Abstract
Background
Aspirin is widely used for secondary prevention after stroke. Cilostazol has shown promise as an alternative to aspirin in Asian people with stroke.
Objectives
To determine the relative effectiveness and safety of cilostazol compared directly with aspirin in the prevention of stroke and other serious vascular events in patients at high vascular risk for subsequent stroke, those with previous transient ischaemic attack (TIA) or ischaemic stroke of arterial origin.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched September 2010), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 4), MEDLINE (1950 to May 2010) and EMBASE (1980 to May 2010). In an effort to identify further published, ongoing and unpublished studies we searched journals, conference proceedings and ongoing trial registers, scanned reference lists from relevant studies and contacted trialists and Otsuka Pharmaceutical Co Ltd.
Selection criteria
We selected all randomised controlled trials (RCTs) comparing cilostazol with aspirin where participants were treated for at least one month and followed systematically for development of vascular events.
Data collection and analysis
Data extracted from eligible studies included: (1) a composite outcome of vascular events (stroke, myocardial infarction or vascular death) during follow up (primary outcome); (2) separate outcomes of stroke (ischaemic or haemorrhagic, fatal or non‐fatal), myocardial infarction (MI) (fatal or non‐fatal), vascular death and death from all causes; and (3) main outcomes of safety including any intracranial, extracranial or gastrointestinal (GI) haemorrhage and other outcomes during treatment follow up (secondary outcomes). We computed an estimate of treatment effect and performed a test for heterogeneity between trials. We analysed data on an intention‐to‐treat basis and assessed bias for all included studies.
Main results
We included two RCTs with 3477 Asian participants. Compared with aspirin, cilostazol was associated with a significantly lower risk of composite outcome of vascular events (6.77% versus 9.39%, risk ratio (RR) 0.72, 95% confidence interval (CI) 0.57 to 0.91), and lower risk of haemorrhagic stroke (0.53% versus 2.01%, RR 0.26, 95% CI 0.13 to 0.55). In terms of outcome of safety compared with aspirin, cilostazol was significantly associated with minor adverse effects (8.22% versus 4.95%, RR 1.66, 95% CI 1.51 to 1.83).
Authors' conclusions
Cilostazol is more effective than aspirin in the prevention of vascular events secondary to stroke. Cilostazol has more minor adverse effects, although there is evidence of fewer bleeds.
Keywords: Humans, Asian Continental Ancestry Group, Aspirin, Aspirin/adverse effects, Aspirin/therapeutic use, Brain Ischemia, Brain Ischemia/complications, Cause of Death, Cilostazol, Gastrointestinal Hemorrhage, Gastrointestinal Hemorrhage/chemically induced, Myocardial Infarction, Myocardial Infarction/prevention & control, Platelet Aggregation Inhibitors, Platelet Aggregation Inhibitors/adverse effects, Platelet Aggregation Inhibitors/therapeutic use, Randomized Controlled Trials as Topic, Secondary Prevention, Secondary Prevention/methods, Stroke, Stroke/complications, Stroke/ethnology, Stroke/prevention & control, Tetrazoles, Tetrazoles/adverse effects, Tetrazoles/therapeutic use
Cilostazol versus aspirin for secondary prevention of vascular events after a stroke of arterial origin
Stroke is a public health problem. As lower and middle income countries make rapid economic progress they face the additional health burden of diseases of affluence like stroke and heart attacks. Unlike heart attack, stroke is a disease caused by more than one mechanism. In Asians, a larger proportion of ischaemic stroke is due to narrowing of the arteries at the base of the brain. Compared to Caucasians, Asians are more likely to have bleeds into their brain matter causing stroke, because of uncontrolled high blood pressure. The medication cilostazol thins the blood by blocking platelet accumulation and appears, from early reports, to be more effective than aspirin in the prevention of stroke, heart attacks and death from vascular causes in patients with stroke. This may be due to its inherent effectiveness, as well as chances of fewer brain bleeds. In this review of two randomised trials involving 3477 participants, we found that cilostazol was more effective for the prevention of stroke, heart attack and death from vascular causes in Asian patients with stroke. In terms of safety, it causes more side effects than aspirin but less serious bleeding in the brain and the body.
Background
Description of the condition
Stroke is the leading cause of sustained disability in the world today. Two‐thirds of all strokes now occur in the developing world (Lopez 2006). It is important to study interventions that are relevant to the Asian population as it bears the brunt of the burden of global stroke mortality (Feldmann 1990). Moreover the distribution of types of strokes is different in this region, with a significantly higher proportion of intracranial haemorrhages (ICH) than in the developed world (Liu 2007). Individuals suffering from stroke are already at a very high risk of developing subsequent stroke (Wong 2002). In addition, they are at higher risk of morbidity from other clinical manifestations of atherosclerotic disease such as myocardial infarction (MI), angina or peripheral arterial disease (PAD) (Burke 1995). Although aspirin is beneficial for the secondary prevention of a wide spectrum of cardiovascular incidents, including stroke, it is also known to be associated with a risk of ICH. Cilostazol, a phosphodiesterase type 3 (PDE3) inhibitor, has been tested in this population for secondary stroke prevention and appears to contribute to fewer intracranial haemorrhages than with aspirin while maintaining a significant reduction in the risk of recurrent strokes. In the Cilostazol Stroke Prevention Study, a phase III clinical trial involving more than 1000 Japanese patients, cilostazol was found to reduce the risk of secondary stroke by 41.7% compared with placebo (Matsumoto 2005). In a phase II clinical trial comparing the efficacy of cilostazol versus aspirin among 720 Chinese patients, stroke recurrence was reported in 12 patients in the cilostazol group and in 20 patients in the aspirin group. The estimated hazard ratio was 0.62 (95% confidence interval (CI) 0.30 to 1.26; P = 0.185). Also, cerebral haemorrhagic events were significantly more common in the aspirin group than in the cilostazol group (7 versus 1; P = 0.034) (Huang 2008).
Description of the intervention
Cilostazol is a selective and potent phosphodiesterase type 3 (PDE3) inhibitor (Minami 1997) that is both an antiplatelet and a vasodilating agent. PDE 3 increases the breakdown of cyclic adenosine monophosphate (cAMP) (Ikeda 1999). Inhibition of PDE3 increases the levels of cAMP. Since both platelets and vascular smooth muscle cells contain PDE 3A, inhibition leads to decreased platelet aggregation. Cilostazol inhibits the uptake of adenosine (Liu 2000). This leads to an enhanced adenosine action via A1 and A2 receptors. In platelets and vascular smooth muscle cells A2 mediated increases in cAMP enhance the consequences of PDE inhibition, that is result in additional increases in cAMP. Aspirin is a non‐selective irreversible inhibitor of cyclooxygenase (COX) and has anti‐inflammatory and antiplatelet effects. It decreases the formation of prostaglandins (PGs) and thromboxanes, which leads to decreased platelet aggregation and stabilization (Abramson 1989).
How the intervention might work
In addition to platelet inhibition, cilostazol has other effects on the circulatory system that may be relevant to stroke prevention. Both PDE inhibition and possibly inhibition of adenosine uptake act in concert to relax vascular smooth muscle cells and lead to vasodilatation. Monocyte chemoattractant protein 1 (MCP‐1) plays a significant role in mediating monocyte recruitment in atherosclerotic lesions. Interestingly, cilostazol also inhibits the cytokine induced expression of MCP‐1 probably due to cAMP elevation, which might contribute to an anti‐inflammatory action (Nishio 1997).
Cilostazol acts as an antimitogenic agent by several mechanisms. It blocks the surface expression of the platelet fibrinogen receptor (G2b/3a) as well as alpha‐granule secretion of P‐selectin (Inoue 1999). P‐selectin is assumed to be involved in platelet dependent mitogenesis. This effect might contribute to inhibition of re‐stenosis. Heparin binding epidermal growth factor (HBEGF), which is also inhibited by cilostazol, is one the most potent mitogens for vascular smooth muscle cells and is found in macrophages and vascular smooth muscle cells (Kayanoki 1997). Cilostazol used for a period of 12 weeks has been shown to increase high density lipoproteins (HDL) by 10% and decrease triglycerides by 15% (Elam 1998). These multiple potential mechanisms of action may explain the efficacy of cilostazol.
Why it is important to do this review
Cilostazol has shown promise as an alternative to aspirin for Asian populations with ischaemic stroke (Shinohara 2008). The risk of primary intracranial haemorrhage (ICH) in people from these regions is 30% compared to 10% in the developed world (Liu 2007). Cilostazol appears to prevent more ischaemic strokes and cause less ICH than aspirin when used for secondary prevention of ischaemic stroke (Huang 2008).
Aspirin is the most widely prescribed agent for the prevention of stroke in the world today (Rother 2008). Aspirin overall reduces the risk of major vascular events by 13% (95% CI 6% to 19%) (Algra 1996). A study of 720 Chinese patients that compared treatment with a standard dose of aspirin at 100 mg per day to cilostazol 100 mg twice a day was associated with a reduction of recurrent stroke by 30% (95% CI ‐26% to 70%) (Huang 2008). A systematic review is necessary to evaluate the strength of these claims.
Objectives
The objective of this review was to determine the relative effectiveness and safety of the phosphodiesterase inhibitor (PDE 3A) cilostazol, compared directly with aspirin (frequently used as monotherapy for secondary prevention), in the prevention of stroke and other serious vascular events in patients at high vascular risk for subsequent stroke. That is, those people with previous transient ischaemic attack (TIA) and ischaemic stroke of arterial origin.
Methods
Criteria for considering studies for this review
Types of studies
We included all truly randomised trials in which cilostazol was compared directly with aspirin and in which patients were followed up prospectively and systematically for at least one month for the occurrence of serious vascular events.
Types of participants
Eligible patients were those at high risk of stroke and other serious vascular events due to previous clinical manifestations of TIA or ischaemic stroke of arterial origin.
Types of interventions
Orally administered cilostazol at a minimum dose of 50 mg twice a day compared with aspirin at a minimum dose of 81 mg once a day.
Types of outcome measures
Primary outcomes
The main outcome measure of effectiveness was the composite outcome of stroke, myocardial infarction (MI), and vascular death.
Secondary outcomes
The secondary outcomes of effectiveness were the separate outcomes of stroke (ischaemic or haemorrhagic, non‐fatal or fatal), MI (non‐fatal or fatal), vascular death, and death from all causes. We classified deaths as due to ischaemic stroke, intracranial haemorrhage (ICH), MI, other vascular causes (for example sudden cardiac death, cardiac failure, pulmonary embolism, extracranial haemorrhage), non‐vascular causes, and unknown causes (deaths that could not be assigned to any of the foregoing categories).
The main outcomes of safety were any ICH, extracranial haemorrhage, and gastrointestinal haemorrhage. Other outcomes measuring safety were headache, gastrointestinal intolerance, palpitation, dizziness, tachycardia, precipitation of angina, and cardiac failure.
Search methods for identification of studies
See the 'Specialized Register' section in the Cochrane Stroke Group module.
Electronic searches
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Managing Editor in September 2010, the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 4), MEDLINE (1950 to May 2010) (Appendix 1), and EMBASE (1980 to May 2010) (Appendix 2).
Searching other resources
In an effort to identify further published, unpublished, and ongoing trials we undertook the following.
-
Handsearched journals and conference proceedings:
Proceedings of the International Stroke Conference 2010, 24 to 26 February 2010, USA, San Antonio Texas (July 2010);
Proceedings of the 18th European Stroke Conference 2009, 26 to 29 May 2009, Sweden, Stockholm (May 2010);
Proceedings of the International Stroke Conference 2009, 18 to 20 February 2009, USA, San Diego, California (May 2010);
Proceedings of the 2nd International Conference on Hypertension, Lipids, Diabetes and Stroke Prevention 2008, 6 to 8 March 2008, Czech Republic, Prague (May 2010).
-
Searched the following international trials registers:
ClinicalTrials.gov (http://www.clinicaltrials.gov/) (May 2010);
Current Controlled Trials (www.controlled‐trials.com) (May 2010);
Stroke Trials Registry (www.strokecenter.org/trials/) (May 2010).
Contacted authors of identified trials and other researchers in the field (April 2010).
Contacted Otsuka Pharmaceutical Co Ltd, the manufacturer of cilostazol (May 2010).
Screened reference lists from relevant articles.
We searched for trials in all languages and arranged translation of potentially relevant trial reports that were published in languages other than English.
Data collection and analysis
We reviewed all potentially eligible studies. We extracted data on types of patients enrolled; entry and exclusion criteria; method of randomisation; process of treatment allocation concealment; the original numbers of patients assigned to the treatment and aspirin groups; number of patients lost to follow up in each treatment group; degree of adherence to treatment; method and duration of follow up; whether patients and trial staff were blinded to treatment allocation and, if so, what methods of blinding to treatment of patients, treating clinicians, and outcome assessors allocation were employed; definitions of outcome events; and treatment‐specific side effects. We analysed data on the basis of an intention‐to‐treat analysis.
Selection of studies
We considered that all RCTs comparing cilostazol with aspirin in patients with a qualifying stroke or TIA were eligible for inclusion in this review. These patients should have been on treatment for at least one month and followed systematically for the development of vascular events.
Data extraction and management
We extracted data from published reports or from the original researchers by using a pre‐designed data collection form. One review author (IN) independently extracted data and reviewed studies. Another review author (AKK) reviewed the studies, verified data entry and numbers to double check for errors. We resolved all disagreements by discussion and with a third review author as needed (BAK).
Assessment of risk of bias in included studies
We used the ’Risk of bias’ table, part of the ’Characteristics of included studies’ table, to delineate assessment of risk of bias in the included studies. This helped to address issues of bias including selection, allocation concealment, blinding, performance, attrition, detection, incomplete outcome data processing, and selective reporting. The content expert (AKK) reviewed the table. We scored each entry as 'low risk', 'high risk' or 'unclear risk' of bias. For each entry, we provided text detailing the description of design, conduct, or observation leading to the judgement. We incorporated bias assessment in the analyses by reporting an estimated intervention effect based on all available studies, with a narrative discussion on the risk of bias in individual domains.
Measures of treatment effect
We determined treatment effect primarily by calculating effect estimators for signal trials, which were then combined by means of the Mantel‐Haenszel (MH) method. The treatment effect was measured in terms of risk ratio (RR). The absolute risk reduction (ARR) and number needed to treat (NNT) were also calculated. For this purpose we used the calculator available on the Cochrane Stroke Group website (http://www.dcn.ed.ac.uk/csrg/NNT2.asp).
Unit of analysis issues
We did not include studies with crossover design or cluster randomisation in this analysis.
Dealing with missing data
We performed an intention‐to‐treat analysis; we included all those participants that were 'lost' in the appropriate assigned arm.
Assessment of heterogeneity
We used the I2 statistic to test for heterogeneity between trials.
Assessment of reporting biases
We constructed a funnel plot and looked for asymmetry to assess reporting bias.
Data synthesis
We used a fixed‐effect model to perform meta‐analysis. Since there was no extreme heterogeneity within trials, we were able to perform meta‐analysis.
Subgroup analysis and investigation of heterogeneity
Pre‐specified subgroups included investigating any differences in the primary outcome in Caucasians versus Asians. For analysis of the differences of effect between subgroups, we planned to compare confidence intervals between subgroups and look for overlap. Since there were no studies from Caucasian populations, this could not be carried out.
We determined statistical significance with the Chi2 test for heterogeneity.
Sensitivity analysis
We performed sensitivity analysis by removing studies that appeared to dominate the data set in terms of the primary outcome and analysed the results without them.
Results
Description of studies
Results of the search
We retrieved a total of 15 hits by the search strategies employed. After screening we considered two trials relevant for inclusion, and we excluded the remaining 13 that did not meet the inclusion criteria.
Included studies
See: Characteristics of included studies
We identified two completed trials that fulfilled our eligibility criteria and these have been included in this review (CASISP 2008; CSPS II 2010). The trials included a total of 3477 participants, of which 2757 participated in CSPS II 2010.
Both studies were RCTs conducted in Asia: CASISP 2008 in China and CSPS II 2010 in Japan. The patients on average were 62 years of age and approximately two‐thirds were men.
Cilostazol was compared with aspirin in 3477 patients with a history of ischaemic stroke of arterial origin within the previous one to 6.5 months, of which 1697 patients belonged to the cilostazol arm and 1694 patients belonged to the aspirin arm in the intention‐to‐treat analysis. All medication was given orally, though dosage differed for each trial. Cilostazol was given at 100 mg twice a day and aspirin at 81 mg once a day in the CSPS II 2010 trial, and at 100 mg twice a day and 100 mg once a day respectively in the CASISP 2008 trial.
The duration of follow up varied from one year (CASISP 2008) to five years (CSPS II 2010).
Both trials recorded stroke (of all types) as a primary outcome, while CASISP 2008 also included MI and vascular death as major outcome events.
A summary of the details of each trial is described in the Characteristics of included studies table.
Excluded studies
See: Characteristics of excluded studies
We excluded 13 studies as they did not meet the eligibility criteria for this review. Of these, four studies compared cilostazol with placebo (Ahn 2001; CSPS 2000; Mitsuhashi 2004; TOSS 2005) and six other studies used a confounded comparison of interest (CATHARSIS 2009; ECLIPse 2009; Kohriyama 1994; Kikuchi 1985; Terayama 2008; TOSS II 2009). Moreover, eight of the excluded studies did not examine a clinical vascular event as the primary outcome (Ahn 2001; DAPC 2006; ECLIPse 2009; Kikuchi 1985; Kohriyama 1994; TOSS 2005; TOSS II 2009; Yasunaga 1985). In one excluded study the patient population did not present with ischaemic stroke or TIA (Suzuki 2010).
Risk of bias in included studies
See: Characteristics of included studies
Methodological quality of the two included studies (CASISP 2008; CSPS II 2010) was generally high in terms of treatment allocation and blinding. In these trials both the cilostazol and aspirin arms were well balanced for major prognostic factors at baseline (such as age, history of stroke, and use of diagnostic tools).
Allocation
Treatment allocation was randomised in both studies, and the methods of randomisation and allocation concealment were stated. In both trials (CASISP 2008; CSPS II 2010) double‐dummy concealment was performed and all pills, placebo and other made to look alike.
Blinding
Blinding was adequately addressed in each trial, with reference to participant, caregiver, investigator, and outcome assessor. The CASISP study (CASISP 2008) stated that radiologists assigned to report diagnoses for the enrolled patients were blinded to clinical data. The CSPS II trial (CSPS II 2010) ensured blinding by sealing assignment lists immediately after assignment until the designated time of unmasking.
Incomplete outcome data
Both trials addressed incomplete outcome data, reporting on participants that were lost to follow up for different reasons.
Selective reporting
All patients that were selected were accounted for and all the data recorded were presented in both studies.
Other potential sources of bias
No other potential sources of bias were noted.
In the CSPS II 2010 trial the sponsor, Otsuka Pharmaceutical Ltd, had a role in the study design, data collection, and data analysis but was not involved in the data interpretation or writing of the report.
Effects of interventions
Of the 3477 randomised participants with a history of previous stroke in the past one to 6.5 months, intention‐to‐treat analysis included 1697 patients in the cilostazol group and 1694 patients in the aspirin group.
The results given below are a culmination of those presented in the two trials (CASISP 2008; CSPS II 2010). They address the composite outcome of vascular events (stroke, MI, or vascular death) and each of these separately as specific outcome events.
Stroke, myocardial infarction (MI), or vascular death during follow up
Data indicated that cilostazol was associated with a significantly lower risk of composite outcome of vascular events (stroke, MI, or vascular death): cilostazol 115/1697 (6.77%) versus aspirin 159/1694 (9.39%), RR 0.72 (95% CI 0.57 to 0.91). This corresponds to the avoidance of about 26 vascular events (95% CI 9 to 40) per 1000 patients treated for an average of three years when compared to aspirin. It meant that for each vascular event prevented by cilostazol 39 patients (95% CI 26 to 117) needed to be treated for an average of three years (Analysis 1.1).
Analysis 1.1.
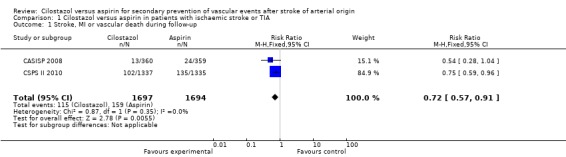
Comparison 1 Cilostazol versus aspirin in patients with ischaemic stroke or TIA, Outcome 1 Stroke, MI or vascular death during follow‐up.
Stroke (all types) during follow up
The two trials collectively showed that cilostazol significantly decreased stroke of all types compared to aspirin, and therefore it is an even more effective intervention for the secondary prevention of stroke: cilostazol 94/1697 (5.54%) versus aspirin 141/1694 (8.32%), RR 0.67 (95% CI 0.52 to 0.86). This corresponds to the avoidance of about 27 strokes (95% CI 12 to 41) per 1000 patients treated for an average of three years. For each stroke to be prevented 37 patients (95% CI 25 to 87) needed to be treated with cilostazol for an average of thee years (Analysis 1.2).
Analysis 1.2.
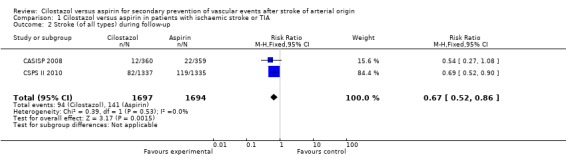
Comparison 1 Cilostazol versus aspirin in patients with ischaemic stroke or TIA, Outcome 2 Stroke (of all types) during follow‐up.
Ischaemic stroke during follow up
Both trials provided data on stroke subtypes. With respect to reduction of ischaemic stroke (fatal and non‐fatal) there was a non‐significant trend in favour of cilostazol: cilostazol 83/1697 (4.89%) versus aspirin 103/1694 (6.08%), RR 0.80 (95% CI 0.61 to 1.07) (Analysis 1.3).
Analysis 1.3.
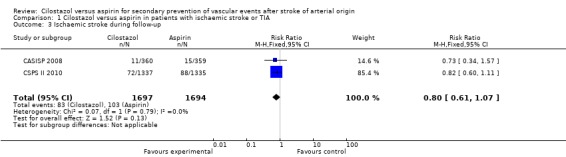
Comparison 1 Cilostazol versus aspirin in patients with ischaemic stroke or TIA, Outcome 3 Ischaemic stroke during follow‐up.
Haemorrhagic stroke (ICH) during follow up
Data pooled from both trials identified a significant trend towards a reduction of haemorrhagic stroke among patients treated with cilostazol: 9/1697 (0.53%) versus aspirin 34/1694 (2.01%), RR 0.26 (95% CI 0.13 to 0.55) (Analysis 1.4).
Analysis 1.4.
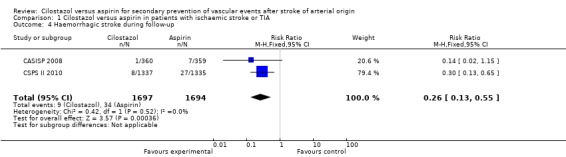
Comparison 1 Cilostazol versus aspirin in patients with ischaemic stroke or TIA, Outcome 4 Haemorrhagic stroke during follow‐up.
Myocardial infarction (MI) during follow up
Available data from both studies provided inconclusive evidence of a reduction in MI among patients treated with cilostazol: 15/1697 (0.88%) versus aspirin 13/1694 (0.77%), RR 1.15 (95% CI 0.55 to 2.41) (Analysis 1.5).
Analysis 1.5.
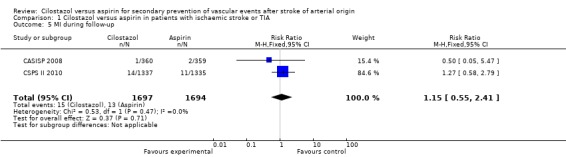
Comparison 1 Cilostazol versus aspirin in patients with ischaemic stroke or TIA, Outcome 5 MI during follow‐up.
Vascular death during follow up
Data from the CCSPS II 2010 study did not indicate a significant reduction in vascular death during follow up: cilostazol 6/1697 (0.35%) versus aspirin 5/1694 (0.30%), RR 1.20 (95% CI 0.37 to 3.92). There were no data reported on this outcome event in the CASISP study (Analysis 1.6).
Analysis 1.6.
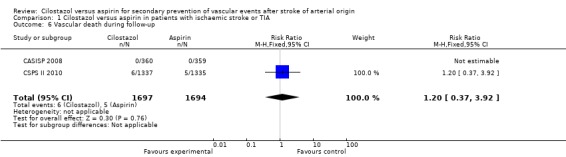
Comparison 1 Cilostazol versus aspirin in patients with ischaemic stroke or TIA, Outcome 6 Vascular death during follow‐up.
Death from any cause during follow up
Both trials reported death during the follow‐up periods. However, these results did not point towards a difference in mortality: cilostazol 16/1697 (0.94%) versus aspirin 18/1694 (1.06%), RR 0.89 (95% CI 0.45 to 1.74) (Analysis 1.7).
Analysis 1.7.
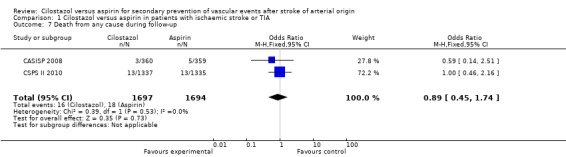
Comparison 1 Cilostazol versus aspirin in patients with ischaemic stroke or TIA, Outcome 7 Death from any cause during follow‐up.
Extracranial haemorrhage during follow up
In both trials, patients that were given cilostazol had significantly fewer extracranial haemorrhagic events than those on aspirin: cilostazol 155/1697 (9.10%) versus aspirin 209/1694 (12.3%), RR 0.74 (95% CI 0.61 to 0.90) (Analysis 1.8).
Analysis 1.8.
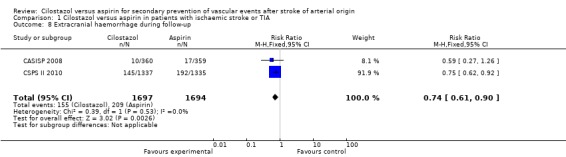
Comparison 1 Cilostazol versus aspirin in patients with ischaemic stroke or TIA, Outcome 8 Extracranial haemorrhage during follow‐up.
Gastrointestinal (GI) haemorrhage during follow up
Patients in both arms of treatment reported GI haemorrhage events: cilostazol 17/1697 (1.00%) versus aspirin 30/1694 (1.77%), RR 0.57 (95% CI 0.31 to 1.02) (Analysis 1.9). The results were statistically inconclusive. In the CASISP 2008 study more people reported GI bleeding versus fecal occult blood on treatment with cilostazol than for those on aspirin, where only one event of upper GI bleeding was reported.
Analysis 1.9.
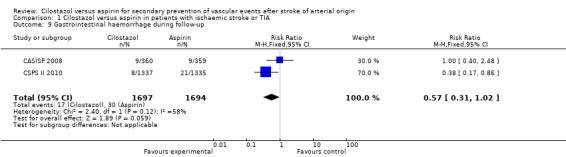
Comparison 1 Cilostazol versus aspirin in patients with ischaemic stroke or TIA, Outcome 9 Gastrointestinal haemorrhage during follow‐up.
Other outcome measures of safety during follow up
Adverse effects of the interventional treatments were addressed in both studies. There was statistically significant evidence to indicate that more adverse effects were experienced by participants enrolled in the cilostazol arm: cilostazol 977/11,879 (8.22%) versus aspirin 587/11,858 (4.95%), RR 1.66 (95% CI 1.51 to 1.83) (Analysis 1.10).
Analysis 1.10.
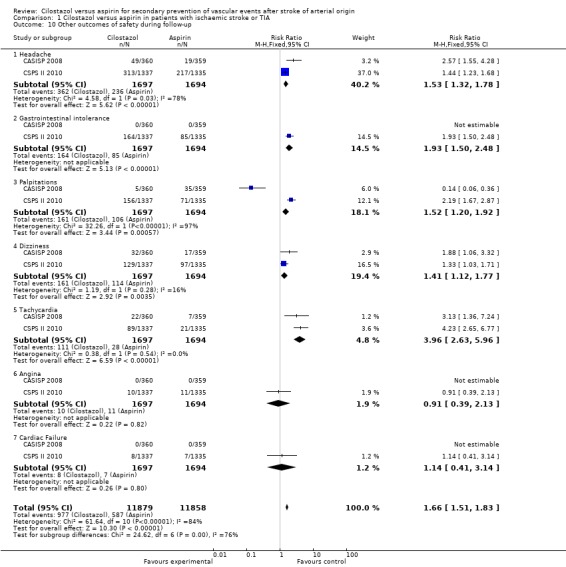
Comparison 1 Cilostazol versus aspirin in patients with ischaemic stroke or TIA, Outcome 10 Other outcomes of safety during follow‐up.
Cilostazol was significantly more likely to cause each of the following adverse effects.
1.10.1. Headaches: cilostazol events 362/1697 (21.3%) versus aspirin 236/1694 (13.9%), RR 1.53 (95% CI 1.32 to 1.78). 1.10.2. Gastrointestinal intolerance: cilostazol 164/1697 (9.66%) versus aspirin 85/1694 (5.02%), RR 1.93 (95% CI 1.50 to 2.48). 1.10.3. Palpitations: cilostazol 161/1697 (9.49%) versus aspirin 106/1694 (6.26%), RR 1.52 (95% CI 1.20 to 1.92). 1.10.4. Dizziness: cilostazol 161/1697 (9.49%) versus aspirin 114/1694 (6.73%), RR 1.41 (95% CI 1.12 to 1.77). 1.10.5. Tachycardia: cilostazol 111/1697 (6.54%) versus aspirin 28/1694 (1.65%), RR 3.96 (95% CI 2.63 to 5.96).
With respect to cardiac adverse effects, results were inconclusive. Angina was reported in both arms: cilostazol 10/1697 (0.59%) versus aspirin 11/1694 (0.65%), RR 0.91 (95% CI 0.39 to 2.13); along with cardiac failure: cilostazol 8/1697 (0.47%) versus aspirin 7/1694 (0.41%), RR 1.14 (95% CI 0.41 to 3.14).
Sensitivity analysis
We performed sensitivity analysis by excluding the trial that appeared to dominate the data set (CSPS II 2010). This did not definitively change treatment effect but did render it imprecise. Outcome events were reduced in patients given cilostazol compared with aspirin but results were no longer statistically significant: cilostazol 13/360 (3.61%) versus aspirin 24/359 (6.69%), RR 0.54 (95% CI 0.28 to 1.04) (Analysis 2.1).
Analysis 2.1.
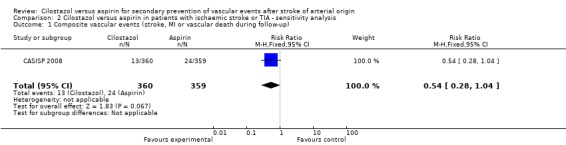
Comparison 2 Cilostazol versus aspirin in patients with ischaemic stroke or TIA ‐ sensitivity analysis, Outcome 1 Composite vascular events (stroke, MI or vascular death during follow‐up).
We did not note any statistically significant heterogeneity between the two included trials except in terms of palpitations as an outcome measure of safety, where the larger study (CSPS II 2010) dominated and changed the outcome of safety in favour of the aspirin arm.
We detected no evidence of reporting bias after analysing a funnel plot constructed for this purpose (Figure 1).
Figure 1.
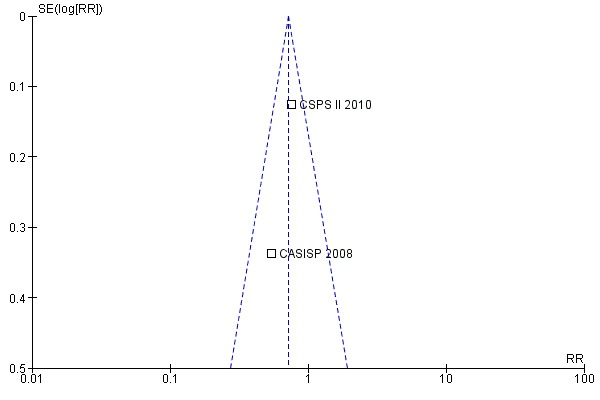
Funnel plot of comparison: 1 Cilostazol versus aspirin in patients with ischaemic stroke or TIA, outcome: 1.1 Stroke, MI or vascular death during follow‐up.
Discussion
Summary of main results
We undertook this review to determine if, compared to aspirin, cilostazol is a better alternative for secondary prevention of vascular events in patients with a previous ischaemic stroke or TIA. We analysed the available data from two randomised trials directly comparing cilostazol to aspirin. The larger trial (CSPS II 2010) contributed about 80% of the patients randomised. It studied patients at high vascular risk (those with previous TIA or ischaemic stroke of arterial origin) and primarily evaluated the outcome of stroke of all types, with ischaemic stroke, death from all causes and a composite outcome as secondary endpoints. It also examined safety endpoints in terms of all significant haemorrhagic events. The smaller study, contributing about 20% of patients (CASISP 2008), assessed the composite outcome of vascular events (stroke, MI, and vascular death) and provided adequate data on each subtype of vascular events, along with outcomes of safety.
Combining the main outcome of serious vascular events into a composite outcome of stroke, MI and vascular death not only increases the statistical power and reliability of the analysis, but also provides a more cohesive measure of effectiveness. Analysis revealed that, compared to aspirin, cilostazol is significantly more effective in preventing vascular events (stroke, MI and vascular death) and stroke of all types, in patients with a history of stroke or TIA. Cilostazol showed an overall reduction in the composite outcome of 28%, ranging between 9% to 43% (95% CI), which corresponds to comparative avoidance of 26 events (ranging between nine to as high as 40 events) per 1000 patients treated for an average of three years. Thus for each vascular event to be prevented, 39 patients needed to be treated with cilostazol for an average of three years compared with aspirin, with a wider range of between 26 and 117 patients per event (95% CI).
In patients with a previous history of stroke or TIA, the proportional benefit of cilostazol over aspirin on the outcome of strokes of all type was very similar to that of the composite outcome of vascular events. Cilostazol demonstrated a reduction of about 33% (14% to 48%) compared with aspirin, corresponding to comparative avoidance of 27 events (95% CI 12 to 41) per 1000 patients treated for an average of three years. Thus for each stroke event to be prevented, the number needed to treat (NNT) for an average of three years with cilostazol was 37 patients (95% CI 25 to 87) when compared to aspirin. Since it is known that in patients with previous history of stroke or TIA (that is patients at highest risk for subsequent vascular events) the greatest risk is of stroke, the composite outcome is bound to heavily reflect that outcome in terms of stroke of all types.
On subgroup analysis, cilostazol showed a 20% reduction in recurrence of ischaemic stroke subtypes compared with aspirin. Although this result was not statistically significant, it did indicate non‐inferiority of cilostazol compared with aspirin in terms of secondary prevention of ischaemic stroke. In relation to haemorrhagic stroke during follow up, cilostazol showed an outstanding risk reduction of 74% (95% CI 45% to 87%) compared to aspirin, thereby demonstrating its safety and tolerability in a population that is inherently at higher risk of intracerebral haemorrhage.
In terms of adverse effects, the results of the review showed that cilostazol had a significantly higher adverse effect profile than aspirin, in terms of all other outcomes of safety including headache, gastrointestinal intolerance, palpitations, dizziness and tachycardia. In both trials (CASISP 2008; CSPS II 2010) it was noted that more recruited patients discontinued cilostazol compared with aspirin as a consequence of adverse drug reactions. Results from the CSPS II trial (CSPS II 2010) were inconclusive in terms of cardiac adverse effects, namely angina and cardiac failure, while CASISP (CASISP 2008) did not note any such events.
In safety analyses, aspirin caused more intracranial haemorrhage, extracranial haemorrhage and GI haemorrhage but, evaluated as separate outcomes, only extracranial haemorrhage was significantly higher in patients on aspirin compared with cilostazol. All of these outcome events were addressed specifically in both included trials. The CASISP 2008 trial reported symptomatic intracerebral haemorrhage along with two events of asymptomatic intracerebral haemorrhage with aspirin that were included in our analysis. Observational studies conclude that cilostazol shows no evidence of an increase in any bleeding abnormality (CSPS 2000). Therefore, it can be stated with a certain degree of reliability that cilostazol is associated with a lesser risk of bleeding events than aspirin.
Overall completeness and applicability of evidence
The two studies included in the review were double‐blind randomised controlled trials that reported relevant outcome data. In CSPS II 2010 outcomes were assessed via clinical record review carried out by an independent data monitoring committee. These patients were regularly reviewed at six‐month intervals for safety, adherence, and drug tolerability. The participants were all Asians and the maximum age at enrolment was 79 years. Importantly, the included strokes were all atherothrombotic (large vessel atherosclerosis and lacunes) in origin, and there were no patients with cardioembolic strokes recruited in these trials. A relevant patient exclusion criterion was the absence of associated cardiovascular disease.
These studies show us that in the above populations and settings, cilostazol is relatively superior to aspirin in terms of a composite outcome of stroke, MI, and vascular death in Asian patients with stroke of arterial origin. Since Asians are at higher risk of intracerebral haemorrhage, and cilostazol‐treated Asians had significantly fewer intracerebral haemorrhages than their aspirin‐treated counterparts, cilostazol is a safer option in this setting.
With regard to patients with concomitant cardiovascular disease, the outcome of safety in terms of cardiac adverse effects of cilostazol compared with aspirin cannot be assessed in this review since patients with cardiovascular disease were excluded in both trials. Hence, it is clinically important to exclude cardiovascular disease in stroke patients prior to initiating cilostazol.
No comparator groups were available to compare for subgroup analysis. Stroke studies from other ethnic populations, including Caucasians, are needed to provide general applicability.
The dose used in the CASISP 2008 trial was at 100 mg twice daily and the same dose of 100 mg twice daily was used in CSPS II 2010. Studies show that lower doses of cilostazol are associated with fewer headaches requiring discontinuation, where 3.7% of patients on 100 mg twice daily required hospitalisation compared to1.3% on 50 mg twice daily (Robless 2008). Thus, to reduce the side‐effect profile of cilostazol, it could be recommended to administer cilostazol in incremental doses starting from a minimum of 50 mg twice daily.
Cilostazol is more expensive than aspirin. Each cilostazol tablet costs 10 times more than that of aspirin, and bearing in mind that cilostazol requires double dosing, this makes each dose of cilostazol 20 times more costly compared with aspirin. To prevent one extra vascular event, 39 patients (95% CI 26 to 117 patients) need to be treated with cilostazol for a period of three years compared to aspirin. Whether this justifies prolonged treatment in resource‐strapped settings needs further cost‐benefit analysis.
Quality of the evidence
Overall the included evidence is based on well‐designed trials.
Potential biases in the review process
The potential bias is that the data are restricted to Asians.
Agreements and disagreements with other studies or reviews
This review is in line with the general studies and reviews on this topic.
Authors' conclusions
Aspirin is a well established monotherapy for the secondary prevention of stroke. For cilostazol to be on a par with aspirin in terms of clinical practice, it needs to not only be considerably more effective than aspirin in the prevention of serious vascular events after stroke but also to confer a tangible benefit in terms of safety and cost.
Current data indicate that cilostazol‐treated patients experience a greater proportional reduction in risk of composite vascular events after stroke, including recurrent stroke, compared with aspirin. In terms of safety, cilostazol is clearly associated with fewer major bleeding events than aspirin. For long‐term use, physicians must be aware that in the Asian stroke population cardiovascular disease must be excluded. In addition, physicians must be familiar with the minor adverse‐effect profile of cilostazol. Moreover, the present cost of cilostazol is too high compared with aspirin for it to be considered for prolonged use in secondary prevention following stroke in many resource‐poor Asian settings.
Further randomised trials with intention‐to‐treat design are required in diverse populations with ischaemic stroke to determine whether the benefit observed in reduction of vascular events after stroke is global or region specific.
Acknowledgements
We would like to thank the following individuals for their time and contribution to this review: Hazel Fraser (Cochrane Stroke Group Managing Editor), Dr Peter Sandercock, Dr Graeme Hankey and Dr Ale Algra (Cochrane Stroke Group Editors); and for technical assistance, Brenda Thomas. Additionally we would like to thank Afshan Dhanani, secretarial assistant in the Division of Neurology, and the entire Neurology staff for their support and provision of protected time to complete this review.
Appendices
Appendix 1. MEDLINE search strategy
We used the following search strategy for MEDLINE and adapted it for CENTRAL.
MEDLINE (Ovid)
1. cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or exp brain ischemia/ or carotid artery diseases/ or carotid artery thrombosis/ or intracranial arterial diseases/ or cerebral arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp stroke/ 2. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. tia$1.tw. 5. 1 or 2 or 3 or 4 6. (cilostazol or pletal or pletaal or OPC‐13013 or OPC 13013 or OPC‐21 or OPC 21).tw. 7. Aspirin/ 8. (aspirin or acetylsalicylic acid or acetyl salicylic acid or acetosalicylic acid).tw. 9. 7 or 8 10. 5 and 6 and 9 11. limit 10 to humans
Appendix 2. EMBASE search strategy
We used the following search strategy for EMBASE.
EMBASE (Ovid)
1. cerebrovascular disease/ or cerebral artery disease/ or cerebrovascular accident/ or stroke/ or vertebrobasilar insufficiency/ or carotid artery disease/ or exp carotid artery obstruction/ or exp brain infarction/ or exp brain ischemia/ or exp occlusive cerebrovascular disease/ 2. stroke patient/ or stroke unit/ 3. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw. 4. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 5. tia$1.tw. 6. 1 or 2 or 3 or 4 or 5 7. cilostazol/ 8. (cilostazol or pletal or pletaal or OPC‐13013 or OPC 13013 or OPC‐21 or OPC 21).tw. 9. 7 or 8 10. acetylsalicylic acid/ 11. (aspirin or acetylsalicylic acid or acetyl salicylic acid or acetosalicylic acid).tw. 12. 10 or 11 13. 6 and 9 and 12 14. limit 13 to human
Data and analyses
Comparison 1.
Cilostazol versus aspirin in patients with ischaemic stroke or TIA
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stroke, MI or vascular death during follow‐up | 2 | 3391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.91] |
| 2 Stroke (of all types) during follow‐up | 2 | 3391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.52, 0.86] |
| 3 Ischaemic stroke during follow‐up | 2 | 3391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.61, 1.07] |
| 4 Haemorrhagic stroke during follow‐up | 2 | 3391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.55] |
| 5 MI during follow‐up | 2 | 3391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.55, 2.41] |
| 6 Vascular death during follow‐up | 2 | 3391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.37, 3.92] |
| 7 Death from any cause during follow‐up | 2 | 3391 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.45, 1.74] |
| 8 Extracranial haemorrhage during follow‐up | 2 | 3391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.61, 0.90] |
| 9 Gastrointestinal haemorrhage during follow‐up | 2 | 3391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.31, 1.02] |
| 10 Other outcomes of safety during follow‐up | 2 | 23737 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.51, 1.83] |
| 10.1 Headache | 2 | 3391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.32, 1.78] |
| 10.2 Gastrointestinal intolerance | 2 | 3391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.50, 2.48] |
| 10.3 Palpitations | 2 | 3391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.20, 1.92] |
| 10.4 Dizziness | 2 | 3391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.12, 1.77] |
| 10.5 Tachycardia | 2 | 3391 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.96 [2.63, 5.96] |
| 10.6 Angina | 2 | 3391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.39, 2.13] |
| 10.7 Cardiac Failure | 2 | 3391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.41, 3.14] |
Comparison 2.
Cilostazol versus aspirin in patients with ischaemic stroke or TIA ‐ sensitivity analysis
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Composite vascular events (stroke, MI or vascular death during follow‐up) | 1 | 719 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.28, 1.04] |
What's new
| Date | Event | Description |
|---|---|---|
| 30 August 2011 | Amended | The dose of cilostazol given in the CASISP trial has been corrected in the Results section of the text. |
History
Protocol first published: Issue 4, 2009 Review first published: Issue 1, 2011
| Date | Event | Description |
|---|---|---|
| 15 July 2011 | Amended | The conclusions are unchanged. The erratum for CASISP has been incorporated and addressed. |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | R: computer‐based stratified block randomisation on basis of pre‐established scheme, stratified according to each site, aspirin‐controlled trial C: double dummy: aspirin and cilostazol pills made to look the same B: double‐blind: patients and clinicians, radiologists blinded to clinical data | |
| Participants | Multicentre, China 720 patients randomised (2004 to 2005) Mean age: 60.2 years (18 to 75 years) Sex: 495 male, 225 female Inclusion criteria: cerebral infarction 1 to 6 months before entry, confirmed with neuroimaging, modified Rankin scale score of less than 4 at enrolment Exclusion criteria: history of intracranial haemorrhage, stroke secondary to cardiogenic embolism, serious damage of motorial function, dementia, serious complications or comorbidity (uncontrolled accelerated type of hypertension, BP > 180/120 mmHg, diabetic acidosis, heart failure, renal failure, hepatocirrhosis, malignant tumour), contraindication of cilostazol and aspirin, need for co‐medication of other antiplatelet agents, anticoagulants or fibrinolytic drugs, active peptic ulcer, pregnancy or breast feeding Patients with hypertension or high lipid concentrations were given antihypertensive drugs or statins, respectively, and were seen by doctors every month during the follow‐up period. Liver and kidney function, electrocardiogram, and lipid profiles were monitored in all patients every 3 months | |
| Interventions | Rx: cilostazol 100 mg twice daily oral versus aspirin 100 mg once daily oral Duration of treatment: 12 months | |
| Outcomes | Primary outcomes: stroke recurrence (ischaemic, intracerebral haemorrhage, subarachnoid haemorrhage) Secondary outcomes: > 1 stroke, new myocardial infarction, transient ischaemic attack, vascular event (pulmonary embolism, deep vein thrombosis, peripheral arterial occlusive disease), death from vascular causes, death from all causes | |
| Notes | 360 participants in aspirin arm intention‐to‐treat analysis and 359 participants in cilostazol arm Compliance on aspirin: 47 discontinued, 3 died; reasons for withdrawal: 25 with adverse effects, 8 did not follow protocol, 6 lost to follow up, 8 withdrew due to other reasons, 3 died (1 myocardial infarction, 1 unknown, 1 drowned). Cilostazol: 35 discontinued, 5 died; reasons for withdrawal: 15 with adverse effects, 3 lack of effect, 7 did not follow protocol, 3 lost to follow up, 7 due to other causes, 5 died (2 myocardial infarction, 1 liver cancer, 1 pancreatitis, 1 suicide) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Double‐dummy, all pills made to look the same |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind (patients, clinicians) and radiologists reviewing reports |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients lost to follow up accounted for |
| Selective reporting (reporting bias) | Low risk | All selected patients accounted for |
| Other bias | Low risk | Free of detection bias, appropriate analysis |
| Methods | R: computer‐generated randomisation sequence by means of a dynamic balancing method with stratification by age, sex, and study institution using patient information obtained at registration. Randomization number was assigned to every drug pack. Patients were given treatment number identical to the numbered drug pack C: double dummy method. Parallel assignment, with placebo tablets identical to drug that patient was not assigned. The person responsible for drug allocation sealed assignment list immediately after assignment, and kept it sealed until designated point of unmasking B: all participants, study personnel, investigators, and sponsors were masked to treatment allocation | |
| Participants | 279 centres in Japan (2003 to 2006) 2757 patients Mean age: 63.5 years (20 to 80 years) Sex: 71.7% men Inclusion criteria: cerebral infarction within 26 weeks prior to enrolment and whose symptoms had remained stable, confirmed by neuroimaging, patients with no cardiac diseases that may be associated with cardiogenic cerebral embolism (mitral stenosis, prosthetic heart valve, endocarditis, myocardial infarction within 6 weeks after occurrence, ventricular aneurysm, endocardial thrombosis, mitral valve prolapse, atrial fibrillation, sick sinus syndrome, idiopathic cardiomyopathy, and patent foramen ovale), patients without asymptomatic cerebral infarction, those who had not undergone or scheduled for percutaneous transluminal angioplasty or revascularization for the treatment of cerebral infarction), without severe disturbances/impairment following cerebral infarction Exclusion criteria: patients with haemorrhage or bleeding tendency (haemophilia, capillary fragility, intracranial haemorrhage, haemorrhage in the digestive tract, haemorrhage in the urinary tract, haemoptysis, and haemorrhage in the vitreous body, pregnant, possibly pregnant, or nursing women, ischaemic heart failure, peptic ulcer, severe blood disorders, severe hepatic or renal disease, malignant neoplasm or patients who have received any therapy for malignant neoplasm within 5 years prior, history of hypersensitivity to salicylic acid formulations or ingredients of cilostazol tablets, aspirin asthma (asthma attacks induced by non‐steroidal anti‐inflammatory analgesic agents) or a history of aspirin asthma, treatment with ticlopidine hydrochloride | |
| Interventions | Cilostazol oral tablet, 100 mg twice a day and placebo of aspirin once daily versus aspirin oral tablet, 81 mg once a day and placebo of cilostazol twice a day Duration of treatment: 1 to 5 years | |
| Outcomes | Primary outcome: occurrence of stroke (cerebral infarction, cerebral haemorrhage, or subarachnoid haemorrhage) Secondary outcomes: first recurrence of cerebral infarction; ischaemic cerebrovascular events including cerebral infarction or transient ischaemic attack; death from any cause; and composite of completed stroke (cerebral infarction, cerebral haemorrhage, or subarachnoid haemorrhage), transient ischaemic attack, angina pectoris, myocardial infarction, heart failure, or haemorrhage requiring hospital admission (excluding cerebral haemorrhage and subarachnoid haemorrhage) | |
| Notes | Of the 2757 participants enrolled and randomly allocated, 23 in the cilostazol arm and 18 in the aspirin arm did not receive treatment due to withdrawal, recurrence of cerebral infarction, or met exclusion criteria Overall, 2716 participants received study drug treatment, of which 19 were ineligible for analysis in the cilostazol arm and 25 were ineligible in the aspirin arm Of these, a further 457 participants discontinued drug in the cilostazol arm, of which 267 were due to adverse drug reactions In the aspirin arm 336 participants discontinued treatment, of which 166 were due to adverse drug reactions Eventually 2672 were included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Double‐dummy method and parallel assignment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinding (participant, caregiver, investigator, sponsor) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients lost to follow up accounted for |
| Selective reporting (reporting bias) | Low risk | All selected patients accounted for |
| Other bias | Low risk | All outcomes addressed |
B: blinding C: control R: randomisation Rx: treatment
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahn 2001 | Cilostazol compared with placebo, not aspirin. Patient population not selected on basis of history of ischaemic stroke or TIA (diabetes type II). Outcome measure (intima‐media thickness (IMT)) was not a vascular event, it is an intermediate biologic phenotype |
| CATHARSIS 2009 | Confounded comparison of interest (aspirin versus aspirin + cilostazol). Secondary outcome measure (stenosis progression) was not a clinical vascular event, it is an intermediate biologic phenotype |
| CSPS 2000 | Cilostazol compared with placebo, not aspirin |
| DAPC 2006 | Outcome measure was not a vascular event (IMT changes), patient population not selected on basis of history of stroke or TIA (diabetes type II and atherosclerosis) |
| ECLIPse 2009 | Confounded comparison of interest (aspirin + placebo versus aspirin + cilostazol), primary outcome not a clinical vascular event (pulsatility indices of transcranial doppler) |
| Kikuchi 1985 | Compares ticlopidine versus aspirin versus cilostazol. Outcome measure was not a clinical vascular event (platelet aggregation) |
| Kohriyama 1994 | Compares ticlopidine versus ticlopidine + aspirin versus cilostazol. Primary outcome measure not a clinical vascular event (platelet aggregation, coagulation and fibrinolysis) |
| Mitsuhashi 2004 | Patient population not selected for stroke (diabetes type II). Cilostazol compared with placebo, not aspirin |
| Suzuki 2010 | Patient population not selected for ischaemic stroke or TIA (subarachnoid haemorrhage) |
| Terayama 2008 | Confounded for comparison of interest (aspirin versus aspirin + cilostazol) |
| TOSS 2005 | Cilostazol compared with placebo, not aspirin. Primary outcome measure not a clinical vascular event (progression of symptomatic intracranial atherosclerosis) |
| TOSS II 2009 | Confounded comparison of interest (cilostazol+aspirin versus clopidogrel+aspirin). Primary outcome measure was not a clinical vascular event (progression of symptomatic intracranial atherosclerosis) |
| Yasunaga 1985 | Not a randomised, double‐blind trial. Outcome measure not a clinical vascular event (platelet aggregation) |
IMT: intima media thickness TIA: transient ischaemic attack
Contributions of authors
AKK conceived and designed the review, reviewed the studies, assisted in review writing and wrote the protocol. IN performed review of studies, data analysis and review writing, and assisted in protocol writing. MRH assisted in the protocol development stage. BAK assisted in review of relevant studies.
Sources of support
Internal sources
The Aga Khan University Hospital, Pakistan.
External sources
No sources of support supplied
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
- Huang Y, Cheng Y, Wu J, Li Y, Xu E, Hong Z. Cilostazol as an alternative to aspirin after ischaemic stroke: a randomised, double‐blind, pilot study. Lancet Neurology 2008;7:494‐9. [DOI] [PubMed] [Google Scholar]; Huang Y, Cheng Y, Wu J, Li Y, Xu E, Hong Z, et al. Cilostazol as an alternative to aspirin after ischaemic stroke: a randomised, double‐blind, pilot study. Lancet Neurology 2008;7(8):675. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, et al. Cilostazol for prevention of secondary stroke (CSPS 2):an aspirin‐controlled, double‐blind, randomised non‐inferiority trial. Lancet Neurology 2010;9(10):959‐68. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Ahn CW, Lee HC, Park SW, Song YD, Huh KB, Oh SJ, et al. Decrease in carotid intima media thickness after 1 year of cilostazol treatment in patients with type 2 diabetes mellitus. Diabetes Research and Clinical Practice 2001;52:45‐53. [DOI] [PubMed] [Google Scholar]
- Uchiyama S, Sakai N, Kimura Y, Ezura M, Okada Y, Takagi M, et al. Cilostazol‐aspirin therapy against recurrent stroke with intracranial artery stenosis (CATHARSIS): current status. Proceedings of the 18th European Stroke Conference 2009. Sweden, Stockholm, 26‐29 May 2009. [Google Scholar]
- Gotoh F, Tohgi H, Hirai S, Terashi A, Fukuuchi Y, Otomo E, et al. Cilostazol stroke prevention study: a placebo‐controlled double‐blind trial for secondary prevention of cerebral infarction. Journal of Stroke and Cerebrovascular Diseases 2000;9(4):147‐57. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Kim YS, Kawamori R. Rationale and protocol of a trial for prevention of diabetic atherosclerosis by using antiplatelet drugs: Study of Diabetic Atherosclerosis Prevention by Cilostazol (DAPC study). Cardiovascular Diabetology 2006;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Han SW, Lee JY, Kim GS, Lee JH, Kim OJ, et al. Study for the multi‐centre placebo‐controlled double‐blind clinical trial for the evaluation of the effect of cilostazol on pulsatility index of transcranial doppler in the acute lacunar infarction patients. Proceedings of the International Stroke Conference 2009. USA, San Diego, California, 18‐20 February 2009. [Google Scholar]
- Ikeda Y, Kikuchi M, Murakami H, Satoh K, Murata M, Watanabe K, et al. Comparison of the inhibitory effects of cilostazol, acetylsalicylic acid and ticlopidine on platelet functions ex vivo. Randomized, double‐blind cross‐over study. Arzneimittel‐Forschung 1987;37:563‐6. [PubMed] [Google Scholar]
- Kohriyama T, Tanaka E, Katayama S, Yamamura Y, Nakamura S. Antiplatelet therapy in patients with cerebral thrombosis at the chronic phase ‐ assessment of its effect on coagulation and fibrinolytic parameters. Rinsho Shinkeigaku Clinical Neurology (Tokyo) 1994;34(8):771‐6. [PubMed] [Google Scholar]
- Mitsuhashi N, Tanaka Y, Kubo S, Ogawa S, Hayashi C, Uchino H, et al. Effect of cilostazol, a phosphodiesterase inhibitor, on carotid IMT in Japanese type 2 diabetic patients. Endocrine Journal 2004;51(6):545‐50. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Sayama T, Ohta M, Nakamura T, Onaka S, Inoue T, et al. Cilostazol improves outcome after subarachnoid hemorrhage. Stroke 2010;41(4):e236 (Abst.129). [DOI] [PubMed] [Google Scholar]
- Terayama Y, Ishibashi Y, Suzuki M. Aspirin and cilostazol vs aspirin alone for the prevention of progressing stroke. International Journal of Stroke 2008;3 Suppl 1:315. [Google Scholar]
- Cho Y‐J, Koo J‐S, Bae H‐J, Lee Y‐S, Hong K‐S. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis. The multicenter double‐blind placebo‐controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke 2005;36:782‐6. [DOI] [PubMed] [Google Scholar]
- Kwon SU, Kang DW, Park JM, Cho YJ, Hong KS, Yu KH, et al. Trial of efficacy and safety of cilostazol on the progression of symptomatic intracranial stenosis comparing clopidogrel: trial of cilostazol in symptomatic intracranial stenosis‐2 (TOSS‐2). Cerebrovascular Diseases 2009;27 Suppl 6:10‐1 (Abst. 5). [Google Scholar]
- Yasunaga K, Mase K. Clinical effects of oral cilostazol on suppression of platelet function in patients with cerebrovascular disease. Arzneimittel‐Forschung 1985;35(II 7a):1186‐8. [PubMed] [Google Scholar]
Additional references
- Abramson SB, Weissmann G. The mechanisms of action of nonsteroidal antiinflammatory drugs. Arthritis and Rheumatism 1989;32(1):1‐9. [DOI] [PubMed] [Google Scholar]
- Algra A, Gijn J. Aspirin at any dose above 30 mg offers only modest protection after cerebral ischaemia. Journal of Neurology, Neurosurgery and Psychiatry 1996;60(2):197‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke GL, Evans GW, Riley WA, Sharrett AR, Howard G, Barnes RW, et al. Arterial wall thickness is associated with prevalent cardiovascular disease in middle‐aged adults. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke 1995;26(3):386‐91. [DOI] [PubMed] [Google Scholar]
- Elam MB, Heckman J, Crouse JR, Hunninghake DB, Herd JA, Davidson M, et al. Effect of the novel antiplatelet agent cilostazol on plasma lipoproteins in patients with intermittent claudication. Arteriosclerosis, Thrombosis, and Vascular Biology 1998;18(12):1942‐7. [DOI] [PubMed] [Google Scholar]
- Feldmann E, Daneault N, Kwan E, Ho KJ, Pessin MS, Langenberg P, et al. Chinese‐white differences in the distribution of occlusive cerebrovascular disease. Neurology 1990;40(10):1541‐5. [DOI] [PubMed] [Google Scholar]
- Huang Y, Cheng Y, Wu J, Li Y, Xu E, Hong Z, et al. Cilostazol as an alternative to aspirin after ischaemic stroke: a randomised, double‐blind, pilot study. Lancet Neurology 2008;7(6):494‐9. [DOI] [PubMed] [Google Scholar]
- Ikeda Y. Antiplatelet therapy using cilostazol, a specific PDE3 inhibitor. Thrombosis and Haemostasis 1999;82(2):435‐8. [PubMed] [Google Scholar]
- Inoue T, Sohma R, Morooka S. Cilostazol inhibits the expression of activation‐dependent membrane surface glycoprotein on the surface of platelets stimulated in vitro. Thrombosis Research 1999;93(3):137‐43. [DOI] [PubMed] [Google Scholar]
- Kayanoki Y, Che W, Kawata S, Matsuzawa Y, Higashiyama S, Taniguchi N. The effect of cilostazol, a cyclic nucleotide phosphodiesterase III inhibitor, on heparin‐binding EGF‐like growth factor expression in macrophages and vascular smooth muscle cells. Biochemical and Biophysical Research Communications 1997;238(2):478‐81. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fong M, Cone J, Wang S, Yoshitake M, Kambayashi J. Inhibition of adenosine uptake and augmentation of ischaemia‐induced increase of interstitial adenosine by cilostazol, an agent to treat intermittent claudication. Journal of Cardiovascular Pharmacology 2000;36(3):351‐60. [DOI] [PubMed] [Google Scholar]
- Liu M, Wu B, Wang WZ, Lee LM, Zhang SH, Kong LZ. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurology 2007;6(5):456‐64. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367(9524):1747‐57. [DOI] [PubMed] [Google Scholar]
- Matsumoto M. Cilostazol in secondary prevention of stroke: impact of the Cilostazol Stroke Prevention Study. Atherosclerosis Supplements 2005;6(4):33‐40. [DOI] [PubMed] [Google Scholar]
- Minami N, Suzuki Y, Yamamoto M, Kihira H, Imai E, Wada H, et al. Inhibition of shear stress‐induced platelet aggregation by cilostazol, a specific inhibitor of cGMP‐inhibited phosphodiesterase, in vitro and ex vivo. Life Sciences 1997;61(25):383‐9. [DOI] [PubMed] [Google Scholar]
- Nishio Y, Kashiwagi A, Takahara N, Hidaka H, Kikkawa R. Cilostazol, a cAMP phosphodiesterase inhibitor, attenuates the production of monocyte chemoattractant protein‐1 in response to tumour necrosis factor‐alpha in vascular endothelial cells. Hormone and Metabolic Research 1997;29(10):491‐5. [DOI] [PubMed] [Google Scholar]
- Robless P, Mikhailidis DP, Stansby GP. Cilostazol for peripheral arterial disease. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD003748.pub3] [DOI] [PubMed] [Google Scholar]
- Rother J, Alberts MJ, Touze E, Mas JL, Hill MD, Michel P, et al. Risk factor profile and management of cerebrovascular patients in the REACH Registry. Cerebrovascular Diseases 2008;25(4):366‐74. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Gotoh F, Tohgi H, Hirai S, Terashi A, Fukuuchi Y, et al. Antiplatelet cilostazol is beneficial in diabetic and/or hypertensive ischaemic stroke patients. Subgroup analysis of the cilostazol stroke prevention study. Cerebrovascular Diseases 2008;26(1):63‐70. [DOI] [PubMed] [Google Scholar]
- Wong KS, Li H, Lam WW, Chan YL, Kay R. Progression of middle cerebral artery occlusive disease and its relationship with further vascular events after stroke. Stroke 2002;33(2):532‐6. [DOI] [PubMed] [Google Scholar]


