Abstract
This roadmap outlines the role semiconductor-based materials play in understanding the complex biophysical dynamics at multiple length scales, as well as the design and implementation of next-generation electronic, optoelectronic, and mechanical devices for biointerfaces. The roadmap emphasizes the advantages of semiconductor building blocks in interfacing, monitoring, and manipulating the activity of biological components, and discusses the possibility of using active semiconductor–cell interfaces for discovering new signaling processes in the biological world.
Keywords: materials, semiconductors, biointerfaces, biophotonics, bioelectronics
Introduction
Research from the last few decades has made it apparent that in addition to biochemical cues, cellular systems also rely on at least bioelectric and biomechanical components to carry out their complex functions. Indeed, bio-integrated electronics, optoelectronics, microelectromechanical systems (MEMS) have already offered the potential for either recording or modulating the electric and mechanical signaling in biology, from organelle to whole body levels. For example, deep brain stimulators have been used for the treatment of Parkinson’s disease and tremor. Although the traditional tools have been effective in the intended patient populations, they are typically rigid and bulky, and the fundamental mechanisms by which they are able to elicit therapeutic effects at the cellular and subcellular levels still remain elusive.
Semiconductors are becoming an emerging system as biophysical tools and biomedical devices. Compared to conventional biointerface materials, semiconductors exhibit a broad spectrum of device configurations (e.g. field effect transistors (FETs), light emitting diodes) and considerably more physical processes that could be coupled to biology. In this regard, semiconductor-based biological interfaces may be better suited for altering the biochemical or biophysical signal flow in single cells or tissues for fundamental studies and eventually therapeutic benefits in patients.
Biological systems are organized hierarchically, with unique characteristics and functionalities spanning multiple length scales. This points to the importance of selecting the right organizational length scale for semiconductor-based biointerface designs. For example, if an animal behaviour study is a goal, one may implement large scale and highly flexible and stretchable device arrays for sensing and modulation. In the case of sub-cellular biophysical studies, nanoscale semiconductor-based materials and devices are particularly promising as conventional metal-based electronics has limits at this length scale. Finally, if biomolecular signaling is a target of interest, one could explore the hybrid information processing that combines synthetic biology with semiconductor-based micro- and nanoelectronics, an area that just starts to grow.
In this roadmap, we will discuss the role semiconductor-based materials play in understanding biophysical dynamics at multiple length scales. We will propose several designs for next-generation electronic, optoelectronic and mechanical devices that can couple to biology more efficiently. In addition, we will address how researchers may overcome the many challenges and limitations that current technologies are facing, such as fabricating mechanically compliant building block materials, designing readily implantable devices, and exerting fine control over three-dimensional (3D) cellular interfaces. We will also suggest the possibility of using active semiconductor–cell interfaces for discovering new signaling processes in the biological world. Finally, our roadmap will cover experimental, theoretical and computational aspects around the semiconductor–biology interfaces, where the semiconductor materials can be inorganic, organic and even biological.
Transient electronics and the future of medicine
Shuai Xu 1,2 and John A Rogers 2,3
1 Department of Dermatology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, United States of America
2 Center for Bio-Integrated Electronics, Northwestern University, Evanston, IL 60208, United States of America
3 Northwestern University, McCormick School of Engineering, Evanston, IL 60208, United States of America
Status
One of the most attractive attributes of modern electronic devices is their ability to operate in an almost perfectly reliable fashion, without physical change, almost indefinitely. By contrast, biological systems in general, and human cells in particular, undergo constant temporal evolution, via repeated cycles of growth, proliferation, division, death and resorption. Certain powerful classes of passive biomedical devices adopt similar physically transient characteristics to achieve desired function in clinical medicine; resorbable sutures and drug release vehicles based on biodegradable polymers represent prominent examples. Recent research findings dramatically expand the range of biodegradable materials from passive polymers to high performance semiconductors, conductors and dielectrics, thereby enabling the construction of sophisticated classes of solid state sensors, integrated circuits, power supply systems and radio frequency components for transient device platforms that can reproduce, even in mechanically flexible forms, the most advanced forms of function found in state-of-the-art consumer and medical electronic systems.
The resulting technologies bridge the dichotomy between the permanence and rigidity of traditional electronics and the transience and soft mechanics of living systems, thereby creating exciting new opportunities to elucidate fundamental biological processes, diagnose disease, and deliver novel and adaptive therapies. In recently reported examples of immediate clinical relevance, biodegradable electronic devices provide stable, targeted function with temporal duration matched to a time dependent biological process such as wound healing, and then dissolve at a molecular level to biocompatible, water soluble end products. This mode of operation negates the need for secondary surgical removal procedures, and minimizing the risks for infection and immunological rejection.
Although some early work explored organic materials as the foundations for partly degradable electronic devices [1–4], the field of transient electronics took its current form upon the discovery that device-grade, monocrystalline silicon will dissolve in water to benign end products, thereby immediately establishing the basis for a high performance, completely biodegradable form of semiconductor technology [5]. This advance in understanding represents a critical milestone because it aligns this emergent technology with device designs, circuit layout tools and manufacturing methods already in widespread use for the production of conventional silicon devices. Specifically, full, integrated systems built around ultrathin sheets of silicon exhibit controlled and predictable dissolution in biofluids, in a biocompatible manner as established in cellular assays and animal model studies. Broad sensing capabilities (e.g. electrical activity, pressure, temperature, pH, flow and others), wireless data transmission and power harvesting schemes, and various electronic digital and analog processes are possible. Over the past 5 years, transient electronics has evolved from an academic curiosity, focused on isolated components and fundamental research in biodegradable materials, to an emerging technology platform for integrated systems with clinical-grade function and demonstrated utility in targeted use cases with extensive evaluations in live animal models. Application examples range from neurophysiologic recording systems to cardiac and post-surgical monitors.
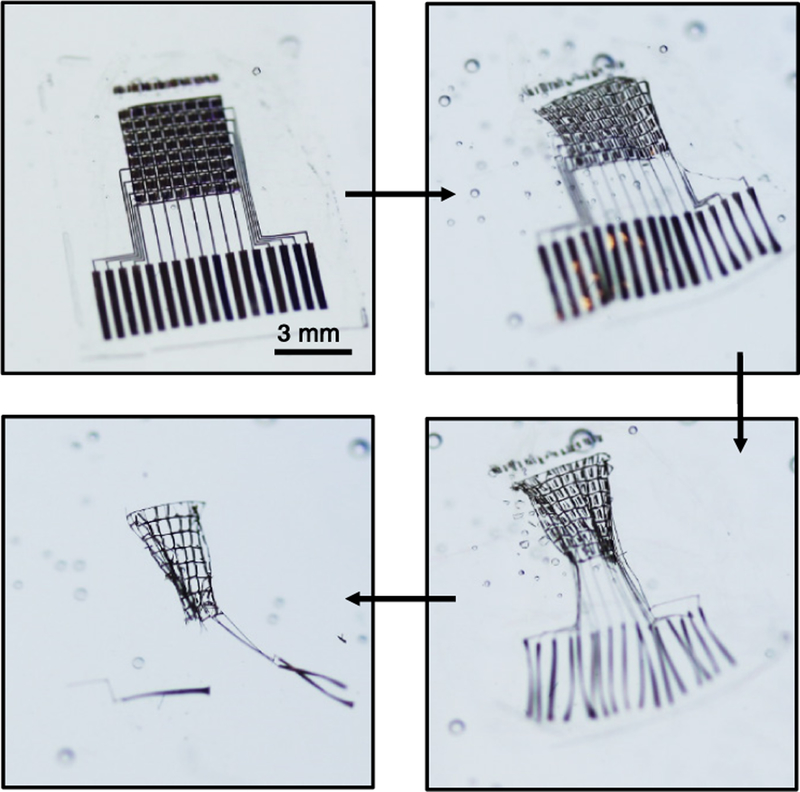
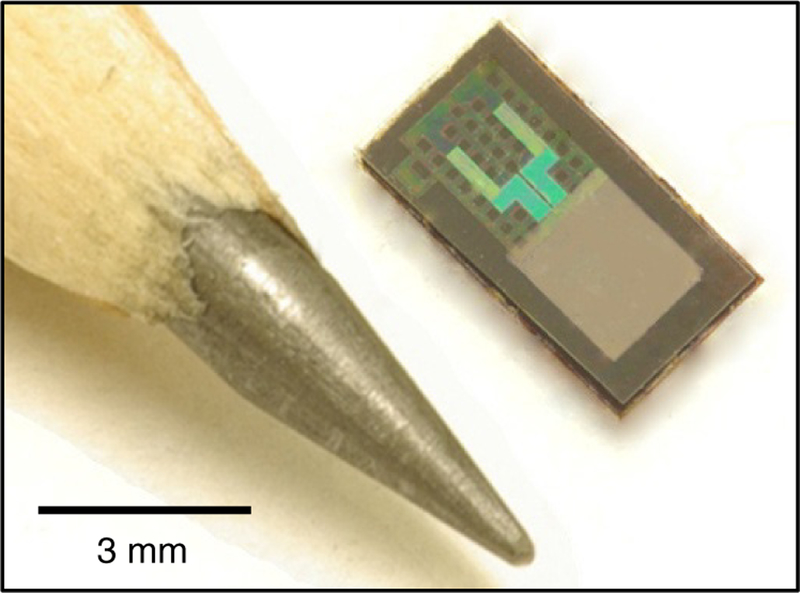
In one case, silicon transistors form the foundations of transient systems for electrocorticography (ECoG) and encephalography (EEG) (figure 1) [6]. Here, the electronics provide capabilities in multiplexed addressing and active, per-channel amplification, to enable high quality recordings in live, awake animals. The system provides reproducible and accurate measurements for up to 33 d of use with no evidence of adverse events. In other work, silicon serves as the active material in transient electromechanical systems (figure 2) based on membranes of poly(lactic-coglycolic acid) for precision intracranial pressure and temperature monitoring as an alternative to conventional, non-transient devices used in tracking recovery from severe traumatic brain injury [7]. At 8 weeks, confocal fluorescence images of the brain cortical surface failed to reveal evidence of inflammation.
Figure 1.
Transient, biodegradable electronic system designed for high-resolution electrophysiological mapping of activity on the surface of the brain, i.e. ECoG. These images show a time sequence corresponding to accelerated dissolution in an aqueous environment. Reprinted from [6] with permission from Macmillan Publishers Ltd: Nature Materials, copyright 2016.
Figure 2.
Image of a transient micro-electromechanical system designed for intracranial pressure monitoring (upper right). The pencil tip (lower left) provides a size comparison. Reprinted from [7] with permission from Macmillan Publishers Ltd: Nature, copyright 2016.
As the field evolves, additional frontiers will emerge. Further miniaturization, again leveraging many of the sophisticated processes in manufacturing that is already available in the consumer electronics industry, will enable cellular, and even sub-cellular, scale devices capable of intracellular monitoring with high specificity for selected targets (e.g. cancer tumor cells). The defining features of degradation and dissolution offer further opportunities for advanced function. Bioresorption could be configured, for example, to occur with a trigger event correlated to the responses of internal sensors, clocks or external signals. Biological activity associated with an immunological action, ligand-receptor linkage, or specific enzymatic process could provide additional avenues for highly specific electronic behaviors, whereby biodegradation might not only eliminate the device hardware over time but also transform its function in ways time-synchronized to changing biological processes. These and related systems can be designed with wireless communication capabilities, optical responsivity at various wavelengths, and physical or biochemical triggers (e.g. pressure, heat, enzymatic activity) to allow the behaviors to adapt to user requirements or biological responses.
Beyond highly specific sensing, transient electronics can perform therapeutic functions in devices such as temporary pacemakers to enhance outcomes in recovery from cardiac surgery, nerve stimulators to treat pain or promote neuro-regeneration, adaptive vehicles to allow programmable drug release and many others. Further work can be directed towards seamlessly integrating sensing and therapy. Through triggered dissolution, the biodegradable electronic device could be transformed by transience to elute dissolution products that perform useful functions either through chemical means or as a drug depot. Dissolution or degradation could, for instance, promote healing via a modulation in the pH of the local environment or a release of heat or light. Similarly, degradation byproducts could stimulate (e.g. fight infection, surveil cancer) or suppress (e.g. prevent fibrosis, retard rejection) immune function depending on the desired effect.
With additional advances in materials science, transient electronics for medicine could form an entirely new class of drug-device technology. By analogy, one of the most sophisticated technologies used in clinical medicine today is the larvae of Phaenicia sericata (green bottle fly). These FDA-cleared medical devices selectively sense and debride only non-viable areas of a wound leaving viable tissue completely undisturbed. Transient diagnostic and therapy systems could also, similarly, sense local wound environments and selectively deliver antibiotics or therapeutic heat. Conceptually similar cardiac systems could detect arrhythmias in a spatially specific way on the myocardium and deliver targeted electrical impulses to suppress this condition over a critical risk period after a myocardial infarction. Transient devices designed to be digestible might embed sensors and signal processing algorithms that release systemic medications only at certain stomach pH conditions (e.g. proton pump inhibitor) or in the presence of abnormal bleeding (e.g. anticoagulant). The same principles have relevance to patients recovering from neurosurgical procedures, where the detection of foci of seizure activity could trigger therapeutic forms of deep brain stimulation. In these and other advanced embodiments, sensing and therapy couple seamlessly to allow adaptation to changing biological stimuli, personalized to the patient and capable of operating in highly targeted modes.
Current and future challenges
Developing device options for the safe, biocompatible generation and/or storage of electrical power represents a major area of opportunity for research in transient electronics. Traditional batteries are bulky. They contain many toxic materials and their failure modes can lead to serious injury, particularly in the context of implantable systems. Limitations in operating range and total available power via wireless harvesting might require novel solutions in antenna design and optical engineering. Mechanical, thermal and chemical harvesting of power from natural body processes associated with motions of the heart, lung or diaphragm, with thermo-regulatory responses or metabolic reactions, respectively, are also be of interest.
In these and other contexts, materials options (e.g. semiconductor, metals, polymers) determine not only the performance, but also the temporal variations in performance through the kinetics of chemical reactions associated with biodegradation. Many aspects of the fundamental chemistry, and in particular the effects of local biofluid composition, are unknown. Currently, magnesium (Mg) and zinc (Zn) represent attractive choices for conductors, partly because elemental Mg and Zn are both essential nutrients. A drawback is that their degradation occurs on timescales that are short relative to many biological processes of interest. Other metals such as tungsten (W) and molybdenum (Mo), also essential nutrients, offer comparatively slow degradation kinetics. Organic polymers are obvious choices for passive elements, such as the substrate and encapsulation layers, due to the wide range of established chemistries (e.g. poly-lactic acid, silk) that are known to degrade harmlessly into naturally occurring byproducts that do not elicit a cytotoxic response. Organic materials as semiconductors have promise in electronic and ionic interfaces to targeted tissues. By comparison, for active electronic function, inorganic semiconductor materials (e.g. silicon) offer greatly superior intrinsic performance characteristics (e.g. field effect mobility) and they leverage a deep base of knowledge and technical capabilities associated with their use in conventional electronic devices. The dissolution chemistry and bio-compatibility of silicon, germanium and silicon-germanium are known to an empirical level across various aqueous solutions at a range of temperatures and pH levels [8]. Nevertheless, these semiconductors are not well suited for light emitting devices or for efficient, thin photodetectors due to their indirect bandgaps. New materials that bypass this limitation could enable biodegradable light emitting diodes for phototherapy and optical diagnostic tools (e.g. blood oximetry).
In all cases, strategies for encapsulation are critically important because, in the most powerful design approaches, they determine the overall functional lifetime, where requirements can demand operation for a few days or a few months. The intrinsic challenge here is in developing materials that biodegrade completely over sufficiently long times but also act as perfect biofluid barriers during the operating period. Most polymers do not offer sufficiently low rates of water permeation for such purposes. New, designer hydrophobic chemistries might be necessary. Metal foils, biodegradable glasses and nanoporous silicon might represent attractive alternatives.
Independent of materials choices, broader clinical acceptance relies on large scale clinical trials demonstrating safety and efficacy in order to obtain regulatory approval. Such trials demand scalable and reliable manufacturing processes capable of producing high volumes of biodegradable sensors with consistency and stability for storage. Thus, advances in manufacturing must occur in parallel with transient electronic device design.
Recent approaches to address these challenges
In terms of power supply, schemes for wirelessly delivered power, either via radio frequency (RF) transmission or visible/infrared light illumination [5], have demonstrated promise as battery-free solutions. For RF power, open air tests illustrate feasibility in the near gigahertz regime. Frequencies in the megahertz range can deliver power to devices implanted deep within the body, obstructed by layers of skin, fat, muscle, fascia and even bone. Recent results suggest that biodegradable batteries based on Mg and Mo foils sealed in biodegradable polymer packages could offer attractive options [9]. Other opportunities include harvesting energy from mechanical motions (e.g. beating heart) using piezoelectric devices (e.g. ZnO as a biodegradable piezoelectric material) [10], near infrared illumination (e.g. Si solar cells) or natural fuels in biofluids (e.g. glucose). A particularly novel possibility is in the capture of power derived from chemical reactions associated with the degradation process itself.
Materials for encapsulation and substrate support offer some of the most significant areas for innovation. Naturally occurring materials such as starches, gelatin and hydrocarbon waxes offer excellent biocompatibility and low cost. Further optimization, in terms of water permeation and biodegradation kinetics, enabled by advanced organic synthetic techniques could be of significant value. New dielectric materials might include biologically active agents such as DNA and sugars, in which cellular signaling could modulate the degradation kinetics thereby creating measurable electrical signatures.
The broader deployment of transient electronics requires parallel innovations in manufacturing processes. The most attractive approaches will leverage, to the extent possible, tooling and facilities that form the basis of commercial CMOS technologies, including device and circuit design tools. Additional advances in heterogeneous integration will be needed to allow integration of transient CMOS devices with bioresorbable packages, interface hardware and other supporting sub-systems.
Concluding remarks
Although biodegradable electronics, as a subset of transient microsystems technologies, is still in its infancy, there now exists a critical mass of demonstrated capabilities with diverse modes of clinical utility. In all cases, the impact and adoption of the materials and devices will require parallel considerations of manufacturability, cytotoxicity testing, and regulatory science. Remaining challenges afford many associated opportunities for research and innovation. The future of the field depends on an integrated and collaborative approach that leverages expertise in molecular biology, materials science, advanced fabrication, and clinical medicine.
Acknowledgments
The authors acknowledge support from the Center for Bio-Integrated Electronics, Simpson/Querrey Institute, Northwestern University.
Semiconductor–microorganism catalytic biohybrid systems for artificial photosynthesis
Stefano Cestellos-Blanco1 and Peidong Yang1,2,3,4
1 Department of Materials Science and Engineering, University of California, Berkeley, CA 94720, United States of America
2 Department of Chemistry, University of California, Berkeley, CA 94720, United States of America
3 Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, United States of America
4 Kavli Energy Nanosciences Institute, Berkeley, CA 94720, United States of America
Status
Modern society is entirely reliant on accessible and affordable sources of energy and yet energy consumption is only expected to rise as the global population expands and standards of living improve. However, this energy is largely derived from fossil fuels whose utilization presents significant economic and societal issues. Namely, the consumption of fossil fuels produces greenhouse gases, which contribute to climate change, while the finite supply of fossil fuels is exacerbated by their loss of usefulness once consumed [11]. In order to keep up with growing energy dependence and overcome the limitations of fossil fuels we must seek new approaches to energy production that are renewable and carbon-neutral.
The most abundant source of renewable energy is the sun, as it can provide up to 105 TW of energy [11]. Accordingly, photovoltaic devices employing semi-conducting materials that convert solar power into electricity have been developed to capitalize on solar energy. While the efficiency of photovoltaic devices is improving, their potential to unseat fossil fuels as a primary source of energy is limited by inadequate electrical storage technology [12]. In addition, a majority of our existing infrastructure is geared toward the utilization of carbon fuels.
In photosynthesis nature has found a way to reliably store solar energy in chemical bonds. Initiatives aiming to mimic photosynthesis by converting H2O and CO2 to value-added multi-carbons are underway. The fixation of CO2 could increase the supply of fuels and reduce atmospheric CO2. The realization of artificial photosynthesis depends on efficient capture of solar power and the improvement of catalytic conversion of H2O and CO2 to fuels. Encouragingly, solid-state semiconductor light absorbers have achieved more efficient light capture than biological organisms [13]. However, these materials suffer from poor transduction of photoexcited electrons into carbon bonds whereas biology achieves CO2 fixation to multi-carbon targets with unparalleled specificity. Therefore, integrating light-absorbing semiconductors with CO2-reducing microorganisms would offer an avenue to create biohybrid systems that elegantly maximize the transduction of solar energy to carbon fuels.
This roadmap focuses on advances in novel biohybrid systems that combine light-absorbing, electron-donating solid-state materials with biological whole-cell catalysts to enhance artificial photosynthesis. We highlight successful studies that have implemented inorganic materials both inter- and intracellularly. Furthermore, investigations of the mechanisms surrounding the inorganic–biological interface are noted. Lastly, we discuss the advances in technology that need to be addressed in order to increase the viability of catalytic biohybrid systems.
Current and future challenges
The success of artificial photosynthesis is predicated upon efficient capture of solar energy and adequate catalytic reduction of CO2. Although solid-state materials have achieved high solar-to-energy efficiencies, they have been plagued by slow progress in the conversion of CO2 to more complex multi-carbons. The catalytic synthesis of multi-carbon products requires an electron transfer to CO2 and the subsequent formation of carbon bonds. However, CO2 anions are highly energetically unfavorable. Even if the energy barrier is overcome, albeit inefficiently, by an applied overpotential, the formation of carbon bonds is further limited by low local concentrations of reactive carbon intermediates.
Appropriately, anaerobic carbon-fixing microorganisms have evolved to solve these challenges [13]. Enzymatic active sites achieve electron transfer to CO2 while sustaining anions through electrophilic interactions. Furthermore, carbonic anhydrases establish high local CO2 concentration. Additionally, organisms employ enzymatic pathways assisted by steric hindrance and electronic stabilization to ensure reaction specificity. As progress to emulate these strategies in solid-state devices has been limited, it would be beneficial to directly combine whole-cell organisms and semiconducting light harvesters in situ.
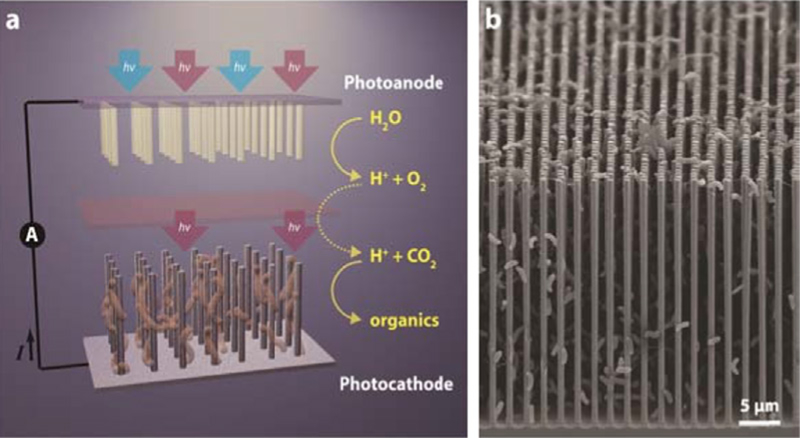
Electrotrophs are a class of microorganisms that can directly accept electrons from an electrode and integrate them into metabolic processes [14]. This allows for the use of light-capturing semiconducting electrodes to transfer electrons to electrotrophic CO2-fixing organisms. Liu et al realized a solar-to-chemical device by loading acetogenic Sporomusa ovata on a light-harvesting nanowire photoelectrochemical cell (figure 3) [15]. Si and TiO2 nanowires comprise photoactive electrodes that provide S. ovata with electrons to drive CO2 reduction. S. ovata employs the donated electrons at the Si photoanode in metabolic acetogenesis while an ion-permeable membrane separates the strict anaerobe from the water-oxidizing TiO2 nanowire electrode. The nanowire substrate is able to accommodate much higher concentrations of S. ovata than planar substrates and the high-surface-area nanowire platform allows for increased contact interfaces with bacteria. Furthermore, the nanowire electrodes create an anaerobic environment that maintains the viability of S. ovata even if oxygen-rich gas is fed into the system. Moreover, this design offers the modularity to convert CO2 into a variety of multi-carbons as S. ovata produces acetate, which can be further upgraded by genetically engineered Escherichia coli. Importantly, the reaction operates at a faradaic efficiency of 90% with 200 h of stability. Ultimately, this study demonstrated, for the first time, the capability to interface an acetogen with semiconductor photoelectrodes for solar-powered CO2 reduction.
Figure 3.
(a) Schematic of a nanowire photoelectrochemical cell loaded with S. ovata for CO2 reduction. (a) SEM image of synergistic nanowire-bacteria network [15]. Reproduced with permission. Copyright 2015 American Chemical Society.
It should be noted that microorganisms incorporate other reducing equivalents generated by electro-chemical catalysts. Exemplarily, genetically engineered Ralstonia eutropha produces isopropanol using H2 created by a water-splitting catalyst and CO2 [16]. Accordingly, a water-splitting cobalt phosphorous catalyst tandem has been reported which operates at neutral pH, limits production of toxic reactive oxygen species (ROS) and reduces the required overpotential [17].
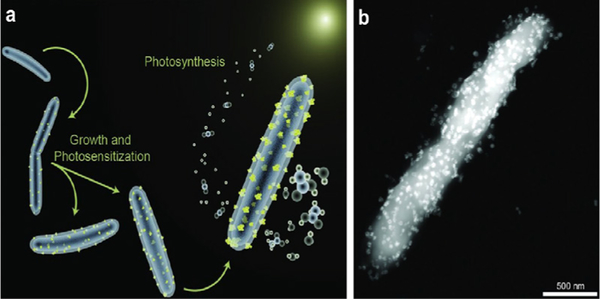
Most recently, a different semiconductor–cell junction has been reported in which semiconducting nanometric light-absorbers supply electrons intracellularly [18]. Moorella thermoacetica, a CO2-fixing acetogen, induces the precipitation of cadmium sulfide (CdS) nanoparticles within its body upon addition of Cd2+ and cysteine (figure 4). CdS has a band structure suitable for light capture and delivers photoexcited electrons to bacterial metabolic pathways that convert CO2 to acetate with high efficiency (~90% yield). The remaining cysteine acts as a hole scavenger and is oxidized to cystine during illumination. M. thermoacetica becomes self-photosensitized as light capture and CO2 fixation transpire within the microorganism. However, the fixed concentration of sacrificial cysteine limits the overall production of acetate and checks the viability of the microorganisms.
Figure 4.
(a) Schematic of the M. thermoacetica–CdS construct including early growth stage, CdS nanoparticle precipitation (yellow) and CO2 reduction through photosynthesis. (b) STEM image of M. thermoacetica–CdS hybrid [18]. Reproduced with permission. Copyright 2016 The American Association for the Advancement of Science.
In order to complete a self-sustaining cycle, cysteine needs to be replenished. As a result, Sakimoto and coworkers devised a strategy in which cystine is reduced back to cysteine by photoactive TiO2 nanocatalysts [19]. TiO2 nanoparticles are loaded with a metal phthalocyanine cocatalyst that increases their affinity toward reduction of disulfide bonds. A ‘Z-scheme’ mimic where photoreduction and photooxidation occur at two distinct light-harvesters, TiO2 nanoparticles and CdS-M. thermoacetica respectively, was actualized with cysteine/cystine serving as a molecular redox mediator. This system achieves acetate yields that exceed the theoretical limit based on the starting concentration of cysteine.
Finally, fundamental questions regarding the mechanism of electron transfer to CO2-reducing metabolic pathways were investigated in CdS-M. thermoacetica hybrids [20]. Transient absorption uncovered that photoexcited electron transfer rates increase with hydrogenase enzyme activity. Additionally, high hydrogenase activity also translates to high quantum efficiency. Hydrogenase is responsible for the generation of H2 intermediates from photoexcited electrons and its activity increases with the duration of photo-synthesis. Paradoxically, CdS-M. thermoacetica with low hydrogenase activity yielded the highest rate of CO2-reduction. This suggests that there is an alternate direct electron transfer mechanism that circumvents hydrogenase. The electron uptake pathway, either direct transduction or H2-mediated, is dependent on the time scale of photosynthesis.
Advances in science and technology to meet challenges
The realization of semiconductor–microorganism interfaces has launched research in several areas: selection and delivery of an appropriate inorganic light harvester, exploration of the synergistic effects of the inorganic–biological hybrid system, and detailed study of the fundamental mechanisms at the newly formed biotic–abiotic interfaces. Although there have been significant improvements in each of these areas, there are some practical challenges that need to be addressed.
Firstly, the ‘Z-scheme’ mimic devised by Sakimoto et al incorporating TiO2 to replenish the redox molecular shuttle generates oxygen and ROS. This restricts the production of acetate as ROS poison M. thermoacetica. Furthermore, the rate of cystine reduction decreases as O2 begins to accumulate. Fortunately, the use of a redox molecular shuttle allows for the physical separation of the oxidative and reductive photocatalysts. A selective membrane can be engineered that quells ROS while continuing to permit diffusion of CO2 and the redox molecular shuttle. Another solution calls for the design of a compartmentalized structure that enables gas purging. Moreover, TiO2 is a large bandgap semiconductor that likely diminishes the efficiency of the system. It is of interest to identify a lower bandgap photocatalyst that is also more selective toward disulfide bond reduction. The objective is to create a system that can operate without the continual addition of sacrificial hole quenchers and maintains chemical production over several bacterial generations.
The Cds-M. thermoacetica construct conceptualized the possibility of intracellular electron transfer. Although CdS nanoparticles are precipitated in vivo, cadmium is a known environmental hazard and exhibits cellular toxicity. Therefore this system can be improved by the discovery of a highly biocompatible light harvester. Its desired characteristics include high quantum efficiency, aqueous stability and a suitable bandgap. Additionally, the method of delivery of the light-harvesting nanostructures into the biological organisms needs to be explored. Phagocytosis could be exploited as the delivery method.
In order to realize viable semiconductor–cell hybrids for CO2 fixation, their solar-to-chemical conversion efficiency needs to be improved. Combinations of CO2-reducing organisms and photoelectrochemical cells achieve solar-to-chemical efficiency of 0.4%, which is an order of magnitude lower than desired [15]. This could be due to a mismatch of the organism’s turnover frequency (TOF) and the flux of photogenerated electrons. A better understanding of the organism’s TOF at different overpotential regions would inform material design and organism loading density. Further elucidations of the charge transfer mechanisms in the biological components are needed as well. A deep molecular understanding of the interactions between semiconductor, bacterium, and light will enable a guided search of microbes and enzymes to optimize the efficiency and performance of hybrid solar-to-chemical platforms. Simultaneously, these insights will inform the genetic engineering of bacteria to maximize the use of photoexcited electrons. When combined with metabolic engineering, this hybrid technology will result in adaptable, rationally-designed solar-to-chemical platform technology.
Concluding remarks
Artificial photosynthesis proposes to convert CO2 to value-added chemicals using solar energy. Opportunely, this has been made possible by advances in the understanding and engineering of catalytic biohybrid systems. The microorganisms use H2 generated by electrochemical catalysts or more advantageously directly incorporate electrons from semiconducting light harvesters into CO2-reducing metabolic pathways. This synergistic approach leverages the exceptional solar capture of semiconductors and the specificity, replication and self-healing of biology. Although there are opportunities for improvement, with steady progress, we can envision a future in which engineered inorganic materials work in cooperation with the natural world.
Acknowledgments
This work was supported by Director, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, & Biosciences Division, of the U.S. Department of Energy under Contract No. DEAC02–05CH11231, FWP No. CH030201 (Catalysis Research Program).
Optocapacitance: photostimulation without cell modification
João L Carvalho-de-Souza and Francisco Bezanilla
Department of Biochemistry and Molecular Biology, The University of Chicago, Chicago, IL 60637, United States of America
Status
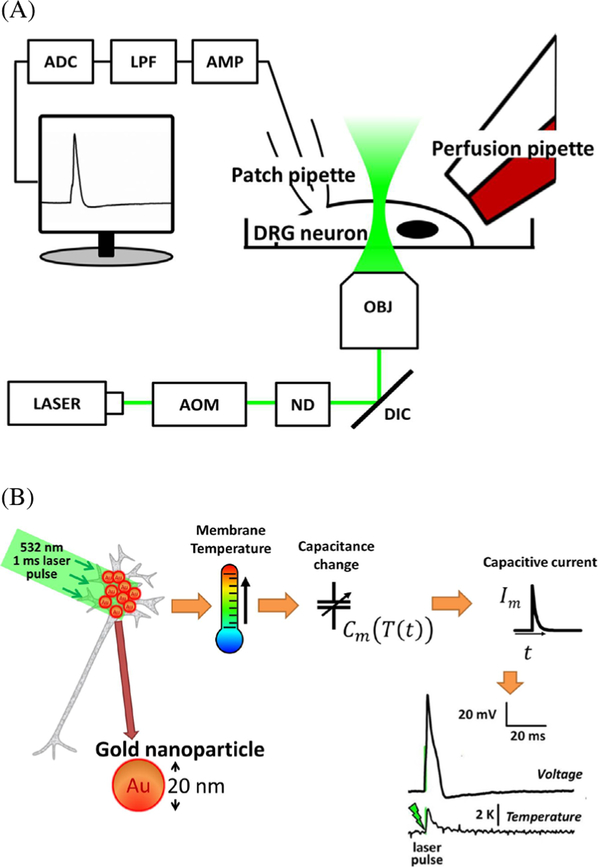
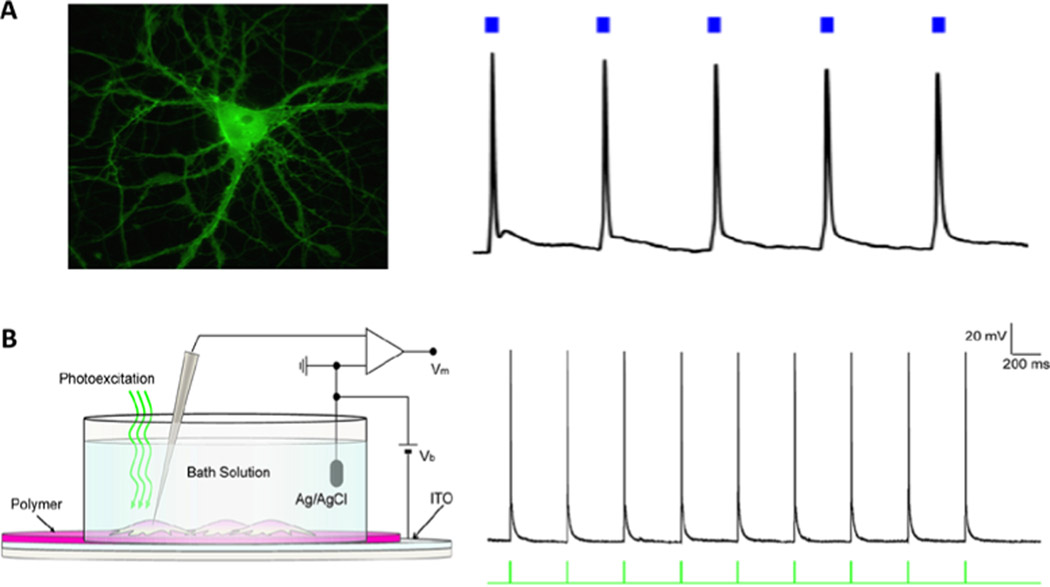
Back in the 18th century Luigi Galvani demonstrated that an electrical spark was able to twitch the muscles of dead frog’s legs. Today it is known that excitable cells such as myocytes, neurons and gland cells have in their membranes voltage-gated channels that working in concert produce stereotyped transients in membrane voltage called action potentials (APs), and most likely these molecules were unintentionally targeted during Galvani’s experiments. In nature, APs are initiated by receptor potentials that arise from many types of chemical and physical stimuli. Experimentally, one can study cell excitability by passing current through cell membranes to trigger APs. By using external electrodes to apply voltage between two points in the tissue, a fraction of the current between the electrodes penetrates the membrane, depolarizing it to the level of the threshold voltage for an AP firing. This type of stimulation produces an electric stimulus artifact that can mask part of the electric signal produced by the tissue upon excitation, in addition to the intrinsic invasiveness of placing electrodes in the tissue. Optical stimulation has been successfully accomplished by making the target cells to express light-gated ion channels from green algae. This technique, called optogenetics, a very valuable tool for neuroscience, suffers from the necessity to interfere genetically with the host cells being studied, which becomes a major problem for its applicability in humans [21]. Photostimulation of non-modified cells can be accomplished with infra-red (IR) radiation. Shapiro and collaborators discovered that IR radiation used frequently in therapeutic applications, changes the temperature of the cell membrane, increases membrane electric capacitance and generates depolarizing capacitive current proportional to the rate of change in temperature [22]. Once the mechanism was defined, the technique was named optocapacitance. As IR radiation is well absorbed by water, the heat generation is diffused, lacking spatial and temporal resolution. Localized heat generation can be achieved by using materials that can absorb light at wavelengths water does not. Gold nanoparticles (AuNPs) serve that purpose especially by their plasmonic-enhanced absorption, making it possible to photostimulate neurons with less than 100 μJ of light delivered in 1 ms (as opposed to 1 mJ with IR radiation) (figure 5(A)). Using nanoparticles as radiation absorbers, the temperature near the membrane changes by less than 2 K, with a millisecond duration 100 mW laser pulse (figure 5(B)). Chemically functionalized AuNPs can be attached close to specific cell types by small peptides or antibodies (figure 6), producing stably photosensitive cells for several tens of minutes [23]. Amorphous silicon-based mesoporous materials, 1–2 μm in size also work for optocapacitance [24]. For further applications and deep stimulation, length-to-diameter ratio-tuned gold nanorods (AuNRs) with peak plasmonic absorption in near-IR (NIR) range are also successfully used. Currently, many independent research laboratories have being successful in using this technique to study cultured neuronal cell excitability in all optic setups [25–29]. Antibody-functionalized AuNP or AuNR have been shown successful in providing photosensitivity to cells and a fluorescence signal proportional to the intracellular Ca2+ concentration in most of the cases serves an indirect readout of the cell electrical activity. In isolated tissue such as brain slices, we have been able to study excitability in all optical setups as well. In this particular case we use as cell electric activity readout the fluorescence signal from indocyanine green, a dye that absorbs in the near-IR range whose fluorescence is linearly dependent on membrane voltage [30].
Figure 5.
(A) Experimental setup for using optocapacitance technique to stimulate single cells. (B) General optocapacitive mechanism whereby the rate of capacitance change determines the amount of depolarization (Reprinted from [23], copyright 2015, with permission from Elsevier).
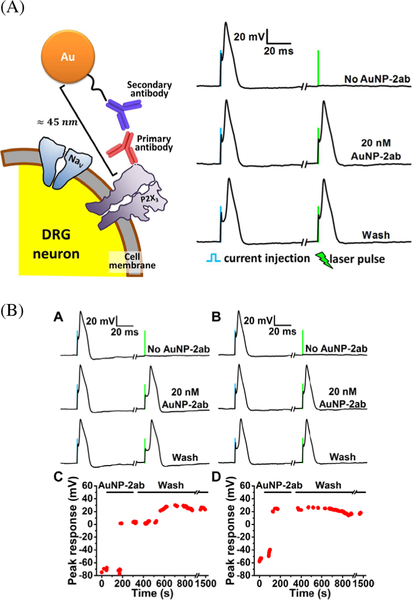
Figure 6.
(A) left, AuNP bound to a DRG neuron to stably make the cells photosensitive for optocapacitance stimulation; right, representative traces recorded from DRG neurons labelled with primary antibodies against P2X3 receptors before and after AuNPs attachments using secondary antibodies. (B) Representative traces and peak responses over a period of time from DRG neurons from neurons labeled with primary antibodies against TRPV1 (A and C) and P2X3 (B and D) receptors respectively. In both cases DRG neurons labelled with AuNPs functionalized with secondary antibodies become stably photosensitive. Reprinted from [23], copyright 2015, with permission from Elsevier.
Current and future challenges
Currently, optocapacitance has been successfully used as a tool to photostimulate cells in culture dishes in many studies, taking advantage that a certain cell phenotype can be selected by choosing the right antibody that functionalizes the nanoparticles. One of the next challenges for the kind of approach described above is the total energy required to produce enough optocapacitive current and trigger an AP. Because of the capacitive nature of the currents (I) generated by light at a cell membrane decorated with nanoparticles (I ~ V dC/dt), the rate of temperature change—and not the temperature itself—is crucial. If dC/dt is small, the amplitude of the optocapacitive current is low and one can only achieve weaker depolarization with light. Therefore the variable to control is the speed of the onset laser power. We are currently tackling this problem by using an acousto-optic modulator, which raises the power in the microsecond or even nanosecond range. Diode lasers are intrinsically faster in raising their output power and lasers that reach their maximum power from 10–90% in less than a microsecond should be adequate. For tests in isolated tissues the same rule described above applies. For this latter case the elaboration and optimization procedures to label specific cells in the tissue is yet to be determined since diffusion, especially through the layer of dead cells produced by slicing the original tissue, plays an important role. Lastly, what this technique really promises, is to contribute greatly to the scientific knowledge of neural tissue function, as it is a reliable and precise wireless neural stimulation in vivo. To that end several features have to be developed as described next.
Advances in science and technology to meet challenges
An obvious match for in vivo application of optocapacitance stimulation comes with AuNRs and near-IR laser pulses. In the optical window, as it is also known the range of wavelengths from 700 to 1300 nm, living tissue allows a penetration depth of 2 cm. Within this range, 785 nm laser diodes work well with 3.8 ratio gold AuNRs for deep stimulation. Next, the targets in the membrane of specific cells should be selected. The choosing process should consider the binding, achieved with a small peptide or an antibody that would not affect the biological response the optocapacitance technique is aimed to activate. For instance, in the single cells optocapacitance studies, we have chosen the membrane receptors TRPV1 and P2×3 since they do not seem to interfere with the APs we were triggering with optocapacitance stimulation. On the matter of the biological system to be used in these experiments, one should consider the following: (i) the readout kind of response, being electrical, behavioral or metabolic; (ii) the depth of the cells relative to the body’s surface, to be targeted by a laser beam coming from outside the animal’s body; (iii) the promptness of the system to respond to the stimulus. On the side of the light source, the laser should be able to: (i) cover with the proper irradiance, an area larger than the targeted cells in order to compensate for the lack of precision when illuminating the organ serving as target tissue; (ii) be powerful enough to produce the desired radiation; (iii) to turn on and off fast enough enabling close to microsecond pulse duration in order to allow for an optocapacitance stimulation at low energy. All these parameters are already quite optimized for studies in isolated cells.
Concluding remarks
Optocapacitance has the great potential to become an alternative technique to stimulate cells by using light pulses delivered remotely, instead of electric pulses delivered from touching or implanted electrodes.
In many cases the well-established techniques fail to deliver stimulation to excitable cells such as neurons or myocytes. Problems may include failure due to species-specific problems in transfecting/infecting and ultimately with the channelrhodopsin-2 (or its analogs) expression by the target cells. The wavelength able to deliver the energy to stimulate cells is also a limiting factor for optogenetics and optopharmacology. With optocapacitance, many already commercially available materials such as silicon-based materials and also AuNR absorb at the tissue window wavelengths, from 700 to 1300 nm, better known as near-IR radiation. Lastly, the promptness of optocapacitance technique, with what one can make a cell/tissue photosensitive without previous prepping the sample/animal, opens the possibility for new experimental designs in virtually whatever cell or tissue from whatever species, never possible before. Therefore, optocapacitance arises as a novel general technique to make cells photosensitive.
Acknowledgments
We thank David R Pepperberg and Bozhi Tian for collaborating with us in developing this technique. This work was supported by NIH (R01-GM030376), ASFOR (FA9550-14-1-0175) and NIH (R21-EY023430).
Roadmap of polymer bioelectronics–cell interface
Jia Liu and Zhenan Bao
Department of Chemical Engineering, Stanford University, Stanford, CA 94305, United States of America
Status
The development of high-performance bioelectronics could enable high-speed and precise interrogation, control and ultimately the enhancement of the biological systems, which is essential to the study of fundamental biology, development of biomedical devices and enhancement of human performance through human–electronics interface [31, 32]. Specifically, designs enabling the seamless, noninvasive, biocompatible and chronically stable electronic interfaces with biological systems are important. Device implementations at the single cellular or subcellular level have been particularly pursued for brain interfaces, precision medicine and tissue engineering applications. While high-performance micro- and nano-scale inorganic materials have made a great initial progress for such cellular interface, the organic, especially the polymer electronic materials have shown their unique promises and advantages in this area. For examples, flexible polymers that can support the sparsely distributed, nanoscale, rigid electronic components have been used as the substrate of bioelectronics to reduce the mechanical mismatches between the soft cellular systems enabling a conformal surface three-dimensional (3D) integration and chronically stable implantation; conductive polymers that possess mixed ionic and electronic conductivities have been used as the coating to reduce the impedance at the electronics–cell interface for enhanced electrical signal collection and stimulation; photoactive polymers that can convert the optical illuminations to electrochemical signals have been used for enabling a non-genetic optical stimulation [32]. In addition, after half-century development, the performance of polymer electronic materials have been significantly enhanced such that polymer conductors can achieve metallic transport behavior and polymer semiconductors can reach the charge-carrier mobility that is comparable to that of poly-Si [32], which paved the way for building high-performance bioelectronics by using polymers, while with additional attributes, such as mechanical flexibility, stretchability, self-healing, stimulus responsive, self-adaptive and biodegrdability.
Current and future challenges
The cellular systems are primarily formed and regulated by organic biomolecules and biopolymers, featuring low modulus, stretchability, self-healing, degradability and involving a distinct biological signal system controlled by the macromolecular interaction and ionic transport. Synthetic polymers can have the similar physicochemical properties through rational and diverse molecular designs. Given this advantage, we envision the polymer electronics, through the incorporation of biomimetic functional units into the existing electronic components, will play an increasingly important role in building the future bioelectronics. Previous studies mainly focused on the development of biomimetic polymer substrate for the cell interface. For the next generation bioelectronics, substrate materials that traditionally occupy >90% volume of the devices will be significantly reduced. This change is desirable because future bioelectronics should be portable, highly integrated and ultralightweight, and with cellular-size to reduce the acute and chronic impact when interfaced with the cellular components. In this regard, we need to enable the biomimetic functions, such as stretchability, self-healing, biodegradability and biomimetic signal transduction, in the active device components including conductive and semiconductive polymers. However, implementation of biomimetic functions while maintaining the overall electrical performance needed for bioelectronics has been a challenge. Here, we review some of the recent advances in the chemical synthesis and device fabrication to meet this challenge.
Advances in science and technology to meet challenges Stretchability
Stretchability is essential for enabling electronics to have an intimate interface with tissue involving 3D curved surface and dynamically moving parts. For instance, strain tolerance of 10%–80% is required for devices that are mounted on the joint, attached to the beating heart and implanted into the brain. In addition, incorporation of intrinsically stretchable elements to the electronics could further enhance its overall complexity and density by avoiding the special geometric design for the strain engineering that typically occupies a large volume. For the stretchable conductive materials, plasticizers can reduce the elastic modulus and increase the stretchability of conductive polymers such as poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS). To further improve this method, we have recently reported that using ionic liquids as the plasticizer and doping component, the PEDOT:PSS can maintain >4100 S cm−1 under 100% strain, and >3600 S cm−1 after 1000 cycles to 100% strain [33]. This superior conductivity and stretchability make it as an ideal candidate for building interconnect as well as contacting electrodes for single-cell recording. For the semiconducting materials, while some conjugated semiconductors can be stretched to 100% strain, their performance such as charge-carrier mobility can only maintain around 10−2 cm2 V−1 s−1. Recently, blending the conjugated semiconductors with elastomer showed the promise to significantly enhance the transistor’s stretchability and maintain its mobility >1 cm2 V−1 s−1 even under 100% strain [34]. Notably, using the semiconductor as the field-effect transistors for the bioelectronics requires low operation voltage, a challenge for the polymer semiconductor. Therefore, stretchable dielectrics with high dielectric constant have been introduced to lower the operation voltage [35].
Self-healing
Incorporation of self-healing, a distinct character in biological systems, into bioelectronics would dramatically enhance the durability and robustness of electronics in a dynamic and usually chronically implanted environment. Additionally, self-healing property can also allow the electronics to establish constant and seamless integration with the motile tissues and cells. Self-healing can be readily achieved by incorporating dynamic bonds into polymers, such as hydrogen bonds, electrostatic interactions, and metal–ligand bonds and applied to a variety of conductive and dielectric materials. For the semiconducting materials, incorporation of dynamic bonds such as 2,6-pyridine dicarboxamide into the backbone of the 3,6-di(thiophen-2-yl)-2,5-dihydropyrrolo[3,4-c] pyrrole-1,4-dione semiconductor polymer shows the successful reduction of elastic modulus, enhanced stretchability and healability facilitated by the heating and solvent treatment, while the device could maintain high mobility >1 cm2 V−1 s−1 over 100% strain and after the healing process [36]. Future study can focus on enabling self-healing for electronic devices in the physiological environment to facilitate the recovering of intact device structure and adaptable interfaces with cells.
Biodegradability
Biodegradability is important to clinical applications. Previous research has been mainly focused on the biodegradable substrate. For the active material, blending polysaccharide with conductive and semiconducting polymers can effectively enable biodegradability in the device, yet the performance is limited. We have recently shown that introducing imine bond (–C=N–) as a stable conjugated linker into dike-topyrrolopyrrole polymer can allow the materials to be readily hydrolyzed in a catalytic amount of acid while maintaining the performance at neutral pH environment [37]. In the future, we envision the biodegradable component in polymer electronics that can be regulated by the signals from in vivo cellular system will enable an ‘on-demand’ biodegradable electronics, which could allow the integrated electronic systems to self-decompose after achieving certain function such as neural network regulation, tissue regeneration or drug release.
Biomimetic signal conversion
Using conductive polymer materials as the bio-interface can lower the impedance of microelectrodes for high-quality recording and electrical stimulation, and enhance transconductance of the electrochemical transistor for the high signal-to-noise ratio amplification by allowing ions from the biological environment to move into the transistor’s channel [38]. In addition, integration of the soft polymer electronic circuits for analogy-to-digital conversion with biological system could directly generate biomimetic signals and enable the in situ, distributed data collection, localized computation and biomimetic stimulation for neural interface [39, 40]. Furthermore, incorporation with biological responsible components, future polymer electronics are expected to be built into a closed-loop control system regulated by the cellular signals.
Concluding remarks
Ultimately, we expect polymer bioelectronics not only to function as a seamless, bidirectional interface to connect and merge the high-performance inorganic machine with biological systems but also to act as artificial components to enhance and introduce new functionality to the biological systems including human-being.
Acknowledgments
We acknowledge the support from Stanford Bio-X Interdisciplinary Initiatives Seed Grant.
Engineering cell access
Martin Hjort, Yuhong Cao and Nicholas Melosh
Materials Science and Engineering, Stanford University, Stanford, CA 94305, United States of America
Status
Manipulation of biological cells has become the forefront of medicine, with striking results in cancer treatment, regenerative medicine, and gene editing. The advent of CRISPR/CAS9 gene editing tools, transformation of autologous cells into new cells types, and training immune cells to recognize cancer are starting to address key issues in biology and health sciences [41, 42]. However, delivery of the exogenous materials into the cells to induce these behaviors, whether proteins, mRNA, or DNA, remains a key limitation in a field were billions of dollars have been invested.
Historically, intracellular delivery centered around DNA cargo delivery into cell lines, generally using cationic delivery vehicles (TAT, lipofectamine), electroporation, or viral transfection (adenovirous, lentivirus). With the rapidly increasing importance and availability of primary cells derived from pluripotent stem cells or patient-derived T-cells, researchers discovered these techniques are no longer sufficient. In particular, the safety of viral-based delivery for humans is still a major roadblock for new therapies [43].
Semiconductor and engineering technology provides an alternative toolset to approach this problem. These mechanisms generally involve physically disrupting the lipid membrane, allowing chemicals in the surrounding media to enter the cell [44]. The lipid membrane is a robust, quasi-2D fluid that easily bends, but is difficult to tear (>5 mN m−1 tension). The principle challenge is to create holes through the membrane that are not too large, which will kill the cell, and not too small, which will be ineffective. Secondarily, physical transport of the cargo through the hole while it is still open is a non-trivial problem, especially for large DNA/RNA molecules or proteins. This method must be scalable to >106–108 cells, not perturb normal cell function, and able to deliver a variety of cargoes. Non-biochemical approaches avoid much of the sophisticated cellular defense mechanisms, thus may be more effective.
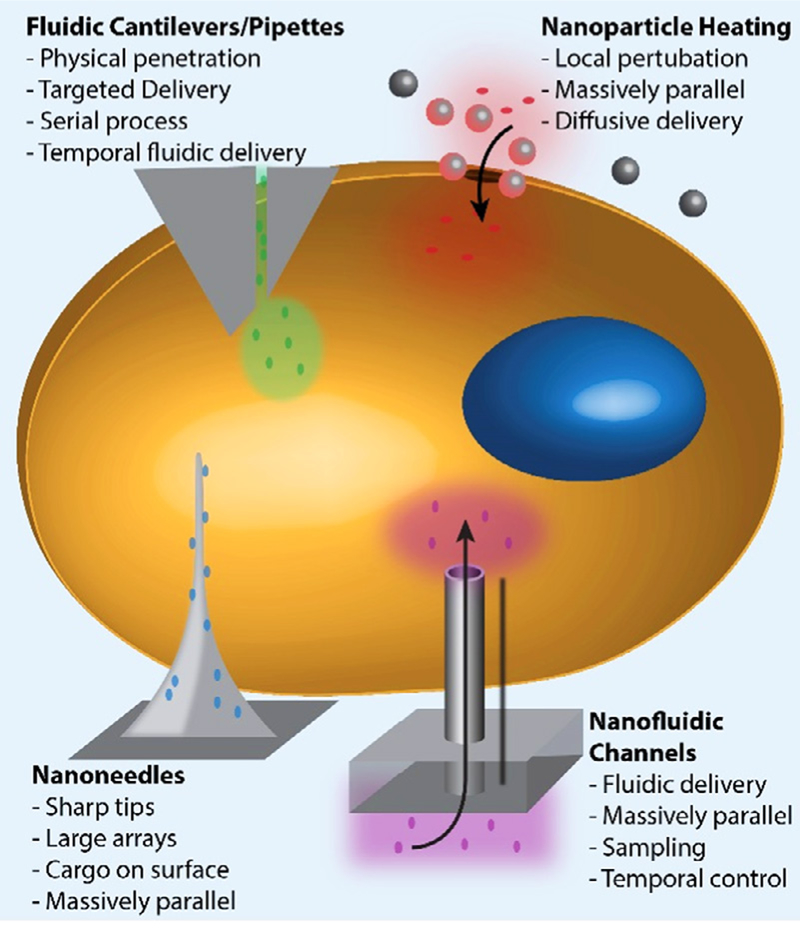
Engineered approaches to cell access have focused on nanoscale materials or devices to provide a highly localized membrane rupture (figure 9). These include penetration with sharp nanowires [45], magnetic nanoparticles [46], thermal disruption [47], microchannel shearing [48], AFM tips [49], or delivery through nanochannels [50, 51]. These rely on mechanical or thermal methods to induce local stress on the membrane, causing a small pore to form. These inorganic approaches are becoming increasing sophisticated, with recent demonstrations of delivery and even single-cell sampling [52] in a wide variety of cell types.
Figure 9.
Engineered devices for cellular delivery.
Current and future challenges
There are still numerous challenges to overcome to make engineered delivery systems fully effective. Current methods can load the cytoplasm with DNA or other cargo, yet effective expression and cell-to-cell variability are still significant obstacles. Most involve one-step injections that introduce an unknown amount of DNA into the cell, with little influence after delivery. The process by which cytoplasmic DNA migrates into the nucleus is still uncertain, with recent studies estimating <1% of injected DNA are expressed. Molecular shepherding mechanisms could greatly improve the efficacy even of current devices. While nuclear localization factors appear effective for peptides, an equivalent for nucleotides is still lacking.
There is also a significant distribution in how much DNA each cell receives; some cells receive a high dosage, and others very little. Increasing the cargo concentration in solution can increase the average delivery level, but DNA/RNA is cytotoxic, leading to higher rates of cell death. In addition, some processes such as CRISPR/Cas9 appear to be dosage dependent. Dosage control, ideally at the single cell level, is a critical issue that is not well addressed.
Cell ‘health’ after delivery is not well quantified at the moment. Clinical applications require a distinct cell phenotype; unhealthy or undifferentiated cells can increase the risk of teratomas and cancer. However, most delivery results assay only the presence of the desired cargo, molecular housekeeping activity, and/or surface markers as an indication of health. More sophisticated analyses are necessary to measure the distribution of actual cell phenotype and functionality. These will provide better assessment to avoid ‘high efficiency methods’, which also dramatically alter cell identity and function.
Scalability and form factor are also critical factors for widespread implementation. CAR-T and IPSC therapies generally require >108 cells, ideally transfected on the order of an hour. For clinical applications, safely producing these cells at the clinic, rather than a remote laboratory, will be essential for widespread patient use. This suggests the part of the system in contact with cells must be disposable to avoid cross-contamination, fast, and simple to operate.
Advances in science and technology to meet challenges
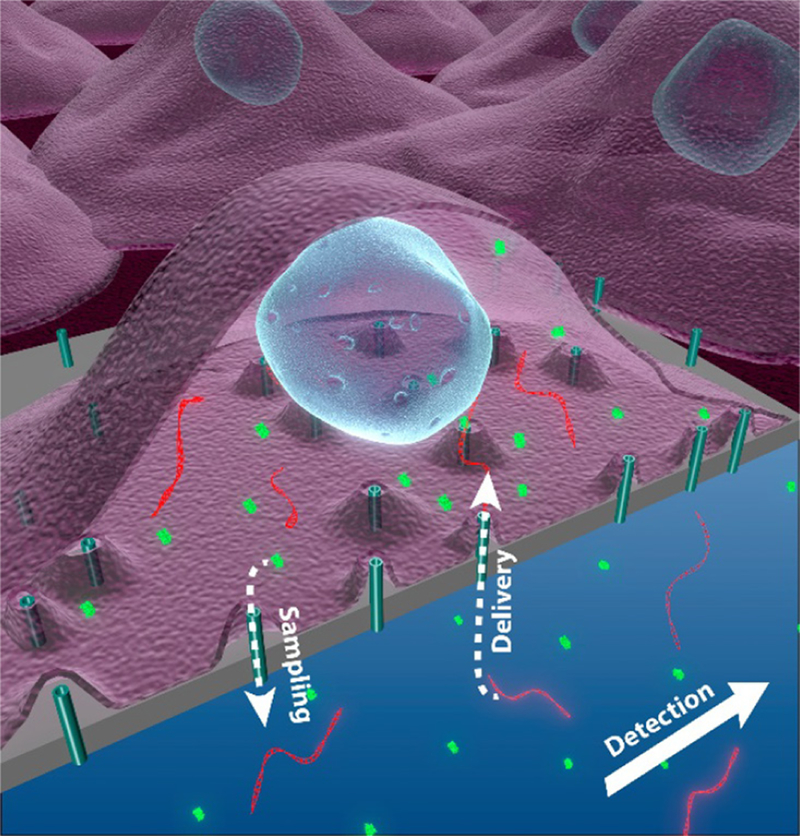
Future cellular interface systems must go beyond simple molecular delivery, and enable full two-way communication with individual cells (figure 10). This exciting vision requires dosage-controlled delivery of a wide variety of cargos into the cell, in conjunction with means of non-destructively ‘listening’ to the cell and responding appropriately. Such ‘closed-loop’ feedback would allow for temporal control of cellular differentiation, re-programming, and development. Careful observation of the cellular response to defined dosages of specific cargoes would accelerate our understanding of internal molecular pathways, and development of therapies.
Figure 10.
A vision for a two-way nano-fluidic communication system with living cells. Nanoconduits allow both delivery and sampling between the cell and the fluidic chamber.
These goals require substantial new technical developments. First, a method to reliably deliver precise quantities of materials into the cell with temporal control must be developed. Many current systems only offer one-time delivery, and need for endosomal escape reduce the temporal precision and control. Recent advances which directly deliver ‘bare’ cargo into the cytoplasm using nanofluidic platforms are promising. Multiple cargo types would provide additional benefits, for example small molecules to interact with protein cascades in the cytoplasm together with DNA for modulating protein expression.
Secondly, the platform must measure signals from the cells. These could range from electrical signals, excreted messengers, metabolites, mRNA, or other relevant parameter. The ultimate goal would be single-cell, non-destructive readout of cell phenotype and behavioral state in real-time. Since a cell may only have a few thousand copies of a given molecule, this would require analytical sensitivity beyond current single cell methods. New nanoscale sensing and detection methods are thus critical.
The need for integration of multiple modalities at the single cell size scale (~10–20 μm) make semiconductor-processing with it’s electronic and fluidic integration capabilities particularly powerful. Biological reagents, detectors, and actuators must all be connected together, with corresponding read-out and control mechanisms.
Concluding remarks
Cell access and communication is more important than ever. Substantial societal benefits are on the horizon, yet new enabling technologies to realize this potential are needed. Engineering approaches are particularly promising with their ability to localize cellular perturbation and integrate multiple different technologies on a single platform. With further innovation, these methods could supplant viral transfection and become the mainstay of cell manipulation at the research bench as well as in the clinic.
Acknowledgments
The authors acknowledge NSF STTR 1549696, and the Joint Institute for Metrology in Biology (JIMB).
Organic opto-biointerfaces
Guglielmo Lanzani1 and Fabio Benfenati2
1 Center for Nanoscience and Technology—Istituto Italiano di Tecnologia e Politecnico di Milano, Italy
2 Center for Synaptic Neuroscience and Technology—Istituto Italiano di Tecnologia, Genova, Italy
Status
By and large cells do not directly respond to light, being essentially transparent. Since light is a fundamental drive for cell metabolism and behavior, nature has developed specific strategies to make cells light sensitive by developing molecular sensor-actuators that provide a broad range of photo-induced functions from simple photoreactions (like ion transport or phototaxis) to vision. In an attempt to imitate Nature, since long ago scientists have explored possibilities to artificially induce light sensitivity in living tissues in order to control physiological functions by photons [53]. This would indeed provide a number of advantages in biotic/abiotic interfacing, such as space and time resolution, addressability, lack of cumbersome wiring, and reduced invasiveness. Applications could span from fundamental studies in cell networks, tissues or even organs to restoring lost functions, notably vision. Light sensitivity is achieved by introducing ‘probes’ in the cells, in a variety of shapes, composition and volume. Most commonly we can distinguish between three approaches, namely: (i) use genetic modification of the cells to express light-sensitive actuators (ion channels/pump or molecular switches), an approach named ‘optogenetics’ [54]; (ii) the use of extended planar interfaces between cells and suitable materials [55–58]; and (iii) the use of nanoparticles [59]. We will concentrate on the two latter strategies that do not imply genetic modification of organisms. In particular, cell/tissue interfacing employs devices, such as capacitors or transistors, on top of which living cells are grown or put in contact with. It can also regard using of planar structures, typically multilayers, that are absorbing light and transducing somehow the stimulus to the cells [55–57]. The other approach deals with the internalization of nanoparticles in living cells or tissues, and their photoexcitation through resonant excitation [59]. Here we restrict our interest to interfaces based on organic semiconductors that allow some kind of functional control, while we refrain to enter the very broad field of imaging and also drug delivery and phototherapy.
Current and future challenges
Albeit optogenetics allows controlling in a very selective and specific way transmembrane as well as intracellular events, it requires the introduction of exogenous genetic material through the use of viral vectors [54]. In addition to the problems related to the choice of the suitable viral vector (diffusion, inflammation, gene incorporation in the genome), the expression of heterologous proteins from very distant species can trigger immune reactions. Avoiding these problems would open up a much broader field of applications, notably extending it to humans. Existing non-genetic actuators are based on nanoparticles, molecules and more rarely on prosthetic implants. These suffer of a number of limitations. Nanoparticles are hardly selective and rather distributed nonspecifically in cells or tissues. They most often exploit a thermal effect that is difficult to localize and requires high incident light power. They are not stable and can be toxic either due to their composition, interaction or biological diffusion. Small molecules, such as photochromic probes or photoactive ligands, are vastly tested in vitro on model systems, and very often requires UV actinic light. This poses a limit to their exploitation in vivo, where UV may be heavily absorbed and result harmful. As a consequence these approaches have rarely been tested in vivo. All these challenges should be addressed and overcome by a new generation of opto-bio interfaces. Their action should be as specific as possible, triggering the required physiological response or function with sub-micron space resolution and sub-millisecond time resolution. The interface coupling should be as seamless as possible, and the transduction mechanism should in principle be the same as those occurring in the natural system. This a very hard challenge, as in many cases the physiological mechanisms are not fully understood in detail and because artificial devices are working on different principles. One example is electronic versus ionic signaling. Mimicking the biological mechanisms in artificial devices is a major challenge for the future. Addressability is an additional challenge. While optical excitation has a number of advantages, as stated above, the major limitation is the reduced penetration depth of the electromagnetic radiation in the optical frequency range.
Advances in science and technology to meet challenges
The first requirement is on materials, both substrates and active layers. Most often substrates are 99% of the mass of the devices, and determine the mechanical properties and thus the conformability and bio affinity of the system. Soft materials based on carbon molecules do have a special attitude for such an application, as they share the very same chemical nature of natural systems and display a similar Young’s modulus that make them compliant to the biological tissue. Yet hybrid systems or even engineered inorganic systems can play a role [57]. The latter systems are in principle less biocompatible, but do exploit an extremely well established and powerful technology that allows overcoming many limitations, such as solubility, mechanical stiffness, reduced conformation adaptability. Active layers may be integrated in the substrates or coincide with it, or can be only a thin layer in the structure. Depending on the function to be carried out, active layers can belong to very different families of materials. Delivery of the interfaces in ‘in vivo’ conditions requires a development, in order to be less invasive with respect to present day electrodes and implants. Localization at the right site is a challenge to be addressed that requires developing and integrating novel bio-recognition methods. In principle, one would like to avoid invasive surgery and be able, for instance, to inject nano-devices that spontaneously locate in the appropriate site to exert their functional effects. However, we are still far from these targets at the moment. Some of the advances in science and technology that may allow meeting these challenges in the near future deal with integration, compliance and biological tolerability, multiple functionalities, targeting, biological fate and light powering of the organic interfaces. A deep understanding of the coupling between an artificial probe with the biological entity is in demand. Controlling this interaction and the ability to predict the coupling dynamics would allow to design better systems in terms of efficiency and specificity. The effect should last a suitable time for achieving the goal. It could be a finite time range in case of drug delivery or local healing or sensing, but it can require long times for prosthesis aimed at substituting lost functions. At their end devices should be easily disposed, either removed or dissolved, without turning toxic or dangerous. Indeed, the biological tolerability seems to be very high for conjugated polymers, partly because their intrinsic resemblance to the backbones of cell macromolecules and partly because they are very conformable and can be easily coated on flexible and compliant supports, resulting in a very weak or absent foreign body reaction after implantation. Another topic to be developed is multiple functionalities. Illumination of conjugated polymers per se can trigger several processes, including capacitive charging, Faradaic currents, heat, local pH changes that can modulate cell functions. In addition, if assembled in nanoparticles, photosensitive polymers can be in principle combined with tracers for localization or drugs to improve their tolerability by the tissues, according to a theranostic strategy. The development of non-invasive light sources for excitation is also mandatory to improve transferability of these strategies to cure diseases. When targeting internal regions of the body, new concepts of powering and stimulating should be developed, that allow for instance to locally exploit the use of light, while avoiding travelling through tissue with light or with optical fibers. Delivery of local light sources is an interesting approach to be explored. This could be potentially achieved in several ways. The most straightforward manner is to engineer suitable conjugated polymers with red-to-infrared shifted absorption spectrum to allow for noninvasive illumination with external infrared sources characterized by high tissue penetration. Alternatively, the optoelectronic properties of the organic materials can be properly exploited to emit light when subjected to specific conditions, such as high temperature or low pH. Finally, tissues can be genetically engineered to express high yield luciferases that can provide endogenous illumination of various wavelengths to the biotic/abiotic interface once the appropriate luciferase substrate is provided.
Concluding remarks
The use of functional materials to make cells and tissues smarter is a very fascinating, but also demanding, endeavor. The strict contact between nanomaterials and live cells at the nanoscale can open new avenues in the physical, chemical and biological interactions at the biotic/abiotic interface. The final aims can be manifold: from readout/interrogation of cell and tissue functions, to tissue prosthetics and theranostics. Opposite to optogenetics in which cell specificity is obtained via genetic targeting exploiting the transcriptional heterogeneity of distinct cell population, the action specificity of organic optobiointerfaces is attained by specific contact with the target issue in case of 2D devices or by local injection or specific surface targeting in the case of nanoparticles. While for excitable cells the biological effects of illumination of the bio-interface occur at the plasma membrane level, a series of potential light-dependent intracellular effects of organic nanoparticles on signal transduction and/or gene transcription and translation have to be explored. This represents a very attractive perspective to investigate in the next future organic electronics in biomedicine.
Acknowledgments
We thank the EU project FP7-PEOPLE-212-ITN 316832 ‘OLIMPIA’, Telethon-Italy (grants GGP12033 and GGP14022), Fondazione Cariplo (project ONIRIS 2013–0738), Compagnia di San Paolo (project ID 4191) and Italian Ministry of Health (project RF-2013-02358313). The support of Istituto Italiano di Tecnologia (Genova, Italy), Ra.Mo. Foundation (Milano, Italy) and Rare Partners srl (Milano, Italy) is also acknowledged.
Predicting interfacial properties from first principles simulations: semiconductors in aqueous media
Giulia Galli1 and Francois Gygi2
1 Institute for Molecular Engineering, University of Chicago, Chicago, IL 60637, United States of America
2 Department of Computer Science, University of California, Davis, CA 95616, United States of America
Status
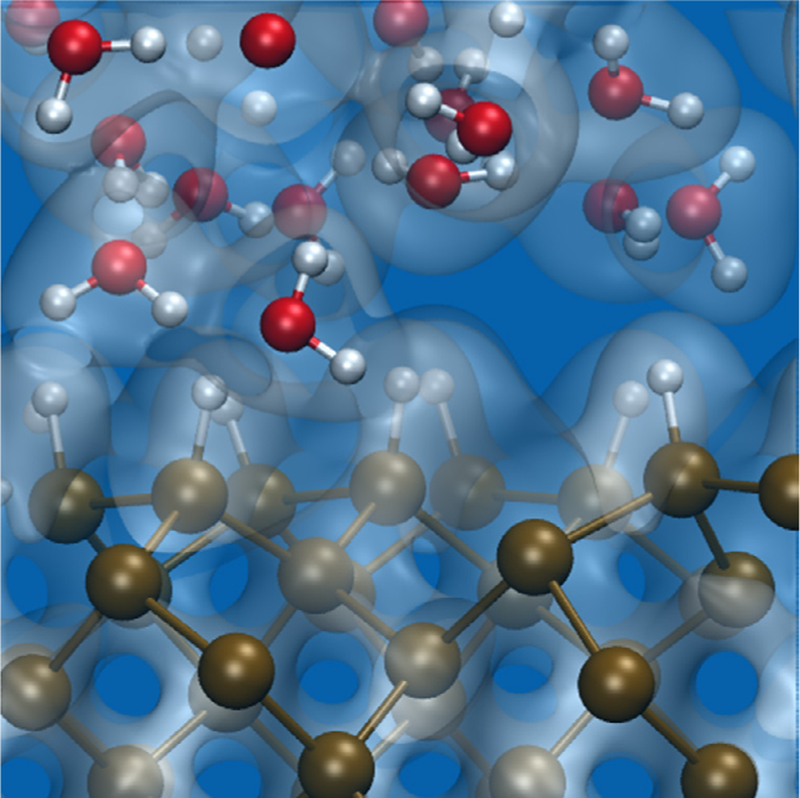
Numerous investigations during the past two decades have pointed at semiconductor-based biological interfaces as promising platforms for fundamental studies of cell functions. Hence understanding the physical and chemical properties of these interfaces, with the goal of designing and engineering their properties for target functions, represents a pressing need. We consider here the role of theory and computation, with focus on first-principles simulations of simple semiconductors in aqueous media [60]. In the last fifty years, the condensed matter physics community has developed methods to study solid/liquid interfaces and to identify, at least for simple systems, atomic-electronic structure relationships (see figures 12 and 13). The latter may in turn be used to understand complex phenomena such as electronic and mass transport across interfaces or photo-electrochemical reactions [60]. The method of choice to describe interatomic interaction for both the solid and liquid has been density functional theory (DFT) [61], at various levels of sophistication, combined, in the last twenty to thirty years with methods that originated in the quantum chemistry community [62]. About three decades ago, DFT was coupled to molecular dynamics, a simulation technique used to investigate both dynamical and thermodynamic properties of matter (ordered and ordered solids, and liquids); this coupling [63] (now known as first-principles or ab initio MD) has enabled studies of the structural and vibrational properties of water and aqueous solutions interfaced with solid surfaces, including surfaces of bio-compatible semiconductors such as Si [60] and SiC [64] and bio-compatible insulators such as titanium oxides [65]. Recently, the use of advanced electronic structure theories beyond DFT [66] has made possible accurate calculations of opto-electronic properties of interfaces and hence the interpretation of complex experiments (for example experimental photoelectron spectra of water and solutions) and the prediction of spectroscopic signatures of aqueous solutions interfaced with semiconductors. These predictions may provide key information about spectroscopic monitoring of ions or more complex species at solid interfaces. It is now conceivable to broaden the scope of first-principles simulations of semiconductor interfaces with aqueous solutions to include semiconductor-based biological interfaces. However a number of remarkable challenges must be faced before this can be accomplished.
Figure 12.
Atomistic configuration extracted from a first principles MD simulation of a Si-water interface. The Si surface is hydrogenated. Brown, white and red spheres represent Si, H and O atoms, respectively. The electronic charge density is represented by grey iso-surfaces.
Figure 13.
Ball and stick representation (lower panel) of a SiC surface with alternating hydrophilic and hydrophobic geometrical arrangements. Chemical reactions occurring between water and the hydrophilic part of the surface, as studied from first principles, are schematically shown on the upper panel. Red, white, grey and blue spheres represent O, H, C and Si atoms, respectively.
Current and future challenges
One outstanding challenge is represented by the size of systems to be simulated, together with the time scale of the simulations. An essential prerequisite to a microscopic understanding of semiconductor-based biological interfaces is the determination of realistic atomistic models of systems consisting of several hundreds, possibly several thousands atoms. These sizes are still out of reach for current quantum mechanical methods and computer codes, as are the time scales (at least nanoseconds) required for meaningful simulations of biological systems. These major challenges may be addressed by developing novel algorithms and codes at the quantum level, and by coupling those with codes operating at different length and time scales, including classical and coarse-grained simulations [67]. Once an interfacial structural model (see, e.g. figure 12) is obtained via simulations, its validation needs to be carefully planned, e.g. by comparing with experiments, computed vibrational spectra or x-ray reflectivity measurements, when available. These comparisons are already challenging for inorganic materials having a well-known composition and experimental surface structure, and they are expected to be even more challenging in the case of biological matter. Another open issue to be addressed is the determination of reaction barriers for chemical reactions occurring at, or close to, the semiconductor surface (see figure 13). These reaction barriers may in principle be determined by free energy calculations based on advanced sampling methods [68], e.g. metadynamics and basis function methods. However the coupling of such methods with first principles MD is in its infancy and its availability, together with that of related codes, will be critical to properly sample the complex energy landscape of a realistic semiconductor-bio-interface. We expect the realization of this coupling between sampling methods and quantum codes to be particularly demanding in the case of aqueous and biological interfaces, as we anticipate that the use of advanced DFT methods (for example computationally demanding hybrid functionals [62]) will be necessary to describe proper electronic charge density distributions at interfaces. In addition, in order to obtain rate constants and dynamical information for proton-coupled electron transfer (PCET) reactions [69] that are often central to processes occurring at bio-interfaces, a theoretical and computational framework needs to be built to compute rate constants in the condensed phase. Such framework will encompass simulations of non-adiabatic processes and quantum effects of the electrons and protons. Again, validation strategies of these complex simulation frameworks will be necessary and critical; given their anticipated complexity, it may be useful to start building them by first investigating simple aqueous solutions interfaced with semiconductors. Numerous input are required to study reaction barriers, including free energy difference between the reactant and product states, reorganization energies, and the coupling between reactant and product vibronic states. In principle, all of these quantities can be determined from first-principles calculations by extending the approaches used for solvated molecular species to solid/liquid interfaces. Such a simulation framework for condensed phases remains to be developed and validated. Even further in the future is the development of simulation techniques capable of handling, in a predictive manner and from first principles, transport processes of large ions in aqueous media. This section has briefly summarized some of the grand challenges that we face, in order to build robust predictive computational methods to simulate semiconductor-bio-interfaces. In addition to simulation tools, open problems regarding availability of experimental data for well-defined samples and corresponding validated, computational data should be solved. Almost no such data are available at present, and efforts to build public databases similar to those for inorganic materials have yet to be started. Finally we note that fundamental, theoretical problems in the description of hydrophobic interactions, hydrogen bonding and the very structure of water are still open. Nevertheless, as of today, approximate methods are available to describe such interactions, based on which predictive simulations may be further improved.
Advances in science and technology to meet challenges
Advances are needed on multiple, interconnected fronts: the development of methods and algorithms for quantum calculations with better size scaling, the development of coupling between simulation techniques operating at multiple length and time scales and the development and optimization of computer codes for constantly changing high-performance computing architectures. In addition, the development of theoretical and computational methods to describe complex chemical reactions in condensed phases are required, as well as available reference data to enable faster computational progress. Not to be underestimated is the effort necessary to train a new generation of scientists with expertise at the interface of multiple fields, encompassing physics, chemistry and biology, and knowledgeable of multiple computational and data science techniques.
Concluding remarks
Perhaps the biggest challenge faced at present is that of integration. Clearly the investigation of semiconductor-based biological interfaces for fundamental cell studies requires developing sophisticated feedback loops involving theory, computation and experiment, including synthesis and preparation of well defined samples and their characterization, and studies that cut across multiple disciplines. A close integration will require sharing of samples, codes and data between various research groups in a concerted manner yet to be fully invented and experimented. Eventually the establishment of a robust feedback loop should lead to new paradigms allowing, for example, for characterization and even modification of interfacial properties by on-the-fly coupled experimental and computational analyses.
Acknowledgments
Work supported by Midwest Integrated Center for Computational Materials (MICCoM) as part of the Computational Materials Sciences Program funded by the DOE, Office of Science, Basic Energy Sciences, Materials Sciences and Engineering Division.
Revisiting a classic inspiration source: cephalopod-derived materials for bioelectronics
Rylan Kautz1 and Alon A Gorodetsky1,2
1 Department of Chemical Engineering and Materials Science, University of California, Irvine, Irvine, CA 92697, United States of America
2 Department of Chemistry, University of California, Irvine, Irvine, CA 92697, United States of America
Status
Cephalopods (e.g. squid, octopuses, and cuttlefish) are widely considered to be the most intelligent invertebrates with cognitive abilities that rival those of some vertebrates [70]. These animals possess highly developed nervous systems, which have made them excellent models in comparative neuroscience research over the past century [70]. For example, the study of cephalopods has provided invaluable insight into neural signaling, behavioral plasticity, nociception, nerve regeneration, neuromotor control, sensory system physiology, and brain development, as depicted in figure 14 [70]. Specifically, squid have directly enabled one of the most famous known electrophysiology experiments, wherein Hodgkin and Huxley plugged electrodes directly into squid giant axons in order to understand and model how action potentials are generated and propagated in neurons [70]. Given their significance in neurobiology, cephalopods are well positioned to contribute to the bioelectronics field.
Figure 14.
Classic neuroscience studies performed in cephalopods that have helped advance analogous scientific understanding in humans.
More recently, squid (and by extension, other cephalopods) have somewhat unexpectedly emerged as a well-spring of functional materials with interesting electrical properties. For example, melanins, which are ubiquitous natural pigments found in squid ink, have been shown to function as mixed protonic–electronic conductors [71]. In addition, maleic chitosan, which is obtained via functionalization of chitin (one of the primary components of the squid beak and pen), has demonstrated excellent performance as the active material in protonic transistors [72]. Furthermore, reflectin, which is an unusual protein found in squid skin, has been discovered to conduct protons, also enabling its application in voltage-gated protonic devices [73]. Interestingly, protonic conductivity in some of these materials has been conceptualized and described via frameworks drawn from classic semiconductor concepts, facilitating the development of various unconventional device architectures [71–73]. Such exciting findings by different groups have suggested that cephalopod-derived biopolymers are promising as active materials for bio-interfaced electronics.
Relative to other potential conductive materials, cephalopod-derived biopolymers hold the critically-important advantage of exceptional biocompatibility, as demonstrated in a number of cell culture experiments. For example, films from melanins, which are found in all animals, have been shown to support the growth of Schwann cells and neurite extension in rat adrenal gland pheochromocytoma (PC12) cells [74]. In addition, films from chitosan derivatives, which have broad commercial applicability in biotechnology, agriculture, cosmetics, and other areas, have been demonstrated to support the growth of chick dorsal root ganglion (DGR) neurons [75]. Furthermore, films from reflectins, which are implicated in cephalopod development, have been discovered to promote the attachment, proliferation, and differentiation of relatively difficult-to-culture human neural stem/progenitor cells (hNSPCs) with an efficacy rivaling that of common neural stem cell growth matrices [76]. These and a number of other reports have underscored the value of cephalopod-derived materials for bioelectronics applications.
The promise of conductive and biocompatible cephalopod-derived materials for interfacing with biological systems can be conceptualized via a thought experiment. Indeed, one could envision fabricating a device featuring an active layer from a proton-conducting biopolymer (i.e. a melanin variant, a chitosan derivative, or a reflectin isoform) and electrical contacts from a metallic alloy (i.e. palladium hydride), as illustrated in figure 15. The active layer could then be interfaced directly with a single cell containing membrane-embedded voltage-gated proton channels, which are involved in maintaining cellular proton homeostasis, play a wide variety of critical physiological roles, and constitute exciting drug targets [77]. The device would not only facilitate monitoring of minuscule currents but might also allow for stimulation of the trans-membrane proton channels (by selectively generating localized proton gradients), thereby potentially affording a measure of control over specific intracellular processes and/or cellular activity. Excitingly, variants of this experiment could provide new fundamental insight into cellular signaling processes as well as guide the development of relevant therapeutic strategies.
Figure 15.
A protonic device, wherein the active layer is composed of a proton-conducting biopolymer, such as a melanin variant, a chitosan derivative, or a reflectin isoform (inset, bottom left), and the electrodes are composed of a proton-injecting alloy, such as palladium hydride (inset, bottom right). Note that representative generalized structures are shown for the constituent materials. The device is interfaced with a single living cell that features trans-membrane proton channels.
Current and future challenges
For devices with active layers from proton-conducting cephalopod-derived biopolymers (such as the one shown in figure 15), the systematic investigation of the constituent materials’ structures constitutes a major scientific challenge. Melanins are notoriously insoluble, redox active, chemically diverse, and generally amorphous as solids, so their definitive characterization has only been possible for a limited number of specific model systems [71]. Chitosan and its derivatives are more tractable materials and have been studied extensively as proton conductors, but efforts aimed at forging connections between their structure and electrical functionality remain in a relatively nascent stage [78]. Reflectins possess an unusual amino acid sequence, a significant degree of structural disorder, an exquisite sensitivity to their environment, and a high propensity for aggregation, and thus, the structural understanding of these proteins has progressed slowly, when compared to studies of their in vivo biological roles and in vitro device applications [79]. Consequently, a great deal of scientific research and exploration will be necessary in order to forge definitive structure-electrical function relationships for melanins, chitosans, and reflectins.
For devices with electrodes from a proton-injecting palladium hydride alloy (such as the one shown in figure 15), the continued improvement of their component electrical contacts constitutes a major technological challenge. In these devices, palladium is exposed to a hydrogen atmosphere, transforming it into palladium hydride [71–73]. The resulting electrodes then enable direct transduction of protonic currents into electronic currents and allow for the interrogation of proton-conducting materials in transistor- or diode-type architectures [71–73]. However, the electrodes feature a number of possible drawbacks intrinsic to palladium and some other metal hydrides, including mechanical fragility, a need for continuous reloading with hydrogen gas, potential electrochemical reactivity, and uncertain stability during repeated electrical cycling [71, 72, 80]. Such disadvantages, while not insurmountable in some applications, indicate that the engineering of improved proton-injecting electrodes will be important in the future.
Advances in science and technology to meet challenges
With regard to the challenges inherent to melanins, chitosans, and reflectins, several distinct but related scientific advances will be necessary to forge robust structure-function relationships for each materials class. The ambiguous nature and difficult processability of melanins will require the introduction of broadly-generalizable chemical synthesis techniques that will enable their robust production. The relatively incomplete understanding of the structure-electrical function relationships forged for chitosans will benefit from the development of more advanced computational strategies that continue to leverage the available structural information. The unusual composition and demanding physical properties of reflectins will necessitate the tandem application of multiple classic techniques from both biochemistry and materials science, in order to fully understand the interplay between order and disorder for this protein class. The aforementioned specific advances will enhance the utility of cephalopod-derived biopolymers as conductive materials for bio-interfaced electronics.
With regard to the challenges inherent to palladium hydride, extensive technological advances will be necessary to improve the nature of the electrical contacts. Historically, the introduction of hydrogen into metals has facilitated the observation and study of interesting physical phenomena related to the metals’ electrical, magnetic, optical, and mechanical properties [80]. Indeed, various metal hydrides have been explored for hydrogen storage as well as for other applications, including thermal regulation, biomedical devices, smart optics, and sensors [80]. However, the discovery of new metal hydrides will inherently require the exploration of a daunting parameter space with integrated materials discovery methodologies that leverage both computational and experimental techniques [80]. The associated efforts encompass a diverse suite of disciplines and will be critical for developing metal hydrides as efficacious proton-injecting electrodes for bio-interfaced electronics.
Concluding remarks
Cephalopods have played a critical historical role in neuroscience, contributing to many seminal studies and greatly enriching modern understanding of the human nervous system. These animals have now been validated as a source of materials with interesting electrical properties, enabling a variety of unconventional devices. Such materials and devices are complementary to more traditional semiconductor-based technologies, and their development encompasses a scientifically-exciting set of distinct challenges. Overall, cephalopods may hold new secrets that are waiting to be unlocked and thus could inspire exciting new discoveries within the bioelectronics community.
Acknowledgments
We are grateful for support from the Air Force Office of Scientific Research Young Investigator Program (FA9550-14-1-0144), the Presidential Early Career Award for Scientists and Engineers (FA9550-17-1-0024), and the National Science Foundation Graduate Research Fellowship (DGE-1321846) Program.
Accelerating synthetic biology with approaches and technologies from semiconductor engineering
Samuel S Kim and Timothy K Lu
Research Laboratory of Electronics, Massachusetts Institute of Technology, Cambridge, MA 02139, United States of America
Status
Based on the idea that living cells can be programmed in rational ways to have novel functions [81], synthetic biology is an emerging interdisciplinary field that focuses on modeling, constructing, probing, and modulating biological control circuits. Synthetic biology is in a developmental stage that is analogous to the early days of semiconductor technology when the first foundational technologies were developed but very-large-scale integration (VLSI) still remained a significant challenge. While many individual biological parts, such as genetic switches [82], oscillators [83], counters [84], logic gates [85], memory [86–89] analog computers [90], and state machines [91], have been described, the rational assembly of these into higher-order circuits with increasingly complexity is difficult due to context-dependent behaviors and non-modularity seen in biological systems [85]. On the other hand, our raw ability to program biological ‘code’ continues to improve as DNA synthesis and sequencing, the underlying drivers of genetic engineering, are advancing at rates that in some cases can exceed Moore’s Law [92]. Thus, there continues to be a need to close the gap between reading/writing arbitrary sequences of DNA and using this power to implement reliable, complex, and useful genetic programs.
Although cells cannot compete with silicon processors in terms of computational speed and complexity, engineered synthetic circuits can enable a wide range of innovative and useful applications. For example, living cells with memory could be scattered into the environment, used to sense and record toxins or other signals of interest in a distributed fashion, and then collected to read out the stored information.
Bacteria and fungi that synthesize materials can be designed to build complex materials, such as fabrics, nanowires, and others, from the ground-up in an environ mentally friendly fashion. Such applications can benefit from the ability to program cells to self-organize into patterns and controllably produce novel biologically templated materials.
Engineered probiotic bacteria could be designed to detect markers of disease inside the gut, including bleeding, inflammation, and early signs of colon cancer, and then trigger the production of easily detectable signals (e.g. colorimetric chemical changes) that can be read out for non-invasive monitoring of health. Such functionality would be enhanced by the ability to build amplifiers that can convert input signals into easily detectable output signals.
Human T cells could be designed to detect multiple antigens that are specific for cancer before triggering a strong anti-tumor response. This behavior would be enabled by cellular logic gates that can integrate multiple inputs and perform Boolean computation.
Finally, by inserting sensors and memory units into living cells within animal models, biologists can gain a deeper understanding over the extracellular and intra-cellular signals that dictate the development of disease in situ. For example, state machines can be used to map and then program transcription factor cascades that are critical to cellular differentiation. Thus, we can achieve a plethora of applications where biocompatibility is important by bringing computing and memory into biology.
Current and future challenges
Much progress has been made in the field of synthetic gene circuit engineering over the last two decades. Large libraries of artificial gene parts, including transcriptional, translational, and post-translational control elements, are now available to biological engineers for use in a few model organisms. Various distinct strategies that leverage transcription factors, RNA, and recombinases have been described for implementing logic functions, analog computing, and memory. Multi-layer gene circuits have now been constructed and shown to be functional [93, 94]. The applicability of engineered gene circuits to diagnostic and therapeutic applications has been demonstrated in proof-of-concept studies performed in animal models across several different disease areas.
However, significant challenges in designing robust synthetic gene circuits still remain. First, it is very difficult to build synthetic gene circuits that work on the first try. Modeling strategies have generally been applied in a post hoc fashion and are not accurate enough to enable forward engineering. Despite efforts to enhance modularity, biological parts are inherently non-modular and thus assembling them together to achieve higher-order functions often fails due to unexpected interactions between components. Due to these limitations, an efficient design-build-test cycle is needed to shorten the time it takes to arrive at a functional construct. However, most synthetic biologists still rely on manual molecular biology protocols rather than automated strategies for building and testing gene circuits.
Second, most academic publications on biological circuits aim at demonstrating novel functionalities rather than testing robustness across important parameters such as multiple conditions and strains, as well over time. The eventual use of these circuits in real-world applications will necessitate strategies for validating the generalizability and robustness of circuits in many conditions.
Third, the metrics by which to judge success when designing biological circuits are often poorly defined. Quantitative, rather than qualitative, performance specifications need to be used when measuring the functionality of gene circuits.
Fourth, the majority of synthetic gene circuits have been built in model organisms (e.g. E. coli, Saccharomyces cerevisiae, and mammalian cell lines), even though their eventual usage would require implementation in less domesticated or well understood cell types (e.g. microbes from the human gut, bacteria and fungi from soil, human immune cells, plants). New strategies are needed to enhance the genetic domestication of new organisms and enhance the portability of gene circuits between organisms.
Advances in science and technology to meet challenges in synthetic biology
The fields of electrical engineering and computer science created the information revolution by providing new technologies and approaches for building silicon-based devices that can be massively scaled and rationally programmed. Concepts of feedback, analog circuit design, modularity, and abstraction, coupled with persistent innovation in device physics and systems design, have been critical to the success of silicon-based computing. Although biological systems have been guided by the messy hand of evolution and are not inherently modular, tools and approaches from electrical engineering and computer science may be leveraged to advance the field of synthetic biology.
Most artificial genetic circuits have been designed in an open-loop fashion and are thus susceptible to noise and have poor robustness. Natural biological circuits do not operate in an open-loop fashion. Rather, natural biological systems incorporate the important concept of homeostasis, whereby multiple competing feedback loops and signals work together to maintain balance but allow for transient perturbations in response to immediate inputs. Control theory approaches [95] are needed to analyze the behavior of natural biological systems and apply closed-loop design principles to synthetic gene circuits.
Most biological components are not inherently digital, and even relatively small changes in concentrations of biological parts can play important roles in modulating biological function. Although much focus has been placed on designing artificial gene circuits to act digitally, analog circuit design may be a suitable design strategy for biological systems [90].
Electronic design automation (EDA), robotics, and microfluidics can be used to build and test biological circuits in greater throughput and shorter timescales. Optical or electronic strategies [96] to modulate biological processes and to read out biological function should allow for dynamic and spatial mapping of cellular functions in greater detail. By speeding up the design-build-test cycle and using machine learning strategies to extract design motifs and sequences that are correlated with successful gene circuits, the prospect of forward design using computer-aided circuits may become feasible.
Finally, integrating genetically engineered cells with semiconductor technologies has the potential to address important applications. For example, distributed biosensing in the environment or in the gut could be achieved by using cells to sense biological signals and convey this information to electronic systems that can process the information and transmit it wirelessly over large distances [97].
Concluding remarks
Synthetic biology offers exciting new opportunities for introducing computing and memory into novel scenarios where semiconductor-based technologies are unable to access due to limitations in power, form factor, and/or biocompatibility. However, synthetic gene circuit design as a discipline is in relative infancy compared to silicon-based circuit design. New strategies are needed to accelerate the design-build-test cycle, to improve design success rates, to enhance robustness and portability between organisms, and to scale in complexity. Design concepts and strategies, as well as concrete tools, from electrical engineering and computer science have the potential to advance synthetic biology and to enable rational biological engineering. We envision that interdisciplinary research efforts and teams will be needed to realize this vision, with the potential to make major impacts across innumerable fields, including diagnostics, therapeutics, materials science, environmental engineering, agriculture, and more.
Addressing signaling complexity of the nervous system
Polina Anikeeva
Department of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, United States of America
Nervous system is composed of billions of cells, among them thousands of types of neurons as well as several types of glia [98]. Each neuron can support up to approximately 6000 synaptic connections with other neurons. Glia are also connected into networks via ion-permeable gap junctions [99, 100]. A diversity of chemical signals such as excitatory and inhibitory neurotransmitters produced by specific neurons, or growth factors and inflammatory cytokines released by glia facilitate communication within the nervous system. Chemical signaling is coupled to electrical signals in the form of action potentials across neuronal membranes as well as oscillations of local electric fields. In addition, glia are known to depend on and modulate local mechanical environment within the brain or spinal cord. This complexity of signaling modalities within the nervous system demands engineering of neural interfaces capable of simultaneously probing and delivering many stimuli.
While at its dawn in the late 1980s, the field of neural engineering has focused on applying the advances of semiconductor processing to fabrication of devices for electrical recording of neural activity such as Michigan probes [101] and Utah arrays [102], decades of physiological data have revealed the long-term reliability and biocompatibility challenges associated with probes based on hard materials [103, 104]. Unlike semiconductors, glasses and metals with Young’s moduli of tens to hundreds of gigapascals, the neural tissue exhibits elastic moduli in the kilo to megapascal range. Along with differences in chemical properties, this elastic mismatch between implanted probes and neural tissues is often implicated in damage to the neurons surrounding the probes, formation of protective glial scars, and the breach of the blood-brain barrier, which collectively contribute to reduction of neural interface utility over time [103, 105–107]. Consequently, in recent years materials and chemistry innovation has come to the forefront of probe engineering delivering a diversity of devices based on polymers, composites, and nanostructures [108–110].
The introduction of optogenetics in 2005, has enabled neuroscientists to optically excite and inhibit activity of genetically-identifiable cells with millisecond precision [21, 111]. This, in turn, has initiated the drive for integration of optical components into neural probes [112–117]. Similarly, pharmacogenetic neuromodulation with designer receptors exclusively activated by designer drugs (DREADDs) has posed a need for delivery of chemicals into specific brain regions [118]. Finally, the majority of genetic methods for manipulation of neural activity rely on delivery of viral vectors into the tissues of interest, which requires integration of microfluidics.
Multifunctionality of neural probes demanded by the complexity of neural signaling and the availability of powerful genetic tools is at odds with the need for miniaturization and flexibility, which are critical to negate the foreign body response and extend functional lifetime of neural interfaces. A number of approaches have recently been proposed to achieve these disparate goals. These include extreme miniaturization and/or micro-contact printing of semiconductor circuits [119, 120] and optoelectronics onto flexible substrates [121, 122], development of transparent flexible electronics using organic materials and grapheme [38, 123], as well as multi-material fiber fabrication [124, 125].
Fiber-based neural probes are produced via thermal drawing process conventionally used in optical telecommunications industry [126, 127]. These devices are designed as macroscale models, preforms, of desired neural probes and fabricated by common machining [127]. The macroscale preforms are then heated above the glass transition and melting temperatures of the constituent materials and stretched into hundreds of meters of fibers with microscale to nanoscale features and cross sectional patters matching those of the preforms. Multiple drawing steps can be performed to achieve feature dimensions down to tens of nanometers [128, 129]. While commercial fibers are composed of glasses, a decade of advances in multimaterial fiber processing now enables simultaneous processing of polymers, metals, and nanomaterials composites [127, 130]. Using this approach, Canales et al have produced flexible neural recording arrays integrating tens of 5 μm electrodes, which allowed for extracellular neural recording with signal-to-noise ratio up to 20, as well as multifunctional probes including optical waveguides, microfluidic channels and conductive composite electrodes [117]. These devices have enabled simultaneous electrophysiological recording, optogenetic stimulation and drug delivery into the brain of moving mice, and the technology was extended by Park et al to permit viral transfection [124]. Furthermore, the application of fiber drawing to elastomers by Lu et al has expanded the utility of fiber-based probes to modulation and monitoring of spinal cord circuits in moving rodents [125, 131]. Initial biocompatibility studies indicate minimal tissue response to these probes up to 3 months following implantation, which is likely due to their miniature dimensions and low bending stiffness; future work, however, will reveal the utility of the polymer fibers in long-term experiments.
Despite being arbitrarily scalable and multifunctional fiber-based probes face two notable challenges. One challenge is the symmetry of the fiber structure in the axial direction, which implies that the functional interface with the tissue is limited to a fiber tip. This limitation may in future be overcome by combining fibers into arrays, and by combining fiber-fabrication with laser milling, micromachining, or photolithography to expose features along the fiber length albeit at the expense of throughput. Furthermore, in future it may be possible to leverage the microfluidic channels within fibers to deliver not only chemical and biological agents but electronic components to the fiber tip or even to the site of interest with the nervous system. For example, highly miniaturized wireless modules for recording and stimulation (e.g. neural dust [132, 133]) could, in principle, be injected through the flexible fibers, and both tools can act synergistically to investigate local electrophysiology combined with optical and chemical interrogation.
Another significant challenge, which applies to all neurotechnologies under development including fiunder development including opmenconnectorization and interfacing with the external circuits. As the number of functional features increases so does the size and the complexity of the connectors to the external recording circuits, light sources, and fluid delivery systems. Although increasing availability of wireless data and power transmission may reduce the connector burden, the thermal management and the dimensions of the radio-frequency antennas may restrict the application of these technologies to larger exper imental subjects [119, 120, 134, 135]. The development of robust and inexpensive interfaces between neural probes and backend electronics, while frequently overlooked, will likely play a significant role in adoption of specific tools by the neuroscience community.
High-frequency nanoscale semiconductor devices for electric sensing and control of proteins
Michal Cifra, Ondrej Krivosudský and Daniel Havelka
Institute of Photonics and Electronics, Czech Academy of Sciences, Czechia
Status
The very fundamental function of brain, heart and muscles is dependent on the electric phenomena of biological cells. They are essential for cell metabolism, shape formation [136] and communication [137] at the cellular scale. Therefore, our ability to measure biological electric signals is vital for understanding both physiological and pathological life processes. Monitoring electrical signals of heart (electrocardiogram (ECG)) and brain (electroencephalogram (EEG)) are great examples of such achievements, as both are standard diagnostic applications on the organismal level. On a level of a single cell, the characteristics of electrical activity reflects various internal processes such as differentiation along with responses to external environment stimuli. To acquire the ultimate understanding of electric phenomena within the cell, one need to understand the interactions on the level of molecules. Practically all intra- and intermolecular interactions are based on electric or electrodynamic forces or lack of them.
To obtain such understanding and employ it in development of future biomedical diagnostic and therapeutic methods, the tool to be used has to match not only the spatial but also the time scales of the bio-molecular phenomena. On the one hand, devices for sensing should have broad bandwidth up to kHz–GHz range to be able to follow even the fastest ns and sub ns processes exhibited by rapidly fluctuating protein dynamics [138] and protein structures such as micro-tubules (MTs) [141]. On the other hand, to accurately modulate the function of these structures the devices has to be able to also deliver the electric signals as ultra-short pulses in the same time (bandwidth) region as they sense.
Current and future challenges
The current challenges for the high frequency semiconductor interface sensing devices are in ability to detect localized electric field, created by fluctuating electric charges of single protein with high precision in space and time. In principle, a single protein can be trapped or attached to a nanostructured semiconductor interface such as nanowire [140] via various biochemical approaches. An electric current is then flowing through the nanowire and modulated by the interaction with the protein electric charges, creating a field-effect-transistor-like structure. The conformational changes of the single protein, which can give unique insight into enzymatic function, can be then tracked in a label-free and real-time manner for unlimited time, which is a major advantage over classical fluorescence based methods. However, the challenge is to tether a protein in such a manner that conformational changes cause observable variation in the current flowing through the nanowire while not restricting the natural protein motions [138]. Future challenges for sensing using semiconductor high-frequency biointerfaces are in detection of very high frequency electric fluctuations (>1 GHz) related to time scale of normal modes of protein and protein complexes and structures such as MTs [141]. Another exciting challenge unmet so far is to detect these high frequency electric fluctuations of the protein structures within the cell—this could give rise to a new family of biosensing approaches.
Another significant challenge for high-frequency semiconductor biointerfaces is in nanolocalized control of proteins via intense pulsed electric field [142]. Electric field delivered in short pulses (ñs range) can exert direct effect on protein function without appreciable thermal effects. So far, mostly metal electrodes with patterning on micron scale have been used to modulate protein-based processes such as motor protein facilitated MT migration on surface [143]. However, nanoscale electrode arrays enable delivery of very high field strengths with applications of moderate voltages. In contrast to metals, seminconductor nanointerfaces enable tuning of conductivity via doping across many orders of magnitude and fabrication of complex 3D nanostructured geometries [144].
The challenge for both localized sensing and control is in the nanoscale patterning of electric field—there are apparent physical limitations due to finite electromagnetic wavelength. Electrical interconnects from macroscopic devices to individual nanoelectrodes and nanostructures remain a great challenge especially for high frequencies where parasitic capacitance among electrodes becomes significant.
Advances in science and technology to meet challenges
To enable localized sensing and control of biological structures by electric field, nanostructures and materials which enable concentration of the energy are fundamental. Slow-wave structures, spoof surface plasmon polaritons and potentially 2D plasmons on van der Waals materials in radiofrequency and microwave bands enable localization of the electromagnetic energy to deep-subwavelength scales [145].
Another possible route for meeting the challenge of localization and nanostructuring of the high frequency electric field will be in employing nanoelectromechanical systems (NEMS), for example based on nanowire with strong intrinsic electric polarization along is long axis. Coupling high frequency electric signals to polar longitudinal vibration modes of the nanowire squeezes electromagnetic wavelength roughly million-fold. In the case of standing electromechanical wave, electric field with local minima and maxima is formed around the nanostructure. We recently showed that depending on the electromechanical wave velocity, frequencies of few GHz could form local minima and maxima with spacing on the submicron scale, similar to what was predicted for electrically polar vibrations of biological nanostructures [139].
Challenges with interconnects can be solved by developing, implementing and integrating nanoscopic high frequency transmission lines which contain the field within until the point of delivery. Nanowires with coaxial waveguide topology (conductor–dielectric-conductor) are one of the options to shield away capacitance coupling of nanoscopically spaced electrodes.
Theoretical approaches based on modelling (both molecular dynamics and coarse grained-based) to predict temporal and spatial distribution of electric field around the protein and electric field effects on protein structures will need to be further developed. Such knowledge will enable to set requirements of the field distribution to be generated or sensed and consequently foster rational design of nanointerfaces and their parameters.
Concluding remarks
Nanoscale high-frequency semiconductor interfaces can be used for sensing at the time scales of electric fluctuations at the level of proteins, protein structures either in vitro or inside the cell. Such sensing will enable direct electronic monitoring of single protein function, enzyme switching or cellular activity in a label-free real-time manner and integrable on chip by its very nature. Nanoscale interfaces will be also employed to deliver pulsed electric fields which will enable modulation of cellular function with important focus on protein function or cytoskeleton dynamics.
Acknowledgments
MC, DH, OK acknowledge support from the Czech Science Foundation, projects no. 15–17102S, 17–11898S and participate in COST Actions BM1309, CA15211 and bilateral exchange project between Czech and Slovak Academies of Sciences, no. SAV-15–22.
Silicon-based intracellular biointerfaces
Yuanwen Jiang and Bozhi Tian
Department of Chemistry, University of Chicago, Chicago, IL 60637, United States of America
Status
Intracellular signaling is the basis of biological activities and actions [146–148]. Traditionally, our knowledge of intracellular dynamics has been limited to biochemical and transcriptional pathways. It is now known that single cells also use electrical [147] and mechanical [148] signals for processing intracellular information. These signals can manifest as rapid voltage changes across cellular membranes [147], or as localized force generation within the cytoskeleton network [148]. Additionally, the intracellular electrical and mechanical properties and dynamics tend to be rather inhomogeneous. For example, in the nerve cells, action potentials are typically initiated in the axon hillocks and the resultant voltage pulses travel down the axons to the nerve terminals. However, the existing chemical and physical tools are not sufficient for studying these cellular heterogeneities.
Current and future challenges
Recent studies show that single organelles (figure 18(A)) may be capable of generating strong electromagnetic fields (EMFs) [149, 150]. For example, microtubules (MTs), composed of electrically polar tubulin heterodimer subunits, have been suggested as one source of cellular EMFs [150]. However, convincing experimental evidence for intracellular EMFs is surprisingly sparse [149]. Additionally, despite numerous extracellular experiments showing that EMFs can control important cell behaviors, studies in the intracellular domain are challenging to execute. The sensing and modulation of intracellular EMFs represent one critical area in which nanoscale semiconductor-based materials and devices can be explored.
Figure 18.
Intracellular biophysical interfaces. (A) Organelles are the new target for silicon-enabled cellular biophysics studies. (B) Modulation by the light-induced capacitive effect. (C) Modulation by photogalvanic process, where oxidation/reduction (Ox/Red) reactions happen near the silicon surface. (D) Fast photothermal effect from the silicon (Ts) can change the electrical capacitance of the lipid membrane. In these situations, light illumination could change Xo (intra-organelle ion concentration) or Vo (intra-organelle potential).
As previously mentioned, intracellular mechanical force transduction also plays an important role in regulating physiological processes, with cytoskeletal filaments providing mechanical cues for intracellular signaling. Understanding these processes is an important step in designing new therapeutic systems, but thus far our ability to probe these mechanisms has been rather limited. Semiconductor nanostructures, given their existing utilities in NEMS and MEMS, may serve as a class of minimally invasive and organelle-like intracellular devices that are useful for both sensing and modulating local intracellular force dynamics in real time.
Finally, to advance the field of cellular engineering, new concepts and techniques need to be developed for rapid, minimally invasive, reliable and time-lapse detection or modulation of the outputs from synthetic cellular circuits. In particular, nonconventional components of orthogonal inputs and outputs can be used to manipulate multiple behaviors simultane ously within single cells. Given that the active parts of nanoscale semiconductors have dimensions that approximate those of macromolecules or organelles, the use of these materials and devices to control intra-cellular behavior is feasible. However, significant basic science studies are still needed to address the challenges of remote electrical, optoelectronic and mechanical interfaces with intracellular components (figure 18(A)). If successful, such research could provide new insights into cellular biophysics and translational medicine.
Advances in science and technology to meet challenges
Recent developments by various groups have showed that nanoscale silicon-based materials and devices can efficiently probe biological systems [151]. Some specific advances and strategies for addressing the aforementioned challenges are as follows.
Bioelectric interfaces
Regarding intracellular electrical sensing, we can expand the use of nanoscale FETs [151] for electrical potential recordings from individual organelles. However, we still need to solve the challenges of placing freely moving electrical interconnects (i.e. used as the source and drain electrodes for FET) that are minimally invasive, and the challenges associated with intracellular targeting and penetration of multiple cellular membranes. Success in this area requires new interconnect designs for FET devices, surface chemistry exploration to enable organelle targeting, and techniques for device manipulation and alignment with respect to the typically mobile organelles.
As for modulating intracellular bioelectric activities, there are a few mechanisms we could implement. First and foremost, nanoscale photodiodes can yield changes in surface potentials upon light illumination. These nanostructures, when placed intracellularly, could produce light-induced capacitive (figure 18(B)) or faradaic (figure 18(C)) currents that modify the original intracellular electrical or chemical microenvironments. Intracellular proteins and organelles can sense such a perturbation [147], leading to a modulative cellular effect. So far, such interfaces have not been achieved in any convincing manner; however, earlier photophysical studies on dopamine-modified intracellular quantum dots [152] suggest that this is feasible. Second, a rapid photothermal effect can induce capacitive current generation across lipid bilayers (figure 18(D)), which can be explored for intra-cellular bioelectric interfaces as well. For example, we recently used a deformable composition of silicon to establish bioelectric interfaces with the plasma membrane [24]. After illuminating the lipid-supported silicon particles, the quick photothermal effect from the silicon induced a local temperature elevation, which subsequently caused an electrical capacitance increase in the lipid bilayer and a depolarization of the bilayer due to the capacitive currents (figure 18(D)). Since the bilayers are the key components of intracellular organelle membranes, one would expect a similar capacitive current generation across organelles whose intraorganelle potential is different from that of cytosol. This thermally-induced bioelectric effect can serve as an alternative mechanism for intracellular bioelectric modulation.
Biomechanical interfaces
Our group recently demonstrated that label-free silicon nanowires can be internalized in multiple cell lines (up to 96% uptake rate), undergoing an active ‘burst-like’ transport process [153]. Label-free silicon nanowires are internalized primarily through an endogenous phagocytosis pathway, rather than through exogenous manipulations. With phagocytized silicon nanostructures, our lab demonstrated that kinked silicon nanowires can serve as mechanical probes for monitoring intracellular force dynamics [154]. Initial results show that cells exhibit unexpected ratcheting-like behavior during intercellular interactions with force peaks of ~69.6 pN per nanowire, while during smooth muscle contraction, intracellular forces of ~116.9 pN were measured as experienced by a single internalized nanowire. We chose kinked structures for intracellular force measurements because they can act both as visual and physical anchors, limiting rotational and translational device transport, and ensuring that the force is transduced primarily to mechanical strain. Future research in this area could be focused on developing multiplexed sensing of intracellular force dynamics. In principle, this can be achieved by recording deformation dynamics from multiple silicon nanowires that are precisely positioned in different locations within a single cell.
Regarding remote intracellular mechanical stimulation, one area of interest is to develop stimuli-response semiconductors that are capable of conformational, thermal and chemical changes upon receiving an external signal (e.g. light or ultrasound). These changes can lead to either direct or indirect mechanical perturbation intracellularly, e.g. the deformation of cytoskeleton filaments.
New components for cellular engineering
We recently showed that when a photothermally-induced bioelectric effect was applied to neurons, action potentials were generated [24]. Importantly, at certain light-input frequencies (e.g. 30–40 Hz), an interesting output pattern was produced with alternating action potentials and sub-threshold depolarizations. This emergent output behaviour was likely a result of dynamic feedback among Si-induced thermal and ionic effects, ion channel activities and membrane potentials. This complex neuronal output through silicon-based interfaces suggests a cellular engineering platform where programmed multi-site inputs could be integrated and computed by biological cells. Additionally, this engineered output could bias the direction of certain intracellular stochastic behaviours, which are currently unknown. Additionally, we can explore this universal biophysical process in other cellular systems, such as cardiomyocyte, skeletal muscle and microbial systems. The exploration of this hybrid system can also uncover fundamental biophysical aspects of the intercellular communications, where one can selectively activate part of the cell populations and study the signal propagation dynamics in a mesoscale cellular network.
Concluding remarks
To understand and then modulate intracellular biophysical activities, it is necessary to have tools that are minimally invasive, displaying high spatiotemperal resolutions and a large signal-to-noise ratio. Although optical methods have been broadly used to address these questions, many new opportunities exist for semiconductor-based materials and devices given their diverse material structures and much more device capabilities. With all the recent advances in nanoscale semiconductor synthesis and device applications, we are now in a position to integrate semiconductor materials and devices with biological systems at the molecular and organelle levels. New nanoscale semiconductor materials—both inorganic and organic, must be designed and developed to enable their integration with cellular architectures in a minimally invasive manner, to have biophysical signal transductions within the cellular circuitry, and to eventually build an integrated platform for controlling cellular dynamics intracellularly.
Figure 7.
Roadmap of incorporation of physicochemical biomimetic components into polymer electronics for cell interfacing. Blue: cells, red: conjugated polymer electronic materials, purple: crosslinker and dynamic bonds, yellow: biomarkers.
Figure 8.
Roadmap of development and incorporation of advanced biomimetic signaling and computational components into polymer electronics for cell interfacing.
Figure 11.
Comparison between neuronal excitation by optogenetics (ChR2; A) and by seamless contact with photosensitive conjugated polymers (P3HT; B). Figure 11B © 2017 Macmillan Publishers Limited, part of Springer Nature. All rights reserved.
Figure 16.
Spectral view on current and future challenges in sensing of bioelectric activity on the level of proteins, subcellular and cellular electric fluctuations. Currently explored versus unexplored frequency region of the cellular electromagnetic activity in logarithmic scale. In terms of bandwidth, the frequency region being explored by classical electrophysiology techniques represents only 10 kHz out of whole electromagnetic spectrum.
Figure 17.
Ultrafast sub-nanosecond scale electric phenomena predicted in MTs (cytoskeleton protein fibers), image adapted from [139]. (a) Propagation of the electric pulse along the MT. Normalized intensity of the electric field is depicted in a logarithmic scale according to the presented color-bar. The shape of the pulse, as an electric field intensity along the line parallel to the MT axis in the distance of 43 nm from the wall of the MTs, is shown by the white line, on a linear scale. (b) An isosurface of the electric field intensity is used to visualize the shape of the pulse and its propagation in 3D.
Acknowledgments
This work is supported by the Air Force Office of Scientific Research (AFOSR FA9550-15-1-0285).
References
- [1].Kim DH. et al. Silicon electronics on silk as a path to bioresorbable, implantable devices. Appl. Phys. Lett. 2009;95:133701. doi: 10.1063/1.3238552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bettinger CJ and Bao Z 2010. Organic thin-film transistors fabricated on resorbable biomaterial substrates Adv. Mater 22 651–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Irimia-Vladu M et al. 2010. Biocompatible and biodegradable materials for organic field-effect transistors Adv. Funct. Mater 20 4069–76 [Google Scholar]
- [4].Kim DH et al. 2010. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics Nat. Mater 9 511–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hwang SW et al. 2012. A physically transient form of silicon electronics Science 337 1640–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yu KJ et al. 2016. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex Nat. Mater 15 782–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kang SK et al. 2016. Bioresorbable silicon electronic sensors for the brain Nature 530 71–6 [DOI] [PubMed] [Google Scholar]
- [8].Hwang SW et al. 2014. Dissolution chemistry and biocompatibility of single-crystalline silicon nanomembranes and associated materials for transient electronics ACS Nano 8 5843–51 [DOI] [PubMed] [Google Scholar]
- [9].Yin L et al. 2014. Materials, designs, and operational characteristics for fully biodegradable primary batteries Adv. Mater 26 3879–84 [DOI] [PubMed] [Google Scholar]
- [10].Dagdeviren C, Hwang S-W, Su Y, Kim S, Cheng H, Gur O, Haney R, Omenetto FG, Huang Y and Rogers JA 2013. Transient, biocompatible electronics and energy harvesters based on ZnO Small 9 3398–404 [DOI] [PubMed] [Google Scholar]
- [11].Kim D, Sakimoto KK, Hong D and Yang P 2015. Artificial photosynthesis for sustainable fuel and chemical production Angew. Chem., Int. Ed 54 3259–66 [DOI] [PubMed] [Google Scholar]
- [12].Cook TR, Dogutan DK, Reece SY, Surendranath Y, Teets TS and Nocera DG 2010. Solar energy supply and storage for the legacy and nonlegacy worlds Chem. Rev 110 6474–502 [DOI] [PubMed] [Google Scholar]
- [13].Sakimoto KK, Kornienko N and Yang P 2017. Cyborgian material design for solar fuel production: the emerging photosynthetic biohybrid systems Acc. Chem. Res 50 476–81 [DOI] [PubMed] [Google Scholar]
- [14].Lovley DR 2011. Powering microbes with electricity: direct electron transfer from electrodes to microbes Environ. Microbiol. Rep 3 27–35 [DOI] [PubMed] [Google Scholar]
- [15].Liu C, Gallagher JJ, Sakimoto KK, Nichols EM, Chang CJ, Chang MCY and Yang P 2015. Nanowire-bacteria hybrids for unassisted solar carbon dioxide fixation to value-added chemicals Nano Lett 15 3634–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Torella JP, Gagliardi CJ, Chen JS, Bediako DK, Colón B, Way JC, Silver PA and Nocera DG 2015. Efficient solar-to-fuels production from a hybrid microbial-water-splitting catalyst system Proc. Natl Acad. Sci. USA 112 2337–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu C, Colón BC, Ziesack M, Silver PA and Nocera DG 2016. Water splitting–biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis Science 352 1210–3 [DOI] [PubMed] [Google Scholar]
- [18].Sakimoto KK, Wong AB and Yang P 2016. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production Science 351 74–7 [DOI] [PubMed] [Google Scholar]
- [19].Sakimoto KK, Zhang SJ and Yang P 2016. Cysteine-cystine photoregeneration for oxygenic photosynthesis of acetic acid from CO2 by a tandem inorganic-biological hybrid system Nano Lett 16 5883–7 [DOI] [PubMed] [Google Scholar]
- [20].Kornienko N, Sakimoto KK, Herlihy DM, Nguyen SC, Alivisatos AP, Harris CB, Schwartzberg A and Yang P 2016. Spectroscopic elucidation of energy transfer in hybrid inorganic-biological organisms for solar-to-chemical production Proc. Natl Acad. Sci. USA 113 11750–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Boyden ES, Zhang F, Bamberg E, Nagel G and Deisseroth K 2005. Millisecond-timescale, genetically targeted optical control of neural activity Nat. Neurosci 8 1263–8 [DOI] [PubMed] [Google Scholar]
- [22].Shapiro MG, Homma K, Villarreal S, Richter CP and Bezanilla F 2012. Infrared light excites cells by changing their electrical capacitance Nat. Commun 3 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Carvalho-de-Souza JL, Treger JS, Dang B, Kent SB, Pepperberg DR and Bezanilla F 2015. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles Neuron 86 207–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jiang Y et al. 2016. Heterogeneous silicon mesostructures for lipid-supported bioelectric interfaces Nat Mater 15 1023–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lavoie-Cardinal F, Salesse C, Bergeron É, Meunier M and De Koninck P 2016. Gold nanoparticle-assisted all optical localized stimulation and monitoring of Ca2? Signaling in neurons Sci Rep 6 20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gentemann L, Kalies S, Coffee M, Meyer H, Ripken T, Heisterkamp A, Zweigerdt R and Heinemann D 2016. Modulation of cardiomyocyte activity using pulsed laser irradiated gold nanoparticles Biomed. Opt. Express 8 177–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sanchez-Rodriguez SP, Sauer JP, Stanley SA, Qian X, Gottesdiener A, Friedman JM and Dordick JS 2016. Plasmonic activation of gold nanorods for remote stimulation of calcium signaling and protein expression in HEK 293T cells Biotechnol. Bioeng 113 2228–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Eom K, Hwang S, Yun S, Byun KM, Jun SB and Kim SJ 2017. Photothermal activation of astrocyte cells using localized surface plasmon resonance of gold nanorods J. Biophotonics 10 486–93 [DOI] [PubMed] [Google Scholar]
- [29].Eom K, Im C, Hwang S, Eom S, Kim TS, Jeong HS, Kim KH, Byun KM, Jun SB and Kim SJ 2016. Synergistic combination of near-infrared irradiation and targeted gold nanoheaters for enhanced photothermal neural stimulation Biomed. Opt. Express 7 1614–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Treger JS, Priest MF, Iezzi R and Bezanilla F 2014. Real-time imaging of electrical signals with an infrared FDA-approved dye Biophys. J 107 L09–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Borton D, Micera S, Millán RJ and Courtine G 2013. Personalized neuroprosthetics Sci. Transl. Med 5 210rv2. [DOI] [PubMed] [Google Scholar]
- [32].Someya T, Bao Z and Malliaras GG 2016. The rise of plastic bioelectronics Nature 540 379–85 [DOI] [PubMed] [Google Scholar]
- [33].Wang Y. et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 2017;3:3. doi: 10.1126/sciadv.1602076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Xu J et al. 2017. Highly stretchable polymer semiconductor films through the nanoconfinement effect Science 355 59–64 [DOI] [PubMed] [Google Scholar]
- [35].Wang C. et al. Significance of the double-layer capacitor effect in polar rubbery dielectrics and exceptionally stable low-voltage high transconductance organic transistors. Sci. Rep. 2015;5:17849. doi: 10.1038/srep17849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Oh JY et al. 2016. Intrinsically stretchable and healable semiconducting polymer for organic transistors Nature 539 411–5 [DOI] [PubMed] [Google Scholar]
- [37].Lei T. et al. Biocompatible and totally disintegrable semiconducting polymer for ultrathin and ultralightweight transient electronics. Proc. Natl Acad. Sci. USA (Early Edition) 2017 doi: 10.1073/pnas.1701478114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Khodagholy D, Gelinas JN, Thesen T, Doyle W, Devinsky O, Malliaras GG and Buzsáki G 2015. NeuroGrid: recording action potentials from the surface of the brain Nat. Neurosci 18 310–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].van de Burgt Y, Lubberman E, Fuller EJ, Keene ST, Faria GC, Agarwal S, Marinella MJ, Talin AA and Salleo A 2017. A non-volatile organic electrochemical device as a low-voltage artificial synapse for neuromorphic computing Nat. Mater 16 414–8 [DOI] [PubMed] [Google Scholar]
- [40].Chortos A, Liu J and Bao Z 2016. Pursuing prosthetic electronic skin Nat. Mater 15 937–50 [DOI] [PubMed] [Google Scholar]
- [41].Doudna JA and Charpentier E 2014. Genome editing. The new frontier of genome engineering with CRISPR-Cas9 Science 346 1258096. [DOI] [PubMed] [Google Scholar]
- [42].Grupp SA et al. 2013. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia New Engl. J. Med 368 1509–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Seow Y and Wood MJ 2009. Biological gene delivery vehicles: beyond viral vectors Mol. Ther.: J. Am. Soc. Gene Ther 17 767–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Stewart MP, Sharei A, Ding X, Sahay G, Langer R and Jensen KF 2016. In vitro and ex vivo strategies for intracellular delivery Nature 538 183–92 [DOI] [PubMed] [Google Scholar]
- [45].Shalek AK et al. 2010. Vertical silicon nanowires as a universal platform for delivering biomolecules into living cells Proc. Natl Acad. Sci 107 1870–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Plank C, Zelphati O and Mykhaylyk O 2011. Magnetically enhanced nucleic acid delivery. Ten years of magnetofection—Progress and prospects Adv. Drug Deliv. Rev 63 1300–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yao C, Qu X, Zhang Z, Huttmann G and Rahmanzadeh R 2009. Influence of laser parameters on nanoparticle-induced membrane permeabilization J. Biomed. Opt 14 054034. [DOI] [PubMed] [Google Scholar]
- [48].Sharei A et al. 2013. A vector-free microfluidic platform for intracellular delivery Proc. Natl Acad. Sci. USA 110 2082–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kang W, Yavari F, Minary-Jolandan M, Giraldo-Vela JP, Safi A, McNaughton RL, Parpoil V and Espinosa HD 2013. Nanofountain probe electroporation of single cells Nano Lett 13 2448–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Vandersarl JJ, Xu AM and Melosh NA 2011. Nanostraws for direct fluidic intracellular access Nano Lett 12 3881–6 [DOI] [PubMed] [Google Scholar]
- [51].Chang L et al. 2015. Dielectrophoresis-assisted 3D nanoelectroporation for non-viral cell transfection in adoptive immunotherapy Lab Chip 15 3147–53 [DOI] [PubMed] [Google Scholar]
- [52].Cao Y, Hjort M, Chen H, Birey F, Leal-Ortiz SA, Han CM, Santiago JG, Paşca SP, Wu JC and Melosh NA 2017. Nondestructive nanostraw intracellular sampling for longitudinal cell monitoring Proc. Natl Acad. Sci 114 E1866–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Arvanitaki A and Chalazonitis N 1949. Inhibition ou excitation des potentiels neuroniques a la photoactivation distincte de deux chromoproteides (carotenoide et chlorophillien) Arch. Sci. Physiol 3 45 [Google Scholar]
- [54].Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 1949;18:1213. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Antognazza MR, Martino N, Ghezzi D, Feyen P, Colombo E, Endeman D, Benfenati F and Lanzani G 2015. Shedding light on living cells Adv. Mater 27 7662. [DOI] [PubMed] [Google Scholar]
- [56].Fromherz P. Electrical interfacing of nerve cells and semiconductor chips. Chemphyschem. 2002;3:276. doi: 10.1002/1439-7641(20020315)3:3<276::AID-CPHC276>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- [57].Bareket-Keren L and Hanein Y 2014. Novel interfaces for light directed neuronal stimulation: advances and challenges Int. J. Nanomed 9 65–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Maya-Vetencourt JF. et al. A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness. Nat. Mater. 2017;16:681. doi: 10.1038/nmat4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Colombo E, Feyen P, Antognazza MR, Lanzani G and Benfenati F 2016. Nanoparticles: a challenging vehicle for neural stimulation Front. Neurosci 10 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Anh Pham T, Ping Y and Galli G 2017. Modelling heterogeneous interfaces for solar water splitting Nat. Mater 16 401–8 [DOI] [PubMed] [Google Scholar]
- [61].Kohn W and Sham LJ 1965. Self-consistent equations including exchange and correlation effects Phys. Rev 140 A1133 [Google Scholar]
- [62].Becke AD. A new mixing of Hartree–Fock and local density functional theories. J. Chem. Phys. 1993;98:1372. [Google Scholar]
- [63].Car R and Parrinello M 1983. Unified approach for molecular dynamics and density-functional theory Phys. Rev. Lett 55 2471. [DOI] [PubMed] [Google Scholar]
- [64].Cicero G, Grossman JC, Catellani A and Galli G 2005. Water at a hydrophilic solid surface probed by ab initio molecular dynamics: inhomogeneous thin layers of dense fluid J. Am. Chem. Soc 127 6831. [DOI] [PubMed] [Google Scholar]
- [65].Selcuk S and Selloni A 2016. Facet-dependent trapping and dynamics of excess electrons at anatase TiO2 surfaces and aqueous interfaces Nat. Mater 15 1107–12 [DOI] [PubMed] [Google Scholar]
- [66].Ping Y, Rocca D and Galli G 2013. Electronic excitations in light absorbers for photoelectrochemical energy conversion: first principles calculations based on many body perturbation theory Chem. Soc. Rev 42 2437. [DOI] [PubMed] [Google Scholar]
- [67].Voth GA ed 2008. Coarse-Graining of Condensed Phase and Biomolecular Systems (Boca Raton, FL: CRC Press; ) [Google Scholar]
- [68].Patrick S, Valsson O and Parrinello M 2016. Enhanced, targeted sampling of high-dimensional free-energy landscapes using variationally enhanced sampling, with an application to chignolin Proc. Natl Acad. Sci 113 1150–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Blumberger J 2015. Recent advances in the theory and molecular simulation of biological electron transfer reactions Chem. Rev 115 11191–238 [DOI] [PubMed] [Google Scholar]
- [70].Fiorito G et al. 2014. Cephalopods in neuroscience: regulations, research and the 3Rs invertebrate Neuroscience 14 13–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Di Mauro E, Xu R, Soliveri G and Santato C 2017. Natural melanin pigments and their interfaces with metal ions and oxides: emerging concepts and technologies MRS Commun 7 141–51 [Google Scholar]
- [72].Strakosas X, Selberg J, Hemmatian Z and Rolandi M 2017. Taking electrons out of bioelectronics: from bioprotonic transistors to ion channels Adv. Sci 4 1600527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ordinario DD, Phan L, Walkup WG IV, Jocson J-M, Karshalev E, Hüsken N and Gorodetsky AA 2014. Bulk protonic conductivity in a cephalopod structural protein Nat. Chem 6 596–602 [DOI] [PubMed] [Google Scholar]
- [74].Bettinger CJ, Bruggeman JP, Misra A, Borenstein JT and Langer R 2009. Biocompatibility of biodegradable semiconducting melanin films for nerve tissue engineering Biomaterials 30 3050–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Freier T, Koh HS, Kazazian K and Shoichet MS 2005. Controlling cell adhesion and degradation of chitosan films by N-acetylation Biomaterials 26 5872–8 [DOI] [PubMed] [Google Scholar]
- [76].Phan L, Kautz R, Arulmoli J, Kim IH, Le DT, Shenk MA, Pathak MM, Flanagan LA, Tombola F and Gorodetsky AA 2016. Reflectin as a material for neural stem cell growth ACS Appl. Mater. Interfaces 8 278–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].DeCoursey TE 2015. The voltage-gated proton channel: a riddle, wrapped in a mystery, inside an enigma Biochemistry 54 3250–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Fischer SA, Dunlap BI and Gunlycke D 2017. Proton transport through hydrated chitosan-based polymer membranes under electric fields J. Polym. Sci. B 55 1103–9 [Google Scholar]
- [79].Naughton KL et al. 2016. Self-assembly of the cephalopod protein reflectin Adv. Mater 28 8405–12 [DOI] [PubMed] [Google Scholar]
- [80].Roennebro ECE and Majzoub EH 2013. Recent advances in metal hydrides for clean energy applications MRS Bull 38 452–8 [Google Scholar]
- [81].Cameron DE, Bashor CJ and Collins JJ 2014. A brief history of synthetic biology Nat. Rev. Microbiol 12 381–90 [DOI] [PubMed] [Google Scholar]
- [82].Gardner TS, Cantor CR and Collins JJ 2000. Construction of a genetic toggle switch in Escherichia coli Nature 403 339–42 [DOI] [PubMed] [Google Scholar]
- [83].Elowitz MB and Leibler S 1999. A synthetic oscillatory network of transcriptional regulators Nature 403 335–8 [DOI] [PubMed] [Google Scholar]
- [84].Friedland AE, Lu TK, Wang X, Shi D, Church G and Collins JJ 2009. Synthetic gene networks that count Science 324 1199–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Nielsen AK, Der BS, Shin J, Vaidyanathan P, Paralanov V, Strychalski EA, Ross D, Densmore D and Voigt CA 2016. Genetic circuit design automation Science 352 53. [DOI] [PubMed] [Google Scholar]
- [86].Siuti P, Yazbek J and Lu TK 2013. Synthetic circuits integrating logic and memory in living cells Nat. Biotechnol 31 448–53 [DOI] [PubMed] [Google Scholar]
- [87].Yang C, Tibbitt MW, Basta L and Anseth KS 2014. Mechanical memory and dosing influence stem cell fate Nat. Mater 13 645–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Farzadfard F and Lu TK 2014. Genomically encoded analog memory with precise in vivo DNA writing in living cell populations Science 346 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Perli S, Cui C and Lu TK 2016. Continuous genetic recording with self-targeting CRISPR-Cas in human cells Science 353 1115. [DOI] [PubMed] [Google Scholar]
- [90].Daniel R, Rubens JR, Sarpeshkar R and Lu TK 2013. Synthetic analog computation in living cells Nature 497 619–23 [DOI] [PubMed] [Google Scholar]
- [91].Roquet N, Soleimany AP, Ferris AC, Aaronson S and Lu TK 2016. Synthetic recombinase-based state machines in living cells Science 353 1–13 [DOI] [PubMed] [Google Scholar]
- [92].Stein L 2010. The case for cloud computing in genome informatics Genome Biol 11 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Nielsen AK and Voigt CA 2014. Multi-input CRISPR/Cas genetic circuits that interface host regulatory networks Mol. Syst. Biol 10 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kiani S, Beal J, Ebrahimkhani MR, Huh J, Hall RN, Xie Z, Li Y and Weiss R 2014. CRISPR transcriptional repression devices and layered circuits in mammalian cells Nat. Methods 11 723–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Vecchio D, Abdallah H, Qian Y and Collins JJ 2017. A blueprint for a synthetic genetic feedback controller to reprogram cell fate Cell Syst 4 109–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Na Y, Kim SY, Gaublomme JT, Shalek AK, Jorgolli M, Park H and Yang EG 2013. Probing enzymatic activity inside living cells using a nanowire-cell ‘sandwich’ array Nano Lett 13 153–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Nadeau P, Mimee M, Carim S, Lu TK and Chandrakasan AP 2017. Nanowatt circuit interface to whole-cell bacterial sensors ISSCC Digest of Technical Papers pp 352–3 [Google Scholar]
- [98].Kandel E, Schwartz J and Jessell T 2000. Principles of Neural Science 4th edn (McGraw-Hill; ) [Google Scholar]
- [99].Haydon PG 2001. Glia: listening and talking to the synapse Nat. Rev. Neurosci 2 185–93 [DOI] [PubMed] [Google Scholar]
- [100].Barres BA 2008. The mystery and magic of glia: a perspective on their roles in health and disease Neuron 60 430–40 [DOI] [PubMed] [Google Scholar]
- [101].Drake KL, Wise KD, Farraye J, Anderson DJ and BeMent SL 1988. Performance of planar multisite microprobes in recording extracellular single-unit intracortical activity IEEE Trans. Biomed. Eng 35 719–32 [DOI] [PubMed] [Google Scholar]
- [102].Campbell PK, Jones KE, Huber RJ, Horch KW and Normann RA 1991. A silicon-based, three-dimensional neural interface: manufacturing processes for an intracortical electrode array IEEE Trans. Biomed. Eng 38 758–68 [DOI] [PubMed] [Google Scholar]
- [103].Polikov VS, Tresco PA and Reichert WM 2005. Response of brain tissue to chronically implanted neural electrodes J. Neurosci. Methods 148 1–18 [DOI] [PubMed] [Google Scholar]
- [104].Ward MP, Rajdev P, Ellison C and Irazoqui PP 2009. Toward a comparison of microelectrodes for acute and chronic recordings Brain Res 1282 183–200 [DOI] [PubMed] [Google Scholar]
- [105].Lee H, Bellamkonda RV, Sun W and Levenston ME 2005. Biomechanical analysis of silicon microelectrode-induced strain in the brain J. Neural Eng 2 81–9 [DOI] [PubMed] [Google Scholar]
- [106].Saxena T, Karumbaiah L, Gaupp EA, Patkar R, Patil K, Betancur M, Stanley GB and Bellamkonda RV 2013. The impact of chronic blood-brain barrier breach on intracortical electrode function Biomaterials 34 4703–13 [DOI] [PubMed] [Google Scholar]
- [107].Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, Turner JN and Shain W 2003. Brain responses to micro-machined silicon devices Brain Res 983 23–35 [DOI] [PubMed] [Google Scholar]
- [108].Jeong J-W, Shin G, Park SI, Yu KJ, Xu L and Rogers JA 2015. Soft materials in neuroengineering for hard problems in neuroscience Neuron 86 175–86 [DOI] [PubMed] [Google Scholar]
- [109].Tian B and Lieber CM 2013. Synthetic nanoelectronic probes for biological cells and tissues Annu. Rev. Anal. Chem 6 31–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Chen R, Canales A and Anikeeva P 2017. Neural recording and modulation technologies Nat. Rev. Mater 2 16093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Fenno L, Yizhar O and Deisseroth K 2011. The development and application of optogenetics Annu. Rev. Neurosci 34 389–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Anikeeva P, Andalman AS, Witten IB, Warden MR, Goshen I, Grosenick L, Gunaydin LA, Frank L and Deisseroth K 2011. Optetrode: a multichannel readout for optogenetic control in freely moving mice Nat. Neurosci 15 163–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zhang J, Laiwalla F, Kim JA, Urabe H, Van Wagenen R, Song YK, Connors BW, Zhang F, Deisseroth K and Nurmikko AV 2009. Integrated device for optical stimulation and spatiotemporal electrical recording of neural activity in light-sensitized brain tissue J. Neural Eng 6 055007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kim TI et al. 2013. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics Science 340 211–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Buzsáki G, Stark E, Berényi A, Khodagholy D, Kipke DR, Yoon E and Wise KD 2015. Tools for probing local circuits: high-density silicon probes combined with optogenetics Neuron 86 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Lee J, Ozden I, Song Y-K and Nurmikko AV 2015. Transparent intracortical microprobe array for simultaneous spatiotemporal optical stimulation and multichannel electrical recording Nat. Methods 12 1157–62 [DOI] [PubMed] [Google Scholar]
- [117].Canales A, Jia X, Froriep UP, Koppes RA, Tringides CM, Selvidge J, Hou C, Wei L, Fink Y and Anikeeva P 2015. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo Nat. Biotechnol 33 277–84 [DOI] [PubMed] [Google Scholar]
- [118].Urban DJ and Roth BL 2015. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility Annu. Rev. Pharmacol. Toxicol 55 399–417 [DOI] [PubMed] [Google Scholar]
- [119].Jeong J-W et al. 2015. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics Cell 162 662–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Park SI et al. 2015. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics Nat. Biotechnol 33 1280–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Minev IR et al. 2015. Electronic dura mater for long-term multimodal neural interfaces Science 347 159–63 [DOI] [PubMed] [Google Scholar]
- [122].Rubehn B, Wolff SB, Tovote P, Lüthi A and Stieglitz T 2013. A polymer-based neural microimplant for optogenetic applications: design and first in vivo study Lab Chip 13 579–88 [DOI] [PubMed] [Google Scholar]
- [123].Kuzum D. et al. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat. Commun. 2014;5 doi: 10.1038/ncomms6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Park S et al. 2017. One-step optogenetics with multifunctional flexible polymer fibers Nat. Neurosci 20 612–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Lu C. et al. Flexible and stretchable nanowire-coated fibers for optoelectronic probing of spinal cord circuits. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1600955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Izawa T and Sudo S 1987. Optical Fibers: Materials and Fabrication (Berlin: Springer; ) [Google Scholar]
- [127].Tao G, Stolyarov AM and Abouraddy AF 2012. Multimaterial fibers Int. J. Appl. Glass Sci 3 349–68 [Google Scholar]
- [128].Yaman M, Khudiyev T, Ozgur E, Kanik M, Aktas O, Ozgur EO, Deniz H, Korkut E and Bayindir M 2011. Arrays of indefinitely long uniform nanowires and nanotubes Nat. Mater 10 494–501 [DOI] [PubMed] [Google Scholar]
- [129].Kaufman JJ, Tao G, Shabahang S, Deng DS, Fink Y and Abouraddy AF 2011. Thermal drawing of high-density macroscopic arrays of well-ordered sub-5-nm-diameter nanowires Nano Lett 11 4768–73 [DOI] [PubMed] [Google Scholar]
- [130].Abouraddy AF, Bayindir M, Benoit G, Hart SD, Kuriki K, Orf N, Shapira O, Sorin F, Temelkuran B and Fink Y 2007. Towards multimaterial multifunctional fibres that see, hear, sense and communicate Nat. Mater 6 336–47 [DOI] [PubMed] [Google Scholar]
- [131].Lu C, Froriep UP, Canales A, Koppes RA, Caggiano V, Selvidge J, Bizzi E and Anikeeva P 2014. Polymer fiber probes enable optical control of spinal cord and muscle function in vivo Adv. Funct. Mater 24 6594–600 [Google Scholar]
- [132].Seo D et al. 2015. Model validation of untethered, ultrasonic neural dust motes for cortical recording J. Neurosci. Methods 244 114–22 [DOI] [PubMed] [Google Scholar]
- [133].Seo D et al. 2016. Wireless recording in the peripheral nervous system with ultrasonic neural dust Neuron 91 529–39 [DOI] [PubMed] [Google Scholar]
- [134].Montgomery KL et al. 2015. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice Nat. Methods 12 969–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Muller R et al. 2015. A minimally invasive 64-channel wireless μECoG implant IEEE J. Solid-State Circuits 50 344–59 [Google Scholar]
- [136].Levin M 2014. Molecular bioelectricity: how endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo Mol. Biol. Cell 25 3835–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Cifra M, Fields JZ and Farhadi A 2011. Electromagnetic cellular interactions Prog. Biophys. Mol. Biol 105 223–46 [DOI] [PubMed] [Google Scholar]
- [138].Akhterov MV, Choi Y, Olsen TJ, Sims PC, Iftikhar M, Tolga Gul O, Corso BL, Weiss GA and Collins PG 2015. Observing lysozyme’s closing and opening motions by high-resolution single-molecule enzymology ACS Chem. Biol 10 1495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Havelka D, Cifra M and Kucera O 2014. Multi-mode electro-mechanical vibrations of a microtubule: in silico demonstration of electric pulse moving along a microtubule Appl. Phys. Lett 104 243702 [Google Scholar]
- [140].Li J, He G, Ueno H, Jia C, Noji H, Qi C and Guo X 2016. Direct real-time detection of single proteins using silicon nanowire-based electrical circuits Nanoscale 8 16172–6 [DOI] [PubMed] [Google Scholar]
- [141].Havelka D, Deriu MA, Cifra M and Kucera O 2017. Deformation pattern in vibrating microtubule: structural mechanics study based on an atomistic approach Sci. Rep 7 4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Hekstra DR, Ian White K, Socolich MA, Henning RW, Šrajer V and Ranganathan R 2016. Electric-field-stimulated protein mechanics Nature 540 400–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Van den Heuvel MGL, De Graaff MP and Dekker C 2006. Molecular sorting by electrical steering of microtubules in kinesin-coated channels Science 312 910–4 [DOI] [PubMed] [Google Scholar]
- [144].Luo Z et al. 2015. Atomic gold–enabled three-dimensional lithography for silicon mesostructures Science 348 1451–5 [DOI] [PubMed] [Google Scholar]
- [145].Basov DN, Fogler MM and Garcia de Abajo FJ 2016. Polaritons in van Der Waals materials Science 354 aag1992. [DOI] [PubMed] [Google Scholar]
- [146].Spiller DG, Wood CD, Rand DA and White MRH 2010. Measurement of single-cell dynamics Nature 465 736–45 [DOI] [PubMed] [Google Scholar]
- [147].Bezanilla F 2008. How membrane proteins sense voltage Nat. Rev. Mol. Cell Biol 9 323–32 [DOI] [PubMed] [Google Scholar]
- [148].Fletcher DA and Mullins D 2010. Cell mechanics and the cytoskeleton Nature 463 485–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Tyner KM, Kopelman R and Philbert MA 2007. ‘Nanosized voltmeter’ enables cellular-wide electric field mapping Biophys. J 93 1163–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Cifra M, Pokorny J, Havelka D and Kucera O 2010. Electric field generated by axial longitudinal vibration modes of microtubule Biosystems 100 122–31 [DOI] [PubMed] [Google Scholar]
- [151].Zimmerman J, Parameswaran R and Tian BZ 2014. Nanoscale semiconductor devices as new biomaterials Biomater. Sci 2 619–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Clarke SJ et al. 2006. Photophysics of dopamine-modified quantumdots and effects on biological systems Nat. Mater 5 409–17 [DOI] [PubMed] [Google Scholar]
- [153].Zimmerman JF, Parameswaran R, Murray G, Wang YC, Burke M and Tian BZ 2016. Cellular uptake and dynamics of unlabeled freestanding silicon nanowires Sci. Adv 2 e1601039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Zimmerman JF, Murray GF, Wang Y, Jumper JM, Austin JR and Tian BZ 2015. Free-standing kinked silicon nanowires for probing inter- and intracellular force dynamics Nano Lett 15 5492–8 [DOI] [PubMed] [Google Scholar]