Abstract
Purpose:
To describe spectral domain optical coherence tomography (SD-OCT) findings in an Amish cohort to assess SD-OCT markers for early age-related macular degeneration (AMD).
Methods:
The authors performed a family-based prospective cohort study of 1,146 elderly Amish subjects (age range 50–99 years) (2,292 eyes) who had a family history of at least 1 individual with AMD. All subjects underwent complete ophthalmic examinations, SD-OCT using both Cirrus and Spectralis (20 × 20° scan area) instruments, fundus auto-fluorescence, infrared imaging, and color fundus photography. Spectral domain optical coherence tomography characteristics were analyzed in subjects with AMD (with and without subretinal drusenoid deposits [SDDs]) and normal healthy cohorts.
Results:
Participants’ mean age was 65.2 years (SD ± 11). Color fundus photographic findings in 596 (53%) subjects (1,009 eyes) were consistent with AMD; the remaining 478 (43%) subjects showed no signs of AMD. The choroid was significantly thinner on OCT (242 ± 76 μm, P < 0.001) in those with AMD compared with those without (263 ± 63 μm). Subretinal drusenoid deposits were found in 143 eyes (7%); 11 of the 143 eyes (8%) had no other manifestations of AMD. Drusen volume (P < 0.001) and area of geographic atrophy (P < 0.001) were significantly greater, and choroid was significantly (P < 0.001) thinner in subjects with SDDs versus those without SDDs.
Conclusion:
The authors describe spectral domain optical coherence tomography characteristics in an elderly Amish population with and without AMD, including the frequency of SDD. Although relatively uncommon in this population, the authors confirmed that SDDs can be found in the absence of other features of AMD and that eyes with SDDs have thinner choroids.
Keywords: Amish population, spectral domain optical coherence tomography, subretinal drusenoid deposits
Age-related macular degeneration (AMD) is influenced by genetic, environmental, and dietary factors and is the leading cause of irreversible vision loss in individuals aged 65 years and older in the developed world.1,2 The rising prevalence of AMD is presumably driven by an aging population. Vision loss caused by AMD is divided into two categories: nonneovascular or dry AMD and neovascular or wet AMD.3 Dry AMD is characterized by the presence of drusen, hyper-or hypopigmentation of the retinal pigment epithelium (RPE), and in later stages, geographic atrophy (GA).4,5 Drusen, particularly large soft drusen, are a distinguishing feature and a characteristic physical sign of nonneovascular AMD.6,7 Neovascular AMD occurs when new blood vessels invade Bruch’s membrane and produce exudation in the macula.3,4
There is substantial variation in the progression from early to late AMD8,9; consequently, the factors influencing this progression are not completely understood. Dietary supplements provide some reduction in the progression of AMD.10 Although anti-VEGF therapy can be an effective treatment for the neovascular form of AMD, it is not a cure. In addition, a significant minority of those with choroidal neovascularization fail to respond to anti-VEGF treatments.11–13 Thus, it is critical to understand the underlying causes of early AMD to enable discoveries of new therapies directed at the earlier stages of the disease.
A potential impediment to the identification of new therapies is the current AMD classification. The clinical disease classification of AMD, obtained by phenotyping the anatomical changes detected by clinical examination and/or fundus photographs do not reflect the biological underpinnings of AMD, which are poorly understood. In addition, these older methods of classifying AMD are not always sensitive enough to detect some of the anatomical changes of AMD. For example, the presence and location of subretinal drusenoid deposits (SDDs) are difficult to detect on a fundus photograph or clinical examination. Today, SDDs can be detected during the early stages of AMD with fundus autofluorescence (FAF), infraed reflectance (IR), or optical coherence tomography (OCT).
The high resolution, sensitivity, and speed of spectral domain optical coherence tomography (SDOCT) have enabled detailed visualization of the outer retinal and subretinal substructures.14,15 The combined use of color fundus photographs and SD-OCT is routine for clinical trials and population-based studies.5,16,17
The Amish Eye Study was initiated to identify biomarkers of OCT for earlier stages of AMD in a family-based cohort; to assess the heritability of identified OCT biomarkers in the families; and to sequence the DNA from these families to identify rare/ moderate genetic variants that associate with the identified AMD biomarkers. Our family-based approach to quantifying SD-OCT biomarkers associated with AMD will provide information on the heritability of that marker and its potential for genetic studies.18 We have intentionally focused on the Amish population because it is a founder population and they are genetically and culturally isolated, and thus experience a relatively uniform environment and reduced genetic diversity. That represents an isolate with less genetic and environmental variation than the general population. As a result, the background heterogeneity between individuals will be reduced. In addition, the Amish do not indulge in significant smoking behavior, thereby reducing the amount of AMD risk attributable to smoking in this sample.
The Amish Eye Study is recruiting Amish subjects from the three largest Amish communities in the United States.: Holmes County, Ohio; Elkhart and LaGrange Counties, Indiana19,20 and Lancaster Counties, Pennsylvania.21 These communities are collectively an isolated founder population originating from two waves of immigration of Swiss Anabaptists into the United States in the 1700s and 1800s. The first wave of immigration brought the Anabaptists to Lancaster County, Pennsylvania. In the early 1800s, some of these immigrants moved to Holmes County, Ohio,22 whereas a second wave of immigration from Europe established Amish communities in Ohio (including Wayne County but not Holmes County) and Indiana (including Adams County).21 Starting in 1841, the Elkhart and LaGrange Counties Amish community was founded by Amish families primarily from Somerset County, Pennsylvania, and from Holmes and Wayne Counties, Ohio, who were seeking a new farmland.23
The present study describes our recruitment and phenotyping strategy, particularly focused on OCT for screening a family-based prospective cohort that can be used to identify SD-OCT markers for early AMD.
Methods
Ascertainment and Eligibility
Participants were identified from three settlements in Ohio, Indiana, and Pennsylvania. Human subject protocols have been approved by the Institutional Review Boards of the University of Pennsylvania, University of Miami, Case Western Reserve University, and University of California—Los Angeles. All study procedures followed the tenets of the Declaration of Helsinki. Written informed consent was obtained from all subjects after explanation of the nature and possible consequences of the study. Inclusion criteria for a subject to the study were age ≥50 years, self-identification as Amish, and membership in a sibship with at least 1 individual reported to have AMD. Exclusion criteria included inadequate image quality or any previous or concomitant ophthalmological condition that could confound the interpretation of AMD features on imaging. Data reported in this manuscript were collected between February 2013 and July 2016.
Study Procedures
The clinical examination included an on-site ophthalmic examination, interview, and venipuncture. Examination data were recorded on examination forms and then entered directly into the Amish Eye Study database at the University of Miami. Data collection was standardized and included measurements taken by clinical research coordinators, ophthalmic technicians, and board-certified ophthalmologists in respective study sites. The examination included 1) name, age, date of birth, sex, address, parents’ and siblings’ names and addresses, family history of AMD, and the presence of any other eye diseases; 2) visual acuity, measured using a Snellen chart at 20 feet; 3) flash color fundus photography with a Topcon camera; 4) fundus examination by direct and indirect ophthalmos-copy; and 5) SD-OCT.
Spectral domain optical coherence tomography imaging at all sites was performed on two instruments: Cirrus OCT (Carl Zeiss Meditec Inc, Dublin, CA) and Spectralis (HRA + OCT; Heidelberg Engineering, Inc, Heidelberg, Germany) using macular cube scan protocols on both eyes. Images were acquired from both devices to take advantage of unique capabilities of each, automated quantification of features such as drusen by the Cirrus, and better visualization of the outer retinal substructures and choroid by the averaged scans of the Spectralis.
The Cirrus OCT scans were acquired using a macular cube protocol of 512 by 128 (128 B-scans and 512 A-scans per B-scan) over a 6 mm × 6 mm area centered on the fovea. The Spectralis acquisition protocol consisted of a 512 × 97 macular volume cube (97 B-scans and 512 A-scans per B-scan, ART of nine frames) over a 20 × 20° (6 mm × 6 mm) field centered on the fovea. Sub-retinal drusenoid deposits have been described to commonly be found outside the central macula, often along the arcades or even nasal to the optic nerve. Thus, sim ilar volume cube scans were also obtained with both devices centered on the optic nerve. Enhanced depth imaging was not obtained; however, the operators did orient the OCT image closer to the vitreous side to minimize distance from the zero delay line. Enhanced depth imaging imaging was not obtained to simplify procedures for the operator and patient and because the choroid was expected to be relatively thinner in this elderly population. Subfoveal choroidal thickness was measured using instrument calipers in both the Spectra-lis and Cirrus acquisitions. However, a priori, only the Spectralis-derived choroidal thickness was used for further analysis. Eyes in which the choroidal-scleral boundary could not be identified were marked “could not be graded” and were not included in the quantitative analysis. Choroidal thickness could not be graded in 96 (4%) eyes by the Spectralis and 211 (9%) eyes by the Cirrus.
To assess the reproducibility of our readings, we evaluated inter-and intragrader correlation by randomly selecting 240 eyes form 120 subjects and having 2 independent graders who were masked to the previous results of choroidal thickness measurements.
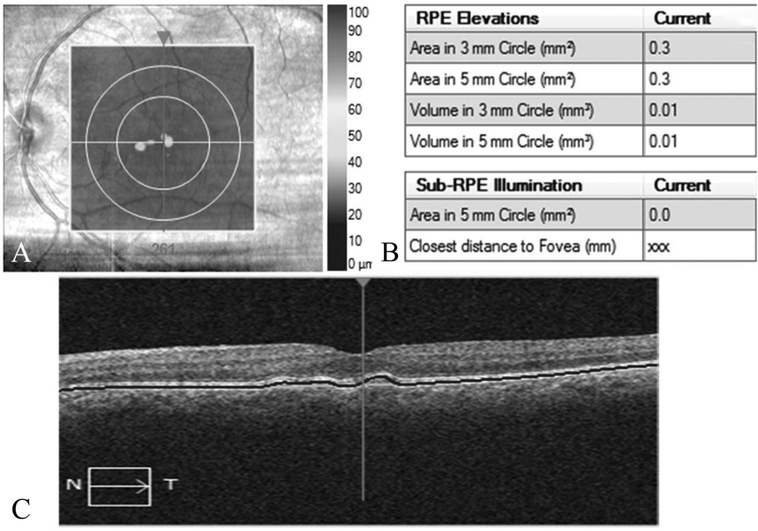
Automated quantification of drusen area, volume, and GA area within the macular cube was performed using the Cirrus 6.2 software RPE analysis segmentation algorithms.14,24 The automated GA segmentation was inspected, and segmentation errors were manually corrected to generate GA areas. Geographic atrophy on SD-OCT was identified as a region where choroidal hypertransmission was detected with RPE loss. Drusen measurements were analyzed in 3-mm and 5-mm rings centered on the fovea (Figure 1). Geographic atrophy area was measured in a 5-mm ring centered on the fovea.
Fig. 1.
Cirrus-automated RPE analysis algorithm providing quantitative measures of drusen area, volume in center 3 mm and 5 mm circular zones, and GA area in central 5 mm zone. A. Infrared image with RPE thickness profile, drusen map, (B) quantitative measures generated by the automated algorithm, and (C) OCT B-scan with automated software–segmented boundaries.
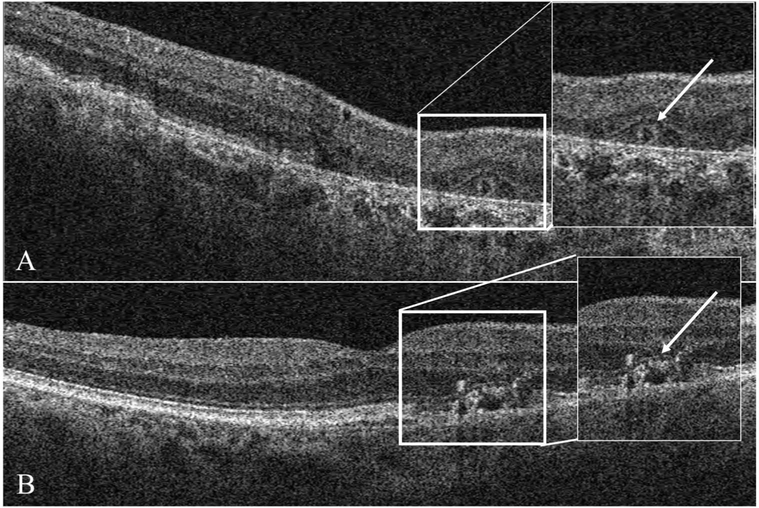
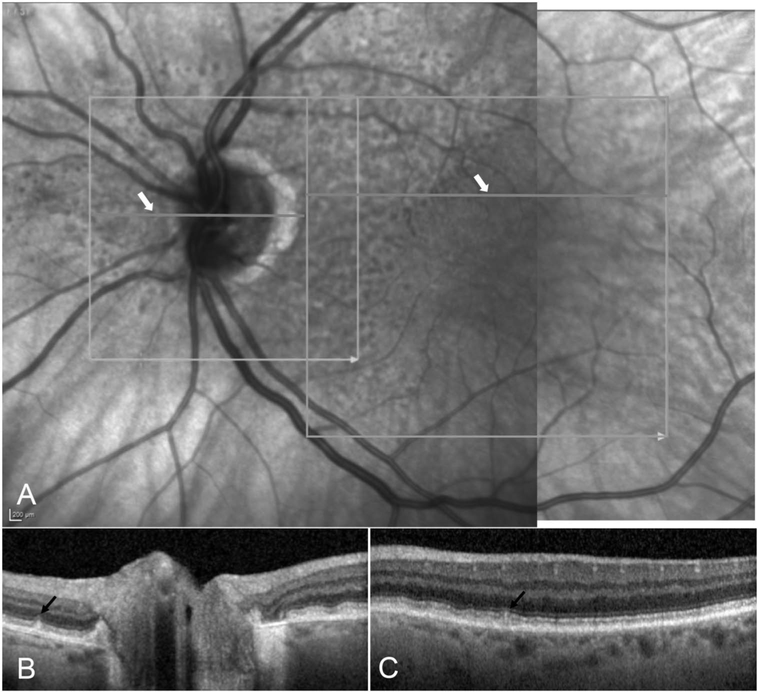
Qualitative parameters such as the presence or absence of intra-and subretinal fluid (hyporeflective pockets of fluid), subretinal hyperreflective material, outer retinal tubulations, SDDs, and presence and axial extent of intraretinal hyperreflective foci (HRF) were analyzed by evaluating all the B-scans in SD-OCT volumes. Of note, subretinal hyperreflective material was diagnosed if there was any bright material between the outer border of the photoreceptors and the RPE (Figure 2). Because no fluorescein angiography was obtained, it was not possible to determine with certainty whether subretinal hyperreflective material corresponded to vitelliform-like accumulations or was related to an exudative process. If HRF were present, the innermost retinal layer to which the HRF extended was documented as LHRF (level of HRF). Retinal layers included in the LHRF classification were the photoreceptor segment (inner and outer) layers, outer nuclear layer, outer plexiform layer, inner nuclear layer, inner plexiform layer, and ganglion cell layer.25 Qualitative assessment of the presence or absence of pigment epithelial detachment and the pigment epithelial detachment subtype based on internal reflectivity characteristics (drusenoid, fibrovascular, or serous) was assessed in each eye.26,27 Subretinal drusenoid deposits were identified as hyperreflective elevations above the RPE or in line with the photoreceptor outer segments. Our OCT scanning area for the detection of SDDs was more limited in the field of view (despite inclusion of both macula-centered and optic nerve–centered scans) (Figure 3) as compared to the recommended regions we scanned by IR or FAF imaging.28
Fig. 2.
Spectral domain optical coherence tomography B-scan showing (white arrow) outer retinal tubulation (A) and HRF over drusen (B).
Fig. 3.
Illustration of subretinal drusenoid deposits (SDDs) on infrared reflectance (IR) and OCT images. The OCT-scanned regions are outlined in light grey boxes on the IR images (A). The horizontal grey lines, indicated by white arrows, denote the locations of selected B-scans (B,C). The black arrows on the OCT B-scans identify individual SDD lesions.
Blue-light FAF and IR imaging were performed using the Spectralis HRA + OCT with a 30° image centered on the fovea (Field 2) and the optic nerve position at the nasal edge of the image. Images with poor quality because of focus, exposure, or artifacts were excluded from the study analysis. All images were analyzed by a trained, certified, senior Doheny Image Reading Center grader. The OCT data from the two devices were graded separately in a masked fashion to identify any discrepancies in assessment between instruments.
Color fundus photographs were evaluated for the presence, number, and size of drusen, as well as for pigment alterations. The diagnosis of AMD in an eye was determined according to the Beckman classification,29 which requires a minimum of five mediumsized drusen (≥63 μm) on a color fundus photograph. Eyes with no abnormalities, only small hard drusen, or fewer than five medium drusen were considered to be unaffected.
Statistical Analysis
The descriptive statistics were performed using all eyes for the main outcome measures for this study including drusen area, drusen volume, GA choroidal thickness, and qualitative parameters as described in Table 1. The correlations of quantitative parameters were assessed using all subjects available after adjusting for the age at examination and sex. To adjust for the influence of sibship among subjects, the multivariate familial correlations were estimated using the method by Keen and Elston30 and Matthew et al,31 as implemented in the FCOR program in the Statistical Analysis for Genetic Analysis (S.A.G.E.) package.32 To compare age-and sex-adjusted phenotype mean values with different groups defined by SDD status or by AMD status, the mean of two measurements from the right and left eyes was used and the generalized estimating equation method was used to adjust for correlations because of the related subjects. A P value of <0.05 was considered statistically significant. All statistical analyses besides familial correlations were performed using SPSS 18 statistical software (SPSS version 18.0, IBM, Chicago, IL).
Table 1.
Frequencies of Spectral Domain Optical Coherence Tomography Findings in an Elderly Amish Population
| SD-OCT Parameter N = 2,191 Eyes | Present in n (%) |
|---|---|
| Subretinal fluid* | 30 (1.4) |
| Subretinal hyperreflective material* | 30 (1.4) |
| Intraretinal cysts* | 78 (3.9) |
| Outer retinal tubules* | 27 (1.2) |
| Subretinal drusen* | 68 (3.1) |
| Geographic atrophy† | 99 (4.5) |
| Hyperreflective foci over drusen* | 167 (7.6) |
| Hyperreflective foci extent (n = 190 eyes) | |
| Photoreceptor cell layer | 12 (7.2) |
| Outer nuclear layer | 112 (67.1) |
| Outer plexiform layer | 43 (25.7) |
| Pigment epithelial detachment* | |
| Drusenoid | 324 (14.8) |
| Fibrovascular | 30 (1.4) |
| Serous | 11 (0.5) |
Cirrus-derived thickness values.
Spectralis-derived thickness values.
Results
Recruitment and Ascertainment
A total of 1,146 (2,292 eyes) participants were recruited to the Amish Eye Study from February 2013 and July 2016. A total of 2,191 eyes (95.6%) of 1,124 subjects met the OCT-including criteria for the qualitative analysis after excluding 101 eyes (4.4%) because of the poor quality scans or significant (>10% of drusen area) drusen segmentation errors, or missing scans on the SD-OCT. For each quantitative variable, additional eyes were excluded. For example, choroidal thickness could not be graded in 96 eyes (4%) by the Spectralis and 211 eyes (9%) by the Cirrus. Among 2,191 eyes, 1,009 eyes (46.1%) had fundus photo graph grades consistent with AMD and 1,048 eyes(49.4%) had no AMD. Age-related macular degeneration status could not be determined for 98 eyes(4.5%). In addition, 444 of 1,124 subjects (40%) had AMD in both eyes (affected cohort), 152 subjects (14%) had AMD in 1 eye only, and 478 subjects (43%) had no AMD (unaffected cohort). There were 2,282 sibpairs from 377 sibships of size 1 to 13 in which 158 of them were without any additional sibs, that is, 14% of data were of independent subjects.
Baseline Characteristics
The mean age of the 1,146 participants was 65.2 ± 11 (mean ± SD) years with a slight majority being women (60%). The mean age of the 478 unaffected cohort was 62.1 ± 9.5 years, whereas AMD-affected subjects were older with an average age of 68.1 ± 11.4 years (P < 0.001).
Table 1 shows frequencies of abnormalities found by SD-OCT. Subretinal fluid and fibrovascular pigment epithelial detachments were relatively uncommon (less than 2%), reflecting the low prevalence of neovascular disease in our population. Aside from drusenoid lesions, the most common abnormalities on OCT in this cohort were intraretinal hyperreflective features, which are believed to correspond to pigment migration on color photographs and can be seen over-lying some drusen. Hyperreflective foci over drusen were seen in nine of the eyes (Figure 2B) and had extended to the outer nuclear layer or further internally in the vast majority of cases.
The SD-OCT measurements for drusen area, volume, and choroidal thickness in subjects with AMD are described in Table 2. The mean drusen area was 0.22 mm2 and 0.39 mm2 for the 3- and 5-mm circles, respectively. The mean drusen volume was 0.01 mm3 for the 3-mm circle and 0.02 mm3 for the 5-mm circle. The mean GA area in the 5-mm circle zone was 0.47 mm2 (Table 2). Considering only those eyes with GA (N = 99 eyes), the mean GA area was 5.32 ± 5.91 mm2. The mean choroidal thickness for all eyes was 255 ± 70 μm (range: 14–439 μm). After adjusting for age, in subjects with AMD, the mean choroidal thickness was significantly less (242 ± 76 μm, P < 0.001) when compared with those with no AMD (263 ± 63 μm).
Table 2.
Descriptive Details of Quantitative Study Parameters in an Age-Related Macular Degeneration Cohort of the Study
| Variable | All Eyes n = 1,009 Eyes (Mean ± SD; Range) | Right Eyes n = 499 Eyes (Mean ± SD; Range) | Left Eyes n = 510 Eyes (Mean ± SD; Range) | Intraclass Correlation in Sibpairs | Correlation of all Subjects | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pair Cnt | Eqv Pair Cnt | r ± SE (P) | Pair Cnt | Eqv Pair Cnt | r ± SE (P) | ||||
| Drusen area in 3 mm* | 0.22 ± 0.68 (0–6.7) | 0.20 ± 0.64 (0–6.4) | 0.23 ± 0.73 (0–6.7) | 358 | 144.3 | 0.0507 ± 0.0833 (0.5464) | 413 | 171.5 | 0.5429 ± 0.0544 (<0.0001) |
| Drusen area in 5 mm* | 0.39 ± 1.25 (0–13.3) | 0.37 ± 1.25 (0–13.3) | 0.41 ± 1.21 (0–12.7) | 355 | 80.7 | 0.0846 ± 0.1078 (0.4403) | 412 | 121.4 | 0.7325 ± 0.0433 (<0.0001) |
| Drusen volume in 3 mm* | 0.01 ± 0.08 (0–1.63) | 0.01 ± 0.08 (0–1.63) | 0.02 ± 0.09 (0–1.04) | 358 | 110.7 | 0.0255 ± 0.0945 (0.7912) | 413 | 109.8 | 0.3616 ± 0.0837 (<0.0001) |
| Drusen volume in 5 mm* | 0.02 ± 0.12 (0–2.19) | 0.02 ± 0.12 (0–2.19) | 0.03 ±0.11 (0–1.5) | 358 | 85.6 | 0.0438 ± 0.1085 (0.6906) | 413 | 93.9 | 0.5021 ± 0.0783 (<0.0001) |
| GA area 5 mm* | 0.47 ± 2.30 (0–19.1) | 0.49 ± 2.37 (0–19.1) | 0.45 ± 2.22 (0–19.1) | 362 | 78.3 | 0.0413 ± 0.1135 (0.7196) | 415 | 427.1 | 0.8451 ± 0.0141 (<0.0001) |
| Cirrus-choroidal thickness† | 246 ± 75 (23–439) | 247 ± 75 (26–439) | 245 ± 76 (23–432) | 333 | 165.1 | 0.2881 ± 0.0717 (0.0002) | 380 | 395.8 | 0.8398 ± 0.0151 (<0.0001) |
| Spectralis-choroidal thickness‡ | 244 ± 78 (23–478) | 245 ± 78 (23–478) | 243 ± 77 (23–428) | 336 | 168.4 | 0.2778 ± 0.0714 (0.0002) | 404 | 483.1 | 0.8279 ± 0.0145 (<0.0001) |
n-overall sample (eye) count, note that the actual sample size is slightly smaller because of missing values.
From the center of fovea.
Cirrus-derived thickness values.
Spectralis-derived thickness values.
Eqv Pair Cnt, equivalent pair count; r, correlation between the right and left eye.
There was a significant correlation (P < 0.001) between the right and left eyes with respect to drusen area, drusen volume, GA, and choroidal thickness measurements (Table 2). Only choroidal thickness measurements had significant intraclass correlations (P < 0.001) among sibpairs but not on drusen area and volume variables in subjects with AMD.
Reproducibility Analysis
Intragrader Reproducibility.
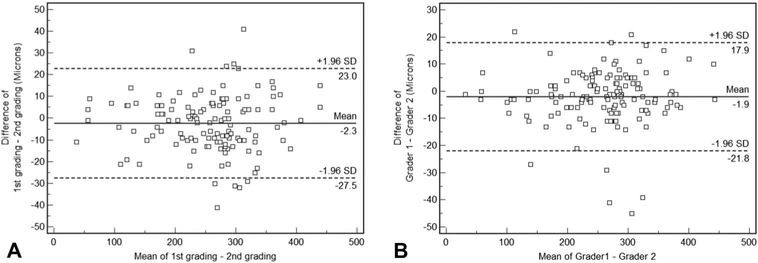
The mean choroidal thickness for the first and second gradings were 250 ± 79 μm and 253 ± 79 μm, respectively, with a mean absolute difference of 10 ± 8 μm (maximum; 41). The ICC was 0.994 (P < 0.001) (95% confidence interval:0.993–0.995) (Figure 4).
Fig. 4.
Bland–Altman plots demonstrating intra-(A) and intergrader (B) reproducibility of subfoveal choroidal thickness. Dashed lines: 95% limits of agreement.
Intergrader Reproducibility.
The mean cystoid space volumes computed by grader 1 and grader 2 were 250 ± 79 μm and 253 ± 78 μm, respectively, with a mean absolute difference of 7 ± 7 (maximum;45). The ICC was 0.996 (P < 0.001) (95% confidence interval: 0.995–0.997) (Figure 4).
The presence of SDDs was assessed in all study eyes using both the Cirrus and Spectralis OCT and both the macula and optic nerve–centered cube scans combined. Subretinal drusenoid deposits could be observed in 32(1.5%) eyes with the Cirrus and in 68 (3.1%) eyes with the Spectralis, with discordant findings between devices for 41 (1.9%) eyes. Of note, when assessing for SDDs with IR and FAF imaging, which also covered a larger total field of view than the OCT scans, the prevalence rose to 7% or 143 of eyes. Of 143 eyes with SDDs, 11 were classified as normal. Drusen volume (P < 0.001) and GA area (P < 0.001) were significantly greater in eyes with SDDs compared with those without (Table3). The choroid was significantly (P < 0.001) thinner in subjects with SDDs compared with those without SDDs (Table 3). There was no meaningful correlation between drusen volume, GA area, and choroidal thickness measurements in eyes with SDDs (Table 4), although there was a weak correlation between choroidal thickness and GA area. Interestingly, however, a statistically significant correlation (r = 0.45, P < 0.001) was observed between drusen volume and GA area in eyes without SDDs.
Table 3.
Quantitative Measures in Subjects With and Without SDD/RPD
| Variable | No RPD Cohort, n = 2,046 Eyes (Mean ± SD; Range) | RPD Cohort, n = 143 Eyes (Mean ± SD; Range) | P |
|---|---|---|---|
| Drusen area in 5 mm* | 0.14 ± 0.73 (0–13.3) | 1.22 ± 2.06 (0–12.8) | <0.001 |
| Drusen volume in 5 mm* | 0.01 ± 0.05 (0–1.00) | 0.08 ± 0.25 (0–2.19) | <0.001 |
| GA area 5 mm* | 0.15 ± 1.41 (0–19.1) | 1.55 ± 3.70 (0–19.1) | <0.001 |
| Spectralis-choroidal thickness† | 259 ± 69 (23–488) | 172 ± 82 (31–367) | <0.001 |
n-overall sample (eye) count, note that the actual sample size is slightly smaller because of missing values.
From the center of fovea.
Spectralis-derived thickness values.
RPD, reticular pseudodrusen.
Table 4.
Correlations Between Study Parameters in Subjects With and Without SDD/RPD
| Variable 1 | Variable 2 | r ± SE | P | Pair Cnt | Eqv Pair Cnt |
|---|---|---|---|---|---|
| SDD cohort | |||||
| Drusen volume | GA area | 0.1746 ± 0.1581 | 0.2913 | 60 | 38.8 |
| Drusen volume | Choroidal thickness | −0.0704 ± 0.1865 | 0.7168 | 54 | 29.5 |
| Choroidal thickness | GA area | −0.2066 ± 0.1847 | 0.2936 | 53 | 28.1 |
| No SDD cohort | |||||
| Drusen volume | GA area | 0.4507 ± 0.0279 | <0.0001 | 986 | 815.4 |
| Drusen volume | Choroidal thickness | −0.1962 ± 0.0382 | <0.0000 | 918 | 635.9 |
| Choroidal thickness | GA area | −0.1315 ± 0.0391 | <0.0001 | 920 | 632.4 |
r, correlation; Eqv Pair Cnt, equivalent pair count.
Discussion
We describe the SD-OCT characteristics of AMD in an elderly Amish population that has not been reported previously. We have qualitatively characterized AMD features beyond automated quantitative measures on SD-OCT. Rather than analyzing a single scan, we have reviewed all B-scans in the macular cubes. This provides a complete survey of the morphological characteristics on SD-OCT and their prevalence in this population.
The qualitative analysis of AMD features from SDOCT imaging as described in Table 1 was guided by previous studies on AMD.5,24–26 Using the high-resolution cross-sectional SD-OCT scans, we were able to identify intra-or subretinal fluid, subretinal tissue, outer retinal tubulation, SDDs, intraretinal HRF (including their axial extent), and pigment epithelial detachment. Outer retinal tubulations are branching tubular structures in the outer nuclear layer seen on en face OCT scans that appear as round or ovoid hyporeflective spaces with hyperreflective borders on OCT B-scans (Figure 2A). Twenty-seven eyes (1.2%) in this study had outer retinal tubulations, which have been associated with a slower growth rate of GA lesions.33 In future analyses, it will be interesting to explore the heritability of these lesions.
Previous reports have found that the presence of HRF over drusen portends a high risk for the development and progression of GA over time.25 Hyperreflective foci are believed to represent the migration of RPE cells into the neurosensory retina.25,34 In this study, 18% of subjects with AMD had HRF. In previous reports,25 the innermost extent of the HRF within the retina was not a significant independent risk factor for developing GA. However, because the HRF may migrate internally over time, the extent of migration may reflect the time since the initial migration event. In the present study, the HRF were found to extend as far as the outer plexiform layer in 26% of eyes.
Previous publications on OCT-based automated quantification of drusen parameters have shown the accuracy and reproducibility of the automated algorithm for detecting and measuring drusen area, volume, and GA area.14,35–37 In this study, we have used the algorithm to analyze and study drusen characteristics in elderly Amish patients. Traditionally, drusen quantification has been performed by color imaging methods, but there is imprecision with respect to measuring drusen area or size, a known indicator of AMD progression.16,38,39 Similar to previous studies,24,40 our study showed comparable drusen measurements between both eyes of subjects, with a significant correlation for drusen area and volume. In this report, we are describing only drusen metrics at a specific point in time. It is our intent in the future to remeasure drusen metrics in this population to calculate drusen growth and turnover rate. These end points could be useful for genetic association studies or targets for therapeutics.
Subretinal drusenoid deposits are a distinct OCT feature found in many diseases,41 including AMD, which appear to correspond to reticular pseudodrusen (RPD) lesions evident on IR or blue-light FAF imaging.42,43 Our results do confirm the superiority of SDOCT, IR, and FAF over color fundus photography (CFP) for detection of SDD and RPD. Analysis of our CFP for RPD/SDD on 2,191 eyes showed a detection rate of only 1.5% (34 eyes). We also found an increased sensitivity to detect these lesions with IR/ FAF compared with SD-OCT (7% vs. 3.1%). This difference could be partially explained by the fact that many of these SDD lesions may be located outside the field of view of the obtained OCT scans.44 This is perhaps not surprising because SDDs are known to have a higher prevalence along the outer border of the macula, whereas regular drusen tend to accumulate more centrally. Hopefully, with faster OCT devices in the future (e.g., swept-source OCT), larger areas can be scanned easily, improving the detection sensitivity of OCT for these lesions. Regardless, our observed OCT prevalence rates have merit because they reflect the current commonly obtained scan patterns in practice.
Previous studies estimating the prevalence of SDDs have used varying inclusion criteria, case definitions, and imaging modalities. The Beaver Dam and Blue Mountains Eye Studies both evaluated eyes (albeit using different metrics) for SDDs from color fundus photographs (CFP) and reported SDD prevalences of0.7% and 1.95%, respectively.45,46 Recently, Zarubina et al47 compared their SDD prevalence in the AL-STAR cohort based on CFPs to the Beaver Dam and Blue Mountain study using the same metrics and found a prevalence of 1.48% and 1.97%, respectively. Our prevalence in the Amish cohort using CFPs was1.5%. Our prevalence of SDDs increased to 7.0% when including the results of multimodal imaging. Still, this prevalence is lower than the reported prevalence (32%) in the ALSTAR cohort.47 We considered five possible explanations for this different result: 1) the ALSTAR cohort evaluated the peripapillary area more extensively than our study. Of note is their finding that 47% or persons with SDDs were detected exclusively on the optic nerve head SD-OCT; 2) the Amish cohort is twice the size of the ALSTAR cohort, making it less prone to statistical errors; 3) the AL-STAR cohort is a clinic-based sample recruited from two primary care ophthalmology practices at a major teaching hospital, whereas the Amish cohort was more population based although more focused on families;4) the Amish cohort was younger than the ALSTAR cohort (mean age = 65 years vs. 69 years); 5) the ALSTAR cohort is mostly white and some are non-whites, whereas our cohort is selected for the Amish; and 6) the Amish do not smoke, whereas 46% of the ALSTAR participants were either former/current smokers.
Careful analysis of previous studies reporting SDD prevalence requires rigorous interpretation. For example, reports assessing only AMD subjects with SDOCT and IR have reported a prevalence as high as 58%,48 whereas studies using SD-OCT, IR, and FAF have reported estimates as high as 69% and as low as 18%.49 These estimates can vary with different study population samples and imaging techniques and may not reflect the population rates. Interestingly, we found SDDs in eight individuals (11 eyes) who had no evidence of drusen or pigment alterations suggestive of AMD. One limitation to our study is that it is a cross-sectional assessment. Subretinal drusenoid deposits have been shown28,41 to evolve over time requiring a progression study to assess their overall progression with the AMD disease process.
Arnold et al50 found a marked decrease in choroidal thickness in subjects with SDDs. Similarly, we found a significantly thinner choroid in eyes with SDDs. It has been suggested that choroidal thinning may have occurred because of the fibrotic replacement of the middle layer of the choroid.50 Studies44 have shown a high prevalence of late AMD among patients with SDDs. We also found that drusen volume and GA area were significantly higher in subjects with SDDs compared with those without SDDs.
In summary, we have described SD-OCT parameters in an elderly Amish population with AMD. We have observed that SDDs can be found in the absence of other AMD features and may influence the thickness of the choroid.
Acknowledgments
Supported by the National Eye Institute, Bethesda, Maryland (Grant #RO1 EY023164), the Department of Ophthalmology at the Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, John P. Hussman Institute of Human Genomics at the University Of Miami Miller School Of Medicine, Miami, Florida and the Institute for Computational Biology, Case Western Reserve University, Cleveland, Ohio. Funds also were received from the F.M. Kirby Foundation and Research to Prevent Blindness.
None of the authors has any financial/conflicting interests to disclose.
References
- 1.Friedman DS, O’Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564–572. [DOI] [PubMed] [Google Scholar]
- 2.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004;82:844–845. [PMC free article] [PubMed] [Google Scholar]
- 3.Danis RP, Lavine JA, Domalpally A. Geographic atrophy in patients with advanced dry age-related macular degeneration: current challenges and future prospects. Clin Ophthalmol 2015;9:2159–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fritsche LG, Fariss RN, Stambolian D, et al. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet 2014;15:151–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meleth AD, Wong WT, Chew EY. Treatment for atrophic macular degeneration. Curr Opin Ophthalmol 2011;22: 190–193. [DOI] [PubMed] [Google Scholar]
- 6.Sarks SH, Arnold JJ, Killingsworth MC, Sarks JP. Early drusen formation in the normal and aging eye and their relation to age related maculopathy: a clinicopathological study. Br J Ophthalmol 1999;83:358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spraul CW, Grossniklaus HE. Characteristics of drusen and Bruch’s membrane in postmortem eyes with age-related macular degeneration. Arch Ophthalmol 1997115:267–273. [DOI] [PubMed] [Google Scholar]
- 8.Tikellis G, Robman LD, Dimitrov P, et al. Characteristics of progression of early age-related macular degeneration: the cardiovascular health and age-related maculopathy study. Eye (Lond) 2007;21:169–176. [DOI] [PubMed] [Google Scholar]
- 9.Klein BE, Klein R. Lifestyle exposures and eye diseases in adults. Am J Ophthalmol 2007;144:961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartlett H, Eperjesi F. Age-related macular degeneration and nutritional supplementation: a review of randomised controlled trials. Ophthalmic Physiol Opt 2003;23:383–399. [DOI] [PubMed] [Google Scholar]
- 11.Brantley MA Jr, Fang AM, King JM, et al. Association of complement factor H and LOC387715 genotypes with response of exudative age-related macular degeneration to intravitreal bevacizumab. Ophthalmology 2007;114:2168–2173. [DOI] [PubMed] [Google Scholar]
- 12.Lee AY, Raya AK, Kymes SM, et al. Pharmacogenetics of complement factor H (Y402H) and treatment of exudative age-related macular degeneration with ranibizumab. Br J Ophthalmol 2009;93:610–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKibbin M, Ali M, Bansal S, et al. CFH, VEGF and HTRA1 promoter genotype may influence the response to intravitreal ranibizumab therapy for neovascular age-related macular degeneration. Br J Ophthalmol 2012;96:208–212. [DOI] [PubMed] [Google Scholar]
- 14.Nittala MG, Ruiz-Garcia H, Sadda SR. Accuracy and reproducibility of automated drusen segmentation in eyes with nonneovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 2012;53:8319–8324. [DOI] [PubMed] [Google Scholar]
- 15.Freeman SR, Kozak I, Cheng L, et al. Optical coherence tomography-raster scanning and manual segmentation in determining drusen volume in age-related macular degeneration. Retina 2010;30:431–435. [DOI] [PubMed] [Google Scholar]
- 16.Davis MD, Gangnon RE, Lee LY, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol 2005;123: 1484–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maguire MG, Alexander J, Fine SL; Complications of Age-related Macular Degeneration Prevention Trial (CAPT) Research Group. Characteristics of choroidal neovascularization in the complications of age-related macular degeneration prevention trial. Ophthalmology 2008;115:1468–1473. [DOI] [PubMed] [Google Scholar]
- 18.Sardell RJ, Nittala MG, Adams LD, et al. Heritability of choroidal thickness in the Amish. Ophthalmol 2016;123: 2537–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahs DW, McCauley JL, Crunk AE, et al. A genome-wide linkage analysis of dementia in the Amish. Am J Med Genet B Neuropsychiatr Genet 2006;141B:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCauley JL, Hahs DW, Jiang L, et al. Combinatorial mismatch scan (CMS) for loci associated with dementia in the Amish. BMC Med Genet 2006;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hostetler JA. Amish Society. 4th ed. Baltimore, MD: Johns Hopkins University Press; 1993. [Google Scholar]
- 22.Beachy L Unser Leit: The Story of the Amish. Millersburg, OH: Goodly Heritage Books; 2011. [Google Scholar]
- 23.Amish Heritage Committee. Amish HeritageCommittee. Elkhart & Lagrange Counties: Amish Heritage Committee; 1990. [Google Scholar]
- 24.Diniz B, Rodger DC, Chavali VR, et al. Drusen and RPE atrophy automated quantification by optical coherence tomography in an elderly population. Eye (Lond) 2015;29:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouyang Y, Heussen FM, Hariri A, et al. Optical coherence tomography-based observation of the natural history of drusenoid lesion in eyes with dry age-related macular degeneration. Ophthalmology 2013;120:2656–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zayit-Soudry S, Moroz I, Loewenstein A. Retinal pigment epithelial detachment. Surv Ophthalmol 2007;52:227–243. [DOI] [PubMed] [Google Scholar]
- 27.Lee SY, Stetson PF, Ruiz-Garcia H, et al. Automated characterization of pigment epithelial detachment by optical coherence tomography. Invest Ophthalmol Vis Sci 2012;53: 164–170. [DOI] [PubMed] [Google Scholar]
- 28.Curcio CA, Messinger JD, Sloan KR, et al. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina 2013;33:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferris FL III, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology 2013;120:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keen KJ, Elston RC. Robust asymptotic sampling theory for correlations in pedigrees. Stat Med 2003;22:3229–3247. [DOI] [PubMed] [Google Scholar]
- 31.Matthew G, Song Y, Elston R. Interval estimation of familial correlations from pedigrees. Stat Appl Genet Mol Biol 2011; 10:1–29. [DOI] [PubMed] [Google Scholar]
- 32.S.A.G.E. 6.4, statistical analysis for genetic epidemiology. 2016. Available at: http://darwin.cwru.edu/sage/. Accessed August 2017.
- 33.Hariri A, Nittala MG, Sadda SR. Outer retinal tubulation as a predictor of the enlargement amount of geographic atrophy in age-related macular degeneration. Ophthalmology 2015;122: 407–413. [DOI] [PubMed] [Google Scholar]
- 34.Keane PA, Patel PJ, Liakopoulos S, et al. Evaluation of age-related macular degeneration with optical coherence tomography. Surv Ophthalmol 2012;57:389–414. [DOI] [PubMed] [Google Scholar]
- 35.Gregori G, Wang F, Rosenfeld PJ, et al. Spectral domain optical coherence tomography imaging of drusen in nonexudative age-related macular degeneration. Ophthalmology 2011;118: 1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu SJ, Izatt JA, O’Connell RV, et al. Validated automatic segmentation of AMD pathology including drusen and geographic atrophy in SD-OCT images. Invest Ophthalmol Vis Sci 2012;53:53–61. [DOI] [PubMed] [Google Scholar]
- 37.Yehoshua Z, Garcia Filho CA, Penha FM, et al. Comparison of geographic atrophy measurements from the OCT fundus image and the sub-RPE slab image. Ophthalmic Surg Lasers Imaging Retina 2013;44:127–132. [DOI] [PubMed] [Google Scholar]
- 38.Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration: AREDS Report No.18. Arch Ophthalmol 2005;123:1570–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein R, Klein BE, Knudtson MD, et al. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology 2007;114:253–262. [DOI] [PubMed] [Google Scholar]
- 40.Gregori G, Yehoshua Z, Garcia Filho CA, et al. Change in drusen area over time compared using spectral-domain optical coherence tomography and color fundus imaging. Invest Ophthalmol Vis Sci 2014;55:7662–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zweifel SA, Imamura Y, Spaide TC, et al. Prevalence and significance of subretinal drusenoid deposits (reticular pseudo-drusen) in age-related macular degeneration. Ophthalmology 2010;117:1775–1781. [DOI] [PubMed] [Google Scholar]
- 42.Zweifel SA, Spaide RF, Curcio CA, et al. Reticular pseudo-drusen are subretinal drusenoid deposits. Ophthalmology 2010; 117:303–312. [DOI] [PubMed] [Google Scholar]
- 43.Steinberg JS, Göbel AP, Fleckenstein M, et al. Reticular drusen in eyes with high-risk characteristics for progression to late-stage age-related macular degeneration. Br J Ophthalmol 2015; 99:1289–1294. [DOI] [PubMed] [Google Scholar]
- 44.De Bats F, Mathis T, Mauget-Faÿsse M, et al. Prevalence of reticular pseudodrusen in age-related macular degeneration using multimodal imaging. Retina 2016;36:46–52. [DOI] [PubMed] [Google Scholar]
- 45.Klein R, Meuer SM, Knudtson MD, et al. The epidemiology of retinal reticular drusen. Am J Ophthalmol 2008;145:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joachim N, Mitchell P, Rochtchina E, et al. Incidence and progression of reticular drusen in age-related macular degeneration: findings from an older Australian cohort. Ophthalmology 2014;121:917–925. [DOI] [PubMed] [Google Scholar]
- 47.Zarubina AV, Neely DC, Clark ME, et al. Prevalence of sub-retinal drusenoid deposits in older persons with and without age-related macular degeneration, by multimodal imaging. Ophthalmology 2016;123:1090–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finger RP, Wu Z, Luu CD, et al. Reticular pseudodrusen: a risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularization. Ophthalmology 2014; 121:1252–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ueda-Arakawa N, Ooto S, Tsujikawa A, et al. Sensitivity and specificity of detecting reticular pseudodrusen in multimodal imaging in Japanese patients. Retina 2013;33:490–497. [DOI] [PubMed] [Google Scholar]
- 50.Arnold JJ, Sarks SH, Killingsworth MC, Sarks JP. Reticular pseudodrusen: a risk factor in age-related maculopathy. Retina 1995;15:183–191. [PubMed] [Google Scholar]