Abstract
Background
According to consensus, initiation of therapy is best based on CD4 cell count, a marker of immune status, rather than on viral load, a marker of virologic replication. For patients with advanced symptoms, treatment should be started regardless of CD4 count. However, the point during the course of HIV infection at which antiretroviral therapy (ART) is best initiated in asymptomatic patients remains unclear. Guidelines issued by various agencies provide different initiation recommendations according to resource availability. This can be confusing for clinicians and policy‐makers when determining the best time to initiate therapy. Optimizing the initiation of ART is clearly complex and must, therefore, be balanced between individual and broader public health needs.
Objectives
To assess the evidence for the optimal time to initiate ART in treatment‐naive, asymptomatic, HIV‐infected adults
Search methods
We formulated a comprehensive and exhaustive search strategy in an attempt to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress). In August 2009, we searched the following electronic journal and trial databases: MEDLINE, EMBASE, and CENTRAL. We also searched the electronic conference database of NLM Gateway, individual conference proceedings and prospective trials registers. We contacted researchers and relevant organizations and checked reference lists of all included studies.
Selection criteria
Randomized controlled trials that compared the effect of ART consisting of three drugs initiated early in the disease at high CD4 counts as defined by the trial. Early initiation could be at levels of 201‐350, 351‐500, or >500 cells/µL, with the comparison group initiating ART at CD4 counts below 200 x 106 cells/µL or as defined by the trial.
Data collection and analysis
Two review authors independently assessed study eligibility, extracted data, and graded methodological quality. Data extraction and methodological quality were checked by a third author who resolved differences when these arose. Where clinically meaningful to do so, we meta‐analysed dichotomous outcomes using the relative risk (RR) and report the 95% confidence intervals (95% CIs).
Main results
One completed trial (N = 816) and one sub‐group (N = 249) of a larger trial met inclusion criteria. We combined the mortality data for both trials comparing initiating ART at CD4 levels at 350 cells/µL or between 200 and 350 cells/µL with deferring initiation of ART to CD4 levels of 250 cells/µL or 200 cells/µL. There was a statistically significant reduction in death when starting ART at higher CD4 counts. Risk of death was reduced by 74% (RR = 0.26; 95% CI: 0.11, 0.62; P = 0.002). Risk of tuberculosis was reduced by 50% in the groups starting ART early; this was not statistically significant, with the reduction as much as 74% or an increased risk of up to 12% (RR = 0.54; 95% CI: 0.26, 1.12; P = 0.01). Starting ART at enrollment (when participants had CD4 counts of 350 cells/µL) rather than deferring to starting at a CD4 count of 250 cells/µL reduced the risk of disease progression by 70%; this was not statistically significant, with the reduction in risk as much as 97% or an increased risk of up to 185% (RR = 0.30; 95% CI: 0.03, 2.85; P = 0.29).
One RCT found no statistically significant difference in the number of independent Grade 3 or 4 adverse events occurring in the early and standard ART groups when we conducted an intention‐to‐treat analysis (RR = 1.72; 95% CI: 0.98, 3.03; P = 0.06). However, when analyzing only participants who actually commenced ART in the deferred group (n = 160), the trial authors report a statistically significant increase in the incidence of zidovudine‐related anaemia (8.1%) compared with those in the early initiation group (3.4%) (RR = 0.42; 95% CI: 0.20, 0.88; P = 0.02).
Authors' conclusions
There is evidence of moderate quality that initiating ART at CD4 levels higher than 200 or 250 cells/µL reduces mortality rates in asymptomatic, ART‐naive, HIV‐infected people. Practitioners and policy‐makers may consider initiating ART at levels ≤ 350 cells/µL for patients who present to health services and are diagnosed with HIV early in the infection.
Plain language summary
When is the best time to start antiretroviral therapy in people with HIV infection, who have not received antiretroviral treatment before and who do not have any symptoms of HIV illness?
Antiretroviral therapy (ART) has been shown to be effective in slowing down the progression of AIDS and in reducing HIV‐related illnesses and death. Traditionally, therapy is administered based on a patient’s CD4 cell count, where the number of CD4 cells reflects the body’s immune (defense) system. An HIV‐infected individual with a CD4 cell count of 500 cells/µL is considered healthy enough not to need ART. When a patient’s cell count reaches 200 cells/ µL, however, the immune system is severely weakened and ART is necessary. A patient with advanced symptoms receives treatment regardless of CD4 count.
Recommendations on the timing for ART initiation differ based on availability of resources, leading to confusion amongst clinicians and policy‐makers in determining the most favorable point to begin treatment. The objective of this review is to assess the evidence for the optimal time to initiate ART in HIV‐infected adults who have not previously received therapy and who do not have symptoms of HIV illness.
The authors reviewed two trials which involved 1,065 participants. Both studies compared the effect of ART initiation at high CD4 counts (350 cells/µL) with ART initiation at low CD4 counts (250 cells/µL). Results showed that starting ART at higher levels of CD4 reduces mortality rates in HIV‐infected individuals who have not received antiretroviral treatment before and who do not have any symptoms of HIV illness.
Summary of findings
Summary of findings for the main comparison. Early ART versus standard or deferred ART (CD4 <= 200 or CD4 <= 250 cells/µl) for asymptomatic, HIV‐infected, treatment naive adults.
| Early ART versus standard or deferred ART (CD4 ⤠200 or CD4 ⤠250 cells/µl) for asymptomatic, HIV‐infected, treatment naive adults | ||||||
| Patient or population: patients with asymptomatic, HIV‐infected, treatment naive adults Settings: Intervention: Early ART versus standard or deferred ART (CD4 ⤠200 or CD4 ⤠250 cells/µl) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Early ART versus standard or deferred ART (CD4 ⤠200 or CD4 ⤠250 cells/µl) | |||||
| Death | Study population | RR 0.26 (0.11 to 0.62) | 1065 (2 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 46 per 1000 | 12 per 1000 (5 to 29) | |||||
| Medium risk population | ||||||
| 32 per 1000 | 8 per 1000 (4 to 20) | |||||
| Tuberculosis | Study population | RR 0.54 (0.26 to 1.12) | 1065 (2 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 68 per 1000 | 37 per 1000 (18 to 76) | |||||
| Medium risk population | ||||||
| 44 per 1000 | 24 per 1000 (11 to 49) | |||||
| Disease progression measured by opportunistic disease Opportunistic disease events Follow‐up: mean 18 months | Study population | OR 0.29 (0.03 to 2.87) | 249 (1 study) | ⊕⊕⊝⊝ low1,2,3 | ||
| 25 per 1000 | 7 per 1000 (1 to 69) | |||||
| Medium risk population | ||||||
| 25 per 1000 | 7 per 1000 (1 to 69) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The SMART study is a post hoc analysis of a sub‐set of a larger trial 2 As the SMART sub‐set is a post hoc analysis there may be other trials which did not conduct or publish similar analyses of potential sub‐sets within the original trials. This is a form of publication bias and we have therefore downgraded the results accordingly 3 This result is a post hoc subset analysis from only one trial and the evidence is therefore not directly able to answer the outcome of disease progression
Background
Provision of combination antiretroviral treatment (ART) to people infected with human immunodeficiency virus (HIV) reduces both progression to the acquired immunodeficiency syndrome (AIDS) and the morbidity and mortality associated with advanced HIV infection. According to consensus, initiation of therapy is best based on CD4 count, a marker of immune status, rather than on viral load, a marker of virologic replication (Sterling 2001). For patients with advanced symptoms, treatment should be started regardless of CD4 count; however, the point during the course of HIV infection at which ART is best initiated in asymptomatic patients remains unclear, and in a 2006 BMJ review, Deeks clearly articulated optimal timing of ART initiation as a key unanswered question for people infected with HIV, clinicians, and policy‐makers (Deeks 2006).
Guidelines issued by various agencies provide different initiation recommendations according to resource availability. This can be confusing for clinicians and policy‐makers when they are determining the best time to initiate therapy. In 2008, the United States Panel of the International AIDS Society recommended that ART in adults with HIV infection should not be initiated before CD4 cell count declines to less than 350 cells/µL (Hammer 2008). In patients with 350 CD4 cells/µL or more, the decision to begin therapy should be individualized based on the presence of comorbidities, risk factors for progression to AIDS and non‐AIDS defining diseases (Hammer 2008). In comparison, in resource‐constrained settings, the World Health Organization recommends that ART should not be initiated at concentrations of CD4 counts above 200 cells/µL in asymptomatic patients (WHO 2006) and does not address initiation at higher concentration of CD4 cells. Optimizing the initiation of ART is clearly complex and must, therefore, be balanced between individual and broader public health needs.
Historically, clinicians determined initiation of ART by balancing the risks and benefits of delaying treatment (Sabin 2009). Original ART formulations had high pill counts, inconvenient dosing instructions and often substantial toxicities which favoured a delay in treatment to avoid poor adherence early in the disease. Currently there are better‐tolerated ART formulations and an increase in the number of treatment options available (Schrader 2008). Additionally, targeted adherence interventions have been shown to be associated with high adherence rates (Orrell 2007). Initiating early treatment has the benefit of reducing or avoiding the irreversible damage done by HIV and opportunistic infections (OIs) (Day 2002). Treating patients at higher CD4 counts may reduce infectivity and so play an important role in community prevention, although this has not been proved conclusively (Granich 2009). Delaying ART until later risks a deteriorating immune function, development of OIs, declining quality of life and progression to AIDS and death, but starting ART too early has the disadvantage of exhausting drug options (as viral resistance is more likely to occur the longer treatment progresses) and the patient’s ability to tolerate drugs (Day 2002).
Several studies have modeled the risks and benefits of starting ART at different CD4 counts using cost‐effectiveness models and have found benefit for starting ART earlier (Palella 2003; Schackman 2002; Walensky 2009). A recent study using validated computer simulation to weigh important harms from earlier initiation of ART (toxicity, side effects, and resistance accumulation) against important benefits (decreased HIV‐related mortality) found that earlier initiation of ART is often favoured compared with current recommendations but cautioned that the findings may not be generalizable to women (Braithwaite 2008). Two recent cohort studies from the USA and Canada recommended that initiation of ART begin at levels at least over 350 cells/µL and possibly over 500 cells/µL (Kitahata 2009) after analyses found improved survival in patients begun on ART at higher levels. To our knowledge no similar studies have been conducted in resource‐poor settings.
Ideally, randomised controlled trials that compare clinical, virologic and immunologic outcomes in asymptomatic patients initiating ART at different CD4 levels provide the best evidence to determine at what levels initiation of treatment is optimised. This systematic review of such trials will provide a much‐needed evidence base to assist clinicians, policy‐makers and consumers in their decision‐making and was used by the World Health Organization (WHO) to develop its 2009 ART treatment guidelines for adults and adolescents (WHO 2009).
Objectives
To assess the evidence for the optimal time to initiate ART in treatment‐naive, asymptomatic, HIV‐infected adults
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials
Types of participants
Asymptomatic, HIV‐infected, treatment‐naive adults (15 years of age and older)
Trials of participants co‐infected with hepatitis B or C were not excluded from this review. Trials of participants who are symptomatic regardless of CD4 counts were excluded from this review. Trials of initiation of ART in participants co‐infected with tuberculosis (TB) were excluded from this review as a concurrent Cochrane review is being conducted on this topic.
Types of interventions
ART consisting of three drugs initiated early in the disease at high CD4 counts, as defined by the trial. In adults, the definition of high count may be at levels of 201‐350, 351‐500 or >500 cells/µL. The comparison group will be when ART is initiated at CD4 counts below 200 cells/µL, or as defined by the trial.
Types of outcome measures
Primary outcomes
Death (all cause)
-
Responses to ART as measured by:
Clinical occurrence of new HIV‐related events (death or AIDS‐defining illness)
Proportion of patients achieving and maintaining an undetectable viral load, as defined by the trial
Time to event of new HIV‐related events (death or AIDS‐defining illness)
Immunologic response (change in mean CD4+ cell count (mean relative change (percent) or mean absolute change compared with baseline), and standard deviation)
Virologic response (proportion of patients maintaining an undetectable viral load and/or change in HIV‐RNA levels (mean relative change (percent) or mean absolute change, compared with baseline), and standard deviation
Secondary outcomes
Proportion of patients discontinuing or switching ART due to virologic failure, as defined by the trial
Development of ART resistance
Proportion of participants remaining on therapy as originally assigned at the end of the trial
Quality of life indicators as reported in the studies
ADVERSE EVENTS Severe adverse events are reported. If classified according to grade 1 to 4 of the Adverse Event Toxicity Scale, we report grade 3 and 4 events. Using this scale, grade 1 and 2 denote mild to moderate symptoms, grade 3 denotes serious symptoms and grade 4 denotes life‐threatening events requiring significant clinical intervention. Grade 5 denotes death. (DAIDS 2009)
Search methods for identification of studies
See: HIV/AIDS Collaborative Review Group search strategy
Electronic searches
We developed the search strategy with the assistance of the HIV/AIDS Review Group Trials Search Co‐ordinator. We formulated a comprehensive and exhaustive search strategy in an attempt to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress). Full details of the Cochrane HIV/AIDS Review Group methods and the journals hand‐searched are published in the section on Collaborative Review Groups in The Cochrane Library. We combined the RCT strategy developed by The Cochrane Collaboration and detailed in the Cochrane Reviewers' Handbook (Higgins 2008) in combination with terms specific to initiation of ART. We limited the date‐of‐publication year to 1996 onwards because triple‐drug ART was not used before this year. The search was iterative and a number of trial searches were run first as there are no database‐specific terms for 'initiation' of treatment and so we used many free‐text terms. This increased the yield and hence the search sensitivity but reduced the precision. We searched the following electronic databases:
1. Journal and trial databases
MEDLINE This search was conducted on 4 August 2009 using the strategy outlined in Table 3. This yielded 1389 records of which we identified 42 records for full article retrieval.
1. Search strategy for PUBMED.
| ID | Search | Hits |
| #5 | Search #1 AND #2 AND #3 AND #4 Limits: Publication Date from 1996 to 2009 | 1389 |
| #4 | Search "THERAPY INITIATION" OR "TREATMENT INITIATION" OR "DRUG THERAPY INITIATION" OR"WHEN TO START" OR "EARLY INITIATION" OR DRUG ADMINISTRATION SCHEDULE[MeSH Terms] OR "DRUG ADMINISTRATION SCHEDULE" | 71826 |
| #3 | Search Antiretroviral Therapy, Highly Active[MeSH] OR Anti‐Retroviral Agents[MeSH] OR Antiviral Agents[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])) OR ((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])) | 99790 |
| #2 | Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MESH:noexp]) | 247596 |
| #1 | Search randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) | 3130518 |
EMBASE This search was conducted on 4 August 2009 using the strategy outlined in Table 4. This yielded 547 records of which we identified 12 records for full article retrieval.
2. Search strategy for EMBASE.
| ID | Search | Hits |
| #5 | #1 AND #2 AND #3 AND #4 | 838 |
| #4 | random*:ti OR random*:ab OR factorial*:ti OR factorial*:ab OR cross?over*:ti OR cross?over:ab OR crossover*:ti OR crossover*:ab OR placebo*:ti OR placebo*:ab OR (doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab) OR (singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab) OR assign*:ti OR assign*:ab OR volunteer*:ti OR volunteer*:ab OR 'crossover procedure'/exp OR 'double‐blind procedure'/exp OR 'single‐blind procedure'/exp OR 'randomized controlled trial'/exp OR allocat*:ti OR allocat*:ab AND [1990‐2009]/py | 751,259 |
| #3 | 'drug administration schedule'/exp OR 'when to start' OR 'drug therapy initiation' OR 'treatment initiation' OR 'therapy initiation' AND [1990‐2009/py | 478,436 |
| #2 | 'human immunodeficiency virus vaccine'/exp OR 'anti human immunedeficiency':ti OR 'anti human immunedeficiency':ab OR 'anti human immunodeficiency':ti OR 'anti human immunodeficiency':ab OR 'anti human immuno‐deficiency':ti OR 'anti human immuno‐deficiency':ab OR 'anti human immune‐deficiency':ti OR 'anti human immune‐deficiency':ab OR 'anti acquired immune‐deficiency':ti OR 'anti acquired immune‐deficiency':ab OR 'anti acquired immunedeficiency':ti OR 'anti acquired immunedeficiency':ab OR 'anti acquired immunodeficiency':ti OR 'anti acquired immunodeficiency':ab OR 'anti acquired immuno‐deficiency':ti OR 'anti acquired immuno‐deficiency':ab OR 'anti hiv':ti OR 'anti hiv':ab OR antiretrovir*:ti OR antiretrovir*:ab OR 'anti retroviral':ti OR 'anti retroviral':ab OR 'anti retrovirals':ti OR 'anti retrovirals':ab OR 'anti retrovirus':ti OR 'anti retrovirus':ab OR haart:ti OR haart:ab OR 'aids vaccine':ti OR 'aids vaccine':ab OR 'aids vaccines':ti OR 'aids vaccines':ab OR 'anti human immunodeficiency virus agent'/exp OR 'antiretrovirus agent'/exp OR 'antivirus agent'/exp OR 'highly active antiretroviral therapy'/exp AND [1990‐2009]/py | 369,711 |
| #1 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus'/exp OR 'b cell lymphoma'/exp OR hiv:ti OR hiv:ab OR 'hiv‐1':ti OR 'hiv‐1':ab OR 'hiv‐2':ti OR 'hiv‐2':ab OR 'human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab OR 'human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab OR 'human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab OR 'human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab OR 'acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab OR 'acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab OR 'acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab OR 'acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab AND [1990‐2009]/py | 275,043 |
Cochrane Central Register of Controlled Trials (CENTRAL) This search of CENTRAL, published in Issue 3 of The Cochrane Library (2009), was conducted on 4 August 2009 using the strategy outlined in Table 5. The search yielded 424 records of which we identified 11 records for full article retrieval.
3. Search strategy for CENTRAL.
| ID | Search | Hits |
| #1 | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (VIRAL SEXUALLY TRANSMITTED DISEASES), from 1996 to 2009 | 6956 |
| #2 | "THERAPY INITIATION" OR "TREATMENT INITIATION" OR "DRUG THERAPY INITIATION" OR "WHEN TO START" OR "EARLY INITIATION" OR DRUG ADMINISTRATION SCHEDULE", from 1996 to 2009 | 14455 |
| #3 | "Highly Active Antiretroviral Therapy" OR "Anti‐Retroviral Agents" OR ((anti) AND (hiv)) OR antiretroviral* OR ((anti) AND (retroviral*)) OR HAART OR ((anti) AND (acquired immunodeficiency)) OR ((anti) AND (acquired immunedeficiency)) OR ((anti) AND (acquired immuno‐deficiency)) OR ((anti) AND (acquired immune‐deficiency)) OR ((anti) AND (acquired immun*) AND (deficiency)), from 1996 to 2009 | 3092 |
| #4 | (#1 AND #2 AND #3) | 424 |
2. Conference databases:
We searched NLM Gateway on 4 August 2009 using the strategy outlined in Table 6. NLM Gateway covers abstracts from a number of relevant international conferences, including the International AIDS Conference, Conference on Retroviruses and Opportunistic Infections, The British HIV Association Conference and the International Congress on Drug Therapy in HIV infection. The search yielded 2666 records of which 94 records were categorised as Meeting Abstracts and of which eight were identified for full article retrieval.
4. Search strategy for NLM Gateway.
| Search Number | Search | Items Found |
| #10 | Search: #8 AND #9 | 2666* |
| #9 | Search: Antiretroviral Therapy, Highly Active[MeSH] OR Anti‐Retroviral Agents[MeSH] OR Antiviral Agents[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])) OR ((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])) | 123249 |
| #8 | Search: #1 AND #7 | 3599 |
| #7 | Search: #2 OR #3 OR #4 OR #5 OR #6 | 86724 |
| #6 | Search: DRUG ADMINISTRATION SCHEDULE[MeSH] OR "DRUG ADMINISTRATION SCHEDULE" | 70915 |
| #5 | Search: "WHEN TO START" | 12948 |
| #4 | Search: "DRUG THERAPY INITIATION" | 126 |
| #3 | Search: "TREATMENT INITIATION" | 2581 |
| #2 | Search: "THERAPY INITIATION" | 1710 |
| #1 | Search: ((HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] )) OR ((acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MESH:noexp])) | 352167 |
* Of the 2666 records, 94 were identified as Meeting Abstracts and these were the records searched.
The Cochrane HIV/AIDS Assistant Managing Editor also searched all the abstract records from the following major related conferences: 1st‐5th IAS Pathogenesis (2001‐2009); 10th‐17th IAC (1994‐2008); 1st‐16th CROI (1994‐2009); US National HIV Prevention Conference ('99, '03, '05); 7th‐14th BHIVA (2001‐2008); and 8th‐9th European AIDS Society Conference (2001, 2003), using the search terms "when to start" OR ("early" AND "initia*") in any field. This retrieved 89 records from which we identified no RCTs (we attempted to retrieve full reports for nine abstract records but only for the purposes of background literature).
We also attended the International AIDS Society conference held in Cape Town, South Africa in July 2009 and identified one relevant study presented as a late‐breaker study (CIPRAHT001 2009).
3. Ongoing trials:
We searched ClinicalTrials.gov (http://clinicaltrials.gov/) (70 records identified and five for download) and the Pan‐African Clinical Trials Registry (www.pactr.org) for HIV‐related records (seven in total) but found no relevant records for download.
Searching other resources
1. Researchers and relevant organizations
We were in close contact with individual researchers working in the field and policymakers based in inter‐governmental organizations including the WHO.
2. Reference lists
We also checked the reference lists of all studies identified by the above methods and examined any systematic reviews, meta‐analyses, or prevention guidelines we identified during the search process for references.
Data collection and analysis
Selection of studies
NS and OU read the titles, abstracts and descriptor terms of all downloaded material from the electronic searches to identify potentially eligible reports. Full text articles were obtained for all citations identified as potentially eligible and NS and OU independently inspected these to establish the relevance of the article according to the pre‐specified criteria. Where there was any uncertainty as to the eligibility of the record, we obtained the full article.
NS and UO independently applied the inclusion criteria, and any differences arising were resolved by discussions with the third reviewer, GR. Studies were reviewed for relevance based on study design, types of participants, exposures and outcome measures.
Data extraction and management
NS and UO independently extracted data into a standardised data extraction form. The following characteristics were extracted from each included study:
Administrative details: Trial identification number; author(s); published or unpublished; year of publication; number of studies included in paper; year in which study was conducted; details of other relevant papers cited;
Details of the study: study design; type, duration and completeness of follow‐up; country and location of study (e.g. higher‐income vs. lower‐income country); informed consent and ethics approval;
Details of participants: setting, numbers, relevant baseline characteristics including CD4 count and viral load;
Details of intervention: CD4 count at which treatment was initiated; drug combinations; additional co‐interventions; and
Details of outcomes: mortality; HIV‐related morbidity; HIV‐RNA viral load measurements and proposed levels for suppression, as defined by the authors; CD4+ cell counts; adverse events and toxicity.
Assessment of risk of bias in included studies
NS and OU independently examined the components of each included trial for risk of bias using a standard form. This included information on the sequence generation, allocation concealment, blinding (participants, personnel and outcome assessor), incomplete outcome data, selective outcome reporting and other sources of bias. The methodological components of the trials were assessed and classified as adequate, inadequate or unclear as per the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2008). Where differences arose, they were resolved by discussions with the third reviewer, GR.
Sequence generation
Adequate: investigators described a random component in the sequence generation process such as the use of random number table, coin tossing, cards or envelops shuffling, etc
Inadequate: investigators described a non‐random component in the sequence generation process such as the use of odd or even date of birth, algorithm based on the day/date of birth, hospital or clinic record number
Unclear: insufficient information to permit judgment of the sequence generation process
Allocation concealment
Adequate: participants and the investigators enrolling participants cannot foresee assignment (e.g. central allocation; or sequentially numbered, opaque, sealed envelopes)
Inadequate: participants and investigators enrolling participants can foresee upcoming assignment (e.g. an open random allocation schedule (e.g. a list of random numbers); or envelopes were unsealed or nonopaque or not sequentially numbered)
Unclear: insufficient information to permit judgment of the allocation concealment or the method not described
Blinding
Adequate: blinding of the participants, key study personnel and outcome assessor, and unlikely that the blinding could have been broken. Or lack of blinding unlikely to introduce bias. No blinding in the situation where non‐blinding is not likely to introduce bias.
Inadequate: no blinding, incomplete blinding and the outcome is likely to be influenced by lack of blinding
Unclear: insufficient information to permit judgment of adequacy or otherwise of the blinding
Incomplete outcome data
Adequate: no missing outcome data, reasons for missing outcome data unlikely to be related to true outcome, or missing outcome data balanced in number across groups
Inadequate: reason for missing outcome data likely to be related to true outcome, with either imbalance in number across groups or reasons for missing data
Unclear: insufficient reporting of attrition or exclusions
Selective Reporting
Adequate: a protocol is available which clearly states the primary outcome as the same as in the final trial report
Inadequate: the primary outcome differs between the protocol and final trial report
Unclear: no trial protocol is available or there is insufficient reporting to determine if selective reporting is present
Other forms of bias
Adequate: there is no evidence of bias from other sources
Inadequate: there is potential bias present from other sources (e.g. early stopping of trial, fraudulent activity, extreme baseline imbalance or bias related to specific study design)
Unclear: insufficient information to permit judgment of adequacy or otherwise of other forms of bias
Measures of treatment effect
Data analysis was conducted using Review Manager (RevMan) version 5.0.15 (2008). Outcome measures for dichotomous data (e.g. death, virologic suppression) were calculated as a relative risk with 95% confidence intervals. We had planned to calculate continuous data (e.g. CD4+ cell counts, HIV‐RNA viral loads) using the weighted mean difference and standard deviations but at time of writing we did not have access to any of these data.
Assessment of heterogeneity
Where trials were found to be methodologically or clinically comparable, we pooled trial results in a meta‐analysis. As we anticipated the presence of statistical heterogeneity we combined data using the random effects model . We formally tested for statistical heterogeneity using the Chi‐square test for statistical homogeneity with a 10% level of significance as the cut‐off. The impact of any statistical heterogeneity was quantified using the I² statistic (Higgins 2002). Where studies do not have combinable outcomes, we provide the data in a narrative form.
Subgroup analysis and investigation of heterogeneity
We anticipated statistical heterogeneity due to differences between trials conducted in resource‐constrained compared with resource‐rich settings, and planned to present the results according to these sub‐groups. However, as only two trials were identified we did not undertake this analysis. We also planned to present trials of participants co‐infected with hepatitis B or C viruses as a sub‐group, but no such trials were identified.
Sensitivity analysis
We planned to explore the effect of trial quality on the results by excluding those trials where allocation concealment was unclear or inadequate from the meta‐analysis and assessing the effect of this on the overall results.
Results
Description of studies
Two completed trials were identified. One published RCT (SMART 2008) was identified from the electronic database searching (see Figure 1) and one unpublished trial (CIPRAHT001 2009) from consultation with experts in the field. Two ongoing studies were identified from searching prospective trials registries. Full details for each are provided in the Tables of Included Studies and of Ongoing Studies.
1.
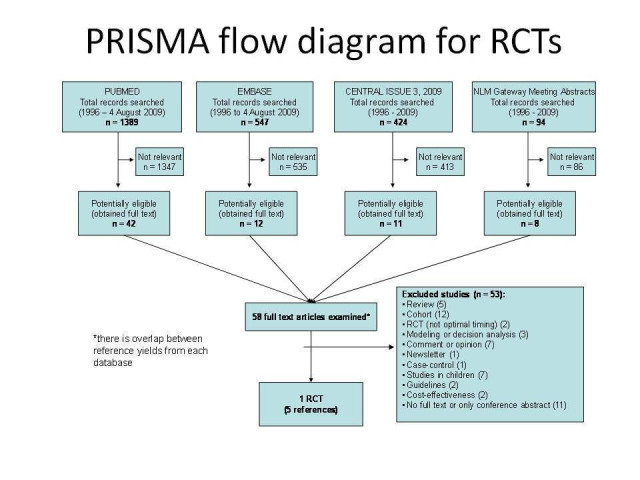
PRISMA flow diagram for search for RCTs
Included studies
The CIPRAHT001 2009 trial was conducted in Haiti and aimed to directly answer the question of whether starting ART at CD4 counts between 200 and 350 cells/µL improved mortality and morbidity significantly more than commencing ART at CD4 count of 200 cells/µL or below. The trial was conducted in one centre in a resource‐poor country and provides the best evidence to date to determine the optimal time of initiation of ART determined by CD4 count levels. The trial began enrollment in August 2005 and was stopped early in May 2009.
The SMART 2008 trial included 318 sites in 33 countries and enrollment commenced in January 2002 and was stopped early on 10 January 2006 by the study's Data Safety and Monitoring Board. The trial compared a viral suppression strategy, with an experimental drug conservation strategy. The results for a sub‐set of those participants within the larger trial who were treatment‐naive are included in this review. This analysis was reported as post‐hoc and included a group of participants who were either ART‐naive or who had received ART and ceased to take it 6 months prior to enrollment. For this review we report the results only for those ART‐naive participants. The analysis of the sub‐set differs slightly from that of the CIPRAHT001 2009 trial in that it compared starting ART at 350 cells/µL with starting ART at 250 cells/µL.
Excluded studies
Reasons for excluding studies during the search are summarised in Figure 1. One study (Erhabor 2006) was reported as a randomised trial but further email discussion with the authors confirmed it was a stratified cohort study.
Risk of bias in included studies
See Figure 2 and Figure 3 for a graphical representation of the risk of bias in both trials.
2.
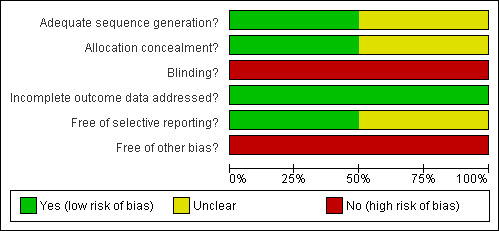
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
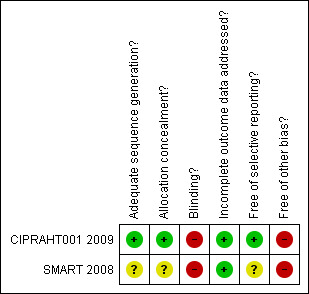
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Generation of the random sequence was by computer and allocation concealment was done centrally so we judged both random generation and allocation concealment to be adequate for the CIPRAHT001 2009 and not likely to introduce bias. Neither the method of generation nor the method of allocation concealment was clearly reported for the SMART 2008 trial, although blocked stratification was used and was likely to be done by computer. As the analysis reported her is for a sub‐group within randomized groups, there is a possible potential for bias but this is unlikely to be due to the method of randomization.
Blinding
In both trials the deferred groups were not provided with placebo and the participants and providers were therefore not blinded. Although in the CIPRAHT001 2009 trial, the investigators and members of the protocol team were blinded to the randomisation groups, we assessed the risk for bias from blinding to be moderate due to the lack of placebo. Similarly in the SMART 2008 trial the assessors in the end‐point committee were blinded to the randomized groups and so we assessed the risk to be moderate.
Incomplete outcome data
Attrition was low and less than 10% in both trials at the time the trials were stopped. However, we rated the risk of bias due to incomplete outcome reporting as moderate in both trials as acceptable statistical survival analysis techniques were used to estimate HIV event distribution over time by accumulating for staggered enrolment and incomplete discrete follow‐up.
Selective reporting
Both trial reports compare favourably with the protocols published on www.clinicaltrials.gov and so the risk of bias is likely to be low from selective reporting.
Other potential sources of bias
The CIPRAHT001 2009 trial was stopped early due to significant benefits in the early ART group. Although the reported results make use of survival analyses in an attempt to reduce the risk from bias due to early stopping, we assessed the bias to be moderate because of the early stopping.
The results reported here for the SMART 2008 trial could be susceptible to publication bias as the analysis of the ART‐naive was post‐hoc and it is possible that investigators of other trials may not have conducted post‐hoc analyses of similar nested sub‐groups within their trials. We assessed the risk of bias from this as high.
Effects of interventions
See: Table 1
Results were not available for all the outcomes we wished to include, and we only report outcomes which were available in the trials below.
PRIMARY OUTCOMES:
1. Death
We combined the mortality data for both trials comparing initiating ART at CD4 levels at 350 cells/µL (SMART 2008) or between 200 and 350 cells/µL (CIPRAHT001 2009) with deferring initiation of ART to CD4 levels of 250 cells/µL (SMART 2008) or 200 cells/µL (CIPRAHT001 2009). We found a statistically significant reduction in death when starting ART at higher CD4 counts. Risk of death was reduced by 74% and could be reduced by between 38% and 89% (RR = 0.26; 95% CI: 0.11, 0.62; P = 0.002). There was little statistical heterogeneity between the trial results (Chi² = 0.01, df = 1; P = 0.93) with the degree of heterogeneity quantified by the I² at 0%. See Analysis 1.1. The SMART 2008 trial added only one event to the analysis and contributes an appropriately low weight in the meta‐analysis.
1.1. Analysis.
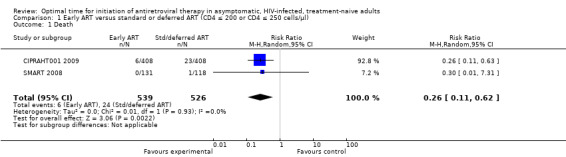
Comparison 1 Early ART versus standard or deferred ART (CD4 ≤ 200 or CD4 ≤ 250 cells/µl), Outcome 1 Death.
2. Responses to ART as measured by:
a. Clinical occurrence of new HIV‐related events (Tuberculosis)
We combined the data for TB for both trials although the SMART 2008 trial only contributed one incident case to the meta‐analysis. Risk of TB was reduced by 50% in the groups starting ART early; this was not statistically significant with the reduction as much as 74% or an increased risk of up to 12% (RR = 0.54; 95% CI: 0.26, 1.12; p = 0.01). There was little statistical heterogeneity between the trial results (Chi² = 1.05, df = 1; p = 0.31) with the degree of heterogeneity quantified by the I² at 4%. See Analysis 1.2.
1.2. Analysis.
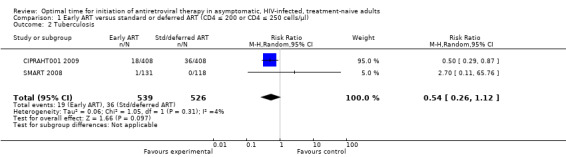
Comparison 1 Early ART versus standard or deferred ART (CD4 ≤ 200 or CD4 ≤ 250 cells/µl), Outcome 2 Tuberculosis.
b. Disease progression measured by opportunistic infections:
This outcome was only reported for SMART 2008 and only four events in total were recorded. Starting ART at enrollment (when participants had CD4 counts of 350 cells/µL) rather than deferring to starting at a CD4 count of 250 cells/µL reduced the risk of disease progression by 70%; this was not statistically significant with the reduction in risk as much as 97% or an increased risk of up to 185% (RR = 0.30; 95% CI: 0.03, 2.85; P = 0.29). See Analysis 1.3.
1.3. Analysis.
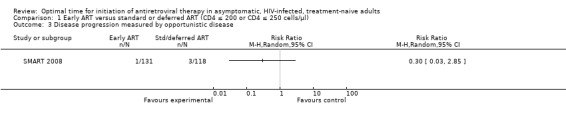
Comparison 1 Early ART versus standard or deferred ART (CD4 ≤ 200 or CD4 ≤ 250 cells/µl), Outcome 3 Disease progression measured by opportunistic disease.
Other anticipated outcomes we identified in our protocol as primary outcomes, were not reported in the two trials and we therefore are not able to provide information on these.
SECONDARY OUTCOMES:
None of the anticipated secondary outcomes identified in our protocol were reported in the two trials.
ADVERSE EFFECTS:
Results were only available for CIPRAHT001 2009. For the SMART 2008 trial, the adverse events were not categorized according to the ART‐naive participants, and so it was not possible to extract data specific to the sub‐group included in this review
In CIPRAHT001 2009 there was no statistically significant difference in the number of independent Grade 3 or 4 adverse events occurring in the early and standard ART groups when we conducted an intention‐to‐treat analysis (i.e. we used the total number of participants randomised into each group as the denominators in both groups with both = 408) (RR = 1.72; 95% CI: 0.98, 3.03; P = 0.06). See Analysis 1.4. When analysing only participants who actually commenced ART in the deferred group (n = 160), the authors report a statistically significant increase in the incidence of zidovudine‐related anaemia (8.1%) compared with those in the early initiation group (3.4%) (RR = 0.42; 95% CI: 0.20, 0.88; P = 0.02). See Analysis 1.5.
1.4. Analysis.
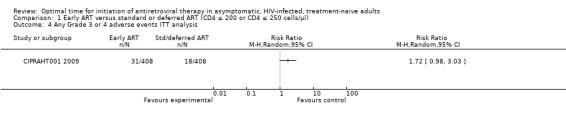
Comparison 1 Early ART versus standard or deferred ART (CD4 ≤ 200 or CD4 ≤ 250 cells/µl), Outcome 4 Any Grade 3 or 4 adverse events ITT analysis.
1.5. Analysis.
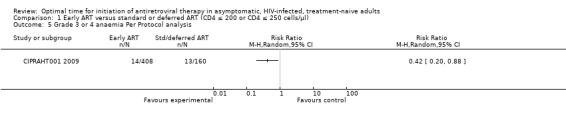
Comparison 1 Early ART versus standard or deferred ART (CD4 ≤ 200 or CD4 ≤ 250 cells/µl), Outcome 5 Grade 3 or 4 anaemia Per Protocol analysis.
GRADE ASSESSMENT:
As this review was done as part of a report to inform the WHO's 2009 adults and adolescent ART guideline revisions, we also conducted a GRADE assessment, although this was not stipulated in the protocol. See Table 1.
Using the GRADE tool, we evaluated the evidence provided by the RCTs and rated this for each outcome identified as critical or important to determining whether or not to change the current WHO guidelines for timing of initiation of ART. For the critical outcomes of death and TB, we rated the quality of the evidence as moderate. Although the data come from two RCTs, they were down‐graded to moderate due to the possibility of publication bias and because the data were obtained from only one high‐quality RCT directly aimed at answering the question combined with data from a sub‐group nested within a larger trial not directly aimed at answering the question. For the critical outcome of disease progression we rated the evidence as low given that the data were only available from the sub‐group nested within the larger trial.
For the critical adverse events we rated the evidence as moderate. In this case, despite the data coming from a high‐quality trial, we downgraded the quality of the evidence because it was only obtained from one RCT and could therefore be imprecise. Ideally additional trials would be needed to improve precision. The important outcomes of sexual immunological and virologic response, adherence, tolerance and retention, and HIV drug resistance were not measured in either of the trials.
Discussion
Summary of main results
Pooled data from one trial of 816 participants and one sub‐group analysis of 248 participants provide moderate‐quality evidence that starting ART at CD4 levels higher than 200 or 250 cells/µL but below 350 cells/µL reduces mortality rates in asymptomatic, ART‐naive, HIV‐infected people. Evidence regarding a reduction in morbidity is less strong and incidence of severe adverse events is apparently low, but this data is available from only one trial and so must be viewed with caution.
Overall completeness and applicability of evidence
Only the CIPRAHT001 2009 trial directly answered the question of whether starting ART at CD4 levels above 200 cells/µL improves mortality and morbidity (as measured by incidence of TB) compared with the current WHO guideline recommendations of starting at 200 cells/µL (WHO 2006). This trial was located in a resource‐constrained setting and therefore has a high applicability to low‐income countries. Full results from this trial have yet to be published, however, and must therefore be viewed as incomplete. There are no available data on follow‐up beyond the median of 21 months and no available data on development of possible resistance in those initiating ART. Although we included the results from the SMART 2008 trial in the meta‐analysis, these were from a post‐hoc analysis of ART‐naive participants nested within a larger trial which aimed to provide evidence regarding treatment interruptions rather than optimal timing of ART. For this reason the evidence from the SMART 2008 trial must be interpreted with caution.
Ideally, additional trials would strengthen the nature and quality of the above evidence. We identified two ongoing trials (START 2009; NCT00491556). Both of these trials are being conducted in high‐income settings and are comparing initiation of ART at CD4 levels above either 350 cells/µL (NCT00491556) or 500 cells/µL (START 2009). Although these trials will provide useful data to inform initiating ART at CD4 levels above current guidelines operational in many high‐income countries, the data will not add to the evidence base for determining whether initiating ART at levels above current WHO guidelines is optimal compared with initiating at CD4 counts <200 cells/µL. We did not identify and are not aware of any ongoing trials aiming to answer this question apart from the CIPRAHT001 2009 trial, although a number of trials aimed at timing of ART in patients co‐infected with TB are underway.
We did not identify any trials which evaluated the effects of optimal initiation of ART in people co‐infected with either hepatitis B or C, or both, and evidence for these populations remains limited.
Quality of the evidence
The quality of the methodological conduct for both trials was moderate to high and the risk of bias was likely to be low to moderate for both trials. Caution must be exercised when interpreting the results from the SMART 2008 trial, however, as this was a post‐hoc analysis and, as such, may be prone to the effects of publication bias. This would occur if other trials did not conduct or publish similar analyses of potential sub‐sets within the original trials. We, however, have no knowledge that this occurred in other trials.
Both trials employed early stopping rules considered acceptable statistical practice (Kim 1987). Use of survival analysis which incorporates the results from all those who completed the trial and who are censored due to loss‐to‐follow‐up or early stopping of the trial, will have reduced the potential for attrition bias in each trial. This was done in both trials, but survival data were not available for the sub‐set in the SMART 2008 trial. For this reason, in our meta‐analysis we present the proportions at the time of stopping the trial. It is important to note that in a systematic review of RCTs stopped early for benefit, such RCTs were found to overestimate treatment effects (Montori 2005). When trials with events fewer than the median number (n=66) were compared with those with event numbers above the median, the odds ratio for a magnitude of effect greater than the median was 28 (95% CI: 11,73) (Montori 2005). Both trials included in our review yielded fewer than 66 events and may thus overestimate the treatment effect. The magnitude of effect was consistent across both trials, however, which strengthens the evidence in favour of starting ART earlier than standard WHO guidelines recommend.
Potential biases in the review process
We conducted comprehensive searches of both journal and conference databases to ensure all relevant published and unpublished trials were identified. We did not limit the searches to a specific language. Our ongoing interaction with the investigator of the CIPRAHT001 2009 trial allowed us access to preliminary and unpublished data. Given the high‐profile nature of the intervention and the complexity of conducting ART initiation trials, it is unlikely that our search strategy failed to detect existing current trial evidence. Potential bias in the conduct of our review was also minimised by having two independent researchers extract data and assess the methodological quality of each study. This detailed process allows for a thorough assessment of trial conduct and an exploration of the possible biases that may be present in each trial.
When we pooled data in the meta‐analysis we combined the arms from both trials although these were slightly different because the analysis of the SMART 2008 sub‐set compared starting ART at CD4 levels of 350 cells/µL with starting ART at 250 cells/µL, whereas the CIPRAHT001 2009 trial compared starting at CD4 levels of between 200 and 350 cells/µL with starting ART at 200 cells/µL. Given that both trials compared initiating ART at higher levels with deferring the start of ART, we did not consider this difference to be a source of bias, but the evidence must be viewed as less direct.
Agreements and disagreements with other studies or reviews
These results are consistent with previous cohorts from both high‐ and low‐income studies which showed that early initiation of ART may reduce morbidity and mortality associated with HIV/AIDS (Sterne 2009; Moh 2007; Badri 2004; Wong 2007; Erhabor 2006). For high‐income countries, the When To Start Consortium (Sterne 2009) analysed data from 18 cohort studies done in Europe and North America and provided evidence that deferring combination therapy until a CD4 cell count of 251‐350 cells/µL was associated with higher rates of AIDS and death than starting therapy in the range 351‐450 cells/µL. Sterne 2009 suggested that a CD4 cell count of 350 cells/µL should be the minimum threshold for initiation of ART and should help to guide physicians and patients in deciding when to start treatment.
We found four cohort studies conducted in resource‐constrained settings that examined the optimal threshold of CD4 cell count for starting ART (Moh 2007; Badri 2004; Wong 2007; Erhabor 2006). Most of the studies provided evidence in support of early initiation of ART (Moh 2007; Badri 2004; Wong 2007). Moh 2007 found that incidence of mortality decreased with increasing pre‐ART CD4 cell count. Badri 2004 also provided evidence that baseline CD4 cell count <200 cells/µL was associated with increased mortality and risk of developing AIDS compared with patients with baseline CD4 cell count >350 cells/µL. Wong 2007 examined cohorts of Chinese people in Hong Kong and found that CD4 cell count <100 cells/µL was associated with increased risk of progression to new AIDS‐defining illness or death, and new AIDS‐defining illness or non‐accidental death. Contrary to other studies, Erhabor 2006 found that there is no long‐term advantage in CD4+ response in initiating highly active ART (HAART) at a pre‐therapeutic CD4 count of >350 cells/µL rather than at 200 – 350 cells/µL among 100 HIV‐infected, previously ART‐naïve individuals in Nigeria. Three cost‐effectiveness studies on when to start ART in resource‐limited settings have been published, but there is conflicting evidence (Walensky 2009; Loubiere 2008; Badri 2004). Walensky 2009 conducted a cost‐effectiveness analysis using a computer simulated model of HIV disease. Published data from randomised trials and observational studies in South Africa were used to populate the model. This study showed that if HIV‐infected patients are identified and linked to care, a CD4 cell count threshold for ART initiation of 350 cells/µL would reduce severe diseases substantially during the next five years compared with ART initiation at CD4 counts of 250 cells/µL. Walensky 2009 concluded that earlier initiation of ART in South Africa would likely reduce morbidity and mortality, improve long‐term survival, and would be cost‐effective. Another cost‐effectiveness analysis was conducted from a public health perspective using primary treatment outcomes, healthcare utilization and cost data derived from the Cape Town AIDS cohort (Badri 2004). Badri 2004 found that HAART is reasonably cost‐effective for HIV‐infected patients in South Africa, and most effective if initiated when CD4 count >200 cells/µL. Deferring treatment to <200 cells/µL would reduce the aggregate cost of the treatment, but this should be balanced against the significant clinical benefits associated with early therapy. Loubiere 2008 from Morocco assessed the cost‐effectiveness of HIV treatment alternatives based on the CD4 T‐cell count at the initiation of treatment using data from 286 HIV‐positive individuals. The study demonstrated a statistical significant difference in mean survival time between patients with baseline CD4 cell counts < 200 cells/µL compared with those with baseline CD4 <100 cells/µL (58.8 versus 16.75 months; P<.0001). However, the incremental cost‐effectiveness ratio was 100 times higher for patients who started ART with CD4 cell count >200 cells/µL compared with patients who started at CD4+ T‐ cell count <100 cells/µL. Loubiere 2008 concluded that, in the Moroccan context, ART is more cost‐effective when the CD4 cell count drops to <200 cells/µL.
Authors' conclusions
Implications for practice.
There is moderate‐quality evidence derived from trials that initiating ART at CD4 levels higher than 200 or 250 cells/µL reduces mortality rates in asymptomatic, ART‐naive, HIV‐infected people. Practitioners and policy‐makers may consider initiating ART at levels ≤350 cells/µL for patients who present to health services and are diagnosed with HIV early in the infection. Evidence for initiating ART in patients co‐infected with TB and hepatitis patients remains unclear.
Implications for research.
Given that there are two RCTs in the field, future research should focus on long‐term cohort studies to collect data on the incidence and nature of adverse effects, and the development of viral resistance, in those initiating ART at higher CD4 levels than previous standard practice. In many resource‐constrained settings a remaining challenge is to identify patients sufficiently early in the course of the infection to benefit from early treatment. Research efforts (ideally clinical trials) should investigate interventions to promote voluntary counseling and testing, to reduce the stigma of testing for HIV and to encourage people to present for testing. Cost‐effectiveness remains an area requiring ongoing investigation. In high‐income countries, the results from two ongoing trials comparing initiation of ART at CD4 levels above either 350 cells/µL or 500cells/µL with standards of care will provide necessary evidence for practice in these settings.
What's new
| Date | Event | Description |
|---|---|---|
| 6 January 2011 | Amended | minor edit to sources of support |
Acknowledgements
We thank the GHESKIO‐Cornell team for providing us with details of their trial and are grateful for their willingness to share these with us.
We thank the World Health Organization (WHO) and the CDC Global AIDS Program for funding. This review was already underway in July 2009 at the time the WHO required evidence to update ART guidelines. Funding from the WHO allowed the review to be expedited.
We are grateful to Elizabeth Pienaar for conducting the searches and assisting with procurement of articles and for her support. We also thank Joy Oliver and Vicki Badenhorst for article procurement and prompt administration support and for their encouragement.
NS wishes to acknowledge the assistance and patience of Eliza Humphreys in co‐ordinating the review process and Larry Chang for his careful review.
Data and analyses
Comparison 1. Early ART versus standard or deferred ART (CD4 ≤ 200 or CD4 ≤ 250 cells/µl).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | 1065 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.11, 0.62] |
| 2 Tuberculosis | 2 | 1065 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.26, 1.12] |
| 3 Disease progression measured by opportunistic disease | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Any Grade 3 or 4 adverse events ITT analysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Grade 3 or 4 anaemia Per Protocol analysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
CIPRAHT001 2009.
| Methods | A single‐centred trial which commenced enrollment in August 2005 in a large clinic providing HIV voluntary counseling and testing to more than 25,000 people a year in Port au Prince in Haiti. The trials was stopped early on 28 May 2009 by the Data Safety and Monitoring Board. Follow‐up took place monthly and all participants were seen by a clinician. Retention was encouraged by home visits, 24 hour/day on call clinician, free phone cards, peer counseling by people living with HIV/AIDS. Median duration of follow‐up was 21 months with a range of one to 44 months. Loss to follow‐up was 4.5% (19/408) in the early ART group and 4.4% (18/408) in the deferred ART group. |
|
| Participants | 1,066 HIV‐infected, treatment‐naive adults with CD4 cell count of 200 ‐ 350 cells/µL were referred for study screening. 150 were excluded and 816 adults were enrolled. Inclusion criteria: HIV‐infected, antiretroviral‐naive, age ≥ 18 years of age, CD4 T cell count between 200 and 350 cells/µL. Exclusion criteria: History of AIDS‐defining illness, prior ART use, pregnant or breast‐feeding, or needed ART in the next three months based on the judgment of the primary care clinician Median age was 40 with males and females distributed similarly in both groups: 41% males and 59% females in the EARLY intervention group and 44% males and 56% females in the STANDARD intervention group. |
|
| Interventions | Intervention: EARLY: Start ART (lamivudine 150mg and zidovudine 300mg in a fixed‐dose combination twice daily and efavirenz 600mg at night) within two weeks of enrollment. Comparison: STANDARD: Start ART (lamivudine 150mg and zidovudine 300mg in a fixed‐dose combination twice daily and efavirenz 600mg at night) when the CD4 cell count is ≤ 200 cells/µL or the patient develops an AIDS‐defining illness. Both groups received Trimethoprim‐sulfamethoxazole prophylaxis and daily multi‐vitamins and monthly food baskets). |
|
| Outcomes | PRIMARY OUTCOME Death ‐ documented by one of the following: obituary, autopsy report, hospital death certificate, or contact report documenting verbal communication with the participant's healthcare provider, family member, or significant other. SECONDARY OUTCOME incidence of TB ‐ HIV infected patients with a cough or other symptoms suggestive of tuberculosis are routinely screened at the clinic with a chest radiograph and three sputum smears for acid fast bacilli (AFB) by Ziehl‐Neelsen staining and Mycobacterium tuberculosis culture on Lowenstein Jensen media. TB case definition was based upon American Thoracic Society with diagnosis requiring symptoms consistent with tuberculosis and microbiologic confirmation of disease, or symptoms, a chest radiograph consistent with tuberculosis, and a positive response to anti‐tuberculosis therapy. ADVERSE EVENTS Information not presented and outstanding at current time. |
|
| Notes | The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, is the study sponsor. NIAID funded the study through the Comprehensive International Program of Research on AIDS (CIPRA). Other support came from The Global Fund Against AIDS, Tuberculosis, and Malaria, Glaxo Smith Kline, Abbott and Fondation Merieux. The trial was carried out by the Haitian Group for the Study of Kaposi's Sarcoma and Immune Deficiency Disorders (GHESKIO) Centers in Port‐au‐Prince, Haiti. The principal investigator is Jean William Pape, M.D., the director of the GHESKIO Centers and a professor of medicine at Weill Medical College of Cornell University in New York. The trial was approved by the Instititional Review Board at the GHESKIO Centres in Haiti and at Cornell University (information provided Prof Dan Fitzgeral). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization was by a computer‐generated random numbers list in blocks of eight in a 1:1 ratio (information provided by Prof Dan Fitzgerald). |
| Allocation concealment? | Low risk | Randomization was performed by Frontier Science and Technology Research Foundation in New York (central randomization) and transmitted to the clinical site electronically (information provided by Prof Dan Fitzgerald). |
| Blinding? All outcomes | High risk | Open‐label and the standard (deferred) group did not receive a placebo so primary care clinicians and participants were aware of their treatment status. Investigators and members of the protocol team were blinded to the data stratified by randomization group. |
| Incomplete outcome data addressed? All outcomes | Low risk | Attrition was less than 5% in both intervention and comparison groups so risk of bias due to high loss to‐follow‐up judged as being low. |
| Free of selective reporting? | Low risk | Compares favourably with protocol on www.clinicaltrials.gov |
| Free of other bias? | High risk | Stopped early but used survival analysis to overcome effects of attrition. |
SMART 2008.
| Methods | The trial included 318 sites in 33 countries and enrollment commenced in January 2002 and was stopped early on 10 January 2006 by the Board. The trial compared a Viral Suppression (VS) strategy, with an experimental Drug Conservation (DC) strategy. The results for a sub‐set of those participants within the larger VS‐DC trial who were ARV treatment‐naive are included in this review. This analysis was reported as post‐hoc and included a group of participants who were either ART‐naive or who had received ART and ceased to take it 6 months prior to enrollment. For this review we report the results only for those ART‐naive participants. Routine visits occurred at 1 and 2 months, every 2 months thereafter for the first year, and every 4 months in subsequent years. Visits included clinical assessments, and samples were obtained for measurement of CD4 cell count and plasma HIV RNA level. At baseline and annually, a 12‐lead electrocardiogram was obtained, and data were electronically transmitted to a central reading facility for assessment of silent myocardial infarction. Median period of follow‐up was 18 months for entire RCT and for the sub‐set (including those participants who had recieved ART 6 months earlier). Follow‐up is not reported specifically for the ART‐naive participants only. Median follow‐up for the subset is reported as 15 months. |
|
| Participants | 5.472 HIV‐infected participants with CD4 cell count > 350 cells/µL were randomly assigned to VS or DC. Of these, 477 participants were either treatment‐naive (n = 249) or had not received ART in the preceding 6 months (n = 228). Inclusion criteria: HIV‐infected, age ≥ 13 years of age, CD4 T cell count > 350 cells/µL, willing to initiate, modify, or stop antiretroviral therapy according to study guidelines. Exclusion criteria: pregnant or breast‐feeding. Additional criteria for post‐hoc sub‐set analysis were that participants were antiretroviral‐naive or not on treatment for greater than 6 months. We report the results for only those participants who were ART‐naive. Median age was 39 in the ART‐naive Viral Suppression group and 40 years in the ART‐naive Drug Conservation group. There were 27.5% females in the ART‐naive VS group and 20.3% in the ART‐naive DC group. Median log viral load at baseline was 4.3 copies/ml in the ART‐naive VS group and 4.6 in the ART‐naive DC group. Median CD4 count at baseline was 432 cells/ul in the ART‐naive VS group and 441 cells/ul ART‐naive DC group. |
|
| Interventions | Intervention: EARLY: In the Viral Suppression strategy available antiretroviral regimens were to be used in an uninterrupted manner with the goal of maximal and continuous suppression of HIV replication. Comparison: DEFERRED: The Drug Conservation strategy entailed the episodic use of antiretroviral therapy according to CD4+ count thresholds: the use of antiretroviral therapy was deferred until the CD4+ count decreased to less than 250 cells per cubic millimeter, at which time antiretroviral therapy was to be initiated (or reinitiated) and continued until the CD4+ count increased to more than 350 cells per cubic millimeter. The protocol also permitted antiretroviral therapy to be initiated (or reinitiated) if symptoms of disease from HIV infection (e.g., oral thrush) developed or the percentage of CD4+ lymphocytes (CD4+ percentage) was less than 15%. On confirmation that the CD4+ count was more than 350 cells per cubic millimeter, antiretroviral therapy was to be stopped and then resumed when the CD4+ count was less than 250 cells per cubic millimeter. The sub‐set of 477 participants were those participants who were treatment‐naive or not on ART for greater than 6 months. Of these 131 were ART‐naive in the Viral Suppressoin Group and 118 in the Drug Conservation Group. We report the results for this group. |
|
| Outcomes |
|
|
| Notes | The study was approved by the institutional review board at each site, and written informed consent was obtained from all participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stratified according to clinical site with the use of permeated blocks of random sizes. Assume computer‐generated |
| Allocation concealment? | Unclear risk | As for above |
| Blinding? All outcomes | High risk | Investigators and participants were aware of treatment assignments. For primary outcome, death or Opportunistic Disease, an end‐point review committee reviewed the events classification unaware of treatment assignments. |
| Incomplete outcome data addressed? All outcomes | Low risk | Eleven were lost to follow‐up of subset of 477 and median follow‐up was 15 months. |
| Free of selective reporting? | Unclear risk | Compares favourably with protocol on www.clinicaltrials.gov but outcomes not clearly reported in the online protocol (possibly due to trial registration on the registry prior to World Health Organization mandatory 20 item minimum dataset criteria for prospective trial registration). We will need to contact investigators to confirm reported outcomes conform to those in protocol. |
| Free of other bias? | High risk | Stopped early but acceptable statistical stopping rules applied to reduce effects of attrition bias. An O'Brien‐Fleming boundary and the Lan‐DeMets alpha spending function was used to determine whether to terminate the trial early. The results reported here are not free of bias as the analysis of the ART‐naive was post‐hoc and it is possible that other studies may not have conducted similar post‐hoc analysis so there is a threat of publication bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Erhabor 2006 | Although the text of the article refers to randomizing patients, email communication with the first author confirmed that this is a stratified cohort study and not an RCT |
Characteristics of ongoing studies [ordered by study ID]
NCT00491556.
| Trial name or title | Early initiation of HAART |
| Methods | This is a randomized, proof of concept study of youth 18‐ 24 years of age with confirmed HIV after age 9 with CD4+ T cells above 350 cells/mm3 who are randomized 3:1 to begin HAART consisting of TDF/FTC/ATV/r (preferred), AZT/3TC/ATV/r, or other recommended NRTI backbone with ATV/r upon entry or to begin treatment under current DHHS guidelines. Subjects in the experimental group who achieve virologic control by week 24 and maintain good control through 48 weeks will then de‐intensify to ATV/r alone and will be followed for two years. Subjects randomized to the standard care arm will begin HAART with TDF/FTC/ATV/r (preferred), AZT/3TC/ATV/r, or other recommended ATV/r based HAART regimen according to current DHHS standard of care. |
| Participants | Age 18 yrs and 0 days to 24 yrs and 364 days with CD4+ T cells >350/mm3 as determined by two consecutive measures within 6 months of entry, with second measure being collected at pre‐entry. Infected with HIV after age 9 |
| Interventions |
Experimental: Subjects in the experimental group will begin HAART consisting of TDF/FTC/ATV/r (preferred), AZT/3TC/ATV/r or other recommended NRTI backbone with ATV/r upon entry or to begin treatment under current DHHS guidelines. Subjects in the experimental group who achieve virologic control by week 24 and maintain good control through 48 weeks will then de‐intensify to ATV/r alone and will be followed for an additional two years Control: Subjects randomized to the standard care arm will begin HAART with TDF/FTC/ATV/r (preferred), AZT/3TC/ATV/r, or other recommended ATV/r based HAART regimen according to current DHHS standard of care and will be followed for a total of three years. Under these guidelines and under current clinical standards, subjects on the standard care arm will begin therapy when the CD4+ T cell count drops below 350 cells/mm3 or other clinical criteria necessitating treatment as determined by the site clinician occur. |
| Outcomes |
Primary Outcome: Ability to maintain or enhance HAART‐associated quantitative changes in CD4+ T cell percentages achieved during HAART following therapy de‐intensification to ATV/r in adolescents and young adults who began treatment prior to meeting DHHS guidelines. Secondary Outcomes:
|
| Starting date | October 2007 |
| Contact information | |
| Notes |
START 2009.
| Trial name or title | Strategic Timing of Antiretroviral Treatment (START) |
| Methods | Treatment, Randomized, Open Label, Dose Comparison, Parallel Assignment, Safety/Efficacy Study |
| Participants | Patients 18 years of age and older who are infected with HIV, have CD4+ cell counts of greater than 500 cells/mm3, and who have never had antiretroviral therapy to treat HIV. |
| Interventions | To determine whether initiation of ART in HIV‐infected, treatment‐naive persons with CD4 counts > 500 cells/mm3 is superior in terms of mortality and morbidity to deferral of treatment until the CD4 count declines to < 350 cells/mm3. |
| Outcomes |
Primary Outcomes: Composite endpoint of AIDS, serious non‐AIDS diagnoses, and all‐cause mortality Secondary Outcomes:
|
| Starting date | March 2009 |
| Contact information | University of Minnesota (James D. Neaton, Ph.D/Principal Investigator) |
| Notes | NCT00867048 |
Differences between protocol and review
We produced and describe a Summary of Findings table in the review which we did not stipulate in the protocol.
Contributions of authors
NS and OU conducted eligibility of the searches, data extraction, and quality assessment. GR resolved differences when needed. NS entered the data and conducted the analyses and wrote the first draft of the review. OU and GR provided feedback into the overall results and their interpretation.
Sources of support
Internal sources
South African Cochrane Centre and Cochrane HIV/AIDS Mentoring Programme, South Africa.
Cochrane HIV/AIDS Review Group, San Francisco, USA.
External sources
-
World Health Organization, Switzerland.
World Health Organization #200106621. Systematic reviews and development of GRADE profiles, based on the new WHO GRC guidelines, for the "WHO Guidelines on antiretroviral therapy for HIV infection in adults and adolescents ‐ 2009 revision."
-
Centers for Disease Control and Prevention, USA.
Cooperative Agreement #U2GPS001468, "Atlanta HQ UCSF Technical Assistance to Support the President's Emergency Plan for AIDS Relief" from the Centers for Disease Control and Prevention (CDC), with funds from National Center for HIV, Viral Hepatitis, STDs and TB Prevention (NCHSTP). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC.
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
CIPRAHT001 2009 {unpublished data only}
- Fitzgerald D. A randomized clinical trial of early versus strandard antiretroviral therapy for HIV‐infected patients with a CD4 T cell count of 200 ‐ 350 cells/ml (CIPRA HT 001). International AIDS Society Conference, Cape Town 2009.
SMART 2008 {published data only}
- El‐Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count‐guided interruption of antiretroviral treatment. The New England journal of medicine 2006;355(22):2283‐96. [PUBMED: 17135583] [DOI] [PubMed] [Google Scholar]
- Lundgren JD, Babiker A, El‐Sadr W, Emery S, Grund B, Neaton JD, et al. Inferior clinical outcome of the CD4+ cell count‐guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ Cell counts and HIV RNA levels during follow‐up. The Journal of infectious diseases 2008;197(8):1145‐55. [PUBMED: 18476293] [DOI] [PubMed] [Google Scholar]
- The Strategies for Management of Antiretroviral Therapy (SMART) Study Group. Major clinical outcomes in antiretroviral therapy (ART)‐naive participants and in those not receiving ART at baseline in the SMART study. The Journal of Infectious Diseases 2008;197(15 April):1133‐44. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Erhabor 2006 {published data only}
- Erhabor O, Ejele OA, Uko EK. HAART – Dependent CD4+ Lymphocyte Response Based onPre‐Therapeutic CD4 Lymphocyte Count in HIV‐Infected Nigerians. Annals of African Medicine 2006;5(3):153‐7. [Google Scholar]
References to ongoing studies
NCT00491556 {published data only}
- Early initiation of HAART. Ongoing study October 2007.
START 2009 {published data only}
- Strategic Timing of Antiretroviral Treatment (START). Ongoing study March 2009.
Additional references
Badri 2004
- Badri M, Bekker LG, Orrell C, Pitt J, Cilliers F, Wood R. Initiating highly active antiretroviral therapy in sub‐Saharan Africa: an assessment of the revised World Health Organization scaling‐up guidelines. AIDS (London, England) 2004;18(8):1159‐68. [PUBMED: 15166531] [DOI] [PubMed] [Google Scholar]
Braithwaite 2008
- Braithwaite RS, Roberts MS, Chang CC, Goetz MB, Gibert CL, Rodriguez‐Barradas MC, et al. Influence of alternative thresholds for initiating HIV treatment on quality‐adjusted life expectancy: a decision model. Annals of internal medicine 2008;148(3):178‐85. [PUBMED: 18252681] [DOI] [PMC free article] [PubMed] [Google Scholar]
DAIDS 2009
- Division of AIDS. Table for grading the severity of Adult and Pediatric Adverse Events. Accessed 28 October 2009 http://rcc.tech‐res.com/Document/safetyandpharmacovigilance/DAIDS_AE_GradingTable_Clarification_August2009_Final.pdf. [Google Scholar]
Day 2002
- Day J, Brink B, Charalambous S, Churchyard G, Grant A, Morris D, et al. Clinical and operational guidelines for use of antiretroviral therapy in adults. Aurum Health Research 2002. [Google Scholar]
Deeks 2006
- Deeks SG. Antiretroviral treatment of HIV infected adults. BMJ (Clinical research ed.) 2006;332(7556):1489. [PUBMED: 16793811] [DOI] [PMC free article] [PubMed] [Google Scholar]
Erhabor 2006
- Erhabor O, Ejele OA, Uko EK. HAART ‐ Dependent CD4+ Lymphocyte Response Based on Pre‐Therapeutic CD4 Lymphocyte Count in HIV‐Infected Nigerians. Annals of African Medicine 2006;5(3):153‐57. [PUBMED: 17319344] 17319344 [Google Scholar]
Granich 2009
- Granich RM, Gilks CF, Dye C, Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009;373(9657):48‐57. [DOI] [PubMed] [Google Scholar]
Hammer 2008
- Hammer SM, Eron JJ Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, Fischl MA, Gatell JM, Hirsch MS, Jacobsen DM, Montaner JS, Richman DD, Yeni PG, Volberding PA, International AIDS Society‐USA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society‐USA panel. JAMA 2008;300(5):555‐70. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgens JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. [Available from www.cochrane‐handbook.org] [Google Scholar]
Kim 1987
- Kim K, DeMets DL. Design and analysis of group sequential tests based on the type 1 error spending rate function. Biometrika 1987;74:149‐54. [Google Scholar]
Kitahata 2009
- Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of Early versus Deferred Antiretroviral Therapy for HIV on Survival. The New England journal of medicine 2009. [PUBMED: 19339714] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loubiere 2008
- Loubiere S, Filal KM, Sodqi M, Loundou A, Luchini S, Cleary S, et al. When to initiate highly active antiretroviral therapy in low‐resource settings: the Moroccan experience. Antiviral therapy 2008;13(2):241‐51. [PUBMED: 18505175] [PubMed] [Google Scholar]
Moh 2007
- Moh R, Danel C, Messou E, Ouassa T, Gabillard D, Anzian A, et al. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV‐infected adults in West Africa. AIDS (London, England) 2007;21(18):2483‐91. [PUBMED: 18025885] [DOI] [PubMed] [Google Scholar]
Montori 2005
- Montori VM, Devereaux PJ, Adhikari NK, et al. Randomized trials stopped early for benefit: a systematic review. JAMA 2005;294:2203‐09.. [DOI] [PubMed] [Google Scholar]
Orrell 2007
- Orrell C, Harling G, Lawn SD, Kaplan R, McNally M, Bekker L, Wood R. Conservation of first‐line antiretroviral treatmentregimen where therapeutic options are limited. Antiviral Therapy 2007;12. [PubMed] [Google Scholar]
Palella 2003
- Palella FJ Jr, Deloria‐Knoll M, Chmiel JS, Moorman AC, Wood KC, Greenberg AE, Holmberg SD, HIV Outpatient Study Investigators. Survival benefit of initiating antiretroviral therapy in HIV‐infected persons in different CD4+ cell strata. Ann Intern Med 2003;138(8):620‐6.. [DOI] [PubMed] [Google Scholar]
Sabin 2009
- Sabin CA, Phillips AN. Should HIV therapy be started at a CD4 cell count above 350 cells/microl in asymptomatic HIV‐1‐infected patients?. Current opinion in infectious diseases 2009;22(2):191‐7. [PUBMED: 19283914] [DOI] [PubMed] [Google Scholar]
Schackman 2002
- Schackman BR, Freedberg KA, Weinstein MC, Sax PE, Losina E, Zhang H, Goldie SJ. Cost‐effectiveness implications of the timing of antiretroviral therapy in HIV‐infected adults. Arch Intern Med 2002;162(21):2478‐86. [DOI] [PubMed] [Google Scholar]
Schrader 2008
- Schrader S, Chuck SK, Rahn LW, Parekh P, Emrich KG. Significant improvements in self‐reported gastrointestinal tolerability, quality of life, patient satisfaction, and adherence with lopinavir/ritonavir tablet formulation compared with soft gel capsules. AIDS research and therapy 2008;5:21. [PUBMED: 18799008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterling 2001
- Sterling TR, Chaisson RE, Moore RD. HIV‐1 RNA, CD4 T‐lymphocytes, and clinical response to highly active antiretroviral therapy. AIDS (London, England) 2001;15(17):2251‐7. [PUBMED: 11698698] [DOI] [PubMed] [Google Scholar]
Sterne 2009
- Sterne JA, May M, Costagliola D, Wolf F, Phillips AN, Harris R, et al. Timing of initiation of antiretroviral therapy in AIDS‐free HIV‐1‐infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet 2009;373(9672):1352‐63. [PUBMED: 19361855] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walensky 2009
- Walensky RP, Wolf LL, Wood R, Fofana MO, Freedberg KA, Martinson NA, et al. When to start antiretroviral therapy in resource‐limited settings. Annals of internal medicine 2009;151(3):157‐66. [PUBMED: 19620143] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2006
- Charles Gilks, Marco Vitorio and the World Health Organisation guidelines development group. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach ‐ 2006 revision. http://www.who.int/hiv/pub/guidelines/adult/en/index.html (accessed July 2007). [Google Scholar]
WHO 2009
- World Health Organization. Rapid advice: Antiretroviral therapy for HIV infection in adults and adolescents. Geneva: World Health Organization November 2009. [Google Scholar]
Wong 2007
- Wong KH, Chan KC, Cheng KL, Chan WK, Kam KM, Lee SS. Establishing CD4 thresholds for highly active antiretroviral therapy initiation in a cohort of HIV‐infected adult Chinese in Hong Kong. AIDS patient care and STDs 2007;21(2):106‐15. [PUBMED: 17328660] [DOI] [PubMed] [Google Scholar]


