Abstract
Introduction
The consequences of low human papillomavirus (HPV) vaccination in Census regions with higher incidence of cervical cancer may contribute to continued disparities. Our purpose was to evaluate regional variations in HPV prevalence across time.
Methods
Repeated cross-sectional data from the National Health and Nutrition Examination Survey (NHANES), 2003–2014 were examined. Participants included females 14 to 34 years old who provided adequate vaginal samples for HPV DNA typing (N=6,387). Region of residence and HPV vaccination status associations with HPV prevalence were examined using chi-square and multivariable logistic regression. HPV types were grouped according to vaccine-type HPV (types 6, 11, 16, 18) and risk (high or low-risk). Time and vaccination status were included in subsequent models for post-licensure survey cycles (2007–2014) to assess their effects on observed associations.
Results
No decreases in vaccine-type HPV prevalence were found between the prevaccine cycles (2003–2006) and early post-licensure cycles (2007–2010, p>0.05). Vaccine-type HPV prevalence decreased in late post-licensure years (2011–2014) compared to prevaccine years (2003–2006, p=0.001). The highest prevalence of vaccine-type HPV occurred in the South (8.6%) and Midwest (8.6%), followed by the West (4.8%), and the Northeast (3.5%) in late post-licensure years. Lower odds of vaccine-type HPV across time in post-licensure survey cycles were found to be attributable to time, and more strongly to HPV vaccination.
Conclusions
There were regional variations in vaccine-type HPV prevalence between prevaccine and post-licensure years. These decreases appeared to be at least partially attributable to HPV vaccination. Programs are needed to address geographical disparities in HPV vaccination.
Keywords: HPV prevalence, vaginal HPV, HPV vaccination, geographic disparities, cancer prevention
INTRODUCTION
Human papillomavirus (HPV) is a common infection with several types that can cause anogenital cancers as well as oropharyngeal cancer [1–3]. The most common HPV types associated with cancer can be prevented through vaccination of young adolescents, preferably before exposure. The most widely used HPV vaccine in the U.S. until 2014, the quadrivalent HPV vaccine (4vHPV), protects against 2 high risk HPV types (16 and 18) responsible for 70% of cervical cancer cases and 2 types responsible for 90% of genital warts (types 6 and 11) [4–7]. In late 2014, the 9-valent HPV vaccine (9vHPV), which protects against 5 additional types of high risk HPV (31, 33, 45, 52, 58), was approved by the Food and Drug Administration (FDA) and has replaced the 4vHPV in the US [8]. Although the vaccine is effective in preventing HPV infections and precancerous lesions, vaccination rates among adolescents and young adults are inadequate in the U.S. due to a combination of factors, including lack of strong health provider recommendation, lack of knowledge or awareness about the HPV vaccine, and concerns with vaccine safety [9–12]. Moreover, regional disparities in HPV vaccination may exist, with Southern states reporting lower initiation and completion rates of the 3-dose series among adolescents and young adults [13–15]. Little is known about how HPV vaccination has affected variations in HPV prevalence between the 4 major Census regions of the United States.
Regional variations in HPV vaccination are concerning, as cervical cancer is particularly high in several Southern states, which have high rates of cervical cancer [16]. As of yet, it may be too early to determine whether regional disparities in cervical cancer incidence will be affected by a geographic variation in vaccine uptake, as cervical cancer develops a decade or more after HPV infection [17]. Thus, potential consequences of low HPV vaccination by region can be investigated through the examination of variation in HPV prevalence to determine whether there is potential for continued or widening geographic disparities in future HPV-related diseases.
The purpose of this study was to: (1) examine geographic variation in HPV prevalence across time and (2) examine the effect of HPV vaccination on associations of region and time with vaccine-type HPV prevalence. Data from 6 survey cycles of the National Health and Nutrition Examination Survey (NHANES) were used to examine regional variation in HPV prevalence in the U.S. across time.
METHODS
Study sample
The NHANES survey is a complex, stratified, multistage probability sample with ongoing cross-sectional surveys of the civilian noninstitutionalized U.S. population. Details about sampling and methodology can be found on the National Center for Health Statistics’ (NCHS) website [18]. Briefly, participants take a household interview followed by a physical examination in a mobile examination center (MEC), which includes self-collected cervicovaginal swab samples among females 14–59 years of age. Cervicovaginal samples were tested for 37 HPV DNA types at the Centers for Disease Control and Prevention (CDC) using the Linear Array HPV genotyping Assay (Roche Diagnostics) [18]. The survey and MEC methods were approved by the NCHS/ CDC research ethics board, and this secondary analysis of NHANES data was exempted by the University of Texas Medical Branch Institutional Review Board.
Data from 2-year cycles between 2003 and 2014 were examined and analyzed. Pre-vaccine years included 2003– 2006 because the 4vHPV was approved by the FDA late in 2006, and the Advisory Committee on Immunization Practices (ACIP) recommended it in early 2007 [19]. Post-licensure years included 2007–2014, with early post-licensure years including 2007–2010 and late post-licensure years including 2011–2014. Regions included states in the Northeast, Midwest, South, and West, as defined by the Census [20]. The number of HPV vaccine doses was assessed among those that reported it. Demographics and sexual history were evaluated. Sexual history was included as a count of the number of lifetime male sex partners, which was categorized as “0” for those who had never had sex, “1–2”, and “≥3” for those that reported 3 or more lifetime partners.
We developed 4 HPV infection groupings, based on type. “Any HPV” included positive values for any of the 37 types tested for. Other categories included: 4 “vaccine-types” (6, 11, 16, 18), 2 “high-risk vaccine-types” (16, 18), and “nonvaccine-types” (33 types, not including 6, 11, 16, 18). Women were included if they: 1) were between 14 and 34 years of age, 2) participated in both the interview and the MEC examination 3) had an adequate cervicovaginal swab sample (inadequate sample were negative for both β-globin and HPV), and 4) either participated in the pre-vaccine cycle, or responded, “yes” or “no” to a question about HPV vaccination status in the post-licensure cycles. HPV vaccination was defined as women who reported they received 1 or more doses. NHANES data included information about HPV vaccination, including number of doses, and age at first dose has been collected since 2011. We did not analyze age at first dose because it was not available for all cycles, and not included in the original data request from the NCHS. Although participants 20–30 years of age may have a lower rate of HPV vaccination compared to younger women, we included those 14–34 years of age because a higher prevalence of HPV vaccination in post-licensure years was anticipated among this group compared to those greater than 35 years of age. Participant samples with inadequate cervicovaginal swab samples were still tested for HPV DNA, and consistent with previous NHANES studies on HPV prevalence, were included if they tested positive for HPV DNA. Participants from prevaccine cycles were considered to be unvaccinated.
Statistical analyses
Demographic characteristics were calculated and compared for the overall sample, and by region. HPV infection prevalence was examined between pre-vaccine and post-licensure periods for each region. HPV type category prevalence was then examined between prevaccine and post-licensure years, stratified by region. In order to examine general trends in vaccine-type HPV prevalence across the NHANES cycles, the prevalence was calculated for each cycle and graphed by region. HPV vaccination rates were also graphed for each NHANES cycle across time for each region. Bivariate comparisons were made using Rao-Scott Chi-square tests. All analyses were weighted using MEC weights provided in the dataset, using methods described in detail elsewhere [18].
Multivariable binary logistic regression models were used to calculate population adjusted odds ratios (PaOR) to examine the effects of time and HPV vaccination on vaccine-type HPV and any HPV types. We developed 3 models for vaccine-type HPV. The first model included age, race/ ethnicity, number of lifetime male sexual partners, and region. Model 2 added a NHANES cycles (2003–2014) to model 1 to assess effects of the vaccine licensure period on associations observed in model 1. We then excluded time (NHANES cycles) in Model 3 and included HPV vaccination to observe its effects on associations from Model 1. We did not include time in model 3, as HPV vaccination and vaccination status during the prevaccine period were strongly associated because all values would have been set to zero.
To evaluate how HPV vaccination affected the association between time and HPV prevalence, multivariable binary logistic regression models were used to calculate PaORs for vaccine-type HPV during the post-licensure period (2007–2014) only. We excluded women who participated in the prevaccine period, as they were all unvaccinated. Model 1 assessed the effect of region on HPV prevalence in post-licensure years (2007–2014), controlling for age, race/ ethnicity, and number of lifetime male sexual partners. Model 2 added the 3 post-licensure NHANES cycles. Model 3 added HPV vaccination to Model 2. Analyses were carried out using a remote access system to the NHANES dataset at the CDC. All analyses were done using SAS® statistical software (SAS Institute, Inc., Cary, NC).
RESULTS
A total of 6,387 participants were included in this study. Regional differences were observed in some demographic variables, including age, and race/ ethnicity (Table 1). There were regional variations in the number of lifetime male sex partners, with the highest proportion of women reporting 3 or more male partners residing in Northeast states (63% ), and the lowest proportion in the West (51%). No regional variations in HPV vaccine initiation or number of HPV doses were found. However, HPV vaccination was low in all regions. Among women in the post-licensure period only (n=3,732), HPV vaccination was 26.3%.
Table 1.
Demographic characteristics of 14–34 year old women with HPV results by region, NHANES data 2003–2014 (N=6,387)
| Overall (N=6,387) | Northeast a (n=931) | Midwest a (n=1,259) | South a (n=2,458) | West a (n=1,739) | |||
|---|---|---|---|---|---|---|---|
| Weighted n | w% (95% CI) | w% (95% CI) | w% (95% CI) | w% (95% CI) | w% (95% CI) | p-value | |
| Age | 0.13 | ||||||
| 14–19 | 9,588,789 | 26.9 (25.4–28.4) | 25.4 (22.6–28.3) | 30.2 (26.4–33.9) | 26.4 (23.7–29.1) | 25.7 (23.1–28.3) | |
| 20–29 | 17,483,814 | 49.1 (47.2–50.9) | 47.3 (43.3–51.2) | 46.9 (42.9–50.9) | 49.4 (46.4–52.4) | 51.6 (48.3–55.0) | |
| 30–34 | 8,551,157 | 24.0 (22.5–25.5) | 27.3 (23.0–31.6) | 22.9 (19.8–26.0) | 24.2 (21.5–26.8) | 22.7 (20.2–25.2) | |
| Race/ethnicity | <0.001 | ||||||
| Non-Hispanic white | 21,583,883 | 60.6 (57.4–63.8) | 68.3 (60.1–76.6) | 75.8 (70.0–81.6) | 51.0 (45.2–56.8) | 55.6 (48.9–62.3) | |
| Non-Hispanic black | 5,050,542 | 14.2 (12.2–16.2) | 10.9 (6.5–15.3) | 11.1 (7.4–14.9) | 24.1 (19.7–28.5) | 5.0 (3.4–6.7) | |
| Mexican American | 4,124,698 | 11.6 (9.8–13.4) | 1.9 (0.8–2.9) | 5.0 (3.3–6.8) | 12.5 (8.6–16.4) | 22.1 (17.4–26.8) | |
| Other Hispanic | 2,298,628 | 6.4 (5.3–7.6) | 12.8 (8.0–17.6) | 1.8 (0.7–2.9) | 6.8 (5.1–8.6) | 6.2 (4.6–7.8) | |
| Other | 2,566,009 | 7.2 (6.2–8.2) | 6.1 (4.2–8.0) | 6.2 (4.2–8.2) | 5.5 (4.0–7.1) | 11.1 (8.5–13.7) | |
| Marital Status | 0.25 | ||||||
| Never married | 18,032,758 | 52.2 (49.8–54.5) | 51.5 (46.9–56.0) | 53.8 (50.1–57.4) | 53.7 (49.0–58.5) | 48.9 (45.4–52.5) | |
| Living together | 4,175,129 | 12.1 (10.9–13.3) | 13.3 (9.9–16.7) | 12.9 (10.7–15.1) | 11.4 (9.2–13.6) | 11.4 (9.2–13.7) | |
| Married | 10,561,770 | 30.6 (28.4–32.7) | 28.9 (24.8–33.1) | 29.6 (25.9–33.2) | 29.8 (25.8–33.9) | 33.4 (29.4–37.4) | |
| Widowed/divorced/ separated | 1,801,280 | 5.2 (4.4–6.0) | 6.3 (4.7–7.8) | 3.7 (2.4–5.0) | 5.0 (3.7–6.3) | 6.2 (4.3–8.1) | |
| Age at first sex | 0.04 | ||||||
| Never | 5,843,936 | 17.8 (16.6–19.0) | 16.6 (14.1–19.1) | 18.9 (16.5–21.3) | 16.6 (14.4–18.6) | 19.3 (16.8–21.8) | |
| <14 y | 2,456,010 | 7.5 (6.6–8.3) | 7.0 (5.3–8.8) | 7.9 (5.3–10.4) | 8.8 (7.6–10.1) | 5.5 (4.3–6.7) | |
| 14–18 y | 19,250,352 | 58.6 (56.6–60.6) | 62.6 (59.0–66.2) | 57.0 (53.1–60.9) | 59.4 (56.5–62.4) | 56.6 (51.6–61.5) | |
| 19+ y | 5,286,360 | 16.1 (14.6–17.6) | 13.8 (10.5–17.0) | 16.2 (12.9–19.5) | 15.2 (12.9–17.4) | 18.6 (14.9–22.4) | |
| Lifetime male sex partners | <0.001 | ||||||
| 0 | 5,985,675 | 18.5 (17.2–19.8) | 17.2 (14.5–20.0) | 19.4 (16.9–21.9) | 17.5 (15.4–19.6) | 19.9 (17.3–22.4) | |
| 1–2 | 8,015,800 | 24.8 (23.3–26.3) | 19.8 (16.7–23.0) | 23.8 (21.1–26.5) | 24.2 (22.1–26.4) | 29.4 (25.8–33.0) | |
| >3 | 18,315,613 | 56.7 (54.9–58.4) | 62.9 (59.5–66.3) | 56.8 (53.9–59.7) | 58.2 (55.6–60.9) | 50.7 (46.4–55.1) | |
| Time period | |||||||
| Pre-vaccine cycle (2003–2006) | 11,763,709 | 33.0 (30.5–35.5) | 32.8 (23.0–42.6) | 35.0 (26.0–44.1) | 32.6 (27.8–37.4) | 32.0 (23.8–40.2) | 0.96 |
| Post-vaccine cycle (2007–2014) | 23,860,051 | 67.0 (64.5–69.5) | 67.2 (57.4–77.0) | 65.0 (55.9–77.0) | 67.4 (62.6–72.2) | 68.0 (59.8–76.2) | |
| HPV vaccine in post-licensure years (2007–2014, n=3,732) | 0.06 | ||||||
| 0 doses | 17,635,474 | 75.4 (73.7–77.9) | 69.8 (64.2–75.3) | 74.8 (70.2–79.5) | 77.5 (74.0–81.0) | 77.9 (74.0–81.7) | |
| >1 dose | 5,635,365 | 24.2 (22.1–26.3) | 30.2 (24.7–35.7) | 25.2 (20.5–29.8) | 22.5 (19.0–25.9) | 22.1 (18.3–26.0) | |
| HPV vaccine dose numberbin post-licensure years (2007–2014, n=3,696) | 0.05 | ||||||
| 1 dose | 851,584 | 3.7 (3.2–4.2) | 3.0 (2.3–3.6) | 3.4 (2.4–4.4) | 4.0 (3.1–5.0) | 3.9 (2.7–5.1) | |
| 2 dose | 1,098,454 | 4.8 (3.8–5.7) | 6.6 (4.0–9.2) | 5.2 (2.3–8.0) | 3.5 (2.2–4.8) | 5.0 (3.5–6.5) | |
| 3 dose | 3,482,258 | 15.1 (13.3–16.9) | 19.7 (14.7–24.7) | 16.4 (12.6–20.2) | 14.0 (11.0–17.0) | 12.7 (9.2–16.2) | |
w% = weighted prevalence, 95% CI = 95% confidence interval
Northeast states=Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, Vermont; Midwest states=Indiana, Illinois, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, Wisconsin; South states=Arkansas, Alabama, Delaware, District of Columbia, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, West Virginia; West=Alaska, Arizona, California, Colorado, Hawaii, Idaho, Nevada, New Mexico, Oregon, Washington, Wyoming.
Some data were missing due to non-response among respondents who had received at least 1 HPV vaccine dose. HPV=human papillomavirus; NHANES=National Health and Nutrition Examination Survey
The highest HPV vaccination rate was found in the Northeast (30.2%) and the lowest in the West (22.1%) and the South (22.5%), although HPV vaccination did not vary significantly by region (p=0.06). Rates of HPV vaccination (≥1 dose) increased at a faster rate in the Northeast, followed by the Midwest, South, then West regions (supplemental figure 1), although there were no significant differences between regional frequencies during any of the cycles. HPV vaccination rates appear to be leveling off in all regions, with the exception of the South, which shows a continued but possibly slowed increase in HPV vaccine initiation.
Regional differences in vaginal HPV prevalence were found for any HPV type (p=0.01), vaccine-type (p<0.05), and non-vaccine HPV types (p<0.05) (Supplemental Table 1). Stratification by prevaccine and post-licensure years revealed regional variations of nonvaccine-type HPVprevalence (p<0.05) in prevaccine years. In post-licensure years, only vaccine-type HPV prevalence differed between regions (p=0.01), with the highest prevalence in the South.
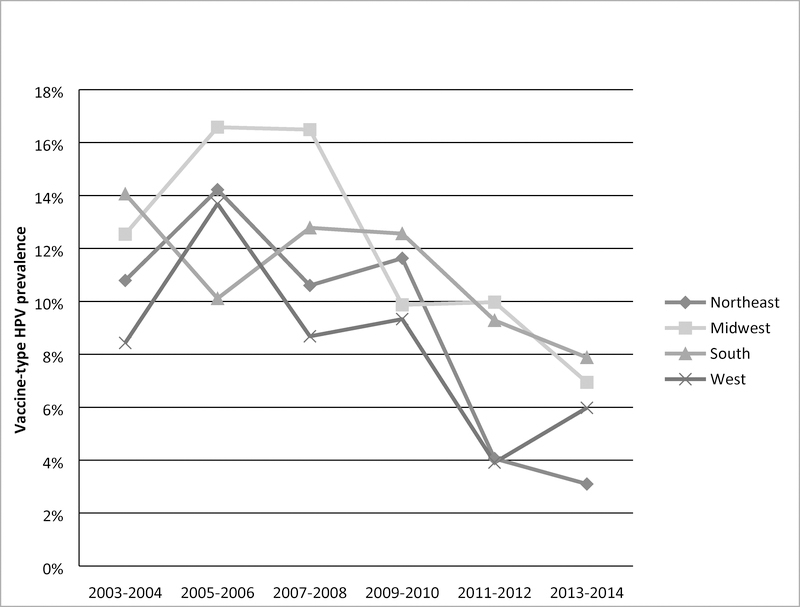
The prevalence in vaccine-type HPV prevalence for each region across the 6 survey cycles were calculated (Figure 1). After the first 2 survey cycles, there was a general downward trend in the Northeast and West. Declines in vaccine-type HPV occurred in the South after the first 4 cycles. Vaccine-type HPV prevalence declined between the 3rd and 4th survey cycles in the Midwest, and prevalences were similar to those in the South and West regions in the 2013–2014 cycle.
Figure 1.
Prevalence of vaccine-type HPV (6, 11, 16, 18) among 14–34 year old women across time, by region, NHANES 2003–2014 (N=6,387)
Interactions between region and survey cycle were significant (p=0.03, analysis not shown), indicating vaccine-type HPV prevalence decreased at different rates between regions.
Overall, HPV prevalence did not decrease significantly between prevaccine cycles and early post-licensure cycles (Table 2). Vaccine-type HPV and high-risk vaccine-type HPV decreased significantly between prevaccine and late post-licensure cycles. Similar reductions between these 2 time periods were observed in the Northeast and West regions. However, there were no changes in the prevalence of high-risk vaccine-type HPV in the Midwest between prevaccine and late post-licensure years (p>0.05), and there were modest reductions in both vaccine-type HPV (p=0.05) and high-risk vaccine-type HPV (p=0.03) in the South between the same 2 time periods.
Table 2.
Comparison of prevaccine and post-licensure prevalence of vaginal HPV by region, NHANES 2003–2014 (N=6,387)
| Prevaccine cycles (2003–2006) | Early post-licensure cycles (2007–2010) | Prevaccine &early PL p-value |
Late post-licensure cycles (2011–2014) | Prevaccine and late PL p-value |
|
|---|---|---|---|---|---|
| w% (95% CI) | w% (95% CI) | w% (95% CI) | |||
| Overall (n=6,387) | |||||
| Any HPV types | 44.4 (41.5–47.2) | 43.5 (40.6–46.4) | 0.68 | 41.7 (38.2–45.2) | 0.23 |
| Vaccine-type (6, 11, 16, 18) | 12.6 (11.2–14.0) | 11.6 (10.1–13.0) | 0.31 | 6.7 (5.2–8.2) | <0.001 |
| High-risk vaccine-type (16, 18) | 9.3 (7.9–10.6) | 9.1 (7.6–10.6) | 0.90 | 5.5 (4.2–6.8) | <0.001 |
| Nonvaccine-type (non-6, 11, 16, 18) | 41.7 (39.0–44.4) | 41.7 (38.6–44.8) | 0.99 | 40.6 (37.1–44.0) | 0.62 |
| Northeast (n=931) | |||||
| Any HPV types | 44.7 (39.0–50.4) | 45.3 (36.0–54.6) | 0.90 | 38.6 (33.9–43.4) | 0.05 |
| Vaccine-type (6, 11, 16, 18) | 12.3 (7.6–16.9) | 11.1 (7.2–15.0) | 0.66 | 3.5 (3.0–4.0) | <0.001 |
| High-risk vaccine-type (16, 18) | 8.1 (5.3–10.9) | 9.0 (4.6–13.4) | 0.69 | 3.3 (2.8–3.7) | <0.001 |
| Nonvaccine-type (non-6, 11, 16, 18) | 40.4 (35.1–45.8) | 41.2 (35.6–54.4) | 0.40 | 38.2 (33.4–43.0) | 0.46 |
| Midwest (n=1,259) | |||||
| Any HPV types | 40.6 (34.4–46.9) | 46.0 (40.2–51.8) | 0.17 | 38.9 (35.8–42.1) | 0.57 |
| Vaccine-type (6, 11, 16, 18) | 14.4 (12.7–16.1) | 12.6 (9.9–15.4) | 0.24 | 8.6 (5.2–12.0) | 0.002 |
| High-risk vaccine-type (16, 18) | 8.2 (6.7–9.7) | 10.1 (7.5–12.8) | 0.16 | 7.3 (4.0–10.6) | 0.57 |
| Nonvaccine-type (non-6, 11, 16, 18) | 38.8 (33.8–43.9) | 43.2 (37.7–48.6) | 0.20 | 38.0 (35.0–41.1) | 0.78 |
| South (n=2.458) | |||||
| Any HPV types | 48.7 (42.9–54.6) | 44.1 (39.5–48.7) | 0.20 | 47.6 (42.2–53.0) | 0.77 |
| Vaccine-type (6, 11, 16, 18) | 12.2 (9.6–14.8) | 12.7 (9.9–15.4) | 0.78 | 8.6 (6.2–11.1) | 0.05 |
| High-risk vaccine-type (16, 18) | 10.0 (7.3–12.7) | 9.4 (6.6–12.1) | 0.72 | 6.8 (5.2–8.5) | 0.03 |
| Nonvaccine-type (non-6, 11, 16, 18) | 47.0 (40.8–53.2) | 42.3 (37.0–47.5) | 0.23 | 45.9 (40.4–51.4) | 0.79 |
| West (n=1,739) | |||||
| Any HPV types | 41.6 (37.7–45.6) | 38.8 (34.0–43.6) | 0.37 | 37.9 (29.9–46.0) | 0.39 |
| Vaccine-type (6, 11, 16, 18) | 11.8 (9.9–13.7) | 9.0 (5.5–12.5) | 0.18 | 4.8 (2.6–7.0) | <0.001 |
| High-risk vaccine-type (16, 18) | 10.0 (8.5–11.6) | 7.9 (4.2–11.6) | 0.31 | 3.9 (2.1–5.6) | <0.001 |
| Nonvaccine-type (non-6, 11, 16, 18) | 37.7 (33.5–41.8) | 37.3 (32.4–42.3) | 0.90 | 37.0 (28.9–45.1) | 0.87 |
Any HPVgenotype includes types 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89, and IS39.
PL=post-licensure; HPV=human papillomavirus; NHANES=National Health and Nutrition Examination Survey
In models that examined the association between HPV vaccination and vaccine-type HPV prevalence after adjusting for demographics and number of lifetime male sex partners, no associations between region and vaccine-type HPV infection were observed (Table 3, Model 1). When vaccine cycle was included in the model (Model 2), the odds of vaccine-type HPV was lower post-licensure (PaOR: 0.66, 95% CI: 0.54–0.81) compared to the prevaccine period. After including HPV vaccination and removing time from the model, HPV vaccination was associated with lower odds (PaOR: 0.32, 95% CI: 0.20–0.53) of vaccine-type HPV infection (Model 3). This model also demonstrated elevated prevalence of vaccine-type HPV in the Midwest (PaOR: 1.59, 95% CI: 1.00–2.52) compared to the West, and there was an increase in the PaOR for the South (PaOR: 1.33, 95% CI: 0.88–2.02) compared to the West, although it remained non-significant.
Table 3.
Effect of time period and HPV vaccination on vaccine type HPV (6, 11, 16, 18), NHANES data 2003–2014 a(N=6,387)
| Model 1 b | Model 2 c | Model 3 d | |
|---|---|---|---|
| PaOR (95% CI) | PaOR (95% CI) | PaOR (95% CI) | |
|
Age | |||
| 14–19 | 2.07 (1.46–2.95) | 1.98 (1.40–2.81) | 1.90 (1.14–3.18) |
| 20–29 | 1.76 (1.35–2.28) | 1.80 (1.38–2.32) | 1.95 (1.41–2.70) |
| 30–34 | Reference | Reference | Reference |
| Race/ethnicity | |||
| Non-Hispanic white | Reference | Reference | Reference |
| Non-Hispanic black | 1.35 (1.07–1.69) | 1.35 (1.07–1.71) | 1.69 (1.24–2.29) |
| Mexican American | 1.02 (0.68–1.51) | 0.99 (0.67–1.46) | 1.21 (0.77–1.90) |
| Hispanic | 0.95 (0.63–1.44) | 1.06 (0.68–1.66) | 1.49 (0.93–2.38) |
| Other | 1.44 (0.93–2.24) | 1.64 (1.04–2.60) | 2.15 (1.29–3.58) |
|
Lifetime male sex partners | |||
| 0 | 0.06 (0.03–0.11) | 0.06 (0.03–0.11) | 0.05 (0.02–0.11) |
| 1–2 | 0.24 (0.17–0.34) | 0.24 (0.17–0.34) | 0.22 (0.15–0.33) |
| 3+ | Reference | Reference | Reference |
| NHANES cycle | |||
| 2003–2004 | Reference | ||
| 2005–2006 | 1.24 (0.93–1.66) | ||
| 2007–2008 | 1.13 (0.80–1.59) | ||
| 2009–2010 | 0.87 (0.64–1.18) | ||
| 2011–2012 | 0.54 (0.38–0.79) | ||
| 2013–2014 | 0.48 (0.30–0.76) | ||
| Region | |||
| Northeast | 0.94 (0.60–1.45) | 0.93 (0.63–1.39) | 1.01 (0.55–1.84) |
| Midwest | 1.30 (0.89–1.90) | 1.30 (0.92–1.83) | 1.59 (1.00–2.52) |
| South | 1.14 (0.82–1.60) | 1.15 (0.85–1.56) | 1.33 (0.88–2.02) |
| West | Reference | Reference | Reference |
| HPV vaccination | |||
| 0 vaccines | Reference | ||
| 1+ doses | 0.32 (0.20–0.53) | ||
Vaccine types include 4 HPV types (6, 11, 16, and 18) that the quadrivalent HPV vaccine protects against.
Model 1 included age, race/ethnicity, number of lifetime male sex partners, and region.
Model 2 included variables from Model 1 + vaccine cycle.
Model 3 included variables from Model 1 + HPV vaccine initiation.
aOR=adjusted odds ratio; 95% CI=95% confidence interval; HPV=human papillomavirus; NHANES=National Health and Nutrition Examination Survey
In an examination of the effects of both time and HPV vaccination on regional variations in vaccine-type HPV during post-licensure cycles, regional differences were non-significant (Table 4, Model 1). However, we wanted to examine the effect of HPV vaccination on the association between time and vaccine-type HPV prevalence. Therefore, in the 2nd Model, after including time, we observed a small but significant decrease in the odds of infection across time during the 2011–2012 (PaOR: 0.48, 95% CI: 0.32–0.71) and 2013–2014 survey cycles(PaOR: 0.38, 95% CI: 0.24–0.62). After adding HPV vaccination (PaOR: 0.48, 95% CI: 0.30–0.77) into the model (Model 3), the association between time and infection became smaller, but remained significant in the last 2 survey cycles. This indicates that part of the association between time and HPV prevalence was attributable to HPV vaccination. We also noted that the PaORs for region, even though non-significant, changed slightly between models, indicating that vaccination, and to lesser extent, time, was partially responsible for the observed changes.
Table 4.
Multivariable logistic regression to examine the effect of time and vaccination on vaccine-specific HPV types (6, 11, 16, 18) detected through vaginal swab during post-licensure years, NHANES data 2007–2014 (N=3,188)a
| Model 1 b | Model 2 c | Model 3 d | |
|---|---|---|---|
| PaOR (95% CI) | PaOR (95% CI) | PaOR (95% CI) | |
| Region | |||
| Northeast | 0.95 (0.53–1.71) | 0.92 (0.54–1.59) | 0.96 (0.54–1.72) |
| Midwest | 1.60 (0.99–2.56) | 1.55 (0.97–2.46) | 1.54 (0.97–2.46) |
| South | 1.38 (0.91–2.10) | 1.38 (0.92–2.07) | 1.32 (0.87–1.99) |
| West | Reference | Reference | Reference |
| NHANES cycles | |||
| 2007–2008 | Reference | Reference | |
| 2009–2010 | 0.76 (0.54–1.06) | 0.80 (0.57–1.12) | |
| 2011–2012 | 0.48 (0.32–0.71) | 0.54 (0.36–0.82) | |
| 2013–2014 | 0.38 (0.24–0.62) | 0.48 (0.30–0.77) | |
| HPV vaccination | |||
| 0 doses | Reference | ||
| 1+ doses | 0.48 (0.30–0.77) | ||
Vaccine-specific HPV types include 4 HPV types (6, 11, 16, and 18) that the quadrivalent HPV vaccine protects against.
Model 1 controlled for age, race/ethnicity, and number of lifetime male sex partners.
Model 2 controlled for variables from Model 1 + NHANES cycles in post-licensure period.
Model 3 controlled for variables from Model 2 + HPV vaccine initiation.
All models controlled for age, race/ ethnicity, and number of male sexual partners in the past year.
HPV=human papillomavirus; NHANES=National Health and Nutrition Examination Survey
DISCUSSION
Significant reductions in prevalence of vaccine-type HPV were found between prevaccine and late post-licensure cycles in all regions but decreases in the South were marginal. Vaccine-type HPV prevalence has been shown to have decreased significantly between prevaccine and post-licensure cycles of NHANES [21, 22]. However, our results indicate that reductions in vaccine-type HPV, particularly high-risk types, may be occurring somewhat unevenly in the U.S. If changes in high-risk vaccine-type HPV prevalence continue to occur unevenly between regions, then cervical cancer disparities may persist in areas that do not experience similar decreases in those HPV types. In particular, Southern states had higher rates of cervical cancer before the HPV vaccine was introduced [23]. Even if these differences were originally due to lower cervical cancer screening rates or increased smoking rates, these disparities could remain if HPV vaccination rates remain similar or lower than the rest of the U.S.
We found the Midwest and South had almost twice the prevalence of vaccine-type HPV during the late post-licensure years compared to Northeastern and Western regions. In particular, we found that high-risk vaccine-types (types 16 and 18) did not decrease significantly in the Midwest and was about 2 times more prevalent in the South and Midwest in late post-licensure years compared to the Northeastern and Western regions during the same time period. This is concerning, as these types are associated with a high proportion of HPV-related cancers, including cervical cancer, oropharyngeal cancer, anal cancer, and vulvar and vaginal cancers [24–26]. Southern states are the most populous in the U.S., and they also include a high proportion of sub-groups considered at higher risk of HPV infection, persistence, cervical cancer incidence, and related mortality [27–29]. For example, Hispanic women have an elevated risk of developing cervical cancer, with an incidence of 9.9 per 100,000, while black women also have higher rates of both cervical cancer incidence (9.2 per 100,000) and cervical cancer mortality (4.0 per 100,000) compared to non-Hispanic white women (incidence, 7.7 per 100,000; mortality 2.1 per 100,000) [30]. Although we did not find variations in HPV vaccination by region, HPV vaccination prevalence has been found in other studies to be lower in Southern states among both adolescent and young women compared to other regions in the U.S [13–15]. The variations in age groups that were assessed in previous studies could account for these differences, as they examined HPV vaccination among younger age groups (9–17, 13–17, and 18–26 year olds) and used different methods to collect the data. It is also possible that erroneous HPV vaccine parental/self-report in this study, particularly for vaccine series completion, affected the findings [31, 32]. Although HPV vaccination report has been assessed in a small sample of 128 participants in NHANES and was found to have an agreement of 0.70 (95% CI 0.56–0.83) with provider records, there was no evaluation of differences in regional or ethnic variations in the results. Therefore, it is unknown what level of bias this issue may have presented [32]. It is also possible that vaccination after exposure occurs more frequently in Southern states, as there were differences in age at sexual initiation in this study, with the South reporting the highest proportion of women who initiated sex before or at age 14 (8.8%). Without improving vaccination rates at the recommended age, geographic disparities in cervical cancer may persist.
Although odds of HPV infection did not vary by region after controlling for potential confounders, the odds of vaccine-type HPV infection in the entire sample were lower in the late post-licensure cycles compared to prevaccine cycles. Further, vaccine-type HPV prevalence decreased across time in the 3 post-licensure cycles, which appeared to be at least partially attributable to HPV vaccination. Reductions across time in the post-licensure period that were not attributable to HPV vaccination may be related to herd immunity effects. HPV vaccination has been shown to be efficacious at preventing new vaccine-type HPV infections in clinical trials [33, 34]. Further, observational studies in Australia, which has vaccinated more than 70% of 12–13 year olds, have shown that high HPV vaccination rates have resulted in significant reductions in vaccine-type HPV infections, cervical lesions, and genital warts, even among unvaccinated populations in the vaccinated age cohort [35, 36]. The U.S. had not achieved such high rates of HPV vaccine coverage, with close to 63% of females and 50% males 13–17 years old having initiated the series and 42% of females and 28% of males in the same age group completing the series by 2015 [37]. It is possible that decreases over time in vaccine-type HPV prevalence not attributable to vaccination could be partially explained by herd immunity, which has previously been found to exist using NHANES data among unvaccinated women [38]. Another previous analysis of NHANES data indicated that HPV prevalence decreased by 34% among sexually active unvaccinated women 14–24 years of age between prevaccine years (2003–2006) and late post-licensure years (2011–2014) [39]. It should be noted that although the vaccine is expected to provide significant levels of herd protection and even cross-protection against other cancer-causing types of HPV [40], low vaccination rates in one region of the US may create a geographic reservoir for these viruses and contribute to a continuing source of potential disease.
The primary strength of this study is that it utilized repeated cross-sectional survey data representative of the U.S. from the NHANES survey. Pooling data to observe prevaccine and post-licensure years allowed us to examine variations in HPV prevalence changes between regions as well as within each region. There were also some limitations. First, while the data are representative of the U.S., they may not be representative of each region. HPV vaccine report was limited to self-report, which is subject to recall bias, as accuracy of adolescent HPV vaccination reports have been found to vary by race/ ethnicity, and could potentially vary across region [31]. Recall bias may have contributed to a conservative estimate of the effect of vaccination on the association between time and vaccine-type HPV infection, as vaccination may have been underreported, particularly among racial/ ethnic minorities who would have been more strongly represented in some regions compared to others. We also restricted the post-licensure sample to only those who responded to the question about vaccination, while the prevaccine years was only restricted to those who had adequate cervicovaginal swab samples.
This may have introduced some bias, but it is unknown whether those who failed to respond to the question about vaccination would have been vaccinated or not. However, the frequency of non-response to the question about HPV vaccine initiation was low (0.4%). In addition, we did not consider age when the HPV vaccine was administered, which may affect outcomes due to increased prevalence of teens receiving the vaccine at younger ages. Previous research found that more than 40% of females who reported their age when they received the first dose of HPV vaccine reported having sex before or during the same year as vaccination [41]. It is possible that regional differences in age at HPV vaccination could affect the findings.
In conclusion, there were regional variations in vaccine-type HPV prevalence between the prevaccine and post-licensure years, with decreases in high-risk vaccine types occurring primarily in the Northeast and Western states. Further, the observed decreases are at least partially attributable to HPV vaccination. These results indicate that observed reductions in HPV may not be occurring evenly across the U.S. More efforts to improve HPV vaccination, particularly in the Southern and Midwestern states, could decrease differences in HPV prevalence observed in post-licensure years. Increased efforts, including strengthening of vaccine policy and provider recommendation to all eligible young adults, with an emphasis on providing the vaccine to adolescents during the recommended age of 11–12 years, will contribute to reductions in geographic disparities in cervical cancer incidence and mortality in the future.
Supplementary Material
Acknowledgment
The authors would like to acknowledge Patricia Barnes, an analyst at the Centers for Disease Control and Prevention, who merged limited use datasets with public use datasets for remote analyses.
Funding
This study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a Clinical and Translational Science Award (UL1 TR001439) from the National Center for Advancing Translational Sciences, National Institutes of Health. J.M. Hirth was a Scholar supported by a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women’s Health Program –BIRCWH; Principal Investigator: Berenson) from the Office of Research on Women’s Health (ORWH), the Office of the Director (OD), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) at the National Institutes of Health during data analyses for this study. The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Research Data Center, the National Center for Health Statistics, or the Centers for Disease Control and Prevention. The sponsors did not have any role in the study design, collection, analysis, and interpretation of the data or any other aspect of this study or manuscript.
Footnotes
Conflicts of interest:
The authors have no conflicts of interest to report.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jacqueline M. Hirth, Center for Interdisciplinary Research in Women’s Health, Department of Obstetrics & Gynecology, University of Texas Medical Branch, Galveston, TX.
Yong-Fang Kuo, Department of Biostatistics and Epidemiology, University of Texas Medical Branch, Galveston, TX.
Jonathan M. Starkey, Institute for Translational Sciences, University of Texas Medical Branch, Galveston, TX.
Richard E. Rupp, Department of Pediatrics, University of Texas Medical Branch, Galveston, TX.
Tabassum H. Laz, Department of Radiology, St. Luke’s International Hospital, Tokyo, Japan.
Mahbubur Rahman, Division of Epidemiology, Graduate School of Public Health, St. Luke’s International University, Tokyo, Japan.
Abbey B. Berenson, Center for Interdisciplinary Research in Women’s Health, Department of Obstetrics & Gynecology, University of Texas Medical Branch, Galveston, TX.
References
- [1].Serrano B, Brotons M, Bosch FX, Bruni L. Epidemiology and burden of HPV-related disease. Best Practice & Research Clinical Obstetrics & Gynaecology. 2018;47:14–26. [DOI] [PubMed] [Google Scholar]
- [2].Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human Papillomavirus is a Necessary Cause of Invasive Cervical Cancer Worldwide. Journal of Pathology. 1999;189:12–9. [DOI] [PubMed] [Google Scholar]
- [3].de Sanjosé S, Bruni L, Alemany L. HPV in genital cancers (at the exception of cervical cancer) and anal cancers. La Presse Médicale. 2014;43:e423–e8. [DOI] [PubMed] [Google Scholar]
- [4].Kasting ML, Shapiro GK, Rosberger Z, Kahn JA, Zimet GD. Tempest in a teapot: A systematic review of HPV vaccination and risk compensation research. Hum Vaccin Immunother. 2016;12:1435–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Oltean HN, Lofy KH, Goldoft MJ, DeBolt CA. Human Papillomavirus Vaccination in Washington State: Estimated Coverage and Missed Opportunities, 2006–2013. Public Health Rep. 2016;131:474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mesher D, Panwar K, Thomas SL, Beddows S, Soldan K. Continuing reductions in HPV 16/18 in a population with high coverage of bivalent HPV vaccination in England: an ongoing cross-sectional study. BMJ Open. 2016;6:e009915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].de Sanjosé S, Bruni L, Alemany L. HPV in genital cancers (at the exception of cervical cancer) and anal cancers. Presse Med. 2014;43:e423–8. [DOI] [PubMed] [Google Scholar]
- [8].U.S. Food & Drug Administration, Gruber MF. December 10, 2014. Approval Letter - GARDASIL 9. https://www.fda.gov/biologicsbloodvaccines/vaccines/approvedproducts/ucm426520.htm2014.
- [9].Vadaparampil ST, Malo TL, Sutton SK, Ali KN, Kahn JA, Casler A, et al. Missing the Target for Routine Human Papillomavirus Vaccination: Consistent and Strong Physician Recommendations Are Lacking for 11- to 12-Year-Old Males. Cancer Epidemiology Biomarkers & Prevention. 2016;25:1435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bratic JS, Seyferth ER, Bocchini JA. Update on barriers to human papillomavirus vaccination and effective strategies to promote vaccine acceptance. Current Opinion in Pediatrics. 2016;28:407–12. [DOI] [PubMed] [Google Scholar]
- [11].Berenson AB. An update on barriers to adolescent human papillomavirus vaccination in the USA. Expert Review of Vaccines. 2015;14:1377–84. [DOI] [PubMed] [Google Scholar]
- [12].Garland SM, Kjaer SK, Muñoz N, Block SL, Brown DA, DiNubile MJ, et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: A systematic review of 10 years of real-world experience. Clinical Infectious Diseases. 2016;63:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rahman M, McGrath CJ, Berenson AB. Geographic variation in human papillomavirus vaccination uptake among 13–17 year old adolescent girls in the United States. Vaccine. 2014;32:2394–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hirth JM, Rahman M, Smith JS, Berenson AB. Regional variations in HPV vaccination among 9–17 year old adolescent females from the BRFSS, 2008–2010. Hum Vaccin Immunother. 2014;10:3475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rahman M, Islam M, Berenson AB. Differences in HPV Immunization Levels Among Young Adults in Various Regions of the United States. J Community Health. 2015;40:404–8. [DOI] [PubMed] [Google Scholar]
- [16].Centers for Disease Control and Prevention. Cervical cancer rates by state In: Centers for Disease Control and Prevention, editor. Cancer prevention and control; 2013. [Google Scholar]
- [17].Schiffman M, Castle PE, Jeronimo J, Rodríguez AC, Wacholder S. Human papillomavirus and cervical cancer. The Lancet. 2007;370:890–907. [DOI] [PubMed] [Google Scholar]
- [18].National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey: Questionnaires, datasets, and related documentation.
- [19].Advisory Committee on Immunization Practices, Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, et al. Quadrivalent human papillomavirus vaccine: Recommendations of teh Advisory Committee on Immunization Practices (ACIP). MMWR. 2007;56:1–24. [PubMed] [Google Scholar]
- [20].United States Census Bureau. Census divisions and Census regions.
- [21].Berenson AB, Laz TH, Rahman M. Reduction in Vaccine-Type Human Papillomavirus Prevalence Among Women in the United States, 2009–2012. Journal of Infectious Diseases. 2016;214:1961–4. [DOI] [PubMed] [Google Scholar]
- [22].Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, et al. Reduction in Human Papillomavirus (HPV) Prevalence Among Young Women Following HPV Vaccine Introduction in the United States, National Health and Nutrition Examination Surveys, 2003– 2010. Journal of Infectious Diseases. 2013;208:385–93. [DOI] [PubMed] [Google Scholar]
- [23].Saraiya M, Ahmed F, Krishnan S, Richards TB, Unger ER, Lawson HW. Cervical Cancer Incidence in a Prevaccine Era in the United States, 1998–2002. Obstetrics & Gynecology. 2007;109:360–70. [DOI] [PubMed] [Google Scholar]
- [24].Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. New England Journal of Medicine. 2003;348:518–27. [DOI] [PubMed] [Google Scholar]
- [25].De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: A meta-analysis. International Journal of Cancer. 2009;124:1626–36. [DOI] [PubMed] [Google Scholar]
- [26].Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human Papillomavirus Types in Head and Neck Squamous Cell Carcinomas Worldwide: A Systematic Review. Cancer Epidemiology Biomarkers & Prevention. 2005;14:467–75. [DOI] [PubMed] [Google Scholar]
- [27].U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2013 incidence and mortality web-based report In: U.S. Department of Health and Human Services, editor. Atlanta: Centers for Disease Control and Prevention, National Cancer Institute; 2016. [Google Scholar]
- [28].Bureau USC. 2011–2015 American Community Survey 5-year estimates. American Fact Finder: U.S. Census Bureau. [Google Scholar]
- [29].Banister CE, Messersmith AR, Cai B, Spiryda LB, Glover SH, Pirisi L, et al. Disparity in the Persistence of High-Risk Human Papillomavirus Genotypes Between African American and European American Women of College Age. Journal of Infectious Diseases. 2015;211:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Institute NC. SEER Stat Fact Sheets: Cervix uteri cancer. 2015.
- [31].Hirth JM, Kuo Y-F, Laz TH, Starkey JM, Rupp RE, Rahman M, et al. Concordance of adolescent human papillomavirus vaccination parental report with provider report in the National Immunization Survey-Teen (2008–2013). Vaccine. 2016;34:4415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lewis RM, Markowitz LE. Human papillomavirus vaccination coverage among females and males, National Health and Nutrition Examination Survey, United States, 2007–2016. Vaccine. 2018;36:2567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].The FUTURE II Study Group. Quadrivalent Vaccine against Human Papillomavirus to Prevent High-Grade Cervical Lesions. New England Journal of Medicine. 2007;356:1915–27. [DOI] [PubMed] [Google Scholar]
- [34].Erickson BK, Landers EE, Huh WK. Update on Vaccination Clinical Trials for HPV-Related Disease. Clinical Therapeutics. 2014;36:8–16. [DOI] [PubMed] [Google Scholar]
- [35].Garland SM. The Australian Experience With the Human Papillomavirus Vaccine. Clinical Therapeutics. 2014;36:17–23. [DOI] [PubMed] [Google Scholar]
- [36].Brotherton JML, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. The Lancet. 2011;377:2085–92. [DOI] [PubMed] [Google Scholar]
- [37].Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans LD, Curtis CR, MacNeil J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2015. MMWR. 2016;65:850–8. [DOI] [PubMed] [Google Scholar]
- [38].Berenson AB, Hirth JM, Chang M. Change in Human Papillomavirus Prevalence Among U.S. Women Aged 18–59 Years, 2009–2014. Obstetrics & Gynecology. 2017;130:693–701. [DOI] [PubMed] [Google Scholar]
- [39].Oliver SE, Unger ER, Lewis R, McDaniel D, Gargano JW, Steinau M, et al. Prevalence of Human Papillomavirus Among Females After Vaccine Introduction—National Health and Nutrition Examination Survey, United States, 2003–2014. The Journal of Infectious Diseases. 2017;216:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Baussano I, Lazzarato F, Ronco G, Lehtinen M, Dillner J, Franceschi S. Different Challenges in Eliminating HPV16 Compared to Other Types: A Modeling Study. The Journal of Infectious Diseases. 2017;216:336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Petrosky EY, Liu G, Hariri S, Markowitz LE. Human Papillomavirus Vaccination and Age at First Sexual Activity, National Health and Nutrition Examination Survey. 2017;56:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.