Abstract
Background
The widely‐accepted treatment outcome for chronic hepatitis C is the sustained viral response (that is, no measurable viral RNA in blood six months after treatment). However, this surrogate outcome (as well as the previously employed biochemical and histologic ones) has never been validated. This situation exists because there are very few randomized clinical trials that have used clinical events (mortality or manifestations of decompensated cirrhosis) as outcomes, because those clinical events only occur after many years of infection. Patients in whom initial therapy fails to produce sustained viral responses do become potential candidates for retreatment; some of these individuals are not candidates for ribavirin or protease inhibitors and consideration could be given to retreatment with interferon alone.
Objectives
To assess the benefits and harms of interferon monotherapy retreatment in chronic hepatitis C patients and to validate the currently employed surrogate outcomes in this group of patients.
Search methods
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded until 16 August 2012.
Selection criteria
Randomized trials comparing interferon versus placebo or no treatment in chronic hepatitis C nonresponders and relapsers to previous interferon.
Data collection and analysis
The primary outcomes were mortality (all‐cause and hepatic), quality of life, and adverse events. Secondary outcomes were liver‐related morbidity, sustained viral responses, biochemical responses, histologic improvements, and costs. We used both fixed‐effect and random‐effects model meta‐analyses, reporting only the former if no difference existed.
Main results
Seven trials were identified. Two of them were at low risk of bias (the HALT‐C and EPIC3 trials) and included 1676 patients. Both of these trials addressed the role of long‐term low‐dose pegylated interferon therapy in patients with severe fibrosis (demonstrated on liver biopsy) and were designed to assess the clinical outcomes. The remaining five trials included 300 patients and were at high risk of bias. Based on all trials reporting the outcomes, no significant difference was observed in either all‐cause mortality (78/843 (9.3%) versus 62/867 (7.2%); risk ratio (RR) 1.30, 95% confidence interval (CI) 0.95 to 1.79; 3 trials) or hepatic mortality (41/532 (7.7%) versus 40/552 (7.2%); RR 1.07, 95% CI 0.70 to 1.63; 2 trials); however, when only the two trials at low risk of bias were combined, all‐cause mortality was significantly higher in the recipients of the pegylated interferon (78/828 (9.4%) versus 57/848 (6.7%); RR 1.41, 95% CI 1.02 to 1.96) although trial sequential analysis could not exclude the possibility of random error. There was less variceal bleeding in the recipients of the interferon (4/843 (0.5%) versus 18/867 (2.1%); RR 0.24, 95% CI 0.09 to 0.67; 3 trials), although again trial sequential analysis could not exclude the presence of a type I error and the effect could not be confirmed in a random‐effects model meta‐analysis. No significant differences were seen with regard to the development of ascites, encephalopathy, hepatocellular carcinoma, or the need for liver transplantation. One trial reported quality of life data; the pain score was significantly worse in the recipients of the pegylated interferon. Adverse effects tended to be more common in the interferon recipients; the ones that were significantly more common included hematologic complications, infections, flu‐like symptoms, and rash. The recipients of interferon had significantly more sustained viral responses (20/557 (3.6%) versus 1/579 (0.2%); RR 15.38, 95% CI 2.93 to 80.71; 4 trials) and a type I error was excluded by trial sequential analysis. The METAVIR activity score also improved (36/55 (65%) versus 20/46 (43.5%); RR 1.49, 95% CI 1.02 to 2.18; 2 trials). No significant differences were seen with regard to histologic fibrosis assessments.
Authors' conclusions
The clinical data were limited to patients with histologic evidence of severe fibrosis who were retreated with pegylated interferon. In this scenario, retreatment with interferon did not appear to provide significant clinical benefit and, when only the trials at low risk of bias were considered, retreatment for several years may even have increased all‐cause mortality. Such treatment also produced adverse events. On the other hand, the treatment did result in improvement in some surrogate outcomes, namely sustained viral responses and histologic evidence of inflammation. Interferon monotherapy retreatment cannot be recommended for these patients. No clinical data are available for patients with less severe fibrosis. The sustained viral response cannot be used as a surrogate marker for hepatitis C treatment in this clinical setting with low sustained viral response rates and needs to be validated in others in which higher sustained viral response rates are reported.
Keywords: Humans; Antiviral Agents; Antiviral Agents/adverse effects; Antiviral Agents/therapeutic use; Hepatitis C, Chronic; Hepatitis C, Chronic/drug therapy; Hepatitis C, Chronic/mortality; Hepatitis C, Chronic/virology; Interferon alpha‐2; Interferon‐alpha; Interferon‐alpha/therapeutic use; Interferons; Interferons/adverse effects; Interferons/therapeutic use; Liver Cirrhosis; Liver Cirrhosis/drug therapy; Liver Cirrhosis/etiology; Polyethylene Glycols; Polyethylene Glycols/therapeutic use; Randomized Controlled Trials as Topic; Recombinant Proteins; Recombinant Proteins/therapeutic use; Recurrence; Viral Load
Plain language summary
Interferon for interferon nonresponding and relapsing patients with chronic hepatitis C
Antiviral treatment for chronic hepatitis C infections is currently judged as being successful if, at least six months after therapy, blood tests for hepatitis C viral RNA are negative; this has been called a sustained viral response. In the past, other outcomes for treatment have included improvements in biochemical tests (especially liver enzyme tests such as the serum alanine aminotransferase) or evidence of reduced inflammation and/or fibrosis on subsequent liver biopsies. All of these outcomes are tests, and it has been assumed that if the test gets better the patient will as well. However, there is no direct evidence that has proven that these outcomes are valid because there have been no long‐term trials that have shown that an improvement in these tests translates into reduced mortality or morbidity. Patients who fail to have sustained viral responses after an initial course of therapy do become potential candidates for retreatment; some of them may be intolerant to ribavirin, and possibly even the newer protease inhibitors, so retreatment would have to be with interferon alone. It has also been speculated that long‐term treatment (namely treatment for several years) might be beneficial; such long‐term therapy would be further complicated if multiple drugs were used because of the additional drug toxicities and costs, so interferon alone could be considered. This review addressed the ability of interferon monotherapy to favorably alter the clinical course of chronic hepatitis C when it is used to retreat patients who failed at least one previous course of therapy. Seven trials were identified, including two large ones (a total of 1676 patients), known as "HALT‐C" and "EPIC3", that specifically were designed to use low‐dose pegylated interferon for three to five years in patients with evidence on liver biopsy of severe fibrosis and who had failed to have a sustained viral response to a course of standard combination (pegylated interferon plus ribavirin) therapy in the past. Both trials were at low risk of bias. A third trial designed to address the use of pegylated interferon monotherapy for 48 weeks in improving survival in patients with cirrhosis (Childs A or B) was terminated early because of the results of the HALT‐C and EPIC3 trials, so three trials have provided mortality and hepatic morbidity data. When all three trials were considered, there was no significant effect of the treatment on either all‐cause mortality (78/843 (9.3%) versus 62/867 (7.2%); risk ratio (RR) 1.30, 95% confidence interval (CI) 0.95 to 1.79; 3 trials) or hepatic mortality (41/532 (7.7%) versus 40/552 (7.2%); RR 1.07, 95% CI 0.70 to 1.63; 2 trials); however, all‐cause mortality was higher in the recipients of the pegylated interferon (78/828 (9.4%) versus 5 7/848 (6.7%); RR 1.41, 95% CI 1.02 to 1.96) when only the two low risk of bias trials were considered. The excess deaths appeared to be from non‐liver causes. Variceal bleeding occurred less often in the treated patients (4/843 (0.5%) versus 18/867 (2.1%); RR 0.24, 95% CI 0.09 to 0.67), but there were no differences seen with regard to the subsequent development of other manifestations of end‐stage liver disease (that is, encephalopathy, ascites, hepatocellular carcinoma, liver transplantation). One trial reported quality of life data; the treated patients had increases in their pain scores. No cost data were available. The recipients of the pegylated interferon generally had more adverse events; statistically significant differences were seen for the occurrence of hematologic complications, infections, flu‐like symptoms, and rashes. Those receiving interferon were more likely to have sustained viral responses (20/557 (3.6%) versus 1/579 (0.2%); RR 15.38, 95% CI 2.93 to 80.71) and were also more likely to have improvements in markers of inflammation. No difference was demonstrated regarding the effect of the treatment on markers of fibrosis. The use of longer‐term (several years) interferon monotherapy in patients with severe underlying hepatic fibrosis who have failed previous courses of treatment is not supported by the evidence; no trials providing data regarding clinical outcomes were identified in other potential treatment scenarios. Two of the commonly employed surrogate markers, sustained viral response and markers of inflammation, failed to be validated since they improved even though the clinical outcomes did not (or may even have become worse). This failure to validate the sustained viral response in this group of patients with a low sustained viral response rate suggests that the presumed validity of the use of sustained viral responses in other groups of patients with chronic hepatitis C viral infections who receive treatment must be formally validated.
Summary of findings
Summary of findings for the main comparison. Summary of findings ‐ mortality.
| Maintenance interferon monotherapy compared with no therapy for patients with chronic hepatitis C and severe histologic disease (grade 3 or 4 fibrosis) who have failed previous antiviral therapy | ||||||
|
Patient or population: patients with chronic hepatitis C who have failed prior antiviral therapy and who have severe histologic disease (grade 3 or 4 fibrosis) but compensated liver disease. Settings: outpatients. Intervention: maintenance (usually half dose) pegylated interferon monotherapy for 3.5 and 5 years in the two large, low risk of bias trials (Di Bisceglie 2008; Bruix 2011) and 48 weeks of standard dose in the third trial (Tanwar 2012) Comparison: no treatment. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Pegylated interferon monotherapy | |||||
|
All‐cause mortality 5 years |
Low risk population: no data available | RR 1.3 (0.95 to 1.79) | 1710 (3 studies) | ⊕⊕⊕⊝ moderate1 | When only the two low risk of bias trials were considered, the treated group had a significantly higher mortality (RR 1.41, 95% confidence interval 1.02 to 1.45). | |
| Medium risk population: no data available | ||||||
| High risk population | ||||||
| 72 per 1000 | 93 per 1000 (68 to 128) | |||||
|
Liver related mortality 5 years |
Low risk population:no data available | RR 1.07 (0.7 to 1.63) | 1084 (2 studies) | ⊕⊕⊕⊝ moderate2 | No effect on liver‐related mortality was observed in one trial with low risk of bias or when that trial was combined with a trial with high risk of bias. | |
| Medium risk population: no data available | ||||||
| High risk population | ||||||
| 72 per 1000 | 78 per 1000 (51 to 118) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is calculated from the data by the software program. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Two of the three trials were at low risk of bias; one of them showed a significant increase in all‐cause mortality in the treated group and the other showed no difference. The third trial, which was at high risk of bias, showed a trend for better survival in the treated group.
2 One large trial with no risk of bias showed nonsignificant increase in hepatic mortality in the treated group and one trial with high risk of bias showed nonsignificant decreased hepatic mortality in the treated group.
Summary of findings 2. Summary of findings ‐ hepatic morbidity.
| Maintenance interferon monotherapy compared with no therapy for patients with chronic hepatitis C and severe histologic disease (grade 3 or 4 fibrosis) who have failed previous antiviral therapy | ||||||
|
Patient or population: patients with chronic hepatitis C who have failed prior antiviral therapy and who have severe histologic disease (grade 3 or 4 fibrosis) but compensated liver disease. Settings: outpatients. Intervention: maintenance (usually half dose) pegylated interferon monotherapy for 3.5 and 5 years in the two large, low risk of bias trials (Di Bisceglie 2008; Bruix 2011) and 48 weeks of standard dose in the third trial (Tanwar 2012) Comparison: no treatment. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Pegylated interferon monotherapy | |||||
|
Hepatic encephalopathy 5 years |
12 per 1000 | 11 per 1000 (4 to 27) | RR 0.92 (38 to 2.26) | 1676 (2 studies) | ⊕⊕⊕⊕ high | No effect was seen on hepatic encephalopathy in two trials with low risk of bias. |
|
Variceal bleeding 5 years |
21 per 1000 | 5 per 1000 (2 to 15) | RR 0.26 (0.09 to 0.71) | 1710 (3 studies) | ⊕⊕⊕⊕ high | Since there was no difference in hepatic mortality, only non‐fatal variceal bleeding prevented. Since number needed to treat is 67, and since cost of treatment is in tens of thousands of dollars per patient, this does not appear to be a cost‐effective intervention. |
|
Ascites 5 years |
25 per 1000 | 28 per 1000 (15 to 50) | RR 1.12 (0.62 to 2) | 1676 (2 studies) | ⊕⊕⊕⊕ high | No effect was seen on ascites in two trials with low risk of bias. |
|
Spontaneous bacterial peritonitis 5 years |
4 per 1000 | 1 per 1000 (0 to 13) | RR 0.38 (04 to 3.54) | 1084 (2 studies) | ⊕⊕⊕⊕ high | No effect was seen on spontaneous bacterial peritonitis in one trial with low risk of bias or when that trial was combined with a trial at high risk of bias. |
|
Hepatocellular carcinoma 5 years |
62 per 1000 | 50 per 1000 (34 to 74]) | RR 0,81 (0.55 to 1.19) | 1710 (3 studies) | ⊕⊕⊕⊕ high | No effect was seen on the occurrence of hepatocellular carcinoma when the two trials at low risk of bias were combined or when all three trials were combined. |
|
Liver transplantation 5years |
13 per 1000 | 6 per 1000 (1 to 35) | RR 0.51 (0.09 to 2.74) | 626 (1 study) | ⊕⊕⊕⊕ high1 | No difference in the need for liver transplantation was seen in one low risk of bias trial. |
|
Decompensated cirrhosis 3.4 years |
53 per 1000 | 22 per 1000 (1 to 503) | RR 0.42 (0.02 to 9.55) | 34 (1 study) | ⊕⊕⊝⊝ low2 | No difference was seen in the total number of patients who developed at least one manifestation of decompensated cirrhosis, but these data are very limited and only available in one high risk of bias trial. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is calculated from the data by the software program. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Data from a single trial (the smaller of the two low risk of bias trials).
2 There was no blinding of the assessor, the analysis only considered a subset (34 of 40) of the patients who were enrolled, and the trial was stopped early.
Background
Infection with the hepatitis C virus (HCV) affects an estimated 170 million people worldwide (Lauer 2001). Once infection occurs, spontaneous clearance of the virus is infrequent, occurring in only about 15% to 25% of patients, although this rate varies depending on age, sex, race, and immune status (Hoofnagle 2002). Chronic hepatitis C is defined as the continued demonstration of HCV‐RNA in the blood for at least six months after the onset of infection. The major long‐term complications of chronic hepatitis C are decompensated cirrhosis and/or hepatocellular carcinoma, although it should be appreciated that the large majority of patients who are infected do not develop these problems (Kenny‐Walsh 1999; Seeff 1999; Vogt 1999; Wiese 2000; Barrett 2001; Seeff 2001; Casaraghi 2004). On the other hand, because of the large number of infected patients, even though only a minority of them will get into trouble, HCV infection causes 27% of cirrhosis and 25% of hepatocellular carcinoma worldwide (Perz 2006). Once cirrhosis is established, the ultimate prognosis is poor (Fattovich 1997). At that point, the mortality associated with chronic hepatitis C is usually due to end‐stage liver disease (Poynard 2003a), namely portal hypertension, hepatocellular failure, and/or hepatocellular carcinoma, the latter perhaps being the most frequent cause of death (Benvegnu 2004). It has been postulated that the best strategy to prevent hepatocellular carcinoma in HCV‐infected patients is to prevent cirrhosis or, if cirrhosis is already present, to suppress viral replication and hepatocyte necroinflammation and thereby inhibit, or at least delay, further progression (Nishiguchi 1995).
In the late 1980s, reports emerged describing the treatment of non‐A, non‐B, presumably viral‐infected patients (subsequently largely found to be HCV infections) with alfa‐interferon (Davis 1989). This treatment improved biochemical and histologic markers of inflammation, resulting in alfa‐interferon becoming the first licensed treatment for this disorder. Indeed, interferon monotherapy has proven effective in normalising liver biochemistry and improving histology in up to half of treatment‐naive patients (Myers 2002). While antiviral treatment has, as its primary objective, the reduction in the subsequent incidence of hepatic morbidity and mortality, randomized trials comparing treatment with no treatment using these outcomes have never been done in the average‐risk patient because of the long period of time that is required before the clinical manifestations become apparent. Instead, the efficacy of treatment with interferon is measured by its ability to achieve viral clearance with cessation of disease activity (Marcellin 1997), referred to as the "sustained virologic response" (SVR). The SVR is defined by the absence of detectable HCV‐RNA in serum 24 weeks after the end of treatment. It has even been suggested that patients with chronic hepatitis C who do not achieve HCV‐RNA clearance after interferon therapy but have a sustained biochemical response, defined as normalisation of serum alanine aminotransferase during interferon therapy and for at least six months after the end of the therapy, could have a lower risk of hepatocellular carcinoma (Alric 2001; Arase 2007).
Assessing the capacity for interferon monotherapy to achieve clinical benefit is limited in three major respects. First, as we just noted, the outcomes of treatment that are employed are all surrogate or intermediate ones that have never been validated, so it is only a hypothesis that achieving them will translate into improved clinical outcomes (Gluud 2007). Second, even if these surrogate outcomes are important, the rate of virologic response at the end of treatment is suboptimal. Although approximately 30% of all patients clear the virus at the end of 48 weeks of therapy with interferon monotherapy, the response rate in patients infected with genotype 1, which comprise approximately 70% of infected patients in North America and Western Europe, is much lower, about 10% (McHutchison 1998; Poynard 1998). Third, in patients who do manifest a response at the end of treatment, the rate of relapse upon discontinuation of therapy is extremely high (approximately 50%) (McHutchison 1998; Poynard 1998; Brok 2005a). Thus, only 15% to 20% of patients treated with interferon monotherapy ultimately achieve SVRs.
The advent of interferon and ribavirin combination therapy led to a doubling of SVR rates in treatment‐naive patients with chronic hepatitis C (McHutchison 1998; Poynard 1998; Brok 2005b). Data from several large clinical trials have shown that pegylated interferons (peg‐interferon) are more effective than standard interferon with or without ribavirin (Lindsay 2001). The addition of ribavirin to peg‐interferon increases the SVR rate to 60% (Manns 2001; Fried 2002). As a result, the combination of peg‐interferon plus ribavirin has been the standard therapy since 2001 (Manns 2001) and, in general, this combination is recommended for treating hepatitis C. However, many patients still do not achieve SVRs despite these improvements, and their disease presumably continues to progress; these individuals may be the ones who are most at risk of developing cirrhosis and hepatocellular carcinoma. Patients with cirrhosis clear serum HCV‐RNA less frequently, and this impaired response is further confounded by their more advanced age and by greater difficulty in achieving optimal dosing (Wright 2002).
Several approaches for the retreatment of the remaining patients, so‐called "nonresponders" and "relapsers", have been tested in randomized clinical trials. Retreatment with the same doses of alfa‐interferon is rarely effective (Alberti 1997). This has prompted trials with higher doses, prolonged treatment duration, and different formulations of interferon, but responses have been variable (Bonkovsky 1996; Gaeta 1997; Poynard 1999). The achievement of higher response rates with combination therapy with interferon and ribavirin, as well as with the use of peg‐interferon in naive patients, has led to using this combination in nonresponders (Cheng 2001) and relapsers (Davis 1998; Camma 1999). Nevertheless, ribavirin therapy frequently causes anemia and should be used carefully in the elderly, in anemic or pregnant young patients, and in those who require long‐term treatment (Maddrey 1999; McHutchison 2002). More recently, a third class of antiviral agents (protease inhibitors) have become available, and their usage has resulted in still higher SVR rates (Jacobson 2011; Poordad 2011); however, these agents are expensive and some patients do not tolerate them. Finally, given the model of the human immunodeficiency virus (HIV) and AIDS, one might speculate that treatment, regardless of the regimen, should be lifelong rather than just for 24 or 48 weeks.
Thus, while interferon monotherapy is not a primary choice for most clinicians, there are a small number of patients who will not be candidates for either ribavirin or protease inhibitors at this time; in such patients interferon would remain as the only option. Furthermore, if one is going to consider long‐term maintenance treatment, it may be desirable from the perspectives of both cost and toxicity to consider only using one agent. In fact, two large randomized trials comparing several years of peg‐interferon therapy to no treatment have been completed, the HALT‐C trial (Di Bisceglie 2008) and the EPIC3 trial (Bruix 2011). Both of these trials focused on the effect of the treatment on clinical outcomes.
The purpose of this review is to update a previous systematic review (Myers 2002) to analyse the beneficial and harmful effects of interferon monotherapy in the retreatment of patients with chronic hepatitis C who failed to achieve SVRs in the past. Also, since we will have both clinical and surrogate outcomes, this review will consider the validation of the surrogate ones (Gluud 2007). The previous version of this systematic review included trials that compared interferon to no therapy as well as trials that compared different regimens of interferon therapy to each other. The latter trials cannot establish the absolute efficacy of the intervention. In fact, they were done primarily to find the optimal dosing of interferon that would result in better surrogate outcomes and virtually no clinical information was available. Since the primary outcomes of interest in this review are the effect of treatment on mortality and morbidity, it was decided to remove the comparative trials from this review. For historical purposes, as well as potential future clinical needs (if the surrogate outcomes are validated), the comparative trials will be the subject of a separate Cochrane review.
Objectives
To assess the benefits and harms of interferon monotherapy retreatment in chronic hepatitis C patients who are nonresponders and relapsers to previous interferon therapy. The following specific questions were addressed based on the results of randomized clinical trials.
What is the effect of a repeated course of interferon monotherapy, versus placebo or no intervention, in nonresponders and relapsers in terms of clinical, virologic, biochemical, and histologic outcomes?
Does treatment affect the clinical and surrogate outcomes in the same direction; in other words, are the surrogate outcomes valid?
Methods
Criteria for considering studies for this review
Types of studies
Randomized clinical trials, unpublished or published as an article, abstract, or letter, were included. No language limitations were used.
Types of participants
Trials that compared interferon monotherapy with no treatment in nonresponding and relapsing patients with chronic hepatitis C were included. Patients either had ribavirin contraindications or intolerance, or ribavirin was deselected due to other reason(s). Similarly, protease inhibitors were not used, either because they were not available at the time of the trial or there was some clinical reason not to use them.
Chronic hepatitis C was defined as the presence of HCV‐RNA by polymerase chain reaction (PCR) assay in serum for more than six months, or the presence of HCV‐RNA in serum with elevated aminotransferases for more than six months and/or histological evidence of chronic hepatitis (including cirrhosis).
Patients who had undergone liver transplantation, were coinfected with HBV and/or HIV, and/or had evidence of hepatic decompensation (for example, ascites, hepatic encephalopathy, esophageal varices, etc.) were excluded.
The previous version of this review (Myers 2002) only provided data regarding the impact of treatment on virologic, biochemical, and histologic (all surrogate) outcomes. In that version, the trials were divided into two categories, those assessing previous nonresponders and those assessing previous relapsers. This separation of trials has been abandoned in the present analyses because there is no evidence that relapsers and nonresponders behave differently in terms of clinical outcomes and because the current trials did not provide data in this fashion.
Types of interventions
Only randomized clinical trials comparing interferon monotherapy with placebo or no treatment were included. Trials assessing interferon in combination with various cointerventions (for example, ribavirin, amantadine, ursodeoxycholic acid, phlebotomy, etc.) were excluded. There were no exclusions based on the type, dose, or duration of interferon therapy. Trials comparing different regimens of interferon to each other will be included in a separate systematic review. Since alfa‐interferon is the type of interferon that is commonly employed and commercially available, trials employing other types of interferon were excluded.
Types of outcome measures
Primary outcome measures
Mortality (all‐cause and liver‐related).
Quality of life: however defined by authors.
Adverse events: defined as any untoward medical occurrence not necessarily having a causal relationship with the treatment, but resulting in a dose reduction or discontinuation of treatment (ICH‐GCP 1997).
Secondary outcome measures
The development of liver‐related morbidity (decompensated liver disease (gastrointestinal bleeding from varices, ascites, hepatic encephalopathy), liver transplantation, hepatocellular carcinoma).
Achievement of an SVR. (SVR was defined as the disappearance of HCV‐RNA from serum at least six months following the end of treatment.)
Achievement of a sustained biochemical response. (Sustained biochemical response was defined as the normalisation of alanine aminotransferase (alanine transaminase) at least six months following the end of treatment.)
Progression of liver disease to cirrhosis (as assessed by histology).
Histologic response: improvement of the histologic activity index or fibrosis score (for example, METAVIR score).
Costs and/or cost‐effectiveness (the latter being defined as cost per quality‐adjusted life year gained as a consequence of the intervention).
Total hospital admissions during the trial.
Search methods for identification of studies
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2012), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded (Royle 2003) until 16 August 2012. We have given the search strategies in Appendix 1 with the time spans for the searches.
We also searched the references of the identified trials to identify further relevant trials.
Data collection and analysis
Trials selection and extraction of data
Identified trials were listed as well as whether inclusion criteria were fulfilled, as assessed by at least two of four of the authors (RK, MP, KSG, VA). Excluded trials were listed with the reason for exclusion. The decision for inclusion or exclusion of studies was independent of trial results.
Data were extracted by RK and validated by MP or extracted by MP and validated by VA, PB, or RC. Disagreements were resolved by discussion between the review authors.
The following characteristics were extracted from each trial.
Authors.
Year and language of publication.
Country.
Inclusion and exclusion criteria.
Previous response to interferon therapy (nonresponse versus relapse).
Definition of prior nonresponse or relapse (biochemical; virologic or combined biochemical and virologic).
Previous interferon therapy.
Retreatment regimens.
Duration of follow‐up after the end of treatment.
Population characteristics such as mean age, percentage of males, mode and duration of infection, percentage of patients with cirrhosis, percentage of patients infected with HCV‐genotype 1.
Number of patients in the study groups.
Outcomes in the study groups (mentioned above).
Methodological quality (described below as "risk of bias").
Assessment of risk of bias
Risk of bias refers to the confidence one can have that the design and the report of the randomized clinical trial would limit the introduction of external influences (biases) that could affect the perceived effect of the intervention (Moher 1998). According to empirical evidence (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008), the domains of bias that can produce false estimates of effects include sequence generation, allocation concealment, blinding (of participants, personnel, and outcome assessors), incomplete outcome data, selective outcome reporting, and others. Investigators can use various methodologic techniques to guard against the introduction of such biases. The degree to which the investigators employed these techniques were assessed as follows.
Generation of the allocation sequence
Adequate, sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards and throwing dice are adequate if performed by an independent adjudicator.
Unclear, the trial is described as randomized but the method of sequence generation was not specified.
Inadequate, the sequence generation method is not, or may not be, random. Quasi‐randomized studies, those using dates, names, or admittance numbers in order to allocate patients, are inadequate and will be excluded for the assessment of benefits but not for harms.
Allocation concealment
Adequate, allocation was controlled by a central and independent randomisation unit, opaque and sealed envelopes, or similar, so that intervention allocations could not have been foreseen in advance of, or during, enrolment.
Unclear, the trial was described as randomized but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment.
Inadequate, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomized. Quasi‐randomized studies will be excluded for the assessment of benefits but not for harm
Blinding of participants, personnel, and outcome assessors
It is difficult to blind the patient and healthcare provider to the allocated treatment. However, it is possible to blind the outcome assessors to this. We do not expect the trials to have blinding of the evaluators nor manuscript writers. We do not believe that lack of blinding influenced the primary outcome of mortality or the secondary outcome of sustained virologic response. If the trial authors used objective definitions for other outcomes, even if not blinded, we do not believe that lack of blinding influenced these outcomes. So, only the outcome assessor blinding was used as a measure to assess bias risk for outcomes, such as for adverse effects, which could be affected by lack of blinding. However, we obtained information on whether the blinding of any other groups or outcomes was undertaken.
Low risk of bias (blinding was performed adequately, or the outcome measurement is not likely to be influenced by lack of blinding).
Uncertain risk of bias (there is insufficient information to assess whether the type of blinding used is likely to induce bias on the estimate of effect).
High risk of bias (no blinding or incomplete blinding, and the outcome or the outcome measurement is likely to be influenced by lack of blinding).
Incomplete outcome data
Low risk of bias (the underlying reasons for missing data are unlikely to make treatment effects depart from plausible values, or proper methods have been employed to handle missing data).
Uncertain risk of bias (there is insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data is likely to induce bias on the estimate of effect).
High risk of bias (the crude estimate of effects (eg, complete case estimate) will clearly be biased due to the underlying reasons for missing data, and the methods used to handle missing data are unsatisfactory).
Selective outcome reporting
Low risk of bias (the trial protocol is available and all of the trial's pre‐specified outcomes that are of interest in the review have been reported, or similar).
Uncertain risk of bias (there is insufficient information to assess whether the magnitude and direction of the observed effect is related to selective outcome reporting).
High risk of bias (not all of the trial's pre‐specified primary outcomes have been reported, or similar).
Other bias
Baseline imbalance
Low risk of bias (there was no baseline imbalance in important characteristics).
Uncertain risk of bias (the baseline characteristics were not reported).
High risk of bias (there was an baseline imbalance due to chance or due to imbalanced exclusion after randomisation).
Early stopping
Low risk of bias (sample size calculation was reported and the trial was not stopped or the trial was stopped early by a formal stopping rule at a point where the likelihood of observing an extreme intervention effect due to chance was low).
Uncertain risk of bias (sample size calculations were not reported and it is not clear whether the trial was stopped early or not).
High risk of bias (the trial was stopped early due to an informal stopping rule or the trial was stopped early by a formal stopping rule at a point where the likelihood of observing an extreme intervention effect due to chance was high).
Academic bias
Low risk of bias (the author of the trial has not conducted previous trials addressing the same interventions).
Uncertain risk of bias (It is not clear if the author has conducted previous trials addressing the same interventions).
High risk of bias (the author of the trial has conducted previous trials addressing the same interventions).
Source of funding bias
Low risk of bias (the trial's source(s) of funding did not come from any parties that might have a conflicting interest (eg, drug manufacturer).
Uncertain risk of bias (the source of funding was not clear).
High risk of bias (the trial was funded by a manufacturer with a conflicting interest).
We considered trials which were classified as having a low risk of bias in sequence generation, allocation concealment, blinding, incomplete data, and selective outcome reporting as low risk of bias trials.
Presentation of risk of bias
We tabulated the risk of bias in the ‘Risk of bias’ table as part of the table 'Characteristics of included studies’. We also illustrated the risk of bias of each trial using the ‘Risk of bias' summary (Figure 1) and cross‐tabulated all the judgements of risk on a ‘Risk of bias' graph (Figure 2).
1.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
2.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Statistical methods
We performed the meta‐analyses according to the recommendations of The Cochrane Collaboration (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2012). We used the software package Review Manager 5.1 (RevMan 2011) provided by The Cochrane Collaboration. For dichotomous outcomes, we calculated the risk ratio (RR) with 95% confidence interval (CI). For continuous outcomes, we planed to calculate mean difference (MD) or standardised mean difference (SMD) with 95% CI. We used a random‐effects model (DerSimonian 1986) and a fixed‐effect model (DeMets 1987). In case of discrepancy between the two models we reported both results; otherwise we have reported only the results from the fixed‐effect model. Heterogeneity was explored by the Chi2 test with significance set at a P value of 0.10, and the quantity of heterogeneity was measured by the I2 statistic (Higgins 2002).
We performed the analysis on an 'intention‐to‐treat' basis (Newell 1992) whenever possible. Otherwise we adopted the 'available patient analysis'. A statistical assessment of publication bias was planned if an adequate number of trials (at least 10) existed (Egger 1997). For significant differences identified in mortality or morbidity outcomes, trial sequential analysis (CTU 2011; Thorlund 2011) was planned to assess for the presence of random error (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010). In order to avoid the confounding of bias (systematic error), only trials with low risks of bias were used in these trial sequential analyses. Because of its wide‐spread use, a trial sequential analysis was done for SVRs.
The following sensitivity analyses were planned to determine the impact on the primary outcome measure (mortality, quality of life, adverse events) and the secondary outcome of liver‐related morbidity.
Risk of bias: comparison of trials with low risk of bias to those failing to meet the criteria for low risk of bias.
Publication status: comparison of trials published as abstracts and letters versus full papers.
Results
Description of studies
Search results
We identified 7367 records through the electronic searches. In addition, we identified five studies (five records) (one of these being another record from a previously identified study) from other references in the electronic searches and five studies (seven records including two from a previously identified study) from the personal files of RK. The details of how we ultimately excluded and included trials are displayed in the study flow diagram (Figure 3).
3.

Study flow diagram.
Fifteen trials comparing different regimens of interferon without an untreated group had been previously included (Arase 1994; Bonkovsky 1996; Ferenci 1996; Lindsay 1996; Chemello 1997; Gaeta 1997; Rolachon 1997; Heathcote 1998; Payen 1998; Scotto 1998; Almasio 1999; Gross 1999; Poynard 1999; Arase 2003; Nomura 2004). An additional nine (Hadziyannis 1997; Wartelle 1997; Davis 1999; Fong 2000; Zeuzem 2000; Suzuki 2001; Iino 2002; Jensen 2009; Neuman Manuela 2010) were identified during the updated search. These trials are the ones that are potentially to be included in another systematic review assessing trials that compare different regimens of interferon therapy to each other.
Two trials (two records) are awaiting classification (Cho 1992; Testino 2002).
A total of seven trials (16 records) from these searches met our inclusion criteria and are included in this present review (Bresci 1995; Vaccaro 1997; Shiffman 1999; Alric 2001; Di Bisceglie 2008; Bruix 2011; Tanwar 2012). These trials included a total of 1976 patients. The duration of treatment was 24 weeks in three of them (Bresci 1995; Vaccaro 1997: Alric 2001), 48 weeks in one (Tanwar 2012), 96 weeks in one (Shiffman 1999), and 3.5 years (Di Bisceglie 2008) and 5 years (Bruix 2011) in the other two. Three trials only randomized patients with severe fibrosis (Di Bisceglie 2008) or cirrhosis (Di Bisceglie 2008; Bruix 2011; Tanwar 2012) and were specifically designed to assess the effect of treatment on clinical outcomes; they were the only trials to report such data. Two of these trials were the largest ones identified; the EPIC3 trial contained 626 participants (Bruix 2011) and the HALT‐C trial 1050 participants (Di Bisceglie 2008). One trial only provided details regarding end‐of‐treatment responses (Bresci 1995) and did not contribute data to any of the analyses.
One of these trials requires some special discussion (Tanwar 2012). This trial was designed to assess the utility of full‐dose peg‐interferon provided for 48 weeks, compared with no treatment, in patients with cirrhosis. Initially 18 patients were randomized to the peg‐interferon arm and 22 to the control group. The paper indicated that two patients were treatment‐naive and five had Childs class B cirrhosis; such individuals were not eligible for inclusion in this review. When requested, the investigator provided us with the pertinent details. The Childs B classification was based, at least, on the presence of ascites that was controlled with diuretics in all five patients; one of these individuals was also treatment‐naive. Thus, we excluded six patients from this analysis, three from each intervention arm. In so doing, we eliminated one treated patient who had developed an SVR and another treated patient who had developed hepatocellular carcinoma. In addition, we excluded two control patients who had either died from a variceal bleeding episode or required a liver transplant for further decompensation manifested at least by the development of encephalopathy.
Risk of bias in included studies
Generation of the allocation sequence and concealment of allocation was adequately performed in three of the trials (Di Bisceglie 2008; Bruix 2011; Tanwar 2012) and was unclear in the others.
While none of the trials were blinded, the assessments of objective outcomes (death, liver transplantation, biochemical, and virologic assessments) were believed to be at low risk of bias anyway, so these trials were all considered to be at low risk of bias. Subjective outcomes were considered to be adequately protected from potential bias if the assessors were unaware of what treatment the patient received; this was the case for all of the outcomes in two trials (Di Bisceglie 2008; Bruix 2011) and for the histologic interpretations in two others (Shiffman 1999; Alric 2001).
Incomplete outcome reporting per se was not a problem in any trial, but for the reasons noted above we wound up with a subgroup analysis of one of the trials (Tanwar 2012).
Selective outcome reporting was judged to have been adequate in the three trials that reported details about the primary clinical outcomes (mortality, adverse events) or hepatic morbidity (Di Bisceglie 2008; Bruix 2011; Tanwar 2012). In the remaining trials, selective outcome reporting cannot be excluded.
Four of the trials did provide sufficient details so that it was clear that the trial was not stopped early (that is, provided a sample size calculation) (Shiffman 1999; Alric 2001; Di Bisceglie 2008; Bruix 2011); a fifth trial also provided a sample size calculation but it was stopped early by the overseeing safety committee after the HALT‐C and EPIC3 results became known (Tanwar 2012). In two trials, no sample size calculation was provided, so it was not clear why either trial was stopped when it was (Bresci 1995; Vaccaro 1997).
There were no baseline differences in any of the trials. There were no vested academic or financial interests in four trials (Shiffman 1999; Alric 2001; Di Bisceglie 2008; Tanwar 2012). The other large trial that intended to assess clinical outcomes primarily did report a sample size calculation, had no baseline differences between the two groups, and had no apparent potential academic interest (Bruix 2011); however, it was funded by one of the companies that manufactures pegylated interferon and was judged to be unclear with regard to "other biases".
Since this latter category did not have to be graded as low risk in order for the trial to be considered such, two trials, which also enrolled the largest numbers of patients (Di Bisceglie 2008; Bruix 2011), were at low risk of bias. The trial by Tanwar et al (Tanwar 2012), while maintaining good methodology for a number of these risks of bias, could not be classified as low risk of bias because of the lack of blinding, the occurrence of early stopping, and the unknown effect that our subgroup analysis created on the randomization (resulting in an unclear assessment of incomplete outcome reporting). A summary of these risks of bias can be seen in Figure 1 and Figure 2.
Effects of interventions
Primary outcomes
Data on all‐cause mortality were available from only three trials (Di Bisceglie 2008; Bruix 2011; Tanwar 2012). When all three trials were combined to assess all‐cause mortality, no significant difference was observed (RR 1.30, 95% CI 0.95 to 1.79, Analysis 1.1). Two of the trials provided data regarding hepatic mortality (Di Bisceglie 2008; Tanwar 2012) and no significant difference was seen (RR 1.07, 95% CI 0.70 to 1.63, Analysis 1.2).
1.1. Analysis.

Comparison 1 Interferon versus control mortality, Outcome 1 All‐cause mortality.
1.2. Analysis.

Comparison 1 Interferon versus control mortality, Outcome 2 Liver‐related mortality.
It did appear, from a perusal of the archived database, that quality of life data were obtained in the HALT‐C trial; however, because of the difficulty in interpreting the coding and abbreviations that were employed, this information could not be quantitatively abstracted or qualitatively determined. Quality of life scores were also obtained by Tanwar et al (Tanwar 2012) in the majority of patients (but data were missing for 1 treated and 8 control participants out of the 40 in the trial). Levels of pain were, on average, "significantly higher, P < 0.001" in the treated patients but no numerical values were provided.
There was a great deal of information regarding adverse events, predominantly from the two large trials (Di Bisceglie 2008; Bruix 2011) with some information from a third (Shiffman 1999). Another trial did describe some events in the treated patients but the information was limited because it was not clear that the controls had been similarly followed for such problems (Tanwar 2012). There was a trend for serious adverse events to occur more commonly in the pegylated interferon arm (Shiffman 1999; Di Bisceglie 2008) (RR 1.18, 95% CI 0.99 to 1.41, P = 0.07, Analysis 3.2). Neutropenia and thrombocytopenia more commonly occurred in the pegylated interferon recipients in one trial (Bruix 2011) (RR 2.42, 95% CI 1.43 to 4.10 and RR 2.63, 95% CI 1.61 to 4.30, Analysis 3.3) although there was no significant difference in "hematological adverse events" in the other large trial (Di Bisceglie 2008) (Analysis 3.3). No significant differences were seen in psychiatric adverse events (Di Bisceglie 2008; Bruix 2011) (Analysis 3.4). Infections were more common in the recipients of pegylated interferon in both large trials (Di Bisceglie 2008; Bruix 2011) (RR 1.51, 95% CI 1.05 to 2.16, Analysis 3.5). There were significant differences found in nine of the other 43 categories of adverse events that were reported. One favored the recipients of the pegylated interferon (fewer esophageal varices, Analysis 3.6) and the other eight adverse effects (fatigue, headaches, myalgia, pyrexia, flu‐like illness, irritability, rash, and erythema at the injection site) were more common in the treated patients (Analysis 3.7; Analysis 3.10). No data were provided regarding subsequent hospital admissions.
3.2. Analysis.

Comparison 3 Interferon versus control ‐ adverse events, Outcome 2 Serious adverse events.
3.3. Analysis.

Comparison 3 Interferon versus control ‐ adverse events, Outcome 3 Hematologic.
3.4. Analysis.

Comparison 3 Interferon versus control ‐ adverse events, Outcome 4 Psychiatric events.
3.5. Analysis.
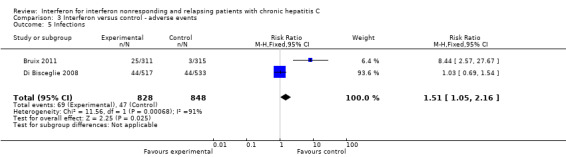
Comparison 3 Interferon versus control ‐ adverse events, Outcome 5 Infections.
3.6. Analysis.

Comparison 3 Interferon versus control ‐ adverse events, Outcome 6 Gastrointestinal.
3.7. Analysis.

Comparison 3 Interferon versus control ‐ adverse events, Outcome 7 Systemic symptoms.
3.10. Analysis.

Comparison 3 Interferon versus control ‐ adverse events, Outcome 10 Dermatologic.
Secondary outcomes
No differences were seen between the two groups with regard to the subsequent development of hepatic encephalopathy (Analysis 4.1), ascites (Analysis 4.3), and spontaneous bacterial peritonitis (Analysis 4.4). The recipients of the pegylated interferon were less likely to have a variceal bleeding episode (RR 0.26, 95% CI 0.09 to 0.71, Analysis 4.2). No differences were seen with regard to the subsequent development of hepatocellular carcinoma (Analysis 4.5) or liver transplantation (Analysis 4.6). One trial provided data for "decompensated cirrhosis" (which was not clearly defined); no significant difference was seen (Analysis 4.7) (Tanwar 2012).
4.1. Analysis.

Comparison 4 Interferon versus control ‐ liver‐related morbidity, Outcome 1 Hepatic encephalopathy.
4.3. Analysis.

Comparison 4 Interferon versus control ‐ liver‐related morbidity, Outcome 3 Ascites.
4.4. Analysis.

Comparison 4 Interferon versus control ‐ liver‐related morbidity, Outcome 4 Spontaneous bacterial peritonitis.
4.2. Analysis.

Comparison 4 Interferon versus control ‐ liver‐related morbidity, Outcome 2 Variceal bleeding.
4.5. Analysis.

Comparison 4 Interferon versus control ‐ liver‐related morbidity, Outcome 5 Hepatocellular carcinoma.
4.6. Analysis.

Comparison 4 Interferon versus control ‐ liver‐related morbidity, Outcome 6 Liver transplantation.
4.7. Analysis.

Comparison 4 Interferon versus control ‐ liver‐related morbidity, Outcome 7 Decompensated cirrhosis.
Both large trials reported data regarding the development of more advanced Child‐Turcotte‐Pugh scores (that is, progression to a more severe disease state than Childs A) (Di Bisceglie 2008; Bruix 2011); no significant effect was seen (RR 1.12, 95% CI 0.84 to 1.50, Analysis 5.1).
5.1. Analysis.

Comparison 5 Interferon versus control ‐ progression of Child‐Pugh‐Turcotte score, Outcome 1 Progression of score.
Although four trials assessed SVRs, none occurred in two of them (Vaccaro 1997; Alric 2001). When the other two trials were combined, SVRs were more commonly seen with interferon (RR 14.73, 95% CI 2.78 to 77.97, Analysis 6.1) (Di Bisceglie 2008; Tanwar 2012).
6.1. Analysis.

Comparison 6 Interferon versus control ‐ surrogate outcomes, Outcome 1 Sustained viral response.
One of the two large trials included 622 patients without cirrhosis (Di Bisceglie 2008); in this subgroup, pegylated interferon did not significantly reduce the incidence of subsequent progression to cirrhosis (as manifested on liver biopsies) (RR 0.93, 95% CI 0.69 to 1.25, Analysis 6.4). Two of the smaller trials, conducted for 24 and 96 weeks (Shiffman 1999; Alric 2001), provided data regarding the degree of inflammation and stage of fibrosis on the pre‐ and post‐treatment liver biopsies; the former was improved by treatment (RR 1.49, 95% CI 1.02 to 2.18, Analysis 6.3) but no significant difference was seen in the latter (RR 1.43, 95% CI 0.76 to 2.68, Analysis 6.5). While the data were not presented in a manner that could be employed in a meta‐analysis, one trial described a significantly greater reduction in the abnormal alanine aminotransferase levels and the histologic necroinflammatory scores in the recipients of the pegylated interferon (Di Bisceglie 2008). In the other large trial (Bruix 2011), which provided about 2/3s of the weight regarding the calculation for the occurrence of variceal bleeding, there was also a treatment‐associated beneficial effect on the appearance or enlargement of esophageal varices. In addition, an abstract from that EPIC3 trial indicated that there was a beneficial effect of the pegylated interferon treatment on non‐invasive markers of necroinflammatory activity and fibrosis (Poynard 2009). Finally, one other trial also described (in a qualitative manner) a significant improvement in two non‐invasive fibrosis scores as a result of treatment (Tanwar 2012).
6.4. Analysis.

Comparison 6 Interferon versus control ‐ surrogate outcomes, Outcome 4 Progression to cirrhosis.
6.3. Analysis.

Comparison 6 Interferon versus control ‐ surrogate outcomes, Outcome 3 Improvement in METAVIR activity score.
6.5. Analysis.

Comparison 6 Interferon versus control ‐ surrogate outcomes, Outcome 5 Improvement in METAVIR fibrosis score.
Sensitivity analyses
The two trials judged to be at low risk of bias (Di Bisceglie 2008; Bruix 2011) were the largest ones in the analyses. When only these two trials were considered, most of the observations remained the same. However, the difference in all‐cause mortality was now significant and identified more deaths in the interferon group (RR 1.41, 95% CI 1.02 to 1.95, Analysis 8.2). However, trial sequential analysis could not exclude the possibility that this was a chance finding (Figure 4). While there was still a significant benefit associated with the use of interferon with regard to variceal bleeding in the fixed‐effect model (R 0.26, 95% CI 0.09 to 0.76), there was statistical heterogeneity (I2 = 44%) and when the random‐effects model was employed the significant difference disappeared (RR 0.27, 95% CI 0.05 to 1.36). Trial sequential analysis could not exclude the possibility that this was a chance finding either (Figure 5).
8.2. Analysis.

Comparison 8 Interferon versus control mortality ‐ low risk of bias trials, Outcome 2 All‐cause mortality.
4.

Trial sequential analysis for low risk of bias trials reporting all‐cause mortality. Assumptions were 1% mortality in control arm, RR = 0.50, alpha error 5%, power 80%. The required information size was 9349 patients.
5.

Trial sequential analysis for low risk of bias trials reporting rates of variceal bleeding in both groups. Assumptions were 2% incidence in control arm, RR = 0.50, alpha error 5%, power 80%.The required information size was 8537 patients.
The contribution from two of the other trials (Shiffman 1999; Alric 2001) were largely in analyses that only included them (Analysis 6.3; Analysis 6.5), so excluding them simply left no trials to consider. Three of the smaller trials (Vaccaro 1997; Alric 2001; Tanwar 2012) were included in the analysis of SVRs (Analysis 6.1); no SVRs were observed in any patients in two of these trials (Vaccaro 1997; Alric 2001) so they did not contribute to the actual data combination. When the third small trial (Tanwar 2012) was removed, a significant difference in the occurrence of SVRs remained (RR 18.56, 95% CI 2.49 to 138.54, Analysis 13.1). Even assuming that the SVR rate was only 3% in the treated patients, the information size (the number of patients required to be sure that a significant finding was not due to chance) was 864; the 1050 patients in the HALT‐C trial exceeded that number (Figure 6). The only other time when a sensitivity analysis was even an issue was in the single analysis of serious adverse events (Analysis 3.2) in which one small trial of 53 patients (Shiffman 1999) was combined with a trial including 1050 patients (Di Bisceglie 2008); removing the small trial had no material effect on the result (Analysis 11.2).
13.1. Analysis.

Comparison 13 Interferon versus control ‐ surrogate outcomes ‐ low risk of bias trials, Outcome 1 Sustained viral response.
6.

Trial sequential analysis in trials reporting sustained viral response rates in both arms. Assumptions were 0.5% in controls, 3% in treated arms, alpha error 5%, power 80%. However, the required information size (864) was exceeded and using lower assumption of rate in controls or higher in treated patients only further reduced the required information size.
11.2. Analysis.

Comparison 11 Interferon versus control ‐ adverse events ‐ low risk of bias trials, Outcome 2 Serious adverse events.
Only one trial was published as an abstract (Vaccaro 1997); that trial only provided data regarding SVRs. Removing it had no effect on the analysis regarding SVRs (Analysis 6.6). This was not surprising as there were no SVRs in either intervention group in this trial.
6.6. Analysis.

Comparison 6 Interferon versus control ‐ surrogate outcomes, Outcome 6 Sustained viral response ‐ only full papers.
Discussion
Our review found that peg‐interferon monotherapy is not an effective treatment for patients with chronic hepatitis C and liver fibrosis who have failed at least one previous course of antiviral treatment. Of course, interferon monotherapy, whether or not patients have been previously treated, is widely regarded as a therapy that no longer has much meaning because of the advent of combination therapy, especially with the availability of the protease inhibitors. However, three observations from this review have important implications with regard to any kind of antiviral treatment program.
The first relates to the observation that the treatment may be effective in preventing variceal bleeding. This benefit was seen when all of the trials were combined and, at least in the fixed‐effect model, when only trials with low risk of bias were considered. This benefit was also observed in another long‐term maintenance trial, the COPILOT study (COPILOT 2008), that compared interferon with colchicine (since there was no true untreated control group, that trial was not included in this meta‐analysis). However, even if interferon therapy does reduce the incidence of variceal bleeding, could we justify using it for that reason? The absolute reduction in the incidence of variceal bleeding appeared to be about 1.5% (an incidence of 2% falling to an incidence of 0.5%). This translates into a number needed to treat of about 67. Since no improvement in mortality was seen, we are considering using an agent that costs tens of thousands of dollars per year per patient in 67 people just to prevent one hospitalization for non‐fatal variceal bleeding. It is unlikely that we could make a cost‐effective argument for such an intervention.
The second observation is troubling, namely that when only the low risk of bias trials were considered (that is, trials which are likely to provide the best effect estimates), there was an increased all‐cause mortality in the recipients of the peg‐interferon. Three trials provided mortality data (Di Bisceglie 2008; Bruix 2011; Tanwar 2012) and, when one looks at the actual numbers, it becomes clear that three different phenomena were being observed. The HALT‐C trial (Di Bisceglie 2008), which largely drove the mortality analysis (especially the one when only the low risk of bias trials were considered), found an increase in the nonhepatic mortality, perhaps suggesting that there was some long‐term adverse effect from peg‐interferon alfa‐2a when being used in half‐dose quantities for several years. No such effect was seen with a seemingly comparable use of peg‐interferon alfa‐2b in the EPIC3 trial (Bruix 2011), although there were slightly more deaths in the treatment group. When all of the participants in the third trial (Tanwar 2012), which was not at low risk of bias, were considered, a significant improvement in both hepatic and all‐cause mortality was observed; the investigators in this trial tried to provide peg‐interferon alfa‐2a at full dose for 48 weeks. When the six patients who were not eligible for inclusion in this review were excluded, the differences in mortality rates were no longer significant, but the arithmetical trend was still present. Since trials that are not at low risk of bias tend to overestimate benefit and/or underestimate harm, the observation from the meta‐analysis of the two low risk of bias trials should be concerning. However, since this harm was largely seen in only one of the two low risk of bias trials it is possible that the HALT‐C finding is simply a chance finding or some effect limited to peg‐interferon alfa‐2a. Nonetheless, since we are considering a very consequential outcome (excessive mortality), this information should be provided to patients who are being counselled about the risks and benefits of treatment.
The third observation is perhaps the most concerning as it was a consistent finding in this review. As we have noted, the best evidence that we have about the clinical impact of interferon monotherapy is that there is no apparent benefit over the first few years in patients who have substantial hepatic fibrosis and are being retreated. Perhaps reflecting the danger of relying on expert opinion, this group was the one that was specifically identified in the first National Institutes of Health (NIH) Consensus Conference as being most in need of treatment with interferon (NIH 1997). Moreover, there is harm with regard to the production of adverse effects as well as cost considerations. There is even a suggestion that all‐cause mortality may be increased with treatment. In spite of this overall unfavorable clinical profile, treatment resulted in a beneficial effect on the surrogate outcomes, especially SVRs and markers of inflammation. This disconnect between the effect of treatment on clinical outcomes and laboratory tests represents a failure of the surrogate outcome to be validated (Gluud 2007), at least in this clinical scenario. While the SVR may be a useful surrogate in other scenarios of hepatitis C treatment, the failure of validation in this setting tells us that the SVR is not universally reliable and should be validated before being viewed as the goal of any therapy. SVR can only regain the status of being the goal for assessing treatment in particular scenarios when it is validated in those scenarios.
There are several reasons why SVRs might have been expected to fail as a surrogate marker.
1) Using viral clearance as an outcome has permeated AIDS treatment strategies, perhaps for good reason (if the serum is the means whereby the AIDS virus reaches its target cell, the lymphocyte). However, as discussed above, chronic hepatitis C is not analogous to AIDS with regard to the ultimate mortality rate in infected individuals who are not treated. In fact, the serum level of the hepatitis C virus may only be an epiphenomenon, especially if the hepatocyte is infected directly from its neighbor.
2) It is becoming apparent that some patients who develop SVRs are still relapsing (Ciancio 2006) or even developing complications of end‐stage liver disease (Chavilitdhamrong 2006; Hung 2006; Innes 2011); therefore SVRs cannot be considered "'cures".
3) While observational studies have shown that patients who develop SVRs have better long‐term outcomes than do patients who fail to develop SVRs (Marcellin 1997; Camma 2001; Papatheodoridis 2001; Everson 2008; Maylin 2008), it does not follow that the treatment actually had anything to do with it. After all, the patients who did not develop SVRs were also treated. We know that there are prognostic features regarding who is more or less likely to have an SVR after treatment; those features associated with the development of SVRs include little or no fibrosis on biopsy, female sex, shorter duration of infection, and normal body weight (Koretz 1995; Zeuzem 2000). These are also factors that would predict a lower likelihood of developing end‐stage liver disease. If responders simply come from the pool of patients who were not very likely to get into trouble in the first place, they would be unlikely to get into trouble just because they received antiviral agents (that is, the treatment had nothing to do with the better long‐term outcomes).
There have been a number of small randomized clinical trials comparing interferon to no therapy in patients with severe fibrosis (Ikeda 1998; Mura 1999; Valla 1999; Planas 2002; Testino 2002; Fartoux 2007). These were not included in this review because the trials were undertaken only in treatment‐naive patients or were not restricted to previously treated individuals and we were not able to separate out the subgroups that would have been eligible for this analysis. The failure of the trials in this review to find a benefit from treatment is consistent with the findings of most of these smaller trials. One of the trials observed benefit (better survival, fewer hepatocellular carcinomas, and fewer episodes of hepatic decompensation) from the interferon (Mura 1999) and that trial is still only available as an abstract 13 years later. One other trial reported that there were fewer patients who developed one or more of several manifestations of end‐stage liver disease (a composite outcome), but the reported percentages were not consistent with the numbers of patients in the two treatment groups (Planas 2002). There is one other trial that was alleged to be randomized (Nishiguchi 1995) that found that treatment reduced the subsequent incidence of hepatocellular carcinoma; however, a closer inspection of the data indicates that the controls, in spite of having worse outcomes, were followed for longer periods of time (Koretz 1996), a situation that is inconsistent with all patients having been randomized at the same time.
Interferon therapy is associated with a variety of adverse events including fatigue, influenza‐like symptoms, psychiatric disturbances, and cytopenias. Not surprisingly, this harm was demonstrated in our included trials.
Our systematic review has several limitations. The number of included trials is small (n = 7) and only some of them provided any clinical data. This leaves open the risks of outcome reporting bias. While the two large trials were at low risk of bias, the included patients all had advanced fibrosis. Thus, it would not necessarily follow that the outcomes would be similar in patients with less severe liver disease. The therapy being assessed, namely interferon alone, is one that may not be commonly employed in the future, so its lack of effect on clinical outcomes may be a moot point. On the other hand, the two lessons that do extrapolate into the therapeutic arena of today (net harm and a surrogate outcome that failed validation) should caution us to stop advocating antiviral interventions of any kind until we have evidence of clinical efficacy and cost‐efficacy.
In conclusion, interferon monotherapy has not been shown to be effective when used to retreat patients, especially those with severe fibrosis. In fact, it only caused net harm. The sustained virological response did not fulfil the criteria needed (Gluud 2007) to be considered as a valid surrogate outcome that can be used in the treatment of patients with chronic hepatitis C. Interferon monotherapy has no established role in nonresponders and relapsers and the alternative treatments still need to be validated with randomized trials assessing both surrogate (to validate them) and clinical (to assess efficacy) outcomes.
Authors' conclusions
Implications for practice.
Retreatment with peg‐interferon, while possibly reducing the subsequent incidence of variceal bleeding, may increase mortality and results in the occurrence of a number of other adverse events. As such, it cannot be recommended. Furthermore, since retreatment with interferon does improve surrogate outcomes, especially rates of SVR occurrence and reduction in markers of inflammation, these surrogates are not adequate outcomes to use for treatment.
Implications for research.
Given the failure of low‐dose peg‐interferon to improve clinical outcomes, and rather to produce harm (increased adverse events, even including death), there is little to be gained by undertaking more trials of interferon monotherapy retreatment. Given the concern about the short‐ and long‐term safety of this agent, we need long‐term randomized trials of other anti‐viral regimens to determine if any efficacy exists and, if so, if the benefit will outweigh the harms. The failure of the biochemical and virologic surrogate outcomes to be validated in this scenario challenges the use of such surrogate outcomes in general and emphasizes the need to use clinical outcomes (mortality and morbidity) in all trials of anti‐viral therapies in patients with hepatitis C infection.
What's new
| Date | Event | Description |
|---|---|---|
| 28 August 2012 | New search has been performed | A new search has been performed. The present update includes seven randomised trials involving 2070 nonresponders and relapsers. |
| 28 August 2012 | New citation required and conclusions have changed | In the present review clinical outcomes have replaced surrogate ones as the primary outcomes, only interferon versus no treatment trials have been considered, and the following conclusions have been made:
|
| 13 January 2009 | Amended | A new team of authors have prepared this update. |
Acknowledgements
We thank the Cochrane Hepato‐Biliary Group for the support that they have provided. Special appreciation is due to Sarah Klingenberg for her guidance, expertise, and provision of the computer database searches.
Peer Reviewers: Christoph Welsch, Germany. Contact Editor: Christian Gluud, Denmark.
We would also acknowledge the assistance of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The HALT‐C trial was conducted by the HALT‐C Investigators and supported by the NIDDK. The NIDDK subsequently provided RK with the data from the HALT‐C trial; this material came from the NIDDK Central Repositories. While all of the numerical data employed in this systematic review actually came from the published papers, our ability to review the raw data did provide us with some insights into that trial. This manuscript was not prepared in collaboration with Investigators of the HALT‐C study and does not necessarily reflect the opinions or views of the HALT‐C study, the NIDDK Central Repositories, or the NIDDK.
We would also like to thank Drs Sudeep Tanwar and Dominique Valla for their prompt and detailed answers to our questions about their trials.
Appendices
Appendix 1. Search strategies
| Database | Period of Search | Search Strategy |
| Cochrane Hepato‐Biliary Group Controlled Trials Register | August 16, 2012. | (interferon and 'chronic hepatitis C') AND NOT ('hepatitis B' or HIV or 'human immune deficiency virus' or 'human immunodeficiency virus' or 'liver transplant*') |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library | Issue 8, 2012. | #1 MeSH descriptor Interferons explode all trees #2 (interferon*) #3 (#1 OR #2) #4 MeSH descriptor Hepatitis C, Chronic explode all trees #5 (chronic hepatitis c) #6 (#4 OR #5) #7 (#3 AND #6) #8 (HIV) or (human immunodeficiency virus) or (hepatitis B) or (liver transplant*) or(human immune deficiency virus) #9 (#7 AND NOT #8) |
| MEDLINE (OvidSP) | 1950 to August 16, 2012. | 1. exp Interferons/ 2. interferon*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 3. 1 or 2 4. exp Hepatitis C, Chronic/ 5. chronic hepatitis c.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 6. 4 or 5 7. 6 and 3 8. (hepatitis B or HIV or human immune deficiency virus or human immunodeficiency virus or liver transplant*).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 9. 7 not 8 10. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 11. 10 and 9 |
| EMBASE (OvidSP) | 1945 to August 16, 2012. | 1. exp Interferon/ 2. interferon*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 3. 1 or 2 4. exp Hepatitis C/ 5. chronic hepatitis c.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 6. 4 or 5 7. 6 and 3 8. (hepatitis B or HIV or human immune deficiency virus or human immunodeficiency virus or liver transplant*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 9. 7 not 8 10. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 11. 10 and 9 |
| Science Citation Index Expanded (http://apps.isiknowledge.com) | 1900 to August 16, 2012. | # 7 #6 AND #5 # 6 TS=(random* or blind* or placebo* or meta‐analysis) # 5 #3 NOT #4 # 4 TS=(hepatitis B or HIV or human immune deficiency virus or human immunodeficiency virus or liver transplant*) # 3 #2 AND #1 # 2 TS=(chronic hepatitis C) # 1 TS=interferon* |
Data and analyses
Comparison 1. Interferon versus control mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 3 | 1710 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.95, 1.79] |
| 2 Liver‐related mortality | 2 | 1084 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.70, 1.63] |
Comparison 3. Interferon versus control ‐ adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any adverse events | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.99, 1.05] |
| 2 Serious adverse events | 2 | 1103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.99, 1.41] |
| 3 Hematologic | 2 | 2302 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.41 [1.71, 3.39] |
| 3.1 Neutropenia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.42 [1.43, 4.10] |
| 3.2 Thrombocytopenia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.61, 4.30] |
| 3.3 "Hematologic" | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.46, 4.52] |
| 4 Psychiatric events | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.94, 2.19] |
| 5 Infections | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.05, 2.16] |
| 6 Gastrointestinal | 2 | 11733 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.87, 1.14] |
| 6.1 Nausea | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.84, 1.81] |
| 6.2 Diarrhea | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.92, 2.38] |
| 6.3 Abdominal pain | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.94, 2.69] |
| 6.4 Dyspepsia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.65, 1.38] |
| 6.5 Esophageal varices | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.42, 0.92] |
| 6.6 Hepatomegaly | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.62, 1.29] |
| 6.7 Splenomegaly | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.81, 1.57] |
| 6.8 Non‐variceal bleeding | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.14, 1.48] |
| 6.9 Hernia or obstruction | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.19, 1.70] |
| 6.10 Gallbladder | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.86] |
| 6.11 Other pancreatico‐biliary | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.46, 4.52] |
| 6.12 Other gastrointestinal | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.35, 1.63] |
| 6.13 Other liver | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.06, 16.44] |
| 6.14 Liver biopsy complications | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.30, 1.85] |
| 7 Systemic symptoms | 1 | 6260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.61, 2.05] |
| 7.1 Fatigue | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.04, 1.72] |
| 7.2 Headache | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.63, 3.02] |
| 7.3 Myalgia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.05 [2.51, 6.53] |
| 7.4 Arthralgia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.81, 1.48] |
| 7.5 Pyrexia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.92 [2.27, 6.76] |
| 7.6 Insomnia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.78, 1.63] |
| 7.7 Flu‐like illness | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.65 [4.24, 31.97] |
| 7.8 Asthenia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.82, 1.85] |
| 7.9 Irritability | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.42, 4.21] |
| 7.10 Pruritus | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.00, 2.49] |
| 8 Cardiopulmonary | 2 | 4402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.74, 1.25] |
| 8.1 Atherosclerosis | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.45, 1.67] |
| 8.2 Arrhythmia | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.10, 1.45] |
| 8.3 "Other cardiovascular" | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.22, 3.05] |
| 8.4 Hypertension | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.75, 1.67] |
| 8.5 Cough | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.63, 1.55] |
| 9 Musculoskeletal | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.82, 1.56] |
| 9.1 "Musculoskeletal" | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.68, 1.98] |
| 9.2 Back pain | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.75, 1.66] |
| 10 Dermatologic | 2 | 2928 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [1.95, 3.97] |
| 10.1 Rash | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [1.61, 5.77] |
| 10.2 Injection erythema | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 73.94 [4.56, 1199.35] |
| 10.3 Alopecia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.84, 2.17] |
| 11 Metabolic | 1 | 3150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.35, 2.03] |
| 11.1 Electrolyte/water | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.30, 2.61] |
| 11.2 Diabetes | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.67] |
| 11.3 Thyroid | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.15, 7.29] |
| 12 Neoplasms | 1 | 2100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.33, 1.65] |
| 12.1 Benign neoplasm | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.29] |
| 12.2 Cancer | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.35, 2.02] |
| 13 Other system adverse events | 1 | 4200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.47, 1.41] |
| 13.1 Genitourinary | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.57, 3.50] |
| 13.2 Gynecologic | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.08, 2.12] |
| 13.3 Neurologic | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.33, 3.18] |
| 13.4 Injury | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.13, 1.31] |
| 14 Hospital admission | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.

Comparison 3 Interferon versus control ‐ adverse events, Outcome 1 Any adverse events.
3.8. Analysis.

Comparison 3 Interferon versus control ‐ adverse events, Outcome 8 Cardiopulmonary.
3.9. Analysis.

Comparison 3 Interferon versus control ‐ adverse events, Outcome 9 Musculoskeletal.
3.11. Analysis.

Comparison 3 Interferon versus control ‐ adverse events, Outcome 11 Metabolic.
3.12. Analysis.

Comparison 3 Interferon versus control ‐ adverse events, Outcome 12 Neoplasms.
3.13. Analysis.

Comparison 3 Interferon versus control ‐ adverse events, Outcome 13 Other system adverse events.
Comparison 4. Interferon versus control ‐ liver‐related morbidity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hepatic encephalopathy | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.38, 2.26] |
| 2 Variceal bleeding | 3 | 1710 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.09, 0.71] |
| 3 Ascites | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.62, 2.00] |
| 4 Spontaneous bacterial peritonitis | 2 | 1084 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.04, 3.54] |
| 5 Hepatocellular carcinoma | 3 | 1710 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.55, 1.19] |
| 6 Liver transplantation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Decompensated cirrhosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Interferon versus control ‐ progression of Child‐Pugh‐Turcotte score.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Progression of score | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.84, 1.50] |
Comparison 6. Interferon versus control ‐ surrogate outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sustained viral response | 4 | 1175 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.73 [2.78, 77.97] |
| 2 Failure to achieve a sustained biochemical response | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Improvement in METAVIR activity score | 2 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.02, 2.18] |
| 4 Progression to cirrhosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Improvement in METAVIR fibrosis score | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.76, 2.68] |
| 6 Sustained viral response ‐ only full papers | 3 | 1137 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.73 [2.78, 77.97] |
Comparison 8. Interferon versus control mortality ‐ low risk of bias trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Liver‐related mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 All‐cause mortality | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.02, 1.95] |
8.1. Analysis.

Comparison 8 Interferon versus control mortality ‐ low risk of bias trials, Outcome 1 Liver‐related mortality.
Comparison 9. Interferon versus control ‐ liver‐related morbidity ‐ low risk of bias trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hepatic encephalopathy | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.38, 2.26] |
| 2 Variceal bleeding | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.09, 0.76] |
| 3 Ascites | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.62, 2.00] |
| 4 Spontaneous bacterial peritonitis | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.42] |
| 5 Hepatocellular carcinoma | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 6 Liver transplantation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
9.1. Analysis.

Comparison 9 Interferon versus control ‐ liver‐related morbidity ‐ low risk of bias trials, Outcome 1 Hepatic encephalopathy.
9.2. Analysis.

Comparison 9 Interferon versus control ‐ liver‐related morbidity ‐ low risk of bias trials, Outcome 2 Variceal bleeding.
9.3. Analysis.

Comparison 9 Interferon versus control ‐ liver‐related morbidity ‐ low risk of bias trials, Outcome 3 Ascites.
9.4. Analysis.
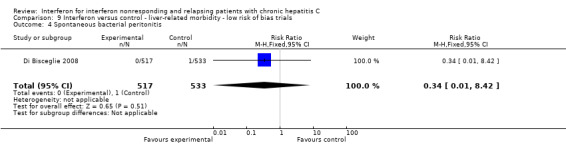
Comparison 9 Interferon versus control ‐ liver‐related morbidity ‐ low risk of bias trials, Outcome 4 Spontaneous bacterial peritonitis.
9.5. Analysis.

Comparison 9 Interferon versus control ‐ liver‐related morbidity ‐ low risk of bias trials, Outcome 5 Hepatocellular carcinoma.
9.6. Analysis.

Comparison 9 Interferon versus control ‐ liver‐related morbidity ‐ low risk of bias trials, Outcome 6 Liver transplantation.
Comparison 11. Interferon versus control ‐ adverse events ‐ low risk of bias trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Haematologic | 2 | 2302 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.41 [1.71, 3.39] |
| 3.1 Neutropenia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.42 [1.43, 4.10] |
| 3.2 Thrombocytopenia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.61, 4.30] |
| 3.3 "Haematologic" | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.46, 4.52] |
| 4 Psychiatric events | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.94, 2.19] |
| 5 Infections | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.05, 2.16] |
| 6 Gastrointestinal | 2 | 11733 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.87, 1.14] |
| 6.1 Nausea | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.84, 1.81] |
| 6.2 Diarrhea | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.92, 2.38] |
| 6.3 Abdominal pain | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.94, 2.69] |
| 6.4 Dyspepsia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.65, 1.38] |
| 6.5 Esophageal varices | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.42, 0.92] |
| 6.6 Hepatomegaly | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.62, 1.29] |
| 6.7 Splenomegaly | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.81, 1.57] |
| 6.8 Non‐variceal bleeding | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.14, 1.48] |
| 6.9 Hernia or obstruction | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.19, 1.70] |
| 6.10 Gallbladder | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.43, 1.86] |
| 6.11 Other pancreatico‐biliary | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.46, 4.52] |
| 6.12 Other gastrointestinal | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.35, 1.63] |
| 6.13 Other liver | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.06, 16.44] |
| 6.14 Liver biopsy complications | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.30, 1.85] |
| 7 Systemic symptoms | 1 | 6260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.61, 2.05] |
| 7.1 Fatigue | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.04, 1.72] |
| 7.2 Headache | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.63, 3.02] |
| 7.3 Myalgia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.05 [2.51, 6.53] |
| 7.4 Arthralgia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.81, 1.48] |
| 7.5 Pyrexia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.92 [2.27, 6.76] |
| 7.6 Insomnia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.78, 1.63] |
| 7.7 Flu‐like illness | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.65 [4.24, 31.97] |
| 7.8 Asthenia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.82, 1.85] |
| 7.9 Irritability | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.42, 4.21] |
| 7.10 Pruritus | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.00, 2.49] |
| 8 Cardiopulmonary | 2 | 4402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.74, 1.25] |
| 8.1 Atherosclerosis | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.45, 1.67] |
| 8.2 Arrhythmia | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.10, 1.45] |
| 8.3 "Other cardiovascular" | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.22, 3.05] |
| 8.4 Hypertension | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.75, 1.67] |
| 8.5 Cough | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.63, 1.55] |
| 9 Musculoskeletal | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.82, 1.56] |
| 9.1 "Musculoskeletal" | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.68, 1.98] |
| 9.2 Back pain | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.75, 1.66] |
| 10 Dermatologic | 2 | 2928 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [1.95, 3.97] |
| 10.1 Rash | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [1.61, 5.77] |
| 10.2 Injection erythema | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 73.94 [4.56, 1199.35] |
| 10.3 Alopecia | 1 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.84, 2.17] |
| 11 Metabolic | 1 | 3150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.35, 2.03] |
| 11.1 Electrolyte/water | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.30, 2.61] |
| 11.2 Diabetes | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.67] |
| 11.3 Thyroid | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.15, 7.29] |
| 12 Neoplasms | 1 | 2100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.33, 1.65] |
| 12.1 Benign neoplasm | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.29] |
| 12.2 Cancer | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.35, 2.02] |
| 13 Other system adverse events | 1 | 4200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.47, 1.41] |
| 13.1 Genitourinary | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.57, 3.50] |
| 13.2 Gynecologic | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.08, 2.12] |
| 13.3 Neurologic | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.33, 3.18] |
| 13.4 Injury | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.13, 1.31] |
| 14 Hospital admission | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
11.1. Analysis.

Comparison 11 Interferon versus control ‐ adverse events ‐ low risk of bias trials, Outcome 1 Any adverse events.
11.3. Analysis.

Comparison 11 Interferon versus control ‐ adverse events ‐ low risk of bias trials, Outcome 3 Haematologic.
11.4. Analysis.

Comparison 11 Interferon versus control ‐ adverse events ‐ low risk of bias trials, Outcome 4 Psychiatric events.
11.5. Analysis.

Comparison 11 Interferon versus control ‐ adverse events ‐ low risk of bias trials, Outcome 5 Infections.
11.6. Analysis.

Comparison 11 Interferon versus control ‐ adverse events ‐ low risk of bias trials, Outcome 6 Gastrointestinal.
11.7. Analysis.

Comparison 11 Interferon versus control ‐ adverse events ‐ low risk of bias trials, Outcome 7 Systemic symptoms.
11.8. Analysis.

Comparison 11 Interferon versus control ‐ adverse events ‐ low risk of bias trials, Outcome 8 Cardiopulmonary.
11.9. Analysis.

Comparison 11 Interferon versus control ‐ adverse events ‐ low risk of bias trials, Outcome 9 Musculoskeletal.
11.10. Analysis.

Comparison 11 Interferon versus control ‐ adverse events ‐ low risk of bias trials, Outcome 10 Dermatologic.
11.11. Analysis.

Comparison 11 Interferon versus control ‐ adverse events ‐ low risk of bias trials, Outcome 11 Metabolic.
11.12. Analysis.

Comparison 11 Interferon versus control ‐ adverse events ‐ low risk of bias trials, Outcome 12 Neoplasms.
11.13. Analysis.

Comparison 11 Interferon versus control ‐ adverse events ‐ low risk of bias trials, Outcome 13 Other system adverse events.
Comparison 12. Interferon versus control ‐ progression of Child‐Pugh‐Turcotte score ‐ low risk of bias trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Progression of score | 2 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.84, 1.50] |
12.1. Analysis.

Comparison 12 Interferon versus control ‐ progression of Child‐Pugh‐Turcotte score ‐ low risk of bias trials, Outcome 1 Progression of score.
Comparison 13. Interferon versus control ‐ surrogate outcomes ‐ low risk of bias trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sustained viral response | 1 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.56 [2.49, 138.50] |
| 2 Failure to achieve a sustained biochemical response | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Improvement in METAVIR activity score | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Progression to cirrhosis | 1 | 622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.69, 1.25] |
| 5 Improvement in METAVIR fibrosis score | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
13.4. Analysis.

Comparison 13 Interferon versus control ‐ surrogate outcomes ‐ low risk of bias trials, Outcome 4 Progression to cirrhosis.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alric 2001.
| Methods | Randomized trial comparing interferon versus no treatment Intention‐to‐treat analysis: yes Sample size calculation: yes. |
|
| Participants | Country: France Nonresponders (n = 57) ‐ Previous nonresponse: virologic ‐ Groups (interferon/C): 29/28 ‐ Excluded: 0/0 ‐ Mean age (y): 46/46 ‐ Male (%): 45/43 ‐ Transfusion (%): N/A ‐ Drug abuse (%): N/A ‐ Genotype 1 (%): 45/43 ‐ Cirrhosis (%): 3/4 Inclusion criteria: Chronic hepatitis C (positive Anti‐HCV, positive HCV‐RNA and data of chronic hepatitis on liver biopsy), previous treatment with interferon alfa‐2b (3 MU three times per week x 48 wk) with normalization of alanine transaminase level and positive HCV‐RNA after the end of the therapy. Exclusion criteria: Other causes of chronic liver disease, HIV positive, active intravenous drugs use and alcohol intake ≥ 50 g/d. |
|
| Interventions | ‐ Schedule: Experimental: Interferon alfa‐2b x 48 wk (2MU three times per week x 12 wk; 1 MU three times per week x 12 wk; 1 MU 2/ wk x12 wk; 1 MU / wk x 12 wk) Control: no treatment ‐ Follow‐up (F/U): 24 wk. | |
| Outcomes | ‐ Virologic end of treatment response ‐ Sustained virologic response ‐ Histology ‐ Biochemical sustained response was also reported. But as this trial included only patients with biochemical response after the first interferon, this outcome was not included for meta‐analysis. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) Objective outcomes (Biochemical or virologic response) | Low risk | The review authors consider that knowledge of the group to which the patient belongs to, will not increase the bias as these outcomes are objective. |
| Blinding (performance bias and detection bias) Subjective outcomes (Clinical events other than mortality, Histology) | Low risk | The authors stated: "The pathologist were unaware of the clinical and biological data or the biopsy order". |
| Blinding (performance bias and detection bias) Adverse effects | High risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient in the treatment group stopped the therapy for adverse effects. |
| Selective reporting (reporting bias) | Unclear risk | While it may be that no clinical events occurred in the 24 weeks of the trial, this was not specified in the paper. |
| Other bias | Low risk | There was no imbalance in important characteristics. Sample size calculation was reported and the trial was not stopped early. The author of the trial has not conducted previous trials addressing the same intervention. The trial's source of funding did not come from any parties that might conflicting interest. |
Bresci 1995.
| Methods | Randomized four‐groups trial comparing different doses and type of interferon with no treated control group Intention‐to‐treat analysis: yes Sample size calculation: no. |
|
| Participants | Country: Italy Nonresponders (n = 112) ‐ Previous nonresponse: biochemical ‐ Groups (interferon/interferon/interferon/C): ‐ Excluded: 0/0/0/0 ‐ Mean age (y): 47/48/45/46 ‐ Male (%): 64/57/61/54 ‐ Transfusion (%): 32/29/29/25 ‐ Drug abuse (%): N/A ‐ Genotype 1 (%): N/A ‐ Cirrhosis (%): 0/0 Inclusion criteria: Chronic hepatitis (clinical, serological and histological data), alanine transaminase levels at least twice the ULN, positive Anti‐HCV, lack of response to previous interferon‐alfa (3 MU three times per week x 24 wk). Exclusion criteria: Consumption of > 40 g/d of alcohol, drug‐induced liver disease, HBV, HDV, HIV, metabolic disorders, autoimmune factors and histological diagnosis of cirrhosis. |
|
| Interventions | ‐ Schedule: Experimental 1: Interferon alfa 3 MU three times per week x 24 wk Experimental 2: Interferon alfa 6 MU three times per week x 24 wk Experimental 3: LYMPH interferon alfa 3 MU three times per week x 24 wk Control: no treatment ‐ Follow‐up (F/U): 24 wk. | |
| Outcomes | ‐ Biochemical end of treatment response. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) Objective outcomes (Biochemical or virologic response) | Low risk | The review authors consider that knowledge of the group to which the patient belongs to, will not increase the bias as these outcomes are objective. |
| Blinding (performance bias and detection bias) Subjective outcomes (Clinical events other than mortality, Histology) | High risk | Not reported. |
| Blinding (performance bias and detection bias) Adverse effects | High risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients discontinued the therapy for adverse events. |
| Selective reporting (reporting bias) | Unclear risk | While it may be that no clinical events occurred in the 24 weeks of the trial, this was not specified in the paper. |
| Other bias | Unclear risk | Unclear. |
Bruix 2011.
| Methods | Randomized trial comparing pegylated interferon with no therapy in 626 patients who were still hepatitis C positive after prior therapy. Intention to treat analysis ‐ unclear (Dropouts who had not had an event were assumed not to have had one afterward (modified ITT); however, 116 and 118 patients in the treatment and control arms failed to complete the trial. Even so, not all of the patients could be counted because 5 were excluded from the analyses because of the failure of the two sites to comply with "Good Clinical Practice"; according to an abstract published in Gastroenterology in 2009, there were actually 631 patients randomized.) (All of the tables indicated that 626 were analysed.) Sample size calculation ‐ yes (Trial was to stop when 98 events had occurred (90% power to detect hazard ratio of 2.0); however, the trial was stopped after 5 years when only 63 patients had experienced a clinical event, as the other decision to stop was after 5 years.) |
|
| Participants | Countries: Spain, France, Italy, United States, Canada, Germany, Mexico, Brazil, Argentina Participants ‐ Both nonresponders and relapsers included. Previous lack of sustained viral response was to combination therapy (pegylated interferon plus ribavirin). ‐ Previous relapse or nonresponse: Virologic. ‐ Groups (interferon/interferon): 311/315 ‐ Excluded: 0/0 ‐ Mean age (y): 52.3 (7.5 SD)/52.0 (7.6 SD) ‐ Male (%): 66/68 ‐ Transfusion (%): N/A ‐ Drug abuse (%): N/A ‐ Genotype 1 (%): 89/90 ‐ Cirrhosis (%): 100/100 Inclusion criteria: HCV‐RNA positive, age 18‐65, biopsy confirmed cirrhosis that was clinically compensated (Childs‐Pugh A), failure to respond to combination therapy in past, no hepatocellular carcinoma (AFP < 100 ng/ml and no evidence of HCC on ultrasound). Some patients failed treatment with combination therapy just prior to randomisation; these patients had to have neutrophil count > 750 and platelet count > 50,000 at end of treatment. The remaining patients were directly enrolled into the trial; this cohort had to have hemoglobin > 9 gm%, neutrophil count > 1200, WBC count > 2500, and platelet count > 70,000. Exclusion criteria: Hepatitis B HIV coinfection, status post liver transplant, decompensated cirrhosis,hepatocellular carcinoma, prothrombin time prolonged > 3 seconds over control, ascites, encephalopathy, other liver disease, “certain pre‐existing psychiatric conditions, use of medications known to decrease portal hypertension. |
|
| Interventions | ‐ Schedule:
Experimental 1: Pegylated Interferon alfa‐2b, 0.5 mcg weekly up to 5 years (up to 130 mcg) (average duration of exposure 31.4 months) Experimental 2: No treatment (average duration of "exposure" 30.2 months) ‐ Follow‐up (F/U): Up to 5 years. |
|
| Outcomes | ‐ Mortality (all‐cause and hepatic) ‐ Liver morbidity (ascites, variceal bleeding, encephalopathy, hepatocellular carcinoma) ‐ Liver transplantation ‐ Childs‐Pugh‐Turcotte score ‐ Adverse events ‐ Noninvasive markers of necroinflammation. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code stratified according to age (more than 50 or not) and retreatment/direct enrollee status). |
| Allocation concealment (selection bias) | Low risk | Centralized. |
| Blinding (performance bias and detection bias) Objective outcomes (Biochemical or virologic response) | Low risk | Trial was not blinded, but outcomes were objective. |
| Blinding (performance bias and detection bias) Subjective outcomes (Clinical events other than mortality, Histology) | Low risk | Committee of experts adjudicated outcomes; they were blinded to the treatment arm. (The same was true for the clinical outcomes.) |
| Blinding (performance bias and detection bias) Adverse effects | Low risk | Committee of experts adjudicated outcomes; they were blinded to the treatment arm. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See comments above regarding intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Pertinent clinical outcomes reported. |
| Other bias | Unclear risk | Sample size calculation performed, no baseline differences between groups, and no apparent academic bias. Study sponsored by Schering‐Plough. |
Di Bisceglie 2008.
| Methods | Randomized trial comparing pegylated interferon with no therapy in 1150 patients who were still hepatitis C positive after prior therapy Intention to treat analysis ‐ yes Sample size calculation ‐ yes. |
|
| Participants | Country: USA Participants ‐ Both nonresponders and relapsers included (63.7% treatment/62.5% control nonresponders, 13.7% treatment/15.0% control relapsers, 22.6% treatment/22.5% control “express’ [only known not to have had SVR to prior therapy in NEJM paper, but Cont Clin Trials paper suggests that these were all nonresponders; however, the official protocol states, in section E7B, that the HCV‐RNA test may be positive after treatment, suggesting that some of these individuals in the express group could have been relapsers]). Previous lack of sustained viral response was to combination therapy (pegylated interferon plus ribavirin). ‐ Previous relapse or nonresponse: Virologic ‐ Groups (interferon/interferon): 517/533 ‐ Excluded: 0/0 ‐ Mean age (y): 51.1 (7.3 SD) in interferon arm, 50.1 (7.0 SD) in controls ‐ Male (%): 70/71.9 ‐ Transfusion (%): N/A ‐ Drug abuse (%): N/A ‐ Genotype 1 (%): 95.2/91.6 ‐ Cirrhosis (%): 40.2/41.3 Inclusion criteria: HCV‐RNA positive after at least 12 weeks of anti‐viral therapy,Ishak fibrosis score >3, age >18 years. Exclusion criteria: Hepatitis B HIV coinfection, status post liver transplant, decompensated cirrhosis,hepatocellular carcinoma, other coexistent liver disease, other uncontrolled medical or psychiatric condition, contraindication to (or intolerance to) interferon, unwillingness to use contraception, WBC count <1000, platelet count <50,000, anemia (hct/hgb < 33/11), use of immunosuppressive medication or coumadin, pregnancy or breast‐feeding (or their partners), illicit drug use within past 2 years, inability to provide informed consent, participation in another trial. |
|
| Interventions | ‐ Schedule:
Experimental 1: Interferon alfa‐2a (Pegasys®, Roche). 90 mcg weekly up to 3.5 years (up to 16380 mcg) Experimental 2: No treatment ‐ Follow‐up (F/U): Initial plan for 1400 days, but trial follow up continued for up to 6.1 years from time of randomisation. |
|
| Outcomes | ‐ Mortality (all‐cause and hepatic) ‐ Liver morbidity (ascites, variceal bleeding, encephalopathy, hepatocellular carcinoma, spontaneous bacterial peritonitis ‐ Childs‐Pugh‐Turcotte score ‐ Adverse events ‐ Virologic response (SVR) ‐ Histologic progression to cirrhosis (in subgroup without cirrhosis at time of entry) ‐ Quality of life (see Notes) |
|
| Notes | Archived data set received from NIDDK at request of RLK. However, because of difficulties in interpreting abbreviations and coding, as well as changes in upper limits of normal for some laboratory tests during the trial, the data could not be extracted. Thus, only published data used in analyses. In adverse events, "drug reaction" included as "rash". Quality of life data available in archived data set, but could not be interpreted due to abbreviations and coding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer using permuted blocks of random size; stratified by center and presence/absence cirrhosis. |
| Allocation concealment (selection bias) | Low risk | Centralized allocation. |
| Blinding (performance bias and detection bias) Objective outcomes (Biochemical or virologic response) | Low risk | Trial was not blinded to investigators or subjects, but outcomes were objective. |
| Blinding (performance bias and detection bias) Subjective outcomes (Clinical events other than mortality, Histology) | Low risk | Outcomes assessed by independent committee whose members were unaware of treatment assignment; this same situation existed for the assessment of the clinical outcomes. |
| Blinding (performance bias and detection bias) Adverse effects | Low risk | Outcomes assessed by independent committee whose members were unaware of treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were patient dropouts, but intention‐to‐treat analyses performed by carrying last value forward. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in protocol were reported and no pertinent ones were not reported. |
| Other bias | Low risk | Sample size calculation performed, trial funded by government agency (National Institutes of Health), no baseline differences between groups, no apparent academic bias. |
Shiffman 1999.
| Methods | Randomized trial comparing maintenance treatment with interferon versus no treatment Intention‐to‐treat analysis: yes Sample size calculation: yes. |
|
| Participants | Country: USA Nonresponders (n = 53) ‐ Previous nonresponse: virologic ‐ Groups (interferon/C): 26/27 ‐ Excluded: 0/0 ‐ Mean age (y): 48/49 ‐ Male (%): 58/52 ‐ Transfusion (%): N/A ‐ Drug abuse (%): N/A ‐ Genotype 1 (%): N/A ‐ Cirrhosis (%): 27/22 Inclusion criteria: Chronic hepatitis C (alanine transaminase > ULN, positive anti‐HCV, positive HCV‐RNA and histological evidence of chronic hepatitis), virological nonresponse to previous interferon alfa‐2b (5 MU three times per week x 24 wk), histological response to previous treatment with interferon, histological evidence of cirrhosis. Exclusion criteria: Other cause of chronic hepatitis, positive HBsAg, positive HIV, elevated antinuclear or antismooth muscle antibodies, abnormal alfa1‐antitrypsin or ceruloplasmin levels, significant stainable iron in biopsy, significant cytopenia, abnormal bilirubin, prothrombin time, albumin or alfa‐fetoprotein levels, active use of intravenous drugs, regular alcohol consumption, chronic renal failure, previous organ transplant. |
|
| Interventions | ‐ Schedule: Experimental 1: Interferon alfa‐2b 3 MU three times per week x 96 wk Control: No treatment for 96 wk ‐ Follow‐up (F/U): 0 to 96 wk. | |
| Outcomes | ‐ Biochemical end of treatment response ‐ Adverse events (number of patients having to discontinue therapy considered as serious adverse events) ‐ Histology. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) Objective outcomes (Biochemical or virologic response) | Low risk | The review authors consider that knowledge of the group to which the patient belongs to, will not increase the bias as these outcomes are objective. |
| Blinding (performance bias and detection bias) Subjective outcomes (Clinical events other than mortality, Histology) | Low risk | The authors stated: "The histological specimens were scored by two pathologist who were blinded to the patient´s participation in this study". |
| Blinding (performance bias and detection bias) Adverse effects | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The treatment was terminated in 5/26 (19%) and 2/27 (7%) patients in the interferon and control group respectively. |
| Selective reporting (reporting bias) | Unclear risk | While it may be that no clinical events occurred in the 96 weeks of the trial, this was not specified in the paper. |
| Other bias | Low risk | There was no imbalance in important characteristics. Sample size calculation was reported and the trial was not stopped early. The author of the trial has not conducted previous trials addressing the same intervention. The trial's source of funding did not come from any parties that might conflicting interest. |
Tanwar 2012.
| Methods | Randomized trial comparing pegylated interferon for 48 weeks with no treatment Intention‐to‐treat analysis ‐ Yes for most outcomes, no for QOL and adverse events Sample size calculation ‐ yes, but trial stopped early by Safety Committee because of results from HALT‐C and EPIC‐3 trials. |
|
| Participants | Country: UK Participants ‐ Both nonresponders and relapsers included (9/18 nonresponders and 7/18 relapsers in treatment arm with an additional 2 patients in treatment arm who were treatment naive/13/22 nonresponder and 9/22 relapsers in control arm. Previous lack of sustained viral response was to any therapy and not specified ‐ Previous relapse or nonresponse: Virologic in the 38 who were previously treated ‐ Groups (interferon/no treatment): 18/22 ‐ Excluded: 0/0 (1 patient in interferon arm withdrew before any treatment received, but appears to have been accounted for in most of outcomes ‐ Mean age (y): 54.9 (8.5 SD) in interferon arm, 52.1 (8.5 SD) in controls ‐ Male (%): 72/77 ‐ Transfusion (%): N/A ‐ Drug abuse (%): N/A ‐ Genotype 1 (%): 50/54.9 ‐ Cirrhosis (%): 100 (16 Child A/2 Child B)/100 (19 Child A/3 Child B) Inclusion criteria: Anti‐HCV and HCV‐RNA positive, age > 18 years, previous failure to therapy or unwilling take ribavirin or were believed not to be able to tolerate ribavirin, Child’s A or B cirrhosis (see above), no evidence hepatocellular carcinoma on imaging of AFP monitoring. Exclusion criteria: Other forms of liver disease, hepatitis B infection, severe pre‐existing depression or other conditions preventing se of interferon (including psychiatric disease, cardiac disease, renal disease, seizure disorders, pregnancy, severe retinopathy), neutrophil count < 1000, platelet count < 60,000. |
|
| Interventions | Interferon arm: Peginterferon α‐2a 90 mcg/week on weeks 1‐4, then dose escalated to 135 mcg/week X 4 weeks, then 180 mcg/week for 40 additional weeks (a total of 48 weeks) – If intolerant, dose decreased by 45 mcg/week intervals until tolerated. Patients then followed for 48 weeks in formal trial, but total follow‐up almost 4 years. Controls ‐ Standard care (unclear if seen as often during the first 48 weeks or if monitored as closely for toxicity‐like events). |
|
| Outcomes | Primary outcomes: SVR, all‐cause and hepatic mortalities. Secondary outcomes: Liver related events (variceal hemorrhage, new ascites and SBP, HCC and, although not listed in Methods, "decompensated cirrhosis"), quality of life scores (SF‐36 and Fatigue Severity Scale). |
|
| Notes | RLK sent an e‐mail to the first author at sudeep.tanwar@nhs.net on 4/12/12 with following questions:1. What were outcomes in two patients who were treatment naive [since plan was to eliminate those two from data]; 2. How was decompensation defined in Child B patients; 3. How often were controls seen, especially in first 48 weeks; 4‐6. How was randomisation sequence generated, allocation concealed, and any blinding performed; 7. Obtaining copy of protocol; 8. Details about support from Roche. A response was received on 4/16/12 indicating that records were all in storage and that it would be a few days before Dr Tanwar could get answers. We responded back to him on 4/16/12 asking a further question about how the patient who dropped out before receiving any treatment was followed so long‐term data could be obtained (or if it was just a matter of the last observation being carried forward). Upon rereading the protocol on 4/17/12, RLK was reminded of the provision that patients with decompensated cirrhosis also were to be excluded, and 5 patients in this trial were Childs B; an email was sent to Dr Tanwar requesting the outcome details on these five patients as well. Dr Tanwar responded back on 4/17/12; he sent us a protocol for the trial as well as information that allowed us to consider both the generation of the randomisation sequence and the concealment of allocation to be adequate. The details about the excluded patients are in the text. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randmization was stratified on the basis of HCV genotype. Patients were randomised..." Further details indicated that the sequence was constructed using a random number generator. |
| Allocation concealment (selection bias) | Low risk | Subsequent details from the investigator indicated that patients were assigned via a central computer, and the allocation was concealed from the investigator and patient. |
| Blinding (performance bias and detection bias) Objective outcomes (Biochemical or virologic response) | Low risk | Trial was not blinded to investigators or subjects, but outcomes were objective. |
| Blinding (performance bias and detection bias) Subjective outcomes (Clinical events other than mortality, Histology) | High risk | No blinding done (confirmed by investigator in his response to us). |
| Blinding (performance bias and detection bias) Adverse effects | High risk | No blinding done (confirmed by the investigator in his response to us). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Alhough there was one dropout in treatment arm, that patient was still followed in accordance with the protocol. Most of data presented as outcomes in all randomized patients; high risk of bias for quality of life scores and adverse events, as the data were incomplete and patients in the treatment arm were seen more frequently than the patients in the control group for the first 48 weeks of the trial. Most importantly, the trial was not limited to previously‐treated patients with compensated cirrhosis (see text), resulting in the removal of 6 patients from the original database, with an unknown effect on the randomisation. |
| Selective reporting (reporting bias) | Low risk | All pertinent outcomes were reported (as documented in the trial protocol provided by the investigator). |
| Other bias | Unclear risk | Sample size calculation done, but trial stopped early by Safety Committee after HALT‐C and EPIC3 trials available. No important differences in baseline characteristics. As noted earlier, there was more intense follow up of the treated patients than the controls during the first year. Some support from Roche was obtained but, according to the information received from the author, it did not appear to be such that there was any industry influence. |
Vaccaro 1997.
| Methods | Randomized trial comparing treatment with interferon versus no treatment in 38 nonresponders Intention‐to‐treat analysis: yes Sample size calculation: no. |
|
| Participants | Country: Italy Nonresponders (n = 38) ‐ Previous nonresponse: N/A ‐ Groups (interferon/C): 19/19 ‐ Excluded: 0/0 ‐ Mean age (y): N/A ‐ Male (%): N/A ‐ Transfusion (%): N/A ‐ Drug abuse (%): N/A ‐ Genotype 1 (%): N/A ‐ Cirrhosis (%): 0/0 Inclusion criteria: Chronic hepatitis C in the liver biopsy, positive HCV‐RNA and non‐response to previous interferon alfa‐2b (6 MU three times per week x 24 wk). Exclusion criteria: N/A. |
|
| Interventions | ‐ Schedule:
Experimental: LEUK‐interferon x 24 wk
Control: No treatment ‐ Follow‐up (F/U): 24 wk. |
|
| Outcomes | ‐ Virologic end of treatment response ‐ Sustained virologic response. | |
| Notes | Although the primary outcome sustained response (defined as sustained virologic and biochemical response), we have included it as sustained virologic response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) Objective outcomes (Biochemical or virologic response) | Low risk | The review authors consider that knowledge of the group to which the patient belongs to, will not increase the bias as these outcomes are objective. |
| Blinding (performance bias and detection bias) Subjective outcomes (Clinical events other than mortality, Histology) | High risk | Not reported. |
| Blinding (performance bias and detection bias) Adverse effects | High risk | No. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the intended course of retreatment. |
| Selective reporting (reporting bias) | Unclear risk | While it may be that no clinical events occurred in the 24 weeks of the trial, this was not specified in the paper. |
| Other bias | Unclear risk | Unclear. |
y = years d = days wk = weeks F/U = follow‐up N/A = not available MU = million international units LYMPH = lymphoblastoid LEUK = leukocyte CON = consensus ULN = upper limit of normal.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adinolfi 2003 | RCT comparing different ribavirin plus interferon regimens. |
| Aghemo 2012 | Editorial review of Lok paper from HALT‐C trial |
| Akham 2011 | Uncontrolled trial. |
| Alaimo 2006 | RCT comparing different ribavirin plus interferon regimens. |
| Alberti 1997 | Meta‐analysis. |
| Almasio 1999 | Previously included but moved because only RCTs comparing IFN to no treatment with clinical data now being considered. |
| Andreone 1999 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Andreone 2000 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Arase 1994 | Previously included but moved because only RCTs comparing IFN to no treatment with clinical data now being considered. |
| Arase 2003 | Previously included but moved because only RCTs comparing IFN to no treatment with clinical data now being considered. |
| August‐Jörg 2003 | RCT comparing different ribavirin plus interferon regimens. |
| Azzaroli 2004 | RCT comparing combination therapy to no treatment in treatment naive patients. |
| Bapin 2004 | RCT comparing different ribavirin plus interferon regimens. |
| Barbaro 1998 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Barbaro 1999 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Barbaro 1999 b | RCT comparing interferon versus interferon and ribavirin regimen. |
| Bekkering 1998 | Uncontrolled study. |
| Bell 1999 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Bellobuono 1997 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Berg 2006 | RCT comparing interferon plus ribavirin in treatment‐naive patients. |
| Bergmann 2007 | RCT comparing different ribavirin plus interferon regimens. |
| Bernardinello 1999 | RCT comparing beta‐interferon to no therapy. |
| Bonkovsky 1996 | Previously included but moved because only RCTs comparing IFN to no treatment with clinical data now being considered. |
| Bresci 2000 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Bresci 2000b | RCT comparing interferon versus interferon and ribavirin regimen. |
| Brillanti 1994 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Brillanti 1995 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Buti 2000 | RCT comparing different ribavirin plus interferon regimens. |
| Böcher 2006 | RCT comparing different ribavirin plus interferon regimen. |
| Cagnoni 1999 | Uncontrolled study. |
| Carr 2007 | Two RCTs comparing different ribavirin plus interferon regimens. |
| Carrara 1998 | Uncontrolled study. |
| Cavalletto 2000 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Chapman 2001 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Chemello 1997 | Previously included but moved because only RCTs comparing IFN to no treatment with clinical data now being considered. |
| Chousterman 2003 | RCT comparing different ribavirin plus interferon regimen. |
| COPILOT 2008 | COPILOT RCT; however, control group received colchicine, not no therapy or placebo |
| Cornberg 2006 | RCT comparing different ribavirin plus interferon regimens. |
| Cotler 1997 | Observational study. |
| Cuccorese 2000 | Uncontrolled study. |
| Davis 1998 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Davis 1999 | Selected subpopulation of patients randomized (only complete and partial responders to a repeat course of interferon were randomized; nonresponders were not randomized). |
| Di Biceglie 2000 | RCT comparing interferon plus iron reduction versus interferon. |
| Di Bisceglie 2001 | RCT comparing different ribavirin plus interferon regimens. |
| Di Marco 2000 | RCT comparing different ribavirin plus interferon regimens. |
| Diago 2007 | RCT comparing different ribavirin plus interferon regimens. |
| Dollinger 2005 | RCT comparing different ribavirin plus interferon regimens. |
| Enriquez 2000 | RCT comparing different ribavirin plus interferon regimens. |
| Erdem 2002 | RCT comparing different ribavirin plus interferon regimens. |
| Fargion 2006 | RCT comparing different ribavirin and amantadine plus interferon regimens. |
| Fartoux 2007 | RCT comparing IFN to no treatment, but not all patients were being retreated and unable to obtain information regarding data just for retreated ones. |
| Fattovich 2003 | RCT comparing different ribavirin plus interferon regimens. |
| Ferenci 1996 | Previously included but moved because only RCTs comparing IFN to no treatment with clinical data now being considered. |
| Fong 2000 | Insufficient data for inclusion. The dose and duration of previous interferon therapy is not described. Also, only 12‐week response rates are reported, but no end of treatment (48‐week) data are available. |
| Fontagnes 2007 | Non‐randomized trial. |
| Gaeta 1997 | Previously included but moved because only RCTs comparing IFN to no treatment with clinical data now being considered. |
| Gerken 1995 | Uncontrolled study. |
| Getachew 2004 | RCT comparing different ribavirin plus interferon regimens. |
| Giudici‐Cipriani 1993 | Non‐randomized study. |
| Gross 1999 | Previously included but moved because only RCTs comparing IFN to no treatment with clinical data now being considered. |
| Guerret 1999 | Observational study. |
| Hadziyannis 1997 | Insufficient data for inclusion. The dose and duration of previous interferon therapy are not described, nor is the number of patients in each intervention group. |
| Hasan 2001 | Uncontrolled study. |
| Hass 2005 | Uncontrolled study. |
| Heathcote 1998 | Previously included but moved because only RCTs comparing IFN to no treatment with clinical data now being considered. |
| Horiike 1994 | Non‐randomized study. |
| Iino 2002 | RCT including both naive patients and patients who had previously received interferon. |
| Ikeda 1998 | Not all patients were being retreated and unable to obtain information regarding data just for retreated ones. |
| Ikeda 2000 | RCT including both naive and relapsers. |
| Imai 1997 | Randomized trial of treatment‐naive patients only. |
| Iyoda 2000 | Uncontrolled study. |
| Jensen 2009 | Randomized trial comparing different regimens of peginterferon. |
| Kakumu 1994 | Non‐randomized study. |
| Katayama 2001 | Uncontrolled study. |
| Kishihara 1995 | Non‐randomized trial. |
| Kumar 2001 | RCT comparing different ribavirin plus interferon regimens. |
| Le 1996 | Uncontrolled study. |
| Leroy 2001 | RCT comparing different ribavirin plus interferon regimens. |
| Lindsay 1996 | Previously included but moved because only RCTs comparing IFN to no treatment with clinical data now being considered. |
| Lodato 2005 | RCT comparing different ribavirin plus interferon regimens. |
| Mangia 2005 | RCT comparing different ribavirin and amantadine plus interferon regimens. |
| Mangia 2008 | Trial compared interferon plus ribavirin. |
| Marcellin 1994 | Uncontrolled study. |
| Marco 2000 | RCT comparing different ribavirin plus interferon regimens. |
| Marriott 1992 | Uncontrolled study. |
| Mathew 2006 | RCT comparing different ribavirin plus interferon regimens. |
| Milella 1999 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Min 2001 | RCT comparing different ribavirin plus interferon regimens. |
| Moreno‐Otero 2003 | RCT comparing different ribavirin plus interferon regimens. |
| Mura 1999 | RCT comparing interferon to no treatment, but not stated if patients treatment‐naive or previously treated or both; communication with Dr. Realdi indicated that records were not available so information could not be retrieved. |
| Neuman Manuela 2010 | Different does of peg‐interferon compared; no untreated control group. |
| Nevens 2005 | RCT comparing different ribavirin plus interferon regimens. |
| Nishiguchi 1995 | Probably non‐randomized trial, did not necessarily include previously treated patients only, and subgroups could not be differentiated. |
| Nomura 2004 | Previously included but moved because only RCTs comparing IFN to no treatment with clinical data now being considered. |
| Pardo 1994 | Uncontrolled study. |
| Payen 1998 | Previously included but moved because only RCTs comparing IFN to no treatment with clinical data now being considered. |
| Perasso 1999 | Uncontrolled study. |
| Picciotto 1997 | Non‐randomized study. |
| Planas 2002 | Prior treatment was reason for exclusion |
| Pockros 2007 | RCT of gamma‐interferon. |
| Pol 1999 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Portal 2003 | RCT comparing different ribavirin plus interferon regimens. |
| Poynard 1999 | Previously included but moved because only RCTs comparing IFN to no treatment with clinical data now being considered. |
| Poynard 2003 | RCT comparing different ribavirin plus interferon regimens. |
| Puoti 2001 | RCT comparing different ribavirin plus interferon regimens. |
| Qu 2012 | Meta‐analysis of trials comparing interferon to no therapy and describing HCC outcome; not all trials in this meta‐analysis included retreated patients and one of the trials was probably not even randomized. |
| Reichard 1994 | Observational study. |
| Rodriguez‐Torres 2011 | Trial compares interferon lambda to interferon alfa in treatment naive individuals. |
| Rolachon 1997 | Previously included but moved because only RCTs comparing IFN to no treatment with clinical data now being considered. |
| Romero‐Gomez 2012 | Meta‐analysis of trials comparing interferon alfa2a to alfa2b; unclear if patients treatment naive or previously treated. |
| Rustgi 2005 | RCT comparing different ribavirin plus interferon regimens. |
| Salmeron 1999 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Saracco 1994 | Uncontrolled study. |
| Saracco 2001 | RCT comparing different ribavirin plus interferon regimens. |
| Saracco 2002 | RCT comparing different ribavirin plus interferon regimens. |
| Sarrazin 2007 | RCT comparing different SCH 503034 plus interferon regimen. |
| Scotto 1996 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Scotto 1998 | Previously included but moved because only RCTs comparing IFN to no treatment with clinical data now being considered. |
| Shiffman 2000 | RCT comparing different ribavirin plus interferon regimens. |
| Singal 2010 | Meta‐analysis. |
| Soza 2005 | Trial of gamma interferon. |
| Sporea 2006 | Quasi‐randomized trial comparing different ribavirin plus interferon regimens. |
| Steindl‐Munda 2003 | RCT comparing different ribavirin plus interferon regimens. |
| Suzuki 2001 | RCT including naive patients and patients who had received interferon previously. |
| Tassopoulos 2003 | RCT comparing different ribavirin plus interferon regimens. |
| Toyota 2002 | RCT comparing interferon versus interferon and ribavirin regimen. |
| Valla 1999 | Randomized trial comparing interferon to no interferon, but unclear if patients previously treated or not; further information requested from Dr. Valla via email (dominique.valla@bjn.ap‐hop‐paris.fr) on 4/22/12. Received response on April 24, 2012 indicating that all of the patients in the trial were treatment‐naive, so trial excluded. |
| Wartelle 1997 | Insufficient data for inclusion. The dose and duration of previous interferon therapy are not described. |
| Weiland 1993 | Uncontrolled study. |
| Yao 1997 | RCT comparing consensus interferon to alfa‐interferon; unclear if patients were previously treated or not. |
| Zeuzem 2000 | Trial compared different regimens of interferon; no untreated control group. |
| Zeuzem 2005 | RCT comparing different ribavirin plus interferon regimens. |
RCT = randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Cho 1992.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Trial identified in October 2011 search, but journal not readily available. |
Testino 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Randomized trial comparing interferon to no interferon; unknown if any patients previously treated. Article in Italian and not readily available. |
Differences between protocol and review
The type of outcome measures in the present updated review differ from the outcome measures listed in the previous review protocol. Mortality (all‐cause, hepatic), quality of life, and adverse events have become the primary outcomes in compliance with Cochrane Collaboration policies (Higgins 2011). The biochemical, histologic, and virologic outcomes have all become secondary outcomes and liver‐related morbidity (hepatic encephalopathy, variceal bleeding, ascites, spontaneous bacterial peritonitis, hepatocellular carcinoma, need for liver transplantation) was also added. The effort to validate the surrogate outcomes was also a new objective. The search methods for identification of studies have been changed: Science Citation Index Expanded (Royle 2003) has been included in the search. As to the statistical methods, the meta‐analyses have been performed according to the recommendations of The Cochrane Collaboration (Higgins 2011). The software package Review Manager 5 (RevMan 2011) provided by The Cochrane Collaboration has been used. Heterogeneity was explored by Chi2 test with significance set at P value of 0.10, and the quantity of heterogeneity was measured by I2 (Higgins 2002). Only trials of alfa‐interferon were included; other types of interferon (for example, beta, gamma, lambda, consensus) were excluded because of the issue of heterogeneity and paucity of trials with these agents. Because clinical data became available, and because the intervention was not shown to provide any clinical benefit, all of the comparisons of different interferon regimens have been removed from this systematic review; for historical purposes, the comparison of different regimens will be the subject of a separate review. The plan to deal differently with analyses containing trials with no events was removed since this issue rarely arose. Trial sequential analysis (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010; CTU 2011; Thorlund 2011) was performed to explore observations of significant differences in primary outcomes (except for adverse events, since so many arose and the effect was consistently in favour of the control patients) or the secondary outcomes of hepatic morbidity and sustained viral response (since this is the principle surrogate outcome used in practice and since observations in this outcome were compared to the primary outcomes).
Contributions of authors
RK, MP, KG, VA, PB, and RC performed the literature search and data extraction. RK and MP performed all statistical calculations and wrote the review. KG provided advice on calculations and methodology assessment. AKB and BD offered suggestions regarding improvement of the review.
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
The authors have no permanent financial contracts with companies producing interferon.
Dr Barrera's research activity is partially supported by the Centro de Investigacion Biomedica en Red en Enfermedades Hepaticas y Digestivas (CIBERehd).
The authors have no other relevant affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Alric 2001 {published data only}
- Alric L, Duffaut M, Selves J, Sandre K, Mularczyck M, Izopet J, et al. Maintenance therapy with gradual reduction of the interferon dose over one year improves histological response in patients with chronic hepatitis C with biochemical response: results of a randomized trial. Journal of Hepatology 2001;35:272‐8. [DOI] [PubMed] [Google Scholar]
Bresci 1995 {published data only}
- Bresci G, Parisi G, Banti S, Capzia A. Re‐treatment of interferon‐resistant patients with chronic hepatitis C with interferon‐alpha. Journal of Viral Hepatitis 1995;2(3):155‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bruix 2011 {published data only}
- Bruix J, Poynard T, Colombo M, Schiff E, Burak K, Heathcote EJ, et al. Maintenance therapy with peginterferon alfa‐2b does not prevent hepatocellular carcinoma in cirrhotic patients with chronic hepatitis C. Gastroenterology 2011;140(6):1990‐9. [DOI: 10.1053/j.gastro.2011.03.010] [DOI] [PubMed] [Google Scholar]
- Bruix J, Poynard T, Colombo M, Schiff ER, Reichen J, Burak KW, et al. Final results of the EPIC3 cirrhosis maintenance trial: pegintron maintenance therapy in cirrhotic (METAVIR F4) HCV patients, who failed to respond to interferon/ribavirin (IR) therapy. Gastroenterology 2009;136 Suppl 1:A798. [Google Scholar]
- Poynard T, Munteanu M, Colombo M, Bruix J, Schiff ER, Terg R, et al. Impact of peginterferon maintenance therapy on biomarkers (Fibrotest [FT]‐Acitest [AT]) in cirrhotic (METAVIR F4) HCV prior non‐responders. Results from the EPIC3 cirrhosis maintenance trial. Hepatology 2009;50(4 Suppl):711A. [Google Scholar]
Di Bisceglie 2008 {published data only}
- Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, et al. Prolonged therapy of advanced chronic hepatitis C with low‐dose peginterferon. New England Journal of Medicine 2008;359(23):2429‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisceglie AM, Stoddard AM, Dienstag JL, Shiffman ML, Seeff LB, Bonkovsky HL, et al. Excess mortality in patients with advanced chronic hepatitis C treated with long‐term peginterferon. Hepatology 2011;53(4):1100‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefs JC, Everson GT, Shiffman ML, Morgan T, Chen W‐P, Gillen D. Is the platelet count determined by hepatic function or hypersplenism: Results of the HALT‐C trial?. Hepatology 2011;54(Suppl 1):582a‐3a. [Google Scholar]
- Hoefs JC, Shiffman ML, Goodman ZD, Kleiner DE, Dienstag JL, Stoddard AM, HALT‐C Trial Group. Rate of progression of hepatic fibrosis in patients with chronic hepatitis C: results from the HALT‐C Trial. Gastroenterology 2011;141(3):900‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok AS, Everhard JE, Wright EC, Bisceglie AM, Kim H‐Y, Sterling RK, et al. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis C. Gastroenterology 2011;3:840‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman ML, Bisceglie AM, Lindsay KL, Morishima C, Wright EC, Hepatitis C Antiviral Long‐Term Treatment Against Cirrhosis Trial Group. Peginterferon alfa‐2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology 2004;126:1015‐23. [DOI] [PubMed] [Google Scholar]
Shiffman 1999 {published data only}
- Shiffman ML, Hofmann CM, Contos MJ, Luketic VA, Sanyal AJ, Sterling RK, et al. A randomized, controlled trial of maintenance interferon therapy for patients with chronic hepatitis C virus and persistent viremia. Gastroenterology 1999;117(5):1164‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tanwar 2012 {published data only (unpublished sought but not used)}
- Tanwar S, Wright M, Foster G, Ryder S, Mills P, Cramp M, et al. PACIFIC: A phase III, randomized, multicentre, dose escalation, efficacy and safety study examining the effects of treatment with peginterferon alfa‐2a in patients with Child's A or B cirrhosis in chronic hepatitis C virus infection. Gut 2010;59 Suppl 2:A38‐9. [Google Scholar]
- Tanwar S, Wright M, Foster GR, Ryder SD, Mills PR, Cramp ME, et al. PACIFIC: A phase III, randomized, multicenter, dose escalation, efficacy and safety study examining the effects of treatment with peginterferon alfa‐2a in patients with Child's A or B cirrhosis in chronic hepatitis C virus infection. Hepatology 2010;52(4 Suppl):710A. [Google Scholar]
- Tanwar S, Wright M, Foster GR, Ryder SD, Mills PR, Cramp ME, et al. Randomized clinical trial: a pilot study investigating the safety and effectiveness of an escalating dose of peginterferon α‐2a monotherapy for 48 weeks compared with standard clinical care in patients with hepatitis C cirrhosis. European Journal of Gastroenterology and Hepatology 2012;24(5):543‐50. [DOI] [PubMed] [Google Scholar]
Vaccaro 1997 {published data only}
- Vaccaro A, Giunta M, Lo Iacono O, Craxi A. Retreatment with leukocite interferon of chronic hepatitis C patients non responders to recombinant interferon. Journal of Hepatology 1997;26 Suppl 1:S197. [Google Scholar]
References to studies excluded from this review
Adinolfi 2003 {published data only}
- Adinolfi LE, Utili R, Tonziello A, Ruggiero G. Effects of alpha interferon induction plus ribavirin with or without amantadine in the treatment of interferon non‐responsive chronic hepatitis C: a randomized trial. Gut 2003;52:701‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aghemo 2012 {published data only}
- Aghemo A, Colombo M. Peginterferon maintenance therapy in patients with advanced hepatitis C to prevent hepatocellular carcinoma: The plot thickens. Journal of Hepatology 2012;56(1):276‐8. [DOI] [PubMed] [Google Scholar]
Akham 2011 {published data only}
- Akham SC, Gurel E, Sayan M. The sustained virologic response of nonresponder hepatitis C virus patients with retreatment. Indian Journal of Pathology and Microbiology 2011;54(1):81‐4. [DOI] [PubMed] [Google Scholar]
Alaimo 2006 {published data only}
- Alaimo G, Marco V, Ferraro D, Stefano R, Porrovecchio S, D'Angelo F, et al. Different doses of consensus interferon plus ribavirin in patients with hepatitis C virus genotype relapsed after interferon monotherapy: a randomized controlled trial. World Journal of Gastroenterology 2006;12(42):6861‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alberti 1997 {published data only}
- Alberti A, Chemello L, Noventa F, Cavalletto L, Salvo G. Therapy of hepatitis C: re‐treatment with alpha interferon. Hepatology 1997;26(3 Suppl 1):137S‐42S. [DOI] [PubMed] [Google Scholar]
Almasio 1999 {published data only}
- Almasio P, DiMarco V, Bonura C, Fuschi P, Camma C, LoIacono O, et al. Retreatment of relapsers with chronic hepatitis C: the effect of dose doubling and of host and viral factors. Hepatology 1997;4(Pt 2):416A. [Google Scholar]
- Almasio PL, Marco V, Bonura C, Fuschi P, Camma C, Lo Iacono O, et al. Viral and host factors in determining response of relapsers with chronic hepatitis C to retreatment with interferon. Digestive Diseases and Sciences 1999;44(5):1013‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fuschi P, Almasio P, Marco V, Camma C, Bonnura C, Lo Iacono O, et al. Should patients with chronic hepatitis C who relapse after interferon (IFN) be retreated?. Journal of Hepatology 1996;26 Suppl 1:S192. [Google Scholar]
Andreone 1999 {published data only}
- Andreone P, Gramenzi A, Cursaro C, Sbolli G, Fiorino S, Giammarino L, et al. Interferon‐alpha plus ribavirin in chronic hepatitis C resistant to previous interferon‐alpha course: results of a randomized multicenter trial. Journal of Hepatology 1999;30(5):788‐93. [DOI] [PubMed] [Google Scholar]
Andreone 2000 {published data only}
- Andreone P, Cusaro C, Gramenzi M, Margotti M, Ferr E, Talarico S, et al. High dose of interferon plus ribavirin for 6 or 12 months in non responder patients with chronic hepatitis C: results of a randomized trial. Journal of Hepatology 2000;32 Suppl 2:115. [Google Scholar]
Arase 1994 {published data only}
- Arase Y, Kumada H, Chayama K, Tsubota A, Koida I, Ikeda K, et al. Interferon retreatment of nonresponders with HCV‐RNA‐positive chronic hepatitis C. Journal of Gastroenterology 1994;29(3):299‐304. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arase 2003 {published data only}
- Arase Y, Ikeda K, Tsubota A, Suzuki Y, Saitoh S, Kobayashi M, et al. Efficacy of prolonged interferon therapy for patients with chronic hepatitis C with HCV‐genotype 1b and high virus load. Journal of Gastroenterology 2003;38(2):158‐63. [DOI] [PubMed] [Google Scholar]
- Arase Y, Ikeda K, Tsubota A, Suzuki Y, Saitoh S, Kobayashi M, et al. Randomized trial of prolonged interferon retreatment for chronic hepatitis C patients with HCV‐genotype 1b and high virus load. Hepatology Research 2003;25:364‐70. [DOI] [PubMed] [Google Scholar]
August‐Jörg 2003 {published data only}
- August‐Jörg BS, Borivicka J, Dufour JF, Gonvers JJ, Henz S, Hermann S, et al. Twenty‐four vs. forty‐eight weeks of re‐therapy with interferon alpha 2b and ribavirin in interferon alpha monotherapy relapsers with chronic hepatitis C. Swiss Medical Weekly 2003;133:455‐60. [DOI] [PubMed] [Google Scholar]
Azzaroli 2004 {published data only}
- Azzaroli F, Accogli E, Nigro G, Trere D, Giovanelli S, Miracolo A, et al. Interferon plus ribavirin and interferon alone in preventing hepatocellular carcinoma: a prospective study on patients with HCV related cirrhosis. World Journal of Gastroenterology: WJG 2004;10(21):3099‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bapin 2004 {published data only}
- Bapin C, Fabbro‐Peray P, Hachemane S, Blanc F, Diaz D, Pueyo P, et al. Retreatment with pegylated interferon alpha‐2b and ribavirin in patients with chronic hepatitis C non responders to interferon monotherapy or interferon and ribavirin combination. A prospective randomized pilot study of two regimens: induction versus Peg. Journal of Hepatology 2004;40 Suppl 1:135. [Google Scholar]
Barbaro 1998 {published data only}
- Barbaro G, Lorenzo G, Soldini M, Giancaspro G, Bellomo G, Belloni G, et al. Interferon‐alpha‐2B and ribavirin in combination for chronic hepatitis C patients not responding to interferon‐alpha alone: an Italian multicenter, randomized, controlled, clinical study. American Journal of Gastroenterology 1998;93(12):2445‐51. [DOI] [PubMed] [Google Scholar]
Barbaro 1999 {published data only}
- Barbaro G, Lorenzo G, Soldini M, Giancaspro G, Pellicelli A, Grisorio B, et al. Intravenous recombinant interferon‐beta versus interferon‐alpha‐2b and ribavirin in combination for short‐term treatment of chronic hepatitis C patients not responding to interferon‐alpha. Multicenter Interferon Beta Italian Group Investigators. Scandinavian Journal of Gastroenterology 1999;34(9):928‐33. [DOI] [PubMed] [Google Scholar]
Barbaro 1999 b {published data only}
- Barbaro G, Lorenzo G, Belloni G, Ferrari L, Paiano A, Poggio P, et al. Interferon alpha‐2B and ribavirin in combination for patients with chronic hepatitis C who failed to respond to, or relapsed after, interferon alpha therapy: a randomized trial. American Journal of Medicine 1999;107(2):112‐8. [DOI] [PubMed] [Google Scholar]
Bekkering 1998 {published data only}
- Bekkering FC, Brouwer JT, Leroux‐Roels G, Vlierberghe H, Elewaut A, Schalm SW. Ultrarapid hepatitis C virus clearance by daily high‐dose interferon in non‐responders to standard therapy. Journal of Hepatology 1998;28(6):960‐4. [DOI] [PubMed] [Google Scholar]
Bell 1999 {published data only}
- Bell H, Hellum K, Harthug S, Myrvang B, Ritland S, Maeland A, et al. Treatment with interferon‐alpha2a alone or interferon‐alpha2a plus ribavirin in patients with chronic hepatitis C previously treated with interferon‐alpha2a. CONSTRUCT Group. Scandinavian Journal of Gastroenterology 1999;34(2):194‐8. [DOI] [PubMed] [Google Scholar]
Bellobuono 1997 {published data only}
- Bellobuono A, Mondazzi L, Tempini S, Silini E, Vicari F, Ideo G. Ribavirin and interferon‐alpha combination therapy vs interferon‐alpha alone in the retreatment of chronic hepatitis C: a randomized clinical trial. Journal of Viral Hepatitis 1997;4(3):185‐91. [DOI] [PubMed] [Google Scholar]
Berg 2006 {published data only}
- Berg T, Wagner M, Nasser S, Sarrazin C, Heintges T, Gerlach T, et al. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon‐alfa‐2a plus ribavirin. Gastroenterology 2006;130(4):1086‐97. [DOI] [PubMed] [Google Scholar]
Bergmann 2007 {published data only}
- Bergmann JF, Vrolijk JM, Schaar P, Vroom B, Hoek B, Sluys Veer A, et al. Gamma‐glutamyltransferase and rapid virological response as predictors of successful treatment with experimental or standard peginterferon‐alfa‐2b in chronic hepatitis C non‐responders. Liver International 2007;27(9):1217‐25. [DOI] [PubMed] [Google Scholar]
Bernardinello 1999 {published data only}
- Bernardinello E, Cavalletto L, Chemello L, Mezzocolli I, Donada C, Benvegnú L, et al. Long‐term clinical outcome after beta‐interferon therapy in cirrhotic patients with chronic hepatitis C. TVVH Study Group. Hepato‐Gastroenterology 1999;46:3216‐22. [PubMed] [Google Scholar]
Böcher 2006 {published data only}
- Böcher WO, Schuchmann M, Link R, Heribert H, Rahman F, Sprinzl M, et al. Consensus interferon and ribavirin for patients with chronic hepatitis C and failure of previous interferon alfa therapy. Liver International 2006;26:319‐25. [DOI] [PubMed] [Google Scholar]
Bonkovsky 1996 {published data only}
- Bonkovsky HL, Clifford BD, Smith LJ, Allan C, Banner B. High‐dose interferon‐alpha 2b for re‐treatment of nonresponders or relapsing patients with chronic hepatitis C. A controlled randomized trial. Digestive Disease and Sciences 1996;41(1):149‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bresci 2000 {published data only}
- Bresci G, Parisi G, Bertoni M, Capria A. High‐dose interferon plus ribavirin in chronic hepatitis C not responding to recombinant alpha‐interferon. Digestive and Liver Disease 2000;32(8):703‐7. [DOI] [PubMed] [Google Scholar]
Bresci 2000b {published data only}
- Bresci G, Parisi G, Bertoni M, Scatena F, Capria A. Interferon plus ribavirin in chronic hepatitis C non‐responders to recombinant alpha‐interferon. Journal of Viral Hepatitis 2000;7(1):75‐8. [DOI] [PubMed] [Google Scholar]
Brillanti 1994 {published data only}
- Brillanti S, Garson J, Foli M, Whitby K, Deaville R, Masci C, et al. A pilot study of combination therapy with ribavirin plus interferon alfa for interferon alfa‐resistant chronic hepatitis C. Gastroenterology 1994;107(3):812‐7. [DOI] [PubMed] [Google Scholar]
Brillanti 1995 {published data only}
- Brillanti S, Miglioli M, Barbara L. Combination antiviral therapy with ribavirin and interferon alfa in interferon alfa relapsers and non‐responders: Italian experience. Journal of Hepatology 1995;23 Suppl 2:13‐5. [PubMed] [Google Scholar]
Buti 2000 {published data only}
- Buti M, Olive G, Stalgis C, Estaban R, Guardi J. Quantification of serum hepatitis C virus RNA with daily or standard interferon doses plus ribavirin in nonresponder patients with chronic hepatitis C. Digestive Diseases and Sciences 2000;45(4):685‐9. [DOI] [PubMed] [Google Scholar]
Cagnoni 1999 {published data only}
- Cagnoni C, Pancotti D, Carrara G. The retreatment with natural leukocyte interferon alfa‐n3 of chronic hepatitis C patients: the intolerant and nonresponsive results to prior treatment with recombinant alfa interferon. Annali of Italiani di Medicina Interna 1999;14(3):159‐65. [PubMed] [Google Scholar]
Carr 2007 {published data only}
- Carr C, Blaine‐Hollinger F, Yoffe B, Wakil A, Phillips J, Bzowej N, et al. Efficacy of interferon alpha‐2b induction therapy before retreatment for chronic hepatitis C. Liver International 2007;27(8):1111‐8. [DOI] [PubMed] [Google Scholar]
Carrara 1998 {published data only}
- Carrara M, Azzurro M, Adamo S. Retreatment with human leukocyte interferon alpha of chronic hepatitis C, recurring after a first cycle with recombinant interferon (Letter). Italian Journal of Gastroenterology and Hepatology 1998;30(2):234‐5. [PubMed] [Google Scholar]
Cavalletto 2000 {published data only}
- Cavalletto L, Chemello L, Donada C, Casarin P, Belussi F, Bernardinello E, et al. The pattern of response to interferon alpha (alpha‐IFN) predicts sustained response to a 6‐month alpha‐IFN and ribavirin retreatment for chronic hepatitis C. TVVH Study Group. Journal of Hepatology 2000;33(1):128‐34. [DOI] [PubMed] [Google Scholar]
Chapman 2001 {published data only}
- Chapman BA, Stace NH, Edgar CL, Bartlett SE, Frampton CM, Scahill SL, et al. Interferon‐alpha2a/ribavirin versus interferon‐alpha2a alone for the retreatment of hepatitis C patients who relapse after a standard course of interferon. New Zealand Medical Journal 2001;114(1128):103‐4. [PubMed] [Google Scholar]
Chemello 1997 {published data only}
- Chemello L, Cavalletto L, Donada C, Bonetti P, Casarin P, Urban F, et al. Efficacy of a second cycle of interferon therapy in patients with chronic hepatitis C. Gastroenterology 1997;113(5):1654‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chousterman 2003 {published data only}
- Chousterman M, Auray‐Cartier V, Hagege H, Arpurt JP, Cassan P, Denis J, et al. Efficacy of pegylated interferon alfa‐2b in combination with ribavirin in patients with chronic hepatitis C non‐responders to a previous treatment. Journal of Hepatology 2003;38 Suppl 2:133. [Google Scholar]
COPILOT 2008 {published data only}
- Afdahl N, Freilich B, Levine R, Black M, Monsour H, O'Brien M. Colchicine versus peg‐intron long term (COPILOT) trial: interim analysis of clinical outcomes at year 2. (Abstract). Hepatology 2004;4 Suppl 1:239A. [Google Scholar]
- Afdahl NH, Levine R, Brown R, Freilich B, O'Brien M, Brass C, et al. Colchicine versus peg‐interferon alfa 2b long term therapy: results of the 4 year COPILOT trial. Journal of Hepatology 2008;48 Suppl 2:S4. [Google Scholar]
- Cardenas A, Pritchett S, Brown RS, Levine RA, Curry MP, Afdahl NH. The effects of long term PEG‐Interferon therapy on the development of esophageal varices and variceal bleeding in patients with hepatitis C and advanced fibrosis: Final results from the Copilot trial (Abstract). Gastroenterology 2009;136 Suppl 1:259. [Google Scholar]
Cornberg 2006 {published data only}
- Cornberg M, Hadem J, Herrmann E, Schuppert F, Schmidt HJ, Reiser M, et al. Treatment with daily consensus interferon plus ribavirin in non‐responder patients with chronic hepatitis C: a randomized open‐label pilot study. Journal of Hepatology 2006;44:291‐301. [DOI] [PubMed] [Google Scholar]
Cotler 1997 {published data only}
- Cotler SJ, Albert DG, Rosenblate HJ, Ganger DR, Jensen DM. Interferon therapy for chronic hepatitis C is associated with sustained histological improvement in virological nonresponders (NR) and responders with relapse (RR) (EASL abstract). Journal of Hepatology 1997;26 Suppl 1:S225. [Google Scholar]
Cuccorese 2000 {published data only}
- Cuccorese G, Tursi A, Spinazzola AM, Modeo ME, Miglietta A. Biochemical and virological changes in serum of nonresponder or relapser chronic hepatitis C patients retreated with interferon and ribavirin followed by interferon alone. American Journal of Gastroenerology 2000;95(9):2399‐400. [DOI] [PubMed] [Google Scholar]
Davis 1998 {published data only}
- Davis GL, Esteban‐Mur R, Rustgi V, Hoefs J, Gordon SC, Trepo C, et al. Interferon alfa‐2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. New England Journal of Medicine 1998;339(21):1493‐9. [DOI] [PubMed] [Google Scholar]
Davis 1999 {published data only}
- Davis GL, Schiff E, Marcellin P, Esteban Mur R, Goodman Z, Harvey J, et al. Long‐term continuous recombinant interferon alfa‐2b (Intron A) versus repeated 24‐week cycles for disease suppression in IFN‐relapsers with chronic hepatitis C (Abstract). Hepatology 1999;30(4 Pt 2):317A. [Google Scholar]
Diago 2007 {published data only}
- Diago M, Crespo J, Olveira A, Perez R, Barcena R, Sanchez‐Tapias JM, et al. Clinical trial: pharmacodynamics and pharmacokinetics of re‐treatment with fixed‐dose induction of peginterferon alfa‐2a in hepatitis C virus genotype 1 true non‐responder patients. Alimentary Pharmacology & Therapeutics 2007;26:1131‐8. [DOI] [PubMed] [Google Scholar]
Di Biceglie 2000 {published data only}
- Bisceglie A, Bonkovsky HL, Chopra S, Flamm S, Reddy RK, Grace N, et al. Iron reduction as an adjuvant to interferon therapy in patients with chronic hepatitis C who have previously not responded to interferon: a multicenter, prospective, randomized, controlled trial. Hepatology 2000;32(1):135‐8. [DOI] [PubMed] [Google Scholar]
Di Bisceglie 2001 {published data only}
- Bisceglie AM, Thompson J, Smith‐Wilkaitis N, Brunt EM, Bacon BR. Combination of interferon and ribavirin in chronic hepatitis C: re‐treatment of nonresponders to interferon. Hepatology 2001;33(3):704‐7. [DOI] [PubMed] [Google Scholar]
Di Marco 2000 {published data only}
- Marco V, Almasio P, Vaccaro A, Ferraro D, Pietro P, Cataldo MG, et al. Combined retreatment of relapse of chronic hepatitis C with high‐dose alfa‐2‐b interferon plus ribavirin for 6 or 12 months. Journal of Hepatology 2000;33:456‐62. [DOI] [PubMed] [Google Scholar]
Dollinger 2005 {published data only}
- Dollinger MM, Dridi Y, Lesske J, Behl S, Fleig WE. Efficacy of daily consensus interferon and ribavirin compared to peg‐interferon‐2b and ribavirin in non‐responders with chronic hepatitis C. Hepatology 2005;42(4):691A‐2A. [Google Scholar]
Enriquez 2000 {published data only}
- Enriquez J, Gallego A, Torras X, Perez‐Olmeda T, Diago M, Soriano V, et al. Retreatment for 24 vs 48 weeks with interferon‐alpha2b plus ribavirin of chronic hepatitis C patients who relapsed or did not respond to interferon alone. Journal of Viral Hepatitis 2000;7(6):403‐8. [DOI] [PubMed] [Google Scholar]
Erdem 2002 {published data only}
- Erdem L, Akbayir N, Cakaloglu Y, Cevikbas U, Badur S. Combination of interferon induction therapy and ribavirin in primary interferon non‐responders infected with genotype 1 hepatitis C virus. Journal of Hepatology 2002;36 Suppl 1:234. [Google Scholar]
Fargion 2006 {published data only}
- Fargion S, Borzio M, Maraschi A, Cargnel A. Triple antiviral therapy in HCV positive patients who failed prior combination therapy. World Journal of Gastroenterology 2006;12(33):5293‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fartoux 2007 {published data only}
- Fartoux L, Degos F, Trepo C, Goria O, Cales P, Tran A, et al. Effect of prolonged Interferon therapy on the outcome of hepatitis C virus‐related cirrhosis: a randomized trial. Clinical Gastroenterology and Hepatology 2007;5:502‐7. [DOI] [PubMed] [Google Scholar]
Fattovich 2003 {published data only}
- Fattovich G, Zagni I, Minola E, Fabris P, Boccia S, Giusti, et al. Efficacy of prolonged 5 millions unit of interferon in combination with ribavirin for relapser patients with chronic hepatitis C. Journal of Viral Hepatitis 2003;10:111‐7. [DOI] [PubMed] [Google Scholar]
- Fattovich G, Zagni I, Ribero L, Castagnetti E, Minola E, Lomonaco L, et al. A randomized trial of prolonged high dose of interferon plus ribavirin for hepatitis C patients nonresponders to interferon alone. Journal of Viral Hepatitis 2004;11:543‐51. [DOI] [PubMed] [Google Scholar]
Ferenci 1996 {published data only}
- Ferenci P, Stauber R, Propst A, Fiedler R, Muller C, Gschwantler M, et al. Dose increase augments response rate to interferon‐alpha in chronic hepatitis C. Digestive Diseases and Sciences 1996;41(12 Suppl):103S‐8S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fong 2000 {published data only}
- Fong TL, Pauly MP, Zacks S, Kahn JA, Sze G, Schulman D, et al. Multicenter randomized trial of 15 mcg Infergen administered daily vs. thrice weekly in patients with chronic hepatitis C who did not respond to interferon (IFN) therapy (abstract). Hepatology 2000;32(4 Pt 2):349A. [Google Scholar]
Fontagnes 2007 {published data only}
- Fontagnes T, Beorchia S, Douvin C, Delassalle P, Combis J.M, Hanslik B, et al. Safety and efficacy of combination therapy with peginterferon alfa‐2a (40kD) and ribavirin in the outpatient setting: prospective analysis of 197 patients with chronic hepatitis C viral infection. Gastroenterologie Clinique et Biologique 2007;31:566‐72. [DOI] [PubMed] [Google Scholar]
Gaeta 1997 {published data only}
- Gaeta GB, Virgilio D, Russo G, Stornaiuolo G, Nicolella U, Colella F, et al. Human leucocyte interferon‐alpha in chronic hepatitis C resistant to recombinant or lymphoblastoid interferon‐alpha: a randomized controlled trial. Journal of Viral Hepatitis 1997;4(3):209‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gerken 1995 {published data only}
- Gerken G, Teuber G, Goergen B, Meyer zum Buschenfelde KH. Interferon‐alpha retreatment in chronic hepatitis C. Journal of Hepatology 1995;22(1 Suppl):118‐21. [PubMed] [Google Scholar]
Getachew 2004 {published data only}
- Getachew Y, Browning JD, Prebis M, Rogers T, Brown GR. Combination therapy for the treatment of hepatitis C in the veteran population: higher than expected rates of therapy discontinuation. Alimentary Pharmacology & Therapeutics 2004;20:629‐36. [DOI] [PubMed] [Google Scholar]
Giudici‐Cipriani 1993 {published data only}
- Giudici‐Cipriani A, Azzola E, Varagona E, Vitali A, Marenco G, Conca V, et al. Usefulness of re‐treatment with interferon (IFN) in relapsing and non‐responder patients with chronic hepatitis C (Abstract). Journal of Hepatology 1993;18 Suppl 1:S123‐4. [Google Scholar]
Gross 1999 {published data only}
- Gross JB, Brandhagen DJ, Gossard AA, Poterucha JJ, Douglas DD, Spivey JR, et al. Daily interferon to suppress HCV viremia among interferon non‐responders (Abstract). Hepatology 1998;28(4 Pt 2):573A. [Google Scholar]
- Gross JB, Brandhagen DJ, Poterucha JJ, Lindor KD, Douglas DD, Spivey JR, et al. Interferon alpha 2b 5MU TIW, +/‐ 4‐week daily interferon induction, +/‐ ribavirin, for re‐treatment of interferon non‐responders with chronic hepatitis C (Abstract). Hepatology 1999;30(4 Pt 2):634A. [Google Scholar]
Guerret 1999 {published data only}
- Guerret S, Desmouliere A, Chossegros P, Costa AM, Badid C, Trepo C, et al. Long‐term administration of interferon‐alpha in non‐responder patients with chronic hepatitis C: follow‐up of liver fibrosis over 5 years. Journal of Viral Hepatitis 1999;6(2):125‐33. [DOI] [PubMed] [Google Scholar]
Hadziyannis 1997 {published data only}
- Hadziyannis AS, Papaioannou C, Spanou E, Manesis E, Hadziyannis SJ. Daily administration of interferon‐alpha for 1 month followed by a TIW standard regimen is associated with high response rates in chronic hepatitis C (Abstract). Hepatology 1997;26(4 Pt 2):420A. [Google Scholar]
Hasan 2001 {published data only}
- Hasan F, Asker H, al Shamali M, al Kalaoui M, al Nakib B. Interferon‐alpha plus ribavirin combination therapy for the treatment of chronic hepatitis C in interferon non‐responders. Hepato‐Gastroenterology 2000;47(36):1642‐4. [PubMed] [Google Scholar]
Hass 2005 {published data only}
- Hass HG, Kreysel C, Fischinger J, Menzel J, Kaiser S. High‐dose interferon alfa‐2b induction therapy in combination with ribavirin for treatment of chronic hepatitis C in patients with non‐response or relapse after interferon alfa monotherapy. World Journal of Gastroenterology 2005;11(34):5342‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heathcote 1998 {published data only}
- Heathcote EJ, Keeffe EB, Lee SS, Feinman SV, Tong MJ, Reddy KR, et al. Re‐treatment of chronic hepatitis C with consensus interferon. Hepatology 1998;27(4):1136‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Heathcote JL, James S, Mullen KD, Hauser SC, Rosenblate H, Albert DG, et al. Chronic hepatitis C virus patients with breakthroughs during interferon treatment can successfully be retreated with consensus interferon. Hepatology 1999;30(2):562‐6. [DOI] [PubMed] [Google Scholar]
- Lee WM, Reddy KR, Tong MJ, Black M, Leeuwen DJ, Hollinger FB, et al. Early hepatitis C virus‐RNA predict interferon treatment outcomes in chronic hepatitis C. Hepatology 1998;28(5):1411‐5. [DOI] [PubMed] [Google Scholar]
Horiike 1994 {published data only}
- Horiike N, Kurose K, Ohkura I, Masumoto T, Nakanishi K, Michitaka K, et al. Retreatment with interferon in chronic hepatitis C (Letter). Journal of Hepatology 1994;21(6):1155. [DOI] [PubMed] [Google Scholar]
Iino 2002 {published data only}
- Iino S, Ichida F, Sakuma A. A randomized clinical trial with natural IFN‐alfa monotherapy for 24 or 48 weeks on patients with chronic hepatitis C having genotype 1b infection in high viral titers. Hepatology Research 2002;24(4):338‐45. [DOI] [PubMed] [Google Scholar]
Ikeda 1998 {published data only}
- Ikeda K, Kumada H, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, et al. A randomized controlled trial of interferon‐alpha in patients with cirrhosis caused by 2a/2b genotype hepatitis C virus. Journal of Hepatology 1998;28:910‐1. [DOI] [PubMed] [Google Scholar]
Ikeda 2000 {published data only}
- Ikeda F, Shimomura H, Miyake M, Fujioka SI, Itoh M, Takahashi A, et al. Early clearance of circulating hepatitis C virus enhanced by induction therapy with twice‐a‐day intravenous injection of IFN‐beta?. Journal of Interferon & Cytokine Research 2000;20(9):831‐6. [DOI] [PubMed] [Google Scholar]
Imai 1997 {published data only}
- Imai Y, Kawata S, Tamura S, Ito N, Seki K, Nishiuchi M, et al. Recombinant interferon‐α‐2a for treatment of chronic hepatitis C: results of a multicenter randomized controlled dose study. Liver 1997;17:88‐92. [DOI] [PubMed] [Google Scholar]
Iyoda 2000 {published data only}
- Iyoda K, Yuki N, Kato M, Sugiyasu Y, Komori M, Fujii E, et al. Retreatment with interferon for chronic hepatitis C after transient response. Journal of Clinical Gastroenterology 2000;31(4):297‐301. [DOI] [PubMed] [Google Scholar]
Jensen 2009 {published data only}
- Jensen DM, Marcellin P, Frelich B, Andreone P, Bisceglie A, Brando CE, et al. Re‐treatment of patients with chronic hepatitis C who do not respond to peginterferon α2b. A randomized trial. Annals of Internal Medicine 2009;150(8):528‐40. [DOI] [PubMed] [Google Scholar]
Kakumu 1994 {published data only}
- Kakumu S, Yoshioka K. Retreatment with interferon in patients with chronic hepatitis C. Journal of Hepatology 1994;21(3):483. [DOI] [PubMed] [Google Scholar]
Katayama 2001 {published data only}
- Katayama K, Kasahara A, Sasaki Y, Kashiwagi T, Naito M, Masuzawa M, et al. Immunological response to interferon‐gamma priming prior to interferon‐alpha treatment in refractory chronic hepatitis C in relation to viral clearance. Journal of Viral Hepatitis 2001;8(3):180‐5. [DOI] [PubMed] [Google Scholar]
Kishihara 1995 {published data only}
- Kishihara Y, Hayashi J, Ohmiya M, Nakashima K, Ikematsu H, Kashiwagi S. A preliminary study of retreatment of chronic hepatitis C with interferon. Fukuoka Igaku Zasshi 1995;86(4):113‐20. [PubMed] [Google Scholar]
Kumar 2001 {published data only}
- Kumar KS, Brown M. Daily vs. thrice weekly interferon in combination with ribavirin for patients with chronic hepatitis C infection: preliminary results of a multicenter randomized trial. Gastroenterology 2001;120(5):A571. [Google Scholar]
Le 1996 {published data only}
- Le X, Zhou X, Dai X. Evaluation of interferon (alpha)‐2b for the treatment of relapsed hepatitis C. Hepatology 1996;24(4 Pt 2):536A. [Google Scholar]
Leroy 2001 {published data only}
- Leroy V, Dutertre N, Tran A, Naveau S, Abergel A, Bronwicki JP, et al. High‐daily induction dose of interferon in combination with ribavirin in chronic hepatitis C non responder patients: a randomised controlled trial. Journal of Hepatology 2001;34(1):146. [Google Scholar]
Lindsay 1996 {published data only}
- Lindsay KL, Davis GL, Schiff ER, Bodenheimer HC, Balart LA, Dienstag JL, et al. Response to higher doses of interferon alfa‐2b in patients with chronic hepatitis C: a randomized multicenter trial. Hepatitis Interventional Therapy Group. Hepatology 1996;24(5):1034‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lodato 2005 {published data only}
- Lodato F, Azzaroli F, Brillanti S, Colecchia A, Tame MR, Montagnani M, et al. Higher doses if peginterferon alpha‐2b administered twice weekly improve sustained virological response in difficult‐to‐treat patients with chronic hepatitis C: results of a pilot randomized study. Journal of Viral Hepatitis 2005;12:536‐42. [DOI] [PubMed] [Google Scholar]
Mangia 2005 {published data only}
- Mangia A, Cimino L, Persico M, Dernelia M, Rumi M, Spinzi O, et al. Enhanced response to peginterferon alfa‐2a‐based triple therapy in previously non‐responsive chronic hepatitis C: final results of PRETTY study. Journal of Hepatology 2005;42 Suppl 2:200‐1. [Google Scholar]
Mangia 2008 {published data only}
- Mangia A, Minerva N, Bacca D, Cozzolongo R, Ricci GL, Carretta V, et al. Individualized treatment duration for hepatitis C genotype 1 patients: a randomized controlled trial. Hepatology 2008;47(1):43‐50. [DOI] [PubMed] [Google Scholar]
Marcellin 1994 {published data only}
- Marcellin P, Boyer N, Pouteau M, Benhamou JP, Erlinger S. Retreatment with interferon‐alpha of chronic hepatitis C virus infection. Lancet 1994;344(8923):690‐1. [DOI] [PubMed] [Google Scholar]
Marco 2000 {published data only}
- Marco VD, Almasio P, Vaccaro A, Ferraro D, Parisi P, Cataldo MG. Combined treatment of relapse of chronic hepatitis C with high‐dose alpha2b interferon plus ribavirin for 6 or 12 months. Journal of Hepatology 2000;33(3):456‐62. [DOI] [PubMed] [Google Scholar]
Marriott 1992 {published data only}
- Marriott E, Quiroga JA, Carreno V. Retreatment of chronic hepatitis C with interferon‐alpha. Journal of Infectious Diseases 1992;166(5):1200‐1. [DOI] [PubMed] [Google Scholar]
Mathew 2006 {published data only}
- Mathew A, Peiffer LP, Rhoades K, McGarrity T. Sustained viral response to pegylated interferon alpha‐2b and ribavirin in chronic hepatitis C refractory to prior treatment. Digestive Diseases and Sciciences 2006;51:1956‐61. [DOI] [PubMed] [Google Scholar]
- Mathew A, Peiffer LP, Rhoades K, McGarrity TJ. Improvement in quality of life measures in patients with refractory hepatitis C, responding to retreatment with pegylated interferon alfa‐2b and ribavirin. Health and Quality of Life Outcomes 2006;4(30):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milella 1999 {published data only}
- Milella M, Santantonio T, Pietromatera G, Maselli R, Casalino C, Mariano N, et al. Retreatment of non‐responder or relapser chronic hepatitis C patients with interferon plus ribavirin vs interferon alone. Italian Journal of Gastroenterology and Hepatology 1999;31(2):211‐5. [PubMed] [Google Scholar]
Min 2001 {published data only}
- Min AD, Jones JL, Esposito S, Lebovics E, Jacobson IM, Klion FM, et al. Efficacy of high‐dose interferon in combination with ribavirin in patients with chronic hepatitis C resistant to interferon alone. American Journal of Gastroenterology 2001;96(4):1143‐9. [DOI] [PubMed] [Google Scholar]
Moreno‐Otero 2003 {published data only}
- Moreno‐Otero R. Controlled study of interferon alpha‐2b plus ribavirin in patients with chronic hepatitis C non‐responders to interferon. Journal of Hepatology 2003;38 Suppl 2:158. [Google Scholar]
- Moreno‐Otero R. Prospective, randomized and controlled study of the efficacy and tolerance of interferon alpha plus ribavirin in chronic hepatitis C non responders to prior interferon. Hepatology 2001;34(4):596A. [Google Scholar]
Mura 1999 {published data only}
- Mura D, Deliperi R, Fastame L, Cugia L, Cossu PA, Pisanu G. Interferon therapy of HCV cirrhosis reduces the incidence of HCC, and decompensation, and significantly improves survival: a 5 year comparative trial. Hepatology 1999;29:A1251. [Google Scholar]
Neuman Manuela 2010 {published data only}
- Neuman Manuela G, Katz Gadi G, Patel A, Izabell M, Izabella Malkiewicz M, Esguerra R. HCV‐RNA and inflammasome is modulated by PEGylated interferon (PEG‐IFNalpha‐2b) monotherapy in chronic hepatitis C patients. Clinical Biochemistry. 2010; Vol. Meeting of the Canadian Society of Clinical Chemists, CSCC 2010 Saskatoon, SK Canada.June 13‐16, 2010.
Nevens 2005 {published data only}
- Nevens F, Vlierberghe V, D'heygere F, Delwaide J, Adler M, Henrion J, et al. Peginterferon alfa‐2a (40 kDa) plus ribavirin is as effective in patients relapsing after conventional interferon based therapy as in naive patients: results from the BERNAR‐1 trial. Journal of Hepatology 2005;42 Suppl 2:214. [Google Scholar]
Nishiguchi 1995 {published data only}
- Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, et al. Randomised trial of effects of interferon‐alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet 1995;346(8982):1051‐5. [DOI] [PubMed] [Google Scholar]
- Nishiguchi S, Kuroki T, Nakatani S, Takeda T, Fukuda K, Tamori A, et al. Prevention of hepatocellular carcinoma in patients with chronic active hepatitis C and cirrhosis. Lancet 2001;357:196‐7. [DOI] [PubMed] [Google Scholar]
Nomura 2004 {published data only}
- Nomura H, Sou S, Nagahama T, Hayashi J, Kashiwagi S, Ishibashi H. Efficacy of early retreatment with interferon β for relapse in patients with genotype 1b chronic hepatitis C. Hepatology Research 2004;28:36‐40. [DOI] [PubMed] [Google Scholar]
Pardo 1994 {published data only}
- Pardo M, Cotonat T, Herrero M, Marriott E, Artillo S, Quiroga JA, et al. Retreatment of chronic hepatitis C virus infection (Letter). Lancet 1994;343(8912):1568‐9. [DOI] [PubMed] [Google Scholar]
Payen 1998 {published data only}
- Payen JL, Izopet I, Galindo V, Zarski JP, Seigneurin JM, Dussaix E, et al. A comparison of three interferon alpha‐2b regimens for retreatment (RTT) of patients with chronic hepatitis C with prior complete response followed by relapse: a controlled, randomized trial (AASLD abstract). Hepatology 1996;24(4 Pt 2):273A. [Google Scholar]
- Payen JL, Izopet J, Galindo‐Migeot V, Lauwers‐Cances V, Zarski JP, Seigneurin JM, et al. Better efficacy of a 12‐month interferon alfa‐2b retreatment in patients with chronic hepatitis C relapsing after a 6‐month treatment: a multicenter, controlled, randomized trial. Le Groupe D'etude et De Traitement du Virus De L'hepatite C (Get.Vhc). Hepatology 1998;28(6):1680‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Perasso 1999 {published data only}
- Perasso A, Testino G, Ansaldi F, Venturino V, Icardi GC. Recombinant interferon alfa‐2b/ribavirin combined therapy followed by low dose recombinant interferon alfa‐2b in chronic hepatitis C interferon nonresponders. Hepatology 1999;29(1):297‐8. [DOI] [PubMed] [Google Scholar]
Picciotto 1997 {published data only}
- Picciotto A, Sinelli N, Brizzolara R, Campo N, Lapertosa G, Celle G. Long‐term interferon alfa‐2b retreatment of relapsing patients with chronic hepatitis C. Journal of Hepatology 1997;26(2):447‐8. [DOI] [PubMed] [Google Scholar]
Planas 2002 {published data only}
- Planas R, Quer JC, Enriquez J, Barrera JM, Dalmau B, Casanovas T, et al. Induction therapy with interferon alfa‐2a in compensated hepatitis C virus‐related cirrhosis. Randomized, multicenter study [Terapia de induccion con interferon alfa‐2a en la cirrosis por el virus de la hepatitis C compensada. Estudio multicentrico aleatorizado]. Medicina Clinica 2002;118(17):641‐4. [DOI] [PubMed] [Google Scholar]
Pockros 2007 {published data only}
- Goodman ZD, Becker RL, Pockros PJ, Afdhal NH. Progression of fibrosis in advanced chronic hepatitis C: evaluation by morphometric image analysis. Hepatology 2007;45(4):886‐94. [DOI] [PubMed] [Google Scholar]
Pol 1999 {published data only}
- Pol S, Couzigou P, Bourliere M, Abergel A, Combis JM, Larrey D, et al. A randomized trial of ribavirin and interferon‐alpha vs. interferon‐alpha alone in patients with chronic hepatitis C who were non‐responders to a previous treatment. Multicenter Study Group under the coordination of the Necker Hospital, Paris, France. Journal of Hepatology 1999;31(1):1‐7. [DOI] [PubMed] [Google Scholar]
Portal 2003 {published data only}
- Portal I, Botta‐Fridlund D, Bourliere M, Rotily M, Halfon P, Couzigou P, et al. Treatment with peg‐interferon alfa‐2b in relapsers to standard interferon plus ribavirin in chronic hepatitis C: efficacy and safety results from a randomized multicentric french study. Hepatology 2003;38(4):311A. [Google Scholar]
Poynard 1999 {published data only}
- Poynard T, Daurat V, Chevret S, Moussalli J, Degos F, Bailly F, et al. A short induction regimen of interferon‐alpha is not effective for treatment of relapse in chronic hepatitis C: a randomized trial. For the multicentre GER‐CYT‐01 group. Journal of Viral Hepatitis 1999;6(5):381‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Poynard 2003 {published data only}
- Poynard T, Marcellin P, Bissery A, Myers P, Moussalli J, Degos F, et al. Reinforced interferon alpha‐2b and ribavirin is more effective than standard combination therapy in he retreatment of chronic hepatitis C previously nonresponsive to interferon: a randomized trial. Journal of Viral Hepatitis 2003;10:197‐204. [DOI] [PubMed] [Google Scholar]
Puoti 2001 {published data only}
- Puoti M, Cadeo GP, Forleo M.A, Barni M.C, Cristini G, Rossi S, et al. Pilot dose‐finding trial on interferon alpha in combination with ribavirin for the treatment of chronic hepatitis C in patients not responding to interferon alone. Digestive and Liver Disease 2001;33:163‐72. [DOI] [PubMed] [Google Scholar]
Qu 2012 {published data only}
- Qu L‐S, Chen H, Kuai X‐L, Xu Z‐F, Jin F, Zhou G‐X. Effects of interferon therapy on development of hepatocellular carcinoma in patients with hepatitis C‐related cirrhosis: A meta‐analysis of randomized controlled trials. Hepatology Research 2012;42(8):782‐9. [DOI] [PubMed] [Google Scholar]
Reichard 1994 {published data only}
- Reichard O, Glaumann H, Weiland O. Long‐term histological outcome in patients with chronic hepatitis C treated repeatedly with interferon alpha‐2b without sustained response. Scandinavian Journal of Infectious Diseases 1994;26(4):383‐9. [DOI] [PubMed] [Google Scholar]
Rodriguez‐Torres 2011 {published data only}
- Rodriguez‐Torres M, Hillson JL, Bacon BR, Box TD, Hassanein T, Greenbloom S, et al. Safety and efficacy of pegylated interferon lambda (PEG‐LAMBDA) compared to pegylated interferon alfa‐2A (PEG‐alfa) in HCV‐infected patients (G1/2/3) with compensated cirrhosis: emerge phase IIB efficacy and safety results through week 12. Hepatology 2011;54(4 Suppl):994A‐6A. [Google Scholar]
Rolachon 1997 {published data only}
- Rolachon A, Kezachian G, Causse X, Baud M, Fournet J, Zarski JP. Interest of high dose of interferon in non‐responder CHC patients. Journal of Hepatology 1996;25 Suppl 1:S149. [Google Scholar]
- Rolachon A, Kezachian G, Causse X, Baud M, Leroy V, Maynard‐Muet M, et al. Value of high‐dose interferon‐alpha in chronic viral hepatitis C patients non‐responder to a 1st treatment. Pilot study prospective and randomized trial. Gastroenterologie Clinique et Biologique 1997;21(12):924‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Romero‐Gomez 2012 {published data only}
- Romero‐Gomez M, Planas R, Sola R, Garcia‐Samaniego J, Diago M, Crespo J, et al. Peginterferon alpha‐2A achieves higher early virological responses (RVR and CEVR) than peginterferon alpha‐2B in chronic Hepatitis C: Meta‐analysis of randomized clinical trials (RCT). Journal of Hepatology 2012;56 Suppl 2:S456. [Google Scholar]
Rustgi 2005 {published data only}
- Rustgi V, Nelson D, Balan V, Sulkowski M, Lambiase L, Davis G, et al. Phase 2 dose‐escalation study of albuferon combined with ribavirin in non‐responders to prior interferon‐based therapy for chronic hepatitis C infection. Journal of Hepatology 2005;44 Suppl 2:S50. [Google Scholar]
Salmeron 1999 {published data only}
- Salmeron J, Ruiz‐Extremera A, Torres C, Rodriguez‐Ramos L, Lavin I, Quintero D, et al. Interferon versus ribavirin plus interferon in chronic hepatitis C previously resistant to interferon: a randomized trial. Liver 1999;19(4):275‐80. [DOI] [PubMed] [Google Scholar]
Saracco 1994 {published data only}
- Saracco G, Rosina F, Abate ML, Carucci P, Solinas A, Chiandussi L, et al. Long‐term response to interferon‐alfa 2b re‐treatment in chronic hepatitis C. Journal of Hepatology 1994;21(2):278‐9. [DOI] [PubMed] [Google Scholar]
Saracco 2001 {published data only}
- Saracco G, Ciancio A, Olivero A, Smedile A, Roffi L, Croce G, et al. A randomized 4‐arm multicenter study of interferon alfa‐2b plus ribavirin in the treatment of patients with chronic hepatitis C not responding to interferon alone. Hepatology 2001;34(1):133‐8. [DOI] [PubMed] [Google Scholar]
Saracco 2002 {published data only}
- Saracco G, Olivero A, Ciancio A, Carenzi S, Smedile A, Cariti G, et al. A randomized 4‐arm multicenter study of interferon alfa‐2b plus ribavirin in the treatment of patients with chronic hepatitis C relapsing after interferon monotherapy. Hepatology 2002;36(4):955‐66. [DOI] [PubMed] [Google Scholar]
Sarrazin 2007 {published data only}
- Sarrazin C, Rouzier R, Wagner F, Forestier N, Larrey D, Gupta SK, et al. SCH 503034, a novel hepatitis C virus protease inhibitors, plus pegilated interferon alfa‐2b for genotype 1 nonresponders. Gastroenterology 2007;132:1270‐8. [DOI] [PubMed] [Google Scholar]
Scotto 1996 {published data only}
- Scotto G, Fazio V, Tantimonaco G. Pilot study of a short course of ribavirin and alpha interferon in the treatment of chronic active hepatitis C not responding to alpha‐interferon alone. Italian Journal of Gastroenterology 1996;28(9):505‐11. [PubMed] [Google Scholar]
Scotto 1998 {published data only}
- Scotto G, Grimaldi M. Effectiveness of Leukocyte Interferon‐alfa treatment in patients with chronic hepatitis C not responsive to recombinant Interferon. Digestive Diseases and Sciences 1998;43(10):2173‐6. [DOI] [PubMed] [Google Scholar]
Shiffman 2000 {published data only}
- Shiffman ML, Hofmann CM, Gabbay J, Luketic VA, Sterling RK, Sanyal AJ. Treatment of chronic hepatitis C in patients who failed interferon monotherapy: effects of higher doses of interferon and ribavirin combination therapy. The Virginia Cooperative Hepatitis Treatment Group. American Journal of Gastroenterology 2000;95(10):2928‐35. [DOI] [PubMed] [Google Scholar]
Singal 2010 {published data only}
- Singal AG, Waljee AK, Shiffman M, Bacon BR, Schoenfeld PS. Meta‐analysis: re‐treatment of genotype I hepatitis C nonresponders and relapsers after failing interferon and ribavirin combination therapy. Alimentary Pharmacology and Therapeutics 2010;32:969‐83. [DOI] [PubMed] [Google Scholar]
Soza 2005 {published data only}
- Soza A, Heller T, Ghany M, Lutchman G, Liang TJ, Germain J, et al. Pilot study of interferon gamma for chronic hepatitis C. Journal of Hepatology 2005;43:67‐71. [DOI] [PubMed] [Google Scholar]
Sporea 2006 {published data only}
- Sporea I, Danila M, Sirli R, Popescu A, Laza A, Baditoiu L. Comparative study concerning the efficacy of peg‐IFN alfa‐2a versus peg‐IFN alfa‐2b on the early virological response in patients with chronic viral C hepatitis. Journal of Gastrointestinal and Liver Diseases 2006;15(2):125‐30. [PubMed] [Google Scholar]
Steindl‐Munda 2003 {published data only}
- Steindl‐Munda P, Ferenci P, Brunner H, Nachbaur K, Datz C, Gschwantler M, et al. Impact of high‐dose interferon induction and ribavirin therapy in patients with chronic hepatitis C relapsing after or not responding to interferon monotherapy. Liver International 2003;23:269‐75. [DOI] [PubMed] [Google Scholar]
Suzuki 2001 {published data only}
- Suzuki F, Chayama K, Tsubota A, Akuta N, Someya T, Kobayashi M, et al. Twice‐daily administration of interferon‐beta for chronic hepatitis C is not superior to once‐daily regimen. Journal of Gastroenterology 2001;36(4):242‐7. [DOI] [PubMed] [Google Scholar]
Tassopoulos 2003 {published data only}
- Raptopoulou M, Tsantoulas D, Vafiadi I, Ketikoglou I, Paraskevas E, Vassiliadis T, et al. The effect of adherence to therapy on sustained response in daily or three times a week interferon alpha‐2b plus ribavirin treatment of naïve and nonresponder chronic hepatitis C patients. Journal of Viral Hepatitis 2005;12:91‐5. [DOI] [PubMed] [Google Scholar]
- Tassopoulos NC, Tsantoulas D, Raptopoulou M, Vassiliadis T, Kanatakis S, Paraskevas E, et al. A randomized trial to assess the efficacy of interferon alpha in combination with ribavirin in the treatment of interferon alpha nonresponders with chronic hepatitis C: superior efficacy of high daily dosage of interferon alpha in genotype 1. Journal of Viral Hepatitis 2003;10:189‐96. [DOI] [PubMed] [Google Scholar]
Toyota 2002 {published data only}
- Iino S, Tomita E, Kumada H, Suzuki H, Toyota J, Kendo K, et al. Prediction of treatment outcome with daily high‐dose interferon alfa‐2b plus ribavirin in patients with chronic hepatitis C with genotype 1b and high HCV RNA levels: relationship of baseline viral levels and viral dynamics during and after therapy. Hepatology Research 2004;30:63‐70. [DOI] [PubMed] [Google Scholar]
- Toyoda J, Sainokami S, Yasuda K, Izumi N, Ota H, Sato Y, et al. Comparision of interferon alfa‐2b and ribavirin (SCH18908) combination therapy and interferon alfa‐2b monotherapy in chronic hepatitis C patients who have not responded or relapsed to previous interferon therapy: a double‐blind comparative study to examine concomitant efficacy. Journal of Clinical and Therapeutical Medicine 2002;4:539‐63. [Google Scholar]
Valla 1999 {published data only}
- Valla DC, Chevallier M, Marcellin P, Payen JL, Trepo C, Fonck M, et al. Treatment of hepatitis C virus‐related cirrhosis: a randomized, controlled trial of interferon alfa‐2b versus no treatment. Hepatology 1999;29(6):1870‐5. [DOI] [PubMed] [Google Scholar]
Wartelle 1997 {published data only}
- Wartelle CF, Larson AM, Cotler SJ, Gretch DR. Higher dose interferon does not increase end‐of‐treatment virological response in nonresponders to interferon therapy (AASLD abstract). Hepatology 1997;4(Pt 2):415A. [Google Scholar]
Weiland 1993 {published data only}
- Weiland O, Zhang YY, Widell A. Serum HCV RNA levels in patients with chronic hepatitis C given a second course of interferon alpha‐2b treatment after relapse following initial treatment. Scandinavian Journal of Infectious Diseases 1993;25(1):25‐30. [PubMed] [Google Scholar]
Yao 1997 {published data only}
- Yao GB, Fu XX, Xu DZ, Hao LI, Su CX, Huangpu YS. A randomized, comparative, multicenter study of consensus interferon (CIFN) with interferon alpha‐2a in patients with chronic hepatitis C virus (HCV) infection (Abstract). Hepatology 1997;26(4S):1174. [Google Scholar]
Zeuzem 2000 {published data only}
- Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, Lai MY, Gane E, et al. Peginterferon alfa‐2a in patients with chronic hepatitis C. New England Journal of Medicine 2000;343(23):1666‐72. [DOI] [PubMed] [Google Scholar]
Zeuzem 2005 {published data only}
- Zeuzem S, Pawlotsky J‐M, Lukasiewicz, Wagner M, Goulis I, Lurie Y, et al. International, multicenter, randomized, controlled study comparing dynamically individualized versus standard treatment in patients with chronic hepatitis C. Journal of Hepatology 2005;43:250‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Cho 1992 {published data only}
- Cho HJ, Dong SH, Lee MS, Kim HY, Park CK, Yoo JY, et al. Interferon alpha therapy in patients with chronic type C hepatitis: changes of serum ALT, anti‐HCV, & HCV‐RNA. Korean Journal of Internal Medicine 1992;7(1):13‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Testino 2002 {published data only}
- Testino G. Hepatocarcinoma in HCV compensated correlated liver cirrhosis: role of treatment with interferon. Recenti Progressi in Medicina 2002;93:302‐7. [PubMed] [Google Scholar]
Additional references
Arase 2007
- Arase Y, Ikeda K, Suzuki F, Suzuki Y, Kobayashi M, Akuta N, et al. Interferon‐induced prolonged biochemical response reduces hepatocarcinogenesis in hepatitis C virus infection. Journal of Medical Virology 2007;79(10):1485‐90. [DOI] [PubMed] [Google Scholar]
Barrett 2001
- Barrett S, Goh J, Coughlan B, Ryan E, Stewart S, O'Keane JC, et al. The natural course of hepatitis C virus infection after 22 years in a unique homogenous cohort: spontaneous viral clearance and chronic HCV infection. Gut 2001;49:423‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benvegnu 2004
- Benvegnu L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut 2004;53(5):744‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brok 2005a
- Brok J, Gluud LL, Gluud C. Ribavirin monotherapy for chronic hepatitis C. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005527] [DOI] [PubMed] [Google Scholar]
Brok 2005b
- Brok J, Gluud LL, Gluud C. Ribavirin plus interferon versus interferon for chronic hepatitis C. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005445] [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Camma 1999
- Camma C, Giunta M, Chemello L, Alberti A, Toyoda H, Trepo C, et al. Chronic hepatitis C: interferon retreatment of relapsers. A meta‐analysis of individual patient data. European Concerted Action on Viral Hepatitis (EUROHEP). Hepatology 1999;30(3):801‐7. [DOI] [PubMed] [Google Scholar]
Camma 2001
- Camma C, Giunta M, Andreone P, Craxi A. Interferon and prevention of hepatocellular carcinoma in viral cirrhosis: an evidence‐based approach. Journal of Hepatology 2001;34(4):593‐602. [DOI] [PubMed] [Google Scholar]
Casaraghi 2004
- Casiraghi MA, Paschale M, Romano L, Biffi R, Assi A, Binelli G, et al. Long‐term outcome (35 years) of hepatitis C after acquisition of infection through mini transfusions of blood given at birth. Hepatology 2004;39:90‐6. [DOI] [PubMed] [Google Scholar]
Chavilitdhamrong 2006
- Chavalitdhamrong D, Tanwandee T. Long‐term outcomes of chronic hepatitis C patients with sustained virological response at 6 months after the end of treatment. World Journal of Gastroenterology 2006;12:5532‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2001
- Cheng SJ, Bonis PA, Lau J, Pham NQ, Wong JB. Interferon and ribavirin for patients with chronic hepatitis C who did not respond to previous interferon therapy: a meta‐analysis of controlled and uncontrolled trials. Hepatology 2001;33(1):231‐40. [DOI] [PubMed] [Google Scholar]
Ciancio 2006
- Ciancio A, Smedile A, Giordanino C, Colletta C, Croce G, Pozzi M, et al. Long‐term follow‐up of previous hepatitis C virus positive nonresponders to interferon monotherapy successfully retreated with combination therapy: are they really cured?. American Journal of Gastroenterology 2006;101:1811‐6. [DOI] [PubMed] [Google Scholar]
CTU 2011
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. http://ctu.dk/tsa/ (accessed 29 September 2012).
Davis 1989
- Davis GL, Balart LA, Schiff ER, Lindsay K, Bodenheimer HC Jr, Perrillo RP, et al. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. New England Journal of Medicine 1989;321(22):1501‐6. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods of combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6:341‐8. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Everson 2008
- Everson GT, Balart L, Lee SS, Reindollar RW, Shiffman ML, Minuk GY, et al. Histological benefits of virological response to peginterferon alfa‐2a monotherapy in patients with hepatitis C and advanced fibrosis or compensated cirrhosis. Alimentary Pharmacology & Therapeutics 2008;27:542‐51. [DOI] [PubMed] [Google Scholar]
Fattovich 1997
- Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow‐up study of 384 patients. Gastroenterology 1997;112(2):463‐72. [DOI] [PubMed] [Google Scholar]
Fried 2002
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus infection. New England Journal of Medicine 2002;347(13):975‐82. [DOI] [PubMed] [Google Scholar]
Gluud 2007
- Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. Journal of Hepatology 2007;46:734‐42. [DOI] [PubMed] [Google Scholar]
Gluud 2012
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2012, Issue 5. Art. No.: LIVER.
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoofnagle 2002
- Hoofnagle JH. Course and outcome of Hepatitic C. Hepatology 2002;36(5 Suppl1):S21‐S29. [DOI] [PubMed] [Google Scholar]
Hung 2006
- Hung CH, Lee CM, Lu SN, Wang JH, Hu TH, Tung HD, et al. Long‐term effect of interferon alpha‐2b plus ribavirin therapy on incidence of hepatocellular carcinoma inpatients with hepatitis C virus‐related cirrhosis. Journal of Viral Hepatitis 2006;13:409‐14. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [MEDLINE: ] [Google Scholar]
Innes 2011
- Innes HA, Hutchinson SJ, Allen S, Bhattacharyya D, Bramley P, Delahooke TES, et al. Excess liver‐related morbidity of chronic hepatitis C patients, who achieve a sustained viral response, and are discharged from care. Hepatology 2011;54:1547‐58. [DOI] [PubMed] [Google Scholar]
Jacobson 2011
- Jacobson IM, McHutchison JG, Dusheiko G, Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. New England Journal of Medicine 2011;364(25):2405‐16. [DOI] [PubMed] [Google Scholar]
Kenny‐Walsh 1999
- Kenny‐Walsh E, for the Irish Hepatology Research Group. Clinical outcomes after hepatitis C infection from contaminated anti‐D immune globulin. New England Journal of Medicine 1999;340:1228‐33. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between small and large randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Koretz 1995
- Koretz RL. Chronic hepatitis: more quotes and misquotes. In: Gitnick G editor(s). Current Hepatology. Vol. 15, St. Louis: Mosby‐Year Book, Inc., 1995:49‐84. [Google Scholar]
Koretz 1996
- Koretz RL. Interferon and hepatocellular carcinoma. Lancet 1996;347:194. [DOI] [PubMed] [Google Scholar]
Lauer 2001
- Lauer GM, Walker BD. Hepatitis C virus infection. New England Journal of Medicine 2001;345(1):41‐52. [DOI] [PubMed] [Google Scholar]
Lindsay 2001
- Lindsay KL, Trepo C, Heintges T, Shiffman ML, Gordon SC, Hoefs JC, et al. A randomized, double‐blind trial comparing pegylated interferon alfa‐2b to interferon alfa‐2b as initial treatment for chronic hepatitis C. Hepatology 2001;34(2):395‐403. [DOI] [PubMed] [Google Scholar]
Maddrey 1999
- Maddrey WC. Safety of combination interferon alfa‐2b/ribavirin therapy in chronic hepatitis C‐relapsed and treatment‐naive patients. Seminars in Liver Disease 1999;19 Suppl 1:67‐75. [PubMed] [Google Scholar]
Manns 2001
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa‐2b plus ribavirin compared with interferon alfa‐2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358(9286):958‐65. [DOI] [PubMed] [Google Scholar]
Marcellin 1997
- Marcellin P, Boyer N, Gervais A, Martinot M, Pouteau M, Castelnau C, et al. Long‐term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferon‐alpha therapy. Annals of Internal Medicine 1997;127(10):875‐81. [DOI] [PubMed] [Google Scholar]
Maylin 2008
- Maylin S, Martinot‐Peignoux M, Moucari R, Boyer N, Ripault MP, Cazals‐Hatem D, et al. Eradication of hepatitis C virus in patients successfully treated for chronic hepatitis C. Gastroenterology 2008;135(3):821‐9. [DOI] [PubMed] [Google Scholar]
McHutchison 1998
- McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, et al. Interferon alfa‐2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. New England Journal of Medicine 1998;339(21):1485‐92. [DOI] [PubMed] [Google Scholar]
McHutchison 2002
- McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, et al. Adherence to combination therapy enhances sustained response in genotype‐1‐infected patients with chronic hepatitis C. Gastroenterology 2002;123(4):1061‐9. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
NIH 1997
- Anonymous. Management of Hepatitis C. NIH Consensus Statement 1997;15(3):1‐41. [PubMed] [Google Scholar]
Papatheodoridis 2001
- Papatheodoridis GV, Papadimitropoulos VC, Hadziyannis SJ. Effect of interferon therapy on the development of hepatocellular carcinoma in patients with hepatitis C virus‐related cirrhosis: a meta‐analysis. Alimentary Pharmacology & Therapeutics 2001;15(5):689‐98. [DOI] [PubMed] [Google Scholar]
Perz 2006
- Perz JF, Armstrong GL, Farrington LA, Hutin YL, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. Journal of Hepatology 2006;45(4):529‐38. [DOI] [PubMed] [Google Scholar]
Poordad 2011
- Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. New England Journal of Medicine 2011;364(13):1195‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poynard 1998
- Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Gaetano I, et al. Randomised trial of interferon alfa‐2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alfa‐2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet 1998;352(9138):1426‐32. [DOI] [PubMed] [Google Scholar]
Poynard 2003a
- Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet 2003;362(9401):2095‐100. [DOI] [PubMed] [Google Scholar]
Poynard 2009
- Poynard T, Munteanu M, Colombo M, Bruix J, Schiff E, Terg R, et al. Impact of PegIntron maintenance therapy on biomarkers (Fibro Test[FT]‐ActiTest[AT]) in cirrhotic (METAVIOR F4) HCV prior nonresponders: results from the EPIC3 cirrhosis maintenance trial. Hepatology 2009;50(4 Suppl):711A. [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Seeff 1999
- Seeff LB, Miller RN, Rabin CS, Buskell‐Bales Z, Straley‐Eason KD, Smoak BL, et al. 45‐year follow‐up of hepatitis C virus infection in healthy young adults. . Annals of Internal Medicine 1999;132:105‐11. [DOI] [PubMed] [Google Scholar]
Seeff 2001
- Seeff LB, Hollinger FB, Alter HJ, Wright EC, Cain CM, Buskell ZJ, et al. Long‐term mortality and morbidity of transfusion‐associated non‐A, non‐B and type C hepatitis: a National Heart, Lung, and Blood Institute Collaborative Study. Hepatology 2001;33:455‐63. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual forTrial Sequential Analysis (TSA). http://ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 15 June 2012).
Vogt 1999
- Vogt M, Lang T, Frosner G, Klingler C, Sendl AF, Zeller A, et al. Prevalence and clinical outcome of hepatitis C infection in children who underwent cardiac surgery before the implementation of blood‐donor screening. New England Journal of Medicine 1999;341:866‐70. [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61:64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random‐effects meta‐analysis. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiese 2000
- Wiese M, Berr F, Lafrenz M, Porst H, Oesen U. Low frequency of cirrhosis in a hepatitis C (genotype 1b) single‐source outbreak in Germany: a 20‐year multicenter study. Hepatology 2000;32:91‐6. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [PUBMED: 18316340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2002
- Wright TL. Treatment of patients with hepatitis C and cirrhosis. Hepatology 2002;36(5 Suppl 1):S185‐94. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Myers 2002
- Myers RP, Poynard T. Interferon for interferon nonresponding and relapsing patients with chronic hepatitis C. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD003617] [DOI] [PubMed] [Google Scholar]


