Abstract
Background
Intimate partner violence (IPV) damages individuals, their children, communities, and the wider economic and social fabric of society. Some governments and professional organisations recommend screening all women for IPV rather than asking only women with symptoms (case‐finding). Here, we examine the evidence for whether screening benefits women and has no deleterious effects.
Objectives
To assess the effectiveness of screening for IPV conducted within healthcare settings on identification, referral, re‐exposure to violence, and health outcomes for women, and to determine if screening causes any harm.
Search methods
On 17 February 2015, we searched CENTRAL, Ovid MEDLINE, Embase, CINAHL, six other databases, and two trial registers. We also searched the reference lists of included articles and the websites of relevant organisations.
Selection criteria
Randomised or quasi‐randomised controlled trials assessing the effectiveness of IPV screening where healthcare professionals either directly screened women face‐to‐face or were informed of the results of screening questionnaires, as compared with usual care (which could include screening that did not involve a healthcare professional).
Data collection and analysis
Two authors independently assessed the risk of bias in the trials and undertook data extraction. For binary outcomes, we calculated a standardised estimation of the odds ratio (OR). For continuous data, either a mean difference (MD) or standardised mean difference (SMD) was calculated. All are presented with a 95% confidence interval (CI).
Main results
We included 13 trials that recruited 14,959 women from diverse healthcare settings (antenatal clinics, women's health clinics, emergency departments, primary care) predominantly located in high‐income countries and urban settings. The majority of studies minimised selection bias; performance bias was the greatest threat to validity. The overall quality of the body of evidence was low to moderate, mainly due to heterogeneity, risk of bias, and imprecision.
We excluded five of 13 studies from the primary analysis as they either did not report identification data, or the way in which they did was not consistent with clinical identification by healthcare providers. In the remaining eight studies (n = 10,074), screening increased clinical identification of victims/survivors (OR 2.95, 95% CI 1.79 to 4.87, moderate quality evidence).
Subgroup analyses suggested increases in identification in antenatal care (OR 4.53, 95% CI 1.82 to 11.27, two studies, n = 663, moderate quality evidence); maternal health services (OR 2.36, 95% CI 1.14 to 4.87, one study, n = 829, moderate quality evidence); and emergency departments (OR 2.72, 95% CI 1.03 to 7.19, three studies, n = 2608, moderate quality evidence); but not in hospital‐based primary care (OR 1.53, 95% CI 0.79 to 2.94, one study, n = 293, moderate quality evidence).
Only two studies (n = 1298) measured referrals to domestic violence support services following clinical identification. We detected no evidence of an effect on referrals (OR 2.24, 95% CI 0.64 to 7.86, low quality evidence).
Four of 13 studies (n = 2765) investigated prevalence (excluded from main analysis as rates were not clinically recorded); detection of IPV did not differ between face‐to‐face screening and computer/written‐based assessment (OR 1.12, 95% CI 0.53 to 2.36, moderate quality evidence).
Only two studies measured women's experience of violence (three to 18 months after screening) and found no evidence that screening decreased IPV.
Only one study reported on women's health with no differences observable at 18 months.
Although no study reported adverse effects from screening interventions, harm outcomes were only measured immediately afterwards and only one study reported outcomes at three months.
There was insufficient evidence on which to judge whether screening increases uptake of specialist services, and no studies included an economic evaluation.
Authors' conclusions
The evidence shows that screening increases the identification of women experiencing IPV in healthcare settings. Overall, however, rates were low relative to best estimates of prevalence of IPV in women seeking healthcare. Pregnant women in antenatal settings may be more likely to disclose IPV when screened, however, rigorous research is needed to confirm this. There was no evidence of an effect for other outcomes (referral, re‐exposure to violence, health measures, harm arising from screening). Thus, while screening increases identification, there is insufficient evidence to justify screening in healthcare settings. Furthermore, there remains a need for studies comparing universal screening to case‐finding (with or without advocacy or therapeutic interventions) for women's long‐term wellbeing in order to inform IPV identification policies in healthcare settings.
Plain language summary
Screening women for intimate partner violence in healthcare settings
Background
We carried out this review to find out if asking (screening) all women attending healthcare settings about their experience of domestic violence from a current or previous partner helps to recognise abused women so that they may be provided with a supportive response and referred on to support services. We were also interested to know if this would reduce further violence in their lives, improve their health, and not cause them any harm compared to women's usual healthcare.
Women who have experienced physical, psychological, or sexual violence from a partner or ex‐partner suffer poor health, problems with pregnancy, and early death. Their children and families can also suffer. Abused women often attend healthcare settings. Some people have argued that healthcare professionals should routinely ask all women about domestic violence. They argue that 'screening' might encourage women who would not otherwise do so, to disclose abuse, or to recognise their own experience as 'abuse'. In turn, this would enable the healthcare professional to provide immediate support or refer them to specialist help, or both. Some governments and health organisations recommend screening all women for domestic violence. Others argue that such screening should be targeted to high‐risk groups, such as pregnant women attending antenatal clinics.
Study characteristics
We examined research up to 17 February 2015. We included research studies that had women over 16 years of age attending any healthcare setting. Our search generated 12,369 studies and we eventually included 13 studies that met the criteria described above. In all, 14,959 women had agreed to be in those studies. Studies were in different healthcare settings (antenatal clinics, women's health/maternity services, emergency departments, and primary care centres). They were conducted in mainly urban settings, in high‐income countries with domestic violence legislation and developed support services to which healthcare professionals could refer. Each of the included studies was funded by an external source. The majority of the funding came from government departments and research councils, with a small number of grants/support coming from trusts and universities.
Key results and quality of the evidence
Eight studies with 10,074 women looked at whether healthcare professionals asked about abuse, discussed it, and/or documented abuse in participating women's records. There was a twofold increase in the number of women identified in this way compared to the comparison group. The quality of this evidence was moderate. We looked at smaller groups within the overall group, and found, for example, that pregnant women were four times as likely to be identified by a screening intervention as pregnant women in a comparison group. We did not see an increase in referral behaviours of healthcare professionals but only two studies measured referrals in the same way and there were some shortcomings to these studies. We could not tell if screening increased uptake of specialist services and no studies examined if it is cost‐effective to screen. We also looked to see if different methods were better at picking up abuse, for example, you might expect that women would be more willing to disclose to a computer, but we did not find one method to be better than another. We found an absence overall of studies examining the recurrence of violence (only two studies looked at this, and saw no effect) and women's health (only one study looked at this, and found no difference 18 months later). Finally, many studies included some short‐term assessment of adverse outcomes, but reported none.
There is a mismatch between the increased numbers of women picked up through screening by healthcare professionals and the high numbers of women attending healthcare settings actually affected by domestic violence. We would need more evidence to show screening actually increases referring and women's engagement with support services, and/or reduces violence and positively impacts on their health and wellbeing. On this basis, we concluded that there is insufficient evidence to recommend asking all women about abuse in healthcare settings. It may be more effective at this time to train healthcare professionals to ask women who show signs of abuse or those in high‐risk groups, and provide them with a supportive response and information, and plan with them for their safety.
Summary of findings
Background
Description of the condition
Intimate partner violence (IPV)
Intimate partner violence (IPV) is a violation of a person's human rights and is now recognised as a global public health issue. For the purpose of this review, we adopt the definition of IPV (often termed domestic violence) of the World Health Organization (WHO), that is, any behaviour within an intimate relationship that causes physical, psychological, or sexual harm to those in the relationship (Krug 2002; WHO 2013a). Intimate partner violence often involves a combination of abuse behaviours. These include threats of and actual physical violence, sexual violence, emotionally abusive behaviours, economic restrictions, and other controlling behaviours. Many survivors of IPV report that the physical violence is not the most damaging: it is the relentless psychological abuse that leaves the person with long‐lasting adverse effects (Campbell 2002; WHO 2013b).
Intimate partner violence against men is a social problem with potential adverse outcomes for victims (Coker 2002). Data from the British Crime Survey suggested that 4.4% of men experienced IPV in the 2012/13 period compared to 7.1% of women (Office for National Statistics 2014). In this review, however, we do not include IPV against men because the majority of abuse with serious health and other consequences is that committed by men against their female partners (Coker 2002), with women being three times more likely than men to sustain serious injury and five times more likely to fear for their lives (CCJS 2005), which is why most screening interventions target women (Taft 2001). We also exclude abuse towards women that is perpetrated by other family members such as in‐laws or children. We do include in this review, women who experience violence by female partners, and by ex‐partners given the increased risks of violence associated with separation (Wilson 1993; Campbell 2004; WHO 2013a).
Prevalence of IPV
Abuse of women by their partners or ex‐partners is a common worldwide phenomenon (Garcia‐Moreno 2006). Latest figures from the WHO indicate that one in three women globally experiences physical or sexual violence, or both, by a partner, or non‐partner sexual violence, in their lifetime (WHO 2013a). Based on 48 population‐based surveys across low‐, middle‐, and high‐income countries, the 2002 World Report on Violence and Health revealed rates of between 10% and 69% for lifetime physical violence by a partner (Krug 2002). Definitions used in prevalence studies ranged from physical abuse in current relationships to the inclusion of physical, emotional, sexual, or a combination of abuses in past relationships (Hegarty 2006). Estimates of the magnitude of IPV are obtained from community surveys, clinical samples, and public records. Discrepancies in prevalence rates arise from differences in definitions of IPV, sensitivity of tools, modes of data collection, reporting time frames, and risk variation in the populations sampled (WHO 2013a).
Impacts of IPV
Intimate partner violence can have short‐term and long‐term negative health consequences for survivors, even after the abuse has ended (Campbell 2002). World Development reports (World Bank 2006) and statements from the United Nations (Ingram 2005) emphasise that IPV is a significant cause of death and disability on a worldwide scale (Ellsberg 2008), and the WHO highlights violence against women as a priority health issue (WHO 2013a). The high incidence of psychosocial, physical, sexual, and reproductive health problems in women exposed to IPV leads to frequent presentations at health services and the need for wide‐ranging health services (Bonomi 2009). In addition, IPV is associated with enormous economic and social costs, including those related to social, criminal justice, housing and health services, lost productivity, and human suffering (CDC 2003; Walby 2004; EIGE 2014).
Psychosocial health
The most prevalent mental health sequelae of IPV for female victims are depression, anxiety, post‐traumatic stress disorder (PTSD), and substance use (Golding 1999; Hegarty 2004; Rees 2011; Trevillion 2012; WHO 2013a), and women often suffer from low self esteem and hopelessness (Kirkwood 1993; Campbell 2002). Suicide and attempted suicide are also associated with IPV in both industrialised and non‐industrialised countries (Golding 1999; Ellsberg 2008; WHO 2013a). Moreover, these effects impact detrimentally on women's ability to parent and thus impact on their children (McCosker‐Howard 2006). Exposure to IPV during childhood has been linked with poor emotional, social, and attainment outcomes (Kitzmann 2003), with around six in 10 IPV‐exposed children exhibiting difficulties. Early exposure to interparental violence has also been associated with increased risk of IPV perpetration or victimisation during adolescence and adulthood (Heyman 2002).
Physical health
Abused women often experience many chronic health problems (WHO 2013a), including chronic pain and central nervous system symptoms (Díaz‐Olavarrieta 1999; Campbell 2002), self reported gastrointestinal symptoms, diagnosed functional gastrointestinal disorders (Coker 2000), and self reported cardiac symptoms (Tollestrup 1999). Intimate partner violence is also one of the most common causes of injury in women (Stark 1996; Richardson 2002), including oral‐maxillofacial trauma treated in dental, emergency, and surgical settings (Clark 2014; Ferreira 2014; Wong 2014). Over 50% of all female murders in the UK and USA are committed by partners or ex‐partners (Brock 1999; Shackelford 2005; Home Office 2010). Worldwide, 38% of female homicides are perpetrated by partners (WHO 2013a). In Australia, as elsewhere, a far higher percentage of indigenous compared with non‐indigenous women are murdered by their partners (Mouzos 2003).
Sexual and reproductive health
The most consistent and largest physical health difference between abused and non‐abused women is the experience of gynaecological symptoms (McCauley 1995; Campbell 2002). Women and their fetuses and babies are also at risk, before, during, and after pregnancy (Martin 2001; Silverman 2006). The most serious outcome is the death of the mother or the fetus (Jejeebhoy 1998; Parsons 1999). Violence by a partner is also associated with high rates of pregnancy at a young age (Moore 2010), miscarriage and abortion (Taft 2004; Pallitto 2013), low birth weight (Murphy 2001), and premature birth and fetal injury (Mezey 1997). High rates of symptoms of antenatal and postnatal depression, anxiety, and post‐traumatic stress disorder (PTSD) are also associated with exposure to IPV during adulthood and pregnancy (Howard 2013).
Description of the intervention
Interventions by healthcare practitioners to improve the health consequences for women experiencing IPV
Healthcare services play a central role in abused women's care (García‐Moreno 2014), but the quality of healthcare professionals' responses has been a focus of concern since the 1970s (Stark 1996; Feder 2006). Over the last few decades there has been a concerted effort by women's and justice organisations and the voluntary sector to respond to the needs of women who have experienced IPV. In contrast, the response of health services has been slow (Feder 2009). While most health professionals believe that IPV is a healthcare issue (Richardson 2001), there are a number of barriers to identification and response on the part of practitioners (Hegarty 2001). These include a perceived lack of time and support resources, fear of offending the woman, a lack of knowledge and training about what to do for the woman, and a belief that the woman will not leave the abusive relationship (Waalen 2000). A further barrier is the lack of evidence for effective interventions (García‐Moreno 2014).
Despite these barriers, there has been progress in the overall response of health systems to IPV with many health professional associations around the world publishing guidelines for clinicians on how to identify women who have been abused (Davidson 2000; Family Violence Prevention Fund 2004; Hegarty 2008). Implicit in many of these recommendations is the assumption that screening or asking routinely about abuse will increase identification of women who are experiencing violence, lead to appropriate interventions and support, and ultimately decrease exposure to violence and its detrimental health consequences, both physical and psychological (Taft 2004; WHO 2013b). Screening is predicated on the assumptions that identifying and responding supportively to, and referring on, women experiencing IPV is fulfilling health professionals' duty of care. However, advocacy or ongoing therapy requires appropriate training and time that clinicians may not have in routine care. Further, clinicians are part of a wider system of response and need to be able to identify and refer to domestic violence services that have more time, and have specialist training and connections to other community‐based services such as housing. Training and knowledge of referral services should improve clinicians' motivation to identify when they are not responsible for ongoing domestic violence counselling and advocacy. This review, an update of an earlier review (Taft 2013; O'Doherty 2014a), is focused on screening with a brief response only; it does not include advocacy or psychotherapeutic interventions, which are the topics of separate reviews.
Screening
Screening aims to identify women who have experienced, or are experiencing, IPV from a partner or ex‐partner in order to offer interventions leading to beneficial outcomes. However, within the field of domestic/family violence, both the immediate‐ and longer‐term benefit of screening such women remains unproven (Taket 2004; Spangaro 2009; WHO 2013b), despite some recommendations for screening in particular countries (e.g. USA) (Nelson 2012). Many factors, such as fear or readiness to take action, influence whether or not women choose to disclose their abuse (Chang 2010), and will affect accurate measurement of screening rates. Screening for IPV, therefore, is a problematic concept when traditional screening criteria are applied (Hegarty 2006), as it is a complex social phenomenon rather than a disease. However, it still requires rigorous evidence for its effectiveness if it is to be implemented as policy.
It is important to distinguish between universal screening (the application of a standardised question to all symptom‐free women according to a procedure that does not vary from place to place), selective screening (where high‐risk groups, such as pregnant women or those seeking pregnancy terminations are screened), routine enquiry (when all women are asked but the method or question varies according to the healthcare professional or the woman's situation), and case‐finding (asking questions if certain indicators are present).
For this review, screening is defined as any method that aims for every woman patient in a healthcare setting to be asked about her experiences of IPV, both past and present. Screening may be conducted directly by a healthcare professional or indirectly through a self completed questionnaire (often by computer) with the healthcare professional informed of the questionnaire results. This may include the use of screening tools (Rabin 2009), which vary in their validity and reliability and therefore in their effectiveness to accurately detect abuse. These tools are reviewed in CDC 2007 and Feder 2009. Alternatively, clinicians may ask one or a range of questions related to IPV only at one time point or at several. It is very unlikely that one single question will address the range of women's experiences of IPV. Whether a woman is currently experiencing IPV from a current partner or an ex‐partner (e.g. harassment) or has previously experienced IPV, the goal of screening is the same ‐ to identify her and offer support appropriate to her needs that will prevent any further abuse (e.g. advocacy, legal or police help) and reduce any consequent problems she is experiencing (e.g. offering therapeutic support) or a combination of these.
There has long been debate about the value of screening per se (Taket 2004; Feder 2009), with some arguing that asking questions can raise awareness in women experiencing IPV who are contemplating their situation. Generally, most women are in favour of universal screening, although this varies with abuse status and age (Feder 2009). However, studies have found that women's preferences vary according to the method of screening used (MacMillan 2006; Feder 2009); readers are referred to several studies that have examined this question but were excluded from this review (Furbee 1998; Bair‐Merritt 2006; Chen 2007; Rickert 2009). Bair‐Merritt 2006 found a similar rate of disclosure in audiotaped (11%) compared with written questionnaires (9%) with both methods preferred to direct physician enquiry. Chen 2007 found that there was little difference between self completion and healthcare professional enquiry in terms of participant comfort, time taken, and effectiveness, but that women who had experienced IPV were less comfortable with physician screening. MacMillan and colleagues reported that women found self completion methods easier and more private and confidential (MacMillan 2006). However, women's preferences for how they are asked about IPV needs to be examined in the context of outcomes beyond disclosure. In other words, self interview methods may yield higher disclosure rates, but does this translate into increased awareness about IPV, better uptake of services, reduced re‐exposure to IPV, and improvements in health?
Identifying IPV is only the first step in intervention. Among women receiving care in US primary care clinics, Klevens 2012b tested computer‐assisted screening accompanied by a brief video in which an advocate provided support and information and encouraged women to seek help and referral information versus no screening with referral information only, versus usual care. One year later they found no difference between the three groups in physical or mental health status. Women may have experienced long‐standing abuse or it may have commenced recently; they may be unaware that the behaviour constitutes abuse or be actively seeking support for change, and therefore responses to their needs may need to differ (Chang 2010; Reisenhofer 2013), and may require involvement of a healthcare professional rather than a list of resources.
Two reviews of studies addressing the UK National Screening Committee criteria found that screening by healthcare professionals leads to a modest increase in the number of abused women being identified following screening, but that screening was not acceptable to the majority of health professionals surveyed (Ramsay 2002; Feder 2009). Hegarty 2006 outlines the many clinician barriers (e.g. time, lack of ongoing or effective training and resources) and system barriers (e.g. different health priorities, lack of referral options in the community) that impede effective screening and routine enquiry, and that need to be addressed before clinicians will feel comfortable asking women about their experiences of abuse. In addition, women experience barriers to disclosure, especially during pregnancy, with the presence of abusive partners or monitoring of her attendance at healthcare services where she might disclose. Most reviews to date have concluded that there is no evidence that women experience better outcomes from screening interventions (Ramsay 2002; Wathen 2003; Taft 2013). This lack of evidence has not deterred many governments around the world implementing universal IPV screening, or selective screening in high‐risk populations. Previous US and Canadian Task Forces on Preventive Health Care conducted thorough systematic reviews of the evidence and concluded that there was insufficient evidence to recommend for or against routine screening for violence against women (Wathen 2003; Nelson 2004); however, the US Preventive Services Task Force revised their decision (Nelson 2012) and now recommend screening based on scant evidence from one effectiveness study (MacMillan 2009). The WHO reviewed the evidence in 2013 and recommended screening women only when they are pregnant (WHO 2013b). In some countries, screening is advocated in the absence of sufficient resources or referral options, and where there is a lack of training and resources, clinicians may undertake screening inappropriately. Some would further argue that it is unethical to implement screening for IPV in the absence of evidence of effectiveness as it may cause harm (Jewkes 2002; Wathen 2012).
How the intervention might work
Universal screening aims for 100% of women to be asked about IPV and those experiencing IPV to disclose it. Universal screening may apply to all women in a healthcare setting, such as a hospital, while selective screening could be applied to those in high‐risk groups such as those in antenatal or abortion clinics or pregnant women attending community‐based family practice clinics. Screening women using face‐to‐face methods implies the clinician is directly asking all women who attend for a given consultation whether they are experiencing or have ever experienced abusive behaviours from their partner or ex‐partner, providing women with the choice to disclose or not. Women who disclose abuse may then be offered a response such as safety assessment and planning, emotional support, referral to specialist services, or information on appropriate local/national resources. Another model might offer all women attending a given health service the option of self completing screening (through written or computer‐based methods) where a woman can choose whether or not to disclose abusive behaviour from a partner or ex‐partner. Positive screen results would then be assessed by the consulting healthcare professional who could exercise their own clinical judgement in how to respond to a positive result. The option of administrative or computerised follow‐up has been explored where the clinician is bypassed, and instead, for example, a print‐out of resources is generated. Klevens and colleagues found no effect of this type of screening intervention on outcomes for women (Klevens 2012b).
Why it is important to do this review
This review was originally published two years ago (Taft 2013). However, the international debate on whether or not screening in healthcare settings is beneficial to women has continued. Given that the evidence presented in the previous review was appraised as low to moderate quality and there were few studies that examined medium‐ and long‐term health and abuse outcomes, it is important to search for and synthesise new research and, where possible, combine studies of similar outcomes in a meta‐analysis. We have incorporated another review 'Domestic violence screening and intervention programmes for adults with dental or facial injury' into this update (Coulthard 2010), please see section on Differences between protocol and review. The reasons for doing this work have not changed since the original review. There is an urgent need to assess and identify health sector screening interventions for IPV (Davidson 2000; Feder 2009), in order to: have clear evidence about what health professionals can do safely and effectively to decrease the impact of IPV on women; determine what is cost‐effective; and inform health professionals and policy‐makers about the cost/benefit of screening interventions. In particular, this systematic review examines the most rigorous evidence around health service screening interventions for IPV to ascertain whether the potential benefits of IPV screening for women's health and wellbeing outweigh any potential for harm.
Objectives
To assess the effectiveness of screening for IPV conducted within healthcare settings on identification, referral, re‐exposure to violence, and health outcomes for women, and to determine if screening causes any harm.
Methods
Criteria for considering studies for this review
Types of studies
Any study that allocated individual women, or clusters of women, by a random or quasi‐random method (such as alternate allocation, allocation by birth date, etc.) to a screening intervention compared with usual care or to a condition where healthcare professionals were not aware of women's screening results.
Types of participants
Women (aged 16 years and over) attending a healthcare setting. We define a 'healthcare setting' as any health setting where health services are delivered (such as those listed below), and home visits by these services.
General (family) practice
Antenatal and postnatal services
Hospital emergency, inpatient or outpatient services
Private specialists (e.g. obstetrics and gynaecology, psychiatry, ophthalmology)
Community health services
Drug and alcohol services
Mental health services
Dental services
Types of interventions
Any IPV screening in a healthcare setting as listed above. Screening is defined as any of a range of methods (face‐to‐face, survey or other method, specific to IPV or where IPV was included as part of general psychosocial screening) that aims for all women patients in a healthcare setting to be asked about current or past IPV, including the use of screening tools as well as asking one or a range of screening questions related to IPV on one or more occasions. We only included studies where, in one arm of the trial, the treating healthcare professional conducted the screening or was informed of the screening result at the time of the relevant consultation.
We excluded extended interventions that went beyond screening and an immediate response to disclosure, for example, interventions that include clinical follow‐up or offer further counselling or psychological treatment. We made this an exclusion factor as it is rarely feasible for health professionals to deliver intensive treatments due to lack of time and skill. Furthermore, we wanted to isolate the effect of screening in order to provide evidence on the independent contribution of this particular response to IPV.
Screening was compared to usual care, implying no screening in the comparative arm. However, we did include studies where an eligible screening intervention was compared to a condition of 'screening' that involved no healthcare professionals or face‐to‐face interaction.
Types of outcome measures
We did not use outcomes measured by studies as a criterion for inclusion or exclusion.
Primary outcomes
A. Identification of IPV by health professionals (data based on clinical encounter).
Identification was defined as any form of acknowledgement by a healthcare professional during a consultation that the woman had experienced exposure to IPV. Identification therefore assumes communication between healthcare professional and participant that acknowledges the abuse. Studies use different terms such as identification, discussion, and patient disclosure of IPV. We carefully assessed how stated outcomes were operationalised across trials in order to determine if they met our definition of identification. Studies could collect identification data using a variety of methods (e.g. audio‐recordings of encounters, surveying women and healthcare professionals about what was discussed during the encounter, and medical record review). Identification of IPV through face‐to‐face interviews with researchers was dealt with separately on the basis that it did not properly represent the clinical context and may threaten the validity of the primary identification data.
B. Information‐giving and referrals to support agencies by healthcare professionals (including take‐up rates when available).
We included in this category any recording, documentation or organisational validation that women had been given information about, or referral to, support agencies.
Secondary outcomes
C. Intimate partner violence as measured by:
validated instruments (e.g. Composite Abuse Scale (CAS), Index of Spouse Abuse (ISP); and
self reported IPV, even if using a non‐validated scale.
D. Women's perceived and diagnosed physical health outcomes, using measures of:
physical health (e.g. Short‐Form health survey ‐ 36 (SF‐36) physical subscale, General Health Questionnaire (GHQ));
physical injuries, such as fractures and bruises (self reported or documented in medical records); and
chronic health disorders, such as gynaecological problems, chronic pain, and gastrointestinal disorders (self reported or clinical symptoms, or both, documented in medical records).
E. Women's psychosocial health, using measures of:
depression (e.g. Beck Depression Inventory (BDI), Center for Epidemiologic Studies Depression Scale (CES‐D));
post‐traumatic stress (e.g. Impact of Events Scale (IES), Post‐traumatic Stress Disorder Checklist (PCL));
anxiety (e.g. Spielberger's State‐Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI));
self efficacy (e.g. Generalized Perceived Self‐Efficacy Scale (GSE), Sherer's Self‐Efficacy Scale (SES));
self esteem (e.g. Rosenberg Self‐Esteem Scale (SES), Coopersmith Self‐Esteem Inventory (CSEI));
quality of life (e.g. WHO Quality of Life‐Bref)
perceived social support (e.g. Medical Outcomes Scale (MOS), Sarason's Social Support Questionnaire (SSQ)); and
alcohol or drug abuse (e.g. Addiction Severity Index (ASI), Alcohol and Other Drug Abuse (AOD) scale).
F. Occurrence of adverse outcomes such as:
increased deaths, all‐cause or IPV‐related (documented in medical records or routinely collected data);
increase of IPV as measured by any of the above;
increase of physical or psychosocial morbidity as listed above; and
false negatives and false positives of screening tests.
G. Services and resource use:
family/domestic violence services;
police/legal services;
counselling or therapeutic services;
health service use; and
other services.
H. Cost/benefit outcomes, using measures of:
health service use;
days out‐of‐role; and
medication use.
Timing of outcome assessment
We documented the duration of follow‐up in all included studies. For the purposes of this review, we defined short‐term follow‐up as less than six months since baseline or delivery of the screening intervention, medium‐term follow‐up as between six and 12 months, and long‐term follow‐up as more than 12 months.
Selecting outcomes for 'Summary of findings' table
We included the results of outcomes that could be pooled together in a meta‐analysis in the 'Summary of findings' tables (Table 1 and Table 2). These were the primary outcomes of clinical identification of IPV, and referral. We also included an outcome that was not indicated a priori, an alternative identification outcome, which we refer to as non‐clinical identification (these data were not drawn from documentation of abuse; medical records etc. within the clinical context) (see Differences between protocol and review).
for the main comparison.
| Screening for intimate partner violence (IPV) compared with usual care or screening without health professional involvement | ||||||
| Patient or population: women attending healthcare settings for any health‐related reason Settings: healthcare Intervention: face‐to‐face screening or written/computerised screening with result passed to the healthcare professional Comparison: non‐screened women or those whose screening result was not passed on to the healthcare professional or those screened for issues other than IPV | ||||||
| Outcomes | Universal screening for IPV | Control | Effect | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Relative effect (95% CI) | Absolute effect (95% CI) | |||||
| Identification of IPV by health professionals (assessed immediately or up to 1 month) | 259/5006 (5.2%) | 86/5068 (1.7%) | OR 2.95 (1.79 to 4.87) | 31 more per 1000 (from 13 more to 61 more) | 10,074 (8 studies) | ⊕⊕⊕⊝ Moderate1 |
| 1.7% | 31 more per 1000 (from 13 more to 60 more) | |||||
| Identification of IPV by type of healthcare setting ‐ Antenatal clinics | 24/317 (7.6%) | 6/346 (1.7%) | OR 4.53 (1.82 to 11.27) | 57 more per 1000 (from 14 more to 149 more) | 663 (2 studies) | ⊕⊕⊕⊝ Moderate2 |
| 1.7% | 55 more per 1000 (from 13 more to 145 more) | |||||
| Identification of IPV by type of healthcare setting ‐ Maternal health services | 51/594 (8.6%) | 9/235 (3.8%) | OR 2.36 (1.14 to 4.87) | 48 more per 1000 (from 5 more to 124 more) | 829 (1 study) | ⊕⊕⊕⊝ Moderate3 |
| 3.8% | 48 more per 1000 (from 5 more to 124 more) | |||||
| Identification of IPV by type of healthcare setting ‐ Emergency departments | 71/1218 (5.8%) | 36/1390 (2.6%) | OR 2.72 (1.03 to 7.19) | 42 more per 1000 (from 1 more to 135 more) | 2608 (3 studies) | ⊕⊕⊕⊝ Moderate1 |
| 1.2% | 20 more per 1000 (from 0 fewer to 67 more) | |||||
| Identification of IPV by type of healthcare setting ‐ Hospital‐based primary care | 25/144 (17.4%) | 18/149 (12.1%) | OR 1.53 (0.79 to 2.94) | 53 more per 1000 (from 23 fewer to 167 more) | 293 (1 study) | ⊕⊕⊕⊝ Moderate3 |
| 12.1% | 53 more per 1000 (from 23 fewer to 167 more) | |||||
| Referrals (assessed immediately) | 7/555 (1.3%) | 4/743 (0.5%) | OR 2.24 (0.64 to 7.86) | 7 more per 1000 (from 2 fewer to 35 more) | 1298 (2 studies) | ⊕⊕⊝⊝ Low4 |
| 0.6% | 7 more per 1000 (from 2 fewer to 39 more) | |||||
| CI: confidence interval; GRADE: Grades of Recommendation, Assessment, Development, and Evaluation; IPV: intimate partner violence; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. | ||||||
1Downgraded due to heterogeneity. 2Downgraded due to imprecision. 3Downgraded due to risk of bias. 4Downgraded due to imprecision and risk of bias.
2.
| Face‐to‐face screening compared with written/computer‐based screening for IPV | |||||||
|
Patient or population: women attending healthcare settings for any health‐related reason Settings: healthcare Intervention: face‐to‐face screening for IPV Comparison: written/computer‐based screening | |||||||
| Outcomes | Face‐to‐face screening for IPV | Written/computer‐based screening | Effect | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Relative effect (95% CI) | Absolute effect (95% CI) | ||||||
|
Identification of IPV (non‐clinically based, assessed immediately) |
139/806 (17.2%) | 247/1959 (12.6%) | OR 1.12 (0.53 to 2.36) | 13 more per 1000 (from 55 fewer to 128 more) | 2765 (4) | ⊕⊕⊕⊝ Moderate1 | |
| 24.8% | 22 more per 1000 (from 99 fewer to 190 more) | ||||||
| CI: Confidence interval; GRADE: Grades of Recommendation, Assessment, Development, and Evaluation; IPV: intimate partner violence; OR: odds ratio. | |||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||
1Downgraded due to heterogeneity.
Search methods for identification of studies
We searched the international literature for peer‐reviewed and non‐peer‐reviewed studies and published and unpublished studies. We did not apply any date or language restrictions to our search strategies. We chose not to use a randomised controlled trial (RCT) filter as we wanted the search to be as inclusive as possible; an initial check of the differences between using and not using a RCT filter uncovered a trial not captured when the RCT filter was applied. Our previous search strategies were not limited to any healthcare setting, and so did not require any revisions as they already captured records relevant to oral and maxillofacial injury clinics. The previous version of this review included studies up to July 2012. The searches for this update cover the period from 2012 to 17 February 2015.
Electronic searches
We searched the following databases on 17 February 2015.
Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 1), which includes the Specialised Register of the Cochrane Developmental, Psychosocial and Learning Problems Group (CDPLPG).
Ovid MEDLINE(R) 1946 to February Week 2 2015.
Ovid MEDLINE(R) In–process and other non‐indexed citations 13 February 2015.
Embase (Ovid) 1980 to 2015 Week 7.
CINAHL PLUS (EBSCOhost) 1937 to current.
PsycINFO (Ovid) 1806 to February Week 2.
Sociological Abstracts (ProQuest) 1952 to current.
Conference Proceedings Citation Index ‐ Social Science and Humanities (CPCI‐SS&H; Web of Science) 1990 to 17 February 2015.
Database of Abstracts of Reviews for Effectiveness (DARE) 2012, Issue 2, part of the Cochrane Library.
Cochrane Database of Systematic Reviews (CDSR) 2015, Issue 2, part of theCochrane Library.
WHO International Clinical Trials Registry Platform (ICTRP) (who.int/ictrp/en/).
ClinicalTrials.gov (clinicaltrials.gov).
The search strategies used for this update are in Appendix 1. Search strategies used for earlier versions of the review are in Appendix 2. The searches were originally run by Joanne Abbott, former Trials Search Co‐ordinator (TSC) of CDPLPG. Subsequent searches were conducted by Margaret Anderson, current TSC of CDPLPG.
We also searched the website of the World Health Organization (WHO) (who.int/topics/violence/en/) and the Violence Against Women (VAW) Online Resources (vaw.umn.edu/).
Searching other resources
Handsearching
Due to insufficient resources, we were unable to undertake planned handsearching of the Journal of Family Violence, Journal of Interpersonal Violence, Violence and Victims, Women's Health, American Journal of Preventive Medicine, American Journal of Public Health, Annals of Emergency Medicine, Archives of Internal Medicine, Australian & New Zealand Journal of Public Health, and Journal of the American Medical Association. We are confident that any major screening trials involving healthcare professionals would have been identified through our other search strategies, including our electronic searches and searches of trials registers, citation tracking, networks of the review authors, and communication with authors of included studies.
Citation tracking
We examined the reference lists of acquired papers and tracked citations forwards and backwards.
Personal communication with the first authors of all included articles
We emailed the authors of all primary studies included in the review about any omissions (and, in particular omissions of non‐peer‐reviewed studies). We contacted the WHO Violence and Injury Programme to inquire about any screening studies that might fit our inclusion criteria of which we were unaware, especially in low‐ and middle‐income countries (García‐Moreno 2015 [pers comm]).
Data collection and analysis
Selection of studies
We ran searches four times for this review (September 2009, September 2011, July 2012, and February 2015; see Figure 1). In the original review, two review author pairs (LOD and AT, LOD and KH) independently reviewed abstracts. For this update, TL and EC independently reviewed studies by title and abstract. LOD and AT reviewed studies independently from the point at which full‐text articles had been retrieved (n = 42 in this update).
1.
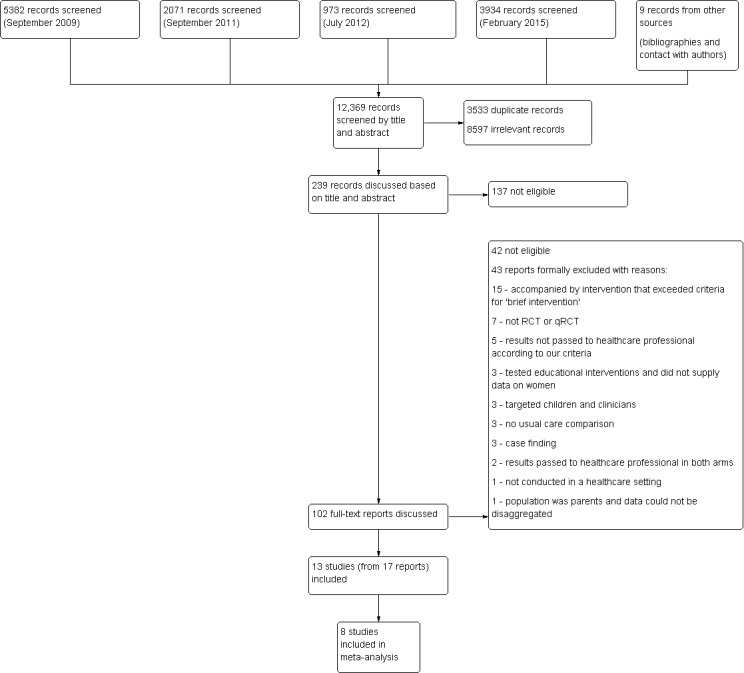
Flow diagram for selection of studies
Where possible, we resolved disagreement about abstract inclusion between any review authors by reading the full study followed by discussion. When agreement could not be reached, a third review author outside that author pairing (GF, LD, JR or KH) assessed whether or not the study fulfilled the inclusion criteria.
Originally, the complex nature of the 'screening' definition required that the entire team met in order to discuss at length and finalise the revised definition of a screening intervention now governing criteria for this review. Two review authors (LOD and AT) independently assessed each study included to this stage against the inclusion criteria with KH also assessing the 42 full‐text articles in the 2015 update. As with the earlier stage of the study review process, we resolved any disagreement by discussing studies in‐depth with other review authors (GF, LD or JR). Where additional information was required to adequately understand the nature of the screening intervention and design, we contacted the first author of the study in question. This led to all outstanding issues being resolved. The reasons behind decisions to exclude otherwise plausible studies are offered in the 'Characteristics of excluded studies' table.
Data extraction and management
Two review authors (LOD and AT prior to 2015, or LOD and TL in 2015) independently extracted the data from the included studies and entered data into electronic data collection forms. We requested any missing information or clarification from the first or corresponding authors of papers, and of the nine authors that we contacted, eight replied (Rhodes 2002; Carroll 2005; MacMillan 2009; Koziol‐McLain 2010; Humphreys 2011; Klevens 2012a; Fraga 2014; Fincher 2015). We resolved any disagreements between the two review authors as regards data extraction through discussion; no adjudication by a third review author was necessary. We noted all instances where additional statistical data were provided by study investigators and we distinguished these data as such in the text (Effects of interventions). Once agreed, we entered all relevant data into Review Manager (RevMan) software, Version 5.3 (RevMan 2014).
We recorded the following information in the 'Characteristics of included studies' table.
Method: randomisation or quasi‐randomisation method, intention‐to‐treat analysis, power calculation, and study dates.
Participants: setting, country, inclusion and exclusion criteria, numbers recruited, numbers dropped out, numbers analysed, age, marital status, ethnicity, socioeconomic status, and educational background.
Interventions: brief description of intervention, including screening tool and method, and method of usual care.
Outcomes: timing of follow‐up events, outcomes assessed, and scales used.
Notes: further information to aid understanding of the study such as source of funding.
Assessment of risk of bias in included studies
Two review authors (LOD and AT prior to 2015, or LOD, AT, and TL in 2015) independently assessed the risk of bias of all included studies using the criteria outlined below and cross‐checked in accordance with the updated methodological criteria in Section 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We rated each domain, for each included study, as either 'high', 'low' or 'unclear' risk of bias.
Sequence generation
Description: the method used to generate the allocation sequence was described in sufficient detail so as to enable an assessment to be made as to whether it should have produced comparable groups.
Review authors' judgement: was there selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence?
Allocation concealment
Description: the method used to conceal allocation sequences was described in sufficient detail to assess whether intervention schedules could have been foreseen in advance of, or during, recruitment.
Review authors' judgement: was there selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment?
Blinding
Blinding of participants and personnel
Description: any measures used to blind healthcare professionals or participants to their randomisation status were described to enable us to know whether the outcomes may have been affected by this knowledge.
Review authors' judgement: was there performance bias due to knowledge of the allocated interventions by participants and personnel during the study?
Blinding of outcome assessment
Description: any measures used to blind outcome assessors were described in sufficient detail so as to enable us to assess possible knowledge of which intervention a given participant might have received.
Review authors' judgement: was there detection bias due to knowledge of the allocated interventions by outcome assessors?
Incomplete outcome data
Description: the study reported data on attrition and the numbers involved (compared with total randomised) as well as the reasons for attrition or these were obtained from investigators.
Review authors' judgement: was there attrition bias due to the amount, nature, or handling of incomplete outcome data?
Selective outcome reporting
Description: attempts were made to assess the possibility of selective outcome reporting by authors. Where available, we checked protocols and trial databases for prior outcome specification. Where a protocol was not available, we searched the databases of registered trials to check pre‐specified outcome measures. Where neither were available, we were unable to assess this and therefore nominated this as 'uncertain'.
Review authors' judgement: were reports of the study free of suggestion of selective outcome reporting?
Other sources of bias
Description: the study was apparently free of other problems that could put the outcomes at high risk of bias. In common with our associated review on advocacy (Ramsay 2009) ‐ update currently under way and due to be published soon ‐ we specified the following three criteria under this heading.
Baseline measurement of outcome measures
Review authors' judgement: were baseline data (if available) evenly distributed?
Reliability of outcome measures
Review authors' judgement: were outcome measures validated and referenced?
Protection against contamination
Review authors judgement: was there adequate protection against the study being contaminated?
Measures of treatment effect
Continuous outcomes
We analysed continuous data if (i) means and standard deviations (SDs) were available in the report or obtainable from the authors of studies, and (ii) the data were said to be normally distributed. If the second standard was not met then we did not enter such data into RevMan (RevMan 2014) (as it assumes a normal distribution). (More detail on the treatment of continuous data is available in Appendix 3).
Binary outcomes
For binary outcomes (e.g. woman identified/not identified, referred/not referred), we calculated a standard estimation of the odds ratio (OR) and 95% confidence intervals (CI) using a random‐effects model (Higgins 2011). Where data required to calculate the OR were neither reported nor available from the authors of studies, we did not try to calculate these but have provided the findings as published by the authors.
Unit of analysis issues
We anticipated both individual‐ and cluster‐randomised controlled trials would be identified. With regard to cluster trials, we examined studies to assess whether they had accounted for the effects of clustering using the CONSORT (Consolidated Standards of Reporting Trials) recommendations (Campbell 2012). We have archived methods for re‐analysing cluster trials in future updates of this review (Appendix 3).
We did not use indirect comparisons as all included studies compared the intervention to a suitable comparison condition (usual care or no involvement of healthcare professionals).
Dealing with missing data
We assessed missing data and dropout rates for each of the included studies. If studies were required to impute missing data in published articles, and tables of outcomes with and without imputation were provided, we used the imputed figures. The 'Characteristics of included studies' tables specify the number of women who were included in the final analysis in each group as a proportion of all women randomised in the study. Where available, we provided the reasons given for missing data in the narrative summary along with an assessment of the extent to which the results may have been influenced by missing data. We planned to use sensitivity analysis to deal with missing data. No study conformed to all intention‐to‐treat analysis criteria. We included those in which all completed cases were analysed in the groups to which they were randomised (available case analysis, Higgins 2011, Section 16.2), irrespective of whether or not they received the screening intervention. More detail on the treatment of missing data is available in Appendix 3.
Assessment of heterogeneity
We assessed the consistency of results visually and by examining the I² statistic ‐ a quantity that describes approximately the proportion of variation in point estimates that is due to heterogeneity rather than sampling error (Higgins 2002). Where significant statistical heterogeneity was detected (I² > 50%), we explored differences in clinical characteristics (participants, interventions, outcomes) and methodological characteristics (risk of bias, study design) with modified analyses. We then summarised any differences in the narrative synthesis.
Assessment of reporting biases
There were not enough studies to assess reporting biases. Methods for assessing reporting bias, archived for future updates of this review, are available in Appendix 3.
Data synthesis
We only performed a meta‐analysis where there were sufficient data and it was appropriate to do so. The decision to pool data in this way was determined by the compatibility of populations, denominators, and screening methods (clinical heterogeneity), duration of follow‐up (methodological heterogeneity), and outcomes. As fixed‐effect models ignore heterogeneity, we have used the random‐effects models to take account of the identified heterogeneity of the screening interventions. The Mantel‐Haenszel method, a default program in RevMan (RevMan 2014), can take account of few events or small study sizes and can be used with random‐effects models. Where it was inappropriate to combine the data in a meta‐analysis, we provided a narrative description of the effect sizes as specified in the original study and 95% CIs or SDs for individual outcomes in individual studies. We did not access individual patient data (IPD) as we did not encounter unpublished studies or studies whose data could not be included in our analyses. The main issue with studies included in this review was risk of bias and the IPD approach cannot, generally, help avoid bias associated with study design or conduct (Higgins 2011).
'Summary of findings' table
We used the online Guideline Development Tool (GDT; GRADEpro GDT) to develop 'Summary of findings' tables (Table 1 and Table 2). These tables summarise the amount of evidence, typical absolute risks for screened and non‐screened women, estimates of relative effect, and the quality of the body of evidence.
We used the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach to classify the review findings: high quality (further research is unlikely to change our confidence in the estimate of effect); moderate quality (further research is likely to have an important impact on our confidence in the estimate of effect, and might change it), and low or very low quality (further research is likely to have an important impact on our confidence in the estimate of effect and is likely to change it). The quality of a body of evidence involves considering risk of bias within studies (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and the risk of publication bias.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses for type of healthcare setting, and analysed data from a subset of studies that measured prevalence (or non‐clinically based identification) rather than clinical identification.
Not enough studies were identified to perform all subgroup analyses planned in the protocol for this review (Taft 2008). Please also see Appendix 3 for subgroup analyses archived for future updates of this review.
Sensitivity analysis
We based our primary analyses on available data from all included studies relevant to the comparison of interest. To assess the robustness of conclusions to quality of data and approaches to analysis, we conducted the following sensitivity analyses:
study quality;
differential dropout.
We have archived additional analyses for future updates of this review. Please see Appendix 3.
Results
Description of studies
Results of the search
Our searches of the listed electronic databases (see Figure 1) generated 12,369 records (including nine records identified from the reference lists of included studies and from contact with authors) of which 3533 were duplicates; we therefore screened 8836 abstracts. Authors agreed that 8597 abstracts were irrelevant and that 239 required joint review. Following discussions, we excluded a further 137. We subsequently retrieved full‐text papers for 102 records. We determined that 42 were ineligible. A further 43 articles, which appeared as though they could meet inclusion criteria, ultimately did not and we excluded them (reasons for their exclusion are detailed in the 'Characteristics of excluded studies' tables). Thirteen studies (that were published in 17 papers) met the inclusion criteria.
Included studies
Study designs
Thirteen randomised controlled trials (Carroll 2005; MacMillan 2006; Rhodes 2006; Ahmad 2009; MacMillan 2009; Kataoka 2010; Koziol‐McLain 2010; Humphreys 2011; Klevens 2012a; Fraga 2014; Fincher 2015), of which, two were quasi‐randomised controlled trials (Rhodes 2002; Trautman 2007), met the criteria for inclusion in this review. All 13 studies were reported in peer‐reviewed journals.
Location
Four studies were conducted in Canada (Carroll 2005; MacMillan 2006; Ahmad 2009; MacMillan 2009), six in the USA (Rhodes 2002, Rhodes 2006; Trautman 2007; Humphreys 2011; Klevens 2012a; Fincher 2015), one in Japan (Kataoka 2010), one in Portugal (Fraga 2014), and one in New Zealand (Koziol‐McLain 2010). Several were cluster‐randomised trials, which accounted for clustering in their analyses, and were conducted in diverse healthcare settings (Carroll 2005; MacMillan 2006; MacMillan 2009). Rhodes 2006 stratified by clinic location (inner urban or suburban) and randomised within location.
Healthcare settings
In three studies, women were recruited from antenatal clinics (Carroll 2005; Kataoka 2010; Humphreys 2011), while Fraga 2014 enrolled women who were one year postpartum at a hospital obstetrics department and MacMillan 2009 included an obstetrics and gynaecology clinic. Four were located in emergency departments (EDs) only (Rhodes 2002; Rhodes 2006; Trautman 2007; Koziol‐McLain 2010). Ahmad 2009 was conducted in a hospital‐affiliated family practice, and both MacMillan 2006 and MacMillan 2009 combined primary and tertiary care sites (family practices, EDs, and women's health services). Klevens 2012a was conducted in assorted women's health clinics in a hospital. Fincher 2015 screened women participating in a Special Supplemental Nutrition Program at a Women, Infants, and Children's (WIC) service. We identified no eligible trials in dental or ophthalmology settings or in maxillofacial injury or fracture clinics.
Characteristics of participants
Both clinicians and their patients participated in all included studies.
Healthcare professionals
In two studies, the first type of participant to be recruited was the clinician (Carroll 2005; Ahmad 2009). They were trained prior to the recruitment of patient participants.
Ahmad 2009 recruited 11/14 eligible family physicians from urban academic hospital‐affiliated family practice clinics. Seven were white female clinicians who had an average age of 46 years and averaged 16 years in practice. Carroll 2005 recruited 48 family physicians, obstetricians, and midwives from four practices diverse in location and populations, which provided antenatal and postpartum care. These different clinicians were paired by age, sex, clinician type, and health service location where possible and then randomised in pairs. Thirty‐six of 48 (75%) were family physicians; the mean age was 42 years and 50% were female. They averaged 13.5 years in practice.
Participants
The 14,959 women recruited to the 13 studies were very diverse in sociodemographic characteristics, and while some studies described the entire screened population, others only described those whose abuse status was identified through screening. The majority of women were Canadian, with over 9000 recruited to MacMillan 2006 and MacMillan 2009.
Pregnant women screened in antenatal settings were aged 30 years or less (Carroll 2005; Kataoka 2010; Humphreys 2011). In Carroll 2005, among the 253 women, 84% were Canadian born; the majority were married with an even income spread and no or minor concerns about their pregnancy. Similarly, although located in an urban Japanese clinic, the 323 women in Kataoka 2010 were overwhelmingly married (over 90%); around 60% were having their first child; and around 80% had post secondary school qualifications, with 42% having college graduate or postgraduate qualifications.
In contrast, Humphreys 2011 described only those 50/410 pregnant women assessed as 'at risk' for IPV at five San Francisco bay antenatal clinics; their profile is consistent with disadvantage. These 50 women were ethnically diverse: 17 were Hispanic, 11 were black or African‐American, 15 were white, and seven were from other backgrounds. Twenty‐three had never married and 29/50 had only high school education or less. The mean age was 28 years and 38 women had been previously pregnant. Women's mean gestational age was 20 weeks and 14 had smoked tobacco in the past 30 days. Forty‐three had been abused in the year before pregnancy and 19 since pregnancy. Twelve had been abused one to three times; four had been abused four to six times; and one more than six times (two had missing data for frequency). Fraga 2014 involved women in a maternity setting who were one year postpartum and had consented to be contacted a year earlier around the time their baby was born. Although they do not provide sociodemographic information for the 915 women in this rapid report, the sample from which women were drawn involved 2660 white women, 9.7% of whom had experienced physical abuse during pregnancy. Women who were abused were more likely to experience preterm birth compared to non‐abused women (21% versus 6.8% respectively), and they were less educated and more likely to be under 20 years of age, not cohabiting, have lower incomes, and have received less antenatal care (Rodrigues 2008).
Klevens 2012a recruited 126 predominantly disadvantaged black women (78.6%) from diverse women's health clinics (obstetric, gynaecological, and family planning) of a Chicago public hospital. The women had a mean age of 35.8 years; either a high school education or less (42.4%) or vocational/college (41.9%); and were uninsured (57.1%) or had Medicaid (37.3%).
Women in emergency settings only were recruited from urban hospitals with ethnically diverse populations (Rhodes 2002; Rhodes 2006; Trautman 2007; Koziol‐McLain 2010). These women tended to be older. In the New Zealand study (Koziol‐McLain 2010), 37.6% of 399 women were Maori and their median age was 40 years. The women's incomes were evenly spread but tended to be in a low‐income bracket; just under half (45.6%) had completed a post‐school qualification other than a university degree (8.3%). About 67.4% currently had partners; and 64.9% were from the main urban area. In a Baltimore Level 1 trauma hospital, the 411 women in Trautman 2007 were overwhelmingly 'non‐white' (83.9%); 41% were aged 35 to 54 years; the majority (50.9%) had children at home; and 34.8% were on Medicaid insurance. While 42.3% were high school graduates, 30.5% had not graduated from high school and 42.4% had an income in the lowest quintile. Around one‐half had physical and mental health summary scores one or two standard deviations (SDs) below norms. The 323 women recruited in Rhodes 2002 had similar characteristics to the urban women in Rhodes 2006. The 1281 women in Rhodes 2006 were very diverse according to whether they were recruited in an urban or suburban ED setting. In the urban ED, 86% of 883 women were African‐American (90% in 2002); had a mean age of 32 years (37 years in 2002); 35% had a high‐school diploma or less and 38% qualifications after high school, but 53% had an income in the lowest quartile; 46% relied on Medicaid (39% in 2002); and 51% were single (59% in 2002). By contrast, in the suburban ED clinic, the median age of the 398 women was 36 years; 80% were white; 71% had post high‐school qualifications; the income spread was more even; 65% had private insurance; and 43% were married with only 31% single.
Ahmad 2009 was the only study to be based solely in a family practice clinic affiliated with an urban academic hospital in Toronto, Canada. The mean age of the 293 women was 44 years; 34.5% of women were born outside Canada; over half were married with 29% having children under 15 years of age living at home. Two‐thirds were employed full‐ or part‐time with an even spread of income, although just under one‐third were in the lowest quintile.
MacMillan 2006 recruited 2461 women from mixed settings: two family practices, two EDs, and two women's health clinics. The women's mean age was 37.1 years; 87% were born in Canada; 55% were married; 46.6% had children at home; 52.2% were educated for more than 14 years; 46.9% were working full‐ or part‐time; and 17.6% had incomes in the lowest quintile.
In the MacMillan 2009 study, 6743 women were also recruited from mixed settings: 12 primary care clinics, 11 EDs, and three obstetric/gynaecology clinics. Characteristics were only described for the 411 women retained and 296 women lost to follow‐up (LTFU) since recruitment, but there was a clear trend to greater abuse and disadvantage among those LTFU compared with those retained. Compared to those LTFU, women retained were more educated, less likely to be single, and had lower scores on the Women Abuse Screen Tool (WAST) and Composite Abuse Scale (CAS).
Fincher 2015 recruited 402 African‐American women, with a mean age of 27 years, who were attending a Women, Infants, Children's (WIC) clinic in Atlanta, Georgia USA. This is an area of high disadvantage with 19% of families living below the federal poverty line and one in four of these families has a child under five years of age. Nearly half of families receive food stamps; the majority are African‐American households. The majority of respondents were single (40%) or in an unmarried relationship (45%). Fourteen per cent of respondents completed some high school education, and 30% had received a high school degree.
Screening intervention
Screening tools
The screening tools applied in these studies as part of the intervention were very heterogeneous. The majority employed an IPV‐specific validated screening instrument, with some studies using more than one tool. Included interventions always consisted of face‐to‐face or healthcare professional‐involved screening. Ideally this was compared to usual care (with no enquiry about IPV). However, there were instances of a screening instrument being applied in the control arm through, for example, computerised or written enquiry, which was tolerated providing those results were not processed by any clinical staff. The tools used in one or more arms of trials were: Woman Abuse Screening Tool (WAST) (MacMillan 2006; MacMillan 2009); Abuse Assessment Screen (AAS) (Rhodes 2006; Ahmad 2009; Koziol‐McLain 2010; Humphreys 2011; Fraga 2014); Partner Violence Screen (PVS) (MacMillan 2006; Trautman 2007; Ahmad 2009; Koziol‐McLain 2010; Klevens 2012a); Violence Against Women Screen (VAWS) (Kataoka 2010); and Revised Conflict Tactics Scale (CTS) ‐ Short Form (Fincher 2015). Rhodes 2002 adapted questions from the AAS and PVS and others. In several cases, omnibus screening aimed to assess a range of psychosocial problems (e.g. to assess a range of health issues in pregnancy or to diminish stigma around the true purpose of the study), of which IPV was only one (e.g. Ahmad 2009; Humphreys 2011). In Carroll 2005, the Antenatal Psychosocial Health Assessment (ALPHA) tool assessed a range of psychosocial issues such as child abuse and depression; the IPV questions contained in the ALPHA are derived from the WAST (Carroll 2005). The validity of these tools is also heterogeneous and thoroughly reviewed in Feder 2009 (p 29). Often, data collected through the screening intervention fed into the primary identification outcome data.
Screening methods and strategies
Studies used different modes of applying the screening tools indicated above in intervention and comparison groups. Five interventions involved a computer‐assisted self completion screening process with positive results being conveyed to providers (Rhodes 2002; Rhodes 2006; Trautman 2007; Ahmad 2009; Humphreys 2011). MacMillan 2009 used written methods in their intervention arm before conveying results to healthcare professionals. Carroll 2005, MacMillan 2006, Kataoka 2010, Koziol‐McLain 2010, and Fraga 2014 included face‐to‐face screening where the healthcare professionals themselves screened the women. Kataoka 2010 selected a written enquiry method as the comparison compared with face‐to‐face screening, but since it was face‐to‐face, this method guaranteed the result was processed by a healthcare professional; in this study we treated face‐to‐face screening as the intervention. Klevens 2012a compared healthcare professional screening with audio computer‐assisted self interviews (A‐CASI) screening. Fraga 2014 had three groups, but we combined the two arms that involved social worker screening (face‐to‐face and telephone) and compared it to a group that received a questionnaire by post. In Fincher 2015, women attending a community health programme (WIC services) received face‐to‐face screening by trained healthcare professional researchers who provided information and resources on issues, including healthy relationships. As this was the only included study that had researchers, as opposed to healthcare professionals, deliver the face‐to‐face screening, we excluded it from our primary analysis as, ultimately, the data were not part of the clinical context.
Comparisons
Six studies compared IPV screening with usual care (Rhodes 2002; Carroll 2005; Rhodes 2006; Ahmad 2009; MacMillan 2009; Koziol‐McLain 2010). Humphreys 2011 compared IPV screening and clinician follow‐up with researcher‐based IPV screening where results were not provided to the clinician. Written self completion was used in one arm of MacMillan 2006 (which we used as a comparison arm) and they used computerised self completion in another (which we also treated as comparison). Trautman 2007 compared screening that included questions about IPV with screening for other issues that did not include IPV, and both sets of results were passed on to clinicians. Kataoka 2010 compared face‐to‐face screening interview by a healthcare professional with a self administered questionnaire and Klevens 2012a compared A‐CASI screening with the same screen administered by the clinician. Fraga 2014 compared screening by social workers to a group that received a questionnaire by post.
We treated groups where women self completed IPV questions but with no follow‐up or involvement of clinicians, and screening for health issues without reference to IPV, as 'usual care' conditions.
Outcomes and outcome measures
Identification (including discussion or detection)
All but one study, Koziol‐McLain 2010, in some way measured the identification of IPV using various screening modes and tools. However, this was not always a form of clinical identification, with some studies gathering what was more akin to prevalence data according to different modes of screening (MacMillan 2006; Kataoka 2010; Fincher 2015), rather than information for use in the clinical domain. There were instances where clinical identification data were recorded but did not lend themselves to meta‐analysis because they were not measured consistently across arms of the trial (Klevens 2012a). Thus, we combined these four studies in a meta‐analysis of non‐clinically based identification based on face‐to‐face screening versus other screening techniques.
Eight studies measured identification such that it could be defined as clinical identification of IPV from screening and we used this in our primary analyses (Rhodes 2002; Carroll 2005; Rhodes 2006; Trautman 2007; Ahmad 2009; MacMillan 2009; Humphreys 2011; Fraga 2014). These data were gathered through providers' and women's self report about what had occurred during the consultation, chart review/clinical documentation, and audio‐recordings of clinical encounters.
Information‐giving, referral, and uptake of services
While most studies included some assessment of the provision of information, referral, and women's service use, measurement of these outcomes varied enormously. First, the provision of information or resources was already linked to the majority of interventions and received by women who took up the intervention. For example, a computer print‐out of resources or information pamphlets commonly occurred as part of the intervention. Thus, it was not appropriate for us to treat it as an outcome of screening interventions. Another example can be found in Rhodes 2006, where provision of services was defined as safety assessment, counselling by the healthcare providers, and provision of information on resources; to measure these would be more in keeping with an assessment of fidelity since these are features of the intervention, rather than outcomes of a screening intervention.
Studies also varied greatly in how they defined referral. For example, Klevens 2012a made reference to three types of 'referral' ‐ healthcare professional, A‐CASI plus provider support, and A‐CASI alone, but this was more about how women in the different arms self referred based on the list of resources provided to them in each trial arm. We were interested to know if screening interventions increased women's formal referral to other internal and external support services, with this information being derived from medical records or self report by participants or even data from services to indicate the number of women referred to them. However, only two studies treated referral in this way and were included in a meta‐analysis (Trautman 2007; Ahmad 2009). Trautman 2007 examined differences in the numbers referred to social work by the treating staff at the ED and Ahmad 2009 used audio‐recordings of consultations to determine if women were referred. Ahmad also checked if any arrangements were made for follow‐up appointments but this was not included in the referral data.
Uptake of services suffered similar difficulties, encompassing different variables for different studies where some looked at specific uptake based on the resources that were flagged (Trautman 2007; Klevens 2012a), and others look at a more general uptake of community services (Koziol‐McLain 2010). Consequently, we were unable to include data on uptake of services in a meta‐analysis.
Intimate partner violence
MacMillan 2009 and Koziol‐McLain 2010 included level of exposure to IPV (using the CAS, Hegarty 2005) as a primary outcome.
Women's health and quality of life
MacMillan 2009 included quality of life as a primary outcome (assessed with the WHO Quality of Life‐Bref), but included in their secondary outcomes: general health (Short Form health survey ‐ 12 (SF‐12)), depressive symptoms (Center for Epidemiologic Studies ‐ Depression Scale (CES‐D)), post‐traumatic stress disorder (Startle, Physiological arousal, Anger, and Numbness (SPAN)), alcohol use or dependency (Tolerance, Worried, Eye‐opener, Amnesia, K/Cut Down (TWEAK), and Drug Abuse Screening Test (DAST)).
Adverse and other outcomes
Ahmad 2009 included advice for follow‐up and patient comfort with screening, and need to consult with the nurse after screening. Carroll 2005, MacMillan 2006, Kataoka 2010, and Klevens 2012a measured comfort and preference for mode or satisfaction with the screening process.
Koziol‐McLain 2010 measured safety behaviours and resource use.
Rhodes 2006 measured provision of domestic violence services, and Trautman 2007, MacMillan 2009 and Klevens 2012a measured services usage rates.
MacMillan 2009 measured potential harms using the Consequences of Screening Tool (COST) (MacMillan 2009).
Excluded studies
We excluded 43 studies for the following reasons.
Fifteen studies because screening was accompanied by an intervention that exceeded our criteria for a 'brief intervention' (Duggan 2004; Green 2005; Curry 2006; Jewkes 2008; Gillum 2009; Cripe 2010; Kiely 2010; Tiwari 2010; Florsheim 2011; Taft 2011; Subramanian 2012; Hegarty 2013; Kiely 2013; Saftlas 2014; Wagman 2015).
Seven studies because they were not randomised or quasi‐randomised controlled trials (Furbee 1998; Larkin 1999; Knight 2000; Bonds 2006; Halpern 2009; Hewitt 2011; Kapur 2011).
Five studies because the results were not passed on to the healthcare professional according to our criteria (Bair‐Merritt 2006; Houry 2011; Klevens 2012b; Beatty 2014; Hoelle 2014).
Three studies because they tested educational interventions and did not supply data on women (Coonrod 2000; Brienza 2005; Fernández Alonso 2006).
Three studies because they targeted children and clinicians (Dubowitz 2011; Feigelman 2011; Dubowitz 2012).
Three studies because there was no usual care comparison (Chen 2007; Ernst 2007; Rickert 2009).
Three studies because they were case‐finding not screening trials (Thompson 2000; Campbell 2001; Feder 2011).
Two studies because screening results were passed on to the healthcare professional in both usual care and intervention groups (Hollander 2001; Taft 2012).
One study because it was not conducted in a healthcare setting (Robinson‐Whelen 2010).
One study because the population were parents and data could not be disaggregated (Garg 2007).
Risk of bias in included studies
Our risk of bias judgements are summarised below and in Figure 2 and Figure 3. Further detail can also be found in the 'Risk of bias' tables beneath the Characteristics of included studies tables.
2.
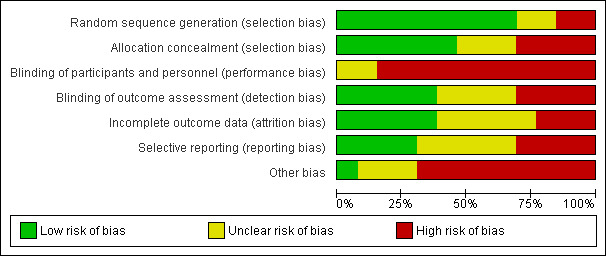
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
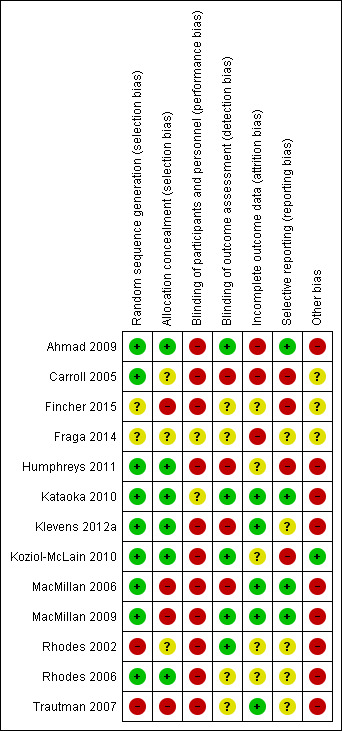
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
Random sequence generation
Nine studies described reliable low‐risk random sampling strategies (Carroll 2005; MacMillan 2006; Rhodes 2006; Ahmad 2009; MacMillan 2009; Kataoka 2010; Koziol‐McLain 2010; Humphreys 2011; Klevens 2012a), but two used methods with a high likelihood of systematic bias: Trautman 2007 used consecutive enrolment periods and Rhodes 2002 used alternate allocation. Fincher 2015 and Fraga 2014 provided no description of the sequence generation in their report so we contacted them for information but were unable to gain a comprehensive account of the procedures.
Allocation concealment
Rhodes 2006, Ahmad 2009, Koziol‐McLain 2010, Humphreys 2011, Kataoka 2010, and Klevens 2012a described reliable procedures to conceal the allocation of participant status. In Carroll 2005, Fraga 2014, and Rhodes 2002, there was inadequate information to judge whether or not bias could have been introduced. In MacMillan 2006 and MacMillan 2009, monthly calendars showing shift allocation for site co‐ordinators was the chosen method. Recruiters with knowledge of this allocation could have introduced bias with selective recruitment. A process with similar potential for bias was used in Trautman 2007. Fincher 2015 was also considered at high risk of bias because there was no description of how the assignment was managed/concealed, and recruiters were also the interviewers for the face‐to‐face group so it is possible that they knew the allocation at the time of recruiting women.
Blinding
Blinding of participants and personnel (performance bias)
It is very difficult to blind healthcare professionals in a screening trial, especially when IPV screening results are attached to the patients' files. All studies suffered from high risk of performance bias with the exception of Kataoka 2010 and Fraga 2014, where we judged that it was unclear whether bias could have affected outcomes. Protocols to minimise performance bias (Koziol‐McLain 2010) and blinding clinicians to the overall purpose of the study (Ahmad 2009) were stated strategies to minimise this type of bias, but similar to the remaining studies, knowledge that they were in a trial, and patients' screening results attached to their files increased the possibility of performance bias. The problem of performance bias differentially affecting outcomes in the women participants was likely greater where an intervention was compared to standard or usual care (Ahmad 2009; Koziol‐McLain 2010), as opposed to using an alternative screening approach.
Blinding of outcome assessment (detection bias)
We judged detection bias to be low in four trials where steps had been taken to actively blind: interviewers (MacMillan 2009; Koziol‐McLain 2010), chart reviewers (Rhodes 2002), and those coding audio‐recordings (Ahmad 2009). In Kataoka 2010, the likelihood of detection bias was reduced as there was no clear intervention or comparison group (it was questionnaire versus face‐to‐face screening) or study hypothesis, and we only used data from the first screening (there had been three in total). The risk of detection bias was unclear in Rhodes 2006, Fincher 2015, Fraga 2014, and Trautman 2007. Healthcare professionals aware of participant IPV status gave estimates of their levels of concern in Carroll 2005 and may have overestimated their levels of concern. In Humphreys 2011 and Klevens 2012a, research staff collecting outcome data may have been able to detect which study arm a woman was in (as there was no indication that they were blinded) and this may have biased outcome detection.
Incomplete outcome data
Three studies were at high risk for attrition differentially affecting groups (Carroll 2005; Ahmad 2009; Fraga 2014). In Ahmad 2009, LTFU was low, but sensitivity analyses suggest missing data potentially affected the results; this was further confirmed by imputation in an intention‐to‐treat analysis. Unbalanced provider attrition (nine in the intervention group versus three in the control group) in Carroll 2005 risks bias, even though participant data loss was low and evenly spread (7.5%). In Fraga 2014, the postal questionnaire group suffered from high attrition (70/305; 23%).
Trautman 2007 attained 100% retention as data were collected immediately. Kataoka 2010 had no more than 10% LTFU across both intervention and control groups but this was across all three screenings, and there was no attrition when data were collected for the first screening (the data used in the review). MacMillan 2006 had approximately 5% attrition depending on the screening tool used. We judged these studies, along with Klevens 2012a, to be at low risk of bias from LTFU. The study conducted by MacMillan 2009 resulted in 42% attrition overall, with participants missing not at random (more severely abused women likely to be lost) suggesting the observed effect may be biased. Multiple imputation in MacMillan 2009 for missing data did reduce the effect size and given that the study accounted for missing data, we judged it to be low risk.
Humphreys 2011, while making conservative assumptions about missing data, did not give the reasons for attrition making it difficult to judge whether assumptions were appropriate. Koziol‐McLain 2010 reported 13.8% LTFU missing at random but did not provide reasons for LTFU, making it difficult to judge. Rhodes 2002 gave an inadequate account of the reasons for the 20% missing chart reviews and the 32% patient attrition, and 21% providers refusing recording probably biased the effects found in Rhodes 2006. In Fincher 2015, 12% of women dropped out of the computer group compared to 4% in the face‐to‐face group but we were unable to obtain any information on these women and it is unclear at what stage that occurred. At two weeks, 31.8% of women completed a follow‐up suggesting high attrition but we do not know if there was differential dropout and, in any case, we were unable to incorporate the two‐week data as they were not reported.
Selective reporting
Publication of protocols and trial registration reduce the risk of selective reporting. MacMillan 2006, Ahmad 2009, Kataoka 2010, Koziol‐McLain 2010, Humphreys 2011, and MacMillan 2009 were registered, but the lack of study protocols across studies made the analysis and primary outcomes difficult to access. Registered trials were considered low risk if there was no indication of selective reporting in the report (e.g. we checked that all outcomes were reported at all time points). These included Ahmad 2009, Kataoka 2010, MacMillan 2006, and MacMillan 2009. There was indication of selective reporting in Koziol‐McLain 2010 based on an inconsistency between the outcomes as registered and reported. In three additional studies, certain outcomes were omitted (Carroll 2005; Humphreys 2011; Fincher 2015), and the risk was unclear in five studies (Rhodes 2002; Rhodes 2006; Trautman 2007; Klevens 2012a; Fraga 2014).
Other potential sources of bias
We judged the potential for contamination in comparison groups of women to be high across a large proportion of studies (Rhodes 2002; Rhodes 2006; Trautman 2007; Ahmad 2009; MacMillan 2009; Humphreys 2011; Klevens 2012a), and low in Koziol‐McLain 2010. A high proportion (21.5% compared to 15.7%) of low‐income women in the computer‐based group may have biased screening results in MacMillan 2006, and Kataoka 2010 acknowledges her measurement had psychometric property limitations with low specificity. The extent of bias from other sources was unclear in Carroll 2005, Fincher 2015, and in Fraga 2014, where there were imbalances at baseline and we found no reference to account for these in analyses. Also, there had been prior research involvement of the cohort of women in a study about IPV during pregnancy 12 months earlier (Rodrigues 2008), but this was not addressed.
Effects of interventions
Screening versus control (no screening/usual care; clinician not notified of screening results)
Primary outcomes
A. Identification of intimate partner violence (IPV) by health professionals
Eight of 13 included studies measured identification of female patients experiencing IPV in ways that could be combined (Rhodes 2002; Carroll 2005; Rhodes 2006; Trautman 2007; Ahmad 2009; MacMillan 2009; Humphreys 2011; Fraga 2014). In most studies, the proportion of women identified was small, and ranged from 3% to 17%. With the exception of Fraga 2014 (where 9% (n = 86) of women were lost to follow‐up between recruitment/randomisation and delivery of the intervention a year later), we used the number of women randomised as the denominator to establish identification rates, rather than the number of women who received the intervention or the number with abuse at baseline. We also made conservative assumptions about identification in four cases. In Ahmad 2009, we have taken cases as detected, rather than the broader 'discussion opportunity' as the measure of identification. In Trautman 2007, we were not able to distinguish identification by healthcare professionals from those detected by research staff in the study report, and therefore have only included the numbers of cases documented in patient records, as these were entered by healthcare professionals only. While MacMillan 2009 did not specify identification as an outcome, we were able to estimate figures using the reported proportions of women who discussed IPV with their clinicians, based on self report following clinical encounters (88/199 screened women (44%) compared to 17/212 (8%) of non‐screened women). To allow the data to be comparable to other studies in the meta‐analysis, we expressed the cases of women that discussed abuse as a proportion of all women randomised to the screened or non‐screened groups. However, it is important to point out that these denominators included women with negative and mixed results on the Women's Abuse Screening Tool and we do not know how many of these women had discussions about abuse with clinicians. In Rhodes 2002, we have only included those detected by chart review. We confirmed our calculations of the women included in the study with the author.
On average, screening interventions more than doubled the identification of women experiencing abuse compared with control groups (odds ratio (OR) 2.95, 95% confidence interval (CI) 1.79 to 4.87, eight studies, n (number of women) = 10,074, I² = 66%; Analysis 1.1). We downgraded the quality of the evidence to moderate due to heterogeneity. This moderate level of (methodological) heterogeneity owed to the MacMillan 2009 study being very large relative to other studies in the analysis. Removing it in sensitivity analysis, the OR was 2.35 (95% CI 1.53 to 3.59, seven studies, n = 4393, I² = 38%; analysis not shown).
1.1. Analysis.
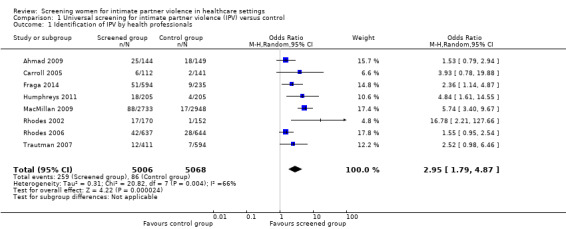
Comparison 1 Universal screening for intimate partner violence (IPV) versus control, Outcome 1 Identification of IPV by health professionals.
Rates of missing data were low for the identification outcome among studies included in the meta‐analysis, with nearly all randomised women being included in the analysis (see Characteristics of included studies). There was one exception where just 66% of women in the intervention group and 70% of women in the control group were successfully audiotaped (Rhodes 2006). Thus, there may have been participation bias with women who found the recording uncomfortable declining it. Removing this study in a sensitivity analysis slightly increased the odds of identifying abused women through screening (OR 2.74, 95% CI 1.65 to 4.53, six studies, n = 3112, I² = 33%; analysis not shown).
Subgroup analyses: type of healthcare setting
We excluded one of the eight studies that reported clinical identification data from the analysis by setting ‐ MacMillan 2009 included multiple healthcare settings and we did not have access to the disaggregated data.
Antenatal clinics
Two studies tested screening in antenatal settings (Carroll 2005; Humphreys 2011). The OR for screening to identify victims of abuse compared to no screening was 4.53 (95% CI 1.82 to 11.27, two studies, n = 663, I² = 0%; Analysis 1.2). In this setting, we estimated that there could be over 300% likelihood of increased identification by healthcare professionals in screened pregnant populations. However, the studies were small and therefore more subject to sampling variation. We downgraded the quality of the evidence to moderate on account of imprecision.
1.2. Analysis.
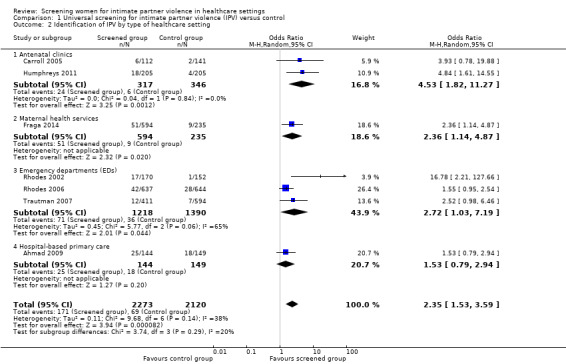
Comparison 1 Universal screening for intimate partner violence (IPV) versus control, Outcome 2 Identification of IPV by type of healthcare setting.
Maternal health services
In one study, based in an obstetrics department with women who were one year postpartum (Fraga 2014), the OR for screening to identify victims of abuse compared to no screening was 2.36 (95% CI 1.14 to 4.87, n = 829; Analysis 1.2). We downgraded the quality of this evidence to moderate on the basis of risk of bias (see 'Characteristics of included studies').
Emergency departments (EDs)
Three studies evaluated identification from screening in emergency department (ED) settings (Rhodes 2002; Rhodes 2006; Trautman 2007). In this setting, the OR was 2.72 (95% CI 1.03 to 7.19, three studies, n = 2608, I² = 65%; Analysis 1.2). We downgraded the quality of the evidence to moderate due to statistical heterogeneity (I² > 50%). With regards to the source of this heterogeneity, there was clinical diversity in Rhodes 2002 as the intervention had also targeted men and a notably high proportion (> 90%) of participants were African‐American. Further, it was a relatively small study, highlighting its methodological diversity. In a sensitivity analysis, we removed Rhodes 2002, which reduced the odds that screening identifies women in this setting, but improved the precision of effect estimates and reduced heterogeneity (OR 1.72, 95% CI 1.11 to 2.66, two studies, n = 2286, I² = 0%; analysis not shown).
Hospital‐based primary care
One moderate quality study evaluated identification from screening in primary care (Ahmad 2009). In this setting, screening did not increase identification (OR 1.53, 95% CI 0.79 to 2.94, n = 293; Analysis 1.2).
Subgroup differences
Results were fairly consistent across location subgroups, suggesting that screening was similarly effective in all of the healthcare settings studied (Chi² = 3.74, df = 3 (P value = 0.29), I² = 19.7%; Analysis 1.2).
Individual studies not included in the meta‐analysis
We excluded five studies from the primary meta‐analysis of identification of exposure to IPV. Koziol‐McLain 2010 did not assess it. Kataoka 2010 reported prevalence (as opposed to data on the clinical encounter) for written (29%, 48/163) versus face‐to‐face (19%, 32/165) enquiry. We excluded Fincher 2015 from the meta‐analysis for similar reasons. However, in contrast with Kataoka 2010, they found that face‐to‐face screening in women one year postpartum increased disclosure of prior‐year IPV (44%, 84/191 versus 28%, 50/177) and lifetime exposure (54%, 103/191 versus 44%, 76/177) compared to computer‐assisted screening. MacMillan 2006 also used this design, although their study contained many more possible interactions as they used different tools, settings, and methods. They reported 12‐month prevalence rates that ranged from 4% to 18% across primary care, emergency departments, and women's clinics. The highest proportions were identified in emergency settings (n = 768), ranging from 10.9% of women when the Partner Violence Screen (PVS) was used in face‐to‐face interviews up to 17.7% for the computerised version of the PVS. In primary care (n = 814), proportions ranged from 5.4% on the paper‐based Women's Abuse Screen Tool (WAST) to 11.6% on the face‐to‐face PVS. Women's health clinics (n = 879) reported the lowest prevalence, from 4.1% (face‐to‐face PVS) to 10.0% (face‐to‐face WAST). In Klevens 2012a, disclosure to healthcare professionals (8.7%, 4/46) was compared to CASI (21.3%, 17/80). Although three women (of 80) in the CASI group later discussed abuse with the healthcare professional, we excluded it as identification was not consistently measured as clinical data across groups.
Given that there were four studies that investigated the identification of abused women using a non‐clinically based approach (more consistent with investigating prevalence rates), we compared face‐to‐face enquiry with computer‐based (Klevens 2012a; Fincher 2015), or written assessment of IPV (Kataoka 2010), or both (MacMillan 2006). For Kataoka 2010, we used data from the first screening only (it was followed up by two additional screening interventions). For MacMillan 2006, we used the data reported on the PVS only (the computer and paper‐based groups completed both the PVS and WAST with the face‐to‐face consisting of one or the other) as it was more conservative estimate than the data derived from the WAST. We combined the two groups of women that had computer‐ or paper‐based screening and compared them to the women who had the face‐to‐face screening on the PVS. Neither face‐to‐face screening nor written/computer‐based techniques were favoured for identifying abused women (OR 1.12, 95% CI 0.53 to 2.36, four studies, n = 2765, I² = 83%; Analysis 2.1). We downgraded this evidence to moderate quality due to statistical heterogeneity. One study favoured face‐to‐face screening (Fincher 2015). The other three suggested no difference between face‐to‐face and written/computer techniques for identifying women (MacMillan 2006; Kataoka 2010; Klevens 2012a). The risk of bias was greatest for the Fincher 2015 study. In removing this study in a sensitivity analysis, heterogeneity remained high and one technique was not favoured above the other (OR 0.88, 95% CI 0.36 to 2.15, three studies, n = 2397, I² = 73%; analysis not shown). The heterogeneity in this analysis was likely due to clinical diversity across studies (different countries, healthcare settings, and participant characteristics) and methodological differences (large variation in sample sizes and study quality).
2.1. Analysis.
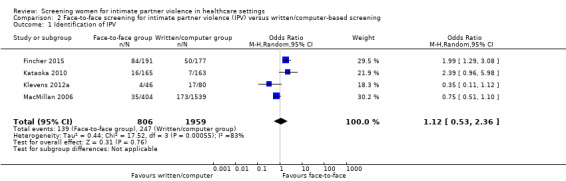
Comparison 2 Face‐to‐face screening for intimate partner violence (IPV) versus written/computer‐based screening, Outcome 1 Identification of IPV.
B. Information‐giving and referrals to support agencies by healthcare professionals (including uptake rates)
We were able to include two studies in our investigation of healthcare professional referrals (Trautman 2007; Ahmad 2009). In the case of Trautman 2007, a narrow definition of referral was adopted (social work assistance only), suggesting that referral to other support services was not counted. Furthermore, we only included cases verified by medical records, which may be an underestimate of the number of women who were referred by healthcare professionals without verification in charts. Ahmad 2009 audio‐recorded all consultations for women in both arms of the trial. There was no evidence of an effect of screening interventions on increasing referrals to supportive services (OR 2.24, 95% CI 0.64 to 7.86, I² = 0%, n = 1298; Analysis 1.3). We downgraded the evidence to low quality due to imprecision and risk of bias issues, particularly in the Trautman 2007 study.
1.3. Analysis.
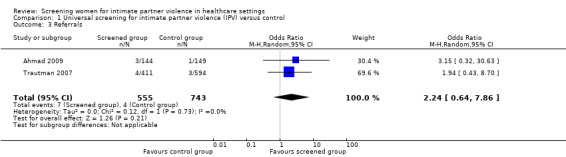
Comparison 1 Universal screening for intimate partner violence (IPV) versus control, Outcome 3 Referrals.
Data on information‐giving and uptake of services were too heterogeneous to be pooled in a meta‐analysis. We treated healthcare professionals discussing safety with abused women as a form of information‐giving. Only Ahmad 2009 reported on the extent to which physicians assessed patient safety following identification, and confirmed that physicians discussed safety with nine of the 25 women detected in the screened group and with only one woman in the control group. Ahmad 2009 reported that, of the 25 women detected, 20 (80%) were asked for follow‐up appointments in the intervention arm, whereas only eight (67%) of the 12 women identified in the comparison arm were invited for follow‐up appointments. Klevens 2012a found that, after one week, 4/36 (11%) who were screened by a healthcare professional had taken up services from the printout provided to women who screened positive compared to 2/66 (3%) of women in the computer‐based screening groups. No participant had contacted the domestic violence advocacy programme in the hospital, but the study was unable to investigate the reasons. Rhodes 2006 assessed IPV‐related services provided during the visit (which combined safety assessment, counselling by the healthcare professional provider or social worker, and referrals to domestic violence resources) to women in the screened group compared to usual care group, in separate groups of urban and suburban women. Of screened women, 25/421 (5.9%) received services compared to 10/443 (2.3%) of unscreened women. Trautman 2007 found that 18/411 (4.4%) of screened women received social work assistance for IPV compared to 2/194 (1%) in the comparison arm.
Secondary outcomes
C. Intimate partner violence
Two studies measured the effect of screening on reduction of IPV among screened compared to non‐screened women and used the same measure (Composite Abuse Scale; CAS) (MacMillan 2009; Koziol‐McLain 2010). However, the denominators and timelines were different: Koziol‐McLain 2010 measured IPV reduction at three months post‐baseline among all women, while MacMillan 2009 measured reduction of IPV among abused women at six, 12, and 18 months following screening. Therefore, we have presented their results separately. Both studies reported point estimates for ORs that were consistent with a decrease in IPV as a result of screening, however, results did not reach statistical significance. At 18 months, MacMillan 2009 reported an OR of 0.88 (CI 0.43 to 1.82, n = 707 (multiple imputation used to account for women lost to follow‐up (LTFU))). At three months, Koziol‐McLain 2010 found an adjusted OR of 0.86 (95% CI 0.39 to 1.92, n = 344).
Koziol‐McLain 2010 also assessed whether women reported using more safety behaviours in the screened versus control group and found an OR of 1.41 (CI 0.71 to 2.81), suggesting no significant difference between groups.
D. Women's perceived and diagnosed physical health outcomes
Only one study measured physical health (SF‐12) after screening (MacMillan 2009). At 18 months, self reported physical health had increased in the screened group, but it was not significant, with a mean difference (MD) of 1.57 (95% CI ‐0.59 to 3.73, n = 707).
E. Women's psychosocial health
MacMillan 2009 is the only study to have measured our other secondary outcomes in the important area of psychosocial health. While the study measured most of the factors of interest (depression and mental health in general, post‐traumatic stress disorder (PTSD), alcohol problems, drug problems, and quality of life) at each time point, we only report those at 18 months, which was the study's final measurement point. We cite the imputed figures and more conservative figures. The study suffered considerable attrition (42%). While the complete case (n = 411) effect sizes are greater than those imputed (n = 707) in the published paper, the imputation method (requested from the author (MacMillan 2011 [pers comm])) assumed missing‐at‐random, however those LTFU had higher scores on the CAS, which suggests a potential underestimate of effect.
Depression ‐ the observed figures found a MD of ‐2.32 (95% CI ‐4.61 to ‐0.03) among screened versus unscreened abused women, consistent with a decrease in depression as a result of screening. However, this reduced to ‐1.97 (95% CI ‐4.33 to 0.39) with imputation for LTFU and was no longer statistically significant.
PTSD ‐ the data suggested no difference between screened and non‐screened women for PTSD (OR 0.63, 95% CI 0.36 to 1.10).
Mental health in general (as assessed by the SF‐12) ‐ screening did not significantly improve the mental health of screened abused women as the mean improvement of 1.05 in SF‐12 scores (95% CI ‐1.70 to 3.79) crossed the line of no significance in both observed and imputed analyses.
Quality of life (as assessed by the WHO Quality of Life‐Bref) ‐ screened women showed more rapid improvement in quality of life (3.74 points higher; 95% CI 0.47 to 7.00), however the imputed data suggested that there was no difference between screened and non‐screened women (MD 2.29, 95% CI ‐1.71 to 6.28).
Alcohol problems ‐ the data suggested no difference between screened and non‐screened women as regards risk of alcohol problems (OR 1.23, 95% CI 0.62 to 2.44).
Drug problems ‐ the data suggested no difference between screened and non‐screened women as regards risk of drug problems (OR 0.83, 95% CI 0.41 to 1.71).
F. Occurrence of adverse outcomes
Included studies measured women's preferences for screening method (MacMillan 2006), acceptability (Ahmad 2009; Koziol‐McLain 2010), comfort levels (Kataoka 2010), positive and negative reactions (Klevens 2012a), overall satisfaction (Rhodes 2006), and harm from screening (MacMillan 2009). Negative reactions to screening at one week were reported as negligible in Klevens 2012a. At three months, Koziol‐McLain 2010, situated in an emergency setting, reported that 82% of women agreed that "health care providers should routinely ask all women about difficulties in home life and relationships". They found no adverse effects in participants, clinicians, or researchers. Rhodes 2006 found that enquiry about and disclosures of IPV were associated with higher patient satisfaction with care. Fincher 2015, in seeking to understand contextual issues that enable disclosure, reported that race‐matching of women and interviewers had no impact on disclosure rates of IPV. In the Ahmad 2009 hospital‐based primary care study, acceptance of computer‐assisted screening was measured using the Computerized Assessment Lifestyle Scale (CLAS) (Ahmad 2008). It examines patient perceptions of screening for a number of health and lifestyle issues and the quality of the subsequent medical consultation. Although women had some concerns about privacy and interruptions to their interaction with the healthcare professional, on average, women agreed that screening was beneficial (mean CLAS score 3.8, standard deviation (SD) 0.67). Scores were not influenced by IPV status.
The most rigorous assessment of harm from IPV screening was undertaken by MacMillan 2009. Across various health settings, they used a specifically developed tool ‐ the Consequences of Screening Tool (COST) (MacMillan 2009) ‐ to assess the effects of being asked IPV screening questions. Among the COST questions, they analysed the eight‐item Effects on Quality of Life subscale as it applies to women who received the screening intervention regardless of their abuse status. Items are scaled from two to minus two (range 16 to ‐16), with negative scores reflecting harm. The COST was administered to a subset of 591 women interviewed at baseline only (within 14 days of the index visit), comprising 227 women who screened positive for abuse, 206 with mixed screen results, and 158 who screened negative. The mean score of 3.52 (SD 3.24) on the eight‐item Effects on Quality of Life subscale supported the view that being asked IPV screening questions was not harmful to women, in the short term at least. There was no variation by abuse group; the mean scores were 3.7 (SD 3.2) for women who scored negative on both the WAST and CAS, 3.3 (SD 3.3) for those who had mixed results, and 3.5 (SD 3.4) for those who scored positive on both measures (data obtained from the authors). No study examined harm or adverse outcomes beyond three months, with the majority of studies measuring these outcomes on the day that screening took place, and up to two weeks later.
G. Services and resource use
There was overlap between this set of outcomes and women's take‐up of services as presented in B above. In B, we described service use/uptake that was linked to the healthcare visit (e.g. services to which they were referred or were prompted to access from information provided), whereas here we present women's use of general services. Koziol‐McLain 2010 found no differences in resource use based on the Community Resource Checklist after three months. MacMillan 2009 presented women's self reported use of violence‐related services ("for descriptive purposes only") using a six‐month time frame at baseline, six‐, 12‐, and 18‐months. Rates for service use by screened versus non‐screened women were not significantly different: 75% versus 71% at baseline and 65% versus 64% at 18 months.
H. Cost‐benefit outcomes
We found no studies that reported any data on cost‐benefit or any other economic evaluation of interventions.
Discussion
Summary of main results
We identified 13 controlled studies of screening for intimate partner violence (IPV) in healthcare settings. These recruited 14,959 women. Studies were conducted in diverse healthcare settings (antenatal and women's health clinics, emergency departments (ED), primary care centres) in predominantly urban settings, in high‐income countries. These were countries with domestic violence legislation and developed support services to which healthcare professionals could refer. Follow‐up periods also varied, from immediately to one month post‐intervention for identification outcomes, and up to 18 months post‐intervention for violence and health outcomes. A range of different screening tools and techniques were applied but the review inclusion criteria stipulated inclusion of interventions that involved screening by, or notification of positive results to, healthcare professionals. Five studies involved computer‐based screening with positive results conveyed to healthcare professionals. One study used paper‐based screening before notifying treating physicians. Seven involved face‐to‐face or telephone screening by the healthcare professional. Of the 13 studies, eight measured clinical identification in both the intervention and comparison arm and four studies compared screening techniques based on identification rates that were not embedded in the clinical context. In these studies, women's data were managed by researchers only, or the clinical encounter/records were not accessible in the two groups (or both), and therefore we dealt with these studies separately. Only one study discussed the implications of non‐disclosure or false measurement on the outcomes.
Screening in healthcare settings is a complex intervention in a complex context, and an optimal evaluation requires multi‐methods to illuminate the reasons for any successes or failures (Spangaro 2009; May 2011; Catallo 2013a; Catallo 2013b). Globally, the barriers to screening by healthcare professionals may reside at the individual professional level (lack of training and resources, fear of inadequate skills to address the problem, lack of time, unfavourable attitudes to the problem), at the clinic or team level (lack of systems for safety, supervision, and links with referral agencies), or at the wider political level (violence‐tolerant societies, other healthcare priorities for funding, and services such as lack of funding for law enforcement or domestic violence services) (Colombini 2008; García‐Moreno 2014). This understanding of an intervention was not adequately acknowledged in the included studies and is often overlooked in trial reporting. There was variability in the description provided about the wider organisational contexts and how healthcare professionals were trained and supported to undertake screening. Very few conducted or reported process evaluations. Similarly, the sustainability of healthcare professional screening behaviours in the future (Taft 2015), and after screening studies are complete, have been rarely addressed since earlier literature (McLeer 1989).
In surveys, qualitative studies and the studies reported here, women report that screening for intimate partner violence (IPV) is acceptable (Koziol‐McLain 2008), although this can vary according to their abuse status (Feder 2009). While some governments and healthcare policymakers are in favour, the majority of healthcare professionals are not as supportive of screening policies, and many barriers to screening have been identified (Hegarty 2006; Feder 2009).
Does screening for intimate partner violence increase identification of victims?
Based on the studies in this review, we found moderate evidence that screening in high‐income countries with developed referral services increases identification of women exposed to IPV compared to usual care. However, the numbers and proportions of women identified are modest when considered against the estimated prevalence of IPV among women in healthcare settings. We are mindful that many women will be not be ready to disclose (Chang 2010; Reisenhofer 2013), nor perhaps willing to disclose to that specific provider or in that setting (Catallo 2013a). The odds of identifying victims/survivors of IPV in antenatal settings were four times higher in screened women compared to those who received usual care. However, we downgraded the quality of this evidence to moderate on account of imprecision, reflected in wide confidence intervals around intervention effect estimates (likely due to the small sample sizes of these studies). Clinical identification was also increased in maternal health services and emergency departments but not in hospital‐based primary care. Further rigorous studies are needed to test these findings in different settings. A gap in the identified studies is that only one report (Wathen 2008), associated with the MacMillan 2006 study, directly addressed the issue of how false positives and false negatives are managed and their impact on women and on screening effectiveness.
What kind of screening technique is preferred in the identification of abused women?
Previous studies have suggested that women have a preference for screening methods that do not involve healthcare professionals, which is understandable given the sensitive nature of IPV and women's preferences for privacy to disclose (MacMillan 2006; Catallo 2013a; Catallo 2013b). A recent Australian trial found, through process and outcome evidence, that both women (and nurses) preferred a self completion maternal health checklist that included IPV screening questions (Hooker 2015; Taft 2015). Although preference for screening technique was not a central question in this review, our evaluation of adverse outcomes across studies suggested that, on the whole, the women included in this review were strongly in favour of being asked about violence in healthcare settings, regardless of the technique used.
An alternative question concerns which techniques and methods (as distinct from which tools) produce more accurate prevalence rates. While this was not an a priori review question, a subset of the included studies did address it. Four studies compared screening techniques based on prevalence rates (or identification rates that were not embedded clinically). Findings suggested that neither health professional/face‐to‐face screening nor written/computer‐based screening is favoured for identifying abused women. High levels of statistical heterogeneity were observed in this four‐study analysis, suggesting clinical diversity across studies (different countries, healthcare settings, and participant characteristics such as education, preferences for privacy, and age) and methodological differences (large variation in sample sizes and study quality). These factors have the potential to moderate the effect of different techniques on disclosure; indeed MacMillan 2006 has highlighted the extent of the variability in prevalence rates depending on settings, instruments, and techniques.
The clinical identification rates in this review ranged from 3% to 17% with a median of just 8%. It would appear that women and/or the providers remained reluctant to raise IPV. For example, there was a mismatch between disclosures via computer/written pre‐assessments and discussions about IPV in consultations afterwards across the studies using this approach. In Rhodes 2002, 58/170 (34%) women indicated exposure to abuse in the pre‐consultation computer self assessment yet just 19/170 (11%) of those cases were documented in patients' charts by the providers. In Trautman 2007, 68/411 women (17%) were detected in the computer pre‐screen; just 12 (3%) women had IPV documented in their charts. We acknowledge that chart documentation may underestimate clinical identification and discussion of abuse. However, using women's self reports, MacMillan 2009 found that, in encounters where physicians had been prompted that abuse was present, under half involved a discussion about violence between the woman and her doctor. This was consistent with Rhodes 2006, where just 48% of health provider prompts that abuse had been reported led to a discussion about IPV. There was more consistency between the disclosure rate in pre‐screening and with the healthcare professional in Ahmad 2009 and Humphreys 2011. In Ahmad 2009, prevalence was reported in exit surveys as 20% (29/144) among screened women with 17% (25/144) having had a discussion during the consultation. In Humphreys 2011, 25/205 (12%) were identified as at‐risk in computer pre‐screening, with 18/205 (9%) indicating in an interview afterwards that they had talked about domestic violence with their doctor. Thus, future studies need to look at how interventions can be enhanced to increase the rate of discussion about IPV (e.g. greater emphasis on training health care professionals).
The relative success of computerised and other distal techniques for eliciting disclosures from women has led to studies that bypass healthcare professionals altogether and instead assess a participant's risk by computer and then provide support and links to services, via a printout for instance. However, when looking beyond the rate of disclosure, these methods appear to have little impact. For example, Klevens 2012b found no evidence of effect of computer‐only screening and a list of resources on women's mental and physical health status at 12 months. Thus, while provider and patient preferences for screening techniques must be understood as yet another potential barrier (or facilitator) to implementation of screening interventions, it remains important to examine pragmatic screening interventions that will offer abused women the best chance of finding a pathway to increased safety and better health.
Does screening increase referral to support services?
Based on the studies that assessed formal referral following clinically‐based identification, screening did not increase referral to support services compared to usual care. However, to date, we only found and included two studies (one from primary care and one from emergency departments) and the assessment of referral was unreliable, for example, referral rates may have been underestimated in Trautman 2007 as they only included referrals to social work. Thus, we judged the evidence on the effect of screening on referrals as low quality and further research is likely to have an important impact on our confidence in the effect estimate. In fact, in the Ahmad 2009 study, where only three women were reported as having received referrals, 20 women were asked to make follow‐up appointments with same provider. In the comparison group, follow‐up appointments were made with eight women. It supports the notion that referring women, particularly in certain settings like primary care, may not be the optimal response as abused women may not yet be ready to take up a referral at the time of immediate disclosure (Chang 2010; Reisenhofer 2013). Alternative provider practices, such as safety‐planning and arranging for follow‐up, may be more appropriate, with measurement of safety behaviours and take‐up of subsequent appointments in follow‐up (Wathen 2012; Taft 2015). An important distinction needs to be made between provider behaviours that occur as part of the consultation (i.e. 'process' variables of referring, safety‐planning, providing emotional support, making follow‐up appointments), and women's later uptake of the specific referrals and follow‐up appointments along with their more general service use. Poor definition of these various processes and outcomes was a key obstacle to the synthesis of evidence in the current review.
Does screening reduce intimate partner violence?
The only two studies that measured the impact of screening on a reduction of partner violence over time did not report an effect. The studies used different time frames for the outcome. More studies would be required to reach a conclusion on the impact of screening on recurrence of violence. Also, further work is necessary to evaluate the effectiveness of screening linked with a range of interventions, advocacy (Ramsay 2009), social support (Taft 2011), and healthcare professional interventions (Hegarty 2013), for impacting on IPV recurrence.
Is screening beneficial for women's health?
One study assessed mental and physical health outcomes and reported no impact of screening at 18 months (MacMillan 2009). Given that there was only one study, we are unable to conclude if screening interventions lead to improvements in women's psychosocial health. Future studies need to incorporate a broader range of health outcomes (including general health and pregnancy outcomes) as part of the evaluation of screening interventions in healthcare settings.
Does screening harm women?
One of the criticisms commonly raised against the implementation of screening is that we do not know whether or not it is harmful (Jewkes 2002). Most studies in this review incorporated a non‐validated set of questions related to women's experiences of participating in a screening programme, with none reporting adverse effects. MacMillan 2009 conducted the most comprehensive assessment of harm from screening and found no evidence of harm. However, it was undertaken immediately after the health visit only. Three months was the longest follow‐up of possible adverse outcomes (Koziol‐McLain 2010), with no evidence of harmful effects in the 86% of women interviewed from both arms of the trial. Two recent Australian primary care trials, which used the same tool as in the MacMillan 2009 trial (Valpied 2014; Taft 2015), also found no evidence of harms over a more extended period of follow‐up. Comprehensive assessment of harm needs to be incorporated into future trials, with greater focus on the weeks and months following delivery of the screening intervention. It needs to be borne in mind that the studies in this review have been undertaken in high‐income countries, which may offer women more legal and social protections in the event that a woman chooses to disclose. Screening interventions may pose a more substantial risk to women's safety and wellbeing in other settings, such as those that are resource‐poor and lack comprehensive training for healthcare professionals, and in environments characterised by higher levels of gender inequality.
Overall completeness and applicability of evidence
The studies in this review are from high‐income countries and the conclusions cannot be generalised to medium‐ and low‐income settings where the care context and culture may be very different. For example, support services for healthcare professional referrals may be absent or underdeveloped, and the problem of violence in women's lives may be much less visible where legal rights for women and criminal sanctions against perpetrators are lacking (Garcia‐Moreno 2006). In such settings, without appropriate safeguards, screening may confer significant harm on women.
There is a need for studies that can investigate the differential impact of screening on women experiencing different severity or types of abuse. Also, the evidence for the effectiveness of screening in specific healthcare settings is scant and more studies are required to confirm whether there is a differential effect (e.g. the finding in antenatal care, which was compatible with an increase in identification following screening). Having incorporated a review on 'Domestic violence screening and intervention programmes for adults with dental or facial injury' (Coulthard 2010), we identified no studies in oral and maxillofacial injury settings, an area that warrants attention in future studies.
Given the costs to healthcare systems to provide support for sustainable and effective screening programmes, it would be helpful to have studies that compared screening to case‐finding strategies (such as Feder 2011), including economic analyses and longer‐term outcomes. Nevertheless, there are sufficient studies to suggest that screening is effective in raising identification rates. It must be acknowledged that the actual number of eligible women in any healthcare setting who are screened has been found to be well below 50% (Stayton 2005) (although the number of eligible women screened across the trials included in this review ranged from 41% to 94% with a median of 69%). The proportions of women asked, those choosing not to disclose, and the impact of false identification on women's lives need further investigation before we can fully understand the effectiveness of screening.
To date, the evidence reviewed here cannot demonstrate that screening involving clinical assessment and referral alone reduces violence, improves health, and does not cause harm. However, we acknowledge that reported outcomes were in the desired direction (less violence, less depression, more referrals), suggesting that linking screening interventions with support, advocacy, or psychological therapies may achieve positive outcomes with significant public health implications. We need larger studies to investigate these outcomes. While the increased rate of identification from screening is encouraging, it is unclear whether the healthcare professionals would continue to screen if they were not part of a study and for how long. The question of sustainability of screening for IPV as in other healthcare behaviour change interventions is a vexed one and calls for greater understanding if we hope to implement such programmes effectively at a state or national level (Colombini 2008; May 2011). A study aimed at improving maternal and child health care for vulnerable mothers provides some evidence that a nurse‐designed, systems approach to screening was sustained with the outcome of safety planning increasing at two years follow‐up (Taft 2015).
Quality of the evidence
Overall in this review, studies performed random sequence generation effectively. Allocation concealment was more open to bias. Steps taken to conceal the sequence prior to assignment of interventions was generally poorly described, and the risk of selection bias could have been reduced by adopting CONSORT guidance. A further difficulty was identified. Ideally all provider‐level (e.g. training interventions) and patient interventions are delivered after baseline assessment and randomisation has occurred. However, with a screening intervention, it is unlikely to be feasible to assign patients and providers simultaneously as the patient‐level intervention needs to be delivered immediately so training needs to have already occurred. Achieving full allocation concealment is made difficult where there is a two‐stage allocation process as knowledge about provider training activities among personnel responsible for recruiting patient participants could influence the enrolment process.
In regards to post‐assignment, it is widely accepted that blinding of staff and participants to minimise performance bias is hard to achieve with complex interventions. This was the greatest threat to validity across studies. Screening women for a range of health issues or withholding full information about the trial aims until a debriefing afterwards, or both, could help to reduce performance bias among patient participants (e.g. Ahmad 2009). However, the challenge of non‐blinding providers remains, which may lead to an overestimate of effect (e.g. due to inappropriate administration of another 'co‐intervention' and other differential behaviours) or underestimate of effect (e.g. due to contamination bias in comparison arms). Cluster trials were uncommon in the studies, however this design may offer some solution to issues of allocation concealment and performance bias. Blinding of outcome assessment was very complex, given that for our primary outcomes ‐ identification and referral ‐ we mainly used clinical documentation and self report. Thus, we may have underestimated the levels of these outcomes. Selecting a reliable measure of identification of IPV is a persistent challenge in screening trials and warrants much planning. In regards to selective reporting, around half of trials had been registered, but protocols were uncommon and there was widespread indication that not all outcomes listed a priori were addressed in trial reports.
We made 91 judgements about the quality of evidence using seven domains across 13 studies. We considered less than one‐third of domains at low risk of bias, whereas we judged 40% to be at high risk and the remainder to be at unclear risk. Therefore, most information is from studies at high or unclear risk of bias. We downgraded evidence quality in response to risk of bias in studies and imprecision arising mainly from small studies/sample sizes. We observed high levels of statistical heterogeneity in some analyses (though not in the main identification analysis). Although interventions were similar, clinical diversity across studies arose from factors such as studies being set in different countries and healthcare settings and variability in participant characteristics. It is likely that the large variation in sample sizes and study quality contributed to methodological heterogeneity. Where possible, we used sensitivity analyses to assess the robustness of the findings. We considered the evidence on identification to be of moderate quality suggesting further research is likely to have an important impact on our confidence in the estimate of effect (and may change it). While we detected no evidence of an effect on referrals, this evidence was of low quality; further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change it.
Undertaking trials of complex interventions in a sensitive area is challenging not least because there is a constant need to balance ethical concerns against methodological and practical issues. For example, ensuring the safety of the comparison arm demands some basic training for providers, however, this may lead to an underestimate of a true effect. Future studies need to incorporate guidance, such as that supplied by CONSORT, in designing, implementing, and evaluating their trials. Understanding the context of a complex intervention, such as screening, requires better theoretical underpinning. It also requires detailing (in process evaluation (Moore 2015) and protocols) of the steps leading to the establishment, implementation, and evaluation of a screening programme, so that those wishing to replicate or scale‐up a given intervention have adequate information.
Potential biases in the review process
We believe that our review process allowed us to identify all published randomised or quasi‐randomised controlled trials of screening interventions, as defined in the review and published up to the most recent search date (February 2015). All of the authors included in the review and other experts in the field responded to our requests for knowledge of other trials, which we may have missed, but they did not identify any further trials that met our inclusion criteria. We scoured all trial databases for those that may be about to be published. At least two review authors made decisions about inclusion or exclusion of studies and we made any changes to the protocol with all authors' involvement. Two review authors also independently assessed study quality.
Agreements and disagreements with other studies or reviews
This review reinforces the findings of our original review, Taft 2013, and is consistent with other major systematic reviews (Wathen 2003; U.S. Preventive Services Task Force 2004; Feder 2009), and guidance from the UK National Institute for Health and Care Excellence (NICE 2014) and from the WHO (Feder 2013), which state that insufficient evidence exists to justify universal screening for IPV in healthcare settings on the basis of demonstrated benefit to women. We do not agree with the Nelson 2012 update on U.S. Preventive Services Task Force 2004, which concluded, mainly from MacMillan 2009, that screening is effective; the evidence does not yet warrant this conclusion. The earlier reviews of screening for IPV found no evidence of either direct harm or benefit to women, despite evidence that it may increase identification and referral. By conducting more recent searches and combining the results of those few studies where feasible, this review has confirmed the modest effects of screening on increasing identification of IPV, though there remains limited evidence of a positive impact of screening on referral by healthcare professionals, on other key outcomes related to women's health and wellbeing, and on any possible harm to women from the screening process.
Authors' conclusions
Implications for practice.
In this update of our review, our conclusions remain that there is insufficient evidence to justify implementation of intimate partner violence (IPV) screening for all women in healthcare settings. It would be equally or more effective to train healthcare professionals in effective case‐finding for IPV as part of the routine social history, to ask women who show signs of abuse or those in high‐risk groups, and provide them with a supportive response, safety‐planning, and information. This review cannot reach any conclusions about the benefits of screening combined with advocacy or other interventions by healthcare professionals. Further trials are required to test these hypotheses.
Implications for research.
Further research is required to extend the limited evidence identified in this review. More studies that examine the complexities of screening in diverse settings (including low‐ or middle‐income countries), with diverse populations, and that examine the social, health, and economic benefits for the differing strategies of identifying women are needed. We need further pragmatic trials of what proportion of women are successfully screened in real‐world settings and over what period can they be sustained as well as systems‐levels interventions to address the manifold barriers that exist to enquiry about IPV by healthcare professionals and disclosure by victims. The question of which subgroups of women, at which stage of their journeys, may benefit from screening programmes also remains.
In addition to emphasising trial registration, publication of protocols, and parallel process evaluation studies, we make a number of recommendations for future studies. We recommend trials compare:
screening all women in particular health settings or from high‐risk groups (e.g. mental health services, antenatal clinics) versus a comparison intervention that also includes basic training for all healthcare providers in asking about and responding to IPV (it would be unethical to conduct such a trial using a usual care arm where the health practitioners have not received basic education/training) (the extent and nature of the training should be clearly stated or available online);
screening plus intensive support intervention in any healthcare setting versus comparison (as described above);
case‐finding plus intensive support intervention versus comparison (as described above);
screening plus intensive support intervention in any healthcare setting versus case‐finding plus intensive support intervention; and
the above applied in low‐ and middle‐resource settings.
Outcome selection and measurement recommendations include:
improving the clarity around definition, operationalisation, and data collection methods for clinical identification and formal referral (short‐term, 'process' outcomes);
explicit timelines to improve the comparability of data across studies (e.g. three months, six months, one year, two years);
measurement of take‐up of referrals and follow‐up appointments (specific) and health service use (general) (short‐ to medium‐term);
assessment of violence and health and wellbeing outcomes (medium‐ to long‐term);
outcomes for children;
economic evaluation; and
systematic harm assessment.
Although the number of eligible women randomised across studies was acceptable and there was little dropout prior to the delivery of interventions, studies that featured follow‐up beyond the day of screening were affected by the loss of more vulnerable women. Given our recommendation for assessing important outcomes such as violence, women's health, and quality of life over the long term, studies will need to develop recruitment and follow‐up protocols that maximise the retention of disadvantaged women as part of further testing of identification programmes in conjunction with other interventions for IPV.
What's new
| Date | Event | Description |
|---|---|---|
| 12 August 2015 | Amended | Information added to sources of support |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 4, 2013
| Date | Event | Description |
|---|---|---|
| 7 May 2015 | New citation required but conclusions have not changed | Two new studies included in the review. |
| 17 February 2015 | New search has been performed | The review was updated following a new search in February 2015. |
| 16 September 2008 | Amended | Converted to new review format. |
Acknowledgements
The review was produced within the Cochrane Developmental, Psychosocial and Learning Problems Group.
The authors acknowledge, with thanks, the support of the Australasian Cochrane Centre for training and support; the Cochrane Developmental, Psychosocial and Learning Problems Group (CDPLPG) for their support and assistance with searches; and La Trobe University; the Cochrane Collaboration; UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP); Department of Reproductive Health and Research (RHR); and the World Health Organization, Switzerland for funding support.
We would like to particularly thank Liesje Toomey who assisted in the original search strategy, and Jo Abbott, Trial Search Co‐ordinator (TSC) of CDPLPG at that time. Jo ran the initial search and the current TSC, Margaret Anderson, updated the searches in 2015. We thank Joanne Wilson (CDPLPG) and Sonja Henderson (WHO) for their ongoing support and co‐ordination through the process of updating the review.
We would also like to acknowledge Evelina Chapman for her assistance on the screening of studies, and Tess Lawrie who assisted with screening studies, data extraction, 'Risk of bias' assessment, and advised on data analyses and interpretation. Their work for the 2015 update was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP); Department of Reproductive Health and Research (RHR); and the World Health Organization. The named authors alone are responsible for the views expressed in this publication.
Finally, we acknowledge the work of Paul Coulthard, Sin Leong Yong, Linda Adamson, Alison Warburton, Helen V Worthington, Marco Esposito and Mohammad O Sharif, the author team responsible for ‘Domestic violence screening and intervention programmes for adults with dental or facial injury’, a related Cochrane review that has now been merged with our review.
Appendices
Appendix 1. Search strategies used for 2015 update
Cochrane Central Register of Controlled Trials (CENTRAL)
CENTRAL 2015, Issue 1, searched 17 February 2015. Limited to publication year = 2012 to 2015 [41 records]
#1 MeSH descriptor: [Battered Women] explode all trees #2 MeSH descriptor: [Domestic Violence] this term only #3 MeSH descriptor: [Spouse Abuse] this term only #4 "domestic violence" #5 abuse* near/3 spous* #6 abuse* near/3 partner* #7 wife near/3 abuse* #8 wives near/3 abuse* #9 wife near/3 batter* #10 wives near/3 batter* #11 batter* near/3 wom*n #12 partner* near/3 violen* #13 spous* near/3 violen* #14 domestic next violence #15 gender near/3 violenc* #16 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 #17 MeSH descriptor: [Mass Screening] this term only #18 MeSH descriptor: [Medical History Taking] this term only #19 screen* #20 identif* #21 routine* near/3 ask* #22 routine* near/3 question* #23 (medical history) or (history near/1 tak*) #24 disclos* #25 detect* #26 #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 #27 MeSH descriptor: [Women] explode all trees #28 MeSH descriptor: [Female] explode all trees #29 MeSH descriptor: [Adolescent] explode all trees #30 wom*n or female* #31 adolescen* #32 teen* #33 #27 or #28 or #29 or #30 or #31 or #32 #34 #16 and #26 and #33 Publication Year from 2012 to 2015, in Trials
Ovid MEDLINE(R)
Ovid MEDLINE(R) 1946 to February Week 2 2015, searched 17 February 2015. Limited to ed=20120601 to 20150205 [777 records]
1 Battered Women/ 2 Domestic Violence/ 3 Spouse Abuse/ 4 (abuse$ adj3 wom#n).tw. 5 (abuse$ adj3 spous$).tw. 6 (abuse$ adj3 partner$).tw. 7 (abuse$ adj3 (wife or wives)).tw. 8 (batter$ adj3 (wife or wives)).tw. 9 (batter$ adj3 wom#n).tw. 10 domestic violence.tw. 11 (partner$ adj3 violen$).tw. 12 (spous$ adj3 violen$).tw. 13 (gender adj3 violen$).tw. 14 or/1‐13 15 Mass Screening/ 16 Medical History Taking/ 17 screen$.tw. 18 identif$.tw. 19 detect$.tw. 20 disclos$.tw. 21 (medical history or (history adj1 tak$)).tw. 22 (routine$ adj3 (ask$ or question$)).tw. 23 or/15‐22 24 exp Women/ 25 Female/ 26 Adolescent/ 27 (wom#n or female$).tw. 28 adolescen$.tw. 29 teen$.tw. 30 or/24‐29 31 14 and 23 and 30 32 limit 31 to ed=20120601‐20150205
Ovid MEDLINE In‐process and other non‐indexed citations
Ovid MEDLINE In‐process February 13 2015, last searched 17 February 2015 [253 records]
1 (abuse$ adj3 wom#n).tw. 2 (abuse$ adj3 spous$).tw. 3 (abuse$ adj3 partner$).tw. 4 (abuse$ adj3 (wife or wives)).tw. 5 (batter$ adj3 (wife or wives)).tw. 6 (batter$ adj3 wom#n).tw. 7 (partner$ adj3 violen$).tw. 8 (spous$ adj3 violen$).tw. 9 domestic violence.tw. 10 (gender adj3 violen$).tw. 11 or/1‐10 12 screen$.tw. 13 identif$.tw. 14 (routine$ adj3 (ask$ or question$)).tw. 15 ((history adj3 tak$) or medical history).tw. 16 detect$.tw. 17 disclos$.tw. 18 or/12‐17 19 (wom#n or female$).tw. 20 adolescen$.tw. 21 teen$.tw. 22 or/19‐21 23 11 and 18 and 22
Embase (Ovid)
Embase 1980 to 2015 Week 07, searched 17 February 2015. Limited to publication year=2012 to current [1187 records]
1 partner violence/ 2 Domestic Violence/ 3 marital rape/ 4 (abuse$ adj3 wom#n).tw. 5 (abuse$ adj3 spous$).tw. 6 (abuse$ adj3 partner$).tw. 7 (abuse$ adj3 (wife or wives)).tw. 8 (batter$ adj3 (wife or wives)).tw. 9 (batter$ adj3 wom#n).tw. 10 domestic violence.tw. 11 (partner$ adj3 violen$).tw. 12 (spous$ adj3 violen$).tw. 13 (gender adj3 violen$).tw. 14 or/1‐13 15 Mass Screening/ 16 Screening/ 17 anamnesis/ 18 screen$.tw. 19 identif$.tw. 20 (routine$ adj3 (ask$ or question$)).tw. 21 ((medical history or history) adj1 tak$).tw. 22 detect$.tw. 23 disclos$.tw. 24 or/15‐23 25 exp Women/ 26 Adolescent/ 27 (wom#n or female$).tw. 28 adolescen$.tw. 29 teen$.tw. 30 or/25‐29 31 14 and 24 and 30 32 limit 31 to yr="2012 ‐Current" (1187)
CINAHL Plus (EBSCOhost)
CINAHL Plus 1937 to current searched 17 February 2015. Limited to EM = 20120601 onwards [532 records]
S26 S24 AND S25 S25 EM 20120601‐ S24 S8 AND S17 AND S23 S23 S18 OR S19 OR S20 OR S21 OR S22 S22 TI (adolescen* or teen*) OR AB (adolescen* or teen*) S21 (MH "Adolescence") S20 TI(wom?n or female*) OR AB(wom?n or female*) S19 (MH "Women") S18 (MH "Female") S17 S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 S16 TI(routine* N3 (ask* or question*)) OR AB(routine* N3 (ask* or question*)) S15 TI(detect* or identif* or disclos*) OR AB(detect* or identif* or disclos*) S14 TI(medical history) or AB(medical history) S13 TI(history N1 tak*) or AB(history N1 tak*) S12 TI(screen*) or AB(screen*) S11 (MH "Patient History Taking") S10 (MH "Patient Assessment") S9 (MH "Health Screening") S8 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 S7 TI(domestic violence) or AB (domestic violence) S6 TI(partner* or spouse* or gender) N3 (violen*)) or AB(partner* or spouse* or gender) N3 (violen*)) S5 TI(batter* N3 (wom?n or wife or wives)) OR AB(batter* N3 (wom?n or wife or wives)) S4 TI(abuse* N3 (wom?n or spouse* or partner* or wife or wives ))or AB(abuse* N3 (wom?n or spouse* or partner* or wife or wives )) S3 (MH "Battered Women") S2 (MH "Domestic Violence") S1 (MH "Intimate Partner Violence")
PsycINFO (Ovid)
PsycINFO 1806 to February Week 2 2015, searched 17 February 2015. Limited to up=20120604 to 20150209 [726 records]
1 Battered Females/ 2 Domestic Violence/ 3 Partner Abuse/ 4 Intimate Partner Violence/ 5 (abuse$ adj3 wom#n).tw. 6 (abuse$ adj3 spous$).tw. 7 (abuse$ adj3 partner$).tw. 8 (abuse$ adj3 (wife or wives)).tw. 9 (batter$ adj3 wom#n).tw. 10 (batter$ adj3 (wife or wives)).tw. 11 (partner$ adj3 violen$).tw. 12 (spous$ adj3 violen$).tw. 13 domestic violence.tw. 14 (gender adj3 violen$).tw. 15 or/1‐14 16 Screening/ 17 Patient history/ 18 screen$.tw. 19 identif$.tw. 20 detect$.tw. 21 disclos$.tw. 22 (routine$ adj3 (ask$ or question$)).tw. 23 (medical history or (history adj1 tak$)).tw. 24 or/16‐23 25 exp Women/ 26 (wom#n or female$).tw. 27 adolescen$.tw. 28 teen$.tw. 29 or/25‐28 30 15 and 24 and 29 31 limit 30 to up=20120604‐20150209
Sociological Abstracts (ProQuest)
Sociological Abstracts 1952 to current, searched 17 February 2015. Limited by year=2012 to 2015 [287 records]
(SU.EXACT("Females") OR SU.EXACT("adolescents") OR (wom*n OR female*) OR (adolescent* OR teen*)) AND ((screen*) OR (identif*) OR ((routine* NEAR/3 question*) OR (routine* NEAR/3 ask*)) OR (detect*) OR (disclos*)) AND (SU.EXACT(("Family violence")) OR SU.EXACT(("Partner Abuse") OR ("Battered Women")) OR (abuse NEAR/3 wom*n) OR (abuse NEAR/3 spouse*) OR (abuse NEAR/3 partner*) OR (wife NEAR/3 abuse*) OR (wives NEAR/3 abuse*) OR (wife NEAR/3 batter*) OR (wives NEAR/3 batter*) OR (women NEAR/3 batter*) OR (partner* NEAR/3 violen*) OR (spouse* NEAR/3 violen*) OR (gender NEAR/3 violen*) OR ("domestic violence")) Limits applied Narrowed by Entered date: 2012 to 2015
Conference Proceedings Citation Index ‐ Social Science and Humanities (CPCI‐SS&H; Web of Science)
CPCI‐SS&H 1990 to 17 February, searched 17 February 2015. No date limits [73 records]
# 8 #7 AND #6 # 7 TS=(women* or female* or adolescen* or teen*) Indexes=CPCI‐SSH Timespan=All years # 6 #5 AND #4 Indexes=CPCI‐SSH Timespan=All years # 5 TS=(screen* or identif* or disclos* or detect* or (routin* NEAR/3 question*) or ( tak* NEAR/1 history) or "medical history") Indexes=CPCI‐SSH Timespan=All years # 4 #3 OR #2 OR #1 Indexes=CPCI‐SSH Timespan=All years # 3 TS=(batter* NEAR/3 ( wife or wives or women )) Indexes=CPCI‐SSH Timespan=All years # 2 TS=(abuse* NEAR/3 ( spous* or partner* or wife or wives )) Indexes=CPCI‐SSH Timespan=All years # 1 TS=(((gender* or spous* or partner*) NEAR/3 violen*) or "domestic violence" ) Indexes=CPCI‐SSH Timespan=All years
Cochrane Database of Systematic Reviews (CDSR), part of the Cochrane Library
CDSR 2015 Issue 2, searched 17 February 2015. No date limits [4 records]
#1 MeSH descriptor: [Battered Women] explode all trees #2 MeSH descriptor: [Domestic Violence] this term only #3 MeSH descriptor: [Spouse Abuse] this term only #4 (abuse* near/3 wom*n):ti,ab #5 (abuse* near/3 spous*):ti,ab #6 (abuse* near/3 partner*):ti,ab #7 (wife near/3 abuse*):ti,ab #8 (wives near/3 abuse*):ti,ab #9 (wife near/3 batter*):ti,ab #10 (wives near/3 batter*):ti,ab #11 (batter* near/3 wom*n):ti,ab #12 (partner* near/3 violen*):ti,ab #13 (spous* near/3 violen*):ti,ab #14 (domestic next violence):ti,ab #15 gender near/3 violenc*:ti,ab #16 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 #17 MeSH descriptor: [Mass Screening] this term only #18 MeSH descriptor: [Medical History Taking] this term only #19 screen*:ti,ab #20 identif*:ti,ab #21 ((medical history) or (history near/1 tak*)):ti,ab #22 routine* near/3 ask*:ti,ab #23 routine* near/3 question*:ti,ab #24 disclos*:ti,ab #25 detect*:ti,ab #26 #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 #27 MeSH descriptor: [Women] explode all trees #28 MeSH descriptor: [Female] explode all trees #29 MeSH descriptor: [Adolescent] explode all trees #30 (wom*n or female*):ti,ab #31 adolescen*:ti,ab #32 teen*:ti,ab #33 #27 or #28 or #29 or #30 or #31 or #32 #34 #16 and #26 and #33 in Cochrane Reviews (Reviews and Protocols)
Database of Abstracts of Reviews of Effects (DARE), part of the Cochrane Library
DARE 2015 Issue 1, searched 17 February 2015. Limited to year=2012 to 2015 [3 records]
#1 MeSH descriptor: [Battered Women] explode all trees #2 MeSH descriptor: [Domestic Violence] this term only #3 MeSH descriptor: [Spouse Abuse] this term only #4 (abuse* near/3 wom*n):ti,ab #5 (abuse* near/3 spous*):ti,ab #6 (abuse* near/3 partner*):ti,ab #7 (wife near/3 abuse*):ti,ab #8 (wives near/3 abuse*):ti,ab #9 (wife near/3 batter*):ti,ab #10 (wives near/3 batter*):ti,ab #11 (batter* near/3 wom*n):ti,ab #12 (partner* near/3 violen*):ti,ab #13 (spous* near/3 violen*):ti,ab #14 (domestic next violence):ti,ab #15 gender near/3 violenc*:ti,ab #16 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 #17 MeSH descriptor: [Mass Screening] this term only #18 MeSH descriptor: [Medical History Taking] this term only #19 screen*:ti,ab #20 identif*:ti,ab #21 ((medical history) or (history near/1 tak*)):ti,ab #22 routine* near/3 ask*:ti,ab #23 routine* near/3 question*:ti,ab #24 disclos*:ti,ab #25 detect*:ti,ab #26 #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 #27 MeSH descriptor: [Women] explode all trees #28 MeSH descriptor: [Female] explode all trees #29 MeSH descriptor: [Adolescent] explode all trees #30 (wom*n or female*):ti,ab #31 adolescen*:ti,ab #32 teen*:ti,ab #33 #27 or #28 or #29 or #30 or #31 or #32 #34 #16 and #26 and #33 Publication Year from 2012 to 2015, in Other Reviews
International Clinical Trials Registry Platform (who.int/ictrp/en/)
ICTRP searched 18 February 2015 No date limits applied [34 records]
CONDITION: intimate partner violence Or domestic violence OR battered women AND Intervention: screen OR screening OR identify OR identification OR detect OR detection OR disclose OR disclosure AND Recruitment status is ALL
ClinicalTrials.gov (clinicaltrials.gov)
ClinicalTrials.gov, searched 18 February 2015. No date limits applied [17 records]
Interventional Studies | intimate partner violence OR domestic violence OR battered women | screen OR screening OR identify OR identification OR disclose OR disclosure OR detect OR detection | Studies with Female Participants
Appendix 2. Search strategies used for previous version of review
Cochrane Central Register of Controlled Trials (CENTRAL)
Last searched 5 July 2012
#1 MeSH descriptor Battered Women explode all trees #2 MeSH descriptor Domestic Violence, this term only #3 MeSH descriptor Spouse Abuse, this term only #4 abuse* near/3 wom*n #5 abuse* near/3 spous* #6 abuse* near/3 partner* #7 wife near/3 abuse* #8 wives near/3 abuse* #9 wife near/3 batter* #10 wives near/3 batter* #11 partner* near/3 violen* #12 spous* near/3 violen* #13 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) #14 MeSH descriptor Mass Screening, this term only #15 screen* #16 identif* #17 routine* near/3 ask* #18 routine* near/3 question* #19 detect* #20 (#14 OR #15 OR #16 OR #17 OR #18 OR #19) #21 MeSH descriptor Women explode all trees #22 MeSH descriptor Adolescent explode all trees #23 wom*n or female* #24 adolescen* #25 teen* #26 (#21 OR #22 OR #23 OR #24 OR #25) #27 (#13 AND #20 AND #26)
Ovid MEDLINE(R)
Last searched 5 July 2012
1 Battered Women/ 2 Domestic Violence/ 3 Spouse Abuse/ 4 (abuse$ adj3 wom#n).tw. 5 (abuse$ adj3 spous$).tw. 6 (abuse$ adj3 partner$).tw. 7 ((wife or wives) adj3 abuse$).tw. 8 ((wife or wives) adj3 batter$).tw. 9 (partner$ adj3 violen$).tw. 10 (spous$ adj3 violen$).tw. 11 or/1‐10 12 Mass Screening/ 13 screen$.tw. 14 identif$.tw. 15 detect$.tw. 16 (routine$ adj3 (ask$ or question$)).tw. 17 or/12‐16 18 exp Women/ 19 Adolescent/ 20 (wom#n or female$).tw. 21 adolescen$.tw. 22 teen$.tw. 23 or/18‐22 24 11 and 17 and 23
Ovid MEDLINE(R) In‐Process and Other Non‐Indexed Citations
Last searched 5 July 2012
1 (abuse$ adj3 wom#n).tw. 2 (abuse$ adj3 spous$).tw. 3 (abuse$ adj3 partner$).tw. 4 ((wife or wives) adj3 abuse$).tw. 5 ((wife or wives) adj3 batter$).tw. 6 (partner$ adj3 violen$).tw. 7 (spous$ adj3 violen$).tw. 8 or/1‐7 9 screen$.tw. 10 identif$.tw. 11 (routine$ adj3 (ask$ or question$)).tw. 12 detect$.tw. 13 or/9‐12 14 (wom#n or female$).tw. 15 adolescen$.tw. 16 teen$.tw. 17 or/14‐16 18 8 and 13 and 17
Embase (Ovid)
Last searched 5 July 2012
1 Battered Women/ 2 Domestic Violence/ 3 Spouse Abuse/ 4 (abuse$ adj3 wom#n).tw. 5 (abuse$ adj3 spous$).tw. 6 (abuse$ adj3 partner$).tw. 7 ((wife or wives) adj3 abuse$).tw. 8 ((wife or wives) adj3 batter$).tw. 9 (partner$ adj3 violen$).tw. 10 (spous$ adj3 violen$).tw. 11 or/1‐10 12 Mass Screening/ 13 screen$.tw. 14 identif$.tw. 15 (routine$ adj3 (ask$ or question$)).tw. 16 detect$.tw. 17 or/12‐16 18 exp Women/ 19 Adolescent/ 20 (wom#n or female$).tw. 21 adolescen$.tw. 22 teen$.tw. 23 or/18‐22 24 11 and 17 and 23
CINAHL Plus (EBSCOhost)
Last searched 5 July 2012
S24 S17 and S23 S23 S18 or S19 or S20 or S21 or S22 S22 adolescen* or teen* S21 AG adolescent S20 women or woman or female* S19 (MH "Women+") S18 (MH "Female") S17 S9 and S16 S16 S10 or S11 or S12 or S13 or S14 or S15 S15 (MH "Health Screening") S14 identif* S13 MH "Experimental Studies" S12 detect* S11 (routin* N3 ask*) or (routin* N3 question*) S10 screen* S9 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 S8 (partner* N3 violen*) or (spouse* N3 violen*) S7 (wife N3 batter*) or (wives N3 batter*) S6 abuse* N3 spouse* S5 abuse* N3 partner* S4 abuse* N3 wom?n S3 MH "Intimate Partner Violence" S2 MH "Domestic Violence" S1 MH "Battered Women"
PsycINFO (Ovid)
Last searched 5 July 2012
1 Battered Women/ (2689) 2 Domestic Violence/ (7821) 3 Spouse Abuse/ (4154) 4 (abuse$ adj3 wom#n).tw. (2995) 5 (abuse$ adj3 spous$).tw. (908) 6 (abuse$ adj3 partner$).tw. (1266) 7 ((wife or wives) adj3 abuse$).tw. (570) 8 ((wife or wives) adj3 batter$).tw. (316) 9 (partner$ adj3 violen$).tw. (3626) 10 (spous$ adj3 violen$).tw. (351) 11 or/1‐10 (15402) 12 Screening/ (5344) 13 screen$.tw. (48857) 14 identif$.tw. (287698) 15 (routine$ adj3 (ask$ or question$)).tw. (244) 16 detect$.tw. (73953) 17 or/12‐16 (383820) 18 exp Women/ (97549) 19 (wom#n or female$).tw. (380933) 20 adolescen$.tw. (157334) 21 teen$.tw. (13531) 22 or/18‐21 (535027) 23 11 and 17 and 22 (2087)
Sociological Abstracts (ProQuest)
Last searched 5 July 2012
((SU.EXACT("Females") or SU.EXACT("adolescents") or (wom*n or female*) or (adolescent*or teen*)) AND ((screen*)or (identif*) or ((routine* NEAR/3 question*) or (routine* NEAR/3 ask*)) or (detect*))) AND (SU.EXACT(("Familyviolence")) or SU.EXACT(("Partner Abuse") or ("Battered Women")) or (abuse NEAR/3 wom*n) or (abuse NEAR/3spouse*) or (abuse NEAR/3 partner*) or (wife NEAR/3 abuse*) or (wives NEAR/3 abuse*) or (wife NEAR/3 batter*) or(wives NEAR/3 batter*) or (partner* NEAR/3 violent*) or (spouse* NEAR/3 violent*))
Database of Abstracts of Reviews of Effects (DARE), part of the Cochrane Library
Last searched 5 July 2012
#1 MeSH descriptor Battered Women explode all trees #2 MeSH descriptor Domestic Violence, this term only #3 MeSH descriptor Spouse Abuse, this term only #4 abuse* near/3 wom*n #5 abuse* near/3 spous* #6 abuse* near/3 partner* #7 wife near/3 abuse* #8 wives near/3 abuse* #9 wife near/3 batter* #10 wives near/3 batter* #11 partner* near/3 violen* #12 spous* near/3 violen* #13 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) #14 MeSH descriptor Mass Screening, this term only #15 screen* #16 identif* #17 routine* near/3 ask* #18 routine* near/3 question* #19 detect* #20 (#14 OR #15 OR #16 OR #17 OR #18 OR #19) #21 MeSH descriptor Women explode all trees #22 MeSH descriptor Adolescent explode all trees #23 wom*n or female* #24 adolescen* #25 teen* #26 (#21 OR #22 OR #23 OR #24 OR #25) #27 (#13 AND #20 AND #26)
metaRegister of Controlled Trials (mRCT)
Last searched July 2012
Search string: intimate partner violence OR domestic violence
Sociological Abstracts (CSA)
Searched up to 2009
Query: ((DE="domestic violence") or(DE="battered women") or(abuse* within 3 wom*n) or(abuse* within 3 spous*) or(abuse* within 3 partner*) or((wife within 3 abuse*) or (wives within3 abuse*)) or((wife within 3 batter*) or (wives within 3 batter*)) or(partner* within 3 violen*) or(spous* within 3 violen*)) and((screen*) or(identif*) or((routine* within 3 question*) or (rountine* within 3 ask*)) or(detect*)) and((DE="women") or(DE="adolescents") or(wom*n or female*) or(adolescen*) or(teen*))
ASSIA (CSA)
Searched up to 2009 only
Query: ((DE="domestic violence") or(DE="battered women") or(abuse* within 3 wom*n) or(abuse* within 3 spous*) or(abuse* within 3 partner*) or((wife within 3 abuse*) or (wives within3 abuse*)) or((wife within 3 batter*) or (wives within 3 batter*)) or(partner* within 3 violen*) or(spous* within 3 violen*)) and((screen*) or(identif*) or((routine* within 3 question*) or (routine* within 3 ask*)) or(detect*)) and((DE="women") or(DE="adolescents") or(wom*n or female*) or(adolescen*) or(teen*))
Appendix 3. Additional methods
| Analysis | Method |
| Measures of treatment effect | Where measurements were comparable and on the same scale, we intended to combine them to obtain mean differences. Where scales measured the same clinical outcomes in different ways (e.g. depression, quality of life), mean differences were to be standardised in order to combine results across scales. There have been insufficient data in studies in the review and update to undertake these analyses. These methods will be retained for subsequent updates. |
| Unit of analysis issues | There was one included cluster‐RCT to date, which did account for clustering. For future updates, where studies have not appropriately accounted for clustering, we will re‐analyse data using methods recommended by Donner 1980. |
| Dealing with missing data | Rates of missing data on the primary outcome have not required thus far that we undertake best‐case and worst‐case scenario analyses to estimate the effect of the missing data on the results of pooled studies. Such analyses would enable us to ascertain if observed effect sizes increased or decreased as a function of the extent of attrition in the two arms of the trial. These methods will be retained for subsequent updates. |
| Assessment of reporting biases | We planned to draw funnel plots to investigate any relationship between effect size and study precision (closely related to sample size) (Egger 1997) to investigate a relationship that could be due to publication or related biases or due to systematic differences between small and large studies. Funnel plots (estimated differences in treatment effects against their standard error) were not drawn because there was an insufficient number of included studies (more than 10 are recommended), to identify asymmetry due to publication bias. |
| Subgroup analyses | We planned to conduct subgroup analysis for type of healthcare setting (which was done) and the type of screening intervention (based on types of tools, questions), which could be done in a future update with more studies. We also stated in the protocol that we would undertake subgroup analysis based on screening intervention only or where it was embedded as part of a larger multi‐component intervention. However, the implications of our altered criterion for assessing inclusion of interventions/comparisons, which explicitly excludes interventions that extended beyond an immediate response and referral phase following screening, meant that this subgroup analysis has not been relevant to date. |
| Sensitivity analysis | Our original protocol stated our intention to use sensitivity analysis to deal with study quality and differential dropout, which has been undertaken in this review. However, we have not used sensitivity analysis for intention‐to‐treat issues and duration of follow‐up as neither have applied to date. These methods will be retained for subsequent updates. |
RCT: randomised controlled trial.
Data and analyses
Comparison 1. Universal screening for intimate partner violence (IPV) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Identification of IPV by health professionals | 8 | 10074 | Odds Ratio (M‐H, Random, 95% CI) | 2.95 [1.79, 4.87] |
| 2 Identification of IPV by type of healthcare setting | 7 | 4393 | Odds Ratio (M‐H, Random, 95% CI) | 2.35 [1.53, 3.59] |
| 2.1 Antenatal clinics | 2 | 663 | Odds Ratio (M‐H, Random, 95% CI) | 4.53 [1.82, 11.27] |
| 2.2 Maternal health services | 1 | 829 | Odds Ratio (M‐H, Random, 95% CI) | 2.36 [1.14, 4.87] |
| 2.3 Emergency departments (EDs) | 3 | 2608 | Odds Ratio (M‐H, Random, 95% CI) | 2.72 [1.03, 7.19] |
| 2.4 Hospital‐based primary care | 1 | 293 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [0.79, 2.94] |
| 3 Referrals | 2 | 1298 | Odds Ratio (M‐H, Random, 95% CI) | 2.24 [0.64, 7.86] |
Comparison 2. Face‐to‐face screening for intimate partner violence (IPV) versus written/computer‐based screening.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Identification of IPV | 4 | 2765 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.53, 2.36] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmad 2009.
| Methods | Study design: randomised controlled trial Randomisation method: random‐number sampling scheme stratified by participating physicians. Before recruitment, the randomisation assignment was computer‐generated using varying block sizes of 2 and 4. Women were individually randomised Power calculation: reported Study dates: March to September 2005 |
|
| Participants | Setting: urban, hospital‐affiliated, academic, family practice clinic Country: Canada Inclusion criteria: women, 18 years and over, in relationship in last 12 months, able to read and write English Exclusion criteria: none stated Number (%) of eligible recruited: 314/586 (53.6%) Numbers recruited: 314; intervention group 156, control group 158 Number of dropouts: 34; intervention group 17, control group 17 Numbers analysed (% of recruited): 280; intervention group 139 (89%), control group 141 (89%) Numbers analysed (sensitivity analysis): 293; intervention group 144, control group 149 Age: mean 44 years (SD 14 years) Marital status: married/living with a partner 74%, single 21%, separated/divorced/widowed 5% Ethnicity: outside Canada 34% Socioeconomic status: ≤ USD 40,000 28%, USD 40,001 to 60,000 18%, USD 60,001 to 80,001 14%, USD 80,001 to 100,001 16%, > USD 100,000 24% Education background: 18% ≤ high school, 33% ≤ college, 34% ≤ university, 15% ≤ postgraduate Children: children at home aged < 15 years 58% Positive IPV result exit survey 62/286; intervention group 28/140, control group 34/146 |
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Primary outcomes
Secondary outcomes
Discussion and detection of other health risks were also measured but not relevant to this study Data collected through audio‐recording (short‐term) |
|
| Notes | Funding: Canadian Institutes of Health Research (grants IGF 63976 and FOW 68219), Institute of Gender and Health, Ontario Women's Health Council, and Strategic Training on Health Care, Place and Technology Program | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random‐number sampling scheme for eligible women stratified by participating physician was used. The randomisation assignment was computer‐generated by an off‐site biostatistician using varying block sizes of 2 and 4 |
| Allocation concealment (selection bias) | Low risk | Women who had provided informed consent were randomly assigned to the intervention group or control group: "Patient assignments were sealed in opaque envelopes that were marked on the outside with a physician number and sequence number. The envelopes were opened by the recruiter after patients' written consent" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All physicians initially received study information and those willing to participate provided written consent. Training was provided during clinical team meetings at the time of consent. Although "Physician participants were blinded to the study's primary purpose throughout the trial by emphasizing all health risks included in the multirisk computer survey and by using a nonspecific study title", they would not have been entirely blinded to the intervention. For example, the prompt in the women's records of the intervention group would have alerted providers to who was in the intervention group and conceivably have influenced their performance. Women were blinded to the study's primary purpose by using strategies similar to those used for physician participants and embedding questions about women's risk for IPV allowed the authors to conceal the study focus from both physician and patient participants. However, the patients were still aware that the computer survey was part of the intervention that could have influenced their behaviour. Awareness of being a control group participant (i.e. not doing the computer survey) may have altered the control group participants' behaviour in some way that related to the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 2 people undertook primary outcome assessment, working independently to code the audio‐recordings of the clinical encounters. Although efforts were made to blind coders to the patients' group assignments, this may have been compromised by what they heard (i.e. some information during the consultation that revealed the patient's allocation). However, this was unlikely to have affected their observation of the primary outcomes (initiation of discussions on IPV and detection of IPV). After their visit, women completed a pencil‐and‐paper exit survey and received brochures on cancer screening, cardiac and mental health, and IPV, at which time the research staff disclosed the purpose of the study to women. Although women were not blinded in answering the exit surveys, the outcomes measured via the exit survey were not primary to this study or our review |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Immediately following randomisation, 12/156 women in the intervention group and 9/158 women in the control group were excluded/withdrew. In the intervention group, 9 did not complete the computer assessment; 2 had their visits cancelled and 1 withdrew. In the control group, 2 women had their visit cancelled, and 6 women withdrew and 1 physician withdrew 1 woman who had mental health issues. The authors observed that women generally showed interest in the computer screening, and some expressed disappointment when they were not assigned to the computer‐screened group, which may have explained the higher number of withdrawals in the control group. It is unclear what the actual level of attrition was, given that individual participant numbers for analyses are inconsistent across the flowchart depiction (intervention 141, control 144), and the results text (intervention 143, control 144). Numbers in Table 3 (RR analyses: intervention 139, control 141) also differed but this was due to missing data (missing covariate values for three visits and outcomes coded as "other" in 2 cases). In the final analysis, reasons for exclusions of participants appear balanced across the 2 groups occurring due to missing data, recording failures, and language barriers. Overall the attrition rate was low at 10.8% (34/314). The sensitivity analyses suggest that the missing data were enough to potentially affect the results: "Sensitivity analyses were conducted to gauge the potential effect of missing values. 2 extreme situations were considered in which each missing value was replaced with an extreme value of the variable that was most likely to diminish the observed RR toward the null value or most likely to accentuate the observed RR away from the null. These 2 extremes provide a range of likely values for each effect." Other imputed missing data were accounted for in the appendicised reanalysed outcome data, which was undertaken by ITT |
| Selective reporting (reporting bias) | Low risk | None noted. Trial registered (NCT00385034) but study protocol not available |
| Other bias | High risk | Protection against contamination: there was a high risk of bias in terms of what the participants in the control group received. Given that the same providers delivered both conditions to different women, this suggests the way in which they interacted with women from the control group may have been influenced by their experience of delivering the intervention and thus underestimated the effect |
Carroll 2005.
| Methods | Study design: cluster‐randomised controlled trial Randomisation method: to obtain a balanced sample, each participating provider was paired to the greatest extent possible with another provider by practice location, type of provider, sex, and age. 1 member of each pair was randomly assigned to the ALPHA group (intervention group) or control group by a biostatistician using computer‐generated random numbers Power calculation: reported Study dates: from 1998 to 2002 |
|
| Participants | Setting: 4 communities in Ontario, including urban, suburban, and rural practices with women from diverse socioeconomic and ethnic backgrounds Country: Canada Inclusion criteria (providers): any HCP (e.g. physicians, obstetricians, midwives) who practised antenatal and intrapartum care, or antenatal plus transfer at 28 weeks, saw at least 10 antenatal women a year, and were not using any antenatal psychosocial screening tool other than the standard Ontario Antenatal Record Inclusion criteria (individuals): female; 12 to 30 weeks' gestation; able to read and write English; able to provide informed consent Exclusion criteria: high obstetric risk as defined by Ontario Antenatal Record Numbers recruited (providers): 60; intervention group 30, control group 30 Number of dropouts (providers): 12; intervention group 9, control group 3 Numbers (%) of eligible individuals recruited: 253/273 (92.7%) Numbers recruited (individuals): 253; intervention group 112, control group 141 Number of dropouts (individuals): 26; intervention group 14, control group 12 Numbers analysed (% of recruited): 227; intervention group 98 (88%), control group 129 (91%) Age: intervention group mean 29.1 (range 17 to 47 years), control group mean 29.4 (range 17 to 44 years) Ethnicity: born in Canada; intervention group 85.7%, control group 84.5% Socioeconomic status
Education background: high school or less intervention group 19.4%, control group 26.6%; some college or university intervention group 25.5%, control group 20.3%; degree intervention group 55.1%, control group 53.1% Pregnancy problems: no concerns intervention group 55.1%, control group 50%; minor concerns intervention group 39.8%, control group 46.9%; major concerns intervention group 5.1%, control group 3.1% |
|
| Interventions | Intervention group
Control group
|
|
| Outcomes |
|
|
| Notes | Data on psychosocial outcomes at 4 months' postpartum were not reported. Data on sample characteristics only reported for the people who completed Funding: Ontario Ministry of Health and Long Term Care and the Ontario Women's Health Council |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | To obtain a balanced sample, each participating provider was paired to the greatest extent possible with another provider by practice location, type of provider, sex, and age. 1 member of each pair was randomly assigned to the ALPHA group (intervention group) or control group by a biostatistician using computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | There is a lack of information about the level of allocation knowledge of those who enrolled the provider. Presumably providers recruited women after their randomisation had occurred. If providers knew their status, this could have influenced how and which women were recruited based on their own allocation status |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Provider participants were aware of the purpose of the study and their status as intervention or control group, which may have influenced their performance. Providers were also responsible for first telling women about the study. Interested women received an explanatory brochure and consent form from their provider and a telephone call from the study nurse to further explain the study and secure consent. We are not told in the report what level of awareness women had about the purpose of the trial. Knowing that the trial included a focus on IPV could have influenced how they responded to their treatment or non‐treatment. However, IPV was just 1 of 15 psychosocial issues and therefore may have not been singled out. Individual women in the intervention group may have been aware that they were in a treatment group based on the introduction of the ALPHA tool into the consultation, which may have influenced their responses to the ALPHA tool. There is no mention about the blinding of other study personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Healthcare professional participants provided the primary outcome data in that they reported back on their level of concern with their participating patients. Both intervention and control group providers may have overestimated their level of concern as they would have been prompted by the questions asked in the data collection form. We are told a nurse undertook a follow‐up of women but are not given information on level of awareness of women's allocations. The women themselves would not have been blinded in outcome reporting |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/30 (30%) intervention group providers compared to 3/30 (10%) control group providers withdrew from the study. Intervention group: withdrawn because of illness, maternity leave or ineligibility because of language barrier (n = 5); no reason given for withdrawal (n = 4); control group: withdrawn because of illness (n = 1); no reason given for withdrawal (n = 2). 6 family physicians withdrew from the intervention group compared to 1 in the control group. There were no data reported on participants of the 12 providers that withdrew. This high level of attrition in the intervention group provider could indicate deliberate withdrawal associated with the outcome (creating high risk of bias). Among providers who remained in the study, and in terms of the primary outcome, attrition of individual women was low ‐ providers did not complete/return data collection forms on 7.5% of participants. No data were reported on the numbers of women who were assessed at the 4‐month postpartum point to allow us to evaluate bias at the participant reporting level. Only 14/21 intervention group providers gave feedback on experience of using the ALPHA tool. Analysis included sensitivity analysis to accommodate loss of provider participants. Results were not robust enough to withstand the loss of providers |
| Selective reporting (reporting bias) | High risk | Importantly, there is an absence of information on the postpartum psychosocial outcomes of women. Data on characteristics of the sample are only reported on those who were included in the final analysis. The reporting of results highlighted the one significant finding (family violence, including child abuse) as the great majority of others were non‐significant |
| Other bias | Unclear risk | Protection against contamination: women in the control group may have seen intervention group providers during subsequent consultations, which may have contaminated women's psychosocial outcome data. There is a lack of information about how the situation of the control group using the ALPHA tool was avoided Reliability of outcome measures: while the primary outcome (akin to identification/detection) was adequately measured as 'some' or 'high' concern about a particular psychosocial issue, the time lapse between the delivery of the intervention and the data collection may have introduced bias through recall bias. Intervention group might have had more notes on which to base recall than that the control group providers |
Fincher 2015.
| Methods | Study design: randomised controlled trial Randomisation method: computer‐generated Power calculation: reported. However, it was based on combining data from 2 clinics. We report on just 1 clinic here Study dates: from 17 July 2012 to 21 September 2012 |
|
| Participants | Setting: Women, Infants, Children's (WIC) clinic in the large metropolitan city of Atlanta. WIC clinics provide supplemental food, healthcare referrals and nutrition education to low‐income women and their children up to five years of age Country: USA Inclusion criteria: females, at least 18 years of age, eligible for WIC services, English‐speaking and literate. Only African‐American women were included in analyses Exclusion criteria: none stated Number (%) of eligible recruited at 'Clinic 2': 402/648 (61.9%; this percentage was based on overall participation rate reported across the 2 clinics) Numbers randomised: 402; face‐to‐face interview 200, CASI 202 Number of dropouts: 34; face‐to‐face interview 9, CASI 25 Numbers analysed (% recruited): 368; face‐to‐face interview 191 (95.5%), CASI 177 (87.6%) Age: mean 27.4 years (SD 7.8) Ethnic background: all African‐American women in this analysis Marital status: unmarried relationship 45%, single 40%, married 15% Education: up to high school 44%, some college 33%, completed college 22% Employment: working outside the home 45% Experience of IPV: lifetime experience of any IPV 49%, prior‐year experience of any IPV 36% |
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Primary outcomes
Other variables measured were health behaviours, including smoking, alcohol and substance use, and contraceptive use (data not reported) Timing of measurement 117 women (31.8%) completed the 2‐week follow‐up but no data are reported here |
|
| Notes | We were unable to obtain the follow‐up data from the authors (Fincher 2015 [pers comm]) Funding: the Georgia Department of Public Health, Maternal and Child Health Program; Emory Center for Injury Control (Grant – R49 CE001494 and PH2011120G); and the Hubert Department of Global Health, Rollins School of Public Health, Emory University |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | We were informed directly by authors that there was "computer generated randomization to FTFI or CASI within clinic strata." (Fincher 2015 [pers comm]). No further detail was supplied in the report or personal communications. There were a number of differences between the groups on sociodemographic characteristics, calling into question the success of the randomisation process |
| Allocation concealment (selection bias) | High risk | We were unable to obtain information on the steps taken to conceal the allocation or on how the personnel/interviewers moved from the recruitment/consenting stage to allocating women and delivering the face‐to‐face screening or CASI. 34/402 cases were omitted from the analysis due to incomplete data and it is unclear at what stage they dropped out. We were informed that those recruiting women also did interviews and recruiters would likely have had knowledge of the allocation prior to inviting individuals into the study, which could have influenced their behaviours differentially suggesting the potential for selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel conducting the face‐to‐face interview were not blinded to the purpose of the study, were aware that they were delivering the intervention condition, and had a high level of interaction with women from recruitment through to follow‐up. Participants were not blinded: "Potential participants were informed that the survey asked questions about their general health and about their relationship with their partner," however women in both arms received this information making it less likely to influence the outcomes differentially in the groups. However, we judge that the lack of blinding for personnel could have interacted with outcomes differentially |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Women's disclosure data were used as the primary outcome data and it is unlikely that the 2 arms behaved differently in this regard. The follow‐up at 2 weeks was conducted by the same interviewers, which could have led to detection bias, however it appears the 2‐week data were not reported here. The latter could have differentially affected outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 34/402 cases were omitted and the rate was higher in the CASI group (12%) compared to face‐to‐face group (4%) as pointed out above, but is unclear at what stage that occurred. At 2 weeks, 117/368 (31.8%) of women completed a follow‐up suggesting high attrition but we do not know if there was differential dropout and, in any case, we were unable to incorporate the 2‐week data as they were not reported |
| Selective reporting (reporting bias) | High risk | No protocol available. The second clinic's data are being reported elsewhere. Data on the 2‐week outcomes for women were not reported. "Telephone follow‐up interviews were conducted with study participants at 2 weeks to ask about their experience with and preference for screening method." |
| Other bias | Unclear risk | The authors were contacted for additional information on how randomisation was conducted and on flow of participants in the study. However, we were not able to obtain clarity on a number of methodological queries. It is unclear if steps were taken to protect against contamination/cross‐over |
Fraga 2014.
| Methods | Study design: 3‐arm, randomised controlled trial Randomisation method: not described Power calculation: used EpiInfo software with 95% confidence interval level and 80% power, assuming prevalence of 7% in control Study dates: reported in Rodrigues 2008 that women were recruited over a 10‐month period during 1999 and 2000. Women were contacted again 1 year postpartum, and although specific dates have not been reported in Fraga 2014, authors confirmed that between 2000 and 2001 women were re‐contacted and received the screening intervention Fraga 2015 [pers comm]. |
|
| Participants | Setting: maternity/maternal health services at a university hospital Country: Portugal Inclusion criteria: consenting women who had delivered a baby at the hospital 1 year prior Exclusion criteria: not reported Numbers recruited (12 months prior): 915 women Numbers randomised: intervention group one 305, intervention group two 305, control group 305 Number dropouts (lost since recruitment): intervention group one 13, intervention group two 3, control group 70 Numbers analysed (% of recruited): 829; intervention group one 292 (96%), intervention group two 302 (99%), control group 235 (77%) Age: not reported for the subset in this study Marital status: not reported Ethnicity: not reported Education: reported as "did not differ between randomization groups" |
|
| Interventions | Intervention groups
We combined the 2 conditions involving the social worker and compared them to postal screening Control group
Timing of measurement: interventions were conducted at 1 year postpartum |
|
| Outcomes | Primary outcome
Referral rates were not reported |
|
| Notes | This was a brief report. Women who did not respond to the postal questionnaire were followed up using one of the other screening methods (face‐to‐face or telephone), which increased the IPV detection rate from 9/235 (3.8%) to 19/235 (8.1%) in this group. Similarly, alternative methods were used for women in the face‐to‐face and telephone groups if they did not respond. We only used the initial screening data from this study, which may have underestimated detection rates for the postal group (as the one with the low participation rate) Funding: Fundacao para a Ciencia e a Tecnologia Fundação para a Ciência e a Tecnologia (FCOMP‐01‐0124‐FEDER‐021439) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed in the ratio 1:1:1 but method was unclear |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described and not likely due to the nature of the intervention but potentially high risk for interviewers/outcome assessors as it seems that data were collected on antenatal abuse at enrolment. It is not clear whether the interviewers/outcome assessors had access to this information, which could have biased the outcome assessor to conduct more rigorous follow‐up interviews of women identified at enrolment as 'at risk' due to abuse in the prenatal phase |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described but potentially high risk for interviewers/outcome assessors as it seems that data were collected on antenatal abuse at enrolment. It is not clear whether the interviewers/outcome assessors had access to this information, which could have biased the outcome assessor to conduct more rigorous follow‐up interviews of women identified at enrolment as 'at risk' due to antenatal abuse |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The postal questionnaire group suffered from high attrition (70/305; 23%) and 81 women (27%) received alternative screening interventions (protocol deviations), which increased the IPV detection rate in the ITT analysis. We excluded these protocol deviations from the ITT data for our meta‐analyses |
| Selective reporting (reporting bias) | Unclear risk | The protocol for this study was not available and therefore we were not able to determine whether selective reporting was present. Results were reported by ITT and per protocol but as percentages only |
| Other bias | Unclear risk | This was a brief report and the authors were contacted for additional information; missing data for the primary outcome were obtained, however we were not able to obtain clarity on most methodological queries. Baseline data were reported to be similar between groups, however the numerical data were not published. It was simply stated that "Age, education, income, smoking, prenatal visits and abuse during pregnancy did not differ across the three randomised groups". "Abuse during pregnancy" was measured using the Abuse Assessment Screen as part of a hospital survey preceding this RCT, and this may have affected the collection and interpretation of outcome data |
Humphreys 2011.
| Methods | Study design: randomised controlled trial Randomisation method: women reporting risks for smoking, alcohol, drug use, and IPV were stratified by risk combination and randomly assigned by the computer (on which they completed a risk assessment) to intervention or usual care Power calculation: none reported Study dates: from June 2006 to June 2007 |
|
| Participants | Setting: 5 antenatal clinics in San Francisco Country: USA Inclusion criteria: females aged 18 years and over, English speaking, < 26 weeks pregnant, receiving antenatal care at one of the participating clinics, and not presenting for first antenatal visit Exclusion criteria: none stated Numbers recruited/assessed for IPV risk: 410 Numbers randomised: 50; intervention group 25, control group 25 Number dropouts at exit interview: intervention group 3, control group 1 Numbers analysed (% recruited) at exit interview: intervention group 22 (88%), control group 24 (96%) Number dropouts at second interview: intervention group 5, control group 8 Numbers analysed (and % recruited) at second interview: 20 intervention group (80%), control group 17 (68%) Numbers analysed (sensitivity analysis): 50; intervention group 25, control group 25 Age: mean 27.7 years (SD 7.1), range 18 to 43 years Marital status: married/living with partner 38%, never married 46%, divorced/separated 16% Ethnicity: Latino 34%, Black 22%, White 30%, Other 14% Education: < high school 22%, high school 36%, some college 28%, college degree 12% |
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Primary outcomes
Timing of measurement: short‐term assessment of outcomes (immediately after the intervention and again following antenatal visit 1 month later; data collected from women) |
|
| Notes | Analysis: no adjustment for clustering Funding: US Department of Health and Human Services National Institute on Drug Abuse (R01 DA 15597). The preparation of this manuscript was supported, in part, by a NIDA Center grant (P50 DA 009253) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomly assigned by the computer (on which they completed a risk assessment) to the intervention or control group: "Women reporting risks were stratified by risk combination and randomly assigned by the computer to intervention or usual care groups" |
| Allocation concealment (selection bias) | Low risk | Allocation was adequately concealed as only the computer had knowledge of the assignment and there was no opportunity to influence what groups women went into as the computer did the allocation immediately |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | While some personnel may have become aware of the participant allocation (e.g. in order to place computer reports in medical records), the review authors judged that the outcome is not likely to be influenced by this lack of blinding. However, the printed report would have alerted physicians to the status of the woman in the intervention group and may have enhanced performance above and beyond how they might otherwise perform if they were to observe such a report but not be part of a research study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | During post‐visit interviews at baseline and 1‐month follow‐up, participants were asked "Did you talk about domestic violence with your doctor today?" which was used to indicate that a patient–provider discussion of IPV occurred. We were not given information on the level of blinding of the research assistant and, in any case, the allocation of the woman could very easily have been revealed during the outcome evaluation potentially biasing the research assistant's observations |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of the 25 women in the intervention group, 3 (12%) did not provide baseline data and 5 (20%) did not provide data at 1‐month follow‐up. Of the 25 women in the control group, 1 (4%) did not provide baseline data and 8 (32%) did not provide data at 1‐month follow‐up. The sensitivity of the results to LTFU was assessed "by making the assumption that in the absence of outcome data, no discussion occurred." Reasons for dropout were not provided and it is therefore difficult to judge if there was a differential dropout across the groups |
| Selective reporting (reporting bias) | High risk | There were differences between the outcomes as reported in the trial registry (NCT00540319) and those reported here |
| Other bias | High risk | Protection against contamination: women assigned to the control group could have received an 'enhanced' usual care given that providers were consulting with women from both the intervention and control groups |
Kataoka 2010.
| Methods | Study design: randomised controlled trial Randomisation method: random numbers table Power calculation: none reported Study dates: from February to November 2003 |
|
| Participants | Setting: antenatal clinic of an urban general hospital Country: Japan Inclusion criteria: women < 25 weeks pregnant Exclusion criteria: none stated Number (%) of eligible recruited: 328/355 (92.4%) Numbers randomised 328: interview 165, questionnaire 163 Numbers analysed (% recruited) at first time point 328: interview 165 (100%), questionnaire 163 (100%) Number of dropouts at second time point: interview 10, questionnaire 3 Numbers analysed (% recruited) at second time point: 315; interview 155 (93.9%), questionnaire 160 (98.2%) Number of dropouts at third time point: interview 7, questionnaire 11 Numbers analysed (% recruited) at third time point: 297; interview 148 (89.7%), questionnaire 149 (91.4%) Age: 20 to 29 years 30.5%, 30 to 39 years 66.2%, ≥ 40 years 3% Marital status: married 96.3%, single 2.1% Education: high school 13.4%, junior college 43.6%, university degree 41.8% Employment: full‐time 33.8%, part‐time 17.7%, not working 46.9% Lifetime experience of physical violence by male partner: 20 (5.8%); interview 8 (4.8%); questionnaire 11 (6.8%) |
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Funding: Grant‐in‐Aid for COE (Centre of Excellence) Research, provided by the Ministry of Education, Culture, Sports, Science and Technology of Japan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Researchers "used a random number table in blocks of four to ensure that approximately equal numbers of women were allocated to each group" |
| Allocation concealment (selection bias) | Low risk | Although it was indicated that numbered, sealed envelopes were used, it was unclear whether opaque envelopes were used. However, since there was no clear intervention/comparison group, the likelihood that selection bias was introduced is low |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The researchers indicate "because of the nature of the screening methods, participants could not be blinded to the group assignment." However any such bias was likely distributed equally across the 2 groups. Although the extent of the knowledge about participants' assignment, especially given the repeat visits among personnel, is unclear it is unlikely to have influenced the outcomes differentially in the groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding of outcome assessment; however, the outcome measurement is not likely to be differentially influenced in the 2 groups by lack of blinding as there was not a clear intervention or comparison group. Also the "same researcher performed the allocation procedure and data analysis" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were low and balanced in the 2 groups (intervention group 10.3%; control group 8.6%). 2 people in the interview group refused to continue compared to 0 in the questionnaire group |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported as specified in protocol. The trial was registered (UMIN‐CTRC000000353) |
| Other bias | High risk | "Measurements of primary and secondary outcomes had psychometric property limitations" |
Klevens 2012a.
| Methods | Study design: randomised controlled trial Randomisation method: the audio computer‐assisted self interview computer program applied simple randomisation (simple randomisation was written into the code of the software program), which facilitated individual randomisation of women to 1 of 3 trial arms Power calculation: none provided Study dates: from 22 April 2008 to 26 September 2008 |
|
| Participants | Setting: women's health clinics (obstetrical, gynaecological, and family planning clinics) at a public hospital Country: USA Inclusion criteria: females, at least 18 years of age Exclusion criteria: women who did not speak English; were accompanied by their partner or a child over 3 years of age; who were visually, hearing, or mentally impaired; women who had no access to a telephone or were over 36 weeks pregnant Number (%) of eligible recruited: 126/228 (55%) Numbers recruited 126: intervention group 46, control group 80 Number of dropouts: 24; intervention group 10, control group 14 Numbers analysed (% recruited): 102; intervention group 36 (78%), control group 66 (83%) Age: mean 35.8 years (SD 14.4 years) Ethnicity: 6.3% White, 78.6% Black, 11.9% Latino, 3.2% Asian Education background: ≤ high school 42.4%, ≤ college/vocational training 41.9% Insurance status: Medicaid/care 37.3%, private insurance 5.6%, uninsured 57.1% |
|
| Interventions | Intervention group
Control group The study authors combined the 2 A‐CASI arms
|
|
| Outcomes | Primary outcomes 3 screening outcomes:
Referral outcomes:
|
|
| Notes | Funding: Centers for Disease Control and Prevention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation was written into the code of the computer program used to screen women and individually assigned participants to 1 of the 3 trial arms |
| Allocation concealment (selection bias) | Low risk | Research assistants obtained informed consent from participants prior to any knowledge of the allocation. The allocation was revealed to the participant directly via the computer program used to conduct the health interview |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. While any impact of non‐blinding on performance was likely to have been low in the pure A‐CASI condition, the potential for involvement of HCPs in the other 2 arms may have influenced the performance of participants especially in the face‐to‐face arm |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Except for data on women's contact with local advocacy services, which was provided by blinded advocacy staff, assessment of outcomes was not blinded. The research assistant collecting the data was aware of the assignment of individuals and therefore there was potential for introducing a bias into the assessment of outcomes. Also "HCPs were asked to respond to a checklist for compliance with the screening and referral protocol, HCPs were not actually observed to establish the validity of this checklist and the accuracy of their reporting" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Participants lost to follow‐up were similar in level of education and insurance status, but were significantly younger. However, there were no differences between assigned study groups for demographic characteristics among the 24 women lost to follow‐up" |
| Selective reporting (reporting bias) | Unclear risk | None noted but study protocol not available. Study was not registered |
| Other bias | High risk | Protection against contamination: there was a high risk of potential for contamination across conditions given that all 3 conditions were delivered in the same clinics. Also, a decision was made to combine data from the 2 A‐CASI (A‐CASI with HCP endorsement and A‐CASI alone) arms in the analysis; it is unclear if this was a decision made a priori. It is possible that such a measure could have led to contamination given the similarities between A‐CASI with HCP endorsement and the HCP alone conditions |
Koziol‐McLain 2010.
| Methods | Study design: randomised controlled trial Randomisation method: randomly assigned individually 1:1 to intervention or control group Power calculation: sample size was calculated to detect a 50% treatment effect for 1 or more physically abusive events occurring in the follow‐up period Study dates: from 16 April 2007 to not reported |
|
| Participants | Setting: North Island New Zealand hospital ED Inclusion criteria: women aged 16 years and over, presenting to the ED for care during selected shifts were eligible Exclusion criteria: acute presentations precluding informed consent, functional or organic impairment based on clinician assessment, emergency health needs, non‐English speaking or entered study during previous visit Number (%) of eligible recruited: 399/983 (40.6%) Numbers randomised: 399; intervention group 199, control group 200 Number of dropouts at exit interview: intervention group 32, control group 23 Numbers analysed (% of randomised): 344; intervention group 167 (84%), control group 177 (88.5%) Age: median 40 years, range 16 to 94 years, interquartile range 27 to 59 years Relationship status: current relationship 67.4%, relationship within past year 8.3%, no relationship in past year 22.3%, never had a partner 2% Ethnicity: Maori 37.6%, New Zealand European 60.4%, other 2% Socioeconomic status (annual individual income): NZD 0 to 10,000 15.2%, NZD 10,001 to 20,000 32.1%, NZD 20,001 to 35,001 26.1%, > NZD 35,000 20.3%, do not know 5.8% Employed: yes 49.1%, no 31.6%, retired 19.3% Education: < high school 23.3%, high school 22.8%, other completed qualification 45.6%, college degree 8.3% Depression (CES‐D): mean 14.0 Mental health (SF‐12): mean 64.8 (SD 24.6) General health (SF‐12): mean 61.9 (SD 30.9) Acute injury: 79 (19.9%); intervention group 34 (17.3%), control group 45 (22.9%) One or more children in household: 73.4% Level of violence (treatment group only): 18% screen result positive, 51% lifetime result positive |
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Primary outcome
Secondary outcomes
Other outcomes
Timing of measurement/follow‐up: 3 months after index ED visit women had a face‐to‐face structured follow‐up interview |
|
| Notes | Analysis: by ITT Funding: Health Research Council of New Zealand |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The biostatistician i) computer‐generated a series of randomly selected shifts across 7 days of the week and times of the day during which recruitment was to be undertaken and ii) provided a computer‐generated randomised sequence for group assignment within those periods |
| Allocation concealment (selection bias) | Low risk | The concealment of allocation followed strict protocols. The randomisation schedule was not available to anyone other than the biostatistician. The biostatistician oversaw the preparation of sealed, opaque, tamper‐proof, sequentially numbered envelopes containing the randomised treatment allocation. Research log sheets were used for the real‐time documentation of recruitment and the use of envelopes to provide a clear audit trail that was closely monitored by the site project leader |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not feasible to blind participants in the intervention group from the purpose of the intervention. Also, personnel may have become aware of the participant's allocation (e.g. through medical record), which may have influenced their treatment of that participant. The study did employ strict protocols in order to attempt to reduce the risk of differential behaviour by participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All follow‐up staff were blinded to group assignment" at 3 months in collecting the primary and secondary outcome data. Medical records were abstracted by a nurse blinded to group assignment to determine if it was documented that there was an IPV screen or diagnosis |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 32/199 (16.1%) LTFU in the intervention group; 23/200 (11.5%) LTFU in the control group. There is a lack of information about whether or not reasons for withdrawal/loss to follow‐up differed between the groups. However, the researchers indicate "logistic regression of missing data because of attrition demonstrated no significant associations with variables associated with the primary outcome measure, supporting their being missing completely at random" |
| Selective reporting (reporting bias) | High risk | There is no reference to a trial protocol. However, the trial was registered (ACTRN12607000210471) and some pre‐selected outcomes (e.g. SF‐12) were not reported here |
| Other bias | Low risk | No evidence of contamination, measures are valid and reliable but some baseline differences reported. "There were some potentially important group differences: compared with women in the usual care group, women in the treatment group were somewhat older (42 versus 38.5); more likely to be New Zealand European (63% versus 58%) and more likely to have been admitted to hospital (43% versus 36%)." They were also less likely to be poorly educated (with less than secondary school) (17.1% versus 29.5%) but study analysis tested and adjusted for baseline differences. "Age and ethnicity were individually associated with violence in the follow‐up period and included in the final model as design effects caused by differences at baseline... the final best subset model included measures of socioeconomic status... Hosmer and Lemeshow test statistic was NS" |
MacMillan 2006.
| Methods | Study design: quasi‐randomised controlled trial Randomisation method: randomised clinic days or shifts Power calculation: yes Study dates: from May 2004 to January 2005 |
|
| Participants | Setting: 2 EDs, family practices, and women's health clinics Country: Canada Inclusion criteria: women were eligible for participation if they were: (1) 18 to 64 years old, (2) at the site for their own healthcare visit, (3) able to separate themselves from individuals who accompanied them, (4) able to speak and read English, (5) able to provide informed consent Exclusion criteria: too ill to participate Number (%) of eligible recruited: 2461/2602 (94.4%) Numbers assigned: 2461 intervention group 853, control group one 769, control group two 839 Number of dropouts (varied by screening tool): intervention group 3.7% to 5.2%, control group one 3.5% to 5.7%, control group two 1.5% to 3.0% Numbers analysed (varied by screening tool): intervention group 788, control group one 741, control group two 810 (CAS); intervention group 404, control group one 725, control group two 814 (PVS); intervention group 411, control group one 742, control group two 826 (WAST) Marital status: single/never married 41% Ethnicity: born outside Canada 11% Employment: working full‐ or part‐time 52% Income: annual income < CAD 25,000 47% Education: achieved education > 14 years 52% Children: ≥ 1 child at home 52% |
|
| Interventions | Intervention group
Control groups
|
|
| Outcomes | Primary outcomes
|
|
| Notes | Funding: the Ontario Women's Health Council (Ontario Ministry of Health and Long‐term Care). Authors MacMillan and Wathen held Canadian Institutes of Health Research grants/fellowships; Dr Boyle held a Canada Research Chair in the Social Determinants of Child Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number table was used to assign clinic shifts |
| Allocation concealment (selection bias) | High risk | "The research coordinator created calendars that informed site coordinators of the assignments." There is, therefore, a risk that advance awareness of shift/day allocations may have introduced selection bias in intervention assignment by not protecting the allocation sequence before and until assignment, for example, recruiters appear to have had knowledge of the allocation prior to inviting individuals into the study, which could have influenced their behaviours differentially |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study did not specify a control condition and it was not feasible to blind participants from the method of screening they would receive. While any impact of non‐blinding on performance was likely to have been distributed similarly across the written and computerised groups (who were told their HCPs would be unaware of their responses), it may have influenced the performance of participants in the face‐to‐face arm since their providers conducted the screening and therefore "would necessarily be aware of women's responses." In this arm, it was also not feasible to blind personnel to the allocation following assignment as they would have been informed by the recruiter of the woman's participation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment was unable to be blinded and based on women's responses to the screening instruments, self completion of the CAS, and their evaluation of the method. It was therefore subjective, although the extent of any systematic differences in responses is likely to be randomly distributed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data collection was conducted immediately following the treatment. Although there was slightly higher attrition (4%) in the face‐to‐face arm of the trial, overall attrition was low at 5%. Reasons for missing data were not supplied |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported. Trial was registered (NCT00336297) |
| Other bias | High risk | A higher proportion of women in the computer group were from the lowest income quintile and may have been more likely to both be abused and to disclose by computer |
MacMillan 2009.
| Methods | Study design: randomised controlled trial Randomisation method: a table for each day/shift of the week was created for an 8‐week period and a random number table was used to determine the order of weeks 1 through 8 in the cells Power calculation: yes Study dates: from July 2005 to December 2006. Individual women were each followed up for 18 months, starting in July 2005 and ending in July 2008 |
|
| Participants | Setting: 12 primary care sites (family practices and community health centres), 11 acute care sites (EDs) and 3 speciality care sites (obstetrics/gynaecology) Country: Canada Inclusion criteria: women aged 18 to 64 years, had a male partner at some time in the last 12 months, presented for their own healthcare visit, able to separate themselves from individuals who accompanied them, were living with 120 km of the site, were able to speak and read English, and able to provide informed consent Exclusion criteria: too ill to participate Number (%) of eligible recruited: 6743/8293 (81.3%) Numbers assigned: 6743; intervention group 3271, control group 3472 Number (%) of assigned that completed all healthcare visit questionnaires: 5681/6743 (84.3%); intervention group 2733, control group 2948 Number (%) with positive results and followed up: 707 (12.4%); intervention group 347, control group 360 Number of dropouts: intervention group 48, control group 148 Numbers analysed (and % with positive result) 411; intervention group 199 (57%), control group 212 (59%) *Age: intervention group mean 33.8 years (SD 10.8), control group mean 33.9 years (SD 10.7) Marital status: single/never married 41% Ethnicity: born outside Canada 11% Employment: working full‐ or part‐time 52% Income: annual income < CAD 25,000 47% Education: intervention group mean 13.7 years (SD 2.8), control group mean 13.5 years (SD 2.8) Children: ≥ 1 child at home 52% |
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Primary outcomes
Timing of measurement: followed up baseline (< 14 days), 6, 12, 18 months post‐intervention (collected through self report by women) We obtained clarification about the number of participants who discussed abuse with their provider (MacMillan 2015 [pers comm]) |
|
| Notes | *Characteristics of participants are provided for the 707 women who had positive results for IPV in last 12 months. Age and education details for the group were obtained through personal communication (MacMillan 2011 [pers comm]) Funding: the Ontario Women's Health Council (Ontario Ministry of Health and Long‐Term Care) with investigator grants from Canadian Institutes of Health Research. Author Boyle held a Canada Research Chair in the Social Determinants of Child Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by day or shift. "A table for each day of the week was created for an 8‐week period, and a random number table was used to determine the order of weeks 1 through 8 in the cells." This suggests there was balance across shifts and days of the week, and that systematic differences in presentation by day or shift were avoided |
| Allocation concealment (selection bias) | High risk | "The research coordinator created monthly calendars showing shift allocations for site coordinators." There is, therefore, a risk that advance awareness of shift/day allocations may have introduced selection bias in intervention assignment by not protecting the allocation sequence before and until assignment. For example, recruiters would likely have had knowledge of the allocation prior to inviting individuals into the study, which could have influenced their behaviours differentially |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to protect the allocation sequence after assignment given that participants "were told that they might be asked questions about their relationships by completing a form that may be passed on during this visit to the clinician, who might discuss their situation in more detail." Thus, participants may have had awareness that they were receiving an intervention (or not), which could have affected their performance. It was also not feasible to blind personnel to the allocation following assignment as they would have been prompted by the questionnaire placed in the patient record |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Interviewers blinded to group assignment met with women within 14 days of the index visit to conduct a baseline interview and again at 6, 12, and 18 months" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Participant loss to follow‐up was high but evenly balanced: 43% (148/347) in screened women and 41% (148/360) in non‐screened women" over 18 months making a true ITT analysis difficult. It was noted by authors that women in the screened group who were LTFU reported higher scores on the WAST and CAS. Such differences between retained and lost were not observed in the non‐screened group. Thus, there is a possibility that the observed effect estimate is biased. In contrast, there were no group differences in proportions lost, or reasons for dropout, although those LTFU in the intervention group were more likely to be more severely abused. To deal with missing data, average growth measures were estimated from 5 complete files generated through multiple imputation to test the robustness of the observed findings for all enrolled women |
| Selective reporting (reporting bias) | Low risk | All outcomes for all time points reported. Trial was registered (NCT00182468) |
| Other bias | High risk | Protection against contamination: sites involved both screening (intervention group) and non‐screening (control group) shifts/days and therefore there is a risk that those who were in the control group could have received care that was influenced by physicians' prior experience of delivering the intervention |
Rhodes 2002.
| Methods | Study design: quasi‐randomised controlled trial Randomisation method: alternate allocation of individual patients Power calculation: none reported Study dates: none reported |
|
| Participants | Setting: 1 urban university hospital emergency department Country: USA Inclusion criteria: English‐speaking women and men, aged 18 to 65 years, who presented for emergency care with a non‐urgent complaint, and triaged into lowest 2 categories of 5‐level system Exclusion criteria: those in pain, blind, overtly psychotic, or unable to read Number (%) of eligible recruited: 470/542 (86.7%) of which 322 (68.5%) were female Numbers (of women) assigned: intervention group 170, control group 152 Number of dropouts: 20% of charts were missing, differences by arm unspecified Numbers analysed (by groups into which they were allocated): intervention group 170, control group 152 Age: mean (women) 33 years (intervention group), 41 years (control group) Marital status (men and women): married 19% (intervention group), 27% (control group); single 60% (intervention group), 58% (control group); widowed/separated or divorced 21% (intervention group), 15% (control group) Ethnicity (all patients): Black; intervention group 91%, control group 90% Insurance status (all patients): Medicaid, intervention group 37%, control group 40%; Medicare, intervention group 17%, control group 19%; private, intervention group 34%, control group 27%; none, intervention group 12%, control group 14% Reason for visit (all patients): medical, intervention group 50%, control group 58%; injury, intervention group 27%, control group 23%; gynaecologic or urinary, intervention group 20%, control group 18%; other, intervention group 3%, control group 1% |
|
| Interventions | Intervention group
Control group
|
|
| Outcomes |
This study also examined other psychosocial risks for both victimisation and perpetration |
|
| Notes | Funding: the Chicago Community Trust (#6‐35467), the Robert Wood Johnson Clinical Scholars Program, and the Section of Emergency Medicine, University of Chicago | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation method: "Patients were alternately assigned to a computer‐based intervention or usual care." This method is open to selection bias and there is inadequate description of protection from such bias |
| Allocation concealment (selection bias) | Unclear risk | Not all eligible patients were enrolled due to limited computer availability. Furthermore, "to avoid selection bias, when the computer was available, the patient to be recruited was the one most recently arrived and assigned as non‐urgent at triage." This method remains fallible to bias, but it is unclear whether it would have biased selection |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Results of screening were attached to the patient file in order to alert the treating physician to psychosocial issues as part of the intervention. This meant that the treating physician was also made aware that the patient was in the intervention group. There was therefore a high risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Chart reviewers were blinded to whether a patient had participated in the computer screening and whether these results were shared with the treating physician and were blinded to the assessment of the other chart reviewer" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Findings are based on a review of 80% of charts. The percentage did not vary by whether the patient had received computer screening" ‐ but detailed figures of and reasons for the missing 20% are not given |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered so we were unable to check the selected outcomes, but as it is a screening trial, identification, documentation and information‐giving are expected outcome measures |
| Other bias | High risk | Characteristics of participants both male and female were evenly distributed across intervention and control groups, but it is unclear how this applied to females. There is a high risk of contamination as the participants were screened or not screened alternately and then saw their physician at the 1 clinic visit, with physicians seeing both intervention and control participants |
Rhodes 2006.
| Methods | Study design: randomised controlled trial Randomisation method: consenting women were randomly assigned in a 1:1 ratio. Treatment assignment was ascertained by the research assistant by opening sealed randomisation envelopes in sequential order. The envelopes were prepared from a randomisation list generated by computer in blocks of size 10 to ensure balance between groups over short time spans such as shifts and days of the week as well as over the entire course of the study Power calculation: no Study dates: from June 2001 to December 2002 |
|
| Participants | Setting: 2 socio‐economically diverse EDs – an urban academic medical centre serving mainly publicly insured inner city African‐American population and a suburban community hospital serving a privately insured suburban white population Country: USA Inclusion criteria: consenting women, aged 18 to 65 years, triaged as medically non‐emergent Exclusion criteria: none stated Number (%) of eligible recruited: 1281/2165 (59.2%) Numbers recruited: 1281; intervention group 637, control group 644 Number of dropouts: intervention group 216, control group 194 Numbers analysed (% recruited): 871; intervention group 421 (66.1%), control group 450 (70%) (based on audio‐recording data) Age: mean 33.3 years (SD 12 years) Marital status: married 21%, single 45%, divorced/separated/widowed 13%, unknown 21% Ethnicity: African‐American 60%, white 29%, other 7%, unknown 4% Socioeconomic status: < USD 20,000 40%, USD 20,000 to 39,999 24%, USD 40,000 to 79,999 16%, ≥ USD 80,000 8% Education: 1 < high school diploma 10%, high school or equivalent 18%, > high school 48%, unknown 24% Positive IPV screen result on exit questionnaire: 218/903 (24%); urban 151/578 (26%), suburban 67/325 (20.6%) |
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Primary outcomes
Data were collected through audio‐recording of consultations (primary method). Data were also abstracted from medical records and collected directly from participants |
|
| Notes | Funding: Grant RO1 HS 11096‐03 from the Agency for Healthcare Research and Quality. Dr Rhodes was also supported by grant K23/ MH64572 from the National Institute of Mental Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation for participating patients was generated by "computer in blocks of size 10 to ensure balance between groups over short time spans, such as shifts and days of the week, as well as over the entire course of the study." |
| Allocation concealment (selection bias) | Low risk | Women were recruited and consented prior to the "research assistant opening sealed randomization envelopes in sequential order." "Consenting patients were then randomly assigned" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Providers were not blinded to the purpose of the study or the intervention: "health care providers were informed that the study objective was to study the effect of a computer prompt on IPV communication and were encouraged to screen all women for abuse." However, this was unlikely to have led to benefits extraneous to the intended effect of the intervention for women in the intervention group; the outcome of interest is unlikely to have been influenced by lack of blinding. Women were blinded to the purpose of the study being told it was a "study of physician‐patient communication." Women in the intervention group may have realised that the computer‐based health risk assessment was part of the intervention thus influencing how they behaved. However, we would not expect that the outcome would have been influenced by this incomplete blinding. For example, changes in women's behaviour such as being more encouraged to discuss IPV with the HCP would not differ from what would be expected to arise from the intervention. Lack of blinding in the situation where participants in the control group inadvertently became aware of the intervention through interactions with other women or staff could conceivably have influenced the outcome. We are not given sufficient information about the degree of awareness of other staff regarding women's allocations, which could have influenced their interactions with women and, therefore, the outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | We are not told about who edited the audio‐recordings of the ED visits "a 7‐hour ED visit might be edited to 20 minutes of actual health care provider–patient interaction"; it would have been important for them to be blinded as knowing the allocation could have affected the editing process. Although research assistants who were to undertake the primary data collection via coding of audio‐recordings of both intervention and control group consultations were said to be blinded, the allocation of participants could have been revealed during the remaining audio data and thereby influenced coders' interpretation of what they heard. It is also not known if the person who edited differed from the coders. If the coder was also the editor then it would have increased the likelihood that the allocation of the participant would have become known. "Charts of all enrolled patients were coded using a structured chart abstraction form to assess evidence of DV documentation;" however, there is no indication of blinding of assessors. It is likely that the allocations of women in the intervention group were quite evident by virtue of presence of the IPV risk report and it is unclear if presence of a report was considered different to other documentation of IPV. Finally, both groups of women self completed an exit survey and were not blinded; however, any effect was likely equal in both groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 21/101 (21%) providers did not consent to having their consultations recorded and thus there was incomplete outcome data for their participants. However, this lack of recording should have been equal in both groups since providers were seeing participants in both the intervention and the control groups. The overall attrition of participants was 32% and we are not given clear information about the extent to which the providers' refusal to audio‐record sessions accounted for this rate (i.e. what proportion of patients declined the audio‐recording post‐randomisation). While the attrition levels in audio‐recording appear balanced across the two groups: 216/637 (intervention group 34%), 194/644 (control group 30.1%), there was no sensitivity analysis included in the report to ascertain the impact of those missing data on the robustness of the effect. Attrition rates on chart review were similarly spread and low at 8%, and moderately high but spread on the exit survey. There is a lack of information on reasons for these (albeit low) attrition rates in the chart review. There were four cases in the control group that appear in the participant flowchart but are absent from the observed rates in Table 3 |
| Selective reporting (reporting bias) | Unclear risk | Data on medical records were not furnished except that it is indicated in the text that there was no difference between groups on the documentation of IPV. No reference to a trial protocol and thus no confirmation that the original trial aims and primary outcomes were as reported here |
| Other bias | High risk | Protection against contamination: the same providers delivered the intervention or usual care to participants. While they should have remained unaware of who the participants were in the control group, their experience of consulting with participants in the intervention group could have influenced their performance with the participants in the control group |
Trautman 2007.
| Methods | Study design: quasi‐experimental control study Randomisation method: there were 3 distinct consecutive 2‐week enrolment periods. In the second enrolment period all eligible women who presented to the ED were assigned to the intervention group. During the first and third enrolment periods all eligible presenting women were allocated to a TCG Power calculation: yes Study dates: enrollment occurred between April and May 2003 |
|
| Participants | Setting: adult urban ED of a large university hospital serving a primarily socio‐economically disadvantaged, minority population Country: USA Inclusion criteria: women aged ≥ 18 years who presented to the ED for medical treatment Exclusion criteria: acute or critically ill presentation, illiteracy, impaired mental status, disorientation or apparent intoxication, would not separate from their partner; or already enrolled Numbers (%) of eligible recruited: 1005/1395 (72%) Numbers recruited: 1005; intervention group 411, control group 594 Number of dropouts: intervention group 0, control group 0 Numbers analysed (% recruited): 1005; intervention group 411 (100%), control group 594 (100%) Age range (years): 18 to 24 years 22.9%, 25 to 34 years 23.3%, 35 to 54 years 41.4%, ≥ 55 years 12.4% Marital status: married/living with partner 20%, never married 53.8%, divorced/separated 21%, widowed 5.2% Ethnicity: White 16.1%, Non‐white 83.9% Socioeconomic status (annual household income): < USD 10,000 42.4%, USD 10,000 to 15,999 20.6%, USD 16,000 to 20,999 12.2%, USD 21,000 to 35,999 14.8%, ≥ USD 36,000 10% Education: < high school 30.5%, high school or equivalent 42.3%, > high school 27.2% Children in household: yes 50.9% |
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Primary outcomes
Timing of measurement: immediate abstraction of data from medical records |
|
| Notes | Funding: no external funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | During 3 distinct, consecutive, 2‐week enrolment periods, all eligible women were asked to complete a computer‐based health survey. During the first and third enrolment periods, the computer‐based health survey did not include any IPV items. During the second enrolment period, it did include IPV screening items. It is likely that this type of allocation process introduced a high risk of bias due to systematic differences between the intervention group and the control group |
| Allocation concealment (selection bias) | High risk | Patient service co‐ordinators recruited and obtained consent from participants. There was no blinding of recruiters to the potential allocation of women as the allocation for the period during which women presented to the ED was defined in advance and not concealed in any way explained in the report. Therefore, awareness of the allocation could have influenced how women were recruited. Furthermore, the experimental allocation for that period may have become inadvertently known to some women through interactions with other participants and staff influencing their decision to participate or not |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women approached were told that this was a study about women's health whereby they would be "asked to answer questions about themselves on a computer and to allow their medical record to be reviewed by study personnel." Thus, there was some blinding of women to the purpose of the study. However, healthcare personnel were unblinded as "the medical records of all subjects were attached by coordinators to participants medical records to alert treating staff" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated that research assistant was unblinded as "the medical records of all subjects were reviewed by a research assistant to determine whether there was any documentation in the record" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts although we acknowledge that it was unclear how trialists dealt with missing data within variables |
| Selective reporting (reporting bias) | Unclear risk | There was no reference to a study protocol and therefore insufficient information to permit a judgement of 'low' or 'high' risk of bias |
| Other bias | High risk | Protection against contamination: the process of the providers consulting with women in the control group in the third, 2‐week block following the 2‐week intervention group block could have contaminated their interactions with participants in the control group. In fact, the authors state, "Three study periods were used to determine whether usual care related to intimate partner violence would return to baseline (i.e. first enrolment period) in the third enrolment period when the intimate partner violence questions were removed or whether it would be higher as a result of the computerized intimate partner violence screening during the second study period". There is some risk that an insensitive instrument was used to measure referrals with referrals applying to social workers only (which could have led to an underestimate of physician referring) |
A‐CASI: audio computer‐assisted self interviews; ALPHA: Antenatal Psychosocial Health Assessment; CAS: Composite Abuse Scale; CASI: computer‐assisted screening interview; CES‐D: Centre for Epidemiological Studies ‐ Depression; ED: emergency department; FTFI: face‐to‐face interview screening; HCP: healthcare professional; IPV: intimate partner violence; ITT: intention‐to‐treat; LTFU: loss to follow‐up; NS: non‐significant; PVS: Partner Violence Screen; CTS2S: Revised Conflict Tactics Scale‐Short Form; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; SF: Short Form; TCG: treatment control group; VAWS: Violence Against Women Scale; WAST: Woman Abuse Screening Tool; WIC: Women, Infants, Children's (clinic)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bair‐Merritt 2006 | Screening results not passed on to healthcare professional |
| Beatty 2014 | Screening results not passed on to healthcare professional |
| Bonds 2006 | Not a randomised or quasi‐random method |
| Brienza 2005 | Educational intervention targeted to clinicians with no data on women |
| Campbell 2001 | Case‐finding not screening |
| Chen 2007 | No usual care group comparison |
| Coonrod 2000 | Educational intervention targeted to clinicians with no data on women |
| Cripe 2010 | Intervention exceeded 'brief' intervention |
| Curry 2006 | Intervention exceeded 'brief' intervention |
| Dubowitz 2011 | Intervention targeted to children and clinicians |
| Dubowitz 2012 | Intervention targeted to children and clinicians |
| Duggan 2004 | Intervention exceeded 'brief' intervention |
| Ernst 2007 | No usual care group comparison |
| Feder 2011 | Case‐finding not screening trial |
| Feigelman 2011 | Intervention targeted to children and clinicians |
| Fernández Alonso 2006 | Educational intervention targeted to clinicians with no data on women |
| Florsheim 2011 | Intervention exceeded 'brief' intervention |
| Furbee 1998 | Not a randomised or quasi‐random method |
| Garg 2007 | Participant data included both sexes and could not be disaggregated |
| Gillum 2009 | Intervention exceeded 'brief' intervention |
| Green 2005 | Intervention exceeded 'brief' intervention |
| Halpern 2009 | Not a randomised or quasi‐randomised method |
| Hegarty 2013 | Intervention exceeded 'brief' intervention |
| Hewitt 2011 | Not a randomised or quasi‐randomised method |
| Hoelle 2014 | Screening results not passed on to healthcare professional |
| Hollander 2001 | Usual care included screening results given to healthcare professional |
| Houry 2011 | Screening results not passed on to healthcare professional |
| Jewkes 2008 | Intervention exceeded 'brief' intervention |
| Kapur 2011 | Not a randomised or quasi‐randomised method |
| Kiely 2010 | Intervention exceeded 'brief' intervention |
| Kiely 2013 | Not a comparison of screening with usual care |
| Klevens 2012b | Screening results not passed on to healthcare professional |
| Knight 2000 | Not a randomised or quasi‐randomised method |
| Larkin 1999 | Not a randomised or quasi‐randomised method |
| Rickert 2009 | No usual care group comparison |
| Robinson‐Whelen 2010 | Not in a healthcare setting |
| Saftlas 2014 | Intervention exceeded 'brief' intervention |
| Subramanian 2012 | Intervention exceeded 'brief' intervention |
| Taft 2011 | Intervention exceeded 'brief' intervention |
| Taft 2012 | Screening result passed on to healthcare professional in both arms |
| Thompson 2000 | Case‐finding not screening |
| Tiwari 2010 | Intervention exceeded 'brief' intervention |
| Wagman 2015 | Intervention exceeded 'brief' intervention |
Characteristics of ongoing studies [ordered by study ID]
NCT01207258.
| Trial name or title | Social Health Intervention Project (SHIP) |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention
Control
|
| Outcomes | Primary outcomes
Timing of measurement: assessed weekly by Interactive Voice Response System for 12 weeks, and at 3, 6, and 12 months by interviewers blinded to group assignment |
| Starting date | Trial is complete and under review |
| Contact information | Karin.rhodes@uphs.upenn.edu |
| Notes | Funding: National Institute on Alcohol Abuse and Alcoholism (NIAAA) Award R01‐AA018705 |
NCT01661504.
| Trial name or title | Evaluating a health care provider delivered intervention to reduce intimate partner violence and mitigate associated health risks: study protocol for a randomized controlled trial in Mexico City |
| Methods | Cluster‐randomised controlled trial |
| Participants | Inclusion criteria
Exclusion criteria: cognitive impairment (e.g. slurred speech), seeking treatment for life‐threatening emergency care, and intending to relocate within 2 years |
| Interventions | Intervention
Control
|
| Outcomes | Primary outcomes
Timing of measurement: surveys conducted at baseline, 3 months, and 15 months from baseline |
| Starting date | Trial was conducted between 2012 and 2015 |
| Contact information | Jhumka Gupta Yale University jhumka.gupta@yale.edu |
| Notes | The study is funded by an anonymous donor. This work was supported, in part, by Yale University's Center for Interdisciplinary Research on AIDS (CIRA), through grants from the National Institute of Mental Health (P30MH062294) |
ED: emergency department; IPV: intimate partner violence.
Differences between protocol and review
1. Altered objective
We made it explicit in the objective for the review that we would also examine the impact of screening in health settings on women's re‐exposure to violence and to determine if screening causes any harms.
2. Altered criterion for assessing inclusion of interventions/comparisons
The treating healthcare professional must have been informed of the result of the screening assessment undertaken at the time of the relevant consultation if they did not conduct the screening themselves face‐to‐face. Essentially, there must be some involvement of a healthcare professional in the intervention arm.
The comparison condition was also considered to determine if the overall comparison was valid for inclusion. Originally we defined the comparison as usual care. We acknowledge, however, that 'treatment‐as‐usual' (TAU) arms may involve some kind of screening technique such as computer or paper‐based screening. Providing that there was no healthcare professional involvement, we considered it to be a comparison consistent with other included studies.
Some studies compared face‐to‐face screening with other techniques of identification, but the way in which identification was operationalised differed from the main body of studies, reflecting prevalence rather than clinical identification.
We excluded interventions where the timing of these consultations went beyond an immediate response and referral phase, and included further counselling or therapeutic sessions as we wanted to isolate the effect of screening only.
3. Amendments to outcomes
We added the outcome below as it has bearing on the potential for beneficial support to women at a later date
G. Services and resource use: i. family/domestic violence services; ii. police/legal services; iii. counselling or therapeutic services; iv. other services.
In this update, we conducted a meta‐analysis on an outcome that was not pre‐specified but represents an alternative definition of 'identification', which was more research‐based than clinical. The methods used to gather data on this outcome were more consistent with prevalence studies. It was necessary to treat this outcome separately to clinical identification given that women may be more inclined to disclose abuse when the enquiry occurs outside the clinical encounter and context. Thus, it would be expected that non‐clinical identification rates would exceed rates of clinical identification, and more closely reflect best estimates of IPV in clinical populations.
4. Search strategy amendment
We were unable to complete the planned handsearching of several journals and we were unable to search 'Domestic Violence Data source' as the webpage was no longer available. However, given the extent of the alternative searching, we believe the likelihood of overlooking an eligible trial was low.
5. Incorporation of a separate review
Although the 2015 update incorporates another review (Coulthard 2010), we have only included studies of screening interventions for women. Screening interventions for men might be addressed in a future review.
Contributions of authors
AT originally developed the search strategy. AT and LOD selected the studies prior to 2009; AT, LOD and KH selected studies for the 2009 to 2012 period and AT and LOD extracted the data; AT undertook the analysis with the help of LOD and KH and drafted the original review. All authors provided topic expertise and contributed to writing and editing the original review. LOD led the 2015 update with guidance from all co‐authors. AT, LOD, and KH independently selected studies from the point at which full‐text articles had been retrieved. Extraction and 'Risk of bias' assessment was done by LOD with Tess Lawrie (not author). All authors provided topic expertise and contributed to writing and editing the update of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
La Trobe University, Australia.
Financial (salary) and technical support
-
UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Provided financial support for the work carried out by Evelina Chapman and Tess Lawrie
Declarations of interest
Lorna O'Doherty ‐ the La Trobe funding went towards contracting Lorna from the University of Melbourne to work on the original Cochrane Review. She is responsible for the 2015 update to the review and conducted the 2015 work within the remit of her current position at Coventry University. The National Health and Medical Research Council (NHMRC) funded her former position as research fellow at the University of Melbourne. She does not have any competing interest to declare. She was first author of an abridged version of the original review published in the BMJ in 2014. Kelsey Hegarty* ‐ has been funded through a NHMRC Grant to undertake a randomised controlled trial in the field of intimate partner violence (IPV) ‐ women's evaluation of abuse and violence care in general practice (WEAVE) trial (Hegarty 2013), which was completed by the time of the 2015 update. Angela Taft, Lorna O'Doherty, and Gene Feder were also involved in the trial. The author team examined the WEAVE trial against inclusion criteria and it was judged as ineligible on the basis that the intervention was too intensive to be considered a screening‐only trial. Kelsey participated in the World Health Organization (WHO) Guideline Group on health practitioners' responses to IPV and received payment from the General Practice Victoria for training provided to general practitioners in how to manage partner violence. Jean Ramsay ‐ none known. Leslie Davidson ‐ National Institutes of Health (NIH) Fogarty International Center paid travel, accommodation, and meeting expenses for Leslie to attend the Society of Neuro Scientists of Africa (SONA) and a NIH workshop in March 2015 in South Africa unrelated to IPV. Gene Feder ‐ Gene chaired the WHO IPV and sexual violence guideline development group. Gene's institution received funds from the National Institute for Health and Care Excellence (NICE) for him to chair the Domestic Violence and Abuse guidelines development group. Gene's institution also receives funds from Safer Lives for his consultancy work and from a National Institute of Health Research applied research programme grant on domestic violence and abuse. Angela Taft* ‐ received an Australian Research Council Grant to undertake a screening trial (Taft 2015). Kelsey Hegarty was also involved in the trial. Its eligibility was considered for the 2015 update. The author team examined the Improving maternal and child health care for vulnerable mothers (MOVE) trial against inclusion criteria and it was judged as ineligible on the basis that screening was conducted by healthcare professionals in both arms of the trial. Kelsey Hegarty* and Angela Taft* were involved in (Taft 2012), which was previously examined by the author team and was judged ineligible for inclusion in the review as it was case‐finding and not a screening trial.
* Kelsey Hegarty and Angela Taft were excluded from making decisions about the trials they led.
Edited (no change to conclusions)
References
References to studies included in this review
Ahmad 2009 {published data only}
- Ahmad F, Hogg‐Johnson S, Stewart DE, Skinner HA, Glazier RH, Levinson W. Computer‐assisted screening for intimate partner violence and control: a randomized trial. Annals of Internal Medicine 2009;151(2):93‐103. [PUBMED: 19487706] [DOI] [PubMed] [Google Scholar]
Carroll 2005 {published and unpublished data}
- Blackmore ER, Carroll JC, Reid AJ, Biringer A, Glazier RH, Midmer D, et al. The use of the Antenatal Psychosocial Health Assessment (ALPHA) tool in the detection of psychosocial risk factors for postpartum depression: a randomized controlled trial. Journal of Obstetrics and Gynaecology Canada 2006;28(10):873‐8. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Reid AJ, Biringer A, Midmer D, Glazier RH, Wilson L, et al. Effectiveness of the Antenatal Psychosocial Health Assessment (ALPHA) form in detecting psychosocial concerns: a randomized controlled trial. Canadian Medical Association Journal 2005;173(3):253‐9. [PUBMED: 16076821] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fincher 2015 {published and unpublished data}
- Fincher D, VanderEnde K, Colbert K, Houry D, Smith LS, Yount KM. Effect of face‐to‐face interview versus computer‐assisted self‐interview on disclosure of intimate partner violence among African American women in WIC clinics. Journal of Interpersonal Violence 2015;30(5):818‐38. [DOI: 10.1177/0886260514536280] [DOI] [PubMed] [Google Scholar]
Fraga 2014 {published and unpublished data}
- Fraga S, Lucas R, Barros H. A comparison of methods to assess intimate partner abuse 1 year postpartum. Annals of Epidemiology 2014;24(5):404‐6. [DOI: 10.1016/j.annepidem.2014.02.005] [DOI] [PubMed] [Google Scholar]
- Rodrigues T, Rocha L, Barros H. Physical abuse during pregnancy and preterm delivery. American Journal of Obstetrics and Gynecology 2008;198(2):171.e1‐6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Humphreys 2011 {published and unpublished data}
- Calderón SH, Gilbert P, Jackson R, Kohn MA, Gerbert B. Cueing prenatal providers: effects on discussions of intimate partner violence. American Journal of Preventive Medicine 2008;34(2):134‐7. [DOI: 10.1016/j.amepre.2007.09.029] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys J, Tsoh JY, Kohn MA, Gerbert B. Increasing discussions of intimate partner violence in prenatal care using Video Doctor plus Provider Cueing: a randomized, controlled trial. Women's Health Issues 2011;21(2):136‐44. [PUBMED: 21185737] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kataoka 2010 {published and unpublished data}
- Kataoka Y, Yaju Y, Eto H, Horiuchi S. Self‐administered questionnaire versus interview as a screening method for intimate partner violence in the prenatal setting in Japan: a randomised controlled trial. BMC Pregnancy and Childbirth 2010;10(84):1‐7. [PUBMED: 21182802] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klevens 2012a {published data only}
- Klevens J, Sadowski L, Kee R, Trick W, Garcia D. Comparison of screening and referral strategies for exposure to partner violence. Women's Health Issues 2012;22(1):e45‐52. [PUBMED: 21798763 ] [DOI] [PubMed] [Google Scholar]
Koziol‐McLain 2010 {published and unpublished data}
- Koziol‐McLain J, Garrett N, Fanslow J, Hassall I, Dobbs T, Henare‐Toka TA, et al. A randomized controlled trial of a brief emergency department intimate partner violence screening intervention. Annals of Emergency Medicine 2010;56(4):413‐23. [PUBMED: 20538369] [DOI] [PubMed] [Google Scholar]
MacMillan 2006 {published and unpublished data}
- MacMillan HL, Wathen CN, Jamieson E, Boyle M, McNutt LA, Worster A, et al. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA 2006;296(5):530‐6. [PUBMED: 16882959] [DOI] [PubMed] [Google Scholar]
MacMillan 2009 {published and unpublished data}
- MacMillan HL, Wathen CN, Jamieson E, Boyle MH, Shannon HS, Ford‐Gilboe M, et al. Screening for intimate partner violence in health care settings: a randomized trial. JAMA 2009;302(5):493‐501. [DOI] [PubMed] [Google Scholar]
Rhodes 2002 {published data only}
- Rhodes KV, Lauderdale DS, He T, Howes DS, Levinson W. 'Between me and the computer': increased detection of intimate partner violence using a computer questionnaire. Annals of Emergency Medicine 2002;40(5):476‐84. [PUBMED: 12399790] [DOI] [PubMed] [Google Scholar]
- Rhodes KV, Lauderdale DS, Stocking CB, Howes DS, Roizen MF, Levinson W. Better health while you wait: a controlled trial of a computer‐based intervention for screening and health promotion in the emergency department. Annals of Emergency Medicine 2001;37(3):284‐91. [DOI: 10.1067/mem.2001.110818] [DOI] [PubMed] [Google Scholar]
Rhodes 2006 {published and unpublished data}
- Rhodes KV, Drum M, Anliker E, Frankel RM, Howes DS, Levinson W. Lowering the threshold for discussions of domestic violence: a randomized controlled trial of computer screening. Archives of Internal Medicine 2006;166(10):1107‐14. [PUBMED: 16717173] [DOI] [PubMed] [Google Scholar]
Trautman 2007 {published and unpublished data}
- Trautman DE, McCarthy ML, Miller N, Campbell JC, Kelen GD. Intimate partner violence and emergency department screening: computerized screening versus usual care. Annals of Emergency Medicine 2007;49(4):526‐34. [PUBMED: 17276547] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bair‐Merritt 2006 {published data only}
- Bair‐Merritt MH, Feudtner C, Mollen CJ, Winters S, Blackstone M, Fein JA. Screening for intimate partner violence using an audiotape questionnaire: a randomized clinical trial in a pediatric emergency department. Archives of Pediatrics & Adolescent Medicine 2006;160(2):311‐6. [PUBMED: 16520452] [DOI] [PubMed] [Google Scholar]
Beatty 2014 {published data only}
- Beatty JR, Chase SK, Ondersma SJ. A randomized study of the effect of anonymity, quasi‐anonymity, and Certificates of Confidentiality on postpartum women's disclosure of sensitive information. Drug and Alcohol Dependence 2014;134:280‐4. [DOI: 10.1016/j.drugalcdep.2013.10.016] [DOI] [PubMed] [Google Scholar]
Bonds 2006 {published data only}
- Bonds DE, Ellis SD, Weeks E, Palla SL, Lichstein P. A practice‐centred intervention to increase screening for domestic violence in primary care practices. BMC Family Practice 2006;7(63):1‐8. [DOI: 10.1186/1471-2296-7-63] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brienza 2005 {published data only}
- Brienza RS, Whitman L, Ladouceur L, Green ML. Evaluation of a women's safe shelter experience to teach internal medicine residents about intimate partner violence: a randomized controlled trial. Journal of General Internal Medicine 2005;20(6):536‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2001 {published data only}
- Campbell JC, Coben JH, McLoughlin E, Dearwater S, Nah G, Glass N, et al. An evaluation of a system‐change training model to improve emergency department response to battered women. Academic Emergency Medicine 2001;8(2):131‐8. [PUBMED: 11157288] [DOI] [PubMed] [Google Scholar]
Chen 2007 {published data only}
- Chen P‐H, Rovi S, Washington J, Jacobs A, Vega M, Pan K‐Y, et al. Randomized comparison of 3 methods to screen for domestic violence in family practice. Annals of Family Medicine 2007;5(5):430‐5. [DOI: 10.1370/afm.716] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coonrod 2000 {published data only}
- Coonrod DV, Bay RC, Rowley BD, Mar NB, Gabriele L, Tessman TD, et al. A randomized controlled study of brief interventions to teach residents about domestic violence. Academic Medicine 2000;75(1):55‐7. [PUBMED: 10667876] [DOI] [PubMed] [Google Scholar]
Cripe 2010 {published data only}
- Cripe SM, Sanchez SE, Sanchez E, Quintanilla BA, Alarcon CH, Gelaye B, et al. Intimate partner violence during pregnancy: a pilot intervention program in Lima, Peru. Journal of Interpersonal Violence 2010;25(11):2054‐76. [DOI: 10.1177/0886260509354517] [DOI] [PMC free article] [PubMed] [Google Scholar]
Curry 2006 {published data only}
- Curry MA, Durham L, Bullock L, Bloom T, Davis J. Nurse case management for pregnant women experiencing or at risk for abuse. Journal of Obstetric, Gynecologic & Neonatal Nursing 2006;35(2):181‐92. [PUBMED: 16620243] [DOI] [PubMed] [Google Scholar]
Dubowitz 2011 {published data only}
- Dubowitz H, Lane WG, Semiatin JN, Magder LS, Venepally M, Jans M. The Safe Environment for Every Kid model: impact on paediatric primary care professionals. Pediatrics 2011;127(4):e962‐70. [DOI: 10.1542/peds.2010-1845] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dubowitz 2012 {published data only}
- Dubowitz H, Lane WG, Semiatin JN, Magder LS. The SEEK model of pediatric primary care: can child maltreatment be prevented in a low‐risk population?. Academic Pediatrics 2012;12(4):259‐68. [PUBMED: 22658954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duggan 2004 {published data only}
- Duggan A, Fuddy L, Burrell L, Higman SM, McFarlane E, Windhma A, et al. Randomized trial of a statewide home visiting program to prevent child abuse: impact in reducing parental risk factors. Child Abuse & Neglect 2004;28(6):623‐43. [PUBMED: 15193852] [DOI] [PubMed] [Google Scholar]
Ernst 2007 {published data only}
- Ernst A, Weiss S, Goldstein L, Hall J, Clark R. Computer versus paper format for intimate partner violence (IPV) screening. Academic Emergency Medicine. 2007 SAEM Annual Meeting Absracts 2007;14(5 Suppl s1):S44. [Google Scholar]
Feder 2011 {published data only}
- Feder G, Agnew Davies R, Baird K, Dunne D, Eldridge S, Griffiths C, et al. Identification and Referral to Improve Safety (IRIS) of women experiencing domestic violence with a primary care training and support programme: a cluster randomised controlled trial. Lancet 2011;378(9805):1788‐95. [DOI: 10.1016/S0140-6736(11)61179-3] [DOI] [PubMed] [Google Scholar]
Feigelman 2011 {published data only}
- Feigelman S, Dubowitz H, Lane W, Grube L, Kim J. Training paediatric residents in a primary care clinic to help address psychosocial problems and prevent child maltreatment. Academic Paediatrics 2011;11(6):474‐80. [PUBMED: 21959095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernández Alonso 2006 {published data only}
- Fernández Alonso, Herrero Velázquez S, Cordero Guevara JA, Maderuelo Fernández JA, González Castro ML, Grupo ISFVIDAP. Protocol to evaluate the effectiveness of a consciousness‐raising and training intervention for primary care professionals, in order to improve detection of domestic violence. Atencion Primaria 2006;38(3):168‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Florsheim 2011 {published data only}
- Florsheim P, McArthur L, Hudak C, Heavin S, Burrow‐Sanchez J. The Young Parenthood Program: preventing intimate partner violence between adolescent mothers and young fathers. Journal of Couple & Relationship Therapy 2011;10(2):117‐34. [DOI: 10.1080/15332691.2011.562823] [DOI] [Google Scholar]
Furbee 1998 {published data only}
- Furbee PM, Sikora R, Williams JM, Derk SJ. Comparison of domestic violence screening methods: a pilot study. Annals of Emergency Medicine 1998;31(4):495‐501. [DOI] [PubMed] [Google Scholar]
Garg 2007 {published data only}
- Garg A, Butz AM, Dworkin PH, Lewis RA, Thompson RE, Serwint JR. Improving the management of family psychosocial problems at low‐income children's well child visits: the WE CARE Project. Pediatrics 2007;120(3):547‐58. [DOI: 10.1542/peds.2007-0398] [DOI] [PubMed] [Google Scholar]
Gillum 2009 {published data only}
- Gillum TL, Sun CJ, Woods AB. Can a health clinic‐based intervention increase safety in abused women? Results from a pilot study. Journal of Women's Health 2009;18(8):1259‐64. [PUBMED: 19627223] [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2005 {published data only}
- Green CEJ. Intimate Partner Violence and Health Care Utilisation: A Randomised Controlled Trial [thesis]. Houston: University of Houston, 2005. [Google Scholar]
Halpern 2009 {published data only}
- Halpern LR, Parry BA, Hayward G, Peak D, Dodson TB. A comparison of 2 protocols to detect intimate partner violence. Journal of Oral and Maxillofacial Surgery 2009;67(7):1453‐9. [DOI: 10.1016/j.joms.2009.03.003] [DOI] [PubMed] [Google Scholar]
Hegarty 2013 {published data only}
- Hegarty K, O'Doherty L, Taft A, Chondros P, Brown S, Valpied J, et al. Screening and counselling in the primary care setting for women who have experienced intimate partner violence (WEAVE): a cluster randomised controlled trial. Lancet 2013;382(9888):249‐58. [DOI: 10.1016/S0140-6736(13)60052-5] [DOI] [PubMed] [Google Scholar]
Hewitt 2011 {published data only}
- Hewitt LN, Bhavsar P, Phelan HA. The secrets women keep: intimate partner violence screening in the female trauma patient. Journal of Trauma, Injury, Infection and Critical Care 2011;70(2):320‐3. [PUBMED: 21307728] [DOI] [PubMed] [Google Scholar]
Hoelle 2014 {published data only}
- Hoelle RM, Weeks E, Sellers S, Carden D. A comparison of two brief intimate partner violence screening tools in the emergency department. Academic Emergency Medicine. Proceedings of the Annual Meeting of the Society for Academic Emergency Medicine; 2014 May 14‐17; Dallas, (TX), United States. 2014; Vol. 21 (Suppl s1):S135‐6. [DOI: 10.1111/acem.12365] [DOI]
Hollander 2001 {published data only}
- Hollander JE, Schears RM, Shofer FS, Baren JM, Moretti LM, Datner EM. The effect of written informed consent on detection of violence in the home. Academic Emergency Medicine 2001;8(10):974‐9. [PUBMED: 11581084] [DOI] [PubMed] [Google Scholar]
Houry 2011 {published data only}
- Houry D, Hankin A, Daugherty J, Smith LS, Kaslow N. Clinical study: effect of a targeted women's health intervention in an inner‐city emergency department. Emergency Medicine International 2011;2011:1‐7. [DOI: 10.1155/2011/543493] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jewkes 2008 {published data only}
- Jewkes R, Nduna M, Levin J, Jama N, Dunkle K, Puren A, et al. Impact of Stepping Stones on incidence of HIV and HSV‐2 and sexual behaviour in rural South Africa: a cluster randomised controlled trial. BMJ 2008;337:a506. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kapur 2011 {published data only}
- Kapur NA, Windish DM. Optimal methods to screen men and women for intimate partner violence: results from an internal medicine residency continuity clinic. Journal of Interpersonal Violence 2011;26(12):2335‐52. [PUBMED: 21712340] [DOI] [PubMed] [Google Scholar]
Kiely 2010 {published data only}
- Kiely M, El‐Mohandes AAE, El‐Khorazaty MN, Gantz MG . An integrated intervention to reduce intimate partner violence in pregnancy. Obstetrics & Gynecology 2010;115(2 Pt 1):273‐83. [PUBMED: 20093899] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiely 2013 {published data only}
- Kiely M, Gantz MG, El‐Khorazaty MN, El‐Mohandes AA. Sequential screening for psychosocial and behavioural risk during pregnancy in a population of urban African Americans. British Journal of Obstetrics and Gynaecology 2013;120(11):1395‐402. [DOI: 10.1111/1471-0528.12202] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klevens 2012b {published data only}
- Klevens J, Kee R, Trick W, Garcia D, Angulo FR, Jones R, et al. Effect of screening for partner violence on women's quality of life: a randomized controlled trial. JAMA 2012;308(7):681‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knight 2000 {published data only}
- Knight RA, Remington PL. Training internal medicine residents to screen for domestic violence. Journal of Women's Health & Gender‐Based Medicine 2000;9(2):167‐74. [PUBMED: 10746520] [DOI] [PubMed] [Google Scholar]
Larkin 1999 {published data only}
- Larkin GL, Hyman KB, Mathias SR, D'Amico F, MacLeod BA. Universal screening for intimate partner violence in the emergency department: importance of patient and provider factors. Annals of Emergency Medicine 1999;33(6):669‐75. [PUBMED: 10339682] [DOI] [PubMed] [Google Scholar]
Rickert 2009 {published data only}
- Rickert VI, Davison LL, Breitbart V, Jones K, Palmetto NP, Rottenberg L, et al. A randomized trial of screening for relationship violence in young women. Journal of Adolescent Health 2009;45(2):163‐70. [DOI: 10.1016/j.jadohealth.2008.12.012] [DOI] [PubMed] [Google Scholar]
Robinson‐Whelen 2010 {published data only}
- Robinson‐Whelen S, Hughes RB, Powers LE, Oschwald M, Renker P, Swank PR, et al. Efficacy of a computerized abuse and safety assessment intervention for women with disabilities: a randomized controlled trial. Rehabilitation Psychology 2010;55(2):97‐107. [PUBMED: 20496965] [DOI] [PubMed] [Google Scholar]
Saftlas 2014 {published data only}
- Saftlas AF, Harland KK, Wallis AB, Cavanaugh J, Dickey P, Peek‐Asa C. Motivational interviewing and intimate partner violence: a randomized trial. Annals of Epidemiology 2014;24(2):144‐50. [DOI: 10.1016/j.annepidem.2013.10.006] [DOI] [PubMed] [Google Scholar]
Subramanian 2012 {published data only}
- Subramanian S, Katz KS, Rodan M, Gantz MG, El‐Khorazaty NM, Johnson A, et al. An integrated randomized intervention to reduce behavioral and psychosocial risks: pregnancy and neonatal outcomes. Maternal and Child Health Journal 2012;16(3):545‐54. [DOI: 10.1007/s10995-011-0875-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taft 2011 {published data only}
- Taft A, Small R, Hegarty KL, Watson LF, Gold L, Lumley JA. Mothers' AdvocateS In the Community (MOSAIC) – non‐professional support to reduce intimate partner violence and depression in mothers: a cluster randomised trial in primary care. BMC Public Health 2011;11:178. [DOI: 10.1186/1471-2458-11-178] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taft 2012 {published data only}
- Taft AJ, Small R, Humphreys C, Hegarty K, Walter R, Adams C, et al. Enhanced maternal and child health nurse care for women experiencing intimate partner/family violence: protocol for MOVE, a cluster randomised trial of screening and referral in primary health care. BMC Public Health 2012;12:811. [DOI: 10.1186/1471-2458-12-811] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2000 {published data only}
- Thompson RS, Rivara FP, Thompson DC, Barlwo WE, Sugg NK, Maiuro RD, et al. Identification and management of domestic violence: a randomised controlled trial. American Journal of Preventive Medicine 2000;19(4):253‐63. [PUBMED: 11064229] [DOI] [PubMed] [Google Scholar]
Tiwari 2010 {published data only}
- Tiwari A, Fong DYT, Yuen KH, Yuk H, Pang P, Humphreys J. Effect of an advocacy intervention on mental health in Chinese women survivors of intimate partner violence: a randomized controlled trial. JAMA 2010;304(5):536‐43. [DOI: 10.1001/jama.2010.1052] [DOI] [PubMed] [Google Scholar]
Wagman 2015 {published data only}
- Wagman JA, Gray RH, Campbell JC, Thoma M, Ndyanabo A, Ssekasanvu J, et al. Effectiveness of an integrated intimate partner violence and HIV prevention intervention in Rakai, Uganda: analysis of an intervention in an existing cluster randomised cohort. Lancet Global Health 2015;3(1):e23‐33. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01207258 {published data only}
- Rhodes KV, Rodgers M, Sommers M, Hanlon A, Crits‐Christoph P. The Social Health Intervention Project (SHIP): protocol for a randomized controlled clinical trial assessing the effectiveness of a brief motivational intervention for problem drinking and intimate partner violence in an urban emergency department. BMC Emergency Medicine 2014;14:10. [DOI: 10.1186/1471-227X-14-10] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01661504 {published data only}
- Falb KL, Diaz‐Olavarrieta C, Campos PA, Valades J, Cardenas R, Carino G, et al. Study protocol: evaluating a health care provider delivered intervention to reduce intimate partner violence and mitigate associated health risks: study protocol for a randomized controlled trial in Mexico City. BMC Public Health 2014;14:772. [DOI: 10.1186/1471-2458-14-772] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ahmad 2008
- Ahmad F, Hogg‐Johnson S, Skinner HA. Assessing patient attitudes to computerized screening in primary care: psychometric properties of the Computerized Lifestyle Assessment Scale. Journal of Medical Internet Research 2008;10(2):e11. [DOI: 10.2196/jmir.955] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonomi 2009
- Bonomi AE, Anderson ML, Rivara FP, Thompson RS. Health care utilization and costs associated with physical and nonphysical‐only intimate partner violence. Health Services Research 2009;44(3):1052‐67. [DOI: 10.1111/j.1475-6773.2009.00955.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brock 1999
- Brock K, Stenzel A. When men murder women: an analysis of 1997 homicide data. www.vpc.org/studies/dv2cont.htm (accessed 11 February 2013).
Campbell 2002
- Campbell JC. Health consequences of intimate partner violence. Lancet 2002;359(9314):1331‐6. [PUBMED: 11965295 ] [DOI] [PubMed] [Google Scholar]
Campbell 2004
- Campbell JC. Helping women understand their risk in situations of intimate partner violence. Journal of Interpersonal Violence 2004;19(12):1464‐77. [DOI: 10.1177/0886260504269698] [DOI] [PubMed] [Google Scholar]
Campbell 2012
- Campbell MK, Piaggio G, Elbourne DR, Altman DG, CONSORT Group. Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345:e5661. [DOI: ] [DOI] [PubMed] [Google Scholar]
Catallo 2013a
- Catallo C, Jack SM, Ciliska D, MacMillan HL. Mixing a grounded theory approach with a randomized controlled trial related to intimate partner violence: what challenges arise for mixed methods research?. Nursing Research and Practice 2013;2013:798213. [DOI: 10.1155/2013/798213] [DOI] [PMC free article] [PubMed] [Google Scholar]
Catallo 2013b
- Catallo C, Jack SM, Ciliska D, MacMillan HL. Minimizing the risk of intrusion: a grounded theory of intimate partner violence disclosure in emergency departments. Journal of Advanced Nursing 2013;69(6):1366‐76. [DOI: 10.1111/j.1365-2648.2012.06128.x] [DOI] [PubMed] [Google Scholar]
CCJS 2005
- Canadian Centre for Justice Statistics. Family violence in Canada: a statistical profile. bit.ly/U3GxPX (accessed 28 May 2015).
CDC 2003
- Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Costs of intimate partner violence against women in the United States. www.cdc.gov/violenceprevention/pdf/IPVBook‐a.pdf (accessed 11 May 2015).
CDC 2007
- Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Intimate partner violence and sexual violence victimization assessment instruments for use in healthcare settings: version 1.0. http://www.cdc.gov/violenceprevention/pdf/ipv/ipvandsvscreening.pdf (accessed 11 May 2015).
Chang 2010
- Chang JC, Dado D, Hawker L, Cluss PA, Buranosky R, Slagel L, et al. Understanding turning points in intimate partner violence: factors and circumstances leading women victims towards change. Journal of Women's Health 2010;19(2):251‐9. [DOI: 10.1089/jwh.2009.1568] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 2014
- Clark TJ, Renner LM, Sobel RK, Carter KD, Nerad JA, Allen RC, et al. Intimate partner violence: an underappreciated etiology of orbital floor fractures. Ophthalmic Plastic and Reconstructive Surgery 2014;30(6):508‐11. [PUBMED: 24833455] [DOI] [PubMed] [Google Scholar]
Coker 2000
- Coker AL, Smith PH, Bethea L, King MR, McKeown RE. Physical health consequences of physical and psychological intimate partner violence. Archives of Family Medicine 2000;9(5):451‐7. [DOI] [PubMed] [Google Scholar]
Coker 2002
- Coker AL, Davis KE, Arias I, Desai S, Sanderson M, Brandt HM, et al. Physical and mental health effects of intimate partner violence for men and women. American Journal of Preventive Medicine 2002;23(4):260‐8. [DOI] [PubMed] [Google Scholar]
Colombini 2008
- Colombini M, Mayhew S, Watts C. Health‐sector responses to intimate partner violence in low‐ and middle‐income settings: a review of current models, challenges and opportunities. Bulletin of the World Health Organization 2008;86(8):635‐42. [PUBMED: 18797623] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coulthard 2010
- Coulthard P, Yong SL, Adamson L, Warburton A, Worthington HV, Esposito M. Domestic violence screening and intervention programmes for adults with dental or facial injury. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD004486.pub3; Art No.: CD004486] [DOI] [PubMed] [Google Scholar]
Davidson 2000
- Davidson L, King V, Garcia J, Marchant S. Briefing note: reducing domestic violence...what works? Health services. http://bit.ly/1J5Js0E (accessed 11 February 2013).
Donner 1980
- Donner A, Koval JJ. The estimation of intraclass correlation in the analysis of family data. Biometrics 1980;36(1):19‐25. [PubMed] [Google Scholar]
Díaz‐Olavarrieta 1999
- Díaz‐Olavarrieta, Campbell J, García de la Cadena C, Paz F, Villa AR. Domestic violence against patients with chronic neurological disorders. Archives of Neurology 1999;56(6):681‐5. [PUBMED: 10369306] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
EIGE 2014
- European Institute for Gender Equality. Estimating the costs of gender‐based violence in the European Union: report. bit.ly/1yEZ7jR (accessed 28 May 2015).
Ellsberg 2008
- Ellsberg M, Jansen HA, Heise L, Watts CH, Garcia‐Moreno C, WHO Multi‐country Study on Women's Health and Domestic Violence against Women Study Team. Intimate partner violence and women's physical and mental health in the WHO multi‐country study on women's health and domestic violence: an observational study. Lancet 2008;371(9619):1165‐72. [PUBMED: 18395577] [DOI] [PubMed] [Google Scholar]
Family Violence Prevention Fund 2004
- Family Violence Prevention Fund. National consensus guidelines on identifying and responding to domestic violence victimisation in health care settings. bit.ly/1G5L9u4 (accessed 11 February 2013).
Feder 2006
- Feder G, Hutson M, Ramsay J, Taket AR. Women exposed to intimate partner violence: expectations and experiences when they encounter health care professionals: a meta‐analysis of qualitative studies. Archives of Internal Medicine 2006;166(1):22‐37. [DOI] [PubMed] [Google Scholar]
Feder 2009
- Feder G, Ramsay J, Dunne D, Rose M, Norman R, Kuntze S, et al. How far does screening women for domestic (partner) violence in different health‐care settings meet criteria for a screening programme? Systematic reviews of nine UK National Screening Committee criteria. NIHR Health Technology Assessment Programme 2009;13(16):156. [DOI: 10.3310/hta13160] [DOI] [PubMed] [Google Scholar]
Feder 2013
- Feder G, Wathen CN, MacMillan HL. An evidence‐based response to intimate partner violence: WHO guidelines. JAMA 2013;310(5):479‐80. [DOI: 10.1001/jama.2013.167453] [DOI] [PubMed] [Google Scholar]
Ferreira 2014
- Ferreira MC, Batista AM, Ferreira Fde O, Ramos‐Jorge ML, Marques LS. Pattern of oral‐maxillofacial trauma stemming from interpersonal physical violence and determinant factors. Dental Traumatology 2014;30(1):15‐21. [PUBMED: 23675634] [DOI] [PubMed] [Google Scholar]
Fincher 2015 [pers comm]
- Fincher D (Rollins School of Public Health, Atlanta, USA). [personal communication]. Conversation with: L O'Doherty (Coventry University, UK) 27 April 2015.
Fraga 2015 [pers comm]
- Fraga S (Institute of Public Health, University of Porto, Portugal). [personal communication]. Conversation with: L O'Doherty (Coventry University, UK) 29 April 2015.
Garcia‐Moreno 2006
- Garcia‐Moreno C, Jansen HAFM, Ellsberg M, Watts C, on behalf of the Multi‐country Study on Women's Health and Domestic Violence against Women Study Team. Prevalence of intimate partner violence: findings from the WHO multi‐country study on women's health and domestic violence. Lancet 2006;368(9543):1260‐9. [DOI] [PubMed] [Google Scholar]
García‐Moreno 2014
García‐Moreno 2015 [pers comm]
- García‐Moreno C (Department of Reproductive Health and Research, World Health Organization). [personal communication]. Conversation with: L O'Doherty (Coventry University UK) 26 May 2015.
Golding 1999
- Golding JM. Intimate partner violence as a risk factor for mental disorders: a meta‐analysis. Journal of Family Violence 1999;14(2):99‐132. [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group/Evidence Prime. GRADEpro. Guideline development tool (GDT). GRADE Working Group/Evidence Prime, 2015. [http://gdt.guidelinedevelopment.org/]
Hegarty 2001
- Hegarty KL, Taft AJ. Overcoming the barriers to disclosure and inquiry of partner abuse for women attending general practice. Australian and New Zealand Journal of Public Health 2001;25(5):433‐7. [PUBMED: 11688623] [PubMed] [Google Scholar]
Hegarty 2004
- Hegarty K, Gunn J, Chondros P, Small R. Association between depression and abuse by partners of women attending general practice: descriptive, cross‐sectional survey. BMJ 2004;328(7440):621‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hegarty 2005
- Hegarty K, Bush R, Sheehan M. The Composite Abuse Scale: further development and assessment of reliability and validity of a multidimensional partner abuse measure in clinical settings. Violence and Victims 2005;20(5):529‐47. [PubMed] [Google Scholar]
Hegarty 2006
- Hegarty K. Identification of intimate partner abuse in health care settings ‐ should health professionals be screening?. In: Roberts G, Hegarty K, Feder G editor(s). Intimate Partner Abuse and Health Professionals: New Approaches To Domestic Violence. London: Churchill Livingstone, 2006. [Google Scholar]
Hegarty 2008
- Hegarty KL, Taft A, Feder G. Clinical review: violence between intimate partners: working with the whole family (clinical review). BMJ 2008;337(a839):484‐7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Heyman 2002
- Heyman RE, Smith Slep AMS. Do child abuse and interparental violence lead to adulthood family violence?. Journal of Marriage and Family 2002;64(4):864‐70. [DOI: 10.1111/j.1741-3737.2002.00864.x] [DOI] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Home Office 2010
- Smith K, Flatley J (editors), Coleman K, Osborne S, Kaiza P, Roe S. Homicides, firearm offences and intimate violence 2008/09. Supplementary Volume 2 to crime in England and Wales 2008/09 (third edition). http://bit.ly/1J923Jl (accessed 11 February 2013).
Hooker 2015
- Hooker L, Small R, Humphreys C, Hegarty K, Taft A. Applying normalization process theory to understand implementation of a family violence screening and care model in maternal and child health nursing practice: a mixed method process evaluation of a randomised controlled trial. Implementation Science 2015;10(1):39. [DOI: 10.1186/s13012-015-0230-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 2013
- Howard LM, Oram S, Galley H, Trevillion K, Feder G. Domestic violence and perinatal mental disorders: a systematic review and meta‐analysis. PLoS Medicine 2013;10(5):e1001452. [DOI: 10.1371/journal.pmed.1001452] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ingram 2005
- Ingram JK. Statement to the UN on violence against women. http://bit.ly/1QF6fTb (accessed 11 February 2013).
Jejeebhoy 1998
- Jejeebhoy SJ. Associations between wife‐beating and fetal and infant death: impressions from a survey in rural India. Studies in Family Planning 1998;29(3):300‐8. [PUBMED: 9789323] [PubMed] [Google Scholar]
Jewkes 2002
- Jewkes R. Intimate partner violence: causes and prevention. Lancet 2002;359(9315):1423‐9. [PUBMED: 11978358] [DOI] [PubMed] [Google Scholar]
Kirkwood 1993
- Kirkwood C. Leaving Abusive Partners: From the Scars of Survival to the Wisdom for Change. London: Sage Publications Ltd, 1993. [Google Scholar]
Kitzmann 2003
- Kitzmann KM, Gaylord NK, Holt AR, Kenny ED. Child witnesses to domestic violence: a meta‐analytic review. Journal of Consulting and Clinical Psychology 2003;71(2):339‐52. [PUBMED: 12699028] [DOI] [PubMed] [Google Scholar]
Koziol‐McLain 2008
- Koziol‐McLain J, Giddings L, Rameka M, Fyfe E. Intimate partner violence screening and brief intervention: experiences of women in two New Zealand health care settings. Journal of Midwifery & Women's Health 2008;53(6):504‐10. [DOI: 10.1016/j.jmwh.2008.06.002; PUBMED: 18984506] [DOI] [PubMed] [Google Scholar]
Krug 2002
- Krug EG, Dahlberg LL, Mercy JA, Zwi AB, Lozano R. World Report on Violence and Health. Geneva: World Health Organization, 2002. [Google Scholar]
MacMillan 2011 [pers comm]
- MacMillan H (Offord Centre for Child Studies, McMaster University, Canada). [personal communication]. Conversation with: A Taft (La Trobe University) 2011.
MacMillan 2015 [pers comm]
- MacMillan H (Offord Centre for Child Studies, McMaster University, Canada). [personal communication]. Conversation with: L O'Doherty (Coventry University, UK) 5 May 2015.
Martin 2001
- Martin SL, Mackie L, Kupper LL, Buescher PA, Moracco KE. Physical abuse of women before, during and after pregnancy. JAMA 2001;285(12):1581‐4. [DOI] [PubMed] [Google Scholar]
May 2011
- May CR, Finch T, Ballini L, MacFarlane A, Mair F, Murray E, et al. Evaluating complex interventions and health technologies using normalization process theory: development of a simplified approach and web‐enabled toolkit. BMC Health Services Research 2011;11(1):245. [PUBMED: 21961827] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCauley 1995
- McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, et al. The 'Battering Syndrome': prevalence and clinical characteristics of domestic violence in primary care internal medicine practices. Annals of Internal Medicine 1995;123(10):737‐46. [DOI] [PubMed] [Google Scholar]
McCosker‐Howard 2006
- McCosker‐Howard H, Woods AB. How is intimate partner abuse experienced by childbearing women. In: Roberts G, Hegarty K, Feder G editor(s). Intimate Partner Abuse and Health Professionals: New Approaches to Domestic Violence. London, UK: Churchill Livingstone, 2006:111‐26. [Google Scholar]
McLeer 1989
- McLeer SV, Anwar RA, Herman S, Maquiling K. Education is not enough: a systems failure in protecting battered women. Annals of Emergency Medicine 1989;18(6):651‐3. [PUBMED: 2729689] [DOI] [PubMed] [Google Scholar]
Mezey 1997
- Mezey GC, Bewley S. Domestic violence in pregnancy. British Journal of Obstetrics and Gynaecology 1997;104(5):528‐31. [DOI] [PubMed] [Google Scholar]
Moore 2010
- Moore AM, Frohwirth L, Miller E. Male reproductive control of women who have experienced intimate partner violence in the United States. Social Science & Medicine 2010;70(11):1737‐44. [PUBMED: 20359808] [DOI] [PubMed] [Google Scholar]
Moore 2015
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350:h1258. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mouzos 2003
- Mouzos J, Rushworth C. Family Homicide in Australia. Canberra: Australian Institute of Criminology, 2003. [Google Scholar]
Murphy 2001
- Murphy CC, Schei B, Myhr TL, Du Mont J. Abuse: a risk factor for low birth weight? A systematic review and meta‐analysis. Canadian Medical Association Journal 2001;164(11):1567‐72. [PMC free article] [PubMed] [Google Scholar]
Nelson 2004
- Nelson HD, Nygren P, McInerney Y, Klein J, US Preventive Services Task Force. Screening women and elderly adults for family and intimate partner violence: a review of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2004;140(5):387‐96. [DOI: 10.7326/0003-4819-140-5-200403020-00015] [DOI] [PubMed] [Google Scholar]
Nelson 2012
- Nelson HD, Bougatsos C, Blazina I. Screening women for intimate partner violence: a systematic review to update the U.S. Preventive Services Task Force recommendation. Annals of Internal Medicine 2012;156(11):796‐808. [DOI: 10.7326/0003-4819-156-11-201206050-00447] [DOI] [PubMed] [Google Scholar]
NICE 2014
- National Institute of Health and Care Excellence (NICE). Domestic violence and abuse: how health services, social care and the organisations they work with can respond effectively. www.nice.org.uk/guidance/ph50 (accessed 28 May 2015).
Office for National Statistics 2014
- Office for National Statistics. Chapter 4 ‐ intimate personal violence and partner abuse. Crime Statistics, Focus on Violent Crime and Sexual Offences, 2012/13. London: Office for National Statistics, 2014. [Google Scholar]
Pallitto 2013
- Pallitto CC, García‐Moreno C, Jansen HA, Heise L, Ellsberg M, Watts C, et al. Intimate partner violence, abortion, and unintended pregnancy: results from the WHO Multi‐country Study on Women's Health and Domestic Violence. International Journal of Gynaecology and Obstetrics 2013;120(1):3‐9. [PUBMED: 22959631] [DOI] [PubMed] [Google Scholar]
Parsons 1999
- Parsons LT, Harper MA. Violent maternal deaths in North Carolina. Obstetrics & Gynecology 1999;94(6):990‐3. [PUBMED: 10576188] [DOI] [PubMed] [Google Scholar]
Rabin 2009
- Rabin R, Jennings J, Campbell J, Bair‐Merritt M. Intimate partner violence screening tools: a systematic review. American Journal of Preventive Medicine 2009;36(5):439‐45. [PUBMED: 19362697] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramsay 2002
- Ramsay J, Richardson J, Carter YH, Davidson LL, Feder G. Should health professionals screen for domestic violence? Systematic review. BMJ 2002;325:314‐8. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramsay 2009
- Ramsay J, Carter Y, Davidson L, Dunne D, Eldridge S, Hegarty K, et al. Advocacy interventions to reduce or eliminate violence and promote the physical and psychosocial well‐being of women who experience intimate partner abuse. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD005043.pub2] [DOI] [PubMed] [Google Scholar]
Rees 2011
- Rees S, Silove D, Chey T, Ivancic L, Steel Z, Creamer M, et al. Lifetime prevalence of gender‐based violence in women and the relationship with mental disorders and psychosocial function. JAMA 2011;306(5):513‐21. [PUBMED: 21813429] [DOI] [PubMed] [Google Scholar]
Reisenhofer 2013
- Reisenhofer S, Taft A. Women's journey to safety ‐ the Transtheoretical model in clinical practice when working with women experiencing Intimate Partner Violence: a scientific review and clinical guidance. Patient Education & Counseling 2013;93(3):536‐48. [DOI: 10.1016/j.pec.2013.08.004] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richardson 2001
- Richardson J, Feder G, Eldridge S, Chung WS, Coid J, Moorey S. Women who experience domestic violence and women survivors of childhood sexual abuse: a survey of health professionals' attitudes and clinical practice. British Journal of General Practice 2001;51(467):468‐70. [PMC free article] [PubMed] [Google Scholar]
Richardson 2002
- Richardson J, Coid J, Petruckevich A, Chung WS, Moorey S, Feder G. Identifying domestic violence: cross sectional study in primary care. BMJ 2002;324:274‐9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rickert 2009
- Rickert V, Davison L, Breitbart V, Jones K, Palmetto N, Rottenberg L, et al. A randomized trial of screening for relationship violence in young women. Journal of Adolescent Health 2009;45(2):163‐70. [DOI] [PubMed] [Google Scholar]
Rodrigues 2008
- Rodrigues T, Rocha L, Barros H. Physical abuse during pregnancy and preterm delivery. American Journal of Obstetrics and Gynecology 2008;198(2):171.e1‐6. [DOI: 10.1016/j.ajog.2007.05.015] [DOI] [PubMed] [Google Scholar]
Shackelford 2005
- Shackelford TK, Goetz AT, Buss DM, Euler HA, Hoier S. When we hurt the ones we love: predicting violence against women from men's mate retention. Personal Relationships 2005;12(4):447‐63. [DOI: 10.1111/j.1475-6811.2005.00125.x] [DOI] [Google Scholar]
Silverman 2006
- Silverman JG, Decker MR, Reed E, Raj A. Intimate partner violence victimization prior to and during pregnancy among women residing in 26 U.S. states: associations with maternal and neonatal health. American Journal of Obstetrics and Gynecology 2006;195(1):140‐8. [PUBMED: 16813751] [DOI] [PubMed] [Google Scholar]
Spangaro 2009
- Spangaro J, Zwi AB, Poulos R. The elusive search for definitive evidence on routine screening for intimate partner violence. Trauma Violence & Abuse 2009;10(1):55‐68. [PUBMED: 19056688] [DOI] [PubMed] [Google Scholar]
Stark 1996
- Stark E, Flitcraft A. Women At Risk: Domestic Violence and Women's Health. London: Sage Publications Ltd, 1996. [Google Scholar]
Stayton 2005
- Stayton CD, Duncan MM. Mutable influences on intimate partner abuse screening in health care settings: a synthesis of the literature. Trauma Violence & Abuse 2005;6(4):271‐85. [DOI: 10.1177/1524838005277439] [DOI] [PubMed] [Google Scholar]
Taft 2001
- Taft A. To screen or not to screen ‐ is this the right question? Quality care, intervention and women's agency in health care responses to partner violence and abuse. Women Against Violence: An Australian Feminist Journal 2001;10:41‐6. [Google Scholar]
Taft 2004
- Taft A, Watson L, Lee C. Violence against young Australian women and associated reproductive events: a cross sectional analysis of a national population sample. Australian and New Zealand Journal of Public Health 2004;28(4):324‐9. [PUBMED: 15704695] [DOI] [PubMed] [Google Scholar]
Taft 2015
- Taft A, Hooker L, Humphreys C, Hegarty K, Walter R, Adams C, et al. Maternal and child health nurse screening and care for mothers experiencing domestic violence (MOVE): a cluster randomised trial. BMC Medicine 2015;13:150. [DOI: 10.1186/s12916-015-0375-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taket 2004
- Taket A, Wathen CN, MacMillan H. Should health professionals screen all women for domestic violence?. PLoS Medicine 2004;1(1):7‐10. [DOI: 10.1371/journal.pmed.0010004] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tollestrup 1999
- Tollestrup K, Sklar D, Frost FJ, Olson L, Weybright J, Sandvig J, et al. Health indicators and intimate partner violence among women who are members of a managed care organization. Preventive Medicine 1999;29(5):431‐40. [DOI: ] [DOI] [PubMed] [Google Scholar]
Trevillion 2012
- Trevillion K, Oram S, Feder G, Howard LM. Experiences of domestic violence and mental disorders: a systematic review and meta‐analysis. PloS One 2012;7(12):e51740. [DOI: 10.1371/journal.pone.0051740] [DOI] [PMC free article] [PubMed] [Google Scholar]
U.S. Preventive Services Task Force 2004
- U.S. Preventive Services Task Force. Screening for family and intimate partner violence: recommendation statement. Annals of Family Medicine 2004;2(2):156‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valpied 2014
- Valpied J, Cini A, O'Doherty L, Taket A, Hegarty K. “Sometimes cathartic. Sometimes quite raw”: benefit and harm in an intimate partner violence trial. Aggression and Violent Behavior 2014;19(6):673‐85. [DOI: 10.1016/j.avb.2014.09.005] [DOI] [Google Scholar]
Waalen 2000
- Waalen J, Goodwin MM, Spitz AM, Petersen R, Saltzman LE. Screening for intimate partner violence by health care providers: barriers and interventions. American Journal of Preventive Medicine 2000;19(4):230‐7. [DOI] [PubMed] [Google Scholar]
Walby 2004
- Walby S. The Cost of Domestic Violence. London: Women and Equality Unit (Department of Trade and Industry), 2004. [www.devon.gov.uk/de/text/cost_of_dv_report_sept04.pdf] [Google Scholar]
Wathen 2003
- Wathen CN, MacMillan HL. Prevention of violence against women: recommendation state from the Canadian Task Force on Preventative Healthcare. Canadian Medical Association Journal 2003;169(6):582‐4. [PMC free article] [PubMed] [Google Scholar]
Wathen 2008
- Wathen CN, Jamieson E, MacMillan HL, McMaster Violence Against Women Research Group. Who is identified by screening for intimate partner violence?. Women's Health Issues 2008;18(6):423‐32. [DOI] [PubMed] [Google Scholar]
Wathen 2012
- Wathen CN, MacMillan HL. Health care’s response to women exposed to partner violence: moving beyond universal screening. JAMA 2012;308(7):712‐3. [PUBMED: 22893169] [DOI] [PubMed] [Google Scholar]
WHO 2013a
- World Health Organization. Global and regional estimates of violence against women: prevalence and health effects of intimate partner violence and non‐partner sexual violence. Geneva: World Health Organization, 2013. [9789241564625] [Google Scholar]
WHO 2013b
- World Health Organization. Responding to Intimate Partner Violence and Sexual Violence Against Women: WHO Clinical and Policy Guidelines. Geneva: World Health Organization, 2013. [ISBN: 9789241548595] [PubMed] [Google Scholar]
Wilson 1993
- Wilson M, Daly M. Spousal homicide risk and estrangement. Violence and Victims 1993;8(1):3‐16. [PUBMED: 8292563] [PubMed] [Google Scholar]
Wong 2014
- Wong JY, Choi AW‐M, Fong DY‐T, Wong JK‐S, Lau C‐L, Kam C‐W. Patterns, aetiology and risk factors of intimate partner violence‐related injuries to head, neck and face in Chinese women. BMC Women's Health 2014;14:6. [DOI: 10.1186/1472-6874-14-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
World Bank 2006
- World Bank. World development report: equity and development. http://bit.ly/1EA2je2 (accessed 11 February 2013).
References to other published versions of this review
O'Doherty 2014a
- O'Doherty LJ, Taft A, Hegarty K, Ramsay J, Davidson LL, Feder G. Screening women for intimate partner violence in healthcare settings: abridged Cochrane systematic review and meta‐analysis. British Medical Journal 2014;348:g2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taft 2008
- Taft A, Hegarty K, Ramsay J, Feder G, Carter Y, Davidson L, et al. Screening women for intimate partner violence in health care settings. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD007007] [DOI] [Google Scholar]
Taft 2013
- Taft A, O'Doherty L, Hegarty K, Ramsay J, Davidson J, Feder G. Screening women for intimate partner violence in healthcare settings. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD007007.pub2] [DOI] [PubMed] [Google Scholar]


