Abstract
Background
Postoperative vitreous cavity haemorrhage (POVCH) is a significant complication following vitrectomy for proliferative diabetic retinopathy (PDR). It delays visual recovery and can make further treatment difficult if the view of the fundus is significantly obscured. A number of interventions to reduce the incidence of POVCH have been proposed, including the perioperative use of anti‐vascular endothelial growth factor (anti‐VEGF). Anti‐VEGFs reduce vascular proliferation and the vascularity of neovascular tissue, which is often the source of bleeding following vitrectomy.
Objectives
This updated review aimed to summarise the effects of anti‐VEGF use to reduce the occurrence of POVCH after vitrectomy surgery for PDR.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2015, Issue 4), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to May 2015), PubMed (January 1966 to May 2015), EMBASE (January 1980 to May 2015), Latin American and Caribbean Health Sciences (LILACS) (January 1982 to May 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 26 May 2015.
Selection criteria
We included all randomised controlled trials (RCTs) and quasi‐RCTs that looked at the use of anti‐VEGFs and the incidence of POVCH in people undergoing vitrectomy for PDR.
Data collection and analysis
Both review authors independently assessed and extracted the data. We used standard methodological procedures expected by Cochrane.
The primary outcomes of the review were the incidence of early and late POVCH following perioperative anti‐VEGF administration. Secondary outcomes included best‐corrected visual acuity at six months following surgery, the incidence of vitreous cavity washout or revision vitrectomy at six months, adverse effects of intervention (cataract, iris rubeosis and rubeotic glaucoma, retinal detachment, increased inflammation and systemic side effects), quality of life measures performed at least six months following vitrectomy, and density of POVCH.
Main results
The current review included 12 RCTs that looked at the pre‐ or intraoperative use of intravitreal bevacizumab to prevent postoperative vitreous haemorrhage during pars plana vitrectomy for complications of PDR. The studies were conducted in a variety of countries (three from Iran, two from Italy, two from Egypt, and the remaining from South Korea, USA, Mexico, Pakistan, and Japan). The inclusion criteria for entry into the studies were standard complications of proliferative retinopathy: non‐clearing vitreous haemorrhage, tractional retinal detachment involving the macula, or combined tractional rhegmatogenous detachment. The included studies randomised a total of 654 eyes. The average age of the participants was 54 years.
We identified methodological issues in all included studies. Risk of bias was highest for masking of participants and investigators (four studies were an 'open label' design), and a number of studies were unclear when describing randomisation methods and sequence allocation.
Participants receiving intravitreal bevacizumab in addition to pars plana vitrectomy were less likely to experience early POVCH (grade 2) compared to people undergoing pars plana vitrectomy alone (risk ratio (RR) 0.28, 95% confidence interval (CI) 0.08 to 0.96, 2 studies, 144 eyes, high‐quality evidence). This corresponds to an absolute effect of 130 fewer people (95% CI 167 fewer to 7 fewer) with early POVCH per 1000 people when treated with intravitreal bevacizumab. We saw similar results for all grades of POVCH (RR 0.35, 95% CI 0.23 to 0.53, 9 studies, 512 eyes) and when excluding cases where assessment of outcome was impossible due to presence of silicone oil (RR 0.34, 95% CI 0.19 to 0.60, 6 studies, 302 eyes).
The effect of pre‐ or intraoperative intravitreal bevacizumab on the incidence of late postoperative haemorrhage was uncertain (RR 0.72, 95% CI 0.30 to 1.72, 3 studies, 196 eyes, low‐quality evidence). The absolute effect was 55 fewer people (95% CI 138 fewer to 143 more) with late POVCH per 1000 people when treated with intravitreal bevacizumab. This outcome was rarer and was only reported in a few studies. We are currently unable to provide an estimate of the effect of intravitreal bevacizumab on postoperative visual acuity due to significant study heterogeneity.
No local or systemic complications of intravitreal bevacizumab were reported by the RCTs. The risk of postoperative retinal detachment was lower in the participants treated with pre‐ or intraoperative bevacizumab (RR 0.46, 95% CI 0.19 to 1.08, 7 studies, 372 participants, low‐quality evidence); the absolute effect was 49 fewer people (95% CI:73 fewer to 8 more) with postoperative retinal detachment per 1000 people when treated with intravitreal bevacizumab.
Authors' conclusions
The use of pre‐ or intraoperative bevacizumab lowers the incidence of early POVCH. The reported complications from its use appear to be low. Futher randomised studies that look at other anti‐VEGF medications are ongoing and will strengthen the current review findings, giving both surgeons and patients evidence to guide treatment choices in the management of proliferative retinopathy.
Plain language summary
Anti‐VEGF for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy
Review question Does anti‐vascular endothelial growth factor (anti‐VEGF) reduce the occurrence of posterior vitreous cavity haemorrhage (POVCH) after vitrectomy surgery for proliferative diabetic retinopathy?
Background POVCH is a significant complication following vitrectomy (removal of the vitreous gel from the posterior chamber of the eye) for the treatment of proliferative retinopathy (the growth of abnormal blood vessels from the retina, a layer of tissue at the back of the eye), occurring in approximately 30% of cases. POVCH has two main forms: early, when haemorrhage (bleeding) is present in the first few postoperative days, and late, when haemorrhage occurs a number of months after surgery. The presence of POVCH delays visual recovery, can lead to elevated pressure within the eye, and can make further treatment for diabetic retinopathy difficult. Ten per cent of patients require revision surgery, which has significant implications for resources, time, and cost. The use of anti‐VEGF before surgery (preoperatively) has been proposed as an intervention to reduce the incidence of POVCH.
Search date The evidence is up to date to May 2015.
Key results The electronic database searches identified 12 randomised controlled trials that met the inclusion criteria. We performed a number of analyses that suggest that pre‐ or intraoperative anti‐VEGF may reduce the incidence of early POVCH. The effect on late POVCH was unclear. We are currently unable to comment on the effect of anti‐VEGF treatment on postoperative visual acuity due to significant differences in the studies' design and outcomes.
The risk of adverse events when using preoperative anti‐VEGF appears small.
Quality of evidence We are reasonably certain that anti‐VEGF reduces the incidence of early POVCH (high‐quality evidence) but less certain about its effects on late POVCH and risk of adverse effects.
Summary of findings
for the main comparison.
| Anti‐VEGF compared with control for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy | ||||||
|
Patient or population: People with proliferative diabetic retinopathy undergoing vitrectomy Settings: Hospital Intervention: Anti‐VEGF Comparison: No anti‐VEGF | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Anti‐VEGF | |||||
|
Early POVCH 4 weeks |
182 per 1000 | 52 per 1000 (15 to 175) | RR 0.28 (0.08 to 0.96) | 144 (2) | ⊕⊕⊕⊕ high | POVCH grade 2 or worse |
|
Early POVCH 4 weeks |
310 per 1000 |
109 per 1000 (71 to 164) |
RR 0.35 (0.23 to 0.53) | 512 (9) |
⊕⊕⊕⊕ high | POVCH all grades |
|
Late POVCH 6 months |
198 per 1000 | 143 per 1000 (60 to 341) | RR 0.72 (0.30 to 1.72) | 196 (3) | ⊕⊕⊝⊝ low1 |
POVCH all grades |
|
Visual acuity logMAR acuity 6 months |
The mean visual acuity ranged across control groups from 0.51 to 2.07 logMAR | Due to substantial heterogeneity between studies (I2 = 88%), we could not estimate a treatment effect and hence a corresponding risk | Mean differences ranged from 1.31 logMAR in favour of anti‐VEGF to 0.14 logMAR in favour of control | 289 (5) |
We did not GRADE this as we do not have an estimate of effect | |
|
Vitreous cavity washout 6 months |
70 per 1000 | 15 per 1000 (3 to 74) | RR 0.19 (0.06 to 0.67) | 291 (5) | ⊕⊕⊝⊝ low2 | |
|
Adverse effects: retinal detachment at any time |
91 per 1000 | 42 per 1000 (18 to 99) | RR 0.46 (0.19 to 1.08) | 372 (7) |
⊕⊕⊝⊝ low3 | Other adverse events considered included: raised intraocular pressure, rubeosis, neovascular glaucoma, cataract progression, systemic adverse events. Not much data available/reported |
|
Quality of life 6 months |
Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RR: Risk ratio; POVCH: Postoperative vitreous cavity haemorrhage; VEGF: Vascular endothelial growth factor. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded for imprecision (confidence intervals include 1 (no effect)) and indirectness (the analysis includes all grades of POVCH, but we planned to consider only grade 2 or worse).
2Downgraded for risk of bias (none of the trials contributing to this analysis reported methods of sequence generation and allocation concealment in enough detail to judge risk of bias) and imprecision (confidence intervals include 1 (no effect)).
3Downgraded for risk of bias (all except one of the trials contributing to this analysis reported methods of sequence generation and allocation concealment in enough detail to judge risk of bias) and imprecision (confidence intervals include 1 (no effect)).
Background
Description of the condition
Pars plana vitrectomy is an established and successful treatment for the complications of proliferative diabetic retinopathy (PDR) (Ho 1992; McLeod 1991). Up to 10% of people presenting with PDR require the treatment within one year (Kaiser 2000). The most common indication for surgery is non‐clearing vitreous haemorrhage (Ho 1992; McLeod 1991). Unfortunately, postoperative vitreous cavity haemorrhage (POVCH) is a significant complication occurring in approximately 20% to 30% of cases, although the reported range is large: 5% to 80% of cases (Benson 1988; Blankenship 1986; Liggett 1987; Novak 1984; Tolentino 1989; Virata 2001; Yorston 2008).
Although there can be overlap, POVCH occurs in two main forms.
Early POVCH, being present from the first few postoperative days and delaying visual recovery by non‐clearance.
Late POVCH, occurring later during follow‐up, commonly at two to six months postoperatively, after a postoperative period during which the vitreous cavity was clear.
Aetiology of POVCH
Persistent haemorrhage can result from operative and postoperative oozing of the remnants of new vessels or dissected tissue, or directly from the sclerostomies used to perform surgery. It can also occur from clot lysis in the first few postoperative days. Leaching of red blood cells can occur from retained old haemorrhages in residual anterior vitreous, causing apparent POVCH (McLeod 1991; Novak 1984; Tolentino 1989).
Recurrent haemorrhage can result from late haemorrhage from dissected tissue, recurrent traction on residual new vessels, or indeed postoperative new vessel growth in the posterior retina. Recent studies have shown that a common cause of recurrent haemorrhage is new anterior vessel growth at the inner sclerostomy sites associated with fibrous traction (Bhende 2000; Hershberger 2004; Hotta 2000; Kreiger 1993; Sawa 2000; Steel 2008; West 2000). Termed ‘entry site neovascularisation' (ESNV) (McLeod 2000), this is thought to be an aberrant wound‐healing response related to the presence of retinal and pars plana ischaemia (Kreiger 1993; Yeh 2005). The presence of ESNV is difficult to observe clinically because of the extreme anterior location, but can be confirmed at the time of revision surgery with deep scleral indentation or endoscopic techniques (West 2000). It can also be localised, with anterior segment high‐resolution ultrasonography of the inner sclerostomy sites (Bhende 2000; Hershberger 2004; Hotta 2000; Steel 2008).
Consequences and management of POVCH after occurrence
People with POVCH suffer a delay in their visual recovery which, in some cases, results in a worse level of visual acuity than preoperatively. Intraocular pressure can become raised from trabecular meshwork obstruction. If maculopathy is present, then additional laser treatment cannot be applied, with the risk of worsening foveal function in the long term.
The initial treatment for POVCH is observation (Tolentino 1989). Spontaneous clearance occurs in many cases. Because they are no longer trapped in the gel structure of the vitreous, red blood cells can circulate and clear more freely from the vitreous cavity. Clearance is related to the amount of haemorrhage, its frequency, and the degree of communication between anterior and posterior segments, allowing red blood cells to enter the anterior chamber and be cleared via the trabecular meshwork (McLeod 2000).
Non‐clearing POVCH necessitates revision surgery in approximately one‐third to one‐half of those who experience POVCH and approximately 10% of all patients undergoing surgery (Blankenship 1986; Blumenkranz 1986; Brown 1992; Han 1991; Martin 1992; Novak 1984; Schachat 1983; Tolentino 1989). Non‐clearing POVCH is treated with surgery to remove haemorrhage and to treat any underlying cause that may have been identified. The timing of the surgery will depend on a variety of factors, including social situation, fellow‐eye status, maculopathy needing treatment, intraocular pressure, etc.
In some patients, spontaneous clearing or revision surgery is followed by further haemorrhage, frustrating visual rehabilitation further, especially if this occurs in the better eye.
Description of interventions to reduce the incidence of POVCH and how the interventions may work
At the time of the initial vitrectomy surgery, several strategies may have been used to prevent POVCH and avoid the need for repeat surgery. We can divide these into two main groups.
-
Established surgical interventions that are generally regarded as standard clinical practice.
Ensuring adequate haemostasis at the time of vitrectomy with laser or intraocular bipolar coagulation to reduce postoperative oozing of dissected blood vessels.
Removal of peripheral haemorrhagic vitreous to reduce leaching of sequestered red blood cells postoperatively into the vitreous cavity.
Identification and removal of all posterior vitreoretinal traction. Vitreoschisis is also known to occur in people with PDR, and identification of this and dissection in the true vitreoretinal plane are important in order to avoid recurrent traction and postoperative bleeding from neovascular tissue (Schwatz 1996).
Applying supplementary posterior panretinal photocoagulation if required.
Other strategies to prevent POVCH that have been reported but not routinely adopted.
Surgical
Applying additional anterior retinal photocoagulation up to the ora serrata to ablate retro‐oral ischaemic retina and reduce the production of postoperative neovascular growth factors (Liggett 1987; Mason 1978; Yeh 2005).
Direct treatment to the sclerostomy sites themselves with either cryotherapy or laser (Yeh 2005). This is thought to reduce the occurrence of entry site neovascularisation by inhibiting cellular migration through the sclerostomy wounds and causing focal atrophy of the ciliary epithelia.
Removal of Wieger’s ligament and thorough anterior vitrectomy, especially around the inner sclerostomy wounds (McLeod 2000; McLeod 2003). This may be effective by:
increasing the egress of red blood cells and growth factors from the posterior segment to the anterior segment for rapid clearance of any POVCH;
reducing any putative concentration of growth factors around the sclerostomy sites themselves;
removing the vitreous scaffold along which anterior new vessels could grow.
Some clinicians advocate simultaneous or even pre‐emptive cataract surgery to achieve these aims and reduce the risk of POVCH (Schiff 2007).
Agents with physical actions thought to possibly reduce the rate of POVCH inserted into the eye during surgery, such as air (Joondeph 1989), gas (Koutsandrea 2001; Yang 2007), or viscoelastic substances (Packer 1989).
Pharmacologic
Preoperative
The vascular endothelial growth factor (VEGF) inhibitor bevacizumab has been used preoperatively to reduce vascular proliferation and reduce the vascularity of neovascular tissue (Romano 2009a; Yang 2008).
Intraoperative
Triamcinolone has been used intraoperatively by intraocular injection to reduce inflammation and vascular proliferation (Faghihi 2008).
Bevacizumab has been used intraoperatively to reduce vascular proliferation following surgery (Romano 2009b).
Postoperative
Oral tranexamic acid, which inhibits fibrinolysis and hence clot dissolution, administered to the patient postoperatively (Laatikainen 1987; Ramezani 2005).
Why it is important to do this review
Diabetes mellitus is an increasing health problem. It is estimated that 6% (3.2 million) of the UK population are currently diabetic and that this will increase to 5 million by 2025. Ten per cent (10 billion UK pounds) of the total NHS budget is spent treating the acute costs of diabetes and its complications and this is predicted to increase to 17 billion by 2035 (Diabetes UK 2014). Diabetic retinopathy is one of the leading cause of blindness in the working age group in the UK accounting for 14.4% of blind and partially sighted registrations (Liew 2014).
Within 20 years of diagnosis nearly all people with Type 1 and almost two thirds of people with Type 2 diabetes have some degree of retinopathy (Scanlon 2008) and after 15 years, 30% and 10% of type 1 and type 2 diabetics respectively develop PDR (Klein 1984a; Klein 1984b). These patients are at risk of severe visual loss resulting from the complications of PDR. A study of patients at a large eye unit in the USA suggested that approximately 10% of patients presenting with PDR require vitrectomy surgery within one year (Kaiser 2000). Estimates based upon data from our region (Vaideanu 2014) suggest that approximately 4000 vitrectomies for the complications of PDR are currently performed annually in the UK. If 10% require revision surgery, this equates to 400 patients per annum in the UK. POVCH after vitrectomy surgery is certainly a significant problem and its occurrence is distressing for patients as well as delaying visual recovery. Repeat interventions expose the patient to further operative risk and anxiety and add to the overall cost of care. The use of anti‐VEGF treatment is becoming increasingly common for many ophthalmic conditions and has significant revenue consequences. The result of anti‐VEGF use, both preoperatively and intraoperatively, to reduce the occurrence of POVCH is uncertain, and a review of the literature will aid in optimum patient management and design of future studies.
The use of anti‐VEGF treatment is becoming increasingly common for many ophthalmic conditions and has significant revenue consequences. However, the effectiveness of anti‐VEGF use, both preoperatively and intraoperatively, in reducing the occurrence of POVCH is uncertain. A review of the literature will aid in optimum patient management and in the design of future studies.
Objectives
This review aimed to summarise the effects of anti‐VEGF use to reduce the occurrence of POVCH after vitrectomy surgery for PDR.
Methods
Criteria for considering studies for this review
Types of studies
This review aimed to include all randomised controlled trials (RCTs) and quasi‐RCTs. If we had not found any RCTs that met our inclusion criteria, we would have provided a description of the evidence for current practice from non‐randomised comparative trials along with the nature of evidence on which the review was based. We imposed no language or date restrictions.
Types of participants
Participants in the trials had to be people with PDR undergoing vitrectomy for the complications of diabetic retinopathy for the first time.
Types of interventions
Intervention: any use of anti‐VEGF designed to reduce POVCH. We considered the following anti‐VEGF treatments when given pre‐ or intraoperatively.
Bevacizumab 1.25 mg/0.05 ml
Ranibizumab 0.5 mg/0.05 ml
Pegaptanib 0.3 mg/0.05 ml
Aflibercept 2.0 mg/0.05 ml
Comparator: no treatment, sham treatment, or any other anti‐VEGF.
Types of outcome measures
As the aim of this review was to look at the prevention of POVCH, we excluded studies that did not report POVCH.
Primary outcomes
The incidence of early POVCH after surgery. We defined early POVCH as haemorrhage present from the first postoperative day or recurrent within four weeks postoperatively, either being at least grade 2 in severity as measured by the Diabetic Retinopathy Vitrectomy Study criteria (Anonymous 1985).
The incidence of late POVCH after surgery. We defined late POVCH as haemorrhage occurring more than four weeks postoperatively after a period during which the vitreous cavity was clear. The minimum length of follow‐up for this outcome was six months.
Secondary outcomes
Visual acuity at six months following the primary vitrectomy.
The incidence of vitreous cavity washout or revision vitrectomy at six months.
Adverse effects of intervention including cataract, iris rubeosis and rubeotic glaucoma, retinal detachment, increased inflammation and systemic side effects.
Quality of life measures performed at least six months following vitrectomy.
Density of POVCH, as measured by the Diabetic Vitrectomy Study criteria (Anonymous 1985).
Follow‐up
Follow‐up for the reviewed studies' outcome measures varied from 1 month to 12 months postvitrectomy. We looked at studies with any follow‐up period, using six‐month follow‐up data as a minimum for late POVCH and visual acuity. Follow‐up periods for the remaining secondary outcomes varied and are described in the Characteristics of included studies section.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2015, Issue 4), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to May 2015), PubMed (January 1966 to May 2015), EMBASE (January 1980 to May 2015), Latin American and Caribbean Health Sciences (LILACS) (January 1982 to May 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 26 May 2015.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), PubMed (Appendix 3), EMBASE (Appendix 4), LILACS (Appendix 5), ISRCTN (Appendix 6), ClinicalTrials.gov (Appendix 7), and the ICTRP (Appendix 8).
We ran the initial searches both with and without RCT search filters, as we wished to identify as much literature as possible on POVCH. For subsequent updates, however, we will only run searches with RCT filters, as the review is focusing on evidence from RCTs only.
After editorial input, we decided to revise and broaden the search strategies for the review. The searches were constructed of terms for the following four components: diabetic retinopathy, vitrectomy, haemorrhage, anti‐VEGF drugs. The searches have now been amended whereby the terms for vitrectomy and for haemorrhage have been combined into one search set of terms. This has made the search broader and will help us to identify additional relevant studies.
Searching other resources
We manually searched the reference lists of the trials included in the review for additional trials. We did not specifically handsearch journals or conference proceedings for this review.
Data collection and analysis
Selection of studies
Both review authors independently assessed abstracts to ascertain which studies met the inclusion criteria for the review. We labelled the abstracts as included, unclear, or excluded. We obtained full‐text copies of all included and unclear studies, and we independently determined which studies met the inclusion criteria. We again labelled each study as included, unclear, or excluded. We discussed any unclear articles and contacted the authors of the relevant article for further details when necessary.
Data extraction and management
Both review authors independently extracted data using standard methodological procedures expected by Cochrane. We compared each data set and resolved any discrepancies by discussion. We independently entered data into Review Manager and then rechecked the data (RevMan 2014).
Assessment of risk of bias in included studies
We structured the criteria for assessment of risk of bias per Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed each domain area, giving a judgement of 'low' risk of bias, 'high' risk of bias, or 'unclear' risk of bias. A judgement of 'unclear' indicated we were uncertain of the risk of bias. We looked specifically at the following domain areas:
Methods used to generate the study groups.
Methods used to conceal the allocation of the study groups.
Methods used to mask (blind) study participants and personnel.
Review of the study outcome data, looking specifically at the completeness of each study group outcome and selective outcome reporting.
Measures of treatment effect
We presented dichotomous outcomes as risk ratios and continuous outcomes as mean difference.
The review's primary outcomes included dichotomous data on the incidence of persistent or early POVCH and the incidence of late POVCH following the primary surgery. The secondary outcome data contained dichotomous data on the incidence of vitreous cavity washout or revision vitrectomy at six months and the adverse effects of the intervention. We intended to analyse visual acuity at six months following the primary vitrectomy as continuous data.
POVCH density is graded into four categories: grades 0 to 3. Rather than analysing the data as ordinal outcomes, we proposed to group together grade 0 with grade 1 POVCH and grade 2 with grade 3 POVCH in order to generate two larger groups, which we planned to analyse as dichotomous data in a meta‐analysis.
Unit of analysis issues
Ahn 2011 and Manabe 2015 included a small number of participants who had both eyes randomised. We included these participants in the analysis. Due to the number being small, we considered that the effect of interpersonal variance on any outcome would be negligible.
Dealing with missing data
Where data were missing in the included studies, we contacted the authors of the trials to request the missing data. In studies for which the trial authors could not provide missing data, we assessed whether the missing data were of 'low' or 'high' risk of bias, as per Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We intended to include studies missing data considered to be of 'low' risk of bias in a meta‐analysis. We would also have included studies thought to be at 'high' risk of bias in the meta‐analysis, but we would have performed a sensitivity analysis in order to assess their impact on the outcome of the meta‐analysis.
Assessment of heterogeneity
We assessed clinical heterogeneity by careful review of the study papers and presented a descriptive summary of results. We assessed heterogeneity between studies included in the meta‐analysis using the I2 statistic. We took an I2 result of more than 50% as an indication of high study heterogeneity.
Assessment of reporting biases
To avoid the presence of reporting biases within the review, we searched trial registry databases, which allowed us to identify all registered trials, published and unpublished. We contacted investigators of registered trials that were listed as having completed participant recruitment to ask for any unpublished data that may be relevant to the review outcomes.
Data synthesis
We performed analysis of data from the included RCTs for the two primary outcomes, secondary outcomes 1 and 2, and also for rate of postoperative retinal detachment. We used a random‐effects model for any analysis containing three or more RCTs.
Sensitivity analysis
We excluded trials that included participants with silicone oil tamponade because we felt that the tamponade could affect the assessment of the outcomes.
Summary of findings table
We prepared a 'Summary of findings' table presenting relative and absolute risks. We graded the overall quality (certainty) of the evidence for each outcome using the GRADE classification (GRADEpro 2014). We included the following outcomes in the 'Summary of findings' table.
Early POVCH (four weeks)
Late POVCH (six months)
Visual acuity, logMAR (six months)
Vitreous cavity washout (six months)
Adverse events: retinal detachment (at any time)
Quality of life
These outcomes were not defined a priori because the original protocol and first edition of this review were published before the summary of findings methodology was adopted by the Cochrane Eyes and Vision Group.
Results
Description of studies
Results of the search
The electronic searches yielded a total of 472 titles and abstracts and 9 reports of ongoing studies. After deduplication, the Trials Search Co‐ordinator scanned 397 records and discarded 311 records that were not relevant to the scope of the review. We screened the titles and abstracts of the remaining 86 references. We rejected 70 abstracts as not eligible for inclusion in the review. We obtained and screened full‐text copies of 16 references, of which we included 4 studies and excluded 12 studies from the review.
The amended searches run in May 2015 retrieved a total of 1414 records (Figure 1). After removing duplicate records, we screened 1138 references and excluded 1110 records that were not relevant to the scope of the review. We assessed 28 full‐text reports of studies for potential inclusion in the review. In addition to the four previously included studies (Ahmadieh 2009; di Lauro 2009; Modarres 2009; Rizzo 2008), we have now included eight new studies (Ahn 2011; El Batarny 2008; Elwan 2013; Farahvash 2011; Hernández‐Da Mota 2010; Manabe 2015; Sohn 2012; Zaman 2013). We excluded 12 studies (Berk Ergun 2014; Bhavsar 2014; Demir 2013; Goncu 2014; Gupta 2008; Gupta 2012a; Gupta 2012b; Jirawison 2012; Li 2010; Pakzad‐Vaezi 2014; Pokroy 2011; Sato 2013). We previously excluded da R Lucena 2009 and Yeh 2009 at the initial stage of screening abstracts. However, these two studies have been included in a meta‐analysis by Zhao 2011, so in the interests of transparency we have now added these studies to our list of excluded trials with reasons for exclusion.
1.
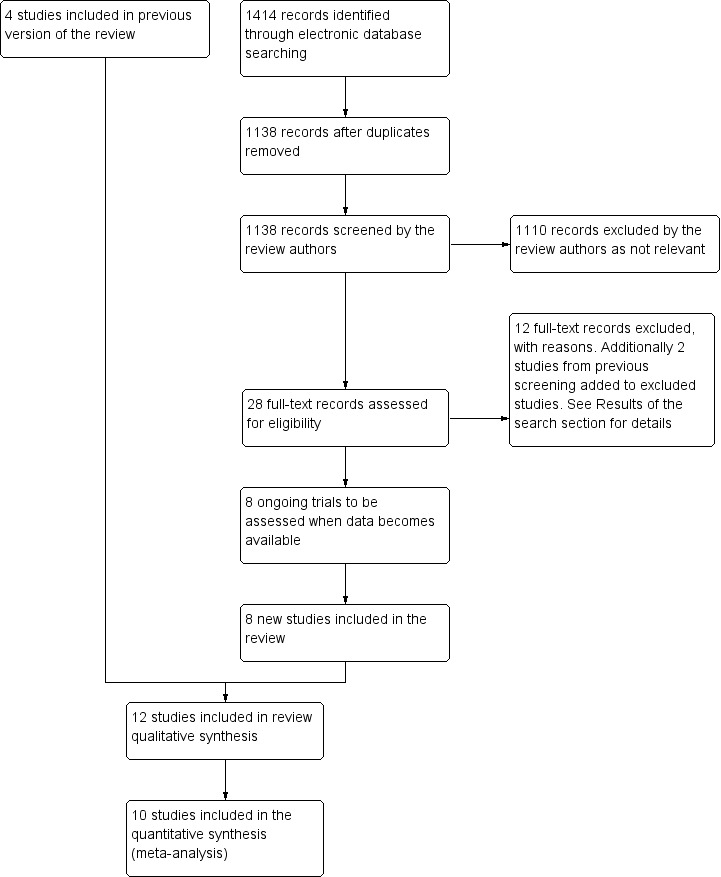
Results from searching for studies for inclusion in the review.
We also added the following eight reports of ongoing trials, which we will assess for potential inclusion in the review when data becomes available (ISRCTN79120387; NCT00931125; NCT01091896; NCT01151722; NCT01306981; NCT01589718a; NCT01805297NCT01854593).
Included studies
We identified 12 completed studies from the electronic database searches that met our inclusion criteria; see the Characteristics of included studies table for details.
Setting and participants
The included studies were conducted in a number of countries. Three were from Iran, two were from Italy, two were from Egypt, and the remaining were from South Korea, USA, Mexico, Pakistan, and Japan. The included studies randomised a total of 654 eyes. Sample size varied from 20, in Sohn 2012, to 107, in Ahn 2011. The mean age of the participants included was 54.2 years.
Intervention
The majority of included studies gave 1.25 mg intravitreal bevacizumab (IVB) (0.05 ml) within one week of pars plana vitrectomy. The studies with a different protocol were the following.
Ahn 2011 had an intervention group in which 1.25 mg IVB was given at the end of surgery (37 participants).
di Lauro 2009 had an intervention group that received 1.25mg IVB three weeks before vitrectomy.
Hernández‐Da Mota 2010 gave 1.25 mg IVB 48 hours before surgery.
Manabe 2015 gave 0.16 mg (0.05 ml) IVB one day before surgery.
Modarres 2009 gave 2.5 mg IVB three to five days before surgery.
Outcomes
The main outcomes in the trials included best‐corrected visual acuity (BCVA), feasibility of surgery (operating time, intraoperative bleed, and type of surgical steps required), and postoperative complications. The main postoperative complications were rate of POVCH, iris rubeosis, and retinal detachment. Follow‐up varied significantly across the trials, ranging from one month, in Ahmadieh 2009 and Manabe 2015, to one year, in Elwan 2013. The majority of trials had a final follow‐up visit at six months.
A number of trials reporting the incidence of POVCH have included participants who received silicone oil endotamponade, which has made interpretation of the trial results difficult. The presence of silicone oil within the vitreous cavity precludes an accurate diagnosis of POVCH, as any blood would be localised to the far periphery of the posterior chamber. Grading of POVCH is not possible with silicone endotamponade present. In light of this, we have carried out sensitivity analyses on the incidence of POVCH excluding participants with silicone oil endotamponade. Two trials excluded participants who received silicone oil and gave an accurate grading system for POVCH (Ahmadieh 2009; Ahn 2011).
In addition, we identified nine ongoing RCTs and have provided details in the Characteristics of ongoing studies table. We cannot comment on whether the these studies' characteristics meet the inclusion criteria until they are published and we can undertake a review of their methodology.
Excluded studies
We excluded 26 studies identified by the search that were either non‐randomised prospective studies or retrospective studies; see the Characteristics of excluded studies table. We excluded two trials that did not collect data on POVCH (da R Lucena 2009; Pakzad‐Vaezi 2014).
Risk of bias in included studies
See also Figure 2 and Figure 3.
2.
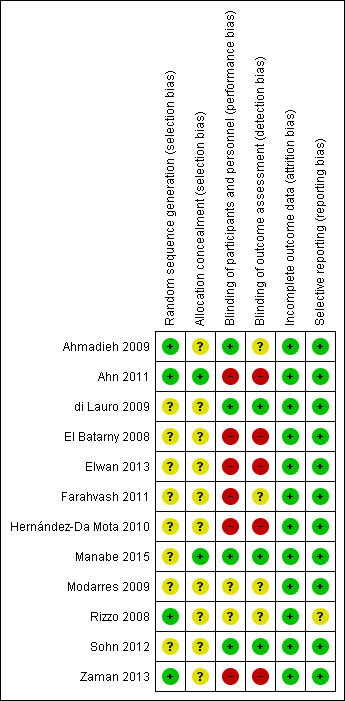
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
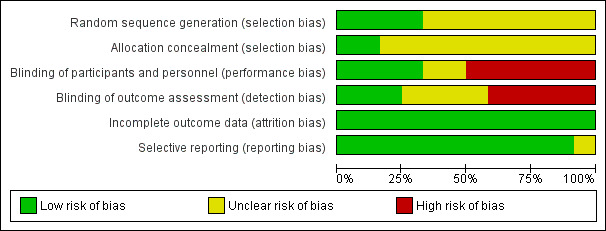
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Ahmadieh 2009 and Ahn 2011 clearly stated how the random sequence generation was performed. In both studies, a biostatistician was used to allocate participants and ensure that investigators were unaware of the series details. We graded the methods as 'low' risk for randomisation and allocation. di Lauro 2009 stated that participants were randomly assigned into the three groups, but provided no description on how the randomisation or sequence allocation was performed. El Batarny 2008, Elwan 2013, Farahvash 2011, Hernández‐Da Mota 2010, Modarres 2009, Sohn 2012, and Zaman 2013 did not state how participants were allocated into each group and provided no details about the randomisation methods. Rizzo 2008 used a table of random numbers to assign each study participant to group one or group two, but provided no details of the sequence generation. We graded the methods used for allocation as 'unclear' for di Lauro 2009, El Batarny 2008, Elwan 2013, Farahvash 2011, Hernández‐Da Mota 2010, Modarres 2009, Rizzo 2008, Sohn 2012, and Zaman 2013. In Manabe 2015, the allocation was done by the "envelop" methods, but the generation of the allocation sequence was not clearly described.
Blinding
We assessed Ahmadieh 2009 and Sohn 2012 as at 'low' risk of bias for masking (blinding), as a clear description of how this process was performed was provided. We graded Ahn 2011, El Batarny 2008, Elwan 2013, Farahvash 2011, and Hernández‐Da Mota 2010, which had an open‐label design, as 'high' risk. We graded di Lauro 2009, Rizzo 2008, Modarres 2009, and Zaman 2013, which did not comment on the masking of participants or investigators, as 'unclear'.
Incomplete outcome data
Ahmadieh 2009, Ahn 2011, Farahvash 2011, and Sohn 2012 clearly accounted for each participant who failed to complete the study protocol. It should be noted that in Ahmadieh 2009, less than half the participants (16 of 35) within the treatment group failed to complete the protocol, and only 18 of 35 participants within the control group completed the protocol. Manabe 2015 reported participant flow; all participants were followed up to 1 month. Although the remaining included RCTs did not comment directly on the attrition rate, it is clear from the results that all participants completed follow‐up.
Selective reporting
All the included studies reported the stated primary and secondary outcomes.
Other potential sources of bias
Ahmadieh 2009 recorded a high number of participants who failed to complete the trial in both the control and treatment groups: 15 of 35 in the control group and 19 of 35 in the treatment group. The main reasons for failing to complete follow‐up were vitreous haemorrhage reabsorption, silicone oil endotamponade, gas tamponade, and lost to follow‐up.
Ahn 2011 included 107 eyes of 91 participants. The 16 bilateral participants were included in the data analysis; no comment was made as to whether adjustment for within‐person correlation was performed during statistical analysis.
In the IVB group of Manabe 2015, participants with bilateral proliferative diabetic retinopathy (PDR) received IVB in one eye only, and the other eye was excluded from the study. The authors considered that IVB injected in one eye may pass to the contralateral eye. In the control group, both eyes of bilateral cases were included in the study.
Effects of interventions
See: Table 1
We have described the effect of each intervention in order of outcome type.
Primary outcomes
1. The incidence of early POVCH after surgery
Eight included RCTs commented on the incidence of early POVCH following pre‐ or intraoperative anti‐VEGF administration (Ahmadieh 2009; Ahn 2011; di Lauro 2009; El Batarny 2008; Farahvash 2011; Hernández‐Da Mota 2010; Modarres 2009; Zaman 2013). As many of the RCTs included participants who received silicone oil endotamponade in their results, making it difficult to establish the true incidence and grade of POVCH, we have performed a number of sensitivity analyses for this outcome excluding trials that included silicone oil cases. They are as follows.
Analysis 1.1: Contained only data from Ahmadieh 2009 and Ahn 2011, as these two RCTs excluded participants who received silicone oil and gave an accurate grading system for POVCH. The analysis included only grade 2 POVCH or worse. The use of pre‐ or intraoperative IVB was shown to reduce the incidence of early POVCH (risk ratio (RR) 0.28, 95% confidence interval (CI) 0.08 to 0.96). This analysis contained 144 participants, 89 in the intervention arm and 55 in the control arm.
Analysis 1.2: Included all trials that reported the incidence of POVCH (all grades). The use of pre‐ or intraoperative IVB was shown to reduce the incidence of POVCH (RR 0.35, 95% CI 0.23 to 0.53; eyes = 512; studies = 9; I2 = 0%) (Figure 4). Thirty‐seven participants in the intervention group received intraoperative IVB.
1.1. Analysis.
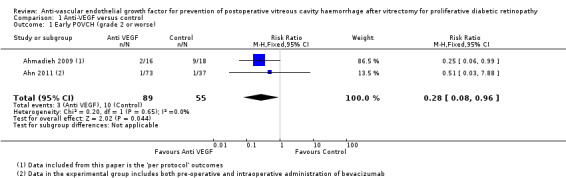
Comparison 1 Anti‐VEGF versus control, Outcome 1 Early POVCH (grade 2 or worse).
1.2. Analysis.
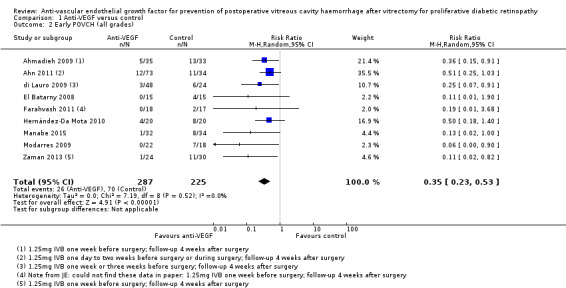
Comparison 1 Anti‐VEGF versus control, Outcome 2 Early POVCH (all grades).
4.
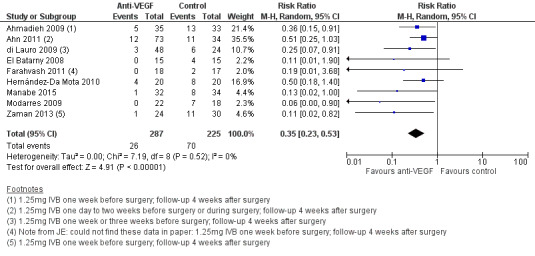
Forest plot of comparison: 1 Anti ‐VEGF vs control, outcome: 1.2 Early POVCH (all grades).
Analysis 1.3: Included all trial results that reported the incidence of POVCH (all grades) with participants who received silicone oil endotamponade excluded. The use of pre‐ or intraoperative IVB was shown to reduce the incidence of POVCH (RR 0.34, 95% CI 0.19 to 0.60; eyes = 302; studies = 6; I2 = 7%).
1.3. Analysis.
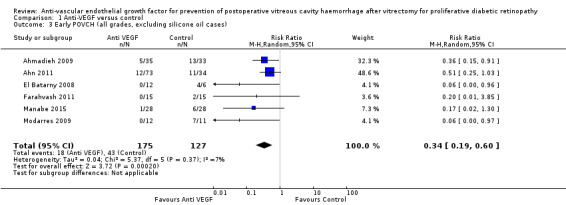
Comparison 1 Anti‐VEGF versus control, Outcome 3 Early POVCH (all grades, excluding silicone oil cases).
Sohn 2012 did comment on one case of POVCH found at three‐month follow‐up in the control group. The corresponding author could not provide a clear definition of early or late POVCH. We have therefore not included this result within the above analyses.
2. The incidence of late POVCH after surgery
Three included studies commented on the effect of IVB on the incidence of late POVCH (Ahn 2011: Farahvash 2011;Zaman 2013). Only Ahn 2011 gave a grade for the severity of late POVCH. Analysis 1.4 contained the reported data from all the trials that commented on late POVCH (any grade) and found no reportable effect of pre‐ or intraoperative IVB on late POVCH (RR 0.72, 95% CI 0.30 to 1.72). This analysis contained 196 participants, 115 in the intervention arm and 81 in the control arm (Figure 5).
1.4. Analysis.
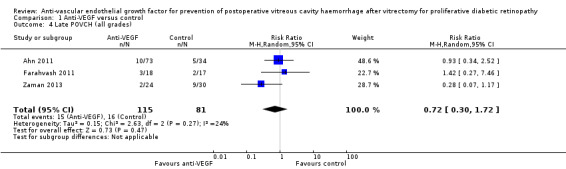
Comparison 1 Anti‐VEGF versus control, Outcome 4 Late POVCH (all grades).
5.
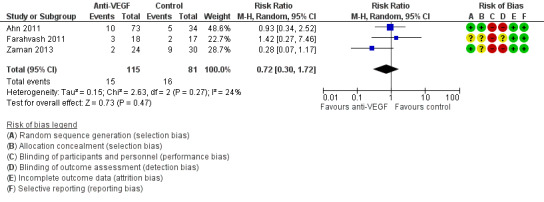
Forest plot of comparison: 1 Anti‐VEGF versus control, outcome: 1.4 Late POVCH (all grades).
Ahn 2011 found no statistical difference between the rate of late POVCH in the control group and combined pre‐ and intraoperative IVB groups, P = 0.813.
Hernández‐Da Mota 2010 included "recurrent haemorrhage" as an outcome, however provided no definition. We attempted to contact the corresponding author, but received no reply. These data are therefore currently excluded.
Secondary outcomes
1. Visual acuity at six months following the primary vitrectomy
Seven included studies commented on BCVA at six months or longer (Ahn 2011; di Lauro 2009; El Batarny 2008; Elwan 2013; Modarres 2009; Rizzo 2008; Zaman 2013). Six of the studies used LogMAR method for measuring acuity, while Elwan 2013 reported decimal acuity. Zaman 2013 provided a comparison of pre‐ and postoperative BCVA; the study provided no specific values.
We found considerable heterogeneity between studies (I2 = 88%) with mean difference in acuity ranging from 1.31 logMAR units better vision in the anti‐VEGF group, Hernández‐Da Mota 2010, to 0.14 logMAR units better vision in the control group, Ahn 2011 (Analysis 1.5) (Figure 6). This means that a pooled estimate of effect may not be informative.
1.5. Analysis.
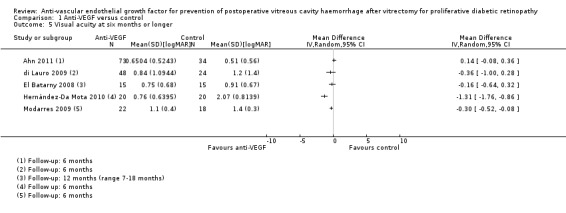
Comparison 1 Anti‐VEGF versus control, Outcome 5 Visual acuity at six months or longer.
6.
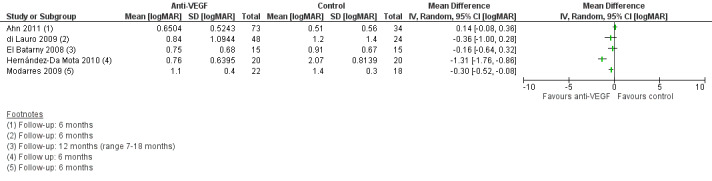
Forest plot of comparison: 1 Anti ‐VEGF vs control, outcome: 1.5 Visual acuity at six months or longer [logMAR].
2. The incidence of vitreous cavity washout or revision vitrectomy at six months
Five RCTs commented on the rate of vitreous cavity washout within six months (di Lauro 2009; El Batarny 2008; Elwan 2013; Manabe 2015; Modarres 2009). Analysis 1.6 showed that people receiving IVB were less likely to receive vitreous cavity washout (RR 0.19, 95% CI 0.06 to 0.67; eyes = 291; studies = 5; I2 = 0%).
1.6. Analysis.
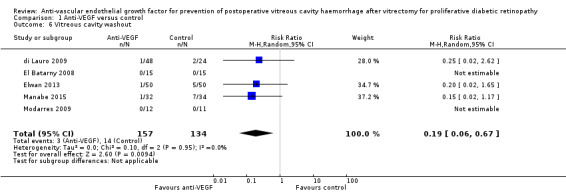
Comparison 1 Anti‐VEGF versus control, Outcome 6 Vitreous cavity washout.
3. Adverse effects of intervention including cataract, iris rubeosis and rubeotic glaucoma, retinal detachment, increased inflammation and systemic side effects
We have provided all reported adverse events in detail in Table 2. No systemic complications of IVB use were reported.
1. Adverse effects.
| Adverse effect | Injection‐related complications | Raised intraocular pressure | Iris neovascularisation | Neovascular glaucoma | Cataract progression | Retinal detachment | Systemic adverse effects | Other local effects |
| Study | ||||||||
| Ahmadieh 2009 | No cases | 1 person only | No cases | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ahn 2011 | ‐ | ‐ | ‐ | Anti‐VEGF: 5/73 (7%) Control: 1/34 (3%) |
Anti‐VEGF: 5/60 (8%) Control: 5/32 (16%) |
Anti‐VEGF: 0/73 (0%) Control: 1/34 (3%) |
No cases | ‐ |
| di Lauro 2009 | ‐ | ‐ | ‐ | ‐ | ‐ | 3/72 (4%) but unclear which group | No cases | ‐ |
| El Batarny 2008 | ‐ | ‐ | Anti‐VEGF: 0/20 Control: 1/15 (6.7%) |
‐ | Anti‐VEGF: 4/15 (26.6%) Control: 7/15 (46.7%) |
Anti‐VEGF: 1/15 (6.7%) Control: 2/15 (13.3%) |
No cases | ‐ |
| Elwan 2013 | ‐ | ‐ | Anti‐VEGF: Regressed in 10/50 (20%) Control: Persisted in 6/50 (12%) No new post‐op cases reported |
‐ | ‐ | Anti‐VEGF: 3/50 (6%) Control: 6/50 (12%) |
No cases | ‐ |
| Farahvash 2011 | ‐ | ‐ | No cases | No cases | “During the follow‐up, all but 2 phakic patients with significant cataract and best‐corrected visual acuity < 20/200 underwent cataract surgery". | Anti‐VEGF: 1/18 (6%) Control: 1/17 (6%) |
Mortality (range 3‐15 months follow‐up) Anti‐VEGF: 1/18 (6%) Control: 1/17 (6%) |
‐ |
|
Hernandez‐ Da Mota 2010 |
‐ | Anti‐VEGF: 4/20 (20%) Control: 0/20 |
‐ | Anti‐VEGF: 0/20 Control: 2/20 (10%) |
Anti‐VEGF: 3/20 (15%) Control: 4/20 (20%) |
Anti‐VEGF: 1/20 (5%) Control: 4/20 (20%) |
‐ | ‐ |
| Manabe 2015 | No cases | Anti‐VEGF: 2/32 (6%) Control: 6/34 (18%) |
‐ | Anti‐VEGF: 0/32 Control: 3/34 (9%) |
‐ | ‐ | No cases | ‐ |
| Modarres 2009 | ‐ | ‐ | ‐ | ‐ | ‐ | Anti‐VEGF: 1/22 (5%) Control: 1/18 (6%) |
No cases | No cases |
| Sohn 2012 | ‐ | ‐ | ‐ | Anti‐VEGF: 0/10 Control: 1/10 (10%) |
Anti‐VEGF: 1/10 (10%) Control: 1/10 (10%) |
No cases | ‐ | ‐ |
| Zaman 2013 | ‐ | ‐ | Anti‐VEGF: 3/24 (13%) Control: 7/30 (23%) |
‐ | ‐ | ‐ | ‐ | ‐ |
VEGF: vascular endothelial growth factor
As many of the included RCTs commented on the rate of postoperative retinal detachment, we elected to analyse this data. We did not list this analysis on the review protocol and undertook it at the update stage. Analysis 1.7 showed the risk of postoperative retinal detachment was lower within the intervention arm, but the result was not statistically significant, the confidence intervals were wide and include 1 (no effect) (RR 0.46, 95% CI 0.19 to 1.08). This analysis contained 372 participants, 208 in the intervention arm and 164 in the control arm.
1.7. Analysis.

Comparison 1 Anti‐VEGF versus control, Outcome 7 Retinal detachment.
4. Quality of life measures performed at least six months following vitrectomy
There are currently no studies that comment on this outcome.
5. Density of POVCH, as measured by the Diabetic Vitrectomy Study criteria
Ahmadieh 2009 clearly stated the grading for the density of POVCH within the results section. Four participants had grade 2 or 3 POVCH in the treatment group at one‐week follow‐up (4/16 (25%)), while 10 participants had grade 2 or 3 POVCH at the same follow‐up within the control group (10/18 (55.5%)). At one‐month follow‐up this had changed to two participants within the treatment group (2/16 (12.5%)) and nine within the control group (9/18 (50%)).
Ahn 2011 defined POVCH in a similar manner to the Diabetic Vitrectomy Study criteria. At one month following surgery, 2/36 (5.6%), 0/37, and 1/34 (2.9%) of participants had grade 2 or worse POVCH in study group 1 (preoperative IVB), group 2 (intraoperative IVB), and group 3 (control), respectively.
The incidence of late POVCH (grade 2 or worse) occurred in 3/36 (8.3%) in the preoperative IVB group, 3/37 (8.1%) in the intraoperative IVB group, and 3/34 (8.8%) in the control group.
di Lauro 2009 commented that grade 3 POVCH (no fundal view) occurred in 6/24 (25%) of eyes in the control group and 1/24 (4%) and 2/24 (8.3%) of eyes that received bevacizumab 7 and 20 days preoperatively, respectively, when assessed three months postoperatively.
El Batarny 2008, Farahvash 2011, Hernández‐Da Mota 2010, Modarres 2009, and Zaman 2013 did not clearly state the grading of POVCH.
Discussion
Summary of main results
The aim of this review was to establish whether the use of anti‐VEGFs as an adjunct to pars plana vitrectomy, either pre‐ or intraoperatively, is of benefit. Currently the review includes 12 RCTs, all of which look at the use of IVB. The main outcomes were the effect of IVB on early and late POVCH, BCVA, vitreous cavity washout rates, and postoperative complications. We summarised the effect of IVB by outcome type.
Primary outcomes
1. The incidence of early POVCH after surgery
Nine RCTs commented on the effect of IVB on the incidence of early POVCH. Analysis 1.1, Analysis 1.2, and Analysis 1.3 all showed a beneficial effect of IVB in reducing the rate of early POVCH following vitrectomy. The inclusion of participants who received silicone oil endotamponade may well have biased the true incidence of early POVCH, and so we advise caution when interpreting the results of Analysis 1.2 and Analysis 1.3. However, as silicone oil is commonly used following complex delamination surgery, the inclusion of this group is important and may provide a more practical guide to the effect of IVB.
2. The incidence of late POVCH after surgery (defined as occurring more than four weeks postoperatively after a period during which the vitreous cavity was clear)
Three RCTs reported the effect of IVB on late POVCH. Analysis 1.4 found no evidence of an effect of pre‐ or intraoperative IVB on the rate of late POVCH. This analysis contained two RCTs that included participants with silicone oil endotamponade in their results. Again, this may bias the true incidence of late POVCH. Ahn 2011 was the only study that excluded these participants before reporting their results, and found no effect of IVB on late POVCH.
Secondary outcomes
1. Visual acuity at six months following the primary vitrectomy
The current analysis (Analysis 1.5, Figure 6) suggested that use of IVB has no significant effect on average BCVA at six months. However, due to substantial heterogeneity between studies, we could not estimate an accurate pooled effect.
Many factors could affect BCVA within this heterogenous group of participants, particularly clarity of the media and vitreous cavity. The presence of silicone oil within the vitreous cavity will ensure it is clear in situations when potential recurrent haemorrhage may well have caused reduced vision. Further RCTs that exclude silicone oil participants are needed before a more accurate result can be reported.
2. The incidence of vitreous cavity washout or revision vitrectomy within six months
Two of the five studies within this analysis (Analysis 1.6) had no estimable effect. The incidence of vitreous cavity washout was reduced with the use of IVB.
3. Adverse effects of intervention including cataract, iris rubeosis and rubeotic glaucoma, retinal detachment, increased inflammation and systemic side effects
All included RCTs reported the adverse effects of preoperative IVB (Table 2). The results suggested that the risk of complications and adverse side effects appears to be small.
4. Quality of life measures performed at least six months following vitrectomy
There are currently no studies that comment on this outcome.
5. Density of POVCH, as measured by the Diabetic Vitrectomy Study criteria
Ahmadieh 2009, Ahn 2011, and di Lauro 2009 commented on the density of POVCH. Five other studies that commented on the density of POVCH did not clearly state the grading of POVCH, and the trial authors were unable to provide further information when contacted. We can draw no conclusion about the relative density of POVCH using preoperative IVB compared with controls.
Overall completeness and applicability of evidence
This review included 12 trials conducted in Europe, North America, Africa, and Asia. The results of these trials are likely to be applicable to standard clinical practice. The trials consistently showed that IVB applied around the time of vitrectomy to reduce the occurrence of complications of proliferative diabetic retinopathy decreased the risk of POVCH. However, the evidence was less complete with respect to other outcomes; for example the effect of IVB on visual acuity and adverse effects was less certain.
All the trials used IVB and most trials used a similar dose of (1.25 mg), but a variety of regimens were used in terms of whether the dose was given before, immediately before, or during surgery. No data were available on other anti‐VEGF agents.
A number of ongoing RCTs are currently looking at the effects of ranibizumab, pegaptanib sodium, and aflibercept on POVCH and BCVA (see Characteristics of ongoing studies). The results of these trials will help clarify the effect of anti‐VEGF on late POVCH and BCVA.
Quality of the evidence
The design of RCTs looking at the effect of adjunctive use of anti‐VEGF in diabetic vitrectomy is difficult. There are a number of indications for vitrectomy in proliferative retinopathy. These vary from non‐clearing vitreous haemorrhage in older patients with previously treated inactive proliferative diabetic retinopathy, through to younger patients with active untreated proliferative diabetic retinopathy and severe tractional changes. Many of the included RCTs had a heterogenous group of participants, as above, and the effect of IVB on POVCH and BCVA may differ depending on the patient type.
Five of the included trials appear to be or state that they are 'open label'. This is a significant proportion and may limit confidence in the estimation of the treatment effect of IVB.
We restricted the meta‐analysis to published RCT data, and it may be possible that bias exists if smaller trials not accepted for publication found no effect on early POVCH. A number of the included trials had a low number of participants, which reduces the power of the current analysis.
Potential biases in the review process
The review authors followed the key criteria listed within the Assessment of risk of bias in included studies section of the review to ensure that risk of bias was minimised during trial selection.
Agreements and disagreements with other studies or reviews
Zhao 2011 performed a systematic review looking at the potential benefits of preoperative IVB on both intraoperative (including intraoperative bleeding, endodiathermy, iatrogenic retinal tears, and mean surgical time) and postoperative (including BCVA, recurrent vitreous haemorrhage, reabsorption time of blood and other complications) outcomes.
Data analysis of included studies within the Zhao 2011 review, which looked at the incidence of early POVCH, showed an almost statistically significant result in favour of the treatment group (odds ratio (OR) 5.48, 95% CI 0.97 to 31.02; P = 0.05). This result is in agreement with the data analysis of this review. However, it should be noted that many of the included studies within the Zhao 2011 data analysis contained participants who received silicone oil endotamponade, which may be a significant source of bias. Also, the Zhao 2011 authors commented that due to varying definitions of POVCH, study heterogeneity within the data analysis was high (I2 = 93.6%), and so the results should be interpreted with caution.
Zhang 2013 reported similar outcomes in a meta‐analysis of the reported data on preoperative IVB use in vitrectomy for proliferative diabetic retinopathy. The study reported a beneficial effect of IVB on the rate of early POVCH (OR 0.35, 95% CI 0.21 to 0.58) and no effect on the rate of late POVCH (OR 0.55, 95% Cl 0.25 to 1.21).
Veliz 2014 summarised the results of a previously published version of this review. Zhang 2013, Zhao 2011, and Veliz 2014 concluded that bevacizumab probably decreased intraoperative bleeding, but its effect on visual acuity is uncertain. They concluded that it might increase the risk of cardiovascular events, but this was based on a RR of 2.2 with 95% CIs from 0.11 to 44.3, so there is some uncertainty with this estimate as well.
Authors' conclusions
Implications for practice.
This review provides support for the use of perioperative IVB to prevent POVCH after vitrectomy for proliferative diabetic retinopathy. The current analysis suggests that pre‐ or intraoperative IVB reduces the risk of early POVCH, but the effect on late POVCH is uncertain. The number of reported severe complications from IVB use was low.
Implications for research.
Further randomised studies looking at the effect of anti‐VEGF agents on the incidence and management of POVCH will strengthen the current evidence base. These studies need to address the effect on both early and late POVCH, while ensuring that the use of endotamponade, which may be required during the postoperative period, is clearly stated within the results and allowed for in the power calculations prior to the study. Furthermore, the spontaneous clearance of vitreous haemorrhage or clearance following the injection of an anti‐VEGF should be factored in for the power calculation. A minimum follow‐up period of at least six months is important to ensure that late POVCH can be recognised. Visual acuity should be analysed at defined time points and measured before and after further surgery to remove POVCH. We recommend grading of POVCH using the criteria within the Diabetic Retinopathy Vitrectomy Study, making the outcome of RCTs more comparable and easier to subject to further analysis.
What's new
| Date | Event | Description |
|---|---|---|
| 22 October 2014 | New citation required and conclusions have changed | Issue 8, 2015: Eight new RCTs (Ahn 2011; El Batarny 2008; Elwan 2013; Farahvash 2011; Hernández‐Da Mota 2010; Manabe 2015; Sohn 2012; Zaman 2013) were identified that met the inclusion criteria |
| 22 September 2014 | New search has been performed | Issue 8, 2015: Electronic searches were updated |
Acknowledgements
The electronic searches were developed and executed by the Cochrane Eyes and Vision Group editorial team. We thank Tim Jackson and Catey Bunce for their comments on the 2015 update, Jennifer Evans for her assistance on the review and Anupa Shah for her help throughout the review process.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Diabetic Retinopathy] explode all trees #2 diabet* #3r etinopath* #4 #1 or #2 or #3 #5 MeSH descriptor: [Vitrectomy] explode all trees #6 vitrectom* #7 PPV* #8 MeSH descriptor: [Sclerostomy] explode all trees #9 sclerostom* or sclerectom* #10 MeSH descriptor: [Vitreous Hemorrhage] this term only #11 MeSH descriptor: [Vitreous Body] this term only #12 hemorrhag* or haemorrhag* #13 MeSH descriptor: [Postoperative Complications] explode all trees #14 MeSH descriptor: [Recurrence] this term only #15 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 #16 MeSH descriptor: [Angiogenesis Inhibitors] explode all trees #17 MeSH descriptor: [Angiogenesis Inducing Agents] explode all trees #18 MeSH descriptor: [Endothelial Growth Factors] explode all trees #19 MeSH descriptor: [Vascular Endothelial Growth Factors] explode all trees #20 macugen* or pegaptanib* or lucentis* or rhufab* or ranibizumab* or bevacizumab* or avastin or aflibercept #21 anti near/2 VEGF #22 endothelial near/2 growth near/2 factor #23 #16 or #17 or #18 or #19 or #20 or #21 or #22 #24 #4 and #15 and #23
Appendix 2. MEDLINE (Ovid) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp diabetic retinopathy/ 14. diabet$.tw. 15. retinopath$.tw. 16. or/13‐15 17. exp vitrectomy/ 18. vitrectom$.tw. 19. PPV$.tw. 20. sclerostomy/ 21. (sclerostom$ or sclerectom$).tw. 22. vitreous hemorrhage/ 23. vitreous body/ 24. (hemorrhag$ or haemorrhag$).tw. 25. exp postoperative complications/ 26. or/17‐25 27. exp angiogenesis inhibitors/ 28. exp angiogenesis inducing agents/ 29. exp endothelial growth factors/ 30. exp vascular endothelial growth factors/ 31. (macugen$ or pegaptanib$ or lucentis$ or rhufab$ or ranibizumab$ or bevacizumab$ or avastin or aflibercept).tw. 32. (anti adj2 VEGF$).tw. 33. (endothelial adj2 growth adj2 factor$).tw. 34. or/27‐33 35. 16 and 26 and 34 36. 12 and 35
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. PubMed search strategy
((diabetic retinopathy[mesh]) OR (diabetes OR diabetic) OR (retinopathy)) AND ((vitrectomy[mesh]) OR (vitrectomy) OR (PPV) OR (sclerostomy[mesh]) OR (sclerostomy OR sclerectomy)) OR (vitreous hemorrhage[mesh]) OR (vitreous body[mesh]) OR (hemorrhage OR haemorrhage) OR (postoperative complications[mesh]) OR (recurrence[mesh])) AND ((angiogenesis inhibitors[mesh]) OR (angiogenesis inducing agents[mesh]) OR (endothelial growth factors[mesh]) OR (vascular endothelial growth factors[mesh]) OR (macugen OR pegaptanib OR lucentis OR rhufab OR ranibizumab OR bevacizumab OR avastin OR aflibercept) OR (endothelial growth factor) OR (anti VEGF))
Appendix 4. EMBASE (Ovid) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp diabetic retinopathy/ 34. diabet$.tw. 35. retinopath$.tw. 36. or/33‐35 37. exp vitrectomy/ 38. vitrectom$.tw. 39. PPV$.tw. 40. sclerostomy/ 41. (sclerostom$ or sclerectom$).tw. 42. vitreous hemorrhage/ 43. vitreous body/ 44. (hemorrhag$ or haemorrhag$).tw. 45. exp postoperative complication/ 46. recurrent disease/ 47. or/37‐46 48. exp angiogenesis/ 49. exp angiogenesis inhibitors/ 50. exp angiogenic factor/ 51. exp endothelial cell growth factor/ 52. exp vasculotropin/ 53. (macugen$ or pegaptanib$ or lucentis$ or rhufab$ or ranibizumab$ or bevacizumab$ or avastin or aflibercept$).tw. 54. (anti adj2 VEGF$).tw. 55. (endothelial adj2 growth adj2 factor$).tw. 56. or/48‐55 57. 36 and 47 and 56 58. 32 and 57
Appendix 5. LILACS search strategy
diabet$ or retinopath$ and vitrectom$ or PPV$ or sclerostom$ or sclerectom$ and vitreous or hemorrhag$ or haemorrhag$ or postoperat$ or recur$
Appendix 6. ISRCTN search strategy
retinopathy AND vitrectomy AND haemorrhage retinopathy AND vitrectomy AND hemorrhage
Appendix 7. ClinicalTrials.gov search strategy
Retinopathy AND Vitrectomy AND (Haemorrhage or Hemorrhage)
Appendix 8. ICTRP search strategy
Vitrectomy OR Haemorrhage OR Hemorrhage OR Vitroretinal OR Vitreous = Title AND Diabetic Retinopathy = Condtion AND Macugen OR Pegaptanib OR Lucentis OR Rhufab OR Ranibizumab OR Bevacizumab OR Avastin OR Aflibercept = Intervention
Data and analyses
Comparison 1. Anti‐VEGF versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early POVCH (grade 2 or worse) | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 0.96] |
| 2 Early POVCH (all grades) | 9 | 512 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.23, 0.53] |
| 3 Early POVCH (all grades, excluding silicone oil cases) | 6 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.19, 0.60] |
| 4 Late POVCH (all grades) | 3 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.30, 1.72] |
| 5 Visual acuity at six months or longer | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Vitreous cavity washout | 5 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.06, 0.67] |
| 7 Retinal detachment | 7 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.19, 1.08] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmadieh 2009.
| Methods | Prospective, randomised, double‐masked clinical trial Country: Iran | |
| Participants | Number: 68 eyes of 68 participants undergoing PPV Age: Mean (± SD) age was 55.2 ± 11.1 years (range 21 to 76 years) Sex: 34 (50%) males; 34 (50%) females Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
|
|
| Outcomes | Primary outcome measure:
Secondary outcome measures:
Follow‐up: 1 day, 1 week, and 1 month after surgery |
|
| Notes | Trial registration number: NCT00524875 Date study conducted: January 2007 to December 2007 Funding: not reported Conflict of interest: reported that there were none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by random block permutation according to a computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were masked to the treatment method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Visual acuity was measured by an optometrist who was masked to the groups. All pre‐ and postoperative examinations were performed by one of the authors (NS), who was also masked to the study group identification |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A flow chart included within the results section accounted for all participants who failed to complete the study protocol |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported, based on both the intention‐to‐treat analysis and per‐protocol analysis |
Ahn 2011.
| Methods | Prospective, randomised, open‐label trial Country: South Korea |
|
| Participants | Number: 107 Age: Mean age (± SD). Preoperative IVB group 51.0 (± 9.5), intraoperative IVB group 55.6 (± 10.6), and control 55.0 (± 11.4) Sex: (Male/female). Preoperative IVB group 23/13, intraoperative IVB group 21/16, and control 16/18 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
|
|
| Outcomes | Primary outcome measures:
Secondary outcome measures:
Follow‐up: 1 day, 1 week, 1, 3, and 6 months after surgery if there were no postoperative events |
|
| Notes | A total of 107 eyes of 91 participants, of which there were 16 bilateral participants, were included for analysis. Trial registration number: NTC00745498 Date study conducted: June 2008 to October 2010 (from trials registry entry) Funding: not reported Conflict of interest: reported that there were none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using a permuted block analysis |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by a biostatistician |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was open label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The distribution and attrition rate of study participants was clearly described in a flow chart |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
di Lauro 2009.
| Methods | Prospective interventional randomised controlled trial Country: Italy | |
| Participants | Number: 72 eyes of 68 participants Age: Not given Sex: Not given Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
|
|
| Outcomes | Primary outcome measures:
Secondary outcome measures:
Follow‐up: 1, 6, 12, and 24 weeks after surgery, and ultrasonography was performed before and 24 weeks after the injection |
|
| Notes | Trial registration number: NCT01025934 Date study conducted: October 2005 to May 2007 Funding: not reported Conflict of interest: reported that there were none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "We randomly assigned eligible patients... " and "The patients were randomly divided into three treatment groups (A, B, C)". |
| Allocation concealment (selection bias) | Unclear risk | Not stated in the report |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A sham injection was used in the control group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A sham injection was used in the control group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not stated in the report, however clear from the results that all participants completed follow‐up |
| Selective reporting (reporting bias) | Low risk | Data for all main outcomes were analysed and presented |
El Batarny 2008.
| Methods | Prospective, randomised, open‐label control trial Country: Egypt |
|
| Participants | Number: 30 Age: Mean (± SD). Control group 46 (± 12) and intervention group 44 (± 11) Sex: Not given Inclusion criteria:
Exclusion criteria not stated |
|
| Interventions | Intervention:
Comparator:
|
|
| Outcomes | Primary outcomes:
Follow‐up: 1 day, 7 days, 2 weeks, 1 month, and then monthly until end of study (all participants completed a minimum of 6 months) |
|
| Notes | Trial registration number: not reported Date study conducted: not reported Funding: not reported Conflict of interest: reported that there were none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised by diagnosis |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not masked to their study group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned, and treatment groups were different |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
Elwan 2013.
| Methods | Prospective, randomised, open‐label study Country: Egypt |
|
| Participants | Number: 100 Age: Mean (± SD). Control group 61.42 (± 5.49) and intervention group 61.94 (± 5.96) Sex: 61 male and 39 female Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
|
|
| Outcomes | Primary outcomes:
Follow‐up: 1 day, 1 week, 1, 3, 6, 9 months, and 1 year |
|
| Notes | No response from authors to correspondence asking for clarification of POVCH definitions. Trial registration number: not reported Date study conducted: November 2008 to November 2010 Funding: not reported Conflict of interest: reported that there were none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No masking of participants |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned, and treatment groups were different |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No comment was made. However, it is clear from the results that all participants completed follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
Farahvash 2011.
| Methods | Randomised, single‐masked (surgeon), controlled trial Country: Iran |
|
| Participants | Number: 35 Age: Mean (range) 58.5 (37 to 73) Sex: M/F. 18/17 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
|
|
| Outcomes | Primary outcomes:
Secondary outcomes
Follow‐up: 1 day, 1 week, every 3 months (minimum follow‐up 3 months) |
|
| Notes | We contacted the authors to further discuss the severity of POVCH in each arm of the study. Although no formal grading scale was used to assess the POVCH, the authors described the clinical findings as follows: "We had two cases of early vitreous cavity haemorrhage (within 4 weeks of surgery), both in non injection group. The haemorrhage was dense and fundus obscuring, but resolved spontaneously during follow up. Both of them have been undergone vitrectomy for vitreous haemorrhage without TRD, with no air, SF6 or silicone as a tamponade. We had five cases of late vitreous cavity haemorrhage before writing of paper (occurring after more than 4 weeks), two in non injection group and three in injection group. Only in one participant in injection group, vitreous cavity haemorrhage was dense and fundus obscuring. In other participants, it presented as recurrent bouts of non fundus obscuring vitreous cavity haemorrhage". Trial registration number: not reported Date study conducted: January 2008 to January 2009 Funding: not reported Conflict of interest: reported that there were none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to injection of bevacizumab (injection group) or not (control group), within each subgroup |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants were not masked to which group they had been randomised to |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | All surgeons were masked regarding treatment group, but it was unclear if the outcome assessors were masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants who failed to complete the study follow‐up are clearly accounted for in the results |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
Hernández‐Da Mota 2010.
| Methods | Prospective, randomised, open‐label study Country: Mexico |
|
| Participants | Number: 40 Age: Mean 55.7 (both groups) Sex: Not given Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
|
|
| Outcomes | Primary outcomes:
Follow‐up: 1 week, 3 and 6 months |
|
| Notes | No response from authors to correspondence asking for clarification of POVCH definitions. Trial registration number: not reported Date study conducted: not reported Funding: reported none Conflict of interest: reported that there were none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not masked to treatment group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "For all patients, visual acuity and intraocular pressure were measured and recorded before PPV in a standardized, masked manner" However, not clearly stated whether outcome assessment was always masked and whether other outcomes, such as intraocular bleeding, were masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although not directly stated, it is clear from the results that all participants completed the stated follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
Manabe 2015.
| Methods | Prospective, double‐masked randomised controlled trial Country: Japan |
|
| Participants | Number: 62 people (66 eyes) Age: average 60 years (range 20 to 82) Sex: 82% male Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
|
|
| Outcomes | Primary outcome:
Secondary outcome measures:
|
|
| Notes | Trial registration number: NCT01854593 Date study conducted: June 2012 to August 2013 Funding: not reported Conflict of interest: reported "none" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | "...were randomised using the envelop method" "Both the investigators and patients were masked to the treatment assignment". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both the investigators and patients were masked to the treatment assignment". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All postoperative examinations were performed by three vitreoretinal surgeons (H.S., T.H., H.N.) who also were masked to the study group allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were followed up for 1 month |
| Selective reporting (reporting bias) | Low risk | All outcomes on trial registry entry reported |
Modarres 2009.
| Methods | Prospective, single‐masked (surgeon), randomised clinical trial Country: Iran | |
| Participants | Number: 40 Age: Mean (± SD). Intervention group 55.8 (± 11.3) and control group 53.2 (± 11.7) Sex: Not given Inclusion criteria:
Exclusion criteria:
Follow‐up: Mean follow‐up 7 ± 3.6 months |
|
| Interventions | Intervention:
Comparator:
|
|
| Outcomes | Primary outcome measure:
Secondary outcome measure:
Follow‐up: Mean follow‐up 7 ± 3.6 months |
|
| Notes | Trial registration number: not reported Date study conducted: not reported Funding: not reported Conflict of interest: reported that there were none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "40 eyes of 40 diabetic participants who were candidates for vitrectomy were randomly assigned to receive ..." |
| Allocation concealment (selection bias) | Unclear risk | Not stated in report |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated in report |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The surgeons were masked as to the injection", but not clear if the outcome assessors were masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not stated in report, however clear from the results that all participants completed follow‐up |
| Selective reporting (reporting bias) | Low risk | All data discussed within the primary outcome for facilitation of surgery were analysed |
Rizzo 2008.
| Methods | Interventional, consecutive, randomised, prospective study Country: Italy | |
| Participants | Number: 22 Age: Mean (range). 52 years (24 to 63) Sex: Not given Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
|
|
| Outcomes | Primary outcome measure:
Secondary outcome measure:
Follow‐up: up to 6 months |
|
| Notes | Trial registration number: not reported Date study conducted: not reported Funding: not reported Conflict of interest: reported that there were none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We used a table of random numbers in order to assign each study participant to group 1 or 2" |
| Allocation concealment (selection bias) | Unclear risk | Not stated in report |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated in report |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated in report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not stated in report, however it is clear from the results that all participants completed follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Results for the feasibility of surgery were stated, but no analysis was performed |
Sohn 2012.
| Methods | Prospective, double‐masked, interventional study Country: USA |
|
| Participants | Number: 20 eyes of 19 participants Age: Median 52 years Sex: M/F. 12/7 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
|
|
| Outcomes | Clinical outcomes:
Follow‐up: up to 3 months |
|
| Notes | We contacted the corresponding author to clarify if the POVCH was early or late. The author confirmed that the haemorrhage had occurred in the 3‐month follow‐up, but could not give more detail of when it occurred. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated in report |
| Allocation concealment (selection bias) | Unclear risk | Not stated in report |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants in the control group underwent a sham injection |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants in the control group underwent a sham injection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants lost to follow‐up was clearly stated |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
Zaman 2013.
| Methods | Randomised, open‐label, controlled trial Country: Pakistan |
|
| Participants | Number: 54 Age: Mean (± SD). 52.07 (± 5.54). Range 39 to 67 Sex: M/F. 32/22 Inclusion criteria:
Exclusion criteria: not stated |
|
| Interventions | Intervention:
Comparator:
|
|
| Outcomes | Primary outcome measures:
Follow‐up: days 1, 7, 14 and then monthly for up to 6 months |
|
| Notes | We attempted to contact the corresponding author to clarify details in the methodology and results, but have received no response. Trial registration number: not reported Date study conducted: September 2010 to August 2011 Funding: not reported Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised into two categories" |
| Allocation concealment (selection bias) | Unclear risk | Not commented on within the article |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although no specific comment was made, the study appears to be open label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Although no specific comment was made, the study appears to be open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study protocol |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were presented in the results |
BCVA: best‐corrected visual acuity IVB: intravitreal bevacizumab PDR: proliferative diabetic retinopathy PPV: pars plana vitrectomy SD: standard deviation TRD: tractional retinal detachment VEGF: vascular endothelial growth factor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelhakim 2011 | This prospective case series included 20 eyes and found the following results:
The study was excluded as it was non‐randomised |
| Berk Ergun 2014 | This study was excluded as it is retrospective |
| Bhavsar 2014 | Vitrectomy was an outcome rather than an intervention |
| Cheema 2010 | This study was excluded as it is retrospective |
| da R Lucena 2009 | Did not assess POVCH |
| Demir 2013 | This study was excluded as it is retrospective |
| Doganay 2010 | This prospective case‐controlled study was excluded due to lack of randomisation. Participants were divided into two groups: Group 1 (n = 32) received 1.25 mg of IVB 1 week before surgery; Group 2 (n = 50) did not. Results:
|
| Goncu 2014 | Excluded as not randomised. |
| Gupta 2008 | Excluded as not randomised. |
| Gupta 2012a | This study was excluded due to its retrospective design |
| Gupta 2012b | Excluded as this is a retrospective observational study |
| Ishikawa 2009 | This study was excluded because of small numbers and no control group. Also no comment made on POVCH |
| Jirawison 2012 | Excluded as this is a retrospective observational study |
| Li 2010 | This study was excluded due to its retrospective design |
| Lo 2009 | This study was excluded due to its retrospective design |
| NCT01589718 | Withdrawn prior to enrolment. |
| Oshima 2009 | This study was excluded because of the retrospective enrolment of participants, the absence of randomisation, and the different study periods of the two groups |
| Pakzad‐Vaezi 2014 | Did not assess POVCH |
| Park 2010 | This study was excluded due to its retrospective design |
| Pokroy 2011 | This study was excluded due to its retrospective design |
| Romano 2009a | Found the following results:
This prospective study was excluded due to the lack of a control group. Participants also received an additional dose of bevacizumab at the end of surgery, which makes interpretation of the effect of the preoperative bevacizumab on POVCH difficult |
| Romano 2009b | Found the following results:
This prospective study was excluded due to the lack of a control group |
| Sato 2013 | This study was excluded due to its retrospective design |
| Yang 2008 | Found the following results:
This study was excluded due to the small numbers and lack of randomisation (participants in the control group were selected retrospectively). Also C3F8 was used in addition to preoperative anti‐VEGF in all participants in the treatment group. |
| Yeh 2009 | Excluded as participants allocated by alternation |
| Yeoh 2008 | Found the following results:
This study was excluded due to the lack of a control group. |
| Yeung 2010 | This study was excluded as it is retrospective. |
BCVA: best‐corrected visual acuity logMAR: logarithm of minimal angle of resolution POVCH: postoperative vitreous cavity haemorrhage SD: standard deviation VEGF: vascular endothelial growth factor
Characteristics of ongoing studies [ordered by study ID]
ISRCTN79120387.
| Trial name or title | A randomised, single‐masked, phase IV pilot study of the efficacy and safety of adjunctive intravitreal Avastin® (bevacizumab) in the prevention of early postoperative vitreous haemorrhage following diabetic vitrectomy |
| Methods | Randomised, single‐masked, controlled trial |
| Participants | 30 |
| Interventions | Avastin® will be administered intravitreally in a single‐ or dual‐dose regimen of 1.25 mg (in 0.05 ml) 2 weeks prior to vitrectomy and at the end of vitrectomy if internal tamponade (oil, air, or gas) was not used. No sham intravitreal injections will be given before or after vitrectomy if participant is randomised to the usual treatment group. |
| Outcomes | Primary: Postoperative vitreous haemorrhage based on 2 masked clinical assessments at 6 weeks and 6 months Secondary:
|
| Starting date | 2007 |
| Contact information | Mr Lyndon da Cruz Moorfields Eye Hospital, 162 City Road, London |
| Notes | No reply from lead investigator |
NCT00516464.
| Trial name or title | Evaluation of ranibizumab on the ease of procedure and complication rate in proliferative diabetic retinopathy (PDR) requiring vitrectomy |
| Methods | Randomised, single‐masked, controlled trial |
| Participants | 40 |
| Interventions | 40 participants will be enrolled and randomised 3:1 to ranibizumab or vitrectomy alone. 30 consented, enrolled participants will receive 3 intravitreal injections of 0.5 mg ranibizumab administered 1 to 3 weeks pre‐procedure, intraoperatively, and 1 month postvitrectomy |
| Outcomes | To evaluate the effect of ranibizumab on vitrectomy complications such as recurrent vitreous haemorrhage, postoperative retinal detachment rates, and development of intraoperative retinal breaks. (Time frame: 6 months) |
| Starting date | August 2007 |
| Contact information | Retina Associates, PA. Shawnee Mission, Kansas, United States, 66204 Contact: Lexie Manning 913‐831‐7400 lmanning@kcretina.com |
| Notes | Principal Investigator: Gregory M Fox, MD. Contacted: Lead author clarified that they are unlikely to have any data relevant to this review and will probably not go on to publish data from this trial |
NCT00931125.
| Trial name or title | Randomized, double blinded, controlled, two‐center study assessing the safety and efficacy of intravitreal ranibizumab as a preoperative adjunct treatment before vitrectomy surgery in proliferative diabetic retinopathy (PDR) compared to vitrectomy alone |
| Methods | Randomised, double‐masked, controlled trial |
| Participants | Active, not recruiting. Estimate 70 |
| Interventions | This is a randomised, double‐masked, controlled, 2‐centre study assessing the feasibility, efficacy, and safety of intravitreal ranibizumab injection applied as a preoperative adjunct treatment before vitrectomy surgery in severe PDR. Comparator arm consists of participants receiving standard vitrectomy alone with sham intravitreal injection preoperatively. |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | March 2009 Estimated study completion date: December 2013 |
| Contact information | University of Debrecen, Medical and Health Science Center, Faculty of Medicine, Department of Ophthalmology |
| Notes | Debrecen, Hungary, H‐4012 |
NCT01091896.
| Trial name or title | Adjuvant intravitreal bevacizumab in pars plana vitrectomy for vitreous hemorrhage secondary to diabetic retinopathy |
| Methods | Randomised, single‐masked, controlled trial |
| Participants | Not stated |
| Interventions | No intervention: 1 ‐ no bevacizumab. Participants will neither receive bevacizumab before nor during vitrectomy Experimental: 2 ‐ bevacizumab before vitrectomy. Participants will receive intravitreal injection of 1.25 mg of bevacizumab (0.05 ml) 7 days before vitrectomy Experimental: 3 ‐ bevacizumab after vitrectomy. Participants will receive intravitreal injection of 1.25 mg of bevacizumab (0.05 ml) at the end of vitrectomy |
| Outcomes | Primary outcome measure: recurrent vitreous haemorrhage incidence after vitrectomy Secondary outcome measure: visual outcome |
| Starting date | 2010 Estimated study completion date: March 2011 |
| Contact information | felipeppalmeida@yahoo.com.br |
| Notes | Principal Investigator: Felipe Almeida |
NCT01151722.
| Trial name or title | Adjuvant intravitreal bevacizumab in pars plana vitrectomy for diabetic vitreous hemorrhage (ABeVi) |
| Methods | Randomised, single‐masked, controlled trial |
| Participants | Not stated |
| Interventions | No intervention: no injection, no bevacizumab Experimental 2: bevacizumab before vitrectomy Experimental 3: bevacizumab after vitrectomy |
| Outcomes | Primary outcome measure: vitreous haemorrhage recurrence [ Time Frame: 3 months ] |
| Starting date | 2009 Estimated study completion date: December 2009 |
| Contact information | felipeppalmeida@yahoo.com.br |
| Notes | Principal Investigator: Felipe Almeida, MD |
NCT01306981.
| Trial name or title | A prospective, randomised controlled trial of ranibizumab pre‐treatment in diabetic vitrectomy ‐ a pilot study |
| Methods | Randomised, double‐masked, controlled study |
| Participants | Not stated |
| Interventions | Participants will be randomised 1:1 to receive either ranibizumab intravitreal injection (0.5 mg in 0.05 ml) or saline subconjunctival injection at the time of surgery |
| Outcomes | Primary outcome measures: BCVA [ Time Frame: 12 weeks post‐op ] Secondary outcome measures:
|
| Starting date | 2011 Estimated study completion date: March 2013 |
| Contact information | Moorfields Eye Hospital NHS Foundation Trust |
| Notes | Principal Investigator: Mr James W Bainbridge MA, PhD, FRCOphth. Contacted: Currently the study is in the write‐up stages. Unpublished data on the rate of POVCH are as follows: Vitreous cavity haemorrhage occurred in 4/15 in the ranibizumab (Lucentis®) arm and 6/15 in the control arm. At 12‐weeks post‐op persistent vitreous cavity haemorrhage was observed in 0/15 in the ranibizumab arm and 2/15 in the control arm. |
NCT01805297.
| Trial name or title | Intravitreal aflibercept injection as a surgical adjuvant in severe proliferative diabetic retinopathy |
| Methods | Randomised, open‐label, controlled trial |
| Participants | 12 |
| Interventions |
|
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | 2013 Estimated study completion date: April 2014 |
| Contact information | Robert‐Leonard@dmei.org |
| Notes | Principal Investigator: Robert Leonard MD |
NCT01854593.
| Trial name or title | Prospective randomized controlled study on the efficacy of 0.16 mg intravitreal bevacizumab injection for proliferative diabetic retinopathy |
| Methods | Randomised, double‐masked, controlled trial |
| Participants | 66 (recruitment completed August 2013) |
| Interventions | Control group: sham injection and 25 gauge vitrectomy Intervention group: 0.16 mg/0.05 ml bevacizumab (single injection 1 day before operation) and 25 gauge vitrectomy |
| Outcomes |
|
| Starting date | May 2012 |
| Contact information | Ayumu Manabe, Nihon University |
| Notes | Study results on ClinicalTrials.gov |
BCVA: Best‐corrected visual acuity ETDRS: Early Treatment Diabetic Retinopathy Study PDR: proliferative diabetic retinopathy TRD: tractional retinal detachment VEGF: vascular endothelial growth factor
Differences between protocol and review
Due to the importance of postoperative retinal detachment, we included an additional analysis to enable patients and surgeons to make a more informed choice when deciding to use IVB as an adjunct to vitrectomy.
Our protocol specified that we would exclude participants who received silicone oil endotamponade in the review analysis. Peer review comments on our review questioned whether this would give rise to bias, and so we presented analyses of all participants and sensitivity analyses excluding silicone oil cases. These additional analyses were not originally planned within the protocol.
Our protocol specified that we would present odds or risk ratios. We have chosen to present risk ratios as they are easier to interpret.
Contributions of authors
Jonathan Smith and David Steel were responsible for:
Conceiving the review
Designing the review
Co‐ordinating the review
Screening search results
Data management
Screening retrieved papers against inclusion criteria
Appraising quality of papers
Abstracting data from papers
Analysis of data
Interpretation of data
Entering data into RevMan
Writing the review
Updating the review
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision Group (CEVG), acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEVG Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
JS: Received money as an an unconditional grant by Novartis to cover the expense of accommodation for an international ophthalmology meeting in 2012.
DS: Acted as a consultant to Alcon, and my institution has received grant funding from Novartis.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Ahmadieh 2009 {published data only}
- Ahmadieh H, Shoeibi N, Entezari M, Monshizadeh R. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: a randomized clinical trial. Ophthalmology 2009;116(10):1943‐8. [DOI] [PubMed] [Google Scholar]
Ahn 2011 {published data only}
- Ahn J, Woo SJ, Chung H, Park KH. The effect of adjunctive intravitreal bevacizumab for preventing postvitrectomy hemorrhage in proliferative diabetic retinopathy. Ophthalmology 2011;118(11):2218‐26. [DOI] [PubMed] [Google Scholar]
di Lauro 2009 {published data only}
- Lauro R, Ruggiero P, Lauro R, Lauro MT, Romano MR. Intravitreal bevacizumab for the surgical treatment of severe proliferative retinopathy. Graefe's Archive for Clinical and Experimental Ophthalmology 2010;248(6):785‐91. [DOI] [PubMed] [Google Scholar]
El Batarny 2008 {published data only}
- Batarny AM. Intravitreal bevacizumab as an adjunctive therapy before diabetic vitrectomy. Clinical Ophthalmology 2008;2(4):709‐16. [PMC free article] [PubMed] [Google Scholar]
Elwan 2013 {published data only}
- Elwan MM, Ghanem AA, Abousamra WA. Outcome of a single intravitreal bevacizumab injection on the visual acuity and course of pars plana vitrectomy in proliferative diabetic retinopathy. Current Eye Research 2013 Sep 27 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Farahvash 2011 {published data only}
- Farahvash MS, Majidi AR, Roohipoor R, Ghassemi F. Preoperative injection of intravitreal bevacizumab in dense diabetic vitreous hemorrhage. Retina 2011;31(7):1254‐60. [DOI] [PubMed] [Google Scholar]
Hernández‐Da Mota 2010 {published data only}
- Hernández‐Da Mota SE, Nuñez‐Solorio SM. Experience with intravitreal bevacizumab as a preoperative adjunct in 23‐G vitrectomy for advanced proliferative diabetic retinopathy. European Journal of Ophthalmology 2010;20(6):1047‐52. [DOI] [PubMed] [Google Scholar]
Manabe 2015 {published data only}
- Manabe A, Shimada H, Hattori T, Nakashizuka H, Yuzawa M. Randomized controlled study of intravitreal bevacizumab 0.16 mg injected one day before surgery for proliferative diabetic retinopathy. Retina 2015 Apr 29. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Modarres 2009 {published data only}
- Modarres M, Nazari H, Falavarjani KG, Naseripour M, Hashemi M, Parvaresh MM. Intravitreal injection of bevacizumab before vitrectomy for proliferative diabetic retinopathy. European Journal of Ophthalmology 2009;19(5):848‐52. [DOI] [PubMed] [Google Scholar]
Rizzo 2008 {published data only}
- Rizzo S, Genovesi‐Ebert F, Bartolo E, Vento A, Miniaci S, Williams G. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR). Graefe's Archive for Clinical and Experimental Ophthalmology 2008;246(6):837‐42. [DOI] [PubMed] [Google Scholar]
Sohn 2012 {published data only}
- Sohn EH, He S, Kim LA, Salehi‐Had H, Javaheri M, Spee C, et al. Angiofibrotic response to vascular endothelial growth factor inhibition in diabetic retinal detachment: report no. 1. Archives of Ophthalmology 2012;130(9):1127‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaman 2013 {published data only}
- Zaman Y, Rehman A, Memon AF. Intravitreal Avastin as an adjunct in patients with proliferative diabetic retinopathy undergoing pars plana vitrectomy. Pakistan Journal of Medical Science 2013;29(2):590‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdelhakim 2011 {published data only}
- Abdelhakim MA, Macky TA, Mansour KA, Mortada HA. Bevacizumab (Avastin) as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy: a prospective case series. Ophthalmic Research 2011;45(1):23‐30. [DOI] [PubMed] [Google Scholar]
Berk Ergun 2014 {published data only}
- Berk Ergun S, Toklu Y, Cakmak HB, Raza S, Simsek S. The effect of intravitreal bevacizumab as a pretreatment of vitrectomy for diabetic vitreous hemorrhage on recurrent hemorrhage. Seminars in Ophthalmology 2015;30(3):177‐80. [DOI] [PubMed] [Google Scholar]
Bhavsar 2014 {published data only}
- Bhavsar AR, Torres K, Glassman AR, Jampol LM, Kinyoun JL, Diabetic Retinopathy Clinical Research Network. Evaluation of results 1 year following short‐term use of ranibizumab for vitreous hemorrhage due to proliferative diabetic retinopathy. JAMA Ophthalmology 2014;132(7):889‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheema 2010 {published data only}
- Cheema RA, Mushtaq J, Al‐Khars W, Al‐Askar E, Cheema MA. Role of intravitreal bevacizumab (Avastin) injected at the end of diabetic vitrectomy in preventing postoperative recurrent vitreous hemorrhage. Retina 2010;30(10):1646‐50. [DOI] [PubMed] [Google Scholar]
da R Lucena 2009 {published data only}
- R Lucena D, Ribeiro JA, Costa RA, Barbosa JC, Scott IU, Figueiredo‐Pontes LL, et al. Intraoperative bleeding during vitrectomy for diabetic tractional retinal detachment with versus without preoperative intravitreal bevacizumab (IBeTra study). British Journal of Ophthalmology 2009;93(5):688‐91. [DOI] [PubMed] [Google Scholar]
Demir 2013 {published data only}
- Demir M, Oba E, Can E, Kara O, Cinar S. Effect of bevacizumab injection before vitrectomy on intravitreal hemorrhage in pseudophakic patients with proliferative diabetic retinopathy. Ophthalmology and Eye Diseases 2013;5:11‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doganay 2010 {published data only}
- Doganay S, Koc B, Cankaya C, Duz C, Bilak S. The effect on surgical success of intravitreal bevacizumab given before pars plana vitrectomy in diabetic patients [Diyabetik olgularda pars plana vitrektomiden once uygulanan intravitreal bevacizumabın cerrahi başarıya etkisi]. Retina‐Vitreus 2010;18(4):310‐3. [Google Scholar]
Goncu 2014 {published data only}
- Goncu T, Ozdek S, Unlu M. The role of intraoperative bevacizumab for prevention of postoperative vitreous hemorrhage in diabetic vitreous hemorrhage. European Journal of Ophthalmology 2014;24(1):88‐93. [DOI] [PubMed] [Google Scholar]
Gupta 2008 {published data only}
- Gupta A, Gupta V, Gupta P, Dogra M. Preoperative intravitreal bevacizumab improves visual outcome in diabetic vitreous surgery. American Academy of Ophthalmology 2008:252. [Google Scholar]
Gupta 2012a {published data only}
- Gupta B, Sivaprasad S, Wong R, Laidlaw A, Jackson TL, McHugh D, et al. Visual and anatomical outcomes following vitrectomy for complications of diabetic retinopathy: the DRIVE UK study. Eye 2012;26(4):510‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2012b {published data only}
- Gupta A, Bansal R, Gupta V, Dogra MR. Six‐month visual outcome after pars plana vitrectomy in proliferative diabetic retinopathy with or without a single preoperative injection of intravitreal bevacizumab. International Ophthalmology 2012;32(2):135‐44. [DOI] [PubMed] [Google Scholar]
Ishikawa 2009 {published data only}
- Ishikawa K, Honda S, Tsukahara Y, Negi A. Preferable use of intravitreal bevacizumab as a pretreatment of vitrectomy for severe proliferative diabetic retinopathy. Eye 2009;23(1):108‐11. [DOI] [PubMed] [Google Scholar]
Jirawison 2012 {published data only}
- Jirawison C, Ittipunkul N. Intravitreal bevacizumab at the end of diabetic vitrectomy for prevention of postoperative vitreous hemorrhage: a comparative study. Journal of the Medical Association of Thailand 2012;95(Suppl 4):S136‐42. [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li CR, Sun SG, Hong W. Effect of intravitreal bevacizumab injection before vitrectomy on proliferative diabetic retinopathy. International Journal of Ophthalmology 2010;3(3):261‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lo 2009 {published data only}
- Lo WR, Kim SJ, Aaberg TM Sr, Bergstrom C, Srivastava SK, Yan J, et al. Visual outcomes and incidence of recurrent vitreous hemorrhage after vitrectomy in diabetic eyes pretreated with bevacizumab (Avastin). Retina 2009;29(7):926‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01589718 {unpublished data only}
- NCT01589718. A phase III randomized 1:1, masked, study of the safety, tolerability, and efficacy of intravitreal pre‐op 0.3mg pegaptanib sodium versus sham, for adjuvant management of TRD and vit hem associated with PDR. clinicaltrials.gov/show/NCT01589718 (accessed 22 June 2015).
Oshima 2009 {published data only}
- Oshima Y, Shima C, Wakabayashi T, Kusaka S, Shiraga F, Ohji M, et al. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology 2009;116(5):927‐38. [DOI] [PubMed] [Google Scholar]
Pakzad‐Vaezi 2014 {published data only}
- Pakzad‐Vaezi K, Albiani DA, Kirker AW, Merkur AB, Kertes PJ, Eng KT, et al. A randomized study comparing the efficacy of bevacizumab and ranibizumab as pre‐treatment for pars plana vitrectomy in proliferative diabetic retinopathy. Ophthalmic Surgery Lasers and Imaging Retina 2014;45(6):521‐4. [DOI] [PubMed] [Google Scholar]
Park 2010 {published data only}
- Park DH, Shin JP, Kim SY. Intravitreal injection of bevacizumab and triamcinolone acetonide at the end of vitrectomy for diabetic vitreous hemorrhage: a comparative study. Graefes Archive for Clinical and Experimental Ophthalmology 2010;248(5):641‐50. [DOI] [PubMed] [Google Scholar]
Pokroy 2011 {published data only}
- Pokroy R, Desai UR, Du E, Li Y, Edwards P. Bevacizumab prior to vitrectomy for diabetic traction retinal detachment. Eye 2011;28(8):989‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romano 2009a {published data only}
- Romano MR, Gibran SK, Marticorena J, Wong D, Heimann H. Can a preoperative bevacizumab injection prevent recurrent postvitrectomy diabetic vitreous haemorrhage?. Eye 2009;23(8):1698‐701. [DOI] [PubMed] [Google Scholar]
Romano 2009b {published data only}
- Romano MR, Gibran SK, Marticorena J, Wong D. Can an intraoperative bevacizumab injection prevent recurrent postvitrectomy diabetic vitreous hemorrhage?. European Journal of Ophthalmology 2009;19(4):618‐21. [DOI] [PubMed] [Google Scholar]
Sato 2013 {published data only}
- Sato T, Morita S, Bando H, Sato S, Ikeda T, Emi K. Early vitreous hemorrhage after vitrectomy with preoperative intravitreal bevacizumab for proliferative diabetic retinopathy. Middle East African Journal of Ophthalmology 2013;20(1):51‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2008 {published data only}
- Yang CM, Yeh PT, Yang CH, Chen MS. Bevacizumab pretreatment and long‐acting gas infusion on vitreous clear‐up after diabetic vitrectomy. American Journal of Ophthalmology 2008;146(2):211‐7. [DOI] [PubMed] [Google Scholar]
Yeh 2009 {published data only}
- Yeh PT, Yang CM, Lin YC, Chen MS, Yang CH. Bevacizumab pretreatment in vitrectomy with silicone oil for severe diabetic retinopathy. Retina 2009;29(6):768‐74. [DOI] [PubMed] [Google Scholar]
Yeoh 2008 {published data only}
- Yeoh J, Williams C, Allen P, Buttery R, Chiu D, Clark B. Avastin as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy: a prospective case series. Clinical and Experimental Ophthalmology 2008;36(5):449‐54. [PubMed] [Google Scholar]
Yeung 2010 {published data only}
- Yeung L, Liu L, Wu WC, Kuo YH, Chao AN, Chen KJ, et al. Reducing the incidence of early postoperative vitreous haemorrhage by preoperative intravitreal bevacizumab in vitrectomy for diabetic tractional retinal detachment. Acta Opthalmologica 2010;88(6):635‐40. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN79120387 {unpublished data only}
- ISRCTN79120387. A randomised, single‐masked, phase IV pilot study of the efficacy and safety of adjunctive intravitreal Avastin® (bevacizumab) in the prevention of early postoperative vitreous haemorrhage following diabetic vitrectomy. www.controlled‐trials.com/ISRCTN79120387 (accessed 22 June 2015).
NCT00516464 {unpublished data only}
- NCT00516464. Evaluation of ranibizumab in proliferative diabetic retinopathy (PDR) requiring vitrectomy. clinicaltrials.gov/ct2/show/NCT00516464 (accessed 22 June 2015).
NCT00931125 {unpublished data only}
- NCT00931125. Randomized, double blinded, controlled, two‐center study assessing the safety and efficacy of intravitreal ranibizumab as a preoperative adjunct treatment before vitrectomy surgery in proliferative diabetic retinopathy (PDR) compared to vitrectomy alone. clinicaltrials.gov/show/NCT00931125 (accessed 22 June 2015).
NCT01091896 {unpublished data only}
- NCT01091896. Adjuvant intravitreal bevacizumab in pars plana vitrectomy for vitreous hemorrhage secondary to diabetic retinopathy. clinicaltrials.gov/show/NCT01091896 (accessed 22 June 2015).
NCT01151722 {unpublished data only}
- NCT01151722. Adjuvant intravitreal bevacizumab in pars plana vitrectomy for diabetic vitreous hemorrhage. clinicaltrials.gov/show/NCT01151722 (accessed 22 June 2015).
NCT01306981 {unpublished data only}
- NCT01306981. A prospective, randomised controlled trial of ranibizumab pre‐treatment in diabetic vitrectomy ‐ a pilot study. clinicaltrials.gov/show/NCT01306981 (accessed 22 June 2015).
NCT01805297 {unpublished data only}
- NCT01805297. Intravitreal aflibercept injection as a surgical adjuvant in severe proliferative diabetic retinopathy. clinicaltrials.gov/show/NCT01805297 (accessed 22 June 2015).
NCT01854593 {unpublished data only}
- NCT01854593. Prospective randomized controlled study of intravitreal injection of 0.16 mg bevacizumab one day before surgery for proliferative diabetic retinopathy. clinicaltrials.gov/show/NCT01854593 (accessed 22 June 2015).
Additional references
Anonymous 1985
- Anonymous. Two‐year course of visual acuity in severe proliferative diabetic retinopathy with conventional management. Diabetic Retinopathy Vitrectomy Study (DRVS) report #1. Ophthalmology 1985;92(4):492‐502. [DOI] [PubMed] [Google Scholar]
Benson 1988
- Benson WE, Brown GC, Tasman W, McNamara JA. Complications of vitrectomy for non‐clearing vitreous hemorrhage in diabetic patients. Ophthalmic Surgery 1988;19(12):862‐4. [PubMed] [Google Scholar]
Bhende 2000
- Bhende M, Agraharam SG, Gopal L, Sumasri K, Sukumar B, George J, et al. Ultrasound biomicroscopy of sclerotomy sites after pars plana vitrectomy for diabetic vitreous hemorrhage. Ophthalmology 2000;107(9):1729‐36. [DOI] [PubMed] [Google Scholar]
Blankenship 1986
- Blankenship GW. Management of vitreous cavity hemorrhage following pars plana vitrectomy for diabetic retinopathy. Ophthalmology 1986;93(1):39‐44. [DOI] [PubMed] [Google Scholar]
Blumenkranz 1986
- Blumenkranz M, Gardner T, Blankenship G. Fluid‐gas exchange and photocoagulation after vitrectomy. Indications, technique, and results. Archives of Ophthalmology 1986;104(2):291‐6. [DOI] [PubMed] [Google Scholar]
Brown 1992
- Brown GC, Tasman WS, Benson WE, McNamara JA, Eagle RC Jr. Reoperation following diabetic vitrectomy. Archives of Ophthalmology 1992;110(4):506‐10. [DOI] [PubMed] [Google Scholar]
Diabetes UK 2014
- Diabetes UK. Diabetes:facts and stats. www.diabetes.org.uk/Documents/About%20Us/Statistics/Diabetes‐key‐stats‐guidelines‐April2014.pdf (accessed 10 July 2015).
Faghihi 2008
- Faghihi H, Taheri A, Farahvash MS, Esfahani MR, Rajabi MT. Intravitreal triamcinolone acetonide injection at the end of vitrectomy for diabetic vitreous hemorrhage: a randomized, clinical trial. Retina 2008;28(9):1241‐6. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- Macmasters University. Computer program on www.gradepro.org. 2014: Macmasters University, (accessed 21 April 2015).
Han 1991
- Han DP, Murphy ML, Mieler WF, Abrams GW. Outpatient fluid‐air exchange for severe postvitrectomy diabetic vitreous hemorrhage. Long‐term results and complications. Retina 1991;11(3):309‐14. [DOI] [PubMed] [Google Scholar]
Hershberger 2004
- Hershberger VS, Augsburger JJ, Hutchins RK, Raymond LA, Krug S. Fibrovascular ingrowth at sclerotomy sites in vitrectomized diabetic eyes with recurrent vitreous hemorrhage: ultrasound biomicroscopy findings. Ophthalmology 2004;111(6):1215‐21. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ho 1992
- Ho T, Smiddy WE, Flynn HW Jr. Vitrectomy in the management of diabetic eye disease. Survey of Ophthalmology 1992;37(3):190‐202. [DOI] [PubMed] [Google Scholar]
Hotta 2000
- Hotta K, Hirakata A, Ohi Y, Yamamoto R, Shinoda K, Oshitari K, et al. Ultrasound biomicroscopy for examination of the sclerotomy site in eyes with proliferative diabetic retinopathy after vitrectomy. Retina 2000;20(1):52‐8. [DOI] [PubMed] [Google Scholar]
Joondeph 1989
- Joondeph BC, Blankenship GW. Hemostatic effects of air versus fluid in diabetic vitrectomy. Ophthalmology 1989;96(12):1701‐6. [DOI] [PubMed] [Google Scholar]
Kaiser 2000
- Kaiser RS, Maguire MG, Grunwald JE, Lieb D, Jani B, Brucker AJ, et al. One‐year outcomes of panretinal photocoagulation in proliferative diabetic retinopathy. American Journal of Ophthalmology 2000;129(2):178‐85. [DOI] [PubMed] [Google Scholar]
Klein 1984a
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Archives of Ophthalmology 1984;102(4):520‐6. [DOI] [PubMed] [Google Scholar]
Klein 1984b
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Archives of Ophthalmology 1984;102(4):527‐32. [DOI] [PubMed] [Google Scholar]
Koutsandrea 2001
- Koutsandrea CN, Apostolopoulos MN, Chatzoulis DZ, Parikakis EA, Theodossiadis GP. Hemostatic effects of SF6 after diabetic vitrectomy for vitreous hemorrhage. Acta Ophthalmologica Scandinavica 2001;79(1):34‐8. [DOI] [PubMed] [Google Scholar]
Kreiger 1993
- Kreiger AE. Wound complications in pars plana vitrectomy. Retina 1993;13(4):335‐44. [DOI] [PubMed] [Google Scholar]
Laatikainen 1987
- Laatikainen L, Summanen P, Immonen I. Effect of tranexamic acid on postvitrectomy haemorrhage in diabetic patients. International Ophthalmology 1987;10(3):153‐5. [DOI] [PubMed] [Google Scholar]
Liew 2014
- Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16‐64 years), 1999‐2000 with 2009‐2010. BMJ Open 2014;12(4):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liggett 1987
- Liggett PE, Lean JS, Barlow WE, Ryan SJ. Intraoperative argon endophotocoagulation for recurrent vitreous hemorrhage after vitrectomy for diabetic retinopathy. American Journal of Ophthalmology 1987;103(2):146‐9. [DOI] [PubMed] [Google Scholar]
Martin 1992
- Martin DF, McCuen BW 2nd. Efficacy of fluid‐air exchange for postvitrectomy diabetic vitreous hemorrhage. American Journal of Ophthalmology 1992;114(4):457‐63. [DOI] [PubMed] [Google Scholar]
Mason 1978
- Mason G, Peyman GA. The role of vitrectomy in diabetic retinopathy. International Ophthalmology Clinics 1978;18(4):133‐64. [PubMed] [Google Scholar]
McLeod 1991
- McLeod D. Microsurgical management of neovascularisation secondary to posterior segment ischaemia. Eye 1991;5(Pt 2):252‐9. [DOI] [PubMed] [Google Scholar]
McLeod 2000
- McLeod D. Entry site neovascularisation after diabetic vitrectomy. British Journal of Ophthalmology 2000;84(8):810‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
McLeod 2003
- McLeod D. Wieger's ligament [republished in Ophthalmology. 2003 Jun;110(6):1265]. Ophthalmology 2003;110(4):628. [DOI] [PubMed] [Google Scholar]
Novak 1984
- Novak MA, Rice TA, Michels RG, Auer C. Vitreous hemorrhage after vitrectomy for diabetic retinopathy. Ophthalmology 1984;91(12):1485‐9. [DOI] [PubMed] [Google Scholar]
Packer 1989
- Packer AJ, McCuen BW 2nd, Hutton WL, Ramsay RC. Procoagulant effects of intraocular sodium hyaluronate (Healon) after phakic diabetic vitrectomy. A prospective, randomized study. Ophthalmology 1989;96(10):1491‐4. [DOI] [PubMed] [Google Scholar]
Ramezani 2005
- Ramezani AR, Ahmadieh H, Ghaseminejad AK, Yazdani S, Golestan B. Effect of tranexamic acid on early postvitrectomy diabetic haemorrhage; a randomised clinical trial. British Journal of Ophthalmology 2005;89(8):1041‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sawa 2000
- Sawa H, Ikeda T, Matsumoto Y, Niiya A, Kinoshita S. Neovascularization from scleral wound as cause of vitreous rebleeding after vitrectomy for proliferative diabetic retinopathy. Japanese Journal of Ophthalmology 2000;44(2):154‐60. [DOI] [PubMed] [Google Scholar]
Scanlon 2008
- Scanlon PH. The English national screening programme for sight threatening diabetic retinopathy. Journal of Medical Screening 2008;15(1):1‐4. [DOI] [PubMed] [Google Scholar]
Schachat 1983
- Schachat AP, Oyakawa RT, Michels RG, Rice TA. Complications of vitreous surgery for diabetic retinopathy. II. Postoperative complications. Ophthalmology 1983;90(5):522‐30. [DOI] [PubMed] [Google Scholar]
Schiff 2007
- Schiff WM, Barile GR, Hwang JC, Tseng JJ, Cekic O, Priore LV, et al. Diabetic vitrectomy: influence of lens status upon anatomic and visual outcomes. Ophthalmology 2007;114(3):544‐50. [DOI] [PubMed] [Google Scholar]
Schwatz 1996
- Schwatz SD, Alexander R, Hiscott P, Gregor ZJ. Recognition of vitreoschisis in proliferative diabetic retinopathy. A useful landmark in vitrectomy for diabetic traction retinal detachment. Ophthalmology 1996;103(2):323‐8. [DOI] [PubMed] [Google Scholar]
Steel 2008
- Steel DH, Habib MS, Park S, Hildreth AJ, Owen RI. Entry site neovascularization and vitreous cavity hemorrhage after diabetic vitrectomy. The predictive value of inner sclerostomy site ultrasonography. Ophthalmology 2008;115(3):525‐32. [DOI] [PubMed] [Google Scholar]
Tolentino 1989
- Tolentino FI, Cajita VN, Gancayco T, Skates S. Vitreous hemorrhage after closed vitrectomy for proliferative diabetic retinopathy. Ophthalmology 1989;96(10):1495‐500. [DOI] [PubMed] [Google Scholar]
Vaideanu 2014
- Vaideanu D, Sandhu SS, Ling J, Richardson J, Steel DH. Rate of diabetic vitrectomy in a defined geographical part of North East England. Ophthalmic Epidemiology 2014;21(3):178‐83. [DOI] [PubMed] [Google Scholar]
Veliz 2014
- Veliz D, Rada G. Does preoperative intravitreal bevacizumab reduce complications of vitrectomy for proliferative diabetic retinopathy? [El uso preoperatorio de bevacizumab reduce lascomplicaciones en pacientes con retinopatía diabéticasometidos a vitrectomía?]. Medwave 2014;14(11):e6052. [DOI] [PubMed] [Google Scholar]
Virata 2001
- Virata SR, Kylstra JA. Postoperative complications following vitrectomy for proliferative diabetic retinopathy with sew‐on and noncontact wide‐angle viewing lenses. Ophthalmic Surgery and Lasers 2001;32(3):193‐7. [PubMed] [Google Scholar]
West 2000
- West JF, Gregor ZJ. Fibrovascular ingrowth and recurrent haemorrhage following diabetic vitrectomy. British Journal of Ophthalmology 2000;84(8):822‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2007
- Yang CM, Yeh PT, Yang CH. Intravitreal long‐acting gas in the prevention of early postoperative vitreous hemorrhage in diabetic vitrectomy. Ophthalmology 2007;114(4):710‐5. [DOI] [PubMed] [Google Scholar]
Yeh 2005
- Yeh PT, Yang CM, Yang CH, Huang JS. Cryotherapy of the anterior retina and sclerotomy sites in diabetic vitrectomy to prevent recurrent vitreous hemorrhage: an ultrasound biomicroscopy study. Ophthalmology 2005;112(12):2095‐102. [DOI] [PubMed] [Google Scholar]
Yorston 2008
- Yorston D, Wickham L, Benson S, Bunce C, Sheard R, Charteris D. Predictive clinical features and outcomes of vitrectomy for proliferative diabetic retinopathy. British Journal of Ophthalmology 2008;92(3):365‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2013
- Zhang ZH, Liu HY, Hernandez‐Da Mota SE, Romano MR, Falavarjani KG, Ahmadieh H, et al. Vitrectomy with or without preoperative intravitreal bevacizumab for proliferative diabetic retinopathy: a meta‐analysis of randomized controlled trials. American Journal of Ophthalmology 2013;156(1):106‐15. [DOI] [PubMed] [Google Scholar]
Zhao 2011
- Zhao LQ, Zhu H, Zhao PQ, Hu YQ. A systematic review and meta‐analysis of clinical outcomes of vitrectomy with or without intravitreal bevacizumab pretreatment for severe diabetic retinopathy. British Journal of Ophthalmology 2011;95(9):1216‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Smith 2011
- Smith JM, Steel DHW. Anti‐vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD008214.pub2] [DOI] [PubMed] [Google Scholar]
Steel 2010
- Steel DHW, Smith JM. Anti‐vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD008214] [DOI] [Google Scholar]


