ABSTRACT
Background
Preclinical evidence suggests that modulation of the gut microbiome could represent a new therapeutic target in nonalcoholic fatty liver disease (NAFLD).
Objectives
The aim of this study was to evaluate the most current evidence for liver-specific and metabolic effects of microbiome-targeted therapies (MTTs) in persons with NAFLD.
Methods
We searched multiple electronic databases for randomized controlled trials (RCTs) published from January 1, 2005 to December 1, 2018 that enrolled persons with NAFLD who received MTT rather than placebo or usual care. MTT was defined as antibiotics, probiotics, synbiotics, or fecal microbiota transplantation (FMT). Clinical outcomes were pooled with the use of random-effects models and heterogeneity was assessed with the I2 statistic. A random-effects meta-regression was performed to determine sources of heterogeneity in prevalence estimates between studies.
Results
Twenty-one RCTs (1252 participants) were included; 9 evaluated probiotics and 12 evaluated synbiotics, with treatment duration ranging from 8 to 28 wk. No RCTs examined the efficacy of antibiotics or FMT. Probiotics/synbiotics were associated with a significant reduction in alanine aminotransferase activity [ALT, weighted mean difference (WMD): −11.23 IU/L; 95% CI: −15.02, −7.44 IU/L] and liver stiffness measurement (LSM) by elastography (reflecting inflammation and fibrosis) (WMD: −0.70 kPa; 95% CI: −1.00, −0.40 kPa), although analyses showed heterogeneity (I2 = 90.6% and I2 = 93.4%, respectively). Probiotics/synbiotics were also associated with increased odds of improvement in hepatic steatosis, as graded by ultrasound (OR: 2.40; 95% CI: 1.50, 3.84; I2 = 22.4%). No RCTs examined sequential liver biopsy findings. Probiotics (WMD: −1.84; 95% CI: −3.30, −0.38; I2 = 23.6%), but not synbiotics (WMD: −0.85; 95% CI: −2.17, 0.47; I2 = 96.6%), were associated with a significant reduction in body mass index.
Conclusions
The use of probiotics/synbiotics was associated with improvement in liver-specific markers of hepatic inflammation, LSM, and steatosis in persons with NAFLD. Although promising, given the heterogeneity in pooled analyses, additional well-designed RCTs are needed to define the efficacy of probiotics/synbiotics for treatment of NAFLD. This study was registered with PROSPERO as CRD42018091455.
Keywords: nonalcoholic fatty liver, microbiome, probiotics, synbiotics, fecal microbiota transplantation
Introduction
As a result of the obesity pandemic, nonalcoholic fatty liver disease (NAFLD) has emerged as the leading cause of chronic liver disease in the developed world, and its prevalence is rapidly increasing (1). NAFLD, which affects both children and adults, encompasses a spectrum of histopathologic abnormalities ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), a more severe phenotype of disease. NASH is associated with cirrhosis, hepatocellular carcinoma, and liver-related mortality, highlighting the serious consequences of overnutrition and the metabolic syndrome on liver health (2). Despite the overwhelming burden of NAFLD, there are no approved pharmacologic therapies for NAFLD in the United States (3). Lifestyle modification, involving exercise and a change in diet, remains the mainstay of therapy, but achievement of sustained weight loss is difficult for most patients (4).
An ever-increasing body of literature suggests that perturbation to the gut microbiome, a complex and diverse community of microbes residing in the human intestine, is implicated in the pathogenesis of NAFLD (5). Numerous clinical studies have demonstrated that NAFLD is associated with a decrease in overall bacterial diversity and enrichment in certain Gram-negative bacterial taxa of the gut microbiome (6). Preclinical studies have demonstrated that therapies targeting the gut microbiome, including probiotics and antibiotics, inhibit the development of diet-induced obesity and hepatic steatosis and improve insulin resistance in murine models (7–11). As such, there is great interest in the potential for the human gut microbiome to serve as a target for therapeutic intervention in NAFLD.
Microbiome-targeted therapies (MTTs) have been proposed as a way to manipulate the gut microbiome and can be considered in several categories, namely probiotics, synbiotics, antibiotics, and fecal microbiota transplantation (FMT). Probiotics are defined as a culture of living microorganisms which could have health benefits for the human host if consumed in adequate amounts (12). Synbiotics are a combination of probiotics and prebiotics, in which prebiotics are composed of fermentable dietary fibers (e.g., inulin, fructo-oligosaccharides) that stimulate the growth and survival of probiotics (12). FMT is a procedure in which stool is collected from a healthy donor and transferred to a patient, via a range of delivery routes including colonoscopy, nasogastric tube, and enema (13).
In 2007, a Cochrane Review did not identify any randomized clinical trials with probiotics in NAFLD and determined that there was no evidence to support or refute the use of probiotics in the NAFLD patient population (14). Over the past decade, there has been an increase in the number of clinical trials examining the role of probiotics in NAFLD. A few recent meta-analyses have examined the role of probiotics in NAFLD, albeit with conflicting results and limitations. These limitations include limiting analysis to a single type of MTT (e.g., only probiotics), focusing on outcomes of insulin resistance and lipid profiles (and not liver-specific outcomes), and inclusion of studies that enrolled obese adults with metabolic risk factors but without definite NAFLD (15–18). Given the limitations in the current literature, we aimed to conduct a systematic review, meta-analysis, and meta-regression in order to evaluate the most current evidence for both liver-specific effects and metabolic effects of all MTTs in adults and children with NAFLD.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement was used to guide the conduct and reporting of the study (Supplemental Methods). We established a protocol for the review, which was registered with PROSPERO prior to commencing the study (https://www.crd.york.ac.uk/prospero/, CRD42018091455, and Supplemental Methods).
Study selection
Randomized controlled trials (RCTs) that compared MTT with placebo, usual care, or no intervention in patients with NAFLD were eligible for inclusion. MTT was defined as interventions in any of the following 4 categories: antibiotics, probiotics, synbiotics, or FMT. Prebiotics were not included as MTT for the purpose of this review. To be eligible, RCTs needed to meet the following criteria: 1) NAFLD was defined by either liver histology or noninvasive imaging modality (MRI, ultrasound, or elastography); 2) duration of therapy was ≥4 wk (excluding FMT trials), and 3) one of the following outcomes was assessed: serum alanine aminotransferase (ALT) concentration, BMI, insulin resistance (HOMA-IR or glycated hemoglobin), triglycerides (TGs), or assessment of change in liver disease severity (steatosis or fibrosis, or a combination of the two) by any of the following modalities: MRI, elastography, or liver biopsy. Specifically, MRI outcomes could include estimated fibrosis stage and MRI-determined proton density fat fraction. Elastography outcomes could include liver stiffness measurement (LSM), reflecting the combined effects of hepatic inflammation and fibrosis, or the controlled attenuation parameter, which estimates hepatic steatosis (19). Liver biopsy outcomes could include scoring of fibrosis stage, ballooning degeneration, steatosis, or lobular inflammation. No restrictions were placed on the setting or context of the included studies. Multiarm trials were eligible for inclusion if there were MTT and placebo groups. Only studies performed in human subjects and published in English were considered. Unpublished studies and studies published only in abstract format were excluded.
Identification and selection of studies
The electronic databases PubMed/MEDLINE, EMBASE, and the Cochrane Library were searched from January 1, 2005 to December 1, 2018. A secondary search was performed where reference lists of all relevant reviews and trials were screened for additional studies. The search strategy was peer-reviewed by an information specialist (Supplemental Methods). During the first screening, 2 reviewers (SRS and BM) evaluated the titles and abstracts of each citation and excluded clearly irrelevant studies. For each potentially eligible study, 2 reviewers (SRS and BM) independently examined the full-text article and assessed whether the study fulfilled the inclusion criteria. Disagreements were resolved through consensus or referral to a third reviewer (NAT). The number of studies identified by the search and excluded at various stages was recorded in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses study flow diagram.
Data extraction and study appraisal
Two independent reviewers (SRS and EH-T) extracted data from each trial with the use of a predesigned, standardized data-extraction form. The data sought included: 1) study characteristics (year of publication, dates of enrollment, inclusion and exclusion criteria, total number of patients enrolled, country where study performed); 2) baseline patient characteristics (age, liver disease, obesity); 3) intervention characteristics (type of MTT, duration of therapy); and 4) clinical outcomes, as previously defined.
All studies were assessed for methodologic quality with the use of the Cochrane Risk of Bias tool (20). If ≥1 of the domains was rated as high, the trial was considered at high risk of bias. If all domains were judged as low, the trial was considered at low risk of bias. Otherwise, the trial was considered as having an unclear risk of bias.
Synthesis and statistical analysis
Clinical heterogeneity was assessed by grouping studies by intervention and outcome. If there were ≥3 trials within a single grouping, data were pooled through the use of random-effects meta-analysis. Analyses were stratified by type of MTT. Primary outcomes were liver-related outcomes, specifically serum ALT level, change in hepatic fibrosis via elastography (LSM), and change in hepatic steatosis via ultrasound. Secondary outcomes included metabolic outcomes, specifically change in BMI, TG, and insulin resistance (as measured by HOMA-IR). The following continuous outcomes were assessed: serum ALT, LSM, BMI, TG, and HOMA-IR. For continuous outcomes, we analyzed the difference in means and 95% CIs. Among studies that assessed change in hepatic steatosis by ultrasound grading and categorization (none, mild, moderate/severe), the number of patients with moderate/severe steatosis at baseline who experienced improvement by ≥1 grade at the end of the trial was analyzed. For this dichotomous outcome, we calculated the OR and 95% CI. For categoric data, we extracted details about each category assessed and the numbers of patients with an outcome in each category. Heterogeneity was investigated through the use of forest plots and the I2 statistic. Where data were considered too heterogeneous to pool or not reported in a format suitable for pooling (e.g., data reported as medians or in different units of measurement), we used a narrative synthesis.
Analyses were stratified based on type of MTT. Sensitivity analyses were performed to account for methodologic quality differences, in which trials with high risk of bias were excluded. To explain heterogeneity between studies and to examine the influence of patient-level and intervention-level factors on clinical outcomes, meta-regression was performed. The following factors were studied when feasible: duration of MTT (≤8 wk, >8 wk), type of MTT, age of enrolled patient population [children and adolescents (<18 y), adults (≥18 y)], and liver disease (NASH as defined by liver histology). When feasible, subgroup analyses were performed with exclusion of RCTs that enrolled pediatric patients (<18 y). Publication bias was assessed through the use of Begg's rank correlation test and Egger's regression test. All reported results of publication bias are Begg's rank correlation test unless otherwise specified.
Statistical significance was assessed at an α level of 0.05. All reported P values are 2-sided. All statistical analyses were performed with Stata version 14.2 (College Station, TX).
Results
Included studies
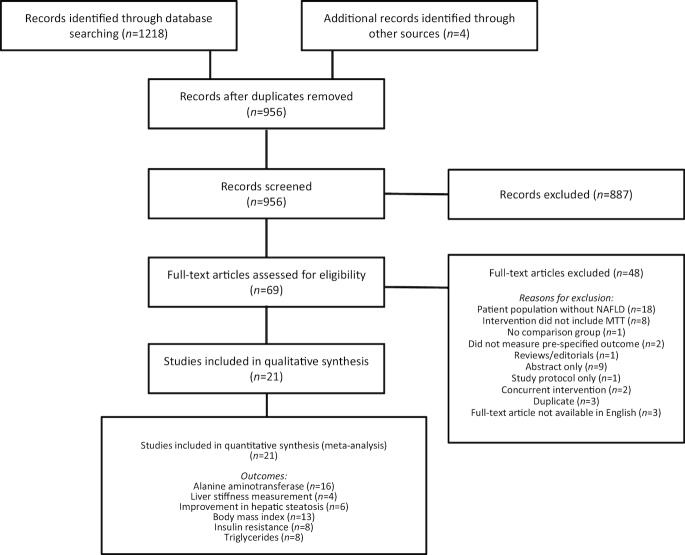
The study selection process is summarized in Figure 1. The search identified 1,218 records, of which 266 were duplicates (identified by >1 electronic database). Based on title and abstract screening, 69 records were considered potentially relevant and were obtained as full-text studies. After full-text review, a total of 21 RCTs (1,252 participants) that evaluated MTT in NAFLD were included (21–41). The characteristics of included trials are summarized in Table 1. Nine RCTs evaluated probiotics in the management of NAFLD, among which 3 enrolled only pediatric patients (<18 y) (21–29). Twelve RCTs evaluated synbiotics in the management of NAFLD, all of which were performed in adults (29–38, 40, 41). No studies that evaluated antibiotics or FMT for treatment of NAFLD fulfilled the inclusion criteria. Probiotic formulations, including the probiotic component of synbiotics, predominantly comprised multiple bacterial species. The duration of probiotic or synbiotic intervention ranged from 8 to 28 weeks. The majority of studies (16 of 21, 67%) utilized placebo as a comparator. One study evaluated a synbiotic as an adjunctive treatment to metformin, where both the intervention and control groups received metformin (36). Another study evaluated a synbiotic as an adjunctive treatment to sitagliptin, where both the intervention and control groups received sitagliptin (40).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram: flow of studies through the review process. MTT, microbiome-targeted therapy; NAFLD, nonalcoholic fatty liver disease.
TABLE 1.
Characteristics of included trials, stratified by probiotics and synbiotics1
| First author (publication year) | Patient population | Total sample size | Intervention of experimental group | Duration, wk | Comparison | Outcome1 | Risk of bias2 |
|---|---|---|---|---|---|---|---|
| Probiotic intervention | |||||||
| Alisi (21) (2014) | Pediatric | 48 | VSL#3 (multispecies) | 16 | Placebo | Steatosis on US*, ALT, TGs, HOMA-IR, BMI | Low |
| Aller (22) (2011) | Adult | 30 | Lactobacillus bulgaricus and Streptococcus thermophilus | 12 | Placebo | ALT*, TB, BMI, weight, TC, TGs, FPG, HOMA-IR, IL-6, TNFα | Low |
| Behrouz (23) (2017) | Adult | 111 | Webber Naturals (multispecies) | 16 | Placebo | BMI, weight, FPG, insulin, HOMA-IR, leptin, % body fat, adiponectin | Low |
| Famouri (24) (2016) | Pediatric | 64 | Prokid (multispecies) | 12 | Placebo | Steatosis on US*, ALT, AST, BMI, weight, TC, WC | Low |
| Kobyliak (25) (2018) | Adult | 58 | Symbiter (multispecies) | 8 | Placebo | LSM*, FLI*, AST, ALT, TC, TNFα, IL-6, IL-8, IFNγ | Low |
| Monem (26) (2017) | Adult | 30 | Lactobacillus acidophilus | 4 | Usual care | ALT, AST, albumin, TB | High |
| Nabavi (27) (2014) | Adult | 72 | Probiotic yogurt (multispecies) | 8 | Placebo (conventional yogurt) | ALT, AST, TC, steatosis on US | Unclear |
| Sepideh (28) (2016) | Adult | 50 | Lactocare (multispecies) | 8 | Placebo | FPG, insulin, HOMA-IR, TNFα, IL-6, HbA1c | Unclear |
| Vajro (29) (2011) | Pediatric | 20 | Lactobacillus rhamnosus | 8 | Placebo | ALT*, steatosis on US, TNFα | Unclear |
| Synbiotic intervention | |||||||
| Asgharian (30) (2016) | Adult | 80 | Familact (multispecies + FOS) | 8 | Placebo | ALT*, AST*, steatosis on US, BMI, weight, CRP | Low |
| Bakhshimoghaddam (41) (2018) | Adult | 102 | Synbiotic yogurt (Bifidobacterium animalis + inulin) | 24 | Usual care or conventional yogurt | Steatosis on US*, ALT, AST, GGT, TC, FPG, insulin, GLP-2 | Unclear |
| Ekhlasi (31) (2016) | Adult | 30 | Protexin (multispecies + FOS) | 8 | Placebo | ALT*, AST, TC, TG, BMI, FPG, HOMA-IR, leptin, Apo A-1 | Low |
| Eslamparast (39) (2014) | Adult | 52 | Protexin (multispecies + FOS) | 28 | Placebo | ALT*, AST, TB, GGT, ALP, LSM, BMI, CRP, TNFα | Low |
| Ferolla (32) (2016) | Adult | 50 | Lactobacillus reuteri + inulin | 12 | Usual care | MRI PDFF*, ALT, AST, BMI, TC, TG, WC, uric acid | High |
| Javadi (33) (2017) | Adult | 36 | Bifidobacterium longum + Lactobacillus acidophilus + inulin | 12 | Placebo | ALT*, BMI, weight, TB, ALP | Low |
| Malaguarnera (34) (2011) | Adult | 66 | Bifidobacterium longum + FOS | 24 | Placebo | NAS*, ALT, AST, GGT, TB, TC, TG, FPG, insulin, CRP, TNFα | Low |
| Manzhalii (35) (2017) | Adult | 75 | Multispecies probiotic + FOS | 12 | Usual care | ALT*, AST, LSM, TC, TG, BMI | High |
| Mofidi (38) (2016) | Adult | 50 | Protexin (multispecies + FOS) | 28 | Placebo | CAP*, LSM, ALT, AST, FPG, HOMA-IR, TC, TG | Unclear |
| Sayari (40) (2018) | Adult | 138 | Familact (multispecies + FOS) | 16 | Placebo (sitagliptin in both groups) | BMI, FPG, ALT, AST, TC, TG | Low |
| Shavakhi (36) (2013) | Adult | 70 | Protexin (multispecies + FOS) | 24 | Placebo (metformin in both groups) | ALT*, AST*, steatosis on US*, BMI, FPG, TC, TG | Unclear |
| Wong (37) (2013) | Adult | 20 | Lepicol (multispecies + FOS) | 24 | Usual care | IHTG*, ALT, AST, BMI, FPG, LSM, TC, TG | High |
The prespecified primary outcome of each study is labeled with an asterisk (*). ALP, alkaline phosphatase; ALT, alanine aminotransferase; Apo, apolipoprotein; AST, aspartate aminotransferase; CAP, controlled attenuation parameter; CRP, C-reactive protein; FPG, fasting plasma glucose; FLI, fatty liver index; FOS, fructo-oligosaccharides; GGT, γ-glutamyltransferase; GLP-2, glucagon-like peptide 2; HbA1c, glycated hemoglobin; IFNγ, interferon γ; IHTG, intrahepatic triglyceride content; LSM, liver stiffness measurement; NAS, NAFLD activity score; PDFF, proton density fat fraction; TB, total bilirubin; TC, total cholesterol; TG, triglyceride; US, abdominal ultrasound; WC, waist circumference.
Assessed via the Cochrane Risk of Bias Tool.
Additional study characteristics are listed in Supplemental Table 1. Studies were conducted in a wide range of countries, including Iran, Italy, Germany, Spain, Ukraine, Egypt, Brazil, and China (Supplemental Table 1). Among the 21 included studies, 7 (33%) utilized histologic criteria (i.e., performance of liver biopsy) to define NAFLD. All included RCTs utilized a parallel group design; 10 of 21 (47%) studies utilized a BMI cutoff as part of the study inclusion criteria.
Overall, 11 (52%) trials were judged at low risk of bias; 4 (19%) were judged at high risk of bias; and 6 (29%) were judged at unclear risk of bias (Supplemental Table 2). A common potential source of bias in trials was blinding of participants. All studies (n = 47) that were excluded after full-text review, along with the primary reason for exclusion, are listed in Supplemental Table 3.
ALT activity
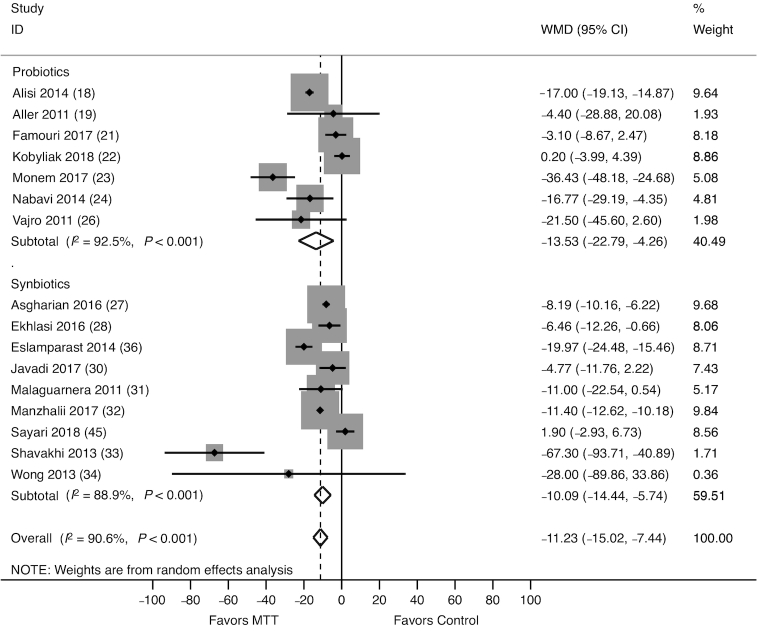
The effect of probiotics/synbiotics on ALT activity was assessed in 16 studies (889 participants), of which 7 evaluated probiotics (21, 22, 24–27, 29) and 9 evaluated synbiotics (30, 31, 33–37, 39, 40). Altogether, probiotics/synbiotics were associated with a significant reduction in ALT [weighted mean difference (WMD): −11.23 U/L; 95% CI: −15.02, −7.44 U/L; Figure 2]. Both probiotics (WMD: −13.53 U/L; 95% CI: −22.79, −4.26 U/L) and synbiotics (WMD: −10.09 U/L; 95% CI: −14.44, −5.74 U/L) were associated with a greater reduction in ALT compared with control.
FIGURE 2.
Forest plot of the effect of MTT on serum ALT, stratified by probiotics and synbiotics, and measured by the WMD. Probiotics/synbiotics were associated with a significant reduction in ALT compared with control. ALT, alanine aminotransferase; MTT, microbiome-targeted therapy; WMD, weighted mean difference.
Significant heterogeneity among the studies was identified (I2 = 90.6%, P < 0.001). In a sensitivity analysis that excluded RCTs with high risk of bias, probiotics/synbiotics remained significantly associated with a reduction in ALT compared with control (WMD: −11.32 U/L; 95% CI: −16.22, −6.34 U/L). A subgroup analysis including only trials that enrolled adult patients revealed that probiotics/synbiotics remained significantly associated with reduction in ALT (WMD: −11.16 U/L; 95% CI: −15.44, −6.87 U/L). Meta-regression analysis revealed no significant relation of MTT type (probiotics compared synbiotics, P = 0.82), MTT duration >8 wk (P = 0.75), and patient age >18 y (P = 0.90) on heterogeneity. However, there was a significant effect of NASH on heterogeneity (P = 0.01). A subgroup analysis limited only to studies that enrolled patients with NASH revealed that probiotics/synbiotics were associated with a significant reduction in ALT (WMD: −35.81 U/L; 95% CI: −56.64, −14.93 U/L). There was no evidence of publication bias (P = 0.28).
LSM
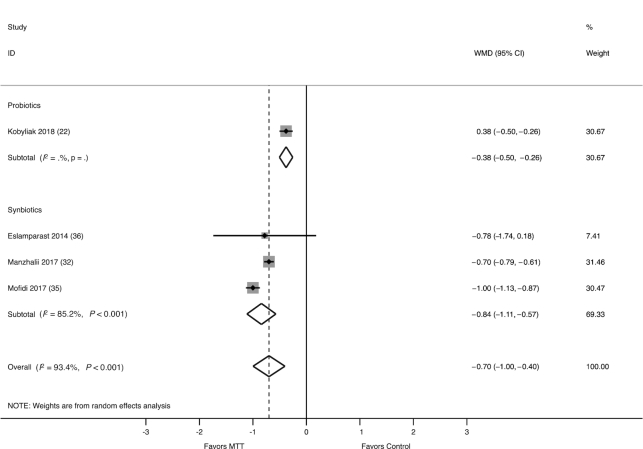
The effect of probiotics/synbiotics on LSM, as measured by elastography, was assessed in 4 studies (235 participants), of which 1 evaluated probiotics (25) and 3 evaluated synbiotics (35, 38, 39). The combined WMD in LSM was −0.70 kPa (95% CI: −1.00, −0.40 kPa), favoring benefit with probiotics/synbiotics as compared with control (Figure 3). There was significant heterogeneity between studies (I2 = 93.4%, P < 0.001), and meta-regression analysis was not feasible due to the limited number of studies. A sensitivity analysis excluding 1 study deemed at high risk of bias did not change the results (WMD: −2.12 kPa; 95% CI: −3.96, −0.28 kPa) and there was no evidence of publication bias (P = 0.95).
FIGURE 3.
Forest plot of the effect of MTT on LSM, as measured by elastography, stratified by probiotics and synbiotics, and measured by the WMD. Probiotics/synbiotics were associated with a significant reduction in LSM compared with control. LSM, liver stiffness measurement; MTT, microbiome-targeted therapy; WMD, weighted mean difference.
Hepatic steatosis
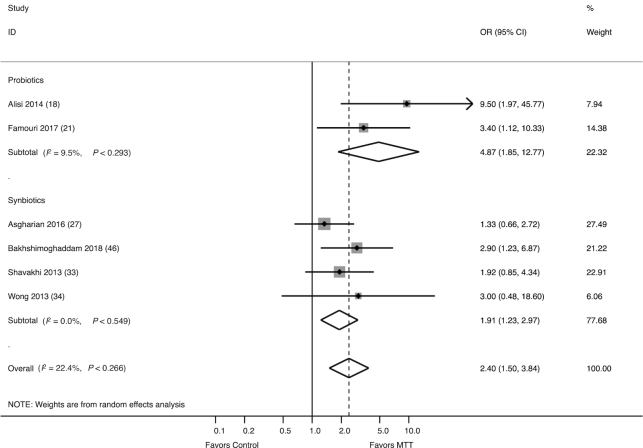
The effect of probiotics/synbiotics on improvement in hepatic steatosis, as graded by liver ultrasound, was assessed in 6 studies (384 participants), of which 2 evaluated probiotics (21, 24) and 4 evaluated synbiotics (30, 36, 37, 41). Together, probiotics/synbiotics were associated with increased odds of having improvement from moderate/severe hepatic steatosis (OR: 2.40; 95% CI: 1.50, 3.84) compared with control (Figure 4). This association remained evident in a sensitivity analysis in which 1 study with high risk of bias was excluded (OR: 2.42; 95% CI: 1.21, 4.82), and there was no significant heterogeneity between studies (I2 = 22.4%, P = 0.27).
FIGURE 4.
Forest plot of the effect of MTT on improvement in hepatic steatosis, as graded by ultrasound, stratified by probiotics and synbiotics. Probiotics/synbiotics were associated with increased odds of having improvement from moderate/severe hepatic steatosis compared with control. MTT, microbiome-targeted therapy.
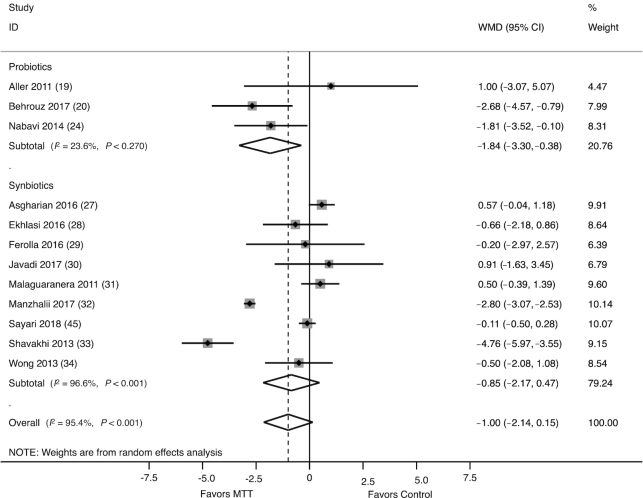
BMI
The effect of probiotics/synbiotics on BMI was assessed in 12 studies (778 participants), of which 3 evaluated probiotics (21–23, 27) and 9 evaluated synbiotics (30–37, 40). Pediatric studies were excluded from analyses examining changes in BMI. Probiotics (WMD: −1.84 kg/m2; 95% CI: −3.30, −0.38 kg/m2), but not synbiotics (WMD: −0.85 kg/m2; 95% CI: −2.17, 0.47 kg/m2), were associated with a significantly greater reduction in BMI compared with control (Figure 5). There was significant heterogeneity between synbiotic studies (I2 = 96.6%, P < 0.001) but not amongst probiotic studies (I2 = 23.6%, P = 0.12). In a sensitivity analysis that excluded RCTs with high risk of bias, the overall effect of probiotics/synbiotics on BMI was attenuated but remained significant (WMD: −0.99 kg/m2: 95% CI: −1.96, −0.01 kg/m2). Meta-regression analysis revealed no significant effect of MTT type (P = 0.65), MTT duration >8 wk (P = 0.73), patient age >18 y (P = 0.66), or NASH (P = 0.84) on heterogeneity. There was no evidence of publication bias (P = 0.92).
FIGURE 5.
Forest plot of the effect of MTT on BMI, stratified by probiotics and synbiotics, and measured by the WMD. Probiotics/synbiotics were associated with a significant reduction in BMI compared with control. MTT, microbiome-targeted therapy; WMD, weighted mean difference.
TGs
The effect of probiotics/synbiotics on TG was assessed in 8 studies (504 participants), of which 4 evaluated probiotics (21–23, 28) and 4 evaluated synbiotics (31, 34, 38, 40). Neither probiotics (WMD: 3.30 mg/dL; 95% CI: −9.36, 15.96 mg/dL) nor synbiotics (WMD: −15.78 mg/dL; 95% CI: −33.16, 1.60 mg/dL) were associated with a greater reduction in TG compared with control (Supplemental Figure 1). No studies that examined TG were deemed to have a high risk of bias, and therefore a sensitivity analysis was not performed. There was no significant heterogeneity in probiotic analyses (I2 = 44.9%, P = 0.14), but there was significant heterogeneity in synbiotic analyses (I2 = 83.9%, P < 0.00). No publication bias was detected (P = 0.23).
Insulin resistance: HOMA-IR
The effect of probiotics/synbiotics on insulin resistance, as measured by HOMA-IR, was assessed in 8 studies (487 participants), of which 4 evaluated probiotics (21–23, 28) and 4 evaluated synbiotics (31, 34, 38, 40). Altogether, probiotics/synbiotics were not associated with a significant improvement in HOMA-IR (WMD: −0.41 mg/dL × μmol/mL/405; 95% CI: −1.37, 0.55 mg/dL × μmol/mL/405) compared with control (Supplemental Figure 2), and this did not vary by type of MTT. There was significant heterogeneity between studies (I2 = 99.3%, P < 0.001); however, this was not explained by MTT duration >8 wk (P = 0.74) or patient age >18 y (P = 0.56). No studies were deemed at high risk of bias, and no evidence of publication bias was found (P = 0.76).
Discussion
NAFLD, the hepatic manifestation of the metabolic syndrome, is anticipated to become the leading worldwide cause of end-stage liver disease in the coming decades, but effective treatments are lacking. Given mounting preclinical evidence that supports a strong association between the gut microbiome and NAFLD, there is increasing interest in the use of MTTs in the management of NAFLD. In this meta-analysis, a comprehensive assessment of the effect of MTTs on a wide array of liver-specific and metabolic outcomes was conducted. We identified a total of 21 RCTs that evaluated probiotics or synbiotics in NAFLD. Our findings suggest that both probiotics and synbiotics may have beneficial effects on key liver-specific outcomes. Specifically, probiotics/synbiotics were associated with a significant reduction in LSM as well as greater odds of a reduction in hepatic steatosis. Although there was also an association between probiotics/synbiotics and reduction in ALT, persons with definite NASH experienced the greatest improvement in ALT with these MTTs.
The association of probiotics/synbiotics with components of the metabolic syndrome was less consistent. Probiotics/synbiotics were associated with a significant reduction in BMI, although this effect was predominantly driven by probiotic and not synbiotic trials. However, probiotics/synbiotics were not associated with a significant improvement in insulin resistance, as measured by HOMA-IR, and the favorable effect of probiotics/synbiotics on TGs was only seen with synbiotics compared with control. Given the reduced levels of ALT and improvement in hepatic steatosis, the lack of effect of probiotics/synbiotics on insulin resistance and the less-apparent effect on serum TGs was unexpected. This could be reflecting differential effects of probiotics/synbiotics on hepatic insulin resistance compared with other tissues. Future studies may benefit from additional measures of insulin sensitivity and resistance to better delineate the underpinnings of the probiotic/synbiotic association with glucose homeostasis.
There were no meaningful differences in the efficacy of probiotics in children and adults with NAFLD, although this comparison was limited by the relative paucity of trials examining probiotics in the pediatric population (and no trials examined synbiotics in children/adolescents). Moreover, subgroup analyses including only trials that enrolled adult participants revealed consistent associations with probiotics and liver and metabolic outcomes. Although the composition of the gut microbiota stabilizes in adulthood, the gut microbial community is dynamic during childhood (12). Several studies have found perturbations in the gut microbiota in children with NAFLD, which may differ from gut microbial dysbiosis noted in adults with this disease (42–44). As a result, one would expect that response to probiotics/synbiotics could vary based on patient age, but there were limited data to draw any conclusions about differential response to probiotics/synbiotics in childhood compared with adulthood. Nevertheless, further studies examining the role of MTTs in the management of NAFLD in the pediatric patient population are important.
There was significant overlap in the probiotic and synbiotic formulations utilized in included studies. The majority of probiotics were multispecies formulations predominantly comprising Lactobacillus or Bifidobacteria strains, or combinations of these. However, it is worth noting that there is a lack of standardization in the formulation and viability of currently marketed probiotic/synbiotic supplements (45), which makes direct comparisons amongst formulations difficult. Although a distinct gut microbial signature has yet to be discovered in NAFLD (5), changes in the relative abundance of Lactobacillus, Bifidobacterium, and Streptococcus genera have been consistently reported in varying clinical phenotypes of NAFLD (46), providing biological plausibility for the positive effects noted with the probiotic/symbiotic formulations utilized in the majority of included RCTs. However, host factors such as diet and genetics can both interact with the gut microbiome composition and elicit varying host responses to MTT (12), and, ultimately, our analyses did not yield specific insights into the ideal composition or duration of MTT for the treatment of NAFLD. Additional mechanistic studies to decipher the exact role of the gut microbiota in the pathogenesis of NAFLD, and how this may differ based on patient-specific factors and environmental exposures, may guide the development of MTTs that are more precisely formulated for the management of NAFLD.
The number of trials examining the efficacy of MTTs in NAFLD has increased over the past 10 y, but there are limitations in the current evidence. First, we did not identify RCTs that have examined the efficacy of either antibiotics or FMT for the treatment of NAFLD. To date, FMT trials in human subjects have been limited to obese adults with metabolic syndrome but without defined NAFLD. FMT from lean to obese donors was shown to improve insulin sensitivity, albeit with only short-term improvement (47, 48). These intriguing results suggest that FMT may provide therapeutic benefit in NAFLD, and at least 2 clinical trials examining FMT in adults with biopsy-confirmed NASH are actively recruiting subjects (Clinicaltrials.gov, NCT02469272 and CT02469272). Second, the number of trials including patients with biopsy-proven NAFLD was modest, and even fewer focused only on NASH, the histologic type at highest risk of liver-related complications (3). As such, there was likely significant variation in the severity of baseline liver disease amongst studies and we could not fully compare the efficacy of probiotics/synbiotics in different histologic phenotypes of NAFLD. Although heterogeneity in the analyses could in part be attributed to liver disease phenotype (histologic-confirmed NASH compared with not), this could not be explained by additional patient-level factors such as the age of the patient population (pediatric compared with adult) or by intervention-level factors including duration of probiotic/synbiotic therapy. Additionally, the majority of trials did not perform sequential liver biopsies or evaluate other measures of hepatic steatosis, such as the controlled attenuation parameter via transient elastography or proton density fat fraction via MRI, and such measures should be included in future trials (49). Although the liver-specific outcomes measured in our analyses may serve as surrogate markers of heptic necroinflammation and liver fibrosis, longitudinal histologic assessment of NAFLD remains standard, when compared with noninvasive measurements. Change in hepatic steatosis as graded by ultrasound is not a well-validated measure of disease improvement and is subject to interobserver variability. However, improvement in hepatic steatosis by ultrasound was reported on a similar categoric scale in 6 RCTs (with grading performed by blinded evaluators in all 6 studies), and therefore this was the most reliable measure of change in hepatic steatosis that could be pooled for analysis. Despite these limitations, we believe these results are encouraging and support the consideration of larger, well-designed studies to evaluate MTT as treatment for NAFLD. This is especially relevant given the low cost of probiotics and synbiotics and the relative paucity of major adverse effects (50).
In summary, modulation of the gut microbiome through administration of probiotics or synbiotics could represent a promising new therapeutic strategy in NAFLD. Our results corroborate findings from preclinical studies (6–10) and should prompt larger trials in patients with biopsy-proven NAFLD to further delineate the efficacy of MTTs in NAFLD.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—SRS, NAT: study concept and design; SRS, BM, and EH-T: data collection; SRS and EV: statistical analysis; SRS, EV, and NAT: interpretation of data: SRS: drafting of the manuscript; and all authors: have critically revised and approved the final version. NAT has institutional grant support from Allergan and Gilead Sciences; all other authors have no conflicts of interest.
Notes
SRS is supported by a Hepatology Training Grant (2T32DK060414-16) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). NAT is supported by NIDDK U01.
Supplemental Methods, Supplemental Tables 1–3, and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ALT, alanine aminotransferase; FMT, fecal microbiota transplantation; LSM, liver stiffness measurement; MTT, microbiome-targeted therapy; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; RCT, randomized controlled trial; TG, triglyceride; WMD, weighted mean difference.
REFERENCES
- 1. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–72. [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM, Loomba R, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L, Negro F, Caldwell SH, Ratziu V et al.. Current and future therapeutic regimens for non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Hepatology. 2018;68(1):361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marek RJ, Coulon SM, Brown LD, Lydecker JA, Marek S, Malcolm R, O'Neil PM. Characteristics of weight loss trajectories in a comprehensive lifestyle intervention. Obesity. 2017;25(12):2062–7. [DOI] [PubMed] [Google Scholar]
- 5. Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412–25. [DOI] [PubMed] [Google Scholar]
- 7. Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, Song XY, Diehl AM. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–50. [DOI] [PubMed] [Google Scholar]
- 8. Mencarelli A, Cipriani S, Renga B, Bruno A, D'Amore C, Distrutti E, Fiorucci S. VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation. PLoS One. 2012;7:e45425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okubo H, Sakoda H, Kushiyama A, Fujishiro M, Nakatsu Y, Fukushima T, Matsunaga Y, Kamata H, Asahara T, Yoshida Y et al.. Lactobacillus casei strain Shirota protects against nonalcoholic steatohepatitis development in a rodent model. Am J Physiol Gastrointest Liver Physiol. 2013;305:G911–8. [DOI] [PubMed] [Google Scholar]
- 10. Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A et al.. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–94. [DOI] [PubMed] [Google Scholar]
- 11. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. [DOI] [PubMed] [Google Scholar]
- 12. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375:2369–79. [DOI] [PubMed] [Google Scholar]
- 13. Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, Moore T, Wu G. Update on FMT 2015: indications, methodologies, mechanisms and outlook. Gastroenterology. 2015;149:223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lirussi F, Orando S, Orlando R. Probiotics for non-alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst Rev. 2007 Jan 24;(1):CD005165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. S Lavekar A, V Raje D, Manohar T, A Lavekar A. Role of probiotics in the treatment of nonalcoholic fatty liver disease: a meta-analysis. Euroasian J Hepatogastroenterol. 2017;7:130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao X, Zhu Y, Wen Y, Liu G, Wan C. Efficacy of probiotics in non-alcoholic fatty liver disease in adult and children: a meta-analysis of randomized controlled trials. Hepatol Res. 2016;46:1226–33. [DOI] [PubMed] [Google Scholar]
- 18. Loman BR, Hernandez-Saavedra D, An R, Rector RS. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Nutr Rev. 2018;76(11):822–39. [DOI] [PubMed] [Google Scholar]
- 19. Sasso M, Miette V, Sandrin L, Beaugrand M. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol. 2012;36(1):13–20. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39:1276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aller R, Luis D, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, De La Fuente B, Gonzalez J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15:1090–5. [PubMed] [Google Scholar]
- 23. Behrouz V, Jazayeri S, Aryaeian N, Zahedi MJ, Hosseini F. Effects of probiotic and prebiotic supplementation on leptin, adiponectin, and glycemic parameters in non-alcoholic fatty liver disease: a randomized clinical trial. Middle East J Dig Dis. 2017;9:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Famouri F, Shariat Z, Hashemipour M, Keikha M, Kelishadi R. Effects of probiotics on nonalcoholic fatty liver disease in obese children and adolescents. J Pediatr Gastroenterol Nutr. 2017;64:413–7. [DOI] [PubMed] [Google Scholar]
- 25. Kobyliak N, Abenavoli L, Mykhalchyshyn G, Kononenko L, Boccuto L, Kyriienko D, Dynnyk O. A multi-strain probiotic reduces the fatty liver index, cytokines and aminotransferase levels in NAFLD patients: evidence from a randomized clinical trial. J Gastrointestin Liver Dis. 2018;27:41–49. [DOI] [PubMed] [Google Scholar]
- 26. Monem SM. Probiotic therapy in patients with nonalcoholic steatohepatitis in Zagazig University Hospitals. Euroasian J Hepatogastroenterol. 2017;7:101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nabavi S, Rafraf M, Somi MH, Homayouni-Rad A, Asghari-Jafarabadi M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci. 2014;97:7386–93. [DOI] [PubMed] [Google Scholar]
- 28. Sepideh A, Karim P, Hossein A, Leila R, Hamdollah M, Mohammad EG, Mojtaba S, Mohammad S, Ghader G, Seyed Moayed A. Effects of multistrain probiotic supplementation on glycemic and inflammatory indices in patients with nonalcoholic fatty liver disease: a double-blind randomized clinical trial. J Am Coll Nutr. 2016;35:500–5. [DOI] [PubMed] [Google Scholar]
- 29. Vajro P, Mandato C, Licenziati ME, Franzese A, Vitale DF, Lenta S, Caropreso M, Vallone G, Meli R. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2011;52:740–3. [DOI] [PubMed] [Google Scholar]
- 30. Asgharian A, Askari G, Esmailzade A, Feizi A, Mohammadi V. The effect of symbiotic supplementation on liver enzymes, C-reactive protein and ultrasound findings in patients with non-alcoholic fatty liver disease: a clinical trial. Int J Prev Med. 2016;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ekhlasi G, Mohammadi RK, Agah S, Zarrati M, Hosseini AF, Arabshahi SS, Shidfar F. Do symbiotic and vitamin E supplementation have favorite effects in nonalcoholic fatty liver disease? A randomized, double-blind, placebo-controlled trial. J Res Med Sci. 2016;21:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferolla SM, Couto CA, Costa Silva L, Armiliato GNA, Pereira CAS, Martins FS, Ferrari MdLA, Vilela EG, Torres HOG, Cunha AS et al.. Beneficial effect of synbiotic supplementation on hepatic steatosis and anthropometric parameters, but not on gut permeability in a population with non-alcoholic steatohepatitis. Nutrients. 2016;8(7):397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Javadi L, Ghavami M, Khoshbaten M, Safaiyan A, Barzegari A, Gargari BP. The effect of probiotic and/or prebiotic on liver function tests in patients with nonalcoholic fatty liver disease: a double blind randomized clinical trial. Iranian Red Crescent Medical Journal. 2017;19(4):e46017. [Google Scholar]
- 34. Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, Mastrojeni S, Malaguarnera G, Mistretta A, Li Volti G et al.. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57:545–53. [DOI] [PubMed] [Google Scholar]
- 35. Manzhalii E, Virchenko O, Falalyeyeva T, Beregova T, Stremmel W. Treatment efficacy of a probiotic preparation for non-alcoholic steatohepatitis: a pilot trial. J Dig Dis. 2017;18:698–703. [DOI] [PubMed] [Google Scholar]
- 36. Shavakhi A, Minakari M, Firouzian H, Assali R, Hekmatdoost A, Ferns G. Effect of a probiotic and metformin on liver aminotransferases in non-alcoholic steatohepatitis: a double blind randomized clinical trial. Int J Prev Med. 2013;4:531–7. [PMC free article] [PubMed] [Google Scholar]
- 37. Wong VWS, Wong GLH, Chim AML, Chu WC, Yeung DK, Li KC, Chan HL. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol. 2013;12:256–62. [PubMed] [Google Scholar]
- 38. Mofidi F, Poustchi H, Yari Z, Nourinayyer B, Merat S, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br J Nutr. 2017;117:662–8. [DOI] [PubMed] [Google Scholar]
- 39. Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014;99:535–42. [DOI] [PubMed] [Google Scholar]
- 40. Sayari S, Neishaboori H, Jameshorani M. Combined effects of synbiotic and sitagliptin versus sitagliptin alone in patients with nonalcoholic fatty liver disease. Clin Mol Hepatol. 2018;24(3):331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bakhshimoghaddam F, Shateri K, Sina M, Hashemian M, Alizadeh M. Daily consumption of synbiotic yogurt decreases liver steatosis in patients with nonalcoholic fatty liver disease: a randomized controlled clinical trial. J Nutr. 2018;148(8):1276–84. [DOI] [PubMed] [Google Scholar]
- 42. Del Chierico F, Nobili V, Vernocchi P, Russo A, Stefanis C, Gnani D, Furlanello C, Zandonà A, Paci P, Capuani G et al.. Gut microbiota profiling of pediatric NAFLD and obese patients unveiled by an integrated meta-omics based approach. Hepatology. 2017;65(2):451–64. [DOI] [PubMed] [Google Scholar]
- 43. Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–9. [DOI] [PubMed] [Google Scholar]
- 44. Michail S, Lin M, Frey MR, Fanter R, Paliy O, Hilbush B, Reo NV. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol Ecol. 2015;91:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Quigley E. Prebiotics and probiotics in digestive health. Clin Gastroenterol Hepatol. 2019;17(2):333–44. [DOI] [PubMed] [Google Scholar]
- 46. Sharpton SR, Ajmera V, Loomba R. Emerging role of gut microbiome in nonalcoholic fatty liver disease: from composition to function. Clin Gastroenterol Hepatol. 2019;17(2):296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R et al.. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–6..e7. [DOI] [PubMed] [Google Scholar]
- 48. Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ et al.. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metabolism. 2017;26:611–619.e6. [DOI] [PubMed] [Google Scholar]
- 49. Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L, Negro F, Caldwell SH et al.. Diagnostic modalities for non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH) and associated fibrosis. Hepatology. 2018;68(1):349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60 Suppl 2:S129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.