Abstract
Kwok, A. T., Moore, J. E., Rosas, S., Kerr, B. A., Andrews, R. N., Nguyen, C. M., Lee, J., Furdui, C. M., Collins, B. E., Munley, M. T. and Willey, J. S. Knee and Hip Joint Cartilage Damage from Combined Spaceflight Hazards of Low-Dose Radiation Less than 1 Gy and Prolonged Hinlimb Unloading. Radiat. Res. 191, 497–506 (2019).
Reduced weight bearing, and to a lesser extent radiation, during spaceflight have been shown as potential hazards to astronaut joint health. These hazards combined effect to the knee and hip joints are not well defined, particularly with low-dose exposure to radiation. In this study, we examined the individual and combined effects of varying low-dose radiation (≤1 Gy) and reduced weight bearing on the cartilage of the knee and hip joints. C57BL/6J mice (n = 80) were either tail suspended via hindlimb unloading (HLU) or remained full-weight bearing (ground). On day 6, each group was divided and irradiated with 0 Gy (sham), 0.1 Gy, 0.5 Gy or 1.0 Gy (n = 10/group), yielding eight groups: ground-sham; ground-0.1 Gy; ground-0.5 Gy; ground-1.0 Gy; HLU-sham; HLU-0.1 Gy; HLU-0.5 Gy; and HLU-1.0 Gy. On day 30, the hindlimbs, hip cartilage and serum were collected from the mice. Significant differences were identified statistically between treatment groups and the ground-sham control group, but no significant differences were observed between HLU and/or radiation groups. Contrast-enhanced micro-computed tomography (micro-CECT) demonstrated decrease in volume and thickness at the weight-bearing femoral-tibial cartilage-cartilage contact point in all treatment groups compared to ground-sham. Lower collagen was observed in all groups compared to ground-sham. Circulating serum cartilage oligomeric matrix protein (sCOMP), a biomarker for ongoing cartilage degradation, was increased in all of the irradiated groups compared to ground-sham, regardless of unloading. Mass spectrometry of the cartilage lining the femoral head and subsequent Ingenuity Pathway Analysis (IPA) identified a decrease in cartilage compositional proteins indicative of osteoarthritis. Our findings demonstrate that both individually and combined, HLU and exposure to spaceflight-relevant radiation doses lead to cartilage degradation of the knee and hip with expression of an arthritic phenotype. Moreover, early administration of low-dose irradiation (0.1, 0.5 or 1.0 Gy) causes an active catabolic response in cartilage 24 days postirradiation. Further research is warranted with a focus on the prevention of cartilage degradation from long-term periods of reduced weight bearing and spaceflight-relevant low doses and qualities of radiation.
INTRODUCTION
Astronauts must maintain their musculoskeletal health during spaceflight to ensure mission success. Joint loading on Earth is necessary to maintain structural integrity, proper function and homeostasis of cartilage and other tissues constituting load-bearing synovial joints (1, 2). Thus, the reduced weight bearing experienced with spaceflight represents a substantial challenge to maintaining the health of synovial joints (3–6). Clinically relevant structural degradation of cartilage has been documented after periods of prescribed partial- or nonweight-bearing (3–5). Radiation, a frequently undetermined threat present during spaceflight, also affects joint health and induces an arthritic phenotype, observed at higher doses in clinical applications (7–10), and at lower doses in preclinical studies (7–10). Chronic knee damage has been observed in rats after acute exposure to 1 Gy X rays (6), which is relevant to spaceflight scenarios (e.g., solar flare). To our knowledge, the combined effects of low-dose exposure (< 1 Gy) and reduced weight bearing have not been studied together.
The goal of this ground-based study was to determine if prolonged periods (~30 days) of reduced weight-bearing with or without exposure to spaceflight-relevant low doses of radiation (<1 Gy) causes or enhances knee and hip joint damage. Of particular interest were the effects of low-dose radiation on cartilage as a single and combined challenge with reduced weight bearing (hindlimb unloading; HLU) via tail suspension, as we hypothesized a priori that HLU would result in cartilage degradation (6,11–13). It has been unclear if low-dose radiation (0.1 and 0.5 Gy) (6, 12, 14) is damaging, as a single challenge or part of a combined challenge.
MATERIALS AND METHODS
Animals and Study Design
Eighty female C57BL/6 mice from Charles River Laboratories (Wilmington, MA) were purchased at 16 weeks of age and housed under a 12:12 h light-dark schedule at 26°C. After one week of acclimation, mice were divided into eight groups of 10 mice per group. Four groups were randomly selected to undergo HLU via tail suspension, while four groups remained full-weight bearing in cages (ground). HLU was initiated on day 1 after acclimation. On day 6, mice were either nonirradiated (sham) or received 0.1, 0.5 or 1.0 Gy total-body irradiation (TBI) of 10 MV X rays, yielding the following 8 groupings (n = 10/group): ground-sham; ground-0.1 Gy; ground-0.5 Gy; ground-1.0 Gy; HLU-sham; HLU-0.1 Gy; HLU-0.5 Gy; and HLU-1.0 Gy). This study was performed as two individual experiments at different times with one half of the animals (n = 5) per group, with no observable differences in terms of behavior, HLU tolerance or body weight changes over time. Tissues were harvested at day 30. All experiments followed the NIH Guide for Care and Use of Laboratory Animals and were approved by the Wake Forest School of Medicine Institutional Animal Care and Use Committee.
Hindlimb Unloading Procedure
On day 1, all mice (HLU and ground) were lightly anesthetized with isoflurane. The tail was prepared by cleaning with 70% alcohol, treated with an adhesive benzoin tincture and allowed to dry and become sticky. A 25-cm strip of traction tape (3M HealthCare, Two Harbors, MN) was then cut and braided around the tail, leaving space for breathability. The end of the tape was tied to a ball-bearing swivel with interlock snap, which clasped onto a repair clamp with a hollowed cylindrical barrel for free access of motion when attached to the custom-designed mouse tail-suspension cage angled 30° off the flooring. The HLU animals were housed in pairs with an open area for improving animal companionship; rodents could see each other and have nose-to-nose contact.
Irradiation Procedure
On day 6, mice in the HLU groups were transferred to a delivery box specifically constructed for irradiating five animals simultaneously with a clinical linear accelerator (LINAC, Varian 2100SC; Palo Alto, CA) emitting a 10-MV photon beam. The corresponding TBI (0.1, 0.5 or 1.0 Gy) were delivered while tail suspension was maintained and without the use of anesthesia, at a dose rate of ~1 Gy/min (6). Irradiations for the ground mice were also performed with 5 mice per exposure in the irradiation box, but without HLU. Animals were irradiated with two parallel, opposed beams for each irradiation, each providing one half the dose (0.05, 0.25 or 0.5 Gy, respectively). Uniform water-equivalent material (5 cm, Solid-Water®; Gammex RMI/Sun Nuclear Corp., Middleton, WI) was placed on each side of the box perpendicular to the photon beam paths to establish electronic equilibrium and obtain dose homogeneity (6). All necessary dosimetry was validated throughout the irradiation box with a Farmer ionization chamber as well as optically stimulated luminescent dosimeters (OSLDs). Sham-irradiated animals were positioned in similar cages as HLU animals on the LINAC for approximately the same amount of time, but were not irradiated (6).
Tissue Harvest
The HLU animals remained unloaded until time of euthanasia on day 30. The blood was collected by cardiac puncture while animals were under anesthesia. Serum was isolated from blood and stored at –80°C. The right hindlimb was carefully removed and placed in 10% neutral buffered formalin for fixation. After day 3, the hindlimbs were transferred to 70% EtOH with 0.1% w/v phosphotungstic acid for contrast-enhanced micro-computed tomography (microCECT). The articular cartilage from both distal femoral condyles was removed using a mini rongeur, pooled and stored at –80°C for LC-MS/MS analysis. Because of low sample size and based on validation studies, the femoral head cartilage samples were pooled (n = 3 samples per treatment) in the following manner: femoral head cartilage from n = 3 mice (2 heads per mouse; 6 femoral head cartilage samples) were pooled per group.
Contrast-Enhanced Micro-Computed Tomography
The right hindlimb incubated with 1% phosphotungstic acid was analyzed using micro-computed tomography (GE Phoenix Nanotom-180M instrument; GE Inspection Technologies, Cincinnati, OH), with isotropic voxels of 2.5 im/side, and X-ray emission parameters of 90 kV and 70 μA. Explanted knee joints were secured in a sponge substrate within a 15-ml enclosed conical tube. Enough fixation solution was removed to ensure the knee joint was surrounded by air but still moist during imaging. The solution was replaced upon scan completion. The 2D X-ray data was collected as an average of three images at each of 1,400 positions, each separated by 9/35°. Reconstruction was performed with GE phoenix datos2 software, and the subsequent 3D rendered volumes were visualized and saved with VGStudio Max version 2.1 software (Volume Graphics, Heidelberg, Germany).
Image Processing and Analysis
The resulting micro-CT images were reformatted so they were all oriented spatially in the same manner (frontal view) and then imported into Mimics® Innovation Suite version 18.0 ×64 (Materialise, Leuven, Belgium) to measure structural properties of the articular cartilage. A rectangular area of 250,000 im2 was mapped out on the tibial medial articular cartilage with the center of the area located at the cartilage-cartilage contact point as a standard reference location for each animal. The mapped area was outlined and compiled into a 3D reconstruction of the articular cartilage, providing volumetric data. The articular cartilage within this region of interest (medial tibial plateau area) was measured between the surface and bone for thickness at five locations, specifically, 250 im to the lateral, medial, anterior and posterior points away from and at the femoral-tibial cartilage-cartilage contact point.
Histology and Molecular Probing
After micro-CT imaging was performed, the fixed right knees were decalcified and embedded in paraffin for histologic analysis, as described elsewhere (6). Knees from each group were sectioned coronally at 5 im. Prepared sections (n = 5–7/group) were stained with hematoxylin and eosin (H&E) to qualitatively assess general morphology, and Russel-Movat Pentachrome (American MasterTech, Lodi, CA) justified with Masson’s Trichrome was used to assess sulfated glycosaminoglycan content and arthritis. The Osteoarthritis Research Society International (OARSI) scoring system was used to evaluate arthritis severity on histology. Immunostaining was per formed to identify matrix metalloproteinase-13 (MMP-13; Abcam®, Cambridge, MA) presence and activity by probing for the metalloproteinase-generated neoepitope. Measurements of collagen with Russel-Movat Pentachrome were performed using a ratio of stained collagen to articular cartilage above the tidemark within the growth plate (margin of calcified cartilage). Quantification of MMP-13 within the articular cartilage was performed to determine the ratio of cells positive for pericellular staining to those with negative staining, within the entire tibial plateau per slide (6). Image capture was performed using an Axioplan 2 microscope with an AxioCam image capture system (Carl Zeiss AG, Oberkochen, Germany). Image analysis was performed using BIOQUANT® OSTEO version 17 (Nashville, TN).
Extracted serum from the animals was then used to analyze for cartilage oligomeric matrix protein (COMP), a biomarker of cartilage turnover, with a sandwich ELISA (LifeSpan BioSciences Inc., Seattle, WA). The samples were then normalized to protein concentration measured with Pierce™ Bicinchoninic Acid Protein Assay Kit (Thermo Scientific™ Inc., Waltham, MA).
Proteomics and Pathway Analysis
The articular cartilage lining the femoral heads was processed for proteomic analysis, using the techniques and resources of the Proteomics and Metabolomics Shared Resource at the Wake Forest School of Medicine (Winston-Salem, NC).
Tissues were first lysed in PBS with protease/phosphatase inhibitor using a Bead Mill Homogenizer (Bead Ruptor, Omni International, Kennesaw, GA), followed by addition of an equal volume of 2× radioimmunoprecipitation (RIPA) buffer. The resulting lysates were incubated on ice for 30 min and centrifuged at 18,000g for 10 min. The supernatant was further processed for proteomics analysis using reductive alkylation (10 mM dithiothreitol and 30 mM iodoacetamide), protein precipitation by adding four times the sample volume of cold (–20°C) acetone and incubation at –20°C overnight, separation of protein fraction by centrifugation at 14,000g for 10 min, resuspension of protein pellet in 50 mM ammonium bicarbonate and enzymatic digestion with trypsin at 1:50 enzyme-to-substrate ratio at 37°C overnight. The resulting peptides were desalted using a C18 spin column prior to LC-MS/MS analysis.
The LC-MS/MS analysis was performed using a Q Exactive™ HF Hybrid Quadrupole-Orbitrap Mass Spectrometer coupled with a Dionex UltiMate™ 3000 nano-UPLC system (Thermo Scientific). An Acclaim™ PepMap™ 100 (C18, 5 μm, 100 Å, 100 μm × 2 cm) trap column and an Acclaim PepMap RSLC (C18, 3 μm, 100 Å, 75 μm × 15 cm) analytical column were used for nano-LC analysis. Peptides were separated using a linear gradient of mobile phases A (water with 0.1% formic acid) and B (acetonitrile with 0.1% formic acid) where the gradient was from 5% B at 0 min to 40% B at 170 min. Data acquisition was performed using a top-20 data-dependent method with a 10-s dynamic exclusion enabled.
MS data were processed using Proteome Discoverer™ version 2.1 (Thermo Scientific), Sequest™ HT as search engine, and the mouse UniProt protein FASTA database (annotated 16,747 entries, December 2015). Search parameters were as follows: FT-trap instrument, parent mass error tolerance of 10 ppm, fragment mass error tolerance of 0.02 Da (monoisotopic), variable modifications of 16 Da (oxidation) on methionine and fixed modification of 57 Da (carbamidomethylation) on cysteine.
Mass spectrometry results were imported into the Ingenuity® Pathway Analysis (IPA®; QIAGEN®, Redwood City, CA) to map specific canonical pathways, diseases and biological functions chosen a priori, namely those that affect osteoarthritic responses and endoplasmic reticulum stress, including the unfolded protein response, osteoarthritis pathway, NRF2-mediated oxidative stress and mTOR signaling canonical pathways. The canonical pathways were clustered by hierarchy and listed in order of ascending P values of each individual group, followed by individual protein expression measured by peptide spectrum matches.
Statistics
Data were primarily analyzed using two-way analysis of variance (ANOVA), for the following main effects and interactions: Load condition (HLU or ground) and radiation condition (sham, 0.1, 0.5 or 1.0 Gy). Tukey’s post hoc tests for multiple comparisons among all groups were performed after identification of main effects; assumptions of normality and equal variance were tested. Significance was set at an α of 0.05. Protein assays were compared from the n = 3 pooled samples using unpaired t tests due to low sample quantity. All data are presented as mean (SD).
RESULTS
Cartilage Volume and Thickness Analysis
A main effect of radiation (F3,68 = 4.52; P = 0.006) on cartilage volume was identified, with a marginal (F1,68 = 2.66; P = 0.11) load effect. Relative to ground-sham, the volume of cartilage was significantly reduced (Fig. 1) in the following manner: ground-0.1 Gy (−23%); ground-0.5 Gy (−20%); ground-1 GY (−23%); HLU-0.5 Gy (−27%); and HLU-1.0 Gy (−22%). Volume was marginally lower compared to ground-sham in HLU-sham (−19%; P = 0.07) and HLU-0.1 Gy (−19%; P = 0.06). Both significant radiation (F3,67 = 5.97; P = 0.001) and load (F1,67 = 12.57; P < 0.001) effects on cartilage thickness were identified. Cartilage thickness throughout the majority of the knee was unchanged in the treatment groups compared to ground-sham (Fig. 2), with the exception of the tibial-femoral cartilage-cartilage contact point, which supports the majority of loading across the knee. At this point of highest-load bearing across the knee, cartilage was significantly thinner after both HLU and/or irradiation at all doses (Fig. 2). A significant interaction between load and radiation was identified (F3,67 = 4.02; P = 0.011); an interaction plot (data not shown) indicated that the decrease with radiation in the ground group was greater than in the HLU group.
FIG. 1.
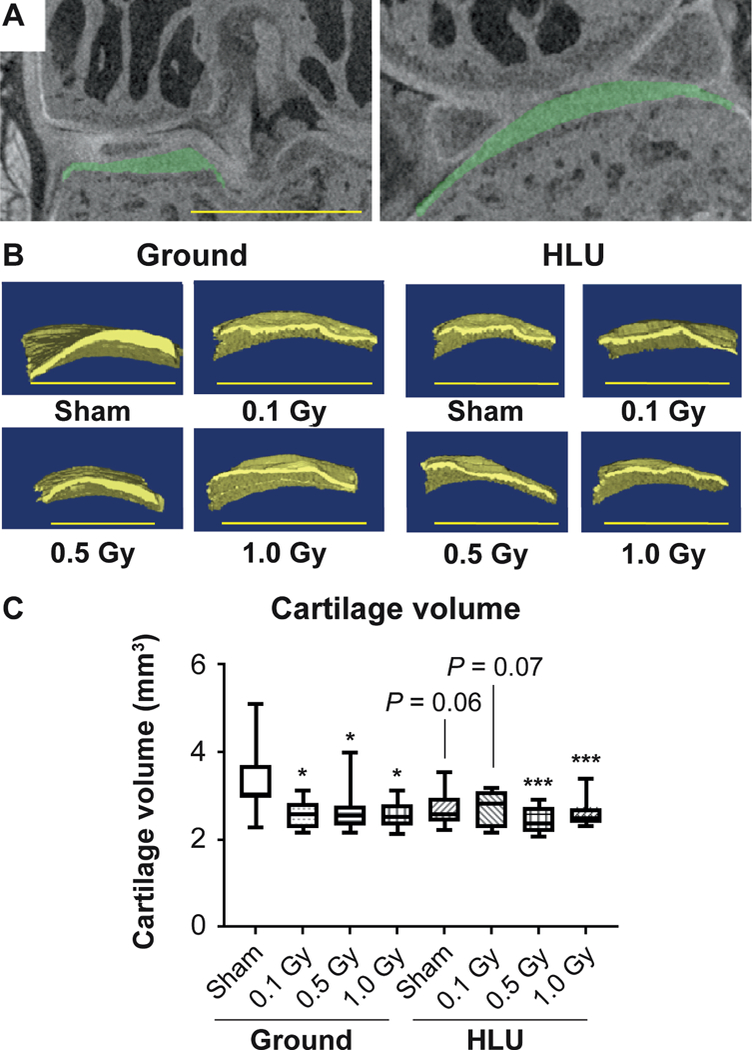
The volume of cartilage lining the medial tibial plateau is reduced compared to ground-sham after HLU and/or low-dose irradiation. Panel A: Region of interest indicating articular cartilage lining the medial tibial plateau (green). Panel B: Representative reconstructed 3D images of cartilage lining the tibial plateau used for biometric analysis. Panel C: Cartilage volume measurements in treatment groups (n = 10/group) compared using two-way ANOVA, with P values from Tukey’s post hoc analysis shown (*P < 0.05, **P < 0.01, ***P < 0.001). Scale bar = 500 μm. Other than treatments vs. ground-sham, no inter-group comparisons showed significant differences.
FIG. 2.
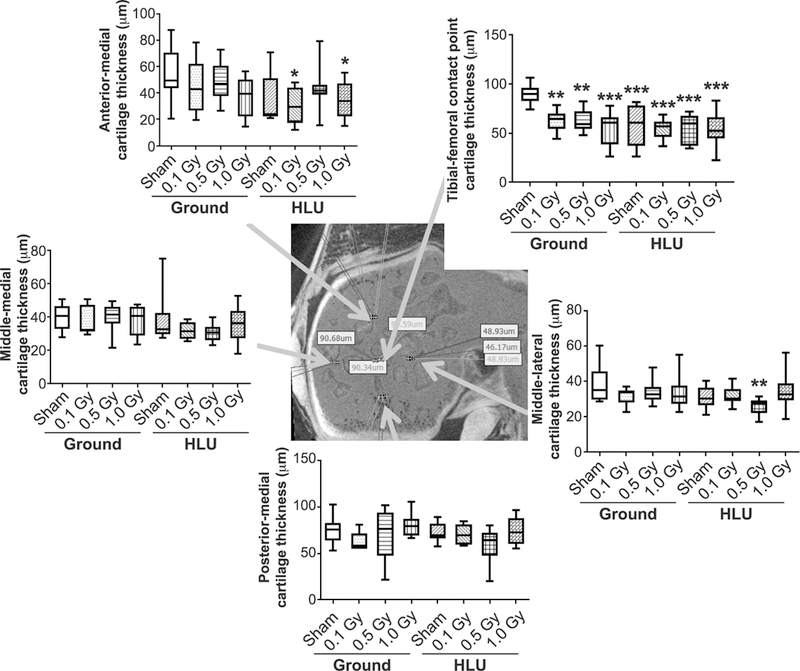
Thinning of cartilage associated with reduced weight bearing and/or low-dose irradiation vs. ground-sham is localized to the anatomic point of greatest load-bearing in the medial tibial plateau, specifically the tibial-femoral cartilage-cartilage contact point (graph, upper right; arrow indicates spatial location in contrast-enhanced microCT image of knee cartilage in cross section) compared using two-way ANOVA, with P values from Tukey’s post hoc analysis presented (*P < 0.05, **P < 0.01, ***P < 0.001). Other than treatments vs. ground-sham, no inter-group comparisons showed significant differences.
Molecular Probing and Histology Findings
Both significant radiation (F3,37 = 5.86; P = 0.002) and load (F1,37 = 6.19; P = 0.018) effects on collagen stained cartilage measured histologically were identified. Collagen area was reduced within the medial tibial plateau articular cartilage after HLU and/or irradiation at all doses relative to ground-sham (Fig. 3A). Relative to ground-sham, the collagen area was reduced in the following manner: ground-0.1 Gy (−81%; P < 0.0001); ground-0.5 Gy (−68%); ground-1.0 Gy (−47%); HLU-sham (−73%); HLU-0.1 Gy (−70%); HLU-0.5 Gy (−49%); and HLU-1.0 Gy (−71%). (Fig. 3C). A significant interaction between load and radiation (F3,37 = 9.46; P < 0.001) was identified; an interaction plot (data not shown) indicated that the greatest decrease in collagen in ground occurred at lower radiation doses, while in HLU this occurred at higher radiation doses.
FIG. 3.
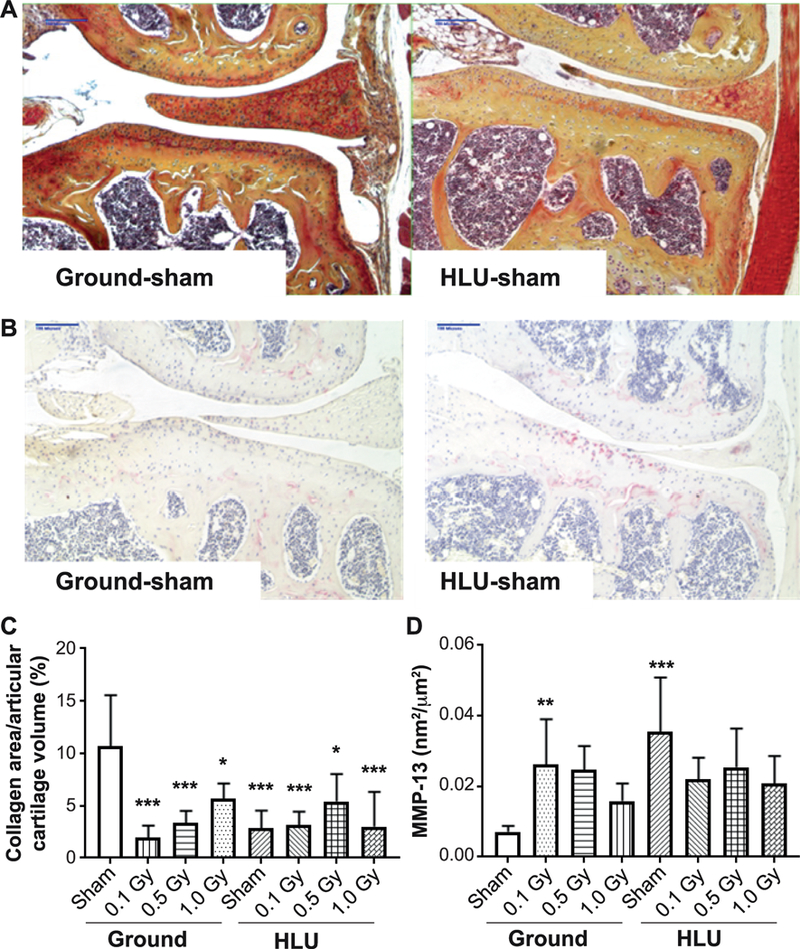
Reduced weight-bearing and/or low-dose radiation lowers collagen content (panels A and C) and increases MMP-13 presence (panels B and D) within articular cartilage vs. ground-sham. (n = 5–7 individuals per group), compared using two-way ANOVA, with P values from Tukey’s post hoc analysis presented (*P < 0.05, **P < 0.01, ***P < 0.001). Other than treatments vs. ground-sham, no inter-group comparisons showed significant differences.
A significant load effect on MMP-13 concentration within the cartilage lining the medial tibial plateau was identified (F1,44 = 7.353; P < 0.01). MMP-13 concentration was higher in HLU (P < 0.01) and ground-0.1 Gy (P < 0.05) relative to ground sham (Fig. 3B and D). OARSI scoring did not identify pronounced structural breakdown of cartilage (P > 0.05). A significant interaction between load and radiation (F3,44 = 6.393; P = 0.001) was identified; an interaction plot (data not shown) indicated that MMP-13 decreased with higher radiation dose for ground groups, but not for HLU.
A significant radiation effect (F3,69 = 20.56; P < 0.001) on circulating serum COMP (sCOMP), a biomarker for ongoing cartilage catabolism, was identified. Significantly more circulating sCOMP was observed in the irradiated groups compared to ground-sham, regardless of weight-bearing condition (Fig. 4). A significant interaction between load and radiation (F3,69 = 5.11; P = 0.003) was identified; an interaction plot (data not shown) indicated that COMP increased with higher radiation dose for ground groups, but not for HLU.
FIG. 4.
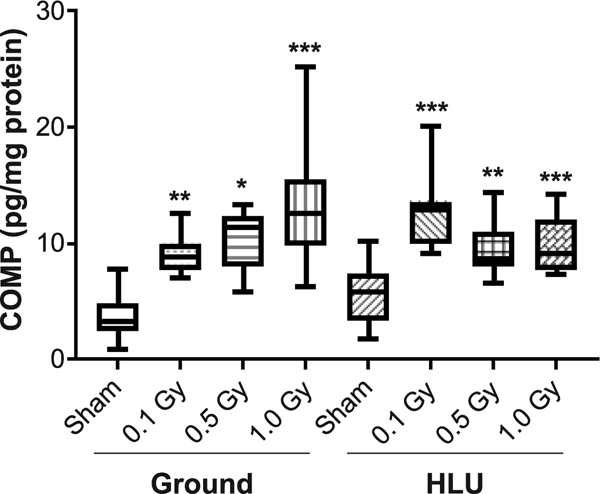
Circulating sCOMP is increased at day 25 postirradiation regardless of weight-bearing status, measured using ELISA and compared using two-way ANOVA, with P values from Tukey’s post hoc analysis shown (**P < 0.01, ***P < 0.001). Other than treatments vs. ground-sham, no inter-group comparisons showed significant differences.
Pathway Analysis
Relative quantification of proteins identified by mass spectrometry analysis was performed for each treatment group using ground-sham as control. The levels of several protein biomarkers suggestive of cartilage matrix damage (a subset of mass spectrometry analysis) were affected by HLU and radiation (Fig. 5). Aggrecan core protein, a major proteoglycan component of cartilage extracellular matrix, had a statistically significant decrease of 71% in animals receiving ground-0.1 Gy, 68% in the HLU-0.1 Gy group, and 66% in the HLU-0.5 Gy group. Hyaluronan link proteoglycan, a proteoglycan aggregate stabilizer, shared the same trend of decrease with aggrecan core protein in the ground-0.1 Gy, HLU-0.1 Gy and HLU-0.5 Gy groups (67–77%; HLU-0.1, P = 0.08 and HLU-0.5, P = 0.09). Chondroadherin, a promoter of chondrocyte attachment, had a statistically significant decrease in the ground-0.1 Gy (−68%; P = 0.05) and HLU-0.1 Gy (−66%; P = 0.057) groups and a borderline significant decrease in the HLU-0.5 Gy group (−63%; P = 0.066) and HLU-1.0 Gy (−67%; P = 0.06). The SPARC protein, which regulates cell interactions within the extracellular matrix, was significantly decreased in ground-0.1 Gy (−62%; P = 0.03), HLU-0.1 Gy (−68%; P = 0.02) and HLU-0.5 Gy (−61%; P = 0.03) groups, and a decreased trend in ground animals receiving 1.0 Gy (−51%; P = 0.09) and HLU-1.0 Gy (51–68%; P = 0.09). Lumican, a glycoprotein found in the extracellular matrix of cartilage, was statistically significantly decreased in ground-0.1 Gy (−74%; P = 0.0069), HLU-0.1 Gy (−68%; P = 0.0078), HLU-0.5 Gy (−61%; P = 0.01) and HLU-1.0 Gy (−57%; P = 0.02). Vimentin, involved with LARP6 in stabilizing type I collagen, displayed a statistically significant decrease in ground-0.1 Gy (−75%; P = 0.026) and HLU-0.5 Gy (−60%; P = 0.04) with a trend of decrease in HLU-0.1 Gy (−56%; P = 0.095) and HLU-1.0 Gy (−50%; P = 0.067).
FIG. 5.
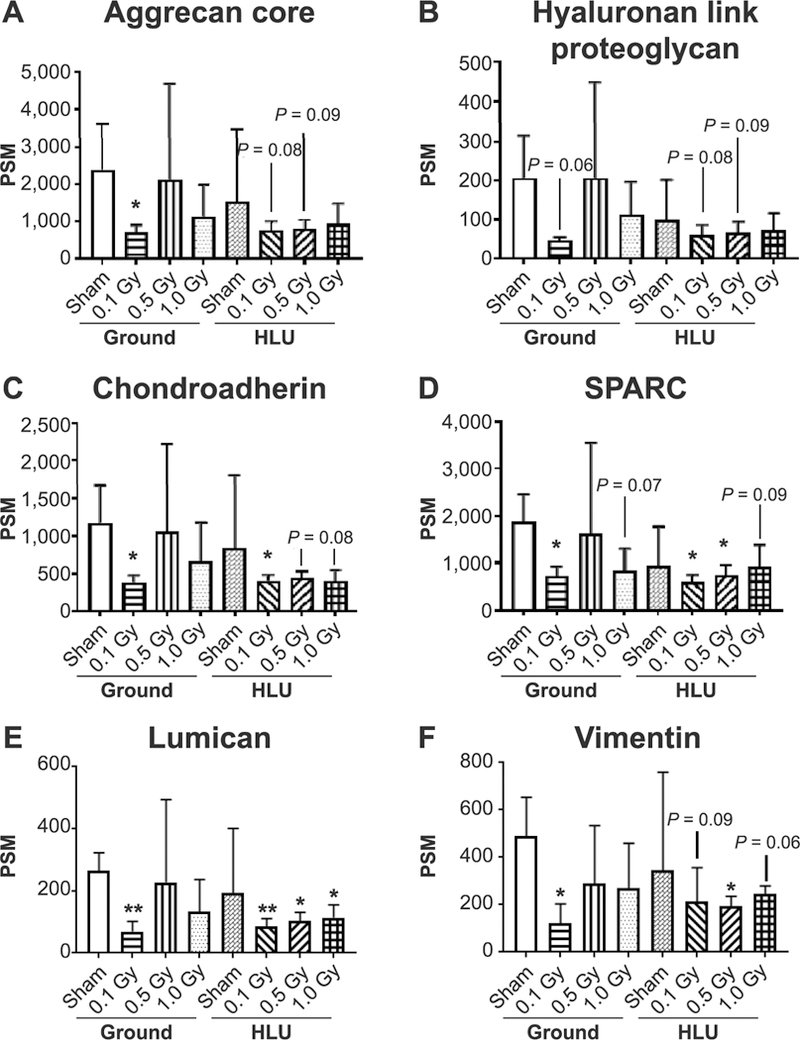
Protein biomarkers of femoral head articular cartilage matrix degradation after reduced weight bearing or low-dose irradiation. The concentration of target proteins that are known to be associated with arthritic changes were characterized a priori. Changes in these biomarkers vs. ground-sham per group are plotted as a function of peptide spectrum matches (PSM) (n = 3/pooled, *P < 0.05, **P < 0.01).
Relevant canonical pathways are shown in Fig. 6. The UPR pathway exhibited a general decreased level of proteins in animals receiving HLU-0.5 Gy (P < 0.05) and a trend of decrease for the ground-0.1 Gy group (P = 0.09). Osteoarthritis pathway showed significant decrease in protein levels for ground-0.1 Gy and HLU-sham groups. The NRF2 pathway had a significant reduction in ground-0.1 Gy (P < 0.05) animals and a trend of decrease in the HLU-sham group. mTOR pathway analysis showed a significant decrease in 0.1 Gy (P < 0.05) treated animals and a trend of decrease in HLU-sham animals (P = 0.09). Cell death and necrosis were noted with a similar trend; i.e., every group had a decrease in expression levels for CNPY2, and an increase in S100A6, particularly the HLU-irradiated groups.
FIG. 6.
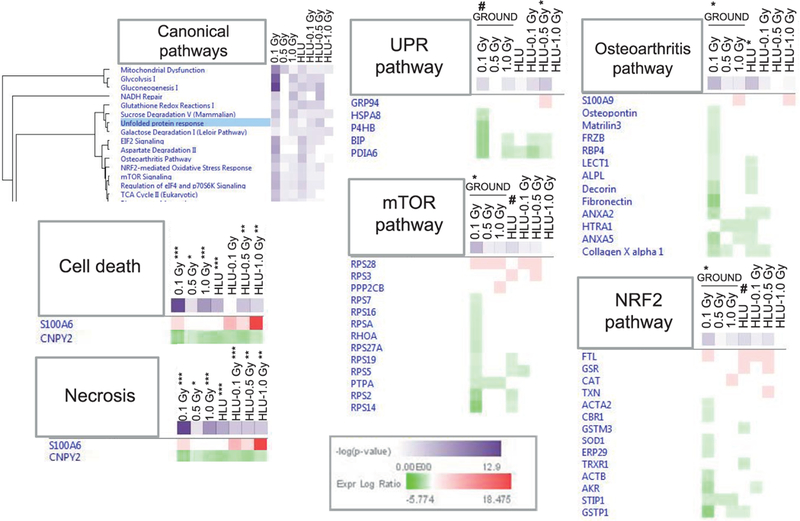
Canonical pathway analysis chosen a priori from the top three pathway clusters shows that femoral head cartilage-related pathways are affected by HLU and low-dose radiation. The green-to-red gradient represents the severity of expression log ratio from decrease to increase, respectively, vs. ground-sham (*P < 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, #P = 0.09).
DISCUSSION
The main findings of this study are that HLU and exposure to low-dose radiation causes degradation and an arthritic phenotype in the knees and hips of mice, with histologic/imaging and serum biomarker evidence suggesting reduced weight-bearing and low-dose radiation cause an early, catabolic response. Our findings support the hypothesis that reduced weight bearing represents a potential hazard for astronaut joint health during and after long-duration space flight (3–5, 12, 15–18). The cartilage degradation observed in mice in this study is consistent with previously published studies (6, 12, 14), thus further validating our model. Moreover, our results demonstrate that low-dose radiation (<1 Gy) presents an additional challenge in combination with HLU to musculoskeletal tissues (19, 20). Importantly, no additive effects or differences between any treatment groups were identified statistically; therefore, both radiation and reduced weight bearing, under these experimental conditions, represented individual challenges to joint health.
In agreement with other preclinical studies (21–25), single fraction radiation exposure resulted in cartilage degradation. It is not surprising that radiation can lead to cartilage damage, despite the misconception that cartilage is a radiation-resistant tissue because the cells are non-dividing (25). Clinically, total-body irradiation results in degeneration of the knee and hip joints due to calcification, degradation and arthritis of the knees and hips (8) coinciding with clinical pain at higher doses (~12 Gy, fractionated) (9). The catabolic effects of radiation exposure on the skeleton appear to be a primary cause of this arthritis (7, 26) with several reported studies demonstrating changes in cartilage with single fractions of ~ 1–2 Gy, both in vivo and in vitro (6, 23, 27). These irradiated chondrocytes, either in culture or in matrix, exhibit a senescent, arthritic phenotype (27, 28).
Surprisingly, the extent of cartilage degradation observed 24 days postirradiation at low doses of <1 Gy, e.g., 0.1 Gy, was evident across all ground and HLU groups. In these mice, a catabolic response was most clearly characterized by thinning of cartilage, loss of volume, and decreased collagen and generally increased levels of the collagen type II-degrading enzyme MMP-13 content measured histologically. Pathway analysis was consistent with an arthritic phenotype, characterized by reduced normal matrix protein constituents (e.g., ColXα1, aggrecan core, chondroadherin; Figs. 5 and 6) and increased S100A9 (29) at radiation doses of 0.1 and 1.0 Gy, with high variance in the 0.5 Gy irradiated group, perhaps due to limited samples and pooling. Interestingly, these responses manifest to the greatest degree within the joint at the anatomic location of greatest weight bearing, the tibial-femoral cartilage-cartilage contact point (Figs. 1 and 2). While reduced volume, increased MMP-13 presence and less collagen are disadvantageous to articular cartilage, OARSI scoring did not yet indicate erosion of the surface, although this response could manifest later due to the indolent nature of arthritis progression. In agreement with previously published studies (6), radiation induced a catabolic response in both HLU and ground animals, as indicated by elevated circulating sCOMP in radiation groups (regardless of HLU) at the 30-day end point (Fig. 4). This was not observed in the HLU-sham group despite these animals exhibiting thinned, degraded cartilage; thus damage from the HLU challenge may have occurred earlier than the 30-day post-HLU end-point. Additionally, at day 30 the following osteoarthritic responses were noted: 1. Increased S100A6 concentration with combined HLU and radiation, suggestive of elevated oxidative stress within cartilage (30–33); and 2. Decreased CPNY2 concentration with all treatments, suggestive of increased apoptosis of chondrocytes vs. necrotic death (34–36). Increased oxidative stress has been previously shown to promote apoptosis of chondrocytes and an osteoarthritic phenotype (37). Thus, we have demonstrated that cartilage is sensitive to radiation injury at low doses. While this study utilized acute exposure to low-linear energy transfer (LET) X rays versus the primarily charged particle, high-energy and charge (HZE) radiation characteristic of the spaceflight environment, this preliminary evidence suggests that radiation present in the spaceflight environment may negatively affect the joint health of astronauts during periods of reduced weight bearing.
As partial weight bearing has been shown to cause cartilage loss in the clinic (3), and given that low-dose radiation appears to cause cartilage damage, low-dose radiation during prolonged spaceflight could contribute to accelerated arthritis induction in astronauts. Furthermore, knee articular cartilage that has undergone degradation does not fully recover after return to full weight-bearing and normal ambulation (38, 39). A similar lack of complete recovery occurs in rodents exposed to radiation during HLU (6). Thus, reduced weight bearing and low-dose radiation simulating a spaceflight environment, both individually and combined, elevates the risk of joint damage in mice, and should be further studied.
LIMITATIONS
This study is not without limitations. Limited sample size necessitated pooling of samples for certain analyses. As samples were pooled across individuals and then statistics were performed on n = 3 pooled samples per group, we refrained from interpreting the radiation dose effects in terms of altered osteoarthritic pathways; however, it should be noted that these observations exist. Also, our brief follow-up period of 30 days (time to euthanasia) may not allow for evaluation of late-appearing degenerative changes. Future studies will be performed to determine if long-term follow-up is required. Moreover, the radiation applied here was low-LET X rays, rather than HZE or proton exposures characteristic of spaceflight scenarios, and administered by acute versus protracted exposure. Since our analysis detected that low dose, low- LET X-ray irradiation induces cartilage damage, future investigations should apply mixed beam charged particle radiation (or protons) that more accurately represent the spaceflight environment.
CONCLUSION
Joint health appears to be affected not only by unloading but also by spaceflight-simulated doses of radiation. The effects of these challenges to joint health require further study to elucidate the long-term consequences that may occur after long-duration spaceflight.
ACKNOWLEDGMENTS
This research was supported by the National Aeronautics and Space Administration (NASA grant no. NNX15AB50G to JSW). Additionally, this work was supported in part by the National Institutes of Health (NIH grant nos. U19 AI67798 and T35 OD010946) and a National Cancer Institute (NCI) Cancer Center Support Grant (P30 CA012197) to the Comprehensive Cancer Center of Wake Forest Baptist Medical Center, as well as the North Carolina Biotechnology Center (no. 2015-IDG-1006). We also acknowledge the support of the Comparative Pathology Laboratory Shared Resources and the Proteomics and Metabolomics Shared Resources, which is partly supported by Comprehensive Cancer Center of Wake Forest Baptist Medical Center (NCI Cancer Center Support Grant No. P30CA012197).
REFERENCES
- 1.Bader DL, Salter DM, Chowdhury TT. Biomechanical influence of cartilage homeostasis in health and disease. Arthritis 2011; 2011:979032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yokota H, Leong DJ, Sun HB. Mechanical loading: bone remodeling and cartilage maintenance. Curr Osteoporos Rep 2011; 9:237–42. [DOI] [PubMed] [Google Scholar]
- 3.Hinterwimmer S, Krammer M, Krotz M, Glaser C, Baumgart R, Reiser M, et al. Cartilage atrophy in the knees of patients after seven weeks of partial load bearing. Arthritis Rheum 2004; 50:2516–20. [DOI] [PubMed] [Google Scholar]
- 4.Liphardt AM, Mundermann A, Koo S, Backer N, Andriacchi TP, Zange J, et al. Vibration training intervention to maintain cartilage thickness and serum concentrations of cartilage oligometric matrix protein (COMP) during immobilization. Osteoarthritis Cartilage 2009; 17:1598–603. [DOI] [PubMed] [Google Scholar]
- 5.Souza RB, Baum T, Wu S, Feeley BT, Kadel N, Li X, et al. Effects of unloading on knee articular cartilage T1rho and T2 magnetic resonance imaging relaxation times: a case series. J Orthop Sports Phys Ther 2012; 42:511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willey JS, Kwok AT, Moore JE, Payne V, Lindburg CA, Balk SA, et al. Spaceflight-relevant challenges of radiation and/or reduced weight bearing cause arthritic responses in knee articular cartilage. Radiat Res 2016; 186:333–44. [DOI] [PubMed] [Google Scholar]
- 7.Armenian SH, Sun CL, Kawashima T, Arora M, Leisenring W, Sklar CA, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood 2011; 118:1413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collis CH, Dieppe PA, Bullimore JA. Radiation-induced chon-drocalcinosis of the knee articular cartilage. Clin Radiol 1988; 39:450–1. [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki O, Nishimura G, Okamoto R, Masaki H, Kumagai M, Shioda Y, et al. Induction of systemic bone changes by preconditioning total body irradiation for bone marrow transplantation. Pediatr Radiol 2009; 39:23–9. [DOI] [PubMed] [Google Scholar]
- 10.Rueegg CS, Gianinazzi ME, Rischewski J, Beck Popovic M, von der Weid NX, Michel G, et al. Health-related quality of life in survivors of childhood cancer: the role of chronic health problems. J Cancer Surviv 2013; 7:511–22. [DOI] [PubMed] [Google Scholar]
- 11.Provenzano PP, Martinez DA, Grindeland RE, Dwyer KW, Turner J, Vailas AC, et al. Hindlimb unloading alters ligament healing. J Appl Physiol 2003; 94:314–24. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald J Cartilage breakdown in microgravity-a problem for long-term spaceflight? NPJ Regen Med 2017; 2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol (1985) 2002; 92:1367–77. [DOI] [PubMed] [Google Scholar]
- 14.Luan HQ, Sun LW, Huang YF, Wu XT, Niu H, Liu H, et al. Use of micro-computed tomography to evaluate the effects of exercise on preventing the degeneration of articular cartilage in tail-suspended rats. Life Sci Space Res (Amst) 2015; 6:15–20. [DOI] [PubMed] [Google Scholar]
- 15.Djurasovic M, Aldridge JW, Grumbles R, Rosenwasser MP, Howell D, Ratcliffe A. Knee joint immobilization decreases aggrecan gene expression in the meniscus. Am J Sport Med 1998: 26:460–6. [DOI] [PubMed] [Google Scholar]
- 16.Provenzano PP, Alejandro-Osorio AL, Grorud KW, Martinez DA, Vailas AC, Grindeland RE, et al. Systemic administration of IGF-I enhances healing in collagenous extracellular matrices: evaluation of loaded and unloaded ligaments. BMC Physiol. 2007;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun HB. Mechanical loading, cartilage degradation, and arthritis. Ann N Y Acad Sci 2010; 1211:37–50. [DOI] [PubMed] [Google Scholar]
- 18.Vanwanseele B, Eckstein F, Knecht H, Spaepen A, Stussi E. Longitudinal analysis of cartilage atrophy in the knees of patients with spinal cord injury. Arthritis Rheum 2003; 48:3377–81. [DOI] [PubMed] [Google Scholar]
- 19.Alwood JS, Kumar A, Tran LH, Wang A, Limoli CL, Globus RK. Low-dose, ionizing radiation and age-related changes in skeletal microarchitecture. J Aging Res 2012; 2012:481983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreurs AS, Shirazi-Fard Y, Shahnazari M, Alwood JS, Truong TA, Tahimic CG, et al. Dried plum diet protects from bone loss caused by ionizing radiation. Sci Rep 2016; 6:21343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hugenberg ST, Myers SL, Brandt KD. Suppression of glycosaminoglycan synthesis by articular cartilage, but not of hyaluronic acid synthesis by synovium, after exposure to radiation. Arthritis Rheum 1989; 32:468–74. [DOI] [PubMed] [Google Scholar]
- 22.Jikko A, Hiranuma H, Iwamoto M, Kato Y, Okada Y, Fuchihata H. Effects of X irradiation on metabolism of proteoglycans. Radiat Res 1996; 146:93–9. [PubMed] [Google Scholar]
- 23.Lindburg CA, Willey JS, Dean D. Effects of low dose X-ray irradiation on porcine articular cartilage explants. J Orthop Res 2013; 31:1780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto T, Iwasaki K, Sugihara H. Effects of radiation on chondrocytes in culture. Bone 1994; 15:97–100. [DOI] [PubMed] [Google Scholar]
- 25.Willey JS, Long DL, Vanderman KS, Loeser RF. Ionizing radiation causes active degradation and reduces matrix synthesis in articular cartilage. Int J Radiat Biol 2013; 89:268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchinson ID, Olson J, Lindburg CA, Payne V, Collins B, Smith TL, et al. Total-body irradiation produces late degenerative joint damage in rats. Int J Radiat Biol 2014; 90:821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saintigny Y, Cruet-Hennequart S, Hamdi DH, Chevalier F, Lefaix JL. Impact of therapeutic irradiation on healthy articular cartilage. Radiat Res 2015; 183:135–46. [DOI] [PubMed] [Google Scholar]
- 28.Hamdi DH, Chevalier F, Groetz JE, Durantel F, Thuret JY, Mann C, et al. Comparable senescence induction in three-dimensional human cartilage model by exposure to therapeutic doses of X-rays or C-ions. Int J Radiat Oncol Biol Phys 2016; 95:139–46. [DOI] [PubMed] [Google Scholar]
- 29.Cremers NAJ, van den Bosch MHJ, van Dalen S, Di Ceglie I, Ascone G, van de Loo F, et al. S100A8/A9 increases the mobilization of pro-inflammatory Ly6C(high) monocytes to the synovium during experimental osteoarthritis. Arthritis Res Ther 2017; 19:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao Y, Song JY, de Vries TJ, Fatmawati C, Parreira DB, Langenbach GE, et al. Osteoclast precursors in murine bone marrow express CD27 and are impeded in osteoclast development by CD70 on activated immune cells. Proc Natl Acad Sci USA 2013; 110:12385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8:315–7. [DOI] [PubMed] [Google Scholar]
- 32.Housman G, Havill LM, Quillen EE, Comuzzie AG, Stone AC. Assessment of DNA methylation patterns in the bone and cartilage of a nonhuman primate model of osteoarthritis. Cartilage 2018: Epub ahead of print. (doi: 10.1177/1947603518759173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghayor C, Weber FE. Epigenetic regulation of bone remodeling and its impacts in osteoporosis. Int J Mol Sci 2016; 17 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farris M, McTyre ER, Okoukoni C, Dugan G, Johnson BJ, Blackstock AW, et al. cortical thinning and structural bone changes in non-human primates after single-fraction whole-chest irradiation. Radiat Res 2018; 190:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arpornmaeklong P, Brown SE, Wang Z, Krebsbach PH. Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell-derived mesenchymal stem cells. Stem Cells Dev 2009; 18:955–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trentz OA, Arikketh D, Sentilnathan V, Hemmi S, Handschin AE, de Rosario B, et al. Surface proteins and osteoblast markers: characterization of human adipose tissue-derived osteogenic cells. Eur J Trauma Emerg Surg 2010; 36:457–63. [DOI] [PubMed] [Google Scholar]
- 37.Marini F, Cianferotti L, Brandi ML. Epigenetic mechanisms in bone biology and osteoporosis: Can they drive therapeutic choices? Int J Mol Sci 2016; 17 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akella SV, Regatte RR, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med 2001; 46:419–23. [DOI] [PubMed] [Google Scholar]
- 39.Haapala J, Lammi MJ, Inkinen R, Parkkinen JJ, Agren UM, Arokoski J, et al. Coordinated regulation of hyaluronan and aggrecan content in the articular cartilage of immobilized and exercised dogs. J Rheumatol 1996; 23:1586–93. [PubMed] [Google Scholar]


