Abstract
Statement of problem.
Treatment and timing considerations for patients seeking oral rehabilitation after marginal or segmental mandibulectomy (with osseous reconstruction) are not well understood.
Purpose.
The purpose of this retrospective review study was to report the type and timing of oral rehabilitation for mandibular defects without discontinuity and describe additional treatment considerations for rehabilitation.
Material and methods.
The records were reviewed of all patients who had a mandibular resection prosthesis after marginal mandibulectomy, marginal mandibulectomy with fasciocutaneous free flap reconstruction, and segmental mandibulectomy with fibula free flap reconstruction between 2000 and 2017 in the tertiary cancer care institution. Patients not treated by the Dental Service in the institution were excluded. The specific type of rehabilitation was noted, as was the time interval between primary surgery and prosthesis delivery.
Results.
During the study period, 111 consecutive patients were treated by the Dental Service for mandibular rehabilitation. Forty-three patients had marginal mandibulectomy, 9 patients had marginal mandibulectomy and fasciocutaneous free flap reconstruction, and 59 patients had segmental mandibulectomy with fibula free flap reconstruction. Most patients in all 3 groups received mandibular resection prostheses without the use of endosseous implants. Only 8% (4) of patients who had marginal mandibulectomy underwent endosseous implant placement, all of which followed marginal mandibulectomy in anterior mandibular segments without free flap reconstruction. Patients with marginal mandibulectomy and fasciocutaneous free flap reconstruction were only restored with removable mandibular resection prostheses, and none had endosseous implants. In patients with segmental mandibulectomy, 22% (13) were rehabilitated with endosseous implants. The majority in this cohort (>50%) received radiation therapy as part of their treatment. The median time to oral rehabilitation was 8 months after marginal mandibulectomy, 14 months after marginal mandibulectomy and fasciocutaneous flap, and 12 months after segmental mandibulectomy and fibula free flap reconstruction.
Conclusions.
Timing for oral rehabilitation may differ depending upon the treatment modality for mandibular tumors in the patient with oral cancer. However, most patients in this cohort had rehabilitation with removable mandibular resection prostheses regardless of the timing of care. Endosseous implants were used infrequently, but research is needed to better understand their potential role and indication in the patient with oral cancer.
INTRODUCTION
Preservation or restoration of the mandibular arch is an important element to facilitate intraoral rehabilitation after oncologic treatment for oral malignancies.1 Esthetic and functional deficits caused by cancer or cancer-related therapy can diminish a patient’s quality of life, and therefore expeditious rehabilitation after marginal or segmental mandibulectomy is desirable.2 Prosthetic intraoral rehabilitation is achieved with a mandibular resection prosthesis. The timing and effectiveness of prosthetic intraoral rehabilitation is highly dependent on pretreatment planning and interdisciplinary coordination of care.
Mandibular defects can be present in various configurations, including marginal and segmental mandibulectomy.3 Marginal mandibulectomy defects involve the resection of a single cortex of the mandible (either superior or inferior cortex), while segmental mandibulectomy defects result from the removal of an entire segment (both cortices and medullary space inclusive of all dental elements) of the mandible. Segmental mandibulectomy defects can be present in the anterior arch, lateral segment of the body, or the ascending ramus of the mandible. The resulting mandibular defects can be restored with osseocutaneous microvascular free flaps, when possible, to avoid the functional and esthetic challenges associated with a mandibular discontinuity defect. These flaps can be obtained from a variety of donor sites (fibula, iliac crest, radius, metatarsal, rib, scapula). The fibula free flap is the most popular flap used because of its long pedicle length, ease of contouring with multiple osteotomies, and suitability as a recipient site for endosseous implants.4 Osseocutaneous free flap defect closure is preferred since the functional and esthetic outcomes are generally better than alternative approaches.5 The rehabilitation of a mandible reconstructed with an osseocutaneous free flap can be challenging because of the altered anatomy and sensory deficits following reconstructive surgery.6 From a restorative perspective, patients with marginal mandibulectomy and osseocutaneous reconstructed segmental mandibulectomy defects are typically good candidates for prosthetic intraoral rehabilitation with mandibular resection prostheses. The restoration of an edentulous mandible is conventionally approached with endosseous implants.7 Patients who undergo marginal mandibulectomy in the posterior region are generally not candidates for implant placement because of the proximity of the alveolar crest to the inferior alveolar canal and the likelihood of causing injury to the inferior alveolar nerve during implant surgery.8,9 However, if the patient has previously received radiotherapy, the placement of endosseous implants may not be advisable because of the risk of osteoradionecrosis of the jaw.10,11
Although these prostheses are commonly provided, data on their outcomes are lacking. Therefore, the purpose of this study was to review retrospectively a consecutive series of patients who had had successful mandibular resection prostheses fabricated by the Dental Service at Memorial Sloan Kettering Cancer Center (MSKCC) following marginal mandibulectomy or segmental mandibulectomy with osseocutaneous free flap reconstruction over a 17-year period. Additionally, treatment concepts that facilitated the management of such patients were reviewed.
MATERIAL AND METHODS
A retrospective review was completed (IRB #16–1132) of consecutive patients who underwent intraoral rehabilitation following marginal or segmental mandibulectomy between 2000 and 2017. Patients who had mandibular discontinuity defects which were not restored with osseocutaneous free flaps were excluded. Additionally, patients who were intermittently seen by the Dental Service at MSKCC but were primarily under the care of non-MSKCC oral health providers were not included in the study cohort. Patient records were reviewed to obtain patient demographics, tumor data, tumor treatment data, the date of oral rehabilitation (delivery of mandibular resection prosthesis), and the date of death (if applicable). Additionally, the time interval between primary surgery and mandibular resection prosthesis delivery was recorded. To better quantify follow-up after prosthesis delivery, the number of patient appointments during the first 90 days following mandibular resection prosthesis delivery was also recorded at 30-day intervals. The last date of follow-up was also recorded. Data were then compiled and analyzed on a spreadsheet (Excel; Microsoft Corp) for descriptive presentation.
RESULTS
The study cohort included 111 patients. They were divided into 3 groups based on the primary mode of ablative and reconstructive surgery: marginal mandibulectomy without free flap (39%; n=43), marginal mandibulectomy with fasciocutaneous free flap (8%; n=9), and segmental mandibulectomy with osseocutaneous free flap (53%; n=59). All fasciocutaneous free flaps in this cohort were radial forearm free flaps. Patient demographics and tumor information are presented in Table 1.
Table 1.
Patient demographics
| Clinical variables | Marginal mandibulectomy n=43 % (n) | Marginal mandibulectomy with fasciocutaneous free flap n=9 % (n) | Segmental mandibulectomy with osseocutaneous free flap n=59 % (n) |
|---|---|---|---|
| Clinical T stage | |||
| T1 | 54 (23) | 11 (1) | 11 (6) |
| T2 | 37 (16) | 55 (5) | 20 (12) |
| T3 | 0 (0) | 11 (1) | 5(3) |
| T4 | 7 (3) | 23 (2) | 38 (22) |
| NA | 2 (1) | 0 (0) | 26 (16) |
| Clinical N stage | |||
| N0 | 82 (35) | 66 (6) | 54 (31) |
| N1 | 7 (3) | 23 (2) | 3 (2) |
| N2 | 9 (4) | 11 (1) | 17 (10) |
| NA | 2 (1) | 0(0) | 26 (16) |
| Site | |||
| Mandibular gingiva | 54 (23) | 23 (2) | 24 (15) |
| Floor of mouth | 24 (11) | 55 (5) | 15 (9) |
| Tongue | 9 (3) | 11 (1) | 5 (3) |
| Retromolar trigone | 7 (3) | 0 (0) | 11 (6) |
| Buccal mucosa | 4 (2) | 0 (0) | 1 (1) |
| Mandible | 2 (1) | 0(0) | 44 (25) |
| Tonsil | 0 (0) | 11 (1) | 0 (0) |
| Pathology | |||
| Squamous cell carcinoma | 96 (41) | 100 (9) | 74 (42) |
| Dysplasia | 2 (1) | 0 (0) | 0 (0) |
| Sarcoma | 0 (0) | 0 (0) | 14 (10) |
| Mucoepidermoid carcinoma | 0(0) | 0 (0) | 1 (1) |
| Benign lesions | 2 (1) | 0 (0) | 11 (6) |
| Postoperative radiotherapy | |||
| Yes | 23 (10) | 55 (5) | 63 (37) |
| No | 77 (33) | 45 (4) | 37 (22) |
| Postoperative chemotherapy | |||
| Yes | 9 (4) | 23 (2) | 20 (12) |
| No | 81 (39) | 77 (7) | 79 (47) |
The median age of the patients who had marginal mandibulectomy with no flap reconstruction was 66 years (range: 30–88 years) and 56% were male (n=24 male, 19 female). The median age of the patients who had marginal mandibulectomy and radial forearm free flap reconstruction was 59 years (range: 47–74 years) and 33% were male (n=3 male, 6 female). The median age of the patients who had segmental mandibulectomy was 56 years (range 16–83 years) and 61% were male (n=36 male, 23 female). In this study cohort, 82% (n=91) of the patients were dentate and 13% (n=14) were edentulous in the mandibular arch. The use of endosseous implants is presented in Table 2. The median time from primary surgery to mandibular resection prosthesis is presented in Table 3. Mandibular resection prosthesis delivery time was 25% longer in patients who had segmental mandibulectomy (12 months) compared with patients who had marginal mandibulectomy with no free flap reconstruction (8 months). The median time for patients who had marginal mandibulectomy and radial forearm free flap reconstruction was 14 months. The average and median number of visits during the first 90 days following mandibular resection prosthesis delivery in 30-day intervals is presented in Table 4. The median number of follow-up visits in all groups for the first 90 days following prosthesis delivery was 2 visits.
Table 2.
Use of endosseous implants in mandibular resection prostheses
| Marginal mandibulectomy n=52 % (n) | Segmental mandibulectomy n=59 %(n) | |
|---|---|---|
| Resection prosthesis with endosseous implants | 92(48) | 78(46) |
| Without endosseous implants | 8(4) | 22(13) |
Table 3.
Mandibular resection prosthesis delivery and follow-up
| Marginal Mandibulectomy | Marginal mandibulectomy and fasciocutaneous free flap | Segmental mandibulectomy and osseocutaneous free flap | ||||
|---|---|---|---|---|---|---|
| Time from surgery to prosthesis delivery | Median ± SD (months) 8 ±17.3 | Range (months)1–107 | Median ±SD (months) 14 ±40.9 | Range (months) 4–134 | Median ±SD (months) 12 ±17.87 | Range (months) 1–65 |
| Time from prosthesis delivery to last prosthesis follow-up | 28 ±47.1 | 7 days-218 | 4 ±25.5 | 7 days-64 | 25.5 ±48.62 | 7 days-178 |
Median time for implant-supported/retained mandibular resection prosthesis delivery =19 months (range 1–107) (SD: 28.95) SD, standard deviation.
Table 4.
Mandibular resection prosthesis follow-up visits (30-day intervals)
| Marginal mandibulectomy | Marginal mandibulectomy and fasciocutaneous free flap | Segmental mandibulectomy and osseocutaneous free flap | ||||
|---|---|---|---|---|---|---|
| Median visits | Range | Median visits | Range | Median visits | Range | |
| 0–30 days | 1 | 0–3 | 1 | 0–4 | 1 | 0–4 |
| 31–60 days | 1 | 0–4 | 0 | 0–2 | 0 | 0–4 |
| 61–90 days | 0 | 0–4 | 0 | 0–1 | 0 | 0–3 |
| 0–90 days | 2 | 0–7 | 2 | 0–5 | 2 | 0–8 |
DISCUSSION
Oral rehabilitation of patients with a history of marginal mandibulectomy or segmental mandibulectomy with osseocutaneous free flap reconstruction is a treatment goal for most patients undergoing oncologic treatment. Rehabilitation challenges are often complicated because of trismus, radiation fibrosis, xerostomia, altered intraoral anatomy, and/or soft tissue changes that require careful evaluation by the treating oral health provider. The study reports a consecutive series of 111 patients with oral cancer who underwent either marginal mandibulectomy or segmental mandibulectomy with reconstruction to establish mandibular continuity.
Most of the mandibular defects in the cohort were from lesions from the mandibular gingiva or floor of the mouth. As is consistent with the principles of oncological surgery, the marginal mandibulectomy approach was used primarily for lower T staged tumors, while segmental mandibulectomy was used in patients with higher T staged tumors. The time to rehabilitation varied, but the data indicated that patients with marginal mandibulectomy had the shortest median time to rehabilitation (8 months), as these sites were primarily closed and rehabilitated after healing. The small cohort of patients who had marginal mandibulectomy and free flap overlying the marginal mandibulectomy site had the longest median time to rehabilitation of 14 months. This may be explained by the complex nature of their surgical defects, which required a greater amount of time to heal before being deemed ready for intraoral prosthetic rehabilitation. These differences are likely multifactorial, as they can be related to the reconstructive surgeon’s preference to delay rehabilitation because of delayed intraoral healing or the need for postoperative adjuvant therapy (chemotherapy and/or radiation therapy). Physicians and oral health providers can better set patient expectations by providing this information before treatment.
Additionally, the cohort of patients had only 1 median follow-up appointment during the first 30 days following delivery of the mandibular resection prosthesis regardless of the surgical approach. Patients who had a marginal mandibulectomy had 1 median follow-up visit during the second month after prosthesis delivery. There were 0 follow-ups (median) for all groups in the third month. This information can give patients a better understanding of the general follow-up that will be required following the delivery of prostheses, as well as assist providers in scheduling patients for recommended follow-up appointments.
Additionally, the cohort was an oncologic population in which many patients were irradiated, deemed inappropriate for implant surgery by their oncology team, or were not interested in additional surgery in previously resected areas. If implant surgery is to be considered in an oncologic population, this approach should be coordinated by the multidisciplinary treatment team to maximize implant treatment availability.
Ultimately, management of the patient after a mandibulectomy requires a sound understanding of removable prosthodontic principles. Patients with such defects often have adequate osseous support for a removable mandibular resection prosthesis and some may be candidates for endosseous implants in the interforaminal area of the anterior mandible (Fig. 1). These prosthetics may have altered contours to accommodate the surgically altered anatomy as compared with conventional removable dentures (Fig. 2). Impression making to generate these portions of the prosthesis can be completed either through static or functional approaches. As shown in Figure 3, the osseous structure can largely resemble an edentulous atrophic mandible. Additionally, the location of rigid fixation screws may prevent implant placement, and these screws would need to be removed before placement of an endosseous implant. In such patients, restoration with a removable mandibular resection prosthesis can be considered, and, as noted, this was the most common modality of rehabilitation in the cohort of patients studied.
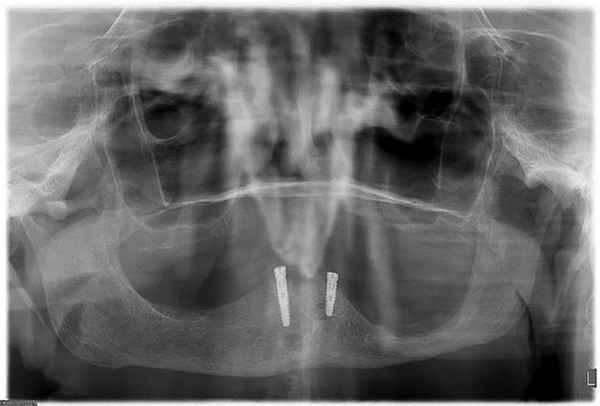
Figure 1.
Panoramic radiograph of patient following left marginal mandibulectomy.
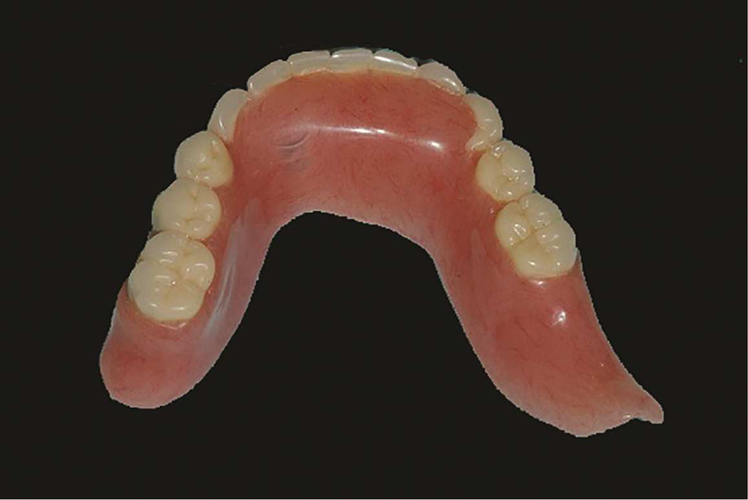
Figure 2.
Mandibular resection prosthesis for patient after left marginal mandibulectomy showing altered prosthesis extension.
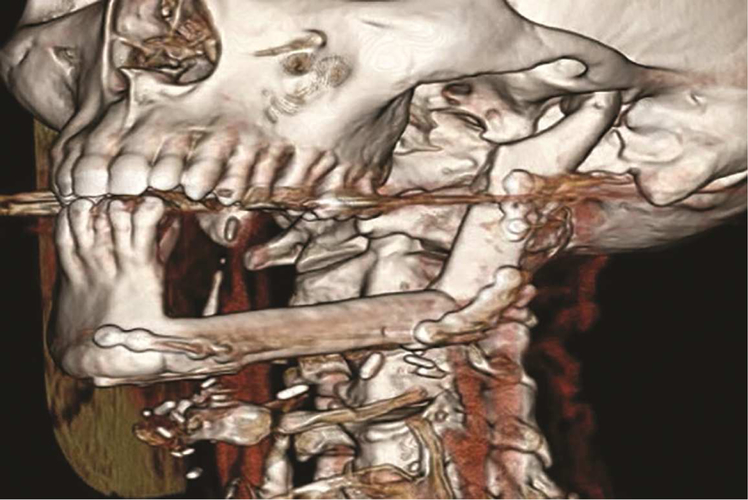
Figure 3.
Three-dimensional reconstruction of patient following left segmental mandibulectomy and reconstruction with fibula free flap.
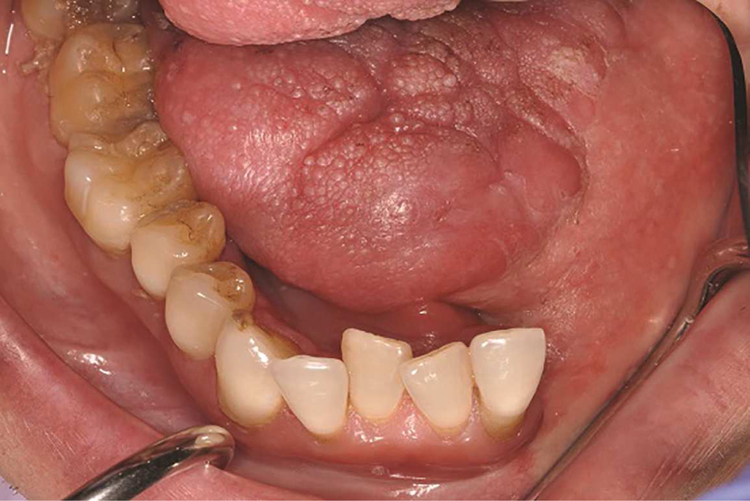
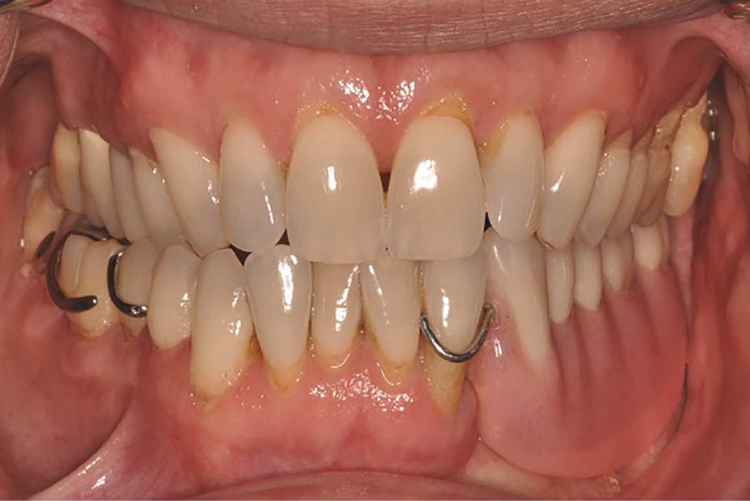
Altered intraoral anatomy following surgery can create challenges for intraoral mandibular resection prosthesis fabrication. Unfavorable location of suture lines can result in the edentulous ridge being contiguous with moveable structures of the oral cavity (tongue or buccal mucosa) (Fig. 4), which can dislodge the prosthesis or cause prosthesis instability. Additionally, skin islands may be more than a centimeter in thickness in some situations; these can become depressed during impression making and result in an ill-fitting prosthesis. Ultimately, static impressions may not be desirable to generate the intaglio surface of the prosthesis in these areas, regardless of the impression material used. Mandibular resection prostheses for partially edentulous patients can be predictably fabricated using conventional removable partial denture prosthesis framework design principles and a functionally generated approach for the edentulous area (Fig. 5). Patients who are completely edentulous following mandibulectomy with osseocutaneous free flap reconstruction can be restored with the aid of endosseous implants or conventional removable prosthetics. However, patients without implants should be cautioned that their restorative prognosis can be compromised because of the lack of prosthesis retention or stability.
Figure 4.
Mandibular occlusal view of patient following left segmental mandibulectomy and reconstruction with fibula free flap.
Figure 5.
Maximum intercuspation view of patient with mandibular resection prosthesis following left segmental mandibulectomy and reconstruction with fibula free flap.
This study has limitations. As this was a retrospective study, the data were limited to the information present in the patient records. Future investigations should obtain data prospectively for a more thorough evaluation of these types of prostheses. Additionally, this study was limited to patients who had mandibular resection prostheses fabricated at the MSKCC Dental Service. The etiology of the mandibular defects was primarily cancer related, and, as a result, the generalizability of the study is limited to such a cohort. Future studies should obtain data from centers with defects of multiple etiologies to better understand the timing and utility of this treatment approach.
CONCLUSIONS
Within the limitations of this retrospective clinical study, the following conclusions were drawn
Oral rehabilitation with mandibular resection prostheses following marginal mandibulectomy or segmental mandibulectomy with osseocutaneous free flap reconstruction is an attainable treatment goal for the oncologic patient population.
Interdisciplinary presurgical planning and detailed discussion and counseling of the patient are essential in managing appropriate oral rehabilitation following oncologic treatment.
Alternative paradigms for placing osseointegrated implants should be developed for patients undergoing either radiation and/or segmental mandibulectomy to enable a more comprehensive oral rehabilitation option.
CLINICAL IMPLICATIONS.
Satisfactory oral rehabilitation after marginal or segmental mandibulectomy should be a viable treatment goal for these patients. Multidisciplinary planning and counseling on the projected timing for rehabilitation and expected follow-up may be helpful information for patient management before the initiation of treatment.
Acknowledgments
Supported in part by NIH/NCI Cancer Center Support Grant P30 CA008748. The Straumann Maxillofacial Dental Implantology Research Fellowship is supported in part by the Straumann SUPER Grant award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Shaw RJ, Sutton AF, Cawood JI, Howell RA, Lowe D, Brown JS, Rogers SN, Vaughan ED. Oral rehabilitation after treatment for head and neck malignancy. Head Neck 2005;27:459–70. [DOI] [PubMed] [Google Scholar]
- 2.Rogers SN. Quality of life perspectives in patients with oral cancer. Oral Oncol 2010;46:445–7. [DOI] [PubMed] [Google Scholar]
- 3.Curtis TA, Cantor R. The forgotten patient in maxillofacial prosthetics. J Prosthet Dent 1974;31:662–80. [DOI] [PubMed] [Google Scholar]
- 4.Hidalgo DA. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg 1989;84:71–9. [PubMed] [Google Scholar]
- 5.Cordeiro PG, Disa JJ, Hidalgo DA, Hu QY. Reconstruction of the mandible with osseous free flaps: a 10-year experience with 150 consecutive patients. Plast Reconstr Surg 1999;104:1314–20. [DOI] [PubMed] [Google Scholar]
- 6.Jackson RS, Price DL, Arce K, Moore EJ. Evaluation of clinical outcomes of osseointegrated dental implantation of fibula free flaps for mandibular reconstruction. JAMA Facial Plast Surg 2016;18:201–6. [DOI] [PubMed] [Google Scholar]
- 7.Granstrom G, Bergstrom K, Tjellström A, Brånemark PI. A detailed study of titanium fixture implants lost in irradiated tissues. Int J Oral Maxillofac Implants 1994;9:653–62. [Google Scholar]
- 8.Levine MH, Goddard AL, Dodson TB. Inferior alveolar nerve canal position: a clinical and radiographic study. J Oral Maxillofac Surg 2007;65:470–4. [DOI] [PubMed] [Google Scholar]
- 9.van Steenberghe D, Lekholm U, Bolender C, Folmer T, Henry P, Herrmann I. Applicability of osseointegrated oral implants in the rehabilitation of partial edentulism: a prospective multicenter study on 558 fixtures. Int J Oral Maxillofac Implants 1990;5:272–81. [PubMed] [Google Scholar]
- 10.Chen H, Liu N, Xu X, Qu X, Lu E. Smoking, radiotherapy, diabetes and osteoporosis as risk factors for dental implant failure: A meta-analysis. PLoS One 2013;8:e71955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adell R, Lekholm U, Rockler B, Brånemark PI. 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg 1981;10:387–416. [DOI] [PubMed] [Google Scholar]