Abstract
Summary
Background:
The role of hepatitis C (HCV) eradication on the long-term complications of type 2 diabetes mellitus remains incompletely studied.
Aim:
To investigate whether antiviral treatment impacted risk of acute coronary syndrome, end-stage renal disease, ischaemic stroke, and retinopathy among diabetic patients from the four US health systems comprising the Chronic Hepatitis Cohort Study (CHeCS).
Methods:
We included CHeCS HCV patients with diagnosis codes for type 2 diabetes who were on antidiabetic medications. Patients were followed until an out-come of interest, death, or last health system encounter. The effect of treatment on outcomes was estimated using the competing risk analysis (Fine-Gray subdistribution hazard ratio [sHR]), with death as a competing event.
Results:
Among 1395 HCV-infected patients with type 2 diabetes, 723 (52%) were treated with either interferon-based or direct-acting antivirals (DAAs); 539 (75% of treated) achieved sustained virological response (SVR). After propensity score adjustment to address treatment selection bias, patients with SVR demonstrated significantly decreased risk of acute coronary syndrome (sHR = 0.36; P < 0.001), end-stage renal disease (sHR = 0.46; P < 0.001), stroke (sHR = 0.34; P < 0.001), and retinopathy (sHR = 0.24; P < 0.001) compared to untreated patients. Results were consistent in subgroup analyses of DAA-treated patients and interferon-treated patients, an analysis of cirrhotic patients, as well as in sensitivity analyses considering cause-specific hazards, exclusion of patients with on-treatment retinopathy, and treatment status as a time-varying covariate.
Conclusion:
Successful HCV treatment among patients with type 2 diabetes significantly reduces incidence of acute coronary syndrome, end-stage renal disease, ischaemic stroke, and retinopathy, regardless of cirrhosis. Our findings support the importance of HCV antiviral therapy among patients with type 2 diabetes to reduce the risk of these extrahepatic outcomes.
1 |. INTRODUCTION
The relationship between hepatitis C (HCV) infection and type 2 diabetes (T2D) is complex. Both chronic HCV itself and HCV-related cirrhosis may increase risk for the subsequent development of type 2 diabetes, while existing diabetes appears to accelerate the progression of chronic HCV infection toward cirrhosis.1–4 Antiviral treatment introduces a further layer of complexity; interferon-based therapies, for example, have been implicated as a mediator for development of type 2 diabetes.5 We recently observed that sustained virological response (SVR) to antiviral treatment, among nondiabetics, reduced the subsequent incidence of diabetes.6
Despite strong evidence of the intricate interrelationship between HCV and type 2 diabetes, the impact of antiviral treatment status and outcome on various long-term diabetes-related complications among HCV patients remains largely unknown. A single study from Taiwan demonstrated that treatment with pegylated interferon improved renal and cardiovascular outcomes among HCV patients with diabetes.7 However, this study was limited by a lack of data regarding newer, direct-acting antiviral (DAA) therapies and treatment outcomes; it is also unclear whether these results are general-isable to other demographic groups. There remain no published reports regarding the impact of successful antiviral treatment—particularly DAAs—on risk of acute coronary syndrome (ACS), end-stage renal disease (ESRD), ischaemic stroke, or retinopathy among diabetic HCV patients in the United States.
Using comprehensive longitudinal electronic health record-based data from the geographically-and racially-diverse Chronic Hepatitis Cohort Study (CHeCS)—which includes over 10 000 HCV patients drawn from four large health systems in the United States—we investigated the impact of HCV treatment status and outcome on long-term complications of T2D.
2 |. METHODS
2.1 |. Patient population
CHeCS is a retrospective/prospective, observational study that includes patients from four large US health systems—Geisinger Clinic (Danville, PA, USA); Henry Ford Health System (Detroit, MI, USA); Kaiser Permanente Hawai’i (Honolulu, HI, USA); and Kaiser Permanente Northwest (Portland, OR, USA). CHeCS follows all guidelines of the US Department of Health and Human Services regarding protection of human subjects; study protocols were approved by the institutional review board at each participating site. The CHeCS study design has been described previously.8 Briefly, electronic administrative data and electronic health records for patients ≥18 years that received health services at any study site from January 1, 2006 through December 31, 2016 were used to identify study candidates; eligibility was confirmed with medical chart abstraction.
For this analysis, the start of the observation period (“index date”) was defined as the date of last treatment initiation for treated patients. For untreated patients, a proxy index date was randomly assigned within the dates of HCV diagnosis and last encounter, so that the distribution of index date for untreated patients is similar to that of treated patients. We included CHeCS HCV patients with the International Classification of Diseases, 9th edition or 10th edition (ICD9-CM and ICD10-CM) diagnosis codes for type 2 diabetes (250.-and E11.-respectively) in their electronic health record. Using prescription claims data, we excluded patients who were not on antidiabetic medications at the index date. In addition, patients were excluded if they had hepatitis B virus co-infection.
2.2 |. HCV treatment status and response
Detailed antiviral medication data (drug name, start/stop dates) were collected via chart abstraction. Data on routine HCV RNA quantification tests were obtained via the electronic health record. Patients with ongoing HCV therapy without sufficient follow-up to assess SVR were excluded from analyses. Patients were classified into one of three treatment status groups: (a) SVR (undetectable viral RNA loads ≥12 weeks post-therapy initiation); (b) treatment failure (TF); and (c) untreated.
2.3 |. Outcomes of interest
All eligible patients were followed for four extra-hepatic outcomes: (a) acute coronary syndrome (ACS); (b) end-stage renal disease (ESRD)9,10; (c) ischaemic stroke; and (d) retinopathy; outcomes were defined using the primary ICD9/ICD10 codes (detailed in Table S1). Follow-up continued through the earliest date of an outcome of interest, patient death, or last encounter. Patients with a history of acute coronary syndrome, end-stage renal disease, ischaemic stroke, or retinopathy prior to index were excluded from the analyses for each of these outcomes. For the analysis of retinopathy, we excluded patients who developed retinopathy within 12 months after index date to eliminate the possible effect of interferon-associated retinopathy and reduce bias when comparing treated and untreated patients.
2.4 |. Adjustment for confounding factors
Index date demographic information included patient age, sex, race/ ethnicity, and study site. Clinical risk factors included: Charlson-Deyo comorbidity indices (calculated from in-patient, out-patient, and claims data for 12 months prior to the index date)11; haemoglobin A1c laboratory results; Fibrosis-4 Index (FIB4; a biomarker for liver fibrosis and cirrhosis); hyperlipidaemia; hypertension; ever use of statins; and cirrhosis at any time prior to index date. Hyperlipidaemia and hypertension within one year pre-/post-index date were ascertained using ICD9/10 codes (Table S1). Pharmacy order and fill data were used to define statin use. Due to the observational nature of the study, availability of cirrhosis data varied. Roughly 20% of our sample had liver biopsy/vibration controlled transient elastography data; 60%−70% had laboratory data for calculation of FIB4. To overcome this variation, we implemented a hierarchical classification algorithm to identify cirrhosis: (a) decompensated cirrhosis identified using our validated Classification and Regression Tree (CART) model12; (b) “F4” liver biopsy or transient elastrography results >12.5 kPa13; (c) FIB4 >5.8814; and (d) presence of ICD9/10 diagnosis codes for cirrhosis in the electronic health record.
2.5 |. Statistical analysis
To account for confounding due to treatment selection bias, we used a propensity score approach based on multiple logistic regression analyses, with treatment as the outcome variable, and indexdate demographic variables and clinical risk factors as covariates. We used the strategy proposed by Ali et al15 for selection of possible confounders and assessment of balance. Briefly, significant variables related to treatment selection and outcomes were retained in the propensity score calculation. Each patient’s probability of receiving the treatment (propensity score) was then estimated from the multiple logistic regression. Stabilised weight was calculated based on the propensity score, using a method proposed by Robins et al.16–18 Balance of index-date covariates between treated and untreated patients was assessed before and after weighting using the standardised difference.19,20 A standardised difference with an absolute value <0.1 indicates balance between the groups.21 The treatment effect on risk of the outcomes of interest was tested using Fine-Gray subdistribution hazards models,22 adjusted for stabilised propensity weights. Death was considered as a competing risk.
We also conducted several subgroup and sensitivity analyses: (a) We performed subgroup analyses confined to patients receiving DAA therapy or interferon-based therapy compared to those who were untreated. As treatment selection is different between DAA and interferon-based therapy, propensity scores were calculated separately for the subgroups. (b) Two subgroup analyses were performed based on patients’ cirrhotic status at index date. Patients were stratified by cirrhotic status using the definition described above, and stratified using FIB4 cut-offs of ≤1.45 and ≥3.25.23 In order to ensure an appropriate understanding of the relationship between HCV treatment and outcomes, we also calculated cause-specific hazards for each outcome, because the effect of a covariate on the cause-specific hazard for a particular cause may differ from its effect on the cumulative incidence when competing risks are present.24 (c) We compared the results when patients who developed retinopathy within 12 months after index date were included and excluded from the analysis. (d) In order to account for the possible time-varying nature of treatment status (i.e., patients may be exposed as “untreated” for some time prior to achieving SVR), we performed an analysis that classified patients as “treatment failure or ongoing treatment” (TF/ OT) during the period from date of treatment initiation to the date of SVR. (e) We omitted both the Charlson-Deyo comorbidity score and the indicator for cirrhosis to determine whether the treatment effect observed in the main analysis was consistent if some important con-founders were omitted; a robust treatment effect indicates that unobserved confounding can be disregarded.25
3 |. RESULTS
We identified 1395 HCV patients with evidence of type 2 diabetes who were receiving antidiabetic medications at index date (Figure 1); 723 (52%) received antiviral treatment for HCV. Average patient age at index was 59.4 years. The following index-date covariates were included in the estimation of propensity scores to control for treatment selection bias: study site; age; sex; race; BMI; HCV genotype; hypertension; hyperlipidaemia; statin use; Charlson-Deyo comorbidity score; cirrhotic status; FIB4; and haemoglobin A1c. Patient characteristics were balanced and within the desired range of standardised difference after propensity score weighting (Table 1). After excluding patients who had experienced an outcome of interest prior to index date, 1242 patients were included in our analysis; 721 (58.0%) were treated. Among treated patients, 540 (75%) achieved SVR. During the post-index date observation period, 143 patients developed acute coronary syndrome, 159 developed endstage renal disease, and 97 experienced ischaemic stroke. After excluding patients who developed retinopathy within 12 months after index date, 187 developed retinopathy (n = 1160). Median follow-up was 2.7 years (interquartile range [IQR] 1.7–5.5 years) for acute coronary syndrome, 2.7 years (IQR 1.8–5.8 years) for end-stage renal disease, 2.7 years (IQR 1.8–5.7 years) for ischaemic stroke, and 2.4 years (IQR 1.3–4.4 years) for retinopathy (Figure 1).The subgroup analysis of DAA therapy included 988 patients who did not experience an outcome of interest prior to index date, including 467 (47%) who were treated; 428 (92%) of these treated patients achieved SVR. Median follow-up ranged from 2.2 years to 2.5 years across the outcomes of interest. The subgroup analysis of interferon-based therapy included 733 patients who did not experience an outcome of interest prior to index date, including 212 (29%) who were treated; 109 (51%) of these treated patients achieved SVR. Median follow-up ranged from 3.5 years to 4.7 years across the outcomes of interest.
FIGURE 1.
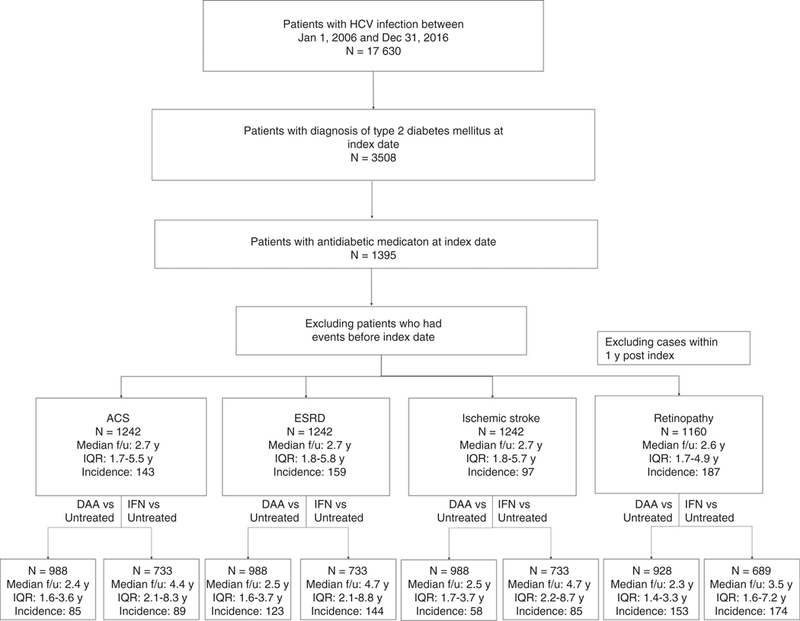
Flow chart of patient selection process. ACS: acute coronary syndrome; ESRD: end-stage renal disease; SVR: sustained virological response; TF: treatment failure; DAA: direct-acting antiviral; f/u: follow-up; y: year; IQR: inter-quartile range
TABLE 1.
Characteristics at index date before and after stabilised inverse probability of treatment weighting and follow‐up status
| Before weighting |
After weighting |
||||||
|---|---|---|---|---|---|---|---|
| Variable name | Response | Untreated (N = 672) |
Treated (N = 723) |
Abs Stddiff |
Untreated (N = 639) |
Treated (N = 720) |
Abs Stddiff |
| Study site | GHS | 110 (16%) | 157 (22%) | 0.159 | (21%) | (20%) | 0.057 |
| HFHS | 315 (47%) | 292 (40%) | (44%) | (42%) | |||
| KPHI | 45 (7%) | 52 (7%) | (7%) | (8%) | |||
| KPNW | 202 (30%) | 222 (31%) | (28%) | (30%) | |||
| Sex | Female | 230 (34%) | 234 (32%) | 0.014 | (33%) | (34%) | 0.046 |
| Male | 442 (66%) | 489 (68%) | (67%) | (66%) | |||
| Race | ASINPI | 42 (6%) | 57 (8%) | 0.293 | (7%) | (7%) | 0.057 |
| Black/African American |
298 (44%) | 243 (34%) | (39%) | (37%) | |||
| White | 291 (43%) | 401 (55%) | (50%) | (50%) | |||
| Unknown | 41 (6%) | 22 (3%) | (5%) | (6%) | |||
| Cirrhosis | Yes | 157 (23%) | 333 (46%) | (33%) | (34%) | 0.020 | |
| FIB4 | ≤1.21 | 137 (20%) | 88 (12%) | 0.290 | (17%) | (16%) | 0.026 |
| 1.21–5.88 | 412 (61%) | 488 (67%) | (63%) | (64%) | |||
| >5.88 | 81 (12%) | 123 (17%) | (15%) | (14%) | |||
| Missing | 42 (6%) | 24 (3%) | (5%) | (5%) | |||
| FIB4 | <3.25 | 433 (64%) | 422 (58%) | 0.220 | (63%) | (61%) | 0.040 |
| ≥3.25 | 197 (29%) | 277 (38%) | (32%) | (34%) | |||
| Missing | 42 (6%) | 24 (3%) | (5%) | (5%) | |||
| BMI | <25 | 144 (21%) | 109 (15%) | 0.304 | (19%) | (19%) | 0.009 |
| 25 < 30 | 181 (27%) | 205 (28%) | (28%) | (28%) | |||
| ≥30 | 271 (40%) | 370 (51%) | (44%) | (44%) | |||
| Missing | 76 (11%) | 39 (5%) | (8%) | (8%) | |||
| HCV Genotype | 1 | 350 (52%) | 535 (74%) | 0.609 | (64%) | (64%) | 0.013 |
| 2 | 51 (8%) | 84 (12%) | (9%) | (10%) | |||
| 3/Unknown | 271 (40%) | 104 (14%) | (27%) | (27%) | |||
| Weighted Charlson-Deyo | 0 | 71 (11%) | 32 (4%) | 0.238 | (8%) | (8%) | 0.012 |
| Comorbidity score | 1 | 163 (24%) | 176 (24%) | (24%) | (24%) | ||
| ≥2 | 438 (65%) | 515 (71%) | (68%) | (69%) | |||
| Hypertension | Yes | 273 (41%) | 146 (20%) | <.001 | (31%) | (31%) | 0.011 |
| Hyperlipidaemia | Yes | 128 (19%) | 258 (36%) | <.001 | (26%) | (28%) | 0.033 |
| Statins (ever used) | Yes | 357 (53%) | 385 (53%) | 0.963 | (54%) | (53%) | 0.014 |
| Age (mean ± SD) | 59.7 ± 9.9 | 59.1 ± 7.9 | 0.066 | 59.2 ± 10.6 | 58.8 ± 9.6 | 0.043 | |
| HbAlc (mean ± SD) | 7.3 ± 1.9 | 7.2 ± 1.5 | 0.025 | 7.3 ± 2.1 | 7.2 ± 1.7 | 0.027 | |
| ACS | |||||||
| F/U in years (median, IQR) | 3.1 (1.6–6.7) | 2.6 (1.8–4.4) | |||||
| Event (n) | 105 | 38 | |||||
| End-stage renal disease | |||||||
| F/U in years (median, IQR) | 3.3 (1.7–7.0) | 2.6 (1.8–4.2) | |||||
| Event (n) | 117 | 42 | |||||
| Ischaemic stroke | |||||||
| F/U in years (median, IQR) | 3.3 (1.8–6.8) | 2.6 (1.8–4.4) | |||||
| Event (n) | 72 | 25 | |||||
| Retinopathya | |||||||
| F/U in years (median, IQR) | 2.5 (1.2–5.4) | 2.6 (1.8–3.9) | |||||
| Event (n) | 141 | 46 | |||||
Abs Stddiff, absolute value of standardised difference; FIB4, fibrosis-4 index; HbAlc, haemoglobin A1c; ASINPI, Asian American, American Indian, and Pacific Islander; GHS, Geisinger Clinic; HFHS, Henry Ford Health System; KPHI, Kaiser Permanente Hawai'I; KPNW, Kaiser Permanente Northwest; F/U, follow-up.
Retinopathy cases within 1 year post index were excluded.
3.1 |. Impact of HCV treatment on incidence of type 2 diabetes-related outcomes
We observed differences in risk across the three treatment groups for all outcomes, with untreated patients demonstrating the highest risks and patients who achieved SVR demonstrating the lowest risks (Table 2). Patients who achieved SVR were at decreased risk of all outcomes of interest compared to untreated patients (Table 2: acute coronary syndrome: sHR = 0.36, 95% CI 0.25–0.54; end-stage renal disease: sHR = 0.46, 95% CI 0.31–0.67; ischaemic stroke: sHR = 0.34, 95% CI 0.21–0.56; retinopathy: sHR = 0.24, 95% CI 0.17–0.36). There were also significant differences between treated patients without SVR and untreated patients for acute coronary syndrome and retinopathy (acute coronary syndrome: sHR = 0.59, 95% CI 0.40–0.87; retinopathy: sHR = 0.37, 95% CI 0.25–0.55). These results are consistent with those provided by the cumulative incidence analyses (Figure 2) and cause-specific hazard model (Table S2). Moreover, patients who achieved SVR demonstrated lower risk of all outcomes of interest compared to untreated patients in the cause-specific hazard model with death treated as a censoring event (acute coronary syndrome: cause-specific hazard ratio [csHR] = 0.30, 95% CI 0.20–0.45; end-stage renal disease: csHR = 0.39, 95% CI 0.27–0.57; ischaemic stroke: csHR = 0.29, 95% CI 0.18–0.49; retinopathy: csHR = 0.20, 95% CI 0.14–0.30).
TABLE 2.
Estimated subdistribution hazard ratios (sHR) for acute coronary syndrome (ACS), end-stage renal disease (ESRD), ischaemic stroke, and retinopathy by antiviral treatment status and outcome. sHR analysis included adjustment for inverse probability of treatment weighting and death as a competing risk
| ACS |
ESRD |
Ischaemic stroke |
Retinopathya |
|||||
|---|---|---|---|---|---|---|---|---|
| sHR (95% CI) | P | sHR (95% CI) | P | sHR (95% CI) | P | sHR (95% CI) | P | |
| Full cohort (n = 1242) | ||||||||
| SVR vs untreated | 0.36 (0.25–0.54) | <0.001 | 0.46 (0.31–0.67) | <0.001 | 0.34 (0.21–0.56) | <0.001 | 0.24 (0.17–0.36) | <0.001 |
| TF vs untreated | 0.59 (0.40–0.87) | 0.007 | 1.11 (0.80–1.54) | 0.521 | 0.64 (0.39–1.03) | 0.068 | 0.37 (0.25–0.55) | <0.001 |
| SVR vs TF | 0.62 (0.38–1.02) | 0.057 | 0.41 (0.27–0.63) | <0.001 | 0.53 (0.29–0.98) | 0.044 | 0.66 (0.40–1.10) | 0.110 |
| DAA-treated subcohort vs untreated (n = 988) | ||||||||
| SVR vs untreated | 0.30 (0.19–0.48) | <0.001 | 0.36 (0.22–0.58) | <0.001 | 0.20 (0.10–0.38) | <0.001 | 0.16 (0.08–0.30) | <0.001 |
| TF vs untreated | 1.08 (0.49–2.38) | 0.842 | 0.38 (0.09–1.57) | 0.181 | 0.44 (0.11–1.77) | 0.248 | 0.12 (0.01–1.12) | 0.063 |
| SVR vs TF | 0.28 (0.12–0.66) | 0.004 | 0.94 (0.22–4.10) | 0.935 | 0.45 (0.10–2.02) | 0.297 | 1.29 (0.13–12.76) | 0.826 |
| IFN-treated subcohort vs untreated (n = 733) | ||||||||
| SVR vs untreated | 0.32 (0.17–0.60) | <0.001 | 0.36 (0.19–0.66) | <0.001 | 0.44 (0.21–0.92) | 0.029 | 0.58 (0.34–0.97) | 0.037 |
| TF vs untreated | 0.54 (0.35–0.82) | 0.005 | 0.97 (0.69–1.36) | 0.841 | 0.68 (0.41–1.13) | 0.138 | 0.63 (0.42–0.94) | 0.025 |
| SVR vs TF | 0.60 (0.29–1.23) | 0.162 | 0.37 (0.19–0.71) | 0.003 | 0.65 (0.29–1.49) | 0.313 | 0.92 (0.50–1.67) | 0.777 |
| Cirrhotic (N = 441) | ||||||||
| SVR vs untreated | 0.10 (0.04–0.27) | <0.001 | 0.31 (0.14–0.67) | 0.003 | 0.26 (0.09–0.70) | 0.008 | 0.39 (0.20–0.76) | 0.006 |
| TF vs untreated | 0.18 (0.05–0.59) | 0.005 | 0.72 (0.34–1.53) | 0.392 | 0.54 (0.17–1.69) | 0.286 | 0.38 (0.14–1.01) | 0.052 |
| SVR vs TF | 0.56 (0.12–2.57) | 0.455 | 0.43 (0.17–1.13) | 0.088 | 0.48 (0.12–1.87) | 0.288 | 1.02 (0.36–2.89) | 0.963 |
| Noncirrhotic (N = 801) | ||||||||
| SVR vs untreated | 0.54 (0.35–0.83) | 0.005 | 0.52 (0.34–0.81) | 0.004 | 0.37 (0.21–0.67) | <0.001 | 0.30 (0.18–0.52) | <0.001 |
| TF vs untreated | 0.78 (0.51–1.20) | 0.251 | 1.24 (0.86–1.79) | 0.243 | 0.65 (0.38–1.10) | 0.109 | 0.54 (0.34–0.85) | 0.007 |
| SVR vs TF | 0.70 (0.41–1.17) | 0.172 | 0.42 (0.26–0.68) | <0.001 | 0.58 (0.29–1.15) | 0.120 | 0.56 (0.30–1.06) | 0.075 |
T2D, type II diabetes; ACS, acute coronary syndrome; ESRD, end‐stage renal disease; SVR, sustained virological response; TF, treatment failure; IFN, interferon‐based therapy; sHR, subdistribution hazard ratio.
Retinopathy cases within 1 year post index were excluded.
FIGURE 2.
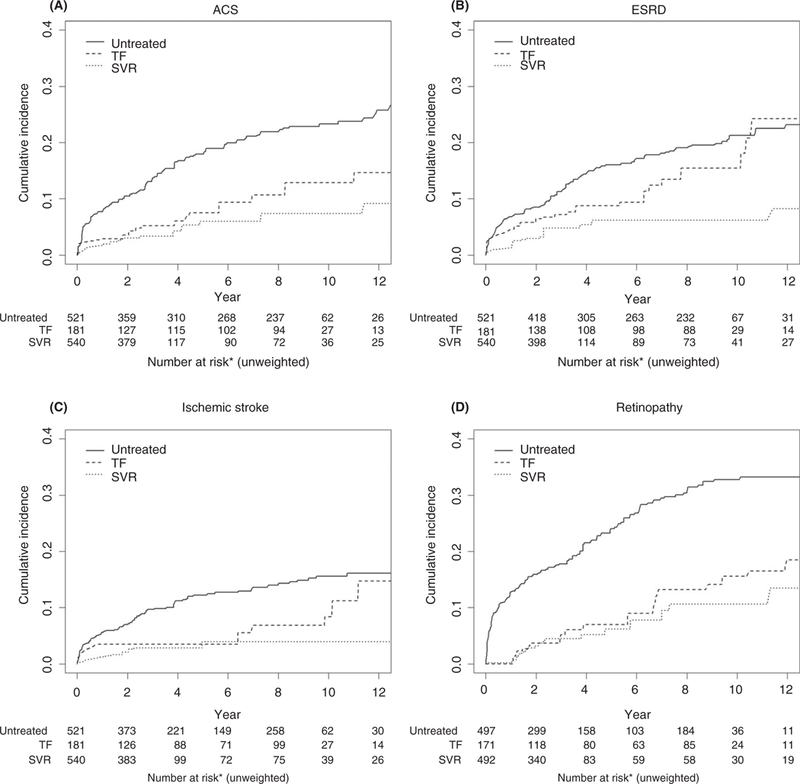
Cumulative incidence function for acute coronary syndrome (ACS, A), end-stage renal disease (ESRD, B), ischemic stroke (C), and retinopathy (D) estimated by Kaplan-Meier estimates with death adjusted as a competing risk and stabilized inverse propensity weights for treatment imbalance. *The number of subjects at risk without weighting for competing risk and stabilized inverse propensity weights is provided for illustration purpose. Please see Table 2 for formal analysis.
Results were consistent in our analysis confined to patients receiving DAA therapy (Table 2 and Tables S2 and S3). Compared to untreated patients, patients who achieved SVR after DAA therapy demonstrated reduced risk of acute coronary syndrome, end-stage renal disease, ischaemic stroke, and retinopathy (Table 2: acute coronary syndrome: sHR = 0.30, 95% CI 0.19–0.48; end-stage renal disease: sHR = 0.36, 95% CI 0.22–0.58; ischaemic stroke: sHR = 0.20, 95% CI 0.10–0.38; retinopathy: sHR = 0.16, 95% CI 0.08–0.30). Notably, patients with DAA treatment failure (TF) did not demonstrate reduced risk of acute coronary syndrome compared to untreated patients, as was seen in the main analysis. This lack of a significant result is not unexpected, given the small number of patients who experienced DAA treatment failure. The confidence intervals for the risk estimates were wide due to small number of events. Associations were similar when the analyses were restricted to patients who had cirrhosis and patients who did not have cirrhosis (Table 2).
Similar conclusions were reached when our analysis was confined to patients receiving interferon-based therapy. Compared to untreated patients, patients who achieved SVR after interferon-based therapy demonstrated reduced risk of acute coronary syndrome, end-stage renal disease, ischaemic stroke, and retinopathy (Table 2: acute coronary syndrome: sHR = 0.32, 95% CI 0.17–0.60; end-stage renal disease: sHR = 0.36, 95% CI 0.19–0.66; ischaemic stroke: sHR = 0.44, 95% CI 0.21–0.92; retinopathy: sHR = 0.58, 95% CI 0.34–0.97). When we included patients with retinopathy that occurred within 1 year of index date, the significance of the association between SVR and retinopathy was no longer maintained (Table S2; sHR = 0.84, 95% CI 0.58–1.19).
Results also remained consistent when treatment status was considered as a time-varying variable. Risk of acute coronary syndrome, end-stage renal disease, ischaemic stroke, and retinopathy were all lowest among SVR patients (Table S3). In addition, we found that the risk of ischaemic stroke was significantly reduced in treatment failure/ongoing treatment patients (sHR = 0.47, 95% CI 0.29–0.75) compared to untreated patients. We also observed a consistent treatment effect in analyses that omitted several key covariates for the calculation of propensity scores (Table S4). The results remained largely unchanged when fibrosis/cirrhosis at baseline was defined using cut-offs FIB4 ≤ 1.45 and ≥3.25, except that the significant association between SVR and retinopathy was no longer observed for the subgroup of patients with FIB4 ≥ 3.25. We note, however, the number of events in this subgroup is very small (n = 30).
4 |. DISCUSSION
In a large, racially diverse cohort of HCV patients with type 2 diabetes, we found that patients who achieved SVR were at significantly reduced risk of acute coronary syndrome, end-stage renal disease, ischaemic stroke, and retinopathy compared to untreated patients. Given consistent results from both cause-specific hazard and subdistribution hazard models, the effect of SVR on the cumulative incidence of acute coronary syndrome, end-stage renal disease, ischaemic stroke, and retinopathy can be interpreted as an actual effect (without being necessarily causal). We found that SVR reduced risk of acute coronary syndrome by 64%, end-stage renal disease by 54%, stroke by 66%, and retinopathy by 76%, compared to absence of treatment. Subgroup analyses showed that the effect of SVR on these outcomes was independent of patients’ cirrhotic status.
We also observed that treatment without SVR reduced risk of acute coronary syndrome by 41% and retinopathy by 63%, compared to no treatment. When patients’ treatment response was treated as time-varying (ie, patients were classified as treatment failure/ ongoing treatment for the period before they achieved SVR) we found that these patients also demonstrated reduced risk of ischaemic stroke compared to untreated patients (sHR = 0.47, 95% CI 0.29–0.75). This finding is consistent with a recent report that treatment even without SVR reduced risk of stroke in a large sample of US veterans,26 although these analyses were based on time-dependent treatment variables among a HCV cohort that was not limited to patients with T2D. Likewise, our overall findings are consistent with a recent meta-analysis that showed decreased risk in a number of extrahepatic outcomes among HCV patients who achieved SVR, although this analysis was also not limited to patients with dia-betes,27 as well as a review that reported improvement across a number of metabolic parameters among diabetic patients.28
A recent study from Taiwan7 demonstrated a strong association between HCV treatment and reduced risk of renal and circulatory outcomes in diabetic patients. Because the comparison groups used in that study were “ever treated” (both SVR and treatment failure) versus “untreated,” it was not possible to distinguish between the impact of successful treatment (viral eradication) versus receipt of antiviral therapy. Our results suggest that both receipt of treatment and viral eradication (via either interferon-based therapy or the newer direct-acting all-oral regimens) play a role in improved diabetic long-term outcomes. Likewise, given geographic differences in the predominant HCV genotypes29 and known racial/ethnic differences in type 2 diabetes outcomes in the US,30 it is not clear that results from the Taiwan study are generalisable to an ethnically diverse American population, particularly African Americans. We expand upon these findings to show the impact of treatment outcome and DAA regimens on long-term complications of diabetes among a racially diverse cohort of HCV patients in the United States. We also performed an interferon-treated subgroup analysis and the conclusions are similar. Interestingly, when we included patients who developed retinopathy within 1 year of index date, the significant association between SVR and risk of retinopathy was no longer statistically significant; this finding may reflect the fact that the well-recognised phenomenon of interferon-associated retinopathy during treatment could have diluted the beneficial effect of SVR.
All HCV patients in this study were prescribed antidiabetic medications at index date, and were assumed to be on continuous medical therapy for T2D throughout the study period. A limitation of the present study is that we do not have data regarding lifestyle modifications, such as increased physical activity or dietary changes, that may impact glucose control over time, nor were we able to analyse whether antidiabetic medication type or dose changed during follow-up. This analysis assumed that patients’ health-related behaviours and medication patterns did not differ between treatment groups after completing their treatment regimens. We also do not have data on a number of known risk factors for cardiovascular outcomes, such as tobacco use. However, our analysis is designed to compare the effect of HCV treatment on the risk of these outcomes. It has been shown that, while the inclusion or exclusion of outcome-related factors may change the efficiency of a treatment effect estimate, it will not change the bias31; therefore, the exclusion of some risk factors is unlikely to impact the validity of our findings. In addition, we performed a sensitivity analysis that omitted several treatment-selection and prognostic covariates; results of this sensitivity analysis were consistent with those of the main analysis, indicating that unobserved confounders are unlikely to have impacted our observed treatment effect.
There are also limitations inherent in the use of observational data drawn from “real world” patients. We used a number of methods to minimise the impact of missing data and possible confounding. To address differences in availability of cirrhosis data, cirrhosis status was captured through a hierarchical classification algorithm that included liver biopsy/transient elastography results, FIB4 indices, and ICD9/10 diagnosis codes for compensated and decompensated cirrhosis (using a validated algorithm for identification of decompensated cirrhosis).32 Propensity score weighting was used to control for treatment selection bias. We also performed a number of sensitivity analyses (a cause-specific hazards analysis; inclusion and exclusion of patients who developed retinopathy while on treatment; an analysis of the impact of cirrhosis; and an analysis that considered treatment status as a time varying covariate) to ensure that appropriate conclusions were drawn given the complexities and limitations of our data. The results of all sensitivity analyses were consistent with the main analysis and support our conclusions. Moreover, the small point estimates and the narrow confidence intervals provides strong evidence of association of SVR and risk of outcomes.
The present analysis from a large and diverse cohort of patients with type 2 diabetes shows that successful HCV treatment reduced the risks of acute coronary syndrome, end-stage renal disease, ischaemic stroke, and retinopathy by 39%−66%; this effect was independent of patients’ baseline fibrosis status, and consistent in subgroup analyses restricted to patients with and without cirrhosis. Chronic HCV infection, independent of diabetic status, is known to confer an increased risk of extrahepatic complications; treatment status and outcome have been associated with improvement in some, but not all, of these conditions. The magnitude of risk reduction we observed within HCV patients with T2D supports the importance of antiviral therapy among diabetic patients to reduce risk of these extrahepatic outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
The CHeCS Investigators include the following investigators and sites: Scott D. Holmberg, Eyasu H. Teshale, Philip R. Spradling, Anne C. Moorman, Jian Xing, and Yuna Zhong, Division of Viral Hepatitis, National Centers for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), Centers for Disease Control and Prevention (CDC), Atlanta, Georgia; Stuart C. Gordon, David R. Nerenz, Mei Lu, Lois Lamerato, Jia Li, Loralee B. Rupp, Nonna Akkerman, Talan Zhang, Sheri Trudeau, and Yueren Zhou, Henry Ford Health System, Detroit, Michigan; Joseph A. Boscarino, Zahra S. Daar, and Robert E. Smith, Center for Epidemiology & Health Services Research, Geisinger Clinic, Danville, Pennsylvania; Yihe G. Daida, Connie Mah Trinacty, and Carmen P. Wong, The Center for Health Research, Kaiser Per-manente-Hawaii, Honolulu, Hawaii; Mark A. Schmidt and Judy L. Donald, The Center for Health Research, Kaiser Permanente-North-west, Portland, OR.
Funding information
CHeCS is funded by Centers for Disease Control and Prevention and Gilead Pharmaceuticals. Gilead and other granting corporations do not have access to CHeCS data and do not contribute to the design of the study, the collection, analysis, interpretation of the data, or writing of manuscripts, nor the decision to approve publication of the finished manuscript.
Declaration of personal interests: Stuart C. Gordon receives grant/ research support from AbbVie Inc, Conatus Pharmaceuticals, Cyma-Bay Therapeutics, Gilead Pharmaceuticals, Intercept Pharmaceuticals, and Merck. He serves as an ad hoc consultant/advisor for Abbvie Inc, Dova Pharmaceuticals Gilead Sciences, Intercept Pharmaceuticals, and Merck & Co. Mei Lu, Jia Li, Lora Rupp, Sheri Trudeau, Talan Zhang, Yueren Zhou, Yihe G. Daida, Mark A. Schmidt, and Joseph A. Boscarino receive grant/research support from Gilead Sciences.
Footnotes
SUPPORTING INFORMATION
Additional supporting information will be found online in the Supporting Information section at the end of the article.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
All authors have approved the final version of the manuscript.
REFERENCES
- 1.White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Negro F, Sanyal AJ. Hepatitis C virus, steatosis and lipid abnormalities: clinical and pathogenic data. Liver Int. 2009;29(Suppl 2):26–37. [DOI] [PubMed] [Google Scholar]
- 3.Stepanova M, Lam B, Younossi Y, Srishord MK, Younossi ZM. Association of hepatitis C with insulin resistance and type 2 diabetes in US general population: the impact of the epidemic of obesity. J Viral Hepat. 2012;19:341–345. [DOI] [PubMed] [Google Scholar]
- 4.Negro F, Forton D, Craxi A, Sulkowski MS, Feld JJ, Manns MP. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149:1345–1360. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Hamid N, Jubori TA, Farhan A, et al. Underlying pathways for interferon risk to type II diabetes mellitus. Curr Diabetes Rev. 2013;9:472–477. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Zhang T, Gordon SC, et al. Impact of sustained virologic response on risk of type 2 diabetes among hepatitis C patients in the United States. J Viral Hepat 2018;25:952–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu YC, Lin JT, Ho HJ, et al. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular out-comes in diabetic patients. Hepatology. 2014;59:1293–1302. [DOI] [PubMed] [Google Scholar]
- 8.Moorman AC, Gordon SC, Rupp LB, et al. Baseline characteristics and mortality among people in care for chronic viral hepatitis: the chronic hepatitis cohort study. Clin Infect Dis. 2013;56:40–50. [DOI] [PubMed] [Google Scholar]
- 9.Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150:1599–1608. [DOI] [PubMed] [Google Scholar]
- 10.Lai TS, Lee MH, Yang HI, et al. Hepatitis C viral load, genotype, and increased risk of developing end-stage renal disease: REVEAL-HCV study. Hepatology. 2017;66(3):784–793. [DOI] [PubMed] [Google Scholar]
- 11.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 12.Lu M, Chacra W, Rabin D, et al. Validity of an automated algorithm using diagnosis and procedure codes to identify decompensated cirrhosis using electronic health records. Clin Epidemiol. 2017;9:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Gordon SC, Rupp LB, et al. The validity of serum markers for fibrosis staging in chronic hepatitis B and C. J Viral Hepat. 2014;21:930–937. [DOI] [PubMed] [Google Scholar]
- 15.Ali MS, Groenwold RH, Belitser SV, et al. Reporting of covariate selection and balance assessment in propensity score analysis is suboptimal: a systematic review. J Clin Epidemiol. 2015;68:112–121. [DOI] [PubMed] [Google Scholar]
- 16.Robins JM. Marginal structural models. 1997. Proceedings of the American Statistical Association, Section on Bayesian Statistical Science. 1998:1–10. [Google Scholar]
- 17.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology (Cambridge, Mass.) 2000;11:550–560. [DOI] [PubMed] [Google Scholar]
- 18.Robins JM. Marginal structural models versus structural nested models as tools for causal inference In: Halloran ME, Berry D, eds. Statistical Models in Epidemiology, the Environment, and Clinical Trials. The IMA Volumes in Mathematics and its Applications. New York, NY: Springer; 2000. [Google Scholar]
- 19.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS®. In: Presented at the SAS Global Forum, Orlando; 2012: 1–6. [Google Scholar]
- 21.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recom-mendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. [DOI] [PubMed] [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 23.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/ HCV coinfection. Hepatology. 2006;43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 24.Latouche A, Allignol A, Beyersmann J, Labopin M, Fine JP. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol. 2013;66:648–653. [DOI] [PubMed] [Google Scholar]
- 25.Steventon A, Grieve R, Sekhon JS. A comparison of alternative strategies for choosing control populations in observational studies. Health Serv Outcomes Res Method. 2015;15:157–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahale P, Engels EA, Li R, et al. The effect of sustained virological response on the risk of extrahepatic manifestations of hepatitis C virus infection. Gut. 2018;67:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cacoub P, Desbois AC, Comarmond C, Saadoun D. Impact of sustained virological response on the extrahepatic manifestations of chronic hepatitis C: a meta-analysis. Gut 2018;67:2025–2034. [DOI] [PubMed] [Google Scholar]
- 28.Adinolfi LE, Rinaldi L, Marrone A, Giordano M. The effect of sustained virological response by direct-acting antivirals on insulin resistance and diabetes mellitus in patients with chronic hepatitis C. Expert Rev Anti Infect Ther. 2018;16:595–597. [DOI] [PubMed] [Google Scholar]
- 29.Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22:7824–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao H, Wu E, Fu S, et al. The higher prevalence of truncal obesity and diabetes in American than Chinese patients with chronic hepatitis C might contribute to more rapid progression to advanced liver disease. Aliment Pharmacol Ther. 2017;46:731–740. [DOI] [PubMed] [Google Scholar]
- 31.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones D, Boudes PF, Swain MG, et al. Seladelpar (MBX-8025), a selective PPAR-delta agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a doubleblind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol. 2017;2:716–726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


