Abstract
As an important gasotransmitter, hydrogen sulfide (H2S) plays crucial roles in cell signaling. Incorporation of p-azidophenylalanine (pAzF) into fluorescent proteins (FPs) via genetic code expansion has been a successful strategy in developing intensity-based, genetically encoded fluorescent biosensors for H2S. To extend this strategy for ratiometric measurement which eliminates many detection uncertainties via self-calibration at two wavelengths, we modified the chromophore of a circularly permutated, superfolder green fluorescent protein (cpsGFP) with pAzF to derived cpsGFP-pAzF, which subsequently served as a Förster resonance energy transfer (FRET) acceptor to EBFP2, an enhanced blue fluorescent protein. The resultant construct, namely hsFRET, is the first ratiometric, genetically encoded fluorescent biosensor for H2S. Both in vitro and in mammalian cells, H2S reduces the azido functional group of hsFRET to amine, leading to an increase of FRET from EBFP2 to cpsGFP. Our results collectively demonstrated that hsFRET could be used to selectively and ratiometrically monitor H2S.
Keywords: genetic code expansion, fluorescent protein-based biosensor, Förster resonance energy transfer, unnatural amino acid, ratiometric measurement, hydrogen sulfide
Graphical Abstract
Hydrogen sulfide (H2S), which was long considered a toxic gas with a wide range of cytotoxic effects, has been recognized as the third biosignaling gasotransmitter following nitric oxide (NO) and carbon monoxide (CO).1 H2S has a pKa1 value of 6.6. At neutral pH and 37 °C, it equilibrates mainly with HS−.2,3 Endogenous H2S can be produced enzymatically or non-enzymatically during sulfur metabolism via the reduction of thiol and thiol-containing molecules. Enzymes that catalyzes H2S production include cystathionine β-synthase (CBS), cystathionine γ-lyase (CGL), and cysteine aminotransferase/3-mercaptopyruvate sulfurtransferase (3-MST).4,5
H2S is involved in sulfhydration, a post-translational modification forming persulfides (-S-SH) on cysteine residues of proteins. S-sulfhydration plays important roles in the regulation of inflammation, endoplasmic reticulum stress, and vascular tension.6,7 H2S has also been shown to mediate cardiovascular functions,2,8 and to regulate ATP-sensitive K+ (KATP) channels.9,10 Furthermore, accumulating evidence suggests that H2S functions as a signaling molecule in biological processes such as insulin release, neuromodulation, anti-oxidation, and angiogenesis.5,11–13 Dysregulation of H2S is related to diseases such as the Down syndrome,14 Alzheimer’s disease,15 diabetes,16 and hypertension.17 Accordingly, dissection of the complex roles H2S plays in biological and pathophysiological processes is highly needed. Yet traditional H2S detection methods such as chromatographic,18 colorimetric,19 and electrochemical analysis20 methods do not allow for real-time and noninvasive detection.
Fluorescent biosensors are powerful research tools that allow rapid and noninvasive investigation of physiological and pathological processes of interest with high spatiotemporal resolution.21 To date, based on distinct chemical principles including the reduction of azido groups to amino groups,22–25 reduction of nitro groups to amines,26–28 nucleophilic reactions of H2S,29,30 and copper sulfide precipitation,19 many H2S-responsive, synthetic fluorescent biosensors have been developed.31,32 Recently, two genetically encoded fluorescent biosensors, cpGFP-pAzF and hsGFP, which both contain an unnatural amino acid p-azidophenylalanine (pAzF), were developed for H2S detection in mammalian cells.33,34 These studies used a genetic code expansion technology35 to replace the chromophore-forming Tyr residue of circularly permutated green fluorescent proteins with pAzF. Upon reaction with H2S, the azido group of pAzF in cpGFP-pAzF or hsGFP is reduced into an amino group, resulting in a fluorescent, p-aminotyrosine (pAmF) derived chromophore.36 In addition to their large dynamic ranges, the genetic encodability of these biosensors allows them to be precisely localized to subcellular domains where H2S signaling occurs.
Because ratiometric biosensors have less dependence on sensor concentrations, excitation intensity, and photobleaching,37,38 we herein present our design, engineering, and characterization of hsFRET (Figure 1), which is, to the best of our knowledge, the first genetically encoded, Förster resonance energy transfer (FRET)-based biosensor for H2S. hsFRET shows selective and sensitive response to H2S and allows ratiometric imaging of H2S in live mammalian cells.
Figure 1.
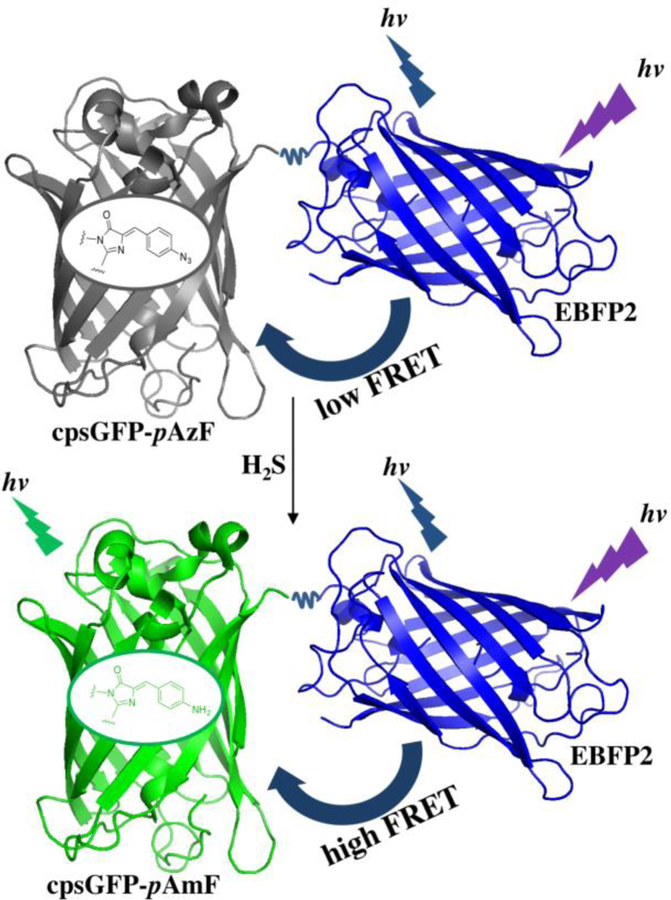
Illustration of the FRET change of hsFRET, a genetically encoded, ratiometric, dual-emission fluorescent biosensor. Upon addition of H2S, the azido functional group of the modified cpsGFP chromophore is reduced to an amino group, resulting a green fluorescent chromophore for enhanced FRET from EBFP2. The chemical structures of the modified cpsGFP chromophore before and after conversion are shown. The basic tertiary structure schematic is adopted from Protein Data Bank entries 3EVP (grey and green) and 2B3P (blue).
EXPERIMENTAL SECTION
Materials.
All materials were obtained and used as previously described.33,39 Sodium hydrosulfide (NaHS) from Acros Organics was dissolved in DPBS, adjusted to pH 7.4 right before use, and added to cells as an H2S donor. To detect endogenous H2S, L-cysteine from Sigma-Aldrich was used to stimulate cellular H2S production. DL-Propargylglycine (DL-PPG) from Acros Organics was used to further inhibit cellular H2S production.
Construction of Bacterial Expression Plasmids and Libraries.
Polymerase chain reactions (PCRs) were utilized to amplify the gene fragments of the FRET donor and acceptor.39,40 In particular, oligos BFP-For and BFP-Rev were utilized to amplify the EBFP2 gene fragment, and the PCR product was digested with HindIII and XhoI restriction enzymes and ligated into a predigested, compatible pCDF-1b expression vector to afford pCDF-1b-EBFP2. Next, oligos GFP-For and GFP-Rev were used to amplify the cpsGFP gene fragment. The resultant PCR product was then digested with NcoI and HindIII and ligated into the predigested pCDF-1b-EBFP2 plasmid to afford FRET-0.1. To generate FRET-0.2, two separate PCR reactions were utilized. In the first PCR reaction, oligos BFP-For2 and BFP-Rev were utilized to amplify the EBFP2 fragment. The resulted PCR product was digested with HindIII and XhoI and ligated into a predigested pCDF-1b to afford pCDF-1b-EBFP2a. In the second PCR reaction, oligos GFP-For, and GFP-Rev2 were used to amplify the cpsGFP fragment. The PCR product was digested with NcoI and HindIII and ligated into the predigested pCDF-1b-EBFP2a. Next, we constructed a library to fully randomize residues His9, Thr64, and Ser67 (Figure 2). Briefly, two separate PCR reactions were performed with cpsGFP. In the first reaction, H148X-For and TX-SX-Rev oligonucleotides containing degenerate NNK codons (in which N = A, T, G, or C, and K = G or T) were used, while in the second reaction TX-SX-For (containing degenerate NNK codons) and GFP-Rev2 oligonucleotides were used. An overlap PCR-based strategy was next used to assemble the products of these two reactions to produce the full-length gene. The amplified DNA fragment was then treated with NcoI and HindIII and ligated into a predigested pCDF-1b-EBFP2a. After screening the generated library for mutants that respond to H2S, FRET-0.3 was selected, amplified, and further inserted into pBAD/His B between NcoI and XhoI restriction sites. To generate a library using error-prone PCR (ep-PCR), pBAD-FRET-0.2 and pBAD-FRET-0.3 were mixed and ep-PCR reaction were carried out using oligos pBAD-For and pBAD-Rev. The reaction was carried out with Tag DNA polymerase for 38 cycles in the presence of 200 μM MnCl2 as previously described.41 Mutagenic PCR products were combined, purified by agarose gel electrophoresis, digested with NcoI and XhoI, and ligated into pBAD/ His B.
Figure 2.
Sequence alignment of hsFRET and other different designs developed in this study. Residues forming the modified cpsGFP chromophores are boxed in green. pAzF is shown as Z (colored green). The mutations of hsFRET from FRET-0.1 are colored in cyan. The full sequence of EBFP2 is not shown since there is no mutation in this fragment.
Library screening.
To screen the library generated by randomizing residues His9, Thr64, and Ser67, we followed a previously described protocol.34 FRET-0.3 was then selected based on the emission ratio of cpsGFP to EBFP2. To screen the library generated from ep-PCR, the gene library in the pBAD/ His B was used to co-transform E. coli DH10B electrocompetent cells with pEvol-pAmF, which was prepared by introducing 5 mutations (Y32T, E107T, D158P, I159L, and V164A) in both copies of the M. jannaschii aminoacyl-tRNA synthetase (aaRS) of pEvol-pAzF.42 After each round of screening, the best mutant was selected for the next round of ep-PCR.
Protein Expression, Purification, and In Vitro Characterization.
We followed previously described procedures.34 In particular, we used a final protein concentration of 1 μM in all in vitro assays. Selectivity assays were performed after incubation of hsFRET with various redox-active molecules (all solutions were prepared as previously described39) at room temperature for 30 min. FRET ratios of fluorescence emission intensities at 500 nm over emission intensities at 450 nm were calculated and represented as means and s.d. from three independent measurements.
Construction of Mammalian Expression Plasmids.
The cpsGFP gene sequence of pBAD-hsFRET was amplified using hsFRET-HindIII-F and hsFRET-EcoRI-R oligonucleotides. After digestion with HindIII and EcoRI, the product was ligated into a predigested pcDNA3 plasmid to afford pcDNA3-cpsGFP. Next, the EBFP gene sequence of pBAD-hsFRET was amplified with oligonucleotides hsFRET-EcoRI-F and hsFRET-ApaI-R. The PCR product was treated with EcoRI and ApaI and ligated into the predigested pcDNA3-cpsGFP to afford pcDNA3-hsFRET. To further improve the mammalian expression of hsFRET, we cloned hsFRET into the pMAH-POLY plasmid39 by digesting pcDNA3-hsFRET with HindIII and ApaI. The digested product was then separated on agarose gel electrophoresis, and ligated into a predigested pMAH-POLY vector to afford POLY-hsFRET.
Mammalian Cell Culture and Imaging.
Human Embryonic Kidney (HEK) 293T cells were cultured and transfected as previously described.34 Typical DMEM contains 0.4 mM cysteine and 0.2 mM methionine. To minimize pre-conversion of hsFRET, cells were cultured in special DMEM containing 1 mM pAzF but no L-methionine or L-cysteine for 48 h, and next in fresh special growth medium without pAzF for an additional 12 h to deplete free pAzF. Cells were then imaged in DPBS under a Leica fluorescence microscope. Time-lapse imaging experiment was set at one frame per min. The excitation laser was set at 405 nm, and emission was collected at 420–465 nm and 495–570 for blue and green channels, receptively.
Cell Viability Assays.
HEK 293T cells were transfected with pMAH-POLY and POLY-hsFRET. 24 hours after transfection, a Promega RealTime-Glo™ MT Cell Viability Assay kit was used to determine the number of viable cells. Briefly, the RealTime-Glo™ reagent was mixed with cells for 30 min. After incubation, the bioluminescence of each sample was measured by using a Synergy Mx Microplate Reader. HEK 293T cells transfected with pMAH-pAzF but cultured in the absence of pAzF were used as the control. Three independent samples in each group were analyzed.
RESULTS AND DISCUSSION
Laboratory Engineering of hsFRET.
To construct a FRET-based sensor for H2S, we selected cpsGFP as the acceptor because of its favorable spectral properties and efficiency in protein folding and maturation.39 We hypothesized that these favorable properties make it an excellent FRET acceptor when fused to an EBFP2 donor,40 which is an improved version of blue fluorescent proteins and has been shown to be an excellent FRET donor for GFP. We envisioned if we could incorporate a H2S-reactive functional group into cpsGFP such as pAzF, an efficient FRET from the donor EBFP2 to the genetically modified cpsGFP acceptor could be achieved upon reaction with H2S and subsequent illumination of EBFP2 (Figure 1). For this reason, we constructed FRET-0.1 by fusing cpGFP with EBFP2 using a short floppy peptide linker (Figure 2). The construct was cloned into a pCDF-1b vector and expressed in BL21(DE3) E. coli bacterial cells along with pEvol-pAzF in the presence of pAzF. Crude proteins were extracted with B-PER and tested with 1 mM H2S. Results of this reaction showed a small FRET ratio change upon reaction with H2S (Figure S1, Supporting Information). Because the sensor is a reaction-based probe, we speculated that reducing the length between the two FPs would increase FRET. We thus cut the small floppy peptide linker and the first few amino acid residues at N-terminal of EBFP2 (FRET-0.2 in Figure 2). FRET-0.2 was then co-expressed with pEvol-pAzF in BL21 E. coli cells in the presence of pAzF. The reaction of crude proteins extracted from E. coli cells with 1 mM H2S showed only a small FRET ratio improvement (Figure S1). Next, we aimed to improve the FRET efficiency by improving the spectral properties of the cpsGFP acceptor. Consequently, we generated a library by fully randomizing three amino acid residues in FRET-0.2 namely His9, Thr64, and Ser67 (aligned to His148, Thr203, and Ser205 of EGFP, Figure 2). These residues appear to stabilize the phenolate ion of the chromophore in EGFP through H-bonds interactions as shown in the crystal structure of EGFP.43 pAzF was incorporated into the library to synthesize pAzF-containing proteins, and the library was screened for H2S-induced FRET changes in bacterial colonies. Bacterial colonies were randomly picked and cultured in liquid media for protein expression. Their FRET ratios were quantitatively measured before and after reaction with H2S. After screening, FRET-0.3 (Figure 2) was chosen for the next step, since the reaction of FRET-0.3 with 1 mM H2S showed a substantial FRET enhancement compared to FRET-0.2 (Figure S1). We then use FRET-0.3 as the template to build another library using a random error-prone mutagenesis technology. The library was cloned into a modified pBAD/His B vector instead of pCDF-1b used before to avoid cell toxicity generated by adding IPTG. We introduced pAmF to the generated library instead of pAzF by co-expressing the library in DH10B cells with pEvol-pAmF, a pEvol-vector containing two copies of M. jannaschii aminoacyl -tRNA synthetase (AARS) with 5 mutations (Y32T, E107T, D158P, I159L, and V164A) in both copies compared to pEvol-pAzF.42 The green to blue emission ratios of the pAmF-incorporated proteins, upon excitation at 385 nm, were used as the measures to evaluate the maximum FRET signals we could get upon H2S-induced conversion of the azido groups to amino groups. Through the screening process, we identified a promising mutant showing a large green/blue emission ratio. This mutant was selected and tested with H2S after incorporating pAzF. Results showed a > 1.7- fold of FRET ratio change (ΔR/R0) in response to 1 mM H2S. We named this mutant hsFRET and performed more experiments to characterize it in vitro and in live cells.
Spectroscopic Responses of hsFRET to H2S.
We next measured fluorescence spectral of hsFRET in response to various concentrations of H2S (Figure 3A). Upon reaction with H2S, hsFRET showed a decrease in emission intensity at 450 nm, and a concomitant increase of emission intensity at 500 nm, suggesting an increase of FRET from the donor to the acceptor in response to H2S. The new peak formed at 500 nm upon addition of H2S suggests that the p-azidobenzylideneimidazolidone chromophore was reduced to p-aminobenzylideneimidazolidone, and that excitation of EBFP2 at 385 nm caused an efficient energy transfer to this newly formed chromophore. This fact also suggests that the reduced p-aminobenzylideneimidazolidone chromophore is highly fluorescent.
Figure 3.
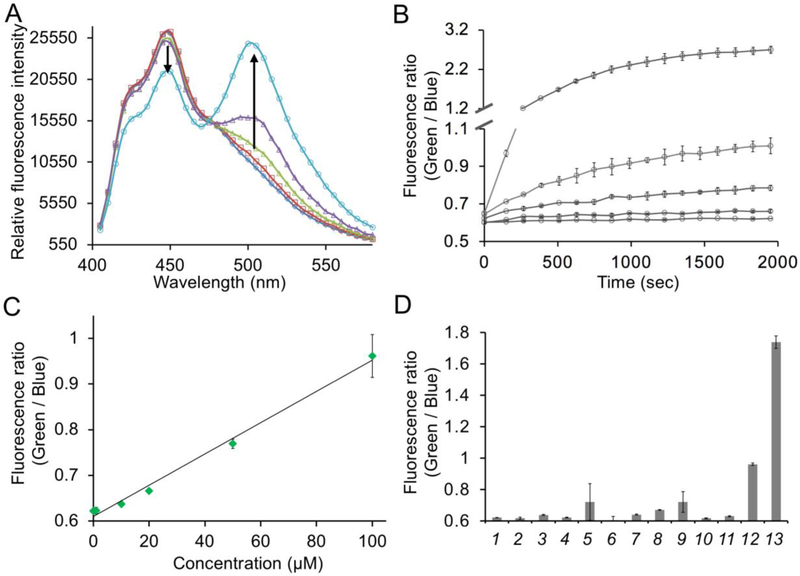
Spectroscopic responses of hsFRET to H2S. (A) Fluorescence emission spectra of hsFRET (1 μM) in response to various concentrations of H2S (H2S concentrations are 1 mM, 100 μM, 50 μM, 20 μM, and 0 μM from top to bottom) with arrows indicating the decrease of emission at 450 nm and the increase of emission at 500 nm. (B) Kinetic changes of fluorescence ratios (green emission/blue emission) of hsFRET (1 μM) in response to 1 mM H2S, 100 μM, 50 μM, 20 μM, and 0 μM from top to bottom (C) Dose-dependent changes of FRET ratios (green emission/blue emission) of hsFRET (1 μM ) to H2S. Measurements were performed at 30 min post addition of H2S. (D) Chemoselectivity of hsFRET against various redox-active chemicals: (1) Tris-HCl, (2) 1 mM H2O2, (3) 100 μM HOCl, (4) 100 μM HOOtBu, (5) •OH (1 mM Fe2+ and 100 μM H2O2), (6) •OtBu (1 mM Fe2+ and 100 μM HOOtBu), (7) 1 mM Vitamin C, (8) 5 mM L-cysteine, (9) 100 μM O2•−, (10) 100 μM ONOO−, (11) 100 μM NOC-5 (NO• donor), (12) 100 μM H2S, and (13) 1 mM H2S.
To measure the kinetics of the reaction and determine the sensitivity of hsFRET to H2S, we monitored the FRET ratio (green emission/blue emission) of hsFRET in response to various concentrations of H2S. We found that the reaction was completed within 30 minutes (Figure 3B). The result also showed that upon reaction of hsFRET with 100 μM or 1 mM H2S, the ratio increased from 0.62 to 0.96 and 1.73, respectively (corresponding to ΔR/R0 of 54 % and 179%, respectively). To further investigate the relationship between the concentration of H2S and the FRET signal, we plotted the ratio change of hsFRET upon treating it with different concentrations of H2S from 1 to 100 μM (Figure 3C). The graph showed a linear relationship between FRET ratios and the concentrations of H2S, suggesting that the formation of the chromophore and the subsequent FRET are concentration-dependent.
Next, we tested the selectivity of hsFRET in response to different redox-active molecules commonly generated by cells, including thiols, and reactive oxygen, nitrogen, and sulfur molecules at physiological or even higher concentrations (Figure 3D). In addition to H2S, only hydroxyl radical and superoxide caused small responses. There was essentially no response observed for hsFRET to all other tested redox-active species, suggesting that hsFRET is a quite selective biosensor for H2S.
hsFRET in live live Mammalian Cells.
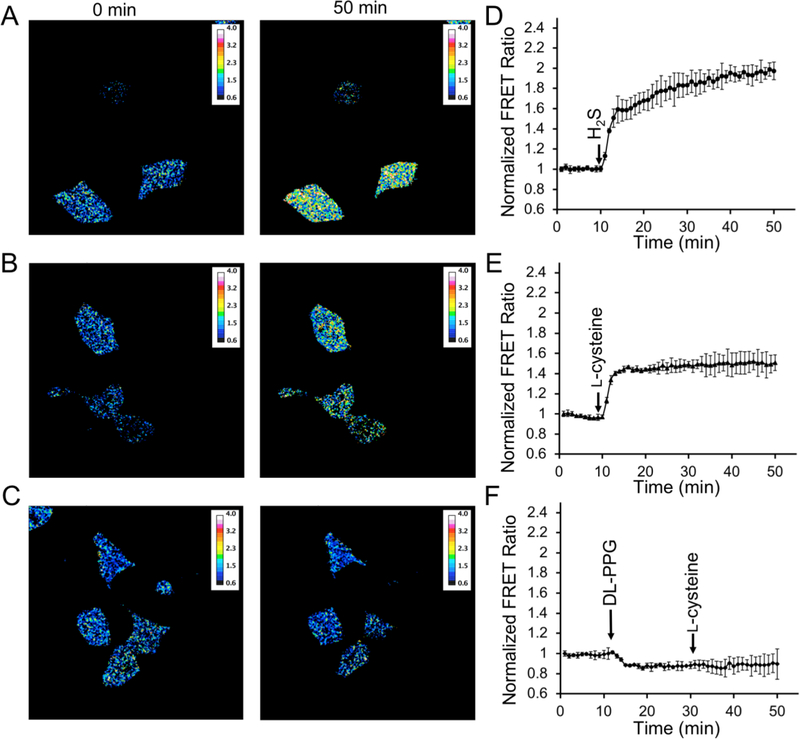
To validate the use of hsFRET for H2S imaging in living mammalian cells, we transiently co-transfected HEK293T cells with pMAH-hsFRET and pMAH-POLY that contains an orthogonal tRNA/aminoacyl-tRNA synthetase pair for incorporation of pAzF into hsFRET in the presence of pAzF. The fluorescence of hsFRET filled both the cytosol and the nucleus, suggesting that there is no preferential accumulation of hsFRET in any of the other subcellular compartments (Figure S2). Upon treating cells with 100 μM H2S, a large increase in the FRET ratio of hsFRET was observed (Figures 4AD and Movie S1). Most changes occurred within 3 min and then the FRET ratio continued to rise slightly, resulting in a total of 100% change (ΔR/R0) in the FRET ratio. The larger dynamic range of the biosensor in mammalian cells as compared to the in vitro experiments, is also observed in the previously reported H2S probes, hsGFP and cpGFP-pAzF.32,33 Better protein folding and maturation of pAzF-derived chromophore, in addition to higher fidelity of mammalian pAzF synthetase, have been proposed to account for this observation. Moreover, the responses of hsFRET reached the plateau faster in mammalian cells than in vitro, likely because H2S degradation pathways were quickly activated in mammalian cells, resulting in no or slow changes after the initial, fast hsFRET responding phase.
Figure 4.
Use of hsFRET to image H2S in mammalian cells. (A–C) Pseudocolor ratiometric images of HEK 293T cells treated with (A) 100 μM H2S (NaHS in DPBS, pH 7.4 as the donor), (B) 100 μM L-cysteine, or (C) 50 mg/L DL-PPG for 20 min, followed by 100 μM L-cysteine. (D–F) Quantification results for panels A–C as the mean and s.d. of all imaged cells from three independent replicates. FRET ratios were normalized to the value at t = 0 min.
We further treated cells with L-cysteine, which is a major precursor for biological production of H2S. We observed a quick and robust, 50% increase (ΔR/R0) in FRET (Figures 4BE and Movie S2), supporting that hsFRET can detect biologically generated H2S in mammalian cells. For comparison, we first treated cells with 50 mg/L DL-PPG, an inhibitor for H2S producing enzymes,44 and then applied L-cysteine to the cells. Not surprisingly, no obvious, L-cysteine-induced fluorescence increase was observed under this condition (Figures 4CF). In addition, we performed prolonged imaging of hsFRET-expressing HEK 293T cells that were untreated with any chemical (Figure S3), and the fluorescence showed excellent stability under our experimental conditions. Taken together, we demonstrate that hsFRET is a robust ratiometric probe for H2S both in vitro and in mammalian cells.
CONCLUSION
To conclude, we developed hsFRET, the first genetically-encoded ratiometric biosensor for H2S by combining genetic code expansion and the FP-based FRET technology. hsFRET showed selective, H2S-induced FRET ratio change by modulation of the acceptor fluorescence intensity. When tested in live mammalian cells, hsFRET exhibited a large ratiometric response to both extracellular addition and endogenous generation of H2S. hsFRET thus represents a valuable addition to the toolbox for H2S detection and imaging. In addition, our sensor design and optimization strategy may be applied to derive similar FRET-based probes for other cellular reactive chemical species, such as peroxynitrite.39
hsFRET, however, still has several limitations. First, short-wavelength violet light (405 nm) is used for excitation, so precautions should be taken to minimize light dosage and avoid phototoxicity. Also, although expression of hsFRET does not cause morphological changes of mammalian cells, cell viability and growth were slightly reduced likely due to the toxicity of suppression of amber codons (Figure S4). Moreover, the response of hsFRET is irreversible and accumulative, despite that hsFRET has its merits as ratiometric biosensors, including normalization to biosensor expression levels and insensitivity to excitation intensity or donor photobleaching. Furthermore, we used plasmid transfection to express hsFRET in mammalian cells. This protocol is incompatible with cells or cell lines that are difficult to transfect. To address this issue, our laboratory is working to optimize a baculoviral system45 for expression of various unnatural-amino-acid-containing fluorescent protein biosensors and their subcellular localization variants.
Supplementary Material
ACKNOWLEDGMENT
Research reported in this publication was supported in part by the National Science Foundation (CHE-1750660) and the National Institute of General Medical Sciences of the National Institutes of Health (R01GM118675 and R01GM129291).
Footnotes
REFERENCES
- (1).Elrod JW; Calvert JW; Morrison J; Doeller JE; Kraus DW; Tao L; Jiao X; Scalia R; Kiss L; Szabo C; Kimura H; Chow CW; Lefer DJ Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proceedings of the National Academy of Sciences of the United States of America 2007, 104 (39), 15560–15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Liu YH; Lu M; Hu LF; Wong PT; Webb GD; Bian JS Hydrogen sulfide in the mammalian cardiovascular system. Antioxid Redox Signal 2012, 17 (1), 141–185. [DOI] [PubMed] [Google Scholar]
- (3).Whitfield NL; Kreimier EL; Verdial FC; Skovgaard N; Olson KR Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol Regul Integr Comp Physiol 2008, 294 (6), R1930–1937. [DOI] [PubMed] [Google Scholar]
- (4).Abe K; Kimura H The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 1996, 16 (3), 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Kamoun P Endogenous production of hydrogen sulfide in mammals. Amino Acids 2004, 26 (3), 243–254. [DOI] [PubMed] [Google Scholar]
- (6).Paul BD; Snyder SHH (2)S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol 2012, 13 (8), 499–507. [DOI] [PubMed] [Google Scholar]
- (7).Zanardo RC; Brancaleone V; Distrutti E; Fiorucci S; Cirino G; Wallace JL Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. Faseb j 2006, 20 (12), 2118–2120. [DOI] [PubMed] [Google Scholar]
- (8).Elsey DJ; Fowkes RC; Baxter GF Regulation of cardiovascular cell function by hydrogen sulfide (H(2)S). Cell Biochem Funct 2010, 28 (2), 95–106. [DOI] [PubMed] [Google Scholar]
- (9).Fitzgerald RS; Shirahata M; Chang I; Kostuk E; Kiihl S The impact of hydrogen sulfide (H(2)S) on neurotransmitter release from the cat carotid body. Respir Physiol Neurobiol 2011, 176 (3), 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Wang R Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? Faseb j 2002, 16 (13), 1792–1798. [DOI] [PubMed] [Google Scholar]
- (11).Kimura H; Shibuya N; Kimura Y Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antioxid Redox Signal 2012, 17 (1), 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Li L; Rose P; Moore PK Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol 2011, 51, 169–187. [DOI] [PubMed] [Google Scholar]
- (13).Peng H; Cheng Y; Dai C; King AL; Predmore BL; Lefer DJ; Wang B A fluorescent probe for fast and quantitative detection of hydrogen sulfide in blood. Angew Chem Int Ed Engl 2011, 50 (41), 9672–9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Kamoun P; Belardinelli MC; Chabli A; Lallouchi K; Chadefaux-Vekemans B Endogenous hydrogen sulfide overproduction in Down syndrome. Am J Med Genet A 2003, 116a (3), 310–311. [DOI] [PubMed] [Google Scholar]
- (15).Eto K; Asada T; Arima K; Makifuchi T; Kimura H Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem Biophys Res Commun 2002, 293 (5), 1485–1488. [DOI] [PubMed] [Google Scholar]
- (16).Wu L; Yang W; Jia X; Yang G; Duridanova D; Cao K; Wang R Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Lab. Invest 2009, 89 (1), 59–67. [DOI] [PubMed] [Google Scholar]
- (17).Yang G; Wu L; Jiang B; Yang W; Qi J; Cao K; Meng Q; Mustafa AK; Mu W; Zhang S; Snyder SH; Wang R H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322 (5901), 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Radford-Knoery J; Cutter GA Determination of carbonyl sulfide and hydrogen sulfide species in natural waters using specialized collection procedures and gas chromatography with flame photometric detection. Analytical Chemistry 1993, 65 (8), 976–982. [Google Scholar]
- (19).Choi MG; Cha S; Lee H; Jeon HL; Chang SK Sulfide-selective chemosignaling by a Cu2+ complex of dipicolylamine appended fluorescein. Chem Commun (Camb) 2009, (47), 7390–7392. [DOI] [PubMed] [Google Scholar]
- (20).Searcy DG; Peterson MA Hydrogen sulfide consumption measured at low steady state concentrations using a sulfidostat. Anal Biochem 2004, 324 (2), 269–275. [DOI] [PubMed] [Google Scholar]
- (21).Yu F; Han X; Chen L Fluorescent probes for hydrogen sulfide detection and bioimaging. Chem. Commun. (Camb.) 2014, 50 (82), 12234–12249. [DOI] [PubMed] [Google Scholar]
- (22).Lippert AR; New EJ; Chang CJ Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J Am Chem Soc 2011, 133 (26), 10078–10080. [DOI] [PubMed] [Google Scholar]
- (23).Lin VS; Lippert AR; Chang CJ Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (18), 7131–7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Zhang H; Wang P; Chen G; Cheung H-Y; Sun H A highly sensitive fluorescent probe for imaging hydrogen sulfide in living cells. Tetrahedron Letters 2013, 54 (36), 4826–4829. [Google Scholar]
- (25).Ji A; Fan Y; Ren W; Zhang S; Ai HW A Sensitive Near-Infrared Fluorescent Sensor for Mitochondrial Hydrogen Sulfide. ACS sensors 2018, 3 (5), 992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Wang R; Yu F; Chen L; Chen H; Wang L; Zhang W A highly selective turn-on near-infrared fluorescent probe for hydrogen sulfide detection and imaging in living cells. Chem. Commun. (Camb.) 2012, 48 (96), 11757–11759. [DOI] [PubMed] [Google Scholar]
- (27).Wu MY; Li K; Hou JT; Huang Z; Yu XQ A selective colorimetric and ratiometric fluorescent probe for hydrogen sulfide. Org Biomol Chem 2012, 10 (41), 8342–8347. [DOI] [PubMed] [Google Scholar]
- (28).Montoya LA; Pluth MD Selective turn-on fluorescent probes for imaging hydrogen sulfide in living cells. Chem. Commun. (Camb.) 2012, 48 (39), 4767–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Qian Y; Karpus J; Kabil O; Zhang SY; Zhu HL; Banerjee R; Zhao J; He C Selective fluorescent probes for live-cell monitoring of sulphide. Nat Commun 2011, 2, 495 DOI: 10.1038/ncomms1506. [DOI] [PubMed] [Google Scholar]
- (30).Liu C; Pan J; Li S; Zhao Y; Wu LY; Berkman CE; Whorton AR; Xian M Capture and visualization of hydrogen sulfide by a fluorescent probe. Angew Chem Int Ed Engl 2011, 50 (44), 10327–10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Cao X; Lin W; He L A near-infrared fluorescence turn-on sensor for sulfide anions. Org Lett 2011, 13 (17), 4716–4719. [DOI] [PubMed] [Google Scholar]
- (32).Sasakura K; Hanaoka K; Shibuya N; Mikami Y; Kimura Y; Komatsu T; Ueno T; Terai T; Kimura H; Nagano T Development of a highly selective fluorescence probe for hydrogen sulfide. J. Am. Chem. Soc 2011, 133 (45), 18003–18005. [DOI] [PubMed] [Google Scholar]
- (33).Chen S; Chen ZJ; Ren W; Ai HW Reaction-based genetically encoded fluorescent hydrogen sulfide sensors. J. Am. Chem. Soc 2012, 134 (23), 9589–9592. [DOI] [PubMed] [Google Scholar]
- (34).Chen ZJ; Ai HW A Highly Responsive and Selective Fluorescent Probe for Imaging Physiological Hydrogen Sulfide. Biochemistry 2014, 53 (37), 5966–5974. [DOI] [PubMed] [Google Scholar]
- (35).Ai HW Biochemical analysis with the expanded genetic lexicon. Anal. Bioanal. Chem 2012, 403 (8), 2089–2102. [DOI] [PubMed] [Google Scholar]
- (36).Ren W; Ai HW Genetically encoded fluorescent redox probes. Sensors (Basel) 2013, 13 (11), 15422–15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Liu B; Zeng F; Wu G; Wu S A FRET-based ratiometric sensor for mercury ions in water with multi-layered silica nanoparticles as the scaffold. Chem Commun (Camb) 2011, 47 (31), 8913–8915. [DOI] [PubMed] [Google Scholar]
- (38).Yu C; Li X; Zeng F; Zheng F; Wu S Carbon-dot-based ratiometric fluorescent sensor for detecting hydrogen sulfide in aqueous media and inside live cells. Chem Commun (Camb) 2013, 49 (4), 403–405. [DOI] [PubMed] [Google Scholar]
- (39).Chen ZJ; Ren W; Wright QE; Ai HW Genetically encoded fluorescent probe for the selective detection of peroxynitrite. J. Am. Chem. Soc 2013, 135 (40), 14940–14943. [DOI] [PubMed] [Google Scholar]
- (40).Ai HW; Shaner NC; Cheng Z; Tsien RY; Campbell RE Exploration of new chromophore structures leads to the identification of improved blue fluorescent proteins. Biochemistry 2007, 46 (20), 5904–5910. [DOI] [PubMed] [Google Scholar]
- (41).Griesbeck O; Baird GS; Campbell RE; Zacharias DA; Tsien RY Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J Biol Chem 2001, 276 (31), 29188–29194. [DOI] [PubMed] [Google Scholar]
- (42).Kolev JN; Zaengle JM; Ravikumar R; Fasan R Enhancing the efficiency and regioselectivity of P450 oxidation catalysts by unnatural amino acid mutagenesis. Chembiochem 2014, 15 (7), 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Wang Q; Shui B; Kotlikoff MI; Sondermann H Structural basis for calcium sensing by GCaMP2. Structure 2008, 16 (12), 1817–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Asimakopoulou A; Panopoulos P; Chasapis CT; Coletta C; Zhou Z; Cirino G; Giannis A; Szabo C; Spyroulias GA; Papapetropoulos A Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). British Journal of Pharmacology 2013, 169 (4), 922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Chatterjee A; Xiao H; Bollong M; Ai HW; Schultz PG Efficient viral delivery system for unnatural amino acid mutagenesis in mammalian cells. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (29), 11803–11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.