Abstract
Background and Objective:
Metabolic syndrome (MetS) exacerbates periodontitis. Since saturated fatty acid (SFA) is increased in MetS and enhances lipopolysaccharide (LPS)-induced proinflammatory cytokine expression in macrophages, it has been considered to play a role in MetS-exacerbated periodontitis. However, it remains unknown how fatty acid receptors, which mediate the interaction of cells with SFA and uptake of SFA, are expressed and regulated in the periodontal tissue. In this study, we tested our hypothesis that the periodontal expression of fatty acid receptors GPR40 and CD36 is increased in patients with both MetS and periodontitis. We also determined the effect of SFA and LPS on GPR40 and CD36 expression in vitro.
Material and Methods:
Periodontal tissue specimens were collected from 11 participants without MetS and periodontitis, 12 participants with MetS, 11 participants with periodontitis, and 14 participants with both MetS and periodontitis after surgeries. The tissues were processed, and GPR40 and CD36 were detected by immunohistochemistry. Furthermore, cultured macrophages and gingival fibroblasts were treated with LPS, palmitate, a major SFA, or LPS plus palmitate and the expression of GPR40 and CD36 was then quantified.
Results:
Analysis of clinical data showed that age, smoker, gender and race/ethnicity were not significantly different among 4 groups. Immunohistochemistry showed that GPR40 and CD36 were expressed by epithelial cells, fibroblasts and immune cells. Quantitative data showed that GPR40 expression is increased in patients with periodontitis, MetS or both periodontitis and MetS while CD36 expression is increased only in patients with both periodontitis and MetS. The in vitro studies showed that the expression of GPR40 and CD36 in macrophages and fibroblasts was upregulated by the combination of LPS and palmitate.
Conclusion:
Periodontal expression of GPR40 and CD36 was upregulated in patients with both MetS and periodontitis, and GPR40 and CD36 in macrophages and fibroblasts were upregulated in vitro by the combination of LPS and palmitate, suggesting that GPR40 and CD36 may be involved in MetS-exacerbated periodontitis.
Keywords: Metabolic syndrome, Periodontitis, GPR40, CD36, Inflammation
1. INTRODUCTION
Periodontitis is a disease of the supporting structures of the teeth, characterized by tissue inflammation and destruction (1, 2). Periodontitis is caused by periodontal bacteria and exacerbated by systemic diseases such as diabetes and metabolic syndrome (MetS) (3–6). MetS is a cluster of cardiovascular risk factors including obesity, high triglycerides, low HDL, high blood pressure, insulin resistance and increased glucose (7, 8). The research finding that MetS is associated with periodontitis is of importance since 34% of the US population have MetS as contrasted to 9.3% of the US population having diabetes (9). Since MetS is considered as a pre-diabetic state (10), early treatment of periodontitis in patients with MetS may reduce the severity of periodontitis after pre-diabetes advances to diabetes. However, in contrast to the extensive studies on the mechanisms involved in diabetes-associated periodontitis (11), the investigation on the pathogenesis of MetS-related periodontitis is lacking.
It is known that MetS is associated with increased free fatty acid, in particular saturated fatty acid (SFA) (12), and dietary SFA is a key factor involved in the pathogenesis of MetS (13). Therefore, we hypothesized that SFA may boost host inflammatory response triggered by periodontal pathogen-derived virulence factors such as lipopolysaccharide (LPS). Interestingly, our in vitro study supported our hypothesis as it showed that palmitate, the most abundant SFA in animal and human (14), robustly amplified LPS-stimulated proinflammatory cytokine expression in macrophages (15).
Long-chain free fatty acids including SFA interact with cells through free fatty acid receptors such as GPR40 (16), GPR120 (17, 18) and CD36 (19). Studies have shown that GPR120 is a fatty acid receptor engaged preferentially by omega-3 (ω−3) polyunsaturated fatty acids (PUFAs) and mediates ω−3 PUFA-stimulated antiinflammatory actions (17, 18), but GPR40 and CD36 have proinflammatory properties (19, 20). Therefore, GPR40 and CD36 may contribute to MetS-associated periodontitis by mediating the proinflammatory effect of SFA. However, it is uninvestigated if GPR40 and CD36 are expressed on periodontal tissue and if the periodontal expression of GPR40 and CD36 is regulated by periodontitis and MetS.
In this study, we performed immunohistochemistry to detect GPR40 and CD36 expression in periodontal tissues and determine if the periodontal expression of GPR40 and CD36 is altered in patients with MetS and periodontitis. Furthermore, we also determined if the in vitro expression of GPR40 and CD36 is upregulated by LPS and palmitate, a major SFA, and if GPR40 or CD36 expression is involved in palmitate-enhanced proinflammatory cytokine expression.
2. MATERIAL & METHODS
2.1. Study Participants
Forty-eight participants, including 11 control participants without periodontitis and MetS (group 1), 12 with periodontitis alone (group 2), 11 with MetS alone (group 3) and 14 with both periodontitis and MetS (group 4), were enrolled in the study. Participants in group 1 who had oral surgeries such as crown lengthening, extractions, and periodontal plastic surgery served as controls. All the tissue specimens collected were periodontal tissues. The participants in groups 2 and 4 met the diagnostic criteria for chronic periodontitis: periodontal pocket depth (PPD) ≥ 6 mm in 2 or more teeth or clinical attachment loss (CAL) ≥ 5 mm in 2 or more teeth (21). Radiographic evidence of alveolar bone loss was apparent. The oral examination was conducted as previously described (22). The participants in groups 3 and 4 met the diagnostic criteria for MetS by International Diabetes Foundation (23): Elevated waist circumference (40 inches for men and 35 inches for women) with any two of the following - Elevated triglycerides (equal to or greater than 150 mg/dL); Reduced HDL cholesterol (less than 40 mg/dL for men and 50 mg/dL for women); Elevated blood pressure (equal to or greater than 130/85 mm Hg or use of medication for hypertension; Elevated fasting glucose (equal to or greater than 100 mg/dL or use of medication for hyperglycemia.
The exclusion criteria were: serum creatinine ≥ 1.6 mg/dl; abnormal hepatic function; hemoglobinopathy; unwillingness to sign the informed consent form or enter the study; aggressive periodontitis; any platelet and coagulation disorders; diabetes. The participants in groups 2 and 4 had periodontal surgery and specimens were obtained from the greatest PPD and/or CAL sites. All participants provided informed consents for the specimen collection. The study protocol and consent form were approved by the Medical University of South Carolina Institutional Review Board.
2.2. Immunohistochemical Analysis of GPR40 and CD36 Expression
Periodontal tissue samples were embedded in Tissue-Tek® OCT™ compound (EMS, Hatfied, PA) and frozen immediately after surgery and stored at −80°C. Using a cryostat, 6 μm sections were cut and fixed in 10% of buffered formalin for 10 minutes and then washed with 0.1 M PBS. The sections were incubated with 5% normal goat serum for 1 hour. The sections were then incubated with antibody against GPR40 (1:300) (Santa Cruz Biotechnology, Santa Cruz, CA) or CD36 (1:300) (Novus Biologicals, Littleton, CO) overnight at 4°C. The sections were incubated with secondary biotinylated antibody (1:250) from the ABC Elite kit (Vector Laboratories, Burlingame, CA) for 1 h and then the ABC reagent (Vector Laboratories) for 30 min. Slides were rinsed in 0.01 M PBS, covered with diaminobenzidine peroxidase substrate solution from the Impack DAB kit (Vector Laboratories) for 2 min and then rinsed with water. Counterstaining was performed with hematoxylin solution, Gill No. 2 (Sigma–Aldrich, St. Louis, MO). Slides were then dehydrated using increasing concentrations of ethanol and xylenes and mounted. Staining with normal IgG was used as a negative control. Photomicrographs of tissue sections were taken using an Olympus BX53 digital microscope with Cellsens digital image software (Olympus American Inc., Center Valley, PA). The area with positive staining was quantified using a computer based morphometry software (Image-Pro Plus 6, Media Cybernetics, Bethesda, MD) as described previously (24).
2.3. Cell Culture Study
Human gingival fibroblasts were purchased from American Type Culture Collection (ATCC, Manassas, VA). The cells were cultured in a 5% CO2 atmosphere in RPMI 1640 medium (GIBCO, Invitrogen Cop. Carlsbad, CA) containing 10% fetal calf serum, 1% MEM non-essential amino acid solution, and 0.6 g/100 ml of HEPES. The medium was changed every 2–3 days. RAW264.7 macrophages were purchased from ATCC and grown in DMEM (ATCC, Manassas, VA) supplemented with 10% heat-inactivated fetal calf serum (HyClone, Logan, UT). The cells were maintained in a 37 °C, 90% relative humidity, 5% CO2 environment. Fibroblasts and RAW264.7 macrophages were grown to 80% confluence before treatments with LPS, palmitate or LPS plus palmitate. LPS was isolated from Porphyromonas gingivalis (Pg) (InvivoGen, San Diego, CA). Palmitate was prepared from palmitic acid (Sigma, St. Louis, MO). Palmitic acid was dissolved in 0.1 N NaOH and 70% ethanol at 70 °C to make 50 mM. The solution was kept at 55°C for 10 min, mixed, and brought to room temperature. GW1100, an antagonist of GPR40 (25) and Sulfo-N-succinimidyl oleate (SSO), an inhibitor of CD36 (26), were purchased from Sigma-Aldrich.
2.4. Real-time Polymerase Chain Reaction (PCR)
Total RNA was isolated from cells using the RNeasy minikit (Qiagen, Santa Clarita, CA). First-strand complementary DNA (cDNA) was synthesized with the iScript™ cDNA synthesis kit (Bio-Rad, Hercules, CA) using 20 μl of reaction mixture containing 1 μg of total RNA, 4 μl of 5x iScript reaction mixture, and 1 μl of iScript reverse transcriptase. The complete reaction was cycled for 5 minutes at 25 °C, 30 minutes at 42 °C and 5 minutes at 85°C using a PTC-200 DNA Engine (MJ Research, Waltham, MA). The reverse transcription (RT) reaction mixture was then diluted 1:10 with nuclease-free water and used for PCR amplification of cDNA in the presence of the primers. The human IL-6 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers for real time PCR were purchased from Qiagen [IL-6, catalog #: PPM03015A-200; GAPDH, catalog #: PPM02946E-200]. The Beacon designer software (PREMIER Biosoft International, Palo Alto, CA) was used for human GPR40 and mouse CD36 primers designing (human GPR40: 5’ primer sequence, TCAGCCTCTCTCTCCTGCTC; 3’ primer sequence, CGCACACACTGTCTTCAGGC; mouse CD36: 5’ primer sequence, TGCTGGAGCTGTTATTGGTG; 3’ primer sequence, CATGAGAATGCCTCCAAACA). Primers were synthesized (Integrated DNA Technologies, Inc., Coralville, IA) and real-time PCR was performed in duplicate using 25 μl of reaction mixture containing 10 μl of RT mixture, 0.2 ◻M of both primers, and 12.5 μl of iQ™ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). Real-time PCR was run in the iCycler™ real-time detection system (Bio-Rad) with a two-step method. The hot-start enzyme was activated (95°C for 2 min) and cDNA was then amplified for 40 cycles consisting of denaturation at 95°C for 10 sec and annealing/extension at 52.5°C for 45 sec. A melt-curve assay was then performed (55°C for 1 min and then temperature was increased by 0.5°C every 10 sec) to detect the formation of primer-derived trimers and dimers. Data were analyzed with the iCycler iQ™ software. The average starting quantity (SQ) of fluorescence units was used for analysis. Quantification was calculated using the SQ of targeted cDNA relative to that of GAPDH cDNA in the same sample.
2.5. Statistical Analysis
The differences of demographics were assessed using Fisher’s Exact Test. The GraphPad Instat 3 software (GraphPad Software, Inc., San Diego, CA) was used for statistical analysis. Nonparametric analyses test using the Mann-Whitney procedure were performed to detect significant differences in continuous variable between two groups based on the two-tailed p value. A value of p<0.05 was considered significant.
3. RESULTS
3.1. Study Population
Demographic data of participants including age, smoker, gender and race/ethnicity are presented in Table 1. The average ages in four groups had no significant difference (p>0.05), ranging from 57 to 60. The smoker, gender and race/ethnicity also had no significant difference for all groups (p>0.05). Furthermore, the CAL for Group 2 (periodontitis) and Group 4 (periodontitis and MetS) was 5.86 ± 0.99 and 6.27 ± 1.10 mm, respectively. Although the CAL for Group 4 appeared higher than that for Group 2, the difference was not statistically significant (p=0.30).
Table 1.
Demographic Data for the Study Population
| Group 1 | Group 2 | Group 3 | Group 4 | P value | |
|---|---|---|---|---|---|
| Non-periodontitis, non-MetS | Periodontitis | MetS | Periodontitis and MetS | ||
| Total | 11 | 12 | 11 | 14 | |
| Age | 57 ± 6 | 59 ± 4 | 58 ± 3 | 60 ± 3 | P=0.5096 |
| Smoker | 2 (18.2%) | 1 (8.3%) | 2 (18.2%) | 2 (14.3%) | P=0.9013 |
| Gender (M/F) | 2/9 (22%) | 3/9 (33%) | 6/5 (120%) | 5/9 (55%) | P=0.3183 |
| Race/Ethnicity | P=0.3892 | ||||
| White (Non-Hispanic) | 41 (77.4%) | 21 (75%) | 5 (45.5%) | 11 (44%) | |
| Black (Non-Hispanic) | 12 (22.7%) | 6 (21.4%) | 6 (54.6%) | 14 (56%) | |
| Hispanic | 0 (0%) | 1 (3.6%) | 0 (0%) | 0 (0%) |
The age was presented as mean ± SD. Other parameters were presented as percentile.
3.2. Periodontal Expression of GPR40 and CD36 in Participants with Periodontitis, MetS or both Periodontitis and MetS
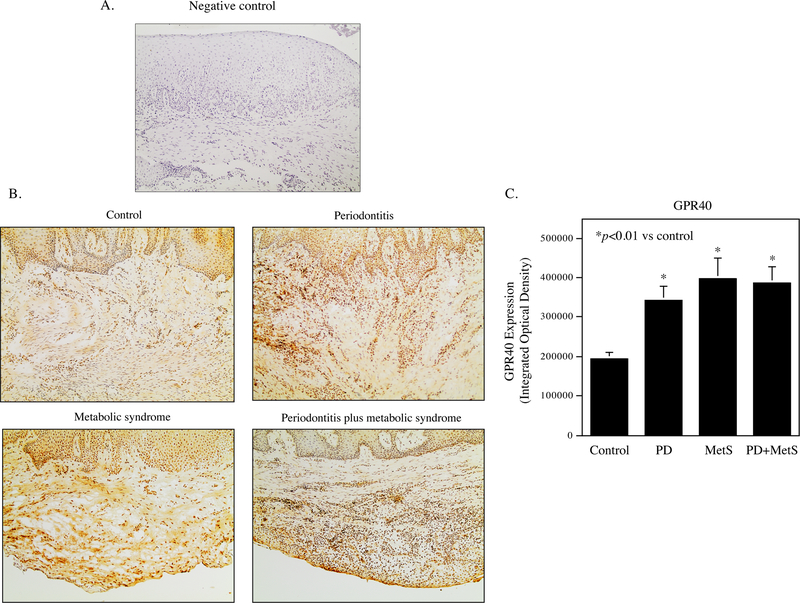
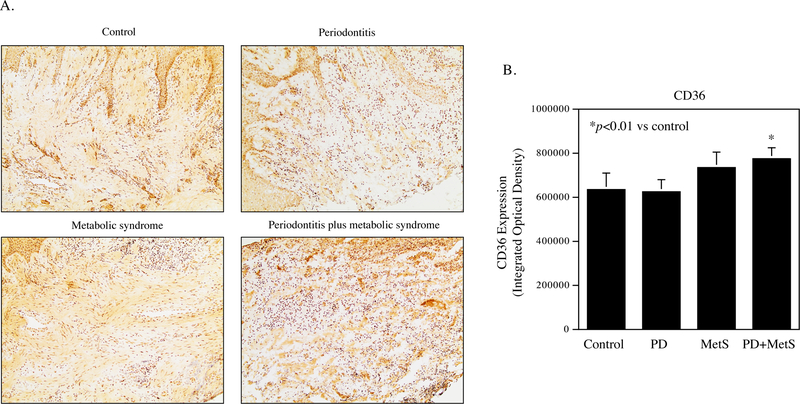
Expression of GPR40 and CD36 protein in periodontal tissue specimens was detected by immunohistochemistry. Results showed that GPR40 and CD36 proteins were expressed by gingival epithelial cells, fibroblasts and immune cells in periodontal tissue (Fig. 1A and Fig. 2A). Quantification of GPR40 and CD36 showed that GPR40 expression is increased in patients with periodontitis, MetS or both periodontitis and MetS (Fig. 1B) while CD36 expression is increased in patients with both periodontitis and MetS (Fig. 2B).
Figure 1.
The expression of GPR40 in periodontal tissues of participants without periodontitis and MetS, patients with periodontitis, MetS or both periodontitis and MetS. A. The negative control for immunohistochemical staining of GPR40 and CD36 in periodontal tissues. The tissue section incubated with normal human IgG as primary antibody was used as negative control. B. The GPR40 expression in periodontal tissues of control participants, patients with periodontitis, MetS, or both periodontitis and MetS. C. Quantification of GPR40 expression in periodontal tissues of control participants, patients with periodontitis (PD), MetS, or both PD and MetS.
Figure 2.
The expression of CD36 in periodontal tissues of participants without MetS and periodontitis (control), patients with periodontitis, MetS or both periodontitis and MetS. A. The CD36 expression in periodontal tissues of control participants, patients with periodontitis, MetS, or both periodontitis and MetS. B. Quantification of CD36 expression in periodontal tissues of control participants, patients with periodontitis (PD), MetS, or both PD and MetS.
3.3. GPR40 and CD36 Are Upregulated by LPS and Palmitate, and Involved in the Stimulation of Proinflammatory Gene Expression by LPS and Palmitate
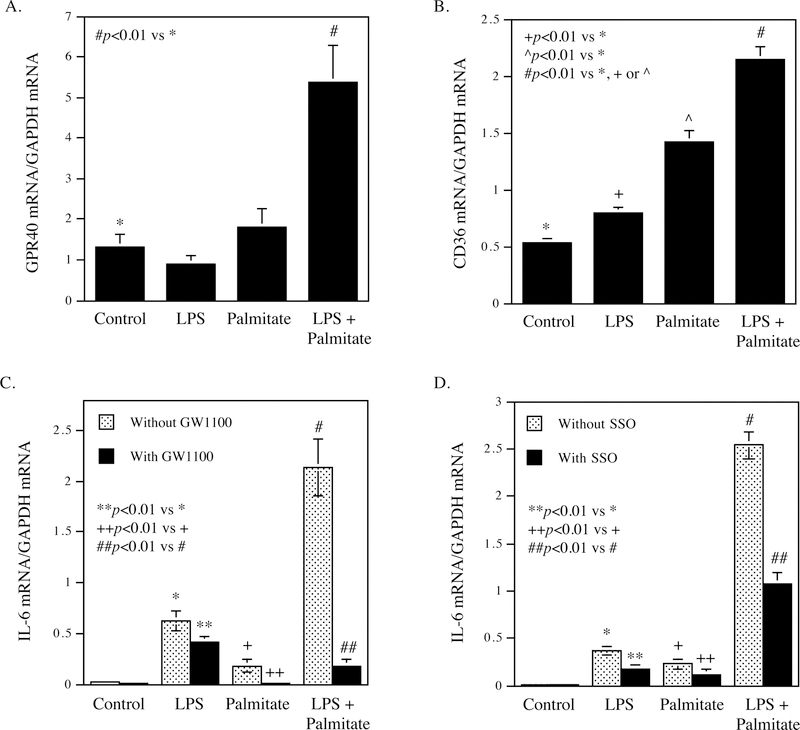
To understand the mechanisms involved in the upregulation of GPR40 and CD36 in periodontal tissues of patients with periodontitis and MetS, we hypothesized that the pathological factors associated with periodontitis or MetS may play a role in the upregulation of GPR40 and CD36. Since it is known that LPS is associated with periodontitis (27, 28) and SFA is associated with MetS (13), we determined the effect of LPS and palmitate, a major SFA, on GPR40 and CD36 expression in fibroblasts and macrophages in vitro. Results showed that GPR40 was upregulated by the combination of LPS and palmitate in gingival fibroblasts (Fig. 3A) and CD36 was upregulated by LPS, palmitate and the combination of LPS and palmitate in RAW264.7 macrophages (Fig. 3B). Furthermore, results showed that the GPR40 antagonist GW1100 or CD36 inhibitor SSO effectively inhibited the expression of IL-6 mRNA in gingival fibroblasts stimulated with LPS, palmitate or LPS plus palmitate (Fig. 3C and D), suggesting that GPR40 and CD36 play an essential role in the stimulation of IL-6 expression by LPS and palmitate in gingival fibroblasts.
Figure 3.
Upregulation of GPR40 and CD36 mRNA expression by LPS and palmitate, and the involvement of GPR40 and CD36 in the stimulation of IL-6 expression by LPS and palmitate. A. Human gingival fibroblasts were treated with 1 μg/ml of Pg LPS, 100 μM of palmitate or both 1 μg/ml of Pg LPS and 100 μM of palmitate for 24 h. After the treatment, GPR40 mRNA was quantified using real-time PCR. B. RAW264.7 macrophages were treated with 1 μg/ml of Pg LPS, 100 μM of palmitate or both 1 μg/ml of Pg LPS and 100 μM of palmitate for 24 h. After the treatment, CD36 mRNA was quantified using real-time PCR. C and D. Human gingival fibroblasts were treated with 1 μg/ml of Pg LPS, 100 μM of palmitate or both 1 μg/ml of Pg LPS and 100 μM of palmitate in the absence or presence of 5 μM of GW1100 (C) or 100 μM of SSO (D) for 24 h. After the treatment, IL-6 mRNA was quantified using real-time PCR. The data (mean ± SD) presented is from representative of three experiments with similar results.
4. DISCUSSION
Our previous studies have shown that SFA augments LPS-induced proinflammatory gene expression in macrophages and vascular endothelial cells by amplifying TLR4-mediated inflammatory signaling such as mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NFκB) cascades (15, 25). These findings strongly suggest that free fatty acid receptors such as GPR40 and CD36 are involved in SFA-enhanced proinflammatory gene expression since SFA engages GPR40 and CD36, which mediate MAPK and NFκB signaling activation (29, 30). The role of GPR40 and CD36 in the upregulation of proinflammatory cytokine expression was further supported by our findings that inhibition of GPR40 or CD36 attenuated proinflammatory cytokine expression in gingival fibroblasts stimulated by palmitate and LPS (Fig. 3). Consistently, studies from other laboratories have also demonstrated a vital role of free fatty acid receptors GPR40 and CD36 in SFA-promoted inflammation (31, 32).
Regulation of surface receptor expression is a well-known cell function to control cellular response to ligands or uptake of ligands (33, 34). Under certain conditions, cells either upregulate or downregulate the receptor expression, resulting in increased or decreased cell response to ligands and ligand uptake, respectively. In our current study, we found that GPR40 and CD36 were upregulated in cells in periodontal tissues of patients with both periodontitis and MetS, suggesting a potentially increased interaction between the cells in periodontal tissue and SFA, leading to an enhanced inflammatory response to SFA and SFA uptake.
It has been shown that GPR40 expression is upregulated by mild hyperlipidemia associated with obesity-prone diabetes (35). Furthermore, it has been also reported that GPR40 expression is upregulated by peroxisome proliferator-activated receptor-γ, a nuclear receptor associated with fatty acids and glucose (36), in pancreatic β-cells (37). These studies indicate that GPR40 expression is upregulated by hyperlipidemia and hyperglycemia-related factors. Given that the participants in groups 3 and 4 had MetS that is associated with hyperlipidemia and hyperglycemia, it is plausible that the hyperlipidemia and hyperglycemia in patients in groups 3 and 4 may promote GPR40 upregulation in periodontal tissue. Moreover, as differing from the upregulation of GPR40 by hyperlipidemia and hyperglycemia, it has been reported that CD36 expression is upregulated by proinflammatory cytokines such as IL-1β, macrophage colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (38). Since proinflammatory cytokines in periodontal tissue are increased in patients with MetS (39), it is possible that CD36 is upregulated in periodontal tissues by increased proinflammatory cytokines. Our in vitro study showed that GPR40 and CD36 were upregulated by the palmitate and LPS, supporting the roles of dyslipidemia and inflammation in the upregulation of GPR40 and CD36 expression.
GPR40 is a G protein-coupled receptor and mediates the proinflammatory effect of SFA (31). Previous studies have shown that SFA has a higher affinity than unsaturated fatty acid to bind GPR40 (40). It has been shown that GPR40 mediate palmitate-enhanced amyloid-β production by activating inflammatory signaling pathways in neuronal cells (29). It has been also shown that GPR40 mediated thiazolidinediones-activated proinflammatory signaling pathways (20). Furthermore, we have shown that GPR40 mediates palmitate-enhanced TLR4 inflammatory signaling in vascular endothelial cells (25). However, a number of studies have shown that GPR40 also had antiinflammatory properties. Interestingly, it was shown that GPR40 activation by GPR40 agonist GW9506 suppressed chemokine expression in keratinocytes and attenuated cutaneous immune inflammation (41). To understand why GPR40 has both proinflammatory and antiinflammatory effects, it is important to know that while GPR40 is coupled to Gq/11, the specific G protein coupled by GPR40 receptor, it is also functionally linked to a β-arrestin 2-mediated signaling cascade that is G protein-independent (42). Since different GPR40 natural ligands such as saturated and unsaturated fatty acids as well as synthetic ligands have different effects on Gq/11 and/or β-arrestin-mediated signaling pathways, they can exert either proinflammatory or antiinflammatory actions.
CD36 is present on the surface of a number of cells including monocytes, macrophages, endothelial cells, smooth muscle cells and adipocytes, and is a scavenger receptor for oxidized LDL, oxidized phospholipids, collagen, thrombospondin and parasitized erythrocytes (19). CD36 has been shown to play a pivotal role in mediating inflammation, insulin resistance and atherosclerosis through signaling activation, transport of fatty acids and uptake of oxidized lipids (30). Studies have also shown that CD36 signaling pathways lead to activation of N-terminal kinase (JNK), a signaling cascade involved in the cell growth, differentiation, survival, apoptosis, and inflammation (30). CD36 also interacts with TLRs, which contribute to proinflammatory cytokine expression in monocytes and macrophages (30).
In conclusion, we demonstrated in this study that the periodontal expression of GPR40 and CD36 is upregulated in patients with periodontitis and MetS. Moreover, we also demonstrated that the expression of GPR40 and CD36 expression in macrophages and gingival fibroblast cultured in vitro is upregulated by LPS and palmitate, and involved in the stimulation of proinflammatory cytokine expression by LPS and palmitate. All these findings suggest that GPR40 and CD36 are potential targets for MetS-associated periodontitis.
ACKNOWLEDGEMENT
We thank Abigail Kelly for statistical analysis. This work was supported by NIH grant DE016353 and Merit Review Grant from the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs (to Y.H.).
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- (1).Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol 1996; 67: 1123–1137. [DOI] [PubMed] [Google Scholar]
- (2).Offenbacher S Periodontal diseases: pathogenesis. Ann Periodontol 1996; 1: 821–878. [DOI] [PubMed] [Google Scholar]
- (3).Katz PP, Wirthlin MR Jr., Szpunar SM, Selby JV, Sepe SJ, Showstack JA. Epidemiology and prevention of periodontal disease in individuals with diabetes. Diabetes care 1991; 14: 375–385. [DOI] [PubMed] [Google Scholar]
- (4).Yalda B, Offenbacher S, Collins JG. Diabetes as a modifier of periodontal disease expression. Periodontology 2000 1994; 6: 37–49. [DOI] [PubMed] [Google Scholar]
- (5).Nibali L, Tatarakis N, Needleman I, et al. Clinical review: Association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J Clin Endocrinol Metab 2013; 98: 913–920. [DOI] [PubMed] [Google Scholar]
- (6).Gomes-Filho IS, das Merces MC, de Santana Passos-Soares J, et al. Severity of Periodontitis and Metabolic Syndrome: Is There an Association? J Periodontol 2016; 87: 357–366. [DOI] [PubMed] [Google Scholar]
- (7).Grundy SM, Brewer HB Jr., Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004; 109: 433–438. [DOI] [PubMed] [Google Scholar]
- (8).Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120: 1640–1645. [DOI] [PubMed] [Google Scholar]
- (9).Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015; 313: 1973–1974. [DOI] [PubMed] [Google Scholar]
- (10).Reisin E, Alpert MA. Definition of the metabolic syndrome: current proposals and controversies. Am J Med Sci 2005; 330: 269–272. [DOI] [PubMed] [Google Scholar]
- (11).Mealey BL. Periodontal disease and diabetes. A two-way street. J Am Dent Assoc 2006; 137 Suppl: 26S–31S. [DOI] [PubMed] [Google Scholar]
- (12).Cascio G, Schiera G, Di Liegro I. Dietary fatty acids in metabolic syndrome, diabetes and cardiovascular diseases. Curr Diabetes Rev 2012; 8: 2–17. [DOI] [PubMed] [Google Scholar]
- (13).Phillips CM, Goumidi L, Bertrais S, et al. Dietary saturated fat, gender and genetic variation at the TCF7L2 locus predict the development of metabolic syndrome. J Nutr Biochem 2012; 23: 239–244. [DOI] [PubMed] [Google Scholar]
- (14).Kageyama A, Matsui H, Ohta M, et al. Palmitic acid induces osteoblastic differentiation in vascular smooth muscle cells through ACSL3 and NF-kappaB, novel targets of eicosapentaenoic acid. PLoS One 2013; 8: e68197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Jin J, Zhang X, Lu Z, et al. Acid sphingomyelinase plays a key role in palmitic acid-amplified inflammatory signaling triggered by lipopolysaccharide at low concentrations in macrophages. Am J Physiol Endocrinol Metab 2013; 305: E853–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Itoh Y, Kawamata Y, Harada M, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 2003; 422: 173–176. [DOI] [PubMed] [Google Scholar]
- (17).Oh DY, Walenta E, Akiyama TE, et al. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat Med 2014; 20: 942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Im DS. Functions of omega-3 fatty acids and FFA4 (GPR120) in macrophages. Eur J Pharmacol 2016; 785: 36–43. [DOI] [PubMed] [Google Scholar]
- (19).Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal 2009; 2: re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Smith NJ, Stoddart LA, Devine NM, Jenkins L, Milligan G. The action and mode of binding of thiazolidinedione ligands at free fatty acid receptor 1. J Biol Chem 2009; 284: 17527–17539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Flemmig TF. Periodontitis. Ann Periodontol 1999; 4: 32–38. [DOI] [PubMed] [Google Scholar]
- (22).Salvi GE, Yalda B, Collins JG, et al. Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. J Periodontal 1997; 68: 127–135. [DOI] [PubMed] [Google Scholar]
- (23).Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech 2009; 2: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Patel S, Chung SH, White G, Bao S, Celermajer DS. The “atheroprotective” mediators apolipoprotein A-I and Foxp3 are over-abundant in unstable carotid plaques. Int J Cardiol 2010; 145: 183–187. [DOI] [PubMed] [Google Scholar]
- (25).Lu Z, Li Y, Jin J, Zhang X, Hannun YA, Huang Y. GPR40/FFA1 and neutral sphingomyelinase are involved in palmitate-boosted inflammatory response of microvascular endothelial cells to LPS. Atherosclerosis 2015; 240: 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Coort SL, Willems J, Coumans WA, et al. Sulfo-N-succinimidyl esters of long chain fatty acids specifically inhibit fatty acid translocase (FAT/CD36)-mediated cellular fatty acid uptake. Mol Cell Biochem 2002; 239: 213–219. [PubMed] [Google Scholar]
- (27).Jain S, Darveau RP. Contribution of Porphyromonas gingivalis lipopolysaccharide to periodontitis. Periodontol 2000 2010; 54: 53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Engebretson S, Chertog R, Nichols A, Hey-Hadavi J, Celenti R, Grbic J. Plasma levels of tumour necrosis factor-alpha in patients with chronic periodontitis and type 2 diabetes. J Clin Periodontol 2007; 34: 18–24. [DOI] [PubMed] [Google Scholar]
- (29).Kim JY, Lee HJ, Lee SJ, et al. Palmitic Acid-BSA enhances Amyloid-beta production through GPR40-mediated dual pathways in neuronal cells: Involvement of the Akt/mTOR/HIF-1alpha and Akt/NF-kappaB pathways. Sci Rep 2017; 7: 4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Kennedy DJ, Kashyap SR. Pathogenic role of scavenger receptor CD36 in the metabolic syndrome and diabetes. Metab Syndr Relat Disord 2011; 9: 239–245. [DOI] [PubMed] [Google Scholar]
- (31).Oh DY, Lagakos WS. The role of G-protein-coupled receptors in mediating the effect of fatty acids on inflammation and insulin sensitivity. Curr Opin Clin Nutr Metab Care 2011; 14: 322–327. [DOI] [PubMed] [Google Scholar]
- (32).Abumrad NA, Goldberg IJ. CD36 actions in the heart: Lipids, calcium, inflammation, repair and more? Biochim Biophys Acta 2016; 1861: 1442–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Locati M, Riboldi E, Otero K, et al. Regulation of the chemokine system at the level of chemokine receptor expression and signaling activity. Immunobiol 2001; 204: 536–542. [DOI] [PubMed] [Google Scholar]
- (34).Pascal V, Stulberg MJ, Anderson SK. Regulation of class I major histocompatibility complex receptor expression in natural killer cells: one promoter is not enough! Immunol Rev 2006; 214: 9–21. [DOI] [PubMed] [Google Scholar]
- (35).Meidute Abaraviciene S, Muhammed SJ, Amisten S, Lundquist I, Salehi A. GPR40 protein levels are crucial to the regulation of stimulated hormone secretion in pancreatic islets. Lessons from spontaneous obesity-prone and non-obese type 2 diabetes in rats. Mol Cell Endocrinol 2013; 381: 150–159. [DOI] [PubMed] [Google Scholar]
- (36).Jones JR, Barrick C, Kim KA, et al. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A 2005; 102: 6207–6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Kim HS, Hwang YC, Koo SH, et al. PPAR-gamma activation increases insulin secretion through the up-regulation of the free fatty acid receptor GPR40 in pancreatic beta-cells. PLoS One 2013; 8: e50128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Choromanska B, Mysliwiec P, Choromanska K, Dadan J, Chabowski A. The role of CD36 receptor in the pathogenesis of atherosclerosis. Adv Clin Exp Med 2017; 26: 717–722. [DOI] [PubMed] [Google Scholar]
- (39).Torumtay G, Kirzioglu FY, Ozturk Tonguc M, Kale B, Calapoglu M, Orhan H. Effects of periodontal treatment on inflammation and oxidative stress markers in patients with metabolic syndrome. J Periodontal Res 2016; 51: 489–498. [DOI] [PubMed] [Google Scholar]
- (40).Miyamoto J, Hasegawa S, Kasubuchi M, Ichimura A, Nakajima A, Kimura I. Nutritional Signaling via Free Fatty Acid Receptors. Int J Mol Sci 2016; 17: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Fujita T, Matsuoka T, Honda T, Kabashima K, Hirata T, Narumiya S. A GPR40 agonist GW9508 suppresses CCL5, CCL17, and CXCL10 induction in keratinocytes and attenuates cutaneous immune inflammation. J Invest Dermatol 2011; 131: 1660–1667. [DOI] [PubMed] [Google Scholar]
- (42).Mancini AD, Bertrand G, Vivot K, et al. beta-Arrestin Recruitment and Biased Agonism at Free Fatty Acid Receptor 1. J Biol Chem 2015; 290: 21131–21140. [DOI] [PMC free article] [PubMed] [Google Scholar]