Abstract
Background
Benign Epilepsy with Centro Temporal Spikes (BECTS) is a common epilepsy syndrome with onset in childhood which almost always remits by adolescence. It is characterised by focal seizures associated with motor signs and somatosensory symptoms, at times progressing to become generalised. The characteristic interictal EEG shows normal background activity with centrotemporal spikes which are more prominent in sleep. The prognosis is good though subtle cognitive impairment has been implicated. Antiepileptic drug (AED) treatment is used if seizures are frequent or occurring in the daytime.
Objectives
To evaluate whether or not treatment with AEDs changes the short‐ or long‐term outcome of children with BECTS or both.
Search methods
We searched the following databases: the Cochrane Epilepsy Group Specialized Register (30 April 2013), the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2013, Issue 4: (April 2013)), MEDLINE (Ovid, 1946 to 30 April 2013), SCOPUS (30 April 2013), ClinicalTrials.gov (30 April 2013) and the WHO International Clinical Trials Registry Platform ICTRP (30 April 2013). We also handsearched the reference lists of articles that were considered for inclusion in the review.
Selection criteria
All randomised controlled trials (RCTs) that compared the use of different AEDs, or compared the use of AEDs with no treatment, or placebo in children with BECTS.
Data collection and analysis
Data were independently extracted by all four of the review authors and discrepancies were resolved by discussion. Analysis included assessment of risk of bias, quality of evidence of individual studies, heterogeneity, and statistical analysis of the effects on seizure remission and cognition.
Main results
There were six eligible studies but only four had sufficient data at the time of this review. The four RCTs included in this review reported on a total of 262 participants. One study, a placebo‐controlled trial with a low risk of bias, found that individuals on sulthiame were significantly more likely to remain in seizure remission during the three and six months from commencement of treatment than those on placebo (3 months: RR 2.26, 95% CI 1.48 to 3.44; 6 months: RR 2.63, 95% CI 1.43 to 4.86, 66 participants, moderate quality evidence). The other three trials, all open‐labelled studies, had a high risk of bias and did not show any significant differences in terms of seizure remission between AEDs. One compared levetiracetam with oxcarbazepine (3 months: RR 1.13, 95% CI of 0.93 ‐ 1.36; 12 months: RR of 1.29 with 95% CI of 0.89 ‐ 1.86, 39 participants, low to very low quality evidence), one clobazam with carbamazepine (4‐40 weeks: RR of 1.04, 95% CI of 0.67 ‐ 1.62; last 9 months: RR of 1.06 with 95% CI of 0.84, 1.34, 45 participants, low quality evidence), and one carbamazepine with topiramate (28 weeks: RR 1.02 with 95% CI of 0.8 ‐ 1.3, 112 participants, low quality evidence).
Other outcome measures assessed included time to first seizure after randomisation which was only obtained in the sulthiame versus placebo study as a hazard ratio of 7.8 (95% CI 2.66 ‐ 22.87). There were no significant differences between the proportion of participants who had adverse events, apart from a higher incidence of rash in the carbamazepine group (14.8%) when compared with topiramate (1.7%), or the proportion who withdrew from treatment due to adverse events, when this was reported. Two trials (carbamazepine versus topiramate, and clobazam versus carbamazepine) evaluated the effects on cognition. The studies were of low to very low quality evidence showing no clear difference in cognition at the end of the study periods between the AEDs compared. A meta‐analysis was not performed as the RCTs evaluated different therapies.
Authors' conclusions
There is evidence from one trial reviewed that sulthiame is effective for seizure remission in the short term in children with BECTS although the precision of the effect estimate is uncertain due to its small sample size. There were no significant differences in the proportion of adverse events between treatment groups studied, including those resulting in withdrawal of treatment. There is insufficient evidence about the medium to longer term effects on seizure control, the optimum antiepileptic drug treatment and the effects of AED treatment on cognition. There is a need for more good quality randomised controlled trials to address these questions to aid the management of children with BECTS.
Plain language summary
Antiepileptic drugs versus no treatment or placebo for children with benign epilepsy with centro temporal spikes
Benign epilepsy with centro temporal spikes is one of the most common childhood seizure disorders . Treatment for this disorder has been controversial as almost all individuals achieve seizure freedom by adolescence. However, this seizure disorder may not be as benign as the name suggests as children may have specific cognitive impairment. Treatment is started if seizures are felt to be frequent and intrusive.
There were few studies found (searches conducted on 30th April 2013) with few antiepileptic drugs compared. One of the four studies included showed evidence that the antiepileptic drug, sulthiame, may have a positive effect in reducing seizure frequency in BECTS in the short term. There were no significant differences in the number of patients with adverse events apart from a higher risk of rash when carbamazepine was compared to topiramate. The number of patients who discontinued treatment as a result of adverse events was also not significant in the studies reviewed. There is insufficient evidence about whether or not treating with antiepileptic drugs has any effect on seizure freedom in the longer term or on a child’s cognition. The optimum treatment has yet to be identified. More research is needed to look into the effectiveness of treatment versus no treatment on seizure control and intellect, and compare the existing treatments.
Summary of findings
for the main comparison.
| Clobazam compared with Carbamazepine for patients with BECTS | ||||||
|
Patient or population: Patients with a clinical and EEG diagnosis of BECTS Settings: Single centre in Cuba Intervention: Clobazam Comparison: Carbamazepine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk on Carbamazepine | Risk on Clobazam | |||||
| Proportion of patients who are seizure free between 4‐40 weeks | 64 per 1001 | 67 per 1001 |
RR 1.04 (0.67 ‐ 1.62) |
432 (1 study) |
⊕⊝⊝⊝3 very low | |
| Proportion of patients who had seizure remission in the last 9 months of the study | 84 per 1004 | 89 per 1004 |
RR 1.06 (0.84 ‐ 1.34) |
43 (1 study) |
⊕⊝⊝⊝5 very low | |
|
Proportion of patients who discontinued treatment due to adverse events (96 weeks trial period) |
40 per 10006 | 56 per 10006 | RR 0.72 (0.05, 10.76) | 43 (1 study) |
⊕⊝⊝⊝7 very low | One patient on clobazam discontinued due to rash whilst one on carbamazepine discontinued due to somnolence. |
| Proportion of patients who reported adverse events | 32 per 1008 | 17 per 1008 |
RR 1.92 (0.59 ‐ 6.25) |
43 (1 study) |
⊕⊝⊝⊝7 very low | |
|
Proportion of patients with a particular or serious adverse event: 1. Vertigo 2. Headache 3. Somnolence 4. Nausea/Vomiting 5. Tremor 6. Fatigue |
17 per 1009 11 per 1009 17 per 1009 56 per 10009 17 per 1009 17 per 1009 |
20 per 1009 16 per 1009 12 per 1009 20 per 10009 4 per 1009 8 per 1009 |
See comment | 43 (1 study) | ⊕⊝⊝⊝8 very low | The numbers in each group range from 1 to 5 patients. These are too small to compare if any statistical differences exist. |
| Changes in cognitive function testing from baseline (96 weeks trial period) | See10 | 39 baseline, 38 at end of trial | ⊕⊝⊝⊝8 very low | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; [other abbreviations, e.g. OR, etc] | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The proportion of patients who were seizure free between 4 ‐ 40 weeks of the trial were 64% for those on carbamazepine and 66.7% for those on clobazam. We have rounded these off to 64 and 68 per 100 respectively.
2 The study had 45 eligible participants but two were not randomised (no reasons given).
3The study was an open‐label study. There were inconsistencies in the reports regarding the numbers of excluded subjects e.g. in the carbamazepine group.
4 The proportion of patients who had seizure remission during the last 9 months of the trial were 84% for those on carbamazepine and 88.9% for those on clobazam. We have rounded these off to 89 and 84 per 100 respectively.
5 The study was an open‐label study. There were inconsistencies in the reports regarding the numbers of excluded subjects e.g. in the carbamazepine group.
6 The proportion of patients who discontinued treatment due to adverse events was 4% for those in the carbamazepine group and 5.6% for those in the clobazam group. We have rounded these off to 40 and 56 per 1000 respectively.
7 Reporting of adverse effects may have been affected as participants were not blinded. Elsewhere in the paper (footnotes of Table 1 in original paper), it was reported that five patients were excluded due to adverse events: three due to a severe rash, and two due to a combination of increasing seizures, the appearance of cognitive deficits, and reports from parents and teachers on a change in behaviour. It was not mentioned which arm of treatment these patients were randomised to, if this was done, and we were uncertain about whether they were considered to have dropped out.
8 The proportion of patients who reported adverse events were 32% for those on carbamazepine and 16.7% for those on clobazam. We have rounded these off to 32 and 17 per 100 respectively.
9 The proportion of patients reporting specific adverse events are listed under the subtitle Effects of Interventions in this review.
10 The neuropsychometric evaluation explain how the patients were grouped into no, mild, moderate and severe learning difficulties. Four of 25 patients randomised to carbamazepine did not have baseline evaluations which could affect the estimates of the results. The results show that three patients of 18 in the clobazam and three of 21 in the carbamazepine groups had moderate to severe learning difficulties at baseline. At the end of the trial, no patients of 17 evaluated in the clobazam group and one of 21 in the carbamazepine group had moderate to severe learning difficulties. Unfortunately, this interesting report of an improvement in cognition did not benefit from adequate reporting and there were no mean cognitive scores provided. The cause of the apparent improvement was not corroborated with seizure remission.
2.
| Levetiracetam compared with Oxcarbazepine for patients with BECTS | ||||||
|
Patient or population: Patients between three and 12 years old with clinical and EEG diagnosis of BECTS Settings: Epilepsy outpatient clinics from three tertiary centres in Italy Intervention: Levetiracetam Comparison: Oxcarbazepine | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk on Oxcarbazepine | Risk on Levetiracetam | |||||
| Proportion of patients who are seizure free at 3 months | 89 per 1001 | 100 per 1001 (83 ‐ 121) |
RR 1.13 (0.93 to 1.36) | 39 (1 study) | ⊕⊝⊝⊝ very low2 | |
| Proportion of patients who are seizure free at 12 months | 67 per 1003 | 86 per 1003 (60 ‐ 111) |
RR 1.29 (0.89 to 1.86) | 39 (1 study) | ⊕⊝⊝⊝ very low2 | |
|
Proportion of patients who discontinued treatment due to adverse events (mean follow‐up period of 18.5 months) |
56 per 1,0004 | 48 per 1,0004 (3 ‐ 612) |
RR 0.86 (0.06 to 12.75) | 39 (1 study) | ⊕⊕⊝⊝ low5 | |
| Proportion of patients with an adverse event | 11 per 1006 (2 ‐ 46) |
14 per 1006 |
RR 0.78 (0.15 to 4.15) |
39 (1 study) | ⊕⊕⊝⊝ low5 |
|
| Changes in cognitive function | Not measured | Not measured | Not measured | 39 (1 study) | See comment | The outcome was not measured in the study. |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The proportion of patients who remained seizure free at three months after randomisation was 89% for those on oxcarbazepine and 100% for those on levetiracetam. We have rounded these off to 89 and 100 per 100 respectively.
2 The study was unblinded. The age at randomisation in the two intervention groups differed causing heterogeneity that would influence the reported results. The small sample size produced a wide confidence interval resulting in imprecision of the estimated effect.
3 The proportion of patients who remained seizure free at three months after randomisation was 67% for those on oxcarbazepine and 86% for those on levetiracetam. We have rounded these off to 67 and 86 per 100 respectively.
4 The proportion of patients who discontinued treatment due to adverse events were 5.6% for those on oxcarbazepine and 4.8% for those levetiracetam. We have rounded these off to 56 and 48 per 1,000 respectively.
5 The study was unblinded and the duration when interventions were used were not fixed. This makes comparison of adverse events difficult.
6 The proportion of patients who reported adverse events were 11.1% for those on oxcarbazepine and 14.3% for those on levetiracetam. We have rounded these off to 11 and 14 per 100 respectively.
3.
| Carbamazepine compared with Topiramate for patients with BECTS | ||||||
|
Patient or population: Patients between five and 15 years old with a diagnosis of BECTS Settings: 12 centres in Korea Intervention: Carbamazepine Comparison: Topiramate | ||||||
| Outcomes | Illustrative comparative risks (CI 95%) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk on Topiramate | Risk on Carbamazepine | |||||
| Proportion of patients who are seizure free at 28 weeks | 69 per 1001 | 70 per 1001 | RR 0.98 (0.77 to 1.25) | 112 (1 study) | ⊕⊕⊝⊝ low2 | |
|
Proportion of patients who discontinued treatment due to adverse events (28 weeks trial period) |
10 per 1003 | 9 per 1003 | RR 1.12 (0.36 to 3.45) | 112 (1 study) | ⊕⊕⊝⊝ low4 | |
| Proportion of patients with an adverse event: | Not reported | Not reported | See comment | 112 (1 study) | See comment | This outcome was not reported in the study. |
|
Proportion of patients with a particular or serious adverse event: 1. Somnolence 2. Rash |
12 per 1005 2 per 1005 |
9 per 1005 15 per 1005 |
1.3 (0.4‐3.9) 8.59 (1.1 ‐ 66.5) |
112 (1 study) 112 (1 study) |
⊕⊕⊝⊝ low6 | |
|
Changes in cognitive function testing from baseline (28 weeks trial period) |
See comment | 88 (1 study) |
low ⊕⊕⊝⊝7 |
The pattern of neuropsychometric changes with topiramate seemed to be slightly worse than carbamazepine. When only those on minimum doses of both drugs were considered (n = 70), the scores for cognitive function were similar. | ||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The proportion of patients who were seizure free at 28 weeks of the trial were 68.9% for those on topiramate and 70.3% for those on carbamazepine. We have rounded these off to 69 and 70 per 100 respectively.
2The study was observer‐blinded only. It had a large proportion of patients who were lost to follow‐up in whom the results were carried forward from the last observation.
3 The proportion of patients who discontinued treatment due to adverse effects was 10.3% for those on topiramate and 9.3% for those on carbamazepine. We have rounded these off to 10 and 9 per 100 respectively.
4 The study was observer blinded though, in this case, the incomplete outcome data does not affect the outcome. The wide confidence interval makes results imprecise.
5 The proportion of patients who had somnolence as an adverse event was 12.1% for those on topiramate and 9.3% for those on carbamazepine. The proportion of patients who had rash as an adverse event was 1.7% for those on topiramate and 14.8% for those on carbamazepine. We have rounded these off to 12, 9, 2, and 15 per 100 respectively.
6 Reporting of adverse effects may have been affected as participants were not blinded. Adverse events were not documented actively by caregivers
7 There was a high loss to follow‐up which could affect the estimates of changes in cognitive function. Moreover, results were described according to the P value with no reports of the differences in mean change scores.
4.
| Sulthiame compared with placebo for patients with BECTS | ||||||
|
Patient or population: Patients between three and 10 years old with a diagnosis of BECTS Settings: 26 centres in Europe Intervention: Sulthiame Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk (Placebo) |
Corresponding risk (Sulthiame) |
|||||
| Proportion of patients who are seizure free at 3 months | 40 per 1001 | 90 per 1001 (59 ‐ 138) |
RR 2.26 (1.48 to 3.44) | 66 (1 study) | ⊕⊕⊕⊝2 moderate |
|
| Proportion of patients who are seizure free at 6 months | 26 per 1002 | 68 per 1003 (37 ‐ 126) |
RR 2.63 (1.43 to 4.86) | 66 (1 study) | ⊕⊕⊕⊝2 moderate |
|
| Time to first seizure after randomisation | Hazard ratio 7.8 (2.66 to 22.87) | 66 (1 study) |
⊕⊕⊕⊝2 moderate |
|||
| Proportion of patients who discontinued treatment due to adverse events (over 6 months trial period) | See comment | See comment | Not estimable | 66 (1 study) | See comment | 0 patients discontinued treatment due to adverse events |
| Proportion of patients with an adverse event | 43 per 1004 | 58 per 1004 (36 ‐ 95) |
RR 1.35 (0.83 to 2.2) | 66 (1 study) | ⊕⊕⊕⊝5 moderate |
|
| Changes in cognitive function | Not measured | Not measured | Not measured | 66 (1 study) | See comment | This outcome was not measured in the study. |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The proportion of patients who remained seizure free at three months after randomisation was 40% for those on placebo and 90% for those on sulthiame. We have rounded these off to 40 and 90 per 100 respectively.
2 The quality of evidence was downgraded given the limitations in the study of the small sample size affecting precision of results.
3 The proportion of patients who remained seizure free at six months after randomisation was 26% for those on placebo and 68% for those on sulthiame. We have rounded these off to 26 and 68 per 100 respectively.
4 The proportion of patients who reported adverse events were 58.1% for those on sulthiame and 42.9% for those on placebo. We have rounded these off to 58 and 43 per 100 respectively.
5 Adverse events were recorded during assessments and not actively by caregivers.
Background
Benign Epilepsy with Centro Temporal Spikes (BECTS), also called Benign Rolandic Epilepsy, is one of the most common epilepsy syndromes in children, with an annual incidence between 6.2 and 10.7 per 100,000 children. BECTS is a well recognised seizure syndrome, with onset generally between the ages of four and 10 years (Luders 1987).
Children with BECTS have normal development, and no neurological deficits. However, as BECTS is very common, it can also coincidentally present in children with neurological problems. Seizures originate from the somatosensory and motor area in the lower rolandic region. Seizures tend to occur at night, and are brief.
An attack typically starts with a somatosensory aura around the mouth, with unilateral paraesthesia of the tongue and mouth, followed by unilateral twitching of face, mouth, pharynx and larynx (Loiseau 1973). These can be associated with drooling and dysarthria. The seizure can propagate and involve the upper limb, and Todd's paralysis can follow. Consciousness is usually maintained, unless there is secondary generalisation. Secondary generalisation is rare in the day, but common at night (ILAE 1989). The focal onset of seizures during sleep can easily be missed. Seizures are generally brief, lasting from a few seconds to a few minutes. Seizure frequency is low and around 10% to 20% cases experience only a single seizure. However, in about 20% of cases, seizures occur frequently (Bouma 1997). Although in general children do not have long‐term problems associated with the seizures, some children show subtle cognitive problems (Deonna 2000). The transient, cognitive impairment seems to correlate with epileptic activity on the electroencephalogram (EEG) (Massa 2001). Some people consider BECTS as a mild form of epileptic encephalopathy in the spectrum of Landau‐Kleffner syndrome, and Electrical Status Epilepticus in Slow Wave Sleep.
The EEG background is normal, though slower rhythms can be seen in the same areas as spikes. The characteristic EEG shows high voltage spikes in the centro temporal region on the left, right or bilaterally. These spikes can also be seen in children with no epilepsy, and tend to disappear spontaneously. A family history of epilepsy is common, and siblings and parents can show sharp waves or focal epileptiform activity on their EEGs, indicating a genetic predisposition in these children.
Treatment is controversial, as the prognosis is deemed to be good. Antiepileptic drugs (AEDs) may control generalised seizures, but do not always help with the focal seizures. In most children with BECTS the epilepsy will resolve, whether treated or not. By 12 years 92% of children are in remission, and by 18 years 99.8% (Bouma 1997). Many parents and children will choose not to start medication, especially if the seizures are infrequent or only occur at night. If treatment is required, most clinicians will choose carbamazepine as a first drug of choice, although clinical experience suggests that other AEDs may be effective.
In this review, we planned to address the following questions.
Does AED treatment make a difference to seizure remission rate and cognition?
Which AED is the most effective in controlling seizures in children with BECTS?
Objectives
To evaluate whether or not treatment with AEDs changes the short‐ or long‐term outcome of children with BECTS, or both.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) that compared the use of different AEDs, or compared the use of AEDs with placebo, or both were included.
Types of participants
See: Differences between protocol and review
Children up to the age of 15 years old who presented with BECTS.
Studies that reported participants with a diagnosis of BECTS.
Types of interventions
Trials were included if they compared one treatment with another or with placebo. Specific drugs included carbamazepine, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, phenobarbital, phenytoin, sodium valproate, sulthiame, topiramate, and vigabatrin.
Types of outcome measures
The outcome measures included:
Short‐term outcome: (a) percentage of children who achieved seizure remission (i.e. seizure freedom) throughout the early period (e.g. three months) after randomisation; (b) time to first seizure after randomisation.
Medium‐term outcome: (c) percentage of children who achieved seizure remission (i.e. seizure freedom) throughout the medium term (e.g. 12 months) after randomisation.
Long‐term outcome: (d) percentage of children who remained seizure free after cessation of medication.
Adverse effects of medication: (e) percentage of children who discontinued medication because of adverse effects, (f) percentage of children with a particular adverse event occurring e.g. increased day time sleepiness, rash, or other adverse effects which are considered to be serious.
Cognitive functioning: (g) mean differences in neuropsychometric scores or resolution of specific cognitive difficulties e.g. dysgraphia on medication.
Resolution of EEG abnormalities was not included as an outcome as the clinical manifestation of seizures is probably not related to persisting EEG abnormality, though cognitive functioning may be.
Search methods for identification of studies
We searched the following databases:
Cochrane Epilepsy Group Specialized Register (30 April 2013) using the search strategy outlined in Appendix 1;
CENTRAL (The Cochrane Central Register of Controlled Trials The Cochrane Library, 2013, Issue 4: (April 2013)), using the search strategy outlined in Appendix 2;
MEDLINE (Ovid, 1946 to 30 April 2013) using the search strategy outlined in Appendix 3;
SCOPUS (30 April 2013) using the search strategy outlined in Appendix 4;
ClinicalTrials.gov (30 April 2013) using the search terms: BECTS OR ((roland* OR sylvian OR centralopathic OR centrotemporal) AND (epilep* OR seizure*))
WHO International Clinical Trials Registry Platform ICTRP (30 April 2013) using the search terms: BECTS OR rolandic OR sylvian OR centralopathic OR centrotemporal in the Title
In addition, the references from articles relevant for inclusion were handsearched by all four review authors to ensure no studies were missed from the electronic searches.
Data collection and analysis
We independently assessed all trials that appeared potentially relevant for inclusion. All four review authors independently extracted the data collected from the individual studies and three authors (HJT,RG, CDG) assessed the risk of bias according to the domains of allocation concealment, blinding, and incomplete outcome data. We compared results and resolved any disagreements by conference. Where necessary, two of the review authors (JS, HJT) contacted the original authors for clarification and obtained individual patient data. We extracted the following data from the articles.
Methodology
Case definition used. The diagnosis of BECTS is made clinically and electrophysiologically. There was variability in the studies reviewed on the descriptions of the case definition. We assumed a clinical diagnosis of BECTS was made when stated, and documented the definitions used.
Method of randomisation and concealment.
Method of (double) blinding.
Whether or not patients had been excluded from the analysis, and if so the reasons for exclusion. If data were missing, we contacted the original authors for this information.
Patient information
Number of participants allocated to each treatment group.
Mean age of participants when seizures started.
Mean age of participants when randomised.
Distribution of sex of participants.
Average frequency of seizures on randomisation (severity).
Proportion of participants who remained seizure free for three months, 12 months or other identifiable length of time from time of initiation of randomised treatment.
Time to first seizure after randomisation.
Proportion of children who remained seizure free after cessation of randomised treatment.
Proportion of participants who discontinued randomised treatment because of adverse effects.
Proportion of participants with serious or specific adverse effects, such as day time sleepiness.
Cognitive assessment, such as mean differences in cognitive function tests.
Data analysis plan The primary analysis was intention‐to‐treat, and we included all randomised participants analysed in the treatment group to which they were allocated, irrespective of the treatment they actually received. The studies were assessed as to their risk of bias according to The Cochrane Collaboration's tool. The domains assessed were: selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), and attrition bias (incomplete outcome data).
Clinical heterogeneity was assessed by reviewing clinical and methodological differences across trials, for example age and sex distribution, differences in seizure frequency/severity, and recruitment of participants in a general paediatric versus tertiary neurology setting. Subgroup regression analysis for heterogeneity was planned if there were at least 10 studies in a meta‐analysis and the pre‐specified characteristics included differences in age, drug dosages and length of treatment. The trial level treatment effect was analysed using risk ratio for dichotomous outcomes, difference in means for continuous outcomes and hazard ratio for time to event outcomes. Each estimate was presented with 95% confidence intervals. Meta‐analysis was performed for dichotomous outcomes using the Mantel‐Haenszel fixed‐effect methods, for continuous outcomes using the mean difference (MD) approach, and time‐to‐event data using the generic inverse variance method. In the case of missing data, a sensitivity analysis was performed for dichotomous outcomes where 'best‐case' and 'worst‐case' scenarios are considered with calculation of their respective relative risks.
Results
Description of studies
See: Characteristics of included studies.
The literature search of the databases retrieved 77 references as follows: 19 from the Cochrane Epilepsy Group's Specialized Register, 29 from CENTRAL, 19 from MEDLINE, seven from SCOPUS, two from Clinical Trials, and one from WHO ICTRP. After eliminating duplicates, we found nine references to six studies that met our inclusion criteria. Out of these six studies, two studies were categorised as "awaiting further classification" and will be reviewed at the next update if sufficient information is obtained. Both these studies, Bourgeois 1998 and Pelliccia 2006, were published abstracts which appeared suitable for inclusion. However, the information given was limited and the studies were not subsequently published as full‐length original articles. We contacted the authors for information about the study methodology and patient information required for this review but did not receive a reply. We intend to contact the authors before the next update of this review. This gave a total of four RCTs for inclusion in this present review with a total of 262 participants treated with six different AEDs, which were clobazam, carbamazepine, levetiracetam, oxcarbazepine, sulthiame, and topiramate.
The characteristics of the participants in each of the studies are summarised under Table 5.
1. Participant characteristics.
| Study ID | Treatment | Participant numbers | Male:Female | *Age when seizures started (years) | *Age when randomised (years) | Frequency of seizures on randomisation |
| Andrade 2009 | Clobazam | 18 | 12:6 | Not clearly stated | 9.22 +/‐ 1.3 (7‐11) | Not given (6 or more per month) |
| Carbamazepine | 25 | 10:15 | Not clearly stated | 8.4 +/‐ 1.3 (7‐10) | Not given (6 or more per month) | |
| Coppola 2007 | Levetiracetam | 21 | 11:10 | 10.2 | 10.5 | Mean 1.8 per month |
| Oxcarbazepine | 18 | 10:8 | 7.7 | 8.4 | Mean 1.5 per month | |
| Kang 2007 | Carbamazepine | 54 | 32:22 | 8.0 | 8.7 | Not given |
| Topiramate | 58 | 32:26 | 8.0 | 8.7 | Not given | |
| Rating 2000 | Sulthiame | 31 | 16:15 | 7.6 (1.9 ‐10.4) | 8.2 (3.9 – 10.7) | Median 3 (2 – 10) during 6‐months |
| Placebo | 35 | 24:11 | 7.7 (3 – 10.2) | 8.4 (3.1 – 10.3) | Median 2 (2 – 20) during 6‐months |
* Mean age (except study by Rating 2000, where median age reported)
Andrade 2009 was a randomised, open‐label, parallel trial of 45 children with a diagnosis of BECTS following the clinical and electrographical criteria of the International League Against Epilepsy (ILAE) (ILAE 1989). Children with six seizures per month or those in whom there was parental anxiety necessitating treatment were included. Exclusion criteria included children with metabolic conditions; those with a specific neurological diagnosis different to BECTS; pseudoseizures; patients on psychotropic medication; patients with active infection or cancer; and patients in whom previous treatment with either clobazam or carbamazepine was discontinued due to an adverse event (AE). Patients were randomised to treatment with either clobazam or carbamazepine. The study duration was 96 weeks, with an initial six weeks when medication was gradually increased, followed by 90 weeks on a maintenance dose. Patients were randomised to either receive clobazam with a starting dose of 1 mg/kgm/day, increased as tolerated to a maximum of 2 mg/kgm/day, or carbamazepine starting at 10 mg/kgm/day divided in three doses, and increased up to a maximum of 30 mg/kgm/day. Doses were reduced in the presence of non‐tolerable adverse effects. There were no significant differences in age or weight between the two groups. The criteria for exit from the study included patients on the maximum dosage without control of seizures, inability to tolerate the lowest dose required, recurrence of seizures after reducing the dose because of side effects, or patients/families wishing to discontinue the study. Non‐adherent patients also had the doses of the treatment drug gradually reduced until discontinuation after four weeks. Forty‐five patients (31 male, 14 female) were eligible but two were not randomised for reasons that were unclear. One of the patients randomised to clobazam, and three patients randomised to carbamazepine did not complete the study. Outcomes reported included seizure freedom during the first four weeks, between week four and week 40, and at nine months of treatment. Other outcomes were the number of patients with a reduction in seizure frequency of 50% or more at four weeks, adverse events, educational, neuropsychological, and behavioural assessments, and satisfaction with treatment.
Coppola 2007 was a randomised, open‐label, parallel, multicentre group trial involving 39 children. The inclusion criteria were patients between three and 12 years of age with a new diagnosis of BECTS according to the ILAE classification and who had not been previously treated. All participants had frequent or recurrent partial motor seizures, with or without generalisation during wakefulness in the last six months, an EEG consisting of focal or multifocal centrotemporal spikes increasing in frequency during sleep with normal background activity, and brain magnetic resonance imaging (MRI) with normal or slightly abnormal findings, and no neurological or mental deficits. Exclusion criteria included: poor compliance by parents/caregivers in keeping records of seizure frequency; adverse events or in undergoing requested clinical controls; progressive neurological and/or systemic disease; and associated pseudoseizures. Patients were seen every three months prior to randomisation for recording of awake and sleep EEGs, monitoring of drug levels, haematological, renal and liver function tests. Randomisation to levetiracetam or oxcarbazepine monotherapy was performed and the dose initiated at 5 mg/kg/day, followed by titration at 5 mg/kg every three days up to a dose of 20 mg/kg/day according to tolerance. Additional increments were allowed up to 30 mg/kg once or twice daily for levetiracetam and 35 mg/kg once or twice a daily for oxcarbazepine. From additional data supplied by the authors, two participants (one from each group) were lost to follow‐up after three months without any specified reasons, but they did not have any seizures during the treatment period. These patients' data were imputed as seizure occurrences by the authors. Patients were followed up for a mean period of 18.5 months (range three to 24 months). Outcomes reported were the percentage of patients with complete seizure freedom after the follow‐up period (mean time of 18.5 months, range 12 ‐ 24 months), and adverse effects of drug treatment. The authors provided us with details regarding the proportion of patients who were seizure free three and 12 months after randomisation.
Kang 2007 was a randomised, observer‐blinded, open‐label, parallel, multicentre trial of 112 patients. Participants, aged between five to 15 years old, were eligible if they had clinical and EEG findings compatible with benign rolandic epilepsy, at least two partial‐onset seizures during six months at baseline, a normal MRI which confirmed the absence of a progressive lesion, and at least one of the following criteria: parent and/or patient wanted to take AEDs; daytime seizures; one or more episodes of a convulsive seizure during six months. Amongst the exclusion criteria were patients previously on topiramate or carbamazepine; cognitive impairment interfering with the cognitive testing procedure; history of poor compliance with AED treatment or inability to maintain a seizure calendar. The study included a baseline phase of six months followed by a one week screening phase during which eligibility was determined. The AED doses were then escalated to the minimum target doses over a period of four weeks. Topiramate was introduced at a dose of 12.5 mg/day with the minimum target dose of 50 mg/day in patients < 30 kg and 75 to 100 mg/day in patients > 30 kg. Carbamazepine was started at a dose of 10 mg/kg/day and the minimum target dose was 20 mg/kg/day. Additional dose increments were allowed up to a maximum of 4 mg/kg/day for topiramate and 30 mg/kg/day for carbamazepine until 22 weeks. The drugs were maintained at a stable dose until completion of the study at 28 weeks. Patients in both groups were similar with regard to known prognostic factors. Thirteen participants dropped out from the topiramate group (six due to adverse effects, seven due to non‐drug‐related causes) and 11 from the carbamazepine group (five due to adverse effects, six due to non‐drug‐related causes). For patients who withdrew early from the study, the last observation reported was carried forward in the analysis. The primary study objective was to evaluate cognitive and behavioural effects of these AEDs by measuring changes in neuropsychological test batteries and behaviour‐rating scales performed at baseline and 28 weeks after treatment. Data regarding treatment‐emergent adverse effects and the percentage of patients who were seizure free after 28 weeks were recorded. This study was supported by a grant of Janssen, Korea Limited, manufacturers of topiramate.
Rating 2000 was a randomised, double‐blind, placebo‐controlled multicentre trial of 66 patients with a diagnosis of BECTS. The case definition was not specifically defined. All patients were between three and 11 years of age, weighed 10 to 50 kilograms, and had experienced two or more seizures six months prior to the study admission. Amongst the exclusion criteria were patients with severe organic diseases; a history of mental illness; relevant hypersensitivity; or relevant renal, thyroid, or hepatic dysfunction; as well as those who had prior treatment for epilepsy after the sixth month of life. Eligible patients were randomised to receive either sulthiame or a placebo. The study consisted of a six‐month historic baseline period and a six‐month double‐blind treatment phase. The dose of sulthiame was approximately 5 mg/kg/day in three divided doses and this was given without titration. Patients in the treatment and control groups were similar with regard to known prognostic factors. Both the recipients and assessors were blinded. Two patients withdrew from the sulthiame group whilst four withdrew from the placebo group. Two from the placebo group withdrew within the first week due to parental wishes and in four patients (two from each group), the trial was terminated in advance after a planned adaptive interim analysis demonstrated superiority of the intervention. This analysis was performed on an intention‐to‐treat basis after 60 patients had completed the trial with an alpha value of 0.05 and P values of <= 0.0299 or > 0.3. The missing data were imputed as seizure reoccurrences. On top of this, four patients from the sulthiame group and one patient from the placebo group had final data from the last visit assessed just days prior to the end of the experimental period. These patients were seizure free but their data have also been imputed as seizure occurrences by the authors. Outcomes reported were 1) the treatment failure events (TFEs) during the period of the trial, defined as first seizure after a seven‐day run‐in period post randomisation, intolerable adverse events, development of another epileptic syndrome, and termination from the trial by their parents or themselves, and 2) changes in EEG recordings over time.
Risk of bias in included studies
The overall risk of bias in three of the four included studies for the outcomes of seizure remission, adverse effects and cognition was judged to be high. Only one of the three included studies (Rating 2000) was found to have a low risk of bias for the outcomes of seizure remission and adverse effects. Although there was an adequate method of randomisation described, the results in the Coppola 2007 study showed that the ages at seizure onset and at randomisation were greater in those receiving levetiracetam (mean age of 10.5 years) than oxcarbazepine (mean age of 8.4 years) probably due to the small sample size. This could lead to an overestimation of the effects of levetiracetam on seizure remission.
Allocation
In the study by Rating 2000, there was adequate sequence generation (block randomisation) and allocation concealment (sealed envelopes). The sequence generation of patients to their respective interventions (computer‐generated randomisation) was considered to have a low risk of bias in the studies by Andrade 2009 and Coppola 2007 though information about allocation concealment was not provided in both studies. Additionally, Andrade 2009 reports that two patients were not randomised but reasons for this were not provided. In the study by Kang 2007, the randomisation process and allocation concealment were not described.
Blinding
One study was double‐blinded (Rating 2000), one observer‐blinded (Kang 2007), and two were open‐label unblinded studies (Andrade 2009 and Coppola 2007). The method in Kang 2007 where recipients were not blinded would have minimal influence on the study's primary outcome measure of cognition. However, the risk of bias on reported outcomes of seizure remission and adverse drug effects from participants and caregivers would be high.
Incomplete outcome data
All participants were analysed in the groups to which they were randomised. All four studies reported patients withdrawing from their allocated treatments. The number of patients with incomplete outcome data was small and balanced across intervention groups in Coppola 2007 and Rating 2000. Kang 2007 reported 24 (21.4%) patients lost to follow‐up due to adverse effects and non‐drug‐related causes. There were discrepancies within the report of the number of patients who withdrew from their assigned treatment group in Andrade 2009 and five patients were reported to have been excluded although it is unclear when in the study this took place. In Andrade 2009, Coppola 2007, and Rating 2000, participants who withdrew early were imputed by the authors as having had seizures. In Kang 2007, the last observation reported was carried forward and the participants were considered as seizure free.
A sensitivity analysis (reported in Table 6) was performed with best, reported, and worst scenarios to determine the effect of incomplete outcome data on estimates of seizure remission. In Coppola 2007, patients only withdrew three months after randomisation and hence, the outcome at three months would not be affected.
2. Sensitivity analysis.
| Study | Outcome reported | Intervention | Best outcome | Best outcome RR (CI) | Reported outcome | Reported outcome RR (CI) |
Worst outcome | Worst outcome RR (CI) |
| Andrade 2009 | Seizure free at 4‐40 weeks | Clobazam Carbamazpine |
13/18 = 72% 16/25 = 64% |
1.13 (0.75 ‐ 1.7) |
12/18 = 66.7% 16/25 = 64% |
1.04 (0.67 ‐ 1.62) |
12/18 = 66.7% 18/25 = 72.2% |
0.93 (0.62 ‐ 1.39) |
| Andrade 2009 | Seizure remission in last 9 months of study | Clobazam Carbamazepine |
17/18=94.4% 21/25 = 84% |
1.12 (0.92 ‐ 1.38) |
16/18 = 88.9% 21/25 = 84% |
1.06 (0.84, 1.34) |
16/18 = 88.9% 22/25 = 88% |
1.01 (0.81 ‐ 1.26) |
| Coppola 2007 | Seizure remission at 3 months | Levetiracetam Oxcarbazepine |
21/21 = 100% 16/18 = 88.9% |
1.13 (0.93 ‐ 1.36) |
21/21 = 100% 16/18 = 88.9% |
1.13 (0.93 ‐ 1.36) |
21/21 = 100% 16/18 = 88.9% |
1.13 (0.93 ‐ 1.36) |
| Coppola 2007 | Seizure remission at 12 months | Levetiracetam Oxcarbazepine |
19/21 = 90.5% 12/18 = 66.7% |
1.36 (0.95 ‐ 1.94) |
18/21=85.7% 12/18 = 66.7% |
1.29 (0.89 ‐ 1.86) |
18/21 = 85.7% 13/18 = 72.2% |
1.19 (0.85 ‐ 1.66) |
| Kang 2007 | Seizure remission at 28 weeks | Carbamazepine Topiramate |
38/54 = 70.3% 27/58 = 46.6% |
1.51 (1.09 ‐ 2.09) |
38/54 = 70.3% 40/58 = 68.9% |
1.02 (0.8 ‐ 1.3) |
27/54 = 50% 40/58 = 68.9% |
0.72 (0.53 ‐ 1) |
| Rating 2000 | Seizure remission at 3 months | Sulthiame Placebo |
29/31 = 93.5% 14/35 = 40% |
2.34 (1.54 ‐ 3.55) |
28/31 = 90.3% 14/35 = 40% |
2.26 (1.48 ‐ 3.44) |
28/31 = 90.3% 16/35 = 45.7% |
1.98 (1.35 ‐ 2.89) |
| Rating 2000 | Seizure remission at 6 months | Sulthiame Placebo |
27/31 = 87.1% 9/35 = 25.7% |
3.39 (1.9 ‐ 6.04) |
21/31 = 67.7% 9/35 = 25.7% |
2.63 (1.43 ‐ 4.86) |
21/31 = 67.7% 14/35 = 40% |
1.69 (1.06 ‐ 2.72) |
In the sensitivity analysis, "best outcome" imputes an outcome for missing data with replacement values favouring the intervention reported to have the higher relative risk of seizure remission, whilst "worst outcome" imputes values for missing data favouring the intervention reported to have the lower relative risk of seizure remission. The analysis shows that seizure remission was not influenced by the withdrawal of participants in the studies by Andrade 2009, Coppola 2007 and Rating 2000. This was because the numbers were small and were balanced between the groups. In Kang 2007, the excessive drop‐out rate in this study could affect the estimate of seizure remission in the topiramate and carbamazepine groups.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
The characteristics of the participants are summarised under Table 5.
Clobazam versus Carbamazepine
There was one study (Andrade 2009).
Effects on seizure remission from 4 ‐ 40 weeks after randomisation: 12 (66.7%) of 18 patients treated with clobazam were seizure free during this period compared with 16 (64%) of 25 patients treated with carbamazepine, giving an RR (relative risk) of 1.04 with 95% CI of 0.67 ‐ 1.62.
Effects on seizure remission in the last nine months of the study period (total 96 weeks): 16 (88.9%) of 18 patients treated with clobazam had no seizures during this period of the study compared to 21 (84%) of 25 patients treated with carbamazepine, giving an RR of 1.06 with 95% CI of 0.84 ‐ 1.34.
Time to first seizure after randomisation was not reported as an outcome in this study.
Effects on seizure remission after cessation of medication were not reported as an outcome in this study.
Adverse effects of medication:three (16.7%) of the 18 patients on clobazam and eight (32%) of the 25 patients on carbamazepine reported 35 and 76 different adverse effects respectively. For clobazam and carbamazepine groups respectively, these include vertigo (16.7% vs. 20%), headaches (11.2% vs. 20%), somnolence (16.7% vs. 12%), nausea/vomiting (16.7% vs. 12%), diarrhoea (5.6% vs. 4%), tremor (16.7% vs. 4%), and fatigue (16.7% vs. 8%). One patient each from the clobazam (5.6%) and carbamazepine groups (4%) withdrew from the study due to rash and somnolence respectively.
Effects on cognitive functioning: Neuropsychometric evaluation was performed using the Weschler intelligence scale for children at baseline and at the end of the study. The numbers of patients categorised as having no, mild, moderate or severe learning difficulties were reported at both time points though three patients in the group assigned to carbamazepine did not have an evaluation. At baseline, there were three (16.7%) of 18 patients in the clobazam group with moderate to severe learning difficulties and three (14.3%) of 21 in the carbamazepine group. This difference was not significant (Mann Whitney test, p=0.4). There was no significant difference observed at the end of study between groups, although an improvement was noted: none of 17 patients assessed in the clobazam group assessed and one (4.8%) of 21 patients in the carbamazepine group were shown to have moderate to severe learning difficulties.
Levetiracetam versus Oxcarbazepine
There was one study (Coppola 2007).
Effects on seizure remission (seizure freedom) for three months after randomisation: all 21 (100%) patients treated with levetiracetam were seizure free during the three months after randomisation compared with 16 (88.9%) out of 18 patients treated with oxcarbazepine, giving a RR of 1.13 with 95% CI of 0.93 ‐ 1.36.
Effects on seizure remission (seizure freedom) for 12 months after randomisation: 18 (88.7%) out of 21 patients treated with levetiracetam were seizure free during the 12 months after randomisation compared with 12 (66.7%) of 18 patients treated with oxcarbazepine, giving a RR of 1.29 with 95% CI of 0.89 ‐ 1.86.
Time to first seizure after randomisation was not reported as an outcome in this study.
Effects on seizure remission after cessation of medication were not reported as an outcome in this study.
Adverse effects of medication: one (4.8%) of 21 patients on levetiracetam and one (5.6%) of 18 on oxcarbazepine discontinued medication because of adverse effects. Three (14.3%) children on levetiracetam reported adverse effects, which included mild decrease in appetite (2), and moderate decrease in appetite with daily frontal headaches (1). Two (11.1%) patients on oxcarbazepine reported adverse effects, which included headache (1), and sedation (1).
Effects on cognitive functioning were not reported as an outcome in this study.
Carbamazepine versus Topiramate
There was one study (Kang 2007).
Effects on seizure remission (seizure freedom) for 28 weeks after randomisation: 40 (68.9%) of the 58 patients randomised to receive topiramate and 38 (70.3%) of the 54 patients randomised to receive carbamazepine were seizure free during the experimental period of 28 weeks, giving a RR 1.02 with 95% CI of 0.8 ‐ 1.3.
Effects on seizure remission for three months after randomisation were not reported as an outcome in this study.
Effects on seizure remission for 12 months after randomisation were not reported as an outcome in this study. The double‐blind phase was only for 28 weeks.
Time to first seizure after randomisation was not reported as an outcome in this study.
Effects on seizure remission after cessation of medication were not reported as an outcome in this study.
Adverse effects of medication: six (10.3%) of the 58 patients treated with topiramate and five (9.3%) of the 54 patients treated with carbamazepine discontinued medication because of adverse effects. The adverse effects in the six on topiramate were anhidrosis (3), anorexia (1), enuresis (1), and rash (1), whilst the adverse effects in the five on carbamazepine were rash (4) and tiredness (1). Amongst all 58 patients treated with topiramate, seven had somnolence, three anhidrosis, two psychomotor slowing, two anorexia, two gastrointestinal disturbance, two paresthesia, and one each had dizziness, tiredness, enuresis, and rash. Amongst all 54 patients treated with carbamazepine, eight had a rash, five somnolence, five tiredness, five increased appetite, and one dizziness.
Effects on cognitive functioning: a neuropsychological test battery consisted of Bender Gestalt Test (BGT) and KEDI‐WISC (Korean Educational Developmental Institute – Wechsler Intelligence Scale for Children), the Korean version of WISC‐R. Eighty eight patients completed the trial and had neuropsychological testing; 45 in the topiramate group and 43 in the carbamazepine group.The results were reported as either an improvement or a worsening of scores from baseline to end point with comparisons made between topiramate and carbamazepine. Statistical analyses was made using the Student's t‐test to compare changes over time for the two treatment groups. The authors reported p values and 95% confidence intervals based on the t‐distribution and pooled estimate of variance. Although there were negative and positive trends in the cognitive variables measured for the two AEDs, only two out of twelve subtests showed statistical significance. The arithmetic subtest showed more deterioration in those on topiramate (p = 0.037; 95% CI: 0.07–2.12) whilst the maze test showed a greater improvement in those on carbamazepine (p = 0.026; 95% CI 0.20‐3.10). When the 30 patients on topiramate and the 40 on carbamazepine who had maintained the minimum target doses were compared, the scores were similar between the two groups, with only object assembly showing statistically significant improvement in those on topiramate compared to worsening scores in those on carbamazepine (p=0.006; 95% CI: 0.20‐4.10).
Sulthiame versus placebo
There was one study (Rating 2000).
Effects on seizure remission (seizure freedom) for three months after randomisation: The study reported treatment failure events as the primary effectiveness variable. The study authors kindly provided individual patient data on seizure remission at three and six months. Twenty eight (90.3%) out of 31 patients treated with sulthiame were seizure free during the first three months compared with 14 (40%) out of 35 patients on placebo, giving a RR of 2.26 with 95% CI of 1.48 ‐ 3.44.
Effects on seizure remission (seizure freedom) for six months after randomisation: 21 (67.7%) out of 31 patients treated with sulthiame were seizure free during the six‐month trial period compared with nine (25.7%) out of 35 patients on placebo, giving a RR of 2.63 with 95% CI of 1.43 ‐ 4.86.
Effects on seizure remission for 12 months after randomisation were not reported as an outcome in this study. The double‐blind phase was only for six months.
Time to first seizure after randomisation: The authors provided us with the estimated log hazard ratio and standard error using the Cox analysis model. The hazard ratio was 7.8 with 95% CI of 2.66 ‐ 22.87.
Effects on seizure remission using Kaplan‐Meier analysis: Following our correspondence with the authors, they used the Kaplan‐Meier analysis to account for patients who terminated or were analysed prior to the end of the study. Please refer to Figure 1. The proportion of patients treated with sulthiame who remained seizure free were 1.000 on Day 28, 0.935 at three months, and 0.902 at six months whilst that of patients on placebo were 0.800 on Day 28, 0.457 at three months, and 0.396 at six months.
1.

Sulthiame versus placebo (Rating 2000): Kaplan‐Meier plot of seizure free patients
Effects on seizure remission after cessation of medication were not reported as an outcome in this study.
Adverse effects of medication: None of the patients in this study discontinued medication because of adverse effects. Eighteen (58.1%) of the 31 children on sulthiame and fifteen (42.9%) of the 35 children on placebo reported adverse events. The adverse effects which were reported more than once per group included two on sulthiame who reported fatigue, two others on sulthiame who reported loss of strength, and two on placebo who reported leukopenia.
Effects on cognitive functioning were not reported as an outcome in this study.
Discussion
Despite the fact that BECTS is one of the most common epilepsy syndromes in school‐aged children, we found only four RCTs suitable for our analysis containing 262 participants.
All four RCTs in this review used different methodologies. The quality of the evidence ranged from moderate to very low quality. Only the trial by Rating 2000 was judged to be of a sufficient quality. However, the weakness in Rating 2000 was its small sample size (66 patients) which affects the precision of the result. The other three trials (Andrade 2009, Coppola 2007 and Kang 2007) did not have or report allocation concealment and were not double‐blinded. The study by Andrade 2009 had a small sample size (43 patients) and was an open‐label unblinded trial with some inaccuracies in the reporting. The study by Coppola 2007 had a small sample size (39 patients) and was an open‐label unblinded trial without a fixed follow‐up period. The study by Kang 2007 was an open‐label, observer‐blinded study with a high drop‐out rate. All three trials compared different AED treatments. Due to the varying treatments and methodologies, a meta‐analysis could not be performed.
After extracting the required data, the trial by Rating 2000 comparing sulthiame with placebo found that significantly more patients on sulthiame were seizure free for three and six months after treatment compared to those on placebo. The time to first seizure after randomisation was also longer for patients on sulthiame. Sulthiame was well tolerated with no significant difference between sulthiame and placebo for withdrawal due to adverse effects.
The other three trials comparing carbamazepine with clobazam, levetiracetam with oxcarbazepine, and topiramate with carbamazepine, were of a low to very low quality. The wide confidence intervals in the point estimates for seizure remission show that there is insufficient information about the superiority of the AEDs compared. The effects on cognitive functioning were assessed in the trial between topiramate and carbamazepine and in the study between carbamazepine and clobazam. In the former, this found changes in a few subtests to be significantly worse in those on topiramate but outcomes were similar when only individuals on minimum target doses were compared. This study did not report the actual differences between the neuropsychometric scores. In the latter study, there was no significant difference found between the groups in the moderate/severe categories of learning disability and neuropsychometric scores were not reported.
The assessment of adverse events reported whilst on AEDs taken from RCTs alone, as performed in this review, does not provide a comprehensive range of adverse events especially those that may be rare but important. The possibility of AEDs resulting in worsening of seizures was not examined in this review. Reliable data about cognitive effects is currently lacking. When considering the cognitive effects in BECTS, it would be useful to assess data from studies which compare no treatment/placebo with an effective AED. There was a lag‐time of more than one year between the search date and the publication of the review. This may introduce some bias as there may be additional studies not included in this review. However, it is our aim to perform frequent updates as we expect an increase in research in this area in the coming years.
In summary, there is some evidence that sulthiame is effective in controlling seizures in the short‐term in children with BECTS. However, the study had a small sample size which can affect precision of the results. Currently, use of sulthiame is uncommon in many parts of the world thus limiting its applicability. The trials which compared two AEDs did not inform us of which AED was superior as far as seizure remission in BECTS is concerned. There is still no evidence from the RCTs reviewed regarding whether choice of AED treatment in BECTS makes a difference to seizure remission in the medium or longer term. Regarding the area of cognitive effects, there is also insufficient evidence comparing those on different AED treatments and to date no RCTs have been performed comparing the effects of treatment with no treatment/placebo.
Authors' conclusions
Implications for practice.
Treatment with antiepileptic drugs can be effective in producing seizure remission in children with BECTS and there is some evidence for remission in the short term with sulthiame. There were no significant differences in adverse effects which resulted in discontinuation of treatment. Currently, there is insufficient evidence about the use of AEDs upon which to base practice. The decisions about when to treat and which antiepileptic drugs to use need careful consideration for each individual patient and an informed discussion with parents and care‐givers. Until further research is conducted, this decision should take into account the intrusiveness of seizures, whilst acknowledging the uncertainties of the effects of treatment on cognition.
Implications for research.
There is a need for good quality randomised‐controlled trials to provide evidence for the optimum management of children with BECTS. Factors that reduced the quality of studies in this review included inadequate blinding of participants and/or personnel, inconsistencies in the reporting of results, large numbers of patients lost to follow‐up (in one study), and small sample sizes. There are difficulties with arranging RCTs of longer duration. However, future trials could aim at measuring seizure remission in the medium term to longer term, including after withdrawal of antiepileptic drugs. Cognitive function is an important outcome measure due to the concerns of cognitive impairment with BECTS. Antiepileptic drugs can contribute positively or negatively towards cognitive performance and this effect would be best measured in placebo‐controlled trials performed over a period of at least 12 months.
Notes
Prior to the publication of this review, the literature searches were re‐run (28th June 2014). One potentially relevant study was identified (Borggraefe 2013). Due to time constraints we were unable to consider this for inclusion in this review version. We will address it for the next update.
Acknowledgements
The authors would like to thank Rachael Kelly, Managing Editor, and Diane Horsley, former Managing Editor of the Cochrane Epilepsy Group; Ellen Dougan, Editorial Assistant; Alison Beamond and Graham Chan, Trial Search Co‐ordinators; Catrin Tudur‐Smith , statistician for the Cochrane Review Group; for all their support in this review and Ivan Sola Arnau, administrator from the Iberoamerican Cochrane Centre in Barcelona for his help in translating a Spanish paper. We would also like to thank the authors of the included studies for clarifying information on their studies.
Appendices
Appendix 1. Cochrane Epilepsy Group Specialized Register search strategy
#1 MeSH DESCRIPTOR Epilepsy, Rolandic Explode All WITH BL CF CI CL CO CN DI DH DT EC EM EN EP EH ET GE HI IM ME MI MO NU PS PA PP PC PX RA RI RT RH SU TH US UR VE VI
#2 BECTS
#3 (roland* OR sylvian OR centralopathic OR centrotemporal) AND (epilep* OR seizure*)
#4 (#1 OR #2 OR #3)
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor Epilepsy, Rolandic explode all trees
#2 (benign rolandic epilep*)
#3 (sylvian epilep*)
#4 (centralopathic epilep*)
#5 (centrotemporal epilep*)
#6 roland* NEXT epilep*
#7 roland* NEXT seizure*
#8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7)
Appendix 3. MEDLINE search strategy
This strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomized trials published in Lefebvre 2009.
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. clinical trials as topic.sh.
6. randomly.ab.
7. trial.ti.
8. 1 or 2 or 3 or 4 or 5 or 6 or 7
9. exp animals/ not humans.sh.
10. 8 not 9
11. exp Epilepsy, Rolandic/
12. benign rolandic epilep$.tw.
13. sylvian epilep$.tw.
14. centralopathic epilep$.tw.
15. centrotemporal epilep$.tw.
16. (roland$ adj (epilep$ or seizure$)).tw.
17. 11 or 12 or 13 or 14 or 15 or 16
18. 10 and 17
Appendix 4. SCOPUS search strategy
(TITLE‐ABS‐KEY(antiepilep* or anticonvulsant* or AED* or Acetazolamide or Alodorm or Arem or Ativan or Barbexaclone or Beclamide or Brivaracetam or Carbagen or Carbamazepine or Celontin or Cerebyx or Chloracon or Clobazam or Clonazepam or Clonex or Clorazepate or Convulex or Depacon or Depak* or Depamide or Desitin or Diacomit or Diamox or Diastat or Diazepam or Dilantin or Diphenin* or Diphenylhydantoin or Divalpr* or Dormicum or Ecovia or Emeside or Epanutin or Epiject or Epilim or Episenta or Epival or Eptoin or Ergenyl or Erimin or Eslicarbazepine or Ethadione or Ethosuximide or Ethotoin or Ethylphenacemide or Exalief or Excegran or Ezogabine or Fanatrex or Felbamate or Felbatol or Fosphenytoin or Frisium or Fycompa or Gabapentin or Gabarone or Gabitril or Gabrene or Gralise or Hibicon or Hypnovel or Inovelon or Insoma or Intensl or Keppra or Klonopin or Kriadex or Lacosamide or Lamict* or Lamitor or Lamitrin or Lamogine or Lamotrigine or Lamotrine or Levetiracetam or Liskantin or Loraz or Lorazepam or Luminal or Lyrica or Mebaral or Mephenytoin or Mephobarbit* or Mephyltaletten or Mesantoin or Mesuximide or Methazolamide or Methsuximide or Methylphenobarbit* or Midazolam or Mogadon or Mylepsinum or Mysoline or Neogab or Neptazane or Neurontin or Nimetazepam or Nitrados or Nitrazadon or Nitrazepam or Normison or Novo‐Clopate or Nupentin or Nydrane or Onfi or Orfiril or Orlept or Ormodon or Ospolot or Oxcarbazepine or Pacisyn or Paraldehyde or Paramethadione or Paxadorm or Paxam or Peganone or Perampanel or Petinutin or Petril or Phemiton or Phenacemide or Pheneturide or Phenobarbit* or Phensuximide or Phenytek or Phenytoin or Posedrine or Potiga or Pregabalin or Primidone or Prodilantin or Progabide or Prominal or Prysoline or Ravotril or Remacemide or Remnos or Resimatil or Restoril or Retigabine or Riv?tril or Rufinamide or Sabril or Seclar or Selenica or Seletracetam or Sertan or Somnite or Stavzor or Stedesa or Stiripentol or Sulthiam* or Sultiam* or Talampanel or Tegretol or Temazepam or Temesta or Teril or Tiagabine or Timonil or Topamax or Topiramate or Tranxene or Tridione or Trileptal or Trimethadione or Trobalt or Urbanol or Valance or Valcote or Valium or Valnoctamide or Valparin or Valpro* or Versed or Vigabatrin or Vimpat or Zalkote or Zarontin or Zebinix or Zonegran or Zonisamide)) AND (TITLE((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") PRE/2 (trial OR method OR procedure OR study)) OR ABS((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") PRE/2 (trial OR method OR procedure OR study))) AND (TITLE‐ABS‐KEY(BECTS OR ((roland* OR sylvian OR centralopathic OR centrotemporal) AND (epilep* OR seizure*))))
Data and analyses
Comparison 1. Clobazam versus Carbamazepine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of patients who are seizure free from 4‐40 weeks | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.67, 1.62] |
| 2 Proportion of patients who had seizure remission in the last 9 months of the study | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.84, 1.34] |
| 3 Proportion of patients who discontinued due to adverse events | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.05, 10.76] |
| 4 Proportion of patients who reported adverse events | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.59, 6.25] |
1.1. Analysis.

Comparison 1 Clobazam versus Carbamazepine, Outcome 1 Proportion of patients who are seizure free from 4‐40 weeks.
1.2. Analysis.

Comparison 1 Clobazam versus Carbamazepine, Outcome 2 Proportion of patients who had seizure remission in the last 9 months of the study.
1.3. Analysis.
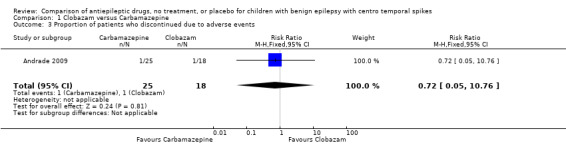
Comparison 1 Clobazam versus Carbamazepine, Outcome 3 Proportion of patients who discontinued due to adverse events.
1.4. Analysis.

Comparison 1 Clobazam versus Carbamazepine, Outcome 4 Proportion of patients who reported adverse events.
Comparison 2. Levetiracetam versus Oxcarbazepine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of patients who are seizure free at 3 months | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.93, 1.36] |
| 2 Proportion of patients who are seizure free at 12 months | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.89, 1.86] |
| 3 Proportion of patients who discontinued treatment due to adverse events | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.06, 12.75] |
| 4 Proportion of patients who reported adverse events | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.24, 6.86] |
2.1. Analysis.

Comparison 2 Levetiracetam versus Oxcarbazepine, Outcome 1 Proportion of patients who are seizure free at 3 months.
2.2. Analysis.

Comparison 2 Levetiracetam versus Oxcarbazepine, Outcome 2 Proportion of patients who are seizure free at 12 months.
2.3. Analysis.

Comparison 2 Levetiracetam versus Oxcarbazepine, Outcome 3 Proportion of patients who discontinued treatment due to adverse events.
2.4. Analysis.
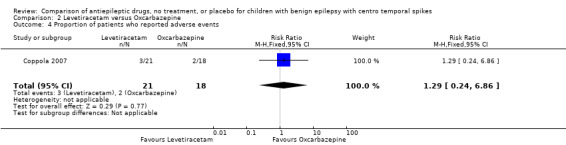
Comparison 2 Levetiracetam versus Oxcarbazepine, Outcome 4 Proportion of patients who reported adverse events.
Comparison 3. Carbamazepine versus Topiramate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of patients who are seizure free at 28 weeks | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.80, 1.30] |
| 2 Proportion of patients who discontinued treatment due to adverse events | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.29, 2.76] |
| 3 Proportion of patients who have somnolence as adverse event | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.44, 3.86] |
| 4 Proportion of patients who have rash as an adverse event | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.59 [1.11, 66.45] |
3.1. Analysis.

Comparison 3 Carbamazepine versus Topiramate, Outcome 1 Proportion of patients who are seizure free at 28 weeks.
3.2. Analysis.

Comparison 3 Carbamazepine versus Topiramate, Outcome 2 Proportion of patients who discontinued treatment due to adverse events.
3.3. Analysis.

Comparison 3 Carbamazepine versus Topiramate, Outcome 3 Proportion of patients who have somnolence as adverse event.
3.4. Analysis.

Comparison 3 Carbamazepine versus Topiramate, Outcome 4 Proportion of patients who have rash as an adverse event.
Comparison 4. Sulthiame versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of patients who are seizure free at 3 months | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.48, 3.44] |
| 2 Proportion of patients who are seizure free at 6 months | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.43, 4.86] |
| 3 Proportion of patients who reported adverse events | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.83, 2.20] |
| 4 Time to first seizure after randomisation | 1 | Hazard Ratio (Fixed, 95% CI) | 7.80 [2.66, 22.87] |
4.1. Analysis.

Comparison 4 Sulthiame versus placebo, Outcome 1 Proportion of patients who are seizure free at 3 months.
4.2. Analysis.
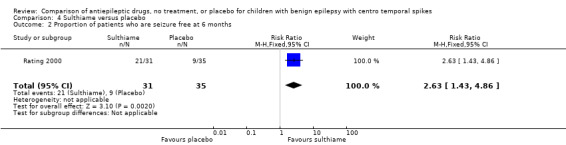
Comparison 4 Sulthiame versus placebo, Outcome 2 Proportion of patients who are seizure free at 6 months.
4.3. Analysis.

Comparison 4 Sulthiame versus placebo, Outcome 3 Proportion of patients who reported adverse events.
4.4. Analysis.
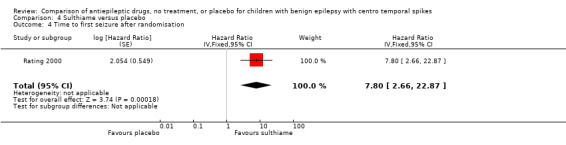
Comparison 4 Sulthiame versus placebo, Outcome 4 Time to first seizure after randomisation.
Characteristics of studies
Characteristics of included studies [author‐defined order]
Andrade 2009.
| Methods | Randomised‐controlled, open‐label trial, 96‐weeks treatment phase | |
| Participants | 43 children with a diagnosis of BECTS, six or more seizures in a month or if parental anxiety | |
| Interventions | Clobazam monotherapy starting dose of 1 mg/kg/day (maximum of 5 mg/kg/day) versus Cabamazepine monotherapy starting does 10 mg/kg/day (maximum of 30 mg/kg/day) | |
| Outcomes | Outcomes considered in the review: effects on seizure freedom between 4‐40 weeks treatment; effects on seizure remission in the last 9 months of study; adverse effects, effects on cognition. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | This was generated using a computer programme using a minimisation procedure. |
| Allocation concealment (selection bias) | Unclear risk | Information about allocation concealment was not provided. |
| Incomplete outcome data (attrition bias) Objectively‐measured outcomes (e.g. seizure remission, cognition) | High risk | The missing data were imputed as seizure occurrence. However, there were some inconsistencies in the reporting of numbers of patients who had dropped out. Five patients were reported to have been excluded and these were not accounted for in the groups to which they had been allocated. Also, two patients were not randomised to the trial for reasons that were unclear. The above reasons lend a high risk of bias to the accuracy of reporting for the objectively‐measured outcomes. |
| Incomplete outcome data (attrition bias) Subjectively‐measured outcomes (e.g. adverse effects) | High risk | The reasons above affect the subjectively‐measured outcomes similarly. |
| Blinding of participants and personnel (performance bias) Objectively‐measured outcomes (e.g. seizure remission, cognition) | High risk | The study was an open‐label one with no blinding performed. There is a high risk of bias both for patients reporting seizure outcome. |
| Blinding of participants and personnel (performance bias) Subjectively‐measured outcomes (e.g. adverse effects) | High risk | The study was an open‐label one with no blinding performed. There is a high risk of bias for patients and personnel reporting adverse effects. |
| Blinding of outcome assessment (detection bias) Objectively‐measured outcomes (e.g. seizure remission, cognition) | High risk | For seizure remission and cognition, the personnel assessing these were unblinded and this would introduce a high risk of bias. |
| Blinding of outcome assessment (detection bias) Subjectively‐measured outcomes (e.g. adverse effects) | High risk | As participants/families who were unblinded were involved in reporting on these outcomes, this is likely to affect outcome. |
Coppola 2007.
| Methods | Randomised, prospective, open‐label trial; mean follow‐up period 18.5 months (range 12‐ 24 months) | |
| Participants | 39 participants with a diagnosis of BECTS according to the ILAE classification and with consistent EEG features; ages three to 14 years old | |
| Interventions | Levetiracetam (target dose 20 mg/kg/day, maximum 30 mg/kg/day) versus Oxcarbazepine (target dose 20 mg/kg/day, maximum 35 mg/kg/day) monotherapy | |
| Outcomes | 1) Outcomes and time‐points considered in the review: effects on seizure remission at three and 12 months, time to first seizure after randomisation, adverse effects of medication. 2) Outcomes and time‐points measured (reported) in the study: complete cessation of seizures and adverse effects of drugs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random Sample Generator from the Epistat program". |
| Allocation concealment (selection bias) | Unclear risk | Comment: Information about allocation concealment was not provided. |
| Incomplete outcome data (attrition bias) Objectively‐measured outcomes (e.g. seizure remission, cognition) | Low risk | There were no missing data for the outcome measure of seizure remission. |
| Incomplete outcome data (attrition bias) Subjectively‐measured outcomes (e.g. adverse effects) | Low risk | There were no reasons for missing data provided for two participants (one from each group). Even if one of the participants withdrew secondary to adverse effects, this would not have a clinically relevant impact on the intervention effect estimate. |
| Blinding of participants and personnel (performance bias) Objectively‐measured outcomes (e.g. seizure remission, cognition) | High risk | Quote: " prospective, open‐label, pilot trial". Comment: Neither the participants or personnel were blinded. This is likely to influence their expectations of the allocated treatment in producing seizure remission |
| Blinding of participants and personnel (performance bias) Subjectively‐measured outcomes (e.g. adverse effects) | High risk | Participants and personnel were not blinded. This is likely to affect their perception of adverse effects. |
| Blinding of outcome assessment (detection bias) Objectively‐measured outcomes (e.g. seizure remission, cognition) | High risk | The participants' parents and/or caregivers who were recording seizure frequency/relapse were not blinded. This is likely to affect outcome. |
| Blinding of outcome assessment (detection bias) Subjectively‐measured outcomes (e.g. adverse effects) | High risk | The participants' parents and/or caregivers who were recording adverse events were not blinded. This is likely to affect outcome. |
Kang 2007.
| Methods | Randomised, open‐label, observer‐blinded, parallel group trial; 28 weeks treatment phase | |
| Participants | 112 participants with clinical and EEG findings compatible with benign rolandic epilepsy from 12 centres in Korea, ages five to 15 years old | |
| Interventions | Topiramate (minimum dose 50‐75 mg/day, maximum 4 mg/kg/day) versus Carbamazepine (minimum dose 20 mg/kg/day, maximum 30 mg/kg/day) monotherapy | |
| Outcomes | 1) Outcomes and time‐points considered in the review: effects on seizure remission at 28 weeks, adverse effects of medication, effects on cognitive functioning. 2) Outcomes and time‐points measured (reported) in the study: changes on neuropsychological test battery after 28 weeks maintenance treatment; percentage of patients seizure free, treatment‐emergent adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each study center received a separate and independent randomization plan". Comment: The method of randomisation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | Comment: The method of allocation concealment was not described. |
| Incomplete outcome data (attrition bias) Objectively‐measured outcomes (e.g. seizure remission, cognition) | High risk | Quote: "the statistical analysis was based on the intent‐to‐treat population...the last observation...carried forward". Comment: Even though seizure freedom was analysed on an intention‐to‐treat basis, there is a risk of bias with carrying forward the last observation. The significant proportion of missing outcomes compared to observed event risk would be enough to induce clinically relevant bias in measurement of seizure remission. See sensitivity analysis Table 1. Quote: "none of the 25 patients that dropped out had cognitive complaints". Comment: The proportion of patients who had missing cognitive outcome measures is significant and although these patients had no complaints, cognitive testing detects some of the subtle domains of cognitive function. |
| Incomplete outcome data (attrition bias) Subjectively‐measured outcomes (e.g. adverse effects) | Low risk | The risk of bias for adverse effects is low having been accounted for in those who withdrew. |
| Blinding of participants and personnel (performance bias) Objectively‐measured outcomes (e.g. seizure remission, cognition) | High risk | Quote: "open‐label, observer‐blinded". Commment: The participants were not blinded. It was not specified if the observers who were blinded were those assessing effects on seizure outcome as well as those performing the neuropsychological tests. There is a high risk of differential behaviour which can impact on outcome. |
| Blinding of participants and personnel (performance bias) Subjectively‐measured outcomes (e.g. adverse effects) | High risk | The participants were not blinded. This is likely to affect their perception of adverse effects. |
| Blinding of outcome assessment (detection bias) Objectively‐measured outcomes (e.g. seizure remission, cognition) | Unclear risk | For seizure remission, the participants/families reporting seizure occurrences were not blinded and it is unclear if the observers assessing this were blinded. For cognition, the observers performing the neuropsychological tests were blinded and there would be a low risk of bias here. |
| Blinding of outcome assessment (detection bias) Subjectively‐measured outcomes (e.g. adverse effects) | High risk | As participants/families who were unblinded were involved in reporting on these outcomes, this is likely to affect outcome. |
Rating 2000.
| Methods | Randomised‐controlled trial, six months double‐blind treatment phase | |
| Participants | 66 children with a diagnosis of BECTS from 26 centres in Europe aged between three and 10 years | |
| Interventions | Sulthiame monotherapy 5 mg/kg/day in three divided doses versus placebo | |
| Outcomes | 1) Outcomes and time‐points considered in the review: effects in seizure remission at three and six months; time to first seizure after randomisation, adverse effects of medication; 2) Outcomes and time‐points measured (reported) in the study: Rate of Treatment Failure Events (TFEs); changes in EEG recording over time | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...by dividing patients in blocks of four according to a pre‐prepared list." Comment: Blocked randomisation was used and the authors informed us that a random sequence generator was used. |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed, coded envelopes...held by the investigator for emergency access only." Comment: The authors informed us that there was adequate allocation concealment which includes sequentially numbered (and opened), opaque envelopes. |
| Incomplete outcome data (attrition bias) Objectively‐measured outcomes (e.g. seizure remission, cognition) | Low risk | The missing data were imputed as seizure occurrence. Even considering the various possibilities of seizure freedom or occurrence in those with missing data, there would be no clinically relevant impact on the intervention effect estimate. |
| Incomplete outcome data (attrition bias) Subjectively‐measured outcomes (e.g. adverse effects) | Low risk | The numbers were small and equally distributed between the intervention arms. |
| Blinding of participants and personnel (performance bias) Objectively‐measured outcomes (e.g. seizure remission, cognition) | Low risk | Quote: "6‐month double blind treatment phase" Participants and personnel involved in outcome assessment were blinded to intervention arm. |
| Blinding of participants and personnel (performance bias) Subjectively‐measured outcomes (e.g. adverse effects) | Low risk | Participants and personnel were blinded to intervention arm. |
| Blinding of outcome assessment (detection bias) Objectively‐measured outcomes (e.g. seizure remission, cognition) | Low risk | Participants and personnel were blinded to intervention arm. |
| Blinding of outcome assessment (detection bias) Subjectively‐measured outcomes (e.g. adverse effects) | Low risk | Participants and personnel were blinded to intervention arm. |
Characteristics of studies awaiting assessment [ordered by study ID]
Bourgeois 1998.
| Methods | Randomised, double‐blind, placebo‐controlled study; 36‐week treatment period |
| Participants | 225 children four to 13 years old with BECTS |
| Interventions | Gabapentin (30 mg/kg/day) versus placebo |
| Outcomes | Time to Treatment Failure Event (TFE) |
| Notes |
Pelliccia 2006.
| Methods | Randomised study; abstract does not state blinding or length of study |
| Participants | Children with rolandic epilepsy |
| Interventions | Sodium valproate versus Carbamazepine versus allergen‐free diet |
| Outcomes | Complete seizure remission, EEG normalisation, adverse effects |
| Notes |
BECTS: benign epilepsy with centro temporal spikes EEG: electroencephalogram
Differences between protocol and review
The title of the review was altered from that of the original proposal. In the protocol, we had set out to compare the use of different AEDs, or the use of AEDs and placebo. In writing the review, we decided to include studies which compared AEDs with no treatment as well. This did not alter our search methods and did not lead to the inclusion of any new study.
The protocol had stated that the review would include children up to the age of 12 years old who have presented with BECTS. Due to the small number of RCTs found, we decided that the criteria for the age of participants could be extended to include children up to the age of 15 years old.
The protocol had also stipulated that studies would be included if the case definition of participants included a history suggestive of BECTS and an EEG showing centro temporal spikes. As not all studies had a clear case definition of participants or reported compatible EEG changes, we accepted the diagnosis of BECTS made.
We also carried out a sensitivity analysis for dichotomous data by looking at 'best‐case' and 'worst‐case' scenarios and considered if missing data influenced the results.
We did not carry out searches on the EMBASE, Pharmline, and Micromedex databases as initially reported in our protocol.
Contributions of authors
Christian DeGoede was responsible in designing the review as well as writing the protocol. Rajat Gupta assisted in the design of the review and writing the protocol. Jaspal Singh helped analyse trials suitable for inclusion. Hui Jeen Tan was responsible for writing the review. All authors were also involved in screening search results and retrieved papers, collecting and analysing data, and editing the review.
Declarations of interest
Hui Jeen Tan received an educational travel scholarship from UCB Pharma in 2011.
New
References
References to studies included in this review
Andrade 2009 {published data only}
- Andrade R, Garcia‐Espinosa A, Machado‐Rojas A, Garcia‐Gonzalez ME, Trapaga‐Quincoses O, Morales‐Chacon LM. A prospective, open, controlled and randomised study of clobazam versus carbamazepine in patients with frequent episodes of rolandic epilepsy. [Estudio prospectivo, abierto, controlado y aleatorizado de clobazam frente a carbamacepina en pacientes con crisis frecuentes de epilepsia rolandica.]. Revista de Neurologia 2009;49(11):581‐6. [PubMed] [Google Scholar]
Coppola 2007 {published data only}
- Coppola G, Franzoni E, Verrotti A, Garane C, Sarajlija J, Operto F, et al. Levetiracetam or oxcarbazepine as monotherapy in newly diagnosed benign epilepsy of childhood with centrotemporal spikes (BECTS): an open‐label, parallel group trial. Brain and Development 2007;29:281‐4. [DOI] [PubMed] [Google Scholar]
- Coppola G, Franzoni E, Verrotti A, Garane C, Sarajlija J, Operto F, et al. Levetiracetam or oxcarbazepine as monotherapy in newly diagnosed benign rolandic seizures in children: an open‐label, parallel group study. Epilepsia 2006;47 Suppl 3:179‐80. [Google Scholar]
Kang 2007 {published data only}
- Kang H‐C, Eun B‐L, Lee CW, Moon HK, Kim J‐S, Kim DW, et al. A multicenter, randomized, open‐labeled, clinical study to evaluate the effect on cognitive and behavioral function of topiramate compared with carbamazepine as monotherapy in children with benign rolandic epilepsy. Epilepsia 2006;47 Suppl 4:138. [Google Scholar]
- Kang HC, Eun BL, Wu Lee C, Ku Moon H, Kim JS, Wook Kim D, et al. The effects on cognitive function and behavioural problems of topiramate compared to carbamazepine as monotherapy for children with benign rolandic epilepsy. Epilepsia 2007;48(9):1716‐23. [DOI] [PubMed] [Google Scholar]
- Lim K, Kim HD, Korean BRE Study Group. Low‐dose topiramate compared with carbamazepine in treating benign rolandic epilepsy. Epilepsia 2004;45 Suppl 7:322‐3. [Google Scholar]
Rating 2000 {published data only}
- Rating D, Wolf C, Bast T. Sulthiame as monotherapy in children with benign childhood epilepsy with centrotemporal spikes: a 6‐month randomized, double‐blind, placebo‐controlled study. Epilepsia 2000;41(10):1284‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bourgeois 1998 {published data only}
- Bourgeois B, Brown LW, Pellock JM, Buroker M, Greiner M, Garofalo EA, et al. Gabapentin (Neurontin) monotherapy in children with benign childhood epilepsy with centrotemporal spikes (BECTS): a 36‐week, double‐blind, placebo‐controlled study. Epilepsia 1998;39(Suppl 6):163, Abstract no: 5.067. [Google Scholar]
Pelliccia 2006 {published data only}
- Pelliccia A, Frediani S, Frediani T, Lucarelli S. Rolandic epilepsy: comparison of allergen‐free diet and conventional treatments. Epilepsia 2006;47(Suppl 3):68, Abstract no: p256. [Google Scholar]
Additional references
Borggraefe 2013
- Borggraefe I, Bonfert M, Bast T, Neubauer BA, Schotten KJ, Maßmann K, et al. Levetiracetam vs. sulthiame in benign epilepsy with centrotemporal spikes in childhood: a double‐blinded, randomized, controlled trial (German HEAD Study). European journal of paediatric neurology 2013;5:507‐14. [DOI] [PubMed] [Google Scholar]
Bouma 1997
- Bouma PA, Bovenkerk AC, Westendorp RG, Brouwer OF. The course of benign partial epilepsy of childhood with centrotemporal spikes: a meta‐analysis. Neurology 1997;48(2):430‐7. [DOI] [PubMed] [Google Scholar]
Deonna 2000
- Deonna T, Zesiger P, Davidoff V. Benign partial epilepsy of childhood, a longitudinal neuropsychological and EEG study of cognitive function. Developmental Medicine and Child Neurology 2000;42:595‐603. [DOI] [PubMed] [Google Scholar]
ILAE 1989
- Commission of the International League against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30:389‐99. [DOI] [PubMed] [Google Scholar]
Lefebvre 2009
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009). The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Loiseau 1973
- Loiseau P, Beaussart M. The seizures of benign childhood epilepsy with Rolandic paroxysmal discharges. Epilepsia 1973;14(4):381‐9. [DOI] [PubMed] [Google Scholar]
Luders 1987
- Luders H, Lesser RP, Dinner DS, Morris HH. III Benign focal epilepsy of childhood. In: Luders H, Lesser RP editor(s). Electroclinical Syndromes. Berlin: Springer Verlag, 1987:609‐619. [Google Scholar]
Massa 2001
- Massa R, Saint‐Martin A, Carcangiu R. EEG criteria predictive of cognitive complications in idiopathic focal epilepsy with rolandic spikes. Neurology 2001;57:1071‐9. [DOI] [PubMed] [Google Scholar]


