Abstract
Background
Various pharmacologic and non‐pharmacologic interventions have been used to suppress lactation after childbirth and relieve associated symptoms. Despite the large volume of literature on the subject, there is currently no universal guideline on the most appropriate approach for suppressing lactation in postpartum women.
Objectives
To evaluate the effectiveness and safety of interventions used for suppression of lactation in postpartum women (who have not breastfed or expressed breastmilk) to determine which approach has the greatest comparative benefits with least risk.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 June 2012).
Selection criteria
Randomised trials evaluating the effectiveness of treatments used for suppression of postpartum lactation.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data.
Main results
We included 62 trials (6428 women). Twenty‐two trials did not contribute data to the meta‐analyses. The trials were generally small and of limited quality. Three trials (107 women) indicated that bromocriptine significantly reduced the proportion of women lactating compared with no treatment at or within seven days postpartum (three trials, 107 women; risk ratio (RR) 0.36, 95% confidence interval (CI) 0.24 to 0.54). Seven trials involving oestrogen preparations (diethylstilbestrol, quinestrol, chlorotrianisene, hexestrol) suggested that they significantly reduced the proportion of lactating women compared with no treatment at or within seven days postpartum (RR 0.40, 95% CI 0.29 to 0.56). We found no trials comparing non‐pharmacologic methods with no treatment. Trials comparing bromocriptine with other pharmacologic agents such as methergoline, prostaglandins, pyridoxine, carbegoline, diethylstilbestrol and cyclofenil suggested similarity in their effectiveness. Side effects were poorly reported in the trials and no case of thromboembolism was recorded in the four trials that reported it as an outcome.
Authors' conclusions
There is weak evidence that some pharmacologic treatments (most of which are currently unavailable to the public) are better than no treatment for suppressing lactation symptoms in the first postpartum week. No evidence currently exists to indicate whether non‐pharmacologic approaches are more effective than no treatment. Presently, there is insufficient evidence to address the side effects of methods employed for suppressing lactation. When women desire treatment, bromocriptine may be considered where it is registered for lactation suppression in those without predisposition to its major side effects of public concerns. Many trials did not contribute data that could be included in analyses. Large randomised trials are needed to compare the effectiveness of pharmacologic (especially bromocriptine) and non‐pharmacologic methods with no treatment. Such trials should consider the acceptability of the intervention and lactation symptoms of concern to women and be large enough to detect clinically important differences in major side effects between comparison groups.
Plain language summary
Treatments for suppression of lactation
Women cannot always breastfeed after birth. Reasons may be because the infant dies or is adopted, or the mother is too ill, or for the well being of the mother or infant. HIV‐positive mothers, particularly those not on antiretroviral drugs during pregnancy, avoid breastfeeding to reduce the risk of passing on the virus to their infants. Some mothers do not breastfeed on personal or social grounds. Without an infant suckling, milk production (lactation) eventually stops of its own accord. In the meantime, women can experience breast engorgement, leakage of milk, discomfort and pain. Clinicians may provide treatment to suppress lactation and reduce these symptoms. Binding the breasts or wearing a tight brassiere, applying an infra‐red lamp, fluid and diet restrictions, external application of jasmine flower and ice packs are tried non‐drug approaches. Drug treatments include oestrogens and bromocriptine which lowers prolactin levels. However, increased risks of thromboembolism, cerebral accident and myocardial infarction have been reported with their use.
The evidence to support treatments for preventing lactation is limited. The review authors identified 62 controlled trials that randomised a total of 6428 mothers to receive the treatment under investigation, no treatment or another treatment. Twenty‐two trials did not contribute data to the meta‐analyses. The trials were generally of limited quality and most were conducted among healthy women who chose not to breastfeed for personal reasons at hospitals in industrialised countries before 1980. Half of the trials involved bromocriptine. Two trials (107 women) reported that taking bromocriptine was better than no treatment in suppressing lactation in the first week after giving birth. The 11 trials using oestrogen preparations (diethylstilbestrol, quinestrol, chlorotrianisene, hexestrol) also showed suppression of lactation. A combination of testosterone and oestrogen preparations was of some benefit in reducing symptoms in three trials (436 women). Other pharmacologic agents (clomiphene, tamoxifen, prostaglandins, pyridoxine, oxytocin, L‐dopa and homeopathic preparation) were tested in single small trials. Generally, side effects were poorly reported and no case of thromboembolism was recorded among trials that included it as an adverse treatment outcome. Most of the drugs tested are currently not available or registered for suppressing lactation. No trials compared non‐drug approaches with no treatment and none of the included trials provided reliable data on women’s satisfaction with the treatment.
Background
Description of the condition
Indications for lactation suppression
For many years, the importance of breastfeeding to both infants and mothers has been emphasised by healthcare providers and various strategies have been employed to promote it globally. In spite of the well‐known advantages of breastfeeding (for example, infant protection against diarrhoeal morbidity and mortality), there are instances when the well being of the mother or infant requires suppression of lactation. Suppression of lactation becomes essential when breastfeeding is no longer required (as in the events of perinatal death and infant adoption) or when the mother is too ill to breastfeed (as in cases of severe obstetric morbidity). Besides medical indications, some mothers in circumstances where alternatives to breastfeeding exist may seek lactation suppression on personal or social grounds. It is estimated that over 30% of women in the United States and United Kingdom do not breastfeed their infants, while a larger proportion discontinue breastfeeding within two weeks of childbirth (Hamlyn 2002; Ryan 2002). Although physiologic cessation of lactation eventually occurs in the absence of physical stimulus such as infant suckling, a variable proportion of women experience moderate to severe milk leakage and discomfort, before lactation ceases. Up to two‐thirds of non‐breastfeeding women experience moderate to severe engorgement and breast pain when no treatment is applied (Spitz 1998). Almeida and Kitay (Almeida 1986) showed that breast engorgement was responsible for puerperal fever in 13.3% of 75 non‐breastfeeding mothers. However, the prevalence, characteristics and health implications of these symptoms have not been well described in the literature.
Lactation suppression and prevention of vertical transmission of HIV
Unlike in the 1970s, when a social reason was the most common indication for lactation suppression (Eastham 1976), the need for complete avoidance of breastfeeding by HIV‐positive mothers to reduce the risk of vertical transmission of HIV has offered a more compelling reason in the last decade. Postnatal transmission through breastfeeding accounts for one‐third to one‐half of all cases of vertical HIV transmission worldwide, with an estimated 16.2% rate of transmission for infants of women untreated with antiretroviral drugs during pregnancy (Nduati 2000). Although breastfed infants of HIV‐positive mothers who receive antiretroviral treatment during pregnancy are less likely to be infected with HIV (Wiktor 1999), the risk is further reduced when such infants are fed with substitutes of breastmilk (Shaffer 1999). Therefore, as the global prevalence of HIV continues to rise, the need for supervised inhibition of lactation may likely become increasingly relevant, especially in developed countries where safe alternatives of infant feeding are available. The symptoms associated with physiologic cessation of lactation may further compromise the physical and emotional status of the HIV‐positive mothers and an effective method of suppressing lactation is desirable to avoid additional morbidity.
Description of the intervention
Non‐pharmacologic methods of lactation suppression
Interventions to suppress lactation in non‐breastfeeding women have evolved for centuries. Healthcare providers have used different non‐pharmacologic approaches to suppress lactation and relieve the associated symptoms. Before the 20th century, these approaches included breast binding or strapping, emptying of the breast by massage, fluid and diet restrictions and application of external products such as belladonna ointment to the breast and nipples. Later, the avoidance of tactile breast stimulation and application of external agents such as cabbage leaves, jasmine flower and ice packs were included. Although many of these methods are still in use today, data on their efficacy are few and inconclusive. A review by Spitz et al (Spitz 1998) showed that up to one‐third of women may experience severe breast pain for most of the first postpartum week when these methods of lactation suppression are employed.
Pharmacologic methods of lactation suppression
In the 1960s, oestrogen preparations given alone or in combination with androgens were demonstrated to be effective in 40% to 100% of women (Llewellyn‐Jones 1968; Senior 1969) but their reported association with a high rate of rebound lactation (resurgence of lactation following cessation of treatment) and increased risk of thrombosis and pulmonary embolism discouraged their use (Jeffcoate 1968). After it was demonstrated that postpartum lactation depends primarily on pituitary prolactin secretion, the synthetic dopamine agonist and strong prolactin inhibitor bromocriptine was introduced in 1972. Its efficacy in the suppression of postpartum lactation is well documented (Bhardwaj 1979; Dewhurst 1977; Duchesne 1981; Van der Heijden 1991). It is, however, associated with some unpleasant side effects and requires administration for about 10 to 14 days to prevent rebound lactation. It has also been implicated in serious puerperal complications such as cerebral accident and myocardial infarction (Iffy 1996; Ruch 1989). In 1989, the United States Food and Drug Administration recommended against the routine use of bromocriptine for suppression of postpartum lactation, noting that while there was no clear proof of adverse effects, there were also no proven health benefits (US FDA 1989). In spite of this development, bromocriptine is still being used in many countries. Since then, many other drugs have been used for suppression of lactation, including those with recognised prolactin‐lowering activity and those with uncertain mechanism of action. These include different preparations of oestrogens, oestrogens in combination with androgens or progestogens, or both, clomiphene, pyridoxine, prostaglandin E2, other dopamine agonists (cabergoline and lisuride) and serotonin antagonists (cyproheptadine, methysergide and methergoline). All these drugs have demonstrated variable effectiveness in the inhibition of postpartum lactation.
The search for another ergot derivative with clinical efficacy similar to bromocriptine but with better compliance and tolerability profile led to the trials on cabergoline. Various randomised studies in the late 1980s described similar effectiveness and a better side‐effect profile of cabergoline, administered as a single dose compared with the conventional bromocriptine dose in the prevention of postpartum lactation (European 1991; Giorda 1991). Recent reports suggest that a new drug, which belongs to the sulfhydryl compound, is also an effective inhibitor of lactation and breast engorgement (Akrivis 2000).
Why it is important to do this review
It is clear from this background that the evolution of lactation suppressants is not over. Thus, while the need for specific medical prevention of lactation in non‐nursing mothers is being questioned from time to time, many clinicians still apply some kind of treatment. Besides, clinicians appear unclear about the most appropriate method for suppressing lactation when an intervention is indicated. In view of the numerous approaches of lactation inhibition and the continuous search for new drugs, it becomes necessary to synthesise previous research findings to determine the most effective intervention to suppress lactation in non‐breastfeeding mothers. An ideal method would be one that has close to 100% efficacy, with minimal or no side effects and good acceptability profile. A systematic review of the previous studies was therefore conducted to understand whether further trials on new approaches or drugs, or both, are justified.
Objectives
The objective of this review was to evaluate the effectiveness and safety of interventions used for suppression of lactation in postpartum women (who have not breastfed or expressed breastmilk) to determine which approach has the greatest comparative benefits with least risk.
Methods
Criteria for considering studies for this review
Types of studies
All published randomised trials evaluating the effectiveness of treatments used for suppression of postpartum lactation. We excluded studies that evaluated the effectiveness of interventions after establishment of lactation (e.g. women who have breastfed or expressed breastmilk). We excluded quasi‐randomised trials (e.g. allocation by date of birth or hospital record number).
Types of participants
Postpartum women (who have not breastfed or expressed breastmilk) with indication(s) for suppression of lactation, irrespective of parity and mode of delivery.
Types of interventions
We assessed pharmacologic (drug) and non‐pharmacologic (breast binding or strapping, firm breast support, fluid restriction, application of ice packs and external products) interventions specifically aimed at suppressing lactation after childbirth.
We considered the following comparisons in this review:
any pharmacologic treatment versus no treatment or placebo;
any non‐pharmacologic treatment versus no treatment or placebo;
comparison of two different non‐pharmacologic treatments;
comparison of non‐pharmacologic versus pharmacologic treatments;
comparison of two different pharmacologic treatments;
comparison of two different pharmacologic combinations;
comparison of different doses of the same agent.
We excluded studies without any of the above comparisons.
Types of outcome measures
Primary outcomes
Failure to suppress lactation as indicated by breast pain, engorgement and or milk secretion (or as described by trial authors) at or within seven days postpartum and at or within 14 days postpartum. In trials where data for two or more breast symptoms or signs were reported, data for failure to suppress lactation were derived from the least suppressed of these symptoms or signs.
Minor adverse events including nausea, vomiting, headache, dizziness and major adverse events including thromboembolism, myocardial infarction and maternal death.
Acceptability of the treatment to the woman.
Secondary outcomes
Rebound lactation (resurgence of lactation after cessation of suppressant).
Percentage of women who require a second line drug or method, or both, to achieve lactation suppression.
Percentage of women who require analgesics to relieve breast pain or discomfort.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 June 2012).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. Disagreements were resolved through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, both review authors independently extracted the data using the agreed form. We resolved discrepancies through discussion. We entered data into Review Manager software (RevMan 2011) and checked them for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details. There was no masking of authors or journals.
Assessment of risk of bias in included studies
We independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3) Blinding (checking for possible performance and detection bias)
We described for each included study the methods used, if any, to blind study participants and key personnel (including outcome assessors) from knowledge of which intervention a participant received. Blinding was assessed separately for different classes of outcomes.
We assessed the methods as:
low risk of bias (blinding of participants and key study personnel including outcome assessors ensured, and unlikely that the blinding could have been broken; participants and key study personnel not blinded but outcome assessors blinded);
high risk of bias (no blinding or incomplete blinding; likely that blinding of participants and key study personnel could have been broken);
unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups.
We assessed the methods as:
low risk of bias (e.g. no missing data; missing data for less than 20% of randomised participants; balanced missing outcome data across groups; reason for missing data unrelated to true outcome; appropriate imputing of missing data; intention‐to‐treat analysis);
high risk of bias (e.g. reason for missing data related to true outcome; missing data for more than 20% of randomised participants; unbalanced missing outcome data across groups; ‘as treated’ analysis);
unclear risk of bias.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where all the study’s prespecified outcomes have not been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was apparently free of other problems that could put it at risk of bias and indicated this as:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
We carried out statistical analysis using RevMan 2011. We used fixed‐effect meta‐analysis for combining data in the absence of significant heterogeneity if trials were sufficiently similar. Where there was significant heterogeneity, we used a random‐effects model.
Dealing with missing data
For included studies, we noted levels of attrition. We planned but could not explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect using sensitivity analysis as few trials which addressed different interventions had missing data greater than 20%.
For all outcomes we carried out analyses, as far as possible, on an intention‐to‐treat basis i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. We planned but could not explore substantial heterogeneity (exceeding 50%) by subgroup analysis as few studies reported the criterion (gestational age) that was pre‐specified in the protocol.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used fixed‐effect inverse variance meta‐analysis for combining data where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. Where we suspected clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects may differ between trials, we used random‐effects meta‐analysis. For studies with results in formats that cannot be included in meta‐analysis, findings were succinctly reported as described by the trialists without attempting to derive a summary effect estimate.
Where substantial heterogeneity was identified in a fixed‐effect meta‐analysis, we repeated the analysis using a random‐effects method.
Subgroup analysis and investigation of heterogeneity
There was no subgroup analysis as few studies reported the criterion (gestational age) that was pre‐specified in the protocol.
Sensitivity analysis
We planned but could not conduct sensitivity analyses to explore the effect of trial quality as most of the trials were generally of low and similar methodological quality and in addition addressed different interventions.
Results
Description of studies
Out of 132 potentially relevant studies considered for inclusion in this review, 62 trials involving 6428 women met our inclusion criteria, with outcome data on efficacy variables for interventions available for 6137 women. Twenty‐two trials did not contribute data that could be included in meta‐analyses (Berrebi 2001; Bhardwaj 1979; Biggs 1978; Binns 1967; Caballero‐Gordo 1991; Cooke 1976; Defoort 1987; Hutchison 1981; Kremer 1990; Kulski 1978; Mann 1971; Martinez 1994; McNicol 1972; Menczer 1969; Mizuno 1990; Niebyl 1979; O'Donoghue 1977; Paggi 1975; Phillips 1975; Swift 2002; Van der Heijden 1991; Winter 1964). Two trials (Caballero 1996; Varga 1974) are awaiting further assessment. All of the included trials are relatively small (the largest trial having 800 women) and 42 of them have fewer than 100 participants. The majority (52/62) of the trials were conducted in industrialised countries and all but six trials were single‐centre studies conducted in a private, general or university hospital. Thirty‐eight of the trials were conducted before 1980 and only four after 2000. Seven of the trials were published in languages other than English.
Participants
Most of the trials included healthy postpartum women who elected not to breastfeed for personal reasons and a few recruited women who could not breastfeed as a result of stillbirth or child adoption. Participants were women who delivered vaginally at term in most cases. Giorda 1991 randomised only women who were delivered by caesarean section while Piya‐Anant 2004 randomised healthy HIV‐positive puerperal women. Exclusion criteria were not specified in many of the trials. In the few trials that specified exclusion criteria, these include abnormal findings that are relevant to prolactin secretion, liver disorders, agalactia (inability to lactate), previous breast surgery, tumour of the pituitary gland, use of drugs that might interfere with prolactin secretion and unwillingness to participate in the study. Recruitment of women was not limited to any parity group in any of the trials and parity ranged between one and eight in studies where it was reported.
Interventions
Pharmacologic treatments were compared with placebo in 29 trials and another pharmacologic treatment in 30 trials. No trial was included for the comparison of non‐pharmacologic treatments with placebo. Three trials compared non‐pharmacologic approaches [Jasmine flower (Shrivastav 1988), tight brassiere with intermittent infrared application (De Gezelle 1979), breast binding (Bhardwaj 1979)] with pharmacologic treatments. Only one trial compared two non‐pharmacologic treatments (breast binding versus wearing of support bra) (Swift 2002).
The classes of pharmacologic agents evaluated in these trials include ergot derivatives (bromocriptine, lisuride, methergoline, cabergoline and terguride); synthetic oestrogen preparations (quinestrol, diethylstilbestrol, ethinyl estradiol, chlorotrianisene and hexestrol); antioestrogenic preparations (tamoxifen, clomiphene and cyclofenil); oxytocics (intranasal oxytocin); androgen preparations (testosterone propionate); combined oestrogen and androgen preparations (testosterone and estradiol esters); combined oestrogen, progestogen and androgen preparations; dopamine agonists/precursors; prostaglandins and pyridoxine. Thirty‐one trials involved bromocriptine. Bromocriptine was compared with placebo in nine trials (Bhardwaj 1979; Biggs 1978; Cooke 1976; Dewhurst 1977; Hutchison 1981; Kulski 1978; Rolland 1973; Walker 1975; Weinstein 1976), other pharmacologic treatments in 20 trials (Biggs 1978; Boes 1980; Defoort 1987; England 1988; European 1991; Fischer 1995; Giorda 1991; Kremer 1990; Nilsen 1976; O'Donoghue 1977; Piya‐Anant 2004; Purkayastha 1991; Scapin 1982; Steenstrup 1977; Thorbert 1983; Utian 1975; Van der Heijden 1991; Varga 1972; Venturini 1981; Yuen 1977) and non‐pharmacologic methods in three trials (Bhardwaj 1979; De Gezelle 1979; Shrivastav 1988).
Most of the pharmacologic agents were given orally and a few were given by intramuscular injections. No agent was given intravenously. Dosage varied widely between trials in both amount and duration of treatment. Except in two trials (England 1988; Giorda 1991), treatments were commenced shortly after delivery (less than 12 to 24 hours). Of the drugs tested, only seven are currently included in the World Health Organization model list of essential medicines. These are ethinyl estradiol, clomiphene, tamoxifen, pyridoxine, oxytocin, testosterone and L‐dopa. None of these drugs was listed for lactation suppression.
Outcomes
The definition of lactation suppression was not consistent among the trials. The primary outcome measures and the descriptions used in most of the trials were suppression of milk secretion or leakage, breast engorgement and/or breast pain. Some trials did not describe what was meant by suppression of lactation and only referred to it as such. For most of the trials, failure to achieve suppression of between one and three breast symptoms or signs (milk secretion or leakage, breast pain, breast engorgement) was presented as a measure of treatment failure. Other outcome measures prespecified for the review such as major and minor side effects, rebound lactation, use of analgesics and need for secondary treatment to achieve suppression were poorly reported in many of the trials. The method of outcome assessments varied widely across studies. In 15 trials, breast symptoms or signs were rated on a visual analogue scale or an ordinal scale of zero to between three and six to describe increasing severity of symptoms or signs (e.g., none, mild, moderate and severe). Most trials used dichotomous variables, or ones that could be dichotomised, to describe clinical efficacy of treatments while 10 trials described outcome measures in terms of means of the breast symptom 'scores', mean number of days or mean change in the degree of breast symptoms. There was no evidence that any of these scoring systems were previously validated. Assessments of clinical efficacy were based on physical examination of the breast by the clinicians or women in the trials, or both, while in the hospital and subsequently by the women at home after hospital discharge. Women were asked to document breast symptoms on a questionnaire or data card that was collected at a specified follow‐up period. The duration and the frequency of outcome assessment also varied widely between studies. Follow‐up varied from 72 hours to eight months postpartum although most assessments were conducted during the first one or two weeks.
As a result of the diversity of the interventions and the method, duration and frequency of outcome assessments, few trials could be included in meta‐analyses for each comparison. It was not possible to conduct sensitivity analyses based on trial quality as most trials were generally at high risk of bias (e.g., allocation concealment was considered adequate in 8.1% of the included trials, all of which evaluated different interventions).
Risk of bias in included studies
Overall, the risk of bias for most reports was uncertain as they contained little methodological description (Figure 1 and Figure 2). Details for each trial are given in the Characteristics of included studies table.
1.
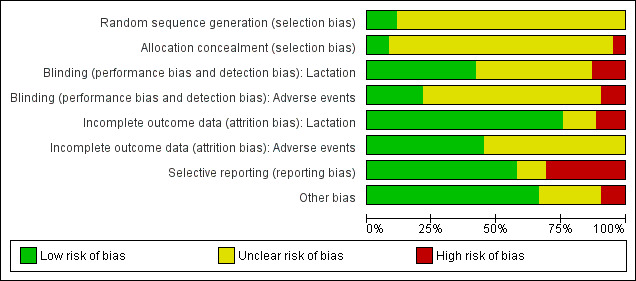
'Risk of bias' graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
2.
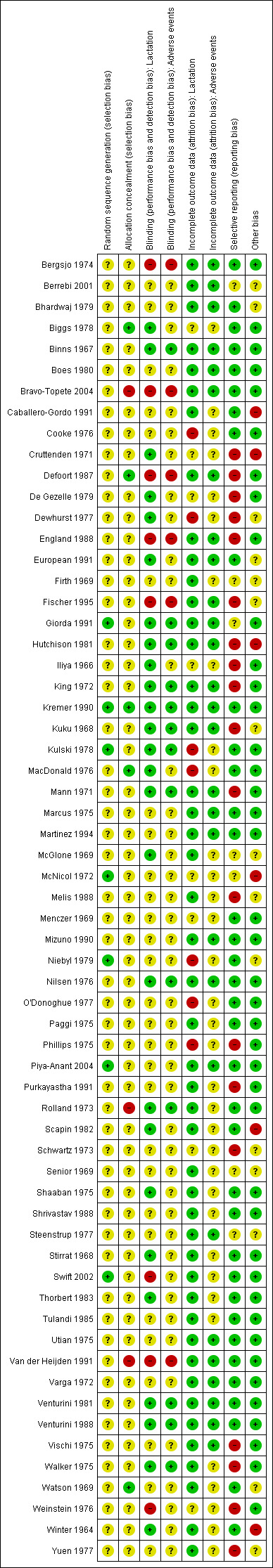
'Risk of bias' summary: review authors' judgments about each risk of bias item for each included study.
Allocation
The risk of selection bias was uncertain for most trials as generation and concealment of allocation sequence were considered adequate in only a few of the included studies. Generation of allocation sequence was considered adequate in seven trials (computer randomisation in three trials (Kremer 1990; McNicol 1972; Piya‐Anant 2004) and use of table of random numbers in four trials (Giorda 1991; Kulski 1978; Niebyl 1979; Swift 2002). Other studies did not describe the method by which random allocation sequence was generated and only reported that participants were "randomised" or "randomly allocated" into treatment groups. Allocation concealment was considered adequate in only five (8.1%) of the included studies (Biggs 1978; Defoort 1987; Kremer 1990; MacDonald 1976; Watson 1969). The majority did not provide adequate information to permit appropriate judgement on allocation concealment, while three trials (Bravo‐Topete 2004; Rolland 1973; Van der Heijden 1991) did not conceal their allocation sequences.
Blinding
With regard to blinding of participants, key study personnel and outcome assessors, we considered 26 trials to be at low risk and eight trials at high risk of bias for the main outcome (lactation) of the review. The risk of bias was uncertain for 28 trials, principally as a result of unclear description of blinding of outcome assessors. Eight of these trials only described their blinding method as "double blinded" with no further information to permit appropriate judgements. Of the 27 trials that addressed adverse events as outcome measures, we considered 12 to be at low risk of bias, five at high risk of bias and the rest at uncertain risk of bias.
Incomplete outcome data
For the main outcome (lactation), we considered 47 trials to be at low risk of attrition bias, seven trials at high risk of bias and eight trials at uncertain risk of bias. Loss to follow‐up or exclusions from the initial cohort entered into the trials varied between 0% and 46.5%. For adverse events, 28 trials adequately addressed incomplete outcome data. It was unclear whether incomplete data were adequately addressed in 34 trials; 31 of these did not address adverse events as outcome measures.
Selective reporting
We considered 36 trials to be at low risk of selective reporting bias, 19 trials at high risk and seven at uncertain risk of bias. In most of the reports at high risk of bias, the trialists did not include results for adverse events especially thromboembolism in spite of prior studies implicating oestrogen preparations in this complication.
Other potential sources of bias
Forty‐one trials were apparently free of other problems that could put them at a risk of bias. We considered six trials to be at high risk of other sources of bias. These sources of bias included extreme imbalance in the number of participants across groups (Caballero‐Gordo 1991), statistical analyses by supplier of tested intervention (Cruttenden 1971; Hutchison 1981, McNicol 1972) and prolonged data collection period involving 25 study personnel and outcome assessors (Winter 1964).
Effects of interventions
Primary outcomes
Pharmacologic treatment versus no treatment or placebo
Ergot derivatives versus no treatment or placebo (comparison 1)
Five trials (206 women) compared bromocriptine with placebo during the first postpartum week (Dewhurst 1977; Hutchison 1981; Rolland 1973; Walker 1975; Weinstein 1976), although only three reported data that could be used in meta‐analysis. These three trials (107 women) (Dewhurst 1977; Rolland 1973; Weinstein 1976) indicated that bromocriptine reduced the risk of failure of lactation suppression during the first seven days postpartum when compared with placebo (risk ratio (RR) 0.36, 95% confidence interval (CI) 0.24 to 0.54) (Analysis 1.1). The two other trials, which did not contribute data to the analysis because they were not in a format suitable for inclusion, indicated that bromocriptine resulted in significantly less milk secretion and breast engorgement compared to placebo during the first seven days postpartum (Hutchison 1981; Walker 1975).
1.1. Analysis.
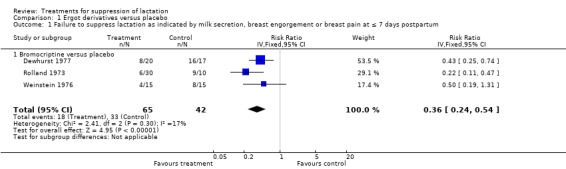
Comparison 1 Ergot derivatives versus placebo, Outcome 1 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 7 days postpartum.
Six trials (258 women) reported the cumulative efficacies of bromocriptine compared to placebo over 14 days postpartum (Bhardwaj 1979; Binns 1967; Cooke 1976; Dewhurst 1977; Kulski 1978; Varga 1972), although only two reported data that could be used in meta‐analysis. These two trials (76 women) (Dewhurst 1977; Varga 1972) indicated that this ergot derivative was similar to placebo in the suppression of lactation (RR 0.18, 95% CI 0.03 to 1.08; Analysis 1.2). However, the four other trials (Bhardwaj 1979; Binns 1967; Cooke 1976; Kulski 1978) that did not provide data in a suitable format for inclusion in an analysis, indicated that bromocriptine resulted in significantly less milk secretion, breast engorgement and discomfort compared to placebo.
1.2. Analysis.
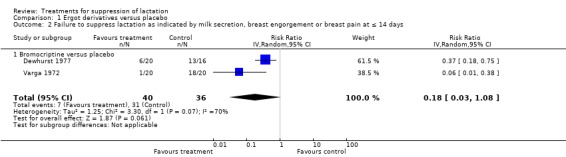
Comparison 1 Ergot derivatives versus placebo, Outcome 2 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 14 days.
Melis 1988 (32 women) indicated that cabergoline reduced the risk of failure to suppress lactation compared to placebo at 14 days postpartum (RR 0.19, 95% CI 0.07 to 0.48; Analysis 1.3).
1.3. Analysis.
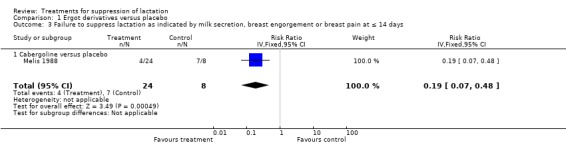
Comparison 1 Ergot derivatives versus placebo, Outcome 3 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 14 days.
Oestrogen preparations versus no treatment or placebo (comparison 2)
There were 11 trials in which oestrogen preparations were compared with placebo. Seven of the trials where nine comparisons were made are presented in the data and analysis table. Diethylstilbestrol was compared with placebo in a total of five trials. Four of these trials (376 women) (Schwartz 1973; Senior 1969; Stirrat 1968; Weinstein 1976), which are included the analyses, indicate a reduced risk of treatment failure when diethylstilbestrol was compared with placebo at or within seven days postpartum (RR 0.33, 95% CI 0.12 to 0.89); Analysis 2.1.1). This analysis indicates significant heterogeneity among the trials as evident by the very high I² statistic. Although data from Winter 1964 (800 women) did not contribute to the analysis because they were not in a suitable format for inclusion, the report indicated that milk secretion, breast congestion and breast pain affected fewer women who were treated with diethylstilbestrol compared to those treated with placebo during the first postpartum week.
2.1. Analysis.
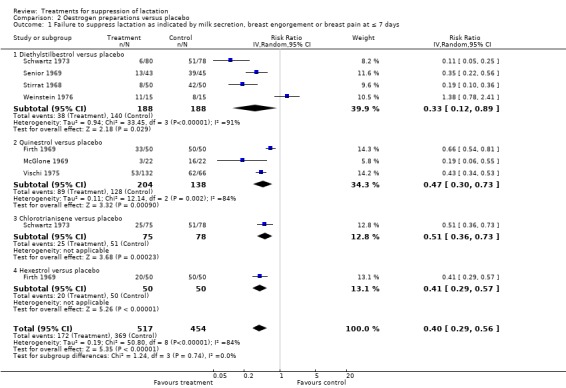
Comparison 2 Oestrogen preparations versus placebo, Outcome 1 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 7 days.
Three trials (342 women) (Firth 1969; McGlone 1969; Vischi 1975) comparing quinestrol with placebo suggested that quinestrol was associated with less risk of treatment failure (RR 0.47, 95% CI 0.30 to 0.73; Analysis 2.1.2). The I² statistic of 93%, however, suggests significant heterogeneity among the trials. Schwartz 1973 (153 women) indicated that chlorotrianisene was associated with less risk of treatment failure when compared with placebo (RR 0.51, 95% CI 0.36 to 0.73; Analysis 2.1.3). Two trials (Binns 1967 (85 women); Phillips 1975 (196 women)), which did not contribute data to the analysis because they were not in a suitable format for inclusion, reported results that were in agreement with the results of this analysis (Analysis 2.1.3). However, another trial (Niebyl 1979 (99 women), which again did not provide data in a suitable format for inclusion in an analysis, indicated similarity in the risks of treatment failure between chlorotrianisene compared with placebo at day three postpartum. One trial (100 women) (Firth 1969) comparing hexestrol with placebo suggested that it was significantly associated with less risk of treatment failure (RR 0.41, 95% 0.29 to 0.57; Analysis 2.1.4), at or within seven days postpartum.
Overall, seven trials (971 women) (Firth 1969; McGlone 1969; Schwartz 1973; Senior 1969; Stirrat 1968; Vischi 1975; Weinstein 1976) with nine comparisons indicated that oestrogen preparations are associated with reduced risk of failure to suppress lactation when compared with placebo at or less than seven days postpartum (RR 0.40, 95% CI 0.29 to 0.56; Analysis 2.1).
Two small trials (Cruttenden 1971; Varga 1972) suggested similarity in the risk of treatment failure between oestrogen preparations (quinestrol and diethylstilbestrol) and placebo up to day 14 postpartum (RR 0.19, 95% CI 0.03 to 1.34; Analysis 2.2). Two trials (130 women), which did not provide data in a suitable format for inclusion in an analysis (Stirrat 1968; Weinstein 1976), reported thromboembolism as an outcome measure and reported no occurrence of this complication in women treated with diethylstilbestrol and placebo.
2.2. Analysis.
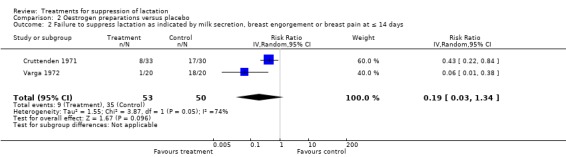
Comparison 2 Oestrogen preparations versus placebo, Outcome 2 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 14 days.
Antioestrogens versus no treatment or placebo (comparison 3)
One trial (30 women) (Weinstein 1976) suggested no difference between clomiphene and placebo (RR 1.00, 95% CI 0.51 to 1.95; Analysis 3.1) when used for lactation suppression in the first postpartum week. The trial also reported no occurrence of thromboembolism in women treated with clomiphene and placebo.
3.1. Analysis.
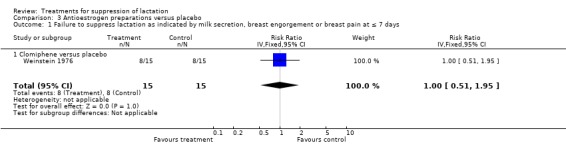
Comparison 3 Antioestrogen preparations versus placebo, Outcome 1 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 7 days.
In the Shaaban 1975 trial (140 women), tamoxifen was significantly less likely to be associated with failure to suppress lactation compared to placebo over a period of 14 days postpartum (RR 0.71, 95% CI 0.62 to 0.82; Analysis 3.2). The trial reported no occurrence of thromboembolism in women treated with tamoxifen and placebo.
3.2. Analysis.
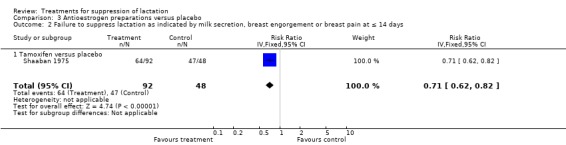
Comparison 3 Antioestrogen preparations versus placebo, Outcome 2 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 14 days.
Pyridoxine versus no treatment or placebo (comparison 4)
MacDonald 1976 and Marcus 1975 (258 women) indicated that the risk of failure to achieve lactation suppression at or within seven days postpartum was similar between pyridoxine and placebo (RR 0.98, 95% CI 0.89 to 1.09; Analysis 4.1).
4.1. Analysis.
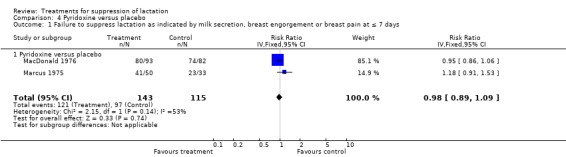
Comparison 4 Pyridoxine versus placebo, Outcome 1 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 7 days.
Combined oestrogen and androgen preparations versus no treatment or placebo (comparison 5)
Three trials (436 women) (Iliya 1966; Marcus 1975; Schwartz 1973) compared testosterone enanthate‐estradiol valerate combination with placebo for inhibition of lactation. This combination was found to significantly reduce the risk of treatment failure when compared with placebo at or within seven days postpartum (RR 0.15, 95% CI 0.10 to 0.22; Analysis 5.1).
5.1. Analysis.
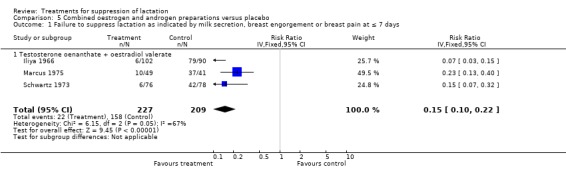
Comparison 5 Combined oestrogen and androgen preparations versus placebo, Outcome 1 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 7 days.
Androgen preparations versus no treatment or placebo (comparison 6)
In the comparison of testosterone propionate with placebo, a small trial (30 women) (Weinstein 1976) indicated no evidence of an association between testosterone propionate and risk of treatment failure (RR 1.13 95% 0.60 to 2.11; Analysis 6.1). There were no reports of thromboembolism in women who received testosterone propionate and placebo. Another trial that did not provide data in a suitable format for inclusion in an analysis (Biggs 1978), indicated similarity regarding breast discomfort, congestion or milk production between methyl testosterone and placebo at two weeks postpartum.
6.1. Analysis.
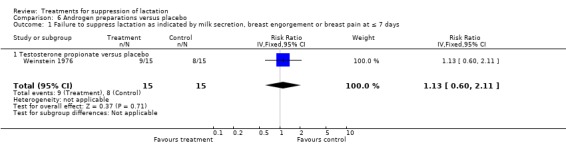
Comparison 6 Androgen preparations versus placebo, Outcome 1 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 7 days.
Prostaglandins versus no treatment or placebo
One trial provided data for this comparison, but they were not in a suitable format for inclusion in an analysis. Tulandi 1985 compared prostaglandin E2 with placebo in a trial involving 16 women. The trial indicated similarity in the degree of milk leakage, breast engorgement and breast pain between women who received prostaglandin E2 and those who received placebo.
Oxytocics versus no treatment or placebo
One trial provided data for this comparison, but they were not in a suitable format for inclusion in an analysis. One trial (98 women) (Winter 1964) indicated that intranasal oxytocin was similar to placebo in the suppression of lactation symptoms.
Dopamine precursors versus no treatment or placebo
One trial provided data for this comparison, but they were not in a suitable format for inclusion in an analysis. Paggi 1975 compared L‐dopa with placebo in a trial involving 40 women. The trial indicated a lower risk of treatment failure among women who received L‐dopa compared to those that received placebo. Although the treatment was reported to last for six days after delivery, the timing of outcome assessment was not specified.
Homeopathic preparations versus no treatment or placebo
One trial provided data for this comparison, but they were not in a suitable format for inclusion in an analysis. Berrebi 2001 (71 women) suggested a lower risk of treatment failure when homeopathic preparation (with anti‐inflammatory and analgesic properties) was compared with placebo on days two and four postpartum.
Non‐pharmacologic treatments versus no treatment or placebo
No trial was included for this comparison.
Pharmacologic treatments versus non‐pharmacologic treatments (comparison 7)
Only three trials compared a pharmacologic agent with non‐pharmacologic agent. De Gezelle 1979 (90 women) was a five‐arm study, which had bendrofluazide (a diuretic), diethylstilbestrol, estradiol/testosterone ester and bromocriptine in the intervention arms and tight brassiere with intermittent application of infra‐red lamp as the control arm. The trial suggested that the risks of treatment failure at or within seven days postpartum were significantly reduced by all the studied pharmacologic treatments compared to wearing of tight brassiere and application of infra‐red lamp (Analysis 7.1). Two trials did not provide data in a suitable format for inclusion in an analysis (Bhardwaj 1979; Shrivastav 1988). Shrivastav 1988 (60 women) indicated that the risk of treatment failure was similar between women who used bromocriptine and those who applied jasmine flowers to the breasts for suppression of postpartum lactation. Bhardwaj 1979 (20 women) suggested that the risk of treatment failure was significantly reduced by bromocriptine compared to breast binding when used over a period of 14 postpartum days.
7.1. Analysis.
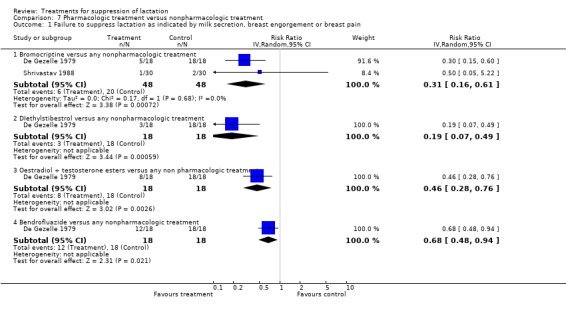
Comparison 7 Pharmacologic treatment versus nonpharmacologic treatment, Outcome 1 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain.
Comparison of two non‐pharmacologic treatments
One trial provided data for this comparison, but they were not in a suitable format for inclusion in an analysis. Swift 2002 indicated that breast binding was associated with higher risk of treatment failure compared to use of a well‐fitting support bra.
Comparison of two pharmacologic treatments (comparison 8)
Bromocriptine versus other pharmacologic treatment (at ≤ seven days postpartum)
Bromocriptine versus oestrogen preparations: bromocriptine was compared with diethylstilbestrol (Nilsen 1976, 38 women; Steenstrup 1977, 41 women), ethinyl estradiol (Piya‐Anant 2004; 230 women), and chlorotrianisene (Utian 1975; 31 women). No significant difference was demonstrated in the risks of failure to suppress lactation in any of the trials in the first postpartum week (RR 0.58, 95% CI 0.25 to 1.38; Analysis 8.1.1). One trial (O'Donoghue 1977), which did not provide data in a suitable format for inclusion in an analysis, indicated that the percentage of women showing failure of lactation suppression was significantly greater in women receiving quinestrol compared with those who received bromocriptine at seventh day postpartum.
8.1. Analysis.
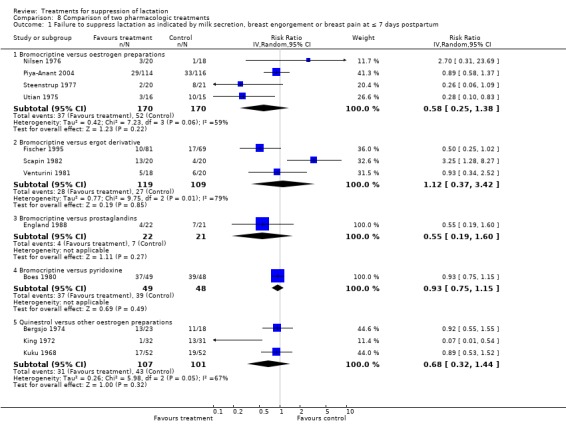
Comparison 8 Comparison of two pharmacologic treatments, Outcome 1 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 7 days postpartum.
Bromocriptine versus other ergot derivatives: two trials (Scapin 1982, 40 women; Fischer 1995, 150 women) comparing bromocriptine and methergoline and Venturini 1981 (38 women) comparing it with lisuride, did not indicate any difference in the risks of treatment failure in the first postpartum week (RR 1.12, 95% CI 0.37 to 3.42; Analysis 8.1.2).
Bromocriptine versus prostaglandins: England 1988 (43 women) compared bromocriptine with prostaglandin E2 for suppression of lactation. The trial suggested no significant difference between the risks of treatment failure of the two agents at or within seven days postpartum (RR 0.55, 95% CI 0.19 to 1.60; Analysis 8.1.3). Bromocriptine versus pyridoxine: Boes 1980 (97 women) suggested no difference in the risks of treatment failure between bromocriptine and pyridoxine in the suppression of postpartum lactation at or less than seven days postpartum (RR 0.93, 95% CI 0.75 to 1.15; Analysis 8.1.4).
Bromocriptine versus other pharmacologic treatment (at ≤ 14 days postpartum)
At day 14 postpartum, bromocriptine has similar risks of treatment failure compared to cabergoline (European 1991; Giorda 1991, 308 women; RR 1.38, 95% CI 0.93 to 2.05, Analysis 8.4.1), diethylstilbestrol (Nilsen 1976; 38 women, RR 0.30, 95% CI 0.07 to 1.30, Analysis 8.4.2) and cyclofenil (Thorbert 1983; 24 women, RR 3.50, 95% CI 0.16 to 78.19, Analysis 8.4. 8.4.3). Yuen 1977 (39 women) suggested that bromocriptine was associated with reduced risk of treatment failure compared to chlorotrianisene (RR 0.35, 95% CI 0.19 to 0.66) (Analysis 8.4.4). One trial (Biggs 1978; 32 women) that did not provide data in a suitable format for inclusion in an analysis, indicated that milk secretion, breast congestion and discomfort were significantly worse with methyl testosterone compared with bromocriptine during 14 postpartum days.
8.4. Analysis.
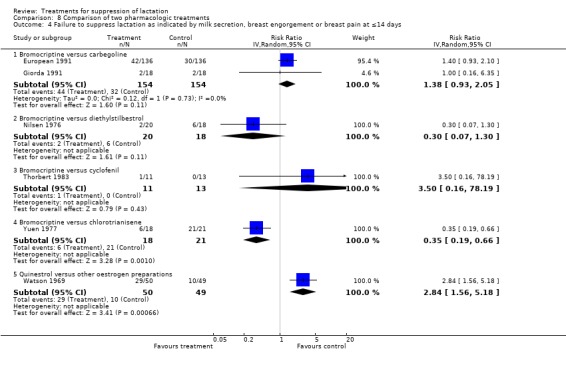
Comparison 8 Comparison of two pharmacologic treatments, Outcome 4 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤14 days.
Bromocriptine versus other pharmacologic treatment (at > 14 days postpartum)
Two trials provided data for this comparison, but they were not in a suitable format for inclusion in an analysis (Purkayastha 1991; Van der Heijden 1991). When used for up to 21 days, Van der Heijden 1991 (30 women) indicated that the efficacy of bromocriptine in suppressing postpartum lactation was similar to that of a non‐ergot dopamine agonist CV 205‐502 (not in data and analysis table). At 28 days of assessment, Purkayastha 1991 (48 women) indicated that bromocriptine was more effective than oestradiol/testosterone ester.
Quinestrol versus other oestrogen preparations (comparison 8)
Quinestrol was compared with ethinyl estradiol (Kuku 1968), diethylstilbestrol (Bergsjo 1974) and chlorotrianisene (King 1972). These trials (208 women) suggested similarity in the risks of treatment failure between quinestrol and other oestrogen preparations in the first postpartum week (RR 0.68, 95% CI 0.32 to 1.44; Analysis 8.1.5).
Watson 1969 (99 women) indicated that quinestrol was associated with increased risk of treatment failure when compared with diethylstilbestrol over a period of one to 10 days (RR 2.84, 95% CI 1.56 to 5.18; Analysis 8.4.5).
Comparison of different dosages of the same drug
High‐ versus low‐dose quinestrol: Vischi 1975 (132 women) suggested that the risk of failure of treatment was significantly lower with 4 mg quinestrol compared with 2 mg quinestrol in lactation suppression (RR 0.51, 95% CI 0.33 to 0.81; Analysis 9.1).
9.1. Analysis.
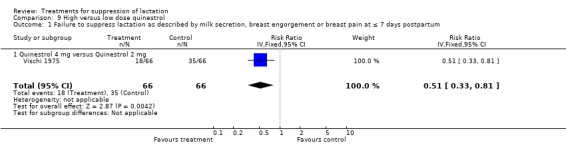
Comparison 9 High versus low dose quinestrol, Outcome 1 Failure to suppress lactation as described by milk secretion, breast engorgement or breast pain at ≤ 7 days postpartum.
Low‐ versus high‐dose terguride: Venturini 1988 (45 women) indicated that terguride 0.5 to 1 mg significantly reduced the risk of failure to suppress postpartum lactation over a period of 15 days when compared with terguride 0.25 mg (RR 0.50, 95% CI 0.29 to 0.88; Analysis 10.1). The risks of dizziness following use were similar between women who received a high dose of terguride and those who received low‐dose terguride (RR 1.55, 95% 0.07 to 35.89; Analysis 10.2).
10.1. Analysis.
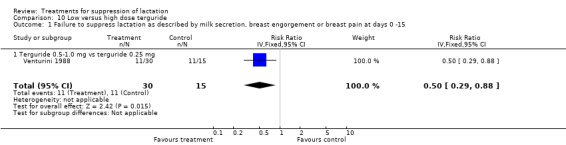
Comparison 10 Low versus high dose terguride, Outcome 1 Failure to suppress lactation as described by milk secretion, breast engorgement or breast pain at days 0 ‐15.
10.2. Analysis.
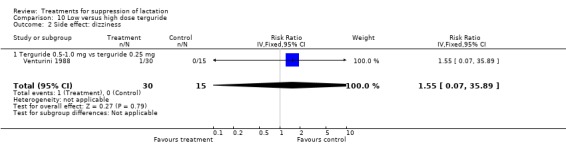
Comparison 10 Low versus high dose terguride, Outcome 2 Side effect: dizziness.
High‐ versus low‐dose cabergoline: Bravo‐Topete 2004 (80 women) indicated that cabergoline 1 mg reduced the risk of failure to suppress lactation when compared with cabergoline 0.5 mg (RR 0.14, 95% CI 0.03, 0.59; Analysis 11.1).
11.1. Analysis.
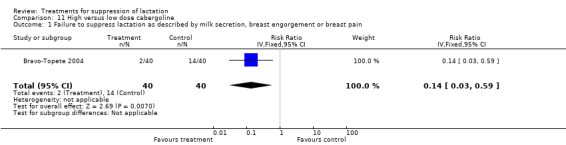
Comparison 11 High versus low dose cabergoline, Outcome 1 Failure to suppress lactation as described by milk secretion, breast engorgement or breast pain.
Long course versus short course of tamoxifen: Shaaban 1975 (65 women) indicated that long‐course tamoxifen (14 days administration) reduced the risk of failure to suppress lactation compared with short‐course tamoxifen (six days administration) when assessed over two weeks postpartum (RR 0.75, 95% CI 0.61 to 0.92, Analysis 12.1).
12.1. Analysis.
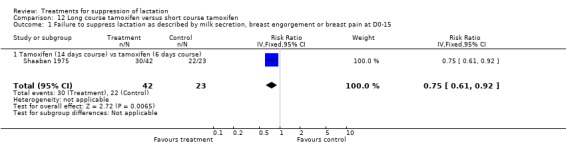
Comparison 12 Long course tamoxifen versus short course tamoxifen, Outcome 1 Failure to suppress lactation as described by milk secretion, breast engorgement or breast pain at D0‐15.
Modified release bromocriptine capsule versus normal bromocriptine tablets: Mizuno 1990 (155 women) indicated similarity in the efficacy of 5 mg once a day modified release capsule and 2.5 mg twice daily normal tablets of bromocriptine when used for suppression of postpartum lactation over a period of two weeks. Data from this trial were not in a suitable format for inclusion in an analysis.
High‐versus low‐dose lisuride: Martinez 1994 (60 women) indicated similarity in the clinical efficacy of lisuride 0.6 mg per day and 0.4 mg per day when used for suppression of postpartum lactation over a period of two weeks. Again, data from this trial were not in a suitable format for inclusion in an analysis.
Comparison of two different pharmacologic combinations
One trial involving 213 women (McNicol 1972) compared a combination of oestradiol benzoate, oestradiol valerate, norethisterone acetate and testosterone enanthate with stilbestrol. Although the trial indicated similarity in the effects of the drugs compared on lactation suppression, the data were not in a format suitable for inclusion in an analysis.
Secondary outcomes
Rebound lactation
We did not include data from many trials on rebound lactation in the results because of inadequate reporting of data. One trial (40 women) (Rolland 1973) comparing bromocriptine with placebo indicated that bromocriptine increased the risk of rebound lactation but the CI was too wide to give a reliable estimate (RR 15.26, 95% CI 1.01 to 231.20, Analysis 1.4). No other trial comparing pharmacologic agents with placebo provided reliable data on rebound lactation. In the comparison of pharmacologic versus non‐pharmacologic treatment, Shrivastav 1988 found no significant difference between the risks of rebound lactation in women treated with bromocriptine compared with those who applied jasmine flower to the breasts (RR 5.00, 95% CI 0.25 to 99.95; Analysis 7.2). Stirrat 1968 (100 women) comparing diethylstilbestrol with placebo did not show any difference between the two with respect to rebound lactation (not in data and analysis table). In the comparison of bromocriptine with other pharmacologic agents, four trials (149 women) (England 1988; Steenstrup 1977; Utian 1975; Venturini 1981) suggested similarity in the risks of rebound lactation between the study and control groups in the trials (RR 0.65, 95% CI 0.39 to 1.10, Analysis 8.2).
1.4. Analysis.
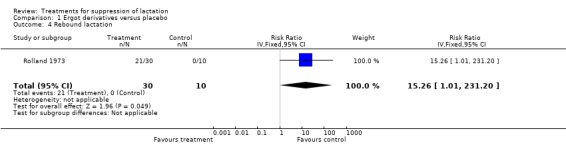
Comparison 1 Ergot derivatives versus placebo, Outcome 4 Rebound lactation.
7.2. Analysis.
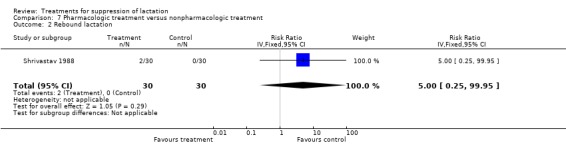
Comparison 7 Pharmacologic treatment versus nonpharmacologic treatment, Outcome 2 Rebound lactation.
8.2. Analysis.
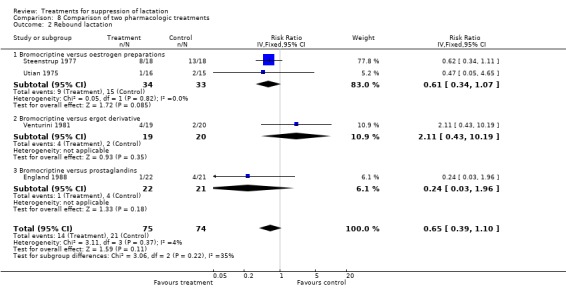
Comparison 8 Comparison of two pharmacologic treatments, Outcome 2 Rebound lactation.
Use of second line drugs or method to achieve lactation suppression
The use of second line drug or methods to achieve suppression was poorly reported in the trials. In the comparison of bromocriptine with any other pharmacologic treatment, there was no statistically significant difference between the risks of using a second line drug or method to achieve lactation suppression when bromocriptine was compared with oestrogen preparations (Utian 1975, 31 women, RR 0.31, 95% CI 0.01 to 7.15, Analysis 8.3.1) and other ergot derivatives (Scapin 1982, 40 women, RR 2.67, 95% CI 0.82 to 8.62, Analysis 8.3.2). Boes 1980 (97 women) suggested that bromocriptine was associated with reduced proportion of women in need of second line methods to suppress lactation compared to pyridoxine (RR 0.07, 95% CI 0.01 to 0.51; Analysis 8.3.3). Phillips 1975 (196 women) suggested that women who received chlorotrianisene were less likely to use supplemental or concurrent therapy (breast binders, ice bags or analgesics, or both) compared with women who had placebo at or within four days after admission (not in data and analysis table).
8.3. Analysis.
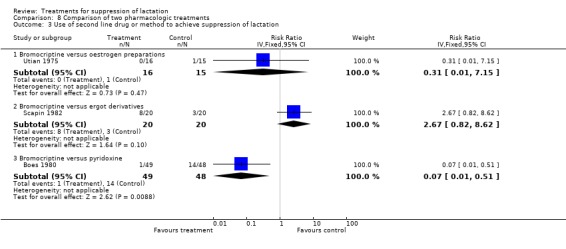
Comparison 8 Comparison of two pharmacologic treatments, Outcome 3 Use of second line drug or method to achieve suppression of lactation.
Discussion
In spite of the questions on the need to apply treatment for suppressing lactation, a lot of research has gone into finding the most effective treatment. This review indicates that the emphasis for this search has been on pharmacologic treatments (compared with either no treatment or each other). The review included comparisons of orally and intramuscularly administered pharmacologic agents with placebo, other pharmacologic agents and non‐pharmacologic methods. In terms of outcome, we used the persistence of one of the three common clinical symptoms or signs of postpartum lactation (i.e., milk secretion, breast engorgement and breast pain) as evidence of failure of lactation suppression. We also explored the consistency of results obtained from this definition with those obtained from separate consideration of each lactation symptom or sign as an indicator of treatment failure. In an attempt to reduce clinical heterogeneity, we extracted results on efficacy of treatment effects within a specified postpartum period.
This review shows that the search for the most effective lactation suppressant has relied on small trials, most of which have low methodological quality. The lack of recent randomised controlled trials on this subject may imply a supposed exhaustiveness and conclusion on this research topic. It may also be attributed to the lack of motivation of potential researchers because of the increasing number of questions on the need for treatments to suppress lactation. The gross variation in the dosage, interventions, duration of treatments and outcome assessments suggests a general lack of a clear understanding of the physiology of lactation and mechanisms of action of the treatments tested among researchers.
This is the first systematic review assessing the effectiveness of all forms of treatments for lactation suppression. Assessment of methodological quality of studies was based on stringent criteria, as evident by the number of relevant trials excluded from the review. In spite of this measure, however, the robustness of the results is diminished by the fact that the majority of included studies are of uncertain methodological quality, as evident in the proportion of trials with adequate allocation concealment (8.1%) and those that blinded their outcome assessments. Blinding of outcome assessment is particularly important for this topic, considering the subjective nature of main outcome measures (secretion, congestion and pain). The extent to which outcome measures are blinded in double‐blinded trials where the women served the dual role of participant and outcome assessor is uncertain. Combining data on outcomes assessed by different observers (clinicians and women) is likely to contribute to ascertainment bias in included studies that employed such method. In addition, the validity of the results interpreted by the trialists could not be examined in included trials that reported data that were unsuitable for inclusion in the analyses table. Another major limitation of this review is the fact that a significant proportion of drugs tested in the included trials are currently no longer registered for use in most countries, either as lactation suppressants or otherwise. This may significantly limit the applicability of the findings of the review.
About half of the trials included compared drugs with placebo. In spite of its popularity, only five trials involving 206 women comparing bromocriptine with placebo met the inclusion criteria. All these trials used uncertain methods of generating allocation sequence and allocation concealment, none had outcome assessment blinded and all were conducted over two decades ago. It can thus be concluded that there is weak evidence that bromocriptine is better than nothing for suppressing the symptoms of lactation during the first seven days postpartum. Contrary to a number of case reports, there is insufficient evidence from this review to indicate whether or not bromocriptine is associated with an increased risk of major side effects (notably thromboembolism, myocardial infarction and maternal death) in the first postpartum week.
Although there is small evidence that oestrogen preparations may be better than nothing in the first postpartum week, none of the tested agents is presently available in the market. In addition, available data on their risks of major side effects are insufficient to make a reliable conclusion on their safety when used for lactation suppression. Of all the pharmacological agents compared with placebo, only bromocriptine is still registered for use in most countries although not necessarily for lactation suppression. It needs to be stated that bromocriptine appeared to be the gold standard in the 1970s and 1980s as shown by the number of trials where it was tested. The possibility of publication bias therefore exists in the face of seemingly 'overwhelming' evidence. This review did not show sufficient evidence to indicate if other pharmacologic agents (clomiphene, tamoxifen, prostaglandins, pyridoxine, oxytocin, L‐dopa and homeopathic preparation) are useful in suppressing the symptoms of lactation postpartum, as they are all based on individual small trials. However, the combination of testosterone and oestrogen preparations appear to be somewhat effective in suppressing the symptoms of lactation.
Evidence on the comparative effectiveness of pharmacologic agents and non‐pharmacologic methods is based on three trials, each testing different non‐pharmacologic methods (Bhardwaj 1979; De Gezelle 1979; Shrivastav 1988). It can be concluded from their results that there is not enough evidence to indicate which of the approaches is better than the other. The comparative effectiveness of jasmine flower with that of bromocriptine can only be useful once there is strong evidence that bromocriptine is better than placebo. There is currently no evidence on whether non‐pharmacologic methods are better than placebo in lactation suppression.
There are mixed views on the subject of lactation suppression in most current obstetric textbooks, although the need for applying some kind of treatment is seldom disputed. Most authors refer to research findings indicating the effectiveness of previously tried approaches, many of which were excluded from this review as a result of low methodological quality and high potential for bias (seeCharacteristics of excluded studies). Generally, non‐pharmacologic approaches such as use of a well‐supporting brassiere and avoidance of nipple stimulation, are often recommended based on the presumed safety and effectiveness of these methods. This policy, however, is not supported by the findings of this review. While the methodological limitations of conducting high‐quality trials involving non‐pharmacologic methods are understandable, they should not compromise the need to provide evidence‐based guidelines on their application.
It is unlikely that the findings from this review would inform any change in the recommendations of the United States' Food and Drug Administration on the routine use of bromocriptine for suppression of postpartum lactation, as the evidence indicating its effectiveness is weak even though the review also did not show any clear evidence of adverse effects.
Three issues related to the data and studies included in this review need to be discussed for a better understanding of the implications for practice and research regarding lactation suppression. The review addresses treatments for lactation suppression in postpartum women who do not desire to breastfeed their infant right from birth. Therefore, the findings are not directly applicable to women who had initiated breastfeeding but later wish to discontinue, or to women who lactate due to other pathology (e.g., hyperprolactinaemia). It is possible that the effectiveness as well as the side‐effect profile of tested agents remote from delivery may be different from that in the immediate postpartum period. Secondly, participants in the included trials were healthy postpartum women (including healthy HIV‐positive mothers) and it is uncertain if similar results, especially regarding side effects, would be found in postpartum women with a higher baseline risk of morbidity, e.g., those who are ill, or taking medications, or both, including antiretroviral drugs. It is important to note that this category of mothers for whom suppression of lactation may have particular health benefits, is likely to have increased risk of side effects, including drug interactions, which were not explored in the studies included in this review. Lastly, a fundamental question that is yet to be answered is whether postpartum women desire treatment for lactation suppression and, if so, which of the symptoms women are most concerned about. This would definitely go a long way in better interpretation of the effectiveness of the tested approaches. It is clear from this review that women's views about the treatment received was not a priority, as only four trials with unreliable data explored the acceptability profile of the tested approach.
With respect to side effects, there is insufficient evidence to show that pharmacologic agents are more associated with a higher risk of major adverse effects (notably thromboembolism) compared with no treatment or each other. Several case reports have been published on thromboembolism, myocardial infarction and cerebral angiopathy following the use of bromocriptine, although a causal relationship has not been established. It is interesting to note that, in spite of these concerns, emphasis was not laid on reporting these side effects in trials conducted subsequently. However, these findings should be interpreted against the background of our review of only published data as exploration of unpublished data, especially adverse outcome data from drug company trials, may provide a clearer picture.
Authors' conclusions
Implications for practice.
There is weak evidence that some pharmacologic treatments (most of which are currently unavailable to the public) are better than no treatment for suppressing the symptoms of lactation in the first week postpartum. There is currently no evidence to show that non‐pharmacologic approaches are more effective than no treatment. Presently, there is insufficient evidence to address the issue of side effects of the pharmacologic and non‐pharmacologic methods that are employed for suppressing lactation. When women desire treatment for suppressing lactation, consideration may be given to bromocriptine where it is still registered for such use in healthy mothers with no predisposition to major side effects of public concern. In spite of its importance, there is inadequate evidence to comment on the acceptability of approaches for suppressing postpartum lactation to women.
Implications for research.
For settings in which application of some forms of treatment for suppressing lactation is the norm, there is a need for well conducted and large randomised controlled trials to compare the effectiveness of pharmacologic treatment, notably, bromocriptine to no treatment. Future research should also focus on comparison of non‐pharmacologic approaches with no treatment since they presently do not appear to have any safety concerns for the public. The most important symptoms that concern women who desire not to breastfeed should be studied in large observational studies to ensure that effective treatment is sought by researchers on the inclination of clients' needs. Such studies should also be large enough to detect clinically important differences between interventions with respect to major side effects that have been reported through less rigorous research. Priority should be given to studying fewer dose regimens and cheap approaches in view of women in low‐resource countries.
What's new
| Date | Event | Description |
|---|---|---|
| 23 July 2012 | New search has been performed | Search updated. Sixteen additional trials included (four previously excluded trials are now included but they do not contribute any data to the review (Bhardwaj 1979; Binns 1967; Kulski 1978; Van der Heijden 1991). Two trials added to Studies awaiting classification. No change to conclusions. |
| 23 July 2012 | New citation required but conclusions have not changed | Review updated. |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 4 January 2010 | Amended | Search updated. Nineteen reports added to Studies awaiting classification. |
Acknowledgements
The authors acknowledge the mentorship of Metin Gulmezoglu, Department of Reproductive Health and Research (RHR), World Health Organization, Geneva, Switzerland and the contributions of Regina Kulier (GFMER, Geneva), Asa Cuzin and Mario Merialdi of Department of Reproductive Health and Research (RHR), World Health Organization, Geneva, Switzerland. The authors are also grateful to those who provided translations for the non‐English language articles considered for this review: Christopher Binns (Martinez 1994); Bianca Garcia (Foukas 1972); Georgia Gallavin (Mizuno 1990); Elena Intra (Lo Dico 1980; Paggi 1975; Polatti 1982); Lorna Kerr (Bravo‐Topete 2004); Pernille Lau (Poulsen 1976); Ayla Pariyar (Foukas 1972; Gerstner 1978; Kaiser 1952); Melissa Slavick (Cantis 1977); Michelle Staniczenko (Momberg 1976); Michał Konieczny and Kamila Stępowska (Varga 1972).
Data and analyses
Comparison 1. Ergot derivatives versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 7 days postpartum | 3 | 107 | Risk Ratio (IV, Fixed, 95% CI) | 0.36 [0.24, 0.54] |
| 1.1 Bromocriptine versus placebo | 3 | 107 | Risk Ratio (IV, Fixed, 95% CI) | 0.36 [0.24, 0.54] |
| 2 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 14 days | 2 | 76 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.03, 1.08] |
| 2.1 Bromocriptine versus placebo | 2 | 76 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.03, 1.08] |
| 3 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 14 days | 1 | 32 | Risk Ratio (IV, Fixed, 95% CI) | 0.19 [0.07, 0.48] |
| 3.1 Cabergoline versus placebo | 1 | 32 | Risk Ratio (IV, Fixed, 95% CI) | 0.19 [0.07, 0.48] |
| 4 Rebound lactation | 1 | 40 | Risk Ratio (IV, Fixed, 95% CI) | 15.26 [1.01, 231.20] |
Comparison 2. Oestrogen preparations versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 7 days | 7 | 971 | Risk Ratio (IV, Random, 95% CI) | 0.40 [0.29, 0.56] |
| 1.1 Diethylstilbestrol versus placebo | 4 | 376 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.12, 0.89] |
| 1.2 Quinestrol versus placebo | 3 | 342 | Risk Ratio (IV, Random, 95% CI) | 0.47 [0.30, 0.73] |
| 1.3 Chlorotrianisene versus placebo | 1 | 153 | Risk Ratio (IV, Random, 95% CI) | 0.51 [0.36, 0.73] |
| 1.4 Hexestrol versus placebo | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.41 [0.29, 0.57] |
| 2 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 14 days | 2 | 103 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.03, 1.34] |
Comparison 3. Antioestrogen preparations versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 7 days | 1 | 30 | Risk Ratio (IV, Fixed, 95% CI) | 1.0 [0.51, 1.95] |
| 1.1 Clomiphene versus placebo | 1 | 30 | Risk Ratio (IV, Fixed, 95% CI) | 1.0 [0.51, 1.95] |
| 2 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 14 days | 1 | 140 | Risk Ratio (IV, Fixed, 95% CI) | 0.71 [0.62, 0.82] |
| 2.1 Tamoxifen versus placebo | 1 | 140 | Risk Ratio (IV, Fixed, 95% CI) | 0.71 [0.62, 0.82] |
Comparison 4. Pyridoxine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 7 days | 2 | 258 | Risk Ratio (IV, Fixed, 95% CI) | 0.98 [0.89, 1.09] |
| 1.1 Pyridoxine versus placebo | 2 | 258 | Risk Ratio (IV, Fixed, 95% CI) | 0.98 [0.89, 1.09] |
Comparison 5. Combined oestrogen and androgen preparations versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 7 days | 3 | 436 | Risk Ratio (IV, Fixed, 95% CI) | 0.15 [0.10, 0.22] |
| 1.1 Testosterone oenanthate + oestradiol valerate | 3 | 436 | Risk Ratio (IV, Fixed, 95% CI) | 0.15 [0.10, 0.22] |
Comparison 6. Androgen preparations versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 7 days | 1 | 30 | Risk Ratio (IV, Fixed, 95% CI) | 1.13 [0.60, 2.11] |
| 1.1 Testosterone propionate versus placebo | 1 | 30 | Risk Ratio (IV, Fixed, 95% CI) | 1.13 [0.60, 2.11] |
Comparison 7. Pharmacologic treatment versus nonpharmacologic treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Bromocriptine versus any nonpharmacologic treatment | 2 | 96 | Risk Ratio (IV, Random, 95% CI) | 0.31 [0.16, 0.61] |
| 1.2 DIethylstibestrol versus any nonpharmacologic treatment | 1 | 36 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.07, 0.49] |
| 1.3 Oestradiol + testosterone esters versus any non pharmacologic treatment | 1 | 36 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.28, 0.76] |
| 1.4 Bendrofluazide versus any nonpharmacologic treatment | 1 | 36 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.48, 0.94] |
| 2 Rebound lactation | 1 | 60 | Risk Ratio (IV, Fixed, 95% CI) | 5.0 [0.25, 99.95] |
Comparison 8. Comparison of two pharmacologic treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤ 7 days postpartum | 12 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Bromocriptine versus oestrogen preparations | 4 | 340 | Risk Ratio (IV, Random, 95% CI) | 0.58 [0.25, 1.38] |
| 1.2 Bromocriptine versus ergot derivative | 3 | 228 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.37, 3.42] |
| 1.3 Bromocriptine versus prostaglandins | 1 | 43 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.19, 1.60] |
| 1.4 Bromocriptine versus pyridoxine | 1 | 97 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.75, 1.15] |
| 1.5 Quinestrol versus other oestrogen preparations | 3 | 208 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.32, 1.44] |
| 2 Rebound lactation | 4 | 149 | Risk Ratio (IV, Fixed, 95% CI) | 0.65 [0.39, 1.10] |
| 2.1 Bromocriptine versus oestrogen preparations | 2 | 67 | Risk Ratio (IV, Fixed, 95% CI) | 0.61 [0.34, 1.07] |
| 2.2 Bromocriptine versus ergot derivative | 1 | 39 | Risk Ratio (IV, Fixed, 95% CI) | 2.11 [0.43, 10.19] |
| 2.3 Bromocriptine versus prostaglandins | 1 | 43 | Risk Ratio (IV, Fixed, 95% CI) | 0.24 [0.03, 1.96] |
| 3 Use of second line drug or method to achieve suppression of lactation | 3 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Bromocriptine versus oestrogen preparations | 1 | 31 | Risk Ratio (IV, Fixed, 95% CI) | 0.31 [0.01, 7.15] |
| 3.2 Bromocriptine versus ergot derivatives | 1 | 40 | Risk Ratio (IV, Fixed, 95% CI) | 2.67 [0.82, 8.62] |
| 3.3 Bromocriptine versus pyridoxine | 1 | 97 | Risk Ratio (IV, Fixed, 95% CI) | 0.07 [0.01, 0.51] |
| 4 Failure to suppress lactation as indicated by milk secretion, breast engorgement or breast pain at ≤14 days | 6 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Bromocriptine versus carbegoline | 2 | 308 | Risk Ratio (IV, Random, 95% CI) | 1.38 [0.93, 2.05] |
| 4.2 Bromocriptine versus diethylstilbestrol | 1 | 38 | Risk Ratio (IV, Random, 95% CI) | 0.30 [0.07, 1.30] |
| 4.3 Bromocriptine versus cyclofenil | 1 | 24 | Risk Ratio (IV, Random, 95% CI) | 3.5 [0.16, 78.19] |
| 4.4 Bromocriptine versus chlorotrianisene | 1 | 39 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.19, 0.66] |
| 4.5 Quinestrol versus other oestrogen preparations | 1 | 99 | Risk Ratio (IV, Random, 95% CI) | 2.84 [1.56, 5.18] |
Comparison 9. High versus low dose quinestrol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to suppress lactation as described by milk secretion, breast engorgement or breast pain at ≤ 7 days postpartum | 1 | 132 | Risk Ratio (IV, Fixed, 95% CI) | 0.51 [0.33, 0.81] |
| 1.1 Quinestrol 4 mg versus Quinestrol 2 mg | 1 | 132 | Risk Ratio (IV, Fixed, 95% CI) | 0.51 [0.33, 0.81] |
Comparison 10. Low versus high dose terguride.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to suppress lactation as described by milk secretion, breast engorgement or breast pain at days 0 ‐15 | 1 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 0.5 [0.29, 0.88] |
| 1.1 Terguride 0.5‐1.0 mg vs terguride 0.25 mg | 1 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 0.5 [0.29, 0.88] |
| 2 Side effect: dizziness | 1 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 1.55 [0.07, 35.89] |
| 2.1 Terguride 0.5‐1.0 mg vs terguride 0.25 mg | 1 | 45 | Risk Ratio (IV, Fixed, 95% CI) | 1.55 [0.07, 35.89] |
Comparison 11. High versus low dose cabergoline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to suppress lactation as described by milk secretion, breast engorgement or breast pain | 1 | 80 | Risk Ratio (IV, Fixed, 95% CI) | 0.14 [0.03, 0.59] |
Comparison 12. Long course tamoxifen versus short course tamoxifen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to suppress lactation as described by milk secretion, breast engorgement or breast pain at D0‐15 | 1 | 65 | Risk Ratio (IV, Fixed, 95% CI) | 0.75 [0.61, 0.92] |
| 1.1 Tamoxifen (14 days course) vs tamoxifen (6 days course) | 1 | 65 | Risk Ratio (IV, Fixed, 95% CI) | 0.75 [0.61, 0.92] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bergsjo 1974.
| Methods | Randomised trial. | |
| Participants | 41 women in whom inhibition of lactation was planned. Indications included late abortion or fetal death, missed abortion, previous mastitis and adoption. Women were included in the study if staying in the hospital for at least 5 days. Exclusion criteria were not specified. Setting: a university hospital in Norway. | |
| Interventions | Quinesterol tablet 4 mg (on day 0) and if necessary, an additional 4 mg tablet on day 4 (n = 23) versus diethylstilbestrol 5 mg tablet thrice daily for 5 days (n = 18). | |
| Outcomes | Milk secretion, breast pain, additional oestrogen medication, symptoms suggestive of mastitis and thrombosis. | |
| Notes | Quinestrol experimental tablets were supplied by Apothekernes Laboratorium for Special preparater, Oslo, Norway. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Participants were divided into 2 groups "by random allocation". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | High risk | No information on blinding of participants, study personnel and outcome assessors. Differences in the dosage regimen between the 2 interventions suggested no blinding. |
| Blinding (performance bias and detection bias) Adverse events | High risk | No information on blinding of participants, study personnel and outcome assessors. Differences in the dosage regimen between the 2 interventions suggested no blinding. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | 2 participants (4.9%) were lost to follow‐up after leaving the hospital (1 from the quinestrol group and the other from the diethylstilbestrol group). Outcome data were available for all participants for the 5 days in the hospital. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Outcome data were available for all participants for the 5 days in the hospital. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Berrebi 2001.
| Methods | Randomised trial. | |
| Participants | 71 postpartum women who elected not to breastfeed. Setting: Department of Obstetrics and Gynaecology, Federation de Gynecologie‐Obstetrique, CHU La Grave, Toulouse Cedex. | |
| Interventions | 5 homeopathic pills twice daily for 10 days (n = 36) versus placebo (n = 35). All patients received an anti‐inflammatory treatment (naproxine‐Apranax) for 5 days. | |
| Outcomes | Milk secretion, breast engorgement and breast pain. Outcome assessment recorded on visual analogue scale. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Study described as "double blind trial". No further information to permit judgement on blinding. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | Study described as "double blind trial". No further information to permit judgement on blinding. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing outcome data. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | Insufficient information to assess whether other important risk of bias exists. |
Bhardwaj 1979.
| Methods | Randomised trial. | |
| Participants | 50 postpartum women who elected not to breastfeed. Women having operative deliveries under general anaesthesia were excluded, as were those requiring concomitant drug therapy with a possible inhibitory or augmentative effect on lactation, e.g. diuretics, pyridoxine and phenothiazine. Setting: community hospital, Melbourne. | |
| Interventions | Bromocriptine 2.5 mg within 2 hours of delivery, then twice daily for 14 days (n = 25) versus placebo with or without breast binding (n = 25). | |
| Outcomes | Milk secretion, breast engorgement, breast pain and side effects. Observations on milk secretion and breast engorgement was rated on a 4‐point scale. Side effects were recorded on a 3‐point scale. | |
| Notes | "The study began on 20 patients as single blind comparison between two parallel groups, 1 receiving bromocriptine and the other placebo plus breast binding. Thereafter the study was conducted as a double‐blind randomised trial involving 30 patients." Breast binding was excluded from the placebo arm in the second part of the study. Outcome data were not separately presented for comparisons with and without breast binding. Data were presented mainly in means, and were not presented in a form suitable for extraction and inclusion in a meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. "The randomisation schedule was prepared in blocks of 10." |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Although the study used "identically‐appearing buff tablets, 7 mm in diameter containing bromocriptine 2.5 mg or placebo (made up of lactose), it is unclear how blinding was ensured in the initial part of the study (described as "single blind comparison") when breast binding was combined with placebo. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | Although the study used "identically‐appearing buff tablets, 7 mm in diameter containing bromocriptine 2.5 mg or placebo (made up of lactose), it is unclear how blinding was ensured in the initial part of the study (described as "single blind comparison") when breast binding was combined with placebo. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | 5 women (10%) did not complete the trial. "Scrutiny of their background data did not indicate that they differed significantly from the other patients". 4 (16%) of the women in the bromocriptine arm and 1 (4%) of those in the placebo arm. "Information on the dropouts has been included in the analysis up to the time of leaving the trial." |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | 5 women (10%) did not complete the trial. "Scrutiny of their background data did not indicate that they differed significantly from the other patients". 4 (16%) of the women in the bromocriptine arm and 1 (4%) of those in the placebo arm. "Information on the dropouts has been included in the analysis up to the time of leaving the trial." |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Unclear risk | The study used placebo plus breast binding in the first part of the study and placebo alone in the latter part of the study. Thus, the possibility of intervention bias in the placebo arm exists. |
Biggs 1978.
| Methods | Randomised trial. | |
| Participants | 60 women who elected not to breastfeed. Setting: Department of Obstetrics and Gynaecology, Royal Brisbane Hospital, Australia. | |
| Interventions | Methyl testosterone tablets (5 mg) thrice daily for 6 days and bromocriptine tablets (2.5 mg) twice daily for 14 days versus placebo. Medication was commenced within 24 hours of delivery. | |
| Outcomes | Milk production, breast congestion and breast discomfort. Outcomes were graded on a scale of 0 to 2 for each symptom and effectiveness of interventions was reported as mean of daily scores for 14 days. Outcomes were recorded by 1 of the researchers while the women were in the hospital and by the women themselves after hospital discharge. | |
| Notes | The drugs used in the trial were supplied by a staff of Sandoz Products (Pty) Ltd. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. "Three sets of medication were prepared according to random numbers". |
| Allocation concealment (selection bias) | Low risk | The code of random numbers was "held at the hospital pharmacy". |
| Blinding (performance bias and detection bias) Lactation | Low risk | Participants and study personnel were blinded to the interventions using a double‐dummy technique. Each participant received small white tablets containing either methyl testosterone or placebo; and capsules containing bromocriptine or placebo, taken according to similar dosage regimen such that the tested drugs are the active elements in 2 arms and the placebo in the third arm of the trial. Study personnel (1 of the researchers) and participants also doubled as outcome assessors. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Unclear risk | Outcome data not available for 11 (18.3%) of the 60 women recruited into the trial. Women completing the trial included 17 in the bromocriptine treated group, 17 in the methyl testosterone group and 15 in the placebo group. The attrition rate for each of the 3 arms of the trial is unclear as no information was given on the number of women primarily recruited into each arm. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Binns 1967.
| Methods | Double‐blind randomised trial. | |
| Participants | 90 women not wishing to breastfeed. Setting: a general practitioner maternity unit at Bridgnorth, Shropshire, United Kingdom. | |
| Interventions | Chlorotrianisene 24 mg tablets versus placebo. Two tablets thrice daily for 4 days starting from the day of delivery. | |
| Outcomes | Milk leakage, breast pain and congestion; side effects. The assessment of degree of severity of breast symptoms was on a 4‐point scale. Observations were made daily for a minimum of 6 and usually 7 or more days until the symptoms ceased. | |
| Notes | Data were not included because the results were not presented in a usable form. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. "The order of administration (of the interventions) was random." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about allocation concealment to permit judgement. "One of each consecutive pairs of numbered bottles contained active chlorotrianisene tablets and the other dummy tablets". |
| Blinding (performance bias and detection bias) Lactation | Low risk | Participants, study personnel and outcome assessors were blinded. "None of those involved in the trial knew at the time whether the patient was taking active or dummy tablets. Assessment of record cards was made, before breaking the code, by an independent statistical adviser who was not directly involved in the trial." |
| Blinding (performance bias and detection bias) Adverse events | Low risk | Participants, study personnel and outcome assessors were blinded. "None of those involved in the trial knew at the time whether the patient was taking active or dummy tablets. Assessment of record cards was made, before breaking the code, by an independent statistical adviser who was not directly involved in the trial." |
| Incomplete outcome data (attrition bias) Lactation | Low risk | 5 (5.6%) of the women initially recruited into the trial "were prematurely discharged from the hospital and were therefore excluded from the within‐pair comparison." |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | 5 (5.6%) of the women initially recruited into the trial "were prematurely discharged from the hospital and were therefore excluded from the within‐pair comparison." |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Boes 1980.
| Methods | Randomised trial. | |
| Participants | 97 non‐breastfeeding mothers. Indications for suppression included adoption, stillbirth and too small or too ill baby. Setting: a university Hospital in Pretoria, South Africa. | |
| Interventions | Oral bromocriptine 2.5 mg twice daily for 14 days (n = 49) versus oral pyridoxine 200 mg thrice daily for 6 days (n = 48). | |
| Outcomes | Milk secretion, breast engorgement, and side effects (nausea, dizziness and blood pressure changes). Main outcome measures were graded on scale of 0 to 4; 0 = no secretion or congestion while 4 = profuse secretion or congestion. Outcome assessment were on days 7 and 14. | |
| Notes | Sandoz Products (Pty) Ltd. supplied the materials used in the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Participants were "randomly assigned" to treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Participants and study personnel were blinded to the interventions. Each participant received "identical bubble packs", supplemented with inactive tablets so that each participant took 1 tablet thrice daily for 14 days. Uncertain whether outcome assessors (2 doctors) were blinded. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | Uncertain whether outcome assessors (2 doctors) were blinded. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | 3 participants were excluded after randomisation (3%): 2 from the pyridoxine group and 1 from bromocriptine group. 2 patients left the hospital within 24 hours of commencing treatment, while the result of 1 patient failed to reach the statistician. Outcome data were available for all participants besides those excluded after randomisation. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Outcome data were available for all participants besides 3 participants excluded after randomisation. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Bravo‐Topete 2004.
| Methods | Randomised trial. | |
| Participants | 80 women with indications for inhibition of lactation. Setting: specialist hospital, Mexico. |
|
| Interventions | Single dose cabergoline 1 mg (n = 40) versus single dose cabergoline 0.5 mg (n = 40). | |
| Outcomes | Milk secretion, breast engorgement, nausea and headache. | |
| Notes | There was no indication of the day of assessment of breast symptoms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Treatments were given orally "at random". |
| Allocation concealment (selection bias) | High risk | Allocation of intervention was dependent on patient's choice suggesting that no sequence was generated that required concealment. |
| Blinding (performance bias and detection bias) Lactation | High risk | Participants, study personnel and outcome assessors were not blinded. Participants had to chose between 2 bottles containing a label with indication of the dose (either 1 mg or 0.5 mg). |
| Blinding (performance bias and detection bias) Adverse events | High risk | Participants, study personnel and outcome assessors were not blinded. Participants had to chose between 2 bottles containing a label with indication of the dose (either 1 mg or 0.5 mg). |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing outcome data. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Caballero‐Gordo 1991.
| Methods | Randomised trial. | |
| Participants | 140 healthy postpartum women who did not wish or were unable to breastfeed and who gave informed consent. Exclusion criteria included women with history of agalactia (inability to lactate), intolerance or allergy to drugs, stillbirth, hepatic or renal disorders, those undergoing treatment that might interfere with prolactin secretion and those unwilling to cooperate with study protocol. Setting: a university hospital in Spain. | |
| Interventions | Single cabergoline tablet 1 mg (n = 40); single cabergoline tablet 0.75 mg (n = 40); single cabergoline tablet 0.5 mg (n = 40); placebo were 20 "additional" women. | |
| Outcomes | Milk secretion, breast engorgement and breast pain. Results were described as excellent if there were no breast symptoms (milk secretion, engorgement and pain) "during hospitalisation or up to 14 days". Specific time of outcome assessment uncertain. | |
| Notes | Financial and substantive support by Farmitalia Carlo Erba, Milan, Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Study was "prospective and randomised". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Study described as "double blind trial". No further information to permit judgement on blinding. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | 9 participants (12.9%) were excluded because of protocol violation, 6 because they did not return for an examination on day 14 and 3 because they used bromocriptine after treatment. Missing data have been imputed using appropriate methods. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | High risk | Had extreme baseline imbalance in the number of participants in intervention arms and the placebo arm (40 versus 20). |
Cooke 1976.
| Methods | Randomised trial. | |
| Participants | 94 healthy patients who elected not to breastfeed during antenatal care. Setting: a single hospital setting in the United Kingdom. | |
| Interventions | Bromocriptine 2.5 mg twice daily for 14 days (plus further supplies for 6 days in case of rebound lactation) versus placebo. | |
| Outcomes | Milk secretion, breast congestion and pain recorded by women on an evaluation score card using a 4‐point scale of 0, 1, 2 and 3. | |
| Notes | 1 of the authors of the paper was a staff of the Pharmaceutical division of Sandoz Products Ltd, London. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Within each strata (or group) of patients, "active agents and placebo preparations were randomised and presented as serially numbered bottles". |
| Allocation concealment (selection bias) | Unclear risk | Unclear information about allocation concealment to permit judgement. "Active agents and placebo preparations were presented as serially numbered bottles the content of which were unknown to the investigators". |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Study described as "double blind trial". No further information to permit judgement on blinding. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | High risk | 82 (86%) of women recruited into the trial returned their symptom evaluation cards. Thrice as much attrition rate in the placebo group compared to the bromocriptine group (19% versus 6%). Reasons for attrition in both groups not stated. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Cruttenden 1971.
| Methods | Randomised trial. | |
| Participants | 63 mothers who did not wish to breastfeed. Setting: Cameron Hospital, Hartlepool, United Kingdom. | |
| Interventions | Oral quinestrol 4 mg as single dose (n = 33) versus placebo (n = 30). | |
| Outcomes | Milk secretion and breast discomfort. | |
| Notes | William R. Warner & Co. Ltd. supplied the materials used and helped with statistical analysis of the findings. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Treatments were given "randomly". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Study was conducted in a "double blind manner", neither the medical nor nursing staff knowing the identity of the coded tablets. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Unclear risk | Outcome data not available for 7 participants (10%) out of the 70 women randomised. Reason for missing data not stated. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | High risk | The trialists did not include results for adverse events especially thromboembolism in spite of published studies implicating oestrogen preparations in this complication before the trial. |
| Other bias | High risk | Statistical analysis by the supplier of the tested drug was a potential threat to the validity of results of the study. |
De Gezelle 1979.
| Methods | Randomised trial. | |
| Participants | 90 healthy postpartum women (in 5 groups of 18) who chose not to breastfeed. Setting: a university hospital in Belgium. | |
| Interventions | Interventions: oral diethylstilboestrol 5 mg thrice daily for 5 days (n = 18); oral bendroflumethiazide 5 mg thrice daily for 5 days (n = 18); intramuscular single dose of a mixture of estradiol esters (10 mg) and the testosterone esters (200 mg) in olive oil (Estandron Prolongatum ®) (n = 18); oral bromocriptine 5 mg daily for 14 days (n = 18). Control: wearing of tight fitting brassiere night and day with application of infra‐red lamp thrice daily for 10 minutes each time (n = 18). | |
| Outcomes | Milk secretion, breast engorgement and breast tenderness. Outcome measures were graded as absent, mild, moderate and severe. | |
| Notes | The NIH kit (VLSI) used for determination of serum prolactin in the same study was provided by the National Institute of Health, Bethesda, Md., USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Treatment regimens were "randomly assigned to five groups of 18 women each". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Only the outcome assessors were blinded. Outcome assessor was the same nurse, who had no knowledge of the type of treatment applied. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Unclear risk | Outcome data for breast engorgement were not provided for 2 participants in the Estandron Prolongatum ® group and no reasons for missing data were provided. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | High risk | The study failed to include thromboembolism as an outcome measure in spite of published studies implicating oestrogen preparations in this complication before the trial. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Defoort 1987.
| Methods | Randomised trial. | |
| Participants | 54 healthy mothers who delivered at term through uncomplicated vaginal deliveries (except for 3 women who had caesarean section) and elected not to breastfeed their infants. Exclusion criteria: women with abnormal physical findings or abnormal laboratory results related to vital functions. Setting: a university hospital in Belgium. | |
| Interventions | Single intramuscular injection of long acting bromocriptine 50 mg (n = 26) versus single 3 mL injection of estandrom prolongatum ® (estradiol/testosterone ester combination) (n = 28). | |
| Outcomes | Milk flow, breast engorgement, breast pain, untoward systemic effects, coagulation profile and rebound lactation. Both investigators and patients reported outcomes. Overall efficacy at the end of 28 days was presented. Patient data were extracted. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not stated though on "nonalternating" basis. |
| Allocation concealment (selection bias) | Low risk | Serially numbered closed envelopes provided by the manufacturer were used to conceal allocation of intervention. |
| Blinding (performance bias and detection bias) Lactation | High risk | Participants were blinded to the intervention, study personnel were not blinded as they had to prepare the injections before administration to the participants. Outcome assessors were not blinded. |
| Blinding (performance bias and detection bias) Adverse events | High risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | 3 (5.3%) of the 57 participants originally "retained for the study did not complete the study" and their data were not included in the analysis. 1 of the patients was disqualified after inadvertently taking prohibited drugs (an oral contraceptive and acetylsalicylate) during the observation period while the remaining 2 were lost to follow‐up. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Outcome data available for participants who completed the study. |
| Selective reporting (reporting bias) | High risk | The trialists did not include thromboembolism as an outcome measure in spite of published studies implicating oestrogen preparations in this complication before the trial. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Dewhurst 1977.
| Methods | Randomised trial. | |
| Participants | 37 participants who did not desire to breastfeed. Setting: Queen Charlotte's Hospital, London. | |
| Interventions | Bromocriptine (2.5 mg) twice daily for 14 days and 1 daily for a further 7 days (n = 20) versus placebo (n = 17). | |
| Outcomes | Milk production, breast engorgement and breast pain reported on data cards over 28 days by participants. Rebound lactation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not stated. Participants were "randomly allocated" treatments. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Patients were randomly allocated indistinguishable capsules of "active" bromocriptine or pharmacologically inactive placebo. Blinding of participants, study personnel and outcome assessors ensured. Participants also doubled as outcome assessors. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | High risk | 15/52 (30.7%) of the participants who embarked on the trial did not return sufficient data for analysis. Distribution of missing data across groups and reasons not reported. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | High risk | The trialists set out to determine the optimum dosage to minimise side effects but did not report side effects of tested interventions. |
| Other bias | Unclear risk | The study appears free of other sources of bias. |
England 1988.
| Methods | Randomised trial. | |
| Participants | 43 healthy postpartum women who requested lactation suppression. Setting: a university hospital in Johannesburg, South Africa. | |
| Interventions | Oral prostaglandin E2: 2 mg on puerperal day 3 or 4, then 2 mg 6 hourly at 3 doses, followed after another 6 hours by a 4 mg dose (n = 21) versus oral bromocriptine 2.5 mg 12 hourly for 14 days (n = 22). | |
| Outcomes | Breast discomfort, congestion and tenderness, volume of breastmilk expressed. Severity of breast tenderness were assessed on a linear analogue scale. Record of women after discharge were collected at 6 weeks postnatal clinic. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Women were allocated "by randomised cards". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | High risk | No blinding was employed. |
| Blinding (performance bias and detection bias) Adverse events | High risk | No blinding was employed. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing data. |
| Selective reporting (reporting bias) | High risk | Data on side effects were reported incompletely. Only provided data on 2 side effects. |
| Other bias | Low risk | The study appears free of other sources of bias. |
European 1991.
| Methods | Randomised trial. | |
| Participants | 272 postpartum women who delivered at term and did not wish to lactate for personal or medical reasons. Partipants were expected to be at the hospital for at least 3 days after delivery and to be visited on day 15 in the hospital or at home. Exclusion criteria: women with history of agalactia or hypogalactia, drug allergy, intrauterine fetal death, pre‐eclampsia, liver or kidney impairment and those with concomitant acute diseases. Setting: university or hospital departments of obstetrics and gynaecology in 12 European centres. | |
| Interventions | Cabergoline 1 mg as single dose (n = 136) versus bromocriptine 2.5 mg twice daily for 14 days (n = 136). First treatment dose had to be given within 27 hours after delivery. | |
| Outcomes | Milk secretion, breast engorgement, breast pain, frequency of adverse events and rebound lactation. Women assessed their breasts daily using a self evaluation form. Success of treatment (complete or partial) was evaluated on both day 3 (before hospital discharge) and day 15 postpartum. Adverse events were monitored daily while in hospital and on day 15. Presented data on efficacy refers to day 15 assessment. Presence of breast symptoms from days 16 to 21 in subjects with complete success by day 15 was defined as rebound lactation. | |
| Notes | The drugs were supplied by Farmitalia Carlo Erba, Milan, Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Participants were "randomised into each treatment arm". Treatments were given according to "a randomised sequence balanced within each centre". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about allocation concealment to permit judgement. "The drugs were provided by Farmitalia Carlo Erba in individualised patient kits and assigned by the doctor according to the participant's order of entry". Comment: unclear whether patients' kits were sequentially numbered and tamper proof. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Participants, study personnel and outcome assessors were blinded. Participants also doubled as outcome assessors. Used "double dummy technique" for blinding. Placebo was used to make up the for the difference in the duration of treatments between the 2 arms of the trial. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | Blinding of participants and study personnel ensured. Adverse events outcome included blood pressure and heart rate monitoring. Blinding of outcome assessors uncertain. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | Before day 14: 6/136 missing from cabergoline group (1 due to intolerance, 2 were lost to follow‐up and 3 for other reasons); 8/136 missing from the bromocriptine group (3 due to intolerance, 3 were lost to follow‐up and 2 had other reasons). Analysis was by intention‐to‐treat. Efficacy variables were described for all randomised participants regardless of their adherence to study protocol. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | 4 women (3 from bromocriptine and one from cabergoline arm) who stopped treatments because of adverse events were included in the result for adverse events. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Unclear risk | 34 women (12.5% of randomised women), 18 taking cabergoline and 16 taking bromocriptine, received concomitant treatment that may have interfered with lactation; ergot derivatives in 28 and oral contraceptives in 6, equally dispensed over the 2 groups. In spite of the similar distribution across both groups, the concurrent use of these classes of drugs could have exaggerated the efficacy and side effects of both interventions. |
Firth 1969.
| Methods | Randomised trial. | |
| Participants | 150 postpartum women who elected not to breastfeed. Setting: St George's Hospital, London, United Kingdom. | |
| Interventions | Oral quinestrol 0.8 mg as single dose (n = 50), intramuscular injection 45 mg hexoestrol (n = 50) and oral placebo (n = 50). Treatment was given within 2 hours of delivery. | |
| Outcomes | Milk secretion and breast congestion. | |
| Notes | Report was presented as correspondence. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Participants were "randomly selected" into groups. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Study described as "double blind trial". Interventions included both orally and intramuscularly administered drugs. No further information to permit judgement on blinding. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | Insufficient information to assess whether other important risk of bias exists. |
Fischer 1995.
| Methods | Randomised trial. | |
| Participants | 150 postpartum women who did not nurse their baby. Setting: a university hospital, Nurnberg, Germany. | |
| Interventions | Oral bromocriptine at 2.5 mg twice daily for 14 days (n = 81) and oral metergoline at 4 mg thrice daily for 10 days (n = 69). | |
| Outcomes | Milk secretion, breast tension, minor side effects, acceptability to the woman. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | High risk | No blinding was employed. |
| Blinding (performance bias and detection bias) Adverse events | High risk | No blinding was employed. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | Data were obtained during the first 5 days of postpartum admission and 7 months later. No missing data for outcomes assessed while on admission. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing data for outcomes assessed while on admission. |
| Selective reporting (reporting bias) | High risk | Trialists did not provide data for a key outcome (thromboembolism) that would be expected to have been reported for such a study. |
| Other bias | Unclear risk | Insufficient information to assess whether other important risk of bias exists. |
Giorda 1991.
| Methods | Randomised trial. | |
| Participants | 36 women who were delivered by caesarean section and elected to suppress postpartum lactation. Setting: Clinics of Obstetrics and Gynaecology, Milan, Italy. | |
| Interventions | Oral cabergoline 1 mg as single dose (n = 18) versus oral bromocriptine 2.5 mg twice daily for 14 days (n = 18). | |
| Outcomes | Milk secretion, breast engorgement and breast pain. | |
| Notes | 25% of the women had signs of breast engorgement before cabergoline was administered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generation of allocation sequence with tables of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Outcome assessors were blinded to intervention. |
| Blinding (performance bias and detection bias) Adverse events | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing data. |
| Selective reporting (reporting bias) | Unclear risk | Specific side effects being evaluated were not stated in the 'Methods' section of the report. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Hutchison 1981.
| Methods | Randomised trial. | |
| Participants | 40 postpartum women who volunteered. Setting: Women's Hospital, Auckland. | |
| Interventions | Oral bromocriptine 2.5 mg twice daily for 14 days (n = 20) versus oral placebo (n = 20). | |
| Outcomes | Milk secretion, breast engorgement, side effects and acceptability of treatment to the woman. Milk secretion, breast engorgement and side effects were scored daily on a scale of 0 to 3. Milk secretion was expressed as "mean score while in hospital". | |
| Notes | Personnel of Sandoz Pharma Ltd. conducted the statistical analysis and supplied the tablets used in the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Study participants, personnel and outcome assessors were blinded using active and matching placebo tablets for interventions. |
| Blinding (performance bias and detection bias) Adverse events | Low risk | Study participants, personnel and outcome assessors were blinded using active and matching placebo tablets for interventions. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing data. |
| Selective reporting (reporting bias) | High risk | Although data on acceptability of treatments to the participants were collected as 1 of the outcome measures, they were not included in the presented result. |
| Other bias | High risk | Statistical analysis by the supplier of the tested drug was a potential threat to the validity of results of the study. |
Iliya 1966.
| Methods | Randomised trial. | |
| Participants | 192 postpartum women who desired to bottle feed. Setting: Lying‐In Hospital, Boston, MA, USA. | |
| Interventions | Intramuscular deladumone ® (testosterone oenanthate 180 mg/cc plus oestradiol valerate 8mg/cc) given at 2 cc as a single dose (n = 102) versus intramuscular placebo (n = 90). | |
| Outcomes | Breast engorgement and breast pain. Outcome assessments were made on days 2 to 4. All observations were made by the same physician. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Treatments were given to the patients "at random". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Participants, study personnel and outcome assessors were blinded. Treatments were allocated using identical dose vials containing active drug or placebo and coded in random fashion. Contents and the code unknown until the end of the study. Code was broken 6 months after the completion of the study. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Unclear risk | Initially, 203 were included in the study, but only 192 were available for satisfactory analysis. Reasons for exclusions not stated. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | High risk | The trialists did not include thromboembolism as an outcome measure in spite of published studies implicating oestrogen preparations in this complication before the trial. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
King 1972.
| Methods | Randomised trial. | |
| Participants | 63 women who had voluntarily decided not to breastfeed. Setting: a hospital setting in the United Kingdom. | |
| Interventions | Quinestrol 4 mg versus chlorotrianisene 12 mg 4 times daily for 5 days. | |
| Outcomes | Milk secretion, breast consistency and discomfort and side effects. Observations made by both medical and nursing staff of breast consistency and milk secretion and women for subjective symptoms of discomfort or otherwise. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Patients "were randomly allocated to treatment with quinestrol or chlorotrianisene". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Study participants, personnel and outcome assessors (participants and personnel) were blinded using green active and matching placebo tablets for interventions. |
| Blinding (performance bias and detection bias) Adverse events | Low risk | Study participants, personnel and outcome assessors (participants and personnel) were blinded using green active and matching placebo tablets for interventions. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing data. |
| Selective reporting (reporting bias) | High risk | Data on side effects were reported incompletely. Only provided data on 2 side effects in the discussion section of the paper. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Kremer 1990.
| Methods | Randomised trial. | |
| Participants | 61 healthy postpartum women, who were delivered at term and not ready to breastfeed their infants. Exclusion criteria were "relevant abnormal findings on physical examination, abnormal laboratory values and the use of concomitant treatment that would influence the hormonal and metabolic state". Setting: a university hospital in Nijmegen, The Netherlands. | |
| Interventions | Depot bromocriptine injection 40 mg (n = 30) versus depot bromocriptine injection 50 mg (n = 31). | |
| Outcomes | Breast symptoms and side effects. | |
| Notes | Sandoz BV (Uden, The Netherlands) supplied the study medication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation was performed. |
| Allocation concealment (selection bias) | Low risk | The dosages were put in numbered envelopes, which were opened after the formal registration of the participants. |
| Blinding (performance bias and detection bias) Lactation | Low risk | The woman did not know the dosage, but the investigator was aware of it because he prepared the injection just before the injection. |
| Blinding (performance bias and detection bias) Adverse events | Low risk | Participants who also doubled as outcome assessors in the trial were blinded. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Kuku 1968.
| Methods | Randomised trial. | |
| Participants | 104 women who did not wish to breastfeed or in whom breastfeeding was contraindicated. Setting: Kingston Hospital, United Kingdom. | |
| Interventions | Oral quinestrol 2 mg as single dose (n = 52) versus oral ethinyl estradiol 0.2 mg within 6 hours, and 0.2 mg twice daily for 5 days (n = 52). | |
| Outcomes | Milk secretion, breast engorgement and breast pain. The condition of the breast was examined daily for 8 days. Case recorded as successful if the breasts are soft in consistency and without discomfort and no milk, or only a little colostrum expressed on the fifth day. | |
| Notes | William R. Warner and Co. Ltd. supplied the drugs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. The preparation were allocated "at random to patients entering the trial". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Study participants, personnel and outcome assessors were blinded to the interventions. Identical looking capsules were used. Placebo was used to make up for the difference in the duration of treatments between the 2 arms of the trial. |
| Blinding (performance bias and detection bias) Adverse events | Low risk | Study participants, personnel and outcome assessors were blinded to the interventions. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing data. |
| Selective reporting (reporting bias) | High risk | Report on side effects was only limited to that of the tested drug. |
| Other bias | Unclear risk | Insufficient information to assess whether other important risk of bias exists. |
Kulski 1978.
| Methods | Double‐blind randomised trial. | |
| Participants | 26 women who elected not to breastfeed their infants. Setting: King Edwward Memorial Hospital, Western Australia. | |
| Interventions | Bromocriptine 2.5 mg 2 to 3 hours after delivery and then twice daily for 14 days (n = 13) versus placebo (n = 13). | |
| Outcomes | Milk leakage, breast engorgement, changes in the composition of milk constituents. | |
| Notes | The primary outcome was the composition of mammary secretion due to ingestion of bromocriptine 2.5 mg twice daily for 14 days in women who elected not to breastfeed. Therefore, < 5 mL of breast secretion was manually collected from each woman daily for 14 days. Although milk leakage and breast engorgement were part of the outcome measures, data are not presented in a form that can be extracted for the meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were "assigned to 1 of 2 groups according to a schedule prepared from a table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Study participants, personnel and outcome assessors were blinded to the interventions. Identical looking capsules were used. |
| Blinding (performance bias and detection bias) Adverse events | Low risk | Study participants, personnel and outcome assessors were blinded to the interventions. Identical looking capsules were used. |
| Incomplete outcome data (attrition bias) Lactation | High risk | 3 women (23.1%) dropped out of the bromocriptine group. 1 of the women decided to breastfeed her baby while the other 2 discontinued therapy because of side effects. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
MacDonald 1976.
| Methods | Randomised trial. | |
| Participants | 131 postpartum women wishing to bottle feed their babies. Setting: St. Mary Hospital, Leeds, United Kingdom. | |
| Interventions | Oral pyridoxine 200 mg thrice daily for 6 days (n = 93) versus oral placebo (lactose) (n = 82). | |
| Outcomes | Milk secretion, breast engorgement and breast discomfort. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Interventions were issued "in random order". |
| Allocation concealment (selection bias) | Low risk | Treatment allocation using identical tablets containing either lactose or pyridoxine in numbered packs dispensed by the hospital pharmacy, which retained the identifying code until completion of the study. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Participants, study personnel and outcome assessors were blinded to the interventions. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | High risk | Of the 191 women randomised, 14 withdrew as they discharged themselves early from the hospital (9 were on pyridoxine and 5 were on placebo), 1 woman decided to breastfeed and another woman was withdrawn for skin rash. Outpatient assessment after discharge on the 9 to10th day was possible for 131 (68.6%) of those randomised. Total exclusion and attrition rate was 31.4%. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Mann 1971.
| Methods | Randomised trial. | |
| Participants | 95 postpartum women who desired not to breastfeed. Setting: Lewis Hospital, Isle of Lewis, UK. | |
| Interventions | Oral quinestrol 4 mg single dose (n = 45) versus oral stilbestrol 15 mg per day for 7 days (n = 50). | |
| Outcomes | Milk secretion, breast engorgement and rebound lactation. | |
| Notes | Personnel of William R. Warner & Co. Ltd. provided the preparations and helped with the preparation of the report of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Participants, study personnel and outcome assessors were blinded to interventions. |
| Blinding (performance bias and detection bias) Adverse events | Low risk | Participants, study personnel and outcome assessors were blinded to interventions. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing data. |
| Selective reporting (reporting bias) | High risk | The trialists did not include thromboembolism as an outcome measure in spite of published studies implicating oestrogen preparations in this complication before the trial. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Marcus 1975.
| Methods | Randomised trial. | |
| Participants | 97 patients were recruited into the study. Setting: a university hospital in Johannesburg, South Africa. | |
| Interventions | 1 tablet of pyridoxine thrice daily for 7 days (n = 52) versus placebo (n = 43). | |
| Outcomes | Breast discomfort and consistency recorded on a form by nursing staff. Discomfort was graded as soft, moderate or hard. | |
| Notes | 44 women in the treatment group and 40 in the control group also had their breasts bound with crepe bandages. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. "The patients were randomly selected." |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Little information regarding blinding of intervention although the study was referred to as "double‐blind trial". Interventions were "two identical tablets", 1 containing a placebo and the other pyridoxine. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | Inadequate information to permit judgement regarding blinding of reported side effects. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | "Two records were spoilt and 95 patients were therefore studied" indicating an attrition rate of 2.1%. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | "Two records were spoilt and 95 patients were therefore studied" indicating an attrition rate of 2.1%. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Martinez 1994.
| Methods | Randomised trial. | |
| Participants | 60 women who requested lactation inhibition after giving birth. Those with previous history of agalactiae were excluded from the study along with those who received any type of treatment that could alter or interfere with the protocol and the results. Setting: General Hospital, Yagüe, Burgos, Spain. | |
| Interventions | Lisuride tablet 0.2 mg taken orally every 12 hours for 14 days versus lisuride tablet 0.2 mg taken orally every 8 hours for 14 days. | |
| Outcomes | Milk secretion, breast engorgement and breast pain. Breast symptoms were rated as nil, light (+), moderate (++) and severe (+++). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. "The group was divided at random into two smaller groups." |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | No information on blinding. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | No information on blinding. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | Of the 60 women who started the study, 4 from each group (13.3%) failed to complete the study, all of them leaving after being discharged from the clinic. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Attrition rates are balanced between both arms of the trial. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
McGlone 1969.
| Methods | Randomised trial. | |
| Participants | 44 mothers who did not wish to breastfeed. Setting: Dryburn Hospital, Durham, United Kingdom. | |
| Interventions | Oral quinestrol 4 mg as single dose (n = 22) versus oral placebo tablets (n = 22). | |
| Outcomes | Milk leakage, breast engorgement and discomfort. | |
| Notes | The Medical Director of William R. Warner & Co. Ltd. supplied the materials and provided advice on the statistical analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Participants, study personnel and outcome assessors were blinded to the interventions. Coded tablets were assigned randomly and the key to the tablet code was broken after all records had been completed and comparisons made. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address adverse events related to the interventions. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | 6 participants (12%) dropped out of the 50 women who entered the trial (3 from each group). |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address adverse events related to the interventions. |
| Selective reporting (reporting bias) | Unclear risk | The trialists did not include thromboembolism as an outcome measure in spite of published studies implicating oestrogen preparations in this complication before the trial. |
| Other bias | Unclear risk | Advice on statistical analysis by the supplier of the tested drug constituted an uncertain threat to the validity of the trial results. |
McNicol 1972.
| Methods | Randomised trial. | |
| Participants | Women who had expressed a wish to have lactation inhibited. "A total of 213 patients was included in the analysis". Setting: 2 general hospitals in Glasgow, United Kingdom. | |
| Interventions | Intramuscular injection containing in 1 mL, a combined preparations of oestradiol benzoate (5 mg), oestradiol valerate (8 mg), norethisterone acetate (20 mg) and testosterone enanthate (100 mg) given as a single dose (n = 99) versus stilbestrol given orally at 10 mg thrice daily for 2 days, 10 mg twice daily for 2 days and 5 mg daily for a further 5 days (n = 114). | |
| Outcomes | Milk leakage, breast consistency and pain. Observations were made for 21 days and completed on a questionnaire by the woman under the supervision of experienced medical and nursing staff while she was in the hospital, and at home with the aid of her general practitioner, district midwife or health visitor. Outcomes were reported as median number of days on which there was milk leakage or when breast symptoms were present. | |
| Notes | Schering Chemicals supplied the combined preparation and conducted the statistical analysis of the results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 2 interventions were administered after "random computer selection". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | No information on blinding. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not report this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Unclear risk | The initial number of women recruited into the trial was not stated in the report. The number rejected due to incomplete records "was a small proportion of the whole". |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not report this outcome. |
| Selective reporting (reporting bias) | Unclear risk | The trialists did not include thromboembolism as an outcome measure in spite of published studies implicating oestrogen preparations in this complication before the trial. |
| Other bias | High risk | Statistical analysis by the supplier of 1 of the tested drugs constituted an uncertain threat to the validity of the trial results. |
Melis 1988.
| Methods | Randomised trial. | |
| Participants | 32 healthy postpartum women who elected not to breastfeed. All women had an uncomplicated pregnancy and spontaneous delivery at term. Exclusion criteria included use of general anaesthetic agents. Setting: a university hospital in Italy. | |
| Interventions | Oral cabergoline 400 mcg (n = 8), oral cabergoline 600 mcg (n = 8), oral cabergoline 800 mcg (n = 8) and oral placebo (n = 8). | |
| Outcomes | Breast tension, breast tenderness, milk secretion and rebound engorgement. | |
| Notes | Drugs were provided by Farmitalia Carlo Erba, Medical Division, Milan, Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Women were "randomly allocated to four treatment groups of eight subjects". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Participants and study personnel were blinded to the interventions. Uncertain whether outcome assessors were blinded. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | High risk | Method of assessment of reported side effects in the cabergoline group was not stated. Non‐reporting of side effects in the placebo group did not permit inclusion of result in meta‐analysis. |
| Other bias | Unclear risk | Women who showed signs of lactation from day 2 of treatment were also given bromocriptine to suppress lactation. The impact of the such additional intervention on the efficacy variables after day 2 of the treatment and its distribution across groups is unclear. |
Menczer 1969.
| Methods | Randomised trial. | |
| Participants | 90 women who had vaginal deliveries, uncomplicated postpartum course and chose not to breastfeed their babies. Setting: a medical centre in Philadelphia, USA. | |
| Interventions | Testosterone enanthate and estradiol valerate administered in a single injection (2 mL intramuscularly) versus placebo. | |
| Outcomes | Breast firmness as determined by registered nurse was recorded twice daily. No attempt was made to grade the findings. | |
| Notes | The primary aim of the study was to determine whether thermography could be used for objective evaluation of venous engorgement in the postpartum breast. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Interventions were administered to the women "on a random" basis. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | No information on blinding. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Unclear risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Mizuno 1990.
| Methods | Randomised trial. | |
| Participants | 155 postpartum women in 42 national institutions in Japan. | |
| Interventions | Bromocriptine modified release capsule (5 mg once a day for 2 weeks) (n = 79) versus bromocriptine normal tablet (2.5 mg twice daily for 2 weeks) (n = 76) | |
| Outcomes | Milk secretion and breast congestion. Clinical efficacy was reported as percentages. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. "Trial medications were randomly allocated." |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information on allocation concealment to permit judgement. "The key code was held in secret by the two controllers separately until the official vote." |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Method of blinding not stated. Study was described as "double‐blind". |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | Method of blinding not stated. Study was described as "double‐blind". |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Niebyl 1979.
| Methods | Randomised trial. | |
| Participants | 99 women who had undergone vaginal delivery and who elected lactation suppression. Setting: John Hopkins Hospital, Maryland, USA. | |
| Interventions | Oral chlorotrianisene 72 mg every 12 hours for 4 doses versus placebo. | |
| Outcomes | Breast congestion, milk secretion, breast pain, haematologic and coagulation factors and satisfaction with drug used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Participants and study personnel were blinded to the interventions. Used identical‐appearing capsules containing corn oil as placebo. Uncertain whether outcome assessors were blinded. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | High risk | Loss to follow‐up as at day 8 postpartum was 46.5%. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Unclear risk | 50 participants were initially randomised and an "additional 49 patients were evaluated". Unclear what the initial sample size for the study was. |
Nilsen 1976.
| Methods | Randomised trial. | |
| Participants | 38 puerperal women who did not desire to breastfeed. Exclusion criteria included women with severe metabolic disturbance or on concomitant therapy, e.g. corticoids, thyroid and antithyroid therapy, diuretics and phenothiazines, which might influence the results of the study. Setting: a university hospital in Oslo, Norway. | |
| Interventions | Oral bromocriptine 2.5 mg twice daily for 14 days (n = 20) versus oral diethylstilbestrol 10 mg twice daily for 7 consecutive days (n = 18). | |
| Outcomes | Milk secretions, breast congestion, side effects, blood pressure changes, clotting profile and rebound lactation. Symptoms were scored on a scale of 0 and 3. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. "A randomisation list' was employed. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Participants, study personnel and outcome assessors were blinded to the interventions. |
| Blinding (performance bias and detection bias) Adverse events | Low risk | Participants, study personnel and outcome assessors were blinded to the interventions. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data for the duration of follow‐up considered in the review. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing data for the duration of follow‐up considered in the review. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
O'Donoghue 1977.
| Methods | Randomised trial. | |
| Participants | 60 women who had elected not to breastfeed during the antenatal period. Setting: a maternity hospital in Cork, Ireland. | |
| Interventions | Bromocriptine 2.5 mg twice daily for 14 days versus 1 tablet of quinestrol immediately after delivery. | |
| Outcomes | Milk secretion, breast congestion and breast pain rated on a 4‐point scale and assessed by observations made by nurses on record cards while in hospital and by women themselves after hospital discharge. | |
| Notes | Bromocriptine and placebo tablets were provided by personnel of Sandoz A.G., Basle. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Women were "randomly allocated to 1 of 2 treatment groups". |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information about allocation concealment to permit judgement. Women "received contents of a numbered envelope containing 1 capsule and 1 tablet. Subsequently, patients received 1 capsule twice daily for 14 days from a similarly numbered bottle". |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Inadequate information about blinding to permit judgement. "The content of the envelope and the bottle were unknown to the patients and staff conducting the trial". |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | High risk | 16 women (26.7%) failed to return their record cards. The attrition rates were not separately reported for the 2 treatment groups. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Paggi 1975.
| Methods | Randomised trial. | |
| Participants | 40 women who elected not to breastfeed. Setting: a University hospital in Torino, Italy. | |
| Interventions | L‐DOPA 1 g/day for 6 days in fractioned dose (n = 20) versus placebo (n = 20). | |
| Outcomes | Milk secretion, breast tension and breast pain as subjectively described by the women. Severity of symptoms was scored as 0, +, ++ and +++. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Women were "randomly allocated to one of two treatment groups". Women were "randomised and submitted to a double blind trial". |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | No information on blinding. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not report this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not report this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting |
| Other bias | Low risk | The study appears free of other sources of bias. |
Phillips 1975.
| Methods | Randomised trial. | |
| Participants | 196 private postpartum women who chose not to breastfeed. Setting: USA. | |
| Interventions | Oral chlorotrianisene 72 mg twice daily for 2 days for a total dose of 4 capsules (n = 98) versus placebo (n = 98) | |
| Outcomes | Breast engorgement, milk secretion and discomfort, use of concurrent supplemental therapy ‐ breast binders, ice bags and or analgesics. | |
| Notes | Partly funded by Merrell‐National Laboratories, Merrell Inc., Cincinnati, Ohio. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Women were randomly assigned on a "double blind basis". No further information to permit judgement on blinding. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | High risk | Out of the 200 participants who entered the trial, 4 were excluded from the analysis as they "took fewer than the specified protocol". |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | High risk | The trialists did not include thromboembolism as an outcome measure in spite of published studies implicating oestrogen preparations in this complication before the trial. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Piya‐Anant 2004.
| Methods | Randomised trial. | |
| Participants | 230 asymptomatic HIV‐positive mothers aged 18 to 35 years. Normal delivery at 37 to 42 weeks. Exclusion criteria included women at high risk for taking a combined pill with a high dose of oestrogen, women older than 35 years, overweight or with a past history of a thromboembolic episode, women at high risk for taking bromocriptine, those with pregnancy induced hypertension, seizures, stroke and myocardial infarction. Seting: a university hospital in Thailand. | |
| Interventions | Oral ethinyl oestradiol 50 µg twice daily for 5 days (n = 116) versus oral bromocriptine dose was not stated (n = 114). | |
| Outcomes | Breast engorgement. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generation of allocation sequence was by computer randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Study participants and personnel were blinded to the interventions. Uncertain whether outcome assessors were blinded to the interventions. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | Study participants and personnel were blinded to the interventions. Uncertain whether outcome assessors were blinded to the interventions. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Purkayastha 1991.
| Methods | Randomised trial. | |
| Participants | 48 women delivering fresh stillborn or extremely premature infants. Women with abnormal physical findings or laboratory reports were not included in the trial. | |
| Interventions | Bromocriptine 5 mg thrice daily for 5 days (n = 28) versus 3 mL single intramuscular injection of oestradiol/testosterone ester. | |
| Outcomes | Milk flow, breast heaviness and pain. Blood pressure, condition of the breasts and uterine involution were checked daily. Observations were made while the women were in the hospital and on day 28. Relief of breast symptoms was rated as good, moderate, poor and nil. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. The only mention of "randomisation" is in the title of the study. |
| Allocation concealment (selection bias) | Unclear risk | No information to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Insufficient information to permit judgement on blinding. Although the study was labelled as "single blind", it is unclear who was blinded and what measures were taken to ensure blinding. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | High risk | The study failed to include results for adverse events directly related to the interventions (especially thromboembolism which was associated with oestrogen containing preparations) in published studies before the trial. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Rolland 1973.
| Methods | Randomised trial. | |
| Participants | 40 women who had given sufficient proof of lactation in a previous puerperium. Exclusion criteria include women on medication that might influence their hormonal state and patients with hypertension (diastolic pressure more than 100 mmHg). Setting: The Wever Hospital, Heerlen, Netherland. | |
| Interventions | Oral bromocriptine 7.5 mg daily for 7 days (n = 10), oral bromocriptine 5 mg daily for 7 days (n = 10) and oral bromocriptine 2.5 mg daily for 7 days (n = 10), oral placebo (n = 10). First capsule given within the 2 to 6 hours after delivery. | |
| Outcomes | Milk secretion, breast engorgement, breast pain, rebound lactation and satisfaction with intervention. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Participants received treatment "at random". |
| Allocation concealment (selection bias) | High risk | No information about allocation concealment to permit judgement. Participants who had to discontinue medication within the first 72 hours after delivery were excluded and "another patient with the same dosage schedule was admitted into the study". Comment: unlikely that allocation sequence was concealed. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Capsules of identical appearance were used for bromocriptine and placebo. Participants, study personnel and outcome assessors were blinded to the interventions. |
| Blinding (performance bias and detection bias) Adverse events | Low risk | Participants, study personnel and outcome assessors were blinded to the interventions. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | Analysis was by intention‐to‐treat. Outcome data for 8 participants who stopped treatment due to mammary activity within 90 hours of delivery were included in the analysis. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | Completeness of data unclear as the result was inadequately presented. "No side effects occurred as a result of CB154 medication; mild symptoms were noted just as frequently in the placebo group as in the treatment groups". |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Scapin 1982.
| Methods | Randomised trial. | |
| Participants | 40 healthy postpartum women who did not want to breastfeed for personal reasons. Evidence of adequate mammary activity in the previous pregnancy. Setting: a university hospital in Milan, Italy. | |
| Interventions | Metergoline 4 mg thrice daily for 7 days versus bromocriptine 2.5 mg twice daily for 7 days. Additional 7 days treatment when there was evidence of mammary activity. 1 capsule containing placebo was added to make up similar treatment schedule. All women also had methylergometrine hydrogen maleate 0.2 mg as part of the routine management after delivery and 0.2 mg daily p.o. thrice daily for 7 days. | |
| Outcomes | Milk excretion, mammary engorgement and pain. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Participants were randomly allocated to 1 of the 2 treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Participants, study personnel and outcome assessors were blinded to the interventions.The 2 drugs were contained in identical sealed capsule. Capsules were packed in small plastic bags with a label specifying the day and time of administration. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | High risk | There were 2 periods of treatment, the second depending on the outcome of the first treatment in the first week. The second period of treatment was not consistent across studies. |
Schwartz 1973.
| Methods | Randomised trial. | |
| Participants | 346 women who elected not to breastfeed. Setting: a university hospital, Pennsylvannia, USA. |
|
| Interventions | 360 mg of intramuscular testosterone oenanthate plus16 mg of oestradiol valerate (n = 89), diethylstilbestrol 5 mg orally thrice daily for 3 days (n = 89), chlorotrianisene 72 mg orally every 12 hours at 4 doses (n = 85), injectable and oral placebo (n = 83). | |
| Outcomes | Breast tenderness, consistency and milk leakage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Participants and study personnel were blinded to the interventions. Uncertain whether outcome assessors were blinded. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Unclear risk | 7 participants (1.9%) out of the 353 who entered the trial were excluded from the analysis of outcome data on day 3. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | High risk | The study failed to include results for adverse events directly related to the interventions especially thromboembolism which was associated with oestrogen preparations in published studies before the trial. |
| Other bias | Unclear risk | The study appears free of other sources of bias. |
Senior 1969.
| Methods | Randomised trial. | |
| Participants | 88 women who did not wish to breastfeed. Setting: a hospital in Penzance, Cornwall, United Kingdom. | |
| Interventions | Stilbestrol 10 mg 4 times daily for 4 days, 5 mg 4 times daily for 4 days and 5 mg twice daily for 2 days (n = 43) versus placebo (n = 45). | |
| Outcomes | Milk leakage, breast consistency and pain. | |
| Notes | Report was presented as correspondence. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Women received the interventions "at random". |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Inadequate information to permit judgement on blinding. Study was referred to as "double blind trial". |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not include this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not include this outcome. |
| Selective reporting (reporting bias) | Unclear risk | The study was reported as a correspondence and did not include separate 'Methods' and 'Results' sections to permit assessment of outcomes reporting bias. |
| Other bias | Unclear risk | Inadequate information in this correspondence to determine other potential sources of bias. |
Shaaban 1975.
| Methods | Randomised trial. | |
| Participants | 150 postpartum women who elected to bottle feed their babies and those whose babies were stillborn or were nursed in incubators on account of prematurity. Setting: 2 tertiary care centres in Egypt. |
|
| Interventions | Short course tamoxifen 10 mg: 3 tabs twice daily for 2 days, followed by 2 tabs twice daily for 2 days and 1 tab twice daily for 2 days, long course tamoxifen 10 mg: 1 tab twice daily for 14 days, placebo equivalent to long course and short course of tamoxifen. Treatments were commenced within 2 hours of delivery. | |
| Outcomes | Breastmilk secretion, engorgement and tenderness, thromboembolic complications. | |
| Notes | The Clinical Research Department of Imperial Chemical Industry supplied tamoxifen. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Participants, study personnel and outcome assessor were blinded to the interventions. The courses of treatment were coded and the code was only broken at the end of the trial. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not adequately report this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | 10 women who did not attend for complete follow‐up were dropped from the trial. Loss to follow‐up was 6.7%. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not adequately report this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Shrivastav 1988.
| Methods | Randomised trial. | |
| Participants | 60 women who required suppression of puerperal lactation following a fresh stillbirth or an early neonatal death. Setting: a university hospital in Vellore, India. | |
| Interventions | Jasmine flower: 50 cm of stringed flowers from the same vendor and applied to each breast and replaced every 24 hours for 5 days (n = 30) versus bromocriptine mesylate 2.5 mg 8 hourly for 5 days (n = 30). | |
| Outcomes | Serum prolactin, milk secretion and breast engorgement. Milk secretion and engorgement were evaluated by manual pressure on the nipple and observations recorded on a 4‐point scale (0, 1, 2 and 3). The 2 scores were combined to give an aggregate score of 0 to 6. Scores greater than or equal to 4 at the end of 72 hours were considered unsuccessful. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Women were "randomly divided into groups". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Blinding was not possible for participants and study personnel. Uncertain whether outcome assessors were blinded. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Steenstrup 1977.
| Methods | Randomised trial. | |
| Participants | 41 women who wished to prevent postpartum lactation either because of maternal wish, adoption, illness of mother, illness of the child, child death and fetal death in utero. Exclusion criteria: women with metabolic disturbances and concomitant therapy, e.g. corticoid, thyroid, antithyroid therapy, diuretics, phenothiazines, which might influence the results of the study. Setting: a university hospital in Denmark. | |
| Interventions | Bromocriptine 2.5 mg twice daily for 14 days (n = 20), diethylstilbestrol capsules containing 10 mg of active compound were employed twice daily for 7 days (and made up with placebo to last for 14 days) (n = 21). | |
| Outcomes | Milk secretion, breast engorgement, spotting per vaginam, rebound lactation, failure to suppress lactation at 14 days. Symptoms were scored on a scale of 0 to 3. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Allocation of patients to 2 groups was made with the "help of a randomisation list". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Participants and study personnel were blinded to the interventions. Uncertain whether outcome assessors were blinded. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | Participants and study personnel were blinded to the interventions. Uncertain whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | 3 participants (7.3%) were lost to follow‐up. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | 3 participants (7.3%) were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | Insufficient information to assess whether other important risk of bias exists. |
Stirrat 1968.
| Methods | Randomised trial. | |
| Participants | 100 mothers delivered consecutively and who chose to bottle feed their babies. Setting: a general hospital in Glasgow, UK. | |
| Interventions | Stilbesterol 5 mg (day 1: 3 tablets thrice daily, day 2: 2 tablets thrice daily, day 3: 1 tablet thrice daily, day 4: 1 tablet twice daily and day 5: 1 tablet statum (n = 50) versus placebo (n = 50). | |
| Outcomes | Milk secretion, breast pain, thromboembolism, number of women who require second line drug or method to achieve suppression. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. "Patients were randomly assigned to either A or B". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Participants and outcome assessors were blinded to the interventions. The study personnel were not blinded. The tablets were placed in identical containers labelled A and B, the content being known to 1 of the authors. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not adequately report this outcome. Only thromboembolic episodes were reported in the result. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not adequately report this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study was free of other sources of bias. |
Swift 2002.
| Methods | Randomised trial. | |
| Participants | 60 non‐breastfeeding mothers who gave birth to viable newborns of singleton gestations, had an uncomplicated postpartum and had not received hormonal lactation suppressants. Setting: a private hospital in south‐central USA. | |
| Interventions | Use of breast binders (n = 30) versus wearing of support bra (n = 30) for the first 10 days postpartum. | |
| Outcomes | Breast engorgement, breast tenderness, breast leakage and use of pain relief measures. Outcomes were rated on a scale of 1 to 4 for each of the 5 data collection periods (postpartum days 1, 3, 4, 9 and 10. Assessments were recorded through telephone on days 3, 4, 9 and 10. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A table of random numbers was used to determine group assignment before the first contact. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | High risk | Impossible to blind participants and study personnel. Outcome assessors (woman herself) were not blinded. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not report this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not report this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study was free of other sources of bias. |
Thorbert 1983.
| Methods | Randomised trial. | |
| Participants | 24 women who wished to inhibit lactation after childbirth. Setting: Central Hospital, Kalmar and University Hospital, Lund, Sweden. | |
| Interventions | Cyclofenil 300 mg twice daily for 14 days (n = 13) versus bromocriptine 2.5 mg twice daily for 14 days (n = 11). | |
| Outcomes | Breast engorgement, rebound lactation, number of women who require second line drug or method to achieve suppression. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. "Allocation of treatment was randomised." |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Participants, study personnel and outcome assessors were blinded to interventions. Double‐blind conditions were achieved by double dummy technique. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not adequately report this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not adequately report this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Tulandi 1985.
| Methods | Randomised trial. | |
| Participants | 16 postpartum women between 20 and 28 years of age, who had normal deliveries and decided not to breastfeed. Setting: a university and a general hospital in Canada. |
|
| Interventions | Prostaglandin E2 at 2 mg daily for 4 days (n = 8) versus placebo (n = 6). | |
| Outcomes | Breast leakage, breast swelling and pain, serum prolactin. Symptoms were scored on a scale of 0 to 3. | |
| Notes | Upjohn Company of Canada supplied the drugs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. "Each subject received PGE2 or placebo orally in random order." |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Participants and study personnel were blinded to interventions. Uncertain whether outcome assessors were blinded. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | 2 participants dropped out from the placebo group because of severe breast engorgement and pain. Missing data were imputed using appropriate methods. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Utian 1975.
| Methods | Method of randomisation not stated. Participants, clinicians and outcome assessors were blinded. | |
| Participants | 31 postpartum non‐breastfeeding women who were free from metabolic or surgical conditions which might interfere with the absorption, metabolism or excretion of the drugs, absence of concurrent medication. Setting: a university hospital in Cape Town, South Africa. | |
| Interventions | Oral bromocriptine 2.5 mg twice daily for 14 days (n = 16) versus chlorotrianisene 24 mg twice daily for 14 days (n = 15). | |
| Outcomes | Milk production, breast congestion and side effects (blood pressure changes, pulse rate, rebound lactation, 24 hour urinary output and thromboembolism. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Study participants and personnel were blinded to interventions. Uncertain whether outcome assessors were blinded. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | Uncertain whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | 7 (18.4%) of the 38 participants who entered the trial were lost to follow‐up. Drop‐out rates were similar for both groups. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Drop‐out rates were similar for both groups. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Van der Heijden 1991.
| Methods | Randomised trial. | |
| Participants | 30 women who elected to bottle feed their babies. Setting: University hospital, Nijmegen, The Netherlands. | |
| Interventions | Dopamine agonist CV 205‐502 given as a daily oral dose at bedtime of 0.05 mg (day 1), 0.075 mg (day 2‐14) (n = 20) and 0.05 mg (day 15‐21) versus bromocriptine 2.5 mg twice daily (day 1‐14) and once daily (day 15‐21) (n = 10). | |
| Outcomes | Breast symptoms (milk secretion, breast congestion and breast pain) and side effects. Daily observations of breast symptoms were graded according to the severity and duration of the symptoms as very good; good and poor. The safety of CV 205‐502 was tested by routine physical examinations, ECG and laboratory tests (haemoglobin, platelets, leucocytes, urea, creatinine and liver enzymes) on days 0 and 42. Coagulation tests, fibrinogen, activated partial thromboplastin time, prothrombin time and anti‐thrombin III were performed on days 0, 1, 12 and 42. The women were visited for assessment between days 0 and 42. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Women were "randomly assigned into a 2 to 1 ratio to receive either CV 205‐502 or bromocriptine for lactation inhibition." |
| Allocation concealment (selection bias) | High risk | There was no concealment of allocation. "An open model was chosen to examine the two aspects of the suppression of postpartum lactation." |
| Blinding (performance bias and detection bias) Lactation | High risk | Study participants and personnel were not blinded to the intervention. "The patients and investigative staff were aware of the treatment of each patient." |
| Blinding (performance bias and detection bias) Adverse events | High risk | Study participants and personnel were not blinded to the intervention. "The patients and investigative staff were aware of the treatment of each patient." |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Varga 1972.
| Methods | Randomised trial. | |
| Participants | 60 consecutive non‐breastfeeding women. Setting: a university hospital in Switzerland. | |
| Interventions | Oestrogen 20 mg twice daily for the first 3 days, 10 mg twice daily for the second 3 days, and 10 mg daily for the last 3 days, ergocryptine 5 mg twice daily for the first 6 days and 5 mg daily for the last 3 days, placebo was initiated within 24 hours after delivery, 12 hours on average. | |
| Outcomes | Breast congestion, rebound lactation and thromboembolic disease. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Women were "randomly assigned" to 1 of 3 groups. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Participants and study personnel were blinded to interventions. Uncertain whether outcome assessor (same observer) was blinded. 3 identical cachets were used containing either a placebo, stilbestrol or bromocriptine. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | Uncertain whether outcome assessor (same observer) was blinded. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Venturini 1981.
| Methods | Randomised trial. | |
| Participants | 38 women with indications for suppression of lactation in the puerperium. All gave informed consent. All patient delivered vaginally. Exclusion criteria: Use of drugs that might interfere with results and non co‐operative women. Setting: a university hospital in Italy. | |
| Interventions | Lisuride 0.2 mg 3 times daily for 15 days (n = 20) versus bromocriptine 2.5 mg 3 times daily for 15 days (n = 18). | |
| Outcomes | Milk secretion, breast engorgement, breast pain, rebound lactation and side effects. Assessment of mammary activity was on a scale of 0 to 3. | |
| Notes | Schering AG, Berlin‐Bergkamen supplied the drugs used in the trial. 1 of the authors of the paper was a staff of Schering AG, Berlin‐Bergkamen. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Participants, study personnel and outcome assessors were blinded to the interventions. The participants also doubled as outcome assessors. |
| Blinding (performance bias and detection bias) Adverse events | Low risk | Participants, study personnel and outcome assessors were blinded to the interventions. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | 4 (9.5%) out of the initial 42 women who entered the trial dropped out of the study because of side effects (1 from the lisuride group and 3 from bromocriptine group). |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | 4 (9.5%) out of the randomised participants dropped out of the study because of side effects (1 from the lisuride group and 3 from bromocriptine group). |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Venturini 1988.
| Methods | Randomised trial. | |
| Participants | 45 women who for medical or personal reason did not want to breastfeed. Setting: a university hospital in Italy. | |
| Interventions | Terguride 0.5 mg (n = 15), terguride 1 mg (n = 15), terguride 0.25 mg capsules were taken orally twice daily for 15 days (n = 15). | |
| Outcomes | Milk secretion, congestion and breast pain, side effects during the first 5 days of treatment. Clinical assessments were scored according to a rating scale of 0 to 4 indicating increasing severity. | |
| Notes | Schering AG, Berlin‐Bergkamen supplied the drugs used in the trial. 3 of the authors of the paper were staff of Schering AG, Berlin‐Bergkamen. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Participants, study personnel and outcome assessors were blinded to the interventions. Capsules of identical appearance containing 3 different doses of terguride were given to the participants. The participants also doubled as outcome assessors. |
| Blinding (performance bias and detection bias) Adverse events | Low risk | Participants, study personnel and outcome assessors were blinded to the interventions. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing data. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Low risk | The authorship of the trial report was a potential threat to the validity of the results. |
Vischi 1975.
| Methods | Randomised trial. | |
| Participants | 198 women unwilling to breastfeed or in whom lactation was not advised on medical grounds. Setting: obstetric departments of 3 hospitals in Northern Italy. |
|
| Interventions | Experiment groups: Oral quinestrol 2 mg single dose (n = 66), oral quinestrol 4 mg single dose (n = 66). Control group: oral placebo (n = 66) | |
| Outcomes | Milk leakage, discomfort, and engorgement. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Participants were allocated by a "random code" to 1 of the treatments. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Participants and personnel were blinded to the interventions. Uncertain whether outcome assessor was blinded. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | Uncertain whether outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data in the first week of assessment. Loss to follow‐up was 59.6% after the first week assessment. Extracted data were restricted to that obtained in first week of assessment. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | No missing data in the first week of assessment |
| Selective reporting (reporting bias) | High risk | The study failed to include results for adverse events directly related to the interventions especially thromboembolism which was associated with oestrogen preparations in published studies before the trial. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Walker 1975.
| Methods | Randomised trial. | |
| Participants | 87 women who were delivered vaginally and who did not wish to breastfeed. Those who had general anaesthesia or were receiving concomitant therapy which might influence the results (e.g. diuretics, corticosteroids, phenothiazines) were excluded. Setting: Obstetrics and Gynaecology Department and General Practice Unit, Welsh National School of Medicine, Cardiff, UK. | |
| Interventions | Bromocriptine 2.5 mg twice daily for 14 days (n = 32), quinestrol 4 mg immediately after delivery, followed by placebo twice daily (n = 28), placebo twice daily (n = 27). | |
| Outcomes | Breast discomfort, congestion, milk leakage (scored on linear analogue scales by the woman), side effects, use of analgesic and rebound lactation. | |
| Notes | Sandoz Ltd. provided financial support. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Participants were given the content of a "numbered envelope" which contained the intervention drug and placebo. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Participants, study personnel and outcome assessors were blinded to the interventions. The participants also doubled as outcome assessors. |
| Blinding (performance bias and detection bias) Adverse events | Low risk | Participants, study personnel and outcome assessors were blinded to the interventions. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | 3 participants (1 from quinestrol arm and 2 from placebo arm) were withdrawn due to breast congestion and pain which was severe enough by the 4th or 5th day postpartum. Outcome data were presented for all participants. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not adequately report this outcome. |
| Selective reporting (reporting bias) | High risk | The study failed to include results for adverse events directly related to the interventions especially thromboembolism which was associated with oestrogen preparations in published studies before the trial. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Watson 1969.
| Methods | Randomised trial. | |
| Participants | 99 women who had elected not to breastfeed as a statement of intent and those who could not breastfeed because of stillbirth. No cases of already established breastfeeding. Setting: Queen Elizabeth II Hospital, Welwyn Garden City, UK. | |
| Interventions | 50 women received 4 mg of quinestrol (plus 24 white tablets of placebo) while 50 other women received (control group) 5 mg stilbestrol after delivery, then each tablets twice daily for 2 days, then 1 tablet thrice daily for 2 days, then 1 tablet twice daily for 2 days, then 1 tablet daily for 2 days. | |
| Outcomes | Breast engorgement., number of women who required second line drug or method to achieve suppression, disturbance of menstrual pattern. | |
| Notes | Personnel of William R. Warner & Co. Ltd. supplied the treatment packs and "arranged" the statistical compilation of the results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Low risk | The tablets were made up of Identical packs identified by a code number the key to which was retained by the hospital pharmacist. Patients were allocated a sequential code number 1 to 100. The corresponding numbered treatment pack was opened by the labour ward Sister after entry into the trial. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Study participants and personnel were blinded to the interventions. Uncertain whether outcome assessors were blinded. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not report this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | 1 participant (1%) among the 100 women who entered the trial was lost to follow‐up. The participant was in stilbestrol arm. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not report this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | Unclear risk | Arrangement of statistical compilation of the results by the supplier of the treatment packs constituted a potential threat to validity of the results. |
Weinstein 1976.
| Methods | Randomised trial. | |
| Participants | 75 women were randomly assigned to 5 groups including a placebo group. Setting: a university hospital in Israel. | |
| Interventions | Interventions: stilbestrol 5 mg thrice daily for 14 days, clomiphene citrate 50 mg twice daily for 14 days, testosterone propionate as a single injection of 75 mg, bromocriptine 2.5 mg twice daily for 14 days. Control: placebo | |
| Outcomes | Milk secretion, breast pain, engorgement, breast tenderness and rebound lactation. Clinical responses were graded as good, fair and poor. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. Participants were "randomly assigned to one of five treatment groups". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | High risk | There was no evidence of blinding. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not report this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Unclear risk | No missing data. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not report this outcome. |
| Selective reporting (reporting bias) | High risk | The study failed to include results for adverse events directly related to the interventions especially thromboembolism which was associated with oestrogen preparations in published studies before the trial. |
| Other bias | Low risk | The study appears free of other sources of bias. |
Winter 1964.
| Methods | Randomised trial. | |
| Participants | 800 (first part of the study) and 98 (second part of the study) non‐nursing postpartum women. Setting: a university hospital and a maternity hospital, Halifax, Nova Scotia, Canada. |
|
| Interventions | First part intervention group: stilbestrol 5 mg; control group: indistinguishable placebo. Second part of the study ‐ intervention group: synthetic oxytocin 40 I.U as nasal spray; control group: indistinguishable placebo. | |
| Outcomes | Breast engorgement, breast pain, milk leakage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Low risk | Study participants, personnel and outcome assessors were blinded to the interventions. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not report this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | No missing data while in hospital. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not report this outcome. |
| Selective reporting (reporting bias) | Low risk | Comparison of outcome measures in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting. |
| Other bias | High risk | Study was conducted over a period of 2 years, and 7 residents and 18 interns participated over this period. |
Yuen 1977.
| Methods | Randomised trial. | |
| Participants | 39 women who delivered at term after normal pregnancies and who had elected not to breastfeed their infants. Setting: a general hospital in Vancouver, British Columbia, Canada. |
|
| Interventions | 18 women received bromocriptine 2.5 mg twice daily for 14 days while 21 received chlorotrianisene 24 mg twice daily for 7 days (made up to 14 days as chlorotrianisene placebo). Therapy started within 2 hours of delivery and continued for 14 days. | |
| Outcomes | Breast leakage, breast swelling and breast pain. Nurses and participants assessed outcomes in hospital and at home respectively. | |
| Notes | Personnel of Sandoz Pharmaceuticals provided medications, financial assistance and data analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment to permit judgement. |
| Blinding (performance bias and detection bias) Lactation | Unclear risk | Participants and study personnel were blinded to the interventions. The capsules containing 2.5 mg bromocriptine were identical to those containing 24 mg chlorotrianisene or chlorotrianisene placebo. Uncertain whether outcome assessors were blinded. |
| Blinding (performance bias and detection bias) Adverse events | Unclear risk | The study did not adequately address this outcome. |
| Incomplete outcome data (attrition bias) Lactation | Low risk | 1 participant (2.5%) did not return for follow‐up on day 14. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | The study did not adequately address this outcome. |
| Selective reporting (reporting bias) | High risk | The study failed to include results for adverse events directly related to the interventions especially thromboembolism, which was associated with oestrogen preparations in published studies before the trial. |
| Other bias | Unclear risk | Data analysis by personnel of the pharmaceutical company that supplied medications and medical assistance constituted a threat to the validity of the results. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Almeida 1986 | This is a randomised double blind study evaluating the effect of bromocriptine mesylate on suppression of puerperal fever resulting from breast engorgement. |
| Bare 1960 | This study was conducted to determine the efficacy and the optimum dosage of fluoxymesterone in the suppression of lactation. There was no evidence of randomisation. The patients were divided into 4 groups. Group I consisted of ward patients who received placebo and other groups were all private patients. The dosage of the intervention was administered on "a strict alternating basis". |
| Barns 1961 | This study compared the effectiveness of a combination of testosterone enanthate and estradiol valerate in the suppression of postpartum lactation. There was no indication of randomisation. |
| Booker 1970 | This study compared combined and sequential type of oral contraceptive pills for suppressing lactation. Alternate allocation method was used. |
| Brett 1971 | This study compared stilbestrol and quinestrol with placebo. Although the study was described as a "double‐blind trial", there was no indication of randomisation. |
| Bristol 1966 | This study compared the effectiveness of compression breast binder and supporting brassiere in the suppression of postpartum lactation. There was no indication of randomisation. The final study group consisted of 38 women who were "divided into two groups of 19 patients each". |
| Brooten 1983 | This study compared 3 non‐pharmacologic measures (compression binder, standardised support bra and fluid restriction) with bromocriptine. The assignment of women to bromocriptine group was "by virtue of their physicians' treatment". |
| Caballero 1987 | This is a double‐blind study comparing methergoline with bromocriptine in the suppression of lactation. Although the study was described as randomised, allocation of interventions was by alternation. |
| Canales 1977 | This study evaluated the effect of clomiphene in the suppression of puerperal milk secretion and serum prolactin. There was no indication of randomisation. |
| Cantis 1977 | This was a single‐blind study carried out in 42 women to assess the lactation suppressing activity of piribedil. There was no evidence of randomisation. Treatments were assigned to unbalanced groups of women (33 women received the active agent while 9 received placebo). |
| Cicinelli 1996 | This study evaluated the effectiveness of nasal bromocriptine on serum prolactin. |
| David 1977 | This study compared the effectiveness of stilbestrol with placebo on prolactin level. |
| De Aloysio 1988 | This study compared the effect of dihydroergocristine to that of bromocriptine on prolactin secretion and postpartum lactation. All the puerperae enrolled in the study had a physiological delivery and wished to interrupt breastfeeding after at least 3 months of nursing. |
| De Cecco 1979 | This study evaluated the effect of Lisuride on lactation and postpartum serum prolactin level. There was no indication of randomisation. |
| Del Pozo 1975 | This study evaluated the action of methergoline on plasma prolactin and milk secretion in the first 7 postpartum days. There was no indication of randomisation and women were "divided" into 2 groups. |
| Duthie 1990 | This randomised trial compared the efficacy, tolerance and effect on prolactin level of 4 different dosages of intramuscular bromocriptine retard. Patients were randomised into 4 equal groups. Loss to follow‐up was 10.8%. Variable numbers of women in the groups developed mild to moderate breast engorgement and milk flow prior to administration of bromocriptine. |
| Fleming 1977 | This double‐blind trial compared the efficacy of pyridoxine, stilbestrol and a placebo in the inhibition of puerperal lactation. There was no indication of randomisation. |
| Foukas 1972 | This double‐blind trial compared diethylstilbestrol and pyridoxine with placebo in the suppression of lactation. There was no indication of any randomised comparison between the groups. Patients were divided into 4 unbalanced groups (86, 68, 75 and 25). |
| Garry 1956 | This study was undertaken to evaluate the effectiveness of oestrogen‐androgen preparation in the suppression of lactation. There was no indication of randomisation. The patients were divided into 2 unequal groups (100 and 50). |
| Gerstner 1978 | This study compared bromocriptine with hexestrol for suppressing lactation. Alternate allocation method was used. |
| Gillibrand 1968 | Data to evaluate the validity of the methods used are not available in this published correspondence that described 2 clinical trials. It has been excluded because no full publication of the study could be located. |
| Gopalan 1997 | This randomised double‐blind study assessed the effectiveness of pyridoxine in inhibition of lactation and serum prolactin. The report is only available in abstract form. |
| Grant 1978 | Although this trial was described as a "double blind evaluation of quinestrol, chlorotrianisene and placebo", there was no indication of randomisation. |
| Hale 2004 | This study evaluated the effects of pseudoephedrine on milk production, plasma prolactin and breastmilk levels following a maternal dose of 60 mg in 8 breastfeeding mothers. There was no indication of randomisation. |
| Kaiser 1952 | This study was conducted to evaluate the effect of combined oestradiol and progesterone on lactation suppression. There was no indication of randomisation. |
| Kalir 1975 | This study was conducted to evaluate the effect of clomiphene citrate on lactation suppression. There was no indication of randomisation. |
| Kee 1989 | This study evaluated the usefulness of serrapeptase (Dansen) in postpartum women with breast engorgement in a double blind randomised controlled trial. |
| King 1958 | Study comparing chlorotrianisene with placebo. There was no indication of randomisation. |
| Kirkland 1960 | This "randomised" study involving 160 women had the allocation concealment deciphered for the placebo group during the study resulting in unequal allocation of subjects to the placebo arm and consequent discontinuation of the arm. This questions adherence to the initially generated allocation sequence till the end of the trial. |
| Koshiishi 1971 | This study evaluated the effect of a proteolytic enzyme (bromelain) on breast engorgement. |
| Lee 1971 | This study compared chlormezanone (a non‐hormonal tranquillizer) with stilbesterol. There was no evidence of randomisation. Patients were allocated alternately into 1 of 2 groups. |
| Lee 1979 | This study compares Ginsenocide triol with bromocriptine. The paper is only available in abstract form. |
| Llewellyn‐Jones 1963 | This double‐blind trial compared the effectiveness of stilbestrol with placebo in the suppression of lactation. There was no indication of randomisation. |
| Lo Dico 1980 | This study evaluated the effectiveness of pyridoxine, 2‐ Br‐ alpha‐ergocriptine and piribedil in lactation suppression. There was no indication of randomisation. Patients were "divided" into 4 uneven groups. |
| Louviere 1975 | The primary purpose of this study was to evaluate the effectiveness of Deladumone OB in the suppression of postpartum breast engorgement and lactation. There was no indication of randomisation. |
| MacDonald 1965 | This double‐blind trial compared stilbestrol with placebo. Alternate allocation method was used. |
| MacLeod 1977 | This study evaluated the dose response and timing of administration of bromocriptine. There was no evidence of randomisation. Patients were divided into 3 groups. |
| Markin 1960 | This study compared the effectiveness of 5 preparations (diethylstilbestrol, dienestrol plus methyltestosterone, conjugated equine oestrogen plus methyltestosterone, testosterone propionate plus diethylstilbestrol and testosterone enanthate plus estradiol valerate) with placebo. Alternation method of allocation was used. "Drug E" or "Drug O" was administered by the nursing staff to the patients delivering on the even‐numbered or the odd‐numbered days of the month, respectively. |
| Masala 1978 | This study was designed to assess the effect of tamoxifen on the inhibition of puerperal lactation. There was no indication of randomisation. Treatments were assigned to 2 unbalanced groups (60: experimental group and 20: placebo group). |
| McLachlan 1991 | This randomised double‐blind placebo controlled trial tested the efficacy of thermal ultrasound therapy as a treatment for severe postpartum breast engorgement. |
| Momberg 1976 | This "double‐blind study" was "based on the order of admittance to the clinic and a randomisation list" suggesting the inclusion of an alternate allocation method. |
| Morris 1967 | This study was designed to evaluate the effectiveness of quinestrol in suppressing puerperal lactation. There was no indication of randomisation. |
| Morris 1970 | This double‐blind study evaluated the effectiveness of 3 preparations; oral chlorotrianisene in 3 different dosage strengths; an intramuscular combination of 2 steroids, testosterone enanthate and estradiol valerate; and identical placebos in the inhibition of puerperal breast engorgement, discomfort and milk secretion. Allocation of patients does not suggest randomisation. For the chlorotrianisene study, an unbalanced number of patients received the active agent and placebo (75 vs 25). |
| Nappi 1987 | This study examined whether the side effects of bromoergocriptine could be prevented by combining bromoergocriptine treatment with the antiemetic domperidone, without affecting the prolactin lowering effect and subsequent inhibition of lactation. |
| Nappi 1990 | This study assessed the effect of Ibopamine, a peripheral agonist on prolactin and milk production. 80 participants were admitted into the study including 30 nursing mothers. Participants were randomly "divided" into 6 groups. |
| Nappi 1993 | This study assessed the effects of dihydroergocriptine on serum prolactin levels and lactation in postpartum women. Women were "divided" into 6 unequal groups. Although it was stated that women were "randomly assigned" to 4 groups of non‐nursing mothers and 2 groups of nursing mothers, the non‐nursing mothers received the active agents while the nursing mothers received placebo. . |
| Ng 1972 | This study compared Quinestrol with Ablacton. Alternate allocation method was used. |
| Nisha 2006 | This "prospective observational study" compared oral carbegoline with intramuscular injection of combined oestrogens and androgens. Women were "randomly divided" into 2 groups. The study was only available in abstract form. |
| Osbourne 1978 | This study compared the effect of bromocriptine and quinestrol on coagulation and fibrinolysis. |
| Polatti 1982 | This study evaluated the inhibitory effect of cyclofenil on prolactin. There was no indication of randomisation. |
| Poulsen 1976 | This study evaluated the effect of bromocriptine on established lactation. |
| Primrose 1957 | This study compared TACE, Premarin and methyltestosterone, stibesterol with placebo. There was no indication of randomisation. |
| Reisfield 1966 | A "double‐blind" study using hydrochlorothiazide and an identical placebo in 100 consecutive postpartum women. There was no indication of randomisation. |
| Robuschi 1987 | This study evaluated the effect of maternal administration of bromocriptine on fetal and maternal serum growth hormone concentrations. |
| Rolland 1978 | This study describes 2 double‐blind studies on the effect of bromocriptine compared with placebo and an oestrogen/androgen compound. There was no indication of randomisation. |
| Roser 1966 | This study compares the effect of testosterone enanthate and estradiol valerate (Deladumone 2X) on suppression of symptoms of postpartum lactation. There was no indication of randomisation. Women were "divided" into 2 groups. |
| Ryan 1962 | This study evaluated the effectiveness of intranasal syntocinon compared to a placebo for relief of postpartum breast discomfort. Patients were instructed to begin assigned treatment when they first felt discomfort in their breasts. A total of 38.3% of women included in the study were excluded from the analysis as they did not experience enough discomfort to require the assignment of treatment. |
| Schneider 1964 | Double‐blind study comparing depot types of oestrogen and androgen together with a rapidly acting oestrogen against placebo. Treatments were allocated to alternative patients. |
| Seppala 1975 | This double‐blind study compared the effect of CB 154 (2‐Br‐alpha‐ergocriptine methane sulphonate) with diethylstilbestrol on established mammary secretion and congestion. There was no indication of randomisation. 39 patients were "divided into two groups". |
| Shapiro 1984 | Compares bromocriptine with breast binders and analgesics for inhibiting lactation. Alternate allocation method was used. |
| Steele 1968 | This study described the comparison of stibestrol with placebo in a double‐blind trial. Paper was presented as correspondence and full paper could not be located. There was no indication of randomisation in the published correspondence. |
| Stenchever 1962 | There was no evidence of randomisation. |
| Tyson 1966 | This study was designed to test the efficacy of a 3‐day course of chlorotrianisene for prevention and treatment of postpartum breast engorgement. There was no evidence of randomisation. |
| Van Dam 1981 | This study compared the lactation‐inhibiting effects of lisuride and bromocriptine. Although the study was labelled as a "double‐blind trial", there was no indication of randomisation. |
| Varga 1972b | This study evaluated the lactation‐inhibitory effects of bromocriptine, diethylstilbestrol and placebo. There was no indication of randomisation. |
| Walker 1980 | This is the same study as Walker 1975. |
| Willmott 1977 | This double‐blind placebo controlled study was undertaken to evaluate the clinical effectiveness of bromoergocrytine in suppressing lactation and observe any side effects over 28 days. There was no evidence of randomisation. |
| Zuckerman 1973 | This study was designed to determine the effectiveness of clomiphene in inhibiting postpartum lactation. There was no indication of randomisation. Patients were divided into 4 uneven groups (110, 26, 31 and 10). |
ITT: intention to treat vs: versus
Characteristics of studies awaiting assessment [ordered by study ID]
Caballero 1996.
| Methods | Not known. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Varga 1974.
| Methods | Not known. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Differences between protocol and review
The definition of lactation suppression as pre‐specified in the protocol for this review was revised in view of the definitions used by most trialists and the need to significantly reduce the clinical heterogeneity that would be introduced by the variable duration of outcome assessment when summarising effects of interventions. We evaluated the methodological quality of included trials by assessing the risk of bias for each study using the criteria outlined in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Contributions of authors
Both review authors extracted, entered and double checked data. Both authors contributed to the writing of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction‐HRP, Switzerland.
The Effective Health Care Alliance Programme (EHCAP) of the Liverpool School of Tropical Medicine, funded by the Department for International Health, UK.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bergsjo 1974 {published data only}
- Bergsjo P, Brodtkorb C. Comparison between quinestrol and diethylstilbestrol for the inhibition of lactation. Acta Obstetricia et Gynecologica Scandinavica 1974;53:77‐80. [DOI] [PubMed] [Google Scholar]
Berrebi 2001 {published data only}
- Berrebi A, Parant O, Ferval F, Thene M, Ayoubi JM, Connan L, et al. Treatment of pain due to unwanted lactation with a homeopathic preparation given in the immediate post‐partum period. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2001;30:353‐7. [PubMed] [Google Scholar]
Bhardwaj 1979 {published data only}
- Bhardwaj N. Inhibition of puerperal lactation: evaluation of bromocriptine and placebo. Australian and New Zealand Journal of Obstetrics and Gynaecology 1979;19:154‐7. [DOI] [PubMed] [Google Scholar]
Biggs 1978 {published data only}
- Biggs JS, Hacker N, Andrews E, Munro C. Bromocriptine, methyl testosterone and placebo for inhibition of physiological lactation: a controlled study. Medical Journal of Australia 1978;2(3 Suppl):23‐5. [DOI] [PubMed] [Google Scholar]
Binns 1967 {published data only}
- Binns DT. A double‐blind trial of chlorotrianisene in the suppression of lactation. Practitioner 1967;199:685‐8. [PubMed] [Google Scholar]
Boes 1980 {published data only}
- Boes EGM. Inhibition of puerperal lactation: a comparative study of bromocryptine and pyridoxine. South African Medical Journal 1980;57:900‐3. [PubMed] [Google Scholar]
Bravo‐Topete 2004 {published data only}
- Bravo‐Topete EG, Mendoza‐Hernandez F, Cejudo‐Alvarez J, Briones‐Garduno C. Cabergoline for inhibition of lactation. Cirugia y Cirujanos 2004;72:5‐9. [PubMed] [Google Scholar]
Caballero‐Gordo 1991 {published data only}
- Caballero‐Gordo A, Lopez‐Nazareno N, Calderay M, Caballero JL, Mancheno E, Sghedoni D. Oral cabergoline. Single‐dose inhibition of puerperal lactation. Journal of Reproductive Medicine 1991;36:717‐21. [PubMed] [Google Scholar]
Cooke 1976 {published data only}
- Cooke I, Foley M, Lenton E, Preston E, Millar D, Jenkins A, et al. The treatment of puerperal lactation with bromocriptine. Postgraduate Medical Journal 1976;52 Suppl 1:75‐80. [PubMed] [Google Scholar]
Cruttenden 1971 {published data only}
- Cruttenden LA. Inhibition of lactation. Practitioner 1971;206:248. [PubMed] [Google Scholar]
Defoort 1987 {published data only}
- Defoort P, Thiery M, Baele G, Clement D, Dhont M. Bromocriptine in an injectable retard form for puerperal lactation suppression: comparison with estandron prolongatum. Obstetrics & Gynecology 1987;70:866‐9. [PubMed] [Google Scholar]
De Gezelle 1979 {published data only}
- Gezelle H, Dhont M, Thiery M, Parewyck W. Puerperal lactation suppression and prolactin. Acta Obstetricia et Gynecologica Scandinavica 1979;58:469‐72. [DOI] [PubMed] [Google Scholar]
Dewhurst 1977 {published data only}
- Dewhurst CJ, Harrison RF, Biswas S. Inhibition of puerperal lactation: a double blind study of a bromocriptine and placebo. Acta Obstetricia et Gynecologica Scandinavica 1977;56:327‐31. [DOI] [PubMed] [Google Scholar]
England 1988 {published data only}
- England MJ, Tjallinks A, Hofmeyr GJ. Suppression of lactation: a comparison between bromocriptine and prostaglandin E2. Proceedings of 23rd Congress of Obstetrics and Gynaecology; 1986 Sept 23‐26; South Africa. 1986:45.
- England MJ, Tjallinks A, Hofmeyr GJ, Harber J. Suppression of lactation: a comparison of bromocriptine and prostaglandin E2. Journal of Reproductive Medicine 1988;33:630‐2. [PubMed] [Google Scholar]
European 1991 {published data only}
- European Multicentre Study Group for Cabergoline in Lactation Inhibition. Single dose cabergoline versus bromocriptine in inhibition of puerperal lactation: randomised, double blind, multicentre study. BMJ 1991;302:1367‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Firth 1969 {published data only}
- Firth PS. Inhibition of lactation. British Medical Journal 1969;1:254‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fischer 1995 {published data only}
- Fischer T, Streitmatter A, Gerede A, Frauendorf A, Krause M, Feige A. Puerperal inhibition of lactation with metergoline or bromocriptine. Zeitschrift fur Geburtshilfe und Neonatologie 1995;199:111‐5. [PubMed] [Google Scholar]
Giorda 1991 {published data only}
- Giorda G, Vincentiis S, Motta T, Casazza S, Fadin M, D'Alberton A. Cabergoline vs bromocriptine in suppression of lactation after cesarean delivery. Gynecologic and Obstetric Investigation 1991;31:93‐6. [DOI] [PubMed] [Google Scholar]
Hutchison 1981 {published data only}
- Hutchison P, Sill H. Lactation suppression with bromocriptine. New Zealand Medical Journal 1981;94:309‐10. [PubMed] [Google Scholar]
Iliya 1966 {published data only}
- Iliya FA, Safon L, O'Leary JA. Testosterone enanthate (180 mg) and estradiol valerate (8 mg) for suppression of lactation: a double‐blind evaluation. Obstetrics & Gynecology 1966;27:643‐5. [PubMed] [Google Scholar]
King 1972 {published data only}
- King MK. A comparative study of two fat‐stored oestrogens in inhibiting lactation. British Journal of Clinical Practice 1972;26:267‐70. [PubMed] [Google Scholar]
Kremer 1990 {published data only}
- Kremer JAM, Rolland R, Heijden PFM, Schellekens LA, Vosmar MBJG, Lancranjan I. Lactation inhibition by a single injection of a new depot‐bromocriptine. British Journal of Obstetrics and Gynaecology 1990;97:527‐32. [DOI] [PubMed] [Google Scholar]
Kuku 1968 {published data only}
- Kuku SB. Inhibition of lactation with Quinestrol. Journal of Obstetrics and Gynaecology of the British Commonwealth 1968;75:103‐4. [DOI] [PubMed] [Google Scholar]
Kulski 1978 {published data only}
- Kulski JK, Hartmann PE, Martin JD, Smith M. Effects of bromocriptine mesylate on the composition of the mammary secretion in non‐breast‐feeding women. Obstetrics & Gynecology 1978;52:38‐42. [PubMed] [Google Scholar]
MacDonald 1976 {published data only}
- MacDonald HN, Collins YD, Tobin MJW, Wijayratne DN. The failure of pyridoxine in the suppression of puerperal lactation. British Journal of Obstetrics and Gynaecology 1976;83:54‐5. [DOI] [PubMed] [Google Scholar]
Mann 1971 {published data only}
- Mann CW. Lactation inhibition in the Outer Hebrides. Practitioner 1971;206:246‐7. [PubMed] [Google Scholar]
Marcus 1975 {published data only}
- Marcus RG. Suppression of lactation with high doses of pyridoxine. South African Medical Journal 1975;49(52):2155‐6. [PubMed] [Google Scholar]
Martinez 1994 {published data only}
- Martínez‐Guisasola J, Guerrero M, Ayuso F, Alonso F, Arnaiz M, Blanco M. Supression of breastfeeding with lisuride. Experience and results [Supresión de la lactancia con lisuride. Experiencia y resultados]. Toko‐Ginecología Práctica 1994;53:392‐6. [Google Scholar]
McGlone 1969 {published data only}
- McGlone J. An assessment of quinestrol in the inhibition of lactation. Practitioner 1969;203:187‐8. [PubMed] [Google Scholar]
McNicol 1972 {published data only}
- McNicol E, Struthers JO. A combined/oestrogen/progestogen/testosterone agent for the inhibition of lactation. British Journal of Clinical Practice 1972;26:567‐8. [PubMed] [Google Scholar]
Melis 1988 {published data only}
- Melis GB, Mais V, Paoletti AM, Beneventi F, Gambacciani M, Fioretti P. Prevention of puerperal lactation by a single oral administration of the new prolactin‐inhibiting drug, Cabergoline. Obstetrics & Gynecology 1988;71:311‐4. [PubMed] [Google Scholar]
Menczer 1969 {published data only}
- Menczer J, Eskin BA. Evaluation of postpartum breast engorgement by thermography. Obstetrics & Gynecology 1969;33(2):260‐3. [PubMed] [Google Scholar]
Mizuno 1990 {published data only}
- Mizuno M, Taketani Y, Aono T, Iizuka R, Tamada T, Tominaga T, et al. Double‐blind study evaluating the efficacy of parlodel modified release Capsule (PLO‐MR) in the suppression of puerperal lactation. Rinsho Hyoka (Clinical Evaluation) 1990;18:341‐55. [Google Scholar]
Niebyl 1979 {published data only}
- Niebyl JR, Bell WR, Schaaf ME, Blake DA, Dubin NH, King TM. The effect of chlorotrianisene as postpartum lactation suppression on blood coagulation factors. American Journal of Obstetrics and Gynecology 1979;134:518‐22. [DOI] [PubMed] [Google Scholar]
Nilsen 1976 {published data only}
- Nilsen PA, Meling AB, Abildgaard U. Study of the suppression of lactation and the influence on blood clotting with bromocriptine (CB 154) (Parlodel): a double blind comparison with diethylstilboestrol. Acta Obstetricia et Gynecologica Scandinavica 1976;55:39‐44. [DOI] [PubMed] [Google Scholar]
O'Donoghue 1977 {published data only}
- O'Donoghue RF. The suppression of puerperal lactation with bromocriptine. Irish Medical Journal 1977;70(5):172‐3. [PubMed] [Google Scholar]
Paggi 1975 {published data only}
- Paggi G, Coggiola F, Fiorentino F, Andreoli C. A new method for the inhibition of lactogenesis [Un nuovo metodo per l'inibizione della lattogenesi]. Minerva Ginecologica 1975;27(11):823‐9. [PubMed] [Google Scholar]
Phillips 1975 {published data only}
- Phillips WP. Prevention of postpartum breast engorgement: double‐blind comparison of chlorotrianisene 72mg and placebo. Journal of the Arkansas Medical Society 1975;72:163‐7. [PubMed] [Google Scholar]
Piya‐Anant 2004 {published data only}
- Piya‐Anant M, Worapitaksanond S, Sittichai K, Saechua P, Nomrak A. The combined oral contraceptive pill versus bromocriptine to suppress lactation in puerperium: a randomized double blind study. Journal of the Medical Association of Thailand 2004;87:670‐3. [PubMed] [Google Scholar]
Purkayastha 1991 {published data only}
- Purkayastha S, De KS. A single blind randomized trial of bromocryptine in inhibiting puerperal lactation. Journal of Obstetrics and Gynaecology of India 1991;41(6):771‐3. [Google Scholar]
Rolland 1973 {published data only}
- Rolland R, Schellekens LA. A new approach to the inhibition of puerperal lactation. Journal of Obstetrics and Gynaecology of the British Commonwealth 1973;80:945‐51. [DOI] [PubMed] [Google Scholar]
Scapin 1982 {published data only}
- Scapin F, Buonaccorsi S, Tronconi G, Pellicciotta G, Pontiroli AE. Metergoline versus bromocriptine in the prevention of puerperal lactation. A double‐blind clinical trial. European Journal of Clinical Pharmacology 1982;22:181‐3. [DOI] [PubMed] [Google Scholar]
Schwartz 1973 {published data only}
- Schwartz DJ, Evans PC, Garcia C, Rickels K, Fisher E. A clinical study of lactation suppression. Obstetrics & Gynecology 1973;42:599‐606. [DOI] [PubMed] [Google Scholar]
Senior 1969 {published data only}
- Senior RE. Stilboestrol inhibition of lactation. BMJ 1969;1(638):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaaban 1975 {published data only}
- Shaaban MM. Suppression of lactation by an antiestrogen, tamoxifen. European Journal of Obstetrics & Gynecology and Reproductive Biology 1975;4:167‐9. [DOI] [PubMed] [Google Scholar]
Shrivastav 1988 {published data only}
- Shrivastav P, George K, Balasubramaniam N, Jasper P, Thomas M, Kanagasabhapathy AS. Suppression of puerperal lactation using jasmine flowers (jasminum sambac). Australian and New Zealand Journal of Obstetrics and Gynaecology 1988;28:68‐71. [DOI] [PubMed] [Google Scholar]
Steenstrup 1977 {published data only}
- Steenstrup EK, Steenstrup OR. Prevention of puerperal lactation with bromocriptine (CB 154): a double‐blind comparison with diethylstilbestrol. Current Therapeutic Research, Clinical and Experimental 1977;21:327‐32. [Google Scholar]
Stirrat 1968 {published data only}
- Stirrat GM, Anderson GE, Grant O. The effectiveness of stilbestrol in the suppression of postpartum lactation. Journal of Obstetrics and Gynaecology of the British Commonwealth 1968;75:313‐5. [DOI] [PubMed] [Google Scholar]
Swift 2002 {published data only}
- Swift K, Janke J. Breast binding... is it all that it's wrapped up to be?. JOGNN ‐ Journal of Obstetric, Gynecologic, & Neonatal Nursing 2003;32(3):332‐9. [DOI] [PubMed] [Google Scholar]
Thorbert 1983 {published data only}
- Thorbert G, Akerlund M. Inhibition of lactation by cyclofenil and bromocriptine. British Journal of Obstetrics and Gynaecology 1983;90:739‐42. [DOI] [PubMed] [Google Scholar]
Tulandi 1985 {published data only}
- Tulandi T, Gelfand MM, Maiolo LM. Effect of prostaglandin E2 on puerperal breast discomfort and prolactin secretion. Journal of Reproductive Medicine 1985;30:176‐8. [PubMed] [Google Scholar]
Utian 1975 {published data only}
- Utian WH, Begg G, Vinik AI, Paul M, Schuman L. Effect of bromocriptine and chlorotrianisene on inhibition of lactation and serum prolactin. A comparative double‐blind study. British Journal of Obstetrics and Gynaecology 1975;82:755‐9. [DOI] [PubMed] [Google Scholar]
Van der Heijden 1991 {published data only}
- Heijden PFM, Kremer JAM, Brownell J, Rolland R. Lactation inhibition by the dopamine agonist CV 205‐502. British Journal of Obstetrics and Gynaecology 1991;98:270‐6. [DOI] [PubMed] [Google Scholar]
Varga 1972 {published data only}
- Varga L, Lutterbeck PM, Pryor JS, Wenner R, Erb H. Suppression of puerperal lactation with an ergot alkaloid: a double‐blind study. British Medical Journal 1972;2:743‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Venturini 1981 {published data only}
- Venturini PL, Horowski R, Maganza C, Morano S, Pedretti E, Ragni N, et al. Effects of lisuride and bromocriptine on inhibition of lactation and on serum prolactin levels: comparative double‐blind study. European Journal of Obstetrics & Gynecology and Reproductive Biology 1981;11:395‐400. [DOI] [PubMed] [Google Scholar]
Venturini 1988 {published data only}
- Venturini PL, Horowski R, Fasce V, Valenzano M, Ferreri C, Badino G, et al. Suppression of puerperal lactation by terguride: a double‐blind study. Gynecologic and Obstetric Investigation 1988;26:33‐8. [DOI] [PubMed] [Google Scholar]
Vischi 1975 {published data only}
- Vischi F, Mandruzzato GP, Dell'Acqua S, Bruni G. Further evaluation of quinestrol in the inhibition of lactation: a double‐blind comparison of two dose levels against placebo. Archives Internationales de Pharmacodynamie et de Therapie 1975;214:62‐7. [PubMed] [Google Scholar]
Walker 1975 {published data only}
- Walker S. A comparison of 2 bromo alpha ergocryptine, quinoestrol and placebo in suppression of puerperal lactation. British Journal of Clinical Pharmacology 1975;2(4):368P‐369P. [DOI] [PubMed] [Google Scholar]
- Walker S, Hibbard BM, Groom G, Griffiths K, Davis RH. Controlled trial of bromocriptine, quinoestrol and placebo in suppression of puerperal lactation. Lancet 1975;2:842‐5. [DOI] [PubMed] [Google Scholar]
Watson 1969 {published data only}
- Watson PS. Instant inhibition of lactation. Practitioner 1969;203:184‐6. [PubMed] [Google Scholar]
Weinstein 1976 {published data only}
- Weinstein D, Ben‐David M, Polishuk WZ. Serum prolactin and the suppression of lactation. British Journal of Obstetrics and Gynaecology 1976;83:679‐82. [DOI] [PubMed] [Google Scholar]
Winter 1964 {published data only}
- Winter RW, Robinson SC. Prevention of lactation. Obstetrics & Gynecology 1964;23:906‐9. [PubMed] [Google Scholar]
Yuen 1977 {published data only}
- Yuen BH, Pendleton HJ, Blair S. Efficacy of bromocriptine and chlorotrianisene in preventing postpartum lactation. Canadian Medical Association Journal 1877;177:919‐21. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Almeida 1986 {published data only}
- Almeida OD, Kitay DZ. Lactation suppression and puerperal fever. American Journal of Obstetrics and Gynecology 1986;154:940‐1. [DOI] [PubMed] [Google Scholar]
Bare 1960 {published data only}
- Bare WW, Lippo FL. Suppression of lactation with fluoxymesterone. American Journal of Obstetrics and Gynecology 1960;80:138‐44. [DOI] [PubMed] [Google Scholar]
Barns 1961 {published data only}
- Barns DH. Suppression of postpartum lactation and prevention of breast engorgement in nonnursing mothers. American Journal of Obstetrics and Gynecology 1961;81:339‐43. [DOI] [PubMed] [Google Scholar]
Booker 1970 {published data only}
- Booker DE, Pahl IR, Forbes DA. Control of postpartum breast engorgement with oral contraceptives. II. American Journal of Obstetrics and Gynecology 1970;108:240‐2. [DOI] [PubMed] [Google Scholar]
Brett 1971 {published data only}
- Brett I, Douglas CP, Walker EH. Inhibition of lactation‐‐a triple study. British Journal of Clinical Practice 1971;25:379‐80. [PubMed] [Google Scholar]
Bristol 1966 {published data only}
- Bristol WM. Comparative effectiveness of compressional and supporting breast binders in suppressing lactation. Nursing Research 1966;15(3):203‐6. [PubMed] [Google Scholar]
Brooten 1983 {published data only}
- Brooten DA, Brown LP, Hollingsworth AO, Tanis JL, Donlen J. A comparison of four treatments to prevent and control breast pain and engorgement in nonnursing mothers. Nursing Research 1983;32:225‐9. [PubMed] [Google Scholar]
Caballero 1987 {published data only}
- Caballero A, Mena P, Caballero‐Diaz JL, Caballero‐Asensi A. Metergoline as an inhibitor of prolactin release. Journal of Reproductive Medicine 1987;32:115‐9. [PubMed] [Google Scholar]
Canales 1977 {published data only}
- Canales ES, Lasso P, Soria J, Zarate A. Effect of clomiphene on prolactin secretion and lactation in puerperal women. British Journal of Obstetrics and Gynaecology 1977;84:758‐9. [DOI] [PubMed] [Google Scholar]
Cantis 1977 {published data only}
- Cantis MS, Guitelman A, Razumny J, Baudini R, Rubel H. Piribedil and its use in the suppression of lactation. Pharmatherapeutica 1977;1:610‐20. [Google Scholar]
Cicinelli 1996 {published data only}
- Cicinelli E, Petruzzi D, Ragno G, Schonauer LM, Ruccia C, Matteo G. Nasal spray bromocriptine: effects on serum prolactin in puerperal women. Acta Obstetricia et Gynecologica Scandinavica 1996;75:730‐3. [DOI] [PubMed] [Google Scholar]
David 1977 {published data only}
- David A, Romem I, Lunenfeld B, Kaufman M, Serr DM. Stilbestrol administration in the puerperium and its effect on the prolactin excretion of non‐lactating patients. Acta Obstetricia et Gynecologica Scandinavica 1977;56:211‐5. [DOI] [PubMed] [Google Scholar]
De Aloysio 1988 {published data only}
- Aloysio D, Pamparana F, Zanotti A, Fabiani AG, Bottiglioni F. Dihydroergocristine in stopping lactation: double‐blind study vs bromocriptine. Gynecological Endocrinology 1988;2:67‐71. [DOI] [PubMed] [Google Scholar]
De Cecco 1979 {published data only}
- Cecco L, Venturini PL, Ragni N, Rossato P, Maganza C, Gaggero G, Horowski R. Effect of lisuride on inhibition of lactation and serum prolactin. British Journal of Obstetrics and Gynaecology 1979;86:905‐8. [DOI] [PubMed] [Google Scholar]
Del Pozo 1975 {published data only}
- Pozo E, Brun Del Re R, Hinselman M. Lack of effect of methyl‐ergonovine on postpartum lactation. American Journal of Obstetrics and Gynecology 1975;123:845‐6. [DOI] [PubMed] [Google Scholar]
Duthie 1990 {published data only}
- Duthie SJ, Fuk‐Him DL, Fang AHS, O'Hoy KM, Pak‐Chung H. Comparison of four different dosages of injectable bromocriptine retard for puerperal ablactation. European Journal of Obstetrics & Gynecology and Reproductive Biology 1990;34:223‐8. [DOI] [PubMed] [Google Scholar]
- Duthie SJ, Li DFH, Fang AHS, O'Hoy KMKY, Ho PC. Comparison of four different dosages of injectable bromocriptine retard for puerperal ablactation. Proceedings of Silver Jubilee British Congress of Obstetrics and Gynaecology; 1989 July 4‐7; London, UK. 1989:183.
Fleming 1977 {published data only}
- Fleming JS. Inhibition of puerperal lactation: pyridoxine of no benefit. Australian and New Zealand Journal of Obstetrics and Gynaecology 1977;17:131. [Google Scholar]
Foukas 1972 {published data only}
- Foukas M. An antilactogenic effect of pyridoxine. Journal of Obstetrics and Gynaecology of the British Commonwealth 1973;80:718‐20. [DOI] [PubMed] [Google Scholar]
- Foukas M. Lactation inhibiting action of pyridoxine [Laktationshemmende Wirkung des Pyridoxins]. Deutsche Medizinische Wochenschrift 1972;97(10):396‐7. [PubMed] [Google Scholar]
- Foukas M. The lactation‐inhibiting effect of pyridoxine [Uber die laktationshemmende Wirkung des Pyridoxins]. Zentralblatt fur Gynakologie 1973;95(40):1433‐6. [PubMed] [Google Scholar]
Garry 1956 {published data only}
- Garry J. Estrogen‐androgen preparation for prevention of postpartum breast engorgement and lactation. Obstetrics & Gynecology 1956;7:422‐4. [PubMed] [Google Scholar]
Gerstner 1978 {published data only}
- Gerstner G, Mick R, Reinold E. Inhibition of postpartal lactation with 2‐bromo‐alpha‐ergocryptine [Postpartale Laktationshemmung mit 2‐Brom‐alpha‐Ergocryptin]. Wiener Medizinische Wochenschrift 1978;128:704‐6. [PubMed] [Google Scholar]
Gillibrand 1968 {published data only}
- Gillibrand PN, Huntingford PJ. Inhibition of lactation with combined oestrogen and progestogen. British Medical Journal 1968;4:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gopalan 1997 {published data only}
- Gopalan S, Geeta, Jain V, Dash RJ. Effectiveness of pyridoxine in unwanted lactation. Acta Obstetricia et Gynecologica Scandinavica 1997;76(167):78. [Google Scholar]
Grant 1978 {published data only}
- Grant K, Rabello Y, Freeman AG, Freeman RK, Baumgardner AS. Comparison of quinestrol and TACE for relief of postpartum breast discomfort. Obstetrics & Gynecology 1978;51:636‐9. [DOI] [PubMed] [Google Scholar]
Hale 2004 {published data only}
- Hale T, Ilett K, Hartmann P, Aljazaf K, Mitoulas L, Kristensen J, et al. Pseudoephedrine effects on milk production in women and estimation of infant exposure via human milk. Advances in Experimental Medicine and Biology 2004;554:437‐8. [DOI] [PubMed] [Google Scholar]
Kaiser 1952 {published data only}
- Kaiser R, Regensburger E. Experiences with combined ovarian hormones to prevent lactation [Erfahrungen mit kombinierten Ovarialhormonen zur Laktationsverhinderung]. Munchener Medizinische Wochenschrift 1952;94(40):2029‐32. [PubMed] [Google Scholar]
Kalir 1975 {published data only}
- Kalir R, David MP, Kraicer PF. Clomiphene citrate in suppression of puerperal lactation. American Journal of Obstetrics and Gynecology 1975;122:570‐2. [DOI] [PubMed] [Google Scholar]
Kee 1989 {published data only}
- Kee WH, Tan SL, Lee V, Salmon YM. The treatment of breast engorgement with serrapeptase (Danzen): a randomized double‐blind controlled trial. Singapore Medical Journal 1989;30:48‐54. [PubMed] [Google Scholar]
- Tan SL, Kee WH, Lee V, Salmon YM. The use of serratiopeptidase (Danzen) for the treatment of breast engorgement ‐ a randomised double‐blind controlled trial. Proceedings of the 24th British Congress of Obstetrics and Gynaecology;1986 April 15‐18; Cardiff, UK. 1986:246.
King 1958 {published data only}
- King AG. Prevention of puerperal breast engorgement with large doses of long‐acting estrogen. American Journal of Obstetrics and Gynecology 1958;78:80‐5. [DOI] [PubMed] [Google Scholar]
Kirkland 1960 {published data only}
- Kirkland JA, Greenberg BG, Flowers CE. Suppression of lactation: a double blind hormone study. Obstetrics & Gynecology 1960;15:292‐8. [PubMed] [Google Scholar]
Koshiishi 1971 {published data only}
- Koshiishi T, Furusawa Y, Iseki H, Iwasaki Y. A double blind study of the effects of Kimotab on engorgement of the breast. Acta Obstetrica et Gynaecologica Japonica 1971;18:222‐8. [PubMed] [Google Scholar]
Lee 1971 {published data only}
- Lee KH. A trial of chlormezanone, a non‐hormonal tranquillizer for inhibition of postpartum lactation. Australian and New Zealand Journal of Obstetrics and Gynaecology 1971;11:99‐102. [DOI] [PubMed] [Google Scholar]
Lee 1979 {published data only}
- Lee JY, Chang YS, Noh HI. Effect of ginseng saponin on puerperal lactation. 9th World Congress of Gynecology and Obstetrics; 1979 October 26‐31; Tokyo, Japan. 1979:304.
Llewellyn‐Jones 1963 {published data only}
- Llewellyn‐Jones D. Inhibition of lactation using hormones. Medical Journal of Malaysia 1963;18:13‐5. [PubMed] [Google Scholar]
Lo Dico 1980 {published data only}
- Lo Dico G, Milia S, Firinu C, Pes F, Masala A, Alagna S, et al. Use of dopaminergic drugs (pyridoxine, 2‐bromo‐alpha‐ergocriptine, piribedil) in blocking lactation [Impiego di farmaci dopaminergici (piridossina, 2‐bromo‐alfa‐ergocriptina, piribedil) nel blocco della lattazione]. Minerva Ginecologica 1980;32(9):771‐9. [PubMed] [Google Scholar]
Louviere 1975 {published data only}
- Louviere RL, Upton RT. Evaluation of Deladumone OB in the suppression of postpartum lactation. American Journal of Obstetrics and Gynecology 1975;121:641‐2. [DOI] [PubMed] [Google Scholar]
MacDonald 1965 {published data only}
- MacDonald D, O'Driscoll K. Suppression of lactation. A double‐blind trial. Lancet 1965;2:623‐5. [DOI] [PubMed] [Google Scholar]
MacLeod 1977 {published data only}
- MacLeod SC, Scott J, Lord L, Brodie G, Perlin I, Simpson AA. Prevention and suppression of post‐partum lactation with 2‐bromo‐alpha‐ergocryptine (CB‐154). Clinical Endocrinology 1977;6:65S‐70S. [DOI] [PubMed] [Google Scholar]
Markin 1960 {published data only}
- Markin KE, Wolst MD. A comparative controlled study of hormones used in the prevention of postpartum breast engorgement and lactation. American Journal of Obstetrics and Gynecology 1960;80:128‐37. [DOI] [PubMed] [Google Scholar]
Masala 1978 {published data only}
- Masala A, Delitala G, Lo Dico G, Stoppelli I, Alagna S, Devilla L. Inhibition of lactation and inhibition of prolactin release after mechanical breast stimulation in puerperal women given tamoxifen or placebo. British Journal of Obstetrics and Gynaecology 1978;85:134‐7. [DOI] [PubMed] [Google Scholar]
McLachlan 1991 {published data only}
- McLachlan Z, Milne EJ, Lumley J, Walker BL. Ultrasound treatment for breast engorgement: a randomised double blind trial. Australian Journal of Physiotherapy 1991;37:23‐9. [DOI] [PubMed] [Google Scholar]
Momberg 1976 {published data only}
- Momberg M, Schneider L. Hormonal limitation of lactation [Hormonale Hemmung der Laktation]. Zeitschrift fur Geburtshilfe und Perinatologie 1976;180:84‐7. [PubMed] [Google Scholar]
Morris 1967 {published data only}
- Morris JA. Lactation inhibition with quinestrol. International Journal of Fertility 1967;12:261‐5. [PubMed] [Google Scholar]
Morris 1970 {published data only}
- Morris JA, Creasy RK, Hohe PT. Inhibition of puerperal lactation. Double‐blind comparison of chlorotrianisene, testosterone enanthate with estradiol valerate and placebo. Obstetrics & Gynecology 1970;36:107‐14. [PubMed] [Google Scholar]
Nappi 1987 {published data only}
- Nappi C, Colace G, Renzo GF, Taglialatela M, Amoroso S, Annunziato L, et al. Domperidone antagonizes bromoergocriptine‐induced nausea and vomiting without affecting its inhibition of prolactin secretion in puerperal women. European Journal of Clinical Pharmacology 1987;32:457‐60. [DOI] [PubMed] [Google Scholar]
Nappi 1990 {published data only}
- Nappi C, Colace G, Affinito P, Taglialatela M, Renzo GF, Montemagno U, et al. Ibopamine‐induced reduction of serum prolactin level and milk secretion in puerperal women. European Journal of Clinical Pharmacology 1990;39:133‐5. [DOI] [PubMed] [Google Scholar]
Nappi 1993 {published data only}
- Nappi C, Colace G, Carlo C, Affinito P, Ruotolo C, Montemagno R, et al. Effect of dihydroergocryptine on serum prolactin levels and milk secretion in puerperal women. Gynecological Endocrinology 1993;7(2):129‐33. [DOI] [PubMed] [Google Scholar]
Ng 1972 {published data only}
- Ng KH, Lee KH. Inhibition of postpartum lactation with single‐dose drugs. Australian and New Zealand Journal of Obstetrics and Gynaecology 1972;12:59‐61. [DOI] [PubMed] [Google Scholar]
Nisha 2006 {published data only}
- Nisha, Singh U, Sachan V, Sankhwar P. Role of newer drug cabergoline in suppression and inhibition of lactation. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:48.
Osbourne 1978 {published data only}
- Osbourne GK, Whigham KAE, Howie PW, England P, Kelly A, Prentice CRM. The effects of quinestrol and bromocriptine on blood coagulation, serum prolactin and serum FSH levels in puerperal women. British Journal of Obstetrics and Gynaecology 1978;85:687‐91. [DOI] [PubMed] [Google Scholar]
Polatti 1982 {published data only}
- Polatti F, Nava C, Zara C. Cyclofenil inhibits the secretion and release of prolactin in the puerperium [Il ciclofenile inibisce la secrezione e il "release" della PRL in puerperio]. Minerva Ginecologica 1982;34(1‐2):7‐11. [PubMed] [Google Scholar]
Poulsen 1976 {published data only}
- Poulsen EF, Nygaard B, Larsen S, Lenstrup C. Bromergocriptine for suppression of puerperal lactation. Ugeskrift for Laeger 1976;138(32):1927‐9. [PubMed] [Google Scholar]
Primrose 1957 {published data only}
- Primrose T, Tremblay P. Studies on the suppression of lactation by hormones. American Journal of Obstetrics and Gynecology 1957;73:1218‐24. [DOI] [PubMed] [Google Scholar]
Reisfield 1966 {published data only}
- Reisfield DR, Paret FL. Value of a diuretic in suppressing breast engorgement. Journal of the Medical Society of New Jersey 1966;63:458‐61. [PubMed] [Google Scholar]
Robuschi 1987 {published data only}
- Robuschi G, Montermini M, Chiodera P, Gardini E, Salvi M, Alboni A, et al. Dopaminergic regulation of fetal growth hormone (GH) secretion: study with maternal administration of bromocriptine. Journal of Perinatal Medicine 1987;15:345‐9. [DOI] [PubMed] [Google Scholar]
Rolland 1978 {published data only}
- Rolland R, Schellekens LA. Inhibition of puerperal lactation by bromocriptine. Acta Endocrinologica. Supplementum 1978;88:119‐30. [PubMed] [Google Scholar]
Roser 1966 {published data only}
- Roser DM. Breast engorgement and postpartum fever. Obstetrics & Gynecology 1966;27:73‐7. [PubMed] [Google Scholar]
Ryan 1962 {published data only}
- Ryan GM Jr, Brown DAJ. Intranasal syntocinon and postpartum breast engorgement. Obstetrics & Gynecology 1962;20:582‐4. [PubMed] [Google Scholar]
Schneider 1964 {published data only}
- Schneider J, MacArthur JL, Patrick JW, Burton GV. The suppression of lactation: an objective study. Obstetrics & Gynecology 1964;24:294‐7. [PubMed] [Google Scholar]
Seppala 1975 {published data only}
- Seppala M, Ylinen O, Sternthal V, Soiva K, Vara P. Suppression of established puerperal lactation with 2‐Br‐alpha‐ergocryptine methane sulphonate (CB 154). International Journal of Gynecology & Obstetrics 1975;13:1‐5. [Google Scholar]
Shapiro 1984 {published data only}
- Shapiro AG, Thomas L. Efficacy of bromocriptine vs breast binders as inhibitors of postpartum lactation. Southern Medical Journal 1984;77:719‐21. [DOI] [PubMed] [Google Scholar]
Steele 1968 {published data only}
- Steele SJ. Inhibition of lactation by oestrogens. British Medical Journal 1968;4:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stenchever 1962 {published data only}
- Stenchever MA. Evaluation of chlorprophenpyridamine for prevention of postpartum breast engorgement. American Journal of Obstetrics and Gynecology 1962;840:969‐71. [PubMed] [Google Scholar]
Tyson 1966 {published data only}
- Tyson JEA. A high‐dosage estrogen for lactation suppression. Obstetrics & Gynecology 1966;27:729‐32. [PubMed] [Google Scholar]
Van Dam 1981 {published data only}
- Dam LJ, Rolland R. Lactation‐inhibiting and prolactin‐lowering effect of lisuride and bromocriptine: a comparative study. European Journal of Obstetrics & Gynecology and Reproductive Biology 1981;12:323‐30. [DOI] [PubMed] [Google Scholar]
Varga 1972b {published data only}
- Varga L, Luttierbeck PM, Pryor JS, Wenner R, Erb H. Inhibition of puerperal lactation with ergot alkaloids [Unterdruckung der puerperalen Laktation mit einem Mutterkornalkaloid.]. Schweizerische Medizinische Wochenschrift 1972;102:1284‐5. [PubMed] [Google Scholar]
Walker 1980 {published data only}
- Walker SM. Inhibition of puerperal lactation. Scottish Medical Journal 1980;25:S95‐S99. [Google Scholar]
Willmott 1977 {published data only}
- Willmott MP, Colhoun EM, Bolton AE. The suppression of puerperal lactation with bromocryptine. Acta Obstetricia et Gynecologica Scandinavica 1977;56:145‐9. [DOI] [PubMed] [Google Scholar]
Zuckerman 1973 {published data only}
- Zuckerman H, Carmel S. The inhibition of lactation by clomiphene. Journal of Obstetrics and Gynaecology of the British Commonwealth 1973;80:822‐3. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Caballero 1996 {published data only}
- Caballero GA, Caballero DJL. Cabergoline: A new dopaminergic at a dingle dose for lactation inhibition. Acta Ginecologica. 1996;53:172‐9. [Google Scholar]
Varga 1974 {published data only}
- Varga L, Radielovic P. Postpartal lactation inhibition by 2‐Br‐alpha‐ergocryptine: I. A comparative study with 2‐Br‐alpha‐ergocryptine in different dosages and placebo [Postpartale Laktationshemmung durch 2‐Br‐Alpha‐Ergokryptin: I. Eine Vergleichsuntersuchung mit 2‐Br‐Alpha‐Ergokryptin in unterschiedlicher Dosierung und Plazebo]. Geburtshilfe und Frauenheilkunde 1974;34:882‐4. [PubMed] [Google Scholar]
Additional references
Akrivis 2000
- Akrivis C, Vezyraki P, Kiortsis DN, Fotopoulos A, Evangelou A. Inhibition of puerperal lactation with 2‐mercaptopropinyl‐glycerine. European Journal of Clinical Pharmacology 2000;56(9‐10):621‐3. [DOI] [PubMed] [Google Scholar]
Duchesne 1981
- Duchesne C, Leke R. Bromocriptine mesylate for prevention of postpartum lactation. Obstetrics & Gynecology 1981;57(4):464‐7. [PubMed] [Google Scholar]
Eastham 1976
- Eastham E, Smith D, Poole D, Neligan G. Further decline of breast‐feeding. British Medical Journal 1976;1(6005):305‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamlyn 2002
- Hamlyn B, Brooker S, Oleinikova K, Wands S. Infant Feeding. London: The Stationery Office, 2002. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Iffy 1996
- Iffy L, Lindenthal J, Mcardle JJ, Ganesh V. Severe cerebral accidents postpartum in patients taking bromocriptine for milk suppression. Israel Journal of Medical Sciences 1996;32(5):309‐12. [PubMed] [Google Scholar]
Jeffcoate 1968
- Jeffcoate TN, Miller J, Roos RF, Tindall VR. Puerperal thromboembolism in relation to the inhibition of lactation by oestrogen therapy. British Medical Journal 1968;3(622):19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Llewellyn‐Jones 1968
- Llewellyn‐Jones D. Inhibition of lactation by oestrogens. British Medical Journal 1968;4(627):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nduati 2000
- Nduati R, John G, Mbori‐Ngacha D, Richardson B, Overbaugh J, Mwatha A, et al. Effects of breastfeeding and formula feeding on transmission of HIV‐1: a randomised clinical trial. JAMA 2000;283:1167‐74. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ruch 1989
- Ruch A, Duhring JL. Postpartum myocardial infarction in a patient receiving bromocriptine. Obstetrics & Gynecology 1989;74(3 Pt 2):448‐51. [PubMed] [Google Scholar]
Ryan 2002
- Ryan AS, Wenjun Z, Acosta A. Breastfeeding continues to increase into the new millennium. Pediatrics 2002;110:1103‐9. [DOI] [PubMed] [Google Scholar]
Shaffer 1999
- Shaffer N, Chuachoowong R, Mock PA, Bhadrakom C, Siriwasin W, Young NL, et al. Short‐course zidovudine for perinatal HIV‐1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet 1999;353(9155):773‐80. [DOI] [PubMed] [Google Scholar]
Spitz 1998
- Spitz AM, Lee NC, Peterson HB. Treatment for lactation suppression: little progress in one hundred years. American Journal of Obstetrics and Gynecology 1998;179(6 Pt 1):1485‐90. [DOI] [PubMed] [Google Scholar]
US FDA 1989
- US Food, Drug Administration. Fertility and Maternal Health Drugs Advisory Committee. Summary minutes. Prevention of postpartum breast engorgement with sex hormones and bromocriptine. Washington: US Food and Drug Administration, 1989. [Google Scholar]
Wiktor 1999
- Wiktor SZ, Ekpini E, Karon JM, Nkengasong J, Maurice C, Severin ST, et al. Short‐course oral zidovudine for prevention of mother‐to‐child transmission of HIV‐1 in Abidjan, Cote d'Ivoire: a randomised trial. Lancet 1999;353(9155):781‐5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Oladapo 2009
- Oladapo OT, Fawole B. Treatments for suppression of lactation. Treatments for suppression of lactation. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005937.pub2] [DOI] [PubMed] [Google Scholar]


