepsis and Critical Illness Research Center investigators: protocols and standard operating procedures for a prospective cohort study of sepsis in critically ill surgical patients. BMJ Open. 2017;7(7):e015136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–6. [DOI] [PubMed] [Google Scholar]
- 21.Sung J, Bichiccio GV, Joshi M, et al. Admission hyperglycemia is predictive of outcomes in critically ill trauma patients. J Trauma 2005; 59: 80–83. [DOI] [PubMed] [Google Scholar]
- 22.Bochicchio GV, Salzano L, Joshi M, et al. Admission preoperative glucose is predictive of morbidity and mortality in trauma patients who require immediate operative intervention. Am Surg 2005; 71: 171–174. [DOI] [PubMed] [Google Scholar]
- 23.Chi A, Lisauer ME, Kirchoffner J, Scalea TM, Johnson SB. Effect of glycemic state on hospital mortality in critically ill surgical patients. Am Surg 2011; 77: 1483–1489. [DOI] [PubMed] [Google Scholar]
- 24.Dosset LA, Cao H, Mowery NT, Dortch MJ, Morris JM, May AK. Blood glucose variability is associated with mortality in the surgical intensive care unit. Am Surg 2008; 74: 679–685. [DOI] [PubMed] [Google Scholar]
- 25.Dupre J, Ross SA, Watson D, et al. Stimulation of insulin secretion by gastric inhibitory peptide in man. J Clin Endocrinol Metab 1973; 37: 826–28. [DOI] [PubMed] [Google Scholar]
- 26.Nauck MA, Heimesaat MM, Behle K, et al. Effects of glucagon-like peptide 1 on counter regulatory hormone responses , cognitive functions, and insulin secretion during hyperinsulinemic , stepped hypo glycemic clamp experiments in healthy volunteers . J Clin Endocrinol Metab 2002; 87: 1239–46. [DOI] [PubMed] [Google Scholar]
- 27.Scrocchi LA, Brown TJ, McClusky N, et al. Glucose intolerance but not satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 1996; 2: 1254–58. [DOI] [PubMed] [Google Scholar]
- 28.Nauck MA, Meier JJ. Glucagon-like peptide 1 (GLP-1) and its derivatives in the treatment of diabetes.Regul Pept 2005; 124 (Suppl): 135–148. [DOI] [PubMed] [Google Scholar]
- 29.Schirra J, Nicolaus M, Roggel R, Katschinski R, Storr M, Woerle HJ, Goke B. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and anteropyloro-duodenal motility in humans. Gut 55: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamanoto H, Lee CE, Marcus JN, et al. Glucagon-like peptide 1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 2002; 110: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abu-Hamdah R, Rabiee A, Meneilly GS, Shannon RP, Anderson DK, Elahi D. The extrahepatic effects of glucagon-like peptide 1 and related peptides. J Clin Endocrinol Metab 2009; 94: 1843–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deane AM, Chapman MJ, Fraser RJL, et al. Effects of exogenous glucagon-like peptide-1 on gastric emptying and glucose absorption in the critically ill: relationship to glycemia. Crit Care Med 2010; 38: 1261–1269. [DOI] [PubMed] [Google Scholar]
- 33.Deane AM, Summers MJ, Zaknic AV, et al. Exogenous glucagon-like peptide-1 attenuates the glycaemic response to postpyloric nutrient infusion in critically ill patients with type 2 diabetes. Critical Care 2011; 15: R35, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deane AM, Chapman MJ, Fraser RJL, et al. The effect of exogenous glucagon-like peptide-1 on the glycaemic response to small intestinal nutrient in the critically ill: a randomized double-blind placebo-controlled crossover study. Critical Care 2009; R67 (doi: 10.1186/cc7874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellingaard H, Hauselmann I, Schuler B, et al. Interleuken −6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nature Medicine 2011; 17: 1481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeBrun LJ, Lenaerts k, Kiers D, et al. Enteroendocrine L cells sense LPS gut barrier injury to enhance GLP-1 secretion. Cell Rep 2017; 21: 1160–1168. [DOI] [PubMed] [Google Scholar]
- 37.Lebherz C, Schlieper G, Mollmann J, et al. GLP-1 levels predict mortality in patients with critical illness as well as end-stage renal disease. Am J Med 2017; 130: 833–841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.