Abstract
Background
Colorectal cancer is one of the most common types of cancer in the Western world. Apart from surgery ‐ which remains the mainstay of treatment for resectable primary tumours ‐ postoperative (i.e., adjuvant) chemotherapy with 5‐fluorouracil (5‐FU) based regimens is now the standard treatment in Dukes´ C (TNM stage III) colon tumours i.e. tumours with metastases in the regional lymph nodes but no distant metastases. In contrast, the evidence for recommendations of adjuvant therapy in rectal cancer is sparse. In Europe it is generally acknowledged that locally advanced rectal tumours receive preoperative (i.e., neoadjuvant) downstaging by radiotherapy (or chemoradiotion), whereas in the US postoperative chemoradiotion is considered the treatment of choice in all Dukes´ C rectal cancers. Overall, no universal consensus exists on the adjuvant treatment of surgically resectable rectal carcinoma; moreover, no formal systematic review and meta‐analysis has been so far performed on this subject.
Objectives
We undertook a systematic review of the scientific literature from 1975 until March 2011 in order to quantitatively summarize the available evidence regarding the impact of postoperative adjuvant chemotherapy on the survival of patients with surgically resectable rectal cancer. The outcomes of interest were overall survival (OS) and disease‐free survival (DFS).
Search methods
CCCG standard search strategy in defined databases with the following supplementary search. 1. Rect* or colorect* ‐ 2. Cancer or carcinom* or adenocarc* or neoplasm* or tumour ‐ 3. Adjuv* ‐ 4. Chemother* ‐ 5. Postoper*
Selection criteria
Randomised controlled trials (RCT) comparing patients undergoing surgery for rectal cancer who received no adjuvant chemotherapy with those receiving any postoperative chemotherapy regimen.
Data collection and analysis
Two authors extracted data and a third author performed an independent search for verification. The main outcome measure was the hazard ratio (HR) between the risk of event between the treatment arm (adjuvant chemotherapy) and the control arm (no adjuvant chemotherapy). The survival data were either entered directly in RevMan or extrapolated from Kaplan‐Meier plots and then entered in RevMan. Due to expected clinical heterogeneity a random effects model was used for creating the pooled estimates of treatment efficacy.
Main results
A total of 21 eligible RCTs were identified and used for meta‐analysis purposes. Overall, 16,215 patients with colorectal cancer were enrolled, 9,785 being affected with rectal carcinoma. Considering patients with rectal cancer only, 4,854 cases were randomized to receive potentially curative surgery of the primary tumour plus adjuvant chemotherapy and 4,367 to receive surgery plus observation. The mean number of patients enrolled was 466 (range: 54‐1,243 cases). 11 RCTs had been performed in Western countries and 10 in Japan. All trials used fluoropyrimidine‐based chemotherapy (no modern drugs ‐ such as oxaliplatin, irinotecan or biological agents ‐ were tested).
Overall survival (OS) data were available in 21 RCTs and the data available for meta‐analysis regarded 9,221 patients: of these, 4854 patients were randomized to adjuvant chemotherapy (treatment arm) and 4,367 patients did not receive adjuvant chemotherapy (control arm). The meta‐analysis of these RCTs showed a significant reduction in the risk of death (17%) among patients undergoing postoperative chemotherapy as compared to those undergoing observation (HR=0.83, CI: 0.76‐0.91). Between‐study heterogeneity was moderate (I‐squared=30%) but significant (P=0.09) at the 10% alpha level.
Disease‐free survival (DFS) data were reported in 20 RCTs, and the data suitable for meta‐analysis included 8,530 patients. Of these, 4,515 patients were randomized to postoperative chemotherapy (treatment arm) and 4,015 patients received no postoperative chemotherapy (control arm). The meta‐analysis of these RCTs showed a reduction in the risk of disease recurrence (25%) among patients undergoing adjuvant chemotherapy as compared to those undergoing observation (HR=0.75, CI: 0.68‐0.83). Between‐study heterogeneity was moderate (I‐squared=41%) but significant (P=0.03).
While analyzing both OS and DFS data, sensitivity analyses did not find any difference in treatment effect based on trial sample size or geographical region (Western vs Japanese). Available data were insufficient to investigate on the effect of adjuvant chemotherapy separately in different TNM stages in terms of both OS and DFS. No plausible source of heterogeneity was formally identified, although variability in treatment regimens and TNM stages of enrolled patients might have played a significant role in the difference of reported results.
Authors' conclusions
The results of this meta‐analysis support the use of 5‐FU based postoperative adjuvant chemotherapy for patients undergoing apparently radical surgery for non‐metastatic rectal carcinoma. Available data do not allow us to define whether the efficacy of this treatment is highest in one specific TNM stage. The implementation of modern anti‐cancer agents in the adjuvant setting is warranted to improve the results shown by this meta‐analysis. Randomized trials of adjuvant chemotherapy for patients receiving preoperative neoadjuvant therapy are also needed in order to define the role of postoperative chemotherapy in the multimodal treatment of resectable rectal cancer.
Keywords: Humans; Antineoplastic Agents; Antineoplastic Agents/therapeutic use; Biological Products; Biological Products/administration & dosage; Camptothecin; Camptothecin/administration & dosage; Camptothecin/analogs & derivatives; Chemotherapy, Adjuvant; Chemotherapy, Adjuvant/methods; Fluorouracil; Fluorouracil/administration & dosage; Irinotecan; Organoplatinum Compounds; Organoplatinum Compounds/administration & dosage; Oxaliplatin; Postoperative Period; Randomized Controlled Trials as Topic; Rectal Neoplasms; Rectal Neoplasms/drug therapy; Rectal Neoplasms/surgery
Plain language summary
Postoperative adjuvant chemotherapy in rectal cancer operated for cure
The use of chemotherapy after curative surgery for non metastatic rectal cancer is widely used in the US, but not in Europe. This systematic review and meta‐analysis, which is the first in this field, shows a significant beneficial effect on both overall (OS) and disease‐free survival (DFS) for patients undergoing postoperative chemotherapy after removal of their primary rectal tumour. Further investigation is needed to define the role of postoperative chemotherapy in the multimodal treatment of patients with rectal carcinoma: for instance, modern anti‐cancer agents (including so called "smart drugs") and integration with neoadjuvant therapy (such as preoperative chemoradiation) should be taken into consideration in order to improve the encouraging findings of this meta‐analysis.
Background
Although colorectal cancer is one of the most common cancers in the Western world, the current practice of postoperative adjuvant chemotherapy of resectable but advanced rectal cancer (i.e. Dukes´ C adenocarcinoma of the rectum which is defined as the lower 16 cm of the large bowel down to the anal verge or as the bowel at or below the sacral promontory) is not based on solid evidence and thus varies around the world (NCCN 2008, Glimelius 2010, Watanabe 2008, Wills 2001, Glimelius 2004). The local recurrence rate without adjuvant therapy of resected Tany, N+, M0 (Dukes´ C) rectal cancer (for TNM and Dukes´classification see table 2 and 3 (SBU 2001)) is reported as high as 45‐65% (before the surgical procedure with total mesorectal excision (TME) was generally applied) (Minsky 2001). When local failure does occur, life expectancy and quality of life is affected, so preventing local failure is an important end point in the treatment of rectal cancer. The staging of tumours according to Dukes´ or the TNM classification is used for prognostic assessment, but has been modified as described by Astler‐Coller (SBU 2001) as tumour penetrating into adjacent organs but classified as Dukes´ B seems to have as bad a prognosis as the localized tumour with regional lymphatic metastasis classified as Dukes´ C. Thus in many recent papers Astler‐Coller stage B2 and C are considered and analysed as a prognostic entity. Different surgical techniques have been used, and total mesorectal excision (TME) has reduced the recurrence rate considerably, but cannot stand alone (Beart 2001, Kronborg 1998). The use of pre‐ or postoperative radiation and chemoradiation as adjuvant therapy has lowered the incidence of local recurrence (Wolpin 2007) as well as mortality (Wong 2007).
Despite these improvements there might be potential for adjuvant postoperative chemotherapy to eliminate circulating tumour cells and micro‐ metastases. During the 1970´s several trials with 5‐Fluorouracil (5‐FU), semustine and vincristine (MOF) given intravenously were reported to increase survival in advanced colon cancer, whereas it failed to show any improvement compared with surgery alone in localized resectable colorectal tumours (SBU 2001). In the 1980´s the NSABP C‐03 protocol compared MOF treatment with 6 months of 5‐FU combined with the 5‐FU modulating agent Leucovorin in Dukes´ B and C colon cancers. A significant improvement in disease free survival and overall survival, more pronounced in Dukes´C than in Dukes´B cancers, was found. Later trials have confirmed this effect on Dukes´ C cancers compared to surgery alone, whereas the difference in Dukes´ B cancers is less certain (Beart 2001). Another 5‐FU modulating agent, the anti‐parasitic drug levamisole has been tested and with a treatment for 12 months showed an advantage in survival of 68% versus 48% compared with 5‐FU alone (SBU 2001). These results correspond with the results of 6 months with 5‐FU and Leucovorin. Leucovorin or levamisole in combination with 5‐FU has now become the standard treatment for Dukes´ C colon cancer on both sides of the Atlantic Ocean. In later years 5‐FU has been combined with oxaliplatin (FOLFOX). It is a treatment which is well tolerated and with few side effects. However, none of these trials found a significant effect on rectal cancer. Some chemotherapy agents e.g. 5‐FU have been shown to sensitize the malignant cells to radiotherapy (Dobelbower 1991). A combination of postoperative radiation and chemotherapy with the MOF regimen has been tested only in small series in rectal cancer. It has shown little or no difference in 5‐year survival but is none the less recommended as a standard treatment in the USA (SBU 2001). In the later years the preoperative neoadjuvant treatment has become increasingly popular ‐ especially in Europe reducing local recurrence and probably cancer related death (Wong 2007).
In earlier trials colon and rectal cancers were treated and analysed as a unity, even though trials showed a very different behaviour of seemingly identical tumours depending on location. In the rectum there are more local recurrences and fewer distant metastases (Compton 2001). Moreover rectal cancer responds differently to chemotherapy than does colon cancer. This has been attributed to the differences of the lymphatic drainage in the two parts of the large bowel and to the anatomy, with the rectum placed in the tight pelvis surrounded with loose connective tissue, and the colon in free peritoneum draining directly into the large vessels (Compton 2001). Since 1990 the Gastrointestinal Tumour Study group and the Mayo/North Central Cancer Treatment Group have recommended postoperative 5‐FU alone or in combination to all patients with T3, N1‐2 rectal cancer. In Europe preoperative radiation was primarily reserved for tumours of the rectum that have extended into adjacent tissue and thus are too fixed to be removed by immediate surgery. Preoperative radiotherapy is now given to many patients in a number of European countries. This preoperative downstaging consists of a short but potent course of radiotherapy which usually makes the tumour shrink so that it can be removed by subsequent surgery. In Europe postoperative chemotherapy regimens are reserved for patients with colon cancer (Wills 2001) and centres with experimental protocols for postoperative adjuvant therapy for rectal cancer.
In recent decades, substantial progress has been made in the treatment of rectal cancer. Improved preoperative staging and new surgical techniques have played a major role in improving treatment outcome (NCCN 2008, Enker 1995,Kapiteijn 2002). Furthermore, in locally advanced stages of rectal carcinoma, i.e. stage II (node‐negative disease with transmural invasion. T3‐4, N0, M0) and stage III (node‐positive disease. Any T, N1‐2, M0), surgery is often supported by combined modality therapy to further reduce the risk of local and distant recurrence.
Precise anatomical excision of the rectum and its mesentery (total mesorectal excision (TME)) is now considered the cornerstone of adequate treatment for rectal cancer and has led to improvements in local recurrence rate and long‐term outcomes (Kapiteijn 2002).
Several studies have shown a further decrease in local failure rates when adding radiotherapy or chemoradiation before or after surgery, and some of these studies have been able to show a benefit regarding overall survival (OS) and disease free survival (DFS) when surgery is combined with radiation (Kapiteijn 2001, SECT 1997, Skibber 2001) or chemoradiation (GTSG 1985, Krook 1991, Mohiuddin 2006, Janjan 2001, Bosset 2005).
In most cases chemotherapy when combined with radiation remains fluoropyrimidine‐based (NCCN 2008). Often a continuous infusion of 5‐FU is recommended although capecitabine, an oral 5‐FU prodrug, is considered a valid alternative during radiotherapy (Glynne‐Jones 2006, Dunst 2008, Kim 2005).
There is evidence that preoperative chemoradiation therapy as compared with postoperative chemoradiation therapy in patients with advanced rectum cancer, improves local control, and is associated with reduced toxicity and at least similar survival endpoints (Sauer 2004, Roh 2004). Based on these results, preoperative treatment with chemoradiation in patients with stage II and III rectal cancer has recently become the preferred approach in Europe and in the USA.
With the improved local control, locoregional relapse is now exceeded by the rate of development of systemic metastases (Gerard 2006, Bujko 2004, Bosset 2006). The major question arising is whether patients with locally advanced rectum cancer should be offered postoperative adjuvant chemotherapy, which is regarded by some as standard treatment for all patients with resectable stage II and III rectal cancer after preoperative treatment (NCCN 2008, Guillem 2008, NIH CC 1990), while others suggest adding postoperative chemotherapy to a selected group of patients with negative prognostic markers (Das 2006, Fietkau 2006).
The efficacy of adjuvant chemotherapy in rectal cancer is still unclear, partly because many studies addressing this subject include patients with colon cancer, despite important differences in the clinical behaviour of these two distinct diseases (Madoff 2008).
Although the effect of adjuvant chemotherapy is assumed to be similar in rectal and colon cancer, there is little direct randomized evidence to support this.
The Quasar trial (Quasar CG 2007) found a small significant improvement in survival when patients with stage II colorectal cancer were treated with adjuvant 5‐FU and folinic acid. None of the patients had been treated with chemotherapy prior to the operation and approximately 50% of the patients had received radiotherapy. The EORTC trial 22921 (Bosset 2006 (EORTC),Collette 2007) showed no significant benefit in terms of DFS or OS from the addition of postoperative 5‐FU and leucovorin when given to patients with cT3‐4, M0 rectal cancer. A subgroup analysis was able to show that delivery of adjuvant chemotherapy prolonged both time to relapse and the survival time when given to patients whose disease had been down‐staged to pT0‐2 by preoperative treatment. Accordingly Nora et al. (Janjan 2001) showed that the administration of adjuvant 5‐FU chemotherapy improved cancer specific survival, but only among patients who responded to preoperative chemoradiation.
In contrast Japanese studies of Uracil‐Tegafur (UFT, an oral fluoropyrimidine) did suggest an advantage to postoperative adjuvant chemotherapy in rectal cancer (Sakamoto 2007 (JFMTC15‐1), Tanaka 2007). The patients in this study did not receive neoadjuvant treatment.
Though the evidence of additional postoperative adjuvant chemotherapy remains controversial, the idea of using non‐cross resistant chemotherapy in the postoperative setting has emerged and the combination of 5‐FU and oxaliplatin (FOLFOX), which has shown superiority over 5‐FU and Leucovorin in adjuvant treatment of colon cancer, is now widely used as adjuvant treatment in locally advanced rectum cancer as well (NCCN 2008, Rödel 2007).
In order to clarify perspectives and establish evidence‐based recommendations for future postoperative treatment of resectable rectal cancer, a thorough critical review of the literature is performed.
Objectives
Since no consensus exists on this subject, we undertook a systematic review of the scientific literature from 1975 till now (March 2011) in order to quantitatively summarize the available evidence regarding the impact of postoperative adjuvant chemotherapy on the survival of patients with surgically resectable rectal cancer. The main outcomes of interest were overall survival (OS) and disease‐free survival (DFS). Treatment related toxicity was also evaluated.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCT) comparing patients undergoing radical surgery for non‐metastatic rectal cancer (Tany, Nany, M0) who received no adjuvant chemotherapy with those receiving any postoperative chemotherapy regimen. Only RCT where the selection criteria are thoroughly reported were considered.
Types of participants
Patients of both genders and of all ages who were surgically treated with curative intent for non‐metastatic rectal cancer (Tany, Nany, M0) and received any kind of postoperative adjuvant chemotherapy compared to patients with the same disease but receiving no postoperative treatment. Patients with locally resectable cancers, with no local spread or regional metastasis (Dukes´ A and B1) and patients with distant metastasis (Dukes´ D) were only included if the different stagegroups could not be separated.
Types of interventions
All regimens of postoperative chemotherapy (IV, (bolus or continuous infusion) intraportal infusion and oral administration of single or multi agent chemotherapy) were compared to control group, receiving no treatment or placebo. Patients receiving preoperative radiotherapy who were subsequently randomized to postoperative chemotherapy or a control group both with or without radiotherapy were included and results assessed separately for the effect of chemotherapy only.
Types of outcome measures
The main outcome measures were hazard ratios (HR) for both overall (OS) and disease‐free survival (DFS).
Adverse effects of the treatment (e.g., nausea, vomiting, stomatitis, anorexia, diarrhoea, alopecia, leucopenia, thrombocytopenia and anaemia) were also recorded, if available.
Finally, we verified whether the reports described Quality‐of‐Life and cost‐effectiveness.
Search methods for identification of studies
We searched the following databases from 1970 and prospectively up to March 2011:
a) EMBASE
b) Medline via PUBMED
c) Cochrane Collaboration Central Register
d) CancerLit
e) CCCG specialized register
We used the following search terms:
1. Rect* or colorect*
2. Cancer or carcinom* or adenocarc* or neoplasm*
3. Adjuv*
4. Chemother*
5. Postoper*
Searching Pubmed and CancerLit, the above search strategy was combined with the Cochrane Collaboration optimally sensitive Medline search strategy for identifying randomised clinical trials. EMBASE was searched manually for randomised clinical trials. Literature references in reviews and papers are searched manually for additional papers.
Data collection and analysis
Eligibility of the retrieved articles was assessed by title and abstract. If this information were insufficient for eligibility assessment, the full article was reviewed. All authors participated in the dataextraction proces and independent search for verification. After all eligible studies had been identified, the reviewers independently performed a quality evaluation of the heterogeneity between studies, randomization, blinding and follow‐up as well as data‐extraction. The reviewers achieved consensus on all results considered for the final analysis.
Meta‐analysis was performed following the Cochrane Handbook (Higgins 2008) and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines (PRISMA). Standard meta‐analysis methods (Sutton 2000) were applied to evaluate the overall effect of postoperative chemotherapy on DFS and OS.
The main outcome measure was the hazard ratio (HR) between the risk of event in the treatment arm (adjuvant chemotherapy) and the control arm (no adjuvant chemotherapy). 95% confidence intervals (CI) were used as a measure of estimate uncertainty. Survival data (HR) were either entered directly in RevMan or extrapolated from Kaplan‐Meier plots using the methods described by Parmar (Parmar 1998). Due to expected clinical heterogeneity a random effects model was used for creating the pooled estimates of efficacy.
The consistency of results (effect sizes) among studies was assessed using the two standard heterogeneity tests, that is, the chi‐squared based Cochran Q‐test and the I‐squared statistic (Higgins 2002). To be more conservative, we considered that heterogeneity was statistically significant when the Cochran Q‐test P‐value was less than 0.1 (i.e., the alpha level of significance for this test was set at 10%). In addition, inconsistency across studies was considered low, moderate and high for I‐squared values lower than 25%, between 25% and 50% and greater than 50%, respectively.
Sensitivity analyses (i.e., subgroup analysis and leave‐one‐out procedure) were used to reveal potential sources of heterogeneity. Predefined subgroups were defined by geographical origin of the trial (ethnicity) and sample size. In order to assess the effect of inadequate sample size, we conducted a sensitivity analysis by excluding small studies. The definition of "small studies" was based on the minimum sample size needed to identify an HR equal to 2 with a statistical power equal to 80% in an ideal trial where patients are allocated to treatment or observation with a 1:1 randomization ratio: with these settings, studies enrolling fewer than 100 patients per arm were excluded. The extent to which the combined risk estimate might be affected by individual studies was assessed by consecutively omitting every study from the meta‐analysis (leave‐one‐out procedure).
Funnel plot was used to detect the so‐called "small‐study effect". Publication and selection biases in meta‐analysis are more likely to affect small studies, which also tend to be of lower methodological quality: this may lead to a smaller study effect, where the smaller studies in a meta‐analysis show larger treatment effects; furthermore, small‐study effect may also arise because of between‐trial heterogeneity (Sterne 2000). Funnel plot asymmetry was formally investigated with the Egger linear regression approach and the Begg rank correlation test: to be more conservative, we considered these tests statistically significant when the P‐value was less than 0.1. The impact of small‐study effect bias on the summary effects was formally assessed by means of the the trim‐and‐fill method described by Duval and Tweedie (Duval 2000).
Statistical analyses were performed using the Cochrane dedicated software RevMan and STATA (version SE 11.1, STATA, College Station, TX, USA).
Quality of life assessments were included only if assessed by acknowledged QoL scoring systems such as SF 36 or EORTC QLQ C‐30 (Ware 1992, Aaronson 1993).
Protocol and review were peer reviewed by the Cochrane Colorectal Cancer Group for publication in the Cochrane Library.
Results
Description of studies
A total of 21 eligible RCTs were identified and used for meta‐analysis purposes. Overall, 16,215 patients with colorectal cancer were enrolled, 9,785 being affected with rectal carcer. Considering patients with rectal cancer only (the target population of this meta‐analysis), 4,854 cases were randomized to receive potentially curative surgery of the primary tumour plus adjuvant chemotherapy and 4,367 to receive surgery plus observation. The mean number of patients enrolled was 466 (range: 54‐1243 cases). 11 RCTs had been performed in Western countries and 10 in Japan. All trials used fluoropyrimidine‐based chemotherapy (no modern drugs ‐ such as oxaliplatin, irinotecan or biological agents ‐ were tested), the systemic route of administration being virtually always adopted.
The main features of these RCT are described below and in the dedicated Tables (see Characteristics of Included Studies section).
1) US trial: this multicentre RCT was carried out on behalf of the Central Oncology Group (COG) and its findings were published in 1981 (Grage 1981). Patients with colorectal cancer (n=134) received apparently radical surgery of the primary tumour and were randomized to one of the following two arms:
‐ observation (n=67)
‐ postoperative adjuvant chemotherapy (n=67)
Systemic chemotherapy regimens consisted of 5‐FU alone. The authors provided separate data for colon and rectal cancer (n=64), which enabled us to include this study in the present meta‐analysis.
A survival advantage was observed for patients with stage III colorectal cancer and those with rectal cancer.
2) NSABP trial: this US multicentre RCT was carried out on behalf of the National Surgical Adjuvant Breast and Bowel Project (NSABP) and the results were published in 1988 (Fisher 1988 (NSABP)). Patients with rectal cancer only received apparently radical surgery of the primary tumour and were randomized to one of the following three arms:
‐ observation (n=191)
‐ adjuvant radiotherapy (n=190)
‐ adjuvant chemoradiation (n=193)
Systemic chemotherapy was a combination of 5‐FU, semustine (methyl‐CCNU)and vincristine (MOF). Chemotherapy provided a survival advantage (both OS and DFS) only in males. Radiotherapy improved DFS but not OS.
3) GTSG trial: this US national RCT was performed on behalf of the Gastrointestinal Tumor Study Group (GTSG) and the results were presented in 1985 (GTSG 1985) and 1988 (Thomas 1988 (GTSG)). Patients with rectal cancer only underwent radical surgery for the primary tumour and were randomized to one of the following four arms:
‐ observation (n=58)
‐ adjuvant chemotherapy (n=48)
‐ adjuvant radiotherapy (n=50)
‐ adjuvant radio‐chemotherapy (n=46)
Systemic chemotherapy consisted of a combination of 5‐FU with semustine. In this meta‐analysis we compared patients randomized to adjuvant chemotherapy (n=48) versus those randomized to observation (n=58)
4) Swedish trial: the results of this multicentre RCT were published in 1990 (Hafström 1990). Patients with colorectal cancer undergoing radical surgery for the primary tumour (n=331) were randomized to one of the following two arms:
‐ observation (n=176)
‐ adjuvant chemotherapy (n=155)
These investigators used a combination systemic chemotherapy regimen including 5‐FU, lomustine (CCNU) and vincristine. The authors provided separate data for colon and rectal cancer (n=99), which enabled us to include this study in the present meta‐analysis.
No survival advantage was reported. 29 patients (out of 147 who started chemotherapy) discontinued the treatment due to toxicity (mainly gastrointestinal); in 30 patients treatment was interrupted due to early disease recurrence.
5) NCCTG trial: this US multicentre RCT was carried out on behalf of the North Central Cancer Treatment Group (NCCTG) and the results were published in 1991 (Krook 1991 (NCCTG)). Patients with rectal cancer only received apparently radical surgery of the primary tumor and were randomized to one of the following two arms:
‐ adjuvant radiotherapy (n=100)
‐ adjuvant radiotherapy associated with chemotherapy (n=104)
These investigators used a combination systemic chemotherapy regimen including 5‐FU and semustine (methyl‐CCNU). The combination of postoperative local therapy with radiation plus fluorouracil and systemic therapy with a fluorouracil‐based regimen significantly improved the outcome of patients with locally advanced rectal cancer as compared with postoperative radiation alone.
6) SGACCS trial: this Japanese multicentre RCT was carried out on behalf of the Cooperative Study Group of Surgical Adjuvant Chemotherapy for Colorectal Cancer (SGACCS) and the authors published the results in 1991 (Matsuda 1991 (SGACCS)). Patients undergoing curative surgery for colorectal cancer were randomized to one of the following two arms:
‐ observation (n=1182)
‐ adjuvant chemotherapy (n=1295)
Chemotherapy consisted of Tegafur (an oral fluoropyrimidine) plus intravenous mitomycin‐C (MMC). The original article is in Japanese: for this meta‐analysis, the data were retrieved from an individual patient data (IPD) meta‐analysis (Sakamoto 1999) where patients with rectal cancer treated with surgery alone (n=598) were compared with those treated with adjuvant chemotherapy (n=645).
7) CCCSGJ trial: this Japanese multicentre RCT was carried out on behalf of the Colorectal Cancer Chemotherapy Study Group of Japan (CCCSGJ) and its findings were published in 1995 (CCCSGJ 1995). Patients with rectal cancer only received apparently radical surgery of the primary tumour and were randomized to one of the following three arms:
‐ observation (n=335)
‐ adjuvant systemic chemotherapy (n=323)
‐ adjuvant intra‐arterial chemotherapy (with MMC) + adjuvant systemic chemotherapy (n=346)
The systemic chemotherapy regimen consisted of MMC combined with 5‐FU.
The authors concluded that adjuvant use of long term oral 5‐FU and intermittent MMC (i.v.) improves the survival rate of patients with curatively resected rectal cancer; regional chemotherapy made no contribution to reducing hepatic recurrence of colon cancer or local recurrence of rectal cancer.
8) Austrian trial: in this single centre RCT, whose results were published in 1996 (Kornek 1996), patients with rectal cancer only underwent radical surgery and were randomized to one of the following two arms:
‐ observation (n=29)
‐ adjuvant chemotherapy (n=28)
In this trial, adjuvant treatment consisted of a combination of locoregional chemotherapy (mitoxantrone, administered within the pelvic cavity) and systemic chemotherapy with leucovorin + 5‐FU. An advantage for the chemotherapy arm was observed in terms of both OS and DFS; this benefit appeared greater in stage II rectal cancer.
9) TSGHCFU trial: this Japanese multicentre RCT was conducted on behalf of the Tokai Study Group on HCFU (TSGHCFU), the findings being published in 1996 (Ito 1996 (TSGHCFU)). Patients undergoing curative surgery for colorectal cancer (n=170) were randomized to one of the following two arms:
‐ observation (n=83)
‐ postoperative chemotherapy (n=87)
Systemic chemotherapy consisted of HCFU (1‐hexylcarbamoyl‐5‐fluorouracil, an oral fluoropyrimidine). The authors provided separate data for colon and rectal cancer (n=77), which enabled us to include this study in the present meta‐analysis.
10) JFMTC 7‐2 trial: this Japanese multicentre RCT was performed on behalf of the Japanese Foundation of Multidisciplinary Treatment for Cancer (JFMTC) and the results were published in 1997 (Yasutomi 1997 (JFMTC 7‐2)). Patients with rectal cancer only underwent radical surgery and were randomized to one of the following two arms:
‐ observation (n=356)
‐ adjuvant chemotherapy (n=357)
The chemotherapy regimen was a combination of HCFU (oral) with MMC (intravenously). The original article is in Japanese: the relevant data for this meta‐analysis were retrieved from an individual patient data (IPD) meta‐analysis conducted in 2004 by the Meta‐analysis Group in Cancer (see Sakamoto 2004).
11) JFMTC 7‐1 trial: this Japanese multicentre RCT was performed on behalf of the Japanese Foundation of Multidisciplinary Treatment for Cancer (JFMTC) and the results were published in 1998 (Kodaira 1998 (JFMTC 7‐1)). Patients with rectal cancer only underwent radical surgery and were randomized to one of the following two arms:
‐ observation (n=398)
‐ postoperative adjuvant chemotherapy (n=396)
Systemic chemotherapy was a combination of MMC (i.v.) and UFT (uracil‐tegafur, an oral fluoropyrimidine).
12) NACCP trial: this Dutch multicentre RCT was conducted on behalf of the Netherlands Adjuvant Colorectal Cancer Project (NACCP), the findings being published in 2001 (Taal 2001 (NACCP)). Patients undergoing curative surgery for colorectal cancer were randomized to one of the following two arms:
‐ observation (n=515)
‐ adjuvant chemotherapy (n=514)
Systemic chemotherapy consisted of 5‐FU plus levamisole. More than 50% of patients with rectal cancer underwent radiotherapy. The authors provided separate data for colon and rectal cancer (n=299), which enabled us to include this study in the present meta‐analysis. Compliance to chemotherapy was 69% and severe toxicity was not observed. The authors conclude that one year 5‐FU plus levamisole was of benefit in stage II and III colon cancer, whereas in rectal cancer a significant positive effect could not be demonstrated.
13) TACSG trial: this Japanese multicentre RCT was conducted on behalf of the Tokai Adjuvant Chemotherapy Study Group (TACSG) and its findings were published in 2002 (Kato 2002 (TACSG)). Patients undergoing curative surgery for colorectal cancer (n=320) were randomized to one of the following two arms:
‐ observation (n=145)
‐ postoperative chemotherapy (n=144)
Systemic chemotherapy consisted of UFT (uracil‐tegafur, an oral fluoropyrimidine). The authors provided separate data for colon and rectal cancer (n=143), which enabled us to include this study in the present meta‐analysis.
14) Italian trial: in this national RCT, whose findings were published in 2003 (Cafiero 2003), patients with rectal cancer only received apparently radical surgery of their primary tumour and were randomized to one of the following two arms:
‐ adjuvant radiotherapy (n=108)
‐ adjuvant radiotherapy + adjuvant chemotherapy (n=110)
The systemic chemotherapy regimen consisted of 5‐fluorouracil (5‐FU) and levamisole. The authors conclude that postoperative treatment does not improve patient clinical outcome, but also underscore the fact that low compliance to the chemotherapy regimen might have compromised an adequate comparison of the two arms (i.e., adjuvant chemotherapy might have resulted falsely ineffective due to the low compliance to treatment).
15) JFMTC 15‐2 trial: this Japanese multicentre RCT was performed on behalf of the Japanese Foundation of Multidisciplinary Treatment for Cancer (JFMTC) and the results were published in 2004 (Watanabe 2004 (JFMTC15‐2)). Patients with rectal cancer only received apparently radical surgery of their primary tumour and were randomized to one of the following three arms:
‐ observation (n=229)
‐ adjuvant chemotherapy (n=218)
‐ adjuvant chemotherapy combined with immunotherapy (n=222)
Systemic chemotherapy: UFT (oral) + MMC and 5‐FU (intravenously). Immunotherapy: OK‐432. For this meta‐analysis, data were retrieved from an individual patient data (IPD) meta‐analysis (Sakamoto 2007 (JFMTC15‐1)) where patients treated with surgery alone (n=122) were compared to patients undergoing surgery plus any adjuvant treatment (n=269)
16) NGTATG trial: the data, which were published in 2005 (Glimelius 2005 (NGTATG)), are presented as a pooled analysis of RCT conducted in Northern Europe on behalf of the Nordic Gastrointestinal Tumor Adjuvant Therapy Group (NGTADG). Patients underwent curative resection of colorectal cancer (n=2224) and were randomized to one of the following two arms:
‐ observation (n=1121)
‐ postoperative adjuvant chemotherapy (n=1103)
Systemic chemotherapy regimens consisted of 5‐FU combined with leucovorin, levamisole or both. The authors provided separate data for colon and rectal cancer (n=691), which enabled us to include this study in the present meta‐analysis.
17) EORTC trial: this European multicentre RCT was carried out on behalf of the European Organization for Research and Treatment of Cancer (EORTC), the findings being published in 2006 (Bosset 2006 (EORTC)). Patients with rectal cancer only received apparently radical surgery of the primary tumour and were randomized to one of the following four arms:
‐ preoperative radiotherapy (n=252)
‐ preoperative chemoradiation (n=253)
‐ preoperative radiotherapy and postoperative chemotherapy (n=253)
‐ preoperative chemoradiation and postoperative chemotherapy (n=253)
Systemic chemotherapy consisted of 5‐FU plus leucovorin. In patients with rectal cancer who receive preoperative radiotherapy, adding fluorouracil‐based chemotherapy preoperatively or postoperatively had no significant effect on survival; chemotherapy, regardless of whether it was administered before or after surgery, conferred a significant benefit with respect to local disease control.
18) JFMTC 15‐1 trial: this Japanese multicentre RCT was performed on behalf of the Japanese Foundation of Multidisciplinary Treatment for Cancer (JFMTC) and the results were published in 2007 (Sakamoto 2007 (JFMTC15‐1)). Patients with rectal cancer only received apparently radical surgery of the primary tumour and were randomized to one of the following four arms:
‐ observation (n=229)
‐ adjuvant chemotherapy (n=218)
Chemotherapy consisted of Uracil‐Tegafur (UFT, an oral fluoropyrimidine) plus intravenous 5‐FU and MMC. The data are from an individual patient data (IPD) meta‐analysis of RCT performed in Japan on the use of adjuvant chemotherapy for the treatment of rectal cancer (no original article on this specific RCT was published).
19) QUASAR trial: this UK national RCT was carried out on behalf of the QUick And Simple And Reliable (QUASAR) Collaborative Group, the findings being published in 2007 (QUASAR 2007). Patients undergoing curative surgery for colorectal cancer were randomized to one of the following two arms:
‐ observation (n=1617)
‐ adjuvant chemotherapy (n=1622)
Systemic chemotherapy consisted of 5‐FU + folinic acid. The authors provided separate data for colon and rectal cancer (n=948), which enabled us to include this study in the present meta‐analysis. The authors conclude that chemotherapy with fluorouracil and folinic acid could improve survival of patients with stage II colorectal cancer, although the absolute improvements are small (assuming 5‐year mortality without chemotherapy is 20%, the relative risk of death seen in this RCT translates into an absolute improvement in survival of 3.6%).
20) Japanese monocentric trial: in this single centre RCT, whose results were published in 2009 (Koda 2009), patients with colorectal cancer (n=124) underwent radical surgery for the primary tumour and were randomized to on the following two arms:
‐ observation (n=67)
‐ postoperative chemotherapy (n=57)
The systemic chemotherapy regimen was a combination of HCFU (oral) with 5‐FU (i.v.). The authors provided separate data for colon and rectal cancer, which enabled us to include this study in the present meta‐analysis.
21) Japanese national trial: this study, whose findings were published in 2011 (Hamaguchi 2011), is an update of the trial described by Akasu in 2006 (Akasu 2006). In this multicentre RCT, patients with rectal cancer only received apparently radical surgery of the primary tumour and were randomized to one of the following two arms:
‐ observation (n=135)
‐ postoperative chemotherapy (n=139)
Systemic chemotherapy consisted of UFT (uracil‐tegafur, an oral fluoropyrimidine).
Risk of bias in included studies
See the dedicated Risk of Bias Tables below.
In general most methodological problems occurred in the randomisation procedures and the reporting of these procedures. In eight studies the allocation was considered concealed (CCCSGJ 1995, Fisher 1988 (NSABP), Kato 2002 (TACSG), Kodaira 1998 (JFMTC 7‐1), Kornek 1996, Taal 2001 (NACCP), Thomas 1988 (GTSG)) and in two it remains unclear (Hafström 1990, Krook 1991 (NCCTG)). The timing of randomisation was only adequately described in three studies (Kodaira 1998 (JFMTC 7‐1), Kornek 1996, Taal 2001 (NACCP)) and was unclear in the rest of the included studies.
None of the studies included any Quality of Life data. None of the studies used an intention to treat design or reported it in a useful manner.
Quality of follow up: In all but one study (Kornek 1996) the exact number of patients followed up was reported. In two studies the exact number of survivors were reported (CCCSGJ 1995, Fisher 1988 (NSABP)). In one study the number of survivors were reported for 5 years, but the 3 years survival data had to be extracted from the Kaplan‐Meier plots (Grage 1981). In the rest of the included studies the survival data were extracted from the survival plots.
The included studies showed considerably clinical heterogeneity in terms of chemotherapy regimens.
Effects of interventions
Overall Survival (OS)
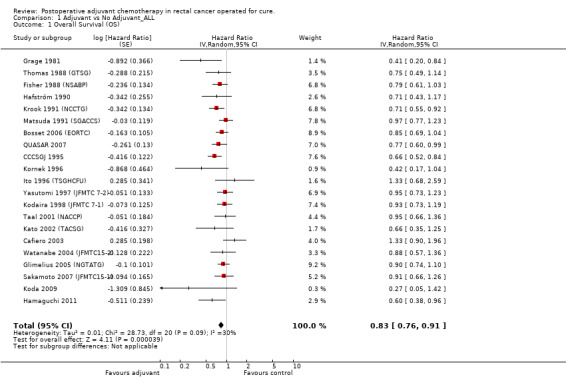
OS data were available in 21 RCTs enrolling 9,785 patients with rectal cancer. The data available for meta‐analysis regarded 9,221 patients. Of these, 4,854 patients were randomized to adjuvant chemotherapy (treatment arm) and 4,367 patients did not receive adjuvant chemotherapy (control arm).
Meta‐analysis of these RCT (see Figure 1) showed a significant risk reduction (17%) in patients undergoing postoperative chemotherapy as compared to those undergoing observation (HR=0.83, CI: 0.76‐0.91). Between‐study heterogeneity was moderate (I‐squared=30%) but significant (P=0.09) at the 10% alpha level.
1.
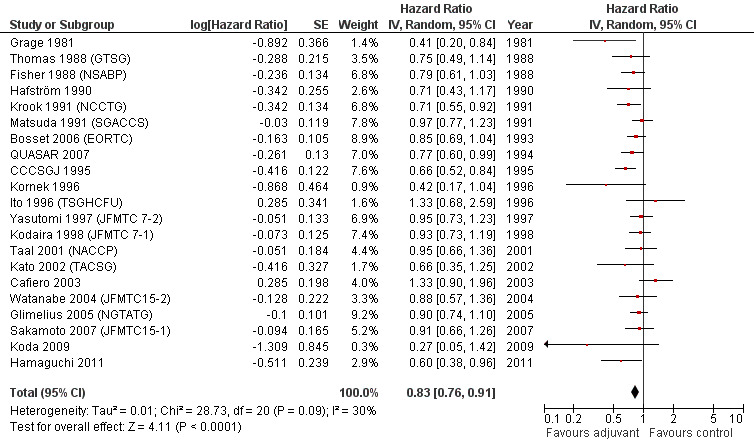
Forest plot of comparison: 1 Adjuvant vs No Adjuvant_ALL, outcome: 1.1 Overall Survival (OS).
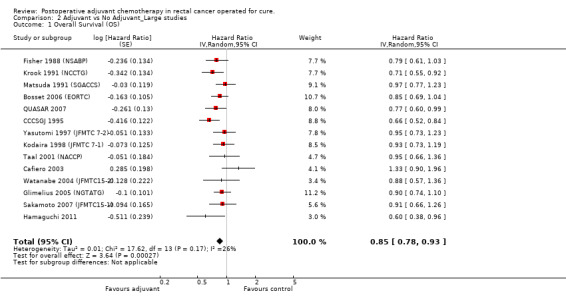
The leave‐one‐out sensitivity analysis showed no dominant RCT, which demonstrates that the overall effect estimate is not affected by the findings of a single RCT. As shown in Figure 2, a small‐study effect was likely to be present (Begg test P=0.09, Egger test P=0.13), and the trim & fill procedure revealed that 4 studies might be missing: however, their addition to the meta‐analysis would not significantly change the meaning of our findings (adjusted HR=0.86; CI: 0.78‐0.94). On the other hand, exclusion of RCT enrolling fewer than 100 patients per arm yielded very similar results (HR=0.85; CI: 0.78‐0.93) (see Figure 3).
2.
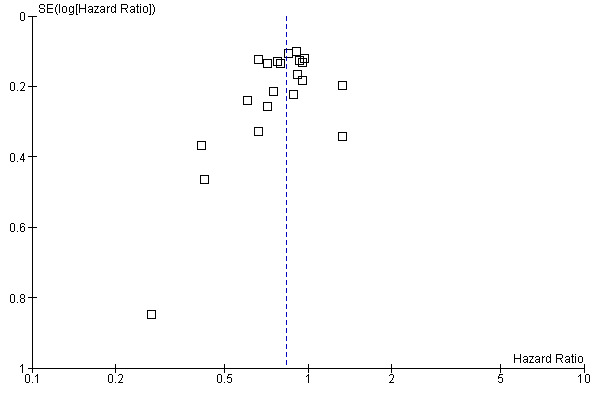
Funnel plot of comparison: 1 Adjuvant vs No Adjuvant_ALL, outcome: 1.1 Overall Survival (OS).
3.
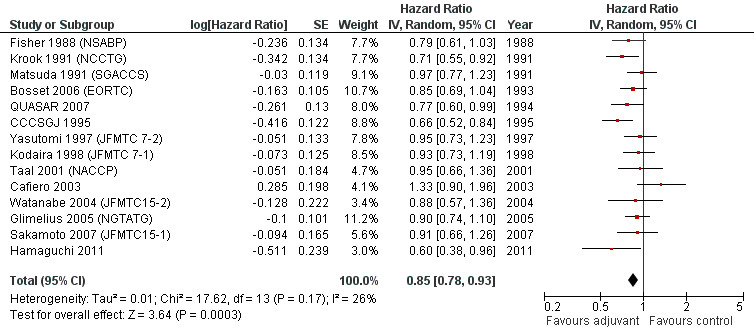
Forest plot of comparison: 2 Adjuvant vs No Adjuvant_Large studies, outcome: 2.1 Overall Survival (OS).
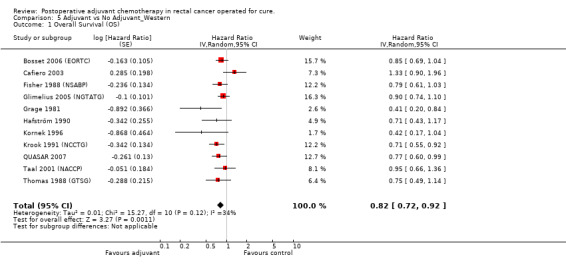
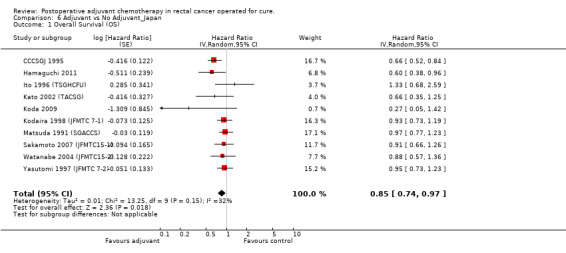
When the geographic/ethnic origin of the trial was taken into consideration (Western vs Japanese), no substantial differences were observed in terms of both overall effect and heterogeneity (see Figure 4 and Figure 5): in particular, adjuvant chemotherapy resulted associated with better survival both among Western (HR=0.82, CI: 0.72‐0.92) and Japanese trials (0.85, CI: 0.74‐0.97).
4.
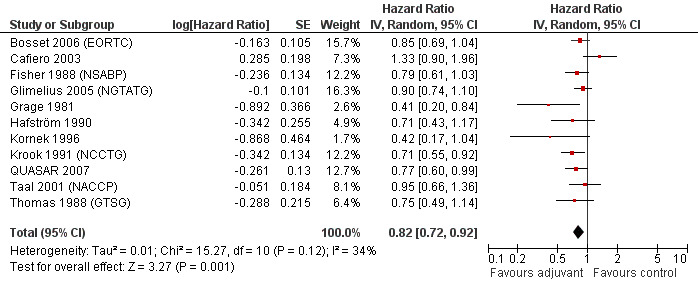
Forest plot of comparison: 5 Adjuvant vs No Adjuvant_Western, outcome: 5.1 Overall Survival (OS).
5.
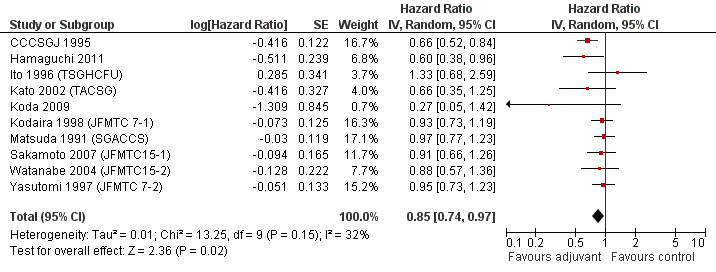
Forest plot of comparison: 6 Adjuvant vs No Adjuvant_Japan, outcome: 6.1 Overall Survival (OS).
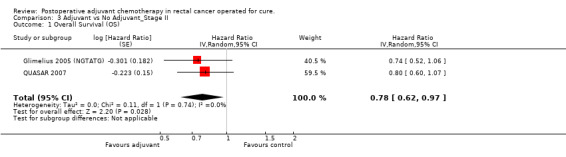
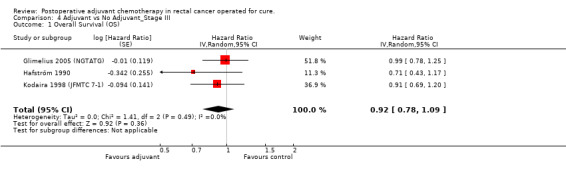
Finally, we considered the RCT that reported the data separately for patients with TNM stage II (lymph node negative) and stage III (lymph node positive) rectal cancer. Unfortunately, only two and three RCTs were available, respectively, which makes the results of their meta‐analysis questionable (see Figure 6 and Figure 7, respectively).
6.
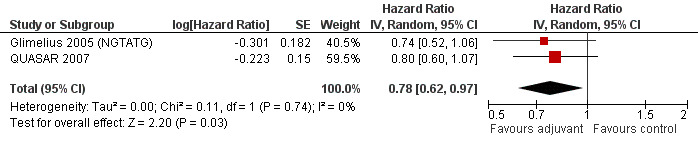
Forest plot of comparison: 3 Adjuvant vs No Adjuvant_Stage II, outcome: 3.1 Overall Survival (OS).
7.
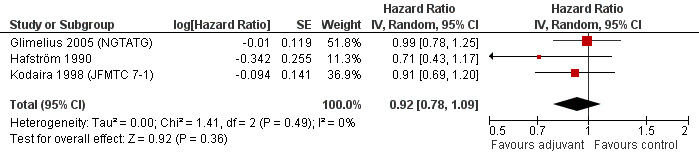
Forest plot of comparison: 4 Adjuvant vs No Adjuvant_Stage III, outcome: 4.1 Overall Survival (OS).
Disease Free Survival (DFS)
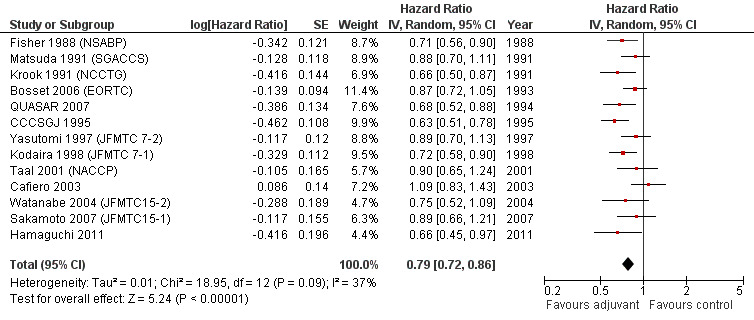
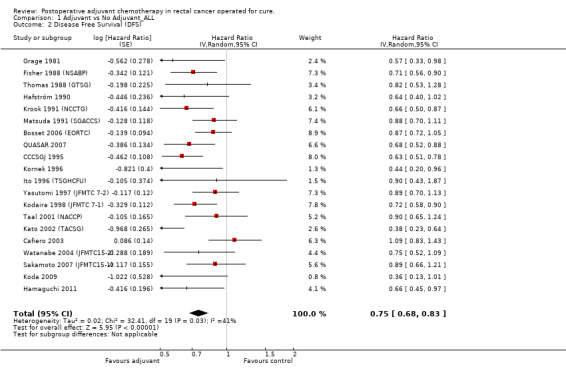
DFS data were reported in 20 RCTs enrolling 9094 patients with rectal cancer. For one study (Glimelius 2005 (NGTATG)) DFS data were not reported: therefore, the data available for meta‐analysis included 8,530 patients. Of these, 4,515 patients were randomized to postoperative chemotherapy (treatment arm) and 4,015 patients received no postoperative chemotherapy (control arm). Meta‐analysis of these RCTs (see Figure 8) showed a significant risk reduction (25%) in patients undergoing adjuvant chemotherapy as compared to those undergoing observation (HR=0.75, CI: 0.68‐0.83). Between‐study heterogeneity was moderate (I‐squared=41%) but significant (P=0.03).
8.
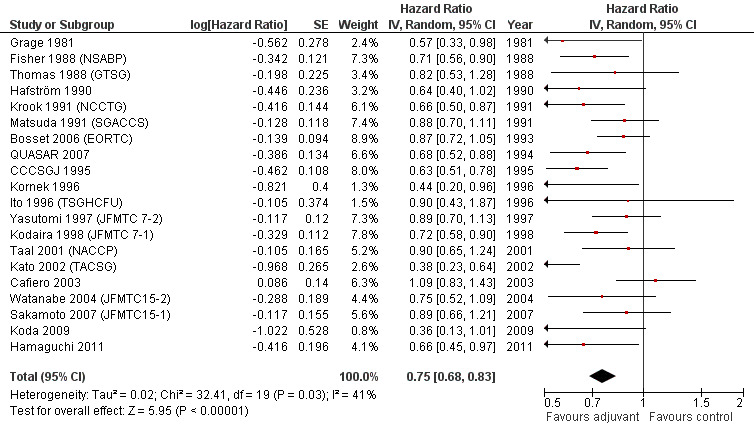
Forest plot of comparison: 1 Adjuvant vs No Adjuvant_ALL, outcome: 1.2 Disease Free Survival (DFS).
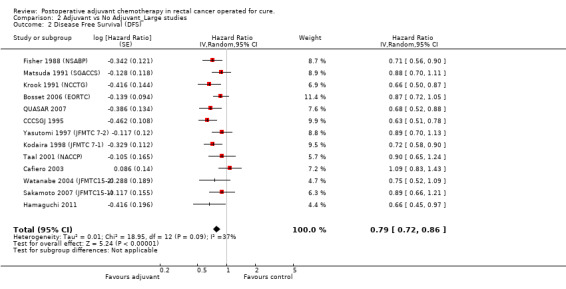
The leave‐one‐out sensitivity analysis showed no leading RCT, which supports the fact that the overall effect estimate is not affected by the findings of a single trial. As shown in Figure 9, a small‐study effect was likely to be present (Begg test P=0.04, Egger test P=0.06), and the trim & fill procedure showed that 4 studies might be missing: nevertheless, their addition to the meta‐analysis would leave virtually unchanged our findings (adjusted HR=0.78; CI: 0.71‐0.87). Moreover, upon exclusion of small size RCT (< 100 patients per arm) we obtained very similar results (HR=0.79; CI: 0.72‐0.86) (see Figure 10).
9.
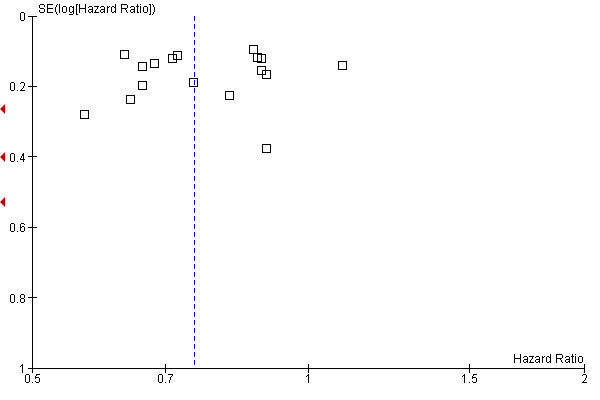
Funnel plot of comparison: 1 Adjuvant vs No Adjuvant_ALL, outcome: 1.2 Disease Free Survival (DFS).
10.
Forest plot of comparison: 2 Adjuvant vs No Adjuvant_Large studies, outcome: 2.2 Disease Free Survival (DFS).
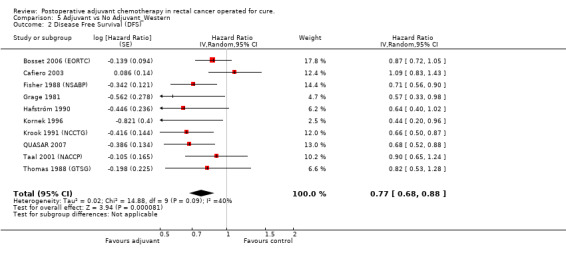
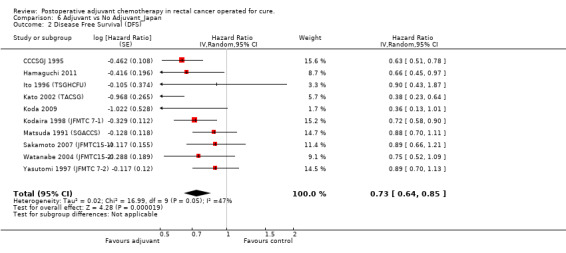
Taking into consideration the geographic/ethnic origin of the trial (Western vs Japanese), no substantial differences were observed in terms of both overall effect and heterogeneity (see Figure 11 and Figure 12): in fact, postoperative chemotherapy was associated with better survival both among Western (HR=0.77, CI: 0.68‐0.88) and Japanese trials (0.73, CI: 0.64‐0.85).
11.
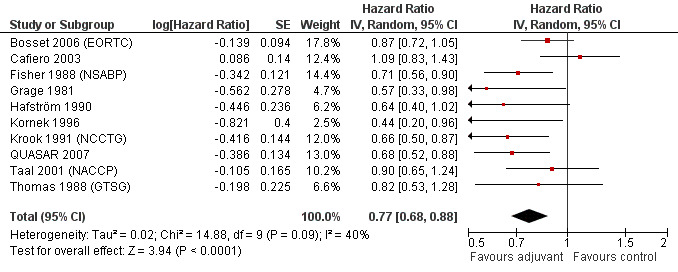
Forest plot of comparison: 5 Adjuvant vs No Adjuvant_Western, outcome: 5.2 Disease Free Survival (DFS).
12.
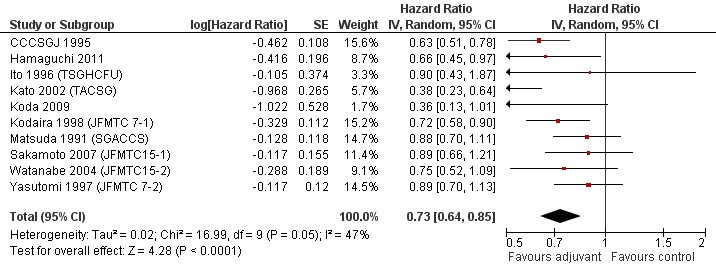
Forest plot of comparison: 6 Adjuvant vs No Adjuvant_Japan, outcome: 6.2 Disease Free Survival (DFS).
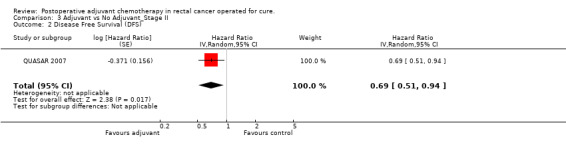
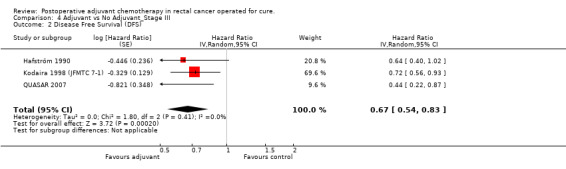
Finally, we considered the RCT that reported the data separately for patients with TNM stage II (lymph node negative) and stage III (lymph node positive) rectal cancer. Only one report provided DFS data for stage II (QUASAR 2007), as shown in Figure 13; the results of the meta‐analysis of the three studies reporting DFS data for stage III are illustrated in Figure 14: given the scarce number of available RCTs, these findings are poorly reliable.
13.
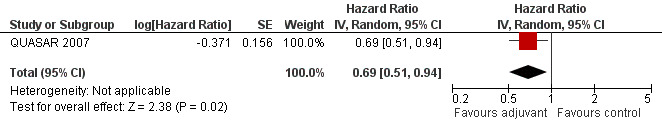
Forest plot of comparison: 3 Adjuvant vs No Adjuvant_Stage II, outcome: 3.2 Disease Free Survival (DFS).
14.
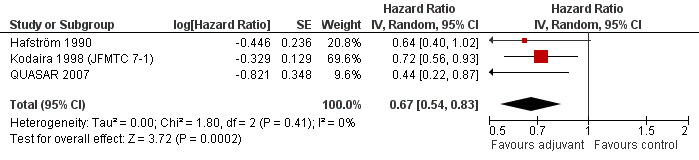
Forest plot of comparison: 4 Adjuvant vs No Adjuvant_Stage III, outcome: 4.2 Disease Free Survival (DFS).
Discussion
The therapeutic management of patients with non‐metastatic rectal cancer (TNM stage I‐III [TanyNanyM0], Dukes´ A‐C) has significantly improved over the past 20 years. From the surgery viewpoint, technical advancements such as stapling devices (to perform very distal colo‐rectal anastomosis) and diffusion of total mesorectal excision (TME) have contributed to improve quality of life and increase the rates of local disease control. Unlike colon cancer, chemotherapy after radical surgery for rectal cancer is not a standardized treatment. This is at least in part due to the fact that preoperative (also known as neoadjuvant) chemoradiation is currently considered the standard of care for stage II‐III rectal cancer, especially in European countries (Glimelius 2010; Van Cutsem 2008; Wolpin 2007; Watanabe 2008). Therefore the role of adjuvant chemotherapy in rectal cancer has remained uncertain despite many randomized controlled trials (RCTs) have been performed that address this issue, a situation that has recently led some authors to advocate the conduction of a meta‐analysis (Bujko 2010). In the present article we present the results of the first systematic review and meta‐analysis on this subject in order to quantitatively summarize the available evidence on the efficacy of adjuvant (i.e., postoperative) chemotherapy for the treatment of patients with surgically resectable rectal carcinoma.
Overall, pooling the summary data of 21 RCTs enrolling almost 10,000 patients, we found that 5‐FU based postoperative chemotherapy does improve both overall (OS) and disease‐free (DFS) survival. The risk reductions (17% and 25% for OS and DFS, respectively) are lower than those observed in TNM stage III colon cancer with similar chemotherapy regimens (Moertel 1990), but still they are of undeniable clinical relevance. Interestingly, at subgroup analysis these findings remained virtually unchanged if we considered only large trials (> 100 patients per arm) or only Western (or Japanese) trials, which supports the robustness of the results. Between‐study heterogeneity was never high (i.e., I‐squared was always < 50%) but we could not identify a specific source, although it is likely that the heterogeneity of TNM stages (from Dukes A‐C to Dukes C only) and chemotherapy regimens (from oral fluoropyrimidines to combinations of intravenous drugs) played a non‐marginal role in determining different outcomes.
Considering that no chemotherapy regimen tested in the RCT eligible for this meta‐analysis included modern drugs such as oxaliplatin, irinotecan or biologicals (e.g., anti‐VEGF antibody bevacizumab) (Wagner 2009; Mocellin 2005), our findings are particularly encouraging: in fact, an even greater survival advantage might be expected with the implementation of more effective anti‐cancer agents in the adjuvant therapy for rectal cancer. In this regard, new trials testing this hypothesis are warranted and might further support the use of postoperative chemotherapy in these patients.
On the other hand, the wide use of neoadjuvant chemoradiation makes things more complicated: in fact, in the light of the known beneficial effects of neoadjuvant treatment, designing trials of adjuvant therapy without taking into consideration preoperative chemoradiation may pose ethical problems. All but one RCT so far performed (i.e., those included in this meta‐analysis) compared adjuvant chemotherapy (associated with radiotherapy in one case Krook 1991 (NCCTG)) with observation (or radiotherapy in one case Krook 1991 (NCCTG)): only the EORTC trial (Bosset 2006 (EORTC)) included an arm of preoperative chemoradiation and showed that in patients receiving preoperative radiotherapy, adding fluorouracil‐based chemotherapy preoperatively or postoperatively had no significant effect on survival, and that chemotherapy, regardless of whether it was administered before or after surgery, conferred a significant benefit only with respect to local disease control. Although the EORTC RCT is a well designed trial, we cannot reply on the results of this single study to draw definitive conclusions on this aspect: clearly, more trials addressing this specific issue are needed. In this regard, it is interesting to note that only five out of 21 RCT showed a nominally significant positive association between adjuvant chemotherapy and patient survival, and only pooling all trials with the present meta‐analysis revealed the therapeutic benefit due to the effect of a larger sample size on the precision of the estimated treatment effect. Furthermore, although neoadjuvant treatments do improve the control of local disease, the issue of controlling distant metastasis remains largely unaddressed by this therapeutic approach (Ceelen 2009), which should further prompt oncologists to investigate in this area. Finally it should be remembered that a key advantage of designing trials that combine neoadjuvant with adjuvant chemotherapy is that tumour response to preoperative chemotherapy might be a useful guide in selecting patients who might best benefit from postoperative chemotherapy, in line with the principles of personalized cancer medicine.
Unfortunately, only very few studies reported separate data for different TNM stages, which made virtually impossible to ascertain whether observed heterogeneity was linked to disease staging; consequently, the lack of these data did not enable us to define whether adjuvant chemotherapy might be more effective in a specific disease stage. Therefore, while for colon cancer adjuvant chemotherapy is widely accepted to represent the standard treatment selectively for TNM stage III patients, for rectal cancer we could not identify a subset of patients more likely to benefit from this treatment.
The lack of data reported within an acknowledged QoL scoring system and the trial design (mainly treatment versus observation) made it practically unfeasible to study this outcome.
Authors' conclusions
Implications for practice.
The results of this meta‐analysis support the use of 5‐FU based postoperative adjuvant chemotherapy for patients undergoing apparently radical surgery for resectable rectal cancer. Available data do not allow us to define whether the efficacy of this treatment is highest in one specific TNM stage.
Implications for research.
The implementation of modern chemotherapy regimens in the adjuvant setting is warranted to improve the encouraging results shown by this meta‐analysis. Randomised trials of adjuvant chemotherapy for patients receiving preoperative neoadjuvant therapy are also needed in order to define the role of postoperative chemotherapy in the multimodal treatment of resectable rectal cancer.
What's new
| Date | Event | Description |
|---|---|---|
| 26 April 2011 | New search has been performed | revman 5.1 converted |
| 12 July 2009 | Feedback has been incorporated | alternative background included, two studies Bossett 2006 and QUASAR 2007 incl., but not in analysis. |
| 24 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 3, 2012
| Date | Event | Description |
|---|---|---|
| 14 September 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The initiation of this study was supported by a grant from the Bispebjerg Hospital Research Foundation
Data and analyses
Comparison 1. Adjuvant vs No Adjuvant_ALL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall Survival (OS) | 21 | Hazard Ratio (Random, 95% CI) | 0.83 [0.76, 0.91] | |
| 2 Disease Free Survival (DFS) | 20 | Hazard Ratio (Random, 95% CI) | 0.75 [0.68, 0.83] |
1.1. Analysis.
Comparison 1 Adjuvant vs No Adjuvant_ALL, Outcome 1 Overall Survival (OS).
1.2. Analysis.
Comparison 1 Adjuvant vs No Adjuvant_ALL, Outcome 2 Disease Free Survival (DFS).
Comparison 2. Adjuvant vs No Adjuvant_Large studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall Survival (OS) | 14 | Hazard Ratio (Random, 95% CI) | 0.85 [0.78, 0.93] | |
| 2 Disease Free Survival (DFS) | 13 | Hazard Ratio (Random, 95% CI) | 0.79 [0.72, 0.86] |
2.1. Analysis.
Comparison 2 Adjuvant vs No Adjuvant_Large studies, Outcome 1 Overall Survival (OS).
2.2. Analysis.
Comparison 2 Adjuvant vs No Adjuvant_Large studies, Outcome 2 Disease Free Survival (DFS).
Comparison 3. Adjuvant vs No Adjuvant_Stage II.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall Survival (OS) | 2 | Hazard Ratio (Random, 95% CI) | 0.78 [0.62, 0.97] | |
| 2 Disease Free Survival (DFS) | 1 | Hazard Ratio (Random, 95% CI) | 0.69 [0.51, 0.94] |
3.1. Analysis.
Comparison 3 Adjuvant vs No Adjuvant_Stage II, Outcome 1 Overall Survival (OS).
3.2. Analysis.
Comparison 3 Adjuvant vs No Adjuvant_Stage II, Outcome 2 Disease Free Survival (DFS).
Comparison 4. Adjuvant vs No Adjuvant_Stage III.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall Survival (OS) | 3 | Hazard Ratio (Random, 95% CI) | 0.92 [0.78, 1.09] | |
| 2 Disease Free Survival (DFS) | 3 | Hazard Ratio (Random, 95% CI) | 0.67 [0.54, 0.83] |
4.1. Analysis.
Comparison 4 Adjuvant vs No Adjuvant_Stage III, Outcome 1 Overall Survival (OS).
4.2. Analysis.
Comparison 4 Adjuvant vs No Adjuvant_Stage III, Outcome 2 Disease Free Survival (DFS).
Comparison 5. Adjuvant vs No Adjuvant_Western.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall Survival (OS) | 11 | Hazard Ratio (Random, 95% CI) | 0.82 [0.72, 0.92] | |
| 2 Disease Free Survival (DFS) | 10 | Hazard Ratio (Random, 95% CI) | 0.77 [0.68, 0.88] |
5.1. Analysis.
Comparison 5 Adjuvant vs No Adjuvant_Western, Outcome 1 Overall Survival (OS).
5.2. Analysis.
Comparison 5 Adjuvant vs No Adjuvant_Western, Outcome 2 Disease Free Survival (DFS).
Comparison 6. Adjuvant vs No Adjuvant_Japan.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall Survival (OS) | 10 | Hazard Ratio (Random, 95% CI) | 0.85 [0.74, 0.97] | |
| 2 Disease Free Survival (DFS) | 10 | Hazard Ratio (Random, 95% CI) | 0.73 [0.64, 0.85] |
6.1. Analysis.
Comparison 6 Adjuvant vs No Adjuvant_Japan, Outcome 1 Overall Survival (OS).
6.2. Analysis.
Comparison 6 Adjuvant vs No Adjuvant_Japan, Outcome 2 Disease Free Survival (DFS).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bosset 2006 (EORTC).
| Methods | RCT (Randomised Controlled Trial), multicentre (Europe) | |
| Participants | Patients (n=1011) with cT3‐cT4, cM0 (Nx) resectable rectal carcinoma (cT: clinical T stage) | |
| Interventions | 4‐arm trial: patients randomised to receive A) preoperative radiotherapy (n=252) or B) preoperative chemoradiotherapy (n=253) or C) preoperative radiotherapy and postoperative chemotherapy (n=253) or D) preoperative chemoradiotherapy and postoperative chemotherapy (n=253). All patients received surgery |
|
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | In this meta‐analysis of adjuvant chemotherapy, arms C and D (adjuvant chemotherapy) were compared to arms A and B (no adjuvant chemotherapy). At subgroup analysis, patients responding to preoperative treatment (ypT0‐T2) had a significant advantage if received adjuvant chemotherapy (as compared to patients who responded but did not receive adjuvant chemotherapy) in terms of both OS (HR=0.64; 95%CI: 0.42‐0.96) and DFS (HR=0.63; CI: 0.44‐0.90) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | Mininimization technique and stratification, before random assignment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Cafiero 2003.
| Methods | RCT (Randomised Controlled Trial), multicentre (Italy) | |
| Participants | Patients (n=218) with TNM stage II‐III resectable rectal carcinoma | |
| Interventions | 2‐arm trial: patients randomized to: either A) surgery + adjuvant radiotherapy (n=108), or B) surgery + adjuvant radiotherapy + adjuvant chemotherapy with 5‐FU and levamisole (n=110) | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | Low compliance to the chemotherapy regimen (59%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
CCCSGJ 1995.
| Methods | RCT (Randomised Controlled Trial); multicentre (Japan) | |
| Participants | Patients (n=1004) with TNM stage II‐III resectable rectal carcinoma | |
| Interventions | 3‐arm trial: patients randomized to either A) surgery alone (n=335), or B) surgery + adjuvant systemic chemotherapy (n=323) with MMC + 5‐FU, or C) surgery + adjuvant intra‐arterial chemotherapy (with MMC) + adjuvant systemic chemotherapy with MMC + 5‐FU (n=346) | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | No stage stratification. In this meta‐analysis, arms B and C (adjuvant chemotherapy) were compared to arm A (no adjuvant chemotherapy) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Fisher 1988 (NSABP).
| Methods | RCT (Randomised Controlled Trial), multicentre (USA) | |
| Participants | Patients with resectable Dukes B, C (TNM stage II‐III) rectal cancer (n=574) | |
| Interventions | 3‐arm trial: patients were randomized to receive: A) surgery alone (n=191), or B) surgery + adjuvant radiotherapy (n=190), or C) surgery + adjuvant chemotherapy with 5‐FU, semustine, and vincristine (n=193). | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | In this meta‐analysis of adjuvant chemotherapy, arm C (adjuvant chemotherapy) was compared to arm A (no adjuvant chemotherapy). At subgroup analysis, the authors reported that both DFS and OS were significantly improved by adjuvant chemotherapy in males |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Glimelius 2005 (NGTATG).
| Methods | RCT (Randomised Controlled Trial); multicentre (Northen Europe: Sweden, Norway, Denmark) | |
| Participants | Patients undergoing curative resection of TNM stage II‐III colorectal cancer (n=2224) | |
| Interventions | 2‐arm trials: patients randomized to: A) surgery alone (n=1121), or B) postoperative adjuvant chemotherapy with 5‐FU based regimens (n=1103) | |
| Outcomes | Overall survival (OS) | |
| Notes | Data are presented as a meta‐analysis of RCT conducted in Northen Europe. In this meta‐analysis we compared patients with rectal cancer randomized to adjuvant chemotherapy (n=339) versus those with rectal cancer randomized to observation (n=352) Chemotherapy regimens: 1) 5‐FU + levamisole; 2) 5‐FU + leucovorin; 3) 5‐FU + leucovorin + levamisole |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Grage 1981.
| Methods | RCT (Randomised Controlled Trial); multicentre (USA) | |
| Participants | Patients undergoing curative or palliative resection of Dukes´B and C (TNM stage II‐III) colorectal cancer (n=134) | |
| Interventions | 2‐arm trial: patients randomized to: A) surgery alone (n=67), or B) postoperative adjuvant chemotherapy with 5‐FU (n=67) | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | No stratification between B and C. In this meta‐analysis we compared patients with rectal cancer randomized to adjuvant chemotherapy (n=33) versus those with rectal cancer randomized to observation (n=31) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Hafström 1990.
| Methods | RCT (Randomised Controlled Trial); multicentre (Sweden) | |
| Participants | Patients with Dukes´C (TNM stage III) colorectal cancer undergoing surgery for the primary tumour (n=334). | |
| Interventions | 2‐arm trial: A) surgery alone (n=176), or B) postoperative adjuvant combination chemotherapy (5‐FU, lomustine [CCNU] and vincristine) | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | In 30 patients treatment was discontinued because of recurrent disease. In 27 patients surgery was not radical. Patients were stratified based on primary tumour site (colon and rectum). In this meta‐analysis we compared patients with rectal cancer randomized to adjuvant chemotherapy (n=43) versus those with rectal cancer randomized to observation (n=56) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Hamaguchi 2011.
| Methods | RCT (Randomised Controlled Trial); multicentre (Japan) | |
| Participants | Patients with Dukes C (TNM stage III) rectal cancer undergoing apparently radical surgery of the primary tumour (n=274) | |
| Interventions | 2‐arm trial: patients were randomly assigned to: A) surgery alone (n=135), or B) postoperative chemotherapy (n=139) with UFT (uracil‐tegafur, an oral fluoropyrimidine) | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | Update of trial described by Akasu in 2006 (Akasu 2006) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | Minimization technique |
| Blinding (performance bias and detection bias) All outcomes | High risk | Non‐blind study |
| Other bias | Low risk | |
Ito 1996 (TSGHCFU).
| Methods | RCT (Randomised Controlled Trial); multicentre (Japan) | |
| Participants | Patients with TNM stage II‐III colorectal cancer undergoing apparently radical surgery of the primary tumour (n=170) | |
| Interventions | 2‐arm trial: patients were randomly assigned to: A) surgery alone (n=83), or B) postoperative chemotherapy (n=87) with HCFU (1‐hexylcarbamoyl‐5‐fluorouracil, an oral fluoropyrimidine) | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | For this meta‐analysis, patients with rectal cancer treated with surgery alone (n=40) were compared to those undergoing surgery + adjuvant chemotherapy (n=37) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Randomization by telephone |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Kato 2002 (TACSG).
| Methods | RCT (Randomised Controlled Trial); multicentre (Japan) | |
| Participants | Patients with Dukes´B and C (TNM stage II‐III) colorectal cancer undergoing apparently radical surgery of the primary tumour (n=289) | |
| Interventions | 2‐arm trial: patients were randomly assigned to: A) surgery alone (n=145), or B) postoperative chemotherapy (n=144) with UFT (uracil‐tegafur, an oral fluoropyrimidine) | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | Patients were stratified based on primary tumour site (colon and rectum). UFT was administered for one year or more in 101/145 patients (70%). For this meta‐analysis, patients with rectal cancer treated with surgery alone (n=72) were compared to those undergoing surgery + adjuvant chemotherapy (n=71) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
Koda 2009.
| Methods | RCT (Randomised Controlled Trial); monocentre (Japan) | |
| Participants | Patients with TNM stage I‐II‐III colorectal cancer undergoing apparently radical surgery of the primary tumour (n=124) | |
| Interventions | 2‐arm trial: patients were randomly assigned to: A) surgery alone (n=67), or B) postoperative chemotherapy (n=57) with HCFU (oral) + 5‐FU (i.v.) | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | In this meta‐analysis we compared patients with rectal cancer randomized to adjuvant chemotherapy (n=25) versus those with rectal cancer randomized to observation (n=29) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Other bias | Low risk | |
Kodaira 1998 (JFMTC 7‐1).
| Methods | RCT (Randomised Controlled Trial); multicentre (Japan) | |
| Participants | Patients with radically resected Dukes A, B or C rectal cancer (n=794) | |
| Interventions | 2‐arm trial: patients were randomized to: A) surgery alone (n=398), or B) postoperative adjuvant chemotherapy with mitomycin + UFT (uracil‐tegafur, an oral fluoropyrimidine) (n=396) | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | Five Dukes´ D patients are included in the Dukes´ C group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Other bias | Low risk | |
Kornek 1996.
| Methods | RCT (Randomised Controlled Trial). Monocentre (Austria) | |
| Participants | Curative resection of Dukes´B & C (TNM stage II, III) rectal cancer (n=57) | |
| Interventions | 2‐arm trial: patients were randomized to: A) surgery alone (n=29), or B) surgery + adjuvant chemotherapy (combination of locoregional [mitoxantrone] and systemic chemotherapy with leucovorin + 5‐FU) (n=28). | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | No stratification between Dukes B and C. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Krook 1991 (NCCTG).
| Methods | RCT (Randomised controlled trial); multicentre (USA) | |
| Participants | Patients undergoing curative resection of high‐risk (T3‐4 or N+) rectal cancer (n=204) | |
| Interventions | 2‐arm trial: patients were randomized to: A) surgery + adjuvant radiotherapy (n=100), or B) surgery + adjuvant radiotherapy associated with chemotherapy with 5‐FU + semustine (n=104) | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | No stratification between B and C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Other bias | Low risk | |
Matsuda 1991 (SGACCS).
| Methods | RCT (Randomised Controlled Trial), multicentre (Japan) | |
| Participants | Patients with colorectal cancer (unspecified TNM stage) undergoing radical surgery (n=2477) | |
| Interventions | 2‐arm trial: patients randomly assigned to: A) surgery alone (n=1182), or B) surgery + adjuvant chemotherapy (n=1295) with Tegafur (an oral fluoropyrimidine) plus intravenous MMC | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | The original article is in Japanese. For this meta‐analysis, the data were retrieved from an individual patient data (IPD) meta‐analysis (Sakamoto 1999) where patients with rectal cancer treated with surgery alone (n=598) were compared with those treated with adjuvant chemotherapy (n=645) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | By envelop method |
| Selective reporting (reporting bias) | Unclear risk | Data from an IPD meta‐analysis, not the original article |
QUASAR 2007.
| Methods | RCT (Randomised Controlled Trial), multicentre (UK) | |
| Participants | Patients with stage II‐III colon or rectal cancer undergoing radical surgery (n=3239). 91% with stage II (node negative) disease, 71% with colon cancer |
|
| Interventions | 2‐arm trial: all patients were randomly assigned to receive chemotherapy with fluorouracil and folinic acid (n=1622) or to observation (n=1617, with chemotherapy considered on recurrence) after apparently radical surgery of the primary tumour. Rectal cancer (stage II‐III): adjuvant (n=474) vs no adjuvant (n=474) Rectal cancer (stage II): adjuvant (n=410) vs no adjuvant (n=407) Rectal cancer (stage III): adjuvant (n=64) vs no adjuvant (n=67) |
|
| Outcomes | Stage II‐III: DFS and OS. Subgroup analysis: stage II: DFS and OS; stage III: DFS |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | Minimised randomisation to secure balanced allocation. Telephone call to central office. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Sakamoto 2007 (JFMTC15‐1).
| Methods | RCT (Randomised Controlled Trial); multicentre (Japan) | |
| Participants | Patients with Dukes A, B and C (TNM stage I, II, III) rectal cancer undergoing radical surgery of the primary tumor (n=447) | |
| Interventions | 2‐arm trial: patients randomly assigned to: A) surgery alone (n=229), or B) surgery + adjuvant chemotherapy (n=218) with Uracil‐Tegafur (UFT, an oral fluoropyrimidine) plus intravenous 5‐FU and MMC | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | The data are from an individual patient data (IPD) meta‐analysis (Sakamoto 2007 (JFMTC15‐1)) since no original article has been published on this RCT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Selective reporting (reporting bias) | High risk | Data from an IPD meta‐analysis (no original article) |
| Other bias | Low risk | |
Taal 2001 (NACCP).
| Methods | RCT (Randomised Controlled Trial); multicentre (Netherlands) | |
| Participants | Patients with Dukes´B and C (TNM stage II‐III) colon (n=730) and rectal (n=299) cancer undergoing radical surgery of the primary tumour (n=1029) | |
| Interventions | 2‐arm trial: patients were randomly assigned to: A) surgery alone (n=515), or B) surgery + adjuvant chemotherapy (n=514) with 5‐FU plus levamisole | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | More than 50% of patients with rectal cancer underwent radiotherapy. No stratification between Dukes B and C. For this meta‐analysis, patients with rectal cancer treated with surgery alone (n=150) were compared to those undergoing surgery + adjuvant chemotherapy (n=149) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Other bias | Low risk | |
Thomas 1988 (GTSG).
| Methods | RCT (Randomised Controlled Trial); multicentre (USA) | |
| Participants | Patients undergoing curative resection of Dukes´B and C (TNM stage II‐III) rectal cancer (n=202) | |
| Interventions | 4‐arm trial: patients were randomized to: A) surgery alone (n=58), or B) adjuvant chemotherapy (n=48), or C) adjuvant radiotherapy (n=50), or D) adjuvant radio‐chemotherapy (n=46) Chemotherapy: 5‐FU + semustine |
|
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | No stratification between B and C In this meta‐analysis we compared patients randomized to adjuvant chemotherapy (n=48) versus those randomized to surgery alone (n=58) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Watanabe 2004 (JFMTC15‐2).
| Methods | RCT (Randomised Controlled Trial); multicentre (Japan) | |
| Participants | Patients with Dukes B and C (TNM stage II, III) rectal cancer undergoing radical surgery of the primary tumor (n=669) | |
| Interventions | 3‐arm trial: patients were randomly assigned to: either A) surgery alone (n=229), or B) surgery + adjuvant chemotherapy (n=218) with UFT (oral) + MMC and 5‐FU (intravenously), or C) surgery + adjuvant chemotherapy with UFT + MMC and 5‐FU combined with immunotherapy (OK‐432) (n=222) | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | For this meta‐analysis, relevant data were retrieved from an individual patient data (IPD) meta‐analysis (Sakamoto 2007 (JFMTC15‐1)) where patients treated with surgery alone (n=122) were compared to patients undergoing surgery plus adjuvant treatment (n=269) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The registered cases were randomly assigned to the treatment groups according to the assignment table. |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data were retrieved from an individual patients data (IPD) meta‐analysis (Sakamoto 2007 (JFMTC15‐1)) and were relative to 381 patients, whereas the original article was about 669 patients |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Yasutomi 1997 (JFMTC 7‐2).
| Methods | RCT (Randomised Controlled Trial); multicentre (Japan) | |
| Participants | Patients with Dukes A, B and C (TNM stage I, II, III) rectal cancer undergoing radical surgery of the primary tumor (n=713) | |
| Interventions | 2‐arm trial: patients were randomly assigned to: A) surgery alone (n=356), or B) surgery + adjuvant chemotherapy (n=357) with HCFU (oral) + MMC (mitomycin‐C, intravenously) | |
| Outcomes | Disease‐free survival (DFS) and overall survival (OS) | |
| Notes | The original article is in Japanese: data were retrieved from an individual patient data (IPD) meta‐analysis conducted in 2004 by the Meta‐analysis Group in Cancer (see Sakamoto 2004) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Selective reporting (reporting bias) | High risk | Data from an IPD meta‐analysis, not the original article |
| Other bias | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdi 1989 | Lacks stratification between colon and rectum for Dukes´C . DFS (disease‐free survival) and OS (overall survival). |
| Focan 2000 | Lacks CG (Control Group/ no further treatment). |
| Fountazilas 1999 | Lacks CG. |
| Gelber 1996 | Lacks CG. |
| Gennatas 2003 | Lacks CG. |
| Grage 1978 | Data included in Grage 1981 |
| GTSG 1992 | Lacks CG. |
| Hartung 1997 | Lacks CG. |
| Ito 2000 | Lacks CG. |
| Iwagaki 2001 | Lacks CG. |
| Koda 2003 | Lacks CG. |
| Lawrence 1978 | Lacks stratification between either Dukes´A, B or C, or Colon and Rectum. Ref. to earlier publication: Lawrence w, et al: Chemotherapy as an Adjuvant to surgery for Colorectal Cancer. An of Surg 1975; 18(5):616‐23 |
| Maehara 1998 | Lacks CG. |
| Min 2000 | Lacks CG. |
| Mitomi 1992 | Lacks CG. |
| O´Connell 1994 | Lacks CG. |
| Quisser 2000 | Lacks CG. |
| Robinson 1979 | Lacks CG. |
| Robinson 1980 | Lacks CG. |
| Sugimachi 1996 | Lacks CG. |
| Takahashi 1996 | Same trial as CCCSGJ 1995 |
| Tepper 1997 | Lacks CG. |
| Tepper 2002 | Lacks CG, and Chemotherapy followed by Chemoradiation therapy. |
| Tveit 1997 | CG vs. Radio‐& Chemotherapy. |
| Wolmark 2000 | Lacks CG. |
Contributions of authors
Kirkeby LT.: Protocol, Literature search
Harling H.: Advice, data extraction
Wille‐Jørgensen P.: Secondary draft, data extraction, data analysis
Petersen SH: Data extraction, analysis, primary draft, literature search
Mocellin S: Literature search, data extraction, statistical analysis
Sources of support
Internal sources
HS: Bispebjerg Research Fund,, Denmark.
External sources
No sources of support supplied
Declarations of interest
None declared
New, comment added to review
References
References to studies included in this review
Bosset 2006 (EORTC) {published data only}
- Bosset JF, Collette L, Calais G, et al. Chemotherapy with pre‐operative radiotherapy in rectal cancer. NEJM 2006;355(11):1114‐23. [DOI] [PubMed] [Google Scholar]
Cafiero 2003 {published data only}
- Cafiero F, Gipponi M, Lionetto R. Randomised clinical trial of adjuvant postoperative RT vs. sequential postoperative RT plus 5‐FU and levamisole in patients with stage II‐III resectable rectal cancer: a final report. J Surg Oncol 2003;83:140‐6. [DOI] [PubMed] [Google Scholar]
CCCSGJ 1995 {published data only}
- The Colorectal Cancer Chemotherapy Study Group Of Japan. Five‐year Results of a Randomized Controlled Trial of Adjuvant Chemotherapy for Curatively Resected Colorectal Carcinoma. Jpn J Clin Oncol 1995;25:91‐103. [PubMed] [Google Scholar]
Fisher 1988 (NSABP) {published data only}
- Fisher B, Wolmark N, Rockette H, Redmond C, Deutsch M, Wickerham DL, Fisher ER, Caplan R, Jones J, Lerner H, Gordon P, Feldman M, Cruz A, Legault‐Poisson, Wexler M, Lawrence W, Robidoux A, et al. Postoperative Adjuvant Chemotherapy or Radiation Therapy for Rectal Cancer: Results From NSABP Protocol R‐01. Journal of the National Cancer Institute 1988;80(1):21‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Glimelius 2005 (NGTATG) {published data only}
- Glimelius B, Dahl O, Cedermark B, Jakobsen A, Bentzen SM, Starkhammar H, Grönberg H, Hultborn R, Albertsson M, Pahlman L, Tveit KM. Adjuvant chemotherapy in colorectal cancer: a joint analysis of randomised trials by the Nordic Gastrointestinal Tumour Adjuvant Therapy Group. Acta Oncol 2005;44(8):904‐12. [DOI] [PubMed] [Google Scholar]
Grage 1981 {published data only}
- Grage TB, Moss SE. Adjuvant Chemotherapy in Cancer of the Colon and Rectum: Demostration of Effectiveness Prolonged 5‐FU Chemotherapy in a Prospectively Controlled Randomized Trial. Surgical Clinics of North America 1981;61(6):1321‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hafström 1990 {published data only}
- Hafström L, Domellöf L, Rudenstam CM, Norryd C, Bergman L, Nilsson T, Hansson K, Wählby L, Asklöf G, Kugelberg C, and the Swedish Gastrointestinal Tumor Adjuvant Therapy Group. Adjuvant chemotherapy with 5‐fluorouracil, vincristine and CCNU for patients with Dukes´C colorectal cancer. British Journal of Surgery 1990;77:1345‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hamaguchi 2011 {published data only}
- Hamaguchi T, Shirao K, Moriya Y, Yoshida S, Kodaira S, Ohashi Y. Final results of randomized trials by the National Surgical Adjuvant Study of Colorectal Cancer (NSAS‐CC).. Cancer Chemother Pharmacol 2011;67(3):587‐96. [PUBMED: 20490797] [DOI] [PubMed] [Google Scholar]
Ito 1996 (TSGHCFU) {published data only}
- Ito K, Yamaguchi A, Miura K, Kato T, Baba S, Matsumoto S, Ishii M, Takagi H. Oral adjuvant chemotherapy with carmofur (HCFU) for colorectal cancer: five‐year follow‐up. Tokai HCFU Study Group‐‐third study on colorectal cancer. J Surg Oncol 1996;63(2):107‐11. [DOI] [PubMed] [Google Scholar]
Kato 2002 (TACSG) {published data only}
- Kato T, Ohashi Y, Nakazato H, Koike A, Saji S, Suzuki H, Takagi H, Nimura Y, Hasumi A, Baba S, Manabe T, Maruta M, Miura K, Yamaguchi A. Efficacy of oral UFT as adjuvant chemotherapy to curative resection of colorectal cancer: multicenter prospective randomized trial. Lagenbeck´s Archives of Surgery 2002;386:575‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koda 2009 {published data only}
- Koda K, Saito N, Ochia T, Miyazaki M, Sarashina H, Nakajima N. Randomized, controlled study of continuous 5‐FU infusion starting immediately after curative surgery for advanced colorectal cancer. Hepatogastroenterology 2009;56(89):116‐9. [PubMed] [Google Scholar]
Kodaira 1998 (JFMTC 7‐1) {published data only}
- Kodeira S, Kikuchi K, Yasutomi m, Takahashi T, Hojo K, Tomoyuki K, Tominaga T, Kunii Y. Postoperative adjuvant chemotherapy with mitomycin C and UFT for curatively resected rectal cancer. Results from the Cooperative Project No.7 Group of the Japanese Foundation for Multidisciplinary Treatment of Cancer. International Journal of Clinical Oncology 1998;3:357‐64. [Google Scholar]
Kornek 1996 {published data only}
- Korneck GV, Dapisch D, Salem G, Karall M, Rorhbacher M, Scheithauer W. Combined Locoregional and Systemic Adjuvant Chemotherapy of Stage II and III Rectal Carcinoma. Onkologie 1996;19:147‐51. [CN‐00183899] [Google Scholar]
Krook 1991 (NCCTG) {published data only}
- Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, et al. Effective surgical adjuvant therapy and chemotherapy for high‐risk rectal carcinoma.. N Eng J Med 1991;324:709‐15. [DOI] [PubMed] [Google Scholar]
Matsuda 1991 (SGACCS) {published data only}
- Matsuda T, Yasutomi M, Kikuchi K, Kasai Y, Abe O, Kondo T, Taguchi T, Hattori T, Inokuchi K, Komi N. Cooperative study of surgical adjuvant chemotherapy for colorectal cancer (third report): five‐year results. Cooperative Study Group of Surgical Adjuvant Chemotherapy for Colorectal Cancer in Japan. Gan To Kagaku Ryoho 1991;18(3):461‐9. [PubMed] [Google Scholar]
QUASAR 2007 {published data only}
- Quasar Collaborative Group. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370(9604):2020‐29. [DOI] [PubMed] [Google Scholar]
Sakamoto 2007 (JFMTC15‐1) {published data only}
- Sakamoto J, Hamada C, Yoshida S, Kodaira S, Yasutomi M, Kato T, Oba K, Nakazato H, Saji S, Ohashi Y. An individual patient data meta‐analysis of adjuvant therapy with uracil‐tegafur (UFT) in patients with curatively resected rectal cancer. Br J Cancer 2007;96(8):1170‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taal 2001 (NACCP) {published data only}
- Taal BG, Tinteren H, Zoetmulder FAN, on behalf of the NCCAP group. Adjuvant 5‐FU plus levamisol in colonic or rectal cancer: improved survival in stage II and III. British Journal of Cancer 2001;85(10):1437‐43. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 1988 (GTSG) {published data only}
- Thomas PRM, Lindblad AS. Adjuvant postoperative radiotherapy and chemotherapy in rectal carcinoma: A review of the Gastrointestinal Tumor Study Group Experience. Radiotherapy and Oncology 1988;13:245‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Watanabe 2004 (JFMTC15‐2) {published data only}
- Watanabe M, Nishida O, Kunii Y, Kodaira S, Takahashi T, Tominaga T, Hojyo K, Kato T, Niimoto M, Kunitomo K, Isomoto H, Ohashi Y, Yasutomi M. Randomized controlled trial of the efficacy of adjuvant immunochemotherapy and adjuvant chemotherapy for colorectal cancer, using different combinations of the intracutaneous streptococcal preparation OK‐432 and the oral pyrimidines 1‐hexylcarbamoyl‐5‐fluorouracil and uracil/tegafur. Int J Clin Oncol 2004;9(2):98‐106. [DOI] [PubMed] [Google Scholar]
Yasutomi 1997 (JFMTC 7‐2) {published data only}
- Yasutomi M, Takahashi T, Kodaira S, Hojo K, Kato T, Ogawa M, Ichihashi H, Tominaga T, Tamada R, Kunii Y, Kikuchi K. Prospective controlled study on the usefulness of Carmofur as a postoperative adjuvant chemotherapy for colorectal cancer. Gan To Kagaku Ryoho 1997;24(13):1953‐60. [PubMed] [Google Scholar]
References to studies excluded from this review
Abdi 1989 {published data only}
- Abdi EA, Hanson J, Harbora DE, Young DG, McPherson TA. Adjuvant Chemoimmuno‐ and Immunnotherapy in Dukes' Stage B2 and C Colorectal Carcinoma: A 7‐year follow‐up Analysis. Journal of Surgical Oncology 1989;40:205‐13. [DOI] [PubMed] [Google Scholar]
Focan 2000 {published data only}
- Focan B, Bury J, Beauduin M, Herman ML, Vindevoghel A, Lecomte M Brohee D, Conan JL, Focan‐Henrard D. Importance of 5‐flourouracil dose‐intensity in a double randomised trial on adjuvant portal and systemic chemotherapy for Dukes´ B2 and C colorectal cancer. Anticancer research 2000;20(6C):4665‐75. [MEDLINE: ] [PubMed] [Google Scholar]
Fountazilas 1999 {published data only}
- Fountazilas G, Zisiadis A, Defni U, Konstantaras C, Hatzitheoharis G, Liaros A, Athanassiou E, Drombros N, Dervenis N, Dervernis C, Basdanis G, Gamvros O, Souparis A, Briasoulis E, Samantas E, Kappas A, Kosmidis P, Skarlos D, Pavlidis N. Postoperative radiation and concomitant bolus fluorouracil with or without additionel chemotherapy with fluorouracil and high‐dose leucovorin in patients with high‐risk cancer: a randomized phase III study conducted by the Helllenic Cooperative Oncology Group. Annals of Oncology 1999;10(6):671‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gelber 1996 {published data only}
- Gelber RD, Goldhirsch A, Cole BF, Wieland HS, Schroeder G, Krook JE. A quality‐adjusted time without symptoms or toxicity (Q‐TWIST) analysis of adjuvant radiation therapy and chemotherapy for resectable rectal cancer.. Journal of the National Cancer Institute 1996;88(15):1039‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gennatas 2003 {published data only}
- Gennatas G, Dardoufas C, Mouratidou D, Tsavaris N, Pouli A, Androulakis G, et al. Surgical adjuvant therapy or rectal carcinoma: a controlled evaluation of leucovorin, 5‐fluorouracil and radiation therapy with og without interferon‐@2b. Annals of Oncology 2003;14:378‐82. [DOI] [PubMed] [Google Scholar]
Grage 1978 {published data only}
- Grage TB, Hill GJ, Cornell GN, Frelick RW, Moss SE. Adjuvant chemotherapy in large bowel cancer: demonstration of effectiveness of single agent in a prospectively controlled, randomised trial. Recent Results Cancer Res 1978;68:222‐30. [DOI] [PubMed] [Google Scholar]
GTSG 1992 {published data only}
- Gastrointestinal Tumor Study Group. Radiation therapy and fluorouracil with or without semustine for the treatment of patientents with surgical advanced adenocarcinoma of the rectum.. Journal of Clinical Oncology 1992;10(4):549‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hartung 1997 {published data only}
- Hartung G, Queisser W, Maas K, Diezler P, Hagmüeller E, Edler E, et al. Adjuvant radio‐chemotherapy with 5 FU and folonic acid in stage II and III rectal cancer: interim analysis. Onkologie 1997;20:231‐4. [Google Scholar]
Ito 2000 {published data only}
- Ito K, Kato T, Koike A, Miura K, Yamaguchi A, Sakou T, et al. Optimum duration of oral adjuvant chemotherapy of HCFU for colorectal cancer.. Anticancer Research 2000;20(6C):4681‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Iwagaki 2001 {published data only}
- Iwagaki H, Tanaka N, Esato K, Kaibara N, Sano K, Dohi K, et al. POst‐operative adjuvant chemotherapy for colorectal cancer with 5‐fluorouracil (5‐FU) infusion combined with 1‐hexylcarbamoyl‐5‐fluorouracil (HCFU) oral administration after curative resection.. Anticancer Research 2001;21(6A):4163‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Koda 2003 {published data only}
- Koda K, Miyazaki M, Sarashina H, Suwa T, Saito N, Suzuki M, et al. A randomized controlled trial of postoperative adjuvant immunochemotherapy for colorectal cancer with oral medicines. International Journal of Oncology 2003;23:165‐72. [CN‐00472836] [PubMed] [Google Scholar]
Lawrence 1978 {published data only}
- Lawrence W jr, Terz JJ, Horsley S III, Brown PW, Romero C. Chemotherapy as an Adjuvant to Surgery for Colorectal Cancer: A follow‐up Report. Archives or Surgery 1978;113:164‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maehara 1998 {published data only}
- Maehara Y, Sugimachi K, Ogawa M, Kakegawa T, Tomita M, Akiyoshi T. Postoperative adjuvant chemotherapy with 1‐hexylcarbamoyl‐5‐fluorouracil in patients with colorectal cancer and at a high risk for recurrence.. Anticancer Research 1998;18(6B):4629‐34. [MEDLINE: ] [PubMed] [Google Scholar]
Min 2000 {published data only}
- Min JS, Kim NK, Park JK, Yun SH, Noh JK. A prospective randomized trial comparing intravenous 5‐fluorouracil and oral doxifluridine as postoperative adjuvant treatment for advanced rectal cancer.. Annals of Surgical Oncology 2000;7(9):374‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mitomi 1992 {published data only}
- Mitomi T, Tsuchiya S, Iijima N, Aso K, Suzuki K, Nishiyama K, et al. Randomized controlled study on adjuvant immunochemotherapy with PSK in curatively resected colorectal cancer. The Cooperative Study Group of Surgical Adjuvant immunochemotherapy for Cancer of Colon and Rectum (Kanagawa).. Diseases of the Colon and Rectum 1992;35(2):123‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
O´Connell 1994 {published data only}
- O´Connell MJ, Martenson JA, Wieand HS, Krook JE, Macdonald JS, Haller DG, et al. Improving adjuvant therapy for rectal cancer by combining protracted‐infusion fluorouracil with radiation therapy after curative surgery.. New England Journal of Medicin. 1994;331(8):502‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Quisser 2000 {published data only}
- Quisser W, Hartung G, Kopp‐Schneider A, Diezler P, Hagmüeller E, Bauer A, et al. Adjuvant readio‐chemotherapy with 5‐fluorouracil and leucovorin in stage II and III rectal cancer: 12 months vs. 6 months of therapy.. Onkologie 2000;23:334‐9. [Google Scholar]
Robinson 1979 {published data only}
- Robinson E, Bartal A, Cohen Y, Milstein D, Mekori T. Adjuvant therapy in colorectal cancer. ( A randomized trial comparing radio‐chemotherapy and radio‐chemotherapy combined with the methanol extraction residue of BCG, MER).. Biomedicin 1979;31(1):8‐10. [MEDLINE: ] [PubMed] [Google Scholar]
Robinson 1980 {published data only}
- Robinson E, Bartal A, Mekori T. Results of postoperative treatment of colorectal cancer by radiotherapy, chemotherapy and immunotherapy.. Recent Results Cancer Res. 1980;75:80‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sugimachi 1996 {published data only}
- Sugimachi K, Maehara Y, Ogawa M, Kakegawa T, Tomita M, Akiyoshi T. Postoperative chemotherapy for colorectal cancer by combining 5‐fluorouiracil infusion and 1‐hexylcarbamoyl‐5‐fluorouracil administration after curative resection.. 1996 77;1:36‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Takahashi 1996 {published data only}
- Takahashi T, Kato T, Kodeira S, Koyama Y, Sakabe T, Tominaga T, Hamano K, Yasutomi M, Ogawa N. Prognostic Factors Of Colorectal Cancer. American Journal of Clinical Oncology 1996;19(4):408‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tepper 1997 {published data only}
- Tepper JE, O´Connell MJ, Petroni GR, Hollis D, Crooke E, Benson AB III, et al. Adjuvant postoperative fluorouracil‐modulated chemotherapy combined with pelvic radiation therapy for rectal cancer: inital results of intergroup 0114.. Journal of Clinical Oncology 1997;15(5):2030‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tepper 2002 {published data only}
- Tepper JE, O´Connell MJ, Niedzwiecki D, Hollis DR, Benson AB 3rd, Cummings B, et al. Adjuvant Therapy in Rectal Cancer: Analysis of Stage , Sex, and Local Control‐Final Report of intergroup 0114.. Journal of Clinical Oncology 2002;20(7):1744‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tveit 1997 {published data only}
- Tveit KM, Guldvog I, Hagen S, Trondsen E, Harbitz T, Nygarrd K, et al. Randomized controlled trial of postoperative radiotherapy and short‐term time‐scheduled 5‐fluorouracil against surgery alone in treatment of Dukes´ B and C rectal cancer. Norwegian Adjuvant Rectal Cancer Project Group.. British Journal of Surgery 1997;84(8):1130‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Wolmark 2000 {published data only}
- Wolmark N, Wieand HS, Hyams DM, Colangelo L, Dimitrov NV, Romond EH, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R‐02.. Journal of National Cancer Institute. 2000;92(5):388‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Aaronson 1993
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Fletchner H, Fleishman SB, Haes JC, et al. The European organization for research and treatment of cancer QLQ‐ Quality of Life instrument for use in international clinical trials in oncology. Journal of the national cancer institute 1993;85(5):365‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Akasu 2006
- Akasu T, Moriya Y, Ohashi Y, Yoshida S, Shirao K, Kodaira S. Adjuvant chemotherapy with uracil‐tegafur for pathological stage III rectal cancer after mesorectal excision with selective lateral pelvic lymphadenectomy: a multicenter randomized controlled trial. Jpn J Clin Oncol 2006;36(4):237‐44. [DOI] [PubMed] [Google Scholar]
Beart 2001
- Beart BW Jr. Current surgical management of colorectal cancer. In: R.W.Beart, Jr editor(s). American Society of Clinical Oncology educational book 2001. 37. annual meeting. San Francisco: Am. soc. Clin. Oncol, 2001:174‐177. [Google Scholar]
Borly 2001
- Borly L, Wille‐Jørgensen P, Rasmussen MS, Andersen BR. Problems with retriving original data; is it a selection bias. Poster 146, Cochrane Colloqium, Lyon. October 2001.
Bosset 2005
- Bosset JF, Calais G, Mineur L, et al. Enhanced Tumorocidal Effect of Chemotherapy with Preoperative Radiotherapy for Rectal Cancer: Preliminary Results‐EORTC 22921. J Clin Oncol 2005;23(24):5620‐27. [DOI] [PubMed] [Google Scholar]
Bosset 2006
- Bosset JF, Collette L, Calais G, et al. Chemotherapy with pre‐operative radiotherapy in rectal cancer. NEJM 2006;355(11):1114‐23. [DOI] [PubMed] [Google Scholar]
Bujko 2004
- Bujko K, Nowacki MP, Nasierowska‐Guttmejer A, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomized trial comparing short‐term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol 2004;72(1):15‐24. [DOI] [PubMed] [Google Scholar]
Bujko 2010
- Bujko K, Glynne‐Jones R, Bujko M. Does adjuvant fluoropyrimidine‐based chemotherapy provide a benefit for patients with resected rectal cancer who have already received neoadjuvant radiochemotherapy? A systematic review of randomised trials.. Ann Oncol 2010;21:1743‐50. [DOI] [PubMed] [Google Scholar]
Ceelen 2009
- Ceelen WP, Nieuwenhove Y, Fierens K. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev 2009, (1):CD006041. [DOI] [PubMed] [Google Scholar]
Collette 2007
- Collette L, Bosset JF, Dulk M, et al. Patients with Curative Resection of cT3‐4 rectal Cancer after preoperative Radiotherapy or Radiochemotherapy: Does anybody benefit From Adjuvant Fluorouracil‐Based Chemotherapy? A Trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol 2007;25(28):4379‐86. [DOI] [PubMed] [Google Scholar]
Compton 2001
- Compton CC. Predicting risk of recurrence in rectal cancer. American Society of Clinical Oncology. San Francisco: ASCO, 2001; Vol. 37. ann. meeting:200‐209.
Das 2006
- Das P, Skibber JM, Rodriguez‐Bigas MA, et al. Clinical and Pathologic Predictors of Locoregional Recurrence, Distant Metastasis, and Overall Survival in Patients Treated With Chemoradiation and Mesorectal Excision for Rectal Cancer. Am J Clin Oncol 2006;29(3):219‐24. [DOI] [PubMed] [Google Scholar]
Dobelbower 1991
- Dobelbower RR, Loeffler RK, MerrickIII HW, Milligan AJ, Bagne FR, Bronn DG, Lokshina AM. Radiation therapy in cancer management. In: Moossa AR, Schimpf SC, Robson MC editor(s). Comprehensive textbook of oncology. 2. Vol. 1, Baltimore: Williams and Wilkins, 1991:519. [ISBN0‐683‐06147‐X] [Google Scholar]
Dunst 2008
- Dunst J, Debus J, Rudat V, et al. Neoadjuvant Capecitabine Combined with Standard Radiotherapy in Patients with Locally Advanced Rectal Cancer. Strahlenther Onkol 2008;184(9):450‐56. [DOI] [PubMed] [Google Scholar]
Duval 2000
- Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56(2):455‐63. [DOI] [PubMed] [Google Scholar]
Enker 1995
- Enker WE, Thaler HT, Cranor ML, Polyak T. Total mesorectal excision in the operative treatment of the rectum [Total mesorectal excision in the operative treatment of the rectum]. J Amer Coll Surgeon 1995;181:335‐46. [PubMed] [Google Scholar]
Fietkau 2006
- Fietkau R, Barten M, Klautke G, et al. Postoperative Chemotherapy May Not Be Necessary for Patients With ypN0‐Category After Neoadjuvant Chemoradiotherapy of Rectal Cancer. Dis Colon Rectum 2006;49(9):1284‐92. [DOI] [PubMed] [Google Scholar]
Gerard 2006
- Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3‐4 rectal cancers: results of FFCD 9203. J Clin Oncol 2006;24:4620‐25. [DOI] [PubMed] [Google Scholar]
Glimelius 2004
- Glimelius B. Adjuvant chemotherapy in rectal cancers: why a standard in th US and not in Europe. Nat Clin Pract Oncol 2004;1(2):58‐9. [DOI] [PubMed] [Google Scholar]
Glimelius 2010
- Glimelius B, Pahlman L, Cervantes A, ESMO Guidelines Working Group. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2010;21(Suppl 5):v82‐6. [DOI] [PubMed] [Google Scholar]
Glynne‐Jones 2006
- Glynne‐Jones R, Dunst J, Sebag‐Montefiore D. The integration of oral capecitabine into chemoradiation regimens for locally advanced rectal cancer: how successful have we been?. Annals of Oncology 2006;17:361‐71. [DOI] [PubMed] [Google Scholar]
GTSG 1985
- Gastrointestinal Tumor Study Group. Prolongation of the disease‐free survival in surgically treated rectal carcinoma [Prolongation of the disease‐free survival in surgically treated rectal carcinoma]. NEJM 1985;312:1465‐72. [DOI] [PubMed] [Google Scholar]
Guillem 2008
- Guillem J, Diaz‐Gonzàles JA, Minsky BD, et al. cT3N0 Rectal Cancer: Potential Overtreatment with Preoperative Chemoradiotherapy Is Warranted. J Clin Oncol 2008;26:368‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & sons, 2008. [Google Scholar]
Janjan 2001
- Janjan NA, Crane C, Feig BW, et al. Improved Overall Survival Among Responders to Preoperative Chemoradiation for Locally Advanced Rectal Cancer. J Clin Oncol 2001;24(2):107‐12. [DOI] [PubMed] [Google Scholar]
Kapiteijn 2001
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer [Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer]. NEJM 2001;345:638‐646. [DOI] [PubMed] [Google Scholar]
Kapiteijn 2002
- Kapiteijn E, Putter H, Velde CJH, et al. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands [Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands]. Brittish journal of Surgery 2002;89(9):1142‐49. [DOI] [PubMed] [Google Scholar]
Kim 2005
- Kim JC, Kim TW, Kim JH, et al. Preoperative concurrent radiotherapy with capecitabine before total mesorectal excision in locally advanced rectal cancer. Int J Radiation Oncology Biol. Phys 2005;63(2):346‐53. [DOI] [PubMed] [Google Scholar]
Kronborg 1998
- Kronborg O, Burcharth F, Bülow S, Christiansen J, Gandrup P, Hanberg F, Harling H, Rasmussen PC, Jakobsen A, Mejer J, Fenger C. Guidelines for diagnosing and treating of colorectal cancer [Retningslinier for diagnostik og behandling af kolorekral cancer]. Klaringsrapport, Dansk Kirurgisk Selskab 1998; Vol. 4:1‐29. [ISSN: 1398‐1560]
Krook 1991
- Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high‐risk rectal carcinoma [Effective surgical adjuvant therapy for high‐risk rectal carcinoma]. NEJM 1991;324(1):709‐15. [DOI] [PubMed] [Google Scholar]
Madoff 2008
- Madoff RD. Chemotherapy for rectal Cancer‐ When, Why and How?. NEJM 2008;351(17):1790‐92. [DOI] [PubMed] [Google Scholar]
Minsky 2001
- Minsky BD. Approaches to adjuvant therapy for rectal cancer. American Society of Clinical Oncology. San Francisco: ASCO, 2001:187‐194.
Mocellin 2005
- Mocellin S, Lise M, Nitti D. Targeted therapy for colorectal cancer: mapping the way. Trends Mol Med 2005;11(7):327‐35. [DOI] [PubMed] [Google Scholar]
Moertel 1990
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, Ungerleider JS, Emerson WA, Tormey DC, Glick JH. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322(6):352‐8. [DOI] [PubMed] [Google Scholar]
Mohiuddin 2006
- Mohiuddin M, Winter K, Mitchell E, et al. Randomized Phase II Study of Neoadjuvant Combined‐Modality Chemoradiation for Distal Rectal Cancer: Radiation Therapy Oncology Group Trial 0012. J Clin Oncol 2006;24(4):650‐55. [DOI] [PubMed] [Google Scholar]
NCCN 2008
- NCCN. Practice Guidelines in Oncology v.3 [Practice Guidelines in Oncology v.3]. National Comprehensive Cancer Network 2008.
NIH CC 1990
- NIH Consensus Conference. Adjuvant Therapy for Patients with Colon and rectal Cancer. JAMA 1990;264(11):1444‐50. [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Stat Med 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
PRISMA
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. PLoS Med 2009;6(6):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quasar CG 2007
- Quasar Collaborative Group. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370(9604):2020‐29. [DOI] [PubMed] [Google Scholar]
Roh 2004
- Roh MS, Colangelo L, Wieand S, et al. Response to preoperative multimodality therapy predicts survival in patients with carcinoma of the rectum. Proc Am Soc Clin Oncol 2004;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rödel 2007
- Rödel C, Sauer R. Integration of Novel Agents into Combined‐Modality Treatment for Rectal Cancer Patients. Strahlenther Onkol 2007;183(5):227‐35. [DOI] [PubMed] [Google Scholar]
Sakamoto 1999
- Sakamoto J, Hamada C, Kodaira S, Nakazato H, Ohashi Y. Adjuvant therapy with oral fluoropyrimidines as main chemotherapeutic agents after curative resection for colorectal cancer: individual patient data meta‐analysis of randomized trials. Jpn J Clin Oncol 1999;29(2):78‐86. [DOI] [PubMed] [Google Scholar]
Sakamoto 2004
- Sakamoto J, Ohashi Y, Hamada C, Buyse M, Burzykowski T, Piedbois P. Efficacy of oral adjuvant therapy after resection of colorectal cancer: 5‐year results from three randomized trials. J Clin Oncol 2004;22(3):484‐92. [DOI] [PubMed] [Google Scholar]
Sauer 2004
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative Chemoradiotherapy for rectal Cancer. NEJM 2004;351(17):1731‐40. [DOI] [PubMed] [Google Scholar]
SBU 2001
- Swedish Council on Technology assessment in Health Care. Chemotherapy for Cancer. Chemotherapy for Cancer. Vol. II, Section 8, Stockholm: Swedish Council on Technology assessment in Health Care, 2001, Feb.:299‐362. [Google Scholar]
SECT 1997
- Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer [Improved survival with preoperative radiotherapy in resectable rectal cancer]. NEJM 1997;336:980‐87. [DOI] [PubMed] [Google Scholar]
Skibber 2001
- Skibber JM, Hoff PM, Minsky BD. Cancer of the Rectum [Cancer of the Rectum]. Cancer: Principles and practice of Oncology. 6 th edition. Philadelphia: Lippincott, Williams and Wilkins, 2001:1271‐318. [Google Scholar]
Sterne 2000
- Publication, related bias in meta‐analysis: power of statistical tests, prevalence in the literature. Sterne JA, Gavaghan D, Egger M.. J Clin Epidemiol 2000;53(11):1119‐29. [DOI] [PubMed] [Google Scholar]
Sutton 2000
- Sutton A, Abrams K, Jones D, Sheldon T, Song. Methods for Meta‐analysis in Medical Research. Methods for Meta‐analysis in Medical Research. John Wiley & Sons, 2000. [Google Scholar]
Tanaka 2007
- Tanaka F. UFT (Tegafur and Uracil) as Postoperative Adjuvant Chemotherapy for Solid Tumors (Carcinoma of the Lung, Stomach, Colon/Rectum, and Breast): Clinical Evidence, Mechanism of Action, and Future Direction. Surg Today 2007;37(11):923‐43. [DOI] [PubMed] [Google Scholar]
Van Cutsem 2008
- Cutsem E, Dicato M, Haustermans K, Arber N, Bosset JF, Cunningham D, Gramont A, Diaz‐Rubio E, Ducreux M, Goldberg R, Glynne‐Jones R, Haller D, Kang YK, Kerr D, Labianca R, Minsky BD, Moore M, Nordlinger B, Rougier P, Scheithauer W, Schmoll HJ, Sobrero A, Tabernero J, Tempero M, Velde C, Zalcberg J. The diagnosis and management of rectal cancer: expert discussion and recommendations derived from the 9th World Congress on Gastrointestinal Cancer, Barcelona, 2007. Ann Oncol 2008;19(Suppl 6):vi1‐8. [DOI] [PubMed] [Google Scholar]
Wagner 2009
- Wagner AD, Arnold D, Grothey AA, Haerting J, Unverzagt S. Anti‐angiogenic therapies for metastatic colorectal cancer. Cochrane Database Syst Rev 2009, (3):CD005392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36 item health survey (SF‐36). Medical care 1992;30:473‐483. [PubMed] [Google Scholar]
Watanabe 2008
- Watanabe T. Chemoradiotherapy and adjuvant chemotherapy for rectal cancer. Int J Clin Oncol 2008;13:488‐97. [DOI] [PubMed] [Google Scholar]
Wills 2001
- Wils J, Dwyer PO, Labianca R. Adjuvant treatment of colorectal cancer at the turn of the century. Annals of Oncology 2001;12:13‐22. [DOI] [PubMed] [Google Scholar]
Wolpin 2007
- Wolpin BM, Meyerhardt JA, Mamon HJ, Mayer RJ. Adjuvant therapy of colorectal cancer. CA Cancer J Clin 2007;57:168‐85. [DOI] [PubMed] [Google Scholar]
Wong 2007
- Wong RK, Tandan V, Silva S, Figueredo A. Pre‐operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database of Systematic Reviews 2007 Apr 18, Issue 2. [DOI: 10.1002/14651858.CD002102.pub2] [DOI] [PubMed] [Google Scholar]


