Abstract
Background:
Adjuvant immunotherapy has improved outcomes in patients with advanced melanoma; however, the potential benefit for patients with pancreatic ductal adenocarcinoma (PDAC) remains unknown. The aim of this study is to determine the impact of adjuvant chemotherapy and immunotherapy (CTx-IT) compared to CTx alone on patient survival following resection of PDAC.
Study Design:
Patients who underwent resection of PDAC from 2004 to 2015 were identified from the National Cancer Database (NCDB). Univariate and multivariate Cox proportional hazards models were utilized to determine predictors of overall survival (OS) based on the type of adjuvant therapy received. Patients who received adjuvant immunotherapy were compared to those who received adjuvant CTx alone by propensity score matching.
Results:
Of 21,313 patients who received curative-intent resection for PDAC followed by adjuvant systemic therapy, 269 patients (1.3%) were treated with adjuvant CTx-IT. Propensity-score matching resulted in a cohort of 477 patients: (229 CTx only and 248 CTx-IT). The 5-year OS was higher in the CTx-IT group compared with CTx alone (29.2% vs. 18.3%, P=0.0045). On multivariate analysis, the addition of adjuvant immunotherapy was associated was improved overall survival (HR 0.74, P=0.007).
Conclusion:
The addition of adjuvant immunotherapy to chemotherapy is associated with improved survival compared to chemotherapy alone after curative-intent resection of pancreatic adenocarcinoma. Future research is warranted to match specific immunotherapy agents with susceptible patient populations to improve outcomes for this aggressive disease.
Keywords: Immunotherapy, Pancreatic adenocarcinoma, chemotherapy, Pancreatic cancer
PRÉCIS:
Patients who underwent curative-intent resection of pancreatic adenocarcinoma experienced improved overall survival after receipt of adjuvant immunochemotherapy compared to chemotherapy alone. Receipt of immunotherapy was associated a survival advantage even in patients with adverse risk factors including R1 margins, node positive, and poorly differentiated disease.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy and is the fourth leading cause of cancer-related mortality in the United States. The incidence and mortality rates are nearly equal, with 55,440 diagnoses and 44,330 cancer-associated deaths projected in 2018.(1) Complete surgical resection remains the mainstay of curative-intent treatment; however, only approximately 10% of patients have disease amenable to complete resection.(2-4) Despite aggressive multidisciplinary care, the 5-year survival for PDAC remains less than 25% even after complete resection with microscopically negative (R0) margins. What is most concerning for the future is that while patient selection, perioperative care, and operative mortality have improved over time, cancer-related mortality has remained largely unchanged.(5) Locoregional and distant recurrence rates approach 80%, which is likely secondary to the presence of occult micro-metastatic disease at the time of resection. (6, 7) The high rate of recurrence underscores the need for more effective systemic adjuvant therapies in this disease.
Based on randomized controlled trials, adjuvant systemic chemotherapy after pancreatectomy has been shown to improve survival and is the standard of care in medically fit patients. CONKO-001 established the role of adjuvant gemcitabine in improving overall survival compared to the observation,(8) and more recent trials have built on this backbone demonstrating further improvements in survival outcomes with multi-drug chemotherapy regimens.(9-12)
The role of adjuvant immunotherapy in PDAC, however, remains unclear. It has rapidly emerged as a novel effective therapy in multiple malignancies in both the metastatic and adjuvant setting, therefore there is great excitement for utilization after pancreatic resection for adenocarcinoma.(13-19) Further, there has been increasing evidence that certain chemotherapies may enhance anti-tumor immune responses. As a result, the premise that combination chemo-immunotherapy may enhance outcomes in PDAC is well-formulated. Given the paucity of data and small single institution studies on combination chemo-immunotherapy in patients with PDAC, the objective of this study was to evaluate outcomes of adjuvant chemotherapy and immunotherapy compared to chemotherapy alone using a large population-based database in a propensity score matched study following resection of PDAC.
METHODS
Patient population and study design
The National Cancer Database (NCDB) was utilized to identify patients who underwent curative intent resection of PDAC. The NCDB is part of a joint program between the American College of Surgeons Commission on Cancer (COC) and the American Cancer Society consisting of approximately 70% of all newly diagnosed malignancies in the United States.(20, 21) The database captures clinicopathologic characteristics from more than 1,500 COC-accredited hospitals in the United States. Patients with primary diagnosis of adenocarcinoma combined with site-specific code for pancreatic tumors (C25.1-C25.4, C25.7-C25.9) were identified using relevant International Classification of Oncology, 3rd edition (ICD-O-3) histology codes. Only patients with pathologically confirmed PDAC who underwent curative-intent resection were included. Patients with R2 resection and distant metastases were excluded. Given previously established Level 1 data on the association between adjuvant chemotherapy and improved survival after resection of PDAC, patients who did not receive adjuvant chemotherapy were excluded from the study.(8, 22, 23)
Patients were classified according to first line adjuvant therapy: chemotherapy alone (CTx) or chemotherapy plus immunotherapy (CTx-IT). NCDB defines and captures immunotherapy as a treatment using a “biological or chemical agent that alter[s] the immune system or change[s] the host’s response to tumor cells”. eTable 1 includes drugs classified as biologic response modifiers (BRM) or immunotherapy for pancreatic cancer in the SEER-Rx database.
Statistical Analysis
Continuous variables were presented as medians with interquartile range (IQR) and compared using Mann-Whitney U test. Categorical variables were presented as frequency and percentages and compared using Pearson chi-square or Fisher’s exact test, where appropriate. Overall survival (OS) was calculated using Kaplan Meier method and compared using log-rank test. Univariate and multivariate survival analyses were performed using Cox proportional hazard models and expressed as hazard ratios (HR) with 95% confidence intervals (CI).
Propensity score matching was performed using the nearest-neighbor algorithm with a caliper of 0.01 to estimate a propensity score and to create a comparable matched cohort. Patients who underwent adjuvant CTx-IT were matched to those who received adjuvant CTx only. The propensity score was estimated using a multivariate logistic regression model with adjuvant CTx-IT as the treatment of interest. Standardized difference was calculated to evaluate balance in the covariates after matching.
All statistical analyses were performed using SPSS version 24.0 (IBM, Chicago, IL) and STATA 13.0 statistical software package (STATA Corp, College Station, TX). Significance was set at a P value of <0.05 (two-tailed).
RESULTS
Baseline Characteristics of the adjuvant CTx and CTx-IT Groups
Between 2004 to 2015, 21,313 patients received curative-intent resection for PDAC followed by adjuvant systemic therapy. Of these patients, 21,044 received adjuvant CTx only and 269 patients (1.3%) received first-line adjuvant CTx-IT. Table 1 details clinicopathologic characteristics of these patients. Compared with those who received adjuvant CTx alone, patients who received CTx-IT tended to be younger (median 65 vs 62 years, P<0.001) and have a Charlson Deyo Score of 0 (80.3% vs. 68.9%, P<0.001). The majority of patients treated with CTx-IT were at academic centers (78% vs. 48.9%, P<0.001) and had private insurance (58.4 vs. 41.9%, P<0.001). There were no significant differences in tumor size, lymphovascular invasion, grade, number of lymph nodes retrieved, margin status, stage, or type of operation performed.
Table 1.
Clinicopathologic Characteristics of Chemotherapy Alone, and Chemotherapy and Immunotherapy in the Entire Cohort and Propensity Score Matched Cohort
| Characteristic | Entire cohort | Matched cohort | ||||
|---|---|---|---|---|---|---|
| CTx (n=21,044) |
CTx+IT (n=269) |
p Value |
CTx (n=229) |
CTx+IT (n=248) |
p Value |
|
| Female sex, n (%) | 10,182 (48.4) | 122 (45.4) | 0.237 | 102 (44.5) | 112 (45.2) | 0.992 |
| Age, y (median, IQR) | 65 (5-72) | 65 (55-68) | <0.001 | 61 (54-68) | 62 (55-68) | 0.715 |
| Race, n (%) | ||||||
| White | 18,216 (86.6) | 240 (89.2) | 0.006 | 206 (89.9) | 220 (88.7) | 0.490 |
| Black | 1,986 (9.4) | 12 (4.5) | 14 (6.1) | 12 (4.8) | ||
| Asian | 368 (1.8) | 8 (2.9) | 3 (1.3) | 8 (3.2) | ||
| Other | 474 (2.3) | 9 (3.4) | 6 (2.6) | 8 (3.2) | ||
| Facility, n (%) | ||||||
| Academic | 10,204 (48.9) | 206 (78.0) | <0.001 | 108 (51.9) | 195 (79.9) | <0.001 |
| Community | 1,112 (5.3) | 1 (0.4) | 9 (4.3) | 1 (0.4) | ||
| Comprehensive | 6,954 (33.4) | 47 (17.8) | 65 (31.3) | 39 (16) | ||
| Integrated network | 2,580 (12.4) | 10 (3.8) | 26 (12.5) | 9 (3.7) | ||
| Insurance status, n (%) | ||||||
| None | 507 (2.5) | 3 (1.1) | <0.001 | 10 (4.7) | 3 (1.2) | 0.036 |
| Private | 8,666 (41.9) | 157 (58.4) | 110 (51.2) | 145 (58.5) | ||
| Government | 11,140 (53.9) | 98 (36.4) | 92 (42.8) | 92 (37.1) | ||
| Unknown | 373 (1.8) | 11 (4.1) | 3 (1.4) | 8 (3.2) | ||
| Charlson Comorbidity Index, n (%) | ||||||
| 0 | 14,245 (68.9) | 216 (80.3) | 0.001 | 183 (79.9) | 200 (80.6) | 0.979 |
| 1 | 5,113 (24.7) | 44 (16.4) | 37 (16.2) | 40 (16.2) | ||
| 2 | 1,016 (4.9) | 7 (2.6) | 7 (3.1) | 6 (2.4) | ||
| 3 | 213 (15) | 2 (0.7) | 2 (0.9) | 2 (0.8) | ||
| Tumor size, mm (median, IQR) | 31 (25-40) | 30 (23-40) | 0.774 | 31 (23-40) | 30 (23-40) | 0.857 |
| Lymphovascular invasion (n=5,024) | 5,024 (48.9) | 44 (47.3) | 0.835 | 55 (56.1) | 43 (47.3) | 0.223 |
| Grade, n (%) | ||||||
| Well | 1,602 (7.6) | 26 (9.67) | 0.056 | 14 (16.1) | 23 (9.3) | 0.591 |
| Moderate | 9,166 (43.6) | 97 (36.06) | 90 (39.3) | 95 (38.3) | ||
| Poor | 6,812 (32.4) | 54 (34.20) | 79 (34.5) | 86 (34.7) | ||
| Unknown | 3,464 (16.5) | 54 (20.07) | 46 (20.1) | 44 (17.7) | ||
| Total lymph nodes retrieved, median (IQR) | 14 (8-20) | 15 (9-21) | 0.079 | 14 (9-21) | 15 (10-22) | 0.156 |
| Margin, n (%) | ||||||
| R0 | 15,695 (78.45) | 200 (77.22) | 0.648 | 171 (74.7) | 189 (76.2) | 0.697 |
| R1 | 4,312 (21.55) | 59 (22.78) | 58 (25.3) | 59 (23.8) | ||
| T stage, n (%) | ||||||
| T1 | 1,294 (6.7) | 14 (6.2) | 0.995 | 13 (6.4) | 13 (6.0) | 0.826 |
| T2 | 2,663 (13.8) | 33 (14.6) | 23 (11.3) | 31 (14.3) | ||
| T3 | 14,512 (75.4) | 171 (75.7) | 161 (78.9) | 165 (76.0) | ||
| N stage, n (%) | ||||||
| N0 | 6,586 (34.4) | 66 (29.2) | 0.105 | 68 (33.3) | 64 (49.5) | 0.396 |
| N1 | 12,547 (65.6) | 160 (70.8) | 136 (66.7) | 153 (70.5) | ||
| Stage, n (%) | ||||||
| I | 2,492 (12.2) | 25 (9.7) | 0.289 | 19 (8.3) | 24 (9.7) | 0.856 |
| II | 16,858 (82.7) | 215 (83.7) | 195 (85.2) | 209 (84.3) | ||
| III | 1,048 (5.1) | 17 (6.6) | 15 (6.6) | 15 (6.0) | ||
| Type of operation, n (%) | ||||||
| Distal pancreatectomy | 2,572 (12.22) | 28 (10.41) | 0.665 | 23 (10.0) | 27 (10.9) | 0.468 |
| Pancreaticoduodenectomy | 14,884 (70.73) | 191 (71) | ||||
| Total pancreatectomy | 2,785 (13.23) | 41 (15.24) | 26 (11.4) | 40 (16.1) | ||
| Pancreatectomy NOS | 803 (3.82) | 9 (3.35) | 4 (1.8) | 4 (1.6) | ||
| Radiotherapy, n (%) | ||||||
| No | 9,468 (45.2) | 64 (23.8) | <0.001 | 53 (23.1) | 57 (22.98) | 0.967 |
| Yes | 11,497 (54.8) | 205 (76.2) | 176 (76.9) | 191 (77.0) | ||
CTx, chemotherapy; IQR, interquartile range; IT, immunotherapy; NOS, not otherwise specified.
Survival Analysis
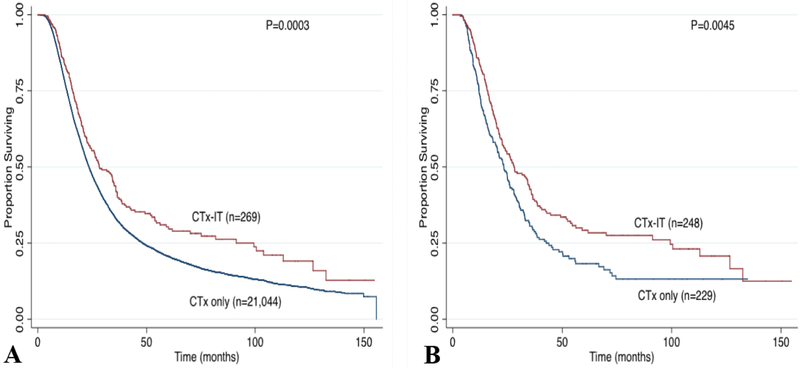
Median time from surgery to receipt of adjuvant therapy was 55 days. The median follow-up time was 21.8 months. Survival analysis of the entire cohort demonstrated that the 5-year OS was significantly higher in patients treated with adjuvant CTx-IT compared to CTx alone (30.3% vs. 20.6%, P=0.003, Figure 1A). When stratified by stage, the 5-year OS after adjuvant CTx-IT was similar in Stage I (38.5% vs. 38.4%, P=0.534) and Stage III disease (41% vs. 12%, P=0.0824), but was associated with improved survival in Stage II patients (27.6% vs. 18.6%, P=0.0011).
Figure 1.
Kaplan Meier survival curve stratified by type of adjuvant therapy: (A) unmatched cohort and (B) matched cohort. CTx, chemotherapy; IT, immunotherapy.
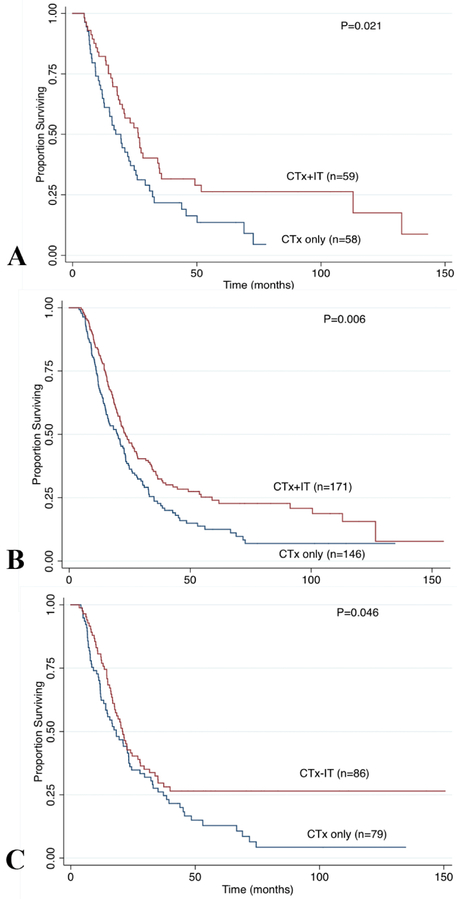
Propensity-score matching based on age, sex, race, tumor characteristics, comorbidity index, and treatment resulted in a cohort of 477 patients: 229 CTx only and 248 CTx-IT (Table 1). The median follow-up was 23.3 months in the matched cohort (22.7 months for CTx only group and 24.6 months for CTx-IT). The 5-year OS remained higher in the CTx-IT group compared with CTx alone (29.2% vs. 18.2%, P=0.0045; Figure 1B). Subgroup survival analyses demonstrated improvement in 5-year OS in the CTx-IT group even among those with positive margins (26.3% vs. 13.6%; P=0.021), node positive disease (24% vs. 12.5%; P=0.006), and poorly differentiated histology (26.5% vs. 12.8%; P=0.046) (Figure 2). When stratified by stage, the 5-year OS after adjuvant CTx-IT was improved in Stage II (5-year OS 27.1% vs. 17.6%, P=0.020), but not Stage I (40.0% vs. 24%, P=0.327) or Stage III (50% vs. 20%, P=0.222).
Figure 2.
Subgroup analysis on overall survival comparing CTx (chemotherapy) only vs CTx+IT (immunotherapy) in the propensity matched cohort factors with high risk factors: (A) R1 margin status, (B) positive lymph node, and (C) poorly differentiated disease.
After adjusting for patient, pathologic, and treatment characteristics; multivariate survival analysis of the entire unmatched cohort revealed that adjuvant CTx-IT was an independent predictor of improved OS (HR 0.75, 95% CI 0.643-0.885, P=0.001) while older age, R1 margins, advancing stage, and high-grade histology were poor prognostic factors (Table 2). Multivariable analysis of the propensity-matched cohort demonstrated that only non-poorly differentiated histology (HR 1.90, 95% CI 1.18-3.05; P=0.008) and receipt of immunotherapy were associated with improved survival (HR 0.74, 95% CI 0.59-0.92; P=0.007)(Table 3).
Table 2.
Multivariate Analysis of Factors Associated with Overall Survival after Resection of Pancreatic Adenocarcinoma in Entire Cohort (n=17,496)
| Characteristic | Hazard ratio | 95% CI | p Value |
|---|---|---|---|
| Age, y | 1.007 | 1.005-1.009 | <0.001 |
| Sex | |||
| Male | Ref | ||
| Female | 0.970 | 0.935-1.002 | 0.069 |
| Race | |||
| White | Ref | ||
| Black | 1.045 | 0.984-1.110 | 0.149 |
| Asian | 0.912 | 0.795-1.045 | 0.186 |
| Other | 0.785 | 0.694-0.889 | <0.001 |
| Charlson Comorbidity Index | |||
| 0 | Ref | ||
| 1 | 1.258 | 1.172-1.30 | <0.001 |
| 2 | 1.601 | 1.490-1.719 | <0.001 |
| 3 | 1.227 | 1.131-1.331 | <0.001 |
| Grade | |||
| Well | Ref | ||
| Moderate | 1.265 | 1.179-1.358 | <0.001 |
| Poor | 1.613 | 1.501-1.733 | <0.001 |
| Unknown | 1.259 | 1.160-1.367 | <0.001 |
| Margin status | |||
| R0 | |||
| R1 | 1.550 | 1.487-1.614 | <0.001 |
| Adjuvant therapy | |||
| CTx | Ref | ||
| CTx+IT | 0.766 | 0.653-0.897 | 0.001 |
| Stage | |||
| I | Ref | ||
| II | 1.590 | 1.500-1.686 | <0.001 |
| III | 2.002 | 1.821-2.200 | <0.001 |
| Radiotherapy | 0.924 | 0.892-0.958 | <0.001 |
| Type of operation | |||
| Distal pancreatectomy | Ref | ||
| Pancreaticoduodenectomy | 1.029 | 0.974-1.087 | 0.309 |
| Total pancreatectomy | 1.027 | 0.959-1.100 | 0.441 |
| Pancreatectomy NOS | 1.284 | 0.145-1.440 | <0.001 |
CTx, chemotherapy; IT, immunotherapy; NOS, not otherwise specified.
Table 3:
Multivariate Cox Regression Survival Analysis of Factors Associated with Overall Survival after Resection of Pancreatic Adenocarcinoma in Matched Cohort (n=447)
| Characteristic | Hazard ratio | 95% CI | p Value |
|---|---|---|---|
| Grade | |||
| Well | Ref | ||
| Moderate | 1.556 | 0.969-2.499 | 0.067 |
| Poor | 1.901 | 1.184-3.050 | 0.008 |
| Unknown | 1.082 | 0.634-1.848 | 0.773 |
| Margin status | |||
| R0 | Ref | ||
| R1 | 1.240 | 0.966-1.593 | 0.092 |
| Adjuvant therapy | |||
| CTx | Ref | ||
| CTx+IT | 0.737 | 0.590-0.919 | 0.007 |
| Stage | |||
| I | Ref | ||
| II | 1.492 | 0.944-2.360 | 0.086 |
| III | 1.686 | 0.879-3.231 | 0.115 |
CTx, chemotherapy; IT, immunotherapy.
DISCUSSION
The current study examined the impact of adjuvant immunotherapy on survival after curative-intent resection for PDAC. Receipt of adjuvant CTx-IT was associated with improved survival and this association persisted when controlled for age, sex, pathology, and treatment in a propensity-matched analysis. Adjuvant CTx-IT was also associated with prolonged survival compared to CTx-alone among patients with adverse risk factors such as positive margins, node positive disease, and poorly differentiated histology.
PDAC is traditionally considered an immuno-resistant disease. Tumors traditionally reflect a lack of tumor infiltrating lymphocytes and a plethora of suppressor T-cells. This may be one of the reasons that immune-based monotherapy has not resulted in the same clinical responses in PDAC compared to other tumor histologies. However, multiple strategies are being pursued to sensitize cells to anti-tumor immune responses. In particular, chemo-immunotherapy may synergize to improve outcomes over chemotherapy alone given increasing evidence that some chemotherapies may enhance anti-tumor immune responses. Gemcitabine has been shown to improve host immune recognition of malignant cells by stimulating dendritic cell maturation, increasing epitope presentation on tumor cells, and decreasing tumor infiltrating myeloid-deprived suppressor cells.(24-26) Oxaliplatin has been shown to upregulate cancer death associated modules and immunogenic cell death, while cyclophosphamide has been shown to promote dendritic cell maturation and upregulate HLA molecules on tumor cells.(27, 28) Thus, the combination of immunotherapies with chemotherapy represents a promising strategy to stimulate immunogenicity, inhibit tumor-mediated immunosuppression, and improve survival.
Checkpoint blockade immunotherapy has resulted in impressive responses in the metastatic setting of various tumor histologies, and more recently has been tested in the adjuvant setting. Specifically, FDA-approval has already been granted for the use of either adjuvant nivolumab or pembrolizumab in advanced melanoma(29, 30), cervical cancer(31), bladder cancer(19, 32), and renal cancer(33) based on the results of recent clinical trials. In addition to checkpoint blockade, tumor vaccines, monoclonal antibodies, adoptive cell transfer, and immune modulators have shown impressive efficacy when delivered systemically or, in some cases, intratumoral (15, 17-19, 34-36). Checkpoint blockade, either alone or in combination with chemotherapy, has been studied in early stage PDAC trials, but to date have not led to FDA-approval in metastatic pancreas cancer, and use has been limited in the adjuvant setting (37). Similarly, though adjuvant vaccines have been shown to stimulate anti-tumor T-cell responses and have been employed to a greater degree in the PDAC, clear efficacy in substantial numbers of patients has been elusive(38-40) IL-2, interferon alpha-2b, and IL-10 have also been used as immunostimulatory agents in combination with chemotherapy for PDAC with measurable activity in the adjuvant setting, though larger numbers of patients will need to be treated to determine efficacy.(41-46)
The immunotherapy group represents only 1.3% of patients who received adjuvant therapy for PDAC, indicating that this is a very highly select group of patients with resectable PDAC, and many of these patients may have been enrolled in clinical trials. There have been several small and underpowered studies on the efficacy of chemo-immunotherapy in the adjuvant setting for PDAC. In a study of 12 patients who underwent resection for PDAC, Aguilar et al. reported that adjuvant gene-mediated cytotoxic immunotherapy resulted in an OS of 9-30 months.(47) In another study consisting of 43 patients, Matsui et al demonstrated that adjuvant adoptive immunotherapy combined with gemcitabine resulted in a disease-free survival of 15.8 months and overall survival of 24.7 months.(48) Furthermore, a Phase II study of 70 patients treated with gemcitabine and plus algenpantucel-L immunotherapy demonstrated a 12 month disease-free survival of 62% and 12-month overall survival of 86%.(49) This led to a multicenter Phase III randomized controlled trial (IMPRESS trial, NCT01072981) evaluating the impact of algenpantucel-L immunotherapy with gemcitabine in patients with surgically resected PDAC. Other ongoing trials using chemo-immunotherapy remain under investigation including combining gemcitabine with nab-paclitaxel, nivolumab and a CD40 agonistic monoclonal antibody.
To our knowledge, the current study represents the largest retrospective study on adjuvant immunotherapy with chemotherapy compared to chemotherapy alone for PDAC. However, there remain several limitations to the present study. While chemo-immunotherapy was associated with improved survival compared to chemotherapy alone, these patients were younger, had a better performance status, and were more likely to be treated at academic cancer centers. These patient and treatment factors were addressed in the propensity score analysis where age and Charleston comorbidity index were matched, nonetheless inherent selection biases may remain. Furthermore, although this is a large, national dataset, the immunotherapy group still represents a highly selective group likely enrolled in clinical trials as biases could not be controlled given the retrospective nature of the database. While we attempted to reduce bias and created a balanced cohort by accounting for covariates that predict receipt of treatment by propensity score matching based on patient and tumor characteristics, unknown confounders not captured in the database might result in biases not accounted for in the propensity score matched cohort as any hidden or latent biases may remain even after matching and multivariable analysis. In addition, the NCDB does not provide granular data on the specific type of chemotherapy or immunotherapy used, or on microsatellite-instability status for PDAC patients. Thus, it was not possible to compare outcomes from those who received vaccine therapy to those who received monoclonal antibodies or check-point inhibitors that would allow subgroup survival analyses based on type of immunotherapy used or microsatellite-instability status. Nevertheless, using this large national database provided sufficient patient numbers as a whole in order to identify patterns of response to chemoimmunotherapy that have been difficult to quantify from small retrospective series or single-arm early stage prospective trials. Clearly, the next steps will be to match specific immunotherapy and chemotherapy agents with susceptible patient populations to identify the optimal chemoimmunotherapy strategies to improve outcomes in this aggressive disease.
CONCLUSION
Although primary surgical resection followed by systemic chemotherapy remains the standard of care for localized pancreatic adenocarcinoma, the combination of adjuvant chemotherapy and immunotherapy was associated with improved survival compared to chemotherapy alone. Clinical trials on the feasibility, durability, and long-term survival benefit of chemo-immunotherapy after resection of pancreatic adenocarcinoma are warranted.
ABBREVIATIONS
- ACS
American College of Surgeons
- BRM
biologic response modifiers
- COC
Commission on cancer
- CTx
chemotherapy
- IT
immunotherapy
- NCDB
National Cancer Database
- OS
overall survival
- PDAC
pancreatic adenocarcinoma
- SEER
Surveillance, Epidemiology, End Results
eTable 1. Immunotherapy Drugs Used in Pancreatic Cancer in Surveillance, Epidemiology, and End Results-Rx Database
| Drug name | Alternative names | Category |
|---|---|---|
| Pancreatic tumor cell vaccine | Vaccine | |
| CEA (CAP1-6D) peptide | Vaccine | |
| Antibody ganitumab (AMG-479) | Monoclonal antibody inhibitor of IGF-IR | |
| Dalotuzumab | MK-0646 | Humanized monoclonal antibody |
| Avicine | Vaccine | |
| GVAX vaccine | CG 8123 | Vaccine |
| RAS 5-17 peptide vaccine | Vaccine | |
| Oncophage | Heat shock protein-peptide complex (HSPPC-96) | Vaccine |
| RC-3095 | Bombesin/gastrin releasing peptide antagonist | |
| Cetuximab | Erbitux, C-225/IMC-C225 | Monoclonal antibody, anti-EGFR antibody |
| TNFerade | Gene therapy | |
| Gastrimmune | Vaccine | |
| P53 and RAS vaccine | Vaccine | |
| CEA-Vac | Vaccine | |
| BrevaRex | Passive monoclonal antibody | |
| O-Vax | Vaccine | |
| Recombinant soluable PSMA vaccine | Vaccine | |
| Telomerase cancer vaccine | Vaccine | |
| Anti-gastrin therapeutic vaccine | Vaccine | |
| Virulizin | Macrophage activator | |
| Vaccinia-MUC-1 vaccine | Vaccine | |
| PanVac | Vaccine | |
| P16 program | Gene therapy | |
| CEA-cide | Anti-CEA monoclonal antibody | |
| Peripheral blood lymphocytes transduced with a gene encoded chimeric T-cell receptor | Gene therapy | |
| Trastuzumab | Anti-erbB2 monoclonal antibody, Anti-HER2/c-erbB2 monoclonal antibody | Targeted therapy—epidermal growth factor receptor |
| Keytruda | MK-3475, SCH-900475, Lambrolizumab, Pembrolizumab | Monoclonal antibody |
CAP1-6D, carcinoembryonic antigen peptide 1-6D; EGFR, epidermal growth factor receptor; erbB, erythroblastic oncogene B; GVAX, GM-CSF gene transduced autologous pancreatic cancer vaccine; IGF-IR, insulin-like growth factor receptor; MUC-1, mucin 1; PSMA, prostate specific cancer antigen human recombinant; TNF, tumor necrosis factor.
Footnotes
Disclosure Information: Nothing to disclose.
Presented at the Western Surgical Association 126th Scientific Session, San Jose del Cabo, Mexico, November 2018.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018. January;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Kooby DA, Gillespie TW, Liu Y, et al. Impact of adjuvant radiotherapy on survival after pancreatic cancer resection: an appraisal of data from the national cancer data base. Ann Surg Oncol. 2013. October;20(11):3634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004. March 27;363(9414): 1049–57. [DOI] [PubMed] [Google Scholar]
- 4.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995. June;221(6):721–31; discussion 31–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012. January;19(1):169–75. [DOI] [PubMed] [Google Scholar]
- 6.Smeenk HG, Tran TC, Erdmann J, et al. Survival after surgical management of pancreatic adenocarcinoma: does curative and radical surgery truly exist? Langenbecks Arch Surg. 2005. April;390(2):94–103. [DOI] [PubMed] [Google Scholar]
- 7.Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013. September;63(5):318–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013. October 9;310(14):1473–81. [DOI] [PubMed] [Google Scholar]
- 9.de WMR, Talamonti MS, Baker MS, et al. Primary systemic therapy in resectable pancreatic ductal adenocarcinoma using mFOLFIRINOX: A pilot study. J Surg Oncol. 2018. March;117(3):354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinn M, Bahra M, Liersch T, et al. CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J Clin Oncol. 2017. October 10;35(29):3330–7. [DOI] [PubMed] [Google Scholar]
- 11.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017. March 11;389(10073): 1011–24. [DOI] [PubMed] [Google Scholar]
- 12.Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018. December 20;379(25):2395–406. [DOI] [PubMed] [Google Scholar]
- 13.Elkord E, Dangoor A, Drury NL, et al. An MVA-based vaccine targeting the oncofetal antigen 5T4 in patients undergoing surgical resection of colorectal cancer liver metastases. J Immunother. 2008. Nov-Dec;31(9):820–9. [DOI] [PubMed] [Google Scholar]
- 14.Geevarghese SK, Geller DA, de Haan HA, et al. Phase I/II study of oncolytic herpes simplex virus NV1020 in patients with extensively pretreated refractory colorectal cancer metastatic to the liver. Hum Gene Ther. 2010. September;21(9): 1119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013. July 11;369(2):134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrop R, Connolly N, Redchenko I, et al. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin Cancer Res. 2006. June 1;12(11 Pt 1): 3416–24. [DOI] [PubMed] [Google Scholar]
- 17.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017. September;18(9): 1182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez MC, Miura JT, Naqvi SMH, et al. Talimogene Laherparepvec (TVEC) for the Treatment of Advanced Melanoma: A Single-Institution Experience. Ann Surg Oncol. 2018. October 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014. November 27;515(7528):558–62. [DOI] [PubMed] [Google Scholar]
- 20.Menck HR, Cunningham MP, Jessup JM, et al. The growth and maturation of the National Cancer Data Base. Cancer. 1997. December 15;80(12):2296–304. [DOI] [PubMed] [Google Scholar]
- 21.Partridge EE. The National Cancer Data Base: ten years of growth and commitment. CA Cancer J Clin. 1998. May-Jun;48(3):131–3. [DOI] [PubMed] [Google Scholar]
- 22.Neoptolemos JP, Stocken DD, Tudur Smith C, et al. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and −3(v1) trials. Br J Cancer. 2009. January 27;100(2):246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueno H, Kosuge T, Matsuyama Y, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009. September 15;101(6):908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghansah T, Vohra N, Kinney K, et al. Dendritic cell immunotherapy combined with gemcitabine chemotherapy enhances survival in a murine model of pancreatic carcinoma. Cancer Immunol Immunother. 2013. June;62(6):1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackaman C, Majewski D, Fox SA, et al. Chemotherapy broadens the range of tumor antigens seen by cytotoxic CD8(+) T cells in vivo. Cancer Immunol Immunother. 2012. December;61(12):2343–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu WM, Fowler DW, Smith P, Dalgleish AG. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010. January 5;102(1): 115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010. January 28;29(4):482–91. [DOI] [PubMed] [Google Scholar]
- 28.Schiavoni G, Sistigu A, Valentini M, et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2011. February 1;71(3):768–78. [DOI] [PubMed] [Google Scholar]
- 29.Eggermont AMM, Robert C, Suciu S. Adjuvant Pembrolizumab in Resected Stage III Melanoma. N Engl J Med. 2018. August 9;379(6): 593–5. [DOI] [PubMed] [Google Scholar]
- 30.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017. November 9;377(19):1824–35. [DOI] [PubMed] [Google Scholar]
- 31.Saglam O, Conejo-Garcia J. PD-1/PD-L1 immune checkpoint inhibitors in advanced cervical cancer. Integr Cancer Sci Ther. 2018;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hakenberg OW. Nivolumab for the treatment of bladder cancer. Expert Opin Biol Ther. 2017. October;17(10):1309–15. [DOI] [PubMed] [Google Scholar]
- 33.Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol. 2018. March;19(3):405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kemeny N, Brown K, Covey A, et al. Phase I, open-label, dose-escalating study of a genetically engineered herpes simplex virus, NV1020, in subjects with metastatic colorectal carcinoma to the liver. Hum Gene Ther. 2006. December;17(12):1214–24. [DOI] [PubMed] [Google Scholar]
- 35.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015. June 25;372(26):2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012. June 28;366(26):2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg A, Mahalingam D. Immunotherapy in pancreatic adenocarcinoma-overcoming barriers to response. J Gastrointest Oncol. 2018. February;9(1):143–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. Lancet. 2009. February 21;373(9664):673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamphorst AO, Araki K, Ahmed R. Beyond adjuvants: immunomodulation strategies to enhance T cell immunity. Vaccine. 2015. June 8;33 Suppl 2:B21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanyi JL, Bobisse S, Ophir E, et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci Transl Med. 2018. April 11;10(436). [DOI] [PubMed] [Google Scholar]
- 41.Bellone G, Novarino A, Vizio B, et al. Impact of surgery and chemotherapy on cellular immunity in pancreatic carcinoma patients in view of an integration of standard cancer treatment with immunotherapy. Int J Oncol. 2009. June;34(6):1701–15. [DOI] [PubMed] [Google Scholar]
- 42.Buzaid AC, Legha SS. Combination of chemotherapy with interleukin-2 and interferon-alfa for the treatment of advanced melanoma. Semin Oncol. 1994. December;21(6 Suppl 14):23–8. [PubMed] [Google Scholar]
- 43.Davis M, Conlon K, Bohac GC, et al. Effect of pemetrexed on innate immune killer cells and adaptive immune T cells in subjects with adenocarcinoma of the pancreas. J Immunother. 2012. October;35(8):629–40. [DOI] [PubMed] [Google Scholar]
- 44.Hoffmann K, Mehrle S, Schmidt J, et al. Interferon-alpha restitutes the chemosensitivity in pancreatic cancer. Anticancer Res. 2008. May-Jun;28(3A):1499–507. [PubMed] [Google Scholar]
- 45.Schmidt J, Abel U, Debus J, et al. Open-label, multicenter, randomized phase III trial of adjuvant chemoradiation plus interferon Alfa-2b versus fluorouracil and folinic acid for patients with resected pancreatic adenocarcinoma. J Clin Oncol. 2012. November 20;30(33):4077–83. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt J, Jager D, Hoffmann K, et al. Impact of interferon-alpha in combined chemoradioimmunotherapy for pancreatic adenocarcinoma (CapRI): first data from the immunomonitoring. J Immunother. 2007. January;30(1):108–15. [DOI] [PubMed] [Google Scholar]
- 47.Aguilar LK, Shirley LA, Chung VM, et al. Gene-mediated cytotoxic immunotherapy as adjuvant to surgery or chemoradiation for pancreatic adenocarcinoma. Cancer Immunol Immunother. 2015. June;64(6):727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsui H, Hazama S, Sakamoto K, et al. Postoperative Adjuvant Therapy for Resectable Pancreatic Cancer With Gemcitabine and Adoptive Immunotherapy. Pancreas. 2017. September;46(8):994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardacre JM, Mulcahy M, Small W, et al. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg. 2013. January;17(1):94–100; discussion p −1. [DOI] [PubMed] [Google Scholar]