Abstract
Background
Patients with chronic heart failure (heart failure) are at risk of thromboembolic events, including stroke, pulmonary embolism and peripheral arterial embolism, whilst coronary ischaemic events also contribute to the progression of heart failure. Long‐term oral anticoagulation is established in certain patient groups, including patients with heart failure and atrial fibrillation, but there is wide variation in the indications and use of oral anticoagulation in the broader heart failure population.
Objectives
To determine whether long‐term oral anticoagulation reduces total deaths, cardiovascular deaths and major thromboembolic events in patients with heart failure.
Search methods
We updated the searches in June 2013 in the electronic databases CENTRAL (Issue 6, 2013) in The Cochrane Library, MEDLINE (OVID, 1946 to June week 1 2013) and EMBASE (OVID, 1980 to 2013 week 23). Reference lists of papers and abstracts from national and international cardiovascular meetings were studied to identify unpublished studies. Relevant authors were contacted to obtain further data. No language restrictions were applied.
Selection criteria
Randomised controlled trials (RCTs) comparing oral anticoagulants with placebo in adults with heart failure, and with treatment duration at least one month. Non‐randomised studies were also included for assessing side effects. Inclusion decisions were made in duplicate and any disagreement between review authors was resolved by discussion or a third party.
Data collection and analysis
Two review authors independently assessed trials for inclusion and assessed the risks and benefits of antithrombotic therapy using relative measures of effects, such as odds ratio, accompanied by the 95% confidence intervals.
Main results
Two RCTs were identified. One compared warfarin, aspirin and no antithrombotic therapy and the second compared warfarin with placebo in patients with idiopathic dilated cardiomyopathy. Three small prospective controlled studies of warfarin in heart failure were also identified, but they were over 50 years old with methods not considered reliable by modern standards. In both WASH 2004 and HELAS 2006, there were no significant differences in the incidence of myocardial infarction, non‐fatal stroke and death between patients taking oral anticoagulation and those taking placebo. Four retrospective non‐randomised cohort analyses and four observational studies of oral anticoagulation in heart failure included differing populations of heart failure patients and reported contradictory results.
Authors' conclusions
Based on the two major randomised trials (HELAS 2006; WASH 2004), there is no convincing evidence that oral anticoagulant therapy modifies mortality or vascular events in patients with heart failure and sinus rhythm. Although oral anticoagulation is indicated in certain groups of patients with heart failure (for example those with atrial fibrillation), the available data does not support the routine use of anticoagulation in heart failure patients who remain in sinus rhythm.
Plain language summary
There is not enough evidence to determine if anticoagulants safely prevent blood clots in patients with chronic heart failure who are in normal heart rhythm
Blood clots (thromboembolism) in the lungs, legs and brain (ischaemic stroke) contribute to disability and the death of patients with heart failure. Although anticoagulants such as warfarin are of proven benefit in patients in certain subgroups of patients with heart failure, such as those with atrial fibrillation, there is little evidence that warfarin works well in the wider heart failure population. There may also be serious side effects such as bleeding (causing ulcers and haemorrhagic stroke). At present there are no data to recommend the routine use of anticoagulants to prevent thromboembolism in patients with heart failure who are in normal heart rhythm.
Background
Chronic heart failure (heart failure) is an increasing clinical and social problem. It is associated with high morbidity rates and annual mortality rates of greater than 30% in patients with severe symptoms (CONSENSUS 1987).
Heart failure has long been recognised to predispose individuals to stroke and thromboembolism, including pulmonary embolism and peripheral arterial embolism. These thromboembolic events contribute to the high morbidity in heart failure (Fuster 1981; Kyrle 1985). In addition, ischaemic and thromboembolic events, particularly stroke, myocardial ischaemia and myocardial infarction, contribute to the high hospital admission rates of these patients (Brown 1998). The incidence of ischaemic and thromboembolic events, and the risk factors associated with a high thromboembolic risk, have been addressed in numerous small and large scale studies, although the reported incidence of these events appears to vary between studies, depending on the study methodologies and populations. Nevertheless, as an example, mild to moderate heart failure appears to be associated with an annual stroke risk of approximately 1.5% (V‐HeFT 1993; SOLVD 1998) compared with an annual stroke risk in the general population of less than 0.5%, whilst the annual risk of stroke increases to almost 4% in patients with severe heart failure (CONSENSUS 1987; PROMISE 1993).
There is evidence of benefit from long‐term oral anticoagulation in certain groups of patients as oral anticoagulation has been proven to be extremely effective in reducing stroke and other embolic events in patients with atrial fibrillation and heart failure (BAATAF 1990; Petersen 1989; SPAF 1991), but the role of anticoagulation in the broader heart failure population is less well established. Indeed, there is wide variation in the use of oral anticoagulants in patients with heart failure (Edep 1997). In addition, although oral anticoagulation has been associated with a reduction in the number of thromboembolic events in various cardiovascular disease states, the potential risks of bleeding must also be considered. Importantly, the control of anticoagulation is reported to be more difficult, and bleeding complications more frequent, in heart failure (Davis 1977; Husted 1976) as a result of hepatic congestion and potential drug interactions which occur in these patients (Landefeld 1989).
Objectives
To determine whether long‐term oral anticoagulation reduces total deaths, cardiovascular deaths and major thromboembolic events in patients with heart failure.
Methods
Criteria for considering studies for this review
Types of studies
Parallel group randomised controlled trials (RCTs) comparing oral anticoagulants with control or placebo.
Inclusion criteria
Treatment with oral anticoagulants
Duration of treatment at least one month
Trials including adults over the age of 16 years
Patients with heart failure due to any underlying cause
Exclusion criteria
No clinical events recorded or available
Short duration of treatment, for example less than one month
Additional active treatments in the intervention arm (for example beta‐blockers, ACE inhibitors)
Participants with various diagnoses and in different diagnostic subgroups not distinguished in analyses or not available from the investigators
Participants with atrial fibrillation only
To assess any adverse effects we will also examine:
cohort studies and non‐randomised controlled studies;
decision analysis studies.
Data from the non‐randomised studies have been included in the discussion to provide additional information to aid interpretation of data on the effectiveness of the therapy.
Types of participants
Patients with heart failure defined clinically and, if possible, by more objective evidence (for example echocardiography, radionuclide ventriculography) of left ventricular systolic dysfunction.
Types of interventions
Administration of (low‐dose and full‐dose) oral anticoagulation.
The type of therapy and duration of treatment was recorded.
Types of outcome measures
a) All cause deaths b) Cardiovascular deaths (stroke, myocardial infarction, pulmonary embolism, peripheral arterial embolism) and sudden deaths c) Non‐fatal cardiovascular events (non‐fatal stroke, myocardial infarction, pulmonary embolism, peripheral arterial embolism) d) Major bleeding events (fatal, non‐fatal)
Complications of the active therapy (when compared to placebo) were also recorded.
Search methods for identification of studies
We updated the searches done in 2005 (Appendix 1) by re‐running them in February 2010 (Appendix 2) and again in June 2013 (Appendix 3):
the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 6, 2013) on The Cochrane Library,
MEDLINE (OVID, 1946 to June week 1 2013),
EMBASE (OVID, 1980 to 2013 week 23),
Database of Abstracts of Reviews of Effects (DARE) (Issue 1, 2010) on The Cochrane Library (not updated in 2013).
The Cochrane sensitive‐maximising RCT filters were used to search MEDLINE and EMBASE (Lefebvre 2011). No language restrictions were applied to the searches.
Using a similar timeline we searched abstracts from national and international cardiology meetings (American Heart Congress, American College of Cardiology Congress, European Society of Cardiology Congress, Congress of the International Society on Thrombosis and Haemostasis, European Stroke Conference, International Stroke Conference, World Stroke Congress, UK Stroke Forum Conference and the Heart Failure Congress). These were searched to identify unpublished studies and relevant authors of these studies were contacted to obtain further details.
Relevant foreign language papers were translated and reference lists of identified papers were checked.
Data collection and analysis
Updating this review
Over the course of the original review and updates, five authors (GYHL, IC, BJW, RP and ES) reviewed the inclusion criteria, the search strategies, the methodology criteria and methods for pooling the data for this systematic review.
Trial selection and data extraction
Over the course of the original review and updates, five authors (GYHL, IC, BJW, RP, ES) independently selected suitable trials for inclusion in the review. We identified RCTs that compared the use of oral anticoagulation to placebo for the thrombo‐prophylactic management of patients with heart failure and subsequently extracted data on patient characteristics and concomitant treatments as well as data relating to study eligibility, quality and outcomes. We extracted data from non‐randomised studies in order to assess possible side effects of anticoagulants.
We expressed the dichotomous data on outcomes as odds ratios (OR) with 95% confidence intervals (CI).
We assessed statistical heterogeneity appropriately in each meta‐analysis using the T², I² and Χ² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either T² was greater than zero or there was a low P value (less than 0.10) in the Χ² test for heterogeneity. If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was we used a random‐effects meta‐analysis to produce it. We conducted sensitivity analyses to explore the effect of trial quality for each comparison by restricting the analyses to those trials rated as 'low risk of bias' for random sequence generation and allocation concealment. For each comparison, we limited the analyses to the primary outcomes.
Risk of bias
Using the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) we assessed six aspects to determine the trial's risk of bias: randomization sequence generation, allocation concealment, incomplete outcome data, selective reporting, blinding of participants and personnel, and blinding of outcome assessment.
Contacting authors
For unpublished studies or where data were incomplete in published papers, attempts were made to contact authors or researchers to obtain further details. Where relevant, the pharmaceutical industry was contacted to attempt to obtain unpublished trial data on newer antiplatelet drugs that may have been used in patients with heart failure.
Resolution of differences
In the rare instances where the four authors disagreed over the grading and inclusion of studies, recourse was made to a fifth author. When resolving the disagreement was not possible the article was added to those ‘awaiting assessment’ and the authors were contacted for clarification.
Results
Description of studies
The previous searches in 2001 and 2005 retrieved 1100 records of which 1082 were excluded based on the screening of titles and abstracts. The remaining 18 records were assessed in full text. Four studies (four records) were found that met the inclusion criteria. Eleven studies (11 records) were excluded and three studies were ongoing.
The updated search in 2010 retrieved 1219 records of which 1201 were excluded based on screening the titles and abstracts. Eighteen records were obtained in full text. Based on the full texts, one previously ongoing study was included (HELAS 2006) and another previously ongoing study was excluded (WATCH 2009). Sixteen studies (16 references) were excluded and one study (WARCEF) remained ongoing.
The updated search in 2013 retrieved 1460 new records of which 1452 were excluded based on screening the titles and abstracts. Four records were abstracts of interest based on two retrospective analyses. However, they did not specify the proportion of patients with atrial fibrillation nor provide information on adverse outcomes and they were thus excluded. Four records were obtained in full text but did not meet the analysis criteria. The completed Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF 2012) trial compared warfarin to aspirin and thus did not meet the inclusion criteria.
In total this review includes five studies (five references) (see Characteristics of included studies).
The mean age of the predominantly male (65%) and Caucasian population of 1266 patients with heart failure was 59.9 years (range 20 to 89 years). The two latest studies (HELAS 2006; WASH 2004) (WASH and HELAS) exclusively or predominantly involved European countries whereas the three older studies (Anderson 1950; Griffith 1952; Harvey 1950) were carried out in the USA. Only one trial (Griffith 1952) reported receiving relevant industry sponsorship.
Included studies
We included five studies. WASH 2004 randomised 89 patients to receive oral anticoagulation with warfarin and 99 patients to no antithrombotic therapy. HELAS 2006 randomised 82 patients with idiopathic dilated cardiomyopathy to receive either warfarin (38 patients) or placebo (44 patients). Three small prospective controlled studies of warfarin versus control in hospitalised patients with heart failure were identified (Anderson 1950; Griffith 1952; Harvey 1950). It would be difficult to describe these as true RCTs by modern criteria but they were considered to be randomised and controlled when they were published.
Two randomised controlled trials (HELAS 2006; WASH 2004) met our trial selection criteria. WASH 2004 was a pilot study of 279 patients with heart failure randomised to anticoagulation (target international normalized ratio (INR) 2.5), aspirin (300 mg) or no antithrombotic therapy. This was an open label trial design and performance bias could therefore not be excluded (see 'Risk of bias' table). HELAS 2006 was a study of 115 patients with ischaemic heart disease randomised to anticoagulation (target INR 2.0 to 3.0) or aspirin, and a group of 82 patients randomised to anticoagulation (target INR 2.0 to 3.0) or placebo. This was a double‐blind trial with an independent data and safety monitoring committee.
The earlier prospective controlled studies (Anderson 1950; Griffith 1952; Harvey 1950) were performed over 50 years ago in hospitalised patients with a high prevalence of rheumatic heart disease and atrial fibrillation. Although described as 'randomised', the trial methodologies in these studies are more properly described as quasi‐randomised and cannot be seen as entirely reliable by modern standards. Allocation of patients to anticoagulation or control in these studies may be biased. Patients with heart failure in the Harvey 1950 study (n = 180) were allocated to dicumarol or control depending on whether their hospital admission was on an even or odd day. In the Anderson 1950 study (n = 297) the first 61 patients were alternatively allocated to treated and control groups, and for the rest the treated and control groups were alternated weekly between different medical units and rotated between the wards. Thus there was patient alternation, service alternation and ward rotation in allotting patients to anticoagulation or control. In the Griffith 1952 study (n = 465), during the first year of this study all admitted patients with heart failure were serially allocated to control, dicumarol or depo‐heparin; although in the second year of the study all patients 'on certain designated wards' were used as controls whilst others were assigned anticoagulants.
Anticoagulation monitoring in the older studies was performed using prothrombin activity but was variable in the different studies: 30% by Quick's method (Harvey 1950), 10% to 30% by an unstated method (Anderson 1950), and 20% by variable methods (Griffith 1952).
In summary, we can distinguish three kinds of study investigating anticoagulation for heart failure in sinus rhythm: a) RCTs (HELAS 2006; WASH 2004); b) older quasi‐randomised controlled trials (Anderson 1950; Griffith 1952; Harvey 1950); c) non‐randomised observational studies and post hoc analyses of the effects of warfarin in non‐randomised comparisons, including from large trials of ACE inhibitors in heart failure (CONSENSUS 1987; EPICAL 2002; Fuster 1981; Kyrle 1985; Natterson 1993; PROMISE 1993; SAVE 1997; SOLVD 1998; V‐HeFT 1993; Wishart 1948).
Excluded studies
We excluded 13 studies (see Characteristics of excluded studies). Four observational studies were excluded but were assessed for possible adverse effects from treatment with warfarin (EPICAL 2002; Fuster 1981; Kyrle 1985; Natterson 1993). Adverse event information is presented in the discussion. Fuster 1981 was a retrospective study of 104 patients with idiopathic dilated cardiomyopathy followed up for a total of 725 patient‐years. Kyrle 1985 was a study of 38 patients with non‐ischaemic cardiomyopathy followed for a total of 72 patient‐years. Natterson 1993 was a more recent study of 224 patients awaiting cardiac transplantation. EPICAL 2002 was a study of 417 patients with an average follow‐up period of five years. EPICAL 2002 was eventually excluded as 24% had concomitant atrial fibrillation. Only survival was reported and event rates were not reported separately for the sinus rhythm group or those treated with aspirin, warfarin or no therapy. Aspirin was used in 31% of patients, warfarin in 28% of patients, and warfarin plus aspirin in 2% of patients. Patients given any antithrombotic treatment compared to none had a better survival at five years (40.4% versus 31%, P = 0.01).
One case series, conducted over 50 years ago in an heterogeneous heart failure population (n = 61), was excluded but it did report that the prevalence of thromboembolism on dicumarol (6.5%) was lower compared to previously published reports (22%) (Wishart 1948).
Five publications from RCTs were excluded because the participants in the analyses were not randomised to anticoagulant or control in the original study (CONSENSUS 1987; PROMISE 1993; SAVE 1997; SOLVD 1998; V‐HeFT 1993). The analyses were post hoc analyses of participants treated with oral anticoagulation at the discretion of the investigators. The V‐HeFT 1993 trials included patients with symptomatic heart failure with radiological, echocardiographic or radionuclide evidence of left ventricular systolic dysfunction (V‐HeFT 1993). The SOLVD 1998 trials included patients with left ventricular systolic dysfunction, defined as a left ventricular ejection fraction of 0.35 or more, who were symptomatic and enrolled into the treatment trial (SOLVD 1998‐treatment) or asymptomatic and enrolled into the prevention trial (SOLVD 1998). Limited information from a retrospective analysis of one trial has been presented in abstract form only (PROMISE 1993). The SAVE 1997 study included patients post‐myocardial infarction with a left ventricular ejection fraction of 40% or more and no overt heart failure, that is, asymptomatic patients. One further study (Visser 2004) was a cohort study of people attending an outpatient anticoagulation clinic for a variety of comorbidities. Two studies (WARCEF 2012, WATCH 2009) were excluded as the control group received aspirin or clopidogrel, but not placebo.
Risk of bias in included studies
Risk of bias is shown in Figure 1, Figure 2 and in Table 1.
1.
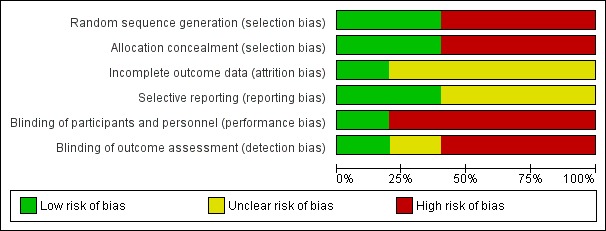
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
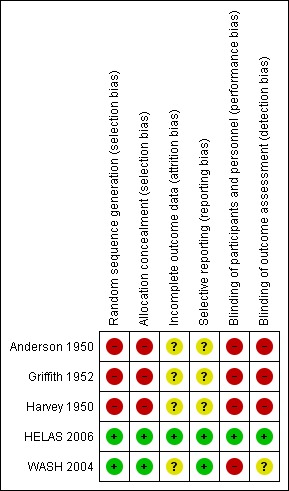
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
1. Risk of bias summary table.
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Anderson 1950 | High | High | Unclear | Unclear | High | High |
| Griffith 1952 | High | High | Unclear | Unclear | High | High |
| Harvey 1950 | High | High | Unclear | Unclear | High | High |
| HELAS 2006 | Low | Low | Low | Low | Low | Low |
| WASH 2004 | Low | Low | Unclear | Low | High | Unclear |
1: Random sequence generation (selection bias); 2: Allocation concealment (selection bias); 3: Incomplete data outcome (attrition bias); 4: Selective reporting (reporting bias); 5: Blinding of participants and personnel (performance bias); 6: Blinding of outcome assessment (detection bias)
Only one study (HELAS 2006) had a low risk of bias for blinding of participants, personnel and outcome assessment. All other studies had a high risk of bias for blinding of participants and personnel. One study had an unknown risk of bias for blinding of outcome assessment (WASH 2004), and all other studies had a high risk.
The data from the non‐randomised observational studies and post hoc analyses of the effects of warfarin in non‐randomised comparisons are potentially confounded by a number of factors, including confounding, selection and information biases, the substantial and uncontrolled use of anticoagulation in these patients, and the influence of time on the risk of embolisation following thrombus development, particularly post‐myocardial infarction.
Effects of interventions
Randomised and quasi‐randomised controlled trials
The main results are summarized in forest plots for the randomised (Analysis 2.1; Analysis 2.2; Analysis 2.3) and quasi‐randomised (Analysis 1.1; Analysis 1.2; Analysis 1.3) controlled trials.
2.1. Analysis.
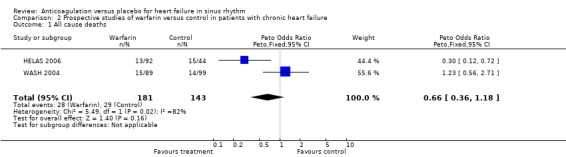
Comparison 2 Prospective studies of warfarin versus control in patients with chronic heart failure, Outcome 1 All cause deaths.
2.2. Analysis.
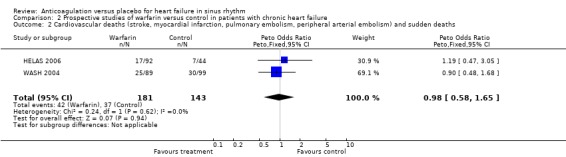
Comparison 2 Prospective studies of warfarin versus control in patients with chronic heart failure, Outcome 2 Cardiovascular deaths (stroke, myocardial infarction, pulmonary embolism, peripheral arterial embolism) and sudden deaths.
2.3. Analysis.

Comparison 2 Prospective studies of warfarin versus control in patients with chronic heart failure, Outcome 3 Non‐fatal cardiovascular events (non‐fatal stroke, myocardial infarction, pulmonary embolism, peripheral arterial embolism).
1.1. Analysis.

Comparison 1 Quasi‐randomised studies of warfarin versus control in patients with chronic heart failure, Outcome 1 All cause deaths.
1.2. Analysis.
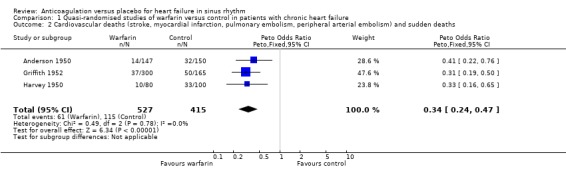
Comparison 1 Quasi‐randomised studies of warfarin versus control in patients with chronic heart failure, Outcome 2 Cardiovascular deaths (stroke, myocardial infarction, pulmonary embolism, peripheral arterial embolism) and sudden deaths.
1.3. Analysis.
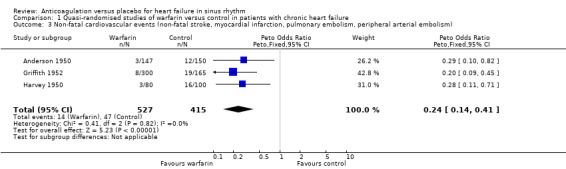
Comparison 1 Quasi‐randomised studies of warfarin versus control in patients with chronic heart failure, Outcome 3 Non‐fatal cardiovascular events (non‐fatal stroke, myocardial infarction, pulmonary embolism, peripheral arterial embolism).
In the WASH 2004 trial, 99 patients received no treatment compared to 89 patients receiving warfarin and they were followed up for a mean of 27 months. No significant difference was evident in the occurrence of the above mentioned primary outcomes.
In the HELAS 2006 study, the target recruitment of 6000 patients was not achieved. Eighty two patients with idiopathic dilated cardiomyopathy were randomised to receive either warfarin or placebo. Again, no significant differences were observed in the primary outcomes.
Overall, the pooled analysis of the RCTs did not show a statistically significant difference in all cause deaths (2 studies, 324 participants, OR 0.66, 95% CI 0.38 to 1.18) (Analysis 2.1), cardiovascular death (2 studies, 324 participants, OR 0.98, 95% CI 0.58 to 1.65) (Analysis 2.2) and non‐fatal cardiovascular events (2 studies, 324 participants, OR 0.59, 95% CI 0.22 to 1.59) (Analysis 2.3) between the group receiving no treatment and those receiving warfarin. However, major bleeding was statistically more often observed in the group treated with warfarin compared to those in the control group (2 studies, 324 participants, OR 5.98, 95% CI 1.71 to 20.93) (Analysis 2.4).
2.4. Analysis.
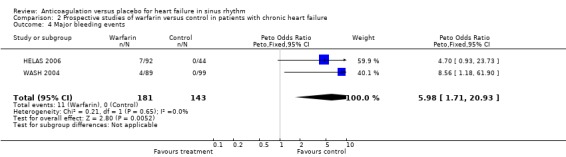
Comparison 2 Prospective studies of warfarin versus control in patients with chronic heart failure, Outcome 4 Major bleeding events.
In contrast, in the three quasi‐randomised trial (Anderson 1950; Griffith 1952; Harvey 1950) a statistically significant difference favouring the use of warfarin was observed for all cause death (OR 0.48, 95% CI 0.32 to 0.71; RR 2.0) (Analysis 1.1), cardiovascular death (OR 0.34, 95% CI 0.14 to 0.41; RR 2.30) (Analysis 1.2) and non‐fatal cardiovascular events (OR 0.24, 95% CI 0.14 to 0.41; RR 4.68) (Analysis 1.3). Major bleeding was not significantly different between those treated with warfarin and patients on placebo (Analysis 1.4). The high level of observed heterogeneity for major bleeding was likely due to differences in methodology such as the definitions used for bleeding events (clinical heterogeneity).
1.4. Analysis.
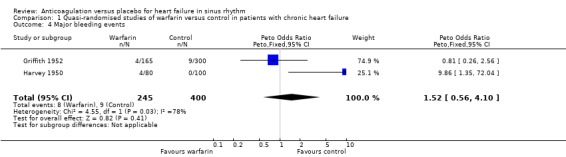
Comparison 1 Quasi‐randomised studies of warfarin versus control in patients with chronic heart failure, Outcome 4 Major bleeding events.
Discussion
Evidence from randomised and quasi‐randomised controlled trials
There have been five prospective controlled studies of oral anticoagulation in patients with heart failure (Anderson 1950; Griffith 1952; HELAS 2006; Harvey 1950; WASH 2004). Three of these studies were performed over 50 years ago in hospitalised patients with a high prevalence (up to 30%) of rheumatic disease and atrial fibrillation. The methods of randomisation used were open to bias and methods used to monitor the patients and determine patient inclusion and exclusion cannot be seen as reliable by modern standards.
Patient outcomes in the control arm of the WASH study were better (fewer deaths, cardiovascular events and adverse events such as bleeding) than for patients given active treatment with warfarin in the older, quasi‐randomised trials (Anderson 1950; Griffith 1952; Harvey 1950). This reflects changes both in the contemporary management of heart failure and in biases likely to be found in the older quasi‐randomised controlled trials (Anderson 1950; Griffith 1952; Harvey 1950) compared to the more modern design of WASH 2004.
The results from the only two randomised trials (HELAS 2006; WASH 2004) showed that the primary outcomes (mortality and cardiovascular events) were not affected by oral anticoagulation when compared to placebo. Therefore, these two studies do not support the routine use of oral anticoagulation therapy for patients with heart failure in sinus rhythm.
Adverse event data from non‐randomised studies
Four observational studies and four large scale non‐randomised cohort analyses of oral anticoagulation in heart failure or left ventricular systolic dysfunction were also assessed. No major haemorrhage was reported in the observational studies (Fuster 1981; Kyrle 1985; Natterson 1993) though non‐fatal bleeding was reported in two of the 82 patients treated with warfarin in the Naterson study. There was no record of bleeding complications in the EPICAL study (EPICAL 2002). No report of bleeding complications was made in the four large scale non‐randomised cohort analyses as these studies were originally designed to assess the value of ACE inhibitors and not antithrombotic therapy use per se (PROMISE 1993; SAVE 1997; SOLVD 1998; V‐HeFT 1993).
Evidence from non‐randomised sources
Evidence from the observational studies conflicted with that from non‐randomised (post hoc) comparisons from RCTs of heart failure.
Post hoc, non‐randomised comparisons from large trials on heart failure
The largest post hoc trial analysis included a high proportion of patients with ischaemic heart disease as the cause of left ventricular dysfunction. In this analysis warfarin therapy was associated with a significantly lower risk of all cardiovascular and sudden deaths (SOLVD 1998).
In a multivariate analysis, the point estimate for the overall risk reduction of sudden death was 32% for warfarin when compared to 25% for beta‐blockers, 24% for aspirin, and 11% for enalapril. In addition, multivariate analysis in patients considered to have non‐ischaemic heart failure also demonstrated a 70% risk reduction (SOLVD 1998). Similarly, observations in CONSENSUS 1987 suggested that (non‐randomised) long‐term anticoagulation with warfarin was associated with a 40% lower mortality (CONSENSUS 1987). Interestingly, 75% of the deaths in CONSENSUS 1987 were classified as due to progressive heart failure.
The Vasodilator Heart Failure Studies (V‐HeFT 1993) also provided detailed observational data regarding the effects of long‐term oral anticoagulation (V‐HeFT 1993). In V‐HeFT 1993 I, during 1068 patient‐years of follow‐up without antithrombotic therapy (aspirin or warfarin) there were 21 strokes, four recorded events of pulmonary embolism and four recorded events of peripheral embolism, with an overall incidence of 2.7 events in 100 patient‐years. In 208 patient‐years of follow‐up in patients receiving chronic oral anticoagulation with warfarin there were four strokes, one recorded pulmonary embolism and one recorded peripheral embolism, with an incidence of 2.9 events in 100 patient‐years (V‐HeFT 1993). There was no significant difference in the rates of thromboembolism between patients on long‐term warfarin therapy and those not on anticoagulation.
In V‐HeFT 1993 II, during 1188 patient‐years of follow‐up without antithrombotic therapy there were 23 strokes, one pulmonary embolism and one peripheral embolism, with an incidence of 2.1 events in 100 patient‐years. In the 247 patient‐years of follow‐up in patients receiving warfarin there were seven strokes, four pulmonary embolic events and one peripheral embolism, an overall incidence of 4.9 events per 100 patient‐years. Interestingly, the difference between the incidence of thromboembolic events in patients with and without warfarin was significantly higher in those receiving warfarin (P = 0.01) (V‐HeFT 1993). In addition, although data from the SOLVD 1998 trials suggest that anticoagulation was associated with a reduction in sudden cardiovascular deaths and all cause deaths, long‐term warfarin was not associated with a reduction in the total number of (fatal and non‐fatal) thromboembolic events (SOLVD 1998).
Similarly, in the SAVE 1997 trial warfarin use was associated with a 81% reduction in stroke risk (RR 0.19, 95% CI 0.13 to 0.27), but no direct comparison against aspirin (56% reduction) was made. One retrospective analysis of limited data from the PROMISE 1993 trial found that warfarin was used in 324 patients with a significant reduction in stroke in only those who had very severe heart failure (ejection fraction ≥ 20%, 0.6% versus 3.3% in the controls, P < 0.05).
Observational studies
Of the observational studies, the first was a retrospective study of 104 patients with idiopathic dilated cardiomyopathy, followed‐up for a total of 725 patient‐years. This study observed an 18% incidence of thromboembolic events (including those demonstrated at post‐mortem) in patients who were not receiving chronic oral anticoagulation (624 patient‐years with an estimated annual incidence of 3.5%) although no events were recorded in those who were anticoagulated (101 patient‐years) (Fuster 1981). In the second, a study of 38 patients with non‐ischaemic cardiomyopathy who were followed for a total of 72 patient‐years, the estimated incidence of thromboembolic events was 45 per 100 patient‐years in patients not receiving oral anticoagulation while no events were recorded in patients who were anticoagulated (Kyrle 1985). In contrast, a third more recent study of 224 patients awaiting cardiac transplantation reported an annual incidence of thromboembolism of 3.2% and failed to demonstrate a statistically significant difference in the rate of thromboembolism in the 37% of patients receiving (non‐randomised) warfarin therapy (Natterson 1993). The latter study reported an actual one year survival for patients receiving warfarin of 78% compared with 86% for patients not receiving warfarin (P = 0.30).
Importantly the positive early small studies (Fuster 1981; Kyrle 1985), which suggested an overall benefit from oral anticoagulation, were limited to patients with non‐ischaemic cardiomyopathy whilst approximately half of the patients in both V‐HeFT 1993 studies and 70% to 80% of those in SOLVD 1998 had definite coronary artery disease. The patient groups also differed in severity of heart failure. For example, in the PROMISE 1993 study (PROMISE 1993) the etiology of heart failure (ischaemic versus non‐ischaemic) did not predict stroke risk. In V‐HeFT 1993, patients were New York Heart Association (NYHA) class II or III (mean LVEF = 30%) (V‐HeFT 1993), whilst in the SOLVD 1998 prevention trial over 99% had NYHA class I or II but in the treatment trial two‐thirds had NYHA class I or II (SOLVD 1998). Of note, the EPICAL 2002 study (EPICAL 2002) found that all of the 417 patients with a mean left ventricular ejection fraction of 22%, NYHA class III or IV, 45% of ischaemic origin demonstrated a significant reduction in thromboembolic events. It is, therefore, possible that the efficacy of oral anticoagulation may differ according to the cause of heart failure, as patients with idiopathic cardiomyopathy may have a greater risk of cardiogenic thromboembolism whilst patients with atherosclerosis are also at risk of other vascular events including in situ coronary artery thrombosis. The PROMISE 1993 and EPICAL 2002 studies suggested that anticoagulation with warfarin was beneficial in patients with severe heart failure, in particular those with NYHA class III or IV.
Clearly, substantial problems exist in interpreting data from non‐randomised studies. Indeed, these four large scale non‐randomised cohort analyses and four observational studies of oral anticoagulation in heart failure included differing populations of heart failure patients (including asymptomatic left ventricular dysfunction) and reported contradictory results. Nevertheless, it is important to note that data from the SOLVD 1998 and VeHeFT studies were observational, without randomisation or a control with respect to oral anticoagulation. The decision to treat with warfarin was made by the study investigator, whilst the target INR, the average degree of anticoagulation, and the INR at the time of thromboembolic events were not reported in either of these studies. The diagnosis of peripheral and pulmonary embolism and stroke was made by participating investigators rather than according to the study protocol. In addition, the interpretation of these data are potentially confounded as it is possible that patients who were considered to be at the highest risk of thromboembolism were treated with warfarin and that this substantially reduced the long‐term risk of thromboembolic events in these patients.
In conclusion, although oral anticoagulation is indicated in certain groups of patients with heart failure (for example those with atrial fibrillation), the present (limited) data does not support its routine use in heart failure patients who remain in sinus rhythm, although full data from a modern randomised trial of warfarin in heart failure patients in sinus rhythm are awaited (WARCEF 2012).
Authors' conclusions
Implications for practice.
As anticoagulation therapy is itself not without risk, clinicians contemplating antithrombotic therapy for prophylaxis against stroke and thromboembolic events in patients with heart failure have to balance the benefit of risk reduction against the risks of potentiating haemorrhage with warfarin therapy. Data from large, randomised controlled trials are lacking but the two existing small randomised studies (HELAS 2006; WASH 2004) do not support the routine use of oral anticoagulation over no therapy.
Based on current evidence, patients with heart failure with poor cardiac function or idiopathic dilated cardiomyopathy, atrial fibrillation and a protruding, mobile left ventricular thrombus on cardiac imaging are probably at highest risk and require anticoagulant therapy. If patients are in sinus rhythm, anticoagulants should perhaps be reserved especially for patients with severe cardiac impairment, the presence of intracardiac thrombus, and previous thromboembolism or stroke. Thus, until more evidence becomes available clinical decisions to treat patients with heart failure with anticoagulants must be made on an individual basis, based upon individual benefits and risks.
Implications for research.
The clinical question of comparing anticoagulation with placebo for heart failure in patients in sinus rhythm is now an outdated scope and future research will be directed towards randomised trials comparing antiplatelet agents versus control or anticoagulation. Data from large scale randomised controlled trials in ambulant patients with heart failure are needed to evaluate the effectiveness of anticoagulant therapy and antiplatelet therapy.
Feedback
Cochrane Editorial Unit's report on feedback on anticoagulants reviews, 15 February 2011
Summary
Feedback received on this review, and other reviews and protocols on anticoagulants, is available on the Cochrane Editorial Unit website at http://www.editorial‐unit.cochrane.org/anticoagulants‐feedback.
Reply
N/A
Contributors
N/A
What's new
| Date | Event | Description |
|---|---|---|
| 20 April 2020 | Amended | Minor correction of search date in abstract. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 2 March 2020 | Amended | Clarification message from the Co‐ordinating Editor added to the Declarations of interest Declarations of interest statement about the review’s compliance with the Cochrane conflict of interest policy, which includes the relevant parts of the Cochrane Commercial Sponsorship Policy. |
| 6 January 2014 | New search has been performed | An updated search has been performed to 14 June 2013. No new trial has been included in this update and the overall conclusion of the review has not changed. |
| 15 February 2011 | Feedback has been incorporated | Added a link to the Cochrane Editorial Unit's report on feedback on anticoagulants reviews. |
| 15 February 2011 | New citation required but conclusions have not changed | New author added. |
| 11 February 2010 | Amended | Error in dates: 'assessed as up‐to‐date' and 'date of search' due to RevMan conversion has been corrected. |
| 20 April 2009 | Amended | Author details error due to RevMan conversion has been corrected. |
| 8 September 2008 | Amended | Converted to new review format. |
| 8 February 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We acknowledge the support of the Sandwell and West Birmingham Hospitals NHS Trust Research and Development Programme for the Haemostasis Thrombosis and Vascular Biology Unit. The contributions of Dr C Gibbs, Dr I Chung, Dr R Pisters and Dr B Wrigley to a previous version of this review are also acknowledged.
Appendices
Appendix 1. Search strategies 2005
CENTRAL
1 HEART‐FAILURE‐CONGESTIVE*:ME 2 (CARDIAC near FAILURE) 3 (HEART near FAILURE) 4 ((#1 or #2) or #3) 5 ANTICOAGULANTS*:ME 6 ANTICOAGULANT* 7 ANTI‐COAGULANT* 8 ANTITHROMBINS*:ME 9 ANTITHROMB* 10 ANTI‐THROM* 11 COUMARINS*:ME 12 COUMARIN* 13 WARFARIN 14 WARFARIN*:ME 15 DICOUMAROL 15 (#5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15) 16 (#4 and #15)
MEDLINE
1 exp Heart Failure, Congestive/ 2 heart failure.tw. 3 cardiac failure.tw. 4 or/1‐3 5 exp ANTICOAGULANTS/ 6 exp Coumarins/ 7 warfarin.tw. 8 dicoumarol.tw. 9 coumarin$.tw. 10 or/5‐9 11 4 and 10 12 randomized controlled trial.pt. 13 controlled clinical trial.pt. 14 Randomized controlled trials/ 15 random allocation.sh. 16 double blind method.sh. 17 single‐blind method.sh. 18 or/12‐17 19 exp animal/ not human/ 20 18 not 19 21 clinical trial.pt. 22 exp Clinical trials/ 23 (clin$ adj25 trial$).ti,ab. 24 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. 25 placebos.sh. 26 placebo$.ti,ab. 27 random$.ti,ab. 28 research design.sh. 29 or/21‐28 30 29 not 19 31 30 not 20 32 comparative study.sh. 33 exp evaluation studies/ 34 follow up studies.sh. 35 prospective studies.sh. 36 (control$ or prospectiv$ or volunteer$).ti,ab. 37 or/32‐36 38 37 not 19 39 38 not (20 or 31) 40 20 or 31 or 39 41 11 and 40
EMBASE
1 exp Heart Failure/ 2 heart failure.tw. 3 cardiac failure.tw. 4 or/1‐3 5 Anticoagulant Agent/ 6 exp Coumarin Anticoagulant/ 7 warfarin.tw. 8 dicoumarol.tw. 9 coumarin$.tw. 10 or/5‐9 11 4 and 10 12 random$.ti,ab. 13 factorial$.ti,ab. 14 (crossover$ or cross over$ or cross‐over$).ti,ab. 15 placebo$.ti,ab. 16 (double$ adj blind$).ti,ab. 17 (singl$ adj blind$).ti,ab. 18 assign$.ti,ab. 19 allocat$.ti,ab. 20 volunteer$.ti,ab. 21 Crossover Procedure/ 22 Double Blind Procedure/ 23 Randomized Controlled Trial/ 24 Single Blind Procedure/ 25 or/12‐24 26 exp animal/ 27 nonhuman/ 28 exp animal experiment/ 29 or/26‐28 30 exp human/ 31 29 not 30 32 25 not 31 33 11 and 32
Appendix 2. Search strategies 2010
CENTRAL
#1 MeSH descriptor heart failure explode all trees #2 heart next failure in All Text #3 cardiac next failure in All Text #4 (#1 or #2 or #3) #5 MeSH descriptor anticoagulants this term only #6 MeSH descriptor coumarins this term only #7 MeSH descriptor 4‐Hydroxycoumarins explode all trees #8 warfarin in All Text #9 dicoumarol in All Text #10 dicumarol in All Text #11 coumarin* in All Text #12 anticoagulant* in All Text #13 anti‐coagulant* in All Text #14 (#5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13) #15 MeSH descriptor platelet aggregation inhibitors explode all trees #16 antiplatelet* in All Text #17 anti‐platelet* in All Text #18 aspirin in All Text #19 ticlopidine in All Text #20 clopidogrel in All Text #21 dipyridamole in All Text #22 antithrombocytic in All Text #23 "acetyl salicylic acid" in All Text #24 acetylsalicylic in All Text #25 (#15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24) #26 (#14 or #25) #27 (#4 and #26)
MEDLINE
1 exp Heart Failure/ 2 heart failure.tw. 3 cardiac failure.tw. 4 or/1‐3 5 Anticoagulants/ 6 Coumarins/ 7 exp 4‐Hydroxycoumarins/ 8 warfarin.tw. 9 dicoumarol.tw. 10 dicumarol.tw. 11 coumarin$.tw. 12 or/5‐11 13 exp Platelet Aggregation Inhibitors/ 14 antiplatelet$.tw. 15 anti‐platelet$.tw. 16 aspirin.tw. 17 ticlopidine.tw. 18 clopidogrel.tw. 19 dipyridamole.tw. 20 or/13‐19 21 4 and (12 or 20) 22 randomized controlled trial.pt. 23 controlled clinical trial.pt. 24 randomized.ab. 25 placebo.ab. 26 exp Clinical Trials as Topic/ 27 randomly.ab. 28 trial.ti. 29 or/22‐28 30 exp animal/ not humans/ 31 29 not 30 32 21 and 31
EMBASE
1 exp Heart Failure/ 2 heart failure.tw. 3 cardiac failure.tw. 4 or/1‐3 5 anticoagulant agent/ 6 exp coumarin anticoagulant/ 7 warfarin.tw. 8 dicoumarol.tw. 9 dicumarol.tw. 10 coumarin$.tw. 11 or/5‐10 12 exp antithrombocytic agent/ 13 antiplatelet$.tw. 14 anti‐platelet$.tw. 15 aspirin.tw. 16 ticlopidine.tw. 17 clopidogrel.tw. 18 dipyridamole.tw. 19 or/12‐18 20 4 and (11 or 19) 21 random$.tw. 22 factorial$.tw. 23 (crossover$ or cross‐over$).tw. 24 placebo$.tw. 25 (doubl$ adj blind$).tw. 26 (singl$ adj blind$).tw. 27 assign$.tw. 28 allocat$.tw. 29 volunteer$.tw. 30 Crossover Procedure/ 31 Double‐blind Procedure/ 32 Randomized Controlled Trial/ 33 Single‐blind Procedure/ 34 or/21‐33 35 (animal/ or nonhuman/) not human/ 36 34 not 35 37 20 and 36
Appendix 3. Search strategies 2013
CENTRAL
#1MeSH descriptor: [Heart Failure] explode all trees #2heart near/2 failure* #3cardiac near/2 failure* #4myocardial near/2 failure* #5heart near/2 decompensat* #6#1 or #2 or #3 or #4 or #5 #7MeSH descriptor: [Anticoagulants] this term only #8MeSH descriptor: [Coumarins] this term only #9MeSH descriptor: [4‐Hydroxycoumarins] explode all trees #10warfarin #11dicoumarol #12dicumarol #13coumarin* #14anticoagulant* or anti‐coagulant* #15indirect next thrombin next inhibitor* #16benzopyron* or benzopyran* #17#7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 #18MeSH descriptor: [Platelet Aggregation Inhibitors] explode all trees #19antiplatelet* or anti‐platelet* #20antithrombocytic or "acetyl salicylic acid" or acetylsalicylic #21aspirin #22ticlopidine #23clopidogrel #24dipyridamole #25platelet near/2 (antagonist* or inhibit* or antiaggregant*) #26#18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 #27#17 or #26 #28#6 and #27
MEDLINE
1. exp Heart Failure/ 2. (heart adj2 failure*).tw. 3. (cardiac adj2 failure*).tw. 4. (myocardial adj2 failure*).tw. 5. (heart adj2 decompensat*).tw. 6. heart failure.tw. 7. cardiac failure.tw. 8. 1 or 6 or 7 9. 1 or 2 or 3 or 4 or 5 10. Anticoagulants/ 11. Coumarins/ 12. exp 4‐Hydroxycoumarins/ 13. warfarin.tw. 14. dicoumarol.tw. 15. dicumarol.tw. 16. coumarin$.tw. 17. anticoagulant*.tw. 18. indirect thrombin inhibitor*.tw. 19. benzopyr?n*.tw. 20. 10 or 11 or 12 or 13 or 14 or 15 or 16 21. 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 22. exp Platelet Aggregation Inhibitors/ 23. antiplatelet$.tw. 24. anti‐platelet$.tw. 25. aspirin.tw. 26. ticlopidine.tw. 27. clopidogrel.tw. 28. dipyridamole.tw. 29. (platelet adj2 (antagonist* or inhibit* or antiaggregant*)).tw. 30. 22 or 23 or 24 or 25 or 26 or 27 or 28 31. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 32. 20 or 30 33. 21 or 31 34. 8 and 32 35. 9 and 33 36. randomized controlled trial.pt. 37. controlled clinical trial.pt. 38. randomized.ab. 39. placebo.ab. 40. drug therapy.fs. 41. randomly.ab. 42. trial.ab. 43. groups.ab. 44. 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 45. exp animals/ not humans.sh. 46. 44 not 45 47. 34 and 46 48. 35 and 46 49. ((2010* or 2011* or 2012* or 2013*) not 201001*).ed. 50. 47 and 49 51. 48 not 47 52. 50 or 51
EMBASE
1. exp heart failure/ 2. heart failure.tw. 3. cardiac failure.tw. 4. (heart adj2 failure*).tw. 5. (cardiac adj2 failure*).tw. 6. (myocardial adj2 failure*).tw. 7. (heart adj2 decompensat*).tw. 8. 1 or 2 or 3 9. 1 or 4 or 5 or 6 or 7 10. anticoagulant agent/ 11. exp coumarin anticoagulant/ 12. warfarin.tw. 13. dicoumarol.tw. 14. dicumarol.tw. 15. coumarin$.tw. 16. anticoagulant*.tw. 17. indirect thrombin inhibitor*.tw. 18. benzopyr?n*.tw. 19. 10 or 11 or 12 or 13 or 14 or 15 20. 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 21. exp antithrombocytic agent/ 22. antiplatelet$.tw. 23. anti‐platelet$.tw. 24. aspirin.tw. 25. ticlopidine.tw. 26. clopidogrel.tw. 27. dipyridamole.tw. 28. (platelet adj2 (antagonist* or inhibit* or antiaggregant*)).tw. 29. 21 or 22 or 23 or 24 or 25 or 26 or 27 30. 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 31. 19 or 29 32. 20 or 30 33. 8 and 31 34. 9 and 32 35. random$.tw. 36. factorial$.tw. 37. crossover$.tw. 38. cross over$.tw. 39. cross‐over$.tw. 40. placebo$.tw. 41. (doubl$ adj blind$).tw. 42. (singl$ adj blind$).tw. 43. assign$.tw. 44. allocat$.tw. 45. volunteer$.tw. 46. crossover procedure/ 47. double blind procedure/ 48. randomized controlled trial/ 49. single blind procedure/ 50. 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 51. (animal/ or nonhuman/) not human/ 52. 50 not 51 53. 33 and 52 54. 34 and 52 55. ((2010* or 2011* or 2012* or 2013*) not 201001*).dd. 56. 53 and 55 57. 54 not 53 58. 56 or 57 59. limit 58 to embase
Data and analyses
Comparison 1. Quasi‐randomised studies of warfarin versus control in patients with chronic heart failure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All cause deaths | 3 | 942 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.32, 0.71] |
| 2 Cardiovascular deaths (stroke, myocardial infarction, pulmonary embolism, peripheral arterial embolism) and sudden deaths | 3 | 942 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.24, 0.47] |
| 3 Non‐fatal cardiovascular events (non‐fatal stroke, myocardial infarction, pulmonary embolism, peripheral arterial embolism) | 3 | 942 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.24 [0.14, 0.41] |
| 4 Major bleeding events | 2 | 645 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.52 [0.56, 4.10] |
Comparison 2. Prospective studies of warfarin versus control in patients with chronic heart failure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All cause deaths | 2 | 324 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.36, 1.18] |
| 2 Cardiovascular deaths (stroke, myocardial infarction, pulmonary embolism, peripheral arterial embolism) and sudden deaths | 2 | 324 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.58, 1.65] |
| 3 Non‐fatal cardiovascular events (non‐fatal stroke, myocardial infarction, pulmonary embolism, peripheral arterial embolism) | 2 | 324 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.22, 1.59] |
| 4 Major bleeding events | 2 | 324 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.98 [1.71, 20.93] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anderson 1950.
| Methods | Controlled, prospective | |
| Participants | Heart failure | |
| Interventions | Dicoumarol | |
| Outcomes | Death, stroke, pulmonary and peripheral embolism | |
| Notes | 297 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised |
| Allocation concealment (selection bias) | High risk | Not randomised |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
Griffith 1952.
| Methods | Controlled prospective | |
| Participants | Heart failure | |
| Interventions | Tromexan, dicoumarol ± heparin | |
| Outcomes | Death, stroke, pulmonary and peripheral embolism | |
| Notes | 465 participants ‐ patients with rheumatic heart disease (n=90) were excluded from this analysis in view of marked benefit in this group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised |
| Allocation concealment (selection bias) | High risk | Not randomised |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
Harvey 1950.
| Methods | Controlled prospective | |
| Participants | Heart failure | |
| Interventions | Warfarin | |
| Outcomes | Death, stroke, pulmonary and peripheral embolism | |
| Notes | 180 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised |
| Allocation concealment (selection bias) | High risk | Not randomised |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
HELAS 2006.
| Methods | Randomised, controlled | |
| Participants | Heart failure, sinus rhythm | |
| Interventions | Warfarin versus aspirin (underlying ischaemic etiology): warfarin versus placebo (underlying idiopathic dilated cardiomyopathy). Only the 'warfarin versus placebo' trial arm(s) were used in this review | |
| Outcomes | Non‐fatal stroke, peripheral or pulmonary embolism, MI, re‐hospitalisation, exacerbation of heart failure, death | |
| Notes | n= 197 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patients were randomised to receive' Comment: probably done |
| Allocation concealment (selection bias) | Low risk | 'patients were randomised to receive' Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient were included in the follow‐up analysis and an independent data committee conducted interim analyses to determine whether continuation of any of the treatment arms might be detrimental to the patients Comment: probably done |
| Selective reporting (reporting bias) | Low risk | The results of the study showed that treatment had no effect on outcome. This was therefore a negative study Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'double‐blind study' 'the investigator‐supervisor was blinded to the study' 'placebo tablets were given daily or according to sham adjustment' Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'the study was monitored by an independent data and safety monitoring committee' Comment: probably done |
WASH 2004.
| Methods | Pilot RCT of WATCH study | |
| Participants | Heart failure (ejection fraction < 40%) | |
| Interventions | Warfarin versus aspirin versus no antithrombotic therapy. Only the warfarin versus no antithrombotic therapy was used in this review | |
| Outcomes | Death, cardiovascular events (including hospitalisations) | |
| Notes | n=279 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Patients were randomised to receive no treatment, aspirin or warfarin Comment: probably done |
| Selective reporting (reporting bias) | Low risk | This was a negative trial in terms of showing no differences in primary endpoint between treatment versus no treatment Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open label trial and is therefore open to performance bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| CONSENSUS 1987 | post hoc retrospective analysis of ACE inhibitor trial |
| EPICAL 2002 | outcomes for patients in sinus rhythm were not reported separately |
| Fuster 1981 | observational, non‐randomised or controlled, cohort study |
| Kyrle 1985 | observational, non‐randomised or controlled, cohort study |
| Natterson 1993 | observational, non‐randomised or controlled, cohort study |
| PROMISE 1993 | observational, non‐randomised or controlled, cohort study |
| SAVE 1997 | post hoc retrospective analysis of ACE inhibitor trial |
| SOLVD 1998 | post hoc retrospective analysis of ACE inhibitor trial |
| V‐HeFT 1993 | post hoc retrospective analysis of ACE inhibitor trial |
| Visser 2004 | cohort study of people on anticoagulants for varied diagnoses |
| WARCEF 2012 | a study comparing warfarin to aspirin, not to placebo |
| WATCH 2009 | a study comparing warfarin to aspirin or clopidogrel, not to placebo |
| Wishart 1948 | case series, conducted over 50 years ago in heterogeneous CHF population (n=61), and methodology considered inappropriate |
Contributions of authors
Professor GYH Lip Conceived the review and obtained funding. Helped interpret data and provided a methodological, policy and clinical perspective on the data Participated in writing the review For purposes of dual data collection screened papers for inclusion or exclusion and extracted data from papers that were to be included Co‐ordinated the review process
Dr I Chung, Dr R Pisters, Dr B J Wrigley, and Dr E Shantsila Developed and ran the search strategy, screened the results. Organised the retrieval of papers. Appraised papers and extracted data.
The contributions of Dr C Gibbs, Dr I Chung, Dr R Pisters and Dr B Wrigley to previous versions of this review are acknowledged.
Sources of support
Internal sources
Sandwell and West Birmingham Hospitals NHS Trust, Birmingham, UK.
External sources
No sources of support supplied
Declarations of interest
The study unit was a centre for the WATCH trial for which drugs and unrestricted grants were provided by Bristol Myers Squibb, Sanofi‐Synthelabo and Dupont pharmaceutical companies. It was also a centre for the WARCEF study, as well as other trials of antithrombotic therapy in CVD and stroke.
Clarification statement added from the Co‐ordinating Editor on 2 March 2020: This review was found by the Cochrane Funding Arbiters, post‐publication, to be noncompliant with the Cochrane conflict of interest policy, which includes the relevant parts of the Cochrane Commercial Sponsorship Policy. It will be updated within a year. The update will have a majority of authors and lead author free of conflicts.
Edited (no change to conclusions)
References
References to studies included in this review
Anderson 1950 {published data only}
- Anderson GM, Hull E. The effects of dicumarol upon the mortality and incidence of thromboembolic complications in congestive heart failure. American Heart Journal 1950;39:697‐702. [DOI] [PubMed] [Google Scholar]
Griffith 1952 {published data only}
- Griffith GC, Stragnell R, Levinson DC, Moore FJ, Ware AG. A study of the beneficial effects of anticoagulant therapy in congestive heart failure. Annals of Internal Medicine 1952;37:867‐87. [DOI] [PubMed] [Google Scholar]
Harvey 1950 {published data only}
- Harvey WP, Finch CA. Dicumarol prophylaxis of thromboembolic disease in congestive heart failure. New England Journal of Medicine 1950;242:208‐11. [DOI] [PubMed] [Google Scholar]
HELAS 2006 {published data only}
- Cokkinos DV, Haralabopoulos GC, Kostis JB, Toutouzas PK. Efficacy of antithrombotic therapy in chronic heart failure: The HELAS study. The European Journal of Heart Failure 2006;8:428‐432. [DOI] [PubMed] [Google Scholar]
WASH 2004 {published data only}
- Cleland JG, Findlay I, Jafri S, Sutton G, Falk R, Bulpitt C, et al. The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure. American Heart Journal 2004;148:157‐64. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
CONSENSUS 1987 {published data only}
- Swedberg K, for the CONSENSUS trial study group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). New England Journal of Medicine 1987;316:1429‐35. [DOI] [PubMed] [Google Scholar]
EPICAL 2002 {published data only}
- Echemann M, Alla F, Briancon S, Juilliere Y, Virion JM, Mertes PM, et al. Antithrombotic therapy is associated with better survival in patients with severe heart failure and left ventricular systolic dysfunction (EPICAL study) [Epidemiologie de l'insuffisance cardiaque avancee en Lorraine]. European Journal of Heart Failure 2002;4(5):647‐54.. [DOI] [PubMed] [Google Scholar]
Fuster 1981 {published data only}
- Fuster V, Gersh BJ, Giuliani ER, Tajik AJ, Bradenburg RO, Frye RL. The natural history of idiopathic dilated cardiomyopathy. American Journal of Cardiology 1981;47:525‐31. [DOI] [PubMed] [Google Scholar]
Kyrle 1985 {published data only}
- Kyrle PA, Korninger C, Gossinger H, Glogar D, Lechner K, Niessner H, et al. Prevention of arterial and pulmonary embolism by oral anticoagulants in patients with dilated cardiomyopathy. Thrombosis and Haemostasis 1985;54:521‐3. [PubMed] [Google Scholar]
Natterson 1993 {published data only}
- Natterson PD, Stevenson WG, Saxon LA, Middlekauff HR, Stevenson LW. Low risk of arterial embolization in outpatients awaiting cardiac transplantation [abstract]. Circulation 1993;88 Suppl I:603. [Google Scholar]
PROMISE 1993 {published data only}
- Falk R, Pollack A, Tandon PK, Packer M. The effect of warfarin on prevalence of stroke in severe heart failure. Journal of the American College of Cardiology 1993;21 Suppl A:218A. [Google Scholar]
SAVE 1997 {published data only}
- Loh E, Sutton MS, Wun CC, Rouleau JL, Flaker GC, Gottlieb SS, et al. Ventricular dysfunction and the risk of stroke after myocardial infarction. New England Journal of Medicine 1997;336:251‐7. [DOI] [PubMed] [Google Scholar]
SOLVD 1998 {published data only}
- Al‐Khadra AS, Salem DN, Rand WM, Udelson JE, Smith JJ, Konstam MA. Warfarin anticoagulation and survival: a cohort analysis from the Studies of Left Ventricular Dysfunction. Journal of the American College of Cardiology 1998;31:749‐53. [DOI] [PubMed] [Google Scholar]
V‐HeFT 1993 {published data only}
- Dunkman WB, Johnson GR, Carson PE, Bhat G, Farrell L, Cohn JN, for the V‐HeFT VA Cooperative Studies Group. Incidence of thromboembolic events in congestive heart failure. Circulation 1993;87 Suppl VI:94‐101. [PubMed] [Google Scholar]
Visser 2004 {published data only}
- Visser LE, Bleumink GS, Trienekens PH, Vulto AG, Hofman A, Stricker BH. The risk of overanticoagulation in patients with heart failure on coumarin anticoagulants. British Journal of Haematology. 2004;127(1):85‐9. [DOI] [PubMed] [Google Scholar]
WARCEF 2012 {published data only}
- Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, et al. WARCEF Investigators. Warfarin and aspirin in patients with heart failure and sinus rhythm. New England Journal of Medicine 2012;366:1859‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
WATCH 2009 {published data only}
- Messie BM, Collins JF, Ammon SE, Armstrong PW, Cleland JG, Ezekowitz M, et al. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation Mar 31;119(12):1616‐24. [DOI] [PubMed] [Google Scholar]
Wishart 1948 {published data only}
- Wishart JH, Chapman CB. Dicumarol therapy in congestive heart failure. New England Journal of Medicine 1948;239:701‐4. [DOI] [PubMed] [Google Scholar]
Additional references
BAATAF 1990
- The Boston Area Anticoagulation Trial for Atrial Fibrillation (BAATAF) Investigators. The effect of low‐dose warfarin on the risk of stroke in patients with non‐rheumatic atrial fibrillation. New England Journal of Medicine 1990;323:1505‐11. [DOI] [PubMed] [Google Scholar]
Brown 1998
- Brown A, Cleland JGF. Influence of concomitant disease on patterns of hospitalisation in patients with heart failure discharged from Scottish hospitals in 1995. European Heart Journal 1998;19:1063‐9. [PubMed] [Google Scholar]
Davis 1977
- Davis FB, Estruch MT, Samson‐Corvera EB, Voigt GC, Tobin JD. Management of anticoagulation in outpatients: experience with an anticoagulation service in a municipal hospital setting. Archives of Internal Medicine 1977;137:197‐202. [PubMed] [Google Scholar]
Edep 1997
- Edep ME, Shah NB, Massie BM. Differences between primary care physicians and cardiologists in the management of congestive heart failure: relationship to practice guidelines. Journal of the American College of Cardiology 1997;30:518‐26. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Reviewers’ Handbook 5.1.0 [updated March 2011] The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org..
Husted 1976
- Husted S, Andraesen F. Problems encountered in long‐term treatment with anticoagulants. Acta Medica Scandinavica 1976;200:379‐84. [DOI] [PubMed] [Google Scholar]
Landefeld 1989
- Landefeld CS, Goldman L. Major bleeding in outpatients treated with warfarin. Incidence and prediction by factors known at the start of outpatient therapy. American Journal of Medicine 1989;87:144‐52. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lebvre C, Manheimer E, Glanville J. Chapter 6: Searching for Studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Petersen 1989
- Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo‐controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation: the Copenhagen AFASAK Study. Lancet 1989;I:175‐9. [DOI] [PubMed] [Google Scholar]
SPAF 1991
- Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Stroke Prevention in Atrial Fibrillation Investigators. Circulation 1991;84:527‐39. [DOI] [PubMed] [Google Scholar]


