Abstract
This pilot study tested a novel human laboratory model for estimating the extent to which electronic cigarettes (e-cigarettes) serve as motivational substitutes for combustible cigarettes. The model assesses 3 parameters of substitutability, including the: (a) increase in motivational reward value of e-cigarettes after tobacco deprivation; (b) reduction in the reward value of combustible cigarettes after e-cigarette administration; and (c) comparability of the withdrawal-suppressing effects of e-cigarettes versus combustible cigarettes. Dual users (daily smokers, vaped 4+ days/week, M age = 35.3) attended 4 visits after 16-h tobacco product abstinence. For 2 visits, participants completed withdrawal measures and a task assessing smoking’s reward value preceded by either: satiation by vaping or sustained tobacco product deprivation. For 2 other visits, participants completed withdrawal measures and a task assessing vaping’s reward value preceded by either: continued deprivation versus satiation by smoking. Tobacco product deprivation (vs. satiation by smoking) increased vaping’s reward value, indicated by nonsignificantly greater likelihood of initiating vaping versus abstaining for money (d = −.26; adjusted p [padj] = .08) and more vaping episodes purchased (β = .22; padj = .08). Satiation by vaping (vs. deprivation) nonsignificantly reduced smoking initiation (d = .46; padj = .09) and significantly decreased number of cigarettes purchased (β = −.29; padj = .04). Relative to deprivation, vaping suppressed withdrawal-related negative affect, smoking and vape urge, and anhedonia (ds ≥ .54). The magnitude of vaping-induced and smoking-induced withdrawal suppression did not significantly differ for all outcomes other than smoking urge, which was more strongly reduced by smoking (d = −;1.57) than vaping (d = −.64). Future application and extension of this model may advance tobacco regulatory science and policy addressing e-cigarette use among smokers.
Keywords: e-cigarette, dual-use, smoking, vaping, substitution
Electronic cigarettes (e-cigarettes) deliver fewer toxicants than combustible cigarettes (Goniewicz et al., 2017). Therefore, e-cigarettes may be a useful harm reduction method for smokers provided that they facilitate substantial reductions in or cessation of combustible cigarette smoking (National Academies of Sciences, 2018). The majority of U.S. adult e-cigarette users concurrently smoke combustible cigarettes (Coleman et al., 2017; Schoenborn & Gindi, 2015; Sharapova, Singh, Agaku, Kennedy, & King, 2018). Reasons for dual use of these tobacco products are varied and complex (Maglia, Caponnetto, Di Piazza, La Torre, & Polosa, 2017). Some smokers vape e-cigarettes with the intent to reduce combustible cigarette consumption or to ultimately quit combustible cigarettes altogether, using both products during a transitional stage that may (or may not) eventually lead to smoking reduction or cessation. By contrast, other smokers adopt e-cigarettes as an additional or alternative method of nicotine delivery (with no intentions of quitting smoking) to maintain nicotine intake in situations where it is not permissible or appropriate to smoke (Coleman et al., 2017; Patel et al., 2016).
Brief periods of nicotine deprivation elicit tobacco withdrawal symptoms including affective disturbance, craving, anhedonia, and impulsivity (Cook et al., 2015; Paolini & De Biasi, 2011; Shiffman et al., 2006). In instances when dual users wish to momentarily avoid smoking combustible cigarettes or do not have the impending opportunity to smoke, e-cigarettes may serve as a pharmacological and sensory substitute that satiates withdrawal symptoms elicited by tobacco deprivation and temporarily suppress the motivation to smoke combustible cigarettes. The motivational “substitutability” of e-cigarettes for combustible cigarettes may be dependent upon the extent to which: (a) the motivation to vape e-cigarettes increase after acute combustible cigarette deprivation, (b) e-cigarette vaping subsequently reduces the motivation to smoke combustible cigarettes, and (c) the magnitude of the withdrawal-suppressing effects of e-cigarette vaping approximate the corresponding withdrawal-suppressing effects of combustible cigarette smoking.
The availability of laboratory methods to test the substitutability of e-cigarettes is critical for mechanistic research aimed to elucidate the motivational processes underlying dual use. Such methods can also be leveraged to study e-cigarette product characteristics, user behaviors, and user characteristics that moderate the substitutability of e-cigarettes—data that are essential to informing regulatory policy aimed to maximize the harm reduction potential of noncombustible tobacco products. Although some laboratory methods have been used to study e-cigarette effects on tobacco withdrawal symptoms (Lechner et al., 2015; Vansickel & Eissen-berg, 2013), a comprehensive methodological design aimed to address the three parameters of substitutability described above is absent from the literature.
Here we report the results from a pilot test of a novel four-visit within-subject crossover design laboratory model aimed to study the three substitution processes. This methodology permits estimation of the effect of overnight tobacco deprivation on the relative reward value of e-cigarette vaping versus a monetary reward using a novel adaptation of an existing behavioral economics-based task used in the combustible cigarette literature (McKee, 2009). Additionally, the dampening effects of e-cigarette vaping on the reward value of combustible cigarette smoking is also examined using this laboratory model. Leveraging the two aforementioned tests, this model also permits comparison of the withdrawal-suppressing effects of e-cigarette vaping (vs. continued tobacco product deprivation) and combustible cigarette smoking (vs. continued tobacco product deprivation).
The primary aim of this pilot study was to estimate the effect of experimental manipulations aimed to model the three substitution processes among dual users involving their preferred brand of combustible cigarettes and preferred e-cigarette device and e-liquid. A secondary aim was to establish feasibility and assess process factors by reporting retention rates and other descriptive methodological information (e.g., how we arrived at eligibility criteria for “dual use;” see Thabane et al. [2010] for further information regarding uses of pilot studies). By doing so, this study aimed to provide information regarding whether further application of this laboratory model of substitutability was warranted and how the methods could be refined for future research in dual users or the study of substitution processes in other populations and products (e.g., vaping-naive combustible cigarette smokers interested in transitioning to e-cigarettes).
Method
Participants
Participants (aged 18–58) were nontreatment seeking dual users recruited beginning in February, 2015 through September, 2015 via online advertisements in the Los Angeles metropolitan area announcing the opportunity to participate in a study of individuals who both vape and smoke. Because there is no established definition of dual use from which to base study eligibility criteria, we conducted exploratory analyses of the distribution of smoking and vaping behaviors reported among an initial sample of potential participants responding to the advertisement who completed a telephone screening. The final inclusion criteria were based on natural breaks observed in the distribution of vaping and smoking characteristics and previous research among dual tobacco product users (Klesges et al., 2011), in addition to our intention to capture the target population of interest, and were as follows: (a) ≥18 years old; (b) regular cigarette smoking for ≥2 years; (c) currently smoking ≥4 cigs/day on a daily basis; (d) regular electronic cigarette use for ≥3 months; (e) use of e-cigarettes ≥4 days/week; (f) report vaping at least ≥4 times (i.e., vaping sessions) per day on vaping days; and (g) use of e-liquid with a nicotine concentration of ≥6 mg/mL, as we were concerned that abstinence from an e-cigarette with a nicotine concentration below 6 mg/mL may not induce withdrawal.1 Participants were required to bring in their devices and e-liquids, and the manufacturer-provided nicotine concentration level reported on the device, packaging, or e-liquid label was verified by research staff. Exclusion criteria were: (a) indication of very low cigarette smoke exposure based on breath carbon monoxide (CO) levels (<4 ppm at intake); (b) indication of very low overall nicotine exposure via semiquantitative salivary cotinine reading at intake (value of 0 on NicAlert test strips; see below); (c) breath alcohol >0 BrAC; and (d) currently pregnant or breast feeding. Participants were compensated approximately $325 USD for completing the study. The study protocol was approved by the University of Southern California Institutional Review Board.
Design
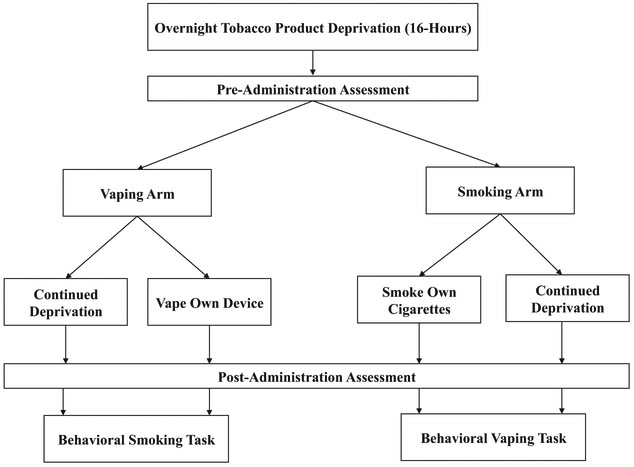
After a baseline visit, participants attended four experimental visits according to a 2 × 2 within-subject design each following 16-h of tobacco product abstinence (i.e., no combustible cigarette or e-cigarette use) as presented in Figure 1: (a) e-cigarette administration with a subsequent behavioral (combustible cigarette) smoking task; (b) continued tobacco product deprivation with a subsequent behavioral smoking task; (c) combustible cigarette administration with a subsequent behavioral (e-cigarette) vaping task; and (d) continued deprivation with a subsequent behavioral vaping task.
Figure 1.
Study design. Participants attended four experimental sessions each after 16-h of nicotine deprivation (i.e., no cigarette or e-cigarette use). There were two arms: (a) satiation by smoking versus continued deprivation (participants completed a behavioral vaping task); and (b) satiation by vaping versus continued deprivation (participants completed a behavioral smoking task).
Vaping arm.
One arm of the study design included two visits—one subcondition involving e-cigarette administration during the first portion of the visit and a second subcondition involving a continuation of deprivation of all tobacco products during the first portion of the visit (Figure 1, left side). At both visits, participants first completed pre- or postsubjective measures of nicotine withdrawal before beginning a behavioral task that assessed motivation to smoke combustible cigarettes during the latter portion of the visit (see Behavioral Smoking Task described below). This arm permits testing of the effects of vaping (vs. deprivation) on the suppression of motivation to smoke.
Smoking arm.
The other study arm included two visits—one subcondition involving continuation of deprivation of all tobacco products during the first portion of the visit and a second subcondition involving combustible cigarette administration (Figure 2, right side). At both visits, participants completed pre- or postwithdrawal measures and then began a vaping motivation task during the latter portion of the visit (described below). This arm permits testing of the effects of deprivation (vs. satiation by combustible cigarette smoking) on increasing motivation to vape.
Figure 2.
Combustible cigarette smoking task and electronic cigarette vaping task outcomes, by deprived versus sated conditions. Panel A: Prevalence (% ± SE) of immediately initiating vaping during 50 min delay period and forgoing opportunity to earn monetary reward for delaying smoking. Panel B: Number (M ± SE) of vaping episodes purchased during the 60 min self-administration period. Panel C: Prevalence (% ± SE) of immediately initiating smoking during 50 min delay period and forgoing opportunity to earn monetary reward for delaying vaping. Panel D: Number (M ± SE) of combustible cigarettes purchased during the 60 min self-administration period.
Comparison of the withdrawal suppressing effects of vaping versus smoking.
Leveraging the abovementioned procedures that occur before the smoking and vaping motivation tasks across all four conditions, the withdrawal-suppressing effects of e-cigarette vaping (vs. deprivation) can be compared with the corresponding withdrawal-suppressing effects of combustible cigarette smoking (vs. deprivation).
Procedure
Baseline visit.
At the baseline session participants completed informed consent before providing a breath sample for CO analysis, a saliva sample for salivary cotinine assessment, and a breath alcohol sample to determine study eligibility. Eligible participants then completed self-report measures of demographics, tobacco product use, and other factors (see Baseline Session Measures).
Experimental visits.
After the baseline session, participants attended four experimental visits each commencing at approximately 12 p.m. after 16 h of tobacco product deprivation. Participants who were not observed to be compliant with the required tobacco product abstinence or who did not demonstrate objective reductions in nicotine product use at visit outset (i.e., CO ≤8 ppm and a reduction of 1 point from baseline on the semiquantitative salivary cotinine index) were given one opportunity to reschedule their session and return after successful compliance with the deprivation instructions (N = 3) as in previous research (Pang, Bello, Liautaud, Weinberger, & Leventhal, 2018). All procedures occurred in a biobehavioral research facility with ventilation to clear smoke and aerosol.
Participants then completed a preadministration assessment that included a battery of subjective withdrawal and physiological measures. Subsequently, participants either were directed to self-administer their preferred e-cigarette for 8 min at their own pace to approximate an exposure equivalent to smoking one combustible cigarette (1 visit), smoke one cigarette of their preferred at their own pace (1 visit), or rest with no smoking or vaping for 8 min to continue the tobacco product deprivation period (2 visits; 1 comparison for vaping arm, 1 comparison for smoking arm; see Figure 1). After the self-administration or continued deprivation period, participants completed a postadministration assessment involving the same measures as the preadministration battery.
Following the postadministration measures, participants rested for 1 h with no smoking or vaping and then began behavioral economics-based tasks that measured their motivation to initiate smoking versus earn money (2 visits) or motivation to initiate vaping versus earn money (2 visits). Following completion of the behavioral smoking or vaping task was a rest period, which lasted between 60 and 110 min depending on participant’s decision in the behavioral task, before dismissal.
Measures
Baseline visit.
Combustible cigarette dependence severity was measured with The Fagerström Test of Cigarette Dependence (FTCD; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), a well-validated six-item measure of heavy smoking and compulsion to smoke (range = 0–10). The Penn State Electronic Cigarette Dependence Index (PSECD; Foulds et al., 2015), a 10-item measure similar to the FTCD, adapted for e-cigarette dependence was also administered (range = 0–20). Investigator-constructed measures of demographics, smoking and vaping history, and use characteristics were administered to characterize the sample, and included questions that assessed e-cigarette nicotine concentration and flavor preferences (i.e., tobacco, menthol, fruit, sweet, and spice). Participants e-cigarette devices (i.e., first-, second-, or third-generation) were coded by model type (generation) according to commonly accepted nomenclature (Barrington-Trimis et al., 2018).
Experimental visit.
Subjective measures.
The 20-item PANAS Positive and Negative Affect Scale (PANAS; Watson, Clark, & Tellegen, 1988) measures the extent to which various emotions are experienced “right now.” Items were rated on a 5-point Likert scale (1 = not at all to 5 = extremely). Each subscale (i.e., positive and negative; 10 items each) was calculated as a mean score (range = 1 to 5).
The Brief Questionnaire of Smoking Urges (QSU; Cox, Tiffany, & Christen, 2001) is a 10-item measure that instructs respondents to rate their level of agreement with statements describing smoking urge experiences “right now” (e.g., “I have a desire for a cigarette”) on 6-point response scale (0 = strongly disagree to 5 = strongly agree).
The Brief Questionnaire of Vaping Urges (QVU; Dowd, Motschman, & Tiffany, 2018) utilizes 10 items with identical wording to the QSU, with the exception of minor adaptations in original terminology oriented toward combustible cigarette to new e-cigarette-specific terminology.
The Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1986) involves rating the severity of eight different withdrawal symptoms (i.e., anger, anxiety, depression, craving, concentration problems, increased appetite, restlessness, and impatience) on 6-point scales (0 = none to 5 = severe), yielding a mean composite score.
The Tripartite Pleasure Inventory (TPI; Leventhal, 2010) responsiveness (TPI-R) and desire (TPI-D) scales were utilized to assess two components of reduced reward—anhedonia (i.e., diminished pleasure) and diminished interest in response to rewarding activities. The TPI presents 12 normally pleasurable activities (e.g., foods, personal hobbies, socializing, etc.) and (Hughes, Dash, & Callas, 2015) instructs participants to make individual ratings of anticipated pleasure in response to (TPI-R; 0 = no pleasure to 4 = extreme pleasure) and desire to engage in (TPI-D; 0 = no desire to 4 = extreme desire) each activity based on how they are feeling right now.
The Current Impulsivity Scale (CIS; Pang & Leventhal, 2014) assesses subjective impulsive state by instructing respondents to rate the extent to which they feel impulsive in 19 ways (e.g., “Not worried about consequences” and “Feel easily distracted”) on a 5-point scale (0 = not at all to 4 = extremely) with the mean response composite used. A self-report measure of impulsivity was included as evidence suggests impulsivity may be a symptom of initial nicotine withdrawal syndrome (Hughes, Dash, & Callas, 2015).
Physiological measures.
Breath CO was assessed via a Vitalograph CO monitor. Systolic and diastolic blood pressure and heart rate were assessed via a digital blood pressure monitor. A NicAlert strip 7-level semiquantitative reading was used to test for salivary cotinine concentration (0 = 0–10, 1 = 10–30, 2 = 30–100, 3 = 100–200, 4 = 200–500, 5 = 500–1,000, and 6 ≥ 1,000 ng/mL) at the beginning of each experimental session to verify nicotine deprivation.
Behavioral smoking and vaping tasks (McKee, 2009).
The smoking task began with the “delay period” in which participants were informed they could begin smoking their preferred brand cigarettes at any point over the next 50 min; however, they would receive $0.20 for each 5 min they delayed smoking up to a maximum of 50 min ($2 maximum). When the participant informed the experimenter that they would like to start smoking (or at the end of 50 min if they chose not to smoke), the delay period portion of the task ended and the “self-administration period” portion of the task commenced. Participants were presented with eight of their own combustible cigarettes and instructed that they could smoke as many or as few as they wished over the next 60 min, but for each cigarette smoked $0.20 would be deducted from their total initial tab of $1.60 for the day.2 The task ended at the termination of the 60-min self-administration period, which was followed by a 60 to 110 min rest period (depending on the length of delay), during which no smoking or vaping occurred. The rest period was included to prevent minimization of smoking or vaping during the task because of expecting an impending opportunity to smoke/vape directly afterward.
The behavioral vaping task applied identical temporal parameters, financial reward, and format as the smoking task. However, for this task participants were given the option to vape their own device or delay vaping for money during the delay period (at $0.20 per every 5 min; identical to the delay price for smoking). When the self-administration period began, participants could choose to purchase 8-min vaping episodes, which cost $0.20 each (identical to the price per combustible cigarette). The 8-min discrete “vaping” episode parameters were selected based on: (a) survey data indicating that vapers use e-cigarettes in similar patterns to combustible cigarettes (Dawkins, Turner, Roberts, & Soar, 2013); (b) laboratory studies in which 10 puffs of an e-cigarette approximated a combustible cigarette (Vansickel & Eissenberg, 2013); and (c) and research suggesting that the time interval it takes to complete 10 puffs is approximately 8 min (Hua, Yip, & Talbot, 2013; St. Helen et al., 2016). Although between-person variation in naturalistic vaping topography has been reported (Dawkins et al., 2013), a common time interval for taking 10 e-cigarette puffs is approximately 8 min (Hua et al., 2013; St. Helen et al., 2016).
For both delay tasks, the primary outcome was a binary classification of whether or not participants chose to smoke or vape immediately versus delaying smoking or vaping to earn additional money (0 = no delay [0 min], 1 = any delay [≥ 1 min]) during the delay period, indicative of the reward value of initiating tobacco product use relative to earning money. A binary classification was used because the minutes delayed continuous variable distributions (0–50) were bimodal (see Figure 3). The self-administration period outcome was the number of cigarettes or vaping episodes purchased during the 60-min period, which is indicative of the reward value of tobacco product use after being given the opportunity to initiate use of the product.
Figure 3.
Histograms of behavioral smoking and vaping task delay times by deprivation condition. N = 25–28.
Data Analysis
After descriptive analyses, we conducted a manipulation check of compliance with abstinence by testing the change in CO and salivary cotinine from baseline to experimental sessions (collapsed across all four experimental sessions) with multilevel linear models (i.e., participants had four lines of data [observations] for the experimental level of the variables and one line of data for the baseline). Generalized linear mixed models were used for the primary analyses. The two smoking task outcomes (delay [yes/no] and number of cigarettes purchased) were tested in two separate models including the within-subject predictor of vaping satiation versus deprivation as the sole regressor. Similarly, the two vaping task outcomes (delay [yes/no] and number of vaping episodes purchased) were tested in two separate models including the within-subject predictor of smoking satiation versus deprivation. Both delay tasks were analyzed utilizing binary logistic models and the number of cigarettes/vaping episodes purchased were analyzed with Poisson distribution models.
For the subjective withdrawal study outcomes, two Gaussian distribution models were conducted, each with its own pairwise contrast effects: (a) the effect of satiation by combustible cigarette administration (vs. deprivation); and (b) the effect of e-cigarette administration (vs. deprivation). In these models, the postadministration assessment served as the primary outcome and, in addition to the within-subject pairwise experimental contrast regressor, the preadministration score was simultaneously entered as a covariate. For each subjective withdrawal and physiological outcome, the difference in the magnitude of satiation effects (vs. respective deprivation control conditions) between vaping and smoking were compared using all of the observations across the four sessions with an interaction term of deprivation versus Satiation × Product administered to satiate (e-cigarette vs. combustible cigarette).
All models were tested in SAS (SAS Institute Inc., Cary, NC). Results from the behavioral smoking and vaping tasks are reported as standardized regression parameter estimates (β) with 95% confidence intervals (CIs) or Cohen’s d standardized effect size estimates. As this was an initial test of this experimental methodology and the sample was modest, effect size estimates were of primary interest. Benjamini-Hochberg multiple testing corrections were applied to adjust the p values of each test to control false discovery rate at .05 (Benjamini & Hochberg, 1995), leading to an adjusted p value (padj) significance threshold of .05 for all tests.
Results
Participant Accrual, Retention, and Sample Characteristics
Among the 44 participants who passed a phone screen indicating preliminary eligibility and attended a baseline visit, 40 (90.9%) were deemed eligible after the in-person screening assessment and enrolled. Of eligible participants, 29 (72.5%) completed at least 1 experimental visit and constituted the sample available for analysis. The 11 participants who did not complete an experimental session reported the time commitment or insufficient compensation as primary reasons for not completing the study or were lost to follow-up (i.e., unable to be contacted by the experimenters). Twenty-five participants completed all 4 visits, one completed 3 visits, two completed 2 visits, and one completed a single visit. The baseline CO (M [SD] = 15.07 [8.15]) and salivary cotinine levels (M [SD] = 3.81 [1.21]) of the 29 participants who completed at least experimental session did not significantly differ from the CO (M [SD] = 17.92 [13.70]) and cotinine (M [SD] = 4.00 [1.13]) of the 11 participants who did not complete an experimental session (psadj > 0.42). The mixed model methodology used to test the primary aims of the study (described below) permits missing data and utilized all 29 participants. Supplemental analyses utilizing the 25 who completed all four study visits yielded comparable results are available upon request to the second author NG.
Descriptive statistics for demographics and smoking and vaping characteristics for the analytic sample (N = 29) are reported in Table 1. On average, the sample reported moderate levels of combustible cigarette and e-cigarette dependence, and was heterogeneous with regards to its vaping characteristics and e-cigarette product preferences. Participants demonstrated a significant decrease in CO from the baseline to experimental sessions (M difference baseline—experimental [SE] = 10.45 [.30]; padj < .001) as well as salivary cotinine (M difference baseline—experimental [SE] = .94 [.05]; padj < .001). There were no participants whose end-tidal CO at either of the experimental sessions exceeded their CO at baseline.
Table 1.
Sample Demographics, Smoking, and Vaping Characteristics
| Variables | M (SD) or % |
|---|---|
| Age | 35.3 (11.7) |
| Female gender | 31.0% |
| Race/ethnicity | |
| Black | 44.8% |
| White | 44.8% |
| Multi-racial/ethnic | 6.9% |
| Other | 3.5% |
| Duration of years smoking | 15.1 (12.5) |
| Combustible cigarette dependence (FTCD) | 5.2 (2.0) |
| Cigarettes/day | 11.4 (7.7) |
| Duration of years vaping | 3.6 (6.3) |
| Vaping frequency | |
| Everyday | 50.0% |
| 5 to 6 days/week | 16.7% |
| 3 to 4 days/week | 33.3% |
| Number of vaping episodes per vaping day | 12.5 (12.7) |
| e-cigarette dependence (PSECD) | 10.5 (4.8) |
| e-cigarette nicotine strength (mg/mL) | 18.7 (9.3) |
| Use of e-cigarettes to reduce smoking | 75.9% |
| Preferred e-cigarette flavor | |
| Tobacco | 20.7% |
| Menthol | 24.1% |
| Fruit | 37.9% |
| Sweet | 6.9% |
| Spice | 3.4% |
| Other | 7.0% |
| e-cigarette device type | |
| First generation (cig-a-like) | 10.3% |
| Second generation (tank) | 75.9% |
| Third generation (mod) | 13.8% |
Note. N = 29. FTCD = Fagerström Test of Cigarette Dependence (0–2 = very low, 3–4 = low, 5 = moderate, 6–7 = high, 8–10 = very high); PSECD = Penn State Electronic Cigarette Dependence (0–3 = not dependent, 4–8 = low dependence, 9–12 = medium dependence, 13+ = high dependence). E-cigarette device type = E-cigarette model used by participants.
Effect of Tobacco Product Deprivation on Reward Value of Vaping
In the deprived condition requiring sustained tobacco product deprivation before the beginning of the behavioral vaping task, 13 of the 27 (48.1%) participants with data available for that condition initiated vaping their preferred e-cigarette product immediately at the outset of the 50-min delay period and forgoed the opportunity to earn money for delaying vaping (Figure 2, panel A). In the condition in which participants were satiated by smoking a combustible cigarette before the task, 10 of 28 (35.7%) participants initiated vaping immediately during the 50-min interval. While the likelihood of vaping initiation was higher in the deprived versus cigarette-sated condition, this difference was not statistically significant (d = −.26; padj = .08; Figure 2, panel A). During the 60-min self-administration portion of the task, participants purchased a greater number of vaping episodes in the deprived (M [SD] = 1.85 [1.61]) versus cigarette sated (M [SD] = 1.46 [1.17]) condition; however, this difference was also not statistically significant (β [95% CI] = .22 [− .02, .45]; padj = .08; Figure 2, panel B).
Effect of e-Cigarette Administration on Reward Value of Smoking
In the condition in which participants self-administered their e-cigarette device before the behavioral smoking task, 12 of 25 (48.0%) participants initiated smoking their preferred brand combustible cigarettes immediately at the outset of the 50-min delay period and forgoed the opportunity to earn money for delaying smoking (Figure 2, panel C). In the deprived condition, 17 of 25 (68.0%) participants initiated smoking immediately at the outset of the 50-min interval. While the likelihood of immediate smoking initiation was lower after vaping (vs. deprivation), this difference was not statistically significant (Cohen’s d = .46; padj = .09; Figure 2, panel C). During the 60 min self-administration portion of the task, participants purchased significantly fewer cigarettes in the condition in which they vaped before the task (M [SD] = 1.92 [1.15]) as compared with the deprived condition (M [SD] = 2.56 [1.66]) condition (β [95% CI] = −.29 [− .55, −.03]; padj = .04; Figure 2, panel D).
Subjective Withdrawal Symptoms
Vaping-induced withdrawal suppression.
Adjusting for preassessment scores, postassessment subjective withdrawal symptoms were significantly improved after participants self-administered their own e-cigarette product (vs. the condition involving continued deprivation over this interval) for negative affect (d = −0.63), urge to smoke (d = −0.64), urge to vape (d = −1.09), consummatory anhedonia (d = 0.54), and anticipatory anhedonia (d = 0.65; all psadj < .05; Table 2). Other outcomes were not significantly affected by vaping, but exhibited small effect sizes, paralleling evidence that certain withdrawal-related symptoms demonstrate smaller changes after acute combustible cigarette deprivation (Leventhal, Waters, Moolchan, Heishman, & Pickworth, 2010). One exception was positive affect for which the withdrawal-suppression effect magnitude was nearly zero (see Table 2 for details).
Table 2.
Vaping- and Smoking-Induced Satiation Effects on Withdrawal Symptoms and Other Outcomes
| Measure | Vaping-induced satiation effect |
Smoking-induced satiation effect |
Difference between vaping- and smoking- induced satiation |
||||
|---|---|---|---|---|---|---|---|
| Sustained deprivation M (SE) |
Satiation by vaping M (SE) |
Contrast d [95% CI] |
Sustained deprivation M (SE) |
Satiation by smoking M (SE) |
Contrast d [95% CI] |
Interaction term B [95% CI] |
|
| Subjective withdrawal measures | |||||||
| PANAS-NA | 1.48 (.30) | 1.20 (.32) | −.63 [−.47, −.11]** | 1.46 (.34) | 1.16 (.22) | − .73 [−.46, −.15]† | − .05 [−.25, .15] |
| PANAS-PA | 2.57 (.39) | 2.56 (.54) | −.01 [−.23, .21] | 2.63 (.58) | 2.62 (.62) | − .01 [−.38, .36] | − .01 [−.28, .26] |
| QSU | 3.19 (.53) | 2.22 (1.34) | −.64 [− 1.56, −.38]** | 2.97 (.55) | 1.14 (.89) | − 1.57 [−2.26, −1.41]† | .84 [.18, 1.49]* |
| QVU | 2.59 (.73) | 1.27 (.80) | − 1.09 [− 1.81, − .85]† | 2.49 (.60) | 1.50 (.88) | − .94 [−1.38, −.61]† | − .26 [−.74, .22] |
| MNWS | 1.55 (.41) | 1.34 (.46) | −.28 [−.44, .07] | 1.53 (.47) | 1.10 (.78) | − .45 [−.77, −.09]* | 1.56 [−1.59, 4.71] |
| TPI-R | 2.52 (.61) | 2.90 (.45) | .54 [.05, .70]* | 2.73 (.33) | 2.98 (.65) | .39 [−.03, .54] | .26 [−.13, .65] |
| TPI-D | 2.37 (.50) | 2.63 (.61) | .65 [.08, .45]** | 2.53 (.26) | 2.77 (.68) | .35 [−.06, .54] | .15 [−.12, .42] |
| CIS | 1.72 (.20) | 1.62 (.21) | −.36 [−.24, .03] | 1.71 (.32) | 1.47 (.32) | − .60 [−.42, −.05]* | .16 [−.04, .35] |
| Physiological measures | |||||||
| CO (ppm) | 5.14 (.66) | 5.02(1.26) | −.11 [−.58, .33] | 4.13 (1.01) | 9.22 (2.26) | 1.92 [4.12, 6.05† | −5.20 [−6.18, −4.22]† |
| BP-SYS (mmHg) | 125.6 (14.04) | 122.8 (15.64) | −.16 [−9.37,3.86] | 114.2(10.69) | 126.4 (10.72) | .84 [6.95, 17.49]† | − 14.96 [−21.27, −8.65]† |
| BP-DIA (mmHg) | 76.23 (9.39) | 79.0 (10.36) | .26 [−1.37, 6.81] | 73.26 (8.46) | 83.81 (12.50) | .77 [5.55, 15.56]† | −7.91 [−13.40, −2.42]** |
| HR (bpm) | 68.07 (7.46) | 73.73 (6.75) | .60 [1.96, 9.37]** | 71.38 (6.25) | 78.46 (6.26) | .67 [3.22, 10.93]† | − 1.42 [2.69, −6.69] |
Note. PANAS = Positive and Negative Affect Scale (possible range: 1 = very slightly or not at all to 5 = extremely); PA = Positive Affect Subscale; NA = Negative Affect subscale; QSU/QVU-Brief Questionnaire of Smoking/Vaping Urges (possible range: 0 = strongly disagree to 5 = strongly agree); TPI-R = Tripartite Pleasure Inventory-Responsivity scale for anhedonia (possible range: 0 = no pleasure to 4 = extreme pleasure); TPI-D = Tripartite Pleasure Inventory-Desire subscale for reduced interest (possible range: 0 = no desire to 4 = extreme desire). CIS = Current Impulsivity Scale (possible range: 0 = not at all to 4 = extremely); CO = Breath Carbon Monoxide (ppm = parts per million); BP-SYS = Blood Pressure Systolic; BP-DIA = Blood Pressure Diastolic; HR = heart rate (bpm = beats per minute); MNWS = Minnesota Nicotine Withdrawal Scale total score (possible range: 0 = not at all to 5 = extremely). Results from generalized linear mixed models testing either satiation by vaping versus sustained deprivation, satiation smoking versus sustained deprivation, or the interaction of satiation (sated vs. sustained deprivation) × tobacco product type (satiation by vaping vs. satiation by smoking). All estimates statistically significant after Benjamini-Hochberg corrections for multiple testing to control false discovery rate at .05 (based on two-tailed adjusted p-value).
padj < .001.
padj < .05.
padj < .01.
Smoking-induced withdrawal suppression.
Adjusting for preassessment scores, postassessment subjective withdrawal symptoms were improved after participants self-administered their preferred combustible cigarette (vs. continued deprivation) for negative affect (d = −0.73), urge to smoke (d = −1.57), urge to vape (d = −0.94), overall withdrawal on the MNWS composite (d = −0.45), and impulsivity (d = −0.60; all psadj < .05). Other outcomes were not significantly affected by smoking but exhibited a range of effect sizes comparable with the literature (Leventhal et al., 2010), except for a nearly zero effect on positive affect (see Table 2 for details).
Comparison of vaping-induced and smoking-induced withdrawal suppression.
Interaction tests used to test for differences between the vaping (vs. deprived) contrast and smoking (vs. deprived) contrast found significant differences for one subjective withdrawal outcome. The smoking-induced suppression of urges to smoke combustible cigarettes was significantly stronger than the corresponding vaping-induced suppression of urge to smoke combustible cigarettes (ds = −1.57 vs. −0.64; interaction padj < .001).
Physiological Outcomes
Combustible cigarette smoking (vs. deprivation) produced characteristic physiological increases in CO, systolic and diastolic blood pressure, and heart rate in postadministration outcomes adjusted for preadministration values (psadj < .001; Table 2). With the exception of heart rate, which was the only physiological outcome significantly impacted by vaping versus deprivation (psadj < 0.01), smoking-induced increases (vs. deprivation) were significantly greater than vaping-induced increases (vs. deprivation) on each physiological outcome (interaction psadj < .01; Table 2).
Discussion
This pilot study provides preliminary support of a novel laboratory model for testing the substitutability of e-cigarette vaping for combustible cigarette smoking. This model utilizes a four-visit experimental design (plus an additional baseline visit) capable of testing three key parameters indicative of whether e-cigarettes can serve as a motivational substitute that reduce smoking: (a) vaping’s heightened motivational value after tobacco product deprivation; (b) vaping’s dampening effect on the motivational value of combustible cigarettes; and (c) vaping’s tobacco withdrawal-suppressing effects. The overall results of this study, including the effect size estimates yielded and descriptive study feasibility information, suggest that this model was fairly sensitive to each of the three aforementioned substitution processes, can be implemented without major barriers and, therefore, warrants further application.
The sample size was modest in this pilot study (N = 29), and was selected to satisfy the primary study aim of providing initial estimates of deprivation and satiation effects to indicate the possible sensitivity of the paradigm, as well as determining whether certain features of the design require further iteration to enhance sensitivity. The sample was sufficient for addressing the secondary study aim of providing descriptive feasibility and process information about the method. Specifically, we found that attrition was fairly moderate for a five-visit paradigm (27.5%) and inline with previous comparable three-visit paradigms involving nonvaping smokers required to abstain overnight from combustible cigarettes (Aguirre, Madrid, & Leventhal, 2015). More important, there were not systematic biases in probability of attrition as a function of baseline nicotine and combustible tobacco smoke exposure. This is notable given the concern that participants with more baseline tobacco product intake could find compliance with the abstinence conditions prohibitive from participating or have other unique characteristics that could systematically influence likelihood of study dropout.
Testing Whether the Reward Value of Vaping Is Enhanced During Tobacco Deprivation
An often-overlooked indicator of whether e-cigarettes are useful substitutes for combustible cigarettes is whether smokers are motivated to use this alternative product when cigarettes are unavailable. If smokers are unmotivated to vape when tobacco deprived, they may be at increased risk of selecting a combustible cigarette instead of an e-cigarette, which would vitiate any harm-reducing potential of e-cigarettes. Furthermore, if smokers remain equally motivated to vape regardless of recent tobacco exposure, vaping may not be serving as a substitute for combustible cigarettes. Rather, vaping may merely reflect a means of “topping off’ the amount of nicotine smokers would normally attain from combustible cigarettes alone, which may accelerate nicotine dependence and unnecessarily increase exposure to toxins in e-cigarette aerosol without a harm reduction benefit.
Two visits in this laboratory model were specifically designed to address this vital question and the results were suggestive that the motivational reward value of vaping was enhanced in states of tobacco product deprivation relative to satiation by combustible cigarettes. Deprivation nonsignificantly increased the likelihood of initiating vaping in lieu of earning money for delaying vaping and purchases of vaping episodes (psadj = .08). Future extension of this result to other populations and products can provide further evidence of the validity of the task and the conditions under which e-cigarettes may provide the substitutional property of increased motivational value during tobacco product deprivation.
Testing Whether e-Cigarette Administration Reduces the Reward Value of Smoking
Two of the study visits within this model permit testing whether vaping reduces the motivational reward value of smoking. This arm of the study design represents an extension of a laboratory analogue smoking cessation medication development methodology. Developed by McKee and colleagues, this methodology has shown that Bupropion, Varenicline, and other interventions reduce the motivational value of initiating and continuing combustible cigarette smoking after a period of tobacco product abstinence (McKee, 2009). Here, we provide suggestive evidence that e-cigarettes may also suppress the motivational value of smoking, as exemplified by a statistically significant reduction in self-administration of combustible cigarettes for money after vaping initiation (padj = .04), and a nonsignificant trend indicative of vaping-induced enhancement in the ability to delay the initiation of smoking for money (padj = .09). In the medication development arena, this task has been proposed to represent an efficient method of screening the potential efficacy of novel medications before moving ahead with a resource-intensive clinical trial (McKee, 2009). An extension to screening the smoking reduction or cessation promotion potential of novel vaping products may warrant consideration.
Testing the Comparative Withdrawal Suppressing Effects of e-Cigarettes Versus Combustible Cigarettes
A key intermediate marker of the potential of e-cigarettes to facilitate reduction or cessation of combustible cigarettes is the extent to which vaping can suppresses tobacco withdrawal symptoms. Here, two questions are paramount: (a) Does vaping measurably reduce withdrawal symptoms (i.e., is the effect estimate of vaping vs. continued deprivation on withdrawal greater than zero?); and (b) What is the relative comparability of withdrawal suppression by vaping versus withdrawal suppression by combustible cigarettes (i.e., is the effect magnitude of withdrawal suppression from vaping different than the effect magnitude of withdrawal suppression for smoking?) While extant studies have applied designs capable of testing one of these two questions (Lechner et al., 2015; Vansickel & Eissenberg, 2013), the current article tests the only model to our knowledge that is capable with addressing both of these questions.
Two visits within the four-visit paradigm can be used to isolate whether vaping-induced withdrawal suppression is greater than zero. The additional two visits permit comparison of whether the vaping-induced withdrawal suppression is significantly different than the magnitude of smoking-induced withdrawal suppression. The inclusion of continued deprivation control conditions provide a key methodological advantage to prior work utilizing single visit prepost administration test sessions to determine vaping- and smoking-induced withdrawal suppression. The single-visit pre versus post administration design confounds order with tobacco product administration (i.e., postadministration always follows what would be the control condition testing before product administration). Our design including a deprivation control condition permits parsing tobacco product administration effects from “testing” or “practice” effects whereby the mere readministration of measure can change a person’s responses regardless of any change in their “true score.”
Using this novel model and a battery of subjective withdrawal symptom assessments, we present some of the most comprehensive evidence to date on the breadth of impact of vaping on the tobacco withdrawal syndrome. We present new evidence that vaping alleviates two components of reward functioning (i.e., reduced interest and reduced pleasure [anhedonia] from rewards). This result is important given extant evidence that anhedonic symptoms during tobacco abstinence predict relapse back to combustible cigarette smoking during a quit attempt (Cook et al., 2015). We also replicate existing literature that vaping reduces negative affect and urge to vape and smoke during tobacco product deprivation (Lechner et al., 2015).
With the exception of urge to smoke, for which smoking-induced suppression was significantly stronger than vaping-induced suppression, the relative magnitude of withdrawal suppression from vaping and smoking were not significantly different. On one hand, this pattern indicates that e-cigarettes may possess adequate substitutability on the parameter of withdrawal suppression. However, the null comparison of withdrawal suppression between e-cigarettes and combustible cigarettes could merely be a result of insufficient statistical power and, thus, should be interpreted with some caution and extended using larger samples.
Limitations and Future Directions
This study was underpowered; however, a significance correction was applied for multiple tests, and the absence of some significant results may result from Type-II errors. We suggest the reader interpret the global pattern of findings as indicating the promise of the new laboratory model of testing e-cigarette substitutability rather than make conclusions regarding the specific withdrawal symptoms or behavioral outcomes significantly or nonsignificantly affected by the study manipulations.
A key restriction on generalizability is that this was a fairly small sample of dual users who utilized their own e-cigarette and cigarette products for the study procedures. The sampling of dual users and use of their own products was purposeful for this preliminary study. There is a range of different e-cigarette products available, with variation in device type, size, design, power, and other features and key variation in e-liquid flavoring, strength, vehicle, and nicotine formulation (e.g., free-base vs. protonated). There is likely large interindividual variability in users’ preferences that may cause certain products to be less useful motivational substitutes for combustible cigarettes for some smokers, yet more useful substitutes for other smokers, depending on personal preferences. For these reasons, recruiting dual users and allowing the participant to utilize their own products for the study procedures likely maximized the probability that substitution effects would be detected.
There was a sizable proportion of participants who chose to smoke or vape immediately during the behavioral task and forgoe the chance to earn money for delaying initiation of tobacco product use during the delay period. It is possible that the monetary values for reinforcing delaying tobacco product use tested here are insufficient for generating optimal sensitivity of the paradigm in future applications. The behavioral smoking and vaping task incentive values may also be specific to the sociocultural characteristics of the population. Dual-users (vs. vaping-naïve smokers) and individuals from different regions of the country may require different financial incentives to be motivated to delay tobacco product use. Hence, further refinement of the paradigm, including testing whether increasing the monetary reward for delaying initiation increases the proportion of individuals who delay and the spread in scores of delay values, may be fruitful to optimize sensitivity of this aspect of the methodology.
The relative substitutability of e-cigarettes for cigarettes in vaping-naive smoking is not clear from this study. Additional limitations, including the absence of certain withdrawal measures (e.g., cognitive performance), test of only a single duration of deprivation (i.e., overnight), utilization of convenient but potentially imprecise biochemical markers of nicotine exposure (e.g., NicAlert) also impact study findings. Although nicotine product deprivation was verified using biochemical measures, the salivary cotinine measure lacks precision, and future research should utilize plasma nicotine measurement or observed abstinence in a laboratory setting to verify abstinence. We focused on dual-users as they comprise the majority of adult e-cigarette users, and are an important population to consider from a public health perspective; however, it is possible that substitution effects could be larger among other populations, including past smokers who successfully transitioned to exclusive e-cigarette use.
Implications and Conclusions
Public agencies, including the U.S. Food and Drug Administration and Public Health England, have expressed interest in policies that encourage combustible cigarette smokers who are unwilling to quit smoking to transition to reduced risk noncombustible tobacco products, such as e-cigarettes (Gottlieb & Zeller, 2017; McNeil et al., 2016; Royal College of Physicians, 2016). Additionally, clinicians and smokers from the general public are interested in whether and under what conditions e-cigarettes may be useful smoking cessation aids. In addition to other avenues of evidence, such as observational surveillance and clinical trials with long-term follow-up, human laboratory models can play a key role in answering questions of interest to policymakers, clinicians, and the general public about the substitutability of e-cigarettes for cigarettes. Human laboratory models can provide rapid results of causal associations under well-controlled conditions with strong internal validity and can be used to compliment the clinical and external validity of other approaches.
Pending further validation, there are many important questions that can be answered using the model presented herein. Applying the four-visit design with the inclusion of between-subjects (or potentially within-subject) moderator variables can provide essential information regarding the conditions under which e-cigarettes may be effective substitutes for combustible cigarettes. Key moderators include individual difference characteristics (e.g., gender, length of tobacco deprivation, age, race, ethnicity, level of cigarette dependence, and previous experience with e-cigarettes) and product characteristics (e.g., device type [cig-a-like vs. tank vs. mod], device power, e-liquid nicotine concentration, and vehicle composition [e.g., vegetable glycerin/propylene glycol ratio]). Ultimately, cross population-product interactions would be ideal to understand the populations who may derive the strongest substitutive properties from certain e-cigarette products. To this end, behavioral pharmacology human laboratory paradigms, such as the model presented here, have the potential to be paramount in a new era of tobacco control that aims to maximize the potential public health benefit of reduced risk noncombustible products, such as e-cigarettes.
Public Health Significance.
This experimental study of dual electronic cigarette (e-cigarette) and combustible cigarette users suggests that the administration of e-cigarettes during tobacco product deprivation may reduce the motivational value of combustible cigarettes and suppress some tobacco withdrawal symptoms. The study also provides preliminary validation of a novel laboratory model for testing the motivation substitutability of vaping for smoking. Pending future validation and extension, the model can be used to garner evidence to inform regulatory policies addressing e-cigarettes.
Acknowledgments
This research was supported by National Institutes of Health Grant P50-DA036106. The funding source had no role other than financial support. All authors contributed to the manuscript in a significant way and approved the final manuscript. All authors report no conflicts of interest and have no competing financial interests to disclose.
Footnotes
Data from the telephone eligibility screen from a previous e-cigarette administration study displayed a break in the distribution of self-reported nicotine concentration, such that few users reported nicotine concentrations less than 6 mg/mL, with the exception of those who vaped nicotine-free e-cigarette solutions. Hence, 6 mg/mL was selected as the eligibility criteria.
Our previous studies in nonvaping combustible cigarette smokers found that higher monetary incentives (e.g., $2 per cigarette) led to an overwhelming majority of participants choosing not to smoke to earn additional money. In that work, $0.20 was ultimately identified to produce sufficient sensitivity to combustible cigarette deprivation amongst smokers (Aguirre et al., 2015). Thus, we selected this same monetary value for the current pilot study in dual users; this value may benefit from further refinement given the distributions of smoking and vaping were skewed (see Figure 3).
Contributor Information
Adam M. Leventhal, Department of Preventive Medicine, University of Southern California Keck School of Medicine, and Department of Psychology, University of Southern California
Nicholas I. Goldenson, Department of Preventive Medicine, University of Southern California Keck School of Medicine.
Claudia G. Aguirre, Department of Preventive Medicine, University of Southern California Keck School of Medicine.
Jimi Huh, Department of Preventive Medicine, University of Southern California Keck School of Medicine..
Matthew G. Kirkpatrick, Department of Preventive Medicine, University of Southern California Keck School of Medicine.
References
- Aguirre CG, Madrid J, & Leventhal AM (2015). Tobacco withdrawal symptoms mediate motivation to reinstate smoking during abstinence. Journal of Abnormal Psychology, 124, 623–634. 10.1037/abn0000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington-Trimis JL, Gibson LA, Halpern-Felsher B, Harrell MB, Kong G, Krishnan-Sarin S, … Weaver SR (2018). Type of e-cigarette device used among adolescents and young adults: Findings from a pooled analysis of eight studies of 2166 vapers. Nicotine & Tobacco Research, 20, 271–274. 10.1093/ntr/ntx069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B: Methodological, 57, 289–300. [Google Scholar]
- Coleman BN, Rostron B, Johnson SE, Ambrose BK, Pearson J, Stanton CA, … Hyland A (2017). Electronic cigarette use among U.S. adults in the Population Assessment of Tobacco and Health (PATH) Study, 2013–2014. Tobacco Control, 26, e117–e126. 10.1136/tobaccocontrol-2016-053462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Piper ME, Leventhal AM, Schlam TR, Fiore MC, & Baker TB (2015). Anhedonia as a component of the tobacco withdrawal syndrome. Journal of Abnormal Psychology, 124, 215–225. 10.1037/abn0000016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, & Christen AG (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research, 3, 7–16. 10.1080/14622200020032051 [DOI] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Roberts A, & Soar K (2013). ‘Vaping’ profiles and preferences: An online survey of electronic cigarette users. Addiction, 108, 1115–1125. 10.1111/add.12150 [DOI] [PubMed] [Google Scholar]
- Dowd AN, Motschman CA, & Tiffany ST (2018). Development and validation of the questionnaire of vaping craving. Nicotine & Tobacco Research, nty046. 10.1093/ntr/nty046 [DOI] [PubMed] [Google Scholar]
- Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, & Eissenberg T (2015). Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine & Tobacco Research, 17, 186–192. 10.1093/ntr/ntu204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P III, & Benowitz NL (2017). Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: A longitudinal within-subjects observational study. Nicotine & Tobacco Research, 19, 160–167. 10.1093/ntr/ntw160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S, & Zeller M (2017). A nicotine-focused framework for public health. The New England Journal of Medicine, 377, 1111–1114. 10.1056/NEJMp1707409 [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerström KO (1991). The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hua M, Yip H, & Talbot P (2013). Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tobacco Control, 22, 103–106. 10.1136/tobaccocontrol-2011-050226 [DOI] [PubMed] [Google Scholar]
- Hughes JR, Dash M, & Callas PW (2015). Is impulsivity a symptom of initial tobacco withdrawal? A meta-analysis and qualitative systematic review. Nicotine & Tobacco Research, 17, 503–509. 10.1093/ntr/ntu220 [DOI] [PubMed] [Google Scholar]
- Hughes JR, & Hatsukami D (1986). Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry, 43, 289–294. 10.1001/archpsyc.1986.01800030107013 [DOI] [PubMed] [Google Scholar]
- Klesges RC, Ebbert JO, Morgan GD, Sherrill-Mittleman D, Asfar T, Talcott WG, & Debon M (2011). Impact of differing definitions of dual tobacco use: Implications for studying dual use and a call for operational definitions. Nicotine & Tobacco Research, 13, 523–531. 10.1093/ntr/ntr032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner WV, Meier E, Wiener JL, Grant DM, Gilmore J, Judah MR, … Wagener TL (2015). The comparative efficacy of first-versus second-generation electronic cigarettes in reducing symptoms of nicotine withdrawal. Addiction, 110, 862–867. 10.1111/add.12870 [DOI] [PubMed] [Google Scholar]
- Leventhal AM (2010). The tripartite pleasure inventory: A mulidimensional measure of anhedonia. Los Angeles, CA: University of Southern California. [Google Scholar]
- Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, & Pickworth WB (2010). A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addictive Behaviors, 35, 1120–1130. 10.1016/j.addbeh.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglia M, Caponnetto P, Di Piazza J, La Torre D, & Polosa R (2017). Dual use of electronic cigarettes and classic cigarettes: A systematic review. Addiction Research and Theory, 26, 330–338. [Google Scholar]
- McKee SA (2009). Developing human laboratory models of smoking lapse behavior for medication screening. Addiction Biology, 14, 99–107. 10.1111/j.1369-1600.2008.00135.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil A, Brose L, Calder R, Hitchman S, Hajek P, & McRobbie H (2016). E-cigarettes: An evidence update. A report commissioned by Public Health England. Public Health England, 111. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems, Eaton DL, Kwan LY, & Stratton K (Eds.). (2018). Public health consequences of e-cigarettes. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Pang RD, Bello MS, Liautaud MM, Weinberger AH, & Leventhal AM (2018). Gender differences in negative affect during acute tobacco abstinence differ between African American and White adult cigarette smokers. Nicotine & Tobacco Research, nty122. [Advance online publication.] 10.1093/ntr/nty122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang RD, & Leventhal AM (2014). The current impulsivity scale. Los Angeles, CA: University of Southern California. [Google Scholar]
- Paolini M, & De Biasi M (2011). Mechanistic insights into nicotine withdrawal. Biochemical Pharmacology, 82, 996–1007. 10.1016/j.bcp.2011.07.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, Davis KC, Cox S, Bradfield B, King BA, Shafer P, … Bunnell R (2016). Reasons for current E-cigarette use among U.S. adults. Preventive Medicine, 93, 14–20. 10.1016/j.ypmed.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal College of Physicians. (2016). Nicotine without smoke—Tobacco harm reduction. London, United Kingdom: RCP. [Google Scholar]
- Schoenborn CA, & Gindi RM (2015). Electronic cigarette use among adults: United States, 2014. National Center for Health Statistics Data Brief, 217, 1–8. [PubMed] [Google Scholar]
- Sharapova SR, Singh T, Agaku IT, Kennedy SM, & King BA (2018). Patterns of e-cigarette use frequency-National Adult Tobacco Survey, 2012–2014. American Journal of Preventive Medicine, 54, 284–288. 10.1016/j.amepre.2017.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Patten C, Gwaltney C, Paty J, Gnys M, Kassel J, … Balabanis M (2006). Natural history of nicotine withdrawal. Addiction, 101, 1822–1832. 10.1111/j.1360-0443.2006.01635.x [DOI] [PubMed] [Google Scholar]
- St. Helen G, Ross KC, Dempsey DA, Havel CM, Jacob P III, & Benowitz NL (2016). Nicotine delivery and vaping behavior during ad libitum e-cigarette access. Tobacco Regulatory Science, 2, 363–376. 10.18001/TRS.2.4.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, … Goldsmith CH (2010). A tutorial on pilot studies: The what, why and how. BMC Medical Research Methodology, 10, 1 10.1186/1471-2288-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, & Eissenberg T (2013). Electronic cigarettes: Effective nicotine delivery after acute administration. Nicotine & Tobacco Research, 15, 267–270. 10.1093/ntr/ntr316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]