Abstract
Background:
Opioid formulations with properties to deter abuse (abuse-deterrent formulations; ADFs) have been developed as one response to the prescription opioid ‘epidemic’. As for all medicines, ADFs undergo evaluation of safety and efficacy prior to registration for marketing. Yet, reduced extra-medical use (the primary intended outcome of ADFs and reason for their introduction) can only be established in post-marketing observational studies, comparing them to opioid formulations without abuse-deterrent properties. This has implications for various features of study design and analysis, as outlined in this manuscript.
Methods:
We discuss a series of proposals for the design, conduct, governance, and reporting of post-marketing studies on the effectiveness of pharmaceutical opioid ADFs based on current guidance documents, public workshops and forums, and our own experience with post-marketing studies of ADFs.
Findings:
Research questions should reasonably be framed around detecting any likely intended or unintended clinical and/or meaningful changes in specific aspects of extra-medical use (e.g., injection use) and harms. Outcomes reported by prevalence and frequency of occurrence and disaggregated by specific product and route of administration can illustrate magnitude of ADF impact. We argue that a multi-faceted approach is required, using data from both general population and sentinel high-risk cohorts, and from primary and secondary data sources. The comparator (historical non-ADF formulation of that opioid, equivalent current generic or similar opioid product), duration of monitoring, and analytical approach require justification and be sufficient to add weight to conclusions of causality. To maximise transparency, we recommend explicit declarations of funding and conflict of interest, establishment of advisory committee, publication of study protocol, and access to all study results.
Conclusions:
Abuse-deterrent formulations are a relatively new strategy to curb opioid-related harm and post-marketing monitoring is complex. The proposals outlined here might not be feasible in all research contexts and without appropriate resourcing. Yet, we believe there is scope for more detailed guidelines with maturing of this field of research.
INTRODUCTION
Pharmaceutical opioid use carries a risk of dependence, and this risk is increased when pharmaceutical opioids are used extra-medically (i.e., outside of a medical professional’s advice; 1). Development of abuse-deterrent formulations (ADFs) of pharmaceutical opioids is one response to increased pharmaceutical opioid extra-medical use and harms in North America (2, 3). The primary intent of ADFs is to reduce specific aspects of extra-medical use by targeting certain behaviours, such as tampering and use via routes of administration other than as intended (e.g., crushing to snort or inject tablet formulations intended for oral consumption).
Definition and Mechanisms of ADFs
ADFs make these behaviours less rewarding or more difficult through:
physical or chemical barriers (e.g., formulations containing hydrogelling agents which resist crushing or dissolving);
antagonist/agonist combinations (e.g., opioids with corresponding antagonist such as naloxone or naltrexone released on tampering);
specific delivery systems (e.g., opioid implants or depot injections);
prodrugs (e.g., opioid released after parent drug ingested and metabolised through stomach enzyme, i.e., orally); and/or
aversive components (e.g., aversive agent like sodium lauryl sulfate released on tampering) (4).
Abuse deterrence does not infer complete prevention of extra-medical use and ADFs are unlikely to deter the most common form of extra-medical use, where opioids are used via the intended route of administration but in excess of the prescribed dose (4, 5). Thus, ADFs form one strategy in what needs to be a multifaceted response targeted at curbing pharmaceutical opioid use and harms (6). Indeed, each ADF must be individually evaluated in terms of risk-benefit, reinforced by recent removal from the market of reformulated products with ADF properties with insufficient evidence to support ADF labelling, and post-marketing data indicating greater harms from extra-medical use (7). Critical to evaluating the possible role of ADFs as part of this response is having high-quality robust data regarding the nature and extent of impact these formulations have in reducing extra-medical use and associated harms.
Contemporary Post-Marketing Surveillance and ADFs
Like any prescribed medicine, ADFs must undergo post-marketing assessment. Traditional post-marketing surveillance of activities focus on pharmacovigilance: safety as typically assessed by adverse event reporting (8). Although safety remains a primary concern, there is increasing recognition that passive systems of adverse event detection (like adverse event reporting) fall short, in that they do not have the capacity to quantify the true magnitude of risk from a particular medicine (9). Contemporary systems that harness administrative and other sources of data (e.g., prescription monitoring programs, analyses of administrative and other data) have the capacity to quantify risk, with certain regulatory bodies (e.g., the US Food and Drug Administration; FDA) requiring active and extensive monitoring of outcomes after the medicine is approved for marketing (10).
Unlike other medicines, ADFs are developed specifically to minimise unintended patterns of opioid use that are known to be harmful. Randomised controlled trials can establish pharmaceutical opioid safety and efficacy. Yet, the latter is limited to the assessment of the pharmacological impact of the opioid. Reductions in extra-medical use associated with a specific formulation can only be established in post-marketing observational studies and require comparison to opioid formulations without abuse-deterrent properties. This also has implications for the design and conduct of ADF post-marketing studies.
Current Post-Marketing Surveillance Guidance for ADFs
To our knowledge, only the US has a regulatory framework regarding the level of pre- and post-marketing evidence required for industry to label a product as an ADF (4). Two major documents provide guidance: the FDA guidance (4) and the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT), written by a group comprising representatives from government organisations, pharmaceutical companies and research organisations (11). Both sources outline recommendations for study design. However, neither addresses issues around study conduct, governance, and reporting. This issue is particularly pertinent given the need for rigorous review of evidence used to inform medicine regulation, and emphasis on transparency and reproducibility of findings.
‘For Debate’ Objectives and Sources of Information
In this ‘For Debate’ piece, we examine the complexities of this issue, proposing a framework for study design, conduct, governance, and reporting of pharmaceutical opioid ADF post-marketing studies. ADFs are a relatively new strategy (the US FDA has approved labelling describing abuse-deterrent properties for eight opioids, including oxycodone and morphine formulations; 12), and there has been substantial maturing of this field of research in the wealth and quality of studies conducted (13–31). Yet, the opportunities for new developments are great, and there is growing momentum for more comprehensive guidance for such studies. This call for further guidance is also driven by government regulators charged with making decisions regarding product registration and public subsidy (10, 32, 33), and heightened by the withdrawal of ADFs from the market based on post-marketing evidence of increased harms (7, 34). Thus, the discussion outlined in this manuscript is timely, and integral to the shaping of this field of research moving forward. Prior and current post-marketing studies may use some components of the suggestions below, but we believe it is important to synthesise current viewpoints and provide clarity in highlighting the best approaches for the field into the future.
The proposals we make here draw upon the existing guidance noted above (4, 11), public workshops and forums on opioid ADFs (35), discussions of transparency for pharmaceutical clinical trials and observational studies (36), and our own experience conducting post-marketing studies of pharmaceutical opioid ADFs (20, 37–39). We also summarise key features of post-marketing studies of ADFs identified in a recent systematic review of ADF effectiveness and value (5), which is available online in full (https://icer-review.org/announcements/final-adf-report/). Our commentary does not cover all aspects of the research process; instead, we have focused on the domains related to study design, conduct, and reporting where guidance and standards are being debated in the aforementioned contexts.
ADF POST-MARKETING STUDY DESIGN COMPONENTS
ADF Post-Marketing Study Research Question
Post-marketing studies of pharmaceutical opioid ADFs assess whether an ADF results in meaningful changes in extra-medical use and harms. Given the intent of ADFs, the research questions and hypotheses might reasonably be framed around detecting a reduction in specific practices, such as insufflation or injection, other aspects of extra-medical use (e.g., attractiveness in the illicit opioid market), and harms (4). Some studies have identified the intended consequence of ADF introduction (i.e., lower levels of tampering with the ADF relative to the comparator) coupled with unintended consequences (e.g., switching to extra-medical use of other pharmaceutical opioids and/or heroin use; 5). Consequently, it may be appropriate to operationalise the research question to detect any likely intended or unintended clinical and/or practically significant changes associated with ADF introduction (i.e. an increase or decrease in these indicators, not necessarily restricted to the use and harms of the ADF product itself).
Outcomes
As aforementioned, the core outcome of post-marketing studies comprises the patterns of opioid use targeted by the ADF, yet other aspects of extra-medical use and harms are also important to monitor for demonstrating both ADF effectiveness and safety (for elaboration, see Figure 1). In terms of monitoring broader unintended consequences of ADFs, we argue that post-marketing studies should also measure total population-level opioid exposure given possible changes in overall utilisation, and market features (e.g., street price) given the association with rates of extra-medical use and harms (5, 40). These outcomes can be split into various facets (see Figure 1). Each of these can contribute to evidence regarding ADF impact, and we believe are important in weighing up risk and benefit (4, 11).
Figure 1.
Key outcomes
The outcomes specified in Figure 1 should be disaggregated where possible by: i) the specific ADF and relevant comparators, and ii) use via any route and route-specific (e.g., intravenous versus intranasal use) (4). There may be instances where outcomes cannot be disaggregated by specific product or route of administration, and it may be appropriate to describe data by the smallest possible unit (e.g., by active ingredient). To illustrate magnitude of ADF impact, outcomes can be quantified by their prevalence and frequency of occurrence where possible. This depth of information captures the scale of change associated with the ADF and facilitates comparison to comparator non-ADF formulations.
Design, data sources and populations
Although not an exhaustive list, key study designs for post-marketing studies of pharmaceutical opioids comprise i) time series: where exposures and outcomes are observed at multiple time points from the same or different individuals (e.g. the number of prescriptions per month); ii) cross-sectional: where exposures and outcomes are collected at a single point in time or serially (not necessarily at equally-spaced intervals) from the same or different individuals (e.g. surveys of people who inject drugs); and iii) cohort: where exposures and outcomes are observed across multiple points of time (not necessarily at equally-spaced intervals) from the same individuals (e.g. people prescribed pharmaceutical opioids followed longitudinally). Other study designs (e.g., self-controlled designs) might also be considered (41)
These study designs can utilise different types of data, specifically primary data collected and collated for the purpose of the study, or secondary data collected for other purposes that can provide information on the key outcomes of interest (typically administrative data, e.g., hospital separations, police drug seizures). They can also use data from different samples: representative of the general population or of sentinel high-risk populations (i.e., a surveillance group in which emerging trends are most likely to be observed). The latter may comprise those populations most likely to engage in extra-medical opioid use, where we are most likely to observe the impacts of ADFs (e.g. people who are opioid-dependent, people who use illicit drugs, people who inject drugs).
Study design, data type and population can determine the degree to which a post-marketing study can: 1) identify events in the total population (signal detection) versus individuals at elevated risk (risk management); 2) identify rare events; 3) detect change in events; 4) identify changes in events at the population- versus individual-level, and 5) provide timely information. In Table 1, we provide a matrix of proposals regarding the degree to which these five objectives can be achieved using various study designs, data types, and populations. Indeed, ADF post-marketing studies have an additional level of complexity given the range of outcomes and levels of disaggregation by product and route of administration, as well as potential jurisdiction-level differences in ADF policy and data availability. Thus, a multi-faceted approach using various study designs, population and sources may be appropriate.
Table 1.
Example Study Designs, Data Types, Populations, and their Utility
| Design | Secondary or primary data | General or sentinel population | Sensitivity | Capacity to identify rare events | Signal detection or risk quantification | Group-level or individual-level change | Timeliness |
|---|---|---|---|---|---|---|---|
| Time series | Primary | General | Low | Low | Risk quantification | No | Moderate |
| Sentinel high-risk | High | Moderate | |||||
| Secondary | General | Low | Low | Signal detection | High | ||
| Sentinel high-risk | High | Moderate | |||||
| Cross-sectional | Primary | General | Low | Low | Risk quantification | Moderate | |
| Sentinel high-risk | High | ||||||
| Secondary | General | Low | Low | Signal detection | High | ||
| Sentinel high-risk | High | Moderate | |||||
| Cohort | Primary | General | Low | Low | Risk quantification | Yes | Moderate |
| Sentinel high-risk | High | Moderate | |||||
| Secondary | General | Low | Low | High | |||
| Sentinel high-risk | High | Moderate |
Note. Signal detection or risk quantification: the former is defined as measurement of any occurrence of the event, whereas risk quantification is defined as measurement of the rate of the event amongst the total sample who were at risk of the event. Capacity to identify rare events: is determined based on whether it targets high risk groups for the event, sample size (statistical power) to identify events, and the variable level of detail in secondary data sources to capture data related to these events. Sensitivity: ability to detect change in events. Timeliness: ability to access data regularly to quickly detect change in events. This table lists several example study designs; other study designs (e.g., self-controlled designs) not listed here could also be considered for post-marketing studies of ADFs.
We argue that data may need to be obtained from both general population and sentinel high-risk samples, and from primary and secondary data sources. General population data permit estimates of population level exposures and risks of outcomes; sentinel high-risk population data reflects outcomes in the group at greatest risk of reporting extra-medical use of ADFs or outcomes. Primary data collection can be designed to permit disaggregation by drug, dose, and route, providing detailed pharmaceutical opioid exposure and extra-medical use data. This level of information is typically not available in secondary data. Despite its limitations (42, 43), secondary data (particularly population-wide datasets) are also important to post-marketing studies, having greater capacity to capture rare events relative to primary data. For example, changes in opioid overdose are more likely to be detected in routinely-collected ambulance data than in survey data given the large sample size necessary to detect change in this low incidence event (38). The proposed capacity of various secondary data sources to measure the core outcomes in ADF post-marketing studies is outlined further in Table 2.
Table 2.
Proposed Capacity to Measure Core Outcomes for ADF Post-Marketing Studies using Secondary Data Sources
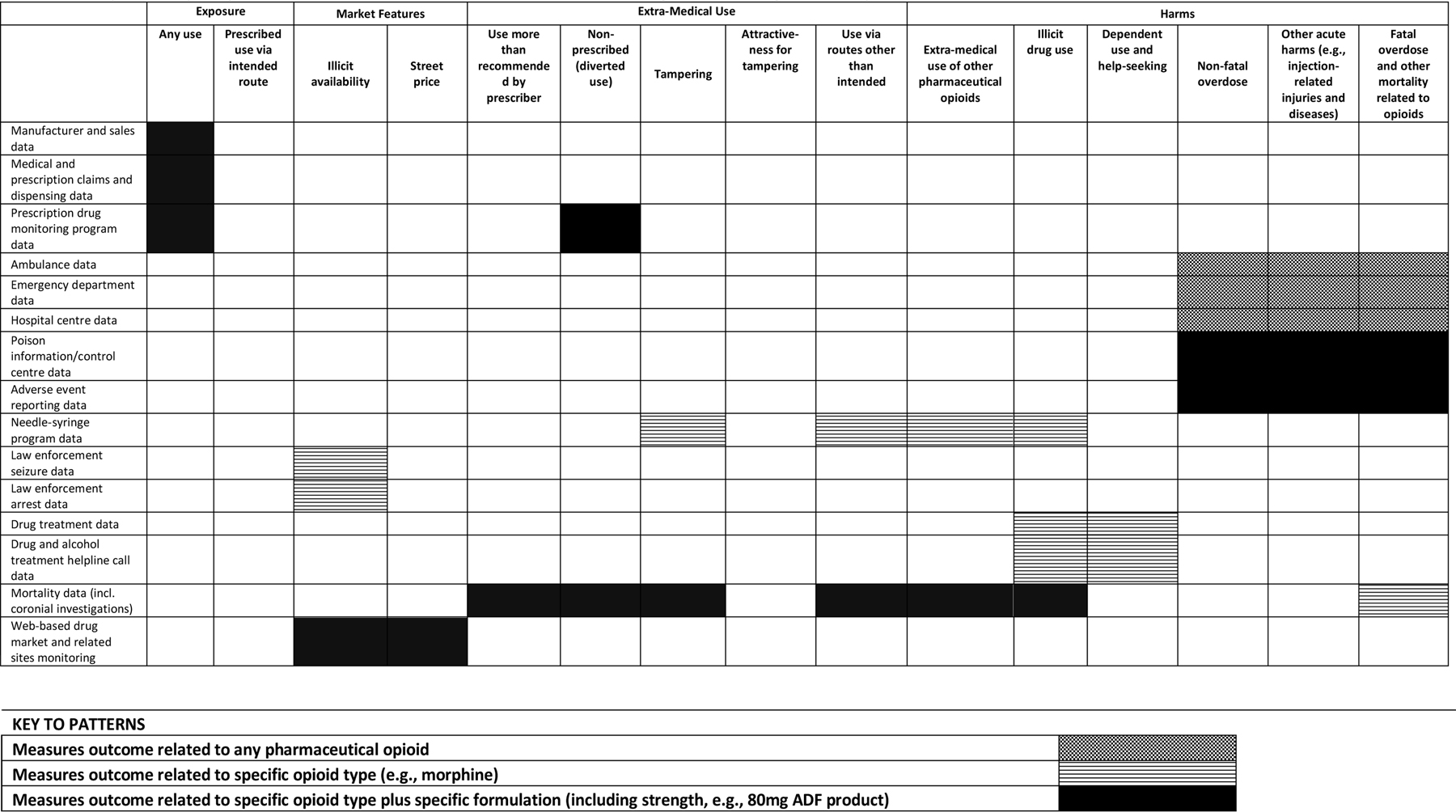 |
In considering a multi-faceted approach to ADF, we highlight two particular combinations of study design, data type, and population: i) time-series study of the general population using secondary data; and ii) cohort study of sentinel high-risk population using primary and/or secondary data (Table 1). Both capture events over time, critical where the comparator may comprise a counterfactual scenario (i.e., an alternative scenario where the ADF was not available, e.g., prior to introduction). One advantage of prospective cohort studies of sentinel high-risk populations is that they can collect rich information on clinical characteristics, other health service use, and adverse outcomes to contextualise changes apparent with ADF introduction. Adapting existing general population cross-sectional surveys and establishing online sentinel high-risk sample surveys have also been discussed as key components of ADF post-marketing monitoring. However, concerns around representativeness of sentinel high-risk samples have been noted given the diversity of groups at high-risk for extra-medical use (e.g., populations reporting chronic non-cancer pain; opioid dependence; illicit opioid use; injecting opioid use; see 35 for further discussion).
Choice of comparators
To answer the research question, it is ideal to have a comparator from which to identify change. Changes may be measured relative to the outcomes for: non-ADF formulations of the same opioid prior to introduction (a counterfactual scenario, as in the case of a reformulation); a generic product equivalent in dosage, strength, route of administration, intended use, effects, and safety but lacking ADF properties; or to a similar but non-ADF opioid comparator (in the case of a new formulation) following ADF introduction. Where no historical or current non-ADF equivalent exists, multiple comparators with the same active moiety and/or similar levels of utilisation and rates of extra-medical use at baseline may be appropriate (44). Consideration should be given to comparison of equianalgesic doses given variation in potency between various opioids (4, 45).
There are a range of issues to consider when selecting a comparator. People who report extra-medical use often report polysubstance use, making it difficult to attribute acute (e.g., overdose) and chronic (e.g., opioid dependence) outcomes to a single substance. Further, the comparator in secondary data can only be as specific as the smallest possible unit. For example, data sources which code according to International Classification of Disease (46) (e.g., hospital separations) record only ‘pharmaceutical opioid’, failing to differentiate opioid type, formulation and strength (Table 2). Consequently, a priori selection and justification of comparators is critical, and multiple comparators may be appropriate where measuring multiple outcomes and using various data sources (4).
Duration of monitoring
The duration of monitoring may depend on the study design, data source(s), outcome(s) of interest, and time for uptake of the ADF (including substitution of the non-ADF with the ADF in dispensing where a reformulation is released). In the event of a reformulation, the FDA (4) states that that the baseline ‘pre’ introduction and observational ‘post’ introduction periods should be long enough to detect outcomes which may be rare or which have large uncertainty (due to bias or variability). Whilst the aforementioned factors (i.e., study design and time for uptake of the ADF) make it difficult to propose a uniform minimum monitoring period, the duration of the pre- and post-periods should allow sufficient time to evaluate whether extra-medical opioid use occurs with exposure to the ADF, with clear justification of the duration of the monitoring period. Yet, it is acknowledged that assessing appropriate duration of monitoring is complex, particularly with low-uptake in prescribing of ADFs and the implications of this for detecting low-frequency outcomes.
Statistical analytic plan
Both guidance documents (4, 11) highlight that the statistical analysis plan should be written before commencing data collection or before commencing analysis where using existing data (4, 45). To maximise transparency, this analytic plan should include a priori power and sample size analyses calculated on detecting a change in primary outcomes. It is acknowledged though that a priori identification of power for studies aimed at signal detection (i.e., measurement of any occurrence of an event in the population) may be challenging. Efforts to pre-specify and justify the denominator used to calculate change in each outcome across each data source will also enhance transparency. To illustrate, in the case of primary data collection it may be appropriate to pre-specify whether risk of extra-medical use will be estimated based on the total group exposed to pharmaceutical opioids or on the group reporting extra-medical use. In both instances, these groups can be defined; in the first instance, as total prescriptions, number of individuals receiving prescriptions, or number of dosage units dispensed (or another unit of total exposure); and in second instance, as number reporting using a greater dose than recommended, non-prescribed use, or tampering of the specific formulation or any opioid.
The key aim is to identify outcomes associated with, and attributable to, ADF introduction (44). This is difficult when there are contemporaneous changes in the pharmaceutical opioid market, illicit drug market, and in prescribing practices which also may have impacts on extra-medical opioid use and harms. Further, there is some debate around how to identify a ‘meaningful’ reduction in extra-medical use and harms, including whether there should be guidance on a minimum effect size observed. In evaluating possible causality, results could be framed using the logic of Bradford Hill as a guide (see 47 as an example), including: strength of associations (strength of relationship between exposure and outcome), consistency of findings (reproducibility across different people, settings, and samples), temporality of exposure and outcome (exposure precedes occurrence of outcome) and biological gradient (greater or lesser exposure leading to greater incidence of the outcome – noting that in some instances the exposure-outcome relationship can be more or less complex) (48). It is important that these criteria are not treated as a ‘checklist’ for causality, and rather as a guide for considering findings within and across studies (49). Indeed, there should be some consideration of competing interventions as an explanation given deployment of other strategies to address pharmaceutical opioid extra-medical use and harms (50).
STUDY CONDUCT, GOVERNANCE AND REPORTING
The following sections overview proposals specific to the conduct, governance and reporting of post-marketing studies of ADFs (Table 3). We summarised published post-marketing studies of ADFs identified in a recent systematic review (5) to identify key components of conduct, governance and reporting which are recommended for observational study designs (51) (see Table 4). As can be seen there was variation across studies, and across the different facets of study conduct and reporting, in the extent to which six features of those were noted in the studies. We explore these features further below.
Table 3.
Proposals for Study Conduct, Governance, and Reporting of Post-Marketing Studies of Pharmaceutical Opioid ADFs
| Funding, role of funders, and conflicts of interest | • Funded by industry but the design, conduct and reporting should occur independent of industry where feasible (e.g., untied educational grant) • All dissemination related to the study should explicitly declare funding, and the role of funders • All dissemination related to the study should explicitly declare any conflicts of interest, including any other sponsorship or support from industry |
| Advisory committee | • Establish an advisory committee including membership that is independent of the research team and funders who advise on study design, conduct and reporting • Ensure representation from researchers, clinicians, and consumers • Meet prior to data collection to overview study protocol and data collection materials, and analytic plan. • Meet subsequent to data collection (with other meetings as needed) to discuss protocol variations, publication plan, and dissemination of findings |
| Protocol | • Include full details and justification of each element of study design • Include power analyses to confirm sufficient statistical power to detect changes in primary outcomes prior to data collection where relevant • Ensure the protocol undergoes independent peer-review • Publish the protocol prospectively (open-access where possible) in an independent public registry and/or in a journal article where it undergoes peer-review • Link to protocol in all subsequent dissemination |
| Access to study results | • Ensure findings are reported and in an open-access or in a publicly-accessible format where undergo peer-review • Report findings according to relevant guidelines (e.g., STrengthening the Reporting of Observational studies in Epidemiology (STROBE) statement (51), Reporting of studies Conducted using Observational Routinely-collected Data (RECORD) statement (62)) • Adopt standard definitions of authorship (e.g., International Committee of Medical Journal Editors authorship guidelines (63)) • Disclose individual investigator contributions to study design, conduct and reporting |
Table 4.
Review of Reporting of ADF Post-Marketing Studies Identified in a Recent Systematic Review
| Study | Publication Year | Funded by industry | Declares role of funding source1 | Declares author conflicts of interest | Declares protocol publication or published priori analysis plan | Declares a priori study registration | Published findings open-access |
|---|---|---|---|---|---|---|---|
| Butler, et al. (13) | 2013 | Yes | Partly (reporting only) | Yes | Not specified | Not specified | No |
| Cassidy, et al. (14) | 2014 | Not specified | Not specified | Yes | Not specified | Not specified | No |
| Chilcoat, et al. (15) | 2016 | Yes | Partly (reporting only) | Yes | Not specified | Not specified | Yes |
| Cicero , et al. (18) | 2012 | No | Yes | Yes | Not specified | Not specified | Yes |
| Cicero and Ellis (16) | 2015 | Yes | Yes | Yes | Not specified | Not specified | Yes |
| Cicero, et al. (17) | 2016 | Yes (indirectly) | Not specified | Yes | Not specified | Not specified | No |
| Coplan, et al. (19) | 2013 | Yes | Not specified | Yes | Not specified | Not specified | Yes |
| Coplan, et al. (64) | 2016 | Yes | Not specified | Yes | Not specified | Not specified | Yes |
| Degenhardt, et al. (20) | 2015 | Yes | Yes | Yes | Yes | Not specified | No |
| Havens, et al. (21) | 2014 | Yes | Yes | Yes | Not specified | Not specified | No |
| Hwang, et al. (22) | 2015 | No | Yes | Yes | Not specified | Not specified | No |
| Jones, et al. (23) | 2016 | Not specified | Not specified | Yes | Not specified | Not specified | No |
| Larochelle, et al. (24) | 2015 | No | Yes | Yes | Not specified | Not specified | Yes |
| Larance, et al. (38)* | 2018 | Yes | Yes | Yes | Yes | Not specified | No |
| Michna, et al. (25) | 2014 | Yes | Not specified | Yes | Not specified | Not specified | Yes |
| Peacock, et al. (26) | 2015 | Yes | Yes | Yes | Yes | Not specified | No |
| Rossiter, et al. (27) | 2014 | Yes | Not specified | Yes | Not specified | Not specified | Yes |
| Sankey, et al. (28) | 2016 | Not specified | Not specified | Not specified | Not specified | Not specified | No |
| Schaffer, et al. (39) | 2018 | No | Yes | Yes | Not specified | Not specified | Yes |
| Sessler, et al. (29) | 2014 | Yes | Not specified | Not specified | Not specified | Not specified | Yes |
| Severtson, et al. (30) | 2013 | Yes (indirectly) | Not specified | Yes | Not specified | Not specified | No |
| Severtson, et al. (31) | 2016 | Yes (indirectly) | Yes | Yes | Not specified | Not specified | Yes |
Note. We wish to acknowledge the work of the authors of a recently published systematic review who identified the studies listed here (5); papers notated with a
were published subsequent to the review and identified by the current authorship team. Poster abstracts were not included in the current analysis.
The authors must declare at a minimum the role the funding source played in study design, conduct, and reporting. STROBE: STrengthening the Reporting of OBservational Studies in Epidemiology guidelines (51).
Funding and Industry Involvement
All products which have been approved for an ADF label in the US must be evaluated post-market introduction, and the findings reported to the FDA (52). This regulatory requirement places the onus for establishing an evidence base regarding safety and effectiveness on the pharmaceutical company. However, there is an inherent conflict of interest where the company conducts such research themselves (53, 54). We acknowledge that regulatory processes have been put in place, in part, to address this issue. Ideally though, ADF post-marketing studies would be conducted at arms-length from commercial interests. Regardless of funding source, it is advisable that study design, conduct and reporting for ADFs should undergo independent review (as detailed below) at multiple stages throughout the study. As per standard reporting guidelines (51), the name of the funder, their role in all aspects of study design, conduct, analysis, and reporting, and any other perceived conflicts of interests (including any other industry support received by the investigators) should be explicitly disclosed in all dissemination using standard reporting forms.
Study advisory committee
An advisory (or expert) committee is a common component of clinical trials or large-scale observational studies. An advisory committee sits separate from the project team: questioning, advising, and troubleshooting to ensure rigorous study design, conduct, and reporting under agreed upon terms of reference (55, 56). This group may form an important component of studies of pharmaceutical opioid ADFs by providing an additional level of monitoring and quality assurance; lending expertise in various study designs and data sources; and ensuring concerns and priorities at the community level are raised through consumer representation.
Ideally, the committee would represent the interests of all parties involved in the research, including the research team, independent experts, consumers, and broader community. If feasible, the committee should include members who are independent of industry and the research team, and who provide a mix of statistical, clinical, epidemiological and lay/consumer backgrounds, including individuals with training in pharmaceutical science. This committee can review the study protocol, statistical analysis plan, data monitoring plan, questionnaires and other data collection tools prior to commencement; amendments to the aforementioned throughout the project; and the dissemination and publication plan at the conclusion of data collection.
Protocol publication
Increasingly, peer-review journals are now requesting submission of a prospectively published protocol or, at a minimum, a prospective statistical plan, for observational studies (57, 58). A study protocol prospectively details the data sources, populations of interest, study outcomes, choice of denominators, choice of opioid comparators, duration of monitoring, and statistical analytic plan (including power analyses). Although protocols for post-marketing studies of opioid ADFs may need to be submitted to regulatory bodies (4), ideally they should also be published in a public registry and/or in a legitimate independent peer-reviewed academic journal prior to study commencement (59). Published study protocols improve ethical conduct, minimise selective publication and selective reporting of results by comparison against the original study aims and analytic plan, and avoid unnecessary duplication of research (60). This transparency is particularly critical for research involving pharmaceutical drugs, where there is additional concern around publication bias and selective reporting. Essential to this process is inclusion of a link to the published protocol in all subsequent dissemination; justification of deviations from the protocol; and progress updates and reports of findings in any databases where the study protocol is available.
Reporting study findings
Investment in research, participant burden, and possible impacts on practice, policy, and future research underlie an ethical obligation to publish study findings (60). This requires dissemination of research findings to the broader community, and not just to regulatory bodies as per ADF reporting requirements. However, reviews of pharmaceutical research have indicated that a substantial number of post-marketing studies are never published or, where results are not positive, published at a delay or with selective reporting of findings (61). Findings should ideally be communicated to the research community via a legitimate independent peer-reviewed (and where possible, open access) academic journal (authored according to standard criteria for authorship; 51, 62), and by making study reports submitted to regulatory bodies publicly accessible.
RECOMMENDATIONS
We outline a series of proposals for ADF post-marketing research. We advocate that all parties should be working towards more detailed guidelines on both the conduct and reporting of ADF post-marketing studies, building on existing guidance documents (4, 45), public workshops and forums on opioid ADFs (35), and the proposals raised in this paper. These reporting guidelines could include a checklist that studies are reported against, including justification of choice of study design, data source, population, comparator, duration of monitoring, analytic plan, and weighting of causal evidence within all dissemination related to the study. Guidelines can only improve research quality and enhance transparency. Thus, we believe that this recommendation can only serve to strengthen this field of research.
CONCLUSIONS
ADFs are a relatively new strategy in the response to increasing pharmaceutical opioid extra-medical use and harms. The best approaches for monitoring effectiveness have not been clearly delineated and are subject to considerable debate. Yet, the importance of progressing guidance for this field is essential given: increasing pharmaceutical opioid extra-medical use and harms; greater push to employ ADFs as a strategic response (10); insufficient evidence to conclude a net health benefit or harm from ADFs (5); and, of concern, preliminary economic modelling suggesting that healthcare costs associated with ADFs may actually be higher than non-ADF products (5). Monitoring of ADFs is complex and challenging, particularly when attempting to detect low-frequency events. The proposals outlined here might not be feasible in all research contexts and without appropriate resourcing, and other approaches not outlined here could also be borrowed from traditional and contemporary post-marketing surveillance approaches. Yet, we believe that this manuscript outlines useful recommendations in striving for best practice in study methods, conduct, design and governance. This work also reinforces the necessity for more detailed guidelines for ADF post-marketing studies, to enhance study quality, transparency, and confidence in research findings.
Acknowledgments
Declaration of interests: AP, BL, and LD are supported by NHMRC research fellowships (#1109366, #1073858 and #1041472/#1135991). The National Drug and Alcohol Research Centre at UNSW Australia is supported by funding from the Australian Government under the Substance Misuse Prevention and Service Improvements Grant Fund.
Footnotes
Some of the investigators have received investigator-initiated untied educational grants from Reckitt Benckiser/Indivior for studies of buprenorphine-naloxone (BL, LD), buprenorphine depot (BL, LD, MF), naloxone (LD, MF), the development of an opioid-related behavior scale (BL, LD, RB) and a study of opioid substitution therapy uptake among chronic non-cancer pain patients (BL, LD, RB). LD, AP, BL and MF have received an untied educational grant from Seqirus for a post-marketing study of tapentadol, and LD, AP, BL, RB, and MF have received an untied educational grant from Mundipharma for a post-marketing study of oxycodone. SAP and NAB have no conflicts of interest to declare.
References
- 1.Larance B, Degenhardt L, Lintzeris N, Winstock A, Mattick R Definitions related to the use of pharmaceutical opioids: Extramedical use, diversion, non‐adherence and aberrant medication‐related behaviours, Drug and alcohol review 2011: 30: 236–245. [DOI] [PubMed] [Google Scholar]
- 2.Wisniewski AM, Purdy CH, Blondell RD The epidemiologic association between opioid prescribing, non-medical use, and emergency department visits, Journal of Addictive Diseases 2008: 27: 1–11. [DOI] [PubMed] [Google Scholar]
- 3.Fischer B, Jones W, Rehm J High correlations between levels of consumption and mortality related to strong prescription opioid analgesics in British Columbia and Ontario, 2005–2009, Pharmacoepidemiology and drug safety 2013: 22: 438–442. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. Abuse-Deterrent Opioids — Evaluation and Labeling; 2015.
- 5.Institute for Clinical and Economic Review. Abuse-deterrent formulations of opioids: Effectiveness and value. Final evidence report ; 2017.
- 6.Degenhardt L, Larance B, Peacock A, Farrell M Reducing extramedical use and harms of pharmaceutical opioids: the potential role of abuse-deterrent formulations, The Lancet Psychiatry 2015: 2: 957–959. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration. FDA requests removal of Opana ER for risks related to abuse; June 8, 2017.
- 8.World Health Organization. The importance of pharmacovigilance: Safety monitoring of medicinal products, Geneva: WHO; 2002. [Google Scholar]
- 9.Coste J Diverging approaches of pharmacovigilance and pharmacoepidemiology to assessing drug safety: epistemological and ethical implications, Pharmacoepidemiology and Drug Safety 2017: 26: 600–602. [DOI] [PubMed] [Google Scholar]
- 10.Califf RM, Woodcock J, Ostroff S A proactive response to prescription opioid abuse, New England Journal of Medicine 2016: 374: 1480–1485. [DOI] [PubMed] [Google Scholar]
- 11.Turk DC, O’Connor AB, Dworkin RH, Chaudhry A, Katz NP, Adams EH et al. Research design considerations for clinical studies of abuse-deterrent opioid analgesics: IMMPACT recommendations, PAIN® 2012: 153: 1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Food and Drug Administration. Abuse-deterrent opioid analgesics: US Food and Drug Administration,; 2018.
- 13.Butler SF, Cassidy TA, Chilcoat H, Black RA, Landau C, Budman SH et al. Abuse rates and routes of administration of reformulated extended-release oxycodone: initial findings from a sentinel surveillance sample of individuals assessed for substance abuse treatment, The Journal of Pain 2013: 14: 351–358. [DOI] [PubMed] [Google Scholar]
- 14.Cassidy TA, DasMahapatra P, Black RA, Wieman MS, Butler SF Changes in prevalence of prescription opioid abuse after introduction of an abuse-deterrent opioid formulation, Pain Medicine 2014: 15: 440–451. [DOI] [PubMed] [Google Scholar]
- 15.Chilcoat HD, Coplan PM, Harikrishnan V, Alexander L Decreased diversion by doctor-shopping for a reformulated extended release oxycodone product (OxyContin), Drug and Alcohol Dependence 2016: 165: 221–228. [DOI] [PubMed] [Google Scholar]
- 16.Cicero TJ, Ellis MS Abuse-deterrent formulations and the prescription opioid abuse epidemic in the United States: lessons learned from OxyContin, JAMA psychiatry 2015: 72: 424–430. [DOI] [PubMed] [Google Scholar]
- 17.Cicero TJ, Ellis MS, Kasper ZA A tale of 2 ADFs: differences in the effectiveness of abuse-deterrent formulations of oxymorphone and oxycodone extended-release drugs, Pain 2016: 157: 1232–1238. [DOI] [PubMed] [Google Scholar]
- 18.Cicero TJ, Ellis MS, Surratt HL Effect of Abuse-Deterrent Formulation of OxyContin, New England Journal of Medicine 2012: 367: 187–189. [DOI] [PubMed] [Google Scholar]
- 19.Coplan PM, Kale H, Sandstrom L, Landau C, Chilcoat HD Changes in oxycodone and heroin exposures in the National Poison Data System after introduction of extended‐release oxycodone with abuse‐deterrent characteristics, Pharmacoepidemiology and drug safety 2013: 22: 1274–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Degenhardt L, Bruno R, Ali R, Lintzeris N, Farrell M, Larance B The introduction of a potentially abuse deterrent oxycodone formulation: early findings from the Australian National Opioid Medications Abuse Deterrence (NOMAD) study, Drug and alcohol dependence 2015: 151: 56–67. [DOI] [PubMed] [Google Scholar]
- 21.Havens JR, Leukefeld CG, DeVeaugh-Geiss AM, Coplan P, Chilcoat HD The impact of a reformulation of extended-release oxycodone designed to deter abuse in a sample of prescription opioid abusers, Drug and alcohol dependence 2014: 139: 9–17. [DOI] [PubMed] [Google Scholar]
- 22.Hwang CS, Chang H-Y, Alexander GC Impact of abuse-deterrent OxyContin on prescription opioid utilization, Pharmacoepidemiology and Drug Safety 2015: 24: 197–204. [DOI] [PubMed] [Google Scholar]
- 23.Jones CM, Muhuri PK, Lurie PG Trends in the Nonmedical Use of OxyContin, United States, 2006 to 2013, The Clinical journal of pain 2017: 33: 452–461. [DOI] [PubMed] [Google Scholar]
- 24.Larochelle MR, Zhang F, Ross-Degnan D, Wharam J Rates of opioid dispensing and overdose after introduction of abuse-deterrent extended-release oxycodone and withdrawal of propoxyphene, JAMA Internal Medicine 2015: 175: 978–987. [DOI] [PubMed] [Google Scholar]
- 25.Michna E, Kirson NY, Shei A, Birnbaum HG, Ben-Joseph R Use of prescription opioids with abuse-deterrent technology to address opioid abuse, Current medical research and opinion 2014: 30: 1589–1598. [DOI] [PubMed] [Google Scholar]
- 26.Peacock A, Degenhardt L, Hordern A, Larance B, Cama E, White N et al. Methods and predictors of tampering with a tamper-resistant controlled-release oxycodone formulation, International Journal of Drug Policy 2015: 26: 1265–1272. [DOI] [PubMed] [Google Scholar]
- 27.Rossiter LF, Kirson NY, Shei A, White AG, Birnbaum HG, Ben-Joseph R et al. Medical cost savings associated with an extended-release opioid with abuse-deterrent technology in the US, Journal of Medical Economics 2014: 17: 279–287. [DOI] [PubMed] [Google Scholar]
- 28.Sankey C, Setnik B, Harsanyi Z, Michalko K, Yang Z, Geoffroy P Opioid use following the introduction of an extended-release oxycodone formulation with tamper-resistant properties: Prospective historical chart review in methadone-maintained patients, Journal of opioid management 2016: 12: 149–159. [DOI] [PubMed] [Google Scholar]
- 29.Sessler NE, Downing JM, Kale H, Chilcoat HD, Baumgartner TF, Coplan PM Reductions in reported deaths following the introduction of extended-release oxycodone (OxyContin) with an abuse-deterrent formulation, Pharmacoepidemiology and Drug Safety 2014: 23: 1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Severtson SG, Bartelson BB, Davis JM, Muñoz A, Schneider MF, Chilcoat H et al. Reduced abuse, therapeutic errors, and diversion following reformulation of extended-release oxycodone in 2010, The Journal of Pain 2013: 14: 1122–1130. [DOI] [PubMed] [Google Scholar]
- 31.Severtson SG, Ellis MS, Kurtz SP, Rosenblum A, Cicero TJ, Parrino MW et al. Sustained reduction of diversion and abuse after introduction of an abuse deterrent formulation of extended release oxycodone, Drug and alcohol dependence 2016: 168: 219–229. [DOI] [PubMed] [Google Scholar]
- 32.Drug Utilisation Sub-Committee (DUSC). Opioid analgesics: An overview; 2014.
- 33.Patented Medicines Price Review Board. Utilization of Prescription Opioids in Canada’s Public Drug Plans, 2006/07 to 2012/13; 2014.
- 34.Dyer O FDA pulls opioid from market over misuse concerns, BMJ 2017: 357. [DOI] [PubMed] [Google Scholar]
- 35.Food and Drug Administration. Data and Methods for Evaluating the Impact of Opioid Formulations with Properties Designed to Deter Abuse in the Postmarket Setting: A Scientific Discussion of Present and Future Capabilities; 2017.
- 36.Food and Drug Administration. Guidance for industry, E6 good clinical practice: consolidated guidance, Federal Register 1997: 10: 691–709. [Google Scholar]
- 37.Degenhardt L, Larance B, Bruno R, Lintzeris N, Ali R, Farrell M Evaluating the potential impact of a reformulated version of oxycodone upon tampering, non‐adherence and diversion of opioids: the National Opioid Medications Abuse Deterrence (NOMAD) study protocol, Addiction 2014. [DOI] [PubMed]
- 38.Larance B, Dobbins T, Peacock A, Ali R, Bruno R, Lintzeris N et al. The effect of a potentially tamper-resistant oxycodone formulation on opioid use and harm: main findings of the National Opioid Medications Abuse Deterrence (NOMAD) study, The Lancet Psychiatry 2018. [DOI] [PubMed]
- 39.Schaffer AL, Buckley NA, Degenhardt L, Larance B, Cairns R, Dobbins TA et al. Person-level changes in oxycodone use after the introduction of a tamper-resistant formulation in Australia, Canadian Medical Association Journal 2018: 190: E355–E362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smithson M, McFadden M, Mwesigye SE, Casey T The impact of illicit drug supply reduction on health and social outcomes: the heroin shortage in the Australian Capital Territory, Addiction 2004: 99: 340–348. [DOI] [PubMed] [Google Scholar]
- 41.Hallas J, Pottegård A Use of self‐controlled designs in pharmacoepidemiology, Journal of internal medicine 2014: 275: 581–589. [DOI] [PubMed] [Google Scholar]
- 42.Schneeweiss S, Avorn J A review of uses of health care utilization databases for epidemiologic research on therapeutics, Journal of Clinical Epidemiology 2005: 58: 323–337. [DOI] [PubMed] [Google Scholar]
- 43.Harpe SE Using secondary data sources for pharmacoepidemiology and outcomes research, Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 2009: 29: 138–153. [DOI] [PubMed] [Google Scholar]
- 44.McAninch JK, Keeton SL, Secora A, Kornegay CJ, Hwang CS, Ly T et al. Important statistical considerations in the evaluation of post‐market studies to assess whether opioids with abuse‐deterrent properties result in reduced abuse in the community, Pharmacoepidemiology and Drug Safety 2017. [DOI] [PubMed]
- 45.Center for Drug Evaluation and Research U. F. a. D. A. Data and Methods for Evaluating the Impact of Opioid Formulations with Properties Designed to Deter Abuse in the Postmarket Setting: Issues Paper; 2017.
- 46.Organization W. H. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines: World Health Organization; 1992. [Google Scholar]
- 47.Dart RC, Iwanicki JL, Dasgupta N, Cicero TJ, Schnoll SH Do abuse deterrent opioid formulations work?, Journal of opioid management 2017: 13: 365–378. [DOI] [PubMed] [Google Scholar]
- 48.Shakir SA, Layton D Causal association in pharmacovigilance and pharmacoepidemiology, Drug Safety 2002: 25: 467–471. [DOI] [PubMed] [Google Scholar]
- 49.Rothman KJ, Greenland S Causation and causal inference in epidemiology, American journal of public health 2005: 95: S144–S150. [DOI] [PubMed] [Google Scholar]
- 50.Ranapurwala SI, Naumann RB, Austin AE, Dasgupta N, Marshall SW Methodologic limitations of prescription opioid safety research and recommendations for improving the evidence base, Pharmacoepidemiology and drug safety 2018. [DOI] [PubMed]
- 51.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies, International Journal of Surgery 2014: 12: 1495–1499.25046131 [Google Scholar]
- 52.Food and Drug Administration. Postmarket Requirements and Commitments database; 2017.
- 53.Spelsberg A, Prugger C, Doshi P, Ostrowski K, Witte T, Hüsgen D et al. Contribution of industry funded post-marketing studies to drug safety: survey of notifications submitted to regulatory agencies, bmj 2017: 356: j337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.London AJ, Kimmelman J, Carlisle B Rethinking Research Ethics: The Case of Postmarketing Trials, Science (New York, NY) 2012: 336: 544–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harman NL, Conroy EJ, Lewis SC, Murray G, Norrie J, Sydes MR et al. Exploring the role and function of trial steering committees: results of an expert panel meeting, Trials 2015: 16: 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daykin A, Selman LE, Cramer H, McCann S, Shorter GW, Sydes MR et al. What are the roles and valued attributes of a Trial Steering Committee? Ethnographic study of eight clinical trials facing challenges, Trials 2016: 17: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The Plos Medicine Editors. Observational Studies: Getting Clear about Transparency, PLOS Medicine 2014: 11: e1001711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas L, Peterson ED The value of statistical analysis plans in observational research: defining high-quality research from the start, Jama 2012: 308: 773–774. [DOI] [PubMed] [Google Scholar]
- 59.Dal-Ré R, Ioannidis JP, Bracken MB, Buffler PA, Chan A-W, Franco EL et al. Making prospective registration of observational research a reality, Science translational medicine 2014: 6: 224cm221–224cm221. [DOI] [PubMed] [Google Scholar]
- 60.Chan A-W, Song F, Vickers A, Jefferson T, Dickersin K, Gøtzsche PC et al. Increasing value and reducing waste: addressing inaccessible research, Lancet (London, England) 2014: 383: 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waller P, Wood S, Langman M, Breckenridge A, Rawlins M Review of company postmarketing surveillance studies, Bmj 1992: 304: 1470–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicholls SG, Quach P, von Elm E, Guttmann A, Moher D, Petersen I et al. The reporting of studies conducted using observational routinely-collected health data (RECORD) statement: methods for arriving at consensus and developing reporting guidelines, PLoS One 2015: 10: e0125620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.International Committee of Medical Journal Editors. International Committee of Medical Journal Editors (ICMJE): Uniform Requirements for Manuscripts Submitted to Biomedical Journals: writing and editing for biomedical publication, Haematologica 2004: 89: 264. [PubMed] [Google Scholar]
- 64.Coplan PM, Chilcoat HD, Butler SF, Sellers EM, Kadakia A, Harikrishnan V et al. The effect of an abuse‐deterrent opioid formulation (OxyContin) on opioid abuse‐related outcomes in the postmarketing setting, Clinical Pharmacology & Therapeutics 2016: 100: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]



