ABSTRACT
Background
Maternal obesity is a risk factor for childhood obesity; this is a major public health concern given that ∼40% of pregnant women are either overweight or obese. Whether differences in milk composition in lean compared with obese women contribute to childhood obesity is unclear.
Objectives
We aimed to analyze relationships between maternal obesity and human milk metabolites, infant body composition, and postnatal weight gain.
Methods
This was a prospective study in which mothers intending to breastfeed exclusively, and their newborn infants, were enrolled at delivery (n = 35 mother–infant pairs). We excluded mothers with diabetes, other medical conditions, or pregnancy complications. Participants were grouped by maternal prepregnancy BMI <25 (lean) or ≥25 kg/m2 (overweight/obese). We analyzed infant body composition by dual-energy X-ray absorptiometry and used untargeted liquid chromatography–gas chromatography–mass spectrometry to measure the milk content of 275 metabolites at 1 and 6 mo postpartum.
Results
At 1 mo postpartum, 10 metabolites differed between overweight/obese and lean groups with nominal P < 0.05, but none was altered with a false discovery rate <0.25. Many differentially abundant metabolites belonged to the same chemical class; e.g., 4/10 metabolites were nucleotide derivatives, and 3/10 were human milk oligosaccharides. Milk adenine correlated positively with both continuously distributed maternal BMI and with infant adiposity and fat accrual. Analysis of milk composition at 6 mo postpartum revealed 20 differentially abundant metabolites (P < 0.05) in overweight/obese compared with lean women, including 6 metabolites with a false discovery rate of <0.25. At both 1 and 6 mo, human milk abundance of 1,5-anhydroglucitol, which has not previously been described in milk, was positively associated with maternal BMI.
Conclusions
Maternal obesity is associated with changes in the human milk metabolome. While only a subset of metabolites correlated with both maternal and infant weight, these point to potential milk-dependent mechanisms for mother–child transmission of obesity. This trial was registered at www.clinicaltrials.gov as NCT02535637.
Keywords: human milk, breast milk, maternal obesity, metabolomics, body composition, human milk oligosaccharides, infant
Introduction
Childhood obesity has reached unprecedented levels in the United States and worldwide (1–3). Maternal obesity is one of the strongest predictors of childhood obesity, increasing risk more than 2-fold (4). Approximately 40% of women in North America are overweight or obese (ov-ob) at pregnancy onset (5), and maternal obesity accounts for 12–15% of the population attributable risk of childhood obesity (6, 7). While the mechanisms by which obesity risk is transmitted from mother to child are not fully understood, differences in infant feeding may play a role. Indeed, maternal obesity is linked to lower initiation and shorter duration of breastfeeding (8), and obesity risk is highest among non-breastfed infants of obese mothers (9). Thus, breastfeeding promotion has been a major focus of public health efforts to curb childhood obesity (10).
While breastfeeding positively impacts many aspects of maternal and child health (11–13), the literature on offspring obesity risk is inconclusive. A large meta-analysis of breastfeeding compared with formula feeding found only a small protective effect on adult BMI (14). When confounders such as socio-economic status, maternal BMI, and maternal smoking are controlled, associations between duration and exclusivity of breastfeeding and later obesity are attenuated or abolished (15). Some studies have even suggested increased obesity risk: in a randomized controlled trial of breastfeeding promotion in Belarus, the intervention group had a slightly higher BMI in adolescence (16).
One possible reason for the lack of clear benefit of breastfeeding on obesity risk is that human milk might not play a mechanistic role in childhood obesity. Another possibility is that milk composition may vary according to maternal weight status (17, 18). For example, milk from obese mothers might contain differential amounts of “obesogenic” compared with “protective” constituents. Substantial evidence indicates that total fat, saturated fatty acids, insulin, leptin, TNF-α, and adiponectin are increased in milk from obese mothers; some studies also have suggested that differences in milk ω-3 and ω-6 (n-3, n-6) fatty acids, and in microbiome diversity, may be associated with maternal weight status (19–25). Despite intriguing recent studies suggesting that milk insulin and leptin may alter the development of the infant microbiome (26), it is not clear whether such differences in composition contribute to differences in weight gain in infants of obese women. For example, human milk insulin content is positively associated with maternal BMI, but inversely correlated with infant BMI; similar associations have been reported for leptin and IL-6 (22). Thus, it remains an open question whether differences in milk composition contribute to mother–child transmission of obesity.
With the current study, we used a metabolomics approach to comprehensively analyze metabolites, nutrients, and small molecules in human milk that differ according to both maternal and infant weight status. We prospectively analyzed infant body composition, in parallel with maternal milk composition, at 1 and 6 mo postpartum. We hypothesized that human milk content of individual metabolites, and groups of metabolites belonging to specific pathways, would be associated with maternal and infant weight status.
Methods
Mother–infant participants and study procedures
All research procedures and protocols were approved by the hospital committee for human subjects research and were conducted in accordance with the Helsinki Declaration. The original purpose of this pilot study was to assess the cross-sectional associations of appetite-regulating hormones and growth factors (leptin, insulin, glucose) and inflammatory factors (IL-6 and TNF-α) in human breast milk with infant size, adiposity, and lean tissue at 1 and 6 mo of age in healthy term infants in women across a broad range of BMI; analysis of the milk metabolome was done as a secondary analysis. We enrolled 31 mother–infant pairs from Oklahoma University Health Sciences Center. Eligibility criteria included intent to exclusively breastfeed from 0 to 6 mo of age and willingness to provide expressed milk samples. We excluded mothers with gestational or pregestational diabetes and/or any medical conditions in the mother or child with the potential to influence weight gain. Participants were grouped according to maternal prepregnancy BMI, with BMI <25 kg/m2 defined as “lean,” and ≥25 kg/m2 defined as overweight/obese ("ov-ob"). Clinical information was obtained from medical records and questionnaires. Milk was collected at 1 and 6 mo. Mother–infant pairs reported to the study site at 1 and 6 mo (±5 d) postpartum, between 08:00 and 10:00, >1.5 h since the last infant feeding, and with the mother fasted >1 h. Upon arrival, a pre-feeding infant weight was obtained using a high-sensitivity scale (Seca 728). Mothers then breastfed the infant ad libitum from both breasts. The study visit continued, including dual-energy X-ray absorptiometry (DXA), anthropometrics, questionnaires, etc., for 2–2.5 h. After the above, based on their discretion, mothers chose the breast they felt could provide the most complete milk expression (though the right was encouraged). The mother was encouraged to completely empty the entire contents of a single breast using an electric hospital-grade breast pump (Symphony, Medela, Inc.). Milk was collected into 150-mL bisphenol A-free polypropylene containers (Medela, Inc.), mixed, and centrifuged at 3000 × g for 15 min at 4°C. The fat layer was skimmed from the top using a microspatula and discarded. Samples were mixed, centrifuged, and scraped 2 more times. The aqueous phase of the sample was transferred to a new microcentrifuge tube, taking care to avoid any remaining lipid and pelleted material. Skimmed milk samples were divided into aliquots and stored at −80°C until analysis.
Infant body composition analysis
At 1 and 6 mo, we measured infant weight using a Seca 728 scale and length (crown-to-heel) with a Seca 416 infantometer (Seca). We estimated infant adiposity (relative percentage of fat, i.e., %fat), total fat mass, total lean mass, and central obesity (trunk fat mass) by DXA (Lunar scanner, GE Healthcare), as described (22, 27). To minimize variability, the same investigator (DAF) positioned the infants and performed all the DXA scans.
Metabolomic analysis
We performed untargeted metabolomics analysis using liquid chromatography–gas chromatography–mass spectrometry, as described (28–30) (Metabolon, Inc.). Two solutions of isotopically labeled reference standards were spiked at a constant level into every experimental and quality control sample, either at the beginning or at the end of the extraction process. These compounds were carefully chosen for each data stream, such that they did not interfere with measurement of endogenous compounds, and were used to monitor assay performance in every batch. Recovery standards, added at the beginning of the extraction, allowed us to monitor extraction efficiency and reproducibility. In addition, internal standards, prepared in the reconstitution solution and added in the final step, allowed for chromatographic peak alignment and were used to monitor instrument performance and data quality over the course of the run. These internal standards included: deuterated d7-glucose, d3-leucine, d8-phenylalanine, and d5-tryptophan (Cambridge Isotope Laboratories); d5-hippuric acid, d5-indole acetic acid, and d9-progesterone (C/D/N Isotopes, Inc.); bromophenylalanine (Sigma-Aldrich); and amitriptyline (MP Biomedicals, LLC). To confirm chromatographic peak identities, all metabolite identities were checked against a reference library. We assessed process variability from the beginning to the end of the run in technical replicate samples comprising a small aliquot of every experimental sample within the study. This sample was injected periodically throughout the run. Upon measuring metabolite peaks emerging from these samples, we calculated 11% variance across all the metabolites measured.
The assay detected a total of 275 metabolites including 223 known metabolites from diverse chemical classes, including amino acids, lipids, carbohydrates, vitamins, cofactors, etc., and 52 metabolites of unknown identity (X-11616, X-11618, etc.). Metabolites undetectable in >50% of the samples were excluded from analysis and undetectable values replaced by half the minimum value detected, using Metaboanalyst 3.0 (31, 32). We tested whether any KEGG (Kyoto Encyclopedia of Genes and Genomes) metabolic pathways were over-represented among the differentially regulated metabolites through the pathway analysis modality of Metaboanalyst 3.0, using a list of detected metabolites as the reference group.
Statistical analysis
We initially enrolled N = 37 mother–infant pairs. We excluded n = 4 due to missing data on weight gain during pregnancy, n = 1 due to insufficient milk, and n = 1 due to missing metabolite data; thus, n = 31 (16 ov-ob, 15 lean) were included in the analysis of human milk at 1 mo. We excluded n = 6 due to missing measures at 6 mo; thus, n = 26 (14 ov-ob, 12 lean) were included in the analysis of human milk at 6 mo (see Study Flowchart in Supplemental Figure 1). We calculated group means, SDs, and other descriptive statistics using JMP Pro v13 (SAS). To identify metabolites that differ in ov-ob compared with lean mothers, we calculated fold changes for each metabolite and performed two-sided unpaired Student's t-tests. To account for multiple hypothesis testing, we calculated false discovery rate (FDR)-adjusted P values using the Benjamini–Hochberg procedure (33, 34), with Metaboanalyst 3.0. We used an FDR threshold of 0.25, which corresponded to a nominal P value of <0.01 for the human milk metabolite data set at 6 mo. (No metabolites reached this significance threshold at 1 mo.) We also calculated Pearson correlation coefficients between levels of individual metabolites and clinical characteristics including maternal prepregnancy BMI, infant weight for age percentile, and infant % fat. We used standard least-squares linear regression to adjust for potential confounders, including infant sex, gestational age, and parity, using the Fit Model function in JMP Pro.
Results
Clinical characteristics of mother–infant pairs
Demographic and clinical information for the mother–infant pairs is presented in Table 1. As expected, women in the ov-ob group had a significantly higher pre-pregnancy BMI than the lean group (30.9 ± 5.1 kg/m2, compared with 21.7 ± 1.9 kg/m2, P < 0.0001). Women in the ov-ob group were less likely to be primiparous (12.5% compared with 46.7%, P = 0.05; parity 2.9 + 1.7 compared with 2.0 + 1.1, P = 0.11). Race and ethnicity were similar in the 2 groups. The ov-ob group had a higher BMI at the 1-mo postpartum visit (30.9 ± 3.6 kg/m2, compared with 23.5 ± 2.4 kg/m2, P < 0.0001) and greater triceps skinfold thickness at 1 mo (31.4 ± 10.1 mm, compared with 19.1 ± 7.3 mm, P = 0.002). Gestational age, birth weight, and birth weight percentile (for gestational age and sex) were similar in infants of ov-ob compared with lean mothers.
TABLE 1.
Demographic and clinical characteristics of mothers and infants1
| Clinical and demographic characteristics | Lean (n = 15) | Overweight-obese (n = 16) | P value2 |
|---|---|---|---|
| Mothers | |||
| Age, y | 27.5 ± 5.7 (15) | 30.5 ± 4.7 (16) | 0.12 |
| Prepregnancy BMI, kg/m2 | 21.7 ± 1.9 (15) | 30.9 ± 5.1 (16) | <0.0001 |
| Parity | 2.0 ± 1.1 (15) | 2.9 ± 1.7 (16) | 0.11 |
| Race, % white | 73.3 (15) | 75 (16) | 1 |
| Gestational weight gain, kg | 15.1 ± 6.5 (13) | 11.9 ± 7.0 (14) | 0.2 |
| Excessive gestational weight gain3, % | 33.3 (13) | 50 (14) | 0.47 |
| Postpartum BMI at 1 mo, kg/m2 | 23.5 ± 2.4 (15) | 30.9 ± 3.6 (16) | <0.0001 |
| Triceps skinfold thickness at 1 mo postpartum, mm | 19.1 ± 7.3 (15) | 31.4 ± 10.1 (13) | 0.002 |
| Infants | |||
| Sex, % males | 66.7 (15) | 50 (16) | 0.47 |
| Birth weight, kg | 3.45 ± 0.50 (15) | 3.68 ± 0.46 (16) | 0.2 |
| Birth weight percentile | 49 ± 29 (15) | 63 ± 26 (16) | 0.18 |
| Gestational age, wk | 39.5 ± 1.0 (15) | 40.0 ± 1.1 (16) | 0.17 |
| Weight for age percentile, 1 mo | 47 ± 29 (15) | 66 ± 23 (16) | 0.06 |
| Body composition (dual-energy X-ray absorptiometry), 1 mo | |||
| Total fat mass, kg | 1.1 ± 0.3 (15) | 1.3 ± 0.3 (16) | 0.02 |
| Total fat, % | 23.1 ± 3.2 (15) | 25.0 ± 2.5 (16) | 0.07 |
| Trunk fat mass, kg | 0.37 ± 0.14 (15) | 0.45 ± 0.11 (16) | 0.09 |
| Weight for age percentile, 6 mo | 32.3 ± 30.5 (12) | 45.5 ± 38.4 (14) | 0.34 |
| Body composition (dual-energy X-ray absorptiometry), 6 mo | |||
| Total fat, % | 32.0 ± 3.5 (12) | 32.6 ± 4.0 (14) | 0.67 |
| Total fat mass, kg | 2.38 ± 0.55 (12) | 2.50 ± 0.74 (14) | 0.63 |
| Trunk fat mass, kg | 0.77 ± 0.29 (12) | 0.82 ± 0.36 (14) | 0.7 |
Continuous data are presented as means ± SDs (n); categorical variables are presented as a percentage (n).
P value calculated using Student's 2-sided t-test for continuous data; Fisher's exact test used for categorical data.
“Excessive gestational weight gain” refers to the percentage of participants exceeding 2009 Institute of Medicine (35) guidelines for gestational weight gain.
Infant weight gain and body composition
At 1 mo postpartum, infants of overweight-obese mothers tended to have a higher weight for age (WFA) percentile (66% ± 23%, compared with 47% ± 29%, P = 0.06). Infants in the ov-ob group had a significantly higher absolute fat mass (DXA) (1.3 ± 0.3 kg, compared with 1.1 ± 0.3 kg, P = 0.02) and trends for higher adiposity, expressed as percentage of body weight (25.0% ± 2.5%, compared with 23.1% ± 3.2%, P = 0.07), and truncal fat (0.45 ± 0.11 kg, compared with 0.37 ± 0.14 kg, P = 0.09) (Table 1) at 1 mo. By 6 mo, however, differences in WFA percentile and body composition were attenuated.
Associations between maternal obesity and milk metabolites at 1 mo postpartum
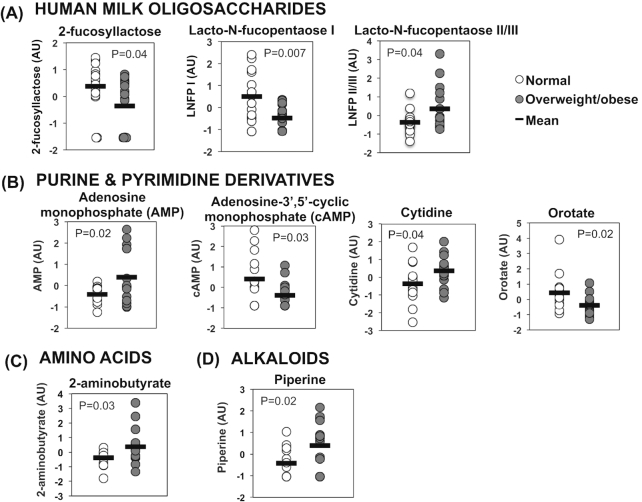
At 1 mo postpartum, 10 human milk metabolites differed between the ov-ob and lean groups (P < 0.05, Table 2 ). None of these metabolites survived adjustment for multiple comparisons (FDR < 0.25). However, many of the top-ranking metabolites belonged to the same chemical class. For example, 3 of the 10 top-ranking differentially abundant metabolites were human milk oligosaccharides, and 4 were purine and pyrimidine derivatives (Figure 1 and Table 2). Pathway analysis indicated that metabolites related to pyrimidine metabolism were over-represented among the 10 differentially abundant metabolites (P = 0.035), as compared with the reference group of 168 detected metabolites with KEGG pathway annotations.
TABLE 2.
Top-ranking differentially regulated human milk metabolites at 1 mo postpartum1
| Name | Class | Fold-change overweight or obese/lean | P value | False discovery rate |
|---|---|---|---|---|
| Lacto-N-fucopentaose I | Oligosaccharide | 0.383 | 0.007 | 0.86 |
| Lacto-N-fucopentaose II or III | Oligosaccharide | 1.674 | 0.04 | 0.86 |
| 2-Fucosyllactose | Oligosaccharide | 0.619 | 0.04 | 0.86 |
| AMP | Nucleosides, nucleotides, and analogues | 1.761 | 0.02 | 0.86 |
| Cyclic adenosine 3′,5′-monophosphate | Nucleosides, nucleotides, and analogues | 0.433 | 0.03 | 0.86 |
| Cytidine | Nucleosides, nucleotides, and analogues | 1.335 | 0.04 | 0.86 |
| Orotate | Pyrimidines and pyrimidine derivatives | 0.704 | 0.02 | 0.86 |
| Piperine | Alkaloids and derivatives | 2.277 | 0.02 | 0.86 |
| 2-Aminobutyrate | Carboxylic acids and derivatives | 1.547 | 0.03 | 0.86 |
| X-19659 | Unknown | 0.510 | 0.03 | 0.86 |
“Fold-change” refers to mean abundance in overweight or obese/lean. The table includes only metabolites with P < 0.05 (2-sided t-test). N = 15–16/group.
FIGURE 1.
Differentially abundant milk metabolites at 1 mo postpartum: selected top-ranking metabolites with levels differing significantly (P < 0.05, 2-sided t-test) between overweight or obese (“ov-ob”) compared with lean women, 1 mo postpartum, n = 15–16/group. (A) Human milk oligosaccharides. (B) Purine and pyrimidine derivatives. (C) Amino acids. (D) Alkaloids. AU, arbitrary units; LNFP, lacto-N-fucopentaose.
Given that mothers in the ov-ob group had a higher parity than controls, and that gestational age and infant sex can influence milk composition (36–38), we adjusted for these potential confounders using linear regression analysis. Three human milk oligosaccharides, 2-fucosyllactose [effect estimate (EE): −0.066 ± 0.030, P = 0.03], lacto-N-fucopentaose II/III (EE: 0.068 ± 0.027, P = 0.02), and lacto-N-fucopentaose I (EE: −0.072 ± 0.027, P = 0.01), remained significantly associated with maternal BMI after adjustment, as did the purine and pyrimidine derivatives orotate (EE: −0.084 ± 0.029, P = 0.008) and adenine (EE: 0.068 ± 0.030, P = 0.03, Supplemental Table 1). To test whether infant birth weight, which was numerically higher in infants ov-ob mothers, was related to the associations between maternal weight status and milk composition, we further adjusted for birth weight and noted that associations between maternal BMI and human milk oligosaccharides, orotate, and 1,5-anhydroglucitol persisted after birth weight adjustment, whereas the association between maternal BMI and milk adenine content was attenuated.
Associations between maternal BMI and milk metabolites at 6 mo postpartum
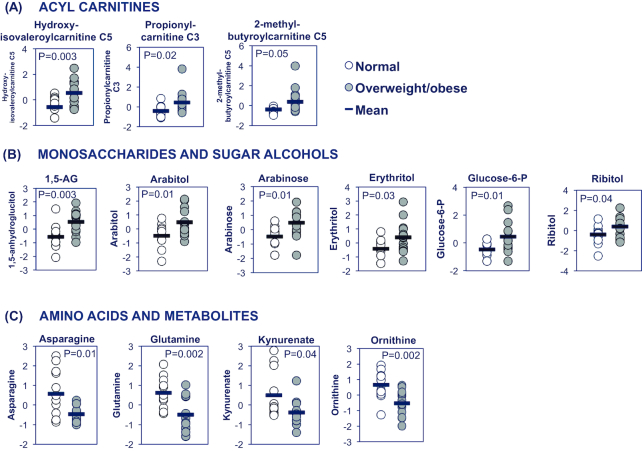
Analysis of human milk at 6 mo revealed 20 metabolites differing significantly between the ov-ob and lean groups (P < 0.05), Table 3; 6 of these survived adjustment for multiple comparisons (FDR < 0.25). The differentially abundant metabolites belonged to similar chemical classes. For example, several acylcarnitines involved in branched chain amino acid metabolism were increased in human milk from ov-ob mothers, including hydroxyisovaleroylcarnitine, propionylcarnitine, and 2-methylbutyroylcarnitine [increased 123%, 45%, and 128%, respectively (P < 0.05), Figure 2A,Supplemental Table 2]. Several sugar alcohols and monosaccharides were more abundant in milk from ov-ob mothers, including 1,5-anhydroglucitol, arabitol, arabinose, glucose-6-phosphate, erythritol, and ribitol (Figure 2B). Moreover, several amino acids, including ornithine, glutamine, and asparagine, were reduced by 17%, 32%, and 49%, respectively (P < 0.05), as were levels of the tryptophan metabolite kynurenate (reduced 39%, P = 0.036, Figure 2C). Pathway analysis (Metaboanalyst) indicated that metabolites belonging to the “Citrate cycle,” “Glyoxylate/dicarboxylate metabolism,” and “Alanine, aspartate and glutamate metabolism” pathways were significantly over-represented (Supplemental Table 2) among the list of differentially regulated metabolites at age 6 mo.
TABLE 3.
Top-ranking differentially regulated human milk metabolites at 6 mo postpartum1
| Name | Class | Fold-change overweight or obese/lean | P value | False discovery rate |
|---|---|---|---|---|
| Hydroxyisovaleroylcarnitine C5 | Acylcarnitine | 2.23 | 0.003 | 0.15 |
| 2-Methylbutyroylcarnitine C5 | Acylcarnitine | 2.28 | 0.045 | 0.56 |
| Propionylcarnitine C3 | Acylcarnitine | 1.45 | 0.02 | 0.35 |
| Glucose 6-phosphate | Monosaccharide; hexose phosphate | 2.07 | 0.01 | 0.29 |
| 1,5-Anhydroglucitol | Monosaccharide | 1.37 | 0.003 | 0.15 |
| Arabinose | Monosaccharide; pentose | 1.72 | 0.01 | 0.28 |
| Arabitol | Sugar alcohol | 1.53 | 0.01 | 0.28 |
| Erythritol | Sugar alcohol | 1.25 | 0.03 | 0.45 |
| Ribitol | Sugar alcohol | 1.38 | 0.04 | 0.52 |
| Glycerate | Sugar acids and derivatives | 0.63 | 0.005 | 0.18 |
| Asparagine | Amino acid | 0.51 | 0.01 | 0.29 |
| Ornithine | Amino acid | 0.83 | 0.002 | 0.15 |
| Glutamine | Amino acid | 0.68 | 0.002 | 0.15 |
| Kynurenate | Amino acid metabolite; uinoline carboxylic acid | 0.61 | 0.04 | 0.51 |
| 3-4-Hydroxyphenyllactate | Amino acid metabolite; phenylpropanoic acid | 1.59 | 0.01 | 0.28 |
| Phosphocholine | Phosphocholine | 1.44 | 0.03 | 0.44 |
| Malate | β-Hydroxyacids and derivatives | 1.80 | 0.002 | 0.15 |
| Fumarate | Dicarboxylic acid | 1.47 | 0.050 | 0.57 |
| Citrate | Tricarboxylic acid | 0.78 | 0.047 | 0.56 |
| X—12,565 | Unknown | 1.71 | 0.02 | 0.33 |
“Fold-change” refers to mean abundance in overweight or obese/lean. The table includes only metabolites with P < 0.05 (2-sided t-test). N = 15–16/group.
FIGURE 2.
Differentially abundant milk metabolites at 6 mo postpartum: selected top-ranking metabolites with levels differing significantly (P < 0.05, 2-sided t-test) between overweight or obese compared with lean women, 6 mo postpartum, n = 12–14/group. (A) Acylcarnitines. (B) Monosaccharides and sugar alcohols. (C) Amino acids and metabolites. 1,5-AG, 1,5-anhydroglucitol.
These metabolomic signatures largely persisted in a linear regression analysis of associations between maternal pre-pregnancy BMI and milk metabolite abundance at 6 mo postpartum, adjusting for maternal age and infant sex; further adjustment for birth weight attenuated associations with glutamine, arabinose, and ribitol (Supplemental Table 3). Interestingly, abundance of 1,5-anhydroglucitol (1,5-AG), which was not previously described in human milk, was positively associated with maternal BMI at both 1 mo (EE: 0.089 ± 0.025, P = 0.002) and 6 mo (EE: 0.108 ± 0.028, P = 0.001), with or without adjustment for birth weight.
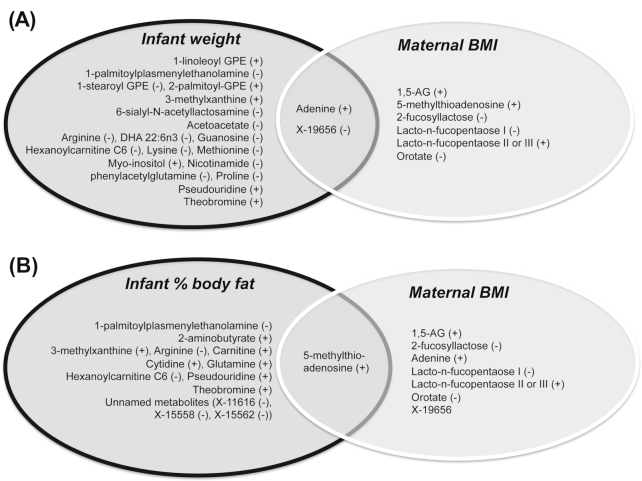
Overlap between milk metabolites associated with maternal BMI and those associated with infant obesity
We next asked whether human milk metabolites associated with maternal weight status were also associated with infant weight status. We looked for overlap between milk metabolites correlated with maternal BMI (Pearson correlation, P < 0.05, Supplemental Table 1) and milk metabolites correlated with infant weight status at 1 mo, assessed by infant WFA percentile, adjusting for infant sex, gestational age, and parity. As shown in Figure 3A, we found minimal overlap between the 2 sets of metabolites, with the exception of adenine, which was positively correlated with both infant weight and with maternal BMI, and the unidentified metabolite X-19656, which was negatively correlated with both infant weight and maternal weight. We next examined the overlap between milk metabolites associated with infant adiposity (% body fat assessed by DXA) at 1 mo (Supplemental Table 4) and metabolites associated with mother's BMI. The metabolite 5-methylthioadenosine was noted to be positively correlated with both maternal BMI and infant adiposity (Figure 3B).
FIGURE 3.
Overlap between milk metabolites correlating with maternal weight status and those correlating with infant weight status at 1 mo postpartum. (A) Venn diagram depicting the overlap between breast milk metabolites at 1 mo postpartum that correlate with infant weight (assessed by weight for age and sex percentile at 1 mo) and metabolites correlating with maternal pre-pregnancy BMI, with all metabolites adjusted for gender, parity, and gestational age. (B) Venn diagram depicting the overlap between breast milk metabolites at 1 mo postpartum that correlate with infant adiposity (assessed by total fat % measured by dual-energy X-ray absorptiometry at 1 mo) and metabolites correlating with maternal prepregnancy BMI. All metabolites adjusted for gender, parity, and gestational age. Significant correlation defined as effect estimate with adjusted P < 0.05. (+) denotes positive correlation, and (–) denotes negative correlation. 1,5-AG, 1,5-anhydroglucitol; GPE, glycerylphosphorylethanolamine.
By 6 mo of age, we found even less overlap between the sets of metabolites correlating with maternal BMI and those correlating with infant WFA and adiposity (Supplemental Table 5). No metabolites were correlated with both maternal BMI and infant WFA at 6 mo or with maternal BMI and infant adiposity.
Associations between milk metabolites and fat accrual in infancy
Because weight gain and fat accrual during infancy are important risk factors for obesity, insulin resistance, and cardiovascular disease risk in later life, we next examined associations between milk metabolites and the change in fat mass from 1 to 6 mo of age, as assessed by DXA. We used linear regression to adjust for infant sex, gestational age, and maternal age. We noted a positive correlation between adenine levels in human milk at 1 mo and change in fat mass from 1 to 6 mo (EE: 1.091 ± 0.369, P = 0.01, Supplemental Table 6); as noted above, human milk adenine was also positively correlated with maternal BMI (Supplemental Table 1).
Discussion
In the current study, we combined detailed metabolomic analysis of human milk from lean compared with overweight or obese women, together with longitudinal analysis of weight gain and body composition in infants, to test whether differentially abundant metabolites in milk are associated with infant obesity. Previous analyses of the human milk metabolome have focused on differences in milk composition according to gestational age (39–41), postnatal age (39), and maternal health status [e.g., diet/lifestyle (42), pre-eclampsia (43), chemotherapy (44)], but our analysis is the first comprehensive analysis of the milk metabolome in relation to both maternal and infant obesity. We demonstrate that maternal obesity is associated with modest differences in the human milk metabolome; metabolite differences did not survive adjustment for multiple corrections at 1 mo postpartum, whereas maternal obesity was modestly associated with milk metabolite content at 6 mo postpartum. We also report that levels of many human milk metabolites are associated with infant weight status and identify a subset of metabolites correlating with both maternal BMI and infant adiposity. Together, these data raise the possibility that obesity-associated differences in human milk composition might contribute to early childhood obesity, although this hypothesis would need to be confirmed in additional studies.
Breastfeeding promotion is widely advocated as a means of improving both maternal and child health outcomes, but surprisingly little is known about the bioactive compounds in human milk responsible for its beneficial health effects. Human milk contains not only nutrients, but also antibodies, cytokines, hormones, adipokines, and a distinct microbiome (45, 46) that could potentially influence metabolic outcomes in the infant. These data raise the question of whether differences in human milk composition might contribute to mother–child transmission of obesity risk. This possibility is suggested by rodent studies, in which lactational exposure to maternal obesity imparts obesity risk in the offspring (47, 48). Similarly, a study of infants of mothers fed either mother's milk or banked human milk from nondiabetic donors found that consumption of diabetic mothers’ milk during the first week of life was associated with a higher weight at 2 y (49). Previous studies have demonstrated that milk from obese women has higher levels of insulin, leptin, TNF-α, and IL-6, as compared with lean women (22, 50). However, it is not clear whether these substances play a pathogenic versus protective role, or no role at all, in childhood obesity risk. If human milk composition does play a mechanistic role in childhood obesity, it is likely to be one of many additive risk factors for childhood obesity and not the sole determinant. For example, human milk may be protective as compared with formula, but milk from obese mothers might contain a lower abundance of protective factors than lean mothers’ milk. This possibility is suggested by the observations of Li et al. (9), who reported that the risk of childhood obesity was highest in non-breastfed infants of obese mothers, but also demonstrated that risk of childhood overweight was increased by maternal obesity among breastfed infants.
We found a relatively small degree of overlap between milk metabolites correlating with maternal BMI and those correlating with infant adiposity. However, levels of adenine were associated with both maternal and infant weight status, and with fat accumulation from 1 to 6 mo of age. Moreover, several nucleoside and nucleotide derivatives (e.g., AMP, orotate, adenosine 3ʹ,5ʹ-cyclic monophosphate, cytidine) were differentially abundant in milk from ov-ob compared with normal weight women at 1 mo. Human milk has a higher abundance of nucleotides than cow milk (51). Infant formulas are typically supplemented with nucleotides, which may increase weight, length, and head circumference during infancy (52). Human milk nucleotides may also promote development of cellular and humoral immune responses (53, 54) and differentiation and growth of the intestinal epithelium (53, 55). More recently, the pyrimidine nucleotide uridine has been discovered to regulate thermogenesis during the fasting–feeding transition (56), raising the question of whether human milk nucleotide content may affect systemic metabolism in the infant. Our overlap analysis also identified 5-methylthioadenosine—a sulfur-containing nucleoside and precursor to methionine—as positively associated both with maternal BMI and with infant fat percentage at 1 mo, which is intriguing in light of recent reports that this metabolite is increased in adolescents with metabolic syndrome and adults with type 2 diabetes (57, 58).
At 1 mo postpartum, we found that maternal obesity was linked to differences in human milk oligosaccharide (HMO) content and composition. HMOs are a family of structurally diverse carbohydrate polymers that represent the third most abundant constituent in human milk (after lactose and lipids) (59). Human milk contains >150 types of oligosaccharides, with biological functions differing according to their structure. Distinct from other carbohydrates, HMOs are not digested or metabolized for energy, instead remaining intact through the small intestine. Upon reaching the colon, HMO can be metabolized by specific gastrointestinal bacterial species. HMOs may thus function as “prebiotics,” selectively fueling the growth of specific bacteria and shaping infant gut microbiome development (13). HMOs can also act as receptor decoys and block attachment of enteric pathogens to the host, and they may protect the infant from group B streptococcus and other neonatal infections (60). We have previously described an association between milk content of lacto-N-fucopentaose I and infant fat mass (61) independent of maternal BMI. It will be essential for future studies to test whether HMO content and composition play a causal role in infant obesity. We speculate that alterations in HMO in obese mothers may contribute to observed differences in gut microbiome composition and diversity in infants of obese mothers (24, 26).
By 6 mo postpartum, we noted that maternal obesity was associated with increased milk abundance of acylcarnitines, monosaccharides, and sugar alcohols, and reductions in amino acids and their metabolites. These patterns are similar to metabolomics signatures in plasma from individuals with obesity or type 2 diabetes: elevations in short-chain acylcarnitines (62, 63), increased hexoses and sugar alcohols [e.g., erythritol (64), glucose (65), mannose (65)], and reduced glutamine:glutamate ratio (66) have been previously reported in adults with diabetes or at risk of the disease. One of the most significant changes in human milk was the positive association between maternal BMI and milk 1,5-AG at both 1 and 6 mo postpartum. This is intriguing in light of a large body of literature demonstrating that 1,5-AG is a marker of glycemic control (67) and that reductions in plasma 1,5-AG predict incident diabetes (65); 1,5-AG has not previously been described in human milk. Taken together, these patterns raise important questions about the degree to which the maternal plasma metabolome might influence the human milk metabolome. Unfortunately, we did not assess correlations between maternal plasma and human milk metabolites, but this will be a key question for future studies.
We acknowledge the limitations of our study. First, our analysis relied on cross-sectional associations, so we cannot infer causality. Second, our sample size was relatively small, so it will be important to replicate our findings in other populations to assess generalizability. Third, we did not assess whether milk components other than metabolites (e.g., essential micronutrients, immune cells, bacteria, exosomes, cytokines, etc.) may be linked to maternal obesity. Finally, we did not obtain detailed dietary questionnaires in the participants, so it is unclear to what degree variation in dietary patterns may contribute to human milk metabolite abundance. For example, piperine, a xenobiotic metabolite found in peppers, was increased in ov-ob mothers at 1 mo, suggesting that diet may play a role. Some of the strengths of our approach include the longitudinal assessments at 1 and 6 mo and the simultaneous analysis of weight status in both mothers and infants.
In summary, we found that maternal obesity is associated with metabolomic signatures in human milk. At 1 mo postpartum, maternal BMI was modestly associated with the abundance of HMOs known to function as prebiotics, raising the possibility that obesity-associated changes in maternal milk composition may modulate infant microbiome acquisition, a hypothesis that would need to be confirmed with additional studies. By 6 mo postpartum, maternal BMI was associated with acyl carnitines, sugar alcohols, and amino acid metabolites in human milk, a pattern reminiscent of plasma metabolomics signatures in obesity and type 2 diabetes. While we found only a modest degree of overlap between human milk metabolites that correlated with maternal obesity and those correlating with infant obesity, the identified association between milk adenine and both maternal and infant weight status raises the possibility that some milk constituents might play a pathogenic role in mother-to-child transmission of obesity.
Supplementary Material
Acknowledgments
We are grateful to Dr Mary-Elizabeth Patti for reviewing the manuscript and providing insightful suggestions.
The authors’ responsibilities were as follows—DAF: designed the study, led the clinical assessments, and reviewed/edited the manuscript; EI: interpreted and analyzed the metabolomics data and wrote the manuscript; EWD: assisted with designing the study and with statistical considerations; CL: assisted with interpretation of metabolomics data; TJM and SV: assisted with preparation of tables and figures and all authors: read and approved the final manuscript. None of the authors reports a conflict of interest related to research presented in this article.
Notes
This study was funded by R01 HD080444 and R00 HD064793 from NICHD, MINECO, Graetz Foundation, Mead Johnson Nutrition, and Abbott Nutrition. The nongovernmental funders were not involved in the design, implementation, analysis, or interpretation of the study.
Supplemental Tables 1–6 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: 1,5-AG, 1,5-anhydroglucitol; DXA, dual-energy X-ray absorptiometry; EE, effect estimate; FDR, false discovery rate; GPE, glycerylphosphorylethanolamine; HMO, human milk oligosaccharides; KEGG, Kyoto Encyclopedia of Genes and Genomes; ov-ob, overweight or obese; WFA, weight for age.
References
- 1. Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315(21):2292–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Obesity and Overweight Fact Sheet. 2016. [Google Scholar]
- 4. Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, Amini SB. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90(5):1303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yogev Y, Catalano PM. Pregnancy and obesity. Obstet Gynecol Clin North Am. 2009;36(2):285–300., viii. [DOI] [PubMed] [Google Scholar]
- 6. Kuhle S, Allen AC, Veugelers PJ. Prevention potential of risk factors for childhood overweight. Can J Public Health. 2010;101(5):365–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toschke AM, Ruckinger S, Bohler E, Von Kries R. Adjusted population attributable fractions and preventable potential of risk factors for childhood obesity. Public Health Nutr. 2007;10(9):902–6. [DOI] [PubMed] [Google Scholar]
- 8. Donath SM, Amir LH. Does maternal obesity adversely affect breastfeeding initiation and duration?. J Paediatr Child Health. 2000;36(5):482–6. [DOI] [PubMed] [Google Scholar]
- 9. Li C, Kaur H, Choi WS, Huang TT, Lee RE, Ahluwalia JS. Additive interactions of maternal prepregnancy BMI and breast-feeding on childhood overweight. Obes Res. 2005;13(2):362–71. [DOI] [PubMed] [Google Scholar]
- 10. U.S. Department of Health and Human Services OotSG. The Surgeon General's Call to Action to Support Breastfeeding. 2011. Pubmed ID: 21452448. [Google Scholar]
- 11. Victora CG, Bahl R, Barros AJ, Franca GV, Horton S, Krasevec J, Murch S, Sankar MJ, Walker N, Rollins NC; Lancet Breastfeeding Series Group. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–90. [DOI] [PubMed] [Google Scholar]
- 12. Grimshaw K, Logan K, O'Donovan S, Kiely M, Patient K, van Bilsen J, Beyer K, Campbell DE, Garcia-Larsen V, Grabenhenrich L et al.. Modifying the infant's diet to prevent food allergy. Arch Dis Child. 2017;102(2):179–86. [DOI] [PubMed] [Google Scholar]
- 13. Hinde K, Lewis ZT. Microbiota. Mother's littlest helpers. Science. 2015;348(6242):1427–8. [DOI] [PubMed] [Google Scholar]
- 14. Owen CG, Martin RM, Whincup PH, Davey-Smith G, Gillman MW, Cook DG. The effect of breastfeeding on mean body mass index throughout life: A quantitative review of published and unpublished observational evidence. Am J Clin Nutr. 2005;82(6):1298–307. [DOI] [PubMed] [Google Scholar]
- 15. Kramer MS, Oken E, Martin RM. Infant feeding and adiposity: Scientific challenges in life-course epidemiology. Am J Clin Nutr. 2014;99(6):1281–83. [DOI] [PubMed] [Google Scholar]
- 16. Martin RM, Kramer MS, Patel R, Rifas-Shiman SL, Thompson J, Yang S, Vilchuck K, Bogdanovich N, Hameza M, Tilling K et al.. Effects of promoting long-term, exclusive breastfeeding on adolescent adiposity, blood pressure, and growth trajectories: A secondary analysis of a randomized clinical trial. JAMA Pediatr. 2017;171(7):e170698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fields DA, George B, Williams M, Whitaker K, Allison DB, Teague A, Demerath EW. Associations between human breast milk hormones and adipocytokines and infant growth and body composition in the first 6 months of life. Pediatr Obes. 2017;12 Suppl 1:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whitaker KM, Marino RC, Haapala JL, Foster L, Smith KD, Teague AM, Jacobs DR, Fontaine PL, McGovern PM, Schoenfuss TC et al.. Associations of maternal weight status before, during, and after pregnancy with inflammatory markers in breast milk. Obesity (Silver Spring). 2017;25(12):2092–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nommsen LA, Lovelady CA, Heinig MJ, Lonnerdal B, Dewey KG. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: The DARLING Study. Am J Clin Nutr. 1991;53(2):457–65. [DOI] [PubMed] [Google Scholar]
- 20. Makela J, Linderborg K, Niinikoski H, Yang B, Lagstrom H. Breast milk fatty acid composition differs between overweight and normal weight women: The STEPS Study. Eur J Nutr. 2013;52(2):727–35. [DOI] [PubMed] [Google Scholar]
- 21. Ley SH, Hanley AJ, Sermer M, Zinman B, O'Connor DL. Associations of prenatal metabolic abnormalities with insulin and adiponectin concentrations in human milk. Am J Clin Nutr. 2012;95(4):867–74. [DOI] [PubMed] [Google Scholar]
- 22. Fields DA, Demerath EW. Relationship of insulin, glucose, leptin, IL-6 and TNF-alpha in human breast milk with infant growth and body composition. Pediatr Obes. 2012;7(4):304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doneray H, Orbak Z, Yildiz L. The relationship between breast milk leptin and neonatal weight gain. Acta Paediatr. 2009;98(4):643–7. [DOI] [PubMed] [Google Scholar]
- 24. Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr. 2012;96(3):544–51. [DOI] [PubMed] [Google Scholar]
- 25. Martin LJ, Woo JG, Geraghty SR, Altaye M, Davidson BS, Banach W, Dolan LM, Ruiz-Palacios GM, Morrow AL. Adiponectin is present in human milk and is associated with maternal factors. Am J Clin Nutr. 2006;83(5):1106–11. [DOI] [PubMed] [Google Scholar]
- 26. Lemas DJ, Young BE, Baker PR 2nd, Tomczik AC, Soderborg TK, Hernandez TL, de la Houssaye BA, Robertson CE, Rudolph MC, Ir D et al.. Alterations in human milk leptin and insulin are associated with early changes in the infant intestinal microbiome. Am J Clin Nutr. 2016;103(5):1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chandler-Laney PC, Gower BA, Fields DA. Gestational and early life influences on infant body composition at 1 year. Obesity (Silver Spring). 2013;21(1):144–48. [DOI] [PubMed] [Google Scholar]
- 28. Lawton KA, Berger A, Mitchell M, Milgram KE, Evans AM, Guo L, Hanson RW, Kalhan SC, Ryals JA, Milburn MV. Analysis of the adult human plasma metabolome. Pharmacogenomics. 2008;9(4):383–97. [DOI] [PubMed] [Google Scholar]
- 29. Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009;81(16):6656–67. [DOI] [PubMed] [Google Scholar]
- 30. Evans CR, Karnovsky A, Kovach MA, Standiford TJ, Burant CF, Stringer KA. Untargeted LC-MS metabolomics of bronchoalveolar lavage fluid differentiates acute respiratory distress syndrome from health. J Proteome Res. 2014;13(2):640–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xia J, Wishart DS. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat Protoc. 2011;6(6):743–60. [DOI] [PubMed] [Google Scholar]
- 32. Xia J, Wishart DS. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr Protoc Bioinf. 2016;55:14.10.1–14.10.91. [DOI] [PubMed] [Google Scholar]
- 33. Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003;19(3):368–75. [DOI] [PubMed] [Google Scholar]
- 34. Benjamini YH, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol). 1995;57(1):289–300. [Google Scholar]
- 35. Rasmussen KM, Yaktine AL, Institute of Medicine (U.S.). Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight gain during pregnancy: Reexamining the guidelines. Washington, DC: National Academies Press; 2009. xiv, 854 p. [PubMed] [Google Scholar]
- 36. Hahn WH, Song JH, Song S, Kang NM. Do gender and birth height of infant affect calorie of human milk? An association study between human milk macronutrient and various birth factors. J Matern Fetal Neonatal Med. 2017;30(13):1608–12. [DOI] [PubMed] [Google Scholar]
- 37. Gidrewicz DA, Fenton TR. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014;14:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Powe CE, Knott CD, Conklin-Brittain N. Infant sex predicts breast milk energy content. Am J Hum Biol. 2010;22(1):50–54. [DOI] [PubMed] [Google Scholar]
- 39. Sundekilde UK, Downey E, O'Mahony JA, O'Shea CA, Ryan CA, Kelly AL, Bertram HC. The effect of gestational and lactational age on the human milk metabolome. Nutrients. 2016;8(5):e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Longini M, Tataranno ML, Proietti F, Tortoriello M, Belvisi E, Vivi A, Tassini M, Perrone S, Buonocore G. A metabolomic study of preterm and term human and formula milk by proton MRS analysis: Preliminary results. J Matern Fetal Neonatal Med. 2014;27 Suppl 2:27–33. [DOI] [PubMed] [Google Scholar]
- 41. Spevacek AR, Smilowitz JT, Chin EL, Underwood MA, German JB, Slupsky CM. Infant maturity at birth reveals minor differences in the maternal milk metabolome in the first month of lactation. J Nutr. 2015;145(8):1698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smilowitz JT, O'Sullivan A, Barile D, German JB, Lonnerdal B, Slupsky CM. The human milk metabolome reveals diverse oligosaccharide profiles. J Nutr. 2013;143(11):1709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dangat K, Upadhyay D, Kilari A, Sharma U, Kemse N, Mehendale S, Lalwani S, Wagh G, Joshi S, Jagannathan NR. Altered breast milk components in preeclampsia; An in-vitro proton NMR spectroscopy study. Clin Chim Acta. 2016;463:75–83. [DOI] [PubMed] [Google Scholar]
- 44. Urbaniak C, McMillan A, Angelini M, Gloor GB, Sumarah M, Burton JP, Reid G. Effect of chemotherapy on the microbiota and metabolome of human milk, a case report. Microbiome. 2014;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Catli G, Olgac Dundar N, Dundar BN. Adipokines in breast milk: An update. J Clin Res Pediatr Endocrinol. 2014;6(4):192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hunt KM, Foster JA, Forney LJ, Schutte UM, Beck DL, Abdo Z, Fox LK, Williams JE, McGuire MK, McGuire MA. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS One. 2011;6(6):e21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C, Soeda J, Fernandez-Twinn DS, Martin-Gronert MS, Ozanne SE et al.. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. 2010;52(6):913–20. [DOI] [PubMed] [Google Scholar]
- 48. Vogt MC, Paeger L, Hess S, Steculorum SM, Awazawa M, Hampel B, Neupert S, Nicholls HT, Mauer J, Hausen AC et al.. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell. 2014;156(3):495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Plagemann A, Harder T, Franke K, Kohlhoff R. Long-term impact of neonatal breast-feeding on body weight and glucose tolerance in children of diabetic mothers. Diabetes Care. 2002;25(1):16–22. [DOI] [PubMed] [Google Scholar]
- 50. Fields DA, George B, Williams M, Whitaker K, Allison DB, Teague A, Demerath EW. Associations between human breast milk hormones and adipocytokines and infant growth and body composition in the first 6 months of life. Pediatr Obes. 2017;12 Suppl 1:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schlimme E, Martin D, Meisel H. Nucleosides and nucleotides: Natural bioactive substances in milk and colostrum. Br J Nutr. 2000;84 Suppl 1:S59–68. [DOI] [PubMed] [Google Scholar]
- 52. Singhal A, Kennedy K, Lanigan J, Clough H, Jenkins W, Elias-Jones A, Stephenson T, Dudek P, Lucas A. Dietary nucleotides and early growth in formula-fed infants: A randomized controlled trial. Pediatrics. 2010;126(4):e946–53. [DOI] [PubMed] [Google Scholar]
- 53. Carver JD. Dietary nucleotides: Cellular immune, intestinal and hepatic system effects. J Nutr. 1994;124(1 Suppl):144S–48S. [DOI] [PubMed] [Google Scholar]
- 54. Gil A. Modulation of the immune response mediated by dietary nucleotides. Eur J Clin Nutr. 2002;56 Suppl 3:S1–4. [DOI] [PubMed] [Google Scholar]
- 55. Sato N, Kawakami H, Idota A. Nucleotide and nucleoside supplementation may morphologically promote the differentiation of human caco-2 cells. J Nutr Sci Vitaminol (Tokyo). 2000;46(4):175–9. [DOI] [PubMed] [Google Scholar]
- 56. Deng Y, Wang ZV, Gordillo R, An Y, Zhang C, Liang Q, Yoshino J, Cautivo KM, De Brabander J, Elmquist JK et al.. An adipo-biliary-uridine axis that regulates energy homeostasis. Science. 2017;355(6330):eaaf5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Perng W, Hector EC, Song PXK, Tellez Rojo MM, Raskind S, Kachman M, Cantoral A, Burant CF, Peterson KE. Metabolomic determinants of metabolic risk in Mexican adolescents. Obesity (Silver Spring). 2017;25(9):1594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tam ZY, Ng SP, Tan LQ, Lin CH, Rothenbacher D, Klenk J, Boehm BO, SPC Team & ActiFE Study Group. Metabolite profiling in identifying metabolic biomarkers in older people with late-onset type 2 diabetes mellitus. Sci Rep. 2017;7(1):4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bode L. The functional biology of human milk oligosaccharides. Early Hum Dev. 2015;91(11):619–22. [DOI] [PubMed] [Google Scholar]
- 60. Lin AE, Autran CA, Szyszka A, Escajadillo T, Huang M, Godula K, Prudden AR, Boons GJ, Lewis AL, Doran KS et al.. Human milk oligosaccharides inhibit growth of group B Streptococcus. J Biol Chem. 2017;292(27):11243–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Alderete TL, Autran C, Brekke BE, Knight R, Bode L, Goran MI, Fields DA. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am J Clin Nutr. 2015;102(6):1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Villarreal-Perez JZ, Villarreal-Martinez JZ, Lavalle-Gonzalez FJ, Torres-Sepulveda Mdel R, Ruiz-Herrera C, Cerda-Flores RM, Castillo-García ER, Rodríguez-Sánchez IP, Martínez de Villarreal LE. Plasma and urine metabolic profiles are reflective of altered beta-oxidation in non-diabetic obese subjects and patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2014;6:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, DeLany JP. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring). 2010;18(9):1695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hootman KC, Trezzi JP, Kraemer L, Burwell LS, Dong X, Guertin KA, Jaeger C, Stover PJ, Hiller K, Cassano PA. Erythritol is a pentose-phosphate pathway metabolite and associated with adiposity gain in young adults. Proc Natl Acad Sci U S A. 2017;114(21):E4233–E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yu D, Moore SC, Matthews CE, Xiang YB, Zhang X, Gao YT, Zheng W, Shu XO. Plasma metabolomic profiles in association with type 2 diabetes risk and prevalence in Chinese adults. Metabolomics. 2016;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D, Palma MJ, Roberts LD, Dejam A, Souza AL et al.. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125(18):2222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang Y, Yuan Y, Zhang Y, Lei C, Zhou Y, He J, Sun Z. Serum 1,5-anhydroglucitol level as a screening tool for diabetes mellitus in a community-based population at high risk of diabetes. Acta Diabetol. 2017;54(5):425–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.