Abstract
Objective
The fecal occult blood (FOB) test is commonly used for colorectal cancer screening; however, it is uncertain if further diagnostic interventions, such as a colonoscopy, should be performed based on its results.
Method
To better understand patient behavior following the FOB test, 6,414 patients (3,807 men and 2,607 women) who underwent colonoscopy between August 2015 and March 2016 at any of the 26 medical institutions throughout Hiroshima Prefecture were invited to participate in the study. All patients provided their written consent, after which they completed a questionnaire, and their colonoscopy results were obtained. These datasets were analyzed in a blinded manner, and the unique codes linking the records were revealed at the end of the analysis.
Results
Of the total study population, 4,749 patients (74.0%) had previously undergone FOB testing. After classification of common behavioral responses that the patients displayed following their FOB test, the group who had undergone the test several times, who had not had positive test results in the past, and whose latest FOB test results were positive had a significantly higher diagnosis rate of both early- and advanced-stage cancer than the other groups. Furthermore, patients in whom several previous FOB test results had been negative whose previous colonoscopy was positive were associated with a higher diagnosis rate of early-stage cancer than other groups.
Conclusion
These results suggested that colonoscopy should be performed immediately for patients with positive FOB test results due to their association with colorectal cancer and the possible detection of cancer at an early stage.
Keywords: colorectal cancer, fecal occult blood test
Introduction
In Japan, colorectal cancer (CRC) has become the leading cause of cancer mortality in women and the third-leading cause in men; however, the rate of CRC screening and close examination has not exceeded 70%. Furthermore, in Hiroshima Prefecture, these rates remain stubbornly below 70%. As cancer mortality is significantly reduced when cancer is detected and treated early, we examined the common behavioral patterns following a standard CRC screening using the fecal occult blood (FOB) test and explored which patterns led to the best patient outcomes. A better understanding of these factors can help design programs to increase the early detection of CRC among individuals undergoing screening through education as well as to increase the rates of CRC screening.
Materials and Methods
A retrospective cohort study was designed with the patient population including all patients who underwent colonoscopy at the 26 medical institutions in Hiroshima Prefecture, Japan, from August 2015 to March 2016. Following the provision of written informed consent, patients were administered a questionnaire, and their colonoscopy results were obtained. When lesions were detected on colonoscopy, benign tumors were diagnosed with the help of a biopsy or diagnostic imaging. Malignant tumors, both early- and advanced-stage, were diagnosed based on the histopathology of either the resected specimen in operated cases (surgical or endoscopic treatment) or a biopsy in unresectable cases.
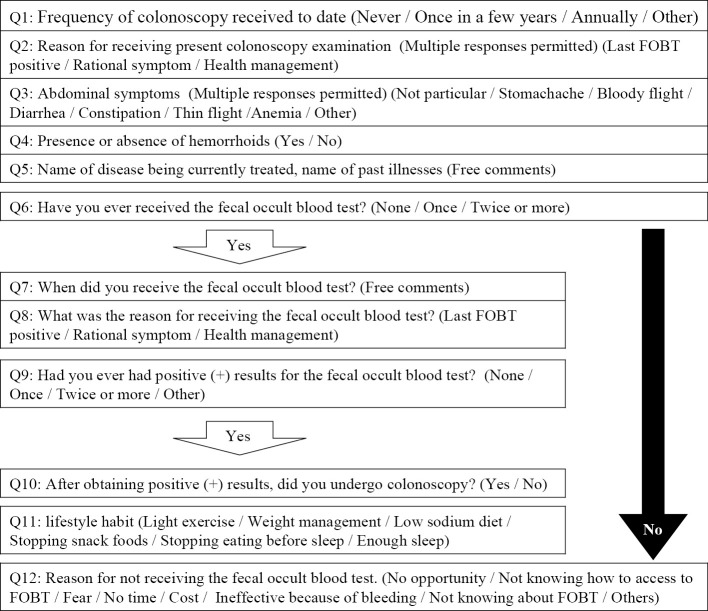
The questionnaire included 12 multiple-choice questions regarding circumstances, subjective symptoms, and occurrence of hemorrhoids prior to colonoscopy. Patients who previously had had a positive FOB test (immunochemical) results were asked about the number of tests conducted, and if recommended, colonoscopy was performed. The detailed contents of the questionnaire survey are shown in Fig. 1. Patient data were also used to measure the time interval from the date of colonoscopy to either the date of the patient’s last FOB test performed for routine CRC screening or the appearance of the first clinical reason requiring the patient to undergo colonoscopy.
Figure 1.
Content of the questionnaire survey.
The trends in the age for prevalence of advanced colon cancer or early colon cancer were analyzed by the Cochran-Armitage trend test. A chi-squared test and post-hoc analysis were used to compare different behavioral circumstances following an FOB test. A multivariate logistic regression analysis model was used to assess the age- and gender-adjusted risk of advanced colon cancer under different behavioral circumstances following an FOB test. Statistical analyses were performed with the JMP software program, version 11 (SAS Institute, Cary, USA). P values less than 0.05 were considered significant.
The study was conducted with the approval of the ethics review board of Hiroshima University and the ethics review boards of each participating institution. This study also formed part of the Hiroshima Prefecture Cancer Screening Accuracy Management Promotion Project.
Results
Study population and demographics
During the study period, the 26 participating medical institutions had 6,789 patients who underwent colonoscopy. From these, 6,414 patients met all the study inclusion criteria (written consent to participate, completed study questionnaire, and corresponding colonoscopy result). Demographically, there were approximately 1.5 times more men (n=3,807) than women (n=2,607) in the study population. Most patients (approximately 60%) who participated in the study were 60-80 years of age (Table).
Table.
Sex and Age Class of Patients.
| n (%) | ||||||
|---|---|---|---|---|---|---|
| Male | 3,807 | |||||
| Female | 2,607 | |||||
| Age | 39> | Male | 166 (4.4) | |||
| Female | 152 (5.8) | |||||
| 40-49 | Male | 364 (9.6) | ||||
| Female | 287 (11.0) | |||||
| 50-59 | Male | 594 (15.6) | ||||
| Female | 378 (14.5) | |||||
| 60-69 | Male | 1,197 (31.4) | ||||
| Female | 769 (29.5) | |||||
| 70-79 | Male | 1,148 (30.2) | ||||
| Female | 767 (29.4) | |||||
| 80< | Male | 338 (8.9) | ||||
| Female | 254 (9.7) |
Questionnaire results
In the patient population at the time of questionnaire completion, 30.5% of patients had never previously undergone colonoscopy, 27.6% had undergone colonoscopy once every 2-3 years, and 20.4% had undergone colonoscopy annually. The clinical rationale for the current colonoscopy request in half of the participants was a positive FOB test result during routine screening for CRC (50.2%), followed by referrals from general practitioners for a further examination (29.8%), the presence of a reported subjective symptom (16.9%), and overall patient health management without subjective symptoms (15%). Current abdominal symptoms were absent in most patients (32.5%), followed by constipation (19.7%), diarrhea (9.8%), abdominal pain (8.4%), and bloody stool (8.4%).
FOB testing was prevalent within the population, with 4749 patients (74.0%) having undergone the test at least once. This was most commonly performed based on screening for CRC (76.5%), general practitioner recommendation (16.0%), and overall patient health management (11.1%). For the remaining 1,665 patients (26.0%) who had never undergone this test, the following reasons were provided: not finding a reason to undergo such a test (55.3%), lack of knowledge about the test (21.4%), and considering it to be cumbersome (8.6%).
Of the 2,464 patients who had previously undergone an FOB test, 320 (13%) reported not undergoing colonoscopy despite being aware of the need to follow up after a positive test result by undergoing a closer examination. This was typically attributed to the patient being very busy (28.1%), a belief that the positive test results were due to hemorrhoids (21.3%), a negative FOB test result (15.0%), and unwillingness to undergo colonoscopy (14.7%). Of these 320 patients, 169 had never undergone colonoscopy, 50 underwent colonoscopies once every few years, and 101 did not respond or were unsure. Subjective symptoms were present in 155 of the 320 patients, while 202 patients had positive FOB test results once, 115 had positive FOB test results twice, and 3 had other results.
The analysis of colonoscopy findings
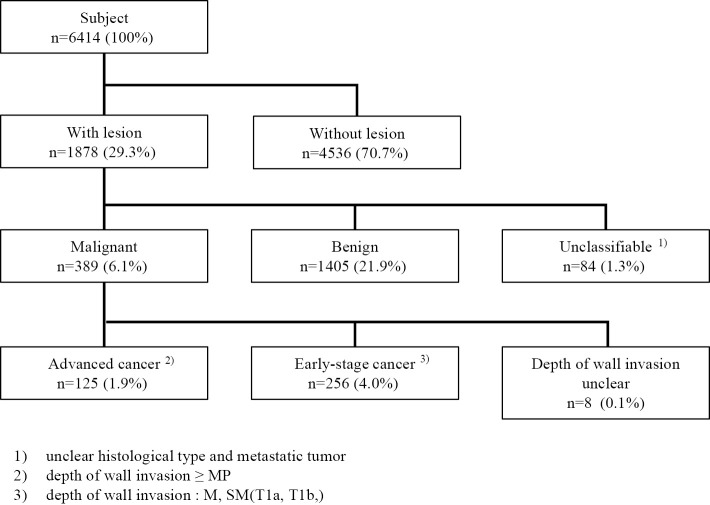
Using the general algorithm (Fig. 2), we analyzed and classified the colonoscopy results of 6,414 patients. Adenocarcinomas and carcinoid tumors were considered malignant and were further split into early- and advanced-stage cancer according to the depth of wall invasion; 125 patients had advanced-stage cancer, and 256 had early-stage cancer. A breakdown of lesions revealed malignant lesions in 389 patients (6.1%), of which early-stage cancer was observed in 256 (4.0%) and advanced-stage cancer in 125 (1.9%).
Figure 2.
Colonoscopy flow chart to identify lesions.
Regardless of staging, there were no significant differences in the incidence of CRC between the sexes. The proportion of patients with advanced-stage CRC significantly increased with age (trend p=0.0107) (Fig. 3).
Figure 3.
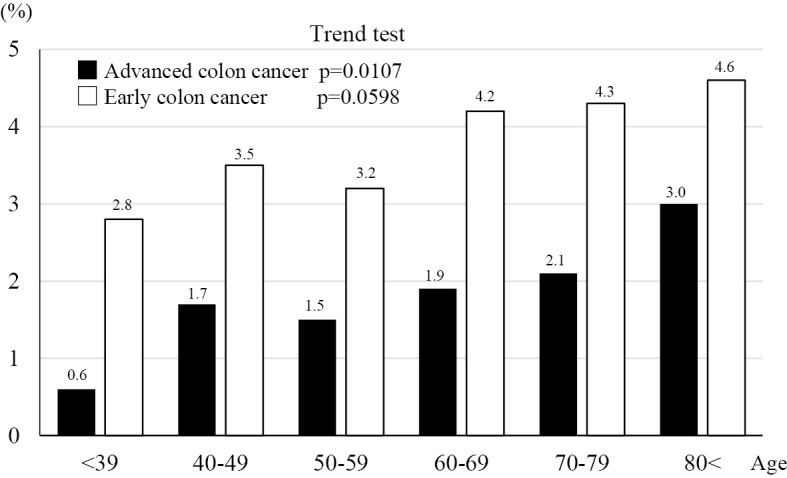
The proportion of patients with advanced or early stage colon cancer.
Classifying behavioral responses following FOB test
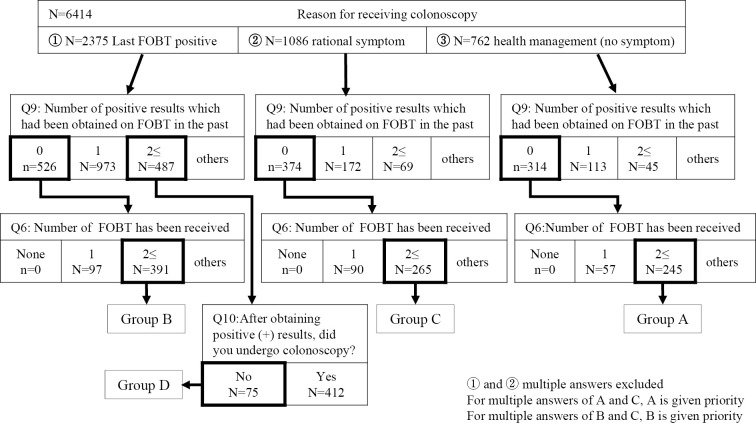
Next, we identified and grouped the common behavioral responses that, when exhibited after the FOB test, resulted in a follow-up colonoscopy being performed (Fig. 4).
Figure 4.
Classification of the behavioral circumstances following fecal occult blood testing.
A. For overall health management of the patient, despite negative results in several FOB tests and no subjective symptoms
B. For the further diagnosis of a patient with a positive FOB test result, despite having negative test results previously and no subjective symptoms
C. For the further diagnosis of a patient with active subjective symptoms, despite negative test results in several FOB test attempts
D. For the further diagnosis of a patient with a positive FOB test result, despite previous positive FOB test results not being followed up by a closer examination and no subjective symptoms.
When the colonoscopy and questionnaire data sets were combined to create this model, the distribution of CRC cases was distinct among the four groups. Group D (75 patients), in which colonoscopy was only performed due to a current positive FOB test result and failure to act on previous FOB results, had a significantly higher rate of early and advanced CRC than those in other groups. Even in the absence of subjective symptoms, repeated positive FOB test results may indicate the presence of a malignant lesion and should be investigated further. Group B (391 patients), which had positive results when the FOB test was administered for the first time but no subjective symptoms, was found to considerably benefit from undergoing a close examination, as the detection rate of early-stage cancer was high at 5.6% (22 patients), whereas that of advanced-stage cancer was low at 0.8% (3 patients). Group C (265 patients), which had had several previous negative FOB test results but presented with subjective symptoms and therefore were sent for a further examination, had a detection rate of early-stage cancer of 1.9% (5 patients) and that of advanced-stage cancer of 1.5% (4 patients). Based on the multivariate logistic regression analysis, the risk of advanced-stage cancer was significantly higher in group D than in group B after adjusting for age and sex (adjusted odds ratio 17.3, 95% confidence interval 4.5-85.4, p<0.0001) (Fig. 5).
Figure 5.
Lesion distribution according to different behavioral circumstances following FOBT. A: For overall health management of the patient, despite negative results in several FOB tests and absence of subjective symptoms. B: For further diagnosis of a patient with a positive FOB test result, despite having negative test results previously and absence of subjective symptoms. C: For further diagnosis of a patient with active subjective symptoms, despite negative test results in several FOB test attempts. D: For further diagnosis of a patient with a positive FOB test result, despite previous positive FOB test results not followed up by closer examination and absence of subjective symptoms. *** p<0.001, ** p<0.01
Discussion
From 1995 in Japan, the age-adjusted mortality due to CRC began to slowly decline; however, this trend seems to have reversed in recent years. Furthermore, with the aging of the general population of Japan, the number of individuals with CRC will continue to increase. Fortunately, CRC is completely treatable but shows a favorable prognosis only if it is detected and treated early. According to the Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines, if a patient is diagnosed with CRC below stage II and without lymph node or distal metastasis, a 5-year survival rate of approximately 85% can be expected (1). Therefore, the most effective way of reducing the mortality from CRC is the utilization of this characteristic of CRC, with efforts focused on secondary prevention (early detection and early treatment).
Based on the evidence collected from randomized controlled trials (RCTs), the only diagnostic methods that have been shown to effectively curtail CRC-associated mortality were FOB testing and colonoscopy of the sigmoid colon (2-6). The highly accurate results of a large subject sample in a cohort study have provided evidence that colonoscopy greatly reduces CRC-associated mortality (7,8). Furthermore, several RCTs conducted to assess chemical methods of FOB testing have consistently demonstrated that FOB screening lowers the CRC-associated mortality due to earlier diagnoses. Although not used in a formal RCT, the effectiveness of immunochemical FOB testing (FIT) developed in Japan has been substantiated by data published in many reports. The findings indicated that FIT had higher sensitivity than that of chemical methods and was found to be superior to other chemical methods (9-13).
Many countries recommend a screening interval of 1-2 years for FOB testing (14). In Japan, since 1992, CRC screening has been performed for the entire Japanese population ≥40 years of age at 1-year intervals using FIT due to cost-effectiveness, accuracy, and safety. In recent years, screening programs for CRC have been provided in more diverse forms, such as voluntary screenings, health checkups, and individual/workplace health checkups.
However, despite the impact it has on CRC mortality, population-based screening is problematic because there is a major difference in the rate of screening among prefectures; the overall rate of screening is low at approximately 20%, and the rate of close examinations of patients with positive FOB test results is low. In Japan, the average annual rate of positive FOB test results is approximately 7%, and only approximately 60-70% of individuals with such results undergo colonoscopy for a closer examination. In those patients, CRC is detected in approximately 4-6% of cases (15). Furthermore, in Hiroshima Prefecture, the rate of screening and close examinations for CRC has not exceeded 70%, despite the high mortality attributed to CRC. Reasons for not having undergone colonoscopy despite having a positive FOB result included lack of time and the perceived difficulty of the pretreatment procedure. Reports indicate that computed tomography colonography enabled the detection of lesions in patients with a positive FOB result with the same sensitivity and specificity as colonoscopy (16). Thus, investigations using modalities other than endoscopy should be considered.
Based on the results of this study, individuals who underwent CRC screening had also undergone FOB tests in addition to health checkups and community screening programs. However, 13% of individuals do not undergo colonoscopy despite having a positive FOB test result; it is important to find ways to increase the rate of colonoscopy in this patient population. Based on the analysis of motivating factors in common behavioral patterns following FOB testing in the present study, we suggest that the possibility of malignant lesions should be considered when FOB test results are repeatedly positive, even in the absence of subjective symptoms. A striking result of this study was that early-stage CRC was detected in several patients who previously had had negative FOB test results that subsequently became positive. There are reports mentioning cases of early detection of CRC through the additional measurement of transferrin, which is more stable in feces than in hemoglobin, along with an FOB test (17). Bleeding is commonly seen from an early stage of CRC, and the amount of bleeding is thought to increase as the cancer progresses. Therefore, our study indicates that many patients who develop positive FOB test results after several negative FOB test results may have early-stage CRC. Furthermore, when negative FOB test results subsequently become positive, colonoscopy should be performed without delay; if not performed, the individual will be at a significantly higher risk of both early- and advanced-stage CRC.
Conclusion
For the early detection of CRC, if the results of the FOB test are positive, a colonoscopy examination should be immediately performed. This procedure should be followed for the early detection of CRC, irrespective of the presence of subjective symptoms or the patient's schedule. No previous studies have analyzed the prevalence of CRC according to behavioral actions and motivating factors following FOB testing, and we believe that broad awareness of these study results will help to improve the rate of screening and close examination for CRC.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank the following doctors for participating in this study: Yoji Sanomura (Hiroshima University Hospital), Yuko Hiraga (Hiroshima Prefectural Hospital), Toru Kawamura (Kawamura Hospital), Shinji Nagata (Hiroshima City Asa Citizens Hospital), Masaki Kunihiro (Hiroshima City Hiroshima Citizens Hospital), Hiroyuki Kanao (Hiroshima Red Cross Hospital&Atomic-bomb Survivors Hospital), Toshio Kuwai (National Hospital Organization Kure Medical Center and Chugoku Cancer Center), Shosuke Kitamura (Chugoku Rosai Hospital), Seiji Onogawa (JA Onomichi General Hospital), Masaharu Sumii (Hiroshima Memorial Hospital), Yoshiaki Matsumoto (Kure Medical Association Hospital), Tsuyoshi Kuroda (Mazda Hospital), Shiro Okamoto (Kure Kyosai Hospital), Tatsuro Tanimoto (Saiseikai Hiroshima Hospital), Hiroko Todo (Hiroshima-Nishi Medical Center), Ryo Yamauchi (Mitsubishi Mihara Hospital), Hiroshige Hamada (Higashihiroshima Medical Center), Sonde Cho (Miyoshi Central Hospital), Kim Sunjin (Chuden Hospital), Tomohiko Kono (JA Yoshida General Hospital), Shigeto Yoshida (JR Hiroshima Hospital), Hironao Komatsu (JA Hiroshima General Hospital), Morihisa Akagi (Akitsu Prefectural Hospital), Katsutoshi Tsuga (Saiseikai Kure Hospital), Kayoko Kunihiro (Hiroshima City Funairi Citizens Hospital), Masahiro Kawanishi (Hiroshima Atomic Bomb Casualty Council).
References
- 1.Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncology 20: 207-239, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 369: 1106-1114, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 366: 2345-2357, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 328: 1365-1371, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 348: 1472-1477, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 375: 1624-1633, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 366: 687-696, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 369: 1095-1105, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 135: 82-90, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Saito H. Screening for colorectal cancer by immunochemical fecal occult blood testing. Japanese journal of cancer research. Gann 87: 1011-1024, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakama H, Kamijo N. Accuracy of immunological fecal occult blood testing for colorectal cancer screening. Prevent Med 23: 309-313, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology 129: 422-428, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med 334: 155-159, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Benson VS, Atkin WS, Green J, et al. Toward standardizing and reporting colorectal cancer screening indicators on an international level: The International Colorectal Cancer Screening Network. Int J Cancer 130: 2961-2973, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Matusda T, Sekiguchi M, Kakugawa Y, et al. Colorectal cancer prevention and early diagnosis using endoscopy. Nihon Shokakibyo Gakkai Zasshi (Jpn J Gastro-entrol) 113: 1176-1185, 2016(in Japanese). [DOI] [PubMed] [Google Scholar]
- 16.Umashima K, Fujiwara T, Wada A, et al. Diagnostic accuracy of computed tomography colonography in patients with a positive fecal occult blood test. J Gastrointest Cancer Screening 56: 120-129, 2018(in Japanese). [Google Scholar]
- 17.Oshima Y, Yamashita K, Nakajima M, et al. [Utility of transferrin measurement in faeces using two-step determination method of fecal occult blood test in colon cancer screening]. Rinsho to Kenkyu (Jpn J Clin Exp Med) 91: 1076-1080, 2014(in Japanese). [Google Scholar]