Abstract
Background
Attention deficit hyperactivity disorder (ADHD) is a major problem in children and adolescents, characterised by age‐inappropriate levels of inattention, hyperactivity and impulsivity, and is associated with long‐term social, academic and mental health problems. The stimulant medications methylphenidate and amphetamine are the most frequently used treatments for ADHD, but these are not always effective and can be associated with side effects. Clinical and biochemical evidence suggests that deficiencies of polyunsaturated fatty acids (PUFA) could be related to ADHD. Children and adolescents with ADHD have been shown to have significantly lower plasma and blood concentrations of PUFA and, in particular, lower levels of omega‐3 PUFA. These findings suggest that PUFA supplementation may reduce the attention and behaviour problems associated with ADHD.
Objectives
To compare the efficacy of PUFA to other forms of treatment or placebo in treating the symptoms of ADHD in children and adolescents.
Search methods
We searched the following databases in August 2011: CENTRAL (The Cochrane Library 2011, Issue 2), MEDLINE (1948 to July Week 3, 2011), EMBASE (1980 to 2011 Week 29), PsycINFO (1806 to current), CINAHL (1937 to current), BIOSIS (1969 to 30 July 2011), Science Citation Index (1970 to 30 July 2011), Social Science Citation Index (1970 to 30 July 2011), Conference Proceedings Citation Index ‐ Science (1990 to 30 July 2011), Conference Proceedings Citation Index ‐ Social Science and Humanities (1990 to 30 July 2011), Cochrane Database of Systematic Reviews (2011, Issue 7), DARE (2011 Issue 2), Dissertation Abstracts (via Dissertation Express) and the metaRegister of Controlled Trials (mRCT). In addition, we searched the following repositories for theses on 2 August 2011: DART, NTLTD and TROVE. We also checked reference lists of relevant studies and reviews for additional references.
Selection criteria
Two review authors independently assessed the results of the database searches. We resolved any disagreements regarding the selection of studies through consensus or, if necessary, by consultation with a third member of the review team.
Data collection and analysis
Two members of the review team independently extracted details of participants and setting, interventions, methodology and outcome data. If differences were identified, we resolved them by consensus or referral to a third member of the team. We made all reasonable attempts to contact the authors where further clarification or missing data were needed.
Main results
We included 13 trials with 1011 participants in the review. After screening 366 references, we considered 23 relevant and obtained the full text for consideration. We excluded five papers and included 18 papers describing the 13 trials.
Eight of the included trials had a parallel design: five compared an omega‐3 PUFA supplement to placebo; two compared a combined omega‐3 and omega‐6 supplement to placebo, and one compared an omega‐3 PUFA to a dietary supplement. Five of the included trials had a cross‐over design: two compared combined omega‐3/6 PUFA to placebo; two compared omega‐6 PUFA with placebo; one compared omega‐3 to omega‐6 PUFA, and one compared omega‐6 PUFA to dexamphetamine. Supplements were given for a period of between four and 16 weeks.
There was a significantly higher likelihood of improvement in the group receiving omega‐3/6 PUFA compared to placebo (two trials, 97 participants; risk ratio (RR) 2.19, 95% confidence interval (CI) 1.04 to 4.62). However, there were no statistically significant differences in parent‐rated ADHD symptoms (five trials, 413 participants; standardised mean difference (SMD) ‐0.17, 95% CI ‐0.38 to 0.03); inattention (six trials, 469 participants; SMD ‐0.04, 95% CI ‐0.29 to 0.21) or hyperactivity/impulsivity (five trials, 416 participants; SMD ‐0.04, 95% CI ‐0.25 to 0.16) when all participants receiving PUFA supplements were compared to those receiving placebo.
There were no statistically significant differences in teacher ratings of overall ADHD symptoms (four trials, 324 participants; SMD 0.05, 95% CI ‐0.18 to 0.27); inattention (three trials, 260 participants; SMD 0.26, 95% CI ‐0.22 to 0.74) or hyperactivity/impulsivity (three trials, 259 participants; SMD 0.10, 95% CI ‐0.16 to 0.35).
There were also no differences between groups in behaviour, side effects or loss to follow‐up.
Overall, there were no other differences between groups for any other comparison.
Authors' conclusions
Overall, there is little evidence that PUFA supplementation provides any benefit for the symptoms of ADHD in children and adolescents. The majority of data showed no benefit of PUFA supplementation, although there were some limited data that did show an improvement with combined omega‐3 and omega‐6 supplementation.
It is important that future research addresses current weaknesses in this area, which include small sample sizes, variability of selection criteria, variability of the type and dosage of supplementation, short follow‐up times and other methodological weaknesses.
Keywords: Adolescent; Child; Humans; Male; Dietary Supplements; Attention Deficit Disorder with Hyperactivity; Attention Deficit Disorder with Hyperactivity/drug therapy; Fatty Acids, Omega‐3; Fatty Acids, Omega‐3/administration & dosage; Fatty Acids, Omega‐6; Fatty Acids, Omega‐6/administration & dosage; Fatty Acids, Unsaturated; Fatty Acids, Unsaturated/administration & dosage
Polyunsaturated fatty acids (PUFA) supplements for attention deficit hyperactivity disorder (ADHD) in children and adolescents
Attention deficit hyperactivity disorder (ADHD) is a major problem in children and adolescents and can result in long‐term social, academic and mental health problems. Stimulant medications, such as methylphenidate and amphetamine, are the most frequently used treatments for ADHD but are not always effective and can be associated with side effects. There is evidence that ADHD could be related to deficiencies of polyunsaturated fatty acids (PUFA) and, in particular, omega‐3 PUFA; therefore, PUFA supplementation may improve ADHD symptoms and associated problems. The aim of this review was to evaluate whether PUFA supplements are an effective treatment for children and adolescents with ADHD. Although there were some limited data that did indicate there may be some improvement, overall there was little evidence that PUFA supplementation is beneficial. Further high‐quality research needs to be done.
Background
Description of the condition
Attention deficit hyperactivity disorder (ADHD) is a developmental disorder characterised by age‐inappropriate levels of inattention, hyperactivity and impulsivity. According to the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV) (APA 1994) and the more recent text revision (DMS‐IV‐TR) (APA 2000), there are three categorical subtypes of ADHD: a predominantly inattentive subtype (ADHD‐Inattentive), a predominantly hyperactive‐impulsive subtype (ADHD‐Hyperactive‐Impulsive) and a combined inattentive and hyperactive‐impulsive subtype (ADHD‐Combined). The International Classification of Diseases (ICD‐10) definition of hyperkinetic disorders similarly includes developmentally inappropriate levels of inattention, hyperactivity and impulsivity (WHO 2010). However, the diagnosis for hyperkinetic disorders is more restrictive, which may explain why it is less prevalent than ADHD (Biederman 2005).
ADHD is a major public health problem (NIH 2000; Pelham 2007). The worldwide prevalence of ADHD is 5% (Polanczyk 2007) with 3% to 16% of children displaying symptoms of ADHD (Cantwell 1996; Faraone 2003). Boys are more commonly identified than girls (Cantwell 1996; Faraone 2003), with an approximate ratio of between two to nine boys to one girl, depending on the ADHD type (APA 2000) and whether prevalence is based on clinical or epidemiological populations (Cantwell 1996).
Children and adolescents with ADHD can have academic impairments, social dysfunction and poor self esteem (Cantwell 1996; Daley 2004; Furman 2005). ADHD is frequently comorbid with other mental health disorders, such as anxiety and depression (Cantwell 1996; Daley 2004), and is associated with a higher risk of negative effects such as substance abuse (Daley 2004). Between 50% to 75% of children with ADHD will continue to have symptoms into adulthood (Cantwell 1996; Daley 2004; Biederman 2005; Harpin 2005).
Aetiology
It is claimed that there are several factors that contribute to the onset and maintenance of ADHD symptoms, including: genetic factors (Goodman 1989; Gill 1997; Swanson 2000; Swanson 2007); neuroanatomical abnormalities (Raskin 1981; Barkley 1991; Biederman 2005); psychosocial factors (Morrell 2003; Biederman 2005); pregnancy and delivery complications (Zappitelli 2001; Biederman 2005) and environmental factors, such as prenatal cigarettes or alcohol (Linnet 2003; Langley 2005) and artificial food colouring (Schab 2004). However, the most significant factor associated with ADHD is heritability, which contributes approximately 75% to the aetiology of ADHD (Biederman 2005; Froehlich 2011).
There is good evidence that the neurotransmitter dopamine may be implicated in the pathogenesis of ADHD. Stimulant drugs that are used in the management of ADHD symptoms increase the availability of dopamine in the brain (Biederman 2005), and a number of genes related to the dopamine pathway have been implicated in ADHD, in particular, the dopamine D4 and D5 receptor, dopamine‐β‐hydroxylase and dopamine transporter genes (Faraone 2000; Li 2006). In addition, imaging studies have shown differences in the dopamine pathway in children and adults with ADHD (Krause 2003; Biederman 2005; Swanson 2007).
Diagnosis of ADHD
The diagnosis of ADHD in children and adolescents should be based on validated criteria such as DSM‐IV (APA 1994) or DSM‐IV‐TR (APA 2000) for ADHD or ICD‐10 for hyperkinetic disorder (WHO 2010). Assessment should be through diagnostic interviews and supported by behaviour rating scales, direct observations of behaviour and clinic‐based testing (Barkley 1998).
Treatment for ADHD
The most common approaches to the treatment of ADHD are medication and/or psychological or behavioural interventions. However, behavioural therapies do not appear to be effective in the absence of medication (Klassen 1999) and other psychological therapies have limited success (Shaywitz 1997). The most frequently used treatments for ADHD are the stimulant medications methylphenidate and amphetamine (Biederman 2005). Meta‐analyses of stimulant medications have shown them to be effective in improving inattention and behavioural symptoms, although their effectiveness for improving cognition and achievement is more modest (Swanson 1993; Klassen 1999; NIH 2000). However, the positive effects do not appear to last once stimulants are no longer used (Swanson 1993) and as many as 30% of children do not respond to stimulants (Banaschewski 2004). Stimulants are associated with side effects such as decreased appetite, weight loss, insomnia, stomachache, headache and irritability (Cantwell 1996; Biederman 2005), although most will dissipate with time (Simeon 1993; Findling 1998). There are also concerns that stimulants may cause longer‐term adverse effects such as decreased growth (Daley 2004), increased risk of substance abuse (Daley 2004; Wilens 2004) and worsening of comorbid symptoms such as tics (Daley 2004).
Non‐stimulant medications can be advantageous in the treatment of ADHD as they are longer acting than stimulants, have limited abuse potential and may be more effective in the treatment of comorbid symptoms such as depression and anxiety (Daley 2004); however, they are less likely to be effective in treating the core symptoms of ADHD (Biederman 2005). Non‐stimulants include: antidepressants (tricyclics such as imipramine and desipramine, and selective serotonin reuptake inhibitors such as fluoxetine); anti‐anxiety agents (such as clonidine and guanfacine); bupropion (which acts on noradrenaline pathways) and atomoxetine (which acts on dopamine and noradrenaline pathways) (Cantwell 1996; Daley 2004). Some of these non‐stimulant medications are also associated with severe side effects. The tricyclic antidepressant desipramine has been linked to sudden death (Findling 1998; Biederman 2005), and selective serotonin reuptake inhibitors such as fluoxetine have been associated with suicidality in some children and adolescents (Hammad 2006).
Description of the intervention
Use of polyunsaturated fatty acids (PUFA) in ADHD
The limitations associated with the available treatments for ADHD, particularly the stimulant medications, mean that families often look for alternative treatments (NIH 2000; Daley 2004). The use of PUFA is one such alternative (Brue 2001; Daley 2004). Clinical and biochemical evidence suggests that functional deficiency of certain PUFA could be related to ADHD (Richardson 2000; Haag 2003). There is growing evidence that behaviour and attention problems are related to omega‐3 deficiencies (Richardson 2000; Richardson 2004). Children with ADHD have been shown to have significantly lower plasma and blood concentrations of PUFA (Mitchell 1987; Stevens 1995; Chen 2004) and, in particular, lower levels of omega‐3 PUFA (Mitchell 1987; Stevens 1995; Burgess 2000; Chen 2004). These findings suggest that PUFA supplementation may reduce the attention and behaviour problems associated with ADHD. There is some evidence that PUFA supplementation can be effective in improving neurodevelopmental indices. Meta‐analyses of the literature have identified some evidence that PUFA formula supplementation improves neurodevelopmental outcomes in preterm infants (Simmer 2008a), although data for full‐term infants are limited (Simmer 2008b).
How the intervention might work
Physiology of polyunsaturated fatty acids (PUFA)
Human infants require omega‐6 and omega‐3 PUFA for neural development and to maintain neural integrity and function (Wainright 1992; Innis 2000; Haag 2003). The omega‐3 PUFA, which include alpha‐linolenic acid (ALA), eicosapentanoic acid (EPA) and docosahexanoic acid (DHA), are associated with brain development (Innis 2000; Richardson 2000; Haag 2003) and DHA is the major PUFA in the adult mammalian brain (Innis 2000). Arachidonic acid is the most abundant omega‐6 PUFA in the human brain (Agostoni 2008). The omega‐3 PUFA, ALA is found in green‐leaved plants while EPA and DHA are found in high concentrations in fish oil; the omega‐6 PUFA precursor linoleic acid is found in vegetable oils (Simopoulos 1991; Haag 2003; Assisi 2006). The ratio of omega‐3 to omega‐6 PUFA is also considered important to normal development and function of the human brain (Simopoulos 1991; Innis 2000; Haag 2003) with a ratio of 1:4 considered optimal (Assisi 2006; Borsonelo 2008).
Why it is important to do this review
Although there had been trials of PUFA supplementation in children and adolescents with ADHD or symptoms of ADHD (for example, Aman 1987; Voigt 2001; Richardson 2002; Stevens 2003; Hirayama 2004), there were no systematic reviews in this area when this review was undertaken.
Objectives
To compare the efficacy of polyunsaturated fatty acids in treating ADHD in children and adolescents compared to other treatments or no treatment.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled trials. Quasi‐randomised trials are trials that use allocation methods with no apparent association with participant characteristics; for example, allocation based on the last number of medical identifier numbers or last number of the date of birth.
Types of participants
Children or adolescents (up to and including 18 years of age) who have been diagnosed with ADHD using validated criteria such as the ICD‐10 or DSM‐IV TR or scores on related scales with high sensitivity and specificity for a diagnosis of ADHD. Children and adolescents with comorbidities were included.
Types of interventions
The four main comparisons are:
PUFA versus placebo, no treatment or waiting list control;
PUFA + medication versus medication, for example, methylphenidate, amphetamine;
PUFA + behavioural therapy versus behavioural therapy*;
PUFA + psychotherapy versus psychotherapy*.
* We included trials that used medication in both groups.
Types of outcome measures
Primary outcomes
A change in ADHD symptoms measured by validated scales such as the Conners Rating Scales (Conners 1998) or Child Behavior Checklist (Achenbach 1983).
Secondary outcomes
Severity or incidence of behavioural problems, for example, oppositional behaviour or conduct disorder (measured by such scales as the Child Behavior Checklist (Achenbach 1983)).
Quality of life (for example, Pediatric Quality of Life Inventory Version 4.0 (Varni 2001)).
Severity or incidence of depressive symptoms (for example, Children's Depression Inventory (Kovacs 1992)).
Severity or incidence of anxiety symptoms (for example, State‐Trait Anxiety Inventory for Children (Spielberger 1973)).
Side effects, such as gastrointestinal symptoms, allergies, changes in weight, changes in appetite or sleep pattern.
Loss to follow‐up.
Cost.
We analysed data for parent, teacher, clinician and self reported outcomes separately.
Search methods for identification of studies
Electronic searches
The first searches for this review were run on the following databases in November 2008 by the Trials Search Co‐ordinator (TSC) of the Cochrane Developmental, Psychosocial and Learning Problems Group. The searches were re‐run to find new studies in September 2009 and again in August 2011.
Cochrane Central Register of Controlled Trials (CENTRAL) 2011, Issue 2, part of The Cochrane Library (last searched 1 August 2011)
Ovid MEDLINE (R) , 1948 to July Week 3 2011 (last searched 1 August 2011)
EMBASE (Ovid),1980 to 2011 Week 29 (last searched 1 August 2011)
PsycINFO (EBSCOhost) 1887 to current (last searched 1 August 2011)
PsycINFO (Ovid) 1806 to August Week 5 2009 (last searched 9 September 2009)
CINAHL, 1937 to current (last searched 1 August 2011)
BIOSIS, 1969 to 30 July 2011 (last searched 1 August 2011)
Science Citation Index, 1970 to 30 July 2011 (last searched 1 August 2011)
Social Science Citation Index, 1970 to 30 July 2011 (last searched 1 August 2011)
Dissertation Abstracts (via Dissertation Express) (last searched 1 August 2011)
The following databases were not listed at protocol stage but were added to improve the coverage of conference papers and theses literature, and to capture studies found by other systematic reviews:
Cochrane Database of Systematic Reviews, 2011, Issue 7 (last searched 1 August 2011)
Database of Abstracts of Review of Effects (DARE) 2011, Issue 2, part of the Cochrane Library (last searched 1 August 2011)
Conference Proceedings Citation Index ‐ Science ,1990 to 30 July 2011 (last searched 1 August 2011)
Conference Proceedings Citation Index ‐ Social Science and Humanities ,1990 to 30 July 2011 (last searched 1 August 2011)
NDLTD ‐ Networked Digital Library of Theses and Dissertations (last searched 2 August 2011)
DART ‐ Europe E‐theses portal (last searched 2 August 2011)
TROVE (limited to theses) ‐ National Library of Australia (last searched 2 August 2011)
metaRegister of Controlled Trials (mRCT), all databases selected, (last searched 1 August 2011)
We did not impose any date or language restrictions. Search strategies for each database are listed in Appendix 1
Searching other resources
We checked the reference lists of relevant studies and reviews for additional references to potentially relevant studies. We included trials in languages other than English.
Data collection and analysis
Selection of studies
Two review authors independently assessed the results of the database searches for eligibility to be included in the review. We resolved any disagreements regarding the selection of studies through consensus or, if necessary, we consulted a third member of the review team. If it was not clear from the abstract or title whether a reference met the inclusion criteria, we obtained and read the full article. Where further clarification was needed from trial authors in order to make a decision, we made all reasonable attempts to contact them.
Data extraction and management
All review authors developed and piloted a data extraction form for this review. Two members of the review team independently extracted details of participants, setting, interventions, methodology and outcome data from each trial. We then compared the data for any differences. If differences were identified, we resolved them by consensus or referral to a third member of the team when necessary. Where we needed further clarification or missing data from trial authors, we made all reasonable attempts to contact them.
We only included diagnostic outcome data (including improvement) in this review if the clinician making the diagnosis was blinded to the participant's treatment group membership or it was not clear whether the clinician was blinded. We only included outcome data that were measured using a scale or questionnaire if the scale or questionnaire had been reported to be valid and reliable in a peer‐reviewed journal.
Assessment of risk of bias in included studies
Using the Cochrane Collaboration’s tool for assessing the risk of bias (Higgins 2011), two review authors independently assessed trials on the following five criteria: 1) the adequacy of sequence generation; 2) allocation concealment; 3) the blinding of participants and personnel; 4) the blinding of outcome assessors; 5) incomplete outcome data; and 6) selective outcome reporting. We rated these criteria as 'low risk of bias', 'high risk of bias' or 'unclear risk of bias'. We also collected information on any other sources of bias (for example, baseline imbalance, cross‐over design, differential loss to follow‐up, inappropriate administration of an intervention or co‐intervention, early stopping and selective reporting of subgroups).
We collected details on how each of these criteria were addressed from the trial report. If there was any disagreement regarding risk of bias criteria, we resolved these differences by consensus or by referral to a third member of the review team.
Measures of treatment effect
Binary data
For binary outcomes, we calculated the risk ratio (RR) and 95% confidence interval (CI) using a fixed‐effect model, or a random‐effects model where there was significant heterogeneity.
Continuous data
For continuous outcomes, we used endpoint data in preference to change data if both were available. We calculated the standardised mean difference (SMD) between groups and 95% CI using a fixed‐effect model, or a random‐effects model where there was significant heterogeneity. We used SMDs because more than one scale was used for each of these continuous outcomes.
Unit of analysis issues
As the length of any effect of essential fatty acids is unknown, we only used first‐period data from any cross‐over trials that fitted the inclusion criteria. As first‐period data may increase the risk of bias (Higgins 2011), we reported this under 'other possible sources of bias' in the 'Risk of bias' tables. In all other cross‐over studies, the data from both periods were combined and were therefore not included in a meta‐analysis.
If cluster‐randomised trials that otherwise fit the inclusion criteria had been identified, they would not have been used because using this design for trials of essential fatty acid supplementation may have resulted in biased data (Higgins 2011).
Dealing with missing data
We used intention‐to‐treat data where available. We also collected information on how the intention‐to‐treat analysis was calculated and reported this in Characteristics of included studies. Where the reporting of data appeared to be incomplete, we made all reasonable efforts to contact trial authors to request missing data.
We reported the loss to follow‐up and reasons for missing data where available. If outcome data were reported as a median or range, or as a mean without a variance, we reported it in additional tables.
We considered the potential impact of missing data on the results in the interpretation of the results of the review.
Assessment of heterogeneity
We used a Chi2 test and I2 statistic to evaluate heterogeneity. We interpreted a P value of less than 0.10 for the Chi2 test of heterogeneity and/or an I2 value of greater than 50% as significant heterogeneity. If heterogeneity was significant, we used a random‐effects model for meta‐analysis. It had been proposed that a sensitivity analysis would be done to try and identify the source of heterogeneity; however, due to the low number of included trials, this was not possible.
Assessment of reporting biases
Publication bias
If data from at least 10 trials were available, we had planned to enter primary outcome data into a funnel plot because asymmetry of the plots may indicate publication bias (Higgins 2011). However, we could not do this due to the low number of identified trials.
Data synthesis
Where data were available, we synthesised similar interventions and outcomes in a meta‐analysis.
Data collection intervals
We planned to collect data for all follow‐up periods. It had been proposed that data would be analysed as short‐term (up to three months following completion of the therapy), medium‐term (from three months to one year following completion) and long‐term (greater than one year); however, we could not do this due to the paucity of data.
Skewed data
As a meta‐analysis is based on assumptions of normality, we checked all continuous data for skew before inclusion. We considered data to be skewed if the standard deviation was greater than half the mean (Altman 1996). It was not possible to check change data as this can include positive and negative values. We found no endpoint data to be skewed.
Subgroup analysis and investigation of heterogeneity
If data were available, subgroup analyses had been planned to assess the differential impact of gender, age group (under five years, five to 12 years and 13 to 18 years) and length of treatment. However, we were not able to do these analyses because of the low number of identified trials.
We carried out subgroup analyses for the type of PUFA supplement that was used that is, omega‐3 only, omega‐6 only, or a combination of omega‐3 and omega‐6.
Sensitivity analysis
We had proposed sensitivity analyses for the risk of selection, detection and attrition bias as these are associated with biased estimates of effect size (Moher 1998). However, there were only enough available data to perform sensitivity analysis for selection bias as described in the Results.
The inclusion criteria of some studies were based on scale cut‐off scores, therefore we conducted an additional sensitivity analyses to evaluate whether there was any difference compared to studies which used a clinician diagnosis of ADHD.
Results
Description of studies
Results of the search
We have included 13 trials with 1011 participants in this review. From the database searches, we identified 366 references. We thought 22 of these were potentially relevant and obtained them to read in full. We identified an additional reference when the authors of the trial contacted one of the authors of this review. Therefore, we obtained 23 papers. We included 18 papers describing 13 trials and excluded five papers (Figure 1). Our search of a trials register clinical trials and recent review of the literature by Sinn 2010 identified eight trials currently in progress, which we have detailed in Characteristics of ongoing studies.
Figure 1.
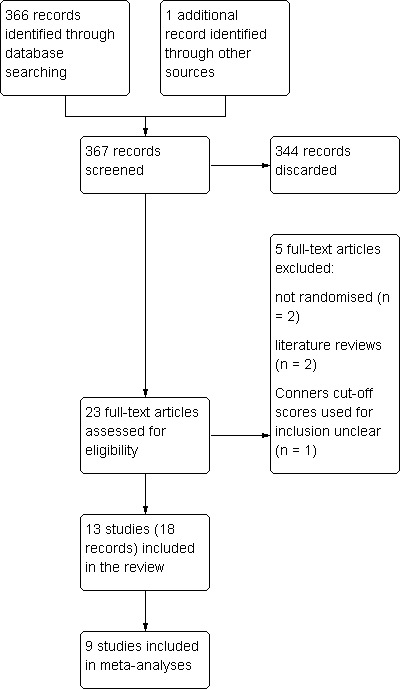
Study flow diagram
Included studies
Five trials (seven references) were of a cross‐over design (Aman 1987; Arnold 1989; Sinn 2007; Belanger 2009; Johnson 2009). Two compared omega‐6 PUFA with a placebo (Aman 1987; Arnold 1989), and one of these also compared omega‐6 PUFA with dexamphetamine (Arnold 1989). One study compared omega‐3 to omega‐6 PUFA (Belanger 2009). Sinn 2007 and Johnson 2009 both compared omega‐3/6 PUFA to placebo in the first phase, followed by PUFA supplement for all, but because first‐phase data were reported, this was integrated with data from parallel trials. We reported data from all other cross‐over trials separately. The eight remaining trials (11 references) were all of a parallel design. Five compared an omega‐3 PUFA supplement to placebo (Voigt 2001; Hirayama 2004; Vaisman 2008; Gustafsson 2010; Manor 2011); two compared a combined omega‐3 and omega‐6 supplement to placebo (Stevens 2003; Raz 2009), and one compared an omega‐3 PUFA to a dietary supplement (Brue 2001).
The omega‐3 PUFA used in supplements were DHA (median dose 187 mg/day, range 2.7 to 1035 mg/day), EPA (median dose 500 mg/day, range 80 to 1000 mg/day) and ALA (120 mg/day). The omega‐6 PUFA were arachidonic acid (40 mg/day), linoleic acid (median dose 1400 mg/day, range 480 to 2800 mg/day) and gamma‐linoleic acid (median dose 96 mg/day, range 60 to 320 mg/day). Supplements were given for a period of between four and 16 weeks.
The number of participants ranged from 18 to 147 and their ages were between six and 18 years. Four trials were done in the US, three in Israel, two in Sweden and one in each of Australia, Canada, Japan and New Zealand. In most trials the diagnosis of ADHD was made by a clinician except for the studies by Aman 1987, Arnold 1989 and Sinn 2007, which used scale cut‐offs with high sensitivity for a diagnosis of ADHD. Only one study (Stevens 2003) used inclusion criteria that could be indicative of PUFA deficiency, that is, thirst or skin symptoms.
We could not include data from Aman 1987 and Belanger 2009, both cross‐over studies, in a meta‐analysis because variances were not reported. We could not include data from two parallel trials in a meta‐analysis because the sample numbers were not clear (Brue 2001), the median number of symptoms were reported (Hirayama 2004). Not all outcomes in Sinn 2007 could be included in meta‐analyses as data for anxiety, oppositional behaviour and social problems were skewed.
Excluded studies
We excluded five references. Two were excluded because they were found to be literature reviews once the full papers were obtained (Doebel 2005; Anon 2009) and two were excluded because they were not randomly allocated trials (Harding 2003; Joshi 2006). Richardson 2002 was excluded because it was not clear which cut‐off scores were used as inclusion criteria despite attempts to contact the authors.
Risk of bias in included studies
See Figure 2 for 'Risk of bias' graph.
Figure 2.
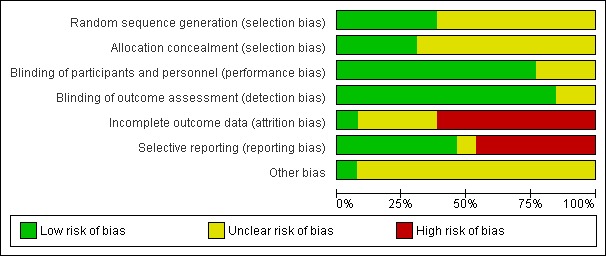
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
In the majority of trials, the methods used to generate a random sequence were not described (Aman 1987; Arnold 1989; Brue 2001; Belanger 2009) or were not described in sufficient detail (Stevens 2003; Sinn 2007; Johnson 2009; Raz 2009) and we therefore rated them as at unclear risk of bias. In the remaining trials (Voigt 2001; Hirayama 2004; Vaisman 2008; Gustafsson 2010; Manor 2011) there was a low risk of bias in the generation of a random sequence.
Allocation concealment
The method of allocation concealment was also unclear in the majority of trials, although there was a low risk of bias in four trials (Hirayama 2004; Sinn 2007; Johnson 2009; Manor 2011).
Blinding
Participants and personnel
The majority (10) of the included trials described methods used to blind participants and personnel and we therefore rated them as at a low risk of performance bias. However, we rated three as at an unclear risk of performance bias. Two of these did not detail how blinding was done (Arnold 1989; Belanger 2009) and in Raz 2009 it may have been possible to detect which intervention was the PUFA.
Outcome assessors
Brue 2001, Raz 2009 and Manor 2011 were the only trials that described how outcome assessment was blinded. However, as the majority of trials described the intervention and placebo as identical, and because parent and/or teacher‐rated measures were used, we also rated these as at a low risk of bias. We rated the remaining two trials (Belanger 2009; Johnson 2009) as at an unclear risk of detection bias because of insufficient information.
Incomplete outcome data
There was a high risk of bias due to attrition in eight included trials (Voigt 2001; Brue 2001; Stevens 2003; Sinn 2007; Vaisman 2008; Belanger 2009; Raz 2009; Manor 2011) because data were only reported for participants who were not lost to follow‐up. In the remainder of trials, we rated the level of bias as unclear. Sinn 2007 also reported that baseline scores for ADHD symptoms were significantly higher in the group lost to follow‐up. The number of participants enrolled and lost to follow‐up was not clear in the trials reported by Arnold 1989 and Johnson 2009. There was only one of 31 participants lost to follow‐up in the trial by Aman 1987, and although Gustafsson 2010 reported that intention‐to‐treat (ITT) analysis was done on 92 participants, this did seem to include "17 dropouts". We rated only one trial (Hirayama 2004) as having a low risk of attrition bias.
Selective reporting
There was a high risk of reporting bias in nine trials (Arnold 1989; Voigt 2001; Sinn 2007; Vaisman 2008; Belanger 2009; Raz 2009; Manor 2011) because the result of at least one outcome was not reported. We rated the risk of bias as unclear in the trial by Johnson 2009 as the authors referred to outcomes that would be published in "future publications". We rated the remaining trials as at a low risk of reporting bias as all outcomes appear to have been reported.
Other potential sources of bias
In the majority of trials (12), there was an unclear risk of other biases. Eight trials were sponsored by the companies that produced the PUFA supplement under investigation (Aman 1987; Arnold 1989; Voigt 2001; Vaisman 2008; Belanger 2009; Johnson 2009; Gustafsson 2010; Manor 2011). In addition, there were potential confounding variables in five studies. In Aman 1987 it was not clear whether the additional five children included in the trial would have met the original inclusion criteria. The PUFA group in Brue 2001 had received an additional 12 weeks of dietary supplementation before the PUFA trial was started. There were fewer children with Asperger's syndrome and more with a learning disorder in the PUFA group in the trial reported by Hirayama 2004, although this was not significant. More than half of the children in the PUFA group in Sinn 2007 also received a multivitamin. Although both groups were comparable over a large range of variables in Stevens 2003, the Conners Parent score and Reaction time were significantly lower in the PUFA group. We rated one trial (Raz 2009) as a low risk of other bias because the authors had comprehensively reported the possibility of confounding variables. Three trials did not report baseline characteristics of each group (Aman 1987; Arnold 1989; Brue 2001).
Effects of interventions
PUFA versus placebo ‐ parallel trials
ADHD symptoms
Two trials reported improvement. Both trials compared omega‐3/6 PUFA to placebo. There was a significantly higher likelihood of improvement in the group receiving PUFA compared to placebo (two trials, 97 participants; risk ratio (RR) 2.19, 95% confidence interval (CI) 1.04 to 4.62, Analysis 1.1).
Analysis 1.1.
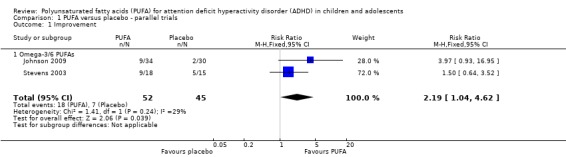
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 1 Improvement.
There were no statistically significant differences between groups in parent‐rated ADHD symptoms (five trials, 413 participants; standardised mean difference (SMD) ‐0.17, 95% CI ‐0.38 to 0.03, Analysis 1.2); inattention (six trials, 469 participants; SMD ‐0.04, 95% CI ‐0.29 to 0.21, Analysis 1.3) or hyperactivity/impulsivity (five trials, 416 participants; SMD ‐0.04, 95% CI ‐0.25 to 0.16, Analysis 1.4). However, there was a significant improvement in parent‐rated ADHD symptoms in the subgroup of studies that compared omega‐3/6 PUFA to placebo (two trials, 120 participants; SMD ‐0.48, 95% CI ‐0.88 to ‐0.08, Analysis 1.2).
Analysis 1.2.
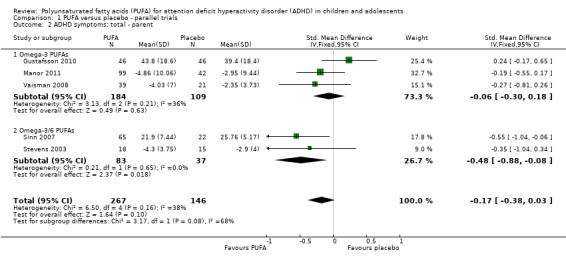
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 2 ADHD symptoms: total ‐ parent.
Analysis 1.3.
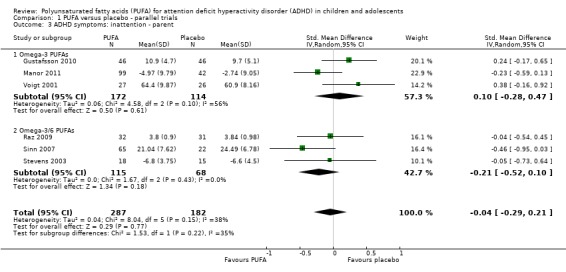
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 3 ADHD symptoms: inattention ‐ parent.
Analysis 1.4.
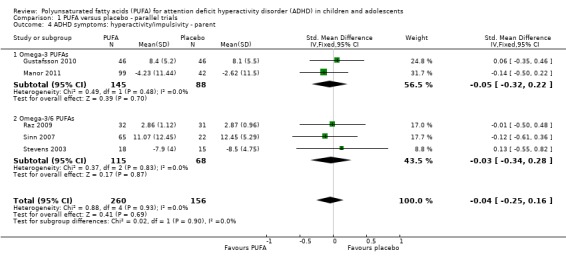
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 4 ADHD symptoms: hyperactivity/impulsivity ‐ parent.
There were also no statistically significant differences in teacher ratings of overall ADHD symptoms (four trials, 324 participants; SMD 0.05, 95% CI ‐0.18 to 0.27, Analysis 1.5); inattention (three trials, 260 participants; SMD 0.26, 95% CI ‐0.22 to 0.74, Analysis 1.6), or hyperactivity/impulsivity (three trials, 259 participants; SMD 0.10, 95% CI ‐0.16 to 0.35, Analysis 1.7).
Analysis 1.5.
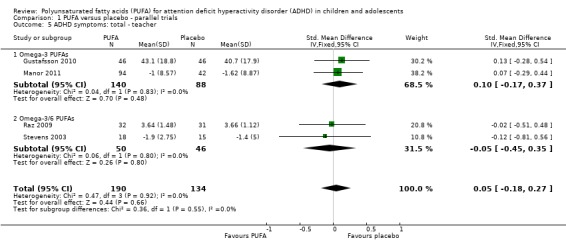
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 5 ADHD symptoms: total ‐ teacher.
Analysis 1.6.
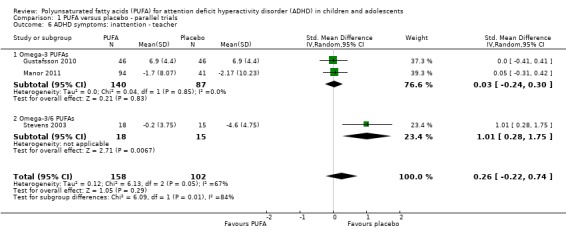
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 6 ADHD symptoms: inattention ‐ teacher.
Analysis 1.7.
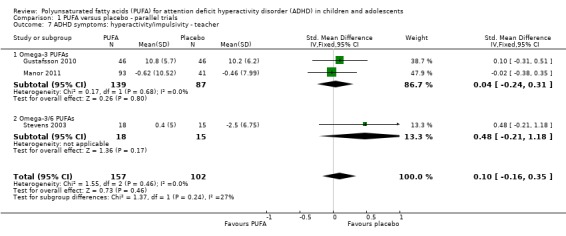
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 7 ADHD symptoms: hyperactivity/impulsivity ‐ teacher.
Only one trial reported clinician ratings of overall ADHD symptoms (64 participants; mean difference (MD) ‐0.30, 95% CI ‐0.80 to 0.19, Analysis 1.8); inattention (64 participants; MD ‐1.01, 95% CI ‐2.59 to 0.57, Analysis 1.9), and hyperactivity/impulsivity (64 participants; MD ‐1.15, 95% CI ‐3.06 to 0.76, Analysis 1.10). There were no differences between groups for any of these measures.
Analysis 1.8.
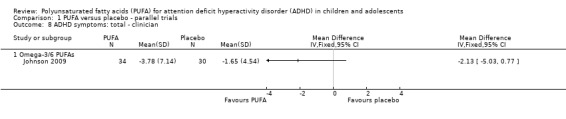
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 8 ADHD symptoms: total ‐ clinician.
Analysis 1.9.
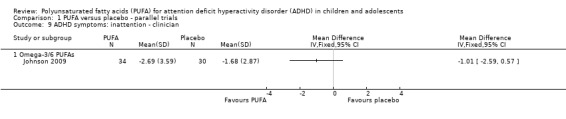
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 9 ADHD symptoms: inattention ‐ clinician.
Analysis 1.10.
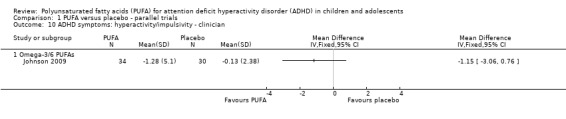
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 10 ADHD symptoms: hyperactivity/impulsivity ‐ clinician.
Behaviour
There were no differences between groups in the one trial that reported parent‐rated internalising behaviour (53 participants; MD 1.90, 95% CI ‐3.16 to 6.96, Analysis 1.11) and externalising behaviour (53 participants; MD 2.20, 95% CI ‐3.76 to 8.16, Analysis 1.12). There were no differences in parent ratings of socialisation (one trial, 53 participants; MD 0.40, 95% CI ‐3.90 to 4.70, Analysis 1.13); conduct (one trial, 33 participants; MD 1.30, 95% CI ‐1.54 to 4.14, Analysis 1.14), and oppositional behaviour (three trials, 266 participants; SMD 0.10, 95% CI ‐0.15 to 0.35, Analysis 1.15) and no differences in teacher ratings of conduct (one trial, 33 participants; MD 0.20, 95% CI ‐2.43 to 2.83, Analysis 1.16) and oppositional behaviour (three trials, 257 participants; SMD 0.11, 95% CI ‐0.15 to 0.36, Analysis 1.17).
Analysis 1.11.
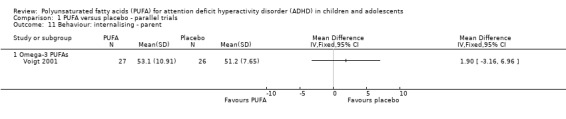
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 11 Behaviour: internalising ‐ parent.
Analysis 1.12.
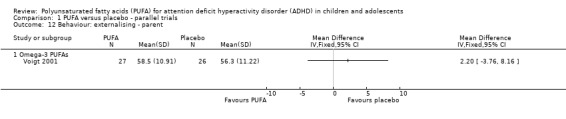
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 12 Behaviour: externalising ‐ parent.
Analysis 1.13.
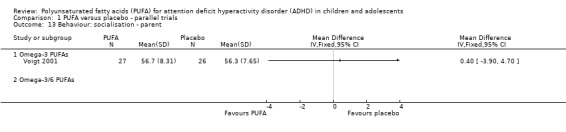
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 13 Behaviour: socialisation ‐ parent.
Analysis 1.14.
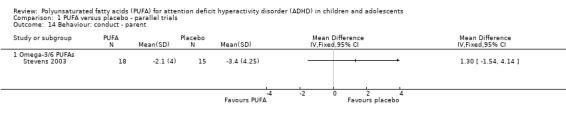
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 14 Behaviour: conduct ‐ parent.
Analysis 1.15.
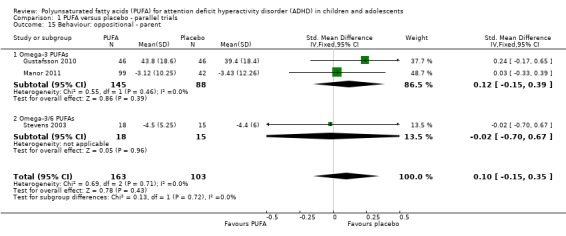
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 15 Behaviour: oppositional ‐ parent.
Analysis 1.16.
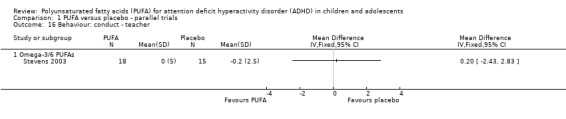
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 16 Behaviour: conduct ‐ teacher.
Analysis 1.17.
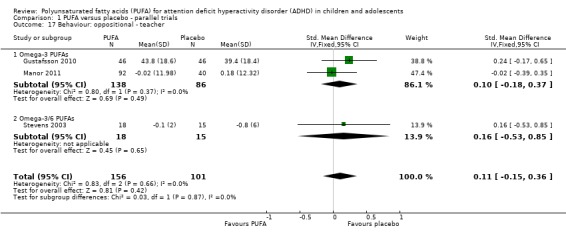
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 17 Behaviour: oppositional ‐ teacher.
Quality of life
There was no difference between participants receiving omega‐3 PUFA and those receiving placebo in the one study that reported a quality of life measure (138 participants; MD ‐0.12, 95% CI ‐3.71 to 3.47, Analysis 1.18).
Analysis 1.18.
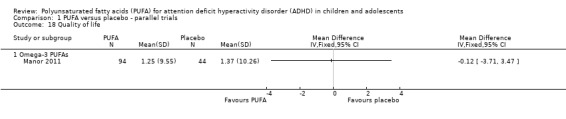
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 18 Quality of life.
Side effects
There were no differences between groups in the incidence of dermatitis (one trial, 147 participants; RR 1.43, 95% CI 0.06 to 34.36), diarrhoea (one trial, 85 participants; RR 0.73, 95% CI 0.17 to 3.08), gastrointestinal discomfort (one trial, 147 participants; RR 0.70, 95% CI 0.21 to 2.38), headache (one trial, 147 participants; RR 0.16, 95% CI 0.01 to 3.82), hyperactivity (one trial, 147 participants; RR 1.43, 95% CI 0.06 to 34.36), nausea (three trials, 306 participants; RR 1.02, 95% CI 0.39 to 2.70), nose bleed (one trial, 88 participants; RR 0.32, 95% CI 0.03 to 2.95), tics (one trial, 147 participants; RR 1.43, 95% CI 0.06 to 34.36) or general side effects (one trial, 63 participants; RR 2.91, 95% CI 0.87 to 9.74) (Analysis 1.19).
Analysis 1.19.
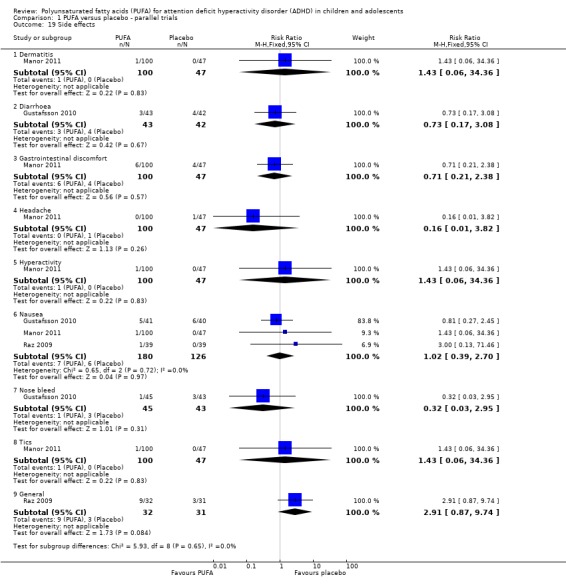
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 19 Side effects.
Loss to follow‐up
Loss to follow‐up in the PUFA and placebo groups was not significantly different (seven trials, 589 participants; RR 0.95, 95% CI 0.69 to 1.31, Analysis 1.20)
Analysis 1.20.
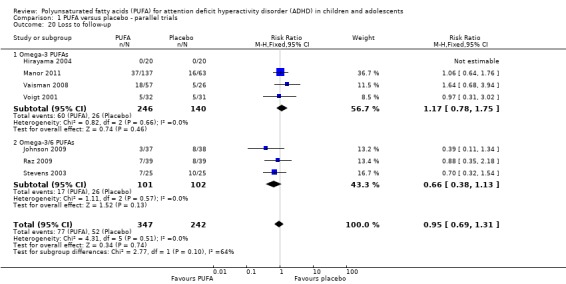
Comparison 1 PUFA versus placebo ‐ parallel trials, Outcome 20 Loss to follow‐up.
PUFA versus placebo ‐ cross‐over trials
Only one trial that compared PUFA with placebo provided enough data for meta‐analysis. In this one trial, which compared an omega‐6 PUFA to placebo, there was no difference in teacher ratings of overall ADHD symptoms (36 participants; MD ‐0.20, 95% CI ‐0.52 to 0.12, Analysis 2.1) or hyperactivity/impulsivity (36 participants; MD 0.29, 95% CI ‐0.17 to 0.75, Analysis 2.2).
Analysis 2.1.
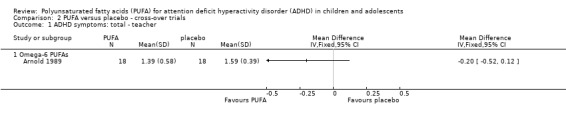
Comparison 2 PUFA versus placebo ‐ cross‐over trials, Outcome 1 ADHD symptoms: total ‐ teacher.
Analysis 2.2.
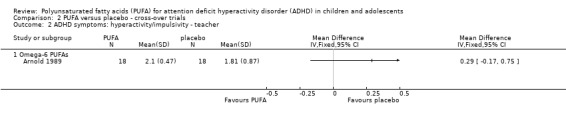
Comparison 2 PUFA versus placebo ‐ cross‐over trials, Outcome 2 ADHD symptoms: hyperactivity/impulsivity ‐ teacher.
Data from the cross‐over trial by Aman 1987 reported that mean scores for parent‐rated inattention and hyperactivity were significantly decreased in the omega‐6 PUFA group compared to the placebo group, but there were no significant differences in teacher ratings of inattention and hyperactivity, and parent‐ or teacher‐rated scores for conduct problems or anxiety (Table 5).
Table 1.
Other data
| Study | Comparison | ADHD symptoms: total | ADHD: inattention | ADHD: hyperactivity | Behaviour | Other outcomes |
| Aman 1987 |
Cross‐over trial of omega‐6 PUFA versus placebo |
Parent Omega‐6: 14.97 Placebo: 17.37 P < 0.01 Teacher Omega‐6: 2.69 Placebo: 2.66 |
Parent ‐ motor excess Omega‐6: 4.48 Placebo: 5.05 P < 0.05 Teacher ‐ motor excess Omega‐6: 2.58 Placebo: 2.58 |
Parent ‐ conduct Omega‐6: 16.30 Placebo: 17.61 Teacher ‐ conduct Omega‐6: 1.62 Placebo: 1.66 |
Parent ‐ anxiety Omega‐6: 6.06 Placebo: 5.73 Teacher ‐ anxiety Omega‐6: 1.73 Placebo: 1.70 |
|
| Belanger 2009 | Cross‐over trial of omega‐3 PUFA versus omega‐6 PUFA – first phase |
Parent Omega‐3: ‐9.1 Omega‐6: ‐7.1 |
Parent Omega‐3: ‐7.2 Omega‐6 ‐3.1 |
Parent Omega‐3: ‐8.8 Omega‐6: ‐5.4 |
||
| Brue 2001 | Parallel trial of omega‐3 PUFA versus dietary supplement |
Parent – non‐Ritalin Omega‐3: 12.0 Supplement: 13.7 Teacher – non‐Ritalin Omega‐3: 19.1 Supplement: 15.3 Parent – Ritalin Omega‐3: 15.6 Supplement: 14.6 Teacher – Ritalin Omega‐3: 16.3 Supplement: 12.2 P = 0.04 |
Parent – non‐Ritalin Omega‐3: 9.4 Supplement: 13.1 P = 0.03 Teacher – non‐Ritalin Omega‐3: 17.9 Supplement: 13.4 P = 0.04 Parent – Ritalin Omega‐3: 13.7 Supplement: 13.5 Teacher – Ritalin Omega‐3: 10.8 Supplement: 12.3 |
Where there were statistical differences between groups, P values are shown.
ADHD: attention deficit hyperactivity disorder; PUFA: polyunsaturated fatty acids
PUFA versus dexamphetamine ‐ cross‐over trials
Only one trial compared PUFA with dexamphetamine. There were no differences between groups in overall ADHD symptoms (36 participants; MD 0.29, 95% CI ‐0.17 to 0.75, Analysis 3.1) or hyperactivity/impulsivity (36 participants; MD 0.28, 95% CI ‐0.10 to 0.66, Analysis 3.2); both of which were teacher‐rated. Omega‐6 PUFA were used in this trial.
Analysis 3.1.
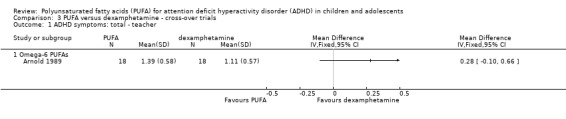
Comparison 3 PUFA versus dexamphetamine ‐ cross‐over trials, Outcome 1 ADHD symptoms: total ‐ teacher.
Analysis 3.2.
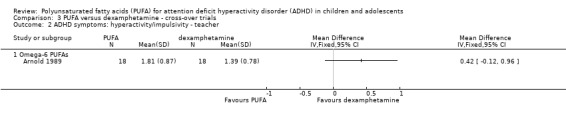
Comparison 3 PUFA versus dexamphetamine ‐ cross‐over trials, Outcome 2 ADHD symptoms: hyperactivity/impulsivity ‐ teacher.
Omega‐3 versus omega‐6 PUFA ‐ cross‐over trials
There were no statistically significant differences in parent ratings of overall ADHD symptoms, inattention or hyperactivity between participants receiving omega‐3 and omega‐6 PUFA (Table 5).
Omega‐3 versus dietary supplement ‐ parallel trials
There were no statistically significant differences between groups in parent or teacher ratings of inattention nor were there any differences in hyperactivity in the subgroup who also received Ritalin (Table 5). There were significant differences in parent and teacher ratings of hyperactivity in the subgroup of participants who were not receiving Ritalin but while parent ratings were lower in the PUFA group, teacher ratings were higher in the group receiving PUFA (Table 5).
Sensitivity analyses
Bias
The only outcomes for which there was enough data to conduct sensitivity analyses for bias were parent and teacher‐rated ADHD symptoms. We rated all of the studies reporting these outcomes as a low risk of detection bias; and we rated all, except Gustafsson 2010 where the risk was unclear, as a high risk of attrition bias. Therefore sensitivity analysis could only be conducted for selection bias. There was no difference between the PUFA and control groups in parent symptom ratings, regardless of whether there was an unclear or high risk of selection bias (Analysis 4.1).
Analysis 4.1.
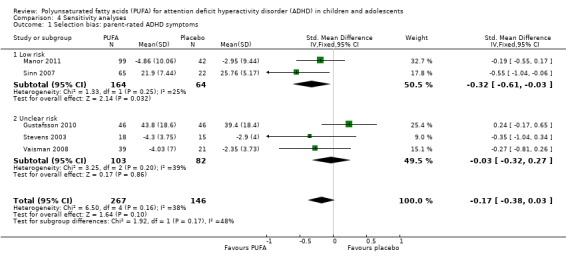
Comparison 4 Sensitivity analyses, Outcome 1 Selection bias: parent‐rated ADHD symptoms.
Inclusion criteria
We conducted an additional sensitivity analysis to evaluate whether there was any difference in findings in studies which used a clinician diagnosis of ADHD as an inclusion criteria compared to those which used scale cut‐offs. As the data from Aman 1987 could not be included in a meta‐analysis, Sinn 2007 was the only study which used scale cut‐offs and was included in a meta‐analysis. There was no difference between the PUFA and control groups in parent‐rated inattention (Analysis 4.2) or hyperactivity‐impulsivity (Analysis 4.3), irrespective of the inclusion of Sinn 2007.
Analysis 4.2.
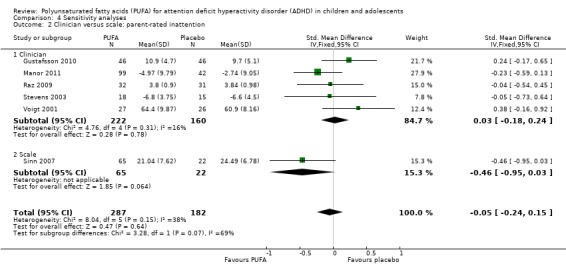
Comparison 4 Sensitivity analyses, Outcome 2 Clinician versus scale: parent‐rated inattention.
Analysis 4.3.
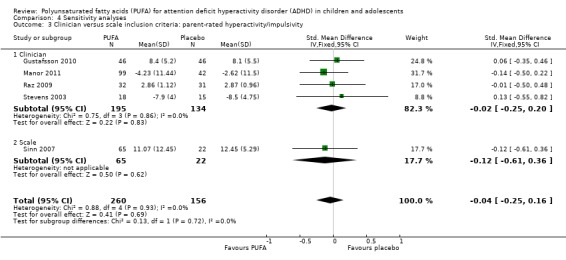
Comparison 4 Sensitivity analyses, Outcome 3 Clinician versus scale inclusion criteria: parent‐rated hyperactivity/impulsivity.
Discussion
Summary of main results
Overall there was little evidence that polyunsaturated fatty acid (PUFA) supplementation provides any benefit for the symptoms of attention deficit hyperactivity disorder (ADHD) in children and adolescents. Although there was some indication that a combination of omega‐3 and omega‐6 PUFA supplementation does result in overall improvement and in improvement in parent ratings of total ADHD symptoms and teacher ratings of attention, these data came from no more than three small trials with a sample size of less than 100. There were no other indications of a beneficial effect of PUFA supplementation, particularly omega‐3 and omega‐6 PUFA supplements only, on any of the ADHD symptom domains, behaviour or quality of life. There was no evidence of use of PUFA leading to harmful side effects or increasing the risk of loss to follow‐up.
Overall completeness and applicability of evidence
At this stage, there are relatively few randomised controlled trials (RCTs) that have investigated the effectiveness of PUFA supplementation in children and adolescents with ADHD and very few that are of high quality. Many of these trials have small sample sizes, highly variable selection criteria, use supplements that are very variable in terms of dosage and constituents, do not address the use or non‐use of stimulant medications and follow up participants for short periods.
There was large variation in the constituents and dosages of PUFA supplements. Supplements used in these trials included omega‐6 alone, omega‐3 alone and combinations of omega‐3 and omega‐6 PUFA. The number of trials that investigated the effects of omega‐6 PUFA (in the absence of omega‐3 PUFA) were surprising given that the evidence for a role of PUFA in ADHD points to a role for omega‐3 supplements rather than omega‐6 PUFA (Richardson 2000; Richardson 2006; Raz 2009b). In addition, pure PUFA supplements were not used in most studies, and non‐PUFA constituents were not replicated in the placebo. For example, PUFA supplements commonly include vitamin E as an antioxidant but in most cases this was not included in the placebo. In future trials, more attention needs to be given to the constituents of PUFA supplements and placebos so that any beneficial effects, or lack thereof, may be more clearly attributed to PUFA supplementation. More attention also needs to be given to the type and dosage of PUFA that is used. It may be most appropriate to base the type of supplement and dosages on supplements that have previously resulted in significant increases in plasma PUFA, for example, see Voigt 2001, Stevens 2003 and Gustafsson 2010.
A particular limitation was that all of the studies were very short. All trials were conducted for periods of 16 weeks or less and the majority followed up participants for 12 weeks or less. Because it may take up to three months for the brain to recover from a chronic PUFA deficiency (Richardson 2000), most of these trials may have been too short to demonstrate a benefit. Therefore, future trials should ensure that follow‐up extends beyond three months and preferably is considerably longer.
Although the premise for the effectiveness of supplementation is a deficiency of PUFA in children and adolescents with ADHD, there is no way of knowing how long‐lasting any effects of supplementation will be. Therefore cross‐over studies, particularly two‐way trials that alternate between a supplement and comparison group, do not seem to be an appropriate design to identify the effectiveness of PUFA in the treatment of ADHD. Nearly half of the trials identified in this review were of a cross‐over design and most of these used a two‐way design. Potential benefits in using a parallel design in future trials would include avoiding any carry‐over effects and identifying how long any beneficial effects of supplementation remain.
Compliance is also likely to be an issue in these trials because participants were commonly expected to take multiple capsules each day (up to eight) and loss to follow‐up was reported because participants could not swallow capsules. Although compliance was quite high in the five trials where it was reported (88% to 97% in the PUFA group and 86% to 100% in the placebo group, Table 6), in future trials it may be preferable to identify supplements that can deliver an appropriate dose in a smaller number of capsules.
Table 2.
Compliance
| Study | Comparison | PUFA group | Control |
| Manor 2011 | Parallel trial of omega‐3 versus placebo | 92% | 90% |
| Raz 2009 | Parallel trial of omega‐3/6 versus placebo | 92% | 86% |
| Richardson 2002 | Parallel trial of omega‐3/6 versus placebo | 90% | 87% |
| Sinn 2007 | Parallel trial of omega‐3/6 versus placebo | 88% across all groups | |
| Voigt 2001 | Parallel trial of omega‐3 versus placebo | 97% | 100% |
PUFA: polyunsaturated fatty acids
Quality of the evidence
Overall, the risk of bias in the trials was unclear because the methods were not adequately described. In the majority of studies, the method of randomisation and allocation concealment was not described. In addition, while most authors reported that studies were double‐blinded, the methods used for blinding were not clearly described. The most obvious source of bias was due to the majority of studies (eight trials) reporting completer analyses and selectively reporting outcomes (nine trials). Other potential biases were also identified in two‐thirds of the included trials. Therefore, there was a high risk of attrition and reporting bias and the potential for other biases, while unclear, could still be high.
Ensuring adequate blinding may be crucially important in this area of research. Despite overall negative findings in a systematic review of essential fatty acids and ADHD (Raz 2009b), positive findings of PUFA supplementation were reported in all four open‐label trials. A major difficulty with omega‐3 PUFA supplementation is masking the distinctive smell and taste of fish oil (Schachter 2005). Therefore, it is likely that parents were aware when their children were receiving an omega‐3 supplement and this may explain why parent ratings were more much likely to show improvement than teacher ratings.
The risk of attrition and reporting bias was high in the majority of included trials and may therefore have resulted in overestimates of benefits associated with PUFA supplementation.
Systematic reviews of the literature have shown that industry‐funded research that identifies a beneficial effect of the industry product is more likely to be published (Lexchin 2003; Golder 2008). Because most of the included trials were funded by the suppliers of the supplement being tested, this may indicate a publication bias in the identified literature.
There was some limited evidence that combined omega‐3 and omega‐6 supplementation may be of some benefit. However, these findings were in the minority and should be treated with caution because of high levels of heterogeneity in these comparisons, the difficulties of blinding families to omega‐3 supplements and the potential biases identified in included trials.
Potential biases in the review process
We do not consider there to be any biases in the conduct of our review.
Agreements and disagreements with other studies or reviews
Despite a considerable body of evidence that implicates PUFA deficiencies in children and adolescents with ADHD (Richardson 2000), there is little evidence at this stage that PUFA supplementation is beneficial. These findings are in common with the narrative syntheses of the literature by Schachter 2005, Richardson 2006 and Raz 2009b. The systematic review of essential fatty acids and ADHD by Raz 2009b concluded that current findings do not support the use of essential fatty acid supplements as a primary or supplementary treatment for children with ADHD, while the US Agency for Healthcare Research and Quality report concluded that no definitive conclusions can be drawn about omega‐3 fatty acids as a primary treatment for ADHD (Schachter 2005). Similarly, in her review of omega‐3 PUFA in ADHD and related neurodevelopmental disorders, Richardson 2006 concluded that evidence of a beneficial role of omega‐3 fatty acids in ADHD is far from definitive at this stage.
While we were completing this review, a further systematic review and meta‐analysis by Bloch 2011 of the effects of omega‐3 supplementation in children with ADHD diagnosis or symptoms was published. This meta‐analysis of 10 studies with 699 participants found a small but significant decrease in ADHD symptoms when omega‐3 supplements were used. This apparent difference from the findings of our review may be related to inclusion of children who were not necessarily diagnosed with ADHD in the review by Bloch 2011 and because these authors pooled data across participant and teacher‐reported ADHD symptom scores and subscores to give one effect estimate.
Authors' conclusions
At this stage, there is insufficient evidence to conclude that polyunsaturated fatty acid (PUFA) supplementation is of benefit in children and adolescents with ADHD. As there were no identified harms associated with PUFA supplementation, there is also insufficient evidence to conclude that it is harmful to children and adolescents with ADHD. Therefore there is little information on which families can base a decision about the use of PUFA supplementation. As there are a number of ongoing trials, there may be better evidence to answer the question of whether PUFA supplementation is effective or not for children and adolescents with ADHD in the near future.
More high‐quality research is needed in this area in order to identify more conclusively the effectiveness or otherwise of PUFA supplementation in children and adolescents with ADHD. It is important that future trials have adequate sample sizes, use valid and reliable selection criteria, and avoid the potential biases identified in the trials included in this review, such as the incomplete reporting of outcomes and follow‐up data. Future studies should use active PUFA supplements at dosages shown to significantly increase circulating PUFA and, in particular, the ratio of omega‐3 to omega‐6 PUFA, and use supplements for considerably longer periods than the maximum of 16 weeks identified in this review.
Acknowledgements
None.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL)
#1MeSH descriptor Fish Oils explode all trees #2MeSH descriptor Fatty Acids, Unsaturated explode all trees #3(polyunsaturated next fatty next acid*) #4(pufa*) #5(essential next fatty next acid*) #6(efa or efas) #7(fish oil*) #8(docosahexaenoic acid*) #9(dha or dhas) #10(alpha‐linolenic acid* or alphalinolenic acid*) #11(ala or alas) #12(omega NEXT 3*) #13(omega NEXT 6*) #14(eicosapentaenoic acid*) #15(epa or epas) #16(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15) #17MeSH descriptor Attention Deficit Disorder with Hyperactivity explode all trees #18adhd #19addh or adhs or "ad/hd" #20hyperactiv* or hyper NEXT activ* #21addh or adhs #22hyperkin* #23(minimal NEAR/3 brain NEAR/3 (disorder* or dysfunc* or damage*)) #24((attention* or behav*) NEAR/3 (defic* or dysfunc* or disorder*)) #25 ((disrupt*) NEAR/3 (disorder* or behav*)) or ((defian*) NEAR/3 (disorder* or behav*) ) #26(#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25) #27MeSH descriptor Adolescent, this term only #28child* NEAR check word #29child* or adolescen* or teen* or pupil* or student* or girl* or boy* or schoolchild* or preschool* or pre‐school* or toddler* #30(#27 OR #28 OR #29) #31(#16 AND #26 AND #30)
Ovid MEDLINE (R)
1 exp Fish Oils/ 2 exp Fatty Acids, Unsaturated/ 3 polyunsaturated fatty acid$.tw. 4 pufa$.tw. 5 essential fatty acid$.tw. 6 efa.tw. 7 fish oil$.tw. 8 efas.tw. 9 docosahexaenoic acid$.tw. 10 (dha or dhas).tw. 11 alpha‐linolenic acid$.tw. 12 alphalinolenic acid$.tw. 13 (ala or alas).tw. 14 omega‐6$.tw. 15 omega‐3$.tw. 16 eicosapentaenoic acid$.tw. 17 (epa or epas).tw. 18 or/1‐17 19 "attention deficit and disruptive behavior disorders"/ or attention deficit disorder with hyperactivity/ or conduct disorder/ 20 ADHD.tw. 21 ADDH.tw. 22 ADHS.tw. 23 "AD/HD".tw. 24 ((attention$ or behav$) adj3 (defic$ or dysfunc$ or disorder$)).tw. (28693) 25 ((disrupt$ adj3 disorder$) or (disrupt$ adj3 behav$) or (defian$ adj3 disorder$) or (defian$ adj3 behav$)).tw. 26 (impulsiv$ or inattentiv$ or inattention$).tw. 27 hyperkinesis/ 28 hyperkine$.tw. 29 (minimal adj3 brain adj3 (disorder$ or dysfunct$ or damage$)).tw. 30 (hyperactiv$ or hyper‐activ$).tw. 31 or/19‐30 32 adolescent/ or child/ or child, preschool/ 33 (child$ or adolescen$ or teen$ or pupil$ or student$ or girl$ or boy$ or schoolchild$ or preschool$ or pre‐school$ or toddler$).tw. 34 32 or 33 35 randomized controlled trial.pt. 36 controlled clinical trial.pt. 37 randomi#ed.ab. 38 placebo$.ab. 39 drug therapy.fs. 40 randomly.ab. 41 trial.ab. 42 groups.ab. 43 or/35‐42 44 exp animals/ not humans.sh. 45 43 not 44 46 18 and 31 and 34 and 45
EMBASE (Ovid)
1 exp Fish Oils/ 2 exp Fatty Acids, Unsaturated/ 3 polyunsaturated fatty acid$.tw. 4 pufa$.tw. 5 essential fatty acid$.tw. 6 efa.tw. 7 fish oil$.tw. 8 efas.tw. 9 docosahexaenoic acid$.tw. 10 (dha or dhas).tw. 11 alpha‐linolenic acid$.tw. 12 alphalinolenic acid$.tw. 13 (ala or alas).tw. 14 omega‐3$.tw. 15 eicosapentaenoic acid$.tw. 16 (epa or epas).tw. 17 omega‐6$.tw. 18 or/1‐17 19 attention deficit disorder/ 20 hyperactivity/ 21 conduct disorder/ 22 ADHD.tw. 23 ADDH.tw. 24 ADHS.tw. 25 "AD/HD".tw. 26 ((attention$ or behav$) adj3 (defic$ or dysfunc$ or disorder$)).tw. 27 ((disrupt$ adj3 disorder$) or (disrupt$ adj3 behav$) or (defian$ adj3 disorder$) or (defian$ adj3 behav$)).tw. 28 (impulsiv$ or inattentiv$ or inattention$).tw. 29 hyperkine$.tw. 30 (minimal adj3 brain adj3 (disorder$ or dysfunct$ or damage$)).tw. 31 (hyperactiv$ or hyper‐activ$).tw. 32 or/19‐31 33 exp Clinical trial/ 34 Randomized controlled trial/ 35 Randomization/ 36 Single blind procedure/ 37 Double blind procedure/ 38 Crossover procedure/ 39 Placebo/ 40 Randomi#ed.tw. 41 RCT.tw. 42 (random$ adj3 (allocat$ or assign$)).tw. 43 randomly.ab. 44 groups.ab. 45 trial.ab. 46 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 47 Placebo$.tw. 48 Prospective study/ 49 (crossover or cross‐over).tw. 50 prospective.tw. 51 or/33‐50 52 18 and 32 and 51
PsycINFO (EBSCOhost) August 2011 searches
S30 S24 and S29 S29 S25 or S26 or S27 or S28 S28 AG preschool or AG school age S27 AG adolescence S26 AG childhood S25 child* or adolescen* or teen* or pupil* or student* or girl* or boy* or schoolchild* or preschool* or pre‐school* or toddler* S24 S14 and S23 S23 S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 S22 ((disrupt* N3 disorder*) or (disrupt* N3 behav*) or (defian* N3 disorder*) or (defian* N3 behav*)) S21 impulsiv* or inattentiv* or inattention* S20 (attention* N3 deficit*) or (attention* N3 dysfunc*) or (attention* N3 disord*) or (behav* N3 deficit*) or (behav* N3 dysfunc*) or (behav* N3 disord*) S19 (minimal N3 brain N3 dysfunct*) or (minimal N3 brain N3 disord*) or (minimal N3 brain N3 damage*) S18 hyperkin* S17 hyperactiv* or hyper‐activ* S16 adhd or addh or adhs or "ad/hd" S15 DE "Attention Deficit Disorder with Hyperactivity" S14 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 S13 (epa or epas) S12 eicosapentaenoic acid* S11 omega‐3* or omega‐6* S10 (ala or alas) S9 alphalinolenic acid* S8 alpha‐linolenic acid* S7 (dha or dhas) S6 docosahexaenoic acid* S5 fish oil* S4 efa or efas S3 essential fatty acid* S2 pufa* S1 polyunsaturated fatty acid*
PsycINFO (Ovid) 2008 and 2009 searches
1 polyunsaturated fatty acid$.tw. 2 pufa$.tw. 3 essential fatty acid$.tw. 4 efa.tw. 5 fish oil$.tw. 6 efas.tw. 7 docosahexaenoic acid$.tw. 8 (dha or dhas).tw. 9 alpha‐linolenic acid$.tw. 10 alphalinolenic acid$.tw. 11 (ala or alas).tw. 12 omega‐3$.tw. 13 omega‐6$.tw. 14 eicosapentaenoic acid$.tw. 15 (epa or epas).tw. 16 Attention Deficit Disorder with Hyperactivity/ 17 adhd.tw. 18 addh.tw. 19 adhs.tw. 20 hyperactiv$.tw. 21 hyperkin$.tw. 22 brain dysfunction.tw. 23 attention deficit$.tw. 24 22 or 18 or 23 or 17 or 19 or 21 or 16 or 20 25 (child$ or adolescen$ or teen$ or pupil$ or student$ or girl$ or boy$ or schoolchild$ or preschool$ or pre‐school$ or toddler$).tw. 26 or/1‐15 27 25 and 24 and 26
CINAHL Plus (EBSCOhost)
S33 S27 and S32 S32 S28 or S29 or S30 or S31 S31 (child* or adolescen* or teen* or pupil* or student* or girl* or boy* or schoolchild* or preschool* or pre‐school* or toddler*) S30 (MH "Child, Preschool") S29 (MH "Child") S28 (MH "Adolescence") S27 S17 and S26 S26 S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 S25 (disrupt* N3 disorder*) or (disrupt* N3 behav*) or (defian* N3 disorder*) or (defian* N3 behav*) S24 impulsiv* or inattentiv* or inattention* S23 (attention* N3 deficit*) or (attention* N3 dysfunc*) or (attention* N3 disord*) or (behav* N3 deficit*) or (behav* N3 dysfunc*) or (behav* N3 disord*) S22 (minimal N3 brain N3 dysfunct*) or (minimal N3 brain N3 disord*) or (minimal N3 brain N3 damage*) S21 hyperkin* S20 hyperactiv* or hyper‐activ* S19 adhd or addh or adhs or "ad/hd" S18 (MH "Attention Deficit Hyperactivity Disorder") S17 S16 or S15 or S14 or S13 or S12 or S11 or S10 or S9 or S8 or S7 or S6 or S5 or S4 or S3 or S2 or S1 S16 epa or epas S15 eicosapentaenoic acid* S14 omega‐6* or omega 6* S13 omega‐3* or omega 3* S12 ala or alas S11 alphalinolenic acid* S10 alpha‐linolenic acid* S9 dha or dhas S8 docosahexaenoic acid* S7 fish oil* S6 efa or efas S5 essential fatty acid* S4 pufa* S3 polyunsaturated fatty acid* S2 (MH "Fatty Acids, Unsaturated+") S1 (MH "Fish Oils+")
BIOSIS (Web of Science)
# 24 #23 AND #22 # 23 TS=(random* or crossover* or placebo* or assign* or control* or trial* or blind*) # 22 #21 AND #20 AND #15 # 21 TS=(child* or adolescen* or teen* or pupil* or student* or girl* or boy* or schoolchild* or preschool* or pre‐school* or toddler*) # 20 #19 OR #18 OR #17 OR #16 # 19 Ts=attention deficit* # 18 TS=brain dysfunction # 17 TS=(hyperactiv* or hyperkin*) # 16 TS=(adhd or addh or adhs) # 15 #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 # 14 Ts=(epa or epas) # 13 TS=eicosapentaenoic acid* # 12 TS=omega‐6* # 11 TS=omega‐3* # 10 TS=(ala or alas) # 9 Ts=alphalinolenic acid* # 8 TS=alpha‐linolenic acid* # 7 TS=(dha or dhas) # 6 Ts=docosahexaenoic acid* # 5 Ts=fish oil* # 4 TS=(efa or efas) # 3 TS=essential fatty acid* # 2 TS=pufa* # 1 TS=(polyunsaturated fatty acid*)
Science Citation Index (Web of Science)
# 24 #23 AND #22 # 23 TS=(random* or crossover* or placebo* or assign* or control* or trial* or blind*) # 22 #21 AND #20 AND #15 # 21 TS=(child* or adolescen* or teen* or pupil* or student* or girl* or boy* or schoolchild* or preschool* or pre‐school* or toddler*) # 20 #19 OR #18 OR #17 OR #16 # 19 Ts=attention deficit* # 18 TS=brain dysfunction # 17 TS=(hyperactiv* or hyperkin*) # 16 TS=(adhd or addh or adhs) # 15 #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 # 14 Ts=(epa or epas) # 13 TS=eicosapentaenoic acid* # 12 TS=omega‐6* # 11 TS=omega‐3* # 10 TS=(ala or alas) # 9 Ts=alphalinolenic acid* # 8 TS=alpha‐linolenic acid* # 7 TS=(dha or dhas) # 6 Ts=docosahexaenoic acid* # 5 Ts=fish oil* # 4 TS=(efa or efas) # 3 TS=essential fatty acid* # 2 TS=pufa* # 1 TS=(polyunsaturated fatty acid*)
Social Science Citation Index (Web of Science)
# 24 #23 AND #22 # 23 TS=(random* or crossover* or placebo* or assign* or control* or trial* or blind*) # 22 #21 AND #20 AND #15 # 21 TS=(child* or adolescen* or teen* or pupil* or student* or girl* or boy* or schoolchild* or preschool* or pre‐school* or toddler*) # 20 #19 OR #18 OR #17 OR #16 # 19 Ts=attention deficit* # 18 TS=brain dysfunction # 17 TS=(hyperactiv* or hyperkin*) # 16 TS=(adhd or addh or adhs) # 15 #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 # 14 Ts=(epa or epas) # 13 TS=eicosapentaenoic acid* # 12 TS=omega‐6* # 11 TS=omega‐3* # 10 TS=(ala or alas) # 9 Ts=alphalinolenic acid* # 8 TS=alpha‐linolenic acid* # 7 TS=(dha or dhas) # 6 Ts=docosahexaenoic acid* # 5 Ts=fish oil* # 4 TS=(efa or efas) # 3 TS=essential fatty acid* # 2 TS=pufa* # 1 TS=(polyunsaturated fatty acid*)
Dissertation Abstracts (searched via Dissertation Express)
Search terms used:
polyunsaturated fatty acid pufa essential fatty acid efa docosahexaenoic acid dha alpha‐linolenic acid alphalinolenic acid ala omega‐3 omega‐6 eicosapentaenoic acid epa
metaRegister of Controlled Trials (http://www.controlled‐trials.com/mrct/)
Search terms used:
fatty acid* AND ADHD
TROVE (http://trove.nla.gov.au/), DART‐Europe e‐theses portal (http://www.dart‐europe.eu/basic‐search.php) , Networked Digitial Library of Theses and Dissertations (NDLTD) (http://www.ndltd.org/
acids OR pufas OR omega AND adhd OR hyperactiv*
Data and analyses
Comparison 1.
PUFA versus placebo ‐ parallel trials
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.04, 4.62] |
| 1.1 Omega‐3/6 PUFAs | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.04, 4.62] |
| 2 ADHD symptoms: total ‐ parent | 5 | 413 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.38, 0.03] |
| 2.1 Omega‐3 PUFAs | 3 | 293 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.30, 0.18] |
| 2.2 Omega‐3/6 PUFAs | 2 | 120 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.88, ‐0.08] |
| 3 ADHD symptoms: inattention ‐ parent | 6 | 469 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.29, 0.21] |
| 3.1 Omega‐3 PUFAs | 3 | 286 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.28, 0.47] |
| 3.2 Omega‐3/6 PUFAs | 3 | 183 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.52, 0.10] |
| 4 ADHD symptoms: hyperactivity/impulsivity ‐ parent | 5 | 416 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.25, 0.16] |
| 4.1 Omega‐3 PUFAs | 2 | 233 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.32, 0.22] |
| 4.2 Omega‐3/6 PUFAs | 3 | 183 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.34, 0.28] |
| 5 ADHD symptoms: total ‐ teacher | 4 | 324 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.18, 0.27] |
| 5.1 Omega‐3 PUFAs | 2 | 228 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.17, 0.37] |
| 5.2 Omega‐3/6 PUFAs | 2 | 96 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.45, 0.35] |
| 6 ADHD symptoms: inattention ‐ teacher | 3 | 260 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.22, 0.74] |
| 6.1 Omega‐3 PUFAs | 2 | 227 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.24, 0.30] |
| 6.2 Omega‐3/6 PUFAs | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | 1.01 [0.28, 1.75] |
| 7 ADHD symptoms: hyperactivity/impulsivity ‐ teacher | 3 | 259 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.16, 0.35] |
| 7.1 Omega‐3 PUFAs | 2 | 226 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.24, 0.31] |
| 7.2 Omega‐3/6 PUFAs | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐0.21, 1.18] |
| 8 ADHD symptoms: total ‐ clinician | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Omega‐3/6 PUFAs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 ADHD symptoms: inattention ‐ clinician | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Omega‐3/6 PUFAs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 ADHD symptoms: hyperactivity/impulsivity ‐ clinician | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Omega‐3/6 PUFAs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Behaviour: internalising ‐ parent | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Omega‐3 PUFAs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Behaviour: externalising ‐ parent | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Omega‐3 PUFAs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Behaviour: socialisation ‐ parent | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Omega‐3 PUFAs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Omega‐3/6 PUFAs | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Behaviour: conduct ‐ parent | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 Omega‐3/6 PUFAs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Behaviour: oppositional ‐ parent | 3 | 266 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.15, 0.35] |
| 15.1 Omega‐3 PUFAs | 2 | 233 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.15, 0.39] |
| 15.2 Omega‐3/6 PUFAs | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.70, 0.67] |
| 16 Behaviour: conduct ‐ teacher | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 Omega‐3/6 PUFAs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Behaviour: oppositional ‐ teacher | 3 | 257 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.15, 0.36] |
| 17.1 Omega‐3 PUFAs | 2 | 224 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.18, 0.37] |
| 17.2 Omega‐3/6 PUFAs | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.53, 0.85] |
| 18 Quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18.1 Omega‐3 PUFAs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Side effects | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Dermatitis | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.06, 34.36] |
| 19.2 Diarrhoea | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.17, 3.08] |
| 19.3 Gastrointestinal discomfort | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.21, 2.38] |
| 19.4 Headache | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.82] |
| 19.5 Hyperactivity | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.06, 34.36] |
| 19.6 Nausea | 3 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.39, 2.70] |
| 19.7 Nose bleed | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 2.95] |
| 19.8 Tics | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.06, 34.36] |
| 19.9 General | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.87, 9.74] |
| 20 Loss to follow‐up | 7 | 589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.69, 1.31] |
| 20.1 Omega‐3 PUFAs | 4 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.78, 1.75] |
| 20.2 Omega‐3/6 PUFAs | 3 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.38, 1.13] |
Comparison 2.
PUFA versus placebo ‐ cross‐over trials
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADHD symptoms: total ‐ teacher | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Omega‐6 PUFAs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 ADHD symptoms: hyperactivity/impulsivity ‐ teacher | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Omega‐6 PUFAs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3.
PUFA versus dexamphetamine ‐ cross‐over trials
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADHD symptoms: total ‐ teacher | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Omega‐6 PUFAs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 ADHD symptoms: hyperactivity/impulsivity ‐ teacher | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Omega‐6 PUFAs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4.
Sensitivity analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Selection bias: parent‐rated ADHD symptoms | 5 | 413 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.38, 0.03] |
| 1.1 Low risk | 2 | 228 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.61, ‐0.03] |
| 1.2 Unclear risk | 3 | 185 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.32, 0.27] |
| 2 Clinician versus scale: parent‐rated inattention | 6 | 469 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.24, 0.15] |
| 2.1 Clinician | 5 | 382 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.18, 0.24] |
| 2.2 Scale | 1 | 87 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.95, 0.03] |
| 3 Clinician versus scale inclusion criteria: parent‐rated hyperactivity/impulsivity | 5 | 416 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.25, 0.16] |
| 3.1 Clinician | 4 | 329 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.25, 0.20] |
| 3.2 Scale | 1 | 87 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.61, 0.36] |
What's new
| Date | Event | Description |
|---|---|---|
| 10 September 2012 | Amended | Reference amended |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 7, 2012
| Date | Event | Description |
|---|---|---|
| 11 August 2009 | Amended | Correction to spelling in the author line |
| 28 June 2008 | Amended | Substantive amendment |
| 24 March 2008 | Amended | Converted to new review format |
Differences between protocol and review
We removed the outcomes of suicide and self harm stated in the protocol from this review. This was because the appropriateness of these data were considered questionable on the completion of this review by the editors and authors and no such data had been reported in any included study.
Because the inclusion criteria of some studies were based on scale cut‐off scores, we conducted an additional sensitivity analyses to evaluate whether there was any difference compared to studies which used a clinician diagnosis of ADHD.
The need for the inclusion of learning‐related outcomes was identified by one of the editors during completion of this review. Therefore it is our intention to include these outcomes in updates of this review.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Cross‐over trial of omega‐6 PUFA versus placebo | |
| Participants | Inclusion criteria: children with scores on the Attention Problem subscale of the Revised Behavior Problem Checklist (RBPC) and Inattention subscale of the Conners Teacher questionnaire above the 90th percentile (n = 26). Five additional children from another study diagnosed with DSM III ADD by a child psychiatrist were also included. Exclusion criteria: not stated. Mean age: 8.86 years; gender: 27 boys and 4 girls; loss to follow‐up: 1/31 Baseline IQ 101.07; Conners inattention subscale: 3.08, hyperactivity: 2.80; RBPC attention problem scale: 20.42, motor excess: 6.23 Setting: Auckland, New Zealand |
|
| Interventions | Cross‐over trial of 4 weeks of omega‐6 PUFA or placebo, 1 week washout and 4 weeks of alternate. Three capsules of PUFA or placebo were to be taken twice daily. Omega‐6 PUFA: Efamol capsules contained 360 mg linoleic acid and 45 mg gamma‐linoleic acid Placebo: 500 mg of liquid paraffin |
|
| Outcomes | ADHD symptoms Parent rated RBPC subscales: attention; motor excess Teacher rated Conners Questionnaire subscales: inattention; hyperactivity and ADD‐H Comprehensive Teacher Rating Scale (ACTeR) subscales: attention, hyperactivity Behaviour Parent‐rated RBPC subscales: conduct; socialised aggression; anxiety‐withdrawal; psychotic behaviour Teacher‐rated Conners Questionnaire subscales: conduct; tension/anxiety and ACTeR subscales: social skills, oppositional behaviour All scales were measured at 4 weeks |
|
| Notes | Variances were not reported therefore data could not be included in a meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors stated that the trial was double‐blind and that the intervention and control were given as "indistinguishable capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Capsules "indistinguishable" and parent and teacher‐rated scales used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One child lost to follow‐up in the placebo group |
| Selective reporting (reporting bias) | Low risk | Data were reported for all outcomes |
| Other bias | Unclear risk | It was not clear whether the additional 5 children included in the trial would have met the original inclusion criteria. Baseline characteristics of each group were not reported. The trial was funded by Efamol Research Inc. |
| Methods | Cross‐over trial of omega‐6 PUFA, dexamphetamine and placebo | |
| Participants | Inclusion criteria: children of normal intelligence aged between 6 and 12 years with a DSM III diagnosis of ADHD by a child psychiatrist, a score of at least 18 on the Conners hyperactivity index and a score of at least 24 on the first 6 items of David's Hyperkinetic Rating scale Median age: 9 years, gender: all 18 were boys, loss to follow‐up: unclear, 18 participants completed Exclusion criteria: psychoactive drug use in the preceding week or history of seizures Baseline scores: not stated Setting: Ohio, US |
|
| Interventions | Cross‐over trial of omega‐6 PUFA, d‐amphetamine and placebo for 1 month each. All were given as 4 capsules twice daily for 1 month. Omega‐6 PUFA Efamol® capsules: contained 500 mg evening primrose oil which provides 350 mg linoleic acid and 40 mg gamma linolenic acid, 13 IU vitamin E as preservative Dextroamphetamine: a 10 or 15 mg time‐released capsule Placebo: paraffin oil |
|
| Outcomes | ADHD symptoms Teacher‐rated Conners total score and hyperactivity index at 2 and 4 weeks |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo was described as matched |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Behavioural ratings were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | High risk | Unclear what other outcomes were used. A parent rating of the Conners scale appears to have been used but was not reported because it was "noncontributory". |
| Other bias | Unclear risk | Baseline characteristics of each group were not reported. Results for a Psychiatrists' Global Rating were reported but this scale was not described. Study funded by Efamol Research Institute. |
| Methods | Cross‐over trial of omega‐3 versus omega‐6 PUFA followed by omega‐6 PUFA for all | |
| Participants | Inclusion criteria: children aged 6 years 11 months to 11 years 11 months with DSM IV diagnosis based on parent and teacher questionnaires and clinical evaluation and a IQ above 85 on the Wechsler Intelligence Scale for Children Exclusion criteria: children with mental health disorders (except ADHD comorbidity); taking ADHD medication, sedatives, anxiolytics, or antipsychotics; with a medical condition requiring long‐term treatment, allergy to sunflower or fish oil; or coagulation disorders. Children who consumed natural medicine products, fish, flaxseed oil and omega‐3 enriched food during the trial were also excluded. Mean age: 9.2 years, gender (at completion of both phases): 18 boys and 8 girls, loss to follow‐up (at the end of the first phase): 8/37 Baseline DSM IV symptoms: omega‐3 74.2, omega‐6 71.1; hyperactive‐impulsive: omega‐3 67.7, omega‐6 66.9; inattentive omega‐3 76.4, omega‐6 71.3 Setting: ADHD clinic, Montreal, Canada |
|
| Interventions | One group received omega‐3 PUFA and the other omega‐6 PUFA supplements for the first 8 weeks of the trial; both groups received omega‐3 PUFA over the following 8 weeks Omega‐3 PUFA (Nutri‐Santé, Canada): each capsule contained 25 mg phospholipids, 250 mg EPA, 100 mg DHA, 3.75 U vitamin E Based on body weight, children received between 2 and 4 capsules daily Omega‐6 PUFA (described as placebo, Nutri‐Santé, Canada): each capsule contained 500 mg sunflower oil which included 70% linoleic acid, 20% oleic acid and 5% palmitic and stearic acid, 3.75 U vitamin E. It was not clear how many of these capsules were given. |
|
| Outcomes | ADHD symptoms Parent and teacher‐rated Strengths and Weaknesses in ADHD and Normal Behaviours (SWAN), parent and teacher‐rated Conners questionnaires. All were measured at 8 weeks. Side effects |
|
| Notes | First‐phase data were collected for this review. Variances were not reported therefore data could not be included in a meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The authors stated that this was a double‐blind trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as a double‐blind trial but no further detail was given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data was reported for 26 of the 37 enrolled participants at the end of the 8‐week trial |
| Selective reporting (reporting bias) | High risk | Data from the teacher‐rated Conners and SWAN questionnaires were not reported |
| Other bias | Unclear risk | The trial was funded by Nutri‐Santé |
| Methods | Parallel trial of omega‐3 PUFA versus a dietary supplement | |
| Participants | Inclusion criteria: children diagnosed with DSM IV ADHD‐combined type by a physician or psychologist Exclusion criteria: serious pre‐existing medical or psychological condition or stimulant medication other than Ritalin Mean age: 8.4 years, gender (at follow‐up): 44 boys and 7 girls, loss to follow‐up: 9/60 Baseline scores: not stated Setting: US |
|
| Interventions | This was the second of 2 12‐week trials. In this second trial an omega‐3 PUFA in combination with a dietary supplement (given to the group who received a single dietary supplement in trial 1) was compared to a double dose of the dietary supplement (placebo group in trial 1). Both were given twice a day for 12 weeks. Omega‐3 PUFA Flax seed (rich in omega‐3 PUFA) 100 mg plus single dietary supplement: Ginkgo biloba 10 mg, Melissa officialis 200 mg, grapine 30 mg, dimethylaminoethanol 35 mg, l‐glutamine 100 mg Double dietary supplement: Ginkgo biloba 20 mg, Melissa officialis 400 mg, grapine 60 mg, dimethylaminoethanol 70 mg, l‐glutamine 200mg |
|
| Outcomes | ADHD symptoms Parent and teacher‐rated Conners Rating Scales (revised: long form): inattentive and hyperactive‐impulsive subscales measured at 12 weeks |
|
| Notes | Participant numbers were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Parents and teachers did not know what the participant was allocated until the trial was completed. Only the trial physician had access to this information. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collectors did not know what the participants were allocated until the trial was completed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9 children were lost to follow‐up but it is not clear what sample numbers were used in the analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to have been reported |
| Other bias | Unclear risk | Baseline characteristics of each group were not reported. The PUFA group had received an additional 12 weeks of dietary supplement before this second trial was started. |
| Methods | Parallel trial of omega‐3 PUFA versus placebo | |
| Participants | Inclusion criteria: children aged between 7 and 12 years with a diagnosis of ADHD combined type meeting DSM IV criteria, inclusive of any neuropsychiatric comorbidity, and had been evaluated for pharmacological treatment Exclusion criteria: mental retardation (IQ < 70), autism, major depression, epileptic seizure during the preceding 2 years, other neurological or endocrine disorders, fish allergy, severely impaired hearing or vision, severe sleeping disorder, psychotic symptoms or ongoing psychoactive, anticonvulsant or stimulant medication. If the child had taken a PUFA, a washout period of 10 weeks was required. Age: 7 to 12 years, gender: not stated, loss to follow‐up: 17/109 Baseline parent‐rated Conners score: placebo 46.0; PUFA 51.0; teacher‐rated: placebo 43.5, PUFA 49.7 Setting: 8 Child and Adolescent Psychiatric and Paediatric clinics, Sweden |
|
| Interventions | Omega‐3 PUFA (PlusEPA® Minami Nutrition, Belgium): 1 capsule daily containing 500 mg eicosapentaenoic acid (EPA), 2.7 mg docosahexaenoic acid (DHA), 10 mg vitamin E for 15 weeks. All took the supplement for at least 70 days. Placebo: 1 placebo capsule (rape seed oil and medium chain triglycerides) daily for 15 weeks |
|
| Outcomes | ADHD symptoms Parent and Teacher Conners Rating Scale total, inattentive and hyperactive‐impulsive subscales Behaviour Parent and Teacher Conners Rating Scale oppositionality subscales Side effects: nausea, diarrhoea, nose bleed All scales measured at 15 weeks |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned a study number and randomised in blocks of 4 according to a computer‐generated code |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and PUFA were in "identical" capsules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Capsules "identical" and parent and teacher‐rated scales used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors reported that ITT analysis was done on 92 participants but this did not include "17 drop‐outs" |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to have been reported |
| Other bias | Unclear risk | Minami Nutrition sponsored the study |
| Methods | Parallel trial of omega‐3 PUFA versus placebo | |
| Participants | Inclusion criteria: children aged 6 to 12 years attending summer camp for psychiatric disorders diagnosed or suspected to have ADHD according to DSM‐IV criteria, diagnostic interviews and behavioural observation by psychiatrists Exclusion criteria: not stated Median age: 9 years, gender: 32 boys and 8 girls, loss to follow‐up: 0/40 Baseline attention deficit symptoms: PUFA 11, placebo 8; hyperactivity symptoms: PUFA 2, placebo 2; impulsivity symptoms: PUFA 0, placebo 2 Setting: Japan |
|
| Interventions | Omega‐3 PUFA: the fish oil supplemented diet provided a total 3.6 g DHA and 0.7 g EPA per week for 2 months. Fish oil enriched food included fermented soybean milk (600 mg DHA/125 ml, 3 times per week), bread rolls (300 mg DHA/45 g, 2 times per week) and steamed bread (600 mg DHA/60 g, 2 times per week). Placebo: indistinguishable foods containing olive oil |
|
| Outcomes | ADHD symptoms Parent and teacher‐rated number of DSM‐IV defined attention deficit, hyperactivity and impulsivity symptoms measured at 8 weeks |
|
| Notes | Median number of symptoms (and range) reported therefore data could not be included in a meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was randomly assigned using a telephone book as a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was conducted by a third party in a double‐blind manner |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors described the trial as double‐blind. The fish oil taste in supplemented foods was masked. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interventions were "masked" and parent and teacher‐rated scales used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All subjects enrolled in the trial were included in the data analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to have been reported |
| Other bias | Unclear risk | The number of symptoms were different at baseline though this was not significant. The number of children with Asperger's syndrome was lower (2 versus 7) and learning disorder was higher (10 versus 5) in the PUFA group. |
| Methods | Cross‐over trial of omega‐3/6 PUFA versus placebo | |
| Participants | Inclusion criteria: children and adolescents aged 8 to 18 years; diagnosed with ADHD of any type (according to DSM‐IV criteria); scoring at least 1.5 standard deviations above the age norm for their diagnostic subtype using the parent ADHD Rating Scale IV. Exclusion criteria: autism, psychosis, bipolar disorder, mental retardation, uncontrolled seizure disorder, hyper‐ or hypo‐thyroidism, significant other medical conditions, weight below 20 kg, alcohol or drug abuse, use of psychoactive drugs or omega‐3 preparations in the last 3 months Mean age: 12 years, gender: 54 boys and 11 girls, loss to follow‐up: 11/75 Baseline ADHD Rating Scale IV‐total: PUFA 33.5 placebo 32.4, inattention: PUFA 19.8 placebo 19.5, hyperactivity/impulsivity: PUFA 14.2, placebo 12.5 Setting: 3 child neurology/psychiatry clinics in Southwest Sweden |
|
| Interventions | Cross‐over trial of omega‐3/6 versus placebo followed by supplement for all Omega‐3/6 PUFA (Equazen eye q); 3 capsules twice daily corresponding to 558 mg EPA, 174 mg DHA, 60 mg GLA, 10.8 mg vitamin E for 3 months Placebo: olive oil, 3 capsules twice daily |
|
| Outcomes | Improvement Defined as more than 25% improvement in ADHD symptoms ADHD symptoms Clinician rated ADHD‐RS score ‐ total, inattention and hyperactive/impulsive score All measured at 12 weeks |
|
| Notes | Authors described as cross‐over because supplement was given to all in 2nd phase. First‐phase data was used in the meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were assigned to treatment in random order using a code list but it is not clear how the code list sequence was generated |
| Allocation concealment (selection bias) | Low risk | Treatment assignment was determined using a code list, which not accessible to investigators and was not broken until all patients had completed the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors described this trial as double‐blind. Also "identical capsules" were used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | The authors state that several outcomes will be reported in future publications |
| Other bias | Unclear risk | Study funded by Equazen UK |
| Methods | Parallel trial of omega‐3 PUFA versus placebo | |
| Participants | Inclusion criteria: Children aged between 6 and 13 years, of normal weight and height, regularly attended school and had a confirmed DSM‐IV ADHD diagnosis following assessment by the Schedule for Affective Disorders and Schizophrenia for School‐Age Children‐Present and Lifetime (K‐SADS‐PL) Version 1 assessed by qualified and experienced psychiatrist (K‐SADS‐Pl or CGI‐S) or psychiatric social worker (K‐SADS‐PL); a score of at least 1.5 standard deviations above the normal for the patient’s age and gender in the Teacher‐rated ADHD Rating Scale‐IV (RS‐IV) School Version; a score of 4 or higher (moderately ill or worse) in the Clinical Global Impression of Severity of Illness (CGI‐S) test; and willingness of the parent and a teacher who is familiar with the child to participate Exclusion criteria: Girls with 3 previous regular menstrual cycles; history or diagnosis of serious systemic or neurological condition; failure to respond to 2 or more adequate courses of stimulant therapy; pervasive developmental disorder; diagnosed psychotic disorders; suicidal risk or current psychiatric comorbidity requiring pharmacotherapy; use of potent psychotropics, including ADHD treatments and dietary supplements, 4 weeks prior to study; history of DSM‐IV alcohol or substance abuse; more than 250 mg/day of caffeine; allergic reactions or sensitivity to marine products, soy or corn; any illness that could jeopardise their health or prevent them from completing the trial Mean age: 9.2 years, gender (at follow‐up): 104 boys and 43 girls, loss to follow‐up: 53/200 Baseline DSM‐IV Total: PUFA 63.65, placebo 64.43, inattention: PUFA 63.66, placebo 64.80, hyperactivity/impulsivity: PUFA 61.15, placebo 60.9 Setting: Israel |
|
| Interventions | Parallel trial of 15 weeks of omega‐3 PUFA or placebo which was followed by a 15‐week open‐label trial of 15 weeks in which both groups received omega‐3 PUFA Omega‐3 PUFA (VayarinTM): the daily omega‐3 dosage of 4 capsules (2 capsules twice a day) provided 300 mg phosphatidylserine, 80 mg EPA, 40 mg DHA. For treatment adherence monitoring, participants returned all treatment packs at each visit, and adherence was calculated using the number of remaining capsules. Placebo: 4 capsules (2 capsules twice a day) of an identical‐looking capsule filled with cellulose as placebo |
|
| Outcomes | ADHD symptoms Change in parent and teacher‐rated Conners DSM‐IV total, inattentive and hyperactivity/impulsivity symptoms at 15 weeks Behaviour Change in parent and teacher‐rated Conners oppositional behaviour at 15 weeks Quality of life Change in Child Health Questionnaire Psychosocial summary score at 15 weeks |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A web‐based random allocation procedure was used |
| Allocation concealment (selection bias) | Low risk | A web‐based random allocation procedure was used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and PUFA capsules were described as identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Capsules "identical" and parent and teacher‐rated scales used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Per protocol analysis was used |
| Selective reporting (reporting bias) | High risk | The SDQ was described as an assessment that was used but results were not reported |
| Other bias | Unclear risk | 4 of the 10 authors were employed by the company which produced the omega‐3 supplement |
| Methods | Parallel trial of omega‐3/6 PUFA versus placebo | |
| Participants | Inclusion criteria: participants were recruited through advertisement on a radio health programme, in health newspapers and ADHD clinics. Children aged 7 to 13 years with a written diagnosis of ADHD from a child psychiatrist, neurologist, paediatrician or clinical psychologist were included Exclusion criteria: use of ADHD medication in the past month, use of EFA supplements in the past 3 months, presence of pervasive developmental disorder, seizure disorder, schizophrenia, major depression or bipolar disorder Mean age: 10.5 years, gender (at follow‐up): 38 boys and 25 girls, loss to follow‐up: 15/78 Baseline parent‐rated DSM‐IV attention: PUFA 4.05, placebo 4.43, hyperactivity‐impulsivity: PUFA 3.29, placebo 3.14; teacher‐rated Conners’ ADHD: PUFA 3.85, placebo 3.71 Setting: Bar‐Ilan University,Tel‐Hashomer Israel and Hillel‐Yaffe Medical Center, Hadera, Israel |
|
| Interventions | Omega‐3/6 PUFA: an oral softgel capsule containing 240 mg linoleic acid, 60 mg alpha‐linolenic acid, 95 mg mineral oil and 5 mg alpha‐tocopherol twice daily for 7 weeks Placebo: 500 mg ascorbic acid as an oral tablet twice daily for 7 weeks |
|
| Outcomes | ADHD symptoms Parent‐rated DSM‐IV attention and hyperactivity‐impulsivity, teacher‐rated Conners’ ADHD measured at 7 weeks Side effects, nausea |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not described except that participants were matched for gender and age and then randomised within each pair |
| Allocation concealment (selection bias) | Unclear risk | Assignment of treatments was based on study number |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The authors described the trial as double‐blind. However, active and control interventions were different i.e. softgel capsules and tablets respectively. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The researchers, teachers, parents and children were all directly involved in data collection, and all were blinded to treatment allocation until the end of the study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data were reported for the 63 of 75 enrolled participants who remained in the trial at 7 weeks |
| Selective reporting (reporting bias) | High risk | The authors stated that the Conners subscales were "found to be unreliable" but not how this was demonstrated. These data were not reported. |
| Other bias | Low risk | There were no statistically significant differences between groups in stimulant use, co‐morbidities, inattention, hyperactivity/impulsivity, EFA deficiency score or blood biochemistry |
| Methods | Cross‐over trial of omega‐3/6 PUFA +/‐ multivitamins versus placebo followed by PUFA and multivitamin supplement for all | |
| Participants | Inclusion criteria: children aged 7 to 12 years were recruited via media releases and school newsletters. Children with scores 2 SD or more above the population average on the parent‐rated Conners Abbreviated ADHD index were included. Exclusion criteria: if they taken stimulant medication and any form of omega‐3 supplementation in the previous 3 months Mean age: 9.4 years, gender (at follow‐up): 77 boys and 27 girls, loss to follow‐up: 63/167 Mean baseline parent‐rated Conners ADHD Index: PUFA 26.68, placebo 26.67 Setting: South Australia |
|
| Interventions | One group received omega‐3/6 PUFA, one group received omega‐3/6 PUFA plus multivitamin and one received placebo for the first 15 weeks of the trial. All groups received omega‐3 PUFA plus multivitamin over the following 15 weeks. PUFA: (eye q TM Equazen, UK and Novasel, Australia): each capsule contained 400 mg fish oil, 100 mg primrose oil containing EPA 93 mg, DHA 29 mg and GLA 10 mg, and vitamin E 1.8 mg. Six capsules daily. Multivitamin (Blackmores, Australia): vitamin A, thiamine, vitamin B2, B6, C, D, B12, E, Biotin, B5, E, biotin, B5, CaH P04, Fe fumarate, MgO, MnSO4, ZnO, Cu gluconate, KI. One tablet per day. There was no placebo for the multivitamin but contents of intervention packaging was blinded. Placebo: 6 capsules of palm oil daily |
|
| Outcomes | Symptoms Parent and teacher‐rated Revised Conners Rating Scales |
|
| Notes | First‐phase data were collected for this review. Although adjusted mean data were available for 104 participants at the end of this phase the unadjusted data which were available for 87 participants were used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised within age and gender |
| Allocation concealment (selection bias) | Low risk | Independently held code numbers were used to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors described phase 1 as double‐blind. Packaging was designed to maintain blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Packaging was designed to maintain blinding and parent‐rated scales used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data were replaced with the variable mean value for 1 or 2 missing responses. Cases with 3 or more missing responses were deleted from the data set. |
| Selective reporting (reporting bias) | High risk | Teacher‐rated outcomes which were not significantly different were not reported |
| Other bias | Unclear risk | Children who withdrew had significantly higher baseline scores on the Conners Index (withdrew before starting: 28.92, withdrew during phase 1: 28.74, completed phase 1: 26.27) |
| Methods | Parallel trial of omega‐3/6 PUFA vs placebo | |
| Participants | Inclusion criteria: children aged 6 to 13 years diagnosed with ADHD by a clinical psychologist, psychiatrist or paediatrician with thirst and/or skin symptoms Exclusion criteria: chronic health problems, distance from the test site, inability to swallow capsules, lack of interest Mean age: 9.8 years, gender (at baseline): 41 boys and 6 girls, loss to follow‐up: 17/50 Baseline parent‐rated Conners Abbreviated Questionnaire: PUFA 16.5, placebo 19.9; teacher‐rated: PUFA 10.5, placebo 13.1 Setting: central Indiana, US |
|
| Interventions | Omega‐3/6 PUFA (Efalex, Efamol Ltd. UK): capsules contained 60 mg DHA, 10 mg EPA, 5 mg AA, 12 mg GLA, 3 mg vitamin E. 8 capsules were given daily for 4 months. Placebo: 0.8 g olive oil given as 8 capsules daily for 4 months |
|
| Outcomes | Improvement Defined as no longer meeting the parent criteria for hyperactivity ADHD symptoms Parent and teacher‐rated Conners questionnaire Parent and teacher‐rated Disrupted Behavior Disorders Rating Scale: hyperactivity, attention Behaviour Parent and teacher‐rated Disrupted Behavior Disorders Rating Scale: conduct, oppositional defiant behaviour |
|
| Notes | SDs calculated from range | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was balanced for age and gender |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors described the trial as double‐blind. The odour and appearance of the placebo capsules were described as comparable to the PUFA capsules. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Capsules were "comparable" and parent and teacher‐rated scales used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The authors reported that ITT analysis was done but reported data from the 33 participants who completed the trial |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to have been reported |
| Other bias | Unclear risk | Both groups were comparable over a large range of variables except the Conners parent's score and reaction time which were significantly lower in the PUFA group Not clear how the screening sample was identified. Diagnoses of ADHD were not verified by a clinician and the reliability and validity of the thirst/skin symptom scales were not reported. |
| Methods | Parallel trial of omega‐3 PUFA versus placebo | |
| Participants | Inclusion criteria: aged 8 to 13 years; had received a previous diagnosis of ADHD by a clinical psychiatrist, neurologist or paediatrician Exclusion criteria: significant sensory or neurological limitations, epilepsy, mental retardation, psychosis, pervasive developmental disorder, taking medications with known central nervous system effects (including stimulants or dietary supplements other than vitamins), or a total Test of Variables of Attention Score more than 1.8 SD lower than age and gender means Mean age: 9.3 years, gender (at follow‐up): 45 boys and 15 girls, loss to follow‐up: 23/83 Baseline Conners Rating Scale: PUFA 15.8; placebo 15.1 Setting: medical centre in Tel‐Aviv, Israel |
|
| Interventions | PUFA: 1. Phospholipid supplement enriched with n‐3 fatty acids (Enzymotec Ltd., Israel) containing 95 mg DHA, 156 mg EPA, 300 mg phosphatidylserine, rosemary extract, ascorbyl palmitate and mixed natural tocopherols (0.8% by weight) emulsified to a dairy chocolate‐flavoured spread containing 4 to 7 mg citrus oil extract (to disguise taste) administered daily as 25 g of spread for 3 months 2. Fish oil supplement (Ocean Nutrition) containing 96 mg DHA, 153 mg EPA and tocopherol mixture (0.2% by weight) daily) emulsified to a dairy chocolate‐flavoured spread containing 4 to 7 mg citrus oil extract (to disguise taste) administered daily as 25 g of spread for 3 months Placebo: Rapeseed oil supplement emulsified to a dairy chocolate‐flavoured spread containing 4 to 7 mg citrus oil extract, administered as 25 g of spread per day for 3 months |
|
| Outcomes | Change in parent‐rated abbreviated Conners Rating Scale at 3 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A block randomisation with a block size of 3 was used |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Supplements appeared "identical" therefore participants were probably blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Supplements appeared identical and a parent‐rated scale was used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A per protocol analysis was used |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to have been reported |
| Other bias | Unclear risk | The principal author was a consultant to Enzymotec Pty Ltd and the Director was another author |
| Methods | Parallel trial of omega‐3 PUFA versus placebo | |
| Participants | Inclusion criteria: children aged 6 to 12 years previously given a diagnosis of ADHD by a physician and confirmed by a neurodevelopmental physician, with significant social or academic impairment, and receiving effective stimulant maintenance therapy Exclusion criteria: ineffective treatment, treatment with other psychotropics, diagnosis of other psychiatric disorders, use of dietary supplements other than vitamins, a significant life event within 6 months, head injury or seizure, mental retardation or pervasive developmental disorder, premature birth, exposure to tobacco or other drugs in utero, lipid metabolism disorder Mean age: 9.3 years, gender: 42 boys and 12 girls, loss to follow‐up: 9/63 Baseline scores: mean response time (TOVA) PUFA 1.43, placebo 1.56 Setting: US |
|
| Interventions | Omega‐3 PUFA (DHASCO, Martek Biosciences Corporation): an algae‐derived triglyceride capsule providing 345 mg DHA given as 3 capsules daily for 4 months Placebo: given as 3 capsules daily for 4 months but not described further |
|
| Outcomes | ADHD symptoms Parent‐rated Child Behaviour Checklist: attention Behaviour Parent‐rated Child Behaviour Checklist: internalising, externalising, socialisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The authors described the trial as double‐blind. Placebo described as "identical in appearance" to PUFA capsule. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Capsules appeared "identical" and parent‐rated scales were used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data was reported for the 53 of 63 enrolled participants who completed the trial |
| Selective reporting (reporting bias) | High risk | The authors stated there were no differences in the Conners Rating Scales but these data were not reported |
| Other bias | Unclear risk | Study funded by Martek Biosciences Corporation |
ADD: attention deficit disorder; ADHD: attention deficit hyperactivity disorder; DHA: docosahexanoic acid; EFA: essential fatty acids; EPA: eicosapentanoic acid; ITT: intention‐to‐treat; PUFA: polyunsaturated fatty acids; RBPC: Revised Behavior Problem Checklist; SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anon 2009 | Review of Johnson 2009 |
| Doebel 2005 | Overview of articles and trials of nutrients in children with ADHD |
| Harding 2003 | Not randomised |
| Joshi 2006 | Pre‐post study |
| Richardson 2002 | The Conners cut‐off score used as an inclusion criteria was not clear |
ADHD: attention deficit hyperactivity disorder
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Effects of N‐3 fatty acids (DHA and EPA) on cognitive control and associated brain activity in ADHD |
| Methods | A double‐blind, placebo‐controlled study |
| Participants | Boys with ADHD (n = 40) and typically developing boys (n = 40) aged 8 to 12 years |
| Interventions | Dietary supplementation with omega‐3 fatty acids (EPA and DHA) |
| Outcomes | Cognitive control and associated brain activity in prefrontal and striatal areas |
| Starting date | |
| Contact information | Dienke Bos Department Child and Adolescent Psychiatry Rudolf Magnus Institute University Medical Center Utrecht d.j.bos‐2@umcutrecht.nl |
| Notes | Email sent 7 September 2011 but no response received |
| Trial name or title | Omega‐3 supplementation and attention‐deficit‐hyperactivity disorder (ADHD) |
| Methods | Randomised, placebo‐controlled trial |
| Participants | 40 children, 6 years to 13 years, diagnosed with ADHD will be randomised to consume either ALA (3 grams of ALA‐containing plant oil) or placebo for 2 months Inclusion criteria: ADHD diagnosis Exclusion criteria: any comorbidities, any medication or supplement use |
| Interventions | Dietary supplement: omega‐3 fatty acid alpha‐linolenic acid (ALA) Dietary supplement: placebo |
| Outcomes | Baseline and end assessments will include both ADHD‐related questionnaires and tests, and blood fatty acid composition |
| Starting date | April 2009 |
| Contact information | Gal Dubnov‐Raz The Edmond and Lily Safra Children’s Hospital, Sheba Medical Center, Tel Hashomer 52621, Israel Email: gal.dubnov‐raz@sheba.health.gov.il |
| Notes | Email sent 23 April 2012 to check on progress of study |
| Trial name or title | PAD‐Study ‐ Efficacy of polyunsaturated fatty acids in children and adolescents with attention deficit/hyperactivity disorder (ADHD) |
| Methods | A randomised, double‐blind, parallel, placebo‐controlled study |
| Participants | Children and adolescents aged 6 to 17 years diagnosed with ADHD (n = 438) |
| Interventions | Polyunsaturated fatty acids (PUFA) in combination with zinc and magnesium |
| Outcomes | Attention Deficit Hyperactivity Disorder Rating Scale, Parent Version IV (ADHDRS‐IV) |
| Starting date | Recruiting 1 August 2011 |
| Contact information | Professor Michael Huss University Medicine Mainz, Germany michael.huss@ukmainz.de |
| Notes | Email sent 7 September 2011. Professor Huss responded that the trial completion could not be expected before end of 2012 |
| Trial name or title | Clinical trial to evaluate the efficacy and the tolerance of an omega 3 fatty acids supplementation in ADHD children |
| Methods | A randomised, double‐blind, parallel, placebo‐controlled study |
| Participants | Children aged 6 to 15 years with ADHD (n = 160) |
| Interventions | Omega‐3 fatty acid supplementation |
| Outcomes | ADHD rating scale |
| Starting date | October 2008 |
| Contact information | Dr Olivier Revol Pierre Wertheimer Hospital olivier.revol@chu‐lyon.fr |
| Notes | Email sent 7 September 2011 but no response received |
| Trial name or title | Randomised controlled trial investigating effect of supplementation with omega‐3 fatty acids EPA and DHA versus omega‐6 fatty acid LA on ADHD symptoms and learning difficulties in 7‐12 year old children |
| Methods | Cross‐over, randomised, double‐blind controlled trial of omega‐3 versus omega‐6 PUFA |
| Participants | Inclusion criteria: 120 children aged 7 to 12 years, ADHD diagnosis, parent‐reported learning difficulties Exclusion criteria: on stimulant medication, taken omega‐3 supplements for up to 3 months, haemophilia |
| Interventions | 1 g eicosapentaenoic acid (EPA), 1 g of docosahexaenoic acid (DHA) or linolenic acid (LA; sunflower oil placebo) in 4 capsules per day) in a 3 x 3 cross‐over trial over 12 months |
| Outcomes | Conners’ parent rating scales, cognitive assessments of attention, impulsivity and literacy at 0, 4, 8 and 12 months |
| Starting date | 16 July 2007 |
| Contact information | Dr Natalie Sinn Nutritional Physiology Research Centre, University of South Australia, GPO Box 2471, Adelaide, SA 5001, Australia +61 8 83021757 natalie.sinn@unisa.edu.au |
| Notes | Email sent 23 April 2012 to check on progress of study |
| Trial name or title | Omega‐3 fatty acids supplementation for adolescent boys with attention deficit hyperactivity disorder: a double‐blind, randomized controlled trial |
| Methods | Double‐blind, parallel, randomised, placebo‐controlled trial |
| Participants | Male adolescents aged 12 to 17 years of age from special schools in the UK who meet ADHD diagnosis criteria and have Conners’ Parents ADHD Rating Global Scale and Conners’ Teachers ADHD Rating Global Scale above 65 |
| Interventions | Omega‐3 fatty acids supplementation for 3 months |
| Outcomes | Conners’ teacher rating scale ADHD index |
| Starting date | 20 March 2006 |
| Contact information | Dr Eric Taylor Child and Adolescent Psychiatry Institute of Psychiatry London United Kingdom e.taylor@iop.kcl.ac.uk |
| Notes | Email sent 7 September 2011. Dr Taylor responded that a paper was in preparation. |
| Trial name or title | Omega‐3 fatty acids as an adjunctive therapy for treatment in attention‐deficit hyperactivity disorder (ADHD) |
| Methods | Double‐blind, cross‐over, randomised, placebo‐controlled trial |
| Participants | Children and adolescents aged between 6 and 15 years with a diagnosis of ADHD according to the DSM‐IV‐TR and on current treatment with stimulants (30 to 150) |
| Interventions | Prescription omega‐3 fatty acids as an adjunctive therapy to stimulants |
| Outcomes | Improvement in ADHD symptoms |
| Starting date | June 2009 |
| Contact information | Dr Jose M Torrijos Maimonides Medical Center Brooklyn jtorrijos@maimonidesmed.org |
| Notes | Email sent 7 September 2011 but no response received |
| Trial name or title | Effect of long chain polyunsaturated fatty acids on behavior and cognition in children with attention deficit hyperactivity disorder |
| Methods | Double‐blind, parallel, randomised, placebo‐controlled trial |
| Participants | Children aged 6 to 12 years with attention deficit hyperactivity disorder (n = 100) |
| Interventions | Omega‐3 supplement for 16 weeks |
| Outcomes | Behaviour and cognition |
| Starting date | April 2009 |
| Contact information | Dr Katharina A Widenhorn‐Mueller Sozialpädiatrisches Zentrum und Kinderneurologie Ulm, Germany katharina.mueller@znl‐ulm.de |
| Notes | Email sent 7 September 2011. Dr Widenhorn‐Mueller responded that trial completion was expected at the end of 2012. |
ADHD: attention deficit hyperactivity disorder; ALA: alpha‐linolenic acid; DHA: docosahexanoic acid; EPA: eicosapentanoic acid; LA: linolenic acid; PUFA: polyunsaturated fatty acids
Contributions of authors
Writing the protocol: Donna Gillies, Sagar Lad, Melissa Ross, John Sinn. Selection of studies for review: Donna Gillies, Sagar Lad, Matthew Leach. Data extraction: Donna Gillies, Sagar Lad, Matthew Leach, Melissa Ross, John Sinn. Data entry: Donna Gillies. Writing the review: Donna Gillies. Revising the review: Sagar Lad, Matthew Leach, Melissa Ross, John Sinn.
Declarations of interest
Donna Gillies: none known. Sagar Lad: has been an investigator on a pre‐post trial of PUFA for symptoms of ADHD (Joshi 2006). Matthew Leach: none known. Melissa Ross: none known. John Sinn: none known.
Edited (no change to conclusions)
References
References to studies included in this review
- Aman MG, Mitchell EA, Turbott SH. The effects of essential fatty acid supplementation by Efamol in hyperactive children. Journal of Abnormal Child Psychology 1987;15(1):75‐90. [DOI] [PubMed] [Google Scholar]
- Arnold LE, Kleykamp D, Votolato N, Gibson RA, Horrocks L. Potential link between dietary intake of fatty acids and behavior: pilot exploration of serum lipids in attention‐deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 1994;4(3):171‐82. [Google Scholar]; Arnold LE, Kleykamp D, Votolato NA, Taylor WA, Kontras SB, Tobin K. Gamma‐linolenic acid for attention‐deficit hyperactivity disorder: placebo‐controlled comparison to D‐amphetamine. Biological Psychiatry 1989;25(2):222‐8. [DOI] [PubMed] [Google Scholar]; Arnold LE, Pinkham SM, Votolato N. Does zinc moderate essential fatty acid and amphetamine treatment of attention‐deficit/hyperactivity disorder?. Journal of Child and Adolescent Psychopharmacology 2000;10(2):111‐7. [DOI] [PubMed] [Google Scholar]
- Belanger SA, Vanasse M, Spahis S, Sylvestre MP, Lippe S, L'Heureux F, et al. Omega‐3 fatty acid treatment of children with attention‐deficit hyperactivity disorder: a randomized, double‐blind, placebo‐controlled study. Paediatrics and Child Health 2009;14(2):89‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brue AW, Oakland TD, Evans RA. The use of a dietary supplement combination and an essential fatty acid as an alternative and complementary treatment for children with attention‐deficit/hyperactivity disorder. The Sciientific Review of Alternative Medicine 2001;5(4):187‐94. [Google Scholar]
- Gustafsson PA, Birberg‐Thornberg U, Duchén K, Landgren M, Malmberg K, Pelling H, et al. EPA supplementation improves teacher‐rated behaviour and oppositional symptoms in children with ADHD. Acta Pædiatrica 2010;99(10):1540‐9. [DOI] [PubMed] [Google Scholar]
- Hamazaki T, Hirayama S. The effect of docosahexaenoic acid‐containing food administration on symptoms of attention‐deficit/hyperactivity disorder ‐ a placebo‐controlled double‐blind study. European Journal of Clinical Nutrition 2004;58(5):838. [DOI] [PubMed] [Google Scholar]; Hirayama S, Hamazaki T, Terasawa K. Effect of docosahexaenoic acid‐containing food administration on symptoms of attention‐deficit/hyperactivity disorder ‐ a placebo‐controlled double‐blind study. European Journal of Clinical Nutrition 2004;58(3):467‐73. [DOI] [PubMed] [Google Scholar]
- Johnson M, Ostlund S, Fransson G, Kadesjo B, Gillberg C. Omega‐3/omega‐6 fatty acids for attention deficit hyperactivity disorder: a randomized placebo‐controlled trial in children and adolescents. Journal of Attention Disorders 2009;12(5):394‐401. [DOI] [PubMed] [Google Scholar]
- Manor I, Magen A, Keidar D, Rosen S, Tasker H, Cohen T, et al. The effect of phosphatidylserine containing omega3 fatty‐acids on attention‐deficit hyperactivity disorder symptoms in children: a double‐blind placebo‐controlled trial, followed by an open‐label extension. European Psychiatry2011:In Press. [DOI] [PubMed]
- Raz R, Carasso RL, Yehuda S. The influence of short‐chain essential fatty acids on children with attention‐deficit/hyperactivity disorder: a double‐blind placebo‐controlled study. Journal of Child and Adolescent Psychopharmacology 2009;19(2):167‐77. [DOI] [PubMed] [Google Scholar]
- Sinn N. Polyunsaturated fatty acid supplementation for ADHD symptoms: response to commentary. Journal of Developmental and Behavioral Pediatrics 2007;28(3):262‐3. [DOI] [PubMed] [Google Scholar]; Sinn N, Bryan J. Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. Journal of Developmental and Behavioral Pediatrics 2007;28(2):82‐91. [DOI] [PubMed] [Google Scholar]; Sinn N, Bryan J, Wilson C. Cognitive effects of polyunsaturated fatty acids in children with attention deficit hyperactivity disorder symptoms: a randomised controlled trial. Prostaglandins Leukotrienes and Essential Fatty Acids 2008;78(4‐5):311‐26. [DOI] [PubMed] [Google Scholar]
- Stevens L, Zhang W, Peck L, Kuczek T, Grevstad N, Mahon A, et al. EFA supplementation in children with inattention, hyperactivity, and other disruptive behaviors. Lipids 2003;38(10):1007‐21. [DOI] [PubMed] [Google Scholar]
- Vaisman N, Kaysar N, Zaruk‐Adasha Y, Pelled D, Brichon G, Zwingelstein G, et al. Correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention: effect of dietary n‐3 fatty acids containing phospholipids. American Journal of Clinical Nutrition 2008;87(5):1170‐80. [DOI] [PubMed] [Google Scholar]
- Voigt RG, Llorente AM, Jensen CL, Fraley JK, Berretta MC, Heird WC. A randomized, double‐blind, placebo‐controlled trial of docosahexaenoic acid supplementation in children with attention‐deficit/hyperactivity disorder. Journal of Pediatrics 2001;139(2):189‐96. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Anon. Omega‐3/omega‐6 fatty acids and ADHD. Brown University Child & Adolescent Psychopharmacology Update 2009;11(4):4‐5. [Google Scholar]
- Doebel JK, Keller G, Zierau M‐T. Dietary therapy for children with ADHD. Deutsche Apotheker Zeitung 2005;145(24):54‐7. [Google Scholar]
- Harding KL, Judah RD, Gant C. Outcome‐based comparison of Ritalin versus food‐supplement treated children with AD/HD. Alternative Medicine Review 2003;8(3):319‐30. [PubMed] [Google Scholar]
- Joshi K, Lad S, Kale M, Patwardhan B, Mahadik SP, Patni B, et al. Supplementation with flax oil and vitamin C improves the outcome of attention deficit hyperactivity disorder (ADHD). Prostaglandins Leukotrienes and Essential Fatty Acids 2006;74(1):17‐21. [DOI] [PubMed] [Google Scholar]
- Richardson AJ, Puri BK. A randomized double‐blind, placebo‐controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD‐related symptoms in children with specific learning difficulties. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2002;26(2):233‐9. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Bos D, Belle J, Weusten J, Hoeksma M, Wiseman S, Durston S. Effects of N‐3 fatty acids (DHA and EPA) on cognitive control and associated brain activity in ADHD: a double‐blind placebo controlled study. European Child and Adolescent Psychiatry2010; Vol. 19, issue Suppl 1:S69.
- Omega‐3 supplementation and attention‐deficit‐hyperactivity disorder (ADHD). Ongoing studyApril 2009.
- PAD‐Study ‐ Efficacy of polyunsaturated fatty acids in children and adolescents with attention deficit/hyperactivity disorder (ADHD). Ongoing study Recruiting 1 August 2011.
- Clinical trial to evaluate the efficacy and the tolerance of an omega 3 fatty acids supplementation in ADHD children. Ongoing studyOctober 2008.
- Randomised controlled trial investigating effect of supplementation with omega‐3 fatty acids EPA and DHA versus omega‐6 fatty acid LA on ADHD symptoms and learning difficulties in 7‐12 year old children. Ongoing study 16 July 2007.
- Omega‐3 fatty acids supplementation for adolescent boys with attention deficit hyperactivity disorder: a double‐blind, randomized controlled trial. Ongoing study 20 March 2006.
- Omega‐3 fatty acids as an adjunctive therapy for treatment in attention‐deficit hyperactivity disorder (ADHD). Ongoing studyJune 2009.
- Effect of long chain polyunsaturated fatty acids on behavior and cognition in children with attention deficit hyperactivity disorder. Ongoing studyApril 2009.
Additional references
- Achenbach TM, Edelbrock CS. Manual for the Child Behavior Checklist. Burlington, VT: University of Vermont, Department of Psychiatry, 1983. [Google Scholar]
- Agostoni C. Role of long‐chain polyunsaturated fatty acids in the first year of life. Journal of Pediatric Gastroenterology and Nutrition 2008;47 (Suppl 2):S41‐4. [DOI] [PubMed] [Google Scholar]
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington DC: American Psychiatric Association, 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (text revision). Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
- Assisi A, Banzi R, Buonocore C, Capasso F, Muzio V, Michelacci F, et al. Fish oil and mental health: the role of n‐3 long‐chain polyunsaturated fatty acids in cognitive development and neurological disorders. International Clinical Psychopharmacology 2006;21(6):319‐36. [DOI] [PubMed] [Google Scholar]
- Banaschewski T, Roessner V, Dittmann RW, Santosh PJ, Rothenberger A. Non‐stimulant medications in the treatment of ADHD. European Child & Adolescent Psychiatry 2004;13(Suppl 1):102‐16. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Diagnosis and assessment of attention deficit‐hyperactivity disorder. Comprehensive Mental Health Care 1991;1(1):27‐43. [Google Scholar]
- Barkley R. Attention‐deficit Hyperactivity Disorders: a Handbook for Diagnosis and Treatment. 2nd Edition. New York: Guilford Press, 1998. [Google Scholar]
- Biederman J. Attention‐deficit/hyperactivity disorder: a selective overview. Biological Psychiatry 2005;57(11):1215‐20. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Qawasmi A. Omega‐3 fatty acid supplementation for the treatment of children with attention‐deficit/hyperactivity disorder symptomatology: systematic review and meta‐analysis. Journal of the American Academy of Child and Adolescent Psychiatry 2011;50(10):991‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsonelo EC, Galduroz JCF. The role of polyunsaturated fatty acids (PUFAs) in development, aging and substance abuse disorders: review and propositions. Prostaglandins Leukotrienes and Essential Fatty Acids 2008;78(4‐5):237‐45. [DOI] [PubMed] [Google Scholar]
- Burgess JR, Stevens L, Zhang W, Peck L. Long‐chain polyunsaturated fatty acids in children with attention‐deficit hyperactivity disorder. American Journal of Clinical Nutrition 2000;71(Suppl 1):327‐30. [DOI] [PubMed] [Google Scholar]
- Cantwell DP. Attention deficit disorder: a review of the past 10 years. Journal of the American Academy of Child Adolescent Psychiatry 1996;35(8):978‐87. [DOI] [PubMed] [Google Scholar]
- Chen J‐R, Hsu S‐F, Hsu C‐D, Hwang L‐H, Yang S‐C. Dietary patterns and blood fatty acid composition in children with attention‐deficit hyperactivity disorder in Taiwan. Journal of Nutritional Biochemistry 2004;15(8):467‐72. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JDA, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS‐R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology 1998;26(4):279‐91. [DOI] [PubMed] [Google Scholar]
- Daley KC. Update on attention‐deficit/hyperactivity disorder. Current Opinion in Pediatrics 2004;16(2):217‐26. [DOI] [PubMed] [Google Scholar]
- Faraone SV. Genetics of childhood disorders: XX ADHD Part 4: is ADHD genetically heterogeneous?. Journal of the American Academy of Child Adolescent Psychiatry 2000;39(11):1455‐7. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Wilens T. Does stimulant treatment lead to substance use disorders?. Journal of Clinical Psychiatry 2003;64(Suppl 11):9‐13. [PubMed] [Google Scholar]
- Findling RL, Dogin JW. Psychopharmacology of ADHD in children and adolescents. Journal of Clinical Psychiatry 1998;59(7):42‐9. [PubMed] [Google Scholar]
- Froehlich TE, Anixt JS, Loe IM, Chirdkiatgumchai V, Kuan L, Gilman RC. Update on environmental risk factors for attention‐deficit/hyperactivity disorder. Current Psychiatry Reports 2011;13(5):333‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman L. What is attention‐deficit hyperactivity disorder (ADHD)?. Journal of Child Neurology 2005;20(12):994‐1002. [DOI] [PubMed] [Google Scholar]
- Gill M, Daly G, Heron S, Hawi Z, Fitzgerald M. Confirmation of association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Molecular Psychiatry 1997;2(4):311‐3. [DOI] [PubMed] [Google Scholar]
- Golder S, Loke YK. Is there evidence for biased reporting of published adverse effects data in pharmaceutical industry‐funded studies?. British Journal of Clinical Pharmacology 2008;66(6):767‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R, Stevenson J. A twin study of hyperactivity II: the aetiological role of genes, family relationships and perinatal adversity. Journal of Child Psychology and Psychiatry 1989;30(5):691‐709. [DOI] [PubMed] [Google Scholar]
- Haag M. Essential fatty acids and the brain. Canadian Journal of Psychiatry 2003;48(3):3195‐203. [DOI] [PubMed] [Google Scholar]
- Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Archives of General Psychiatry 2006;63(3):332‐9. [DOI] [PubMed] [Google Scholar]
- Harpin VA. The effect of ADHD on the life of an individual their family and community from preschool to adult life. Archives of Disease in Childhood 2005;90(Suppl 1):12‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Innis SM. The role of dietary n‐6 and n‐3 fatty acids in the developing brain. Developmental Neuroscience 2000;22(5‐6):474‐80. [DOI] [PubMed] [Google Scholar]
- Klassen A, Miller A, Raina P, Lee SK, Olsen L. Attention‐deficit hyperactivity disorder in children and youth: a quantitative systematic review of the efficacy of different management strategies. Canadian Journal of Psychiatry 1999;44(10):1007‐16. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children's Depression Inventory. https://www.mhs.com/ecom/(3jkh5055rnqsekfhnxgzn145)/TechBrochures/CDI.pdf (accessed 18 May 2007).
- Krause KH, Dresel SH, Krause J, Fougere C, Ackenheil M. The dopamine transporter and neuroimaging in attention deficit hyperactivity disorder. Neuroscience Biobehavioral Reviews 2003;27(7):605‐13. [DOI] [PubMed] [Google Scholar]
- Langley K, Rice F, Bree MBM, Thapar A. Maternal smoking during pregnancy as an environmental risk factor for attention deficit hyperactivity disorder behaviour. A review. Minerva Pediatrica 2005;57(6):359‐71. [PubMed] [Google Scholar]
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003;326(7400):1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Sham PC, Owen MJ, He L. Meta‐analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD). Human Molecular Genetics 2006;15(14):2276‐84. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. American Journal of Psychiatry 2003;160(6):1028‐40. [DOI] [PubMed] [Google Scholar]
- Mitchell EA, Aman MG, Turbott SH, Manku M. Clinical characteristics and serum essential fatty acid levels in hyperactive children. Clinical Pediatrics 1987;26(8):406‐11. [DOI] [PubMed] [Google Scholar]
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
- Morrell J, Murray L. Parenting and the development of conduct disorder and hyperactive symptoms in childhood: a prospective longitudinal study from 2 months to 8 years. Journal of Child Psychology and Psychiatry 2003;44(4):489‐508. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health Consensus Development Conference Statement: diagnosis and treatment of attention‐deficit/hyperactivity disorder (ADHD). Journal of the American Academy of Child & Adolescent Psychiatry2000; Vol. 39, issue 2:182‐93. [DOI] [PubMed]
- Pelham WE, Foster EM, Robb JA. The economic impact of attention‐deficit/hyperactivity disorder in children and adolescents. Journal of Pediatric Psychology 2007;32(6):711‐27. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. American Journal of Psychiatry 2007;164(6):942‐8. [DOI] [PubMed] [Google Scholar]
- Raskin LA, Shaywitz SE, Shaywitz BA, Anderson GM, Cohen DJ. Neurochemical correlates of attention deficit disorder. Pediatric Clinics of North America 1981;31(2):387‐96. [DOI] [PubMed] [Google Scholar]
- Raz R, Gabis L. Essential fatty acids and attention‐deficit‐hyperactivity disorder: a systematic review. Developmental Medicine and Child Neurology 2009;51(8):580‐92. [DOI] [PubMed] [Google Scholar]
- Richardson AJ, Puri BK. The potential role of fatty acids in attention‐deficit/hyperactivity disorder. Prostaglandin, Leukotrienes and Essential Fatty Acids 2000;63(1‐2):79‐87. [DOI] [PubMed] [Google Scholar]
- Richardson AJ. Clinical trials of fatty acid treatment in ADHD, dyslexia, dyspraxia and the autistic spectrum. Prostaglandins Leukotrienes and Essential Fatty Acids 2004;70(4):383‐90. [DOI] [PubMed] [Google Scholar]
- Richardson AJ. Omega‐3 fatty acids in ADHD and related neurodevelopmental disorders. International Review of Psychiatry 2006;18(2):155‐72. [DOI] [PubMed] [Google Scholar]
- Schab DW, Trinh NH. Do artificial food colors promote hyperactivity in children with hyperactive syndromes? A meta‐analysis of double‐blind placebo‐controlled trials. Journal of Developmental Behavioral Pediatrics 2004;25(6):423‐34. [DOI] [PubMed] [Google Scholar]
- Schachter H, Kourad K, Merali Z, Lumb A, Tran K, Miguelez M, et al. Effects of Omega‐3 Fatty Acids on Mental Health. Evidence Report/Technology Assessment No 116 (Publication No 05‐E022‐2). Rockville, MD: Agency for Healthcare Research and Quality, July 2005. [PMC free article] [PubMed] [Google Scholar]
- Shaywitz B, Fletcher J, Shaywitz S. Attention‐deficit/hyperactivity disorder. Advances in Pediatrics 1997;44:331‐67. [PubMed] [Google Scholar]
- Simeon JG, Wiggins DM. Pharmacotherapy of attention deficit hyperactivity disorder. Canadian Journal of Psychiatry 1993;38(6):443‐8. [DOI] [PubMed] [Google Scholar]
- Simmer K, Schulzke SM, Patole S. Longchain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD000375.pub4] [DOI] [PubMed] [Google Scholar]
- Simmer K, Patole S, Rao S. Longchain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD000376.pub2] [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Omega‐3 fatty acids in health and disease and in growth and development. American Journal of Clinical Nutrition 1991;54(3):438‐63. [DOI] [PubMed] [Google Scholar]
- Sinn N, Milte C, Howe PRC. Oiling the brain: a review of randomized controlled trials of omega‐3 fatty acids in psychopathology across the lifespan. Nutrients 2010;2(2):128‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State‐Trait Anxiety Inventory for Children. Palo Alto, CA: Consulting Psychologists Press, 1973. [Google Scholar]
- Stevens LJ, Zentall SS, Deck JL, Abate ML, Watkins BA, Lipp SR, et al. Essential fatty acid metabolism in boys with attention‐deficit hyperactivity disorder. American Journal of Clinical Nutrition 1995;62(4):761‐8. [DOI] [PubMed] [Google Scholar]
- Swanson JM, McBumett K, Wigal T. Effect of stimulant medication on children with attention deficit disorder: a "review of reviews". Exceptional Children 1993;60(2):154‐62. [Google Scholar]
- Swanson J, Oosterlaan J, Murias M, Schuck S, Flodman P, Spence MA, et al. Attention deficit/hyperactivity disorder children with a 7‐repeat allele of the dopamine receptor D4 gene have extreme behaviour but normal performance on critical neuropsychology tests of attention. Proceedings of the National Academy of Sciences 2000;97(9):4754‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, et al. Etiologic subtypes of attention‐deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychology Review 2007;17(1):39‐59. [DOI] [PubMed] [Google Scholar]
- Varni JW, Seid M, Kurtin PS. PedsQL™ 4.0: reliability and validity of the Pediatric Quality of Life Inventory™ version 4.0 generic core scales in healthy and patient populations. Medical Care 2001;39(8):800‐12. [DOI] [PubMed] [Google Scholar]
- Wainwright PE. Do essential fatty acids play a role in brain and behavioral development?. Neuroscience and Biobehavioral Reviews 1992;16(2):193‐205. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders. http://apps.who.int/classifications/icd10/browse/2010/en. Geneva: WHO, 2010.
- Wilens TE. Attention‐deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship who is at risk and treatment issues. Primary Psychiatry 2004;11(7):63‐70. [Google Scholar]
- Zappitelli M, Pinto T, Grizenko N. Pre‐ peri‐ and postnatal trauma in subjects with attention‐deficit hyperactivity disorder. Canadian Journal of Psychiatry 2001;46(6):542‐8. [DOI] [PubMed] [Google Scholar]


