Abstract
Objective: Postoperative atrial fibrillation (POAF) is a common complication after cardiac surgery. The aim of this multicenter study was to determine the relationship between POAF and patients' progress in early rehabilitation after heart valve surgery. Methods: We enrolled 302 patients (mean age, 69±10 years) who had undergone heart valve surgery. POAF was monitored using continuous electrocardiogram telemetry, and the Short Physical Performance Battery (SPPB) was used to assess lower-extremity function before surgery and at the time of discharge. Progress in early rehabilitation was evaluated by the duration from the surgery to independent walking. We determined factors associated delayed early rehabilitation and evaluated the interplay of POAF and delayed early rehabilitation in increasing the risk of decline in lower-extremity function from preoperatively to hospital discharge. Results: Multivariate analysis determined POAF to be independent predictors of delayed early rehabilitation after heart valve surgery (OR: 3.906, P = .01). The association between delayed early rehabilitation and decline in lower extremity function was stronger in patients with POAF (OR: 2.73, P = .041) than in those without (OR: 2.22, P = .052). Conclusions: POAF was clinical predictors of delayed early rehabilitation in patients undergoing heart valve surgery. The combination of POAF with delayed early rehabilitation conferred a high risk of decline in lower-extremity function during hospitalization.
Keywords: postoperative atrial fibrillation, heart valve surgery, rehabilitation, lower-extremity function
In patients who have undergone cardiac surgery, prolonged inactivity postoperatively is associated with increased sensation of fatigue and reduced functional capacity1). Recently, early rehabilitation has been shown to play an important role in enhancing postoperative recovery of physical function during hospitalization after cardiac surgery2). Studies have reported that early rehabilitation significantly reduced both the length of hospital stay and medical costs while providing similar clinical outcomes after cardiac surgery3,4).
However, early rehabilitation can be delayed by various postoperative complications, such as heart failure, pulmonary complications, neurologic deficits, and acute kidney dysfunction5,6). Postoperative atrial fibrillation (POAF) is a common complication in patients who have undergone heart valve surgery7). A previous study reported that postoperative median intensive care unit stay and hospital stay were higher in patients with POAF compared with those with sinus rhythm (SR) after cardiac surgery (3.6 days in POAF vs. 2 days in SR; 10 days in POAF vs. 7 days in SR, respectively)8). Because POAF has been demonstrated to increase the likelihood of stroke or myocardial infarction and to be associated with an increased risk of short- and long-term morbidity and mortality9), we speculated that POAF may interfere with progress in postoperative early rehabilitation. However, the association between POAF and progress in early rehabilitation in patients undergoing heart valve surgery has not been fully elucidated. In addition, it is unknown whether delayed early rehabilitation because of POAF affects physical functional recovery during hospitalization.
This study investigated the relationship between POAF and progress in early rehabilitation in patients who have undergone heart valve surgery.
Methods
Patients
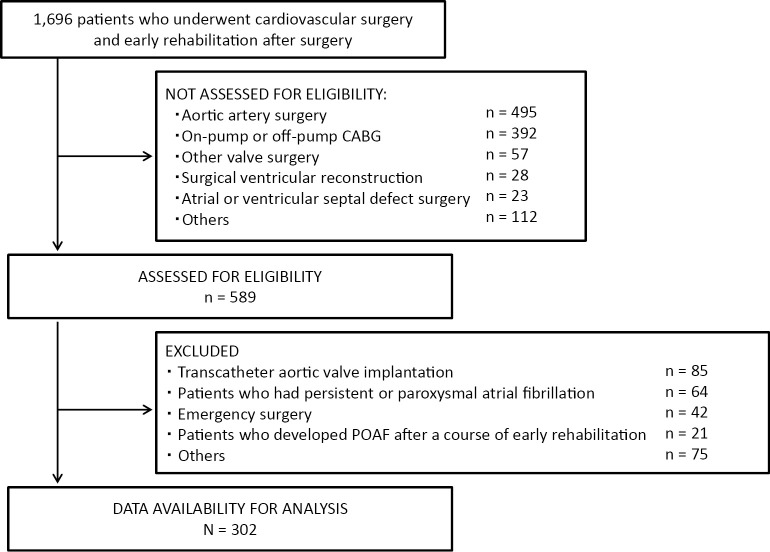
The present investigation was a multicenter study conducted with the approval of the ethics review committee of Hyogo University Health Sciences (chairperson of the ethics committee, Michiaki Baba; protocol number, 12029; date of approval by the ethics committee, 11/6/2014), in addition to the local institutional board at each participating hospital. Because of the retrospective nature of the study, written informed consent to participate was waived. We identified 1,696 patients who, between April 2015 and March 2016, underwent cardiovascular surgery and early rehabilitation after surgery at 10 institutions in Japan (Figure 1). Of these patients, 589 consecutive patients who underwent aortic and mitral valve surgery were enrolled in the study. Patients who had persistent or paroxysmal atrial fibrillation (AF) diagnosed by Holter electrocardiography (ECG) preoperatively were excluded from this study. We also excluded patients who underwent emergency surgery or transcatheter aortic valve implantation. In addition, patients who developed POAF after a course of early rehabilitation were excluded because progress in early rehabilitation was an outcome of the study. After applying inclusion and exclusion criteria, 302 patients were enrolled (ratio of patients in each facility: Sakakibara Heart Institute, 32%; Kishiwada Tokushukai Hospital, 20%; Kobe City Medical Center General Hospital, 7%; The Cardiovascular Institute, 7%; Fukuyama Cardiovascular Hospital, 7%; St. Luke's International Hospital, 6%; Kitano Hospital, 6%; Shizuoka Medical Center, 6%; Higashi Takarazuka Satoh Hospital, 5%; Sakakibara Heart Institute of Okayama, 5%).
Figure 1.
Exclusions
CABG: Coronary artery bypass grafting; POAF: postoperative atrial fibrillation.
Perioperative management and postoperative early rehabilitation
Preoperative antiarrhythmic therapies were not routinely adopted. In general, β-blockers were restarted in the postoperative period as soon as possible unless contraindicated for clinical reasons. At the end of surgery, patients were transferred to a dedicated intensive care unit and managed postoperatively. Amiodarone, digoxin, β-blockers, and diltiazem were constituted as standard pharmacological treatment for rate and rhythm control of POAF10). Warfarin was administered within 48 h of the onset POAF, with the aim of achieving an international normalized ratio of between 2.0 and 3.010).
All patients underwent early rehabilitation from the day following surgery, according to Japanese Circulation Society guidelines for rehabilitation in patients with cardiovascular disease, as part of the standard of care at each participating hospital2). Patients participated in postoperative early rehabilitation consisting of upper- and lower-extremity exercise in bed, sitting on the edge of the bed, standing at the bedside, and walking around the bed and for 100 m on the ward2). Progress in early rehabilitation was carried out according to criteria for evaluating the results of exercise stress tests, and conducted regardless of the day of the week by a physical therapist or nurse. Even if patients developed POAF, early rehabilitation was carried out if their hemodynamic status was stable. After early rehabilitation, patients continued gait training for up to 500 m and performed endurance training using a stationary bike or treadmill in the hospital's inpatient rehabilitation center until they were discharged from the hospital.
Data collection
Preoperative patient characteristics, including age, sex, body mass index (BMI), comorbidities, laboratory values, cardiac functions, and pulmonary function were obtained from medical records at each institution. Cardiac functions were assessed by ultrasound at each participating hospital. Left ventricular (LV) and left atrial dimensions were measured, and LV ejection fraction (LVEF) was assessed using the biplane Simpson method. LV diastolic function was calculated as the ratio of early mitral inflow velocity to mitral annular early diastolic velocity (E/e′). Forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) were measured using a spirometer and used to calculate FEV1/FVC, a parameter of pulmonary function, in accordance with Japanese Respiratory Society guidelines.
Frailty index for elderly Japanese (FI-J) score was assessed as a parameter of frailty11). The FI-J is a 15-item self-report questionnaire that has been reported to have sensitivity and specificity of 70% and 89%, respectively, with the Fried frailty phenotype11). Assessments of FI-J were conducted on the day before surgery.
Participants performed the Short Physical Performance Battery (SPPB), which is a brief performance battery based on a timed 4-m walk test, a test of repeated chair stands, and a set of standing balance tests12). Each test score ranged from 0 to 4. The sum of the three test scores equaled the final SPPB score, with a possible range of 0 to 12. A score of 12 indicated the highest degree of lower-extremity function. The SPPB has been validated as an assessment tool for measuring lower-extremity function and is widely used in clinical settings13); it is also an independent predictor of long-term survival of older patients with heart failure14). In the present study, the SPPB was performed on the day before surgery and on the day of hospital discharge. To assess decline in lower-extremity function in patients during hospitalization, the change in SPPB score from preoperatively to hospital discharge was calculated (ΔSPPB score). It has been reported that a change of 1 point in SPPB score is the best initial estimate of a substantial clinical effect15). Therefore, we defined a decline in lower-extremity function as a decrease in patients' SPPB score of more than 1 point from preoperatively to hospital discharge.
Surgical procedure, operative duration, cardiopulmonary bypass (CPB) duration, and aortic cross-clamping duration were obtained from medical records and used as parameters of intraoperative clinical characteristics.
Detection of POAF
Heart rate and rhythm were monitored, using continuous ECG telemetry, for the occurrence of POAF until postoperative day (POD) 6. In a study by Echahidi et al., POAF tended to occur within 2-4 days after the procedure and 94% of patients developed this dysrhythmia before the end of POD 616). POAF was defined as any documented sustained episodes of AF lasting ≥5 min recorded on continuous telemetry17). The diagnosis of POAF was confirmed using a 12-lead electrocardiogram by a cardiologist at each participating hospital.
Evaluation of progress of early rehabilitation
We examined the duration from the time of surgery to the day when patients were able to complete sitting on the edge of the bed, standing at the bedside, and walking around the bed. We also investigated the duration from the time of surgery until patients were able to complete a 100-m walk without assistance as a parameter of progress in early rehabilitation5). According to Japanese Circulation Society guidelines, patients should complete a 100-m walk on or before POD 5. For this reason, patients were divided into two groups: non-delayed and delayed early rehabilitation. In addition, the length of intensive care unit stay and postoperative hospital stay were also evaluated as measures of early rehabilitation progress.
Sample size
The sample size was calculated based on a significance level of .05, and power equal to .80, using the software EZR (Easy R), which is based on R (http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmed.html). When the rate of delayed early rehabilitation in a previous study was used as a reference value5), the sample size was determined to be 268 patients or more in total.
Statistical analysis
Preoperative and intraoperative clinical characteristics, and parameters of progress in early rehabilitation, were compared between patients who developed POAF (POAF group) and those who maintained sinus rhythm (SR group) to evaluate the characteristics of POAF patients. Variables were compared between groups using the unpaired Student's t-test or Fisher's exact test.
Univariate and multivariate logistic regression analysis using the backward-elimination method with likelihood ratio were carried out using delayed early rehabilitation as the dependent variable. For multivariate analyses, the variables identified as significant in univariate analysis were used as exploratory variables, and odds ratios (ORs) were calculated with 95% confidence intervals (CIs). Statistically significant factors identified in multivariate analyses were considered independent predictors of delayed early rehabilitation.
In a sub-analysis investigating the interplay of POAF and delayed early rehabilitation in increasing the risk of decline in lower-extremity function, we divided our patients into four groups according to heart rhythm and progress in early rehabilitation: SR/non-delayed, SR/delayed early rehabilitation, POAF/non-delayed, and POAF/delayed early rehabilitation. Multivariate logistic regression analyses were carried out for the four groups and age as exploratory variables, with decline in lower-extremity function from preoperatively to hospital discharge as the dependent variable.
Data are presented as mean±standard deviation for continuous variables, and as frequencies and percentages for categorical variables. Differences with a P value of <.05 were considered significant. All analyses were carried out using IBM SPSS Statistics for Windows, Version 19.0 (IBM Corp., Armonk, NY, USA).
Results
Table 1 summarizes the pre- and intraoperative clinical characteristics of the POAF (n = 66; mean age 67±11 years) and SR (n = 236; mean age 73 ± 9 years) patients. POAF occurred most frequently on POD 2 (32%), followed by POD 3 (27%) and POD 1 (17%). Age, proportion of females, LVEF, E/e′, and proportion of complex surgery were significantly higher in POAF patients than in SR patients (P < .001, P = .02, P = .009, P = .037, and P = .027, respectively). There were no significant differences in BMI; the proportion of patients with hypertension, diabetes mellitus, dyslipidemia, peripheral artery disease, myocardial infarction, and cerebrovascular accident; B-type natriuretic peptide, albumin, and C-reactive protein concentrations; estimated glomerular filtration rate (eGFR); glycohemoglobin (HbA1c) percentage; left atrial dimension; FEV1/FVC; SPPB score; FI-J score; and durations of operation, CPB, and aortic cross-clamp.
Table 1.
Pre- and intraoperative clinical characteristics
| Total (N=302) | SR group (n=236) | POAF group (n=66) | P | |
|---|---|---|---|---|
| Data expressed as mean ± standard deviation or n (%). AVP: aortic valvuloplasty; AVR: atrial valve replacement; E/e': early mitral inflow velocity to mitral annular early diastolic velocity; eGFR: estimated glomerular filtration rate; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; HbA1c: glycohemoglobin; LVEF: left ventricular ejection fraction; POAF: postoperative atrial fibrillation; MVP: mitral valvuloplasty; MVR: mitral valve replacement; SR: sinus rhythm. | ||||
| Age (years) | 69 ± 10 | 67 ± 11 | 73 ± 9 | <.001 |
| Male / Female | 190/112 | 155/81 | 35/31 | .020 |
| Body mass index (kg/m2) | 22.6 ± 3.3 | 22.6 ± 3.4 | 22.5 ± 3.1 | .758 |
| Hypertension n (%) | 193 (64) | 153 (65) | 40 (62) | .604 |
| Diabetes mellitus n (%) | 65 (22) | 47 (20) | 18 (28) | .175 |
| Dyslipidemia n (%) | 106 (35) | 78 (33) | 28 (43) | .153 |
| Peripheral artery disease n (%) | 22 (7) | 17 (7) | 5 (8) | .700 |
| Myocardial infarction n (%) | 24 (8) | 19 (8) | 5 (8) | .978 |
| Cerebrovascular accident n (%) | 30 (10) | 25 (11) | 5 (8) | .225 |
| B-type natriuretic peptide (pg/mL) | 301 ± 428 | 314 ± 459 | 248 ± 268 | .338 |
| Albumin (mg/dL) | 4.0 ± 0.5 | 4.0 ± 0.5 | 4.0 ± 0.4 | .813 |
| eGFR (mL/min/1.73 m2) | 55.1 ± 23.1 | 55.8 ± 23.5 | 52.2 ± 21.4 | .287 |
| C-reactive protein (mg/dL) | 0.22 ± 0.34 | 0.22 ± 0.35 | 0.22 ± 0.34 | .892 |
| HbA1c (%) | 5.8 ± 0.9 | 5.7 ± 0.7 | 6.0 ± 1.2 | .128 |
| LVEF (%) | 59.3 ± 12.9 | 58.3 ± 13.3 | 62.6 ± 10.7 | .009 |
| Left atrial dimension (mm) | 41.7 ± 6.9 | 41.5 ± 7.1 | 42.5 ± 6.3 | .338 |
| E/e' | 19.1 ± 9.3 | 18.5 ± 8.6 | 21.5 ± 11.1 | .037 |
| FEV1/FVC (%) | 77.2 ± 9.3 | 77.1 ± 9.4 | 78.1 ± 8.9 | .452 |
| Short physical performance battery score (point) | 11.0 ± 1.8 | 10.9 ± 2.0 | 11.1 ± 1.4 | .615 |
| Frailty index (point) | 2.3 ± 2.2 | 2.3 ± 2.3 | 2.3 ± 1.9 | .940 |
| Surgical procedure | ||||
| Isolated AVR or AVP n (%) | 91 (30) | 78 (33) | 11 (16) | |
| Isolated MVR or MVP n (%) | 45 (15) | 38 (16) | 9 (14) | .027 |
| Complex surgery n (%) | 166 (55) | 120 (51) | 46 (70) | |
| Operative duration (min) | 298 ± 97 | 292 ± 95 | 318 ± 100 | .064 |
| Cardiopulmonary bypass duration (min) | 158 ± 58 | 156 ± 59 | 166 ± 54 | .210 |
| Aortic cross clamping duration (min) | 112 ± 42 | 110 ± 42 | 120 ± 42 | .135 |
Variables indicating progress in early rehabilitation are shown in Table 2. The POAF group took significantly more time to complete standing at the bedside (P = .019), walking around the bed (P = .007), and a 100-m walk without assistance (P = .001). Length of hospital stay after surgery and delayed early rehabilitation were also significantly greater for the POAF group than for the SR group (P = .008 and P < .001, respectively).
Table 2.
Early rehabilitation progress
| SR group (n=236) | POAF group (n=66) | P | |
|---|---|---|---|
| Data expressed as mean ± standard deviation. ICU: intensive care unit; POAF: postoperative atrial fibrillation; SR: sinus rhythm. | |||
| Sitting on edge of bed (days) | 1.3 ± 0.9 | 1.5 ± 1.2 | 0.176 |
| Standing at bedside (days) | 1.6 ± 1.2 | 2.5 ± 2.9 | 0.019 |
| Walking around bed (days) | 2.4 ± 1.9 | 4.1 ± 4.5 | 0.007 |
| 100 m of independent walking (days) | 4.6 ± 3.4 | 7.2 ± 5.1 | 0.001 |
| Length of stay in ICU (days) | 2.9 ± 2.4 | 3.2 ± 2.9 | 0.431 |
| Length of postoperative hospital stay (days) | 17.7 ± 9.6 | 23.0 ± 14.1 | 0.008 |
| Patients with delayed early rehabilitation, n (%) | 73 (31) | 46 (69) | <0.001 |
Table 3 shows the results of univariate analysis of factors related to delayed early rehabilitation. Unadjusted univariate analysis identified age, albumin, eGFR, CRP, HbA1c, E/e', FEV1/FVC, SPPB, durations of operation and CPB, and POAF (absence, 0; presence, 1) as factors related to delayed early rehabilitation. After eliminating CPB duration, which was closely related to operative duration (r = 0.74, P < 0.001), age, albumin, eGFR, CRP, HbA1c, E/e′, FEV1/FVC, SPPB, operative duration, and POAF (absence, 0; presence, 1) were adopted as exploratory variables in the multivariate logistic regression model. Ultimately age, albumin, operative duration, and POAF were identified as independent predictors of delayed early rehabilitation after heart valve surgery in multivariate analysis (Table 4).
Table 3.
Univariate analysis of factors related to the retardation of early rehabilitation
| OR | 95% CI | P | |
|---|---|---|---|
| AVP: aortic valvuloplasty; AVR: atrial valve replacement; CI: confidence interval; E/e'; early mitral inflow velocity to mitral annular early diastolic velocity; eGFR: estimated glomerular filtration rate; FEV1; forced expiratory volume in 1 s; FVC: forced vital capacity; HbA1c: glycohemoglobin; LVEF: left ventricular ejection fraction; MVP; mitral valvuloplasty; MVR; mitral valve replacement; OR: odds ratio; POAF: postoperative atrial fibrillation. | |||
| Age (1 years old increase) | 1.077 | 1.046-1.108 | .001 |
| Sex (male) | 0.614 | 0.371-1.018 | .060 |
| Body mass index (1 kg/m2 increase) | 0.866 | 1.006-1.083 | .866 |
| Hypertension (presence) | 1.147 | 0.684-1.925 | .603 |
| Cerebrovascular accident (presence) | 1.496 | 0.671-3.338 | .325 |
| B-type natriuretic peptide (1 pg/mL increase) | 1.001 | 1.000-1.001 | .155 |
| Albumin (1 mg/dL increase) | 0.588 | 0.364-0.951 | .030 |
| eGFR (1 mL/min/1.73 m2 increase) | 0.986 | 0.975-0.996 | .009 |
| C-reactive protein (1 mg/dL increase) | 2.757 | 1.310-5.800 | .008 |
| HbA1c (1 % increase) | 1.485 | 1.035-2.130 | .032 |
| LVEF (1 % increase) | 0.996 | 0.977-1.015 | .688 |
| Left atrial dimension (1 mm increase) | 0.997 | 0.962-1.033 | .862 |
| E/e' (1 increase) | 1.056 | 1.025-1.088 | <.001 |
| FEV1/FVC (1 % increase) | 0.968 | 0.940-0.997 | .032 |
| Short physical performance battery score (1 point increase) | 0.779 | 0.673-0.902 | .001 |
| Frailty Index (1 point increase) | 1.137 | 0.997-1.297 | .056 |
| Surgical procedure | |||
| Isolated AVR or AVP | Reference | ||
| Isolated MVR or MVP (presence) | 0.602 | 0.261-1.387 | .233 |
| Complex surgery (presence) | 1.063 | 0.611-1.851 | .828 |
| Operative duration (1 min increase) | 1.006 | 1.003-1.009 | <.001 |
| Cardiopulmonary bypass duration (1 min increase) | 1.007 | 1.003-1.011 | .002 |
| POAF (presence) | 4.711 | 2.595-8.552 | <.001 |
Table 4.
Multivariate analysis of factors related to delayed early rehabilitation
| OR | 95% CI | P | |
|---|---|---|---|
| Models χ2, <.001; Hosmer-Lemshow, .704; Negelkerke R2, .438. CI: confidence interval; OR: odds ratio; POAF: postoperative atrial fibrillation. | |||
| Age (1 years old increase) | 1.084 | 1.023-1.148 | .007 |
| Albumin (1 mg/dL increase) | 0.763 | 0.552-0.974 | .011 |
| Operative duration (1 min increase) | 1.013 | 1.007-1.019 | <.001 |
| POAF (presence) | 3.906 | 1.376-11.082 | .010 |
The association between delayed early rehabilitation and decline in lower-extremity function from preoperatively to hospital discharge was significantly stronger in the POAF group (OR 2.73, 95% CI 1.05-7.07, P = .041) than in the SR group (OR 2.22, 95% CI 0.99-5.21, P = .052) (Figure 2).
Figure 2.
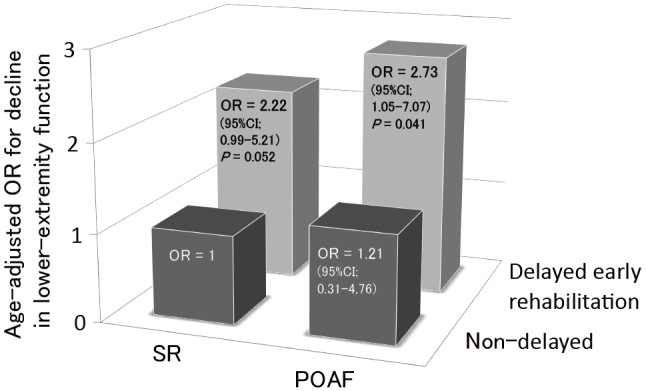
The interplay of POAF and delayed early rehabilitation in increasing the risk of decline in lower extremity function
OR: odds ratio; POAF: postoperative atrial fibrillation; SR: sinus rhythm.
Discussion
POAF is the most common complication after cardiac surgery. The present study has shown POAF to be a clinical predictor of delayed early rehabilitation in patients undergoing heart valve surgery, while also establishing that the combination of POAF with delayed rehabilitation confers a high risk of decline in lower-extremity function during hospitalization.
To confirm the characteristics of POAF patients, we compared characteristics of POAF and SR patients. In the present study, POAF occurred most often on POD 2, which is consistent with the results of another study in which the mean time of onset of POAF was 2.6±2.2 days postoperatively18), and age, E/e′, and proportion of patients undergoing complex surgery were significantly greater in POAF patients. It is well known that the risk of POAF is increased in the elderly, in those with cardiac diastolic dysfunction, or in those who undergo a complex procedure16). However, in the present study the incidence of POAF was 22%, which is low compared with the 40% to 50% reported by a study of the incidence of POAF after valve replacement or repair, or combined procedures19). Previous studies included patients undergoing emergency surgery and with paroxysmal AF, and defined POAF as including other postoperative arrhythmias (e.g., atrial flutter and atrial tachycardia)19). Moreover, duration of POAF was not included as a criterion for diagnosis19). A possible explanation for the results of the present study is that the subjects and definition of POAF differed from those of previous studies.
In the present study, multivariate analysis identified POAF, age, albumin, and operative duration as independent predictors of delayed early rehabilitation after heart valve surgery. POAF has been reported to be a major morbid event associated with increased risks of postoperative thromboembolism and stroke, hemodynamic compromise, ventricular dysrhythmias, and reintubation9,20-22). In addition, weakened atrial and ventricular contraction, characteristic of AF, is well known to cause loss of cardiac output, which can exacerbate heart failure23). We therefore considered these postoperative complications and/or subjective symptoms such as palpitation, shortness of breath, and dizziness resulting from heart failure as reasons for the relationship between POAF and delayed early rehabilitation. Poor preoperative nutritional status and older age have also been shown to be clinical predictors of delayed rehabilitation after cardiac surgery24,25), and intensely invasive surgery can lead to adverse events (pain, nausea/vomiting, ileus, stress-induced catabolism, impaired pulmonary function, increased cardiac demand, and risk of thromboembolism), which in turn result in prolonged mechanical ventilation and disturbed early rehabilitation26). Thus, we consider the results of these studies to be consistent with those of the present study.
With regard to the risk of decline in lower-extremity function from preoperatively to hospital discharge, the present study suggested an interaction between POAF and delayed early rehabilitation. It is important to maintain lower-extremity function during hospitalization because of its association with long-term prognosis in patients who undergo cardiac surgery27). Previous studies have shown that AF alters atrial structure and performance, and an irregular heart rate diminishes LV filling and cardiac output28). Reduced cardiac output impairs peripheral perfusion and ultimately diminishes the capacity for activities of daily living29). For this reason, AF is known to contribute directly to the decline in physical performance22). In addition, early exercise training in critically ill patients was demonstrated to enhance recovery of functional exercise capacity and muscle strength at hospital discharge30). Schweickert et al. reported that patients who underwent early rehabilitation had improved return to independent functional status at hospital discharge when compared with patients who received standard care without early rehabilitation31). Therefore, we considered delayed early rehabilitation also to lead to reduced physical function and capacity. To maintain patients' lower-extremity function, their postoperative complications and progress in early rehabilitation must be carefully monitored, and a different exercise modality or alternative therapy should be considered for those who have POAF and delayed early rehabilitation.
The present study has several limitations. First, it focused on the relationship between POAF and recovery of postoperative physical function only in patients undergoing elective heart valve surgery. Because POAF also manifests in patients undergoing other cardiac or thoracic vascular surgery, these populations should also be investigated. Although we adjusted for confounders such as preoperative and intraoperative clinical characteristics, including physical function, we cannot exclude the possibility that progress in early rehabilitation was affected by other factors, such as psychological aspects or other postoperative complications. Another limitation was the retrospective nature of the study. Further prospective investigations, including these additional confounders in multivariate analysis, should be conducted.
Conclusions
The present study demonstrated POAF to be a clinical predictor of delayed early rehabilitation in patients who underwent heart valve surgery. We also found that the combination of POAF with delayed early rehabilitation conferred a high risk of decline in lower-extremity function during hospitalization.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
We thank the staff members of all the hospitals, institutions, and universities of the Cardiovascular Surgery Physiotherapy Network who collaborated in this study. We are grateful to the following institutions for facilitating this multicenter study: Sakakibara Heart Institute, Kishiwada Tokushukai Hospital, Kobe City Medical Center General Hospital, The Cardiovascular Institute, St. Luke's International Hospital, Higashi Takarazuka Satoh Hospital, Sakakibara Heart Institute of Okayama, Kitano Hospital, Fukuyama Cardiovascular Hospital, Hyogo University of Health Sciences, Tokyo University of Technology, Tokoha University and Shizuoka Medical Center.
References
- 1.da CostaTorres D, Dos Santos PM, et al.: Effectiveness of an early mobilization program on functional capacity after coronary artery bypass surgery: A randomized controlled trial protocol. SAGE Open Med. 2016; 4: 2050312116682256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.JCS Joint Working Group: Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ J. 2014; 78: 2022-2093. [DOI] [PubMed] [Google Scholar]
- 3.Ender J, Borger MA, et al.: Cardiac surgery fast-track treatment in a postanesthetic care unit: six-month results of the Leipzig fast-track concept. Anesthesiology. 2008; 109: 61-66. [DOI] [PubMed] [Google Scholar]
- 4.Cheng DC, Wall C, et al.: Randomized assessment of resource use in fast-track cardiac surgery 1-year after hospital discharge. Anesthesiology. 2003; 98: 651-657. [DOI] [PubMed] [Google Scholar]
- 5.Saitoh M, Takahashi T, et al.: Factors determining achievement of early postoperative cardiac rehabilitation goal in patients with or without preoperative kidney dysfunction undergoing isolated cardiac surgery. J Cardiol. 2013; 61: 299-303. [DOI] [PubMed] [Google Scholar]
- 6.Ota H, Kawai H, et al.: Effect of early mobilization on discharge disposition of mechanically ventilated patients. J Phys Ther Sci. 2015; 27: 859-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunning J, Treasure T, et al.: Guidelines on the prevention and management of de novo atrial fibrillation after cardiac and thoracic surgery. Eur J Cardiothorac Surg. 2006; 30: 852-872. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin EJ, Wolf PA, et al.: Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998; 98: 946-952. [DOI] [PubMed] [Google Scholar]
- 9.Almassi GH, Schowalter T, et al.: Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997; 226: 501-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunning J, Treasure T, et al.: Guidelines on the prevention and management of de novo atrial fibrillation after cardiac and thoracic surgery. Eur J Cardiothorac Surg. 2006; 30: 852-872. [DOI] [PubMed] [Google Scholar]
- 11.Shinkai S, Watanabe N, et al.: Validity of the “Kaigo-Yobo Check-List" as a frailty index. Nihon Koshu Eisei Zasshi. 2013; 60: 262-274. [PubMed] [Google Scholar]
- 12.Guralnik JM, Simonsick EM, et al.: A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994; 49: M85-94. [DOI] [PubMed] [Google Scholar]
- 13.Morisawa T, Ueno K, et al.: Significance of sequential cardiac rehabilitation program through inter-hospital cooperation between acute care and rehabilitation hospitals in elderly patients after cardiac surgery in Japan. Heart Vessels. 2017; 32: 1220-1226. [DOI] [PubMed] [Google Scholar]
- 14.Chiarantini D, Volpato S, et al.: Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Card Fail. 2010; 16: 390-395. [DOI] [PubMed] [Google Scholar]
- 15.Perera S, Mody SH, et al.: Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006; 54: 743-749. [DOI] [PubMed] [Google Scholar]
- 16.Echahidi N, Pibarot P, et al.: Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008; 51: 793-801. [DOI] [PubMed] [Google Scholar]
- 17.Ishida K, Kimura F, et al.: Relation of inflammatory cytokines to atrial fibrillation after off-pump coronary artery bypass grafting. Eur J Cardiothorac Surg. 2006; 29: 501-505. [DOI] [PubMed] [Google Scholar]
- 18.Albahrani MJ, Swaminathan M, et al.: Postcardiac surgery complications: association of acute renal dysfunction and atrial fibrillation. Anesth Analg. 2003; 96: 637-643. [DOI] [PubMed] [Google Scholar]
- 19.Creswell LL, Schuessler RB, et al.: Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993; 56: 539-549. [DOI] [PubMed] [Google Scholar]
- 20.Fuller JA, Adams GG, et al.: Atrial fibrillation after coronary artery bypass grafting. Is it a disorder of the elderly? J Thorac Cardiovasc Surg. 1989; 97: 821-825. [PubMed] [Google Scholar]
- 21.Lauer MS, Eagle KA, et al.: Atrial fibrillation following coronary artery bypass surgery. Prog Cardiovasc Dis. 1989; 31: 367-378. [DOI] [PubMed] [Google Scholar]
- 22.Creswell LL, Schuessler RB, et al.: Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993; 56: 539-549. [DOI] [PubMed] [Google Scholar]
- 23.Magnani JW, Wang N, et al.: Atrial Fibrillation and Declining Physical Performance in Older Adults: The Health, Aging, and Body Composition Study. Circ Arrhythm Electrophysiol. 2016; 9: e003525. (10.1161/CIRCEP.115.003525). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa M, Izawa KP, et al.: Poor preoperative nutritional status is an important predictor of the retardation of rehabilitation after cardiac surgery in elderly cardiac patients. Aging Clin Exp Res. 2017; 29: 283-290. [DOI] [PubMed] [Google Scholar]
- 25.Tobita R, Iwata K, et al.: Clinical characteristics of functional recovery after coronary artery bypass graft surgery in Japanese octogenarians. J Phys Ther Sci. 2016; 28: 621-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kehlet H and Dahl JB: Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003; 362: 1921-1928. [DOI] [PubMed] [Google Scholar]
- 27.Afilalo J, Eisenberg MJ, et al.: Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010; 56: 1668-1676. [DOI] [PubMed] [Google Scholar]
- 28.Lau CP, Leung WH, et al.: Haemodynamics of induced atrial fibrillation: a comparative assessment with sinus rhythm, atrial and ventricular pacing. Eur Heart J. 1990; 11: 219-224. [DOI] [PubMed] [Google Scholar]
- 29.Groenveld HF, Crijns HJ, et al.: Health ABC (Rate Control Efficacy in Permanent Atrial Fibrillation II) study. J Am Coll Cardiol. 2011; 58: 1795-1803. [DOI] [PubMed] [Google Scholar]
- 30.Burtin C, Clerckx B, et al.: Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009; 37: 2499-2505. [DOI] [PubMed] [Google Scholar]
- 31.Schweickert WD, Pohlman MC, et al.: Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009; 373: 1874-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]