Abstract
IMPORTANCE
In 2010, due to a pertussis outbreak and neonatal deaths, the California Department of Health recommended that the tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) be administered during pregnancy. Tdap is now recommended by the Advisory Committee on Immunization Practices for all pregnant women, preferably between 27 and 36 weeks’ gestation. Limited data exist on Tdap safety during pregnancy.
OBJECTIVE
To evaluate whether maternal Tdap vaccination during pregnancy is associated with increased risks of adverse obstetric events or adverse birth outcomes.
DESIGN AND SETTING
Retrospective, observational cohort study using administrative health care databases from 2 California Vaccine Safety Datalink sites.
PARTICIPANTS AND EXPOSURES
Of 123 494 women with singleton pregnancies ending in a live birth between January 1, 2010, and November 15, 2012, 26 229 (21%) received Tdap during pregnancy and 97 265 did not.
MAIN OUTCOMES AND MEASURES
Risks of small-for-gestational-age (SGA) births (<10th percentile), chorioamnionitis, preterm birth (<37 weeks’ gestation), and hypertensive disorders of pregnancy were evaluated. Relative risk (RR) estimates were adjusted for site, receipt of another vaccine during pregnancy, and propensity to receive Tdap during pregnancy. Cox regression was used for preterm delivery, and Poisson regression for other outcomes.
RESULTS
Vaccination was not associated with increased risks of adverse birth outcomes: crude estimates for preterm delivery were 6.3% of vaccinated and 7.8% of unvaccinated women (adjusted RR, 1.03; 95% CI, 0.97–1.09); 8.4% of vaccinated and 8.3% of unvaccinated had an SGA birth (adjusted RR, 1.00; 95% CI, 0.96–1.06). Receipt of Tdap before 20 weeks was not associated with hypertensive disorder of pregnancy (adjusted RR, 1.09; 95% CI, 0.99–1.20); chorioamnionitis was diagnosed in 6.1% of vaccinated and 5.5% of unvaccinated women (adjusted RR, 1.19; 95% CI, 1.13–1.26).
CONCLUSIONS AND RELEVANCE
In this cohort of women with singleton pregnancies that ended in live birth, receipt of Tdap during pregnancy was not associated with increased risk of hypertensive disorders of pregnancy or preterm or SGA birth, although a small but statistically significant increased risk of chorioamnionitis diagnosis was observed.
Bordetella pertussis is a highly contagious human respiratory pathogen. Infants are at highest risk of severe pertussis infections. The tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) was licensed in 2005 for use in nonpregnant adolescents and adults.1 Initially, postpartum administration of Tdap to parents and other care-givers was encouraged to prevent the transmission of pertussis to newborns.1,2 However, recent outbreaks, including infant deaths,3,4 have led to changing Tdap vaccine recommendations.5
In 2010, in response to a widespread pertussis outbreak, California became the first state to recommend Tdap be routinely administered during pregnancy.6 In 2011, the Advisory Committee on Immunization Practices followed with similar recommendations that Tdap be administered during pregnancy, at 20 weeks gestation or later, to women who had not been previously vaccinated. In October 2012, the committee further revised recommendations that Tdap be given to all pregnant women, preferably between 27 and 36 weeks’gestation, even if previously vaccinated.5 These recommendations have been endorsed by the American College of Obstetricians and Gynecologists.7 Recommendations to administer Tdap during pregnancy were based on urgent public health needs3,4,8,9 and available evidence on the safety of other inactivated vaccines during pregnancy.10,11 To date there are limited specific data on whether vaccination with Tdap during pregnancy adversely affects the health of mothers or their offspring.12–14 The goals of this study were to evaluate, among pregnancies ending in a live birth, whether receipt of Tdap during pregnancy was associated with increased risks of selected adverse obstetric or birth outcomes.
Methods
In this observational retrospective cohort study, we used administrative and electronic health record (EHR) data to evaluate adverse events associated with Tdap vaccination during pregnancy.
Study Population
The Vaccine Safety Datalink (VSD) is a collaborative effort between the Centers for Disease Control and Prevention’s Immunization Safety Office and 9 large medical care organizations in the United States. The primary aim of the VSD is to monitor the safety of vaccines routinely administered within the United States.15 Pregnancies for this study were identified using a validated algorithm based on administrative, EHR, and claims data.16 Women were required to be 14 through 49 years of age at the end of pregnancy, continuously insured from 6 months prior to their last menstrual period through 6 weeks postpartum, and to have at least 1 outpatient visit at an affiliated site.
Data for the current analyses were limited to 2 VSD sites, Kaiser Permanente Northern California and Kaiser Permanente Southern California. At these sites, in response to the pertussis outbreak and specific recommendation from the California Department of Health,6 Tdap vaccination during pregnancy increased markedly starting in 2010. Tdap coverage during pregnancy at the other VSD sites remained less than 2% until 2012.17 The preliminary cohort was composed of pregnancies from the California VSD sites with end dates of January 1, 2007, through November 15, 2012, because the initial plan was to evaluate outcomes for the full time period. After observing vaccination during pregnancy to be uncommon in the years 2007–2009,17we further limited our sample to singleton pregnancies ending in a live birth between January 1, 2010, and November 15, 2012, with birth weight and gestational age recorded in the EHR. Gestational age at delivery was based on clinician assessment. The gestational age was then subtracted from the date of birth in order to assign an estimated pregnancy start date, equivalent to the estimated the last menstrual period.
For these analyses, we excluded women receiving 1 or more live virus vaccines during pregnancy and those who received Tdap during washout periods, in the 7 days after the estimated pregnancy start date or in the 7 days before delivery.
This study was approved by the institutional review boards at all participating sites and the Centers for Disease Control and Prevention with a waiver of informed consent.
Exposure
Receipt of Tdap was identified from the VSD vaccine files. These files contain standardized data on vaccinations, captured primarily through site-based registries.15 Tdap vaccines administered to women experiencing 1 or more pregnancies during the study period were then assigned as weeks following the estimated last menstrual period and classified as occurring during pregnancy if recorded starting 8 days after last menstrual period through 8 days before delivery. Consistent with our previous studies, these cut-offs were assigned to account for uncertainty regarding the last menstrual period and specifically to avoid misclassification of postpartum vaccinations as ocurring during pregnancy.18,19
At the 2 California VSD sites, the majority of Tdap doses administered to pregnant women were Adacel (Sanofi Pasteur).
Outcomes
The 2 adverse obstetric outcomes examined were chorioamnionitis and hypertensive disorders of pregnancy. These obstetric outcomes were chosen because they are both common and important markers of maternal health and they represent the leading maternal risks of preterm birth. In addition, these outcomes were selected based on prior work by our group on influenza vaccine safety during pregnancy.18 Both were identified from diagnostic International Classification of Diseases, Ninth Revision (ICD-9) codes assigned during health care visits. Chorioamnionitis (658.41) was included if there were 1 or more inpatient diagnoses occurring at the time of birth. Hypertensive disorders included gestational hypertension (642.3x), hypertension in pregnancy not otherwise specified (642.9), and preeclampsia or eclampsia (642.4x–642.8x). All hypertensive disorders were required to have onset at 20 weeks gestation or later. Gestational hypertension and preeclampsia without severe features (642.3X, 642.4X, 642.9x) were required to have 2 outpatient or 1 inpatient diagnosis. Severe pre-eclampsia or eclampsia (642.5x–642.7x) were only included if diagnosed at an inpatient visit.
The 2 birth outcomes examined were preterm and small-for-gestational-age (SGA) births. These birth outcomes were chosen for evaluation based on public health importance, feasibility given data availability, and expected background rates. In addition, these outcomes are consistent with prior studies by our group20 and others21–23 on influenza vaccine safety during pregnancy. Preterm birth was defined as delivery before 37 weeks’ gestation and was based on clinician estimate, as recorded in the EHR. Birth weights also came from the EHR. Weight for gestational age percentiles were assigned based on reference values derived by Oken et al24 A cut-off of less than the 10th percentile was used to classify a birth as SGA.
Statistical Analyses
Our analytic approach varied for each outcome, accounting for the expected timing of the outcome in pregnancy and presumed pathophysiology. For hypertensive disorders, analyses were limited to comparing women who received Tdap before 20 weeks’ gestation vs women unexposed during pregnancy. The rationale for this was 2-fold. First, by only looking at exposures prior to 20 weeks, we could be assured that all Tdap vaccinations occurred before any hypertensive disorders could be diagnosed. Second, although hypertensive disorders of pregnancy, by definition, occur at 20 weeks’ gestation or later, they are thought to be due to abnormalities in placental development as evidenced by early changes in maternal angiogenic biomarkers.25 We used a Poisson model with robust variance estimate to compare risks of hypertensive disorders between Tdap exposed and unexposed women.
For preterm delivery, in order to reduce bias due to differences in potential exposure periods,20 we limited analyses to women receiving Tdap at 36 weeks’ gestation or earlier. To evaluate risks of preterm delivery, we compared Tdap exposed and unexposed women using a time-dependent exposure Cox model.26 This model adjusts for the potential bias introduced because women who are vaccinated later in pregnancy (eg, at 35 weeks) have less time available to have a preterm delivery. For chorioamnionitis and SGA births, we compared all women who received Tdap during pregnancy to all unexposed women using a Poisson model with robust variance estimate. In secondary analyses, approximating current recommendations regarding optimal timing for Tdap vaccination, we evaluated risks of chorioamnionitis, SGA, and preterm births in the subset of women who received Tdap between 27 and 36 weeks’ gestation vs all women in the cohort who were unexposed to Tdap during pregnancy.
All multivariable models included the following covariates: site, receipt of other vaccines during pregnancy, and propensity score parameterized as a decile categorical variable. A propensity score approach was preferred over other methods to optimize adjustment of multiple potential confounders.27,28 All pregnancies were assigned a propensity score estimating their likelihood of receiving Tdap during pregnancy. Propensity scores were calculated, were stratified by site using logistic regression, and were based on sociodemographic characteristics (neighborhood poverty index and age); presence of maternal comorbidities (hypertension, diabetes, and cardiovascular or renal disease occurring prior to start of pregnancy); receipt of medical care in the first trimester, the Kotelchuck Adequacy of Prenatal Care Utilization Index derived from VSD data29,30; and number of hospitalizations during the first 20 weeks of pregnancy as a surrogate of pregnancy complications. Maternal race/ ethnicity was determined from birth data, based on self-report. As a factor associated with both receipt of Tdap and risk of an adverse birth outcome, it was important to include race/ethnicity in the propensity score. All of the preceding factors were included in the model as main statistical effects. Model fit for the propensity score was evaluated by the C statistic and by evaluating whether risk factors were better balanced after propensity score adjustment.
Based on prior work by our group, all outcomes were expected to have a background rate between 5% and 8%.18,20 Given the available sample of women who received Tdap during pregnancy,17 with α = .05 and using a 2-sided test, the study was powered a priori to detect a 7 per 1000 risk difference for all outcomes.
Following automated data analysis, chart reviews for a random sample of 220 women with an inpatient chorioamnionitis diagnosis (ICD-9 code 658.41) were conducted. Clinical variables, diagnoses, medications, procedures, and pathology findings from labor and delivery recorded in the EHR were collected by trained chart abstractors at both VSD sites and entered into a REDCap data form.31 Chart review data were used to classify cases with ICD-9 code 658.41 as “probable chorioamnionitis” if they had a temperature of 38.0°C (100.4°F) or higher and 2 additional findings consistent with chorioamnionitis (maternal or fetal tachycardia, fundal tenderness, malodorous or puslike amniotic fluid) or had 2 or more clinical findings and placental pathology consistent with chorioamnionitis.32 Possible chorioamnionitis was defined as fever and 1 additional clinical finding. The positive predictive values (PPVs) of the ICD-9 code 658.41 and having EHR documentation of chorioamnionitis, possible chorioamnionitis, and probable chorioamnionitis were calculated. Estimates of PPV were applied to the cohort assuming nondifferential ascertainment between vaccination status. Statistical significance was evaluated according to chorioamnionitis case definition. All analyses were conducted using SAS version 9.3 (SAS Institute Inc).
Results
For the years 2010 through 2012, in the 2 California VSD sites, there were 140 343 pregnancies with continuous enrollment and singleton live births (Figure). We excluded 16 204 (11.5%) with incomplete birth data (birth weight or gestational age not available), 308 (0.2%) who received a live virus vaccine, and 337 (0.2%) who were vaccinated during washout periods, within 7 days of the estimated pregnancy start or within 7 days of delivery. Tdap coverage among excluded women was 19%. Among the remaining 123 494 eligible pregnancies, 26 229 (21.2%) received Tdap during pregnancy, with 92% of vaccines administered during the second and third trimester. Among 97 265 unexposed pregnancies, 46% received Tdap prior to pregnancy. At baseline, the largest difference between women who received Tdap during pregnancy and those who did not was in receipt of another vaccine during pregnancy (53.8% of Tdap exposed vs 36.3% of unexposed; Table 1). The remaining baseline characteristics were included in propensity scores to estimate likelihood of Tdap vaccination during pregnancy, stratified by site. For both sites, propensity score C statistics were 0.54, signifying that baseline factors were not strongly associated with vaccination. Furthermore, the expected distribution of covariates after propensity adjustment is presented in Table 1.
Figure. Flowchart of Pregnancies Identified and Exclusions From 2 California Vaccine Safety Datalink Sites.
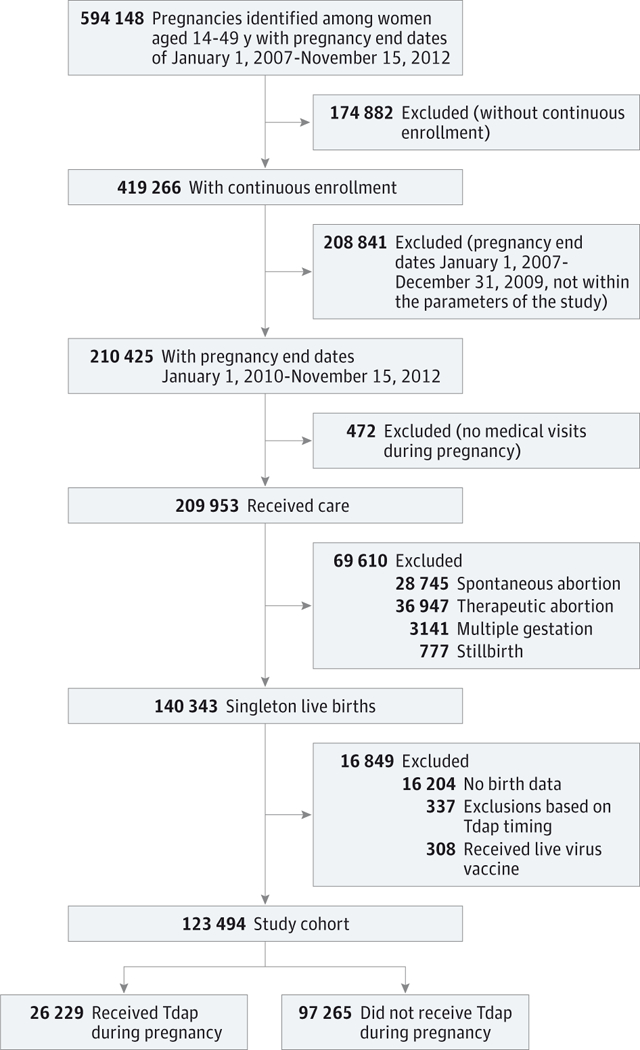
Tdap indicates tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine.
Table 1.
Baseline Characteristics of Pertussis Vaccine (Tdap) Exposed and Unexposed Pregnant Women With a Live Birtha
| Original Sample, No. (%) |
After Propensity Adjustment, % |
||||||
|---|---|---|---|---|---|---|---|
| Tdap Exposed (n = 26 229) |
Unexposedb (n = 97 265) |
P Value | Tdap Exposed | Unexposed | P Value | ||
| Age at delivery, y | |||||||
| <18 | 202 (0.8) | 1344 (1.4) | <.001 | 1.0 | 1.3 | .03 | |
| 18–24 | 3183 (12.1) | 12 825 (13.2) | 12.6 | 13.1 | |||
| 25–34 | 16 423 (62.6) | 59 171 (60.8) | 61.5 | 61.1 | |||
| ≥35 | 6421 (24.5) | 23 925 (24.6) | 24.9 | 24.5 | |||
| Race/ethnicity | |||||||
| Asian | 4975 (19.0) | 18 811 (19.3) | <.001 | 18.9 | 19.4 | .02 | |
| African American | 1903 (7.3) | 8094 (8.3) | 7.8 | 8.2 | |||
| Hispanic | 10 669 (40.7) | 35 105 (36.1) | 40.9 | 36.0 | |||
| Other | 2106 (8.0) | 7453 (7.7) | 7.8 | 7.7 | |||
| White | 6576 (25.1) | 27 802 (28.6) | 24.6 | 28.7 | |||
| Trimester of Tdap exposure | |||||||
| First | 2014 (7.7) | ||||||
| Second | 10 935 (41.7) | ||||||
| Third | 13 280 (50.6) | ||||||
| Year | |||||||
| 2010 | 6548 (25.0) | 35 886 (36.9) | <.001 | ||||
| 2011 | 12 433 (47.4) | 30 118 (31.0) | |||||
| 2012 | 7248 (27.6) | 31 261 (32.1) | |||||
| Medical care in first trimester | 25 372 (96.7) | 93 404(96.0) | <.001 | 96.6 | 96.1 | <.001 | |
| Prenatal Care Index | |||||||
| Adequate/plus | 21 719 (82.8) | 74 485 (76.6) | <.001 | 82.7 | 76.6 | <.001 | |
| Intermediate | 4090 (15.6) | 20 613 (21.2) | 15.5 | 21.2 | |||
| Inadequate | 857 (1.6) | 2167 (2.2) | 1.8 | 2.2 | |||
| Hospitalization <20 wk gestation | |||||||
| None | 25 725 (98.1) | 94 919 (97.6) | <.001 | 97.8 | 97.7 | .12 | |
| 1 | 453 (1.7) | 2119 (2.2) | 2.0 | 2.1 | |||
| ≥2 | 46 (0.2) | 227 (0.2) | 0.2 | 0.2 | |||
| Received other vaccine during pregnancyc | 14 111 (53.8) | 35 307 (36.3) | <.001 | ||||
| Received Tdap prior to pregnancy | 1080 (4.1) | 44 399 (45.6) | <.001 | ||||
| Preexisting comorbiditiesd | 4614 (17.6) | 18 176 (18.7) | <.001 | ||||
| Hypertension | 777 (3.0) | 3320 (3.4) | <.001 | 18.5 | 18.4 | .75 | |
| Pulmonary | 2961 (11.3) | 11 723 (12.1) | <.001 | ||||
| Diabetes | 505 (1.9) | 1870 (1.9) | .97 | ||||
| Heart disease | 442 (1.7) | 1853 (1.9) | .02 | ||||
| Renal complication | 475 (1.8) | 1785 (1.8) | .80 | ||||
| Poverty %e | 17.5 | 16.7 | <.001 | 17.8 | 17.6 | <.001 | |
| Site | <.001 | ||||||
| A | 10 118 (38.6) | 56 821 (58.4) | |||||
| B | 16 106 (61.4) | 40 444 (41.6) | |||||
Unvaccinated may have received Tdap prior to pregnancy or after delivery.
Unexposed corresponds to unique pregnancies.
Other vaccines administered during pregnancy included inactivated influenza vaccines (95.9%), hepatitis A or hepatitis B (1.4%), human papillomavirus vaccine (1.1%), other (1.6%).
Diagnoses at inpatient, outpatient, or emergency visits, from 6 months before pregnancy through the end of the pregnancy.
Percentage of families in census tract whose income was below 150% of the federal poverty level.
Among women who received Tdap at anytime during pregnancy, 6.1% were diagnosed with chorioamnionitis compared with 5.5% of unexposed women. After adjusting for site, receipt of 1 or more other vaccines in pregnancy and the propensity score, the adjusted relative risk (RR) was 1.19 (95% CI, 1.13–1.26). In the subset of women vaccinated between 27 and 36 weeks’ gestation, this risk was still increased but less so (adjusted RR, 1.11; 95% CI, 1.03–1.21). Among preterm births (<37 weeks’ gestation), there was not an elevated risk of chorioamnionitis (adjusted RR, 0.87; 95% CI, 0.64–1.16). Among women with chorioamnionitis, the median gestational week of vaccination was 28 (interquartile range [IQR], 21–34 weeks) vs 28 weeks (IQR, 21–33 weeks) among women without it. In women receiving Tdap before 20 weeks’ gestation, 8.2% developed a hypertensive disorder of pregnancy vs 8.0% of unexposed women (adjusted RR, 1.09; 95% CI, 0.99–1.20; Table 2).
Table 2.
Rates of Adverse Gestational and Birth Outcomes and Relative Risks Associated With Receipt of Pertussis Vaccine (Tdap) During Pregnancy
| No. (%) |
Risk Ratios (95% CI) |
||||
|---|---|---|---|---|---|
| Outcome | Tdap Exposed |
Unexposed | Unadjusted | Adjusteda | P Value |
| Full cohort | 26229 | 97 265 | |||
| Chorioamnionitis | 1596 (6.1) | 5329 (5.5) | 1.11 (1.05–1.17) | 1.19 (1.13–1.26) | <.001 |
| Preterm delivery, 37 wk | 1527 (6.3) | 7544 (7.8) | 1.01 (0.95–1.06) | 1.03 (0.97–1.09) | .33 |
| Small for gestational age, <10th percentile | 2214(8.4) | 8086 (8.3) | 1.02 (0.97–1.06) | 1.00 (0.96–1.06) | .68 |
| Vaccinated at <20 wk gestation | 6083 | 97 265 | |||
| Hypertensive disorders | 497 (8.2) | 7736 (8.0) | 1.03 (0.94–1.12) | 1.09 (0.99–1.20) | .05 |
| Vaccinated at 27-≤36 wk gestation | 11351 | 97 265 | |||
| Chorioamnionitis | 637 (5.6) | 5329 (5.5) | 1.02 (0.95–1.11) | 1.11 (1.03–1.21) | .009 |
| Preterm delivery <37 wk | 602 (5.3) | 7544 (7.8) | 0.88 (0.81–0.96) | 0.88 (0.80–0.95) | .002 |
| Small for gestational age, <10th percentile | 978 (8.6) | 8086 (8.3) | 1.04 (0.97–1.10) | 1.03 (0.96–1.10) | .40 |
Adjusted for propensity score, receipt of influenza vaccine, and site. For chorioamnionitis, small for gestational age and hypertensive disorders, relative risk ratios were estimated using a Poisson model; for preterm delivery outcome relative risk ratios (hazard ratios), in both unadjusted and adjusted models, were estimated using time-dependent Cox model.
Receipt of Tdap during pregnancy was not associated with increased risk of preterm or SGA births. Among all pregnancies, 8.4% of those who received Tdap during pregnancy and 8.3% who were unexposed to the vaccine had an SGA birth (adjusted RR, 1.00; 95% CI, 0.96–1.06). The rate of preterm delivery among women receiving Tdap during pregnancy at 36 weeks’ gestation or earlier was 6.3%, whereas the rate for unexposed women was 7.8% (adjusted hazard ratio [HR], 1.03; 95% CI, 0.97–1.09). The findings for SGA were similar in the subset of women vaccinated between 27 and 36 weeks’ gestation. The preterm delivery rate of 5.3% among women vaccinated between 27 and 36 weeks’ gestation was slightly lower than the rate of 7.8% among the unvaccinated cohort. These differences were statistically significant (adjusted HR, 0.88; 95% CI, 0.80–0.95; Table 2)
In a sample of 220 women with an ICD-9 code for chorioamnionitis, 213 (96.8%) had the diagnosis documented in the EHR and 200 (91%) were treated with antibiotics during the course of labor and delivery. Two hundred nine women (95%) had an epidural. Similar to the full cohort, 14 women (6.4%) had preterm births (<37 weeks’ gestation). See the eTable in the Supplement for additional data from chart review. The PPV of ICD-9 code 658.41 for having an EHR diagnosis of chorioamnionitis was 0.97 (95% CI, 0.94–0.99), for “possible chorioamnionitis” was 0.78 (95% CI, 0.72–0.83), and for “probable chorioamnionitis” was 0.50 (95% CI, 0.43–0.57). After applying the PPVs for possible and probable chorioamnionitis, associations between maternal Tdap and chorioamnionitis for the full cohort remained statistically significant; for the subgroup vaccinated between 27 and 36 weeks, after taking into account the PPVs, associations were no longer significant (P = .07).
Discussion
In this retrospective observational study of more than 26 000 women who received Tdap during pregnancy over a 3-year period, vaccination was not associated with increased risks of selected adverse birth outcomes or maternal hypertensive disorders. In the full cohort, we detected an increased risk of being diagnosed with chorioamnionitis associated with maternal Tdap; this risk was relatively lower in the subgroup vaccinated between 27 and 36 weeks’ gestation. Given limited prior safety data,12,13 continued widespread pertussis transmission,33 and current recommendations to routinely vaccinate during pregnancy,5 our study provides important information on the safety of Tdap vaccination during pregnancy.34
Although there are extensive data demonstrating the safety of influenza vaccination during pregnancy,18,20,21,35–38 relatively few studies have examined the safety of Tdap administered during pregnancy. Between 2008–2012 Munoz et al14 conducted a phase 1 and 2 randomized clinical trial and found no concerning safety signals among 33 women who received Tdap between 30 and 32 weeks.
From 2005–2010, there were 132 reports to the Vaccine Adverse Events Reporting System following Tdap administrations in pregnant women; no unexpected patterns of maternal or neonatal adverse events were observed.12 Shakib et al13 reported on 138 women who received Tdap during pregnancy in Utah between 2005–2009 and compared them with 552 unvaccinated controls. More than half of the Tdap vaccinations were administered early in pregnancy. They found no differences between vaccinated and unvaccinated women in rates of spontaneous abortion, therapeutic abortion, preterm delivery, low birth weight, or congenital anomalies. More recently, Donegan et al39 reported among more than 20 000 women in the United Kingdom who received Tdap during pregnancy, compared with historical rates, that there was no increased risk of stillbirth within 14 days of vaccination. They also observed that premature birth, low birth weight, or pre-eclampsia-eclampsia were not associated with maternal pertussis vaccination.
We detected an increased risk of being diagnosed with chorioamnionitis following vaccination (adjusted RR, 1.19). However, these results should be interpreted with caution because the magnitude of this risk was small, and we did not observe an increased risk of preterm birth, a major sequela of chorioamnionitis. Thus, the chorioamnionitis risk observed may be due to residual confounding. Additional electronic data were not available to adjust for important chorioamnionitis risk factors in the full cohort, including prolonged rupture of membranes, prolonged labor, or genital tract pathogens. Furthermore, we were unable to evaluate for the possibility of differential epidural use between vaccinated and unvaccinated women.40 It is worth noting that our chart review sample showed that 95% of women with chorioamnionitis had received an epidural.
Another potential explanation is that the findings related to chorioamnionitis reflect heterogeneity in the diagnosis. Chart reviews revealed that a diagnosis of chorioamnionitis had only 50% PPV for having a clinical presentation consistent with chorioamnionitis. When this PPV was applied, the association between Tdap vaccination at 27 and 36 weeks’ gestation and chorioamnionitis was no longer significant. Although maternal influenza vaccination is known to induce a brief non-specific inflammatory response,41,42 we are not aware of a biological mechanism for Tdap vaccination during pregnancy to increase a woman’s risk of developing clinical chorioamnionitis during delivery.
The current study included a large cohort of women receiving Tdap during pregnancy and thus greatly expands on the existing literature. An additional strength was the use of validated data sources and outcome measures.15 Similar to prior VSD studies,18,20,35 pregnancy outcomes were identified using an algorithm that has been shown to be 99% accurate for identifying pregnancies that end in a live birth.16 In addition, in a sample of 350 automated mother-infant linkages within the VSD files, 100% were confirmed to be correct with chart review.16
Our assessments of gestational age and birth weight came from EHR data, with gestational age specifically assigned by clinical assessment of the newborn. In a recent validation by Andrade et al,43 of 465 infants from managed care organizations, gestational age based on clinical assessment was confirmed in 94%. Similarly, among 212 infants with birth certificate data showing low birth weight (<2500 g), 99% were confirmed in medical record review.43 Preeclampsia was identified through diagnoses (ICD-9 codes). A recent validation study from 1 California VSD site found these ICD-9 codes to have a PPV of 94%.44 To further ensure high PPV for this outcome, we restricted to diagnoses occurring at 20 weeks’ gestation or more and required eclampsia to be an inpatient diagnosis.
An additional strength was the use of analytic techniques to minimize bias. For example, for assessing preterm delivery we used time-dependent exposure methods to adjust for differential timing of vaccination. Although crude rates for preterm delivery were higher in women unexposed to Tdap during pregnancy, after accounting for differential-time exposure (Table 2) in the full cohort, this risk was no longer apparent. As an example, a woman vaccinated at 35 weeks has limited time following vaccination for a preterm delivery to occur. Alternatively, a woman who delivers at 28 weeks has less time while still pregnant to receive Tdap. Evaluations of pregnancy exposures that do not account for differential exposure times may mistake differences in crude rates as protective effects. For the subset vaccinated between 27 and 36 weeks, the time-dependent Cox model did not fully correct for the bias due to differential exposure times and an apparent protective effect of vaccination remained (HR, 0.88; 95% CI, 0.80–0.95).
Several limitations to these analyses should be noted. First, our population only included pregnancies ending in a live birth. Given that Tdap may be most effective at preventing pertussis in newborns when administered late in pregnancy,45 and current US recommendations are to vaccinate between 27 and 36 weeks’ gestation,5 risks of spontaneous or therapeutic abortion following Tdap may be less of a concern. Stillbirth remains an important outcome but was not feasible for the current study. Our analyses relied heavily on automated EHR data for assigning gestational age at delivery and thus defining pregnancy periods. To accurately assign a gestational age for still-births would require chart reviews. Furthermore, because still-births represented only 0.4% of the cohort, their exclusion was unlikely to bias our study findings.
Second, this study was limited to women from a single state with continuous insurance coverage, complete birth data available, and at least 1 medical visit during pregnancy. Thus, the highest-risk pregnancies, occurring in women with intermittent insurance coverage, were underrepresented. In addition, more than 10% of women were excluded because their complete birth data were not available. During the first 2 years of this study, Tdap was not routinely administered to pregnant women outside of California. Furthermore, despite direct adjustment, residual biases due to variation in receipt of other vaccines during pregnancy may remain. Finally, data presented reflect outcomes associated with a single Tdap dose administered during pregnancy. Because current recommendations from the Advisory Committee on Immunization Practices are to administer Tdap in every pregnancy, continued monitoring of the safety of repeated Tdap doses in a geographically diverse population will be important.
Conclusions
In this cohort of women with singleton pregnancies that ended in live birth, receipt of Tdap during pregnancy was not associated with increased risk of preterm delivery or SGA birth or with hypertensive disorders of pregnancy, although a small but statistically significant increased risk of being diagnosed with chorioamnionitis was observed.
Supplementary Material
Acknowledgments
Funding/Support: This study was funded through contract 200–2012-53526 from the CDC. Additional funding for chart reviews came from contracts 200–2012–53581 and 200–2012-53580 from the CDC Vaccine Safety Datalink infrastructure task order.
Role of the Funder/Sponsor: Both Dr DeStefano and Ms McCarthy assisted with study design, interpretation of findings, and the decision to submit this article for publication. This manuscript underwent the CDC clearance process.
Glossary
- EHR
electronic health record
- ICD-9
International Classification of Diseases, Ninth Revision
- PPV
positive predictive value
- SGA
small for gestational age
- Tdap
tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine
- VSD
Vaccine Safety Datalink
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Klein reported that she has received grant support from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck & Co, Novartis, Nuron Biotech, Protein Science, and MedImmune. Dr Cheetham reported that he receives research support from Merck. Dr Naleway reported that she has received research support from GlaxoSmithKline. Dr DeStefano and Ms McCarthy reported that they are employees of the CDC and served as collaborators on this research. No other disclosures were reported.
Disclaimer: Findings of this study represent those of the authors and do not necessarily represent the official views of the CDC.
Contributor Information
Elyse O. Kharbanda, HealthPartners Institute for Education and Research, Minneapolis,Minnesota.
Gabriela Vazquez-Benitez, HealthPartners Institute for Education and Research, Minneapolis,Minnesota.
Heather S. Lipkind, Obstetrics and Gynecology, Yale University, New Haven, Connecticut.
Nicola P. Klein, Kaiser Permanente of Northern California, Oakland.
T.Craig Cheetham, Kaiser Permanente Southern California, Pasadena.
Allison Naleway, Kaiser Permanente Northwest, Portland, Oregon.
Saad B. Omer, Kaiser Permanente Georgia, Atlanta.
Simon J. Hambidge, Department of Ambulatory Care Services, Denver Health, Denver, Colorado, Institute for Health Research, Kaiser Permanente Colorado, Denver.
Grace M. Lee, Harvard Pilgrim Health Care Institute, Harvard Medical School, Boston, Massachusetts.
Michael L. Jackson, Group Health Cooperative, Seattle,Washington.
Natalie L. McCarthy, Centers for Disease Control and Prevention, Atlanta, Georgia.
Frank DeStefano, Centers for Disease Control and Prevention, Atlanta, Georgia.
James D. Nordin, HealthPartners Institute for Education and Research, Minneapolis, Minnesota.
REFERENCES
- 1.Broder KR, Cortese MM, Iskander JK, et al. ; Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-3):1–34. [PubMed] [Google Scholar]
- 2.Murphy TV, Slade BA, Broder KR, et al. ; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of pertussis, tetanus, and diphtheria among pregnant and postpartum women and their infants recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57(RR-4):1–51. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC). Pertussis epidemic—Washington, 2012. MMWR Morb Mortal Wkly Rep. 2012;61(28):517–522. [PubMed] [Google Scholar]
- 4.Winter K, Harriman K, Zipprich J, et al. California pertussis epidemic, 2010. J Pediatr. 2012;161(6): 1091–1096. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013;62(7):131–135. [PMC free article] [PubMed] [Google Scholar]
- 6.CDPH Broadens recommendations for vaccinating against pertussis: immunization key to controlling whooping cough. http://www.cdph.ca.gov/Pages/PH10-048.aspx.Accessed December 2, 2013.
- 7.ACOG Committee Opinion No. 566: Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2013;121 (6):1411–1414. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Prevention and Control. Notes from the field: pertussis—California, January-June 2010. Morb Mortal Wkly Rep. 2010;59 (26):817. [Google Scholar]
- 9.Cherry JD. Epidemic pertussis in 2012—the resurgence of a vaccine-preventable disease. N Engl J Med. 2012;367(9):785–787. [DOI] [PubMed] [Google Scholar]
- 10.Munoz FM, Greisinger AJ, Wehmanen OA, et al. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2005;192(4):1098–1106. [DOI] [PubMed] [Google Scholar]
- 11.Tamma PD, Ault KA, del Rio C, Steinhoff MC, Halsey NA, Omer SB. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2009;201(6):547–552. [DOI] [PubMed] [Google Scholar]
- 12.Zheteyeva YA, Moro PL, Tepper NK, et al. Adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women. Am J Obstet Gynecol. 2012;207(1):e1–e7. [DOI] [PubMed] [Google Scholar]
- 13.Shakib JH, Korgenski K, Sheng X, Varner MW, Pavia AT, Byington CL. Tetanus, diphtheria, acellular pertussis vaccine during pregnancy: pregnancy and infant health outcomes. J Pediatr. 2013;163(5): 1422–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munoz FM, Bond NH, Maccato M, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA. 2014;311(17):1760–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baggs J, Gee J, Lewis E, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics. 2011;127(suppl 1):S45–S53. [DOI] [PubMed] [Google Scholar]
- 16.Naleway AL, Gold R, Kurosky S, et al. Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine. 2013; 31(27):2898–2903. [DOI] [PubMed] [Google Scholar]
- 17.Kharbanda EO, Vazquez-Benitez G, Lipkind H, et al. Receipt of pertussis vaccine during pregnancy across 7 Vaccine Safety Datalink Sites. Prev Med. 2014;67(Jun):316–319. [DOI] [PubMed] [Google Scholar]
- 18.Kharbanda EO, Vazquez-Benitez G, Lipkind H, Naleway A, Lee G, Nordin JD; Vaccine Safety Datalink Team. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013;122(3):659–667. [DOI] [PubMed] [Google Scholar]
- 19.Kharbanda EO, Vazquez-Benitez G, Shi WX, et al. Assessing the safety of influenza immunization during pregnancy: the Vaccine Safety Datalink. Am J Obstet Gynecol. 2012;207(3)(suppl): S47–S51. [DOI] [PubMed] [Google Scholar]
- 20.Nordin JD, Kharbanda EO, Vazquez Benitez G, Lipkind H, Vellozzi C, Destefano F; Vaccine Safety Datalink. Maternal influenza vaccine and risks for preterm or small for gestational age birth. J Pediatr. 2014;164(5):1051–1057,e2. [DOI] [PubMed] [Google Scholar]
- 21.Pasternak B, Svanström H, M0lgaard-Nielsen D, et al. Risk of adverse fetal outcomes following administration of a pandemic influenza A(H1N1) vaccine during pregnancy. JAMA. 2012;308(2):165–174. [DOI] [PubMed] [Google Scholar]
- 22.Omer SB, Goodman D, Steinhoff MC, et al. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS Med. 2011;8(5):e1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinhoff MC, Omer SB, Roy E, et al. Neonatal outcomes after influenza immunization during pregnancy: a randomized controlled trial. CMAJ. 2012;184(6):645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myatt L, Clifton RG, Roberts JM, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Can changes in angiogenic biomarkers between the first and second trimesters of pregnancy predict development of pre-eclampsia in a low-risk nulliparous patient population? BJOG. 2013;120(10):1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145–157. [DOI] [PubMed] [Google Scholar]
- 27.Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med. 2002;137(8): 693–695. [DOI] [PubMed] [Google Scholar]
- 28.Seeger JD, Williams PL, Walker AM. An application of propensity score matching using claims data. Pharmacoepidemiol Drug Saf. 2005;14 (7):465–476. [DOI] [PubMed] [Google Scholar]
- 29.Kotelchuck M The adequacy of Prenatal Care Utilization Index: its US distribution and association with low birthweight. Am J Public Health. 1994;84 (9):1486–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotelchuck M An evaluation of the Kessner Adequacy of Prenatal Care Index and a proposed Adequacy of Prenatal Care Utilization Index. Am J Public Health. 1994;84(9):1414–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R,Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tita AT, Andrews WW. Diagnosis and management ofclinical chorioamnionitis. Clin Perinatol. 2010;37(2):339–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention (CDC). Notice to readers: final 2012 reports of nationally notifiable infectious diseases. MMWR Morb Mortal Wkly Rep. 2013;62(33):669–682. [PMC free article] [PubMed] [Google Scholar]
- 34.Jiménez-Truque N, Edwards KM. Maternal pertussis immunization: can it help infants? JAMA. 2014;311(17):1736–1737. [DOI] [PubMed] [Google Scholar]
- 35.Nordin JD, Kharbanda EO, Benitez GV, et al. Maternal safety of trivalent inactivated influenza vaccine in pregnant women. Obstet Gynecol. 2013; 121(3):519–525. [DOI] [PubMed] [Google Scholar]
- 36.Irving SA, Kieke BA, Donahue JG, et al. ; Vaccine Safety Datalink. Trivalent inactivated influenza vaccine and spontaneous abortion. Obstet Gynecol. 2013;121(1):159–165. [DOI] [PubMed] [Google Scholar]
- 37.Sheffield JS, Greer LG, Rogers VL, et al. Effect of influenza vaccination in the first trimester of pregnancy. Obstet Gynecol. 2012;120(3):532–537. [DOI] [PubMed] [Google Scholar]
- 38.Pasternak B, Svanström H, Mølgaard-Nielsen D, et al. Vaccination against pandemic A/H1N1 2009 influenza in pregnancy and risk of fetal death: cohort study in Denmark. BMJ. 2012;344:e2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donegan K, King B, Bryan P. Safety of pertussis vaccination in pregnant women in UK: observational study. BMJ. 2014;349:g4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abramovici A, Szychowski JM, Biggio JR, Sakawi Y, Andrews WW, Tita AT. Epidural use and clinical chorioamnionitis among women who delivered vaginally. Am J Perinatol. 2014;31(11): 1009–1014. [DOI] [PubMed] [Google Scholar]
- 41.Christian LM, Iams JD, Porter K, Glaser R. Inflammatory responses to trivalent influenza virus vaccine among pregnant women. Vaccine. 2011;29 (48):8982–8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christian LM, Porter K, Karlsson E, Schultz-Cherry S, Iams JD. Serum proinflammatory cytokine responses to influenza virus vaccine among women during pregnancy versus non-pregnancy. Am J Reprod Immunol. 2013;70(1): 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andrade SE, Scott PE, Davis RL, et al. Validity of health plan and birth certificate data for pregnancy research. Pharmacoepidemiol Drug Saf. 2013;22 (1):7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Getahun D, Rhoads G, Fassett M, et al. Accuracy of reporting maternal and infant perinatal service system coding and clinical utilization coding. J Med Stat Inform. 2013;1(3):1–3. [Google Scholar]
- 45.Healy CM, Rench MA, Baker CJ. Importance of timing of maternal combined tetanus, diphtheria, and acellular pertussis (Tdap) immunization and protection of young infants. Clin Infect Dis. 2013;56 (4):539–544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


