Abstract
Background
Tinnitus is the perception of sound or noise in the absence of an external or internal acoustic stimulation. It is a common and potentially distressing symptom for which no adequate therapy exists.
Objectives
To assess the effectiveness of anticonvulsants in patients with chronic tinnitus.
Search methods
We searched the Cochrane Ear, Nose and Throat Disorders Group Specialised Register, CENTRAL (2010, Issue 2), MEDLINE, EMBASE, bibliographies and additional sources for published and unpublished trials. The date of the most recent search was 26 May 2010.
Selection criteria
We selected randomised controlled trials in patients with chronic tinnitus comparing orally administered anticonvulsants with placebo. The primary outcome was improvement in tinnitus measured with validated questionnaires. Secondary outcomes were improvement in tinnitus measured with self‐assessment scores, improvement in global well‐being or accompanying symptoms, and adverse drug effects.
Data collection and analysis
Three authors assessed risk of bias and extracted data independently.
Main results
Seven trials (453 patients) were included in this review. These studies investigated four different anticonvulsants: gabapentin, carbamazepine, lamotrigine and flunarizine. The risk of bias of most studies was 'high' or 'unclear'. Three studies included a validated questionnaire (primary outcome). None of them showed a significant positive effect of anticonvulsants. One study showed a significant negative effect of gabapentin compared to placebo with an increase in Tinnitus Questionnaire (TQ) score of 18.4 points (standardised mean difference (SMD) 0.82, 95% confidence interval (CI) 0.07 to 1.58). A second study showed a positive, non‐significant effect of gabapentin with a difference compared to placebo of 2.4 points on the Tinnitus Handicap Inventory (THI) (SMD ‐0.11, 95% CI ‐0.48 to 0.25). When the data from these two studies are pooled no effect of gabapentin is found (SMD 0.07, 95% CI ‐0.26 to 0.40). A third study reported no differences on the THI after treatment with gabapentin compared to placebo (exact numbers could not be extracted from the article).
A meta‐analysis of 'any positive effect' (yes versus no) based on a self‐assessment score (secondary outcome) showed a small favourable effect of anticonvulsants (RD 14%, 95% CI 6% to 22%). A meta‐analysis of 'near or total eradication of tinnitus annoyance' showed no effect of anticonvulsants (risk difference (RD) 4%, 95% CI ‐2% to 11%). Side effects of the anticonvulsants used were experienced by 18% of patients.
Authors' conclusions
Current evidence regarding the effectiveness of anticonvulsants in patients with tinnitus has significant risk of bias. There is no evidence from studies performed so far to show that anticonvulsants have a large positive effect in the treatment of tinnitus but a small effect (of doubtful clinical significance) has been demonstrated.
Keywords: Humans; Administration, Oral; Amines; Amines/therapeutic use; Anticonvulsants; Anticonvulsants/therapeutic use; Carbamazepine; Carbamazepine/therapeutic use; Chronic Disease; Cyclohexanecarboxylic Acids; Cyclohexanecarboxylic Acids/therapeutic use; Flunarizine; Flunarizine/therapeutic use; Gabapentin; Lamotrigine; Randomized Controlled Trials as Topic; Tinnitus; Tinnitus/drug therapy; Triazines; Triazines/therapeutic use; gamma‐Aminobutyric Acid; gamma‐Aminobutyric Acid/therapeutic use
Anticonvulsants for tinnitus
Tinnitus is the perception of sound or noise in the absence of external acoustic stimulation. It is a common and potentially distressing symptom for which no adequate therapy exists. The pathophysiology of tinnitus has been compared to phantom limb pain therefore anticonvulsant drugs have been proposed as a possible therapy.
This review includes seven studies (six low‐quality and one high‐quality) of four different anticonvulsants (gabapentin, carbamazepine, flunarizine and lamotrigine). We found that anticonvulsants do not have a beneficial effect in the treatment of tinnitus. Side effects of the anticonvulsants used were experienced by 18% of patients.
Background
Background
This is one of a number of tinnitus reviews produced by the Cochrane Ear, Nose & Throat Disorders Group. The following paragraphs ('Description of the condition') are partially based on earlier work in the following reviews and reproduced with permission: Baldo 2006; Bennett 2007; Hilton 2004; Hobson 2010; Phillips 2010.
Description of the condition
Tinnitus is a phantom auditory perception of meaningless sound in the absence of an external or internal acoustic stimulation. While for the patient this perception of noise is very real, it can be considered a phantom, or false, perception because there is no corresponding external sound. For the patient it may be trivial or it may be a debilitating condition (Luxon 1993). The characteristics of the perceived sound (description, number, frequency, onset, presence and location of the sound) can vary enormously between patients. For example, patients may hear a single sound or multiple sounds, it may be perceived in one ear, both ears, within the head or outside the body and the symptom may be continuous or intermittent.
It is important to distinguish between clinically significant and non‐significant tinnitus (Davis 2000) and several different classifications have been proposed (Dauman 1992; McCombe 2001; Stephens 1991). Severe tinnitus, defined as tinnitus interfering with the normal way of life, is reported in up to 5% of tinnitus patients (Coles 1984; Davis 2000; Nondahl 2002). It is usually associated with other symptoms, such as hyperacusis and many of these patients also suffer from affective disorders and sleeping problems (Crummer 2004; Henry 2005; Jastreboff 2003; Moller 2003).
Differentiation between tinnitus and somatosounds (perceptions of sound caused by an internal acoustic source, due to either a vascular abnormality or a muscular or anatomical cause such as sound generated by blood flow in or around the ear or unusual activity of middle ear muscles within the middle ear) is important because they have different pathophysiologies and therefore different therapeutic approaches. Somatosounds are usually objective; they can be detected by an examiner, either unaided or using a listening aid such as a stethoscope or microphone in the ear canal. Somatasounds are much less common than tinnitus. Tinnitus is by definition always subjective, meaning that it cannot be heard by anyone other than the patient, while for the patient this perception of noise is real.
Aetiology
The most common causes of tinnitus are otological disorders, most frequently noise and age‐induced sensorineural hearing loss, or other types of sensorineural hearing loss. Conductive hearing loss can also cause tinnitus, sometimes transient. Almost any form of disorder involving the outer, middle or inner ear or the auditory nerve may be associated with tinnitus (Brummet 1980; Shea 1981). However, it is possible to have severe tinnitus with no evidence of any aural pathology. Presumably in these cases there is a moderate degree of aural pathology, but not evident enough to be able to diagnose with current diagnostic methods (audiometry only screens a portion of the auditory function). Non‐otological causes of tinnitus have also been described, but the causal relationship is less understood. Conversely, tinnitus can even exist without a peripheral auditory system: when the cochlear nerve is severed patients retain their tinnitus (Baguley 1992). This suggests the fundamental importance of the central auditory pathways in the development or maintenance of the symptom, irrespective of trigger.
Pathophysiology
Over 50 years ago, Heller and Bergman demonstrated that if 'normal' people (with no known cochlear disease) were placed in a quiet enough environment, the vast majority of them would experience sounds inside their head. They concluded that tinnitus‐like activity is a natural phenomenon perceived by many in a quiet enough environment (Heller 1953).
Despite the high prevalence and morbidity of tinnitus, its pathophysiology is poorly understood. It is probable that different processes are involved in the generation of tinnitus; for example, when it is transient or chronic or when it is caused by conductive or sensorineural hearing loss. Possible theories on the pathophysiology focus on dysfunction of hair cells, the auditory nerve or central auditory system. In the 'neurophysiological model' of tinnitus (Jastreboff 1990; Jastreboff 2004) it is proposed that tinnitus results from the abnormal processing of a signal generated in the auditory system. This abnormal processing occurs before the signal is perceived centrally. This may result in 'feedback', whereby the annoyance created by the tinnitus causes the individual to focus increasingly on the noise, which in turn exacerbates the annoyance and so a 'vicious cycle' develops. In this model tinnitus could therefore result from continuous firing of cochlear fibres to the brain, from hyperactivity of cochlear hair cells or from permanent damage to these cells being translated neuronally into a 'phantom' sound‐like signal that the brain 'believes' it is hearing.
It is commonly thought that chronic tinnitus (caused by sensorineural hearing loss) is generated in the brain as a result of functional reorganisation of the primary auditory cortex, following damage to the peripheral auditory system (Eggermont 2004). This functional reorganisation would cause the tonotopic maps in the central auditory cortex to alter. This altering of maps has indeed been shown in humans with tinnitus (Muhlnickel 1998). On the neuronal level, it is thought that this functional reorganisation causes an increased spontaneous firing rate of neurons in the auditory cortex and auditory brainstem, and an increased synchronisation of spontaneous activity of cortical neurons (Eggermont 2004; Norena 2003; Ochi 1997). This increase in firing rate and synchronisation would lead to hyperactivity in the central auditory system. This resulting hyperactivity has been shown in functional magnetic resonance imaging (fMRI) research in tinnitus patients (Giraud 1999; Lockwood 1998; Melcher 2000). The mechanism underlying this increase in spontaneous firing rate and synchronisation is thought to be reduced inhibition, which is the consequence of the decreased output from damaged cochlear regions (Eggermont 2004; Salvi 2000).
The relationship between the symptom of tinnitus and the activity of the prefrontal cortex and limbic system has been emphasised. The limbic system mediates emotions. It can be of great importance in understanding why the sensation of tinnitus is in many cases so distressing for the patient. It also suggests why, when symptoms are severe, tinnitus can be associated with major depression, anxiety and other psychosomatic and/or psychological disturbances, leading to a progressive deterioration of quality of life (Lockwood 1999; Sullivan 1989; Sullivan 1992; Sullivan 1993).
Prevalence
Epidemiological data reports are few. Reports show that tinnitus is common, affecting approximately 7% to 19% of the adult population (Chung 1984; Coles 1984; Davis 1989; Davis 2000; Henry 2005; Nondahl 2002). This substantial variance might be explained by the different definitions and criteria of tinnitus that were used and the different populations that were investigated. The largest single study was undertaken in the UK by the Medical Research Council Institute of Hearing Research and was published in 2000 (Davis 2000). This longitudinal study of hearing questioned 48,313 people; 10.1% described tinnitus arising spontaneously and lasting for five or more minutes at a time and 5% described it as moderately or severely annoying. However, only 0.5% reported tinnitus having a severe effect on their life. This is another of the paradoxes of tinnitus: the symptom is very common but the majority of people who experience it are not particularly concerned by it. These figures from the UK are broadly consistent with data collected by the American Tinnitus Association (ATA) which suggests that tinnitus may be experienced by around 50 million Americans, or 17% of the US population (ATA 2004). Data also exist for Japan, Europe and Australia (Sindhusake 2003), and estimates suggest that tinnitus affects a similar percentage of these populations, with 1% to 2% experiencing debilitating tinnitus (Seidman 1998). Tinnitus can occur at any age, but the prevalence increases with advancing age (peak prevalence between 40 and 70 years) (Baguley 1999; Crummer 2004; Davis 2000; Hegarthy 2000; Henry 2005; Schleuning 1991). The Oregon Tinnitus Data Archive (Oregon 1995) contains data on the characteristics of tinnitus drawn from a sample of 1630 tinnitus patients. The age groups with the greater prevalence are those between 40 and 49 years (23.9%) and between 50 and 59 years (25.6%).
Diagnosis
Firstly a patient with tinnitus may undergo a basic clinical assessment. This will include the relevant otological, general and family history, and an examination focusing on the ears, teeth and neck and scalp musculature. Referral to a specialist is likely to involve a variety of other investigations including audiological tests and radiology. Persistent, unilateral tinnitus may be due to a specific disorder of the auditory pathway and imaging of the cerebellopontine angle is important to exclude, for example, a vestibular schwannoma (acoustic neuroma) ‐ a rare benign tumour of the cochleo‐vestibular nerve. Other lesions, such as glomus tumours, meningiomas, adenomas, vascular lesions or neuro‐vascular conflicts may also be detected by imaging (Marx 1999; Weissman 2000).
Treatment
At present no specific therapy for tinnitus is acknowledged to be satisfactory in all patients. Many patients who complain of tinnitus, and also have a significant hearing impairment, may benefit from a hearing aid (Del Bo 2007). Not only will this help their hearing disability but the severity of their tinnitus may be reduced by masking it through the amplification of ambient sounds. Tinnitus masking can also be achieved with devices which produce a sound that can reduce or eliminate the perception of tinnitus (Hobson 2010).
The role of pharmacotherapy in the treatment of tinnitus is still inconclusive. A wide range of drugs have been proposed for the treatment of tinnitus symptoms since it was shown that intravenous lignocaine may be effective in suppressing tinnitus in some patients (Melding 1978). Pharmacological interventions used include cortisone (Koester 2004), vasodilators, benzodiazepines, lidocaine and spasmolytic drugs. Antidepressants are commonly prescribed for tinnitus. However, two reviews (Baldo 2006; Robinson 2007) have shown that there is no indication that tricyclic antidepressants have a beneficial effect. A Cochrane Review showed that there is no evidence that Ginkgo biloba is effective (Hilton 2004).
Psychological therapies (counselling, cognitive behavioral therapy (CBT) and tinnitus retraining therapy (TRT)) may diminish tinnitus by lessening the distress caused by it or by improving quality of life by teaching coping strategies, relaxation techniques and distraction skills (Andersson 1999; Martinez 2010; Phillips 2010). A Cochrane Review has shown that CBT can have an effect on the qualitative aspects of tinnitus and can improve patients' ability to manage the condition (Martinez 2010).
Other options for the management of patients with tinnitus which have been evaluated, include music therapy (Argstatter 2008), traditional Chinese medicine, including acupuncture (Li 2009) and hyperbaric oxygen therapy (HBOT). Hyperbaric oxygen therapy (HBOT) can improve oxygen supply to the inner ear which, it is suggested, may result in an improvement in tinnitus, however a Cochrane Review found insufficient evidence to support this (Bennett 2007).
Different treatment modalities, working on the assumption that tinnitus is related to central auditory hyperactivity, are being evaluated, including transcranial magnetic stimulation (Meng 2009) and extradural electrical stimulation of the auditory cortex (De Ridder 2007).
Anticonvulsants
Anticonvulsants form an important group of drugs used in the treatment of tinnitus, again because of the assumption that tinnitus is related to central auditory hyperactivity. Several different reviews and non‐randomised controlled trials have been published which mention anticonvulsants (Dobie 1999; Goodey 1981; Melding 1979; Shea 1978; Waddell 2005). Anticonvulsants might diminish this hyperactivity and treat tinnitus in three ways:
they may enhance inhibition in the central auditory system by augmenting the action of gamma‐aminobutyric acid (GABA), an inhibitory neurotransmitter;
they may lower the excitation level in the central auditory system by lessening glutamate transmission, an excitatory neurotransmitter;
they may halt the depolarisation of cells, and thus central activation, by blocking voltage‐dependent sodium channels.
The effectiveness of anticonvulsants in tinnitus patients is, however, not yet clear. A comprehensive systematic review and meta‐analysis of randomised controlled trials evaluating the effectiveness of anticonvulsants in patients with chronic tinnitus is therefore warranted.
Objectives
To assess the effectiveness of anticonvulsants in patients with chronic tinnitus.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised controlled trials and cross‐over trials (if data could be extracted before the cross‐over) in which anticonvulsants were compared with placebo, for inclusion in this review. Desirable time points of outcome assessment were four and eight weeks. We excluded single‐dose studies.
Types of participants
Patients with chronic tinnitus.
We excluded studies on patients with somatosounds (carotid pathology, arteriovenous malformations, high cardiac output, hypertension, aortic murmurs, vascular tumours, atherosclerosis of the subclavian artery, persistent stapedial artery, turbulent stream in the jugular vein, pseudotumour cerebri or myoclonus of the muscles in the palate or within the ear) and patients with auditory hallucinations. Somatosounds were differentiated on the basis of brain imaging or on characteristic features in the patient's history.
Types of interventions
We included studies using orally administered anticonvulsants (without restrictions regarding type of anticonvulsant, dose or frequency) versus placebo.
Types of outcome measures
Primary outcome
Improvement in tinnitus‐specific health‐related quality of life measured with validated questionnaires, such as the Tinnitus Questionnaire (TQ), Tinnitus Handicap Inventory (THI), Tinnitus Handicap Questionnaire (THQ) or Tinnitus Experience Questionnaire (TEQ).
Secondary outcomes
Improvement in self‐assessment of tinnitus severity measured with self‐assessment scores.
Improvement in accompanying symptoms, such as depression, anxiety or sleeping problems measured with validated questionnaires such as the Profile of Mood States, Beck Depression Scale, State‐Trait Anxiety Inventory or Brief Symptom Inventory.
Adverse drug effects.
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials on the effectiveness of anticonvulsants in patients with tinnitus. There were no language, publication year or publication status restrictions. The date of the last search was 26 May 2010.
Electronic searches
We searched the following databases from their inception: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 2); PubMed (1950 onwards); EMBASE (1974 onwards); CINAHL (1982 onwards); PsycINFO; LILACS; KoreaMed; IndMed; PakMediNet; CNKI; MEMR (Index Medicus for WHO Eastern Mediterranean Region); IMSEAR (Index Medicus for WHO South‐East Asia Region); Hellis Metasearch; J‐East (Science Links Japan); UKCRN (the UK Clinical Research Network Portfolio Database); ICTRP (the World Health Organization International Clinical Trials Registry Platform); ClinicalStudyResults.org; mRCT (the metaRegister of Controlled Trials) and Google.
Search strategies
Subject strategies for databases were modelled on the search strategy designed for CENTRAL (see Appendix 1). Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1, Box 6.4.b. (Handbook 2008)).
Searching other resources
We checked the reference lists of identified publications for additional trials. We searched PubMed, TRIPdatabase, NLH ENT & Audiology Specialist Library and Google to retrieve existing systematic reviews possibly relevant to this systematic review, so that we could scan their reference lists for additional trials. We sought abstracts from conference proceedings via the Cochrane Ear, Nose and Throat Disorders Group Trials Register and CENTRAL.
Data collection and analysis
We conducted the review according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 (Handbook 2008).
Selection of studies
Two review authors (CELH and SPR) scanned the retrieved abstracts to identify relevant randomised controlled trials. The same two authors reviewed the full texts of these articles. We assessed the eligibility of the trials independently. We resolved any differences in opinion by discussion.
Data extraction and management
Three authors (CELH, SPR and MMR) independently collected and extracted data. Disagreement was resolved by discussion. We extracted the following data from each study: number of included patients, inclusion and exclusion criteria, intervention and placebo information, trial duration, primary and secondary outcomes, follow up and adverse events. We contacted the original authors for clarification and further data if trial reports were unclear. Where necessary we arranged translations of papers.
Assessment of risk of bias in included studies
We assessed the quality of the included studies using the Cochrane Collaboration's tool for assessing risk of bias ('Risk of bias' table, Cochrane Handbook for Systematic Reviews of Interventions, chapter 8 (Handbook 2008, version 5.0.1). We addressed six specific domains, i.e. sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and 'other biases'. By answering pre‐specified questions we judged the risk of bias for each domain as 'yes' (low risk of bias), 'no' (high risk of bias) or 'unclear'. We resolved disagreement by discussion (CELH, SPR and MMR). We planned to assess publication bias with a scatter plot (funnel plot) of the log rate ratios (x‐axis) versus precision defined as 1/standard error (y‐axis) (Handbook 2008).
Assessment of heterogeneity
If heterogeneity was low (I2 < 25%) we calculated the summary weighted risk differences and 95% confidence intervals (CIs) (random‐effects model) by the Mantel‐Haenszel method, which weighs studies by the number of events in the control group, using the Cochrane statistical package in Review Manager (RevMan) (version 5.1) (RevMan 2011).
Data synthesis
We used RevMan 5.1 to carry out the meta‐analyses for comparable trials and outcomes. For continuous outcomes (questionnaire scores) we calculated standardised mean differences (SMD) and their corresponding 95% confidence intervals (CI). We calculated standardised mean differences by dividing the difference between means by the standard deviation. For dichotomous outcomes, we measured the estimates of effect as risk differences (RD) with their corresponding 95% confidence intervals. We calculated risk differences using: (proportion of patients with improvement in intervention group) ‐ (proportion of patients with improvement in placebo group). Furthermore, we also planned to perform sensitivity analyses excluding the studies with the lowest methodological quality, according to the Cochrane Collaboration’s risk of bias assessment, to establish whether this factor influences the final outcome. We also intended to perform subgroup analyses for cause of tinnitus, duration of tinnitus, patient age, type of anticonvulsant used and outcome measures used. Ultimately it was not possible to perform sensitivity and subgroup analysis, mainly because of lack of data concerning these factors in the original articles.
Results
Description of studies
Results of the search
We found 96 studies through the combined searches. First, we sifted the articles by title/abstract, leaving 15 articles to read in full text. We excluded eight publications from the review: five articles (Bauer 2006; Guth 1990; Marks 1981; Menkes 1998; Shulman 2008) did not fit the criteria for this review (no anticonvulsant, non‐RCT, comments) and three articles (Castagno 1989; Halmos 1982; Viada 1981) were not available through the databases that we used or through the internet. We identified no additional trials by checking the bibliographies of the selected trials.
Included studies
We included seven trials (453 patients) that looked at the effectiveness of anticonvulsants in patients with tinnitus in this review (Bakhshaee 2007; Donaldson 1981; Hulshof 1985; Hulshof 1986; Piccirillo 2007; Simpson 1999; Witsell 2006).
Design
Five trials had a randomised controlled trial design (Bakhshaee 2007; Hulshof 1985; Hulshof 1986; Piccirillo 2007; Witsell 2006). The other two studies were cross‐over trials (Donaldson 1981; Simpson 1999).
Sample size
The average sample size was 62 patients (range 9 to 135 patients).
Settings
Some studies did not describe their settings (Bakhshaee 2007; Hulshof 1985; Piccirillo 2007). The other studies described their settings only broadly. Two studies were performed in a tinnitus clinic population (Donaldson 1981; Simpson 1999). One study included patients from a regular otorhinolaryngology practice and also through public advertisement (Witsell 2006). Another study mentioned only that outpatients were included (Hulshof 1986).
Participants
Participant characteristics and inclusion and exclusion criteria were not reported in great detail in most studies. Four studies mentioned the age groups that were included, ranging from 18 to 81 years (Bakhshaee 2007; Piccirillo 2007; Simpson 1999; Witsell 2006). Four studies gave information on the duration of the tinnitus. Three studies included patients with tinnitus present at the same level at enrolment for more than the preceding six months (Bakhshaee 2007; Piccirillo 2007; Simpson 1999) and one study included patients with tinnitus for more than three months (Witsell 2006). One study described the character of the tinnitus, which needed to be continuous and non‐pulsatile (Bakhshaee 2007). Five studies applied restrictions on the degree of tinnitus. Three studies used a broad description as "annoying tinnitus" (Hulshof 1985; Hulshof 1986) or "sufficient severity to disrupt daily activities" (Piccirillo 2007). Two studies used a minimal score on a questionnaire or visual analogue scale; > 38 on the THI (Piccirillo 2007), > 30 on the TQ (Bakhshaee 2007) or > 5 on a visual analogue scale (VAS) of 0 to 10 (Simpson 1999).
The exclusion criteria most often mentioned were related to the anticonvulsants used (Bakhshaee 2007; Piccirillo 2007; Simpson 1999; Witsell 2006). Two studies restricted patients on their hearing level. One included only patients with a sensorineural hearing loss but patients with deafness were excluded (a definition for deafness was not given) (Donaldson 1981). The other study did not include patients with Ménière's disease, conductive hearing loss, sensorineural hearing loss of "well‐known aetiology" , or more than moderately severe loss (> 50 (no units given)) in at least one frequency (Bakhshaee 2007). The exclusion criterion "sensorineural hearing loss of well‐known etiology" includes noise‐induced hearing loss, but is not explained any further. Three studies excluded participants with cognitive disorders or impairment (Bakhshaee 2007; Piccirillo 2007; Witsell 2006).
Baseline patient characteristics were often not reported, precluding any judgements about the comparability of the patient groups both within and between trials.
Interventions
These studies investigated four different anticonvulsants. Gabapentin was the drug of investigation in three trials in different dosages and duration (four weeks 900 mg per day; four weeks 1800 mg per day; eight weeks 3600 mg per day) (Bakhshaee 2007; Piccirillo 2007; Witsell 2006). Two studies looked at carbamazepine in different dosages and durations (eight weeks 200 mg; four weeks 450 mg) (Donaldson 1981; Hulshof 1985). Lamotrigine and flunarizine were both studied in one trial (Hulshof 1986; Simpson 1999). All studies were placebo‐controlled.
None of the studies included another form of treatment during the study period (such as counselling, for example). All studies had one or more evaluation time points. None of the studies described in detail how or by whom the evaluation was performed and if there was any form of interaction between the clinician and the patients during the study.
Outcomes
Three studies used a validated questionnaire as outcome measurement (our primary outcome measure); the Tinnitus Handicap Inventory (THI) was used in two trials (Piccirillo 2007; Witsell 2006) and the Tinnitus Questionnaire (TQ) in one (Bakhshaee 2007). Four studies used different Likert scales as outcome measurement (our secondary outcome measure) (Donaldson 1981; Hulshof 1985; Hulshof 1986; Simpson 1999).
See: Characteristics of included studies; Characteristics of excluded studies.
Risk of bias in included studies
Figure 1 and Figure 2 show the results of the risk of bias assessment according to the Cochrane Collaboration's tool for assessing risk of bias. Figure 1 shows the judgements about each methodological quality item presented as percentages across included studies, whereas Figure 2 shows the judgement for each included study separately. Detailed information about the assessment can be found in the Characteristics of included studies.
Figure 1.
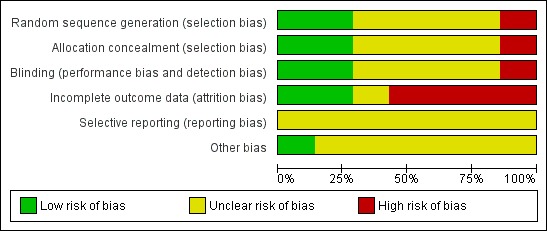
'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Figure 2.
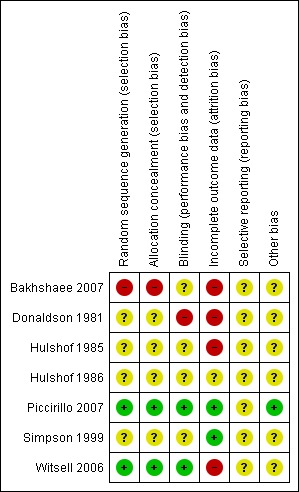
'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study.
Bakhshaee 2007 (gabapentin): this appears to be a cross‐over trial for which the randomisation procedure and treatment protocol are not described clearly. It therefore scored low for sequence generation, allocation concealment and blinding. The drop‐out rate was high (59%) and these incomplete outcome data are not addressed. Tinnitus patients with a sensorineural hearing loss of well‐known aetiology were excluded. This exclusion criterion is not described sufficiently, but it is stated to include noise‐induced hearing loss. As this is one of the main causes of hearing loss, and consequently tinnitus, this will decrease the generalisability of this study.
Donaldson 1981 (carbamazepine): this appears to be a single‐blind trial and therefore scored low for blinding. The drop‐out rate is high (21%) and these incomplete outcome data are not addressed. The other items were not reported clearly in the paper.
Hulshof 1985 (carbamazepine): the drop‐out rate in the intervention group is high (41%) and these incomplete data are not addressed. The other items were not reported clearly in the paper.
Hulshof 1986 (flunarizine): due to incomplete reporting none of the items to assess the risk of bias could be scored.
Piccirillo 2007 (gabapentin): all items were reported and methodologically sound (i.e. low risk of bias).
Simpson 1999 (lamotrigine): blinding appears to have been carried out correctly as identical capsules were used for placebo. The other items were not adequately reported and therefore could not be assessed.
Witsell 2006 (gabapentin): the randomisation process, allocation concealment and blinding are all reported and methodologically sound (i.e. low risk of bias for these domains). The drop‐out rate was high (30%), however, and selective loss to follow up cannot be precluded.
In summary, the overall risk of bias of the included studies is 'high' or 'unclear'.
Effects of interventions
Improvement in tinnitus‐specific health‐related quality of life
The studies included in this review did not show a positive effect of anticonvulsants on the primary outcome. Only three studies, all of gabapentin, evaluated the effect through a validated questionnaire (Bakhshaee 2007; Piccirillo 2007; Witsell 2006). One study showed a negative effect of gabapentin, one study showed a small, statistically non‐significant, positive effect and the third study showed no difference between gabapentin and placebo. When the data of the first two studies are pooled (Witsell could not be included because of lack of exact numbers) no effect of gabapentin is found (standardised mean difference (SMD) 0.07, 95% confidence interval (CI) ‐0.26 to 0.40).
Bakhshaee 2007 showed that treatment with gabapentin for four weeks at 900 mg/d resulted in a negative effect of gabapentin compared to placebo with an increase in Tinnitus Questionnaire (TQ) score of 18.4 points (standardised mean difference (SMD) 0.82, 95% CI 0.07 to 1.58). This standard mean difference is low because of high standard deviations (20.22 and 23.36, with a total possible score of 84). Piccirillo 2007 showed that treatment with gabapentin for eight weeks at 900 to 3600 mg/d resulted in a positive, non‐significant effect for gabapentin with a difference compared to placebo of 2.4 points on the Tinnitus Handicap Inventory (THI) (SMD ‐0.11, 95% CI ‐0.48 to 0.25). The data in this study also included high standard deviations (23.52 and 19.02, with a total possible score of 100). In a subgroup of patients with normal hearing the THI improved significantly more in the gabapentin group than in the placebo group (difference 17.2 points, SMD 0.89, 95% CI ‐1.27 to ‐0.50). Witsell 2006 showed no differences on the THI after a four‐week treatment with gabapentin up to 1800 mg compared to placebo (exact numbers could not be extracted from the article).
Improvement in self‐assessment of tinnitus severity
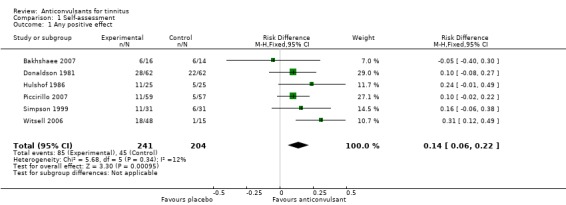
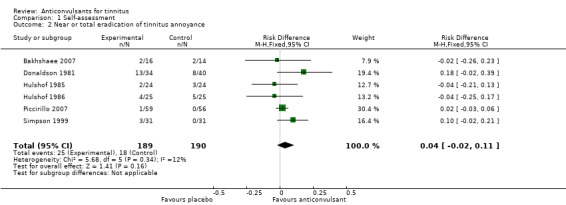
The patient's self‐assessment of their tinnitus, our secondary outcome, was included in all studies as an outcome measurement. Five out of six studies did not show a positive effect of anticonvulsants (Bakhshaee 2007; Donaldson 1981; Hulshof 1986; Piccirillo 2007; Simpson 1999; Witsell 2006). When these data are pooled in a meta‐analysis, however, this shows a small favourable effect of anticonvulsants (risk difference (RD) 14%, 95% CI 6% to 22%). This meta‐analysis includes all levels of improvement on the various Likert scale, and therefore these patients may still be annoyed to some or a large degree by their tinnitus. It can be presumed that the best possible scores on these Likert scales (complete effect, abolition, not annoying, not annoying/disappeared, very much better, much better) entail annulment of annoyance. Summarised as near or total eradication of tinnitus annoyance, a meta‐analysis of these results showed no effect of anticonvulsants (RD 4%, 95% CI ‐2% to 11%) (Bakhshaee 2007; Donaldson 1981; Hulshof 1985; Hulshof 1986; Piccirillo 2007; Simpson 1999). Figure 3 and Figure 4 show the results of these meta‐analyses.
Figure 3.
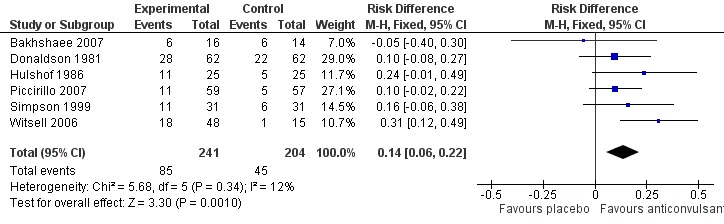
Forest plot of comparison: 1 Self‐assessment, outcome: 1.1 Any positive effect.
Figure 4.
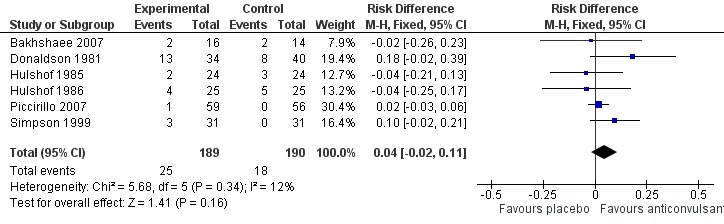
Forest plot of comparison: 1 Self‐assessment, outcome: 1.2 near or total eradication of tinnitus annoyance
Gabapentin
Bakhshaee 2007 showed no effect of gabapentin on a self‐assessment score (RD 5%, 95% CI ‐40% to 30%). Complete response was achieved on this score in 13% in the gabapentin group and in 14% of the placebo group (RD 2%, 95% CI ‐26% to 23%).
Piccirillo 2007 reported a non‐significant improvement in 19% in the gabapentin group compared to 9% in the placebo group (RD 10%, 95% CI ‐2% to 22%) on a self‐assessment score. A large result was achieved in 2% in the gabapentin group and not in the placebo group (RD 2%, 95% CI ‐3% to 6%). This difference is also not significant.
Witsell 2006 found a significant effect for gabapentin using a self‐assessment score: 38% of patients in the gabapentin group reported a positive effect compared to 7% of placebo patients (RD 30%, 95% CI 14% to 48%).
Carbamazepine
Donaldson 1981 showed that treatment with carbamazepine 200 mg twice a day for two months resulted in a non‐significant positive effect in 45% as compared to 21% in the placebo group on a self‐assessment score (RD 10%, 95% CI ‐8% to 27%). A good or excellent result was achieved in 38% in the carbamazepine group and in 20% of the placebo group (RD 18%, 95% CI ‐2% to 39%).
Hulshof 1985 showed that treatment with carbamazepine 150 mg three times a day for 30 days resulted in a non‐significant negative effect: in 8% as compared to 13% in the placebo group on a self‐assessment score (RD ‐4%, 95% CI ‐21% to 13%).
Flunarizine
Hulshof 1986 showed that treatment with 10 mg flunarizine for six weeks resulted in a non‐significant improvement of tinnitus in 44% as compared to 24% in the placebo group using a self‐assessment score (RD 20%, 95% CI ‐6% to 46%). Disappearance of the annoyance of tinnitus was non‐significantly lower in the flunarizine group; 16% in the flunarizine group compared to 20% in the placebo group (RD ‐4%, 95% CI ‐25% to 17%)
Lamotrigine
Simpson 1999 showed that treatment with lamotrigine up to 100 mg for eight weeks resulted in a non‐significant improvement in tinnitus in 36% as compared to 19% in the placebo group using a self‐assessment score (RD 16%, 95% CI ‐6% to 38%). A large improvement was found in 10% of the lamotrigine group and not found in placebo group (RD 10%, 95% CI ‐2% to 21%). This difference is also not significant.
Improvement in accompanying symptoms (e.g. depression, anxiety or sleeping problems)
Only two studies included outcome measurements on accompanying symptoms. Witsell et al did not find a significant difference in the total mood score of the Profile of Mood States (exact numbers could not be extracted from the article) between the gabapentin and the placebo group (Witsell 2006). Piccirillo et al included the Beck Depression Scale and Brief Symptom Inventory in their analysis, but did not include a description of these data in their article (Piccirillo 2007).
Adverse drug effects
In all studies side effects were reported. Fifty‐two of the 286 patients (18%) that received an anticonvulsant experienced side effects. Nausea (12 patients) and dizziness (11 patients) were the most frequently reported. Other side effects reported were: headache (five patients), elevated tiredness (four patients), vomiting during the treatment (three patients), weight gain (two patients), sleep disturbance (two patients), and diarrhoea, mouth sores and decreased libido (one patient). In 14 patients the side effects were not specified.
Discussion
Summary of main results
This review of seven trials (453 patients) shows that current evidence regarding the effectiveness of anticonvulsants has a significant risk of bias. Nevertheless, based on the findings in this review anticonvulsants do not show a beneficial effect on tinnitus, measured through validated questionnaires. The seven included trials investigated four different anticonvulsants: gabapentin, carbamazepine, lamotrigine and flunarizine. Of the three studies that measured improvement with a validated questionnaire (our primary outcome), one study showed a significant negative (adverse) effect of gabapentin compared to placebo with an increase in Tinnitus Questionnaire (TQ) score of 18.4 points (standardised mean difference (SMD) 0.82, 95% confidence interval (CI) 0.07 to 1.58). A second study did not show a significant effect of gabapentin compared to placebo (difference 2.4 points on the Tinnitus Handicap Inventory (THI), SMD ‐0.11, 95% CI ‐0.48 to 0.25). When the data from these two studies are pooled no effect of gabapentin is found (SMD 0.07, 95% CI ‐0.26 to 0.40). A third study comparing gabapentin and placebo did not show a difference using the THI. A meta‐analysis of 'any positive effect' (yes versus no) based on a self‐assessment score (secondary outcome) showed a small beneficial effect (risk difference (RD) 14%, 95% CI 6% to 22%) for anticonvulsants. However, this effect is not large enough to be considered clinically relevant. It shows that in 14% an improvement can be seen, which is in itself a low number. Secondly, in tinnitus a minor improvement is not always enough to obtain the treatment goal: a decrease in annoyance to a level in which it does not interfere with the patient's quality of life. The treatment goal to be aimed at is near or total eradication of tinnitus annoyance: a meta‐analysis of this outcome showed no effect for anticonvulsants (RD 4%, 95% CI ‐2% to 11%). Side effects of the anticonvulsants used were experienced by 18% of patients.
Overall completeness and applicability of evidence
The results of this review are only applicable to the general tinnitus population. Since tinnitus is a diverse symptom, different subgroups of tinnitus patients may exist, potentially leading to different results for different therapies. Due to the lack of data on factors that could potentially modify the effect of anticonvulsants (such as degree of burden, aetiology of hearing loss, duration of tinnitus or whether the patients actively seek help or not), it was not possible to perform subgroup analyses to identify patients that might benefit from anticonvulsants. Piccirillo et al, however, showed a beneficial effect of gabapentin in a subgroup with normal hearing (Piccirillo 2007). It is thus possible that some subgroups might benefit more than others from treatment with anticonvulsants.
Quality of the evidence
Most of the studies included in this review have a moderate or high risk of bias as descriptions of the methodology used are minimal.
Potential biases in the review process
During the review process we identified potential biases both in the individual trials and in the review process itself.
Since tinnitus is a subjective symptom, no gold standard is available to measure the severity of the symptom. Furthermore, there is as yet no consensus regarding the best way to measure treatment effects, making it difficult to interpret and compare results. Validated tinnitus questionnaires are, however, deemed to be more reliable than other subjective measurements, such as visual analogue scales and Likert scales. Audiometric measurements of tinnitus are not regarded as reliable outcome parameters. We therefore used validated questionnaires as our primary outcome measure. Of the included studies one used the TQ and two the THI as outcome measurement. These are the most commonly used questionnaires, but as mentioned above it remains unclear whether these questionnaires are usable for measuring treatment effects (Kamalski 2010).
Due to the large variety of outcome measures used it was not possible to explore publication bias in a funnel plot.
The authors of this review were not blinded to the authorship and origin of the included studies since we knew most of the literature before embarking on this review.
Authors' conclusions
Current evidence regarding the effectiveness of anticonvulsants in patients with tinnitus has significant risk of bias. There is no evidence from studies performed so far to show that the anticonvulsants studied (gabapentin, carbamazepine, flunarizine and lamotrigine) have a large positive effect in the treatment of tinnitus but a small effect (of doubtful clinical significance) has been demonstrated.
Future trials should be methodologically sound. They should be set up as randomised clinical trials. Patients, treatment providers and outcome assessors should be blinded. Randomisation should be performed in a reliable way (e.g. by computer) and the placebo used should be identical to the actual treatment. Results should be analysed by intention‐to‐treat.
Consensus should be reached about evaluation methods so that studies can be compared. A first step towards reaching this consensus had been made by the Tinnitus Research Initiative (Langguth 2007). We would recommend following these guidelines on outcome measurements. The two most commonly used validated tinnitus questionnaires are the Tinnitus Questionnaire (TQ) and the Tinnitus Handicap Inventory (THI), so use of at least one of these in evaluations is recommended.
Study populations should be clearly defined in future trials, including degree of burden, aetiology of hearing loss, duration of tinnitus and other tinnitus characteristics, and whether the patients actively seek help or not.
Future trials should have large enough study populations so that possible effects in subgroups can be evaluated. Smaller trials should only be performed in well‐chosen subgroups. Decisions on the type of subgroups should be based (if possible) on earlier studies showing a possible (better) result in these subgroups.
Acknowledgements
None
Appendices
Appendix 1. Search strategies
| CENTRAL | PubMed | EMBASE (Ovid) |
| #1 MeSH descriptor Tinnitus explode all trees #2 tinnit* #3 (#1 OR #2) #4 MeSH descriptor Anticonvulsants explode all trees #5 MeSH descriptor Carbamazepine #6 MeSH descriptor Vigabatrin #7 MeSH descriptor Phenobarbital #8 MeSH descriptor Ethosuximide #9 MeSH descriptor Clonazepam #10 anticonvul* OR antiepilept* OR anti‐epilept* #11 zonisamide OR AD 810 OR CI 912 OR zonegran OR carbamazepine OR Finlepsin OR Neurotol OR Epitol OR amizepine OR Tegretol OR vigabatrin OR gamma Vinyl OR sabri #12 oxcarbazepine OR GP 47680 OR timox OR trileptal OR Phenobarbital OR phenemal OR phenorbarbitone OR Phenylbarbital OR phenylethylbarbituric acid OR gardenal OR luminal OR methsuximide OR mesuximide #13 N,2‐dimethyl‐2‐phenylsuccinimide OR celontin OR petinutin OR lamotrigine OR lamictal OR lamiktal OR gabapentin OR neurontin OR felbamate OR felbatol OR taloxa OR W‐554 #14 etiracetam OR levetiracetam OR Keppra OR Emeside OR suksilep OR suxilep OR zarontin OR clonazepam OR antelepsin OR rivotril OR Ro 5‐4023 OR clobazam OR frisium OR urbanyl #15 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 #16 #3 AND #15 | #1 Tinnitus [Mesh] #2 tinnit* [tiab] #3 #1 OR #2 #4 Anticonvulsants [Mesh] #5 Carbamazepine [MeSH] #6 Vigabatrin [MeSH] #7 Phenobarbital [MeSH] #8 Ethosuximide [MeSH] #9 Clonazepam [MeSH] #10 Clobazam [Substance Name] #11 Etiracetam [Substance Name] #12 Felbamate [Substance Name] #13 Gabapentin [Substance Name] #14 Lamotrigine [Substance Name] #15 Methsuximide [Substance Name] #16 Oxcarbazepine [Substance Name] #17 Zonisamide [Substance Name] #18 Anticonvulsants[Pharmacological Action] #19 etiracetam[tiab] OR levetiracetam[tiab] OR Keppra[tiab] OR Emeside[tiab] OR suksilep[tiab] OR suxilep[tiab] OR zarontin[tiab] OR clonazepam[tiab] OR antelepsin[tiab] OR rivotril[tiab] OR Ro 5‐4023[tiab] OR clobazam[tiab] OR frisium[tiab] OR urbanyl [tiab] #20 N,2‐dimethyl‐2‐phenylsuccinimide[tiab] OR celontin[tiab] OR petinutin[tiab] OR lamotrigine[tiab] OR lamictal[tiab] OR lamiktal[tiab] OR gabapentin[tiab] OR neurontin[tiab] OR felbamate[tiab] OR felbatol[tiab] OR taloxa[tiab] OR W‐554 #21 oxcarbazepine[tiab] OR GP 47680[tiab] OR timox[tiab] OR trileptal[tiab] OR phenobarbital[tiab] OR phenemal[tiab] OR phenorbarbitone[tiab] OR phenylbarbital[tiab] OR phenylethylbarbituric acid[tiab] OR gardenal[tiab] OR luminal[tiab] OR methsuximide[tiab] OR mesuximide #22 zonisamide[tiab] OR AD 810[tiab] OR CI 912 [tiab] OR zonegran[tiab] OR carbamazepine[tiab] OR finlepsin[tiab] OR neurotol[tiab] OR epitol[tiab] OR amizepine[tiab] OR tegretol[tiab] OR vigabatrin[tiab] OR "gamma vinyl"[tiab] OR sabri #23 anticonvul*[tiab] OR antiepilept*[tiab] OR anti‐epilept* [tiab] #24 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 #25 #3 AND #24 | 1. exp tinnitus/ 2. tinnit*.tw. 3. 1 or 2 4. exp anticonvulsive agent/ 5. exp anticonvulsant activity/ 6. exp anticonvulsant therapy/ 7. (anticonvul* OR antiepilept* OR anti‐epilept*).tw. 8. (zonisamide OR AD 810 OR CI 912 OR zonegran OR carbamazepine OR Finlepsin OR Neurotol OR Epitol OR amizepine OR Tegretol OR vigabatrin OR gamma Vinyl OR sabri).tw. 9. (oxcarbazepine OR GP 47680 OR timox OR trileptal OR Phenobarbital OR phenemal OR phenorbarbitone OR Phenylbarbital OR phenylethylbarbituric acid OR gardenal OR luminal OR methsuximide OR mesuximide).tw. 10. N,2‐dimethyl‐2‐phenylsuccinimide OR celontin OR petinutin OR lamotrigine OR lamictal OR lamiktal OR gabapentin OR neurontin OR felbamate OR felbatol OR taloxa OR W‐554).tw. 11. (etiracetam OR levetiracetam OR Keppra OR Emeside OR suksilep OR suxilep OR zarontin OR clonazepam OR antelepsin OR rivotril OR Ro 5‐4023 OR clobazam OR Frisium OR urbany).tw. 12. 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 13. 3 AND 12 |
Data and analyses
Comparison 1.
Self‐assessment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any positive effect | 6 | 445 | Risk Difference (M‐H, Fixed, 95% CI) | 0.14 [0.06, 0.22] |
| 2 Near or total eradication of tinnitus annoyance | 6 | 379 | Risk Difference (M‐H, Fixed, 95% CI) | 0.04 [‐0.02, 0.11] |
Analysis 1.1.
Comparison 1 Self‐assessment, Outcome 1 Any positive effect.
Analysis 1.2.
Comparison 1 Self‐assessment, Outcome 2 Near or total eradication of tinnitus annoyance.
Comparison 2.
Questionnaires
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tinnitus Handicap Inventory (THI) | 1 | 115 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.48, 0.25] |
| 2 Tinnitus Questionnaire (TQ) | 2 | 145 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.26, 0.40] |
Analysis 2.1.
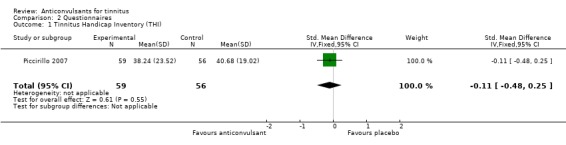
Comparison 2 Questionnaires, Outcome 1 Tinnitus Handicap Inventory (THI).
Analysis 2.2.
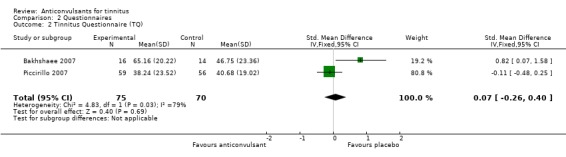
Comparison 2 Questionnaires, Outcome 2 Tinnitus Questionnaire (TQ).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Prospective, double‐blind, placebo‐controlled, cross‐over trial | |
| Participants | 30 participants with moderate to severe idiopathic subjective tinnitus 16 gabapentin, 14 placebo Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention: 900 mg gabapentin per day Control: identical placebo (opaque starch‐filled gel capsules) Duration: 4 weeks |
|
| Outcomes | Primary outcome: psychoacoustically determined tinnitus loudness and TQ score Secondary outcome: Tinnitus Severity Index score |
|
| Notes | Drawbacks:
Adverse events: 3% experienced dizziness |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "every participant received the same treatment sequence, therefore evaluations could be made with each participant serving as their own control" Quote: "16 participants in the study group and 14 in the control group completed the protocol" Comment: this study is described as a RCT and the results show an intervention and a placebo group. The methods section, however, describes every participant receiving the same treatment sequence and serving as their own control. This implies a cross‐over design. As it is stated clearly that everybody received the same treatment sequence we believe that no randomisation was performed |
| Allocation concealment (selection bias) | High risk | Quote: "every participant received same treatment sequence" Comment: a fixed sequence excludes the possibility of allocation concealment |
| Blinding (performance bias and detection bias) All groups | Unclear risk | Quote: "double blind" Quote: "neurontin capsules and placebo were individually enclosed in snap‐lock, opaque starch‐filled gel capsules by an investigational pharmacist" Quote: "every participant received same treatment sequence" Comment: blinding not adequately described 1) Identical pills suggests blinding of patients 2) Same treatment sequence makes blinding of study personnel and outcome assessors doubtful |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "42 patients were noncompliant with the study instructions and one failed to complete the study due to side effects" Quote: "30 patients completed the protocol" Comment: not adequately described; 59% drop‐out is implied (not included in the analysis) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk |
|
| Methods | Placebo‐controlled, randomised, cross‐over trial | |
| Participants | 78 tinnitus clinic patients 62 carbamazepine, 62 placebo (patients who dropped out not included) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention: 100 mg carbamazepine twice a day Control: placebo tablets Duration: 2 times 2 months |
|
| Outcomes | Patients' assessment of tinnitus change on a percentage (not fully described, presumably 0% to 100%) analogue scale: excellent (abolition); good (> 60% reduction); partial (30% to 60% reduction); no significant relief (< 30% reduction) | |
| Notes | Adverse events: types not described 18% withdrew because of side effects from carbamazepine and 3% withdrew because of side effects from placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised system" Comment: randomisation process not explained |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding (performance bias and detection bias) All groups | High risk | Quote: "double blind system" Comment: blinding is not explained. The study date and the fact that no study pharmacy is mentioned mean that adequate blinding is judged highly unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "14 patients withdrew whilst taking carbamazepine and 2 whilst taking placebo" Comment: 21% drop‐out (18% intervention, 3% placebo) is too high and patients that dropped out were excluded from analyses |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk |
|
| Methods | Double‐blind, placebo‐controlled, randomised trial | |
| Participants | 48 patients with annoying tinnitus 24 carbamazepine, 24 placebo No further inclusion/exclusion criteria described |
|
| Interventions | Intervention: 150 mg carbamazepine 3 times a day Control: identical‐looking gelatin placebo capsules Duration: 30 days |
|
| Outcomes | Likert scale (tinnitus disappeared, tinnitus improved, tinnitus did not disappear) | |
| Notes | Adverse events: 63% of carbamazepine patients experienced side effects (8 dizziness, 8 nausea, 4 headache, 2 tiredness, 2 vomiting, 1 diarrhoea). 4% of placebo patients experienced side effects (1 headache). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised controlled trial" Comment: randomisation process not explained |
| Allocation concealment (selection bias) | Unclear risk | Quote: "after randomisation" Randomisation process not explained |
| Blinding (performance bias and detection bias) All groups | Unclear risk | Quote: "double‐blind controlled trial" Quote: "identical‐looking gelatin capsules" Comment: blinding not adequately described
|
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "10 patients withdrew in carbamazepine group because of side effects" Comment: 42% drop‐out in the intervention group is very high; patients were included in analyses |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk |
|
| Methods | Double‐blind, placebo‐controlled, randomised clinical trial | |
| Participants | 50 patients with annoying tinnitus 25 flunarizine, 25 placebo No further inclusion/exclusion criteria described |
|
| Interventions | Intervention: 10 mg flunarizine once a day Control: identical placebo capsules Duration: 6 weeks |
|
| Outcomes | Likert scale (0 tinnitus has disappeared, 1 tinnitus persists but is no longer annoying, 2 tinnitus annoying but less severe, 3 no change, 4 severity increased) | |
| Notes | Adverse events: 8% of flunarizine patients experienced sleepiness during the day. No side effects were mentioned in the placebo group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomisation" Comment: randomisation process not explained |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomisation" Comment: randomisation process not explained |
| Blinding (performance bias and detection bias) All groups | Unclear risk | Quote: "double blind" Comment: blinding process not adequately described
|
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: not adequately described if there were no drop‐outs, or if the 50 patients analysed were the patients left over after exclusion of a number of drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk |
|
| Methods | Double‐blind, placebo‐controlled, randomised trial | |
| Participants | 135 subjects with severe idiopathic subjective tinnitus (1028 screened) 59 gabapentin, 56 placebo Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention: 3600 mg gabapentin per day in 3 doses or highest possible dose reached (titration: week 1, 900 mg/day; week 2, 1800 mg/day; week 3, 2700 mg/day; week 4, 3600 mg/day) Control: identical blue placebo capsule, similar administration Duration: 4 weeks titration period and 4‐week fixed‐dose period |
|
| Outcomes | Primary outcome: change in THI from week 0 to 8 between treatment arms Secondary outcomes: 1) Patient Global Impression of Change score, 2) Brief Symptom Inventory, 3) Beck Depression Scale score |
|
| Notes | Drawbacks:
Adverse events: 7% withdrew because of side effects (3 nausea, 2 weight gain, 2 sleep disturbance, 2 dizziness, 1 seizure). It was not mentioned if the side effects occurred in the gabapentin or the placebo group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "sequentially randomised according to a computer‐generated random code" |
| Allocation concealment (selection bias) | Low risk | Quote: "research pharmacist maintained the randomisation schedule" |
| Blinding (performance bias and detection bias) All groups | Low risk | Quote: "double blind" Quote: "matching placebo capsules", "identical blue capsules" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16% drop out in gabapentin group and 14% in placebo group Modified intention‐to‐treat analyses (inclusion of patients with at least 1 dose of study medication and at least 1 follow‐up assessment) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | — |
| Methods | Double‐blind, placebo‐controlled, cross‐over trial | |
| Participants | 33 subjects from a general tinnitus clinic population No other inclusion criteria mentioned Exclusion criteria
|
|
| Interventions | Intervention: 100 mg/d lamotrigine (titration: week 1 to 2, 25 mg/day; week 3 to 4, 50 mg/day) Control: matching placebo tablets Duration: 4 weeks titration period, 4 weeks fixed‐dose period, followed by same regimen after cross‐over |
|
| Outcomes | Questionnaires (Likert: much better, better, no change, worse, much worse), visual analogue scales (loudness, annoyance, awareness) and audiological measurements (pure tone audiometry, masking audiogram, pitch matching of tinnitus, loudness matching of tinnitus, masking of tinnitus, residual inhibition and uncomfortable loudness levels) at 0 weeks, 4 weeks, 8 weeks, 12 weeks and 16 weeks | |
| Notes | Drawbacks:
Adverse events: 3% of patients withdrew in the lamotrigine group (1 nausea, vomiting and headache) and 3% of patients withdrew in the placebo group (1 dizziness and rash) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" Comment: randomisation process not explained |
| Allocation concealment (selection bias) | Unclear risk | Randomisation process not explained |
| Blinding (performance bias and detection bias) All groups | Unclear risk | Quote: "double‐blind" Quote: "matching placebo tablets" Comment: blinding process not adequately described. Identical pills suggest at least blinding of patients. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6% drop‐out, evenly distributed in placebo and lamotrigine group Left out from results |
| Selective reporting (reporting bias) | Unclear risk | No protocol available No primary outcome measurement or time point stated |
| Other bias | Unclear risk |
|
| Methods | Double‐blind, placebo‐controlled, randomised clinical trial | |
| Participants | 79 patients with moderate tinnitus (102 screened) 53 gabapentin, 26 placebo Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention: 1800 mg gabapentin per day in 3 doses Control: identical placebo capsules in same dosing schedule Duration: 2 week escalating‐dose period (week 1, 300 mg/day; week 2, 900 mg/day), 2 week fixed‐dose period, 2 weeks descending‐dose period (week 5, 900 mg/day; week 6, 300 mg/day) |
|
| Outcomes | Primary outcome: Tinnitus Handicap Inventory (THI), no time period stated (questionnaire was administered at week 1, week 4, week 10) Secondary outcome: Profile of Mood States (POMS) |
|
| Notes | Adverse events: 2% of patients in the gabapentin group experienced side effects (1 mouth sores and decreased libido) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computerised random‐number generator" |
| Allocation concealment (selection bias) | Low risk | Quote: "key to randomisation was held by the pharmacy" |
| Blinding (performance bias and detection bias) All groups | Low risk | Quote: "placebo identical to the gabapentin capsules" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30% drop‐out, selective loss to follow up Unclear handling of drop‐out |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk |
|
RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bauer 2006 | ALLOCATION Not randomised (cross‐over trial with a set regiment) |
| Castagno 1989 | Not available |
| Guth 1990 | ALLOCATION Random; randomisation process not explained Cross‐over design PARTICIPANTS 66 patients (66 AOAA, 66 placebo) Inclusion criteria:
Exclusion criteria:
INTERVENTIONS Intervention: Amino‐oxyacetic acid (AOAA) is not a registered anticonvulsant |
| Halmos 1982 | Not available |
| Marks 1981 | ALLOCATION Not described PARTICIPANTS 9 patients Inclusion criteria:
INTERVENTIONS Intervention: single dose of 200 mg carbamazepine |
| Menkes 1998 | ALLOCATION Article is a letter to the editor and not a randomised controlled trial |
| Shulman 2008 | ALLOCATION Article is a comment and not a randomised controlled trial |
| Viada 1981 | Not available |
Contributions of authors
CEL Hoekstra Designing protocol and search strategy, selection and quality assessment of studies, data extraction and data analysis, drafting of the protocol and review.
SP Rynja Designing protocol and search strategy, selection and quality assessment of studies, data extraction and data analysis, co‐drafting of the protocol and review.
GA van Zanten Co‐drafting of the protocol and review, supervision of review.
MM Rovers Supervision of design, selection of studies, data extraction and production of review, assistance with quality assessment of studies and data analysis, co‐drafting of the protocol and review.
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
None known.
New
References
References to studies included in this review
- Bakhshaee M, Ghasemi M, Azarpazhooh M, Khadivi E, Rezaei S, Shakeri M, et al. Gabapentin effectiveness on the sensation of subjective idiopathic tinnitus: a pilot study. European Archives of Oto‐Rhino‐Laryngology 2007;265(5):525‐30. [DOI] [PubMed] [Google Scholar]
- Donaldson I. Tegretol: a double blind trial in tinnitus. Journal of Laryngology and Otology 1981;95(9):947‐51. [DOI] [PubMed] [Google Scholar]
- Hulshof JH, Vermeij P. The value of carbamazepine in the treatment of tinnitus. ORL: Journal of Oto‐Rhino‐Laryngology and Its Related Specialties 1985;47:262‐6. [DOI] [PubMed] [Google Scholar]
- Hulshof JH, Vermeij P. The value of flunarizine in the treatment of tinnitus. ORL: Journal of Oto‐Rhino‐Laryngology and Its Related Specialties 1986;48:33‐6. [DOI] [PubMed] [Google Scholar]
- Piccirillo JF, Finnell J, Vlahiotis A, Chole RA, Spitznagel E. Relief of idiopathic subjective tinnitus. Archives of Otolaryngology ‐ Head and Neck Surgery 2007;133:390‐7. [DOI] [PubMed] [Google Scholar]
- Simpson JJ, Gilbert AM, Weiner GM, Davies WW. The assessment of lamotrigine, an antiepileptic drug, in the treatment of tinnitus. American Journal of Otology 1999;20:627‐31. [PubMed] [Google Scholar]
- Witsell DL, Hannley MT, Stinnet S, Tucci DL. Treatment of tinnitus with gabapentin: a pilot study. Otology & Neurotology 2006;28:11‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Bauer CA, Brozoski TJ. Effect of gabapentin on the sensation and impact of tinnitus. Laryngoscope 2006;116:675‐82. [DOI] [PubMed] [Google Scholar]
- Castagno LA. Tinnitus: a therapeutic trial with cinnarizine, primidone and placebo. Folha Medica 1989;99(5‐6):279‐84. [Google Scholar]
- Guth PS, Blair P, Norris C, Risey J, Reed H, Housley G, et al. Evaluation of amino‐oxyacetic acid as a palliative in tinnitus. Annals of Otology, Rhinology and Laryngology 1990;99:74‐9. [DOI] [PubMed] [Google Scholar]
- Halmos P, Molnar R, Kormos K. Experiences made with an anticonvulsive in the therapy of tinnitus. HNO‐Praxis 1982;7(1):59‐61. [Google Scholar]
- Marks NJ, Onisiphorou C, Trounce JR. The effect of single doses of amylobarbitone sodium and carbamazepine in tinnitus. Journal of Laryngology and Otology 1981;95:941‐5. [DOI] [PubMed] [Google Scholar]
- Menkes DB, Larson P. Sodium valproate for tinnitus. Journal of Neurology, Neurosurgery and Psychiatry 1998;65(5):803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman A. Gabapentin and tinnitus relief. International Tinnitus Journal 2008;14(1):1‐5. [PubMed] [Google Scholar]
- Viada J, Hess JC, Garcia MT, May A. Assessment of the results of treatment of tinnitus with carbamazepine in patients with strongly positive lidocaine tests. Revista de Otorrinolaringologia y Cirurgia de Cabeza y Cuello 1981;41(2):29‐32. [Google Scholar]
Additional references
- Andersson G, Lyttkens L. A meta‐analytic review of psychological treatments for tinnitus. British Journal of Audiology 1999;33(4):201‐10. [DOI] [PubMed] [Google Scholar]
- Argstatter H, Krick C, Bolay HV. Music therapy in chronic tonal tinnitus. Heidelberg model of evidence‐based music therapy. HNO 2008;56(7):678‐85. [DOI] [PubMed] [Google Scholar]
- American Tinnitus Association. http://www.ata.org 2004 (accessed 12 November 2010).
- Baguley DM, Moffat DA, Hardy DG. What is the effect of translabyrinthine acoustic schwannoma removal upon tinnitus?. Journal of Laryngology and Otology 1992;106:329‐31. [DOI] [PubMed] [Google Scholar]
- Baguley DM, McFerran DJ. Tinnitus in childhood. International Journal of Pediatric Otorhinolaryngology 1999;49:99‐105. [DOI] [PubMed] [Google Scholar]
- Baldo P, Doree C, Lazzarini R, Molin P, McFerran DJ. Antidepressants for patients with tinnitus. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003853.pub2] [DOI] [PubMed] [Google Scholar]
- Bennett MH, Kertesz T, Yeung P. Hyperbaric oxygen for idiopathic sudden sensorineural hearing loss and tinnitus. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004739.pub3] [DOI] [PubMed] [Google Scholar]
- Brummett RE. Drug‐induced ototoxicity. Drugs 1980;19(6):412‐28. [DOI] [PubMed] [Google Scholar]
- Chung DY, Gannon RP, Mason K. Factors affecting the prevalence of tinnitus. Audiology 1984;23:441‐52. [DOI] [PubMed] [Google Scholar]
- Coles RR. Epidemiology of tinnitus: (1) prevalence. Journal of Laryngology and Otology. Supplement 1984;9:7‐15. [DOI] [PubMed] [Google Scholar]
- Crummer RW, Hassan GA. Diagnostic approach to tinnitus. American Family Physician 2004;69:120‐6. [PubMed] [Google Scholar]
- Dauman R, Tyler RS. Some considerations on the classification of tinnitus. Proceedings of the Fourth International Tinnitus Seminar, Bordeaux. 1992:225‐9. [Google Scholar]
- Davis AC. The prevalence of hearing impairment and reported hearing disability among adults in Great Britain. International Journal of Epidemiology 1989;18:911‐17. [DOI] [PubMed] [Google Scholar]
- Davis A, Rafaie EA. Epidemiology of tinnitus. Tinnitus Handbook. San Diego: Singular, Thomson Learning, 2000:3‐21. [Google Scholar]
- Ridder D, Mulder G, Menovsky T, Sunaert S, Kovacs S. Electrical stimulation of auditory and somatosensory cortices for treatment of tinnitus and pain. In: Langguth B, Hajak G, Kleinjung T, Cacace A, Moller AR editor(s). Progress in Brain Research. Vol. 166, Amsterdam: Elsevier BV, 2007:377‐88. [DOI] [PubMed] [Google Scholar]
- Bo L, Ambrosetti U. Hearing aids for the treatment of tinnitus. In: Langguth B, Hajak G, Kleinjung T, Cacace A, Moller AR editor(s). Progress in Brain Research. Vol. 166, Amsterdam: Elsevier BV, 2007:341‐5. [DOI] [PubMed] [Google Scholar]
- Dobie RA. A review of randomized clinical trials in tinnitus. Laryngoscope 1999;109(8):1202‐11. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends in Neurosciences 2004;27:676‐82. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Chery‐Croze S, Fischer G, Fischer C, Vighetto A, Gregoire MC, et al. A selective imaging of tinnitus. Neuroreport 1999;10:1‐5. [DOI] [PubMed] [Google Scholar]
- Goodey RJ. Drugs in the treatment of tinnitus. Ciba Foundation Symposium 1981;85:263‐78. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
- Hegarthy JL, Smith RJH. Tinnitus in children. Tinnitus Handbook. San Diego: Singular, Thomson Learning, 2000:243‐61. [Google Scholar]
- Heller MF, Bergman M. Tinnitus aurium in normally hearing persons. Annals of Otology, Rhinology and Laryngology 1953;62:73‐83. [DOI] [PubMed] [Google Scholar]
- Henry JA, Dennis KC, Schechter MA. General review of tinnitus: prevalence, mechanisms, effects, and management. Journal of Speech, Language, and Hearing Research 2005;48:1204‐35. [DOI] [PubMed] [Google Scholar]
- Hilton M, Stuart E. Ginkgo biloba for tinnitus. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD003852.pub2] [DOI] [PubMed] [Google Scholar]
- Hobson J, Chisholm E, Loveland M. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD006371] [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neuroscience Research 1990;8:221‐54. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Jastreboff MM. Tinnitus retraining therapy for patients with tinnitus and decreased sound tolerance. Otolaryngologic Clinics of North America 2003;36:321‐36. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Hazell JWP. Tinnitus Retraining Therapy. Implementing the Neurophysiological Model. Cambridge: Cambridge University Press, 2004. [Google Scholar]
- Kamalski DM, Hoekstra CE, Zanten BG, Grolman W, Rovers MM. Measuring disease‐specific health‐related quality of life to evaluate treatment outcomes in tinnitus patients: a systematic review. Otolaryngology ‐ Head and Neck Surgery 2010;143(2):181‐5. [DOI] [PubMed] [Google Scholar]
- Koester M, Storck C, Zorowka P. Tinnitus ‐ classification, causes, diagnosis, treatment and prognosis. MMW Fortschritte der Medizin 2004;146(1‐2):23‐4, 26‐8; quiz 29‐30. [PubMed] [Google Scholar]
- Langguth B, Goodey R, Azevedo A, Bjorne A, Cacace A, Crocetti A, et al. Consensus for tinnitus patient assessment and treatment outcome measurement (Tinnitus Research Initiative Meeting, Regensburg, July 2006). Progress in Brain Research 2007;166:525‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zeng RF, Zheng D. Acupuncture for tinnitus. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008149] [DOI] [Google Scholar]
- Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology 1998;50:114‐20. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Alvi RJ, Burkard RF, Galantowicz PJ, Coad ML, Wack DS. Neuroanatomy of tinnitus. Scandinavian Audiology. Supplementum 1991;51:47‐52. [PubMed] [Google Scholar]
- Luxon LM. Tinnitus: its causes, diagnosis and treatment. BMJ 1993;306:1490‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Devesa P, Waddell A, Perera R, Theodoulou M. Cognitive behavioural therapy for tinnitus. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD005233.pub3] [DOI] [PubMed] [Google Scholar]
- Marx SV, Langman AW, Crane RC. Accuracy of the fast spin echo magnetic resonance imaging in the diagnosis of vestibular schwannoma. American Journal of Otolaryngology 1999;20(4):211‐6. [DOI] [PubMed] [Google Scholar]
- McCombe A, Baguley D, Coles R, McKenna L, McKinney C, Windle‐Taylor P. Guidelines for the grading of tinnitus severity: the results of a working group commissioned by the British Association of Otolaryngologists ‐ Head and Neck Surgeons. Clinical Otolaryngology 2001;26:388‐93. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Sigalovsky IS, Guinan JJ, Levine RA. Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. Journal of Neurophysiology 2000;83:1058‐72. [DOI] [PubMed] [Google Scholar]
- Melding PS, Goodey RJ, Thorne PR. The use of intravenous lignocaine in the diagnosis and treatment of tinnitus. Journal of Laryngology and Otology 1978;92(2):115‐21. [DOI] [PubMed] [Google Scholar]
- Melding PS, Goodey RJ. The treatment of tinnitus with oral anticonvulsants. Journal of Laryngology and Otology 1979;93(2):111‐22. [DOI] [PubMed] [Google Scholar]
- Meng Z, Liu S, Zheng Y. Transcranial magnetic stimulation for tinnitus. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007946] [DOI] [PubMed] [Google Scholar]
- Moller AR. Pathophysiology of tinnitus. Otolaryngologic Clinics of North America 2003;36:249‐66, v‐vi. [DOI] [PubMed] [Google Scholar]
- Muhlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proceedings of the National Academy of Sciences of the United States of America 1998;95:10340‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nondahl DM, Cruickshanks KJ, Wiley TL, Klein R, Klein BE, Tweed TS. Prevalence and 5‐year incidence of tinnitus among older adults: the epidemiology of hearing loss study. Journal of the American Academy of Audiology 2002;13:323‐31. [PubMed] [Google Scholar]
- Norena AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hearing Research 2003;183:137‐53. [DOI] [PubMed] [Google Scholar]
- Ochi K, Eggermont JJ. Effects of quinine on neural activity in cat primary auditory cortex. Hearing Research 1997;105(1‐2):105‐18. [DOI] [PubMed] [Google Scholar]
- Meikle MB, Johnson RM, Griest SE, Press LS, Charnell MG. Oregon Tinnitus Data Archive 95‐01. http://www.tinnitusarchive.org 1995 (accessed 12 November 2010).
- Phillips JS, McFerran D. Tinnitus Retraining Therapy (TRT) for tinnitus. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD007330.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
- Robinson S. Antidepressants for treatment of tinnitus. Progress in Brain Research 2007;166:263‐71. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hearing Research 2000;147:261‐74. [DOI] [PubMed] [Google Scholar]
- Schleuning AJ. Management of the patient with tinnitus. Medical Clinics of North America 1991;75:1225‐37. [DOI] [PubMed] [Google Scholar]
- Seidman MD. Glutamate antagonists, steroids and antioxidants as therapeutic options for hearing loss and tinnitus and the use of an inner ear drug delivery system. International Tinnitus Journal 1998;4(2):148‐54. [PubMed] [Google Scholar]
- Shea JJ, Harell M. Management of tinnitus aurium with lidocaine and carbamazepine. Laryngoscope 1978;88(9 Pt 1):1477‐84. [DOI] [PubMed] [Google Scholar]
- Shea A. Otosclerosis and tinnitus. Journal of Laryngology and Otology. Supplement 1981;4:149‐50. [PubMed] [Google Scholar]
- Sindhusake D, Mitchell P, Newall P, Golding M, Rochtchina E, Rubin G. Prevalence and characteristics of tinnitus in older adults: the Blue Mountains Hearing Study. International Journal of Audiology 2003;42(5):289‐94. [DOI] [PubMed] [Google Scholar]
- Stephens D, Hetu R. Impairment, disability and handicap in audiology: towards a consensus. Audiology 1991;20:185‐200. [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Dobie RA, Saki CS, Katon WJ. Treatment of depressed tinnitus patients with nortriptyline. Annals of Otology, Rhinology and Laryngology 1989;98(11):867‐72. [DOI] [PubMed] [Google Scholar]
- Sullivan M, Katon WJ, Russo J, Dobie R, Saki C. Somatization, comorbidity, and the quality of life: measuring the effect of depression upon chronic mental illness. Psychiatric Medicine 1992;10:61‐76. [PubMed] [Google Scholar]
- Sullivan M, Katon W, Russo J, Dobie R, Sakai C. A randomized trial of nortriptyline for severe chronic tinnitus. Archives of Internal Medicine 1993;153:2251‐9. [PubMed] [Google Scholar]
- Waddell A. Tinnitus. Clinical Evidence 2005;14:703‐11. [PubMed] [Google Scholar]
- Weissman JL, Hirsch BE. Imaging of tinnitus: a review. Radiology 2000;216(2):342‐9. [DOI] [PubMed] [Google Scholar]


