Abstract
Background
Dental implants are usually placed by elevating a soft tissue flap, but in some instances, they can also be placed flapless reducing patient discomfort. Several flap designs and suturing techniques have been proposed. Soft tissues are often manipulated and augmented for aesthetic reasons. It is often recommended that implants are surrounded by a sufficient width of attached/keratinised mucosa to improve their long‐term prognosis.
Objectives
To evaluate whether (1a) flapless procedures are beneficial for patients, and (1b) which is the ideal flap design; whether (2a) soft tissue correction/augmentation techniques are beneficial for patients, and (2b) which are the best techniques; whether (3a) techniques to increase the peri‐implant keratinised mucosa are beneficial for patients, and (3b) which are the best techniques; and (4) which are the best suturing techniques/materials.
Search methods
The following electronic databases were searched: Cochrane Oral Health's Trials Register (to 9 June 2011), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 2), MEDLINE via OVID (1950 to 9 June 2011), EMBASE via OVID (1980 to 9 June 2011). Several dental journals were handsearched. There were no language restrictions.
Selection criteria
All randomised controlled trials (RCTs) of root‐form osseointegrated dental implants, with a follow‐up of at least 6 months after function, comparing various techniques to handle soft tissues in relation to dental implants. Outcome measures, according to the different hypotheses, were: prosthetic and implant failures, biological complications, aesthetics evaluated by patients and dentists, postoperative pain, marginal peri‐implant bone level changes on periapical radiographs, patient preference, ease of maintenance by patient, soft tissue thickness changes and attached/keratinised mucosa height changes.
Data collection and analysis
Screening of eligible studies, assessment of the methodological quality of the trials and data extraction were conducted at least in duplicate and independently by two or more review authors. Trial authors were contacted for missing information. Results were expressed using risk ratios for dichotomous outcomes and mean differences for continuous outcomes with 95% confidence intervals.
Main results
Seventeen potentially eligible RCTs were identified but only six trials with 138 patients in total could be included. One study was at low risk of bias, two studies were judged to be at unclear risk of bias and three at high risk of bias. Two trials (56 patients) compared flapless placement of dental implants with conventional flap elevation, one trial (10 patients) compared crestal versus vestibular incisions, one trial (20 patients) Erbium:YAG laser versus flap elevation at the second‐stage surgery for implant exposure, one split‐mouth trial (10 patients) evaluated whether connective tissue graft at implant placement could be effective in augmenting peri‐implant tissues, and one trial (40 patients) compared autograft with an animal‐derived collagen matrix to increase the height of the keratinised mucosa. On a patient, rather than per implant basis, implants placed with a flapless technique and implant exposures performed with laser induced statistically significantly less postoperative pain than flap elevation. Sites augmented with soft tissues connective grafts showed a better aesthetic and thicker tissues. Both palatal autografts or the use of a porcine‐derived collagen matrix are effective in increasing the height of keratinised mucosa at the price of a 0.5 mm recession of peri‐implant soft tissues. There were no other statistically significant differences for any of the remaining analyses.
Authors' conclusions
There is limited weak evidence suggesting that flapless implant placement is feasible and has been shown to reduce patient postoperative discomfort in adequately selected patients, that augmentation at implant sites with soft tissue grafts is effective in increasing soft tissue thickness improving aesthetics and that one technique to increase the height of keratinised mucosa using autografts or an animal‐derived collagen matrix was able to achieve its goal but at the price of a worsened aesthetic outcome (0.5 mm of recession). There is insufficient reliable evidence to provide recommendations on which is the ideal flap design, the best soft tissue augmentation technique, whether techniques to increase the width of keratinised/attached mucosa are beneficial to patients or not, and which are the best incision/suture techniques/materials. Properly designed and conducted RCTs, with at least 6 months of follow‐up, are needed to provide reliable answers to these questions.
Plain language summary
Interventions for replacing missing teeth: management of soft tissues for dental implants
Dental implants are usually placed by elevating a soft tissue flap, but in some instances, they can also be placed without flap elevation to reduce postoperative discomfort. Several flap and suturing techniques have been proposed. Soft tissues are often manipulated and augmented for aesthetic reasons. It is often recommended that implants are surrounded by 'firm' (attached/keratinised) soft tissues rather than 'movable' gums to improve their long‐term prognosis. The review found some weak evidence from only two studies with 56 patients that flapless placement of dental implants reduces postoperative discomfort (pain and swelling), without jeopardising implant success in selected patients. There is insufficient evidence to recommend any specific flap or suturing technique. There is only a small study (10 patients) suggesting that soft tissue grafts from the palate improve gum thickness and aesthetics. There are no studies evaluating whether there is a benefit in increasing the amount of firm gum surrounding dental implants but there is a small trial suggesting that it is possible to increase the amount of firm gum surrounding dental implants using either tissue taken from the palate or a porcine‐derived collagen matrix at the price of considerable postoperative pain/discomfort and aesthetic deterioration (there were several cases where the gum receded exposing the metal of the implant).
Background
Missing teeth and supporting oral tissues have traditionally been replaced with dentures or bridges permitting restoration of chewing function, speech, and aesthetics. Dental implants offer an alternative. These implants are inserted into the jaw bones to support dental prostheses and are retained because of the intimacy of bone growth onto their surface. This direct structural and functional connection between living bone and implant surface, termed osseointegration, was first described by Brånemark (Brånemark 1977) and has undoubtedly been one of the most significant scientific breakthroughs in dentistry over the past 40 years.
Implant treatment is widely known by patients who nowadays have increasing aesthetic expectations. Implants are usually placed after soft tissue flap elevation (Brånemark 1977) to visualise better the bone sites where the implant(s) will be placed. Flap elevation ensures that some anatomical landmarks (e.g. foramina, lingual undercuts or maxillary sinuses) are clearly identified and protected. When the amount of available bone is limited, flap elevation will facilitate implant placement maximising bony contact while minimising the risk of bone fenestrations. However, flap elevation is associated with some degree of morbidity and discomfort, and requires suturing. There are situations where flap elevation may not be necessary to allow a successful implant placement since the amount of bone is more than adequate and the risk of complications is minimal. Under these circumstances, flapless implant placement may be indicated. When placing implants with a flapless procedure the surgeon is working blindly and bone perforations are more likely to occur. Guided surgery aided with customised surgical templates derived from CT scans can help clinicians to minimise the risk of perforation and incorrect implant alignment (van Steenberghe 2005). From a patient perspective it would be ideal to minimise postoperative discomfort, if the risks of complications/implant failures are not increased.
Sometimes the aesthetic outcome of implant therapy is not ideal. Compromised aesthetics are usually caused by lack of sufficient bone after tooth loss. Several techniques have been proposed to augment bone prior or at implant placement (Esposito 2009; Esposito 2010), however, there are situations in which it might be possible to obtain good aesthetic results only by manipulating or augmenting soft tissues. Numerous techniques have been proposed to design flaps, to rebuild the lost interdental papillae, to augment the soft tissues, to suture flaps etc, but it is still unclear which techniques could achieve the best results in a predictable way. Many variations have strong proponents with surgeons claiming that a particular technique offers improved aesthetics. However, there is frequently disagreement and this area is controversial.
Another area of controversy is the need for an adequate band of keratinised/attached mucosa around dental implants to improve their long‐term prognosis. Many authors strongly advocate the use of techniques to increase the width of keratinised mucosa, however, no firm evidence has been provided to support this view (Wennström 1994; Chung 2006). A recent study even found a correlation between the presence of an "adequate width of keratinised mucosa" (> 2 mm) and the incidence of peri‐implant mucositis (Roos‐Jansåker 2006).
Objectives
To evaluate:
(1) Flap designs
(a) Whether and when flap elevation is necessary. (b) Which is the best flap design/technique for specific clinical indications.
(2) Soft tissue management/augmentation techniques to improve aesthetics
(a) Whether and when management/augmentation procedures are necessary. (b) Which is the most effective management/augmentation technique for specific clinical indications.
(3) Techniques to increase the peri‐implant attached/keratinised mucosa
(a) Whether and when the peri‐implant attached/keratinised mucosa has to be increased. (b) Which is the most effective technique to increase the attached/keratinised mucosa for specific clinical indications.
(4) Suturing techniques/materials
Which are the most effective suturing techniques/materials in relation to dental implants.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) with exception of quasi‐randomised controlled trials.
Types of participants
Any patient rehabilitated with implant supported/retained prostheses.
Types of interventions
Any flap design including flapless procedures (manual or computer‐guided), soft tissue augmentation and suture technique/material used in relation with osseointegrated root‐formed dental implants. Trials to be included had to have a minimum of 6‐month follow‐up after implant loading.
Types of outcome measures
All trials to be included have to report the following primary outcomes.
Prosthetic failures
Implant failures
Biological complications
The following additional outcomes were considered.
(1) Flap designs
Postoperative pain
Marginal bone level changes on periapical radiographs
Aesthetics evaluated by patients
Aesthetics evaluated by dentists
Patient preference including aesthetics (only in split‐mouth trials)
Height of the attached/keratinised mucosa changes
(2) Soft tissue management/augmentation techniques to improve aesthetics
Aesthetics evaluated by patients
Aesthetics evaluated by dentists
Soft tissue thickness changes
Postoperative pain
Patient preference including aesthetics (only in split‐mouth trials)
Ease of maintenance by patient
(3) Techniques to increase the peri‐implant attached/keratinised mucosa
Attached/keratinised mucosa height changes (for the study to be included this outcome has to be reported)
Postoperative pain
Ease of maintenance by patient
Marginal bone level changes on periapical radiographs
Patient preference including aesthetics (only in split‐mouth trials)
Aesthetics evaluated by patients
Aesthetics evaluated by dentists
(4) Suturing techniques/materials
Patient preference including aesthetics (only in split‐mouth trials)
Aesthetics evaluated by patients
Aesthetics evaluated by dentists
Postoperative pain
Search methods for identification of studies
For the identification of studies included or considered for this review, detailed search strategies were developed for each database searched. These were based on the search strategy developed for MEDLINE (OVID) but revised appropriately for each database. The search strategy used a combination of controlled vocabulary and free text terms and was run with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] (Higgins 2011). Details of the MEDLINE search are provided in Appendix 1.
Searched databases
Cochrane Oral Health's Trials Register (to 9 June 2011) (seeAppendix 2).
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 2) (seeAppendix 3).
MEDLINE via OVID (1950 to 9 June 2011) (seeAppendix 1).
EMBASE via OVID (1980 to 9 June 2011) (seeAppendix 4).
The most recent electronic search was undertaken on 9 June 2011.
Language
There were no language restrictions.
Unpublished studies
We wrote to all the authors of the identified RCTs, we checked the bibliographies of all identified RCTs and relevant review articles, and we used personal contacts in an attempt to identify unpublished or ongoing RCTs. In the first version of this review we also wrote to more than 55 oral implant manufacturers and we requested information on trials through an Internet discussion group (implantology@yahoogroups.com), however, we discontinued this due to poor yield.
Handsearching
Details of the journals being handsearched by Cochrane Oral Health's ongoing programme are given on the website: www.ohg.cochrane.org. The following journals have been identified as being potentially important to be handsearched for this review: British Journal of Oral and Maxillofacial Surgery, Clinical Implant Dentistry and Related Research, Clinical Oral Implants Research, European Journal of Oral Implantology, Implant Dentistry, International Journal of Oral and Maxillofacial Implants, International Journal of Oral and Maxillofacial Surgery, International Journal of Periodontics and Restorative Dentistry, International Journal of Prosthodontics, Journal of Clinical Periodontology, Journal of Dental Research, Journal of Oral Implantology, Journal of Oral and Maxillofacial Surgery, Journal of Periodontology, Journal of Prosthetic Dentistry. Where these have not already been searched as part of the Cochrane Journal Handsearching Programme, the journals were handsearched by one review author up to the month in which the last electronic search was undertaken.
Data collection and analysis
Selection of studies
The titles and abstracts (when available) of all reports identified through the electronic searches were scanned independently by at least two review authors. For studies appearing to meet the inclusion criteria, or for which there were insufficient data in the title and abstract to make a clear decision, the full report was obtained. The full reports were assessed independently by at least two review authors to establish whether met the inclusion criteria or not. Disagreements were resolved by discussion. Where resolution was not possible, a third review author was consulted. All studies meeting the inclusion criteria then underwent validity assessment and data extraction. Studies rejected at this or subsequent stages were recorded in the 'Characteristics of excluded studies' table, and reasons for exclusion recorded.
Data extraction and management
Data were extracted by at least two review authors independently using specially designed data extraction forms. The data extraction forms were piloted on several papers and modified as required before use. Any disagreement was discussed and a third review author consulted where necessary. Trial authors were contacted for clarification or missing information.
For each trial the following data were recorded.
Year of publication, country of origin and source of study funding.
Details of the participants including demographic characteristics, source of recruitment and criteria for inclusion.
Details of the type of intervention.
Details of the outcomes reported, including method of assessment, and time intervals.
Assessment of risk of bias in included studies
The risk of bias assessment of the included trials was undertaken independently and in duplicate by at least two review authors as part of the data extraction process. In the case that the paper to be assessed had one or more review authors in the authors list, it was independently evaluated only by those review authors not involved in the trial.
This was conducted using the recommended approach for assessing risk of bias in studies included in Cochrane Reviews (Higgins 2011). It is a two‐part tool, addressing the six specific domains (namely sequence generation, allocation concealment, blind outcome assessment, incomplete outcome data, selective outcome reporting and 'other issues'). Each domain includes one specific entry in a 'Risk of bias' table. Within each entry, the first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry.
Summarising risk of bias for a study:
After taking into account the additional information provided by the authors of the trials, studies were grouped into the following categories. We assumed that the risk of bias was the same for all outcomes and each study was assessed as follows:
| Risk of bias | Interpretation | Within a study | Across studies |
| Low risk of bias. | Plausible bias unlikely to seriously alter the results. | Low risk of bias for all key domains. | Most information is from studies at low risk of bias. |
| Unclear risk of bias. | Plausible bias that raises some doubt about the results. | Unclear risk of bias for one or more key domains. | Most information is from studies at low or unclear risk of bias. |
| High risk of bias. | Plausible bias that seriously weakens confidence in the results. | High risk of bias for one or more key domains. | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results. |
Measure of treatment effect
For dichotomous outcomes, the estimate of effect of an intervention was expressed as risk ratios (RR) together with 95% confidence intervals (CIs). For continuous outcomes, mean differences and standard deviations were used to summarise the data for each group using mean differences and 95% CIs.
Unit of analysis issues
The statistical unit was the patient and not the prosthesis, the procedure or the implant.
Dealing with missing data
All trial authors were contacted to retrieve missing data when necessary. Data were excluded until further clarification was available if agreement could not be reached. Methods in section 7.7.3 of the Cochrane Handbook (Higgins 2011) were to be used to estimate missing standard deviations.
Assessment of heterogeneity
The significance of any discrepancies in the estimates of the treatment effects from the different trials was to be assessed by means of Cochran's test for heterogeneity and heterogeneity would have been considered significant if P < 0.1. The I2 statistic, which describes the percentage total variation across studies that is due to heterogeneity rather than chance, was to be used to quantify heterogeneity with I2 over 50% being considered substantial heterogeneity.
Assessment of reporting biases
If there had been sufficient numbers of trials (more than 10) in any meta‐analysis, publication bias would have been assessed according to the recommendations on testing for funnel plot asymmetry (Egger 1997) as described in the Cochrane Handbook (Higgins 2011). If asymmetry was identified we would have examined possible causes.
Data synthesis
Only if there were studies of similar comparisons reporting the same outcome measures a meta‐analysis was to be done. Risk ratios were to be combined for dichotomous data, and mean differences for continuous data, using fixed‐effect models. Random‐effects models were to be used when there were more than three studies in a meta‐analysis. Data from split‐mouth studies were combined with data from parallel group trials with the method outlined by Elbourne (Elbourne 2002), using the generic inverse variance method in RevMan. The techniques described by Follmann (Follmann 1992) were used to estimate the standard error of the difference for split‐mouth studies, where the appropriate data were not presented and could not be obtained. The Cochrane Handbook recommendations (Higgins 2011) were followed for RCTs with parallel design with zero‐cell counts. The fixed value of 0.5 was automatically added to all cells with zero‐cell counts and risk ratios calculated with the RevMan software. If there were no events in both arms, no calculations were undertaken because in this situation the study does not provide any indication of the direction or magnitude of the relative treatment effect.
Subgroup analysis and investigation of heterogeneity
Clinical heterogeneity was to be assessed by examining the types of participants and interventions for all outcomes in each study. No subgroup analyses were planned, however this may be done in future updates of this review.
Sensitivity analyses
It was planned to undertake sensitivity analyses to examine the effect of the study quality on the overall estimates of effect. In addition, the effect of including unpublished literature on the review's findings was also to be examined. There were too few trials in the meta‐analyses to undertake these analyses.
Results
Description of studies
SeeCharacteristics of included studies table. SeeCharacteristics of excluded studies table.
Characteristics of the trial settings and investigators
Of the 17 potentially eligible trials (Kerscher 1993; Hunt 1996; Heydenrijk 2000; Ivanoff 2001; Arnabat‐Dominguez 2003; Fortin 2006; Lai 2006; Cannizzaro 2008; Ozan 2007; Covani 2008; Shahidi 2008; Sanz 2009; Lindeboom 2010; Van de Velde 2010; Wiesner 2010; Cannizzaro 2011; Lorenzo 2012), 11 trials had to be excluded because: five trials had too short follow‐ups (Hunt 1996; Fortin 2006; Lai 2006; Covani 2008; Shahidi 2008); in three trials interventions were confounded (Ivanoff 2001; Cannizzaro 2008; Van de Velde 2010); in one trial the number of participants was unclear (Kerscher 1993); one trial was judged not to be randomised (Ozan 2007); in one trial both teeth and implants were included without the data being described separately (Sanz 2009). Of the six included trials (Heydenrijk 2000; Arnabat‐Dominguez 2003; Wiesner 2010; Lindeboom 2010; Lorenzo 2012; Cannizzaro 2011), two were conducted in Spain (Arnabat‐Dominguez 2003; Lorenzo 2012), two in the Netherlands (Heydenrijk 2000; Lindeboom 2010), one in Italy (Cannizzaro 2011), and one in Austria (Wiesner 2010). Four trials had a parallel group study design (Heydenrijk 2000; Arnabat‐Dominguez 2003; Lindeboom 2010; Lorenzo 2012) and two a split‐mouth study design (Wiesner 2010; Cannizzaro 2011). Four trials were conducted at university dental clinics (Heydenrijk 2000; Arnabat‐Dominguez 2003; Lindeboom 2010; Lorenzo 2012) and two in private practices (Wiesner 2010; Cannizzaro 2011). All trials included only adults. One trial was funded by the industry (Lorenzo 2012). Three trials did not receive financial support from external sources (Lindeboom 2010; Wiesner 2010; Cannizzaro 2011). It is unclear whether support from industry was received for two trials (Heydenrijk 2000; Arnabat‐Dominguez 2003).
Characteristics of the interventions
(1) Flap design
(a) Is flap elevation necessary? ‐ two trials (Lindeboom 2010; Cannizzaro 2011).
One trial (Lindeboom 2010) compared flapless versus flap elevation to place at least six implants in fully edentulous maxillae. The flapless surgery procedure was performed using individually customised surgical templates fabricated with CAD/CAM technology planned with the Procera Software 3D Planning Program (Nobel Biocare AB, Goteborg, Sweden). In the flapless group, soft tissues were punched away and after implant installation, the punch wounds were sutured. Data were reported in the publication up to 1 month after implant placement, however, the authors provided data up to 6 months after loading.
One trial (Cannizzaro 2011) of split‐mouth compared flapless versus flap elevation to place dental implants in partially edentulous patients. Two separate edentulous areas characterised by residual bone least 5 mm thick and 10 mm in height were randomised to receive at least one implant in each side after flap elevation or not. Implants were first placed in one site, and after 2 weeks in the other site with free hand surgery. Surgical templates were used when indicated. Flapless surgery was planned on measurements taken with a bone calliper and intraoral, panoramic or CT scans. It was aimed to place the implants with a minimum insertion torque of 48 Ncm in underprepared sites to be functional loaded the same day with full acrylic restorations which were replaced after 3 months by definitive metal‐ceramic restorations. Data up to 1‐year follow‐up of the implants in function were provided.
(b) Which is the most effective flap design/technique? ‐ two trials (Heydenrijk 2000; Arnabat‐Dominguez 2003).
One trial (Heydenrijk 2000) compared the vestibular incision with the crestal incision using a parallel group design in fully edentulous mandibles. For the crestal incision technique, an incision was made longitudinally along the crest of the alveolar bone through gingiva and periosteum and a vertical incision was made labially in the midline. For the vestibular incision, the incision was made in the vestibular mucosa approximately 10 mm from the top of the alveolar process with the ends extending to the top of the alveolar process. The flap was reflected and two implants were placed. The flap was then replaced and small incisions through the keratinised mucosa were made at the site of the implants. Healing abutments were then connected to the implants through these incisions. All implants were placed with non‐submerged approach. Patients were followed for 1 year after placement of the prostheses.
Another trial (Arnabat‐Dominguez 2003) compared Erbium:YAG laser with flap elevation at implant exposure to connect abutments. An Er:YAG laser (Key laser II, KaVo, Biberach, Germany; wavelength 2940 nm) was used using the 2051 and 2056 handpieces, the former operating in non‐contact mode and the latter in contact mode with a quartz prism tip. Surgery was initiated using the 2051 non‐contact handpiece under a water spray (0.3 mL/minute) at a frequency of 2 Hz and a pulse power rating of 250 mJ, without anaesthesia. The handpiece was used at a focal distance of 14 mm. In those patients who reported no perioperative pain, the parameters were modified, increasing the frequency or pulse energy or both. Local anaesthesia was provided when the intraoperative discomfort (one patient) or bleeding (one patient) prevented the continuation of surgery. When bleeding continued to obstruct the action of the laser beam, the 2056 handpiece (used for periodontal surgery) was used with the quartz prism tip under water drip for contact working. All implants were followed for at least 6 months after abutment connection.
(2) Soft tissue correction/augmentation techniques
(a) Are correction/augmentation procedures necessary? ‐ one trial (Wiesner 2010).
One trial (Wiesner 2010) compared soft tissue grafting at implant placement versus no grafting using a split‐mouth design in the posterior mandible. For both sites a crestal incision was made, a split thickness flap elevated and each patient received one implant in each side. After implant placement one side was augmented with a subepithelial connective tissue graft taken from the palate. The graft was positioned over the implant to cover both buccal and lingual sides and sutured to the periosteum with 6‐0 absorbable suture. Subsequently flaps were closed submerging the implants. Donor sites were sutured and covered with a deep drawing template. For the whole healing period no temporary prostheses was used. After 3 months, the implants were exposed and definitive crowns were fitted 1 month later. Patients were followed for 1 year after loading and were recalled every 3 months for maintenance.
(b) Which is the most effective correction/augmentation? ‐ no trials.
(3) Techniques to increase the peri‐implant attached/keratinised mucosa
(a) The peri‐implant attached/keratinised mucosa has to be increased ‐ no trials.
(b) Which is the most effective technique to increase the attached/keratinised mucosa: one trial (Lorenzo 2012).
One trial (Lorenzo 2012) compared a xenogeneic collagen matrix of porcine origin (Mucograft®; Geistlich Pharma AG, Wolhusen, Switzerland) to augment the keratinised tissue around implants supporting prosthetic restorations with a connective tissue autograft harvested from the palate. An intrasulcular incision was made and a mucosal partial thickness flap was raised. The recipient site was prepared by sharp dissection in order to create a periosteal bed free of any muscle attachment. The resulting flap was excised or sutured at the base of the newly created vestibule with 5‐0 non‐resorbable mattress braided nylon sutures. The graft was then sutured in the recipient bed with 5‐0 non‐resorbable braided nylon interrupted single sutures. Patients were followed for 6 months after grafting.
(4) Suturing techniques
Which is the most effective suturing technique/material? ‐ no trials.
Dental implant types
IMZ cylindrical implants with a titanium plasma sprayed coating (Friedrichsfeld AG, Mannheim, Germany) were used in one trial (Heydenrijk 2000).
Mark II, Branemark System dental implants with turned surface (Nobel Biocare AB, Goteborg, Sweden) were used in one trial (Arnabat‐Dominguez 2003).
Neoss titanium sand‐blasted conical implants (Neoss, Harrogate, UK) were used in one trial (Wiesner 2010).
Zimmer titanium tapered SwissPlus with a grit‐blasted acid‐etched surface (Zimmer Dental, Carlsbad, Ca, USA) were used in one trial (Cannizzaro 2011).
Nobel Replace titanium implants with an oxidised surface (Nobel Biocare AB, Goteborg, Sweden) were used in one trial (Lindeboom 2010).
Unknown implant system was used in one trial (Lorenzo 2012).
Characteristics of outcome measures
Prosthesis failures: all trials.
Implant failures: all trials.
Aesthetics evaluated by patients: one trial (Wiesner 2010).
Aesthetics evaluated by dentists: two trials (Wiesner 2010; Lorenzo 2012), however, the actual data were not presented in one trial (Lorenzo 2012).
Biological complications: all but one trial (Lindeboom 2010).
Postoperative pain: four trials (Arnabat‐Dominguez 2003; Lindeboom 2010; Cannizzaro 2011; Lorenzo 2012), however, data from two trials were not presented in the articles (Lindeboom 2010; Lorenzo 2012).
Patient preference including aesthetics only in split‐mouth studies: two trials (Wiesner 2010; Cannizzaro 2011).
Ease of maintenance by patient: no trials.
Width of the attached/keratinised mucosa: one trial (Lorenzo 2012).
Peri‐implant marginal bone level changes on periapical radiographs: two trials (Wiesner 2010; Cannizzaro 2011).
Main inclusion criteria
Fully edentulous maxillas able to harbour at least six implants (Lindeboom 2010).
Completely edentulous severely resorbed mandibles (Heydenrijk 2000).
Partially or completely edentulous patients (Arnabat‐Dominguez 2003).
Partially edentulous patients (both mandibles and maxillae) allowing the placement of at least two separated 10 mm long implants (Cannizzaro 2011).
Partially edentulous patients requiring one single mandibular implant per side at least 9 mm long (Wiesner 2010).
Patients with implant supported prostheses presenting at least one location with ≤ 1 mm keratinised tissue (Lorenzo 2012).
Main exclusion criteria
Preprosthetic surgery or previous implants (Heydenrijk 2000).
Previously irradiated jaws (Heydenrijk 2000; Lindeboom 2010; Wiesner 2010; Cannizzaro 2011).
Need of bone grafting at implant placement (Lindeboom 2010; Cannizzaro 2011).
Immediate placement of implants in extraction sockets (Wiesner 2010).
Severe bone resorption (Lindeboom 2010; Cannizzaro 2011).
Single implants in the anterior zone (Arnabat‐Dominguez 2003).
Lack of keratinised gingiva around the implant perimeter (Arnabat‐Dominguez 2003).
Uncontrolled diabetes (Wiesner 2010).
Untreated periodontitis and poor oral hygiene (Wiesner 2010; Cannizzaro 2011; Lorenzo 2012).
Smoking (Lindeboom 2010; Wiesner 2010) or smoking > 10 cigarettes/day (Lorenzo 2012).
Treated or under treatment with intravenous amino‐bisphosphonates (Wiesner 2010).
Comparability of control and treatment groups at entry
Insufficient data were presented in the original articles to understand whether the control and treatment groups were comparable at baseline for two trials (Heydenrijk 2000; Arnabat‐Dominguez 2003); for three trials the groups appear to be comparable (Wiesner 2010; Cannizzaro 2011; Lorenzo 2012); in one trial the oral health‐related quality of life measured with the OHIP‐14 was higher in the flap elevation group (Lindeboom 2010).
Duration of the studies (after implant loading)
Six months (Arnabat‐Dominguez 2003; Lindeboom 2010; Lorenzo 2012).
One year (Heydenrijk 2000; Wiesner 2010; Cannizzaro 2011).
Sample size
A priori sample size calculation was performed in two trials (Wiesner 2010; Lorenzo 2012).
Risk of bias in included studies
Allocation
Three of the included studies provided clear information about the methods used to generate the random allocation (Lindeboom 2010; Cannizzaro 2011; Lorenzo 2012). The study by Arnabat‐Dominguez 2003 reported that randomisation was "according to Efron's method" and Heydenrijk 2000 reported that simple randomisation was performed but there was no response to our request for further information, so this was assessed as unclear. Wiesner 2010 did not describe the method used to generate the randomisation sequence.
Two studies (Cannizzaro 2011; Lorenzo 2012) described the methods used to conceal the allocation and the remaining four studies provided no information on allocation concealment.
Blinding
For surgical interventions such as those include in this review, it is not possible to blind the surgeon or the patient to the intervention. We acknowledge that there is a risk of performance bias in surgical trials.
We decided to focus our assessment on the blinding of outcome evaluation which is possible and is the only practical way to minimise detection bias in these trials. Therefore we have only included blinding of outcome assessment in the in the risk of bias. Three studies (Lindeboom 2010; Cannizzaro 2011; Lorenzo 2012) reported that outcome assessors were blinded to allocated treatment and were assessed as low risk for this domain. In the remaining three studies blinding of outcome assessment was unclear.
Incomplete outcome data
One trial (Lorenzo 2012) was assessed at high isk of bias for this domain because data from some participants were excluded. In the remaining five studies the risk of attrition bias was assessed as low.
Selective reporting
Three studies (Arnabat‐Dominguez 2003; Lindeboom 2010; Lorenzo 2012) were assessed as being at high risk of selective reporting and the other three reported insufficient information and were assessed as unclear risk of selective reporting.
Other potential sources of bias
Two trials (Arnabat‐Dominguez 2003; Lorenzo 2012) were assessed as being at high risk of other bias and the remaining four were at low risk for this domain.
Overall risk of bias
As mentioned above it is not possible to blind operators and patients to the allocated intervention in a surgical trial. We end up in a classical paradox in that both a well designed trial in which everything is described in detail, and a poorly reported trial where authors simply did not describe the methodological procedures adopted to minimise bias and did not answer our clarification requests, are both likely to be rated as being at unclear risk of bias. Therefore readers should be cautious when interpreting results from the risk of bias summary table (Figure 1). However, all the necessary information helping readers to make up their own minds is described in detail in the risk of bias tables in the 'Characteristics of included studies' section of the review.
1.
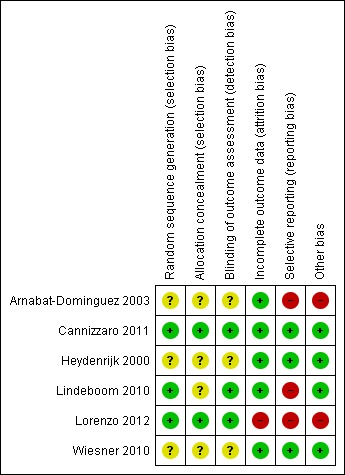
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The final risk of bias assessment after having incorporated the additional information kindly provided by the authors of the included trials, and considering what is possible in surgical trials, is summarised in Figure 2 and Figure 1. Overall, one study was at low risk of bias (Cannizzaro 2011), two studies were judged at unclear risk of bias (Heydenrijk 2000; Wiesner 2010) and the remainder at high risk of bias.
2.
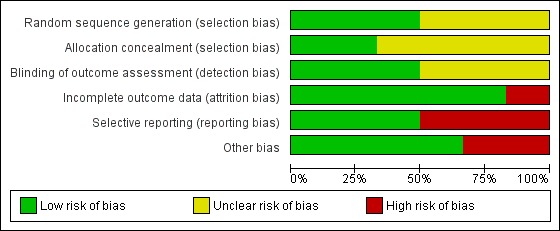
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
(1a) Is flap elevation necessary?
Two trials were included (Lindeboom 2010; Cannizzaro 2011).
Lindeboom 2010 (parallel study design) compared implant placement with or without flap elevation in fully edentulous maxillae. The flapless surgery procedure was performed using individually customised surgical templates fabricated with CAD/CAM technology planned with the Procera Software 3D Planning Program (Nobel Biocare AB, Goteborg, Sweden). In the flapless group, soft tissues were punched away and after implant installation, the punch wounds were then sutured. Eight patients were included in each group. At baseline the patients who were going to receive flapless surgery were less satisfied according to the oral health‐related quality of life measured with the OHIP‐14. There were no withdrawals and complications up to 6 months. Two implants were lost in the flapless group versus none in the flap elevation group (Analysis 1.2), this difference was not statistically significant. It is interesting to observe that the study also reported significantly more pain during and just after surgery in the flapless group than in the open flap group. Swelling was not measured, however the authors stated that more swelling occurred in the flap elevation group.
1.2. Analysis.
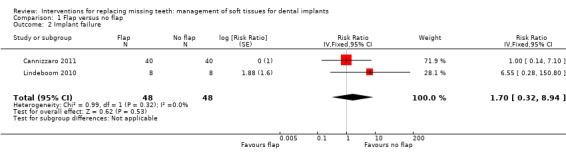
Comparison 1 Flap versus no flap, Outcome 2 Implant failure.
Cannizzaro 2011 (split‐mouth design) compared flapless versus flap elevation implant placement in partially edentulous patients. All implants were placed with an insertion torque > 48 and were immediately functionally loaded. Forty patients were included. No apparent baseline differences were present between the two groups. No patient was lost up to 1 year. In four patients of the flapless group, a flap had to be elevated to properly evaluate the direction of the drills. Two prostheses and two implants failed in each group, all in different patients. There were no statistically significant differences for prosthesis/implant failures (Analysis 1.1; Analysis 1.2) and biological complications (Analysis 1.3) between the groups. One patient had one biological complication (peri‐implantitis with purulent discharge) in the flapless group versus four complications in three patients (one intrasurgical haemorrhage; one intrasurgical fracture of the buccal bone plate, one peri‐implant marginal bone loss exceeding 4 mm, one patient experiencing pain on chewing) in the flap elevation group. Less patients subjected to a flapless procedure experienced postoperative pain than those subjected to flap elevation (19 versus 30 patients) (mean difference ‐1.10; 95% confidence interval (CI) ‐2.02 to ‐0.18; Analysis 1.4). There were no differences for peri‐implant marginal bone levels (Analysis 1.5). Thirty‐one patients preferred the flapless intervention, three patients preferred flap elevation and six patients had no preference. This difference was statistically significant (risk ratio (RR) 106.70; 95% CI 20.97 to 542.82; Analysis 1.6). In the paper it was also reported that patients of the flapless group suffered significantly less postoperative swelling and consumed less analgesics (2.1 versus 5.7) than those in the flap elevation group and that the flapless procedure lasted significantly less (12 versus 29 minutes).
1.1. Analysis.
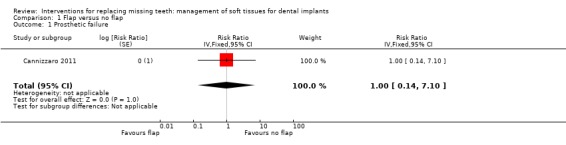
Comparison 1 Flap versus no flap, Outcome 1 Prosthetic failure.
1.3. Analysis.
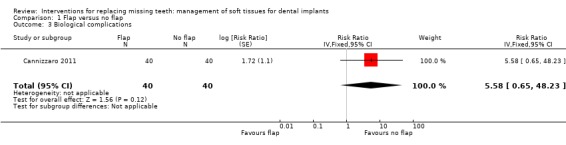
Comparison 1 Flap versus no flap, Outcome 3 Biological complications.
1.4. Analysis.
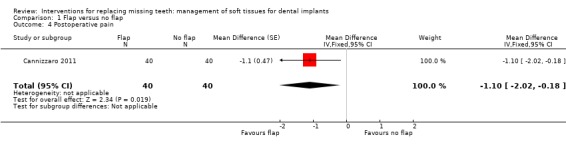
Comparison 1 Flap versus no flap, Outcome 4 Postoperative pain.
1.5. Analysis.
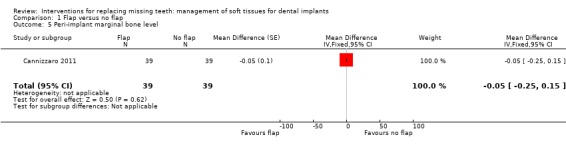
Comparison 1 Flap versus no flap, Outcome 5 Peri‐implant marginal bone level.
1.6. Analysis.
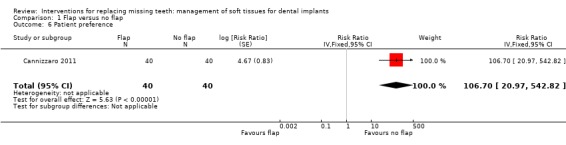
Comparison 1 Flap versus no flap, Outcome 6 Patient preference.
Due to the differences between the two studies it was decided not to attempt a meta‐analysis.
(1b) Which is the most effective flap design/technique?
Two trials were included (Heydenrijk 2000; Arnabat‐Dominguez 2003).
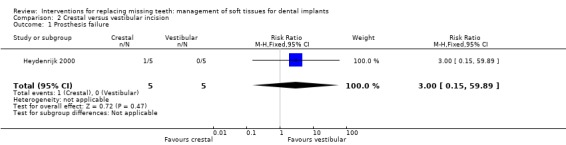
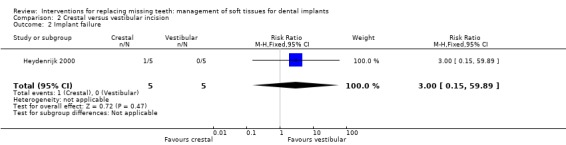
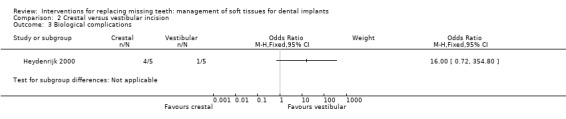
Heydenrijk 2000 (parallel study design) compared crestal versus vestibular incision to place one‐stage dental implants. Five patients were included in each group. It is unclear whether any baseline differences among the two groups existed. No withdrawals but one implant and related prosthesis failed (crestal group) up to 1 year in function. There were no statistically significant differences for prosthesis/implant failures (Analysis 2.1; Analysis 2.2) and complications (Analysis 2.3) between the groups. Four patients of the crestal group and one of the vestibular group suffered from hyperplastic tissue covering the healing abutment after surgery.
2.1. Analysis.
Comparison 2 Crestal versus vestibular incision, Outcome 1 Prosthesis failure.
2.2. Analysis.
Comparison 2 Crestal versus vestibular incision, Outcome 2 Implant failure.
2.3. Analysis.
Comparison 2 Crestal versus vestibular incision, Outcome 3 Biological complications.
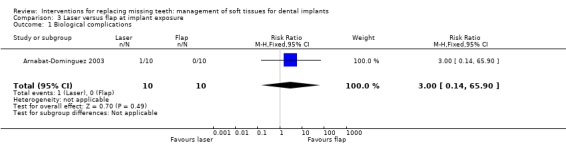
Arnabat‐Dominguez 2003 (parallel study design) compared Erbium:YAG laser with a flap elevation at implant exposure to connect abutments. Ten patients were included in each group. It is unclear whether any baseline differences among the two groups existed. No withdrawal or implant failures occurred up to 6 months after abutment connection. Patients treated with laser did not receive local anaesthesia, though two patients had to be anaesthetised during the procedure: one because of pain and one because of profuse bleeding (complication). In the latter case the 2056 laser handpiece for periodontal surgery was used. Less patients treated with laser experienced postoperative pain than those treated with conventional flap elevation: RR 0.25 (95% CI 0.07 to 0.90; Analysis 3.2). In the article it was also reported that patients of the laser group consumed significantly less analgesics and that the prosthetic procedures could start earlier (after 7.3 days) than in patients of the conventional flap group (after 13.6 days). No statistically significant differences were observed for the duration of surgery.
3.2. Analysis.
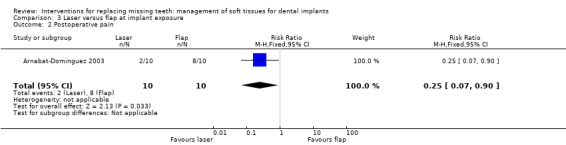
Comparison 3 Laser versus flap at implant exposure, Outcome 2 Postoperative pain.
(2) Soft tissue correction/augmentation techniques
(a) Are correction/augmentation procedures necessary? ‐ one trial was included (Wiesner 2010).
Wiesner 2010 (split‐mouth study design) evaluated the efficacy of connective tissue graft from the palate to thicken the buccal peri‐implant mucosa after implant placement in the posterior mandible versus no augmentation in 10 patients for 1 year. No apparent baseline differences were present between the two groups. No withdrawal, implant failure or complications occurred. Augmented sites showed statistically significant higher pink aesthetic scores (PES) than non‐augmented sites (mean difference ‐2.87; 95% CI ‐3.67 to ‐2.07; Analysis 4.1). Six patients preferred aesthetically the augmented sites whereas four patients had no preference and this difference was statistically significant (exact McNemar significance probability P = 0.031; OR = 0; CI 0 to 0.85; data not shown), though seven patients were uncertain or sure not to undergo the augmentation procedure again. Augmented sites had significantly thicker buccal peri‐implant soft tissues (mean difference ‐1.35 mm; 95% CI ‐1.78 to ‐0.92; Analysis 4.2) but there were no significant differences in peri‐implant marginal bone level changes (Analysis 4.3).
4.1. Analysis.
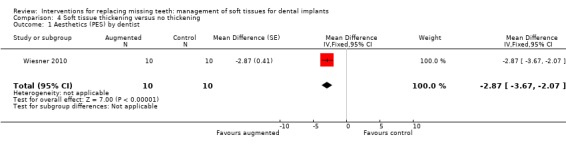
Comparison 4 Soft tissue thickening versus no thickening, Outcome 1 Aesthetics (PES) by dentist.
4.2. Analysis.
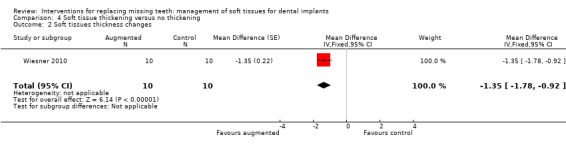
Comparison 4 Soft tissue thickening versus no thickening, Outcome 2 Soft tissues thickness changes.
4.3. Analysis.
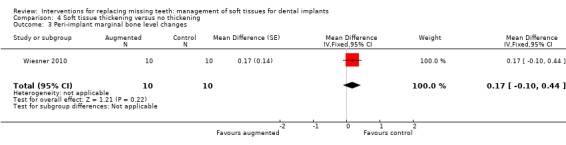
Comparison 4 Soft tissue thickening versus no thickening, Outcome 3 Peri‐implant marginal bone level changes.
(b) Which is the most effective correction/augmentation? ‐ no trials.
(3) Techniques to increase the peri‐implant attached/keratinised mucosa
(a) The peri‐implant attached/keratinised mucosa has to be increased ‐ no trials.
(b) Which is the most effective technique to increase the attached/keratinised mucosa? ‐ one trial was included (Lorenzo 2012).
Lorenzo 2012 (parallel group study) compared two techniques to increase the height of the keratinised mucosa around implants having ≤ 1 mm of keratinised mucosa using a xenogeneic collagen matrix of porcine origin in 12 patients (Mucograft®; Geistlich Pharma AG, Wolhusen, Switzerland) or a connective tissue autograft harvested from the palate in 12 patients for 6 months. No apparent baseline differences were present between the two groups. Despite that it was declared that an intention‐to‐treat was made, the authors withdrew two patients from the Mucograft group with the motivation that they increased the amount of smoked cigarettes above the inclusion threshold of the study (10 cigarettes/day) and did not present pain data of a patient from the autograft group since the patient had a sport trauma and consequently, he was prescribed high doses of ibuprofen. Therefore the trial was judged to be at high risk of bias and results should be viewed with caution. No implant failure or biological complication was reported. Both procedures were able to increase the width of keratinised mucosa (2.4 mm Mucograft versus 2.3 mm autograft) but there were no differences between the two procedures (Analysis 5.1). The actual data on postoperative pain after 10 and 30 days, consumption of analgesics, and aesthetic evaluated by dentist as colour blending of the grafts in the surrounding tissues were not presented in the article, so we could not use them. There was no difference for aesthetics assessed as recession (Analysis 5.2), however, it is interesting to observe that both procedures determined an increase of recession (0.4 mm for the Mucograft and 0.5 mm for the autograft) which exposed some of the titanium abutments determining a worsening in aesthetics from the initial situation.
5.1. Analysis.
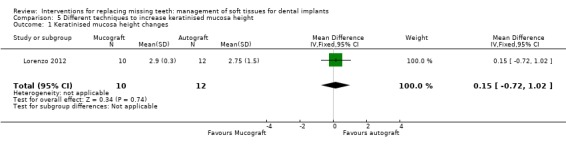
Comparison 5 Different techniques to increase keratinised mucosa height, Outcome 1 Keratinised mucosa height changes.
5.2. Analysis.
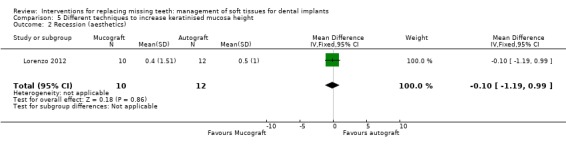
Comparison 5 Different techniques to increase keratinised mucosa height, Outcome 2 Recession (aesthetics).
(4) Suturing techniques
Which is the most effective suturing technique/material? ‐ no trials.
Discussion
Summary of main results
Seventeen randomised controlled trials (RCTs) have been identified on how to manage peri‐implant soft tissues in implant dentistry, however, we were able to include only six of these trials. Even more disappointing was the lack of replies from the authors of some of the trials to our request of information. Therefore some of the included trials may not even be RCTs or on the contrary, useful information could have been collected but not published leaving the reader with a great deal of uncertainty with respect to the interpretation and the transferability of the results to routine clinical practice.
Two trials were designed to assess whether there could be any advantages/additional risks when placing dental implants without flap elevation (Lindeboom 2010; Cannizzaro 2011). The results of these two trials are apparently contradictory and the possible factors influencing these differences ought to be discussed. While the results of one trial (Cannizzaro 2011) suggested that flapless implant placement was almost three times faster than flap elevation and induced less postoperative pain, swelling, consumption of analgesics, and was preferred by the patients, the other trial (Lindeboom 2010) suggested that the flapless procedure required more time and was more painful during and just after surgery. While in one trial (Cannizzaro 2011) the flapless procedure was implemented by free hand in partially edentulous patients, in the other study (Lindeboom 2010), implants were placed with guided surgery using a stent fixed with three intrabony pins to fully edentulous maxillae. The findings of the Cannizzaro 2011 study are supported by the results of two other RCTs (Fortin 2006; Cannizzaro 2008), which were included in the previous version of this systematic review but have been excluded in this update because patients were followed‐up for only 6 days and it was not reported whether implants were successful or not (Fortin 2006), and other interventions, such as underpreparation of the implant sites, higher insertion torques and immediate loading of the flapless placed implants only, confounded the other trial (Cannizzaro 2008). A speculative explanation is that the fully edentulous patients treated with the flapless approach (Lindeboom 2010), received less local anaesthesia to minimise alterations of the soft tissue profiles to avoid possible distortion of the correct positioning of the surgical guide and/or that a freehand approach gives the operator a better feedback enabling him to adjust the procedure and even to elevate a flap when judged to be necessary. In one trial (Cannizzaro 2011) neither increased failure nor complication rates were observed for the flapless placed implants, however, readers should consider that the operator of this trial has significant clinical experience with the flapless procedures and therefore the extrapolation of his results to other settings has to be done with caution. It should be also observed that the only two failures reported by the other trial (Lindeboom 2010) were flapless placed implants. However, despite that 'common sense' would suggest that flapless procedures would induce less postoperative discomfort but there could be a higher risk for complications/failures, it is definitely preferable though that 'common sense' is backed‐up by sound scientific evidence, since sometimes 'common sense' guides towards decisions which may have negative consequences for patients. Therefore more trials are needed to better understand the actual advantages and disadvantages of flapless implant placement.
Only one study (Heydenrijk 2000) was aimed at evaluating which type of surgical incisions could be preferable for implant placement: a crestal or a vestibular approach. Only 10 patients were included in the trial and the number of reported complications was unusually high being 50%. We feel that the very small number of patients is a major issue, since the high number of complications may have occurred simply by chance. It would be prudent not to extrapolate any conclusions from this pilot study, with the exception of the need of conducting larger multicentre trials to answer this question. Such trials are particularly easy to design and conduct since the follow‐up can be rather short and it would be easy to recruit large numbers of patients.
One single trial (Arnabat‐Dominguez 2003) evaluated whether a Erbium:YAG laser could be advantageous over flap elevation at the two‐stage surgery to connect the abutments to the implants. The results suggested that local anaesthesia may not be required for the majority of patients subjected to laser intervention and that postoperative pain and consumption of analgesics were significantly less when the laser was used, and that the prosthetic procedures could start earlier (after 7.3 days) than in patients of the conventional flap group (after 13.6 days). However, we do not share the same 'enthusiasm' of the authors favouring the laser intervention. The only complication occurred in a patient treated with the laser: profuse bleeding which could not be controlled by the vasoconstrictor contained in the local anaesthetic. The authors had to use an alternative laser handpiece to complete the surgery. One of the disadvantages with laser is its cost and the need of additional training for the operators to face various possible situations. Moreover the authors did not use an appropriate control group. In the control group they elevated a flap after surgical incision which was then sutured, but they could have used a 'punch technique' or even better a minimally invasive 'H' incision technique instead. These techniques usually do not require flap elevation and suturing. The 'H' incision technique allows also the preservation of keratinised/attached mucosa which will be lost using the laser or the punch. The authors did not declare or respond concerning whether the study was sponsored by the laser manufacturer or not.
Methodological issues in the studies
What is striking is that for techniques aimed at manipulating or augmenting soft tissues mostly for aesthetic reasons or for those techniques aimed at increasing the width of keratinised/attached mucosa allegedly done to improve the long‐term prognosis of dental implants, there are only two acceptable RCTs published in the world literature (Wiesner 2010; Lorenzo 2012). Another five identified trials which were excluded from this review (Kerscher 1993; Lai 2006; Covani 2008; Shahidi 2008; Sanz 2009), of which one in German (Kerscher 1993) and one in Chinese (Lai 2006), resulted in no clinical value because of the poor way they were reported or for thedue to poor reporting or insufficient minimumduration of follow‐up. Literally hundreds of thousands of patients have been subjected to various procedures to increase the keratinised/attached mucosa and yet stilldespite that this issue is highly controversial (Wennström 1994; Chung 2006; Roos‐Jansåker 2006) and that there is no reliable evidence to back up these procedures. This does not mean that a sufficient width of keratinised/attached mucosa is not beneficial for the patients, but we do need reliable evidence proving or rejecting this hypothesis.
Only one pilot study (Wiesner 2010) evaluated the efficacy of a connective tissue graft from the palate to augment soft tissues to improve aesthetics around dental implants. To avoid issue with the control side of the patients, it was decided only to include posterior mandible areas since these have little aesthetic impact. Despite the small sample size it could be concluded that grafts were effective for augmenting soft tissues, in fact, after 6 months augmented sites were 1.3 mm thicker than untreated control sites, and aesthetics were significantly improved. Despite the fact that patients did prefer the augmented sites, seven patients were uncertain or unwilling to undergo the augmentation procedure again on the contra‐lateral side. A possible explanation is that since augmentations were done in non‐aesthetic areas, patients did not perceive any "visible" advantage motivating them to undergo the same procedure again.
The only trial (Lorenzo 2012) which compared two techniques for increasing the height of the keratinised mucosa provided results which need some careful reflection: while the advantages of increasing the keratinised mucosa height are yet to be demonstrated, both the tested interventions did compromise the aesthetic outcomes by increasing recessions of about 0.5 mm at treated sites which caused in many cases the exposure of the metallic abutment through the soft tissues. This example casts serious doubts on the personal "clinical feeling" commonly used by clinicians to justify the effectiveness of the procedures that they are using and underscores once more the need for proper scientific evaluation of procedures and materials currently used in clinical practice.
Among the excluded studies the one by Ivanoff 2001, comparing resorbable versus non‐resorbable sutures, deserves some additional comments. Patients were allocated to the intervention groups by birth date (quasi‐randomisation) and various types of suture techniques (single, continuous or a combination of both types) were used according to surgeon preference. This created unbalanced groups and heavily confounded the aim of the study, i.e. comparing types of sutures and not the suture techniques. We feel that no reliable conclusions can be made from this trial. Randomisation should be properly performed. By using patient birthday no allocation concealment could be implemented giving the surgeons prior knowledge of group allocation. This has been shown to bias results (Schulz 1995a; Schulz 1995b). In addition, since the suture technique is likely to influence the results, the same type of suture technique should have been used in both groups. Furthermore, the suture technique could have been randomised.
Authors' conclusions
Implications for practice.
1a) There is limited weak evidence suggesting that flapless implant placement can cause less postoperative pain, edema and consumption of analgesics than flap elevation. Flapless surgery performed by skilful clinicians in properly selected cases can be as successful and complication‐free as conventional flap elevation. However, there is still insufficient evidence regarding a potential increased risk of complications/failures using a flapless approach. Clinicians should select patients for flapless implant placement with a great deal of caution in relation to their own clinical skills and experience. The safety and efficacy of customised surgical templates created with the help of planning software on CT scans to facilitate flapless placement of dental implants still needs to be assessed, especially for fully edentulous patients where it might be more difficult to correctly position the stent.
1b) There is insufficient evidence to suggest which is the best flap design.
2a‐b) There is weak evidence suggesting that soft tissue autografts increase soft tissue thickness improving the aesthetic outcome, however, we do not know which technique is the most effective.
3a‐b) There is weak evidence suggesting that grafting, either with an autograft or with a xenogenic collagen matrix, is able to increase the height of the peri‐implant keratinised mucosa but at risk of soft tissue recessions which could compromise the aesthetic outcome if the abutment becomes visible, however, it is still unknown whether an increase of keratinised mucosa is of any benefit for the patient.
4) There is still a lack of evidence about the most effective incision/suturing techniques/materials.
Implications for research.
More properly designed, conducted and reported randomised controlled trials with at least 6 months of follow‐up are needed to evaluate the potential risks/advantages of flapless procedures and the safety and efficacy of implant planning software based on CT scans. There is a need to evaluate whether techniques aimed at increasing the keratinised/attached mucosa are of any benefit for patients, and which could be the most effective techniques to modify/augment soft tissues for aesthetic reasons. Relevant outcome measures should include implant success, complications, and aesthetic parameters evaluated both by patients and dentists.
What's new
| Date | Event | Description |
|---|---|---|
| 10 October 2019 | Review declared as stable | This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future. |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 6 December 2011 | New citation required but conclusions have not changed | Change in review authors; 1 new included study; 8 new excluded studies; no changes to conclusions. |
| 6 December 2011 | New search has been performed | Searches updated to June 2011. |
| 12 June 2008 | Amended | Converted to new review format. |
Notes
This review originates from a previous Cochrane Review entitled 'Interventions for replacing missing teeth: surgical techniques for placing dental implants'. The original review had a very broad scope, so it was decided to split it into several reviews having more focused aims. Outcome measures were adapted to the specificity of the aims.
This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future.
Acknowledgements
We wish to thank Paul Coulthard his contribution to the first version of this review; Anne Littlewood (Cochrane Oral Health) and Sylvia Bickley and for their assistance with literature searching; Tanya Walsh, Luisa Fernandez Mauleffinch and Philip Riley (Cochrane Oral Health) for their help with the preparation of this review; Professor Shi Zongdao and Dr Li Na of the Chinese Center for Evidence Based Medicine for translating the Chinese article; Regina Mitezki for translating the German article; Carl‐Johan Ivanoff, Jerome Lindeboom and Mariano Sanz for providing us with information on their trials. We would also like to thank the following referees: Paul Brunton, Elena Figuero, Alan Payne, Frank Schwarz, and Andrew Tawse‐Smith.
Appendices
Appendix 1. MEDLINE (OVID) search strategy
1. exp Dental Implants/ 2. exp Dental Implantation/ or dental implantation 3. exp Dental Prosthesis, Implant‐Supported/ 4. ((osseointegrated adj implant$) and (dental or oral)) 5. dental implant$ 6. (implant$ adj5 dent$) 7. (((overdenture$ or crown$ or bridge$ or prosthesis or restoration$) adj5 (Dental or oral)) and implant$) 8. "implant supported dental prosthesis" 9. ("blade implant$" and (dental or oral)) 10. ((endosseous adj5 implant$) and (dental or oral)) 11. ((dental or oral) adj5 implant$) 12. OR/1‐11
The above subject search was run with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomized trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of The Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. (Higgins 2011)
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 2. Cochrane Oral Health's Trials Register Search Strategy
(dental‐implants OR "dental implant*" OR "oral implant*" OR dental‐implantation OR dental‐prosthesis‐implant‐supported OR "implant supported" OR "implant supported prosthesis" OR dental‐implantation‐endosseous‐endodontic OR "endosseous implant*" OR blade‐implantation OR "blade implant*" OR (implant* AND (oral OR dental)) or dental‐implantation‐subperiosteal OR "subperiosteal implant" OR (implant* AND overdenture*) OR ((overdenture* OR crown* OR bridge* OR prosthesis OR prostheses OR restoration*) AND ("dental implant*" OR "Oral implant" OR (zygoma* AND implant*))))
Appendix 3. The Cochrane Central Register of Controlled Clinical Trials (CENTRAL) Search Strategy
#1 DENTAL IMPLANTS explode all trees (MeSH) #2 DENTAL IMPLANTATION explode all trees (MeSH) #3 DENTAL PROSTHESIS IMPLANT‐SUPPORTED single term (MeSH) #4 ((osseointegrat* near implant*) and (dental* or oral*)) #5 (dental next implant*) #6 (implant* near dent*) #7 dental‐implant* #8 ((overdenture* near dental*) and implant*) #9 ((overdenture* near oral*) and implant*) #10 ((crown* near dental*) and implant*) #11 ((crown* near oral*) and implant*) #12 ((bridge* near dental*) and implant*) #13 ((bridge* near oral*) and implant*) #14 ((prosthesis near dental*) and implant*) #15 ((prosthesis near oral*) and implant*) #16 ((prostheses near dental*) and implant*) #17 ((prostheses near oral*) and implant*) #18 ((restoration* near dental*) and implant*) #19 ((restoration* near oral*) and implant*) #20 (implant next supported next dental next prosthesis) #21 (blade next implant*) #22 ((endosseous near implant*) and dental) #23 ((endosseous near implant*) and oral*) #24 ((dental* near implant*) or (oral* near implant*)) #25 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24)
Appendix 4. EMBASE via OVID Search Strategy
1. tooth implantation/ 2. ((implant‐supported or implant$) adj support$).mp. 3. ((osseointegrated adj implant$) and (dental or oral)).mp. 4. ((dental implant$ or dental‐implant or implant$) adj (dent$ or oral or tooth)).mp. 5. (((overdenture$ or crown$ or bridge$ or prosthesis or prostheses or restoration$) adj5 (dental or oral)) and implant$).mp. 6. "implant supported dental prosthesis".mp. 7. ("blade implant$" and (dental or oral or tooth or teeth)).mp. 8. ((endosseous adj5 implant$) and (dental or oral or tooth or teeth)).mp. 9. ((dental or oral or tooth or teeth) and implant$).mp. 10. or/1‐9
The EMBASE subject search was run with the Cochrane Oral Health Group Search Strategy for identifying randomized controlled trials in EMBASE:
1. random$.it,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 16. HUMAN/ 17. 16 and 15 18. 15 not 17 19. 14 not 18
Data and analyses
Comparison 1. Flap versus no flap.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prosthetic failure | 1 | Risk Ratio (Fixed, 95% CI) | 1.0 [0.14, 7.10] | |
| 2 Implant failure | 2 | 96 | Risk Ratio (Fixed, 95% CI) | 1.70 [0.32, 8.94] |
| 3 Biological complications | 1 | 80 | Risk Ratio (Fixed, 95% CI) | 5.58 [0.65, 48.23] |
| 4 Postoperative pain | 1 | 80 | Mean Difference (Fixed, 95% CI) | ‐1.1 [‐2.02, ‐0.18] |
| 5 Peri‐implant marginal bone level | 1 | 78 | Mean Difference (Fixed, 95% CI) | ‐0.05 [‐0.25, 0.15] |
| 6 Patient preference | 1 | 80 | Risk Ratio (Fixed, 95% CI) | 106.70 [20.97, 542.82] |
Comparison 2. Crestal versus vestibular incision.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prosthesis failure | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.15, 59.89] |
| 2 Implant failure | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.15, 59.89] |
| 3 Biological complications | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 3. Laser versus flap at implant exposure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Biological complications | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 65.90] |
| 2 Postoperative pain | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.07, 0.90] |
3.1. Analysis.
Comparison 3 Laser versus flap at implant exposure, Outcome 1 Biological complications.
Comparison 4. Soft tissue thickening versus no thickening.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Aesthetics (PES) by dentist | 1 | 20 | Mean Difference (Fixed, 95% CI) | ‐2.87 [‐3.67, ‐2.07] |
| 2 Soft tissues thickness changes | 1 | 20 | Mean Difference (Fixed, 95% CI) | ‐1.35 [‐1.78, ‐0.92] |
| 3 Peri‐implant marginal bone level changes | 1 | 20 | Mean Difference (Fixed, 95% CI) | 0.17 [‐0.10, 0.44] |
Comparison 5. Different techniques to increase keratinised mucosa height.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Keratinised mucosa height changes | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.72, 1.02] |
| 2 Recession (aesthetics) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.19, 0.99] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arnabat‐Dominguez 2003.
| Methods | 6‐month follow‐up after second‐stage implant surgery, parallel group randomised controlled study. Unclear whether outcome assessor was blind or not. No withdrawals. | |
| Participants | Partially or fully edentulous patients needing abutment connection. Patients with single anterior implants and lack of keratinised gingiva were excluded. Adults treated in the Dental School of the University of Barcelona, Spain. 20 patients enrolled (10 in each group) and results given for 20. | |
| Interventions | Erbium:YAG laser with the 2051 and 2056 handpieces versus conventional blade incision and flap elevation. No local anaesthesia given to patients in the laser group. | |
| Outcomes | Prosthesis/implant failures, postoperative complications, pain (VAS and ordinal scale), duration of surgery, consumption of analgesics, need of anaesthesia, conditions of the peri‐implant soft tissues, and days to start the prosthetic rehabilitation, patient satisfaction (not reported). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The article reported: "The patients were randomized according to Efron's method and divided into 2 groups....". No reply to our request for clarifications. |
| Allocation concealment (selection bias) | Unclear risk | Not reported in the study. No reply to our request for clarifications. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported in the study. No reply to our request for clarifications. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals and apparently no data missing. |
| Selective reporting (reporting bias) | High risk | Not all outcomes measures were reported. No reply to our request for clarifications. |
| Other bias | High risk | Misleading study design since the laser was used without flap elevation whereas a flap was elevated after surgical incision in the control group. This was not necessary since the procedure should have been implemented using a punch without flap elevation. |
Cannizzaro 2011.
| Methods | 1‐year follow‐up, split‐mouth design randomised controlled study. Blinded outcome assessors. No withdrawals. | |
| Participants | Partially edentulous patients having 2 separate areas in need of at least one single dental implant with bone of at least 10 mm in height and 5 mm thick. Exclusion criteria were: general contraindications to implant surgery; irradiation in the head and neck area less than 1 year; poor oral hygiene and motivation; untreated periodontal disease; uncontrolled diabetes; pregnancy or lactation; substance abuse; psychiatric problems or unrealistic expectations; lack of opposing occluding dentition in the area intended for implant placement; severe bruxism or clenching; active infection or severe inflammation in the area intended for implant placement; need for bone‐augmentation. Adults treated in an Italian private practice. 40 patients enrolled and results given for 40. | |
| Interventions | According to the randomisation procedures, implants were placed flapless or not. Surgical stents were used when considered useful to better position the implants for an optimal prosthetic aesthetic outcome. For flapless placed implants, 2 mm diameter surgical drills were inserted directly into the mucosa, followed by 2.7 mm diameter surgical drills, otherwise mucoperiosteal flaps were elevated to properly visualise the area after crestal incision. Implants inserted with a torque > 48 Ncm were immediately loaded with full acrylic temporary restorations. After 2 months, definitive metal‐ceramic restorations were delivered. | |
| Outcomes | Prosthesis and implant failures, complications, postoperative swelling and pain, consumption of analgesics, patient preference, surgical time, marginal bone level changes and ISQ values. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The article reported: "A computer generated restricted randomisation list was created". |
| Allocation concealment (selection bias) | Low risk | The article reported: "Only one of the investigators (Marco Esposito), not involved in the selection and treatment of the patients, was aware of the randomisation sequence and could have access to the randomisation list stored in his pass‐word protected portable computer.The information on how to treat first site was enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes were opened sequentially after delivery of local anaesthesia and the surgeon treated in that occasion only the first of the 2 sites, after 2 weeks the second site was treated according to the allocated procedure. Therefore, treatment allocation was concealed to the investigators in charge of enrolling and treating the patients." and "If the upper right side did not need implant(s), the lower right was selected and, if also this side did not need implants either, the upper left jaw was selected. After delivery of local anaesthesia the surgeon was informed whether to raise a flap or not at the selected side by opening a sequentially numbered, sealed, opaque envelope. Only one side was treated during the first session, the second side was treated after 2 weeks according to a split‐mouth design." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The article reported: "The final follow‐up was at 1 year after loading. All outcome measures with the exception of marginal bone levels were assessed by a blinded dentist (Dr Giuseppe Fontana) who was not aware of patient allocation. Marginal bone level changes on periapical radiographs were evaluated by another blinded dentist (Dr Cinzia Torchio)". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or missing data. |
| Selective reporting (reporting bias) | Low risk | All data from all outcome measures presented. |
| Other bias | Low risk | None detected. |
Heydenrijk 2000.
| Methods | 1‐year follow‐up, parallel group randomised controlled trial. Unclear whether outcome assessor was blind or not. No withdrawals. | |
| Participants | Patients were included if they had a severely resorbed mandible resulting in impaired stability and retention of their lower dentures. Patients with a history of radiation therapy of the head and neck or with a previous history of preprosthetic surgery or implantology were excluded. Adults treated in the University Hospital of Groningen, the Netherlands. 10 patients enrolled (5 in each group) and results given for 10 at year 1. | |
| Interventions | Vestibular incision versus crestal incision for flap design during placement of 2 IMZ implants according to a 1‐stage technique. | |
| Outcomes | Prosthesis/implant failure, the degree of peri‐implant inflammation, mucosal level (recession or overgrowth) were measured at 2, 6 and 12 weeks after implant insertion and at 1 year after placement of prosthesis. Other outcome measures at 1 year were plaque, calculus, bleeding score, pocket probing depth, modified 'gingiva' score, recession, width of attached mucosa. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | In the article was reported: "the patients were allocated to 1 of 2 groups, A and B, by simple randomization". No reply to our request for clarifications. |
| Allocation concealment (selection bias) | Unclear risk | No information presented in the article. No reply to our request for clarifications. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No reply to our request for clarifications. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or missing data. |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported. |
| Other bias | Low risk | None perceived. |
Lindeboom 2010.
| Methods | 6‐month follow‐up, parallel group randomised controlled trial. Blinded outcome assessor. No withdrawals. | |
| Participants | Patients were included if they had fully edentulous maxillas allowing the placement of at least 6 implants. Smokers, patients with a history of radiation therapy of the head and neck, metabolic disorders, bleeding disorders and pregnant women were excluded. Adults treated at the Department of Oral and Maxillofacial Surgery at the Academic Medical Center in Amsterdam, the Netherlands. 16 patients enrolled (8 in each group) and results given for 16 at year 3. | |
| Interventions | Implant placement with or without flap elevation. The flapless surgery procedure was performed using individually customised surgical templates fabricated with CAD/CAM technology planned with the Procera Software 3D Planning Program (Nobel Biocare AB, Goteborg, Sweden). In the flapless group, soft tissues were punched away and after implant installation, the punch wounds were then sutured. | |
| Outcomes | Prosthetic and implant failures, complications, the Dutch version of the Impact of Event Scale‐Revised (IES‐R), the short version of the Dental Anxiety Inventory, the oral health‐related quality of life measured with the OHIP‐14, 11‐point numerical visual analogue rating scales (NRS), ranging from 0 to 100, were used to measure anxiety at this moment, pain at this moment, anxiety during treatment, pain felt during treatment and to what extent patients experienced treatment as aversive, duration of the procedure (in min), type of anaesthesia, amount of anaesthesia given, whether any additional anaesthesia had to be given (no, yes, how much more) and how difficult (in a technical sense) the procedure was. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | In the article it was reported: "Patients scheduled for implant placement were randomized using a computer program to implant placement with a flapless or with a flap technique". |
| Allocation concealment (selection bias) | Unclear risk | No information presented in the article. No reply to our request for clarifications. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information presented in the article. Author's reply: "outcome was assessed by assessors blinded for the groups". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals and apparently no missing data. |
| Selective reporting (reporting bias) | High risk | Not all outcome measures were reported and in particular postoperative pain. Author replied that he was unable to provide separate data for some of the outcome measures of interest. |
| Other bias | Low risk | None perceived. |
Lorenzo 2012.
| Methods | 6‐month follow‐up, parallel group randomised controlled trial. Blinded outcome assessor. Two exclusions from the Mucograft group. | |
| Participants | Patients having at least one implant site with < 1 mm of keratinised mucosa. Patients were excluded if they were heavy smokers (more than 10 cigarettes per day), suffering any systemic disease that would negatively influence wound healing, known allergy to collagen and poor oral hygiene (full mouth plaque score > 20%). Adults treated at the Periodontal Postgraduate Clinic at the Faculty of Odontology in the University Complutense of Madrid and the Branemark Osseintegration Center in Madrid, Spain. 24 patients enrolled (12 in each group) and results given for 22. | |
| Interventions | Augmentation of the keratinised tissue around implants supporting prosthetic restorations using a xenogeneic collagen matrix of porcine origin (Mucograft®; Geistlich Pharma AG, Wolhusen, Switzerland) or a connective tissue autograft harvested from the palate. | |
| Outcomes | Prosthetic and implant failures, complications, probing pocket depths, probing "attachment" levels, recessions, full‐mouth bleeding scores (FMBS), full‐mouth plaque scores (FMPS), aesthetics (colour blending of the grafted site with the adjacent soft tissues), height of keratinised mucosa, pain recorded at 10 and 30 days on a visual analogue scale from 0‐10. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | In the article it was reported: "The study monitor generated the allocation sequence by means of a computer‐generated random permuted block (patient) and instructed a different subject (C.M., secondary investigator) to assign a sealed envelope containing the assigned treatment to each site". |
| Allocation concealment (selection bias) | Low risk | In the article it was reported: "The randomization opaque envelope was opened immediately before the application of the graft material, once the recipient was prepared". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In the article it was reported: "All clinical parameters were recorded with a North Carolina University probe by the same blinded examiner to the treatment (V.G.)". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data of 2 patients from the Mucograft group were excluded since the patients increased significantly their smoking habit surpassing the threshold level defined in the protocol. |
| Selective reporting (reporting bias) | High risk | Not all outcome measures/data presented. |
| Other bias | High risk | Trial commercially funded and data of 2 patients from the sponsored intervention excluded by the authors. |
Wiesner 2010.
| Methods | 1‐year follow‐up, split‐mouth randomised controlled trial. The outcome assessor was blinded for the radiographic and aesthetic assessment but not for the measurement of the thickness of soft tissue. No withdrawals. | |
| Participants | 10 adult patients were treated in three different private practices in Austria. Results given for all 10 patients over a year period. | |
| Interventions | Crestal incisions and split thickness flap design at placement of Neoss implants. Soft tissue augmentation took place in one side of posterior mandible using a subepithelial connective tissue graft taken from the palate. The graft was positioned over the implant to cover both buccal and lingual sides and sutured to the periosteum with 6‐0 absorbable suture. Subsequently flaps were closed submerging the implants. | |
| Outcomes | Prosthesis/implant failures, any biological or prosthetic complications, peri‐implant marginal bone levels evaluated on standardised intraoral digital radiographs, soft tissue thickness for lingual and buccal sites, aesthetics (pink aesthetic scores), patient satisfaction and preference. All assessments were made at year after loading. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study clarified: "Two identical, opaque sealed envelops were prepared for each patient. One envelop contained a paper indicating the right side and the other the left side. Just prior to the initiation of the initiation of implant placement procedure, the patient was asked to choose one of the two envelopes. The graft was performed on the side indicated by the envelope chosen by the patient." |
| Allocation concealment (selection bias) | Unclear risk | The study clarified: "Just prior to the initiation of the initiation of implant placement procedure, the patient was asked to choose one of the two envelops. The graft was performed on the side indicated by the envelope chosen by the patient." Reviewers comment: The envelope should have only been opened after the flap elevation and the implant placement to make sure that the surgeon technique does not get influenced by the allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The article reported that peri‐implant marginal bone level, aesthetics evaluation, patient preference and patient satisfaction were assessed by a blinded assessor. Implant/crown failure, biological complications and soft tissue thickness were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study clarified: "Data from all the patients were included in the statistical analysis". |
| Selective reporting (reporting bias) | Low risk | Reviewers comment: All outcomes considered at the beginning of the study were reported in the publication. |
| Other bias | Low risk | Reviewers comment: Nothing could be found. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cannizzaro 2008 | Trial comparing flapless implant placement with flap elevation. Implants placed flapless were inserted in underprepared sites and were loaded immediately whereas implants placed after flap elevation were inserted in conventionally prepared sites and were conventionally loaded. |
| Covani 2008 | Follow‐up limited to abutment connection. |
| Fortin 2006 | Follow‐up limited to 6 days after implant placement. |
| Hunt 1996 | Follow‐up limited to abutment connection. |
| Ivanoff 2001 | Quasi‐random study: group allocation was undertaken using birth date. Methodological problem: type of suture material (intervention) confounded by suture technique. Letter received from authors confirmed that the 3 suture techniques were not randomly distributed between the 2 suture type groups. |
| Kerscher 1993 | 10 patients were included in each group, however, only data from 11 patients were presented and it was unclear from which group. |
| Lai 2006 | No outcomes of interest and too short follow‐up (12 weeks). |
| Ozan 2007 | Authors replied that this was a quasi‐randomised trial with randomisation based on the birth date. The 2 groups were not comparable. |
| Sanz 2009 | Both teeth and implants subjected to the interventions under evaluation and data presented for both implant and teeth together. |
| Shahidi 2008 | Follow‐up limited to abutment connection. |
| Van de Velde 2010 | Trial comparing flapless implant placement with flap elevation. Implants placed flapless were loaded immediately whereas implant placed after flap elevation were early loaded. |
Differences between protocol and review
In this review update, the outcome measures of radiographic marginal bone level changes and soft tissue thickness changes were added, and the requirement of a minimum follow‐up of 6 months after functional loading of the implants was extended to all trials to be eligible for inclusion in this review.
Contributions of authors
Conceiving, designing and co‐ordinating the review (Marco Esposito (ME)). Developing search strategy and undertaking searches (ME, Paul Coulthard (PC)). Screening search results and retrieved papers against inclusion criteria (ME, Maria Gabriella Grusovin (MG), Hassan Maghaireh (HM)). Appraising quality (ME, MG, HM, Yannis Ziounas (YZ)). Extracting data from papers (ME, MG, Helen Worthington (HW), HM, YZ). Writing to authors for additional information (ME, HM, HW). Data management for the review and entering data into RevMan (HW, ME). Analysis and interpretation of data (ME, HW, MG, HM). Writing the review (ME). Providing general advice on the review (MG, PC). Performing previous work that was the foundation of current study (ME, HW, PC).
Sources of support
Internal sources
Division of Dentistry, The University of Manchester, UK.
-
MAHSC, UK.
Cochrane Oral Health is supported by the Manchester Academic Health Sciences Centre (MAHSC) and the NIHR Manchester Biomedical Research Centre
External sources
-
British Orthodontic Society (BOS), UK.
The BOS have provided funding for Cochrane Oral Health Global Alliance.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed are those of the authors and not necessarily those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; and the Swiss Society for Endodontology, Switzerland.
Declarations of interest
Marco Esposito is among the authors of three of the eligible trials, however, he was not involved in the inclusion/exclusion decision process or the quality assessment of these trials. Marco Esposito is working as independent methodological consultant in various implant related projects for some of the companies whose implants were used both in the included and excluded trials, however, in this review, implant brands were not under evaluation.
From April 2011 Marco Esposito became a full‐time freelance consultant in dentistry specialising in implantology. Therefore he receives funding for conducting clinical trials and presenting the results of trials/Cochrane reviews at international dental meetings. This funding comes from universities, companies (in alphabetical order: Apollonia e Fama Implant, Biomax, Biomet 3i, Bioteck, Bone System, Branemark Integration, CMS Dental, Dentsply‐Friadent, Geistlich Pharma, Geass, Keystone Dental, MegaGen Implant, Mozo‐Grau, Nano Bridging molecules, Nobel Biocare, Ricerfarma, Saint Jude Medical, Southern Implants, Supercharched production, Techoss Dental Thommen Medical, Tutogen Medical, Zimmer Dental, Z‐Systems), scientific societies, publishing companies, and private dentists. This list of companies was provided by Marco on Friday 4th November 2011 and the funders will change all the time.
This is to certify that: a) Marco does not own stock in companies that produce products included in the reviews; b) he does not have patents on any of the products included in the reviews; c) his salary will not be affected by company sales of any of the products included in the reviews.
Marco's authorship has been authorised by The Cochrane Collaboration Funding Arbiter (reference 071111/057: Oral Health Group).
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Arnabat‐Dominguez 2003 {published data only}
- Arnabat‐Domínguez J, España‐Tost AJ, Berini‐Aytés L, Gay‐Escoda C. Erbium:YAG laser application in the second phase of implant surgery: a pilot study in 20 patients. The International Journal of Oral and Maxillofacial Implants 2003;18(1):104‐12. [PubMed] [Google Scholar]
Cannizzaro 2011 {published data only}
- Cannizzaro G, Felice P, Leone M, Checci V, Esposito M. Flapless versus open flap implant surgery in partially edentulous patients subjected to immediate loading: 1‐year results from a split‐mouth randomised controlled trial. European Journal of Oral Implantology 2011;4(3):177‐88. [PubMed] [Google Scholar]
Heydenrijk 2000 {published data only}
- Heydenrijk K, Raghoebar GM, Batenburg RHK, Stegenga B. A comparison of labial and crestal incisions for the 1‐stage placement of IMZ implants: a pilot study. Journal of Oral and Maxillofacial Surgery 2000;58(10):1119‐23. [DOI] [PubMed] [Google Scholar]
Lindeboom 2010 {published data only}
- Lindeboom JA, Wijk AJ. A comparison of two implant techniques on patient‐based outcome measures: a report of flapless vs. conventional flapped implant placement. Clinical Oral Implants Research 2010;21(4):366‐70. [DOI] [PubMed] [Google Scholar]
Lorenzo 2012 {published data only}
- Lorenzo R, García V, Orsini M, Martin C, Sanz M. Clinical efficacy of a xenogeneic collagen matrix in augmenting keratinized mucosa around implants: a randomized controlled prospective clinical trial. Clinical Oral Implants Research 2012;23(3):316‐24. [DOI] [PubMed] [Google Scholar]
Wiesner 2010 {published data only}
- Wiesner G, Esposito M, Worthington H, Schlee M. Connective tissue grafts for thickening peri‐implant tissues at implant placement. One‐year results from an explanatory split‐mouth randomised controlled clinical trial. European Journal of Oral Implantology 2010;3(1):27‐35. [PubMed] [Google Scholar]
References to studies excluded from this review
Cannizzaro 2008 {published data only}
- Cannizzaro G, Leone M, Consolo U, Ferri V, Esposito M. Immediate functional loading of implants placed with flapless surgery versus conventional implants in partially edentulous patients. a 3‐year randomized controlled clinical trial. The International Journal of Oral & Maxillofacial Implants 2008;23(5):867‐75. [PubMed] [Google Scholar]
Covani 2008 {published data only}
- Covani U, Cornelini R, Barone A. Buccal bone augmentation around immediate implants with and without flap elevation: a modified approach. The International Journal of Oral & Maxillofacial Implants 2008;23(5):841‐6. [PubMed] [Google Scholar]
Fortin 2006 {published data only}
- Fortin T, Bosson JL, Isidori M, Blanchet E. Effect of flapless surgery on pain experienced in implant placement using an image‐guided system. The International Journal of Oral and Maxillofacial Implants 2006;21(2):298‐304. [PubMed] [Google Scholar]
Hunt 1996 {published data only}
- Hunt BW, Sandifer JB, Assad DA, Gher ME. Effect of flap design on healing and osseointegration of dental implants. The International Journal of Periodontics and Restorative Dentistry 1996;16(6):582‐93. [PubMed] [Google Scholar]
Ivanoff 2001 {published data only}
- Ivanoff CJ, Widmark G. Nonresorbable versus resorbable sutures in oral implant surgery: a prospective clinical study. Clinical Implant Dentistry and Related Research 2001;3(1):57‐60. [DOI] [PubMed] [Google Scholar]
Kerscher 1993 {published data only}
- Kerscher A, Kreusch T. Implants in the edentulous mandible with vestibulum plastic ‐ Edlan or Kazanjian? [Implantate im zahnlosen unterkiefer mit vestibulumplastik ‐ Edlan oder Kazanjian?]. Deutsche Zahnärztliche Zeitschrift 1993;48:797‐9. [Google Scholar]
Lai 2006 {published data only}
- Lai HC, Xu YY, Zhang ZY, Huang W, Wu YQ. [Buccal soft tissue augmentation using acellular dermal matrix in implant therapy]. Zhonghua Kou Qiang Yi Xue Za Zhi 2006;41(7):395‐6. [PubMed] [Google Scholar]
Ozan 2007 {published data only}
- Ozan O, Turkyilmaz I, Yilmaz B. A preliminary report of patients treated with early loaded implants using computerized tomography‐guided surgical stents: flapless versus conventional flapped surgery. Journal of Oral Rehabilitation 2007;34(11):835‐40. [DOI] [PubMed] [Google Scholar]
Sanz 2009 {published data only}
- Sanz M, Lorenzo R, Aranda JJ, Martin C, Orsini M. Clinical evaluation of a new collagen matrix (Mucograft prototype) to enhance the width of keratinized tissue in patients with fixed prosthetic restorations: a randomized prospective clinical trial. Journal of Clinical Periodontology 2009;36(10):868‐76. [DOI] [PubMed] [Google Scholar]
Shahidi 2008 {published data only}
- Shahidi P, Jacobson Z, Dibart S, Pourati J, Nunn ME, Barouch K, et al. Efficacy of a new papilla generation technique in implant dentistry: a preliminary study. The International Journal of Oral & Maxillofacial Implants 2008;23(5):926‐34. [PubMed] [Google Scholar]
Van de Velde 2010 {published data only}
- Velde T, Sennerby L, Bruyn H. The clinical and radiographic outcome of implants placed in the posterior maxilla with a guided flapless approach and immediately restored with a provisional rehabilitation: a randomized clinical trial. Clinical Oral Implants Research 2010;21(11):1223‐33. [DOI] [PubMed] [Google Scholar]
Additional references
Brånemark 1977
- Brånemark PI, Hansson BO, Adell R, Breine U, Lindström J, Hallén O, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10‐year period. Scandinavian Journal of Plastic and Reconstructive Surgery. Supplementum 1977;16:1‐132. [PubMed] [Google Scholar]
Chung 2006
- Chung DM, Oh TJ, Shotwell JL, Misch CE, Wang HL. Significance of keratinized mucosa in maintenance of dental implants with different surfaces. Journal of Periodontology 2006;77(8):1410‐20. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Esposito 2009
- Esposito M, Grusovin MG, Felice P, Karatzopoulos G, Worthington HV, Coulthard P. Interventions for replacing missing teeth: horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003607.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Esposito 2010
- Esposito M, Grusovin MG, Polyzos IP, Felice P, Worthington HV. Interventions for replacing missing teeth: dental implants in fresh extraction sockets (immediate, immediate‐delayed and delayed implants). Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD005968.pub3] [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lekholm 1985
- Lekholm U, Zarb GA. Patient selection and preparation. In: Brånemark P‐I, Zarb GA, Albrektsson T editor(s). Tissue integrated prostheses ‐ osseointegration in clinical dentistry. Chicago: Quintessence Publishing Co., 1985:199‐209. [Google Scholar]
Roos‐Jansåker 2006
- Roos‐Jansåker AM, Renvert H, Lindahl C, Renvert S. Nine‐ to fourteen‐year follow‐up of implant treatment. Part III: factors associated with peri‐implant lesions. Journal of Clinical Periodontology 2006;33(4):296‐301. [DOI] [PubMed] [Google Scholar]
Schulz 1995a
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schulz 1995b
- Schulz KF. Subverting randomization in controlled trials. JAMA 1995;274(18):1456‐8. [PubMed] [Google Scholar]
van Steenberghe 2005
- Steenberghe D, Glauser R, Blomback U, Andersson M, Schutyser F, Pettersson A, et al. A computed tomographic scan‐derived customized surgical template and fixed prosthesis for flapless surgery and immediate loading of implants in fully edentulous maxillae: a prospective multicenter study. Clinical Implant Dentistry and Related Research 2005;7 Suppl 1:S111‐20. [DOI] [PubMed] [Google Scholar]
Wennström 1994
- Wennström JL, Bengazi F, Lekholm U. The influence of the masticatory mucosa on the peri‐implant soft tissue condition. Clinical Oral Implants Research 1994;5(1):1‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Esposito 2007
- Esposito M, Grusovin MG, Maghaireh H, Coulthard P, Worthington HV. Interventions for replacing missing teeth: management of soft tissues for dental implants. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006697] [DOI] [PubMed] [Google Scholar]


