Abstract
Within animals, oxygen exchange occurs within vascular transport networks containing potentially billions of microvessels that are distributed throughout the body. By comparison, large blood vessels are theorized to minimize transport costs, leading to tree-like networks that satisfy Murray’s law. We know very little about the principles underlying the organization of healthy micro-vascular networks. Indeed capillary networks must also perfuse tissues with oxygen, and efficient perfusion may be incompatible with minimization of transport costs. While networks that minimize transport costs have been well-studied, other optimization principles have received much less scrutiny. In this work we derive the morphology of networks that uniformize blood flow distribution, inspired by the zebrafish trunk micro-vascular network. To find uniform flow networks, we devise a gradient descent algorithm able to optimize arbitrary differentiable objective functions on transport networks, while exactly respecting arbitrary differentiable constraint functions. We prove that in a class of networks that we call stackable, which includes a model capillary bed, the uniform flow network will have the same flow as a uniform conductance network, i.e. in which all edges have the same conductance. This result agrees with uniform flow capillary bed network found by the algorithm. We also show that the uniform flow completely explains the observed radii within the zebrafish trunk vasculature. In addition to deriving new results on optimization of uniform flow in micro-vascular networks, our algorithm provides a general method for testing hypotheses about possible optimization principles underlying real microvascular networks, including exposing tradeoffs between flow uniformity and transport cost.
Keywords: zebrafish, capillary, blood flow, gradient descent, constrained optimization, phase transition
1. Introduction.
The large vessels within animal ardiovascular networks are widely believed to be shaped primarily by the necessity of minimizing the cost of transport. This minimization leads to Murray’s law for the relationship between a vessel’s radius and the flow of blood through it, which has been verified in dog intestine microvasculature and plant bundle network [51, 35]. The idea that networks should be organized to optimize transport costs for given amount of material investment provides a powerful tool for understanding the organization of networks, and underpins use of CFD models to optimize vascular geometries in cardiac surgery [33], as well as studies explaining the scaling of metabolic needs with organism size [54, 19]
Work on cardiovascular network optimization has focused primarily on the largest vessels in the network. Do the same optimization principles and constraints apply for finer vessels – arterioles and capillaries? Indeed if we divide the cardiovascular network into levels, we can estimate the total dissipation within each level as the product of the pressure drop between the level and the next and the total volume of blood passing through all vessels in that level per unit time. Now since we expect the same volume blood to be cleared through every level of the network in its passage from the heart to the finest vessels of the network, pressure drops alone become a proxy for the relative dissipations occurring at different levels. In the pressure measurements summarized in guyton and Hall [21], we find the pressure drop from the arteriole level to the capillary level (~ 60 mmHg) accounts for half of the total drop from the heart to the capillary level (~ 120 mmHg).
Mathematical modeling of microvascular networks has illuminated how networks with statistically predictable properties may emerge from the growth of vessels following rules for oxygen taxis and self avoidance [28, 23], the disconnection and reconnection of capillaries [40], and the remodeling of small vessels in response to the blood flows they carry [10]. However, despite the success of these mechanistic models for network formation, there are, to our knowledge, no works that have identified physical principles that may explain microvascular network organization and vessel geometries in the same way that Murray’s law describes the largest vessels within the network. For example, while Hu and Cai [22] have hypothesized that capillary networks minimize transport costs, we are aware of no data that shows that principles of dissipation minimization extend to these vessels. Indeed microvascular networks typically contain abundant loops [7, 55, 5, 52] (also see Fig. 1), but minimally dissipative networks, when subject to constant loads, can be proven to never contain loops [17, 8], at least when supplying steady flows of blood. Hence, the minimization of dissipation alone can not explain organization in microvascular networks [20, 42]. Our study of zebrafish trunk vasculature suggests that blood flows are uniformly distributed among fine vessels at the cost of transport efficiency, that is red blood cells are delivered at identical rates to the finest vessels of the trunk (i.e. the intersegmental vessels) [9]. Maintaining uniform flows across each vessel requires precise tuning of vessel radii. To show that tuning is needed we compare the real zebrafish trunk flow with flows in a ensemble of theoretical zebrafish trunk vasculatures. These theoretical vasculatures are created by simply permuting the radii measured in the real zebrafish trunk vessels. In all of 10, 000 theoretical trunk vasculatures, we found that the partitioning of blood flows between finest vessels, measured by the coefficient of variation (CV) of flow was less uniform than in the real zebrafish trunk (Fig. 2). The same analysis reveals a trade-off between transport efficiency and flow uniformity: All of the simulated networks had lower dissipation than the real network, and in general dissipation decreased as CV of flows increased. Thus, uniform flow is bought at the cost of decreased transport efficiency. Why might microvascular networks, like the zebrafish trunk, be adapted for uniform flow? Blood flow may serve as a proxy for oxygen perfusion; since red blood cells carry oxygen that then diffuses from the vessel into the tissue that surrounds it, uniform delivery of red blood cells to vessels may be an important precondition for uniform perfusion (but see Discussion). Indeed when we simulated oxygen perfusion in a model zerafish trunk in which every fine vessel was assigned the same radius, we found that oxygen entering the trunk from the blood was strongly concentrated around the fine vessels closest to the fish’s heart [9]. The evolutionary adaptation to vessels to create oxygen perfusion [31] may result in microvascular networks with optimally uniform blood flow, but a detailed comparison between networks with uniform blood flow with real microvascular networks is lacking. To determine whether optimization for uniform flow can explain the structure of real microvascular networks, we develop in this paper an algorithm for generating networks that optimize arbitrary functions of flow, going beyond previous works (summarized in Table 1) that have computed networks that optimize transport costs (and in the case of [26] resistance to damage).
Fig. 1.
Examples of complex microvascular networks and the corresponding model networks. (A) Capillary bed in salamander skin [36]. (B) Microvascular network of zebrafish 7.5 days post fertilization (dpf) embryo [25, 9].
Fig. 2.
Zebrafish trunk microvascular network (red square) optimizes uniform flow in fine vessels at a high transport cost, compared to untuned networks (blue dots). The untuned networks are obtained by randomly permuting the conductances of fine vessels in a real zebrafish trunk network [9]. The transport cost is characterized by dissipation [6, 1], and the flow variation is quantified by the coefficient of variation of flows in the fine vessels.
Table 1.
Comparison between previous works on optimal transport networks and the results presented in this work. Previous works focus on optimizing dissipation either alone or in combinations with damage resistance or flow fluctuations (first two rows). They constrain the network by imposing a fixed material cost of vessels. Our work agrees with previous work on the morphologies of minimally dissipative networks under material constraint (first row), but extends the classes of the target functions and constraints. Specifically we study uniform flow networks, under both material constraint and a Murray constraint that includes dissipation (last four rows).
| Target functional | Constraint | Method |
|---|---|---|
| local topological optimization [16], global optimization [6], structural adaptation [22], growth and structural adaptation [44], Section 3.1 | ||
| with damage and flow fluctuations | global optimization [26], fluctuating source [13] | |
| Section 3.2 | ||
| Section 3.3 | ||
| Section 3.4 | ||
| Section 3.4 |
To find the uniform flow networks we devise a gradient descent algorithm with Lagrange multipliers to tune the hydraulic conductances of vessels and derive uniform flow networks under fixed network topologies. At each step gradient descent algorithms calculate the optimal direction to perturb the variables in a system to optimize a target function, and approach the optimum after several small steps [11]. Lagrange multipliers are used widely to solve constrained optimization problems [39]. In our algorithm they are implemented to impose the laws of flow conservation in the network and to constrain the cost of maintaining the network. We use the algorithm to study uniform flow networks (1) on a model capillary bed consisting of a rectangular grid with single inflow (arteriole) and outflow (venule) (Fig. 1A), and (2) on a model of the zebrafish trunk that includes both main trunk vessel (dorsal aorta) and the fine (intersegmental) vessels that branch off from it (Fig. 1B).
In particular, we compare the morphologies of minimally dissipative and uniform flow networks. We find that uniform flow capillary beds contain loops that are not present in minimally dissipative networks. We are able to analytically show that, in a class of networks we call stackable, the blood flows in uniform flow networks agree with those in uniform conductance networks (See Section 3.2), hence they provably contain loops. Stackable networks include other popular models for capillary beds branching trees and honeycomb grids, as well as square grids. We show that, in zebrafish trunk topology, the real conductances of vessels are in almost exact accordance with the conductances required to create uniform flow. These results suggest that principle of uniform flow explains the zebrafish trunk network geometry. By contrast, minimization of dissipation produces networks with unrealistic morphologies. Our algorithm will then allow the uniform flow to be evaluated as an optimization principle for general microvascular networks.
To explore how uniform flow networks may yet be constrained by transport costs, we calculate networks in the zebrafish trunk while constraining both the total cost of the vessel material and the blood flow dissipation. When the relative importance of transport cost is gradually increased, we observed an abrupt change in both the flow uniformity and network morphology, resembling a phase transition: in particular, up until dissipation reaches a critical point, the optimal network is apparently unconstrained by dissipation. Thus even when a network is actually subject to multiple constraints, it may apparently be organized to ignore all but a subset of these constraints. Thus optimizing a single target function (e.g. dissipation) may be endowed with surprising power to predict shapes of real networks that are, in reality, targeting multiple functions [38].
The rest of this paper is organized as follows: In Section 2.1 we introduce the mathematical notations for describing microvascular networks. In Section 2.2 we describe a gradient descent algorithm to find general optimal networks. In Sections 2.3 - 2.7 we write down the detailed formulation of the algorithm when finding minimally dissipative and uniform flow networks under different topologies and constraints. In Section 3.1 we use the algorithm to calculate minimally dissipative networks and show that they agree with previous work. In Section 3.2 we compare minimally dissipative and uniform flow networks on the capillary bed model (a square grid). In Section 3.3 we extend our results to uniform flow zebrafish trunk networks, and show that the principle of uniform flow produces networks that match experimental data. In Section 3.4 we impose both material cost and dissipation as constraints on uniform flow networks, and describe a phase transition in network morphology that occurs as the relative weight of the two constraints is varied. Finally in Section 4 we summarize our work and discuss its consequences for microvascular network organization and for methods for finding optimal transport networks generally.
2. Methods.
2.1. Physical modeling and notation.
First we mathematically frame the problem of finding optimal networks for general network topology. Consider an undirected graph ( , ) with V vertices k = 1,…, V. For any given 2 vertices k, l we write ⟨k, l⟩ = 1 if there is a edge linking k and l and ⟨k, l⟩ = 0 if k and l are not linked. Each edge in the network is assigned a conductance κkl; the flow Qkl in the edge is then determined by Qkl = (pk – pl)κkl, where pk and pl are respectively the pressures at the vertices k and l. In typical microvascular networks vessel diameters are on the order of 10 μm, and blood flow velocities are on the order of 1 mm/s, so the Reynolds number, which represents the relative importance of inertia to viscous stresses, is Re = UL/ν ≈ 4 × 10−3, using the viscosity of whole blood ν ≈ 2.74 mm2/s. Since Re ≪ 1 inertial effects may be neglected, and the conductances of individual vessels will be obtained by Hagen-Poiseuille’s law [1]:
| (1) |
where κ is the conductance, μ is the blood viscosity, ℓ is the vessel length, and r is the vessel radius. In ascribing a well-defined pressure to each vertex within the graph, and applying the Hagen-Poiseuille law to compute edge flows from pressures, we assume that there are unidirectional flows within each vessel, ignoring the entrance and exit effects that occur when vessels branch or merge [9]. In capillary networks hydraulic conductances might change with concentration of red blood cells, a physiological effect known as Fahraeus-Lindqvist effect [43], which can change our expression here. Although our method can be generalized to include this effect (see Discussion), we will use our default model (1) throughout this work.
The networks we consider consist of vertices and predescribed edges where conductance may be positive (or zero if required by the algorithm, in which case the vessel is pruned) along with two kinds of boundary conditions on vertices (Fig. 3). At any vertex in the network we can either impose Kirchoff’s first law (conservation of flux)
| (2) |
where qk is the total flow of blood entering the network (or leaving it if qk < 0) at vertex k, or we impose (i.e. pressure is specified). We say a vertex is in (or is Dirichlet) if pressure is specified, or in (is Neumann) if Kirchhoff’s first law is imposed (possibly with inflow or outflow). This system of V linear equations forms a discretized Poisson equation with Neumann and Dirichlet boundary conditions imposed on selected vertices, and the flow is uniquely solvable if and only if each connected component of the network (connected by edges with positive conductances) either has at least one Dirichlet vertex or with sum restricted to the component [29, 8]. The uniform flow network has the flows on all edges being as uniform as possible, that is the flows κkl(pk – pl) on some subset of the edges are close to being identical (See Section 2.4 for a detailed discussion). However, to understand the tradeoffs between perfusion and other network objectives, we may also add e.g. total viscous dissipation as an additional cost. We therefore have the general goal of how to tune the conductances within the network to minimize arbitrary predetermined objective function f({pk}, {κkl}), where {pk} means the set of all pk’s and {κkl} denotes the set of all κkl’s. Previous works (see Table 1) have shown how to generate networks that minimize the total viscous dissipation occurring within the network: .
Fig. 3.
Transport network with the Dirichlet vertices and Newmann vertices . In our representation we imagine Dirichlet vertices connected to fluid reservoirs allowing pressure to be imposed, and Neumann vertices to syringe pumps, allowing inflows or outflows to be imposed [8]. Loops are shown in arrows.
This optimization is complicated by the coupling of the pressures {pk} and conductances {κkl} through Equations (2). Since the relationship between {pk} and {κkl} is holonomic, we may incorporate it into a functional via Lagrange multipliers. Lagrange multipliers are a widely used method to solve optimization problems when the variables are subject to holonomic constraints (i.e. must solve some differentiable equation). They allow us to treat the variables as if they were independent for the purpose of calculating partial derivatives, at the cost of introducing new auxillary variables – one for each constraint [11]. The functional that we want to minimize in this paper will take the form:
| (3) |
which has VN +1 Lagrange multipliers: a set enforcing Kirchoff’s first law on Neumann vertices (the set with ), and a single multiplier λ that constrains the amount of energy that the organism can invest in pushing blood through the network and in maintaining the vessels that make up the network. The transport constraint is made up of two terms: ∑κkl(pk – pl)2 represents the total viscous dissipation within the network, while represents the total cost of maintaining the network (the material constraint) [38, 6, 26], with dkl being the vessel length. K is the total amount of material and dissipation to which the network should adhere. The exponent γ can be altered to embody different models for the cost of maintaining a network. In our default model (Equation (1)) conductance of an edge is proportional to the fourth power of its radius, so if the cost of maintaining a particular vessel is proportional to its surface area (and thus to its radius), then we expect γ = 1/4, while if the cost is proportional to volume then γ = 1/2. In general we need γ < 1 to produce well posed optimization problems (otherwise, the cost of building a vessel can be indefinitely reduced by subdividing the vessel into finer parallel vessels). We initially adopt the same material cost function definition as was used in previous work, i.e. a = 0 [26, 6], but in the latter part of the paper we will incorporate a parameter a > 0 that represents the relative importance of network maintenance and dissipation to the cost of maintaining the network. When presenting optimal networks, we will discuss the effect of varying a (as well as asymptotic limits in which a → 0) upon the network geometry. Since Murray’s work on dissipation-minimizing networks [38, 37] is equivalent to minimizing the constraint function [8], we will adopt the shorthand of calling the network cost term the Murray constraint.
Table 1 gives a systematic description of previous work on minimizing functionals across networks, as well as outlining the new results that will be presented here on the optimization of (3).
2.2. A gradient descent method with extended Lagrange multipliers.
At any local minimum of Θ, each of the partial derivatives of (3) must vanish. In order to locate such points, we adopt a gradient descent approach, in which κkl are treated as adiabatically changing variables. That is: is calculated, and an optimal perturbation of the form is applied to ensure Θ decreases each time the conductances in the network are updated. At the same time, the other variables in the system, namely {pk, μk, λ}, are assumed to vary much more rapidly, to remain at a local equilibrium, so that:
| (4) |
Our ability to perform gradient descent therefore hinges on our ability to solve the system of 2VN + 1 equations (4) for each set of conductances {κkl} that the network passes through on its way to the local minimum. Fortunately it turns out that for general target functions f only one nonlinear equation in a single unknown variable needs to be solved for to solve all of the conditions (4); the other equations are linear and can be solved with relatively low computational cost.
Because we will consider multiple variants of the Murray constraint, in what follows we will write the summand that enforces the Murray constraint in the general form: λg({pk}, {κkl}). Then the condition that , , merely enforces the system of mass conservation statements at each Neumann-vertex in the network (2). These equations represent a discretized form of the Poisson equation and can be solved by inverting a sparse VN × VN matrix with O(E, VN) entries [29]. That is, we write:
| (5) |
where fk = qk is the prescribed inflow at Neumann vertices and , the prescribed pressure at Dirichlet vertices. –D is a form of graph Laplacian:
| (6) |
where κ(1) = 1. (For any κ(1) ≠ 0 D is full rank; we will make use of other positive constant values for κ(1) later.)
To solve for {μk}, we consider the system of equations , :
| (7) |
If λ, {pk} and {κkl} are all known then these equations again take the form of a discrete Poisson equation, however, just as with the solution of the pressure equation, these equations themselves do not admit unique solutions unless a reference value of μk is established. If , i.e. if pressure is specified at least one vertex within ( , E), then and Eqns. (7) admit a unique solution; otherwise the μk’s are determined up to a constant (see Appendix A). For some forms of target function f and constraint function g, we will show that μk’s for the minimizer are directly related to the pressures, with no need to solve the Poisson equation by a separate matrix inversion.
However, to use Equation (7) to solve for μk it is still necessary to know the Lagrange multiplier that enforces the Murray constraint (i.e. λ). The simplest way to derive λ is to dictate that the variational of the constraint function should vanish when κkl is updated, since the constraint function should remain constant under changes in conductances, i.e.:
| (8) |
(we set δpk = 0 if ) where
| (9) |
At this point in our calculation {δpk} and {μk} are undetermined. The Lagrange multipliers {μk} can be solved in terms of the still unknown λ from (7) (see Appendix A). The {μk} are linear functions of λ since (7) is a linear system. To obtain δpk for each we calculate the variational in Kirchhoff’s first law:
| (10) |
When written in matrix form, the matrix multiplying {δpk} is again the negative of the graph Laplacian, –D. Thus {δpk} can be solved in terms of λ so long as the original matrix system is solvable for {pk}. Since {μk} are linear in λ, {δpk} are also linear in λ, which implies that the right hand side of Equation (8) is linear in λ. Therefore λ can be solved in closed form from Equation (8), and the optimal variation δκkl can be determined from equation (9).
With {pk}, {μk}, and λ solvable given {κkl} we can perform gradient descent using Equation (9) and numerically approach a minimizer. However our descent method has the following limitations: 1. For finite step sizes α, conductances may drop below 0 when perturbed according to Equation (9). 2. The method only conserves the Murray function up to terms of O(δκ).
To avoid negative conductances we truncate at a small positive value ϵ at each step, i.e. set:
| (11) |
To ensure that the constraint is exactly obeyed we then project the conductances onto the constraint manifold g({pk}, {κkl}) = 0, via a projection function:
| (12) |
Throughout this work we consider three possible projection functions: One choice is to project according to the normal of the constraint surface:
| (13) |
The value of β must be chosen numerically to ensure that exactly. This entails recomputing the pressure distribution for each β value, and secant search on β to obtain the root. Another approach we have followed is varying the parameter λ. This method has comparable complexity to projection on ; since the {μk} depend linearly on λ via Equation (7), depends linearly on the parameter λ. However, just as with the projection method, we must still recompute the for each trial set of . Moreover, for some target functions f or constraint functions g, it is difficult to derive closed-form expressions for λ (i.e. to calculate the partial derivatives and ). In this case λ may only be computed numerically, by solving . A third approach that we have adopted is to simply scale the conductances:
| (14) |
where β is chosen to satisfy the Murray constraint. This method produces theoretically suboptimal corrections on the conductances, but it is typically easy to compute a value of β that satisfies the Murray constraint. In particular, under certain boundary conditions, e.g. , within each connected component of the network (meaning that all pressure vertices within a single connected component have the same imposed pressures, which is trivially true if there is only one Dirichlet vertex in each connected subcomponent of the graph), a rescaling of the conductances throughout the network leaves the fluxes on each edge unaffected. For a uniform flow network, this rescaling affects the constraint function g, but not the target function f.
2.3. Calculating minimally dissipative networks.
To calculate minimally dissipative networks we set the target function to be the dissipation ∑⟨k,l⟩=1, k>l κkl(pk – pl)2 and the constraint to be the total amount of material. Thus the function to minimize becomes:
| (15) |
Here we ignore dkl since we assume all the vessels have the same length which may be scaled to 1 by choice of units, but different vessel lengths can be readily incorporated by adding dkl back in the following equations.
The equations for adiabatic variation of pk and μk are derived from
| (16) |
and the fixed pressure boundary condition on pressure vertices allows us to specify that:
| (17) |
The μk are therefore solving a variant of the Kirchhoff flux conservation equations:
| (18) |
with D as defined in Equation 6.
This system can be solved for μk under the same conditions as the presure equations being solvable (see Appendix A). In particular if, as in the network topologies we study in this work, the only pressure boundary conditions imposed at vertices in are of the form p = 0, then μk = 2pk, , i.e. μk’s represent the pressures. Now we calculate the derivatives of Θ with respect to the conductances:
| (19) |
In general we determine λ from Equations (8,9,10). However the constraint function g is independent of {pk} in this case, so Equation (8) becomes
| (20) |
and we can solve λ directly in terms of {pk}, {κkl}:
| (21) |
p
As described in Section 2.2 we project {κkl} along after each step of the algorithm. At each step of the algorithm, we solve for the pressures pk from the conductances {κkl}, then the μk, and then descend according to Eqn. (19). Analyzing minimal dissipation on networks allows us to compare the performance of the algorithm described in this paper with previous work [6, 26] (See Section 3.1).
2.4. Calculating microvascular networks with uniform flow.
We now turn to other target functions that have not been extensively studied. At the level of micro-vessels it is likely that uniform flow rather than transport efficiency is the dominant principle underlying network organization, as shown in the comparison between the zebrafish trunk network with its untuned counterparts (Fig. 2). We frame this question more generally, i.e. ask what organization of vessels achieves a given amount of flow on all edges or equivalently, how the flow variation
| (22) |
may be minimized by optimal choice of conductances κkl
To simplify the target function we expand the function f and abandon the constant term:
| (23) |
Under the assumption that the total flow on all edges is conserved, i.e.:
| (24) |
the function f can be reduced to
| (25) |
by ignoring constants. The assumption (24) is valid in networks provided that the network may be divided into levels: that is, a series of control surfaces may be constructed between source (inflow to the network) and sink (outflow from the network), with each edge intersected by exactly one control surface and each path from source to sink intersecting all control surfaces (Fig. 4). We call networks with this property stackable. The stackability of a network depends both on its geometry and the positions of sources and sinks: a honeycomb grid is also stackable when source and sink are arranged across the diameter of the network, but the square-grid network is not stackable if the sink were on the bottom instead of the right corner. Then since the total flow across each control surface is the same, the total flow over all network edges is ∑k>l,⟨k,l⟩=1 Qkl = SF where F is the total sink strength and S is the number of control surfaces. Both symmetric branching trees and quadrilateral grids (such as the one shown in Fig. 4) are examples of stackable networks, and both can be used as simplified models of microvascular transport networks [23].
Fig. 4.
A quadrilateral grid (black) can be divided using a set of non-intersecting control surfaces (red dashed lines) such that each edge in the grid is intersected by exactly one control surface.
For the model capillary bed network we have only one pressure vertex, which means that a multiplicative scaling in all the conductances does not affect the flow. Therefore solving unconstrained optimal networks is equivalent to solving those with material constraints. Without any constraint the function to be optimized can now be written as
| (26) |
To do the gradient descent we calculate
| (27) |
| (28) |
At each step we can solve for μk by setting in Eqn. (27), along with μk = 0, , and we can calculate the gradient from Eqn. (28). Note that here we have neither Murray nor material constraint, so a numerical projection is not required to ensure that the constraint is being exactly met, and the network can be obtained by repeating the procedure until convergence (See Section 3.2).
2.5. Calculating embryonic zebrafish trunk vasculatures with uniform flow.
To compare putative network optimization principles on the zebrafish trunk vasculature, we start by identifying our model for the trunk network. Blood flows into the trunk of the zebrafish from the heart through the dorsal aorta and then passes into minute vessels called intersegmental (Se) vessels. Blood then returns to the heart via the cardinal vein. These vessels are arranged just like rungs (Se) and parallels (cardinal vein and dorsal aorta) of a ladder (Fig. 8A). Most gas exchange in the network is assumed to occur in the Se vessels. As the zebrafish develops further minute vessels form between the Se vessels, converting the trunk into a dense reticulated network [25]. We focus on the mechanisms underlying flow distribution in the main fine vessels. Since the zebrafish trunk network is symmetric, we can consider only half of the network consisting of the aorta and intersegmental arteries, designated by vertices v1, …, v2n+1 and edges e1, …, e2n with n being the number of Se vessels. Since this topology does not satisfy the assumption (24) we have to consider the full uniform flow target function:
| (29) |
where is a predetermined flow for all the capillaries (in the following arguments edge-defined quantities such as Qi are indexed with the edges, and vertex-defined quantities such as pi are indexed with the vertices). Using this indexing scheme, the function to be optimized becomes:
| (30) |
Fig. 8.
Minimal dissipative networks for zebrafish trunk vasculature do not explain observed morphology. (A) The zebrafish trunk vasculature can be simplified into a ladder network with arterial (red) and venous parts (blue). The edges e1, e3, …, e2n-1 are aorta segments and e2, e4, …, e2n are capillaries. We use n = 12 in all the following calculations on zebrafish network. (B) The optimal dissipative network with and fixed inflow does not correctly describe the zebrafish trunk network since all the conductances are concentrated on the first capillary (red circle), and the whole aorta is deleted (blue cross). In this calculation we imposed a fixed inflow on v1 and fixed zero pressure on vn+1,…,v2n+1. We started with κ = 20 for aorta segments and κ = 1 for capillaries to reflect the difference in radii in real zebrafish. This initial condition is used for all the following simulations. (C) The optimal dissipative network with and fixed outflows has a tapering aorta (blue cross) and capillaries with the same conductances (red circle). We imposed zero pressure on v1 and fixed outflows on vn+1, …,v2n+1 with vn+1 taking half of the total outflow (i.e. F) and vn+2,…, v2n+1 evenly dividing the other half of F. (D) However the pressures on the ends of capillaries are decreasing to maintain uniform flows among capillaries, which is non-physical since this means that the blood flows toward the tail in the principal cardinal vein, due to the aorta-vein symmetry.
Just as in Section 2.4 we do not need to introduce a Lagrange multiplier enforcing the material constraint because the target function only depends on flows, and we can scale all conductances to realize any material constraint without affecting the target function. For performing gradient descent method on this function we calculate the partial derivatives of Θ:
| (31) |
| (32) |
Then we impose the physical boundary conditions, i.e. fixed inflow into the network and zero pressure on the ends of the main aorta and the capillaries, and perform gradient descent to find the optimal network (See Section 3.3).
Since no constraint is imposed on the uniform flow zebrafish trunk network the explicit relation between vessel radius and conductance does not need to be specified (see Section 2.1). Therefore when we compare the uniform flow network to experimental data in Section 3.3 we can assume that, instead of the default model (1), the occlusive effect of red blood cells contribute to the effective vessel conductance [9].
2.6. Calculating uniform flow network on capillary beds under Murray’s constraint.
So far we have followed previous work [6, 26] by calculating all of our optimal networks under constraints on the total material (or without constraints if the target function does not involve conductances). However both material investment and transport costs (i.e. dissipation) may contribute to the total cost of a particular network. We modify our cost function, g, to include both costs. In this case depends on both pressure and conductance, and the full mechanism for keeping g constant during the gradient descent needs to be used. Specifically now we are minimizing the function
| (33) |
with and a > 0. To calculate the optimal network by this method we need an explicit formula for λ.
We introduce several notations to be used later. Suppose {bij} is a set of quantities defined on the edges of the network. For any real constant c we define the matrix for the graph Laplacian with specified boundary conditions as
| (34) |
We also abbreviate . In the notation of Equation (6) D = Mκ. We define D(n) = Mκn for notational convenience. For a quantity v that is defined on the vertices of the network (such as pressure) we define the graph difference vector as
| (35) |
where ε denotes the set of ordered pairs of edges so that each edge only appears once in ε. Now we can derive explicit formulas for λ and δκ. From we obtain μ = D−1D(2)p + 2λap (recall here we have , , and so:
| (36) |
We determine λ from the variational:
| (37) |
This formula depends on δp; the change in p produced by the change κ ↦ κ+δκ. If we assume we can write Equation (10) in matrix form as
| (38) |
so
| (39) |
(Equation (38)) can be modified by adding a non-zero vector on the right hand side, if inhomogeneous pressure boundary conditions are applied.) Thus if we define auxiliary variables: β ≐ γκγ−1 – a∇p2, X ≐ κ∇p2 – ∇(D−1 D(2)p)∇p, so that δκ = –α(λβ + X), then:
| (40) |
Finally we can write down the formula for λ as
| (41) |
The value of λ in Eqn. (41) ensures that g remains constant up to O(δκkl) terms. However, we must also adjust {κkl} at each step to exactly maintain the constraint following the method given in Section 2.2. In previous applications since g was a function of κ alone this additional projection step did not require perturbation of pressures. Now both the change in κkl and the change in flow must be considered when adjusting conductances. We calculate here the additional terms created by the pressure variation. To project along the constraint surface normal we need to calculate the normal vector:
| (42) |
To obtain we differentiate Kirchhoff’s first law with respect to κkl:
| (43) |
or:
| (44) |
Notice that since these pi are fixed by the boundary conditions. Then we can solve for , 1 ≤ i ≤ V by solving the linear system (solvability was discussed in Appendix A) and calculate the normal vector.
2.7. Calculating uniform flow zebrafish microvascular network under Murray’s constraint.
The gradient descent method with Murray constraint closely follows Section 2.6 with the target function
| (45) |
where ε = {(k, l : ⟨k, l⟩ = 1, k < l} and the index convention here follows Fig. 8A. Here Ikl = 1 if and only if the edge kl is an intersegmental vessel. To carry out the gradient descent we calculate
| (46) |
Now given {κkl}, {pk} we need to solve for {μk}, λ for the algorithm. Again from we get
| (47) |
where
| (48) |
To solve for lambda we can use Eqn. (41) if we calculate β, X in Eqn. (46). Since the target function here is the same as that in Section 2.6 we have . The χ can be calculated as
| (49) |
With these expressions we can solve for λ by Eqn. (41) and obtain the expression for {μk} by Eqn. (47). Then we can carry out the gradient descent by Eqn. (46). Notice that if we set , Ikl = 1 ∀⟨k,l⟩ = 1 then f is the same as in Section 2.6 and the expression of X agrees with the auxiliary variable defined in that section.
3. Results.
3.1. The algorithm finds known minimally dissipative networks.
To evaluate the ability of our algorithm to find optimal networks we start with minimally dissipative networks that have been thoroughly studied. It has been proven that, when the exponent in material constraint γ is less than one, as is the case for vascular networks, the minimally dissipative networks with flow (Neumann) boundary conditions contain no loops [17] (this result was generalized to both flow and pressure (Dirichlet) boundary conditions in [8]). Those networks are also known to satisfy Murray’s law [38, 51], which states that the sum of third power of vessel radii in the same hierarchy is a constant. Although the original derivation by Murray in 1926 was based on local optimization in a network in which flows are unaffected by changes in conductances, Murray’s law is also necessary for a network to be a global minimizer [8]. To see whether our algorithm recovers these theoretical properties we consider a model for the branching vasculature in animals [54] (Fig. 5A). We impose distributed sinks on the bottom and a single source on top (with pressure imposed to ensure solvability [29]). We apply the algorithm described in Section 2.3 starting from a network with no prior knowledge, i.e. all edges have the same uniformly random distribution of conductances. All 20 networks we simulated are trees (Fig. 5B), agreeing with previous theoretical work. To further validate our algorithm we calculate the Murray exponent by minimizing the coefficient of variation (CV) of sums of vessel radii in the same hierarchy to the exponent (Fig. 5C). The Murray exponents cluster tightly around 3 (3.01 ± 0.03, mean ± sd, 20 networks), which is the exponent in Murray’s law [38]. So our algorithm recovers both properties of minimally dissipative networks [6].
Fig. 5.
Minimally dissipative networks agree with previous work (with target junction Σκkl(pk – pl)2 and material constraint with ). (A) We use a branching grid as our basic topology. There are N = 20 layers of vertices and a total of 380 edges, connecting a single source (red filled circle) with 8 sinks (red open circles). (B) A minimal dissipative network calculated by gradient descent method exhibits tree structure as predicted in [17]. We imposed a fixed zero pressure on the top vertex and 8 evenly distributed outflows on the bottom. Each edge is initially assigned a positive uniformly random conductance to impose no prior knowledge on the algorithm. (C) Murray’s law [38] is obeyed by the minimal dissipative network, indicated by the nearly constant sum of radius to an exponent 3.004 among different hierarchies in network shown in (B).
3.2. Uniform flow qualitatively explains network structure of capillary beds.
Here we compare these organizing principles on the topology of capillary beds [53, 36] (Fig. 6A). We follow Section 2.3 to calculate minimally dissipative networks, and Section 2.4 to calculate uniform flow networks. We assume that the capillary bed is supplied by a single arteriole and blood leaves from a single venule. Networks formed according to the minimal dissipation principle consist of a single pipe following a geodesic route (Fig. 6B, C), which can be shown theoretically to be the optimal solution. Specifically, since the network cannot contain loops, and connects a single source to a single sink, there can only be one pipe in the network. Longer pipes have less material in each segment but the same flows as shorter pipes, so have higher total dissipation. Finally uneven material distribution has been ruled out by [16]. This morphology contrasts sharply with empirically observed capillary structure [53, 30, 36], suggesting that minimizing dissipation is not the dominant factor for capillary systems.
Fig. 6.
Minimally dissipative networks consist of a single conduit on capillary bed topology (with target function Σ κkl (pk – pl)2 and material constraint with on a 10×10 square grid). (A) We represent the capillary bed network by a square grid where a single source and a single sink locate at upper-left and lower-right corners respectively. (B, C) Different initial conductances produce different optimal networks, but all optimal networks are made of a single wide conduit. Here we use a constant step size throughout the process, and at each step we project by surface normal to maintain the material constraint. Each edge is initially assigned a positive uniformly random conductance to impose no prior knowledge on the algorithm.
Uniform flow networks, on the other hand, might explain capillary bed morphology. Starting from a uniformly random configuration, our algorithm finds a network with an apparently random distribution of materials (Fig. 7A). The conductances have a smaller variance compared to the initial configuration, but do not have a clear pattern otherwise (Fig. 7B). While the uniform flow networks more closely resembles a capillary bed than a minimally dissipative network, it is not clear what quantitative characters can be used to compare the networks. As discussed in Section 2.4, the uniform flow target function simplifies to on capillary bed topology, which allows us to analytically derive properties of uniform flow networks.
Fig. 7.
Uniform flow networks have a seemingly random morphology, but can be shown to have the same flows as a uniform conductance network (Here we show a 20×20 square grid network with 400 vertices). (A) An optimal network has an apparently random distribution of conductances. The edge widths are proportional to the conductances. (B) A closer view reveals that the conductances of the optimal network (blue circle) are quite different from uniform (red cross), and do not seem qualitatively different from initial conductances drawn from a uniform random distribution (green star). The conductances are normalized such that are the same. Each edge is initially assigned a positive uniformly random conductance to impose no prior knowledge on the algorithm. (C) The differences of flows from those in a uniform conductance network (blue circles) are uniformly zero, while the differences of initial flows from those in a uniform conductance network (green stars) are not.
Theorem 1. Any critical point of the target function (26) in which has the same set of flows as a uniform, conductance network with the same support on edges. That is, suppose we let κkl, Qkl be the conductances and flows on the stationary network, and , be those on the uniform, conductance network, i.e.
| (50) |
Then
| (51) |
(The proof will be presented in the end of this subsection.) The theorem states that a uniform flow network, i.e. a local minimum of target function (26), will have the same flow as the network where all the edges with positive conductances are assigned unit conductance. In order to evenly distribute the flows, networks with all allowed edges present will have higher uniformity than networks in which some subset of edges have been cut (indeed among 100 uniform flow networks those with all edges present have an average value for the target function of 1.9465, compared to 1.9470 for networks with at least one edge cut). Therefore the uniform conductance network can predict the flows in all uniform flow (Fig. 7C). While the flows in uniform flow networks are tightly constrained, many possible configurations can achieve the same uniformity, and there is no visible pattern in the distribution of conductances for those networks, unlike minimally dissipative networks.
Proof of Theorem 1. The assumption that all pressure vertices have pressure zero is really an assumption that all pressure vertices have the same pressure: In the latter case since a constant shift in all pressures does not change the flows. To find the critical points of Θ we recall the derivatives in Eqns. (27, 28), along with by assumption. First we show that a uniform distribution of conductances would result in a critical point ({pk}, {μk}, {κkl}), by rewriting the equation (27) into the matrix form:
| (52) |
Here Dkl is in Equation (6) and –D(2) is another graph Laplacian:
| (53) |
in which the matrix is made full-rank if κ(2) > 0 (similarly to the κ(1) constant in D). The κ(1) entries in Dkl enforce μk = 0 at each . The entries in D(2) are not needed since pk = 0 at each , but we add values here to emphasize the symmetry between {μk} and {pk}. Now consider uniform conductances, i.e. κkl = a > 0 ∀⟨k, l⟩ = 1. We can set κ(1) = a and κ(2) = a2. Then we have D = aD(2) and since D is invertible (see Appendix A)
| (54) |
Now this set of μk’s and pk’s then also satisfies because
| (55) |
Thus the network with uniform conductances along with pressures solved from the Kirchhoff’s first law is indeed a critical point.
Now we show that any interior critical point, i.e. satisfying κkl > 0 ∀⟨k, l⟩ = 1, has the same flows as the uniform conductance network. We will see that for any such network the μk’s represent the pressures of the uniform conductance network. Since all the conductances are positive we have . Assume for now pk – pl ≠ 0 ∀⟨k, l⟩ = 1. Then from Equation (28) we obtain that the (μk} obey a system of equations
| (56) |
which may be rewritten as
| (57) |
Kirchhoff’s first law in terms of μk’s then reads
| (58) |
In matrix form the equations can be written as
| (59) |
where Fk = qk if and is zero otherwise, and D is defined as for network made up of unit conductances:
| (60) |
Because D is invertible we can solve for μk’s from Eqn. (59). The {μk}’s represent the pressures that would occur at each vertex if all conductances in the network were set equal to 1, creating uniform conductance network. Since the flows Qkl = μk – μl are determined by μk’s we conclude that the locally optimal networks would have flows the same as in the network of uniform conductances.
To derive (56) from (28) we had to assume that pk ≠ pl whenever ⟨k, l⟩ = 1. Consider the case where in the optimal network pk – pl = 0 for some ⟨k, l⟩ = 1. For these (k, l)’s Eqn. (56) no longer holds and we have to set in Eqn. (27) to obtain extra information. We claim that μk = μl if pk – pl = 0. This can be seen from a loop current argument similar to that used in Appendix A to prove existence and uniqueness of the {μk}. Specifically, suppose for contradiction that μk1 ≠ μk2 for some pair of vertices with pk1 – pk2 = 0 and without loss of generosity let μk1 > μk2. If k1 and then μk1 = μk2 = 0; so at least one of the two vertices does not lie in . If then implies:
| (61) |
Since Eqn. (56) holds when pk – pl ≠ 0 we have
| (62) |
Since κkl > 0 ∀⟨k, l⟩ = 1 and the sum includes the negative summand κk2k2 (μk2 – μk1) we can find l for which μl < μk2 and pl = pk2. We let k3 = l and repeat the process to find a neighbor of k3 such that pl = pk3 but μl < pk3. We then can keep repeating this process until we reach a vertex (no vertex may be visited more than once). We have imposed μkN = 0. Now we trace through increasing μk’s starting from k2 and k1 and we get , such that and . By the same reasoning we have and we reach a contradiction since . Therefore μk = μl when pk = pl and Eqn. (56) actually holds for all ⟨k,l⟩ = 1. Again we conclude that the flows of a locally optimal network with non-zero conductances are the same as the flows in the uniform conductance network.
Finally we discuss the boundary case where κkl = 0 for some ⟨k,l⟩ = 1, and we denote this set of edges by I. To avoid ill-posedness of pressures we require that that the matrix D is invertible. In this case we do not have Eqn. (56) for κkl = 0 because need not be zero on these edges. However since there is no flow through edges with κkl = 0 we can write down Kirchhoff’s first law as
| (63) |
where −D is again the graph Laplacian, but with zero conductance edges removed and other edges with conductance 1:
| (64) |
We can safely remove the zero conductance edges from the network because the difference μk – μl no longer represents the flow Qkl, and that we know Qkl = 0 for these edges. By assumption we can solve for μ from Eqn. (63) so {μk} represent the pressures within the uniform conductance network, but with edges κkl = 0 removed from the network.
3.3. Optimal zebrafish microvasculature.
Comparison of the different organizing principles on our model capillary bed network suggests that uniform flow is prioritized more than transport efficiency for these networks. The comparison remains qualitative because although capillary beds in e.g. salamander skin do resemble square grids [36] (and Fig. 1A), this resemblance is only approximate, so it is difficult to test our theoretical predictions. The embryonic zebrafish trunk vasculature, on the other hand, can be completely mapped, down to the finest vessels. Moreover, the blood flows can be measured by tracking the motion of red blood cells ([49, 32], as well as our previous work [9]), thereby directly testing the flows predicted in optimal networks.
First we show how far the embryonic zebrafish network is from minimizing dissipation. The optimal morphology of the network depends on the boundary conditions we impose, and there are two conceivable ways to assign boundary conditions. One way is to assume a fixed inflow from the heart into the trunk from v1. If one thinks of the venous part of the trunk as symmetric to the arterial part then the pressures in the middle of fine vessels need to be the same, and we can set pn+1 = pn+2 = ⋯ = p2n+1 = 0 (since a constant shift in the pressure does not change the flow). Under this boundary condition we can find the minimally dissipative network by methods described in Secion 2.3. The minimally dissipative network eliminates all the edges but e2, on which all the allowed material is used (Fig. 8B). This agrees with prior theoretical predictions since minimally dissipative networks cannot have loops, nor can they connect vertices with the same assigned pressure [8]. Therefore there are only n + 1 possibilities: the flow returns through one of the fine vessels e2,…, e2n or the tail e2n−1. Since the shortest route would be going through the first fine vessel e2, the minimally dissipative network concentrates all the material on e2.
Instead of imposing a fixed pressure in the middle of the fine vessels, one can also impose a fixed flow [6, 26, 16] through each vessel. This boundary condition interprets into a uniform flow at each of the vertices vn+2,…, v2n+1, and we can find a distribution of conductances that minimizes dissipation from Section 2.3. Now equal amount of flow passes through each fine vessels, leading to a decaying flow in the aorta from head to tail. Therefore the material is concentrated on the rostral part of the aorta, with all the fine vessels having equal amount of material (Fig. 8C). The imposed fixed flow then requires a decrease of pressure in the middle of fine vessels from head to tail, to compensate for the pressure drop in the aorta (Fig. 8D). Under symmetry of arterial and venous parts, this pressure distribution then creates an unrealistic blood flow in the vein toward the tail, i.e. away from the heart.
Since the principle of minimal dissipation is unable to generate networks that resemble the observed zebrafish trunk microvasculature, we look at uniform flow networks and see if they match the observed vasculature. To avoid back flow in the vein we impose fixed pressure in the middle of fine vessels, and a fixed flow into the trunk from v1. The zebrafish trunk vasculature is not stackable, so we use the square error target function (29) instead of (25), and we use methods described in Section 2.5 to find the uniform flow network. Instead of concentrating all the materials on the first capillary or tapering the aorta, the uniform flow network has constant conductance along the aorta and conductances on the fine vessels that increase exponentially with the distance from the heart (Fig. 9A). If vessels obeyed the Hagen-Poiseuille law (Eqn. (1)) a 20-fold change in conductance would require a 2-fold change in radii of fine vessels, which is not observed in the real zebrafish trunk [25]. However, these fine vessels have radii that match the size of red blood cells; thus red blood cells almost totally occlude the vessels they pass through, adding a large increment to the vessel’s hydraulic resistance [46]. This increment depends sensitively on the gap distance between the cell and the endothelial wall, which is typically on the scale of 10 ~ 300 nm, and is smaller than the optical resolution for fluorescence microscopy [47]. In our previous work, we proposed an indirect method that measures this increment based on the correlation between the flow speed and the number of cells in each fine vessel [9]. When we compare the measured hydraulic resistance with the resistances of fine vessels in the uniform flow network, we see an excellent agreement between the predicted and measured resistances (Fig. 9B). Our results show that the principle of uniform flow quantitatively reproduces the zebrafish trunk geometry.
Fig. 9.
The uniform flow networks quantitatively explains the zebrafish trunk vascular network morphology. (A) The uniform flow network dictates a constant conductance on aorta segments (blue cross) but assigns conductances to Se vessels that increase exponentially from head to tail (red circle). We scale the conductances such that remains the same for comparison with minimal dissipative networks. We started with κ = 20 for aorta segments and κ = 1 for capillaries to reflect the difference in radii in real zebrafish. (B) The predicted hydraulic resistance (blue dashed curve) agrees well with experimentally measured data (red curve, with 95% confidence intervals). The data is obtained from our previous work [9] under the assumption that the volume fraction of the red blood cells is 0.45 [43]. Theoretical resistances are normalized by the mean since optimization only controls the relative resistances of vessels.
3.4. Murray constraint on optimal networks.
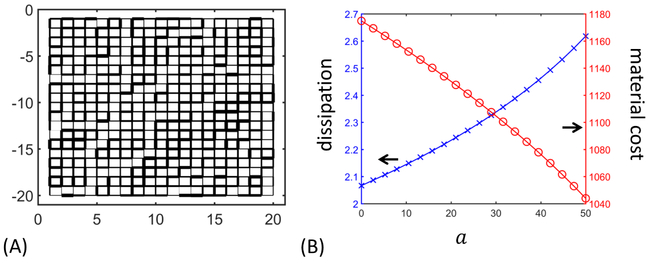
So far we show that capillary networks prioritize uniform flow over minimizing dissipation, but biological networks may be subject to more than one constraint at a time. In capillary networks the total dissipation will be higher when the density of capillaries increases, which in turn requires more energy input. As we noted in the introduction, fine vessels account for half of the cost of blood transport in the human body [21], suggesting that capillaries will be constrained not only in respect to materials but also by dissipation. In this case the function to be optimized takes the full form of (4) with a constant a > 0, representing the relative importance of dissipation compared to material cost. Following Section 2.6 we can calculate uniform flow networks with the model capillary bed geometry under the Murray constraint (Fig. 10A). The uneven distribution of conductances resembles that in Section 3.2, and indeed for 20 networks with a ranges from 0 to 50, the function clusters closely at the optimal value (1.9461 ±9.25 × 10−6, mean ± sd, 20 networks), i.e. that of uniform conductance network. This result is counter-intuitive since we would expect that constraining dissipation would prevent the network from achieving the non-constrained optimal uniformity in flows. However, on the capillary bed topology we can reason by a scaling argument. Suppose we find an optimal network under the material constraint. We calculate the total material cost K of this network. Then we calculate the optimal network in which Murray’s constraint is imposed with allowed total energy K including both material costs and dissipation. Denote by κkl the conductances in the network under Murray constraint, and by the conductances in the optimal network under material constraint. If a is sufficiently close to zero then the target function of Murray network will be lower or equal to that of material network. The reasoning is that although does not hold, we can try to solve for a multiplicative scaling β > 0 that satisfies Notice that Qkl does not change under the scaling for this class of networks, so the value of target function is unaffected by scaling conductances. Now if a > 0 is small enough we expect to be able to find a solution β and is an admissible network in the sense that it obeys the Murray constraint. Thus the optimal network under the Murray constraint must have equal or smaller target function value than the optimal network obeying only the material constraint. By reversing this argument we can see that the optimal networks for small enough a > 0 actually agree with those with a = 0. The question is how large a has to be so that the Murray network is truly constrained by the total energy cost, and the optimal networks under the Murray constraint and under the material constraint diverge. From our numerical simulations we can see that large changes of a can be accommodated with changing the morphology of the uniform flow networks from its unconstrained counterpart. We observe that increasing a decreases material costs (Fig. 10B), which is unintuitive since increasing a adds weight to dissipation, and we might expect this to encourage the network to decrease its dissipation. If we study the curve of as a function of β, the function is U-shaped and diverges when β → 0 or β → ∞. When a increases the total energy increases, and the network has to adjust itself to a low energy state. If the network is on the left side of the U this means increasing β, which increases the material cost to realize the constraint. In contrast when the network is on the right side of the curve, decreasing β will be the only way to lower the total energy, which explains the trends depicted in Fig. 10B. The simulation suggests that, on the capillary bed topology, constraining dissipation seems to have little effect on the morphology of uniform flow network.
Fig. 10.
Uniform flow networks under Murray constraint have the same flows as the analytic solution in Sec 3.2, but exhibit tradeoff between dissipation and material cost as a increases. (A) For small a the uniform flow network with Murray constraint is equivalent to a network with material constraint. The network is constrained with a = 36.8, and the solution is selected from the best network visited during the gradient descent, with relative error in energy cost < 10−4, as in the following simulations. Widths show the relative conductances. (B) When a is increased, the dissipation in the network increases (blue crosses), while the material cost decreases (red circles). The simulations were carried out in the manner of numerical continuation, i.e. the simulation for each a starts with the solution from previous a, and the simulation for a = 0 starts with a random conductance configuration. All the networks have the same fixed total energy cost K = 1174.9.
Would uniform flow models of the zebrafish trunk follow the same trend? Using methods described in Section 2.7, we can calculate uniform flow networks under Murray constraint, with different values in a. When a is close to zero, the constrained networks have optimal flow uniformity, and dissipation increases as in the case of capillary bed topology (Fig. 11A). However, at a critical value around ac = 33.3, a phase change occurs; the flows become less uniform and dissipation starts dropping (Fig. 11A). The network morphology changes dramatically, in particular conductances of the fine vessels become non-monotonic, and the flows are concentrated toward the head side of zebrafish (Fig. 11B, C). Our results suggest that, when a biological network has multiple constraints to meet, the network morphology will conform only to the tightest constraint, and then if the relative weight of these constraints is changed, the network changes dramatically only when another constraint becomes dominant.
Fig. 11.
Uniform flow networks on zebrafish trunk topology exhibit a phase transition when a, the relative cost of dissipation to total material, is varied in the Murray constraint. (A) The target function remains zero for small a until ac = 33.3 where a phase transition occurs and the value of target function suddenly increases (blue crosses). The dissipation (red circles) increases with a for a < ac just as for the capillary bed, but has a sharp decrease right after the critical value ac. Here we adopted numerical continuation as in Fig. 10B, but when a local minimum around previous initial condition does not satisfy Murray constraint the initial configuration at a = 0 is reused for the initial conductances. The minimal value for the total energy cost upon scaling of conductances is used whenever the Murray constraint cannot be maintained. The Murray energy K is maintained to be 70.43 in all simulations by the projection method described in Section 2.6. The total energy cost is fixed to that of initial configuration (with uniform conductances in fine vessels being 1 and those in aorta being 20) when a = 1. The solution is selected from the best network visited during the gradient descent, with relative error in energy cost < 10−4 (B) The conductances of capillaries change qualitatively after the phase transition. The morphology resembles the unconstrained network (Fig. 9A) before the phase transition (blue cross and red circle), but changes qualitatively afterwards (green square). (C) The flows are uniform before the phase transition (blue cross and red circle), but decrease from head to tail afterwards (green square).
4. Discussion and Conclusion.
Microvascular network mapping tools have until recently lagged far behind our ability to measure the morphology of large vessels using e.g. plasticization. However, recent advances in microscopy have created detailed digitization of microvascular networks [7, 5, 18]. For example Knife-Edge Scanning Microscopy (FESM) and Micro-optical Sectioning Tomography (MOST) have been used to map the blood vessels within rodent brains to micron resolution [34, 55]; while mapping the blood vessels in the human brain is one of the central goals of the BRAIN initiative [24]. Meanwhile long working distance two photon microscopes can be used to directly measure blood flows within living rodent brains [7, 15]. But the revolution in microvascular imaging has not been matched by new theorizing about what are the organizing principles for microvascular networks. For large vessels minimization of dissipation and the concomitant laws for vessel radii have proven to be powerful organizing tools with which to understand both the morphology of healthy and pathological cardiovascular networks [42]. By comparison it is not known whether any optimization principles underly real microvascular networks. In this work we explore the principle of uniform flow as a candidate organizing principle for microvascular networks [9] in both an idealized capillary bed geometry and the real embryonic zebrafish trunk. To do so, we devised an algorithm for optimizing general functions on transport networks. On the model capillary bed we showed analytically that the flows in a uniform flow network are identical to a network with constant conductances. In the zebrafish trunk, achieving uniform flow requires careful tuning of vessel conductances, and implies a distribution of conductances that matches the real zebrafish trunk.
Finally we expose a phase transition that occurs if dissipation costs are also modeled, as the relative size of transport and material costs is increased. Surprisingly, networks that are constrained by both material costs and dissipation do not continuously interpolate between optimizing uniformity and optimizing dissipation, but instead are initially invariant under changes in the cost of dissipation, and then undergo a sudden phase transition-like reconfiguration when this cost exceeds a certain threshold. Although further work is needed to show whether such transitions occur for other combinations of functions, it offers two surprising biological insights. First, no two functions will likely shape the network simultaneously, explaining the remarkable power of single target functions to predict the geometry of biological networks. Secondly, the departure of real zebrafish networks from the optimum for creating uniform distributions of fluxes cannot readily be explained as a result of the network needing to balance tradeoffs between multiple target functions. We believe that the non-optimal features of the zebrafish trunk network are more likely due to another cause; for example variability (e.g. due to biological noise) during vessel formation. For example a 10% variation in vessel radius, as has been observed in small arterioles [27], leads to a 40% variation in vessel conductance under the Hagen-Poiseuille model (1), and the variation will be even larger when the occlusion of red blood cells is considered [46]. Under even such mild perturbations, variation in red blood cell flux can reach 0.7 (1/s) standard deviation, comparable to the variations measured for real zebrafish in [9].
The principle of uniform flow may be relevant to other biological transport networks as well. Many organisms build networks for transport, such as plants and slime molds [26, 2]. Previously the loopy structure of leaf vascular networks was attributed to damage resistance [26]. Yet uniform flow also favors loopy structures, and provides an alternative optimal principle for these networks.
Real blood is a multi-phase fluid containing both plasma and cells. By assuming Hagen-Poiseuille law we omit two of the complexities of real blood flow: non-Newtonian viscosities and ZweiFach-Fung effect [43]. Hydraulic conducances depend both on vessel radius (the Fahraeus-Lindqvist effect) and on the concentration of cells due to both the occlusion of red blood cells in narrow capillaries and the disordered flow of cells within large vessels [9, 46, 48]. The Zweifach-Fung effect refers to variation of hematocrit between vessels following uneven partitioning of red blood cells at branching points [56, 14]. Our algorithm has the flexibility to incorporate the Fahraeus-Lindqvist effect by changing the constraint term into a non-power law representation of vessel radius. For simplicity we considered the default model (1) throughout the paper, except when comparing uniform flow zebrafish trunk vasculature to experimental data in Section 3.3. To incorporate the Zweifach-Fung effect the hematocrit has to be re-calculated each time we calculate blood flows which will affect oxygen perfusion. Although [41] proposed an empirical formula for the Zweifach-Fung effect, the effect of vessel radius and cell geometry upon the Zweifach-Fung effect is still under active research [4, 50, 12].
Throughout this work we have used uniformity of flow as a proxy for uniform perfusion. This is defensible if perfusion in limited by the rate of delivery of oxygenated red blood cells to a particular tissue. However it is also affected by the rate at which it subsequently diffuses through the surrounding tissue – which is in turn affected by levels of oxygen saturation and pH in the tissues and blood and may indeed vary in the same tissues due to functional activation of capillaries in response to time varying metabolic demands [3]. Thus although we have previously presented direct evidence of uniform flow across the zebrafish trunk microvasculature, and in this work show that this uniformity results from conductances being close to the unique maximum of a constrained optimization problem, the connection between uniform flow and uniform perfusion is not proven.
Although our algorithm easily handles the relatively small number of vessels in the zebrafish trunk or model capillary, the mouse cortex contains about 14, 000 capillaries per mm3 [5]. Further tool development is needed to determine whether such networks obey the same principles of uniform flow.
There are many other relevant biological functions that have been identified as candidates for optimization in biological transport networks, for example damage resistance [26] and mixing [45]. Our algorithm has the flexibility to find optimal networks for general biological functions that can be represented by closed-form target functions. Previous numerical work focused on dissipation and functions derived from it [6, 26], but we hope that our algorithm will help to catalyze systematic study of other target functions.
Our networks are constrained either by material or total transport cost (i.e. material plus dissipation). However, we could equally impose network cost as a penalty function rather than as a constraint. Indeed, in Murray’s original paper, optimal vessel geometries were derived by minimizing the total energy formed as a sum of material and transport costs (i.e. by a penalty function approach) [38, 51]. In contrast, recent works on minimal dissipation networks impose the material cost as a constraint and minimize dissipation under this constraint. The two approaches carry different physical meanings; and under many, but not all conditions, they produce equivalent networks [8].
Highlights.
Large blood vessels are organized for transport efficiency, but microvascular networks are not – we explore the hypothesis that uniform flow may explain microvascular morphologies.
A gradient descent algorithm is devised to find optimal vascular networks with general functions and constraints, comparing transport efficient and uniform flow networks on different microvasculatures.
On a class of model capillary beds, we prove that uniform flow networks have the same flows as networks in which all vessels are assigned the same conductance.
Optimizing fine vessels in the zebrafish trunk network for uniform flow produces vessel conductances that match experimental measurements.
When the cost of transport is incorporated as a constraint with controlled strength, microvascular network morphologies undergo a phase transition, suggesting that networks that optimize multiple functions may be indistinguishable from networks that optimize only one function.
5. Acknowledgments.
MR and SSC were funded by grants from the NSF (under grant DMS-1351860) and NIGMS (under R01 GM126556). SSC was also supported by the National Institutes of Health, under a Ruth L. Kirschstein National Research Service Award (T32-GM008185). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. MR also thanks Eleni Katifori and Karen Alim for useful discussions, and the American Institute of Mathematics for hosting him during part of the development of this paper.
Appendix A. Solvability of {μk}.
Here we prove that {μk} in Equation (7) are solvable under a general configuration of flow (i.e. Neumann) and pressure (i.e. Dirichlet) boundary conditions (BCs). We assume that κkl > 0 ∀⟨k, l⟩ = 1 (since κkl = 0 is the same as ⟨k, l⟩ = 0) and that the network is connected. It suffices to show that the matrix D is invertible. This is the same matrix in the linear system for solving {pk} with the specified boundary conditions, so we only have to show that there exists a unique flow given any flow and pressure boundary conditions, which is a well-known [29]. Since our derivation makes use of multiple invertibility results for different matrices D, D(2) and so on, we provide a proof in order to highlight under what conditions invertibility is allowed. The problem is equivalent to showing that
| (65) |
The solution p for Eqn. (65) corresponds to a network where we do not have any flows into the system except possibly at vertices with pressure boundary conditions, denoted by . The goal is to show that pk =0 ∀k. Suppose for contradiction that s.t. pi ≠ 0 (since we already have pj = 0 ). Then we would have Qkl ≠ 0 for some ⟨k, l⟩ = 1 since the network is connected, and without loss of generality let Qkl > 0. Now we can trace this flow throughout the network in the following procedure:
Given that Qkn−1kn > 0 first check if , and stop if this is the case.
Consider all vertices l s.t. ⟨kn, l⟩ = 1. According to Kirchhoff’s first law there must be an l s.t. Qknl > 0. Since the network is finite we can pick e.g. the smallest l satisfying these conditions and let kn+1 = l.
Repeat the procedure until for some N and stop.
If we start with k1 = k, k2 = l we can initiate the process since the first condition is satisfied. This procedure has to stop eventually because the network is finite and that k1,…, kn are all distinct for any given n > 1. To see this suppose kn = km with m > n. Then we would have pn > pn+1 > ⋯ > pm = pn, a contradiction. Thus we would end up with a chain of distinct vertices k1, k2,…, kN with ⟨kn, kn+1) = 1, Qknkn+1 > 0 ∀n = 1,…, N – 1, and . Now we repeat the same procedure just with , to trace the flows upstream, and we would end up with another chain , , with , , and . Notice that there is no repetition in the set since would lead to the same contradiction since pressures must be ordered.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Acheson David J. Elementary fluid dynamics. Oxford University Press, 1990. [Google Scholar]
- [2].Alim Karen, Amselem Gabriel, Peaudecerf François, Brenner Michael P, and Pringle Anne. Random network peristalsis in physarum polycephalum organizes fluid flows across an individual. Proceedings of the National Academy of Sciences, 110(33):13306–13311, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ayata Cenk, Andrew K Dunn Yasemin Gursoy-Özdemir, Huang Zhihong, Boas David A, and Moskowitz Michael A. Laser speckle flowmetry for the study of cerebrovascular physiology in normal and ischemic mouse cortex. Journal of Cerebral Blood Flow & Metabolism, 24(7):744–755, 2004. [DOI] [PubMed] [Google Scholar]
- [4].Barber Jared O, Alberding Jonathan P, Restrepo Juan M, and Secomb Timothy W. Simulated two-dimensional red blood cell motion, deformation, and partitioning in microvessel bifurcations. Annals of biomedical engineering, 36(10):1690–1698, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Blinder Pablo, Philbert S Tsai, Kaufhold John P, Knutsen Per M, Suhl Harry, and Kleinfeld David. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nature neuroscience, 16(7):889–897, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bohn Steffen and Magnasco Marcelo O. Structure, scaling, and phase transition in the optimal transport network. Physical review letters, 98(8):088702, 2007. [DOI] [PubMed] [Google Scholar]
- [7].Chaigneau Emmanuelle, Oheim Martin, Audinat Etienne, and Charpak Serge. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. P. Natl. Acad. Sci. USA, 100(22):13081–13086, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chang Shyr-Shea and Roper Marcus. Minimal transport networks with general boundary conditions. SIAM Journal on Applied Mathematics, 78(3):1511–1535, 2018. [Google Scholar]
- [9].Chang Shyr-Shea, Tu Shenyinying, Kyung In Baek Andrew Pietersen, Liu Yu-Hsiu, Savage Van M, Hwang Sheng-Ping L, Hsiai Tzung K, and Roper Marcus. Optimal occlusion uniformly partitions red blood cells fluxes within a microvascular network. PLoS computational biology, 13(12):e1005892, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen Qi, Jiang Luan, Li Chun, Hu Dan, Bu Ji-wen, Cai David, and Du Jiu-lin. Haemodynamics-driven developmental pruning of brain vasculature in zebrafish. PLoS biology, 10(8):e1001374, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chong Edwin KP and Zak Stanislaw H. An introduction to optimization, volume 76 John Wiley & Sons, 2013. [Google Scholar]
- [12].Clavica Francesco, Homsy Alexandra, Jeandupeux Laure, and Obrist Dominik. Red blood cell phase separation in symmetric and asymmetric microchannel networks: effect of capillary dilation and inflow velocity. Scientific Reports, 6, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Corson Francis. Fluctuations and redundancy in optimal transport networks. Physical Review Letters, 104(4):048703, 2010. [DOI] [PubMed] [Google Scholar]
- [14].Doyeux Vincent, Podgorski Thomas, Peponas Sarah, Ismail Mourad, and Coupier Gwennou. Spheres in the vicinity of a bifurcation: elucidating the zweifach–fung effect. Journal of Fluid Mechanics, 674:359–388, 2011. [Google Scholar]
- [15].Andrew K Dunn Anna Devor, Dale Anders M, and Boas David A. Spatial extent of oxygen metabolism and hemodynamic changes during functional activation of the rat somatosensory cortex. Neuroimage, 27(2):279–290, 2005. [DOI] [PubMed] [Google Scholar]
- [16].Durand Marc. Architecture of optimal transport networks. Physical Review E, 73(1):016116, 2006. [DOI] [PubMed] [Google Scholar]
- [17].Durand Marc. Structure of optimal transport networks subject to a global constraint. Physical Review Letters, 98(8):088701, 2007. [DOI] [PubMed] [Google Scholar]
- [18].Francis Cassot, Frederic Lauwers, Sylvie Lorthois, Prasanna Puwanarajah, and Henri Duvernoy. Scaling laws for branching vessels of human cerebral cortex. Microcirculation, 16(4):331–344, 2009. [DOI] [PubMed] [Google Scholar]
- [19].Gillooly James F, Brown James H, West Geoffrey B, Savage Van M, and Charnov Eric L. Effects of size and temperature on metabolic rate. science, 293(5538):2248–2251, 2001. [DOI] [PubMed] [Google Scholar]
- [20].Hacking WJ, VanBavel E, and Spaan JA. Shear stress is not sufficient to control growth of vascular networks: a model study. American Journal of Physiology-Heart and Circulatory Physiology, 270(1):H364–H375, 1996. [DOI] [PubMed] [Google Scholar]
- [21].Hall John E. Guyton and Hall textbook of medical physiology. Elsevier Health Sciences, 2015. [Google Scholar]
- [22].Hu Dan and Cai David. Adaptation and optimization of biological transport networks. Physical review letters, 111(13):138701, 2013. [DOI] [PubMed] [Google Scholar]
- [23].Hu Dan, Cai David, and Aaditya V Rangan. Blood vessel adaptation with fluctuations in capillary flow distribution. PloS one, 7(9):e45444, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Insel Thomas R, Landis Story C, and Collins Francis S. The nih brain initiative. Science, 340(6133):687–688, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Isogai Sumio, Horiguchi Masaharu, and Weinstein Brant M. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Developmental biology, 230(2):278–301, 2001. [DOI] [PubMed] [Google Scholar]
- [26].Katifori Eleni, Szöllősi Gergely J, and Magnasco Marcelo O. Damage and fluctuations induce loops in optimal transport networks. Physical Review Letters, 104(4):048704, 2010. [DOI] [PubMed] [Google Scholar]
- [27].Klitzman Bruce, Damon David N, Gorczynski Richard J, and Duling Brian R. Augmented tissue oxygen supply during striated muscle contraction in the hamster. relative contributions of capillary recruitment, functional dilation, and reduced tissue po2. Circulation Research, 51(6):711–721, 1982. [DOI] [PubMed] [Google Scholar]
- [28].Howard A Levine Serdal Pamuk, Sleeman Brian D, and Nilsen-Hamilton Marit. Mathematical modeling of capillary formation and development in tumor angiogenesis: penetration into the stroma. Bulletin of mathematical biology, 63(5):801–863, 2001. [DOI] [PubMed] [Google Scholar]
- [29].Lyons Russell and Peres Yuval. Probability on Trees and Networks. Cambridge University Press, New York, 2016. Available at http://pages.iu.edu/~rdlyons/. [Google Scholar]
- [30].Makanya Andrew N and Djonov Valentin. Parabronchial angioarchitecture in developing and adult chickens. Journal of Applied Physiology, 106(6):1959–1969, 2009. [DOI] [PubMed] [Google Scholar]
- [31].Makanya Andrew N and Mortola Jacopo P. The structural design of the bat wing web and its possible role in gas exchange. Journal of anatomy, 211(6):687–697, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Michael H Malone Noah Sciaky, Stalheim Lisa, Klaus M Hahn Elwood Linney, and Johnson Gary L. Laser-scanning velocimetry: a confocal microscopy method for quantitative measurement of cardiovascular performance in zebrafish embryos and larvae. BMC biotechnology, 7(1):1, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Marsden Alison L, Bernstein Adam J, Reddy V Mohan, Shadden Shawn C, Spilker Ryan L, Chan Frandics P, Taylor Charles A, and Feinstein Jeffrey A. Evaluation of a novel y-shaped extracardiac fontan baffle using computational fluid dynamics. The Journal of Thoracic and Cardiovascular Surgery, 137(2):394–403, 2009. [DOI] [PubMed] [Google Scholar]
- [34].Mayerich David, Kwon Jaerock, Sung Chul, Abbott Louise, Keyser John, and Choe Yoonsuck. Fast macro-scale transmission imaging of microvascular networks using kesm. Biomedical optics express, 2(10):2888–2896, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McCulloh Katherine A, Sperry John S, and Adler Frederick R. Water transport in plants obeys murray’s law. Nature, 421(6926):939–942, 2003. [DOI] [PubMed] [Google Scholar]
- [36].Miodoński A and Jasiński A. Scanning electron microscopy of microcorrosion casts of the vascular bed in the skin of the spotted salamander, salamandra salamandra l. Cell and tissue research, 196(1):153–162, 1979. [DOI] [PubMed] [Google Scholar]
- [37].Murray Cecil D. The physiological principle of minimum work applied to the angle of branching of arteries. The Journal of general physiology, 9(6):835–841, 1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Murray Cecil D. The physiological principle of minimum work i. the vascular system and the cost of blood volume. Proceedings of the National Academy of Sciences, 12(3):207–214, 1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Neuhauser Claudia and Roper Marcus L. Calculus for biology and medicine. Pearson, 2018. [Google Scholar]
- [40].Nguyen Thi-Hanh, Eichmann Anne, Ferdinand Le Noble, and Fleury Vincent. Dynamics of vascular branching morphogenesis: the effect of blood and tissue flow. Physical Review E, 73(6):061907, 2006. [DOI] [PubMed] [Google Scholar]
- [41].Pries AR, Ley K, Claassen M, and Gaehtgens P. Red cell distribution at microvascular bifurcations. Microvascular research, 38(1):81–101, 1989. [DOI] [PubMed] [Google Scholar]
- [42].Pries Axel R, Höpfner Michael, Le Noble Ferdinand, Dewhirst Mark W, and Secomb Timothy W. The shunt problem: control of functional shunting in normal and tumour vasculature. Nature Reviews Cancer, 10(8):587, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pries Axel R and Secomb Tim W. Microvascular blood viscosity in vivo and the endothelial surface layer. American Journal of Physiology-Heart and Circulatory Physiology, 289(6):H2657–H2664, 2005. [DOI] [PubMed] [Google Scholar]
- [44].Ronellenfitsch Henrik and Katifori Eleni. Global optimization, local adaptation and the role of growth in distribution networks. arXiv preprint arXiv:1606.00331, 2016. [DOI] [PubMed] [Google Scholar]
- [45].Roper Marcus, Simonin Anna, Patrick C Hickey Abby Leeder, and Glass N Louise. Nuclear dynamics in a fungal chimera. Proceedings of the National Academy of Sciences, 110(32):12875–12880, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Savin Thierry, Bandi MM, and Mahadevan L. Pressure-driven occlusive flow of a confined red blood cell. Soft matter, 12(2):562–573, 2016. [DOI] [PubMed] [Google Scholar]
- [47].Schermelleh Lothar, Heintzmann Rainer, and Leonhardt Heinrich. A guide to super-resolution fluorescence microscopy. The Journal of cell biology, 190(2):165–175, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schindler Michael and Ajdari Armand. Droplet traffic in microfluidic networks: A simple model for understanding and designing. Phys. Rev. Lett, 100(4):044501, 2008. [DOI] [PubMed] [Google Scholar]
- [49].Schwerte Thorsten, Überbacher Dietmar, and Pelster Bernd. Non-invasive imaging of blood cell concentration and blood distribution in zebrafish danio rerio incubated in hypoxic conditions in vivo. Journal of Experimental Biology, 206(8):1299–1307, 2003. [DOI] [PubMed] [Google Scholar]
- [50].Shen Zaiyi, Coupier Gwennou, Kaoui Badr, Polack Benoît, Harting Jens, Misbah Chaouqi, and Podgorski Thomas. Inversion of hematocrit partition at microfluidic bifurcations. Microvascular research, 105:40–46, 2016. [DOI] [PubMed] [Google Scholar]
- [51].Sherman Thomas F. On connecting large vessels to small. the meaning of murray’s law. The Journal of general physiology, 78(4):431–453, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shih Andy Y, Rühlmann Charlotta, Blinder Pablo, Devor Anna, Drew Patrick J, Friedman Beth, Knutsen Per M, Lyden Patrick D, Mateo Celine, Mellander Lisa, et al. Robust and fragile aspects of cortical blood flow in relation to the underlying angioarchitecture. Microcirculation, 22(3):204–218, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tuma Pamela L and Hubbard Ann L. Transcytosis: crossing cellular barriers. Physiological reviews, 83(3):871–932, 2003. [DOI] [PubMed] [Google Scholar]
- [54].West Geoffrey B, Brown James H, and Enquist Brian J. A general model for the origin of allometric scaling laws in biology. Science, 276(5309):122–126, 1997. [DOI] [PubMed] [Google Scholar]
- [55].Wu Jingpeng, He Yong, Yang Zhongqin, Guo Congdi, Luo Qingming, Zhou Wei, Chen Shangbin, Li Anan, Xiong Benyi, Jiang Tao, et al. 3d braincv: simultaneous visualization and analysis of cells and capillaries in a whole mouse brain with one-micron voxel resolution. Neuroimage, 87:199–208, 2014. [DOI] [PubMed] [Google Scholar]
- [56].Zweifach Benjamin W and Lipowsky Herbert H. Quantitative studies of microcirculatory structure and function. iii. microvascular hemodynamics of cat mesentery and rabbit omentum. Circ. Res, 41(3):380–390, 1977. [DOI] [PubMed] [Google Scholar]