Abstract
Alterations to the mesolimbic dopamine (DA) system are thought to underlie dysfunctional reward processing in stress-related psychiatric disorders. Using in vivio microdialysis in awake freely moving mice, we assessed the effects of stress on the motivational and neurochemical correlates underlying conditioned approach behavior for palatable food in the non-deprived mouse. Mice trained to approach and consume food in a familiar environment exhibited a 30% increase in nucleus accumbens shell (AcbSh) extracellular dopamine levels coincident with approach towards and consumption of the food reward. This effect was not observed in mice that were presented with the food in an unfamiliar environment or were exposed for the first time and were region specific. The addition of an acute environmental stressor (bright light and novel scent) during food exposure decreased DA release and delayed approach to the food. The disruptive impact of acute novelty stress on DA levels and approach behavior was reversed in animals pretreated with buprenorphine, an opioid drug with antidepressant-like and anxiolytic effects. Together, these data indicate that exposure to mild stress reduces incentive drive to approach palatable food via alterations in AcbSh dopamine responsiveness to food reward. Moreover, they implicate the brain opioid system as a potential pharmacological target for counteracting behavioral and neurochemical elements associated with stress.
Keywords: Dopamine, Stress, Feeding behavior, In vivo microdialysis, Novelty-induced hypophagia
1. Introduction
The most well characterized brain circuit that mediates reward is comprised of dopaminergic neurons originating in the ventral tegmental area (VTA) that project to and innervate limbic and cortical regions, including the nucleus accumbens (NAc), amygdala, hippocampus, and medial prefrontal cortex (mPFC). Multiple lines of evidence support the involvement of dopaminergic projections from the VTA to the NAc in reward seeking behavior. Previous studies have shown that rodents exhibit conditioned place preference for environments in which they received microinjections of DA agonists into the NAc (Carr and White, 1983) and will perform operant responses for NAc infusions of agents that increase extracellular DA (Carlezon et al., 1995; Hoebel et al., 1989; Phillips et al., 1994). Yet, the exact roles of DA in reward processing, e.g., prediction, valuation, motivational effort or learning, remain complex and controversial (Der-Avakian et al., 2016). Recent evidence implicates a role for subcortical DA transmission in reinforcement learning via the attribution of incentive salience, or “wanting”, towards effort worthy goals (Berridge, 2012). In support, studies conducted largely in rats have measured phasic increases in extracellular concentrations of DA in the NAc in response to both initial consumption of a palatable food reward and the instrumental responding for food (Ahn and Phillips, 2007; Bassareo and Di Chiara, 1997). In contrast, depletion or inhibition of DA activity in the NAc reduces goal-directed behavior towards food, but does not diminish hedonic reactions to food (Berridge, 2007). Therefore, dopaminergic signaling in the NAc is likely involved in multiple processes (Der-Avakian et al., 2016), including initiating appetitive behavioral responses and eliciting cue-triggered incentive salience (Berridge and Robinson, 1998; Cardinal et al., 2002; Cone et al., 2016; Parkinson et al., 2002). However, the manner in which altered DA activity influences behavior is still being debated.
Approach-based behavioral adjustments to salient stimuli are one of the most fundamental components of goal-directed behavior (Elliot et al., 2006). Thus, stress-induced disruption of the neural mechanisms that regulate the manifestation of incentive salience can accompany altered behavioral responses towards rewarding stimuli. Indeed, exposure to chronic stressors has been shown to inhibit the DA response to palatable food reward in the NAc and diminish reward processing in rats (Di Chiara et al., 1999; Di Chiara and Tanda, 1997; Gambarana et al., 2003). Additionally, acute stressors, such as exposure to a novel environment, are known to suppress conditioned responses, such as approach behavior for food. The interaction of these elements is modeled in the novelty-induced hypophagia (NIH) test, a situation of considerable importance to psychopharmacology because acute anxiolytic or chronic antidepressant treatments counteract the suppression of food approach behavior produced by exposing rodents to a novel environment (Dulawa and Hen, 2005). In humans, acute stress has been shown to impair reward responsiveness within healthy populations, particularly in individuals who exhibited greater cortisol reactivity to a stressor (Berghorst et al., 2013) or reported higher levels of anhedonia (Bogdan and Pizzagalli, 2006). Moreover, exposure to stressors produce hedonic blunting, predominantly in individuals with a family history of depression, which may involve failures to approach or process rewards (Al'absi et al., 2012; Berenbaum and Connelly, 1993).
In addition to its well-established role in nociception and analgesia (Dickenson, 1991), the opioid system is also implicated in the regulation of reward processing (Koob, 1992) and the stress response (Bruchas et al., 2010). Mu (MOR) and kappa (KOR) opioid receptors respectively are localized to the mesocorticolimbic DA pathway and are thought to mediate the reinforcing properties of rewards (Kieffer and Gaveriaux-Ruff, 2002; Matthes et al., 1996) and the aversive properties of stressful experiences (Lutz and Kieffer, 2013; McLaughlin et al. 2003, 2006a, 2006b). Recent preclinical studies have revealed promising effects of opioid drugs, such as buprenorphine (BPN), in ameliorating anxiety and depressive like behaviors induced by stress (Almatroudi et al., 2015; Browne et al., 2015; Falcon et al. 2015, 2016). Moreover, clinical trials have shown low doses of BPN to have therapeutic effects in patients with treatment-resistant depression (Bodkin et al., 1995; Ehrich et al., 2015; Nyhuis et al., 2008).
The goal of the present study was to measure simultaneously the motivational aspects and neurochemical substrates underlying approach behavior for palatable food reward within a single behavioral paradigm in mice and its interaction with stress. Using in vivo microdialysis, we assessed extracellular DA levels in the AcbSh in 5-min increments during the period before, during and after the presentation of palatable food to non-deprived male C57BL/6 mice. Comparison of DA reactivity and approach behavior to conditioned versus unconditioned food stimuli revealed that a history of repeated exposure to food reward was necessary to produce the food-cued increase of AcbSh DA release and that this response reflected both anticipatory and consummatory signals. Once the core neurochemical phenotype associated with stable approach behavior was established, we employed the novelty-induced hypophagia (NIH) paradigm to determine whether exposure to a novel environmental stressor during food presentation alters AcbSh DA response and approach behavior to a conditioned food stimulus. Lastly, we examined whether pretreatment with opioid drug buprenorphine (BPN), shown previously to block the stress of novelty in the NIH paradigm (Falcon et al., 2015; Robinson et al., 2017), would prevent both the neurochemical and behavioral effects of stress in the NIH paradigm.
2. Materials and methods
2.1. Animals
Male C57BL/6 J mice, aged 7–8 weeks old upon arrival, were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in pairs in polycarbonate cages and maintained under a 12-h light-dark cycle (lights on at 0700 h) in a temperature (20–22 °C)- and humidity-controlled environment. Food and water were available ad libitum. All experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and protocols were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
2.2. Drugs and treatment
Buprenorphine hydrochloride (Sigma, St. Louis, MO) was dissolved in distilled water and injected intraperitoneally (i.p.) at a dose of 0.25 mg/kg. The dose was calculated according to the base weight of the drug and administered in a volume of 10 ml/kg. This dose administered 24 h prior to testing has been shown to prevent suppression of approach behavior by novelty exposure in the NIH paradigm (Falcon et al., 2015).
2.3. Surgery
Microdialysis probes were custom-made and surgically implanted as described previously (Knobelman et al., 2001). Briefly, under isoflurane anesthesia, the probe was targeted at the nucleus accumbens shell [ +1.2 mm anteroposterior (AP), ±0.5 mm mediolateral (ML) from bregma, −4.5 mm dorsoventral (DV) from dura] or at the dorsal striatum (+1.0 mm AP, ±1.7 mm ML from bregma, −4.5 mm DV from dura) (Franklin and Paxinos, 1997) using a Kopf stereotaxic instrument. Probes were constructed so that the length of the sampling area was 2.0 mm for the nucleus accumbens shell and 2.5 mm for the dorsal striatum. Control animals were treated with a topical anesthetic after surgery but did not receive BPN to avoid drug interactions that could confound the results of the experiment. Mice in the BPN treated group were injected with 0.25 mg/kg BPN immediately after completion of surgery while still anesthetized. Following surgery, the mice were placed into a 21.5 cm high, clear polycarbonate cylindrical in vivo microdialysis chamber with a counterbalance arm holding a liquid swivel (Instech Laboratories, Plymouth Meeting, PA) and allowed to recover overnight with the pump flow rate set to 0.9 μl/min. Dialysate samples were collected into polypropylene microcentrifuge vials at 5 min intervals and stored at −80 °C until analyzed for dopamine as described previously (Andrews and Lucki, 2001) by a Shimadzu Prominence HPLC system (including a LC-20 AD pump and Sil-20 AC refrigerated microsampler) using an Unijet microbore column (3 μM ODS/100 × 1 mm) coupled with an Antec Decade II electrochemical detector. DA levels were identified by comparing their elution times with those of reference standards and quantified from their respective peak heights using a linear regression analysis of the peak heights obtained from a series of reference standards.
2.4. Experimental procedures
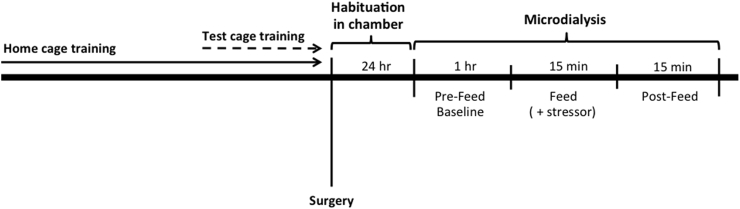
Mice were trained to consume three (∼1.4 g) Reese's peanut butter chips (The Hershey Company, Hershey, PA) presented in a small, clear petri dish in a home cage environment in daily 15-min sessions. Opaque, black, plastic dividers were placed inside each home cage to separate the mice during training sessions. As shown in the schematic (Fig. 1), mice were allowed to habituate to the dividers for 1 h before the start of the training session. Sessions continued until animals approached the food reward in less than 30 s for three consecutive days. Mice assigned to the “test cage” trained group received five additional training sessions in the microdialysis chamber two days prior to testing. Mice assigned to the “home cage” trained group underwent surgery after meeting criteria in the home cage and were tested in the microdialysis procedure without prior food exposure in the microdialysis chamber. Mice assigned to the “no training” group were naïve to the food reward. In the stress exposure experiments, “test cage” trained animals were exposed to a bright light (60 W) and novel scent (vanilla extract) in conjunction with food presentation. All animals were tested in the microdialysis chamber. The body weight of mice in the no training group (23.4 g ± 0.92) was lower than the groups that received training in the test chamber (26.8 ± 0.66) or home cage (28.2 ± 1.34) because of exposure to the peanut butter chips.
Fig. 1.
Timeline of experiment. Mice were given daily sessions (15 min) of exposure to the peanut butter chips in their home cage until animals approached the food reward in less than 30 s for three consecutive days. Test cage trained mice were exposed to five additional training sessions in the microdialysis chamber two days prior to testing. Animals without training (naïve) had no exposure to the food prior to surgery. After surgery, mice were placed in the test chambers for 24 h prior to behavioral testing. Dialysis samples were collected during the Pre-Feed baseline for 1 h, during exposure to the peanut butter chips (15 min) and during a post-feeding period (15 min).
Microdialysis experiments started 20–24 h after surgery. The pump flow rate was increased to 2.2 μl/min 2 h prior to testing. Baseline dialysate samples were collected at 5-min intervals starting 1 h prior to food exposure. The final three samples were used to establish a baseline value before food exposure. During food exposure, a small clear Petri dish with three peanut butter chips was placed in the microdialysis chamber for 15 min and 3 samples were collected. Three additional samples were collected after the food dish had been removed. At the completion of the experiment, the mice were sacrificed and their brains were removed, placed in cold isopentane, and frozen at −80 °C. The brains were then sectioned (35 μm) with a refrigerated cryostat and the tissue examined for the location of the dialysis probe.
2.5. Behavioral recordings
Mice were recorded in the microdialysis chamber throughout the session to gain greater insight into the effects of different testing conditions on behavior before, during and after the presentation of food. After food presentation, the latency to approach and begin eating the peanut butter chips and the amount consumed during the 15-min feeding period was measured for each animal. Mice were also monitored for proactive defensive behaviors, such as defensive burying (aggressive shoveling movement of bedding material with forepaws), rearing (standing on hindpaws), and grooming (nose/face/body washing), and passive behaviors (freezing or inactivity) in 5-min periods throughout the experiment. For each behavioral category, mice received a score of either “1” if they engaged in the behavior within the 5 min sampling time for a minimum of 5 s or “0” if they did not. The scores were averaged among animals in each group for each time interval, thereby reflecting the proportion of animals engaging in a particular behavior at a given time.
2.6. Data analysis
One-way and two-way ANOVA were performed to examine the significance of differences between experimental groups. Significant overall main effects or interactions were followed by Dunnett's or Holm-Sidak's multiple comparisons test where appropriate. Microdialysis data were expressed as a percentage of baseline values, determined by the mean of three samples collected immediately before food presentation. Repeated measures two-way ANOVA was used to compare variations in DA levels between experimental groups over time. Significant differences within groups were followed by Dunnett's test. Variations in DA levels between groups were compared at individual time points using Tukey's test. For all tests, p < 0.05 was considered statistically significant.
3. Results
3.1. The effects of training experience on DA response and approach behavior to palatable food
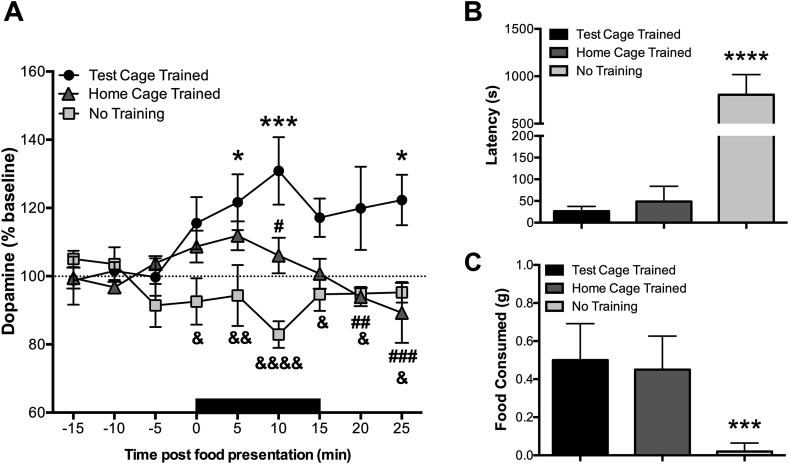
To determine whether familiarity with the food reward and feeding environment impacts DA response and approach behavior to palatable food (peanut butter chips) in C57BL/6 J mice, we first evaluated the effects of training experience on AcbSh DA reactivity and latency to approach and consume the food. Mice trained to consume food in the microdialysis test cage were compared with mice trained to consume the same food in their home cage but not in the microdialysis test cage and mice with no prior exposure to the food (Fig. 2A). A repeated-measures two-way ANOVA revealed a significant effect of training experience [F(2, 15) = 9.884, p = 0.002] and an interaction between training experience and time [F(16, 120) = 2.713, p = 0.001] on extracellular DA levels in response to the food presentation (solid bar). Presentation of the Petri dish containing food significantly increased extracellular DA levels (∼30%) in the AcbSh of mice with prior experience with the food in the microdialysis test cage (p < 0.05). DA levels remained high after the dish with the food was removed. The DA response was attenuated in animals that had been allowed to consume the food in feeding cages but not in the microdialysis test cage (home cage trained animals) and returned to baseline levels after removal of the food. Mice naïve to the peanut butter chips did not demonstrate significant variation of DA levels during or after presentation of the food (Fig. 2A).
Fig. 2.
Effects of training experience on NAc DA response and approach behavior to palatable food. A) NAc DA response to food presentation in test cage trained (n = 7; baseline 15.56 ± 2.36 fmol/5 μl dialysate), home cage trained (n = 6; 5.74 ± 1.72), and naïve animals (n = 5; 14.95 ± 3.62). Black bar denotes duration of food exposure. Test cage trained animals were the only group to exhibit a significant change from baseline in DA levels in response to the food reward. The asterisk denotes significant differences compared to baseline values within the test cage trained group (*p < 0.05, ***p < 0.001), but not for any other groups. The symbol # denotes time points with significant differences in DA release between test cage trained and home cage trained animals (#p < 0.05). The symbol & denotes significant differences in DA release between test cage trained and animals without training (naïve) (&p < 0.05). B) Naïve animals took longer to approach the food and C) consumed less food compared to test cage trained animals. The asterisk denotes a significant difference compared to test cage trained animals (***p < 0.001, ****p < 0.0001). Data is depicted as mean ± SEM.
Differing approach latencies between groups [F(2,15] = 84.98, p < 0.001] paralleled the association between DA levels and food presentation (Fig. 2B). Animals interacting with the food reward for the first time had significantly higher approach latencies compared to test cage and home trained animals (p < 0.001). Similarly, the amount of food consumed differed between groups [F(2,15) = 14.92, p = 0.003]. As illustrated in Fig. 2C, test cage trained and home cage trained animals consumed more of the peanut butter chips compared to untrained animals (p < 0.01).
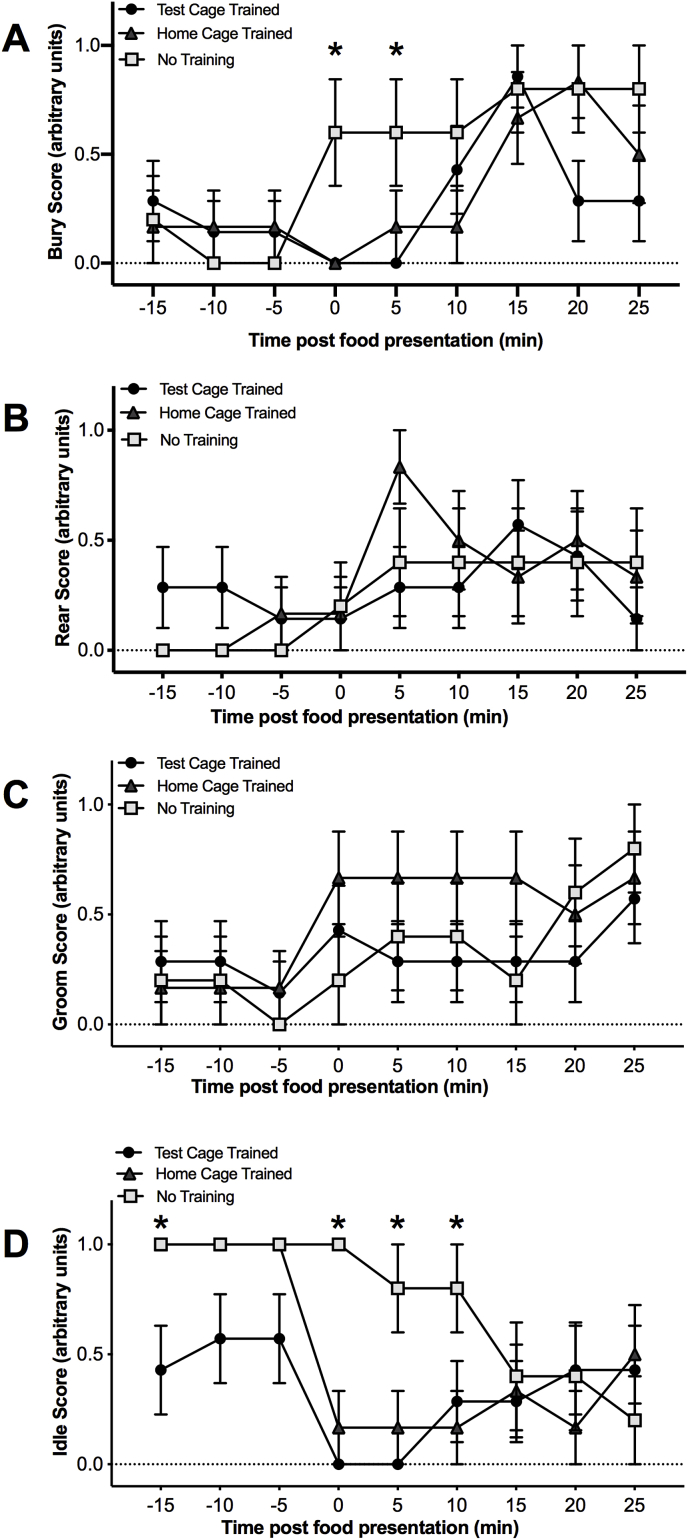
Determination of a behavioral profile, by measuring burying, rearing, grooming, and idle behavior before, during, and after food presentation, provided additional information on the differential effects of training experience. Animals with no previous experience with the food reward showed significantly more burying behavior during food presentation compared to test cage and home cage trained animals. A two-way ANOVA revealed a significant main effect of time [F(8,120) = 8.658, p < 0.0001] and an interaction between training experience and time [F(16, 120) = 2.401, p = 0.016] on the amount of burying behavior (Fig. 3A). A significant main effect of time was also observed for rearing [F(8, 120) = 4.074, p < 0.003] (Fig. 3B) and grooming [F(8,120) = 2.562, p < 0.013] (Fig. 3C) behaviors. Animals from all groups exhibited more rearing and grooming behavior following food presentation. Analysis of the time spent idle showed a significant main effect of time F(8,120) = 12.05, p < 0.0001] and an interaction between groups F(16,120) = 3.072, p < 0.0002]. Home cage trained and animals without training spent more time idle prior to food exposure compared to test cage trained animals (Fig. 3D). Moreover, untrained animals spent more time idle during food presentation compared to test cage and home cage trained animals.
Fig. 3.
Effects of training experience on behavioral profile. Time course of behavioral profiles of test cage trained (n = 7), home cage trained (n = 6), and naïve animals (n = 5). Behavior was scored in blocks of 5 min and measured before, during, and after food exposure. Animals were evaluated for changes in A) burying, B) rearing, C) grooming and D) idle behavior. Naïve animals exhibited more burying behavior and idle behavior during food presentation. The asterisk denotes a significant difference compared to test cage trained animals. (*p < 0.05). Data is depicted as mean ± SEM.
3.2. AcbSh DA is responsive to anticipatory and consummatory cues for palatable food
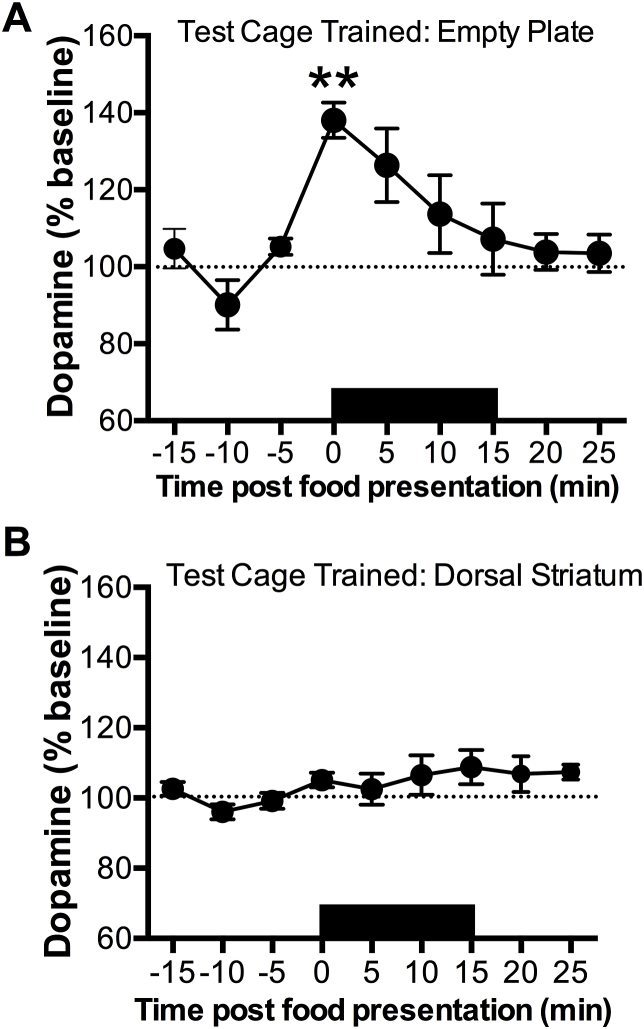
Having demonstrated that mice trained to feed in the test cage exhibit DA reactivity to food exposure, we next sought to address whether the AcbSh DA response is associated with an appetitive signal or dependent on consumption of the food reward. To test this, we presented test cage trained animals with an empty Petri dish (in which the peanut butter chips were previously stored) while sampling DA from the AcbSh (Fig. 4A). In the absence of food, we observed a 40% elevation in DA in response to presentation of the food dish F(8,32) = 5.184, p = 0.003]. However, the DA response had decreased to baseline levels by the end of the exposure period and there was no change in DA during the post-feeding period.
Fig. 4.
DA response to conditioned cues for food reward and effects of food exposure on general striatal activity. Black bar denotes duration of conditioned cue or food exposure. A) NAc DA response to presentation of a conditioned cue (empty food plate) in test cage trained animals (n = 5; 2.02 ± 0.52). Exposure to the conditioned cue significantly increased NAc DA levels from baseline. The asterisk denotes significant differences compared to baseline values (**p < 0.01). B) Dorsal striatum DA response to food presentation in test cage trained animals (n = 4; 7.50 ± 0.76). Food presentation did not alter DA transmission in the dorsal striatum. Data is depicted as mean ± SEM.
3.3. AcbSh DA response to palatable food is not reflective of generalized striatal activity
To examine the regional specificity of the food-induced DA response, a separate cohort of test cage trained animals was implanted with microdialysis probes in the dorsal striatum. As seen in Fig. 4B, food presentation had no effect on extracellular DA release in this region F(8,24) = 1.663, p = 0.160]. Behaviorally, these animals exhibited similar approach latencies and food consumption to animals who received probe implants in the AcbSh (data not shown).
3.4. BPN prevents effects of stress on AcbSh DA response and approach behavior
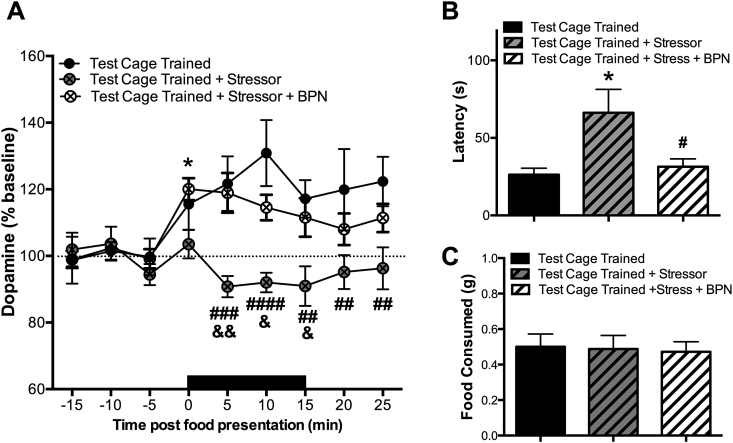
The next experiment measured how stress exposure in the form of novelty influences the AcbSh DA response to a familiar food reward and approach behavior. A repeated measures ANOVA revealed a significant effect of treatment [F(2,19) = 9.848, p = 0.001], time [F(8,152) = 3.454, p = 0.001], and an interaction [F(16, 152) = 2.541, p = 0.002]. Animals exposed to the acute stress, a bright light and novel scent during food presentation, exhibited no change in extracellular DA in response to the food reward (Fig. 5A). In contrast, animals treated with 0.25 mg/kg BPN 24 h prior to testing prevented stress exposure from diminishing the DA response to the palatable food reward.
Fig. 5.
Effects of stress exposure and BPN treatment on NAc DA response and approach behavior to palatable food. A) NAc DA response to food presentation in test cage trained animals exposed to a stressor (bright light and novel vanilla scent) during food presentation (n = 8; 18.74 ± 5.30), and test cage trained animals treated with 0.25 mg/kg BPN 24 h prior to testing and exposed to a stressor during food presentation (n = 7; 9.32 ± 3.52). Data for test cage trained animals for comparison are the same as those shown in Fig. 2. Black bar denotes duration of food and stress exposure. BPN treatment prevented stress-induced inhibition of NAc DA response to food. The asterisk denotes significant differences compared to baseline values within the BPN treated group (*p < 0.05). The symbol # denotes significant differences in DA release between test cage trained animals and untreated test cage trained animals exposed to the stressor (##p < 0.01, ###p < 0.001, ####p < 0.0001, analysis includes data from Fig. 2A). The symbol & denotes significant differences in DA release between untreated and BPN treated test cage trained animals exposed to the stressor (&p < 0.05, &&p < 0.01). B) Untreated test cage trained animals exposed to the stressor took longer to approach the food compared to non-stressed test cage trained animals. Approach latency was restored to control values in BPN treated animals. The asterisk denotes a significant difference compared to test cage trained animals (*p < 0.05, analysis includes data from Fig. 1B). The symbol # denotes a significant difference between untreated and BPN treated test cage trained animals exposed to the stressor (#p < 0.05). C) There was no significant effect of group condition on amount of food consumed. Data is depicted as mean ± SEM.
Exposure to stress was effective at increasing the latency to approach the food compared to unstressed animals (Fig. 5B). A one way ANOVA revealed a significant effect of stress on latency to approach the food reward [F(2,19) = 4.735, p = 0.021]. This effect was prevented in animals pretreated with BPN. Interestingly, there was no significant difference between groups in amount of food consumed [F(2,19) = 0.040, p = 0.961] (Fig. 5B).
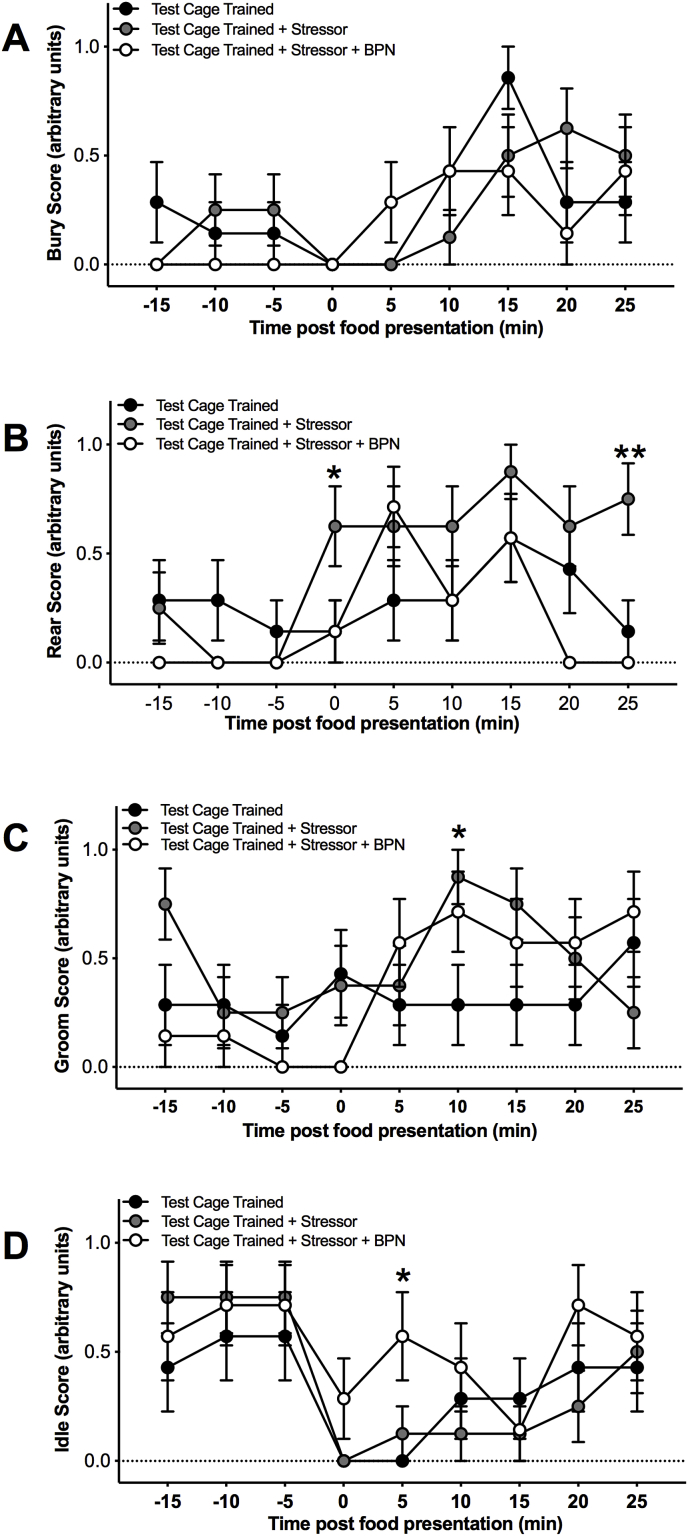
Examination of the behavioral profile indicated differences in burying and rearing behaviors before, during, and after food presentation between groups. There was a significant main effect of time on burying behavior [F(8,152) = 6.068, p = 0.001] (Fig. 6A). For rearing behavior, there was a significant main effect of time [F(8,152) = 6.958, p = 0.001] and an interaction [F(16, 152) = 2.242, p = 0.006]. Exposure to the stressor increased rearing during and after the feeding period compared to unstressed animals (Fig. 6B). There were also significant main effects of time on grooming [F(8,152) = 2.816, p = 0.006] and idle [F(8,152) = 6.848, p = 0.001] behavior.
Fig. 6.
Effects of stress exposure and BPN pretreatment on behavioral profile. Time course of behavioral profiles of test cage trained animals exposed to a stressor during food presentation (n = 8), and test cage trained animals treated with BPN and exposed to a stressor during food presentation (n = 7). Behavior was scored in blocks of 5 min and measured before, during, and after food exposure. Animals were evaluated for changes in A) burying, B) rearing, C) grooming and D) idle behavior. Untreated animals exposed to stress displayed more rearing and grooming behavior during food presentation compared to non-stressed test cage trained animals. BPN treated animals exposed to stress exhibited more idle behavior compared to non-stressed test cage trained animals. The asterisk denotes a significant difference compared to test cage trained animals (*p < 0.05, analysis includes data from Fig. 2). Data is depicted as mean ± SEM.
4. Discussion
The present study established that repeated exposure of mice to palatable food elicits DA efflux in the mouse AcbSh, and that this neurochemical response is associated with the conditioned incentive salience effects as measured by the reduced latency to approach the food. Due to the fact that mice were not food deprived prior to testing, approach behavior likely represented a motivational “wanting” response as opposed to a need to maintain adequate energy stores. Presentation of the palatable food in a feeding environment made unfamiliar, or to mice that were naïve to the food reward, failed to elicit a significant AcbSh DA response. Moreover, the DA response was limited to the AcbSh and did not occur in the dorsal striatum. Altogether, these findings implicate AcbSh DA transmission as a substrate for facilitating the emergence and maintenance of motivated behavioral output for food reward in the mouse. Furthermore, we showed that exposure to aversive environmental stimuli during food presentation diminished the AcbSh DA response and increased approach latency to the conditioned food stimulus, using a similar procedure to the novelty-induced hypophagia (NIH) paradigm. Remarkably, pretreatment with buprenorphine (BPN) 24 h prior to testing blocked the effects of acute stress on AcbSh DA reactivity and restored approach behavior for the food, similar to the effects of other antidepressant treatments. The concordant neurobehavioral and neurochemical response suggests a role for opioid systems in mitigating the aversive effects of stress on incentive salience for natural rewards involving the release of DA.
Empirical evidence supports the involvement of subcortical DA transmission in reinforcement learning and attribution of incentive salience towards effort worthy goals (Berridge, 2012). Specifically, DA activity in the NAc is believed to be important for the promotion of behaviors that aid in the formation of new motivational connections between salient stimuli and environmental cues (Ikemoto and Panksepp, 1999). For example, presentation and consumption of a novel palatable food to rats has been shown to produce robust increases in NAc DA release (Bassareo and Di Chiara, 1997; Gambarana et al., 2003). Even exposure to unconditioned novel stimuli in rats transiently elevates NAc DA and triggers investigatory activity (Legault and Wise, 2001; Rebec et al., 1997). The present study conducted in mice found some important differences from the prior literature collected predominately in rats. Most notable, in comparison to animals familiar with the food reward, naïve mice exhibited no AcbSh DA response and did not approach the novel palatable food. Moreover, naïve animals exhibited more signs of active avoidance during food presentation, exemplified by increased defensive burying and freezing. Interestingly, animals that were made familiar with the food, but received it in an unconditioned feeding environment, displayed attenuated AcbSh DA response during the feeding period and slightly higher (though not reaching significance) approach latencies compared to those receiving the food reward in a familiar feeding environment. It is important to note, however, that although both test cage and home cage trained animals received training to an identical behavioral approach criterion prior to testing, test-cage trained animals received a few additional food exposures in the microdialysis chamber. Although unlikely, the attenuated DA response seen during feeding in home cage trained animals may potentially be due to less experience with the food reward. Nonetheless, these findings highlight the importance of prior experiences and environmental context in the acquisition of conditioned approach behavior and neurochemical response to natural rewards.
Consistent with previous literature in rats (Martel and Fantino, 1996; Salamone, 1994; Salamone et al., 1994), mice exhibited a robust increase in AcbSh DA efflux when presented with a predictive stimulus (empty food dish) after being trained to expect the presentation of food. This finding is in accordance with classical Pavlovian conditioning theory, which theorizes that a neutral stimulus associated with a conditioned stimulus can become a conditioned stimulus in and of itself as it acquires the ability to elicit a conditioned response. However, we also observed a notable distinction in the time course for the AcbSh DA response to the conditioned versus predictive stimulus. DA efflux was elevated throughout the period of food presentation and consumption and this persisted after the food had been removed. In contrast, presenting the food dish alone increased DA efflux only when the dish was present and its removal was followed by a rapid return to baseline. Consequently, DA transmission in the AcbSh may be increased by both anticipatory cues and consummatory cues, with the latter possibly encoding reinforcement learning for subsequent exposures.
To address the question of whether exposure to aversive stimuli alters the AcbSh DA response and motivated behavior towards natural rewards, we employed a hyponeophagia paradigm similar to the NIH test. Exposure to novelty is a mild stressor for rodents, and has been shown to transiently elevate corticosterone levels in mice (Kurumaji et al., 2011). The unconditioned suppression of feeding in a novel or aversive environment is a well-documented phenomenon in rodents and is used to detect the effects of acute anxiolytics and chronic antidepressant treatments (Dulawa and Hen, 2005). The NIH paradigm allowed for the exploration of neural mechanisms underlying stress-induced changes in conditioned approach behavior. The disruptive effects of novelty likely reflect the disruption of incentive salience driving the conditioned approach behavior. The presentation of a bright light and novel scent during food exposure blunted AcbSh DA reactivity to the conditioned food stimulus and increased approach latency, consistent with reflecting alterations in DA-mediated incentive salience. Additionally, animals exhibited increased rearing and grooming behavior during stress exposure, indicating a state of heightened anxiety (Kalueff and Tuohimaa, 2005; Lever et al., 2006). As DA amplifies appetitive behavioral states to more readily engage motor systems involved in approach behavior, mice would not be as immediately energized towards salient stimuli in the absence of the DA response. Alternatively, suppression of the initial DA response may interfere with the prominence of a stimulus so that it no longer elicits “wanting”. The fact that stressor exposed animals do eventually approach and consume comparable amounts of food to control animals lends support to the hypothesis that conditioned approach behaviors are not dependent entirely on DA transmission (Ikemoto and Panksepp, 1999). Further studies are needed to dissociate these mechanisms.
Both appetitive and aversive experiences are reported to evoke mesocorticolimbic DA activity. Brain stress response systems interact with the midbrain DA system to produce complex state-dependent responses to aversive stimuli (Cabib and Puglisi-Allegra, 2012). There is considerable evidence indicating robust DA release and turnover in the mPFC and the NAc following exposure to stressful or aversive stimuli (Abercrombie et al., 1989; Anstrom et al., 2009; Broom and Yamamoto, 2005; Butts et al., 2011; Imperato et al., 1989; Kalivas and Duffy, 1995; McCullough and Salamone, 1992; Salamone, 1994; Thierry et al., 1976; Tidey and Miczek, 1996). In contrast, mesolimbic dopamine neurons have also been reported to become inhibited or unresponsive following exposure to a stressor (Roitman et al., 2008; Ungless et al., 2004). This discrepancy may be due in part to distinct regional subpopulations of dopamine neurons that differ in neuronal firing to appetitive versus aversive stimuli. Indeed, an electrophysiological study revealed DA neurons in the dorsal VTA to be inhibited by footshocks whereas DA neurons in the ventral VTA were excited by the same stimulus (Brischoux et al., 2009). Similarly, Lammel et al. (2011) demonstrated that rewarding experiences selectively modify excitatory synapses on dopaminergic cells projecting to the AcbSh, whereas aversive stimuli modify synapses on DA neurons projecting to the mPFC. Mesocortical DA neurons exert top-down control over striatal DA release (King et al., 1997; Ventura et al., 2002), therefore stress-induced potentiation of mPFC DA activity may facilitate blunted DA release in the NAc. Additionally, GABAergic neurons in the rostromedial tegmental nucleus (RMTg) project to VTA DA neurons and are selectively activated in response to stress (Jhou et al., 2009). Thus, inputs from various brain regions implicated in stress response may converge onto the RMTg to inhibit DA transmission in the NAc. The present study is unique in that animals were presented with an appetitive and aversive stimulus at the same time. Our data suggest that food reward overlaid with stress exposure might selectively activate circuitry involved in the processing of aversive stimuli while also suppressing signaling pathways associated with motivational salience. This hypothesis is supported by a similar study conducted in rats which revealed that intra-VTA injection of CRF, a neuropeptide that activates the hypothalamic-pituitary-adrenal (HPA) axis during stress exposure, reduced operant responding for food reward and attenuated NAc DA release to food reward (Wanat et al., 2013).
Strikingly, pretreatment with BPN, a partial MOR agonist and KOR antagonist, prevented stress-induced suppression of AcbSh DA and restored approach latencies to that seen in non-stressed animals. These data complement previous studies conducted in our laboratory and others showing that acute BPN treatment reduces approach latency in the NIH test (Almatroudi et al., 2015; Falcon et al., 2015), and implicates a role for opioid systems in mitigating the aversive effects of stress on approach behavior for natural rewards. Activation of MORs in the VTA facilitates DA release in the NAc (Johnson and North, 1992; Spanagel et al., 1992), an essential molecular component of reward processing (Contet et al., 2004). MORs mediate the reinforcing properties of both drugs of abuse (Kieffer and Gaveriaux-Ruff, 2002; Matthes et al., 1996) and natural rewards, as evidenced by reduced operant responding for both standard chow and sucrose pellets in MOR knockout animals compared to wildtype (Papaleo et al., 2007). Furthermore, activation of MORs in hedonic “hot-spots” within the AcbSh stimulates positive orofacial reactions to food and increases consumption (Pecina and Berridge, 2005). In contrast, the KOR is implicated in mediating the aversive properties of stress or stimuli with negative emotional valence (McLaughlin et al. 2003, 2006a, 2006b). Activation of KORs induces depressive-like and anxiety-like behaviors in rodents (Carlezon et al., 2006; Van't Veer and Carlezon, 2013). Exposure to environmental stress can lead to increased levels of dynorphin, the endogenous KOR ligand, in key mesocorticolimbic regions involved in the processing of reward and motivation (Flaisher-Grinberg et al., 2012; Shirayama et al., 2004; Svingos et al., 1999). Notably, stimulation of KORs in the NAc region decreases DA transmission (Spanagel et al., 1992) and produces robust conditioned place aversion (Bals-Kubik et al., 1993). Facilitation of stress-like effects by KOR activation is mediated in part by interactions with the neuroendocrine system as evidenced by the finding that the behavioral and neurochemical effects of environmental stress or exogenous CRF exposure are blocked by treatment with KOR antagonists (Land et al., 2008; McLaughlin et al., 2003). Interestingly, BPN treated animals exposed to the stressor selectively exhibited more freezing behavior during stress exposure, suggesting that BPN reduces proactive defensive behaviors while promoting passive coping behaviors. In the present study, animals were tested 24 h after BPN administration, a time point at which the drug is no longer activating MOR. However, BPN may still cause protracted antagonist activities at MORs and KORs (Paronis and Bergman, 2011; Robinson et al., 2017). Furthermore, the dose of BPN used in the current study does not alter extracellular DA levels in the AcbSh when injected 1 h prior to sampling (Falcon et al., 2015); therefore, it is unlikely that DA levels would be altered 24 h later at the time of testing. Thus, BPN's effect in this paradigm may be mediated by indirect effects of blockade of MOR or KOR activity in the AcbSh, however additional studies are needed to confirm this.
Although we detected striking differences in extracellular DA AcbSh release between experimental groups, there are some limitations to the current study that are worth noting. We did not assess levels of DA metabolites, such as homovanillic acid (HVA) and 3,4-dihydroxyphenylacetic acid (DOPAC), which are indicative of DA turnover. There is evidence that both rewarding and aversive stimuli increase DA metabolite levels in the AcbSh (Salamone, 1994; Martel and Fantino, 1996). Interestingly, exposure to novelty stress has been shown to increase DOPAC levels in the NAc without significantly altering DA levels (Jones et al., 1996). Therefore, it is possible that changes in DA synthesis or metabolism in the AcbSh may underlie some of our observed effects, even in the absence of changes in DA levels. A second limitation of this study is the focus on DA alone. Several other neurotransmitter systems, including glutamate, GABA, acetylcholine, norepinephrine, and serotonin, converge onto the NAc to impact motivated behavior. Serotonin activity, in particular, was likely affected by the environmental and pharmacological manipulations employed in this study. Serotonin signaling in the brain is integral to regulating food intake (Lam et al., 2010), and stimulation of selective selective serotonin receptors in the AcbSh specifically has been shown to modulate food intake in both deprived and non-deprived rats (Pratt et al., 2009). Serotonergic projections from the dorsal raphe nucleus (DRN) to the medial prefrontal cortex and basolateral amygdala are believed to modulate glutamatergic and dopaminergic activity in the NAc in response to stress, which in turn can influence stress coping (Puglisi-Allegra and Andolina, 2015). Serotonin signaling is also closely interlinked with the opioid system, as infusions of MOR ligands into the DRN increases extracellular serotonin levels in the nucleus accumbens while infusions of KOR ligands lowers serotonin levels (Tao and Auerbach, 2002). Lastly, the use of only male mice in this study prevented further investigation into how sex influences AcbSh dopaminergic response to stress and reward. Sex differences in stress response (Bangasser and Valentino, 2014) and reward processing systems (Becker and Koob, 2016) at both the behavioral and molecular level are well documented in the literature. Our group has previously shown that female mice respond similarly to males in the NIH paradigm, and also exhibit reduced approach latencies when pretreated with BPN (Browne et al., 2017). Whether or not females display similar context-dependent changes in AcbSh DA response remains to be determined.
In conclusion, our findings with a mouse model measuring the motivational aspects and neurochemical substrates associated with approach behavior for palatable food reward offer greater insight into the interactions between brain stress and reward circuitry. Moreover, these studies provide a method for further investigation into the neural mechanisms underlying affective behavior and antidepressant drug treatments in rodents. The ability of BPN to prevent changes in neurotransmission and behavior caused by stress exposure supports the opioid system as a potential target for the development of novel therapeutic treatments of emotional dysfunction.
Conflicts of interest
The authors declare no competing financial interests.
Acknowledgements
This research was supported by United States Public Health Service grants R01 MH092412, R01 MH105623 and T32 MH14652.
References
- Abercrombie E.D., Keefe K.A., DiFrischia D.S., Zigmond M.J. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J. Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Ahn S., Phillips A.G. Dopamine efflux in the nucleus accumbens during within-session extinction, outcome-dependent, and habit-based instrumental responding for food reward. Psychopharmacology. 2007;191:641–651. doi: 10.1007/s00213-006-0526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al'absi M., Nakajima M., Hooker S., Wittmers L., Cragin T. Exposure to acute stress is associated with attenuated sweet taste. Psychophysiology. 2012;49:96–103. doi: 10.1111/j.1469-8986.2011.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almatroudi A., Husbands S.M., Bailey C.P., Bailey S.J. Combined administration of buprenorphine and naltrexone produces antidepressant-like effects in mice. J. Psychopharmacol. 2015;29:812–821. doi: 10.1177/0269881115586937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews C.M., Lucki I. Effects of cocaine on extracellular dopamine and serotonin levels in the nucleus accumbens. Psychopharmacology. 2001;155:221–229. doi: 10.1007/s002130100704. [DOI] [PubMed] [Google Scholar]
- Anstrom K.K., Miczek K.A., Budygin E.A. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals-Kubik R., Ableitner A., Herz A., Shippenberg T.S. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J. Pharmacol. Exp. Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V., Di Chiara G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J. Neurosci. 1997;17:851–861. doi: 10.1523/JNEUROSCI.17-02-00851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., Koob G.F. Sex differences in animal models: focus on addiction. Pharmacol. Rev. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum H., Connelly J. The effect of stress on hedonic capacity. J. Abnorm. Psychol. 1993;102:474. doi: 10.1037//0021-843x.102.3.474. [DOI] [PubMed] [Google Scholar]
- Berghorst L.H., Bogdan R., Frank M.J., Pizzagalli D.A. Acute stress selectively reduces reward sensitivity. Front. Hum. Neurosci. 2013;7:133. doi: 10.3389/fnhum.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge K.C. From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur. J. Neurosci. 2012;35:1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bodkin J.A., Zornberg G.L., Lukas S.E., Cole J.O. Buprenorphine treatment of refractory depression. J. Clin. Psychopharmacol. 1995;15:49–57. doi: 10.1097/00004714-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Bogdan R., Pizzagalli D.A. Acute stress reduces reward responsiveness: implications for depression. Biol. Psychiatry. 2006;60:1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F., Chakraborty S., Brierley D.I., Ungless M.A. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom S.L., Yamamoto B.K. Effects of subchronic methamphetamine exposure on basal dopamine and stress-induced dopamine release in the nucleus accumbens shell of rats. Psychopharmacology. 2005;181:467–476. doi: 10.1007/s00213-005-0007-6. [DOI] [PubMed] [Google Scholar]
- Browne C.A., Erickson R.L., Blendy J.A., Lucki I. Genetic variation in the behavioral effects of buprenorphine in female mice derived from a murine model of the OPRM1 A118G polymorphism. Neuropharmacology. 2017;117:401–407. doi: 10.1016/j.neuropharm.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne C.A., van Nest D.S., Lucki I. Antidepressant-like effects of buprenorphine in rats are strain dependent. Behav. Brain Res. 2015;278:385–392. doi: 10.1016/j.bbr.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas M.R., Land B.B., Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts K.A., Weinberg J., Young A.H., Phillips A.G. Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proc. Natl. Acad. Sci. Unit. States Am. 2011;108:18459–18464. doi: 10.1073/pnas.1111746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S., Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neurosci. Biobehav. Rev. 2012;36:79–89. doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Cardinal R.N., Parkinson J.A., Hall J., Everitt B.J. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Bobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carlezon W.A., Jr. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J. Pharmacol. Exp. Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carlezon W.A., Jr., Devine D.P., Wise R.A. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology. 1995;122:194–197. doi: 10.1007/BF02246095. [DOI] [PubMed] [Google Scholar]
- Carr G.D., White N.M. Conditioned place preference from intra-accumbens but not intra-caudate amphetamine injections. Life Sci. 1983;33:2551–2557. doi: 10.1016/0024-3205(83)90165-0. [DOI] [PubMed] [Google Scholar]
- Cone J., Fortin S., McHenry J., Stuber G.D., McCutcheon J., Roitman M. Physiological state gates acquisition and expression of mesolimbic reward prediction signals. Proc. Natl. Acad. Sci. Unit. States Am. 2016;113:1943–1948. doi: 10.1073/pnas.1519643113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C., Kieffer B.L., Befort K. Mu opioid receptor: a gateway to drug addiction. Curr. Opin. Neurobiol. 2004;14:370–378. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A., Barnes S.A., Markou A., Pizzagalli D.A. Translational assessment of reward and motivational deficits in psychiatric disorders. Curr Top Behav Neurosci. 2016;28:231–262. doi: 10.1007/7854_2015_5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G.D., Loddo P., Tanda G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol. Psychiatry. 1999;46:1624–1633. doi: 10.1016/s0006-3223(99)00236-x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G., Tanda G. Blunting of reactivity of dopamine transmission to palatable food: a biochemical marker of anhedonia in the CMS model? Psychopharmacology. 1997;134:351–353. doi: 10.1007/s002130050465. [DOI] [PubMed] [Google Scholar]
- Dickenson A.H. Mechanisms of the analgesic actions of opiates and opioids. Br. Med. Bull. 1991;47:690–702. doi: 10.1093/oxfordjournals.bmb.a072501. [DOI] [PubMed] [Google Scholar]
- Dulawa S.C., Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci. Biobehav. Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Ehrich E., Turncliff R., Du Y., Leigh-Pemberton R., Fernandez E., Jones R., Fava M. Evaluation of opioid modulation in major depressive disorder. Neuropsychopharmacology. 2015;40:1448–1455. doi: 10.1038/npp.2014.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot A.J., Gable S.L., Mapes R.R. Approach and avoidance motivation in the social domain. Pers. Soc. Psychol. Bull. 2006;32:378–391. doi: 10.1177/0146167205282153. [DOI] [PubMed] [Google Scholar]
- Falcon E., Browne C.A., Leon R.M., Flietes V.C., Sweeney R., Kirby L.G., Lucki I. Antidepressant-like effects of buprenorphine are mediated by kappa opioid receptors. Neuropsychopharmacology. 2016;41:2344–2351. doi: 10.1038/npp.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon E., Maier K., Robinson S.A., Hill-Smith T.E., Lucki I. Effects of buprenorphine on behavioral tests for antidepressant and anxiolytic drugs in mice. Psychopharmacology. 2015;232:907–915. doi: 10.1007/s00213-014-3723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaisher-Grinberg S., Persaud S.D., Loh H.H., Wei L.-N. Stress-induced epigenetic regulation of κ-opioid receptor gene involves transcription factor c-Myc. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109:9167–9172. doi: 10.1073/pnas.1205565109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K.B.J., Paxinos G. Academic Press; San Diego: 1997. The Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- Gambarana C. Acquisition of a palatable-food-sustained appetitive behavior in satiated rats is dependent on the dopaminergic response to this food in limbic areas. Neuroscience. 2003;121:179–187. doi: 10.1016/s0306-4522(03)00383-x. [DOI] [PubMed] [Google Scholar]
- Hoebel B.G., Hernandez L., Schwartz D.H., Mark G.P., Hunter G.A. Microdialysis studies of brain norepinephrine, serotonin, and dopamine release during ingestive behavior. Ann. N. Y. Acad. Sci. 1989;575:171–191. doi: 10.1111/j.1749-6632.1989.tb53242.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto S., Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res. Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Imperato A., Puglisi-Allegra S., Casolini P., Zocchi A., Angelucci L. Stress-induced enhancement of dopamine and acetylcholine release in limbic structures: role of corticosterone. Eur. J. Pharmacol. 1989;165:337–338. doi: 10.1016/0014-2999(89)90735-8. [DOI] [PubMed] [Google Scholar]
- Jhou T.C., Fields H.L., Baxter M.G., Saper C.B., Holland P.C. The rostomedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.W., North R.A. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.C., Hou X., Cook M.N. Effect of exposure to novelty on brain monoamines in C57BL/6 and DBA/2 mice. Physiol. Behav. 1996;59:361–367. doi: 10.1016/0031-9384(95)02010-1. [DOI] [PubMed] [Google Scholar]
- Kalivas P.W., Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- Kalueff A.V., Tuohimaa P. Mouse grooming microstructure is a reliable anxiety marker bidirectionally sensitive to GABAergic drugs. Eur. J. Pharmacol. 2005;508:147–153. doi: 10.1016/j.ejphar.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Kieffer B.L., Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog. Neurobiol. (N. Y.) 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- King D., Zigmond M.J., Finlay J.M. Effects of dopamine depletion in the medial prefrontal cortex on the stress-induced increase in extracellular dopamine in the nucleus accumbens core and shell. Neuroscience. 1997;77:141–153. doi: 10.1016/s0306-4522(96)00421-6. [DOI] [PubMed] [Google Scholar]
- Knobelman D.A., Hen R., Lucki I. Genetic regulation of extracellular serotonin by 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) autoreceptors in different brain regions of the mouse. J. Pharmacol. Exp. Ther. 2001;298:1083–1091. [PubMed] [Google Scholar]
- Kurumaji A., Umino M., Nishikawa T. Effects of nolvelty stress on hippocampal gene expression, corticosterone and motor activity in mice. Neurosci. Res. 2011;71:161–167. doi: 10.1016/j.neures.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Koob G.F. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol. Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Lam D.D., Garfield A.S., Marston O.J., Shaw J., Heisler L.K. Brain serotonin system in the coordination of food intake and body weight. Pharmacol., Biochem. Behav. 2010;97:84–91. doi: 10.1016/j.pbb.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Lammel S., Ion D.I., Roeper J., Malenka R.C. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B.B., Bruchas M.R., Lemos J.C., Xu M., Melief E.J., Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J. Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legault M., Wise R.A. Novelty-evoked elevations of nucleus accumbens dopamine: dependence on impulse flow from the ventral subiculum and glutamatergic neurotransmission in the ventral tegmental area. Eur. J. Neurosci. 2001;13:819–828. doi: 10.1046/j.0953-816x.2000.01448.x. [DOI] [PubMed] [Google Scholar]
- Lever C., Burton S., O'Keefe J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev. Neurosci. 2006;17:111–133. doi: 10.1515/revneuro.2006.17.1-2.111. [DOI] [PubMed] [Google Scholar]
- Lutz P.E., Kieffer B.L. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36:195–206. doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel P., Fantino M. Mesolimbic dopaminergic system activity as a function of food reward: a microdialysis study. Pharmacol. Biochem. Behav. 1996;53:221–226. doi: 10.1016/0091-3057(95)00187-5. [DOI] [PubMed] [Google Scholar]
- Matthes H.W. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- McCullough L.D., Salamone J.D. Anxiogenic drugs beta-CCE and FG 7142 increase extracellular dopamine levels in nucleus accumbens. Psychopharmacology. 1992;109:379–382. doi: 10.1007/BF02245888. [DOI] [PubMed] [Google Scholar]
- McLaughlin J.P., Land B.B., Li S., Pintar J.E., Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J.P., Li S., Valdez J., Chavkin T.A., Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J.P., Marton-Popovici M., Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J. Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhuis P.W., Gastpar M., Scherbaum N. Opiate treatment in depression refractory to antidepressants and electroconvulsive therapy. J Cin Psychopharmacol. 2008;28:593–595. doi: 10.1097/JCP.0b013e31818638a4. [DOI] [PubMed] [Google Scholar]
- Papaleo F., Kieffer B.L., Tabarin A., Contarino A. Decreased motivation to eat in mu-opioid receptor-deficient mice. Eur. J. Neurosci. 2007;25:3398–3405. doi: 10.1111/j.1460-9568.2007.05595.x. [DOI] [PubMed] [Google Scholar]
- Parkinson J.A. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav. Brain Res. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Paronis C.A., Bergman J. Buprenorphine and opioid antagonism, tolerance, and naltrexone-precipitated withdrawal. J. Pharmacol. Exp. Ther. 2011;336:488–495. doi: 10.1124/jpet.110.173823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S., Berridge K.C. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J. Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G.D., Robbins T.W., Everitt B.J. Bilateral intra-accumbens self-administration of d-amphetamine: antagonism with intra-accumbens SCH-23390 and sulpiride. Psychopharmacology. 1994;114:477–485. doi: 10.1007/BF02249339. [DOI] [PubMed] [Google Scholar]
- Pratt W.E., Blackstone K., Connolly M.E., Skelly M.J. Selective serotonin receptor stimulation of the medial nucleus accumbens causes differential effects on food intake and locomotion. Behav. Neurosci. 2009;123L:1046–1057. doi: 10.1037/a0016882. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra A., Andolina D. Serotonin and stress coping. Behavioral Brain Research. 2015;277:58–67. doi: 10.1016/j.bbr.2014.07.052. [DOI] [PubMed] [Google Scholar]
- Rebec G.V., Christensen J.R., Guerra C., Bardo M.T. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997;776:61–67. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- Robinson S.A., Erickson R.L., Browne C.A., Lucki I. A role for the mu opioid receptor in the antidepressant effects of buprenorphine. Behav. Brain Res. 2017;319:96–103. doi: 10.1016/j.bbr.2016.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman M.F., Wheeler R.A., Wightman R.M., Carelli R.M. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat. Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone J.D. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav. Brain Res. 1994;61:117–133. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Salamone J.D., Cousins M.S., McCullough L.D., Carriero D.L., Berkowitz R.J. Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption. Pharmacol. Biochem. Behav. 1994;49:25–31. doi: 10.1016/0091-3057(94)90452-9. [DOI] [PubMed] [Google Scholar]
- Shirayama Y., Ishida H., Iwata M., Hazama G.I., Kawahara R., Duman R.S. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J. Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R., Herz A., Shippinberg T.A. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc. Natl. Acad. Sci. Unit. States Am. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos A.L., Colago E.E.O., Pickel V.M. Cellular sites for dynorphin activation of k-Opioid receptors in the rat nucleus accumbens shell. J. Neurosci. 1999;19:1804–1813. doi: 10.1523/JNEUROSCI.19-05-01804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R., Auerbach S.B. Opioid receptor subtypes differentially modulate serotonin efflux in the rat central nervous system. J. Pharmacol. Exp. Ther. 2002;303:549–556. doi: 10.1124/jpet.102.037861. [DOI] [PubMed] [Google Scholar]
- Thierry A.M., Tassin J.P., Blanc G., Glowinski J. Selective activation of mesocortical DA system by stress. Nature. 1976;263:242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- Tidey J.W., Miczek K.A. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Ungless M.A., Magill P.J., Bolam J.P. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Van't Veer A., Carlezon W.A., Jr. Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology. 2013;229:435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R., Cabib S., Puglisi-Allegra S. Genetic susceptibility of mesocortical dopamine to stress determines liability to inhibition of mesoaccumbens dopamine and to behavioral despair in a mouse model of depression. Neuroscience. 2002;115:999–1007. doi: 10.1016/s0306-4522(02)00581-x. [DOI] [PubMed] [Google Scholar]
- Wanat M.J., Bonci A., Phillips P.E. CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat. Neurosci. 2013;16:383–385. doi: 10.1038/nn.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]