Abstract
Poor linkage, engagement and retention remain significant barriers in achieving HIV treatment goals in the US. HIV-infected persons entering or re-entering care across three Southern California academic HIV clinics, were randomized (1:1) to an Active, Linkage, Engagement, Retention and Treatment (ALERT) specialist for outreach and health coaching, or standard of care (SOC). The primary outcome of time to loss to follow up (LTFU) was compared using Cox proportional hazards regression modeling. No differences in the median time to LTFU (81.7 for ALERT versus 93.6 weeks for SOC; HR 1.27; p = 0.40), or time to ART initiation was observed (N = 116). Although, ALERT participants demonstrated worsening depressive symptomatology from baseline to week 48 compared to SOC (p = 0.02). The ALERT intervention did not improve engagement and retention in HIV care over SOC. Further studies are needed to determine how best to apply resources to improve retention and engagement.
Keywords: HIV engagement in care, Health coaching, CCTG 594, Adherence, Lost to Follow up
Resumen
Pobre vinculación, la participación activa con el cuidado médico y la retención siguen siendo barreras significantes para lograr los objetivos de tratamiento del VIH en los Estados Unidos. Las personas infectadas por el VIH que ingresan o reingresan a la atención medica en tres clínicas académicas de VIH del sur de California, aleatorizado (1:1) a un Active, Linkage, Engagement, Retention and Treatment (ALERT) especialista para alcance y educación de salud, o estándar de atención (SOC). Se comparó el resultado primario del tiempo hasta la pérdida de seguimiento (LTFU) usando modelado de regresión de riesgos proporcionales Cox. No hubo diferencias en el tiempo mediano para LTFU (81,7 para ALERT versus 93,6 semanas para SOC; HR: 1.27; p = 0.40), tampoco en el tiempo de iniciación los medicamentos del VIH (N = 116). Los participantes en el grupo de ALERT demostraron un empeoramiento de la sintomatología depresiva relativo a la línea basal hasta la semana 48 comparado al SOC (p = 0.02). La intervención ALERT no mejoro la participación activa y la retención en el cuidado del VIH sobre el SOC. Se necesita estudios adicionales para determinar la mejor manera de aplicar recursos para mejorar la retención y la participación activa.
Introduction
HIV infection today is a manageable chronic illness when HIV-infected persons are engaged in care and adhere to antiretroviral therapy (ART). Of those living with HIV in the United States, 86% are diagnosed, 40% are engaged in care (defined as having one clinic visit or one laboratory for CD4/ HIV RNA within the last year), 37% are on ART, and 30% are virologically suppressed [1]. This “cascade of HIV care” identifies clear gaps in care delivery, from identifying HIV-infected people, to linking newly identified HIV-infected persons to a HIV clinic, retaining those persons in clinic, prescription of ART and optimizing adherence to ART [2]. In the United States, much effort has been placed on HIV testing, resulting in a decrease in the number of HIV-infected people who do not know their status from approximately 25% in the early 2000’s [3] to 14% in 2012 [4]. There has also been a lot of effort to link newly HIV-infected persons to care, yet as the cascade shows, only about 40% remain engaged in care. This is particularly important because poor engagement in care is associated with the majority of new HIV transmissions in the U.S [5].
Multiple factors contribute to poor engagement and retention in care for HIV-infected persons including low health literacy [6, 7], difficulty navigating health care [8, 9], HIV stigma [10–12], depression [13], substance use [14], and poor social support [15]. These barriers to care are common in HIV-infected persons [16–21] and associated with poorer psychosocial outcomes, changes in clinic site, lower CD4 counts, higher HIV viral loads, increased hospitalizations, and poorer self-reported health [10–12, 16, 18, 22–26]. African American and Hispanic HIV-infected patients are particularly susceptible to barriers to care [27, 28] because of historically lower levels of health literacy [20, 21]. However, studies suggest that individual targeting of specific barriers to care can impact clinical outcomes. For example, improving health literacy promotes sustained increases in knowledge of ART and results in gains in CD4 T cell counts [23, 24]. Similarly, assisting with system navigation improves use of available resources, communication, and ultimately care engagement [29, 30].
Active participation of HIV-infected patients in their healthcare increases the likelihood of ART initiation, improves virologic response and lowers mortality [31–34]. Thus, several studies have utilized patient education, empowerment and facilitation of access to healthcare in an attempt to keep patients engaged in care but show mixed results [29, 35–37]. Project HOPE enrolled HIV positive patients who were recently hospitalized active drug users and used strength based and motivational interviewing to keep patients engaged in care [35]. In spite of extensive contact with the patient navigator and financial incentives given to participants, project HOPE did not impact lost to follow up (LTFU) rates. Other studies have successfully impacted LTFU rates with similar enhanced contact, skills building [36], and multidisciplinary care coordination teams [29, 37] that provided outreach, patient navigation, and ART directly observed therapy. Despite differing results, these studies suggest that to sustain meaningful engagement in HIV care amongst a diverse population of PLWH, requires addressing multiple barriers to care and very labor-intensive multidisciplinary team efforts. To determine if barriers to care can be overcome in a more time and cost-effective way, the California Collaborative Treatment Group (CCTG) designed a multi-site, structured behavioral intervention that targets multiple patient and clinic level factors to retention and engagement in HIV care that unlike previous studies utilized a single specialist. Specifically, CCTG 594 evaluated the impact of one-on-one coaching sessions delivered by an Active, Linkage, Engagement, Retention and Treatment (ALERT) specialist on engagement and retention in HIV care. We hypothesized that participants randomized to the ALERT arm would demonstrate higher levels of retention in care compared to subjects in the standard of care (SOC) arm.
Patient and Methods
Study Design
CCTG 594 (NCT01957748) was a two-arm, open-label, randomized controlled trial (RCT) evaluating the effect of an enhanced engagement and health coaching strategy using an ALERT specialist compared to SOC on retention in care. This study ran from January 2014 to March 2016 at three CCTG-affiliated clinics in Southern California. Institutional Review Board approval was obtained at all institutions and informed consent was obtained from all participants included in the study.
Standardized assessments collected at baseline and annually were used to evaluate factors associated with successful retention in care and to evaluate the effect of an ALERT specialist. The primary endpoint was time to lost to follow up (LTFU), defined as no visit with a HIV provider at any of the three participating clinical sites for greater than 180 days as captured by chart review. Once the study endpoint was met, staff proceeded down an algorithm to identify the location and status of participants. This algorithm included attempting all forms of contact: phone, email, letter to home, as well as searching public incarceration and death records. If participant were found to be incarcerated, they were censored at the time of incarceration. The protocol team debated on how to treat incarcerated participants and ultimately deemed them to be a separate category from LTFU. Incarcerated participants still have access to care while incarcerated within the prison system, but since incarceration is not volitional, participants cannot keep scheduled clinic appointments within the CCTG network. Because there was no way of knowing if these incarcerated participants would have kept their clinic appointments if not incarcerated, they were censored as stated above. Participants who established care with an outside HIV provider and did not consent for study staff to access outside clinic records were treated as LTFU.
Secondary endpoints were assessed through self-report at baseline, annually, and when primary endpoint was reached. These included known barriers to care such as HIV knowledge [38], PHQ-9 (Depression) [39], Self-Efficacy (adapted from Cancer Behavior Inventory Self-Efficacy scale) [40], Alcohol Use Disorders Identification Test (AUDIT-C) [41], tobacco, marijuana, and other drug use. We also evaluated by self-report endpoints that could contribute to ongoing HIV transmissions including number of sexual partners in past 3 months, number of episodes of unprotected intercourse, intent to use condoms, determination to practice safe sex, and disclosure of HIV serostatus. Lastly, we collected additional clinical HIV outcomes by medical chart review: time to initiation of ART, viral suppression (defined as HIV RNA < 50 copies/mL), percentage and absolute CD4 T cell counts.
Subjects
Eligible participants were HIV-infected individuals 18 years or older, who were receiving HIV care services at one of the CCTG-affiliated clinics. This included persons new to care and returning to care (defined as not having a clinic visit for at least 180 days and a detectable HIV RNA). All participants were randomized and enrolled within 60 days of their initial clinic visit. Participants were either English or Spanish speakers.
Participants were randomized (1:1) to the ALERT Intervention Arm or SOC. Randomization was stratified by participant status as new or returning to care, and by HIV study site.
Intervention
Using the framework of the ‘Behavioral Model for Vulnerable Populations’ [15], we constructed a behavioral intervention aimed to improve engagement in care. This model posits that patient level factors [education (literacy), perception of HIV risk/diagnosis/stigma, social support] and clinic level factors [available services on site (e.g., psychiatric), reminder phone calls] are critical barriers to engagement with healthcare [33, 42].
Participants in the ALERT arm received five coaching interventions. Topics included HIV health literacy, Navigating the Health Care System, Disclosure, Adherence, and Self-Efficacy (Supplement Table 1). The coaching intervention was derived from outreach models previously proven to increase engagement and retention in HIV care [43, 44]. Each ALERT worker was trained to deliver the modules consistently to assure fidelity between sites. Modules were standardized across study sites and there was regular study team calls between ALERT workers and supervising study investigators across study sites throughout the study period to assure consistency in module delivery.
Participants in the ALERT arm received reminder calls about upcoming appointments and follow up calls for missed visits. Engagement and retention efforts followed an algorithm developed in a pilot project [9]. Participants in the SOC arm received on-going clinic standards regarding out-reach and retention activity and once they reached LTFU, an ALERT worker would attempt to engage the participant to offer an End of Study visit. For participants who established care with an outside HIV provider, consent was obtained to access outside medical records for chart review and were not considered LTFU if they continued to engage in care, but if consent was not provided to access outside medical records, then were considered LTFU. Preferences for communication were elicited and included: text, voice message, phone call, and email. Participants also provided secondary contacts and included friends, family, partners, or other persons. All participants received monetary compensation of 20 dollars for completion of baseline visit and week 48 visit.
Statistical Analysis
Descriptive analyses were performed to compare demographic and baseline characteristics between study arms. Associations between categorical variables were analyzed using Fisher’s Exact Test, while associations between continuous variables were analyzed using the Wilcoxon Rank Sum Test.
Sample size calculations were based on a two-sided, two-sample log-rank test to compare the differences in time to the primary end point proportions between the intervention arm and the SOC arm. Since attrition is a component of the composite endpoint, attrition rates were not used as an adjustment in the power calculations. We developed the composite endpoint using data from national and local cascades of care that estimated the proportion of HIV positive patients who are HIV diagnosed and in care (ranging from 40 to 80%) [45, 46]. Based on this, we used 68% as a crude estimate for power. In our protocol, assuming 55 subjects per group (for a total of 110 subjects), a two-year accrual and one-year follow up timeline, we calculated 81% power to detect a meaningful difference of 15%. This assumed a time without a composite endpoint rate of 68% in the SOC arm and that the intervention arm would increase the time without an endpoint to a rate of 83% (hazard ratio of 2.37). Analyses were stratified between persons new to care and returning to care. Secondary hypotheses were considered exploratory and were not a priori powered.
For the primary hypothesis, Kaplan-Meier curves and the log-rank test were used to determine if there was a difference in “time to LTFU” between treatment arms. Cox proportional hazards regression modeling was conducted to compare the time to LTFU between the two study arms adjusting for the following covariates: age, race and ethnicity. The following covariates were identified a priori with intent to include in the model if they were unbalanced by baseline and univariately associated with the outcome: education, income, AUDIT-C Score, alcohol use, tobacco use, marijuana use, any other illicit substance use, PHQ9 mood scale total score, HIV medication prescribed, and HIV Knowledge correct proportion.
Since the study population was primarily men (93.1%), analyses were separately conducted on (a) all study participants and (b) the male subgroup. An analogous approach was applied on the secondary endpoint, “time to initiation of ART.”
For the other secondary outcomes descriptive analyses were conducted to compare study arms. All analyses were performed in the statistical software, R (version 3.1.1.). P-values less than 0.05 were considered statistically significant. As these were secondary outcomes of interest, there were no adjustments made for multiple comparisons.
Results
Baseline Characteristics of Study Participants
The characteristics of the study population are summarized in Tables 1 (for categorical variables) and 2 (for continuous variables), and consort presented in Fig. 1. A total of 116 participants were enrolled, the majority were male 108 (93.1%) with 7 (6%) cis-women and 1 (0.9%) trans-woman. The mean age was 38.3 years (SD = 11). Of the participants, 79 (72.5%) were White, 10 (9%) Black, 3 (2.8%) Asian, 4 (3.7%) multiple race and 13 (11.9%) another race. Seventy-six participants (66.1%) were of Hispanic or Latino(a) ethnicity and 82 (70.7%) said English was their primary language. Many participants had some college education 50 (43.1%), but most had low income with 71 (61.2%) reporting a household monthly average of < $2000, and 38 (32.8%) being unemployed. HIV transmission risk factors among participants included 88 (75.9%) reporting homosexual contact, 44 (37.9%) heterosexual contact, and 14 (12.1%) injection drug use. Risk factors were not mutually exclusive and thus participants were able to report multiple risks. The majority of participants were newly entering HIV care, 93 (80.2%), and many had been prescribed ART 65 (56.5%) by study entry (study protocol allowed enrollment within 60 days of initial clinic visit thus some participants had already started ART).
Table 1.
Baseline characteristics
| ALERT (N = 60) | SOC (N = 56) | Total (N = 116) | p value | |
|---|---|---|---|---|
| Gender | ||||
| Male | 55 (91.7%) | 53 (94.6%) | 108 (93.1%) | > 0.999 |
| Female | 4 (6.7%) | 3 (5.4%) | 7 (6.0%) | |
| Male to femalea | 1 (1.7%) | 0 (0%) | 1 (0.9%) | |
| Female to malea | 0 (0%) | 0 (0%) | 0 (0%) | |
| Race | ||||
| Asian | 2 (3.6%) | 1 (1.9%) | 3 (2.8%) | 0.938 |
| Black | 4 (7.3%) | 6 (11.1%) | 10 (9.2%) | |
| Multiple | 2 (3.6%) | 2 (3.7%) | 4 (3.7%) | |
| Other | 6 (10.9%) | 7 (13.0%) | 13 (11.9%) | |
| White | 41 (74.6%) | 38 (70.4%) | 79 (72.5%) | |
| Ethnicity | ||||
| Hispanic or Latino(a) | 40 (66.7%) | 36 (65.5%) | 76 (66.1%) | > 0.999 |
| Not Hispanic or Latino(a) | 20 (33.3%) | 19 (34.6%) | 39 (34.0%) | |
| Education | ||||
| Less than high school | 10 (16.7%) | 9 (16.1%) | 19 (16.4%) | 0.952 |
| High school | 13 (21.7%) | 12 (21.4%) | 25 (21.6%) | |
| Some college | 27 (45%) | 23 (41.1%) | 50 (43.1%) | |
| Bachelor degree | 7 (11.7%) | 9 (16.1%) | 16 (13.8%) | |
| Some post graduate | 1 (1.7%) | 0 (0%) | 1 (0.9%) | |
| Advanced degree | 2 (3.3%) | 3 (5.4%) | 5 (4.3%) | |
| Household average monthly income | ||||
| < $2000 | 36 (60%) | 35 (62.5%) | 71 (61.2%) | 0.122 |
| $2000 | 14 (23.3%) | 18 (32.1%) | 32 (27.6%) | |
| Refused to answer | 10 (16.7%) | 3 (5.4%) | 13 (11.2%) | |
| Relationship status | ||||
| Married | 2 (3.3%) | 5 (8.9%) | 7 (6.0%) | 0.67 |
| Divorced | 3 (5%) | 3 (5.4%) | 6 (5.2%) | |
| Single | 41 (68.3%) | 34 (60.7%) | 75 (64.6%) | |
| Widowed | 1 (1.7%) | 1 (1.8%) | 2 (1.7%) | |
| In a committed relationship | 8 (13.3%) | 11 (19.6%) | 19 (16.4%) | |
| Separated | 4 (6.7%) | 1 (1.8%) | 5 (4.3%) | |
| Refused to answer | 1 (1.7%) | 1 (1.8%) | 2 (1.7%) | |
| Primary language | ||||
| English | 44 (73.3%) | 38 (67.9%) | 82 (70.7%) | 0.546 |
| Spanish | 16 (26.7%) | 18 (32.1%) | 34 (29.3%) | |
| Employment | ||||
| Full time | 17 (28.3%) | 11 (19.6%) | 28 (24.1%) | |
| Part time | 14 (23.3%) | 18 (32.1%) | 32 (27.6%) | |
| Unemployed | 22(36.7%) | 16 (28.6%) | 38 (32.8%) | 0.359 |
| Retired | 0 (0%) | 1 (1.8%) | 1 (0.9%) | |
| Unable to work | 7 (11.7%) | 8 (14.3%) | 15 (12.9%) | |
| Refused to answer | 0 (0%) | 2 (3.6%) | 2 (1.7%) | |
| Care status | ||||
| Newly diagnosed | 47 (78.3%) | 46 (82.1%) | 93 (80.2%) | 0.648 |
| Returning to care | 13 (21.7%) | 10 (17.9%) | 23 (19.8%) | |
| Had ARV before or on baseline | ||||
| No | 21 (35%) | 29 (52.7%) | 50 (43.5%) | 0.062 |
| Yes | 39 (65%) | 26 (47.3%) | 65 (56.5%) | |
| Disclosure | ||||
| No | 13 (21.7%) | 9 (16.4%) | 22 (19.1%) | 0.489 |
| Yes | 47 (78.3%) | 46 (83.6%) | 93 (80.9%) | |
| Alcohol use | ||||
| No | 21 (35%) | 25 (44.6%) | 46 (39.7%) | 0.344 |
| Yes | 39 (65%) | 31 (55.4%) | 70 (60.3%) | |
| Tobacco use | ||||
| No | 40 (66.7%) | 36 (64.3%) | 76 (65.5%) | 0.846 |
| Yes | 20 (33.3%) | 20 (35.7%) | 40 (34.5%) | |
| Marijuana use | ||||
| No | 40 (66.7%) | 43 (76.8%) | 83 (71.6%) | 0.303 |
| Yes | 20 (33.3%) | 13 (23.2%) | 33 (28.5%) | |
| Any other drug use | ||||
| No | 34 (56.7%) | 28 (50%) | 62 (53.5%) | 0.577 |
| Yes | 26 (43.3%) | 28 (50%) | 54 (46.5%) | |
Results all reported as number (percent)
ALERT active, linkage, engagement, retention, treatment, SOC standard of care
Transgender individuals
Fig. 1.
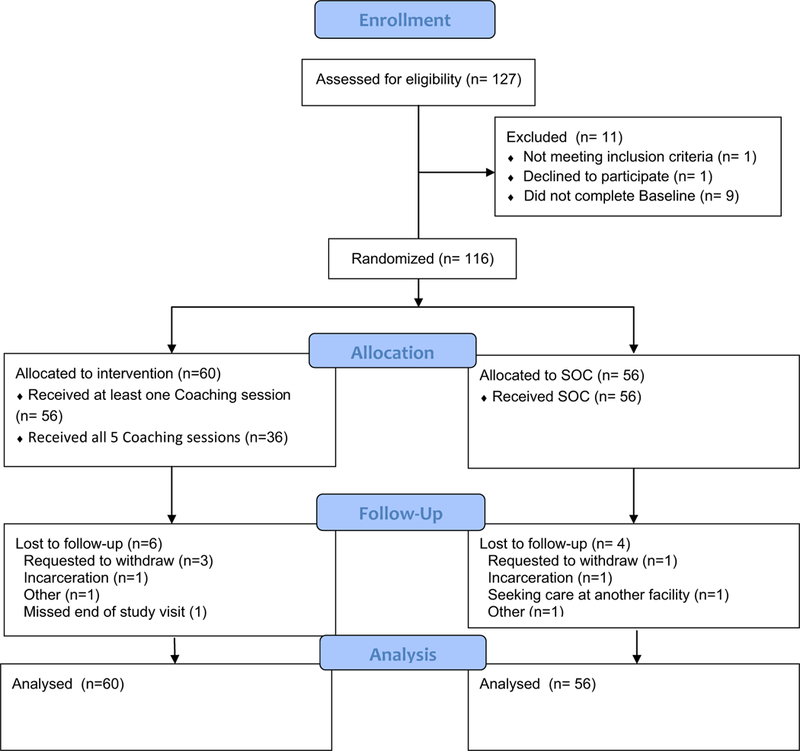
Legend: Consort Figure
Most participants reported their relationship status was “single” [75 (64.7%)] and disclosure to at least one person of HIV status was high (n = 93, 80.9%). Our population had high levels of alcohol (n = 70, 60.3%), tobacco (n=40, 34.5%), marijuana (n = 33, 28.5%) and other substance use (n = 54, 46.6%) (Table 1). However, the median baseline score on AUDIT-C was 3 (IQR: 0–5) suggesting participants were not, on average, hazardous alcohol drinkers (Table 2).
Table 2.
Baseline characteristics (continuous variables)
| N | Mean | SD | P value | |
|---|---|---|---|---|
| Age (years) | ||||
| ALERT | 60 | 39 | 11.0 | 0.316 |
| SOC | 56 | 37.5 | 11.9 | |
| CD4 T cell percent | ||||
| ALERT | 60 | 20.8 | 9.9 | 0.096 |
| SOC | 55 | 17.7 | 10.3 | |
| CD4 T cell absolute count (cells/uL) | ||||
| ALERT | 60 | 361.7 | 244.6 | 0.07 |
| SOC | 56 | 289.6 | 240 | |
| CD8 T cell percent | ||||
| ALERT | 59 | 54.4 | 12.5 | 0955 |
| SOC | 55 | 54.2 | 12.9 | |
| CD8 T cell absolute count (cells/uL) | ||||
| ALERT | 59 | 878.5 | 365 | 0.057 |
| SOC | 55 | 782.7 | 392.9 | |
| HIV RNA (log10 copies/mL) | ||||
| ALERT | 60 | 4.1 | 1.3 | 0.115 |
| SOC | 56 | 4.4 | 1.1 | |
| HIV knowledge correct proportion | ||||
| ALERT | 60 | 0.7 | 0.2 | 0.458 |
| SOC | 56 | 0.8 | 0.2 | |
| Patient Health Questionnaire (PHQ-9) | ||||
| ALERT | 60 | 7.7 | 6.4 | 0.769 |
| SOC | 56 | 7.3 | 6.1 | |
| Self-Efficacy Subscale 1 | ||||
| ALERT | 60 | 2.4 | 0.5 | 0.387 |
| SOC | 56 | 2.3 | 0.6 | |
| Self-Efficacy Subscale 2 | ||||
| ALERT | 60 | 2.3 | 0.7 | 0.294 |
| SOC | 56 | 2.2 | 0.6 | |
| Self-Efficacy Subscale 3 | ||||
| ALERT | 60 | 2.3 | 0.7 | 0.97 |
| SOC | 56 | 2.4 | 0.6 | |
| Alcohol used disorder identification test (AUDIT-C) | ||||
| ALERT | 60 | 2.7 | 2.3 | 0.64 |
| SOC | 56 | 3.4 | 3.5 | |
ALERT active, linkage, engagement, retention, treatment, SOC standard of care, SD standard deviation
At baseline the mean CD4 T cell count was 327 cells/uL (SD = 244) with mean percent being 19.3 (SD = 10.2). Mean HIV viral load was 4.27 log10 copies/mL (SD = 1.22). There were no differences in baseline characteristics between participants randomized to the ALERT intervention and SOC.
Baseline demographics were also evaluated by site and as would be expected based on differences in population distribution in Southern California, some metrics did differ. Most notably one clinic in particular University of Southern California (USC) had a significantly greater proportion of Hispanic participants; 84.3% compared to University of California San Diego (UCSD) at 45.8% and Harbor-University of California Los Angeles (Harbor) at 68.75% (p < 0.001). Participants at USC also demonstrated lower education (p = 0.002) and lower household monthly income (p < 0.001) and were more likely to note Spanish as a primary language (p < 0.001). Participants at USC were also significantly less likely to use drugs 70.6% compared to 38.8% at UCSD and 42.75% at Harbor (p = 0.004) (data not shown).
Interestingly, UCSD had higher CD4 T cell counts at baseline (mean 445 cells/mL) compared to both Harbor and USC (207 and 250 cells/mL respectively, p < 0.001) and lower HIV viral loads (mean 3.73 log10 copies/mL) compared to Harbor and USC (mean 4.43 and 4.73 copies/mL p < 0.001) (data not shown).
Impact of ALERT Specialist on LTFU
Of the 60 participants randomized into ALERT, 29 (48.3%) were LTFU compared to 24 (42.9%) of the 56 participants in SOC. The median time to LTFU of ALERT subjects was 81.7 weeks compared to 93.6 weeks for SOC (p = 0.49) (Fig. 2a). When adjusting for age, race, and ethnicity there was still no difference between the two arms (HR 1.27; 95% CI 0.73, 2.22; p = 0.40). LTFU rates were not significantly different between the new to care and returning to care groups (43 vs. 56.5%, respectively) (P = 0.25). Participants in the ALERT intervention had a median of 3 visits (IQR: 1–4) compared to 2.5 (IQR: 1–3.25) in SOC before being LTFU (p = 0.43). This did not differ if participants were newly diagnosed or retuning to care [median 3 visits (IQR: 1.75–4.25) versus 2 (IQR: 1–3), respectively] (p = 0.12). Of note LTFU rates were balanced between sites (data not shown).
Fig. 2.
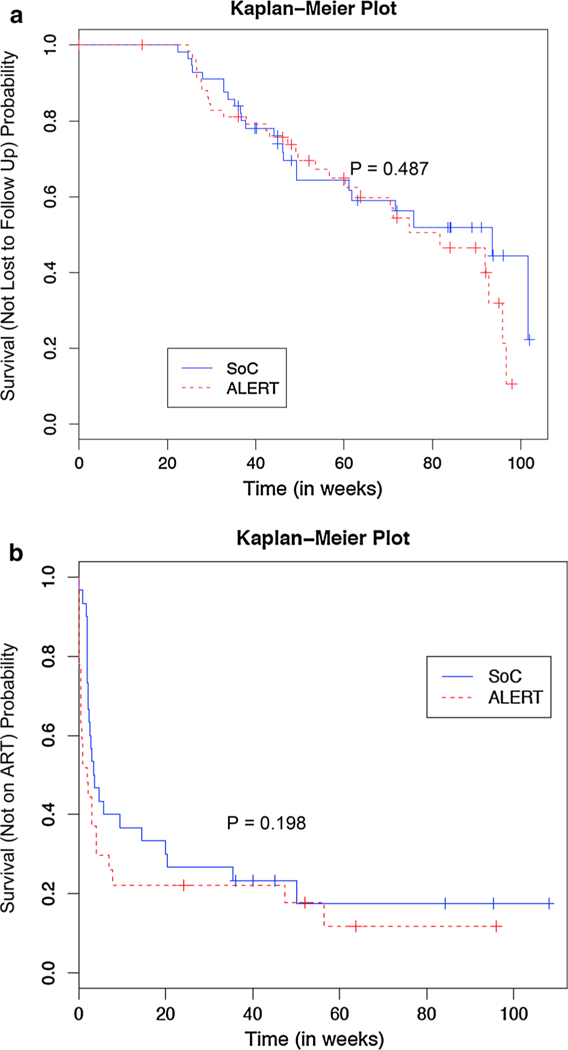
a The primary outcome of time to lost to follow up (LTFU) is represented using a Kaplan–Meier Plot. At end of study there were no differences in the ALERT intervention arm compared to Standard of Care (SOC) (p = 0.487). b The secondary outcome of time to antiretroviral therapy (ART) initiation in persons not on ART at study entry did not differ between persons in the ALERT intervention compared to standard of care (p = 0.198)
Adherence to the intervention was as follows: 93% completed at least one, 83.3% at least two, 77% at least three, 73% at least four, and 60% all five modules.
Impact of ALERT Specialist on Secondary Outcomes of Interest
Fifty-seven participants were not on ART before baseline (27 in ALERT and 30 in SOC), of those 47 (82.5%) were newly diagnosed. In this population, we evaluated whether an ALERT specialist impacted time to ART initiation. The median time to ART in the ALERT group was 2 weeks compared to 3.6 weeks in SOC (p = 0.198) (Fig. 2b). Univariate analysis and multivariable Cox regression model found that the ALERT group initiated ART faster than SOC but not significantly (HR 1.45, 95% CI 0.81–2.6, p = 0.21) (Fig. 2b).
We evaluated the impact of the ALERT intervention on achieving suppressed HIV viral loads (defined as HIV RNA < 50 copies/mL) and the change in the absolute number and percentage of CD4 T cells over time. At week 24 a slightly higher proportion of participants randomized to the ALERT arm achieved an undetectable HIV viral load, but this was not a sustained at 48 weeks (Supplemental Table 2). There was no impact of the ALERT intervention on absolute or percentage of CD4 T cells (data not shown).
To investigate other outcomes of interest, we evaluated the impact of the ALERT intervention on HIV knowledge, depression, self-efficacy, use of alcohol, tobacco, marijuana, other drugs and on sexual risk practices at 48 weeks compared to SOC. No differences between arms were noted in HIV knowledge, self-efficacy, alcohol, tobacco, marijuana or other substances (data not shown).
At 48 weeks, persons in the ALERT arm reported an increase from baseline in depressive symptoms compared to SOC (p = 0.02; Fig. 3).
Fig. 3.
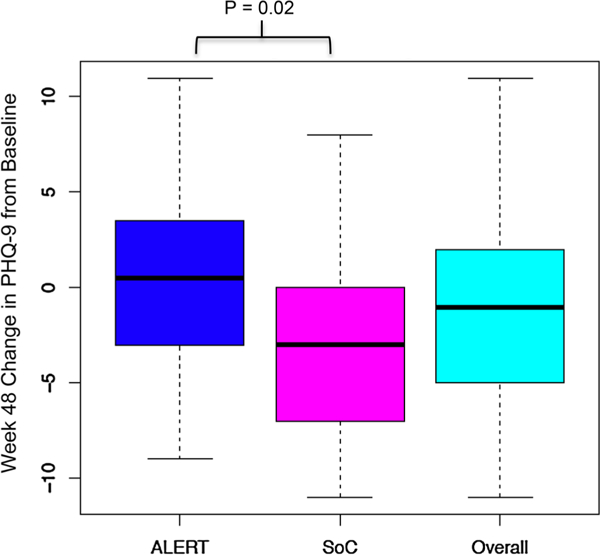
Legend: From baseline to week 48, study participants in the ALERT arm reported slightly increased depression symptomatology based on PHQ-9 score while persons in standard of care (SOC) had decreases in depression symptomatology. The difference between the two arms was statistically significant (p = 0.02)
There were no significant changes in the total number of partners, number of unprotected intercourse events, or attitudes about condom use in both arms from baseline to 48 weeks. Interestingly, persons in the ALERT arm reported more “strong agreement” in their determination to practice safe sex than SOC (p = 0.041) (Fig. 4a) but also lower rates of HIV disclosure to another person (vs. SOC) at week 48 (p = 0.032) (Fig. 4b).
Fig. 4.
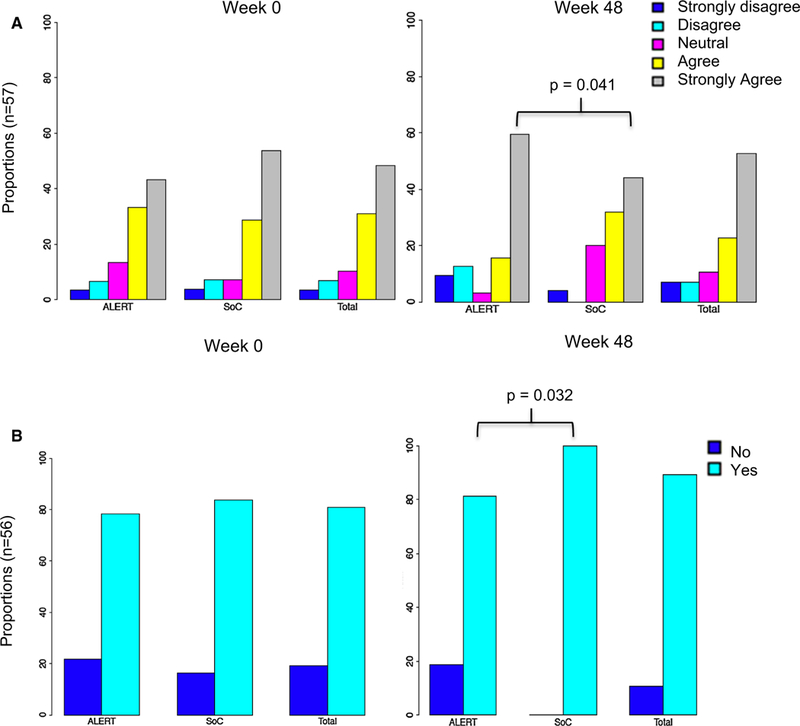
a Proportions of persons in ALERT compared to standard of care (SOC) arm (and overall summary) of attitudes regarding determination to practice safe sex. This was determined by asking the question “I am determined to practice safe sex” with a Likert scale of responses: strongly disagree, disagree, neutral, agree and strongly agree. At week 48 More persons in the ALERT arm were strongly determined to practice safe sex (p = 0.041). b Proportions of persons in ALERT compared to SOC arm (and overall summary) of HIV disclosure practices. At week 48 less persons in the ALERT arm reported disclosure of HIV compared to SOC (p = 0.032)
To determine if the findings were influenced by differential attrition, we evaluated the baseline differences of participants with early discontinuation compared to those who completed the study. The majority of persons enrolled in the study (n = 116) had complete data (n = 106). The only statistically significant baseline demographic that differed between those who discontinued participation in the study early and those that completed the study was that all early discontinuers reported English as a primary language (p = 0.03) (data not shown). Additionally, completer and adherer sensitivity analyses did not impact study findings.
Discussion
This RCT evaluating enhanced outreach and health coaching by an ALERT worker revealed no impact on retention in care in HIV-infected persons new to or returning to care when compared to SOC in these academic HIV-specialty clinics. This was true despite differences between sites in baseline demographics. Of participants not on ART before baseline, there was a tendency to start ART faster in the ALERT arm versus SOC (2 weeks versus 3.6 weeks), (p = 0.21). However, there were no differences in HIV knowledge, self-efficacy, alcohol, tobacco, marijuana or other substances at week 48.
Other studies utilizing different linkage and retention in care strategies demonstrate varying degrees of success. Project HOPE [35] enrolled hospitalized HIV-infected patients with active substance use and randomized them to patient navigation alone, patient navigation with financial incentives or SOC for 6 months. Both patient navigation arms included extensive contact with participants (up to 11 sessions) and like our study, used strength-based and motivational interviewing approaches. However, at end of study there were no differences in viral suppression or death between the three arms at 48 weeks. LTFU rates were also similar between the three groups. Interestingly, a similar study evaluating enhanced contact, enhanced contact with skills building or SOC did show improved retention in both enhanced contact arms in six academically affiliated HIV primary care clinics [36]. This study evaluated significantly more participants (N = 1838) than ours which may suggest our intervention was underpowered to see an effect. Another study evaluated a care coordination program in Ryan White funded clinics in New York [29] that included outreach, multidisciplinary care team communication, patient navigation (including accompaniment to primary care visits), ART adherence support (including directly observed therapy) and structured health promotion. Outcomes in engagement in care and viral load suppression were significantly improved. Lastly, the District of Columbia evaluated the impact of a medical case management (MCM) program and found persons participating in clinics with MCM were more likely to be retained in care, and virally suppressed [37]. All these studies utilized interventions that included patient education, empowerment, and facilitation of access to healthcare. Yet one model that reproduces consistent positive findings across differing populations of HIV infected has yet to be established.
Health coaching has demonstrated success in other chronic diseases such as diabetes mellitus, heart failure and chronic obstructive pulmonary disease [47–53] but did not impact participants in our study. Unlike other chronic diseases, HIV-infection is associated with high rates of co-occurring conditions that impact retention including substance use, mental health disorders and unemployment [54–56]. This was true in our study population that reported high rates of substance use, notably alcohol use (60%), and other drug use (excluding tobacco and marijuana use) (46.6%), although these did not appear problematic in most individuals as indicated by AUDIT-C and were not associated with LTFU. Additionally, nearly 33% of participants were unemployed with 61% reporting a household income of less than $2000 a month, which for a family of 3 or more is below the federal poverty level [57]. Unlike other chronic illnesses, HIV-infection may be asymptomatic, especially when the CD4 count is preserved [58, 59], which was often case in our study population with a mean CD4 count at baseline of 327 cells/uL. Thus, perhaps our participants were not yet symptomatic from HIV, as opposed to other chronic disease states such as heart failure, where poor adherence leads to immediate clinical change that motivates adherence and retention [60]. This study stratified participants according to whether they were new to care or returning to care, and a nonsignificant trend towards higher LTFU was observed in the returning to care group. It is possible that circumstances that caused the returning to care participants to have been LTFU prior to entering the study have not changed (i.e. homeless status, drug use) and thus contributed to continuing poor engagement in care. Lastly, all the clinic sites that participated in this study are multidisciplinary specialty HIV clinics with highly trained and experienced healthcare providers and staff that also provide access to case management and other ancillary services. Thus, the health coaching intervention offered by CCTG 594 may have overlapped with services already rendered in clinic. It is possible that health coaching would be more effective in a HIV-infected population with less specialty knowledge/ experience or access to ancillary services.
Other secondary outcomes of interest such as HIV knowledge, self-efficacy, alcohol, tobacco, marijuana or other substance use did not differ over the 48 weeks of study. Yet participants in the ALERT arm had higher week 48 depressive symptoms compared to those in standard of care. The reason for this change is unclear. It may be due to differences in the study populations that we did not capture. It is possible that repeated communication with research staff about HIV issues was a reminder to participants that they had HIV, which may have been inconsistent with the patient’s changing perspective on HIV and HIV self-identification [59]. Or, participants in the ALERT arm were encouraged to discuss issues such as stigmatization, disclosure, and safe sex, which may have been difficult to process, resulting in higher rates of negative affect [61].
Participants in the ALERT intervention were more likely to report being motivated to practice safe sex, yet less likely to disclose HIV status. Consistent with previous studies, discussing safe sex and risk for transmission may have led ALERT arm participants to strongly agree with practicing safe sex at week 48 [62]. Their hesitancy to disclose may be related to a higher rate of actual safe sex practices, or could have been associated with higher levels of depression as discussed above [63]. Alternatively, lower self-reported disclosure could reflect greater dissemination of the message that “undetectable=untransmittable” which participants in the ALERT arm could have used to justify non-disclosure [64].
This study had several limitations. First, persons in the ALERT arm demonstrated variable participation rates with 60% completing all five modules. Multiple attempts to optimize participation including trying to meet participants during scheduled HIV clinic appointment and modifications to the protocol that allowed module completion by phone were pursued but did not result in 100% completion. Unfortunately, because participants did not respond to multiple attempts at contact we could not capture reasons for not completing modules. It is possible that a more convenient or accessible approach may have had a beneficial effect. For example, a mobile technology approach (e.g. virtual health coach delivered via a mobile device) may address this concern. A second limitation of the study is that the majority of study participants were male, however analyses adjusted for gender did not change outcomes. A third limitation was the disproportionate recruitment of persons newly diagnosed compared to those returning to care which may have diluted the impact of the intervention. A fourth limitation is that during the study, two of the three participating clinics implemented a Medical Care Coordination Team, targeting the highest risk clinic patients, including those previously LTFU during the time of the study. This may have led to medical management burn out for the participants randomized to ALERT, or improved engagement in SOC. A fifth limitation is the possibility of misclassification of patients as LTFU. Lastly, linkage and engagement in care is impacted by multiple and complex factors that may have not been addressed by our intervention or modifiable within the time frame of the study, such as employment, transportation, and income.
Conclusion
It remains unknown how best to engage and retain patients who are new to and returning to care. In our RCT, enhanced contact, coaching and education did not enhance retention over the existing SOC in three HIV-specialty clinics. In fact, despite this very intensive intervention, LTFU rates were very high. Overcoming barriers to care and increasing engagement and retention remain challenges in HIV care. Future work should build on the lessons learned from CCTG 594, and focus on better understanding of clinic-specific and patient-specific factors that that contribute to poor engagement and retention. Delivering viral suppression as a standard of care for all will require more than outreach targeted at retention, but likely draw upon personalized medicine and social determinants to make the most impact.
Supplementary Material
Acknowledgments
Funding This work was funded by the California HIV Research Program fund El11-SD-005. Consent from all co-authors listed in this paper was received prior to submitting for review.
Conflicts of interest KC has received research support from Gilead and ViiV. MPD has served as a consultant to Gilead, Theratec, and Astra Zeneca and receives research support through his university from GileaMerck, Theratec, and ViiV. ESD has received research support from Gilead, Merck and ViiV as well as being a consultant/advisor for Bristol Myers Squibb, Gilead, Janssen, Merck, Teva, Theratechnology and ViiV. RH is an employee of Gilead Sciences. MK has served on an Advisory Board and receives funding to the institution from Gilead Sciences. Her other sources of funding come in part by the National Institutes of Health [R24AG044325, R01MH110057] and she is supported by the University of California San Diego Center For AIDS Research (CFAR) a NIH-funded program (P30 AI036214).
Footnotes
Complaince with Ethical Standards
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards and approved by the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individuals participants included in the study.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10461-018-2132-3) contains supplementary material, which is available to authorized users.
References
- 1.Prevention CfDCa. Undestanding the HIV care continuum 2014. https://stacks.cdc.gov/view/cdc/26481.
- 2.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diagnosis and reporting of HIV and AIDS in states with HIV/ AIDS surveillance-United States, 1994–2000. MMWR Morb Mortal Wkly Rep. 2002;51(27):595–98. [PubMed] [Google Scholar]
- 4.Hall HI, An Q, Tang T, Song R, Chen M, Green T, et al. Prevalence of diagnosed and undiagnosed HIV infection-United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2015;64(24):657–62. [PMC free article] [PubMed] [Google Scholar]
- 5.Skarbinski J, Rosenberg E, Paz-Bailey G, Hall HI, Rose CE, Viall AH, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175(4):588–96. [DOI] [PubMed] [Google Scholar]
- 6.Wawrzyniak AJ, Ownby RL, McCoy K, Waldrop-Valverde D. Health literacy: impact on the health of HIV-infected individuals. Curr HIV/AIDS Rep. 2013;10(4):295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palumbo R Discussing the effects of poor health literacy on patients facing HIV: a narrative literature review. Int J Health Policy Manag. 2015;4(7):417–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wohl AR, Carlos JA, Tejero J, Dierst-Davies R, Daar ES, Khanlou H, et al. Barriers and unmet need for supportive services for HIV patients in care in Los Angeles County, California. AIDS Patient Care STDs. 2011;25(9):525–32. [DOI] [PubMed] [Google Scholar]
- 9.Sitapati AM, Limneos J, Bonet-Vazquez M, Mar-Tang M, Qin H, Mathews WC. Retention: building a patient-centered medical home in HIV primary care through PUFF (patients unable to follow-up found). J Health Care Poor Undeserved. 2012;23(3 Suppl):81–95. [DOI] [PubMed] [Google Scholar]
- 10.Johnson MO, Chesney MA, Neilands TB, Dilworth SE, Remien RH, Weinhardt LS, et al. Disparities in reported reasons for not initiating or stopping antiretroviral treatment among a diverse sample of persons living with HIV. J Gen Intern Med. 2009;24(2):247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolitski RJ, Pals SL, Kidder DP, Courtenay-Quirk C, Holtgrave DR. The effects of HIV stigma on health, disclosure of HIV status, and risk behavior of homeless and unstably housed persons living with HIV. AIDS Behav. 2009;13(6):1222–32. [DOI] [PubMed] [Google Scholar]
- 12.Kalichman SC, Simbayi LC. HIV testing attitudes, AIDS stigma, and voluntary HIV counselling and testing in a black township in Cape Town, South Africa. Sex Transm Infect. 2003;79(6):442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pence BW, O’Donnell JK, Gaynes BN. Falling through the cracks: the gaps between depression prevalence, diagnosis, treatment, and response in HIV care. AIDS (London, England). 2012;26(5):656–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palepu A, Horton NJ, Tibbetts N, Meli S, Samet JH. Uptake and adherence to highly active antiretroviral therapy among HIV-infected people with alcohol and other substance use problems: the impact of substance abuse treatment. Addiction (Abingdon, England). 2004;99(3):361–8. [DOI] [PubMed] [Google Scholar]
- 15.Gelberg L, Andersen RM, Leake BD. The behavioral model for vulnerable populations: application to medical care use and outcomes for homeless people. Health Serv Res. 2000;34(6):1273–302. [PMC free article] [PubMed] [Google Scholar]
- 16.Kalichman SC, Ramachandran B, Catz S. Adherence to combination antiretroviral therapies in HIV patients of low health literacy. J Gen Intern Med. 1999;14(5):267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalichman SC, Benotsch E, Suarez T, Catz S, Miller J, Rompa D. Health literacy and health-related knowledge among persons living with HIV/AIDS. Am J Prev Med. 2000;18(4):325–31. [DOI] [PubMed] [Google Scholar]
- 18.Wolf MS, Davis TC, Cross JT, Marin E, Green K, Bennett CL. Health literacy and patient knowledge in a Southern US HIV clinic. Int J STD AIDS. 2004;15(11):747–52. [DOI] [PubMed] [Google Scholar]
- 19.Paasche-Orlow MK, Cheng DM, Palepu A, Meli S, Faber V, Samet JH. Health literacy, antiretroviral adherence, and HIV-RNA suppression: a longitudinal perspective. J Gen Intern Med. 2006;21(8):835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osborn CY, Paasche-Orlow MK, Davis TC, Wolf MS. Health literacy: an overlooked factor in understanding HIV health disparities. Am J Prev Med. 2007;33(5):374–8. [DOI] [PubMed] [Google Scholar]
- 21.Drainoni ML, Rajabiun S, Rumptz M, Welles SL, Relf M, Rebholz C, et al. Health literacy of HIV-positive individuals enrolled in an outreach intervention: results of a cross-site analysis. J Health Commun. 2008;13(3):287–302. [DOI] [PubMed] [Google Scholar]
- 22.Bird JD, Fingerhut DD, McKirnan DJ. Ethnic differences in HIV-disclosure and sexual risk. AIDS Care. 2011;23(4):444–8. [DOI] [PubMed] [Google Scholar]
- 23.Dowse R, Barford KL, Browne S, editors. Illustrated patient information leaflets on antiretroviral medication significantly increases drud knowledge, seld-efficacy and improves CD4 count. Rome: International AIDS Society; 2011. [Google Scholar]
- 24.Dowse R, Ramela T, Barford KL, Browne S. Developing visual images for communicating information aboutantiretroviral side effects to a low-literate population. Afr J AIDS Res AJAR. 2010;9(3):213–24. [DOI] [PubMed] [Google Scholar]
- 25.Vyavaharkar M, Moneyham L, Tavakoli A, Phillips KD, Mur-daugh C, Jackson K, et al. Social support, coping, and medication adherence among HIV-positive women with depression living in rural areas of the southeastern United States. AIDS Patient Care STDs. 2007;21(9):667–80. [DOI] [PubMed] [Google Scholar]
- 26.Stirratt MJ, Remien RH, Smith A, Copeland OQ, Dolezal C, Krieger D. The role of HIV serostatus disclosure in antiretroviral medication adherence. AIDS Behav. 2006;10(5):483–93. [DOI] [PubMed] [Google Scholar]
- 27.Hall HI, Frazier EL, Rhodes P, Holtgrave DR, Furlow-Parmley C, Tang T, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Inter Med. 2013;173(14):1337–44. [DOI] [PubMed] [Google Scholar]
- 28.Castel AD, Kalmin MM, Hart RL, Young HA, Hays H, Benator D, et al. Disparities in achieving and sustaining viral suppression among a large cohort of HIV-infected persons in care— Washington, DC. AIDS Care. 2016;28(11):1355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irvine MK, Chamberlin SA, Robbins RS, Myers JE, Braunstein SL, Mitts BJ, et al. Improvements in HIV care engagement and viral load suppression following enrollment in a comprehensive HIV care coordination program. Clin Infect Dis. 2015;60(2):298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brennan-Ing M, Seidel L, Rodgers L, Ernst J, Wirth D, Tietz D, et al. The impact of comprehensive case management on HIV client outcomes. PLoS ONE. 2016;11(2):e0148865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giordano TP, Hartman C, Gifford AL, Backus LI, Morgan RO. Predictors of retention in HIV care among a national cohort of US veterans. HIV Clin Trials. 2009;10(5):299–305. [DOI] [PubMed] [Google Scholar]
- 32.Mugavero MJ, Lin HY, Allison JJ, Giordano TP, Willig JH, Raper JL, et al. Racial disparities in HIV virologic failure: do missed visits matter? J Acquir Immune Defic Syndr. 2009;50(1):100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulett KB, Willig JH, Lin HY, Routman JS, Abroms S, Allison J, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDs. 2009;23(1):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horberg MA, Hurley LB, Silverberg MJ, Klein DB, Quesenberry CP, Mugavero MJ. Missed office visits and risk of mortality among HIV-infected subjects in a large healthcare system in the United States. AIDS Patient Care STDs. 2013;27(8):442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metsch LR, Feaster DJ, Gooden L, Matheson T, Stitzer M, Das M, et al. Effect of patient navigation with or without financial incentives on viral suppression among hospitalized patients with HIV infection and substance use: a randomized clinical trial. JAMA. 2016;316(2):156–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardner LI, Giordano TP, Marks G, Wilson TE, Craw JA, Drain-oni ML, et al. Enhanced personal contact with HIV patients improves retention in primary care: a randomized trial in 6 US HIV clinics. Clin Infect Dis. 2014;59(5):725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willis S, Castel AD, Ahmed T, Olejemeh C, Frison L, Kharfen M. Linkage, engagement, and viral suppression rates among HIV-infected persons receiving care at medical case management programs in Washington, DC. J Acquir Immune Defic Syndr. 1999;2013(64 Suppl 1):S33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carey MP, Schroder KE. Development and psychometric evaluation of the brief HIV Knowledge Questionnaire. AIDS Educ Prev. 2002;14(2):172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heitzmann CA, Merluzzi TV, Jean-Pierre P, Roscoe JA, Kirsh KL, Passik SD. Assessing self-efficacy for coping with cancer: development and psychometric analysis of the brief version of the Cancer Behavior Inventory (CBI-B). Psycho-oncology. 2011;20(3):302–12. [DOI] [PubMed] [Google Scholar]
- 41.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory care quality improvement project (ACQUIP). Alcohol use disorders identification test. Arch Intern Med. 1998;158(16):1789–95. [DOI] [PubMed] [Google Scholar]
- 42.Christopoulos KA, Das M, Colfax GN. Linkage and retention in HIV care among men who have sex with men in the United States. Clin Infect Dis. 2011;52(Suppl 2):S214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabral HJ, Tobias C, Rajabiun S, Sohler N, Cunningham C, Wong M, et al. Outreach program contacts: do they increase the likelihood of engagement and retention in HIV primary care for hard-to-reach patients? AIDS Patient Care STDs. 2007;21(Suppl 1):S59–67. [DOI] [PubMed] [Google Scholar]
- 44.Hightow-Weidman LB, Jones K, Wohl AR, Futterman D, Outlaw A, Phillips G 2nd, et al. Early linkage and retention in care: findings from the outreach, linkage, and retention in care initiative among young men of color who have sex with men. AIDS Patient Care STDs. 2011;25(Suppl 1):S31–8. [DOI] [PubMed] [Google Scholar]
- 45.Agency CoSDHaHS. County of San Diego Monthly STD Report. 2014;6(I). [Google Scholar]
- 46.Bradley H, Hall HI, Wolitski RJ, Van Handel MM, Stone AE, LaFlam M, et al. Vital Signs: HIV diagnosis, care, and treatment among persons living with HIV-United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63(47):1113–7. [PMC free article] [PubMed] [Google Scholar]
- 47.Wolever RQ, Dreusicke MH. Integrative health coaching: a behavior skills approach that improves HbA1c and pharmacy claims-derived medication adherence. BMJ Open Diabetes Res Care. 2016;4(1):e000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherifali D, Viscardi V, Bai JW, Ali RM. Evaluating the effect of a diabetes health coach in individuals with type 2 diabetes. Can J Diabetes. 2016;40(1):84–94. [DOI] [PubMed] [Google Scholar]
- 49.Rn DS. Diabetes coaching for individuals with type 2 diabetes: a state-of-the-science review and rationale for a coaching model. J Diabetes. 2017;9(6):547–54. [DOI] [PubMed] [Google Scholar]
- 50.Rosen D, Berrios-Thomas S, Engel RJ. Increasing self-knowledge: utilizing tele-coaching for patients with congestive heart failure. Soc Work Health Care. 2016;55(9):711–9. [DOI] [PubMed] [Google Scholar]
- 51.Stut W, Deighan C, Cleland JG, Jaarsma T. Adherence to self-care in patients with heart failure in the HeartCycle study. Patient Preference Adherence. 2015;9:1195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inglis SC, Clark RA, Dierckx R, Prieto-Merino D, Cleland JG. Structured telephone support or non-invasive telemonitoring for patients with heart failure. The Cochrane Database Syst Rev. 2015;10:007228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rice KL, Dewan N, Bloomfield HE, Grill J, Schult TM, Nelson DB, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2010;182(7):890–6. [DOI] [PubMed] [Google Scholar]
- 54.Dew M, Becker J, Sanchez J, Caldararo R, Lopez O, Wess J, et al. Prevalence and predictors of depressive, anxiety and substance use disorders in HIV-infected and uninfected men: a longitudinal evaluation. Psychol Med. 1997;27(02):395–409. [DOI] [PubMed] [Google Scholar]
- 55.Tobias C, Cunningham WE, Cunningham CO, Pounds MB. Making the connection: the importance of engagement and retention in HIV medical care. AIDS Patient Care STDs. 2007;21(Suppl 1):S3–8. [DOI] [PubMed] [Google Scholar]
- 56.Friedland J, Renwick R, McColl M. Coping and social support as determinants of quality of life in HIV/AIDS. AIDS care. 1996;8(1):15–32. [DOI] [PubMed] [Google Scholar]
- 57.Services USDoHaH. The Poverty Guidelines Updated Periodically in the Federal Register by the U.S. Department of Health and Human Services under the authority of 42 U.S.C.9902(2) 2018. [Google Scholar]
- 58.Opravil M, Ledergerber B, Furrer H, Hirschel B, Imhof A, Gallant S, et al. Clinical efficacy of early initiation of HAART in patients with asymptomatic HIV infection and CD4 cell count > 350 × 106/l. AIDS. 2002;16(10):1371–81. [DOI] [PubMed] [Google Scholar]
- 59.Christopoulos KA, Massey AD, Lopez AM, Geng EH, Johnson MO, Pilcher CD, et al. Taking a half day at a time: patient perspectives and the HIV engagement in care continuum. AIDS Patient Care STDs. 2013;27(4):223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ni H, Nauman D, Burgess D, Wise K, Crispell K, Hershberger RE. Factors influencing knowledge of and adherence to self-care among patients with heart failure. Arch Intern Med. 1999;159(14):1613–9. [DOI] [PubMed] [Google Scholar]
- 61.Vanable PA, Carey MP, Blair DC, Littlewood RA. Impact of HIV-related stigma on health behaviors and psychological adjustment among HIV-positive men and women. AIDS Behav. 2006;10(5):473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelly JA, Murphy DA, Sikkema KJ, McAuliffe TL, Roffman RA, Solomon LJ, et al. Randomised, controlled, community-level HIV-prevention intervention for sexual-risk behaviour among homosexual men in US cities. The Lancet. 1997;350(9090):1500–5. [DOI] [PubMed] [Google Scholar]
- 63.Vanable PA, Carey MP, Blair DC, Littlewood RA. Impact of HIV-related stigma on health behaviors and psychological adjustment among HIV-positive men and women. AIDS Behav. 2006;10(5):473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


