Abstract
Insulin resistance, which characterizes type 2 diabetes, is associated with reduced translocation of glucose transporter 4 (GLUT4) to the plasma membrane following insulin stimulation, and diabetic patients with insulin resistance show a higher incidence of ischaemia, arrhythmias and sudden cardiac death. The aim of this study was to examine whether GLUT4 deficiency leads to more severe alterations in cardiac electrical activity during cardiac stress due to hypoxia. To fulfil this aim, we compared cardiac electrical activity from cardiac-selective GLUT4-ablated (G4H−/−) mouse hearts and corresponding control (CTL) littermates. A custom-made cylindrical ‘cage’ electrode array measured potentials (Ves) from the epicardium of isolated, perfused mouse hearts. The normalized average of the maximal downstroke of Ves(|dVes/dtmin|na), which we previously introduced as an index of electrical activity in normal, ischaemic and hypoxic hearts, was used to assess the effects of GLUT4 deficiency on electrical activity. The d|Ves/dtmin|na of G4H−/− and CTL hearts decreased by 75 and 47%, respectively (P < 0.05),30 min after the onset of hypoxia. Administration of insulin attenuated decreases in values of |dVes/dtmin|na in G4H−/− hearts as well as in CTL hearts, during hypoxia. In general, however, G4H−/− hearts showed a severe alteration of the propagation sequence and a prolonged total activation time. Results of this study demonstrate that reduced glucose availability associated with insulin resistance and a reduction in GLUT4-mediated glucose transport impairs electrical activity during hypoxia, and may contribute to cardiac vulnerability to arrhythmias in diabetic patients.
Heart disease is the major cause of diabetes-related morbidity and mortality, accounting for more than 70% of deaths in patients with diabetes (Gu et al. 1998; Grundy et al. 1999). Diabetic patients also show a higher incidence of arrhythmias and sudden cardiac death (Curb et al. 1995; Balkau et al. 1999). Type 2 diabetes, the most common form, is characterized by an impaired response of cells to insulin (insulin resistance), resulting in increased insulin requirements to maintain glucose homeostasis.
Fatty acids provide ~70% of the energy generation in basal conditions in the heart (Taegtmeyer, 1994; Abel, 2004). However, during anoxic conditions, oxidative metabolism of fatty acids is greatly reduced and glucose becomes the predominant fuel for the heart to maintain ATP production by anaerobic glycolysis (Opie, 1976; Liedtke, 1981; Myears et al. 1987). Elevated rates of glycolysis are required during anoxia because of the low efficiency of ATP generation by glycolysis relative to aerobic metabolism (Dietrich & Elzinga, 1992).
Glucose transport is an important regulator of myocardial glucose utilization and mediates the increase in glucose uptake that is stimulated by insulin and increased extracellular concentrations of glucose (Morgan et al. 1961; Manchester et al. 1994; Clow et al. 2004). Glucose enters myocytes via glucose transporters GLUT1 and GLUT4 (Mueckler, 1990). GLUT1 predominantly resides in the plasma membrane and plays a major role in basal glucose uptake in quiescent cells (Kraegen et al. 1993; Laybutt et al. 1997; Young et al. 1997; Abel et al. 1999; Davey et al. 2007). GLUT4, the most abundant glucose transporter in the heart, cycles to the sarcolemma from an intracellular storage compartment in response to contraction, ischaemia or to hormones such as insulin or catecholamines. Insulin, released from the pancreas in response to high blood glucose levels, stimulates GLUT4 translocation, which contributes to its blood glucose-lowering effect (Slot et al. 1991; Rett et al. 1996; Egert et al. 1997; Fischer et al. 1997; Russell et al. 1998). The underlying mechanisms by which insulin and ischaemia promote GLUT4 translocation are distinct (Rett et al. 1996; Egert et al. 1997; Russell et al. 1999; Bryant et al. 2002) and additive (Russell et al. 1998). Insulin resistance, which characterizes type 2 diabetes, is associated with reduced translocation of GLUT4 to the plasma membrane following insulin stimulation (Gibbs et al. 1995; Desrois et al. 2004; Cook et al. 2010).
Abel et al. (1999) generated mice with myocardium-restricted deletion of GLUT4 (G4H−/−). Abel and coworkers (Abel et al. 1999; Tian & Abel, 2001; Abel, 2005) reported impaired insulin-stimulated glucose uptake in G4H−/− hearts. Those features of G4H−/− hearts reflect characteristics of insulin-resistant diabetic hearts. The deteriorated glucose metabolism of G4H−/− hearts resulted in reduced contractile performance. The hearts maintained normal function in basal conditions, but showed systolic and diastolic contractile dysfunction during ischaemia owing to a marked inability to increase glucose transport (Tian & Abel, 2001). GLUT4 deficiency in the heart has also been linked to hypertrophy, impaired excitation–contraction coupling, and altered Ca2+ and pH handling (Huggins et al. 2008; Domenighetti et al. 2010).
Other studies have linked changes in cardiac cellular electrical activity and ATP derived from glycolysis during anoxia, hypoxia and ischaemia (MacLeod & Daniel, 1965; MacLeod & Prasad, 1969; Prasad & MacLeod, 1969; McDonald et al. 1971; McDonald & MacLeod, 1971, 1973; Beller et al. 1976; Willerson et al. 1977; Winston et al. 1990; Nakaya et al. 1991; Ikenouchiet al. 1993; Jansen et al. 2003). Therefore, we hypothesized that the deficiency of GLUT4 would lead to dysfunction of cardiac electrical activity.
To test our hypothesis, we used a previously established index of electrical activity developed in our laboratory (Sohn et al. 2007, 2009, 2011) to investigate the effects of GLUT4 and insulin on cardiac electrical activity using G4H−/− and corresponding control (CTL)hearts. We measured mouse 64 extracellular surface electrograms from isolated, perfused mouse hearts with a cylindrical electrode array. Using these electrograms, we determined the normalized average of the magnitude of the maximal downstroke of the surface potentials,(|dVes/dtmin |na), total activation time (TAT) and the correlation coefficient of propagation sequences (Cas). The results of our study show electrical impairment of G4H−/− hearts during hypoxia and may provide insight into−arrhythmogenesis and other electrical consequences in individuals with diabetes, who universally exhibit decreased myocardial glucose utilization (Boudina & Abel, 2007).
Methods
Experimental procedure
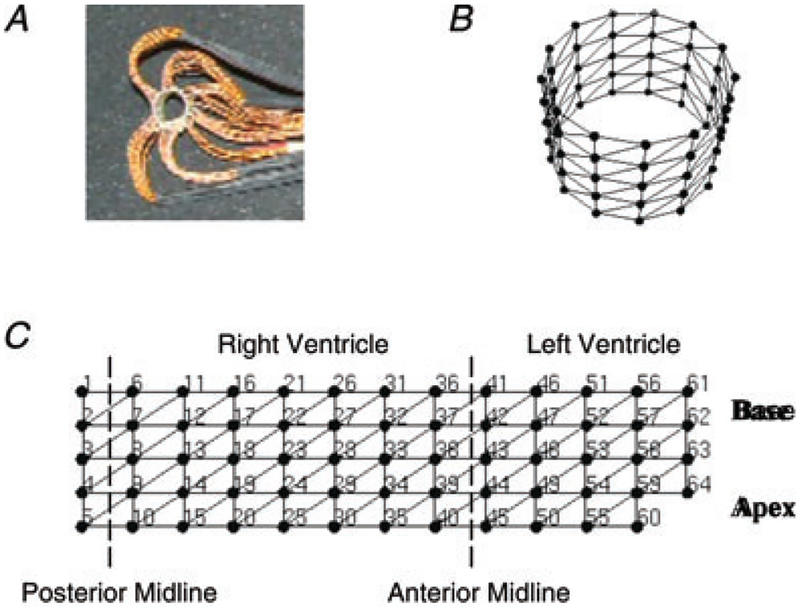
The experimental protocol for these studies was reviewed and approved by the Institutional Animal Care and Use Committee of the University of Utah and conforms to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2010). Female mice at 10–14 weeks of age with cardiac-selective GLUT4 ablation (G4H−/−) or control littermate mice were studied (see Table 1; Abel et al. 1999; Tian & Abel, 2001). Mice were injected with 3 units kg−1 of heparin and 50 mg kg−1 of sodium pentobarbital in sequence at 7 min intervals, and more sodium pentobarbital (25 mg kg−1) was injected as needed. After a deep plane of anaesthesia had been attained, the heart was rapidly excised and Langendorff perfused with a modified Krebs solution, which was composed of (mM): NaCl, 118; NaHCO3, 22; KCl, 4.1;CaCl2, 2.5; MgSO4, 1.2; EDTA, 0.5; and KH2PO4, 1.2. The perfusate was bubbled with a mixture of 95% O2 and 5% CO2 for 100% oxygen saturation. For hypoxic solutions, the concentration of NaHCO3 was lowered to adjust the pH to 7.4, the perfusate was bubbled with a mixture of 95% N2 and 5% CO2, and the temperature was maintained at 37°C. A cylindrical ‘cage’ electrode array fabricated in our laboratory, as shown in Fig. 1, was described in detail previously (Sohn et al. 2007, 2011). This electrode array was slipped over the perfused mouse hearts to measure 64 unipolar epicardial surface potentials referenced to an electrode positioned at the top of the anterior mid-line of the heart. The heart and electrode array were enclosed inside a custom-made Plexiglass chamber, in which the ambient temperature was maintained at 37°C. During hypoxia, a hypoxic solution with 25 mmHg measured PO2 and lacking insulin and glucose was continuously supplied through a syringe bathing the surface of the heart to minimize differences that may occur in oxygen penetration in the tissue (Sohn et al. 2009).
Table 1.
General characteristics of the animals studied and values for |dVes/dtmin (magnitude of the maximum downstroke of the surface potentials, in volts per second), total activation time (in milliseconds) and heart rate (in beats per minute) for each cohort during baseline conditions prior to intervention
| Parameter | i2_CTL | i0_CTL | i2_G4H−/− | i0_G4H−/− | g0_CTL | g0_G4H−/− |
|---|---|---|---|---|---|---|
| No. of mice | 7 | 10 | 6 | 9 | 4 | 4 |
| Age (weeks) | 10.8 ± 0.7 | 12.8 ± 0.3† | 11.6 ± 0.6 | 13.2 ± 0.4† | 11.5 ± 0.3 | 10.9 ± 0.1 |
| Body weight (g) | 19.3 ± 0.7 | 21.9 ± 0.7† | 20.2 ± 1.0 | 21.3 ± 0.7 | 20.8 ± 1.1 | 20.4 ± 1.3 |
| Heart weight (mg) | 126 ± 6 | 132 ± 8 | 160 ± 13* | 161 ± 9* | 117 ± 5 | 182 ± 12* |
| |dVes/dtmin| (V s−1) | 4.0 ± 0.4 | 3.9 ± 0.2 | 4.8 ± 1.0 | 4.1 ± 0.3 | 3.7 ± 0.3 | 4.0 ± 0.5 |
| Total activation time (ms) | 3.1 ± 0.4 | 2.7 ± 0.5 | 3.4 ± 0.2 | 4.1 ± 0.7 | 3.2 ± 0.4 | 4.2 ± 0.4 |
| Heart rate (beats min−1) | 374 ± 12 | 361 ± 10 | 363 ± 9 | 368 ± 11 | 367 ± 13 | 343 ± 13 |
P < 0.05 versus control (CTL).
P < 0.05 versus with insulin.
Figure 1. Cylindrical 64-electrode array.
A, photograph of the electrode array. B, the triangulated mesh geometry of the electrode array in three dimensions. The electrode array formed a cylinder 8.03 mm in diameter and 5.16 mm in length. C, two-dimensional triangulated mesh of the electrode array with electrode numbers. Five electrodes were arranged in each column with 1.29 mm interelectrode distance, and 13 columns were arranged circumferentially with 1.94 mm interelectrode spacing.
Protocols involved varying concentrations of glucose (0 and 10 mM) and insulin (0 and 2 units l−1) and PO2 within the perfusate. There were six experimental conditions labelled as follows: i2_CTL, i2_G4H−/−i0_CTL, i0_G4H−/−, g0_CTL and g0_G4H−/−, where ‘CTL’ represents control, i2 and i0 represent−2−units l−1 and no insulin, respectively, and g0 represents absence of glucose in the perfusate. Studies were conducted in CTL and G4H−/− hearts in the presence and absence of insulin during hypoxia (i2_CTL, i0_CTL, i2_G4H−/− and i0_G4H−/−). Additional studies were conducted in normoxic CTL and G4H−/− the hearts that were studied in absence of glucose (g0_CTL and g0_G4H−/−). Each experimental procedure consisted of the following four parts: heart cannulation and stabilization (1 h); baseline perfusion (30 min); induction of hypoxia or glucose deprivation (30 min); and reoxygenation or glucose (30 min; see Table 2).
Table 2.
Experimental protocols
| 0–30 min | 30–60 min | 60–90 min | ||
|---|---|---|---|---|
| PO2 | i2_CTL | >550 mmHg | ~19 mmHg | >550 mmHg |
| i0_CTL | >550 mmHg | ~19 mmHg | >550 mmHg | |
| i2_G4H−/− | >550 mmHg | ~19 mmHg | >550 mmHg | |
| i0_G4H−/− | >550 mmHg | ~19 mmHg | >550 mmHg | |
| g0_CTL | >550 mmHg | >550 mmHg | >550 mmHg | |
| g0_G4H−/− | >550 mmHg | >550 mmHg | >550 mmHg | |
| Insulin | i2_CTL | 2 units l−1 | 2 units l−1 | 2 units l−1 |
| i2_G4H−/− | 2 units l−1 | 2 units l−1 | 2 units l−1 | |
| i0_CTL | No insulin | No insulin | No insulin | |
| i0_G4H−/− | No insulin | No insulin | No insulin | |
| g0_CTL | No insulin | No insulin | No insulin | |
| g0_G4H−/− | No insulin | No insulin | No insulin | |
| Glucose | i2_CTL | 10 mM | 10 mM | 10 mM |
| i0_CTL | 10 mM | 10 mM | 10 mM | |
| i2_G4H−/− | 10 mM | 10 mM | 10 mM | |
| i0_G4H−/− | 10 mM | 10 mM | 10 mM | |
| g0_CTL | 10 mM | No glucose | 10 mM | |
| g0_G4H−/− | 10 mM | No glucose | 10 mM |
The G4H−/− and control (CTL) hearts in the presence and absence of glucose and insulin were tested during 30 min of hypoxia (PO2 ≈ 19 mmHg). Additional protocols looked separately at the effects of insulin and glucose on electrical activity.
Data acquisition and analysis
Cardiac extracellular potentials from 64 electrodes were simultaneously recorded, amplified and digitized at 4 kHz with 12-bit resolution, and stored on a Macintosh computer. The activation time at each electrode was determined as the time of the minimal slope of the potentials (Punske et al. 2003). To quantify changes in action potential propagation sequence over the epicardial surface, we defined the correlation coefficient of action potential propagation sequences (Cas) as the Pearson product–moment correlation coefficient of two activation maps from the end of baseline and other times during experimental interventions. Total activation time (TAT) was defined by the time difference between the latest activation time and the earliest activation time recorded from the 64 electrodes; TATn denotes TAT normalized to the value at the end of baseline. Values of dVes/dtmin at each electrode were normalized to the corresponding| dVes/dtmin| value at the end of baseline recordings and denoted as |dVes/dtmin|n, and all values were averaged to yield |dVes/dtmin|na. Signals were quality checked and eliminated based on two criteria; if the standard deviation of baseline |dVes/dtmin|n values of a channel exceeded 2.2 or if |dVes/dtmin|n of the channel exceeded 1.5 during experiments. Heart rates (HR) were normalized to the value at the end of baseline and are indicated as HRn.
Statistics
Quantitative results are expressed as means ± SEM. A linear mixed model was applied for statistical comparisons of |d Ves/dtmin|na, because values of |dVes/dtmin|n from electrode sites were correlated with each other. Compound symmetry covariance structure and maximal likelihood were used in the application of the linear mixed model (Littell et al. 2006). Statistical analysis of other indexes, such as Cas, TATn and HRn, was performed by oneway ANOVA. Statistical significance was determined by P < 0.05.
Results
Statistical data on our experimental cohorts and baseline values are presented in Table 1. Consistent with earlier reports, the weight of G4H−/− hearts was significantly greater than that of control hearts (Abel et al. 1999). Values of |dVes/dtmin| (in volts per second), TAT (in milliseconds) and HR (in beats per minute) showed no statistically significant differences during baseline conditions for all experiments (Table 1).
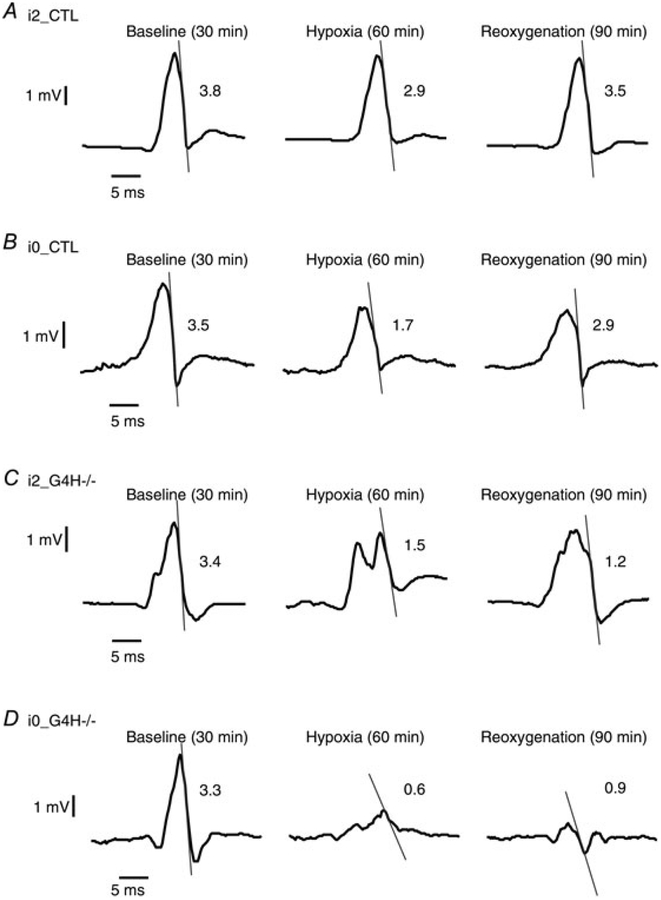
Representative potentials at the time of activation for each set of experimental conditions are shown in Fig. 2. Signal morphology typically consisted of an upstroke followed by downstroke. The G4H−/− hearts showed noticeable changes in signal morphology during hypoxia compared with control hearts. In control hearts, the amplitudes of the 64 extracellular potentials decreased during hypoxia, but each potential still showed a clear downstroke with detectable time of activation. In G4H−/− hearts, however, there were not only larger decreases in the amplitudes but also profound alterations in the signal morphology during hypoxia and the absence of insulin-accentuated decreases in |dVes/dtmin and alterations in the signal morphology. For the example in Fig. 2A, the value of |dVes/dtmin for i2_CTL decreased by 24% during hypoxia but recovered close to the baseline values during reoxygenation. In the absence of insulin, control hearts (i0_CTL) showed a larger decrease in |dVes/dtmin| during hypoxia, and the values did not return to baseline during reoxygenation (Fig. 2B). In i2_G4H/morphologies hearts during hypoxia, electrogram changed from the standard upstroke followed by a downstroke to having multiple peaks, but in general the altered morphology of these potentials recovered to prehypoxia morphologies during reoxygenation (Fig. 2C). The |dVes/dtmin decreased by 56% during hypoxia and did not return to the prehypoxic level during reoxygenation (Fig. 2C). For G4H−/− hearts perfused without/insulin (i0_G4H−/−) during hypoxia, all signals showed profound decreases in amplitude and |dVes/dtmin. A majority of electrodes showed no clear activation times, with some electrodes having multiple downstrokes and altered morphology. This altered morphology and the values of |dVes/dtmin did not recover during reoxygenation.
Figure 2.
Typical examples of recorded potentials with |dVes/dtmin (magnitude of the maximum downstroke of the surface potentials) marked by tangential lines and| values indicated (in volts per second) during baseline conditions, during hypoxia and during reoxygenation for each cohort
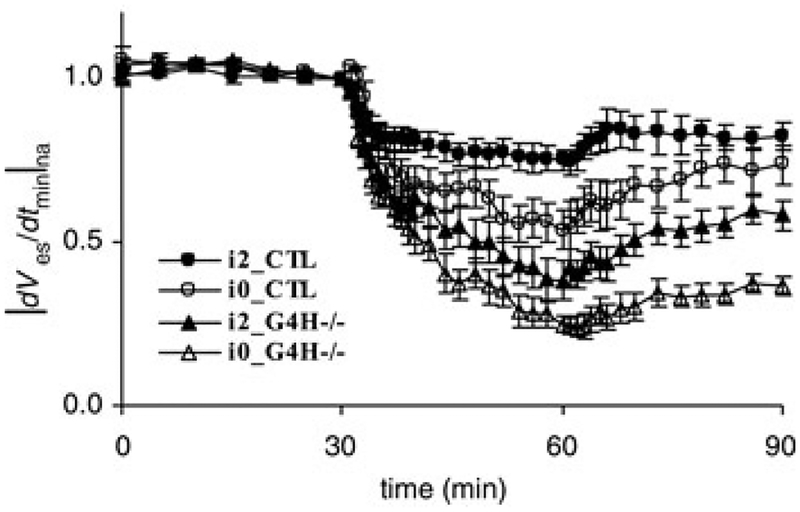
Mean values and standard error of |dVes/dtmin|na from multiple hearts as a function of time during hypoxia are shown in Fig. 3. Initially, values of |dVes/dtmin na decreased for all four cohorts at the onset of hypoxia. |After several minutes of hypoxia, |dVes/dtmin|na values for the G4−/− groups were lower than the CTL groups. In the absence of insulin, values of |dVes/dtmin |na were significantly lower for both CTL and G4H−/− groups (open circles and open triangles). The dVes/dtmin|na for i2_CTL, i0CTL, i2_G4H−/− and i0_G4H−/− decreased to 0.75, 0.53, 0.38and 0.25 at the end of hypoxia, and recovered to 0.82, 0.73,0.58 and 0.37 at the end of reoxygenation, respectively.
Figure 3.
Mean values and standard error of |dVes/dtmin na(normalized average of the magnitude of the maximum | downstroke of the surface potentials) as a function of time during hypoxia for each cohort
Times are as follows: 0–30 min, baseline; 30–60 min, hypoxia; and 60–90 min, reoxygenation.
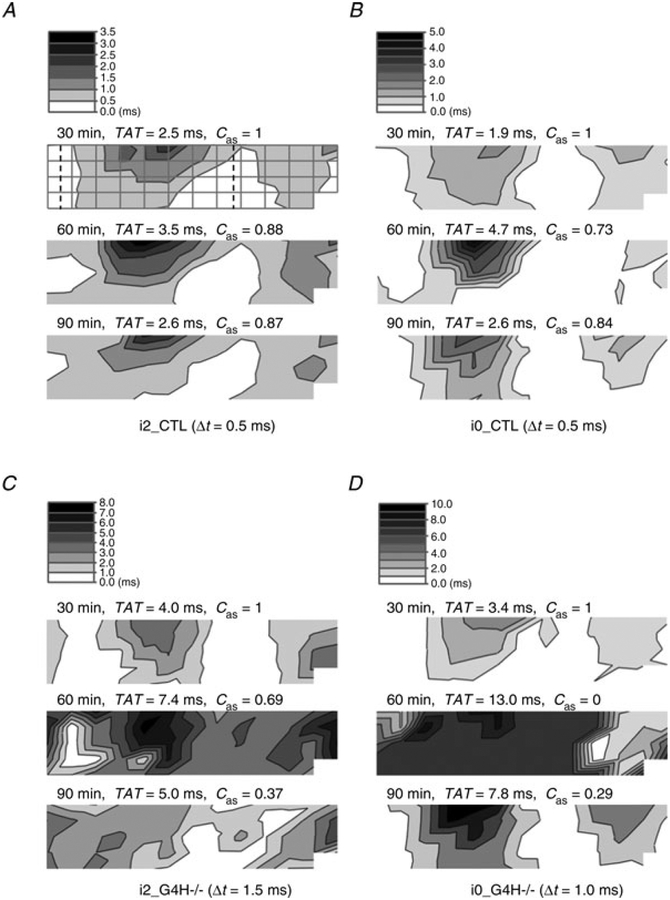
Typical examples of activation time maps for each heart type before, during and after hypoxia are shown in Fig. 4. Typically, the base of the right ventricle was the last part to activate and exhibited the slowest surface propagation speed. To quantify alterations in activation sequence, Cas was computed by comparing maps generated during hypoxia and reoxygenation with baseline maps. The maps of propagation sequence for i2_CTL and i0_CTL hearts remained qualitatively consistent throughout the experimental protocol, with small differences in Cas (Fig. 4A and B). For G4H−/−hearts, propagation sequences changed markedly by−the−end of hypoxia, without recovery of the baseline sequence during reoxygenation. Values of TAT are listed for each map, indicating the increased propagation time associated with hypoxia.
Figure 4. Activation time maps for selected hearts during hypoxia.
For each set of experimental conditions, activation time maps are displayed at the end of baseline (30 min), at the end of hypoxia (60 min) and at the end of reoxygenation (90 min). The total activation time (TAT) and correlation coefficient of action potential propagation sequences (Cas) are also listed above the corresponding activation time map. The electrode positions as shown in Fig. 1C are overlaid on the first map in A. White indicates early activation and black indicates late activation.
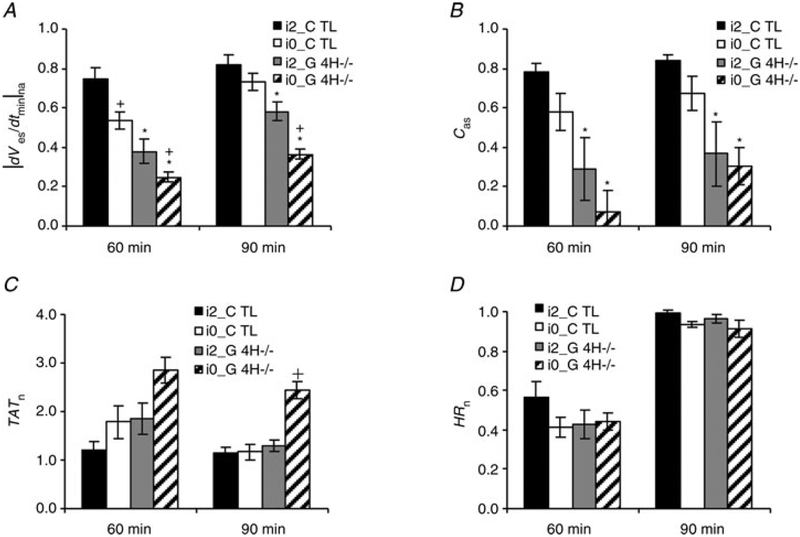
Significant differences in |dVes/dtmin|na values between G4H−/− and corresponding control hearts (i2_G4H−/−versus i2_CTL and i0_G4H−/− versus i0_CTL, P < 0.05) at the end of hypoxia and reoxygenation are indicated in Fig. 5. Insulin availability created significant differences in values of |dVes/dtmin|na measured at the end of hypoxia for each heart type (i2_G4H−/− versus i0_G4H−/− and i2_CTL versus i0_CTL, P < 0.05; Fig. 5A). The decrease of Cas was closely related to the decrease of |dVes/dtmin |na, and there were significant differences in Cas values between G4H−/− and corresponding CTL hearts at the ends of hypoxia and reoxygenation (Fig. 5B). The increase of TATn was also related to the decrease of |dVes/dtmin|na during hypoxia. Values of TATn usually recovered to baseline by the end of reoxygenation, except in the case of i0_G4H−/− (Fig. 5C). The HRn appeared to be independent of heart type and insulin availability, decreasing by ~54% after the onset of hypoxia and recovering to the baseline levels at the onset of reoxygenation (Fig. 5D).
Figure 5.
Values of |dVes/dtmin|na (A), Cas (B), TATn (C) and normalized heart rate (HRn) (D) at the end of hypoxia (60 min) and at the end of reoxygenation (90 min)
*P < 0.05 for G4H−/− versus CTL. +P < 0.05 for with versus without insulin.
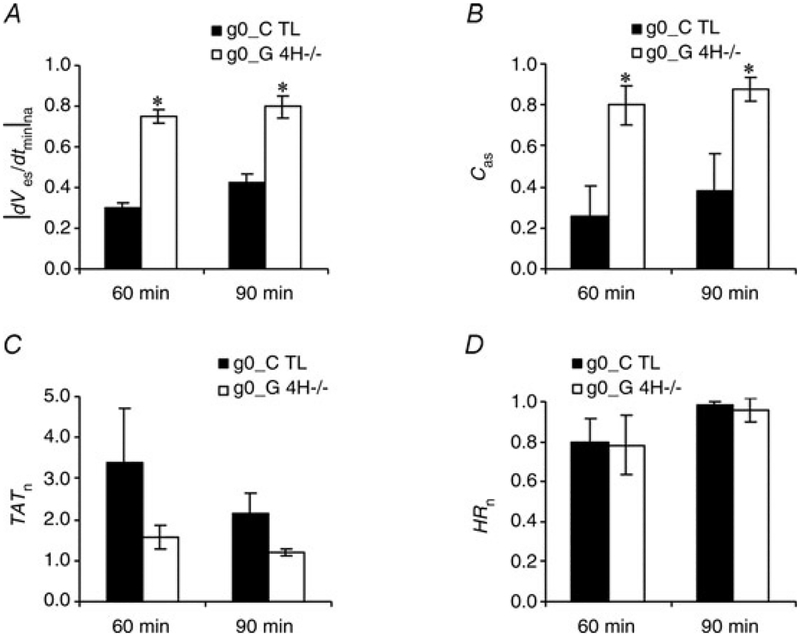
The G4H−/− hearts have previously been shown to have higher basal glycogen levels compared with CTL hearts, as a compensatory effect (Tian & Abel, 2001). To investigate the influence of the higher glycogen levels in G4H−/− hearts, perfusate with no glucose was administered for 30 min (Table 2; g0_G4H−/− and g0_CTL). The CTL hearts showed significantly lower at |dVes/dtmin|n and Cas values than G4H−/− hearts the end of glucose suspension and glucose resupply (Fig. 6). The CTL hearts also showed much larger increases in TATn than G4H−/− hearts. Values of HRn for both G4H−/− and CTL hearts decreased by ~20% in the absence of glucose and recovered after glucose was resupplied, showing no significant differences.
Figure 6.
Values of |dVes/dtmin|na (A), Cas (B), TATn (C) and HRn (D) at the end of glucose withdrawal (60 min) and at the end of glucose resupply (90 min)
*P < 0.05 versus corresponding CTL.
Discussion
The present study reveals a prominent role for GLUT4 in the maintenance of proper electrical responses during hypoxic stress. Previous studies have shown mechanical consequences of impaired glucose transport during stress (Tian & Abel, 2001; Huggins et al. 2008; Domenighetti et al. 2010). Our study shows that the electrical activity of the heart also depends greatly on the ability to transport glucose during hypoxia. We tested the effects of the absence of GLUT4 on electrical activity during hypoxia using control and G4H−/− mouse hearts. This study demonstrated greater alterations in the electrical activity of G4H−/− hearts versus control hearts during hypoxia. Both |dVes/dtmin|na and Cas of G4H−/− hearts were significantly lower than for CTL hearts at the end of hypoxia and even at the end of reoxygenation.
The fast inward sodium current (INa) generates the upstroke of the action potential, and our previous study suggested that decreases in |dVes/dtmin n are related to decreases in INa (Sohn et al. 2011). |A reduction in intracellular pH, as occurs during hypoxia, leads to sodium channel inactivation (Nonner et al. 1980) and was reflected in the reduced values of |dVes/dtmin |na measured during hypoxia in both G4H−/− and CTL hearts. For G4H−/− hearts, where impaired glucose transport reduces available energy for ionic homeostasis via Na+–K+-ATPase, Na+–Ca2+ exchanger and Na+–H+ exchanger (Barth & Tomaselli, 2009), reduced extrusion of H+ from the cells may exacerbate Na+ accumulation and inactivation, as revealed in the lower measured values of |dVes/dtmin|na.
Changes in| Cas represented alterations in activation sequence. In i0_G4H−/− hearts, |dVes/dtmin|n decreased by 75%, Cas reduced to 0.07, TATn increased threefold,| and the morphology of the potential signals frequently showed multiple downstrokes during hypoxia. These suggested decreases in INa and regional inactivation, which lead to slow conduction, conduction block and activation sequence change.
Given that insulin stimulates GLUT4 translocation, we expected to observe improved electrical activity in CTL hearts as measured by |dVes/dtmin |n at the end of hypoxia in the presence of insulin.| We also observed that the electrical activity of G4H−/− hearts was improved by insulin. The |dVes/dtmin|n of G4H−/− hearts was significantly improved at the end of hypoxiaes and reoxygenation by administration of insulin. Without insulin, we observed more frequent atrioventricular conduction blocks, premature ventricular activations and signs of local conduction block. It has previously been demonstrated that there are no changes in the rate of glucose uptake in G4H−/−hearts after insulin administration (Tian & Abel, 2001), which suggests that the improved electrical activity in G4H−/− hearts in the presence of insulin is not due to increased glucose transport. Insulin stimulates not only glucose transport but also glycogen synthesis in CTL hearts. While G4H−/− hearts might not increase glucose transport in response to insulin, the increase in glycogen content could contribute to the improved electrical activity. In addition, insulin also has direct effects on the cardiac electrophysiology, such as L-type Ca2+ current (Aulbach et al. 1999) and the Na+–Ca2+ exchange (Villa-Abrille et al. 2008). The enhanced electrical activity of G4H −/− hearts with insulin may therefore result from the direct effect of insulin on the electrical activity rather than improved energy metabolism.
Abel and co-workers (Abel et al. 1999; Tian & Abel, 2001) reported a threefold increase in GLUT1 expression and 54% higher glycogen content in G4H−/− hearts relative to CTL in basal conditions. The increased GLUT1 expression yields a threefold increase in the rate of glucose transport. The effect of increased glycogen in G4H−/− hearts may be responsible for significantly higher values of |dVes/dtmin|na and Cas when exogenous glucose was not supplied (Fig. 6). Tian & Abel (2001) reported that 20 h of fasting removed the compensatory effects of increased glycogen levels in G4H−/− hearts. We also fasted both G4H−/− and CTL mice For examine 20 h to the effects of depleted glycogen on the electrical activity (data not shown). We found that the majority of the fasted G4H−/− hearts stopped beating and could not provide viable data during hypoxia. This demonstrated the crucial role of GLUT4 during hypoxia. Taken together, these results support the hypothesis that reduced glucose availability may contribute to cardiac vulnerability to arrhythmias in diabetic patients with insulin resistance, many of whom develop reduced GLUT4-mediated glucose transport. In addition to the increased glycogen levels and hypertrophy reported in G4H−/− hearts, partial and total atrioventricular block were also−frequently observed during hypoxia in these studies. These observations provide a phenotype that shares many similarities to Wolff–Parkinson–White syndrome with glycogen storage disease (Light, 2006).
Heart rates did not show a strong dependence on available energy or metabolism like the other parameters, |dVes/dtmin|n, Cas and TATn. Heart rate decreased by ~45% quickly at the onset of hypoxia and recovered immediately following reoxygenation regardless of genotype or insulin administration (Fig. 6). Likewise, when glucose supply was arrested without hypoxia (Fig. 6), there were significant differences in |dVes/dtmin|n and Cas values between G4H−/− and CTL, However HRn values of G4H−/−and CTL hearts were virtually identical.
The epicardial surface potentials were measured using a custom-made cylindrical electrode cage. This method of measurement does not damage or restrict the heart. In addition, this method eliminates the need for voltage-sensitive dyes and electromechanical uncouplers or other methods to reduce the contraction of the heart as required for optical mapping techniques. Di-4-ANEPPS, a commonly used dye in optical mapping studies, has been shown to alter cardiac electrophysiology, including prolongation of the PQ interval and QRS duration, and reduction of conduction velocity (Nygren et al. 2003; Larsen et al. 2010, 2012). Likewise, excitation–contraction uncouplers, such as 2,3-butanedione monoxime, cytochalasin D and diacetyl monoxime, frequently used for optical mapping have been shown to have effects on action potential duration, conduction velocity, electrical restitution curve, calcium transient and ventricular fibrillation activation patterns (Qin et al. 2003; Baker et al. 2004; Cheng et al. 2004; Kettlewell et al. 2004). Additional evidence has shown that uncouplers affect metabolism, where the administration of 2,3-butanedione monoxime before or after ischaemia increased myocardial ATP and reduced myocardial injury (Habazettl et al. 1996; Moriguchi et al. 2010). Hence, the methods used in this study offer the advantage of allowing the heart to contract freely and do not alter characteristics of energy metabolism or electrophysiology. However, the cylindrical shape of the electrode array has limitations in that it does not fit the exact shape of the heart, allowing variable contact for the electrodes, and the fixed size of the cage limits the heart size used for optimal contact with the electrodes. The i0_CTL and i0_G4H−/− mice were slightly older than the i2_CTL and i2_G4H−/− mice. However, we confirmed that the parameters−we−used (|dVes/dtmin|na Cas, TATn and HRn) did not show any clear dependence on the age within the animals used. Our study investigated only global hypoxia. Although partial and total atrioventricular block occurred frequently in this study, ventricular tachycardia and ventricular fibrillation were very rare. Regional anoxic/ischaemic conditions may generate more frequent ventricular tachycardia and ventricular fibrillation by creating electrophysiological heterogeneity in larger hearts.
The experimental design for these studies, while maintaining simplicity, yielded a fundamental relationship between glucose transport and electrical activity, thus warranting further studies that can begin to address the more complex nature of the diabetic condition. A limitation to this work was the choice to provide only glucose as the energy substrate to reduce contributions from allosteric regulation. Glucose becomes the predominant fuel for the heart during hypoxia/ischaemia due to anaerobic ATP production by glycolysis and was therefore the primary target in these studies. The availability of fatty acids may modulate the electrical response through additional energy reserve. Pyruvate has been shown to protect the myocardium from ischaemia–reperfusion injury by providing an energy reserve and suppressing oxidative stress (Crestanello et al. 1995, 1998; Mallet, 2000; Arya et al. 2006). Studies have also shown that exogenous lactate during ischaemia may have adverse effects, such as increased intracellular lactate concentration and decreased glycolysis (Saman & Opie, 1984; Cross et al. 1995). Other studies showed a protective effect of externally supplied lactate against ischaemic injury (Doenst et al. 1996; Tokuno et al. 1999). Future investigations are required to determine whether substrate competition has a significant impact on the electrical activity.
The use of hypoxia to stress the heart reduces the complexities of electrical responses by eliminating the accumulation of potassium and metabolic wastes in the extracellular space that are associated with ischaemia. Our results show that the evaluation of ischaemia and the direct measurement of ionic currents during ischaemia are now warranted.
In conclusion, this study demonstrated the vital role of GLUT4 in maintaining cardiac electrical activity during hypoxia, and illustrates the direct link between metabolism and electrical function. Given the reduction in GLUT4-mediated glucose uptake in the hearts of patients with diabetes, these findings may provide additional insight into arrhythmic complications in this high-risk population, particularly in the context of ischaemia.
New Findings.
●. What is the central question of this study?
The aim of this study was to examine quantitatively whether glucose transporter 4 deficiency leads to more severe alterations in cardiac electrical activity during cardiac stress.
●. What is the main finding and what is its importance?
When compared with hearts from corresponding control littermates, the measured epicardial potentials from the surface of cardiac-selective glucose transporter 4-ablated mouse hearts during hypoxia showed the following differences: (i) significant decreases in the maximal downstroke of the potentials; (ii) increased activation time; and (iii) greater alterations in the activation sequence.
Acknowledgements
This work was supported by the Richard A. and Nora Eccles Fund for Cardiovascular Research and awards from the Nora Eccles Treadwell Foundation. A.R.W. was supported by an American Heart Association Western States Affiliate Postdoctoral Fellowship and an Advanced Postdoctoral Fellowship from the Juvenile Diabetes Research Foundation. E.D.A. was supported by UO1 HL087947. The authors wish to acknowledge Dr Philip Ershler and Mr Bruce Steadman for the design and development of the data acquisition hardware and Mr Ted Dustman for his efforts in designing our data processing software. In addition, we would like to acknowledge the technical expertise and support of Alicja Booth, Jayne Davis and Nancy Allen.
References
- Abel ED (2004). Glucose transport in the heart. Front Biosci 9,201–215. [DOI] [PubMed] [Google Scholar]
- Abel ED (2005). Myocardial insulin resistance and cardiac complications of diabetes. Curr Drug Targets Immune Endocr Metabol Disord 5, 219–226. [DOI] [PubMed] [Google Scholar]
- Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J,Doetschman T, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, Quist W, Lowell BB, Ingwall JS & Kahn BB (1999). Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest 104, 1703–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya DS, Bansal P, Ojha SK, Nandave M, Mohanty I & Gupta SK (2006). Pyruvate provides cardioprotection in the experimental model of myocardial ischemic reperfusion injury. Life Sci 79, 38–44. [DOI] [PubMed] [Google Scholar]
- Aulbach F, Simm A, Maier S, Langenfeld H, Walter U, Kersting U & Kirstein M (1999). Insulin stimulates the L-type Ca2+current in rat cardiac myocytes. Cardiovasc Res 42, 113–120. [DOI] [PubMed] [Google Scholar]
- Baker LC, Wolk R, Choi BR, Watkins S, Plan P, Shah A & Salama G (2004). Effects of mechanical uncouplers, diacetyl monoxime, and cytochalasin-D on the electrophysiology of perfused mouse hearts. Am J Physiol Heart Circ Physiol 287, H1771–H1779. [DOI] [PubMed] [Google Scholar]
- Balkau B, Jouven X, Ducimetiere P & Eschwege E (1999). Diabetes as a risk factor for sudden death. Lancet 354, 1968–1969. [DOI] [PubMed] [Google Scholar]
- Barth AS & Tomaselli GF (2009). Cardiac metabolism and arrhythmias. Circ Arrhythm Electrophysiol 2, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller GA, Conroy J & Smith TW (1976). Ischemia-induced alterations in myocardial (Na+ + K+)-ATPase and cardiac mglycoside binding. J Clin Invest 57, 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudina S & Abel ED (2007). Diabetic cardiomyopathy revisited. Circulation 115, 3213–3223. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Govers R & James DE (2002). Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol 3, 267–277. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Li L, Nikolski V, Wallick DW & Efimov IR (2004). Shock-induced arrhythmogenesis is enhanced by 2,3-butanedione monoxime compared with cytochalasin D. Am J Physiol Heart Circ Physiol 286, H310–H318. [DOI] [PubMed] [Google Scholar]
- Clow KA, Rodnick KJ, MacCormack TJ & Driedzic WR (2004). The regulation and importance of glucose uptake in the isolated Atlantic cod heart: rate-limiting steps and effects of hypoxia. J Exp Biol 207, 1865–1874. [DOI] [PubMed] [Google Scholar]
- Cook SA, Varela-Carver A, Mongillo M, Kleinert C, Khan MT, Leccisotti L, Strickland N, Matsui T, Das S, Rosenzweig A, Punjabi P & Camici PG (2010). Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur Heart J 31, 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestanello JA, Kamelgard J & Whitman GJ (1995). The cumulative nature of pyruvate’s dual mechanism for myocardial protection. J Surg Res 59, 198–204. [DOI] [PubMed] [Google Scholar]
- Crestanello JA, Lingle DM, Millili J & Whitman GJ (1998).Pyruvate improves myocardial tolerance to reperfusion injury by acting as an antioxidant: a chemiluminescence study. Surgery 124, 92–99. [PubMed] [Google Scholar]
- Cross HR, Clarke K, Opie LH & Radda GK (1995). Is lactate-induced myocardial ischaemic injury mediated by decreased pH or increased intracellular lactate? J Mol Cell Cardiol 27, 1369–1381. [DOI] [PubMed] [Google Scholar]
- Curb JD, Rodriguez BL, Burchfiel CM, Abbott RD, Chiu D & Yano K (1995). Sudden death, impaired glucose tolerance, and diabetes in Japanese American men. Circulation 91, 2591–2595. [DOI] [PubMed] [Google Scholar]
- Davey KA, Garlick PB, Warley A & Southworth R (2007).Immunogold labeling study of the distribution of GLUT-1 and GLUT-4 in cardiac tissue following stimulation by insulin or ischemia. Am J Physiol Heart Circ Physiol 292, H2009–H2019. [DOI] [PubMed] [Google Scholar]
- Desrois M, Sidell RJ, Gauguier D, King LM, Radda GK & Clarke K (2004). Initial steps of insulin signaling and glucose transport are defective in the type 2 diabetic rat heart. Cardiovasc Res 61, 288–296. [DOI] [PubMed] [Google Scholar]
- Dietrich DL & Elzinga G (1992). ATP formation and energy demand in anoxic heart muscle of the rabbit. Am J Physiol Heart Circ Physiol 263, H526–H532. [DOI] [PubMed] [Google Scholar]
- Doenst T, Guthrie PH, Chemnitius JM, Zech R & Taegtmeyer H (1996). Fasting, lactate, and insulin improve ischemia tolerance in rat heart: a comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 270, H1607–H1615. [DOI] [PubMed] [Google Scholar]
- Domenighetti AA, Danes VR, Curl CL, Favaloro JM, Proietto J & Delbridge LM (2010). Targeted GLUT-4 deficiency in the heart induces cardiomyocyte hypertrophy and impaired contractility linked with Ca2+ and proton flux dysregulation. J Mol Cell Cardiol 48, 663–672. [DOI] [PubMed] [Google Scholar]
- Egert S, Nguyen N, Brosius FC 3rd & Schwaiger M (1997). Effects of wortmannin on insulin- and ischemia-induced stimulation of GLUT4 translocation and FDG uptake in perfused rat hearts. Cardiovasc Res 35, 283–293. [DOI] [PubMed] [Google Scholar]
- Fischer Y, Thomas J, Sevilla L, Munoz P, Becker C, Holman G, Kozka IJ, Palacín M, Testar X, Kammermeier H & Zorzano A (1997). Insulin-induced recruitment of glucose transporter 4 (GLUT4) and GLUT1 in isolated rat cardiac myocytes. Evidence of the existence of different intracellular GLUT4 vesicle populations. J Biol Chem 272, 7085–7092. [DOI] [PubMed] [Google Scholar]
- Gibbs EM, Stock JL, McCoid SC, Stukenbrok HA, Pessin JE,Stevenson RW, Milici AJ & McNeish JD (1995). Glycemic improvement in diabetic db/db mice by overexpression of the human insulin-regulatable glucose transporter (GLUT4). J Clin Invest 95, 1512–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC Jr & Sowers JR (1999). Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 100, 1134–1146. [DOI] [PubMed] [Google Scholar]
- Gu K, Cowie CC & Harris MI (1998). Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care 21, 1138–1145. [DOI] [PubMed] [Google Scholar]
- Habazettl H, Palmisano BW, Bosnjak ZJ & Stowe DF (1996).Initial reperfusion with 2,3 butanedione monoxime is better than hyperkalemic reperfusion after cardioplegic arrest in isolated guinea pig hearts. Eur J Cardiothorac Surg 10, 897–904. [DOI] [PubMed] [Google Scholar]
- Huggins CE, Domenighetti AA, Ritchie ME, Khalil N, Favaloro JM, Proietto J, Smyth GK, Pepe S & Delbridge LM (2008). Functional and metabolic remodelling in GLUT4-deficient hearts confers hyper-responsiveness to substrate intervention. J Mol Cell Cardiol 44, 270–280. [DOI] [PubMed] [Google Scholar]
- Ikenouchi H, Zhao L, McMillan M, Hammond EM & Barry WH (1993). ATP depletion causes a reversible decrease in Na+ pump density in cultured ventricular myocytes. Am J Physiol Heart Circ Physiol 264, H1208–H1214. [DOI] [PubMed] [Google Scholar]
- Jansen MA, Shen H, Zhang L, Wolkowicz PE & Balschi JA(2003). Energy requirements for the Na+ gradient in the oxygenated isolated heart: effect of changing the free energy of ATP hydrolysis. Am J Physiol Heart Circ Physiol 285, H2437–H2445. [DOI] [PubMed] [Google Scholar]
- Kettlewell S, Walker NL, Cobbe SM, Burton FL & Smith GL(2004). The electrophysiological and mechanical effects of 2,3-butane-dione monoxime and cytochalasin-D in the Langendorff perfused rabbit heart. Exp Physiol 89, 163–172. [DOI] [PubMed] [Google Scholar]
- Kraegen EW, Sowden JA, Halstead MB, Clark PW, Rodnick KJ,Chisholm DJ & James DE (1993). Glucose transporters and in vivo glucose uptake in skeletal and cardiac muscle: fasting, insulin stimulation and immunoisolation studies of GLUT1 and GLUT4. Biochem J 295, 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen AP, Olesen SP, Grunnet M & Poelzing S (2010). Pharmacological activation of IKr impairs conduction in guinea pig hearts. J Cardiovasc Electrophysiol 21, 923–929. [DOI] [PubMed] [Google Scholar]
- Larsen AP, Sciuto KJ, Moreno AP & Poelzing S (2012). The voltage-sensitive dye di-4-ANEPPS slows conduction velocity in isolated guinea pig hearts. Heart Rhythm 9, 1493–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laybutt DR, Thompson AL, Cooney GJ & Kraegen EW (1997). Selective chronic regulation of GLUT1 and GLUT4 content by insulin, glucose, and lipid in rat cardiac muscle in vivo. Am J Physiol Heart Circ Physiol 273, H1309–H1316. [DOI] [PubMed] [Google Scholar]
- Liedtke AJ (1981). Alterations of carbohydrate and lipid metabolism in the acutely ischemic heart. Prog Cardiovasc Dis 23, 321–336. [DOI] [PubMed] [Google Scholar]
- Light PE (2006). Familial Wolff-Parkinson-White Syndrome: a disease of glycogen storage or ion channel dysfunction? J Cardiovasc Electrophysiol 17(Suppl 1), S158–S161. [DOI] [PubMed] [Google Scholar]
- Littell R, Milliken G, Stroup W, Wolfinger R & Schabenberger O (2006). SAS for Mixed Models, 2nd edn. SAS Institute Inc., Cary, NC. [Google Scholar]
- McDonald TF, Hunter EG & MacLeod DP (1971). Adenosinetriphosphate partition in cardiac muscle with respect to transmembrane electrical activity. Pflugers Arch 322, 95–108. [DOI] [PubMed] [Google Scholar]
- McDonald TF & MacLeod DP (1971). Anoxia-recovery cycle in ventricular muscle: action potential duration, contractility and ATP content. Pflugers Arch 325, 305–322. [DOI] [PubMed] [Google Scholar]
- McDonald TF & MacLeod DP (1973). Metabolism and the electrical activity of anoxic ventricular muscle. J Physiol 229, 559–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod DP & Daniel EE (1965). Influence of glucose on the transmembrane action potential of anoxic papillary muscle. J Gen Physiol 48, 887–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod DP & Prasad K (1969). Influence of glucose on the transmembrane action potential of papillary muscle. Effects of concentration, phlorizin and insulin, nonmetabolizable sugars, and stimulators of glycolysis. J Gen Physiol 53, 792–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet RT (2000). Pyruvate: metabolic protector of cardiac performance. Proc Soc Exp Biol Med 223, 136–148. [DOI] [PubMed] [Google Scholar]
- Manchester J, Kong X, Nerbonne J, Lowry OH & Lawrence JC Jr (1994). Glucose transport and phosphorylation in single cardiac myocytes: rate-limiting steps in glucose metabolism. Am J Physiol Endocrinol Metab 266, E326–E333. [DOI] [PubMed] [Google Scholar]
- Morgan HE, Henderson MJ, Regen DM & Park CR (1961). Regulation of glucose uptake in muscle. I. The effects of insulin and anoxia on glucose transport and phosphorylation in the isolated, perfused heart of normal rats. J Biol Chem 236, 253–261. [PubMed] [Google Scholar]
- Moriguchi A, Otani H, Yoshioka K, Shimazu T, Fujita M, Okazaki T, Sato D, Kyoi S & Iwasaka T (2010). Inhibition of contractile activity during postconditioning enhances cardioprotection by restoring sarcolemmal dystrophin through phosphatidylinositol 3-kinase. Circ J 74, 2393–2402. [DOI] [PubMed] [Google Scholar]
- Mueckler M (1990). Family of glucose-transporter genes. Implications for glucose homeostasis and diabetes. Diabetes 39, 6–11. [DOI] [PubMed] [Google Scholar]
- Myears DW, Sobel BE & Bergmann SR (1987). Substrate use in ischemic and reperfused canine myocardium: quantitative considerations. Am J Physiol Heart Circ Physiol 253, H107–H114. [DOI] [PubMed] [Google Scholar]
- Nakaya H, Takeda Y, Tohse N & Kanno M (1991). Effects of ATP-sensitive K+ channel blockers on the action potential shortening in hypoxic and ischaemic myocardium. Br J Pharmacol 103, 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonner W, Spalding BC & Hille B (1980). Low intracellular pH and chemical agents slow inactivation gating in sodium channels of muscle. Nature 284, 360–363. [DOI] [PubMed] [Google Scholar]
- Nygren A, Kondo C, Clark RB & Giles WR (2003). Voltage-sensitive dye mapping in Langendorff-perfused rat hearts. Am J Physiol Heart Circ Physiol 284, H892–H902. [DOI] [PubMed] [Google Scholar]
- Opie LH (1976). Effects of regional ischemia on metabolism of glucose and fatty acids. Relative rates of aerobic and anaerobic energy production during myocardial infarction and comparison with effects of anoxia. Circ Res 38, I52–I74. [PubMed] [Google Scholar]
- Prasad K & MacLeod DP (1969). Influence of glucose on the transmembrane action potential of guinea-pig papillary muscle. Metabolic inhibitors, ouabain, and calcium chloride, and their interaction with glucose, sympathomimetic amines, and aminophylline. Circ Res 24, 939–950. [DOI] [PubMed] [Google Scholar]
- Punske BB, Ni Q, Lux RL, MacLeod RS, Ershler PR, Dustman TJ, Allison MJ & Taccardi B (2003). Spatial methods of epicardial activation time determination in normal hearts. Ann Biomed Eng 31, 781–792. [DOI] [PubMed] [Google Scholar]
- Qin H, Kay MW, Chattipakorn N, Redden DT, Ideker RE&Rogers JM(2003). Effects of heart isolation, voltage-sensitive dye, and electromechanical uncoupling agents on ventricular fibrillation. Am J Physiol Heart Circ Physiol 284, H1818–H1826. [DOI] [PubMed] [Google Scholar]
- Rett K, Wicklmayr M, Dietze GJ & Haring HU (1996). Insulin-induced glucose transporter (GLUT1 and GLUT4) translocation in cardiac muscle tissue is mimicked by bradykinin. Diabetes 45(Suppl 1), S66–S69. [DOI] [PubMed] [Google Scholar]
- Russell RR 3rd, Bergeron R, Shulman GI & Young LH (1999). Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol Heart Circ Physiol 277, H643–H649. [DOI] [PubMed] [Google Scholar]
- Russell RR 3rd, Yin R, Caplan MJ, Hu X, Ren J, Shulman GI, Sinusas AJ & Young LH (1998). Additive effects of hyperinsulinemia and ischemia on myocardial GLUT1 and GLUT4 translocation in vivo. Circulation 98, 2180–2186. [DOI] [PubMed] [Google Scholar]
- Saman S & Opie LH (1984). Mechanism of reduction of action potential duration of ventricular myocardium by exogenous lactate. J Mol Cell Cardiol 16, 659–662. [DOI] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, James DE & Lienhard GE (1991). Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Proc Natl Acad Sci U S A 88, 7815–7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn K, Punske B & Sachse F (2009). Relationship between maximal upstroke velocity of transmembrane voltage and minimum time derivative of extracellular potential In Lecture Notes in Computer Science, (Eds. Ayache N, Delingette H, and Sermesant M) Vol. 5528, pp. 505–512. Springer-Verlag, Berlin. [Google Scholar]
- Sohn K, Sachse FB, Moreno AP, Ershler PR, Wende AR, Abel ED & Punske BB (2011). The maximal downstroke of epicardial potentials as an index of electrical activity in mouse hearts. IEEE Trans Biomed Eng 58, 3175–3183. [DOI] [PubMed] [Google Scholar]
- Sohn K, Sutherland D, Liang Q & Punske B (2007).Experimental measures of the minimum time derivative of the extracellular potential as an index of electrical activity during metabolic and hypoxic stress In Lecture Notes in Computer Science, (Eds, Sachse FB& Seemann G) Vol. 4466, pp. 250–259. Springer-Verlag, Berlin. [Google Scholar]
- Taegtmeyer H (1994). Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol 19, 59–113. [DOI] [PubMed] [Google Scholar]
- Tian R & Abel ED (2001). Responses of GLUT4-deficient hearts to ischemia underscore the importance of glycolysis. Circulation 103, 2961–2966. [DOI] [PubMed] [Google Scholar]
- Tokuno T, Watanabe M & Imaizumi Y (1999). Effects of lactate on intracellular pH and hypercontracture during simulated ischemia and reperfusion in cardiac ventricular myocytes of the guinea pig. Jpn J Pharmacol 80, 343–350. [DOI] [PubMed] [Google Scholar]
- Villa-Abrille MC, Sidor A & O’Rourke B (2008). Insulin effects on cardiac Na+/Ca2+ exchanger activity: role of the cytoplasmic regulatory loop. J Biol Chem 283, 16505–16513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerson JT, Scales F, Mukherjee A, Platt M, Templeton GH, Fink GS & Buja LM (1977). Abnormal myocardial fluid retention as an early manifestation of ischemic injury. Am J Pathol 87, 159–188. [PMC free article] [PubMed] [Google Scholar]
- Winston DC, Spinale FG, Crawford FA & Schulte BA (1990). Immunocytochemical and enzyme histochemical localization of Na+,K+-ATPase in normal and ischemic porcine myocardium. J Mol Cell Cardiol 22, 1071–1082. [DOI] [PubMed] [Google Scholar]
- Young LH, Renfu Y, Russell R, Hu X, Caplan M, Ren J,Shulman GI & Sinusas AJ (1997). Low-flow ischemia leads to translocation of canine heart GLUT-4 and GLUT-1 glucose transporters to the sarcolemma in vivo. Circulation 95, 415–422. [DOI] [PubMed] [Google Scholar]