Abstract
Background
Folate is a B‐vitamin required for DNA synthesis, methylation, and cellular division. Wheat and maize (corn) flour are staple crops consumed widely throughout the world and have been fortified with folic acid in over 80 countries to prevent neural tube defects. Folic acid fortification may be an effective strategy to improve folate status and other health outcomes in the overall population.
Objectives
To evaluate the health benefits and safety of folic acid fortification of wheat and maize flour (i.e. alone or in combination with other micronutrients) on folate status and health outcomes in the overall population, compared to wheat or maize flour without folic acid (or no intervention).
Search methods
We searched the following databases in March and May 2018: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and MEDLINE In Process, Embase, CINAHL, Web of Science (SSCI, SCI), BIOSIS, Popline, Bibliomap, TRoPHI, ASSIA, IBECS, SCIELO, Global Index Medicus‐AFRO and EMRO, LILACS, PAHO, WHOLIS, WPRO, IMSEAR, IndMED, and Native Health Research Database. We searched the International Clinical Trials Registry Platform and ClinicalTrials.gov for ongoing or planned studies in June 2018, and contacted authors for further information.
Selection criteria
We included randomised controlled trials (RCTs), with randomisation at the individual or cluster level. We also included non‐RCTs and prospective observational studies with a control group; these studies were not included in meta‐analyses, although their characteristics and findings were described. Interventions included wheat or maize flour fortified with folic acid (i.e. alone or in combination with other micronutrients), compared to unfortified flour (or no intervention). Participants were individuals over two years of age (including pregnant and lactating women), from any country.
Data collection and analysis
Two review authors independently assessed study eligibility, extracted data, and assessed risk of bias.
Main results
We included 10 studies: four provided data for quantitative analyses (437 participants); five studies were randomised trials (1182 participants); three studies were non‐RCTs (1181 participants, 8037 live births); two studies were interrupted time series (ITS) studies (1 study population of 2,242,438, 1 study unreported). Six studies were conducted in upper‐middle‐income countries (China, Mexico, South Africa), one study was conducted in a lower‐middle‐income country (Bangladesh), and three studies were conducted in a high‐income country (Canada). Seven studies examined wheat flour fortified with folic acid alone or with other micronutrients. Three studies included maize flour fortified with folic acid alone or with other micronutrients. The duration of interventions ranged from two weeks to 36 months, and the ITS studies included postfortification periods of up to seven years. Most studies had unclear risk of bias for randomisation, blinding, and reporting, and low/unclear risk of bias for attrition and contamination.
Neural tube defects: none of the included RCTs reported neural tube defects as an outcome. In one non‐RCT, wheat flour fortified with folic acid and other micronutrients was associated with significantly lower occurrence of total neural tube defects, spina bifida, and encephalocoele, but not anencephaly, compared to unfortified flour (total neural tube defects risk ratio (RR) 0.32, 95% confidence interval (CI) 0.21 to 0.48; 1 study, 8037 births; low‐certainty evidence).
Folate status: pregnant women who received folic acid‐fortified maize porridge had significantly higher erythrocyte folate concentrations (mean difference (MD) 238.90 nmol/L, 95% CI 149.40 to 328.40); 1 study, 38 participants; very low‐certainty evidence) and higher plasma folate (MD 14.98 nmol/L, 95% CI 9.63 to 20.33; 1 study, 38 participants; very low‐certainty evidence), compared to no intervention. Women of reproductive age consuming maize flour fortified with folic acid and other micronutrients did not have higher erythrocyte folate (MD ‐61.80 nmol/L, 95% CI ‐152.98 to 29.38; 1 study, 35 participants; very low‐certainty evidence) or plasma folate (MD 0.00 nmol/L, 95% CI ‐0.00 to 0.00; 1 study, 35 participants; very low‐certainty evidence) concentrations, compared to women consuming unfortified maize flour. Adults consuming folic acid‐fortified wheat flour bread rolls had higher erythrocyte folate (MD 0.66 nmol/L, 95% CI 0.13 to 1.19; 1 study, 30 participants; very low‐certainty evidence) and plasma folate (MD 27.00 nmol/L, 95% CI 15.63 to 38.37; 1 study, 30 participants; very low‐certainty evidence), versus unfortified flour. In two non‐RCTs, serum folate concentrations were significantly higher among women who consumed flour fortified with folic acid and other micronutrients compared to women who consumed unfortified flour (MD 2.92 nmol/L, 95% CI 1.99 to 3.85; 2 studies, 657 participants; very low‐certainty evidence).
Haemoglobin or anaemia: in a cluster‐randomised trial among children, there were no significant effects of fortified wheat flour flatbread on haemoglobin concentrations (MD 0.00 nmol/L, 95% CI ‐2.08 to 2.08; 1 study, 334 participants; low‐certainty evidence) or anaemia (RR 1.07, 95% CI 0.74 to 1.55; 1 study, 334 participants; low‐certainty evidence), compared to unfortified wheat flour flatbread.
Authors' conclusions
Fortification of wheat flour with folic acid may reduce the risk of neural tube defects; however, this outcome was only reported in one non‐RCT. Fortification of wheat or maize flour with folic acid (i.e. alone or with other micronutrients) may increase erythrocyte and serum/plasma folate concentrations. Evidence is limited for the effects of folic acid‐fortified wheat or maize flour on haemoglobin levels or anaemia. The effects of folic acid fortification of wheat or maize flour on other primary outcomes assessed in this review is not known. No studies reported on the occurrence of adverse effects. Limitations of this review were the small number of studies and participants, limitations in study design, and low‐certainty of evidence due to how included studies were designed and reported.
Keywords: Female; Humans; Pregnancy; Flour; Food, Fortified; Folic Acid; Folic Acid/administration & dosage; Neural Tube Defects; Neural Tube Defects/prevention & control; Preconception Care; Randomized Controlled Trials as Topic; Triticum; Vitamin B Complex; Vitamin B Complex/administration & dosage; Zea mays
Plain language summary
The effects of fortification of wheat and maize flour with folic acid on population health outcomes
Background
Folate is an essential vitamin that is needed to make and repair DNA and for cell division. Folate has two main forms: folate, the natural form found in foods, and folic acid, the form that is used in supplements and fortified foods. Wheat and maize (corn) flour are staple crops consumed widely throughout the world. Fortification (i.e. the addition of vitamins and minerals to foods, to increase their nutritional value) of wheat or maize flour with folic acid has been introduced in over 80 countries to prevent neural tube defects among women of reproductive age. However, no previous systematic reviews have been conducted to evaluate the effects of folic acid‐fortified flour on folate status or other health outcomes in the general population.
Review question
This review aimed to determine the benefits and safety of fortification of wheat and maize flour with folic acid (i.e. alone or with other vitamins and minerals), compared to wheat or maize flour without folic acid (or no intervention), on folate status and different measures of health in the general population.
Study characteristics
We conducted the literature search in March and May 2018. We included 10 studies; four studies provided data for meta‐analyses. Six studies were conducted in upper‐middle‐income countries (China, Mexico, South Africa), one study was conducted in a lower‐middle‐income country (Bangladesh), and three studies were conducted in a high‐income country (Canada). Seven studies examined the effects of wheat flour fortified with folic acid alone (3 studies) or with other micronutrients (4 studies). Three studies assessed the effects of maize flour fortified with folic acid alone (1 study) or with other micronutrients (two studies).
Key results and certainty of the evidence
Fortification of wheat flour with folic acid may reduce the likelihood of neural tube defects (i.e. total neural tube defects and two specific types of neural tube defects, spina bifida and encephalocoele (a type of neural tube defect that affects the brain and the membranes that cover it through an opening in the skull). Fortification of wheat or maize flour with folic acid (i.e. alone or with other vitamins and minerals) may increase folate status. There was limited evidence of the effects of folic acid‐fortified wheat flour on haemoglobin levels or anaemia. The effects of folic acid fortification of wheat or maize flour on other main outcomes assessed in this review is not known. No studies reported on the occurrence of adverse effects. Limitations of this review were the small number of studies and participants, and the low‐certainty of evidence due to how included studies were designed and reported.
Summary of findings
Summary of findings for the main comparison. Maize flour or maize flour products fortified with folic acid alone versus no intervention for population health outcomes.
| Maize flour or maize flour products fortified with folic acid alone versus no intervention for population health outcomes | ||||||
| Patient or population: pregnant women Setting: rural hospital in South Africa, 1974 Intervention: corn meal made with folic acid‐fortified maize flour Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with maize flour or maize flour products fortified with folic acid alone | |||||
| Erythrocyte concentrations | MD 238.9 nmol/L higher (149.4 higher to 328.40 higher) | ‐ | 38 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | One study, Colman 1974a | |
| Serum folate concentrations | MD 14.98 higher (9.63 higher to 20.33 higher) | ‐ | 38 (1 RCT) | ⊕⊝⊝⊝ Very low a,b,c | One study, Colman 1974a | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;MD: mean difference;RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded once for high risk of bias; randomisation and allocation concealment were not clear, there was a high risk of bias due to lack of blinding in the only study providing data for this outcome (Colman 1974a). bDowngraded once for directness; this is a study including pregnant women residing at the hospital before delivery where they received folic acid‐fortified maize porridge (Colman 1974a). cDowngraded twice for imprecision; the confidence intervals are wide, it is only one study with few participants and short study duration (10 to 50 days with average 26 days of intervention) (Colman 1974a).
Summary of findings 2. Maize flour or maize flour products fortified with folic acid plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing folic acid nor any other vitamins and minerals) for population health outcomes.
| Maize flour or maize flour products fortified with folic acid plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing folic acid or any other vitamins or minerals) for population health outcomes | |||||
|
Patient or population: women of reproductive age in Mexico Settings: urban Intervention: maize flour fortified with folic acid plus other vitamins and minerals Comparison: unfortified maize flour | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with unfortified maize flour | Risk with maize flour or maize flour products fortified with folic acid plus other vitamins and minerals | ||||
| Erythrocyte folate concentrations (nmol/L) | MD 61.80 nmol/L lower (152.98 lower to 29.38 higher) | 35 (1 RCT) | ⊕⊝⊝⊝ Very low a,b | One study, Sanchez 2011 | |
| Serum folate concentrations (nmol/L) | MD 0.00 nmol/L (0.00 to 0.00 ) | 35 (1 RCT) | ⊕⊝⊝⊝ Very low a,b | One study, Sanchez 2011 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;MD: mean difference;RCT: randomised controlled trial | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded once for risk of bias, randomisation was not clear; allocation concealment and blinding were not reported (Sanchez 2011). bDowngraded twice for imprecision, there was only one study informing this outcome with few patients and few events, and confidence intervals are wide (Sanchez 2011).
Summary of findings 3. Wheat flour or wheat flour products fortified with folic acid alone versus unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals) for population health outcomes.
| Wheat flour or wheat flour products fortified with folic acid alone versus unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals) for population health outcomes | ||||||
|
Patient or population: adult women of reproductive age in Canada Settings: urban Intervention: wheat flour products fortified with folic acid alone Comparison: unfortified wheat flour products | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with wheat flour or wheat flour products fortified with folic acid alone | |||||
| Erythrocyte folate concentrations (nmol/L) | The mean erythrocyte folate concentration was 0 nmol/L | MD 0.66 nmol/L higher (0.13 higher to 1.19 higher) | ‐ | 30 (1 RCT) | ⊕⊝⊝⊝ Very low a,b | 1 study, Green 2013 |
| Plasma folate concentrations (nmol/L) | The mean serum folate concentration was 0 nmol/L | MD 27.00 nmol/L higher (15.63 higher to 38.37 higher) | ‐ | 30 (1 RCT) | ⊕⊝⊝⊝ Very low a,b | 1 study, Green 2013 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;MD: mean difference;RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded once for directness, the participants received a high dose of folic acid (400 mg folic acid/bread) which is not commonly used by the general population (Green 2013). bDowngraded twice for imprecision; only one study informing these outcomes with few patients and few events, wide confidence intervals (Green 2013).
Summary of findings 4. Wheat flour or wheat flour products fortified with folic acid plus other vitamins and minerals versus unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals) for population health outcomes.
| Wheat flour or wheat flour products fortified with folic acid plus other vitamins and minerals versus unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals) for population health outcomes | ||||||
| Patient or population: school‐aged children Setting: rural Bangladesh Intervention: wheat flour fortified with folic acid plus other vitamins and minerals Comparison: unfortified wheat flour | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with unfortified flour | Wheat flour or wheat flour products fortified with folic acid plus other vitamins and minerals | |||||
| Anaemia | Study population | RR 1.07 (0.74 to 1.55) | 334 (1 RCT) | ⊕⊕⊝⊝ Low a,b | 1 study, Rahman 2015 | |
| 245 per 1000 | 262 per 1000 (181 to 379) | |||||
| Haemoglobin concentrations | MD 0.00 g/L (2.08 lower to 2.08 higher) | ‐ | 334 (1 RCT) | ⊕⊕⊝⊝ Low a,b | 1 study, Rahman 2015 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;MD: mean difference;RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded once for directness, the only study informing this outcome included children aged 6 to 15 years who received daily chapatis made with wheat flour fortified with folic acid and other nutrients and minerals, food intake was supervised by an adult (Rahman 2015). bDowngraded once for imprecision, there was only one study informing this outcome, and wide confidence intervals (Rahman 2015).
Summary of findings 5. Wheat flour or wheat flour products fortified with folic acid plus other vitamins and minerals compared to unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals) for population health: non‐randomised studies.
| Wheat flour or wheat flour products fortified with folic acid plus other vitamins and minerals compared to unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals) for population health: non‐randomised studies | ||||||
| Patient or population: women of reproductive age and pregnant women Setting: rural areas of China Intervention: wheat flour or wheat flour products fortified with folic acid plus other vitamins and minerals Comparison: unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamin and minerals) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals) | Risk with wheat flour or wheat flour products fortified with folic acid plus other vitamins and minerals | |||||
| Neural tube defects (spina bifida, encephalocoele, anencephaly‐fatal and non‐fatal) | Study population | RR 0.32 (0.21 to 0.48) | 8037 (1 non‐RCT) | ⊕⊝⊝⊝ Very low a | 1 study, Wang 2016 | |
| 23 per 1000 | 7 per 1000 (5 to 11) | |||||
| Spina bifida | Study population | RR 0.29 (0.16 to 0.56) | 8037 (1 non‐RCT) | ⊕⊝⊝⊝ Very low a | 1 study, Wang 2016 | |
| 10 per 1000 | 3 per 1000 (2 to 5) | |||||
| Encephalocoele | Study population | RR 0.26 (0.13 to 0.56) | 8037 (1 non‐RCT) | ⊕⊝⊝⊝ Very low a | 1 study, Wang 2016 | |
| 8 per 1000 | 2 per 1000 (1 to 5) | |||||
| Fatal anencephaly | Study population | RR 0.47 (0.21 to 1.07) | 8037 (1 non‐RCT) | ⊕⊝⊝⊝ Very low a,b | 1 study, Wang 2016 | |
| 5 per 1000 | 2 per 1000 (1 to 5) | |||||
| Serum folate concentrations (nmol/L) | The mean serum folate (nmol/L) was 19.33 nmol/L | MD 2.92 nmol/L higher (1.99 higher to 3.85 higher) | ‐ | 657 (2 non‐RCTs) | ⊕⊝⊝⊝ Very low a,b | 2 studies, Wang 2016, Huo 2011 |
| Anaemia | Study population | RR 0.87 (0.68 to 1.11) | 657 (2 non‐RCTs) |
⊕⊝⊝⊝ Very low a,b | 2 studies, Huo 2011, Huo 2012 | |
| 35‐70 per 1,000 | 1000 per 1000 (1000 to 1000) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded twice due to high risk of bias. There was no evidence of randomisation, the clustering effect was not taken into consideration in the statistical analysis or outcome blinding (Huo 2011; Huo 2012; Wang 2016). b Downgraded once for indirectness due to wide confidence intervals (Huo 2011; Huo 2012; Wang 2016).
Background
Description of the condition
Folate is an essential nutrient that plays a key role in cell division, DNA repair, and tissue growth (Ulrich 2008). Folate and folic acid are forms of the water soluble vitamin B9. Folate is present in legumes, leafy green vegetables, and some citrus fruits; lower folate intakes are common where the staple diet consists of unfortified cereals, and intake of folate‐rich legumes, vegetables, and fruit is low (Allen 2008; de Benoist 2008). Folic acid is the synthetic and most stable form of folate, and is often used in supplements and fortified foods. Folic acid bioavailability is approximately 70% higher than folate naturally contained in foods, although there are wide variations depending on the method of assessment (McNulty 2004; Yetley 2011). Folate is mainly stored in the liver, and can be assessed in serum, plasma, or erythrocytes (also called red blood cells) via microbiological assay, liquid chromatography‐tandem mass spectrometry (LC‐MS), radioisotope competitive binding, or enzyme‐linked or chemiluminescence assays (Yetley 2011). Red blood cell folate is an indicator of longer‐term folate status, while serum or plasma folate levels are influenced by recent folate intake.
The classic presentation of folate deficiency is haematological: macrocytic anaemia. Inadequate dietary intake of folate decreases erythrocyte folate and serum folate concentrations, and leads to megaloblastic changes in bone marrow and macrocytosis in circulating red blood cells (Stabler 2010).
Folate concentrations lower than 100 ng/mL (less than 226.5 nmol/L) in erythrocytes and lower than 3 ng/mL (less than 6.8 nmol/L) in serum or plasma are associated with increased risk of macrocytic anaemia, but inadequate folate status has also been linked to several other adverse health outcomes (WHO 2015a).
Folate insufficiency during the periconceptional period has been associated with a number of early developmental foetal anomalies, most notably neural tube defects. Neural tube defects comprise a collection of neurodevelopmental abnormalities that arise when the neural folds fail to fuse entirely during early embryogenesis, and include anencephaly, spina bifida, and encephalocoele (Botto 1999; WHO/CDC/ICBDSR 2014). Neural tube defects are a leading cause of infant morbidity and mortality (WHO 2012a; WHO/CDC/ICBDSR 2014); neural tube defects are among the most common structural congenital anomalies worldwide, with over 300,000 cases per year, with most of the burden in low‐ and middle‐income countries (Christianson 2006; Lo 2014). It is estimated that up to 70% of neural tube defects can be prevented by increasing folic acid intake during the periconceptional period (Czeizel 1992; Czeizel 2013; De‐Regil 2015; MRC 1991).
Folate insufficiency also has severe consequences throughout the life cycle. For example, inadequate folate status during pregnancy has been associated with increased risk of low birth weight (less than 2500 g) (Molloy 2008; van Uitert 2013); congenital heart defects, orofacial clefts, and cleft palate (Czeizel 2000); and placental abruption, spontaneous abortion, preterm delivery, small for gestational age, and stillbirth (Molloy 2008; van Uitert 2013). Inadequate folate status has also been associated with increased risk of non‐communicable diseases in studies in men and postmenopausal women, including cancers (e.g. lymphoma, leukaemia; colorectal, breast, and prostate cancer), cardiovascular disease (e.g. hypertension, stroke), depression, and cognitive dysfunction (Bailey 2015). Studies in children and adolescents have also noted an age‐related decline in folate status biomarkers, which suggests higher metabolic demands for growth (Bailey 2015). Together, these findings suggest that the safety and efficacy of folic acid fortification interventions need to be evaluated at the population level.
Lower folate intake has also been associated with impairments in other biomarkers in one‐carbon metabolism, including circulating vitamin B12 and functional biomarkers, methylmalonic acid and total homocysteine (tHcy) (Yetley 2011). The World Health Organization (WHO) published guidelines for optimal red blood cell folate and serum folate concentrations in women of reproductive age for prevention of neural tube defects (WHO 2015b). The recommended cutoffs for prevention of neural tube defects are red cell folate concentrations above 906 nmol/L (greater than 400 ng/mL; WHO 2015b).
Description of the intervention
The association between lower maternal folate status and increased risk of neural tube defects was first reported over 50 years ago (Hibbard 1965; Smithells 1976). Adequate periconceptional maternal folate status is critical for embryonic development and prevention of neural tube defects. Clinical trials have established that periconceptional folic acid supplementation prevents the occurrence and recurrence of neural tube defects by up to 70% (Czeizel 1992; De‐Regil 2015; MRC 1991). This informed the development of dietary guidelines for folate intake for women of reproductive age, and the USA Public Health Service recommended that all women capable of becoming pregnant should consume 400 µg of folic acid daily (CDC 1992). Since it is estimated that approximately half of all pregnancies in the USA are unplanned (Finer 2006), in 1998 the United States Food and Drug Administration (US FDA) mandated that folic acid be added to the flour supply to target women of reproductive age and ensure adequate folate intake (US Preventive Services Task Force 2017).
Fortification is a promising, sustainable, and cost‐effective approach to combat micronutrient deficiencies. It has been defined as “the addition of one or more essential nutrients to a food, whether or not it is normally contained in the food, for the purpose of preventing or correcting a demonstrated deficiency of one or more nutrients in the general population or specific population groups" (WHO/FAO 2006). This process usually takes place during the processing of staple foods at a central level so that it reaches a considerable proportion of the at‐risk populations without requiring their active participation. Although there are different definitions for enrichment, in this systematic review, enrichment and fortification are used interchangeably (WHO/FAO 2006).
Folic acid fortification of flour has since been rapidly scaled up worldwide, and is thought to be one of the most efficacious and cost‐effective public health interventions to date (WHO/FAO 2006). Over 80 countries have adopted mandatory fortification of wheat (Triticum aestivum (T aestivum)) flour with folic acid, iron, or both (FFI 2018a). Sixteen countries have adopted mandatory fortification of maize (also known as corn) (Zea mays subsp Mays) flour or meal with folic acid, iron, or both (FFI 2018a). Fortification of grains with folic acid has substantially reduced the prevalence of neural tube defects in the USA and a number of other countries (Castillo‐Lancellotti 2013). Several studies have noted a decrease in neural tube defects ranging from 19% to 32% following initiation of fortification, with the greatest reduction in the year immediately following fortification (Crider 2011). A systematic review of 27 studies assessed the impact of folic acid fortification on the prevalence of neural tube defects from 2000 to 2011 in nine countries, and revealed a significant reduction in all countries (Castillo‐Lancellotti 2013).
Cereals are the major source of food supplies for direct human consumption. Of the 2.4 billion tonnes of cereals currently produced, approximately 1.1 billion tonnes are destined for food use, and the remainder is used for animal feed, industrial use, seed, or is wasted. Wheat is the third‐largest cereal crop after maize and rice, but ranks second to rice in terms of dietary intake (FAO 2012). With an ability to grow in diverse climates, maize ‐ the world's primary coarse grain ‐ is cultivated in most parts of the world, although most production is concentrated in the Americas, particularly in the USA where genetically modified maize accounts for 85% of plantings (USDA 2014). Currently, approximately 55% of world consumption of coarse grains is used for animal feed, but in many countries (mainly in Sub‐Saharan Africa and Latin America) they are also directly used for human consumption.
Flour is defined as a powder that is made by grinding cereal grains, other seeds, or roots (e.g. cassava). Wheat flour is one of the most important foods in Europe, North America, Middle East, India, and North Africa, and is the defining ingredient in most types of breads and pastries. Maize flour has been important in Mesoamerican cuisine since ancient times, and remains a staple in Latin America and Africa (Ranum 2014). In some parts of the world, maize is called corn (or mielies or mealies), and these terms are often used interchangeably. The term 'maize' is used throughout this review to describe flour or meal derived from Zea mays.
Wheat processing and products
Wheat kernels are comprised of three parts: bran, endosperm, and germ (Khan 2009). The bran is the hard, brown, outer protective skin that surrounds the germ and the endosperm. It consists of seven layers that are a concentrated source of dietary fibre. The endosperm is the inner part of the grain, which contains 8% to 18% protein and 50% to 75% starch. The germ contains the plant embryo and accounts for most of the wheat kernel's fat and vitamin E content.
Raw wheat can be ground into flour or semolina, germinated and dried to create malt, crushed or cut into cracked wheat, and parboiled, dried, crushed, and debranned into bulgur. Wheat flour is a powder made from ground wheat and used to prepare food for human consumption. Refined, white flour is made from the endosperm only; whole grain flour is made from the entire grain, including bran, endosperm, and germ; and germ flour is made from the endosperm and germ. The extraction rate describes the composition of flour, and is the percentage of flour extracted from the grain compared to the weight of grain.
Maize processing and products
Maize kernels are comprised of several components: the outer cover (i.e. pericarp and aleurone); the endosperm, which comprises the largest fraction of the kernel; and the germ which consists of the embryo and scutellum. Genetic background, variety, environmental conditions, plant age, and geographic location can impact kernel composition within and between maize varieties (Nuss 2010). The nutritional properties of maize are located in distinct though overlapping components of the kernel. Maize contains approximately 72% starch (endosperm), 10% protein (endosperm and germ), and 3% to 6% oils.
Following harvest, maize undergoes several initial processing steps. Cobs are dried, hulled, and shelled to remove kernels prior to wet or dry milling (ILO 1984). Some maize products use whole maize, while others use degerminated kernels. In many settings, maize grains undergo nixtamalisation or precooking prior to milling. All of these processes may impact its overall nutritional content. Maize meal or flour derived from dry milling is used in different ways throughout the world (Herbst 2001), such as polenta in Italy, angu in Brazil, mamaliga in Romania, mush in the USA, and sadza, nshima, and ugali in African countries. Corn flakes are also derived from corn meal that has undergone extrusion (Nuss 2010). Fermentation of milled kernels is also common in African and South American countries: derived products, including bread and alcohol, may have improved bioavailability of niacin, and fermented maize gruel has been used as a fluid for replacement of electrolytes in acute diarrhoea for children in low‐ and middle‐income settings (Yartey 1995).
The definitions of maize (corn) flour and maize meal vary widely. The US FDA defines maize flour and maize meal as products obtained from the grinding of dried yellow or white corn grains. These regulations define the size, moisture content, and amount of fibre and fat that is retained in the product. Maize meal and flour may also be included as part of a composite flour in combination with other products, such as tubers (e.g. yam, sweet potato), legumes (e.g. soy, peanut), and cereals (e.g. rice, wheat), to enhance nutritional content and bioavailability (Seibel 2006).
Fortification of maize flour and other products (e.g. porridges, tortillas, tamales, arepas) produced from maize has been implemented in several settings around the world. Although folic acid fortification of maize flour is less common than wheat flour, mass fortification of maize flour with at least iron has been practiced for many years in several countries in the Americas (Dary 2002; García‐Casal 2002), and Sub‐Saharan Africa (GFDx 2018; Peña‐Rosas 2014a). Maize flour and maize meal products vary worldwide, based on local and regional practices (Ranum 2014). Additionally, the legislative (Makhumula 2014), dietary (Fiedler 2014; Guamuch 2014), logistical (Fiedler 2014), economic (Fiedler 2014), risk population (Hamner 2014), and equity contexts (Zamora 2014), need to be considered to evaluate the feasibility and long‐term sustainability of folic acid fortification of maize flour.
How the intervention might work
The WHO recommends fortification of wheat and maize flour with folic acid in doses ranging from 1 part per million (ppm) to 5 ppm, depending on the average per capita flour availability per day, a proxy measure of dietary intake (WHO 2009).
Fortification of wheat and maize flour with folic acid is implemented to increase daily intake of folic acid to meet the existing intake gap, improve folate status, and reduce the risk of neural tube defects and other adverse health outcomes. In addition to the general population, in countries where folate intake is insufficient, population groups such as women of reproductive age and young children are at highest risk of deficiency and of interest in this review. Fortification of grains with folic acid has reduced the prevalence of neural tube defects in several countries, including the USA, Canada, Chile, Australia, and South Africa (Bower 2009; Castillo‐Lancellotti 2013; De Wals 2007; Das 2013; Honein 2001; López‐Camelo 2005; Sayed 2008). Additionally, folic acid fortification has been associated with a reduced risk of other health outcomes, including cardiovascular disease, cancer, and depression (Cui 2010; Rimm 1998), and macrocytic anaemia (Ganji 2009; Odewole 2013).
Fortification of flour with folic acid is considered one of the most efficacious and cost‐effective public health interventions to date. Three countries have compared the costs of adding folic acid to wheat and maize flour with the costs of treating people with spina bifida (Grosse 2016; Llanos 2007; Sayed 2008). Each study showed significant net savings in healthcare expenses when spina bifida is prevented through fortification: 2.3 million international dollars in Chile (Llanos 2007), 40.6 million rand in South Africa (Sayed 2008), and 603 million US dollars in the USA (Grosse 2016). These are annual savings and are considered conservative estimates; every year of fortification leads to these net savings. The study in Chile only included surgical repair and rehabilitation costs through 22 years of age (Llanos 2007). The study in South Africa only accounted for treatment costs during infancy (Llanos 2007). The estimate in the study in the USA is highest partly because it includes costs over the lifetime, including the cost of family care for children with spina bifida. However, the authors of the study in the USA noted that they used conservative assumptions in these analyses (Grosse 2016).
Despite the success of this public health intervention, folic acid fortification has not eliminated neural tube defects (CDC 2004; CDC 2010), due in part to suboptimal coverage and scale of programmes. It is estimated that approximately 70% of neural tube defects are folate‐sensitive (Czeizel 1992; MRC 1991), and preventable if current folic acid interventions were implemented and scaled. The recent WHO guidelines recommend that at the population level, red blood cell folate concentrations should be above 906 nmol/L (i.e. 400 ng/mL) in women of reproductive age for optimal prevention of neural tube defects (WHO 2015b). The remaining neural tube defects are due to other nutritional (e.g. vitamin B12 deficiency) and non‐nutritional (e.g. genetic predisposition) factors, and are not expected to be responsive to folic acid. Some concerns have been noted regarding potential unintended consequences of further increasing intake of folic acid (Cole 2007; Wien 2012), such as in the context of vitamin B12 deficiency (Mills 2003; Molloy 2018a; Molloy 2018b; Qi 2014), cancers (HAWC 2015; Van Guelpen 2006), and unmetabolised folic acid in circulation (Boilson 2012; Kelly 1997b, Morris 2010; Troen 2006), particularly in populations that are not at risk for neural tube defects, such as the elderly and young children. However, there are no data to date of an association between unmetabolised folic acid at any concentration and health outcomes in humans.
In addition to demonstrated benefits on health outcomes, the success of flour fortification with folic acid as a public health intervention will likely be determined by several factors, such as availability of resources, existence of appropriate policies and legislation, production and supply, the development and implementation of delivery systems, external and internal certainty control systems, and strategies for information, education, and communication for consumer behaviour change. Figure 1 presents an overall logic model for micronutrient interventions that depicts the programme theory and the potential relationships between inputs and anticipated changes in health and outcomes that can be adapted to the context of each setting (De‐Regil 2013; WHO/CDC 2011).
1.
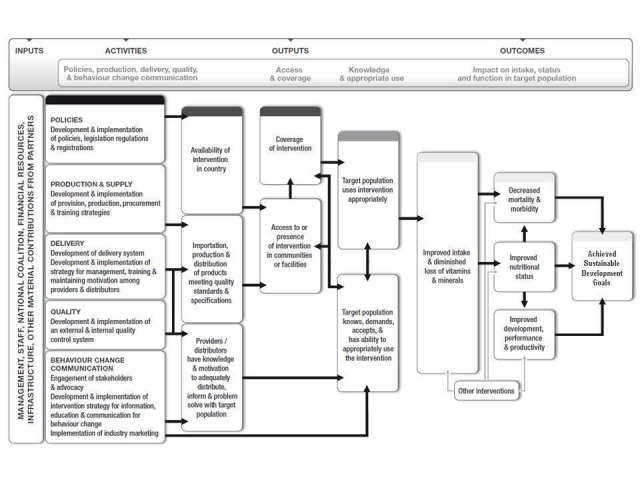
Adapted from WHO/CDC 2011.
Why it is important to do this review
Vitamin and mineral deficiencies are important public health problems worldwide. Among the potential strategies to address these deficiencies, mass fortification is a promising, sustainable, and cost‐effective approach to combat micronutrient deficiencies and improve development, as it leverages existing market and delivery systems, and does not require the active participation of vulnerable populations to increase food intake or dietary diversity. Wheat and maize flour represent suitable vehicles for fortification, as they are considered staple foods in most of the world, particularly in regions where micronutrient deficiencies are common.
Wheat or maize flour fortification with folic acid and other micronutrients may improve population health outcomes, including reducing neural tube defects, anaemia, depression, and cardiovascular disease (Castillo‐Lancellotti 2013; Qi 2014; Zeng 2015). An increasing number of countries across the world are rapidly adopting fortification of wheat and/or maize flour as strategies to target micronutrient deficiencies. In 2004, 33 countries had mandatory wheat flour fortification with folic acid. In 2013, there were 77 countries with legislation for mandatory fortification of wheat or maize flour, and 11 required both to be fortified (CDC 2008). The access to fortified wheat flour by women aged 15 to 60 years increased by 167 million from 2004 to 2007, while the number of births that potentially benefited from flour fortification increased by at least 14 million (Castillo‐Lancellotti 2013; CDC 2008). In 2015, an estimated 35,500 cases of spina bifida and anencephaly were prevented because of wheat or maize flour fortification with folic acid; this represents approximately 13% of the neural tube defects that can be prevented with folic acid globally (Arth 2016).
Folic acid fortification of wheat or maize flour has been implemented in many countries for the prevention of neural tube defects. Previous reviews have been conducted to examine the efficacy or effectiveness of folic acid fortification interventions on health outcomes (including systematic reviews (Atta 2016; Castillo‐Lancellotti 2013; Das 2013), narrative reviews (Berry 2010), meta‐analyses (Atta 2016; Das 2013), and synthesis of evidence from cross‐sectional studies in countries with fortification programmes (Britto 2014; Nazer 2013; Williams 2015)). These reviews have been conducted using data from neural tube defect registries and hospital records from cross‐sectional studies (Atta 2016), a combination of randomised efficacy and effectiveness trials (Das 2013), and synthesising evidence from cross‐sectional studies in countries that mandated flour fortification with folic acid (Berry 2010; Castillo‐Lancellotti 2013). However, to date no systematic reviews have been conducted to examine the safety and efficacy of folic acid fortification of wheat or maize flour ‐ or to examine its effects on folate status and other health outcomes in the general population. A systematic review of the benefits and safety of folic acid fortification of wheat and maize flour is needed to complement the programmatic evidence and inform the development of guidelines and policy making.
There is considerably more variability in processing maize flour and maize meal compared to wheat flour; therefore the evidence and principles of wheat flour fortification may not necessarily apply to maize flour or maize meal fortification (Gwirtz 2014; Peña‐Rosas 2014a). There are limited studies that have evaluated the stability of folic acid and other micronutrients during storage, processing, preparation, and cooking of maize flour and maize meal (Dunn 2014). Available evidence suggests that folic acid offers adequate bioavailability, which is likely independent of food vehicle, and that bioavailability of folic acid in fortified maize flour and maize meal products may be similar to those of fortified wheat products (Moretti 2014). However, some studies have noted significant losses in folic acid and other B‐vitamins during manufacturing, distribution, and cooking of maize products, which warrants investigation (Dunn 2014).
This Cochrane Review complements other Cochrane Reviews investigating the effects of fortification of maize flour (Garcia‐Casal 2018), and wheat flour (Field et al, currently in progress) (Peña‐Rosas 2014b) to improve iron status in the general population. This review will also complement the findings of systematic reviews that examine the effects of interventions that may improve folate status and health‐related outcomes. Two Cochrane Reviews have been conducted to assess the effects of folic acid supplementation during the periconceptional period (De‐Regil 2015), and pregnancy (Lassi 2013). Other related Cochrane Reviews include the combined effects of iron and folic acid supplementation among menstruating women (Fernández‐Gaxiola 2011; Low 2016), pregnant women (Peña‐Rosas 2015a; Peña‐Rosas 2015b), , and the effect of iron and folic acid supplementation on the prevention and treatment of anaemia in children (up to 19 years of age) in malaria‐endemic areas (Okebe 2011). A Cochrane Review on the fortification of rice with folic acid is also currently in progress (Ashong 2012).
Objectives
To evaluate the health benefits and safety of folic acid fortification of wheat and maize flour (i.e. alone or in combination with other micronutrients) on folate status and health outcomes in the overall population, compared to wheat or maize flour without folic acid (or no intervention).
Methods
Criteria for considering studies for this review
Types of studies
We included the following types of studies.
Randomised controlled trials (RCTs), with randomisation at either individual or cluster level
Quasi‐RCTs (where allocation of treatment has been made, for example, by alternate allocation, date of birth, or alphabetical order)
Non‐RCTs
-
Observational studies that are prospective and have a control group:
cohort studies (prospective and retrospective)
controlled before‐and‐after studies
interrupted time series (ITS) with at least three measurement points both before and after the intervention
Fortification of wheat flour, maize flour or maize meal is an intervention that aims to reach the entire population of a country or large sections of the population and is frequently delivered through the market system. We anticipated, therefore, that we would not be able to assess the benefits and potential harms of flour fortification with folic acid if we only included RCTs; thus in addition to RCTs, we examined data from other prospective study designs with a control group.
We included RCTs, non‐RCTs, and observational studies with a control group in this review; however, we did not pool results from these studies together in meta‐analyses; instead, we conducted meta‐analyses for RCTs only. Observational studies without a control group are described in Appendix 1. In addition, we describe uncontrolled before‐and‐after studies (i.e. pre‐ and postintervention studies where participants served as their own control) in Appendix 1, as a narrative assessment of evidence, as we anticipated many studies would include measures of impact at a regional or national level using uncontrolled study designs. We did not include these studies in the meta‐analyses or pool them with randomised studies, and these studies do not inform the overall conclusions of this review. However, these studies provide information on the feasibility and other contextual factors of implementing fortification programmes.
RCTs can provide causal evidence of the effects of folic acid‐fortified wheat or maize flour on health outcomes ‐ and determine if these interventions improve health outcomes (e.g. folate status, anaemia) in individuals who receive the intervention. However, food fortification is a public health intervention that aims to reach the entire population (or the majority of the population) at the country or district level, and is often delivered through national programmes. As folic acid fortification is successfully implemented and scaled up in over 80 countries worldwide, the landscape and equipoise for the design and conduct of RCTs of folic acid fortification has changed ‐ this constrains the feasibility and ethics of conducting additional randomised controlled efficacy trials with a control group. In contrast to the extensive programmatic evidence for folic acid fortification of flour for neural tube defect prevention (which is summarised in other reviews), we therefore anticipated that there would be limited evidence from RCTs on the efficacy of folic acid‐fortified flour interventions on health outcomes, and that evidence from other study designs (e.g. controlled before‐and‐after studies) would also need to be considered in the interpretation and evaluation of the potential benefits and risks of wheat and maize flour fortification on health outcomes. Other recent reviews have been conducted to examine the efficacy and effectiveness of folic acid fortification using programmatic evidence and other types of observational study designs (e.g. cross‐sectional studies); such study designs are not included in this review.
Types of participants
We included participants from the general population, who were two years of age and older (including pregnant and lactating women), and from any country. We excluded children under two years of age, since they are not the intended beneficiaries of maize and wheat flour fortification. We excluded studies of interventions targeted toward participants with critical illnesses or severe comorbidities.
Types of interventions
We included studies in which wheat flour, maize flour, or maize meal were centrally fortified with folic acid, irrespective of the fortification technology used. Interventions included in the review were those in which wheat flour was fortified with any form of folic acid alone or in combination with other vitamins and minerals, and those in which maize flours, or maize subproducts, or both have been fortified with folic acid alone or in combination with other micronutrients.
Maize flour was defined as white or yellow maize (corn) flour or maize meal that was produced by grinding dried maize grains (Codex Alimentarius 1985a; Codex Alimentarius 1985b; FDA 2011). We also included nixtamalised dehydrated maize flour, also known as 'masa flour' or precooked maize flour. We considered any wheat flour for direct human consumption prepared from common wheat, T aestivum or club wheat, Triticum compactum (T compactum), or mixtures thereof (Codex Alimentarius 1995a); durum wheat semolina, including whole durum wheat semolina and durum wheat flour prepared from durum wheat (Triticum durum (T durum)) (Codex Alimentarius 1995b); as well as products prepared with these flours. We included composite flours that contain more than 70% wheat for wheat flours or more than 50% maize for maize flours, within the definitions of either predominantly wheat or maize flour in this review. Wheat flour products included those prepared from wheat flour (e.g. bread, pasta, crackers, cakes). Maize flour products included all products derived from maize meal and flour (e.g. breads, cereals, polenta, porridges, grits, arepas). Studies were eligible for inclusion if the fortification of the wheat flour, maize flour, or maize meal occurred at the flour stage. We excluded studies where fortification occurred at the dough or masa stage.
We considered any form of fortification of wheat flour or maize flour, independently of length of intervention, extraction rate of flour, compounds used, preparation of the folic acid premix, and fortification levels achieved in the wheat flour, maize flour, or derivative foods.
Fortification of the wheat or maize flour must have occurred at the flour stage for the study to have been included. We planned to make the following a priori comparisons.
Maize flour
Maize flour or maize flour products fortified with folic acid alone versus no intervention
Maize flour or maize flour products fortified with folic acid plus other vitamins and minerals versus no intervention
Maize flour or maize flour products fortified with folic acid alone versus unfortified maize flours or maize flour products (not containing folic acid nor any other vitamins and minerals)
Maize flour or maize flour products fortified with folic acid plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing folic acid nor any other vitamins and minerals)
Wheat flour
Wheat flour or wheat flour products fortified with folic acid alone versus no intervention
Wheat flour or wheat flour products fortified with folic acid plus other vitamins and minerals versus no intervention
Wheat flour or wheat flour products fortified with folic acid alone versus unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals)
Wheat flour or wheat flour products fortified with folic acid plus other vitamins and minerals versus unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals)
Combined flour
Combined wheat and maize flour or products fortified with folic acid alone versus no intervention
Combined wheat and maize flour or products fortified with folic acid plus other vitamins and minerals versus no intervention
Combined wheat and maize flour or products fortified with folic acid alone versus unfortified wheat and maize flours or flour products (not containing folic acid nor any other vitamins and minerals)
Combined wheat and maize flour or products fortified with folic acid plus other vitamins and minerals versus unfortified wheat and maize flours or flour products (not containing folic acid nor any other vitamins and minerals)
These comparisons were determined a priori during the scoping of the review to inform WHO guidelines for folic acid fortification of wheat flour and maize flour. We planned to include studies with cointerventions (e.g. fortified flour with education) only if all compared groups received the same cointervention (e.g. folic acid‐fortified flour with education versus education only). We excluded studies that compared flour fortification to other forms of micronutrient interventions, such as micronutrient supplementation, biofortification, point‐of‐use fortification with multiple micronutrient powders or lipid‐based nutrient supplements, or other forms of micronutrient interventions. These are currently the focus of other Cochrane Reviews (Das 2013; Goudet 2015; Garcia‐Casal 2016).
We excluded studies with wheat flour destined for use as a brewing adjunct or for the manufacture of starch, or gluten, or both; flours whose protein content had been reduced; or had been submitted after the milling process to a special treatment other than drying or bleaching. We also excluded studies that evaluated products derived from wet milling of maize, including corn starch (which is often called 'corn flour' in the UK and Australia), and products that were fortified after recomposition of the flour (i.e. dough). For example, if wheat flour or maize flour was used to prepare a bread product or biscuit, and fortification occurred at the level of dough preparation, then this study was excluded. Fortification of the flour must have occurred at the flour stage for the study to have been included. This was to ensure that evidence would be applicable to fortification programmes, in which fortification occurs at the flour stage.
Types of outcome measures
Primary outcomes
We considered the primary outcomes across all populations (children (2 to < 12 years of age), adolescent girls and boys (12 to < 19 years of age), pregnant women (any age), adult males and females (19 years of age to < 60 years of age), and older persons (60 years of age and older) of neural tube defects, folate biomarkers (erythrocyte folate, serum/plasma folate), haemoglobin concentrations, and the presence of anaemia, and any type of cancer. Additional primary outcomes of interest differed by participant group, and we have listed these below by participant group.
Neural tube defects (e.g. total neural tube defects; anencephaly, spina bifida, encephalocoele, meningocele)
Erythrocyte folate concentrations (nmol/L) (continuous, deficiency, and insufficiency, as defined by the study authors)
Serum/plasma folate concentrations (nmol/L) (continuous, deficiency, and insufficiency, as defined by the study authors)
Anaemia (defined as haemoglobin below the WHO cut‐off, adjusted for altitude as appropriate, as defined by the study authors)
Haemoglobin concentrations (g/L)
Any type of cancer (as defined by the study authors)
Additionally, we considered other primary outcomes in different population groups.
Children (2 to < 12 years of age)
Childhood cancers (as defined by the study authors)
Pregnant women (any age)
Low birth weight (less than 2500 g)
Other adverse pregnancy outcomes (as reported by the study authors, including preterm delivery (less than 37 weeks of gestational age), and other congenital anomalies)
Older persons (60 years of age and older)
Cognitive function/decline (as defined by the study authors)
Secondary outcomes
We considered the following secondary outcomes
Serum/plasma homocysteine concentrations (μmol/L) (adjusted for renal function, vitamin B12, as defined by the study authors)
Serum/plasma methylmalonic acid (μmol/L) (adjusted for renal function, vitamin B12, as defined by the study authors)
Depression (as defined by the study authors)
Cognitive function (as defined by study authors, e.g. formal tests addressing intelligence, memory, attention, and other cognitive domains). We accepted any measure of cognitive function that has been previously validated as an appropriate test in this domain
Pernicious anaemia (as defined by the study authors)
Urinary folic acid, 5‐methyltetrahydrofolate (5MTHF), and catabolite concentrations (nmol/L) (adjusted for renal function, as defined by study authors)
Unmetabolised blood folic acid (nmol/L)
Malaria (as defined by the study authors)
Colorectal cancer/polyps (as defined by the study authors)
Cardiovascular disease (as defined by the study authors)
Any adverse side effects (as measured by the study authors, including but not limited to abdominal pain, vomiting, nausea, heartburn, diarrhoea, constipation)
Search methods for identification of studies
We designed and piloted a structured search strategy. We conducted this search strategy in electronic databases and handsearched relevant journals and publications to identify primary studies. We also contacted study authors for unpublished/ongoing studies, as needed, and consulted institutions, agencies, and experts in the field regarding the results of our search and for any additional data.
Electronic searches
We searched the following international and regional sources, no date restrictions were applied to any of the searches.
International databases
The Cochrane Central Register of Controlled Trials (CENTRAL) In the Cochrane Library (searched 26 March 2018)
MEDLINE (searched 02 May 2018)
MEDLINE® In Process (searched 02 May 2018)
Embase (searched 02 May 2018)
Web of Science (both the Social Science Citation Index and the Science Citation Index) (searched 23 March 2018)
CINAHL (searched 23 March 2018)
POPLINE (searched 26 March 2018)
AGRICOLA (agricola.nal.usda.gov) (searched 26 March 2018)
BIOSIS (searched 26 March 2018)
Food Science and Technology Abstracts (FSTA) (searched 26 March 2018)
Regional databases
IBECS (ibecs.isciii.es) (searched 26 March 2018)
Scielo (www.scielo.br) (searched 26 March 2018)
Global Index Medicus ‐ AFRO (includes African Index Medicus); EMRO (includes Index Medicus for the Eastern Mediterranean Region) (searched 26 March 2018)
LILACS (searched 26 March 2018)
PAHO (Pan American Health Library) (searched 26 March 2018)
WHOLIS (WHO Library) (searched 26 March 2018)
WPRO (includes Western Pacific Region Index Medicus) (searched 26 March 2018)
IMSEAR, Index Medicus for the South‐East Asian Region (searched 26 March 2018)
IndMED, Indian medical journals (indmed.nic.in) (searched 26 March 2018)
Native Health Research Database (hscssl.unm.edu/nhd) (searched 26 March 2018)
We also contacted the Information Specialist of the Cochrane Public Health Group to search the Cochrane Public Health Group Specialised Register.
The search used keyword and controlled vocabulary (when available), and the search terms are summarised in Appendix 2. These structured search terms were adapted as appropriate for each database.
We handsearched the five journals with the highest number of included studies in the last 12 months to capture any articles that may not have been indexed in the databases at the time of the search. We did not apply language or date restrictions for any databases. We contacted the authors of included studies and checked reference lists of included papers for identification of additional records.
We searched the WHO International Clinical Trials Registry Platform (apps.who.int/trialsearch), and clinicaltrials.gov databases for any ongoing or planned studies (21 June 2018) using the terms from Appendix 3.
Where we identified articles written in a language other than English, we requested their translations into English. If this was not possible, we sought advice from the Cochrane Public Health Group. We planned to store such articles in the 'Awaiting assessment' section of the review until a translation was available.
Searching other resources
For assistance in identifying ongoing or unpublished studies, we contacted the Departments of Nutrition for Health and Development, Reproductive Health and Research and Maternal, Newborn, Child and Adolescent Health, as well as the regional offices from the WHO, Centers for Disease Control and Prevention (CDC), the nutrition section of the United Nations Children's Fund (UNICEF), the World Food Programme (WFP), Nutrition International (NI), Global Alliance for Improved Nutrition (GAIN), and the Food Fortification Initiative (FFI) (28 July 2016).
Data collection and analysis
Selection of studies
Two review authors (ECT, HG) independently screened the titles and abstracts of articles retrieved by each search to assess eligibility, as determined by the inclusion and exclusion criteria listed above. We retrieved full‐text copies of all eligible articles, for further evaluation when we could not reject a title or abstract with certainty. Two review authors (ECT, HG) independently assessed full‐text articles for eligibility. If we could not obtain the full‐text article, we attempted to contact the authors to obtain further details of the study. Failing this, we classified studies as 'awaiting assessment' until further information was published or made available to us. Any discrepancies at any stage of eligibility assessment process were resolved through discussion and consultation with the senior author (JLF).
Data extraction and management
Two review authors (ECT, HG) independently extracted data using data extraction forms based on those from the Cochrane Public Health Group (Cochrane PHG 2010), and the Cochrane Effective Practice and Organisation of Care (EPOC) Group (Cochrane EPOC 2017).
Most of the review authors were involved in piloting the data extraction form. We used a subset of articles to enhance consistency among review authors and, based on this, we modified the form as necessary. We collected information on study design, study setting and participants (number and characteristics), and provided a full description of the interventions examined. We extracted details of outcomes measured (including a description of how and when outcomes were measured) and findings.
The data extraction form was designed so that we were able to record results for our prespecified outcomes and for other (non‐prespecified) outcomes (although such outcomes did not underpin any of our conclusions). When available, we extracted additional items relating to study recruitment and the implementation of the intervention; these included number of sites for an intervention, whether recruitment was similar at different sites, whether there were protocol deviations, levels of adherence/use of flours in different sites within studies, resources required for implementation, as well as findings from process evaluations conducted.
We used the equity checklist to record if data had been reported by sociodemographic characteristics (PROGRESS ‐ i.e. place of residence, race/ethnicity, occupation, gender, religion/culture, education, socioeconomic status, social capital) known to be important from an equity perspective (Ueffing 2011). We also recorded if studies included specific strategies to address diversity or disadvantage. We extracted data on the costs of the implementation of the intervention where available. This information is summarised in the 'Characteristics of included studies' table in the review.
For eligible studies, two review authors independently extracted data using the data extraction form. Two review authors entered data into Review Manager 5 (RevMan 5) software (Review Manager 2014), and two other review authors carried out checks for accuracy. Any discrepancies were resolved through discussion and consultation with the senior author.
When information regarding any aspect of study design or results was unclear, we attempted to contact the authors of the original reports, and asked them to provide further details.
Assessment of risk of bias in included studies
We used the Cochrane EPOC 'Risk of bias' tool for studies with a control group, to assess the risk of bias of all included studies (EPOC 2009). This tool examines five domains of bias: selection, performance, attrition, detection, and reporting, as well as an 'other' bias category to capture other potential threats to validity.
Two review authors (ECT, HG) independently assessed the risk of bias for each included study. We resolved any disagreements by discussion and consultation with the senior author (JLF).
Assessing risk of bias in RCTs
Random sequence generation (checking for possible selection bias)
We assessed RCTs as one of the following levels of bias.
Low risk of bias if there was a random component in the sequence generation process (any truly random process, e.g. random number table; computer random number generator).
High risk of bias if the trial authors used a non‐random approach (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number).
Unclear.
Allocation concealment (checking for possible selection bias)
We assessed trials as one of the following
Low risk of bias if participants and investigators that enrolled participants could not foresee assignment because an appropriate method was used to conceal allocation (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes). We used this rating for studies where the unit of allocation was by institution and allocation was performed on all units at the start of the study.
High risk of bias if participants and investigators that enrolled participants could possibly foresee assignments and potentially introduce selection bias (e.g. open random allocation; unsealed or non‐opaque envelopes).
Unclear.
Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias)
We assessed studies as one of the following levels of bias.
Low risk of bias if outcomes were measured prior to the intervention, and no important differences were present across intervention groups.
High risk of bias if important differences in outcomes between groups were present prior to the intervention and were not adjusted for in analyses.
Unclear risk of bias if there were no baseline measure of outcome (note: if 'high' or 'unclear' risk of bias, but there was sufficient information to conduct an adjusted analysis, the assessment was determined to be 'low').
Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias)
We assessed studies as follows.
Low risk of bias if baseline characteristics were reported and were similar across intervention groups.
High risk of bias if baseline characteristics were not reported or if there were differences across groups.
Unclear risk of bias if it was not clear (e.g. characteristics were mentioned in the text but no data were presented).
Blinding of participants and personnel (checking for possible performance)
We assessed the risk of performance bias associated with blinding as follows.
Low, high, or unclear risk of bias for participants and personnel
We combined the results into a single evaluation of risk of bias associated with blinding of participants and personnel as follows.
Low risk of bias if there was blinding of participants and key study personnel and it was unlikely to have been broken.
High risk of bias if there was no blinding or incomplete blinding, or if there was blinding that was likely to have been broken.
Unclear risk of bias.
Blinding of outcome assessment (checking for possible detection bias)
We assessed the risk of detection bias associated with blinding as follows.
Low, high, or unclear risk of bias for outcome assessors.
Specifically, we assessed the following.
Low risk of bias if the outcome assessment was blinded and the non‐blinding of others was unlikely to introduce bias.
High risk of bias if the outcome assessment was likely to be influenced by a lack of blinding.
Unclear risk of bias.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, and protocol deviations)
We assessed the outcomes in each included study as one of the following.
Low risk of bias due to incomplete outcome data; either there were no missing outcome data or the missing outcome data were unlikely to bias the results based on the following considerations: study authors provided transparent documentation of participant flow throughout the study, the proportion of missing data was similar in the intervention and control groups, the reasons for missing data were provided and balanced across intervention and control groups, and the reasons for missing data were unlikely to bias the results.
High risk of bias if missing outcome data were likely to bias the results. We used this rating if an 'as‐treated (per protocol)' analysis was performed with substantial differences between the intervention received and that assigned at randomisation, or if potentially inappropriate methods for imputation were used.
Unclear risk of bias.
Contamination (checking for possible performance bias)
We assessed included studies as follows.
Low risk of bias if allocation was by community, institution, or practice and it was unlikely that the control group received the intervention.
High risk of bias if it is likely that the control group received the intervention.
Unclear risk of bias if it is possible that contamination occurred, but the risk of this happening is unclear.
Selective reporting bias
For each included study, we described how potential selective outcome reporting bias was investigated, and what was found. We assessed included studies for this domain as follows.
Low risk of bias, where it was clear that all of the prespecified outcomes in the study and all expected outcomes of interest to the review were reported.
High risk of bias, where not all the prespecified outcomes in the study were reported, one or more reported primary outcomes were not prespecified, outcomes of interest were reported incompletely and so could not be used, or a lack of reporting of results of a key outcome that would have been expected to have been reported.
Unclear risk of bias.
Other sources of bias
We described other possible sources of bias for each included study, and used a rating of either low, high, or unclear risk of bias for this item.
In addition to the above criteria, we also assessed cluster‐RCTs with the following criteria.
Recruitment bias
We assessed included studies as follows
Low risk of bias if individuals were recruited to the trial before the clusters were randomised.
High risk of bias if individuals were recruited to the trial after the clusters were randomised.
Unclear risk of bias.
Baseline imbalance
We assessed included studies as follows
Low risk of bias if baseline characteristics were reported and were similar across clusters or if authors used stratified or pair‐matched randomisation of clusters.
High risk of bias if baseline characteristics were not reported or if there were differences across clusters.
Unclear risk of bias.
Loss of clusters
We assessed included studies as follows
Low risk of bias if no complete clusters were lost or omitted from the analysis.
High risk of bias if complete clusters were lost or omitted from the analysis.
Unclear risk of bias.
Incorrect analysis
We assessed included studies as follows
Low risk of bias if study authors appropriately accounted for clusters in the analysis or provided enough information for review authors to account for clusters in the meta‐analysis.
High risk of bias if study authors appropriately accounted for clusters in the analysis or did not provide enough information for review authors to account for clusters in the meta‐analysis.
Unclear risk of bias.
Compatibility with individual RCTs
We assessed included studies as follows
Low risk of bias if effects of the intervention were likely not altered by the unit of randomisation.
High risk of bias if effects of the intervention were likely altered by the unit of randomisation.
Unclear risk of bias.
Overall risk of bias
For all included studies, we summarised the overall risk of bias by primary outcome across studies. Studies at high risk of bias were those with high or unclear risk of bias in the following domains: allocation concealment, similarity of baseline outcome measurements, and completeness of outcome data. We also considered the likely magnitude and direction of bias and whether it was likely to impact the study findings.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented proportions. For two‐group comparisons, we presented results as a weighted average risk ratio (RR) with 95% confidence intervals (CIs).
Continuous data
We reported the results for continuous outcomes as the mean difference (MD) with 95% CIs if all included trials measured outcomes on the same scale. When some studies reported endpoint data and others reported changes from baseline to endline data (with a measure of dispersion), we combined these in meta‐analyses if the studies reported the outcomes using the same scale.
If a sufficient number of studies did not meet the inclusion criteria, or data for studies could not be pooled, we summarised the results in a narrative form.
Unit of analysis issues
Cluster‐RCTs
We did not combine results from both cluster‐ and individually‐randomised RCTs, since these studies did not report the same outcomes for the same interventions. When the authors of cluster‐RCTs conducted their analyses at a different level to that of allocation and did not appropriately account for the cluster design in their analyses, we calculated trials' effective sample size to account for the effect of clustering in data. We utilised the intracluster correlation coefficient (ICC) derived from the trial (if available), or from another source (e.g. using the ICCs derived from other similar trials) and calculated the design effect with the formula provided in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When we used this approach, we planned to report it and undertake sensitivity analyses to investigate the effect of variations in the ICC on results. Due to the limited number of studies and data obtained in this review, it was not possible to conduct sensitivity analyses to evaluate the potential impact of variations in the ICC on findings.
Studies with more than two treatment groups
When we identified studies with more than two intervention groups (i.e. multiarm studies), where possible, we combined groups to create a single pair‐wise comparison or used the methods described in the Cochrane Handbook for Systematic Reviews of Interventions to avoid double‐counting study participants (Higgins 2011). For subgroup analyses, when the control group was shared by two or more study arms, we planned to divide the control group (i.e. events and total population) over the number of relevant subgroups to avoid double‐counting participants. The included studies in this review had only two treatment groups (i.e. one intervention arm and one comparison group) that met criteria for inclusion in this review.
Dealing with missing data
We attempted to contact the authors if outcome data were missing, unclear, or not fully reported. We planned to capture the missing data in the data extraction form and report it in the 'Risk of bias' tables; however, we did not receive or include unpublished data in the review.
For all outcomes, where possible, we conducted analyses on an intention‐to‐treat (ITT) basis (i.e. for RCTs, we attempted to include all participants randomised to each group in the analyses). Where ITT analysis was not possible, the denominator for each outcome in each study was the number of participants who were randomised minus any participants whose outcomes were known to be missing. For non‐RCTs, where possible, we planned to analyse data according to initial group allocation, irrespective of whether participants received or adhered to the planned intervention.
When assessing adverse events, the principle of ITT has additional considerations. Thus, we related the results to the treatment received ('per protocol' or 'as observed'). This means that for the side effects we planned to base the analyses on the participants who actually received the intervention and the number of adverse events reported in the included studies.
Assessment of heterogeneity
We examined the forest plots from meta‐analyses to visually assess the level of heterogeneity (i.e. in terms of the size or direction of treatment effect) among studies. We used the I² and Tau² statistics, and the Chi² test to quantify the level of heterogeneity among the studies in each analysis. We regarded moderate or substantial heterogeneity as Tau² > 0 and either I² > 30% or a low P value (< 0.10) in the Chi² test. If moderate or substantial heterogeneity were identified, we planned to explore it by prespecified subgroup effects analyses. Due to the limited number of studies and data obtained in this review, it was not possible to conduct sensitivity analyses to evaluate the potential impact of heterogeneity on findings.
Assessment of reporting biases
Where we suspected reporting bias (see 'Selective reporting bias' above), we attempted to contact study authors and requested them to provide data for missing outcomes. Where this was not possible, we considered if the missing data introduced serious bias; if so, we planned to conduct sensitivity analyses to examine the impact of including such studies in the overall assessment of results. Due to the limited number of studies and data obtained in this review, it was not possible to conduct sensitivity analyses.
We planned to generate funnel plots in RevMan 5 if more than 10 studies reporting the same outcome of interest met the inclusion criteria of the review (Review Manager 2014). We planned to visually examine them for asymmetry. When studies were pooled in meta‐analyses, we ordered studies in terms of weight, so that a visual examination of forest plots enabled us to assess whether the results from smaller and larger studies were similar, and if there were any apparent differences in the effect sizes between smaller and larger studies.
Data synthesis
We conducted meta‐analyses to provide an overall estimate of treatment effect when more than one study examined the same intervention, provided that included studies used similar methods, and measured the same outcome in similar ways in similar populations. We did not combine results from RCTs and non‐RCTs together in meta‐analyses, or pool estimates for non‐RCTs with different types of study designs. We considered that evidence on different outcomes may be available from different types of study designs (e.g. it is likely that larger non‐RCTs reported data on less common adverse events). Where there was evidence on a particular outcome from both RCTs and non‐RCTs, we planned to use the evidence from trials that were at lower risk of bias to estimate treatment effect.
In cases where there was evidence from several RCTs, or high‐certainty of evidence non‐RCTs, we conducted statistical analyses using RevMan 5 software (Review Manager 2014). We used a random‐effects meta‐analysis for combining data, as we anticipated that there was likely natural heterogeneity between studies attributable to the different doses, durations, populations, and implementation or delivery strategies. For continuous variables, we used the inverse variance method. For dichotomous variables, we used the method proposed by Mantel‐Haenszel.
For non‐RCTs, where results were adjusted for potential confounding factors, we planned to use the generic inverse variance method in RevMan 5 to conduct meta‐analyses (if included studies provided both adjusted and non‐adjusted figures, we planned to conduct sensitivity analyses using the unadjusted figures, to examine any potential impact on the estimate of treatment effect) (Review Manager 2014).
We also used narrative synthesis, guided by the data extraction form, to group and summarise data for studies and describe the outcomes, explore intervention processes, and describe the impact of interventions by sociodemographic characteristics known to be important from an equity perspective based on the PROGRESS framework (Ueffing 2011), where this information was available. Specifically, we described factors that determined the differential availability, accessibility, acceptability, and effective usage of fortified wheat or maize flour and maize meal among population groups and defined this using prespecified categories. We defined key areas of monitoring, to inform appropriate policy action to promote equity in access to these products. In addition, we described any financial issues related to the implementation of wheat or maize flour and maize meal fortification programmes, considering existing facilities, production, and considerations for implementation of fortifying wheat and maize flour and maize meal in settings with different levels of market development.
Findings from prospective studies without a control group, and uncontrolled before‐and‐after studies are summarised in Appendix 1.
'Summary of findings' tables
We summarised the body of evidence for dichotomous and continuous outcomes as recommended by the GRADE Working Group. We presented data in 'Summary of findings' tables for the primary outcomes (Guyatt 2013a; Guyatt 2013b), using GRADEprofiler software (GRADEpro GDT 2015).
We listed the primary outcomes for each comparison with estimates of relative effects, along with the number of participants and studies that contributed data for those outcomes. For each individual outcome, we assessed the certainty of the evidence using the GRADE approach (Balshem 2011). We downgraded the evidence from 'high certainty' by one level for serious (or by two for very serious) study limitations in risk of bias, directness of evidence, heterogeneity, precision of effect estimates, risk of publication bias, dose‐effect responses, magnitude of effects, and potential residual confounding. We expressed the certainty of evidence as one of four levels (i.e. high, moderate, low, or very low). We assessed certainty of evidence for the following primary outcomes: neural tube defects (i.e. total neural tube defects; anencephaly, spina bifida, encephalocoele, meningocele), erythrocyte folate, serum/plasma folate, haemoglobin, and anaemia.
Subgroup analysis and investigation of heterogeneity
Data did not allow us to conduct sensitivity or subgroup analysis, but the following analyses were planned (and will be considered in updates of this review where data allow).
Range of wheat or maize flour consumption patterns: less than 75 g/day, versus 75 g to 149 g/day, versus 150 g to 300 g/day, versus greater than 300 g/day.
Dose of folic acid in parts per million (ppm): less than 1.5 ppm versus 1.5 ppm to 4.99 ppm versus 5 ppm or more.
Length of intervention: less than six months, six months to 12 months, more than 12 months.
Baseline folate status (as defined by study authors): deficient versus non‐deficient or unknown/unreported.
Malaria endemicity at the time that the trial was conducted: malaria‐endemic setting versus non‐/unknown malaria setting.
Sensitivity analysis
We planned a priori to conduct sensitivity analyses to examine the potential effects of removing studies at high risk of bias (e.g. those with high or unclear risk of bias for allocation concealment, lack of similarity of baseline outcome measurements, or incomplete outcome data) from the analysis. When cluster‐RCTs met the inclusion criteria, we planned to conduct sensitivity analyses considering a range of intracluster correlation values. However, due to the limited number of studies and data obtained in this review, it was not possible to conduct sensitivity analyses for these findings.
Results
Description of studies
Results of the search
Results of the search are summarised in Figure 2 . A total of 1248 articles were reviewed for potential inclusion in this review; 10 studies were included and four of these studies provided data for quantitative analyses. Seven studies were conducted to examine the effects of wheat flour fortified with folic acid (i.e. alone or in combination with other vitamins and minerals) on health outcomes: one study of wheat flour fortified with folic acid alone (Green 2013), and six studies with wheat flour fortified with folic acid and other micronutrients (French 2003; Huo 2011; Huo 2012; Ionescu‐Ittu 2009; Rahman 2015; Wang 2016). Three studies were conducted to examine the effects of maize flour fortified with folic acid (i.e. alone or in combination with other micronutrients) on health outcomes: one study of maize meal fortified with folic acid alone (Colman 1974a), and two studies with maize flour fortified with folic acid and other micronutrients (Carrasco 2013; Sanchez 2011).
2.
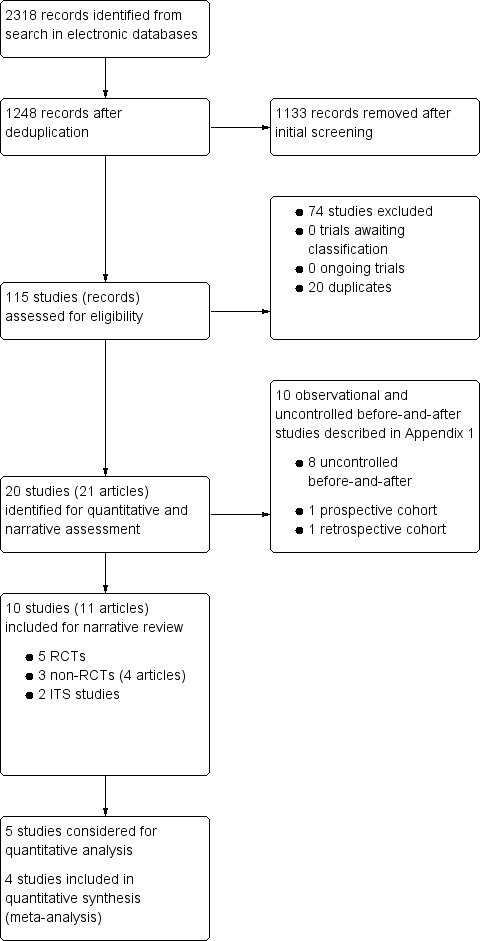
Flow diagram.
A total of four studies provided data for quantitative analyses (Colman 1974a; Green 2013; Rahman 2015; Sanchez 2011). Of the six studies which were not included in quantitative analyses, one study was eligible for inclusion (Carrasco 2013), but did not provide measures of dispersion for outcome data and was not included in the meta‐analyses; three of the studies were non‐randomised controlled trials (non‐RCTs) (Huo 2011; Huo 2012; Wang 2016), and two studies had an ITS design (French 2003; Ionescu‐Ittu 2009), and were not included in quantitative analyses. We identified 10 observational studies without a control group (i.e. participants served as their own control) and results are summarised in Appendix 1. We did not identify any ongoing studies or studies awaiting classification. A detailed description of included and excluded studies are provided in the 'Characteristics of included studies' and 'Characteristics of excluded studies' sections.
Included studies
Study designs
We included a total of seven studies of wheat flour fortified with folic acid (i.e. alone or in combination with other micronutrients) in this review: one study with randomisation at the cluster level (Rahman 2015), one study with randomisation at the individual level (Green 2013), three non‐RCTs (Huo 2011; Huo 2012; Wang 2016), and two ITS studies (French 2003; Ionescu‐Ittu 2009).
We included three studies of maize flour fortified with folic acid (i.e. alone or in combination with other micronutrients) in this review, with randomisation at the individual level (Carrasco 2013; Colman 1974a; Sanchez 2011).
Participants
The studies reporting outcomes for folic acid‐fortified wheat flour included: 45 adult men and women (18 to 45 years; Green 2013), 611 women (20 to 60 years; Huo 2011), 448 women (20 to 60 years; Huo 2012), 334 male and female children (5 to 12 years; Rahman 2015), and 23,685 women (Wang 2016); neural tube defects were reported in one study which included 8037 births (Wang 2016). Congenital heart defects were reported in one ITS study which reported incidence per 1000 births (Ionescu‐Ittu 2009). One ITS study reported incidence of childhood cancers among 1,953,296 children (0 to 17 years) at risk during the prefortification period and 289,142 children at risk during the postfortification period (French 2003).
Studies reporting outcomes for folic acid‐fortified maize flour were primarily conducted among women of reproductive age. These studies included 38 pregnant women (Colman 1974a), and 353 women who were not pregnant or lactating (i.e. 308 women 14 to 64 years of age (Carrasco 2013), and 45 women 12 to 45 years of age (Sanchez 2011)).
Baseline folate status
Among studies evaluating the effects of folic acid‐fortified wheat flour on health outcomes:
one study reported dietary intake of folate at baseline for the intervention (N = 16,648; mean ± SD 237.5 µg/day ± 76.4 µg/day) and control (N = 7037; mean ± SD 168.3 µg/day ± 72.1 µg/day) groups; however, serum folate concentrations were only reported at endline in this study (Wang 2016);
in another study (Green 2013), plasma and erythrocyte folate concentrations were reported at baseline in each of the three groups: wheat bread rolls fortified with folic acid (N = 14; mean ± SD plasma folate: 40 nmol/L ± 12 nmol/L; erythrocyte folate: 0.78 µmol/L ± 0.19 µmol/L), unfortified wheat bread rolls (N = 12; mean ± SD plasma folate: 36 nmol/L ± 9 nmol/L; erythrocyte folate: 0.89 µmol/L ± 0.23 µmol/L), and a third group consuming food products fortified with L‐5‐methyltetrahydrofyolate (L‐5MTHF) (N = 13; mean ± SD plasma folate: 39 nmol/L ± 10 nmol/L; erythrocyte folate: 0.78 µmol/L ± 0.16 µmol/L);
baseline folate status of participants was not reported in the other studies of folic acid‐fortified wheat flour (French 2003; Huo 2011; Huo 2012; Ionescu‐Ittu 2009; Rahman 2015).
Among studies examining the effects of folic acid‐fortified maize flour on health outcomes:
one study reported baseline erythrocyte and serum folate concentrations (assessed via Lactobacillus casei (L casei) microbiological method) in pregnant and lactating women (Colman 1974a). Baseline serum folate concentrations were not significantly different in the intervention (N = 20; mean 10.88 nmol/L) and control (N = 18; mean 11.78 nmol/L) groups;
in a RCT, baseline erythrocyte and plasma folate concentrations were reported in groups receiving folic acid‐fortified flour (N = 18; mean ± SD plasma folate: 0.017 pmol/L ± 0.004 pmol/L; erythrocyte folate: 552.5 nmol/L ± 143.8 nmol/L), unfortified flour (N = 17; mean ± SD plasma folate: 0.016 pmol/L ± 0.004 pmol/L; erythrocyte folate: 583.6 nmol/L ± 175.8 nmol/L), and folic acid supplementation (N = 10; mean ± SD plasma folate: 0.016 pmol/L ± 0.005 pmol/L; erythrocyte folate: 565.2 nmol/L ± 174.0 nmol/L) (Sanchez 2011);
baseline folate status was not reported in Carrasco 2013.
Interventions
Micronutrient composition
In one RCT (Green 2013), men and women consumed bread rolls made with wheat flour that was fortified with folic acid alone, unfortified bread rolls, or wheat flour fortified with L‐5MTHF. Two ITS studies in Canada reported data during the periods before and after initiation of fortification of wheat flour with folic acid (French 2003; Ionescu‐Ittu 2009). In the other four studies of fortified wheat flour (Huo 2011; Huo 2012; Rahman 2015; Wang 2016), wheat flour was fortified with folic acid in combination with other vitamins and minerals.
One maize flour fortification study included maize meal fortified with folic acid alone (Colman 1974a). Two randomised intervention studies included maize flour fortified with folic acid in combination with other vitamins and minerals (Carrasco 2013; Sanchez 2011).
Doses of folic acid in flour
The dose of folic acid administered in fortified flour varied across studies included in this review from 0.5 ppm to 33 ppm.
Wheat flour
In one RCT (Green 2013), wheat flour was fortified with 400 micrograms of folic acid per bread roll (approximately 11.1 ppm).
In three non‐RCTs in rural China, wheat flour was fortified with the following amount of folic acid per kilogram of flour: 1 milligram (1 ppm) (Huo 2011), 1.5 milligrams (1.5 ppm) (Huo 2012), or 2 milligrams (2 ppm) (Wang 2016).
In a cluster‐RCT in Bangladesh (Rahman 2015), wheat flour chapattis (i.e. flat breads) were fortified with 0.15 milligrams of folic acid per 100 grams of flour (1.5 ppm), along with other micronutrients.
The dosage was not specified in two ITS studies (French 2003; Ionescu‐Ittu 2009).
Maize flour
In a RCT of folic acid‐fortified maize flour in South Africa (Colman 1974a), pregnant women (N = 15) received a daily serving of 30 grams (dry weight) of maize meal porridge fortified with approximately 300 micrograms of folic acid per serving; maize meal was fortified with 1 milligram of crystalline folic acid per 30 kilograms of maize meal prior to cooking (33 ppm).
In another RCT (Sanchez 2011), women received maize flour fortified with 0.05 milligrams of folic acid per 110 grams of flour (0.5 ppm), along with other micronutrients. Participants were asked to consume fortified flour in the form of eight tortillas per day.
In the RCT by Carrasco et al, participants received 20 kilograms of maize flour per month, that contained 548 micrograms of folic acid per 100 grams of flour (5.48 ppm), along with other micronutrients (Carrasco 2013).
Duration of intervention
Interventions of folic acid‐fortified wheat flour ranged in duration from 16 weeks in Green 2013 to 36 months in Huo 2011 and Huo 2012. The RCT in Canada of folic acid‐fortified wheat bread rolls was conducted over a 16‐week period (Green 2013). The cluster‐RCT in Bangladesh among children was conducted over a six‐month period (Rahman 2015). The intervention studies in rural China were conducted over 34 months in Wang 2016 and 36 months in Huo 2011 and Huo 2012. The postintervention periods for the ITS studies were up to two years in French 2003 and seven years in Ionescu‐Ittu 2009.
In studies of folic acid‐fortified maize flour, interventions ranged in duration from 26.4 days in Colman 1974a to 10 months in Carrasco 2013. The Colman 1974a intervention study of folic acid‐fortified maize flour in South Africa was conducted from enrolment until delivery, or an average duration of 26.4 days. In contrast, in two RCTs, participants consumed maize flour fortified with folic acid and other vitamins and minerals for a period of three months in Sanchez 2011 and 10 months in Carrasco 2013.
Comparison groups
Several intervention studies were conducted to determine the effects of wheat flour fortified with folic acid and other micronutrients compared to unfortified wheat flour (Huo 2011; Huo 2012; Rahman 2015; Wang 2016). Additionally, in one RCT, the effects of consuming folic acid‐fortified wheat bread rolls (and microencapsulated L‐5MTHF‐fortified bread rolls) were compared to unfortified wheat bread rolls (Green 2013). In two ITS studies, data from the postfortification (folic acid‐fortified wheat flour) period were compared to the prefortification periods (French 2003; Ionescu‐Ittu 2009).
One RCT was conducted to determine the effects of maize flour fortified with folic acid and other vitamins and minerals (Carrasco 2013), compared to unfortified maize flour. Another RCT included three intervention groups (Sanchez 2011): maize flour fortified with folic acid and other micronutrients compared to unfortified maize flour or weekly folic acid supplementation. In the intervention study in South Africa (Colman 1974a), researchers compared maize meal fortified with folic acid alone to no intervention.
None of the included studies evaluated the following comparisons.
Wheat flour
Wheat flour or wheat flour products fortified with folic acid alone versus no intervention
Wheat flour or wheat flour products fortified with folic acid plus other vitamins and minerals versus no intervention
Maize flour
Maize flour or maize flour products fortified with folic acid alone versus unfortified maize flours or maize flour products (not containing folic acid or any other vitamins or minerals)
Maize flour or maize flour products fortified with folic acid plus other vitamins and minerals versus no intervention
Combined ‐ wheat and maize flour
Wheat and maize flour or products fortified with folic acid alone versus no intervention
Wheat and maize flour or products fortified with folic acid plus other vitamins and minerals versus no intervention
Wheat and maize flour or products fortified with folic acid alone versus unfortified wheat and maize flours or flour products (not containing folic acid or any other vitamin or minerals)
Wheat and maize flour or products fortified with folic acid plus other vitamins and minerals versus unfortified wheat and maize flours or flour products (not containing folic acid or any other vitamin or minerals)
Settings
Most of the studies included in this review were conducted in upper‐middle or lower‐middle‐income countries. Six studies were conducted in upper‐middle‐income countries, namely China (Huo 2011; Huo 2012; Wang 2016), Mexico (Carrasco 2013; Sanchez 2011), and South Africa (Colman 1974a); one study was conducted in a lower‐middle‐income country (Bangladesh; Rahman 2015); and three studies were conducted in a high‐income country (Canada; Green 2013; French 2003; Ionescu‐Ittu 2009).
The equity characteristics of the 10 included studies are described in Table 6. Most of these studies were conducted in rural settings, among participants of marginalised or lower socioeconomic status. Most of these studies were conducted among women of reproductive age, and some included pregnant or lactating women as well as children. However, none of the studies examined the potential implications of racial or ethnic, cultural, or religious factors for the prevalence of folate deficiency or other health outcomes; the impact of social inequalities or their determinants were also not analysed in any of these studies.
1. PROGRESS‐Plus equity checklist of included studies.
| Study | Place | Race/ethnicity | Occupation | Gender |
Religion/ culture/education |
Socioeconomic status | Social status |
Others/ disability/ age/ sexual orientation |
Overall PROGRESS‐Plus |
| Carrasco 2013 | Huejutla de Reyes (Hidalgo), Atlacomulco (Estado de México) and Huatusco (Veracruz) rural counties in Mexico | Mexican women including indigenous (speak a language other than Spanish) and non‐indigenous (speak only Spanish) | Not reported | Female | Not reported | Communities are classified as highly marginalised and rural (less than 2500 inhabitants) by the Consejo Nacional de Poblacion | Highly marginalised | Women were 14 to 64 years of age. No details were provided on sexual orientation or disability | The study enrolled 308 rural indigenous (speak a language other than Spanish) and non‐indigenous (speak only Spanish) Mexican women 14 to 64 years of age from highly marginalised communities |
| Colman 1974a | Charles Johnson Memorial Hospital at Nqutu, KawaZulu, Bantu homeland, South Africa, as reported in the paper | African women | Not reported | Female | Not reported | Not reported | Not reported. The study reports that women were encouraged to lodge at the hospital during the last month of pregnancy to avoid hazards of long travel after onset of labor, but there is no indication of social status | Age, disability, or sexual orientation were not reported. All women were pregnant | The study enrolled 45 African pregnant women lodging in a South African hospital during their last month of pregnancy |
| French 2003 | Participants were identified using a hospital database in Ontario, Canada | Not specified | Not reported | Not reported | Not reported | Not reported | Not reported | Participants were 17 years of age or younger | This study assessed the incidence of childhood cancers among 1,953,296 children at 55 quarterly periods before fortification and 289,142 children at 9 quarterly periods after fortification |
| Green 2013 | Participants were recruited from Vancouver, Canada, through advertisement and word‐of‐mouth | Of the 45 enrolled participants; more than 70% of participants were non‐white, with 14 identifying as Chinese, 12 as other Asian, and 7 as other | Not reported | 19 male and 26 female participants | 21 participants had some college/university education and 24 completed college/university | Not reported | Not reported | The age of the participants was 25.6 ± 6.3 years, with the majority (70%) < 30 years | This study enrolled 45 participants; 19 male and 26 female, aged 25.6 ± 6.3 years old to consume wheat bread rolls made with wheat flour fortified with folic acid, L‐5‐MTFH or placebo |
| Huo 2011 | Weichang, a county in Hebei province, located in the northwest of China | Not specified | The study refers to all participants as farmers | The intervention, fortified wheat flour was provided to be consumed at household level, however the study assessments were performed on women | Not reported | Not specifically reported but the intervention was undertaken in rural farm areas | Rural farm areas | Age was reported only for the sample of women included in study assessments: 37.7 ± 7.8 years. Disabilities or sexual orientation were not reported |
This study included 700 farmers in 1233 households receiving fortified wheat flour and 2750 farmers in 751 households receiving unfortified wheat flour. Flour was provided at the household level but outcomes were measured in women of reproductive age (319 women, aged 37.7 ± 7.8 years). This study was part of a reforestation programme where farmers in west provinces could voluntarily reforest or regrass their land with very low production in exchange for grain from the central government |
| Huo 2012 | Lanzhou, a city in Gansu Province, located in the Northwest of China. Aitou Village, suburb of Lanzhou as the intervention village, Ershilipu as a control village | Not specified | The study refers to all participants as farmers | Fortified wheat flour was provided to be consumed at household level; however, the serological assessments were performed on women | The control group had an average of 6.8 ± 2.2 years of education while the intervention group had 6.5 ± 2.2 years of education | The family income was 2348 ± 324 RMB in the control group and 2550 ± 259 RMB in the intervention group 1 RMB = USD 0.16 |
Rural farm areas | Age was reported only for the sample of women included in the study assessments: 37.7 ± 7.8 years. Disabilities or sexual orientation were not reported |
In this study, villages received fortified or unfortified wheat flour as part of government reforestation programme. The population was described as farmers and the wheat flour was distributed for household level consumption; however, the study assessments were performed only on women aged 37.7 ± 7.8 years with 6.5 years of education |
| Ionescu‐Ittu 2009 | Participants were from Quebec, Canada | Not specified | Not reported | Not reported | Not reported | Not reported | Not reported | Age was not reported for the mothers of the infants born with congenital heart defects. Disabilities or sexual orientation were not reported | This study assessed the prevalence of congential heart defects at 9‐yearly periods before and 7‐yearly periods after fortification |
| Rahman 2015 | 7 out of the total 16 unions (approximately 65 villages) of Mirsarai subdistrict in the south‐eastern part of Bangladesh. Composed of baris (usually containing 5 to 6 adjoining households with a population of about 30 to 35 relatives) | Not specified | This study included children. Occupation of parents or caregivers was not described | 95 male and 96 female in the intervention group. 74 male and 69 female in the control group | Not reported | Not reported | Not reported | Children's age was 10.4 ± 2.68 years in the intervention group and 10.3 ± 2.86 years in the control group | This study enrolled 334 male and female children of a mean age of 10.3 years old. Children received wheat fortified flour or unfortified flour to be consumed as chapattis |
| Sanchez 2011 | Dr Arroyo municipality, Nuevo León state, Mexico | Not specified | Not specified | Female | Not reported | The study area was rural and of low socioeconomic status according to the CONAPO‐INEGI classification | Rural areas | Enrolled women were 12 to 45 years of age. Disabilities or sexual preferences were not reported | This study enrolled 45 women of 12 to 45 years old to receive fortified maize flour, unfortified maize flour, or folic acid supplements. Women were residents of rural areas of low socioeconomic status |
| Wang 2016 | Three counties in Shanxi Province, China. Eight villages from Zhongyang and Jiaokou Counties, representing the intervention group, and three villages from Liulin County, representing the control group | Not specified | Not specified | Female | In the control group, from 7037 women, 17.1% had primary education or below, while 57.7% had Junior high school, 16.3% senior high school and 8.9% college or below. In the intervention group, from 16648 women, 12.2% had primary school education or below, 61.1% had junior school education, 17.4% senior school and 9.3% college or below |
The areas were reportedly poor and rural | Poor, rural areas | The age in the control group was 26.3 ± 4.6 years and in the intervention group was 26.4 ± 5.2 years. The area was reported to have high prevalence of NTDs. Other disabilities or sexual preferences were not reported |
In this study, 16,648 women were recruited as the intervention group and 7260 women as the control group, from rural, poor villages in China. Women received fortified or unfortified wheat flour. The level of education was at least primary education, and the area had high prevalence of NTDs |
RMB: Chinese Yuan L‐5‐MTFH: L‐5‐methyltetrahydrofyolate NTD: Neural tube defect
Wheat or maize flour consumption patterns
Wheat flour consumption was reported in three of the included studies. One study reported an average consumption of 117 grams of fortified wheat flour per person per day during the three‐year intervention period (Huo 2011), with no significant differences in flour consumption reported between the intervention and control groups. In another study conducted in rural China (Huo 2012), average flour consumption was 215.8 grams (SD 133.0) per person per day in the intervention village during the 36‐month follow‐up period. In the Wang 2016 study, wheat flour consumption was reported at baseline and endline in the intervention (baseline: mean 405.1 g/day; endline: mean ± SD 390.1 g/day ± 60.9 g/day) and control (baseline: mean 309.29 g/day; endline: mean ± SD 314.8 g/day ± 46.2 g/day) groups. Four studies did not report consumption of wheat flour (French 2003; Green 2013; Ionescu‐Ittu 2009; Rahman 2015).
Maize flour consumption was not reported in the included studies (Carrasco 2013; Colman 1974a; Sanchez 2011), apart from noting that maize is a staple food in South Africa (Colman 1974a).
A limited number of the studies reported dietary intake of folic acid, folate, or folate‐rich foods. Wang 2016 reported dietary intake of folate at baseline (237.5 ± 76.4 µg/day and 168.3 ± 72.1 µg/day for intervention and control groups, respectively). Two studies reported intake of food groups (Huo 2011; Huo 2012), which included sources of folate (i.e. vegetables, fruits, beans). Sanchez 2011 reported folic acid intake at baseline (441.4 µg/day ± 140.8 µg/d and 542.6 µg/day ± 222.1 µg/day for intervention and control groups, respectively).
Malaria endemicity at the time of the trial
Malaria endemicity or malaria infection were not reported in any of the included studies. Canada was the only study setting (French 2003; Green 2013; Ionescu‐Ittu 2009), which has no reported malaria endemicity. There are limited data available for malaria endemicity at the time of the other intervention studies. Based on available information at the country level (WHO 2015c), malaria is prevalent in South Africa and was widespread at the time the Colman 1974a study was conducted. Mexico is currently in the pre‐elimination phase of malaria (Carrasco 2013; Sanchez 2011; WHO 2015c), Bangladesh has had more than a 75% decrease in the incidence of malaria cases between 2000 and 2014 (Rahman 2015), and China is currently in the malaria elimination phase (Huo 2011; Huo 2012; Wang 2016; WHO 2015c). However, specific data regarding malaria endemicity were not provided in the settings at the time when these studies were conducted.
Supervision and Cointerventions
Participants in one study received folic acid‐fortified wheat bread rolls each week and were instructed to eat one roll per day (Green 2013); however, wheat bread roll consumption was not supervised or recorded. In two studies conducted in different villages in rural China (Huo 2011; Huo 2012), the amount of fortified flour allocated was recorded in one notebook per household, and a dietary survey was conducted every six months to assess flour consumption throughout the three‐year study period. In another study (Wang 2016), women receiving fortified wheat flour were asked to record their daily consumption of flour using a dietary survey, which was validated via monthly in‐person interviews. In a study in Bangladesh (Rahman 2015), daily consumption of folic‐acid fortified flatbread was supervised and recorded by a designated person residing in the selected localities. In the two ITS studies, consumption was not observed or recorded (French 2003; Ionescu‐Ittu 2009).
In a RCT (Sanchez 2011), participants were requested to record maize flour intake in a daily diary, which was reviewed by study staff on a monthly basis. In a study in Mexico (Carrasco 2013), consumption of fortified maize flour was evaluated at three time points during the 10‐month study period; however, intake of fortified flour was not directly supervised or recorded. In the Colman 1974a study, pregnant women resided at the hospital from enrolment to delivery, where they received maize porridge prepared with fortified flour.
Setting and healthworker cadre
One study was conducted in a hospital in a rural area in South Africa (Colman 1974a), where pregnant women were recommended to reside during the last month of pregnancy and early postpartum period. Other studies included in this review were conducted in community‐based settings: five studies were conducted in rural communities (Huo 2011; Huo 2012; Rahman 2015; Sanchez 2011; Wang 2016), one study was conducted among indigenous women in Mexico (Carrasco 2013), and one study provided fortified wheat bread rolls to participants to be consumed at home (Green 2013). The two ITS studies were population‐based studies (French 2003; Ionescu‐Ittu 2009).
Outcomes
Seven of the included studies were conducted to evaluate the effects of consuming folic acid‐fortified wheat flour on health outcomes. Erythrocyte and plasma folate concentrations were assessed with an L casei microbiological method in one study (Green 2013). Another study assessed plasma folate and homocysteine concentrations (Wang 2016), but the methods used were not described. In two studies conducted in non‐pregnant women in China, serum folate was measured with an AXSYM kit (Huo 2011; Huo 2012). Three studies assessed haemoglobin concentrations: two studies reported haemoglobin concentrations as assessed by the HemoCue method (HemoCue 2019) (Huo 2011; Huo 2012), and another study used the methaemoglobin method to assess haemoglobin concentrations (Rahman 2015). Anaemia was defined as a haemoglobin concentration less than 115 g/L for children less than 12 years of age and less than 120 g/L for children aged 12 to 15 years (Rahman 2015). In studies among women of reproductive age, one study defined anaemia as haemoglobin concentration less than 120 g/L (Huo 2012); however, the cut‐off for anaemia was not defined in another study by the same authors (Huo 2011). Wang 2016 reported homocysteine concentrations assessed in women and the occurrence of neural tube defects, including anencephaly, spina bifida, encephalocoele, and total neural tube defects, which were evaluated based on their respective ICD‐10 codes (WHO 2019). In one ITS study (Ionescu‐Ittu 2009), the occurrence of congential heart defects was reported, including tetralogy of Fallot, endocardial cushion defects, univentricular hearts, truncus arteriosus, and transposition complex. In the other ITS study (French 2003), the incidence of childhood cancers were reported, including neuroblastoma, acute lymphoblastic leukaemia, and hepatoblastoma using the Pediatric Oncology Group of Ontario registry (POGO 2019). Three studies of folic acid‐fortified maize flour provided data for a priori outcomes in this review. One study reported erythrocyte folate concentrations assessed using the L casei microbiological method (Colman 1974a). In another study (Sanchez 2011), erythrocyte and plasma folate concentrations were assessed by solid‐phase radioimmunoassay; and erythrocyte folate concentrations were calculated by multiplying the data from the standard curve with a dilution factor, and dividing the product by the haematocrit value. One study reported haemoglobin concentration (Carrasco 2013), as assessed by the HemoCue method (HemoCue 2019).
Funding
Studies contributing data to this systematic review were funded by university, government, or international organisations. One study was funded by the Department of Genetics and Endocrinology Services at the Universidad Autónoma de Nuevo León, Mexico (Sanchez 2011). Another study was funded by a government organisation, DICONSA, previously CONASUPO (Compañía Nacional de Subsidios Populares) (Carrasco 2013). One study received funding from Peking University, national and state level grants (National Key Project and State Key Funds of Social Science Project), and international organisations (UNFPA/CPA) (Wang 2016). Two of the studies received funds from UNICEF and the US Centers for Disease Control and Prevention (CDC) (Huo 2011; Huo 2012). One study was funded by USAID (Rahman 2015), and one study was funded by the Advanced Food Materials Network (Green 2013). In one study (Colman 1974a), the fortified maize flour was provided by Upjohn Pharmaceuticals. One of the ITS studies was funded by the Heart and Stroke Foundation of Canada and the Fonds de Recherche en Santé du Québec (FRSQ) (Ionescu‐Ittu 2009); and the other ITS study did not report the source of funding (French 2003).
Excluded studies
A total of 74 studies were excluded (Figure 2). The main reasons for exclusion were that the intervention did not include wheat or maize flour that was fortified with folic acid (i.e. alone or with other micronutrients); or studies examined the effects of folic acid‐fortified breakfast cereals, which were outside the scope of this review. A detailed description of all excluded studies is provided in the table, 'Characteristics of excluded studies', along with reasons for exclusion.
Risk of bias in included studies
We used the standardised domains for ‘Risk of bias’ to evaluate all intervention studies, including individual‐ and cluster‐randomised design, as well as non‐RCTs and studies with an ITS design. We also included potential bias related to cluster‐randomisation in the 'Risk of bias' table for this study design in the Characteristics of included studies section, and a summary of the 'Risk of bias' analyses is depicted in Figure 3 and Figure 4.
3.
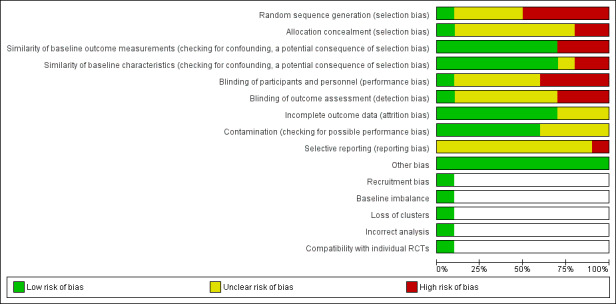
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
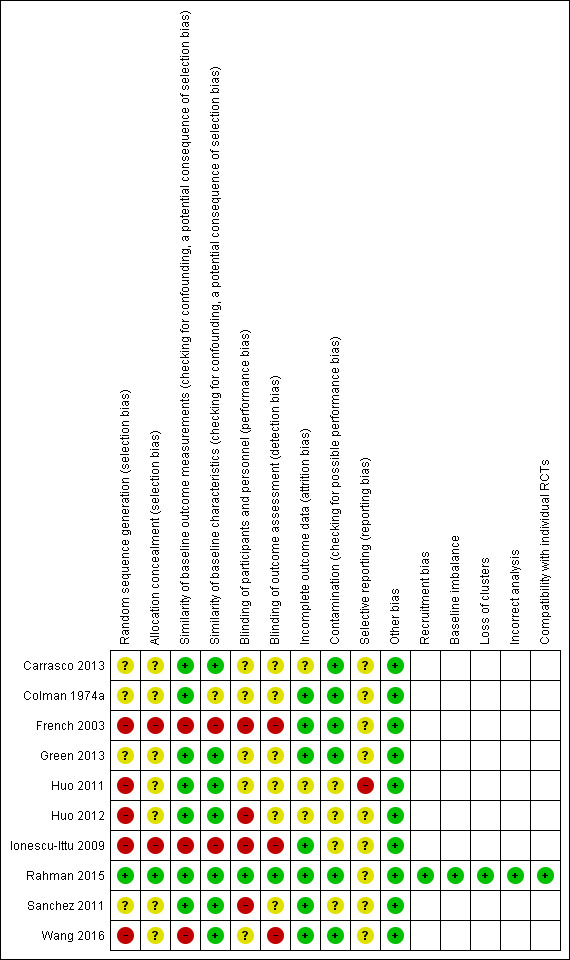
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
One cluster‐randomised study used random number tables to allocate the intervention (Rahman 2015) and we scored this to be a low risk of bias. Other studies were scored as unclear risk of bias since they mentioned randomisation of participants but did not describe the methods used for sequence generation or randomisation (Carrasco 2013; Colman 1974a; Green 2013; Sanchez 2011). Three studies were non‐RCTs (Huo 2011; Huo 2012; Wang 2016) and therefore, were scored as high risk of bias. The two ITS studies were scored as high risk of bias for sequence generation since they did not conduct randomisation or report data on an individual level (French 2003; Ionescu‐Ittu 2009).
Allocation concealment
In one cluster‐randomised study, investigators reported that the intervention (fortified flour) and control (unfortified flour) bags of flour were indistinguishable (Rahman 2015) we scored this as low risk of bias, although specific information on allocation concealment was not provided. None of the other included studies reported methods for allocation concealment and were scored as unclear risk of bias, Two ITS studies were scored as high risk of bias for allocation concealment since there was no intervention or group assignment to conceal (French 2003; Ionescu‐Ittu 2009).
Similarity of baseline characteristics
Six studies showed similar baseline outcome measurements among the included groups, we scored this as a low risk of selection bias (Carrasco 2013; Colman 1974a; Green 2013; Huo 2011; Huo 2012; Rahman 2015; Sanchez 2011). These studies also showed similar baseline characteristics, However, one study (Colman 1974a) was scored as unclear risk of bias since baseline characteristics were limited to outcome measurements. Another study, (Wang 2016) was scored as high risk of selection bias since none of the outcomes of interest were measured at baseline: serum folate, homocysteine concentrations, and neural tube defects but low risk of bias in baseline characteristics since there were similar among the study groups. Two studies (French 2003; Ionescu‐Ittu 2009) were scored as high risk of bias for similarity of baseline outcome and similarity of baseline characteristics since there were interrupted time series with no participants selection
Blinding
In one randomised controlled trial (Rahman 2015), participants and investigators were blinded to the type of flour used throughout the duration of the study and data analyses therefore, it was scored as low risk of performance and detection bias. However, other included studies did not report how blinding was ensured, we scored them as unclear risk of bias (Carrasco 2013; Colman 1974a; Green 2013; Huo 2011 ; Rahman 2015; ). However, two studies were scored as high risk of performance since, the fortified flour assigned to the intervention group was labelled with the nutrients and nutrient concentrations added to the flour (Huo 2012), and no measures were reported to blind towards the use of tablets supplements by one of the intervention groups (Sanchez 2011). Lack of blinding was unlikely to affect ascertainment of laboratory‐assessed serological outcomes, such as erythrocyte or serum folate concentrations, which were the main endpoints reported by these studies in the meta‐analyses. Two studies (French 2003; Ionescu‐Ittu 2009) were scored as high risk of bias since there were interrupted time series with intervention or outcome assessment to blind for.
Incomplete outcome data
Some of the included studies did not report data on attrition (Carrasco 2013; Huo 2011; Sanchez 2011) and were scored as unclear risk of attrition bias. It was unclear if all of the participants completed the studies, what methods were used to identify and report participants who were lost to follow‐up, or if attrition rates were similar in intervention and control groups. Three studies were scored as low risk of incomplete outcome data. Two of the studies reported low attrition rates (Green 2013; Wang 2016). One study was conducted among patients residing at a hospital (Colman 1974a), all of the participants completed the trial. Highattrition was reported in one study and it was unclear if this contributed to risk of bias (Huo 2012).. The two ITS studies had a low risk of attrition bias: in one study the cases were captured by administrative databases with full coverage of births in Quebec (Ionescu‐Ittu 2009), and in one study the database used for outcome assessment captured 95% of all paediatric cancers in Ontario (French 2003).
Selective reporting
The protocols were not provided or available electronically for most of the studies included in this review; this precluded assessment of selective reporting [i.e. reporting bias; rated unclear in 9 studies] (Carrasco 2013; Colman 1974a; Green 2013; Sanchez 2011; Huo 2012; Rahman 2015; Wang 2016). . One study (Huo 2011) was scored as high risk of selective reporting bias, since serum folate concentrations assessment was mentioned in the methods but no data were presented..
Other potential sources of bias
Additional criteria for sources of bias in cluster‐randomised studies (i.e. recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, compatibility with individual RCTs) were evaluated and determined to be low for the cluster‐randomised study included in the review (Rahman 2015). Other sources of bias were not apparent in the remaining studies included in this review. The remaining studies were scored as low risk of other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Seven of the studies included in this review examined the effects of folic acid‐fortified wheat flour on health outcomes, and three studies examined the effects of folic acid‐fortified maize flour on health outcomes. A total of four studies reported data that we included in quantitative analyses.
We described studies without measures of dispersion but did not include them in the quantitative analyses. In studies with more than two intervention groups, all arms were not necessarily included in analyses. We presented results by type of flour that was fortified (i.e. wheat or maize flour), comparison groups, and primary and secondary outcomes. Most of the included studies focused on serological indicators (i.e. erythrocyte folate, plasma or serum folate, or haemoglobin concentrations). Among the non‐RCTs, only one study each reported data for the following outcomes: occurrence of congenital heart defects, neural tube defects, and childhood cancers. No studies reported on the occurrence of any adverse events. The included studies did not contribute to all of the comparisons that were included in the protocol, and some of the a priori outcomes were informed by only one study. Based on the limited number of studies and the heterogeneity across the studies, we used random‐effects models in all meta‐analyses. There was not sufficient information to undertake the subgroup analyses identified a priori for this review.
Maize flour or maize flour products fortified with folic acid alone versus no intervention
Primary outcomes
Neural tube defects
No included RCTs reported data on neural tube defects.
Erythrocyte folate
RCTs
One study contributed data for erythrocyte folate (Colman 1974a). Pregnant women who received folic acid‐fortified maize porridge had significantly higher erythrocyte folate concentrations at endline (i.e. delivery) (N = 20; mean ± SD 589.00 nmol/L ± 151.6 nmol/L), compared to pregnant women who received a regular hospital diet (N = 18; mean ± SD 350.10 nmol/L ± 129.82 nmol/L) (mean difference (MD) 238.90 nmol/L, 95% confidence interval (CI) 149.40 to 328.40; 1 study, 38 participants; very low‐certainty evidence; Analysis 1.1). We downgraded the certainty of evidence once for high risk of bias,and twice for imprecision. Due to the limited number of studies, heterogeneity was not applicable.
1.1. Analysis.
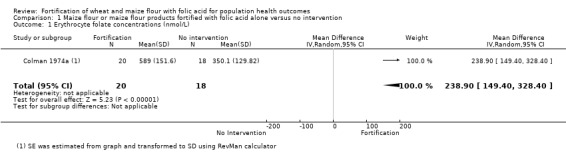
Comparison 1 Maize flour or maize flour products fortified with folic acid alone versus no intervention, Outcome 1 Erythrocyte folate concentrations (nmol/L).
Serum/plasma folate
RCTs
One study contributed data for serum folate (Colman 1974a). Pregnant women receiving folic acid‐fortified maize porridge had significantly higher serum folate concentrations at the end of the study period (N = 20; mean ± SD 22.66 nmol/L ± 10.15 nmol/L), compared to the control group (N = 18; mean ± SD 7.68 nmol/L ± 6.45 nmol/L) (MD 14.98 nmol/L, 95% CI 9.63 to 20.33; 1 study, 38 participants; very low‐certainty evidence; Analysis 1.2). We downgraded the certainty of evidence once for high risk of bias and twice for imprecision. Due to the limited number of studies, heterogeneity was not applicable.
1.2. Analysis.

Comparison 1 Maize flour or maize flour products fortified with folic acid alone versus no intervention, Outcome 2 Serum folate concentrations (nmol/L).
Anaemia
No studies reported data on anaemia.
Haemoglobin
No studies reported data on haemoglobin.
Any type of cancer
No studies reported data on any type of cancer.
Childhood cancers
No studies reported data on childhood cancers.
Low birth weight
No studies reported data on low birth weight.
Other adverse pregnancy outcomes
No studies reported data on other adverse pregnancy outcomes.
Cognitive function/decline
No studies reported data on cognitive function or decline.
Secondary outcomes
No studies reported data for secondary outcomes in this comparison.
Maize flour or maize flour products fortified with folic acid plus other vitamins and minerals versus no intervention
No studies contributed data for this comparison.
Maize flour or maize flour products fortified with folic acid alone versus unfortified maize flours or maize flour products (not containing folic acid nor any other vitamins and minerals)
No studies contributed data for this comparison.
Maize flour or maize flour products fortified with folic acid plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing folic acid nor any other vitamins and minerals)
Primary outcomes
Neural tube defects
No studies reported data on neural tube defects.
Erythrocyte folate
RCTs
One study reported data for erythrocyte folate (Sanchez 2011). Women who received fortified maize flour had increased erythrocyte folate concentrations at endline compared to baseline (N = 18; mean ± SD 577.8 nmol/L ± 132.9 nmol/L at endline versus 552.5 nmol/L ± 143.8 nmol/L at baseline). Women who received unfortified maize flour (i.e. without folic acid or any other micronutrients) also had an increase in erythrocyte folate concentrations during the study (N = 17; mean ± SD 639.6 nmol/L ± 141.8 nmol/L at endline versus 583.6 nmol/L ± 175.8 nmol/L at baseline). However, there were no significant differences in erythrocyte folate concentrations between the intervention and control groups (MD ‐61.80 nmol/L, 95% CI ‐152.98 nmol/L to 29.38 nmol/L; 1 study, 35 participants; low‐certainty evidence; Analysis 2.1). We downgraded the certainty of evidence once for risk of bias and twice for imprecision. Due to the limited number of studies, heterogeneity was not applicable.
2.1. Analysis.
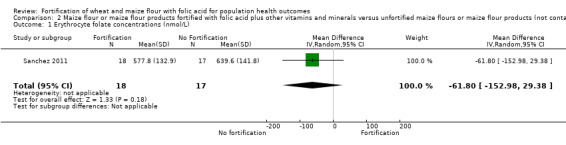
Comparison 2 Maize flour or maize flour products fortified with folic acid plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing folic acid nor any other vitamins and minerals), Outcome 1 Erythrocyte folate concentrations (nmol/L).
Serum/plasma folate
RCTs
One study contributed data for plasma folate (Sanchez 2011). During a three‐month intervention, women who consumed fortified maize flour had a significant increase in plasma folate concentrations (N = 18; mean ± SD 0.022 pmol/L ± 0.006 pmol/L at endline versus 0.017 pmol/L ± 0.004 pmol/L at baseline). Women in the control group who received unfortified maize flour also had an increase in plasma folate concentrations during the follow‐up period (N = 17; mean ± SD 0.020 pmol/L ± 0.003 pmol/L at endline versus 0.016 pmol/L ± 0.004 pmol/L at baseline). However, there were no significant differences in plasma folate concentrations between the intervention and control groups (MD 0.00 nmol/L, 95% CI ‐0.00 nmol/L to 0.00 nmol/L; 1 study, 35 participants; low‐certainty evidence; Analysis 2.2). The units (pmol/L) reported in the original study were confirmed by the study authors via correspondence. We downgraded the certainty of evidence once for risk of bias and twice for imprecision. Due to the limited number of studies, heterogeneity was not applicable.
2.2. Analysis.
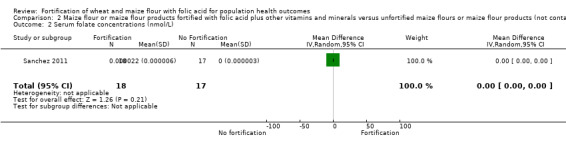
Comparison 2 Maize flour or maize flour products fortified with folic acid plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing folic acid nor any other vitamins and minerals), Outcome 2 Serum folate concentrations (nmol/L).
Anaemia
No studies reported data on anaemia.
Haemoglobin
RCTs
One study reported data for haemoglobin concentrations (Carrasco 2013). During the six‐month intervention, women who consumed fortified maize flour had a significant increase in haemoglobin concentrations (N = 155, mean 133 g/L at endline versus 131 g/L at baseline). However, this study did not report any measures of dispersion, and could not be included in quantitative analyses.
Any type of cancer
No studies reported data on any type of cancer.
Childhood cancers
No studies reported data on childhood cancers.
Low birth weight
No studies reported data on low birth weight.
Other adverse pregnancy outcomes
No studies reported data on other adverse pregnancy outcomes.
Cognitive function/decline
No studies reported data on cognitive function or decline.
Secondary outcomes
No studies reported data for secondary outcomes in this comparison.
Wheat flour or wheat flour products fortified with folic acid alone versus no intervention.
No studies contributed data for this comparison.
Wheat flour or wheat flour products fortified with folic acid plus other vitamins and minerals versus no intervention
Primary outcomes
Neural tube defects
No studies reported data on neural tube defects.
Erythrocyte folate
No studies reported data on erythrocyte folate.
Serum/plasma folate
No studies reported data on serum/plasma folate.
Anaemia
No studies reported data on anaemia.
Haemoglobin
No studies reported data on haemoglobin.
Any type of cancer
No studies reported data on any type of cancer.
Childhood cancers
Other study designs
One ITS study reported data on the incidence of childhood cancers (i.e. neuroblastoma, hepatoblastoma, infant acute lymphoblastic leukaemia) before and after initiation of folic acid fortification of wheat flour in Canada (French 2003). This study reported a significant reduction in the occurrence of neuroblastoma from 1.57 cases per 10,000 to 0.62 cases per 10,000; there were no significant changes in the occurrence of hepatoblastoma or infant acute lymphoblastic leukaemia (1 ITS study; 2,242,438 children at risk).
Low birth weight
No studies reported data on low birth weight.
Other adverse pregnancy outcomes
Other study designs
One ITS study reported data on the incidence of congenital heart defects before and after initiation of folic acid fortification of wheat flour in Canada (Ionescu‐Ittu 2009). This study reported there were no significant changes in the occurrence of congenital heart defects during the period before fortification, and a 6.2% decrease per year in the incidence of congenital heart defects during the period after fortification was initiated (1 study; sample size not reported).
Cognitive function/decline
No studies reported data on cognitive function or decline.
Secondary outcomes
No studies reported data on secondary outcomes.
Wheat flour or wheat flour products fortified with folic acid alone versus unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals)
Primary outcomes
Neural tube defects
No studies reported data on neural tube defects.
Erythrocyte folate
RCTs
One study reported data for erythrocyte folate (Green 2013). Adult women and men (18 to 45 years) who received folic acid‐fortified wheat bread rolls had significantly higher erythrocyte concentrations at endline, compared to the control group that received unfortified wheat bread rolls (MD 0.66 nmol/L, 95% CI 0.13 nmol/L to 1.19 nmol/L; 1 study, 30 participants; very low‐certainty evidence; Analysis 3.1). We downgraded the certainty of evidence once for directness and twice for imprecision. Due to the limited number of studies, heterogeneity was not applicable.
3.1. Analysis.
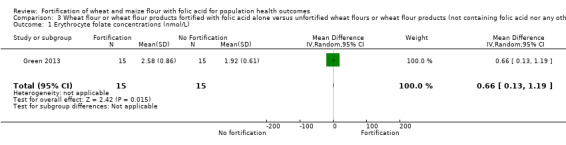
Comparison 3 Wheat flour or wheat flour products fortified with folic acid alone versus unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals), Outcome 1 Erythrocyte folate concentrations (nmol/L).
In the third arm of this study, participants who received wheat bread rolls fortified with microencapsulated L‐5MTHF also had significantly higher erythrocyte folate concentrations at endline, compared to the control group who received unfortified wheat bread rolls (N = 15; mean ± SD L‐5MTHF 1200 nmol/L ± 450 nmol/L versus control N = 15; 850 nmol/L ± 270 nmol/L). However, this comparison was outside the scope of this review.
Serum/plasma folate
RCTs
One study reported data for plasma folate (Green 2013). Adult women and men who received folic acid‐fortified wheat bread rolls had significantly higher plasma folate concentrations at endline, compared to the control group who received unfortified wheat bread rolls (N = 15; mean ± SD folic acid intervention 57 nmol/L ± 19 nmol/L versus control N = 15; 30 nmol/L ± 12 nmol/L; MD 27.00 nmol/L, 95% CI 15.63 nmol/L to 38.37 nmol/L; 1 study, 30 participants; very low‐certainty evidence; Analysis 3.2). We downgraded the certainty of evidence once for directness and twice for imprecision. Due to the limited number of studies, heterogeneity was not applicable.
3.2. Analysis.
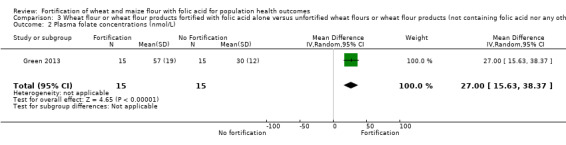
Comparison 3 Wheat flour or wheat flour products fortified with folic acid alone versus unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals), Outcome 2 Plasma folate concentrations (nmol/L).
In the third group in this study, individuals who received wheat bread rolls fortified with microencapsulated L‐5MTHF also had significantly higher plasma folate concentrations at endline, compared to the control group (N = 15; mean ± SD L‐5MTHF 56 nmol/L ± 22 nmol/L versus control N = 15; 30 nmol/L ± 12 nmol/L), although this comparison was outside the scope of this review.
Anaemia
No studies reported data on anaemia.
Haemoglobin
No studies reported data on haemoglobin.
Any type of cancer
No studies reported data on any type of cancer.
Childhood cancers
No studies reported data on childhood cancers.
Low birth weight
No studies reported data on low birth weight.
Other adverse pregnancy outcomes
No studies reported data on other adverse pregnancy outcomes.
Cognitive function/decline
No studies reported data on cognitive function or decline.
Secondary outcomes
No studies reported data on secondary outcomes.
Wheat flour or wheat flour products fortified with folic acid plus other vitamins and minerals versus unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals)
Primary outcomes
Neural tube defects
Other study designs
In a study among 23,685 women (Wang 2016), investigators reported the total number of neural tube defect cases (i.e. a combined endpoint of total neural tube defects: spina bifida, encephalocoele, and anencephaly, including fatal and non‐fatal cases). Wheat flour fortified with folic acid with other micronutrients was associated with significantly lower occurrence of total neural tube defects, compared to unfortified wheat flour (risk ratio (RR) 0.32, 95% CI 0.21 to 0.48; 1 non‐RCT, 8037 births; low‐certainty evidence). We downgraded the certainty of evidence twice due to high risk of bias.
Spina bifida: in one study (Wang 2016), women who received wheat flour fortified with folic acid and other micronutrients had significantly lower occurrence of spina bifida (RR 0.29, 95% CI 0.16 to 0.56; 1 non‐RCT, 8037 births; low certainty evidence), compared to women who received unfortified flour. For this outcome, there were 17 spina bifida cases among 5898 women who received fortified wheat flour, and 21 cases among 2139 women who received unfortified flour. We downgraded the certainty of evidence twice due to high risk of bias.
Encephalocoele: in the same study (Wang 2016), wheat flour fortified with folic acid and other micronutrients was associated with significantly lower occurrence of encephalocoele, compared to unfortified flour (RR 0.26, 95% CI 0.13 to 0.56; 1 non‐RCT, 8037 births; low‐certainty evidence). A total of 13 encephalocoele cases occurred among 5898 women who received fortified wheat flour, compared to 18 cases in 2139 women who received unfortified wheat flour. We downgraded the certainty of evidence twice due to high risk of bias.
Anencephaly: Wang 2016 reported no significant differences in the number of cases of anencephaly among women who received wheat flour fortified with folic acid and other micronutrients, compared to women who received unfortified flour (RR 0.47, 95% CI 0.21 to 1.07; 1 non‐RCT, 8037 births; low‐certainty evidence). A total of 13 fatal anencephaly cases were reported in 5898 women receiving fortified wheat flour, compared to 10 cases among 2139 women who received unfortified flour. We downgraded the certainty of evidence twice due to high risk of bias and once for indirectness.
Erythrocyte folate
No studies reported on erythrocyte folate.
Serum/plasma folate
Other study designs
Two non‐RCTs reported data for serum folate concentrations (Huo 2012; Wang 2016). In a cluster‐randomised study (Wang 2016), women in the group that received fortified wheat flour had significantly higher serum folate concentrations at endline, compared to the control group (1 non‐RCT, 217 participants).
In a non‐RCT among 440 women of childbearing age (Huo 2012), women in the group that received fortified wheat flour had significantly higher serum folate concentrations at endline, compared to women who received unfortified flour (MD 2.92 nmol/L, 95% CI 1.99 nmol/L to 3.85 nmol/L; 1 non‐RCT, 440 participants; very low‐certainty evidence). We did not pool data from these studies, since randomisation procedures were not specified in the second study. We downgraded the certainty of evidence twice due to high risk of bias and once for indirectness.
Anaemia
RCTs
In a cluster‐randomised trial among 334 children (Rahman 2015), there were no significant effects of fortified wheat flour flatbread on risk of anaemia, compared to unfortified flour (RR 1.07, 95% CI 0.74 to 1.55; 1 study, 334 participants; low‐certainty evidence; Analysis 4.1). We downgraded the certainty of evidence once for directness and once for imprecision. Due to the limited number of studies, heterogeneity was not applicable.
4.1. Analysis.
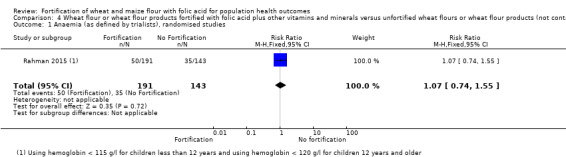
Comparison 4 Wheat flour or wheat flour products fortified with folic acid plus other vitamins and minerals versus unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals), Outcome 1 Anaemia (as defined by trialists), randomised studies.
Other study designs
Two non‐RCTs in rural China reported the prevalence of anaemia in women of reproductive age (Huo 2011; Huo 2012. There were no significant effects of fortified wheat flour on the prevalence of anaemia after a 36‐month intervention, compared to unfortified wheat flour (RR 0.87, 95% CI 0.68 to 1.11; 2 non‐RCTs, 964 participants; very low‐certainty evidence). We downgraded the certainty of evidence twice due to high risk of bias and once for indirectness.
Haemoglobin
RCTs
In a cluster‐randomised trial among 334 children (Rahman 2015), there were no significant effects of fortification on haemoglobin concentrations, compared to the control (MD 0.00 nmol/L, 95% CI ‐2.08 nmol/L to 2.08 nmol/L; 1 study, 334 participants; low‐certainty evidence; Analysis 4.2). We downgraded the certainty of evidence once for directness and once for imprecision. Due to the limited number of studies, heterogeneity was not applicable.
4.2. Analysis.
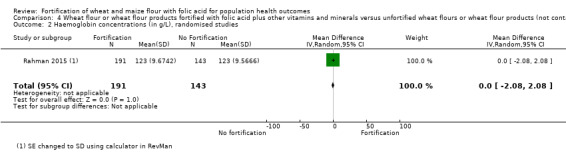
Comparison 4 Wheat flour or wheat flour products fortified with folic acid plus other vitamins and minerals versus unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals), Outcome 2 Haemoglobin concentrations (in g/L), randomised studies.
Other study designs
Two non‐RCTs reported haemoglobin concentrations among 516 and 448 non‐pregnant women of reproductive age (Huo 2011; Huo 2012), respectively. In the fortification groups, haemoglobin concentrations significantly increased from baseline to 36 months in both studies. In one study (Huo 2011), haemoglobin concentrations were significantly higher in the fortification group at endline, compared to the control group. In these non‐RCTs, wheat flour fortified with folic acid and other micronutrients was associated with significantly increased haemoglobin concentrations, compared to unfortified wheat flour (RR 3.26, 95% CI 1.31 to 5.22; 2 non‐RCTs, 964 participants; very low‐certainty evidence). We downgraded the certainty of evidence twice due to high risk of bias and once for indirectness.
Any type of cancer
No studies reported data on any type of cancer.
Childhood cancers
No studies reported data on childhood cancers.
Low birth weight
No studies reported data on low birth weight.
Other adverse pregnancy outcomes
No studies reported data on other adverse pregnancy outcomes.
Cognitive function/decline
No studies reported data on cognitive function or decline.
Secondary outcomes
Homocysteine
Other study designs
In one non‐RCT (Wang 2016), women who consumed wheat flour fortified with folic acid and other micronutrients had significantly lower homocysteine concentrations, compared to women who received unfortified flour (1 study, 217 participants).
No studies reported data for other secondary outcomes.
Combined wheat and maize flour or products fortified with folic acid alone versus no intervention
No studies contributed data for this comparison.
Combined wheat and maize flour or products fortified with folic acid plus other vitamins and minerals versus no intervention
No studies contributed data for this comparison.
Combined wheat and maize flour or products fortified with folic acid alone versus unfortified wheat and maize flours or flour products (not containing folic acid nor any other vitamins and minerals)
No studies contributed data for this comparison.
Combined wheat and maize flour or products fortified with folic acid plus other vitamins and minerals versus unfortified wheat and maize flours or flour products (not containing folic acid nor any other vitamins and minerals)
No studies contributed data for this comparison.
Discussion
Summary of main results
Folic acid fortification of wheat flour in combination with other micronutrients was associated with a decreased occurrence of neural tube defects (i.e. total neural tube defects and two types of neural tube defects: spina bifida, and encephalocoele), compared to unfortified flour in the one non‐randomised controlled trial (non‐RCT) reporting this outcome. Fortification of wheat or maize flour with folic acid (i.e. alone or in combination with other micronutrients) was associated with increased erythrocyte and serum/plasma folate concentrations, compared to unfortified flour or no intervention in two of the three studies included in quantitative analyses and in both of the other studies included in the narrative review that assessed these outcomes. There was limited evidence of the effects of fortification of wheat flour with folic acid on haemoglobin concentrations, and one cluster‐RCT and two non‐RCTs found no association between folic acid‐fortified wheat flour and reduced risk of anaemia. There was limited evidence of an association between folic acid‐fortified wheat or maize flour and the risk of other adverse pregnancy outcomes and childhood cancer. None of the studies reported on the occurrence of low birth weight, cognitive function/decline, or adverse effects. The effects of folic acid fortification of wheat and maize flour on other primary outcomes evaluated in this review are unknown.
Overall completeness and applicability of evidence
Study characteristics ‐ comparability
Flour fortification with folic acid is a promising strategy to improve folate status and reduce the risk of neural tube defects and other adverse health outcomes in populations. This review included 10 studies, four of which reported data for inclusion in quantitative analyses. Five studies provided information about randomisation (i.e. with randomisation at the cluster or individual level) and five studies did not provide any details about a randomisation procedure. Most studies included in this review were comparable in terms of the population studied (i.e. women of reproductive age with non‐deficient baseline folate status), setting (i.e. rural areas of low‐ and middle‐income countries), intervention (i.e. wheat or maize flour fortified with folic acid alone or in combination with other micronutrients), comparison groups (i.e. unfortified flour or no intervention), and the limited number of a priori outcomes (i.e. primarily erythrocyte or serum/plasma folate concentrations) that were reported.
Seven of the 10 studies included in this review were conducted in lower‐middle‐income or upper‐middle‐income countries, namely: South Africa, China, Bangladesh, and Mexico. Participants in seven of the studies included women of reproductive age, one study included children 5 to 12 years of age (Rahman 2015), and one study included children 0 to 17 years of age (French 2003). The emphasis on women of reproductive age in the literature is appropriate, as they are a priority target group for prevention of anaemia and neural tube defects.
Participant folate status at baseline was not reported in six of the included studies. In contrast, the remaining four studies were conducted among women with non‐deficient baseline folate status (Colman 1974a; Green 2013; Huo 2012; Sanchez 2011), based on erythrocyte or serum/plasma folate concentrations (WHO 2012b).
In all of the studies included in this review, folic acid was the fortification compound added to flour; seven studies were conducted using fortified wheat flour, and three studies were conducted with maize flour. Only one included study reported the extraction rate of flour (Rahman 2015), and used high extraction of whole grain wheat flour (80%). Seven of the intervention studies included unfortified flour as the control group; in one study, the control group did not receive an intervention (Colman 1974a). Two interrupted time series (ITS) studies compared the periods before and after initiation of fortification of wheat flour with folic acid (French 2003; Ionescu‐Ittu 2009).
In terms of other primary and secondary outcomes, homocysteine concentrations were reported as an outcome in one included study (Wang 2016). No studies included in this review reported on the occurrence of other a priori primary outcomes, including cancer, cognitive function, or other adverse pregnancy outcomes. No studies reported on the occurrence of other secondary outcomes, including serum/plasma methylmalonic acid, depression, pernicious anaemia, urinary folic acid concentrations, 5‐methyltetrahydrofolate (5MTHF) concentrations, 5‐MTHF catabolite concentrations, unmetabolised folic acid concentrations in blood, malaria infection, colorectal cancer or polyps, or cardiovascular disease, and no studies reported on the occurrence of adverse side effects.
Four studies contributed data to quantitative analyses in this review, and each reported data for a subset of the a priori outcomes. Erythrocyte and serum/plasma folate concentrations were reported as outcomes in three studies (Colman 1974a; Green 2013; Sanchez 2011); haemoglobin concentrations and anaemia were reported as outcomes in one study (Rahman 2015).
Study characteristics ‐ heterogeneity
The studies included in this review were heterogeneous in terms of study characteristics, i.e. study design, population studied (e.g. participant lifecycle stage), daily dose of folic acid (i.e. amount of folic acid that was added to flour and the amount that was consumed by participants), intervention duration, and outcomes reported. This may constrain the generalisability of findings to other populations and settings. Most studies were conducted among women of reproductive age; however, they included a range of pregnant, lactating, and not pregnant or lactating women.
The amount of folic acid added to fortified flour interventions varied considerably across studies. Intervention studies included flour fortified with 0.5 parts per million (ppm) to 33 ppm of folic acid, providing 100 micrograms to 900 micrograms of folic acid per day. Six of the studies included flour fortified with 0.5 ppm to 5.48 ppm of folic acid; this amount of folic acid added to wheat or maize flour is close to the range recommended by the World Health Organization (WHO) for fortification of wheat flour (i.e. 1.0 ppm to 5.0 ppm, based on flour availability or intake; WHO 2009) and maize flour (i.e. 1.3 ppm to 5.0 ppm, based on flour availability or intake; WHO 2016). The estimated daily dose of folic acid provided in fortified flour ranged from one‐quarter to more than twice the amount that is recommended that women of reproductive age consume per day for the prevention of neural tube defects (i.e. 400 µg/day, without a previous pregnancy affected by a neural tube defect; WHO 2007). Due to the heterogeneity in the design of included studies ‐ including the variability in the amount of folic acid added to fortified flours, type and duration of interventions, and lack of assessment of flour consumption ‐ it was not possible to conduct the proposed a priori subgroup analyses based on the length of intervention or daily dosage of folic acid delivered in interventions. Further, the heterogeneity of doses of folic acid in different fortified flour interventions may have limited the ability to detect an effect of folic acid fortification on different health outcomes in this review, since the effects of folic acid would be expected to vary by the daily dose of folic acid (i.e. amount of folic acid that was added to flour and the amount that was consumed by participants).
The micronutrient composition of interventions (i.e. folic acid alone or in combination with other micronutrients) varied in included studies. For example, four studies included flour that was fortified with folic acid alone (Colman 1974a; French 2003; Green 2013; Ionescu‐Ittu 2009). In contrast, flour was fortified with folic acid and other nutrients in the other six included studies, namely vitamin A, niacin, iron, zinc, and protein (Carrasco 2013); vitamin A, niacin, thiamine, riboflavin, iron, and zinc (Huo 2011); vitamin A, niacin, thiamine, riboflavin, iron, and zinc (Huo 2012); vitamin A, niacin, thiamine, riboflavin, iron, and zinc (Rahman 2015); riboflavin and zinc (Sanchez 2011); and thiamine, riboflavin, iron, and zinc (Wang 2016). In one study, participants received additional micronutrient supplementation; iron supplements were provided to all participants during pregnancy (Colman 1974a).
The mode of delivery for fortified flour interventions also varied across studies. Interventions were administered in the form of fortified flour (Carrasco 2013; French 2003; Huo 2011; Huo 2012; Ionescu‐Ittu 2009; Rahman 2015; Sanchez 2011; Wang 2016), porridge (Colman 1974a), and wheat bread rolls (Green 2013). In four studies, the intervention was administered at the individual level (Carrasco 2013; Colman 1974a; Green 2013; Sanchez 2011), and in five studies, interventions were administered at the household (Huo 2011; Huo 2012; Wang 2016) or bari (i.e. 5 to 6 adjoining households with populations of approximately 30 to 35 relatives) level (Rahman 2015). In the two ITS studies that did not contribute data to quantitative analyses, flour was distributed at the national level (French 2003; Ionescu‐Ittu 2009).
Preparation of fortified flour also varied in the different studies. For example, caregivers used flour in preparation of food at home in five studies, where there were no data reported on storage, preparation, or cooking procedures used to prepare the fortified flour (Carrasco 2013; Colman 1974a; Huo 2011; Huo 2012; Wang 2016). In the remaining studies, instructions were provided to participants on preparation of chapattis (Rahman 2015), or tortillas (Sanchez 2011), at home; or fortified flour was used to prepare porridge (Colman 1974a), or bread rolls (Green 2013), by study personnel.
The intervention duration ranged from approximately four weeks to 36 months in included RCTs, and up to seven years during the postintervention period in an ITS study. The duration of folic acid‐fortified wheat flour interventions were longer, compared to the fortified maize flour interventions.
Individual flour consumption was reported in three studies of wheat flour fortification, and was not reported in any of the maize flour fortification studies. These three wheat flour studies were conducted in rural China among women of reproductive age; individual consumption was 117 grams of wheat flour per person per day (Huo 2011), and 215 grams per person per day (Huo 2012), as evaluated by an interviewer‐administered food frequency questionnaire; and 390 grams to 405 grams per person per day (Wang 2016), as assessed by self‐reported questionnaire which was validated by an interview administered monthly by trained staff.
Among the 10 studies included in this review, most studies reported data for one to two of the a priori primary outcomes. Erythrocyte or serum/plasma folate concentrations were reported in six studies (Colman 1974a; Green 2013; Huo 2011; Huo 2012; Sanchez 2011; Wang 2016). Haemoglobin concentrations and anaemia prevalence were reported in three studies (Huo 2011; Huo 2012; Rahman 2015). The occurrence of neural tube defects (i.e. total neural tube defects, spina bifida, encephalocoele, and anencephaly) were reported in one non‐RCT (Wang 2016); and occurrence of congenital heart defects (Ionescu‐Ittu 2009), and occurrence of childhood cancers (French 2003), were reported in ITS studies.
Laboratory methods used to assess folate status varied considerably in the studies included in this review. The L casei microbiological method was used to measure erythrocyte and serum folate concentrations in two studies (Colman 1974a; Green 2013). In other studies, serum folate was measured by a microparticle enzyme immunoassay (Huo 2011), erythrocyte folate concentrations were calculated multiplying the data from the standard curve with the dilution factor, and dividing the product by the haematocrit value (Sanchez 2011), and the laboratory methods to assess serum folate were not specified in one study (Wang 2016). The use of different laboratory methods to evaluate biomarkers of folate status is a major limitation which may lead to inaccurate estimates of folate status and constrain interpretation of findings (Pfeiffer 2016).
Most studies included in this review had small sample sizes ranging from five to 611 individuals with available outcome data. For example, three of the studies included 45 or fewer participants (Colman 1974a; Green 2013; Sanchez 2011), and five studies included 155 to 611 participants (Carrasco 2013; Huo 2011; Huo 2012; Rahman 2015; Wang 2016). Additionally, one of the studies reported the occurrence of neural tube defects among 8037 births (Wang 2016). Three of the four studies included in quantitative analyses had sample sizes of 45 or fewer participants (Colman 1974a; Green 2013; Sanchez 2011).
Two ITS studies assessed population‐level outcomes, including one study that assessed childhood cancers among 1,953,296 children during the prefortification period and 289,142 children during the postfortification period (French 2003), and one study that did not specify the population size (Ionescu‐Ittu 2009).
In terms of time period, one of the included studies was published in the mid‐1970s, and the remainder were published between 2003 and 2016.
Certainty of the evidence
We categorised the majority of the included studies in this review as having unclear bias for random sequence generation (i.e. selection bias), allocation concealment (i.e. selection bias), blinding of participants and personnel (i.e. performance bias), blinding of outcome assessment (i.e. detection bias), and selective reporting (i.e. reporting bias). We categorised seven of the studies as low risk for incomplete outcome data (i.e. attrition bias).
Potential biases in the review process
Studies were reviewed independently by two review authors in order to evaluate if studies met the inclusion criteria, extract information from studies into a structured data extraction form, and evaluate risk of bias based on established a priori criteria. Discrepancies were resolved through discussion and consultation with the senior review author. In order to minimise publication bias, a detailed search was conducted using an extensive list of databases outlined in the Methods section, including the MEDLINE database and Cochrane Library. Additional searches were conducted with the information specialist of the Cochrane Public Health Group, trial registries, the grey literature, and by contacting authors and agencies involved in the conduct of included studies. Language bias was minimised by not restricting searches to any languages; all of the abstracts and full‐text articles except two were translated into English prior to review.
Agreements and disagreements with other studies or reviews
Several reviews have been conducted to examine the efficacy or effectiveness of folic acid fortification interventions on population health outcomes. These include systematic reviews (Atta 2016; Castillo‐Lancellotti 2013; Das 2013), narrative reviews (Berry 2010), meta‐analyses (Atta 2016; Das 2013), and synthesis of evidence from cross‐sectional studies in countries with fortification (Britto 2014; Nazer 2013; Williams 2015). These reviews have been conducted using data from neural tube defect registries and hospital records from cross‐sectional studies (Atta 2016), a combination of randomised efficacy and effectiveness trials (Das 2013), and by synthesising evidence from cross‐sectional studies in countries that mandated flour fortification with folic acid (Berry 2010; Castillo‐Lancellotti 2013). These cross‐sectional studies primarily assessed information from birth registries in the periods before and after national flour fortification programmes and have been conducted in countries such as Brazil, which included mandatory fortification of wheat and maize flour with folic acid (Britto 2014); in Chile, which mandated folic acid fortification of wheat flour (Nazer 2013); and the USA, where mandatory folic acid fortification includes wheat flour, maize flour, and cereal products (Williams 2015).
Previous reviews have focused on outcomes including the occurrence of neural tube defects in settings with and without fortification of grains (Atta 2016; Castillo‐Lancellotti 2013), haematological outcomes in individuals consuming any foods fortified with folic acid (Das 2013), and haematological outcomes and neural tube defects in countries before and after mandatory folic acid‐fortification of wheat flour (i.e. alone or in combination with maize flour) (Berry 2010). None of the previous studies simultaneously evaluated the main endpoints included in this review, such as erythrocyte and serum/plasma folate concentrations, haemoglobin concentrations, anaemia, neural tube defects, low birth weight, small for gestational age and preterm birth. We did not identify any recent reviews, meta‐analyses, or studies that assessed any other a priori primary or secondary outcomes in this review.
Reviews
In Atta 2016, investigators assessed the prevalence of spina bifida from 1985 to 2010 in countries with and without mandatory fortification of grains with folic acid based on studies reporting on birth registries or national surveillance for birth defects. Their findings suggest that the prevalence of spina bifida was lower in countries with mandatory folic acid fortification, compared to those with voluntary fortification. This finding was observed in studies that reported only live births; live births and stillbirths; and live births, stillbirths, and termination of pregnancies. However, this review had several limitations, including heterogeneity of populations, levels of folic acid fortification, vehicles of fortification, and methods for cooking and processing flour in different settings where the studies were conducted. Das 2013 reviewed 31 studies of foods fortified with folic acid (i.e. not limited to flour fortification) and their associations with erythrocyte and serum folate concentrations and the occurrence of neural tube defects. Findings from meta‐analyses suggested there were no significant increases in erythrocyte or serum folate concentrations or reductions in neural tube defect prevalence after the folic acid fortification intervention period. These findings regarding the impact of folic acid fortification on folate status are contrary to the current review and other analyses of the impact of folic acid fortification of wheat or maize flour on health outcomes.
Berry 2010 conducted a review examining changes in population‐level erythrocyte and folate concentrations before and after mandatory folic acid fortification periods in the USA, Canada, Chile, and Costa Rica; national fortification programmes included folic acid‐fortified wheat flour alone or in combination with fortified maize flour. Average erythrocyte and serum folate concentrations increased in these settings between the pre‐ and postfortification periods.
Several reviews have been conducted to examine the occurrence of neural tube defects in countries before and after the initiation of folic acid fortification of flour, based on birth defects registries, with similar findings to the current review. In a systematic review of 27 studies from nine countries, Castillo‐Lancellotti 2013 observed a reduction in the occurrence of total neural tube defects and spina bifida in the postfortification periods, compared to the prefortification periods in all studies. Additionally, 26 of the 27 included studies also reported a reduction in the occurrence of anencephaly between the pre‐ and postfortification periods.
Meta‐analyses
In a meta‐analysis of 123 cross‐sectional studies, Atta 2016 concluded that the prevalence of spina bifida was lower in countries with mandatory fortification of flour with folic acid, compared to countries with either voluntary folic acid fortification or no fortification. Das 2013 also noted that there was a decrease in the prevalence of spina bifida, anencephaly, and neural tube defects in the postfortification period, compared to the prefortification period.
These aforementioned reviews cover an important amount of programmatic evidence and indirect evidence that should be considered in the decision‐making process regarding fortification of flour with folic acid. In particular, the establishment of folic acid supplementation as standard of care during pregnancy and implementation and scale‐up of national fortification programmes in over 80 countries poses ethical and logistical barriers in the conduct of new randomised intervention studies with control groups.
Summary of observational studies without a control group and uncontrolled before‐and‐after studies
Similar to the other studies included in this review, the available evidence from non‐randomised, observational studies without a control group and uncontrolled before‐and‐after studies (Appendix 1), suggests improvements in erythrocyte folate and plasma/serum folate levels after folic acid fortification of flour; they also consistently pointed to reductions in the prevalence of anaemia among women and children after folic acid fortification. However, there were mixed results in terms of reductions in the prevalence of folate deficiency or increases in haemoglobin levels after fortification with folic acid. One study each reported on the occurrence of the following outcomes after folic acid fortification: decrease in homocysteine concentrations, hyperhomocysteinaemia, overall mortality and deaths occurring after hospital discharge, and increase in the incidence of myelomeningocoele. One study observed no differences in deaths occurring before hospital discharge.
However, among the 10 studies reviewed, there were methodological differences in the study designs which constrain interpretation of findings, including different study populations, sample size, setting, baseline prevalence of anaemia and folate deficiency, dose and timing of folic acid fortification exposures, and methods for analysis of erythrocyte and serum/plasma folate concentrations.
Summary of evidence from folic acid fortification programmes
Oman was the first country to implement the fortification of wheat flour with folic acid in 1996 (Zimmerman 2011). Over 80 countries have since established mandatory fortification of wheat flour with folic acid (FFI 2018a), and 15 countries also require fortification of maize flour with folic acid (FFI 2018b). Folic acid is used as the fortification compound in all countries with mandatory fortification of wheat or maize flour (GFDx 2018). In practice, large‐scale flour fortification with folic acid has been implemented in many countries for nearly two decades. This programmatic experience can complement the findings from the current review. Since no countries currently implement mandatory maize flour fortification alone (i.e. mandatory fortification includes either wheat flour fortification alone or fortification of both wheat and maize flour together), these findings cannot be used to determine the efficacy or effectiveness of folic acid fortification of maize flour alone.
Country‐level reports of folic acid fortification of flour have demonstrated higher population‐level folate status and lower prevalence of neural tube defects in the postfortification period, compared to the prefortification period. In a literature review conducted in Brazil of 47 studies, average population‐level serum folate concentrations were higher in the postfortification period compared to the prefortification period (Britto 2014). Nazer 2013 reported the occurrence of neural tube defects in Chile during the postfortification period, compared to the prefortification period. Investigators reported a significantly lower prevalence of total neural tube defects, anencephaly, and spina bifida in the postfortification period compared to the prefortification period, although there were no differences in the prevalence of cephalocoele (Nazer 2013). Similarly, in an analysis in the USA, Williams 2015 reported a lower prevalence of total neural tube defects, spina bifida, and anencephaly in the postfortification period, compared to the prefortification period.
Findings from observational studies without a control group and uncontrolled before‐and‐after studies
Results from observational studies without a control group and uncontrolled before‐and‐after studies are summarised in Appendix 1. Of these 10 studies (Appendix 1), five included fortified wheat flour (Black 2014; Hertrampf 2003; Hirsch 2002; Noor 2017; Tazhibayev 2008), two used fortified maize flour (Colman 1974c; Seal 2008), two included fortified wheat and maize flour (Modjadji 2007; Salomao 2017), and one did not specify the type of flour that was fortified (Margo 1975). As reported by the authors, the flour was fortified with folic acid alone in five studies (Black 2014; Colman 1974c; Hirsch 2002; Margo 1975; Salomao 2017), and folic acid along with other vitamins or minerals in five studies (Hertrampf 2003; Modjadji 2007; Noor 2017; Seal 2008; Tazhibayev 2008). Fortified flour (or foods made with the flour) was the only intervention in nine studies (Colman 1974c; Hertrampf 2003; Hirsch 2002; Margo 1975; Modjadji 2007; Noor 2017; Salomao 2017; Seal 2008; Tazhibayev 2008), and was included as a cointervention with a fruit and vegetable subsidy in one study (Black 2014).
The study designs employed were uncontrolled before‐and‐after for eight studies (Black 2014; Colman 1974c; Hertrampf 2003; Hirsch 2002; Margo 1975; Modjadji 2007; Seal 2008; Tazhibayev 2008), prospective cohort for one study (Noor 2017), and retrospective cohort for one study (Salomao 2017). The study populations included all age groups and different health statuses: women of reproductive age (Hertrampf 2003; Modjadji 2007; Noor 2017), pregnant women (Margo 1975), pregnant or lactating women (Colman 1974c), young children with surgically repaired myelomeningocoele (Salomao 2017), low‐income children (Black 2014), a combination of women of reproductive age and children (Tazhibayev 2008), a combination of children, adolescents and women of reproductive age (Seal 2008), and lower‐income older adults (Hirsch 2002). The sample size ranged from 12 women in Colman 1974c to 751 women in Hertrampf 2003. The study setting included countries of diverse socioeconomic levels, i.e. South Africa (Colman 1974c; Margo 1975; Modjadji 2007), Tanzania (Noor 2017), Zambia (Seal 2008), Chile (Hertrampf 2003; Hirsch 2002), Brazil (Salomao 2017), Australia (Black 2014), and several countries in Central Eurasia: Azerbaijan, Kazakhstan, Kyrgyzstan, Tajikistan, Uzbekistan and Mongolia (Tazhibayev 2008).
Three studies did not specify the baseline prevalence of anaemia and folate deficiency (Black 2014; Margo 1975; Salomao 2017). Those that did, reported baseline prevalence of anaemia between 0% in Colman 1974c and 70% in Tazhibayev 2008, and baseline prevalence of folate deficiency between 1.3% in Hertrampf 2003 and 85% in Tazhibayev 2008. The duration of the interventions ranged from four weeks in Margo 1975 to up to 10 years in Salomao 2017. The flour extraction rate was only specified in two studies: 97% (i.e. high extraction in Seal 2008), and 55% to 72% (i.e. low extraction in Tazhibayev 2008).
Four of the studies did not specify the amount of folic acid that was added to the flour or that was delivered to study participants (Black 2014; Margo 1975; Modjadji 2007; Salomao 2017). Among the five studies that reported the amount of folic acid added to the flour, it ranged from 1.2 ppm in Tazhibayev 2008 to 3 ppm in Noor 2017. One study reported the amount of folic acid delivered in the flour intervention, and described as a target dose of 500 μg folic acid daily for each adult in the household (Colman 1974c). The amount of folic acid delivered was calculated for two studies; it was an average of 427 μg per day in Hertrampf 2003 and 900 μg per day in Margo 1975.
Eight of the studies reported erythrocyte or serum/plasma folate concentrations as an outcome, and described the assay used to measure folate concentrations. The assays used were the microbiological assay with L casei (Colman 1974c; Margo 1975), Architect i2000 immunoassay analyser and ADVIA Centaur XP automated immunoassay platform (Black 2014), QuantaPhase II Folate Assay kit (Hertrampf 2003), anion capture technique (Hirsch 2002), Access ImmunoAssay (Modjadji 2007), Cuba’s e411 automated analyser (Noor 2017), and high‐performance liquid chromatography using a fluorescent detector (Tazhibayev 2008).
With regards to erythrocyte folate concentrations, the main study findings were as follows: mean erythrocyte folate levels increased significantly in one of the three study communities (Black 2014), there was a significant rise in erythrocyte folate concentrations in nine of the 12 participants receiving the fortified food (Colman 1974c), erythrocyte folate concentrations increased from 290 nmol/L ± 102 nmol/L to 707 nmol/L ± 179 nmol/L (Hertrampf 2003), erythrocyte folate concentrations rose weekly (Margo 1975), and median erythrocyte folate levels increased after fortification (Modjadji 2007).
For serum or plasma folate, there was an increase in serum folate concentrations from mean ± SD 9.7 nmol/L ± 4.32 nmol/L to 37.2 nmol/L ± 9.5 nmol/L (Hertrampf 2003), serum folate increased from mean ± SD 16.2 nmol/L ± 6.2 nmol/L to 32.7 nmol/L ± 7.1 nmol/L (Hirsch 2002), median concentrations of serum folate increased after fortification from median (interquartile range (IQR)) 3.58 ng/mL (2.87 to 4.22) to median (IQR) 10.51 ng/mL (8.53 to 13.52) (Modjadji 2007), mean plasma folate increased from 5.44 ng/mL ± 2.30 ng/mL to 9.70 ng/mL ± 3.75 ng/mL (Noor 2017), and mean serum folic acid concentrations increased in Mongolian women and in children from Azerbaijan, Kazakhstan, Mongolia and Tajikistan (Tazhibayev 2008).
The prevalence of folate deficiency based on erythrocyte levels did not change in Hertrampf 2003 and decreased from 26.4% to 1.9% in Modjadji 2007. Based on serum or plasma folate, the prevalence of folate deficiency did not differ in Hertrampf 2003 and decreased from 16.3% to 0% in Modjadji 2007, decreased from 26.9% to 5.0% in Noor 2017, and decreased in Mongolian women and children from Azerbaijan, Kazakhstan, Mongolia and Tajikistan (Tazhibayev 2008).
Haemoglobin levels did not change in two studies among pregnant and lactating women in Colman 1974c and women of reproductive age in Seal 2008. In other studies, haemoglobin levels increased in women of childbearing age (Modjadji 2007), children and adolescents (Seal 2008), women of reproductive age from Kazahkstan and children from Azerbaijan, Kazakhstan, Mongolia, and Tajikistan (Tazhibayev 2008).
The prevalence of anaemia decreased in children from mean 95% CI, 47.7% (39.7 to 55.9) to mean 95% CI, 24.3% (17.3 to 34.4) (Seal 2008), and in women of reproductive age in Kazakhstan and in children from Azerbaijan, Kazakhstan, Mongolia and Tajikistan (Tazhibayev 2008).
Two of these studies reported data for additional outcomes. Hirsch 2002 noted that homocysteine concentrations decreased from mean ± SD 12.95 μmol/L ± 3.7 μmol/L to 11.43 μmol/L ± 3.6 μmol/L; the prevalence of hyperhomocysteinaemia also decreased from 31% to 17%. Salomao 2017 reported that the incidence of myelomeningocoele increased from 1.34% to 1.81%, overall mortality decreased from 13.7% to 5.0%, deaths occurring after hospital discharge decreased from 8.8% to 1.8%, and there were no differences in deaths before hospital discharge.
Authors' conclusions
Implications for practice.
Fortification of staple foods is an important public health strategy to improve intake and health outcomes in populations. Folic acid interventions, including flour fortification and periconceptional folic acid supplementation, have been identified as among the most important and successful public health interventions globally. The association between folate status and neural tube defects has been known for over 50 years; evidence from randomised trials has established that periconceptional folic acid supplementation reduces the risk and recurrence of neural tube defects by over 70% (Czeizel 1992; De‐Regil 2015; MRC 1991), and informed recommendations for dietary guidelines and fortification of flour with folic acid (CDC 1992; US Preventive Services Task Force 2017).
Fortification of wheat or maize flour with folic acid (i.e. alone or with other vitamins and minerals) has been introduced in over 80 countries, based on its likely benefits on folate status and risk of neural tube defects. The data from this review suggest that fortification of wheat or maize flour with folic acid may improve folate status and that fortification of wheat flour with folic acid may reduce the occurrence of neural tube defects. There was limited evidence of the effect of fortification of wheat flour with folic acid on haemoglobin concentrations. There were no significant effects of wheat flour fortification with folic acid on the risk of anaemia, as reported in three studies (Huo 2011; Huo 2012; Rahman 2015). Similarly, there was limited evidence of the effects of fortification of flour on other adverse pregnancy outcomes. The effects of folic acid fortification on other primary outcomes assessed in this review are unknown.
Limitations in these analyses include the variability in the folic acid levels delivered and the duration of the interventions; insufficient folic acid provided over too short a period may lead to null or inconclusive results. For example, in the included studies, the amount of folic acid added to fortified flour ranged from 100 µg to 900 µg of folic acid daily. Further, the fortified foods were provided for durations ranging from four weeks to 36 months; a study duration of four weeks may be too brief to confer any changes in the outcomes assessed, particularly if lower doses of folic acid were administered. This review includes a limited number of randomised controlled trials (RCTs) with heterogeneous study designs evaluating the effects of folic acid fortification on specific health outcomes. However, there is extensive programmatic evidence and observational studies on the benefits of folic acid flour fortification for the prevention of neural tube defects, which are consistent with the demonstrated benefits of periconceptional folic acid supplementation on reduced risk of neural tube defects. In practice, findings from this review should be considered in conjunction with the available evidence on folic acid fortification of flour from programmatic and observational studies.
Implications for research.
Few intervention studies have been conducted to date to examine the efficacy of folic acid fortification of wheat or maize flour on population health outcomes. Findings from this review suggest that fortification of wheat or maize flour with folic acid (i.e. alone or in combination with other vitamins and minerals) may improve erythrocyte and serum/plasma folate concentrations, and fortification of wheat flour with folic acid may reduce the occurrence of neural tube defects (i.e. total neural tube defects and two types of neural tube defects, i.e. spina bifida and encephalocoele). These findings are consistent with programmatic evidence and pre‐ and postintervention studies without a control group conducted to date. There was limited evidence of the effect of fortification of wheat flour with folic acid on haemoglobin concentrations, and there were no significant effects of wheat flour fortification with folic acid on the risk of anaemia, as reported in three studies. Similarly, there was limited evidence of the effects of fortification of flour on other adverse pregnancy outcomes. The effects of folic acid fortification ‐ either alone or in combination with other micronutrients ‐ and other primary outcomes assessed in this review are unknown.
As folic acid fortification is successfully implemented and scaled up in national programmes, policies, and legislation worldwide, the landscape and equipoise for randomised controlled efficacy trials has changed ‐ this constrains the feasibility and ethics of conducting additional randomised efficacy trials with a control group. Evidence from folic acid fortification programmes (e.g. neural tube defects risk pre‐ and postfortification), other types of folic acid interventions and study designs (e.g. folic acid intake and risk of health outcomes), and modelling approaches (e.g. effects of folic acid interventions on blood folate concentrations which predict neural tube defect risk) need to be integrated with the evidence from available RCTs to inform policies and public health programmes for folic acid fortification.
Acknowledgements
The protocol and review were developed during the World Health Organization (WHO)/Cochrane/Cornell University Summer Institute for Systematic Reviews in Nutrition for Global Policy Making hosted at the Division of Nutritional Sciences, Cornell University, Ithaca, USA. The WHO partially supported this programme beginning in 2014. We are grateful to Dr. Juan Pablo Peña‐Rosas and Dr. Luz Maria De‐Regil for their leadership in developing the protocol and their feedback on the review. We thank Dr. Michael Cannon from the US Centers for Disease Control and Prevention (CDC) for his thorough comments on the protocol.
The review authors retain copyrights in their respective contributions to this review manuscript as submitted for publication.
Appendices
Appendix 1. Pre‐ and Postintervention studies without a control group
| Study name | Type of flour | Intervention* | Study design | Population | Sample size (n) | Setting | Prevalence of anaemia/folate deficiency at baseline | Duration of intervention | Flour extraction rate | Amount of folic acid (ppm) | Assay used for folate concentrations | Equivalent dose of folate | Relevant outcomes | Results‐main finding |
| Black 2014 | Wheat | Fruit and vegetable subsidy implemented alongside mandatory folic acid fortification of bread‐making flour | Uncontrolled before‐and‐after (same participants at baseline and follow‐up) | Children (0 to 17 years) from low‐income Aboriginal families who participated in a fruit and vegetable subsidy programme | 125 | New South Wales, Australia | Not specified | 3 to 9 months | Not specified | Not specified | Architect i2000 Immunoassay analyser (Abbott Diagnostics, Australia) in two communities and ADVIA Centaur XP automated immunoassay platform (Siemens Diagnostics, Australia) | Not specified | Erythrocyte folate | Mean erythrocyte folate increased significantly in one of the three communities (Clarence Valley) |
| Colman 1974c | Maize | Maize flour fortified with folic acid | Unontrolled before‐and‐after (same participants at baseline and follow‐up) | Pregnant/lactating women (16 to 33 years), oldest women in household (58 to 76 years) | 5 pregnant women, 1 lactating woman, 6 older adults | Johnson Memorial Hospital at Nqutu, KwaZulu in South Africa. Older participants selected from household of women residing near the hospital | Study reported individual measurements. Erythrocyte folate < 151 ng/mL (WHO): 2/12 participants; erythrocyte folate < 160 ng/mL (author's cut‐off): 3/12 participants; Hb < 12.0 g/100L for nonpregnant women and <11.0 g/100L for pregnant women (WHO): 0/12 participants | 6 weeks | Not specified | Enough so that each adult in the family would receive 500 μg folic acid/day | Microbiological assay with Lactobacillus casei (method of Hoffbrand et al 1966) | 500 μg/day | Haemoglobin, erythrocyte cell folate, serum folate | After 6 weeks, there was a significant rise in the erythrocyte folate concentration in 9 of the 12 subjects receiving the fortified food. Haemoglobin remained unchanged. Serum folate not reported due to wide variations |
| Hertrampf 2003 | Wheat | Wheat flour fortified with folic acid and B‐vitamins | Unontrolled before‐and‐after (same participants at baseline and follow‐up) | Women of reproductive age attending the Maternal and Infant Health Programme of the National Health Service | 751 | 3 outpatient clinics in Santiago, Chile | Low serum folate (< 3.2 nmol/L): 1.3%, low erythrocyte folate (< 181 nmol/L): 10.6% | approximately 1 year | Not specified | 2.2 mg/kg | QuantaPhase II Folate Assay kit (Bio‐Rad Laboratories, Hercules, CA) | 427 ± 18 μg/day | Serum folate, erythrocyte folate, low serum folate (< 3.2 nmol/L), low RBC folate (< 181 nmol/L) | Serum folate increased from mean ± SD 9.7 ± 4.32 nmol/L to 37.2 ± 9.6 nmol/L . Erythrocyte folate increased from mean ± SD 290 ± 102 nmol/L to 707 ± 179 nmol/L. The prevalence of low serum and RBC folate was not significantly different |
| Hirsch 2002 | Wheat | Wheat flour fortified with folic acid | Unontrolled before‐and‐after (same participants at baseline and follow‐up) | Free‐living adults >/= 70 years of age, with low income | 149 | 3 outpatient clinics in Santiago, Chile | Folate deficiency (serum folate < 6.8 nmol/L): 1.8% | 6 months | Not specified | 220 µg/100 g flour | Anion capture technique using Abbott Laboratories, Diagnostic Division, Abbot Park, IL | Not specified | Serum folate, haemoglobin, serum homocysteine, folate deficiency (< 6.8 nmol/L), hyperhomocysteinaemia | Serum folate increased from mean ± SD 16.2 ± 6.2 nmol/L to 32.7 ± 7.1 nmol/L. Homocysteine decreased from mean ± SD 12.95 ± 3.7 μmol/L to 11.43 ± 3.6 μmol/L. The prevalence of hyperhomocysteinaemia decreased from 31% to 17% |
| Margo 1975 | Not specified | Slices of bread with flour fortified with folic acid | Uncontrolled before‐and‐after study (same participants at baseline and follow‐up) | Pregnant women with less than 37 gestational weeks, without any illness and not receiving any folic acid supplement | 15 | Charles Johnson Memorial Hospital located in a rural area at Nqutu, KwaZulu in South Africa | Mean erythrocyte folate level 298 range 177 ng/mL to 568 ng/mL haemoglobin: mean 12.4 SD 1.0 | 4 weeks | Not specified | Not specified | Microbiological assay with Lactobacillus casei (method of Hoffbrand 1966) | 900 μg per 1 slice of bread, daily | Erythrocyte folate, haemoglobin | During the trial there was a weekly rise in the erythrocyte folate concentration and no significant change in haemoglobin concentration |
| Modjadji 2007 | Maize and wheat | Maize and wheat foodstuffs fortified with folic acid, iron, vitamin A, thiamine, riboflavin, niacin, pyridoxine, and zinc | Uncontrolled before‐and‐after (same participants at baseline and follow‐up) | Women of reproductive age (18 to 44 years) | 100 | Dikgale Demographic Surveillance Site, a rural area in the Capricorn district of Limpopo Province, high prevalence of poor socioeconomic status and unemployment, South Africa | 27.6% had low serum folate (< 3 ng/mL), 26.4% had low erythrocyte folate (< 164 ng/mL), 7.5% had low haemoglobin (< 11.0 g/dL) | 9 months | Not specified | Not specified | Access ImmunoAssay (Beckman Coulter, California) | Not specified | Serum folate, erythrocyte folate, haemoglobin, prevalence of folic acid deficiency (serum folate < 3 ng/mL, erythrocyte folate < 164 ng/mL), low haemoglobin (< 11.0 g/dL) | After fortification,median concentrations of serum folate and erythrocyte folate increased from median (IQR) 3.58 (2.87 to 4.22) to 10.51 (8.53 to 13.52) ng/mL and from 227.01 (153.77 to 301.42) to 429.29 (367.26 to 610.33) ng/mL, respectively. The percentage of participants with low serum folate and erythrocyte folate decreased from 16.3% to 0% and from 26.4% to 1.9%, respectively. Concentrations of haemoglobin increased from median (IQR) 13.55 (12.63 to 14.20) to 14.0 (13.10 to 14.60) g/dL. The prevalence of low haemoglobin concentrations did not significantly change |
| Noor 2017 | Wheat | Wheat flour fortified with folic acid, iron, zinc, and vitamin B12 | Prospective cohort | Women of reproductive age (18 to 49 years) | 600 | Temple and Ilala districts of Dar es Salaam, Tanzania (mix of urban and peri‐urban populations) | Folate deficiency (plasma folate < 4 ng/ml): 26.9% at baseline | 1 year | Not specified | 3 ± 2 mg/kg | Cuba's e411 automated analyser (Roche Diagnositcs, Switzerland) | Intake of fortified wheat‐based food: 0.62 (± 0.55) servings at 12 months | Plasma folate, prevalence of folate deficiency (< 4 ng/ml) | Plasma folate concentrations increased from mean ± SD 5.44 ± 2.30 ng/ml to 9.70 ± 3.75 ng/ml. Folate deficiency (< 4 ng/ml) decreased from 26.9% to 5.0% |
| Salomao 2017 | Wheat and maize | Wheat and maize flour fortified with folic acid | Retrospective cohort | Infants/children with surgically repaired myelomeningocoele | 383 | Paediatric hospital in Rio de Janeiro, Brazil | Not specified | Up to 10 years | Not specified | Not specified | n/a | Not specified | Mortality before and after hospital discharge, incidence of myelomeningocoele | The incidence of myelomeningocoele increased from 1.34% to 1.81%. Overall mortality decreased from 13.7% to 5%. Deaths occurring after hospital discharge decreased from 8.8% to 1.8%. There was no difference in deaths occurring before hospital discharge. |
| Seal 2008 | Maize | Maize flour fortified with vitamin A, thiamin, riboflavin, nicotinamide, vitamin B6, vitamin B12, folic acid, iron, and zinc (along with pulses, vegetable oil, salt) | Uncontrolled before‐and‐after study (same participants at baseline and follow‐up) | Adolescents (10 to 19 years), children (6 to 59 months), women (20 to 49 years) | 212 adolescents, 157 children, 118 women | Nangweshi refugee camp, Zambia | Anaemia: 19.2% (14.2 to 25.2) among adolescents, 47.7% (39.7 to 55.9) among children, 16.5% (9.8 to 26.1) among women | 12 months | 97% extraction | 1.5 mg/ kg flour | n/a | Not specified | Haemoglobin, anaemia (Hb < 110 g/L for children aged 6 to 59 months; < 115 g/L for adolescents aged 10 to 11 years; < 120 g/L for adolescents aged 12 to 14 years; < 120 g/L for adolescent females aged 15 to 19 years; < 130 g/L for adolescent males aged 15 to 19 years; < 120 g/L for women aged 20 to 45 years) | Haemoglobin (mean (95% CI)) increased in adolescents (12.9 (12.7 to 13.2) to 13.1 (12.8 to 13.3) g/L), and children (10.9 (10.6 to 11.2) to 11.8 (11.6 to 12.1) g/L), but it did not change in women. The prevalence (95% CI) of anaemia decreased in children from 47.7% (39.7 to 55.9) to 24.3% (17.3 to 34.4). The prevalence (95% CI) of anaemia did not change among adolescents (19.2% (14.2 to 25.2) to 24.4% (18.3 to 31.5)) or among women (16.5% (9.8 to 26.1) to 27.6% (17.1 to 41.1)) |
| Tazhibayev 2008 | Wheat | Wheat flour fortified with iron, zinc, niacin, riboflavin, folic acid and salt fortified with iodine | Unontrolled before‐and‐ after (same participants at baseline and follow‐up) | Women of reproductive age (15 to 45 years), children (2 to 15 years) | 40 households and 120 individuals (80 children and 40 women) in each country | Azerbaijan, Kazakhstan, Kyrgyzstan, Tajikistan, Uzbekistan, and Mongolia | Anaemia: 12.5% to 70% among children, 10% to 69% among women. Folate deficiency: 16.2% to 85% among children, 12.8% to 65% among women | 16 months | Premium grade flour (extraction rate 55% to 60%) was fortified with 150 g of micronutrient fortified first‐grade flour (extraction rate up to 72%) | 1.5 ppm and 1.2 ppm (depending on the grade of flour) | High‐performance liquid chromatography using a fluorescent detector in KAN’s laboratory | Not specified | Haemoglobin, anaemia (Hb < 11.0 g/dL 6 to 59 months, < 11.5 g/dL 5 to 11 years, < 12.0 g/dL > 11 years and in women of reproductive age), plasma folic acid, folic acid deficiency (< 6 μg/L) | Among children, mean haemoglobin increased and the prevalence of anaemia decreased in Azerbaijan, Kazakhstan, Tajikistan, and Uzbekistan, but there were no significant changes in Mongolia. Mean plasma folic acid concentration and the prevalence of folic acid deficiency among children increased in Azerbaijan, Kazakhstan, Mongolia, and Tajikistan, but there were no significant changes in Uzbekistan. Among women, mean haemoglobin concentration increased and prevalence of anaemia decreased in Kazakhstan, but there were no significant changes in Azerbaijan, Mongolia, Tajikistan, and Uzbekistan. Mean plasma folate increased and prevalence of folic acid deficiency decreased among women in Mongolia, but there were no significant changes in Azerbaijan, Kazakhstan, Tajikistan, and Uzbekistan |
Appendix 2. Search strategies
We used the following search strategies to search databases, and adapt these to other databases as necessary.
CENTRAL
#1 MESH DESCRIPTOR Folic Acid EXPLODE ALL TREES
#2 ((folic acid or folate or "vitamin b9" or "vitamin m" or folvite or folacin or pteroylglutamic acid)):TI,AB,KY
#3 #1 OR #2
#4 MESH DESCRIPTOR Flour
#5 MESH DESCRIPTOR Triticum
#6 MESH DESCRIPTOR Zea mays
#7 ((wheat or maize or mielies or mealies or corn or cornmeal or flour* or cornflour*)):TI,AB,KY
#8 #4 OR #5 OR #6 OR #7
#9 ((fortif* or enrich* or enhanc* or boost*)):TI,AB,KY
#10 MESH DESCRIPTOR Food, Fortified EXPLODE ALL TREES
#11 #9 OR #10
#12 #3 AND #8 AND #11
MEDLINE and Medline in Progress (OVID)
1 exp Folic Acid/
2 (folic acid or folate or "vitamin b9" or "vitamin m" or folvite or folacin or pteroylglutamic acid).tw.
3 or/1‐2
4 Flour/
5 Triticum/
6 Zea mays/
7 (wheat or maize or mielies or mealies or corn or cornmeal or flour* or cornflour*).tw.
8 4 or 5 or 6 or 7
9 (fortif* or enrich* or enhanc* or boost*).tw.
10 Food, Fortified/
11 9 or 10
12 3 and 8 and 11
13 exp animals/ not humans/
14 12 not 13
EMBASE (OVID)
1 exp Folic Acid/
2 (folic acid or folate or "vitamin b9" or "vitamin m" or folvite or folacin or pteroylglutamic acid).tw.
3 or/1‐2
4 Flour/
5 Triticum/
6 Zea mays/
7 (wheat or maize or mielies or mealies or corn or cornmeal or flour* or cornflour*).tw.
8 4 or 5 or 6 or 7
9 (fortif* or enrich* or enhanc* or boost*).tw.
10 Food, Fortified/
11 9 or 10
12 3 and 8 and 11
13 exp animals/ not humans/
14 12 not 13
15 limit 14 to embase
CINAHL (EBSCO)
S11 (S3 AND S7 AND S10)
S10 S8 OR S9
S9 (MH "Food, Fortified")
S8 (fortif* or enrich* or enhanc* or boost*)
S7 S4 OR S5 OR S6
S6 (wheat or maize or mielies or mealies or corn or cornmeal or flour* or cornflour*)
S5 (MH "Corn")
S4 (MH "Wheat")
S3 S1 OR S2
S2 (folic acid or folate or "vitamin b9" or "vitamin m" or folvite or folacin or pteroylglutamic acid)
S1 (MH "Folic Acid+")
Web of Science (SCI, SSCI, CPCI & CRCI‐SSH)
#1 ((folic acid or folate or "vitamin b9" or "vitamin m" or folvite or folacin or pteroylglutamic acid))
#2 (Triticum)
#3 (Zea mays)
#4 (wheat or maize or mielies or mealies or corn or cornmeal or flour* or cornflour*)
#5 #4 OR #3 OR #2
#6 (fortif* or enrich* or enhanc* or boost*)
#7 #1 and #5 and #6
BIOSIS (ISI)
#1 ((folic acid or folate or "vitamin b9" or "vitamin m" or folvite or folacin or pteroylglutamic acid))
#2 (Triticum)
#3 (Zea mays)
#4 (wheat or maize or mielies or mealies or corn or cornmeal or flour* or cornflour*)
#5 #4 OR #3 OR #2
#6 (fortif* or enrich* or enhanc* or boost*)
#7 #1 and #5 and #6
#8 Refined by: MAJOR CONCEPTS: ( NUTRITION ) AND SUPER TAXA: ( PRIMATES )
Popline
(fortif* or enrich* or enhanc* or boost*)
and
(folic acid or folate or "vitamin b9" or "vitamin m" or folvite or folacin or pteroylglutamic acid)
and
(wheat or maize or mielies or mealies or corn or cornmeal or flour* or cornflour* or Triticum or "zea mays")
Bibliomap and TRoPHI
Freetext: "folic acid" and wheat
Freetext: "folic acid" and maize
Freetext: "folic acid" and flour
OpenGrey
folic acid and flour
folic acid and maize
folic acid and wheat
IBECS, PAHO, WHOLIS and LILACS (BIRME)
wheat or maize or mielies or mealies or corn or cornmeal or flour$ or cornflour$ or "zea mays" or triticum [Words] and fortif$ or enrich$ or enhanc$ or boost$ [Words] and folic acid or folate or "vitamin b9" or "vitamin m" or folvite or folacin or pteroylglutamic acid [Words]
SCIELO
(folic acid or folate or vitamin b9 or vitamin m or folvite or folacin or pteroylglutamic acid) and (wheat or flour$ or maize or mielies or mealies or corn or cornmeal or zea mays or triticum ) and (fortif$ or enrich$ or enhanc$ or boost$)
WPRO, IMSEAR, AFRO and EMRO (GLOBAL INDEX MEDICUS)
(folic acid or folate or "vitamin b9" or "vitamin m" or folvite or folacin or pteroylglutamic acid) and (fortif* or enrich* or enhanc* or boost*) and (wheat or maize or mielies or mealies or corn or cornmeal or flour* or cornflour* or "Zea mays" or Triticu)
INMED
wheat or flour or flours or maize or mielies or mealies or corn or cornmeal or zea mays or triticum
and
(folic acid or folate or vitamin b9 or vitamin m or folvite or folacin or pteroylglutamic acid)
and
(fortify or fortified or enrich or enriched or enhance or enhanced or boost or boosted or boosts)
Native Health Research database
(folic acid) and (wheat or corn or maize)
Appendix 3. Terms for International Clinical Trials Registry Platform
wheat flour AND fortification; wheat flour AND enrichment; flour AND fortified; maize AND fortified; folic acid AND fortification; folic acid AND fortified; folic acid AND enrichment; folate AND fortification; folate AND enrichment.
Data and analyses
Comparison 1. Maize flour or maize flour products fortified with folic acid alone versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Erythrocyte folate concentrations (nmol/L) | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 238.90 [149.40, 328.40] |
| 2 Serum folate concentrations (nmol/L) | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 14.98 [9.63, 20.33] |
Comparison 2. Maize flour or maize flour products fortified with folic acid plus other vitamins and minerals versus unfortified maize flours or maize flour products (not containing folic acid nor any other vitamins and minerals).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Erythrocyte folate concentrations (nmol/L) | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐61.80 [‐152.98, 29.38] |
| 2 Serum folate concentrations (nmol/L) | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.00, 0.00] |
Comparison 3. Wheat flour or wheat flour products fortified with folic acid alone versus unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Erythrocyte folate concentrations (nmol/L) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.66 [0.13, 1.19] |
| 2 Plasma folate concentrations (nmol/L) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 27.0 [15.63, 38.37] |
Comparison 4. Wheat flour or wheat flour products fortified with folic acid plus other vitamins and minerals versus unfortified wheat flours or wheat flour products (not containing folic acid nor any other vitamins and minerals).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anaemia (as defined by trialists), randomised studies | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.74, 1.55] |
| 2 Haemoglobin concentrations (in g/L), randomised studies | 1 | 334 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐2.08, 2.08] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carrasco 2013.
| Methods | Randomised double‐blind controlled trial, conducted to evaluate the effects of maize flour enriched with soy and micronutrients on the nutritional status of indigenous women | |
| Participants | 308 women (14 to 64 years) from communities classified as highly marginalised and rural; the study included indigenous women (i.e. native speakers of a language other than Spanish) and non‐indigenous women (i.e. native Spanish speakers) | |
| Interventions | The study was 10 months in duration. Each woman received 20 kg of maize flour monthly: 155 women received fortified flour and 153 received unfortified flour. The fortified flour contained per 100 g: 1.5 g soy protein, 42.4 mg iron as ferrous fumarate, 120 µg vitamin A, 548 µg folic acid, 33.3 mg zinc, and 6.5 mg niacin. | |
| Outcomes | Haemoglobin concentrations were reported at baseline, four months, and at 10 months (endline), for both intervention and control groups. Haemoglobin was evaluated by capillary blood by the HemoCue method (HemoCue 2019). Haemoglobin concentrations were reported as mean, minimum, and maximum; however, neither SD nor SE were reported. | |
| Notes | Study was conducted in Mexico
Source of funding: DICONSA, previously CONASUPO (Compañía Nacional de Subsidios Populares). C/COL/2907/2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study design was described as a longitudinal, double‐blind, randomised study; however, there was no description of the methods or process of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | There was no description of allocation concealment. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Low risk | Baseline outcome measures were reported similarly in the intervention and control groups. |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) | Low risk | Baseline characteristics were reported for intervention and control groups, and were presented based on indigenous status of women and across different localities. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was reported that neither participants nor researchers were aware of the type of flour administered (i.e. fortified or not fortified); however, methods to ensure blinding were not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up were not reported; it is unclear if no participants were lost to follow‐up in each group, or if the losses to follow‐up were not reported. |
| Contamination (checking for possible performance bias) | Low risk | 20 kilograms of maize flour was provided in bulk to participants on a monthly basis; procedures to prevent sharing, exchanging, or contamination of flour were not described. No information was reported about possible contamination. The authors reported that the flour for each monthly delivery was analysed to ensure correct fortification levels. |
| Selective reporting (reporting bias) | Unclear risk | According to the methods described, all outcomes were reported. No protocol or trial registry entry was found. |
| Other bias | Low risk | Not apparent |
Colman 1974a.
| Methods | Randomised controlled trial, at the individual level, to assess the efficacy of folic acid‐fortified maize meal compared to non‐fortified maize meal | |
| Participants | 45 pregnant women (< 37 weeks gestation) were recruited during a three‐week period from patients residing at the hospital's lodging facility. Exclusion criteria were: received folic acid or antibiotics, anaemia (i.e. Hb < 110 g/L), or any clinical illness. Seven women were excluded because they delivered within the first week of enrolment in the study. All women received iron supplementation starting from their first prenatal visit. A random number method was used to assign women to the fortification group or non‐fortification group. | |
| Interventions | Porridge maize meal prepared with maize flour fortified with folic acid (as synthetic pteroylglutamic acid). Participants received 30 g (dry weight) daily of fortified meal during 10 to 50 days, for a mean of 26.4 days. The fortified maize flour was prepared with 1 g of folic acid in 30 kg of maize flour (33 ppm folic acid). The daily helping of fortified maize meal contained 1000 µg (1 mg) of folic acid. Authors aimed to deliver at least 300 µg (0.3 mg) folic acid per day. The control group received a non‐fortified maize porridge meal. | |
| Outcomes | Red blood cell folate and serum folate concentrations. Blood samples were collected in the morning (11:00 am) on the following occasions: 2 days before the fortified maize was first administered, and once every week until women gave birth. In all cases, there was a 28‐hour period between the maize meal consumption and the blood draw. At the lodging facility, the serum was separated. It was then transferred to an ascorbic acid‐containing test tube for subsequent folate analysis. Serum and erythrocyte folate concentrations were measured by L. casei microbiological method, as confirmed by reviewing the studies cited in the methods: (Herbert 1961) for serum folate and (Hoffbrand 1966) for erythrocyte folate. Endpoint outcomes were reported in a figure only. Data were extracted from the figure (mean, minimum, maximum values). |
|
| Notes | All women received iron supplements since their first prenatal visit. 1000 µg of pteroylglutamic acid was delivered daily. Data from the control group were not reported. The study was conducted in South Africa.
Sources of funding: Upjohn Pharmaceuticals prepared the fortified meal. The South African Medical Research Council and the Atomic Energy Board were acknowledged for assistance and support. Funds were not acknowledged from any source. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | While all patients were assigned to the fortification and no‐fortification groups using a random number method, it is not clear how the randomisation was undertaken. |
| Allocation concealment (selection bias) | Unclear risk | No information was provided. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Low risk | Baseline concentrations of serum folate and red blood cell folate were similar in the two groups. |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) | Unclear risk | Baseline characteristics were not sufficiently reported for any group. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No attempt was described to blind the participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No attempt was described to blind the personnel. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The only reported attrition was seven patients excluded because of delivery within one week of enrolment. |
| Contamination (checking for possible performance bias) | Low risk | Maize porridge was provided while patients were lodging in the hospital, one serving per day. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes of interest (serum folate and red blood cell folate concentrations) were reported. No protocol or trial registry entry was found. |
| Other bias | Low risk | Not apparent |
French 2003.
| Methods | Interrupted time series analysis of incidence of childhood cancers before and after flour fortification with folic acid, comprising time points 1985‐2000, 55 quarterly periods before, 9 quarterly periods after flour fortification. | |
| Participants | Children 17 years and younger in Ontario, Canada. 1,953,296 children were at risk before fortification and 289,142 were at risk after fortification. | |
| Interventions | This was an observational study with no intervention. The objective of this time trend analysis was to estimate the change of childhood cancer incidence after fortification. | |
| Outcomes | Data on incidence of neuroblastoma, acute lymphoblastic leukaemia, and hepatoblastoma was obtained from the Pediatric Oncology Group of Ontario registry (POGO 2019) made up of 5 large paediatric hospitals in Ontario. This registry captures 95% of all paediatric cancers in Ontario. The registry used the Manchester nomenclature system for classifying outcomes. Time series analysis was conducted using auto‐regressive integrated moving average methods. | |
| Notes | Study was conducted in Canada
Source of funding: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not a RCT. This is an interrupted time series analysis. |
| Allocation concealment (selection bias) | High risk | Not a RCT. There was no treatment allocated. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | High risk | This is an interrupted time series analysis. |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) | High risk | This is an interrupted time series analysis. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This is an interrupted time series analysis; there was no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This is an interrupted time series analysis; there was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The database used for outcome assessment captures 95% of all paediatric cancers in Ontario. |
| Contamination (checking for possible performance bias) | Low risk | This was an observational study using an interrupted time series analysis. Contamination could not occur between the periods before and after the intervention. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Not apparent |
Green 2013.
| Methods | Randomised controlled trial, at the individual level to assess the efficacy of wheat flour fortified with (1) microencapsulated calcium L‐5‐MTHF acid or (2) equimolar folic acid, compared with non‐fortified wheat flour, on plasma folate and erythrocyte folate concentrations. | |
| Participants | 45 healthy men and women, 18 to 45 years. Recruitment was through advertisement and word‐of‐mouth. Exclusion criteria were presence of any of the following: medical conditions (e.g. diabetes, asthma, cancer, cardiovascular disease, high blood pressure, coeliac disease, psychiatric illness), taking medications known to interfere with folate metabolism, known deficiency of vitamin B12, allergy of wheat or cow milk, lactose intolerance, consumption of an average of 4 or more alcoholic drinks daily, pregnancy in the past year, "planning a pregnancy, might become pregnant during the study", or had a previous pregnancy affected by a neural tube defect. | |
| Interventions | Participants received wheat bread rolls fortified at the flour stage with 400 µg of folic acid or 425 µg of L‐5‐MTHF, or wheat bread rolls prepared with non‐fortified wheat flour (control group). Participants were given 7 bread rolls weekly and asked to consume 1 bread roll each day at any time of the day. Bread rolls were provided for 16 weeks. An intention‐to‐treat analysis was conducted. | |
| Outcomes | Erythrocyte folate and plasma folate. Both outcomes were measured and reported at baseline, and at weeks 8 and 16. Intention‐to‐treat analysis is also reported. Blood samples were taken in the morning after an overnight fast. An L. casei microbiological method was used to analyse erythrocyte and plasma folate. | |
| Notes | Study conducted in Canada
Source of funding: supported by the Advanced Food Materials Network and the Canadian Vitamin Class Action settlement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study mentions randomisation, but there is no report on the process |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Low risk | Similar blood folate concentrations reported at baseline |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) | Low risk | Similar general characteristics at baseline between the folic acid and placebo groups (only), shown in table 2 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It only mentions that the study design is double‐blinded, without describing the process. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | While the study design was double‐blind, no details were given on how blinding was implemented for providers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants dropped out: 3 were in the control group and 1 was in the folic acid group. Intention‐to‐treat analysis was reported |
| Contamination (checking for possible performance bias) | Low risk | All participants were given a weekly (7‐day) supply of wheat bread rolls. They were asked to eat one wheat roll daily, at any time of the day. They were also asked to put the wheat rolls in the refrigerator and to keep a diary of their consumption of the wheat bread rolls. |
| Selective reporting (reporting bias) | Unclear risk | According to what was stated in the methodology, all outcomes were reported. No protocol or trial registry entry was found. |
| Other bias | Low risk | None apparent |
Huo 2011.
| Methods | Non‐randomised intervention trial, in Weichang county China, to evaluate the effectiveness of wheat flour fortified with multiple micronutrients on the nutritional status of poor rural adult women. A group of farmers received multiple‐micronutrient fortified wheat flour and the other group received non‐fortified wheat flour. | |
| Participants | The participants were 4700 farmers in 1233 households who received fortified wheat flour and 2750 farmers in 751 households who received unfortified wheat flour. Flour was provided at the household level but outcomes were measured in women of reproductive age: 319 women, aged 37.8 ± 7.5 years, from the intervention group and 302 women, aged 37.7 ± 7.8 years, from the control group. | |
| Interventions | As part of a government reforestation programme, participant farmers received "compensation" wheat flour in exchange for not farming their land. The compensation wheat flour was fortified with folic acid, vitamin A, thiamin, riboflavin niacin, zinc and iron, and was provided to farmers during a period of 3 years, 2004 to 2007, through twice yearly distributions (in January and July). Unfortified flour was provided to the control group. Farmers could purchase additional wheat flour from the local market at any time. The amount of compensation flour provided to all families depended on the amount of land that was given up for reforestation. | |
| Outcomes | Anaemia, haemoglobin and serum folate concentrations were measured. Haemoglobin was measured using the HemoCue method (HemoCue 2019). Serum folic acid was measured using a German AXSYM kit. Although folic acid concentrations are mentioned in the methods and title of results (Table 6), no outcome data were reported on blood folate concentrations; the inclusion of folic acid appears to be an error in the table's title. At baseline and month 36, serum folic acid was measured. At baseline and months 6, 12, 18, 24, 30 and 36, haemoglobin was measured. | |
| Notes |
Source of funding: the project was launched by the Chinese Ministry of Health and funded by UNICEF and CDC from 2003 to 2008. "Sight and Life through DSM contributed nutrient premix for the project and Beijing Vita Sci‐Tech Company contributed NaFeEDTA to the project." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The selection of intervention and control groups was done at the village level. However, there is no mention of any randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | No mention of any attempt of allocation concealment |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Low risk | Baseline outcomes for haemoglobin, free erythrocyte protoporphyrin, serum iron, serum zinc, serum retinol were similar in the control and experimental groups |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) | Low risk | Baseline anthropometric measures were similar for the two groups: height, weight, body fat, BMI, as was women's age |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of any attempt of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of any attempt of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The total number of women who received the intervention was not specified. The selection method was not specified for the women that were assessed in the trial. |
| Contamination (checking for possible performance bias) | Unclear risk | It is unclear how far the intervention and control areas were from each other. While it was possible for farmers to buy more wheat flour from the market, it is not mentioned if they could sell or exchange the flour provided through the project. The methodology does mention that "a record note book was used to record flour sources and amount of consumption in a household." |
| Selective reporting (reporting bias) | High risk | Serum folic acid concentrations were mentioned in the methods but no data were presented. No protocol or trial registry entry was found. |
| Other bias | Low risk | Not apparent |
Huo 2012.
| Methods | Non‐randomised intervention trial to assess the effect of wheat flour fortified with multiple micronutrients on the nutritional status of rural‐dwelling women in China | |
| Participants | In Gansu province, participants were selected from two different villages. 978 households from the intervention village received fortified wheat flour. 640 households from the control village received unfortified wheat flour. Women aged 20 to 60 years were randomly selected from both villages for outcome assessment. From each village, 329 non‐pregnant women were screened and a total of 545 women were enrolled. By the end of the study, 97 women dropped out: 42 from the control group and 55 from the intervention group. | |
| Interventions | This study was carried out in the context of a government reforestation programme whereby farmers received "compensation" flour in exchange for not farming their lands. Farmers residing in the intervention village received wheat flour fortified with 2 mg/kg of vitamin A in the form of retinol acetate (not clear if this nutrient was added to flour), 3.5 mg/kg of thiamin in the form of thiamin hydrochloride, 3.5 mg/kg of riboflavin in the form of riboflavin, 35 mg/kg niacin in the form of niacinamide, 1.5 mg/kg folic acid in the form of folic acid, 25 mg/kg of zinc in the form of zinc oxidate and 20 mg/kg of iron in the form of electrolytic iron. Farmers residing in the control village received wheat flour that was not fortified. All participating farmers received compensation flour twice a year: in January and July. The fortified flour was only available for the intervention families, packaged in 25 kg bags and labelled with the nutrient formulation and dosage of fortificant. The amount of compensation flour provided to all families depended on the amount of land that was given up for reforestation. The intervention was implemented from October 2004 to October 2007. | |
| Outcomes | Anaemia, haemoglobin and serum folate concentrations were measured. Haemoglobin was measured using the HemoCue method (HemoCue 2019). Serum folic acid was measured using a German AXSYM kit. At baseline and month 36, serum folic acid was measured. At baseline and months 6, 12, 18, 24, 30 and 36, haemoglobin was measured. | |
| Notes |
Source of funding: the project was launched by the Chinese Ministry of Health and funded by UNICEF and US CDC from 2003 to 2008. UNICEF also provided financial support. DSM contributed the fortification premix. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The selection of intervention and control groups was done at the village level. However, there is no mention of any randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | No mention of any attempt of allocation concealment |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Low risk | Baseline outcome measurements were similar for serum folic acid, anaemia, haemoglobin, and for serum iron, zinc and retinol |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) | Low risk | The authors noted that "The two villages have similar characteristics in terms of location, economic levels, and ethnic groups. They are both at an altitude of approximately 1,500 m." "There were no significant differences between the control and intervention groups at baseline for the following: women's age, height, weight, BMI, body fat, and educational level, and household size and family income." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The fortified flour the intervention group received was labelled with the nutrients and nutrient concentrations were added to the flour. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of 545 women enrolled, 97 dropped out of the study. Sample sizes varied for the number of women who participated in data collection from baseline to month 36. The authors note that "this was primarily due to personal reasons, such as travel or illness during data collection.” |
| Contamination (checking for possible performance bias) | Unclear risk | It is unclear how far the intervention and control areas are from each other, or if there was any possibility for families to exchange or sell the government‐provided flour. Families were able to buy more flour, as needed, from the market. |
| Selective reporting (reporting bias) | Unclear risk | According to what was stated in the methodology, all outcomes were reported. No protocol or trial registry entry was found. |
| Other bias | Low risk | Not apparent |
Ionescu‐Ittu 2009.
| Methods | Interrupted time series analysis of prevalence of congenital hearth defects before and after flour fortification with folic acid, comprising time points 1990 to 2005, 9‐yearly periods before, 7‐yearly periods after flour fortification | |
| Participants | All infants (live births and stillbirths) born in Quebec, with severe congenital heart defects, including tetralogy of Fallot, endocardial cushion defects, univentricular hearts, truncus arteriosus, and transposition complex. | |
| Interventions | This was an observational study with no intervention. The objective of this time trend analysis was to estimate the change of congenital heart defect prevalence after fortification. | |
| Outcomes | The birth prevalence of severe congenital heart defects was determined annually and defined as the number of infants (live births and stillbirths) born with severe congenital heart defects per 1000 births. Time trends were estimated the period before and after fortification by Poisson regression. The cases of congenital heart defects were identified from administrative databases. | |
| Notes | Study was conducted in Canada
Source of funding: the study was funded in part by the Heart and Stroke foundation of Canada and by the Fonds de Recherche en Santé du Québec. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not a RCT; this is an interrupted time series analysis |
| Allocation concealment (selection bias) | High risk | Not a RCT; there was no treatment allocated |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | High risk | This is an interrupted time series analysis. |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) | High risk | This is an interrupted time series analysis. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This is an interrupted time series analysis; there was no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This is an interrupted time series analysis; there was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The cases were captured by administrative databases with full coverage of births in Quebec. |
| Contamination (checking for possible performance bias) | Unclear risk | This is an interrupted time series analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Low risk | Not apparent |
Rahman 2015.
| Methods | Double‐blind cluster‐randomised controlled trial, to assess the impact of wheat flour fortified with multiple micronutrients on haemoglobin and micronutrient status in children | |
| Participants | Children 6 to 15 years living in 'baris' which have 5 to 6 households containing 30 to 35 individuals. Exclusion criteria were as follows: children younger than 6 years (who receive vitamin A supplementation on a 6‐month basis) or children with a severe illness. Analysis was completed on 191 children (intervention group) with a mean (SD) age 10.4 (2.68) years (SD) and 143 children (control group) with a mean (SD) age of 10.3 (2.86) years. | |
| Interventions | Children residing in intervention baris received chapattis made from wheat flour fortified with (per 100 grams of flour): 150 µg (0.15 mg) folic acid, 212 µg vitamin A as retinyl palmitate, 6.6 mg iron as hydrogen‐reduced elemental iron, 0.64 mg thiamin, 0.40 mg riboflavin, 3.3 mg zinc as zinc oxide, 5.3 mg niacin as niacinamide. Children residing in control baris received chapattis made from unfortified wheat flour. The flour was 80% extraction (usually considered high extraction). On a daily basis (in the mornings) for 6 months, children received chapattis made with 100 grams of wheat flour. Children's consumption was monitored by an adult. | |
| Outcomes | The primary outcome of the study was vitamin A status and the secondary outcomes included haemoglobin. Blood draws were taken at baseline, 3 months and 6 months after initiation of the fortification intervention. For haemoglobin measurement, venous blood was pipetted in a ethylenediaminetetraacetic acid‐coated tube. Haemoglobin from whole blood was measured using the methaemoglobin method. Markers of folate status were not outcomes of interest in the study; they were not measured. | |
| Notes | Study conducted in Bangladesh
Source of funding: grant from the MOST project (Contract No. HRN‐AA‐00–98‐00047‐00) and by support to the Mirsarai field area by USAID Cooperation Agreement number 388‐A‐00–97‐00032‐00 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | As noted by the authors, "using a statistics book‐generated random number table", baris (clusters) were allocated to the intervention or control group; indicating randomisation at the cluster level not at individual participant level. |
| Allocation concealment (selection bias) | Low risk | The flour codes were only known by a person not involved with the study. And the code was unlocked after the analyses were completed. Furthermore, the randomisation of cluster was done all at once. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Low risk | Among 8 child‐specific outcomes compared at baseline between the intervention and control groups, 1 was statistically significantly different (serum transferrin receptor > 5 mg/L) and 7 were not (serum retinol, haemoglobin, 2 biomarkers of iron status and the percentage of children with vitamin A deficiency, anaemia and iron deficiency due to low serum ferritin concentrations). This indicates that there was low risk of baseline imbalance among the control and intervention clusters at baseline. |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) | Low risk | At baseline, between the intervention and control groups, there was a statistically significant difference for BMI for age z‐scores, and no difference for sex, age, weight, and height. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The fortified and unfortified flours were packaged in 700 gram bags that were identical, made from polyethylene, and labelled with a blinded code based on flour type. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The flour codes were only known by a person not involved with the study. And the code was unlocked after the analyses were completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 203 children were enrolled in the intervention group, 12 were lost to follow‐up, and 191 completed the study. 149 children were enrolled in the control group, 6 were lost to follow‐up, and 143 completed the study. There was no intention‐to‐treat analysis or reasons listed for losses to follow‐up. |
| Contamination (checking for possible performance bias) | Low risk | Flour was given to bari family members who were not the index child (who received the chapatti daily), to reduce the sharing of chapattis intended for the child. |
| Selective reporting (reporting bias) | Unclear risk | According to the methodology, all outcomes were reported. No protocol or trial registry entry was found. |
| Other bias | Low risk | Not apparent |
| Recruitment bias | Low risk | It does not mention if individuals were recruited before or after randomisation. However, blinding was ensured during the intervention period so it is unlikely that there was recruitment bias. |
| Baseline imbalance | Low risk | Baseline characteristics were presented for individuals but not for clusters. However, the study authors adjusted for age, sex, and baseline values in their analyses. |
| Loss of clusters | Low risk | No clusters were lost to follow‐up. |
| Incorrect analysis | Low risk | The authors included 'cluster' as a random‐effect in mixed‐model analyses. |
| Compatibility with individual RCTs | Low risk | It is unlikely that the outcomes in this study would be effected by a cluster design. |
Sanchez 2011.
| Methods | Randomised controlled trial to compare the effect of maize flour fortified with folic acid and other micronutrients on micronutrient status, compared with unfortified flour or folic acid supplements | |
| Participants | 45 women aged 21 to 26 years, who had reached menarche, did not have a history of previous birth defects, living in the rural surroundings of the Nuevo Leon state in Mexico, where there is a high birth prevalence of neural tube defects. Women belonged to a low socioeconomic status. Women were selected from a convenience sample. | |
| Interventions | Women were randomised to one of three groups: group A (N = 18) received maize flour fortified with 0.05 mg folic acid/100 grams of flour, zinc and riboflavin; group B (N = 17) received unfortified maize flour; group C (N = 10) received folic acid supplements (5.0 mg folic acid weekly). Flour was meant to be used to prepare 8 tortillas daily during 3 months. In group A, the tortillas were intended to contribute 128 µg of folic acid daily. Women kept their usual diet, which included beans ‐ the largest source of dietary folate. The women receiving fortified flour were trained in cooking the flour. The intervention lasted 3 months. | |
| Outcomes | Plasma vitamin B12, plasma folate, and red blood cell folate were assessed at baseline and endline (after 3 months of intervention). Plasma and erythrocyte folate concentrations and plasma vitamin B12 were assessed by solid‐phase radioimmunoassay. Erythrocyte folate concentrations were calculated by multiplying the data from the standard curve with the dilution factor, and dividing the product by the haematocrit value. DNA analysis was also conducted for the MTHFR polymorphism. | |
| Notes | Study conducted in Mexico
Source of funding: Department of Genetics and Endocrinology Services, Faculty of Medicine, Universidad Autónoma de Nuevo León, Mexico |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There is no detail on how randomisation was undertaken; the authors only mention that it was randomised. |
| Allocation concealment (selection bias) | Unclear risk | No information regarding allocation concealment |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | Low risk | Baseline outcome measures were reported for all outcomes. Table 2 shows that the values for plasma folate are 0.016 pmol/L to 0.017 pmol/L (or 0.000016 nmol/L to 0.000017 nmol/L) at baseline in all three groups, which demonstrates high similarity at baseline. |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) | Low risk | No significant differences among groups at baseline for nutritional status based on weight categories using BMI. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding reported in the groups that received fortified or unfortified flour. No measures for blinding the group receiving folic acid supplement were reported i.e. providing non‐fortified flour, and placebo tablets to other groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses to follow‐up. |
| Contamination (checking for possible performance bias) | Unclear risk | There is no information on how contamination could have been prevented, or if intervention and control participants were from the same village, or if they knew who was receiving fortified flour. |
| Selective reporting (reporting bias) | Unclear risk | According to the methodology, all outcomes seem to be reported. No protocol or trial registry entry was found. |
| Other bias | Low risk | Not apparent |
Wang 2016.
| Methods | Non‐randomised controlled trial to evaluate the effectiveness of fortified wheat flour for the prevention of neural tube defects | |
| Participants | A total of 11 villages from Shanxi province were randomly selected: 8 villages from Zhongyang and Jiaokou counties (intervention group) and 3 villages from Liulin county (control group). The goal was to recruit all women dwelling in the counties who were planning to get married or to get pregnant during a specific time period. 16,811 women were recruited for the intervention group and 7260 women were recruited for the control group. 16,648 and 7037 women from the intervention and control groups, respectively, contributed data to the analysis of flour consumption and the prevalence of neural tube defects at birth. For the serologic indicators serum folate and homocysteine, 217 women who had delivered a baby were randomly selected; however, 46 women from the intervention group were excluded because they consumed fortified flour for fewer than 12 months. | |
| Interventions | Wheat flour was provided to selected villages for a period of 24 months. Fortified wheat flour contained folic acid (2 mg/kg), vitamin B1 (3.5 mg/kg), vitamin B2 (3.5 mg/kg), iron (30 mg/kg), and zinc (25 mg/kg). Women in the intervention group were asked to keep their regular dietary habits but change ordinary flour to fortified flour. In all villages of the intervention group, fortified flour was distributed to families with women of childbearing age (18 to 35 years old) in groups. Unfortified wheat flour was provided to the control group. | |
| Outcomes | Serum folate (nmol/L) and homocysteine concentrations (µmol/L) were reported only at endpoint. No information was presented on how the biomarkers were laboratory measured. Prevalence of neural tube defects (spina bifida, encephalocoele (fatal and non‐fatal), anencephaly (fatal) was also reported at endpoint. Classification of the birth defects was based on ICD‐10 codes (WHO 2019). | |
| Notes | Study conducted in China
Source of funding: this study was supported by the National Key Project (973) of Study on Interaction Mechanism of Environment and Genetic Birth Defect in China (Grant No. 2007CB5119001), State Key Funds of Social Science Project (Research on Disability Prevention Measurement in China, Grant No. 09&ZD072), the Cai Zhai Scholarship of Graduate School of Peking University (Grant No. CZ201316), and the UNFPA/CPA Small Research Grants for Young Scholar. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This was a non‐randomised trial with no details provided on the method used for randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No clear information about allocation concealment was provided. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) | High risk | None of the outcomes of interest were measured at baseline: serum folate, homocysteine concentrations, and neural tube defects. |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) | Low risk | Two sociodemographic factors were statistically compared between the intervention and control groups: women's age and formal education. Neither was statistically different between the intervention and control groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. It could have been possible to implement blinding of the flour package but no information was provided in this regard. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 16,811 women recruited for the intervention group, 16,648 contributed data for the analysis. Of the 7260 women recruited for the control group, 7037 contributed data to the analysis. |
| Contamination (checking for possible performance bias) | Low risk | Selected intervention and control villages did not neighbour each other. Fortified flour was not sold in the control villages; however it is possible that there was unauthorised distribution of fortified flour in the control villages. |
| Selective reporting (reporting bias) | Unclear risk | According to what was described in the methodology, all outcomes were reported. No protocol or trial registry entry was found. |
| Other bias | Low risk | Not apparent |
BMI: body mass index Hb: haemoglobin L‐5‐MTHF: L‐5‐methyltetrahydrofolic ppm: parts per million SD: standard deviation SE: standard error
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aaron 2011a | 132 adult men from Senegal participated in this randomised double‐blind trial to assess the changes in plasma zinc concentration with different vehicles. Participants received: 1) 200 g/day of wheat bread fortified with iron and folic acid, but not zinc, and a liquid MV supplement without zinc between meals; 2) the same bread product and the same MV supplement between meals with 15 mg zinc added to the supplement; 3) the same bread co‐fortified with 7.5 mg zinc and the same MV supplement without zinc between meals; or 4) the same bread co‐fortified with 15 mg zinc and the same MV supplement without zinc between meals. All participants received folic acid fortified wheat bread. This type of intervention is out of the scope of this review. |
| Aaron 2011b | 24 young children (12 to 17 months of age) and their caregivers in Senegal were provided complementary foods fortified with 60 mg iron per kg or 60 mg iron plus 240 mg zinc per kg. Another group of 32 adults (> 18 years of age) were provided wheat bread fortified with either: 1) 15 mg iron and 1.5 µg folic acid per kg of flour; 2) the same amount of iron and folic acid plus 63 mg zinc per kg of flour; 3) the same amount of iron and folic acid plus 126 mg zinc per kg flour. All intervention arms received the same amount of folic acid, and therefore this intervention is outside the scope of this review. |
| Adamson 2001 | This study analyses the impact of intake of folic acid fortified foods after national fortification in Australia. Dietary data from adolescents collected in 1999 were analysed for intake of total folate and white flour from all sources. The amount of folic acid from fortified foods was estimated to establish the amount of folic acid that adolescents were consuming. This study design is outside the scope of this review. |
| Agnoletti 2011 | This is a book chapter without any original data. It is outside the scope of this review. |
| AJP 2012 | This is an editorial article without any original data. It is outside the scope of this review. |
| Bayston 2007 | This in an editorial letter without any original data. It is outside the scope of this review. |
| Bazzano 1978 | Abstract or full‐text not available |
| Bower 2003 | This article discusses the history of folic acid foritifcation and its impact on the incidence of neural tube defects in New Zealand. It does not include primary data and is outside the scope of this review. |
| Bower 2010 | This is correspondence addressesing a study that found an increase in the incidence of cancer rates from pre‐ to postfortification. It does not include primary data and is outside the scope of this review. |
| Brito 2015 | This is a conference abstract published in the European Journal of Nutrition and Food Safety. Folate and vitamin B12 concentrations were assessed in 159 older Chilean adults to determine the association between folate and vitamin B12 status and nerve conduction. All participants were exposed to folic acid fortification. This study design is outside the scope of this review. |
| Bronberg 2016 | Using national level data, this study assessed temporal and spatial distribution of birth outcomes and infant and foetal deaths by phases of folic acid fortification in Brazil. This study design is outside the scope of this review. |
| Callaghan‐Gillespie 2017 | 1828 moderately malnourished pregnant women in Malawi were randomised to receive ready‐to‐use supplememental food, a fortified corn‐soy blend with a multiple micronutrient supplement, or a fortified corn‐soy blend with an iron and folic acid supplement. Newborn anthropometrics were assessed for differences between groups. The study population was malnourished and outside the scope of this review. |
| Carlsson 2002a | This is an abstract from a conference without original full data. It is outside the scope of this review. |
| Carlsson 2002b | This is a letter to the editor of Annals of Internal Medicine. It has no original data and is outside the scope of this review. |
| CIGNIS 2010 | 743 infants (6 months ± 2 weeks of age) were randomised to receive either conventional porridge or micronutrient fortified porridge fortified that contained 9 or 18 micronutrients, respectively. Both porridges were made of mostly maize flour and also included groundnuts, bambaranuts, and beans. The conventional porridge was fortified with 0.65 mg folic acid per kg flour and the micronutrient porridge was fortified with 2.21 mg folic acid per kg flour. This study compared two doses of folic acid fortification instead of comparing folic acid fortification to a control. This intervention is outside the scope of this review. |
| Colman 1974b | The families of 1 lactating woman and 5 women who were 28 weeks pregnant at the Charles Johnson Memorial Hospital at Nqutu, KwaZulu in South Africa were provided folic acid foritifed maize meal for 6 weeks. Folic acid was added to provide approximately 500 µg of folic acid daily to each adult family member. Haemoglobin, red cell folate, and vitamin B12 were measured weekly in the women and the oldest member of each family. This study design is outside the scope of this review. |
| Colman 1974c | 5 lactating women who were patients admitted to the hospital with severe folate‐deficient megaloblastic anaemia were provided with folic acid fortified maize meal porridge for approximately 2 weeks. Patient 1 received 100 µg folic acid per day, patients 2 and 3 received 300 µg folic acid per day, and patients 4 and 5 received 500 ug folic acid per day. Red cell and reticulocyte count were measured daily. This study compared three doses of folic acid fortification instead of comparing folic acid fortification to a control. The comparator is outside the scope of this review. |
| Colman 1975a | 7 adults were given one of three interventions: 1) folic acid fortified maize porridge, 2) folic acid fortified rice, or 3) folic acid fortified bread. The absorption of folic acid from these foods was compared to the absorption of an aqueous solution of pteroylglutamic acid in the same subjuct. The comparator is outside the scope of this review. |
| Colman 1975b | Haemoglobin, red cell folate, and vitamin B12 concentrations, and flour consumption patterns were assessed in 469 adults (> 16 years of age) in Nqutu, KwaZulu in South Africa. 144 of these participants were pregnant women in their third trimester. This survey describes the distribution of these outcomes in this population. No intervention was provided, and this study is outside the scope of this review. |
| Colman 1982 | This is a book chapter without any original data. It is outside the scope of this review. |
| DAZ 2004 | This is a magazine article which describes the results from a study that simulated folic acid fortification. It has no original study and is outside the scope of this review. |
| Doyle 1995 | This article comments on the importance of implementing folic acic fortification of flour in the UK. This study design is out of the scope of this review. |
| Fleischman 2011 | This is a commentary published in the American Journal of Public Health and is outside the scope of this review. |
| Freire 2000 | This article describes preliminary findings from a study in Santiago, Chile, among women of reproductive age with at least one child. Serum and red cell folate were assessed at baseline before folic acid fortification (October ‐ December 1999) and will be assessed again one year after folic acid fortification. Only baseline folate status was reported. This study design is outside the scope of this review. |
| Ganes 2002 | This article is an author's reply. It does not have original data and is outside the scope of this review. |
| Gardiner 2009 | This is an editorial article without original full data. It is outside the scope of this review. |
| Glosz 2016 | This abstract describes a study conducted in moderately malnourished pregnant women in Malawi. Women received either a ready‐to‐use supplementary food, corn‐soy blend flour with a multiple micronutrient supplement, or corn‐soy blend flour with an iron and folic acid supplement. The study population was malnourished, which is outside the scope of this review. |
| Hansen 2001 | This study has a 2 x 2 factorial design to determine the effect of added folic acid on zinc absorption from white bread prepared with wheat flour. 15 healthy women of childbearing age (22‐33 years old) were randomised to receive 4 types of bread meals made with wheat flour fortified with folic acid or zinc or both in a 2 x 2 factorial cross‐over design. Each meal consisted of 2 rolls, and were made with 4 different formulations: 1) low zinc; 2) low zinc + folic acid; 3) high zinc; and 4) high zinc + folic acid. Each formulation was consumed as a single meal spaced 2 weeks apart. The subjects’ habitual daily intakes of folate were 308 ± 60 µg. Reported outcomes include serum folate and erythrocyte folate pooled for all participants exposed to all formulations. The intervention of this study is outside the scope of this review. |
| Hirsch 2011 | This is a review article without any original data. It is outside the scope of this review. |
| Hurrell 2015 | This is a commentary published in the British Journal of Nutrition. It has no original data and is outside the scope of this review. |
| Jacobson 1995 | This is a letter to the editor of the BMJ. It has no original data and is outside the scope of this review. |
| Janmohamed 2016 | This cluster‐randomised trial assessed the effect of a maize‐soy blend plus fortified flour in subsistence rice farming villages in central Cambodia. 75 villages were cluster‐randomised and 547 pregnant women enrolled during their first trimester of pregnancy. Women in the intervention villages received one 6.75 kg bag of maize‐soy blend (CSB) fortified flour composed of 75% to 80% maize and 20% to 25% soybeans from enrolment until delivery. The flour was fortified with folic acid, vitamin A, thiamin, riboflavin, niacin, pantothenic acid, vitamins B6, B12, C, D, E, K, iodine, iron, phosphorus, calcium, potassium,and zinc. The daily CSB Plus ration (200 g of dry flour) provided 760 kcal, 27 g protein (14% of total kcal), and 5 g fat (6% of total kcal). Palmolein oil fortified with vitamin A and vitamin D was provided with the flour with instructions to use during the cooking process. The control group did not receive any flour or oil. Both groups received iron and folic acid supplements and antenatal care. This study was excluded because the intervention group received a cointervention (i.e. fortified oil) that was not also provided to the control group. |
| Jian 2013 | This is an abstract that describes a study in Shanxi province in China among women aged 18 to 39. 155 women received fortified flour for 20 months while 63 women did not receive fortified flour. The full‐text is not available. |
| Jiang 2011 | In this study, 48 random women of reproductive age received wheat fortified flour as part of a government programme. The flour was fortified with 20 mg folic acid, 3.5 mg vitamin B1, 3.5 mg vitamin B2, 30 mg ferric sodium edetate and 25 mg zinc oxide per kilogram. The study reports only on laboratory analysis from those samples conducting a serum metabolomic fingerprinting. No other information on study design or participant characteristics was reported. This study design is outside of the scope of this review. |
| Johansson 2002 | 29 adult healthy women were randomised to consume a wheat‐based breakfast roll with low folic acid content (166 µg/roll) or high folic acid content (355 µg/roll) each day for 3 months. For the low folic acid batch, 367.3 mg folic acid/100 kg dough was added to give a folic acid concentration of 200 µg/roll. For the high folic acid batch, 733.4 mg folic acid/100 kg dough was added to give a concentration of 400 µg folic acid/roll. Folic acid fortification ocurred at the dough stage. The intervention of this study is outside the scope of this review. |
| Jooma 2004 | This is a review article without any original data. It is outside the scope of this review. |
| Kelly 1997 | This study aimed to examine the appearance of unmetabolised folic acid in serum in response to the consumption of fortified foods. 23 participants, male and female aged 18 to 42 years, were exposed to a 5‐day regimen of fortified ready‐to‐eat‐cereal and bread in addition to their normal diet. The bread (commercially available white milk bread) was fortified by applying microlitre amounts of a freshly prepared folic acid solution (10 g/L) to 10 evenly spaced regions of individual bread slices with a micropipette. This study also assessed unmetabolised folic acid in 30 healthy elderly volunteers ager 63 to 82 years who consumed the fortified bread and an isotonic saline solution. The intervention of this study is outside the scope of the present review. |
| Kirby 2000 | This is a letter to the editor of The New England Journal of Medicine. It has no original data and is outside the scope of this review. |
| Landim 2016 | 262 pre‐school children were randomised to receive one of two interventions: 1) cookies made from wheat flour fortified with iron and folic acid; 2) cookies made from a mix of wheat flour fortified with iron and folic acid and cowpea flour fortified with iron and zinc. All participants received fortified wheat flour. The comparator is outside the scope of this review. |
| Langman 2005 | This is a retrospective cohort study that assessed the changes in phenytoin and valproic acid concentrations after folic acid fortification in Canada. This study design is outside the scope of this review. |
| Margo 1975 | 15 pregnant women less than 37 weeks gestation were selected from Charles Johnson Memorial Hospital in South Africa. For four weeks, participants received a 100 g slice of bread daily. Each slice was fortified with 900 µg crystalline folic acid with the intention to provide 300 µg folic acid daily. Each participant received the same intervention. This study design is outside the scope of this review. |
| McNulty 2001 | This is a commentary published in the British Journal of Nutrition and is outside the scope of this review. |
| Metz 1986 | This is an editorial published in the South African Medical Journal and is outside the scope of this review. |
| Mirarefin 2007 | 17 men and women aged 61 ± 5 years with hyperhomocysteinemia received bread fortified with 100 µg folic acid daily for 8 weeks. Plasma homocysteine and serum folate concentrations were measured at baseline and at 8 weeks. This study was excluded because the intervention was targeted towards participants with hyperhomocysteinemia. |
| Monch 2015 | 24 adults participated in a cross‐over design study to examine the bioavaiablity of folate from four different foods. Participants received a meal that included folate from Camembert cheese, wheat germ, heated spinach, or a pteroylmonoglutamic acid solution, and a 14 day washout period between treatments. None of the treatment arms included fortified flour. |
| Morinigo Martinez 2017 | 105 participants with coeliac disease in Paraguay were given two versions (fortified or unfortified) of two types of baked goods (bread and sticks) and asked to evaluate color, odour, taste, hardness, appearance, and overall score using a 9‐point hedonic scale. This study is not among the general population and is outside the scope of this review. |
| Nackers 2010 | 807 moderately malnourished children age 6 to 59 months in Niger were randomised to receive ready‐to‐use therapeutic food or a corn‐soy‐blend flour premix. Anthropometry and haemoglobin were measured. The study population was malnourished which is outside the scope of this review. |
| Northrop‐Clewes 2013a | A national survey was undertaken in Uzbekistan to assess the coverage of fortified flour in the population and its impact on iron and folate levels in women of reproductive age and the knowledge of women of dietary concepts after 5 years of national fortification. This study design is outside the scope of this review. |
| Northrop‐Clewes 2013b | This is an abstract describing a cross‐sectional study in Cote D'Ivoire among households consuming fortified oil and wheat. Pre‐school age children were assessed for haemoglobin, iron, and vitamin A status. Women of reporductive age were assessed for haemoglobin, iron, vitamin A, and folate status. This study design is outside the scope of this review. |
| Paniz 2015 | In this thesis a subgroup of participants receiving 5 g of folic acid per day and participants with thalassemia were assessed for serum and erythrocyte folate, messenger RNA (mRNA) expression of ihydrofolate reductase (DHFR) and methylenetetrahydrofolate reductase (MTHFR) as well as other cytokines, to evaluate if serum concentrations of unmetabolised folic acid affect global DNA methylation. The intervention in this study is outside the scope of this review. |
| Pfeiffer 1997 | In this study the absorption of folic acid in fortified white and whole wheat bread, rice, or pasta or in solution was evaluated in 47 men and women aged 20 to 35 years, using a single‐dose, dual‐label, stable isotope protocol that did not involve prior loading of subjects with non‐labelled folate. The bread was fortified with [13C5 ]folic acid at both the sponge and dough steps to an extent of 70% and 30% of the total amount added. This intervention is outside the scope of this review. |
| Pouraram 2015 | This is an abstract describing a cohort study of 202 adults in Iran. Oxidative stress indices were measured before and after an 8‐month intervention of iron fortified wheat flour. Folic acid was not part of the intervention. |
| Ray 2005 | This is a letter to the editor of The American Journal of Medicine. It has no original data and is outside the scope of this review. |
| SAJCN 2003 | This article is an introduction to an informational programme. It has no original data and is outside the scope of this review. |
| Serdula 2010 | This is a summary of a workshop on wheat and maize flour fortification with iron, folic acid, vitamin B12, vitamin A, and zinc. It has no original data and is outside the scope of this review. |
| Skeaff 1998 | This article describes the debate of folic acid fortification. It has no original data and is outside the scope of this review. |
| Sweeney 2006 | Participants were four adults between the ages of 20 and 30 years. They consumed bread made with fortified flour in four different regimens: 1) one slice of bread, which was fortified with 1 mg folic acid; 2) 2 slices of bread with 500 µg folic acid each and consumed 4 hours apart; 3) three slices of bread fortified with 333 µg folic acid each; 4) five slices of bread fortified with 200 µg folic acid each was consumed by each subject; 5) 10 slices of bread fortified with 100 µg each. After consuming a slice of bread, post‐prandial blood samples were collected for red cell folate and serum folate analyses. This was repeated for each slice of bread in the regimen. Participants were exposed to each of this regimens for only one day with two weeks between each regimen. This study does not assess the health impact of folate fortification of wheat flour and therefore is excluded. |
| Sweeney 2007 | 20 adults in Ireland received folic acid supplements for 14 weeks to become folic acid replete. This was followed with week‐long interventions of consuming 2 slices of bread daily for a total of 400 µg, 200 µg, and 50 µg of folic acid per day for each of the three weeks, respectively. During bread making, the folic acid was added to the water and then mixed with the flour and other ingredients. During 1‐week washout periods between interventions, participants received 400 µg of supplemental folic acid per day. Serum total folate and folic acid were assessed. The folic acid was not added at the flour stage and therefore this intervention is outside the scope of this review. |
| Thurston 1999 | This article describes reasons to support folic acid fortification in New Zealand. It has no original data and is outside the scope of this review. |
| Tinker 2013 | A model was developed to estimate the percentage reduction in the prevalence of spina bifida and anencephaly that could occur with folic acid fortification in the Hispanic population of the USA. This type of study is outside the scope of this review. |
| Vahteristo 2002 | 64 participants were randomised to a four‐week intervention of either rye bread and orange juice or wheat bread fortified with folic acid and apple juice. Serum and red cell folate and homocysteine were assessed. During bread making, the folic acid was added to the water and then mixed with the flour and other ingredients. The folic acid was not added at the flour stage. The intervention and comparator in this study are outside the scope of this review. |
| van den Wijngaart 2013 | This is an article that summarises the results from studies that assessed sensory, physical, and nutritional attributes of foods fortified with folic acid in China, India, Indonesia, Malaysia, the Philippines, and Sri Lanka. It has no original data and is outside the scope of this review. |
| Wald 1995 | This is a letter to the editor with no original data and outside the scope of this review. |
| Wald 2001 | This article summarises the importance of fortifying food with folic acid. It has no original data and is outside the scope of this review. |
| Wald 2002 | This letter to the editor comments on a published study on the management and control of hypertension. It has no original data and is outside the scope of this review. |
| Walker 1983 | 6 adults aged 18 to 25 years in South Africa were given fortified or unfortified maize meal porridge in a triangular design to assess the acceptability of folic acid fortified porridge. To prepare the porridge, folic acid was added to water and then mixed with the maize meal. This intervention is outside the scope of this review. |
| Westerman 1947 | This study was conducted in rats and is outside the scope of this review. |
| Westerman 1948 | This study was conducted in rats and is outside the scope of this review. |
| Wharton 2001 | This article describes the steps that should be followed for folic acid fortification programmes. It has no original data and is outside the scope of this review. |
| Winkels 2008 | In this study, men and women aged 50 to 75 years in the Netherlands were randomly assigned in this 12‐week double‐blind, placebo‐controlled trial to consume bread fortified with 138 µg folic acid and 9.6 µg vitamin B12 daily (N = 72) or unfortified bread (N = 70). Fortification occurred at the dough stage. This intervention is beyond the scope of this review. |
| Wise 1999 | This is a commentary published in the New Zealand Medical Journal. It has no original data and is outside the scope of this review. |
| Yang 2004 | A population‐based cohort study in the USA and Canada assessed the trends in stroke‐related mortality before and after folic acid fortification programmes. This study design was outside the scope of this review. |
| Zavaleta 2004 | 250 infants under six months of age were randomised to receive flour fortified with either 1) iron; 2) iron and zinc; 3) iron and vitamin A; 4) iron and folic acid; or 5) iron, zinc, vitamin A, and folic acid for 9 months. The participants in this study were under two years of age and therefore, this is outside the scope of this review. |
| Zheng 2011 | This was a retrospective cohort study among women in China that compared serum homocysteine, serum folate, and the prevalence of neural tube defects before and after wheat flour fortification with multiple micronutrients. This study design is outside the scope of this review. |
MV: multivitamin
Differences between protocol and review
We had planned to compare any form of folic acid fortification of wheat flour, maize flour, or maize meal, with or without other micronutrients, with other strategies to improve folate status and reduce folate deficiency in the wider population, but restricted the comparisons to fortification with folic acid (i.e. alone or in combination with other micronutrients) versus unfortified flour or no intervention. The efficacy and safety of folic acid supplementation has been reviewed elsewhere.
We planned to jointly assess the risk of bias for blinding of participants, personnel, and outcome assessor. However, in the review, we assessed blinding for outcome assessors separately from participants or personnel, in order to improve the assessment of any potential bias in outcome ascertainment.
We included specific criteria for assessing risk of bias for cluster‐randomised trials (i.e. recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, compatibility with individual RCTs).
Observational studies and uncontrolled before‐and‐after studies were not included in the meta‐analyses since other reviews have been published with a comprehensive assessment of this type of evidence; however, findings from these studies were summarised in Appendix 1 and in the Discussion section, along with programmatic evidence from other published reviews, meta‐analyses, and pre‐ and postintervention studies without a control group, in countries with folic acid fortification programmes.
We were not able to conduct subgroup analyses, including primary outcomes by age group, due to the limited number of included studies reporting data for the same outcome.
Contributions of authors
Julia Finkelstein contributed to the development of the protocol, along with other co‐authors. Elizabeth Centeno Tablante, Heather Guetterman, and Julia Finkelstein screened the studies, assessed eligibility, and extracted the data. Julia Finkelstein and Helena Pachón wrote the first draft of the Discussion section and Authors' conclusions, and all authors provided feedback.
Disclaimers: the findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The review authors alone are responsible for the views expressed in this publication and they do not necessarily represent the official position, decisions, policy or views of their organisations.
Sources of support
Internal sources
-
Centers for Disease Control and Prevention (CDC), Center for Chronic Disease Prevention and Health Promotion, USA.
Helena Pachón was supported by an agreement between the Centers for Disease Control and Prevention (CDC), McKing Consulting Corporation, and Emory University
External sources
-
Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, WHO, Switzerland.
Dr. Julia Finkelstein and Elizabeth Centeno Tablante's time were partially supported by the WHO Department of Nutrition for Health and Development
-
Bill and Melinda Gates Foundation, USA.
The Bill and Melinda Gates Foundation provided support to the WHO for the development of the systematic reviews of the evidence in nutrition interventions
-
Nutrition International, Canada.
Nutritional International provided support to the WHO for the development of the systematic reviews of the evidence in nutrition interventions
Declarations of interest
Elizabeth Centeno Tablante: has no affiliation with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review. Helena Pachón: works for the Food Fortification Initiative which receives funding from private, public and civic sector organisations to support its mission of providing technical assistance to countries in the area of food fortification. Heather Guetterman: has no affiliation with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review. Julia Finkelstein: has no affiliation with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review.
New
References
References to studies included in this review
Carrasco 2013 {published data only}
- Carrasco Quintero MR, Ortiz Hernández L, Roldán AJA, Chávez Villasana A, Aguirre Arenas J, Aguilar Carrasco FR. Effect of consumption of corn flour enriched with soja on nutrition status of indigenous women of Mexico [Efecto del consumo de una harina de maiz enriquecida con soya en el estado de nutricion de mujeres indigenas de Mexico]. Revista Española de Salud Pública 2013;87(3):293‐302. [DOI] [PubMed] [Google Scholar]
Colman 1974a {published data only}
- Colman N, Barker EA, Barker M, Green R, Metz J. Prevention of folate deficiency by food fortification. IV. Identification of target groups in addition to pregnant women in an adult rural population. American Journal of Clinical Nutrition 1975;28(5):471‐6. [DOI] [PubMed] [Google Scholar]
- Colman N, Barker EA, Barker M, Green R, Metz J. Prevention of folate deficiency in pregnancy by food fortification. American Journal of Clinical Nutrition 1974;27:339‐44. [DOI] [PubMed] [Google Scholar]
- Colman N, Green R, Metz J. Prevention of folate deficiency by food fortification. II. Absorption of folic acid from fortified staple foods. American Journal of Clinical Nutrition 1975;28(5):459‐64. [DOI] [PubMed] [Google Scholar]
- Colman N, Larsen JV, Barker M, Barker EA, Green R, Metz J. Prevention of folate deficiency by food fortification. III. Effect in pregnant subjects of varying amounts of added folic acid. American Journal of Clinical Nutrition 1975;28(5):465‐70. [DOI] [PubMed] [Google Scholar]
French 2003 {published data only}
- French AE, Grant R, Weitzman S, Ray JG, Vermeulen MJ, Sung L, et al. Folic acid fortification is associated with a decline in neuroblastoma. Clinical Pharmacology & Therapeutics 2003;74(3):288‐94. [DOI] [PubMed] [Google Scholar]
Green 2013 {published data only}
- Green TJ, Liu Y, Dadgar S, Li W, Böhni R, Kitts DD. Wheat rolls fortified with microencapsulated L‐5‐methyltetrahydrofolic acid or equimolar folic acid increase blood folate concentrations to a similar extent in healthy men and women. Journal of Nutrition 2013;143(6):867‐71. [DOI] [PubMed] [Google Scholar]
Huo 2011 {published data only}
- Huo J, Sun J, Huang J, Li W, Wang L, Selenje L, et al. The effectiveness of fortified flour on micro‐nutrient status in rural female adults in China. Asia Pacific Journal of Clinical Nutrition 2011;20(1):118‐24. [PubMed] [Google Scholar]
- Sun J, Huo J, Li W, Wang L. Observation on effectiveness of fortified flour on nutrition status improvement of poor area women in Weichang County of Hebei Province in China. Journal of Hygiene Research 2008;37(2):199‐202. [PubMed] [Google Scholar]
Huo 2012 {published data only}
- Huo J, Sun J, Huang J, Li W, Wang L, Selenje L, et al. Effectiveness of fortified flour for enhancement of vitamin and mineral intakes and nutrition status in northwest Chinese villages. Food and Nutrition Bulletin 2012;33(2):161‐168. [DOI] [PubMed] [Google Scholar]
Ionescu‐Ittu 2009 {published data only}
- Ionescu‐Ittu R, Marelli AJ, Mackie AS, Pilote L. Prevalence of severe congenital heart disease after folic acid fortification of grain products: time trend analysis in Quebec, Canada. BMJ 2009;338:b1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahman 2015 {published data only}
- Rahman AS, Ahmed T, Ahmed F, Alam MS, Wahed MA, Sack DA. Double‐blind cluster randomised controlled trial of wheat flour chapatti fortified with micronutrients on the status of vitamin A and iron in school‐aged children in rural Bangladesh. Maternal and Child Nutrition 2015;11(Suppl 4):120‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanchez 2011 {published data only}
- Sánchez MA, Carmen Esmer M, Martínez L, Varela N, Valdez R, Torres R, et al. Effect of fortified flour consumption on blood folate levels in women of childbearing age [Efecto del consumo de harina de maíz fortificada con acido fólico sobre los niveles de folatos sanguíneos en mujeres de edad fértil]. Revista Chilena de Nutricion 2011;38(2):178‐85. [Google Scholar]
Wang 2016 {published data only}
- Wang H, Steur H, Chen G, Zhang X, Pei L, Gellynck X, et al. Effectiveness of folic acid fortified flour for prevention of neural tube defects in a high risk region. Nutrients 2016;8(3):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aaron 2011a {published data only}
- Aaron GJ, Lo NB, Hess SY, Guiro AT, Wade S, Brown KH. Plasma zinc concentration increases within 2 weeks in healthy Senegalese men given liquid supplemental zinc, but not zinc‐fortified wheat bread. Journal of Nutrition 2011;141(7):1369‐74. [DOI] [PubMed] [Google Scholar]
- Aaron GJ, Lo NB, Hess SY, Guiro AT, Wade S, Brown KH. Plasma zinc concentration responds within two weeks to additional zinc delivered in a liquid supplement, but not zinc‐fortified wheat bread, in healthy Senegalese adult men. FASEB Journal 2011;25(1):Suppl 779.13. [Google Scholar]
Aaron 2011b {published data only}
- Aaron GJ, Lo NB, Hess SY, Guiro AT, Wade S, Ndiaye NF, et al. Acceptability of complementary foods and breads prepared from zinc‐Fortified cereal flours among young children and adults in Senegal. Journal of Food Science 2011;76(1):S56‐62. [DOI] [PubMed] [Google Scholar]
Adamson 2001 {published data only}
- Adamson AJ, Rugg‐Gunn AJ, Butler TJ, Moynihan PJ. Implications of fortification of white flour with folic acid on total folate intake by adolescents. Proceedings of the Nutrition Society 2001;60(OCA):58A. [Google Scholar]
Agnoletti 2011 {published data only}
- Agnoletti D, Zhang Y, Czernichow S, Galan P, Hercberg S, Safar ME, et al. Fortification of Vitamin B‐12 to Flour and the Metabolic Response. Flour and Breads and their Fortification in Health and Disease Prevention. Elsevier, 2011:437‐49. [DOI: 10.1016/b978-0-12-380886-8.10040-6] [DOI] [Google Scholar]
AJP 2012 {published data only}
- Folic acid flour fortification does not heighten the risk of paediatric cancer. Australian Journal of Pharmacy 2012; Vol. 93, issue 1102:77.
Bayston 2007 {published data only}
- Bayston R, Russell A, Wald N, Hoffbrand A. Folic acid fortification and cancer risk. Lancet 2007;370(9604):2004. [DOI] [PubMed] [Google Scholar]
Bazzano 1978 {published data only}
- Bazzano G, Trosclair G, Carlisle J. The effect of iron fortification of flour‐based products on hematology in two study groups. Clinical Research 1978;26(3):283A. [Google Scholar]
Bower 2003 {published data only}
- Bower C. Fortification of food with folic acid and the prevention of neural tube defects. New Zealand Medical Journal 2003;116:1168. [PubMed] [Google Scholar]
Bower 2010 {published data only}
- Bower C, Milne E. Colon cancer in Chile. European Journal of Gastroenterology and Hepatology 2010;22(7):900. [DOI] [PubMed] [Google Scholar]
Brito 2015 {published data only}
- Brito A, Miller JW, Fedosov SN, Shahab‐Ferdows S, Sanchez H, Albala C, et al. Sensory peripheral nerve function in Chilean elderly consuming folic acid fortified flour: relationship to plasma folate and a novel combined biomarker of B12 status. European Journal of Nutrition and Food Safety 2015;5(5):1067‐8. [Google Scholar]
Bronberg 2016 {published data only}
- Bronberg R, Dipierri J, Alfaro E, Sanseverino MT, Schuler‐Faccini L. Primary prevention of neural tube defects in Brazil: insights into anencephaly. Journal of Community Genetics 2016;7(1):97‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Callaghan‐Gillespie 2017 {published data only}
- Callaghan‐Gillespie M, Schaffner AA, Garcia P, Fry J, Eckert R, Malek S, et al. Trial of ready‐to‐use supplemental food and corn‐soy blend in pregnant Malawian women with moderate malnutrition: a randomized controlled clinical trial. American Journal of Clinical Nutrition 2017;106(4):1062‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlsson 2002a {published data only}
- Carlsson CM, Pharo LM, Stein JH. Effect of folic acid fortification of cereal grain flour on the prevalence of hyperhomocysteinemia in older adults. Journal of the American Geriatrics Society 2002;50(4):S135. [Google Scholar]
Carlsson 2002b {published data only}
- Carlsson CM, Stein JH. Clinical trials testing the homocysteine hypothesis. Annals of Internal Medicine 2002;137(4):295‐6. [DOI] [PubMed] [Google Scholar]
CIGNIS 2010 {published data only}
- The Chilean Infant Growth, Nutrition and Infection (CIGNIS) Study Team. Micronutrient fortification to improve growth and health of maternally HIV‐unexposed and exposed Zambian infants: a randomized controlled trial. Plos One 2010;5(6):e11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colman 1974b {published data only}
- Colman N, Larsen JV, Barker M, Barker EA, Green R, Metz J. Prevention of folate deficiency by food fortification. V. A pilot field trial of folic acid fortified maize meal. South African Medical Journal 1974;48(41):1763‐6. [PubMed] [Google Scholar]
Colman 1974c {published data only}
- Colman N, Green R, Stevens K, Metz J. Prevention of folate deficiency by food fortification. VI. The antimegaloblastic effect of folic acid‐fortified maize meal. South Africa Medical Journal 1974;48(42):1795‐8. [PubMed] [Google Scholar]
Colman 1975a {published data only}
- Colman N, Green R, Metz J. Prevention of folate deficiency by food fortification. II. Absorption of folic acid from fortified staple foods. American Journal of Clinical Nutrition 1975;28(5):459‐64. [DOI] [PubMed] [Google Scholar]
Colman 1975b {published data only}
- Colman N, Barker EA, Barker M, Green R, Metz J. Prevention of folate deficiency by food fortification. IV. Identification of target groups in addition to pregnant women in an adult rural population. American Journal of Clinical Nutrition 1975;28(5):471‐6. [DOI] [PubMed] [Google Scholar]
Colman 1982 {published data only}
- Colman N. Addition of folic acid to staple foods as a selective nutrition intervention strategy. Nutrition Reviews 1982;40(8):225‐33. [DOI] [PubMed] [Google Scholar]
DAZ 2004 {published data only}
- Folic acid deficiency: enriched flour for an improved folic acid supply. Deutsche Apotheker Zeitung 2004; Vol. 144, issue 39:105‐7.
Doyle 1995 {published data only}
- Doyle W. Folic acid in prevention of neural tube defects. Lancet 1995;345:389‐90. [PubMed] [Google Scholar]
Fleischman 2011 {published data only}
- Fleischman AR, Oinuma M. Fortification of corn masa flour with folic acid in the United States. American Journal of Public Health 2011;101(8):1360‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freire 2000 {published data only}
- Freire WB, Hertrampf E, Corets F. Effect of folic acid fortification in Chile: preliminary results. European Journal of Pediatric Surgery 2000;10(1):42‐3. [PubMed] [Google Scholar]
Ganes 2002 {published data only}
- Ganes S, Astrup AV, Stender S. Folic acid fortification of flour. Ugeskrift for Laeger 2002;164(35):4087‐8. [PubMed] [Google Scholar]
Gardiner 2009 {published data only}
- Gardiner HM, Fouron JC. Folic acid fortification and congenital heart disease. BMJ 2009;338(7705):1144. [DOI] [PubMed] [Google Scholar]
Glosz 2016 {published data only}
- Glosz CM, Reaves S, Manary M, Papathakis P. Assessment of micronutrient status in pregnant Malawian women before and after treatment for moderate malnutrition. FASEB Journal 2016;30(Suppl 1):892.9. [Google Scholar]
Hansen 2001 {published data only}
- Hansen M, Samman S, Madsen LT, Jensen M, Sorensen SS, Sandstrom B. Folic acid enrichment of bread does not appear to affect zinc absorption in young women. American Journal of Clinical Nutrition 2001;74(1):125‐9. [DOI] [PubMed] [Google Scholar]
Hirsch 2011 {published data only}
- Hirsch S, Pia de la Maza M, Barrera G, Leiva L, Bunout D. Folic Acid and Colon Cancer: impact of wheat flour fortification with folic acid. San Diego: Elsevier Academic Press Inc, 2011. [Google Scholar]
Hurrell 2015 {published data only}
- Hurrell RF. Flour fortification as a strategy to prevent anaemia. British Journal of Nutrition 2015;114(4):501‐2. [DOI] [PubMed] [Google Scholar]
Jacobson 1995 {published data only}
- Jacobson B. Folic acid and the prevention of neural tube defects. Chapati flour should be fortified as well. BMJ 1995;311(6999):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Janmohamed 2016 {published data only}
- Janmohamed A, Karakochuk CD, Boungnasiri S, Chapman GE, Janssen PA, Brant R, et al. Prenatal supplementation with Corn Soya Blend Plus reduces the risk of maternal anemia in late gestation and lowers the rate of preterm birth but does not significantly improve maternal weight gain and birth anthropometric measurements in rural Cambodian women: a randomized trial. American Journal of Clinical Nutrition 2016;103(2):559‐66. [DOI] [PubMed] [Google Scholar]
Jian 2013 {published data only}
- Jian H, Jing S, Junsheng H, Wenxian L, Chunming C. Effects of fortified flour consumption on serum folate and homocysteine in childbearing‐aged women in high prevalence regions of neurotube defect. Annals of Nutrition and Metabolism 2013;63:1530. [Google Scholar]
Jiang 2011 {published data only}
- Jiang Z, Liang Q, Wang Y, Zheng X, Pei L, Zhang T, et al. Metabonomic study on women of reproductive age treated with nutritional intervention: screening potential biomarkers related to neural tube defects occurrence. Biomedical Chromatography 2011;25(7):767‐74. [DOI] [PubMed] [Google Scholar]
Johansson 2002 {published data only}
- Johansson M, Witthoft CM, Bruce A, Jagerstad M. Study of wheat breakfast rolls fortified with folic acid ‐ The effect on folate status in women during a 3‐month intervention. European Journal of Nutrition 2002;41(6):279‐86. [DOI] [PubMed] [Google Scholar]
Jooma 2004 {published data only}
- Jooma R. Preventing neural tube defects by folic acid fortification of flour. JPMA. The Journal of the Pakistan Medical Association 2004;54:540‐1. [PubMed] [Google Scholar]
Kelly 1997 {published data only}
- Kelly P, McPartlin J, Goggins M, Weir DG, Scott J M. Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements. American Journal of Clinical Nutrition 1997;65(6):1790‐5. [DOI] [PubMed] [Google Scholar]
Kirby 2000 {published data only}
- Kirby RS. Fortification of foods with folic acid. New England Journal of Medicine 2000;343(13):971. [PubMed] [Google Scholar]
Landim 2016 {published data only}
- Landim L, Pessoa M, Brandao A, Morgano MA, Araujo MA, Rocha MD, et al. Impact of the two different iron fortified cookies on treatment of anemia in preschool children in Brazil. Nutricion Hospitalaria 2016;33(5):579. [DOI] [PubMed] [Google Scholar]
Langman 2005 {published data only}
- Langman LJ, Ray JG, Mamdani MM, Cole DEC. Absence of effect of folic acid flour fortification on anticonvulsant drug levels. Therapeutic Drug Monitoring 2005;27(2):232. [DOI] [PubMed] [Google Scholar]
Margo 1975 {published data only}
- Margo G, Barker M, Gernandes‐Costa F, Colman N, Green R, Metz J. Prevention of folate deficiency by food fortification. VII. The use of bread as a vehicle for folate supplementation. The American Journal of Clinical Nutrition 1975;28(7):761‐3. [DOI] [PubMed] [Google Scholar]
McNulty 2001 {published data only}
- McNulty H. Increasing evidence in favour of mandatory fortification with folic acid. British Journal of Nutrition 2001;86(4):425‐6. [DOI] [PubMed] [Google Scholar]
Metz 1986 {published data only}
- Metz J. Fortification of maize meal with folic acid. South African Medical Journal 1986;70(3):132‐3. [PubMed] [Google Scholar]
Mirarefin 2007 {published data only}
- Mirarefin M, Aminpour A, Fakhrzadeh H, Tahbaz F, Abadi A. The effect of folate fortified bread on homocysteine, folate and vitamin B12 status among hyperhomocysteinemic subjects: Tehran homocysteine study. Iranian Journal of Diabetes and Lipid Disorders 2007;7(2):229‐37+E23. [Google Scholar]
Monch 2015 {published data only}
- Monch S, Netzel M, Netzel G, Ott U, Frank T, Rychlik M. Folate bioavailability from foods rich in folates assessed in a short term human study using stable isotope dilution assays. Food and Function 2015;6(1):242‐8. [DOI] [PubMed] [Google Scholar]
Morinigo Martinez 2017 {published data only}
- Morinigo Martinez M, Cubilla Gimenez PV, Monsalvo Benitez MB, Acosta Escobar J. Enrichment of premix of flour suitable for celiac and acceptability of baked products. Annals of Nutrition and Metabolism 2017;Suppl 2:1183‐4. [Google Scholar]
Nackers 2010 {published data only}
- Nackers F, Broillet F, Oumarou D, Djibo A, Gaboulaud V, Guerin PJ, et al. Effectiveness of ready‐to‐use therapeutic food compared to a corn/soy‐blend‐based pre‐mix for the treatment of childhood moderate acute malnutrition in Niger. Journal of Tropical Pediatrics 2010;56(6):407‐13. [DOI] [PubMed] [Google Scholar]
Northrop‐Clewes 2013a {published data only}
- Northrop‐Clewes C, Hund L, Nazario R, Suleymanova D, Mirzoyan L, Irosova M, et al. Association between consumption of fortified first grade flour and iron and folate status of women of reproductive age in Uzbekistan. Annals of Nutrition and Metabolism 2013;63:1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Northrop‐Clewes 2013b {published data only}
- Northrop‐Clewes C, Rohner F, Raso G, Ake O, Tschannen A, Jungjohann S, et al. Effect of a fortified oil and flour program in Cote D'Ivoire on micronutrient status of pre‐school children and women. Annals of Nutrition and Metabolism 2013;63:1036. [Google Scholar]
Paniz 2015 {published data only}
- Paniz C. Efeitos do ácido fólico não metabolizado na metilação global do DNA, na expressão de RNAm dos genes de DHFR, MTHFR, interferon‐gamma, TNF‐alpha; e interleucina‐8, e na citotoxicidade das células NK [Doctorate dissertation]. Universidade de São Paulo, 2015. [Google Scholar]
Pfeiffer 1997 {published data only}
- Pfeiffer CM, Rogers LM, Bailey LB, Gregory JF 3rd. Absorption of folate from fortified cereal‐grain products and of supplemental folate consumed with or without food determined by using a dual‐label stable‐isotope protocol. American Journal of Clinical Nutrition 1997;66(6):1388‐97. [DOI] [PubMed] [Google Scholar]
Pouraram 2015 {published data only}
- Pouraram H, Abtahi M, Neyestani TR, Siassi F, Dorosty AR, Elmadfa I. Long term fortified bread consumption and oxidative stress biomarkers. Annals of Nutrition and Metabolism 2015;67(Suppl 1):448. [Google Scholar]
Ray 2005 {published data only}
- Ray JG, Langman LJ, Mamdani MM, Cole DE. Absence of effect of folic acid flour fortification on anticonvulsant drug levels. The American Journal of Medicine 2005;118(4):444‐5. [DOI] [PubMed] [Google Scholar]
SAJCN 2003 {published data only}
- Food fortification becomes a reality in South Africa. South African Journal of Clinical Nutrition 2003; Vol. 16, issue 2:39.
Serdula 2010 {published data only}
- Serdula M. Maximizing the impact of flour fortification to improve vitamin and mineral nutrition in populations. Food and Nutrition Bulletin 2013;31(Suppl 1):S86‐93. [DOI] [PubMed] [Google Scholar]
Skeaff 1998 {published data only}
- Skeaff M, Mann J. Should folate be added to flour to prevent neural tube defects?. New Zealand Medical Journal 1998;111(1077):417‐8. [PubMed] [Google Scholar]
Sweeney 2006 {published data only}
- Sweeney MR, McPartlin J, Weir DG, Daly L, Scott JM. Postprandial serum folic acid response to multiple doses of folic acid in fortified bread. British Journal of Nutrition 2006;95(1):145‐51. [DOI] [PubMed] [Google Scholar]
Sweeney 2007 {published data only}
- Sweeney MR, McPartlin J, Scott J. Folic acid fortification and public health: report on threshold doses above which unmetabolised folic acid appear in serum. BMC Public Health 2007;22(7):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thurston 1999 {published data only}
- Thurston LG. Should folate be added to flour?. New Zealand Medical Journal 1999;112(1080):20. [PubMed] [Google Scholar]
Tinker 2013 {published data only}
- Tinker S, Devine O, Mai C, Hamner H, Reefhuis J, Gilboa S, et al. Folic acid fortification of corn masa flour and neural tube defect prevention. American Journal of Epidemiology 2013;177(Suppl 11):S20. [Google Scholar]
Vahteristo 2002 {published data only}
- Vahteristo L, Kariluoto S, Barlund S, Karkkainen M, Lamberg‐Allardt C, Salovaara H, et al. Functionality of endogenous folates from rye and orange juice using human in vivo model. European Journal of Nutrition 2002;41(6):271‐8. [DOI] [PubMed] [Google Scholar]
van den Wijngaart 2013 {published data only}
- Wijngaart A, Codling K. Impact of flour fortification on common Asian wheat flour‐based foods. Cereal Foods World 2013;58(5):238‐45. [Google Scholar]
Wald 1995 {published data only}
- Wald NJ, Gilbertson MP. Folic acid in prevention of neural tube defects. Lancet 1995;345(8946):389. [PubMed] [Google Scholar]
Wald 2001 {published data only}
- Wald NJ. Folic acid and neural tube defects. Bibliotheca Nutritio et Dieta 2001;55:22‐3. [DOI] [PubMed] [Google Scholar]
Wald 2002 {published data only}
- Wald D, Law M, Wald N. The serum folate response to the US mandatory fortification of grain products with folic acid. Archives of Internal Medicine 2002;162(19):2254. [DOI] [PubMed] [Google Scholar]
Walker 1983 {published data only}
- Walker AR, Walker BF, Metz J. Acceptability trials of maize meal fortified with niacin, riboflavin and folic acid. South African Medical Journal 1983;64(10):343‐6. [PubMed] [Google Scholar]
Westerman 1947 {published data only}
- Westerman BD, Hall G. Improving the nutritive value of flour; the effect of supplementing enriched flour with other B‐complex vitamins. The Journal of Nutrition 1947;33(3):301‐2. [DOI] [PubMed] [Google Scholar]
Westerman 1948 {published data only}
- Westerman BD, Templeton F. Improving the nutritive value of flour; further studies on the effect of supplementing enriched flour with B‐complex vitamins and some observations on the use of 80% extraction flour. The Journal of Nutrition 1948;36(2):187‐203. [DOI] [PubMed] [Google Scholar]
Wharton 2001 {published data only}
- Wharton B, Booth I. Fortification of flour with folic acid. BMJ 2001;323(7323):1198‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winkels 2008 {published data only}
- Winkels RM, Brouwer IA, Clarke R, Katan MB, Verhoef P. Bread cofortified with folic acid and vitamin B12 improves the folate and vitamin B12 status of healthy older people: a randomized controlled trial. American Journal of Clinical Nutrition 2008;88(2):348‐55. [DOI] [PubMed] [Google Scholar]
Wise 1999 {published data only}
- Wise C. Should folate be added to flour. New Zealand Medical Journal 1999;112(1087):167. [PubMed] [Google Scholar]
Yang 2004 {published data only}
- Yang Q, Botto LD, Erickson JD, Berry RJ, Sambell C, Johansen H, et al. Improvement in Stroke Mortality in Canada and the United States, 1990 to 2002. Circulation 2006;113:1335‐43. [DOI] [PubMed] [Google Scholar]
- Yang QH, Friedman JM, Botto LD. Improvement in cardiovascular and stroke mortality after flour fortification with folic acid in the United States. Circulation 2004;109(15):E190. [Google Scholar]
Zavaleta 2004 {published data only}
- Zavaleta N, Abram S, Lonnerdal B. Effect of wheat flour fortification with iron with and without zinc, vitamin A and folic acid on serum copper in Peruvian infants. Pediatric Research 2004;55(3):530. [Google Scholar]
Zheng 2011 {published data only}
- Zheng X, Pei L, Zhang T, Song X, Hu CH. The change of prevalence of NTD in high prevalence areas of China before and after wheat‐flour fortification with multinutrients 2006‐2007. Intensive Care Medicine 2011;37(Suppl 2):S398‐9. [Google Scholar]
Additional references
Allen 2008
- Allen LH. Causes of vitamin B12 and folate deficiency. Food and Nutrition Bulletin 2008;29(2 Suppl):S20‐34; discussion S35‐7. [PUBMED: 18709879] [DOI] [PubMed] [Google Scholar]
Arth 2016
- Arth A, Kancherla V, Pachon H, Zimmerman S, Johnson Q, Oakley GP Jr. A 2015 global update on folic acid‐preventable spina bifida and anencephaly. Birth Defects Research (Part A) 2016;106:520‐9. [DOI] [PubMed] [Google Scholar]
Ashong 2012
- Ashong J, Muthayya S, De‐Regil LM, Laillou A, Guyondet C, Moench‐Pfanner R, et al. Fortification of rice with vitamins and minerals for addressing micronutrient malnutrition. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD009902] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atta 2016
- Atta CA, Fiest KM, Frolkis AD, Jette N, Pringsheim T, Germaine‐Smith C, et al. Global birth prevalence of spina bifida by folic acid fortification status: a systematic review and meta‐analysis. American Journal of Public Health 2016;106:e24‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bailey 2015
- Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory JF 3rd, Mills JL, et al. Biomarkers of nutrition for development‐folate review. The Journal of Nutrition 2015;145(7):1636S‐80S. [PUBMED: 26451605] [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Berry 2010
- Berry RJ, Mulinare J, Hamner HC. Folic acid fortification: neural tube defect risk reduction—a global perspective. In: LB Bailey editor(s). Folate in Health and Disease. 2nd Edition. Boca Raton: CRC Press, 2010. [Google Scholar]
Black 2014
- Black AP, Vally H, Morris P, Daniel M, Esterman A, Smith F, et al. High folate levels in Aboriginal children after subsidised fruit and vegetables and mandatory folic acid fortification. Australian and New Zealand Journal of Public Health 2014;4(38):241‐6. [DOI] [PubMed] [Google Scholar]
Boilson 2012
- Boilson A, Staines A, Kelleher CC, Daly L, Shirley I, Shrivastava A, et al. Unmetabolized folic acid prevalence is widespread in the older Irish population despite the lack of mandatory fortification program. American Journal of Clinical Nutrition 2012;96(3):613‐21. [DOI] [PubMed] [Google Scholar]
Botto 1999
- Botto LD, Moore CA, Khoury MJ, Erickson JD. Neural‐tube defects. New England Journal of Medicine 1999;341(20):1509‐19. [DOI] [PubMed] [Google Scholar]
Bower 2009
- Bower C, D'Antoine H, Stanley FJ. Neural tube defects in Australia: trends in encephaloceles and other neural tube defects before and after promotion of folic acid supplementation and voluntary food fortification. Birth Defects Research. Part A, Clinical and Molecular Teratolology 2009;85(4):269‐73. [DOI] [PubMed] [Google Scholar]
Britto 2014
- Britto JC, Cançado R, Guerra‐Shinohara EM. Concentrations of blood folate in Brazilian studies prior to and after fortification of wheat and cornmeal (maize flour) with folic acid: a review. Revista Brasileira Hematologia e Hemoterapia 2014;36(4):275‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castillo‐Lancellotti 2013
- Castillo‐Lancellotti C, Tur JA, Uauy R. Impact of folic acid fortification of flour on neural tube defects: a systematic review. Public Health Nutrition 2013;16(5):901‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 1992
- Centers for Disease Control and Prevention. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR. Morbidity and Mortality Weekly Report 1992;41(RR‐14):1‐7. [PubMed] [Google Scholar]
CDC 2004
- Centers for Disease Control and Prevention. Spina bifida and anencephaly before and after folic acid mandate‐‐United States, 1995‐1996 and 1999‐2000. MMWR. Morbidity and Mortality Weekly Report 2004;53(17):362‐5. [PubMed] [Google Scholar]
CDC 2008
- Centers for Disease Control and Prevention. Trends in wheat‐flour fortification with folic acid and iron‐worldwide, 2004 and 2007. MMWR. Morbidity and Mortality Weekly Report 2008;57(1):8‐10. [PubMed] [Google Scholar]
CDC 2010
- Centers for Disease Control and Prevention. CDC Grand Rounds: additional opportunities to prevent neural tube defects with folic acid fortification. MMWR. Morbidity and Mortality Weekly Report 2010;59(31):980‐4. [PubMed] [Google Scholar]
Christianson 2006
- Christianson A, Modell B, Howson C. March of Dimes global report on birth defects: the hidden toll of dying and disabled children. www.marchofdimes.org/materials/global‐report‐on‐birth‐defects‐the‐hidden‐toll‐of‐dying‐and‐disabled‐children‐executive‐summary.pdf (accessed 17 August 2017).
Cochrane EPOC 2017
- Cochrane Effective Practice and Organisation of Care (EPOC) Group. Data collection form. EPOC Resources for Review Authors, 2017. epoc.cochrane.org/resources/epoc‐specific‐resources‐review‐authors (accessed prior to 30 May 2019).
Cochrane PHG 2010
- Cochrane Public Health Group. Guide for developing a Cochrane protocol. ph.cochrane.org/sites/ph.cochrane.org/files/uploads/Guide%20for%20PH%20protocol_Nov%202011_final%20for%20website.pdf (accessed 8 April 2016).
Codex Alimentarius 1985a
- Codex Alimentarius. CODEX standard for whole maize (corn) meal (CODEX STAN 154‐1985). www.fao.org/fao‐who‐codexalimentarius/sh‐proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCODEX%2BSTAN%2B154‐1985%252FCXS_154e.pdf (accessed 26 July 2012):1‐3.
Codex Alimentarius 1985b
- Codex Alimentarius. CODEX standard for degermed maize (corn) meal and maize (corn) grits (CODEX STAN 155‐1985). www.fao.org/fao‐who‐codexalimentarius/sh‐proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCODEX%2BSTAN%2B155‐1985%252FCXS_155e.pdf (accessed 26 July 2012):1‐3.
Codex Alimentarius 1995a
- Codex Alimentarius. Codex standard for wheat flour. Codex Standard 152‐1985. Adopted 1985. Revision 1995. www.fao.org/fao‐who‐codexalimentarius/sh‐proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCODEX%2BSTAN%2B152‐1985%252FCXS_152e.pdf (accessed 17 February 2016).
Codex Alimentarius 1995b
- Codex Alimentarius. Codex standard for durum wheat semolina and durum wheat flour. Codex Standard 178‐1991. Adopted 1991. Revision 1995. www.fao.org/fao‐who‐codexalimentarius/sh‐proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCODEX%2BSTAN%2B178‐1991%252FCXS_178e.pdf (accessed 17 February 2016).
Cole 2007
- Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA 2007;297(21):2351‐9. [DOI] [PubMed] [Google Scholar]
Crider 2011
- Crider KS, Bailey LB, Berry RJ. Folic acid food fortification‐its history, effect, concerns, and future directions. Nutrients 2011;3(3):370‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cui 2010
- Cui R, Iso H, Date C, Kikuchi S, Tamakoshi A, Japan Collaborative Cohort Study Group. Dietary folate and vitamin B6 and B12 intake in relation to mortality from cardiovascular diseases: Japan collaborative cohort study. Stroke 2010;41(6):1285‐9. [DOI] [PubMed] [Google Scholar]
Czeizel 1992
- Czeizel AE, Dudás I. Prevention of the first occurrence of neural‐tube defects by periconceptional vitamin supplementation. New England Journal of Medicine 1992;327(26):1832‐5. [DOI] [PubMed] [Google Scholar]
Czeizel 2000
- Czeizel AE. Primary prevention of neural‐tube defects and some other major congenital abnormalities: recommendations for the appropriate use of folic acid during pregnancy. Paediatric Drugs 2000;2(6):437‐49. [DOI] [PubMed] [Google Scholar]
Czeizel 2013
- Czeizel AE, Dudás I, Vereczkey A, Bánhidy F. Folate deficiency and folic acid supplementation: the prevention of neural‐tube defects and congenital heart defects. Nutrients 2013;5(11):4760‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dary 2002
- Dary O. Staple food fortification with iron: a multifactorial decision. Nutrition Reviews 2002;60(7 Pt 2):S34‐41; discussion S46‐9. [DOI] [PubMed] [Google Scholar]
Das 2013
- Das JK, Salam RA, Kumar R, Bhutta ZA. Micronutrient fortification of food and its impact on woman and child health: a systematic review. Systematic Reviews 2013;2:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Das 2014
- Das JK, Salam RA, Kumar R, Lassi ZS, Bhutta ZA. Food fortification with multiple micronutrients: impact on health outcomes. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Benoist 2008
- Benoist B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food and Nutrition Bulletin 2008;29(2 Suppl):S238‐44. [DOI] [PubMed] [Google Scholar]
De Wals 2007
- Wals P, Tairou F, Allen MI, Uh SH, Lowry RB, Sibbald B, et al. Reduction in neural‐tube defects after folic acid fortification in Canada. New England Journal of Medicine 2007;357(2):135‐42. [DOI] [PubMed] [Google Scholar]
De‐Regil 2013
- De‐Regil LM, Peña‐Rosas JP, Flores‐Ayala R, Socorro Jefferds ME. Development and use of the generic WHO/CDC logic model for vitamin and mineral interventions in public health programmes. Public Health Nutrition 2013;17(3):634‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
De‐Regil 2015
- De‐Regil LM, Peña‐Rosas JP, Fernández‐Gaxiola AC, Rayco‐Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD007950.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunn 2014
- Dunn ML, Jain V, Klein BP. Stability of key micronutrients added to fortified maize flours and corn meal. Annals of the New York Academy of Sciences 2014;1312:15‐25. [DOI] [PubMed] [Google Scholar]
EPOC 2009
- Cochrane Effective Practice and Organisation of Care Group. Risk of bias for studies with a separate control group (RCTs, CCTs, CBAs). EPOC resources for review authors, 2009. epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/Suggested%20risk%20of%20bias%20criteria%20for%20EPOC%20reviews.pdf (accessed 8 April 2016).
FAO 2012
- Food, Agriculture Organization of the United Nations. FAO Statistical Yearbook. World Food and Agriculture. Rome: Food and Agriculture Organization of the United Nations, 2012. [Google Scholar]
FDA 2011
- US Food, Drug Administration. Subpart B‐Requirements for specific standardized cereal flours and related products, 21 C.F.R. § 137 part B. Code of Federal Regulations (CFR) Title 21 Food and Drug Part 137—Cereals flours and related products. Silver Spring, Maryland: US Food and Drug Administration, 2011. [Google Scholar]
Fernández‐Gaxiola 2011
- Fernández‐Gaxiola AC, De‐Regil LM. Intermittent iron supplementation for reducing anaemia and its associated impairments in menstruating women. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009218.pub2] [DOI] [PubMed] [Google Scholar]
FFI 2018a
- Food Fortification Initiative. Global Progress. www.ffinetwork.org/global_progress/index.php (accessed 6 August 2018).
FFI 2018b
- Food Fortification Initiative. Food Fortification Initiative database. www.ffinetwork.org (accessed prior to 3 June 2019).
Fiedler 2014
- Fiedler JL, Afidra R, Mugambi G, Tehinse J, Kabaghe G, Zulu R, et al. Maize flour fortification in Africa: markets, feasibility, coverage, and costs. Annals of the New York Academy of Sciences 2014;1312:26‐39. [DOI] [PubMed] [Google Scholar]
Finer 2006
- Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspectives on Sexual and Reproductive Health 2006;38(2):90‐6. [DOI] [PubMed] [Google Scholar]
Ganji 2009
- Ganji V, Kafai MR. Hemoglobin and hematocrit values are higher and prevalence of anemia is lower in the post‐folic acid fortification period than in the pre‐folic acid fortification period in US adults. The American Journal of Clinical Nutrition 2009;89(1):363‐71. [PUBMED: 19056553] [DOI] [PubMed] [Google Scholar]
Garcia‐Casal 2016
- Garcia‐Casal MN, Peña‐Rosas JP, Pachón H, De‐Regil LM, Centeno Tablante E, Flores‐Urrutia MC. Staple crops biofortified with increased micronutrient content: effects on vitamin and mineral status, as well as health and cognitive function in the general population. Cochrane Database of Systematic Reviews 2016, Issue 8. [Google Scholar]
Garcia‐Casal 2018
- Garcia‐Casal MN, Pena‐Rosas JP, De‐Regil LM, Gwirtz JA, Pasricha SR. Fortification of maize flour with iron for controlling anaemia and iron deficiency in populations. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD010187.pub2; PUBMED: 30577080] [DOI] [PMC free article] [PubMed] [Google Scholar]
García‐Casal 2002
- García‐Casal MN, Layrisse M. Iron fortification of flours in Venezuela. Nutrition Reviews 2002;60(7 Pt 2):S26‐9. [DOI] [PubMed] [Google Scholar]
GFDx 2018
- Global Fortification Data Exchange. www.FortificationData.org (accessed 6 August 2018).
Goudet 2015
- Goudet SM, Griffiths PL, Bogin BA, Madise NJ. Nutritional interventions for preventing stunting in children (0 to 5 years) living in urban slums in low and middle‐income countries (LMIC). Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Grosse 2016
- Grosse SD. Retrospective assessment of cost savings from prevention: folic acid fortification and spina bifida in the U.S. American Journal of Preventive Medicine 2016;5(Suppl 1):S74‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guamuch 2014
- Guamuch M, Dary O, Rambelson Z, Cruz V, Villalpando S, Tom C, et al. Model for estimating nutrient addition contents to staple foods fortified simultaneously: Mexico and Kampala data. Annals of the New York Academy of Sciences 2014;1312:76‐90. [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables‐binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles‐continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [DOI] [PubMed] [Google Scholar]
Gwirtz 2014
- Gwirtz JA, Garcia‐Casal MN. Processing maize flour and corn meal food products. Annals of the New York Academy of Sciences 2014;1312:66‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamner 2014
- Hamner HC, Tinker SC. Fortification of corn masa flour with folic acid in the United States: an overview of the evidence. Annals of the New York Academy of Sciences 2014;1312:8‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
HAWC 2015
- Health Assessment Workspace Collaboration. Folic acid‐cancer pooled and meta‐analyses: draft‐all pooled studies. hawcproject.org/summary/data‐pivot/assessment/94/draft‐all‐pooled‐studies (accessed 6 August 2018).
HemoCue 2019
- HemoCue 2019. HemoCue. www.hemocue.com (accessed 11 June 2019).
Herbert 1961
- Herbert V. The assay and nature of folic acid activity in human serum. Journal Clinical Investigation 1961;40:81‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herbst 2001
- Herbst ST. The Food Lover's Companion. 3rd Edition. New York: Barron's Educational Series, 2001. [Google Scholar]
Hertrampf 2003
- Hertrampf E, Cortés F, Erickson JD, Cayazzo M, Freire W, Bailey LB, et al. Consumption of folic acid‐fortified bread improves folate status in women of reproductive age in Women of Reproductive Age in Chile. Journal of Nutrition 2003;133(10):3166‐69. [DOI] [PubMed] [Google Scholar]
Hibbard 1965
- Hibbard ED, Smithells RW. Folic acid metabolism and human embryopathy. Lancet 1965;1:1254. [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hirsch 2002
- Hirsch S, Maza P, Barrera G, Gattas V, Petermann M, Bunout D. The Chilean flour folic acid fortification program reduces serum homocysteine levels and masks vitamin B12 deficiency in elderly people. Journal of Nutrition 2002;132(2):289‐91. [DOI] [PubMed] [Google Scholar]
Hoffbrand 1966
- Hoffbrand AV, Newcombe BFA, Mollin DL. Method of assay of red cell folate activity and the value of the assay as a test for folate deficiency. Journal of Clinical Pathology 1966;19:17‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Honein 2001
- Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 2001;285(23):2981‐6. [DOI] [PubMed] [Google Scholar]
ILO 1984
- International Labor Organization. Small‐Scale Maize Milling. Geneva: International Labor Organization, 1984. [Google Scholar]
Kelly 1997b
- Kelly P, McPartlin J, Goggins M, Weir DG, Scott JM. Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements.. American Journal of Clinical Nutrition 1997;65(6):1790‐5. [DOI] [PubMed] [Google Scholar]
Khan 2009
- Khan K, Shewry PR. Wheat: Chemistry and Technology. 4th Edition. AACC International, 2009. [Google Scholar]
Lassi 2013
- Lassi ZS, Salam RA, Haider BA, Bhutta ZA. Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD006896.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Llanos 2007
- Llanos A, Hertrampf E, Cortes F, Pardo A, Grosse SD, Uauy R. Cost‐effectiveness of a folic acid fortification program in Chile. Health Policy 2007;83(2‐3):295‐303. [DOI] [PubMed] [Google Scholar]
Lo 2014
- Lo A, Polšek D, Sidhu S. Estimating the burden of neural tube defects in low‐ and middle‐income countries. Journal of Global Health 2014;4(1):010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Low 2016
- Low MS, Speedy J, Styles CE, De‐Regil LM, Pasricha SR. Daily iron supplementation for improving anaemia, iron status and health in menstruating women. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD009747.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
López‐Camelo 2005
- López‐Camelo JS, Orioli IM, Graça Dutra M, Nazer‐Herrera J, Rivera N, Ojeda ME, et al. Reduction of birth prevalence rates of neural tube defects after folic acid fortification in Chile. American Journal of Medical Genetics. Part A 2005;135(2):120‐5. [DOI] [PubMed] [Google Scholar]
Makhumula 2014
- Makhumula P, Dary O, Guamuch M, Tom C, Afidra R, Rambeloson Z. Legislative frameworks for corn flour and maize meal fortification. Annals of the New York Academy of Sciences 2014;1312:91‐104. [DOI] [PubMed] [Google Scholar]
McNulty 2004
- McNulty H, Pentieva K. Folate bioavailability. Proceedings of the Nutrition Society 2004;63(4):529‐36. [DOI] [PubMed] [Google Scholar]
Mills 2003
- Mills JL, Kohorn I, Conley MR, Zeller JA, Cox C, Williamson RE, et al. Low vitamin B‐12 concentrations in patients without anemia: the effect of folic acid fortification of grain. American Journal of Clinical Nutrition 2003;77:1474‐7. [DOI] [PubMed] [Google Scholar]
Modjadji 2007
- Modjadji SE, Alberts M, Mamabolo RL. Folate and iron status of South African non‐pregnant rural women of childbearing, age, before and after fortification of foods. South African Journal of Clinical Nutrition 2007;20(3):89‐93. [Google Scholar]
Molloy 2008
- Molloy AM, Kirke PN, Brody LC, Scott JM, Mills JL. Effects of folate and vitamin B12 deficiencies during pregnancy on fetal, infant, and child development. Food and Nutrition Bulletin 2008;29(2 Suppl):S101‐11. [DOI] [PubMed] [Google Scholar]
Molloy 2018a
- Molloy AM. Should vitamin B12 status be considered in assessing risk of neural tube defects?. Annals of the New York Academy of Sciences 2018;1414(1):109‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Molloy 2018b
- Molloy AM, Mills JL. Fortifying food with folic acid to prevent neural tube defects: are we now where we ought to be?. American Journal of Clinical Nutrition 2018;107(6):857‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moretti 2014
- Moretti D, Biebinger R, Bruins MJ, Hoeft B, Kraemer K. Bioavailability of iron, zinc, folic acid, and vitamin A from fortified maize. Annals of the New York Academy of Sciences 2014;1312:54‐65. [DOI] [PubMed] [Google Scholar]
Morris 2010
- Morris MS, Jacques PF, Rosenberg IH, Selhub J. Circulating unmetabolized folic acid and 5‐methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. American Journal of Clinical Nutrition 2010;91(6):1733‐44. [DOI] [PubMed] [Google Scholar]
MRC 1991
- MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 1991;338(8760):131‐7. [PubMed] [Google Scholar]
Nazer 2013
- Nazer HJ, Cifuentes OL. Effects of wheat flour fortification with folic acid on the prevalence of neural tube defects in Chile [Resultados del Programa de Prevención de Defectos de Tubo Neural en Chile mediante la fortificación de la harina con ácido fólico. Período 2001‐2010]. Revista Medica de Chile 2013;141(6):751‐7. [DOI] [PubMed] [Google Scholar]
Noor 2017
- Noor RA, Abioye AJ, Ulenga N, Msham S, Kaishozi G, Gunaratna NS, et al. Large–scale wheat flour folic acid fortification program increases plasma folate levels among women of reproductive age in urban Tanzania. PLoS ONE 2017;12(8):e0182099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nuss 2010
- Nuss ET, Tanumihardjo SA. Maize: a paramount staple crop in the context of global nutrition. Comprehensive Reviews in Food Science and Food Safety 2010;9(4):417‐36. [DOI] [PubMed] [Google Scholar]
Odewole 2013
- Odewole OA, Williamson RS, Zakai NA, Berry RJ, Judd SE, Qi YP, et al. Near‐elimination of folate‐deficiency anemia by mandatory folic acid fortification in older US adults: Reasons for Geographic and Racial Differences in Stroke study 2003‐2007. American Journal of Clinical Nutrition 2013;98(4):1042‐7. [PUBMED: 23945721] [DOI] [PMC free article] [PubMed] [Google Scholar]
Okebe 2011
- Okebe JU, Yahav D, Shbita R, Paul M. Oral iron supplements for children in malaria‐endemic areas. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD006589.pub3] [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2014a
- Peña‐Rosas JP, Garcia‐Casal MN, Pachón H, McLean MS, Arabi M. Technical considerations for maize flour and corn meal fortification in public health: consultation rationale and summary. Annals of the New York Academy of Sciences 2014;1312:1‐7. [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2014b
- Peña‐Rosas J, Field MS, Burford BJ, De‐Regil L. Wheat flour fortification with iron for reducing anaemia and improving iron status in populations. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011302] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peña‐Rosas 2015a
- Peña‐Rosas JP, De‐Regil LM, Gomez Malave H, Flores‐Urrutia MC, Dowswell T. Intermittent oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD009997.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peña‐Rosas 2015b
- Peña‐Rosas JP, De‐Regil LM, Garcia‐Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD004736.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pfeiffer 2016
- Pfeiffer CM, Sternberg MR, Hamner HC, Crider KS, Lacher DA, Rogers LM, et al. Applying inappropriate cutoffs leads to misinterpretation of folate status in the US population. American Journal of Clinical Nutrition 2016;104(6):1607‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
POGO 2019
- Pediatric Oncology Group of Ontario. POGONIS – Childhood Cancer Database. https://www.pogo.ca/research‐data/pogonis‐childhood‐cancer‐database/ (accessed 11 June 2019).
Qi 2014
- Qi YP, Do AN, Hamner HC, Pfeiffer CM, Berry RJ. The prevalence of low serum vitamin B‐12 status in the absence of anemia or macrocytosis did not increase among older U.S. adults after mandatory folic acid fortification. Journal of Nutrition 2014;144(2):170‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ranum 2014
- Ranum P, Peña‐Rosas JP, Garcia‐Casal MN. Global maize production, utilization, and consumption. Annals of the New York Academy of Sciences 2014;1312:105‐12. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rimm 1998
- Rimm EB, Willett WC, Hu FB, Sampson L, Colditz GA, Manson JE, et al. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA 1998;279(5):359‐64. [DOI] [PubMed] [Google Scholar]
Salomao 2017
- Salomão RM, Cervante TP, Salomão JF, Leon SV. The mortality rate after hospital discharge in patients with myelomeningocele decreased after implementation of mandatory flour fortification with folic acid. Arquivos de Neuro‐Psiquiatria 2017;75(1):20‐4. [DOI] [PubMed] [Google Scholar]
Sayed 2008
- Sayed AR, Bourne D, Pattinson R, Nixon J, Henderson B. Decline in the prevalence of neural tube defects following folic acid fortification and its cost‐benefit in South Africa. Birth Defects Research. Part A, Clinical and Molecular Teratology 2008;82(4):211‐6. [DOI] [PubMed] [Google Scholar]
Seal 2008
- Seal A, Kafwembe E, Kassim I, Hong M, Wesley A, Wood J, et al. Maize meal fortification is associated with improved vitamin A and iron status in adolescents and reduced childhood anaemia in a food aid‐dependent refugee population. Public Health Nutrition 2008;11(7):720‐8. [DOI] [PubMed] [Google Scholar]
Seibel 2006
- Seibel W. Composite flours. In: Popper L, Schäfer W, Freund W editor(s). Future of Flour ‐ a Compendium of Flour Improvement. Clenze: Verlag Agrimedia, 2006:193‐8. [Google Scholar]
Shah 2016
- Shah D, Sachdev HS, Gera T, De‐Regil LM, Peña‐Rosas JP. Fortification of staple foods with zinc for improving zinc status and other health outcomes in the general population. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smithells 1976
- Smithells RW, Sheppard S, Schorah CJ. Vitamin deficiencies and neural tube defects. Archives of Disease in Childhood 1976;51(12):944‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stabler 2010
- Stabler SP. Clinical folate deficiency. In: Bailey LB editor(s). Folate in Health and Disease. 2nd Edition. Boca Raton: CRC Press, 2010:409‐28. [Google Scholar]
Tazhibayev 2008
- Tazhibayev S, Dolmatova O, Ganiyeva G, Khairov K, Ospanova F, Oyunchimeg D, et al. Evaluation of the potential effectiveness of wheat flour and salt fortification programs in five Central Asian countries and Mongolia, 2002‐2007. Food and Nutrition Bulletin 2008;29:255‐65. [DOI] [PubMed] [Google Scholar]
Troen 2006
- Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. Journal of Nutrition 2006;136(1):189‐94. [DOI] [PubMed] [Google Scholar]
Ueffing 2011
- Ueffing E, Tugwell P, Welch V, Petticrew M, Kristjansson E, Campbell and Cochrane Equity Methods Group. Equity checklist for systematic review authors. www.campbellcollaboration.org/artman2/uploads/1/C2_Equity_Checklist.pdf (accessed 8 April 2016).
Ulrich 2008
- Ulrich CM, Reed MC, Nijhout HF. Modeling folate, one‐carbon metabolism, and DNA methylation. Nutrition Reviews 2008;66(Suppl 1):S27‐30. [DOI] [PubMed] [Google Scholar]
US Preventive Services Task Force 2017
- US Preventive Services Task Force. Folic acid supplementation for the prevention of neural tube defects: U.S. Preventive Services Task Force recommendation statement. JAMA 2017;317(2):183–9. [DOI] [PubMed] [Google Scholar]
USDA 2014
- Fernandez‐Cornejo J, Wechsler S, Livingston M, Mitchell L. Genetically Engineered Crops in the United States. www.ers.usda.gov/publications/pub‐details/?pubid=45182 (accessed prior to 3 June 2019).
Van Guelpen 2006
- Guelpen B, Hultdin J, Johansson I, Hallmans G, Stenling R, Riboli E, et al. Low folate levels may protect against colorectal cancer. Gut 2006;55(10):1461‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Uitert 2013
- Uitert EM, Steegers‐Theunissen RP. Influence of maternal folate status on human fetal growth parameters. Molecular Nutrition & Food Research 2013;57(4):582‐95. [DOI] [PubMed] [Google Scholar]
WHO 2007
- World Health Organization. Prevention of neural tube defects: Integrated management of pregnancy and childbirth. Standards for maternal and neonatal care [Programa nacional del Perú de fortificación con ácido fólico y su efecto sobre los defectos del tubo neural en Lima]. www.who.int/reproductivehealth/publications/maternal_perinatal_health/neural_tube_defects.pdf (accessed 7 April 2017).
WHO 2009
- World Health Organization. Recommendations on wheat and maize flour fortification meeting report: interim consensus statement. apps.who.int/iris/bitstream/10665/111837/1/WHO_NMH_NHD_MNM_09.1_eng.pdf?ua=1&ua=1. Geneva: World Health Organization, (accessed 8 April 2016).
WHO 2012a
- March of Dimes, the Partnership for Maternal, Newborn and Child Health, Save the Children, World Health Organization. Born too soon: the global action report on preterm birth. www.who.int/pmnch/media/news/2012/201204_borntoosoon‐report.pdf. Geneva: World Health Organization, (accessed 29 July 2013).
WHO 2012b
- World Health Organization. Serum and red blood cell folate concentrations for assessing folate status in populations. Vitamin and Mineral Nutrition Information System. apps.who.int/iris/bitstream/10665/75584/1/WHO_NMH_NHD_EPG_12.1_eng.pdf (accessed 2 April 2017).
WHO 2015a
- World Health Organization. Serum and red blood cell folate concentrations for assessing folate status in populations. Vitamin and Mineral Nutrition Information System. apps.who.int/iris/bitstream/10665/162114/1/WHO_NMH_NHD_EPG_15.01.pdf?ua=1. Geneva: World Health Organization, (accessed 17 February 2016).
WHO 2015b
- World Health Organization. Guideline: Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects. apps.who.int/iris/bitstream/10665/161988/1/9789241549042_eng.pdf?ua=1. Geneva: World Health Organization, (accessed 8 April 2016).
WHO 2015c
- WHO Global Malaria Programme. World Malaria Report. apps.who.int/iris/bitstream/10665/200018/1/9789241565158_eng.pdf?ua=1 (accessed 4 September 2016).
WHO 2016
- World Health Organization. Guideline: fortification of maize flour and corn meal with vitamins and minerals. apps.who.int/iris/bitstream/10665/251902/1/9789241549936‐eng.pdf?ua=1 (accessed 2 April 2017).
WHO 2019
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision. https://icd.who.int/browse10/2016/en.
WHO/CDC 2011
- World Health Organization, Centers for Disease Control and Prevention. Logic model for micronutrient interventions in public health. Vitamin and Mineral Nutrition Information System (WHO/NMH/NHD/MNM/11.5). www.who.int/vmnis/toolkit/WHO‐CDC‐english_colour.pdf. Geneva: World Health Organization, (accessed 7 July 2011).
WHO/CDC/ICBDSR 2014
- World Health Organization/Centers for Disease Control and Prevention/International Clearinghouse of Birth Defects Surveillance and Research. Birth defects surveillance: Atlas of selected congenital anomalies. Geneva: World Health Organization, 2014. [Google Scholar]
WHO/FAO 2006
- World Health Organization, Food, Agriculture Organization of the United Nations. Guidelines on Food Fortification with Micronutrients. Geneva: World Health Organization, 2006. [Google Scholar]
Wien 2012
- Wien TN, Pike E, Wisløff T, Staff A, Smeland S, Klemp M. Cancer risk with folic acid supplements: a systematic review and meta‐analysis. BMJ Open 2012;2(1):e000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2015
- Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, Ethen M. Updated estimates of neural tube defects prevented by mandatory folic acid fortification — United States, 1995–2011. MMWR: Morbidity and Mortality Weekly Report 2015;64(1):1‐5. [PMC free article] [PubMed] [Google Scholar]
Yartey 1995
- Yartey J, Nkrumah F, Hori H, Harrison K, Armar D. Clinical trial of fermented maize‐based oral rehydration solution in the management of acute diarrhoea in children. Annals of Tropical Paediatrics 1995;15(1):61‐8. [PUBMED: 7598439] [DOI] [PubMed] [Google Scholar]
Yetley 2011
- Yetley EA, Pfeiffer CM, Phinney KW, Bailey RL, Blackmore S, Bock JL, et al. Biomarkers of vitamin B‐12 status in NHANES: a roundtable summary. American Journal of Clinical Nutrition 2011;94(1):313S‐21S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zamora 2014
- Zamora G, De‐Regil LM. Equity in access to fortified maize flour and corn meal. Annals of the New York Academy of Sciences 2014;1312:40‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeng 2015
- Zeng R, Xu CH, Xu YN, Wang YL, Wang M. The effect of folate fortification on folic acid‐based homocysteine‐lowering intervention and stroke risk: a meta‐analysis. Public Health Nutrition 2015;18(8):1514‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zimmerman 2011
- Zimmerman S. Fifteen years of fortifying with folic acid: birth defect are reduced and healthcare expenses are averted. Sight and Life 2011;25(3):54‐61. [Google Scholar]
References to other published versions of this review
De‐Regil 2016
- De‐Regil LM, Finkelstein JL, Sæterdal I, Gaitán D, Peña‐Rosas JP. Fortification of wheat and maize flour with folic acid for population health outcomes. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD012150] [DOI] [PMC free article] [PubMed] [Google Scholar]


