ABSTRACT
Background
Dietary recommendations to limit red meat are based on observational studies linking intake to cardiovascular disease (CVD) risk together with the potential of its saturated fatty acid (SFA) content to raise low-density lipoprotein (LDL) cholesterol. However, the relation of white meat to CVD risk, and the effects of dietary protein source on lipoprotein particle subfractions, have not been extensively evaluated.
Objective
We tested whether levels of atherogenic lipids and lipoproteins differed significantly following consumption of diets with high red meat content compared with diets with similar amounts of protein derived from white meat or nonmeat sources, and whether these effects were modified by concomitant intake of high compared with low SFAs.
Methods
Generally healthy men and women, 21–65 y, body mass index 20–35 kg/m2, were randomly assigned to 1 of 2 parallel arms (high or low SFA) and within each, allocated to red meat, white meat, and nonmeat protein diets consumed for 4 wk each in random order. The primary outcomes were LDL cholesterol, apolipoprotein B (apoB), small + medium LDL particles, and total/high-density lipoprotein cholesterol.
Results
Analysis included participants who completed all 3 dietary protein assignments (61 for high SFA; 52 for low SFA). LDL cholesterol and apoB were higher with red and white meat than with nonmeat, independent of SFA content (P < 0.0001 for all, except apoB: red meat compared with nonmeat [P = 0.0004]). This was due primarily to increases in large LDL particles, whereas small + medium LDL and total/high-density lipoprotein cholesterol were unaffected by protein source (P = 0.10 and P = 0.51, respectively). Primary outcomes did not differ significantly between red and white meat. Independent of protein source, high compared with low SFA increased LDL cholesterol (P = 0.0003), apoB (P = 0.0002), and large LDL (P = 0.0002).
Conclusions
The findings are in keeping with recommendations promoting diets with a high proportion of plant-based food but, based on lipid and lipoprotein effects, do not provide evidence for choosing white over red meat for reducing CVD risk. This trial was registered at Clinicaltrials.gov as NCT01427855.
Keywords: beef, chicken, poultry, vegetable protein, plant protein, dairy fat, dietary recommendations, lipoprotein particle distribution
Introduction
Observational studies suggest that red meat intake is associated with increased cardiovascular disease (CVD) risk (1, 2), whereas no such association has been observed with regular consumption of poultry (3). Conversely, plant protein sources and vegetarian dietary patterns appear to be cardioprotective (4, 5), a finding supported by a systematic review of randomized controlled trials showing decreased LDL cholesterol (0.16 mmol/L), non-HDL cholesterol (0.18 mmol/L), and apolipoprotein B (apoB) (0.05 g/L) when animal protein is replaced with plant protein (6).
Because it is well known that dietary saturated fatty acids (SFAs) increase plasma concentrations of LDL cholesterol, it has been generally assumed that the SFA content of red meat contributes to its association with CVD risk (7, 8). This assumption is supported by the lack of significant differences in total cholesterol, LDL cholesterol, and HDL cholesterol in randomized controlled feeding trials in which lean red meat (beef, pork, or lamb) compared with lean chicken/poultry was consumed as part of a low-SFA diet (7–11% of total energy [E]) (7–13). Equivalent effects of red compared with white meat on plasma lipids and lipoproteins have also been reported in meta-analyses of randomized controlled trials, although these results were not assessed in relation to the SFA content of the diets (14, 15).
In summary, there has to date been no systematic evaluation of the potential interaction of dietary protein source and SFA content on concentrations of LDL cholesterol and related atherogenic lipoprotein measures, including levels of lipoprotein particles. Hence, the primary objective of the present clinical trial (Animal and Plant Protein and Cardiovascular Health: APPROACH) was to test for differences in lipoprotein effects of diets in which the main source of protein is red meat compared with diets with similar total protein content derived from white meat (poultry) or plant protein sources, and to determine whether these effects were modified by high compared with low SFA intake.
Methods
Study participants
Participants were recruited primarily through Internet advertisements, community postings and events, and our database of previous study participants. Eligible participants were healthy men and women aged 21–65 yr who met the following criteria: BMI 20–35 kg/m2; blood pressure <150/90; fasting glucose <7 mmol/L, total and LDL cholesterol ≤95th percentile for sex and age; fasting triacylglycerol <5.65 mmol/L; and willingness to refrain from use of vitamin supplements and alcoholic beverages for the duration of the study. Exclusion criteria included: use of tobacco or recreational drugs; use of lipid-, glucose-, or blood-pressure-lowering medications, blood thinners, or hormones; unwillingness to consume all key study foods; weight loss >3% body weight in the 3 mo preceding the study onset; and history of coronary artery disease, diabetes, or other chronic disorder. We also excluded study participants who were enrolled in one of our previous dietary trials related to the hypothesis being tested in the current study. The study was approved by the Institutional Review Board of the University of California, San Francisco Benioff Children's Hospital Oakland (IRB# 2011-041), and all participants provided written consent to take part.
Study design, randomization, and masking
Participants were randomly assigned to 1 of 2 parallel arms (high or low SFA), and within each arm the effects of food source of protein (red meat, white poultry meat, nonmeat) were tested in a 3-period randomized crossover design (Figure 1). Participants first consumed a 2-wk baseline diet to test their compliance to a controlled dietary protocol before being randomly assigned to either a low-SFA (∼7% total energy, E) or high-SFA (∼14% E) group. Within each SFA group, the 3 experimental diets tested the effects of substituting red meat for white meat or nonmeat sources of protein, which constituted ∼12% E. The diets were assigned in random order and consumed for 4 wk each, separated by a 2–7-wk washout period during which participants consumed their habitual diet (Figure 1). The flexible washout period allowed breaks in the dietary protocol to coincide with longer holidays and vacations. A uniform random-number generator, generated by the study statistician, was used to determine block sizes of 2, 4, 6, or 8 subjects, and the SFA level and sequence of the red meat, white meat, and nonmeat protein sources within the block. Randomized diet codes were kept in sealed numbered envelopes until assigned to study participants by the study coordinator during the second week of the baseline diet. For the last 8% of enrolled subjects, a new set of randomization envelopes was prepared to reflect the remaining available study foods in order to maximize the use of available study diets. Study participants were blinded to assignment of high compared with low SFA, but the nature of red meat, white meat, and nonmeat foods precluded blind assignment to the order of dietary protein sources. Although principal investigators and laboratory staff were blinded to diet order, the staff personnel responsible for provision of research diets and monitoring of compliance were not. For the duration of the study, including washout periods, participants met with clinic staff weekly to pick up study foods, receive dietary counseling, and be weighed. Participants were required to remain weight-stable (±3% baseline weight) and maintain their baseline activity level as measured with a triaxial accelerometer (Actigraph GT3X, Actigraph) and weekly activity logs.
FIGURE 1.
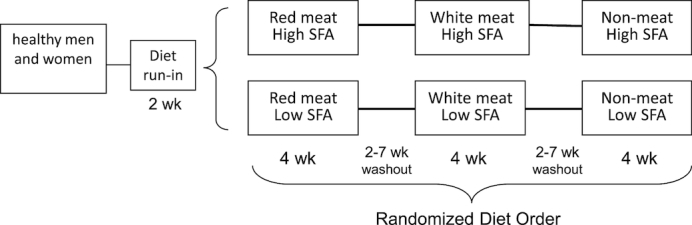
Study design.
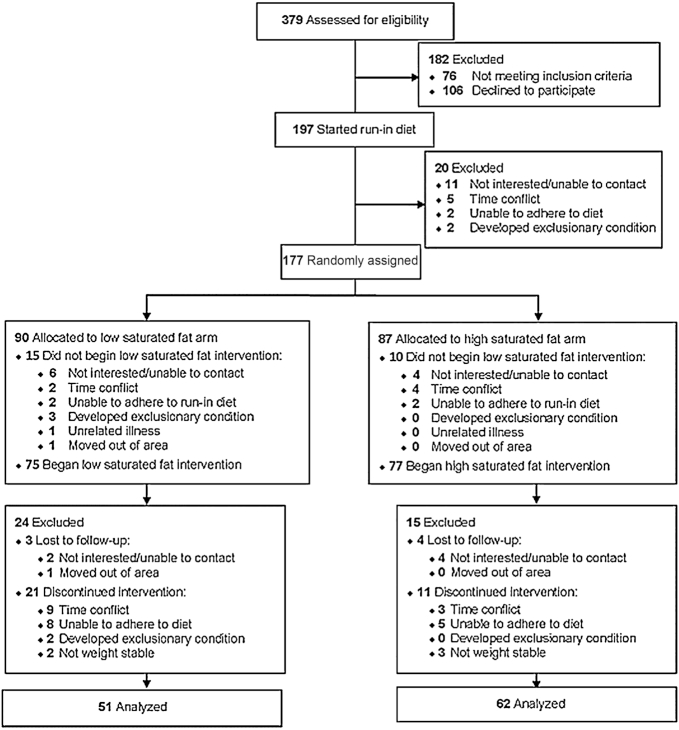
On 2 consecutive days at the end of the baseline diet and after each experimental diet, venous blood samples were collected after a 12–14-h overnight fast, for duplicate analysis of plasma lipids, lipoprotein particle subfractions, apolipoproteins, and glucose. Measurements of body weight, blood pressure, hip and waist circumference, percentage body fat by bioimpedance scale (Tanita TBF-551), and endothelial function measured by finger reactive hyperemia peripheral arterial tonography index (EndoPat2000, Itamar Medical) were also obtained at the end of each dietary period. Participant enrollment is shown inFigure 2.
Figure 2.
Participant enrollment.
Dietary provision
The nutrient composition of the baseline diet (Table 1) reflected that of the typical American diet (16). The moderately high total protein and fat content of the experimental diets was required to achieve the desired variations in protein and SFA composition within and across study arms while remaining in ranges compatible with dietary recommendations that were in place at the time of study onset (17). The high- and low-SFA diets were designed to maximize differences in both SFA content across study arms (∼7% E difference) and dietary protein source within study arms (∼12% E difference). The macronutrient composition of the high- and low-SFA diets was based upon our previous study showing changes in lipoprotein concentrations when SFA was increased from 9 to 15% E in exchange for monounsaturated fatty acids (18). Differences in SFA content between the high- and low-SFA arms were achieved primarily by using high-fat dairy products and butter, with only 2–3% E from SFAs derived from lean red or white meat. For the nonmeat diets, 2–3% E from SFAs was derived from tropical oils and fats, with the remainder provided by high-fat dairy products. The percentage energy contributed by the test protein sources was based upon earlier work showing a preferential increase in smaller LDL particles when high SFAs were consumed in a diet providing ∼12% E from beef (19), but no change in small LDL when moderate amounts of beef (6.5% E) were consumed as part of a mixed protein diet (18). In the present study, food sources of protein were varied by providing ∼12% E from either: lean cuts of red meat (11% E from beef, 1% E from pork); lean white meat (8% E from chicken; 4% E from turkey); or nonmeat sources (legumes, nuts, grains, isoflavone-free soy products). For all experimental diets, the remaining protein (∼13% E) was derived from eggs, dairy, and vegetable sources (Supplemental Table 1; sample menus Supplemental Table 2). The study was not designed to test effects of fish and seafood, which were excluded from all the diets. Grain- rather than grass-finished beef sources were used in the preparation of red meat diets because grain-finished beef currently represents 96% of the total US beef market (20). Processed meats were excluded from the diets to avoid the potential confounding effects of added chemicals on the metabolic variables of interest. Dietary carbohydrates were provided in the form of complex starches and simple sugars in a 60:40 ratio, with total carbohydrate content held constant across experimental diets.
TABLE 1.
Composition of baseline and experimental diets, based on compositional analysis of 10,460 kJ 4-d rotating menus
| High-SFA | Low-SFA | ||||||
|---|---|---|---|---|---|---|---|
| Baseline diet | Red meat | White meat | Nonmeat | Red meat | White meat | Nonmeat | |
| Carbohydrate, % E | 49 | 41 | 42 | 41 | 39 | 46 | 41 |
| Protein, % E | 14 | 24 | 24 | 24 | 26 | 23 | 25 |
| Red meat1 | Mixed2 | 11.5 | 0 | 0 | 12.5 | 0 | 0 |
| White meat1 | — | 0 | 11.5 | 0 | 0 | 11.0 | 0 |
| Vegetable protein1 | — | 3.8 | 3.8 | 15.4 | 4.1 | 3.7 | 16 |
| Dairy1 | — | 6.7 | 6.7 | 6.7 | 7.3 | 6.5 | 7 |
| Eggs1 | — | 2 | 2 | 1.9 | 2.1 | 1.8 | 2 |
| Fat, % E | 37 | 35 | 34 | 35 | 35 | 31 | 34 |
| Saturated fat | 131 | 13 | 14 | 14 | 8 | 7 | 7 |
| Monounsaturated fat | 151 | 12 | 13 | 12 | 21 | 18 | 20 |
| Polyunsaturated fat | 61 | 5 | 5 | 6 | 5 | 6 | 5 |
| Cholesterol, mg/d | 336 | 403 | 473 | 297 | 353 | 424 | 275 |
| Fiber1, g/d | 35 | 35 | 33 | 36 | 34 | 36 | 41 |
Calculated values (Nutrition Data System for Research; University of Minnesota) include adjustments based on compositional analysis of daily menus. % E, percentage of energy.
The baseline diet provided a mixture of all dietary protein sources.
Diets and menus were developed and prepared in collaboration with the Bionutrition Unit of the University of California, San Francisco-based Clinical and Translational Studies Institute. Four-day rotating menus were developed for all diets, and made available at 5 energy levels (6275, 8365, 10,460, 12,550, 14,645 kJ) with provision of 1045 kJ snacks reflecting the nutrient composition of the experimental diets for individuals whose needs fell between energy levels. With the exception of fresh produce (fruits and vegetables), which participants purchased for themselves to ensure freshness at the time of consumption, all menu items (standardized entrees, side dishes, caloric beverages, snacks) were provided for the duration of the study. Abstention from alcohol was required for the duration of the study, including washout periods. Participants came to the outpatient Cholesterol Research Center (Berkeley, CA) weekly to meet with staff nutritionists, pick up study foods, and be weighed. Energy intake was adjusted if changes in body weight exceeded ±3% of baseline weight. The nutrient content of the diets was first assessed using the Nutrition Data System for Research software (NDS2010; Nutrition Coordinating Center, University of Minnesota), and validated by compositional analysis (Covance Laboratories).
Dietary compliance was assessed by measuring 24-h urinary urea nitrogen and creatinine concentrations (Quest Diagnostics) during the second week of the baseline diet and during the third week of each experimental diet. The urea nitrogen to creatinine ratio was used to assess dietary protein intake (21, 22) during each dietary period. As an additional measure of control of dietary intake, menu checklists, grocery receipts, and reported deviations from dietary instructions were collected weekly by staff nutritionists and used to assign compliance scores (1–5 point scale, where 5 = high compliance) to all study participants.
Laboratory measurements
Fasting plasma triglycerides, total cholesterol, HDL cholesterol, and plasma glucose were measured by enzymatic endpoint analysis using enzyme reagent kits (Ciba-Corning Diagnostics Corporation) on a clinical chemistry analyzer (Liasys 330, AMS Diagnostics). LDL cholesterol was calculated using the Friedewald equation (23). These measurements are standardized through the CDC-NHLBI lipid standardization program. ApoB and apolipoprotein A-I (apoA-I) were analyzed by immunoturbidimetric assays (Bacton Assay Systems; AMS Liasys 330 analyzer). Lipoprotein particle concentrations and LDL peak diameter were measured by ion mobility, as previously described (24, 25).
Outcomes
The primary outcomes were LDL cholesterol, apoB, small + medium LDL (sum of small LDL and medium LDL particle concentrations, previously shown to be associated with the greatest CVD risk among LDL subfractions (26)), and total/HDL cholesterol ratio. These measurements were obtained on the last 2 consecutive days of the baseline diet and each of the 3 dietary protein sequences. Secondary outcomes included HDL cholesterol, non-HDL cholesterol, apoA-I, large HDL2b, small HDL2a + 3, and endothelial function measured as reactive hyperemia peripheral arterial tonography index. Other secondary outcomes, to be reported separately, were homeostatic model assessment of insulin resistance, inflammatory markers (C-reactive protein, TNF-α, IL-6, MCP-1), and apolipoprotein A-II (apoA-II).
Statistical analyses
Our primary hypothesis was that on a high-SFA diet, red meat relative to other sources of protein would increase serum concentrations of LDL cholesterol, apoB, small and medium LDL particles, and the ratio of total/HDL cholesterol. An N of 90 participants per group in a crossover design was estimated to provide 80% power (α = 0.0125) to yield detectable differences, as the percentage change from a low-SFA diet, of 5.3% for LDL cholesterol, 4.6% for apoB, 14.3% for small + medium LDL, and 4.8% for total/HDL cholesterol. Based on earlier results (18, 19), these differences were estimated to be adequate to detect the expected changes between protein sources within the high-SFA group. As described in Supplemental Methods, power calculations were revised as a consequence of unanticipated reduction in sample size due to loss of availability of the expected number of experimental diets in the final year of the study. Treatment differences were determined by ANOVA for a parallel arm, 3-treatment crossover design (JMP, version 13.2.0, SAS Inc.), followed by post hoc pairwise comparisons, adjusted by the Bonferroni method for 3 group comparisons. Significance was determined for sequence, period, carry-forward, and treatment effects. The study was designed for a per-protocol analysis, with statistics restricted to participants who completed all 3 dietary protein sequences. As this was a low-risk study, there was no data monitoring committee created to oversee the study.
Results
This controlled, randomized, dietary intervention trial was conducted in an outpatient setting (San Francisco Bay Area, California) between March 26, 2012 and October 27, 2016. The flow of participants through the various phases of the study is illustrated in Figure 2. A total of 62 participants completed the high-SFA arm (27 men, 35 women), and 51 participants completed the low-SFA arm (17 men, 34 women). The baseline characteristics of individuals assigned to the high- and low-SFA diets did not differ significantly between groups (Table 2). Based on current guidelines defining overweight and obesity (27), the majority of participants were either of normal BMI (42%) or overweight (42%), with 16% of the study population being obese. On average, participants were normotensive and displayed plasma glucose and lipid concentrations within normal ranges. Over the course of the study, there were no significant changes in body weight across protein diets in either the low-SFA groups (red meat, −0.36 ± 1.28 kg; white meat, −0.29 ± 1.67 kg; nonmeat, 0.09 ± 1.17 kg; P = 0.59) or high-SFA groups (red meat, −0.16 ± 1.28 kg; white meat, −0.36 ± 1.32 kg; nonmeat, 0.15 ± 1.37 kg; P = 0.10).
TABLE 2.
Participant characteristics after the baseline diet1
| High-SFA (n = 62) | Low-SFA (n = 51) | |
|---|---|---|
| Male/female, n | 27/35 | 17/34 |
| Self-reported race, n (%) | ||
| White | 34 (55%) | 28 (55%) |
| Asian | 9 (15%) | 13 (25%) |
| African American | 10 (16%) | 4 (8%) |
| Other (unknown, >1 race) | 9 (15%) | 6 (12%) |
| Age, y | 45 ± 12 | 42 ± 13 |
| BMI, kg/m2 | 25.9 ± 3.8 | 26.0 ± 3.8 |
| Body fat, % | 30.1 ± 10.1 | 30.4 ± 9.4 |
| Waist circumference, cm | 90 ± 11 | 89 ± 9 |
| Systolic blood pressure, mm Hg | 110 ± 10 | 109 ± 13 |
| Diastolic blood pressure, mm Hg | 70 ± 7 | 68 ± 8 |
| Triglycerides, mmol/L | 1.01 ± 0.54 | 0.88 ± 0.34 |
| Cholesterol, mmol/L | ||
| Total | 4.50 ± 0.80 | 4.53 ± 0.88 |
| LDL | 2.69 ± 0.70 | 2.68 ± 0.69 |
| HDL | 1.35 ± 0.31 | 1.45 ± 0.39 |
| Non-HDL | 3.16 ± 0.85 | 3.08 ± 0.80 |
| Apolipoprotein A-I, g/L | 1.31 ± 0.20 | 1.36 ± 0.21 |
| Apolipoprotein B, g/L | 0.75 ± 0.21 | 0.74 ± 0.21 |
| Total/HDL cholesterol | 3.50 ± 1.01 | 3.27 ± 0.83 |
| Very-low-density lipoprotein, nmol/L | ||
| Total | 93.8 ± 44.9 | 85.2 ± 41.4 |
| Large | 12.4 ± 9.4 | 10.6 ± 7.0 |
| Medium | 38.5 ± 21.6 | 33.8 ± 18.3 |
| Small | 42.8 ± 15.9 | 40.9 ± 18.1 |
| Intermediate-density lipoprotein, nmol/L | 116 ± 39 | 112 ± 46 |
| LDL, nmol/L | ||
| Total | 1025 ± 280 | 1037 ± 340 |
| Large | 588 ± 151 | 614 ± 183 |
| Medium | 162 ± 75 | 155 ± 88 |
| Small | 122 ± 79 | 119 ± 95 |
| Very small | 153 ± 92 | 149 ± 75 |
| HDL, nmol/L | ||
| Total | 18,907 ± 3860 | 19,145 ± 4219 |
| Large | 5234 ± 1759 | 5489 ± 1542 |
| Small | 13,673 ± 2572 | 13,656 ± 3066 |
| LDL peak particle diameter, Å | 223 ± 6 | 225 ± 6 |
1Values are means ± SDs.
As expected, the ratio of 24-h urinary urea nitrogen to creatinine excretion increased significantly from the baseline diet to the higher-protein intervention diets (P < 0.0001 for each protein source) in both the high- and low-SFA arms of the study. The urinary nitrogen/creatinine ratio did not differ significantly between diets in either the high SFA arm (mean ± SE [mg/g per 24 h]: red meat, 9062 ± 292; white meat, 10,034 ± 341; nonmeat, 10,193 ± 457; P = 0.46) or low-SFA arm (mean ± SE: red meat, 9330 ± 391; white meat, 10,859 ± 1347; nonmeat, 10,161 ± 515; P = 0.07).
There were significant effects of both dietary protein source and SFA content on total cholesterol, LDL cholesterol, non-HDL cholesterol, and apoB concentrations, whereas the ratio of total/HDL cholesterol was unaffected by these dietary modifications (Table 3). Moreover, there were no significant interactions between protein source and SFA level on these lipid and lipoprotein measurements. Pairwise comparisons across dietary protein sources showed that concentrations of total cholesterol (P < 0.0001), LDL cholesterol (P < 0.0001), and non-HDL cholesterol (P < 0.001) were significantly higher after either the red meat or white meat diet than after the nonmeat diet (Supplemental Table 3). There was a relatively small effect of protein source on HDL cholesterol (P = 0.004, Table 3) with concentrations slightly higher on the red and white meat diets, particularly in the high-SFA arm. Apolipoprotein responses reflected lipid changes, with significantly higher plasma apoB (P < 0.001) and apoA1 (P < 0.05) following the red and white meat compared with nonmeat diets. The cholesterol-raising effect of the meat diets was associated with increases in large LDL (P < 0.001), whereas small + medium LDL and LDL peak particle diameter were unaffected by dietary protein source (Table 4; Supplemental Table 3). Lipid, lipoprotein, and apolipoprotein concentrations, together with concentrations of large, medium, and small LDL particles, did not differ significantly between the red meat and white meat diets (Supplemental Table 3).
TABLE 3.
Plasma lipid concentrations after 4 wk of diets varying in dietary protein source and saturated fat content1
| High-SFA | Low-SFA | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Red meat | White meat | Nonmeat | Red meat | White meat | Nonmeat | Protein | SFA | Interaction | |
| Total cholesterol, mmol/L | 4.42 ± 0.93 | 4.39 ± 0.83 | 4.22 ± 0.83 | 4.11 ± 0.78 | 4.14 ± 0.80 | 3.98 ± 0.80 | <0.0001 | 0.0002 | 0.69 |
| LDL cholesterol, mmol/L | 2.64 ± 0.80 | 2.61 ± 0.72 | 2.46 ± 0.70 | 2.35 ± 0.59 | 2.38 ± 0.65 | 2.22 ± 0.65 | <0.0001 | 0.0003 | 0.63 |
| HDL cholesterol, mmol/L | 1.34 ± 0.31 | 1.34 ± 0.31 | 1.29 ± 0.31 | 1.40 ± 0.36 | 1.42 ± 0.39 | 1.40 ± 0.41 | 0.004 | 0.07 | 0.24 |
| Non-HDL cholesterol, mmol/L | 3.08 ± 0.93 | 3.05 ± 0.85 | 2.92 ± 0.85 | 2.72 ± 0.70 | 2.74 ± 0.72 | 2.59 ± 0.75 | <0.0001 | 0.0003 | 0.83 |
| Triglycerides, mmol/L | 0.95 ± 0.47 | 0.96 ± 0.49 | 0.99 ± 0.49 | 0.80 ± 0.33 | 0.78 ± 0.34 | 0.80 ± 0.33 | 0.32 | 0.39 | 0.40 |
| apoA-I, g/L | 1.31 ± 0.18 | 1.30 ± 0.18 | 1.28 ± 0.18 | 1.33 ± 0.21 | 1.33 ± 0.23 | 1.31 ± 0.23 | 0.01 | 0.32 | 0.62 |
| apoB, g/L | 0.73 ± 0.23 | 0.74 ± 0.22 | 0.70 ± 0.21 | 0.67 ± 0.18 | 0.67 ± 0.19 | 0.63 ± 0.18 | <0.0001 | 0.0002 | 0.99 |
| apoB/apoA-I | 0.57 ± 0.20 | 0.58 ± 0.21 | 0.57 ± 0.21 | 0.51 ± 0.15 | 0.52 ± 0.17 | 0.49 ± 0.16 | 0.02 | 0.01 | 0.72 |
| Total/HDL cholesterol | 3.41 ± 0.97 | 3.41 ± 0.96 | 3.41 ± 1.0 | 3.07 ± 0.72 | 3.08 ± 0.78 | 3.01 ± 0.79 | 0.51 | 0.15 | 0.60 |
| LDL cholesterol/apoB | 3.61 ± 0.39 | 3.57 ± 0.41 | 3.51 ± 0.38 | 3.57 ± 0.32 | 3.60 ± 0.37 | 3.52 ± 0.38 | 0.09 | 0.44 | 0.52 |
1Values are means ± SD, n = 113. Data were analyzed by ANOVA for a 3-treatment crossover design, adjusted for dietary period. apoA-I, apolipoprotein A-I; apoB, apolipoprotein B.
TABLE 4.
Total mass concentrations of plasma lipoprotein subfractions after 4 wk of diets varying in dietary protein source and saturated fat content1
| High-SFA | Low-SFA | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Red meat | White meat | Nonmeat | Red meat | White meat | Nonmeat | Protein | SFA | Interaction | |
| Very-low-density lipoprotein, nmol/L | |||||||||
| Total | 82.9 ± 41.1 | 85.0 ± 41.3 | 86.6 ± 41.4 | 72.8 ± 37.6 | 71.0 ± 37.8 | 72.6 ± 38.8 | 0.64 | 0.24 | 0.54 |
| Large | 10.5 ± 7.1 | 11.1 ± 7.8 | 11.7 ± 8.6 | 8.9 ± 6.7 | 8.2 ± 6.2 | 8.7 ± 6.3 | 0.20 | 0.48 | 0.14 |
| Medium | 33.8 ± 19.1 | 35.3 ± 19.6 | 36.6 ± 20.3 | 29.1 ± 16.5 | 28.1 ± 16.8 | 29.2 ± 17.2 | 0.22 | 0.34 | 0.31 |
| Small | 38.7 ± 16.9 | 39.2 ± 16.1 | 38.4 ± 14.8 | 34.8 ± 16.4 | 34.7 ± 16.2 | 34.6 ± 16.6 | 0.90 | 0.18 | 0.92 |
| Intermediate-density lipoprotein, nmol/L | 112 ± 52 | 111 ± 41 | 108 ± 48 | 103 ± 53 | 99 ± 44 | 97 ± 41 | 0.36 | 0.12 | 0.93 |
| LDL, nmol/L | |||||||||
| Total | 1019 ± 298 | 1021 ± 262 | 955 ± 273 | 940 ± 295 | 946 ± 307 | 911 ± 292 | 0.004 | 0.005 | 0.37 |
| Large | 590 ± 164 | 593 ± 161 | 547 ± 136 | 561 ± 171 | 546 ± 176 | 519 ± 179 | <0.0001 | 0.0002 | 0.57 |
| Medium | 161 ± 83 | 158 ± 72 | 148 ± 70 | 142 ± 72 | 145 ± 78 | 138 ± 63 | 0.04 | 0.33 | 0.40 |
| Small | 123 ± 83 | 120 ± 75 | 115 ± 76 | 105 ± 74 | 112 ± 73 | 107 ± 62 | 0.28 | 0.28 | 0.24 |
| Small + medium | 284 ± 159 | 278 ± 137 | 263 ± 140 | 247 ± 142 | 257 ± 147 | 245 ± 118 | 0.10 | 0.27 | 0.28 |
| Very small | 145 ± 68 | 149 ± 72 | 144 ± 72 | 132 ± 55 | 142 ± 59 | 147 ± 83 | 0.05 | 0.76 | 0.04 |
| HDL, nmol/L | |||||||||
| Total | 18,881 ± 3334 | 18,891 ± 3741 | 18,313 ± 3554 | 19,016 ± 4144 | 18,800 ± 4632 | 18,969 ± 4661 | 0.27 | 0.99 | 0.12 |
| Large | 5241 ± 1532 | 5318 ± 1651 | 5119 ± 1558 | 5415 ± 1572 | 5568 ± 1871 | 5551 ± 1912 | 0.18 | 0.80 | 0.12 |
| Small | 13,641 ± 2360 | 13,573 ± 2553 | 13,194 ± 2457 | 13,601 ± 2918 | 13,232 ± 3206 | 13,417 ± 3161 | 0.07 | 0.88 | 0.13 |
| LDL peak particle diameter, Å | 223 ± 6 | 223 ± 6 | 224 ± 6 | 222 ± 5 | 224 ± 5 | 223 ± 5 | 0.17 | 0.01 | 0.004 |
1Values are means ± SD, n = 113. Data were analyzed by ANOVA for a 3-treatment crossover design, adjusted for dietary period.
Independent of dietary protein source, diets high in SFA resulted in higher plasma total cholesterol, LDL cholesterol and non-HDL cholesterol concentrations than diets low in SFA (all P < 0.001, Table 3). This was accompanied by higher apoB concentrations (P = 0.0004) and an increase in apoB/apoA-1 with high SFA intake (P < 0.05). The increased LDL cholesterol and apoB with high SFA were associated with higher concentrations of large LDL particles (P < 0.001), whereas small- and medium-sized LDL particles were unaffected by high compared with low SFA intake. Concentrations of very-low-density lipoprotein particles, intermediate-density lipoproteins, and HDL particle subfractions were unaffected by either dietary protein source or SFA content.
Notably, with the exception of LDL peak particle diameter, which responded differently to SFA when combined with white meat compared with other protein sources, and very small LDL, which responded differently to SFA when combined with nonmeat compared with animal protein sources, there were no significant interactions between dietary protein source and SFA level for any of the lipoprotein particle biomarkers. There were no significant sequence or carry-forward effects for any of the lipid or lipoprotein variables (data not shown), but there was a significant period effect for total cholesterol, HDL cholesterol, and apoA-1 (P < 0.05), for which adjustment was made in the ANOVA.
Finally, there were no significant effects of protein source or SFA level on blood pressure, plasma glucose, or endothelial reactivity as assessed by endothelial peripheral arterial tone, EndoPAT (Supplemental Table 4).
Discussion
We have shown here that, compared with nonmeat as the major protein source, diets containing high amounts of either red or white meat, and without differences in other macronutrients, result in higher concentrations of LDL cholesterol and apoB, and that these effects are primarily attributable to increases in large, cholesterol-rich LDL particles. Notably, the effects of red and white meat were similar and were observed with diets containing either low or high levels of SFAs. As expected, the higher SFA level, provided primarily by full-fat dairy products and butter, resulted in higher LDL cholesterol and apoB, and, as demonstrated previously in healthy subjects (18, 28, 29), increases in large but not medium or small LDL particles. There were no interactions between protein source and dietary SFA level on these responses, such that the effects were additive; i.e., the highest concentrations of LDL cholesterol, apoB, and large LDL particles resulted from the combination of high SFA intake with either red or white meat as the major protein source.
The present findings are consistent with previous studies indicating that intake of neither lean red meat nor poultry results in increases in plasma lipid concentrations in the context of a diet low in SFA (7–13, 15), and with earlier studies of primarily plant-based, lacto-ovo-vegetarian, or vegan dietary patterns reporting significantly lower total, LDL-, and HDL cholesterol concentrations than diets including animal protein (30). However, the present study is the first to show that both categories of meat protein result in LDL concentrations that are higher than those resulting from vegetable protein sources in otherwise comparable diets, and that these effects are independent of dietary SFA level.
The present findings contrast with those of a previous report from our laboratory showing that in conjunction with a diet high in beef protein (11% E), consumption of high compared with low SFA resulted in significantly increased concentrations of medium-sized LDL, with a lesser but significant increase in small LDL (19). The basis for the differing results in the earlier study is not known, though factors to consider include a smaller study population (n = 40), the provision of a smaller proportion of the foods in the experimental diets, and differences in dietary macronutrient composition, including lower carbohydrate intake (31% E) with a higher proportion of simple sugars, and slightly higher SFA intake (15% E). In another recent study in 53 individuals selected for a predominance of small LDL (LDL subclass phenotype B), consumption of a moderate-carbohydrate (39% E) very high-SFA diet (18% E) in conjunction with mixed protein sources also resulted in increases in medium- and small-sized LDL, with no significant increase in larger LDL (31). Thus, features of diet composition, including SFA level, as well as characteristics of the study population, may modify the lipoprotein response to variation in protein source and SFA intake.
Given the emphasis placed on lowering LDL cholesterol concentrations in dietary guidelines for reducing risk of CVD in the general population (32, 33), the present findings may have implications for the development of future dietary recommendations. Based on the predicted effects on CVD risk of lowering LDL cholesterol as well as apoB, substitution of vegetable sources of protein for meat, either red or white, together with substitution of unsaturated for SFAs, would be considered to yield some benefit. This inference is consistent with the current US Dietary Guidelines recommendations for dietary patterns including high amounts of vegetables and fruits, and low levels of SFAs (32). However, the present findings also bring into consideration the fact that differing apoB-containing lipoproteins have differing relations to CVD risk. In particular, as summarized elsewhere (34, 35), large LDL particles, measured by several different methodologies, have not been associated with CVD in multiple population cohorts in contrast to the associations observed for concentrations of medium, small, and/or very small LDL (26, 36–38). Although these selective relations have been challenged, in large part due to the effects of adjustment for multiple covariates (38), it is noteworthy that several studies have demonstrated their persistence after adjustment for LDL cholesterol and other lipids (36–38). Thus, the estimated impact of red meat, white meat, and dairy-derived SFA on CVD risk as reflected by their effects on LDL cholesterol and apoB concentrations may be attenuated by the lack of their effects on smaller LDL particles that are most strongly associated with CVD.
Although it is possible that the results of the present study were contingent on higher protein than is typical of US dietary intake (16), the amount provided (25% E) falls within the acceptable macronutrient distribution range for protein (10–35% E) established by the Institute of Medicine (39). The percentages of total protein derived from animal (48% E), dairy (28% E), and plant protein (16% E) in the red and white meat diets were also representative of what is habitually consumed by US adults (40). From the present study, we cannot determine whether inclusion of both red and white meat in the diet would have additive effects on plasma lipids. Likewise, it remains unclear to what extent the lipid-lowering effects of the nonmeat diet can be ascribed to components inherent to plant-based foods (e.g., plant-derived phytochemicals, micronutrients, differences in amount and type of dietary fiber) compared with the removal of red and white meat. Based on the Katan equation (http://www.katancalculator.nl/), the lower dietary cholesterol content of the nonmeat than of animal protein diets would be predicted to reduce total and LDL cholesterol by 0.0021 and 0.0018 mmol/L, respectively, suggesting that the cholesterol-lowering effect of the nonmeat diets cannot be attributed solely to differences in dietary cholesterol across diets. Nevertheless, the present findings provide robust evidence for the equivalent effect of red and white meat on both standard and more detailed plasma lipoprotein measures, and the absence of an interaction of these effects with intake of SFAs in healthy individuals.
Our results indicate that current advice to restrict red meat and not white meat should not be based on their plasma lipid effects. Indeed, other effects of unprocessed red meat consumption could contribute to adverse effects on CVD risk (41, 42). In this regard, while we found no significant effects of dietary protein source on blood pressure, fasting glucose, or endothelial reactivity, we have reported separately that the red meat dietary intervention resulted in significant increases in plasma concentrations of trimethylamine-N-oxide (43), a metabolite derived from intestinal bacterial metabolism of carnitine (44) that has been linked to incidence of CVD (44, 45). Moreover, recent meta-analyses of prospective cohort studies reported increased CVD incidence associated with processed red meat (3, 46) suggesting that preservatives such as sodium, nitrates, and their by-products may contribute to the association between total red meat intake and CVD risk. Dietary intake of heme iron, abundant in red meat, has also been associated with increased CVD risk (47), likely through mechanisms involving lipid peroxidation and inflammation.
Among the strengths of the present study are its provision of all experimental foods except fresh produce, the documentation of excellent compliance by both laboratory and clinical criteria, and the inclusion of a diverse study population. Limitations include its short-term nature, the omission from the experimental design of fish (in order to avoid potential confounding effects on lipoprotein metabolism of long-chain ω-3 fatty acids) and grass-finished beef products, and the provision of SFAs primarily from dairy sources (in order to provide standardization across the different protein sources). Finally, because animal protein sources in the present study were restricted to lean cuts and were matched for saturated fat content (2.6% total E derived from red meat SFA; 2.5% total E derived from white meat SFA), we cannot extrapolate our findings to the lipid and lipoprotein effects of higher-fat red meat products in comparison with generally leaner white meats.
The results of the present study support current dietary recommendations to adopt dietary patterns with high vegetable content, but do not provide evidence for choosing white over red meat for reducing CVD risk on the basis of plasma lipid and lipoprotein effects. Moreover, the weaker association with CVD risk of large LDL than of small LDL (26, 36–38) suggests that the impact of high intakes of red and white meat, as well as SFA from dairy sources, which selectively raised large LDL subfractions, may be overestimated by reliance on LDL cholesterol, as is the case in current dietary guidelines. Future studies should test the effects of SFA content and dietary protein source on atherogenic lipoprotein indices as well as clinical CVD outcomes in individuals with hyperlipidemia.
Supplementary Material
Acknowledgments
The authors wish to thank Cewin Chao MS, RD, MBA; Monique Schloetter RD; Laurie Herraiz RD; and the staff of the Bionutrition Unit (UCSF) for the design and preparation of study diets; Megan Bennett for participant recruitment and coordination of all clinic activities; and Barbara Sutherland PhD and Alison Brown for participant recruitment, and implementation and monitoring of the dietary protocol.
The authors’ responsibilities were as follows—NB, SC, and RMK: designed and conducted the research. NB and SC: conducted the literature searches and collected the data. PTW: carried out the statistical analysis. NB and RMK: interpreted the data and wrote the first draft of the manuscript. SMK: was responsible for managing the lipid and lipoprotein analyses. NB and RMK: accept full responsibility for the finished article, had access to all the data, and controlled the decision to publish; and all authors: read and approved the final manuscript. RMK and NB are recipients of a grant from Dairy Management Inc., but this grant is not for the submitted work; RMK holds a licensed patent for ion mobility analyses. All other authors report no conflicts of interest.
Notes
Research reported in this publication was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health under award number R01HL106003, and by the National Center for Advancing Translational Sciences, NIH, through the University of California, San Francisco Clinical & Translational Science Institute under award number UL1TR000004.
Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: apo, apolipoprotein; apoA-I, apolipoprotein A-I; apoB, apolipoprotein B; CVD, cardiovascular disease; E, energy.
References
- 1. Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122(9):876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: A prospective study of over half a million people. Arch Intern Med. 2009;169(6):562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abete I, Romaguera D, Vieira AR, Lopez de Munain A, Norat T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: A meta-analysis of cohort studies. Br J Nutr. 2014;112(5):762–75. [DOI] [PubMed] [Google Scholar]
- 4. Tharrey M, Mariotti F, Mashchak A, Barbillon P, Delattre M, Fraser GE. Patterns of plant and animal protein intake are strongly associated with cardiovascular mortality: The Adventist Health Study-2 cohort. Int J Epidemiol. 2018;47(5):1603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang T, Yang B, Zheng J, Li G, Wahlqvist ML, Li D. Cardiovascular disease mortality and cancer incidence in vegetarians: A meta-analysis and systematic review. Ann Nutr Metab. 2012;60(4):233–40. [DOI] [PubMed] [Google Scholar]
- 6. Li SS, Blanco Mejia S, Lytvyn L, Stewart SE, Viguiliouk E, Ha V, de Souza RJ, Leiter LA, Kendall CWC, Jenkins DJA et al.. Effect of plant protein on blood lipids: A systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2017;6(12):e006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denke MA. Role of beef and beef tallow, an enriched source of stearic acid, in a cholesterol-lowering diet. Am J Clin Nutr. 1994;60(6 Suppl):1044S–9S. [DOI] [PubMed] [Google Scholar]
- 8. Morgan SA, Sinclair AJ, O'Dea K. Effect on serum lipids of addition of safflower oil or olive oil to very-low-fat diets rich in lean beef. J Am Diet Assoc. 1993;93(6):644–8. [DOI] [PubMed] [Google Scholar]
- 9. Scott LW, Dunn JK, Pownall HJ, Brauchi DJ, McMann MC, Herd JA, Harris KB, Savell JW, Cross HR, Gotto AM Jr.. Effects of beef and chicken consumption on plasma lipid levels in hypercholesterolemic men. Arch Intern Med. 1994;154(11):1261–7. [PubMed] [Google Scholar]
- 10. Davidson MH, Hunninghake D, Maki KC, Kwiterovich PO Jr., Kafonek S. Comparison of the effects of lean red meat vs lean white meat on serum lipid levels among free-living persons with hypercholesterolemia: A long-term, randomized clinical trial. Arch Intern Med. 1999;159(12):1331–8. [DOI] [PubMed] [Google Scholar]
- 11. Beauchesne-Rondeau E, Gascon A, Bergeron J, Jacques H. Plasma lipids and lipoproteins in hypercholesterolemic men fed a lipid-lowering diet containing lean beef, lean fish, or poultry. Am J Clin Nutr. 2003;77(3):587–93. [DOI] [PubMed] [Google Scholar]
- 12. Melanson K, Gootman J, Myrdal A, Kline G, Rippe JM. Weight loss and total lipid profile changes in overweight women consuming beef or chicken as the primary protein source. Nutrition. 2003;19(5):409–14. [DOI] [PubMed] [Google Scholar]
- 13. Mateo-Gallego R, Perez-Calahorra S, Cenarro A, Bea AM, Andres E, Horno J, Ros E, Civeira F. Effect of lean red meat from lamb v. lean white meat from chicken on the serum lipid profile: A randomised, cross-over study in women. Br J Nutr. 2012;107(10):1403–7. [DOI] [PubMed] [Google Scholar]
- 14. O'Connor LE, Kim JE, Campbell WW. Total red meat intake of >/ = 0.5 servings/d does not negatively influence cardiovascular disease risk factors: A systemically searched meta-analysis of randomized controlled trials. Am J Clin Nutr. 2017;105(1):57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maki KC, Van Elswyk ME, Alexander DD, Rains TM, Sohn EL, McNeill S. A meta-analysis of randomized controlled trials that compare the lipid effects of beef versus poultry and/or fish consumption. J Clin Lipidol. 2012;6(4):352–61. [DOI] [PubMed] [Google Scholar]
- 16. Rehm CD, Peñalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999–2012. JAMA. 2016;315(23):2542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th ed Washington, DC: US Government Printing Office; 2010. [Google Scholar]
- 18. Krauss RM, Blanche PJ, Rawlings RS, Fernstrom HS, Williams PT. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am J Clin Nutr. 2006;83(5):1025–31.; quiz 205. [DOI] [PubMed] [Google Scholar]
- 19. Mangravite LM, Chiu S, Wojnoonski K, Rawlings RS, Bergeron N, Krauss RM. Changes in atherogenic dyslipidemia induced by carbohydrate restriction in men are dependent on dietary protein source. J Nutr. 2011;141(12):2180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Back to grass. The market potential for US grassfed beef. Stone Barns Center for Food and Agriculture, 2017:58. [Google Scholar]
- 21. Bingham SA. Urine nitrogen as a biomarker for the validation of dietary protein intake. J Nutr. 2003;133 Suppl 3(3):921S–4S. [DOI] [PubMed] [Google Scholar]
- 22. Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr. 2003;78(4):734–41. [DOI] [PubMed] [Google Scholar]
- 23. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 24. Caulfield MP, Li S, Lee G, Blanche PJ, Salameh WA, Benner WH, Reitz RE, Krauss RM. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin Chem. 2008;54(8):1307–16. [DOI] [PubMed] [Google Scholar]
- 25. Mora S, Caulfield MP, Wohlgemuth J, Chen Z, Superko HR, Rowland CM, Glynn RJ, Ridker PM, Krauss RM. Atherogenic lipoprotein subfractions determined by ion mobility and first cardiovascular events after random allocation to high-intensity statin or placebo: The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) Trial. Circulation. 2015;132(23):2220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Musunuru K, Orho-Melander M, Caulfield MP, Li S, Salameh WA, Reitz RE, Berglund G, Hedblad B, Engstrom G, Williams PT et al.. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol. 2009;29(11):1975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ryan DH, Kahan S. Guideline recommendations for obesity management. Med Clin North Am. 2018;102(1):49–63. [DOI] [PubMed] [Google Scholar]
- 28. Dreon DM, Fernstrom HA, Campos H, Blanche P, Williams PT, Krauss RM. Change in dietary saturated fat intake is correlated with change in mass of large low-density-lipoprotein particles in men. Am J Clin Nutr. 1998;67(5):828–36. [DOI] [PubMed] [Google Scholar]
- 29. Sjogren P, Rosell M, Skoglund-Andersson C, Zdravkovic S, Vessby B, de Faire U, Hamsten A, Hellenius ML, Fisher RM. Milk-derived fatty acids are associated with a more favorable LDL particle size distribution in healthy men. J Nutr. 2004;134(7):1729–35. [DOI] [PubMed] [Google Scholar]
- 30. Ferdowsian HR, Barnard ND. Effects of plant-based diets on plasma lipids. Am J Cardiol. 2009;104(7):947–56. [DOI] [PubMed] [Google Scholar]
- 31. Chiu S, Williams PT, Krauss RM. Effects of a very high saturated fat diet on LDL particles in adults with atherogenic dyslipidemia: A randomized controlled trial. PLoS One. 2017;12(2):e0170664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. US Department of Health and Human Services and US Department of Agriculture Dietary guidelines for Americans, 2015–2020. 8th ed Washington, DC: US Government Printing Office, 2015. [Google Scholar]
- 33. Eckel RH, Jakicic JM, Ard JD, Hubbard VS, de Jesus JM, Lee I-M, Lichtenstein AH, Loria CM, Millen BE, Miller NH et al.. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76–99. [DOI] [PubMed] [Google Scholar]
- 34. Krauss RM. Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol. 2010;21(4):305–11. [DOI] [PubMed] [Google Scholar]
- 35. Krauss RM. All low-density lipoprotein particles are not created equal. Arterioscler Thromb Vasc Biol. 2014;34(5):959–61. [DOI] [PubMed] [Google Scholar]
- 36. St-Pierre AC, Cantin B, Dagenais GR, Mauriege P, Bernard PM, Despres JP, Lamarche B. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Quebec Cardiovascular Study. Arterioscler Thromb Vasc Biol. 2005;25(3):553–9. [DOI] [PubMed] [Google Scholar]
- 37. Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E et al.. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: The Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014;34(5):1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, Hoefner DM, Remaley AT. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: The Multi-ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34(1):196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Institute of Medicine of the National Academies. Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Protein and Amino Acids (Macronutrients). Washington, DC: The National Academies Press; 2002/2005. [Google Scholar]
- 40. Pasiakos SM, Agarwal S, Lieberman HR, Fulgoni VL 3rd. Sources and amounts of animal, dairy, and plant protein intake of US adults in 2007–2010. Nutrients. 2015;7(8):7058–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: Results from 2 prospective cohort studies. Arch Intern Med. 2012;172(7):555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang X, Lin X, Ouyang YY, Liu J, Zhao G, Pan A, Hu FB. Red and processed meat consumption and mortality: Dose-response meta-analysis of prospective cohort studies. Public Health Nutr. 2016;19(5):893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WH et al.. Impact of chronic dietary red meat, white meat and non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;Feb 14; 40(7):583-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L et al.. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur Heart J. 2017;38(39):2948–56. [DOI] [PubMed] [Google Scholar]
- 46. Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes—an updated review of the evidence. Curr Atheroscler Rep. 2012;14(6):515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fang X, An P, Wang H, Wang X, Shen X, Li X, Min J, Liu S, Wang F. Dietary intake of heme iron and risk of cardiovascular disease: A dose-response meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis. 2015;25(1):24–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.