Abstract
Autoimmune pancreatitis (AIP) is now considered a pancreatic manifestation of a newly proposed disease condition, IgG4-related disease (IgG4-RD). IgG4-RD is characterized by enhanced IgG4 antibody responses and multiple organ involvements. Recent epidemiological studies have addressed the incidence of cancer in patients with AIP and/or IgG4-RD. Surprisingly, a significant number of AIP patients were detected with cancer at or within one year of the diagnosis of AIP. Furthermore, around 50% of all cancers detected in AIP patients comprised mainly 3 types (gastric, lung, and prostate cancer). Thus, AIP appears to be associated with cancer of other organs rather than the pancreas itself, which suggests that AIP is not a pre-cancerous condition of the pancreas. Moreover, the simultaneous occurrence of cancer and AIP in many patients has led to the establishment of an attractive concept that AIP might sometimes arise from co-existing cancers as a paraneoplastic syndrome.
Keywords: autoimmune pancreatitis, IgG4-related disease, cancer
Introduction
Autoimmune pancreatitis (AIP) is a unique form of chronic pancreatitis in which autoimmunity against as-yet-unidentified auto antigens (Ags) underlie the chronic fibro-inflammatory responses in the pancreas (1).
AIP is now classified into type 1 AIP and type 2 AIP based on clinical features, pathological findings, and IgG4 antibody (Ab) responses (2). It is generally accepted that type 1 AIP, also called lymphoplasmacytic sclerosing pancreatitis, is a pancreatic manifestation of the systemic IgG4-related disease (IgG4-RD). IgG4-RD is a newly established disease characterized by elevated serum levels of IgG4 Ab and multiple organ involvements (3,4). Massive infiltration of IgG4-expressing plasmacytes into the affected organs, accompanied by storiform fibrosis, is a prominent pathological feature of type 1 AIP (3-5).
Gastroenterologists and clinical immunologists have established the diagnostic criteria based on a substantial expansion of awareness and recognition of IgG4-RD by physicians (3-5). A wide variety of single-organ diseases, such as AIP, sialoadenitis, and retroperitoneal fibrosis, are now regarded as organ-specific manifestations of this systemic autoimmune disorder (3-5). As the number of patients with IgG4-RD and type 1 AIP increases, gastroenterologists and clinical immunologists promote further their investigations of type 1 AIP, and our knowledge regarding the clinicopathological findings is rapidly expanding. At present, however, our knowledge of the clinicopathological findings of type 2 AIP, also called idiopathic duct centric chronic pancreatitis, is limited.
Infiltration of neutrophils, but not IgG4-expressing plasmacytes, is a pathological hallmark of type 2 AIP (5,6). In addition, the co-occurrence of inflammatory bowel diseases is sometimes seen in patients with type 2 AIP. These differences in clinicopathological features between type 1 and type 2 AIP support the idea that two different types of immune responses are involved in the development of AIP, although both diseases are sensitive to corticosteroid treatment.
Chronic inflammatory responses play an indispensable role in the development of inflammation-associated cancer, including Helicobacter pylori (H. pylori)-associated gastric cancer, hepatitis virus-associated hepatocellular carcinoma, and colitis-associated colon cancer (7). The high risk of pancreatic cancer in patients with chronic pancreatitis (CP) suggests the involvement of chronic inflammatory responses (8,9). Given that AIP is a unique form of chronic fibroinflammatory disorder of the pancreas, it is possible that AIP is a pre-cancerous condition. Indeed, recent studies have addressed the relationship between AIP and malignant diseases (10-14). Interestingly, it has been suggested that the presence of AIP is associated with cancers of other organs rather than the pancreas.
In this review, we discuss the risk of cancer in type 1 AIP and IgG4-RD.
Incidence of Cancer in IgG4-RD and AIP
Shiokawa et al. were the first to show that the incidence of cancer after the diagnosis of AIP is significantly higher in patients with AIP than in a sex-, age-, and observation period-matched standard population in a multicenter, retrospective cohort study (10) (Table 1). They investigated the incidence of cancer at or after the diagnosis of AIP in 108 patients. During the follow-up period, 15 AIP patients (13.9%) were diagnosed with cancer. Similar to the findings in earlier studies of IgG4-RD patients (13), the standardized incidence ratio (SIR) for cancer was 2.7, which was significantly higher than that in the general population. Surprisingly, 10 cases were detected at or within 1 year of the diagnosis of AIP. Thus, these data support the idea that the presence of AIP is a strong risk factor for cancer. Such a high incidence of cancer detection within 1 year after the diagnosis was also reproduced in Asano's study in which 9 out of 36 malignancies were detected in patients with IgG4-RD at or within 1 year of the diagnosis (14) (Table 2). Their study included 109 patients with AIP, and 28 patients with AIP developed cancer at or after the diagnosis of AIP (Table 3). Furthermore, a high incidence of cancer in AIP patients with a high level of SIR (17.3) was also reported in a single-center study in Germany (15) (Table 1).
Table 1.
Relationship between Autoimmune Pancreatitis and Cancer.
| Reference | (10) | (11) | (12) | (15) | ||||
|---|---|---|---|---|---|---|---|---|
| Number of patients | 108 | 95 | 116 | 28 | ||||
| Mean observation period (month) | 39.6 | 73.0 | 36.0 | 91.0 | ||||
| Cancer occurrence (%) | 15 (14) | 11 (12) | 19 (16) | 5 (18) | ||||
| SIR | 2.7 | 1.04 | NA | 17.3 | ||||
| Number of cancers | 18 | 12* | 23 | 6 | ||||
| Cancer occurrence | ||||||||
| before the AIP diagnosis | NA | NA | 12/23 | 2/6 | ||||
| at or within one year after the AIP diagnosis | 10/18 | NA | 1/23 | 0/6 | ||||
| more than one year after the AIP diagnosis | 8/18 | NA | 10/23 | 4/6 | ||||
| Type of cancer | Stomach (7) | Lung (4) | Prostate (5) | Breast (2) | ||||
| Lung (3) | Stomach (2) | Lymphoma (5) | Lymphoma (1) | |||||
| Lymphoma (2) | Pancreas (2) | Bladder (4) | Colon (1) | |||||
| Prostate (2) | Bile duct (1) | Renal Cell (2) | Ovary (1) | |||||
| Colon (2) | Tongue (1) | Breast (1) | Bladder (1) | |||||
| Bile duct (1) | Melanoma (1) | Cholangio-carcinoma (1) | ||||||
| Thyroid (1) | Leukemia (1) | Pancreas (1) | ||||||
| Leukemia (1) | ||||||||
| Melanoma (1) | ||||||||
| Ovary (1) | ||||||||
| Salivary (1) | ||||||||
| Number of pancreatic cancers (%) | 0 (0) | 2 (17) | 1 (4.3) | 0 (0) |
AIP: autoimmune pancreatitis, SIR: standardized incidence ratio, NA: not available
* Seven cases with concurrent occurrence of AIP and malignancy were excluded.
Table 2.
Relationship between IgG4-related Disease and Cancer.
| Reference | (13) | (14) | (16) | |||
|---|---|---|---|---|---|---|
| Number of patients | 106 | 158 | 125 | |||
| Mean observation period (month) | 37.2 | 71.4 | NA | |||
| Cancer occurrence (%) | 11 (10) | 34 (22) | 20 (16) | |||
| SIR | 3.83 | 2.01 | 2.51 | |||
| Number of cancers | 11 | 36 | 21 | |||
| Cancer occurrence | ||||||
| before IgG4-RD diagnosis | NA | NA | 21* | |||
| at or within one year after the IgG4-RD diagnosis | NA | 7/34 | NA | |||
| more than one year after the IgG4-RD diagnosis | NA | 27/34 | NA | |||
| Type of cancer | Colon (2) | Lung (5) | Prostate (7) | |||
| Lung (2) | Colon (5) | Lymphoma (4) | ||||
| Lymphoma (2) | Prostate (5) | Breast (2) | ||||
| Prostate (1) | Stomach (4) | Lung (2) | ||||
| Renal cell (1) | Pancreas (4) | Colon (2) | ||||
| Breast (1) | Renal cell (2) | Testis (2) | ||||
| Ovary (1) | Lymphoma (2) | Leukemia (1) | ||||
| Tongue (1) | Bile duct (1) | Uterine (1) | ||||
| Liver (1) | ||||||
| Esophagus (1) | ||||||
| Breast (1) | ||||||
| Ovary (1) | ||||||
| Thyroid (1) | ||||||
| Skin (1) | ||||||
| Tongue (1) | ||||||
| MDS (1) | ||||||
| Number of pancreatic cancers (%) | 0 (0) | 4 (11.1) | 0 (0) |
IgG4-RD: IgG4-related disease, SIR: standardized incidence ratio, MDS: myelodysplatic syndrome, NA: not available
* Patients with IgG4-RD with history of malignancy were analyzed.
Table 3.
Relationship between IgG4-related Autoimmune Pancreatitis and Cancer.
| Reference | (13) | (14) | (16) | |||
|---|---|---|---|---|---|---|
| Number of patients | 10 | 109 | 24* | |||
| Cancer occurrence (%) | 2 (20) | 28 (26) | 4 (17) | |||
| Number of cancers | 2 | 30 | 5 | |||
| Type of cancer | Prostate (1) | Prostate (5) | Prostate (2) | |||
| Renal cell (1) | Lung (4) | Breast (1) | ||||
| Pancreas (4) | Testis (1) | |||||
| Colon (3) | Lymphoma (1) | |||||
| Stomach (3) | ||||||
| Lymphoma (2) | ||||||
| Renal cell (1) | ||||||
| Bile duct (1) | ||||||
| Liver (1) | ||||||
| Breast (1) | ||||||
| Ovary (1) | ||||||
| Thyroid (1) | ||||||
| Skin (1) | ||||||
| Tongue (1) | ||||||
| MDS (1) | ||||||
| Number of pancreatic cancers (%) | 0 (0) | 4 (13.3) | 0 (0) |
IgG4-RD: IgG4-related disease, NA: not available, MDS: myelodysplastic syndrome
* Patients with IgG4-RD with a history of malignancy were analyzed.
Yamamoto et al. also showed that the incidence of cancer after the diagnosis of IgG4-RD is significantly higher in patients with IgG4-RD than in a sex-, age-, and observation period-matched standard population (13). They investigated 106 patients with IgG4-RD, including 10 patients with type 1 AIP, and performed a retrospective observational study to determine the occurrence rate of cancer following the diagnosis of IgG4-RD. They reported that 11 of the 106 IgG4-RD patients developed cancer and that the SIR was 3.83. In addition, 2 of the 10 AIP patients were diagnosed with renal cell cancer and prostate cancer during the follow-up period (Table 3). Based on these data, Yamamoto et al. concluded that IgG4-RD might increase the risk of cancer. Consistent with this finding, Asano et al. reported that 34 of 158 IgG4-RD patients developed cancer and that the SIR of IgG4-RD was 2.01 (14). In addition to the these reports providing evidence that the presence of AIP and/or IgG4-RD increase the risk of cancer after the diagnosis, one report showed an association between IgG4-RD and a history of malignancy (16) (Table 2, 3). Wallace et al. analyzed the presence or absence of a history of malignancy in patients with IgG4-RD and reported that 20 patients with IgG4-RD (16%) had a history of malignancy (16). Therefore, it may be possible that not only does the presence of IgG4-RD and/or AIP increase the risk of cancer but also that the pre-existing malignant disorders are associated with the subsequent development of IgG4-RD and/or AIP (16).
In contrast to the aforementioned studies, two recent studies failed to confirm the association between AIP and cancer (Table 1). Hirano et al. analyzed 113 patients with IgG4-RD, including 95 AIP cases, to determine the occurrence rate of cancer during the follow-up period (11). Although 14 patients (12.4%) were diagnosed with cancer during the follow-up period, the SIR of cancer (1.04) was not statistically significant. Thus, the presence of AIP was not identified as a risk factor for cancer in their study. In agreement with the findings of this study, Hart et al. reported that the cancer risk before and after the diagnosis of AIP was similar to that in the control subjects (12) (Table 1).
Although the reasons for such discrepancy have not been fully elucidated, two possibilities have been discussed (12,17). One possibility is related to dealing with patients in whom cancer and AIP occur concurrently. In the study by Shiokawa et al., eight cases were detected at the diagnosis of AIP (10). This simultaneous occurrence of cancer and AIP is one of the most striking findings in their study. Although Hirano's study also detected seven cases with the simultaneous occurrence of cancer and IgG4-RD, they regarded these cases as institutional bias and excluded them from their investigation (11). Since the aim of Hirano's study was to clarify the oncogenic effects in the presence of established IgG4-RD, they tried to address whether preceding IgG4-RD and/or type 1 AIP could cause malignant disorders. Therefore, the discrepancy between Shiokawa's and Hirano's studies can be partially explained by the fact that significant numbers of cases with concurrent cancer and AIP were included in the former study (10) whereas such cases were excluded in the latter study (12,17). Consistent with this idea, the SIR for cancer was much lower in cancer cases detected at more than one year after the diagnosis of AIP than in those detected within one year (10). Another possibility is related to the routine use of extensive screening tests for cancer in Japan, including positron emission tomography (PET) and endoscopic examinations. Hart et al. showed that the cancer risk before and after the diagnosis of AIP was similar to that of control subjects (12). In Japan, PET is widely used for the detection and evaluation of multiple organ involvement in IgG4-RD and AIP. Thus, as Hart et al. point out (12), the routine use of several kinds of screening tests might increase the detection rate of malignancy at the diagnosis of IgG4-RD and/or AIP in Japan. Taken together, these findings support the association between cancer and AIP if cases with simultaneous occurrence of type 1 AIP and cancer are included in the analysis.
Is Type 1 AIP a Pre-cancerous Condition?
It is well established that long-standing chronic inflammation plays a critical role in the development of cancer through the process of inflammation-associated carcinogenesis (7). If AIP enhances the risk of cancer, then the next question is whether proceeding AIP promotes the development of malignancy through inflammation-associated carcinogenesis.
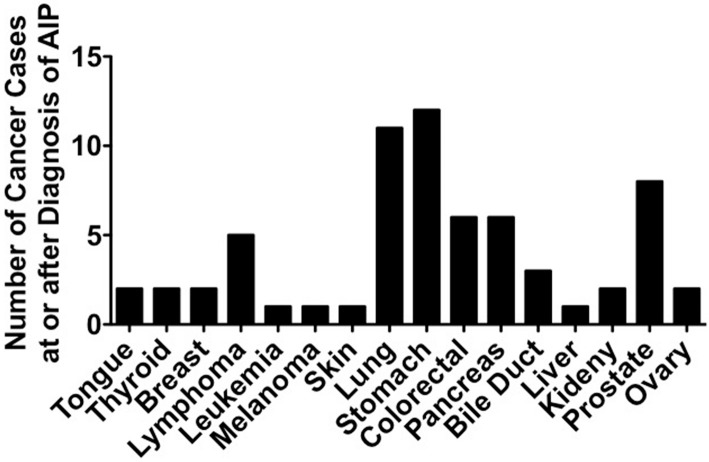
Several case reports regarding the co-existence of AIP and pancreatic cancer are available (18,19). One single-center study showed that the incident rate of pancreatic cancer after the diagnosis of AIP or CP was comparable between the two groups (4.8% vs. 2.4%) (20). Given that CP is one of the strongest risk factors for the development of pancreatic cancer (21), surveillance for the development of pancreatic cancer might also be required for patients with AIP. However, in contrast to these previous findings, the incidence of pancreatic cancer at or after the diagnosis of AIP is not very high, ranging from 0.0% to 17% among all of the detected cancers (Table 1, 2). It therefore seems less likely that AIP increases the risk of pancreatic cancer through inflammation-associated carcinogenesis. To clarify the relationship between AIP and pancreatic cancer, we investigated the types of cancer in patients with AIP at or after the diagnosis of AIP in five reports (10,11,13-15), in which types of cancers at or after the diagnosis of AIP were fully described. As shown in Figure, gastric, lung and prostate cancer are frequently detected cancers in patients with AIP and account for around 50% of all cancers detected at or after the diagnosis of AIP. The numbers of pancreatic cancer cases are smaller than those of gastric, lung, and prostate cancers. These data regarding cancer distribution strongly suggest that the presence of AIP might be more closely associated with extra-pancreatic (gastric, lung, and prostate) cancers rather than pancreatic cancer. Thus, it is unlikely that the presence of AIP promotes the development of pancreatic cancer through inflammation-associated carcinogenesis.
Figure.
Types of malignancies in patients with autoimmune pancreatitis. The numbers of malignant diseases were obtained from five reports that addressed the incidence of cancers at or after the diagnosis of AIP (10, 11, 13-15).
At present, the mechanism underlying the association of AIP and carcinogenesis of the extra-pancreatic organs, such as the stomach, lung, and prostate rather than the pancreas itself, remains unknown. Shiokawa et al. analyzed the expression of IgG4 in cancer tissues in AIP patients with cancer (10). Interestingly, an abundant infiltration of IgG4-expressing plasma cells was seen in the cancer tissues in six cases. Thus, the cancer and pancreatic tissues of AIP patients with cancer might share key immune responses leading to the enhancement of IgG4 Ab production. This interesting finding by Shiokawa et al. provides new insights into the pathogenesis of the co-occurrence of AIP and cancer (10). One possibility is that pancreatic tissue of AIP bearing abundant IgG4-expressing plasmacytes may function as a reservoir of immune cells necessary for IgG4 Ab production. In this scenario, the enhancement of IgG4 Ab responses arises from the pancreas, and IgG4-expressing plasma cells then migrate to the cancer tissue. Another possibility is that the enhancement of IgG4 Ab responses arises from the cancer tissues, and then IgG4-expressing plasma cells cause AIP by migrating to the pancreatic tissue. The identification of the primary sites for enhanced IgG4 Ab responses might provide new insights into the pathogenesis of cancer in AIP patients.
One question that needs to be addressed in Shiokawa's study is whether or not the extra-pancreatic occurrence of cancer in patients with AIP is associated with long-standing inflammation related to IgG4-RD. Given that the stomach, lung, and prostate are candidate target organs of IgG4-RD, extra-pancreatic inflammation related to IgG4-RD may underlie cancer development. Although Shiokawa et al. have demonstrated the presence of IgG4-expressing plasmacytes in cancer tissues of AIP patients, they have not described the chronic inflammation characteristics of IgG4-RD in cancer tissue.
In addition to IgG4 Ab responses, K-ras gene mutations might be involved in the development of gastrointestinal tract cancer in AIP patients. Kamisawa et al. examined K-ras mutations in the gastrointestinal mucosa of patients with AIP (22). Interestingly, K-ras mutations were detected in the gastrointestinal tract in a significant population of AIP patients. Conversely, a mutational K-ras analysis in endoscopic ultrasound-guided fine needle aspiration samples of pancreatic tissue revealed that none of the AIP cases exhibited significant K-ras mutations, whereas most cases of pancreatic cancer bear K-ras mutations (23). Thus, the status of K-ras mutations also supports the idea that the presence of AIP may be associated with cancer of the gastrointestinal tract rather than the pancreas.
Although recent studies have highlighted the association of AIP with extra-pancreatic rather than pancreatic carcinogenesis, we need to be cautious regarding the interpretation of these data. Gastric, lung, and prostate cancer are frequently detected malignancies in patients with AIP and account for around 50% of all cancers detected at or after the diagnosis of AIP. H. pylori infection of the stomach and cigarette smoking are the strongest risk factors for gastric and lung cancers, respectively (24,25). In addition, patients with AIP and IgG4-RD are typically men and elderly, as in the case of prostate cancer (3-5,26).
At present, there are no studies that directly compare the incidence of gastric, lung, and prostate cancers between patients with AIP/IgG4-RD and risk factor-matched controls. Thus, the limitations of the recent epidemiological studies are the lack of analyses of cancer risk factors in both AIP/IgG4-RD patients and control subjects. It is therefore too early to establish the concept that pre-exiting AIP increases the risk of malignancies in the extra-pancreatic organs but not the pancreas itself. Future prospective studies addressing the incidence of pancreatic cancer in AIP patients are absolutely required to confirm this idea.
Is Type 1 AIP a Paraneoplastic Syndrome?
Two Japanese studies reported that a significant population of patients with type 1 AIP already had cancer at the time of the AIP diagnosis (10,11). We must therefore consider the possibility of co-existing cancer upon the diagnosis of AIP. According to the studies by Shiokawa et al., 10 cancer cases were diagnosed at or within 1 year of the AIP diagnosis (10). Strikingly, eight cancer cases were detected at the diagnosis of AIP. A high incidence of cancer early after the diagnosis of AIP was also reported in Asano's study (14). Based on these data, Shiokawa et al. proposed that pre-existing cancer might evoke IgG4-related type 1 AIP and that type 1 AIP can occur as an autoimmune paraneoplastic disease. They therefore speculate that as-yet-undisclosed immune responses, caused by the malignant tumors, might underlie the immuno-pathogenesis of simultaneous occurrence of type 1 AIP and cancer. A high incidence of cancer in AIP patients early after the diagnosis can be fully explained by this novel concept. This idea also supports the findings of Wallace et al., who showed an association between pre-existing malignant disorders and the subsequent development of IgG4-RD (16). As in the case of other autoimmune paraneoplastic disorders, such as polymyositis and dermatomyositis (27,28), remission of AIP has been achieved after the successful treatment of co-existing cancer (10). Collectively, Shiokawa et al. propose a very attractive concept that type 1 AIP and IgG4-RD can occur as a paraneoplastic syndrome. Confirmation of this new concept awaits future prospective studies that address the time window of cancer occurrence in a large number of patients with AIP and IgG4-RD. In addition, elucidation of the molecular mechanisms underlying how co-existing cancer induces AIP and/or IgG4-RD as a paraneoplastic syndrome will help strengthen this concept.
Conclusion
The presence of AIP may be associated with the risk of cancers of extra-pancreatic organs, such as the stomach, lung, and prostate. However, the presence of AIP and/or IgG4-RD may not promote the development of pancreatic cancer. The large number of AIP patients in whom cancers are detected at or within one year of the AIP diagnosis strongly suggests that AIP and/or IgG4-RD sometimes arise as a paraneoplastic syndrome. Confirmation of these findings awaits future-prospective studies in which the incidences of cancer in patients with AIP and/or IgG4-RD are directly compared with those in age-, sex-, and risk factor-matched control subjects.
This article is a review based on retrospective clinical data obtained from human patients.
This study does not involve human subjects and does not require informed consent.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported by the Naito Foundation, SENSHIN Medical Research Foundation, Yakult Bio-Science Foundation, Smoking Research Foundation, Kobayashi Foundation for Cancer Research, Takeda Science Foundation, and Japan Agency for Medical Research and Development (AMED) Grants for Research on Intractable Diseases.
References
- 1.Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med 355: 2670-2676, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Chari ST, Lerch MM, Kim MH, Gress TM, Shimosegawa T. Recent advances in autoimmune pancreatitis: type 1 and type 2. Gut 62: 1373-1380, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol 41: 613-625, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med 366: 539-551, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Hart PA, Zen Y, Chari ST. Recent advances in autoimmune pancreatitis. Gastroenterology 149: 39-51, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Mitsuyama T, Uchida K, Sumimoto K, et al. . Comparison of neutrophil infiltration between type 1 and type 2 autoimmune pancreatitis. Pancreatology 15: 271-280, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology 143: 550-563, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Bansal P, Sonnenberg A. Pancreatitis is a risk factor for pancreatic cancer. Gastroenterology 109: 247-251, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Malka D, Hammel P, Maire F, et al. . Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut 51: 849-852, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiokawa M, Kodama Y, Yoshimura K, et al. . Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol 108: 610-617, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Hirano K, Tada M, Sasahira N, et al. . Incidence of malignancies in patients with IgG4-related disease. Intern Med 53: 171-176, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Hart PA, Law RJ, Dierkhising RA, Smyrk TC, Takahashi N, Chari ST. Risk of cancer in autoimmune pancreatitis: a case-control study and review of the literature. Pancreas 43: 417-421, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M, Takahashi H, Tabeya T, et al. . Risk of malignancies in IgG4-related disease. Mod Rheumatol 22: 414-418, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Asano J, Watanabe T, Oguchi T, et al. . Association between immunoglobulin G4-related disease and malignancy within 12 years after diagnosis: an analysis after longterm followup. J Rheumatol 42: 2135-2142, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Schneider A, Hirth M, Munch M, et al. . Risk of cancer in patients with autoimmune pancreatitis: a single-center experience from germany. Digestion 95: 172-180, 2017. [DOI] [PubMed] [Google Scholar]
- 16.Wallace ZS, Wallace CJ, Lu N, Choi HK, Stone JH. Association of IgG4-related disease with history of malignancy. Arthritis Rheumatol 68: 2283-2289, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirano K, Isayama H, Tada M, Koike K. Association between autoimmune pancreatitis and malignancy. Clin J Gastroenterol 7: 200-204, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Fukui T, Mitsuyama T, Takaoka M, Uchida K, Matsushita M, Okazaki K. Pancreatic cancer associated with autoimmune pancreatitis in remission. Intern Med 47: 151-155, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Loos M, Esposito I, Hedderich DM, et al. . Autoimmune pancreatitis complicated by carcinoma of the pancreatobiliary system: a case report and review of the literature. Pancreas 40: 151-154, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Ikeura T, Miyoshi H, Uchida K, et al. . Relationship between autoimmune pancreatitis and pancreatic cancer: a single-center experience. Pancreatology 14: 373-379, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe T, Kudo M, Strober W. Immunopathogenesis of pancreatitis. Mucosal Immunol 10: 283-298, 2017. [DOI] [PubMed] [Google Scholar]
- 22.Kamisawa T, Horiguchi S, Hayashi Y, et al. . K-ras mutation in the major duodenal papilla and gastric and colonic mucosa in patients with autoimmune pancreatitis. J Gastroenterol 45: 771-778, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Khalid A, Dewitt J, Ohori NP, et al. . EUS-FNA mutational analysis in differentiating autoimmune pancreatitis and pancreatic cancer. Pancreatology 11: 482-486, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Asano N, Imatani A, Watanabe T, et al. . Cdx2 expression and intestinal metaplasia induced by H. pylori infection of gastric cells is regulated by NOD1-mediated innate immune responses. Cancer Res 76: 1135-1145, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornfield J, Haenszel W, Hammond EC, Lilienfeld AM, Shimkin MB, Wynder EL. Smoking and lung cancer: recent evidence and a discussion of some questions. Int J Epidemiol 38: 1175-1191, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Merriel SWD, Funston G, Hamilton W. Prostate cancer in primary care. Adv Ther 35: 1285-1294, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah AA, Casciola-Rosen L, Rosen A. Review: cancer-induced autoimmunity in the rheumatic diseases. Arthritis Rheumatol 67: 317-326, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giat E, Ehrenfeld M, Shoenfeld Y. Cancer and autoimmune diseases. Autoimmun Rev 16: 1049-1057, 2017. [DOI] [PubMed] [Google Scholar]