Abstract
Background
Sudden cardiac death is a significant cause of mortality in both the US and globally. However, 5% to 15% of people with sudden cardiac death have no structural abnormalities, and most of these events are attributed to underlying cardiac ion channelopathies. Rates of cardiac ion channelopathy diagnosis are increasing. However, the optimal treatment for such people is poorly understood and current guidelines rely primarily on expert opinion.
Objectives
To compare the effect of implantable cardioverter defibrillators (ICD) with antiarrhythmic drugs or usual care in reducing the risk of all‐cause mortality, fatal and non‐fatal cardiovascular events, and adverse events in people with cardiac ion channelopathies.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 6), EMBASE, MEDLINE, Conference Proceedings Citation Index ‐ Science (CPCI‐S), ClinicalTrials.gov, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) in July 2015. We applied no language restrictions.
Selection criteria
We included all randomized controlled trials of people aged 18 years and older with ion channelopathies, including congenital long QT syndrome, congenital short QT syndrome, Brugada syndrome, or catecholaminergic polymorphic ventricular tachycardia. Participants must have been randomized to ICD implantation and compared to antiarrhythmic drug therapy or usual care.
Data collection and analysis
Two authors independently selected studies for inclusion and extracted the data. We included all‐cause mortality, fatal and non‐fatal cardiovascular events, and adverse events for our primary outcome analyses and non‐fatal cardiovascular events, rates of inappropriate ICD firing, quality of life, and cost for our secondary outcome analyses. We calculated risk ratios (RR) and associated 95% confidence intervals (CIs) for dichotomous outcomes, both for independent and pooled study analyses.
Main results
From the 468 references identified after removing duplicates, we found two trials comprising 86 participants that met our inclusion criteria. Both trials included participants with Brugada syndrome who were randomized to ICD versus β‐blocker therapy for secondary prevention for sudden cardiac death. Both studies were small, were performed by the same investigators, and exhibited a high risk of bias across multiple domains. In the group randomized to ICD therapy, there was a nine‐fold lower risk of mortality compared with people randomized to medical therapy (0% with ICD versus 18% with medical therapy; RR 0.11, 95% CI 0.01 to 0.83; 2 trials, 86 participants). There was low quality evidence of a difference in the rates of combined fatal and non‐fatal cardiovascular events, and the results were imprecise (26% with ICD versus 18% with medical therapy; RR 1.49, 95% CI 0.66 to 3.34; 2 trials, 86 participants). The rates of adverse events were higher in the ICD group, but these results were imprecise (28% with ICD versus 10% with medical therapy; RR 2.44, 95% CI 0.92 to 6.44; 2 trials, 86 participants). For secondary outcomes, the risk of non‐fatal cardiovascular events was higher in the ICD group, but these results were imprecise and were driven entirely by appropriate ICD‐termination of cardiac arrhythmias (26% with ICD versus 0% with medical therapy; RR 11.4, 95% CI 1.57 to 83.3; 2 trials, 86 participants). Approximately 25% of the ICD group experienced inappropriate ICD firing, all of which was corrected by device reprogramming. No data were available for quality of life or cost. We considered the quality of evidence low using the GRADE methodology, due to study limitations and imprecision of effects.
Authors' conclusions
Among people with Brugada syndrome who have survived a prior episode of sudden cardiac death, ICD therapy appeared to reduce mortality when compared to β‐blocker therapy, but the true magnitude may be substantially different from the estimate of the effect because of study limitations and imprecision. Due to the large magnitude of effect, it is unlikely that there will be additional studies evaluating the role of ICDs for secondary prevention in this population. Further studies are necessary to determine the optimal treatment, if any, to prevent an initial episode of sudden cardiac death in people with cardiac ion channelopathies.
Plain language summary
Implantable cardioverter defibrillators versus medical therapy for people with inherited heart rhythm abnormalities
Review question
What is the evidence about potential benefits and harms of implantable cardioverter defibrillators compared to medical treatment for the prevention of sudden cardiac death in people with inherited heart rhythm abnormalities?
Background
Sudden (unexpected) cardiac death is the leading mechanism of death worldwide. An unknown proportion of these deaths is caused by heart beat abnormalities that are passed on within families (inherited) that can lead to sudden cardiac death in otherwise healthy people. Four inherited diseases that affect the heart's electrical system, called congenital long QT syndrome, congenital short QT syndrome, Brugada syndrome, and catecholaminergic polymorphic ventricular tachycardia, account for most of all inherited cases of sudden cardiac death. These people have abnormalities in the way in which their heart's balance electrolytes (naturally occurring substances in the blood and other body fluids that carry an electric charge) in a way that make them more likely to have higher sudden cardiac death rates than the general population. Two methods are used to prevent sudden cardiac death in these people: 1. implantable cardioverter defibrillators, which are small devices that provide electrical shocks to make the heart rhythm more regular, and 2. medical therapy (treatment with medicines that influence the heart's rhythm).
The aim of this systematic review was to compare the potential benefits and harms of implantable cardioverter defibrillators with medical therapy for the prevention of sudden cardiac death in people with inherited heart rhythm abnormalities. We reviewed the evidence up to July 2015 to answer this question.
Study characteristics
We searched scientific databases and found two randomized controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) including 86 adults that met our inclusion criteria. Both trials compared implantable cardioverter defibrillators with medical therapy in people with Brugada syndrome who had already survived a sudden cardiac arrest event. We found no studies in people who had not experienced such an event or with other abnormalities. These two studies were small, were performed by the same investigators from Thailand, were funded by a device company, and had major methodological limitations, including being stopped early. Nearly all (98%) participants were men who had an average age of 44 years old.
Key results
All‐cause mortality (death from any cause) was lower in people who received the implantable cardioverter defibrillator. Rates of survived sudden cardiac arrest, heart attack, and stroke were higher, primarily due to differences in survived sudden cardiac arrest rates. Implantable cardioverter defibrillators had a higher rate of side effects compared with medical therapy, including receiving inappropriate shocks from their defibrillators, which required reprogramming of these devices.
Quality of evidence
There was low quality evidence that implantable cardioverter defibrillators lower mortality in people with inherited heart rate abnormalities who have survived a sudden cardiac arrest event because of few, small, low quality studies. These devices carry a higher risk of side effects than medical therapy. Further research is very likely to have an important impact on our confidence in the results.
Summary of findings
Summary of findings for the main comparison. Implantable cardioverter defibrillators compared to β‐blocker therapy for prevention of sudden cardiac death in people with cardiac channelopathies.
| Implantable cardioverter defibrillators compared to β‐blocker therapy for prevention of sudden cardiac death in people with cardiac channelopathies | ||||||
| Patient or population: people with cardiac channelopathies Settings: Thailand Intervention: implantable cardioverter defibrillators Comparison: β‐blocker therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with β‐blocker therapy | Risk with ICD | |||||
| All‐cause mortality | Study population | RR 0.11 (0.01 to 0.83) | 86 (2 RCTs) | ⊕⊕⊝⊝ Low 1,2 | All included study participants experienced a previously documented or clinically suspected episode of terminated sudden cardiac arrest due to Brugada syndrome | |
| 18 per 100 | 2 per 100 (1 to 15 per 100) | |||||
| Fatal and non‐fatal cardiovascular events | Study population | RR 1.49 (0.66 to 3.34) | 86 (2 RCTs) | ⊕⊕⊝⊝ Low 1,2 | ‐ | |
| 18 per 100 | 27 per 100 (12 to 61 per 100) | |||||
| Adverse events | Study population | RR 2.44 (0.92 to 6.44) | 86 (2 RCTs) | ⊕⊕⊝⊝ Low 1,2 | Adverse events in the β‐blocker group were mild medication intolerability and did not result in switching of drug therapy, while those in the ICD therapy group consisted of 11 reprogrammable inappropriate ICD shocks, 1 pocket infection, and 1 lead replacement | |
| 10 per 100 | 25 per 100 (10 to 64 per 100) | |||||
| Non‐fatal cardiovascular events | Study population | RR 11.44 (1.57 to 83.36) | 86 (2 RCTs) | ⊕⊕⊝⊝ Low 1,2 | All non‐fatal cardiovascular events in the ICD therapy group were appropriate ICD discharges; no non‐fatal cardiovascular events were documented in the β‐blocker group | |
| 0 per 100 | 26 per 100 (4 to 186 per 100) | |||||
| Inappropriate ICD firing | Study population | RR 10.17 (1.32 to 78.54) | 86 (2 RCTs) | ⊕⊕⊝⊝ Low 1,2 | All ICDs were successfully corrected by device reprogramming | |
| 0 per 100 | 23 per 100 (3 to 178 per 100) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ICD: implantable cardioverter defibrillator; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1These trials were at high risk for performance bias, small‐study bias, and early stopping bias.
2The included studies had wide confidence intervals. Because of this imprecision, we downgraded the quality of evidence.
Background
Description of the condition
Sudden cardiac death causes between 180,000 and 450,000 deaths in the US and between 50,000 and 70,000 deaths in the UK each year, making it the leading mechanism of death in these countries and around the world (Deo 2012; Go 2014; NICE 2014). Sudden cardiac death alone accounts for higher annual mortality than other common diseases such as stroke, lung cancer, and colon cancer combined (Go 2014; Siegel 2013). Though the majority of these deaths are due to ischaemic heart disease, an estimated 5% to 15% of people with sudden cardiac death have no structural abnormalities at autopsy (Bowker 1996; Corrado 2001; Eckart 2011; Puranik 2005). Further investigation into this important subset has revealed a genetic predisposition to sudden cardiac death due to altered cardiac ion channels, collectively known as cardiac ion channelopathies (Goldberger 2014; Napolitano 2012).
Cardiac ion channelopathies are a diverse class of heritable arrhythmias owing to defective ion channels in cardiac cell membranes. These can cause sudden cardiac death in otherwise healthy people (Tester 2011). Defective ion channels lead to abnormal electrical rhythms, with clinical manifestations ranging from asymptomatic carriers to life‐threatening cardiac arrhythmias and death (Martin 2012). Arrhythmias can involve ventricular tachycardia (VT) degenerating into ventricular fibrillation (VF) (Napolitano 2012). If these arrhythmias are not prevented or treated, they can lead to sudden cardiac death, which is the common mechanism of death for the aforementioned cardiac ion channelopathies (Martin 2012).
Beginning in the mid‐1990s, several important reports linked well‐established heritable syndromes with specific ion channel gene mutations (Ackerman 2004). Currently, four types of inherited cardiac ion channelopathies have been strongly linked to sudden cardiac death in people with structurally normal hearts: congenital long QT syndrome, congenital short QT syndrome, Brugada syndrome, and catecholaminergic polymorphic VT (Napolitano 2012). A total of 13 susceptible genes have been linked to congenital long QT syndrome (Abriel 2012); three genes to congenital short QT syndrome (Gollob 2011); 12 genes to Brugada syndrome (Crotti 2012); and two genes to catecholaminergic polymorphic VT (Napolitano 2014). Prevalence data have proven difficult to estimate for each syndrome, but congenital long QT syndrome and Brugada syndrome appear to be the most common, with the prevalence of each estimated to be 1 in every 2000 people (Postema 2012; Schwartz 2009). Congenital short QT syndrome is estimated to be present in 3 in every 10,000 people (Anttonen 2007), and catecholaminergic polymorphic VT is present at a rate of 1 in every 10,000 people (Napolitano 2014).
Susceptibility of people with these cardiac channelopathies to sudden cardiac death is variable due to multiple factors including incomplete penetrance, variable expressivity, and differential exposures to potential arrhythmic triggers, but the potentially fatal natural history of these diseases highlights the importance of sudden cardiac death prevention in this high‐risk population (Napolitano 2012).
Description of the intervention
Previous research has focused on preventing and treating potentially fatal arrhythmias in high‐risk people to prevent sudden cardiac death. Initial prevention strategies included medical management with antiarrhythmic drugs as prophylaxis for prevention of sudden cardiac death. Antiarrhythmic drugs can suppress ventricular arrhythmias, including asymptomatic or mildly symptomatic non‐sustained VT, which may be precursors to sudden cardiac death (Burkart 1990; Friedman 1986). These antiarrhythmic drugs are often classified based on their primary mechanism of action on the cardiac action potential. The Singh‐Vaughan Williams classification scheme is widely used to separate antiarrhythmic drugs into five main categories: class I agents primarily modulate myocardial sodium channels; class II agents inhibit sympathetic activity, primarily via β‐adrenergic blockade; class III agents primarily block myocardial potassium channels; class IV agents primarily block myocardial calcium channels; class V agents work via other or unknown mechanisms (Bonow 2011). However, many of the antiarrhythmic drugs have well‐described adverse effect profiles, which limit their widespread use (Malhotra 2011).
Two early landmark randomized controlled trials (RCTs), the 'Cardiac Arrhythmia Suppression Trials' I and II (CAST I 1989; CAST II 1992), investigated the antiarrhythmic effects of class Ic antiarrhythmic agents in participants with ischaemic heart disease. While these antiarrhythmic drugs were able to suppress arrhythmias, they were associated with increased rates of all‐cause mortality (CAST I 1989; CAST II 1992). β‐Blockers are considered first‐line therapy for many people in the medical management of ventricular tachyarrhythmias and ventricular ectopic beats (Zipes 2006). In fact, registry data have suggested β‐blockers are effective in reducing the risk of a first cardiac event in long QT syndrome, though the specific β‐blocker may be genotype dependent (Abu‐Zeitone 2014). Non‐β‐blocker antiarrhythmic drugs, such as quinidine, amiodarone, and sotalol, have been used primarily as adjunctive therapy in people at higher risk for arrhythmias; however, their benefit remains unclear (Malhotra 2011). Research into additional medical therapy for cardiac channelopathies is ongoing, as evidenced by the creation of registries such as that of quinidine therapy in people with Brugada syndrome (Viskin 2009).
A second prevention strategy utilizes implantable cardioverter defibrillator (ICDs) to detect and treat people with potentially fatal heart rhythms. Originally developed in the 1980s, ICDs are small battery‐powered devices capable of detecting heart rhythms and delivering electrical impulses to the heart capable of terminating arrhythmias with rapid overdrive pacing, cardioversion, or defibrillation. Since 1990, ICD implantation rates have rapidly increased worldwide. In the US, ICD implantation rates have increased nearly 20‐fold, from approximately 30 implants per million people to 577 implants per million people in 2006 (Camm 2010). In Europe, absolute ICD implantation rates lag behind those in the US but have increased from under 10 implants per million to 155 implants per million individuals in 2006 (Camm 2010). However, neither the incidence nor prevalence of ICD implantation for cardiac channelopathies is known.
ICD implantation carries both short‐ and long‐term risks. Procedural adverse events associated with ICD implantation include haematoma (1.1%), lead dislodgment (1.0%), and pneumothorax (0.5%); with a cumulative major complication rate of 1.5% during the post‐implant hospitalization period (Curtis 2009). Long‐term risks outside of the peri‐operative period include inappropriate shocks at rates documented at approximately 11% after three and a half years post‐implantation. Implant‐related complications also include infection, lead failure, and risks associated with generator change, though these occur at lower rates (Alter 2005). Thus, the potential benefits of ICDs need to be carefully weighed against their potential risks for prevention in people with cardiac channelopathies.
How the intervention might work
ICDs are relatively small, 40‐cm3 devices that are usually implanted subcutaneously in the upper chest. ICDs scan a person's intrinsic heart rhythm in real time to detect arrhythmias, if necessary. If pre‐programmed criteria are met for VT or VF, ICDs attempt to terminate the arrhythmia via electrical treatments including rapid overdrive pacing, cardioversion, or defibrillation. In defibrillation, the electrical impulse generated by ICDs induce global cardiac depolarization and halts specific abnormal rhythms using predetermined electrical rhythm evaluation algorithms (DiMarco 2003). ICDs are very effective at converting people's hearts out of shockable rhythms, including VT and VF, with estimated rates as high as 97% (Epstein 2013; Zipes 2006).
Why it is important to do this review
Although the physiology of many cardiac channelopathies has been elucidated since the mid‐2000s, the proper treatment modality is unclear. Clinical practice guidelines recommend genetic testing for certain subsets of people with these cardiac channelopathies and their family members. These guidelines will likely lead to increasing detection of such inheritable conditions (Ackerman 2011). However, the appropriate treatment for individuals diagnosed with these inheritable conditions remains uncertain and lags behind the diagnostic abilities for a variety of reasons. First, risk factors predisposing people with ion channelopathies to sudden cardiac death are not well described, making it difficult to match the intensity of treatment with the level of sudden cardiac death risk. However, it is well recognized that people with prior cardiac arrest or syncope are at high risk for future arrhythmias (Epstein 2013; Gehi 2006). In fact, the 2012 American College of Cardiology, American Heart Association, and Heart Rhythm Society guideline for people with congenital long QT syndrome, congenital short QT syndrome, Brugada syndrome, and catecholaminergic polymorphic VT recommends ICD consideration for people with prior episodes of sustained VT or VF and for primary prevention for some people with a very strong family history of early mortality (Epstein 2008; Epstein 2013). These guidelines, however, are based on expert opinion. Given the increased risk of sudden cardiac death and increasing number of genetic variants discovered in people with cardiac channelopathies, it is important to understand whether ICDs can reduce the risk of sudden cardiac death compared with antiarrhythmic drugs or usual care. We could not find any prior systematic reviews evaluating the effect of these interventions on people with cardiac channelopathies.
Objectives
To compare the effect of implantable cardioverter defibrillators (ICD) with antiarrhythmic drugs or usual care in reducing the risk of all‐cause mortality, fatal and non‐fatal cardiovascular events, and adverse events in people with cardiac ion channelopathies.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs of any length in which participants with diagnosed ion channelopathies were randomized to either ICD implantation versus antiarrhythmic drugs or ICD implantation versus usual care. We excluded non‐randomized studies.
Types of participants
We included adults (aged 18 years or older) who were diagnosed by study investigators with one of the following cardiac ion channelopathies: congenital long QT syndrome, congenital short QT syndrome, Brugada syndrome, or catecholaminergic polymorphic VT.
Types of interventions
We included studies where the intervention group was randomized to receive any ICD, including permanent and temporary, including single chamber, dual chamber, and biventricular pacemakers. We included trials evaluating the effect of any external, wearable, or vest cardioverter defibrillators.
We included studies where the comparator group was randomized to receive usual care or antiarrhythmic drugs from any drug class according to the Singh‐Vaughan Williams classification system: class I, class II (including β‐blockers), class III (including amiodarone), class IV, and class V (including digoxin).
Types of outcome measures
Primary outcomes
All‐cause (total) mortality.
Fatal and non‐fatal cardiovascular events, including sudden cardiac death, survived sudden cardiac arrest, myocardial infarction, and stroke.
Adverse events, including: ICD site, generator, or lead infection; inappropriate firing; ICD malfunction; lead extraction; pocket haematoma; pneumothorax; ICD removal; drug discontinuation due to adverse effects, toxicity, intolerance, or adverse reaction.
Secondary outcomes
Non‐fatal cardiovascular events, including survived sudden cardiac arrest, myocardial infarction, and stroke.
Inappropriate ICD firing.
Quality of life.
Cost.
Search methods for identification of studies
Electronic searches
We searched the Database of Abstracts of Reviews of Effectiveness (DARE), Issue 2 of 4, April 2015 (The Cochrane Library) to identify existing systematic reviews and the following sources to identify published trials:
the Cochrane Central Register of Controlled Trials (CENTRAL), Issue 6 of 12, 2015 (The Cochrane Library);
MEDLINE, 1946 to July week 2 2015 (Ovid);
MEDLINE In‐Process & Other Non‐Indexed Citations 15 July 2015 (Ovid);
EMBASE, 1974 to 16 July 2015 (embase.com);
Conference Proceedings Citation Index ‐ Science (CPCI‐S), 1990 to 16 July 2015 (Thomson Reuters Web of Science).
See Appendix 1 for detailed database search strategies. We adapted search strategies for CENTRAL, EMBASE, and other databases from the MEDLINE search strategy to conform to the differing controlled vocabularies and search syntax associated with each database. We used the sensitivity‐maximizing Cochrane RCT filter for MEDLINE and the methodological terms suggested in the Cochrane Handbook for Systematic Reviews of Interventions for EMBASE (Lefebvre 2011). We did not apply any language limits to the searches. We performed searches from the earliest date available in each database to the present and did not apply any date limits.
Searching other resources
We also searched the following clinical trial registers for ongoing and additional unpublished studies:
ClinicalTrials.gov (clinicaltrials.gov/);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/).
See Appendix 2 for detailed search statements for each trial register. In addition to the above electronic searches, we attempted to discover additional studies by searching the reference lists of included trials as well as reference lists of relevant systematic reviews, and meta‐analyses retrieved during the search process. We planned to contact authors of unpublished or ongoing studies for missing data but this was not necessary. We contacted two trials' authors to gain information regarding the trial's inclusion into our systematic review and participant phenotyping.
Data collection and analysis
Selection of studies
We identified eligible studies by the search results listed above (see Search methods for identification of studies). Two authors (DAM, MDH) reviewed the title and abstract of each paper for potential relevancy. Of the studies initially deemed relevant by either author, we retrieved the full text and evaluated it for inclusion based on our inclusion criteria. In the case of disagreement, we resolved this by consensus, and when necessary, arbitrated by a third author (JG) for final inclusion.
Data extraction and management
Two authors (DAM, MDH) independently abstracted information using standardized data abstraction forms on each study including study design, participant characteristics, intervention and control information, outcome data, cost, quality of life, adverse events, and risks of bias. We resolved disagreements by consensus. When needed, a third author (JG) arbitrated in any disagreements. We used The Cochrane Collaboration's statistical software, Review Manager 5, for our analyses (RevMan 2014).
Assessment of risk of bias in included studies
Two authors (DAM, MDH) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving a third author (JG). We assessed the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias (e.g. industry funding, small study bias).
We graded risk of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the Characteristics of included studies table. We summarized the risk of bias judgements across different studies for each of the domains listed. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the Characteristics of included studies table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Measures of treatment effect
We presented data according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We calculated the risk ratios (RRs) and associated 95% confidence intervals (CIs) for each study with dichotomous outcomes using inverse variance weighting of studies. For studies with continuous variables, we planned to calculate the mean differences (MDs) between the intervention and control groups with associated 95% CIs using inverse variance weighting of studies, but this was not necessary. We calculated and report the number needed to treat for an additional beneficial outcome (NNTB), where possible.
Dealing with missing data
We contacted investigators to verify key study characteristics and obtain missing numerical outcome data, when necessary. Where it was not possible to obtain missing numerical outcome data, and the missing data were thought to introduce serious bias, we planned to explore the potential impact of including such studies in the overall assessment of results by a sensitivity analysis. However, this was not necessary.
Assessment of heterogeneity
We evaluated the presence and degree of heterogeneity between studies by the Mantel‐Haenszel Chi2 test (presence of heterogeneity) and the I2 statistic (degree of heterogeneity), for each outcome. In the presence of no or minimal heterogeneity, we used fixed‐effect analytical models. However, if significant heterogeneity was present (I2 > 50%), we planned to search for possible explanations, including both participants and interventions and planned to use random‐effects models with cautious interpretation, but this was not necessary.
Assessment of reporting biases
We created funnel plots to evaluate for publication bias based on our primary outcomes. We sought to perform tests of asymmetry to assess for publication bias, but this was not applicable to our review given the small number of studies (Egger 1997).
Subgroup analysis and investigation of heterogeneity
If sufficient data existed, we aimed to conduct the following subgroup analyses:
age;
sex;
channelopathy subtype (congenital long QT syndrome, congenital short QT syndrome, Brugada syndrome, or catecholaminergic polymorphic VT);
implanted versus non‐implanted cardioverter defibrillator.
We planned to use the formal test for subgroup interactions in Review Manager 5 (RevMan 2014), but data were insufficient to do so.
Sensitivity analysis
We planned to perform sensitivity analyses by excluding studies with high risk of bias and studies with unclear risk of bias, but the data were limited due to the small number of included studies.
Results
Description of studies
Results of the search
We identified 546 de‐duplicated references. By reviewing titles and abstracts, we excluded 541 references, leaving five studies as potentially eligible after screening. Of the five references remaining after screening, we reviewed the full‐text articles for inclusion criteria. Of these, we excluded all five references. We found one additional reference by handsearching the reference list of the previously mentioned full‐text articles. This single report included two trials and we included it in our analysis (Figure 1).
1.
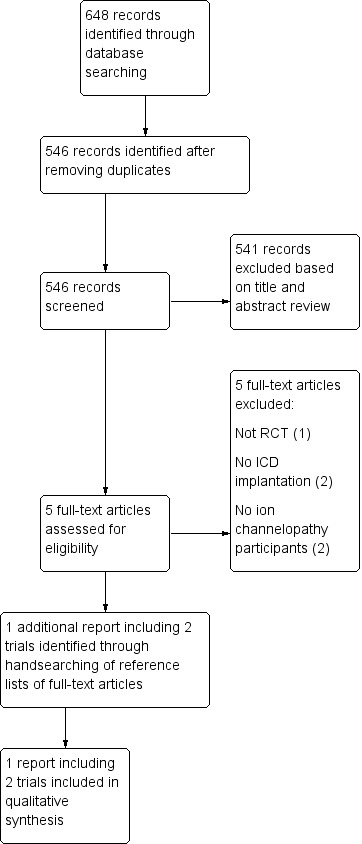
Study flow diagram.
Included studies
The single report contained two studies from the Defibrillator Versus β‐Blockers for Unexplained Death in Thailand (DEBUT) trial, both of which we included in the review (DEBUT 2003; DEBUT Pilot 2003). The DEBUT trial consisted of two phases: a pilot study followed by the main trial. Both phases were multicentre, randomized, controlled clinical trials comparing transvenous ICD to β‐blocker therapy (propranolol 40 to 160 mg daily) in a population of Thai participants classified as surviving either a sudden unexplained death syndrome (SUDS) or probable SUDS episode. Nearly all participants (98%) were men, and the mean age ranged from 40 to 48 years between the groups in each study. The pilot study was conducted from January 1995 to April 1997, and the main phase was conducted from May 1997 to December 2000. The primary endpoint of this study was all‐cause mortality. Secondary endpoints included recurrent VT or VF, or cardiac arrest. Participants were followed after the first month and at three‐month intervals for a maximum of three years' post‐randomization.
For both phases, SUDS survivors were defined as "a healthy subject without structural heart disease who had survived unexpected VF or cardiac arrest after successful resuscitation". Probable SUDS survivors were defined as "a subject without structural heart disease who experienced symptoms indicative of the clinical presentation of SUDS, especially during sleep, including agonal respiration, transient episodes of stress, abnormal respiration associated with grasping and groaning, syncope, or seizure‐like symptoms". Probable SUDS survivors were further defined as having "ECG [electrocardiogram] abnormalities showing a right bundle branch‐like pattern with ST elevation in the right precordial leads (V1 to V3) and inducible ventricular tachycardia/fibrillation in the electrophysiology laboratory before randomisation".
We sought and obtained additional information regarding the study participants' inclusion criteria via email correspondence with study authors, which confirmed participants with probable SUDS were required to have ECG abnormalities consistent with Brugada syndrome. All individual data were obtained from the published studies; no unpublished data were included in this review.
In the pilot study, the 20 eligible participants were classified either as SUDS survivors or probable SUDS survivors. Ten of these participants were randomized to receive ICD therapy, and 10 were randomized to receive β‐blocker therapy. In the main trial, 66 participants with identical inclusion criteria underwent randomization; 37 were allocated to the ICD therapy group and 29 were allocated to the β‐blocker group. The main DEBUT study was terminated early at the first interim analysis by the Data Safety and Monitoring Board due to superior benefit in the ICD group over the β‐blocker group.
Excluded studies
We excluded five full‐text references following abstract and title screening. One report was based on computer modelling and was not an RCT (Wang 2008). Two studies did not have ICD implantation as an intervention or lacked participants with documented or suspected cardiac ion channelopathies (Hohnloser 2006; VAST 2006). The Cardiac Arrest Study Hamburg (CASH) trial was a prospective RCT comparing metoprolol, amiodarone, propafenone, and ICD implantation in people surviving SCD due to VT/VF. However, CASH did not contain anyone known to have a cardiac ion channelopathy. We contacted the study authors who confirmed no further phenotyping of participants was undertaken, and no included participants were documented as having ion channelopathies. Since there were no people with confirmed or suspected ion channelopathy in the CASH study, we excluded this trial from our analysis (CASH 1993). See Characteristics of excluded studies table.
We found no ongoing studies meeting inclusion criteria for our analyses.
Risk of bias in included studies
There was a high risk of bias in two domains, unclear risk of bias in three domains, and low risk of bias in two domains (Figure 2Figure 3).
2.
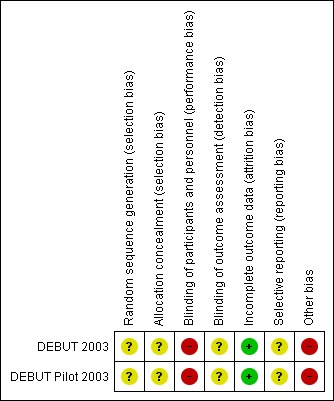
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
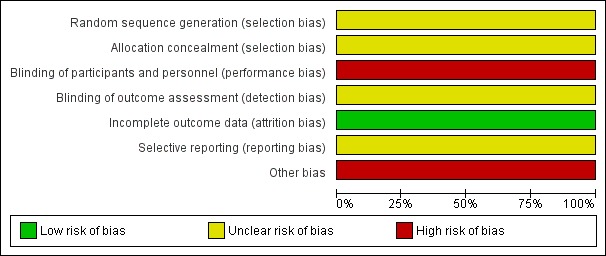
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
In both included studies, the study authors did not describe the method of sequence generation or allocation concealment, making the risk of selection bias unclear. However, the characteristics of each group were similar at baseline.
Blinding
In both studies, neither participants nor personnel were blinded to ICD placement or antiarrhythmic drug therapy. There was inherent difficulty blinding the interventions, because the intervention required a procedure and long‐term device management. Due to the lack of blinding, the included studies had a high risk of performance bias.
Because the included studies did not describe specifically how the outcomes were assessed, how the primary outcome of death was ascertained, or whether study members were blinded to the individual data, the risk of detection bias was unclear.
Incomplete outcome data
While both included studies reported an intention‐to‐treat analysis, one participant in the main DEBUT trial was randomized to ICD therapy but refused implantation. This participant was excluded from the published analyses despite randomization to the intervention group. However, after running sensitivity analyses, this does not appear to have had an effect on the reported outcomes (Analysis 2.1). No participants were lost to follow‐up between the intervention and control groups. Thus, the included studies had a low likelihood of attrition bias.
2.1. Analysis.
Comparison 2 Sensitivity analysis: implantable cardioverter defibrillators (ICD) versus β‐blocker therapy, Outcome 1 All‐cause mortality.
Selective reporting
In both included studies, the endpoints of death and recurrent VF or VT were prespecified outcomes and thus reflected a low risk of reporting bias.
Other potential sources of bias
The main DEBUT trial was stopped after the first interim analysis by the Data and Safety Monitoring Board, which was prespecified after half of the target sample had been recruited (57 participants). According to the study authors, the decision was based on "the cumulative weight of all evidence gained from the data supporting the conclusion that ICD therapy is superior to beta‐blocker treatment". RCTs stopped early are associated with a greater effect of the intervention compared with trials that are not stopped early, suggesting a high risk of bias of the effect size in this domain (Bassler 2010), though it is unlikely to have affected the direction of the effect. As both included studies were relatively small, there was a high possibility of introducing a small‐study effect, in which smaller trials may report greater treatment benefits than larger trials (Sterne 2001).
Effects of interventions
See: Table 1
Primary outcomes
All‐cause mortality
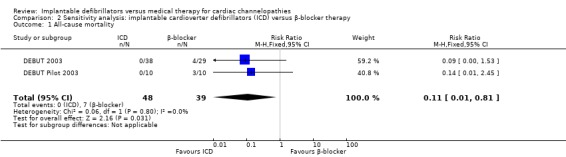
Two trials evaluated all‐cause mortality (DEBUT 2003; DEBUT Pilot 2003). Of the study participants, 0/47 (0%) receiving ICD therapy died compared to 7/39 (18%) receiving β‐blocker therapy. Pooling the two studies using a fixed‐effect model demonstrated a lower risk of death in participants randomized to the ICD group compared with those in the β‐blocker group (RR 0.11, 95% CI 0.01 to 0.83; 2 trials, 86 participants; Analysis 1.1). There was no heterogeneity (I2 = 0%; Figure 4).
1.1. Analysis.
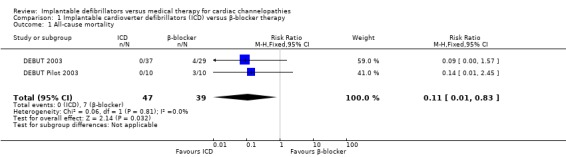
Comparison 1 Implantable cardioverter defibrillators (ICD) versus β‐blocker therapy, Outcome 1 All‐cause mortality.
4.
Funnel plot of comparison: 1 Implantable cardioverter defibrillators versus β‐blocker therapy: 1.1 All‐cause mortality.
Fatal and non‐fatal cardiovascular events
Two trials evaluated combined fatal and non‐fatal cardiovascular events. The study found 12/47 (26%) participants receiving ICD therapy compared to 7/39 (18%) participants receiving β‐blocker therapy had a combined fatal or non‐fatal cardiovascular event. Pooling the two studies using a fixed‐effect model, there was low quality evidence of a difference in the risk of fatal and non‐fatal cardiovascular events in the ICD group compared to the β‐blocker group (RR 1.49, 95% CI 0.66 to 3.34; 2 trials, 86 participants; Analysis 1.2), because the results were imprecise. There was no heterogeneity (I2 = 0%).
1.2. Analysis.
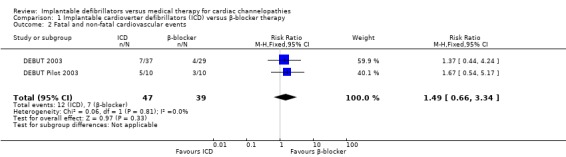
Comparison 1 Implantable cardioverter defibrillators (ICD) versus β‐blocker therapy, Outcome 2 Fatal and non‐fatal cardiovascular events.
Adverse events
Two trials evaluated adverse events. Adverse events occurred in 13/47 (28%) participants receiving ICD therapy compared to 4/39 (10%) participants receiving β‐blocker therapy. In the DEBUT pilot study, one participant in the ICD group experienced inappropriate defibrillation, and a second participant underwent ICD lead replacement because of an insulation break. In the main DEBUT trial, 10 participants experienced inappropriate ICD defibrillation and one additional participant experienced a pocket infection and erosion necessitating explant of the ICD. All inappropriate defibrillations were corrected by reprogramming the ICD and led to no further complications in both the main and pilot DEBUT trials.
In the participants randomized to medical therapy in the main DEBUT trial, four participants (14%) had medication‐induced adverse effects; one participant (3%) experienced each of the following adverse effects: impotence/decrease in libido, fatigue, profound bradycardia, and hypotension/central nervous system adverse effect. None of the adverse effects prompted a changed in drug therapy.
When we pooled the studies, there was a trend towards a greater risk of adverse events in the ICD group compared with the β‐blocker group, but the results were imprecise (RR 2.44, 95% CI 0.92 to 6.44; 2 trials, 86 participants; Analysis 1.3). There was no heterogeneity (I2 = 0%).
1.3. Analysis.
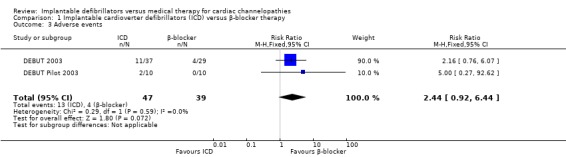
Comparison 1 Implantable cardioverter defibrillators (ICD) versus β‐blocker therapy, Outcome 3 Adverse events.
Secondary outcomes
Non‐fatal cardiovascular events
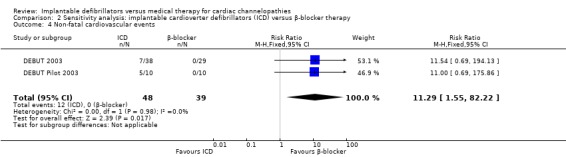
Twelve of 47 (26%) participants randomized to ICD therapy compared with 0/39 (0%) participants receiving β‐blocker therapy experienced a non‐fatal cardiovascular event, all of which were ICD‐terminated ventricular arrhythmias. The pooled risks of non‐fatal cardiovascular events using a fixed‐effect model were 11.4 times higher in the ICD group compared with the β‐blocker group (RR 11.4, 95% CI 1.57 to 83.36; 2 trials, 86 participants; Analysis 1.4). There was no heterogeneity (I2 = 0%).
1.4. Analysis.
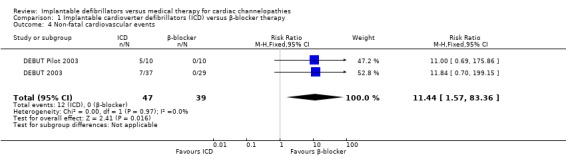
Comparison 1 Implantable cardioverter defibrillators (ICD) versus β‐blocker therapy, Outcome 4 Non‐fatal cardiovascular events.
Inappropriate implantable cardioverter defibrillator firing
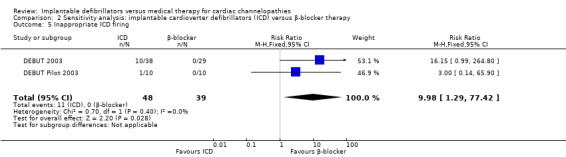
Eleven of 47 (23%) participants receiving ICD therapy compared with 0/39 (0%) participants receiving β‐blocker therapy had inappropriate ICD firing. The pooled risk of inappropriate ICD firing was higher in the ICD group compared with the β‐blocker group (RR 10.17, 95% CI 1.32 to 78.54; 2 trials, 86 participants; Analysis 1.5). There was no heterogeneity (I2 = 0%).
1.5. Analysis.
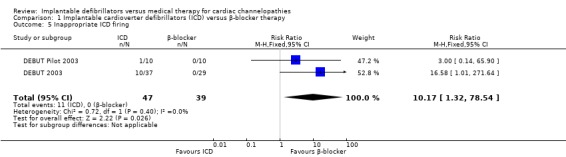
Comparison 1 Implantable cardioverter defibrillators (ICD) versus β‐blocker therapy, Outcome 5 Inappropriate ICD firing.
Quality of life
None of the included studies reported data on quality of life measurements.
Cost
None of the included studies reported data on cost.
Sensitivity analysis
We conducted a sensitivity analysis to determine if including the one participant who was randomized to ICD therapy but subsequently refused implantation affected the observed results. There were no major differences in either the direction or the magnitude of the risk any primary or secondary endpoints.
Subgroup analyses
There was an insufficient number of participants to conduct prespecified subgroup analyses in age, sex, channelopathy subtype, implantable versus non‐implanted cardioverter defibrillators.
Discussion
Summary of main results
In our systematic review evaluating the effects of ICDs compared with medical therapy in people with cardiac ion channelopathies, we found two small studies that demonstrated a reduction in all‐cause mortality in people randomized to ICD therapy. The effect was a nine‐fold decrease in the pooled risk of all‐cause mortality and an absolute risk reduction in mortality of 17.9% in people with ICDs implanted when compared with medical therapy. The consistent direction and magnitude of the benefit in the two studies analysed suggests these differences are unlikely to be explained by chance alone, but we considered the quality of evidence low by GRADE methodology due to study limitations and imprecision of effect. We are confident ICD therapy decreases the risk of mortality when compared with β‐blocker therapy but have limited confidence in the true effect size due to the high risk of performance bias, early stopping bias, and small‐study bias. Together, these potential biases may lead to an overestimation of the effect of ICD over medical therapy. Because of the large magnitude of effect, it is unlikely that there will be additional studies on the effect of ICDs for secondary prevention of sudden cardiac arrest in this population.
This reduction in all‐cause mortality in participants with an ICD versus medical management was paralleled by an 11‐fold increase in the risk of non‐fatal cardiovascular events in participants treated with ICDs. However, the increased risk was explained entirely by appropriate ICD‐terminated arrhythmias. In fact, 12/12 (100%) of the non‐fatal cardiovascular events experienced by participants in the ICD arm were appropriate ICD‐terminated arrhythmias, compared with no non‐fatal cardiovascular events in the medical group. Thus, ICD therapy probably decreases all‐cause mortality and concomitantly increases the incidence of appropriately terminated arrhythmias when compared with medical therapy.
Participants who underwent ICD implantation experienced higher rates of adverse events, with a 2.4 higher risk (95% CI 0.92 to 6.44), though the results were imprecise. The adverse events in the ICD group were generally mild and largely driven by a 23% combined inappropriate ICD firing rate, but 4% of ICD participants experienced more serious complications. There were no data reported on quality of life or cost analysis.
Overall completeness and applicability of evidence
Our review identified only two small studies addressing secondary prevention of sudden cardiac death in people with Brugada syndrome. We found no RCTs in people with congenital long QT syndrome, congenital short QT syndrome, or catecholaminergic polymorphic VT, or in participants with any of the cardiac ion channelopathies for primary prevention of sudden cardiac death. Therefore, our results are applicable to people with Brugada syndrome but may not necessarily be extrapolated to other cardiac channelopathies, neither should they be extrapolated for the primary prevention of sudden cardiac death.
The data were sufficient to comment on our three primary objectives as well as two of our four secondary objectives. However, there were insufficient data to comment on the quality of life and cost analysis of ICD versus medical therapy in people with cardiac ion channelopathies. The outcomes of mortality, fatal and non‐fatal cardiovascular events, and adverse events are important clinical endpoints to understanding and treating people with inherited cardiac ion channelopathies.
Current opinions are variable in the proper treatment modality for primary prevention of sudden cardiac death in people with cardiac channelopathies. US guidelines suggest consideration of ICD therapy for primary and secondary prevention of sudden cardiac death in people with congenital long QT syndrome, short QT syndrome, Brugada syndrome, or catecholaminergic polymorphic VT, though they are expert opinion‐driven recommendations (Epstein 2008; Epstein 2013). Our data provide supporting evidence from RCTs of ICDs for a secondary prevention indication, though these data were low GRADE quality.
Quality of the evidence
The two small trials had a high risk of performance bias, early stopping bias, and small‐study bias. These limitations may lead to an overestimation of the effect of ICD therapy over medical therapy but are difficult to quantify. Limitations in the quality of the evidence are that the two included studies were conducted by the same authors, which has the potential to introduce trial design biases and lack of replication by outside groups. Overall, these data were low GRADE quality.
Potential biases in the review process
We used a prespecified protocol for our review and complemented our search of published literature with handsearching and contacting study authors. We consider that there was a high likelihood that we included all relevant studies. We followed guidelines from the Cochrane Handbook for Systematic Reviews of Interventions to perform our title screening and data extraction to minimize bias and believe the review process has a low risk of introducing bias (Higgins 2011).
Agreements and disagreements with other studies or reviews
To our knowledge, there are no other systematic reviews comparing ICD versus medical therapy in people with cardiac ion channelopathies. Our review demonstrated a probable mortality benefit for secondary prevention of sudden cardiac death in people with Brugada syndrome treated with ICD therapy versus medical therapy. Several prior studies and meta‐analyses supported ICD implantation for secondary prevention of sudden cardiac arrest (Connolly 2000). However, our study provided the first systematic review of the secondary prevention literature in people with cardiac ion channelopathies. These analyses provided additional data to support evidence‐based therapy for people with cardiac ion channelopathies who experienced a prior sudden cardiac arrest.
Our review found no RCTs to address the question of whether ICD versus medical therapy is superior for primary prevention of sudden cardiac death among people with cardiac ion channelopathies. A combination of factors make RCTs investigating the proper treatment modality for the primary prevention of sudden cardiac arrest inherently difficult to conduct in a population with cardiac ion channelopathies. First, each of the studied ion channelopathies has variable penetrance, which inherently leads to difficulties associating a person's genotype with phenotype. This, in turn, makes predicting which people are at relatively higher risk for sudden cardiac death difficult. Second, each ion channelopathy is a heterogeneous genotypic group that may actually necessitate different treatment modalities. This possibility was suggested by registry data suggesting specific β‐blocker therapy may be more beneficial for certain subsets of long QT syndrome (Abu‐Zeitone 2014).
Despite these difficulties, it is important to bridge the current gap between diagnosing an inherited cardiac ion channelopathy and determining the optimal treatment in these people.
Authors' conclusions
Implications for practice.
Our systematic review supported the use of implantable cardioverter defibrillators (ICD) over β‐blocker therapy in the secondary prevention of sudden cardiac arrest in people with Brugada syndrome, but the role for ICDs in primary prevention in this population remains uncertain. However, the large magnitude of effect must be balanced with the study limitations, imprecision of effect, participant preferences, and higher rates of adverse events in participants undergoing ICD implantation. We found no studies addressing if ICD versus medical therapy reduces mortality for the primary prevention of sudden cardiac arrest, or in secondary prevention of sudden cardiac arrest in people with long QT syndrome, short QT syndrome, or congenital polymorphic VT.
Implications for research.
Further trials evaluating the effect of ICDs in the primary prevention of sudden cardiac arrest among people with inherited cardiac ion channelopathies are warranted. These trials should include outcomes on adverse events, health‐related quality of life, and cost. These data would assist patients, physicians, and policymakers in determining effective treatment strategies for the growing number of people with inherited cardiac ion channelopathies.
What's new
| Date | Event | Description |
|---|---|---|
| 2 March 2020 | Amended | Conflict of interest declaration amended (Declarations of interest) for clarification of compliance with the Cochrane conflict of interest policy, which includes the relevant parts of the Cochrane Commercial Sponsorship Policy. |
Acknowledgements
We thank Fiona Taylor, and the entire London‐based Cochrane Heart Group for their assistance at all stages of this review.
Appendices
Appendix 1. Database search strategies
CENTRAL and DARE (The Cochrane Library ‐ Wiley)
ID Search
#1 MeSH descriptor: [Ion Channels] explode all trees
#2 ((cardiac or ion or sodium or calcium or potassium or chloride) near/3 channel*):ti,ab,kw
#3 MeSH descriptor: [Channelopathies] explode all trees
#4 channelopath*:ti,ab,kw
#5 MeSH descriptor: [Long QT Syndrome] explode all trees
#6 (long qt syndrome* or long q‐t syndrome* or lqts):ti,ab,kw
#7 timothy syndrome*:ti,ab,kw
#8 (andersen near/3 (syndrome* or tawil or cardiodysrythmic or paralysis)):ti,ab,kw
#9 ((Jervell‐Lange Nielsen or surdo cardiac or cardioauditory) near/3 syndrome*):ti,ab,kw
#10 ((romano ward or ward romano) near/3 syndrome*):ti,ab,kw
#11 (short qt syndrome* or short‐qt syndrome* sqts):ti,ab,kw
#12 MeSH descriptor: [Brugada Syndrome] this term only
#13 (brugada or sudden unexplained nocturnal death syndrome* or sunds or right bundle branch block):ti,ab,kw
#14 idiopathic ventricular fibrillation:ti,ab,kw
#15 cardiac conduction near/2 (disease* or disorder*):ti,ab,kw
#16 (Catecholaminergic Polymorphic Ventricular Tachycardia or CPVT):ti,ab,kw
#17 Ankyrin‐B Syndrome*:ti,ab,kw
#18 {or #1‐#17}
#19 MeSH descriptor: [Defibrillators, Implantable] this term only
#20 MeSH descriptor: [Electric Countershock] this term only
#21 MeSH descriptor: [Prostheses and Implants] explode all trees
#22 (defibrillator* near/5 implant*):ti,ab,kw
#23 cardioverter*:ti,ab,kw
#24 electric countershock*:ti,ab,kw
#25 electric defibrillat*:ti,ab,kw
#26 electroversion therap*:ti,ab,kw
#27 cardiac electroversion*:ti,ab,kw
#28 cardioversion*:ti,ab,kw
#29 {or #19‐#28}
#30 #18 and #29
Ovid MEDLINE(R) 1946 to July week 2 2015 and Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations 15 July 2015
1. exp ion channels/
2. ((cardiac or ion or sodium or calcium or potassium or chloride) adj3 channel*).tw.
3. Channelopathies/
4. channelopath*.tw.
5. exp Long QT Syndrome/
6. (long qt syndrome* or long q‐t syndrome* or lqts).tw.
7. timothy syndrome*.tw.
8. (andersen adj3 (syndrome* or tawil or cardiodysrythmic or paralysis)).tw.
9. ((Jervell‐Lange Nielsen or surdo cardiac or cardioauditory) adj2 syndrome*).tw.
10. ((romano ward or ward romano) adj syndrome).tw.
11. (short qt syndrome* or short‐qt syndrome* sqts).tw.
12. Brugada Syndrome/
13. (brugada or sudden unexplained nocturnal death syndrome* or sunds or right bundle branch block).tw.
14. idiopathic ventricular fibrillation.tw.
15. cardiac conduction disease.tw.
16. (Catecholaminergic Polymorphic Ventricular Tachycardia or CPVT).tw.
17. Ankyrin‐B Syndrome*.tw.
18. or/1‐17
19. Defibrillators, Implantable/
20. Electric Countershock/
21. "Prostheses and Implants"/
22. (defibrillator* adj5 implant*).tw.
23. cardioverter*.tw.
24. electric countershock*.tw.
25. electric defibrillat*.tw.
26. electroversion therap*.tw.
27. cardiac electroversion*.tw.
28. cardioversion*.tw.
29. or/19‐28
30. randomized controlled trial.pt.
31. controlled clinical trial.pt.
32. randomized.ab.
33. placebo.ab.
34. drug therapy.fs.
35. randomly.ab.
36. trial.ab.
37. groups.ab.
38. or/30‐37
39. exp animals/ not humans.sh.
40. 38 not 39
41. 18 and 29
42. 40 and 41
EMBASE (embase.com)
'single blind procedure'/exp OR 'randomized controlled trial'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR random*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR (cross NEXT/1 over*):ab,ti OR placebo*:ab,ti OR (doubl* NEXT/1 blind*):ab,ti OR (singl* NEXT/1 blind*):ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti AND ('ion channel'/exp OR ((cardiac OR ion OR sodium OR calcium OR potassium OR chloride) NEAR/3 channel*):ab,ti OR 'channelopathy'/exp OR channelopath*:ab,ti OR 'long qt syndrome':ab,ti OR 'long qt syndromes':ab,ti OR 'long q‐t syndrome':ab,ti OR 'long q‐t syndromes':ab,ti OR lqts:ab,ti OR (timothy NEAR/1 syndrome*):ab,ti OR (andersen NEAR/3 (syndrome* OR tawil OR cardiodysrythmic OR paralysis)):ab,ti OR (('jervell‐lange nielsen' OR 'surdo cardiac' OR cardioauditory) NEAR/2 (syndrome OR syndromes)):ab,ti OR (('romano ward' OR 'ward romano') NEAR/1 syndrome*):ab,ti OR 'short qt syndrome':ab,ti OR 'short qt syndromes':ab,ti OR 'short‐qt syndrome':ab,ti OR 'short‐qt syndromes':ab,ti OR sqts:ab,ti OR brugada*:ab,ti OR 'sudden unexplained nocturnal death':ab,ti OR sunds:ab,ti OR 'right bundle branch block':ab,ti OR 'heart right bundle branch block'/de OR 'idiopathic ventricular fibrillation':ab,ti OR ('cardiac conduction' NEAR/2 disease*):ab,ti OR ('cardiac conduction' NEAR/2 disorder*):ab,ti OR 'catecholaminergic polymorphic ventricular tachycardia':ab,ti OR cpvt:ab,ti OR ('ankyrin b':ab,ti AND syndrome*:ab,ti)) AND ('implantable cardioverter defibrillator'/de OR 'cardioversion'/de OR ('defibrillator'/de AND implant*:ab,ti) OR (defibrillator* NEAR/6 implant*):ab,ti OR cardioverter*:ab,ti OR 'electric countershock':ab,ti OR (electric NEAR/1 defibrillat*):ab,ti OR (electroversion NEAR/6 therap*):ab,ti OR (cardiac NEAR/6 electroversion*):ab,ti OR cardioversion*:ab,ti)
Conference Proceedings Citation Index ‐ Science (CPCI‐S) (Web of Science)
| #23 | #22 AND #21 DocType=All document types; Language=All languages; |
| #22 | #20 AND #14 DocType=All document types; Language=All languages; |
| #21 | TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over* or group*) DocType=All document types; Language=All languages; |
| #20 | #19 OR #18 OR #17 OR #16 OR #15 DocType=All document types; Language=All languages; |
| #19 | TS=(cardioversion*) DocType=All document types; Language=All languages; |
| #18 | TS=(electrover*) DocType=All document types; Language=All languages; |
| #17 | TS=(electric near/6 (countershock or defibrillat*)) DocType=All document types; Language=All languages; |
| #16 | TS=(cardioverter*) DocType=All document types; Language=All languages; |
| #15 | TS=(defibrillator* and implant*) DocType=All document types; Language=All languages; |
| #14 | #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 DocType=All document types; Language=All languages; |
| #13 | TS=("Ankyrin‐B Syndrome*" or "Ankyrin B Syndrome*") DocType=All document types; Language=All languages; |
| #12 | TS=("catecholaminergic polymorphic ventricular tachycardia" or CPVT) DocType=All document types; Language=All languages; |
| #11 | TS=("cardiac conduction" and (disease or disorder)) DocType=All document types; Language=All languages; |
| #10 | TS=("idiopathic ventricular fibrillation") DocType=All document types; Language=All languages; |
| #9 | TS=(brugada or "sudden unexplained nocturnal death syndrome*" or sunds or "right bundle branch block") DocType=All document types; Language=All languages; |
| #8 | TS=("short qt" or "short‐qt" or sqts) DocType=All document types; Language=All languages; |
| #7 | TS=(("romano ward" or "ward romano") near/6 syndrome*) DocType=All document types; Language=All languages; |
| #6 | TS=(("Jervell‐Lange Nielsen" or "Jervell Lange Nielsen" or "surdo cardiac" or cardioauditory) near/6 syndrome*) DocType=All document types; Language=All languages; |
| #5 | TS=(andersen near/6 (syndrome* or tawil or cardiodysrythmic or paralysis)) DocType=All document types; Language=All languages; |
| #4 | TS=(timothy near/6 syndrome*) DocType=All document types; Language=All languages; |
| #3 | TS=("long qt" or "long q‐t" or lqts) DocType=All document types; Language=All languages; |
| #2 | TS=channelopath* DocType=All document types; Language=All languages; |
| #1 | TS=((cardiac or ion or sodium or calcium or potassium or chloride) near/3 channel*) DocType=All document types; Language=All languages; |
Appendix 2. Trials register search strategies
ClinicalTrials.gov
Ran two searches due to character limits. Each search retrieved same 32 records.
Search 1 (defibrillator OR defibrillators OR defibrillation) AND (channelopathy OR channelopathies OR ion channel OR ion channels OR sodium channel OR sodium channels OR calcium channel OR calcium channels OR potassium channel OR potassium channels)
Search 2 (defibrillator OR defibrillators OR defibrillation) AND (long qt OR short qt OR brugada OR sudden cardiac OR Catecholaminergic Polymorphic Ventricular Tachycardia OR CPVT OR sudden cardiac death)
WHO International Clinical Trials Registry Platform (ICTRP)
Used simple search interface. Merged search results from two searches and removed one duplicate record. Retrieved 127 records for 117 trials.
Search 1 defibrillator AND channelopathy OR defibrillator AND channelopathies OR defibrillator AND ion channel OR defibrillator AND ion channels OR defibrillator AND sodium channel OR defibrillator AND sodium channels OR defibrillator AND calcium channel OR defibrillator AND calcium channels OR defibrillator AND potassium channel OR defibrillator AND potassium channels OR defibrillators AND channelopathy OR defibrillators AND channelopathies OR defibrillators AND ion channel OR defibrillators AND ion channels OR defibrillators AND sodium channel OR defibrillators AND sodium channels OR defibrillators AND calcium channel OR defibrillators AND calcium channels OR defibrillators AND potassium channel OR defibrillators AND potassium channels OR defibrillation AND channelopathy OR defibrillation AND channelopathies OR defibrillation AND ion channel OR defibrillation AND ion channels OR defibrillation AND sodium channel OR defibrillation AND sodium channels OR defibrillation AND calcium channel OR defibrillation AND calcium channels OR defibrillation AND potassium channel OR defibrillation AND potassium channels
Search 2 defibrillator AND long qt OR defibrillator AND short qt OR defibrillator AND brugada OR defibrillator AND sudden cardiac OR defibrillator AND Catecholaminergic Polymorphic Ventricular Tachycardia OR defibrillator AND CPVT OR defibrillator AND sudden cardiac death OR defibrillators AND long qt OR defibrillators AND short qt OR defibrillators AND brugada OR defibrillators AND sudden cardiac OR defibrillators AND Catecholaminergic Polymorphic Ventricular Tachycardia OR defibrillators AND CPVT OR defibrillators AND sudden cardiac death OR defibrillation AND long qt OR defibrillation AND short qt OR defibrillation AND brugada OR defibrillation AND sudden cardiac OR defibrillation AND Catecholaminergic Polymorphic Ventricular Tachycardia OR defibrillation AND CPVT OR defibrillation AND sudden cardiac death
Data and analyses
Comparison 1. Implantable cardioverter defibrillators (ICD) versus β‐blocker therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 2 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 0.83] |
| 2 Fatal and non‐fatal cardiovascular events | 2 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.66, 3.34] |
| 3 Adverse events | 2 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.92, 6.44] |
| 4 Non‐fatal cardiovascular events | 2 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.44 [1.57, 83.36] |
| 5 Inappropriate ICD firing | 2 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.17 [1.32, 78.54] |
Comparison 2. Sensitivity analysis: implantable cardioverter defibrillators (ICD) versus β‐blocker therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 0.81] |
| 2 Fatal and non‐fatal cardiovascular events | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.65, 3.29] |
| 3 Adverse events | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [0.90, 6.30] |
| 4 Non‐fatal cardiovascular events | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.29 [1.55, 82.22] |
| 5 Inappropriate ICD firing | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.98 [1.29, 77.42] |
2.2. Analysis.
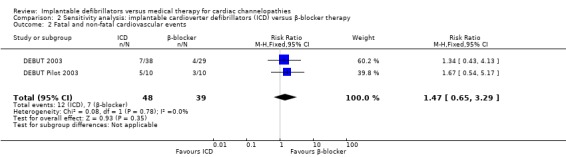
Comparison 2 Sensitivity analysis: implantable cardioverter defibrillators (ICD) versus β‐blocker therapy, Outcome 2 Fatal and non‐fatal cardiovascular events.
2.3. Analysis.
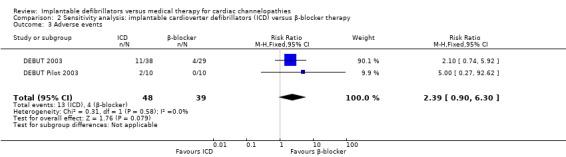
Comparison 2 Sensitivity analysis: implantable cardioverter defibrillators (ICD) versus β‐blocker therapy, Outcome 3 Adverse events.
2.4. Analysis.
Comparison 2 Sensitivity analysis: implantable cardioverter defibrillators (ICD) versus β‐blocker therapy, Outcome 4 Non‐fatal cardiovascular events.
2.5. Analysis.
Comparison 2 Sensitivity analysis: implantable cardioverter defibrillators (ICD) versus β‐blocker therapy, Outcome 5 Inappropriate ICD firing.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
DEBUT 2003.
| Methods | Randomized, controlled clinical trial, not blinded to participants or staff | |
| Participants | SUDS survivors and probable SUDS survivors. SUDS Survivors were defined as "a healthy subject without structural heart disease who had survived unexpected ventricular fibrillation or cardiac arrest after successful resuscitation". Probable SUDS survivors were defined as "a subject without structural heart disease who experienced symptoms indicative of the clinical presentation of SUDS, especially during sleep, including agonal respiration, transient episodes of stress, abnormal respiration associated with grasping and groaning, syncope, or seizure‐like symptoms". Inclusion criteria for probable SUDS survivors were "ECG abnormalities showing a right bundle branch‐like pattern with ST elevation in the right precordial leads (V1 to V3) and inducible ventricular tachycardia/fibrillation in the electrophysiology laboratory before randomization" 37 participants randomized to ICD; 29 participants randomized to β‐blocker 64/66 men Mean (SD) age: 40 (11) years in ICD group; 40 (14) years in β‐blocker group Mean (SD) left ventricular ejection fraction: 66% (10%) in ICD group; 67% (7%) in β‐blocker group CPR: 26 in ICD group; 20 in β‐blocker group Defibrillation: 17 in ICD group; 18 in β‐blocker group Initial rhythm of VF: 9 in ICD group; 11 in β‐blocker group No participants were reported as lost to follow‐up |
|
| Interventions | Group 1: transvenous ICD implantation Group 2: β‐blocker therapy with propranolol (40‐160 mg daily) |
|
| Outcomes | Primary endpoint: all‐cause mortality Secondary endpoints: recurrent VT or VF, or cardiac arrest Participants were followed after the first month and at 3‐month intervals for a maximum of 3 years' post‐randomization |
|
| Notes | The trial was terminated early on 15 December 2000 at the first interim Data and Safety Monitoring Board's analysis, which was prespecified after half of the target sample had been recruited (57 participants). According to the authors, "Although the primary analysis during the first interim look did not reach a level of statistical significance defined by the stopping rules, the DSMB [Data and Safety Monitoring Board] unanimously recommended termination of the DEBUT trial. The DSMB based their decision on the cumulative weight of all evidence gained from the data supporting the conclusion that ICD therapy is superior to beta‐blocker treatment" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment from study members not reported, but the characteristics of each group were similar at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It does not appear that either participants or personnel were blinded to the intervention or the control. Blinding was difficult as 1 of the interventions required a procedure and device implantation (ICD) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The authors did not report how the outcomes were ascertained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no differential loss to follow‐up between the 2 groups |
| Selective reporting (reporting bias) | Unclear risk | The authors described a similar protocol between the main DEBUT trial (DEBUT 2003), and the pilot DEBUT trial (DEBUT Pilot 2003), but the authors do not report a published protocol registration number and there was none available by searching clinical trial registers |
| Other bias | High risk | The trial was stopped early by the Data and Safety Monitoring Board of the trial. This bias may have led to an observed response that is greater than the true response |
DEBUT Pilot 2003.
| Methods | Randomized, controlled, clinical trial, not blinded to participants or staff | |
| Participants | The study population consisted of Thai participants classified as having survived SUDS or probable SUDS. SUDS survivors were defined as "a healthy subject without structural heart disease who had survived unexpected ventricular fibrillation or cardiac arrest after successful resuscitation". Probable SUDS survivors were defined as "a subject without structural heart disease who experienced symptoms indicative of the clinical presentation of SUDS, especially during sleep, including agonal respiration, transient episodes of stress, abnormal respiration associated with grasping and groaning, syncope, or seizure‐like symptoms". Further inclusion criteria for probable SUDS survivors were defined as "ECG abnormalities showing a right bundle branch‐like pattern with ST elevation in the right precordial leads (V1 to V3) and inducible ventricular tachycardia/fibrillation in the electrophysiology laboratory before randomization" 10 participants randomized to ICD; 10 participants randomized to β‐blocker 20/20 men Mean (SD) age: 44 (11) years in ICD group; 48 (15) years in β‐blocker group Mean (SD) left ventricular ejection fraction: 67% (12) in ICD group; 69% (6) in β blocker group CPR: 9 in ICD group; 6 in the β‐blocker group Defibrillation: 8 in ICD group; 5 in β‐blocker group Initial rhythm of VF: 7 in ICD group; 6 in β‐blocker group No participants were reported as lost to follow‐up |
|
| Interventions | Group 1: transvenous ICD implantation Group 2: β‐blocker therapy with propranolol (40‐160 mg daily) |
|
| Outcomes | Primary endpoint: all‐cause mortality Secondary endpoints: recurrent VT or VF, or cardiac arrest Participants were followed after the first month and at 3‐month intervals for a maximum of 3 years' post‐randomization |
|
| Notes | This was the pilot study used for the power calculation for the main trial (DEBUT 2003) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment from study members not reported, but the characteristics of each group were similar at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It does not appear that either participants or personnel were blinded to the intervention or the control. Blinding was difficult as 1 of the interventions required a procedure and device implantation (ICD) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The authors did not report how the outcomes were ascertained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no differential loss to follow‐up between the 2 groups |
| Selective reporting (reporting bias) | Unclear risk | The authors described a similar protocol between the main DEBUT trial and the pilot DEBUT trial, but the authors did not report a published protocol registration number and there was none available by searching clinical trial registers |
| Other bias | High risk | The trial was stopped early by the Data and Safety Monitoring Board of the trial. This bias may have led to an observed response that is greater than the true response |
CPR: cardiopulmonary resuscitation; ECG: electrocardiogram; ICD: implantable cardioverter defibrillator; SD: standard deviation; SUDS: sudden unexplained death syndrome; VF: ventricular fibrillation; VT: ventricular tachycardia.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AVID 1997 | No known ion channelopathy participants were included in the study |
| CASH 1993 | No known ion channelopathy participants were included in the study |
| Hohnloser 2006 | Inappropriate intervention |
| VAST 2006 | Inappropriate intervention and control groups |
| Wang 2008 | Not a randomized controlled trial |
Differences between protocol and review
None.
Contributions of authors
All authors contributed to the development of concept and writing the protocol. The following authors performed each step:
title/abstract and full‐text screening: DAM, MDH;
data extraction: DAM, MDH;
risk of bias assessment: DAM, MDH;
data management and analysis: DAM, MDH;
writing: DAM, MDH;
commenting critically for intellectual content: all authors.
Sources of support
Internal sources
The Cochrane Heart Group US Satellite receives intramural support from the Northwestern University Feinberg School of Medicine, Other.
External sources
No sources of support supplied
Declarations of interest
Dr. McNamara received no grant support and had no declarations of interest.
Dr. Goldberger is the Director of the Path to Improved Risk Stratification, NFP and reports unrestricted educational grants from Boston Scientific, Medtronic, St. Jude Medical, and consulting/Honoraria‐Medtronic, GE Healthcare, Zoll; all of which were unrelated to this review.
Dr. Huffman received grant support from the National Heart, Lung, and Blood Institute and World Heart Federation through unrestricted educational grants from AstraZeneca, Boehringer Ingelheim, and Bupa on research and a research training programme, respectively, both of which are unrelated to this review. Boehringer Ingelheim, AstraZeneca and Bupa had no input or influence over the grants’ nominations, the World Heart Federation controlled the use of the funds.
Mark Berendsen declares no financial or academic conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
DEBUT 2003 {published data only (unpublished sought but not used)}
DEBUT Pilot 2003 {published data only (unpublished sought but not used)}
References to studies excluded from this review
AVID 1997 {published data only}
- AVID Investigators. A comparison of antiarrhythmic‐drug therapy with implantable defibrillators in patients resuscitated from near‐fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. New England Journal of Medicine 1997;337:1576‐83. [DOI] [PubMed] [Google Scholar]
CASH 1993 {published data only}
- Siebels J, Cappato R, Ruppel R, Schneider MA, Kuck KH. Preliminary results of the Cardiac Arrest Study Hamburg (CASH). American Journal of Cardiology 1993;72:109F‐13F. [DOI] [PubMed] [Google Scholar]
Hohnloser 2006 {published data only}
- Hohnloser SH, Al‐Khalidi HR, Pratt CM, Brum JM, Tatla DS, Tchou P, et al. Electrical storm in patients with an implantable defibrillator: incidence, features, and preventive therapy: insights from a randomized trial. European Heart Journal 2006;27:3027‐32. [DOI] [PubMed] [Google Scholar]
VAST 2006 {published data only}
- Friedman PA, Jalal S, Kaufman S, Villareal R, Brown S, Hahn SJ, et al. Effects of a rate smoothing algorithm for prevention of ventricular arrhythmias: results of the Ventricular Arrhythmia Suppression Trial (VAST). Heart Rhythm 2006;3:573‐80. [DOI] [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang K, Yamauchi K, Li P, Kato H, Kobayashi M, Kato K, et al. Cost‐effectiveness of implantable cardioverter‐defibrillators in Brugada syndrome treatment. Journal of Medical Systems 2008;32:51‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Abriel 2012
- Abriel H, Zaklyazminskaya EV. A modern approach to classify missense mutations in cardiac channelopathy genes. Circulation: Cardiovascular Genetics 2012;5:487‐9. [DOI] [PubMed] [Google Scholar]
Abu‐Zeitone 2014
- Abu‐Zeitone A, Peterson DR, Polonsky B, McNitt S, Moss AJ. Efficacy of different beta‐blockers in the treatment of long QT syndrome. Journal of the American College of Cardiology 2014;64:1352‐8. [DOI] [PubMed] [Google Scholar]
Ackerman 2004
- Ackerman MJ. Cardiac channelopathies: it's in the genes. Nature Medicine 2004;10:463‐4. [DOI] [PubMed] [Google Scholar]
Ackerman 2011
- Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm 2011;8:1308‐39. [DOI] [PubMed] [Google Scholar]
Alter 2005
- Alter P, Waldhans S, Plachta E, Moosdorf R, Grimm W. Complications of implantable cardioverter defibrillator therapy in 440 consecutive patients. Pacing and Clinical Electrophysiology 2005;28:926‐32. [DOI] [PubMed] [Google Scholar]
Anttonen 2007
- Anttonen O, Junttila MJ, Rissanen H, Reunanen A, Viitasalo M, Huikuri HV. Prevalence and prognostic significance of short QT interval in a middle‐aged Finnish population. Circulation 2007;116:714‐20. [DOI] [PubMed] [Google Scholar]
Bassler 2010
- Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta‐regression analysis. JAMA 2010;303:1180‐7. [DOI] [PubMed] [Google Scholar]
Bonow 2011
- Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald's Heart Disease: a Textbook of Cardiovascular Medicine. 9th Edition. Philadelphia, PA: Saunders, 2011. [Google Scholar]
Bowker 1996
- Bowker TJ, Clayton TC, Ingham J, McLennan NR, Hobson HL, Pyke SD, et al. A British Cardiac Society survey of the potential for the secondary prevention of coronary disease: ASPIRE (Action on Secondary Prevention through Intervention to Reduce Events). Heart 1996;75:334‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burkart 1990
- Burkart F, Pfisterer M, Kiowski W, Follath F, Burckhardt D. Effect of antiarrhythmic therapy on mortality in survivors of myocardial infarction with asymptomatic complex ventricular arrhythmias: Basel Antiarrhythmic Study of Infarct Survival (BASIS). Journal of the American College of Cardiology 1990;16:1711‐8. [DOI] [PubMed] [Google Scholar]
Camm 2010
- Camm JA, Nisam S. European utilization of the implantable defibrillator: has 10 years changed the 'enigma'?. Europace 2010;12:1063‐9. [DOI] [PubMed] [Google Scholar]
CAST I 1989
- The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. New England Journal of Medicine 1989;321:406‐12. [DOI] [PubMed] [Google Scholar]
CAST II 1992
- The Cardiac Arrhythmia Suppression Trial II Investigators. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. New England Journal of Medicine 1992;327:227‐33. [DOI] [PubMed] [Google Scholar]
Connolly 2000
- Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, et al. Meta‐analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. European Heart Journal 2000;21:2071‐8. [DOI] [PubMed] [Google Scholar]
Corrado 2001
- Corrado D, Basso C, Thiene G. Sudden cardiac death in young people with apparently normal heart. Cardiovascular Research 2001;50:399‐408. [DOI] [PubMed] [Google Scholar]
Crotti 2012
- Crotti L, Marcou CA, Tester DJ, Castelletti S, Giudicessi JR, Torchio M, et al. Spectrum and prevalence of mutations involving BrS1‐ through BrS12‐susceptibility genes in a cohort of unrelated patients referred for Brugada syndrome genetic testing: implications for genetic testing. Journal of the American College of Cardiology 2012;60:1410‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curtis 2009
- Curtis JP, Luebbert JJ, Wang Y, Rathore SS, Chen J, Heidenreich PA, et al. Association of physician certification and outcomes among patients receiving an implantable cardioverter‐defibrillator. JAMA 2009;301:1661‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deo 2012
- Deo R, Albert CM. Epidemiology and genetics of sudden cardiac death. Circulation 2012;125:620‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
DiMarco 2003
- DiMarco JP. Implantable cardioverter defibrillators. New England Journal of Medicine 2003;349:1836‐47. [DOI] [PubMed] [Google Scholar]
Eckart 2011
- Eckart RE, Shry EA, Burke AP, McNear JA, Appel DA, Castillo‐Rojas LM, et al. Sudden death in young adults: an autopsy‐based series of a population undergoing active surveillance. Journal of the American College of Cardiology 2011;58:1254‐61. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Epstein 2008
- Epstein AE, Dimarco JP, Ellenbogen KA, Estes NA III, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: executive summary. Heart Rhythm 2008;5:934‐55. [DOI] [PubMed] [Google Scholar]
Epstein 2013
- Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA III, Freedman RA, Gettes LS, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology 2013;61:e6‐75. [DOI] [PubMed] [Google Scholar]
Friedman 1986
- Friedman LM, Byington RP, Capone RJ, Furberg CD, Goldstein S, Lichstein E. Effect of propranolol in patients with myocardial infarction and ventricular arrhythmia. Journal of the American College of Cardiology 1986;7:1‐8. [DOI] [PubMed] [Google Scholar]
Gehi 2006
- Gehi AK, Duong TD, Metz LD, Gomes JA, Mehta D. Risk stratification of individuals with the Brugada electrocardiogram: a meta‐analysis. Journal of Cardiovascular Electrophysiology 2006;17:577‐83. [DOI] [PubMed] [Google Scholar]
Go 2014
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics ‐ 2014 update: a report from the American Heart Association. Circulation 2014;129:e28‐e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldberger 2014
- Goldberger JJ, Basu A, Boineau R, Buxton AE, Cain ME, Canty JMJ, et al. Risk stratification for sudden cardiac death: a plan for the future. Circulation 2014;129:516‐26. [DOI] [PubMed] [Google Scholar]
Gollob 2011
- Gollob MH, Redpath CJ, Roberts JD. The short QT syndrome: proposed diagnostic criteria. Journal of the American College of Cardiology 2011;57:802‐12. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Malhotra 2011
- Malhotra S, Das MK. Delayed and indirect effects of antiarrhythmic drugs in reducing sudden cardiac death. Future Cardiology 2011;7:203‐17. [DOI] [PubMed] [Google Scholar]
Martin 2012
- Martin CA, Matthews GD, Huang CL. Sudden cardiac death and inherited channelopathy: the basic electrophysiology of the myocyte and myocardium in ion channel disease. Heart 2012;98:536‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Napolitano 2012
- Napolitano C, Bloise R, Monteforte N, Priori SG. Sudden cardiac death and genetic ion channelopathies: long QT, Brugada, short QT, catecholaminergic polymorphic ventricular tachycardia, and idiopathic ventricular fibrillation. Circulation 2012;125:2027‐34. [DOI] [PubMed] [Google Scholar]
Napolitano 2014
- Napolitano C, Priori SG, Bloise R. Catecholaminergic polymorphic ventricular tachycardia. Pagon RA, Adam MP, Ardinger HH, et al. (editors). Seattle, WA: GeneReviews® [Internet], 14 October 2004 [Updated 6 March 2014]. [Google Scholar]
NICE 2014
- National Institute for Health and Care Excellence. Implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure (review of TA95 and TA120). June 2014. www.nice.org.uk/guidance/ta314 (accessed 16 July 2015).
Postema 2012
- Postema PG. About Brugada syndrome and its prevalence. Europace 2012;14:925‐8. [DOI] [PubMed] [Google Scholar]
Puranik 2005
- Puranik R, Chow CK, Duflou JA, Kilborn MJ, McGuire MA. Sudden death in the young. Heart Rhythm 2005;2:1277‐82. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schwartz 2009
- Schwartz PJ, Stramba‐Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, et al. Prevalence of the congenital long‐QT syndrome. Circulation 2009;120:1761‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siegel 2013
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: A Cancer Journal for Clinicians 2013;63:11‐30. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne J A, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta‐analysis. BMJ 2001;323:101‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tester 2011
- Tester DJ, Ackerman MJ. Genetic testing for potentially lethal, highly treatable inherited cardiomyopathies/channelopathies in clinical practice. Circulation 2011;123:1021‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Viskin 2009
- Viskin S, Wilde AA, Tan HL, Antzelevitch C, Shimizu W, Belhassen B. Empiric quinidine therapy for asymptomatic Brugada syndrome: time for a prospective registry. Heart Rhythm 2009;6:401‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zipes 2006
- Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). Journal of the American College of Cardiology 2006;48:e247‐346. [DOI] [PubMed] [Google Scholar]


